Abstract
Background
Cognitive impairment is a frequent consequence of stroke and can impact on a person's ability to perform everyday activities. Occupational therapists use a range of interventions when working with people who have cognitive impairment poststroke. This is an update of a Cochrane Review published in 2010.
Objectives
To assess the impact of occupational therapy on activities of daily living (ADL), both basic and instrumental, global cognitive function, and specific cognitive abilities in people who have cognitive impairment following a stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register, CENTRAL, MEDLINE, Embase, four other databases (all last searched September 2020), trial registries, and reference lists.
Selection criteria
We included randomised and quasi‐randomised controlled trials that evaluated an intervention for adults with clinically defined stroke and confirmed cognitive impairment. The intervention needed either to be provided by an occupational therapist or considered within the scope of occupational therapy practice as defined in the review. We excluded studies focusing on apraxia or perceptual impairments or virtual reality interventions as these are covered by other Cochrane Reviews. The primary outcome was basic activities of daily living (BADL) such as dressing, feeding, and bathing. Secondary outcomes were instrumental ADL (IADL) (e.g. shopping and meal preparation), community integration and participation, global cognitive function and specific cognitive abilities (including attention, memory, executive function, or a combination of these), and subdomains of these abilities. We included both observed and self‐reported outcome measures.
Data collection and analysis
Two review authors independently selected studies that met the inclusion criteria, extracted data, and assessed the certainty of the evidence. A third review author moderated disagreements if consensus was not reached. We contacted trial authors for additional information and data, where available. We assessed the certainty of key outcomes using GRADE.
Main results
We included 24 trials from 11 countries involving 1142 (analysed) participants (two weeks to eight years since stroke onset). This update includes 23 new trials in addition to the one study included in the previous version. Most were parallel randomised controlled trials except for one cross‐over trial and one with a two‐by‐two factorial design. Most studies had sample sizes under 50 participants. Twenty studies involved a remediation approach to cognitive rehabilitation, particularly using computer‐based interventions. The other four involved a compensatory and adaptive approach. The length of interventions ranged from 10 days to 18 weeks, with a mean total length of 19 hours. Control groups mostly received usual rehabilitation or occupational therapy care, with a few receiving an attention control that was comparable to usual care; two had no intervention (i.e. a waiting list). Apart from high risk of performance bias for all but one of the studies, the risk of bias for other aspects was mostly low or unclear.
For the primary outcome of BADL, meta‐analysis found a small effect on completion of the intervention with a mean difference (MD) of 2.26 on the Functional Independence Measure (FIM) (95% confidence interval (CI) 0.17 to 4.22; P = 0.03, I2 = 0%; 6 studies, 336 participants; low‐certainty evidence). Therefore, on average, BADL improved by 2.26 points on the FIM that ranges from 18 (total assist) to 126 (complete independence). On follow‐up, there was insufficient evidence of an effect at three months (MD 10.00, 95% CI −0.54 to 20.55; P = 0.06, I2 = 53%; 2 studies, 73 participants; low‐certainty evidence), but evidence of an effect at six months (MD 11.38, 95% CI 1.62 to 21.14, I2 = 12%; 2 studies, 73 participants; low‐certainty evidence). These differences are below 22 points which is the established minimal clinically important difference (MCID) for the FIM for people with stroke.
For IADL, the evidence is very uncertain about an effect (standardised mean difference (SMD) 0.94, 95% CI 0.41 to 1.47; P = 0.0005, I2 = 98%; 2 studies, 88 participants). For community integration, we found insufficient evidence of an effect (SMD 0.09, 95% CI −0.35 to 0.54; P = 0.68, I2 = 0%; 2 studies, 78 participants). There was an improvement of clinical importance in global cognitive functional performance after the intervention (SMD 0.35, 95% CI 0.16 to 0.54; P = 0.0004, I2 = 0%; 9 studies, 432 participants; low‐certainty evidence), equating to 1.63 points on the Montreal Cognitive Assessment (MoCA) (95% CI 0.75 to 2.52), which exceeds the anchor‐based MCID of the MoCA for stroke rehabilitation patients of 1.22. We found some effect for attention overall (SMD −0.31, 95% CI −0.47 to −0.15; P = 0.0002, I2 = 20%; 13 studies, 620 participants; low‐certainty evidence), equating to a difference of 17.31 seconds (95% CI 8.38 to 26.24), and for executive functional performance overall (SMD 0.49, 95% CI 0.31 to 0.66; P < 0.00001, I2 = 74%; 11 studies, 550 participants; very low‐certainty evidence), equating to 1.41 points on the Frontal Assessment Battery (range: 0–18). Of the cognitive subdomains, we found evidence of effect of possible clinical importance, immediately after intervention, for sustained visual attention (moderate certainty) equating to 15.63 seconds, for working memory (low certainty) equating to 59.9 seconds, and thinking flexibly (low certainty), compared to control.
Authors' conclusions
The effectiveness of occupational therapy for cognitive impairment poststroke remains unclear. Occupational therapy may result in little to no clinical difference in BADL immediately after intervention and at three and six months' follow‐up. Occupational therapy may slightly improve global cognitive performance of a clinically important difference immediately after intervention, likely improves sustained visual attention slightly, and may slightly increase working memory and flexible thinking after intervention. There is evidence of low or very low certainty or insufficient evidence for effect on other cognitive domains, IADL, and community integration and participation.
Given the low certainty of much of the evidence in our review, more research is needed to support or refute the effectiveness of occupational therapy for cognitive impairment after stroke. Future trials need improved methodology to address issues including risk of bias and to better report the outcome measures and interventions used.
Plain language summary
Occupational therapy for cognitive impairment in people who have had a stroke
What was the aim of this review?
The aim of this Cochrane Review was to find out if occupational therapy improves function in everyday activities and cognition after a stroke. Cognition is the information‐processing carried out by the brain such as thinking, paying attention to things you see or hear, learning, remembering, and solving problems. Cochrane researchers collected and analysed all relevant studies to answer this question and found 24 studies.
Key messages
For people with cognition problems after a stroke, occupational therapy may make little to no meaningful difference in the person's ability to do self‐care activities, such as showering and dressing, immediately after occupational therapy and six months later. Occupational therapy may improve these people's general information‐processing skills and ability to pay attention while looking at something, immediately after the intervention. Occupational therapy may slightly improve some aspects of memory and ability to think flexibly.
The quality of the evidence means that our findings are mostly of low or very low certainty. More well‐designed studies that test occupational therapy interventions for cognitive impairment after a stroke are needed.
What did the review study?
Problems with cognition are common after stroke and can affect a person's ability to do everyday self‐care activities such as dressing, feeding, and showering, as well as activities in the home or community, such as housework or grocery shopping.
People who have had a stroke can receive a range of therapies after a stroke in hospital, a rehabilitation centre, or in their home. Occupational therapy is one of these therapies. Occupational therapists work with people who have problems with cognition after a stroke to assist them to become as independent as possible. They do this by teaching people ways to adapt to or compensate for the problems, or with training activities to improve cognition (such as memory training), or a combination of these.
What were the main results of the review?
The review authors found 24 relevant studies from 11 countries. These studies compared occupational therapy with a control group that received usual rehabilitation care for people with problems in cognition following stroke. In most studies, the occupational therapy intervention involved training using a computer that had specially designed games to improve cognition. Some interventions involved training in daily activities, such as dressing.
The review showed that when people with cognitive problems after stroke receive occupational therapy, compared to usual care, it may:
– make little to no meaningful difference in their ability to do self‐care activities after receiving the therapy and little meaningful difference six months later (low‐certainty evidence);
– slightly improve their overall information‐processing ability after receiving the therapy (low‐certainty evidence);
– result in little to no meaningful difference in their overall ability to pay attention (low‐certainty evidence), but likely slightly improves their ability to pay attention to things they see, after receiving therapy (moderate‐certainty evidence);
– slightly improve their working memory (low‐certainty evidence), but may make little to no difference in other aspects of memory, after receiving the therapy;
– increase slightly their ability to think flexibly after receiving therapy (low‐certainty evidence).
The evidence is very uncertain about the effect of occupational therapy on ability to do activities in the home and community, and 'higher‐level' information‐processing skills that co‐ordinate and control other cognitive skills.
There was insufficient evidence of an effect on ability to do self‐care activities three months after receiving the therapy and on getting back into community activities.
How up‐to‐date is this review?
The review authors searched for studies published up to September 2020.
Summary of findings
Summary of findings 1. Occupational therapy compared to usual care for people with stroke with cognitive impairment.
| Occupational therapy compared to usual care for people with stroke with cognitive impairment | ||||
| Patient or population: adults with cognitive impairment after stroke Setting: inpatient and outpatient hospital, rehabilitation centre, and home settings Intervention: occupational therapy Comparison: usual care | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| With occupational therapy | ||||
| 1. BADL (primary outcome) | ||||
| 1a. BADL (postintervention) Assessed with: FIM Scale: 18–126 (higher is better) Follow‐up: 2–12 weeks |
MD 2.26 higher (0.17 higher to 4.22 higher) | 336 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | Occupational therapy may result in little to no meaningful clinical difference in BADL (postintervention). BADL improved by 2.19 points on the FIM scale, which ranges from 18 (total assist) to 126 (complete independence). This difference is well below the MCID for the FIM, which has been established as 22 points for people with stroke (Beninato 2006). |
| 1b. BADL (3‐month follow‐up) Assessed with: FIM Scale: 18–126 (higher is better) Follow‐up: 3 months |
MD 10.00 higher (0.54 lower to 20.55 higher) | 73 (2 RCTs) |
⊕⊕⊝⊝ Lowb,c | There was insufficient evidence of an effect of occupational therapy on BADL at 3‐month follow‐up. |
| 1c. BADL (6‐month follow‐up) Assessed with: FIM Scale: 18–126 (higher is better) Follow‐up: 6 months |
MD 11.38 higher (1.62 higher to 21.14 higher) | 73 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | Occupational therapy may result in little meaningful difference in BADL at 6‐month follow‐up. This MD does not reach the FIM MCID of 22 points (Beninato 2006). |
| 2. IADL and other ADL/IADL | ||||
| 2a. IADL (postintervention) Assessed with: 'IADL scale', Lawton & Brody Instrumental Activities of Daily Living scale (higher is better) Follow‐up: 8–12 weeks |
SMD 0.94higher (0.41 higher to 1.47 higher) | 88 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,d,e | The evidence is very uncertain about the effect of occupational therapy on IADL (postintervention). |
| 2b. Other ADL/IADL (postintervention) Assessed with: 'IADL scale' (higher is better) Follow‐up: 10 days to 2 weeks |
MD 2.61 higher (0.1 higher to 5.12 higher) | 111 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,f | The evidence is very uncertain about the effect of occupational therapy on other IADL (postintervention). |
| 3. Community integration and participation | ||||
| 3a. Self‐reported community integration or participation (postintervention) Assessed with: CIQ, USER‐P (Restriction subscale) (higher is better) Follow‐up: 12–18 weeks |
SMD 0.09 higher (0.35 lower to 0.54 higher) |
78 (2 RCTs) |
⊕⊕⊝⊝ Lowb,g | There was insufficient evidence of an effect on community integration and participation (postintervention). |
| 4. Global cognitive function | ||||
| 4a. Global cognitive functional performance (sensitivity analysis) (postintervention) Assessed with: MoCA, MMSE, BNIS (higher is better) Follow‐up: 10 days to 18 weeks |
SMD 0.35 higher (0.16 higher to 0.54 higher) | 432 (9 RCTs) | ⊕⊕⊝⊝ Lowh | Occupational therapy may slightly increase global cognitive functional performance (postintervention). The difference between groups equates to 1.63 points on the MoCA (95% CI 0.75 to 2.52). Therefore, on average, participants receiving the intervention had improved global cognitive functional performance by 1.63 points on the MoCA scale. This difference exceeds the anchor‐based MCID of the MoCA for stroke rehabilitation patients of 1.22 but not the distribution‐based MCID of 2.15 (Wu 2019). |
| 5. Attention | ||||
| 5a. Visual attention overall (postintervention) Assessed with: VCPT, Schulte's Tables, TMT‐A, Attentive Matrices, Stroop Colour Word, CWIT‐3 (lower is better) Follow‐up: 10 days to 12 weeks |
SMD 0.31 lower (0.47 lower to 0.15 lower) | 620 (13 RCTs) | ⊕⊕⊝⊝ Lowi | Occupational therapy may result in little to no difference in visual attention overall (postintervention). The difference between groups equates to 17.31 seconds (95% CI 8.38 to 26.24). |
| 5b. Visual attention overall (3–6 months' follow‐up) Assessed with: TMT‐A, VCPT, CWIT‐3, Stroop Colour Word (lower is better) Follow‐up: 3–6 months |
SMD 0.32 lower (0.55 lower to 0.09 lower) | 293 (5 RCTs) | ⊕⊕⊝⊝ Lowb,j | Occupational therapy may result in little to no difference in visual attention overall (at 3–6 months' follow‐up). The difference between groups equates to 17.87 seconds (95% CI 5.03 to 30.71). |
| 5c. Sustained visual attention (postintervention) Assessed with: VCPT, Schulte's Tables, TMT‐A (lower is better) Follow‐up: 10 days to 12 weeks |
SMD 0.28 lower (0.47 lower to 0.10 lower) | 463 (10 RCTs) | ⨁⨁⨁⊝ Moderatek | Occupational therapy likely improves sustained visual attention slightly (postintervention). The difference between groups equates to 15.63 seconds (95% CI 5.58 to 26.24). |
| 6. Memory | ||||
| 6a. Working memory (postintervention) Assessed with: Span board reversed, TMT‐B, Visual Span Backwards test, PASAT 2.4 (higher is better) Follow‐up: 4–12 weeks |
SMD 0.45 higher (0.26 higher to 0.65 higher) | 420 (8 RCTs) | ⊕⊕⊝⊝ Lowl | Occupational therapy may increase working memory slightly (postintervention). The difference between groups equates to 59.9 seconds (95% CI 34.60 to 86.5). |
| 6b. Immediate verbal memory span (postintervention) Assessed with: Digit Span Forwards, Listening Span, Verbal paired associates (higher is better) Follow‐up: 4–18 weeks |
SMD 0.35 higher (0.14 higher to 0.56 higher) | 357 (8 RCTs) | ⊕⊝⊝⊝ Very lowb,m | The evidence is very uncertain about the effect of occupational therapy on immediate verbal memory span (postintervention). The difference between groups equates to a difference of 0.76 (95% CI 0.31 to 1.22), equating to a difference of recall of 1 digit on the Digit Span forwards test. |
| 6c. Immediate spatial memory span (postintervention) Assessed with: Block Span Forward, Visual Span test, Span Board Forwards, Spatial Span, Corsi's test (higher is better) Follow‐up: 4–18 weeks |
SMD 0.27 higher (0.03 higher to 0.50 higher) | 292 (7 RCTs) | ⊕⊕⊝⊝ Lowb,n | Occupational therapy may result in little to no difference in immediate spatial memory span. |
| 7. Executive function | ||||
| 7a. Executive functional performance overall (postintervention) Assessed with: FAB, BADS, CWIT‐4, PM47, Mental rotation test (higher is better) Follow‐up: 10 days to 3 months |
SMD 0.49 higher (0.31 higher to 0.66 higher) | 550 (11 RCTs) | ⊕⊝⊝⊝ Very lowe,o | The evidence is very uncertain about the effect of occupational therapy on executive functional performance overall. The difference equates to 1.41 (95% CI 0.89 to 1.89) on the FAB. Therefore, on average, participants receiving the intervention improved executive functional performance by 1.41 points on the FAB scale, which ranges from 0 to 18. |
| 7b. Cognitive flexibility (postintervention) Assessed with: CWIT‐4 (lower is better) Follow‐up: 4 weeks and 3 months |
SMD 1.50 lower (2.20 lower to 0.80 lower) | 43 (2 RCTs) | ⨁⨁⊝⊝ Lowp,q |
Occupational therapy may slightly increase ability to think flexibly (postintervention). The difference equates to 4.5, which may be considered a clinically meaningful change on the CWIT‐4. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; BADL: basic activities of daily living; BADS: Behavioural Assessment of Dysexecutive Syndrome; BNIS: Barrow Neurological Institute Screen for Higher Cerebral Functions; CI: confidence interval; CIQ: Community Integration Questionnaire; CWIT‐3: Color Word Interference Test – Inhibition subscale (Condition 3); CWIT‐4: Color Word Interference Test Cognitive flexibility (switching) subscale (Condition 4); FAB: Frontal Assessment Battery; FIM: Functional Independence Measure; IADL: instrumental activities of daily living; MCID: minimal clinically important difference; MD: mean difference; MoCA: Montreal Cognitive Assessment; MMSE: Mini‐Mental State Examination; PASAT 2.4: Paced Auditory Serial Addition Test; PM47: Raven's Colored Progressive Matrices 47; RCT: randomised controlled trial; SMD: standardised mean difference; TMT‐A: Trail making Test A; TMT‐B: Trail making Test B; USER‐P: Utrecht Scale for Evaluation of Rehabilitation‐Participation; VCPT: Visual Continuous Performance Test. | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded one level due to risk of bias: all studies had no blinding of participants and personnel and some studies were unclear for selection bias but of most concern was Jiang 2016, which was at high risk of bias for attrition bias and it was by far the largest study and had the highest weighting in the analysis. bDowngraded one level due to imprecision: the sample size was below 400 considered a general rule for adequate sample size (Schünemann 2013). cDowngraded one level due to risk of bias: Skidmore 2015a was unclear for allocation concealment and both studies had no blinding of participants and personnel. dDowngraded one level due to risk of bias: both studies were unclear for allocation concealment. eDowngraded one level due to inconsistency: there was substantial heterogeneity that was not clearly explained. fDowngraded two levels due to very serious concerns about risk of bias: all were unclear for allocation concealment, two were unclear for sequence generation, all studies had no blinding of participants and personnel and two were unclear for attrition bias. gDowngraded one level due to risk of bias: both studies were unclear for allocation concealment, one was unclear and the other high risk for blinding of participants and personnel. hDowngraded two levels due to very serious concerns about risk of bias: all were unclear for allocation concealment except Jiang 2016; Jiang 2016, the highest weighted study, had potential for high risk for incomplete outcome data. iDowngraded two levels due to very serious concerns about risk of bias: all were unclear for allocation concealment except Barker‐Collo 2009 and Bo 2019; Bo 2019 was unclear for incomplete outcome data and was one of the highest weighted studies and eight were unclear for incomplete outcome data. jDowngraded one level due to risk of bias: three of the smaller weighted studies were unclear for allocation concealment and Bo 2019, the highest weighted study, was high risk for incomplete outcome data. kDowngraded one level due to risk of bias: all but one study was unclear for allocation concealment, with Barker‐Collo 2009 at low risk. lDowngraded two levels due to very serious concerns about risk of bias: six studies had unclear allocation concealment, two studies with high weight were low risk; Bo 2019, with the highest weighting, was high risk for incomplete outcome data and four were unclear for this criterion. mDowngraded two levels due to very serious concerns about risk of bias: Bo 2019 contributed the most and had high risk for incomplete outcome data and Zuchella 2014, the next heavily weighted was unclear for allocation concealment. Remaining studies were unclear for allocation concealment. All studies had no blinding of participants and personnel. nDowngraded one level due to risk of bias: all studies had unclear allocation concealment and three were unclear for incomplete outcome data. oDowngraded two levels due to very serious concerns about risk of bias: all but the highest weighted study (Bo 2019) were unclear for allocation concealment and Bo 2019 was high risk for incomplete outcome data and four other studies were unclear for this criterion. pDowngraded one level due to imprecision: the sample size is below 400 considered a rule of thumb for adequate sample size (Schünemann 2013). qDowngraded one level due to risk of bias: unclear for allocation concealment and high risk for blinding for both studies and unclear detection bias for Lundqvist 2010.
Background
This is an update of a review published in 2010 (Hoffmann 2007; Hoffmann 2010).
Description of the condition
Stroke is a leading cause of chronic disability worldwide (Feigin 2015; Mozaffarian 2016; Vos 2020). A frequent and persistent consequence of stroke is impairment of cognition (Winstein 2016), with a prevalence of 4 in 10 patients displaying cognitive impairment no dementia (CIND) within one year' poststroke (Sexton 2019), and 22% over a five‐year period after the onset of their first stroke (Douiri 2013). Cognitive impairment is common even in people with seemingly good clinical recovery three months after stroke and typically affects complex cognitive functions across multiple cognitive domains (Jokinen 2015).
Impairment of cognition for people poststroke is associated with less functional independence (Arsic 2015); predicts poorer long‐term functional outcome (Wagle 2011); is strongly associated with greater mortality, depression, dependency, and institutionalisation five years later (Obaid 2020); and is one of the factors with the strongest association with poor social and community participation outcomes (Ezekiel 2018). One systematic review examining the relationship between early poststroke cognition and activities and participation 6 to 12 months later found a predictive relationship, particularly for impairment in cognitive domains such as visual memory and attention or executive functioning (Mole 2020). Another systematic review and meta‐analysis found significant medium association between cognition and basic activities of daily living (BADL) (such as eating, dressing, and toileting) and instrumental activities of daily living (IADL) (such as housework and social interactions) (Stolwyk 2021). Cognitive impairment can reduce the independence of people who have had a stroke when performing BADL (Kihun 2012), and IADL (Akbari 2013). As a result, people with cognitive impairment following stroke often require ongoing care and support which can also impact their caregivers' health and well‐being (Caro 2017). Therefore, it is important for researchers and clinicians to identify effective interventions to assist people with cognitive impairment following stroke to improve patient functional outcomes (Stolwyk 2021).
Cognitive impairments are impairments in the information‐processing functions of the brain including acquisition, processing, and use of information to produce thought and direct behaviour (Korner‐Bitensky 2011). Cognition is multidimensional and hierarchical with some cognitive domains clearly defined while others overlap, making classification challenging (Bernhardt 2019). There is no definitive agreement on the classification of domains (Saa 2019). Commonly, classification of cognition includes primary cognitive domains such as orientation, attention, and memory; and higher level executive functions such as organisational skills, problem‐solving, and reasoning (Korner‐Bitensky 2011; Winstein 2016). Perception is the early stage in the processing of sensory information, sometimes defined as 'making sense of the senses' (Maskill 2017). Impairments of perception, such as unilateral neglect, apraxia, and agnosia, have been viewed as components of cognitive impairments in some literature (e.g. Dirette 2020; van Heugten 2012). The Occupational Practice Framework for occupational therapists cites perception as a specific mental function for discrimination of sensations (AOTA 2014). Although the concept of perception appears to overlap with other cognitive and sensory areas in theory, clinical assessments and interventions for perceptual disorders are usually distinguished from those for cognitive and sensory impairments (Stroke Foundation 2017). As per the protocol (Hoffmann 2007), and original review (Hoffmann 2010), for the purposes of this update, cognitive impairment is considered to encompass impairments in global cognitive function and specifically the cognitive domains or abilities of attention, concentration, memory, orientation, executive function, or a combination of these in accordance with the (Australian) Clinical Guidelines for Stroke Management 2017 (Stroke Foundation 2017). Readers are referred to Cochrane Reviews on "Non‐pharmacological interventions for perceptual disorders following stroke and other adult‐acquired, non‐progressive brain injury" (Bowen 2011), "Cognitive rehabilitation for spatial neglect following stroke" (Bowen 2013), and "Interventions for motor apraxia following stroke" (West 2008), for evidence for perceptual impairments.
Description of the intervention
Occupational therapy plays a unique and important role in a multidisciplinary approach to the management of cognitive impairment (AOTA 2013). The goal of occupational therapy is to maximise individuals' independence and participation in their life roles, habits, and routines at home, school, in the workplace, in the community, and other settings through a collaborative assessment and intervention process that includes a range of skilled services (AOTA 2014). Occupational therapists assess people with stroke for impairment in cognition and work with them to improve the impact of cognitive impairment on the person's performance of their valued occupations, especially their independence in BADL and IADL (AOTA 2013; De Wit 2006; Korner‐Bitensky 2011; Schiavi 2018). To achieve individuals' goals in activities of daily living (ADL) outcomes, a range of interventions can be used alone or in combination in the occupational therapy process. Training in ADL is a commonly provided intervention with the use of functional activities the therapists' common choice of therapeutic activity in the occupational therapy process (e.g. Holmqvist 2014; Koh 2009; Korner‐Bitensky 2011; Kristensen 2016). Other interventions include prescription of assistive technology such as personal digital assistants, and environmental adaptations such as sensor alerting systems and facilitating client awareness of limitations and strategies to compensate for these limitations (Holmqvist 2014; Koh 2009). Commonly targeted cognitive abilities in occupational therapy cognitive rehabilitation after stroke are planning, attention, initiation, structuring or organisation, short‐term memory, and orientation (Holmqvist 2014). In cognitive stroke rehabilitation, occupational therapy interventions take a restorative and remedial approach (also called a cognitive remediation approach) or a compensatory and adaptive approach, or a combination of both (Gillen 2015; Gillen 2018).
Restorative and remedial approach
The restorative and remedial approach in cognitive rehabilitation is a 'bottom‐up' approach that focuses on restoring specific cognitive abilities or deficits (Gillen 2018; Poulin 2020), for example using memory drills, games, or computer‐based technologies that allow targeted and intense cognitive rehabilitation training with automatic adjustment of the level of challenge according to the person's ability level (Toglia 2014). This approach aims to restore the cognitive skill and assumes that training in a specific domain (e.g. memory training), will transfer to improved skill (Gillen 2015; Gillen 2018), and functional ability in activities requiring that domain. The choice of therapeutic activity in the restorative and remedial approach is driven by the domain needing improvement (e.g. memory game to improve memory) (Gillen 2018).
Compensatory and adaptive approach
The compensatory and adaptive approach is a 'top‐down' approach that focuses on intact skill training and environmental or task modifications for adaptation to the deficits (Poulin 2020). This approach is activity specific (Gillen 2018). Examples of interventions in this approach include practice of activities such as preparing a simple dinner or adaptive approaches such as breaking down the steps of the meal preparation with the person, using a basic recipe rather than a complex one, using a virtual assistant to recite the steps of recipe, or involving the assistance of a caregiver, or both practice and adaptive approaches. The choice of therapeutic activity is driven by the challenges in performance of the activity (e.g. remembering the ingredients or sequence of steps in a particular recipe) (Gillen 2018).
Combined occupational therapy approach
A combined approach, such as the cognitive rehabilitation model for occupational therapy (Averbuch 2011), uses different approaches at various stages after injury (Toglia 2014). Compensatory and adaptive training to accommodate cognitive deficits may be used in the earlier stages and later combined with graded cognitive training, which may include intense practice of specific cognitive skills (Toglia 2014). In this approach, the choice of therapeutic activity is driven by how the activity challenges the underlying deficits as well as its relevance to the person's occupational and participation needs (Gillen 2018). Occupational therapists working in stroke cognitive rehabilitation commonly use both remedial and compensatory approaches (Holmqvist 2014; Koh 2009).
How the intervention might work
As noted above, interventions used by occupational therapists to address cognitive impairment can be a restorative and remedial approach (often called a cognitive remediation approach), or a compensatory and adaptive approach, or a combination of both. Based on the concept of the plasticity of the human brain and its ability to reorganise after being damaged (Draaisma 2020), the cognitive remediation approach aims to promote person's function by targeted and intense training of deficits in cognitive skills (Toglia 2014), for example, training patients to make mental associations and visual pictures to remember things such as people's names (Powell 2017), or playing computer games that are personally tailored and motivating to improve cognitive domains with the aim of transferring to daily function (Draaisma 2020).
The compensatory and adaptive approach utilises the person's residual strengths to compensate for their cognitive deficits in everyday activities rather than attempt to remediate them. Teaching and assisting patients and their families to develop strategies or to use assistive devices to overcome performance deficits are common methods, as well as modification of the environment to support functional performance (Gillen 2018). For example, repeated dressing practice using a problem‐solving approach and energy conservation techniques such as putting the affected arm into the sleeve first, crossing the affected leg over the other leg to reach feet, etc. (Walker 2012), or using an electronic memory device with reminder alarms to help people with memory deficits to complete their daily tasks (AOTA 2013). A combination approach, such as the cognitive rehabilitation model for occupational therapy, uses different approaches at various stages after injury (Toglia 2014). Strategy and compensatory training to improve affected function may be used in the earlier stages and later combined with graded domain‐specific cognitive training (Toglia 2014).
Why it is important to do this review
This is an update of a review published in 2010 (Hoffmann 2007; Hoffmann 2010), and aims to specifically examine the effectiveness of occupational therapy interventions for improving the effects of cognitive impairment in people with stroke, especially the impact on BADL, IADL, and cognitive abilities. The original review identified and included only one trial (33 participants), which found no difference between groups for BADL and the cognitive ability of time judgement. Hence, the effectiveness of occupational therapy for the effects of cognitive impairment after stroke was unclear.
Identifying the best ways to improve cognition following stroke has been named first of the top 10 research priorities relating to life after stroke (Pollock 2012). Cognitive impairments can be persistent and are associated with poor long‐term outcomes in disability and survival (Winstein 2016). While recent reviews reported a growing body of evidence related to cognitive interventions or cognitive rehabilitation, most are not specific to occupational therapy in terms of treatment goals and interventions, and some include studies on participants with causes of brain injury other than stroke (e.g. traumatic brain injury (TBI)) (e.g. Chung 2013; Cicerone 2019; Fernandez Lopez 2020). Because people with stroke are the minority in many reviews, the treatment effect of the published evidence to date is difficult to generalise to people with stroke (Winstein 2016). Furthermore, some reviews have methodological issues that may restrict the strength of the evidence level, such as involving studies without randomised controlled design or providing narrative synthesis alone without meta‐analyses. These reviews include literature reviews such as the series by the American Congress of Rehabilitation Medicine (ACRM) to inform evidence‐based guidelines for cognitive rehabilitation of people with TBI and stroke (Cicerone 2000; Cicerone 2005; Cicerone 2011; Cicerone 2019). The latest review made recommendations for practice of interest to our review, including support for cognitive rehabilitation for attention deficits after TBI or stroke, for compensatory strategies for mild memory deficits, for meta‐cognitive strategy training for deficits in executive functioning, and for comprehensive neuropsychological rehabilitation (Cicerone 2019). The review included non‐randomised controlled trials as well as randomised controlled trials (RCTs) and no meta‐analyses. The 2019 update of the Canadian Stroke Best Practice Recommendations for cognitive impairment following stroke made Level B recommendations for considering both remediation interventions (including intensive specific training for impaired cognitive domains, such as drills, mnemonic strategies, or computer‐ or tablet‐based training) and compensation and adaptive strategies (such as strategy training for specific activity limitations or physical or social modification of the environment or activity) (Lanctôt 2020).
A relevant broad review is one that examined the effectiveness of 'cognitive remediation' approaches after stroke alone and on general and domain‐specific cognition, including meta‐analysis (Rogers 2018). Based on 22 RCTs, with 1098 participants, they found that cognitive remediation produced a small significant overall effect moderated by recovery stage, quality of study, and dose, but not type of approach. The effect persisted at follow‐up, which ranged from 2 to 52 weeks. For individual domains of cognition relevant to our review, they found significant small effects on attention, memory, and executive functioning. Another systematic review that focused on the effectiveness of computer‐based training on poststroke cognitive impairment found no superiority of such training over traditional rehabilitation for overall cognition, based on a meta‐analysis of six studies (Ye 2020). There have also been systematic reviews, some including meta‐analyses, of the effects of cognitive rehabilitation on specific cognitive abilities of relevance to our review, including Cochrane Reviews for attention following stroke (Loetscher 2019), memory impairment following stroke (das Nair 2016), and executive function following stroke and other acquired brain injuries (ABI) (Chung 2013). Loetscher 2019 found some limited evidence for improvement in divided attention in the short term but insufficient evidence for supporting or refuting persisting effect on attention more broadly. das Nair 2016 concluded that the evidence was limited to support or refute the effectiveness of rehabilitation for memory impairment after stroke and was of poor quality. Chung 2013 found a lack of high‐quality evidence to make conclusions about the effects of cognitive rehabilitation on executive function or other outcomes.
As occupational therapy is considered an important part of the multidisciplinary management of stroke (Stroke Foundation 2018; Winstein 2016), and improvement of the functional effects of cognitive impairment is a common focus of occupational therapy assessment and intervention (Draaisma 2020; Lanctôt 2020; Stroke Foundation 2018), it is important to review the effectiveness of occupational therapy in assisting people with cognitive impairment after stroke to improve their independence and participation. In one Cochrane Review, Legg 2017 examined the effectiveness of occupational therapy interventions on the functional ability of adults with stroke in ADL and concluded that there was low‐quality evidence for improved performance in ADL and reduced risk of deterioration in these abilities. This review did not specifically address occupational therapy for cognitive impairment as ours does. To our knowledge, there is only one review focusing on the effectiveness of interventions to improve occupational performance of people with cognitive impairment after stroke (Gillen 2015). Based on 46 articles, 26 of which were Level 1 evidence of systematic reviews or RCTs, Gillen 2015 concluded that the evidence for interventions for executive dysfunction and memory loss was limited and that there was insufficient evidence for impairments of attention. However, Gillen 2015 was a narrative synthesis of studies without meta‐analyses, which included lower levels of evidence (e.g. cohort, case‐control, non‐controlled, and cross‐sectional studies) than our review and did not have as strict inclusion criteria such as confirmed cognitive impairment on study inclusion. This may introduce bias and weaken the strength of evidence level for their conclusions. Therefore, there remains the need for a systematic review of higher‐quality studies of occupational therapy specifically for cognitive impairment in people with stroke.
Objectives
To assess the impact of occupational therapy on activities of daily living (ADL), both basic and instrumental, global cognitive function, and specific cognitive abilities in people who have cognitive impairment following a stroke.
Methods
Criteria for considering studies for this review
Types of studies
We restricted the review to RCTs, clinical trials where participants were quasi‐randomly assigned to one of two or more treatment groups, and cross‐over trials.
Types of participants
We included trials if their participants were adults (aged 18 years or over) with a clinically defined stroke and confirmed cognitive impairment as specified in each trial. For the purpose of this review, we focused on global cognitive impairment and impairment of specific cognitive abilities including attention, memory, orientation, executive functions, or a combination of these. We excluded trials with mixed aetiology groups unless participants who had had (and only had) a stroke comprised more than 50% of the participants in the trial and separate data for the participants with stroke were available either in the published article or from the trial authors.
Types of interventions
We included all occupational therapy interventions for cognitive impairment in people with stroke. We included studies where the intervention was delivered by an occupational therapist or under the supervision of an occupational therapist or if the papers reporting an intervention were authored by an occupational therapist. We also included interventions that are considered within occupational therapy scope of practice, which was informed by contemporary occupational therapy texts (e.g. Gillen 2018; Katz 2018; Toglia 2014), and surveys of practice (e.g. Holmqvist 2014; Koh 2009; Korner‐Bitensky 2011; Kristensen 2016). In the case of multicomponent interventions, all components needed to have been or able to have been delivered by occupational therapists.
For the purpose of this review, we focused on interventions for improving impairment in function and cognition generally as well as in specific cognitive abilities including attention and concentration, memory, orientation, executive functions, or a combination of these. We excluded studies that focused on apraxia or perceptual impairments without also containing elements of cognitive retraining. We also excluded trials that examined the effects of change of pharmaceutical interventions on cognitive impairment following stroke. In this update, we excluded virtual reality interventions. This is because, since the original review was conducted, another Cochrane Review has investigated the effectiveness of virtual reality interventions in stroke rehabilitation, which included cognitive function among its outcomes (Laver 2017).
Management of control groups was not specified in the protocol or in the previous review. For this update, we considered all types of comparators, including inactive control interventions (e.g. no intervention, usual care, or a waiting list control) or active control interventions (e.g. a different variant of the same intervention or a different type of intervention).
Types of outcome measures
We included both observed or self‐reported performance measures of the primary and secondary outcomes.
Primary outcomes
BADL, such as dressing, feeding, and bathing. We included both composite measures of BADL (e.g. Functional Independence Measure (FIM) (Stineman 1996), Barthel Index (BI) (Mahoney 1965; see Table 2 and Table 3), and measures of individual activities (e.g. dressing assessment).
1. Outcome measures used in the included studies.
| Author and year | BADL | IADL |
Other ADL/IADL occupational performance and |
Community integration/participation measures | Global cognitive function | Cognitive abilities | |||
| Orientation | Attention | Memory | Executive functions | ||||||
| Akerlund 2013 | — |
AMPS (Fisher 2003) | — | — | BNIS (Prigatano 1995) | — | — | WAIS‐III Digit Span and Span Board Forwards, Backwards; WAIS‐III NI; Working Memory subscale score (Wechsler 1997); RBMT‐II (Wilson 1989); Working Memory questionnaire (Akerlund 2013) |
DEX (Chan 2001) |
| Barker‐Collo 2009 | — | — | mRS (Bamford 1989) | — | CFQ (Broadbent 1982) | — | IVA‐CPT (Sandford 2000); TMT‐A (Strauss 2006) |
TMT‐B (Strauss 2006); PASAT 2.4 and 2.0 (Gronwall 1977) | — |
| Bo 2019 | — | — | — | — | — | — | Stroop colour‐word test (Jensen 1966) | TMT‐B (Bowie 2006); Digit Span Forward (Wechsler 2014) | Mental Rotation Tests (Vandenberg 1978) |
| Carter 1983 | Barthel Index (Mahoney 1965) |
— | — | — | — | — | — | — | Time Judgement Tests (Carter 1980) |
| Chen 2015 | — | — | — | — | MoCA (Nasreddine 2005) | — | — | — | BADS (Wilson 1999) |
| Cho 2015 | — | — | — | — | — | — | VCPT and ACCPT (Bae 2005) | DST; VST (Bae 2005) |
— |
| Cho 2016 | FIM (Stineman 1996) | — | — | — | — | — | — | — | — |
| De Luca 2018 | BADL; Barthel Index (both apparently measured but results not reported) |
IADL (apparently measured but results not reported) | — | — | MMSE (Folstein 1975) | — | Attentive Matrices (Spinnler 1987) | RAVLT (Bean 2018); Digit Span | Raven's Colored Progressive Matrices (Basso 1987) |
| Hasanzadeh Pashang 2020 | — | — | — | — | — | — | IVA + Plus (Sandford 1995) | — | — |
| Jiang 2016 | FIM (Stineman 1996) | — | — | — | MMSE (Folstein 1975); MoCA (Nasreddine 2005) |
— | — | — | — |
| Lin 2014 | — | — | — | — | — | Wechsler Memory Scale (Wechsler 1945) – Orientation subscale | TMT‐A | TMT‐B; Wechsler Memory Scale (Wechsler 1945) – Mental control subscale, Logical memory subscale, Digits Forward and Backward, Memory quotient |
— |
| Lundqvist 2010 | — | — | — | — | — | — | — | PASAT 2.4 (Gronwall 1977); Working Memory Improvement Index (Lundqvist 2010); Listening span; Picture span; Block span Forward and Backward from WAIS R‐NI (Wechsler 1997) | CWIT Cognitive Flexibility (Condition 4) (Delis 2001) |
| Maggio 2020 | ADL | IADL | — | — | MoCA | — | — | — | FAB (Dubois 2000); Weigl's Test |
| Park 2015a | — | — | — | — | LOTCA (Itzkovich 2000) | — | — | — | — |
| Prokopenko 2013 | — | — | IADL scale (Prokopenko 2013) | — | MMSE (Folstein 1975); MoCA (Nasreddine 2005) | — | Schulte's Tables (Prokopenko 2013) | — | FAB (Dubois 2000) |
| Prokopenko 2018 | — | — | IADL | — | MMSE (Folstein 1975); MoCA (Nasreddine 2005) | — | Shulte's Test | — | FAB (Dubois 2000) |
| Prokopenko 2019 | — | — | IADL | — | MMSE (Folstein 1975); MoCA (Nasreddine 2005) |
— | Schulte's Tables | — | FAB (Dubois 2000) |
| Skidmore 2015a | FIM (Stineman 1996) | — | — | — | — | — | Color Word Interference Inhibition (Condition 3) (Delis 2001) | — | Color Word Interference Cognitive Flexibility (Condition 4) (Delis 2001) |
| Skidmore 2017 | FIM (Stineman 1996) | — | — | — | — | — | — | — | — |
| van de Ven 2017 | — | Lawton & Brody Instrumental Activities of Daily Living scale (Lawton 1988) | — | USER‐P (Restriction subscale) (van der Zee 2010; van der Zee 2013) | CFQ (Broadbent 1982) | — | TMT‐A; DSC (Wechsler 2000) | TMT‐B online version; D‐Kefs TMT number‐letter switching condition Letter Number Sequencing (Wechsler 2000); PASAT (Gronwall 1977); RAVL) (Saan 1986); Blokkenreeksen (NeuroTask BV); online modified version of Corsi's test |
TOL (Culbertson 2005); Raven's Colored Progressive Matrices (Raven 1998); Shipley Institute of Living Scale (Zachary 1991); DEX (Burgess 1996) |
| Walker 2012 | NSDA (Walker 1990; Fletcher‐Smith 2010) | — | — | — | — | — | — | — | — |
| Yeh 2019 | — | — | — | CIQ (Willer 1994) | MoCA (Nasreddine 2005) | — | — | Spatial Span Test; Verbal Paired Associates subtest (Wechsler 1997) | — |
| Yoo 2015 | FIM (Stineman 1996) | — | — | — | TMT (not specified if TMT‐A or TMT‐B so unable to classify under a cognitive ability) (Yoo 2015) | — | ACCPT; VCPT (Yoo 2015) | DST; Verbal Learning Test; Visual Span test; Visual Learning Test (Yoo 2015) | — |
| Zuchella 2014 | FIM (Stineman 1996) | — | — | — | MMSE (Folstein 1975) |
— | TMT‐A (Giovagnoli 1996); Attentive Matrices (Spinnler 1987) |
Digit Span Forward (Orsini 1987); Corsi's Test (Orsini 1987); RAVLT (Carlesimo 1996); Logical Memory (Carlesimo 2002); TMT‐B (Giovagnoli 1996) |
Raven's Colored Progressive Matrices 47 (Basso 1987); FAB (Appollonio 2005) |
ACCPT: Auditory Controlled Continuous Performance Test; ADL: activities of daily living; AMPS: Assessment of Motor and Process Skills; BADL: basic activities of daily living; BADS: Behavioural Assessment of Dysexecutive Syndrome; BNIS: Barrow Neurological Institute Screen for Higher Cerebral Functions; CFQ: Cognitive Failures Questionnaire; CIQ: Community Integration Questionnaire; CWIT: Color Word Interference Test; DEX: Dysexecutive Questionnaire; DSC: Digit Symbol Coding; DST: Digit Span test; FAB: Frontal Assessment Battery; FIM: Functional Independence Measure; IADL: instrumental activities of daily living; IVA + Plus: Integrated Visual and Auditory Continuous Performance Test; IVA‐CPT: Integrated Auditory Visual Continuous Performance Test; LOTCA: Lowenstein Occupational Therapy Cognitive Assessment; MMSE: Mini‐mental State Examination; MoCA: Montreal Cognitive Assessment Scale; mRS: modified Rankin scale; NSDA: Nottingham Stroke Dressing Assessment; PASAT: Paced Auditory Serial Addition Test; RAVLT: Rey Auditory Verbal Learning Test; RBMT‐II: Rivermead Behavioural Memory Test – version 2; TMT‐A: Trail Making Test A; TMT‐B: Trail Making Test B; TOL: Tower of London; USER‐P: Utrecht Scale for Evaluation of Rehabilitation‐Participation; VCPT: Visual Continuous Performance Test; VST: Visual Span Test; WAIS‐III: Wechsler Adult Intelligence Scale III.
2. Outcome measures used in included studies by domain and subdomains.
| Outcome | Nature of measure | Domains | Subdomains | Instrument |
Range where applicable or available ↑ higher is better ↓ lower is better |
Studies |
| BADL | Observed performance | Motor | Eating, grooming, bathing, dressing, toileting, bladder and bowel management, transfers, walk/wheelchair, stairs | FIM | 18–26 ↑ | Cho 2016; Jiang 2016; Skidmore 2015a; Skidmore 2017; Yoo 2015; Zuchella 2014 |
| Cognitive | Comprehension, expression, social interaction, problem solving, memory | |||||
| BADL | Feeding, toileting, bathing, dressing, toilet transfer, controlling bladder, controlling bowel, bed transfers, walking/wheelchair, stairs | Barthel Index | 0–100 ↑ | Carter 1983 | ||
| Dressing | NSDA | 0–100 ↑ | Walker 2012 | |||
| Unclear if self‐report of performance | ADL | No description provided | ADL scale | Not reported | Maggio 2020 | |
| IADL/other ADL | Observed performance | Motor and process skills | Related to activities | AMPS | ↑ | Akerlund 2013 |
| Self‐report | IADL | Telephone use, food preparation, grocery shopping, laundry, transport, housework/home maintenance, medication management, finances management | Lawton & Brody Instrumental Activities of Daily Living scale | ↓ 0 (no problems at all) – 22 (highly impaired) |
van de Ven 2017 | |
| Unclear if self‐report or performance | IADL | No description provided | IADL | Not reported, assumed ↑ | Maggio 2020 | |
| ADL and IADL | "walking, feeding, travelling, carrying out hygienic procedures, shopping etc." Prokopenko 2013 | IADL scale | Not reported | Prokopenko 2013; Prokopenko 2018; Prokopenko 2019 | ||
| Other ADL/disability | — | Overall disability | — | mRS | 1–6 ↓ | Barker‐Collo 2009 |
| Community reintegration | Self‐report | Community integration/participation | Home, social, and productive activities | CIQ | 0–25 ↑ | Yeh 2019 |
| IADL and productive activities | Perceived restriction in a range of home, social, and productive activities | USER‐P (Restriction subscale) | 0–100 ↑ | van de Ven 2017 | ||
| Global cognitive function | Performance (total score) | Range of cognitive domains | — | MMSE | 0–30 ↑ | De Luca 2018; Jiang 2016; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Zuchella 2014 |
| MoCA | 0–30 ↑ | Chen 2015; Jiang 2016; Maggio 2020; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Yeh 2019 | ||||
| BNIS | 0–50 ↑ | Akerlund 2013 | ||||
| LOTCA | 26–115 ↑ | Park 2015a | ||||
| Self‐report | Range of memory, perception, and motor functions | — | CFQ | 0–100 ↓ | Barker‐Collo 2009; van de Ven 2017 | |
| Orientation | Performance | Orientation | — | Orientation subscale of Wechsler Memory Scale | ↑ | Lin 2014 |
| Attention | Performance | Sustained | Visual and auditory | IVA‐CPT | Seconds ↓ | Barker‐Collo 2009; Hasanzadeh Pashang 2020 |
| Visual | VCPT | Seconds ↓ | Barker‐Collo 2009; Cho 2015; Yoo 2015 | |||
| TMT‐A | Seconds ↓ / correct (0–24) ↑ |
Barker‐Collo 2009; Lin 2014; van de Ven 2017; Zuchella 2014 | ||||
| Schulte's Tables | Seconds ↓ | Prokopenko 2013; Prokopenko 2018; Prokopenko 2019 | ||||
| Auditory | ACCPT | Seconds ↓ | Barker‐Collo 2009; Cho 2015; Yoo 2015 | |||
| Selective | Visual | Attentive Matrices | 0–60 ↑ | De Luca 2018; Zuchella 2014 | ||
| Stroop Color‐Word test | Seconds ↓ | Bo 2019 | ||||
| CWIT‐3 | Scaled scores ↑ | Skidmore 2015a | ||||
| Memory | Performance | Working memory | — | Digit Span Backwards | 0–12 ↑ | Akerlund 2013; Cho 2015 |
| TMT‐B | Seconds ↓/correct 0‐24 ↑ | Barker‐Collo 2009; Bo 2019; Lin 2014; van de Ven 2017; Zuchella 2014 | ||||
| PASAT | Number correct ↑ | Barker‐Collo 2009; Lundqvist 2010; van de Ven 2017 | ||||
| Span Board reversed | ↑ | Akerlund 2013 | ||||
| Block Span Backwards | ↑ | Lundqvist 2010 | ||||
| VST Backwards | ↑ | Cho 2015 | ||||
| Wechsler Memory Scale – Mental control subscale | ↑ | Lin 2014 | ||||
| Working Memory subscale | ↑ | Akerlund 2013 | ||||
| Memory span | Immediate verbal | Digit Span Forwards | 0–12 ↑ | Akerlund 2013; Bo 2019; Cho 2015; De Luca 2018; Yoo 2015; Zuchella 2014 | ||
| Listening Span | ↑ | Lundqvist 2010 | ||||
| Verbal Paired Associates Test | ↑ | Yeh 2019 | ||||
| Immediate spatial | Span Board Forwards | ↑ | Akerlund 2013 | |||
| Block Span Forwards | ↑ | Lundqvist 2010 | ||||
| Picture Span | ↑ | Lundqvist 2010 | ||||
| Spatial Span | ↑ | Yeh 2019 | ||||
| Corsi's test | ↑ | van de Ven 2017; Zuchella 2014 | ||||
| Visual Span test (Forwards) | ↑ | Cho 2015 | ||||
| Visual Span test | ↑ | Yoo 2015 | ||||
| Episodic memory | Immediate recall | RAVLT Immediate | 0–75 ↑ | De Luca 2018; van de Ven 2017; Zuchella 2014 | ||
| Delayed recall | RAVLT Delayed | 0–15 ↑ | De Luca 2018; van de Ven 2017; Zuchella 2014 | |||
| Logical memory | — | Wechsler Memory Scale – Logical memory subscale | ↑ | Lin 2014 | ||
| Immediate recall | Logical Memory Test | ↑ | Zuchella 2014 | |||
| Delayed recall | Logical Memory Test | ↑ | Zuchella 2014 | |||
| Performance (total score) | Other | Functional tasks | RBMT‐II | 0–24 ↑ | Akerlund 2013 | |
| Combined score | "Digits forward and backward" | ↑ | Lin 2014 | |||
| Composite performance score of improvement in training from baseline | Working Memory Improvement Index | — | Lundqvist 2010 | |||
| Performance | Unclear | — | Visual Learning Test | ↑ | Yoo 2015 | |
| Unclear | — | Verbal Learning Test | ↑ | Yoo 2015 | ||
| Self‐report | Working memory | Everyday situations that place demand on working memory | Working Memory Questionnaire | — | Akerlund 2013 | |
| Executive Function | Performance | Range of executive functions (total score) | Conceptualisations, mental flexibility, motor programming, sensitivity to interference, inhibitory control, and environmental autonomy | FAB | 0–18 ↑ | Maggio 2020; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Zuchella 2014 |
| Planning, organising, initiating, monitoring, and adapting behaviour | BADS | 0–24 ↑ | Chen 2015 | |||
| Specific executive functions | Non‐verbal reasoning | Raven's Colored Progressive Matrices 47‐(PM47) | 0–36 ↑ | De Luca 2018; van de Ven 2017; Zuchella 2014 | ||
| Weigl's Test | 0–4 ↑ | Maggio 2020 | ||||
| Reasoning | Shipley Institute of Living Scale | ↑ | van de Ven 2017 | |||
| Cognitive flexibility | CWIT‐4 | Seconds ↓ Scaled scores ↑ |
Lundqvist 2010; Skidmore 2015a | |||
| Spatial imagination | Mental Rotation Tests | Maximum of 12 ↑ | Bo 2019 | |||
| Problem solving | TOL | ↓ (minimal required moves) |
van de Ven 2017 | |||
| Time judgement (estimation of 1 minute time period) | Time Judgement Test | % improvement ↑ | Carter 1983 | |||
| Self‐report | Range of executive function problems | Emotional, motivational, behavioural, and cognitive changes | DEX | Maximum 80 ↓ | Akerlund 2013; van de Ven 2017 |
ACCPT: Auditory Controlled Continuous Performance Test; ADL: activities of daily living; AMPS: Assessment of Motor and Process Skills; BADL: basic activities of daily living; BADS: Behavioural Assessment of Dysexecutive Syndrome; BNIS: Barrow Neurological Institute Screen for Higher Cerebral Functions; CFQ: Cognitive Failures Questionnaire; CIQ: Community Integration Questionnaire; CWIT: Color Word Interference Test; DEX: Dysexecutive Questionnaire; DSC: Digit Symbol Coding; DST: Digit Span test; FAB: Frontal Assessment Battery; FIM: Functional Independence Measure; IADL: instrumental activities of daily living; IVA + Plus: Integrated Visual and Auditory Continuous Performance Test; IVA‐CPT: Integrated Auditory Visual Continuous Performance Test; LOTCA: Lowenstein Occupational Therapy Cognitive Assessment; MMSE: Mini‐mental State Examination; MoCA: Montreal Cognitive Assessment Scale; mRS: modified Rankin scale; NSDA: Nottingham Stroke Dressing Assessment; PASAT: Paced Auditory Serial Addition Test; RAVLT: Rey Auditory Verbal Learning Test; RBMT‐II: Rivermead Behavioural Memory Test – version 2; TMT‐A: Trail Making Test A; TMT‐B: Trail Making Test B; TOL: Tower of London; USER‐P: Utrecht Scale for Evaluation of Rehabilitation‐Participation; VCPT: Visual Continuous Performance Test; VST: Visual Span Test; WAIS‐III: Wechsler Adult Intelligence Scale III.
Secondary outcomes
IADL (e.g. Lawton & Brody Instrumental Activities of Daily Living scale) (Lawton 1988).
Community integration and participation (e.g. Community Integration Questionnaire (CIQ) (Willer 1994))
-
Global cognitive function:
performance (e.g. Montreal Cognitive Assessment (MoCA) Scale (Nasreddine 2005)), and self‐reported (e.g. CIQ (Willer 1994)).
-
Specific cognitive abilities:
orientation (e.g. Wechsler Memory Scale (Wechsler 1945) – Orientation subscale);
attention (e.g. Integrated Auditory Visual Continuous Performance Test (IVA‐CPT) (Sandford 2000));
memory (e.g. Wechsler Memory Scale (Wechsler 1945)).
-
executive functions:
performance (e.g. Frontal Assessment Battery (FAB) (Dubois 2000), and self‐reported (Dysexecutive Questionnaire (DEX) (Burgess 1996)).
Once eligible studies were identified, we grouped measures of cognitive abilities by cognitive domains, and subdomains where relevant (e.g. sustained attention and selective attention). See Table 2 and Table 3. We were guided, where possible, by the papers as to how they classified the outcome measures, using original descriptions of the instrument where available, and other systematic reviews from the field. Some papers provided no classification of the cognitive domain and different papers classified the same measure differently. As cognition is complex, hierarchical, and multidimensional, classifying cognitive constructs (Bernhardt 2019) and domains is challenging. Agreement on classification of domains and the instruments that measure them can be variable (Saa 2019).
Search methods for identification of studies
We searched for relevant trials in all languages and where possible, translated papers published in languages other than English.
Electronic searches
We searched:
the Cochrane Stroke Group Trials Register, last searched by the Cochrane Stroke Group Information Specialist on 14 September 2020).
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9) in the Cochrane Library (searched 2 September 2020) (Appendix 1);
MEDLINE Ovid (1966 to 2 September 2020) (Appendix 2);
Embase Ovid (1980 to 2 September 2020) (Appendix 3);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 2 September 2020) (Appendix 4);
PsycINFO Ovid (1840 to 2 September 2020) (Appendix 5);
NeuroBITE (previously PsycBITE) (last searched 16 September 2020) (Appendix 6); and
OTseeker (last searched 16 September 2020) (Appendix 7).
We developed the search strategies for each database in consultation with the Cochrane Stroke Group Information Specialist and an experienced medical librarian. The search strategies included four major areas: stroke, cognitive impairment, occupational therapy interventions, and trial methodology.
Searching other resources
To identify further published, unpublished, and ongoing trials, we searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; last searched 20 February 2020; ICTRP was not operating later in 2020 due to the COVID‐19 pandemic), and the US National Institutes of Health ClinicalTrials.gov trial registry (last searched 16 September 2020). See Appendix 8 for search strategies used. We reviewed the reference lists of included studies and any relevant systematic reviews identified. For this update, we did not handsearch relevant occupational therapy journals or track relevant references through the Web of Science Cited Reference search, as in the original review (Hoffmann 2010) and protocol (Hoffmann 2007).
Data collection and analysis
Selection of studies
Two review authors (EG or SE or CK) independently screened the titles and abstracts of articles in the search results and identified those for which full texts needed to be obtained. Two review authors (EG or CK or SE or TH) independently assessed the full texts against the eligibility criteria. If consensus was unable to be reached, a third review author (TH) made the decision. When aspects of the study were unclear (such as whether the intervention was or could be delivered by an occupational therapist, or if separate data for participants with stroke were available) we contacted the study authors for clarification.
Data extraction and management
Two review authors (EG or CK or SE or SB for this review) independently recorded the following information using a self‐developed data extraction form.
Sample characteristics such as: age, level of education, sex, first or recurrent stroke, type and severity of stroke, time since onset of stroke, type of cognitive impairment, sample size, number of dropouts.
Methodological quality: according to Cochrane's risk of bias tool for assessing risk of bias (see Table 4).
Details of the interventions, according to the Template for Intervention Description and Replication (TIDieR) checklist (Hoffmann 2014), which includes the intervention(s) and comparator(s): brief name, rationale, materials, procedure, providers, delivery mode, location, frequency or dosage, tailoring, modification, fidelity, and adherence.
Outcome measures: outcomes used in the trial and when they were administered.
3. Criteria for assessing the methodological quality of trials – Cochrane's tool for assessing risk of bias.
| Domain | Description |
| Selection bias | |
| Random sequence generation | Inadequate generation of the randomisation sequence |
| Allocation concealment | Inadequate concealment of allocations prior to assignment |
| Performance bias | |
| Blinding of participants and personnel | Knowledge of the allocated interventions by participants and personnel during the study |
| Detection bias | |
| Blinding of outcome assessment | Knowledge of the allocated interventions by outcome assessors |
| Attrition bias | |
| Incomplete outcome data | Amount, nature, or handling of incomplete outcome data |
| Reporting bias | |
| Selective reporting | When all prespecified outcomes that are of interest in the review have not been reported |
| Other bias | |
| Other sources of bias | Any other problems not covered elsewhere |
The extractions of the two review authors were reconciled. We resolved differences in data extraction by discussion. If no consensus could be achieved, we consulted a third review author (TH or SB) to arbitrate.
Some data were requested, and in some instances obtained, from trial authors. Some missing data were obtained from the results section on the US National Institutes of Health ClinicalTrials.gov trial registry. Where required, we converted medians to means and standard errors and interquartile ranges to standard deviations (SD) (Wan 2014). One study, Prokopenko 2019, reported medians and 95% confidence intervals (CI) of the median without reporting how they estimated the CIs. We used the medians and 95% CIs to estimate means and SDs using a method described in University College London 2010. Where this study was included in meta‐analyses, we conducted sensitivity analyses to determine the effects with and without this study included. One trial, van de Ven 2017, reported some results for all participants randomised to the intervention and control groups and some results for participants who completed the training according to the protocol (e.g. completed at least 50 sessions) and who completed outcome measures after the intervention and on follow‐up. For this trial, for the purposes of meta‐analyses, where possible, we used the data reported for those participants who completed the protocol and did not drop out and where not available, reported which data were used.
To meta‐analyse outcomes that had opposing directions in the scale, we multiplied the mean values from one set of studies by –1, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 6.5.1.2; Higgins 2021).
We reported the specifics of such data management in the Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors (EG and CK or SE or SB) independently evaluated the methodological quality of eligible trials. If consensus was unable to be reached, a third review author (TH or SB) made the decision. We assessed the risk of bias categories suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Sequence generation (the first contributor to selection bias) refers to an inadequate generation of the randomisation sequence.
Allocation sequence concealment (the second contributor to selection bias) refers to inadequate concealment of allocations prior to assignment.
Blinding of participants and personnel (or performance bias) refers to knowledge of the allocated interventions by participants and personnel during the study (we only rated this low bias if there was blinding of both the participants and the personnel).
Blinding of outcome assessment (or detection bias) refers to knowledge of the allocated interventions by outcome assessors (in this review, we preferred ADL outcomes to make this judgement where possible and applicable).
Incomplete outcome data (or attrition bias) refers to amount, nature, or handling of incomplete outcome data.
Selective outcome reporting (or reporting bias) refers to when all prespecified outcomes that are of interest in the review have not been reported.
Other potential sources of bias refers to any other problems not covered elsewhere (Higgins 2011).
After reviewing the trials, we assigned each of the seven items a rating of 'high', 'low', or 'unclear' risk to indicate the methodological quality of the studies (Table 4).
Measures of treatment effect
For continuous data, we calculated two types of estimate. The measure of the treatment difference for any outcome was the mean difference (MD) when the pooled trials used the same rating scale or test, and the standardised mean difference (SMD) (the absolute MD divided by the SD) when trials used different rating scales or tests. We calculated each one, together with the corresponding 95% CI. For dichotomous data, we planned to compute the risk ratio (RR) or odds ratio (OR) with 95% CI.
Unit of analysis issues
In the case of any cluster‐randomised and cross‐over trials, we planned to identify these, clearly report how these data were included, and conduct sensitivity analyses. For the one cross‐over trial identified, we used the follow‐up data immediately postintervention for both groups in the first period (four weeks after the completion of training), rather than the latest follow‐up time point (20 weeks after intervention) due to concerns about washout period and residual training effects (Lundqvist 2010).
In the case of repeated observations, we planned to perform separate analyses for each outcome, based on the follow‐up periods of up to six months' duration, six to 12 months' duration, and more than 12 months' duration if available. However, due to the various lengths of interventions and the majority at 18 weeks or under and few studies conducting further follow‐up, we grouped analyses by postintervention, three months' follow‐up, six months' follow‐up, and 12 months' follow‐up.
In the case of multiple intervention groups, we planned to either combine groups to create a single pair‐wise comparison (if both intervention groups met our intervention eligibility criteria), or use one pair of groups (i.e. control group and the one intervention group that met our intervention criteria) and exclude the others. The latter was the case for five studies and is reported in detail in the Results (Bo 2019; Cho 2016; Prokopenko 2018; Prokopenko 2019; van de Ven 2017).
Management of the case of more than one control group was not specified in the protocol or the last update. For this review, in the case of one or more control groups, we planned to use the inactive control group where possible and if not, report which control group was used in analysis.
Dealing with missing data
We attempted to contact authors where possible, and searched trial registries of studies (where available) to obtain any missing data and then analysed only the available data.
Assessment of heterogeneity
Where data were sufficient, we pooled the results of trials to present the overall estimate of the treatment effect using a fixed‐effect model. We tested heterogeneity between trial results by using I2 statistic estimates (Higgins 2003). We considered I2 values between 0% and 40% as might not be important, 30% and 60% may represent moderate heterogeneity, 50% and 90% may represent substantial heterogeneity, and 75% and 100% may represent considerable heterogeneity (Deeks 2021).
Assessment of reporting biases
Where more than 10 studies reported outcomes, we created a funnel plot to explore possible reporting biases, interpreting these with other considerations for publication bias within the GRADE evaluation of certainty of evidence (Page 2021; Schünemann 2013).
Data synthesis
Data were pooled where it was clinically homogeneous and we conducted meta‐analyses using Review Manager 2020. We used a fixed‐effect model, except in the cases of substantial heterogeneity, when we applied a random‐effects model, as outlined in Subgroup analysis and investigation of heterogeneity.
Where there were insufficient data to perform a meta‐analysis or the data were unsuitable for inclusion in a meta‐analysis, we reported outcomes using a narrative format.
Subgroup analysis and investigation of heterogeneity
Where there were characteristics not previously specified in the protocol (Hoffmann 2007), but that were later identified as worthy of exploration through subgroup analysis, we clearly identified and reported these as post‐hoc analyses. An example of this is a subgroup analysis for improvement in BADL based on the type of intervention (Analysis 1.1). For all outcomes, in the cases of substantial heterogeneity (i.e. greater than 50%), we applied a random‐effects model to see if homogeneous results could be generated and we conducted sensitivity analyses to examine the studies contributing to the heterogeneity. We considered conducting subgroup analyses for any characteristics of differing populations or interventions contributing to the intervention effect that may explain the heterogeneity (Deeks 2021).
1.1. Analysis.

Comparison 1: Basic activities of daily living (BADL) performance, Outcome 1: BADL (postintervention)
Sensitivity analysis
For any outcomes and where applicable, we carried out a sensitivity analyses to evaluate the effect of trial quality by analysing separately trials with and without adequate randomisation and concealment of treatment allocation, which was possible in a small number of analyses.
We conducted sensitivity analysis for the only cross‐over trial (Lundqvist 2010). We also conducted sensitivity analyses for outcomes including data from Prokopenko 2019, where we converted the data from medians and 95% CIs to means and SDs, as reported in Data extraction and management.
We did not conduct sensitivity analyses, as per the protocol, for trials with and without intention‐to‐treat analyses or for trials with follow‐up periods of less than six months, six to 12 months, and more than 12 months' duration. See Differences between protocol and review.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of evidence (Guyatt 2011). For assessments of the overall quality of evidence for each outcome, we downgraded the evidence from 'high certainty' by one level for serious (or by two for very serious) study limitations in risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. We used the GRADE approach to interpret and communicate findings (Santesso 2020; Schünemann 2013) and GRADEpro GDT software (GRADEpro 2020) to import data from Review Manager 5 (Review Manager 2020) to create the Table 1. This table provides outcome‐specific information concerning the magnitudes of effects of the interventions examined, the amount of available evidence, and the certainty of available evidence (Schünemann 2021a) of the key outcomes of interest. When selecting outcomes for the summary of findings table, we included results for the primary outcome of BADL and secondary outcomes of IADL, community integration and participation, and key cognitive abilities (i.e. global cognitive function, attention, memory, and executive function and subdomains), considering which outcomes would be of most interest to occupational therapists and their patients.
Results
Description of studies
The original review (Hoffmann 2010), included one trial (Carter 1983). We included an additional 23 trials in this update, bringing the total trials included to 24. See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
This search yielded 9384 records and 159 additional records from other sources. After removal of duplicates, we screened the titles and abstracts of 7609 records, then retrieved and reviewed full texts for 246 articles. We identified 36 full texts that met the inclusion criteria that reported on 24 trials, three full texts that are ongoing trials, and 14 full texts awaiting classification. We excluded 175 studies (193 full texts) that did not meet the inclusion criteria. See Characteristics of excluded studies table. See Figure 1 for the study flow diagram. We identified 36 additional trials that may meet the criteria that are ongoing (one) or awaiting classification (35). See Characteristics of ongoing studies and Characteristics of studies awaiting classification tables.
1.
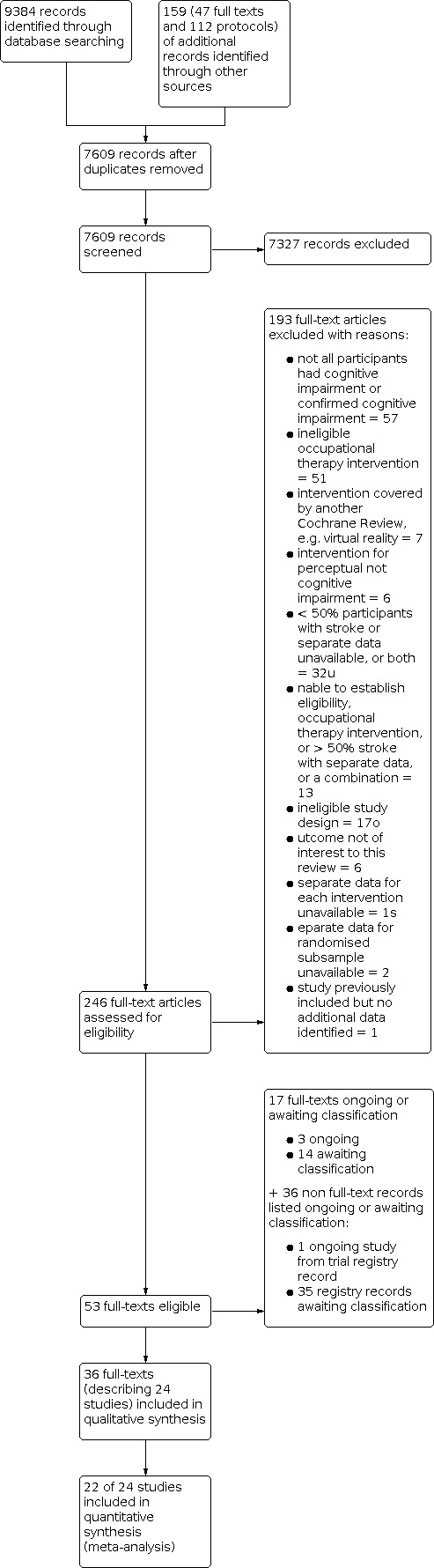
Study flow diagram: original review with 2009–2021 update.
Included studies
We identified 24 trials involving 1205 participants (1142 analysed) that met the inclusion criteria. Two trials had an eligible subsample of participants with stroke (Akerlund 2013; Lundqvist 2010). The authors of these studies provided the data for these subsamples after correspondence with them and we included these data in the analysis. Another author provided the means and SDs needed for analysis after correspondence (Barker‐Collo 2009).
Twenty‐two trials were parallel RCTs (Akerlund 2013; Barker‐Collo 2009; Bo 2019; Carter 1983; Chen 2015; Cho 2015; Cho 2016; De Luca 2018; Hasanzadeh Pashang 2020; Lin 2014; Maggio 2020; Park 2015a; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Skidmore 2015a; Skidmore 2017; van de Ven 2017; Walker 2012; Yeh 2019; Yoo 2015; Zuchella 2014). One was a cross‐over RCT where we used the follow‐up data immediately postintervention for both groups (at four weeks) rather than the latest follow‐up time (of 20 weeks) (Lundqvist 2010). One RCT was a two‐by‐two factorial design where we only included data for two of the four groups (the control group and the group who received the occupational therapy relevant intervention) (Jiang 2016). Bo 2019 randomised participants to four groups, two of which were ineligible for our purposes: a physical exercise group and a combined physical exercise and cognitive training. Cho 2016 also included a group receiving neurofeedback through electroencephalogram training, which we considered an ineligible intervention. Therefore, we only included data for two of the three groups (i.e. the group who received the eligible occupational therapy intervention and the control group). Prokopenko 2018 and Prokopenko 2019 included two comparator groups, one a "passive" control group, the other an "active" control group. We only included data for the passive control group as it equated most to usual care of the other comparators in other studies. van de Ven 2017 had two comparators, an active control of "mock training" using the same online environment as the intervention with different tasks and a waiting‐list group. We used the data from the waiting‐list control group primarily because this had the most complete reporting of data for use in analysis while being comparable to other comparators and as the active control used the same intervention software.
Sample characteristics
The trials were published between 1983 and 2020, and the study duration (where reported) ranged from six weeks (Cho 2016), to 31 months (Akerlund 2013; Zuchella 2014). The sample sizes for the participants with stroke and reported in this review ranged from 13 (Lundqvist 2010), to 100 (Jiang 2016), with 17 studies having fewer than 50 participants. Four trials occurred in the Republic of Korea (Cho 2015; Cho 2016; Park 2015a; Yoo 2015); four in China (Bo 2019; Chen 2015; Jiang 2016; Lin 2014); three trials occurred in Italy (De Luca 2018; Maggio 2020; Zuchella 2014), Russia (Prokopenko 2013; Prokopenko 2018; Prokopenko 2019), and the USA (Carter 1983; Skidmore 2015a; Skidmore 2017); two in Sweden (Akerlund 2013; Lundqvist 2010); and one occurred in each of: Iran (Hasanzadeh Pashang 2020), New Zealand (Barker‐Collo 2009), Taiwan (Yeh 2019), the Netherlands (van de Ven 2017), and the UK (Walker 2012). All but one full text (Li 2016 from Chen 2015 study) were published in English. We partially translated sections of Li 2016 for additional data extraction. Additional data for participants with stroke were requested from and provided by Akerlund 2013, Barker‐Collo 2009, and Lundqvist 2010. Additional data were obtained from trial registry entries for Skidmore 2015a and Skidmore 2017. Additional data were requested from but not provided by De Luca 2018, Prokopenko 2013, Yoo 2015, and Zuchella 2014. Details of each of the studies are summarised in the Characteristics of included studies table. Table 5 provides a summary of key participant and intervention characteristics.
4. Summary of participant and intervention characteristics.
| Author Year | Participant characteristics | Intervention characteristics | |||||||
| Number allocated (analysed) |
Type of participants |
Time since onset of stroke (mean or median or recruitment timeframe) |
% Men |
Age (mean/median years) |
CR/CA |
Duration (session, weeks) |
Frequency (times per week) |
Total dose (maximum possible hours) |
|
| Akerlund 2013 | 34 (29) | Outpatients | 30/28 weeks (7–8 months) |
NR | IG: 47.38 CG: 52.86 |
CR | 30–45 min 5 weeks |
5 | 18.75 |
| Barker‐Collo 2009 | 78 (78) | Inpatients | 18/19 days | 60 | IG: 70.2 CG: 67.7 |
CR | 60 min 4 weeks |
5 | 20 |
| Bo 2019 | 114 (92) | Outpatients | < 6 months' recruitment | 55 | IG: 67.51 CG: 64.36 |
CR | 60 min 12 weeks |
3 | 36 |
| Carter 1983 | 33 (28a, 25b) | Inpatients | 5 days (from admittance)c | 48 | IG: 70.5 CG: 73.4 |
CR | 30–40 min 3–4 weeks |
3 | 8 |
| Chen 2015 | 80 (80) | Inpatients | ≤ 3 months' recruitment | 65 | 57.74 | CR | 30 min 4 weeks |
5 | 10 |
| Cho 2015 | 25 (25) | Inpatients | 5/6 months | 64 | IG: 60 CG: 63.7 |
CR | 30 min 6 weeks |
5 | 15 |
| Cho 2016 | 30 (30) | Inpatients | 5/6.5 months | 53 | IG: 63 CG:64 |
CR | 30 min 6 weeks |
5 | 15 |
| De Luca 2018 | 35 (35) | Not designated | 3.5 months | 51 | 43.1 | CR | 45 min 8 weeks |
3 | 18 |
| Hasanzadeh Pashang 2020 | 20 (20) | Not designated | 12/20 months | 75 | IG: 53.90 CG: 57.70 | CR | 60 min 8 weeks |
1 | 8 |
| Jiang 2016 | 120 (100) | Inpatients and outpatients | 43/44 days | 49 | IG: 62.37 CG: 60.53 |
CR | 30 min 12 weeks |
5 | 30 |
| Lin 2014 | 34 (34) | Not designated | 228 days (8 months) |
59 | IG: 62.4 CG: 63.2 | CR | 60 min 10 weeks |
6 | 60 |
| Lundqvist 2010 | 13 (13) | Outpatients | 51 months | 54 | 45.2 | CR | 45–60 min 5 weeks |
5 | 25 |
| Maggio 2020 | 40 (40) | Not reported | 6 months | 55 | 53.9 | CA | 60 min 8 weeks |
3 | 24 |
| Park 2015a | 30 (30) | Inpatients | 1.5/1.8 months | 47 | IG: 64.7 CG: 65.2 |
CR | 30 min 4 weeks |
5 | 10 |
| Prokopenko 2013 | 43 (43) | Inpatients | < 2 weeks' recruitment | 53 | IG: 61 CG: 66 |
CR | 30 min 2 weeks |
7 | 7 (up to 15) |
| Prokopenko 2018 | 19 (19) | Not reported | < 6 months' recruitment | 72 | IG: 59.5 CG: 62.55 |
CR | 30–40 min 10 days |
5–7 | 6.7 |
| Prokopenko 2019 | 49 (49) | Not reported | "early and late recovery period" recruitment | 65 | IG: 59 CG: 60.5 |
CR | 30–40 min 10 days |
5–7 | 6.7 |
| Skidmore 2015a | 30 (30) | Inpatients | 16/19 days | 67 | IG: 64.87 CG: 71.80 |
CA | 3.75 hours For duration of inpatient therapy |
1 | ND |
| Skidmore 2017 | 43 (43) | Inpatients | 16/22 days | 51 | IG: 65.86 CG: 66.73 |
CA | 45 min 2 weeks |
5 | 7.5 |
| van de Ven 2017 | 97d (97) | Outpatients | 28/29 months | 69e | IG: 57 CG: 61.2 |
CR | 30 min 12 weeks |
5 | 30 |
| Walker 2012 | 70 (64) | Inpatients | 22/26 days | 41 | IG: 77 CG:81 |
CA | 6 weeks | Not reported | Not reported |
| Yeh 2019 | 30 (30) | Not reported | 48/94 months | 70 | IG: 50.63 CG: 60.21 |
CR | 60 min 12–18 weeks |
2–3 | 36 |
| Yoo 2015 | 46 (46) | Inpatients | 11 months | 37 | IG: 53.2 CG: 56.3 |
CR | 30 min 5 weeks |
5 | 12.5 |
| Zuchella 2014 | 92 (87) | inpatients | < 4 weeks recruitment ("time for admission" 11/11.5 days) |
53 | IG: 64 CG:70 |
CR | 60 min 4 weeks |
4 | 16 |
CA: compensatory‐adaptive; CG: control group; CR: cognitive remediation; IG: intervention group; min; minutes; ND: no data. aFor ADL outcome. bFor time judgement outcome. cThis is reported as number of mean days from admittance to stroke programme, so it is unclear if this is duration since stroke onset. dThis number is for all participants randomised, i.e. to 3 groups, intervention, active control, and waiting‐list control groups; data from either the waiting‐list control or the active control groups were used in analysis. eMean of the percentages for each of the 3 groups.
Participants
Twenty studies reported mean ages, ranging from 43 years in Lundqvist 2010 to 74 years in Carter 1983; four included studies reported median ages, which ranged from 59.5 years for the intervention group in Prokopenko 2018 to 81 years in the control group in Walker 2012.
Across all studies, most participants were patients of a hospital or rehabilitation centre. Some studies did not clearly report the duration of time since stroke onset. Recruitment periods or reported duration ranged from within two weeks up to eight years of stroke onset. Nine trials recruited inpatients within one to three months since stroke onset: Carter 1983 (mean time from admission approximately five days; it was not clear if this was five days after stroke onset); Prokopenko 2013 (participants approached within two weeks of onset); Barker‐Collo 2009 (mean onset of 18.5 days); Skidmore 2015a (approximate mean time since onset of 17 in the intervention group and 18 days in the control group); Skidmore 2017 (16.20 in the intervention group and 22.36 days in the control group); Walker 2012 (median time since onset of 26 and in the intervention group and 22 days in the control group); Zuchella 2014 (eligibility criteria of stroke within previous four weeks); Park 2015a (1.5 months for the intervention group and 1.8 months for the control group); and Chen 2015 (eligibility criteria of time since onset of less than three months). Jiang 2016 recruited inpatients or outpatients with mean time since onset of 44.22 days in the intervention group and 42.76 days in the control group (Yang 2014). Cho 2015 and Cho 2016 recruited hospitalised patients with stroke onset within three months to one year. De Luca 2018 enrolled participants who were within three to six months from the acute event. Yeh 2019 recruited "stroke survivors" from rehabilitation units whose stroke occurred at least six months before enrolment (mean onset of 47.8 months for intervention group and 94.43 months for the control group). One trial recruited inpatients with an approximate time since stroke onset of 12 months for the intervention group and 11 months for the control group (Yoo 2015). Three trials recruited outpatients only: Akerlund 2013 (time since stroke onset approximately 30 in the intervention group and 28 weeks in the control group), Bo 2019 (eligibility criterion of less than six months poststroke), and Lundqvist 2010 (eligibility criterion of time since stroke one year or longer with mean onset of 51 months). van de Ven 2017 recruited community‐based participants within three months to five years (mean durations of 28 to 29 months) after stroke from rehabilitation centres and departments of hospitals; some were still outpatients receiving rehabilitation. Five trials were unclear as to whether participants were inpatients or outpatients: Hasanzadeh Pashang 2020, whose participants were patients of a rehabilitation clinic with duration since stroke of 11.9 months in the intervention group and 20.3 months in the control group; Lin 2014, whose participants were people attending a rehabilitation hospital within six to 10 months' poststroke, with a mean of about 7.5 months; Maggio 2020, who recruited people attending a neurorehabilitation unit who were in the chronic phase (i.e. between six and 12 months after the event with a mean of six months); Prokopenko 2018, who recruited people up to six months after stroke; and Prokopenko 2019, who recruited people in the "early and late recovery period".
Many trials reported exclusion criteria related to language, comprehension or communication impairments, or issues, such as aphasia or speaking a language other than that of the country (Akerlund 2013; Barker‐Collo 2009; Cho 2015; Hasanzadeh Pashang 2020; Park 2015a; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Skidmore 2015a; Skidmore 2017; van de Ven 2017; Walker 2012; Zuchella 2014). Many also excluded people due to significant other health conditions that could impact participation, particularly mental disorders including depression and dementia (Akerlund 2013; Chen 2015; De Luca 2018; Jiang 2016; Lin 2014; Maggio 2020; Lundqvist 2010; Skidmore 2015a; Skidmore 2017; van de Ven 2017; Walker 2012; Zuchella 2014). Some trials excluded people with severe or extensive cognitive impairment that would affect participation (e.g. Barker‐Collo 2009 and Prokopenko 2013 (less than 20 on the Mini‐mental Status Examination (MMSE)) (Folstein 1975), Skidmore 2015a (Quick Executive Interview score of 3 or less, Royall 1992), van de Ven 2017 (Telephone Interview Cognitive Status (TICS) score less than 26), or low intelligence quotient (e.g. Lundqvist 2010 (70 or less based on Wechsler Adult Intelligence Scale (WAIS III)) (Wechsler 1997) and Zuchella 2014 (less than 70, not specified) and Prokopenko 2018 and Prokopenko 2019 (not specified)). Some specifically excluded people with perceptual impairments or motor impairments that would affect participation, such as neglect (van de Ven 2017), motor deficits or unsafe for physical activity (Bo 2019), spasticity (De Luca 2018), unable to use a controller (Lundqvist 2010; Park 2015a; Zuchella 2014), inability to walk with or without assistive devices (Yeh 2019), or poor sitting tolerance (Walker 2012). Some excluded people with hearing or vision impairments that may have impacted on participation (e.g. De Luca 2018; Hasanzadeh Pashang 2020; Jiang 2016; Maggio 2020; Prokopenko 2018; Prokopenko 2019; van de Ven 2017; Zuchella 2014). Some excluded people who had received similar interventions within the past year (e.g. Cho 2015; Cho 2016), and one if participants had "computer fear" (van de Ven 2017).
Interventions
Table 6 provides a list of the interventions classified by type of occupational therapy approach. Each intervention is described in more detail in the Characteristics of included studies table and Table 7 according to the TIDieR checklist (Hoffmann 2014).
5. Classification of interventions by occupational therapy approach.
| Cognitive remediation approach (CR) | Compensatory and adaptive approach (CA) | ||
| Computer‐based software materials | Study | Intervention | Study |
| RehaCom | Cho 2015; Cho 2016; Jiang 2016; Lin 2014; Yoo 2015, | ADL Strategy Training | Skidmore 2015a |
| KrasSMU programs | Prokopenko 2013; Prokopenko 2018; Prokopenko 2019 | ADL GUIDE Training | Skidmore 2017 |
| BrainHQ | Chen 2015; Yeh 2019 | Home Automation Training (HAT) | Maggio 2020 |
| Cogmed | Akerlund 2013; Lundqvist 2010 (formerly QM) | DRESS (dressing training) | Walker 2012 |
| COGPACK | Bo 2019 | — | |
| ERICA | De Luca 2018 | ||
| CoTras | Park 2015a | ||
| BrainGymmer | van de Ven 2017 | ||
| A gym for the mind 2 Training of cognitive rehabilitation |
Zuchella 2014 | ||
| Pen and paper materials | Study | ||
| Attention Processing Training (APT) + auditory CD | Barker‐Collo 2009 | ||
| Thinking Skills Workbook | Carter 1983 | ||
| Brain Injury Workbook | Hasanzadeh Pashang 2020 | ||
6. Description of interventions in included studies using the items from the Template for Intervention Description and Replication (TIDieR) checklist.
| Study | Brief name | Recipient | Why | What (materials) | What (procedures) | Who provided | How | Where | When and how much | Tailoring |
Modification of intervention throughout trial |
Strategies to improve or maintain intervention fidelity |
Extent of intervention fidelity |
| Akerlund 2013 | Cogmed QM | Rehabilitation outpatients of working age in the postacute phase after brain injury with identified WM deficits. | To provide an evidence‐based remedial approach using a computerised training program with intensive repetition of tasks for visuospatial and verbal WM that are adapted for the participant at a challenging performance level. | Cogmed WM training. Stockholm: Pearson Assessment AB. 2006. Available online at: www.cogmed.com/; the online version requires a stable broadband Internet connection, preferably 0.5 Mbit/second or better; quiet room. | The Cogmed program includes a battery of visuospatial and verbal auditory WM tasks: 1. visuospatial WM tasks require recall of the position of stimuli in a 4 × 4 grid and then reproduction of the stimuli in the same order, in the reverse order, or in a rotated grid; 2. verbal WM tasks require recall of sequences of letters and digits forwards or backwards 9or both). All parts of the battery must be trained at each session, 90 trials each day. The tasks are introduced with a voice‐over transmitted by the computer's speaker. The person responds by localising and remembering multiple stimuli at the same time. The tasks have a unique sequencing order in each trial and short delays that require the representation of stimuli to be held in the person's WM. |
Occupational therapists with experience in rehabilitation medicine and who were trained as Cogmed coaches. According to the Cogmed website, Cogmed is "currently used by psychologists, speech pathologists, occupational therapists and other clinical specialists working with individuals with attention and learning difficulties" (www.cogmed.com/) and requires providers to undertake Cogmed Coach Training and Accreditation courses, either by self‐paced online coursework, which is free with the purchase of a Cogmed Coach Starter Pack or attending a 1‐day face‐to‐face course offered worldwide. | Face‐to‐face, individually, and independently using the online software. | In a quiet room in the Occupational Therapy department. | 30–45 minutes per session, 5 days per week for 5 weeks (25 sessions) (18.75 possible maximum hours) | The difficulty level of the tasks adapts according to the participant's performance. The software includes direct reinforcement via scores and positive verbal feedback. Participants were able to ask staff for assistance. Once per week the coach gave personal and individual feedback about results. | None reported | There appeared to be no formal assessment of fidelity. The program was reportedly used according to the guidelines and that the coaches provided input to help participants "adhere to the training" but no evaluation of this was reported. | None reported. The researchers commented that the training was tiring and that the schedule of 5 days per week for 5 weeks and the need to attend the outpatient clinic for the training was challenging for many of the participants and was the reason for dropouts. |
| Barker‐Collo 2009 | APT | Inpatient adult survivors of incident stroke with a confirmed attention deficit | To provide a theoretically based, hierarchical, and multilevel treatment, involving cognitive exercises for remediation and improvement of aspects of attention including sustained, selective, alternating, and divided attention (Sohlberg 1987; Sohlberg 2001). Sohlberg 2001 outlines 6 treatment principles for attention process training, including theoretically grounded, hierarchically organised, providing sufficient repetition, based on client performance data, with active facilitation of generalisation throughout treatment, and providing a flexible and adaptable format. | APT package including paper‐and‐pencil tasks, set of CDs including auditory CDs that produced auditory stimuli as well as a distraction (like "white noise") to overlay some tasks where selective attention was needed. For the visual tasks, this distraction was produced by using acetate overlays with patterns on them. The latest version of APT program can be purchased at: lapublishing.com/apt-attention-process-training/, which provides details of latest APT software and training program; materials include a manual and tracking sheets for exercises. See also Sohlberg 1987 for an appendix of materials used for training each aspect of attention and Sohlberg 2001 for an outline of an APT program that included computer activities, auditory tapes, and pen‐and‐paper tasks. | The provider used a hierarchy of treatment tasks targeting different aspects of attention starting at sustained attention then progressing to selective, alternating, and divided attention. Each task was considered "mastered" once the client was able to complete the task with 0 or 1 errors. Current APT program involves "a set of drill based, hierarchically organized exercises that tap different domains of attention that are matched to the client's impairment profile and administered repetitively. They are paired with generalization real world, individualized exercises that are selected to promote generalization" (Barker‐Collo 2009). Sohlberg 2001 has examples of tasks in the APT computer program for addressing each of these aspects and appendices with example recording protocols and a case study. Examples of tasks include listening for target words or sequences on auditory attention tapes, mental math activities, putting words in alphabetical order, placing a visual distractor (e.g. a plastic overhead sheet with distractor lines) over the top of a paper and pencil activity. | A registered clinical neuropsychologist provided APT training; APT can be administered by neuropsychologists, occupational therapists, speech language therapists, and other rehabilitation specialists. There are no training requirements in addition to the manual. | Face‐to‐face and individually. Sohlberg 2001 reported that delivery should be flexible and adaptable including delivery to individuals or groups. | In hospital prior to discharge and then in clients' primary residences after discharge; Sohlberg 2001 reported that delivery should be flexible and adaptable including delivery in clinics or at home. |
Up to 30 hours of individual APT conducted for 1 hour on weekdays for 4 weeks | Because of issues such as fatigue, a 30‐hour maximum was set in this study. Sohlberg 2001 described the hierarchical nature of the program tasks and how clinicians used client performance data to tailor the intervention. The hierarchy of program tasks place increasing demands on attentional control and WM. Client performance data were used to make treatment decisions, such as when to start, stop, or modify a program. For example, clinicians examine the participants' error profiles to assess where errors were occurring, such as at the beginning or end of a task, reflecting a different attentional demand and adjust the training tasks accordingly. Graphs of performance were shown to the client to provide objective and powerful feedback. |
None reported | None reported in the paper and not formally assessed; quote "there was tracking of the order in which tasks were administered to ensure that the protocol was adhered to" (Barker‐Collo 2009). | None reported |
| Bo 2019 | COGPACK | Adult outpatients < 6 months poststroke with vascular cognitive impairment (aged > 18 years) | To provide an effective and safe alternative to established drugs to decrease cognitive impairments in people with stroke in the form of a non‐pharmacological intervention of cognitive training. | COGPACK programme, developed for neurorehabilitation (Marker Software, www.markersoftware.com) delivered on up to 20 tablet computers with touch screens "to avoid training difficulties in computer novices"; 12 of possible 64 exercises were selected, including 4 tasks of memory ('memory for route', 'memory for signs', 'memory for pattern', 'memory for scene'), 4 tasks of execution ('mental arithmetic', 'logical block', 'shortest way', 'continue a series'), and 4 attention and speed tasks ('scanning', 'catch', 'steer', 'assembly line') | Supervised cognitive training in groups using tablet computers | Quote: "experienced therapists with exercise physiology or clinical psychology backgrounds" provided the interventions for the 4 groups. However, the cognitive training group used the COGPACK program, which is a commercially available program and which, according to the website, can be used in occupational therapy as a "concentration, performance and motivation aid". | Face‐to‐face in a supervised group (≤ 20). | Within the rehabilitation centre, further details not provided. | 60 minutes, 3 times per week, for 12 weeks (36 hours in total). | Group training was supervised, so presumably individual and group assistance was provided as needed. | None reported | None reported | None reported |
| Carter 1983 | Cognitive skills remediation training | Inpatient adults with cognitive impairments after acute stroke | To provide formal cognitive remediation training to people within 1‐week poststroke that included (quote) "continuous reinforcement, immediate feedback, cuing, gradually increasing … difficulty level and stressing the importance of the skills being taught to activities of daily living." | Thinking Skills Workbook for pen and paper tasks requiring: visual scanning, visual‐spatial, or time judgement; early versions cited in papers, latest edition: Languirand 2014. Provides pen and paper pre‐ and post‐tests and tasks covering various areas of cognition including paying attention and reading, concentrating on detail, listening, scheduling and time management, memory in everyday living, sorting and classifying, sequencing and logic, verbal skills, maths skills. |
Based on pretest of the 3 main areas of interest, visual scanning, visual‐spatial, or time judgement, trained research assistants provided 1‐to‐1 training sessions in any of these areas where pretest performance was < 80%. | Trained research assistants provided the intervention; Languirand 2014 workbook states that it is for use by professional rehabilitation staff, paraprofessionals, family members, or a combination of these. | Face‐to‐face and individually | Stroke rehabilitation unit; Languirand 2014 workbook recommends a quiet private room with minimal distractions. | 30–40 minutes, 3 times per week for a mean of 3–4 weeks (up to 8 hours); the testing and training took place between 9.00 a.m and 11.45 a.m. before or after other stroke program activities; Languirand 2014 workbook recommends session length of 25–35 minutes, twice per week for 4–6 weeks. | Not reported except that training was given only in areas where the participant scored < 80%. | None reported | Only that the research assistants were trained in activities from the workbook. | None reported |
| Chen 2015 | BrainHQ | Adults within 3 months poststroke with executive disorder | To provide computer‐based training to improve executive functioning in addition to regular or standard rehabilitation and therapy. | BrainHQ (Posit Science) computer‐based training accessed via a computer with Internet access: Double Decision, Target Tracker, Hawk Eye, and Visual Sweep available online; Double Decision: trains attention (www.brainhq.com/why-brainhq/about-the-brainhq-exercises/attention/double-decision); Target Tracker: trains executive function (www.brainhq.com/why-brainhq/about-the-brainhq-exercises/attention/target-tracker); Hawk Eye: trains memory (www.brainhq.com/why-brainhq/about-the-brainhq-exercises/brainspeed/hawk-eye); Visual Sweeps: trains spatial orientation (www.brainhq.com/why-brainhq/about-the-brainhq-exercises/brainspeed/visual-sweeps). |
In addition to "regular/standard occupational therapy, physical therapy, TENS, cognitive rehabilitation training, and acupuncture" BrainHQ computer‐based training was provided, mainly using the 4 games of BrainHQ. Each game includes 10 levels, increasing in difficulty and graded by different shapes, colours, and interferences. In addition, each level has 3 different backgrounds. Each participant had a practice session at the enrolment of the study. The study researcher chose the suitable model for training to ensure the safety of the participants with hemiplegia. The first to fourth week of training all included Double Decision, Target Tracker, Hawk Eye, and Visual Sweep games. | Not specified although the researchers (who appear to be from nursing and rehabilitation backgrounds) provided the demonstration and training and accompanied the participant during training to ensure smooth training. BrainHQ training was completed in addition to regular occupational therapy and other therapies. | Not specified but presumably face‐to‐face and individually. | Not specified but within inpatient rehabilitation ward. | 5 sessions per week for 4 weeks of 30 minutes per session (10 hours in total). | The difficulties of each game were individually adjusted according to the participant's ability. Grading of games: each game included 10 levels, increasing in difficulty. Each level was graded by different shapes, colours, and interferences. The levels of difficulty were raised by increasing the similarity of shapes and colours and by increasing the number of interferences. In addition, each level had 3 different backgrounds. Based on the participant's correct or incorrect responses, the time of the target items shown on the screen decreases or increases. The system automatically adjusted the difficult according to participant's progress. |
None reported | None reported | None reported |
| Cho 2015 | RehaCom | Adults within 3–12 months' poststroke with cognitive dysfunction | To provide objective cognitive training based on neuropsychological patterns to stimulate damaged location of the brain | RehaCom software (Korean version): the awakening, reactivity, attention and concentration, simultaneous attention, and selective attention programs; see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer; joystick and touch screen input devices; reaction board. | Computer‐assisted cognitive rehabilitation | 2 "expert therapists" using commercially available software | Face‐to‐face and individually. | Not specified. | 30 minutes per day, 5 days per week, for 6 weeks (15 hours in total). | Feedback on the result during and after the treatment was provided and training occurred according to each participant's functional ability; the participants could complete the training using a reaction board while seated and watching the screen. | None reported. | None reported. | None reported. |
| Cho 2016 | RehaCom | Adults within 3–12 months' poststroke with cognitive dysfunction | To improve problem‐solving ability using games or other computer‐based programs in a manner that allows different levels of task difficulty for the participant. | RehaCom software (Korean version): the attention, concentration, and memory programs; see hasomed.de/en/products/rehacom/. The software is available in 27 languages at no extra cost; computer; monitor, keyboard. | Computer‐assisted cognitive rehabilitation. | 2 "expert therapists" using commercially available software. | Face‐to‐face and individually. | Not specified. | 30 minutes per day, 5 days per week, for 6 weeks (15 hours in total). | Training at different levels of task difficulty according to the functional level of the participant. | None reported. | None reported. | None reported. |
| De Luca 2018 | ERICA | Adults in chronic phase of stroke (3–6 months) | To implement cognitive training in 5 cognitive domains of attention process, memory abilities, spatial cognition, and verbal and non‐verbal executive functions. | PC‐based ERICA software training (www.erica.giuntios.it/it/) in Italian; personal computer. | Traditional cognitive rehabilitation plus computer‐based ERICA software training provided by a therapist who provided exercises with a growing hierarchy of complexity. | Trained "cognitive therapist"; the ERICA website states that professionals who can use ERICA include: doctors with specialisation in geriatrics, physical medicine and rehabilitation, neurology and neuropsychiatry, psychologists, speech therapists, physiotherapists, occupational therapists. | Face‐to‐face and individually. | Not specified. | 24 sessions of 45 minutes each, 3 times per week for 8 weeks plus same time in traditional cognitive rehabilitation (up to 18 hours) in addition to traditional cognitive rehabilitation of 24 sessions 3 times per week for 8 weeks (for total of 48 sessions of 45 minutes each, total of 36 hours). | The therapist provided the programs within the "growing hierarchy of complexity" through the ERICA platform; the difficulty of the exercises "was flexible to the progressive changes of the patient's performance and consistently ensure(d) effective and pleasant rehabilitation sessions". The website states: "The user [rehabilitation professional] selects the exercise to be administered, sets the parameters (target stimulus, exposure time, background color, etc.), administers the exercise and proceeds with the session." | None reported. | None reported. | None reported. |
| Hasanzadeh Pashang 2020 | Cognitive rehabilitation | Adults with attention impairment | To improve visual and auditory attention performance through group work including focused stimulation, learning compensatory coping strategies, acquiring insight and awareness, emotional adjustment and improved self‐efficacy of feeling more 'in control' (Powell 2017). | The Brain Injury Workbook. Exercises for Cognitive Rehabilitation (Powell 2017). www.routledge.com/The-Brain-Injury-Workbook-Exercises-for-Cognitive-Rehabilitation-2nd/Powell/p/book/9781315172897. | Routine rehabilitation plus cognitive rehabilitation was delivered according to the Brain Injury Workbook (Powell 2017). Table 1 of paper outlines content of 8 sessions: 1: "Determining the purpose and familiarity with stroke and its effects on attention, memory and daily life" 2: "Defining attention and its types. Attention persistence training. Training (backward training)" 3: "Memorizing pictures, names and face, and practicing word listing" 4: "Meaning evocation training, and completing words" 5: "Family name training, and word finding training to promote the divided attention" 6: "Different‐options training, and using memory auxiliaries" 7: "Training how to remember arrangements and gathering training" 8: "Training how to remember numbers and review of some training, solving problems and responding to patient questions". |
Not reported; Powell 2017 stated that the workbook can be used by therapists working with brain‐injured people in groups and can be used by people with brain injuries themselves and their carers. | Face‐to‐face in groups of 2–10 people, as per workbook. | Stroke rehabilitation clinic but not described. | 8 sessions (1 hour per week) (8 hours in total). | None reported, although content appeared amenable to individual tailoring, e.g. family name training. | None reported. | None reported. | None reported. |
| Jiang 2016 | RehaCom | Adults within 6 months poststroke with cognitive dysfunction | To provide computer‐based training with 5 different treatment programs designed to "restore attention, memory, and executive function and to improve the visual field." | RehaCom software package (Chinese version); see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer. | Conventional therapy plus computer software training with RehaCom, which includes 5 different therapeutic programs, each with 1–4 different tasks from which participants chose during each therapy session and 3–5 varying levels of difficulty. The provider chose the program and difficulty level according to each participant's needs and provided guidance or reminders and increased difficulty according to patient feedback. | Physiotherapists using commercially available software. The RehaCom website states that the software is used "extensively by …occupational therapists" and other clinicians in rehabilitation centres, hospitals, and clinics. The protocol (Yang 2014) stated the following requirements of training and experience for all arms of the study: 1. proven record of ≥ 3 years of clinical experience and certified training or education in related fields of rehabilitation or research; 2. participation in a 2‐day training in the standard operating procedures provided by the author of the manualised protocol and the standard operation videos. In the training, the protocol was explained and practised on each other during exercises and role‐plays. |
Face‐to‐face and appears to have been individually. | Not specified but within a rehabilitation hospital. | 30 minutes per day, 5 days per week, for a total of 60 sessions over 3 months (30 hours in total). | The physiotherapists chose different training programs and difficulty levels according to the specific circumstances of each participant and increased the training difficulty according to participant feedback. | None reported in paper, but protocol stated that the investigators could be convened to discuss practical issues such as intervention protocol revisions (Yang 2014). | None reported. | None reported. |
| Lin 2014 | RehaCom | Adults undergoing rehabilitation 6–10 months since stroke with executive function and memory deficits | To improve executive function and memory. | RehaCom software: see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer. | Cognitive training – no further description of procedures provided. | 2 trained psychologists; the RehaCom website states that the software is used "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals and clinics. | Not described but presumably face‐to‐face. | Not described but presumably in the rehabilitation department. | 1 hour per session, 6 sessions per week for 10 weeks (60 hours in total). | None reported. | None reported. | None reported. | None reported. |
| Lundqvist 2010 | WM training (Cogmed QM) | Adult rehabilitation outpatients | To improve WM function using individualised and intense computerised training that occurs in a clinic, at least initially, so that participants receive the benefit of coaching, meeting with other participants, and training in a calm, quiet environment. | QM (formerly called ReMemo and now called Cogmed) for adults, a WM training computerised system with visuo‐spatial and verbal WM tasks developed at the Karolinska Institute and Cogmed Cognitive Medical System AB, Sweden (www.cogmed.com/healthcare). See also Klingberg 2002; Westerberg 2007; Westerberg 2004 for details of the program. | Participants performed their WM training program on a personal computer in pairs in the presence of 1–3 certified coaches who provided special feedback once per week beside the continuous statistics, which the participants could follow themselves on the computer. The training program specified different visuo‐spatial and verbal WM tasks. Each task was introduced by a speaker voice and the person responded by localising and remembering the stimuli. The participants responded using a computer mouse. The visuo‐spatial WM tasks required the participant to remember the position of stimuli in a 4 × 4 grid and then they are asked to reproduce stimuli in the same order, in the reverse order, or in the grid after the grid had been rotated. The verbal WM tasks required the participant to remember sequences of letters and digits forwards or backwards, or both. |
3 certified coaches; author contact confirmed these were occupational therapists. | Face‐to‐face in pairs in the presence of 1–3 certified coaches. | In a separate quiet room in the Rehabilitation Department. | 45–60 minutes of intense training per day, 5 days per week, for 5 weeks (25 hours possible maximum), weekly coach feedback. | The difficulty level of each training task automatically adjusted according to each participant's progress, increasing the WM load according to each participant's performance levels. The coach provided feedback once per week in addition to ongoing statistics provided to the participants on the computer. | None reported. | None reported. | None reported. |
| Maggio 2020 | Home automation training | Adults with cognitive impairments after chronic stroke undergoing rehabilitation | To evaluate how participants reacted and interacted with the environment, and prepare them for a return home. | Home automation technologies within the home automation room (see Figure 1 of photo of original paper of example technologies in use); functional activities in the room facilitated by the therapist; quote: "The room is designed for severely disabled patients who are partially autonomous in their movements. Through a centralized control system, patients can change the environment, monitor some environmental parameters (for example, detect the presence of smoke, water or gas leaks), but also use the alarm bell. Kitchen countertops and other shelves can be adapted in height and depth. The bathroom has an adaptable toilet and shower, which can be changed by the patient." | Small group activities in a home automation room with home automation or domotics technologies where the interaction was mediated by these technologies to achieve patient ADL goals, e.g. cooking and personal care in preparation for returning home. | Not clearly stated in paper; however, 1 of the authors was an occupational therapist and the intervention was clearly within scope of occupational therapy practice. | Face‐to‐face in a group (3–5 participants per group). | In a room with home automation or domotics technologies in neurorehabilitation unit. | 3 sessions per week for 8 weeks (i.e. total of 24 sessions), each session lasting about 60 minutes (24 hours in total). | The adjustability provided within home automation room allowed tailoring of heights and depths of equipment and technologies to suit patient's individual needs, disabilities, and dimensions. | None reported. | None reported. | None reported. |
| Park 2015a | CoTras | Adult rehabilitation patients with stroke | To provide a Korean computer‐based cognitive rehabilitation to enhance attention, concentration, implementation skills and perception‐motor skills | CoTras program (Netblue Co., Ltd, Korea) made for Koreans; see www.netblue.co.kr/eng/doc/product01-01.php. A joystick and a large button on the CoTras panel, which make the training easier for participants who are unfamiliar with computer use. |
All participated in a standard rehabilitation program according to a daily inpatient treatment schedule. In addition to standard rehabilitation, all participants received 30‐minute daily sessions of the computer‐based program treatment. | Not reported; however, the research was conducted by occupational therapists and the software is commercially available. | Unclear but appears it was face‐to‐face. | Local inpatient rehabilitation hospital, no further details provided. | 20 × 30 minute sessions (5 days per week for 4 weeks) (10 hours in total). | The training allows adjusting to individual participant's abilities at all levels of the program. | None reported. | None reported. | None reported. |
| Prokopenko 2013 | Computer training | Inpatient adults with cognitive impairments from a hemisphere stroke | To restore cognitive function using 2‐part computer training focusing on attention and visual and spatial gnosis with built‐in feedback and a help option. Part 1 focused on 4 aspects of attention (sustained, selective, divided and alternating); part 2 focused on figure‐background activities with gradually decreasing intensity of "background noise". The tasks were not aimed at evaluation of cognitive functions, but rather at training of these functions; though task performance speed in the attention task was measured in time and fed back to the participant, the feedback serves only as a reference point for improvement. |
Computer programs: 1. Schulte's Tables for attention training 2. Figure‐background test for visual and spatial gnosis training Other computerised tasks: "remembering a sequence of symbols", "arranging the clock hands", and "the serial count". |
In addition to standard treatment at the inpatient rehabilitation department: 1. attention training Schulte's Table (a 5 × 5 square grid containing numbers from 1 to 25 in a random order) was presented in full screen mode on the computer monitor. The participant was timed while locating numbers from 1 to 25 in ascending order by clicking on the corresponding number with the mouse. Cues were provided after a fixed time period, e.g. the number pulsated or changed colour. The time taken to complete the task was displayed at the end of the session; 2. training of visual and spatial gnosis a. Figure Ground 1 picture image with decreasing intensity of "background noise" was presented on the computer screen. At the top of the screen, several different images, such as objects or letters without background were shown, including a picture of the image shown in the task. The participant needed to identify the picture that corresponded to the image presented in the task with the noise as soon as possible by clicking the mouse cursor on the corresponding image on the top part of the screen. Speed of recognition was assessed on scale of 0–10. Correctness of performance was marked by applause or a signal "incorrect". b. Position Memory A 5 × 5 grid with a gradually increasing number of objects (images of books) was used to train remembering of the position of images. The pictures were first shown, then hidden and the patient clicked on the cells where they remembered the pictures were located until 2 mistakes were made. The paper provides figures with examples of the computer programs. |
Not clearly stated in paper; however, the authors confirmed sessions were conducted by occupational therapists. The paper stated that the approach could possibly be used independently by a person without involvement of the medical personnel. | Face‐to‐face and individually. | Inpatient rehabilitation department. | Daily for 30 minutes for 2 weeks (up to 15 hours). | Schulte's Tables training: Difficulty level could be adjusted by "changing the period of time allowed for the patient to find the number before the hint appears". Visual and spatial gnosis training: figure‐ground training: there is a gradual reduction of "noise" intensity until it completely disappears. Position memory training: an extra object is added with correct answers and training continues until 2 mistakes are made. Information appears on the screen about the speed and correctness of answers and highest amount of information memorised. |
None reported. | Use of a training protocol. | None reported. |
| Prokopenko 2018 | Computer cognitive training | People with vascular cognitive impairments without dementia in early recovery periods of ischaemic hemispheric stroke | To correct poststroke cognitive impairments in acute and early recovery periods based on the "classical neuropsychological approach of Alexander Luria." | KrasSMU complex of neuropsychological computer programs (in Russian):
The software is reportedly available on CD and online. |
An instructor demonstrated how to use a computer and explained the tasks and rules for each training program in the first few sessions then participants could train independently, remaining under the supervision of the instructor. Procedures for Figure‐Background test and Visual and Position test described in depth: Figure‐Background program: "Patient is asked to identify the image in the picture with noise, and click on the corresponding image in the top part of the screen. There is then a gradual reduction of noise intensity, up to its complete disappearance. The patient needs to recognise the image as soon as possible. The speed of recognition is assessed on a scale of 0–10. The accuracy of performance is marked by applause or a signal 'incorrect'." (see Figure 1 in paper). Pattern position program: "After presentation of various pictures arranged in cells, the pictures are hidden, and then the patient is asked to click on the cells where pictures he or she remembers were located. After a correct performance, the number of objects for memorisation is increased by one. Training continues until the patient makes 2 mistakes, and is followed by the appearance of information about the speed and correctness of answers, and the highest volume of information memorized on a screen (Figure 2). Then, the participant goes up to the next level, where the quantity of cells increases." |
Not clearly stated in paper, however the authors confirmed in personal correspondence about the same intervention provided in Prokopenko 2013 that the sessions were conducted by occupational therapists. | Face‐to‐face and individually with an instructor then participants could train independently, while under the supervision of the instructor | In neurorehabilitation centre; also stated that patients could avail themselves of the programs online | 10 daily sessions for 30 to 40 minutes (5 hours to 6 hours 40 minutes in total) | Supervision provided throughout so that individual support could be provided as needed. Levels of complexity could be increased depending on patients' abilities. | None reported | None reported | None reported |
| Prokopenko 2019 | Neuropsychological computer training | Patients in the early and late recovery period following first hemispheric ischaemic stroke, with cognitive impairments | To provide low‐cost stimulation of several cognitive functions "with automatic changes in loadings and assessment of point scores" and ensuring high level compliance through high motivation from play aspects of the programs. | KrasSMU complex of neuropsychological computer programs (in Russian):
|
In addition to complex restorative treatment, patients participated in courses of sessions using the neuropsychological computerised stimulation programs. | Not clearly stated in paper, however the authors confirmed in personal correspondence about the same intervention provided in Prokopenko 2013 that the sessions were conducted by occupational therapists. | Not reported but appears to be face‐to‐face and individually | In neurorehabilitation centre | 10 daily sessions for 30 to 40 minutes (5 hours to 6 hours 40 minutes in total) | Programs provided "automatic changes in loadings" presumably in response to performance | None reported | None reported | None reported |
| Skidmore 2015a | Strategy Training | Inpatients with cognitive impairments after stroke | To harness the ability of the person with cognitive impairments after stroke "to observe, assess, and positively alter" his or her performance in "real‐life" activities; to teach "individuals to identify and prioritize problematic daily activities, identify barriers impeding performance, generate and evaluate strategies addressing these barriers, and generalize learning through practice." | Workbook materials, Canadian Occupational Performance Measure (COPM) (Law 1998), modified CO‐OP manual (Polatajko 2004) for adults with TBI (Dawson 2009), "Goal‐Plan‐Do‐Check" goal sheets (see Appendix 1, Dawson 2009) | Sessions were administered according to standardised procedures described further in Dawson 2009; Skidmore 2011; Skidmore 2014; 4 critical ingredients (self‐selected goals, self‐evaluation of performance, strategy development and implementation, and therapeutic guided discovery) were applied iteratively throughout the sessions in 4 steps via guided discussion and workbook materials. Step 1: self‐selection of goals: using the COPM and indepth interviews over 1 or 2 sessions, therapists helped participants identify activities that were important to them and that were difficult to perform since the stroke. After prioritising these activities, the participants choose 4–6 activities to address. Step 2: self‐evaluation: the participants selected an activity, performed that activity, and identified barriers to performance. Step 3: strategy development: participants learned a global "Goal‐Plan‐Do‐Check" strategy where they set a goal to address identified barriers (i.e. set criterion for performance outcome), developed a plan to address the goal, completed the plan, and checked whether the plan worked or required revising. Step 4: g generalisation and transfer: these steps were repeated iteratively until the goal was met (and thus participants moved on to the next activity). Using guided discovery technique, the therapists prompted the participants to identify key principles that they learned and to discuss ways to apply these key principles. |
Trained rehabilitation personnel; developers of intervention are occupational therapists | Face‐to‐face and individually | Inpatient rehabilitation facility | 45 minute sessions daily 5 days per week for the duration of inpatient rehabilitation (in addition to usual inpatient rehabilitation therapy) | Participants self‐selected their goals and prioritised and choose 4 to 6 activities to address in the sessions; steps were repeated iteratively until the goal was met (and thus participants moved on to the next activity). Using guided discovery technique, the therapists prompted the participants to identify key principles that they learned and to discuss ways to apply these key principles. | None reported | Fidelity procedures were completed using a protocol described elsewhere (Skidmore 2014) which reported that "All research intervention sessions were videotaped and rated for fidelity to the respective manualized procedures … [using] fidelity checklists … to assess treatment integrity and treatment differentiation in a random 20% of sessions in each treatment group". | There is no report of the outcome of the videotaped fidelity rating, as described above. |
| Skidmore 2017 | Guided Training (GUIDE) | Inpatients with cognitive impairment after acute stroke undergoing rehabilitation | To maximise "the expertise of the patient by training patients to actively engage in the direction and focus of their treatment. Therefore, patients learn to identify and prioritize problematic daily activities, identify barriers to performing activities, generate their own individualized strategies for addressing these barriers, and apply this process through iterative practice. In doing so, guided training is designed to equip patients with the ability to generalize knowledge and skills in problem identification and problem‐solving skills to address new but similar problems over time." | Workbooks, Canadian Occupational Performance Measure (COPM) (Law 1998), standardised protocol | In addition to usual care; using the COPM, the therapist asked the participant to describe a typical routine prior to the stroke, focusing on a typical weekday, a typical Saturday and a typical Sunday. Based on this discussion they then asked the participant to identify 4–6 activities thought important and likely to be problematic after the stroke, then focused the subsequent intervention on these activities. The research therapist asked the participant to pick the first activity that he or she wanted to practice that addressed 1 of the participant‐identified goals. The therapist asked the participant to perform that activity and identified barriers to performance, the therapists then taught the participant a global strategy, goal‐plan‐do‐check, and asked the participant to set a goal to address the barriers (i.e. identify a criterion for performance), develop a plan to address the goal, do the plan and check whether the plan worked or required revising. This process was repeated iteratively until the goal was met (and therefore participants moved on to the next activity), or until the end of the 10 sessions. The therapist guided participants using prompting questions and workbooks to facilitate learning and aid the participants in implementing the strategy. | Licensed occupational and physical therapists (research therapists) who were trained to competency on 1 or the other standardised intervention protocols and who were independent contractors and not members of the usual care rehabilitation team. | Face‐to‐face and individually | Inpatient rehabilitation at an academic health centre and at home | 10 sessions of 45 minutes (5 days per week for 2 weeks) (7.5 hours in total) | The research therapist would ask the participant to describe a typical routine prior to the stroke, focusing on a typical weekday, typical Saturday, and typical Sunday. Based on this discussion, the research therapist then asked the participant to identify 4 to 6 activities that the participant thought were important and likely to be problematic after the stroke. Therapists then focused the subsequent intervention program on these activities. | None reported | Fidelity to each protocol and differentiation of elements between protocols was assessed using standardised checklists applied to a random 20% of video recorded sessions in each condition. They also assessed the degree to which elements of direct skill training and guided training protocols were present in usual care sessions (1 occupational therapy session, 1 physical therapy session and 1 speech therapy session – if being followed by speech therapy – for each study participant). | Adherence to intervention procedures was 85% in the guided training group. The 2 protocols were sufficiently differentiated, with guided training intervention elements present in none of the sampled direct skill training sessions. Analysis of the sampled usual care sessions indicated that on average 2% (usual care training) was consistent with guided training. |
| van de Ven 2017 | Cognitive flexibility training (BrainGymmer) | Adults patients who had a stroke within last 5 years with cognitive impairments | To improve cognitive flexibility and thereby executive functioning through cognitive computer‐based training that includes frequent switching between various training tasks. | Online platform BrainGymmer (in Dutch); English version: www.braingymmer.com/en/; daily access to a computer with Internet connection and sound (either through headset or speakers); 9 tasks consisting of 20 levels selected to train 3 cognitive domains: attention, reasoning, and WM (see Additional file 2 van de Ven 2015 for brief descriptions and pictures of tasks; www.ncbi.nlm.nih.gov/pmc/articles/PMC4545547/:
Training videos of each task on individual computers Instruction booklet |
Training instructions were provided at baseline using videos on computers followed by supervised practice by participants and provision of training booklet. Participants trained on daily on several tasks within 1 session and frequently switching between the tasks. Participants were asked to train when they had at least 50 minutes available and when not mentally fatigued (e.g. late at night). Personal feedback was provided based on individuals' scores after each task, using a 3 star rating scale, with more stars for better performance and at the end of each session with more detailed feedback of their scores on each task. Email availability for questions or to address any problems. Weekly or fortnightly contact by phone for participants to ask questions and for discussion about training adherence. Reminder email sent if no training for 2 days and personal contact if 3 days without training. Daily log by participants. Exit questionnaire administered. |
Trained masters students who were familiar with all training tasks and login procedures for remote support and training instruction. Neuropsychologist for weekly or fortnightly contact. |
Individually and independently without face‐to‐face supervision at the time but with remote assistance if required; individual and personal telephone and email contact with trainers / neuropsychologist. | At home for computer tasks; training occurred at a university | 10 tasks daily for 3 minutes each (30 minutes), 5 times per week for 12 weeks; 58 sessions (29 hours); In week 1, participants trained for 10 minutes each day on 3 tasks then from week 2, the number of trials per task was reduced to promote frequent switching between task. Trainer contact weeks 1, 2, 3, 5, 6, 8, and 10. Email contact if no training for 2 days. |
"Difficulty of tasks was adapted individually to the performance of participants. Participants were instructed to go to the next level when 2 or 3 stars were obtained. However, participants could choose to stay at the same level when receiving 2 stars, whereas they were obliged to stay at the same level when one star or less was obtained." Tasks were set up each session for each participant. "The order of tasks ensured that tasks from the same cognitive domain (attention, reasoning, and WM) were not presented immediately after each other." van de Ven 2015 (p.4) Emails were sent as soon as participants did not train for 2 days. | None reported | Weekly or fortnightly discussion by telephone about training adherence. Number of sessions was recorded. Degree of adaptiveness of the cognitive training was assessed. Amount of extra personal contact (telephone or email) due to questions or technical issues during training and level of engagement (i.e. how often a reminder to train was needed) was recorded. Participant daily log of their level of motivation during training, amount of physical exercise at the day of training, how interesting and difficult the tasks of that day were, fatigue level before and after training. Exit questionnaire about subjective training effectiveness; change of strategies during training; check of blinding to experimental condition; changes in cognitive stimulation in daily life besides study related training; and major changes during training. |
The number of sessions did not differ significantly between the intervention training group and the active control group. The degree of adaptiveness was compromised in 17% of the intervention group participants. 5 participants (17%) were slightly less challenged in the last weeks of the training because they reached the highest level and score possible on 1 of the 9 tasks. |
| Walker 2012 | Neuropsychological group | Inpatient adults with cognitive impairments after acute stroke | To select tailored, evidence‐based techniques for dressing impairment from a preprepared neuropsychological treatment manual based on detailed cognitive testing that assesses the impact of deficits on performance using error analysis. | Neuropsychological treatment manual of evidence‐based techniques culled from the wider neuropsychological literature and based on comprehensive literature searches, survey results (Walker 2003) and occupational therapy textbooks (Edmans 2001); standard T‐shirt available in different sizes (Sunderland 2006), error analysis rating form (see Fletcher‐Smith 2010) | Detailed cognitive testing and an assessment of the impact of cognitive deficits on dressing by observation of a standard task of putting on a t‐shirt (Sunderland 2006) with performance scored using an error analysis rating form (Fletcher‐Smith 2010). Based on the test results and observed errors, the occupational therapists selected interventions from a menu of evidence‐based techniques described in the neuropsychological treatment manual. Commonly used techniques included cueing and alerting procedures to combat neglect or attentional difficulties, systematic laying out of clothing to reduce spatial confusion and graded errorless learning strategies to enhance acquisition of dressing skills. | Research occupational therapists experienced in the treatment of people with stroke. | Face‐to‐face and individually | In a stroke rehabilitation ward and in patients' homes if they were discharged from hospital before the end of the treatment period. | The aim was for 3 times per week for 6 weeks based on previous single case experiments (Sunderland 2006). In the study, the DRESS group received a median of 13 sessions (min 0, max 18) of 18 possible sessions. | Interventions were selected on the basis of patients' test results and observed errors in the dressing assessment and provided at home if patients were discharged early. | None reported | A random sample of treatment sessions were observed by an independent researcher to ensure the manuals were adhered to and that they included the actual treatment prescribed in the manual. | A high level of fidelity of treatment was reported without further details. |
| Yeh 2019 | SEQ (sequential) with BrainHQ | Adult rehabilitation patients poststroke | To provide a sequential combination of aerobic exercise and cognitive training to "prepare the brain for the compensatory recruitment process in the cognitive training sessions that follow"; cognitive training aimed to enhance cognitive functions. | Stationary bicycle; BrainHQ (Posit Science) computer‐based software and personal computer | Aerobic exercise followed by cognitive training: 1. aerobic exercise training: using a progressive resistance stationary bicycle, participants performed 3 minutes of warm‐up, 25 minutes of aerobic resistance exercise ending with 2 minutes of cool down; target heart rate of 40 to 70% maximal heart rate (208 – 0.7 × age); vital signs and perceived effort (Borg Perceived Exertion Scale) monitored and recorded each session; 2. computer‐based cognitive training used to facilitate cognitive functions including attention, recognition, colour and shape identification, calculation, visual perception, visuo‐spatial processing, and executive function. |
Certified occupational therapists (according to personal communication with the authors) | Face‐to‐face and individually | Rehabilitation unit | 60 minutes per session consisting of 30 minutes of exercise and 30 minutes of cognitive training, 2 or 3 days per week for 12 to 18 weeks for a total of 36 sessions (36 hours in total) | 1. "exercise intensity was progressed as the participants improved their performance throughout the training". 2. "The (cognitive) training program was adjusted automatically and continuously according to each participant's level of performance". |
None reported | None reported | Not reported except that the intervention was "feasible and safe, with low dropout rates". |
| Yoo 2015 | RehaCom | Rehabilitation inpatients with stroke | To provide computer‐based cognitive rehabilitation to improve attention, focus, memory, spatial imagination, visual impairment and visuomotor coordination. | RehaCom software (Hasomed GmbH, Magdeburg, Germany) see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer | In addition to rehabilitation therapy, a computerised cognitive rehabilitation program using the RehaCom software composed of 20 detailed training programs targeting attention, focus, memory, spatial imagination, visual impairment, and visuomotor co‐ordination. | Not reported however the research was conducted by occupational therapists. The RehaCom website states that the software is used "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals and clinics. | Not reported but appears to be face‐to‐face and individually | Inpatient rehabilitation in local hospital | 30 minutes/day, 5 times/week for 5 weeks (12.5 hours in total) | RehaCom reportedly "enables adjustment of difficulty based on the task performance capacity of the patient, immediate feedback, reduction in time spent by the therapist once the patient learns the therapy task, and maintenance of objective and continuous information concerning performance results." |
None reported | None reported | None reported |
| Zuchella 2014 | Cognitive Training | Inpatient adults with first‐ever stroke and confirmed cognitive deficits | To provide early "comprehensive cognitive rehabilitation… combining computer training and metacognitive strategies". | Computer and 2 software programs (in Italian). 1. "Una palestra per la mente" (Gollin 2011); see A Gym for the Mind 2 for the English version and a description in English. A gym for the mind 2 (KIT: Book + CD‐ROM) New exercises of cognitive stimulation for brain aging and dementia 300 activities for temporal and spatial orientation, visual attention, memory, language, and logic. 2. "Training di riabilitazione cognitiva" (Powell 2009) See www.erickson.it/it/training-di-riabilitazione-cognitiva Training of cognitive rehabilitation (KIT: Book + CD‐ROM) Exercises of memory, thinking skills and executive functions after brain injury The website stated that the activities are also printable. |
In individual sessions, participants performed activities using the software addressing the following cognitive domains. "1) Time orientation: days of the week, months of the year, seasons, holidays and celebrations; anagrams of the days of the week, months and seasons; identification of temporal sequences within a story or in the execution of ADL; temporal sequences with images relating to ADL; 2) Spatial orientation: recognition of right and left; recognition and identification of cities, regions; word search puzzles; positions of objects; observation of scenes and identification of the position of objects; orienteering skills following pathways; 3) Visual attention: searching for targets among distractors (stylized elements of objects); word search puzzles; findings the differences between images/scenes; searching for elements by categories; 4) Logical reasoning: calculation; words in context; searching for intruders within categories; logical completion (metaphors and proverbs); categorization; 5) Memory: recognition of pairs of words with or without logical connections; remembering lists; face recognition; memorization of scenes and stories then answering a questionnaire; object location and object seeking (e.g. memory game with cards); 6) Executive functions; answering questions about a story; identifying the purpose/meaning of a story; following pathways subject to certain rules; recognition of moods; mathematical logic; action planning; re‐ordering the sequence of a story; critical judgment (giving the pros and cons of ethical and social topics); problem‐solving." While the patients performed the activities the psychologist "suggested metacognitive strategies to them in order to develop their awareness and self‐regulation …" e.g. the patient was asked to predict results on tasks and identify factors that were contributing to their successes and failures. In the last 15 minutes of the session, the psychologist "reasoned with the patients about any problems encountered …, explaining how to transfer the learned strategies to everyday situations in order to foster their generalization to real‐world tasks (e.g. patients were encouraged to adopt ‘associative techniques' and to use their imagination to improve their memory …" |
2 psychologists, experts in neuropsychology, provided the sessions in the study; the website states that: the activities can be administered by professionals or family caregivers and refers to "rehabilitation therapists" using features to track progress. |
Apparently individually and face‐to‐face; the website states that activities can be provided individually or in groups. | Neurorehabilitation unit; website says activities can be done at home | 4 × 1‐hour sessions, including 45 minutes of therapist‐guided computer exercises, per week over 4 weeks (16 hours in total) | 3 levels of difficulty; progress occurred to the next level when 70% correct response rate achieved; if the patient failed an activity 3 times, "the presentation was simplified" and made more understandable through examples; Website states that the activities can be expanded and modified to suit the particular needs of the person and that the instructions can be heard or written. |
None reported | "To ensure inter‐therapist reliability" and to ensure "that the patients received the same guidance during the training sessions, the psychologists followed written instructions and examples defined during the drafting of the protocol." | None reported |
ADL: activities of daily living; APT: Attention Process Training; CO‐OP: Cognitive Orientation to Occupational Performance; TENS: transcutaneous electrical nerve stimulation; WM: working memory.
The main component of the eligible occupational therapy intervention in 17 trials was computer‐based training software for a cognitive remediation approach: RehaCom in Cho 2015, Cho 2016, Jiang 2016, Lin 2014, and Yoo 2015; KrasSMU complex of neuropsychological programs in Prokopenko 2013, Prokopenko 2018, and Prokopenko 2019; BrainHQ in Chen 2015 and Yeh 2019; Cogmed QM in Akerlund 2013 and Lundqvist 2010; COGPACK programme in Bo 2019; ERICA in De Luca 2018; CoTras in Park 2015a; BrainGymmer in van de Ven 2017; and two programs in Zuchella 2014, including books and CD‐ROMs (Gollin 2011; Powell 2009). Many of these are commercially available. Many programs were provided in languages other than English, rather of the country where the research was conducted (i.e. Korean, Chinese, Russian, or Italian). See Characteristics of included studies table for details. Three interventions used pen‐and‐paper tasks for cognitive remediation: Barker‐Collo 2009 used "Attention Processing Training (APT)" with addition of distraction auditory CDs, Carter 1983 used tasks from a workbook for "Thinking Skills" training, and Hasanzadeh Pashang 2020 used a workbook of cognitive exercises rehabilitation. One trial that used computer‐based training preceded this with aerobic exercise training using a progressive stationary bicycle, that we confirmed was delivered by occupational therapists (Yeh 2019). Four approaches used a compensatory and adaptive approach focusing on functional activities skills training or environmental adaptation. Skidmore 2015a and Skidmore 2017 used an individualised goal‐directed training approach called "strategy training" (Skidmore 2015a), and "guided training" (Skidmore 2017); and Walker 2012 focused specifically on retraining in the activity of dressing, based on detailed cognitive and dressing assessment and using a menu of evidence‐based interventions. Maggio 2020 used Home Automation (HA or Domotics) training in a "home automation" room where technologies were available to provide an adjustable environment including a kitchen and bathroom.
Not all trials reported the professional qualification of the intervention providers. Many trial authors reported or confirmed on contact that the interventions were or could be delivered by occupational therapists (Akerlund 2013; Lundqvist 2010; Prokopenko 2013; Skidmore 2015a; Skidmore 2017; Walker 2012; Yeh 2019). Other providers included physiotherapists (Jiang 2016), psychologists (Lin 2014; Zuchella 2014), a clinical neuropsychologist (Barker‐Collo 2009), and "experienced therapists with exercise physiology or clinical psychology backgrounds" (Bo 2019). Others were reported as "trained research assistants" (Carter 1983), "expert therapists" (Cho 2015; Cho 2016), and a "trained cognitive therapist" (De Luca 2018). See Characteristics of included studies table for details. Seven trials did not specifically report the providers for their interventions but used commercially available programs or workbooks for occupational therapists' use (Chen 2015; Hasanzadeh Pashang 2020; Park 2015a; Yeh 2019; Yoo 2015), or included occupational therapists in the research team (Maggio 2020; Park 2015a; Yoo 2015). We confirmed with author contact that occupational therapists were, and could be, involved in delivery, as noted above or the intervention was considered within occupational therapy scope of practice, as defined in Methods. See Characteristics of included studies table for details.
Twenty studies delivered interventions within a hospital ward, rehabilitation unit or centre, or occupational therapy department (Akerlund 2013; Bo 2019; Carter 1983; Chen 2015; Cho 2015; Cho 2016; Hasanzadeh Pashang 2020; Jiang 2016; Maggio 2020; Lin 2014; Lundqvist 2010; Park 2015a; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Skidmore 2015a; Walker 2012; Yeh 2019; Yoo 2015; Zuchella 2014). Two studies delivered interventions in hospital and at home after discharge (Barker‐Collo 2009; Skidmore 2017). Interventions were predominantly provided individually using face‐to‐face or computer‐based training modes, or both. Barker‐Collo 2009, Carter 1983, Chen 2015, De Luca 2018, Lin 2014, Skidmore 2015a, Skidmore 2017, Walker 2012, and Yeh 2019 provided their interventions individually and face‐to‐face. Akerlund 2013 reported that intervention group participants worked "… individually and independently using the online software"; the participants in Jiang 2016 received supervision, guidance, or reminders; while the participants in Prokopenko 2013, Prokopenko 2018, and Zuchella 2014 received individual training or supervision using the computer programs. Intervention group participants in Lundqvist 2010 performed their working memory training program "in pairs in a separate quiet room … in the presence of one of three certified coaches who provided special feedback once a week". Bo 2019, delivered face‐to‐face computer training in a group setting (up to 20 participants). Hasanzadeh Pashang 2020 delivered cognitive rehabilitation in groups of two to 10 participants. The home automation training in Maggio 2020 was face‐to‐face in a group of three to five participants. The intervention in van de Ven 2017 was primarily delivered in participants' homes, supervised remotely, and monitored by telephone and email. Cho 2015, Cho 2016, Park 2015a, Prokopenko 2019, and Yoo 2015 did not report mode of delivery, but it appeared to be individual computer‐based delivery.
Dose and scheduling of the interventions
Duration of the interventions ranged from 10 days (Prokopenko 2018; Prokopenko 2019), or two weeks (Prokopenko 2013; Skidmore 2017), to 12 to 18 weeks (Yeh 2019). Carter 1983 delivered the intervention for a mean of three to four weeks. Four interventions were delivered for four weeks (Barker‐Collo 2009; Chen 2015; Park 2015a; Zuchella 2014), three interventions for five weeks (Akerlund 2013; Lundqvist 2010; Yoo 2015), four for six weeks (Cho 2015; Cho 2016; Walker 2012), three for eight weeks (De Luca 2018; Hasanzadeh Pashang 2020; Maggio 2020), one for 10 weeks (Lin 2014), and three for 12 weeks (Bo 2019; Jiang 2016, van de Ven 2017). Skidmore 2015a provided the intervention for the duration of inpatient rehabilitation, which was not reported. The scheduling (frequency) of intervention delivery ranged from two to three days per week (Yeh 2019), and three days per week (Carter 1983; De Luca 2018; Walker 2012), to seven days per week(Prokopenko 2013), with most reporting that sessions occurred five days per week (Akerlund 2013; Barker‐Collo 2009; Chen 2015; Cho 2015; Cho 2016; Jiang 2016; Lundqvist 2010; Park 2015a; Skidmore 2015a; Skidmore 2017; van de Ven 2017; Yoo 2015). Participants in the Zuchella 2014 trial received four sessions per week. Intervention sessions ranged in duration from 30 minutes (Chen 2015; Cho 2015; Cho 2016; Jiang 2016; Park 2015a; Prokopenko 2013; van de Ven 2017; Yoo 2015), 30 to 45 minutes (Akerlund 2013; Carter 1983), 45 minutes (De Luca 2018; Skidmore 2015a; Skidmore 2017), 45 to 60 minutes (Lundqvist 2010), to one hour (Barker‐Collo 2009; Lin 2014; Yeh 2019; Zuchella 2014). Walker 2012 did not report session length for the intervention, but we assumed it to be a minimum of 15 minutes (due to an eligibility criterion of being able to tolerate sitting in a chair for this time period).
The total dose possible of an intervention provided ranged from a possible minimum of five hours (Prokopenko 2018; Prokopenko 2019), to a possible maximum of 60 hours (Lin 2014), with a mean total dose possible (excluding Skidmore 2015a and Walker 2012) of 19 hours. Other low‐dose interventions were six to seven hours (Prokopenko 2013; Prokopenko 2013; Prokopenko 2018), seven hours 30 minutes (Skidmore 2017), and eight hours (Carter 1983; Hasanzadeh Pashang 2020). Seven studies provided a maximum dose possible of between 10 and 18 total hours of intervention (Chen 2015; Cho 2015; Cho 2016; De Luca 2018; Park 2015a; Yoo 2015; Zuchella 2014). The dose in Akerlund 2013 ranged from 12.5 to 18.75 hours, 20 hours in Barker‐Collo 2009, 24 hours in Maggio 2020, 25 hours in Lundqvist 2010, 29 hours in van de Ven 2017, 30 hours in Jiang 2016, and 3.75 hours per week for the duration of inpatient therapy in Skidmore 2015a.
Comparison interventions or controls
Most trials compared the experimental intervention with a standard care control group (considered to be similar to usual poststroke rehabilitation or occupational therapy available at that site) or attention control (Akerlund 2013; Barker‐Collo 2009; Carter 1983; Chen 2015; Cho 2015; Cho 2015; De Luca 2018; Jiang 2016; Lin 2014; Maggio 2020; Park 2015a; Prokopenko 2013; Yoo 2015; Zuchella 2014). Bo 2019 compared the intervention to usual care plus 45‐minute video documentaries to provide a similar dose. Some trials compared the intervention to an attention control group (written material with guidance in Skidmore 2015a, and a functional group in Walker 2012), and one to direct skill training (Skidmore 2017), all of which were comparable to usual occupational therapy care. Lundqvist 2010 was a cross‐over trial where the control group received no training during the intervention period. In Yeh 2019, the control group received an "active" control of non‐aerobic exercise training and "unstructured mental activities". Prokopenko 2018 and Prokopenko 2019 had two comparator groups, one a "passive" control of usual motor rehabilitation, the other an "active" control of distracting or entertaining computer games. For these two studies, we compared the intervention of interest to the passive control group. van de Ven 2017 had two comparators, an active control of "mock training" using the same online environment as the intervention with different tasks and a waiting list group. For this trial, we compared the intervention to the waiting‐list control group because this had the most complete reporting of data for use in analysis while being comparable to other comparators and as the active control used the same intervention software. In some instances, for this trial, only the data for the active control group was reported, so we then compared the intervention to the active control group and noted this accordingly. We considered the comparators in terms of inactive versus active interventions and given that the active interventions in the particular included studies were comparable to usual care, we primarily compared the interventions to inactive control interventions.
More details of the comparison interventions, where provided, are included within the Characteristics of included studies table according to the TIDieR checklist (Hoffmann 2014).
Outcomes
The studies used a range of measures for outcomes of interest in this review. Table 2 lists the measures used in each study classified under the outcomes of interest, according to the key aspect measured by the tool. Table 3 lists the measures grouped by outcome, domain, and subdomain, where applicable. Eight trials measured and reported the primary outcome of BADL. The FIM (Stineman 1996) was the most commonly used ADL measure, used in six studies (Cho 2016; Jiang 2016; Skidmore 2015a; Skidmore 2017; Yoo 2015; Zuchella 2014). De Luca 2018 reportedly measured ADL and IADL, but did not report the data for these outcomes. One study measured IADL and ADL, but provided no description of the scales (Maggio 2020). Prokopenko 2013, Prokopenko 2018, and Prokopenko 2019 used an 'IADL scale' to measure "functional state" with minimal description of the items and scales, except that it measured "independent walking, feeding, travelling, carrying out hygienic procedures, shopping etc" (Prokopenko 2013), so an apparent mix of BADL and IADL. Akerlund 2013 used the Assessment of Motor and Process Skills (AMPS). van de Ven 2017 measured IADL using Lawton & Brody Instrumental Activities of Daily Living scale (Lawton 1988). Two studies measured community integration and participation; Yeh 2019 using the CIQ (Willer 1994), and van de Ven 2017 using the Utrecht Scale for Evaluation of Rehabilitation‐Participation (USER‐P) (Restriction subscale) (van der Zee 2010; van der Zee 2013).
Studies measured global cognitive function, both performance and self‐report, with a variety of instruments, including the MoCA (Nasreddine 2005), used in seven studies (Chen 2015; Jiang 2016; Maggio 2020; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Yeh 2019), and the MMSE (Folstein 1975), used in six studies (De Luca 2018; Jiang 2016; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Zuchella 2014), to assess performance, and the Cognitive Failures Questionnaire (CFQ) (Broadbent 1982), used in two studies (Barker‐Collo 2009; van de Ven 2017), to measure self‐reported global cognitive function. Thirteen trials reported attention (Barker‐Collo 2009; Bo 2019; Cho 2015; De Luca 2018; Hasanzadeh Pashang 2020; Lin 2014; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Skidmore 2015a; van de Ven 2017; Yoo 2015; Zuchella 2014). Eleven studies specifically measured memory, including working memory and memory span (Akerlund 2013; Bo 2019; Barker‐Collo 2009; Cho 2015; De Luca 2018; Lin 2014; Lundqvist 2010; van de Ven 2017; Yeh 2019; Yoo 2015; Zuchella 2014), by a variety of instruments, most commonly the Digit Span tests from the WAIS‐III Neuropsychological Instrument (WAIS‐III NI) (Wechsler 1997). Thirteen studies measured executive function, globally or specifically, by a variety of measures, most commonly the FAB (Appollonio 2005; Dubois 2000), in five studies (Maggio 2020; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Zuchella 2014), and Raven's Colored Progressive Matrices 47 (PM47) (Basso 1987), in three studies (De Luca 2018; van de Ven 2017; Zuchella 2014).
Regarding the timing of outcome measurement, 16 studies measured outcomes immediately after, or close to, the end of delivery of the intervention without any further follow‐up points (Carter 1983; Chen 2015; Cho 2015; Cho 2016; De Luca 2018; Jiang 2016; Lin 2014; Maggio 2020; Park 2015a; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Walker 2012; Yeh 2019; Yoo 2015; Zuchella 2014). Akerlund 2013 also completed a three‐month follow‐up; Barker‐Collo 2009 and Bo 2019 a six‐month follow‐up; Hasanzadeh Pashang 2020 a six‐week follow‐up; Lundqvist 2010 a five‐month follow‐up; Skidmore 2015a a three‐ and six‐month follow‐up from admission; Skidmore 2017 a three‐, six‐, and 12‐month follow‐up; and van de Ven 2017 a four‐week follow‐up after the end of the intervention (i.e. at 16 weeks).
Excluded studies
We excluded trials from this review if the intervention did not address cognitive impairments of people following stroke; if the trials were not RCTs or quasi‐randomised or randomised cross‐over trials; if the studies were conducted with mixed aetiology groups and the participants with stroke were fewer than 50% of the participants or the data were not separately available for participants with stroke; or the intervention in the trial could not have been carried out or supervised by an occupational therapist. We also excluded studies if the focus was on interventions for perceptual impairments or apraxia without also including interventions for cognitive impairments and if the intervention was a virtual reality intervention, as this is now covered by another Cochrane Review (Laver 2017). Studies listed in the Characteristics of excluded studies table are those that appeared to be relevant and focused on interventions for people with cognitive impairment after stroke, but to the best of our knowledge were not or could not be delivered by occupational therapists, did not focus on cognitive rehabilitation, or otherwise meet the eligibility criteria. The most common exclusion reasons were that not all participants had cognitive impairment or confirmed cognitive impairment (57 studies) and the intervention was ineligible as an occupational therapy intervention (51 studies).
Studies awaiting classification
Forty‐five studies are awaiting classification (see Characteristics of studies awaiting classification table).
Ongoing studies
Four studies are ongoing (see Characteristics of ongoing studies table).
Risk of bias in included studies
Individual items for the risk of bias of included studies can be found in Figure 2 and Figure 3. In terms of minimising risk of bias, the studies were strongest in random sequence generation (selection bias) and management of incomplete outcome data (detection bias). The studies were weakest in blinding of participants and personnel for intervention delivery (performance bias) and allocation concealment (selection bias). No study was rated as low bias across all domains. Neither Skidmore 2017 nor Walker 2012 blinded participants, but were low risk in all other domains. Sixteen studies reported adequate random sequence generation (selection bias), with the rest unclear. Five studies reported low‐risk procedures for allocation concealment, but it was unclear in the remainder. All studies were at high risk of performance bias, except van de Ven 2017, which was unclear. Twelve studies were at low risk for detection bias, two at high risk, and 10 were unclear. Management of incomplete data (attrition bias) was low risk in 10 studies, high risk in two, and unclear in the rest. Selective outcome reporting (reporting bias) was high risk in two studies and low risk in the remainder.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eight studies were assessed at unclear risk of bias for random sequence generation (Carter 1983; Cho 2015; Cho 2016; De Luca 2018; Hasanzadeh Pashang 2020; Prokopenko 2013; Prokopenko 2019; Yoo 2015), some did not state the method, and others did not provide sufficient detail (e.g. "method of letters"; Prokopenko 2013). The remainder of the studies were at low risk, using methods such as online randomisation services, drawing of lots, random number generator software, or a random numbers table. Only five of the studies reported adequate allocation concealment using Internet services or an independent researcher or the research co‐ordinator having sole access (Barker‐Collo 2009; Bo 2019; Jiang 2016; Skidmore 2017; Walker 2012). The remaining studies were assessed as unclear risk due to inadequate reporting.
Blinding
Twenty‐three studies (all except van de Ven 2017) were at high risk of performance bias since none were able to blind the participants to group allocation. In van de Ven 2017, there was blinding of the participants of the intervention and active control groups, but the participants of the waiting‐list group would not have been blinded and the person administering the computer tasks and training instructions was not blind to training allocation, so we rated this as unclear.
Twelve studies were at low risk of detection bias. Eleven of these were assessed as low risk because the outcome measurement was conducted by trained assessors blinded to randomisation (Barker‐Collo 2009; Bo 2019; Jiang 2016; Lin 2014; Prokopenko 2013; Skidmore 2015a; Skidmore 2017; van de Ven 2017; Walker 2012; Yeh 2019; Zuchella 2014). Cho 2015 was at low risk as they used a computerised test to assess outcomes, providing a level of objective assessment. Two studies were at high risk (Akerlund 2013; Carter 1983), and the 10 remaining studies were at unclear risk due to no or unclear reporting of this criterion.
Incomplete outcome data
Ten studies were at low risk for attrition bias by adequately managing and reporting incomplete data or reporting about attrition (Carter 1983; Lundqvist 2010; Maggio 2020; Prokopenko 2013; Skidmore 2015a; Skidmore 2017; van de Ven 2017; Walker 2012; Yeh 2019; Zuchella 2014). Two studies were at high risk: one due to a high percentage loss to follow‐up (Bo 2019), and the other due to inadequate management of participants lost to follow‐up in the analysis (Jiang 2016). The remaining 12 studies were at unclear risk due to inadequate or unclear reporting.
Selective reporting
Two studies were at high risk of bias for selective reporting, for example, because of discrepancies between the method and results in reporting outcomes (Carter 1983; De Luca 2018). The remainder were at low risk.
Other potential sources of bias
All studies were at low risk of bias from other potential sources.
Effects of interventions
See: Table 1
We assessed the effect of interventions for each outcome immediately after treatment and at follow‐up, where this occurred. The included studies used a range of relevant outcome measures, some measuring the primary outcome (BADL), some measuring one or more of the secondary outcomes, and some assessing both. The measures we chose for the analysis of intervention effects on ADL and cognitive outcomes are listed by study in Table 2 and Table 3. See Table 2 for the outcome measures categorised by outcome and Table 3 for the outcome measures used listed by outcome, domain, and subdomains of the measure. For the purposes of meta‐analyses, we used the data from the passive control groups in Prokopenko 2018 and Prokopenko 2019, and from the waiting‐list control group in van de Ven 2017 where possible and used the active control when only those data were reported. Where Prokopenko 2019 was included in the analyses, we conducted sensitivity analyses with it removed as we are cautious about conversion of reported CIs of the medians to obtain the SDs of the mean. We also conducted sensitivity analyses where Lundqvist 2010, the only cross‐over trial, was included in meta‐analyses to see the effect of its removal. For sensitivity analyses to evaluate the effect of trial quality by analysing separately trials with and without adequate randomisation and concealment of treatment allocation, no studies were at high risk for these domains, only low or unclear, so the sensitivity analyses effectively examined low versus unclear or low versus unclear and low risk.
See Table 1 for a summary of results for the primary outcome of BADL and for other key secondary outcomes.
Activities of daily living
Eight trials reported on BADL (Carter 1983; Cho 2016; Jiang 2016; Skidmore 2015a; Skidmore 2017; Walker 2012; Yoo 2015; Zuchella 2014), and five trials on IADL or a combination of both BADL and IADL (Akerlund 2013; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; van de Ven 2017). Maggio 2020 reportedly measured ADL and IADL but provided no description of the items included in the two scales, so we could not classify these results under either BADL or IADL. De Luca 2018 reportedly measured ADL and IADL, but did not report the data for these outcomes and this was not provided on contact with the authors.
Basic activities of daily living (primary outcome)
Eight trials reported on BADL or an aspect of BADL that we could ascertain from scale descriptions (Carter 1983; Cho 2016; Jiang 2016; Skidmore 2015a; Skidmore 2017; Walker 2012; Yoo 2015; Zuchella 2014). Five of these used a cognitive remediation approach (Carter 1983; Cho 2016; Jiang 2016; Yoo 2015; Zuchella 2014), and three used a compensatory and adaptive approach (Skidmore 2015a; Skidmore 2017; Walker 2012). Six studies used the FIM (Cho 2016; Jiang 2016; Skidmore 2015a; Skidmore 2017; Yoo 2015; Zuchella 2014).
Effects immediately after intervention
We pooled the postintervention data (12 weeks or less) for the studies that used the FIM and found evidence of a small effect with an MD of 2.20 in favour of the intervention (95% CI 0.17 to 4.22; P = 0.03, I2 = 0%; 6 studies, 336 participants; low‐certainty evidence; Analysis 1.1). Therefore, on average, at completion of the intervention, BADL improved by 2.2 points on the FIM scale that ranges from 18 (total assist) to 126 (complete independence). This could be considered a clinically unimportant gain as the minimal clinically important difference (MCID) for the FIM has been established as 22 points for people with stroke (Beninato 2006). We downgraded the strength of the evidence due to serious concerns about risk of bias and imprecision. We conducted a post‐hoc subgroup analysis of the six studies by type of intervention (i.e. cognitive remediation or compensatory and adaptive) and found no difference between the groups by type of intervention (P = 0.80). We conducted a sensitivity analysis, as planned, of studies only with low risk of randomisation bias for random sequence generation and allocation concealment (Jiang 2016; Skidmore 2017), and found insufficient evidence of an effect (MD 2.04, 95% CI −0.12 to 4.19; P = 0.06, I2 = 0%; 2 studies, 143 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Basic activities of daily living (BADL) performance, Outcome 2: BADL (postintervention) sensitivity analysis
We did not pool the two remaining studies that measured ADL: Carter 1983 used the BI but only reported change scores and Walker 2012 used the Nottingham Stroke Dressing Assessment (NSDA), which measures one aspect of BADL (i.e. dressing) and only reported mean change and SD from baseline scores. Carter 1983 was the only trial included in the 2010 version of this review (Hoffmann 2010). Hoffmann 2010 reported an MD of 10.71 (95% CI −2.41 to 23.83) for the 28 participants, indicating insufficient evidence of an effect of improvement in ADL. Walker 2012 reported that both treatment groups showed significant improvements in dressing ability (improvements of 31% for the intervention group and 22% for the control group on the NSDA), but the groups did not differ significantly.
Effects on follow‐up after intervention
Two studies measured BADL at follow‐up points beyond the intervention. Skidmore 2015a and Skidmore 2017 followed up participants at three and six months after study admission, and Skidmore 2017 also followed participants up at 12 months. Both Skidmore 2015a and Skidmore 2017 used the FIM, so we pooled the results for three and six months. At the three‐month follow‐up, there was insufficient evidence of an effect (MD 10.00, 95% CI −0.54 to 20.55; P = 0.06, I2 = 53%; 2 studies, 73 participants; low‐certainty evidence; Analysis 1.3) (Skidmore 2015a; Skidmore 2017). At six months, there was some evidence of an effect with an MD of 11.38 (95% CI 1.62 to 21.14; P = 0.02, I2 = 12%; 2 studies, 73 participants; low‐certainty evidence; Analysis 1.3) (Skidmore 2015a; Skidmore 2017). We downgraded the strength of the evidence due to concerns about risk of bias and imprecision. This MD does not reach the FIM MCID of 22 points (Beninato 2006). At the 12‐month follow‐up, Skidmore 2017 reported a moderate effect size estimate for the between‐group difference in change scores from baseline to 12 months (Cohen d = 0.53) for 43 participants.
1.3. Analysis.

Comparison 1: Basic activities of daily living (BADL) performance, Outcome 3: BADL (follow‐up)
Other activities of daily living/instrumental activities of daily living outcomes (secondary outcomes)
Six trials reported other measures of ADL/IADL (Akerlund 2013; Maggio 2020; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; van de Ven 2017). Maggio 2020 used an 'ADL scale' and an 'IADL scale', but provided no description of the items and range of the scales. Maggio 2020 reported that an analysis of covariance of group differences was not possible for effects on the ADL scale. They found evidence of an effect on the IADL scale (P < 0.001). van de Ven 2017 also measured IADL using the self‐report Lawton & Brodie Instrumental Activities of Daily Living scale. We pooled these two studies that appeared to measure IADL alone, reversing the scores for van de Ven 2017 as they reported lower scores indicated better performance on the scale. We found evidence of an effect but with substantial heterogeneity (SMD 0.94, 95% CI 0.41 to 1.47; P = 0.0005, I2 = 98%; 2 studies, 88 participants; very low‐certainty evidence; Analysis 2.1). When we applied a random‐effects model, the heterogeneity was the same. We downgraded the strength of the evidence due to serious concerns with rating of bias, inconsistency, and imprecision.
2.1. Analysis.

Comparison 2: Other activities of daily living (ADL)/instrumental activities of daily living (IADL), Outcome 1: IADL (postintervention)
Prokopenko 2018 and Prokopenko 2019 measured "functional state" using the 'IADL scale' measuring "independent walking, feeding, travelling, carrying out hygienic procedures, shopping etc" (Prokopenko 2013), which is broader than the IADL alone. The score range of the IADL scale was not described. We pooled the data for the three Prokopenko studies postintervention (eight weeks or less) and found evidence of an effect with an MD of 2.61 in favour of the intervention (95% CI 0.10 to 5.12; P = 0.04, I2 = 0%; 3 studies, 111 participants; very low‐certainty evidence; Analysis 2.2). Sensitivity analysis with Prokopenko 2019 removed had little effect on the MD. We downgraded the evidence to very low certainty due to very serious concerns about risk of bias and serious concerns about imprecision.
2.2. Analysis.

Comparison 2: Other activities of daily living (ADL)/instrumental activities of daily living (IADL), Outcome 2: Other ADL/IADL (postintervention)
Akerlund 2013 used the AMPS. For the subsample of people with stroke, there were no between‐group differences on the AMPS motor scores at either six‐week (P = 0.784) or three‐month (P = 0.117) follow‐up. There were also no between‐group differences on the AMPS process scores at either six‐week (P = 0.366) or three‐month (P = 0.920) follow‐up (data and analysis provided by the authors).
Barker‐Collo 2009 used the modified Rankin Scale (Bamford 1989), a single‐item subjective global disability scale (rated from 0 to 6) often used in stroke trials, and although it is based on the person's dependence or otherwise in activity is not necessarily a measure of BADL or IADL (Uyttenboogaart 2007). They reported mean between‐group difference in the change at six‐month follow‐up (MD −0.29, 95% CI −0.75 to 0.17; P = 0.261) and provided data for immediately after the intervention (five weeks) (MD 1.94 (SD 1.29) for intervention group and MD 1.97 (SD 1.24) for the control group).
Community integration (secondary outcome)
Yeh 2019 used the 15‐item CIQ (range 0 to 25), a self‐ or carer‐reported questionnaire used to measure participation in home, social, and productive activities, a mixture of IADL and participation activities. Higher scores indicate better functioning (Hirsh 2011). van de Ven 2017 used the Restriction subscale of the USER‐P as a measure of societal participation, which is also a measure of perceived restriction in a range of home, social, and productive activities. Higher scores (range 0 to 100) indicate better function. We pooled the results of these two measures for immediately after the intervention and found insufficient evidence of an effect (SMD 0.09, 95% CI −0.35 to 0.54; P = 0.68, I2 = 0%; 2 studies, 78 participants; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Community reintegration, Outcome 1: Community reintegration (self‐reported, postintervention)
Global cognitive function (secondary outcome)
Thirteen trials reported global cognitive function (Akerlund 2013; Barker‐Collo 2009; Chen 2015; De Luca 2018; Jiang 2016; Maggio 2020; Park 2015a; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; van de Ven 2017; Yeh 2019; Zuchella 2014). All used a cognitive remediation approach except Maggio 2020, which used a compensatory and adaptive approach. Yeh 2019 combined a cognitive remediation approach with sequential exercise. All except Barker‐Collo 2009 and van de Ven 2017, which measured self‐reported global cognitive function, used a performance measure that provided a total score across a range of cognitive domains. Some studies used two measures of global cognitive function, so we chose one per study for analysis, choosing the one most commonly used across other studies. The majority of these measured effects postintervention (18 weeks or less) with two studies also following up participants beyond the intervention: Akerlund 2013 at three months and van de Ven 2017 four weeks after the intervention (i.e. at 16 weeks). Barker‐Collo 2009 only followed up on this outcome at six months.
Global cognitive functional performance
Effects immediately after intervention
We pooled 11 studies reporting global cognitive functional performance outcomes (542 participants): data from the MoCA from Chen 2015, Jiang 2016, Maggio 2020, Prokopenko 2013, Prokopenko 2018, Prokopenko 2019, and Yeh 2019; the MMSE from De Luca 2018 and Zuchella 2014; the Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS) from Akerlund 2013; and the Lowenstein Occupational Therapy Cognitive Assessment (LOTCA) from Park 2015a. For postintervention (18 weeks or less), there was evidence of an effect in favour of the intervention but with substantial heterogeneity (SMD 0.58, 95% CI 0.40 to 0.76; P < 0.00001, I2 = 78%; 11 studies, 542 participants; very low‐certainty evidence; Analysis 4.1). We downgraded the strength of the evidence due to very serious concerns about risk of bias and serious concerns about inconsistency. When we applied a random‐effects model, the heterogeneity was the same. Excluding the studies by Chen 2015 and Park 2015a removed the heterogeneity and the SMD decreased, with some evidence of an effect (SMD 0.35, 95% CI 0.16 to 0.54; P = 0.0004, I2 = 0%; 9 studies, 432 participants; low‐certainty evidence; Analysis 4.2). We downgraded the strength of the evidence due to serious concerns about risk of bias, especially allocation concealment. We considered aspects of these studies, such as chronicity of the stroke, age of participants, and dose of the intervention, may have explained this heterogeneity but found no apparent reason. Removal of Prokopenko 2019 for sensitivity analysis had little effect on the results. To re‐express the SMD from the sensitivity analysis using a familiar instrument, as recommended in Section 15.5.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021b), we chose the MoCA as it was used by seven studies in the analysis, has established MCID values for people receiving rehabilitation for stroke (Wu 2019), and has been used in a large observational cohort study of participants poststroke (Fu 2017). Using the SD of 4.67 of the mean score on the MoCA scale (Beijing version) from the cohort study of 1222 poststroke hospital patients (within 15 days to one month after stroke in 14 hospitals in China) (Fu 2017), and the SMD of 0.35 from the sensitivity analysis, the difference between groups equates to 1.63 points on the MoCA (95% CI 0.75 to 2.52). Therefore, on average, participants who received the intervention had improved global cognitive functional performance by 1.63 points on the MoCA scale. This difference exceeds the anchor‐based MCID of the MoCA for stroke rehabilitation patients of 1.22 found by Wu 2019, but not the distribution‐based MCID of 2.15.
4.1. Analysis.

Comparison 4: Global cognitive function, Outcome 1: Global cognitive performance (postintervention)
4.2. Analysis.

Comparison 4: Global cognitive function, Outcome 2: Global cognitive performance (sensitivity analysis, Park 2015 and Chen 2015 removed)
Effects on follow‐up after intervention
For the participants with stroke in Akerlund 2013, there was a significant difference between the intervention and control groups in the BNIS at three months' follow‐up (P = 0.025) in favour of the control group (analysis provided by authors).
Self‐reported global cognitive function
Two studies measured self‐reported global cognitive function with the CFQ (Barker‐Collo 2009; van de Ven 2017). Barker‐Collo 2009 measured this at six months and van de Ven 2017 measured this postintervention at 12 weeks and four weeks later (at 16 weeks). As these were measured at different time points and data were not available for the 16 weeks' follow‐up for van de Ven 2017, we did not pool these results. For self‐reported global cognitive function with the CFQ at six months' follow‐up, Barker‐Collo 2009 reported a non‐significant between‐group change difference (MD 6.14, 95% CI −0.50 to 12.78; P = 0.070). van de Ven 2017 reported that both the intervention and active control group "improved significantly over time" with P values less than 0.001 for both.
Orientation (secondary outcome)
One study reported orientation, using the subscale of the Wechsler Memory Scale (Lin 2014). They reported only on within‐group differences after computer‐assisted cognitive training, finding no significant differences for the treatment group (mean 4.25 (SD 0.67)) and the control group (mean 4.14 (SD 0.63)).
Attention (secondary outcome)
Thirteen trials reported attention (Barker‐Collo 2009; Bo 2019; Cho 2015; De Luca 2018; Hasanzadeh Pashang 2020; Lin 2014; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; Skidmore 2015a; van de Ven 2017; Yoo 2015; Zuchella 2014), all except Skidmore 2015a using a cognitive remediation approach. Ten trials measured sustained attention (Barker‐Collo 2009; Cho 2015; Hasanzadeh Pashang 2020; Lin 2014; Prokopenko 2013; Prokopenko 2018; Prokopenko 2019; van de Ven 2017; Yoo 2015; Zuchella 2014). Some studies measured visual sustained attention, some measured auditory sustained attention, and some measured both. Four trials measured selective attention (Bo 2019; De Luca 2018; Skidmore 2015a; Zuchella 2014). All of these studies measured attention before and after intervention (up to 12 weeks) with some also measuring it on follow‐up: Barker‐Collo 2009, Bo 2019, and Skidmore 2015a measured attention at six‐month follow‐up; Hasanzadeh Pashang 2020 also reported six‐week follow‐up after the intervention (14 weeks); and van de Ven 2017 reported one‐month follow‐up (at 16 weeks). We pooled studies measuring the same attention abilities and subdomains and at similar time points.
Sustained attention
Effects immediately after intervention
Sustained visual attention
Ten trials measured sustained visual attention. We pooled postintervention data for these studies (463 participants), using the data from the Visual Continuous Performance Test (VCPT) from Barker‐Collo 2009, Cho 2015, Hasanzadeh Pashang 2020, and Yoo 2015; Schulte's Tables from Prokopenko 2013, Prokopenko 2018, and Prokopenko 2019; and Trail Making Test A (TMT‐A) from Lin 2014, van de Ven 2017, and Zuchella 2014. Lower scores indicate better performance as these are timed tests. We found statistical evidence of a small effect (SMD −0.28, 95% CI −0.47 to −0.10; P = 0.003, I2 = 38%; 10 studies, 463 participants; moderate‐certainty evidence; Analysis 5.1; Figure 4). We downgraded the evidence due to serious concerns about risk of bias, particularly unclear allocation concealment for all but one study. Removal of Prokopenko 2019 in a sensitivity analysis gave similar results. Re‐expressing the SMD using the TMT‐A (seconds) given that the highest weighted study (Zuchella 2014) used this outcome measure and using data from a cohort study of 223 people with stroke for performance on the TMT‐A (SD 55.84 seconds) (Hochstenbach 1998), we multiplied the SMD of 0.28 by the SD 55.84 (seconds) for a difference between groups of 15.63 seconds (95% CI 5.58 to 26.24). We know of no standards to which we can compare this result in terms of a clinically important difference.
5.1. Analysis.

Comparison 5: Attention, Outcome 1: Sustained visual attention (postintervention)
4.

Funnel plot of comparison: 5 Attention, outcome: 5.1 Sustained visual attention (postintervention).
Sustained auditory attention
Four studies measured sustained auditory attention using the Auditory Continuous Performance Test (ACPT) (169 participants) (Barker‐Collo 2009; Cho 2015; Hasanzadeh Pashang 2020; Yoo 2015). We pooled the reported results for reaction times (seconds) for Cho 2015 and Yoo 2015 and z scores of reaction times for Barker‐Collo 2009 that we obtained from the authors, and reported results from Hasanzadeh Pashang 2020 for immediately after the intervention and found insufficient evidence of an effect (SMD 0.09, 95% CI −0.22 to 0.39; P = 0.57, I2 = 0%; 4 studies, 169 participants; Analysis 5.2). Lower scores indicate better performance.
5.2. Analysis.

Comparison 5: Attention, Outcome 2: Sustained auditory attention (postintervention)
Effects on follow‐up after intervention
Sustained visual attention
We pooled available data for follow‐up after the intervention between three and six months (VCPT for Barker‐Collo 2009 and Hasanzadeh Pashang 2020, and TMT‐A for van de Ven 2017); for van de Ven 2017, follow‐up data were only reported for the active control group and as intention‐to‐treat so for this outcome we used the active control group data. We reversed the data for Hasanzadeh Pashang 2020 as higher was better for this study contrary to the others. We found insufficient evidence of an effect (SMD −0.17, 95% CI −0.47 to 0.13; P = 0.27, I2 = 46%; 3 studies, 171 participants; Analysis 5.3).
5.3. Analysis.

Comparison 5: Attention, Outcome 3: Sustained visual attention (follow‐up)
Sustained auditory attention
We pooled available data for follow‐up after the intervention between three and six months (ACPT for Barker‐Collo 2009 and Hasanzadeh Pashang 2020), and found insufficient evidence of an effect (SMD −0.22, 95% CI −0.62 to 0.18; P = 0.28, I2 = 0%; 2 studies, 98 participants; Analysis 5.4).
5.4. Analysis.

Comparison 5: Attention, Outcome 4: Sustained auditory attention (follow‐up)
Other sustained attention
Cho 2015 also reported the results of the correct response scores for the VCPT and ACPT. They reported significant within‐groups differences for VCPT only for the intervention group with no "notable changes … observed in the control group". Barker‐Collo 2009 also measured sustained visual attention with the TMT‐A test after intervention at five weeks and at six months' follow‐up, reporting that MDs in change between groups were not significant at either time point (five weeks: MD 0.01, 95% CI −1.64 to 1.65; P = 0.995; six months: MD 0.55, 95% CI −1.17 to 2.28; P = 0.524).
Selective attention
Selective visual attention
Effects immediately after intervention
Two trials measured selective visual attention, using Attentive Matrices at eight weeks (De Luca 2018) and four weeks (Zuchella 2014). When we pooled the results, we found an MD of 5.99 in favour of the intervention (95% CI 1.87 to 10.11; P = 0.004, I2 = 0%; 2 studies, 122 participants; Analysis 5.5). Therefore, on average, participants who received the intervention had improved selective attention by 6 points on the Attentive Matrices, which ranges from 0 to 60, showing an average improvement of 10% on the scale. We know of no standards to which we can compare this result in terms of a clinically important difference. On this test, patients are given three printed sheets containing numbers and they are instructed to cancel as many specified digits as they can in a time limit of 45 seconds per sheet. The score is the number of digits correctly crossed out from a total of 60 (Giovagnoli 2001). Two trials used colour word tests at three months (postintervention) (Bo 2019; Skidmore 2015a). We combined the data from these studies for postintervention with De Luca 2018 and Zuchella 2014 for effect on selective visual attention and found evidence of an effect in favour of the intervention (SMD 0.43, 95% CI 0.17 to 0.68; P = 0.001, I2 = 0%; 4 studies, 244 participants; low‐certainty evidence; Analysis 5.6). We multiplied the data from Bo 2019 by −1 as it is a timed test unlike the others, which are scored. We downgraded the evidence due to concerns about risk of bias and imprecision. We know of no standards to which we can compare this result in terms of a clinically important difference.
5.5. Analysis.

Comparison 5: Attention, Outcome 5: Selective visual attention (Attentive Matrices)
5.6. Analysis.

Comparison 5: Attention, Outcome 6: Selective visual attention (postintervention)
Effects on follow‐up after intervention
We pooled data for Bo 2019 and Skidmore 2015a that used colour word tests at six months' follow‐up and found evidence of an effect (SMD 0.53, 95% CI 0.17 to 0.90; P = 0.004, I2 = 49%; 2 studies, 122 participants; low‐certainty evidence; Analysis 5.7). We downgraded the evidence due to concerns about risk of bias and imprecision. We know of no standards to which we can compare this result in terms of a clinically important difference.
5.7. Analysis.

Comparison 5: Attention, Outcome 7: Selective visual attention (follow‐up)
Attention overall
Effects immediately after intervention
We combined data from the 13 studies that measured attention (504 participants); the 10 that measured sustained visual attention (Analysis 5.1), as well as three that measured selective visual attention: Bo 2019 (Stroop Colour Word timed test), De Luca 2018 (Attentive Matrices), and Skidmore 2015a (Colour Word Interference Test Condition 3 scaled score). This analysis found evidence of an effect in favour of the intervention (lower is better) (SMD −0.31, 95% CI −0.47 to −0.15; P = 0.0002, I2 = 20%; 13 studies, 620 participants; low‐certainty evidence; Analysis 5.8; Figure 5). Given serious concerns about the risk of bias across important domains and highly weighted studies, we downgraded the evidence to low certainty. Re‐expressing the SDM using the TMT‐A (seconds) and data from a cohort study of 223 people with stroke for performance on the TMT‐A (SD 55.84 seconds) (Hochstenbach 1998), we multiplied the SMD of 0.31 by the SD 55.84 (seconds) for a difference between groups of 17.31 seconds (95% CI 8.38 to 26.24). We know of no standards to which we can compare this result in terms of a clinically important difference. We conducted a sensitivity analysis, as planned, of studies only with low risk of randomisation bias for random sequence generation and allocation concealment (Barker‐Collo 2009; Bo 2019), and found insufficient evidence of an effect (SMD −0.17, 95% CI −0.47 to 0.13; P = 0.28, I2 = 0%; 2 studies, 170 participants).
5.8. Analysis.

Comparison 5: Attention, Outcome 8: Visual attention overall (postintervention)
5.

Funnel plot of comparison: 5 Attention, outcome: 5.8 Visual attention overall (postintervention).
Effects on follow‐up after the intervention
We pooled results from the five studies that measured visual attention (sustained and selective) on follow‐up after the intervention between three and six months using TMT‐A, VCPT, Color Word Interference Test – Inhibition subscale (Condition 3) (CWIT‐3), and Stroop Colour Word tests (Barker‐Collo 2009; Bo 2019; Hasanzadeh Pashang 2020; Skidmore 2015a; van de Ven 2017). We found evidence of an effect (lower is better) with moderate heterogeneity (SMD −0.32, 95% CI −0.55 to −0.09; P = 0.007, I2 = 50%; 5 studies, 293 participants; low‐certainty evidence; Analysis 5.9). Applying a random‐effects model did not reduce the heterogeneity. In sensitivity analysis, the heterogeneity was mostly explained by data from Hasanzadeh Pashang 2020, which had significant differences between the experimental and control groups at baseline. We downgraded the evidence due to concerns about imprecision and with risk of bias in important domains and in highly weighted studies. Re‐expressing the SDM using the TMT‐A (seconds) using data from a cohort study of 223 people with stroke for performance on the TMT‐A (SD 55.84 seconds) (Hochstenbach 1998), we multiplied the SMD of 0.32 by the SD 55.84 (seconds) for a difference between groups of 17.87 seconds (95% CI 5.03 to 30.71). We know of no standards to which we can compare this result in terms of a clinically important difference.
5.9. Analysis.

Comparison 5: Attention, Outcome 9: Visual attention overall (follow‐up)
Other attention
van de Ven 2017 also used DSC (online and paper versions) reporting significant within‐group differences after the intervention for the intervention group for the online version but not the waiting‐list control group or both groups for the paper version. van de Ven 2017 also reported four‐week follow‐up results for DSC and TMT‐A noting improvements in all groups that were not training related.
Memory (secondary outcome)
Eleven trials reported the impact of cognitive remediation type interventions on memory (Akerlund 2013; Bo 2019; Barker‐Collo 2009; Cho 2015; De Luca 2018; Lin 2014; Lundqvist 2010; van de Ven 2017; Yeh 2019; Yoo 2015; Zuchella 2014), some on specific aspects of memory such as working memory, memory span, and immediate and delayed recall, using a variety of outcomes measures. We pooled studies measuring the same memory domains, where possible or subdomains.
Working memory
Eight trials measured working memory, some using two measures to measure this domain of cognitive abilities, some measuring it only after the intervention and some also on follow‐up.
Effects immediately after intervention
We pooled eight studies (420 participants) for results after the intervention (12 weeks and less) using data from the Span Board reversed test (Akerlund 2013), Paced Auditory Serial Addition Task (PASAT 2.4) (Barker‐Collo 2009; Lundqvist 2010), Visual Span Backwards test (Cho 2015), and TMT‐B (Bo 2019; Lin 2014; van de Ven 2017; Zuchella 2014). We found statistical evidence of an effect in favour of the intervention (SMD 0.45, 95% CI 0.26 to 0.65; P < 0.00001, I2 = 0%; 8 studies, 420 participants; low‐certainty evidence; Analysis 6.1). We downgraded the evidence to low certainty due to very serious concerns with risk of bias. Re‐expressing the SDM using the TMT‐B (seconds) given that the two highest weighted studies used this outcome measure (Bo 2019; Zuchella 2014), and using data from a cohort study of 185 people with stroke for performance on the TMT‐B (in seconds) (Hochstenbach 1998), we multiplied the SMD of 0.45 by the SD from Hochstenbach 1998 of 133.09 for a difference between groups of 59.90 seconds (95% CI 34.60 to 86.5). We know of no standards to which we can compare this result in terms of a clinically important difference. We conducted a sensitivity analysis as planned, removing Lundqvist 2010, and found similar effects (SMD 0.44, 95% CI 0.24 to 0.63; P < 0.0001, I2 = 0%; 7 studies, 407 participants). We also conducted a sensitivity analysis, as planned, of studies only with low risk of randomisation bias for random sequence generation and allocation concealment (Barker‐Collo 2009; Bo 2019), and found a similar effect in favour of the intervention but with substantial heterogeneity (SMD 0.49, 95% CI 0.19 to 0.80; P = 0.002, I2 = 64%; 2 studies, 170 participants) that was not improved when we applied a random‐effects analysis.
6.1. Analysis.

Comparison 6: Memory, Outcome 1: Working memory (postintervention)
Two of these studies also measured working memory with Digit Span Backwards (Akerlund 2013; Cho 2015). We pooled the postintervention results and found insufficient evidence of an effect (MD 0.21, 95% CI −0.50 to 0.93; P = 0.56, I2 = 0%; 54 participants; Analysis 6.2). Lundqvist 2010 also used the Block‐Span‐board Backwards test, a measure of working memory; the authors provided the data for the subsample of participants with stroke (after the intervention at 4 weeks): intervention group: mean 8.2 (SD 1.48); control group: mean 8.75 (SD 1.28). Barker‐Collo 2009 also used the TMT‐B. They reported that difference between groups in change was not significant at five weeks (mean −0.29, 95% CI −1.84 to 1.26; P = 0.707). Lin 2014 also used the Mental control subscale of the Wechsler Memory Scale. They only reported within‐group differences, with intervention group showing significantly improved scores after training (P < 0.003), but not the control group.
6.2. Analysis.

Comparison 6: Memory, Outcome 2: Working memory (Digit Span backwards) (postintervention)
Effects on follow‐up after intervention
Barker‐Collo 2009 and Bo 2019 used the TMT‐B at six months, and van de Ven 2017 at four months. Using the only reported data for van de Ven 2017 (active control group), we pooled the data from these studies and found insufficient evidence of an effect (SMD −0.18, 95% CI −0.43 to 0.08; P = 0.17, I2 = 0%; 3 studies, 243 participants; Analysis 6.3). At three months' follow‐up, Akerlund 2013 found a significant between‐group difference for Digit Span backwards in favour of the intervention group (P = 0.049) and no significant difference between groups for Span Board reversed (P = 0.980) (analysis of stroke subsample provided by authors). We pooled the four studies that measured working memory on follow‐up after the intervention between three and six months (Akerlund 2013; Barker‐Collo 2009; Bo 2019; van de Ven 2017), using Span Board reversed for Akerlund 2013 and TMT‐B for the rest. We found insufficient evidence of an effect (SMD −0.17, 95% CI −0.40 to 0.07; P = 0.17, I2 = 0%; 4 studies, 272 participants; Analysis 6.4).
6.4. Analysis.

Comparison 6: Memory, Outcome 4: Working memory (follow‐up)
Other
Barker‐Collo 2009 also reported non‐significant between‐group change differences for the PASAT 2.0 at five weeks (P = 0.085) and PASAT 2.4 and 2.0 at six months (PASAT 2.4: P = 0.70; PASAT 2.0: P = 0.609). van de Ven 2017 also reported results for the PASAT postintervention and at one‐month follow‐up, the latter using the active control group only and for Delis‐Kaplan Executive Function System (D‐Kefs) TMT number‐letter switching condition and Letter Number Sequence. Between‐group differences were not reported.
Akerlund 2013 reported on a Working Memory subscale, aggregated from the combined scaled‐scored results of the three tests of the WAIS‐III NI, Digit Span, Arithmetic and Letter‐Number Sequences. Separate results for the subsample of participants with stroke was not reported or provided. Akerlund 2013 also used the Working Memory questionnaire (a self‐report questionnaire) and found no between‐group differences at six weeks (P = 0.651) or three months (P = 0.935) for the subsample of participants with stroke (data provided by study authors). Lundqvist 2010 reported results of a Working Memory Improvement Index, which was a composite measure of the participant's improvement during the working memory training period, calculated by subtracting the "Start index" from the "Max index" based on results from the two days with the best performances during the training period; the authors provided the data for the subsample of participants with stroke (after the intervention at four weeks: intervention group: mean 20.8 (SD 7.29) with 28% improvement; control group: mean 19.63 (SD 8.05) with 27% improvement).
Memory span
Memory span was measured with multiple instruments in some studies and for different aspects of working memory, including immediate verbal memory span and immediate spatial memory span. These outcomes were measured immediately after the interventions and on follow‐up in some studies.
Effects immediately after intervention
Immediate verbal memory span
Several studies measured immediate verbal memory span on completion of the intervention (18 weeks and less). Akerlund 2013, Bo 2019, and Cho 2015 used Digit Span Forwards. Zuchella 2014 used "Digit span", which we assumed to be Digit Span Forwards as the authors reported that it measured verbal immediate memory span. De Luca 2018 reported results for "Digital span" but we were unable to establish with the authors if this was for Digit Span Forwards. Similarly, Yoo 2015 reported results for "Digit Span Test". We assumed these latter two studies used Digit Span Forwards and pooled these six studies with Lundqvist 2010 that used the Listening Span and Yeh 2019 that used Verbal Paired Associates subtest (verbal learning and memory) and found statistical evidence of an effect of very low certainty in favour of the intervention (SMD 0.35, 95% CI 0.14 to 0.56; P = 0.001, I2 = 13%; 8 studies, 357 participants; Analysis 6.5). Re‐expressing the SMD and using data from a cohort study of 199 people with stroke for performance on the Digit Span Forwards (0 to 12) (Hochstenbach 1998), we multiplied the SMD by the SD from Hochstenbach 1998 of 2.18 for a difference between groups of 0.763 (95% CI 0.31 to 1.22), equating to a difference of recall of one digit. We know of no standards to which we can compare this result in terms of a clinically important difference. We downgraded the certainty of the evidence two levels due to very serious concerns about risk of bias and serious concerns about imprecision. Sensitivity analysis removing Lundqvist 2010 found similar effects (SMD 0.34, 95% CI 0.12 to 0.55; I2 = 13%; 344 participants). Bo 2019 also reported on performance on Digit Span Forwards at six months' follow‐up for cognitive training group versus control group: cognitive training: mean 7.69 (SD 1.14); control: mean 7.40 (SD 1.01), reporting a return to baseline levels in the cognitive training group.
6.5. Analysis.

Comparison 6: Memory, Outcome 5: Immediate verbal memory span (postintervention)
Immediate spatial memory span
Five studies measured immediate spatial memory span immediately after the intervention (18 weeks and less) using Span Board Forwards, Block Span Forwards, Spatial Span, and Corsi's test (Akerlund 2013; Lundqvist 2010; van de Ven 2017; Yeh 2019; Zuchella 2014). Cho 2015 and Yoo 2015 used a "Visual Span test" with no further description of the measure provided. We assumed this was similar to the other measures and combined results for these seven studies (292 participants) and found statistical evidence of a small effect (SMD 0.27, 95% CI 0.03 to 0.50; P = 0.03, I2 = 38%; 7 studies, 292 participants; low‐certainty evidence; Analysis 6.6). We downgraded the evidence due to serious concerns with risk of bias and imprecision. Removal of Lundqvist 2010 for sensitivity analysis increased the effect slightly with lower heterogeneity (SMD 0.31, 95% CI 0.07 to 0.55; P = 0.01, I2 = 28%). Lundqvist 2010 also measured spatial memory span with the Picture Span test; the authors provided the data for the subsample of participants with stroke (after the intervention at four weeks: intervention group: mean 10.4 (SD 2.07); control group: mean 7.5 (SD 2.62)).
6.6. Analysis.

Comparison 6: Memory, Outcome 6: Immediate spatial memory span (postintervention)
Immediate and delayed recall
Three studies measured immediate and delayed recall using the Rey Auditory Verbal Learning Test (RAVLT) immediately after the intervention, at four weeks (Zuchella 2014); eight weeks (De Luca 2018), and 12 weeks (van de Ven 2017). When we pooled these studies, there was insufficient evidence of a difference in immediate recall (SMD 0.17, 95% CI −0.12 to 0.46; P = 0.26, I2 = 0%; 184 participants; Analysis 6.7). There was statistical evidence of an effect for delayed recall but with substantial heterogeneity (SMD 0.35, 95% CI 0.05 to 0.66; P = 0.02, I2 = 90%; very low‐certainty evidence; Analysis 6.8). We downgraded the evidence due to concerns with risk of bias, inconsistency, and imprecision. Applying a random‐effects model did not reduce the heterogeneity for delayed recall. Excluding Zuchella 2014 from the analysis removed the heterogeneity, but then there was insufficient evidence of an effect (SMD −0.27, 95% CI −0.68 to 0.13; P = 0.19, I2 = 0%; 97 participants).
6.7. Analysis.

Comparison 6: Memory, Outcome 7: Immediate recall (postintervention)
6.8. Analysis.

Comparison 6: Memory, Outcome 8: Delayed recall (postintervention)
Effects on follow‐up after intervention
Three trials measured memory span on follow‐up; two trials used the Digit Span Forwards at three months (Akerlund 2013) and six months (Bo 2019), and van de Ven 2017 used Corsi's test at four months. We pooled these results using the active control group for van de Ven 2017 and found insufficient evidence of an effect (SMD 0.17, 95% CI −0.11 to 0.45; P = 0.23, I2 = 20%; 3 studies, 194 participants; Analysis 6.9).
6.9. Analysis.

Comparison 6: Memory, Outcome 9: Memory span (follow‐up)
Memory (other)
Several studies reported on other aspects of memory, or memory generally, but data were insufficient or unavailable to be pooled. Akerlund 2013 found non‐significant differences between groups at both six weeks' and three months' follow‐up for the Rivermead Behavioural Memory Test (RBMT) – Profile (six weeks: P = 0.539; three months: P = 0.876) and the RBMT Screen (six weeks: P = 0.401; three months: P = 0.917) (data analysis for stroke subsample provided by study authors). Lin 2014 reported within‐group differences for the Memory quotient and the Logical memory subscale and "Digits forward and backward" of the Wechsler Memory Scale, finding significant changes for the intervention group postintervention but not for the control group. Yoo 2015 reported only within‐group differences for several other memory outcomes at five‐week follow‐up. For the Visual learning test, they reported a significant difference for the intervention group (P < 0.01), but not the control group. For the Verbal learning test, they reported non‐significant differences for both intervention and control groups. Zuchella 2014 reported between‐ and within‐group differences for immediate and delayed Logical Memory scales at baseline and four‐week follow‐up without reporting effect sizes. They reported significant between‐group differences in favour of the intervention group for both immediate (intervention group: median 4.5, interquartile range (IQR) 3.4 to 6.0; control group: median 3.4, IQR 2.6 to 4.6; P = 0.005) and delayed logical memory (intervention group: median 4.4, IQR 3.0 to 6.0; control group: median 3.2, IQR 1.8 to 4.4; P = 0.009).
Executive function (secondary outcome)
Thirteen trials reported outcomes for global or specific executive functions. Most used scored performance measures. Six studies used measures of multiple domains of executive functions combined for a total score: Maggio 2020, Prokopenko 2013, Prokopenko 2018, Prokopenko 2019, and Zuchella 2014 used the FAB, and Chen 2015 used the Behavioural Assessment of Dysexecutive Syndrome (BADS). Eight studies measured specific domains of executive function: non‐verbal reasoning with PM47 (De Luca 2018; van de Ven 2017; Zuchella 2014), or Weigl's Test (Maggio 2020), cognitive flexibility with CWIT‐4 (Lundqvist 2010; Skidmore 2015a), spatial imagination (Bo 2019), time judgement (Carter 1983), and problem‐solving and reasoning (van de Ven 2017). All but two of these trials used a cognitive remediation approach. Maggio 2020 and Skidmore 2015a used a compensatory and adaptive approach. Akerlund 2013 and van de Ven 2017 used a measure of self‐reported executive function, the DEX (Burgess 1996). We pooled studies that measured the same executive functions and executive functions overall. A small number of studies examined executive function on follow‐up after completion of the intervention as well as immediately after the intervention.
Effects immediately after intervention
Non‐verbal reasoning
Three studies measured non‐verbal reasoning immediately after the intervention (12 weeks or less) with the PM47 (De Luca 2018; van de Ven 2017; Zuchella 2014). When we pooled these three studies there was insufficient evidence of an effect (MD 0.46, 95% CI −0.67 to 1.60; P = 0.42, I2 = 0%; 184 participants; Analysis 7.1). When we combined these with Maggio 2020, who measured abstract reasoning with Weigl's Test, there was evidence of an effect but with substantial heterogeneity contributed by Maggio 2020 (SMD 0.40, 95% CI 0.12 to 0.68; P = 0.005, I2 = 93%; 4 studies, 224 participants; Analysis 7.2). When we applied a random‐effects model, the heterogeneity was unchanged. Maggio 2020 showed the greatest improvement in non‐verbal reasoning; this study used a compensatory and adaptive approach, while the others used a cognitive remediation approach. van de Ven 2017 also measured reasoning with Shipley Institute of Living Scale and found significant within‐group differences postintervention for the intervention group (P = 0.02) and the waiting‐list control group (P < 0.01).
7.1. Analysis.

Comparison 7: Executive function, Outcome 1: Non‐verbal reasoning (Raven's Colored Progressive Matrices) (postintervention)
7.2. Analysis.

Comparison 7: Executive function, Outcome 2: Non‐verbal reasoning – standardised mean difference (postintervention)
Cognitive flexibility
We pooled Lundqvist 2010 and Skidmore 2015a who used the CWIT‐4 to measure the specific executive function of cognitive flexibility. As Lundqvist 2010 used a timed score version of this test, with lower scores indicating better performance and Skidmore 2015a used a scaled score version with higher scores indicating better performance, we reversed the direction of Skidmore 2015a data by multiplying the means by −1 to enable pooling of data (see Data extraction and management). We pooled the data for four weeks for Lundqvist 2010 and three months for Skidmore 2015a as it was not measured postintervention and found evidence of an effect and no heterogeneity (SMD −1.50, 95% CI −2.20 to −0.80; P < 0.0001, I2 = 0%; 2 studies, 43 participants; low‐certainty evidence; Analysis 7.3). We downgraded the evidence due to low sample size and concerns about risk of bias from unclear allocation concealment and high risk of treatment blinding. Skidmore 2015a reported that the scaled scores from the CWIT‐4 have a population mean of 10 and an SD of 3 and that "change greater than 3 points could be considered clinically meaningful". Re‐expressing the SMD of 1.50 that we obtained in meta‐analysis by multiplying it by the population SD of 3, which equates to 4.5, may be considered a clinically meaningful change on the CWIT‐4.
7.3. Analysis.

Comparison 7: Executive function, Outcome 3: Cognitive flexibility (postintervention)
Skidmore 2015a also reported on post‐hoc model‐derived change score differences between groups at six months for CWIT‐4. They found significant post‐hoc model‐derived change score differences between groups in favour of intervention (P = 0.004, d = 1.23).
Time judgement
Carter 1983 measured the specific executive function of time judgement and reported MD improvement scores, so we could not include these within the meta‐analysis. There was insufficient evidence of an effect (MD 17.00, 95% CI −2.46 to 36.46; I2 = 0%; 25 participants) (Hoffmann 2010).
Problem‐solving
van de Ven 2017 measured another aspect of executive function, problem‐solving, with the Tower of London test. They reported significant within‐group differences postintervention for the intervention group (P = 0.02) but not the control group (P = 0.24). Between‐group differences were not reported.
Global executive functional performance
We pooled the studies that measured global executive functional performance: Maggio 2020, Prokopenko 2013, Prokopenko 2018, Prokopenko 2019, and Zuchella 2014, which all used the FAB, and Chen 2015, which used the BADS. There was evidence of an effect but substantial heterogeneity (SMD 0.63, 95% CI 0.41 to 0.86; P < 0.00001, I2 = 75%; 6 studies, 318 participants; Analysis 7.4). Applying a random‐effects model did not lower the heterogeneity. Removing Chen 2015, leaving only studies that used the FAB, reduced the heterogeneity to 30%. This gave an MD of 0.82 (95% CI 0.30 to 1.35; P = 0.002, I2 = 30%; 5 studies, 238 participants; low‐certainty evidence; Analysis 7.5), indicating that participants improved by 0.82 on the FAB which has a maximum score of 18. We know of no standards to which we can compare this result in terms of a clinically important difference for the FAB for people with stroke. We downgraded the evidence due to concerns about imprecision and risk of bias from all studies being unclear for allocation concealment, two unclear for incomplete data reporting, and all high risk for blinding of treatment.
7.4. Analysis.

Comparison 7: Executive function, Outcome 4: Global executive functional performance (postintervention)
7.5. Analysis.

Comparison 7: Executive function, Outcome 5: Global executive functional performance (sensitivity analysis, Frontal Assessment Battery, postintervention)
Executive functional performance overall
We pooled the 11 studies that used performance measures of executive function: Bo 2019 used the Mental Rotation test; De Luca 2018 and van de Ven 2017 used the PM47; Lundqvist 2010 and Skidmore 2015a used the CWIT‐4; Chen 2015 used the BADS; and Maggio 2020, Prokopenko 2013, Prokopenko 2018, Prokopenko 2019, and Zuchella 2014 used the FAB. We found evidence of an effect with substantial heterogeneity (SMD 0.49, 95% CI 0.31 to 0.66; P < 0.00001, I2 = 74%; 11 studies, 550 participants; very low‐certainty evidence; Analysis 7.6; Figure 6). We downgraded the certainty of evidence three levels with very serious concerns for risk of bias and consistency. Applying a random‐effects model did not improve the heterogeneity. In sensitivity analysis, removal of Chen 2015 reduced heterogeneity but it was still substantial (SMD 0.35, 95% CI 0.16 to 0.53; P = 0.0003, I2 = 54%). Removal of Prokopenko 2019 had minimal effect. We conducted a post‐hoc subgroup analysis of the 11 studies by type of intervention (i.e. cognitive remediation or compensatory and adaptive) and found a significant difference between the groups by type of intervention with greater effect from the two compensatory and adaptive approaches (P = 0.004). To re‐express this SMD using a familiar instrument as recommended in Section 15.5.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021b), we multiplied the SMD (0.49) by an estimate of the SD associated with the most familiar instrument, in this case, the FAB used in five studies and calculated "a weighted average across all intervention groups of all studies" that used the FAB, using the mean of the postintervention SDs of these studies (i.e. 2.87). The multiplication gave an MD of 1.41 (95% CI 0.89 to 1.89). Therefore, on average, participants receiving the intervention improved executive functional performance by 1.41 points on the FAB scale, which ranges from 0 to 18, but the evidence is very uncertain. We know of no standards to which we can compare this result in terms of a clinically important difference.
7.6. Analysis.

Comparison 7: Executive function, Outcome 6: Executive functional performance overall (postintervention)
6.

Funnel plot of comparison: 7 Executive function, outcome: 7.6 Executive functional performance overall (postintervention).
Self‐reported executive function
Two studies measured perceived executive function (Akerlund 2013; van de Ven 2017), using the DEX, a self‐ or carer‐administered questionnaire measuring a range of problems commonly associated with the dysexecutive syndrome, such as emotional, motivational, behavioural, and cognitive changes (Chan 2001). Lower scores indicate better function. We pooled these two studies and found insufficient evidence of an effect (MD −3.67, 95% CI −8.69 to 1.35; P = 0.15, I2 = 0%; 2 studies, 77 participants; Analysis 7.7).
7.7. Analysis.

Comparison 7: Executive function, Outcome 7: Self‐reported executive function (postintervention)
Effects on follow‐up after intervention
Three studies measured effects on executive functional performance on follow‐up between four and six months after the intervention using Mental Rotation test (Bo 2019), CWIT‐4 (Skidmore 2015a), and Tower of London (van de Ven 2017). We pooled these studies using the active control group for van de Ven 2017 as it was the only control data reported for this outcome, but found insufficient evidence of an effect and substantial heterogeneity (SMD 0.27, 95% CI −0.02 to 0.55; P = 0.07, I2 = 82%; 3 studies, 195 participants; Analysis 7.8). In sensitivity analysis, removal of Skidmore 2015a eliminated the heterogeneity, but there was still insufficient evidence of an effect. When we applied a random‐effects model, the heterogeneity remained and was still significant.
7.8. Analysis.

Comparison 7: Executive function, Outcome 8: Executive functional performance overall (follow‐up)
Other
Yoo 2015 used the Trail Making Test, without specifying whether it was TMT‐A or TMT‐B, so we did not include these data in the analyses. They did not report between‐group differences but reported that there was no significant within‐group difference for both groups.
Discussion
The aim of this review was to determine the effectiveness of occupational therapy for people with cognitive impairment after a stroke, particularly the impact of occupational therapy on BADL and IADL and cognitive abilities poststroke. Twenty‐four trials met the criteria for inclusion.
Summary of main results
See Table 1 for a summary of results for the primary outcome of BADL and other key secondary outcomes.
Basic activities of daily living
We found evidence that occupational therapy may result in little to no clinical difference in BADL for people with stroke with cognitive impairments with, on average, a mean improvement of 2.2 points on the FIM immediately after the intervention, based on six studies (low‐certainty evidence) and a mean improvement of 11.38 points at six‐month follow‐up, based on two studies (low‐certainty evidence). Neither of these improvements meet the established MCID for the FIM with people with stroke of 22 points (Beninato 2006). There was insufficient evidence of an effect at three months' follow‐up (low‐certainty evidence).
Instrumental activities of daily living
The evidence is very uncertain about the effect of occupational therapy on IADL (postintervention) based on two studies (very low‐certainty evidence).
Community integration
There was insufficient evidence of an effect on community integration and participation (postintervention), based on two studies (low‐certainty evidence).
Global cognitive function
We found low‐certainty evidence based on nine studies that occupational therapy may slightly increase global cognitive functional performance immediately after the intervention with, on average, an improvement of 1.63 points on the MoCA. This exceeds the anchor‐based MCID of the MoCA for rehabilitation in people with stroke of 1.22 found by Wu 2019.
Orientation
One study reported on orientation and reported only within‐groups differences after computer‐assisted cognitive training, so we could not make conclusions about the effect of occupational therapy on orientation.
Attention
We found some evidence of an effect for attention overall, immediately after the intervention, when we pooled data for 13 studies, with a possible mean improvement of 17 seconds on tests that measured visual attention. We know of no standards to which we can compare this result in terms of a clinically important difference. We downgraded the evidence to low certainty and concluded that occupational therapy may result in little to no difference in visual attention overall at the end of the intervention. We found low‐certainty evidence of an effect for attention overall at three to six months' follow‐up, based on five studies, concluding that occupational therapy may result in little to no difference equating to 18 seconds. We know of no standards to which we can compare this result in terms of a clinically important difference.
We also examined effects for subdomains of attention, including sustained and selective, visual and auditory. For sustained attention, there was some effect of moderate certainty based on 10 studies that the interventions improved sustained visual attention immediately after the interventions but not on follow‐up. Occupational therapy likely improves sustained visual attention after intervention slightly. The difference equates to 16 seconds. We know of no standards to which we can compare this result in terms of a clinically important difference. There was insufficient evidence of an effect that the interventions improved sustained auditory attention immediately after the intervention or on follow‐up. For selective attention, there was evidence of an effect of low certainty based on four studies for selective visual attention after the intervention and similarly for selective visual attention on six months' follow‐up, based on two studies.
Memory
For improved memory immediately after the intervention, we found some effect of low certainty for working memory equating to a difference of 60 seconds (based on eight studies), some effect of very low certainty for immediate verbal memory span equating to a difference of recall of 1 digit on the Digit Span Forwards test (based on eight studies), and evidence of low certainty of a small effect on immediate spatial memory (based on seven studies). We know of no standards to which we can compare this result in terms of a clinically important difference. We conclude that occupational therapy may increase working memory slightly after intervention, may result in little to no difference in immediate spatial memory span, and that the evidence is very uncertain about the effect on immediate verbal memory span. There was insufficient evidence of an effect on immediate recall and the evidence is very uncertain for delayed recall. We found insufficient evidence of an effect on follow‐up for any aspects of memory.
Executive function
The evidence is very uncertain about the effect of occupational therapy on executive functional performance, based on 11 studies. There was a difference between groups that equates to 1.41 on the FAB. Therefore, on average, participants receiving the intervention improved executive functional performance by 1.41 points on the FAB scale, which ranges from 0 to 18, but the evidence is very uncertain. We know of no standards to which we can compare this result in terms of a clinically important difference. When we examined specific executive functions, we found that occupational therapy may slightly increase cognitive flexibility after the intervention, with a difference equating to 4.5, which may be considered a clinically meaningful change on the CWIT‐4. We found some effect but substantial heterogeneity for non‐verbal reasoning and insufficient evidence of an effect for self‐reported executive function.
Types of intervention
When we ran subanalyses of the effects of different types of occupational therapy interventions classified as either cognitive remediation or compensatory and adaptive, there was no difference between subgroups for BADL, but there was a difference between the groups for executive functional performance overall with greater effect from the two compensatory and adaptive approaches.
Overall completeness and applicability of evidence
Our review included studies from a range of countries, which were published in English and languages other than English, and evaluated various approaches to occupational therapy intervention for adults with cognitive impairment after stroke. Most studies were from countries in Asia, including four from the Republic of Korea and four from China. Seven studies were from European countries, three from Russia, and three from the USA. Research from such a diverse range of countries provides indication of the diversity of clinical practice. Most eligible interventions used computer‐based cognitive training programs, with 17 studies using one of nine programs and three studies using a pen and paper‐based intervention. Only four studies used interventions with a compensatory and adaptive approach.
Although some studies provided detailed accounts of the interventions or referred to supplementary papers or websites to access the intervention, many descriptions were not comprehensive enough to allow replication of the approach in practice. Even if interventions were well‐described or freely or commercially available, the role of the therapist in supervision or tailoring, or both, was not often clearly explained, as also noticed by Rogers 2018 in their review. Many of the interventions lacked a description of a theoretical rationale, as also found by Rogers 2018. Greater attention to description of interventions in trials, such as suggested by the TIDieR guide (Hoffmann 2014), would greatly improve the ability of clinicians to use evidence‐based interventions in practice. Similarly, there was often very limited description of the comparator control or usual care provided, which limits research replication and full interpretation of trial results.
Furthermore, as Rogers 2018 found, there was considerable variation in the outcome measures administered for the same domain, including ADL and cognitive domains and varying classification of different outcome measures for different domains. Having BADL as the primary outcome in our review addresses the need for evidence about interventions for improving function in activity performance in people with stroke (Rogers 2018). Only 10 studies measured BADL and fewer (seven) measured IADL. Not all of these clearly described the measures used and which domains of ADL were assessed by the measures. Only two studies measured community integration or participation. Our review included the evaluation of cognition across multiple domains, as recommended by the recent Stroke Recovery and Rehabilitation Roundtable (McDonald 2019). Thirteen studies measured global cognitive function and aspects of attention and executive function. Eleven studies measured aspects of memory. Only one study measured orientation.
The participants of our review were generally representative of the population of interest, with a balance in gender and chronicity of stroke. We found, in agreement with Rogers 2018, that patient characteristics, such stroke severity, were not always well described making comparisons difficult and that well‐established standardised tools for these purposes, such as the National Institutes of Health Stroke Scale, were not commonly used. Participants' physical status was rarely reported, so we cannot make conclusions about the potentially confounding impact of physical impairments on outcomes such as ADL performance. However, motor impairment was not commonly listed as an exclusion criterion (i.e. one study excluded participants based on motor deficits (Bo 2019), one if they had diplegia (Cho 2015), and another based on participants having spasticity (De Luca 2018)). Three studies excluded people for inability to use a controller (Lundqvist 2010; Park 2015a; Zuchella 2014), one for inability to walk with or without assistive devices (Yeh 2019), and one for poor sitting tolerance (Walker 2012), but these were specific to the interventions and not necessarily excluding people with motor impairment generally. So, it appears that participants may have had concomitant physical impairment affecting intervention performance and outcomes, thereby supporting the likelihood that the participants were generally representative of the population of interest.
Quality of the evidence
We found the certainty of the evidence for most outcomes to be low or very low with all outcomes having concerning risk of bias issues, many outcomes having some concern about imprecision due to insufficient sample sizes, and a few outcomes having issues with inconsistency due to substantial heterogeneity. The studies were strongest in random sequence generation (selection bias) and reporting of outcome data (reporting bias) and lack of other bias. The studies were weakest in blinding of participants and personnel for intervention delivery (performance bias) and minimising or reporting of minimising risk in allocation concealment (selection bias).
Potential biases in the review process
Although we attempted a broad search, there may be small studies from minor occupational therapy journals or unpublished studies that were not in the databases, registers, and registries searched. Where possible, we contacted authors with queries about study eligibility and if data for subsamples of participants with stroke were available; only some authors replied. Another potential bias is the process by which we classified the reported cognitive domains for analysis purposes. There were differences between studies about which aspect of cognition was measured by different outcome measures and there is a general lack of consensus about cognitive domains and their measurement (Bernhardt 2019; Loetscher 2019). We classified these as best we could, guided by original descriptions of the instruments where available, and other systematic reviews in the field. For some outcome measures, we were unable to ascertain the specific nature of the instrument used (e.g. 'Digit Span test' without specifying if it was Digit Span Forwards or Backwards), so in some instances we assumed about which measure it was and pooled it with other like measures, noting this where applicable. For some studies, where the comparator data reported were for one of two possible comparators in the study, we noted which group we used the data from for pooling in meta‐analysis. Given that we treated the control groups as comparable (i.e. most being usual care or comparable to usual care and some no intervention), this may have affected the results given the large variation in usual care plus some receiving no intervention.
Agreements and disagreements with other studies or reviews
As noted in the Background, there are several reviews and guidelines relevant to our review (e.g. Chung 2013; Cicerone 2019; das Nair 2016; Lanctôt 2020; Loetscher 2019; NICE 2013; Poulin 2012; Rogers 2018; Stroke Foundation 2018; Winstein 2016). However, direct comparisons are challenging since some reviews are about cognitive rehabilitation in general, or include people with TBI or other ABI as well as stroke, or are about specific cognitive impairments from stroke. Furthermore, there are some discrepancies in classifications of the outcome measures included in the reviews. For example, the PASAT is classified as an attention outcome in a review on attention (Loetscher 2019), and the Digit Span Forwards and Backwards are classified as attention in Rogers 2018, but all of these are classified as working memory in a review on executive function (Chung 2013), and in our review. As Loetscher 2019 commented in their review, while there "is a consensus that attention is not a unitary process, there is no agreement on the typologies and taxonomies describing the range of attentional processes". This also applies to other cognitive abilities given the complex, hierarchical, and multidimensional nature of cognition (Bernhardt 2019).
Activities of daily living/instrumental activities of daily living
Systematic evaluation of the effect of rehabilitation on the activity performance of people with stroke with cognitive impairments has been lacking to date (Rogers 2018). Of the reviews that have included studies that measured ADL as an outcome measure, there has been limited evidence to indicate improvement. Unlike das Nair 2016, who found no significant effect on functional ADL ability in the short or long term from cognitive rehabilitation for memory impairments, and Chung 2013, who found no high‐quality evidence for effects of cognitive rehabilitation for executive dysfunction on ADL, we found low‐certainty evidence that, on average, at completion of the intervention (12 weeks or less) BADL improved by 2.24 points on the FIM scale (that ranges from 18 (total assist) to 126 (complete independence)). This may not be a clinically important gain as the MCID for the FIM has been established as 22 points for people with stroke (Beninato 2006). However, another study found that a change of 1 point on the total FIM score for people with stroke was equivalent to an average of 2.19 minutes of help from another person per day (Granger 1993); therefore, 2.24 on the FIM equates to 4.91 fewer minutes of help per day.
The interventions evaluated in the trials included both compensatory and adaptive approaches and cognitive remediation approaches, including computer‐based interventions. Some studies included in our review have been published since earlier reviews were conducted. For example, Legg 2017 reviewed RCTs of occupational therapy interventions that focused on ADL for people with stroke and excluded specific treatment approaches (e.g. task‐specific training or cognitive training as included in our study). Based on nine RCTs with 994 participants, they found low‐quality evidence in support of such interventions.
Global cognitive function
Few existing reviews included measures of global cognitive functional performance (e.g. MoCA, MMSE). In their review, Gillen 2015 concluded that evidence supports the use of general cognitive rehabilitation to improve global cognitive function for people with ABI including stroke. In their meta‐analyses of studies examining the effectiveness of 'cognitive remediation' approaches after stroke, Rohling 2009 found modest, statistically significant treatment effects on global cognitive function. Combining measures from 22 studies across cognitive domains, Rogers 2018 concluded that interventions for cognition for people with stroke had a small overall effect (P < 0.01). When they combined data from measures of global cognitive performance, they found a smaller but insignificant effect; this was based on two studies that used the MMSE (Prokopenko 2013; Zuchella 2014), both of which were included in our review. In a meta‐analysis of six studies (all but one included in our review) measuring overall cognition, Ye 2020 found no evidence of an effect (SMD 0.59, 95% CI −0.06 to 1.24) and substantial heterogeneity that was partly explained by Park 2015a in a sensitivity analysis. Our analysis also found substantial heterogeneity, also partly explained by Park 2015a in sensitivity analysis (as well as Chen 2015). However, our review found evidence of an effect for overall cognition, when Park 2015a and Chen 2015 were both included in analysis (SMD 0.58, 95% CI 0.40 to 0.76; very low‐certainty evidence) and removed (SMD 0.35, 95% CI 0.16 to 0.54; low‐certainty evidence). We concluded that there is low‐certainty evidence that, following occupational therapy, cognitive functional performance may improve beyond the established MCID.
Orientation
Only one study measured orientation, so we cannot make any comparisons with other reviews for this cognitive domain.
Attention
We found low‐certainty evidence of some effect for attention overall, equating to an improvement of 17 seconds on visual attention tests and concluded that occupational therapy may result in little to no difference in visual attention overall after the intervention. We found some effect of moderate certainty for sustained visual attention after the intervention and of low certainty for selective visual attention after the intervention and after six months, but not for other aspects of attention measured. Loetscher 2019 found some support for cognitive remediation interventions for divided attention only and only immediately after the intervention. That review included six RCTs of 223 participants and included measures of global and specific aspects of attention. One of the measures of divided attention was the PASAT, which we classified as a measure of working memory; the other measures were not covered in the studies in our review. A systematic review of the effectiveness of cognitive remediation of attention following ABI found support for the improvement of divided attention in a subgroup analysis of participants with stroke (Virk 2015). Other reviews in the field have found some support for attention training, including the Rohling 2009 meta‐analysis, which found support for attention training after TBI; the Rogers 2018 meta‐analysis, which found a small but significant effect (g = 0.40, 95% CI 0.22 to 0.59; P < 0.01); and the Cicerone 2019 evidence‐based guidelines, which recommended direct attention training for specific working memory impairments, including use of computer‐based training, as a practice guideline for people with TBI or stroke. The 10 studies cited for support of the Rogers 2018 review included Barker‐Collo 2009, Cho 2015, Lin 2014, Prokopenko 2013, and Zuchella 2014, which were included in our review; and the Cicerone 2019 practice guideline included Akerlund 2013, Barker‐Collo 2009, and Lundqvist 2010, all included in our review.
Memory
We found some evidence of effect immediately after the intervention for working memory (low certainty), immediate verbal memory span (very low certainty), immediate spatial memory span (low certainty), and delayed recall (very low certainty), but not for immediate recall. Our findings of small or insufficient evidence of effect of cognitive remediation for memory impairment is consistent with the findings of das Nair 2016, Chung 2013, and Rogers 2018. Based on 13 RCTs of 514 participants with stroke, das Nair 2016 found significant effects of cognitive rehabilitation on self‐report of memory function in the short term but not long term, and not for objective tests of memory. Based on two studies (104 participants with stroke or other ABI), Chung 2013 found no significant effect of cognitive rehabilitation on working memory. Gillen 2015 also found that the evidence is limited for occupational therapy improving performance in ADL for people with memory loss following stroke. In contrast, Rogers 2018 found small significant effects (P < 0.05) for memory outcomes for people with stroke based on five studies. We also found some evidence of effects for working memory based on eight studies that used a cognitive remediation approach. A meta‐analysis of the effect of memory rehabilitation therapy for people with TBI and stroke also found some support for memory improvement in people with stroke and that interventions addressing working memory specifically, produced significantly larger effects than rehabilitation addressing other aspects of memory (Elliott 2014). This is supported by a meta‐analysis of studies of working memory training that included people with and without brain injury and that found people with brain injuries, including stroke, benefited the most both immediately after the intervention and on follow‐up (Weicker 2015).
Executive function
Chung 2013 reviewed RCTs of cognitive rehabilitation interventions that addressed executive function after stroke or other ABI including restorative interventions (to improve components of executive functions), compensative interventions (training to compensate for lost executive function), and adaptive interventions (training in adaptive techniques to improve independence in ADL). In the review, executive function component outcomes included initiation, inhibition, concept formation, planning, and flexibility. Based on 13 studies with 770 participants, 304 of whom had stroke, the authors concluded that there was insufficient high‐quality evidence about the effect of cognitive rehabilitation on the primary outcome of global executive function or secondary outcomes, including executive function components, working memory, ADL, mood and anxiety, vocational activities, and quality of life and social isolation. Cicerone 2019 also found insufficient evidence to support computer‐based cognitive remediation of executive function impairments. We found evidence of a small effect of very low certainty of occupational therapy interventions on executive function performance tests. Rogers 2018 also found a small and significant effect (P < 0.01) in an analysis which included two studies from our analysis (Prokopenko 2013; Zuchella 2014).
Types of interventions
Most of the studies in our review used a cognitive remediation approach and the majority of these used computer‐based training. No studies used a combined approach, that is, a cognitive remediation approach combined with a compensatory and adaptive approach. We found some support for both cognitive remediation and compensatory and adaptive approach interventions in terms of ADL performance with no significant difference between the approaches in a post‐hoc analysis. The results for the impact on global cognitive function and cognitive domains apply mostly to cognitive remediation or restoration approaches. For executive functional performance overall, we conducted a post‐hoc subgroup analysis of the 11 studies by type of intervention (i.e. cognitive remediation or compensatory and adaptive) and found a difference between the groups by type of intervention with greater contribution to the effect from the two studies using compensatory and adaptive approaches.
Some surveys of occupational therapy practice indicate that functional approaches are used in preference to cognitive remediation approaches in some countries, for example, Australia and Canada (Koh 2009; Korner‐Bitensky 2011), and that use of computers and technology generally is low in stroke rehabilitation in some countries (Holmqvist 2014; Koh 2009; Langan 2018). This may be because there has been a lack of evidence to date about cognitive remediation approaches within occupational therapy practice (Korner‐Bitensky 2011), and concerns about the generalisation and transfer of skills developed from cognitive remediation approaches to functional performance, including ADL (Gillen 2015). A study of clinicians' rehabilitation practice for people with ABI reported that more attention was given to functionally oriented cognitive rehabilitation than computer‐based working memory and attention training (Poulin 2020), despite guidelines recommending specific cognitive training interventions (e.g. Cicerone 2019; Lanctôt 2020). These evidence‐based guidelines now support computer‐based training in attention, memory, and executive function for people with stroke but only if the training is actively guided by a rehabilitation therapist and follows principles of neuroplasticity (e.g. direct stimulation of the cognitive domain, adjustment of task difficulty informed by performance, and immediate and objective feedback) (Cicerone 2019). There is some evidence that structured, regular support of a supervising health professional can improve the adherence of people with stroke to computer‐based cognitive training (Wentink 2018). Many of the computer‐based training interventions in the studies of our review meet these stipulations. Computerised technologies can be limited in terms of tailoring and personalisation with one‐fits‐all interventional approaches (Draaisma 2020). However, emerging technologies will allow greater personalisation which may enhance efficacy (Draaisma 2020). More research is needed for comparing effectiveness in people with different characteristics such as severity of impairment or concomitant impairments such as physical impairment and to investigate translation of cognitive skills training to daily life (Draaisma 2020).
Support, in the Cicerone 2019 practice guidelines, for goal‐directed, individualised, and client‐centred cognitive and interpersonal therapies to improve function are in line with our findings of the benefits of client‐centred, goal‐directed functional training in some of the occupational therapy interventions covered in our review (e.g. Skidmore 2015a0; Skidmore 2017; Walker 2012). Cicerone 2019 noted the apparent benefits of the use of functional skills training combined with client‐directed goals and activities, such as used in the Cognitive Orientation to Occupational Performance (CO‐OP) approach (e.g. Skidmore 2015a; Skidmore 2017), but that the "specific effective ingredients" of this approach have not been isolated. This supports the conclusions of Gillen 2015 that occupational therapy interventions for people with cognitive impairments from stroke need to take a compensatory, performance‐based, and strategy approach. However, contrary to Gillen 2015, the evidence in our review suggests that either the cognitive remediation or the functional compensatory approach may improve function, at least in the short‐term and that there is also some support for cognitive remediation approaches, but the long‐term carryover effects on functional performance remain unknown. Although there were not enough studies in their review that measured ADL, Rogers 2018 similarly found support for both a restorative, cognitive training approach and a more compensatory, rehabilitative approach on general and domain‐specific cognitive abilities. As noted by Rogers and colleagues, both approaches may show improved cognitive outcomes, especially when delivered within high‐quality studies and our review provides further indication that both approaches may have merit. Further research is needed to compare the approaches (Rogers 2018).
Authors' conclusions
Implications for practice.
The body of evidence for the effectiveness of occupational therapy interventions used in the studies within this review for cognitive impairment poststroke has improved since our first review, with 23 additional eligible studies. However, the effectiveness remains unclear. The potential benefits of occupational therapy interventions on basic activities of daily living (BADL) performance and global cognitive function for people with stroke have some support based on the evidence, albeit of low certainty, from the studies in this review. Only the effect on global cognitive function was of a clinically important difference. There is also some support of moderate certainty for such interventions to improve visual attention slightly after the intervention, although it is not clear if this result has clinical importance or if it could be maintained in the longer term. There is some evidence of low certainty that occupational therapy may increase slightly working memory and flexible thinking after intervention. There may be little to no difference on other cognitive domains and subdomains of attention, memory, and executive function and on instrumental activities of daily living (IADL) and community integration and participation.
The occupational therapy interventions in the studies included in the review used cognitive remediation approaches as well as goal‐directed, compensatory, and adaptive approaches, though cognitive remediation approaches were used in the majority. There was no indication of superiority of either approach in the evidence for performance in BADL. The improvement in global cognitive function was based on all but one intervention (Maggio 2020) taking a cognitive remediation approach. However, for executive functional performance, there was evidence of greater effect from the two compensatory and adaptive approaches (Maggio 2020; Skidmore 2015a), compared with the nine cognitive remediation approaches.
Implications for research.
Given the low certainty of most of the evidence in our review, more research is needed to further support or refute the effectiveness of occupational therapy for cognitive impairment after stroke. Research examining combined occupational therapy approaches is particularly needed (i.e. cognitive remediation and compensatory and adaptive approaches and cognitive interventions combined with physical interventions), as often occurs in practice and as noted in surveys of occupational therapy practice in this area (Holmqvist 2014; Koh 2009). Indeed, more research is needed to establish if there are long‐term benefits on activities of daily living (ADL) and social participation of different approaches on the performance of people with cognitive impairment poststroke (Clarke 2015). Research is needed to discern which elements of approaches are the most effective, along with the minimal and optimum 'dose' of the intervention (Cicerone 2019; Rogers 2018), and what the indications are for specific approaches, for example, depending on the individual profile of the person with stroke and their cognitive impairment (Clarke 2015).
Future research also needs to be of a high quality, and designed and conducted to address the weaknesses found in many of the studies in our review, particularly in selection and performance bias (Stinear 2020). Blinding of rehabilitation interventions can be challenging, including concealment of technological interventions given the physical nature of such interventions (Stinear 2020). Given that delivery of standardised rehabilitation interventions in using blinded methods is complex and time‐intensive, greater resources in stroke research funding are needed for supporting such delivery (Stinear 2020), and to minimise these biases.
What's new
| Date | Event | Description |
|---|---|---|
| 8 March 2021 | New citation required and conclusions have changed | The body of evidence for the effectiveness of occupational therapy interventions for cognitive impairment poststroke has improved since the original review. However the effectiveness remains unclear. The original review found no difference between groups for the two relevant outcomes that were measured: improvement in time judgement skills and improvement in basic activity of daily living (BADL). In this update, we found that the potential benefits of occupational therapy interventions on BADL performance and global cognitive function for people with cognitive impairment after stroke have some support based on the evidence, albeit of low certainty. Only the difference on global cognitive function was of a clinical importance. There is also some support of moderate certainty for such interventions to improve visual attention slightly after the intervention, although if this is of clinical importance and maintained in the longer term is unclear. There may be little to no difference on other cognitive domains of attention, memory and executive function. |
| 2 September 2020 | New search has been performed | We reran the searches to September 2020 and revised the text as appropriate. We included 24 trials involving 1142 participants in this update compared with 1 trial with 33 participants in the original version of the review from 2010. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 10 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We gratefully acknowledge Justin Clark and Joshua Cheyne for assistance with the searches, Jungho Park and Kyung Hwa Seo for assistance with eligibility screening and translation of two studies in Korean to English, Associate Professor Mark Jones for advice about data analysis, and Dr Laura Bergade for assistance with data extraction and cross‐checking.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL) search strategy (April 2016)
#1 [mh “Cerebrovascular Disorders”]
#2 [mh “Basal Ganglia Cerebrovascular Disease”]
#3 [mh “Brain Ischemia”]
#4 [mh “Carotid Artery Diseases”]
#5 [mh “Cerebrovascular Trauma”]
#6 [mh “Intracranial Arterial Diseases”]
#7 [mh “Intracranial Arteriovenous Malformations”]
#8 [mh “Intracranial Embolism and Thrombosis”]
#9 [mh “Intracranial Hemorrhages”]
#10 [mh Stroke]
#11 [mh “Vasospasm, Intracranial”]
#12 [mh “Vertebral Artery Dissection”]
#13 stroke* or post stroke or poststroke or post‐stroke or apoplex* or cerebral vasc* or cerebrovasc* or cva or SAH:ti,ab
#14 (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) near/5 (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*):ti,ab
#15 (brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) near/5 (hemorrhag* or haemorrhag* or hematoma* haematoma* or bleed*):ti,ab
#16 [mh Hemiplegia]
#17 [mh Paresis]
#18 [mh Brain Injuries]
#19 [mh Brain Injury, Chronic]
#20 hempar* or hemipleg* or paresis or paretic or brain injur*:ti,ab
#21 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#22 [mh Cognition Disorders]
#23 [mh Mild Cognitive Impairment]
#24 [mh Confusion]
#25 [mh Neurobehavioral Manifestations]
#26 [mh Memory Disorders]
#27 [mh Agnosia]
#28 agnosia or confusion or inattention:ti,ab
#29 [mh Cognition]
#30 [mh Arousal]
#31 [mh Orientation]
#32 [mh Attention]
#33 [mh Memory]
#34 [mh Perception]
#35 [mh Mental Processes]
#36 [mh Thinking]
#37 [mh Awareness]
#38 [mh Problem Solving]
#39 [mh Generalization (Psychology)]
#40 [mh Transfer (Psychology)]
#41 [mh Comprehension]
#42 [mh Impulsive Behavior]
#43 [mh Learning]
#44 (cogniti* or arous* or orientat* or attention* or concentrat* or memor* or recall or percept* or think* or sequenc* or judgment* or judgement* awareness or problem solving or generaliaztion or generalisation or transfer or comprehension or learning or mental process* or (concept near/5 formation) or executive function*) near/5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem*):ti,ab
#45 dysexecutive syndrome* or impulsive behaviour* or impulsive behaviour* or executive dysfunction*:ti,ab
#46 #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45
#47 [mh Occupational Therapy]
#48 [mh Rehabilitation]
#49 [mh Rehabilitation, Vocational]
#50 [mh Activities of Daily Living]
#51 [mh Self Care]
#52 [mh Self Efficacy]
#53 [mh Automobile Driving]
#54 [mh Transportation]
#55 [mh Task Performance and Analysis]
#56 [mh Work Simplification]
#57 [mh Leisure Activities]
#58 [mh Home Care Services]
#59 [mh Home Care Services, Hospital‐Based]
#60 [mh Recovery of Function]
#61 [mh Self‐Help Devices]
#62 [mh Work]
#63 [mh Therapy, Computer‐Assisted]
#64 [mh Reminder Systems]
#65 activit* near/3 "daily living":ti,ab
#66 ADL or EADL or IADL:ti,ab
#67 (remedial or compensatory) near/5 (approach* or therap* or rehabilitation):ti,ab
#68 occupational therap*:ti,ab
#69 (self or personal) near/5 (care or manage*):ti,ab
#70 (dressing or feeding or eating or cooking or housework or toilet* or bathing or shower* or mobil* or ambulat* or driving or leisure or recreation* or public transport* or computer) near/5 (train* or therap* or teach* or retrain* or assist* or practice* or skill* or task* or rehabilitat* or aid* or remedial or exercise* or educat* or intervention*):ti,ab
#71 meal near/3 prepar*:ti,ab
#72 (assistive or computer or alarm*) near/5 (device* or technology):ti,ab
#73 (cognit* or thinking or judgement) near/5 (training or remediation or workbook):ti,ab
#74 #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73
#75 #21 and #46 and #74
Appendix 2. MEDLINE search strategy
MEDLINE search strategy (Ovid) Revised April 2016
exp Cerebrovascular Disorders/ OR exp Basal Ganglia Cerebrovascular Disease/ OR exp Brain Ischemia/ OR exp Carotid Artery Diseases/ OR exp Cerebrovascular Trauma/ OR exp Intracranial Arterial Diseases/ OR exp Intracranial Arteriovenous Malformations/ OR exp Intracranial Embolism and Thrombosis/ OR exp Intracranial Hemorrhages/ OR exp Stroke/
OR exp Vasospasm, Intracranial/ OR exp Vertebral Artery Dissection/ OR exp Hemiplegia/ OR exp Paresis/ OR exp Brain Injuries/ OR exp Brain Injury, Chronic/
OR
(stroke* or post stroke or poststroke or post‐stroke or apoplex* or cerebral vasc* or cerebrovasc* or cva or SAH OR hempar* or hemipleg* or paresis or paretic or brain injur*).ti,ab.
OR
(brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) adj5 (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*).ti,ab.
OR
(brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) adj5 (hemorrhag* or haemorrhag* or hematoma* haematoma* or bleed*).ti,ab.
AND
exp Cognition Disorders/ OR exp Mild Cognitive Impairment/ OR exp Confusion/ OR exp Neurobehavioral Manifestations/ OR exp Memory Disorders/ OR exp Agnosia/ OR exp Cognition/ OR exp Arousal/ OR exp Orientation/ OR exp Attention/ OR exp Memory/ OR exp Perception/ OR exp Mental Processes/ OR exp Thinking/ OR exp Awareness/ OR exp Problem Solving/ OR exp "Generalization (Psychology)"/ OR exp "Transfer (Psychology)"/ OR exp Comprehension/ OR exp Impulsive Behavior/ OR exp Learning/
OR
(agnosia or confusion or inattention OR dysexecutive syndrome* or impulsive behaviour* or impulsive behaviour* or executive dysfunction*).ti,ab.
OR
(cogniti* or arous* or orientat* or attention* or concentrat* or memor* or recall or percept* or think* or sequenc* or judgment* or judgement* awareness or problem solving or generalization or generalisation or transfer or comprehension or learning or mental process* or (concept adj5 formation) or executive function*) adj5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem*).ti,ab.
AND
exp Occupational Therapy/ OR exp Rehabilitation/ OR exp Rehabilitation, Vocational/ OR exp Activities of Daily Living/ OR exp Self Care/ OR exp Self Efficacy/ OR exp Automobile Driving/ OR exp Transportation/ OR exp Task Performance and Analysis/ OR exp Work Simplification/ OR exp Leisure Activities/ OR exp Home Care Services/ OR exp Home Care Services, Hospital‐Based/ OR exp Recovery of Function/ OR exp Self‐Help Devices/ OR exp Work/ OR exp Therapy, Computer‐Assisted/ OR exp Reminder Systems/
OR
(activit* adj3 "daily living").ti,ab. OR (ADL or EADL or IADL).ti,ab. OR ((remedial or compensatory) adj5 (approach* or therap* or rehabilitation)).ti,ab. OR occupational therap*.ti,ab. OR ((self or personal) adj5 (care or manage*)).ti,ab. OR ((dressing or feeding or eating or cooking or housework or toilet* or bathing or shower* or mobil* or ambulat* or driving or leisure or recreation* or public transport* or computer) adj5 (train* or therap* or teach* or retrain* or assist* or practice* or skill* or task* or rehabilitat* or aid* or remedial or exercise* or educat* or intervention*)).ti,ab. OR (meal adj3 prepar*).ti,ab. OR ((assistive or computer or alarm*) adj5 (device* or technology)).ti,ab. OR ((cognit* or thinking or judgement) adj5 (training or remediation or workbook)).ti,ab.
AND
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or randomised.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.)
Appendix 3. Embase search strategy
Embase search strategy Revised April 2016
'cerebrovascular disease'/exp OR 'basal ganglion hemorrhage'/exp OR 'brain ischemia'/exp OR 'carotid artery disease'/exp OR 'cerebrovascular accident'/exp OR 'cerebral artery disease'/exp OR 'brain arteriovenous malformation'/exp OR 'thromboembolism'/exp OR 'brain hemorrhage'/exp OR 'brain vasospasm'/exp OR 'artery dissection'/exp OR 'hemiplegia'/exp OR 'paresis'/exp OR 'brain injury'/exp
OR
(stroke* or “post stroke” or poststroke or apoplex* or “cerebral vasc*” or cerebrovasc* or cva or SAH OR hempar* or hemipleg* or paresis or paretic or “brain injur*”):ti,ab
OR
((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or “middle cerebr*” or mca* or “anterior circulation” or “basilar artery” or “vertebral artery”) NEAR/5 (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab
OR
((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or “basal ganglia” or putaminal or putamen or “posterior fossa” or hemispher* or subarachnoid) NEAR/5 (hemorrhag* or haemorrhag* or hematoma* OR haematoma* or bleed*)):ti,ab
AND
'cognitive defect'/exp OR 'mild cognitive impairment'/exp OR 'confusion'/exp OR 'cognition'/exp OR 'memory disorder'/exp OR 'agnosia'/exp OR 'arousal'/exp OR 'orientation'/exp OR 'attention'/exp OR 'memory'/exp OR 'perception'/exp OR 'mental function'/exp OR 'thinking'/exp OR 'awareness'/exp OR 'problem solving'/exp OR 'learning'/exp OR 'comprehension'/exp OR 'impulsiveness'/exp
OR
(agnosia or confusion or inattention OR “dysexecutive syndrome*” or “impulsive behaviour*” or “impulsive behaviour*” or “executive dysfunction*”):ti,ab
OR
((cogniti* OR arous* OR orientat* OR attention* OR concentrat* OR memor* OR recall OR percept* OR think* OR sequenc* OR judgment* OR judgement* OR awareness OR 'problem solving' OR generalization OR generalisation OR transfer OR comprehension OR learning OR 'mental process*' OR 'concept formation' OR 'executive function*') NEAR/5 (disorder* OR declin* OR dysfunct* OR impair* OR deficit* OR disabilit* OR problem*)):ab,ti
AND
'occupational therapy'/exp OR 'rehabilitation'/exp OR 'vocational rehabilitation'/exp OR 'daily life activity'/exp OR 'self care'/exp OR 'self concept'/exp OR 'car driving'/exp OR 'traffic and transport'/exp OR 'task performance'/exp OR 'leisure'/exp OR 'home care'/exp OR 'convalescence'/exp OR 'self help device'/exp OR 'work'/exp OR 'computer assisted therapy'/exp OR 'reminder system'/exp
OR
(activit* NEAR/3 "daily living"):ti,ab or (adl or eadl or iadl):ti,ab or ((remedial or compensatory) NEAR/5 (approach* or therap* or rehabilitation)):ti,ab or occupational therap*:ti,ab or ((self or personal) NEAR/5 (care or manage*)):ti,ab or ((dressing or feeding or eating or cooking or housework or toilet* or bathing or shower* or mobil* or ambulat* or driving or leisure or recreation* or “public transport*” or computer) NEAR/5 (train* or therap* or teach* or retrain* or assist* or practice* or skill* or task* or rehabilitat* or aid* or remedial or exercise* or educat* or intervention*)):ti,ab or (meal NEAR/3 prepar*):ti,ab or ((assistive or computer or alarm*) NEAR/5 (device* or technology)):ti,ab or ((cognit* or thinking or judgement) NEAR/5 (training or remediation or workbook)):ti,ab
AND
'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR (random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEAR/1 blind* OR singl* NEAR/1 blind* OR assign* OR allocat* OR volunteer*):de,ab,ti
Appendix 4. CINAHL search strategy
CINAHL search strategy EBSCO April 2016
(MH "Cerebrovascular Disorders+") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Cerebral Ischemia+") OR (MH "Carotid Artery Diseases+") OR (MH "Intracranial Arterial Diseases+") OR (MH "Arteriovenous Malformations+") OR (MH "Intracranial Embolism and Thrombosis+") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke+") OR (MH "Vertebral Artery Dissections+") OR (MH "Hemiplegia+") OR (MH "Mobius Syndrome+") OR (MH "Brain Injuries+") OR stroke* or post stroke or poststroke or post‐stroke or apoplex* or cerebral vasc* or cerebrovasc* or cva or SAH OR hempar* or hemipleg* or paresis or paretic or brain injur* OR (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) N5 (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*) OR (brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) N5 (hemorrhag* or haemorrhag* or hematoma* haematoma* or bleed*) AND (MH "Cognition Disorders+") OR (MH "Cognition Disorders+") OR (MH "Confusion+") OR (MH "Neurobehavioral Manifestations+") OR (MH "Memory Disorders+") OR (MH "Agnosia+") OR (MH "Cognition+") OR (MH "Arousal+") OR (MH "Orientation+") OR (MH "Attention+") OR (MH "Memory+") OR (MH "Perception+") OR (MH "Mental Processes+") OR (MH "Thinking+") OR (MH "Problem Solving+") OR (MH "Transfer (Psychology)+”) OR (MH "Learning+") OR agnosia or confusion or inattention OR dysexecutive syndrome* or impulsive behaviour* or impulsive behaviour* or executive dysfunction* OR (cogniti* or arous* or orientat* or attention* or concentrat* or memor* or recall or percept* or think* or sequenc* or judgment* or judgement* awareness or problem solving or generalization or generalisation or transfer or comprehension or learning or mental process* or “concept formation” or executive function*) N5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem*) AND (MH "Occupational Therapy+") OR (MH "Rehabilitation+") OR (MH "Rehabilitation, Vocational+") OR (MH "Activities of Daily Living+") OR (MH "Self Care+") OR (MH "Self Efficacy+") OR (MH "Automobile Driving+") OR (MH "Transportation+") OR (MH "Task Performance and Analysis+") OR (MH "Work Redesign+") OR (MH "Leisure Activities+") OR (MH "Home Health Care+") OR (MH "Recovery of Function+") OR (MH "Assistive Technology Devices+") OR (MH "Work+") OR (MH "Therapy, Computer‐Assisted+") OR (MH "Reminder Systems+")
OR (activit* N3 "daily living") OR ADL or EADL or IADL. OR ((remedial or compensatory) N5 (approach* or therap* or rehabilitation)) OR occupational therap* OR ((self or personal) N5 (care or manage*)) OR ((dressing or feeding or eating or cooking or housework or toilet* or bathing or shower* or mobil* or ambulat* or driving or leisure or recreation* or public transport* or computer) N5 (train* or therap* or teach* or retrain* or assist* or practice* or skill* or task* or rehabilitat* or aid* or remedial or exercise* or educat* or intervention*)) OR (meal N3 prepar*) OR ((assistive or computer or alarm*) N5 (device* or technology)) OR ((cognit* or thinking or judgement) N5 (training or remediation or workbook))
AND
TX (allocat* random*) OR (MH "Quantitative Studies") OR (MH "Placebos") OR TX placebo* OR TX (random* allocat*) OR (MH "Random Assignment") OR TX (randomi* control* trial*) OR TX ( (singl* n1 blind*) or (singl* n1 mask*)) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) TX (clinic* n1 trial*) OR PT (“Clinical trial”) OR (MH "Clinical Trials+")
Appendix 5. PsycINFO search strategy
PsycINFO search strategy (Ovid)
exp Cerebrovascular Disorders/ OR exp Cerebral Ischemia/ OR exp Carotid Arteries/ OR exp Traumatic Brain Injury/ OR exp Cerebral Hemorrhage/ OR exp Cerebrovascular Accidents/ OR exp Subarachnoid Hemorrhage/ OR exp HEMIPLEGIA/ OR exp GENERAL PARESIS/
OR
(stroke* or post stroke or poststroke or post‐stroke or apoplex* or cerebral vasc* or cerebrovasc* or cva or SAH OR hempar* or hemipleg* or paresis or paretic or brain injur*).ti,ab.
OR
(brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) adj5 (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*).ti,ab.
OR
(brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) adj5 (hemorrhag* or haemorrhag* or hematoma* haematoma* or bleed*).ti,ab.
AND
Exp Cognitive Impairment/ OR exp MENTAL CONFUSION/ OR exp Memory Disorders/ OR exp Agnosia/ OR exp Cognition/ OR exp PHYSIOLOGICAL AROUSAL/ OR exp Attention/ OR exp Memory/ OR exp Perception/ OR exp Judgment/ OR exp Thinking/ OR exp Awareness/ OR exp Problem Solving/ OR exp Comprehension/ OR exp Impulsiveness/ OR exp LEARNING/
OR
(agnosia or confusion or inattention OR dysexecutive syndrome* or impulsive behaviour* or impulsive behaviour* or executive dysfunction*).ti,ab.
OR
(cogniti* or arous* or orientat* or attention* or concentrat* or memor* or recall or percept* or think* or sequenc* or judgment* or judgement* awareness or problem solving or generalization or generalisation or transfer or comprehension or learning or mental process* or (concept adj5 formation) or executive function*) adj5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem*).ti,ab.
AND
exp Occupational Therapy/ OR exp Rehabilitation/ OR exp VOCATIONAL REHABILITATION/ OR exp "Activities of Daily Living"/ OR exp Self Care Skills/ OR exp Self‐Efficacy/ OR exp Transportation/ OR exp Recreation/ OR exp Home Care/ OR exp Self Help Techniques/ OR exp Computer Assisted Therapy/
OR
(activit* adj3 "daily living").ti,ab. OR (ADL or EADL or IADL).ti,ab. OR ((remedial or compensatory) adj5 (approach* or therap* or rehabilitation)).ti,ab. OR occupational therap*.ti,ab. OR ((self or personal) adj5 (care or manage*)).ti,ab. OR ((dressing or feeding or eating or cooking or housework or toilet* or bathing or shower* or mobil* or ambulat* or driving or leisure or recreation* or public transport* or computer) adj5 (train* or therap* or teach* or retrain* or assist* or practice* or skill* or task* or rehabilitat* or aid* or remedial or exercise* or educat* or intervention*)).ti,ab. OR (meal adj3 prepar*).ti,ab. OR ((assistive or computer or alarm*) adj5 (device* or technology)).ti,ab. OR ((cognit* or thinking or judgement) adj5 (training or remediation or workbook)).ti,ab.
AND
control:.tw. OR random:.tw. OR exp treatment/
Appendix 6. NeuroBITE search strategy
NeuroBITE (previously PsycBITE)
Target area: Cognition/Mental (All)
Method: Randomised Controlled Trials
Neurological Group: Stroke / CVA (Cerebrovascular Accidents)
Age group: Adults
Appendix 7. OTseeker search strategy
OTseeker
[Diagnosis/Subdiscipline] like 'Stroke' AND [Method] like 'Randomised controlled trial'
Appendix 8. Registries search strategy
Search strategy for Clinicaltrials.gov:
cognition | Interventional Studies | Stroke | Adult, Older Adult
Search strategy for International Clinical Trials Registry Platform (ICTRP):
cogniti* AND stroke as condition in advanced search
Data and analyses
Comparison 1. Basic activities of daily living (BADL) performance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 BADL (postintervention) | 6 | 336 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [0.17, 4.22] |
| 1.1.1 Cognitive remediation approaches | 4 | 263 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [0.17, 4.35] |
| 1.1.2 Compensatory/adaptive approaches | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | 1.19 [‐6.98, 9.36] |
| 1.2 BADL (postintervention) sensitivity analysis | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | 2.04 [‐0.12, 4.19] |
| 1.3 BADL (follow‐up) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 At 3 months' follow‐up | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | 10.00 [‐0.54, 20.55] |
| 1.3.2 At 6 months' follow‐up | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | 11.38 [1.62, 21.14] |
Comparison 2. Other activities of daily living (ADL)/instrumental activities of daily living (IADL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 IADL (postintervention) | 2 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.94 [0.41, 1.47] |
| 2.2 Other ADL/IADL (postintervention) | 3 | 111 | Mean Difference (IV, Fixed, 95% CI) | 2.61 [0.10, 5.12] |
Comparison 3. Community reintegration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Community reintegration (self‐reported, postintervention) | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.35, 0.54] |
Comparison 4. Global cognitive function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Global cognitive performance (postintervention) | 11 | 542 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.40, 0.76] |
| 4.2 Global cognitive performance (sensitivity analysis, Park 2015 and Chen 2015 removed) | 9 | 432 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.16, 0.54] |
Comparison 5. Attention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Sustained visual attention (postintervention) | 10 | 463 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.47, ‐0.10] |
| 5.2 Sustained auditory attention (postintervention) | 4 | 169 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.22, 0.39] |
| 5.3 Sustained visual attention (follow‐up) | 3 | 171 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.47, 0.13] |
| 5.4 Sustained auditory attention (follow‐up) | 2 | 98 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.62, 0.18] |
| 5.5 Selective visual attention (Attentive Matrices) | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | 5.99 [1.87, 10.11] |
| 5.6 Selective visual attention (postintervention) | 4 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.17, 0.68] |
| 5.7 Selective visual attention (follow‐up) | 2 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.17, 0.90] |
| 5.8 Visual attention overall (postintervention) | 13 | 620 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.47, ‐0.15] |
| 5.9 Visual attention overall (follow‐up) | 5 | 293 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.55, ‐0.09] |
Comparison 6. Memory.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Working memory (postintervention) | 8 | 420 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.45 [0.26, 0.65] |
| 6.2 Working memory (Digit Span backwards) (postintervention) | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.50, 0.93] |
| 6.3 Working memory (Trail Making Test B) (follow‐up) | 3 | 243 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.43, 0.08] |
| 6.4 Working memory (follow‐up) | 4 | 272 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.40, 0.07] |
| 6.5 Immediate verbal memory span (postintervention) | 8 | 357 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.14, 0.56] |
| 6.6 Immediate spatial memory span (postintervention) | 7 | 292 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [0.03, 0.50] |
| 6.7 Immediate recall (postintervention) | 3 | 184 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.12, 0.46] |
| 6.8 Delayed recall (postintervention) | 3 | 184 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.05, 0.66] |
| 6.9 Memory span (follow‐up) | 3 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.11, 0.45] |
6.3. Analysis.

Comparison 6: Memory, Outcome 3: Working memory (Trail Making Test B) (follow‐up)
Comparison 7. Executive function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Non‐verbal reasoning (Raven's Colored Progressive Matrices) (postintervention) | 3 | 184 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐0.67, 1.60] |
| 7.2 Non‐verbal reasoning – standardised mean difference (postintervention) | 4 | 224 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.12, 0.68] |
| 7.3 Cognitive flexibility (postintervention) | 2 | 43 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.20, ‐0.80] |
| 7.4 Global executive functional performance (postintervention) | 6 | 318 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.41, 0.86] |
| 7.5 Global executive functional performance (sensitivity analysis, Frontal Assessment Battery, postintervention) | 5 | 238 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.30, 1.35] |
| 7.6 Executive functional performance overall (postintervention) | 11 | 550 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.31, 0.66] |
| 7.6.1 Cognitive remediation approaches | 9 | 480 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.21, 0.58] |
| 7.6.2 Compensatory/adaptive approaches | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.21 [0.69, 1.73] |
| 7.7 Self‐reported executive function (postintervention) | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐8.69, 1.35] |
| 7.8 Executive functional performance overall (follow‐up) | 3 | 195 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.02, 0.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akerlund 2013.
| Study characteristics | ||
| Methods |
Design: parallel, single‐site RCT Duration of trial: 31 months (March 2008 to December 2010) Unit of randomisation: rehabilitation outpatients of working age (18–65 years) in the postacute phase after a brain injury with identified WM deficits. Recruitment and allocation: of 331 rehabilitation outpatients of working age in the postacute phase after a brain injury, 203 people were screened for WM deficits. 121 met the WM inclusion criteria; 24 of these were excluded due to communication problems or being unfit for testing. Of the remaining number who accepted participation, 45 participants were randomised by lot to either to IG (n = 25) or CG (n = 20). 34 of these participants had stroke, 19 in IG and 15 in CG. |
|
| Participants |
Setting: metropolitan rehabilitation outpatient clinic Country: Sweden Sample size: 34 adults allocated (IG: 19; CG: 15), 29 analysed (gender of subsample with stroke not specified; original sample had 24 men (51%) and 23 women (49%)) Inclusion criteria: ≤ 5 digits/blocks forwards or ≤ 4 digits/blocks reversed in the WAIS‐III Digit Span and WAIS‐III‐NI Span board Exclusion criteria: inability to communicate, medical reasons according to the physician's health assessment (i.e. pronounced fatigue, pain, or depression) Age: mean: IG: 47.38 years; CG: 52.86 years Time since onset of stroke: mean: IG: 30.31 (SD 13.41) weeks; CG: 27.79 (SD 13.94) weeks Types of stroke: 66% of participants with stroke had a right hemisphere stroke, 41% had a left hemisphere stroke, and the rest bilateral. |
|
| Interventions |
Intervention group Brief name: Cogmed QM computerised training program Recipients: rehabilitation outpatients of working age in the postacute phase after a brain injury with identified WM deficits Why: to provide an evidence‐based remedial approach using a computerised training program with intensive repetition of tasks for visuospatial and verbal WM that are adapted for the participant at a challenging performance level. What (materials): Cogmed WM training. Stockholm: Pearson Assessment AB. 2006. Available online at: www.cogmed.com/. Online version requires a stable broadband Internet connection, preferably 0.5 Mbit/second or better. What (procedures): the Cogmed training program in addition to usual rehabilitation routines. The training programme included a battery of visuospatial and verbal auditory WM tasks:
All parts of the battery must be trained at each session, 90 trials each day. The tasks are introduced with a voice‐over transmitted by the computer's speaker. The person responds by localising and remembering multiple stimuli at the same time. The tasks have a unique sequencing order in each trial and short delays that require the representation of stimuli to be held in the person's WM. Who provided: an occupational therapist certified as coach for the Cogmed QM training, confirmed in personal communication with the authors. According to the Cogmed website, Cogmed is "currently used by psychologists, speech pathologists, occupational therapists and other clinical specialists working with individuals with attention and learning difficulties" and requires providers to undertake Cogmed Coach Training and Accreditation courses, either by self‐paced online coursework that is free with the purchase of a Cogmed Coach Starter Pack or attending a 1‐day face‐to‐face course offered around the world. How: the person can work individually and independently using the online software on a personal computer. Participants were able to ask staff for assistance and were provided with personal and individual feedback once per week, by a specially trained coach. Where: in a quiet room in the occupational therapy department. When and how much: 30–45 minutes per session, 5 days per week for 5 weeks (25 sessions) (12.5–18.75 hours in total) Tailoring: the difficulty level of the tasks adapts according to the individual's performance. The software included direct reinforcement via scores and positive verbal feedback. Participants were able to ask staff for assistance. Once per week the coach gave personal and individual feedback about results. Modification: none reported How well (planned): there appeared to be no formal assessment of fidelity. The program was reportedly used according to the guidelines and that the coaches provided input to help participants "adhere to the training" but no evaluation of this was reported. How well (actual): not reported. The researchers commented that the training was tiring and that the schedule of 5 days per week for 5 weeks and the need to attend the outpatient clinic for the training was challenging for many of the participants and was the reason for dropouts. Comparator group Brief name: usual rehabilitation Recipients: rehabilitation outpatients of working age in the postacute phase after a brain injury with identified WM deficits Why: provide usual rehabilitation services What: outpatient rehabilitation in accordance with the usual routines at the clinic, based on their rehabilitation needs Who: several different professionals in the team How: face‐to‐face but not further specified Where: rehabilitation clinic When and how much: 5 weeks Tailoring: rehabilitation services based on individual needs Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Other
Methods of data collection: demographic data were collected from the medical chart; the AMPS was administered by a clinical occupational therapist trained, calibrated, and certificated in the administration of the AMPS; other information not provided. Data collection time points: baseline, after the 5‐week intervention, and at 3 months' follow‐up |
|
| Notes |
Funding: no Conflict of interest: none Published trial protocol: no Trial registration: no Ethics approval: no Author contact: further information and stroke‐specific data from authors requested and provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "randomized by lot". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The IG received Cogmed training from trained occupational therapist coaches and the CG received regular occupational therapy and rehabilitation services but with computerised CT excluded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported and there was no ethical process of informed consent; however, the participants completed the outcome measures and knowledge of receiving the CT or not could have impacted outcome measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For the whole sample, there was 15% attrition after training and 19% after 18 weeks with missing outcome data balanced in numbers across groups. However, reasons for attrition were not provided separately for both groups. About half of the reasons appeared unrelated to outcome, and the other half were unclear. We used data provided by authors for the subsample of stroke participants for which attrition was unclear. |
| Selective reporting (reporting bias) | Low risk | No protocol located but all outcomes reported across 2 papers. |
| Other bias | Low risk | No other identifiable bias. |
Barker‐Collo 2009.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 18 months (dates not specified) Unit of randomisation: inpatient adult survivors of incident stroke with a confirmed attention deficit Recruitment and allocation: 334 survivors of stroke (all pathological subtypes) were approached within 2 weeks after stroke. 107 (32%) consented and were checked for eligibility. Of these 95 (88.8%) were eligible for attention screening, 84 of these (88%) were assessed as having deficits and 78 of these (5 withdrew and 1 moved) were randomised by stratified minimisation (by age, gender, ethnicity (European, non‐European) and Barthel Index (≤ 18, > 18) to 2 groups, APT (n = 38) and SC (n = 40). |
|
| Participants |
Setting: 2 metropolitan hospitals Country: New Zealand Sample size: 78 adults, 60% men; APT: 38; SC: 40 Inclusion criteria: 1 SD below the normative mean on any of the following tests: Bells Test, IVA‐CPT, TMT‐A and TMT‐B, and 2 slowest Paced Auditory Serial Addition Test trials; newly diagnosed stroke (first‐ever‐in‐a‐lifetime stroke) Exclusion criteria: inability to give informed consent, severe cognitive deficit precluding participation (MMSE < 20), medical instability or condition that could impact results (e.g. dementia), not fluent in English as required for standardised assessment Age: mean: APT: 70.2 (SD 15.6) years; SC: 67.7 (SD 15.6) years Time since onset of stroke: mean: APT: 18.48 (SD 11.95) days; SC: 18.58 (SD 7.62) days Types of stroke: 61.5% had had an ischaemic stroke Site of lesion: APT: left hemisphere 14 (43.8%), right 15 (46.9%), other 3 (9.1%); SC: left hemisphere 25 (58.1%), right 17 (39.5%), other 1 (2.3%) |
|
| Interventions |
Intervention group Brief name: APT Recipients: inpatient adult survivors of incident stroke with a confirmed attention deficit Why: to provide a theoretically based, hierarchical, and multilevel treatment, involving cognitive exercises for remediation and improvement of aspects of attention including sustained, selective, alternating, and divided attention (Sohlberg 1987; Sohlberg 2001). Sohlberg 2001 outlined 6 treatment principles for attention process training, including theoretically grounded, hierarchically organised, providing sufficient repetition, based on client performance data, with active facilitation of generalisation throughout treatment, and providing a flexible and adaptable format. What (materials): APT package including paper and pencil tasks, set of CDs including auditory CDs that produced auditory stimuli as well as a distraction (like 'white noise') to overlay some tasks where selective attention was needed. For the visual tasks, this distraction was produced by using acetate overlays with patterns on them (Barker‐Collo 2009). The latest version of APT program can be purchased at: lapublishing.com/apt-attention-process-training/, which provides details of latest APT software and training programme; materials include a manual and tracking sheets for exercises. See also Sohlberg 1987 for an appendix of materials used for training each aspect of attention and Sohlberg 2001 for an outline of an APT programme that included computer activities, auditory tapes, and pen‐and‐paper tasks. What (procedures): provider used a hierarchy of treatment tasks targeting different aspects of attention starting at sustained attention then progressing to selective, alternating, and divided attention. Each task was considered "mastered" once the client was able to complete the task with no more than 1 error (Barker‐Collo 2009). Current APT programme involves "a set of drill based, hierarchically organized exercises that tap different domains of attention that are matched to the client's impairment profile and administered repetitively. They are paired with generalization real world, individualized exercises that are selected to promote generalization" (Barker‐Collo 2009). Sohlberg 2001 has examples of tasks in the APT computer program for addressing each of these aspects and appendices with example recording protocols and a case study. Examples of tasks include listening for target words or sequences on auditory attention tapes, mental math activities, putting words in alphabetical order, placing a visual distractor (e.g. a plastic overhead sheet with distractor lines) over the top of a paper‐and‐pencil activity. Who provided: a registered clinical neuropsychologist provided APT training in this study; APT could be administered by neuropsychologists, occupational therapists, speech language therapists, and other rehabilitation specialists. There were no training requirements in addition to the manual. How: face‐to‐face individual sessions. Sohlberg 2001 reported that delivery should be flexible and adaptable including delivery to individuals or groups. Where: in hospital prior to discharge and then in clients' primary residences after discharge; Sohlberg 2001 reported that delivery should be flexible and adaptable including delivery in clinics or at home. When and how much: up to 30 hours of individual APT conducted for 1 hour on weekdays for 4 weeks (mean 13.5 (SD 9.44) hours) Tailoring: because of issues such as fatigue, a 30‐hour maximum was set in this study. Sohlberg 2001 described the hierarchical nature of the programme tasks and how clinicians use client performance data to tailor the intervention. The hierarchy of programme tasks place increasing demands on attentional control and WM. Client performance data were used to make treatment decisions, such as when to start, stop, or modify a programme. For example, clinicians examine the participants' error profiles to assess where errors were occurring, such as at the beginning or end of a task, reflecting a different attentional demand and adjust the training tasks accordingly. Graphs of performance were shown to the client to provide objective and powerful feedback. Modification: none reported How well (planned): none reported in the paper and not formally assessed; the author advised that "there was tracking of the order in which tasks were administered to ensure that the protocol was adhered to". How well (actual): none reported Comparator group Brief name: SC Recipients: inpatient adult survivors of incident stroke with a confirmed attention deficit Why: to provide standard rehabilitation care poststroke. What: not reported Who: not reported How: not reported except standard inpatient care Where: metropolitan hospital When and how much: not reported Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Other
Methods of data collection: a trained assessor blind to randomisation administered and scored the assessments using standard procedures. Assessments lasted 2.5 hours, occurring over 2 sessions if required. Data collection time points: baseline, 5 weeks (1‐week postintervention), and 6 months |
|
| Notes |
Funding: yes (supported by the New Zealand Health Research Council (HRC Refs 06/063C and 07/070C) and a National Heart Foundation (New Zealand) Fellowship Conflict of interest: none Published trial protocol: not reported Trial registration: yes (ACTRN12607000045415) Ethics approval: yes Author contact: further details about intervention implementation were provided and unpublished data (means and SDs) supplied. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was concealed using an online internet randomization service whose procedures ensure enrollment and check eligibility before allowing randomization. Stratified minimization randomization was used to ensure the balance for possible prognostic factors … across the groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "Implementation of the randomization sequence was via secured online contacting of the treating clinician, who had no access to assessment data. Randomization information was not accessible to any other study staff during the study". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists not blinded. Quote: "All APT sessions were administered by a registered clinical neuropsychologist, who was the only member of the study team (e.g. named investigators, statisticians, data management, assessors) who did not remain blind to randomization status throughout the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were repeated at 5 weeks and 6 months by a trained assessor blind to randomization … Randomization information was not accessible to any other study staff during the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition overall provided, not numbers lost for each group. Reasons for losses not provided separately for both groups. Used last observation carried forward for missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes included in this review were reported as per the protocol. |
| Other bias | Low risk | No other identifiable bias. |
Bo 2019.
| Study characteristics | ||
| Methods |
Design: parallel RCT with 4 groups (note: data only for 2 groups included in this review: CG and CT groups) Duration of trial: about 11 months (first recruitment 10 February 2017 to 22 January 2018 (last follow‐up)) Unit of randomisation: outpatients < 6 months poststroke with vascular cognitive impairment (aged > 18 years) Recruitment and allocation: of 260 people with stroke assessed for eligibility, 225 people were eligible and randomised to 1 of 4 groups: PE + CT; PE; CT; and CG; as noted above, we included only data from the CT (n = 57) and CG (n = 57) |
|
| Participants |
Setting: rehabilitation centre Country: China Sample size: 114 allocated, 92 adults analysed; CT: 45 (53.33% men); CG: 47 (57.44% men) Inclusion criteria: aged > 18 years; medically stable; < 6 months poststroke; able to understand and follow verbal instructions; without severe somatic diseases or mental disorders, including anxiety and depression; without visual or auditory disturbances in recent months; met the diagnostic criteria for vascular cognitive impairment Exclusion criteria: motor deficits, non‐stroke‐related neurological impairments, clinically determined as unsafe for physical activity Age: mean: CT: 67.51 (SD 2.24) years; CG: 64.36 (SD 2.31) years Time since onset of stroke: not reported; however, inclusion criterion of < 6 months poststroke Types of stroke: not reported Site of lesion: not reported |
|
| Interventions |
Intervention group Brief name: CT (CO) Recipients: outpatients < 6 months poststroke with vascular cognitive impairment (aged > 18 years) Why: to provide an effective and safe alternative to established drugs to decrease cognitive impairments in people with stroke in the form of a non‐pharmacological intervention of CT. What (materials): COGPACK programme, developed for neurorehabilitation (Marker Software, www.markersoftware.com) delivered on up to 20 tablet computers with touch screens "to avoid training difficulties in computer novices"; 12 of possible 64 exercises were selected, including 4 tasks of memory ('memory for route', 'memory for signs', 'memory for pattern', 'memory for scene'), 4 tasks of execution ('mental arithmetic', 'logical block', 'shortest way', 'continue a series'), and 4 attention and speed tasks ('scanning', 'catch', 'steer', 'assembly line'). What (procedures): supervised CT in groups using tablet computers Who: experienced therapists with exercise physiology or clinical psychology backgrounds provided the interventions for the 4 groups. However, the CT group used the COGPACK programme, which is a commercially available programme and which, according to the website, can be used in occupational therapy as a "concentration, performance and motivation aid". How: supervised group (up to 20) Where: in the rehabilitation centre, further details not provided When and how much: 60 minutes, 3 times weekly, for 12 weeks (36 hours in total) Tailoring: group training was supervised, so presumably individual and group assistance was provided as needed. Modifications: none reported How well (planned): not reported How well (actual): not reported Comparator group Brief name: usual care and watched 45‐minute video documentaries 3 times per week, for 12 weeks Recipients: outpatients < 6 months poststroke with vascular cognitive impairment (aged > 18 years) Why: to provide usual rehabilitation services to patients poststroke and an equivalent dose through the documentary watching. What: usual care and video documentaries Who provided: not described, presumably usual care rehabilitation staff How: face‐to‐face Where: rehabilitation centre When and how much: length of usual care plus 45‐minute video documentaries 3 times per week, for 12 weeks Tailoring: not reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Methods of data collection: 4 research assistants blinded to group allocation performed a battery of standardised measurements. Data collection time points: baseline, 12 weeks (postintervention), and 6 months after training ended |
|
| Notes |
Funding: none. Authors declared they received no financial support for the research, authorship, or publication of the study. Conflict of interest: the authors declared no potential conflicts of interest with respect to the research, authorship, or publication of the study. Published trial protocol: none reported Trial registration: ISRCTN16009172 Ethics approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "used the on‐line Research Randomizer to generate allocation sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent research assistant who was not involved in the study held the random lists of number" and "contacted the participants". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Incomplete blinding. Single‐blind (investigator‐blinded, not participant blinded); it is not clear if the "investigator" is the researcher or the therapist, may have been only the outcome assessors (research assistants). Therefore, it may have been possible for therapists to discern which therapy a participant was receiving and for the participants to know which group they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Four research assistants performed a battery of standardized measurements … The research assistants were blind to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up and the reasons for losses were reported and these were balanced between groups. However, 12 (21%) missing from CT group and 10 (17.5%) from CG. |
| Selective reporting (reporting bias) | Low risk | Checked against protocol and all outcomes assessed and reported as planned. |
| Other bias | Low risk | No other identifiable bias. |
Carter 1983.
| Study characteristics | ||
| Methods |
Design: parallel RCT; 1988: post‐hoc analysis of data from Carter 1983 Duration of trial: not reported Unit of randomisation: inpatient adults with a clinically defined stroke and cognitive impairment Recruitment and allocation: 33 people with acute stroke were randomised to 2 groups, IG (n = 16) and CG (n = 17) |
|
| Participants |
Setting: metropolitan community hospital Country: USA Sample size: 33 adults (48% men); IG: 16; CG: 17; n = 28 analysed for ADL, n = 25 analysed for time judgement Exclusion criteria: people with tumours, extensive bilateral damage, or prior brain damage Age: mean: IG: 70.5 (SD 11.4) years; CG: 73.4 (SD 9.2) years Types of stroke: neurological Severity Scores mean (of 60): IG: 29.4 (SD 3.9); CG: 28.5 (SD 6.4) Site of lesion: IG: left hemisphere 9 (56%), right 7 (44%); CG: left hemisphere 9 (53%), right 8 (47%) Days from admittance to stroke programme to pretest: mean: IG: 4.8 (SD 1.6) days; CG: 4.6 (SD 2.6) days |
|
| Interventions |
Intervention group Brief name: cognitive skills remediation training Recipients: inpatient adults with cognitive impairments after acute stroke Why: to provide formal cognitive remediation training to acute patients within 1 week poststroke that included "continuous reinforcement, immediate feedback, cuing, gradually increasing … difficulty level and stressing the importance of the skills being taught to activities of daily living" (Carter 1983). What (materials): Thinking Skills Workbook (Carter 1980) for pen‐and‐paper tasks requiring: visual scanning, visual‐spatial or time judgement; early versions cited in papers, latest edition (Languirand 2014) provided pen‐and‐paper pre‐ and post‐tests and tasks covering various areas of cognition including paying attention and reading, concentrating on detail, listening, scheduling and time management, memory in everyday living, sorting and classifying, sequencing and logic, verbal skills, maths skills. What (procedures): based on pretest of the 3 main areas of interest, visual scanning, visual‐spatial, or time judgement, trained research assistants provided 1‐to‐1 training sessions in any of these areas where pretest performance was < 80%, in additional to usual rehabilitation. Who provided: trained research assistants provided the intervention in the study; Languirand 2014 workbook stated that it was for use by professional rehabilitation staff, paraprofessionals, or family members. How: face‐to‐face and individually Where: stroke rehabilitation unit; Languirand 2014 workbook recommended a quiet private room with minimal distractions. When and how much: 30–40 minutes, 3 times per week for a mean of 3–4 weeks (i.e. up to 8 hours in total); the testing and training took place between 9.00 a.m. and 11.45 a.m. before or after other stroke programme activities; Languirand 2014 workbook recommended session length of 25–35 minutes, twice per week for 4–6 weeks Tailoring: not reported except that training was given only in areas where the participant scored < 80%. Modifications: none reported How well (planned): only that the research assistants were trained in activities from the workbook. How well (actual): not reported Comparator group Brief name: rehabilitation as usual Recipients: inpatient adults with cognitive impairments after acute stroke Why: to provide rehabilitation services poststroke What: routine stroke programme activities, including social work consultations, occupational therapy, physical therapy, speech therapy, family visits, and interaction with rehabilitation nursing staff Who: occupational therapists, physical therapists, speech therapists, social workers, and rehabilitation nursing staff How: face‐to‐face Where: stroke rehabilitation unit When and how much: mean length of stroke programme was 3–4 weeks Tailoring: not reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Other
Methods of data collection: attempts were made for blinded pre‐ and postassessment by trained trainers and testers not involved in stroke programme. Data collection time points: before and after training (baseline and 3–4 weeks after baseline) |
|
| Notes |
Funding: yes (research grant) Conflict of interest: not reported Published trial protocol: no Trial registration: no Ethics approval: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Description provided was "randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The stroke program staff was not informed of group assignment for the patients in this project, or of the experimental nature of the project … However, because of the physical layout of the stroke unit, at times it was possible for the tester to see which patients were given training by the other assistant". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Incomplete blinding. Quote: "For the major part of the study, an experimental blind testing procedure was used … However … at times it was possible for the tester to see which patients were given training by the other assistant". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall improvement scores are provided for the IG (n = 16) and CG (n = 17), suggesting that there was no attrition. In the secondary analysis, data from 28/33 participants was available for the Barthel Index with attrition equal across groups. |
| Selective reporting (reporting bias) | High risk | Post‐hoc analysis of ADLs reported in Carter 1988 but not referred to in Carter 1983; Carter 1988 also reported auditory attention, digit span, verbal memory, abstract reasoning, and verbal comprehension (that are not reported in Carter 1983). |
| Other bias | Low risk | No other identifiable bias. |
Chen 2015.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 8 months (June 2014 to January 2015) Unit of randomisation: rehabilitation inpatients with stroke and cognitive dysfunction Recruitment and allocation: 80 inpatients with stroke were prospectively recruited and randomised by computer to IG (n = 40) or CG (n = 40). |
|
| Participants |
Setting: inpatient ward of a rehabilitation centre Country: China Sample size: 80 adults, 65% men; IG: 40; CG: 40 Inclusion criteria: diagnostic criteria of stroke according to 4th National Cerebrovascular Disease Congress 1995 and confirmed by computer tomography or magnetic resonance imaging, first stroke onset, stroke chronicity ≤ 3 months, no signs of moderate brain atrophy or leukaraiosis, no field vision defect of visual neglect; MoCA < 26, no impairment of consciousness, no history of psychological diseases, able to provide written informed consent. Exclusion criteria: "mental retardation", mental diseases or coma; severe vision or auditory impairments, aphasia; severe cardiac, lung, or kidney function impairments; respiratory failure; tumour; drug or alcohol abuse Age: mean: 57.74 (SD 8.5) years; IG: 58 (SD 9) years; CG: 55 (SD 8) years Time since onset of stroke: n: 1–2 months: 38 (48%), 2–3 months: 27 (34%), ≥ 3 months: 15 (19%) Types of stroke: 71% had infarction stroke Site of lesion: 45% right, 39% left, 16% bilateral |
|
| Interventions |
Intervention group Brief name: BrainHQ Recipients: adult rehabilitation inpatients within 3 months of stroke with cognitive dysfunction/executive disorder Why: to provide computer‐based training to improve cognitive and executive functioning in addition to regular or standard rehabilitation and therapy. What (materials): BrainHQ (Posit Science) (www.brainhq.com) computer‐based training accessed via a computer with Internet access: Double Decision, Target Tracker, Hawk Eye and Visual Sweep programs. Double Decision: trains attention – more details at www.brainhq.com/why-brainhq/about-the-brainhq-exercises/attention/double-decision Target Tracker: trains executive function – more details at www.brainhq.com/why-brainhq/about-the-brainhq-exercises/attention/target-tracker Hawk Eye: trains memory – more details at www.brainhq.com/why-brainhq/about-the-brainhq-exercises/brainspeed/hawk-eye Visual Sweeps: trains spatial orientation – more details at www.brainhq.com/why-brainhq/about-the-brainhq-exercises/brainspeed/visual-sweeps What (procedures): in addition to "regular/standard occupational therapy, physical therapy, TENS, cognitive rehabilitation training, and acupuncture" BrainHQ computer‐based training was provided, mainly using the 4 games of BrainHQ listed above. Each game included 10 levels, increasing in difficulty and graded by different shapes, colours, and interferences. In addition, each level had 3 different backgrounds. Each participant had a practice session at the enrolment of the study. The study researcher chose the suitable model for training to ensure the safety of the participants with hemiplegia. The first to fourth week of training all included Double Decision, Target Tracker, Hawk Eye, and Visual Sweep games. Who provided: not specified although the researchers (who appear to be from nursing and rehabilitation backgrounds) provided the demonstration and training and accompanied the participant during training to ensure smooth training. BrainHQ training was completed in addition to regular occupational therapy and other therapies. How: not specified but presumably face‐to‐face and individually Where: inpatient ward of a rehabilitation centre When and how much: 5 sessions per week of 30 minutes per session for 4 weeks (10 hours in total); usually between 9 a.m. and 10 a.m., but depended on participant's schedule of other treatments. Week 1: choice of sublevel of Double Decision; week 2: Target Tracker, week 3: Hawk Eye, week 4: Visual Sweeps. Tailoring: the difficulties of each game were individually adjusted according to the participant's ability. Each game included 10 levels, increasing in difficulty. Each level was graded by different shapes, colours, and interferences. The levels of difficulty were raised by increasing the similarity of shapes and colours and by increasing the number of interferences. In addition, each level had 3 different backgrounds. Based on the participant's correct or incorrect responses, the time of the target items shown on the screen decreases or increases. The system automatically adjusts the difficult according to participant's progress. Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: usual rehabilitation Recipients: rehabilitation inpatients with stroke and cognitive dysfunction/executive disorder Why: to provide usual rehabilitation services What: regular/standard occupational therapy, physical therapy, cognitive rehabilitation training, and acupuncture, including TENS, balance co‐ordination training, gait training Who provided: not stated; however, presumably included occupational therapists, physiotherapists, and nurses How: not reported but implied individually and face‐to‐face Where: inpatient ward of a rehabilitation centre When and how much: 5 sessions per week of 30 minutes per session for 4 weeks (10 hours) Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary:
Methods of data collection: questionnaires were administered by researchers 1‐to‐1; no reporting of blinding of outcome assessment or not. Data collection time points: baseline and after the 4‐week training programme |
|
| Notes |
Funding: not reported Conflict of interest: authors declared no conflicts of interest. Published trial protocol: none located Trial registration: not reported Ethics approval: yes Data: we translated Li 2016 to English and we converted medians and interquartile ratios from both papers. Author contact: further information about nature of the reported data obtained from the research team. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[Participants] were randomly assigned to the intervention group and the control group using a computer". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; however, participants would have been provided with an information sheet that explained about the nature of the study. The participants would have been able to determine if they were receiving a computerised intervention or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No reporting of any blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data of dropouts reported. |
| Selective reporting (reporting bias) | Low risk | No protocol but all outcomes appear to be reported. |
| Other bias | Low risk | No other identifiable bias. |
Cho 2015.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 2 months (December 2013 to January 2014) Unit of randomisation: people with hemiparetic stroke onset within 3 months to 1 year Recruitment and allocation: recruited successively from 30 hospitalised stroke patients who were receiving occupational and physical therapy and randomised to the either CACR (n = 12) or CG (n = 13). |
|
| Participants |
Setting: 1 general hospital Country: Republic of Korea Sample size: 25 adults, 64% men; CARC: 12 (58% men); CG: 13 (69% men) Inclusion criteria: hemiparetic stroke of onset within 3 months to 1 year, able to follow verbal instructions, communicate to a certain level, able to perform all tests and had experienced light cognitive function failures that scored 18–23 on the MMSE. Exclusion criteria: diplegia, had never attended a school, were biased, or had received CACR within past year. Age: mean: CARC: 60.0 (SD 4.7) years; CG: 63.7 (SD 6.3) years Time since onset of stroke, mean: CARC: 5.3 (SD 2.3) months; CG: 6.0 (SD 2.2) months Types of stroke: hemiplegic only description provided Site of lesion: n (right/left hemisphere): CARC: 9/3; CG: 8/5 |
|
| Interventions |
Intervention group Brief name: CARC using RehaCom software Recipients: adults with stroke within 3–12 months poststroke with cognitive dysfunction Why: to provide objective CT based on neuropsychological patterns to stimulate damaged location of the brain. What (materials): RehaCom software (Korean version): the awakening, reactivity, attention and concentration, simultaneous attention, and selective attention programs; see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer; joystick and touch screen input devices; optional reaction board. What (procedures): CACR in addition to occupational therapy and physical therapy Who provided: 2 "expert therapists" provided the CARC and traditional rehabilitation therapy. The RehaCom website states that the software is used "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals, and clinics. How: not described but presumably face‐to‐face and individually Where: not specified but within general hospital When and how much: 30 minutes per day, 5 days per week, for 6 weeks (total of 15 hours) (in addition to usual occupational therapy and physical therapy) Tailoring: feedback on the result during and after the treatment was provided and training occurred according to each participant's functional ability; the participants could complete the training using a reaction board while seated and watching the screen. Modifications: none reported in paper How well (planned): not reported How well (actual): not reported Comparator group Brief name: traditional rehabilitation therapy Recipients: adults with stroke with cognitive dysfunction Why: to provide rehabilitation services to people poststroke What: occupational therapy and physical therapy. Exercise was prescribed and supervised by 2 experienced physiotherapists Who provided: occupational therapists and physiotherapists How: face‐to‐face Where: 1 general hospital When and how much: 30 minutes 5 times per week for 6 weeks (total of 15 hours) Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Methods of data collection: the 4 tests were performed in order from the easiest to the hardest, and they were performed by everyone in the same order. Data collection time points: baseline and 6 weeks (post‐treatment) |
|
| Notes |
Funding: not reported Conflict of interest: not reported Published trial protocol: not reported Trial registration: not reported Ethics approval: quote: "All of the protocols used in this study were approved by Gachon University. Before beginning the study, the procedures, risks and benefits were explained to all of the participants, who gave their informed consent". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated "randomly allocated" in the abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants signed a written consent form after receiving a full explanation of the expected result and adverse effects of the study. 2 expert therapists provided the CACR group and the CG with traditional rehabilitation therapy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A computerised neurocognitive function test was used to assess relevant cognitive outcomes, providing a level of objective assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods were reported in the results. |
| Other bias | Low risk | No other identifiable bias. |
Cho 2016.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 6 weeks (dates not specified) Unit of randomisation: people with hemiparetic stroke onset within 3 months to 1 year Recruitment and allocation: eligible people from among 48 stroke inpatients who were undergoing physical and occupational therapy were randomised to 1 of 3 groups, NFB (not included in this review), CACR (n = 14), and CG (n = 16). |
|
| Participants |
Setting: 1 general hospital Country: Republic of Korea Sample size: 30 adults, 53% men; CARR: 14 (64% men); CG: 16 (48% men) Inclusion criteria: hemiparetic stroke of onset within 3 months to 1 year, able to follow verbal instructions and to communicate, experienced mild cognitive deficit (18–23 on the MMSE), able to perform all tests Exclusion criteria: if had never attended a school or had received CACR within the past year Age: mean: CARC: 63.0 (SD 5.4) years; CG: 64.0 (SD 8.8) years Time since onset of stroke: mean: CARC: 5.1 (SD 2.2) months; CG: 6.5 (SD 1.5) months Types of stroke: not described, except hemiplegic Site of lesion: not reported |
|
| Interventions |
Intervention group Brief name: CARR using RehaCom software Recipients: adults with stroke within 3–12 months poststroke with cognitive dysfunction Why: to improve problem‐solving ability through use of games or other computer‐based programs in a manner that allowed different levels of task difficulty for the participant. What (materials): RehaCom software (Korean version): attention, concentration, and memory programs; see hasomed.de/en/products/rehacom/. The software is available in 27 languages at no extra cost; computer; monitor, keyboard. What (procedures): CACR in addition to occupational therapy and physical therapy. Who provided: 2 "expert therapists" provided the CACR and traditional rehabilitation therapy. The RehaCom website states that the software is used "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals, and clinics. How: face‐to‐face and individually (presumably) Where: not specified but within hospital When and how much: 30 minutes per day, 5 days per week, for 6 weeks (total of 15 hours) (in addition to usual occupational therapy and physical therapy) Tailoring: training at different levels of task difficulty according to the functional level of the participant Modifications: none reported How well (planned): not reported How well (actual): not reported Comparator group Brief name: traditional rehabilitation therapy Recipients: adults with stroke with cognitive dysfunction Why: to provide rehabilitation services to people poststroke. What: occupational therapy and physical therapy Who provided: occupational therapists and physiotherapists How: face‐to‐face Where: 1 general hospital When and how much: 30 minutes 5 times per week for 6 weeks (total of 15 hours) Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Methods of data collection: data collection not described except that FIM Motor and Cognitive subtotal and FIM Total scores were calculated. Data collection time points: baseline and 6 weeks (post‐treatment) |
|
| Notes |
Funding: not reported Conflict of interest: not reported Published trial protocol: not reported Trial registration: not reported Ethics approval: yes "approved by the Institutional Review Board of Gachon University" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could discern which group they were in and therapist could also. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods were reported in the results; no protocol reported. |
| Other bias | Low risk | No other identifiable bias. |
De Luca 2018.
| Study characteristics | ||
| Methods |
Design: single‐blind RCT Duration of trial: 2 years and 4 months (January 2013 to May 2015) Unit of randomisation: adults receiving rehabilitation with ischaemic or haemorrhagic stroke in the chronic phase (3–6 months after acute neurological event). Recruitment and allocation: people diagnosed to need cognitive rehabilitation were enrolled and assigned in order of recruiting to intervention group of CCR (IG) (n = 20) or traditional cognitive rehabilitation (CG) (n = 15). |
|
| Participants |
Setting: cognitive rehabilitation institute Country: Italy Sample size: 35 adults; 51.4% men; IG: 20; CG: 15 Inclusion criteria: diagnosis of vascular brain injury of either haemorrhagic or ischaemic aetiology (the latter involving the middle cerebral artery); presence of moderate cognitive impairment, i.e. MMSE score 12–20; absence of severe spasticity with an Ashworth Scale < 3; absence of disabling sensory alterations (i.e. hearing and visual loss), and severe medical and psychiatric illness according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition and International Classification of Diseases‐10. Exclusion criteria: severe spasticity; disabling sensory alterations, e.g. hearing and visual loss, severe medical and psychiatric illness Age: mean: 43.1 (SD 16.8) years Time since onset of stroke: mean: 3.5 (SD 2.0) months Types of stroke: ischaemic: 24; haemorrhagic: 11 Site of lesion: n: cortical right: 11; subcortical right: 10; cortical left: 8; subcortical left: 6 |
|
| Interventions |
Intervention group Brief name: ERICA PC training Recipients: adults with chronic phase of stroke (3–6 months) Why: to implement CT in 5 cognitive domains of attention process, memory abilities, spatial cognition, and verbal and non‐verbal executive functions. What (materials): computer‐based ERICA software training (www.erica.giuntios.it/it/) in Italian; personal computer What (procedures): traditional cognitive rehabilitation plus computer‐based ERICA software training provided by a therapist who provided exercises with a growing hierarchy of complexity. Who provided: a trained cognitive therapist; the ERICA website states that professionals who can use ERICA include: doctors with specialisation in geriatrics, physical medicine and rehabilitation, neurology and neuropsychiatry, psychologists, speech therapists, physiotherapists, occupational therapists. How: face‐to‐face and individually Where: not specified but within the rehabilitation institute When and how much: 24 sessions of 45 minutes each, 3 times per week for 8 weeks (total of 18 hours) in addition to traditional cognitive rehabilitation of 24 sessions 3 times per week for 8 weeks (for total of 48 sessions of 45 minutes each, total of 36 hours) Tailoring: the therapist provided the programmes within the "growing hierarchy of complexity" through the Erica platform; the difficulty of the exercises "was flexible to the progressive changes of the patient's performance and consistently ensure(d) effective and pleasant rehabilitation sessions". The website states: "The user [rehabilitation professional] selects the exercise to be administered, sets the parameters (target stimulus, exposure time, background color, etc.), administers the exercise and proceeds with the session". Modifications: none reported How well (planned): not reported How well (actual): not reported Comparator group Brief name: cognitive rehabilitation Recipients: adults with stroke Why: to stimulate specific cognitive skills and improve cognitive functional recovery poststroke What: CT using pen‐and‐paper tasks Who provided: a therapist How: face‐to‐face Where: in a rehabilitation institute When and how much: 45‐minute sessions 6 times per week for 8 weeks Tailoring: customised for participants' needs Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary (results not reported)
Secondary
Other
Methods of data collection: reported that each patient "was submitted to a complete neuropsychological evaluation before and after the treatment" to measure the cognitive domains (secondary outcomes in this review) and that the functional scales, BADL and IADL, Levels of Cognitive Functioning, and Barthel Index, were filled with the help of caregivers. The authors stated that it was a single‐blind study but did not report clearly who was blinded and if it was for this outcome assessment. Data collection time points: before and after treatment (8 weeks) |
|
| Notes |
Funding: authors reported no financial support was received for study. Conflict of interest: authors reported no conflicts of interest. Published trial protocol: none located Trial registration: none reported nor located Ethics approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 1 of 2 groups "in order of recruiting". Unclear if this is describing alternate sequence generation or not. |
| Allocation concealment (selection bias) | Unclear risk | The authors did not describe any procedure of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A trained cognitive therapist provided exercises with a growing hierarchy of complexity through the Erica rehabilitative platform". Both the treatment provider and participants were assumed to be aware of their group of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported that each patient "was submitted to a complete neuropsychological evaluation before and after the treatment" to measure the cognitive domains of interest to this review and that it was a single‐blind study but did not report clearly who was blinded in the study and if it was for this outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | High risk | ADL described as an outcome but not reported in the results. |
| Other bias | Low risk | No other identifiable bias. |
Hasanzadeh Pashang 2020.
| Study characteristics | ||
| Methods |
Design: RCT Duration of trial: not reported Unit of randomisation: people with right cerebral cortical ischaemic stroke with attention impairments 6 months to 3 years poststroke Recruitment and allocation: using purposive sampling of participants with attention impairment, 20 participants randomised to IG (n = 10) or CG (n = 10). |
|
| Participants |
Setting: stroke rehabilitation clinic Country: Iran Sample size: 20 adults, 75% men; IG: 10; CG: 10 Inclusion criteria: first‐time ischaemic stroke, thrombotic ischaemia in right cerebral cortical region, confirmed attention impairment, 13–15 consciousness on Glasgow Coma tables, no previous treatment for attention deficits, able to read and write, 6 months to 3 years after stroke Exclusion criteria: white matter lesion of brain, and cerebral atrophy (with no previous symptoms); alcohol and drug addiction; hearing loss; complete memory recovery before the end of intervention; impaired consciousness or brain "re‐attack"; no speech power; the lack of co‐operation of patients' families Age: mean: IG: 53.90 (SD 9.73) years; CG: 57.70 (SD 12.16) years Time since onset of stroke: mean: IG: 11.90 months; CG: 20.3 months Types of stroke: thrombotic ischaemia Site of lesion: right cerebral cortical region |
|
| Interventions |
Intervention group Brief name: cognitive rehabilitation Recipients: adults 6 months to 3 years poststroke with attention impairment Why: to improve visual and auditory attention performance through group work including focused stimulation, learning compensatory coping strategies, acquiring insight and awareness, emotional adjustment, and improved self‐efficacy of feeling more 'in control' (Powell 2017). What (materials): the Brain Injury Workbook. Exercises for Cognitive Rehabilitation (Powell 2017). www.routledge.com/The-Brain-Injury-Workbook-Exercises-for-Cognitive-Rehabilitation-2nd/Powell/p/book/9781315172897 What (procedures): routine rehabilitation plus cognitive rehabilitation delivered according to the Brain Injury Workbook (Powell 2017) Table 1 of paper outlines content of 8 sessions:
Who provided: not reported; Powell 2017 stated that the workbook can be used by therapists working with brain‐injured people in groups and can be used by people with brain injuries themselves and their carers. How: face‐to‐face in groups of 2–10 people, as per workbook Where: stroke rehabilitation clinic with no further description When and how much: 8 sessions, 1 hour per week (total of 8 hours) Tailoring: none reported, although content appears amenable to individual tailoring, e.g. family name training Modifications: none reported How well (planned): none reported How well (actual): none Comparator group Brief name: routine rehabilitation Recipients: adults 6 months to 3 years poststroke with attention impairment Why: to provide routine rehabilitation. What: drug therapy combined with physiotherapy Who provided: not reported, presumably relevant rehabilitation clinic staff How: individual and face‐to‐face Where: stroke rehabilitation clinic When and how much: not reported Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Methods of data collection: not reported Data collection time points: before and after the intervention (8 weeks) and 6 weeks after completion of intervention |
|
| Notes |
Funding: none Conflict of interest: none Published trial protocol: none located Trial registration: none reported Ethics approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but it is apparent that participants could have known which group they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported. |
| Selective reporting (reporting bias) | Low risk | It appears that all planned outcomes were reported. |
| Other bias | Low risk | No other identifiable bias. |
Jiang 2016.
| Study characteristics | ||
| Methods |
Design: 2 × 2 factorial design RCT (note: data only for 2 groups included in this review: CG and RehaCom computer training alone group). Duration of trial: 28 months (August 2013 to November 2015) Unit of randomisation: people within 6 months poststroke with cognitive dysfunction; aged 18–75 years Recruitment and allocation: of 1020 recruited people with stroke being treated (as inpatients or outpatients, Yang 2014), 240 people were eligible and randomised to 4 groups: conventional therapy (CG) (n = 60), acupuncture group (n = 60), RehaCom treatment group (IG) (n = 60), and acupuncture plus RehaCom group (n = 60). |
|
| Participants |
Setting: 1 university traditional Chinese medicine hospital Country: China Sample size: 120 adults allocated, 100 analysed, 49% men; IG: 51; CG: 49 Inclusion criteria: clinical diagnosis of first stroke incident within the preceding 6 months; aged 18–75 years; MMSE score within specific range according to education level; conscious and in stable physical condition Exclusion criteria: existing mental disorder before stroke onset; severe hearing or vision problems affecting computer‐based assessment and training; pregnancy or breastfeeding; bleeding disease; heart, liver, or kidney failure or other serious disease; prior participation in other clinical trials Age: mean: IG: 62.37 (SD 7.89) years; CG: 60.53 (SD 9.19) years Time since onset of stroke: mean: IG: 44.22 (17.00) days; CG: 42.76 (SD 16.00) days Types of stroke: most participants had ischaemic strokes (IG: 61%; CG: 63%) Site of lesion: left (IG: 53%; CG: 51%) |
|
| Interventions |
Intervention group Brief name: RehaCom Recipients: adults within 6 months poststroke with cognitive dysfunction Why: to provide computer‐based training with 5 different treatment programmes designed to "restore attention, memory, and executive function and to improve the visual field". What (materials): RehaCom software (Chinese version). See hasomed.de/en/products/rehacom/ for more details. The software is available in 27 languages at no extra cost; computer. What (procedures): conventional therapy plus computer software training with RehaCom, which includes 5 different therapeutic programmes, each with 1–4 different tasks from which participants chose during each therapy session and 3–5 varying levels of difficulty. The provider chose the program and difficulty level according to each participant's needs and provided guidance or reminders and increased difficulty according to patient feedback. Who provided: physiotherapists using commercially available software. The RehaCom website states that the software is used "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals and clinics. The protocol (Yang 2014) stated the following requirements of training and experience:
How: face‐to‐face and appeared to have been individually Where: not specified but within rehabilitation hospital When and how much: 30 minutes per day, 5 days per week, total of 60 sessions over 3 months (30 hours in total) Tailoring: the physiotherapists chose different training programmes and difficulty levels according to the specific circumstances of each participant and increased the training difficulty according to patient feedback. Modifications: none reported in paper, but protocol stated that the investigators could be convened to discuss practical issues such as intervention protocol revisions (Yang 2014). How well (planned): not reported How well (actual): not reported Comparator group Brief name: conventional therapy Recipients: adults with stroke with cognitive dysfunction Why: to provide rehabilitation services to people poststroke What: traditional rehabilitation therapy, including basic treatment according to Chinese guidelines for cerebrovascular disease, healthy lifestyle education, occupational therapy and physical therapy, medical exercise therapy, hydrotherapy, except CT (Yang 2014) Who provided: medical staff and rehabilitation professionals, such as occupational therapists and physiotherapists How: face‐to‐face Where: rehabilitation hospital When and how much: 12 weeks Tailoring: doctors administered appropriate treatment according to patients' needs. Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Methods of data collection: demographic data were obtained on enrolment. Researchers stated that they "tried to ensure that the same therapist completed each patient’s cognitive assessment and that he/she did not participate in patient treatment"; protocol stated that outcome assessors would not be involved in screening and allocating of participants (Yang 2014). Data collection time points: baseline and 12 weeks (post‐treatment) |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: yes; Yang 2014 Trial registration: yes (Chinese Clinical Trial Registry: ChiCTR‐TRC‐13003704) Ethics approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper referred to study protocol for details of design, which stated that the random allocation sequence would be generated by an independent statistician using statistical software. |
| Allocation concealment (selection bias) | Low risk | Paper referred to study protocol for details of design which described detailed management of blinded allocation by an independent research assistant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported that the researchers "… tried to ensure that the same therapist completed each patient's cognitive assessment and that he/she did not participate in patient treatment …"; reported in protocol as "not possible". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported in paper that researchers "tried to ensure that the same therapist completed each patient's cognitive assessment and that he/she did not participate in patient treatment …"; protocol stated, "Although patients, investigators, and therapists administering treatment will not be blinded, those assessing the patients for primary and secondary endpoints will be blinded to patient treatment assignment. The investigators, therapists and assessors are different people. Patients will be told not to talk to the examiner about the group allocation or therapy content during the post‐intervention assessments" (Yang 2014). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis per‐protocol rather than intention‐to‐treat and 15% missing in IG and 18% in CG. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in protocol were reported. |
| Other bias | Low risk | No other identifiable bias. |
Lin 2014.
| Study characteristics | ||
| Methods |
Design: RCT Duration of trial: 15/16 months (July 2011 to November 2012) Unit of randomisation: people with stroke of 6–10 months' duration with executive function and memory deficits; aged 45–70 years Recruitment and allocation: patients attending a rehabilitation hospital were recruited and randomly allocated to 1 of 2 groups: IG (n = 16) and CG (n = 18) |
|
| Participants |
Setting: rehabilitation department of 1 rehabilitation hospital Country: China Sample size: 34 adults, 59% men; IG: 16; CG: 18 Inclusion criteria: confirmed diagnosis of first stroke; deficits in both executive function and memory (z‐scores < 1.5 in the Wechsler Memory Scale memory quotient and TMT); right handed; education ≥ 8 years; time since stroke 6–10 months (180–300 days); aged 45–70 years; normal hearing and vision Exclusion criteria: mental retardation; history of Alzheimer's disease or mental illness such as schizophrenia before stroke; vital organ failure; concomitant antidepressant, psychoactive drug, or steroid therapy Age: mean: IG: 62.4 (SD 6.0) years; CG: 63.2 (SD 5.7) years Time since onset of stroke: mean: IG: 227.5 (SD 24.0) days; CG: 228.1 (SD 18.4) days Types of stroke: not reported Site of lesion: n (left/right hemisphere): IG: 6/10; CG: 9/9 |
|
| Interventions |
Intervention group Brief name: RehaCom Recipients: adults in rehabilitation 6–10 months since stroke with executive function and memory deficits. Why: to improve executive function and memory. What (materials): RehaCom software; see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer. What (procedures): CT; no further description of procedures provided. Who provided: 2 trained psychologists; the RehaCom website states that the software is used "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals, and clinics. How: not described but presumably face‐to‐face Where: not described but presumably in the rehabilitation department When and how much: 1 hour per session, 6 sessions per week for 10 weeks (60 hours in total) Tailoring: none reported Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: CG Recipients: adults in rehabilitation 6–10 months since stroke with executive function and memory deficits Why: to provide rehabilitation. What: not described except that no CT was provided. Who provided: not reported How: not reported Where: not reported, presumably within the rehabilitation department When and how much: not reported Tailoring: none reported Modifications: none reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: neuropsychological assessments performed by 2 trained psychologists who were blinded to group assignment. Data collection time points: baseline and after intervention (10 weeks) |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: none located Trial registration: none reported Ethics approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding or not was not reported but it was apparent that participants could have known which group they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neuropsychological assessments were performed by 2 trained psychologists who were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised was unclear, just the number who completed the study was reported. |
| Selective reporting (reporting bias) | Low risk | It appears that all planned outcomes were reported. |
| Other bias | Low risk | No other identifiable bias. |
Lundqvist 2010.
| Study characteristics | ||
| Methods |
Design: cross‐over RCT (wait group) Duration of trial: not reported Unit of randomisation: adult outpatients with WM impairments after acquired brain injury Recruitment and allocation: 38 rehabilitation outpatients from 76 people with acquired brain injury met inclusion criteria; 21 accepted and were randomised by drawing of lots to 2 groups; IG: 10; CG: 11. 13 (62%) of these had stroke: IG: 5; CG: 8. |
|
| Participants |
Setting: outpatient hospital rehabilitation department Country: Sweden Sample size: 13 adults with stroke, 54% men; IG: 5; CG: 8 Inclusion criteria: aged 20–65 years, time since injury/illness ≥ 1 year, self‐reported WM impairments and a significantly impaired Working Memory Index compared to the index for verbal comprehension or index for perceptual organization (or both) or a Working Memory Index < 80 using the WAIS III (Wechsler 1997), and a reported motivation for training. Exclusion criteria: IQ ≤ 70 based on WAIS III, depression according to DSM‐IV, perceptual or motor impairments that made computerised training impossible, extensive cognitive impairments and completion of other rehabilitation programmes. Age: mean: IG: 48.4 (SD 8.2) years; CG: 43.25 (SD 9.97) years Time since onset of stroke: mean: IG: 51.4 (SD 46.39) months; CG: 51.13 (SD 36.24) months Types of stroke and sites of lesion: not reported |
|
| Interventions |
Intervention group Brief name: WM training, QM, now called Cogmed Recipients: outpatient adults with WM impairments after acquired brain injury Why: to improve WM function using individualised and intense computerised training that occurred in a clinic, at least initially, so that participants received the benefit of coaching, meeting with other participants, and training in a calm, quiet environment. What (materials): QM (formerly called ReMemo and now called CogMed) for adults, a WM training computerised system with visuo‐spatial and verbal WM tasks developed at the Karolinska Institute and Cogmed Cognitive Medical System AB, Sweden (www.cogmed.com/healthcare). See also Klingberg 2002; Westerberg 2004; Westerberg 2007 for details of the program; personal computer. What (procedures): participants performed their WM training program on a personal computer in pairs in the presence of 1–3 certified coaches who provided special feedback once per week beside the continuous statistics, which the participants could follow themselves on the computer. The training program specified different visuo‐spatial and verbal WM tasks. Each task was introduced by a speaker voice and the person responded by localising and remembering the stimuli. Participants used a computer mouse to respond:
Who provided: 3 certified coaches; authors confirmed that these coaches were occupational therapists. How: face‐to‐face in pairs in the presence of 1–3 certified coaches Where: in a separate quiet room at the Department of Rehabilitation When and how much: 45–60 minutes of intense training per day, 5 days per week, for 5 weeks (up to 25 hours), weekly coach feedback Tailoring: the difficulty level of each training task automatically adjusted according to each participant's progress, increasing the WM load according to each participant's performance levels. The coach provided feedback once per week in addition to ongoing statistics provided to the participant on the computer. Modifications: not reported How well (planned): none reported How well (actual): not reported Comparator group None (received no training during the same period) 4 weeks after the IG participants had had their training, WM functions were assessed in both groups. Then, CG received the same training for 5 weeks. |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: a neuropsychologist assessed all participants for each time point. Data collection time points: before training (baseline) and 4 and 20 weeks after training |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: no Trial registration: no Ethics approval: yes Author Contact: further information and stroke‐specific data from authors requested and supplied. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… randomized into two groups at the beginning of the study, through 'drawing of lots' ". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Certified coaches provided the intervention, including tailored feedback to participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A neuropsychologist assessed all subjects before training (baseline) and at 4 and 20 weeks after training". Comment: COPM semi‐structured interview was administered by an occupational therapist; unclear if either of these people were one of the certified coaches providing the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Personal correspondence reported no participants lost to follow‐up and provided data for same. |
| Selective reporting (reporting bias) | Low risk | No protocol/trial registry reported or located but reports on significant and non‐significant outcome measures. |
| Other bias | Low risk | No other identifiable bias. |
Maggio 2020.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 22/23 months (June 2017 to March 2019) Unit of randomisation: people affected by chronic stroke with mild‐to‐moderate cognitive impairment undergoing neurorehabilitation Recruitment and allocation: not reported in detail except that 40 people with a diagnosis of stroke who attended the Robotic and Behavioral Neurorehabilitation Unit of the IRCCS Centro Neurolesi "Bonino‐Pulejo" (Messina, Italy) from June 2017 to March 2019, were enrolled in this study and randomly divided into 2 groups: IG (n = 20); CG (n = 20). |
|
| Participants |
Setting: robotic and behavioural neurorehabilitation unit of a rehabilitation facility Country: Italy Sample size: 40 adults, 55% men, IG: 20; CG: 20 Inclusion criteria: first‐ever supratentorial stroke in the chronic phase (i.e. 6–12 months after the event); presence of mild‐to‐moderate cognitive impairment (MMSE: 11–26); absence of disabling sensory alterations (i.e. auditory and visual loss) Exclusion criteria: age > 85 years; presence of severe medical and psychiatric illness potentially interfering with the training Age: mean: 53.9 (SD 4.5) years Time since stroke onset: mean: 6 (SD 1) months Types of stroke and site of lesion: (n) cortical right: IG: 12; CG: 11; subcortical right: IG: 6; CG: 7; cortical left: IG: 2; CG: 2; subcortical left: 0 |
|
| Interventions |
Intervention group Brief name: HAT Recipients: adults with cognitive impairments after chronic stroke undergoing rehabilitation Why: to evaluate how people react and interact with this environment, and prepare them for a return home. What (materials): HAT within the home automation room (see Figure 1 of photo in original paper for example technologies in use). The room was designed for severely disabled people who were partially autonomous in their movements. Using a centralised control system, participants can change the environment, monitor some environmental parameters (e.g. detect the presence of smoke, water, or gas leaks), and use the alarm bell. Kitchen countertops and other shelves could be adapted in height and depth. The bathroom had an adaptable toilet and shower, which could be changed by the patient. What (procedures): small group functional activities in a home automation room with home automation or domotics technologies where the interaction was mediated by these technologies to achieve participant ADL goals, e.g. cooking and personal care in preparation for returning home. Who provided: a "therapist" – further specifics not provided; however, 1 of the authors was an occupational therapist and the intervention clearly falls within the scope of occupational therapy practice. How: face‐to‐face in a group (3–5 participants per group) Where: in a room with home automation or domotics technologies in neurorehabilitation unit When and how much: 3 sessions per week for 8 weeks (i.e. total of 24 sessions), each session lasting about 60 minutes (24 hours in total) Tailoring: the adjustability provided within the room allowed tailoring of heights and depths of equipment and technologies to suit participant's individual needs, disabilities, and dimensions. Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: traditional training Recipients: adults with cognitive impairments after chronic stroke undergoing rehabilitation Why: to facilitate homecoming even for severely disabled people who are partially autonomous in their movements. Training allows the use of domestic environments (e.g. kitchens, bathrooms) with strategies that permit an effective use. What: therapist led the participant through a series of exercises promoting autonomy in daily life activities, including positioning of marbles, ball exercises, and manipulation of various objects such as buttons for fastening a jacket or zippers. Conventional training was based on direct interaction and exercises with the participants (often working together), who could also use paper/pencil exercises. Who provided: a "therapist" – further specifics not provided; however, 1 of the authors was an occupational therapist and the intervention clearly falls within scope of occupational therapy practice. How: face‐to‐face activities in small group (3–5 participants) Where: within rehabilitation unit but not further described When and how much: 3 sessions per week for 8 weeks (i.e. total of 24 sessions), each session lasting about 60 minutes (total of 24 hours) Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Other
Methods of data collection: each participant was evaluated by a neuropsychologist before (T0) and after the last training session (T1). Data collection time points: baseline and postintervention (8 weeks) |
|
| Notes |
Funding: no. Quote: "The authors received no financial support for the research, authorship, and/or publication of these authors". Conflict of interest: none Published trial protocol: none reported Trial registration: none reported Ethics approval: yes Data: medians and interquartile ranges converted to means and SDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By tossing a coin. |
| Allocation concealment (selection bias) | Unclear risk | No indication if coin tossing was done independently. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Incomplete blinding. Therapists were blind to the assignment and objectives of the study; however, it may have been possible for therapists to discern which therapy a participant was receiving and for participants to know to which group they were allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The testers were blind to the assignment and objectives of the study, but it is unclear if ADL and IADL was assessed by a tester or participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper stated that all participants completed the intervention and it was likely that all were assessed. |
| Selective reporting (reporting bias) | Low risk | No protocol reported but appears that all planned outcomes were reported. |
| Other bias | Low risk | No other identifiable bias. |
Park 2015a.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 6 months (October 2013 to March 2014) Unit of randomisation: adult inpatients with cognitive impairment after stroke Recruitment and allocation: of 37 inpatients recruited and screened, 30 were eligible and randomised by random numbers table into IG (n = 15) or CG (n = 15). |
|
| Participants |
Setting: rehabilitation hospital Country: Korea Sample size: 30 adults, 47% men; IG: 15; CG: 15 Inclusion criteria: history of 1 stroke; stroke with onset duration of < 3 months; score of ≤ 23 on the Korean version of MMSE; ability to understand instructions; ability to use the controller with the unaffected upper limb; and without unilateral hemispatial neglect and hemianopsia Age: mean: IG: 64.7 (SD 8.9) years; CG: 65.2 (SD 8.0) years Time since stroke onset: mean: IG: 1.5 (SD 0.5) months; CG: 1.8 (SD 0.6) months Types of stroke and lesion: not reported |
|
| Interventions |
Intervention group Brief name: CoTras Recipients: adult inpatients with stroke undergoing rehabilitation within 3 months' onset with cognitive impairment Why: to provide a Korean computer‐based cognitive rehabilitation to enhance attention, concentration, implementation skills, and perception‐motor skills. What (materials): CoTras program (Netblue Co., Ltd, Korea) computerised cognitive rehabilitation training, made for Koreans; a joystick and a large button on the CoTras panel which make the training easier for people who are unfamiliar with computer use; see www.netblue.co.kr/eng/doc/product01-01.php What (procedures): during the sessions, all participants completed a standard rehabilitation programme according to a daily inpatient treatment schedule. In addition to standard rehabilitation, all participants received 30‐minute daily sessions of the computer‐based program treatment. Who provided: not reported; however, the research was conducted by occupational therapists, the software is commercially available, and the intervention is within the scope of practice defined in the review. How: unclear but appeared it was face‐to‐face and individually. Where: local inpatient rehabilitation hospital, no further details provided When and how much: 20 × 30‐minute sessions (i.e. 5 days per week for 4 weeks) (10 hours in total) Tailoring: training allowed adjusting to individual participant's abilities at all levels of the programme Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: conventional cognitive rehabilitation Recipients: inpatient with stroke with cognitive impairment undergoing rehabilitation Why: to provide usual rehabilitation services. What: pencil and paper with emphasis on visual perception ability Who provided: not reported How: not reported, assumed face‐to‐face Where: inpatient rehabilitation hospital When and how much: matched that for the IG participants in terms of duration (in minutes) (i.e. 20 × 30‐minute sessions) Tailoring: not reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: all outcome measures were administered to the participants by the assistant researcher with 5 years' experience in using the measures. Data collection time points: baseline and 4 weeks postintervention |
|
| Notes |
Funding: none stated Conflict of interest: none reported Published trial protocol: none located Trial registration: none reported Ethics approval: unclear, stated, "All subjects provided written informed consent before study inclusion according to the code of ethics of the World Medical Association (Declaration of Helsinki, version 2004)". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used to randomly assign participants to either CG or IG. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Quote: "All subjects gave their voluntary consent to participate after receiving a detailed explanation of the purpose and methods of the study" and "During the sessions, all subjects participated in a standard rehabilitation program according to a daily inpatient treatment schedule. In addition to standard rehabilitation, all subjects received 30‐min daily sessions of either the CG or the EG [IG] treatment". Comment: highly likely that participants and personnel knew which group they were in. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All outcome measures were administered to the patients at baseline and at the end of treatment (after the 4‐week intervention) by the assistant researcher with 5 years' experience in using the measures". Comment: unclear whether this person was blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not address this criterion. |
| Selective reporting (reporting bias) | Low risk | No protocol located but appeared to report all outcomes and data. |
| Other bias | Low risk | No other identifiable bias. |
Prokopenko 2013.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: not reported Unit of randomisation: inpatients with cognitive impairments after a hemisphere stroke Recruitment and allocation: not reported in detail; inpatient stroke survivors were approached within 2 weeks after hemisphere stroke and 43 of those with cognitive impairments and who did not have epilepsy or significant speech pathology were randomised by 'method of letters' to 2 groups, IG (n = 24) and CG (n = 19). |
|
| Participants |
Setting: inpatient facility Country: Russia Sample size: 43 adults, 53% men, IG: 24; CG: 19 Exclusion criteria: unable to give informed consent, not fluent in Russian, significant speech pathology, e.g. aphasia, severe cognitive deficits (MMSE < 20), medically unstable, epilepsy Age: median (quartiles): IG: 61 (25th 57, 75th 69) years; CG: 66 (25th 61, 75th 69) years Time since stroke onset: not reported except people were approached within 2 weeks of stroke Types of stroke and site of lesion: not reported |
|
| Interventions |
Intervention group Brief name: computer training Recipients: inpatient adults with cognitive impairments from a hemisphere stroke Why: to restore cognitive function using 2‐part computer training focusing on attention and visual and spatial gnosis with built‐in feedback and a help option. Part 1 focused on 4 aspects of attention (sustained, selective, divided, and alternating); part 2 focused on figure‐background activities with gradually decreasing intensity of "background noise". Tasks were not aimed at evaluation of cognitive functions, but rather at training of these functions; though task performance speed in the attention task was measured in time and fed back to the participant, the feedback served only as a reference point for improvement. What (materials): computer programs:
Other computerised tasks: "remembering a sequence of symbols", "arranging the clock hands", and "the serial count" What (procedures): in addition to standard treatment at the inpatient rehabilitation department:
Training of visual and spatial gnosis
Who provided: not clearly stated in paper; however, the authors confirmed sessions were conducted by occupational therapists. The paper stated that the approach could possibly be used independently by a participant without involvement of the medical personnel. How: face‐to‐face and individually Where: inpatient rehabilitation department When and how much: daily for 30 minutes per day for 2 weeks (up to 15 hours) Tailoring:
Modifications: none reported How well (planned): use of a training protocol How well (actual): none reported Comparator group Brief name: standard treatment Recipients: inpatient adults with cognitive impairments from a hemisphere stroke Why: to provide standard rehabilitation. What: no details provided Who provided: inpatient rehabilitation department staff How: not described but presumably face‐to‐face Where: inpatient rehabilitation department When and how much: not reported Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: cognitive assessments were conducted by a trained assessor blinded to randomisation. Data collection time points: baseline and postintervention (14–16 days after baseline) |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: no Trial registration: no Ethics approval: yes Author contact: further details about intervention implementation were provided by the authors. Data: median and interquartile ranges converted to means and SDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed using 'method of letters', which was not explained and authors did not reply to email request for explanation of the method. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed with the use of method of letters. Cognitive assessment was conducted on the day of inclusion into the study; assessments were repeated on day 14–16 by a trained assessor blind to randomization". Comment: authors did not specifically state that baseline assessment occurred prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As for allocation concealment above and also the intervention was delivered "in addition to standard treatment at the in‐patient rehabilitation department", so it appeared the staff and participants delivering the computer program intervention would have known it was additional to standard treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "… assessments were repeated on day 14–16 by a trained assessor blind to randomization". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up could be detected. |
| Selective reporting (reporting bias) | Low risk | No protocol/trial registry reported or located but reports on all outcome measures. |
| Other bias | Low risk | No other identifiable bias. |
Prokopenko 2018.
| Study characteristics | ||
| Methods |
Design: parallel RCT (note: data only for 2 groups included in this review: IG and passive CG) Duration of trial: not reported Unit of randomisation: participants aged 40–67 years, with vascular cognitive impairments without dementia in early recovery periods of ischaemic hemispheric stroke (up to 6 months after stroke) Recruitment and allocation: not reported in detail; 25 participants receiving conventional treatment in the Neurorehabilitation Center were randomised into 3 groups: IG (n = 10), passive CG (n = 9), and active CG (n = 6); data only for the intervention and passive CG were used in this review. |
|
| Participants |
Setting: neurorehabilitation centre Country: Russia Sample size: 19 adults, 72% men; IG: 10; passive CG: 9 Exclusion criteria: decompensation of somatic and neurological diseases; epilepsy; severe cognitive dysfunction; severe and moderate aphasia; and severe decrease of vision or hearing Age: median (quantiles): IG: 59.5 (1st 57; 3rd 60) years; CG: 62.55 (1st 61; 3rd 65) years Time since stroke onset: not reported except recruited up to 6 months after stroke Types of stroke: ischaemic hemispheric stroke Side of lesion: not reported |
|
| Interventions |
Intervention group Brief name: computer CT Recipients: people with vascular cognitive impairments without dementia in early recovery periods of ischaemic hemispheric stroke Why: to correct poststroke cognitive impairments in acute and early recovery periods based on the "classical neuropsychological approach of Alexander Luria". What (materials): KrasSMU complex of neuropsychological software programs for cognitive correction (in Russian); the software is reportedly available on CD, and online; includes programs for training:
What (procedures): an instructor demonstrated how to use a computer and explained the tasks and rules for each training program in the first few sessions then participants could train independently, remaining under the supervision of the instructor. Procedures for Figure‐Background test and Visual and Position test described in depth:
Who provided: not clearly stated in paper; however, the authors confirmed in personal correspondence about the same intervention provided in Prokopenko 2013 that the sessions were conducted by occupational therapists. How: individual face‐to‐face training using computer software programs; an instructor demonstrated how to use a computer and explained the tasks and rules for each training program during the first few sessions, then participants could train independently, while under the supervision of the instructor. Where: in a neurorehabilitation centre; also stated that participants could use the programs online. When and how much: 10 daily sessions for 30–40 minutes (5 hours to 6 hours 40 minutes in total) Tailoring: supervision was provided throughout so that individual support could be provided as needed. Levels of complexity could be increased depending on participants' abilities. Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: conventional treatment Recipients: people with vascular cognitive impairments without dementia in early recovery periods of ischaemic hemispheric stroke Why: to provide conventional rehabilitation What: physiotherapy and drug treatment without any cognitive rehabilitation Who provided: neurorehabilitation centre staff How: not described but presumably face‐to‐face Where: not described but presumably in the neurorehabilitation unit When and how much: not reported Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: evaluation of the participants' neurological, cognitive, affective, and functional states was performed before and after the observational period (the first examination during the first/second days on admission, and the second examination on the day after the last day of training). Data collection time points: before and after intervention (baseline and 10 days) |
|
| Notes |
Funding: not reported Conflict of interest: not reported Published trial protocol: not reported Trial registration: not reported Ethics approval: yes Data: median (1st, 3rd quantiles) converted to means and SDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A simple randomization using simple random tables was performed". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but apparent that participants could have known to which group they were allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not address this criterion. |
| Selective reporting (reporting bias) | Low risk | No protocol located but appeared all planned outcomes were reported except data for Clinicians Global Impression Scale and Patient's Global Impression Scale was not provided, which is not of interest to this review. |
| Other bias | Low risk | No other identifiable bias. |
Prokopenko 2019.
| Study characteristics | ||
| Methods |
Design: parallel RCT (note: data only for 2 groups included in this review: study group (IG) and passive CG) Duration of trial: not reported Unit of randomisation: people aged 40–65 years, in the early and late recovery period following first hemispheric ischaemic stroke, with cognitive impairments Recruitment and allocation: not reported in detail; 68 participants receiving restorative treatment at a neurorehabilitation centre were randomised into 3 groups: a study group (IG) (neuropsychological computer programs) (n = 23), an active CG (distracting games) (n = 19), and a passive CG (standard rehabilitation) (n = 26); data only for the IG and passive CG were used in this review. |
|
| Participants |
Setting: neurorehabilitation centre Country: Russia Sample size: 49 adults, 65% men; IG: 23; passive CG: 26 Inclusion criteria: aged 40–65 years, early and late recovery period following first hemispheric ischaemic stroke, cognitive impairments at the stage of mild and moderate disorders, informed consent Exclusion criteria: somatic and neurological diseases in the stage of decompensation, presence of epileptic seizures or epileptic activity on the electroencephalogram, age outside the specified range, profound cognitive or aphatic impairments, visual or auditory pathology preventing sessions Age: mean: IG: 59.0 (95% CI 54.9 to 66.5); CG: 60.5 (95% CI 55.8 to 68.8) Time since stroke onset: not reported except recruited in early and late recovery period Types of stroke: hemispheric ischaemic stroke Side of lesion: not reported |
|
| Interventions |
Intervention group Brief name: neuropsychological computer training Recipients: people in the early and late recovery period following first hemispheric ischaemic stroke, with cognitive impairments Why: to provide low‐cost stimulation of several cognitive functions "with automatic changes in loadings and assessment of point scores" and ensuring high‐level compliance through high motivation from play aspects of the programmes. What (materials): the authors' set of original computerised stimulation programs (KrasSMU complex of neuropsychological computer programs), in Russian including:
What (procedures): in addition to complex restorative treatment, participants completed courses of sessions using the neuropsychological computerised stimulation programs. Who provided: not stated in paper; however, the authors confirmed in personal correspondence about the same intervention provided in Prokopenko 2013 that the sessions were conducted by occupational therapists. How: not reported but appeared to be face‐to‐face and individually Where: neurorehabilitation centre When and how much: daily for 10 days, each session lasting 30–40 minutes (5 hours to 6 hours 40 minutes in total) Tailoring: programmes provided "automatic changes in loadings" presumably in response to performance Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: standard rehabilitation Recipients: people in the early and late recovery period following first hemispheric ischaemic stroke, with cognitive impairments Why: to provide standard rehabilitation. What: motor rehabilitation only Who provided: not reported How: not described but presumably face‐to‐face and individually Where: neurorehabilitation centre When and how much: not reported Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: no details provided except that participants were assessed before and after treatment. Data collection time points: before and after treatment (baseline and 10 days) |
|
| Notes |
Funding: none reported Conflict of interest: none Published trial protocol: no Trial registration: no Ethics approval: none reported Data: median and 95% CI data converted to means and SDs by a method described in University College London 2010. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but apparent that participants could have known to which group they were allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not address this criterion. |
| Selective reporting (reporting bias) | Low risk | No protocol reported but it appeared that all planned outcomes were reported. |
| Other bias | Low risk | No other identifiable bias. |
Skidmore 2015a.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: not reported Unit of randomisation: inpatient adults with a clinically defined stroke and confirmed cognitive impairment Recruitment and allocation: consecutive inpatient admissions of adults with cognitive impairments after acute stroke were invited to participate, with 60 inpatients assessed for eligibility. 27 did not meet eligibility criteria and 3 withdrew, leaving 30 that were randomised by random number table to 2 intervention groups, strategy training (IG) (n = 15) and attention control (CG) (n = 15). |
|
| Participants |
Setting: metropolitan university medical centre rehabilitation facility Country: USA Sample size: 30 adults, 67% men; IG: 15; CG: 15 Exclusions: primary diagnosis of acute stroke; no impairment of cognitive functions (Quick Executive Interview ≤ 3); presence of severe aphasia (Boston Diagnostic Aphasia Examination Severity Rating Scale ≥ 1); diagnosis of dementia (indicated in the medical record); presence of current major depressive disorder, bipolar, or psychotic disorder (Primary Care Evaluation of Mental Disorders); presence of drug and alcohol abuse within 3 months (Mini‐International Neuropsychiatric Interview); anticipated length of stay < 5 days Age: mean: IG: 64.87 (SD 16.59) years; CG: 71.80 (SD 13.19) years Time since stroke onset: mean: IG: 16.80 (SD 15.58) days; CG: 18.47 (SD 21.29) days Types of stroke: ischaemic, n: IG: 10 (67%); CG: 11 (73%) Hemisphere: right, n: IG: 10 (67%); CG: 10 (67%) NIHSS stroke severity: mean: IG: 8.87 (SD 2.77); CG: 5.87 (SD 2.72) |
|
| Interventions |
Intervention group Brief name: strategy training Recipients: inpatient adults with cognitive impairments after acute stroke Why: to harness the ability of the person with cognitive impairments after stroke to observe, assess, and positively alter his or her performance in real‐life activities; to teach participants to identify and prioritise problematic daily activities, identify barriers impeding performance, generate and evaluate strategies addressing these barriers, and generalise learning through practice. What (materials): workbook materials, COPM (Law 1998), modified CO‐OP manual (Polatajko 2004) for adults with TBI (Dawson 2009), "Goal‐Plan‐Do‐Check" goal sheets (see Appendix 1 of Dawson 2009) What (procedures): sessions were administered according to standardised procedures described further in Dawson 2009, Skidmore 2011, and Skidmore 2014. 4 critical ingredients (self‐selected goals, self‐evaluation of performance, strategy development and implementation, and therapeutic guided discovery) were applied iteratively throughout the sessions in 4 steps via guided discussion and workbook materials.
Who provided: trained rehabilitation personnel, including occupational therapists How: face‐to‐face guided discussion sessions Where: inpatient rehabilitation facility When and how much: 45‐minute sessions daily 5 days per week for the duration of inpatient rehabilitation (in addition to usual inpatient rehabilitation therapy) Tailoring: participants self‐selected their goals and prioritised and choose 4–6 activities to address in the sessions; steps were repeated iteratively until the goal was met (and thus participants moved on to the next activity). Using guided discovery technique, the therapists prompted the participants to identify key principles that they learned and to discuss ways to apply these key principles. Modifications: not reported How well (planned): fidelity procedures were completed using a protocol described elsewhere (Skidmore 2014), which reported that "All research intervention sessions were videotaped and rated for fidelity to the respective manualized procedures … [using] fidelity checklists … to assess treatment integrity and treatment differentiation in a random 20% of sessions in each treatment group". How well (actual): there is no report of the outcome of the videotaped fidelity rating, as described above. Comparator group Brief name: attention control Recipients: inpatient adults with cognitive impairments after acute stroke Why: to provide a comparable intervention in addition to usual rehabilitation similar in dose and attention. What (materials): workbook materials, e.g. journal entries and scripted open‐ended questions What (procedures): initial sessions were used to help the participants identify and prioritise self‐selected goals, using same procedure used in the strategy training intervention. "The remaining sessions focused on the use of journal entries in a workbook and discussions using scripted open‐ended questions to stimulate participants' reflections on their rehabilitation goals, activities, and experiences" (p.3). Who provided: trained rehabilitation personnel How: face‐to‐face guided discussion and written materials Where: inpatient rehabilitation facility When and how much: 45‐minute sessions daily, 5 days per week for the duration of inpatient rehabilitation (in addition to usual inpatient rehabilitation therapy) Tailoring: if participants asked direct questions for guidance on addressing their goals, they were directed to the discuss the questions with the rehabilitation team. Modifications: none reported How well (planned): fidelity procedures were completed using a protocol described elsewhere (Skidmore 2014), which reported that "All research intervention sessions were videotaped and rated for fidelity to the respective manualized procedures… [using] fidelity checklists … to assess treatment integrity and treatment differentiation in a random 20% of sessions in each treatment group". How well (actual): there is no report of the outcome of the videotaped fidelity rating, as described above. |
|
| Outcomes |
Primary
Secondary
Methods of data collection: demographic data and medical information were obtained from medical records at study admission; a battery of outcome measures was administered by trained raters masked to study intent and design. Data collection time points: baseline, 3 months, and 6 months later |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: no Trial registration: yes, NCT02755805 Ethics approval: "We … obtained written informed consent from the individual, if cognitive status permitted, or from a proxy, consistent with approved institutional review board procedures". Data: we obtained additional mean and standard error data from the Clinical Trials Registry (NCT02755805); we converted standard errors to SDs in Review Manager 5. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation scheme used derived from a random number table (1:1 ratio). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "… single‐blind study … research intervention sessions were administered according to standardized procedures … by trained rehabilitation personnel who were masked to the opposing intervention protocol". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trained and reliable raters who were masked to study intent and design administered a standardized measure of activities of daily living and a battery of neuropsychological tests at baseline, 3 months, and 6 months later". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data managed appropriately (repeated‐measures mixed modelling, without imputing data) and caution urged for interpretation of results. 20% of participants were missing 6‐month data for the primary outcome due to refusal of assessments (n = 1), withdrawal (n = 2), and loss to follow‐up (n = 3). |
| Selective reporting (reporting bias) | Low risk | All outcomes from method and protocol reported. |
| Other bias | Low risk | No other identifiable sources of bias. |
Skidmore 2017.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 29 months (August 2012 to December 2014) Unit of randomisation: adult inpatients with acute stroke and cognitive impairment undergoing rehabilitation Recruitment and allocation: of 77 potentially eligible people, 27 did not meet inclusion criteria and 7 withdrew prior to randomisation, leaving 43 who were randomised by random generator to 2 interventions, IG (n = 22) or CG (n = 21) |
|
| Participants |
Setting: inpatient academic health centre Country: USA Sample size: 43 adults, 51% men; IG: 22; CG: 21 Inclusion criteria: diagnosis of first‐time stroke within 30 days of rehabilitation admission and demonstrated cognitive impairments (as indicated by 14‐item Executive Interview ≥ 3) Exclusion criteria: severe aphasia, prior cognitive impairment or dementia, current major depressive disorder, recent substance abuse or psychosis Age: mean: IG: 65.86 (SD 11.67) years; CG: 66.73 (SD 14.25) years Time since stroke onset: mean: IG: 16.29 (SD 18.24) days; CG: 22.36 (SD 30.97) days Types of stroke: ischaemic, n: IG: 14 (67%); CG: 14 (64%) Hemisphere: left, n: IG: 10 (62%); CG: 13 (59%) NIHSS stroke severity: mean: IG: 7.00 (SD 3.90); CG: 7.86 (SD 5.10) |
|
| Interventions |
Intervention group Brief name: guided training Recipients: inpatients with acute stroke and cognitive impairment undergoing rehabilitation Why: to maximise the expertise of participant by training them to actively engage in the direction and focus of their treatment. Therefore, they learned to identify and prioritise problematic daily activities, identify barriers to performing activities, generate their own individualised strategies for addressing these barriers, and apply these processes through iterative practice. In doing so, guided training is designed to equip people with the ability to generalise knowledge and skills in problem identification and problem‐solving skills to address new but similar problems over time. What (materials): COPM (Law 1998); guided training standardised intervention protocol What (procedures): in addition to usual care; using the COPM, the therapist asked the participant to describe a typical routine prior to the stroke, focusing on a typical weekday, a typical Saturday, and a typical Sunday. The therapist then asked the participant to identify 4–6 activities that were important to them and that he or she thought likely to be problematic after the stroke. The subsequent intervention was then focused on these activities. The participant picked the first activity that he or she wanted to practise. The participant performed that activity and the therapist identified barriers to performance, and taught the participant a global strategy, 'goal‐plan‐do‐check', by asking the participant to set a goal to address the barriers (i.e. identify a criterion for performance), develop a plan to address the goal, complete the plan, and check whether the plan worked or required revising. This process was repeated iteratively until the goal was met (participants moved on to the next activity), or until the end of the 10 sessions. The therapist guided participants using prompting questions and workbooks to facilitate learning and aid the participants in implementing the strategy. Who provided: licensed occupational therapists and physiotherapists who were independent contractors and not members of the usual care rehabilitation team How: face‐to‐face and individually Where: inpatient rehabilitation at an academic health centre and home When and how much: 10 sessions of 45 minutes, 5 days per week for 2 weeks (7.5 hours in total) Tailoring: the research therapist asked the participant to describe a typical routine prior to the stroke, focusing on a typical weekday, typical Saturday, and typical Sunday. Therapist then asked the participant to identify 4–6 activities that the participant thought were important and likely to be problematic after the stroke. Therapists focused subsequent intervention programme on these activities. Modifications: none reported How well (planned): fidelity to each protocol and differentiation of elements between protocols assessed using standardised checklists applied to a random 20% of video‐recorded sessions in each condition. They also assessed the degree to which elements of direct skill training and guided training protocols were present in usual care sessions (1 occupational therapy session, 1 physical therapy session, and 1 speech therapy session, if being followed by speech therapy, for each study participant). How well (actual): adherence to intervention procedures was 85% in the guided training group. The 2 protocols were sufficiently differentiated, with guided training intervention elements present in none of the sampled direct skill training sessions. On average, direct skill training participants and guided training participants received a similar number of sessions. Comparator group Brief name: direct skill training Recipients: inpatients with acute stroke and cognitive impairment undergoing rehabilitation Why: to promote independence of participants in daily activities; direct skill training maximises the skill of the rehabilitation practitioner who directs treatment. What (materials): COPM (Law 1998); direct training standardised intervention protocol What (procedures): research therapist selected an activity to practice in the research session that addressed 1 of the participant‐identified goals. The direct skill training therapist analysed the activity using an interview and performance‐based assessment, and identified barriers to performance of that activity. The therapist then set criteria for performance, developed strategies to improve performance, and taught the participant those strategies. Participants practiced the identified strategies until the performance criteria were met (research therapist moved on to the next activity), or until the end of the 10 sessions. The direct skill training therapist documented each of these steps using pre‐established forms in a workbook shared with the participants. Who provided: licensed occupational therapists and physiotherapists who were independent contractors and not members of the usual care rehabilitation team How: face‐to‐face Where: inpatient rehabilitation at an academic health centre and home When and how much: 10 sessions of 45 minutes, 5 days per week for 2 weeks Tailoring: activities and performance criteria used for the skill training were based on the individual assessment. Modifications: not reported How well (planned): fidelity to each protocol and differentiation of elements between protocols was assessed using standardised checklists applied to a random 20% of video‐recorded sessions in each condition. They also assessed the degree to which elements of direct skill training and guided training protocols were present in usual care sessions (1 occupational therapy session, 1 physical therapy session, and 1 speech therapy session, if being followed by speech therapy, for each study participant). How well (actual): analysis of the sampled usual care sessions indicated that on average 98% of training in usual care sessions was consistent with direct skill training and 2% (usual care training) was consistent with guided training. |
|
| Outcomes |
Primary
Secondary: none Methods of data collection: FIM administered by 4 independent (blinded) trained evaluators Data collection time points: study admission (baseline); inpatient rehabilitation discharge; and 3, 6, and 12 months after study admission |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: no Trial registration: yes, NCT02766400 Ethics approval: none reported Data: we obtained additional mean and standard error data from the Clinical Trials Registry (NCT02766400); We converted standard errors to SDs within Review Manager 5. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… assigned to an intervention by the research coordinator using a simple randomization scheme (1:1 allocation ratio) developed with a random number generation program …" |
| Allocation concealment (selection bias) | Low risk | Quote: "… maintained in an electronic file accessible only to the research coordinator …" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | *For personnel only: quote: "… research therapists were independent contractors who were not members of the usual care rehabilitation team. To avoid cross contamination, the research therapists were trained in only one protocol and remained naive to the opposite protocol …" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "… FIM was administered by 4 trained independent (ie, blinded) evaluators". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Linear mixed model analysis used. Quote: "all participants regardless of retention were included in the remaining analysis". |
| Selective reporting (reporting bias) | Low risk | As per protocol. |
| Other bias | Low risk | No other identifiable bias. |
van de Ven 2017.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: April 2013 to March 2015; last follow‐up measurement in November 2015 Unit of randomisation: people with stroke 3 months to 5 years prior, aged 30–80 years, and had received inpatient or outpatient rehabilitation therapy Recruitment and allocation: participants were recruited from a patient database of rehabilitation centres and 1 hospital and from advertisements in newsletters and forums of national stroke patients associations. Of 223 assessed for eligibility, 97 were randomised to 1 of 3 groups: IG (n = 38), active CG (n = 35), and waiting list CG (n = 24). Data for the IG and waiting list CG were used in this review. |
|
| Participants |
Setting: participants' homes Country: the Netherlands Sample size: 97 adults, 69% men; IG: 38; waiting list CG: 24 Inclusion criteria: stroke 3 months to 5 years prior, aged 30–80 years, and had received rehabilitation therapy as inpatient or outpatient, cognitive impairments after stroke (as testified by medical records), with cognitive complaints still present at study entry, able to work with a computer and have daily access to a computer with Internet connection Exclusion criteria: presence of neurodegenerative disease; epilepsy; serious psychiatric illness; any disease other than stroke that resulted in severe cognitive impairments; drug or alcohol dependency; severe colour blindness, aphasia, neglect, or computer fear; disabling vision or auditory problems; diagnosed learning disability, not mentally or physically fit enough to be able to complete 12 weeks of training, unable to understand the training instructions or who could not execute the training due to any other unforeseen reason, after instructions or after the first training week (van de Ven 2017a) Age: mean: IG: 57 (SD 9.1) years; waiting list CG: 61.2 (SD 9.0) years Time since stroke onset: mean: IG: 28.3 (SD 16.4) months; waiting list CG: 29.1 (SD 17.0) months Types of stroke: not reported Hemisphere: not reported |
|
| Interventions |
Intervention group Brief name: cognitive flexibility training (BrainGymmer) Recipients: adults with stroke within last 5 years with cognitive impairments Why: to improve cognitive flexibility and executive functioning through cognitive computer‐based training that included frequent switching between various training tasks. What (materials): online platform BrainGymmer (in Dutch); English version: www.braingymmer.com/en/; daily access to a computer with Internet connection and sound (either through headset or speakers); 9 tasks consisting of 20 levels selected to train 3 cognitive domains: attention, reasoning, and WM (see Additional file 2 van de Ven 2015 for brief descriptions and pictures of tasks; www.ncbi.nlm.nih.gov/pmc/articles/PMC4545547/:
Training videos of each task on individual computers Instruction booklet What (procedures): training instructions were provided at baseline using videos on computers followed by supervised practice by participants and provision of training booklet. Participants trained daily on several tasks within 1 session and frequently switching between the tasks. Participants were asked to train when they had ≥ 50 minutes available and when not mentally fatigued (e.g. late at night). Personal feedback was provided based on individuals' scores after each task, using a 3‐star rating scale, with more stars for better performance and at the end of each session with more detailed feedback of their scores on each task. Email availability for questions or to address any problems. Weekly or fortnightly contact by telephone for participants to ask questions and for discussion about training adherence. Reminder email sent if no training for 2 days and personal contact if 3 days without training. Daily log by participants. Exit questionnaire administered. Who provided: trained Master's degree‐level students who were familiar with all training tasks and login procedures for remote support and training instruction. Neuropsychologist for weekly or fortnightly contact. How: individually and independently without face‐to‐face supervision at the time but with remote assistance if required; individual and personal telephone and email contact with trainers/neuropsychologist Where: at home for computer tasks; training at University of Amsterdam When and how much: 10 tasks daily for 3 minutes each (30 minutes), 5 times per week for 12 weeks; 58 sessions (29 hours in total); In week 1, participants trained for 10 minutes each day on 3 tasks then from week 2, the number of trials per task was reduced to promote frequent switching between task. Trainer contact weeks 1, 2, 3, 5, 6, 8, and 10. Email contact if no training for 2 days Tailoring: difficulty of tasks was adapted individually to the performance of participants. Participants were instructed to go to the next level when obtaining 2 or 3 stars. However, participants could choose to stay at the same level when receiving 2 stars, whereas they were obliged to stay at the same level when obtaining no or 1 star. Tasks were set up each session for each participant. Order of tasks ensured that tasks from the same cognitive domain (attention, reasoning, and WM) were not presented immediately after each other (van de Ven 2015). Emails were sent as soon as participants did not train for 2 days. Modifications: none reported How well (planned): weekly or fortnightly discussion by telephone about training adherence. Number of sessions was recorded. Degree of adaptiveness of the CT was assessed. Amount of extra personal contact (telephone or email) due to questions or technical issues during training and level of engagement (i.e. how often a reminder to train was needed) was recorded. Participant daily log of their level of motivation during training, amount of PE at the day of training, how interesting and difficult the tasks of that day were, fatigue level before and after training. Exit questionnaire about subjective training effectiveness; change of strategies during training; check of blinding to experimental condition; changes in cognitive stimulation in daily life besides study related training; and major changes during training. How well (actual): the number of sessions did not differ significantly between the intervention training group and the active control group. The degree of adaptiveness was compromised in 17% of the intervention group participants. 5 participants (17%) were slightly less challenged in the last weeks of the training because they reached the highest level and score possible on 1 of the 9 tasks. Comparator group Brief name: waiting list CG or mock training (active CG) Recipients: adults with stroke within last 5 years with cognitive impairments Why: active CG: to provide comparable computer‐based training selected to be sufficiently challenging but not too difficult and thought to be to unlikely to improve executive functioning; waiting list CG: no intervention What (materials): active CG: non‐adaptive mock training consisting of 4 computer tasks using same online platform braingymmer with 9 levels each (see Additional file 2 van de Ven 2015 for brief descriptions and pictures of tasks); Fuzzle, Sliding Search, Pay Attention, Grid Tracks; daily access to a computer with Internet connection and sound (either through headset or speakers) Training videos of each task on individual computers Instruction booklet; waiting list CG: none What (procedures): active CR completed computer‐based tasks with the same amount of feedback, motivational instructions, visual stimulation, and use of mouse as the intervention training. Email availability for questions or to address any problems. Weekly or fortnightly contact by telephone for participants to ask questions and for discussion about training adherence. Reminder email sent if no training for 2 days. Daily log by participants. Exit questionnaire administered; waiting list AC did not receive training and were not contacted by telephone during the first 12 weeks. Who provided: active CG: trained Master's degree level students who were familiar with all training tasks and login procedures for remote support and training instruction. Neuropsychologist for weekly or fortnightly contact; waiting list CG: no‐one How: active CG: individually and independently without face‐to‐face supervision at the time but with remote assistance if required; individual and personal telephone and email contact with trainers/neuropsychologist; waiting list CG: none Where: active CG: at home; training at University of Amsterdam; waiting list CG: none When and how much: active CG: 3 tasks per day for 10 minutes (30 minutes) switching between tasks every 10 minutes; waiting list CG: none Tailoring: active CG: level increased each week during first 5 weeks, then every 2 weeks during weeks 6–12 unless participant did not master a level (at least 1 star). Emails were sent as soon as participants did not train for 2 days; waiting list CG: none Modifications: none How well (planned): active CG: weekly or fortnightly discussion by telephone about training adherence. Number of sessions was recorded. Degree of adaptiveness of the cognitive training assessed. Amount of extra personal contact (telephone or email) due to questions or technical issues during training and level of engagement (i.e. how often a reminder to train was needed) recorded. Participant daily log of their level of motivation during training, amount of PE at the day of training, how interesting and difficult the tasks of that day were, and fatigue level before and after training. Exit questionnaire about subjective training effectiveness; change of strategies during training; check of blinding to experimental condition; changes in cognitive stimulation in daily life besides study related training; and major changes during training; waiting list CG: none How well (actual): active CG: participants were asked not to train beyond level 9. However, some participants disregarded this and trained at higher levels. The degree of adaptiveness was compromised in 83% of the active control group; waiting list CG: none |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: neuropsychological outcomes assessed by assessor blinded to groups. Some outcomes were measured online without an assessor and remainder in person at the University Data collection time points: baseline (week 1), after 12 weeks' intervention (week 13), 4 weeks after completion of intervention (week 17) |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: yes (van de Ven 2015) Trial registration: yes: Central Committee on Research Involving Human Subjects Register NL4468502913; Netherlands National Trial Register NTR5174 Ethics approval: yes Data: reported results included some for participants randomised to the IG and CG and some for participants who completed the training according to the protocol (e.g. completed ≥ 50 sessions) and who completed outcome measures after the intervention and on follow‐up. Where possible we used the data reported for those participants who completed the protocol and did not drop out. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by randomisation software (Minimpy). |
| Allocation concealment (selection bias) | Unclear risk | Process of allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants of the IG and active CGs were blinded, the participants of the waiting list CG would not have been blinded. The person administering the computer tasks and training instructions was not blind to training allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Groups were coded by the research co‐ordinator such that the assessors were blind to which training condition participants were in. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although the paper indicates loss to follow‐up, the reasons for losses were reported and these were balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported as per protocol. |
| Other bias | Low risk | No other identifiable bias. |
Walker 2012.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 2 years (1 March 2008 to 28 February 2010) Unit of randomisation: people with stroke with persistent dressing problems and accompanying cognitive difficulties at 2 weeks after their stroke Recruitment and allocation: 965 consecutive inpatients were monitored by the ward occupational therapists over 2 weeks of conventional rehabilitation to identify those people with persistent dressing difficulties. Of 110 screened, 70 people met inclusion criteria and were randomised by Internet randomisation to the IG (n = 36) or CG (n = 34). Data from 33 participants from the IG and 31 participants from the CG were analysed. |
|
| Participants |
Setting: stroke rehabilitation wards Country: UK Sample: 70 adults, 41% men; allocated: IG: 36; CG: 34; analysed: IG: 33; CG: 31 Inclusion: scoring < 100% on the Nottingham Stroke Dressing Assessment and having confirmed cognitive impairment from a brief cognitive screening test Exclusion: unable to tolerate sitting in a chair for 15 minutes, premorbid disability (Rankin > 3), known diagnosis of depression or dementia, unable to understand English if it was not their first language Age: median: IG: 77 (range 47–93) years; CG: 81 (range 41–96) years Time since stroke onset: median: IG: 26 (range 12–139) days; CG: 22 (range 13–99) days |
|
| Interventions |
Intervention group Brief name: neuropsychological approach to dressing (DRESS) Recipients: inpatient adults with cognitive impairments after acute stroke Why: to select tailored, evidence‐based techniques for dressing impairment from a preprepared neuropsychological treatment manual based on detailed cognitive testing that assessed the impact of deficits on performance using error analysis. What (materials): neuropsychological treatment manual of evidence‐based techniques culled from the wider neuropsychological literature and based on comprehensive literature searches, survey results (Walker 2003), and occupational therapy textbooks (Edmans 2001); standard T‐shirt available in different sizes (Sunderland 2006); error analysis rating form (see Fletcher‐Smith 2010) What (procedures): participants received further detailed cognitive testing and an assessment of the impact of cognitive deficits on dressing by observation of a standard task of putting on a t‐shirt (Sunderland 2006), with performance scored using an error analysis rating form (Fletcher‐Smith 2010). Based on the test results and observed errors, the occupational therapists selected interventions from a menu of evidence‐based techniques described in the neuropsychological treatment manual. Commonly used techniques included cueing and alerting procedures to combat neglect or attentional difficulties, systematic laying out of clothing to reduce spatial confusion and graded errorless learning strategies to enhance acquisition of dressing skills. Who provided: 2 research occupational therapists experienced in the treatment of people with stroke How: face‐to‐face and individually Where: in a stroke rehabilitation ward and in participants' homes if they were discharged from hospital before the end of the treatment period. When and how much: the aim was for 3 times per week for 6 weeks based on previous single case experiments (Sunderland 2006). They received a median of 13 sessions (minimum 0, maximum 18) of 18 possible sessions. Tailoring: interventions were selected on the basis of participants' test results and observed errors in the dressing assessment and provided at home if participants were discharged early. Modifications: none reported How well (planned): a random sample of treatment sessions were observed by an independent researcher to ensure the manuals were adhered to and that they included the actual treatment prescribed in the manual. How well (actual): the authors reported that they found a high level of fidelity of treatment but details were not specifically reported. Comparator group Brief name: functional group Recipients: inpatient adults with cognitive impairments after acute stroke Why: to provide usual care in addressing dressing difficulties by providing dressing practice using a problem‐solving approach, with assistance when required. What (materials): functional treatment manual What (procedures): dressing interventions included components such as putting the affected arm into the sleeve first, crossing affected leg over other leg to reach feet, energy conservation techniques, etc. There was no attempt to formally assess the participant's cognitive difficulties or relate them to evidence on which approach to training might be the most successful Who provided: 2 research occupational therapists experienced in the treatment of people with stroke How: face‐to‐face and individually Where: in a stroke rehabilitation ward and in participants' homes if they were discharged from hospital before the end of the treatment period When and how much: the aim was for 3 times per week for 6 weeks; received a median number of 12 sessions (minimum 0, maximum 18). Modifications: none reported Tailoring: none reported How well (planned): a random sample of treatment sessions was observed by an independent researcher to ensure the manuals were adhered to and that they included the actual treatment prescribed in the manual. How well (actual): the authors reported that they found a high level of fidelity of treatment but details were not specifically reported. |
|
| Outcomes |
Primary
Secondary: none Other
Methods of data collection: baseline assessments were conducted prior to randomisation and participants were assessed 6 weeks after randomisation by an independent assessor. Data collection time points: at baseline and 6 weeks |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: no Trial registration: yes; Dressing Rehabilitation Evaluation Stroke Study: ISRCTN14430342 Ethics approval: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… using … the University of Nottingham Clinical Trials Unit Internet randomization service, patients were randomized to one of two treatment groups … Patients were stratified by side of stroke and severity of their dressing problem". |
| Allocation concealment (selection bias) | Low risk | Quote: "… concealed allocation via the University of Nottingham Clinical Trials Unit internet randomization service". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The 2 groups continued with their usual rehabilitation therapy and nursing care and only differed in the type of dressing practice provided by the trial occupational therapists. Both interventions were delivered by 2 research occupational therapists experienced in the treatment of people with stroke. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were assessed … by an independent assessor who was masked to the patient's treatment group allocation. Masking of the independent assessor was monitored by completion of a best guess form. Masking of the outcome assessor was tested and found to be compromised for only six patients". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up was reported and managed in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcome measures identified in registered protocol reported on in the paper (ISRCTN14430342). |
| Other bias | Low risk | No other identifiable bias. |
Yeh 2019.
| Study characteristics | ||
| Methods |
Design: single‐blind, multisite RCT Duration of trial: 1 year (September 2016 to September 2017) Unit of randomisation: people with ≥ 6 months after ischaemic or haemorrhagic stroke with cognitive dysfunction stratified by MMSE scores (Strata 1: MMSE score: 19 to 24; Strata 2: MMSE score: 25–30) undergoing rehabilitation and matched for baseline characteristics Recruitment and allocation: of 46 people recruited from rehabilitation units, 30 were stratified by MMSE score then randomised to 1 of 2 groups: IG (n = 15) and active CG (n = 15) |
|
| Participants |
Setting: rehabilitation units Country: Taiwan Sample size: 30 adults, 70% men; IQ: 15 (53% men); CG: 15 (87% men) Inclusion criteria: ischaemic or haemorrhagic stroke occurring ≥ 6 months before enrolment; MMSE score ≥ 19; MoCA score < 26; self‐reported or informant‐reported memory or cognitive complaints or score on the Clinical Dementia Rating scale 0.5; able to follow the study instruction; adequate cardiopulmonary function to perform aerobic exercise; and able to walk with or without assistive devices Exclusion criteria: unstable medical history that might limit participation concomitant with other neurological disorders, such as Parkinson disease, amyotrophic lateral sclerosis, and multiple sclerosis; current participation in another interventional trial Age: mean: IG: 50.63 (SEM 3.99) years; CG: 60.21 (SEM 3.10) years Time since onset of stroke: mean: IG: 47.80 (SEM 11.49) months; CG: 94.43 (SEM 30.80) months Types of stroke: not reported Site of lesion: not reported |
|
| Interventions |
Intervention group Brief name: SEQ (sequential) Recipients: adults > 6 months poststroke with cognitive dysfunction Why: to provide a sequential combination of aerobic exercise and CT to "prepare the brain for the compensatory recruitment process in the cognitive training sessions that follow"; CT aimed to enhance cognitive functions, to facilitate several cognitive functions, including attention, recognition, colour and shape identification, calculation, visual perception, visuospatial processing, and executive function. What (materials): stationary bicycle; BrainHQ (Posit Science) computer‐based software and personal computer What (procedures): aerobic exercise followed by CT:
Who provided: not reported in the paper but confirmed by author contact that the intervention was delivered by certified occupational therapists. How: face‐to‐face individually monitored by staff Where: not specified but within rehabilitation unit When and how much: 60 minutes per session consisting of 30 minutes of exercise and 30 minutes of CT, 2 or 3 days per week for 12–18 weeks for a total of 36 sessions (36 hours in total) Tailoring: exercise intensity was progressed as participants improved their performance throughout the training; CT programme was adjusted automatically and continuously according to each participant's level of performance. Modifications: none reported How well (planned): not reported How well (actual): not reported except that the intervention was "feasible and safe, with low dropout rates". Comparator group Brief name: active control Recipients: adults > 6 months poststroke with cognitive dysfunction Why: to provide comparable active activities What: exercise training followed by mental activities:
Who provided: not reported How: face‐to‐face Where: not specified except within rehabilitation unit When and how much: 60 minutes of training sessions, 2 or 3 days per week for 12–18 weeks for a total of 36 sessions (36 hours in total) Tailoring: not reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary: none Secondary
Other
Methods of data collection: evaluator blinded to the group allocation assessed participants before and after the intervention programmes. Data collection time points: within 1 week before and after intervention (12–18 weeks) |
|
| Notes |
Funding: yes Conflict of interest: none Published trial protocol: no Trial registration: yes; NCT03045991 Ethics approval: yes Author contact: further information requested and provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme was generated with the web‐based Research Randomizer randomisation tool. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel for intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluator for the before and after assessments was blinded to the patient's group allocation" and "… the assessments were conducted by a trained and experienced therapist who was blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram indicated there were no dropouts. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes from method and protocol reported. |
| Other bias | Low risk | No other identifiable bias. |
Yoo 2015.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 6 months (October 2013 to March 2014) Unit of randomisation: inpatients with cognitive impairment after stroke Recruitment and allocation: people with stroke receiving inpatient rehabilitation; 46 participants randomised to IG (n = 23) or CG (n = 23) |
|
| Participants |
Setting: university hospital Country: Republic of Korea Sample: 46 adults, 37% men; IG: 23; CG: 23 Inclusion/exclusion criteria: not reported Age: mean: IG: 53.2 (SD 8.8) years; CG: 56.3 (SD 7.9) years Time since stroke onset: mean: IG: 11.8 (SD 7.5) months; CG: 10.7 (SD 6.2) months |
|
| Interventions |
Intervention group Brief name: RehaCom training Recipients: inpatients with stroke with cognitive impairment undergoing rehabilitation Why: to provide computer‐based cognitive rehabilitation to improve attention, focus, memory, spatial imagination, visual impairment, and visuomotor co‐ordination. What (materials): RehaCom software (Hasomed GmbH, Magdeburg, Germany; see hasomed.de/en/products/rehacom/; the software is available in 27 languages at no extra cost; computer. What (procedures): in addition to rehabilitation therapy, a computerised cognitive rehabilitation programme using the RehaCom software composed of 20 detailed training programs targeting attention, focus, memory, spatial imagination, visual impairment, and visuomotor co‐ordination Who provided: not reported; however, the research was conducted by occupational therapists. The RehaCom website states that the software is used (quote) "extensively by … occupational therapists" and other clinicians in rehabilitation centres, hospitals, and clinics. How: not reported, but appeared to be face‐to‐face and individually Where: inpatient rehabilitation in local hospital When and how much: 25 sessions of 30 minutes per day (5 times per week for 5 weeks) (12.5 hours in total) Tailoring: RehaCom reportedly (quote) "enables adjustment of difficulty based on the task performance capacity of the patient, immediate feedback, reduction in time spent by the therapist once the patient learns the therapy task, and maintenance of objective and continuous information concerning performance results". Modifications: none reported How well (planned): none reported How well (actual): none reported Comparator group Brief name: rehabilitation therapy Recipients: inpatients with stroke with cognitive impairment undergoing rehabilitation Why: to provide usual inpatient rehabilitation. What (materials): not reported What (procedures): included physical and occupational therapy Who provided: included physical and occupational therapists How: not reported, assumed face‐to‐face Where: inpatient rehabilitation in local hospital When and how much rehabilitation: not reported Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary Computerised Neuropsychological Test, including:
Methods of data collection: cognitive assessment was conducted by computerised testing; method of ADL assessment with FIM not described. Data collection time points: baseline and after 5 weeks of computerised cognitive rehabilitation |
|
| Notes |
Funding: not stated Conflict of interest: none reported Published trial protocol: none reported Trial registration: none reported Ethics approval: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into IG and CG with no further description of procedure. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly divided into IG and CG with no description of procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It appeared the participants and personnel would have known which group received the additional cognitive rehabilitation program. Quote: "The participants understood the objective of this study … The training group received rehabilitation therapy and an additional computerized cognitive rehabilitation program using the RehaCom software 30 minutes/day, 5 times/week for 5 weeks. The control group received only rehabilitation therapy, including physical and occupational therapy". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported; unclear who administered the FIM. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not address this criterion. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes from method reported. |
| Other bias | Low risk | No other identifiable bias. |
Zuchella 2014.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration of trial: 31 months (1 June 2010 to 31 December 2012) Unit of randomisation: inpatients with first‐ever stroke and confirmed cognitive deficits Recruitment and allocation: of 288 people consecutively referred, 196 were excluded, leaving 92 who were randomised by random number generator to 2 groups, IG (n = 45) and CG (n = 47); 2 participants from each group did not receive the allocated intervention due to worsening clinical conditions and 1 from the study group had poor compliance, leaving 42 participants in IG and 45 in CG. |
|
| Participants |
Setting: neurorehabilitation unit Country: Italy Sample: 92 participants randomised, 87 adults reported and analysed, 53% men; IG: 42; CG: 45 Inclusion criteria: first‐ever stroke within previous 4 weeks, aged 45–80 years, MMSE score > 10, cognitive deficits defined as test scores below population‐based norms on ≥ 3 neuropsychological tests Exclusion criteria: progressing stroke, neglect, aphasia, additional neuropsychological or psychiatric disorders, depression (> 7 on Hamilton Depression Rating Scale), premorbid IQ < 70 or pre‐existing dementia, visual deficits, motor impairment liable to affect performance on tests Age: median (quartiles): IG: 64 (25th 56.2; 75th 74.2) years; CG: 70 (25th 62.5; 75th 76.5) years Time for admission: median (quartiles): IG: 11.5 (25th 10; 75th 14) days; CG: 11 (25th 9; 75th 14) days Types of stroke: ischaemic: n: IG: 31 (73.8%); CG: 34 (75.6%) Site of lesion: n: IG: right 18 (43%), left 12 (29%), brain stem 8 (19%), cerebellum 3 (7%), bilateral 1 (2%); CG: right 27 (60%), left 14 (31%), brain stem 3 (7%), bilateral 1 (2%) NIHSS stroke severity score: median (quartiles): IG: 15 (25th 14; 75th 17); CG: 15 (25th 13; 75th 15.2) |
|
| Interventions |
Intervention group Brief name: CT Recipients: inpatients with first‐ever stroke and confirmed cognitive deficits Why: to provide early comprehensive cognitive rehabilitation combining computer training and metacognitive strategies. What (materials): computer and 2 software programs (in Italian):
What (procedures): in addition to usual rehabilitation care, including medications and physiotherapy, individual sessions performing activities using the software addressing the following cognitive domains:
While the participants performed the activities the psychologist suggested metacognitive strategies to them to develop their awareness and self‐regulation, e.g. the participant was asked to predict results on tasks and identify factors that were contributing to their successes and failures. In last 15 minutes of session, the psychologist reasoned with participants about any problems encountered, explaining how to transfer the learned strategies to everyday situations to foster their generalisation to real‐world tasks (e.g. participants were encouraged to adopt 'associative techniques' and to use their imagination to improve their memory. Who provided: 2 psychologists, experts in neuropsychology, provided the sessions; the website states that the activities can be administered by professionals or family caregivers and refers to "rehabilitation therapists" using features to track progress How: apparently individually and face‐to‐face Where: neurorehabilitation unit; website states activities can be performed at home When and how much: 4 × 1‐hour sessions per week over 4 weeks (16 hours in total) Tailoring: 3 levels of difficulty; progress occurred to the next level when 70% correct response rate achieved; if the participant failed an activity 3 times, the presentation was simplified and made more understandable through examples. Website states that the activities can be expanded and modified to suit the particular needs of the person and that the instructions can be heard or written. Modifications: not reported How well (planned): to ensure inter‐therapist reliability and to ensure that participants received the same guidance during the training sessions, the psychologists followed written instructions and examples defined during the drafting of the protocol. How well (actual): not reported Comparator group Brief name: usual care (plus sessions with psychologist) Recipients: inpatients with first‐ever stroke and confirmed cognitive deficits Why: to provide usual care and equivalent sessions with a psychologist. What (materials): general topics, news, and participants' recent activities for general discussion What (procedures): the participants spent the same amount of time with a psychologist, discussing general topics, news, and their recent activities and usual rehabilitation care (medications, physiotherapy) Who provided: psychologist and other providers, e.g. medical and physical therapists How: individual, face‐to‐face Where: neurorehabilitation unit When and how much: 16 hours of individual training, divided into 1‐hour sessions spread over 4 weeks (4 sessions per week) Tailoring: none reported Modifications: not reported How well (planned): not reported How well (actual): not reported |
|
| Outcomes |
Primary
Secondary
Other
Methods of data collection: functional evaluation using the FIM on admission and after intervention, neuropsychological assessment performed within first week of admission before randomisation and after intervention. All assessments performed by a psychologist blind to the randomization and not involved in their care. Data collection time points: baseline and after 4 weeks of intervention |
|
| Notes |
Funding: not reported Conflict of interest: none Published trial protocol: no Trial registration: no Ethics approval: yes Data: median and interquartile ranges converted to means and SDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… patients who met the inclusion criteria were enrolled in the study and randomly assigned to the study group (SG) [IG] or to the control group (CG) by means of a computer random number generator". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors reported that the CG received a sham intervention but the difference between the nature of the intervention (cognitive rehabilitation with a therapist leading them through computer activities) and control (discussion with therapist) would still be possible for participants to discern. Similarly, therapists were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the assessments were done by a psychologist blind to the patients' randomization and not involved in their care". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart showed the numbers missing (IG: 3; CG: 2) and who did not receive allocated intervention and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes from method reported. |
| Other bias | Low risk | No other identifiable bias. |
ACCPT: Auditory Controlled Continuous Performance Test; ADL: activities of daily living; AMPS: Assessment of Motor and Process Skills; APT: Attention Process Training; BADL: basic activities of daily living; BADS: Behavioural Assessment of Dysexecutive Syndrome; BNIS: Barrow Neurological Institute Screen for Higher Cerebral Functions; CACR: computer‐assisted cognitive rehabilitation; CCR: computer cognitive rehabilitation; CFQ: Cognitive Failures Questionnaire; CI: confidence interval; CO‐OP: Cognitive Orientation to Occupational Performance; COPM: Canadian Occupational Performance Measure; CT: cognitive training; CWIT‐3: Color Word Interference Test – Inhibition (Condition 3); CWIT 4: Colour Word Interference Test – Inhibition/Switching (Condition 4); DEX: Dysexecutive Questionnaire; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders – 4th Edition; DST: Digit Span Test; FAB: Frontal Assessment Battery; FIM: Functional Independence Measure; FIS: Fatigue Impact Scale; FSAQ: Full‐Scale Attention Quotient; GHQ‐28: 28‐item General Health Questionnaire; HADS: Hospital Anxiety and Depression Scale; HAT: home automation training; HDRS: Hamilton Rating Scale for Depression; IADL: instrumental activities of daily living; IQ: intelligence quotient; IVA‐CPT: Integrated Visual Auditory Continuous Performance Test; LOTCA: Lowenstein Occupational Therapy Cognitive Assessment; MoCA: Montreal Cognitive Assessment; MMSE: Mini‐mental State Examination; mRS: modified Rankin Scale; MVPT‐3: Motor‐free Visual Perception Test‐3; N/n: number of participants; NFB: neurofeedback; NIHSS: National Institutes of Health Stroke Scale; PASAT: Paced Auditory Serial Addition Test; PE: physical exercise; RAVLT: Rey Auditory Verbal Learning Test; RBMT‐II: Rivermead Behavioural Memory Test‐II; RCT: randomised controlled trial; SASS: Social Adaptation Self‐evaluation Scale; SC: standard care; SD: standard deviation; SEM: standard error of the mean; SF‐36 MCS: Medical Outcomes Study 36‐item Short‐Form – Mental Component Score; SF‐36 PCS: Medical Outcomes Study 36‐item Short‐Form – Physical Component Score; SS‐QOL: stroke‐specific quality of life; TBI: traumatic brain injury; TENS: transcutaneous electrical nerve stimulation; TMT‐A: Trail Making Test A; TMT‐B: Trail Making Test B; USER‐P: Utrecht Scale for Evaluation of Rehabilitation‐Participation; VCPT: Visual Continuous Performance Test; VST: Visual Span test; WAIS‐III: Wechsler Adult Intelligence Scale III; WAIS‐III‐NI: Wechsler Adult Intelligence Scale III Neuropsychological Instrument; WM: working memory.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abba 2020 | Ineligible study design. |
| Abd‐Elaziz 2015 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Aben 2014 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| ACTRN12617000009314 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Adomaviciene 2019 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Ahn 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Alipoor 2020 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Aparicio‐López 2015 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Ashman 2011 | < 50% participants with stroke. |
| Atteya 2020 | Outcomes not of interest to this review. |
| Backhaus 2014 | Study with mixed aetiology groups and unable to confirm with authors if > 50% participants with stroke. |
| Baltaduoniene 2019 | Participants not all cognitively impaired. |
| Baylan 2020 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Bertens 2013 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Bertens 2015 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Best 2018 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Bezdenezhnykh 2014 | Unable to establish eligibility from authors. |
| Bezdenezhnykh 2015 | Unable to establish eligibility from authors. |
| Boiko 2008 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Boss 2014 | Participants did not all have cognitive impairment. |
| Brainin 2015 | Participants did not all have cognitive impairment. |
| Bunketorp‐Käll 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Bushnik 2010 | < 50% participants with stroke. |
| Carr 2012 | < 50% participants with stroke. |
| Chan 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Chen 2006a | Unable to establish eligibility of the intervention from the authors. |
| Chen 2006b | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Cheng 2018 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Chuang 2017 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Cuberos‐Urbano 2018 | < 50% participants with stroke. |
| Cumming 2018 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Cumming 2019 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| das Nair 2012 | < 50% participants with stroke. |
| Dean 2018 | Participants did not have confirmed cognitive impairment. |
| Debreceni‐Nagy 2019 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| De Joode 2013 | < 50% participants with stroke. |
| De Luca 2014 | Study with mixed aetiology groups and with > 50% participants with stroke but separate data for participants with stroke not available from authors. |
| Devos 2009 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Dirette 1999 | Study with mixed aetiology groups; < 50% participants diagnosed with stroke. |
| Donkervoort 2001 | Intervention for perceptual not cognitive impairment. |
| Donnelly 2017 | < 50% participants with stroke. |
| Doornhein 1998 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Edmans 2000 | Intervention for perceptual not cognitive impairment. |
| Ehlhardt 2010 | < 50% participants with stroke. |
| Eksteen 2015 | Participants did not all have cognitive impairment. |
| Evans 2009 | < 50% participants with stroke. |
| Faria 2016 | Participants did not all have cognitive impairment. |
| Faria 2018 | Intervention covered by another Cochrane Review. |
| Faria 2020 | Participants did not have confirmed cognitive impairment. |
| Fasoli 2019 | Participants not all cognitively impaired. |
| Feng 2017 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Fernandez‐Solano 2020 | Ineligible study design. |
| Fong 2009 | < 50% participants with stroke. |
| Fotakopoulos 2018 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Fu 2015 | Ineligible study design. |
| Fujioka 2018 | Participants did not have confirmed cognitive impairment. |
| Gamito 2014 | Intervention covered by another Cochrane Review. |
| Gamito 2017 | Intervention covered by another Cochrane Review. |
| Gandy 2020 | < 50% participants with stroke. |
| Gasparinni 1979 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Gocheva 2018 | < 50% participants with stroke. |
| Gong 2017 | Unable to establish with authors if eligible occupational therapy intervention. |
| Gracey 2017 | < 50% participants with stroke. |
| Grau‐Sánchez 2018 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Gray 1992 | < 50% participants with stroke. |
| Guidetti 2015 | Participants did not all have cognitive impairment. |
| Harel‐Katz 2020 | Not all participants had confirmed cognitive impairment. |
| Heyn 2012 | Ineligible study design. |
| Hildebrandt 2011 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Howe 2014 | Intervention for perceptual not cognitive impairment. |
| Hsu 2018 | < 50% participants with stroke. |
| Hu 2003 | Unable to determine if the intervention meets the definition of occupational therapy used in this review. |
| Hung 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Johansson 2015 | Ineligible study design. |
| Jones 2018 | < 50% participants with stroke. |
| Kannan 2019 | Participants did not all have cognitive impairment. |
| Karimian 2017 | Unable to establish eligibility. |
| Karner 2019 | Intervention for perceptual not cognitive impairment. |
| Kersey 2019 | Ineligible outcome (of included study). |
| Kessler 2014 | Participants did not have confirmed cognitive impairment. |
| Kim 2011a | Unclear if randomised or quasi‐randomised study; no response from author to establish eligibility. |
| Kim 2011b | Intervention covered by another Cochrane Review. |
| Koch 2020 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Kyoung‐Hee 2015 | Participants did not all have cognitive impairment. |
| Lannin 2014 | Study with mixed aetiology groups; < 50% participants diagnosed with stroke. |
| Lawson 2020 | Not all participants had confirmed cognitive impairment. |
| Lemoncello 2011 | < 50% participants diagnosed with stroke. |
| Lesniak 2018 | < 50% participants with stroke. |
| Lindelov 2016 | Participants did not all have cognitive impairment. |
| Lindeløv 2017 | < 50% participants with stroke. |
| Liu 2014 | Participants not cognitively impaired. |
| Liu 2017 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Liu 2018 | Unable to confirm eligibility of intervention with authors. |
| Löw 2012 | Ineligible study design. |
| Lyukmanov 2019 | Not all participants had cognitive impairment or confirmed cognitive impairment. |
| Maier 2017 | Intervention covered by another Cochrane Review. |
| Man 2006 | Study with mixed aetiology groups; unable to obtain separate data for participants with stroke from authors. |
| Manuli 2020 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Mao 2020 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Markovic 2019 | Unable to obtain separate data for participants with stroke. |
| McDonald 2011 | < 50% participants diagnosed with stroke. |
| McEwen 2015 | Participants did not all have cognitive impairment. |
| Merchán‐Baeza 2015 | Participants did not all have cognitive impairment. |
| Miller 2014 | Ineligible study design. |
| Moon 2020 | Participants did not have confirmed cognitive impairment. |
| Mount 2007 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| O'Neil‐Pirozzi 2016 | Ineligible study design. |
| Ownsworth 1999 | < 50% participants with stroke. |
| Padua 2020 | Not all participants had cognitive impairment or confirmed cognitive impairment. |
| Pallesen 2018 | Outcomes not of interest to this review. |
| Pallesen 2019 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Park 2013 | < 50% participants with stroke. |
| Park 2015b | Ineligible study design. |
| Park 2018 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Park 2019 | Participants did not meet the inclusion criterion of confirmed cognitive impairment. |
| Park 2020 | Ineligible study design. |
| Peers 2020 | Not all participants had confirmed cognitive impairment. |
| Petruševičienè 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Ploughman 2008 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Ploughman 2017 | Outcome not of interest to this review. |
| Ploughman 2018 | Outcome not of interest to this review. |
| Ploughman 2019 | Outcome not of interest to this review. |
| Polatajko 2012 | Participants did not all have confirmed cognitive impairment. |
| Poulin 2013 | Unable to obtain separate data for the 7 randomised participants. |
| Powell 2012 | < 50% of participants diagnosed with stroke. |
| Powell 2019 | < 50% participants with stroke. |
| Preminger 2016 | Intervention covered by another Cochrane Review. |
| Prokopenko 2012 | Unable to establish eligibility with authors. |
| Prokopenko 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Rackoll 2018 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Ramirez‐Hernandez 2020 | Participants did not all have confirmed cognitive impairment. |
| Ranzani 2020 | Participants did not all have confirmed cognitive impairment. |
| Richard 2020 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Richter 2015 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Russo 2017 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Salaris 2014 | Ineligible study design. |
| Salaris 2016 | Ineligible study design. |
| Salvadori 2016 | < 50% participants diagnosed with stroke. |
| Sampanis 2015 | Ineligible study design. |
| Sarkamo 2008 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Shi 2017 | Participants did not all have cognitive impairment or confirmed cognitive impairment. |
| Shim 2007 | Ineligible study design. |
| Shottke 1997 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Skidmore 2015b | Study previously included but no additional data identified. |
| Soderback 1988 | Not all participants had cognitive impairment as defined in this review. |
| Spahn 2010 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Spikman 2010 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Spikman 2013 | < 50% of participants diagnosed with stroke. |
| Svaerke 2019 | Intervention for perceptual not cognitive impairment. |
| Szepfalusi 2017 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Thickpenny‐Davis 2007 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Tornas 2014 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Tornas 2019 | < 50% participants had stroke. |
| Torrisi 2019 | Intervention covered by another Cochrane Review. |
| Veisi‐Pirkoohi 2020 | Participants were not confirmed to have cognitive impairment. |
| Virk 2015 | Ineligible study design. |
| Visser 2013 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Visser 2016 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Weicker 2016 | Ineligible study design. |
| Weicker 2020 | Participants did not all have confirmed cognitive impairment. |
| Wentink 2014 | Participants had self‐perceived cognitive impairment, not confirmed cognitive impairment on recruitment. |
| Wentink 2016 | Participants had self‐perceived cognitive impairment, not confirmed cognitive impairment on recruitment. |
| Westerberg 2007 | Participants had self‐perceived cognitive impairment, not confirmed cognitive impairment on recruitment. |
| Wilson 2014 | Study with mixed aetiology groups; < 50% participants diagnosed with stroke. |
| Winkens 2009 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Withiel 2018 | Participants had self‐reported memory complaints on study inclusion, not confirmed cognitive impairment. |
| Withiel 2019 | Participants had self‐reported memory complaints on study inclusion, not confirmed cognitive impairment. |
| Wolf 2016 | Participants did not all have cognitive impairment. |
| Wood 2012 | < 50% participants diagnosed with stroke. |
| Yip 2012 | Unable to contact authors to confirm eligibility of intervention and obtain final results. |
| Yu 2019 | Participants did not have confirmed cognitive impairment. |
| Zengin‐Metli 2018 | Participants did not have confirmed cognitive impairment. |
| Zhang 2016a | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
| Zhang 2019 | Participants did not have confirmed cognitive impairment. |
| Zheng 2018 | Intervention did not meet the definition of eligible occupational therapy intervention used in this review. |
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12619001557123.
| Methods | RCT |
| Participants | Adults with ischaemic or haemorrhagic stroke undergoing rehabilitation |
| Interventions | ELEMENTS‐T tablet, an electronic device containing a computer software providing a mix of goal‐directed and exploratory upper limb movement and cognitive interactive tasks compared with conventional rehabilitation |
| Outcomes |
|
| Notes | ACTRN12619001557123; protocol registered 12 November 2019 Principal investigator: Jeffrey Rogers Need to establish eligibility including whether participants had confirmed cognitive impairment. |
Askim 2019.
| Methods | RCT |
| Participants | 490 adults with first‐ever stroke |
| Interventions | Monthly meetings with stroke co‐ordinator compared with usual care |
| Outcomes |
|
| Notes | All participants may not have confirmed cognitive impairment; need to establish if eligible occupational therapy intervention NCT03859063; last verified November 2020 Estimated study completion date: 31 December 2032 Principal investigators: T Askim, A Hokstad, J Helbostad |
Bergfeldt 2019.
| Methods | RCT |
| Participants | 20 people with stroke or TBI with cognitive impairments aged 18–60 years |
| Interventions | Rehabilitation programme plus 30 minutes' aerobic training 3 or 4 times per week on stationary ergometer bicycle |
| Outcomes |
|
| Notes | Conference abstract; may not be eligible intervention and may not have > 50% participants with stroke |
ChiCTR1800015132.
| Methods | Parallel RCT |
| Participants | Adults with first cerebral infarction with cognitive impairment |
| Interventions | Robot rehabilitation compared with traditional rehabilitation |
| Outcomes |
|
| Notes | ChiCTR1800015132 protocol; last refreshed March 2018 Investigators: Yanqing Wu, Hongge Li Need to establish if eligible occupational therapy intervention |
ChiCTR1800018633.
| Methods | Parallel RCT |
| Participants | 46 adults with stroke and clinical comprehensive judgement of definite cognitive dysfunction after stroke |
| Interventions | Routine cognitive training and cognitive training based on PASS compared with control group of 2 routine cognitive training sessions |
| Outcomes |
|
| Notes | ChiCTR1800018633 protocol; last refreshed: October 2018 Investigators: Lei Xingxing, Song Luping Need to confirm establishment of cognitive impairment and eligibility of intervention |
ChiCTR1900025540.
| Methods | 3‐arm RCT |
| Participants | 90 people with ischaemic cerebral infarction and confirmed cognitive impairment MMSE score ≤ 24 |
| Interventions | Internet‐based computer‐assisted cognitive training vs traditional cognitive training combined with general computer‐assisted cognitive training vs traditional cognitive training |
| Outcomes |
|
| Notes | ChiCTR1900025540 protocol; last updated 31 August 2019 Investigator: Xinxin Zhang, Xia Bi |
ChiCTR1900026532.
| Methods | RCT |
| Participants | Adults with stroke with mild cognitive impairment |
| Interventions | Yijinjing group training and routine rehabilitation vs routine rehabilitation |
| Outcomes |
|
| Notes | ChiCTR1900026532 protocol; last refreshed: October 2019 Investigators: Xue Xin, Hu Jun Need to establish if eligible intervention |
ChiCTR‐INR‐17013042.
| Methods | Parallel RCT |
| Participants | 90 adults with first‐ever stroke and confirmed cognitive impairment |
| Interventions | 3 groups
|
| Outcomes |
|
| Notes | ChiCTR‐INR‐17013042 protocol Principal investigators: Jing Tao, Tianshen Xiao, Yaqi Bao |
Chiu 2020.
| Methods | RCT |
| Participants | 24 people with stroke |
| Interventions | Home‐based ADL vs hospital‐based traditional rehabilitation |
| Outcomes | Cognition and visual perception |
| Notes | Conference abstract; need more details to determine eligibility |
CTRI/2017/11/010466.
| Methods | Parallel RCT |
| Participants | 160 stroke survivors |
| Interventions | Art therapy compared with standard hospital therapy |
| Outcomes |
|
| Notes | CTRI/2017/11/010466: www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=18433 Trial underway: last updated November 2019 Investigator: Prabha Participants may not have confirmed cognitive impairment and intervention may not be eligible |
Donnellan 2014.
| Methods | Single‐blind RCT |
| Participants | People with stroke and their carers |
| Interventions | REsources And LIfe Strategy Management (REALISM) intervention using goal setting and attainment care plan |
| Outcomes |
|
| Notes | Conference abstract; need to establish if eligible occupational therapy intervention and if participants have confirmed cognitive impairment |
Gil‐Pages 2018.
| Methods | Cross‐over RCT |
| Participants | 40 chronic‐stage stroke patients with cognitive impairment confirmed by pre‐intervention neuropsychological assessment |
| Interventions | 6 weeks of 5 sessions per week of cognitive training through customised tele‐rehabilitation platform ('Guttmann, NeuroPersonalTrainer') or sham intervention (ictus.online) |
| Outcomes | Attention, memory, executive function for daily activities Attention
Memory
Speed of processing
Visuoconstruction
Executive functions
Administered rating measures
Other
|
| Notes | NCT03326349; last verified May 2019 Correspondence: Macarena Gil‐Pagés, macarenagil@guttmann.com May not be eligible occupational therapy intervention if neuropsychologist required |
ISRCTN10613029.
| Methods | RCT |
| Participants | 50 stroke survivors with mild cognitive function in their chronic stage (≥ 18 points in Korean MMSE) |
| Interventions |
|
| Outcomes |
|
| Notes | Need to establish if occupational therapist eligible intervention ISRCTN10613029: study completed and results to be published Principal investigator: Hee‐Tae Jung |
Janssen 2014.
| Methods | Pilot RCT |
| Participants | 15 stroke patients |
| Interventions | Tablets and Technology (TnT): participants provided with training and access to a tablet during inpatient rehabilitation and after discharge home |
| Outcomes |
|
| Notes | Conference abstract; need to establish eligibility of participants and intervention ACTRN12614000081617 last updated 22 January 2014 |
Jung 2019.
| Methods | RCT |
| Participants | 50 stroke survivors in the chronic stage (≥ 1‐year poststroke) with moderate cognitive impairment |
| Interventions | Standard medical care or standard medical care plus serious games for 3 months |
| Outcomes |
|
| Notes | Conference abstract that appears to be early results of Jung 2019 protocol (ISRCTN10613029); need to establish eligibility of intervention |
NCT01934621.
| Methods | Parallel single‐blind RCT |
| Participants | 128 adult inpatients with primary diagnosis of acute stroke with impairment in higher order cognitive functions (Executive Interview‐14 ≥ 3) |
| Interventions | Intervention group: strategy training is a form of meta‐cognitive instruction that trains people with stroke‐related cognitive impairments to identify and prioritise problematic daily activities, identify the barriers impeding performance, generate and evaluate their own strategies to address barriers, and apply these skills through iterative practice. Participants use printed workbooks to learn and apply this method. Placebo group: attention control for the non‐specific effects of strategy training. The therapists will administer the standardised and dose‐matched protocol, using scripted open‐ended questions to facilitate participants' reflections on their rehabilitation activities and experiences. In lieu of the strategy training workbook materials, participants will complete a daily journal, and discuss their entries during attention control sessions. |
| Outcomes |
Primary
Secondary
|
| Notes | NCT01934621; last verified November 2020 Completion date: September 2020 Contact: Elizabeth R Skidmore, skidmore@pitt.edu |
NCT02384057.
| Methods | Parallel single‐blind RCT |
| Participants | Participants may include brain injury as well as stroke; aged ≥ 18 years (adult, senior), both genders Inclusion criteria: people with stroke or brain injury, cognitive deficits Exclusion criteria: uncontrolled medical comorbidities; unable to perform outcome measurements; aphasia, which hampers communication; prediagnosed psychological or other neurological disease not relevant to stroke or brain injury |
| Interventions | Confirmed intervention is provided by occupational therapists Intervention: cognitive rehabilitation with C8 sciences (1000 minutes of computerised cognitive rehabilitation with C8 sciences program) Active comparator: conventional cognitive rehabilitation (Same amount of conventional cognitive rehabilitation with conventional methods) |
| Outcomes |
|
| Notes | NCT02384057; last verified March 2018 Study completed October 2017 Contact: Joon‐Ho Shin, asfreelyas@gmail.com |
NCT02925637.
| Methods | Single‐blind RCT |
| Participants | 46 adults after mild‐to‐moderate stroke living in the community |
| Interventions | Novel cognitive‐functional intervention (FACoT) in occupational therapy compared with usual care |
| Outcomes |
|
| Notes | Protocol; may not have confirmed cognitive impairment on enrolment NCT02925637; last verified February 2019 Study completion date: February 2020 Principal investigator: Tal Adamit |
NCT03168360.
| Methods | Single‐blind, parallel RCT |
| Participants | 150 adults with first‐ever stroke and Korean MMSE: 11–24 at 7 days after stroke onset |
| Interventions | Intensive cognitive rehabilitation compared with conventional cognitive rehabilitation by cognitive therapist |
| Outcomes |
|
| Notes | NCT03168360; last verified October 2020 Estimated study completion date: 31 December 2021 Principal investigators: Won Hyuk Chang, Yun‐Hee Kim Need to establish if occupational therapist eligible intervention |
NCT03621397.
| Methods | RCT |
| Participants | 40 Spanish‐speaking adults who have sustained a subarachnoid haemorrhage in the last 6 months |
| Interventions | Online Spanish cognitive intervention program (BrainHQ by Posit Science) |
| Outcomes |
|
| Notes | NCT03621397; last verified December 2020 Estimated completion date: August 2022 Principal investigator: Estevis E Need to establish if participants had confirmed cognitive impairment |
NCT03644290.
| Methods | Triple‐blind parallel RCT |
| Participants | 30 adults with chronic vascular Ischaemic lesions (> 6 months) |
| Interventions | Cognitive training vs behavioural information and education control |
| Outcomes |
|
| Notes | NCT03644290; last verified March 2020 Estimated study completion date: December 2021 Principal investigator: Mioto Need to establish if eligible intervention and if confirmed cognitive impairment on enrolment |
NCT03792061.
| Methods | Double‐blind, parallel RCT |
| Participants | 450 adults with stroke and cognitive impairment |
| Interventions | Strategy training in daily activities compared with reflective listening |
| Outcomes |
|
| Notes | NCT03792061; last verified July 2020 Estimated study completion date: 31 December 2023 Principal investigator: Chang FH Appears may be eligible study |
NCT03828851.
| Methods | Single‐blind RCT |
| Participants | 90 adults with stroke |
| Interventions | Home‐based ADL training vs traditional hospital‐based rehabilitation |
| Outcomes |
|
| Notes | NCT03828851; last verified January 2019 Estimated study completion date: 14 February 2021 Principal investigator: Der‐Sheng Han Need to establish if participants have confirmed cognitive impairment on enrolment |
NCT03890159.
| Methods | Double‐blind, parallel RCT |
| Participants | 45 adults with stroke |
| Interventions |
|
| Outcomes |
|
| Notes | NCT03890159; last verified March 2019 Estimated study completion date: 1 April 2020 Principal investigator: Hale Karapolat Need to establish if confirmed cognitive impairment on enrolment |
NCT04012866.
| Methods | RCT |
| Participants | 75 adults with ischaemic or haemorrhagic stroke occurring ≥ 6 months prior to enrolment with MMSE score < 28, or Montreal Cognitive Assessment score < 25 |
| Interventions | Sequential training group of 30‐minutes aerobic exercise training followed by 30‐minutes computerised cognitive training vs dual training group of simultaneous aerobic exercise training and computerised cognitive training vs active comparator of non‐aerobic physical activities and unstructured mental activities |
| Outcomes |
|
| Notes | NCT04012866; last updated June 2020 Estimated study completion date: 31 December 2021 Investigators: Wu Ching‐yi |
NCT04033952.
| Methods | Double‐blind, parallel‐group RCT |
| Participants | 180 participants with acquired brain injury |
| Interventions | OPASS ADL strategy training |
| Outcomes |
|
| Notes | NCT04033952; last verified October 2020 Estimated study completion date: August 2022 Principal investigator: Chang FH (Taiwan) Need to establish if > 50% of participants have stroke |
NCT04038424.
| Methods | RCT |
| Participants | 60 adults after ischaemic or haemorrhagic stroke |
| Interventions | Art therapy (painting, colouring, listening to music, and hand therapy ball exercises) in addition to routine hospital management and conventional rehabilitation therapy vs control of routine hospital management and conventional rehabilitation therapy |
| Outcomes |
|
| Notes |
NCT04038424 protocol; last verified October 2020; suspended (due to COVID‐19 pandemic) Estimated study completion date: December 2020 Investigator: Naglaa Fathy Afifi Youssef Need to establish if eligible intervention and if participants had confirmed cognitive impairment |
NCT04098835.
| Methods | Clinical trial (may not be eligible study design) |
| Participants | 20 adults with stroke |
| Interventions | ASCEND – combination of computer‐based cognitive training and coaching of cognitive strategies to improve daily cognitive functioning in people with stroke |
| Outcomes |
|
| Notes | NCT04098835; last verified December 2020 Estimated study completion: 1 October 2023 Principal investigator: Abhishek Jaywant, Ryan Lowder Need to establish eligibility as may not be eligible design and participants may not have confirmed cognitive impairment ("Subjective or objective evidence of mild cognitive impairment") |
NCT04099511.
| Methods | Single‐blind, parallel RCT |
| Participants | 135 adults < 9 months poststroke |
| Interventions | Cognitive Orientation to daily Occupational Performance (CO‐OP) approach vs usual occupational therapy care |
| Outcomes |
|
| Notes | NCT04099511 protocol; participants may be ineligible as confirmed cognitive impairment may be excluded; last verified December 2020 Estimated study completion date: 30 September 2023 Principal investigator: Timothy Wolf |
NCT04164381.
| Methods | Single group assignment so may not be RCT |
| Participants | 50 consecutive patients with subacute stroke (within 6 months of the event) |
| Interventions | Robotic treatment of the upper limb (30 sessions, 5 times per week) using a set of robotic devices |
| Outcomes |
|
| Notes | Protocol; study completed 30 March 2020; participants may not have confirmed cognitive impairment and study design may not be eligible NCT04164381; last verified 7 May 2020 Principal investigator: Aprile |
NCT04214314.
| Methods | RCT |
| Participants | 46 participants with acquired brain injury aged 18–65 years |
| Interventions | Integral rehabilitation plus NeuronUp APT vs integral rehabilitation |
| Outcomes |
|
| Notes | NCT04214314; last verified January 2020 Estimated study completion date: November 2021 Principal investigator: Juan Carlos Arango Lasprilla |
NCT04229056.
| Methods | RCT |
| Participants | 300 people with impaired working memory after stroke, cardiac arrest of Parkinson's disease |
| Interventions | COMPuter‐assisted Self‐training to Improve EXecutive Function (COMPEX) |
| Outcomes |
|
| Notes | NCT04229056; last verified July 2020 Estimated study completion: 31 December 2024 Principal investigator: H Christensen Need to establish if > 50% of participants have stroke |
NCT04246385.
| Methods | Pilot RCT |
| Participants | 22 rehabilitation day patients from stroke |
| Interventions | Cognitive Orientation to daily Occupational Performance (CO‐OP) vs usual occupational therapy care |
| Outcomes |
|
| Notes | NCT04246385; last verified October 2020 Estimated study completion date: November 2020 Investigators: Sarah Zera, Eileen Wilmsen Need to establish if participants had confirmed cognitive impairment |
NCT04282564.
| Methods | Unclear if RCT or cross‐over trial |
| Participants | Adults with stroke |
| Interventions | Cognitive Orientation to daily Occupational Performance (CO‐OP) approach |
| Outcomes |
|
| Notes | NCT04282564; last verified November 2020 Estimated study completion date: February 2022 Principal investigator: Xavier De Boissezon |
NCT04323501.
| Methods | RCT |
| Participants | 134 adults with stroke |
| Interventions | Intensive rehabilitation treatment of poststroke sensorimotor disability based on a "guided self‐rehabilitation contract" vs conventional outpatient rehabilitation |
| Outcomes |
|
| Notes | NCT04323501; last verified June 2020 Estimated study completion date: March 2025 Principal investigator: Nicole Smania |
NCT04399759.
| Methods | Cross‐over RCT |
| Participants | 40 adults with stroke |
| Interventions | Individualised instrumental activities of daily living training vs general home rehabilitation, not individualised |
| Outcomes |
|
| Notes | NCT04399759; last verified November 2020 Principal investigator: En‐Chi Chiu, Associate Professor, National Taipei University of Nursing and Health Sciences Estimated study completion date: 18 January 2021 May be related to study Chiu 2020 |
NCT04470219.
| Methods | RCT |
| Participants | 16 adults with cognitive impairment after stroke or other neurological diseases/injury |
| Interventions | Interactive web‐based mobile reminder calendar (RemindMe) training with an occupational therapist vs usual rehabilitation |
| Outcomes |
|
| Notes | NCT04470219; last verified July 2020 Principal investigator: Tiny Jaarsma Study appears to be completed but no results posted or cited |
NCT04472351.
| Methods | Pilot RCT |
| Participants | 70 participants with stroke |
| Interventions | "ASCEND‐I" (A Strategy and Computer‐based intervention to ENhance Daily cognitive functioning after stroke – Inpatient), an inpatient intervention that combines computer‐based cognitive training and coaching of cognitive strategies to improve working memory and related executive functions in individuals with stroke vs enhanced usual care control |
| Outcomes |
|
| Notes | NCT04472351; last verified November 2020 Estimated study completion date: August 2023 Principal investigator: Abhishek Jaywant |
Peers 2018.
| Methods | Wait‐list RCT |
| Participants | People with stroke |
| Interventions | 20 days of novel online selective attention training vs online working memory training vs wait list |
| Outcomes | Attention, working memory, and self‐reported everyday function |
| Notes | Conference abstract; participants may not have had confirmed cognitive impairment on enrolment |
Sahakian.
| Methods | Not stated |
| Participants | Adults with brain injury (traumatic injury, stroke, or brain tumour), estimated enrolment 50 participants |
| Interventions | 12 hours of cognitive training on a Neurogame |
| Outcomes |
|
| Notes | Source: das Nair R, Cogger H, Worthington E, Lincoln NB (2016). Cognitive rehabilitation for memory deficits after stroke. Cochrane Database of Systematic Reviews, 2016(9). Contact information: Prof Barbara Sahakian, bjssec@medschl.cam.ac.uk No reply from author to establish eligibility |
Slenders 2019.
| Methods | Multicentre, single‐blind, cluster‐RCT |
| Participants | Patients of 12 hospitals discharged home after ischaemic stroke |
| Interventions | Screening and patient‐tailored care for Emotional and COgnitive problems in people discharged home after ischaemic stroke (ECO‐stroke) including: screening for cognitive and emotional problems using sensitive instruments; active information provision and decision‐making according to the principles of self‐management support; and a protocol for referral if needed |
| Outcomes |
|
| Notes | Protocol: www.trialregister.nl/trial/7295 Intervention provided by nurses so may not be eligible occupational therapy intervention |
Wiseman 2016.
| Methods | RCT |
| Participants | 28 participants who had experienced a haemorrhagic or ischaemic stroke > 6 months pre‐enrolment |
| Interventions | 10 week (30 minutes, 3 times per week) cognitive training of a computerised sequential dual n‐back task (N‐IGMA) that required responding to sequentially presented paired audio and visual stimuli vs an active control group (Blockmaster) |
| Outcomes |
|
| Notes | Conference abstract; no further published result located. Need to establish eligibility of participants and intervention |
Yeh 2017.
| Methods | RCT |
| Participants | 75 stroke survivors with cognitive decline will be recruited |
| Interventions | Cognitive training, aerobic exercise, and sequential combination of aerobic exercise and cognitive training groups of 60 minutes per day, 3 days per week for 12 weeks |
| Outcomes |
|
| Notes | November 2018: authors confirmed this study is underway but awaiting more details to determine eligibility |
Yeh 2018.
| Methods | RCT (protocol) |
| Participants | 75 stroke survivors with mild cognitive decline will be recruited |
| Interventions | Sequential exercise‐cognitive training, dual‐task exercise‐cognitive training, or health‐related rehabilitation programmes of non‐aerobic physical exercise and unstructured cognitive related rehabilitation programmes (control group) |
| Outcomes |
Primary
Cognitive tasks
Secondary Biomarkers Cognitive function
Physical function
Daily function and quality of life
|
| Notes | November 2018: authors confirmed this study is underway but awaiting more details to determine eligibility |
Zhang 2016b.
| Methods | Multicentre RCT |
| Participants | 47 people with attention deficits |
| Interventions | 2 intervention groups of 30‐minute daily training for 6 weeks:
Control group: no attention training |
| Outcomes |
|
| Notes | Conference abstract Location of head researcher: Beijing Charity Hospital China Rehabilitation Research Center Unable to contact to establish eligibility |
ADL: activities of daily living; BADL: basic activities of daily living; BBT: Box and Blocks Test; CANTAB: Cambridge Neuropsychological Test Automated Battery; COPM: Canadian Occupational Performance Measure; CPT‐II: Conners Continuous Performance; DEX: Dysexecutive Questionnaire; FIM: Functional Independence Measure; HADS: Hospital Anxiety and Depression Scale; IADL: Instrumental Activities of Daily Living; MMSE: Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; mRS: modified Rankin Scale; NFI: Neurobehavioural Function Inventory; NIH: National Institutes of Health; NIHSS: National Institutes of Health Stroke Scale; OPASS: Optimising Participation after Stroke through Strategy‐training; PASS: Participation after Stroke through Strategy‐training; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; TBI: traumatic brain injury; TMT: Trail Making Test; TMT‐A: Trail Making Test A; TMT‐B: Trail Making Test B; USER‐P: Utrecht Scale for Evaluation of Rehabilitation‐Participation; WAIS‐III: Wechsler Adult Intelligence Scale.
Characteristics of ongoing studies [ordered by study ID]
Chen 2018.
| Study name | Early tablet‐assisted cognitive rehabilitation for aneurysmal subarachnoid hemorrhage: feasibility of a single‐center randomised controlled trial |
| Methods | Single‐centre RCT |
| Participants | People within 3 weeks after aneurysmal subarachnoid haemorrhage |
| Interventions | Intervention group
Control group
|
| Outcomes | Attention deficits, visual perception, and executive functions measured by:
|
| Starting date | Not applicable |
| Contact information | Bing Yu Chen Faculty of Medicine, Saint‐Laurent QC Canada |
| Notes | Although a feasibility study, check for later updates of intervention effects Access: n.neurology.org/content/90/15_Supplement/P3.227 |
Dawson 2013.
| Study name | Managing executive dysfunction following acquired brain injury and stroke using an ecologically valid rehabilitation approach |
| Methods | RCT (protocol) |
| Participants | 100 community‐dwelling adult survivors of acquired brain injury or stroke of ≥ 6 months from the Greater Toronto area with confirmed executive dysfunction |
| Interventions | 2 × 1‐hour sessions for 8 weeks (maximum of 15 hours of therapy) of either intervention or control Intervention group
Control group
|
| Outcomes | Primary
Secondary
|
| Starting date | March 2012 |
| Contact information | Deirdre R Dawson Rotman Research Institute, Baycrest, 3560 Bathurst Street, Toronto, ON M6A 2E1, Canada ddawson@research.baycrest.org |
| Notes | October 2018: authors confirmed study completed and results being submitted for publication NCT01414348; last verified January 2015 |
NCT02724813.
| Study name | Tele‐rehabilitation study for people with a history of stroke |
| Methods | RCT |
| Participants | 24 community‐dwelling adults ≥ 3 months' poststroke with impairment of executive cognitive functions |
| Interventions | Intervention group
Control group
|
| Outcomes |
|
| Starting date | November 2015 |
| Contact information | Yael Bar, MSW ybar@research.baycrest.org Adora Chui, MScOT achui@research.baycrest.org |
| Notes | NCT02724813; last verified February 2018 October 2018: authors confirmed recruitment is completed and results are being prepared for publication |
Weicker 2013.
| Study name | Computerized training of working memory for patients with acquired brain injury – a randomized controlled trial |
| Methods | RCT |
| Participants | People with stroke or traumatic brain injury of > 3 months and with reduced working memory performance in Germany |
| Interventions | Working memory training 3 times per week for 45 minutes over 1 month Intervention group
Control group
|
| Outcomes | Broad neuropsychological and multiple functional outcomes |
| Starting date | Not reported |
| Contact information | Mrs Juliane Weicker Max‐Planck‐Institute for Human Cognitive and Brain Sciences Leipzig, Neurology, Leipzig, Germany weicker@cbs.mpg.de |
| Notes | Conference abstract of early findings > 50% participants with stroke confirmed by authors March 2019: unable to locate published study and no reply from email to author if complete results published |
CO‐OP: Cognitive Orientation to daily Occupational Performance; COPM: Canadian Occupational Performance Measure; MoCA: Montreal Cognitive Assessment; RCT: randomised controlled trial.
Differences between protocol and review
There are several differences between the protocol (Hoffmann 2007), and this update of the review.
We have clarified the definition of eligible occupational therapy interventions from the original protocol such that in Types of interventions it now states that we "included studies where the intervention was delivered by an occupational therapist or under the supervision of an occupational therapist or where an occupational therapist was involved in the study. We also included interventions that are considered within occupational therapy scope of practice, which was informed by contemporary occupational therapy texts (e.g. Katz 2018; Toglia 2014), and surveys of practice (e.g. Holmqvist 2014; Koh 2009; Korner‐Bitensky 2011)."
Since the original review, another Cochrane Review has investigated the effectiveness of virtual reality interventions in stroke rehabilitation (Laver 2017), which included cognitive function among its outcomes. Therefore, we excluded virtual reality interventions from this update.
For cross‐over trials, we used the follow‐up data immediately postintervention for both groups rather than the latest follow‐up time point due to concerns about washout and residual training effects.
In the case of repeated observations, we planned to perform separate analyses for each outcome, based on the follow‐up periods of up to six months' duration, six to 12 months' duration, and more than 12 months' duration if available. However, due to the various lengths of interventions and the majority at 18 weeks or under and few studies conducting further follow‐up, we grouped analyses by postintervention then by follow‐up times, if any (i.e. three months, six months, and 12 months).
We did not conduct sensitivity analyses on the basis of trials with and without intention‐to‐treat analysis or for trials with follow‐up periods of less than six months' duration, six to 12 months' duration, and more than 12 months' duration.
In most recent searches, we did not search Dissertation Abstracts, handsearch relevant occupational therapy journals, or track relevant references through the Science Citation Index (SCI) and Social Science Citation Index (SSCI), as in the original review and protocol.
Contributions of authors
EG (updated review only): co‐ordinating the review; screening search results; organising the retrieval of papers; screening retrieved papers against inclusion criteria; writing to authors of papers for additional information; obtaining and screening data on unpublished studies; appraising the quality of papers; extracting data from papers; managing and analysing the data for review; interpreting the data (providing methodological, clinical, and policy perspectives); and writing the review.
CK: designing the review; designing search strategies; undertaking searches; screening search results; organising the retrieval of papers; screening retrieved papers against inclusion criteria; writing to authors of papers for additional information; providing additional data about papers; obtaining and screening data on unpublished studies; managing and analysing the data for review; interpreting the data (providing methodological, clinical, and policy perspectives); and writing the review.
SE (updated review only): screening search results; screening retrieved papers against inclusion criteria; organising the retrieval of papers; writing to authors of papers for additional information; obtaining and screening data on unpublished studies; appraising the quality of papers; extracting data from papers; managing and analysing the data for review; interpreting the data (providing methodological, clinical, and policy perspectives); and writing the review.
SB: in original review: screening retrieved papers against inclusion criteria; appraising the quality of papers; extracting data from papers; managing and analysing the data for the review; interpreting the data (providing methodological, clinical, and policy perspectives); and writing the review. Updated review: appraising the quality of papers; extracting data from papers; and writing and editing the review.
AS (updated review only): assisting with extracting data from papers, analysing data for the review, interpreting the data (providing methodological perspectives); and writing the review.
TH: conceiving, designing, and co‐ordinating the review; advising on search strategies; screening search results; screening retrieved papers against inclusion criteria; appraising the quality of papers; extracting data from papers; managing and analysing the data for review; interpreting the data (providing methodological, clinical, and policy perspectives); and writing the review.
Sources of support
Internal sources
-
None, Other
none
External sources
-
None, Other
none
Declarations of interest
EG: none.
CK: work as a health professional: works as an assistant professor of occupational therapy in the Department of Occupational Therapy, National Cheng Kung University, Taiwan.
SE: payment for writing this review: most of the work contributing to the writing of this review was done while employed by Bond University as a paid Research Fellow. Employed 0.2 FTE 2011–2015. Other work was completed in this role (i.e. this systematic review was not the only project worked on).
SB: none.
AS: none.
TH: grant and contacts: various research grants related to evidence‐based healthcare through the National Health and Medical Research Council. Royalties or licenses: royalties for book on evidence‐based practice published by Elsevier.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akerlund 2013 {published and unpublished data}
- Akerlund E, Esbjornsson E, Sunnerhagen KS, Björkdahl A.Can computerized working memory training improve impaired working memory, cognition and psychological health? Brain Injury 2013;27(13-14):1649-57. [DOI] [PubMed] [Google Scholar]
- Björkdahl A, Akerlund E, Svensson S, Esbjornsson E.A randomized study of computerized working memory training and effects on functioning in everyday life for patients with brain injury. Brain Injury 2013;27(13-14):1658-65. [DOI] [PubMed] [Google Scholar]
- Björkdahl A, Esbjornsson E, Svensson S, Akerlund E.Effects of working memory training on functioning in daily life. Brain Impairment 2012;13(1):182. [Google Scholar]
- Björkdahl A.Cochrane Review inclusion [personal communication]. Email to: S Eames 24 October 2014.
- Björkdahl A.Stroke patient data [Remeo\Slutdokument\Cochrane\Cochrane stroke fil.sav (as supplied 2015)]. Data on file.
Barker‐Collo 2009 {published data only}
- Barker-Collo S.Cochrane systematic review on occupational therapy for cognitive impairment in stroke patients [personal communication]. Email to: E Gibson 2 October 2019.
- Barker-Collo S.Details of APT training for Cochrane Review [personal communication]. Email to: S Barker-Collo 9 October 2015.
- Barker-Collo S.Means tables for Cochrane (as supplied 3 October 2019). Data on file.
- Barker-Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A.Reducing attention deficits after stroke using attention process training: a randomized controlled trial. Stroke 2009;40(10):3293-8. [DOI] [PubMed] [Google Scholar]
- Barker-Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A.Reducing post-stroke attention deficits using attention process training: results of the START trial. Brain Impairment 2011;12(S1):63. [Google Scholar]
- Dudley MD, Barker-Collo S, Starkey N.Impact of attention process training on attention in early recovery from stroke. International Journal of Stroke 2010;5(2 Suppl):58. [Google Scholar]
- Sohlberg MM.Attention process training details for Cochrane Review [personal communication]. Email to: E Gibson 19 October 2015.
Bo 2019 {published data only}
- Bo W, Lei M, Tao S, Jie LT, Qian L, Lin FQ, et al.Effects of combined intervention of physical exercise and cognitive training on cognitive function in stroke survivors with vascular cognitive impairment: a randomized controlled trial. Clinical Rehabilitation 2019;33(1):54-63. [DOI: 10.1177/0269215518791007] [DOI] [PubMed] [Google Scholar]
Carter 1983 {published data only}
- Carter LT, Howard BE, O'Neill WA.Effectiveness of cognitive skills remediation in acute stroke patients. American Journal of Occupational Therapy 1983;37(5):320-6. [DOI] [PubMed] [Google Scholar]
- Carter LT, Oliveira DO, Duponte J, Lynch SV.The relationship of cognitive skills performance to activity of daily living in stroke patients. American Journal of Occupational Therapy 1988;42(7):449-55. [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen C-X, Mao R-H, Li S-X, Zhao Y-N, Zhang M.Effect of visual training on cognitive function in stroke patients. International Journal of Nursing Sciences 2015;2(4):329-33. [Google Scholar]
- Chen C-X.Nature of data in Brain HQ study [关于「Brain HQ 视觉训练对卒中患者执行功能的作用」答复 [personal communication]. Email to: K Koh 5 June 2017.
- Li X, Pang H, Tian C, Dong Q, Chen C.Effect of Brain HQ visual training improved executive function in stroke patients. Chinese Journal of Cerebrovascular Diseases 2016;13(4):178-81. [Google Scholar]
Cho 2015 {published data only}
- Cho HY, Kim KT, Jung JH.Effects of computer assisted cognitive rehabilitation on brain wave, memory and attention of stroke patients: a randomized control trial. Journal of Physical Therapy Science 2015;27:1029-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2016 {published data only}
- Cho H-Y, Kim K-T, Jung J-H.Effects of neurofeedback and computer-assisted cognitive rehabilitation on relative brain wave ratios and activities of daily living of stroke patients: a randomized control trial. Journal of Physical Therapy Science 2016;28:2154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Luca 2018 {published data only}
- De Luca R, Leonardi S, Spadaro L, Russo M, Aragona B, Torrisi M, et al.Improving cognitive function in patients with stroke: can computerized training be the future? Journal of Stroke & Cerebrovascular Diseases 2018;27(4):1055-60. [DOI: 10.1016/j.jstrokecerebrovasdis.2017.11.008] [DOI] [PubMed] [Google Scholar]
Hasanzadeh Pashang 2020 {published data only}
- Hasanzadeh Pashang S, Zare H, Alipour A, Sharif-Alhoseini M.The effectiveness of cognitive rehabilitation in improving visual and auditory attention in ischemic stroke patients. Acta Neurologica Belgica 2020;121(4):915-20. [DOI: 10.1007/s13760-020-01288-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jiang 2016 {published data only}
- Jiang C, Yang S, Tao J, Huang J, Li Y, Ye H, et al.Clinical efficacy of acupuncture treatment in combination with RehaCom cognitive training for improving cognitive function in stroke: a 2 × 2 factorial design randomized controlled trial. Journal of the American Medical Directors Association 2016;17(12):1114-22. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only (unpublished sought but not used)}
- Lin Z, Tao J, Gao Y, Yin D, Chen A, Chen L.Analysis of central mechanism of cognitive training on cognitive impairment after stroke: resting-state functional magnetic resonance imaging study. Journal of International Medical Research 2014;42(3):659-68. [DOI] [PubMed] [Google Scholar]
Lundqvist 2010 {published and unpublished data}
- Lundqvist A, Grundstrom K, Samuelsson K, Ronnberg J.Computerized training of working memory in a group of patients suffering from acquired brain injury. Brain Injury 2010;24(10):1173-83. [DOI] [PubMed] [Google Scholar]
- Lundqvist A, Grundstrom K, Samuelsson K, Ronnberg J.Computerized training of working memory in a group of patients suffering from acquired brain injury. Brain Injury 2012;26(4-5):423-4. [DOI] [PubMed] [Google Scholar]
- Samuelsson K.Cochrane Review inclusion [personal communication]. Email to: S Eames 24 October 2014.
- Samuelsson K.Stroke patient data [To review study2.xlsx]. personal communication with author 2015.
Maggio 2020 {published data only}
- Maggio MG, Maresca G, Russo M, Stagnitti MC, Anchesi S, Casella C, et al.Effects of domotics on cognitive, social and personal functioning in patients with chronic stroke: a pilot study. Disability and Health Journal 2020;13(1):100838. [DOI: ] [DOI] [PubMed] [Google Scholar]
Park 2015a {published data only}
- Park J-H, Park J-H.The effects of a Korean computer-based cognitive rehabilitation program on cognitive function and visual perception ability of patients with acute stroke. Journal of Physical Therapy Science 2015;27:1577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prokopenko 2013 {published data only}
- Mozheyko E.Cochrane Review inclusion [personal communication]. Email to: S Eames 19 December 2014.
- Prokopenko SV, Mozheyko EY, Petrova MM, Koryagina TD, Kaskaeva DS, Chernykh TV, et al.Correction of post-stroke cognitive impairments using computer program. Journal of the Neurological Sciences 2013;325:148-53. [DOI] [PubMed] [Google Scholar]
Prokopenko 2018 {published data only}
- Prokopenko SV, Bezdenezhnykh AF, Mozheyko EU, Petrovaa MM.A comparative clinical study of the effectiveness of computer cognitive training in patients with post-stroke cognitive impairments without dementia. Psychology in Russia: State of the Art 2018;11(2):55-66. [DOI: 10.11621/pir.2018.0205] [DOI] [Google Scholar]
Prokopenko 2019 {published data only}
- Prokopenko S, Bezdenezhnykh A, Mozheyko E, Zubrickaya E.Effectiveness of computerized cognitive training in patients with poststroke cognitive impairments. Neuroscience and Behavioral Physiology 2019;49(5):539-43. [DOI: 10.1007/s11055-019-00767-3] [DOI] [Google Scholar]
Skidmore 2015a {published data only}
- NCT02755805.CO-OPerative training for stroke rehabilitation (CO-OP) [CO-OPerative training for stroke rehabilitation: a phase II trial examining meta-cognitive strategy training in acute stroke rehabilitation]. clinicaltrials.gov/ct2/show/study/NCT02755805 (first received April 2016).
- Skidmore ER, Dawson DR, Butters MA, Grattan ES, Juengst SB, Whyte EM, et al.Strategy training shows promise for addressing disability in the first 6 months after stroke. Neurorehabilitation and Neural Repair 2015;29(7):668-76. [NCT02755805] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore ER, Dawson DR, Whyte EM, Holm MB, Becker JT.Closing the gap: early intervention for cognitive disability after stroke. Archives of Physical Medicine and Rehabilitation 2012;93(10):E11. [Google Scholar]
Skidmore 2017 {published data only}
- NCT02766400.Examining rehabilitation training methods (GUIDE) [Guided versus directed training in acute stroke rehabilitation]. clinicaltrials.gov/ct2/show/NCT02766400 (first received May 2016).
- Skidmore E, Butters M, Grattan E, Waterstram L, Terhorst L.Guided discovery offers slight advantages over direct skill training in acute stroke rehabilitation. Brain Injury 2016;30(5-6):616-7. [Google Scholar]
- Skidmore ER, Butters M, Whyte E, Grattan E, Shen J, Terhorst L.Guided training relative to direct skill training for individuals with cognitive impairments after stroke: a pilot randomized trial. Archives of Physical Medicine and Rehabilitation 2017;98(4):673-80. [NCT02766400] [DOI] [PMC free article] [PubMed] [Google Scholar]
van de Ven 2017 {published data only}
- de Ven RM, Buitenweg JI, Schmand B, Veltman DJ, Aaronson JA, Nijboer TC, et al.Brain training improves recovery after stroke but waiting list improves equally: a multicenter randomized controlled trial of a computer-based cognitive flexibility training. PloS One 2017;12(3):e0172993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ven RM, Murre JM, Buitenweg JI, Veltman DJ, Aaronson JA, Nijboer TC, et al.The influence of computer-based cognitive flexibility training on subjective cognitive well-being after stroke: a multi-center randomized controlled trial. PloS One 2017;12(11):e0187582. [DOI: 10.1371/journal.pone.0187582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2012 {published data only}
- Walker MF, Drummond A, Edmans J, Fletcher-Smith J, Garvey K, Horn J, et al.Dressing rehabilitation stroke study: a randomised controlled trial (The DRESS study). International Journal of Stroke 2010;5(3 Suppl):9. [Google Scholar]
- Walker MF, Sunderland A, Fletcher-Smith J, Drummond A, Logan P, Edmans JA, et al.The DRESS trial: a feasibility randomized controlled trial of a neuropsychological approach to dressing therapy for stroke inpatients. Clinical Rehabilitation 2012;26(8):675-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeh 2019 {published data only}
- Wu C-Y, Yeh T-T, Hu Y-T, Chang K-C.The beneficial effects of sequential combination of cognitive training and aerobic exercise in stroke patients with cognitive decline. Stroke 2018;46(Suppl 1):TP140. [DOI: 10.1161/str.49.suppl_1.TP140] [DOI] [Google Scholar]
- Wu CY.Eligibility of stroke research for Cochrane systematic review [personal communication]. Email to: E Gibson 4 September 2019.
- Yeh TT, Chang KC, Wu CY.The active ingredient of cognitive restoration: a multicenter randomized controlled trial of sequential combination of aerobic exercise and computer-based cognitive training in stroke survivors with cognitive decline. Archives of Physical Medicine & Rehabilitation 2019;100(5):821-7. [NCT03045991] [DOI] [PubMed] [Google Scholar]
Yoo 2015 {published data only (unpublished sought but not used)}
- Yoo C, Yong M-H, Chung J-Y, Yang Y.Effect of computerized cognitive rehabilitation program on cognitive function and activities of living in stroke patient. Journal of Physical Therapy Science 2015;27:2487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuchella 2014 {published data only}
- Zucchella C, Capone A, Codella V, Vecchione C, Buccino G, Sandrini G, et al.Assessing and restoring cognitive functions early after stroke. Functional Neurology 2014;29(4):255-62. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abba 2020 {published data only}
- Abba MA, Olaleye OA, Hamzat TK.Effects of over-ground walking and cognitive rehabilitation on cognition, brain-derived neurotrophic factor, participation and quality of life among stroke survivors: a study protocol. European Journal of Physiotherapy 2020. [DOI: 10.1080/21679169.2020.1808056] [DOI]
Abd‐Elaziz 2015 {published data only}
- Abd-Elaziz SA, Khedr EM, Ahmed HA, Ibrahim HD.Effect of cognitive rehabilitation on improving cognitive function and activities of daily living among elderly patients with stroke at Assiut University Hospital. Journal of Education and Practice 2015;6:44-56. [Google Scholar]
Aben 2014 {published data only}
- Aben L, Heijenbrok-Kal M, Ponds R, Busschbach J, Ribbers G.Long term effects of a memory self-efficacy training program for stroke patients in the chronic stage: a randomized controlled trial. Brain Injury 2012;26:4-5. [Google Scholar]
- Aben L, Heijenbrok-Kal MH, Ponds RW, Busschbach JJ, Ribbers GM.Long-lasting effects of a new memory self-efficacy training for stroke patients: a randomized controlled trial. Neurorehabilitation & Neural Repair 2014;28(3):199-206. [DOI] [PubMed] [Google Scholar]
- Aben L, Heijenbrok-Kal MH, Ponds RW, Busschbach JJ, Ribbers GM.Training memory self-efficacy after stroke: a randomized controlled trial [Training van het geheugenzelfvertrouwen na een CVA* EEN RCT]. Nederlands Tijdschrift voor Geneeskunde 2013;157(32):1488-94. [Google Scholar]
- Aben L, Heijenbrok-Kal MH, Loon EM, Groet E, Ponds RW, Busschbach JJ, et al.Training memory self-efficacy in the chronic stage after stroke: a randomized controlled trial. Neurorehabilitation & Neural Repair 2013;27(2):110-7. [DOI] [PubMed] [Google Scholar]
ACTRN12617000009314 {published data only}
- ACTRN12617000009314.Comparison of computer-based training and compensatory memory rehabilitation in acquired brain injury. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371984 (first received 3 January 2017). [CENTRAL: 201808] [ACTRN12617000009314]
Adomaviciene 2019 {published data only}
- Adomaviciene A, Daunoraviciene K, Kubilius R, Varzaityte L, Raistenskis J.Influence of new technologies on post-stroke rehabilitation: a comparison of Armeo Spring to the Kinect System. Medicina 2019;55(4):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahn 2017 {published data only}
- Ahn SN, Yoo EY, Jung MY, Park HY, Lee JY, Choi YI.Comparison of Cognitive Orientation to daily Occupational Performance and conventional occupational therapy on occupational performance in individuals with stroke: a randomized controlled trial. Neurorehabilitation 2017;40(3):285-92. [DOI] [PubMed] [Google Scholar]
Alipoor 2020 {published data only}
- Alipoor L, Moosazadeh M, Esmaeili R, Akbari H, Cheraghmakani H, Shafipour V.Effects of early family-centered sensory stimulation on disability and rehabilitation of stroke patients. Journal of Mazandaran University of Medical Sciences 2020;30(185):61-73. [IRCT20151004024342N6] [Google Scholar]
Aparicio‐López 2015 {published data only}
- Aparicio-López C, García-Molina A, García-Fernández J, Lopez-Blazquez R, Enseñat-Cantallops A, Sánchez-Carrión R, et al.Cognitive rehabilitation with right hemifield eye-patching for patients with sub-acute stroke and visuo-spatial neglect: a randomized controlled trial. Brain Injury 2015;29(4):204-7. [DOI] [PubMed] [Google Scholar]
Ashman 2011 {published data only}
- Ashman T, Tsaousides T.Embedding problem solving and emotional regulation into traditional comprehensive day treatment program for brain injury. Brain Impairment 2011;12:62. [Google Scholar]
Atteya 2020 {published data only}
- Atteya AA, Shaker E, Elsemary MM, Alsaid HM, Elbalawy YM.Effect of cognitive behavioral therapy on functional outcomes in patients with stroke. International Journal of Research in Pharmaceutical Sciences 2020;11(2):2725-30. [Google Scholar]
Backhaus 2014 {published data only}
- Backhaus S, Ibarra S, Parrot D, Malec J.Comparison of a cognitive behavioural coping skills group to a peer support group in improving self-efficacy and neurobehavioural functions after brain injury. Brain Injury 2014;28(5-6):594. [Google Scholar]
Baltaduoniene 2019 {published data only}
- Baltaduoniene D, Kubilius R, Berškiene K, Vitkus L, Petruševičiene D.Change of cognitive functions after stroke with rehabilitation systems. Translational Neuroscience 2019;10(1):118-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baylan 2020 {published data only}
- Baylan S, Haig C, MacDonald M, Stiles C, Easto J, Thomson M, et al.Measuring the effects of listening for leisure on outcome after stroke (MELLO): a pilot randomized controlled trial of mindful music listening. International Journal of Stroke 2020;15(2):149-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylan S, McGinlay M, Macdonald M, Easto J, Cullen B, Haig C, et al.Participants' experiences of music, mindful music, and audiobook listening interventions for people recovering from stroke. Annals of the New York Academy of Sciences 2018;1423:349-59. [DOI] [PubMed] [Google Scholar]
Bertens 2013 {published data only}
- Bertens D, Fasotti L, Boelen DH, Kessels RP.A randomized controlled trial on errorless learning in goal management training: study rationale and protocol. BMC Neurology 2013;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertens 2015 {published data only}
- Bertens D, Kessels RP, Boelen DH, Fasotti L.Transfer effects of errorless goal management training on cognitive function and quality of life in brain-injured persons. Neurorehabilitation 2016;38(1):79-84. [DOI] [PubMed] [Google Scholar]
- Bertens D, Kessels RP, Fiorenzato E, Boelen DH, Fasotti L.Do old errors always lead to new truths? A randomized controlled trial of errorless goal management training in brain-injured patients. Journal of the International Neuropsychological Society 2015;21(8):639-49. [DOI] [PubMed] [Google Scholar]
Best 2018 {published data only}
- Best JR, Eng JJ, Davis JC, Hsiung R, Hall PA, Middleton LE, et al.Study protocol for Vitality: a proof-of-concept randomised controlled trial of exercise training or complex mental and social activities to promote cognition in adults with chronic stroke. BMJ Open 2018;8(3):e021490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bezdenezhnykh 2014 {published data only}
- Bezdenezhnykh A, Prokopenko S, Mozheyko E, Koryagina T, Chernukh T, Shvetsova I, et al.Computer cognitive training for patients with cognitive impairments in acute ischemic stroke. International Journal of Stroke 2014;9(S3):218. [Google Scholar]
Bezdenezhnykh 2015 {published data only}
- Bezdenezhnykh A, Prokopenko S, Mozheiko E, Petrova M, Grippa E, Lipatnikova S, et al.Neuropsychological computer training versus entertaining computer games in patients with post-stroke cognitive impairments: randomized clinical study. International Journal of Stroke 2015;10(Suppl 2):412. [Google Scholar]
- Bezdenezhnykh AF, Prokopenko SV, Mozheyko EY, Koryagina TV.Computer cognitive programs and entertaining computer games: what are differences? Neurodegenerative Diseases 2015;15(Suppl 1):1705. [Google Scholar]
Boiko 2008 {published data only}
- Boiko EA, Kulishova TV, Shumakher GI, Iusupkhodzhaev RV.The role of physical exercises in the improvement of cognitive functions in patients who survived stroke, in the early rehabilitative period. Voprosy Kurortologii, Fizioterapii i Lechebnoi Fizicheskoi Kultury 2008;6:9-12. [PubMed] [Google Scholar]
Boss 2014 {published data only}
- Boss HM, Schaik SM, Deijle IA, Melker EC, den Berg BT, Scherder EJ, et al.A randomised controlled trial of aerobic exercise after transient ischaemic attack or minor stroke to prevent cognitive decline: the MoveIt study protocol. BMJ Open 2014;4(12):e007065. [DOI: 10.1136/bmjopen-2014-007065 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brainin 2015 {published data only}
- Brainin M, Matz K, Nemec M, Teuschl Y, Dachenhausen A, Asenbaum-Nan S.Prevention of poststroke cognitive decline: ASPIS – a multicenter, randomized, observer-blind, parallel group clinical trial to evaluate multiple lifestyle interventions – study design and baseline characteristics. International Journal of Stroke 2015;10:627-35. [DOI] [PubMed] [Google Scholar]
- Brainin M, Matz K, Teuschl Y, Firlinger B, Dachenhausen A, Seyfang L, et al.Randomized, multi-center, multi-intervention study to prevent cognitive decline after ischemic stroke (ASPIS) – main results. International Journal of Stroke 2015;10:70. [Google Scholar]
Bunketorp‐Käll 2017 {published data only}
- Bunketorp-Käll L, Lundgren-Nilsson Å, Samuelsson H, Pekny T, Blomvé K, Pekna M, et al.Long-term improvements after multimodal rehabilitation in late phase after stroke. Stroke 2017;48(7):1916-24. [DOI] [PubMed] [Google Scholar]
Bushnik 2010 {published data only}
- Bushnik TE, Englander J, Oggins J, Levinson R, Halper D.A randomized clinical trial of a cognitive orthotic with executive planning capability in individuals with cognitive dysfunction. Brain Injury 2010;24(2):392-3. [Google Scholar]
Carr 2012 {published data only}
- Carr B, Allaous J, Lannin N, MacKenzie B, Falcon A, Tate R, et al.Efficacy of using handheld computers plus occupational therapy training to compensate for memory and planning difficulties after brain injury: a randomised control trial. Neurorehabilitation and Neural Repair 2012;26(6):687. [Google Scholar]
Chan 2017 {published data only}
- Chan WN, Tsang WW.Effect of Tai Chi training on dual-tasking performance that involves stepping down among stroke survivors: a pilot study. Evidence-based Complementary and Alternative Medicine 2017;2017:9134173. [DOI: 10.1155/2017/9134173] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2006a {published data only}
- Chen SZ, Jiang Q, Liu P, Huang DF, Ding JX.Effect of the cognitive rehabilitation on the functional independence of hemiplegic patients with stroke. Chinese Journal of Clinical Rehabilitation 2006;10(18):14-6. [Google Scholar]
Chen 2006b {published data only}
- Chen X-F.Effect of community-based-rehabilitation on activities of daily life and cognitive function in stroke patients. Chinese Journal of Clinical Rehabilitation 2006;25(10):4-6. [Google Scholar]
Cheng 2018 {published data only}
- Cheng C, Liu X, Fan W, Bai X, Liu Z.Comprehensive rehabilitation training decreases cognitive impairment, anxiety, and depression in poststroke patients: a randomized, controlled study. Journal of Stroke and Cerebrovascular Diseases 2018;27:2613-22. [DOI: 10.1016/j.jstrokecerebrovasdis.2018.05.038] [DOI] [PubMed] [Google Scholar]
Chuang 2017 {published data only}
- Chuang LL, Lin YH, Lien YS, Tsai CY, Yu MH, Hsu AL, et al.Comparative effectiveness research of dual-task and single-task balance training on gait speed and cognition in individuals with stroke. European Journal of Neurology 2017;24:218. [Google Scholar]
Cuberos‐Urbano 2018 {published data only}
- Cuberos-Urbano G, Caracuel A, Valls-Serrano C, Garcia-Mochon L, Gracey F, Verdejo-Garcia A.A pilot investigation of the potential for incorporating lifelog technology into executive function rehabilitation for enhanced transfer of self-regulation skills to everyday life. Neuropsychological Rehabilitation 2018;28(4):589-601. [DOI] [PubMed] [Google Scholar]
Cumming 2018 {published data only}
- Cumming TB, Bernhardt J, Lowe D, Collier J, Dewey H, Langhorne P, et al.Early mobilization after stroke is not associated with cognitive outcome findings from AVERT. Stroke 2018;49(9):2147-54. [DOI] [PubMed] [Google Scholar]
Cumming 2019 {published data only}
- Cumming TB, Churilov L, Collier J, Donnan G, Ellery F, Dewey H, et al.Early mobilization and quality of life after stroke: findings from AVERT. Neurology 2019;93(7):e717-28. [DOI: 10.1212/WNL.0000000000007937] [DOI] [PMC free article] [PubMed] [Google Scholar]
das Nair 2012 {published data only}
- das Nair R, Lincoln, NB.Evaluation of rehabilitation of memory in neurological disabilities (ReMiND): a randomized controlled trial. Clinical Rehabilitation 2012;26(10):894-903. [DOI] [PubMed] [Google Scholar]
Dean 2018 {published data only}
- Dean SG, Poltawski L, Forster A, Taylor RS, Spencer A, James M, et al.Community-based rehabilitation training after stroke: results of a pilot randomised controlled trial (ReTrain) investigating acceptability and feasibility. BMJ Open 2018;8:e018409. [DOI: 10.1136/bmjopen-2017-018409] [DOI] [PMC free article] [PubMed] [Google Scholar]
Debreceni‐Nagy 2019 {published data only}
- Debreceni-Nagy A, Horvath J, Bajuszne Kovacs N, Fulop P, Jenei Z.The effect of low-intensity aerobic training on cognitive functions of severely deconditioned subacute and chronic stroke patients: a randomized, controlled pilot study. International Journal of Rehabilitation Research 2019;42(3):275-9. [DOI] [PubMed] [Google Scholar]
De Joode 2013 {published data only}
- De Joode EA, Heugten CM, Verhey FR, Boxtel MP.Effectiveness of an electronic cognitive aid in patients with acquired brain injury: a multicentre randomised parallel-group study. Neuropsychological Rehabilitation 2013;23(1):133-56. [DOI] [PubMed] [Google Scholar]
De Luca 2014 {published data only}
- De Luca R, Calabro RS, Gervasi G, De Salvo S, BonannoL, Corallo F, et al.Is computer-assisted training effective in improving rehabilitative outcomes after brain injury? A case-control hospital-based study. Disability & Health Journal 2014;7(3):356-60. [DOI] [PubMed] [Google Scholar]
Devos 2009 {published data only}
- Devos H, Akinwuntan AE, Nieuwboer A, RingootI, Berghen K, Tant M, et al.Effect of simulator training on fitness-to-drive after stroke: a 5-year follow-up of a randomized controlled trial. Neurorehabilitation and Neural Repair 2010;24(9):843-50. [DOI] [PubMed] [Google Scholar]
- Devos H, Akinwuntan AE, Nieuwboer A, Tant M, Truijen S, De Wit L, et al.Comparison of the effect of two driving retraining programs on on-road performance after stroke. Neurorehabilitation & Neural Repair 2009;23(7):699-705. [DOI] [PubMed] [Google Scholar]
Dirette 1999 {published data only}
- Dirette DK, Hinojosa J, Carnevale GJ.Comparison of remedial and compensatory interventions for adults with acquired brain injuries. Journal of Head Trauma Rehabilitation 1999;14(6):595-601. [DOI] [PubMed] [Google Scholar]
Donkervoort 2001 {published data only}
- Donkervoort M, Dekker J, Stehmann-Saris FC, Deelman BG.Efficacy of strategy training in left hemisphere stroke patients with apraxia: a randomised clinical trial. Neuropsychological Rehabilitation 2001;11(5):549-66. [Google Scholar]
Donnelly 2017 {published data only}
- Donnelly KZ, Linnea K, Grant DA, Lichtenstein J.The feasibility and impact of a yoga pilot programme on the quality-of-life of adults with acquired brain injury. Brain Injury 2017;31(2):208-14. [DOI] [PubMed] [Google Scholar]
Doornhein 1998 {published data only}
- Doornhein K, DeHaan EH.Cognitive training for memory deficits in stroke patients. Neuropsychological Rehabilitation 1998;8(4):393-400. [Google Scholar]
Edmans 2000 {published data only}
- Edmans JA, Webster J, Lincoln NB.A comparison of two approaches in the treatment of perceptual problems after stroke. Clinical Rehabilitation 2000;14:230-43. [DOI] [PubMed] [Google Scholar]
Ehlhardt 2010 {published data only}
- Ehlhardt L, Glang A, Sohlberg M, Ettel D.Training assistive technology after acquired brain injury: results of a randomized controlled trial. Journal of Head Trauma Rehabilitation 2010;25(5):394. [Google Scholar]
Eksteen 2015 {published data only}
- Eksteen CA, Wyk A.The effect of saccadic eye movement training integrated into task-specific activities on cognitive function, depression and functional ability post-stroke. Physiotherapy 2015;101:eS349. [Google Scholar]
Evans 2009 {published data only}
- Evans JJ, Greenfield E, Wilson BA, Bateman A.Walking and talking therapy: improving cognitive-motor dual-tasking in neurological illness. Journal of the International Neuropsychological Society 2009;15(1):112-20. [DOI] [PubMed] [Google Scholar]
Faria 2016 {published data only}
- Faria AL, Andrade A, Soares L, Bermúdez i Badia S.Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: a randomized controlled trial with stroke patients. Journal of NeuroEngineering and Rehabilitation 2016;13(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faria 2018 {published data only}
- Faria AL, Cameirao MS, Couras JF, Aguiar JR, Costa GM, Bermudez IB.Combined cognitive-motor rehabilitation in virtual reality improves motor outcomes in chronic stroke – a pilot study. Frontiers in Psychology 2018;9:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faria 2020 {published data only}
- Faria AL, Pinho MS, Bermudez i Badia S.A comparison of two personalization and adaptive cognitive rehabilitation approaches: a randomized controlled trial with chronic stroke patients. Journal of NeuroEngineering and Rehabilitation 2020;17:78. [DOI: 10.1186/s12984-020-00691-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria Al, Pinho MS, Bermudez IBS.Clinical impact of a personalized ecologically valid virtual reality system in cognitive rehabilitation: a randomized controlled trial with chronic stroke patients. European Stroke Journal 2019;4(1S):268-9. [Google Scholar]
Fasoli 2019 {published data only}
- Fasoli SE, Adans-Dester CP.A paradigm shift: rehabilitation robotics, cognitive skills training, and function after stroke. Frontiers in Neurology 2019;10:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2017 {published data only}
- Feng H, Li G, Xu C, Ju C, Qiu X.Training rehabilitation as an effective treatment for patients with vascular cognitive impairment with no dementia. Rehabilitation Nursing 2017;42(5):290-7. [DOI] [PubMed] [Google Scholar]
Fernandez‐Solano 2020 {published data only}
- Fernandez-Solano AJ, Del Bano-Aledo ME, Rodriguez-Bailon M.Results of an occupational self-analysis program in people with acquired brain injury. A pilot study. Brain Injury 2020;34(2):253-61. [DOI] [PubMed] [Google Scholar]
Fong 2009 {published data only}
- Fong KN, Howie DR.Effects of an explicit problem-solving skills training program using a metacomponential approach for outpatients with acquired brain injury. American Journal of Occupational Therapy 2009;63(5):525-34. [DOI] [PubMed] [Google Scholar]
Fotakopoulos 2018 {published data only}
- Fotakopoulos G, Kotlia P.The value of exercise rehabilitation program accompanied by experiential music for recovery of cognitive and motor skills in stroke patients. Journal of Stroke and Cerebrovascular Diseases 2018;27(11):2932-9. [DOI: 10.1016/j.jstrokecerebrovasdis.2018.06.025] [DOI] [PubMed] [Google Scholar]
Fu 2015 {published data only}
- Fu MJ, Knutson JS, Chae J.Stroke rehabilitation using virtual environments. Physical Medicine and Rehabilitation Clinics of North America 2015;26(4):747-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujioka 2018 {published data only}
- Fujioka T, Dawson DR, Wright R, Honjo K, Chen JL, Chen JJ, et al.The effects of music-supported therapy on motor, cognitive, and psychosocial functions in chronic stroke. Annals of the New York Academy of Sciences 2018;1423:264-74. [DOI: 10.1111/nyas.13706] [DOI] [PubMed] [Google Scholar]
Gamito 2014 {published data only}
- Gamito P, Oliveira J, Santos N, Pacheco J, Morais D, Saraiva T, et al.Virtual exercises to promote cognitive recovery in stroke patients: the comparison between head mounted displays versus screen exposure methods. International Journal on Disability and Human Development 2014;13(3):337-42. [Google Scholar]
Gamito 2017 {published data only}
- Gamito P, Oliveira J, Coelho C, Morais D, Lopes P, Pacheco J, et al.Cognitive training on stroke patients via virtual reality-based serious games. Disability and Rehabilitation 2017;39(4):385-8. [DOI] [PubMed] [Google Scholar]
Gandy 2020 {published data only}
- Gandy M, Karin E, McDonald S, Meares S, Scott AJ, Titov N, et al.A feasibility trial of an internet-delivered psychological intervention to manage mental health and functional outcomes in neurological disorders. Journal of Psychosomatic Research 2020;136:110173. [DOI] [PubMed] [Google Scholar]
Gasparinni 1979 {published data only}
- Gasparinni B, Satz P.A treatment of memory problems in left hemisphere CVA patients. Journal of Clinical Neuropsychology 1979;1(2):137-50. [Google Scholar]
Gocheva 2018 {published data only}
- Gocheva V, Hund-Georgiadis M, Hediger K.Effects of animal-assisted therapy on concentration and attention span in patients with acquired brain injury: a randomized controlled trial. Neuropsychology 2018;32(1):54-64. [DOI] [PubMed] [Google Scholar]
Gong 2017 {published data only}
- Gong W-J.Effect of motor imagery therapy on cognitive function of patients with stroke. Chinese Journal of Contemporary Neurology and Neurosurgery 2017;17(6):415-20. [DOI: 10.3969/j.issn.1672-6731.2017.06.005] [DOI] [Google Scholar]
Gracey 2017 {published data only}
- Gracey F, Fish JE, Greenfield E, Bateman A, Malley D, Hardy G, et al.A randomized controlled trial of assisted intention monitoring for the rehabilitation of executive impairments following acquired brain injury. Neurorehabilitation and Neural Repair 2017;31(4):323-33. [DOI] [PubMed] [Google Scholar]
Grau‐Sánchez 2018 {published data only}
- Grau-Sánchez J, Duarte E, Ramos-Escobar N, Sierpowska J, Rueda N, Redón S.Music-supported therapy in the rehabilitation of subacute stroke patients: a randomized controlled trial. Annals of the New York Academy of Sciences 2018;1423:318-28. [DOI: 10.1111/nyas.13590] [DOI] [PubMed] [Google Scholar]
Gray 1992 {published data only}
- Gray JM, Robertson I, Pentland B, Anderson S.Microcomputer-based attentional retraining after brain damage: a randomised group controlled trial. Neuropsychological Rehabilitation 1992;2(2):97-115. [Google Scholar]
Guidetti 2015 {published data only}
- Guidetti S, Ranner M, Tham K, Andersson M, Ytterberg C, Koch L.A 'client-centred activities of daily living' intervention for persons with stroke: one-year follow-up of a randomized controlled trial. Journal of Rehabilitation Medicine 2015;47(7):605-11. [DOI] [PubMed] [Google Scholar]
Harel‐Katz 2020 {published data only}
- Harel-Katz H, Adar T, Milman U, Carmeli E.Examining the feasibility and effectiveness of a culturally adapted participation-focused stroke self-management program in a day-rehabilitation setting: a randomized pilot study. Topics in Stroke Rehabilitation 2020;27(8):577-89. [DOI: 10.1080/10749357.2020.1738676] [DOI] [PubMed] [Google Scholar]
Heyn 2012 {published data only}
- Heyn PC, Abreu BC, Ottenbacher KJ, Carollo J.Mixed-reality therapy for adults with cognitive impairments. Gait and Posture 2012;36:S66. [Google Scholar]
Hildebrandt 2011 {published data only}
- Hildebrandt H, Gehrmann A, Modden C, Eling P.Enhancing memory performance after organic brain disease relies on retrieval processes rather than encoding or consolidation. Journal of Clinical & Experimental Neuropsychology 2011;33(2):257-70. [DOI] [PubMed] [Google Scholar]
Howe 2014 {published data only}
- Howe J, Laverick R, Drozdowska B, Rotshtein P, Wing A.RCT of CogWatch system efficacy in rehabilitation of ADL skills. 9th UK Stroke Forum Conference; 2014 Dec 2-4; Harrogate, UK.
- Laverick R, Howe J, Chua W, Evans R, Jean-Baptiste E, Russell M, et al.Activities of daily living (ADL) rehabilitation for stroke apraxia and action disorganization syndrome (AADS): randomised controlled trial of the Cogwatch approach. International Journal of Stroke 2016;11(Suppl 4):61. [Google Scholar]
- Wing A.CogWatch v3 aw. UK Clinical Research Network Portfolio Database (UKCRN) 2014.
Hsu 2018 {published data only}
- Hsu CL, Best JR, Davis JC, Nagamatsu LS, Wang S, Boyd LA, et al.Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. British Journal of Sports Medicine 2018;52(3):184-91. [DOI] [PubMed] [Google Scholar]
Hu 2003 {published data only}
- Hu X, Dou Z, Zhu H, Wan G, Li J.The single blind procedure research of cognitive rehabilitation interventions on cognitive deficits in patients with stroke. Chinese Journal of Clinical Rehabilitation 2003;7(10):1521-3. [Google Scholar]
Hung 2017 {published data only}
- Hung JW, Chou CX, Chang HF, Wu WC, Hsieh YW, Chen PC, et al.Cognitive effects of weight-shifting controlled exergames in patients with chronic stroke: a pilot randomized comparison trial. European Journal of Physical and Rehabilitation Medicine 2017;53(5):694-702. [DOI] [PubMed] [Google Scholar]
Johansson 2015 {published data only}
- Johansson B, Bjuhr H, Ronnback L.Evaluation of an advanced mindfulness program following a mindfulness-based stress reduction program for participants suffering from mental fatigue after acquired brain injury. Mindfulness 2015;6(2):227-33. [Google Scholar]
Jones 2018 {published data only}
- Jones M.Exploring music-based cognitive rehabilitation following acquired brain injury: a randomized control trial comparing attention process training and musical attention control training. Dissertation Abstracts International: Section B: The Sciences and Engineering 2018;79:12-B(E). [Google Scholar]
Kannan 2019 {published data only}
- Kannan L, Vora J, Bhatt T, Hughes SL.Cognitive-motor exergaming for reducing fall risk in people with chronic stroke: a randomized controlled trial. Neurorehabilitation 2019;44(4):493-510. [DOI] [PubMed] [Google Scholar]
Karimian 2017 {published data only}
- Karimian N, Neshat-Doost HT, Asgari K, Orayzi HR, Najafi MR.Comparison of the effectiveness of mindfulness training and memory specificity training on the aspects of memory in patients with ischemic stroke. Journal of Isfahan Medical School 2017;35(441):961-8. [Google Scholar]
Karner 2019 {published data only}
- Karner S, Stenner H, Spate M, Behrens J, Krakow K.Effects of a robot intervention on visuospatial hemineglect in postacute stroke patients: a randomized controlled trial. Clinical Rehabilitation 2019;33(12):1940-8. [DOI: 10.1177/0269215519865993] [DOI] [PubMed] [Google Scholar]
Kersey 2019 {published data only}
- Kersey J, Juengst SB, Skidmore E.Effect of strategy training on self-awareness of deficits after stroke. American Journal of Occupational Therapy 2019;73(3):7303345020p1‐7303345020p7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2014 {published data only}
- Kessler DE, Egan MY, Dubouloz C-J, Graham FP, McEwen SE.Occupational performance coaching for stroke survivors: a pilot randomized controlled trial protocol. Canadian Journal of Occupational Therapy 2014;81(5):279-88. [DOI] [PubMed] [Google Scholar]
Kim 2011a {published data only}
- Kim YG.The effects of Korean computer-based cognitive rehabilitation (CoTras) for the cognition and ADL in stroke. Journal of Korean Society of Occupational Therapy 2011;19:75-87. [Google Scholar]
Kim 2011b {published data only}
- Kim BR, Chun MH, Kim LS, Park JY.Effect of virtual reality on cognition in stroke patients. Annals of Rehabilitation Medicine 2011;35(4):450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koch 2020 {published data only}
- Koch S, Tiozzo E, Simonetto M, Loewenstein D, Wright CB, Dong C, et al.Randomized trial of combined aerobic, resistance, and cognitive training to improve recovery from stroke: feasibility and safety. Journal of the American Heart Association 2020;9(10):e015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetto M, Dong C, Gutierrez CM, Wright CB, Loewenstein D, Sacco RL.Gender disparities in cognitive, motor and mood outcomes: preliminary data from the Bugher Foundation's combined aerobic and resistance exercise training (CARET) program and cognitive training intervention (CTI) study. Neurology 2018;90(15 Suppl):S15.003. [Google Scholar]
Kyoung‐Hee 2015 {published data only}
- Kyoung-Hee LE.Effects of a virtual reality-based exercise program on functional recovery in stroke patients: part 1. Journal of Physical Therapy Science 2015;27(6):1637-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lannin 2014 {published data only}
- Lannin NA, Schmidt J, Carr B, Allaous J, Falcon A, Tate R.Occupational therapy training to use handheld personal digital assistant (PDA) devices to address memory and planning difficulties after acquired brain injury: a randomised controlled trial. Stroke 2014;45(12):e296. [Google Scholar]
Lawson 2020 {published data only}
- Lawson D, Stolwyk R, Ponsford J, Wong D.Telehealth delivery of memory rehabilitation following stroke. Brain Impairment 2018;19(3):334. [DOI] [PubMed] [Google Scholar]
- Lawson DW, Stolwyk RJ, Ponsford JL, McKenzie DP, Downing MG, Wong D.Telehealth delivery of memory rehabilitation following stroke. Journal of the International Neuropsychological Society 2020;26(1):58-71. [DOI] [PubMed] [Google Scholar]
Lemoncello 2011 {published data only}
- Lemoncello R, Sohlberg MM, Fickas S, Prideaux J.A randomised controlled crossover trial evaluating Television Assisted Prompting (TAP) for adults with acquired brain injury. Neuropsychological Rehabilitation 2011;21(6):825-46. [DOI] [PubMed] [Google Scholar]
Lesniak 2018 {published data only}
- Lesniak MM, Mazurkiewicz P, Iwanski S, Szutkowska-Hoser J, Seniow J.Effects of group versus individual therapy for patients with memory disorder after an acquired brain injury: a randomized, controlled study. Journal of Clinical and Experimental Neuropsychology 2018;40(9):853-64. [DOI] [PubMed] [Google Scholar]
Lindelov 2016 {published data only}
- Lindelov JK, Dall JO, Kristensen CD, Aagesen MH, Olsen SA, Snuggerud TR, et al.Training and transfer effects of N-back training for brain-injured and healthy subjects. Neuropsychological Rehabilitation 2016;26(5-6):895-909. [DOI] [PubMed] [Google Scholar]
Lindeløv 2017 {published data only}
- Lindeløv JK, Overgaard R, Overgaard M.Improving working memory performance in brain-injured patients using hypnotic suggestion. Brain 2017;140(4):1100-3. [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu KP, Chan CC.Pilot randomized controlled trial of self-regulation in promoting function in acute poststroke patients. Archives of Physical Medicine & Rehabilitation 2014;95(7):1262-7. [DOI] [PubMed] [Google Scholar]
Liu 2017 {published data only}
- Liu Y, Fang J, Jiang R, Hu N, Pan C, Ye Z, et al.Effects of high frequency repetitive transcranial magnetic stimulation on executive function in patients after stroke. Chinese Journal of Neurology 2017;50(10):745-50. [Google Scholar]
Liu 2018 {published data only}
- Liu X, Huang X, Lin J, Li L, Zhang R, Ding R.Computer aided technology-based cognitive rehabilitation efficacy against patients' cerebral stroke. Neuroquantology 2018;16:86-92. [DOI: 10.14704/nq.2018.16.4.1212] [DOI] [Google Scholar]
Löw 2012 {published data only}
- Löw N, Heydekorn A.Cognitive training according to Stengel doubles success in post-stroke therapy [Kognitives Training nach Stengel® verdoppelt Therapieerfolg nach Schlaganfall]. Ergotherapie & Rehabilitation 2012;51(6):15-7. [Google Scholar]
Lyukmanov 2019 {published data only}
- Lyukmanov RK, Aziatskaya GA, Mokienko OA, Varako NA, Kovyazina MS, Suponeva NA, et al.Brain–computer interfaces in poststroke rehabilitation: a clinical neuropsychological study. Neuroscience and Behavioral Physiology 2019;49(8):1038-46. [Google Scholar]
Maier 2017 {published data only}
Man 2006 {published data only}
- Man DW, Soong WY, Tam SF, Hui-Chan CW.A randomized clinical trial study on the effectiveness of a tele-analogy-based problem-solving programme for people with acquired brain injury (ABI). NeuroRehabilitation 2006;21(3):205-17. [PubMed] [Google Scholar]
Manuli 2020 {published data only}
- Manuli A, Maggio MG, Latella D, Cannavo A, Balletta T, De Luca R, et al.Can robotic gait rehabilitation plus virtual reality affect cognitive and behavioural outcomes in patients with chronic stroke? A randomized controlled trial involving three different protocols. Journal of Stroke & Cerebrovascular Diseases 2020;29(8):104994. [NCT03914313] [DOI] [PubMed] [Google Scholar]
Mao 2020 {published data only}
- Li Y, Mao H, Chen Y, Wang Y, Tang L, Liu L.The clinical effects of mirror neuron training system on upper extremity and cognitive function of stroke patients. Neurorehabilitation and Neural Repair 2018;32(4-5):419-20. [Google Scholar]
- Mao H, Li Y, Tang L, Chen Y, Ni J, Liu L, et al.Effects of mirror neuron system-based training on rehabilitation of stroke patients. Brain and Behavior 2020;10:e01729. [DOI: 10.1002/brb3.1729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Markovic 2019 {published data only}
- Markovic G, Schult ML, Elg M, Bartfai A.Beneficial effects of early attention process training after acquired brain injury: a randomized controlled trial. Journal of Rehabilitation Medicine 2019;52(1):jrm00011. [DOI: 10.2340/16501977-2628] [NCT02091453] [DOI] [PubMed] [Google Scholar]
McDonald 2011 {published data only}
- Haslam C, McDonald A.Google calendar: a memory aid to manage prospective memory deficits following acquired brain injury. Brain Impairment 2012;13(1):180. [DOI] [PubMed] [Google Scholar]
- McDonald A, Haslam C, Yates P, Gurr B, Leeder G, Sayers A.Google Calendar: a new memory aid to compensate for prospective memory deficits following acquired brain injury. Neuropsychological Rehabilitation 2011;21(6):784-807. [DOI] [PubMed] [Google Scholar]
McEwen 2015 {published data only}
- McEwen S, Polatajko H, Baum C, Rios J, Cirone D, Doherty M, et al.Combined cognitive-strategy and task-specific training improve transfer to untrained activities in subacute stroke: an exploratory randomized controlled trial. Neurorehabilitation & Neural Repair 2015;29(6):526-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merchán‐Baeza 2015 {published data only}
- Merchán-Baeza JA, Gonzalez-Sanchez M, Cuesta-Vargas A.Clinical effect size of an educational intervention in the home and compliance with mobile phone-based reminders for people who suffer from stroke: protocol of a randomized controlled trial. JMIR Research Protocols 2015;4(1):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2014 {published data only}
- Miller LA, Radford K.Testing the effectiveness of group-based memory rehabilitation in chronic stroke patients. Neuropsychological Rehabilitation 2014;24(5):721-37. [DOI] [PubMed] [Google Scholar]
Moon 2020 {published data only}
- Moon JH, Jung JH, Cho HY.Effects of cognitive exercise therapy on tactile sensations of the hands and activities of daily living in stroke patients. Medico-Legal Update 2020;20(1):1976-80. [Google Scholar]
Mount 2007 {published data only}
- Mount J, Pierce SR, Parker J, DiEgidio R, Woessner R, Spiegel L, et al.Trial and error versus errorless learning of functional skills in patients with acute stroke. NeuroRehabilitation 2007;22(2):123-32. [PubMed] [Google Scholar]
O'Neil‐Pirozzi 2016 {published data only}
- O'Neil-Pirozzi TM, Hsu H.Feasibility and benefits of computerized cognitive exercise to adults with chronic moderate-to-severe cognitive impairments following an acquired brain injury: a pilot study. Brain Injury 2016;30(13-14):1617-25. [DOI] [PubMed] [Google Scholar]
Ownsworth 1999 {published data only}
- Ownsworth TL, McFarland K.Memory remediation in long-term acquired brain injury: two approaches in diary training. Brain Injury 1999;13(8):605-26. [DOI] [PubMed] [Google Scholar]
Padua 2020 {published data only}
- Padua L, Imbimbo I, Aprile I, Loreti C, Germanotta M, Coraci D, et al.Cognitive reserve as a useful variable to address robotic or conventional upper limb rehabilitation treatment after stroke: a multicentre study of the Fondazione Don Carlo Gnocchi. European Journal of Neurology 2020;27(2):392-8. [DOI] [PubMed] [Google Scholar]
Pallesen 2018 {published data only}
- Pallesen H, Næss-Schmidt ET, Kjeldsen SS, Pedersen SK, Sørensen SL, Brunner I, et al."Stroke – 65 Plus. Continued Active Life": a study protocol for a randomized controlled cross-sectoral trial of the effect of a novel self-management intervention to support elderly people after stroke. Trials 2018;19(1):639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pallesen 2019 {published data only}
- Pallesen H, Bjerk M, Pedersen AR, Nielsen JF, Evald L.The effects of high-intensity aerobic exercise on cognitive performance after stroke: a pilot randomised controlled trial. Journal of Central Nervous System Disease 2019;11:1-10. [DOI: 10.1177/1179573519843493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2013 {published data only}
- Park SW, Bak IH, You SJ.The effects of Korean computer-based cognitive rehabilitation program (CoTras) for the cognition and visual perception and ADL in brain injury. Soci Occup Ther Agged Demen 2013;7:47-57. [Google Scholar]
Park 2015b {published data only}
- Park IS, Yoon JG.The effect of computer-assisted cognitive rehabilitation and repetitive transcranial magnetic stimulation on cognitive function for stroke patients. Journal of Physical Therapy Science 2015;27:773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2018 {published data only}
- Park MO, Lee SH.Effects of cognitive-motor dual-task training combined with auditory motor synchronization training on cognitive functioning in individuals with chronic stroke: a pilot randomized controlled trial. Medicine 2018;97(22):e10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2019 {published data only}
- Park MO, Lee SH.Effect of a dual-task program with different cognitive tasks applied to stroke patients: a pilot randomized controlled trial. NeuroRehabilitation 2019;44(2):239-49. [DOI] [PubMed] [Google Scholar]
Park 2020 {published data only}
- Park HK, Song MK, Kim JH, Han JY.A randomized controlled trial to evaluate the effectiveness and safety of electro acupuncture and transcranial direct current stimulation with computerized cognitive rehabilitation in patients with vascular cognitive impairment. Medicine 2020;99(29):e21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peers 2020 {published data only}
- Peers PV, Astle DE, Duncan J, Murphy FC, Hampshire A, Das T, et al.Dissociable effects of attention vs working memory training on cognitive performance and everyday functioning following fronto-parietal strokes. Neuropsychological Rehabilitation 2020;30(6):1092-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petruševičienè 2017 {published data only}
- Petruševičienè D, Virviciutè D, Savickas R, Lendraitienè E, Mingaila S, Vasilavicius P.The effect of different occupational therapy techniques on post-stroke patients. Ceska a Slovenska Neurologie a Neurochirurgie 2017;80(4):464-9. [Google Scholar]
Ploughman 2008 {published data only}
- Ploughman M, McCarthy J, Bosse M, Sullivan HJ, Corbett D, Ploughman M, et al.Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Archives of Physical Medicine & Rehabilitation 2008;89(11):2041-7. [DOI] [PubMed] [Google Scholar]
Ploughman 2017 {published data only}
- Ploughman M, Eskes GA, Kelly LP, Kirkland MC, Devasayaham AJ, Wallack EM, et al.Aerobic exercise enhances the beneficial effects of cognitive training and reopens the 'window of recovery' in chronic stroke via neurotrophins. International Journal of Stroke 2017;12(4):18-9. [Google Scholar]
Ploughman 2018 {published data only}
- Ploughman M, Eskes GA, Kelly LP, Kirkland MC, Devasahayam AJ, Wallack EM, et al.Combining aerobic exercise and cognitive training in chronic stroke; enhanced cognition predicted by IGF-1. International Journal of Stroke 2018;13(2):32. [Google Scholar]
Ploughman 2019 {published data only}
- Ploughman M, Eskes GA, Kelly LP, Kirkland MC, Devasahayam AJ, Wallack EM, et al.Synergistic benefits of combined aerobic and cognitive training on fluid intelligence and the role of IGF-1 in chronic stroke. Neurorehabilitation & Neural Repair 2019;33(3):199-212. [DOI] [PubMed] [Google Scholar]
Polatajko 2012 {published data only}
- Polatajko HJ, McEwen SE, Ryan JD, Baum CM.Pilot randomized controlled trial investigating cognitive strategy use to improve goal performance after stroke. American Journal of Occupational Therapy 2012;66(1):104-9. [DOI] [PubMed] [Google Scholar]
Poulin 2013 {published data only (unpublished sought but not used)}
- Poulin V, Korner Bitensky N, Dawson D, Lussier M.Comparison of two novel cognitive interventions for adults experiencing executive dysfunction post-stroke. Archives of Physical Medicine and Rehabilitation 2013;94(10):e27. [DOI] [PubMed] [Google Scholar]
Powell 2012 {published data only}
- Powell LE, Glang A, Ettel D, Todis B, Sohlberg MM, Albin R.Systematic instruction for individuals with acquired brain injury: results of a randomised controlled trial. Neuropsychological Rehabilitation 2012;22(1):85-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2019 {published data only}
- Powell LE, Wild MR, Glang A, Ibarra S, Gau JM, Perez A, et al.The development and evaluation of a web-based programme to support problem-solving skills following brain injury. Disability & Rehabilitation: Assistive Technology 2019;14(1):21-32. [DOI] [PubMed] [Google Scholar]
Preminger 2016 {published data only}
- Preminger S, Eliav R, Blumenfeld B, Swartz Y, Maoz S, Rand D, et al.Cognitive training with adaptive motion-based video games for improving executive functions following acquired brain injury. Archives of Physical Medicine and Rehabilitation 2016;97(10):e56. [Google Scholar]
Prokopenko 2012 {published data only}
- Prokopenko SV, Yu E, Mozheyko OS, Levin TD, Koryagina TV, Chernykh MA, et al.Cognitive disorders and its correction in the acute period of ischemic stroke. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2012;8(2):35-9. [PubMed] [Google Scholar]
Prokopenko 2017 {published data only}
- Prokopenko SV, Bezdenezhnykh AF, Mozheyko EY, Zubrickaya EM.A comparative clinical study of the efficacy of computer cognitive training in patients with post-stroke cognitive impairments. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2017;117(8. Vyp. 2):32-6. [DOI] [PubMed] [Google Scholar]
Rackoll 2018 {published data only}
- Rackoll T, Nave A, Grittner U, Mousa H, Villringer K, Ebinger M, et al.Direct and long term influence of cardiovascular training on cognition in subacute stroke patients. Clinical Neurophysiology 2018;129(8):e91-2. [Google Scholar]
Ramirez‐Hernandez 2020 {published data only}
- Ramirez-Hernandez D, Stolwyk RJ, Ownsworth T, Wong D.A comparison of systematic instruction, error-based learning and trial and error to train the use of smartphone memory apps after acquired brain injury: a three-armed phase II randomised controlled trial study protocol. Brain Impairment 2020;First View:1-16. [DOI: 10.1017/BrImp.2020.10] [DOI] [Google Scholar]
Ranzani 2020 {published data only}
- Ranzani R, Lambercy O, Metzger JC, Califfi A, Regazzi S, Dinacci D, et al.Neurocognitive robot-assisted rehabilitation of hand function: a randomized control trial on motor recovery in subacute stroke. Journal of Neuroengineering & Rehabilitation 2020;17(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richard 2020 {published data only}
- Richard G, Kolskår K, Ulrichsen KM, Kaufmann T, Alnæs D, Sanders AM, et al.Brain age prediction in stroke patients: highly reliable but limited sensitivity to cognitive performance and response to cognitive training. NeuroImage: Clinical 2020;25:102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richter 2015 {published data only}
- Richter KM, Modden C, Eling P, Hildebrandt H.Working memory training and semantic structuring improves remembering future events, not past events. Neurorehabilitation and Neural Repair 2015;29(1):33-40. [DOI] [PubMed] [Google Scholar]
Russo 2017 {published data only}
- Russo M, De Luca R, Naro A, Sciarrone F, Aragona B, Silvestri G, et al.Does body shadow improve the efficacy of virtual reality-based training with BTS NIRVANA? A pilot study. Medicine 2017;96(38):e8096. [DOI: 10.1097/MD.0000000000008096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salaris 2014 {published data only}
- Salaris M, Vries R, Jordan LA, Quinn R, Young AD, Paisley E, et al.How frequently and for what purpose is tablet technology being used during stroke recovery? International Journal of Stroke 2014;9(Suppl 2):27-8. [Google Scholar]
Salaris 2016 {published data only}
- Salaris M, Quinn R, Jordans LA, Galvin R, Veitch K, Young A, et al.Tablets are not hard to swallow after stroke! Tablet computers are used frequently for selfdirected therapy and leisure activities despite limited pre-stroke exposure. International Journal of Stroke 2016;11(Suppl 1):21. [Google Scholar]
Salvadori 2016 {published data only}
- Salvadori E, Poggesi A, Valenti R, Della Rocca E, Diciotti S, Mascalchi M, et al.The rehabilitation of attention in patients with mild cognitive impairment and brain subcortical vascular changes using the Attention Process Training-II. The RehAtt Study: rationale, design and methodology. Neurological Sciences 2016;37(10):1653-62. [DOI] [PubMed] [Google Scholar]
Sampanis 2015 {published data only}
- Sampanis DS, Mevorach C, Shalev L, Mohammed S, Humphreys GW.Cognitive deficits after stroke through Computerized Progressive Attentional Training (CPAT): a pilot study. Physical Medicine and Rehabilitation International 2015;2(7):1058. [Google Scholar]
Sarkamo 2008 {published data only}
- Sarkamo T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al.Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 2008;131(3):866-76. [DOI] [PubMed] [Google Scholar]
Shi 2017 {published data only}
- Shi Y, Zhang Z.Effect of comprehensive rehabilitation training on prevention of post-stroke dementia: a randomized controlled trial. International Journal of Clinical and Experimental Medicine 2017;10(5):7760-6. [Google Scholar]
Shim 2007 {published data only}
- Shim JM, Kim HH, Lee YS.Effects of computerized neurocognitive function program induced memory and attention for patients with stroke. Journal of Korean Physical Therapy 2007;19:25-32. [Google Scholar]
Shottke 1997 {published data only}
- Shottke H.Rehabilitation of attention deficits after stroke: efficacy of a neuropsychological training program for attention deficits. Verhaltenstherapie 1997;7:21-33. [Google Scholar]
Skidmore 2015b {published data only}
- Skidmore ER, Whyte EM, Butters MA, Terhorst L, Reynolds CF.Strategy training during inpatient rehabilitation may prevent apathy symptoms after acute stroke. PM&R: the Journal of Injury, Function, and Rehabilitation 2015;7(6):562-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soderback 1988 {published data only}
- Soderback I.The effectiveness of training intellectual functions in adults with acquired brain damage. An evaluation of occupational therapy methods. Scandinavian Journal of Rehabilitation Medicine 1988;20(2):47-56. [PubMed] [Google Scholar]
Spahn 2010 {published data only}
- Spahn V, Kulke H, Kunz M, Thone-Otto A, Schupp W, Lautenbacher S.Is the neuropsychological treatment of memory specific or unspecific? Comparing treatment effects on memory and attention. Zeitschrift fur Neuropsychologie 2010;21(4):239-45. [Google Scholar]
Spikman 2010 {published data only}
- Spikman JM, Boelen DH, Lamberts KF, Brouwer WH, Fasotti L.Effects of a multifaceted treatment program for executive dysfunction after acquired brain injury on indications of executive functioning in daily life. Journal of the International Neuropsychological Society 2010;16(1):118-29. [DOI] [PubMed] [Google Scholar]
Spikman 2013 {published data only}
- Spikman JM.A therapeutic approach to improve self-awareness in brain injury patients with executive dysfunction. Behavioural Neurology 2013;27(3):330. [Google Scholar]
Svaerke 2019 {published data only}
- Svaerke KW, Omkvist KV, Havsteen IB, Christensen HK.Computer-based cognitive rehabilitation in patients with visuospatial neglect or homonymous hemianopia after stroke. Journal of Stroke & Cerebrovascular Diseases 2019;28(11):104356. [DOI] [PubMed] [Google Scholar]
Szepfalusi 2017 {published data only}
- Szepfalusi N, Nemeth V, Vekony T, Imre N, Balogh R, Holczer A, et al.Ten-day cognitive training program combined with transcranial direct current stimulation (tDCS) in stroke rehabilitation-a sham controlled study. 30th European College of Neuropsychopharmacology Congress, ECNP, 2017 France 2017;27(Suppl 4):S1038-9. [Google Scholar]
Thickpenny‐Davis 2007 {published data only}
- Thickpenny-Davis KL, Barker-Collo SL.Evaluation of a structured group format memory rehabilitation program for adults following brain injury. Journal of Head Trauma Rehabilitation 2007;22(5):303-13. [DOI] [PubMed] [Google Scholar]
Tornas 2014 {published data only}
- Tornas S, Lovstad M, Solbakk AK, Host K, Schanke AK, Stubberud J.Goal management training in patients with acquired brain injury – preliminary results. Brain Injury 2014;28(5-6):569-70. [Google Scholar]
Tornas 2019 {published data only}
- Tornas S, Lovstad M, Solbakk A-K, Schanke A-K, Stubberud J.Use it or lose it? A 5-year follow-up study of goal management training in patients with acquired brain injury. Journal of the International Neuropsychological Society 2019;25(10):1082-7. [DOI: 10.1017/S1355617719000626] [DOI] [PubMed] [Google Scholar]
Torrisi 2019 {published data only}
- Torrisi M, Maresca G, De Cola MC, Cannavo A, Sciarrone F, Silvestri G, et al.Using telerehabilitation to improve cognitive function in post-stroke survivors: is this the time for the continuity of care? International Journal of Rehabilitation Research 2019;42(4):344-51. [DOI] [PubMed] [Google Scholar]
Veisi‐Pirkoohi 2020 {published data only}
- Veisi-Pirkoohi S, Hassani-Abharian P, Kazemi R, Vaseghi S, Zarrindast MR, Nasehi M.Efficacy of RehaCom cognitive rehabilitation software in activities of daily living, attention and response control in chronic stroke patients. Journal of Clinical Neuroscience 2020;71:101-7. [DOI] [PubMed] [Google Scholar]
Virk 2015 {published data only}
- Virk S, Williams T, Brunsdon R, Suh F, Morrow A.Cognitive remediation of attention deficits following acquired brain injury: a systematic review and meta-analysis. Neurorehabilitation 2015;36(3):367-77. [DOI] [PubMed] [Google Scholar]
Visser 2013 {published data only}
- Visser MM, Heijenbrok-Kal MH, Van't Spijker A, Busschbach JJ, Ribbers GM.Problem solving therapy during outpatient rehabilitation for stroke: short-term results of a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2014;95(10):e23-4. [Google Scholar]
- Visser MM, Heijenbrok-Kal MH, van't Spijker A, Ribbers GM, Busschbach JJ.The effectiveness of problem solving therapy for stroke patients: study protocol for a pragmatic randomized controlled trial. BMC Neurology 2013;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visser 2016 {published data only}
- Visser M, Heijenbrok-Kal M, Van't Spijker A, Lannoo E, Busschbach J, Ribbers G.Problem-solving therapy during outpatient stroke rehabilitation improves coping and HR-Qol: a randomized controlled trial. Brain Injury 2016;30(5):577. [DOI] [PubMed] [Google Scholar]
- Visser MM, Heijenbrok-Kal MH, Van't Spijker A, Lannoo E, Busschbach JJ, Ribbers GM.Problem-solving therapy during outpatient stroke rehabilitation improves coping and health-related quality of life: randomized controlled trial. Stroke 2016;47(1):135-42. [DOI] [PubMed] [Google Scholar]
Weicker 2016 {published data only}
- Weicker J, Villringer A, Thöne-Otto A.Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. Neuropsychology 2016;30(2):190-212. [DOI] [PubMed] [Google Scholar]
Weicker 2020 {published data only}
- Weicker J, Hudl N, Hildebrandt H, Obrig H, Schwarzer M, Villringer A, et al.The effect of high vs. low intensity neuropsychological treatment on working memory in patients with acquired brain injury. Brain Injury 2020;34(8):1051-60. [DOI] [PubMed] [Google Scholar]
Wentink 2014 {published data only}
- Wentink M, De Kloet A, Berger M, Haastrecht K, Verhoeven I, Jakobs M, et al.The effect of computerized brain training on cognitive impairments and quality-of-life after stroke: a RCT. Brain Injury 2014;289(5-6):588-9. [Google Scholar]
Wentink 2016 {published data only}
- Wentink M, Berger M, De Kloet A, Meesters J, Band G, Wolterbeek R, et al.Cognitive gaming after stroke: a randomized controlled trial. Brain Injury 2016;30(5-6):545-6. [Google Scholar]
- Wentink M, Berger M, Kloet A, Meesters J, Band G, Wolterbeek R, et al.The effects of an 8-week computer-based brain training programme on cognitive functioning, QoL and self-efficacy after stroke. Neuropsychological Rehabilitation 2016;26(5-6):847-65. [DOI] [PubMed] [Google Scholar]
Westerberg 2007 {published data only}
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Östensson ML, Bartfai A, et al.Computerized working memory training after stroke – a pilot study. Brain Injury 2007;21:21-9. [DOI] [PubMed] [Google Scholar]
Wilson 2014 {published data only}
- Wilson BA, Emslie HC, Quirk K, Evans JJ.Reducing everyday memory and planning problems by means of a paging system: a randomised control crossover study (2001). In: The Assessment, Evaluation and Rehabilitation of Everyday Memory Problems: Selected Papers of Barbara A Wilson. Hove (UK): Psychology Press, 2014:96-107. [Google Scholar]
Winkens 2009 {published data only}
- Winkens I, Heugten CM, Wade DT, Habets EJ, Fasotti L.Efficacy of time pressure management in stroke patients with slowed information processing: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2009;90(10):1672-9. [DOI] [PubMed] [Google Scholar]
Withiel 2018 {published data only}
- Withiel T, Wong D, Ponsford J, Cadilhac D, New P, Mihaljcic T, et al.Comparing compensatory and restorative approaches to memory rehabilitation following stroke: a phase II randomised controlled trial. International Journal of Stroke 2018;13(1):5. [Google Scholar]
Withiel 2019 {published data only}
- Withiel TD, Wong D, Ponsford JL, Cadilhac DA, New P, Mihaljcic T, et al.Comparing memory group training and computerized cognitive training for improving memory function following stroke: a phase II randomized controlled trial. Journal of Rehabilitation Medicine 2019;51(5):343-51. [DOI] [PubMed] [Google Scholar]
Wolf 2016 {published data only}
- Wolf TJ, Polatajko H, Baum C, Rios J, Cirone D, Doherty M, et al.Combined cognitive-strategy and task-specific training affects cognition and upper-extremity function in subacute stroke: an exploratory randomized controlled trial. American Journal of Occupational Therapy 2016;70(2):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2012 {published data only}
- Wood A, Scott F, Baylan S, Evans J.Rehabilitation of executive function deficits following acquired brain injury: a randomised controlled trial of goal management training and implementation intentions for the improvement of prospective memory. Brain Injury 2012;26(4-5):557. [Google Scholar]
Yip 2012 {published data only}
- Yip BC, Man DW.Virtual reality-based prospective memory training program for people with acquired brain injury. NeuroRehabilitation 2013;32(1):103-15. [DOI] [PubMed] [Google Scholar]
- Yip CB.An intelligent rehabilitation system for cognitive rehabilitation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2012;73(3-B):1524.
Yu 2019 {published data only}
- Yu HL, Cao DX, Liu J.Effect of a novel designed intensive patient care program on cognitive impairment, anxiety, depression as well as relapse free survival in acute ischemic stroke patients: a randomized controlled study. Neurological Research 2019;41(9):857-66. [DOI] [PubMed] [Google Scholar]
Zengin‐Metli 2018 {published data only}
- Zengin-Metli D, Ozbudak-Demir S, Eraktas I, Binay-Safer V, Ekiz T.Effects of robot assistive upper extremity rehabilitation on motor and cognitive recovery, the quality of life, and activities of daily living in stroke patients. Journal of Back and Musculoskeletal Rehabilitation 2018;31(6):1059-64. [DOI] [PubMed] [Google Scholar]
Zhang 2016a {published data only}
- Zhang J, Chen C.Effect of audio training on executive dysfunction in patients with stroke. Chinese Journal of Cerebrovascular Diseases 2016;13(7):356-9. [Google Scholar]
Zhang 2019 {published data only}
- Zhang L, Zhang T, Sun Y.A newly designed intensive caregiver education program reduces cognitive impairment, anxiety, and depression in patients with acute ischemic stroke. Brazilian Journal of Medical and Biological Research 2019;52(9):e8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2018 {published data only}
- Zheng G, Zheng Y, Xiong Z, Ye B, Tao J, Chen L.Effect of Baduanjin exercise on cognitive function in patients with post-stroke cognitive impairment: study protocol for a randomised controlled trial. BMJ Open 2018;8(6):e020954. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12619001557123 {published data only}
- ACTRN12619001557123.Evaluation of the ELEMENTS tablet for post-stroke rehabilitation. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378298 (first received September 2019). [AUSTRALIAN NEW ZEALAND CLINICAL TRIALS REGISTRY: ACTRN12619001557123]
Askim 2019 {published data only}
- Askim T, Saltvedt I, Hokstad A, Indredavik B, Halsteinli V, Thingstad P, et al.A multimodal individualized intervention to prevent functional decline after stroke. A randomised controlled trial on long-term follow-up after stroke (the LAST-long trial). European Stroke Journal 2019;4(1S):811. [10.1177/2396987319848124] [Google Scholar]
- NCT03859063.Long-term follow-up after stroke (The LAST-long Trial) [A multimodal individualized intervention to prevent functional decline after stroke. A randomized controlled trial on long-term follow-up after stroke (the LAST-long Trial)]. clinicaltrials.gov/ct2/show/NCT03859063 (first received March 2019).
Bergfeldt 2019 {published data only}
- Bergfeldt U, Ingolfsdottir E, Berthold-Lindstedt M, Eriksson M, Julin P.Effects of aerobic training on memory, attention, and working memory in patients with stroke and traumatic brain injury. European Stroke Journal 2019;4(Suppl 1):793. [Google Scholar]
ChiCTR1800015132 {published data only}
- ChiCTR1800015132.The effects of robot rehabilitation on motor function, depression and cognitive impairment in patients with acute ischemic stroke: a randomized control trial. trialsearch.who.int/?TrialID=ChiCTR1800015132 (first received March 2018). [www.chictr.org.cn/showproj.aspx?proj=25549]
ChiCTR1800018633 {published data only}
- ChiCTR1800018633.Clinical controlled study for computer-aided cognitive assessment and training system based on PASS theory for patients with post stroke cognitive impairment. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1800018633 (first received September 2018).
ChiCTR1900025540 {published data only}
- ChiCTR1900025540.The effect and neural mechanism of internet-based cognitive training on post-stroke cognitive impairment. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900025540 (first received August 2019). [ChiCTR1900025540]
ChiCTR1900026532 {published data only}
- ChiCTR1900026532.Effect of Yijinjing group training on cognitive function in elderly stroke patients. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900026532 (first received October 2019). [ChiCTR1900026532]
ChiCTR‐INR‐17013042 {published data only}
- ChiCTR-INR-17013042.Effects of self-regulated learning and computer-assisted cognitive training on cognitive function and activities of daily living in patients with cognitive dysfunction after stroke. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-17013042 (first received October 2019). [www.chictr.org.cn/showproj.aspx?proj=22013]
Chiu 2020 {published data only}
- Chiu En-Chi, Chi Fang-Chi.Effect of home-based activities of daily living (ADL) on cognition and visual perception in patients with stroke: a randomized controlled pilot study. American Journal of Occupational Therapy 2020;74:1-1. [Google Scholar]
CTRI/2017/11/010466 {published data only}
- CTRI/2017/11/010466.Effects of art therapy on cognitive functions among stroke survivors. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=18433 (first received 13 November 2017). [CTRI/2017/11/010466]
Donnellan 2014 {published data only}
- Donnellan C.REsources And LIfe Strategy Management (REALISM) trial: protocol for a stroke rehabilitation intervention using a goal setting and attainment framework. International Journal of Stroke 2014;9(Suppl 3):221. [Google Scholar]
Gil‐Pages 2018 {published data only}
- Gil-Pages M, Solana J, Sanchez-Carrion R, Tormos JM, Ensenat-Cantallops A, Garcia-Molina A.A customized home-based computerized cognitive rehabilitation platform for patients with chronic-stage stroke: study protocol for a randomized controlled trial. Trials 2018;19(1):191. [DOI: 10.1186/s13063-018-2577-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN10613029 {published data only}10613029
- ISRCTN10613029.Effectiveness of a self-administered serious game for cognitive rehabilitation in chronic stroke survivors with mild cognitive impairment: a randomized controlled trial. isrctn.com/ISRCTN10613029 (first received 22 March 2019).
Janssen 2014 {published data only}
- ACTRN12614000081617.Tablets and technology during stroke recovery. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000081617 (first received 22 January 2014). [ACTRN12614000081617]
- Janssen H, Salaris M, Quinn R, Jordan LA, Galvin R, Veitch K, et al.Tablet computers may contribute to better stroke survivor quality of life one month after discharge from inpatient rehabilitation. International Journal of Stroke 2016;11(Suppl 1):15-6. [Google Scholar]
Jung 2019 {published data only}
- Jung HT, Daneault JF, Nanglo T, Lee H, Kim K, Kim B, et al.Preliminary results from a randomized controlled trial of serious games for the improvement of cognitive function in chronic stroke survivors. Journal of the Neurological Sciences 2019;405:79. [Google Scholar]
NCT01934621 {published data only}
- NCT01934621.Adapting daily activity performance through strategy training (ADAPTS). clinicaltrials.gov/ct2/show/NCT01934621 (first received 4 September 2013). [NCT01934621]
NCT02384057 {published data only}
- NCT02384057.Cognitive rehabilitation with C8 sciences [Cognitive rehabilitation for participants with stroke or brain injury using C8 Sciences]. clinicaltrials.gov/ct2/show/NCT02384057 (first received 10 March 2015).
NCT02925637 {published data only}
- NCT02925637.Effectiveness of FACoT for individuals post stroke [Effectiveness of a novel meta-cognitive-functional Intervention (FACoT) for individuals post mild-moderate stroke]. clinicaltrials.gov/ct2/show/NCT02925637 (first received 6 October 2016).
NCT03168360 {published data only}
- NCT03168360.Effect of intensive cognitive rehabilitation in subacute stroke patient. clinicaltrials.gov/ct2/show/NCT03168360 (first received 30 May 2017).
NCT03621397 {published data only}
- NCT03621397.Online Spanish cognitive intervention program for Spanish-speaking Latino/Hispanic subarachnoid hemorrhage patients [The effectiveness of an online Spanish cognitive intervention program for Spanish-speaking Latino/Hispanic subarachnoid hemorrhage patients]. clinicaltrials.gov/ct2/show/NCT03621397 (first received 8 August 2018).
NCT03644290 {published data only}
- NCT03644290.Cognitive training in stroke patients. clinicaltrials.gov/ct2/show/NCT03644290 (first received 23 August 2018).
NCT03792061 {published data only}
- NCT03792061.Enhancing community participation for stroke survivors with cognitive impairments. clinicaltrials.gov/ct2/show/NCT03792061 (first received 3 January 2019).
NCT03828851 {published data only}
- NCT03828851.Development of domiciliary program on improving activities of daily living program for patients with stroke. clinicaltrials.gov/ct2/show/NCT03828851 (first received 4 February 2019). [NCT03828851]
NCT03890159 {published data only}
- NCT03890159.Effects of computer assisted cognitive rehabilitation on patients with stroke. clinicaltrials.gov/ct2/show/NCT03890159 (first received 26 March 2019).
NCT04012866 {published data only}
- NCT04012866.Effects of combined cognitive training with aerobic exercise in stroke patients with MCI. clinicaltrials.gov/show/NCT04012866 (first received 9 July 2019). [NCT04012866]
NCT04033952 {published data only}
- NCT04033952.Strategy training on improving executive functions in persons following acquired brain injury. clinicaltrials.gov/show/NCT04033952 (first received 26 July 2019). [NCT04033952]
NCT04038424 {published data only}
- NCT04038424.The effect of art therapy on patients with stroke. clinicaltrials.gov/show/NCT04038424 (first received 30 July 2019). [NCT04038424]
NCT04098835 {published data only}
- NCT04098835.A pilot study of a strategy and computer-based intervention to enhance daily cognitive functioning after stroke. clinicaltrials.gov/ct2/show/NCT04098835 (first received 23 September 2019). [NCT04098835]
NCT04099511 {published data only}
- NCT04099511.Metacognitive-strategy training in sub-acute stroke. clinicaltrials.gov/show/NCT04099511 (first received 23 September 2019). [NCT04099511]
NCT04164381 {published data only}
- NCT04164381.Robotic rehabilitation and cognitive functions [Use of robotics to improve cognitive functions in subject with subacute stroke: a bicentric pilot study]. clinicaltrials.gov/ct2/show/NCT04164381 (first received 15 November 2019). [NCT04164381]
NCT04214314 {published data only}
- NCT04214314.Efficacy of a computerized program of cognitive rehabilitation of attention in people with acquired brain injury (ABI). clinicaltrials.gov/show/NCT04214314 (first received 2 January 2020).
NCT04229056 {published data only}
- NCT04229056.COMPuter-assisted Self-training to Improve EXecutive Function. clinicaltrials.gov/show/NCT04229056 (first received 14 January 2020). [NCT04229056]
NCT04246385 {published data only}
- NCT04246385.Implementing a CO-OP group in the day rehab setting: a pilot study. clinicaltrials.gov/show/NCT04246385 (first received 29 January 2020). [NCT04246385]
NCT04282564 {published data only}
- NCT04282564.Improvement of executive functions with the CO-OP method in the adult subject after stroke. clinicaltrials.gov/show/NCT04282564 (first received 24 February 2020).
NCT04323501 {published data only}
- NCT04323501.Post-stroke Recovery (PSR_e2020) [Rehabilitation treatment and biomarkers of post-stroke recovery: a pilot study]. clinicaltrials.gov/show/NCT04323501 (first received 25 June 2020).
NCT04399759 {published data only}
- NCT04399759.Instrumental activities of daily living approach in home environment for patients with stroke [Investigation of the effect on Instrumental Activities of Daily Living approach in home environment for patients with stroke]. clinicaltrials.gov/ct2/show/NCT04399759 (first received 22 May 2020).
NCT04470219 {published data only}
- NCT04470219.Digital support for people with cognitive impairment [Digital support for people with cognitive impairment – an intervention to Increase occupational performance and the possibility to live a more independent life]. clinicaltrials.gov/ct2/show/NCT04470219 (first received 14 July 2020).
NCT04472351 {published data only}
- NCT04472351.Cognitive training in stroke rehabilitation [Strategy training for cognitive dysfunction in inpatient stroke rehabilitation]. clinicaltrials.gov/show/NCT04472351 (first received 15 July 2020).
Peers 2018 {published data only}
- Peers PV, Bishop S, Murphy F, Duncan J, Astle DE, Watson P, et al.Home-based cognitive training in stroke: implications for cognition and everyday functioning. International Journal of Stroke 2018;13(3):51. [Google Scholar]
Sahakian {published data only}
- Sahakian B.Investigation into the efficacy of cognitive training on cognition in adults with brain injury. www.ukctg.nihr.ac.uk/trials/trial-details/trial-details?trialId=32055 2016. [IRAS ID 159857]
Slenders 2019 {published data only}
- NL7295.Screening and patient-tailored care for Emotional and COgnitive problems in patients discharged home after ischemic stroke (ECO-stroke). trialregister.nl/trial/7295 (first received 25 September 2018).
- Slenders J, den Berg-Vos R, Heugten C, Visser-Meily J, Kwa V.Screening and patient-tailored care for emotional and cognitive complaints in patients discharged home after ischemic stroke (ECO-stroke trial). European Stroke Journal 2019;4:807. [Google Scholar]
Wiseman 2016 {published data only}
- Wiseman HA, Wallack EM, Downer M, Chatterjee T, Eskes GA, Chaves AR, et al.Does perception of cognitive defects affect improvement during computerized cognitive training in stroke? International Journal of Stroke 2016;11(Suppl 2):85. [Google Scholar]
Yeh 2017 {published data only}
- Wu CY, Yeh TT, Lee YY, Teng CH, Chang KC.Synergistic effects of aerobic exercise and cognitive training on cognition and quality of life in stroke patients with cognitive decline. Archives of Physical Medicine and Rehabilitation 2016;97(10):e12. [Google Scholar]
- Yeh T-T, Wu C-Y, Hsieh Y-W, Chang K-C, Lee L-C, Hung J-W, et al.Synergistic effects of aerobic exercise and cognitive training on cognition, physiological markers, daily function, and quality of life in stroke survivors with cognitive decline: study protocol for a randomized controlled trial. Trials 2017;18(1):405. [DOI: 10.1186/s13063-017-2153-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeh 2018 {published data only}
- Yeh T-T, Chang K-C, Wu C-Y, Lee Y-Y, Chen P-Y, Hung J-W.Effects and mechanism of the HECT study (hybrid exercise-cognitive trainings) in mild ischemic stroke with cognitive decline: fMRI for brain plasticity, biomarker and behavioral analysis. Contemporary Clinical Trials Communications 2018;9:164-71. [DOI: 10.1016/j.conctc.2018.02.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016b {published data only}
- Zhang H, Yun X, Zhang X, Shen M, Pan H, Zhang Y, et al.Efficacy of rehabilitation for impairments of attention. Archives of Physical Medicine and Rehabilitation 2016;97(10):e131-e132. [Google Scholar]
References to ongoing studies
Chen 2018 {published data only}
- Chen BY, Aldhukair H, Cote J, Flinois J, Hoang T, Shen HC.Early tablet-assisted cognitive rehabilitation for aneurysmal subarachnoid hemorrhage: feasibility of a single-center randomized controlled trial. Neurology 2018;90(15 Suppl):3.277. [Google Scholar]
Dawson 2013 {published data only}
- Dawson DR, Anderson ND, Binns MA, Bottari C, Damianakis T, Hunt A, et al.Managing executive dysfunction following acquired brain injury and stroke using an ecologically valid rehabilitation approach: a study protocol for a randomized, controlled trial. Trials 2013;14(1):306. [NCT01414348] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02724813 {unpublished data only}
- NCT02724813.Tele-rehabilitation study for people with a history of stroke. clinicaltrials.gov/ct2/show/NCT02724813 (first received 31 March 2016). [NCT02724813]
Weicker 2013 {published data only}
- Weicker J, Marichal E, Hudl N, Muller K, Lepsien J, Trapp S, et al.Computerized training of working memory for patients with acquired brain injuries – a randomized controlled trial. Behavioural Neurology 2013;27(3):371-2. [Google Scholar]
Additional references
Akbari 2013
- Akbari S, Lyden PD, Kamali M, Fahimi MA.Correlations among impairment, daily activities and thinking operations after stroke. Neurorehabilitation 20103;33(1):153-60. [DOI] [PubMed] [Google Scholar]
AOTA 2013
- American Occupational Therapy Association (AOTA).Cognition, cognitive rehabilitation, and occupational performance. American Journal of Occupational Therapy 2013;67:S9-31. [DOI] [PubMed] [Google Scholar]
AOTA 2014
- American Occupational Therapy Association.Occupational therapy practice framework: domain and process (3rd edition). American Journal of Occupational Therapy 2014;68(Suppl 1):S1-48. [DOI: ] [Google Scholar]
Appollonio 2005
- Appollonio LM, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, et al.The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurological Sciences 2005;26:108-16. [DOI] [PubMed] [Google Scholar]
Arsic 2015
- Arsic S, Eminovic F, Konstantinovic L, Pavlovic D, Kljajic D, Despotovic M.Correlation between functional independence and quality of executive functions in stroke patients. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2015;61(4):333-8. [Google Scholar]
Averbuch 2011
- Averbuch S, Katz N.Cognitive rehabilitation: a retraining model for clients with neurological disabilities. In: Katz N, editors(s). Cognition, Occupation, and Participation Across the Life Span: Neuroscience, Neurorehabilitation, and Models of Intervention in Occupational Therapy. Bethesda (MD): AOTA Press, 2011:277-98. [Google Scholar]
Bae 2005 [Computer program]
- Computerized neurocognitive function test.Bae DS, Lee JB, Ban YK. Seoul (South Korea): Hana Medical, 2005.
Bamford 1989
- Bamford J, Sandercock PA, Warlow CP, Slattery J.Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20:828. [DOI] [PubMed] [Google Scholar]
Basso 1987
- Basso A, Capitani E, Laiacona M.Raven's coloured progressive matrices: normative values in 305 adult normal controls. Functional Neurology 1987;2:189-94. [PubMed] [Google Scholar]
Bean 2018
- Bean J.Rey Auditory Verbal Learning Test, Rey AVLT. In: Kreutzer JS, DeLuca J, Caplan B, editors(s). Encyclopedia of Clinical Neuropsychology. 2nd edition. Cham (Switzerland): Springer, 2018. [Google Scholar]
Beninato 2006
- Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J.Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Archives of Physical Medicine and Rehabilitation 2006;87:32-9. [DOI] [PubMed] [Google Scholar]
Bernhardt 2019
- Bernhardt J, Borschmann KN, Kwakkel G, Burridge JH, Eng JJ, Walker MF, et al.Setting the scene for the Second Stroke Recovery and Rehabilitation Roundtable. International Journal of Stroke 2019;14(5):450–6. [DOI: 10.1177/1747493019851287] [DOI] [PubMed] [Google Scholar]
Bowen 2011
- Bowen A, Knapp P, Gillespie D, Nicolson DJ, Vail A.Non-pharmacological interventions for perceptual disorders following stroke and other adult-acquired, non-progressive brain injury. Cochrane Database of Systematic Reviews 2011, Issue 4. Art. No: CD007039. [DOI: 10.1002/14651858.CD007039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2013
- Bowen A, Hazelton C, Pollock A, Lincoln NB.Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD003586. [DOI: 10.1002/14651858.CD003586.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowie 2006
- Bowie CR, Harvey PD.Administration and interpretation of the Trail Making Test. Nature Protocols 2006;1:2277–81. [DOI: 10.1038/nprot.2006.390] [DOI] [PubMed] [Google Scholar]
Broadbent 1982
- Broadbent D, Cooper PF, Fitzgerald P, Parkes KR.The Cognitive Failures Questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology 1982;21:1-16. [DOI] [PubMed] [Google Scholar]
Burgess 1996
- Burgess PW, Alderman N, Wilson BA, Evans JJ, Emslie H.The Dysexecutive Questionnaire. In: Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ, editors(s). Behavioural Assessment of the Dysexecutive Syndrome. Bury St Edmunds (UK): Thames Valley Test Company, 1996. [Google Scholar]
Carlesimo 1996
- Carlesimo GA, Caltagirone C, Gainotti G, The Group for the Standardization of the Mental Deterioration Battery.The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. European Neurology 1996;36:378-84. [DOI] [PubMed] [Google Scholar]
Carlesimo 2002
- Carlesimo GA, Buccione I, Fadda L, Graceffa A, Mauri M, Lorusso S, et al.Normative data of two memory tasks: Short-Story recall and Rey's Figure [Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey]. Nuova Rivista di Neurologia 2002;12:1-13. [Google Scholar]
Caro 2017
- Caro CC, Mendes PV, Denubila Costa J, Nock LJ, Cezar da Cruz DM.Independence and cognition post-stroke and its relationship to burden and quality of life of family caregivers. Topics in Stroke Rehabilitation 2017;24(3):194-9. [DOI] [PubMed] [Google Scholar]
Carter 1980
- Carter LT, Caruso JL, Languirand MA, Berard MA.The Thinking Skills Workbook: a Cognitive Skills Remediation Manual for Adults. Springfield (IL): Charles C Thomas, 1980. [Google Scholar]
Chan 2001
- Chan RCK.Dysexecutive symptoms among a non-clinical sample: a study with the use of the Dysexecutive Questionnaire. British Journal of Psychology 2001;92:551-65. [PubMed] [Google Scholar]
Chung 2013
- Chung CS, Pollock A, Campbell T, Durward BR, Hagen S.Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD008391. [DOI: 10.1002/14651858.CD008391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicerone 2000
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al.Evidence-based cognitive rehabilitation: recommendations for clinical practice. Archives of Physical and Medical Rehabilitation 2000;81:1596-615. [DOI] [PubMed] [Google Scholar]
Cicerone 2005
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S.Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Archives of Physical Medicine Rehabilitation 2005;86:1681-92. [DOI] [PubMed] [Google Scholar]
Cicerone 2011
- Cicerone K, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al.Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation 2011;92(4):519-30. [DOI] [PubMed] [Google Scholar]
Cicerone 2019
- Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, et al.Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Archives of Physical Medicine and Rehabilitation 2019;100(8):1515-33. [DOI: 10.1016/j.apmr.2019.02.011.] [DOI] [PubMed] [Google Scholar]
Clarke 2015
- Clarke S, Bindschaedler C, Crottaz-Herbette S.Impact of cognitive neuroscience on stroke rehabilitation. Stroke 2015;46:1408-13. [DOI: 10.1161/STROKEAHA.115.007435] [DOI] [PubMed] [Google Scholar]
Culbertson 2005
- Culbertson W, Zillmer E.Tower of London Drexel University. Chicago (IL): Multi-Health Systems, 2005. [Google Scholar]
das Nair 2016
- das Nair R, Lincoln, N.Cognitive rehabilitation for memory deficits following stroke. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD002293. [DOI: 10.1002/14651858.CD002293.pub3] [DOI] [PubMed] [Google Scholar]
Dawson 2009
- Dawson DR, Gaya A, Hunt A, Levine B, Lemsky C, Polatajk HJ.Using the Cognitive Orientation to Occupational Performance with adults with traumatic brain injury. Canadian Journal of Occupational Therapy 2009;76:115-27. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG.Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Delis 2001
- Delis DC, Kaplan EF, Kramer JH.Delis-Kaplan Executive Function System (D-KEFS). London (UK): Harcourt Assessment, 2001. [Google Scholar]
De Wit 2006
- De Wit L, Putman K, Lincoln N, Baert I, Berman P, Beyens H, et al.Stroke rehabilitation in Europe: what do physiotherapists and occupational therapists actually do? Stroke 2006;37(6):1483-9. [DOI: 10.1161/01.STR.0000221709.23293.c2] [DOI] [PubMed] [Google Scholar]
Dirette 2020
- Dirette DP, Gutman SA.Occupational Therapy for Physical Dysfunction. 8th edition. Alphen aan den Rijn (the Netherlands): Wolters Kluwer, 2020. [Google Scholar]
Douiri 2013
- Douiri A, Rudd AG, Wolfe CD.Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke 2013;44(1):138-45. [DOI] [PubMed] [Google Scholar]
Draaisma 2020
- Draaisma LR, Wessel MJ, Hummel FC.Neurotechnologies as tools for cognitive rehabilitation in stroke patients. Expert Review of Neurotherapeutics 2020;20(12):1249-61. [DOI: 10.1080/14737175.2020.1820324] [DOI] [PubMed] [Google Scholar]
Dubois 2000
- Dubois B, Slachevsky A, Litvan I, Pillon B.The FAB: a Frontal Assessment Battery at bedside. Neurology 2000;55:1621-6. [DOI] [PubMed] [Google Scholar]
Edmans 2001
- Edmans J, Champion A, Hill L, Ridley M, Skelly F, Jackson T.Occupational Therapy and Stroke. Oxford (UK): Wiley Blackwell, 2001. [Google Scholar]
Elliott 2014
- Elliott M, Parente F.Efficacy of memory rehabilitation therapy: a meta-analysis of TBI and stroke cognitive rehabilitation literature. Brain Injury 2014;28:1610-6. [DOI: 10.3109/02699052.2014.934921] [DOI] [PubMed] [Google Scholar]
Ezekiel 2018
- Ezekiel L, Collett J, Mayo NE, Pang L, Field L, Dawes H.Factors associated with participation in life situations for adults with stroke: a systematic review. Archives of Physical Medicine and Rehabilitation 2018;100:945-55. [DOI: 10.1016/j.apmr.2018.06.017] [DOI] [PubMed] [Google Scholar]
Feigin 2015
- Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al.Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: the GBD 2013 Study. Neuroepidemiology 2015;45:161-76. [DOI: 10.1159/000441085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez Lopez 2020
- Fernandez Lopez R, Antoli A.Computer-based cognitive interventions in acquired brain injury: a systematic review and meta-analysis of randomized controlled trials. PloS One 2020;15(7):e0235510. [DOI: 10.1371/journal.pone.0235510] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2003
- Fisher A.AMPS – Assessment of Motor and Process Skills. Volume 1 – Development, Standardization and Administration Manual. 5th edition. Vol. 1. Fort Collins (CO): Three Star Press, 2003. [Google Scholar]
Fletcher‐Smith 2010
- Fletcher-Smith J, Walker M, Sunderland A, Garvey K, Wan A, Turner H.An interrater reliability study of the Nottingham Stroke Dressing Assessment. British Journal of Occupational Therapy 2010;73:570-8. [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR."Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189-98. [DOI] [PubMed] [Google Scholar]
Fu 2017
- Fu C, Jin X, Chen B, Xue F, Niu H, Guo R, et al.Comparison of the Mini-Mental State Examination and Montreal Cognitive Assessment executive subtests in detecting post-stroke cognitive impairment. Geriatrics & Gerontology International 2017;17(12):2329-35. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gillen 2015
- Gillen G, Nilsen DM, Attridge A, Banakos E, Morgan M, Winterbottom L, et al.Effectiveness of interventions to improve occupational performance of people with cognitive impairments after stroke: an evidence-based review. American Journal of Occupational Therapy 2015;69:6901180040. [DOI: 10.5014/ajot.2015.012138] [DOI] [PubMed] [Google Scholar]
Gillen 2018
- Gillen G.Part IV intervention applications: cerebrovascular accident (stroke). In: Pendleton, Heidi McHugh, Schultz-Krohn, Winifred, editors(s). Pedretti's Occupational Therapy: Practice Skills for Physical Dysfunction. 8th edition. St Louis (MO): Elsevier Health Sciences, 2018:809-40. [Google Scholar]
Giovagnoli 1996
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E.Trail Making Test: normative values from 287 normal adult controls. Italian Journal of Neurological Sciences 1996;17:305-9. [DOI] [PubMed] [Google Scholar]
Giovagnoli 2001
- Giovagnoli AR.Relation of sorting impairment to hippocampal damage in temporal lobe epilepsy. Neuropsychologia 2001;39(2):140-50. [DOI: 10.1016/S0028-3932(00)00104-4] [DOI] [PubMed] [Google Scholar]
Gollin 2011 [Computer program]
- A gym for the mind 2 (KIT: Book + CD-ROM) New exercises of cognitive stimulation for brain aging and dementia[Una palestra per la mente].Gollin D, Ferrari A, Peruzzi A. Trento (Italy): Erickson, 2011. Book + CD-ROM.
GRADEpro 2020 [Computer program]
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), Accessed July 2021. Available at gradepro.org.
Granger 1993
- Granger CV, Cotter AC, Hamilton BB, Roger C, Fiedler RC.Functional assessment scales: a study of persons after stroke. Archives of Physical Medicine and Rehabilitation 1993;74:133-8. [PubMed] [Google Scholar]
Gronwall 1977
- Gronwall D.Paced auditory serial addition task: a measure of recovery from concussion. Perceptual and Motor Skills 1977;44:367-73. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383-94. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Stenre JAL.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Li T, Deeks JJ.Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hirsh 2011
- Hirsh AT, Braden AL, Craggs JG, Jensen MP.Psychometric properties of the Community Integration Questionnaire in a heterogeneous sample of adults with physical disability. Archives of Physical Medicine and Rehabilitation 2011;92:1602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hochstenbach 1998
- Hochstenbach J, Mulder T, Limbeek J, Donders R, Schoonderwaldt H.Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke. Journal of Clinical and Experimental Neuropsychology 1998;20(4):503-17. [DOI: 10.1076/jcen.20.4.503.1471] [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann T, Glasziou P, Boutron I, Milne R, Perer R.Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:1687. [DOI] [PubMed] [Google Scholar]
Holmqvist 2014
- Holmqvist K, Ivarsson AB, Holmefur M.Occupational therapist practice patterns in relation to clients with cognitive impairment following acquired brain injury. Brain Injury 2014;28(11):1365-73. [DOI: 10.3109/02699052.2014.919529] [DOI] [PubMed] [Google Scholar]
Itzkovich 2000
- Itzkovich M, Averbuch S, Elazar B, Katz N.Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) Battery. 2nd edition. Pequannock (NJ): Maddak, 2000. [DOI] [PubMed] [Google Scholar]
Jensen 1966
- Jensen AR, Rohwer WD.The Stroop color-word test: a review. Acta Psychologica 1966;25:36-93. [DOI: 10.1016/0001-6918(66)90004-7] [DOI] [PubMed] [Google Scholar]
Jokinen 2015
- Jokinen H, Melkas S, Ylikoski R, Pohjasvaara T, Kaste M, Erkinjuntti T, et al.Post‐stroke cognitive impairment is common even after successful clinical recovery. European Journal of Neurology 2015;22:1288-94. [DOI: 10.1111/ene.12743] [DOI] [PubMed] [Google Scholar]
Katz 2018
- Katz N, Toglia J, editor(s).Cognition and Occupation Across the Life Span: Neuroscience, Neurorehabilitation, and Models for Intervention in Occupational Therapy. 4th edition. Bethesda (MD): AOTA Press, 2018. [Google Scholar]
Kihun 2012
- Kihun C, Wanhee L.Cognitive factors associated with activities of daily living in post-stroke patients. Journal of Physical Therapy Science 2012;24(8):779-82. [Google Scholar]
Klingberg 2002
- Klingberg T, Forssberg H, Westerberg H.Training of working memory in children with ADHD. Journal of Clinical and Experimental Neuropsychology 2002;24:781-91. [DOI] [PubMed] [Google Scholar]
Koh 2009
- Koh CL, Hoffmann T, Bennett S, McKenna K.Management of patients with cognitive impairment after stroke: a survey of Australian occupational therapists. Australian Occupational Therapy Journal 2009;56(5):324-31. [DOI: 10.1111/j.1440-1630.2008.00764.x] [DOI] [PubMed] [Google Scholar]
Korner‐Bitensky 2011
- Korner-Bitensky N, Barrett-Bernstein S, Bibas G, Poulin V.National survey of Canadian occupational therapists' assessment and treatment of cognitive impairment post-stroke. Australian Occupational Therapy Journal 2011;58(4):241-50. [DOI: 10.1111/j.1440-1630.2011.00943.x] [DOI] [PubMed] [Google Scholar]
Kristensen 2016
- Kristensen HK, Ytterberg C, Jones DL, Lund H.Research-based evidence in stroke rehabilitation: an investigation of its implementation by physiotherapists and occupational therapists. Disability and Rehabilitation 2016;38(26):2564-74. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lanctôt 2020
- Lanctôt KL, Lindsay MP, Smith EE, Sahlas DJ, Foley N, Gubitz G, et al.Canadian Stroke Best Practice Recommendations: mood, cognition and fatigue following stroke, 6th edition update 2019. International Journal of Stroke 2020;15(6):668-88. [DOI: 10.1177/1747493019847334] [DOI] [PubMed] [Google Scholar]
Langan 2018
- Langan J, Subryanb H, Nwoguc I, Cavuotod L.Reported use of technology in stroke rehabilitation by physical and occupational therapists. Disability and Rehabilitation: Assistive Technology 2018;13(7):641-7. [DOI: 10.1080/17483107.2017.1362043] [DOI] [PubMed] [Google Scholar]
Languirand 2014
- Languirand M, Tondat Ruggeri L.The Thinking Skills Workbook A Cognitive Skills Remediation Manual for Adults. 4th edition. Springfield (IL): Charles C Thomas, 2014. [Google Scholar]
Laver 2017
- Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M.Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD008349. [DOI: 10.1002/14651858.CD008349.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 1998
- Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N.Canadian Occupational Performance Measure. 3rd edition. Ottawa (ON): CAOT Publications, 1998. [Google Scholar]
Lawton 1988
- Lawton MP, Brody EM.Instrumental Activities of Daily Living (IADL) Scale – self-rated version. Psychopharmacology Bulletin 1988;24:789-91. [PubMed] [Google Scholar]
Legg 2017
- Legg L, Lewis SR, Schofield-Robinson OJ, Drummond A, Langhorne P.Occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD003585. [DOI: 10.1002/14651858.CD003585.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loetscher 2019
- Loetscher T, Potter K, Wong D, Das Nair R.Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD002842. [DOI: 10.1002/14651858.CD002842.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW.Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;44:61-5. [PubMed] [Google Scholar]
Maskill 2017
- Maskill L, Tempest S, Grieve JI.Neuropsychology for Occupational Therapists: Cognition in Occupational Performance. 4th edition. Hoboken (NJ): John Wiley & Sons Inc, 2017. [Google Scholar]
McDonald 2019
- McDonald MW, Black SE, Copland DA, Corbett D, Dijkhuizen RM, Farr TD, et al.Cognition in stroke rehabilitation and recovery research: consensus-based core recommendations from the second Stroke Recovery and Rehabilitation Roundtable. International Journal of Stroke 2019;14(8):777-82. [DOI: 10.1177/1747493019873600] [DOI] [PubMed] [Google Scholar]
Mole 2020
- Mole JA, Demeyere N.The relationship between early post-stroke cognition and longer term activities and participation: a systematic review. Neuropsychological Rehabilitation 2020;30(2):346-70. [DOI: 10.1080/09602011.2018.1464934] [DOI] [PubMed] [Google Scholar]
Mozaffarian 2016
- Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, et al.Heart Disease and Stroke Statistics – 2016 Update. Circulation 2016;133(4):e38-e360. [DOI: 10.1161/cir.0000000000000350] [DOI] [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al.The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005;53:695-9. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence.Stroke rehabilitation in adults Clinical Guideline (CG162). www.nice.org.uk/guidance/cg162 (accessed prior to 22 October 2021).
Obaid 2020
- Obaid M, Flach C, Marshall I, Wolfe CD, Douiri A.Long-term outcomes in stroke patients with cognitive impairment: a population-based study. Geriatrics 2020;5(2):E32. [DOI: 10.3390/geriatrics5020032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orsini 1987
- Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G.Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Italian Journal of Neurological Sciences 1987;8:539-48. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA.Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Polatajko 2004
- Polatajko H, Mandich A.Enabling Occupation In Children: the Cognitive Orientation to Daily Occupational Performance (CO-OP) Approach. Ottawa (ON): CAOT, 2004. [Google Scholar]
Pollock 2012
- Pollock A, St George B, Fenton M, Firkins L.Top ten research priorities relating to life after stroke. Lancet Neurology 2012;11(3):209. [DOI: 10.1016/S1474-4422(12)70029-7] [DOI] [PubMed] [Google Scholar]
Poulin 2012
- Poulin V, Korner-Bitensky N, Dawson DR, Bherer L.Efficacy of executive function interventions after stroke: a systematic review. Topics in Stroke Rehabilitation 2012;19:158-71. [DOI: 10.1310/tsr1902-158] [DOI] [PubMed] [Google Scholar]
Poulin 2020
- Poulin V, Jean A, Lamontagne M-E, Pellerin M-A, Viau-Guay A, Ouellet M-C.Identifying clinicians' priorities for the implementation of best practices in cognitive rehabilitation post-acquired brain injury. Disability and Rehabilitation 2020;11:1-11. [DOI: 10.1080/09638288.2020.1721574] [DOI] [PubMed] [Google Scholar]
Powell 2009 [Computer program]
- Training of cognitive rehabilitation (KIT: Book + CD-ROM) Exercises of memory, thinking skills and executive functions after brain injury[Training di riabilitazione cognitiva].Powell T, Malia K. Trento (Italy): Erikson, 2009. Book + CD-ROM.
Powell 2017
- Powell T.The Brain Injury Workbook. Exercises for Cognitive Rehabilitation. 2nd edition. Abingdon (UK): Routledge, 2017. [Google Scholar]
Prigatano 1995
- Prigatano GP, Amin K, Rosenstein LD.Administration And Scoring Manual For The BNI Screen For Higher Cerebral Functions. Phoenix (AZ): Barrow Neurological Institute, 1995. [Google Scholar]
Raven 1998
- Raven J, Raven JC, Court JH.Manual For Raven's Progressive Matrices and Vocabulary Scales – Section 3: Standard Progressive Matrices. Oxford (UK): Oxford Psychologists Press, 1998. [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rogers 2018
- Rogers JM, Foord R, Stolwyk RJ, Wong D, Wilson PH.General and domain-specific effectiveness of cognitive remediation after stroke: systematic literature review and meta-analysis. Neuropsychology Review 2018;28:285-309. [DOI: 10.1007/s11065-018-9378-4] [DOI] [PubMed] [Google Scholar]
Rohling 2009
- Rohling ML, Faust ME, Beverly B, Demakis G.Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.'s (2000, 2005) Systematic Reviews. Neuropsychology 2009;23:20-39. [DOI] [PubMed] [Google Scholar]
Royall 1992
- Royall DR, Mahurin RK, Gray KF.Bedside assessment of executive cognitive impairment: the executive interview. Journal of the American Geriatrics Society 1992;40:1221-6. [DOI] [PubMed] [Google Scholar]
Saa 2019
- Saa JP, Tse T, Baum C, Cumming T, Josman N, Rose M, et al.Longitudinal evaluation of cognition after stroke – a systematic scoping review. PloS One 2019;14(8):e0221735. [DOI: 10.1371/journal.pone.0221735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saan 1986
- Saan R, Deelman B.De 15-woordentest A en B (een voorlopige handleiding). Groningen (the Netherlands): Afdeling Neuropsychologie, AZG (international publication), 1986. [Google Scholar]
Sandford 1995
- Sandford JA, Fine AH, Goldman L.Validity study of the IVA: a visual and auditory CPT. Annual Convention of the American Psychological Association; 1995 June 29-July 2; New York, NY.
Sandford 2000
- Sandford JA, Turner A.Integrated Visual and Auditory Continuous Performance Test Manual. Richmond (VA): Braintrain Inc, 2000. [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al.GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Schiavi 2018
- Schiavi M, Costi S, Pellegrini M, Formisano D, Borghi S, Fugazzaro S.Occupational therapy for complex inpatients with stroke: identification of occupational needs in post-acute rehabilitation setting. Disability and Rehabilitation 2018;40(9):1026-32. [DOI: 10.1080/09638288.2017.1283449] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html. [www.gradeworkinggroup.org]
Schünemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook 2021.
Schünemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Sexton 2019
- Sexton E, McLoughlin A, Williams DJ, Merriman NA, Donnelly N, Rohde D, et al.Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. European Stroke Journal 2019;4(2):160-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skidmore 2011
- Skidmore ER, Holm MB, Whyte EM, Dew MA, Dawson D, Becker JT.The feasibility of meta-cognitive strategy training in acute inpatient stroke rehabilitation: case report. Neuropsychological Rehabilitation 2011;23:208-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skidmore 2014
- Skidmore ER, Dawson DR, Whyte EM, Butters MA, Dew MA, Grattan ES, et al.Developing complex interventions: lessons learned from a pilot study examining strategy training in acute stroke rehabilitation. Clinical Rehabilitation 2014;28:378-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohlberg 1987
- Sohlberg MM, Mateer CA.Effectiveness of an attention-training program. Journal of Clinical and Experimental Neuropsychology 1987;9(2):117-30. [DOI] [PubMed] [Google Scholar]
Sohlberg 2001
- Sohlberg MM, Mateer CA.Management of attention disorders. In: Sohlberg MM, Mateer CA, editors(s). Cognitive Rehabilitation: an Integrative Neuropsychological Approach. New York (NY): Guilford Press, 2001:125-61. [Google Scholar]
Spinnler 1987
- Spinnler H, Tognoni G.Standardizzazione e taratura italiana di test neuropsicologici. Italian Journal of Neurological Sciences 1987;Suppl 8:1-120. [PubMed] [Google Scholar]
Stinear 2020
- Stinear CM, Lang CE, Zeiler S, Byblow WD.Advances and challenges in stroke rehabilitation. Lancet Neurology 2020;19(4):348-60. [DOI: 10.1016/S1474-4422(19)30415-6] [DOI] [PubMed] [Google Scholar]
Stineman 1996
- Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R.The Functional Independence Measure: tests of scaling assumptions, structure and reliability across 20 diverse impairment categories. Archives of Physical Medicine and Rehabilitation 1996;77:1101-8. [DOI] [PubMed] [Google Scholar]
Stolwyk 2021
- Stolwyk RJ, Mihaljcic T, Wong DK, Chapman JE, Rogers JM.Poststroke cognitive impairment negatively impacts activity and participation outcomes: a systematic review and meta-analysis. Stroke 2021;52(2):748-60. [DOI: 10.1161/STROKEAHA.120.032215] [DOI] [PubMed] [Google Scholar]
Strauss 2006
- Strauss E, Sherman EM, Spreen O.A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York (NY): Oxford University Press, 2006. [Google Scholar]
Stroke Foundation 2017
- Stroke Foundation.National Stroke Audit Acute Services Report 2017. informme.org.au/-/media/7D1480925C2046BA914F3F66D392B83A.ashx?la=en (accessed 22 October 2021).
Stroke Foundation 2018
- Stroke Foundation.(Australian) Clinical Guidelines for Stroke Management 2017. www.clinicalguidelines.gov.au/portal/2585/clinical-guidelines-stroke-management-2017 (accessed 22 October 2021).
Sunderland 2006
- Sunderland A, Walker C, Walker MF.Action errors and dressing ability after stroke: an ecological approach to neuropsychological assessment and intervention. Neuropsychological Rehabilitation 2006;16:666-83. [DOI] [PubMed] [Google Scholar]
Toglia 2014
- Toglia JP, Golisz KM, Goverover Y.Cognition, perception, and occupational performance. In: Willard H, Schell B, editors(s). Willard & Spackman's Occupational Therapy. 12th edition. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins, 2014. [Google Scholar]
University College London 2010
- University College London, Centre for Applied Statistics Courses (CASC), UCL Great Ormond Street Institute of Child Health.Confidence intervals for a median, Introduction to Statistics and Research Methods. 2010; Chapter 8. www.ucl.ac.uk/child-health/short-courses-events/about-statistical-courses/research-methods-and-statistics/chapter-8-content-8.
Uyttenboogaart 2007
- Uyttenboogaart M, Luijckx GJ, Vroomen PC, Stewart RE, De Keyser J.Measuring disability in stroke: relationship between the modified Rankin scale and the Barthel index. Journal of Neurology 2007;254(8):1113-7. [DOI] [PubMed] [Google Scholar]
Vandenberg 1978
- Vandenberg SG, Kuse AR.Mental rotations, a group test of three-dimensional spatial visualization. Perceptual and Motor Skills 1978;47:599-604. [DOI] [PubMed] [Google Scholar]
van der Zee 2010
- Zee CH, Priesterbach AR, Dussen L, Kap A, Schepers VP, Visser-Meily JM, et al.Reproducibility of three self-report participation measures: the ICF measure of participation and activities screener, the participation scale, and the Utrecht Scale for Evaluation of Rehabilitation-Participation. Journal of Rehabilitation Medicine 2010;42:752-7. [DOI: 10.2340/16501977-0589] [DOI] [PubMed] [Google Scholar]
van der Zee 2013
- Zee CH, Baars-Elsinga A, Visser-Meily JM, Post MW.Responsiveness of two participation measures in an outpatient rehabilitation setting. Scandinavian Journal of Occupational Therapy 2013;20(3):201-8. [DOI: 10.3109/11038128.2012.754491] [DOI] [PubMed] [Google Scholar]
van de Ven 2015
- de Ven RM, Schmand B, Groet E, Veltman DJ, Murre JMJ.The effect of computer-based cognitive flexibility training on recovery of executive function after stroke: rationale, design and methods of the TAPASS study. BMC Neurology 2015;15:144. [DOI: 10.1186/s12883-015-0397-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Heugten 2012
- Heugten C, Wolters Gregório G, Wade D.Evidence-based cognitive rehabilitation after acquired brain injury: a systematic review of content of treatment. Neuropsychological Rehabilitation 2012;22(5):653-73. [DOI] [PubMed] [Google Scholar]
Virk 2015
- Virk S, Williams T, Brunsdona R, Suha F, Morrowa A.Cognitive remediation of attention deficits following acquired brain injury: a systematic review and meta-analysis. NeuroRehabilitation 2015;36:367-77. [DOI: 10.3233/NRE-151225] [DOI] [PubMed] [Google Scholar]
Vos 2020
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M.Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. [DOI: 10.1016/s0140-6736(20)30925-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagle 2011
- Wagle J, Farner L, Flekkøy K, Bruun Wyller T, Sandvik L, Fure B, et al.Early post-stroke cognition in stroke rehabilitation patients predicts functional outcome at 13 months. Dementia and Geriatric Cognitive Disorders 2011;31(5):379-87. [DOI] [PubMed] [Google Scholar]
Walker 1990
- Walker MF, Lincoln NB.Reacquisition of dressing skills after stroke. International Disability Studies 1990;12:41-3. [DOI] [PubMed] [Google Scholar]
Walker 2003
- Walker CM, Walker MF, Sunderland A.Dressing after a stroke: a survey of current occupational therapy practice. British Journal of Occupational Therapy 2003;66:263-8. [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T.Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: 10.1186/1471-2288-14-135.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wechsler 1945
- Wechsler D.A standardized memory scale for clinical use. Journal of Psychology 1945;19:87-95. [Google Scholar]
Wechsler 1997
- Wechsler D.WAIS III, WMSIII Technical Manual. San Antonio (TX): The Psychological Corporation, 1997. [Google Scholar]
Wechsler 2000
- Wechsler D.Wechsler Adult Intelligence Scale (WAIS-III) Nederlandstaligebewerking. Technische handleiding. Lisse (the Netherlands): Swets & Zeitlinger, 2000. [Google Scholar]
Wechsler 2014
- Wechsler D.Wechsler Adult Intelligence Scale. 4th edition. San Antonio (TX): The Psychological Corporation, 2014. [Google Scholar]
Weicker 2015
- Weicker J, Villringer A, Thöne-Otto A.Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. Neuropsychology 2015;30:190-212. [DOI: 10.1037/neu0000227.] [DOI] [PubMed] [Google Scholar]
Wentink 2018
- Wentink MM, Meesters J, Berger MA, Kloet AJ, Stevens E, Band GP, et al.Adherence of stroke patients with an online brain training program: the role of health professionals' support. Topics in Stroke Rehabilitation 2018;25:359-65. [DOI: 10.1080/10749357.2018.1459362] [DOI] [PubMed] [Google Scholar]
West 2008
- West C, Bowen A, Hesketh A, Vail A.Interventions for motor apraxia following stroke. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004132. [DOI: 10.1002/14651858.CD004132.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westerberg 2004
- Westerberg H.Working Memory: Development, Disorders and Training [Doctoral dissertation]. Stockholm (Sweden): Karolinska Institute, 2004. [Google Scholar]
Willer 1994
- Willer B, Ottenbacher KJ, Coad ML.The Community Integration Questionnaire. A comparative examination. American Journal of Physical Medicine and Rehabilitation 1994;73:103-11. [DOI] [PubMed] [Google Scholar]
Wilson 1989
- Wilson B, Cockburn J, Baddeley A, Hiorns R.The development and validation of a test battery for detecting and monitoring everyday memory problems. Journal of Clinical and Experimental Neuropsychology 1989;11:855-70. [DOI] [PubMed] [Google Scholar]
Wilson 1999
- Wilson BA, Alderman N, Burgess PW, Ernslie H, Evans JJ.Behavioural Assessment of the Dysexecutive Syndrome (BADS). Stockholm (Sweden): Pearson Assessment, 1999. [Google Scholar]
Winstein 2016
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al.Guidelines for adult stroke rehabilitation and recovery. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98-e169. [DOI: 10.1161/STR.0000000000000098] [DOI] [PubMed] [Google Scholar]
Wu 2019
- Wu C-Y, Hung S-J, Lin K-C, Chen K-H, Chen P, Tsay P-K.Responsiveness, minimal clinically important difference, and validity of the MoCA in stroke rehabilitation. Occupational Therapy International 2019;2019:2517658. [DOI: 10.1155/2019/2517658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2014
- Yang S, Ye H, Huang J, Tao J, Jiang C, Lin Z, et al.The synergistic effect of acupuncture and computer-based cognitive training on post-stroke cognitive dysfunction: a study protocol for a randomized controlled trial of 2 × 2 factorial design. BMC Complementary & Alternative Medicine 2014;14:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2020
Zachary 1991
- Zachary RA.Shipley Institute of Living Scale: Revised Manual. Los Angeles (CA): Western Psychological Services, 1991. [Google Scholar]
References to other published versions of this review
Hoffmann 2007
- Hoffmann T, Bennett S, Koh CL, McKenna KT.Occupational therapy for cognitive impairment in stroke patients. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD006430. [DOI: 10.1002/14651858.CD006430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2010
- Hoffmann T, Bennett S, Koh CL, McKenna KT.Occupational therapy for cognitive impairment in stroke patients. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD006430. [DOI: 10.1002/14651858.CD006430.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


