Highlights
-
•
Thymic epithelial tumors(TETs) often require a multimodal approach, including RT.
-
•
RT dose recommendations largely derive from old data, where 2D RT was widely used.
-
•
This systematic review focused on the optimal dose for TETs with modern RT.
-
•
5 eligible studies reporting dose response were analyzed and synthesized.
-
•
Current guidelines remain valid, this work might be an eye-opener fostering new data.
Keywords: Thymoma, Thymic carcinoma, TETs, Radiotherapy, Radiation dose
Abstract
Thymic epithelial tumors (TETs) are rare thoracic tumors, often requiring multimodal approaches. Surgery represents the first step of the treatment, possibly followed by adjuvant radiotherapy (RT) and, less frequently, chemotherapy. For unresectable tumors, a combination of chemotherapy and RT is often used. Currently, the optimal dose for patients undergoing radiation is not clearly defined. Current guidelines on RT are based on studies with a low level of evidence, where 2D RT was widely used. We aim to shed light on the optimal radiation dose for patients with TETs undergoing RT through a systematic review of the recent literature, including reports using modern RT techniques such as 3D-CRT, IMRT/VMAT, or proton-therapy. A comprehensive literature search of four databases was conducted following the PRISMA guidelines. Two investigators independently screened and reviewed the retrieved references. Reports with < 20 patients, 2D-RT use only, median follow-up time < 5 years, and reviews were excluded. Two studies fulfilled all the criteria and therefore were included. Loosening the follow-up time criteria to > 3 years, three additional studies could be evaluated. A total of 193 patients were analyzed, stratified for prognostic factors (histology, stage, and completeness of resection), and synthesized according to the synthesis without meta-analysis (SWIM) method. The paucity and heterogeneity of eligible studies led to controversial results. The optimal RT dose neither for postoperative, nor primary RT in the era of modern RT univocally emerged. Conversely, this overview can spark new evidence to define the optimal RT dose for each TETs category.
Introduction
Thymoma and thymic carcinoma are rare epithelial tumors with a worldwide incidence of 1.3–3.4 million cases per year representing the most frequent neoplasms in the anterosuperior mediastinum in the adult [1], [2]. Thymic epithelial tumors (TETs) are classified according to the World Health Organization (WHO) histopathological classification, which distinguishes thymomas from the more aggressive thymic carcinomas. Thymoma is further divided into subtypes A, AB, B1, B2, and B3, based upon the morphology of epithelial tumor cells, the relative proportion of the non-tumoral lymphocytic component, and resemblance to normal thymic architecture [3], [4]. The prognosis of patients with TETs is mostly favorable with 10-years overall survival (OS) rates of 84% in stage I-II patients, dropping to 70% in stage III and to 42–53% in stage IV patients [5]. The cornerstone of treatment of TET in patients with good performance status is complete surgical resection for resectable tumors, although recurrences occur in approximately 30% of patients undergoing surgical resection [6], [7], [8], [9], [10], [11]. Indication for adjuvant radiotherapy (RT) or chemotherapy depends on the WHO-histopathological classification, local extent of disease (expressed as Masaoka-Koga stages I-IV), and completeness of resection as well as the overall clinical condition of the patient [5]. Radiation reduces the local recurrence rate when incomplete surgery is performed or in locally advanced diseases [12], [13], [14]. However, RT does not influence distant nor pleural out-of-field recurrences, which account for about 2/3 of all recurrences [15]. The role of RT in the post-operative setting after complete resection of TETs has been widely debated, especially in Masaoke-Koga II stage. Recent analyses of pooled large observational studies showed benefit in survival rates of postoperative RT over surgery alone for Masaoka-Koga stages II to IV thymoma [16], [17], [18], [19]. For unresectable tumors and more advanced stages, platinum-based chemotherapy and/or definitive RT is used.
The radiation dose delivered to patients with TETs may differ between medical centers, and guidelines only developed on expert opinions/retrospective cohort studies where 2D RT was largely used (Level of evidence low, grade of recommendation IV-V) [5]. The current ESMO guidelines propose a total radiation dose of 45–50 Gy after complete resection, 50–54 Gy after R1 resection, with a boost to areas of likely residual disease, in daily doses of 1.8–2 Gy. For unresectable tumors, a total radiation dose of 60–66 Gy in 2 Gy fractions is advised. However, given the low level of evidence for the recommendations stipulated in the current guidelines, the optimal dose/fractionation is yet to be defined. These recommendations are based on old and retrospective studies. [20], [13], [21], [13], [6], [22]. Considering the rarity of such cases, the periods of inclusion of patients are usually very long. As a result, a significant number of patients in the series used for the current guidelines received 2D RT with larger fields, less reliable dose calculations and treatment delivery assurance, compared to newer techniques. Therefore, we aimed to review for TETs literature using modern external beam RT techniques (3D-CRT, IMRT, VMAT, SBRT, proton therapy) to find studies that support these recommendations on the RT dose for TETs [23]. Currently, a wide range of doses is used (40–70 Gy). It is advisable to carefully distinguish each category of patients with TETs as well as their RT delivery setting, whether it is neoadjuvant, adjuvant after complete resection (R0), or after incomplete resection (R1, R2), or as a definitive treatment of RT alone with or without chemotherapy. To the best of our knowledge, a systematic review to respond such a question has never been performed before.
Methods and materials
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline [24]. The primary outcomes were OS rates in relation to the radiation dose, accounting for the WHO classification and Masaoka-Koga stage of TET reported in the included studies. The review protocol has been drawn up after the scientific question was clearly defined following the PICO tool. Subsequently, we submitted and registered to the international prospective register of systematic reviews (PROSPERO ID: CRD42021241826) before the search started. A comprehensive search of PUBMED (NLM), EMBASE(Ovid), ClinicalTrials.gov, and Cochrane library of Clinical Trials databases was conducted by two researchers (A.A., S.P.) after consulting a specialized librarian. The literature search included retrospective and prospective studies on humans affected by TETs undergoing external beam radiation. Reports published in languages other than English were excluded. The search was restricted to the timeframe from 01-Jan-1990 to the search date (23-Apr-2021), to include studies with modern RT techniques. Reviews, duplicates, studies with <20 patients recruited, and reports without a clear RT dose–response evaluation or with the use of 2D RT only were excluded. The detailed search strategy and the resume from all the database searches are available in the supplementary materials.
Before screening all the citations found through the search, an interrater reliability test (Cohen’s Kappa) has been carried out [25]. An online random number generator was used to create a random sample of citations (each number corresponded to a title and abstract of a specific reference in the sample). Two authors (A.A., S.P.) independently screened the same sample blinded to authors and journal titles [26]. After the level of agreement was tested, A.A. and S.P. independently screened all titles and abstracts, still blinded to authors and journal titles, using an Excel workbook specifically designed for literature screening [27]. Data were compiled into a single Excel workbook [27]. Disagreements were discussed by the two screeners; if consensus could not be reached, a third person (D.R.) familiar with the project provided final arbitration.
Articles considered for inclusion were independently reviewed by A.A. and S.P. and the consensus was reached by discussion together with a third person (D.R.) on any disagreement. A list of excluded citations from each step may be requested from the first author.
Risk of bias assessment was done for the selected studies using the New Castle-Ottawa scale [28]. A score of 7 or higher indicated studies with a low risk of bias. Since we did not include any randomized clinical trial, the Revised Cochrane risk-of-bias tool for randomized trials was not used.
A.A. extracted the data from eligible full texts in regard to: first author and publication year, study design, sample size, tumor histology and stage, surgery performed, surgical margin status, RT timing, RT technique; RT dose delivered, median follow-up time, main endpoint(s) of the study, OS rates according to the radiation dose, progression-free survival (PFS), treatment toxicity (acute, late). The results were independently checked by S.P. and synthesis without meta-analysis of the collected data was performed according to the “SWiM” (Synthesis without meta-analysis) method [29]. Survival probability at 5 and 10 years was used to analyze results from each group of studies, although a standardized metric was not used. A narrative resume and a critical appraisal of the relevant data collected were carried out.
Results
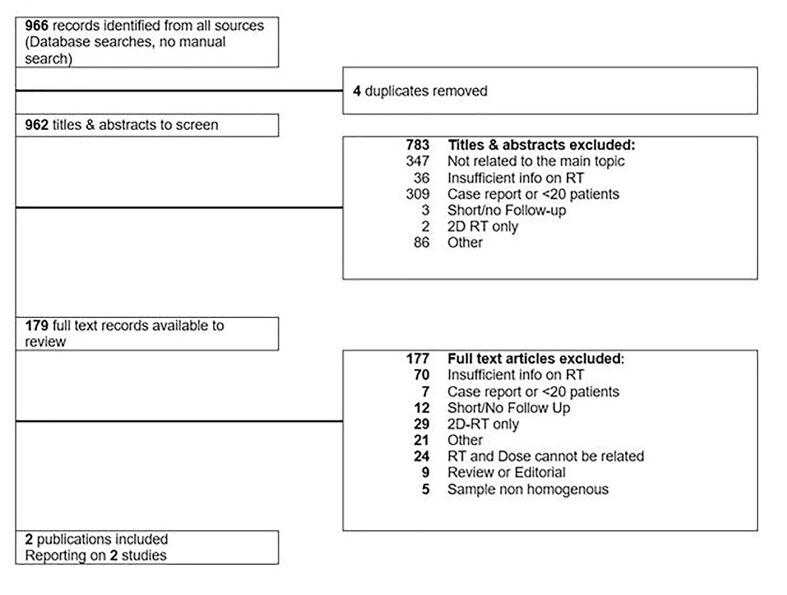
A total number of 966 citations were identified after the online database search. Interrater reliability test based on 66 random titles and abstracts among the retrieved ones, showed a very high agreement between the investigators (Cohen’s K = 0,91, CI 0,8–1; the excel file specifically designed for Cohen’s K calculation including the citations used to test the level of agreement can be requested to the corresponding authors). Four duplicates were removed, and 179 citations were selected for a full-text review. Only two studies, involving 154 patients (113 were treated with RT, of which 69 with modern RT techniques) with thymoma, thymic carcinoma or unspecified TETs, fulfilled the inclusion criteria and were eligible for the first analysis. (Fig. 1; Table 1) The studies were grouped according to the tumor histology and stage of disease, because these two variables, together with the completeness of surgery, have previously been described as independently correlating with the survival rates [30], [31], [32], [33]. With less strict inclusion criteria regarding median follow-up time, allowing a minimum of 3 years instead of 5 years, we were able to analyze data from three more papers (Table 2) including 203 patients (191 were treated with RT, of which 124 with modern RT techniques). In total, radiotherapy data regarding 294 patients, of which 2/3 received modern RT technique treatment, were finally analyzed. The heterogeneous stage, histology, and treatment of the patients (e.g., the differences in the sample and intervention) among the studies in our review were considered, and data were accordingly synthesized. All the main outcomes of the eligible studies, and of the three studies separately evaluated with a slightly shorter median follow up time, are visually compared and summarized in Table 3. In our comparison, a synthesis method was considered (vote counting) but we did not find applicable the use of a standardized metric (e.g., the direction of effect), thus we limited the synthesis of the findings to the narration. The studies were analyzed after we grouped them according to the above-mentioned prognostic factors. The directness or relevance of the evidence addressing the review question was used to prioritize the analysis of the studies when two or more studies were grouped together (“Stage III - TETs”, “TETs – all stages”). We performed an exploration of heterogeneity among studies using tables, by comparing the effect sizes of studies grouped according to tumor histology and Masaoka-Koga stage. These included: surgery and completeness of resection, type of intervention used (RT technique, total doses delivered, and fractionations). We explicitly evaluated the certainty of the findings for each outcome using the GRADE approach [34]; given that all the outcomes did not come from randomized clinical trials, the starting rating of evidence was “low quality”. We downgraded the certainty by one level for concerns and two levels for serious concerns regarding the risk of bias, and the directness of the evidence according to the review question. A table showing the risk of bias for all the studies is available as supplementary material.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. Note: Two studies were included according to all the pre-specified inclusion and exclusion criteria.Three additional studies were separately analyzed after the first synthesis of data; however, they did not satisfy the median follow-up time criteria, thus they are not shown between the included ones.
Table 1.
Main features of the studies eligible for inclusion with median follow-up time > 5 years.
|
Author, Year |
Sample size (Recruitment period) |
Tumor Histology (WHO type) |
Stage (M−K) |
Margin status |
RT intent (n) |
RT technique (n) |
Median RT Dose in Gy (range) |
Median Follow-up months (range) |
|---|---|---|---|---|---|---|---|---|
| RECURRENCES of TETs | ||||||||
| Yang 2019 [39] | 47 (2007–2015) |
TETs (A-B3 + C) |
Recurr. | n/a | dRT (47) |
3D-CRT (29) IMRT (18) |
52 Gy (30–70) |
83 (8–299) |
| THYMOMA (M−K II) | ||||||||
| Chen 2010 [36] |
107* (1964–2006) |
Thymoma (A-B3) |
II | R0 | PORT (66) |
2D-RT (44) 3D-CRT (22) |
60 Gy (22–60) |
63 (2–303) |
Abbreviations: TETs = thymic epithelial tumors; WHO = World health organization; M−K = Masaoka-Koga surgical tumor stage classification; n/a = not applicable; PORT = Post operative RT; dRT = Radiotherapy alone with definitive or curative intent; 3D-CRT = 3D conformal radiotherapy; IMRT: Intensity modulated radiotherapy; R0 = complete resection; Recurr. = recurrence; n = number of patients.
*The original study sample differs from the patients undergoing RT with modern techniques (e.g. the final sample size used for the synthesis).
Table 2.
Main features of the studies eligible for inclusion with median follow-up time >3 years.
| Author, year |
Sample size (Recruitment period) |
Tumor Histology (WHO type) |
Stage (M−K) |
Margin status |
RT intent (n) |
RT technique (n) |
Median RT Dose (range) |
Median Follow-up (Months, range) |
|---|---|---|---|---|---|---|---|---|
| TETs (M−K II to IV) | ||||||||
| Fan X., 2020 [35] |
56 (2011–2018) |
TETs (A-B3 + C) |
Limited advanced III-IVb | n/a (No surgery) |
C-CRT (56) |
IMRT (56) |
60 Gy (32–64) |
46 (7–101) |
| THYMOMA (M−K III) | ||||||||
| Fan C., 2013 [37] |
65* (1982–2010) |
Thymoma (A-B3) |
III | R0 (Complete surgery) |
PORT (53) | 2D-RT (25) 3D-CRT/IMRT (28) |
56 Gy (28–60) |
50 (5–360) |
| Fan C., 2020 [38] |
82* (2000–2017) |
Thymoma (A-B3) |
III Unresectable |
R2 (DS or Biopsy) |
dRT (54) non-dRT (28) |
2D-RT (42) 3D-CRT/IMRT† (40) |
60 Gy (10–70) |
41 (5–166) |
Abbreviations: TETs = thymic epithelial tumors; WHO = World health organization; M−K = Masaoka-Koga surgical tumor stage classification; n/a = not applicable; PORT = Post operative radiotherapy; DS: Debulking surgery; dRT = Definitive Radiotherapy alone with curative intent. 3D-CRT = 3D conformal radiotherapy; IMRT = Intensity modulated radiotherapy; C-CRT = concurrent chemo-radiation; n = number of patients.
*The original study sample differs from the patients undergoing RT with modern techniques (e.g. the final sample size used for the synthesis).
†Exact doses delivered for the modern RT subgroup are unclear.
Table 3.
Insight of data extracted for the dose–response analysis, from the studies’ results pooled in the narrative synthesis.
| Authors, Year | Study design | Study intervention | Results | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Definitive RT | ||||||||
| Fan X., 2020 [35] |
Phase II Trial | IMRT plus etoposide/cisplatin for unresectable TETs | < 54Gy | 54-59 Gy | 60-64 Gy | MVA significant for PFS: stage (p=0.04) and dose (≥54 Gy vs <54 Gy) (p=0.002). MVA trend for OS: dose (≥54 Gy vs <54 Gy) (p=0.06) |
||
| 2y-PFS† | 10% | 50% | 54% | † p<0.01 | ||||
| 3y-OS†† | 39% | 63% | 74% | †† p=0.05 | ||||
| Fan C., 2020 [38] |
Retro-spective | Definitive RT after R1/R2 surgery in unresectable stage III M-K Thymoma | < 54 Gy | 54-70 Gy | No significant differences in dRT dose sub-group analysis: 54-60 Gy vs 60 Gy vs >60 (p=0.5) |
|||
| 5y-OS | 27% | 66% | ||||||
| 10y-OS | 13% | 56% | p<0.01 | |||||
|
Yang, 2019 [39] |
Retro-spective |
Salvage RT for recurrent TETs |
<52Gy | 52-70 Gy | MVA significant for PFS: histology and dose; MVA significant for OS: dose |
|||
| 5y-OS | 59% | 80% | p=0.04 | |||||
| 5y-PFS | 14% | 30% | p=0.02 | |||||
| Postoperative RT | ||||||||
| Chen, 2010 [36] |
Retro-spective | Stage II Thymoma after R0 resection vs S alone | ≤ 50 Gy | 51-60 Gy | No significant difference in PFS between PORT doses (p=0.6). MVA: dose not included as a variable. |
|||
| 5y-DFS | 93% | 92% | p=0.6 | |||||
| 10-DFS | 93% | 74% | ||||||
|
Fan C., 2013 [37] |
Retro-spective | Stage III thymoma after R0 resection vs S alone | ≤ 50 Gy | >50-60 Gy | No significant difference in OS between PORT doses (p=0.7); 5y-OS and DFS values using 3D-CRT/IMRT are 100% and 73% respectively. | |||
| 5y-OS | 95% | 89% | p=0.7 | |||||
| 10y-OS | 65% | 58% | ||||||
Notes: In Bold “First Author,year” of the studies with a lower Risk of Bias.
*studies with a median follow-up time <60 months.
Abbreviations: TETs = thymic epithelial tumors; M−K = Masaoka-Koga surgical tumor stage classification; RT = radiotherapy; Gy = Gray; IMRT = Intensity modulated radiotherapy; S = Surgery; PORT = Post operative radiotherapy; dRT = Definitive radiotherapy with curative intent; OS = Overall survival, PFS = progression free survival; DFS = disease free survival; MVA = Multivariate analysis.
Of the identified studies, only one was a prospective phase II trial study on 56 patients with thymoma and thymic carcinoma [35] (Table 2). The other studies were of retrospective nature with a sample size ranging from 47 to 107 patients (Table 1, Table 2) and included thymoma stages II [36] or III [37], [38], or recurrent TETs [39]. Indications for RT were postoperative for R0 [36], [37], R1 or R2-resection [38], or as a definitive treatment [35], [39]sometimes in combination with chemotherapy. Only two of the five included studies used modern RT techniques for all patients, such as 3D-CRT or intensity-modulated RT (IMRT) but we allowed studies where a proportion of patients had 2D treatments as well [37], [38]. The median RT doses in these 5 studies ranged from 52 to 60 Gy, with minimum delivered dose 15 Gy and a maximum of 70 Gy. (Table 1, Table 2) The single-arm phase II trial from Fan X. et al. included a small, but a rather homogeneous group of 56 patients with limited advanced disease treated with definitive chemoradiation, using IMRT [35]. Limited advanced disease was defined as Masaoka-Koga stages III-IVB which could be encompassed within a single field of radiation treatment. Many patients (75%) had stage IVB disease. Chemotherapy was 4 cycles of a platinum-based regimen; RT prescription dose was 60 Gy in 2-Gy daily fractions, 5 fractions per week, but the total dose could be adjusted according to the normal tissue dose constraints. The delivered median RT dose ranged between 32 Gy and 64 Gy. Five patients who underwent unplanned surgery were excluded from the analysis of RT dose. The overall objective response rate was 86% (no complete response, only partial responses), with 82% for thymoma and 88% for thymic carcinoma, but this difference was not significant. For the whole group, the 5y-OS and 5y-PFS were 56.2% and 29.5%, respectively. OS at 3 years was 74%, 63% and 39% for the high (≥60 Gy), medium (54–59 Gy) and low (<54 Gy) dose group, respectively (log-rank p = 0.05). PFS at 2 years was 54%, 50% and 10% in these three dose groups (log-rank p = 0.008). In the multivariate analysis (adjusted for gender, age, stage, and TET type), a dose–effect was seen with a higher RT dose (≥54 Gy vs. < 54 Gy) resulting in a better PFS (p = 0.002) and OS (p = 0.06). Toxicities observed were grade 3–4 leucopenia in 42% of patients, grade 3 acute esophagitis, as well as pulmonary fibrosis in 3 patients accounting for 5.4% of treated patients; no grade ≥ 3 radiation pneumonitis nor other radiation induced side effect was seen, suggesting that toxic events were most likely attributable to chemotherapy.
One study included in this review focused specifically on post-operative RT (PORT) for stage II thymoma with a clear dose/response analysis [36] (Table 3). It primarily tested the hypothetical benefit of PORT for patients with stage II thymoma who underwent complete tumor resection and concluded that the addition of adjuvant RT did not significantly alter survival, and recurrence rates compared to surgery alone. Further subgroup analyses performed in the same study on the 66 patients treated with adjuvant RT provided information regarding the risks of a dose escalation in this setting. The group treated with a total dose of 50 Gy or less, in daily fractions of 1.8–2 Gy, performed better in terms of disease-free survival, disease specific survival, and toxicity than the group receiving 51 to 60 Gy, with the same dose per fraction. Similar results were shown for patients undergoing 3D-RT when compared with 2D-RT, and for the use of limited fields versus extended fields of treatment, with a lower rate of toxicities (e.g., in this study only 1 radiation pneumonitis was reported in the group of 22 patients treated with 3D-CRT), although none of these results were significant.
In the highly heterogeneous group of TETs stage III, where the potential benefits of PORT in the completely resected stage III thymoma seemed to be clearer than in stage II thymoma, the role of PORT has been widely investigated. Results coming from a retrospective cohort study of patients undergoing PORT after complete surgical resection [37] showed that higher doses (>50 Gy) did not achieve better survival rates. Furthermore, 3D-RT was superior to 2D-RT showing a safer profile in terms of toxicities with a lower probability of recurrences. When surgery was not a viable option to treat stage III TETs, other authors suggested as definitive radiation dose (dRT) of 54 Gy or higher [38]. The differences they observed between the dRT (54 Gy or higher) group and the patients receiving<54 Gy (e.g., non-dRT group), with unresectable disease in both cases, were significantly in favor of the dRT. In the subgroup analysis with patients with ≥ 54 Gy, no significant difference was found between the various total doses administered 54–59 Gy, 60 Gy, or > 60 Gy.
Finally, regarding the role of salvage RT and a focus on the relative doses when recurrences of TETs occur, the inclusion of a retrospective study from Yang et al.[39] added some useful information. In this study, the radiation dose was independently associated with OS and PFS at univariate and multivariate analysis. The dose cut-off identified was 52 Gy. Thus, the same authors considered the high-dose RT group (52 Gy or higher) as the curative group, concluding that the lower doses (<52 Gy) could only be used with a palliative intent.
Discussion
The current guidelines on radiation dose for TETs are mainly based on old and poor data using 2D-RT and large field RT. The aim of this systematic review was to search for data using modern RT techniques, and to determine whether the current guidelines on RT dose should be revised. Because only two studies fulfilled the prespecified criteria, we enlarged the analysis with three more studies, where the median follow-up time was slightly shorter than the prespecified 5 years [15]. Despite a time-span of 30 years, only five small studies with dose–response data could be identified: three on definitive RT for unresectable TETs [36], [39], [40], and two on postoperative RT [37], [38]. All studies on definitive RT showed a significantly better outcome with doses > 52–54 Gy compared to lower doses, whereas in the postoperative group no dose–response could be identified (Table 3). A potential benefit of even higher radiation doses has also been suggested by an interesting prospective study evaluating 32 TET patients with stage II to IVb treated with stereotactic body radiation therapy with curative intent [40]. The doses delivered ranged from 49 to 70 Gy in 10 fractions – corresponding to equivalent doses in 2 Gy fractions of > 84 Gy – showing a very high objective response rate of 97% (34% complete response, 63% partial response), median PFS of 21 months, and a safe toxicity profile. Heterogeneity of intervention and unclear data on dose–response, did not let us include the study in our analysis.
The benefit of postoperative RT in TETs is a matter of debate, especially in Masaoka-Koga stages II, where results are conflicting when comparing postoperative RT and no postoperative RT [12], [41]. It is therefore not surprising that the two studies on postoperative RT included in our systematic review did not detect a potential dose–response with the small dose differences between subgroups.
Low quality recommendations on the dose to deliver in completely resected stage III TETs could also be drawn from a study where 50 to 56 Gy were delivered with 95% 5-y OS rate and 85% 5-y DFS rate [42]. A clear dose–response outcome was not reported, and a heavy selection bias may have affected these results, although the large series of 105 patients remains noteworthy as well as the comparison with a small group of patients suitable for surgery who underwent definitive RT instead of surgery and PORT [42]. However, the study included in the current synthesis indicated as optimal dose 46 to 50 Gy for PORT achieving similar results[37].
Whenever surgery is not feasible or clearly unsuccessful for stage III TETs, the general prognosis is impaired, and the definitive RT (dRT) is meant to be > 56 Gy [38]; whether a dose escalation should be performed there’s no consensus. In the study abovementioned, not eligible according to the review criteria, a small proportion of stage III TETs patients underwent dRT to 66 Gy, seemingly showing acceptable, but markedly inferior, outcomes compared to the PORT group [42].
Important concern when irradiating TET patients is the dose to the heart because of its proximity to the RT target volume. Especially with older RT techniques, larger doses to the heart may have resulted in more cardiac toxicity, abolishing the potential benefit of RT ([43], [44], [45]), and again stressing the need for data with modern RT techniques. The majority of patients (2/3) included in this review were treated with modern RT (3D-CRT or IMRT), except for a subset of patients in three studies, mostly due to the long inclusion period for this rare disease recruitment. [37], [38].
Proton therapy has the potential to even better spare normal tissues such as the heart. A few studies, encountered during our search reported the use of proton therapy with promising results. [23], [46], [47], [48], [49], [50], [51], [52], [53].
Unfortunately, the small sample size, the absence of a dose–response analysis, or a very short follow-up time, did not let us include them in the review.
We wondered about the impact of the new IASLC/ITMIG stage classification system, adopted by some centers but not consistently used in the past to replace M−K classification, to stratify patients’ disease and redefine their treatment accordingly. Some authors already considered of correlating M−K stage and the TNM class [54]. Such a correlation was impractical in our analysis; therefore we kept the reported staging system in the original studies.
Another important issue in modern RT is the delineation of the target, which was not well defined in most of the included papers. Furthermore, it has been shown that large variability can be seen when delineating the target in the postoperative setting [55], which may potentially impact on toxicity and outcome.
The optimal timing of RT (time-to-RT) after surgery might be an important factor affecting patient outcomes as well, and it was only reported in a few studies retrieved in our search, yet not suitable for analysis[56], [57]. PORT performed within a month from surgery seemed to be related to better survival rates, although selection bias could not be neglected [57].
A narrative synthesis was carried out because the data collected did not allow us to perform a proper meta-analysis, despite a very meticulous protocol for search and review. The identified studies were small, retrospective (except one), with heterogenous patient populations, and with long inclusion periods. Furthermore, different definitions of PFS and OS were used, some studies included a small subset of patients treated with 2D, and the dose–response analysis was not the primary outcome in any of the studies of this systematic review. Therefore, there is a need for studies of higher quality with homogeneous patient groups, preferably coming from specialized centers with a relatively high volume of TET patients per year. Planning a randomized controlled study (RCT) or a retrospective study with a very low risk of bias is challenging; thus, larger studies with a homogeneous sample, likely coming from specialized centers with a high volume of patients with TETs per year, would be auspicious to extract robust data (e.g. adopting propensity score methods [58], [59]). However, population-based comparative efficacy research explored possible drawbacks and pitfalls when comparing results from observational studies and RCTs to infer treatment effects in oncology [60]. Nonetheless, biased data on RT could be extracted from RCTs accounting for differences in technology, user skills, operation procedures, length of recruitment and learning curve between the centers involved in the same trial.
Unfortunately, even the biggest registers of patients used in similar analyses such as the Surveillance, Epidemiology and End Results database (SEER) or the Chinese Alliance of Research for Thymoma (ChART), are not useful for evaluating the radiation dose to deliver because information on RT technique, dose fractionation and outcomes are missing [45], [61], [62], [63], [64]. We believe our protocol of search and review might be of help when future studies will be available on this topic.
We expect in the upcoming years the results from the promising and multicenter “Francophone” trial will help in defining the radiation doses appropriate to treating TETs (NCT02724696) [65]. Additional findings on the possible abscopal effect while delivering SBRT together with recombined human granulocyte–macrophage colony stimulating factor (rhGM-CSF) and Peginterferon alfa-2b in the metastatic setting of TETs (NCT04517539) could be diriment [66].
Conclusions
With this systematic review, we could not identify the optimal RT dose for postoperative, nor primary RT in the era of modern RT because only a few small studies with heterogeneity regarding WHO subtype, stage, treatment strategy and RT-technique could be identified. Therefore, to date, current clinical practice guidelines remain comprehensive and valid. This work provides a unique look into modern RT for TETs. It might serve as an eye-opener, to spur and promote homogeneous research in this field, looking for clear findings on the radiation dose and dose–response analyses in different treatment settings of patients with TETs.
Authors’ contributions
SP: Planning, design, acquisition of data, analysis, and manuscript writing; AA: Planning, design, acquisition of data, analysis, and manuscript writing; RH: Acquisition of data and manuscript writing; DR: Design, acquisition of data, analysis, and manuscript writing; FM: Analysis and manuscript writing; MH: Analysis and manuscript writing, JM: Analysis and manuscript writing. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.03.005.
Contributor Information
A. Angrisani, Email: angrisani.antonio.4@gmail.com.
S. Peeters, Email: stephanie.peeters@maastro.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Carter B.W., Okumura M., Detterbeck F.C., Marom E.M. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol. 2014;9:S110–S118. doi: 10.1097/JTO.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 2.Carter B.W., Benveniste M.F., Madan R., Godoy M.C., de Groot P.M., Truong M.T., et al. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics. 2017;37(2):413–436. doi: 10.1148/rg.2017160095. [DOI] [PubMed] [Google Scholar]
- 3.Marx A., Ströbel P., Badve S.S., Chalabreysse L., Chan J.K.C., Chen G., et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9(5):596–611. doi: 10.1097/JTO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 4.Marx A., Chan J.K.C., Coindre J.-M., Detterbeck F., Girard N., Harris N.L., et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol. 2015;10(10):1383–1395. doi: 10.1097/JTO.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girard N., Ruffini E., Marx A., Faivre-Finn C., Peters S. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v40–v55. doi: 10.1093/annonc/mdv277. [DOI] [PubMed] [Google Scholar]
- 6.Zhu G., He S., Fu X., Jiang G., Liu T. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys. 2004;60:1113–1119. doi: 10.1016/j.ijrobp.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Bott MJ, Wang H, Travis W, Riely GJ, Bains M, Downey R, et al. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg 2011;92:1984–91; discussion 1991-1992. Doi: 10.1016/j.athoracsur.2011.07.078. [DOI] [PubMed]
- 8.Huang J., Riely G.J., Rosenzweig K.E., Rusch V.W. Multimodality therapy for locally advanced thymomas: state of the art or investigational therapy? Ann Thorac Surg. 2008;85:365–367. doi: 10.1016/j.athoracsur.2007.10.098. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg D., Port J.L., Weksler B., Delgado R., Rosai J., Bains M.S., et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg. 1995;60(4):908–914. doi: 10.1016/0003-4975(95)00669-c. [DOI] [PubMed] [Google Scholar]
- 10.Wright C.D. Management of thymomas. Crit Rev Oncol Hematol. 2008;65:109–120. doi: 10.1016/j.critrevonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wright C.D., Wain J.C., Wong D.R., Donahue D.M., Gaissert H.A., Grillo H.C., et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg. 2005;130(5):1413–1421. doi: 10.1016/j.jtcvs.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Rimner A., Yao X., Huang J., Antonicelli A., Ahmad U., Korst R.J., et al. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol. 2016;11(10):1785–1792. doi: 10.1016/j.jtho.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akaogi E., Ohara K., Mitsui K., Onizuka M., Ishikawa S., Mitsui T., et al. Preoperative radiotherapy and surgery for advanced thymoma with invasion to the great vessels. J Surg Oncol. 1996;63:17–22. doi: 10.1002/(SICI)1096-9098(199609)63:1<17::AID-JSO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Gao L., Wang C., Fang W., Zhang J., Lv C., Fu S. Outcome of multimodality treatment for 188 cases of type B3 thymoma. J Thorac Oncol. 2013;8:1329–1334. doi: 10.1097/JTO.0b013e31829ceb50. [DOI] [PubMed] [Google Scholar]
- 15.Rimner A., Gomez D.R., Wu A.J., Shi W., Yorke E.D., Moreira A.L., et al. Failure patterns relative to radiation treatment fields for stage II-IV thymoma. J Thorac Oncol. 2014;9(3):403–409. doi: 10.1097/JTO.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 16.A B, A S, M P, P B, N.g L, E D, et al. The role of postoperative radiotherapy for thymomas: A multicentric retrospective evaluation from three Italian centers and review of the literature. J Thorac Dis 7518. [DOI] [PMC free article] [PubMed]
- 17.J M, X S, L H, Z X, M Y, S Z, et al. Postoperative radiotherapy and tumor recurrence after complete resection of stage II /III thymic tumor: A meta-analysis of cohort studies. OncoTargets and Therapy 4517. [DOI] [PMC free article] [PubMed]
- 18.] F Y, J F, Y W, L T, P Z, Y H, et al. The role of postoperative radiotherapy for stage I/II/III thymic tumor-Results of the ChART retrospective database. J Thorac Dis; 2016. [DOI] [PMC free article] [PubMed]
- 19.Tateishi Y., Horita N., Namkoong H., Enomoto T., Takeda A., Kaneko T. Postoperative Radiotherapy for Completely Resected Masaoka/Masaoka-Koga Stage II/III Thymoma Improves Overall Survival: An Updated Meta-Analysis of 4746 Patients. J Thorac Oncol. 2021;16:677–685. doi: 10.1016/j.jtho.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Cowen D., Richaud P., Mornex F., Bachelot T., Jung G.M., Mirabel X., et al. Thymoma: results of a multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. FNCLCC trialists. Fédération Nationale des Centres de Lutte Contre le Cancer. Radiother Oncol. 1995;34(1):9–16. doi: 10.1016/0167-8140(94)01493-m. [DOI] [PubMed] [Google Scholar]
- 21.Takeda S., Sawabata N., Inoue M., Koma M., Maeda H., Hirano H. Thymic carcinoma. Clinical institutional experience with 15 patients. Eur J Cardiothorac Surg. 2004;26:401–406. doi: 10.1016/j.ejcts.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Nonaka T., Tamaki Y., Higuchi K., Katoh H., Nakahashi M., Horikoshi H., et al. The role of radiotherapy for thymic carcinoma. Jpn J Clin Oncol. 2004;34:722–726. doi: 10.1093/jjco/hyh141. [DOI] [PubMed] [Google Scholar]
- 23.Gomez D., Komaki R. Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol. 2010;5:S336–S343. doi: 10.1097/JTO.0b013e3181f20ea2. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–9):W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 25.McHugh M.L. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 26.VonVille HM. Excel workbooks for systematic reviews. 2015, 2015.
- 27.VonVille HM. Excel workbooks for systematic reviews. University of Texas Health Science Center at Houston School of Public Health …; 2015.
- 28.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 29.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020:l6890. Doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed]
- 30.Suk Park M., Young Chung K., Dong Kim K., Ick Yang W., Ho Chung J., Sam Kim Y., et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg. 2004;78(3):992–997. doi: 10.1016/j.athoracsur.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 31.Kondo K., Monden Y. Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg. 2005;79:219–224. doi: 10.1016/j.athoracsur.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 32.D'Angelillo R.M., Trodella L., Ramella S., Cellini N., Balducci M., Mantini G., et al. Novel prognostic groups in thymic epithelial tumors: assessment of risk and therapeutic strategy selection. Int J Radiat Oncol Biol Phys. 2008;71(2):420–427. doi: 10.1016/j.ijrobp.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Regnard J.-F., Magdeleinat P., Dromer C., Dulmet E., De Montpreville V., Levi J.-F., et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112(2):376–384. doi: 10.1016/S0022-5223(96)70265-9. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan X.-W., Yang Y.u., Wang H.-B., Xu Y., Kang M., Xie L.-Y., et al. Intensity Modulated Radiation Therapy Plus Etoposide/Cisplatin for Patients With Limited Advanced Unresectable Thymic Epithelial Tumors: A Prospective Phase 2 Study. Int J Radiat Oncol Biol Phys. 2020;107(1):98–105. doi: 10.1016/j.ijrobp.2019.12.045. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.-D., Feng Q.-F., Lu H.-Z., Mao Y.-S., Zhou Z.-M., Ou G.-F., et al. Role of adjuvant radiotherapy for stage II thymoma after complete tumor resection. Int J Radiat Oncol Biol Phys. 2010;78(5):1400–1406. doi: 10.1016/j.ijrobp.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 37.Fan C., Feng Q., Chen Y., Zhai Y., Zhou Z., Chen D., et al. Postoperative radiotherapy for completely resected Masaoka stage III thymoma: a retrospective study of 65 cases from a single institution. Radiat Oncol. 2013;8(1) doi: 10.1186/1748-717X-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan C., Ge H., Zhang S., Xing W., Ye K.e., Zheng Y., et al. Impact of Definitive Radiotherapy and Surgical Debulking on Treatment Outcome and Prognosis for Locally Advanced Masaoka-Koga stage III Thymoma. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-58692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.A.j Y, S.h C, H.k B, H.j K, C.g L, J C. The role of salvage radiotherapy in recurrent thymoma. Radiat Oncol J; 2019. [DOI] [PMC free article] [PubMed]
- 40.Hao X.-J., Peng B., Zhou Z., Yang X.-Q. Prospective Study of Stereotactic Body Radiation Therapy for Thymoma and Thymic Carcinoma: Therapeutic Effect and Toxicity Assessment. Sci Rep. 2017;7:13549. doi: 10.1038/s41598-017-12909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou D., Deng X.-F., Liu Q.-X., Zheng H., Min J.-X., Dai J.-G. The Effectiveness of Postoperative Radiotherapy in Patients With Completely Resected Thymoma: A Meta-Analysis. Ann Thorac Surg. 2016;101:305–310. doi: 10.1016/j.athoracsur.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Liao J., Liu T., Zhang H., Cai F., Chen J., Dang J. The role of postoperative radiation therapy for completely resected stage III thymoma and effect of higher heart radiation dose on risk of cardiovascular disease: A retrospective cohort study. Int J Surg. 2018;53:345–349. doi: 10.1016/j.ijsu.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Banfill K., Giuliani M., Aznar M., Franks K., McWilliam A., Schmitt M., et al. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J Thorac Oncol. 2021;16(2):216–227. doi: 10.1016/j.jtho.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes A.T., Shinohara E.T., Guo M., Mitra N., Wilson L.D., Rengan R., et al. The role of radiation therapy in malignant thymoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Oncol. 2010;5(9):1454–1460. doi: 10.1097/JTO.0b013e3181e8f345. [DOI] [PubMed] [Google Scholar]
- 46.Barsky A.R., Kim M.M., Williams G.R., Lally B.E., Ingram W.S., Cengel K.A., et al. Proton-Beam Therapy: At the Heart of Cardiac Dose-Sparing in Mediastinal Radiotherapy for Thymic Carcinoma. J Thorac Oncol. 2020;15(7):1240–1242. doi: 10.1016/j.jtho.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Figura N., Hoppe B.S., Flampouri S., Su Z., Osian O., Monroe A., et al. Postoperative proton therapy in the management of stage III thymoma. J Thorac Oncol. 2013;8(5):e38–e40. doi: 10.1097/JTO.0b013e31827a8911. [DOI] [PubMed] [Google Scholar]
- 48.Franceschini D., Cozzi L., Loi M., Franzese C., Reggiori G., Mancosu P., et al. Volumetric modulated arc therapy versus intensity-modulated proton therapy in the postoperative irradiation of thymoma. J Cancer Res Clin Oncol. 2020;146(9):2267–2276. doi: 10.1007/s00432-020-03281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haefner M.F., Verma V., Bougatf N., Mielke T., Tonndorf-Martini E., König L., et al. Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol. 2018;57(12):1713–1720. doi: 10.1080/0284186X.2018.1502467. [DOI] [PubMed] [Google Scholar]
- 50.J.h V, A.t B, T.t P, L.p W, P.e G, S K, et al. Proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma. J Thora Dis; 2015.
- 51.Mercado C.E., Hartsell W.F., Simone C.B., Tsai H.K., Vargas C.E., Zhu H.J., et al. Proton therapy for thymic malignancies: multi-institutional patterns-of-care and early clinical outcomes from the proton collaborative group and the university of Florida prospective registries. Acta Oncol. 2019;58(7):1036–1040. doi: 10.1080/0284186X.2019.1575981. [DOI] [PubMed] [Google Scholar]
- 52.Vogel J., Lin L., Simone C.B., Berman A.T. Risk of major cardiac events following adjuvant proton versus photon radiation therapy for patients with thymic malignancies. Acta Oncol. 2017;56(8):1060–1064. doi: 10.1080/0284186X.2017.1302097. [DOI] [PubMed] [Google Scholar]
- 53.Vogel J., Berman A.T., Lin L., Pechet T.T., Levin W.P., Gabriel P., et al. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol. 2016;118(3):504–509. doi: 10.1016/j.radonc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Ried M., Eicher M.-M., Neu R., Kraus D., Inderhees S., Marx A., et al. Comparison of the Masaoka-Koga Classification with the New TNM Staging of the IASLC/ITMIG for Thymoma and Thymic Carcinoma. Zentralbl Chir. 2018;143:S44–S50. doi: 10.1055/a-0606-5603. [DOI] [PubMed] [Google Scholar]
- 55.Marcuse F., Peeters S., Herman K., Vaassen F., van Elmpt W., Maat A.P.W.M., et al. Optimal delineation of the clinical target volume for thymomas in the post-resection setting: A multi-center study. Radiother Oncol. 2021;165:8–13. doi: 10.1016/j.radonc.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Modh A., Rimner A., Allen P.K., Greenfield B., Marom E.M., Rice D., et al. Treatment Modalities and Outcomes in Patients With Advanced Invasive Thymoma or Thymic Carcinoma: A Retrospective Multicenter Study. Am J Clin Oncol. 2016;39(2):120–125. doi: 10.1097/COC.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C.-F., Yu L., Jing Y., Zhang Y.-F., Ke J. Value of Adjuvant Radiotherapy for Thymoma with Myasthenia Gravis after Extended Thymectomy. Chin Med J (Engl) 2018;131:927–932. doi: 10.4103/0366-6999.229894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leuzzi G., Rocco G., Ruffini E., Sperduti I., Detterbeck F., Weder W., et al. Multimodality therapy for locally advanced thymomas: A propensity score-matched cohort study from the European Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2016;151(1):47–57.e1. doi: 10.1016/j.jtcvs.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 60.Soni P.D., Hartman H.E., Dess R.T., Abugharib A., Allen S.G., Feng F.Y., et al. Comparison of Population-Based Observational Studies With Randomized Trials in Oncology. J Clin Oncol. 2019;37(14):1209–1216. doi: 10.1200/JCO.18.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim Y.J., Kim H.J., Wu H.-G. Role of Postoperative Radiotherapy in Nonlocalized Thymoma: Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. J Thorac Oncol. 2015;10:1357–1363. doi: 10.1097/JTO.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 62.Mou H., Liao Q., Hou X., Chen T., Zhu Y. Clinical characteristics, risk factors, and outcomes after adjuvant radiotherapy for patients with thymoma in the United States: analysis of the Surveillance, Epidemiology, and End Results (SEER) Registry (1988–2013) Int J Radiat Biol. 2018;94:495–502. doi: 10.1080/09553002.2018.1454618. [DOI] [PubMed] [Google Scholar]
- 63.J.-H F, Q.-W L, F Y, W.-T F, K.n C, Z.-T Y, et al. The role of postoperative radiotherapy for stage I/II/III thymic tumor-results of chart database. J Thora Dis; 2015.
- 64.F W, L P, J F, Y W, L T, P Z, et al. Postoperative survival for patients with thymoma complicating myasthenia gravis-Preliminary retrospective results of the ChART database. J Thorac Dis; 2016. [DOI] [PMC free article] [PubMed]
- 65.Intergroupe Francophone de Cancerologie Thoracique. French National Observatory of Patients With Thymic Epithelial Tumor. clinicaltrials.gov; 2020.
- 66.Shanghai Cancer Hospital, China. Abscopal Effect of SBRT in Combination With rhGM-CSF and INF-α 2b for Metastatic Thymic Epithelial Tumors. clinicaltrials.gov; 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.