Abstract
Introduction
The association between thiamine use and clinical outcomes among patients with sepsis and alcohol use disorder (AUD) is unclear.
Methods
In this retrospective cohort study of patients from Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4), we evaluated the association of thiamine use with clinical outcomes in patients with AUD and sepsis. The primary outcome was 28-day survival, and secondary outcomes included ICU, in-hospital, and 90-day mortality, ICU and hospital length of stay, duration of vasopressor use, need and duration of continuous renal replacement therapy (CRRT), and dynamic changes for variables up to day 7 after ICU admission.
Results
A total of 944 patients with sepsis and AUD were included in this cohort [median age, 53.1 years; women, 26.0% (245 of 944)]. Among all patients, 24.6% (233 of 944) received thiamine with a dose of 200 mg (IQR 100–345 mg). The 28-day mortality was 11.2% (26 of 233) in the thiamine use group compared with 18.6% (132 of 711) in the no thiamine use group (P = 0.009). After adjustment for a series of confounders, the mixed-effects Cox proportional hazards models showed that administration of thiamine was associated with a lower risk of 28-day mortality compared with no administration of thiamine.
Conclusions
In critically ill patients with alcohol use disorder admitted for sepsis, treatment with thiamine may be associated with a decreased risk of death. However, the present results should be interpreted with caution due to the limitations of retrospective design. Additional larger, multicenter randomized controlled trials are needed to confirm our findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00603-1.
Keywords: Sepsis, Thiamine, Alcohol use disorder, Critically ill, Mortality
Key Summary Points
| Why carry out this study? |
| Sepsis is a common condition that is associated with unacceptably high mortality. Patients with sepsis and alcohol use disorder (AUD) are frequently thiamine deficient due to malnutrition or malabsorption. |
| Clinical outcomes of thiamine use in patients with sepsis and AUD have not been investigated. |
| We aimed to investigate the association between thiamine administration and the 28-day mortality among patients with sepsis and AUD in a large cohort of patients. |
| What was learned from the study? |
| In critically ill patients with AUD admitted for sepsis, treatment with thiamine may be associated with a decreased risk of death. Additional larger, multicenter trials are needed to confirm our findings. |
| Patients with AUD admitted for sepsis with higher lactate levels and greater severity of illness and need for mechanical ventilation on ICU day 1 may benefit from administration of thiamine. |
Introduction
Among patients with alcohol use disorder (AUD), sepsis is common, leading to adverse outcomes, including death [1, 2]. In addition, critically ill patients with sepsis or AUD are frequently thiamine deficient due to malnutrition or malabsorption [3–5]. Thiamine supplementation is not routinely used for critically ill patients. However, previous studies have suggested that treatment with thiamine may result in improved outcomes in critically ill individuals, especially in patients with sepsis and AUD [6, 7]. Nonetheless, a recent observational cohort study of 68,571 patients with septic shock demonstrated that thiamine administration did not improve the 28-day survival compared with no thiamine administration [8]. On the other hand, among patients with sepsis and AUD, Holmberg et al. [9], in a small cohort (N = 53), reported a decreased mortality rate among those who received thiamine.
In the current study, we aimed to investigate the association between thiamine administration and the 28-day mortality among patients with sepsis and AUD in a large cohort of patients. We hypothesized that administration of thiamine leads to improved 28-day survival compared with no thiamine use.
Methods
Ethics Approval
The establishment of the Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4) was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the original data collection. Therefore, the ethical approval statement and the need for informed consent were waived for this manuscript.
Study Design
This retrospective cohort study was based on the MIMIC-III. The MIMIC-III is a large, single-center, freely accessible critical care database, which comprises over 60,000 individuals admitted to ICU at the Beth Israel Deaconess Medical Center from 2001 to 2012 [10]. We accessed the MIMIC-III after completion of the Protecting Human Research Participants exam (No. 30380922). In the MIMIC-III database for patient privacy, all participants are deidentified. In addition, we followed the Reporting of Studies Conducted Using Observational Routinely Collected Health Data reporting (RECORD) guidelines [11].
Patients
We included adult patients (at least 18 years old) with AUD defined by the International Classification of Diseases, 9th edition [ICD-9] codes (Supplementary Table S1), and admitted to ICU for sepsis identified by the Sepsis 3.0 criteria [12]. Patients with documented or suspected infection with Sequential Organ Failure Assessment [SOFA] [13] score of at least 2 before or within 24 h of ICU admission met the sepsis definition (Supplementary Fig. S1). Among those with multiple ICU admissions, we analyzed only the first ICU admission. Additionally, we excluded patients undergoing cardiothoracic surgery and had missing information regarding the primary outcome.
Clinical and Laboratory Data
We collected baseline demographic information, comorbidities, admission type, primary infection site, the vital sign and laboratory values on day 1, illness severity scores (i.e., Glasgow Coma Scale [GCS] [14], Model for End-Stage Liver Disease [MELD] [15], SOFA, Oxford Acute Severity of Illness Score [OASIS] [16], Logistic Organ Dysfunction score [LODS] [17]) on day 1, and the need for mechanical ventilation or vasopressors on the first day of ICU admission. In addition, the mean values of vital signs in the first 24 h in the ICU were collected, including heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, and body temperature. The maximum laboratory values in the first 24 h of the ICU admission were abstracted, including lactate, white blood cell count, prothrombin time, activated partial thromboplastin time, international normalized ratio, creatine kinase, creatine kinase-MB, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, serum creatinine, blood urea nitrogen, serum sodium, serum potassium, serum chloride, and serum magnesium. The minimum values of hemoglobin, platelet, and albumin during the first 24 h of the ICU admission were also collected. We selected the maximum severity of illness scores in the first 24 h of the ICU admission (MELD, SOFA, OASIS, LODS), except GCS, for which we chose its minimum values. In addition, heart rate, respiratory rate, systolic blood pressure, lactate, white blood cell count, alanine aminotransferase, aspartate aminotransferase, serum creatinine, blood urea nitrogen, serum magnesium, GCS and MELD values were collected from day 1 to day 7 after ICU admission. The cumulative dose of thiamine (defined as the total amount of drug received during the ICU stay) and the daily dose of thiamine (defined as the total amount of drug received in daily) were also collected.
Outcomes
The primary outcome was 28-day mortality. Secondary outcomes included ICU, hospital, and 90-day mortality rates, ICU and hospital length of stay, duration of vasopressor and continuous renal replacement therapy (CRRT) use, and dynamic changes of heart rate, respiratory rate, systolic blood pressure, lactate, white blood cell count, alanine aminotransferase, aspartate aminotransferase, serum creatinine, blood urea nitrogen, serum magnesium, GCS and MELD up to day 7 following ICU admission.
Statistical Analysis
We determined the normality of the distribution for continuous variables by the Kolmogorov–Smirnov test. We reported the normally distributed variables as the means ± standard deviation (SD), the skewed distributed variables as the median and interquartile range (IQR). The categorical variables were expressed as numbers and percentages. Among the groups, we compared continuous variables by Student t test or Mann–Whitney U test, as appropriate. Categorical variables were compared by Pearson’s chi-squared test or Fisher’s exact test, as appropriate. The sample size was calculated to maintain event (28-day mortality) per variable ratio of 10:1 in the regression models.
A priori-defined variables were selected for the multivariate Cox regression analysis because of their plausible association with 28-day mortality. Additionally, considering potential confounders that were not fully balanced between two groups (P < 0.05), we conducted Cox proportional hazard model to adjust for these confounders including primary infection site, heart rate, blood pressure, international normalized ratio, total bilirubin, serum creatinine, blood urea nitrogen, serum potassium, GCS, MELD, and OASIS. To avoid overadjusting for potential confounders (such as MELD score which contains total bilirubin, international normalized ratio, and serum creatinine), we used six models with a gradual degree of adjustment. Model 1 was adjusted for age, gender, admission type, and primary infection site. Model 2 was adjusted for the confounders included in model 1 plus vital signs (heart rate and blood pressure). Model 3 was adjusted for the confounders included in model 2 plus laboratory tests (international normalized ratio, total bilirubin, serum creatinine, blood urea nitrogen, and serum potassium). Model 4 was adjusted for the confounders included in model 3 plus GCS. Model 5 was adjusted for the confounders included in model 2 plus GCS and MELD. Model 6 was adjusted for the confounders included in model 2 plus OASIS. The results were presented as the hazard ratio (HR) and associated 95% confidence interval (CI).
Kaplan–Meier survival curves using the log-rank test were drawn to assess mortality differences between the groups. In addition, we compared trends for heart rate, respiratory rate, systolic blood pressure, lactate, white blood cell count, alanine aminotransferase, aspartate aminotransferase, serum creatinine, blood urea nitrogen, serum magnesium, GCS, and MELD from day 1 to day 7. Generalized linear mixed models were used to estimate the conditional associations of thiamine treatment. We used restricted cubic spline fitted for Cox proportional hazards models relative to the median doses of thiamine therapy. Kernel density plots were used to demonstrate the distribution of quantities of thiamine used.
Subgroup analyses for the primary outcome were performed for (1) patients < 50 vs. ≥ 50 years old, (2) patients with serum lactate of < 2 vs. ≥ 2, (3) patients without need for mechanical ventilation vs. those on mechanical ventilator on the day 1, (4) patients without the need for vasopressors vs. those on vasopressor on day 1, (5) patients with day 1 SOFA score of < 5 vs. ≥ 5, and (6) patients with day 1 OASIS < 34 vs. ≥ 34. We did not impute the missing data as information for the primary outcome was available, and for the secondary outcomes was missing in at most 5% of patients.
Statistical analysis was performed using the statistical package IBM SPSS statistics software (SPSS) (version 24.0, Chicago, IL, USA), and figures were constructed using Origin (version 9.6. Northampton, Massachusetts, USA), R software (version 3.6.0). P values less than 0.05 represented statistical significance in the two-sided hypothesis.
Results
Baseline Characteristics
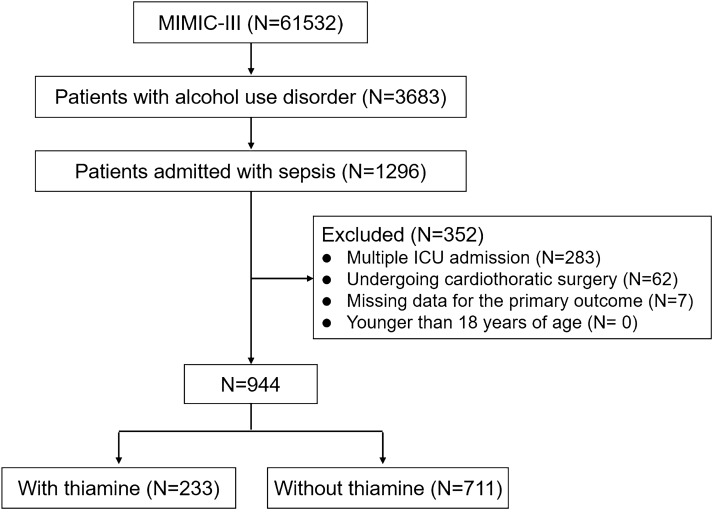
A total of 61,532 individuals from MIMIC-III were screened, and 1296 patients with AUD admitted for sepsis were included in the study. A total of 352 patients were excluded from the analysis (283 recurrent admissions, 62 patients with cardiothoracic surgery, and seven patients with missing data for the primary outcome). Thus, 944 patients (233 and 711 with and without thiamine supplementation, respectively) entered the final analyses (Fig. 1). The patient demographics and baseline characteristics were similar between the groups (the median (IQR) age was 53.1 [44.5–62.2], and 699 patients [74.0%] were male). Approximately 96% of the participants were admitted from the emergency department. There were no significant between-group differences in comorbidities, and the most common comorbidities were liver diseases (31.8%), followed by diabetes mellitus (17.3%) and congestive heart failure (10.5%). The underlying source of infection differed significantly between the two groups, with the bloodstream being the most common site of infection in the thiamine use group (44.1%), followed by lung (30.1%) and urine (21.4%), while with the lung being the most common site of infection in the no thiamine administration group (48.1%), followed by bloodstream (33.9%) and urine (15.9%). The demographic and clinical characteristics of the patients are summarized in Table 1.
Fig. 1.
Detailed process of data extraction. MIMIC-III Medical Information Mart for Intensive Care III, ICU intensive care unit
Table 1.
Baseline characteristics of included patients
| Characteristics | All, n = 944 | No thiamine, n = 711 | Thiamine, n = 233 | P value |
|---|---|---|---|---|
| Age, year | 53.1 (44.5–62.2) | 53.0 (44.1–62.7) | 53.3 (44.6–61.2) | 0.920 |
| Sex, n (%) | 0.346 | |||
| Female | 245 (26.0) | 190 (26.7) | 55 (23.6) | |
| Male | 699 (74.0) | 521 (73.3) | 178 (76.4) | |
| BMI, kg/m2 | 27.1 (24.4–31.9) | 27.6 (24.6–32.3) | 26.1 (23.5–30.7) | 0.068 |
| Ethnicity, n (%) | ||||
| White | 674 (71.4) | 506 (71.2) | 168 (72.1) | 0.784 |
| Hispanic | 27 (2.9) | 21 (3.0) | 6 (2.6) | 0.764 |
| Black | 73 (7.7) | 59 (8.3) | 14 (6.0) | 0.256 |
| Other/unknown | 170 (18.0) | 125 (17.6) | 45 (19.3) | 0.550 |
| Comorbidities, n (%) | ||||
| Liver disease | 300 (31.8) | 234 (32.9) | 66 (28.3) | 0.192 |
| Diabetes | 163 (17.3) | 125 (17.6) | 38 (16.3) | 0.656 |
| Congestive heart failure | 99 (10.5) | 80 (11.3) | 19 (8.2) | 0.181 |
| Renal failure | 70 (7.4) | 56 (7.9) | 14 (6.0) | 0.345 |
| Hypertension | 50 (5.3) | 40 (5.6) | 10 (4.3) | 0.430 |
| Solid tumor | 36 (3.8) | 32 (4.5) | 4 (1.7) | 0.054 |
| Rheumatoid arthritis | 13 (1.4) | 9 (1.3) | 4 (1.7) | 0.535 |
| Metastatic cancer | 13 (1.4) | 12 (1.7) | 1 (0.4) | 0.153 |
| Admission type | 0.205 | |||
| Emergency | 903 (95.7) | 679 (95.5) | 224 (96.1) | |
| Elective | 21 (2.2) | 14 (2.0) | 7 (3.0) | |
| Urgent | 20 (2.1) | 18 (2.5) | 2 (0.9) | |
| Primary infection site | < 0.001 | |||
| Bloodstream | 392 (41.5) | 313 (44.0) | 79 (33.9) | |
| Pneumonia | 326 (34.5) | 214 (30.1) | 112 (48.1) | |
| Renal/urinary tract | 189 (20.0) | 152 (21.4) | 37 (15.9) | |
| Others | 37 (3.9) | 32 (4.5) | 5 (2.1) | |
BMI body mass index
Compared with those who did not receive thiamine, patients who received thiamine had a higher heart rate, systolic and diastolic blood pressures, shorter activated partial thromboplastin time, lower international normalized ratio, total bilirubin, serum creatinine, and blood urea nitrogen (Table 2). Patients who received thiamine had similar severity of illness scores (excluding GCS, MELD, and OASIS) and therapeutic options (included the percentage of mechanical ventilation, vasopressor, and CRRT) on day 1, compared with patients who did not receive thiamine (Table 3).
Table 2.
Vital signs and laboratory findings of included patients
| Characteristics | All, n = 944 | No thiamine, n = 711 | Thiamine, n = 233 | P value |
|---|---|---|---|---|
| Vital sign | ||||
| Heart rate, bpm | 92 (81–104) | 91 (80–102) | 95 (83–107) | 0.004 |
| Respiratory rate | 19 (17–22) | 19 (17–22) | 19 (17–23) | 0.492 |
| Systolic blood pressure, mmHg | 116 (106–130) | 115 (105–129) | 121 (109–133) | 0.001 |
| Diastolic blood pressure, mmHg | 63 (57–71) | 63 (57–70) | 67 (60–75) | < 0.001 |
| Body temperature, °C | 37.0 (36.5–37.5) | 36.9 (36.5–37.5) | 37.0 (36.6–37.6) | 0.053 |
| Laboratory tests | ||||
| Lactate, mmol/L | 2.7 (1.8–4.5) | 2.8 (1.8–4.7) | 2.4 (1.7–3.9) | 0.170 |
| White blood cell count, × 103/μL | 12.3 (8.3–17.8) | 12.5 (8.4–17.9) | 11.9 (8.1–17.6) | 0.212 |
| Hemoglobin, g/dL | 10.2 (8.8–11.9) | 10.1 (8.6–11.9) | 10.3 (9.1–12.0) | 0.175 |
| Platelet, × 103/μL | 139 (82–211) | 138 (80–213) | 143 (89–202) | 0.934 |
| Prothrombin time, s | 15.4 (13.4–19.1) | 15.5 (13.5–19.1) | 15.0 (13.1–18.5) | 0.079 |
| Activated partial thromboplastin time, s | 32.9 (27.8–44.9) | 33.6 (28.1–46.6) | 31.5 (26.9–38.8) | 0.012 |
| International normalized ratio | 1.4 (1.2–1.8) | 1.4 (1.2–1.9) | 1.3 (1.1–1.7) | 0.005 |
| Creatine kinase, U/L | 312 (117–1405) | 319 (107–1351) | 301 (137–1866) | 0.503 |
| Creatine kinase–MB, U/L | 8 (4–20) | 8 (4–20) | 9 (5–20) | 0.301 |
| Lactate dehydrogenase, U/L | 304 (218–495) | 298 (212–491) | 315 (227–517) | 0.223 |
| Alanine aminotransferase, U/L | 45 (24–112) | 47 (24–117) | 35 (23–101) | 0.111 |
| Aspartate aminotransferase, U/L | 82 (41–232) | 81 (41–235) | 87 (38–201) | 0.704 |
| Total bilirubin, mmol/L | 1.5 (0.6–4.4) | 1.6 (0.7–4.6) | 1.3 (0.6–3.4) | 0.043 |
| Serum creatinine, mg/dL | 1.1 (0.8–1.8) | 1.1 (0.8–1.8) | 1.0 (0.7–1.8) | 0.017 |
| Blood urea nitrogen, mg/dL | 20 (13–35) | 21 (13–37) | 17 (11–29) | 0.001 |
| Albumin, g/dL | 2.9 (2.5–3.4) | 2.9 (2.5–3.4) | 3.0 (2.5–3.5) | 0.499 |
| Serum sodium, mmol/L | 140 (137–143) | 141 (137–144) | 140 (137–143) | 0.633 |
| Serum potassium, mmol/L | 4.3 (3.9–4.9) | 4.4 (4.0–5.0) | 4.2 (3.8–4.8) | 0.016 |
| Serum chloride, mmol/L | 107 (103–111) | 107 (103–111) | 108 (103–111) | 0.485 |
| Serum magnesium, mg/dL | 2.1 (1.9–2.4) | 2.1 (1.9–2.4) | 2.1 (1.9–2.4) | 0.595 |
For the vital sign data, the mean value in the first 24 h in the ICU was selected for the following variables: heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, and body temperature. For laboratory data, the maximum value in the first 24 h in the ICU was selected for the following variables: lactate, white blood cell, prothrombin time, activated partial thromboplastin time, international normalized ratio, creatine kinase, creatine kinase-MB, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, serum creatinine, blood urea nitrogen, serum sodium, serum potassium, serum chloride, and serum magnesium. The minimum value in the first 24 h in the ICU was selected for the following variables: hemoglobin, platelet, and albumin
Table 3.
Severity of illness scores, treatments and outcomes of included patients
| Variables | All, n = 944 | Non-thiamine, n = 711 | Thiamine, n = 233 | P value |
|---|---|---|---|---|
| Scores | ||||
| GCS | 15 (13–15) | 15 (13–15) | 14 (12.5–15) | 0.001 |
| MELD | 16 (9–25) | 16 (9–25) | 14 (8–22) | 0.023 |
| SOFA | 5 (4–8) | 6 (4–8) | 5 (3–7) | 0.164 |
| OASIS | 34 (28–40) | 34 (28–40) | 36 (30–41) | 0.003 |
| LODS | 5 (3–7) | 5 (3–7) | 5 (3–7) | 0.582 |
| Interventions on day 1, n (%) | ||||
| Mechanical ventilation | 553 (58.6) | 408 (57.4) | 145 (62.2) | 0.192 |
| Vasopressor | 323 (34.2) | 247 (34.7) | 76 (32.6) | 0.554 |
| CRRT | 39 (4.1) | 30 (4.2) | 9 (3.9) | 0.812 |
| Primary outcome | ||||
| 28-day mortality, n (%) | 158 (16.7) | 132 (18.6) | 26 (11.2) | 0.009 |
| Secondary outcome | ||||
| ICU mortality, n (%) | 106 (11.2) | 91 (12.8) | 15 (6.4) | 0.008 |
| In-hospital mortality, n (%) | 147 (15.6) | 124 (17.4) | 23 (9.9) | 0.006 |
| 90-day mortality, n (%) | 200 (21.1) | 164 (23.1) | 36 (15.5) | 0.014 |
| ICU length of stay, day | 3.7 (1.9–8.5) | 3.2 (1.8–7.4) | 5.0 (2.2–9.8) | < 0.001 |
| Hospital length of stay, day | 10.0 (5.5–17.6) | 9.4 (5.2–17.0) | 11.8 (6.4–18.1) | 0.003 |
| Duration of vasopressor use during ICU stay, hours | 46 (15–113) | 41 (15–108) | 58 (12–121) | 0.456 |
| Duration of CRRT use during ICU stay, h | 78 (36–115) | 73 (34–108) | 96 (47–135) | 0.549 |
For the severity of illness scores, we selected the maximum value in the first 24 h in the ICU for the following variables: MELD, SOFA, OASIS, LODS. We selected the minimum value of GCS in the first 24 h in the ICU
GCS Glasgow Coma Scale, MELD Model for End-stage Liver Disease, SOFA Sequential Organ Failure Assessment, OASIS Oxford Acute Severity of Illness Score, LODS Logistic Organ Dysfunction Score, CRRT continuous renal replacement therapy, ICU intensive care unit
Interventions
Of the 233 patients who received continuous intravenous administration of thiamine, 75.1% (175/223) and 92.3% (215/233) of patients received first thiamine treatment within 24 h and 72 h after ICU admission, respectively. Most patients (179/233, 76.8%) received a dose of 100 mg once daily, and only 12 patients (5.2%) received more than 200 mg. The median cumulative dose of thiamine used during ICU stay was 200 mg (IQR 100–345 mg). Approximately half of the patients (125/233, 53.6%) received thiamine administration in ICU for 1 day, and only 7.7% of patients (18/233) received thiamine on 5 days or more. The median duration of thiamine use in ICU was 1 day (IQR 1–3), and the cumulative median duration of thiamine treatment was 18 h (IQR 7–63 h) (Supplementary Table S2).
Primary Outcome
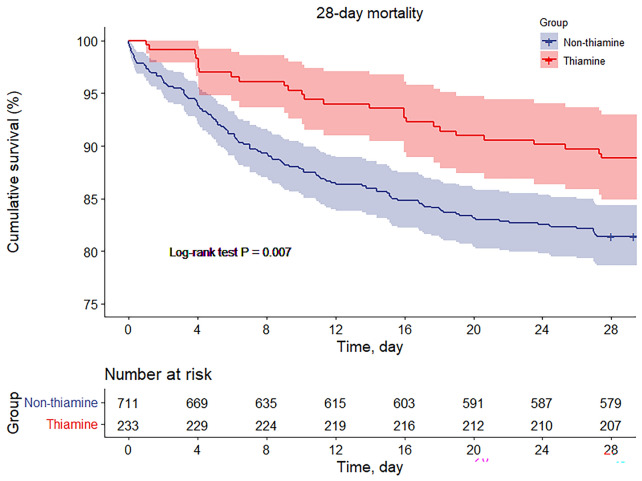
The primary outcome, 28-day mortality, was significantly lower in the thiamine group compared with the no thiamine group (11.2% vs. 18.6%, P = 0.009) (Table 3). Figure 2 shows the cumulative mortality (log-rank test P = 0.007). After adjustment for a series of confounders, such as age, gender, admission type, primary infection site, heart rate, blood pressure, international normalized ratio, total bilirubin, serum creatinine, blood urea nitrogen, serum potassium, GCS, MELD, and OASIS, the mixed-effects Cox proportional hazards models showed that administration of thiamine was associated with a lower risk of 28-day mortality compared with non-thiamine [all adjusted hazard ratio (HR) < 1 and P < 0.05] (Table 4).
Fig. 2.
Cumulative incidence of the primary outcome of 28-day survival for thiamine use vs. no thiamine use
Table 4.
Hazard ratios and 95% CIs for 28-day all-cause mortality associated with the thiamine use in patients with sepsis and alcohol use disorder
| Regression models | Multivariable | |
|---|---|---|
| HR (95% CI) | P value | |
| Unadjusted | 0.566 (0.372–0.862) | 0.008 |
| Model 1 | 0.633 (0.414–0.970) | 0.036 |
| Model 2 | 0.643 (0.418–0.988) | 0.044 |
| Model 3 | 0.610 (0.385–0.967) | 0.035 |
| Model 4 | 0.594 (0.375–0.941) | 0.026 |
| Model 5 | 0.626 (0.407–0.963) | 0.033 |
| Model 6 | 0.561 (0.365–0.863) | 0.008 |
Model 1 was adjusted for age, gender, admission type, and primary infection site. Model 2 was adjusted for the confounders included in model 1 plus vital signs (heart rate and blood pressure). Model 3 was adjusted for the confounders included in model 2 plus laboratory tests (international normalized ratio, total bilirubin, serum creatinine, blood urea nitrogen, and serum potassium). Model 4 was adjusted for the confounders included in model 3 plus GCS. Model 5 was adjusted for the confounders included in model 2 plus GCS and MELD. Model 6 was adjusted for the confounders included in model 2 plus OASIS
GCS Glasgow Coma Scale, MELD Model for End-stage Liver Disease, SOFA Sequential Organ Failure Assessment, OASIS Oxford Acute Severity of Illness Score, ICU intensive care unit
Secondary Outcomes
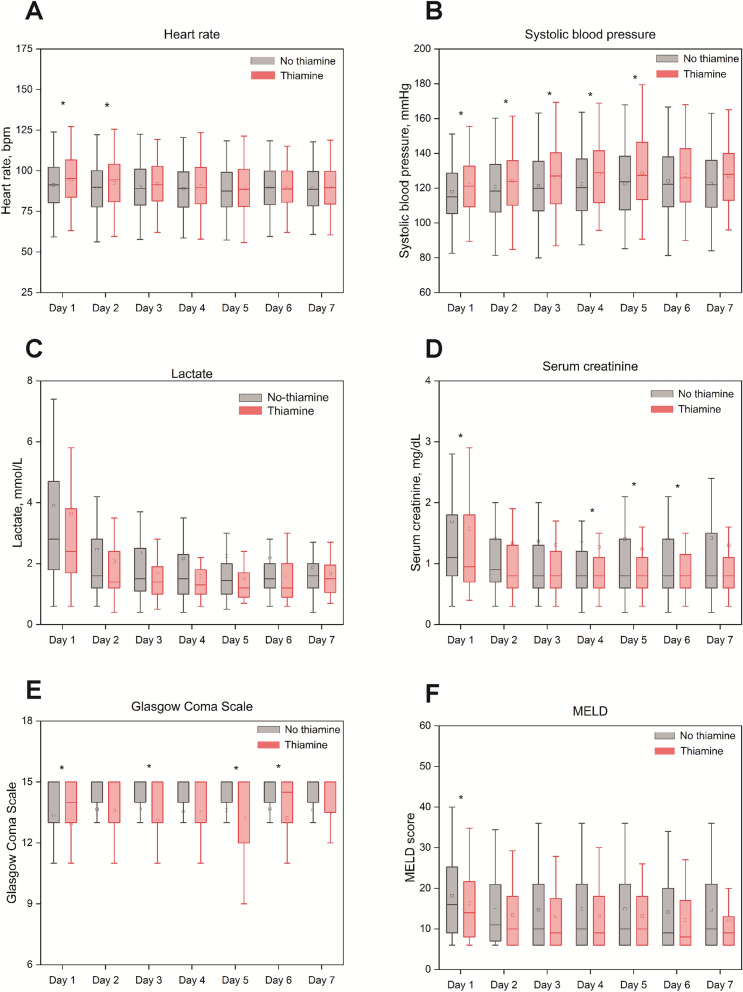
Table 3 shows the secondary outcomes between the two groups. The ICU, in-hospital, and 90-day mortality rates in the treatment group were significantly lower than those in the control arm (6.4% vs. 12.8%, P = 0.008; 9.9% vs. 17.4%, P = 0.006; 15.5% vs. 23.1%, P = 0.014; respectively). The ICU and hospital stay duration for patients with thiamine use were significantly longer than for the no thiamine group (5.0 days vs. 3.2 days, P < 0.001; 11.8 days vs. 9.4 days, P = 0.003; respectively). There were also no differences in the duration of vasopressor use or CRRT use among the two groups (Table 3 and Supplementary Table S3). The trends for heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, lactate, white blood cell count, alanine aminotransferase, aspartate aminotransferase, serum creatinine, blood urea nitrogen, serum magnesium, GCS, and MELD up to day 7 were similar between the two groups (Fig. 3, Supplementary Fig. S2 and Table S4).
Fig. 3.
Dynamic changes for prespecified variables: a heart rate, b systolic blood pressure, c lactate, d serum creatinine, e Glasgow Coma Scale, f MELD. MELD Model for End-stage Liver Disease. The square icon in each group represented the mean value. *P < 0.05
Subgroup Analyses
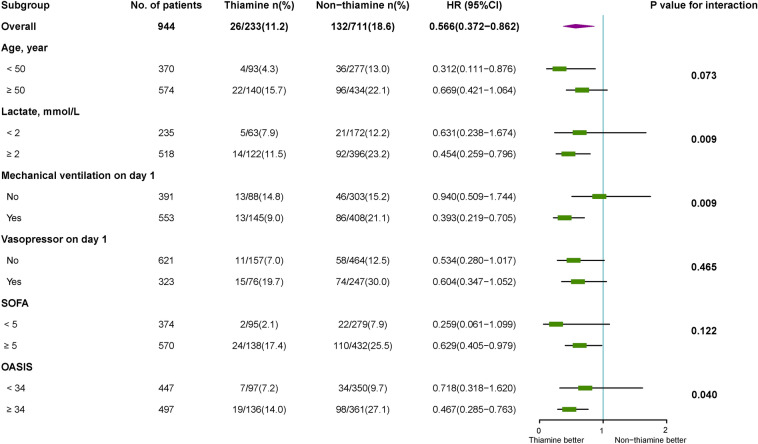
There were statistically significant interactions for three subgroups: (1) those with lower vs. higher lactate levels (HR 0.631 [0.238–1.674] vs. 0.454 [0.259–0.796], P = 0.009 for interaction), (2) those without vs. with the need for mechanical ventilation on ICU day 1 (HR 0.940 [0.509–1.744] vs. 0.393 [0.219–0.705], P = 0.009 for interaction), and (3) those with higher vs. those lower OASIS (HR 0.718 [0.318–1.620] vs. 0.467 [0.285–0.763], P = 0.040 for interaction) (Fig. 4).
Fig. 4.
Subgroup analysis for primary outcome defined by population. SOFA Sequential Organ Failure Assessment, OASIS Oxford Acute Severity of Illness Score, HR hazard ratio
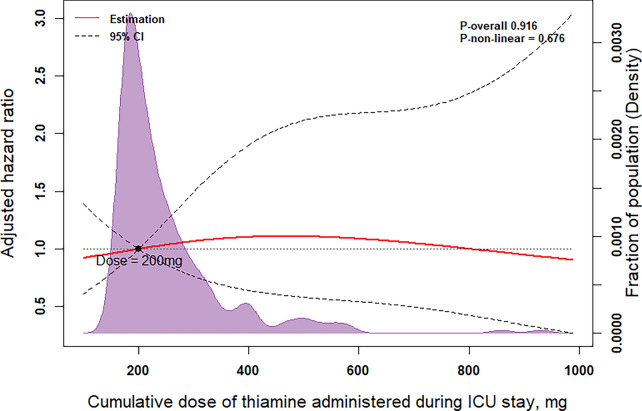
Among patients with thiamine therapy, all-cause 28-day mortality was numerically lower in the cumulative dose of 100 to 200 mg (4.5%) vs. other dose groups (11.8% for cumulative dose with < 100 mg, 10.5% for a cumulative dose of 200–345 mg, 15.5% for cumulative dose > 345 mg), but it did not achieve statistical significance (P = 0.375) (Supplementary Fig. S3). Furthermore, after adjustment for potential confounders, no differences were found in 28-day mortality between patients with different doses of thiamine during ICU stay (Fig. 5). A similar result indicated insignificant association between time of thiamine use and 28-day mortality in patients with sepsis and AUD (P = 0.451) (Supplementary Fig. S4).
Fig. 5.
Restricted cubic spline and kernel density plot of the association between cumulative dose of thiamine administered during ICU stay and 28-day mortality. Results were adjusted for age, gender, admission type, primary infection site, and baseline SOFA score. The models were expressed relative to the median doses, and the dotted lines represent the 95% confidence intervals for the spline model (reference is 200 mg). The area shown in purple is the kernel density plot, which demonstrates the distribution of the cumulative dose of thiamine administered. SOFA Sequential Organ Failure Assessment, ICU intensive care unit
Discussion
To our knowledge, this is the most extensive descriptive study for the association between thiamine administration and the outcomes of patients with sepsis and AUDs. In this study, we found that receiving thiamine was associated with a lower risk of 28-day mortality than no thiamine administration in patients with sepsis and AUDs, especially in patients with higher lactate levels and greater severity of illness and need for mechanical ventilation on ICU day 1.
AUD is often combined with thiamine deficiency that results in some severe complications [18]. The most common and severe complication is Wernicke’s encephalopathy (WE), with a frequency of about 35% in chronic alcoholics [19]. Furthermore, some studies have shown that alcohol dependence is independently associated with sepsis and increased incidence of multiple organ dysfunction and sepsis-related hospital mortality [20, 21]. Sepsis is also associated with thiamine deficiency [22]. Thus, it is likely that thiamine supplementation may provide some benefits to patients with sepsis and AUD. Some previous randomized controlled trials (RCTs) have emphasized the effect of thiamine in sepsis; however, the results are mostly negative [6, 23–25]. The reason is that these studies only focus on the thiamine administration in patients with sepsis, not in patients with sepsis and AUD; on the other hand, these RCTs had a relatively small sample size, which would reduce the confidence of the outcome. A small retrospective cohort study (N = 53) reported that thiamine administration in patients with AUD admitted for septic shock was associated with decreased mortality (thiamine group 44% vs. no thiamine group 79%, P = 0.02) [9]. The result of our study was aligned with this previous research, indicating that administration of thiamine was independently associated with a lower risk of 28-day mortality compared with patients not administered thiamine during sepsis among individuals with AUD. We reported that the mortality differences lasted up to the 90-day follow-up (15.5% vs. 23.1%, P = 0.014).
Despite the potential benefits of thiamine administration, its low side-effect profile, and cost, the percentage of patients who received thiamine was only 24.7% in patients with sepsis and AUD. This is in accordance with Pawar et al.’s recent study [26]. In a nationwide retrospective observational study, the thiamine supplementation was seen in 59% of patients with AUD but the rate was significantly decreased in patients with sepsis and AUD [26]. This obvious difference gap may be associated with care provider’s variability. Among patients with AUD admitted for sepsis, care providers may be more focused on sepsis compared with AUD. This may explain the lower-than-expected rate of thiamine supplementation in patients with sepsis and AUD. Treatment with thiamine in patients with AUD is recommended in the USA [27] and Europe [28]. However, the administration details, including timing, dosing, and route of administration, are still unclear. We noted that 75.1% and 92.3% of patients received first thiamine via intravenous routes within 24 h and 72 h after ICU admission, respectively. However, we did not find any significant difference in 28-day mortality between patients with different doses of thiamine during ICU stay. Further research is needed to evaluate the doses of thiamine for mortality in patients with sepsis and AUD.
Thiamine is an essential cofactor for pyruvate dehydrogenase (PDH), the initial enzyme for Krebs cycle [29]. Severe thiamine deficiency can result in lower PDH function, and it could, in turn, lead to lower pyruvate conversion to acetyl coenzyme A. This phenomenon indirectly promotes the conversion of pyruvate to lactic acid, resulting in mitochondrial aerobic metabolism disturbances and multiple organ dysfunction [30]. Having said that, the exact mechanism of improved survival by thiamine supplementation in this cohort is still unclear. We did not find differences in cardiovascular (systolic blood pressure and need for vasopressors), kidney (serum creatinine and need for CRRT), and liver (MELD score) functions. Despite the statistical differences in daily trends for GCS, slight changes in consciousness did not significantly increase mortality (Supplementary Table S5). These findings may mean that the observed survival benefits of thiamine administration could be independent of organ dysfunction. Although there were no statistically significant differences in lactate levels between the two groups, there was a tendency to lower lactate among those who received thiamine. Notably, in subgroup analysis, higher lactate level (≥ 2 mmol/L) and higher SOFA score (≥ 5) were associated with lower mortality when patients received thiamine. Therefore, it is likely that choosing appropriate target patients for thiamine therapy could lead to higher benefits. Receiving thiamine may reduce the gap between macro- and microcirculation, promote the aerobic metabolism in mitochondria, and restore the impaired tissue perfusion leading to improved patient outcomes. Donnino and colleagues found that thiamine administration in patients with septic shock and baseline thiamine deficiency could significantly decrease the lactate level at 24 h and possibly reduce mortality [25].
Our study has several limitations. First, the wide existence of immortal time bias in observational studies may exaggerate the association between thiamine and survival among patients with sepsis and AUD. We have attempted to partly mitigate this effect by utilizing the Cox model. Second, as a result of its retrospective design, we are not able to imply any causal relationship. Hence, prospective studies are required to verify the results of our hypothesis-generating study. Third, we cannot identify thiamine deficiency because of the lack of baseline thiamine levels in MIMIC-III. Therefore, we cannot infer whether all patients with sepsis and AUD can benefit or only the thiamine-deficient individuals. Fourth, in the thiamine group, 53.6% of patients received thiamine for only 1 day, and 4.3% of patients received thiamine with a cumulative dose of less than 100 mg. The lower thiamine doses used in these individuals make it difficult to assess the dose-dependency of thiamine effects; the heterogeneous doses and time of thiamine administration could limit the application of these results. Fifth, thiamine supplementation should be routinely recommended in today’s management of AUD; however, as only 24.7% of patients received thiamine supplementation in our study, this may be related to the time period of MIMIC-III. Lastly, the adjustments in our study may not sufficiently address all confounding variables, and some confounders may have remained unaccounted for, such as care provider variability. Owing to the aforementioned limitations, although there is a significant difference in mortality, the present results should be interpreted with caution.
Conclusions
In critically ill patients with alcohol use disorder admitted for sepsis, treatment with thiamine may be associated with a decreased risk of death. However, the present results should be interpreted with caution because of the limitations of retrospective design. Additional larger, multicenter randomized controlled trials are needed to confirm our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the journal’s rapid service fee were funded by the Chinese Medical Information and Big Data Association (Bo Hu, No. Z-2019-1-003), and by Translational Medicine and Interdisciplinary Research Joint Fund of Zhongnan Hospital of Wuhan University (Bo Hu, No. ZNJC202011), and by Qinghai Province Key R & D and Transformation Projects (Siqing Ma, No. 2019-SF-132).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CH, TW, SM. The first draft of the manuscript was written by CH, TW, WH, QX, KK, BH and JL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Chang Hu, Tong Wu, Siqing Ma, Weipeng Huang, Qiancheng Xu, Kianoush B. Kashani, Bo Hu and Jianguo Li have nothing to disclose.
Compliance with Ethics Guidelines
The establishment of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the original data collection. Therefore, the ethical approval statement and the need for informed consent were waived for this manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Chang Hu and Tong Wu contributed equally and share the first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chang Hu, Email: huchang@whu.edu.cn.
Tong Wu, Email: annie_wutong@foxmail.com.
Siqing Ma, Email: siqing177@sina.com.
Weipeng Huang, Email: huang7@whu.edu.cn.
Qiancheng Xu, Email: qianchengxu@whu.edu.cn.
Kianoush B. Kashani, Email: Kashani.Kianoush@mayo.edu
Bo Hu, Email: hobbier1979@163.com.
Jianguo Li, Email: drljg5361@163.com.
References
- 1.Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witkiewitz K, Litten RZ, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5(9):eaax4043. doi: 10.1126/sciadv.aax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collet L, Bisch M, Viennet S, Schwan R, Paille F. Thiamin and alcohol use disorder: a national survey of practice. Therapie. 2020;75(3):281–294. doi: 10.1016/j.therap.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25(4):576–581. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382–390. doi: 10.1016/j.amjms.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. 2018;46(11):1747–1752. doi: 10.1097/CCM.0000000000003311. [DOI] [PubMed] [Google Scholar]
- 7.Chou WP, Chang YH, Lin HC, Chang YH, Chen YY, Ko CH. Thiamine for preventing dementia development among patients with alcohol use disorder: a nationwide population-based cohort study. Clin Nutr. 2019;38(3):1269–1273. doi: 10.1016/j.clnu.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto Y, Aso S, Iwagami M, et al. Association between IV thiamine and mortality in patients with septic shock: a nationwide observational study. Crit Care Med. 2020;48(8):1135–1139. doi: 10.1097/CCM.0000000000004394. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg MJ, Moskowitz A, Patel PV, et al. Thiamine in septic shock patients with alcohol use disorders: an observational pilot study. J Crit Care. 2018;43:61–64. doi: 10.1016/j.jcrc.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):1885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–854. doi: 10.1016/S1474-4422(14)70120-6. [DOI] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of Acute Physiology And Chronic Health Evaluation data elements shows comparable predictive accuracy. Crit Care Med. 2013;41(7):1711–1718. doi: 10.1097/CCM.0b013e31828a24fe. [DOI] [PubMed] [Google Scholar]
- 17.Le Gall JR, Klar J, Lemeshow S, et al. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276(10):802–810. doi: 10.1001/jama.1996.03540100046027. [DOI] [PubMed] [Google Scholar]
- 18.Abdou E, Hazell AS. Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res. 2015;40(2):353–361. doi: 10.1007/s11064-014-1430-z. [DOI] [PubMed] [Google Scholar]
- 19.Cook CC, Hallwood PM, Thomson AD. B vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33(4):317–336. doi: 10.1093/oxfordjournals.alcalc.a008400. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien JM, Jr, Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 21.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 22.Costa NA, Gut AL, de Souza DM, et al. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. J Crit Care. 2014;29(2):249–252. doi: 10.1016/j.jcrc.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Qian X, Zhang Z, Li F, Wu L. Intravenous thiamine for septic shock: a meta-analysis of randomized controlled trials. Am J Emerg Med. 2020;38(12):2718–2722. doi: 10.1016/j.ajem.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz A, Andersen LW, Cocchi MN, Karlsson M, Patel PV, Donnino MW. Thiamine as a renal protective agent in septic shock. A secondary analysis of a randomized, double-blind placebo-controlled trial. Ann Am Thorac Soc. 2017;14(5):737–741. doi: 10.1513/AnnalsATS.201608-656BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnino MW, Andersen LW, Chase M, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. 2016;44(2):360–367. doi: 10.1097/CCM.0000000000001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawar RD, Balaji L, Grossestreuer AV, et al. Thiamine supplementation in patients with alcohol use disorder presenting with acute critical illness: a nationwide retrospective observational study. Ann Intern Med. 2021 doi: 10.7326/M21-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders: alcohol, cocaine, opioids. Am J Psychiatry. 1995;152(11 Suppl):1–59. [DOI] [PubMed]
- 28.Galvin R, Brathen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–1418. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- 29.Frank RA, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci. 2007;64(7–8):892–905. doi: 10.1007/s00018-007-6423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34(Pt 2):217–222. doi: 10.1042/BST0340217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.