Abstract
Importance
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have been widely recommended for glucose control and cardiovascular risk reduction in patients with type 2 diabetes, and more recently, for weight loss. However, the associations of GLP-1 RAs with gallbladder or biliary diseases are controversial.
Objective
To evaluate the association of GLP-1 RA treatment with gallbladder and biliary diseases and to explore risk factors for these associations.
Data Sources
MEDLINE/PubMed, EMBASE, Web of Science, and Cochrane Library (inception to June 30, 2021), websites of clinical trial registries (July 10, 2021), and reference lists. There were no language restrictions.
Study Selection
Randomized clinical trials (RCTs) comparing the use of GLP-1 RA drugs with placebo or with non−GLP-1 RA drugs in adults.
Data Extraction and Synthesis
Two reviewers independently extracted data according to the PRISMA recommendations and assessed the quality of each study with the Cochrane Collaboration risk-of-bias tool. Pooled relative risks (RRs) were calculated using random or fixed-effects models, as appropriate. The quality of evidence for each outcome was assessed using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) framework.
Main Outcomes and Measures
The primary outcome was the composite of gallbladder or biliary diseases. Secondary outcomes were biliary diseases, biliary cancer, cholecystectomy, cholecystitis, and cholelithiasis. Data analyses were performed from August 5, 2021, to September 3, 2021.
Results
A total of 76 RCTs involving 103 371 patients (mean [SD] age, 57.8 (6.2) years; 41 868 [40.5%] women) were included. Among all included trials, randomization to GLP-1 RA treatment was associated with increased risks of gallbladder or biliary diseases (RR, 1.37; 95% CI, 1.23-1.52); specifically, cholelithiasis (RR, 1.27; 95% CI, 1.10-1.47), cholecystitis (RR, 1.36; 95% CI, 1.14-1.62), and biliary disease (RR, 1.55; 95% CI, 1.08-2.22). Use of GLP-1 RAs was also associated with increased risk of gallbladder or biliary diseases in trials for weight loss (n = 13; RR, 2.29; 95% CI, 1.64-3.18) and for type 2 diabetes or other diseases (n = 63; RR, 1.27; 95% CI, 1.14-1.43; P <.001 for interaction). Among all included trials, GLP-1 RA use was associated with higher risks of gallbladder or biliary diseases at higher doses (RR, 1.56; 95% CI, 1.36-1.78) compared with lower doses (RR, 0.99; 95% CI, 0.73-1.33; P = .006 for interaction) and with longer duration of use (RR, 1.40; 95% CI, 1.26-1.56) compared with shorter duration (RR, 0.79; 95% CI, 0.48-1.31; P = .03 for interaction).
Conclusions and Relevance
This systematic review and meta-analysis of RCTs found that use of GLP-1 RAs was associated with increased risk of gallbladder or biliary diseases, especially when used at higher doses, for longer durations, and for weight loss.
Trial Registration
PROSPERO Identifier: CRD42021271599
Key Points
Question
What is the association of glucagon-like peptide-1 receptor agonist (GLP-1 RAs) use with the risk of gallbladder or biliary diseases?
Findings
This systematic review and meta-analysis of 76 randomized clinical trials found that use of GLP-1 RAs was associated with increased risk of gallbladder or biliary diseases, especially when used at higher doses, for longer durations, and for weight loss.
Meaning
The findings of this systematic review and meta-analysis indicate that physicians and patients should be concerned about the risks of gallbladder or biliary diseases with using GLP-1 RAs for treatment in clinical practice; future studies should report on associated gallbladder and biliary diseases.
This systematic review and meta-analysis of 76 randomized clinical trials examines the effects of glucagon-like peptide-1 receptor agonist use on the risk of gallbladder and biliary diseases.
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are recommended for patients with type 2 diabetes to control glycemia and reduce cardiovascular risk, and for patients with obesity to reduce weight.1,2,3,4 Given the widespread use of these drugs,3 potential safety concerns deserve attention.
Several randomized clinical trials (RCTs) have shown a higher rate of gallbladder disorders in patients who were randomized to GLP-1 RAs vs a placebo.5,6,7 However, whether increased risk of gallbladder-related events is a class effect of GLP-1 RAs has not been established,8,9 and prescribing information for all GLP-1 RA medications does not provide a warning regarding increased risk of gallbladder disorders.10,11 In addition to gallbladder-related events, a post hoc analysis of the LEADER trial8 found significantly increased risks of acute biliary obstruction in patients randomized to liraglutide compared with placebo. Because GLP-1 RAs are generally prescribed at higher doses for weight loss rather than for control of type 2 diabetes, there may be differential effects on risk for gallbladder or biliary diseases depending on dose.
In response to these knowledge gaps, we conducted a systematic review and meta-analysis to evaluate the associations of GLP-1 RA use with the risk for gallbladder or biliary diseases. We also sought to determine if risks differ by indication (for diabetes vs weight loss), dose, or duration of treatment.
Methods
The protocol of this systematic review and meta-analysis was prospectively registered in the International Prospective Register of Systematic Reviews (CRD42021271599). The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline statement.12 Ethical review and informed consent were waived because the study used secondary data from previous studies.
Data Sources and Searches
The literature search was conducted of MEDLINE (via PubMed), Cochrane Library, EMBASE, and Web of Science, from inception to June 30, 2021, with no language restrictions (details are available in eMethods 1 in the Supplement). The search was supplemented by screening the reference lists of relevant systematic reviews and manually searching for gray literature on clinical trial registries.
Study Selection
Two reviewers (H.L.Y., Z.H.B.) independently searched for randomized clinicals trials of GLP-1 RA medications (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, or semaglutide) that also reported adverse events of gallbladder or biliary diseases according to predefined inclusion and exclusion criteria available in eTable 1 in the Supplement. Eligible studies were identified and selected by 2 reviewers (H.L.Y., W.J.L.) who screened titles, abstracts, and citations, and evaluated full-text records. Disagreements were resolved through discussions with other team members.
Outcome Measures
The primary outcome was a composite of gallbladder or biliary diseases, including gallbladder disorders and biliary-related events. Secondary outcomes were 3 subcategories of gallbladder and biliary diseases, including bile duct obstruction, stenosis, and stone; biliary colic, cyst, and fistula; biliary tract cancer; cholecystectomy, cholecystitis, and cholelithiasis; and cholangitis. Definitions of outcomes are shown in eMethods 2 in the Supplement.
Data Extraction and Quality Assessments
Data extraction was performed independently by 2 reviewers (H.L.Y., H.J.Y.) using a standardized predefined data extraction form. Extracted data included first author or trial name, publication year, indication for treatment, duration of treatments, GLP-1 RA administrations, comparator drugs, incidence of gallbladder or biliary disease outcomes, and characteristics of trial participants (ie, age, weight, body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], and glycated hemoglobin [HbA1c] levels).
The risk of bias in each of the studies included was assessed independently by 3 reviewers (H.L.Y., Z.H.B., W.J.L.) using the revised Cochrane risk-of-bias tool for randomized clinical trials.13 Disagreements were resolved by discussion with other team members.
Data Synthesis and Analysis
Pooled relative risks (RR) and 95% CIs were calculated using random or fixed-effects models. Statistical heterogeneity was assessed using Q tests and I2 statistic.14 Random-effects models with the DerSimonian-Laird method were used when there was no significant heterogeneity (Q tests, P < .05; I2 > 50%); otherwise, Mantel-Haenszel methods and fixed-effects models were used. Absolute risks were estimated based on the calculated RR and mean event rate across control groups for each outcome, and the event rates derived from different treatment durations for each outcome were converted into annual incidences.15,16
In subgroup analyses, we evaluated associations with gallbladder or biliary diseases of specific GLP-1 RA medications, higher and lower doses, shorter and longer treatment durations (≤26 or >26 weeks), indication for treatment (type 2 diabetes/other diseases or obesity), type of control (placebo or active comparator), and baseline BMI. High doses of GLP-1 RAs were defined as equal to or greater than: albiglutide, 50 mg once weekly; dulaglutide, 1.5 mg once weekly; liraglutide, 1.8 mg once daily; subcutaneous semaglutide, 1.0 mg once weekly; and oral semaglutide, 7 mg once daily. Low doses were defined as: albiglutide, 30 mg (<50 mg) once weekly; dulaglutide, 0.75 mg (<1.5 mg) once weekly; liraglutide, 0.6 to 1.2 mg (<1.8 mg) once daily; subcutaneous semaglutide, 0.5 mg (<1.0 mg) once weekly; and oral semaglutide, 3.0 mg (<7.0 mg) once daily. Exenatide and lixisenatide as single doses were not included to assess the dosage-dependent effects. Significant differences between subgroups were estimated using metaregression with the residual maximum likelihood method.
Sensitivity analyses were conducted by omitting eligible trials 1 by 1 and removing studies with albiglutide. Random-effects models were also used to pool results for sensitivity analysis.
Publication bias was assessed visually using funnel plots and statistically using the Egger test. Certainty of the evidence for each outcome in the analysis was evaluated using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) framework,17 which considers risk of bias, imprecision, inconsistency, indirectness, and publication bias of the included studies.
Analyses were repeated and restricted to trials for control of type 2 diabetes or weight loss. Subgroup analyses by dose or duration of treatment were only performed in trials for control of diabetes owing to the small number of trials that included lower doses or shorter duration for weight loss. Finally, among the trials of treatment for diabetes, we assessed risk of gallbladder and biliary diseases according to whether the trial aimed to assess cardiovascular outcome or not.
Statistical analyses were conducted from August 5, 2021, to September 3, 2021, using R, version 4.0.2 (R Foundation for Statistical Computing). Tests were 2-tailed, and P values <.05 were considered statistically significant.
Results
Trial Identification and Characteristics
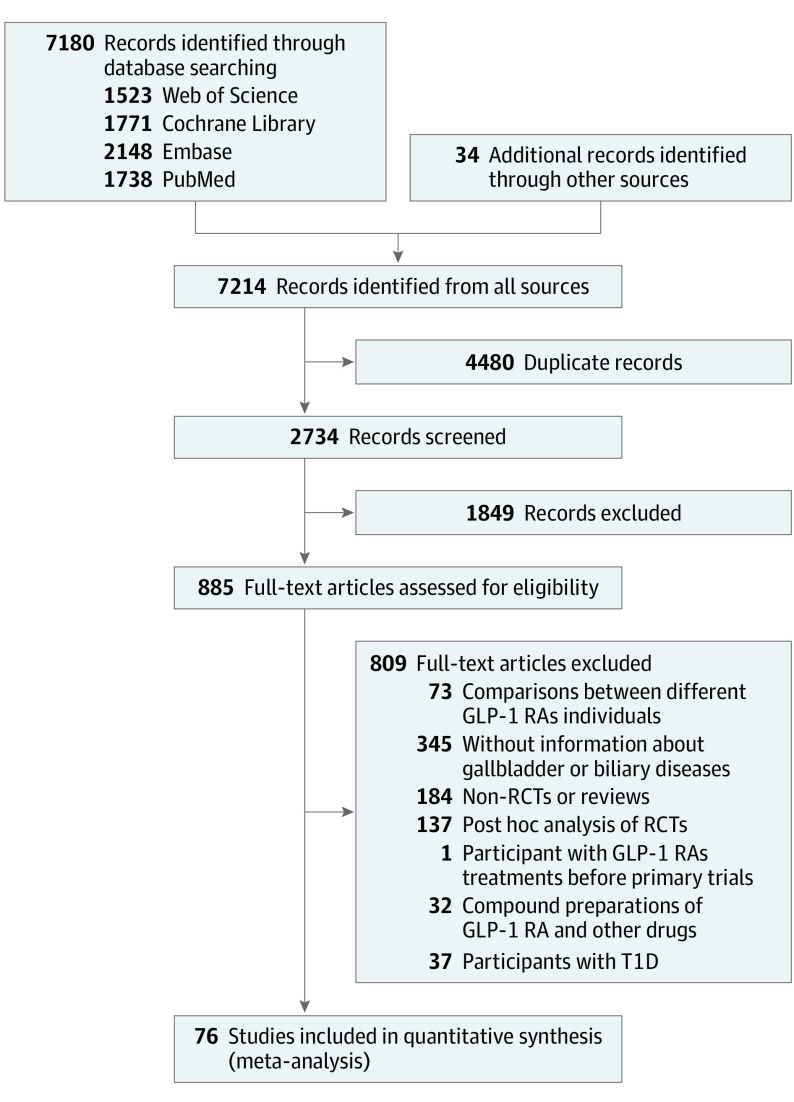
The literature search identified 7214 potentially relevant studies (Figure 1), of which 76 randomized clinical trials with 77 data sets were included in the meta-analysis—the complete list is available in the eReferences of the Supplement. The included studies had a combined total of 103 371 participants (mean [SD] age, 57.8 [6.2] years; 41 868 [40.5%] women; mean BMI, 32.6; mean HbA1c, 7.8%). Most of the trials included participants with type 2 diabetes (84.4%). The baseline characteristics of eligible trials and participants are shown in eTable 2 in the Supplement.
Figure 1. PRISMA Flow Diagram.
GLP-1 RAs denotes glucagon-like peptide-1 receptor agonists; RCTs, randomized clinical trials; and T1D, type 1 diabetes.
Stratified by indication for treatment, there were 60 trials (including 61 data sets) with 91 599 participants randomized to GLP-1 RAs for diabetes, 13 trials with 11 281 participants randomized for weight loss, and 3 trials for nonalcoholic steatohepatitis, polycystic ovary syndrome, and schizophrenia. Three trials conducted in participants with type 2 diabetes were classified with weight loss trials because their primary outcome was change in weight and GLP-1 RA doses were in the weight-loss range. The mean BMI of patients in trials for treatment of diabetes and obesity was 31.6 and 36.9, respectively.
Risk of Bias and GRADE Rating
The risk of bias for each study is shown in eTable 3 and eFigure 1 in the Supplement. Most of the studies had a low risk or some concern of bias across all 5 domains evaluated.
The overall quality of evidence for the comparisons of GLP-1 RAs vs control groups for increased risk of the combined outcome of gallbladder or biliary disease was rated high. For the secondary outcomes of cholelithiasis, cholecystitis, and biliary disease, the quality of evidence was rated high or moderate (eTable 4 in the Supplement).
Association of GLP-1 RAs With Gallbladder or Biliary Disease
Randomization to GLP-1 RAs treatment was associated with a significantly increased risk of gallbladder or biliary diseases (RR, 1.37; 95% CI, 1.23-1.52; I2 = 0%) compared with controls (eResults in the Supplement); the absolute risk difference (range) was an additional 27 (17-38) events per 10 000 patients per year (Figure 2). Treatment with GLP-1 RAs was associated with increased risks of cholelithiasis (RR, 1.27; 95% CI, 1.10-1.47; I2 = 0%), cholecystitis (RR, 1.36; 95% CI, 1.14-1.62; I2 = 0%), and biliary disease (RR, 1.55; 95% CI, 1.08-2.22; I2 = 0%) compared with controls (Figure 2; eFigure 2 in the Supplement). The results from trials of diabetes or weight-loss treatment are presented in eFigures 3 to 6 in the Supplement.
Figure 2. Risks of Cholelithiasis, Cholecystitis, and Biliary Diseases in Patients Randomized to GLP-1 RA Treatment Compared With Controls in All Trials.
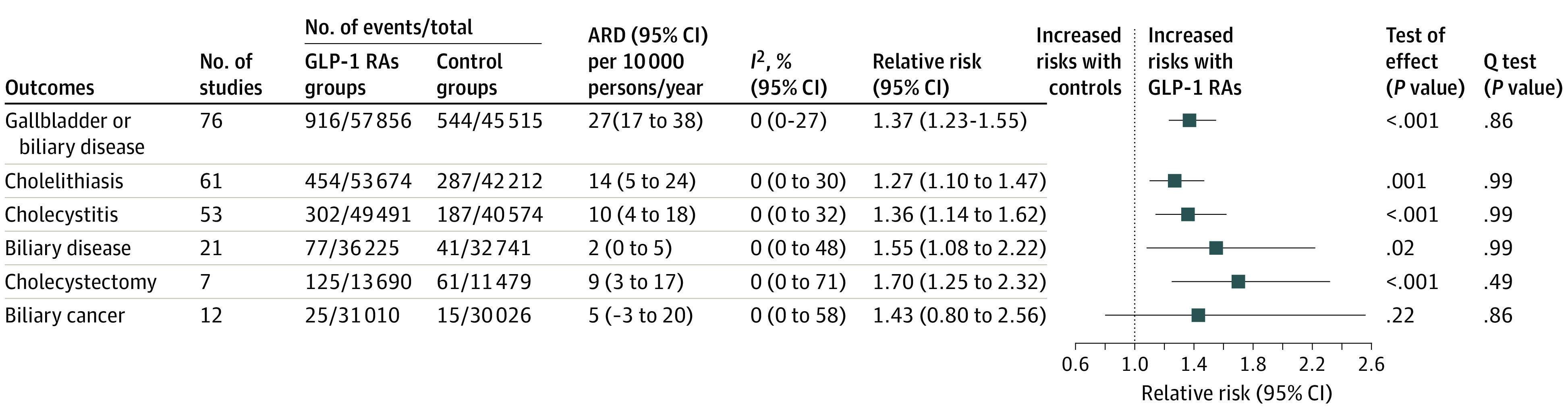
ARD denotes the absolute risk difference and GLP-1 RA, glucagon-like peptide-1 receptor agonist.
In all of the included trials, GLP-1 RAs was associated with a higher risk of cholecystectomy compared with controls (RR, 1.70; 95% CI, 1.25-2.32; I2 = 0%; eFigure 7 in the Supplement); there was no increase in risk of biliary tract cancer (RR, 1.43; 95% CI, 0.80-2.56; I2 = 0%; eFigure 8 in the Supplement).
Specific GLP-1 RA Drugs and Gallbladder or Biliary Disease
Compared with the control group, randomization to liraglutide (RR, 1.79; 95% CI, 1.45-2.25) or dulaglutide (RR, 1.35; 95% CI, 1.06-1.73) treatment was associated with increased risk for gallbladder or biliary diseases. Randomization to subcutaneous semaglutide (RR, 1.28; 95% CI, 0.99-1.65) and exenatide (RR, 1.23; 95% CI, 1.00-1.52) was associated with increased risk, although the increase was not statistically significant. However, oral semaglutide, lixisenatide, and albiglutide did not increase risk (Figure 3; eFigure 9 in the Supplement); higher doses of subcutaneous semaglutide (≥1.0 mg) were associated with increased gallbladder or biliary diseases (RR, 1.58; 95% CI, 1.13-2.22). The results in trials with treatment for diabetes or weight loss are presented in eFigures 10 to 12 in the Supplement.
Figure 3. Risks of Gallbladder or Biliary Diseases Associated With Individual GLP-1 RA Drugs.
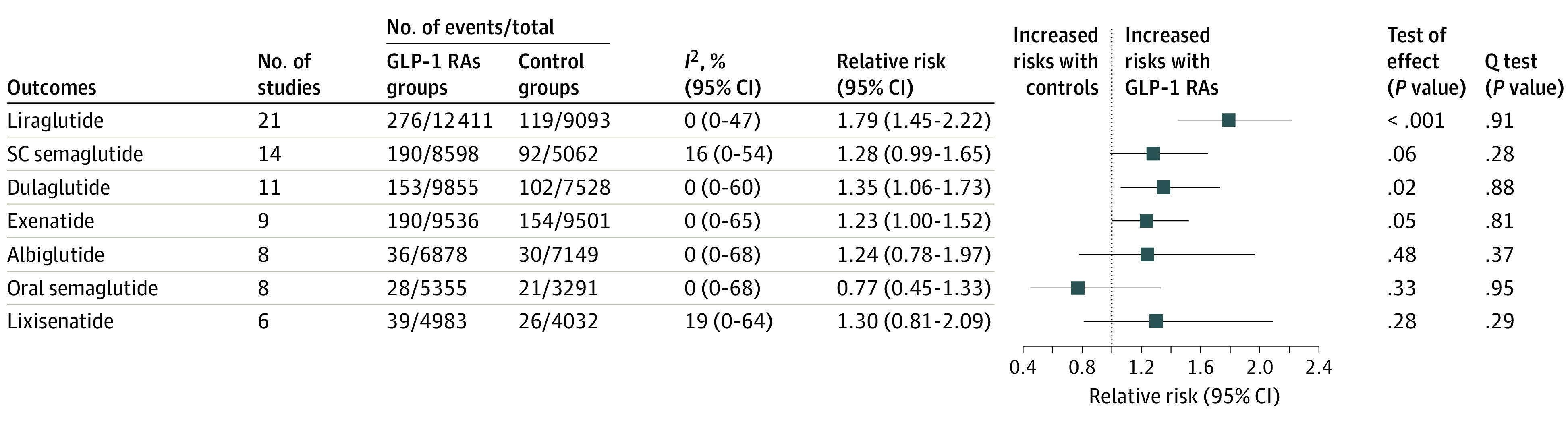
GLP-1 RA indicates glucagon-like peptide-1 receptor agonist; and SC, subcutaneous.
Dose, Duration, and Indication for Treatment
Use of GLP-1 RAs was significantly associated with increased risks of gallbladder or biliary disease at higher doses (RR, 1.56; 95% CI, 1.36-1.78) but not at lower doses (RR, 0.99; 95% CI, 0.74-1.33; P = .006 for interaction; Table). Longer duration of treatment with GLP-1 RAs (>26 weeks) was associated with increased risk for gallbladder or biliary disease (RR, 1.40; 95% CI, 1.26-1.56), but shorter duration (≤26 weeks) of treatment was not (RR, 0.79; 95% CI, 0.48-1.31; P = .03 for interaction; Table). Restricted to trials for diabetes, the effect of dose (P = .08 for interaction) and duration of treatment (P = .07 for interaction) were similar (supporting data reported in eTable 5 and eFigure 13 in the Supplement).
Table. Factors and Risks of Gallbladder or Biliary Diseases in 76 Randomized Clinical Trials of GLP-1 RA Drug Use.
| Factor | No. of patients | No. of trials | Relative risks (95% CI) | Heterogeneity | P value for interactiona | |
|---|---|---|---|---|---|---|
| I2 % | P value | |||||
| Treatment | ||||||
| Doseb | ||||||
| High | 61 962 | 54 | 1.56 (1.36-1.78) | 0 | .99 | .006 |
| Low | 16 952 | 33 | 0.99 (0.74-1.33) | 0 | .67 | |
| Duration, wk | ||||||
| ≤26 | 13 401 | 24 | 0.79 (0.48-1.31) | 0 | .97 | .03 |
| >26 | 90 417 | 53 | 1.40 (1.26-1.56) | 0 | .64 | |
| Indicationc | ||||||
| Weight loss | 11 282 | 13 | 2.29 (1.64-3.18) | 0 | .85 | <.001 |
| T2D/other | 92 090 | 63 | 1.27 (1.14-1.43) | 0 | .94 | |
| Baseline BMId | ||||||
| High | 25 275 | 33 | 1.49 (1.20-1.84) | 0 | .50 | .36 |
| Low | 77 530 | 42 | 1.33 (1.18-1.50) | 0 | .89 | |
| Type of control | ||||||
| Placebo | 80 281 | 45 | 1.41 (1.26-1.58) | 0 | .83 | .08 |
| Active comparator | 25 433 | 36 | 1.03 (0.74-1.44) | 0 | .93 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GLP-1 RA, glucagon-like peptide-1 receptor agonist; T2D, type 2 diabetes.
To test group differences, estimated with metaregression using the residual maximum likelihood method.
Only studies of albiglutide, dulaglutide, liraglutide, and semaglutide (oral and subcutaneous) had different treatment doses (a single trial may have ≥2 dose sizes).
One trial each of nonalcoholic steatohepatitis, polycystic ovary syndrome, and schizophrenia.
The cut point was 32.6 (baseline BMI mean in all trials).
Use of GLP-1 RAs for weight loss showed stronger effects on the risk of gallbladder or biliary disease than the other indications (ie, diabetes/other diseases; P <.001 for interaction; Table). No significant association of baseline BMI or type of control (placebo or active comparators) were observed (Table; eTables 5 and 6 and eFigure 14 in the Supplement). In trials for diabetes, there were no significant differences in effects in trials for cardiovascular or noncardiovascular outcomes (eFigure 15 in the Supplement).
Sensitivity Analyses
After the iterative omission of each trial, removal of all trials of albiglutide or which used random-effects models, the pooled results did not change. Additional details are available in eFigures 16 and 17 in the Supplement.
Publication Bias
There was no evidence of publication bias for studies of gallbladder or biliary diseases (P = .83), cholelithiasis (P = .19), cholecystitis (P = .20), or biliary diseases (P = .57) by the Egger test (supporting data are eFigure 18 in the Supplement). Visually, funnel plots did not demonstrate publication bias.
Discussion
This systematic review and meta-analysis of 76 randomized clinical trials found that randomization to treatment with GLP-1 RAs compared with placebo or active controls was associated with increased risk of the composite outcome of gallbladder or biliary diseases and for cholelithiasis, cholecystitis, and biliary diseases. Risk was increased in trials of patients treated for diabetes and for weight loss and was higher in the trials for weight reduction. Higher doses and longer duration of GLP-1 RAs treatment were also associated with increased risk of gallbladder or biliary diseases, although the association was not statistically significant.
Previous systematic reviews have reported an increased risk of cholelithiasis with GLP-1 RA use,18,19 but these reviews were limited to trials with restrictions on populations and duration of treatment and did not include several important studies that have been published recently.6,7,20
Use of GLP-1 RAs may be associated with increased risk of gallbladder or biliary diseases because GLP-1 inhibits gallbladder motility and delays gallbladder emptying by suppressing the secretion of cholecystokinin.8,21,22,23,24 In addition, marked weight loss, which occurs in some patients using GLP-1 RAs, has been associated with a high risk of gallbladder disorders.9,25
Gallbladder disease has been reported as an adverse event in the published reports and/or the supplemental materials of most of the randomized clinical trials of GLP-1 RAs6,20,26,27,28; however, to our knowledge, biliary diseases have seldom been reported. Only 20 of the 76 trials eligible for this systematic review clearly reported biliary-related events, suggesting potential underreporting. Given the findings of this review and analysis, studies of GLP-1 RAs should fully report biliary-related events.
The risk of gallbladder or biliary diseases was higher in trials using GLP-1 RAs for weight loss than for diabetes control. The increased risk of gallbladder or biliary diseases in weight loss trials may be associated with weight loss produced by GLP-1 RAs, but these trials also used higher doses and/or longer treatment durations than the diabetes control trials used. Liraglutide (3.0 mg)28 and subcutaneous semaglutide (2.4 mg)29 have been approved by the US Food and Drug Administration (FDA) for weight management,9,29 suggesting that GLP-1 RA drugs will increasingly be used at high doses for weight control; the increased risk for gallbladder and biliary diseases should be of concern in these patients.
Liraglutide has drawn the most attention concerning the increased risk of gallbladder-related events,7,8,9,30while other GLP-1 RA medications have received limited attention.9 Previous studies18,19 did not notice a high risk of gallbladder or biliary disease in patients randomized to dulaglutide treatment, and current prescribing information for dulaglutide does not contain warnings about the risks of gallbladder or biliary diseases.31,32 The prescribing information from the FDA and the European Medicines Agency mention the possible increased risk of cholelithiasis for exenatide33,34 and subcutaneous semaglutide35,36 compared with placebo. Our findings showed that GLP-1 RA medications, including dulaglutide, exenatide, and subcutaneous semaglutide (≥1.0 mg), increased the risk of gallbladder or biliary diseases. Given the similar efficacy and effectiveness of oral and subcutaneous semaglutide,37,38 the association of the oral formulation with the risk of gallbladder or biliary diseases requires further evaluation. Although in this meta-analysis, albiglutide and lixisenatide were not associated with a statistically significant increased risk, these findings may have been influenced by low power owing to a small number of studies or to inadequate reporting of gallbladder or biliary diseases in these trials.39,40,41,42
Higher risk of gallbladder or biliary diseases was associated with higher doses of GLP-1 RAs in weight loss trials compared with lower doses in diabetes control trials; however, there was a similar trend to increased risk with higher doses in the diabetes trials. Increasing doses of GLP-1 RAs may be recommended for patients who do not achieve glycemic control goals with lower doses43; therefore, increased risks of gallbladder or biliary diseases should be considered when the doses are escalated.
A previous observational study reported that gallbladder or biliary diseases tended to occur in the first 6 months of GLP-1 RA treatment.25 In contrast, the present meta-analysis found that GLP-1 RA use increased the risk of gallbladder or biliary disease only with longer durations of treatment. Given that GLP-1 RAs may need to be used for the long term, both for control of diabetes and weight, an increased risk associated with long-duration treatment could be clinically important.
Although this meta-analysis showed that the RRs for gallbladder and biliary diseases were elevated, the overall absolute risk increase was small (an additional 27 cases per 10 000 persons treated per year). This absolute risk increase should be weighed against the benefits of treatment with GLP-1 RA drugs.
Strengths and Limitations
This systematic review and meta-analysis was strengthened by the incorporation of recently published studies, by addressing the increased risks of biliary diseases with GLP-1 RA use separately from gallbladder disorders, and by highlighting the increased risk associated with other GLP-1 RA medications, in addition to liraglutide. The present study revealed that GLP-1 RAs used at higher doses for weight control may contribute to a higher risk of gallbladder or biliary diseases. However, this study had several limitations. Information on biliary-related events may not have been fully reported because they were not a predefined safety outcome in most of the included trials. These studies were not specifically designed to evaluate the risk of gallbladder or biliary diseases associated with GLP-1 RAs treatment. Also, because this was a meta-analysis of randomized trials, we were unable to use patient-level data to evaluate outcomes. Finally, the small number of events in subgroups may have allowed for underpowered subgroup analyses.
Conclusions
The findings of this systematic review and meta-analysis indicate that physicians should be concerned about the increased risk of gallbladder or biliary disease associated with GLP-1 RA use, especially at the higher doses recommended for weight loss. In addition, future trials should prespecify gallbladder and biliary diseases as potential adverse events, and fully test for and report on these outcomes.
eMethods 1. Data sources and search strategies
eMethods 2. Identification of the outcomes
eTable 1. Eligibility criteria of included studies
eTable 2. Baseline characteristics of studies and participants included
eTable 3. Assessments of risks of bias of eligible studies according to revised Cochrane risk-of-bias tool for randomized trials
eTable 4. Grade summary of findings for each outcome in the meta-analysis
eTable 5. Effects of factors on the risks of gallbladder or biliary diseases with GLP-1 RAs in trials with treatment for diabetes
eTable 6. Effects of factors on the risks of gallbladder or biliary diseases with GLP-1 RAs in trials with treatment for weight loss
eFigure 1. Summary of risks of bias of all included studies
eFigure 2. Risks of cholelithiasis, cholecystitis, and biliary diseases in patients with GLP-1 RAs treatments compared with controls
eFigure 3. Risks of the composite of gallbladder or biliary diseases, cholelithiasis, cholecystitis, and biliary diseases with glp-1 ras compared with controls in trials with treatment for diabetes
eFigure 4. Overall risks of cholelithiasis, cholecystitis, and biliary diseases in patients with GLP-1 RAs compared with controls in trials with treatment for diabetes
eFigure 5. Risks of the composite of gallbladder or biliary diseases, cholelithiasis, cholecystitis, and biliary diseases with GLP-1 RAs compared with controls in trials with treatment for weight loss
eFigure 6. Overall risks of cholelithiasis, cholecystitis, and biliary diseases in patients with GLP-1 RAs compared with controls in trials with treatment for weight loss
eFigure 7. Risks of cholecystectomy in patients with GLP-1 RAs compared with controls
eFigure 8. Risks of biliary tract cancer in patients with GLP-1 RAs compared with controls
eFigure 9. Risks of gallbladder or biliary diseases in patients with different GLP-1 RAs individuals compared with controls in all trials
eFigure 10. Risks of gallbladder or biliary diseases in patients with different GLP-1 RAs medications compared with controls in trials with treatment for diabetes
eFigure 11. Risks of gallbladder or biliary diseases in patients with different GLP-1 RAs medications compared with controls in trials with treatment for weight loss
eFigure 12. Effects of doses and duration of treatments on the association between GLP-1 RAs and gallbladder or biliary diseases in all trials
eFigure 13. Effects of doses and duration of treatments on the association between GLP-1 RAs and gallbladder or biliary diseases in trials with treatment for diabetes
eFigure 14. Effects of baseline bmi and types of control on the association between GLP-1 RAs and gallbladder or biliary diseases in all trials, trial for diabetes or weight loss
eFigure 15. Effects of types of trials on the risks of gallbladder or biliary diseases in trials with treatment for diabetes
eFigure 16. Sensitivity analyses by omitting each trial one by one and removing studies with albiglutide
eFigure 17. Sensitivity analyses by using random-effect models
eFigure 18. Funnel plot of all eligible studies
eResults. Risks of gallbladder or biliary diseases in patients with GLP-1RAs treatments compared with controls
eReferences
References
- 1.Kalyani RR. Glucose-lowering drugs to reduce cardiovascular risk in type 2 diabetes. N Engl J Med. 2021;384(13):1248-1260. doi: 10.1056/NEJMcp2000280 [DOI] [PubMed] [Google Scholar]
- 2.Liuzzo G, Galiuto L. GLP-1 receptor agonists: fighting obesity with an eye to cardiovascular risk. Eur Heart J. 2021;42(17):1652-1653. doi: 10.1093/eurheartj/ehab175 [DOI] [PubMed] [Google Scholar]
- 3.Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9(1):46-52. doi: 10.1016/S2213-8587(20)30343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx N, Grant PJ, Cosentino F. Compelling evidence for SGLT2 inhibitors and GLP-1 receptor agonists as first-line therapy in patients with diabetes at very high/high cardiovascular risk. Eur Heart J. 2020;41(2):329-330. doi: 10.1093/eurheartj/ehz853 [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, Bailey TS, Billings LK, et al. ; STEP 3 Investigators . Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilding JPH, Batterham RL, Calanna S, et al. ; STEP 1 Study Group . Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 7.Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719-1730. doi: 10.1056/NEJMoa2028198 [DOI] [PubMed] [Google Scholar]
- 8.Nauck MA, Muus Ghorbani ML, Kreiner E, Saevereid HA, Buse JB; LEADER Publication Committee on behalf of the LEADER Trial Investigators . Effects of liraglutide compared with placebo on events of acute gallbladder or biliary disease in patients with type 2 diabetes at high risk for cardiovascular events in the LEADER randomized trial. Diabetes Care. 2019;42(10):1912-1920. doi: 10.2337/dc19-0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen RM, Juhl CR, Torekov SS. Benefit-risk assessment of obesity drugs: focus on glucagon-like peptide-1 receptor agonists. Drug Saf. 2019;42(8):957-971. doi: 10.1007/s40264-019-00812-7 [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration . Drugs@FDA: FDA approved drug products. Accessed February 22, 2022. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- 11.European Medicines Agency . Medicines. Accessed February 22, 2022. https://www.ema.europa.eu/en/medicines
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Accessed February 22, 2022. www.training.cochrane.org/handbook.
- 15.Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021;374(1537):n1537. doi: 10.1136/bmj.n1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veroniki AA, Bender R, Glasziou P, Straus SE, Tricco AC. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J Clin Epidemiol. 2019;111:11-22. doi: 10.1016/j.jclinepi.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes Metab. 2017;19(9):1233-1241. doi: 10.1111/dom.12926 [DOI] [PubMed] [Google Scholar]
- 19.Nreu B, Dicembrini I, Tinti F, Mannucci E, Monami M. Cholelithiasis in patients treated with glucagon-like peptide-1 receptor: an updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2020;161:108087. doi: 10.1016/j.diabres.2020.108087 [DOI] [PubMed] [Google Scholar]
- 20.Davies M, Færch L, Jeppesen OK, et al. ; STEP 2 Study Group . Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 21.Shaddinger BC, Young MA, Billiard J, Collins DA, Hussaini A, Nino A. Effect of albiglutide on cholecystokinin-induced gallbladder emptying in healthy individuals: a randomized crossover study. J Clin Pharmacol. 2017;57(10):1322-1329. doi: 10.1002/jcph.940 [DOI] [PubMed] [Google Scholar]
- 22.Rehfeld JF, Knop FK, Asmar A, Madsbad S, Holst JJ, Asmar M. Cholecystokinin secretion is suppressed by glucagon-like peptide-1: clue to the mechanism of the adverse gallbladder events of GLP-1-derived drugs. Scand J Gastroenterol. 2018;53(12):1429-1432. doi: 10.1080/00365521.2018.1530297 [DOI] [PubMed] [Google Scholar]
- 23.Nexøe-Larsen CC, Sørensen PH, Hausner H, et al. Effects of liraglutide on gallbladder emptying: A randomized, placebo-controlled trial in adults with overweight or obesity. Diabetes Obes Metab. 2018;20(11):2557-2564. doi: 10.1111/dom.13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gether IM, Nexøe-Larsen C, Knop FK. New Avenues in the regulation of gallbladder motility-implications for the use of glucagon-like peptide-derived drugs. J Clin Endocrinol Metab. 2019;104(7):2463-2472. doi: 10.1210/jc.2018-01008 [DOI] [PubMed] [Google Scholar]
- 25.Faillie JL, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of bile duct and gallbladder diseases with the use of incretin-based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med. 2016;176(10):1474-1481. doi: 10.1001/jamainternmed.2016.1531 [DOI] [PubMed] [Google Scholar]
- 26.Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719-1730. doi: 10.1056/NEJMoa2028198 [DOI] [PubMed] [Google Scholar]
- 27.Álvarez-Villalobos NA, Treviño-Alvarez AM, González-González JG. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(18):1797-1798. doi: 10.1056/NEJMc1611289 [DOI] [PubMed] [Google Scholar]
- 28.Holman RR, Bethel MA, Mentz RJ, et al. ; EXSCEL Study Group . Effects of once weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration . FDA approves new drug treatment for chronic weight management, first since 2014. Accessed February 23, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- 30.Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: where do we stand? Curr Obes Rep. 2021;10(1):14-30. doi: 10.1007/s13679-020-00422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency . Trulicity. Accessed February 23, 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/trulicity
- 32.US Food and Drug Administration . Drug approval package Trulicity (dulaglutide) injection. NDA No. 125469. Accessed February 23, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125469Orig1s000TOC.cfm
- 33.AstraZeneca . Bydureon BCise full prescribing information. Accessed February 23, 2022. http://www.azpicentral.com/pi.html?product=bydureon_bcise
- 34.European Medicines Agency . Bydureon, annex I: summary of product characteristics. Accessed February 23, 2022. https://www.ema.europa.eu/en/documents/product-information/bydureon-epar-product-information_en.pdf
- 35.US Food and Drug Administration . Ozempic. NDA No. 209637. Accessed February 23, 2022. http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=209637
- 36.European Medicines Agency . Ozempic: EPAR product information. Accessed February 23, 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozempic#product-information-section
- 37.Meier JJ. Efficacy of semaglutide in a subcutaneous and an oral formulation. Front Endocrinol (Lausanne). 2021;12:645617. doi: 10.3389/fendo.2021.645617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460-1470. doi: 10.1001/jama.2017.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinget M, Goldenberg R, Niemoeller E, Muehlenbartmer I, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in patients with type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Abstracts of the 48th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia. 2012;55:S334. doi: 10.1007/s00125-012-2688-9 [DOI] [Google Scholar]
- 40.Ratner RE, Hanefeld M, Shamanna P, et al. Efficacy and safety of lixisenatide once daily versus placebo in patients with type 2 diabetes mellitus insufficiently controlled on sulfonylurea ± metformin (GetGoal-S). Abstracts of the 48th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia. 2011;54:S317. doi: 10.1007/s00125-011-2276-4 [DOI] [Google Scholar]
- 41.Reusch J, Stewart M, Perkins C, et al. HARMONY 1 results at week 52 primary endpoint: once weekly albiglutide vs placebo in patients with type 2 diabetes mellitus not controlled on pioglitazone ± metformin. Abstracts of the 48th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia. 2013;56:S359-S360. doi: 10.1007/s00125-013-3012-z [DOI] [Google Scholar]
- 42.Green JB, Hernandez AF, D’Agostino RB, et al. Harmony outcomes: a randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus: rationale, design, and baseline characteristics. Am Heart J. 2018;203:30-38. doi: 10.1016/j.ahj.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 43.Frías JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9(9):563-574. doi: 10.1016/S2213-8587(21)00174-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Data sources and search strategies
eMethods 2. Identification of the outcomes
eTable 1. Eligibility criteria of included studies
eTable 2. Baseline characteristics of studies and participants included
eTable 3. Assessments of risks of bias of eligible studies according to revised Cochrane risk-of-bias tool for randomized trials
eTable 4. Grade summary of findings for each outcome in the meta-analysis
eTable 5. Effects of factors on the risks of gallbladder or biliary diseases with GLP-1 RAs in trials with treatment for diabetes
eTable 6. Effects of factors on the risks of gallbladder or biliary diseases with GLP-1 RAs in trials with treatment for weight loss
eFigure 1. Summary of risks of bias of all included studies
eFigure 2. Risks of cholelithiasis, cholecystitis, and biliary diseases in patients with GLP-1 RAs treatments compared with controls
eFigure 3. Risks of the composite of gallbladder or biliary diseases, cholelithiasis, cholecystitis, and biliary diseases with glp-1 ras compared with controls in trials with treatment for diabetes
eFigure 4. Overall risks of cholelithiasis, cholecystitis, and biliary diseases in patients with GLP-1 RAs compared with controls in trials with treatment for diabetes
eFigure 5. Risks of the composite of gallbladder or biliary diseases, cholelithiasis, cholecystitis, and biliary diseases with GLP-1 RAs compared with controls in trials with treatment for weight loss
eFigure 6. Overall risks of cholelithiasis, cholecystitis, and biliary diseases in patients with GLP-1 RAs compared with controls in trials with treatment for weight loss
eFigure 7. Risks of cholecystectomy in patients with GLP-1 RAs compared with controls
eFigure 8. Risks of biliary tract cancer in patients with GLP-1 RAs compared with controls
eFigure 9. Risks of gallbladder or biliary diseases in patients with different GLP-1 RAs individuals compared with controls in all trials
eFigure 10. Risks of gallbladder or biliary diseases in patients with different GLP-1 RAs medications compared with controls in trials with treatment for diabetes
eFigure 11. Risks of gallbladder or biliary diseases in patients with different GLP-1 RAs medications compared with controls in trials with treatment for weight loss
eFigure 12. Effects of doses and duration of treatments on the association between GLP-1 RAs and gallbladder or biliary diseases in all trials
eFigure 13. Effects of doses and duration of treatments on the association between GLP-1 RAs and gallbladder or biliary diseases in trials with treatment for diabetes
eFigure 14. Effects of baseline bmi and types of control on the association between GLP-1 RAs and gallbladder or biliary diseases in all trials, trial for diabetes or weight loss
eFigure 15. Effects of types of trials on the risks of gallbladder or biliary diseases in trials with treatment for diabetes
eFigure 16. Sensitivity analyses by omitting each trial one by one and removing studies with albiglutide
eFigure 17. Sensitivity analyses by using random-effect models
eFigure 18. Funnel plot of all eligible studies
eResults. Risks of gallbladder or biliary diseases in patients with GLP-1RAs treatments compared with controls
eReferences