Abstract
A 9‐year‐old castrated male poodle dog was presented with icterus, anorexia, and lethargy. The dog was diagnosed with hypothyroidism 1 month before and was treated with levothyroxine. Severe anaemia with spherocytes, positive saline agglutination test, and hyperbilirubinemia indicated immune‐mediated haemolytic anaemia (IMHA). Therefore, immunosuppressive therapy with prednisolone, mycophenolate mofetil, and danazol was started. Although the IMHA was well controlled, during tapering of prednisolone, acute multiple joint swelling and oedema suspected immune‐mediated polyarthritis occurred twice. First, clinical symptoms improved as the dosage of prednisolone increased. However, the dog showed severe adverse effects to the steroid. Second time, we added leflunomide as another immunosuppressant, and clinical signs of arthritis disappeared. About 3 weeks later, despite the immunosuppressive therapy, skin lesions resembling an autoimmune dermatologic disorder spread throughout the body. Addition of cyclosporine resolved the skin lesions. This is a case report of a dog showing several sporadic clinical signs related to multiple autoimmune syndromes and their management using different immunosuppressant drugs.
Keywords: dog, immune‐mediated haemolytic anaemia, immune‐mediated polyarthritis, immunosuppressant, multiple autoimmune syndrome
This is case report of a dog showing several sporadic clinical signs related to multiple autoimmune syndromes and their management using different immunosuppressant drugs.

1. INTRODUCTION
Autoimmune diseases occur frequently in patients with a history of other autoimmune diseases; however, coexistence of three or more autoimmune disorders, defined as multiple autoimmune syndrome (MAS), is rare. The pathogenesis of the MAS is unknown, but genetic immunologic or environmental factors are thought to be involved. MAS can be categorised into three types according to the prevalence of disorders occurring together (Anaya et al., 2012; Cojocaru et al., 2010; Humbert & Dupond, 1988; Sarfaraz & Anis, 2020).
Like humans, dogs can also spontaneously develop multiple autoimmune disorders. They are more common in pure breeds than mixed breeds and in female dogs than male dogs (Gorman & Werner, 1986; Pedersen, 1999). In veterinary medicine, genetic factors are implicated in the development of MAS, and several studies have been conducted in certain breeds (Bremer et al., 2018; Day & Penhale, 1992; Pedersen et al., 2012). However, our understanding about MAS in dogs is still inadequate. The classification and treatment of MAS in dogs are based on the criteria for humans (Gorman & Werner, 1986; Pedersen, 1999).
This clinical case report describes the clinical course, treatment, and clinical response to therapy in a poodle dog suspected of developing MAS. Clinical signs of hypothyroidism, immune‐mediated haemolytic anaemia (IMHA), putative immune‐mediated polyarthritis (IMPA), and immune‐mediated dermatologic disorders occurred sequentially and not at the same time. Different immunosuppressants were administered for each clinical sign, resulting in rapid clinical improvement.
2. CASE REPORT
A 9‐year‐old castrated male poodle dog was presented to our hospital with clinical signs of lethargy, anorexia, pale mucous membrane, and icterus. A month earlier, the dog was taken to a local hospital because of alopecia and skin crust without pruritus. At that time, based on the low T4 level [0.7, reference range (RR) 1–4] and high thyroid‐stimulating hormone level, the dog was diagnosed with hypothyroidism and prescribed levothyroxine. During the treatment of hypothyroidism, the dog's condition was well, but 4 days before visiting our hospital, the dog showed lethargy, and a blood test at a local animal hospital revealed severe anaemia [haematocrit (HCT) 11%; RR: 37.3–61] with hyperbilirubinemia. The anaemia was regenerative, and spherocytosis was found on the blood smear. Abdominal ultrasound showed no significant findings, except for splenomegaly. The presumptive diagnosis was IMHA and the dog received a blood transfusion with packed red blood cells. The dog was hospitalised for 3 days, and immunosuppressive prednisolone was prescribed.
Physical examination on the first day of the visit to our hospital revealed a holosystolic murmur (grade 4/6), yellowish pale mucous membrane, and icterus in the abdomen. Generalised alopecia, scale, and multifocal epidermal collarette were also found throughout the body. A complete blood count (CBC) revealed severe anaemia (12.4%, RR: 36.9–55) and leucocytosis (43.7 × 109/L, RR: 6.7–18.3). The anaemia was regenerative, and the blood smear revealed anisocytosis and the presence of numerous spherocytes. The autoagglutination test was positive, and no infectious organisms were found on the blood smear. Polymerase chain reaction (PCR) test was negative for vector‐borne haematopathogens (Anaplasma spp., Babesia spp., Ehrlichia spp., Hepatozoon spp., Leptospira spp., Mycoplasma haematoparvum, and Richettsia spp.). Serum biochemical analysis revealed mild hyperbilirubinemia (0.6 mg/dl, RR: 0.1–0.5).
Based on the results, IMHA was highly suspected, and the immunosuppressive therapy was continued. The dog was hospitalised (day 1) and administered blood transfusion with packed red blood cells. The posttransfusion HCT was 29.4%. The dog was treated with cefotaxime (Cefotaxime sodium: Yungjin Pharmaceutical Co., Korea; 20 mg/kg IV q12h), famotidine (Gaster; Dong‐A Pharmaceutical Co., Korea; 1 mg/kg IV q12h), predinisolone (solondo; Yuhan Pharmaceuticals, Korea; 1 mg/kg PO q12h), doxycyclone (Doxycycline hyclate; Kukje Pharmaceutical Co., Korea; 5 mg/kg PO q12h), and clopidogrel (Lopirel; CMG Pharmaceutical Co., Korea; 1 mg/kg PO q24h).
The HCT was 26.0% and 17.6% on days 2 and 3, respectively. Additional blood transfusion with whole blood was provided on day 3, and the posttransfusion HCT was 31%. Another immunosuppressive drug, danazol (Danazol; Yong Poong Pharmaceutical Co., Korea 5 mg/kg PO q12h) was added to the previous treatment. The HCT declined to 26.4%, and numerous reticulocytes were found on the blood smear. Due to hospitalisation stress, the dog was discharged on oral levothyroxine, antithrombotic, antibiotic, gastroprotectant, and immunosuppressive drugs (i.e. predinisolone, mycophenolate mofetil, and danazol). The tapering of prednisolone was started due to lack of efficacy and adverse effects.
The dog was presented for a recheck on day 6. The owner reported that the general condition of the dog was good, except for the presence of melena. The HCT was 23.7%. Sucralfate (Ulcerlmin; JW Pharmaceutical Co., Korea; 40 mg/kg PO q12h) was prescribed to treat the melena and the HCT decreased to 17.8% on day 8 and increased to 27.9% on day 12. On day 19, the HCT was 38.4%, and the appetite of the dog was good. Prednisolone was tapered by 50% every 7 days and discontinued on day 33.
On day 36 that is, 3 days after stopping prednisolone, the dog developed clinical signs of fever, joint swelling in multiple joints, oedema of the periarticular soft tissue, and lameness. A physical examination identified the presence of fever (40.3°C) and multiple joint swelling (Figure 1). A CBC and blood smear revealed no significant changes. Radiographic changes, such as joint erosion, destruction, and joint effusion were not found. Due to the lack of joint effusion, arthrocentesis and joint fluid analysis were not performed. Since the dog was previously treated with immunosuppressive drugs, additional rheumatoid factor and antinuclear antibody (ANA) tests were not performed. The differential diagnosis of polyarthritis was IMPA, systemic lupus erythematosus (SLE), and bacterial arthritis. Based on the history of clinical signs after steroid discontinuation and the absence of a change in the blood test, the primary differential diagnosis was IMPA. Prednisolone (0.5 mg/kg PO q12h), analgesics, and antibiotics were added to the previous prescription to control the clinical symptoms. The dog was presented for a recheck on day 47. The periarticular soft tissue oedema, joint swelling, and gait had improved upon physical examination, and the HCT was 38.9%. Danazol was discontinued, and prednisolone was tapered by 50%.
FIGURE 1.

Gross findings of the left carpal joint (a and b) and left right tarsal joint (c and d) in the dog with clinical signs of fever, joint swelling, and periarticular soft tissue oedema
On day 54, the clinical signs of arthritis reappeared after tapering prednisolone. Because alopecia, scale, and thinning of the skin aggravated, the dose of prednisolone was quickly reduced, and leflunomide (Rheumide, Chong Kun Dang Pharmaceutical Corp., Korea; 3 mg/kg PO q24h) was started to treat the polyarthritis. The other immunosuppressive, mycophenolate mofetil was tapered by 50% because HCT was in the normal range and was considered to not affect the clinical sings of polyarthritis. On day 70, the clinical sings improved, and the HCT was normal. Mycophenolate mofetil was then discontinued, and prednisolone was tapered by 50%.
The dog was presented on day 84 for a recheck of the clinical signs of arthritis and anaemia. The dog's general condition was stable, and the gait was normal. The owner reported the presence of alopecia, which progressed during the treatment, while erythematous dermatologic lesions appeared 2 days ago. Dermatologic examination revealed generalised alopecia, scale, thinning of the skin, multifocal well‐demarcated erythematous lesions, and multiple yellowish nodules (Figure 2a,b). The appearance of skin lesions resembled an immune‐mediated skin disease. Cytology revealed the presence of numerous Malassezia without inflammatory cells on the dorsum and degenerative neutrophils without infectious agents from the yellow nodules. No organism growth was seen upon culturing the collected yellow nodule on blood agar. The owner refused a skin biopsy because of the poor and thin skin conditions of the dog. Based on the appearance of the lesions and cytology, the differential diagnosis was immune‐mediated dermatologic disease, such as pemphigus complex. Cyclosporin (Cipol‐N soft cap; Chong Kun Dang Pharmaceutical Corp., 25 mg/dog, PO q24h) was instituted as an immunosuppressant, and the dermatologic lesions improved after 7 days (Figure 2c,d). At this point, the dog had stable serum levels of cyclosporine.
FIGURE 2.
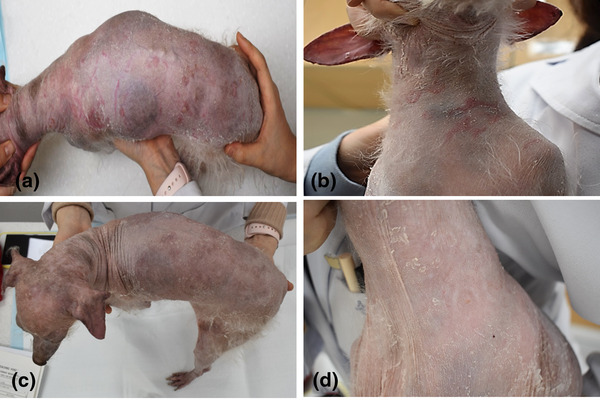
Generalised alopecia, scale, and well‐demarcated erythematous lesions (a and b). Improvement in the lesions 7 days after cyclosporine treatment (c and d)
On day 98, the clinical signs of arthritis and dermatologic lesions remained improved. Leflunomide was discontinued, and the dog was placed on treatment with levothyroxine and cyclosporine. After resolution of the polyarthritis and IMHA, the dog was being treated with cyclosporine for the skin lesions. The clinical course of the dog is depicted in Figure 3.
FIGURE 3.

Clinical course of the dog that had developed anaemia, polyarthritis, and dermatologic lesions. The dog was treated with multiple immunosuppressive drugs
3. DISCUSSION
The dog in the present clinical case sequentially developed hypothyroidism, IMHA, putative IMPA, and an autoimmune dermatologic disorder. To control the clinical symptoms, several immunosuppressive drugs and levothyroxine were administered, and each abnormal condition responded to the different treatments. The IMHA responded to the MMF but not prednisolone, and HCT remained well after the discontinuation of MMF. The polyarthritis responded to the use of leflunomide and the anti‐inflammatory prednisolone. Finally, the dermatologic lesions were improved after the prescription of cyclosporine.
IMHA is an autoimmune disorder characterised by the presence of pathogenic autoantibodies that attack red blood cells (Barker et al., 1992). There is no gold standard for the diagnosis of IMHA, and clinicians rely on the combination of clinical history, clinical signs, results of laboratory tests, and response to immunosuppressants. The American College of Veterinary Internal Medicine (ACVIM) recently proposed consensus guidelines for the diagnosis of IMHA (Garden et al., 2019). Accordingly, IMHA is possible if a dog has anaemia, more than two signs of immune‐mediated destruction, and more than one sign of haemolysis. In this case, the dog showed signs of immune‐mediated destruction, such as spherocytosis and a positive saline autoagglutination test. Serum biochemical analysis revealed icterus and hyperbilirubinemia. These features reflect the presence of a haemolysis. In addition, the PCR test for vector borne haematopathogens was negative. Thus, according to the ACVIM guidelines, the dog was diagnosed with IMHA and treated with several immunosuppressive drugs.
During the clinical treatment for IMHA, IMPA or SLE was suspected twice in the dog. IMPA is characterised by the idiopathic accumulation of immune complexes in the joint space (Johnson & Mackin, 2012). The differential diagnosis of IMPA includes trauma, vaccine or drug induced arthritis, SLE and infectious polyarthritis (Johnson & Mackin, 2012). In this case, radiographic changes such as subchondral bone destruction and irregular joint spaces were not seen. In addition, there was no history of suspected arthritis caused by trauma or vaccine or drug induced arthritis. Although test for rheumatoid factor and ANA are considered important for the diagnosis of immune‐mediated arthritis (Johnson & Mackin, 2012), these tests were not conducted. However, joint swelling was observed in multiple joints, and the dog responded to increasing doses of prednisolone or additional immunosuppressant therapy. Thus, IMPA was highly suspected. In SLE, immune complexes affect multiple tissues, resulting in variable clinical signs depending on the organs involved (Bennett, 1987). Generally, a positive ANA titre is considered important for the diagnosis of SLE (Johnson & Mackin, 2012); however, the ANA test was not conducted in this case. Steroids decrease the ability of immune cells to synthesise antibodies including ANA. The dog had been treated with steroids since a long time, which could have decreased the ANA values. Thus, we considered the primary differential diagnosis as IMPA, and the dog was treated with immunosuppressive drugs. Prednisolone and leflunomide improved the clinical signs of fever and polyarthritis.
The skin lesions of the dog showed multifocal well‐demarcated erythematous lesions and yellow nodules. The cytology of the erythematous lesions and nodules showed no bacteria or the presence of inflammatory cells. Malassezia was observed in the samples taken from the back, but the characteristics of the skin lesions were not the same as Malassezia dermatitis. Blood agar culture revealed no growth of organisms. Although a biopsy was not performed as the skin condition of the dog was very poor, the skin lesions were considered to be immune mediated. The owner was concerned about hepatotoxicity and declined the use of an antifungal agent. Therefore, the dog was administered cyclosporine alone and the dermatologic lesions improved rapidly.
The dog also had underlying hypothyroidism, a common endocrine disorder in dogs (Mooney, 2011). Most cases are of primary hypothyroidism which is classified into idiopathic atrophy and lymphocytic thyroiditis (also called immune‐mediated thyroiditis) (Graham et al., 2007). Lymphocytic thyroiditis is characterised by a lymphocytic infiltration of the thyroid glands, and the miniature poodle (the dog in this case) is predisposed to this condition (Graham et al., 2007).
MAS is a rare condition in which the patient develops at least three autoimmune disorders (Cojocaru et al., 2010; Humbert & Dupond, 1988; Sarfaraz & Anis, 2020). In humans, MAS can be classified into types 1, 2, and 3, and type 1 includes myasthenia gravis, giant cell carditis, thymoma, and polymyositis. Type 2 includes autoimmune thyroid disease, primary biliary cirrhosis, rheumatoid arthritis, Sjögren's syndrome, and scleroderma. Type 3 includes autoimmune thyroid disease, myasthenia gravis, Sjögren's syndrome, pernicious anaemia, idiopathic thrombopenic purpura, Addison's disease, type 1 diabetes mellitus, vitiligo, IMHA, SLE, and dermatitis herpetiformis (Anaya et al., 2012). In human medicine, the classification of MAS corresponds with the prevalence of their being associated with one another in patient with two autoimmune diseases (Anaya et al., 2012). In this study, the dog showed clinical signs of several immune‐mediated suspected condition corresponded to type 3 MAS.
MAS is rare, and it has not been clearly defined in veterinary medicine. However, it can occur commonly in dogs, and the presence of more than two immune‐mediated diseases should alarm clinicians about the possibility of additional autoimmune disorders. Monitoring based on the human classification of MAS may be helpful. Moreover, clinicians should be aware that each autoimmune condition in MAS may respond to different immunosuppressants.
4. CONCLUSION
The dog in this case sequentially developed numerous immune‐mediated diseases. Each disease responded to different immunosuppressive drugs. In human medicine, patients with an autoimmune disease have a tendency to develop additional autoimmune diseases. Therefore, periodic monitoring for the occurrence of new autoimmune diseases is crucial. Further studies are needed to define and classify MAS in veterinary medicine.
AUTHOR CONTRIBUTIONS
Data curation, project administration, and writing—original draft preparation: Dahye Lim. Data curation and project administration: Yunseok Jin and Youngmin Son. Conceptualization and supervision: Taeho Oh. Conceptualization, supervision, and writing—review & editing: Seulgi Bae.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required for this particular case report.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.741
ACKNOWLEDGEMENT
None.
Lim, D. , Jin, Y. , Son, Y. , Oh, T. , & Bae, S. (2022). A suspected case of a multiple autoimmune syndrome in a poodle dog. Veterinary Medicine and Science, 8, 431–436. 10.1002/vms3.741
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
Further enquiries can be directed to the corresponding authors.
REFERENCES
- Anaya, J.‐M. , Rojas‐Villarraga, A. , Pineda‐Tamayo, R. , Levy, R. A. , Gómez‐Puerta, J. , Dias, C. , Mantilla, R. D. , Gallo, J. E. , Cervera, R. , Shoenfeld, Y. , & Arcos‐Burgos, M. (2012). The multiple autoimmune syndromes. A clue for the autoimmune tautology. Clinical Reviews in Allergy and Immunology, 43(3), 256–264. [DOI] [PubMed] [Google Scholar]
- Barker, R. N. , Gruffydd‐Jones, T. J. , Stokes, C. R. , & Elson, C. J. (1992). Autoimmune haemolysis in the dog: relationship between anaemia and the levels of red blood cell bound immunoglobulins and complement measured by an enzyme‐linked antiglobulin test. Veterinary Immunology and Immunopathology, 34(1–2), 1–20. [DOI] [PubMed] [Google Scholar]
- Bennett, D. (1987). Immune‐based non‐erosive inflammatory joint disease of the dog. 1. Canine systemic lupus erythematosus. Journal of Small Animal Practice, 28(10), 871–889. [Google Scholar]
- Bremer, H. D. , Landegren, N. , Sjöberg, R. , Hallgren, Å. , Renneker, S. , Lattwein, E. , Leonard, D. , Eloranta, M.‐.L. , Rönnblom, L. , Nordmark, G. , Nilsson, P. , Andersson, G. , Lilliehöök, I. , Lindblad‐Toh, K. , Kämpe, O. , & Hansson‐Hamlin, H. (2018). ILF2 and ILF3 are autoantigens in canine systemic autoimmune disease. Scientific Reports, 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocaru, M. , Cojocaru, I. M. , & Silosi, I. (2010). Multiple autoimmune syndrome. Maedica, 5(2), 132. [PMC free article] [PubMed] [Google Scholar]
- Day, M. J. , & Penhale, W. J. (1992). Immune‐mediated disease in the old English sheepdog. Research in Veterinary Science, 53(1), 87–92. [DOI] [PubMed] [Google Scholar]
- Garden, O. A. , Kidd, L. , Mexas, A. M. , Chang, Y.‐M. , Jeffery, U. , Blois, S. L. , Fogle, J. E. , Macneill, A. L. , Lubas, G. , Birkenheuer, A. , Buoncompagni, S. , Dandrieux, J. R. S. , Di Loria, A. , Fellman, C. L. , Glanemann, B. , Goggs, R. , Granick, J. L. , Levine, D. N. , Sharp, C. R. , Smith‐Carr, S. , Swann, J. W. , & Szladovits, B. (2019). ACVIM consensus statement on the diagnosis of immune‐mediated hemolytic anemia in dogs and cats. Journal of Veterinary Internal Medicine, 33(2), 313–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman, N. T. , & Werner, L. L. (1986). Immune‐mediated diseases of the dog and cat. I. Basic concepts and the systemic immune‐mediated diseases. British Veterinary Journal, 142(5), 395–402. [DOI] [PubMed] [Google Scholar]
- Graham, P. A. , Refsal, K. R. , & Nachreiner, R. F. (2007). Etiopathologic findings of canine hypothyroidism. Veterinary Clinics of North America: Small Animal Practice, 37(4), 617–631. [DOI] [PubMed] [Google Scholar]
- Humbert, P. , & Dupond, J. (1988). Multiple autoimmune syndromes . Annales de medecine interne, 139, 159–168. [PubMed] [Google Scholar]
- Johnson, K. C. , & Mackin, A. (2012). Canine immune‐mediated polyarthritis: part 1: Pathophysiology. Journal of the American Animal Hospital Association, 48(1), 12–17. [DOI] [PubMed] [Google Scholar]
- Mooney, C. (2011). Canine hypothyroidism: A review of aetiology and diagnosis. New Zealand Veterinary Journal, 59(3), 105–114. [DOI] [PubMed] [Google Scholar]
- Pedersen, N. C. (1999). A review of immunologic diseases of the dog. Veterinary Immunology and Immunopathology, 69(2–4), 251–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, N. C. , Liu, H. , Greenfield, D L. , & Echols, L. G. (2012). Multiple autoimmune diseases syndrome in Italian Greyhounds: Preliminary studies of genome–wide diversity and possible associations within the dog leukocyte antigen (DLA) complex. Veterinary Immunology and Immunopathology, 145(1–2), 264–276. [DOI] [PubMed] [Google Scholar]
- Sarfaraz, S. , & Anis, S. (2020). Multiple autoimmune syndrome: An unusual combination of autoimmune disorders. Reviews on Recent Clinical Trials, 15(3), 240–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Further enquiries can be directed to the corresponding authors.


