Abstract
An increasing amount of evidence has shown critical roles of gut microbiome in host pathophysiology. The gut and the liver are anatomically and physiologically connected. Given the critical role of gut-liver axis in the homeostasis of the liver, gut microbiome interplays with a diverse spectrum of hepatic changes, including steatosis, inflammation, fibrosis, cholestasis, and tumorigenesis. In clinic, cholestasis manifests with fatigue, pruritus, and jaundice, caused by the impairment in bile formation or flow. Studies have shown that the gut microbiome is altered in cholestatic liver disease. In this review, we will explore the interaction between the gut microbiome and the liver with a focus on the alteration and the role of gut microbiome in cholestatic liver disease. We will also discuss the prospect of exploiting the gut microbiome in the development of novel therapies for cholestatic liver disease.
Keywords: Gut microbiome, Liver pathophysiology, Cholestatic liver disease
1. Introduction
Although the gut microbiota has been studied for decades, it has received an increasing level of attention in the past 20 years due to the availability of advanced genetic and metagenomic tools,1 both of which enable the interrogation of the complexity of the microbiome. Indeed, the use of germ-free animal models and human microbiota-associated rodents makes it possible to experimentally prove functions of the gut microbiome in the host.2,3 In humans, the gut microbiota includes 100 trillion bacteria that inhabit the gastrointestinal tract.4 Gut microbiome composition and diversity are varied in different populations, indicating environment-diet-microbe-host interactions.5, 6, 7
Accumulating evidence suggests that gut microbiome not only can be altered by host health conditions but also actively impacts host functions. Diets significantly affect the composition and functions of gut microbiota,8 whereas microbial metabolites in turn impact host health.9,10 For example, the microbial diversity confers resistance or susceptibility to cholera infection through altering the chemical environment of the gut, relying on the enzyme bile salt hydrolase.11 The microbial metabolites can also alter enterocyte lipid metabolism.12 Conversely, colonocyte metabolism leads to high epithelial oxygen consumption, and the consequent epithelial hypoxia helps to maintain a homeostatic gut microbiota dominated by obligate anaerobic bacteria.13 Other factors like defective gut barrier and antibiotic use are also found to be involved in the interaction between gut microbiome and host metabolism.14 Given its strong interplay with the host metabolism, gut microbiome is undoubtedly involved in the development of metabolic syndrome. In mice, maternal gut microbiota in pregnancy can affect the metabolic phenotype of the offspring.15 In humans, a lower bacterium richness is associated with a higher level of overall adiposity, insulin resistance, and dyslipidemia.16 Gut microbial metabolites derived from lipid, carbohydrate, and protein fermentation play important roles in the development of obesity, non-alcoholic fatty liver disease (NAFLD), and type 2 diabetes mellitus.17, 18, 19, 20 Interestingly, the gut microbiota can also regulate host digestion and absorption of dietary lipids, which may contribute to the host adaptability to dietary lipid variations.21
Besides the impact on the host metabolism, gut microbiota is also critical for the regulation of host immune system.22, 23, 24, 25, 26 Evidence has shown that gut microbiome can alter sex hormone levels and contribute to the fate of autoimmune diseases, such as type 1 diabetes.27 The gut microbiome has also been shown to have a control in the thymic development of mucosal-associated invariant T cells by production of 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU).28 Ketogenic diets low in carbohydrate but rich in fat can induce a pronounced shift in the gut microbiome, thereby resulting in the decrease of intestinal pro-inflammatory T helper 17 (Th17) cells.29 Interestingly, host immune system can in turn regulate the gut microbiome. In a study based on age-associated metabolic syndrome, T cell-dependent events are found to be associated with the expansion of Desulfovibrio and loss of Clostridia, key features that are associated with obesity in mouse models and metabolic syndrome in humans.30
The gut microbiome has also been found to be involved in oncogenesis,31,32 cancer therapy,33, 34, 35 drug metabolism,36, 37, 38, 39 and numerous other health conditions.40, 41, 42, 43 The gut and the liver are anatomically and physiologically connected. The “gut-liver axis” is critical for liver homeostasis and pathogenesis of various liver diseases.44, 45, 46 In this review, we will discuss the interaction between the gut microbiome and hepatic pathophysiology in liver disease with a focus on the role of gut microbiome in cholestatic liver disease.
2. Gut microbiome and liver pathophysiology
2.1. Inflammation and liver injury
Gut-liver axis can contribute to liver inflammation by the regulation of microbial products.47 One recent study showed that resident immune cells can display a spatial polarization in the liver, which was attributed to commensal microbiota and was crucial to the prevention of pathogen dissemination.48 In this study, myeloid and lymphoid cells were found to concentrate around the periportal region. The observed asymmetric localization was not controlled by liver development, but related to sustained MYD88-dependent signaling in liver sinusoidal endothelial cells induced by commensal bacteria. Mechanistically, MYD88-dependent signaling formed chemokine gradients by regulating the composition of the pericellular matrix.
Evidence has also shown a potential interplay of gut microbiome with liver injury. Antibiotics pretreatment effectively reduces transplant injury in mice and humans.49 In acetaminophen (APAP)- and thioacetamide (TAA)-induced acute liver failure (ALF), gut microbiome alters Toll-like receptor (TLR) signaling and contributes to a MYC-dependent transcriptional program, which orchestrate the activation of stellate, endothelial, and Kupffer cell during ALF.50 These studies suggest that gut microbiome can contribute to the development of liver injury. However, protective roles of gut microbiome in liver injury have also been reported. Gut-resident Lactobacillus rhamnosus GG (LGG) seems to be able to protect against oxidative liver injury in two models, APAP overdose and acute ethanol toxicity, by producing a small molecule activator of nuclear factor erythroid 2-related factor 2 (NRF2), 5-methoxyindoleacetic acid.51 In hepatic autophagy-deficient mice, the gut microbiota plays a protective role in liver injury via fibroblast growth factor 15 (FGF15)-fibroblast growth factor receptor 4 (FGFR4) signaling.52 These studies indicate that the functional role of gut microbiota in liver injury can vary in different contexts.
2.2. Alcohol-induced liver injury
Alcohol-associated liver disease (ALD) covers a spectrum of diseases ranging from mild reversible hepatic steatosis to severe inflammatory and cirrhotic status.53,54 Alcohol misuse can alter the composition of gut microbiome and intestinal permeability and a significant amount of evidence has shown interplays of gut microbiota with the development of ALD.55,56 One study transplanted human gut microbiota from alcoholic hepatitis (AH) patients into mice, and found that microbiota caused severe liver inflammation in mice following alcohol-feeding.57 Another study investigated mice from two distinct animal facilities with different susceptibility to alcohol, and found that fecal microbiota transplantation (FMT) from alcohol-resistant mice to alcohol-sensitive mice prevented the latter from alcohol-induced liver injury and steatosis.58 These two studies indicate that gut microbiota may contribute to individual susceptibility to ALD and can be a potential therapeutic target. Indeed, one study has shown that restoration of a particular type of bacteria, Akkermansia muciniphila, which is depleted by alcohol consumption can promote intestinal barrier integrity and ameliorate the pathogenesis of ALD in mouse models.59 Another study also shows that alcohol-associated dysbiosis may cause the dysfunction of bile acid homeostasis, thereby reducing intestinal nuclear farnesoid X receptor (FXR) activity and FGF15 expression.60 Restoring FXR activity or overexpressing a nontumorigenic FGF19 variant, a human FGF15 ortholog, in mice can be beneficial to reduce alcohol-induced liver pathology.
2.3. Fatty acids metabolism
The gut microbiome plays a critical role in metabolic homeostasis. Antibiotic-induced microbiome depletion in mice can shift colonocyte energy utilization from short-chain fatty acids (SCFAs) to glucose.61 The gut microbiome ferments dietary fiber and produces acetate, thereby promoting hepatic fatty acid desaturation and elongation in mice.62 Emerging evidence also shows a role of gut microbiota in amino acid metabolism.63,64
The important role of gut microbiome in host metabolism has shed a light on its impacts on NAFLD. NAFLD describes a fatty liver disease that occurs in the absence of significant alcohol consumption. Since the intimate connections between NAFLD and metabolic syndrome, a new nomenclature, metabolic associated fatty liver disease (MAFLD), has been proposed and used in some recent publications.65, 66, 67 Results from multiple study cohorts have shown alterations of gut microbiome at different stages of NAFLD, and identified a few consistent microbiome signatures discriminating healthy individuals from those with different stages of NAFLD and other metabolic diseases.68 In general, gut microbiota contributes to the progression of NAFLD by metabolites and the factors derived from them.68 Gut microbiota-derived SCFAs, such as acetate, proprionate, and butyrate, prevent the progression of NAFLD. However, decrease in total bile acid pool size and gut microbiota-derived lipopolysaccharide (LPS), lactate, ethanol, and trimethylamine are considered as drivers of NAFLD progression.68 Recently reported studies have identified some other microbial metabolites in NAFLD patients. In a cross-sectional study of well-characterized twins and families, one microbial metabolite, 3-(4-hydroxyphenyl)-lactate is identified in the serum, which is associated with both hepatic steatosis and fibrosis.69 3-(4-hydroxyphenyl)-lactate is significantly correlated with the abundance of several gut microbiome species that are reported to be associated with advanced fibrosis, suggesting a potential link between the gut microbiome and its metabolites that shares gene-effect in hepatic steatosis and fibrosis. Another microbial metabolite, N,N,N-trimethyl-5-aminovaleric acid (TMAVA), is found to be increased in liver steatosis in patients and mouse models, which may be due to the change of the intestinal bacteria Enterococcus faecalis and Pseudomonas aeruginosa.70 Further mechanical studies suggest that TMAVA promotes high-fat diet (HFD)-induced liver steatosis in mice, which can be reduced by carnitine supplementation. Knockout of gamma-butyrobetaine hydroxylase, which causes carnitine deficiency and decreases fatty acid oxidation, also exacerbates HFD-induced fatty liver in mice.
2.4. Cirrhosis
Cirrhosis is the late stage of liver fibrosis seen in a wide range of liver diseases. Gut dysbiosis has been demonstrated in both animal models and patients with cirrhosis.71, 72, 73, 74, 75, 76 Early study suggested that gut dysbiosis in patients with cirrhosis was characterized by the overgrowth of potentially pathogenic bacteria, such as Enterobacteriaceae and Streptococcaceae, together with the decrease of beneficial populations such as Lachnospiraceae.76 More recent studies have shown that the progression of cirrhosis is associated with the alteration of gut microbiome, especially in acute-on-chronic liver failure (ACLF).73,77 Changes in the profile of bile acids may link to the alteration of gut microbiome in liver cirrhosis.78,79 These findings also lead to the exploration of gut microbiome as diagnostic and prognostic measures for cirrhotic patients. Indeed, gene markers in gut microbiome may have the potential to be used to identify patients with liver cirrhosis.75 Discrete stool metagenomic and metabolomic signatures in NAFLD patients may offer universal utility as a non-invasive diagnostic test for cirrhosis when combined with age, serum albumin levels, and serum aspartate aminotransferase levels.72
2.5. Tumorigenesis
Patients with chronic cirrhosis may progress to develop hepatocellular carcinoma (HCC). The diversity of fecal microbiota can be increased from cirrhosis to early-stage HCC.80 Several bacteria are found to be enriched in early HCC, including the phylum Actinobacteria and 13 other genera. Decrease of butyrate-producing genera and increase of LPS-producing genera are also observed in early HCC patients. Further analysis suggests that the characteristic microbiome signatures can be useful for the establishment of non-invasive tools for early diagnosis of HCC. Findings from patients with NAFLD-related HCC have also shown that Bacteroides and Ruminococcaceae are enriched in the HCC group, while the abundance of Bifidobacterium is decreased.81 Given the crucial role of bile acids in gut and liver interactions, the crosstalk between bile acid and gut microbiome inevitably contributes to the gastrointestinal inflammation and carcinogenesis.82 Indeed, a gut microbiome-mediated bile acid change has been associated with a liver-selective anti-tumor effect in mice.83 Ma et al.83 showed that CXCL16 expression of liver sinusoidal endothelial cells was correlated with primary bile acid levels, while inversely correlated with secondary bile acid levels. Expression of CXCL16 caused the accumulation of hepatic natural killer T (NKT) cells, thereby mediating the anti-tumor immunosurveillance effect in the liver.
3. Gut microbiome and cholestatic liver diseases
In clinic, cholestasis manifests with fatigue, pruritus, and jaundice that caused by the impairment in bile formation or flow.84 Cholestatic liver disease may occur in a variety of clinical scenarios with etiologies varying in the anatomical location of the defect and in the acuity of presentation.84 Early biochemical evidence of cholestasis includes increased serum alkaline phosphatase (ALP) and gamma-glutamyltranspeptidase (GGT), followed by the onset of hyperbilirubinemia.84 The main acute causes of cholestasis include common bile duct stenosis and cholangitis, drug-induced liver injury, sepsis, pregnancy, and total parenteral nutrition. Causes of chronic cholestasis include primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), secondary sclerosing cholangitis, biliary atresia, and genetic disorders. Notably, some acute cholestasis can develop into chronic presentation, while chronic cholestasis can manifest an acute flare-up.84,85
A number of animal models have been generated to explore the mechanisms of cholestatic liver disease with the goal to identify potential therapeutic targets. These models are developed based on following approaches: (i) bile duct ligation to create experimental biliary obstruction; (ii) chemical-induced cholestasis (e.g., cholestasis caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine, DDC; alpha-naphthylisothiocyanate, ANIT; 2,4,6-trinitrobenzenesulfonic acid, TNBS; lithocholic acid, LCA); (iii) viral infections; and (iv) genetic manipulation (e.g., deletion or mutation of multidrug resistance 2 (Mdr2) or cystic fibrosis transmembrane conductance regulator (Cftr)).86,87 Although the animal models recapitulate some of the main features of human cholestatic liver disease and provide valuable tools to investigate the fundamental mechanisms, they still have limited applicability in the human setting given the significant differences in the primary etiology and the metabolism pattern between rodents and humans.86
Despite cholestasis commonly occurs in various liver diseases, the therapeutic options are still limited and not effective in general. Treatments for cholestatic liver diseases caused by hereditary transporter defects are limited to the use of ursodeoxycholic acid (UDCA), primary bile acid replacement, biliary diversion, and liver transplantation.87 A recent study showed that synthetic human adenosine triphosphate-binding cassette B4 (ABCB4, i.e., MDR2) mRNA could be used to rescue cholestatic liver disease caused by ABCB4-deficiency in mice, suggesting gene therapy as a way for cholestatic liver disease.88 Several types of drugs have shown potentials in improvement of PBC, including UDCA, obeticholic acid, and some immunosuppressive agents, with UDCA being the most effective treatment for PBC.89 On the other hand, therapeutic effects of PSC are limited.89
3.1. Gut microbiome and bile acid metabolism
The gut microbiome plays a central role in the homeostasis of bile acid metabolism. Conjugated bile acids are deconjugated by bile salt hydrolases (BSH) produced by gut microbiota. Primary bile acids are mainly converted into secondary bile acids by microbial 7-dehydroxylation.82,90 Bile acids in turn have direct antimicrobial effects on gut microbiota and indirect effects through FXR-induced antimicrobial peptides.91,92 Decrease of bile acid levels in the gut favors the growth of Gram-negative bacteria, which can include potential pathogens and cause the production of potent LPS.92 Increase of bile acid levels in the gut favors the growth of Gram-positive members of the Firmicutes, which include bacteria with 7-dehydroxylation capability, and leads to a conversion of primary bile acids to toxic secondary bile acids.92
The transformation of bile acids by gut microbiome modulates the signaling properties of bile acids via two major bile acid receptors, the nuclear FXR and the Takeda G protein-coupled receptor 5 (TGR5).90 In mice, gut microbiota deconjugates and reduces the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist.93 This microbial effect alleviates the suppression of FXR in the ileum, thereby leading to enhanced FXR-FGF15 signaling to reduce bile acid synthesis in the liver. The modification of the signaling properties of bile acids by gut microbiome has a great impact on the host. Evidence has shown that bile acid metabolites are linked to the development of host diseases, including NAFLD,94 ALD,95 short bowel syndrome-associated liver disease,96 and gastrointestinal inflammation and carcinogenesis.82 In a recent study, two distinct derivatives of LCA, 3-oxoLCA and isoalloLCA, were identified to be T cell regulators in mice.97 Mechanically, 3-oxoLCA could inhibit the differentiation of Th17 cells through binding with a key transcription factor, retinoid-related orphan receptor-gammat (RORgammat), and isoalloLCA could increase the differentiation of regulatory T (Treg) cells by the production of mitochondrial reactive oxygen species (mitoROS), which resulted in an increased expression of forkhead box protein P3 (FOXP3). Administration of 3-oxoLCA and isoalloLCA can respectively reduce Th17 cell differentiation and increase Treg cell differentiation in the intestinal lamina propria.97
3.2. Changes of gut microbiome in patients with cholestatic liver disease
3.2.1. Gut microbiome and PBC
Gut microbiome is undoubtedly altered in patients with cholestatic liver disease and in such animal models.98 PBC is an autoimmune cholestatic liver disease which represents the most common chronic cholestatic liver disease in the US. PBC can be attributed to a combination of genetic and environmental factors that trigger a T-lymphocyte-mediated destruction of intrahepatic bile ducts.84 PBC is characterized by nonsuppurative cholangitis and destruction of small- and medium-sized bile ducts with a female proportion of nearly 90%.84 PBC patients commonly have autoimmune diseases, such as Sjögren syndrome, hypothyroidism, cutaneous scleroderma, and rheumatoid arthritis.84 Lipid abnormalities are common in PBC patients. The presence of antimitochondrial antibody (AMA) has been considered as the serologic hallmark of PBC, which is present in 95% of patients with PBC.84
Several pieces of studies have shown that gut microbiome is altered in PBC patients,99, 100, 101 which can be partially restored following treatment.100 Some potentially beneficial bacteria are depleted whereas other bacterial taxa containing opportunistic pathogens are enriched in the gut of PBC patients.99 Although the diversity of gut microbiome seems varied in different studies, several bacteria are consistently altered in PBC patients. These changes include the depletion of genus Bacteroides, and the enrichment of genus Enterobacteriaceae, Veillonella, Lactobacillus, Haemophilus, Streptococcus, and Klebsiella.99, 100, 101 Potential interplays of gut microbiome with the metabolism and immunity were observed in PBC patients.99 The alteration of gut microbiome has also been shown to be associated with bile acid compositions in serum and feces of PBC patients.102 It is worth noting that UDCA therapy can partially restore the altered gut microbiome in PBC patients,100 and the gut microbiome in turn is associated with the response of PBC patients to UDCA therapy.103 Furukawa et al.103 found that PBC patients in the UDCA non-responder group tended to have lower abundance of the genus Faecalibacterium, suggesting that gut microbiome could contribute to the efficacy of treatments in PBC patients.
3.2.2. Gut microbiome and PSC
PSC is a chronic cholestatic liver disease with an unknown etiology. It is characterized by inflammation and fibrosis of intra- and extrahepatic bile ducts and can cause multifocal stricture and dilation of bile ducts.84 The male to female ratio of PSC patients is around 2:1. More than 70% of PSC patients are also complicated with inflammatory bowel disease (IBD).84
Compared to PBC, there is more evidence showing the alterations of gut microbiome in PSC patients (Table 1).99, 100, 101,104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115 In general, the diversity of gut microbiome tends to be reduced in PSC patients compared to healthy controls (HC), and geography is contributable to the alteration of gut microbiome. In most of published reports, PSC and IBD had differential effects on gut microbiome, whereas no significant shifts were observed in PSC and PSC with IBD (PSC-IBD), suggesting that PSC is the main contributor to the alteration of gut microbiome. By revisiting a disease-association microbiome data set comprising 106 patients with PSC and/or IBD, Vieira-Silva et al.116 assessed quantitative taxon abundances and studied microbiome alterations. This study identified and validated a near-exclusion pattern between the inflammation-associated Fusobacterium and Veillonella genera, with the formal being detected only in Crohn's disease and Crohn's disease with PSC. Overall, evidence has indicated that the alteration of gut microbiome can be found in PSC patients and can potentially be used as a biomarker to distinguish PSC and IBD.
Table 1.
Alteration of gut microbiome in PBC and PSC patients.
| Disease | Material | Patients | IBD controls | HC | Method | Diversity | Key results | Reference |
|---|---|---|---|---|---|---|---|---|
| PBC |
Stool |
79 UDCA treatment-naïve patients with PBC and 37 patients who underwent analysis before and after 6 months of UDCA treatment |
0 |
114 |
16S rRNA sequencing |
Alpha: reduced Beta: shows significant shifts |
Increased genus:Enterobacteriaceae, Pseudomonas, Veillonella, Clostridium, Lactobacillus, Haemophilus, Streptococcus, Klebsiella Decreased genus:Oscillospira, Sutterella, Bacteroides, Faecalibacterium |
100 |
| PBC |
Stool |
56 patients with autoimmune liver disease (AILD, including 39 PBC and 17 autoimmune hepatitis) |
0 |
15 |
Terminal restriction fragment length polymorphism of 16S rDNA |
Alpha: no significant change Beta: more clustering in the AILD group than in the HC group |
Increased: order Lactobacillales Decreased: genus Clostridium subcluster XIVa |
101 |
| PBC |
Stool |
42 early-stage PBC |
0 |
30 |
16S rRNA sequencing |
Alpha: no significant change Beta: no significant change |
Decreased: some potentially beneficial bacteria, such as Acidobacteria, Lachnobacterium sp., Bacteroides eggerthii, and Ruminococcus bromii Increased: some bacterial taxa containing opportunistic pathogens, such as gamma-Proteobacteria, Enterobacteriaceae, Neisseriaceae, Spirochaetaceae, Veillonella, Streptococcus, Klebsiella, Actinobacillus pleuropneumoniae, Anaeroglobus geminatus, Enterobacter asburiae, Haemophilus parainfluenzae, Megasphaera micronuciformis, and Paraprevotella clara |
99 |
| PSC |
Mucosal biopsies |
10 PSC-IBD |
10 UC |
10 |
16S rRNA sequencing |
Alpha: no significant change Beta: shows significant shifts between different groups |
PSC-IBD vs. HC: PSC-IBD in comparison with HC was associated with significant shifts in taxa which included a reduction in family Lachnospiraceae and increase in class Bacilli, genus Pseudomonas and Streptoccocus, and species Haemophilus parainfluenzae PSC-IBD vs. UC: in comparison with UC, PSC-IBD was characterized by a significant difference in 50 taxa, of which 24 were enriched in PSC-IBD. In PSC-IBD, there were reductions in taxa that included phylum Lentisphaerae, class Gammaproteobacteria, families Enterobacteriaceae, Prevotellacae, Paraprevotellacae, and Myxococcales, and genus Streptococcus. PSC-IBD was associated with a significant increase in taxa that included the class Bacilli, genus Staphylococcus, and species Parvimonas sp. and Bacteroides fragilis |
105 |
| PSC |
Stool (two cohorts, one Norwegian and one German) |
136 patients with PSC (58% with IBD) |
93 |
158 |
Metagenomic shotgun sequencing |
Alpha: reduced in PSC patients compared to HC, concomitant IBD had no effect in PSC patients Beta: shows significant shifts between different groups, geography matters |
Increased: an enrichment of Clostridium asparagiforme and an unclassified Escherichia species in PSC compared to HC Decreased:Coprococcus catus, Roseburia inulinivorans, Ruminococcus obeum, unclassified Subdoligranulum, Eubacterium rectale, Eubacterium siraeum, Bacteroidales bacterium ph8, Barnesiella intestinihominis, Alistipes shahii, Bacteroides intestinalis Increased prevalence: nine Journal Pre-proof species, including Clostridium clostridioforme, Clostridiales bacterium 1 7 47FAA, Clostridium bolteae, Bifidobacterium bifidum, Clostridium symbiosum, Eggerthella lenta, unclassified Escherichia, unclassified Eggerthella, Clostridium citroniae Decreased prevalence: five species in patients with PSC compared to HC, including Coprobacter fastidiosus, Alistipes senegalensis, Eubacterium ramulus, Eubacterium hallii, Lachnospiraceae bacterium 7 158FAA While several species showed similar patterns in IBD, species like Ruminococcus obeum, Bacteroides intestinalis and several Clostridium species did not differ between IBD and HC |
106 |
| PSC |
Stool |
137 patients with PSC (n = 75 with colitis) |
118 UC |
133 |
16S rRNA sequencing |
Alpha: shows geographical difference. In the Norwegian cohort, reduced in PSC when compared with HC, but comparable between PSC and UC; In German cohort, comparable between PSC and HC, but increased in PSC when compared with UC Beta: shows significant shifts between PSC and HC or between PSC and UC. PSC is similar to PSC with colitis |
PSC vs. HC: bacteria with increased relative abundance in patients with PSC include the genera Veillonella, Streptococcus, Lactobacillus, and Enterococcus and the phylum Proteobacteria, represented by the class Gamma proteobacteria, order Lactobacillales and the class Bacilli Bacteria with decreased relative abundance in patients with PSC include the genera Coprococcus, Holdemanella, Desulfovibrio, Faecalibacterium, and Clostridium IV PSC-IBD vs. PSC: decreased prevalences of Bilophila and Bacteroides in patients with PSC-IBD PSC vs. UC: the phylum Firmicutes was significantly increased in patients with PSC |
107 |
| PSC |
Stool |
7 patients with PSC and IBD |
8 |
8 |
16S rRNA sequencing |
Alpha: reduced in PSC-IBD patients compared to either IBD or HC Beta: not shown |
PSC/IBD had a decrease in the relative abundance of Bacteroides compared to HC and IBD |
108 |
| PSC |
Stool |
27 Japanese patients with paediatric-onset PSC |
16 patients with UC |
23 |
16S rRNA sequencing |
Alpha: reduced in PSC patients when compared to HC, but increased in PSC when compared to UC Beta: shows significant shifts between different groups |
PSC vs. HC: the abundance of genus Parabacteroides was significantly decreased; The abundance of Enterococcus was significantly increased PSC vs. UC: the abundance of genus Faecalibacterium, Ruminococcus, and Roseburia was significantly higher in the PSC |
109 |
| PSC |
Stool |
85 (30 PSC, 44 PSC-UC, 11 PSC-CD) |
36 UC |
263 |
16S rRNA sequencing |
Alpha: reduced in PSC patients when compared to HC, comparable between PSC and UC Beta: shows significant shifts between different groups |
Genus Veillonella enriched specifically in PSC |
110 |
| PSC |
Stool |
43 (11 PSC, 32 PSC-IBD) |
32 UC |
31 |
16S rRNA sequencing |
Alpha: no significant change Beta: shows significant shifts between different groups, PSC-IBD is similar to PSC |
Rothia, Enterococcus, Streptococcus, Clostridium, Veillonella, and Haemophilus were markedly overrepresented in PSC regardless of concomitant IBD. PSC was further characterized by decreased abundance of Adlercreutzia equolifaciens and Prevotella copri. Decrease in genus Phascolarctobacterium was linked to presence of colonic inflammation regardless of IBD phenotype. Akkermansia muciniphila, Butyricicoccus pullicaecorum, and Clostridium colinum were decreased in UC along with genus Roseburia |
111 |
| PSC |
Mucosal biopsies |
11 PSC-IBD |
10 |
9 |
16S rRNA sequencing |
Alpha: not shown Beta: shows significant shifts between different groups |
Significant increase in Veillonella, Escherichia, Lachnospiraceae and Megasphera and decrease of Prevotella and Roseburia and a near-absence of Bacteroides in PSC-IBD |
112 |
| PSC |
Mucosal biopsies |
20 (1 PSC, 19 PSC-IBD) |
15 |
9 |
16S rRNA sequencing |
Alpha: no significant change Beta: no significant change |
Significant PSC-associated enrichment in Barnesiellaceae at the family level, and in Blautia and an unidentified Barnesiellaceae at the genus level. At the operational taxa unit (OTU) level, most shifts in PSC were observed in Clostridiales and Bacteroidales orders |
113 |
| PSC |
Stool |
66 (18 PSC, 27 PSC-UC, 21 PSC-CD) |
13 UC, 30 CD |
66 |
16S rRNA sequencing |
Alpha: decreased in PSC especially PSC-IBD Beta: PSC was significantly different from HC, CD, and UC. PSC only was similar to PSC-IBD |
Three genera, including Enterococcus, are increased in patients with PSC regardless of concomitant IBD and UDCA treatment |
114 |
| PSC | Mucosal biopsies | 12 (8 PSC-UC, 4 PSC-CD) | 11 UC | 9 | 16S rRNA sequencing | Alpha: decreased in PSC Beta: no significant change |
At the genus-like level, the relative abundance of uncultured Clostridiales II was significantly lower in PSC compared with UC and HC | 115 |
Abbreviations: AILD, autoimmune liver disease; CD, Crohn's disease; HC, healthy controls; IBD, inflammatory bowel disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PSC-CD, primary sclerosing cholangitis with concomitant Crohn's disease; PSC-IBD, primary sclerosing cholangitis with concomitant inflammatory bowel disease; PSC-UC, primary sclerosing cholangitis with concomitant ulcerative colitis; UC, ulcerative colitis; UDCA, ursodeoxycholic acid; vs, versus.
Interestingly, several bacteria are consistently enriched in both PBC and PSC patients, including Veillonella, Lactobacillus, Streptococcus, Enterococcus, and Haemophilus, whereas the proportion of some other bacteria are decreased in these patients, such as Bacteroides and Faecalibacterium (Table 2).99, 100, 101,105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115 These findings indicate that some gut microbiota can be altered by cholangitis regardless the etiology.
Table 2.
Bacteria reported to be altered in both PBC and PSC patients.
| Enriched | Decreased |
|---|---|
| Lactobacillus | Bacteroides |
| Veillonella | Faecalibacterium |
| Streptococcus | |
| Enterococcus | |
| Haemophilus |
Abbreviations: PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
3.3. Changes and roles of gut microbiome in animal models for cholestatic liver disease
The alteration of gut microbiome in patients with cholestatic liver disease can be associated with the changes of metabolism and inflammation in these patients.99,105 However, causative roles of gut microbiome in the pathogenesis of cholestatic liver disease remain unclear in humans. Studies have been performed in animal models to investigate roles of gut microbiome in cholestatic liver disease.
3.3.1. Genetic models
Mdr2, i.e., Abcb4, is critical for phosphatidylcholine transportation and thereby plays an important role in bile formation. Disruption of Mdr2 in mice (Mdr2−/−) causes the inability of the liver to secrete phospholipid into the bile, which increases the toxic detergent activity of bile acids for hepatocytes and cholangiocytes.117 Mdr2−/− mice have been widely used as a model for PSC. Gut dysbiosis and increased gut permeability have been observed in Mdr2−/− mice by several groups.118,119 In the study by Tedesco et al.,118 serum levels of interleukin (IL)-17 were increased in Mdr2−/− mice. Mechanically, gut dysbiosis caused the enrichment of Lactobacillus gasseri (L. gasseri), which translocated to the liver due to increased gut permeability in Mdr2−/− mice. L. gasseri activated γδ TCR+ cells to produce IL-17 in the liver, which contributed to the development of liver fibrosis and inflammation in Mdr2−/− mice. In another study, Liao et al.119 showed that increased bacterial translocation amplified the innate immune response via hepatic NLR family pyrin domain containing 3 (NLRP3). Transfer of microbiota from Mdr2−/− mice into healthy wild-type mice caused liver injury in recipient mice, whereas a pan-caspase inhibitor, IDN-7314, dampened inflammasome activation and rescued represented cholestasis-associated liver injury, serum bile acid changes, and gut dysbiosis. Furthermore, gut microbiome were shown to deteriorate the pathology of autoimmune cholangitis in dnTGFbetaRII mice and NOD.c3c4 mice.120,121 These studies indicate that gut dysbiosis contributes to the pathogenesis of cholestatic liver disease and appreciation of gut dysbiosis is a potential therapeutic approach.
Interestingly, different from the findings in conventional Mdr2−/− mice, germ-free Mdr2−/− mice exhibited exacerbated biochemical and histological features of PSC, suggesting that gut microbiome may also protect mice against biliary injury.122 Consistently, our recent findings from hepatic autophagy-deficient mice also indicate an adaptive protection against liver injury from gut microbiome.52 Loss of autophagy-related gene Atg5 or Atg7 in the liver leads to autophagy deficiency and causes cholestatic liver injury characterized by hepatomegaly, fibrosis, and ductular reaction. Gut microbiome is altered in hepatic autophagy-deficient mice with an enrichment of bile acid-metabolizing bacteria. The profile of intestinal bile acids is altered and becomes more capable of activating FXR in hepatic autophagy-deficient mice. Unexpectedly, antibiotics treatment exacerbates cholestatic liver injury in Atg5-knockout livers. Further mechanistic studies suggest that altered gut microbiome provides an adaptive protection against the liver injury through the FGF15-FGFR4 signaling in hepatic autophagy-deficient mice.
3.3.2. Chemical-induction models
Studies are limited on the alteration of gut microbiome in chemical-induced mouse cholestasis models. Furuya et al.123 analyzed gut microbiome in mice fed with 0.1% DDC-containing diet for 8-week, followed by a 6-week recovery. Although the diversity of gut microbiome was not altered in DDC-fed mice, the authors observed a significant enrichment of Gram-negative bacteria in these mice compared to control mice, suggesting that DDC may cause the alteration of gut microbiome even after a 6-week recovery.
Role of gut microbiome has been studied in ANIT- and DDC-induced cholestatic liver disease using germ-free mice. ANIT administration or 0.1% DDC diet-feeding induced cholestatic liver injury in germ-free mice and germ-free mice conventionalized with the microbiome from wile-type specific pathogen free (SPF) animals.124 Compared to the conventionalized mice, germ-free mice were resistant to liver injury, hepatic inflammatory changes, and fibrosis following either ANIT treatment or 0.1% DDC diet-feeding, despite the increase of hepatic bile acid levels in both types of mice. In addition, endotoxin was found to sensitize hepatocytes to bile acid-induced cell death. ANIT-treatment caused shift of gut microbiome in conventionalized germ-free mice and exacerbated intestinal permeability, which seemed to be related to macrophages and inflammasome. Indeed, inhibiting macrophage activation by clodronate-liposomes and NLRP3 inhibitors could alleviate chemical-induced cholestatic liver pathology. The evidence suggests that gut microbiome is critical for the development of chemical-induced cholestatic liver disease.
3.3.3. Human microbiota-associated models
The utilization of germ-free mice has led to the development of murine models carrying disease-associated gut microbiota from human patients. Aiming to further understand the role of gut microbiome in the pathogenesis of PSC, Nakamoto et al.125 studied germ-free mice inoculated with microbiota derived from HC and patients with ulcerative colitis (UC) or with PSC-UC (HC mice, UC mice, and PSC-UC mice respectively). Th17 priming was only induced in the livers from PSC-UC mice, whereas it was induced in the colons from both HC and PSC-UC mice, indicating that gut microbiota derived from PSC-UC patients caused differential hepatic immune response. Interestingly, PSC-UC mice did not show obvious histological or serological changes in the liver even after a long colonization period of 90 days. The authors therefore gave these mice DDC-containing diet. DDC diet failed to cause significant hepatobiliary injury in germ-free mice as shown in another study mentioned above,124 but both PSC-UC and HC mice displayed significant cholestatic phenotype with PSC-UC mice being more susceptible to DDC-induced hepatobiliary damage. Intriguingly, a dramatic enhancement of Th17 response was also observed in PSC-UC mice following DDC-feeding. In order to study the potential mechanisms, the authors cultured bacteria recovered from the liver, mesenteric lymph nodes (MLNs), and spleens, and successfully isolated only bacterial clones from the MLNs of PSC-UC mice. Bacteria Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus gallinarum, were identified by 16S rRNA sequencing. These bacteria were also prevalent in PSC-UC patients, especially Klebsiella pneumoniae. Further mechanistic studies showed that PSC-derived Klebsiella pneumoniae was responsible for epithelial damage, which may be associated with bacterial translocation and susceptibility to Th17-mediated hepatobiliary damages. Finally, antibiotics treatment ameliorated the Th17 response in livers of PSC-UC mice. Overall, this study delineates the role of gut microbiome in hepatic immune response, which is associated with the pathogenesis of PSC. The results provide preclinical evidence for a therapeutic strategy for PSC by targeting gut microbiota.
4. Gut microbiome as a potential therapeutic target for cholestatic liver disease
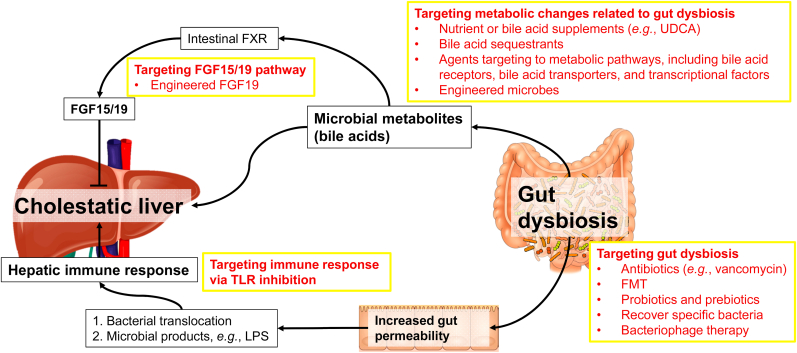
Given the critical role of gut microbiome in liver disease and beyond, gut microbiome-based therapeutic strategies are encouraging as shown by the evidence from pre-clinical studies in animal models and clinical trials.44,126,127 These therapeutics address the following three aspects: (i) maintaining gut barrier integrity; (ii) modulating intestinal dysbiosis by antibiotics, prebiotics, probiotics, synbiotics, FMT, and bacteriophage-based therapy; and (iii) targeting gut microbiota metabolism.126 Accumulating evidence has shown that therapies targeting to gut microbiota can improve PBC or PSC (Table 3).100,103,128, 129, 130, 131, 132, 133 Here, we will discuss some potential therapeutic approaches for cholestatic liver disease by targeting gut dysbiosis and its consequential effects (Fig. 1).
Table 3.
Therapeutic interventions on gut microbiome and outcomes in PBS or PSC patients.
| Therapeutic interventions | Outcomes | References |
|---|---|---|
| FMT | A pilot clinical trial including 10 patients with PSC-IBD showed that FMT in PSC-IBD patients was safe and could improve gut microbiome diversity with no significant change in fecal bile acid profile. | 129 |
| Antibiotics | Several antibiotics have shown significant improvement in serological liver injury markers of PSC patients. Among these antibiotics, vancomycin shows a remarkable efficacy in ameliorating symptoms of PSC patients in several clinical trials. | 130,131 |
| Probiotics | Probiotics (containing four Lactobacillus and two Bifidobacterium strains) did not show significant beneficial effects on symptoms, liver biochemistry or liver function in PSC patients. | 132 |
| A case study suggests that a combination of prednisolone, salazosulfapyridine, and a probiotic (Lactobacillus casei Shirota) treatment significantly improved a 13 years old PSC patient. | 133 | |
| UDCA | UDCA therapy can partially restore the altered gut microbiome in PBC patients, and the gut microbiome in turn is associated with the response of PBC patients to UDCA therapy. | 100,103 |
Abbreviations: FMT, fecal microbiota transplantation; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PSC-IBD, primary sclerosing cholangitis with concomitant inflammatory bowel disease; UDCA, ursodeoxycholic acid.
Fig. 1.
Roles of gut microbiome in cholestatic liver disease and potential therapies. Gut microbiome is altered in cholestatic liver disease and in turn contributes to the pathogenesis of cholestatic liver disease. Modulation of the gut microbiota is feasible via a diverse array of strategies, including antibiotics, fecal microbiota transplantation (FMT), and probiotics. Gut dysbiosis increases gut permeability, which enhances bacterial translocation and transportation of microbial products like lipopolysaccharide (LPS) to the liver, thereby causing hepatic immune response. Inhibition of Toll-like receptors (TLRs) may be a potential therapy. Changes in microbial metabolites, particularly bile acids, by gut dysbiosis affect physiological functions of both the intestine and the liver. Altered bile acid profile in the intestine can alter intestinal farnesoid X receptor (FXR) activity and the expression of fibroblast growth factor (FGF)15/19, which are critical for bile acid homeostasis. In the liver, altered bile acid composition is associated with cholestatic liver disease. Interventions specifically targeted to microbial metabolism in cholestatic liver disease include ursodeoxycholic acid (UDCA) treatment, bile acid sequestrants, and agents targeting to bile acid metabolism. Abbreviations: FGF, fibroblast growth factor; FMT, fecal microbiota transplantation; FXR, farnesoid X receptor; LPS, lipopolysaccharide; TLR, Toll-like receptor; UDCA, ursodeoxycholic acid.
4.1. FMT
As the most straightforward approach, transplantation of gut microbiota from healthy donors to patients seems effective based on several clinical trials. Clostridioides difficile infection (CDI) causes symptoms ranging from diarrhea to life-threatening inflammation in the colon. FMT has been shown as a promising second-line treatment for CDI, especially recurrent CDI, although the treatment is so far still difficult to access with debatable safety concerns.134, 135, 136, 137, 138
According to USA National Institutes of Health (NIH) clinical trial database, numerous clinical trials of FMT for patients with liver diseases are on-going with some of them having been reported. FMT has been reported to improve hepatic encephalopathy,139,140 alcohol use disorder,141 and antibiotic-associated disruption of gut microbiome in cirrhosis patients.142 One pilot clinical trial has shown the role of FMT in PSC patients. In this study, Allegretti et al.129 recruited 10 patients with PSC-IBD and gave them a single FMT by colonoscopy. Their report showed that FMT in PSC-IBD patients was safe and could improve gut microbiome diversity with no significant change in fecal bile acid profile. Although further trials are required to determine whether FMT has a role to play in PSC treatment, the result from this study is encouraging.
4.2. Antibiotics
Other than directly replacing gut microbiome in patients through FMT, controlling gut dysbiosis, particularly the growth of pathogenic bacteria, using antibiotics can be effective and more feasible. Indeed, several antibiotics have shown significant improvement in serological liver injury markers of PSC patients.130 Among antibiotics that have been studied in PSC patients, vancomycin shows a remarkable efficacy in ameliorating symptoms of PSC patients in several clinical trials.131
Although therapies based on bacteriophage have not been reported in patients with cholestatic liver disease, a recent study has found that bacteriophage targeting to cytolytic Enterococcus faecalis can decrease cytolysin in the liver and improve alcohol-induced liver damage in humanized mice.143 This study provides encouraging evidence that bacteriophage-based treatment may be an approach to precisely modify gut microbiota. Indeed, several bacteria are shown to be enriched in both PBC and PSC patients (Table 2), and PSC-derived Klebsiella pneumoniae seems critical to the development of cholestatic liver damage in murine models.125 Thus, a precise editing of gut microbiota can be a potential therapy for cholestatic liver disease.
4.3. Probiotics, prebiotics, and specific bacteria recovery
The recovery from gut dysbiosis can also be achieved by giving probiotics and/or prebiotics to facilitate the growth of bacteria beneficial to host health, which has shown beneficial effects on multiple diseases.144, 145, 146 In mice, probiotics modulate gut microbiome and suppress HCC growth by regulating the T-cell differentiation in the gut.32 Impacts of probiotics on PSC have been studied in clinic. Probiotics (containing four Lactobacillus and two Bifidobacterium strains) did not show significant beneficial effects on symptoms, liver biochemistry or liver function in PSC patients.132 A case study suggests that a combination of prednisolone, salazosulfapyridine, and a probiotic (Lactobacillus casei Shirota) treatment significantly improved a 13 years old PSC patient.133 Although effects of probiotics on PSC are still controversial, probiotics may enhance the efficacy of other frontline therapies for PSC.
Giving specific bacteria supplement is another way to recover the loss of certain bacteria owing to a disease condition. Akkermansia muciniphila is a Gram-negative intestinal commensal, which can promote barrier function partly by enhancing mucus production. Interestingly, one study showed that alcohol consumption caused depletion of Akkermansia muciniphila in both murine models and human patients.59 Akkermansia muciniphila could be recovered by oral supplementation in mouse models for ALD, and its recovery promoted intestinal barrier integrity and improved experimental ALD. One recent study reported that daily dosing SER-287, an oral formulation of Firmicutes spores, improved UC particularly in patients following vancomycin preconditioning.147 Although these studies were not done in patients with cholestatic liver disease, identification and supplementation of specific bacteria can be one future direction to find novel therapies for cholestatic liver disease.
4.4. Therapeutic targets attributable to gut microbiome
Metabolic changes caused by gut dysbiosis play a crucial role in the pathogenesis of liver disease. As mentioned above, bile acid metabolism mediated by gut microbiome is a critical part of gut-liver axis. Given the nature of cholestatic liver disease, targeting bile acid metabolism has shown promising therapeutical potential for cholestatic liver disease. Agents like UDCA and some bile acid sequestrants are widely used in clinic for cholestatic liver disease with high efficacy.130 Several agents targeting bile acid receptors (e.g., FXR, TGR5), bile acid transporters (e.g., apical sodium-dependent bile acid transporter, ASBT; sodium/taurocholate cotransporting polypeptide, NTCP), and other transcriptional factors like peroxisome proliferator-activated receptor (PPAR) have been studied and shown to be hopeful as therapies of cholestatic liver disease.130 A nontumorigenic variant of FGF19 also shows the potential to improve cholestatic liver diseases.148,149 It is worth noting that UDCA therapy can partially restore the altered gut microbiome in PBC patients,100 and the gut microbiome in turn is associated with the response of PBC patients to UDCA therapy,103 suggesting therapies targeting to bile acid homeostasis may improve gut dysbiosis as well.
Besides pharmacological approaches, engineered microbes have been shedding a light on remedying host diseases by engulfing bacteria, which can express factors functioning in metabolism or immune response, in the gut. In a chronic-binge ethanol feeding mouse model, engineered Lactobacillus reuteri, which can produce IL-22, is able to recover the expression of antimicrobial C-type lectin regenerating islet-derived 3 gamma (REG3G) in the small intestine, and thereby improves ethanol-induced liver damage.150 The secondary bile acids deoxycholic acid (DCA) and LCA are produced by gut microbiome and play an important role in regulating host physiology. One recent study has delineated the metabolic pathway for bile acid dihydroxylation by identifying a set of six enzymes that are necessary and sufficient for the conversion of cholic acid to DCA.151 The authors engineered the pathway into Clostridium sporogenes, and successfully conferred production of DCA and LCA on a nonproducing commensal, indicating that this pathway can be expressed and controlled heterologously. This study not only establishes a complete pathway to produce secondary bile acids, but also provides a potential application of engineered microbes as a therapy. Future studies should be carried on developing engineered microbes that modulate bile acid composition in patients with cholestatic liver disease.
5. Conclusion and future perspectives
Role of gut microbiome in gut-liver axis and other physiological functions in the host has been extensively recognized in the past two decades. Interplays of gut microbiome with the liver have been elucidated with critical roles in host metabolic homeostasis and immune response. Gut dysbiosis contributes to various types of liver disease. In cholestatic liver disease, gut microbiome is altered and can be recovered following remedies. Gut microbiome in turn is attributed to the pathogenesis of cholestatic liver disease by its metabolites and its effects in immune regulation. Clinical trials have been started to determine the therapies for cholestatic liver disease by targeting gut microbiome, and FMT has shown encouraging efficacy in mitigating PSC.
Despite a large amount of evidence from animal models showing the functions of gut microbiome in cholestatic liver disease, it is still unclear whether those mechanisms are comparable to the situation in human patients. A better understanding of the interplay of gut microbiome with cholestatic liver disease in human may lead to novel therapies for cholestatic liver disease.
Authors' contributions
S. Yan and X.-M. Yin drafted, revised, and approved this manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported in part by the USA National Institutes of Health (NIH) grants DK116605 (to X.-M. Yin) and LA CaTS Pilot Grant U54 GM104940 (to S. Yan).
Footnotes
Edited by Peiling Zhu and Genshu Wang.
References
- 1.Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter J, Armet AM, Finlay BB, Shanahan F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020;180:221–232. doi: 10.1016/j.cell.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Goel A, Gupta M, Aggarwal R. Gut microbiota and liver disease. J Gastroenterol Hepatol. 2014;29:1139–1148. doi: 10.1111/jgh.12556. [DOI] [PubMed] [Google Scholar]
- 4.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 7.Koppel N, Balskus EP. Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem Biol. 2016;23:18–30. doi: 10.1016/j.chembiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 10.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 11.Alavi S, Mitchell JD, Cho JY, Liu R, Macbeth JC, Hsiao A. Interpersonal gut microbiome variation drives susceptibility and resistance to cholera infection. Cell. 2020;181:1533–1546(e13). doi: 10.1016/j.cell.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araújo JR, Tazi A, Burlen-Defranoux O, et al. Fermentation products of commensal bacteria alter enterocyte lipid metabolism. Cell Host Microbe. 2020;27:358–375(e7). doi: 10.1016/j.chom.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Litvak Y, Byndloss MX, Bäumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362 doi: 10.1126/science.aat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–4057. doi: 10.1172/jci129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura I, Miyamoto J, Ohue-Kitano R, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367 doi: 10.1126/science.aaw8429. [DOI] [PubMed] [Google Scholar]
- 16.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 17.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto J, Igarashi M, Watanabe K, et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun. 2019;10:4007. doi: 10.1038/s41467-019-11978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Jang C, Liu J, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Guryn K, Hubert N, Frazier K, et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe. 2018;23:458–469(e5). doi: 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Chen BD, Zhao LD, Li H. The gut microbiota: emerging evidence in autoimmune diseases. Trends Mol Med. 2020;26:862–873. doi: 10.1016/j.molmed.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Schluter J, Peled JU, Taylor BP, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588:303–307. doi: 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuganbaev T, Mor U, Bashiardes S, et al. Diet diurnally regulates small intestinal microbiome-epithelial-immune homeostasis and enteritis. Cell. 2020;182:1441–1459(e21). doi: 10.1016/j.cell.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Leshem A, Liwinski T, Elinav E. Immune-microbiota interplay and colonization resistance in infection. Mol Cell. 2020;78:597–613. doi: 10.1016/j.molcel.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 28.Legoux F, Bellet D, Daviaud C, et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366:494–499. doi: 10.1126/science.aaw2719. [DOI] [PubMed] [Google Scholar]
- 29.Ang QY, Alexander M, Newman JC, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181:1263–1275(e16). doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen C, Bell R, Klag KA, et al. T cell-mediated regulation of the microbiota protects against obesity. Science. 2019;365:eaat9351. doi: 10.1126/science.aat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadosh E, Snir-Alkalay I, Venkatachalam A, et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586:133–138. doi: 10.1038/s41586-020-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Sung CY, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306–E1315. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Chanona E, Trinchieri G. The role of microbiota in cancer therapy. Curr Opin Immunol. 2016;39:75–81. doi: 10.1016/j.coi.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javdan B, Lopez JG, Chankhamjon P, et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181:1661–1679(e22). doi: 10.1016/j.cell.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pryor R, Norvaisas P, Marinos G, et al. Host-microbe-drug-nutrient screen identifies bacterial effectors of metformin therapy. Cell. 2019;178:1299–1312(e29). doi: 10.1016/j.cell.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang MJ, Kim HG, Kim JS, et al. The effect of gut microbiota on drug metabolism. Expert Opin Drug Metab Toxicol. 2013;9:1295–1308. doi: 10.1517/17425255.2013.807798. [DOI] [PubMed] [Google Scholar]
- 40.Chevalier G, Siopi E, Guenin-Macé L, et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. 2020;11:6363. doi: 10.1038/s41467-020-19931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun. 2017;8:1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Ohtani N, Kawada N. Role of the gut-liver axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship. Hepatol Commun. 2019;3:456–470. doi: 10.1002/hep4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chopyk DM, Grakoui A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology. 2020;159:849–863. doi: 10.1053/j.gastro.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo-Dela Cruz P, Wanek AG, Kumar P, et al. Intestinal IL-17R signaling constrains IL-18-driven liver inflammation by the regulation of microbiome-derived products. Cell Rep. 2019;29:2270–2283(e7). doi: 10.1016/j.celrep.2019.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gola A, Dorrington MG, Speranza E, et al. Commensal-driven immune zonation of the liver promotes host defence. Nature. 2020;589:131–136. doi: 10.1038/s41586-020-2977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura K, Kageyama S, Ito T, et al. Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J Clin Invest. 2019;129:3420–3434. doi: 10.1172/jci127550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolodziejczyk AA, Federici S, Zmora N, et al. Acute liver failure is regulated by MYC- and microbiome-dependent programs. Nat Med. 2020;26:1899–1911. doi: 10.1038/s41591-020-1102-2. [DOI] [PubMed] [Google Scholar]
- 51.Saeedi BJ, Liu KH, Owens JA, et al. Gut-resident lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metab. 2020;31:956–968(e5). doi: 10.1016/j.cmet.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan S, Khambu B, Chen X, Dong Z, Guo G, Yin XM. Hepatic autophagy deficiency remodels gut microbiota for adaptive protection via FGF15-FGFR4 signaling. Cell Mol Gastroenterol Hepatol. 2021;11:973–997. doi: 10.1016/j.jcmgh.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Parker R, Aithal GP, Becker U, et al. Natural history of histologically proven alcohol-related liver disease: a systematic review. J Hepatol. 2019;71:586–593. doi: 10.1016/j.jhep.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 57.Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 58.Ferrere G, Wrzosek L, Cailleux F, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66:806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Grander C, Adolph TE, Wieser V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 60.Hartmann P, Hochrath K, Horvath A, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67:2150–2166. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarrinpar A, Chaix A, Xu ZZ, et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9:2872. doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kindt A, Liebisch G, Clavel T, et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat Commun. 2018;9:3760. doi: 10.1038/s41467-018-05767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7:2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krishnan S, Ding Y, Saedi N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Younossi ZM, Rinella ME, Sanyal AJ, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2020;73:1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 66.Eslam M, Sanyal AJ, George J, International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014(e1). doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 67.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 68.Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 69.Caussy C, Hsu C, Lo MT, et al. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology. 2018;68:918–932. doi: 10.1002/hep.29892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao M, Zhao L, Xiong X, et al. TMAVA, a metabolite of intestinal microbes, is increased in plasma from patients with liver steatosis, inhibits γ-butyrobetaine hydroxylase, and exacerbates fatty liver in mice. Gastroenterology. 2020;158:2266–2281(e27). doi: 10.1053/j.gastro.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 71.De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 72.Oh TG, Kim SM, Caussy C, et al. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab. 2020;32:878–888(e6). doi: 10.1016/j.cmet.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acharya C, Bajaj JS. Altered microbiome in patients with cirrhosis and complications. Clin Gastroenterol Hepatol. 2019;17:307–321. doi: 10.1016/j.cgh.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 77.Solé C, Guilly S, Da Silva K, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis. Gastroenterology. 2021;160:206–218(e13). doi: 10.1053/j.gastro.2020.08.054. [DOI] [PubMed] [Google Scholar]
- 78.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren Z, Li A, Jiang J, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014–1023. doi: 10.1136/gutjnl-2017-315084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 82.Jia W, Xie G, Jia W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360 doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hilscher MB, Kamath PS, Eaton JE. Cholestatic liver diseases: a primer for generalists and subspecialists. Mayo Clin Proc. 2020;95:2263–2279. doi: 10.1016/j.mayocp.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Jüngst C, Berg T, Cheng J, et al. Intrahepatic cholestasis in common chronic liver diseases. Eur J Clin Invest. 2013;43:1069–1083. doi: 10.1111/eci.12128. [DOI] [PubMed] [Google Scholar]
- 86.Mariotti V, Strazzabosco M, Fabris L, Calvisi DF. Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1254–1261. doi: 10.1016/j.bbadis.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury - what is the link? J Hepatol. 2017;67:619–631. doi: 10.1016/j.jhep.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 88.Wei G, Cao J, Huang P, et al. Synthetic human ABCB4 mRNA therapy rescues severe liver disease phenotype in a BALB/c.Abcb4-/- mouse model of PFIC3. J Hepatol. 2021;74:1416–1428. doi: 10.1016/j.jhep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Islam KB, Fukiya S, Hagio M, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 92.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Jiao N, Baker SS, Chapa-Rodriguez A, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 95.Li T, Chiang JYL. Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease. Hepatobiliary Surg Nutr. 2020;9:152–169. doi: 10.21037/hbsn.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pereira-Fantini PM, Lapthorne S, Joyce SA, et al. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J Hepatol. 2014;61:1115–1125. doi: 10.1016/j.jhep.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 97.Hang S, Paik D, Yao L, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kummen M, Hov JR. The gut microbial influence on cholestatic liver disease. Liver Int. 2019;39:1186–1196. doi: 10.1111/liv.14153. [DOI] [PubMed] [Google Scholar]
- 99.Lv LX, Fang DQ, Shi D, et al. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ Microbiol. 2016;18:2272–2286. doi: 10.1111/1462-2920.13401. [DOI] [PubMed] [Google Scholar]
- 100.Tang R, Wei Y, Li Y, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 101.Barbier O, Abe K, Takahashi A, et al. Dysbiosis of oral microbiota and its association with salivary immunological biomarkers in autoimmune liver disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen W, Wei Y, Xiong A, et al. Comprehensive analysis of serum and fecal bile acid profiles and interaction with gut microbiota in primary biliary cholangitis. Clin Rev Allergy Immunol. 2020;58:25–38. doi: 10.1007/s12016-019-08731-2. [DOI] [PubMed] [Google Scholar]
- 103.Furukawa M, Moriya K, Nakayama J, et al. Gut dysbiosis associated with clinical prognosis of patients with primary biliary cholangitis. Hepatol Res. 2020;50:840–852. doi: 10.1111/hepr.13509. [DOI] [PubMed] [Google Scholar]
- 104.Little R, Wine E, Kamath BM, Griffiths AM, Ricciuto A. Gut microbiome in primary sclerosing cholangitis: a review. World J Gastroenterol. 2020;26:2768–2780. doi: 10.3748/wjg.v26.i21.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quraishi MN, Acharjee A, Beggs AD, et al. A pilot integrative analysis of colonic gene expression, gut microbiota, and immune infiltration in primary sclerosing cholangitis-inflammatory bowel disease: association of disease with bile acid pathways. J Crohns and Colitis. 2020;14:935–947. doi: 10.1093/ecco-jcc/jjaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kummen M, Thingholm LB, Rühlemann MC, et al. Altered gut microbial metabolism of essential nutrients in primary sclerosing cholangitis. Gastroenterology. 2021;160:1784–1798(e0). doi: 10.1053/j.gastro.2020.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rühlemann M, Liwinski T, Heinsen FA, et al. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Aliment Pharmacol Ther. 2019;50:580–589. doi: 10.1111/apt.15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaughn BP, Kaiser T, Staley C, et al. A pilot study of fecal bile acid and microbiota profiles in inflammatory bowel disease and primary sclerosing cholangitis. Clin Exp Gastroenterol. 2019;12:9–19. doi: 10.2147/CEG.S186097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iwasawa K, Suda W, Tsunoda T, et al. Characterisation of the faecal microbiota in Japanese patients with paediatric-onset primary sclerosing cholangitis. Gut. 2017;66:1344–1346. doi: 10.1136/gutjnl-2016-312533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kummen M, Holm K, Anmarkrud JA, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 111.Bajer L, Kverka M, Kostovcik M, et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23:4548–4558. doi: 10.3748/wjg.v23.i25.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quraishi MN, Sergeant M, Kay G, et al. The gut-adherent microbiota of PSC–IBD is distinct to that of IBD. Gut. 2017;66:386–388. doi: 10.1136/gutjnl-2016-311915. [DOI] [PubMed] [Google Scholar]
- 113.Torres J, Bao X, Goel A, et al. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;43:790–801. doi: 10.1111/apt.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rossen NG, Fuentes S, Boonstra K, et al. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J Crohns Colitis. 2015;9:342–348. doi: 10.1093/ecco-jcc/jju023. [DOI] [PubMed] [Google Scholar]
- 116.Vieira-Silva S, Sabino J, Valles-Colomer M, et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol. 2019;4:1826–1831. doi: 10.1038/s41564-019-0483-9. [DOI] [PubMed] [Google Scholar]
- 117.Smit JJ, Schinkel AH, Oude Elferink RP, et al. Homozygous disruption of the murine MDR2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 118.Tedesco D, Thapa M, Chin CY, et al. Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic γδ T-cell receptor–positive cells and pathogenesis of cholestatic liver disease. Gastroenterology. 2018;154:2178–2193. doi: 10.1053/j.gastro.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liao L, Schneider KM, Galvez EJC, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. 2019;68:1477–1492. doi: 10.1136/gutjnl-2018-316670. [DOI] [PubMed] [Google Scholar]
- 120.Ma HD, Zhao ZB, Ma WT, et al. Gut microbiota translocation promotes autoimmune cholangitis. J Autoimmun. 2018;95:47–57. doi: 10.1016/j.jaut.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schrumpf E, Kummen M, Valestrand L, et al. The gut microbiota contributes to a mouse model of spontaneous bile duct inflammation. J Hepatol. 2017;66:382–389. doi: 10.1016/j.jhep.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tabibian JH, O’Hara SP, Trussoni CE, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63:185–196. doi: 10.1002/hep.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Furuya S, Argemi J, Uehara T, et al. A novel mouse model of acute-on-chronic cholestatic alcoholic liver disease: a systems biology comparison with human alcoholic hepatitis. Alcohol Clin Exp Res. 2020;44:87–101. doi: 10.1111/acer.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Isaacs-Ten A, Echeandia M, Moreno-Gonzalez M, et al. Intestinal microbiome-macrophage crosstalk contributes to cholestatic liver disease by promoting intestinal permeability in mice. Hepatology. 2020;72:2090–2108. doi: 10.1002/hep.31228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakamoto N, Sasaki N, Aoki R, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019;4:492–503. doi: 10.1038/s41564-018-0333-1. [DOI] [PubMed] [Google Scholar]
- 126.Sharpton SR, Schnabl B, Knight R, Loomba R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 2021;33:21–32. doi: 10.1016/j.cmet.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. 2020;158:1881–1898. doi: 10.1053/j.gastro.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 128.Shah A, Macdonald GA, Morrison M, Holtmann G. Targeting the gut microbiome as a treatment for primary sclerosing cholangitis: a conceptional framework. Am J Gastroenterol. 2020;115:814–822. doi: 10.14309/ajg.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am J Gastroenterol. 2019;114:1071–1079. doi: 10.14309/ajg.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 130.Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16:885–896. doi: 10.1016/j.autrev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 131.Shah A, Crawford D, Burger D, et al. Effects of antibiotic therapy in primary sclerosing cholangitis with and without inflammatory bowel disease: a systematic review and meta-analysis. Semin Liver Dis. 2019;39:432–441. doi: 10.1055/s-0039-1688501. [DOI] [PubMed] [Google Scholar]
- 132.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol. 2008;20:688–692. doi: 10.1097/MEG.0b013e3282f5197e. [DOI] [PubMed] [Google Scholar]
- 133.Shimizu M, Iwasaki H, Mase S, Yachie A. Successful treatment of primary sclerosing cholangitis with a steroid and a probiotic. Case Rep Gastroenterol. 2012;6:249–253. doi: 10.1159/000338834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelly CR, Yen EF, Grinspan AM, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology. 2021;160:183–192(e3). doi: 10.1053/j.gastro.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent clostridioides difficile infection. Gastroenterology. 2021;160:1961–1969(e3). doi: 10.1053/j.gastro.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 136.Cook L, Rees WD, Wong MQ, Peters H, Levings MK, Steiner TS. Fecal microbiota transplantation for recurrent Clostridioides difficile infection enhances adaptive immunity to C difficile toxin B. Gastroenterology. 2021;160:2155–2158(e4). doi: 10.1053/j.gastro.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 137.Blaser MJ. Fecal microbiota transplantation for dysbiosis - predictable risks. N Engl J Med. 2019;381:2064–2066. doi: 10.1056/NEJMe1913807. [DOI] [PubMed] [Google Scholar]
- 138.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 139.Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mullish BH, McDonald JAK, Thursz MR, Marchesi JR. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66:1354–1355. doi: 10.1002/hep.29369. [DOI] [PubMed] [Google Scholar]
- 141.Bajaj JS, Gavis EA, Fagan A, et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology. 2021;73:1688–1700. doi: 10.1002/hep.31496. [DOI] [PubMed] [Google Scholar]
- 142.Bajaj JS, Kakiyama G, Savidge T, et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology. 2018;68:1549–1558. doi: 10.1002/hep.30037. [DOI] [PubMed] [Google Scholar]
- 143.Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 145.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 146.Britton RA, Hoffmann DE, Khoruts A. Probiotics and the microbiome: how can we help patients make sense of probiotics? Gastroenterology. 2021;160:614–623. doi: 10.1053/j.gastro.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 147.Henn MR, O’Brien EJ, Diao L, et al. A phase 1b safety study of SER-287, a spore-based microbiome therapeutic, for active mild to moderate ulcerative colitis. Gastroenterology. 2021;160:115–127(e30). doi: 10.1053/j.gastro.2020.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luo J, Ko B, Elliott M, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:247ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 149.Mayo MJ, Wigg AJ, Leggett BA, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2:1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hendrikx T, Duan Y, Wang Y, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019;68:1504–1515. doi: 10.1136/gutjnl-2018-317232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Funabashi M, Grove TL, Wang M, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582:566–570. doi: 10.1038/s41586-020-2396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]