Abstract
With the increase of global population, people's life expectancy is growing as well. Humans tend to live more active lifestyles and, therefore, trauma generated large defects become more common. Instances of tumour resection or pathological conditions and complex orthopaedic issues occur more frequently increasing necessity for bone substitutes. Composition of calcium phosphate cements (CPCs) is comparable to the chemical structure of bone minerals. Their ability to self-set and resorb in vivo secures a variety of potential applications in bone regeneration. Despite the years-long research and several products already reaching the market, finding the right properties for calcium phosphate cement to be osteoinductive and both injectable and suitable for clinical use is still a sudoku. This article is focused on injectable, porous CPCs, reviewing the latest developments on the path toward finding osteoinductive material, which is suitable for injection.
Keywords: Calcium phosphate cements, Bone cements, Bone regeneration, Osteoinductivity
Abbreviations: Calcium phosphate cements, CPCs
Graphical abstract
Highlights
-
•
Phase separation is an essential factor to be improved to obtain injectable material; several methods have been proposed.
-
•
Osteoinductive bone substitutes – possible solution for bad mechanical performance of CPCs.
-
•
Osteoinductivity of CPC could be attained even without the addition of different supplements.
-
•
Less complex composition of CPC – potentially reduced price of the final product and wider availability on the market.
1. Introduction
Research in application of calcium phosphate cements (CPCs) for bone graft substitution has been conducted extensively since their development in 1986 [1]. Different literature sources mention their use in dental, craniofacial and orthopaedic applications [2,3].
Bone self-repair ability is limited, and typically external intervention is needed to repair and regenerate critical size defects in bones. The present gold standard for bone reconstruction is still the autograft (“transposition of a patient's bone from a distant region of the body to the site of the bony defect” [4]) – anterior iliac crest bone graft, and effectiveness of all osteobiologics is measured against it. Allografts, and xenografts are also used [3,[5], [6], [7], [8], [9]].
With the increase of global population, people's life expectancy is growing as well. Humans tend to live more active lifestyles and, therefore, trauma generated large defects become more common. Instances of tumour resection or pathological conditions and complicated orthopaedic problems occur more frequently [10] and the need for bone grafts increases. Limited availability is not the only disadvantage of bone grafts. There are other drawbacks such as the requirement for prior surgical intervention at the donor site, the risk of the donor site being infected or a low rate of resorption of the graft. Also, the bone graft is of a shape that demands adjustments to it or to the surgical site in order to fit the implant. Ability to eliminate the adjustments to the surgical site would help avoid bone loss, surrounding tissue trauma, incomplete regeneration of bones and extended time for completing the surgery [1,11].
Aforementioned circumstances promote research for new bone substitute materials. The research aims to find bone graft that is of:
-
•
good biocompatibility in order to be accepted by the patient and not lead to adverse consequences,
-
•
bioactivity so that the bone graft is able to successfully bond with the bone tissue surrounding it,
-
•
osteoinductivity to further bone cell differentiation into osteoblasts.
Bone graft must be biodegradable or bioresorbable with a proviso that degradation products are non-cytotoxic. Significance must also be placed on particular physical properties, for example, the bone graft should incorporate a porous network of interconnected macro- and micropores. The aforementioned properties promote resorption of graft and lay the foundation for development of a living tissue, diffusion of nutrients and fluid exchange [12,13]. Bone grafts also require mechanical strength and firmness; and since solid and dense structures are required to provide an increased mechanical strength, a reasonable compromise between interconnected porosity and mechanical properties must be found [12].
Current trend in bone and cartilage regeneration encompases the use of biomaterials to encourage new tissue formation and to advance healing process at the implantation area [14]. Composition of calcium phosphate cements (CPCs) is comparable to the chemical structure of bone minerals. Their ability to self-set through a low-temperature setting reaction and resorb in vivo secures a variety of potential applications in bone regeneration. CPCs are highly biocompatible (degradation products serve as a source of calcium and phosphate ions at the implant site), as well as osteoconductive and osteoinductive. Also, bioactivity, injectability, ability to form a direct bonding with bone [2,3,13,[15], [16], [17], [18]] and limited morbidity after surgical procedures [19] are properties that have drawn attention to CPCs. Also, calcium phosphate is said to lower the infection rate in distal radius fractures [20]. Another advantage is that materials containing calcium phosphate are quite easy to certify as these are already present in human body [21].
Osteoconduction is the ability to act as a scaffold, mechanically supporting ingrowth of vessels and new bone from the borders of the defect into and onto its surfaces, initiating or inducing new bone formation followed by the reabsorbtion of the mentioned scaffold [[22], [23], [24]]. Osteoinduction stimulates osteoprogenitor cells to induce migration and differentiation into bone-forming osteoblasts [24,25]. Calcium and phosphate ion release is necessary for bone mineralization – Ca2+ and PO43− ions regulate bone resorption and bone deposition [18,26]. Furthermore, keeping the bone scaffold similar to that of natural bone provides a desirable environment for cell attachment and proliferation [27]. The degradation rate of the ideal bone graft should be at the same rate as new bone forms [26], which is a crucial challenge [12]. Chemical composition, surface structure morphology, size and porosity of biomaterial can be used to control the inflammatory response and cell-material interactions that occur [18,28]. CPCs can be classified in two main groups – apatites and brushites and the final product of the reaction between powder (dry phase) and liquid phase (cement liquid or hardening liquid) determines to which of the two groups the cement belongs. Apatites can be obtained by a hydrolysis reaction (by using a single CaP component) or by an acid-base interaction (in the case of more than one CaP component). End products of apatitic CPCs are precipitated hydroxyapatite (HAP) or calcium-deficient hydroxyapatite (CDHA), whereas of brushites – dicalcium phosphate dehydrate (DCPD). The type of reaction is determined by different CaP compounds (such as tricalcium phosphate (TCP, Ca3(PO4)2 or HAP (Ca10(PO4)6(OH)2)), which contains crystal modifications α-tricalcium phosphate (α-TCP) and β-tricalcium phosphate (β-TCP, rhombohedral Ca3(PO4)2)) in the powder phase [9,29]. Different forms of calcium phosphates influence properties that modify bioactivity. Due to differences in solubility, ion release, stability, and mechanical strength calcium phosphates with different structure can be applied for different purposes as well as used together or mixed with other materials to retouch their disadvantages and to focus on their advantages [10,30]. Osteoconductivity of β-TCP has proven to have better features than osteoinductivity thereof. It has been observed that in the case of β-TCP a new bone is formed starting from the surface of β-TCP as osteoclasts resorb it and continues to the center of β-TCP [31].
An alternative to the implant materials in hardened form is injectable bone substitutes that can be employed in problematic surgical sites, which have difficult access in an open surgery scenario, and that are able to fill narrow and irregular defects [1,32]. The aim is to provide the treatment by an injection that would be minimally invasive, therefore reducing patient trauma and improving efficacy [33]. Important requirement for said injectable materials is the ability to self-set at physiological temperatures with no heat release [34].
Injectable bone cement (IBC) is a type of material that is in the form of a liquid or paste, which can be injected or moulded and solidifies taking the shape of implantation site as well as providing mechanical support and facilitating the formation of new bone within the defect. An ideal IBC for use in treatment of bone defects, particularly in load-bearing orthopaedic applications, should be easily injectable and with consistent homogeneity throughout the injection, set in appropriate time after mixing, have low risk of necrosis, adequate mechanical strength, similar stiffness to the surrounding bone, high radiopacity, bioactivity and resorption rate similar to that of tissue formation, as well as sufficient amount of microporosity and macroporosity [13,35,36].
Comparing to presently clinically available injectable bone cements CPC shows better bioresorbability rate that facilitates the new bone formation comparing to calcium sulphate cements and are much more favorable than polymethylmethacrylate (PMMA) which is not bioactive and bioresorbable at all [13].
Despite CPC formulations already being present on the market, the research focusing primarily on the improvement of mechanical properties and enhanced resorption rate continues [3]. Although CPCs showing bioactivity and ability to be injected have been approved for clinical use for low load-bearing applications [37] and are successfully used in treatment of benign bone tumors [38] such as enchondroma [[39], [40], [41]] and are said to be useable for osteoporotic patients [42], they do not possess enough tensile and shear strength to be used for high load-bearing applications, such as long bone replacement, kyphoplasty, vertebroplasty, and fixation of articulating prostheses [16,35]. Reconstruction of a large size cortical defect using a bone graft substitute is still accompanied by additional fixation, such as plates, screws, nails and wires [43]. The osteoconductivity of commercially available implants is reported to be low [44]. It also must be mentioned that costs of presently available commercial implants are much higher than costs of allograft [43,45], therefore injectable CPC with good osteoinductivity are promising approach of cost-effective bone graft substitute without necessity to add cells or growth factors, or other components [27,46].
2. Brief history of CPCs as bone substitutes
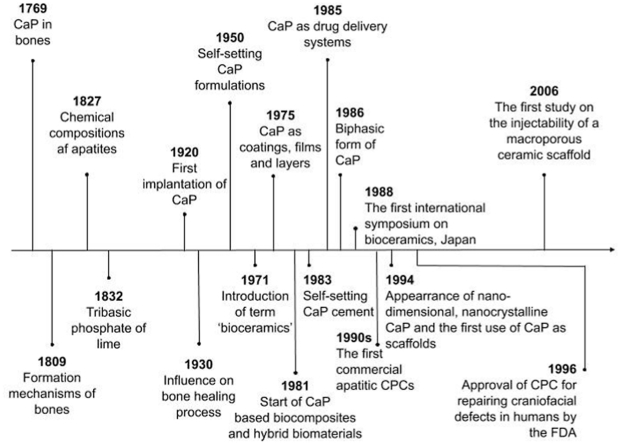
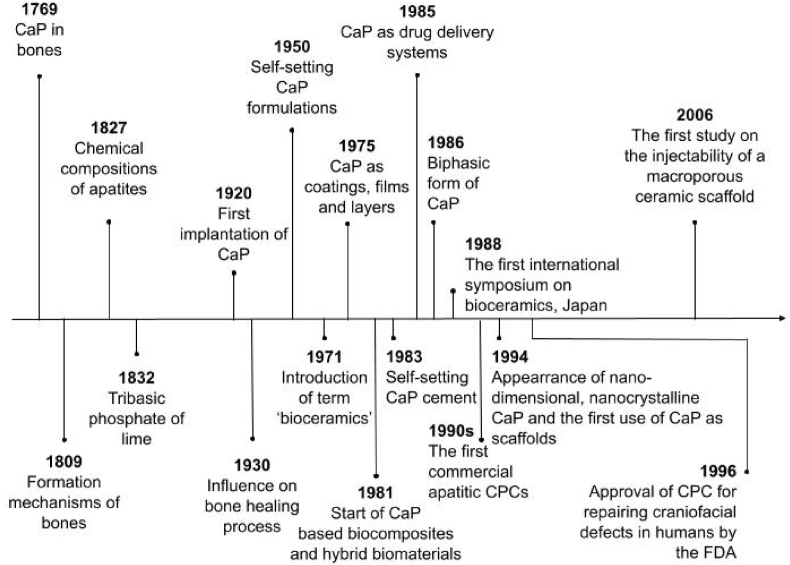
The time scale illustrated in Fig. 1 starts with the earliest available data of study by Gahn, who discovered calcium phosphate in bones in 1769. Research continued and the structure, composition, properties and formation mechanisms of bones were described in detail in the London medical dictionary in 1809. And only 18 years later Rose (German mineralogist) presented the correct view of the apatite chemical composition. In 1832 a chemical term ‘tribasic phosphate of lime’, which fully corresponds to both α-TCP and β-TCP was mentioned for the first time [47]. Compounds of calcium phosphate (CaP) have been investigated as materials for bone generation since 1920, when the first attempt to implant man made calcium orthophosphate (TCP) was performed [47,48].
Fig. 1.
Time scale of CPCs history as bone substitutes.
In 1930s scientists noticed that not all types of calcium orthophosphates have a real impact on the bone healing process [47,49].
1950 was a year when the story of calcium orthophosphate materials that are able to self-set started. The term ‘bioceramics’ showed up in the abstract of a paper for the first time in 1971. 17 years later, in 1988, Kyoto, Japan hosted the first international symposium on bioceramics [47]. CaP as layers, coatings and films were first mentioned in 1975, 6 years later CaP based biocomposites and hybrid biomaterials appeared, nanodimensional and nanocrystalline CaP started and the first paper on use of CaP as scaffolds was published in 1994 [12,47].
In the early 1980s Brown and Chow described new calcium-phosphate setting cement [50], which was a start of a broad commercialization of calcium orthophosphate (primarily, HAP) bioceramics as materials for use in dentistry and surgery [30,47,51]. CaP suitability for drug delivery was mentioned for the first time in 1985 [47]. A year later, in 1986 a mixture of HAP and β-TCP was presented as a biphasic form of calcium phosphates [52]. The commercialization of apatitic CPCs started in the 1990s, and after that brushite cements were also introduced in the market [15]. The field experienced rapid development in 1996, when the Food and Drug Administration approved CPC for repair of craniofacial defects in humans [32]. The first study on the injectability of a macroporous ceramic scaffold appeared in 2006 [1].
If the reader is interested in an extensive study of the history of CPCs, authors of this article suggest reading detailed review by Dorozhkin [47].
3. Porosity
Interconnected macro-pores within bone substitutes plays a significant morphological role of aiding successful and fast bone in-growth [11]. Reader should note that the terms micro and macroporosity used in this field differ from terms proposed by IUPAC in relation to the characterization of porous solids used in other fields such as catalysis [3]. Microporosity refers to pores smaller than 100 μm and macroporosity refers to pores larger than 100 μm in diameter [35]. “Interconnectivity” indicates to what extent pores are connected to each other. Interconnected pores provide an efficient way of fluid flow [9].
The porosity of calcium phosphate influences bioactivity. With the increase of porosity contact with body fluids increases as well, thus improving the dissolution rate. The presence of pores on the surface improves protein adsorption when pore size is between 20 and 500 μm [30,53]. The macroporosity is said to be responsible for promoting both biological growth factors in the implant as well as penetration of cells, while providing for osteogenesis process to take place on the inside surface of the pores [54,55]. It is reported that to be osteoconductive and facilitate bony ingrowth and implant fixation an ideal scaffold should incorporate macropores of 150–500 μm in diameter (the critical low pore size for new bone formation is around 100 μm) [3,56,57] and exhibit 60–80% interconnected porosity [26,32]. Pore interconnections in the range of 60–100 μm with the optimal interconnection size of 130 μm are particularly favorable for bone growth [58]. The lack of macropores limits the extensive use of CPCs [34] due to their slow rate of resorption and slow replacement by new bone mainly starting only from periphery of the defect [59].
Not only the macroporosity is important, microporosity also plays an important role as research shows that CaP ceramics without micropores were not resorbed and bone ingrowth was slower compared with ceramics that had micropores [60]. Also, it is reported that ingrowth of mineralized tissue was observed in micropores in size larger than 1–10 μm, however it must be emphasised that micropores were interconnected. It was concluded that mineralized tissue formation was driven by the coordinated action of cells within the micropores [61].
Presence of both macropores and micropores ensures larger specific surface area which is suitable for application of adsorbable substances with biological activity, such as growth factors or drugs, thus adding functionality to the CPC [53,62,63].
Contrary to the closed porosity, which only decreases the mechanical properties and does not ensure any desirable effect, interconnected porosity with its ability to settle implant to its place by increasing bone bonding and favor cell colonization and adhesion, vascularization, bone ingrowths and bioresorption is beneficial [12] and is a provision for osteoconductivity as well as promotes angiogenesis [26].
Instant porosity enables early formation of new bone [62].
Methods of creating porosity are mentioned in paragraph Porous injectable calcium phosphates of this review.
4. Mechanical strength of CPCs
The content of amorphous phase, porosity and grain size are essential criterions that determine mechanical properties. Perfect implant would not be subjected to the stress forces in the interface of implant-surrounding tissue as it would have mechanical properties that are as close as possible to the properties of said tissue [12], however mechanical properties may differ depending on the mechanical strength the bone is exposed to and age, gender and medical condition of the patient as well as clinical indication [64].
Usually, compressive strength is used as a parameter to determine mechanical properties [65], but in some cases also tensile strength is measured. These parameters vary depending on the CaP phase – apatite-forming CPCs usually have a compressive strength of up to 80 MPa, but brushite-forming CPCs have smaller values of up to 52 MPa [64]. Diverse literature sources reveal that the compressive strength of human cortical bone is in the range of 90–230 MPa and for the cancellous bone – 1.5 to 45 MPa [9,15].
Denser cements may promote adjacent vertebral fractures. This obstacle is especially noteworthy for patients suffering from osteoporosis. Although the stiffness of CPCs is quite like the stiffness of human bone (elastic modulus of trabecular bone: 0.05–0.8 GPa), it is possible that the elastic modulus of CPCs (apatite-forming CPCs: 0.4–8 GPa, brushite-forming CPCs: 2–8 GPa) is still too high [64].
Several methods are claimed to improve mechanical properties for CPCs [13,15,63]:
-
•
reduction of the porosity and pore size of the CPC matrix,
-
•
use of smaller particle size of starting materials,
-
•
use of a high aging temperature,
-
•
addition of accelerators or retarders to the powder or liquid components,
-
•
use of a dual setting cement system involving the cross-linking of polymeric monomers,
-
•
the reinforcement with fibers,
-
•
addition of polymers, such as polyacrylic acid, polyvinyl alcohol, sodium alginate, sodium polyacrylate, polyelectrolytes, polyethylene oxide,
-
•
addition of bovine serum albumin,
-
•
addition of superplasticizers,
-
•
the reduction of the liquid/powder ratio within the limits of workability.
These methods can be classified in two main groups: methods for increasing the fracture energy and methods for reducing the size and severity of defects (including pores). As explained before, pores play a significant role in the performance of bioactive ceramics, therefore improvements of mechanical properties should be directed at increasing the resistance to crack propagation [66].
Baudín et al. suggests the use of 0–3.84 vol% of functionalized graphene nanoplatelets to improve performance of CPCs. They obtained injectable bone substitute and for specimens with graphene addition of up to 1.96 vol% strengthening was observed. It was explained that improvement was achieved due to crack deflection, bridging and branching, the pull-out of the unbroken graphene platelets and interlayer sliding between the graphene sheets that form the platelets before pulling out [66].
A more detailed insight about mechanical properties of CPCs can be found in the article by Schröter et al. [64].
Many researchers and clinicians have often overlooked the condition that using CPCs specifically for bone repair can be completely sufficient although CPCs generally have a low mechanical strength [63]. Due to high strength ionic bonds CaP are brittle [12], much more brittle than cancellous bone, therefore the comparison of the compressive strength of the cementious bone substitute with that of cancellous bone is meaningless. Furthermore, test samples are usually prepared under predetermined conditions in a predetermined shape and then tested using predetermined pressure, so the resulting findings cannot be very helpful in evaluating whether the considered cement strength will be sufficient applying cyclic load in a human body [67,68].
Bending and tensile tests are clinically more relevant; however, they are usually rarely used due to technical limitations. Paknahad et al. developed an experimental protocol to perform the three-point bending and tensile test for a CPC matrix. Using their protocol, it is possible to predict the bending and tensile responses of the CPC matrix [69].
Nevertheless, mechanical properties of the paste, later cement can change a lot of times after the injection [67,68]. Moreover, Schmidt et al. reported that repair process of rat calvaria bone was not interfered by low mechanical strength of the CPC implant [70].
Furthermore, attempts to improve the mechanical strength of the CPCs affect negatively other important properties such as injectability [68], therefore, in order to avoid necessity of the mechanical strength improvement, an alternative approach is to develop rapidly resorbable bone substitute that can be relatively quickly replaced by new mature bone [21,67].
5. Injectability
Even though there already are a lot of known injectable CaP products in the market, injectability could still be improved. For example, it is advertised that 4.5 ml Norian SRS® branded cement paste which is sold as a mixture to be mixed before the operation (monocalcium phosphate monohydrate, α-TCP and CaCO3 as a dry phase (cement powder) and water and Na2HPO4 as a liquid phase) is injectable ∼5 min and it hardens within ∼10 min after injection. Turns out that only ∼3 ml of the Norian SRS® are injectable, the rest is unable to extrude from the syringe [68].
Currently there is no uniform opinion among scientists about the meaning of injectability. Many authors are certain that injectability is related to the injection force that has to be applied to a syringe to deliver the cement paste or the weight percentage of the extruded material over the initial material under given conditions [35,51,63]. In 2005 Bohner and Baroud questioned the existing opinion, stating that previous definition describes the ease of injection, not injectability and published the opinion that creation of a model describing the injectability of CaP cement pastes would make the chances of developing injectable cement, which is suitable for medicinal application, much higher [71]. Ease of injection is strongly dependent on the injection system (e.g. type of syringe, needle size, injection speed) [13,63], for example, a good injectability is favoured by shorter cannulas with a larger diameter, as well as slower injection rates [13,71]. Known definition does not take into account the quality of the extruded paste in which phase separation (also called filter-pressing) may happen, probably causing a deviation of the actual composition of the extruded paste from the initial one, creating threat to clinical acceptability of the set paste. As a result Bohner et al. put forward a concept of the injectability of paste as being able to retain homogeneity throughout the process of injection that is not dependent on the force applied during the injection [13,63].
The main interest why injectable materials have gained attention is their ability of filling inaccessible bone defects with an irregular shape due to viscosity of the material. It is only acceptable for viscosity to change if it is still possible for surgeon to inject the material and after the injection the material preserves its form against the influence of body fluids [72,73]. The use of injectable materials may also allow to reach better clinical results and reduce surgery time, which would be beneficial not only for patient, but also for surgeons who will be no longer required to perform long and complex surgeries [73].
According to the definition by Bohner et al. an essential factor to be improved to obtain injectable material is phase separation. As a paste is biphasic mixture consisting of fine powder or granules and a liquid, it could be subjected to a phase separation when submitted to a pressure gradient (the pressure to be applied to the syringe for the liquid to be filtered through the cement particles is lower than the pressure that is necessary to inject the paste), resulting in local changes of the paste composition, namely, as a pressure is higher closer to the plunger of a syringe a liquid flows in the direction of the syringe tip, changing the consistence of paste – a wet powder stays on the top of the syringe, but close to the tip an excessively liquid paste is created. As mentioned process occurs dynamically, the amount of the wet powder increases resulting in a plugging of the syringe [67]. In a phase separation scenario the liquid content in the extrudate is higher than desirable, which may contribute to discharge of the defect zone as well as to negative effect on the properties of final CPC [51].
Researchers suggest several approaches for phase separation prevention: use of smaller and rounder particles that allows to add lower volume of liquid phase, use of additives and adjustment of plastic limit [32,67] as well as refinement of the L/P ratio of the paste [74]. As it turns out plastic limit measurements could be used to determine the injectability of CaP. Furthermore, these measurements are easier to conduct than measurements of injectability [71].
The injectability adjusting liquid/powder ratio is a factor that affects also the initial plasticity of the paste and consequently its setting times [15]. High L/P ratios may cause a prolonged setting time [32,51].
There have been sugestions to replace the liquid phase with glycerol to improve the injectability [75], but Bohner and Baroud suggests hydrogel for this goal, stating that it appears as the most adequate and applicable way, as CaP cements must be self-setting in an aqueous environment [71]. The use of viscous binders like salts of alginic acid, sodium hyaluronate, hydroxypropyl methylcellulose (HPMC), methylcellulose (MC) and ethylene oxide/propylene oxide block copolymers such as Pluronic has also been suggested to improve injectability [51]. Burguera et al. reported that the incorporation of a HPMC efficaciously improved the injectability of the CPC paste [76]. Replacement of the liquid phase with a polymeric solution of chitosan and polyethylene is reported to give an easily injectable pasty-like consistency material [77]. Furthermore, using adjuvants, transformation of the cement into an injectable paste is possible without fundamental influence on the chemical reactions during setting and hardening [32].
Kim et al. suggests improving the injectability of β-TCP cement paste by the mechano-chemical modification using ball-milling. Spherical particles were obtained, and particle size distribution was broadened, and these two factors are thought as aiding the improvement of injectability [78].
Ideal mixing procedure of CPC paste does not leave air bubbles by the paste. The procedure is significant due to the necessary transfer from mixing vessel to a syringe before the injection [68]. Mixing affects the setting [79] and complicated or non-reproducible mixing procedures limit the use of injectable CaP in surgery [34,55].
6. Setting reaction and time
Another important feature of CPCs is cement setting reaction that not only controls cement setting properties, including setting time, but also determines the largest number of the physical and biological properties of the hardened cement [9,80].
For the most part throughout the setting reaction chemical process two things occur. One is the dissolution, which involves release of calcium and phosphate ions that create supersaturation within the solution, the other is precipitation where the nucleation of the new phase takes place, which begins when concentration in the solution reaches critical value. Cement hardening occurs due to the association of the precipitated crystals. Furthermore, dissolution and the growth of the new phase can transpire simultaneously. Calcium phosphate phases are with divergent stability and since the less stable phase will dissolve to develop a more stable phase it will alter the composition of the precipitated phase. As a result of the CPC setting reaction at pH that is higher than 4,2 the most stable of the calcium phosphate phases is apatite, but at pH that is lower than 4,2 – brushite [63,64,81]. A basic concept of these two phases is clarified in a review article by Ginebra et al., cited here as a reference [81].
The setting of cement is the result of one of three different reactions, which can also be combined: reaction among different CaP compounds in an aqueous environment (mainly dissolution/precipitation reaction, which can be accompanied by possible hydrolysis of CaP compounds and formation of many possible, unstable intermediate products – CaP formulations, which dissolve to form more stable CaP phase, as well as nucleation and growing of the new phase together with ongoing dissolution); reaction between a carboxylic acid and calcium compounds in the case of mixture of the powder phase with a liquid containing a carboxylic acid; reaction between CaP compounds and aqueous solutions containing polymers [9,63,81].
Investigation of the setting of anhydrous dicalcium phosphate cement showed that hardening is a complex process: formation of a set cement starts with a smooth, homogenous, compact structure; afterwards it gradually changes to a smooth, homogenous structure which coexists with porous, stack-plate crystals agglomerated surface; then turns into porous structure and pores continue to grow [82].
Setting time is very important handling property of cements. It directly affects the clinical procedure [13,67]. If the setting time is too long cement pastes may disintegrate when coming into contact with physiological fluids or if bleeding occurs due to the failure to achieve complete hemostasis [83]. Setting time is defined as the time required for the CPC to become strong enough to resist an applied force [13,67].
The initial setting time, tI (when paste deforms only with structure damage and static pressure of 0.3 MPa is maximum the setting cement can support) and the final setting time, tF (when 5 MPa static pressure can be supported and no scratches appears touching the cement) can be determined by Gillmore needle method. tI is an important parameter as during surgical operation the CaP paste must be prepared and delivered to the surgical site before reaching tI, which is advised to be in a range of 3–8 min depending on the type of the procedure. tF should not exceed 15 min [51,84].
The other standardized method to measure the setting time of CPC is Vicat needle method that involves a penetration of a standard needle into a cement paste. According to the Standard Method NM 65:2003 (equivalent to ASTM C191-13), the initial time of setting occurs when the needle stops at 1 mm from the bottom and the final seting time is determined when the needle stops at 38 mm (2 mm from the surface) [85].
More common method used to measure the setting time is setting time measurement with Vicat apparatus according to ISO standard 1566. “Dental zinc phosphate cement”. The cement is considered set when the Vicat needle (weighing 400 g, 50 mm in length, and with tip diameter of 1 mm) no longer leaves a visible circular print on the surface of the paste [86].
Before trying to adjust setting times for injectable CPCs the most important aspect to be considered is phase separation – whether it occurs and, if occurs, at what degree it occurs. In the case of injectable CPCs the delivery process is the one that is affected the most by the setting reaction. This influence could be decreased by developing premixed CPC that sets only after injection into the body [51].
Setting time can be modified changing the particle size of the reagents, adjusting the liquid to powder ratio (L/P), adding a nucleating agent or growth/nucleation inhibitor, or dissolving adequate additives (accelerators or retarders) into the mixing solution, adjusting solubility of reagent, adding ions [67,87]. Bohner suggests that use of fine and monodispersed reagent powders and thought-out selection of additives, especially those that are added to mixing liquid, would aid in better control of CPC setting rate [87].
CaP enrichment with ions, even in small amounts, have a significant impact on the setting time of CPC [88]. For example, β-TCP dissolution is inhibited by zinc and magnesium ions. Less soluble reagents are connected with longer setting and reaction time [87].
Arkin et al. doped magnesium and strontium with synthesized semiamorphous and crystalline HAP. To develop this cement the powder mixture was mixed with Na2HPO4, NaH2PO4, and a carboxymethyl cellulose (CMC) solution. They produced a CPC paste with good injectability and fast setting time – the conversion from paste to solid phase took about 3–4 min after initial setting time. The final product was different phases of HAP. Arkin et al. explain that the fast setting was attained due to response of acid−base as Mg2+ and Sr2+ ions dissolve gradually in the acidic solution and the released cations react with the phosphate anions to give acid−base reactions [83].
Uskoković et al. investigated iron-doped cements undergoing TCP→brushite transformation during hardening, the setting time point was detected at 0.31 ± 0.03 h, the hardening time point – at 2 h, and cement was completely stabilized after 50 h [89].
Optimal self-hardening properties of a cement were obtained by doping whitlockite (β-TCP) with copper ions. During the first minutes of hardening TCP was transformed to brushite and due to the partial replacement of Ca with Cu, formation of apatite as second phase was inhibited [90].
To have a more comprehensive understanding of the processes involved during the various stages of the CPC setting reaction as well as read about the strategies used to monitor and modify CPCs setting rate authors suggest reading the feature article by Bohner under reference [87].
Alteration of setting properties should be viewed in a complex study together with other properties like cohesion, biodegradability and injectability as modification of the first can modify the latter as well [91].
7. Cohesion
Once injected the CaP paste comes in contact with blood or other physiological fluids, therefore it becomes unclear whether the properties of the extruded part of the paste are still clinically acceptable. Thus, a good cohesion is an essential factor of the paste [68].
Cohesion which is otherwise named as non-decay ability, anti-washout, compliance, swelling or stability [15] is the ability of the paste to keep its geometrical integrity and harden in an aqueous solution [63,67]. Consequently, in the context of CaP paste cohesion could be understood as a struggle between forces acting on the particles and forces acting between the paste and the surrounding fluid [63].
A poor cohesion has been linked to a poor biocompatibility that may result in inflammatory reactions [92] due to microparticles being released, as well as lead to setting being prevented [67,84]. The methods used to improve injectability may compromise cohesion [93] and poor cohesion may prevent clinical use of the implant [94].
Conversely, good cohesion is achieved when particles have strong attractive forces among them. Consequently, boosting van der Waals forces (attractive) and diminishing electrostatic forces (repulsive) can be used to improve cohesion. The approach used for this purpose includes a decrease of mean particle size and L/P ratio, and an increase of ionic strength of the mixing solution. Alternatively, good cohesion may be aided using hydrogels i.e., applying them to the mixing liquid to increase viscosity [67,95], or raising viscosity by incorporating water soluble polymers into the CPC paste [9]. Also cohesion promoters, such as sodium alginate, cellulose derivatives, or chitosan derivatives, may be added [84]. However, the use of polymers and hydrogels can prolong the setting time and decrease mechanical properties of CPCs. Zhong et al. suggests the use of oxidized sodium alginate-citric acid (OSA-CA) to build covalent bonds between CPC and bone tissue, which is reported to lead to good tissue adhesion for CPC [94].
8. Osteoinduction, new bone formation and vascularization
With the term osteoinduction one describes a process of chemical stimulation induced conversion of mesenchymal stem cells into osteoblasts [26,96]. Osteoblasts are the main functional cells of bone formation and are involved in the synthesis, secretion, and mineralization of bone matrix [97] and osteoinduction is responsible for causing osteogenesis [26].
Not all material types are osteoinductive. Osteoinductivity of CPCs depends on material properties, both chemical (stoichiometry, solubility) and topographical (microporosity, roughness) properties. Also, the cell type and the presence of osteogenic supplements have an influence on osteoinductivity. Literature demonstrates osteoinductivity in HAP, TCP, blends thereof in form of BCP, carbonated apatite (CA) and octocalcium phosphate (OCP) [21], but there are differences in the osteoinductivities across CaP types: TCP > BCP ∼ HAP > ACP. Some studies reported that calcium phosphates are osteoinductive even in the absence of supplements [53].
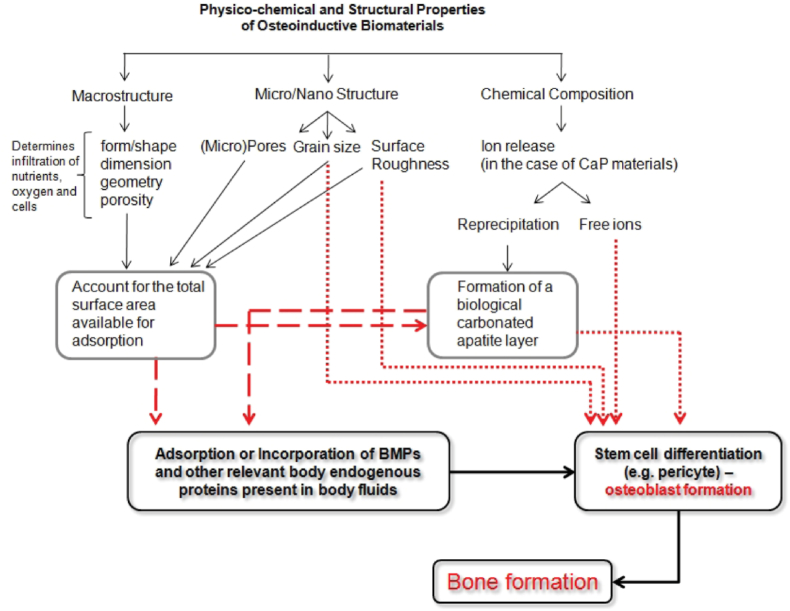
The exact mechanism of osteoinduction by biomaterials is not entirely understood. Review article by Habraken et al. have compiled some possible mechanisms in their review article under reference number [21]. One of the proposed theories is that release of calcium ions during the in vivo degradation/resorption of CPCs promotes osteoinduction and new bone formation. Notwithstanding, for bone to start to form a relatively stable surface is necessary, therefore a compromise between the level of dissolution/reprecipitation process on the material surface and the rate of degradation must be found [54]. Schematic summary of the hypothesised mechanism is reproduced in Fig. 2.
Fig. 2.
Schematic summary of the hypothesised mechanism of osteoinductive biomaterials proposed by Barradas et al. Reproduced from Ref. [54] with permission.
Barradas et al. concluded that macrostructure of the CPC should promote sufficient supply of nutrients, oxygen and infiltration of cells and tissue. Not only macropores are able to facilitate the material replacement by the newly formed bone, but also the presence of other “protective” areas, such as concavities or channels, or spaces between individual particles are beneficial and helps to ensure that bone formation is not disturbed by high body fluid refreshments or mechanical forces due to implant movement [54].
Porosity and pore sizes also impact vascularization [98] and by changing these properties the degree of the immunological reaction of the host tissue can be regulated. It has an influence on the formation of multinucleated giant cells, which have an impact on degradation too [99].
Variations in vascularization behaviour can be obtained by changing the size of channels. Small vessels were formed in channels with a diameter of 250 μm by the expression of the representative angiogenic factors HIF1α, PLGF and migration factor CXCR4 and large vessels were developed in channels with a diameter of 500 μm by the expression of vascular endothelial growth factor (VEGF) [100].
It is intended that due to its bioresorbability the pore size and porosity of CPC will increase over time in vivo [1].
In 2019 Bohner and Miron published a review, proposing mechanism for intrinsic osteoinduction of materials. It includes conclusions that contradicts with previous views: the mechanism of intrinsic osteoinduction is often related to the release of calcium and phosphate ions, but current review claims, that it is not caused by an accumulation of but by a reduction in the local calcium and/or phosphate ion concentration. This is possible if the local consumption of calcium and phosphate ions due to the precipitation of carbonated apatite (dahlite) is larger than the supply of these ions by diffusion and convection processes (blood supply). In summary, Bohner and Miron suggest that material is osteoinductive if: 1) it mineralizes in vivo (a “bioactive” apatite layer forms on the material); 2) it is porous; 3) the pores are large enough to allow blood vessel ingrowth and cell transport into the core of the material; 4) blood supply is insufficient to keep physiological calcium and/or phosphate ion concentration [101].
As the biomimetic apatite layer formation as well as the growth of blood vessels into the CPC (usually with a rate of few hundred micrometers per day) and providing of bone forming cells takes time intrinsic osteoinduction is a very slow process. The formation of bone may take a time from few weeks to one year [101].
The conclusion of work by Bohner and Miron was supported by Maazouz et al., who confirmed that mineralization could be the trigger of osteoinduction by testing the hypothesis by Ref. [102], that a quantitative approach of the mineralization measurement can be used to predict the osteoinduction early in the development process of bone graft substitute. Maazouz et al. studied ectopic implantations of bone graft substitutes, submitting them to experiments proposed in Ref. [102] and comparing their in vivo osteoinductive potential with their in vitro predicted potential [103].
9. Biodegradability
With the formation rate of new bone balanced degradation rate is the most significant property of bone substitute materials intended for use in the clinic [62,68]. If an implant degradates too quickly or too slowly regeneration of bone is slowed down [64].
Biodegradability is one of the main issues regarding CPCs since due to lack of macropores CPCs degradates slowly and biodegradation starts from the outer surface to the central part [9].
Degradation of CPC can occur by two differing routes: active and passive resorption. Active resorption is basically a phagocytosis or an intervention of osteoclasts and macrophages, when pH is locally dropped down at values at which CPC becomes soluble [12,104]. Passive resorption includes dissolution or chemical hydrolysis of CPC [12,62].
Brushite cements due to brushite solubility in body fluids are mainly resorbed by passive mechanism [105], while apatite cements due to HAP limited solubility at physiological pH are mostly resorbed by the active mechanism and this contributes also to the quite slow resorption rate in vivo of these materials [64,104,106]. At pH 7.3 the diminishing pattern of TTCP > α-TCP > β-TCP > OHA > CDHA > HAP applies to the dissolution rates of monophasic CaP [12]. According to reported dissolution rates HAP will remain in human body for a long time after implantation. Contrary to HAP, TCP degradates faster and can be considered a successful replacement for bone tissues [29].
The phase ratios determine the biodegradation kinetics when considering biphasic, triphasic and multiphasic CaP. Moreover, introduction of doping ions can alter solubility and therefore biodegradability. For example, CO32− increases the biodegradability of CDHAP and HAP, by increasing apatite lattice disorder favoring crystal dissolution [12,107], but Mg2+ or Zn2+ ions lower the biodegradability of β-TCP [12]. As mentioned before, porosity also modulates CPC resorption [81]. When pores are distributed homogeneously and the apatite network between the granules is of lower density in vivo degradation occurs faster [108].
Furthermore, external factors such as site of implantation, blood supply or mechanical loads; as well as defect site unrelated factors such as gender and age of a patient, their metabolism rate, health conditions and social habits, can have an impact on the resorbtion [81].
10. Additives
Many academic sources report different additives used to improve the properties of CPCs. Authors of this review article have compiled some of the more frequently mentioned additives together with some new developments in Table 1 below.
Table 1.
Frequently mentioned additives.
| Additive | Effect | Reference |
|---|---|---|
| Citric acid | Fluidificant. Reduced mixing time, decreased compressive strength during initial setting, increased compressive strength in final stages of the setting; retardation of the dissolution precipitation reactions in cements; decreased setting time; improved injectability, decreased porosity | [15,51,68,109] |
| Strontium | Improved strength, promotion of the bone formation, prevention of bone resorption, enhanced proliferation of human-bone-marrow-derived mesenchymal stem cells, enhanced mechanical properties | [83,[110], [111], [112], [113], [114], [115], [116]] |
| Silicon | Formation of bone and cartilage systems (the degree of Si doping is limited to a maximum of about 2 wt% for HAP, in order to prevent the formation of other calcium silicate phases), faster resorption rate and favorable pH | [117] |
| Silicon and sodium | Improved degradation and bone regeneration ability, and favorable pH | [11] |
| Zirconia | Improved strength of the cement compact | [78] |
| Mannitol | Interconnected macroporous structure created | [118] |
| Glycerol | Increased setting time, setting upon contact with body fluids, decreased injection pressure, no disintegration was observed | [119] |
| Glucose | Porogen. Macroporosity with interconnected pores generated. Pores generated instantly upon cement setting. Porosity and pore sizes adjustable by changing weight percent and particle size of glucose. | [120,121] |
| Polivinylalcohol | Increased setting time, improved stability of the cement paste during storage, improved cohesion properties and injectability | [106] |
| Poly(ethylene glycol) (PEG) | Porogen. Pore sizes determined by the size of PEG | [56] |
| Poly(D,L-lactic-co-glycolic acid) | Porogen. Delayed porosity with homogeneously dispersed pores created | [62,122] |
| Hyaluronic acid | Hydroexpansivity caused and improved mechanical stability, regulation of cellular migration and inflammatory processes during wound repair secondary to bone tissue damage, increased rate of bone ingrowth to the defect site, ability to modulate the inflammatory response, osteoconductivity | [28] |
| Collagen | Improved bone regeneration, biocompatibility and osseointegration, controlled adhesion of osteoblasts, promoted osteoblastic differentiation of bone marrow cells, cushioning effect, improved drug delivery capacity – decreased initial release, improved mechanical properties | [123,124] |
| Cellulose | Strengthening of the border of the bone defect cavity, delayed resorption of the implant, promotion of longer implant lifetime | [28] |
| Hydroxypropyl methylcellulose | Enhanced filling of bone defects | [28] |
| Carboxymethyl cellulose | Slowed down initial degradation process, improved cohesion and injectability of CPC, decreased early degradation and hence bone formation | [62,125] |
| Silanized hydroxypropyl methylcelluose | Foaming agent. Shortened initial and final setting time, improved quality of bone healing. | [17,126] |
| Gelatin | Interconnected porosity, good cohesion, injectability | [122,127,128] |
| Growth factors | Bone morphogenetic proteins (BMPs) stimulate osteogenesis and promotes vascularization, transforming growth factor-β regulates cell growth, differentiation and immune function, platelet derived growth factor promotes cellular proliferation and VEGF induces angiogenesis | [18,26,124,129] |
| Xanthan gum | Increased viscosity and imparted elasticity to a cement paste, facilitating injection and delivery | [33] |
| Chitosan or chitooligosaccharide | Improved bone regeneration, antibacterial activity, improved strength of CPC, cement elasticity | [93,[130], [131], [132], [133]] |
| Sodium alginate | Improved cohesion behavior, enabled injection directly after preparation, no delay in the setting reaction in vitro | [3] |
| Chitosan-alginate complex | Controlled setting reaction, better injectability, biocompatibility and wash out resistance, enhanced bone growth around interface of defect site | [134] |
| Alginate – hyaluronic acid microbeads | Improved injectability, washout resistance, and bone formation | [135] |
| Starch together with BaSO4 | Reinforcement of CPC | [136,137] |
| Chondroitin sulphate | Enhanced injectability and improved anti-washout property, no evident effect on the phase, morphology, apparent porosity and compressive strength of hydrated cement products | [138] |
| Nanomaterials | Improved mechanical properties (tensile, compressive and shear strengths and fracture toughness) and osteoinduction abilities of CPCs, improved injectability, enhanced stem cell performance and bone regeneration efficacy, enhanced surface energy and protein adsorption and influenced integrin binding | [35,139,140] |
| Copper ions | Improved formation of new bone and blood vessels, improved bioactivity, antibacterial properties, accelerated angiogenesis | [90,124,141,142] |
| Magnesium | Improved injectability, adhesion of bone marrow mesenchymal stem cells and osteogenic differentiation via an integrin-mediated mechanism, improved biodegradability and promotion of anti-inflammatory immunomodulation | [124,143] |
| Manganese ions | Improved osteogenesis, increased compressive strength | [29,144] |
| Silver ions | Excellent antibacterial properties, increased compressive strength | [145,146] |
| Ovine whole blood | Liquid phase. High cohesiveness and injectability, promoted bone formation, delayed setting time | [147] |
| Basalt fibres | Reinforcement phase. Improved toughness of CPC | [148] |
To further develop this topic readers are advised to read an extensive review on functionalized calcium orthophosphates by Dorozhkin [149].
Some authors even suggest the use of more than one additive, obtaining a hybrid material with improved injectability, anti-washout and self-setting properties [150]. It is clear that addition of different additives can aid calcium phosphate functionality, however it should be noted that additives may have an effect on other aspects of implant properties. For example, the use of growth factors should be carefully asessed due to their protein nature, high molecular weight and spatial arrangement. Furthermore, CaP, especially in nanosize, is reported to being able to promote osteogenesis without the additives [18].
11. Recent developments in patents
Scientific publications are not the only source, where to look for new developments in a particular field of interest. Recent patents with grant date or patent applications with earliest priority date starting from 2017 were chosen, searching in Espacenet by keywords ‘injectable calcium phosphate’, ‘injectable osteoinductive porous calcium phosphate’ and ‘porous calcium phosphate’. Search results were evaluated, and most relevant documents are reviewed in this paragraph.
Since 2019 a significant drop in the count of filed patents was observed, but most probably this is due to the worldwide Covid-19 crisis.
The vast majority of the relevant patent documents are Chinese patent applications (appl.). Especially active in the field of biomaterials is SHANGHAI NATIONAL ENGINEERING RESEARCH CENTER FOR NANOTECHNOLOGY CO., LTD. The company files applications mostly for preparation methods of injectable calcium phosphate bone cements that are modified with different additives [[151], [152], [153], [154], [155], [156], [157]]. Another noticeable player in this field is GUANGZHOU RAINHOME PHARM & TECH CO LTD, who has filed patent applications on rapidly-degradable injectable type bone cement [158], injectable bone cement capable of promoting growth [159] and injectable porous calcium phosphate cement [160]. With the latter rather trying to get protection on preparation method of such cement as well as product directly obtained by this method.
Most popular CaP compound employed in the reviewed patent documents is α-TCP [[144], [145], [146], [147], [148], [149], [150], [151],151,152,[152], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170]], although there are some documents that use β-TCP as well [153,159,[162], [163], [164], [165], [166],172]; in some documents ACP also appears [160,166,169,173]. Tendency to use more than one CaP form can also be observed [153,[158], [159], [160],[162], [163], [164], [165], [166],169,170,172,174]. Pure CaP forms are never used in the newest patent literature; they are, for example, modified with PEG cladding photosensitizer IR780 [152], gelatin [153,154,157,161], chitosan [153,155,159,173], HPMC [153,170], MC carboxymethylcellulose [171], zirconium phosphate [157], chondroitin sulphate [175], lanthanides [153], strontium [158,169], growth factors [159,176], pore generating proteins [177], or mixed with other biocompatible salts and compounds, such as polyanhydride [151], calcium sulphate [158,164,167,168,178], sodium phosphate [162], ammonium salt [163], and bioactive glass [158,168,171], hydrogel [179] and fibers [172]. In some cases, citric acid is used as liquid phase [153,162,169].
Reading patent application documents, it should be noted that everything that is claimed in the application may not be absolutely new. For example, the first independent claim of the Chinese patent application reads as “An injectable calcium phosphate ceramic for promoting tendon healing, characterized in that the material is composed of calcium phosphate ceramics and natural polymers or derivatives thereof; preferably, the natural polymers or derivatives thereof, and the natural polymers or the derivatives thereof are collagens and collagen modified products.” [180]. The claim is very broad and most probably will not be accepted in current wording. Similar situation is with European patent application No. 17722590.1. The first claim of the original set of claims reads as: “An injectable biomaterial comprising: (a) a solid component; and (b) a liquid component comprising a carbohydrate; wherein the injectable biomaterial sets and cures to form an apatitic crystal structure after mixing of the solid component and the liquid component” [181]. Such broad claim may not be accepted in the field that already has quite extensive prior art, which is why amended set of claims has already been filed for this application and examination is still ongoing.
Moreover, descriptions and abstracts and even claims, in the case of patent application documents not yet examined by patent examiners, where it is announced that the respective invention has such relative terms as ‘good injectability’ [[152], [153], [154], [155],160,161,163,168], ‘excellent antibacterial properties’ [155,159], excellent osteoconductivity [179], excellent mechanical properties [163,167], should be looked at with caution, especially in the cases when claims cover a wide range of CaP forms like in European patent No. 3254710 [182] or international patent application CN2019130318W [166]. Although patent documents usually contain specific examples of invention that also cover tests on injectability, mechanical strenght, setting time, porosity and other properties, it is highly questionable, whether with a change of CaP form within the scope of invention the same property would be achieved. Nevertheless, some patent documents have included pore size [165,183], porosity [183], compressive strength [174,181] and injectable time [174,181] in patent claims, which is the most important part of the patent document, determining the scope of protection.
Recent patents and their main benefits that are disclosed and applications for which the patent protection is desired are compiled in Table 2.
Table 2.
Recent patents and patent applications in the field of injectable, osteoinductive CPCs.
| Title | Benefit | Status | Ref |
|---|---|---|---|
| Preparation method of injectable calcium phosphate cement modified with PEG cladding photosensitizer IR780, product and application | Good injectability | Appl. | [152] |
| Preparation method of polyanhydride modified controllable biodegradable calcium phosphate bone cement as well as product and application thereof | Controllable biodegradability | Appl. | [151] |
| Preparation method of lanthanide-doped injectable calcium phosphate bone cement as well as product and application of bone cement | Good injectability, possibility to use as a bone cement with fluorescent label | Appl. | [153] |
| Preparation method of rapid degradation controlled drug release injectable bone cement and product and application of rapid degradation controlled drug release injectable bone cement | Good injectability and prevented brittleness | Appl. | [154] |
| Chitosan quaternary ammonium salt-modified antibacterial calcium phosphate bone cement and preparation method and use thereof | Good injectability, suitable curing time and excellent antibacterial performance | Appl. | [155] |
| Preparation method of injectable bone cement modified by medicine carrying organic zirconium phosphate, and product and application thereof | Improved injectability and the collapse resistance of the bone cement, slow release of drugs | Appl. | [156] |
| Preparation method of nano-zinc oxide modified antibacterial injectable calcium phosphate bone cement as well as products and application thereof | Good injection properties, proper curing time, and excellent antibacterial properties | Patent | [157] |
| Rapidly-degradable injectable type bone cement and application thereof | Porous structure, promoted bone cement degradation and the ingrowth of bone cells, shortened setting time, improved compressive strength, promoted cell proliferation | Appl. | [158] |
| Injectable porous calcium phosphate cement and preparation method thereof | Good injectability, presence of macropores and micropores, appropriate setting time, good biocompatibility, simple preparation method, easy operation | Appl. | [160] |
| Injectable bone cement capable of promoting growth and preparation method of injectable bone cement | Enhanced mechanical performance, good antibacterial performance, promoted regeneration of bone cell tissues | Appl. | [159] |
| Method of making injectable cements | Excellent osteoconductivity and osseointegration, strong bonding to drug molecules | Patent | [179] |
| Resorbable radioopaque cross-calcium phosphate cement for bone plastics | Simplified composition of the radiopaque injected calcium-phosphate cement, optimal flow and radiopacity parameters without the introduction of special improvement additives, increased safety of use | Patent | [184] |
| Solidifying liquid capable of regulating injectability of calcium phosphate bone cement and preparation method and application of solidifying liquid | Simple, rapid and efficient preparation method, the prepared bone cement has strong cohesive force before solidification, the liquid and solid are not separated in the injection process, and the injectability and compressive strength are both good | Patent | [162] |
| Porous brushite-based injectable bone filler for application in vertebroplasty and preparation method of the bone filler | Injectability sufficiently high enough that cement slurry can be injected through a needle having a diameter of 10–15 gauge, a high level of reabsorption | Patent | [177] |
| Injectable continuous antibacterial anti-inflammation bone cement and preparation method thereof | Slow release effect on anti-inflammatory drugs, continuous long-acting antibacterial and anti-inflammation action | Patent | [178] |
| Injection type degradable high-mineralization-activity composite bone cement and preparation method thereof | Improved collapse resistance, excellent compressive strength and injectability | Appl. | [173] |
| Brushite bone cement as well as preparation method and application thereof | Excellent mechanical properties, controllable curing time (can be adjusted to be between 10 min and 1 min), good injectability | Appl. | [163] |
| Bone cement with hyaluronic acid | Significant improvement of the physical and mechanical parameters of cement compositions, as well as other clinically relevant properties | Appl. | [164] |
| Injectable and moldable osteoinductive ceramic materials | Favorable osteoinductive properties | Patent | [165] |
| Flowable bioactive bone void filler | Osteostimulative, bioactive and flowable for injection through a syringe | Appl. | [171] |
| Self-setting calcium phosphate cement with independently adjustable initial setting time and final setting time | Wider adjustable ranges of the initial setting time and the final setting time, wider adjustable range of pH | Appl. | [166] |
| Injectable bone cement composition kit containing calcium phosphate with fiber and preparing method of the same | Compromise between curing time, injecting power, and large porosity | Patent | [172] |
| Porous injectable calcium phosphate bone cement compound | Internal pore size 10 μm–50 μm, porosity of about 60%, favorable pore connectivity, accelerated degradation of bone cement, biocompatibility and bone repair capacity, good fluidity | Appl. | [175] |
| Bone cement with induction and degradation characteristics and preparation method of bone cement with induction and degradation characteristics | Excellent compressive strength, biocompatibility, bone conductivity and bone inductivity, and degradation speed of the bone cement is equivalent to bone growth speed. The bone cement can be made into different dosage forms by control of a liquid-solid ratio | Appl. | [167] |
| Preparation method of high-strength injectable polyphase calcium phosphate-based bone cement | Excellent injectability, collapse resistance, after 7 days of solidification at 37 °C, the mechanical compression strength reaches 113.68+/7.11Mpa | Appl. | [168] |
| Injectable calcium phosphate ceramic for promoting tendon bone healing and preparation method and application thereof | Promoted tendon bone healing, early fixation | Appl. | [180] |
| Novel injection self-condensing composite artificial bone carrying rhBMP_2 micro spheres | Good bone forming osteoinductive performance, and high mechanical strength, good degradation rate | Appl. | [176] |
| Strontium ion-mediated self-curing calcium phosphate bone cement | Enhanced degradation, applicable as a drug carrier | Appl. | [169] |
| Developable and injectable calcium phosphate bone cement, and its preparation and application | Compressive strength ≥9 MPa, injectable time ≤8 min | Appl. | [174] |
| Osteoinductive bone material | Compressive strength greater than at least 1 MPa | Patent | [182] |
| Methods and compositions for the treatment of degenerate bone | Injectable biomaterial | Appl. | [181] |
| Method for producing an osteoinductive calcium phosphate and products thus obtained | Porous, osteoinductive implant material | Patent | [185] |
| Macroporous and highly resorbable apatitic calcium-phosphate cement | Injectable CPC, that may comprise antibiotics, anti-inflammatory drugs, anti-cancer drugs, drugs against osteoporosis, and growth factors | Patent | [170] |
| Osteoinductive calcium phosphates | Porous, osteoinductive implant material having interconnected porosity | Patent | [183] |
Table 2 shows that recently patents have been granted to complex compositions with more than two ingredients. Simpler composition would be desirable to reduce costs and simplify the preparation and/or mixing procedure.
The large amount of patent applications shows that the field is still developing and there is a place left for new research to be done.
12. Porous injectable calcium phosphates
Despite the years-long research and several products already reaching the market [68] degradation rate of porous injectable CPCs is still slower than the formation of new bones [120]. Calcium phosphates are highly porous materials, however, naturally they only contain micropores and micropores are too small to receive osteoid minerals and fibrous tissues [56,186]. To increase the surface area for cell attachment that is needed for new bone growth and vascularization and to accelerate both active and passive degradation it is necessary to incorporate interconnected macropores in the structure of CPC [56,120,186], furthermore, it is preferable that porosity is instantly generated to accelerate the bone formation process [62].
Some adjustments in processing parameters are known to have an impact on the porosity of CPCs. Examples of these parameters are particle size of the powder phase and the liquid to powder ratio (L/P). As particles with smaller size have larger specific surface area, the reactivity of the powder consisting of such particles will be higher. Higher reactivity is one of the factors influencing the degree of supersaturation in the cement paste. A higher supersaturation degree enhances crystal nucleation and therefore more numerous and smaller needle-like crystals are precipitated. When bigger particles are used formation of the larger plate-like crystals occurs. As microstructures differ, pore size distribution in the set cements differs as well. Using powder with smaller particles even with the same L/P ratio smaller pores will be formed [81].
Adjusting the L/P ratio also leads to variations in the porosity and the pore size distribution of the CPCs. The spaces between particles in the blend are smaller when lower L/P ratio is used. In this way a more compact structure of crystal agglomerates is generated. In contrast, when using larger L/P ratio, the augmented separation between aggregates resulting from the larger distance between original α-TCP particles occurs and the total porosity of the cements increases, and larger pores are formed [81].
The macroporosity can be generated before or after the setting of cement. One approach to achieve incorporation of macropores is to add gas-generating compounds, for example, hydrogen peroxide or sodium bicarbonate that will create gas bubbles during setting. For this approach only highly biocompatible porogens shall be used as organism may be harmed due to liberation of gas after the cement paste has been implanted [120,186]. Furthermore, despite the fact porosity is instantaneous, which is positive factor, disadvantages like the lack of control on pore size, distribution and interconnectivity exceed advantages [122].
Another approach is to use biocompatible foaming agents, such as proteins [120,186]. It has been shown by Ginebra et al. that the use of albumen in aforementioned approach has aided in the development of injectable macroporous CPCs, which maintained the open macroporosity after injection [186]. Macropores can be incorporated also by using liquid droplets that can create pores after a mass transport phenomenon and by the integration of solid particles that dissolve or degrade after setting. If the degradation rate of the pores generating agent is faster than the one of CPC, a porous scaffold will be obtained [120].
Grosfeld et al. demonstrated in vivo, that glucose microparticles (GMPs) can be used as fast-acting porogen for CPCs. Furthermore, they suggest the use of GMPs together with a porogen with later onset of forming macroporous structure to provide a gradual replacement of CPC by newly generated bone starting from the injection in the bone defect [121]. A similar approach has been reported by Lodoso-Torrecilla et al. They used polymers as pore generating agents focusing on dual porogen system combining the water-soluble polymer with hydrolytically degrading polymer [187].
Focus is turned on another problem connected with bone substitutes, namely, that to guarantee interconnected pore structure, which is necessary for successful new bone formation in the volume of the implant, large amount (at least 40%) of a porogenic agent must be added [120]. Addition of a large amount of porogen often compromises injectability of the CPC paste [63,186].
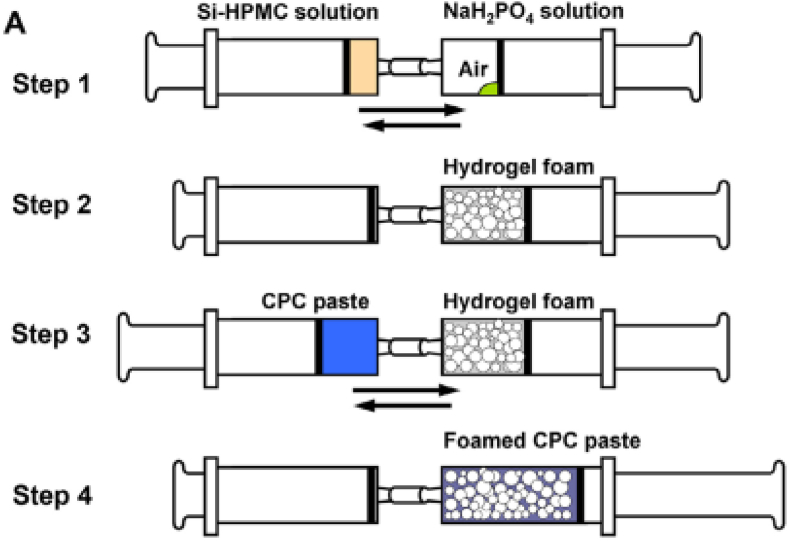
To solve the problem both with gas liberation and/or large amounts of porogenic agents it is suggested to create macroporous CPCs from cement paste that is mixed with pre-prepared foam [63,186]. Improvements in the mixing method is important not only because of already mentioned reasons, but also because porous injectable CPCs are intended for use in surgical operations that would benefit from simple preparation methods. Many macroporosity introducing methods like the use of mannitol particles, glucose, frozen ice crystals or PEG particles, are successful at laboratory, but may not work when done clinically [64]. To relieve the mixing procedure Zhang et al. established preparation method for injectable macroporous CPCs by syringe-foaming via hydrophilic viscous polymeric solution, such as silanized-hydroxypropyl methylcellulose (Si-HPMC), as a foaming agent. Connected syringes are used to foam the viscous liquid phase of CPC and subsequently to mix the resulting foam with the CPC paste. The actions involved in this process can be provided at room temperature. The porosity of the final product can be controlled by regulating the volume of air incorporated in the syringe. Scheme showing the preparing process is reproduced in Fig. 3. As a result, foamed, easily injectable CPC paste with good cohesion properties is obtained. It is also mentioned that this method could still be improved [17]. Mostafa et al. used the same method for filling surgically induced bone defect in femur bone of a rabbit and concluded that “the calcium phosphate cement for injection is biocompatible, has the biological properties of bone conduction, and can be used to fill the cavity of bone defect” [126].
Fig. 3.
(A) Scheme showing the preparation process of the Si-HPMC hydrogel foam and the foamed CPC paste. Reproduced from Ref. [17] with permission.
13. Injectable calcium phosphates as drug delivery systems
A setting reaction in CPCs is not exothermic and CPCs possess porous intrinsic porosity, therefore multiple binding sites are provided, and the incorporation of different drugs and biological molecules is possible and CPCs can be used as a vehicle for drugs [81,131,[188], [189], [190]]. The incorporation of active molecules can aid enhancement of the bone regeneration capacity of the material or targeting specific skeletal disorders or pathologies [81]. Also, such drug delivery approach is useful when systemic delivery of the relevant drug is accompanied by adverse side effects [189].
As implant presence in the human body may result in infections due to bacterial colonization [93,191], addition of antibiotics to CPCs composition is highly desirable. The antibiotic molecule is usually incorporated by adding it directly to the solid or to the liquid phase of the cement, negatively affecting mechanical and setting properties of the cements. Therefore, the amount of drug that could be added is limited [74].
Filippo et al. used gentamicin as a well known additive to enable materials to display a strong inhibition towards the growth of Gram-negative, as well as Grampositive bacteria and to enhance the viability of osteoblast-like cells. They employed spray congealed Cutina® HR (hydrogenated castor oil) microparticles to add gentamicin sulphate to the cement composition. With the protection of microparticles the setting times were not lengthened, and the compressive strength was not worsened. Furthermore, Filippo et al. reported that all the formulations displayed good injectability and cohesion, and no evidence of demixing. Injectable formulation was achieved by suitable modification of the liquid/powder ratio. Addition of BaSO4 established radiopacity [74].
Wu et al. developed an antibacterial and injectable novel CPC-chitosan paste containing penicillin-encapsulated alginate microbeads. They obtained injectable, biocompatible material with a strength matching cancellous bone and potent and lasting antibacterial activity [132].
CPCs can also be used as carriers of anti-tumor drugs or radioactive materials. Injection of such CPCs into patient would give an anti-tumor effect and simplify the treatment process, alleviating pain in patients and reducing side effects [124].
With the incorporation of drugs multi-target therapy is possible – incorporated drugs can be able to not only treat inflammation, but also inhibit bone degeneration [192]. Farbod et al. showed that sustained release drugs can also be engineered. With such instruments designing chemotherapeutically active bone substitutes, suitable for specific and personalized therapeutic needs could become possible. In their reaserch they developed chemotherapeutically active injectable CPCs containing HAP nanoparticles, loaded with platinum-bisphosphonate (Pt-BP) drugs. Farbod et al. concluded that variation of the HAP-binding affinity of the Pt-BP complexes as well as the amount of drug-loaded HAP nanoparticles can be used for adjusting the drug release kinetics [193].
For readers who are looking for a deeper insight into this topic, authors suggest consulting a review by Ginebra et al., cited here as a reference [81], the article presents different methods for drug delivery in the skeletal system and names valuable developments on this field. Another suggestable review [194] provides compilation of studies in controlled release of drugs from CPCs and factors affecting and determining drug release kinetics are revealed.
14. Conclusions
Even though CPCs have been studied already since 1986 there has remained space for improvements – research is still ongoing and new patent applications are filed and protection is granted in this field. The main directions to be improved include incorporation of the desired size of pores, injectability, osteoinductivity and handling properties, particularly mixing.
Biocompatible porogen that generates macropores of at least 100 μm should instantly be used in an amount that is not less than 40% to achieve the desirable structure of macroporous CPC with interconnected pores, which as reported enhances osteoinductivity.
An essential factor in the path to develop injectable material is phase separation. Several methods like use of smaller and rounder particles that allows to add lower volume of liquid phase, use of additives and adjustment of the L/P ratio of the paste and plastic limit have been proposed as a way to avoid the problem of phase separation.
As it is always necessary to attain compromise between porosity and mechanical properties and as negative influence on other properties when trying to improve mechanical properties of CPCs is too significant, a way to avoid the attempts to improve mechanical properties of CPCs could be osteoinductive bone substitutes that would promote rapid resorption of the cement accompanied with new bone formation with balanced rate.
Although the conclusion that CPC could be osteoinductive even without the addition of various additives may seem controversial, it is a good direction for future research and would favor the simpler composition of CPC and could potentially reduce the price of the final product and render it more available and demanded on the market.
In future, advancements such as the recipe of injectable biocompatible, osteoinductive, porous implant material, suitable for high load-bearing applications, with balanced resorbtion and new bone formation ratio, which is individually adjustable by loading different drugs or active principles and sets only after injection into human body, are highly probable since so many research groups are focusing their efforts on these problems. Simpler production methods will be developed to enhance the use of injectable porous osteoinductive CPCs as implants for treating bone fractures and diseases.
Declaration of competing interest
All authors in this study declare no conflict of interest.
Acknowledgements
This research/publication was supported by Riga Technical University's Doctoral Grant programme. The authors acknowledge financial support for granting Open Access from the European Union's Horizon 2020 research and innovation programme under the grant agreement No. 857287.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Agneta Vezenkova, Email: agneta.vezenkova@gmail.com.
Janis Locs, Email: janis.locs@rtu.lv.
References
- 1.Xu H.H.K., Weir M.D., Burguera E.F., Fraser A.M. Injectable and macroporous calcium phosphate cement scaffold. Biomaterials. 2006;27:4279–4287. doi: 10.1016/j.biomaterials.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Meng D., Dong L., Yuan Y., Jiang Q. In vitro and in vivo analysis of the biocompatibility of two novel and injectable calcium phosphate cements. Regen. Biomater. 2019;6:13–19. doi: 10.1093/rb/rby027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natalia V., Mu F., Gonz A., Planell J.A., Ginebra M. In vivo evaluation of an injectable macroporous calcium phosphate cement. J. Mater. Sci. Mater. Med. 2007;18:353–361. doi: 10.1007/s10856-006-0700-y. [DOI] [PubMed] [Google Scholar]
- 4.Valtanen R.S., Yang Y.P., Gurtner G.C., Maloney W.J., Lowenberg D.W. Synthetic and Bone tissue engineering graft substitutes: what is the future? Injury. 2021;52 doi: 10.1016/j.injury.2020.07.040. S72–S77. [DOI] [PubMed] [Google Scholar]
- 5.Marmor M.T., Matz J., McClellan R.T., Medam R., Miclau T. Use of osteobiologics for fracture management: the when, what, and how. Injury. 2021;52 doi: 10.1016/j.injury.2021.01.030. S35–S43. [DOI] [PubMed] [Google Scholar]
- 6.Bai X., Gao M., Syed S., Zhuang J., Xu X., Zhang X. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018;3:401–417. doi: 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt A.H. Autologous bone graft: is it still the gold standard? Injury. 2021;52 doi: 10.1016/j.injury.2021.01.043. S18–S22. [DOI] [PubMed] [Google Scholar]
- 8.Lin X., Chen J., Liao Y., Pathak J.L., Li H., Liu Y. Biomimetic calcium phosphate coating as a drug delivery vehicle for bone tissue engineering: a mini-review. Coatings. 2020;10:1–19. doi: 10.3390/coatings10111118. [DOI] [Google Scholar]
- 9.Lodoso-Torrecilla I., van den Beucken J.J.J.P., Jansen J.A. Calcium phosphate cements: optimization toward biodegradability. Acta Biomater. 2021;119:1–12. doi: 10.1016/j.actbio.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Miculescu M., Machedon-pisu T., Maidaniuc A., Robert C., Ioni M., Pasuk I., Stan G.E., Miculescu F. Internal and external surface features of newly developed porous ceramics with random interconnected 3D channels by a fibrous sacrificial porogen method. Appl. Surf. Sci. 2019;489:226–238. doi: 10.1016/j.apsusc.2019.05.354. [DOI] [Google Scholar]
- 11.Sarkar S.K., Lee B.Y., Padalhin R., Sarker A., Carpena N., Kim B., Paul K., Choi H.J., Bae S., Lee B.T. Brushite-based calcium phosphate cement with multichannel hydroxyapatite granule loading for improved bone regeneration. J. Biomater. Appl. 2015:1–15. doi: 10.1177/0885328215601938. 0. [DOI] [PubMed] [Google Scholar]
- 12.Canillas M., Pena P., De Aza A.H., Rodríguez M.A. Calcium phosphates for biomedical applications. Bol. La Soc. Esp. Ceram. y Vidr. 2017;56:91–112. doi: 10.1016/j.bsecv.2017.05.001. [DOI] [Google Scholar]
- 13.Hablee S., Razali N., Alqap A., Sopyan I. Recent developments on injectable calcium phosphate bone cement. Recent Pat. Mater. Sci. 2016;9:72–94. doi: 10.2174/1874464809666160802145629. [DOI] [Google Scholar]
- 14.Przekora A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019;20:434. doi: 10.3390/ijms20020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginebra M.P. Orthopaedic Bone Cements. 2008. Calcium phosphate bone cements; pp. 206–230. [DOI] [Google Scholar]
- 16.Schickert D.L., Marques S., Herber R., Kucko N.W., Van Den Beuken J.J.J.P., Zuo Y., Leeuwenburgh S.C.G. Tough and osteocompatible calcium phosphate cements reinforced with poly(vinyl alcohol) fibers. ACS Biomater. Sci. Eng. 2019;5:2491–2505. doi: 10.1021/acsbiomaterials.9b00226. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Liu W., Gauthier O., Sourice S., Pilet P., Rethore G., Khairoun K., Bouler J., Tancret F., Weiss P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair : syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016;31:326–338. doi: 10.1016/j.actbio.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 18.Levingstone T.J., Herbaj S., Dunne N.J. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomaterials. 2019;9:1570. doi: 10.3390/nano9111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angadi D.S., Edwards D., Melton J.T.K. Calcium phosphate injection of symptomatic bone marrow lesions of the knee: what is the current clinical evidence? Knee Surg. Relat. Res. 2020;32:10–17. doi: 10.1186/s43019-019-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leighton R., Trask K., Russel T.A., Bhandari M., Buckley R.F. Evidence‐Based Orthop. second ed. 2021. Calcium-based bone substitutes; p. 1. [DOI] [Google Scholar]
- 21.Habraken W., Habibovic P., Epple M., Bohner M. Calcium phosphates in biomedical applications: materials for the future? Mater. Today. 2016;19:69–87. doi: 10.1016/j.mattod.2015.10.008. [DOI] [Google Scholar]
- 22.Oryan A., Alidadi S., Moshiri A., Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014;9:1–27. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uetanabaro L., Claudino M., Mobile R., Zielak J., Garlet G., de Araujo M. Osteoconductivity of biphasic calcium phosphate ceramic improves new bone formation: a histologic, histomorphometric, gene expression, and microcomputed tomography study. Int. J. Oral Maxillofac. Implants. 2020;35:70–78. doi: 10.11607/jomi.7745. [DOI] [PubMed] [Google Scholar]
- 24.Borcherding K., Schmidmaier G., Hofmann G.O., Wildemann B. The rationale behind implant coatings to promote osteointegration, bone healing or regeneration. Injury. 2021;52 doi: 10.1016/j.injury.2020.11.050. S106–S111. [DOI] [PubMed] [Google Scholar]
- 25.Olkowski R., Kaszczewski P., Czechowska J. Cytocompatibility of the selected calcium phosphate based bone cements: comparative study in human cell culture. J. Mater. Sci. Mater. Med. 2015;26:270. doi: 10.1007/s10856-015-5589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denry I., Kuhn L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2015;32:43–53. doi: 10.1016/j.dental.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tebyanian H., Norahan M.H., Eyni H., Movahedin M., Mortazavi S.J., Karami A., Nourani M.R., Baheiraei N. Effects of collagen/β-tricalcium phosphate bone graft to regenerate bone in critically sized rabbit calvarial defects. J. Appl. Biomater. Funct. Mater. 2019;17 doi: 10.1177/2280800018820490. [DOI] [PubMed] [Google Scholar]
- 28.Ghanaati S., Barbeck M., Hilbig U., Hoffmann C., Unger R.E., Sader R.A., Peters F., Kirkpatrick C.J. An injectable bone substitute composed of beta-tricalcium phosphate granules , methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomater. 2011;7:4018–4028. doi: 10.1016/j.actbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Che J., Wang H., Ma Y., Cao F., Liu G., Shang W., Lv X., Sun T., Tong J. Effects of Mn-doping on the structure and in vitro degradation of β-tricalcium phosphate. Ceram. Int. 2021;47:22994–23000. doi: 10.1016/j.ceramint.2021.05.013. [DOI] [Google Scholar]
- 30.Jeong J., Kim J.H., Shim J.H., Hwang N.S., Heo C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019;23:1–11. doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ariizumi T., Kawashima H., Hatano H., Yamagishi T., Oike N., Sasaki T., Umezu H., Xu Y., Endo N., Ogose A. Osteoinduction and osteoconduction with porous beta-tricalcium phosphate implanted after fibular resection in humans. J. Biomaterials Nanobiotechnol. 2019;10:159–173. doi: 10.4236/jbnb.2019.103009. [DOI] [Google Scholar]
- 32.Low K.L., Tan S.H., Zein S.H.S., Roether J.A., Mouriño V., Boccaccini A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94:273–286. doi: 10.1002/jbm.b.31619. [DOI] [PubMed] [Google Scholar]
- 33.Matamoros-Veloza A., Hossain K.M.Z., Scammell B.E., Ahmed I., Hall R., Kapur N. Formulating injectable pastes of porous calcium phosphate glass microspheres for bone regeneration applications. J. Mech. Behav. Biomed. Mater. 2020;102:103489. doi: 10.1016/j.jmbbm.2019.103489. KAS 72!!!! [DOI] [PubMed] [Google Scholar]
- 34.Yousefi A. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019:1–21. doi: 10.1177/2280800019872594. October-De. [DOI] [PubMed] [Google Scholar]
- 35.No Y.J., Roohani-Esfahani S., Zreiqat H. Nanomaterials: the next step in injectable bone cements. Nanomedicine. 2014;9:1745–1764. doi: 10.2217/NNM.14.109. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H., Liang C., Wei Z., Bai Y., Bhaduri S.B., Webster T.J., Bian L., Yang L. Injectable biomaterials for translational medicine, Mater. Today Off. 2019;28:81–97. doi: 10.1016/j.mattod.2019.04.020. [DOI] [Google Scholar]
- 37.Raucci M.G., D'Amora U., Ronca A., Ambrosio L. Injectable functional biomaterials for minimally invasive surgery. Adv. Healthc. Mater. 2020;9:1–20. doi: 10.1002/adhm.202000349. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi T., Yamamoto N., Hayashi K., Takeuchi A., Kimura H., Miwa S., Igarashi K., Abe K., Taniguchi Y., Aiba H., Tsuchiya H. Calcium phosphate cement in the surgical management of benign bone tumors. Anticancer Res. 2018;38:3031–3035. doi: 10.21873/anticanres.12558. [DOI] [PubMed] [Google Scholar]
- 39.Osaka E., Kojima T., Yoshida Y., Uei H. A bent needle tip during irrigation for enchondroma of the distal phalanx: a new curettage tool. J. Int. Med. Res. 2019;48:1–6. doi: 10.1177/0300060519892367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda M., Masada K., Takeuchi E. Treatment of enchondroma of the hand with injectable calcium phosphate bone cement. J. Hand Surg. Am. 2006;31:98–102. doi: 10.1016/j.jhsa.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Al Rajeh M., Diaz J.J.H., Facca S., Matheron A.S., Gouzou S., Liverneaux P. Treatment of hand enchondroma with injectable calcium phosphate cement: a series of eight cases. Eur. J. Orthop. Surg. Traumatol. 2017;27:251–254. doi: 10.1007/s00590-016-1888-2. [DOI] [PubMed] [Google Scholar]
- 42.Heng S., Lu Z., Liu Q., Jiang T., He M., Song F., Zhao J., Zheng L. Injectable calcium phosphate ceramics prevent osteoclastic differentiation and osteoporotic bone loss: potential applications for regional osteolysis. Mater. Sci. Eng. C. 2020;110 doi: 10.1016/j.msec.2020.110691. [DOI] [PubMed] [Google Scholar]
- 43.Pearson R.G., Scammell B.E. Bone graft substitutes currently available in orthopaedic practice. Bone Jt. J. 2013;95:583–597. doi: 10.1302/0301-620X.95B5.30286. [DOI] [PubMed] [Google Scholar]
- 44.Marongiu G., Verona M., Cardoni G., Capone A. Synthetic bone substitutes and mechanical devices for the augmentation of osteoporotic proximal humeral fractures: a systematic review of clinical studies. J. Funct. Biomater. 2020;11 doi: 10.3390/jfb11020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohner M., Santoni B.L.G., Döbelin N. β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater. 2020;113:23–41. doi: 10.1016/j.actbio.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Arisan V. Biodegradation of injectable calcium phosphate bone cements: a dental perspective. Dent. Implantol. Biomater. 2016 doi: 10.5772/62789. [DOI] [Google Scholar]
- 47.Dorozhkin S.V. A detailed history of calcium orthophosphates from 1770s till 1950. Mater. Sci. Eng. C. 2013;33:3085–3110. doi: 10.1016/j.msec.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Albee F.H. Studies in bone growth. Ann. Surg. 1920;71:32–39. doi: 10.1097/00000658-192001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Key J.A. The effect of a local calcium depot on osteogenesis and healing of fractures. J. Bone Joint Surg. 1934;16:176–184. [Google Scholar]
- 50.Brown W., Chow L. A new calcium-phosphate setting cement. J. Dent. Res. 1983;62:672. [Google Scholar]
- 51.O'Neill R., McCarthy H.O., Montufar E.B., Ginebra M.P., Wilson D.I., Lennon A., Dunne N. Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater. 2017;50:1–19. doi: 10.1016/j.actbio.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Ellinger R.F., Nery E., Lynch K. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int. J. Periodontics Restor. Dent. 1986;6:22–33. [PubMed] [Google Scholar]
- 53.Samavedi S., Whittington A.R., Goldstein A.S. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 2013;9:8037–8045. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Barradas A.M.C., Yuan H., Van Blitterswijk C.A., Habibovic P., Medicine T. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur. Cell. Mater. 2011;21:407–429. doi: 10.22203/eCM.v021a31. [DOI] [PubMed] [Google Scholar]
- 55.Turczyn R., Weiss P., Lapkowski M., Daculsi G. In situ self hardening bioactive composite for bone and dental surgery. J. Biomater. Sci. Polym. Ed. 2000;11:217–223. doi: 10.1163/156856200743661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu W., Ma Q., Borg S., Öhman Mägi C., Weng X., Engqvistb H., Xia W. Cemented injectable multi-phased porous bone grafts for the treatment of femoral head necrosis. J. Mater. Chem. B. 2019;7:2997–3006. doi: 10.1039/C9TB00238C. [DOI] [Google Scholar]
- 57.Markovic M., Takagi S., Chow L.C. Formation of macropores in calcium phosphate cements through the use of mannitol crystals. Key Eng. Mater. 2001:773–776. doi: 10.4028/www.scientific.net/kem.192-195.773. 192–195. [DOI] [Google Scholar]
- 58.Flautre B., Descamps M., Delecourt C., Blary M.C. Porous HA ceramic for bone replacement : role of the pores and interconnections ± experimental study in the rabbit. J. Mater. Sci. Mater. Med. 2001;2:679–680. doi: 10.1023/a:1011256107282. [DOI] [PubMed] [Google Scholar]
- 59.Apelt D., Theiss F., El-Warrak A.O., Zlinszky K., Bettschart-Wolfisberger R., Bohner M., Matter S., Auer J.A., Von Rechenberg B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials. 2004;25:1439–1451. doi: 10.1016/j.biomaterials.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 60.Kakuta A., Tanaka T., Chazono M., Komaki H., Kitasato S., Inagaki N., Akiyama S., Marumo K. Effects of micro-porosity and local BMP-2 administration on bioresorption of β-TCP and new bone formation. Biomater. Res. 2019;23:1–9. doi: 10.1186/s40824-019-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bohner M., Baroud G., Bernstein A., Döbelin N., Galea L., Hesse B., Heuberger R., Meille S., Michel P., von Rechenberg B., Sague J., Seeherman H. Characterization and distribution of mechanically competent mineralized tissue in micropores of β-tricalcium phosphate bone substitutes. Mater. Today. 2017;20:106–115. doi: 10.1016/j.mattod.2017.02.002. [DOI] [Google Scholar]
- 62.Grosfeld E.-C., Hoekstra J.W.M., Herber R.-P., Ulrich D.J.O., Jansen John A., van den Beucken J.J.J.P. Long-term biological performance of injectable and degradable calcium phosphate cement. Biomed. Mater. 2017;12 doi: 10.1088/1748-605X/12/1/015009. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Liu W., Schnitzler V., Tancret F., Bouler J.M. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10:1035–1049. doi: 10.1016/j.actbio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Schröter L., Kaiser F., Stein S., Gbureck U., Ignatius A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020;117:1–20. doi: 10.1016/j.actbio.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 65.Ajaxon I., Persson C. Mechanical properties of brushite calcium phosphate cements. Front. Nanobiomed. Res. 2017;9:285–300. doi: 10.1142/9789813205573_0008. [DOI] [Google Scholar]
- 66.Baudín C., Benet T., Pena P. Effect of graphene on setting and mechanical behaviour of tricalcium phosphate bioactive cements. J. Mech. Behav. Biomed. Mater. 2019;89:33–47. doi: 10.1016/j.jmbbm.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cell. Mater. 2010;20:1–12. doi: 10.22203/eCM.v020a01. [DOI] [PubMed] [Google Scholar]
- 68.Dorozhkin S.V. Self-setting calcium orthophosphate formulations: cements, concretes, pastes and putties. Int. J. Mater. Chem. 2012;1:1–48. doi: 10.5923/j.ijmc.20110101.01. [DOI] [Google Scholar]
- 69.Paknahad A., Kucko N.W., Leeuwenburgh S.C.G., Sluys L.J. Experimental and numerical analysis on bending and tensile failure behavior of calcium phosphate cements. J. Mech. Behav. Biomed. Mater. 2020;103:103565. doi: 10.1016/j.jmbbm.2019.103565. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt L.E., Hadad H., De Vasconcelos I.R., Colombo L.T., Da Silva R.C., Santos A.F.P., Cervantes L.C.C., Poli P.P., Signorino F., Maiorana C., De Carvalho P.S.P., Souza F.Á. Critical defect healing assessment in rat calvaria filled with injectable calcium phosphate cement. J. Funct. Biomater. 2019;10 doi: 10.3390/jfb10020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bohner M., Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Karfarma M., Hossein M. Poly(propylene fumarate)/magnesium calcium phosphate injectable bone composite: effect of filler size and its weight fraction on mechanical properties. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2019:1–10. doi: 10.1177/0954411919877277. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz J., Barbeck M., Kirkpatrick C.J., Sader R., Lerner H., Ghanaati S. Injectable bone substitute material on the basis of β-TCP and hyaluronan achieves complete bone regeneration while undergoing nearly complete degradation. Int. J. Oral Maxillofac. Implants. 2018;33:636–644. doi: 10.11607/jomi.6026. [DOI] [PubMed] [Google Scholar]
- 74.Di Filippo M.F., Dolci L.S., Albertini B., Passerini N., Torricelli P., Parrilli A., Fini M., Bonvicini F., Gentilomi G.A., Panzavolta S., Bigi A. A radiopaque calcium phosphate bone cement with long-lasting antibacterial effect: from paste to injectable formulation. Ceram. Int. 2020;46:10048–10057. doi: 10.1016/j.ceramint.2019.12.272. [DOI] [Google Scholar]
- 75.Carey L.E., Xu H.H.K., Simon C.G., Takagi S., Chow L.C. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials. 2005;26:5002–5014. doi: 10.1016/j.biomaterials.2005.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burguera E.F., Xu H.H.K., Weir M.D. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J. Biomed. Mater. Res. B Appl. Biomater. 2006;77B:126–136. doi: 10.1002/jbm.b.30403. [DOI] [PubMed] [Google Scholar]
- 77.Lima D.B., de Souza M.A.A., de Lima G.G., Ferreira Souto E.P., Oliveira H.M.L., Fook M.V.L., de Sá M.J.C. Injectable bone substitute based on chitosan with polyethylene glycol polymeric solution and biphasic calcium phosphate microspheres. Carbohydr. Polym. 2020;245 doi: 10.1016/j.carbpol.2020.116575. [DOI] [PubMed] [Google Scholar]
- 78.Kim Y., Bae J., Uyama E., Sekine K., Kawano F., Hamada K. Effects of zirconia additives on β-tricalcium-phosphate cement for high strength and high injectability. Ceram. Int. 2021;47:1882–1890. doi: 10.1016/j.ceramint.2020.09.017. [DOI] [Google Scholar]
- 79.Barrac M., Fern J.A.M.E. Does mixing affect the setting of injectable bone cement? An ultrasound study. J. Mater. Sci. Mater. Med. 2007;18:347–352. doi: 10.1007/s10856-006-0699-0. [DOI] [PubMed] [Google Scholar]
- 80.Chow L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009;28:1–10. doi: 10.4012/dmj.28.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ginebra M.P., Canal C., Espanol M., Pastorino D., Montufar E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Generosi A., Rau J.V., Komlev V.S., Albertini V.R., Fedotov A.Y., Barinov S.M. Anomalous hardening behavior of a calcium phosphate bone cement. J. Phys. Chem. B. 2010;114:973–979. doi: 10.1021/jp907350u. [DOI] [PubMed] [Google Scholar]
- 83.Arkin V.H.R., Narendrakumar U., Madhyastha H., Manjubala I. Characterization and in vitro evaluations of injectable calcium phosphate cement doped with magnesium and strontium. ACS Omega. 2021;6:2477–2486. doi: 10.1021/acsomega.0c03927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jansen J., Ooms E., Verdonschot N., Wolke J. vol. 36. 2005. pp. 89–95. (Injectable Calcium Phosphate Cement for Bone Repair and Implant Fixation). [DOI] [PubMed] [Google Scholar]
- 85.Romano R.C.O., Cincotto M.A., Pileggi R.G. Hardening phenomenon of Portland cement suspensions monitored by Vicat test, isothermal calorimetry and oscillatory rheometry isotérmica e reometria oscilatória. IBRACON Struct. Mater. J. 2018;11:949–959. [Google Scholar]
- 86.Sopcak T., Medvecky L., Giretova M., Stulajterova R., Durisin J. Hydrolysis, setting properties and in vitro characterization of wollastonite/newberyite bone cement mixtures. J. Biomater. Appl. 2017;32:871–885. doi: 10.1177/0885328217747126. [DOI] [PubMed] [Google Scholar]
- 87.Bohner M. Reactivity of calcium phosphate cements. J. Mater. Chem. 2007;17:3980–3986. doi: 10.1039/B706411J. [DOI] [Google Scholar]
- 88.Laskus A., Kolmas J. Ionic substitutions in non-apatitic calcium phosphates. Int. J. Mol. Sci. 2017;18:2542–2564. doi: 10.3390/ijms18122542. J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uskoković V., Graziani V., Wu V.M., Fadeeva I.V., Fomin A.S., Presniakov I.A., Fosca M., Ortenzi M., Caminiti R., Rau J.V. Gold is for the mistress, silver for the maid: enhanced mechanical properties, osteoinduction and antibacterial activity due to iron doping of tricalcium phosphate bone cements. Mater. Sci. Eng. C. 2019;94:798–810. doi: 10.1016/j.msec.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rau J.V., Wu V.M., Graziani V., Fadeeva I.V., Fomin A.S., Fosca M., Uskoković V. The Bone Building Blues: self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. C. 2017;79:270–279. doi: 10.1016/j.msec.2017.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Irbe Z., Loca D. Soluble phosphate salts as setting aids for premixed calcium phosphate bone cement pastes. Ceram. Int. 2021;47:24012–24019. doi: 10.1016/j.ceramint.2021.05.110. [DOI] [Google Scholar]
- 92.Miyamoto Y., Ishikawa K., Takechi M., Toh T., Yuasa T., Nagayama M., Suzuki K. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J. Biomed. Mater. Res. Appl. Biomater. 1998;48:36–42. doi: 10.1002/(sici)1097-4636(1999)48:1<36::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 93.Saleh A.T., Raheem A.A. Injectable calcium phosphate cements (CPCs), mechanical and biological properties, applications in the drug delivery: a review. Int. Res. J. Mod. Eng. Technol. Sci. 2021;3:887–895. https://www.researchgate.net/publication/349664377 [Google Scholar]
- 94.Zhong W., Sun L., Yu T., Zhou C. Preparation and characterization of calcium phosphate cement with enhanced tissue adhesion for bone defect repair. Ceram. Int. 2021;47:1712–1720. doi: 10.1016/j.ceramint.2020.08.288. [DOI] [Google Scholar]
- 95.Xie Y., Liu J., Cai S., Bao X., Li Q., Xu G. Setting characteristics and high compressive strength of an anti-washout, injectable calcium phosphate cement combined with thermosensitive hydrogel. Materials. 2020;13:1–18. doi: 10.3390/ma13245779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Afewerki S., Bassous N., Harb S., Palo-Nieto C., Ruiz-Esparza G.U., Marciano F.R., Webster T.J., Furtado A.S.A., Lobo A.O. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2020;24 doi: 10.1016/j.nano.2019.102143. [DOI] [PubMed] [Google Scholar]
- 97.Bueno E.M., Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat. Rev. Rheumatol. 2009;5:685–697. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 98.Xu H.H.K., Wang P., Wang L., Bao C., Chen Q., Weir M.D., Chow L.C., Zhao L., Zhou X., Reynolds M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017;5:1–19. doi: 10.1038/boneres.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghanaati S., Barbeck M., Orth C., Willershausen I., Thimm B.W., Hoffmann C., Rasic A., Sader R.A., Unger R.E., Peters F., Kirkpatrick C.J. Influence of b-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010;6:4476–4487. doi: 10.1016/j.actbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Yu T., Dong C., Shen Z., Chen Y., Yu B., Shi H., Zhou C., Ye J. Vascularization of plastic calcium phosphate cement in vivo induced by in-situ-generated hollow channels. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;68:153–162. doi: 10.1016/j.msec.2016.05.106. [DOI] [PubMed] [Google Scholar]
- 101.Bohner M., Miron R.J. A proposed mechanism for material- induced heterotopic ossification. Mater. Today. 2019;22:132–141. doi: 10.1016/j.mattod.2018.10.036. [DOI] [Google Scholar]
- 102.Maazouz Y., Rentsch I., Lu B., Santoni B.L.G., Doebelin N., Bohner M. In vitro measurement of the chemical changes occurring within β-tricalcium phosphate bone graft substitutes. Acta Biomater. 2020;102:440–457. doi: 10.1016/j.actbio.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 103.Maazouz Y., Chizzola G., Döbelin N., Bohner M. Cell-free, quantitative mineralization measurements as a proxy to identify osteoinductive bone graft substitutes. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120912. [DOI] [PubMed] [Google Scholar]
- 104.Wenisch S., Stahl J.P., Horas U., Heiss C., Kilian O., Trinkaus K., Hild A., Schnettler R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: fine structural microscopy. J. Biomed. Mater. Res. 2003;67:713–718. doi: 10.1002/jbm.a.10091. [DOI] [PubMed] [Google Scholar]
- 105.Theiss F., Apelt D., Brand B., Kutter A., Zlinszky K., Bohner M., Matter S., Frei C., Auer J.A., Von Rechenberg B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials. 2005;26:4383–4394. doi: 10.1016/j.biomaterials.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 106.Engel E., Planell J.A., Rajzer I., Castan O. Injectable and fast resorbable calcium phosphate cement for body-setting bone grafts. J. Mater. Sci. Mater. Med. 2010;21:2049–2056. doi: 10.1007/s10856-010-4078-5. [DOI] [PubMed] [Google Scholar]
- 107.Fernandez E., Planell J.A., Best S.M. Precipitation of carbonated apatite in the cement system α-Ca3(PO4)2-Ca(H2PO4)2-CaCO3. J. Biomed. Mater. Res. 1997;47:466–471. doi: 10.1002/(sici)1097-4636(19991215)47:4<466::aid-jbm2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 108.Le Ferrec M., Mellier C., Lefèvre F.X., Boukhechba F., Janvier P., Montavon G., Bouler J.M., Gauthier O., Bujoli B. In vivo resorption of injectable apatitic calcium phosphate cements: critical role of the intergranular microstructure. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108:367–376. doi: 10.1002/jbm.b.34395. [DOI] [PubMed] [Google Scholar]
- 109.Hesaraki S., Zamanian A., Moztarzadeh F. The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: acetic acid versus citric acid. J. Biomed. Mater. Res. B Appl. Biomater. 2008;86:208–216. doi: 10.1002/jbm.b.31008. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Z., Ye D., Liang W., Wang B., Zhu Z. Preparation and characterization of a novel injectable strontium-containing calcium phosphate cement with collagen. Chin. J. Traumatol. - English Ed. 2015;18:33–38. doi: 10.1016/j.cjtee.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 111.Dai J., Fu Y., Chen D., Sun Z. A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: a systematic evaluation. Mater. Sci. Eng. C. 2021;124 doi: 10.1016/j.msec.2021.112052. [DOI] [PubMed] [Google Scholar]
- 112.Yang L., Chen S., Shang T., Zhao R., Yuan B., Zhu X., Raucci M.G., Yang X., Zhang X., Santin M., Ambrosio L. Complexation of injectable biphasic calcium phosphate with phosphoserine-presenting dendrons with enhanced osteoregenerative properties. ACS Appl. Mater. Interfaces. 2020;12:37873–37884. doi: 10.1021/acsami.0c09004. [DOI] [PubMed] [Google Scholar]
- 113.Schumacher M., Lode A., Helth A., Gelinsky M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013;9:9547–9557. doi: 10.1016/j.actbio.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 114.Thormann U., Ray S., Sommer U., ElKhassawna T., Rehling T., Hundgeburth M., Henß A., Rohnke M., Janek J., Lips K.S., Heiss C., Schlewitz G., Szalay G., Schumacher M., Gelinsky M., Schnettler R., Alt V. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials. 2013;34:8589–8598. doi: 10.1016/j.biomaterials.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 115.Schumache M., Henß A., Rohnke M., Gelinsky M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013;9:7536–7544. doi: 10.1016/j.actbio.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Schumacher M., Gelinsky M. Strontium modified calcium phosphate cements - approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B. 2015;3:4626–4640. doi: 10.1039/c5tb00654f. [DOI] [PubMed] [Google Scholar]
- 117.Gibson I.R., Best S.M., Bonfield W. Effect of silicon substitution on the sintering and microstructure of hydroxyapatite. J. Am. Ceram. Soc. 2004;85:2771–2777. doi: 10.1111/j.1151-2916.2002.tb00527.x. [DOI] [Google Scholar]
- 118.Alshaaer M., Kailani M.H., Jafar H., Ababneh N., Awidi A. Physicochemical and microstructural characterization of injectable load-bearing calcium phosphate scaffold. Adv. Mater. Sci. Eng. 2013 doi: 10.1155/2013/149261. 2013. [DOI] [Google Scholar]
- 119.Engel E., Planell J.A., Rajzer I., Castan O. Injectable and fast resorbable calcium phosphate cement for body-setting bone grafts. J. Mater. Sci. Mater. Med. 2010;21:2049–2056. doi: 10.1007/s10856-010-4078-5. [DOI] [PubMed] [Google Scholar]
- 120.Smith B.T., Santoro M., Grosfeld E.C., Shah S.R., Van Den Beucken J.J.J.P., Jansen J.A., Mikos A.G. Incorporation of fast dissolving glucose porogens into an injectable calcium phosphate cement for bone tissue engineering. Acta Biomater. 2017;50:68–77. doi: 10.1016/j.actbio.2016.12.024.Incorporation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grosfeld E.-C., Smith B.T., Santoro M., Lodoso-Torrecilla I., Jansen J.A., Ulrich D.J., Melchiorri A.J., Scott D.W., Mikos A.G., van den Beucken J.J.J.P. Fast dissolving glucose porogens for early calcium phosphate cement degradation and bone regeneration. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/ab5f9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van De Watering F.C.J., Molkenboer-kuenen J.D.M., Boerman O.C., Van Den Beucken J.J.J.P., Jansen J.A. Differential loading methods for BMP-2 within injectable calcium phosphate cement. J. Contr. Release. 2012;164:283–290. doi: 10.1016/j.jconrel.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 123.Seong Y., Song E., Park C., Lee H., Kim H., Jeong S. Porous calcium phosphate–collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater. Sci. Eng. C. 2020;109 doi: 10.1016/j.msec.2019.110480. [DOI] [PubMed] [Google Scholar]
- 124.Wang X.H., Jia S.J., Hao D.J. Advances in the modification of injectable calcium-phosphate-based bone cements for clinical application. Chin. Med. J. 2020;133:2610–2612. doi: 10.1097/CM9.0000000000001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kucko N.W., Li W., García Martinez M.A., ur Rehman I., Ulset A.S.T., Christensen B.E., Leeuwenburgh S.C.G., Herber R.P. Sterilization effects on the handling and degradation properties of calcium phosphate cements containing poly (D,L-lactic-co-glycolic acid) porogens and carboxymethyl cellulose. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107:2216–2228. doi: 10.1002/jbm.b.34306. [DOI] [PubMed] [Google Scholar]
- 126.El Din Mahmoud H., Ezz M., Sayur H., Abdel-Ghany H. Histological assessment of injectable macro-porous calcium phosphate cement (CPC) versus autogenous bone graft on healing of osseous defect (experimental animal study), Egypt. Dent. J. 2021;67:1953–1965. doi: 10.21608/edj.2021.69779.1567. [DOI] [Google Scholar]
- 127.Kovtun A., Goeckelmann M.J., Niclas A.A., Montufar E.B., Ginebra M.P., Planell J.A., Santin M., Ignatius A. In vivo performance of novel soybean/gelatin-based bioactive and injectable hydroxyapatite foams. Acta Biomater. 2015;12:242–249. doi: 10.1016/j.actbio.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nezafati N., Farokhi M., Heydari M., Hesaraki S., Nasab N.A. In vitro bioactivity and cytocompatablity of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. B Eng. 2019;175 doi: 10.1016/j.compositesb.2019.107146. [DOI] [Google Scholar]
- 129.Lode A., Wolf-Brandstetter C., Reinstorf A., Bernhardt A., König U., Pompe W., Gelinsky M. Calcium phosphate bone cements, functionalized with VEGF: release kinetics and biological activity. J. Biomed. Mater. Res. 2007;81A:474–483. doi: 10.1002/jbm.a.31024. [DOI] [PubMed] [Google Scholar]
- 130.Kjalarsdóttir L., Dýrfjörd A., Dagbjartsson A., Laxdal E.H., Örlygsson G., Gíslason J., Einarsson J.M., Ng C.-H., Jónsson H. Bone remodeling effect of a chitosan and calcium phosphate-based composite. Regen. Biomater. 2019;6:241–247. doi: 10.1093/rb/rbz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H., Yang H., Weir M.D., Schneider A., Ren K., Homayounfar N., Oates T.W., Zhang K., Liu J., Hu T., Xu H.H.K. An antibacterial and injectable calcium phosphate scaffold delivering human periodontal ligament stem cells for bone tissue engineering. RSC Adv. 2020;10:40157–40170. doi: 10.1039/d0ra06873j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu S., Lei L., Bao C., Liu J., Weir M.D., Ren K., Schneider A., Oates T.W., Liu J., Xu H.H.K. An injectable and antibacterial calcium phosphate scaffold inhibiting Staphylococcus aureus and supporting stem cells for bone regeneration. Mater. Sci. Eng. C. 2021;120 doi: 10.1016/j.msec.2020.111688. [DOI] [PubMed] [Google Scholar]
- 133.Barinov S.M., Komlev V.S. Calcium phosphate bone cements. Inorg. Mater. 2011;47:1470–1485. doi: 10.1134/S0020168511130024. [DOI] [PubMed] [Google Scholar]
- 134.Lee H., Kim B., Padalhin A.R., Lee B. Incorporation of chitosan-alginate complex into injectable calcium phosphate cement system as a bone graft material. Mater. Sci. Eng. C. 2019;94:385–392. doi: 10.1016/j.msec.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 135.Amirian J., Makkar P., Lee G.H., Paul K., Lee B.T. Incorporation of alginate-hyaluronic acid microbeads in injectable calcium phosphate cement for improved bone regeneration. Mater. Lett. 2020;272 doi: 10.1016/j.matlet.2020.127830. [DOI] [Google Scholar]
- 136.Liu H., Liu B., Gao C., Meng B., Yang H., Yu H. Injectable, biomechanically robust, biodegradable and osseointegrative bone cement for percutaneous kyphoplasty and vertebroplasty. Int. Orthop. 2018;42:125–132. doi: 10.1007/s00264-017-3674-0. [DOI] [PubMed] [Google Scholar]
- 137.Liu H., Zhang Z., Gao C., Bai Y., Liu B., Wang W., Ma Y., Saijilafu, Yang H., Li Y., Chan A., Yang L. Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater. Sci. Eng. C. 2020 doi: 10.1016/j.msec.2020.110904. [DOI] [PubMed] [Google Scholar]
- 138.Shi H., Ye X., Zhang J., Ye J. Enhanced osteogenesis of injectable calcium phosphate bone cement mediated by loading chondroitin sulfate. ACS Biomater. Sci. Eng. 2019;5:262–271. doi: 10.1021/acsbiomaterials.8b00871. [DOI] [PubMed] [Google Scholar]
- 139.Vlad M.D., Del Valle L.J., Barracó M., Torres R., López J., Fernández E. Iron oxide nanoparticles significantly enhances the injectability of apatitic bone cement for vertebroplasty. Spine. 2008;33:2290–2298. doi: 10.1097/BRS.0b013e31817eccab. Phila. Pa. 1976. [DOI] [PubMed] [Google Scholar]
- 140.Xia Y., Zhao Y., Zhang F., Chen B., Hu X., Weir M.D., Schneider A., Jia L., Gu N., Xu H.H.K. Iron oxide nanoparticles in liquid or powder form enhanced osteogenesis via stem cells on injectable calcium phosphate scaffold. Nanomed. Nanotechnol. Biol. Med. 2019;21 doi: 10.1016/j.nano.2019.102069. [DOI] [PubMed] [Google Scholar]
- 141.Lin Z., Cao Y., Zou J., Zhu F., Gao Y., Zheng X., Wang H., Zhang T., Wu T. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater. Sci. Eng. C. 2020;114 doi: 10.1016/j.msec.2020.111032. [DOI] [PubMed] [Google Scholar]
- 142.Barralet J., Gbureck U., Habibovic P., Vorndran E., Gerard C., Doillon C.J. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. 2009;15:1601–1609. doi: 10.1089/ten.tea.2007.0370. [DOI] [PubMed] [Google Scholar]
- 143.Tan S., Wang Y., Du Y., Xiao Y., Zhang S. Injectable bone cement with magnesium-containing microspheres enhances osteogenesis via anti-inflammatory immunoregulation. Bioact. Mater. 2021;6:3411–3423. doi: 10.1016/j.bioactmat.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu T., Shi H., Liang Y., Lu T., Lin Z., Ye J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped β-tricalcium phosphate. Mater. Sci. Eng. C. 2020;109 doi: 10.1016/j.msec.2019.110481. [DOI] [PubMed] [Google Scholar]
- 145.Ewald A., Hösel D., Patel S., Grover L.M., Barralet J.E., Gbureck U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011;7:4064–4070. doi: 10.1016/j.actbio.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 146.Rau J.V., Fosca M., Graziani V., Egorov A.A., Zobkov Y.V., Fedotov A.Y., Ortenzi M., Caminiti R., Baranchikov A.E., Komlev V.S. Silver-doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016;7:10. doi: 10.3390/jfb7020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mellier C., Lefèvre F.X., Fayon F., Montouillout V., Despas C., Le Ferrec M., Boukhechba F., Walcarius A., Janvier P., Dutilleul M., Gauthier O., Bouler J.M., Bujoli B. A straightforward approach to enhance the textural, mechanical and biological properties of injectable calcium phosphate apatitic cements (CPCs): CPC/blood composites, a comprehensive study. Acta Biomater. 2017;62:328–339. doi: 10.1016/j.actbio.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 148.Li G., Zhang K., Pei Z., Liu P., Chang J., Zhang K., Zhao S., Zhang K., Jiang N., Liang G. Basalt fibre reinforced calcium phosphate cement with enhanced toughness. Mater. Technol. 2020;35:152–158. doi: 10.1080/10667857.2019.1659536. [DOI] [Google Scholar]
- 149.Dorozhkin S.V. Functionalized calcium orthophosphates (CaPO4) and their biomedical applications. J. Mater. Chem. B. 2019;7:7471–7489. doi: 10.1039/c9tb01976f. [DOI] [PubMed] [Google Scholar]
- 150.Medvecky L., Štulajterová R., Giretova M., Luptakova L., Sopčák T. Injectable enzymatically hardened calcium phosphate biocement. J. Funct. Biomater. 2020;11 doi: 10.3390/jfb11040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cui D., Xu Y., Yang D., Zhu J. 2020. Chinese Patent Application No. CN202011394591A. [Google Scholar]
- 152.He D., Jin C., Wang P., Yang D., Zhang Z. 2018. Chinese Patent Application No. CN201811620520A. [Google Scholar]
- 153.Cui D., Yang D. 2020. Chinese Patent Application No. CN202010801815A. [Google Scholar]
- 154.He D., Jin C., Liu X., Jan Y., Yang D., Zhu S. 2017. Chinese Patent Application No. CN201711050250A. [Google Scholar]
- 155.He D., Jin C., Jan Y., Yang D., Zhu S. 2017. Chinese Patent Application No. CN201711165829A. [Google Scholar]
- 156.He D., Jin C., Jan Y., Yang D., Zhu S. 2017. Chinese Patent Application No. CN201711230551A. [Google Scholar]
- 157.He D., Jin C., Liu X., Jan Y., Yang D., Zhu S. 2020. Chinese Patent No. CN107812240B. [Google Scholar]
- 158.Che Q. 2018. Chinese Patent Application No. CN201811113125A. [Google Scholar]
- 159.Che Q., Liu S., Xie P. 2018. Chinese Patent Application No. CN201811127192A. [Google Scholar]
- 160.Che Q. 2018. Chinese Patent Application No. CN201811085265A. [Google Scholar]
- 161.He D., Jin C., Liu X., Jan Y., Yang D., Zhu S. 2021. Chinese Patent No. CN107812240B. [Google Scholar]
- 162.Dong Y., Pan Y., Zhan Y. 2021. Chinese Patent No. CN109939261B. [Google Scholar]
- 163.Chen S., Ding L., Li B. 2021. Chinese Patent Application No. CN202110014054A. [Google Scholar]
- 164.Bastianelli E., Bouler J.M., Bujoli B. 2020. International Patent Application No. EP2020070351W. [Google Scholar]
- 165.Barbieri D., Davison N., De Bruijn J.D., De Groot F., Yuan H. 2020. European Patent No. 2456475. [Google Scholar]
- 166.Dong L., Liu S. 2019. International Patent Application CN2019130318W. [Google Scholar]
- 167.Zhang Q., Zhang T. 2018. Chinese Patent Application No. CN201810404802A. [Google Scholar]
- 168.Gao C., Pu Z., Zhu P. 2019. Chinese Patent Application No. CN201910874656A. [Google Scholar]
- 169.Che Q., Liu S., Yu T., Zhao P., Zhou C.N. 2018. Chinese Patent Application No. CN201810706542A. [Google Scholar]
- 170.Khairoun I., Weiss P., Bouler J.M. 2017. US Patent No. US09642939B2. [Google Scholar]
- 171.Bagga C.S., Bondre S.P. 2021. US Patent Application No. US202117168589A. [Google Scholar]
- 172.Kim B.R., Lee B.T., Lee H.J. 2018. Korean Patent KR101905790B1. [Google Scholar]
- 173.Lai X., Li J., Wang H., Wang L., Yang Y., Yin Y., Zhang X., Zou Q. 2020. Chinese Patent Application No. CN202010985069A. [Google Scholar]
- 174.Cao X., Lin G., Yin M., Yue S., Zhu S. 2016. Chinese Patent Application No. CN201610474837A. [Google Scholar]
- 175.Chen X., Hu H., Liao H., Liu H., Qu R., Zhou Q. 2018. Chinese Patent Application No. CN201810068344A. [Google Scholar]
- 176.Fei Z. 2018. Chinese Patent Application No. CN201810329764A. [Google Scholar]
- 177.Lee B.T., Abueva S. 2019. Korean Patent No. KR101978386B1. [Google Scholar]
- 178.Xu H. 2020. Chinese Patent No. CN108144115B. [Google Scholar]
- 179.Ren W., Song W. 2021. European Patent No. 3256177. [Google Scholar]
- 180.Hu H., Jiang J., Jiang Q., Lin H., Lu T., Su J., Wang L., Jan X., Zhang X., Zhao J., Zhu H. 2020. Chinese Patent Application No. CN202010187999A. [Google Scholar]
- 181.Ahn E.S., Lin C.E., White C.D. 2017. European Patent Application No. 17722590; p. 1. [Google Scholar]
- 182.Rosenberg A.D., Gilles De Pelichy L.D., Egan D., Tofighi A.N., Lee D.D. 2019. European Patent No. 3254710. [Google Scholar]
- 183.de Bruijn J.D., Yuan H. 2020. US Patent No. US10532131B2. [Google Scholar]
- 184.Grishchenko D., Medkov M. 2018. Russian Patent No. RU2643337C1. [Google Scholar]
- 185.De Groot-Barrere F., Van Miegem V., Yuan H., Du Bruijn J. 2018. European Patent No. 3021878. [Google Scholar]
- 186.Ginebra M., Delgado J.-A., Harr I., Almirall A., Del Valle S., Planell J.A. Factors affecting the structure and properties of an injectable self-setting calcium phosphate foam. J. Biomed. Mater. Res. 2007;80A:351–361. doi: 10.1002/jbm.a. [DOI] [PubMed] [Google Scholar]
- 187.Lodoso-Torrecilla I., Stumpel F., Jansen J.A., van den Beucken J.J.J.P. Early-stage macroporosity enhancement in calcium phosphate cements by inclusion of poly(N-vinylpyrrolidone) particles as a porogen. Mater. Today Commun. 2020;23 doi: 10.1016/j.mtcomm.2020.100901. [DOI] [Google Scholar]
- 188.Kyllönen L., D'Este M., Alini M., Eglin D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015;11:412–434. doi: 10.1016/j.actbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 189.Bigi A., Boanini E. Calcium phosphates as delivery systems for Bisphosphonates. J. Funct. Biomater. 2018;9 doi: 10.3390/jfb9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ginebra M.P., Traykova T., Planell J.A. Calcium phosphate cements: competitive drug carriers for the musculoskeletal system? Biomaterials. 2006;27:2171–2177. doi: 10.1016/j.biomaterials.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 191.Kaufman M.G., Meaike J.D., Izaddoost S.A. Orthopedic prosthetic infections: diagnosis and orthopedic salvage. Semin. Plast. Surg. 2016;30:66–72. doi: 10.1055/s-0036-1580730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fasolino I., Soriente A., Ambrosio L., Raucci M.G. Osteogenic and anti-inflammatory behavior of injectable calcium phosphate loaded with therapeutic drugs. Nanomaterials. 2020;10:1–15. doi: 10.3390/nano10091743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Farbod K., Sariibrahimoglu K., Curci A., Hayrapetyan A., Hakvoort J.N.W., Van Den Beucken J.J.J.P., Iafisco M., Margiotta N., Leeuwenburgh S.C.G. Controlled release of chemotherapeutic platinum-bisphosphonate complexes from injectable calcium phosphate cements. Tissue Eng. 2016;22:788–800. doi: 10.1089/ten.tea.2016.0001. [DOI] [PubMed] [Google Scholar]
- 194.Fosca M., Rau J.V., Uskoković V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2022;7:341–363. doi: 10.1016/j.bioactmat.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]