Abstract
Serum creatinine concentration, the classical biomarker of chronic kidney disease (CKD) in cats, has important limitations that decrease its value as a biomarker of early CKD. Recently, serum symmetric dimethylarginine concentration was introduced as a novel glomerular filtration rate biomarker for the early detection of CKD in cats. However, data on its specificity are still limited. The limitations of conventional biomarkers and the desire for early therapeutic intervention in cats with CKD to improve outcomes have prompted the discovery and validation of novel renal biomarkers to detect glomerular or tubular dysfunction. Changes in the serum or urinary concentrations of these biomarkers may indicate early kidney damage or predict the progression of kidney before changes in conventional biomarkers are detectable. This review summarizes current knowledge on renal biomarkers in CKD in cats, a field that has progressed substantially over the last 5 years.
Keywords: feline, fibroblast growth factor‐23, kidney injury molecule‐1, liver‐type fatty acid‐binding protein, neutrophil gelatinase‐associated lipocalin, transforming growth factor‐β1
ABBREVIATIONS
- AKI
acute kidney injury
- ALB
albumin
- CKD
chronic kidney disease
- CysB
cystatin B
- CysC
cystatin C
- FGF‐23
fibroblast growth factor‐23
- GFR
glomerular filtration rate
- HMW
high molecular weight
- HSP‐72
heat shock protein‐72
- IMW
intermediate molecular weight
- kDa
kilodalton
- KIM‐1
kidney injury molecule‐1
- L‐FABP
liver‐type fatty acid‐binding protein
- LMW
low molecular weight
- MMP
matrix metalloproteinase
- NAG
N‐acetyl‐b‐d‐glucosaminidase
- NGAL
neutrophil gelatinase‐associated lipocalin
- p
plasma
- PENIA
particle‐enhanced nephelometric immunoassay
- PETIA
particle‐enhanced turbidimetric immunoassay
- PIIINP
procollagen type III amino‐terminal propeptide
- PTH
parathyroid hormone
- RBP
retinal‐binding proteins
- s
serum
- sCr
Serum creatinine
- SDMA
symmetric dimethylarginine
- SDS‐PAGE
sodium dodecyl sulfate‐polyacrylamide gel electrophoresis
- TGF‐β1
transforming growth factor‐β1
- u
urinary
- UPC
urinary protein : creatinine ratio
- USG
urine specific gravity
1. INTRODUCTION
Chronic kidney disease (CKD) is a common diagnosis in older cats. The overall prevalence of CKD in cats is approximately 2% to 4%, 1 , 2 and increases to 30% to 40% in cats >10 years of age. 3 , 4 Data from 3 million dogs also support that the prevalence of CKD increases with age. 5 In cats, several factors contribute to kidney injury are implicated in CKD, 6 and renal damage tends to progress over time at an unpredictable rate. 6 Tubulointerstitial inflammation is the most common histopathological feature of CKD in cats, with fibrotic changes signifying a worse prognosis. 7 , 8
Clinically, CKD in cats usually is diagnosed at a late stage based on a combination of compatible clinical signs, azotemia, and an inappropriate urine specific gravity (USG). 9 , 10 Although a USG <1.035 is regarded as abnormal in cats with dehydration or azotemia, urinary concentrating ability is occasionally intact in cats with CKD. 9 , 11 Furthermore, inappropriately dilute urine also can be non‐renal in origin, for instance secondary to certain drugs (eg, diuretics), glucosuria, electrolyte imbalances, or liver disease. 12
Measurement of glomerular filtration rate (GFR) is the gold standard and most sensitive test for impaired renal function. 13 The GFR can be determined directly by measuring the clearance of an endogenous or exogenous filtration marker. 13 Although limited sampling techniques have been developed to improve the practical use of GFR measurement in cats, none of these current techniques is feasible for routine veterinary practice, and direct GFR measurement is still mostly a research tool. 14 , 15 , 16 , 17
Proteinuria as assessed by the urinary protein : creatinine ratio (UPC) is a routine renal biomarker and traditional hallmark of glomerular disease but also increases with tubular dysfunction in cats. 8 , 18 , 19 Persistent renal proteinuria without azotemia may indicate early glomerular disease in cats. 9 Epidemiologic studies have suggested that UPC is prognostic for survival, progression and the development of CKD in cats. 3 , 20 , 21 , 22 Results of the UPC values in cats commonly are influenced by non‐renal diseases such as hyperthyroidism, viral infections, and lower urinary tract diseases, which substantially decrease the specificity of UPC for kidney disease. 6 , 23 , 24
To delay CKD progression, treatment ideally should be instituted as soon as the diagnosis is achieved. 25 The search for serum or urine renal biomarkers that are more sensitive for detecting early kidney damage or small decreases in kidney function is driven by the limitations and poor sensitivities of traditional biomarkers such as serum creatinine (sCr) concentration. 26
The innovative concept that acute kidney injury (AKI) and CKD in cats and dogs are interconnected processes recently has been proposed, analogous to the situation in humans. This concept states that sustained or serious kidney injury, such as that occurring during an episode of AKI, could lead to the development of CKD and vice versa. This ongoing kidney injury generally occurs before a noticeable decrease in GFR. 6 , 27 Furthermore, “acute kidney stress” was introduced as a concept in humans and dogs to describe a predisposition to AKI or a very early insult to the kidney before AKI. 28 Combining these concepts, it can be hypothesized that kidney stress or early kidney injury may be associated with the development and progression of CKD. Therefore, renal biomarkers that can identify active kidney stress or injury have the potential to detect CKD earlier than biomarkers that are surrogates of decreased GFR. Moreover, these injury biomarkers, especially those in urine, can also specifically localize injury to glomeruli or tubules. Furthermore, these biomarkers might have the potential to predict the development of CKD, monitor recovery, and facilitate prognostication. 6 , 29
In this review, we categorize and discuss renal biomarkers measured in feline blood and urine based on their origin and role in kidney disease. In particular, we describe biomarkers of GFR, metabolic derangement, glomerular vs tubular injury or dysfunction, and renal fibrosis, focusing on biomarkers described using new data obtained the last 5 years.
2. CURRENT STATUS OF CLINICAL DIAGNOSIS OF CKD
2.1. Systemic biomarkers of GFR
The serum or plasma biomarkers under investigation in cats with CKD are summarized in Table 1.
TABLE 1.
Overview of systemic renal biomarkers in cats with CKD
| Biomarker | Origin | Type of molecule | Mechanism underlying increased levels | Validated assays in cats | Changes in cats with azotemic‐CKD | Non‐renal factors to consider |
|---|---|---|---|---|---|---|
| Biomarkers of GFR | ||||||
| SDMA | All nucleated cells | Methylated amino acid | Decreased GFR | Liquid chromatography‐mass spectroscopy 30 | Increased 30 | |
| CysC | All nucleated cells | LMW protein and cysteine protease inhibitor | Decreased GFR |
Human PENIA 31 |
Increased 31 , 32 , 34 , 35 or insignificant 36 | Hyperthyroidism 24 , 36 |
| Biomarker of metabolic derangement | ||||||
| FGF‐23 | Osteoblasts and osteocytes | Phosphaturic hormone | Altered phosphate metabolism | ELISA 37 | Increased 37 , 38 | Hyperthyroidism 39 |
Abbreviations: CysC, cystatin C; FGF‐23, fibroblast growth factor‐23; GFR, glomerular filtration rate; LMW, low molecular weight; PENIA, particle‐enhanced nephelometric immunoassay; PETIA, particle‐enhanced turbidimetric immunoassay; SDMA, symmetric dimethylarginine.
Because of logistical drawbacks, GFR is usually indirectly estimated by measuring serum biomarker concentrations. The traditional GFR biomarker is sCr, which is widely used to diagnose CKD because it is economical, readily available, easy to measure, and shows low intra‐individual variability. 9 , 40 Also when within reference range, serial monitoring of sCr can be used to detect early change in renal function. 10 , 40 However, sCr has important limitations. First, it is influenced by non‐renal factors, especially muscle mass. 30 , 41 , 42 Second, sCr has a low sensitivity to detect an early decrease in GFR and cannot detect kidney damage that does not affect GFR. 6 , 29 Third, its wide reference interval because of high inter‐individual variability and its analytical variability can lead to misinterpretation in clinical practice. 43 , 44 These major limitations hamper the utility of sCr to detect early CKD in cats, resulting in a search for other indirect GFR biomarkers such as symmetric dimethylarginine (SDMA).
Besides the conventional functional biomarker, sCr, which is the main representative of this group, SDMA concentration recently has been described as a novel GFR biomarker for the diagnosis of CKD in cats. 45 , 46 Its main advantage is that it is more sensitive than sCr concentration because it can detect a smaller decrease in GFR, increasing when the GFR decreases by approximately 40% in cats. 25 , 30 Another advantage is that SDMA concentration is not affected by muscle mass. 47 , 48 , 49 However, breed‐related differences in SDMA concentration have been noted and require further exploration. 48 A more important limitation of SDMA concentration is that any increase must be interpreted with caution because data on its specificity are scarce. 46 , 50 International Renal Interest Society (IRIS) has recently incorporated SDMA concentration into guidelines for CKD staging. 51 According to IRIS, persistently increased SDMA concentration may indicate early CKD (IRIS stage 1). 51 Hence, multiple blood samplings are required when interpreting SDMA concentration. Because extensive reviews on the current knowledge of SDMA in dogs and cats have been published recently, 29 , 46 , 52 , 53 SDMA will not be discussed in depth in the present review. To date, the only other surrogate biomarker of GFR that has received attention in cats is serum cystatin C (sCysC). This marker will be reviewed below, but despite extensive analytical validation sCysC has failed to show clinical utility as a marker of GFR. 32 , 36
2.1.1. Serum cystatin C
Cystatin C is a 13 kilodalton (kDa) non‐glycosylated protein produced by all nucleated cells at a constant rate. It is involved in intracellular protein catabolism as a proteinase inhibitor. 54 Circulating CysC generally passes through the glomerular barrier without restriction. 55 In the proximal tubules, CysC is reabsorbed via megalin receptors and then is metabolized completely. 56 There is no proof that CysC is secreted by tubular cells into urine. 57 In humans and dogs, sCysC concentration is used as GFR biomarker and is highly correlated with sCr and GFR. 34 , 57 , 58 , 59 , 60 Also, a GFR equation based on the combination of sCysC and sCr has been shown to be the optimal equation to estimate GFR in humans. 61
No cat‐specific assay is available for CysC measurement. Although particle‐enhanced nephelometric (PENIA) and particle‐enhanced turbidimetric (PETIA) sCysC immunoassays used in humans had acceptable precision and reproducibility in cats, 31 , 32 , 33 the cross‐reactivity between the anti‐human CysC antibody and feline CysC was relatively low based on Western blot analysis. 31 , 32 This questions the reliability of the PETIA and PENIA used in humans for measurement of sCysC in cats. Moreover, sCysC concentrations significantly decrease after storage for only 5 months at both −20°C and −72°C. 62
Age, sex, breed, and body weight do not affect sCysC concentrations in cats, 35 , 63 in contrast to humans and dogs. 64 , 65 No significant differences were found between pre‐ and post‐prandial sCysC concentrations in cats. 62 Hyperthyroidism increases sCysC concentrations in cats independent of decreased renal function. 24 , 36 However, feline immunodeficiency virus infection has no effect on sCysC concentrations. 24
Although initial studies indicated that CKD cats had significantly higher sCysC concentrations than healthy cats, there was overlap between groups. 31 , 35 , 58 Also, despite a significant negative correlation with GFR, sCysC concentrations were not different for different IRIS stages within the group of CKD cats. 35 The sensitivity of sCysC for the detection of decreased GFR was only 22%, and, in recent studies, sCysC concentrations could not differentiate CKD cats and healthy cats. 32 , 36 The correlation between GFR and sCysC was significantly weaker than that between GFR and sCr. 32 Additionally, sCysC could not predict the development of azotemia after treatment of hyperthyroid cats. 36 Overall, despite promising data in humans, sCysC is not a useful biomarker for renal function in cats.
3. BIOMARKERS OF METABOLIC DERANGEMENT
The decrease in phosphate excretion from diseased kidneys results in disturbances in the systemic calcium‐phosphate homeostasis characterized by increasing serum phosphate concentrations, stimulation of parathyroid hormone (PTH) secretion, and osteodystrophy. This systemic condition of calcium‐phosphate homeostasis disturbance caused by CKD is called chronic kidney disease‐mineral and bone disorder (CKD‐MBD). 66 Recently, knowledge of this metabolic derangement in cats with CKD has been improved by studying how the phosphaturic hormone, fibroblast growth factor‐23 (FGF‐23), behaves in CKD cats and geriatric cats (Table 1).
3.1. Fibroblast growth factor‐23
Fibroblast growth factor‐23 is a hormone responsible for the regulation of phosphorus and calcitriol produced by osteocytes and osteoblasts. 67 , 68 Both hyperphosphatemia and increased blood calcitriol (active vitamin D) concentrations stimulate FGF‐23 production, which promotes urinary phosphorus excretion and decreases intestinal phosphorus reabsorption. Urinary phosphorus excretion is increased by the inhibition of proximal tubular sodium‐phosphorus cotransporters, whereas intestinal phosphorus reabsorption is decreased by impaired renal calcitriol production. 69 , 70 , 71 The concentration of FGF‐23 also has been investigated as an early biomarker of CKD‐MBD in humans. 72 , 73 , 74 An increase in FGF‐23 concentration is an early abnormality detected in people with early CKD, preceding increased serum phosphate and PTH concentrations. 74 , 75 Also, FGF‐23 was negatively correlated with estimated GFR (eGFR) in human patients. 76
Different FGF‐23 ELISAs have been used in studies of cats. Initially, a commercial FGF‐23 sandwich ELISA designed for humans was validated to measure plasma FGF‐23 concentration in cats, with acceptable reproducibility, precision, and specificity. 37 Plasma (p)FGF‐23 concentrations were stable for at least 7 days at 22°C and for at least 14 days at −20°C, and 4 freeze‐thaw cycles did not influence its concentration. 37 Recently, a commercial ELISA designed to detect FGF‐23 in cats has been examined. 38
Cats with CKD and hyperphosphatemia have higher pFGF‐23 concentrations than those with normophosphatemia within the same IRIS stage. 37 In contrast, pFGF‐23 concentration is not correlated with phosphate concentration in cats with normal renal function. 77 , 78 It is still unclear whether this difference is specifically related to serum phosphorus concentration. Many other factors (eg, total calcium, magnesium, PTH, and PCV) also are associated with pFGF‐23 concentrations in CKD cats. 37 , 79 Hyperthyroidism seems to decrease pFGF‐23 concentrations in non‐azotemic cats, but its concentration cannot predict the development of azotemia after treatment. 39 Feeding a renal diet decreased pFGF‐23 concentrations in both hyper‐ and normo‐phosphatemic CKD cats. 80 Along with decreased pFGF‐23, significant changes in plasma phosphate and PTH concentrations also were found in the hyperphosphatemic group but not in the normophosphatemic group. 80 Thus, lower pFGF‐23 concentrations may signify improvement of phosphate derangement in CKD cats.
Several studies have suggested that FGF‐23 might be a promising early biomarker for phosphate derangement in CKD in cats. 37 , 38 , 77 , 78 , 80 , 81 Despite similar phosphate, PTH, and sCr concentrations, baseline pFGF‐23 concentrations were significantly higher in non‐azotemic geriatric cats that developed azotemia within 12 months than in cats with stable renal function. 77 Furthermore, pFGF‐23 was increased in non‐azotemic cats with SDMA concentrations >14 μg/dL despite an absence of hyperphosphatemia. 78 These findings indicate that FGF‐23 might have diagnostic potential for early detection of CKD and early phosphate derangement in cats and that phosphate dysregulation may be ongoing in the early stage of CKD before azotemia and hyperphosphatemia occur. Plasma FGF‐23 concentrations were also significantly higher in cats with azotemic CKD than in healthy cats, and these concentrations significantly increased with the severity of CKD. 37 , 38 Also, pFGF‐23 concentration was identified as independent predictor of CKD progression (>25% increase in sCr) within 12 months of diagnosis of CKD on a population basis. 79 , 81 However, the studies described are population‐based studies and overlap identified between groups indicates that it is difficult to extrapolate to an individual cat basis. 79 , 81 In these studies, overlap existed in pFGF‐23 concentrations between the different study groups despite significantly different pFGF‐23 concentrations between groups. 37 , 38 , 77 , 78 , 80 , 81
Overall, FGF‐23 is an earlier biomarker for phosphate derangement as compared to other routine biomarkers such as PTH and phosphate, and could identify CKD cats benefiting from dietary management in an earlier stage. Additional studies are needed to define the true diagnostic potential of pFGF‐23 concentration as marker of early phosphate derangement or marker of CKD progression in the individual cat presented in clinical practice.
4. KIDNEY INJURY BIOMARKERS IN CATS WITH CKD
Biomarkers of glomerular and tubular injury or dysfunction primarily are detected in urine. Glomerular biomarkers are either intermediate (IMW) or high molecular weight (HMW) proteins, the concentrations of which increase in urine when the glomerular barrier becomes more permeable because of glomerular damage. 29 Generally, most tubular biomarkers are LMW proteins, the urinary concentrations of which are low or undetectable when tubular function is normal. Tubular injury or dysfunction can cause defects in the resorptive capacity for these LMW proteins. Additionally, tubular cells can secrete some LMW proteins in response to direct tubular damage. 6 , 29 Tubulointerstitial nephritis commonly is found in CKD cats. 8 This explains why CKD cats usually have mild proteinuria, and mostly changes in tubular biomarkers are expected. On the other hand, marked proteinuria or markedly increased glomerular biomarkers are expected in primary glomerular disease, which is uncommon in cats, except for renal amyloidosis in predisposed cat breeds. 8 , 82 , 83
Other biomarkers also can be present in urine as a consequence of fibrosis or oxidative injury, or both reflecting the pathogenesis of CKD. 84 Urinary biomarker concentrations usually are corrected by determining their ratio to urinary creatinine concentrations to adjust for variation in urinary volume and concentration. 85 An overview of urinary renal biomarkers evaluated in CKD in cats based on their origin in the kidney is presented in Figure 1. Knowledge about urinary renal biomarkers in cats is summarized in Table 2.
FIGURE 1.
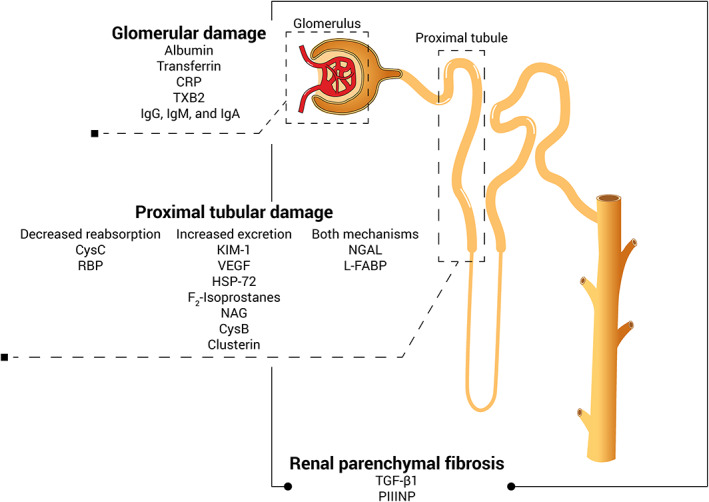
Overview of the urinary biomarkers in CKD in cats based on their origin. CRP, C‐reactive protein; CysB, cystatin B; CysC, cystatin C; HSP‐72, heat shock protein‐72; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; KIM‐1, kidney injury molecule‐1; L‐FABP, liver‐type fatty acid‐binding protein; NAG, N‐acetyl‐b‐d‐glucosaminidase; NGAL, neutrophil gelatinase‐associated lipocalin; PIIINP, procollagen type III amino‐terminal propeptide; RBP, retinol‐binding protein; TGF‐β1, transforming growth factor‐β1; TXB2, thromboxane B2; VEGF, vascular endothelial growth factor
TABLE 2.
Overview of urinary renal biomarkers in cats with CKD
| Biomarker | Origin | Type of molecule | Mechanism underlying increased levels | Validated assays in cats | Changes in cats with azotemic‐CKD | Non‐renal factors to consider |
|---|---|---|---|---|---|---|
| Biomarkers of glomerular damage | ||||||
| Albumin | Hepatocytes | IMW protein | Increased glomerular permeability |
Feline‐specific ELISA 20 Human PETIA 33 Electrophoresis 86 |
Insignificant 33 | Lower urinary tract disease; systemic disease 86 , 87 , 88 , 89 |
| Transferrin | Liver (primarily) and other tissues | IMW protein iron‐transporting protein | Increased glomerular permeability | SDS‐PAGE 90 | Increased 90 | |
| Biomarkers of tubular impairment | ||||||
| L‐FABP | Proximal tubular cells | LMW and fatty acid‐binding proteins | Increased tubular excretion | ELISA 91 | Increased 91 , 92 , 93 | Hyperthyroidism 91 |
| NGAL | Epithelial cells of proximal tubules, neutrophils and other tissues | LMW protein and glycoprotein | Decreased tubular reabsorption and increased tubular excretion | ELISA 91 , 94 | Increased 94 , 95 or insignificant 91 | Pyuria; systemic disease 95 |
| KIM‐1 | Proximal tubular cells | Renal tubular transmembrane glycoprotein | Decreased reabsorption and increased excretion | ELISA 96 | Not assessed | |
| VEGF | Renal proximal tubules | Signaling protein | Decreased production | ELISA 97 | Decreased 97 | Hyperthyroidism 98 |
| CysC | All nucleated cells | LMW protein and cysteine protease inhibitor | Decreased tubular reabsorption |
Human PENIA 31 |
Increased 32 or decreased 36 | Hyperthyroidism 24 |
| HSP72 | Renal tubular cells | Stress‐induced cytoprotective protein | Increased tubular excretion | ELISA 99 | Increased 99 | Urethral obstruction 99 |
| F2‐Isoprostanes | Kidney | Antioxidant | Increased production | ELISA 100 | Decreased 100 | Hyperthyroidism 101 |
| CysB | Proximal tubular cells | LMW protein and cysteine protease inhibitor | Ruptured and death of tubular epithelial cells | ELISA 27 | Not assessed | |
| Clusterin | Renal tubular cells | LMW protein and glycoprotein | Increased production | ELISA 27 | Not assessed | |
| Biomarkers of renal fibrosis | ||||||
| TGF‐β1 | Parenchymal and inflammatory cells | Cytokine and pro‐fibrotic mediator | Increased production | ELISA 102 , 103 | Increased 102 or insignificat 103 | |
| PIIINP | Collagen | Amino‐terminal propeptide of type III collagen | Decreased reabsorption | ELISA 104 | Increased 104 | |
Abbreviations: CysB cystatin B, CysC, cystatin C; HSP‐72, heat shock protein‐72; IMW, intermediate molecular weight; KIM‐1, kidney injury molecule‐1; L‐FABP, liver‐type fatty acid‐binding protein; LMW, low molecular weight; NGAL, neutrophil gelatinase‐associated lipocalin; PENIA, particle‐enhanced nephelometric immunoassay.
Other glomerular biomarkers such as immunoglobulins G, M, and A as well as C‐reactive protein and thromboxane B2 have been studied in dogs, but have not yet been investigated in cats. 29 , 105 Also, no recent information of other tubular biomarkers previously investigated in cats, namely urinary N‐acetyl‐b‐d‐glucosaminidase (NAG) and retinal‐binding proteins (RBP) have been published since previous reviews on this topic. 29 , 105 Therefore, these biomarkers will not be discussed in the present review.
4.1. Biomarkers of glomerular injury
4.1.1. Albumin
Albumin (ALB) is an IMW (65 kDa) protein produced by hepatocytes. In general, circulating ALB cannot freely pass through an intact glomerular barrier because its size exceeds the glomerular permeability threshold. Small quantities of ALB that pass through the glomerulus into tubular fluid are completely reabsorbed by proximal tubular cells. Consequently, healthy cats have <1 mg/dL of ALB in their urine (uALB). Thus, both glomerular and tubular dysfunction can result in albuminuria. 106 Microalbuminuria and overt albuminuria are defined as uALB concentrations between 1 to30 mg/dL and >30 mg/dL, respectively. 106 , 107 Albuminuria and increased uALB/Cr have been evaluated in AKI and CKD in dogs, 87 , 108 , 109 and microalbuminuria was associated with increased risk of death in dogs with critical conditions. 87
In the past, semi‐quantitative techniques including urine dipstick colorimetric testing and sulfosalicylic acid turbidity testing were used as screening tools for albuminuria in cats. However, their poor specificity for the detection of albuminuria in cats decreases the utility of these tests. 86 , 110 , 111 In recent years, more accurate techniques have been validated to quantify uALB concentration in cats, including a feline‐specific sandwich ELISA, a PETIA used in humans, and electrophoresis, all with acceptable precision and reproducibility. 20 , 33 , 86 , 90 However, the availability of these tests to general veterinary practitioners remains limited.
Several studies have demonstrated the diagnostic and prognostic value of uALB concentration for CKD in cats. Correlations between albuminuria and proteinuria were reported to be significant. 20 , 23 , 33 , 110 Similar to proteinuria, albuminuria was negatively associated with survival in a population of client‐owned cats with or without CKD. 20 However, guidelines to predict survival in an individual cat based on severity of albuminuria currently do not exist. Cats with CKD and higher uALB/Cr ratios had shorter survival times than those with lower uALB/Cr ratios. 20 Urinary ALB/Cr ratios in cats with stage 1 CKD were significantly higher (2‐fold) than in healthy cats, but unfortunately the study groups were not age‐matched with a younger healthy group being compared to the CKD cats. 90 The other study showed that uALB/Cr had poor diagnostic value when differentiating cats with azotemic CKD from healthy cats, but it tended to be higher in cats with azotemic CKD. 33 In a more recent study, uALB/Cr was shown to have high sensitivity and specificity for classifying the severity of proteinuria in cats with various diseases. This ratio could distinguish healthy from diseased cats, but it could not differentiate between CKD cats and cats with non‐renal diseases. 86 This result was supported by several studies in cats that detected microalbuminuria in a wide variety of non‐renal diseases, suggesting that albuminuria is not specific for renal diseases in cats. 23 , 87 , 88 , 89 , 110 Moreover, it has been suggested that the clinical importance of the uALB/Cr ratio has not yet surpassed that of the UPC, which is routinely available in commercial laboratories. 9 , 18 Therefore, the clinical implication of albuminuria in cats with renal diseases is unclear.
4.1.2. Transferrin
Transferrin is an ion‐binding glycoprotein synthesized in the liver and other tissues. Because its MW (77 kDa) is similar to that of ALB, transferrinuria is expected to be present when there is glomerular damage. In a study in humans, transferrinuria showed potential as a sensitive indicator of early diabetic nephropathy and a predictor of microalbuminuria development. 112 However, urinary transferrin concentration was not associated with the progression of renal disease in another study in humans. 113
A single study has investigated urinary transferrin concentrations in cats using sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). This study showed that cats with IRIS stage 1 CKD had higher urinary transferrin concentrations than did healthy cats. With a cutoff of 0.93 mg/dL, urinary transferrin concentration was more sensitive and specific than plasma creatinine concentration for the detection of early‐stage CKD. 90 Because of limited data availability, it is currently unknown whether increased urinary transferrin concentration reflects primarily tubulointerstitial nephritis or primary glomerular disease, taking into account that tubulointerstitial nephritis is the most common lesion in CKD cats.
4.2. Biomarkers of tubular injury or dysfunction
4.2.1. Neutrophil gelatinase‐associated lipocalin
Neutrophil gelatinase‐associated lipocalin (NGAL) is produced by neutrophils and epithelial cells in various tissues, including renal tubular cells. 114 It contributes to the innate immune response as a bacteriostatic agent and also aids in limiting damage after renal tubular injury. 115 Neutrophil gelatinase‐associated lipocalin is expressed in response to infection, inflammation, and neoplasia. 116 , 117 Circulating NGAL passes freely through the glomerular barrier and is reabsorbed in the proximal tubules. 115 , 118 Renal injury impairs tubular reabsorption of NGAL and NGAL is released from the damaged renal tubular cells. Consequently, an increased NGAL concentration in the serum or urine may reflect tubular injury. 119 , 120 , 121 In humans, both sNGAL and uNGAL concentrations appear to be promising biomarkers for the detection and prediction of AKI as well as CKD. 122 , 123 , 124 In dogs, sNGAL is prognostic for survival in dogs with CKD and the urinary NGAL : creatinine ratio (uNGAL/Cr) has been suggested to be a useful biomarker for the detection of early‐stage CKD and predicting CKD progression in dogs. 125 , 126 , 127 , 128
An in‐house sandwich ELISA using anti‐canine NGAL antibodies has been established for measuring pNGAL and uNGAL concentrations in cats, with acceptable precision and repeatability. 94 Recently, a commercial ELISA using anti‐human NGAL antibodies has been validated for sNGAL and uNGAL measurements in cats. 91 In humans, uNGAL remains stable for at least 96 hours at 4°C and at least 6 months at −80°C, slowly decreasing with storage at −80°C over 5 to 8 years. 129 Urinary NGAL concentrations in both humans and dogs decrease only slightly after 3 freeze‐thaw cycles. 6 , 130
The effect of biological status on NGAL concentrations has not been reported in cats, except for age, which does not influence uNGAL/Cr. 94 Circulating NGAL concentrations do not affect uNGAL concentrations in cats. 91 , 94 Urinary tract infections (UTI) and pyuria increase uNGAL concentrations in both dogs and cats. 95 , 131 Therefore, lower urinary tract disease or UTI should be ruled out before interpreting uNGAL concentration as a biomarker of renal disease in these species. Again, with respect to specificity, increased uNGAL/Cr ratios also have been found in cats with a variety of non‐renal diseases. 94 , 95 One study compared uNGAL/Cr among CKD cats, cats with AKI, and healthy cats. Higher uNGAL/Cr ratios were found in cats with CKD and AKI compared to healthy cats. The highest uNGAL/Cr ratios were detected in the AKI group, which probably was related to the more severe azotemia in that group compared to the CKD group. 95
To date, studies that evaluate NGAL as early renal biomarker in CKD cats are limited, and conflicting results have been found. In a study evaluating uNGAL concentration in 80 cats with azotemic CKD and 18 healthy cats, uNGAL/Cr ratio was significantly increased in the former group, particularly in cats with IRIS stages 3 and 4 CKD. 94 In contrast, a recent study evaluating 9 CKD cats and 44 healthy cats reported that uNGAL/Cr was not different between the groups. The reason for the discordant findings between these studies is unclear, but might be because of the different assays used. 91 The former study also assessed the prognostic utility of uNGAL concentrations in CKD cats and found higher baseline uNGAL/Cr concentrations in the group with progression compared to the group without progression. 94 Unfortunately, this finding must be interpreted cautiously because of important study limitations such as the different severity of azotemia between both groups at baseline and the short duration of follow‐up (30 days). 94 Additional studies are needed to determine whether uNGAL/Cr indeed can be used to predict progression of CKD in cats in clinical practice.
Similar to humans and dogs, NGAL in cats has 3 different molecular forms including monomeric (25 kDa), dimeric (45 kDa), and NGAL/matrix metalloproteinase‐9 (MMP9) complexes (135 kDa). The monomeric form is mainly detected in azotemic cats with AKI and late‐stage CKD (ie, stages 3 and 4). 95 Its presence may reflect advanced tubular injury or impairment, which may be useful as a prognostic factor in cats with AKI or CKD. The monomer was also the predominant form of uNGAL detected in a group of non‐pyuric cats with non‐renal diseases. 95 The monomeric form is valuable primarily to investigate the diagnostic potential of NGAL in AKI and as biomarker for progression of CKD. The dimeric form is predominantly present in cats with UTI and pyuria, emphasizing that UTI should be ruled out when interpreting uNGAL concentrations in cats. 95 Interestingly, the NGAL/MMP9 complex is commonly found in the urine of cats independent of their health status, which is a notable difference compared to humans and dogs. 95 , 132 , 133 , 134 This finding may infer that healthy cats physiologically express NGAL/MMP9 complex, or unknown pathological conditions are present in these cats.
Based on results of the available studies, uNGAL may relate more to the severity of azotemia or renal impairment rather than the type and progression of kidney disease. Also, the limitation of using non‐feline specific assays for NGAL measurements in cats must be considered.
4.2.2. Liver‐type fatty acid‐binding protein
Liver‐type fatty acid‐binding protein (L‐FABP) is a 14‐kDa protein located in the cytoplasm of hepatocytes and proximal tubular epithelial cells. 135 , 136 It also is expressed in the intestine, lung, and pancreas. 137 Circulating L‐FABP normally passes through the glomerular barrier and is reabsorbed in the proximal tubules. In the kidneys, L‐FABP plays a major role in fatty acid homeostasis, resulting in fatty acid catabolism that provides energy to proximal tubular cells. 136 , 138 Moreover, it has protective effects on the proximal tubules by transporting harmful lipid peroxidation products from the cytoplasm into the tubular lumen. 136 , 139 Ischemic injury and oxidative stress of the proximal tubules result in L‐FABP excretion into the urine in small laboratory animals and humans. 135 , 140 In the clinical setting, uL‐FABP/Cr ratio is a biomarker of early tubular damage that can reflect the severity of tubulointerstitial injury, detect AKI, and predict CKD progression in humans. 141 , 142 , 143 , 144 , 145 , 146 , 147 Furthermore, L‐FABP has been shown to be renoprotective in small laboratory animals. 148 , 149
A commercial feline L‐FABP ELISA based on cross‐reactivity with anti‐human L‐FABP has been used in research on cats and recently has been validated for feline serum and urine. 91 , 92 , 93 Human uL‐FABP was stable at 4°C for 48 hours and at −70°C for at least 18 months. 150 , 151 The stability of L‐FABP in samples from cats has not been reported.
In humans, other non‐renal factors are known to influence uL‐FABP concentrations. 141 , 143 , 152 , 153 , 154 , 155 , 156 In non‐diabetic human patients, age, sex, and serum cholesterol concentration are not associated with uL‐FABP/Cr, whereas this biomarker significantly increases in anemic patients. 154 Proteinuria, albuminuria, and hematuria are strongly associated with uL‐FABP/Cr, whereas pyuria has very little impact. 141 , 143 , 153 , 156 Liver disease significantly influences sL‐FABP concentrations but not uL‐FABP/Cr. Also, sL‐FABP concentration did not correlate with uL‐FABP/Cr in humans with CKD and liver disease. 152 Nevertheless, uL‐FABP/Cr was correlated with various liver enzymes in critically ill humans. 155 In cats, the current information about the effect of non‐renal factors on L‐FABP is limited. It is only known that uL‐FABP/Cr is significantly increased in hyperthyroid cats, and resolved after euthyroidism was established. 91 It remains unknown whether uL‐FABP concentration can predict post‐treatment azotemia in hyperthyroid cats because of a low number of post‐treatment azotemic cats in this study. 91
Currently, only 3 studies have evaluated L‐FABP in cats. 91 , 92 , 93 It was detected in the cytoplasm of proximal tubular cells in healthy feline kidneys using immunohistochemistry with antibodies targeting human L‐FABP, and it was expressed in the tubular lumen in response to ischemic‐reperfusion injury. 92 Most healthy control cats had uL‐FABP/Cr ratios below the limit of detection, 91 , 92 whereas this ratio increased in an AKI model in cats immediately after inducing ischemic‐reperfusion injury. 92 Although uL‐FABP/Cr was significantly increased in CKD cats compared to healthy cats, an overlap between groups was noted. 91 , 93 Urinary L‐FABP/Cr was high (>10 μg/g) in approximately 50% of cats with azotemic CKD and in only 7% of non‐azotemic cats. 93 Also, the cutoff of 0.97 μg/g was able to distinguish azotemic CKD cats from healthy cats with 100% sensitivity and 93% specificity. 91 Because uL‐FABP concentration appears to be a sensitive biomarker for tubular stress or injury, cats having increased uL‐FABP/Cr are thought to have active tubular stress or injury regardless of the presence of renal structural and functional changes. Higher uL‐FABP/Cr may indicate more advanced tubular injury or loss. Increased uL‐FABP/Cr in non‐azotemic cats might suggest increased odds for development of AKI or CKD in the future, but this possibility should be confirmed by additional studies. In particular, studies evaluating non‐renal factors affecting uL‐FABP/Cr in cats and prospective, longitudinal studies of non‐azotemic cats with increased uL‐FABP/Cr are lacking. Also, the reference interval for uL‐FABP/Cr needs to be established in a larger population of healthy cats.
4.2.3. Kidney injury molecule‐1
Kidney injury molecule‐1 (KIM‐1) is a type‐1 membrane glycoprotein. 157 It is a cell surface receptor in epithelial, lymphoid, and myeloid cells that scavenges oxidized circulating low‐density lipoproteins and membrane‐associated phosphatidylserine. 158 No KIM‐1 protein was detectable in the urine of healthy humans. 159 With renal tubular damage, KIM‐1 is upregulated in proximal tubular cells. 160 , 161 Several studies in humans have reported an association between uKIM‐1 and both AKI severity and rapid CKD progression. 159 , 160 , 161 , 162 , 163 , 164 In dogs, uKIM‐1 has been suggested as a promising biomarker for the detection of both naturally occurring AKI and CKD. 165 , 166
Feline KIM‐1 has been detected in urine using the commercially available anti‐rat KIM‐1 lateral flow immunoassay. 167 More recently, the same research group also developed a specific lateral flow assay for the detection of feline uKIM‐1. 96 In mice, uKIM‐1 is stable for 5 days at room temperature and at least for 1 year at −80°C, and multiple freeze–thaw cycles do not significantly alter uKIM‐1 concentrations. 168 The effects of storage time and temperature on uKIM‐1 concentrations in cat samples remain unknown.
Regarding the influence of non‐renal factors, age may positively affect uKIM‐1 concentrations in humans, 169 , 170 as might ethnic origin, with those of white European ancestry having higher uKIM‐1 concentrations than African Americans. 171 A difference in uKIM‐1 concentrations between the sexes in humans is debatable. 169 , 171 In humans, uKIM‐1 concentrations correlate with proteinuria severity but are unaffected by the presence of erythrocytes or leukocytes. 172 No studies have evaluated the influence of non‐renal factors on KIM‐1 concentrations in cats.
One research group performed 3 studies on uKIM‐1 as a biomarker for the detection of kidney disease in cats. The first investigated the presence of feline uKIM‐1 using the urine anti‐rat immunoassay. Kidney injury molecule‐1 was present in the urine of cats with critical conditions associated with AKI but was undetectable in all healthy cats. By immunohistochemistry, KIM‐1 localized specifically to proximal tubules in the outer medulla and in luminal cell debris in feline kidneys. Moreover, positive uKIM‐1 immunoassay results were consistent with KIM‐1 immunohistochemical staining and histopathological findings in kidney tissue sections. Nevertheless, 3 of 6 CKD cats had negative immunohistochemical staining results. The authors suggested that this apparent contradiction might have been associated with fibrotic changes and loss of the specific localization of KIM‐1 in renal tissue sections because of small biopsy sample size. 167 In their second study, the authors found that healthy cats had no positive KIM‐1 immunohistochemical staining, whereas cats with experimental and naturally occurring AKI showed increased expression of KIM‐1 in the proximal tubules. 173 Their recent third study showed an overlap between uKIM‐1 concentrations in cats with suspected AKI and healthy cats using an in‐house feline‐specific KIM‐1 immunoassay. The concentrations in cats with suspected AKI were highly variable, whereas healthy cats had low and non‐variable uKIM‐1 concentrations. Some cats (31%) with conditions associated with AKI had a substantial but transient increase in uKIM‐1. Only 1 CKD cat expressed uKIM‐1 at lower levels than healthy cats. 96 Overall, uKIM‐1 is highly expressed after early severe tubular injury but further tubular loss may result in negative results. Therefore, kinetic monitoring of this biomarker using serial sample analysis might be important in practice. Finally, KIM‐1 seems to have greater potential in the AKI setting compared to the CKD setting in cats, but further evaluation of uKIM‐1 in CKD cats is warranted.
4.2.4. Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is a signaling protein affecting angiogenesis. 174 It is expressed in renal proximal tubular cells in response to hypoxia. 175 In normal human kidneys, VEGF is constantly produced to maintain the glomerular and peritubular vasculature. 176 Urinary VEGF expression is downregulated in progressive CKD in humans. 176 , 177 , 178 , 179 Thus, uVEGF is a potential prognostic indicator for kidney function loss.
Very few studies on VEGF in CKD have been performed in cats. 97 , 98 , 180 The commercially available human VEGF ELISA cross‐reacts with its feline homolog and has been validated for the detection of feline uVEGF. 180 In CKD cats, uVEGF concentrations were significantly lower than in healthy cats, with considerable overlap between groups. The association between uVEGF/Cr and sCr was not significant in these CKD patients. 97 Hyperthyroid cats also had significantly higher uVEGF/Cr ratios than healthy cats. Upon treatment, uVEGF significantly decreased but it remained significantly higher than in healthy cats. Moreover, hyperthyroid cats with post‐treatment renal azotemia had lower uVEGF/Cr both pre‐ and post‐treatment compared to those that remained non‐azotemic after treatment. Additionally, uVEGF/Cr was negatively correlated with sCr but positively correlated with plasma total thyroxine concentrations, plasma renin activity, and UPC in hyperthyroid cats. 98 Consequently, increased uVEGF/Cr in hyperthyroid cats may not reflect renal dysfunction, whereas abnormal uVEGF/Cr may indicate decreased renal mass or function in hyperthyroid cats. 98 Overall, the higher uVEGF/Cr in non‐azotemic compared to CKD cats may indicate a renoprotective effect of VEGF in healthy kidneys, and a low uVEGF/Cr in healthy cats may indicate early CKD. 97 Based on these findings, the exact role of VEGF in the feline kidney remains unclear and additional studies are required to determine the diagnostic and prognostic value of uVEGF in early CKD in cats.
4.2.5. Urinary cystatin C
Serum CysC concentration was discussed in Section 2.1.1 as a GFR biomarker. However, tubular renal damage decreases tubular reabsorption of CysC, resulting in increased excretion. 181 Therefore, uCysC concentration has been suggested as a promising biomarker for the assessment of renal tubular function in humans and dogs. In humans, uCysC/Cr was found to be useful as a tubular damage biomarker in patients with CKD. 181 In dogs, uCysC/Cr can be used to distinguish dogs with renal disease from dogs with non‐renal disease. 182
The anti‐human‐based PETIA and PENIA kits have been validated for uCysC in cats, although the former has poor repeatability in the lower concentration range. 31 , 32 , 33 In humans, uCysC is stable at both −20 and 4°C for 7 days and even at 20°C for 48 hours. 183 Urinary CysC concentration is increased in humans with proteinuria, but the effect of proteinuria on uCysC concentration has not been studied in cats. 184 Urinary CysC/Cr ratios were significantly increased in hyperthyroid cats but not in cats with diabetic mellitus. 24 , 185
One study found that uCysC/Cr was significantly higher in CKD cats compared to healthy cats. Nevertheless, 34% of CKD cats had undetectable uCysC concentrations vs 88% of healthy cats. 32 A more recent study observed that uCysC/Cr was significantly lower in CKD cats compared to healthy cats. 33 These different findings among studies might be a consequence of different CKD stages of the cats included or different analytical methods used. In the former study, CKD cats had more advanced disease and higher median UPC than those in the latter study. 32 , 33 Moreover, the usefulness of uCysC/Cr as a screening test to distinguish azotemic CKD cats from healthy cats was not better than USG. 33 These poor clinical correlations may be a result of the disadvantage of the current assays such as sCysC. Therefore, with the currently available assays, uCysC does not seem to be a reliable biomarker for detection of early CKD in cats.
4.2.6. Heat shock protein‐72
Heat shock protein‐72 (HSP‐72) is a stress‐induced HSP isoform, which plays a role in protecting cell and protein structure upon cellular insult and stress conditions. 186 , 187 It is synthesized by a variety of cells including renal tubular cells. 188 In children undergoing renal transplantation, urinary HSP72 excretion was substantially increased during the early post‐transplant period. 189 In dogs, uHSP72/Cr was shown to be a potential renal biomarker for AKI. 190
A commercial HSP72 ELISA kit for cats has been validated by its manufacturer and used in a recent study in cats. 99 Urinary HSP72 concentrations were higher in cats with AKI, CKD, urethral obstruction, and acute‐on‐chronic kidney disease. Furthermore, uHSP72/Cr could distinguish cats with AKI from healthy cats with 94% sensitivity and 70% specificity using a cutoff of 0.54 ng/mg. However, few cats in the early stages of disease were included, as only 2 of 16 AKI cats had AKI grade 1, and 3 of 15 CKD cats were IRIS stage 1. Also, an overlap was noticed in uHSP72/Cr between CKD and healthy cats. Thus, the clinical value of HSP72 to detect early AKI and CKD in cats remains uncertain and additional investigations in a larger group of cats are warranted. Moreover, non‐renal factors affecting HSP72 in cats have not been studied.
4.2.7. F2 ‐isoprostanes
F2‐isoprostanes are prostaglandin‐like metabolites synthesized locally in the kidney through peroxidation of the common precursor arachidonic acid by glomerular endothelial and mesangial cells. 191 These lipids are reliable indicators of oxidative injury and lipid peroxidation. 192 Oxidative stress can induce tubulointerstitial injury and fibrosis. 193 , 194 Plasma F2‐isoprostane concentrations were increased in human CKD patients, 195 , 196 , 197 , 198 whereas uF2‐isoprostane concentrations in humans with CKD were lower than in healthy humans. 199 Another study demonstrated that uF2‐isoprostane concentrations correlated positively with eGFR in elderly men. 200
Affinity column purification followed by a competitive ELISA has been used to quantify uF2‐isoprostane concentrations in cats. This method weakly correlates with gas chromatography/mass spectrometry, which remains the gold standard for isoprostane measurement. 201 Thus, the validity of the data assessed using the ELISA method remains doubtful. Hypertension and proteinuria were not associated with uF2‐isoprostane concentrations in cats. 100 Urinary F2‐isoprostane concentrations were, however, increased in hyperthyroid cats compared to healthy cats and normalized after treatment. 101
Although cats with stage 1 CKD had higher uF2‐isoprostane concentrations than healthy cats, this difference was not significant. The authors suggested that this finding might be a consequence of insufficient statistical power when comparing CKD stage 1 cats and healthy cats. With advancing CKD stage (stages 3 and 4), uF2‐isoprostane concentrations decreased significantly compared to concentrations in stage 1 CKD and healthy cats. No correlation was observed between uF2‐isoprostane and sCr concentrations. 100 Despite several limitations, these findings suggest that oxidative stress may be transiently active in cats with stage 1 CKD, and may be a diagnostic target for early detection of CKD. Because of limited information and several limitations, further study to demonstrate the potential of uF2‐isoprostane concentration as an early renal biomarker in CKD cats is less compelling compared to other renal biomarkers.
4.2.8. Others
Novel biomarkers in veterinary medicine are cystatin B (CysB) and urinary clusterin. Cystatin B is a low‐molecular weight protein (11 kDA) that functions to inhibit members of the cysteine proteases (family 1). Cystatin B is mainly an intracellular protein with limited concentration in the circulation. 27 Clusterin is a glycoprotein (70‐80 kDA) produced in various tissues during several physiological and pathological processes. 27 , 202 , 203 Normally, clusterin is found in very low concentration in urine. Urinary clusterin (uClust) concentration is increased during tubular injury in dogs. 204 , 205 In people, uClust is part of renal biomarker panels for drug development and toxicity. 27 For both markers, concentrations can be determined in dogs and cats using sandwich immunoassays. 27 To accurately detect active tubular injury in samples from dogs and cats, the kidney‐specific isoform of clusterin is measured. 27
Studies have focused on the diagnostic potential of these markers in dogs and cats with AKI. Urinary CysB is increased in dogs with AKI and is shown not to be affected by UTI. 27 , 206 Similarly, an abstract reported that cats with AKI had significantly higher uCysB concentrations than healthy cats and that uCysB can predict AKI with 90% sensitivity and 92% specificity. 207 In dogs, uClust concentration is increased in AKI. 27 , 206 , 208
The only study evaluating uCysB and uClust concentrations in CKD cats did not find a difference in the concentrations of those and other renal biomarkers between cats treated with low‐dose meloxicam and cats treated with placebo. 209 Current scientific information in cats is too limited to draw conclusions on the clinical utility of CysB and clusterin to diagnose CKD in cats.
4.3. Biomarkers of renal fibrosis
The most common histopathological diagnosis in CKD in cats is interstitial inflammation and fibrosis. 210 Among the histopathological findings in CKD in cats, interstitial fibrosis is most closely associated with azotemia severity, hyperphosphatemia, anemia, and proteinuria. 8 These findings suggest that renal fibrosis and its mediators play crucial roles in CKD pathogenesis. Renal biomarkers indicating renal fibrosis therefore may be of benefit for the early detection and treatment of CKD in cats.
4.3.1. Transforming growth factor‐β1
Transforming growth factor‐β1 (TGF‐β1) is a multifunctional cytokine that potentially plays a role as a profibrotic mediator activating tissue fibrosis. 211 It is produced by parenchymal and inflammatory cells in several organs and especially the kidneys. 212 , 213 , 214 , 215 , 216 In the kidneys, TGF‐β1 initially is released in an inactive form in response to various renal insults such as oxidative stress, hypoxia, proteinuria, and products of the renin‐angiotensin‐aldosterone system. 217 , 218 , 219 , 220 Inactive TGF‐β1 then must be altered to its active form by proteolytic cleavage before promoting renal fibrosis. 221 , 222 The profibrotic effect of TGF‐β1 consists of myofibroblast formation, which promotes extracellular matrix production, decreased extracellular matrix degradation, and tubular injury and cellular apoptosis. 84 , 223 , 224 , 225 , 226 It also has an important role in tissue homeostasis, recruiting stem cells in tissue reparative processes. 227 Increased urinary TGF‐β1 (uTGF‐β1) expression is found in rodents and humans with kidney disease related to interstitial fibrosis. 228 , 229 Overall, studies in humans have shown significant upregulation of uTGF‐β1 expression in patients with glomerular diseases. 228 , 229 , 230 Urinary TGF‐β1 concentrations were significantly correlated with the severity of interstitial fibrosis in renal biopsy samples but did not correlate with sCr and GFR. 228 , 230
In CKD cats, a commercial multispecies total TGF‐β1 ELISA has been used to measure total urinary TGF‐β1 concentrations in cats. However, no validation data have been reported for feline urine using this ELISA. 97 , 102 A commercial active TGF‐β1 (aTGF‐β1) ELISA for humans, which detected an active form of TGF‐β1 in urine, later was validated for cats with acceptable precision and reproducibility. 103
In rodent models, renal interstitial fibrosis was associated with aging, resulting in increased TGF‐β1 expression in the kidneys. 231 However, it is unknown whether this phenomenon occurs in cats. Proteinuria may influence uaTGF‐β1 concentrations, because significant correlation was found between log UPC and uaTGF‐β1 : creatinine ratio (uaTGF‐β1/Cr). 103
Few studies have evaluated TGF‐β1 for assessing renal fibrosis in CKD cats. Cats with CKD had higher urinary total TGF‐β1 concentrations than did healthy cats, whereas serum TGF‐β1 concentration was not different between groups. 97 , 102 Urinary total TGF‐β1/Cr also was positively correlated with sCr concentrations. 97 In contrast, another study was unable to measure total TGF‐β1 concentrations in feline urine using the same ELISA. 103 Moreover, baseline uaTGF‐β1/Cr ratios were not different between CKD cats and healthy cats, suggesting that CKD cats might not express more uaTGF‐β1 than healthy cats. 103 However, non‐azotemic cats that later developed renal azotemia had a 2.6‐fold increase in uaTGF‐β1/Cr from baseline approximately 6 months before azotemia was detected. These results suggest that rather than sampling at a single time point, serial measurements in non‐azotemic geriatric cats of uaTGF‐β1/Cr may be useful to predict azotemic CKD later in life. 103 Additionally, uaTGF‐β1/Cr moderately correlated with the severity of renal interstitial fibrosis in this histopathologic study. 103 In vitro, TGF‐β1 induced the expression of genes associated with the pathogenesis of renal fibrosis in the proximal tubular epithelial cells of cats. 232 These findings support that TGF‐β1 might be involved in the pathogenesis of CKD in cats by inducing pro‐fibrotic factors related to renal fibrosis, and uaTGF‐β1 expression may reflect renal fibrosis severity in cats. Overall, TGF‐β1 may be promising to identify the likelihood of development of azotemic CKD in non‐azotemic geriatric cats.
4.3.2. Procollagen type III amino‐terminal propeptide
Procollagen type III amino‐terminal propeptide (PIIINP), a LMW (44 kDa) peptide, is cleaved and released from collagen into extracellular fluids including blood during the synthesis and deposition of type III collagen. Circulating PIIINPs can pass through glomeruli and are reabsorbed by proximal tubular cells. 233 In the absence of renal damage, only a small amount of PIIINP can be detected in human urine. However, urinary PIIINP expression is increased in renal fibrosis. 234 This molecule has been studied for its utility as a novel biomarker of fibrosis in various organs such as lung, heart, liver, and kidney. Many studies in humans and dogs have shown that plasma PIIINP concentrations are increased in patients with diseases characterized by fibrosis. 235 , 236 , 237 , 238 , 239 The urinary PIIINP : creatinine ratio (uPIIINP/Cr) is negatively correlated with eGFR and positively associated with the progression of CKD and severity of renal fibrosis in humans. 240 , 241 , 242 , 243
A single study has evaluated PIIINP in small groups of cats using a sandwich ELISA. No validation data of the ELISA was reported. The study showed significantly increased uPIIINP/Cr in cats with azotemic CKD compared with healthy cats. The uPIIINP/Cr ratio also was highly correlated with renal parenchyma stiffness as determined by ultrasonic shear‐wave ultrasonography. 104 Currently, information of PIIINP in cats remains scarce. Additional studies using validated assays are required to establish whether PIIINP is a promising biomarker for CKD in cats.
5. CONCLUSIONS
Several biomarkers have shown value or are promising in the diagnostic evaluation of CKD in cats. Rather than detecting decreased GFR, the true power of these biomarkers lies in their other roles, especially the ability to identify acute kidney stress or injury or active pathological changes. Several renal biomarkers show potential for early detection or for determining progression in CKD in cats. Taking these characteristics into account, longitudinal monitoring of these biomarkers may be superior to individual measurements for the determination of the renal status of individual cats.
Before any of these renal biomarkers can be used in clinical practice, steps of validation are needed. First, for the concentration of a biomarker to be valuable in samples from cats, thorough analytical validation of available assays is needed. Second, biological validation is needed by establishing reference intervals in healthy cats and by assessing the potential influence of biological factors. Third, it is important to evaluate whether the biomarkers can reliably differentiate the renal status of the cats (ie, non‐azotemic healthy vs non‐azotemic early CKD vs azotemic CKD), which represents clinical validation. Choosing the decision threshold of certain biomarkers also is required in this step. The true diagnostic value of biomarkers will depend on the combined analytical, biological, and clinical validation. Also, it is important to evaluate whether the additional information provided by the biomarkers results in additional benefits for the diagnosis and clinical‐decision making in cats with CKD. Unfortunately, most of the biomarkers discussed here are not yet fully validated. Important limitations to assess the clinical utility of renal biomarkers in living cats are the difficulty to correlate biomarker concentrations with pathological changes within the kidney and the fact that most of the current data result from cross‐sectional studies. Additional investigations still are needed before determining the usefulness of these biomarkers in clinical practice, preferably by performing longitudinal studies. Also, for daily use in the clinic, easily accessible, reliable, and inexpensive assays should become available for the most promising biomarkers.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Thirawut Kongtasai is supported by a grant from Mahidol University, Thailand, for studying the doctoral program at Ghent University.
Kongtasai T, Paepe D, Meyer E, et al. Renal biomarkers in cats: A review of the current status in chronic kidney disease. J Vet Intern Med. 2022;36(2):379‐396. doi: 10.1111/jvim.16377
Funding information Mahidol University
REFERENCES
- 1. Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med A. 1999;214:1336‐1341. [PubMed] [Google Scholar]
- 2. O'Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary‐care veterinary practices in England. Vet J. 2014;202:286‐291. [DOI] [PubMed] [Google Scholar]
- 3. Jepson RE, Brodbelt D, Vallance C, Syme HM, Elliott J. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med. 2009;23:806‐813. [DOI] [PubMed] [Google Scholar]
- 4. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg. 2014;16:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall JA, Fritsch DA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. A longitudinal study on the acceptance and effects of a therapeutic renal food in pet dogs with IRIS‐stage 1 chronic kidney disease. J Anim Physiol Anim Nutr. 2018;102:297‐307. [DOI] [PubMed] [Google Scholar]
- 6. Cowgill LD, Polzin DJ, Elliott J, et al. Is progressive chronic kidney disease a slow acute kidney injury? Vet Clin North Am Small Anim Pract. 2016;46:995‐1013. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez‐Iturbe B, Garcia G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin Pract. 2010;116:c81‐c88. [DOI] [PubMed] [Google Scholar]
- 8. Chakrabarti S, Syme HM, Brown CA, Elliott J. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol. 2013;50:147‐155. [DOI] [PubMed] [Google Scholar]
- 9. Paepe D, Daminet S. Feline CKD: diagnosis, staging and screening—what is recommended? J Feline Med Surg. 2013;15 (Suppl 1):15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sparkes AH, Caney S, Chalhoub S, et al. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J Feline Med Surg. 2016;18:219‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:78‐85. [DOI] [PubMed] [Google Scholar]
- 12. Graham PA. Urinalysis. In: Ettinger S, Feldman E, Cote E, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 8th ed. St. Louis, Missouri: Elsevier; 2017:283‐288. [Google Scholar]
- 13. Finch N. Measurement of glomerular filtration rate in cats: methods and advantages over routine markers of renal function. J Feline Med Surg. 2014;16:736‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barthez PY, Chew DJ, DiBartola SP. Simplified methods for estimation of 99mTc‐pentetate and 131I‐orthoiodohippurate plasma clearance in dogs and cats. J Vet Intern Med. 2001;15:200‐208. [DOI] [PubMed] [Google Scholar]
- 15. Katayama M, Saito J, Katayama R, et al. A single‐blood‐sample method using inulin for estimating feline glomerular filtration rate. J Vet Intern Med. 2013;27:17‐21. [DOI] [PubMed] [Google Scholar]
- 16. Finch NC, Heiene R, Elliott J, Syme HM, Peters AM. A single sample method for estimating glomerular filtration rate in cats. J Vet Intern Med. 2013;27:782‐790. [DOI] [PubMed] [Google Scholar]
- 17. Paepe D, Lefebvre HP, Concordet D, Van Hoek I, Croubels S, Daminet S. Simplified methods for estimating glomerular filtration rate in cats and for detection of cats with low or borderline glomerular filtration rate. J Feline Med Surg. 2015;17:889‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Syme HM. Proteinuria in cats. Prognostic marker or mediator? J Feline Med Surg. 2009;11:211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grauer GF. Proteinuria: measurement and interpretation. Top Companion Anim M. 2011;26:121‐127. [DOI] [PubMed] [Google Scholar]
- 20. Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med. 2006;20:528‐535. [DOI] [PubMed] [Google Scholar]
- 21. Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000‐2002). J Vet Intern Med. 2008;22:1111‐1117. [DOI] [PubMed] [Google Scholar]
- 22. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26:275‐281. [DOI] [PubMed] [Google Scholar]
- 23. Mardell EJ, Sparkes AH. Evaluation of a commercial in‐house test kit for the semi‐quantitative assessment of microalbuminuria in cats. J Feline Med Surg. 2006;8:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghys LFE, Paepe D, Taffin ERL, et al. Serum and urinary cystatin C in cats with feline immunodeficiency virus infection and cats with hyperthyroidism. J Feline Med Surg. 2016;18:658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall JA, MacLeay J, Yerramilli M, et al. Positive impact of nutritional interventions on serum symmetric dimethylarginine and creatinine concentrations in client‐owned geriatric cats. PloS One. 2016;11:e0153654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quimby JM. Searching for biomarkers in feline chronic kidney disease: a new frontier. Vet J. 2015;206:3‐4. [DOI] [PubMed] [Google Scholar]
- 27. Yerramilli M, Farace G, Quinn J, Yerramilli M. Kidney disease and the nexus of chronic kidney disease and acute kidney injury: the role of novel biomarkers as early and accurate diagnostics. Vet Clin North Am Small Anim Pract. 2016;46:961‐993. [DOI] [PubMed] [Google Scholar]
- 28. Katz N, Ronco C. Acute kidney stress—a useful term based on evolution in the understanding of acute kidney injury. Crit Care. 2016;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hokamp JA, Nabity MB. Renal biomarkers in domestic species. Vet Clin Pathol. 2016;45:28‐56. [DOI] [PubMed] [Google Scholar]
- 30. Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28:1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghys LF, Meyer E, Paepe D, et al. Analytical validation of a human particle‐enhanced nephelometric assay for cystatin C measurement in feline serum and urine. Vet Clin Pathol. 2014;43:226‐234. [DOI] [PubMed] [Google Scholar]
- 32. Ghys LF, Paepe D, Lefebvre HP, et al. Evaluation of cystatin C for the detection of chronic kidney disease in cats. J Vet Intern Med. 2016;30:1074‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams TL, Archer J. Evaluation of urinary biomarkers for azotaemic chronic kidney disease in cats. J Small Anim Pract. 2016;57:122‐129. [DOI] [PubMed] [Google Scholar]
- 34. Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc. 2008;44:131‐138. [DOI] [PubMed] [Google Scholar]
- 35. Poświatowska‐Kaszczyszyn I. Usefulness of serum cystatin C measurement for assessing renal function in cats. Bull Vet Inst Pulawy. 2012;56:235. [Google Scholar]
- 36. Williams TL, Dillon H, Elliott J, Syme HM, Archer J. Serum cystatin C concentrations in cats with hyperthyroidism and chronic kidney disease. J Vet Intern Med. 2016;30:1083‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27:234‐241. [DOI] [PubMed] [Google Scholar]
- 38. Liao YL, Chou CC, Lee YJ. The association of indoxyl sulfate with fibroblast growth factor‐23 in cats with chronic kidney disease. J Vet Intern Med. 2019;33:686‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract. 2012;53:561‐571. [DOI] [PubMed] [Google Scholar]
- 40. Baral RM, Dhand NK, Freeman KP, Krockenberger MB, Govendir M. Biological variation and reference change values of feline plasma biochemistry analytes. J Feline Med Surg. 2014;16:317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reynolds BS, Concordet D, Germain CA, Daste T, Boudet KG, Lefebvre HP. Breed dependency of reference intervals for plasma biochemical values in cats. J Vet Intern Med. 2010;24:809‐818. [DOI] [PubMed] [Google Scholar]
- 42. Reynolds BS, Brosse C, Jeunesse E, Concordet D, Lefebvre HP. Routine plasma biochemistry analytes in clinically healthy cats: within‐day variations and effects of a standard meal. J Feline Med Surg. 2015;17:468‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boozer L, Carter L, Sheldon S, et al. Lack of utility of laboratory “normal” ranges for serum creatinine concentration for the diagnosis of feline chronic renal insufficiency. J Vet Intern Med. 2002;16:354. [Google Scholar]
- 44. Ulleberg T, Robben J, Nordahl KM, Ulleberg T, Heiene R. Plasma creatinine in dogs: intra‐ and inter‐laboratory variation in 10 European veterinary laboratories. Acta Vet Scand. 2011;53:25‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braff J, Obare E, Yerramilli M, Elliott J, Yerramilli M. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med. 2014;28:1699‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract. 2016;46:941‐960. [DOI] [PubMed] [Google Scholar]
- 47. Hall JA, Yerramilli M, Obare E, Yerramilli M, Yu S, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J. 2014;202:588‐596. [DOI] [PubMed] [Google Scholar]
- 48. Paltrinieri S, Giraldi M, Prolo A, et al. Serum symmetric dimethylarginine and creatinine in Birman cats compared with cats of other breeds. J Feline Med Surg. 2018;20:905‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Langhorn R, Kieler IN, Koch J, Christiansen LB, Jessen LR. Symmetric dimethylarginine in cats with hypertrophic cardiomyopathy and diabetes mellitus. J Vet Intern Med. 2018;32:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buresova E, Stock E, Paepe D, et al. Assessment of symmetric dimethylarginine as a biomarker of renal function in hyperthyroid cats treated with radioiodine. J Vet Intern Med. 2019;33:516‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. IRIS (International Renal InterestSociety) : IRIS Staging of CKD (modified 2019). http://www.iris-kidney.com/pdf/IRIS_Staging_of_CKD_modified_2019.pdf. Accessed February 20, 2021. http://iris-kidneycom/guidelines/staging.html
- 52. Kovarikova S. Indirect markers of glomerular filtration rate in dogs and cats: a review. Vet Med. 2018;63:395‐412. [Google Scholar]
- 53. Sargent HJ, Elliott J, Jepson RE. The new age of renal biomarkers: does SDMA solve all of our problems? J Small Anim Pract. 2021;62:71‐81. [DOI] [PubMed] [Google Scholar]
- 54. Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J. 1990;268:287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Inv. 1996;56:409‐414. [DOI] [PubMed] [Google Scholar]
- 56. Kaseda R, Iino N, Hosojima M, et al. Megalin‐mediated endocytosis of cystatin C in proximal tubule cells. Biochem Biopl Res co. 2007;357:1130‐1134. [DOI] [PubMed] [Google Scholar]
- 57. Seronie‐Vivien S, Delanaye P, Pieroni L, et al. Cystatin C: current position and future prospects. Clin Chem Lab Med. 2008;46:1664‐1686. [DOI] [PubMed] [Google Scholar]
- 58. Almy FS, Christopher MM, King DP, Brown SA. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J Vet Intern Med. 2002;16:45‐51. [DOI] [PubMed] [Google Scholar]
- 59. Miyagawa Y, Takemura N, Hirose H. Evaluation of the measurement of serum cystatin C by an enzyme‐linked immunosorbent assay for humans as a marker of the glomerular filtration rate in dogs. J Vet Med Sci. 2009;71:1169‐1176. [DOI] [PubMed] [Google Scholar]
- 60. Ghys L, Paepe D, Smets P, Lefebvre H, Delanghe J, Daminet S. Cystatin C: a new renal marker and its potential use in small animal medicine. J Vet Intern Med. 2014;28:1152‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghys LF, Paepe D, Lefebvre HP, et al. The effect of feeding, storage and anticoagulant on feline serum cystatin C. Vet J. 2015;206:91‐96. [DOI] [PubMed] [Google Scholar]
- 63. Ghys LF, Paepe D, Duchateau L, et al. Biological validation of feline serum cystatin C: the effect of breed, age and sex and establishment of a reference interval. Vet J. 2015;204:168‐173. [DOI] [PubMed] [Google Scholar]
- 64. Galteau MM, Guyon M, Gueguen R, Siest G. Determination of serum cystatin C: biological variation and reference values. Clin Chem Lab Med. 2001;39:850‐857. [DOI] [PubMed] [Google Scholar]
- 65. Braun J‐P, Perxachs A, Pe'Chereau D, et al. Plasma cystatin C in the dog: reference values and variations with renal failure. Comp Clin Path. 2002;11:44‐49. [Google Scholar]
- 66. Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945‐1953. [DOI] [PubMed] [Google Scholar]
- 67. Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419‐37426. [DOI] [PubMed] [Google Scholar]
- 68. Pereira RC, Juppner H, Azucena‐Serrano CE, et al. Patterns of FGF‐23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF‐23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Min Res. 2004;19:429‐435. [DOI] [PubMed] [Google Scholar]
- 70. Saito H, Maeda A, Ohtomo S, et al. Circulating FGF‐23 is regulated by 1alpha,25‐dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543‐2549. [DOI] [PubMed] [Google Scholar]
- 71. Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637‐1647. [DOI] [PubMed] [Google Scholar]
- 72. Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF‐23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272‐2279. [DOI] [PubMed] [Google Scholar]
- 73. Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor‐23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant. 2010;25:993‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Filler G, Liu D, Huang S‐HS, Casier S, Chau LA, Madrenas J. Impaired GFR is the most important determinant for FGF‐23 increase in chronic kidney disease. Clin Biochem. 2011;44:435‐437. [DOI] [PubMed] [Google Scholar]
- 77. Finch NC, Geddes RF, Syme HM, Elliott J. Fibroblast growth factor 23 (FGF‐23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med. 2013;27:227‐233. [DOI] [PubMed] [Google Scholar]
- 78. Sargent HJ, Jepson RE, Chang YM, Biourge VC, Bijsmans ES, Elliott J. Fibroblast growth factor 23 and symmetric dimethylarginine concentrations in geriatric cats. J Vet Intern Med. 2019;33:2657‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van den Broek DHN, Chang Y‐M, Elliott J, Jepson RE. Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. J Vet Intern Med. 2018;32:1359‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med. 2013;27:1354‐1361. [DOI] [PubMed] [Google Scholar]
- 81. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29:1494‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paltrinieri S, Sironi G, Giori L, Faverzani S, Longeri M. Changes in serum and urine SAA concentrations and qualitative and quantitative proteinuria in Abyssinian cats with familial amyloidosis: a five‐year longitudinal study (2009‐2014). J Vet Intern Med. 2015;29:505‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rossi F, Aresu L, Martini V, et al. Immune‐complex glomerulonephritis in cats: a retrospective study based on clinico‐pathological data, histopathology and ultrastructural features. BMC Vet Res. 2019;15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lawson J, Elliott J, Wheeler‐Jones C, Syme H, Jepson R. Renal fibrosis in feline chronic kidney disease: known mediators and mechanisms of injury. Vet J. 2015;203:18‐26. [DOI] [PubMed] [Google Scholar]
- 85. Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ferlizza E, Dondi F, Andreani G, Bucci D, Archer J, Isani G. Validation of an electrophoretic method to detect albuminuria in cats. J Feline Med Surg. 2017;19:860‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vaden SL, Turman CA, Harris TL, Marks SL. The prevalence of albuminuria in dogs and cats in an ICU or recovering from anesthesia. J Vet Emerg Crit Care (San Antonio). 2010;20:479‐487. [DOI] [PubMed] [Google Scholar]
- 88. Whittemore JC, Miyoshi Z, Jensen WA, Radecki SV, Lappin MR. Association of microalbuminuria and the urine albumin‐to‐creatinine ratio with systemic disease in cats. J Am Vet Med Assoc. 2007;230:1165‐1169. [DOI] [PubMed] [Google Scholar]
- 89. Al‐Ghazlat SA, Langston CE, Greco DS, et al. The prevalence of microalbuminuria and proteinuria in cats with diabetes mellitus. Top Companion Anim Med. 2011;26:154‐157. [DOI] [PubMed] [Google Scholar]
- 90. Maeda H, Sogawa K, Sakaguchi K, et al. Urinary albumin and transferrin as early diagnostic markers of chronic kidney disease. J Vet Med Sci. 2015;77:937‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kongtasai T, Meyer E, Paepe D, et al. Liver‐type fatty acid‐binding protein and neutrophil gelatinase‐associated lipocalin in cats with chronic kidney disease and hyperthyroidism. J Vet Intern Med. 2021;35:1376‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Katayama M, Miyazaki T, Ohata K, et al. Temporal changes in urinary excretion of liver‐type fatty acid binding protein (L‐FABP) in acute kidney injury model of domestic cats: a preliminary study. J Vet Med Sci. 2019;81:1868‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Katayama M, Ohata K, Miyazaki T, et al. Renal expression and urinary excretion of liver‐type fatty acid‐binding protein in cats with renal disease. J Vet Intern Med. 2020;34:761‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang IC, Hsu WL, Wu PH, Yin HY, Tsai HJ, Lee YJ. Neutrophil gelatinase‐associated lipocalin in cats with naturally occurring chronic kidney disease. J Vet Intern Med. 2017;31:102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu PH, Hsu WL, Tsai PJ, et al. Identification of urine neutrophil gelatinase‐associated lipocalin molecular forms and their association with different urinary diseases in cats. BMC Vet Res. 2019;15:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bland SK, Clark ME, Côté O, Bienzle D. A specific immunoassay for detection of feline kidney injury molecule 1. J Feline Med Surg. 2019;21(12):1069‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Habenicht LM, Webb TL, Clauss LA, Dow SW, Quimby JM. Urinary cytokine levels in apparently healthy cats and cats with chronic kidney disease. J Feline Med Surg. 2013;15:99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Williams TL, Elliott J, Syme HM. Association between urinary vascular endothelial growth factor excretion and chronic kidney disease in hyperthyroid cats. Res Vet Sci. 2014;96:436‐441. [DOI] [PubMed] [Google Scholar]
- 99. Chen H, Avital Y, Bruchim Y, Aroch I, Segev G. Urinary heat shock protein‐72: a novel marker of acute kidney injury and chronic kidney disease in cats. Vet J. 2019;243:77‐81. [DOI] [PubMed] [Google Scholar]
- 100. Whitehouse W, Quimby J, Wan S, Monaghan K, Robbins R, Trepanier LA. Urinary F2 ‐Isoprostanes in cats with international renal interest society stage 1‐4 chronic kidney disease. J Vet Intern Med. 2017;31:449‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Branter E, Drescher N, Padilla M, Trepanier LA. Antioxidant status in hyperthyroid cats before and after radioiodine treatment. J Vet Intern Med. 2012;26:582‐588. [DOI] [PubMed] [Google Scholar]
- 102. Arata S, Ohmi A, Mizukoshi F, et al. Urinary transforming growth factor‐beta1 in feline chronic renal failure. J Vet Med Sci. 2005;67:1253‐1255. [DOI] [PubMed] [Google Scholar]
- 103. Lawson JS, Syme HM, Wheeler‐Jones CP, et al. Urinary active transforming growth factor beta in feline chronic kidney disease. Vet J. 2016;214:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Thanaboonnipat C, Sutayatram S, Buranakarl C, Choisunirachon N. Renal shear wave elastography and urinary procollagen type III amino‐terminal propeptide (uPIIINP) in feline chronic kidney disease. BMC Vet Res. 2019;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kovarikova S. Urinary biomarkers of renal function in dogs and cats: a review. Vet Med. 2016;60:589‐602. [Google Scholar]
- 106. Grauer GF. Measurement, interpretation, and implications of proteinuria and albuminuria. Vet Clin North Am Small Anim Pract. 2007;37(283–295):vi‐vii. [DOI] [PubMed] [Google Scholar]
- 107. Lees GE, Brown SA, Elliott J, Grauer GE, Vaden SL, American College of Veterinary Internal Medicine . Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med. 2005;19:377‐385. [DOI] [PubMed] [Google Scholar]
- 108. Raila J, Brunnberg L, Schweigert FJ, Kohn B. Influence of kidney function on urinary excretion of albumin and retinol‐binding protein in dogs with naturally occurring renal disease. Am J Vet Res. 2010;71:1387‐1394. [DOI] [PubMed] [Google Scholar]
- 109. Smets PM, Meyer E, Maddens BE, et al. Urinary markers in healthy young and aged dogs and dogs with chronic kidney disease. J Vet Intern Med. 2010;24:65‐72. [DOI] [PubMed] [Google Scholar]
- 110. Lyon SD, Sanderson MW, Vaden SL, Lappin MR, Jensen WA, Grauer GF. Comparison of urine dipstick, sulfosalicylic acid, urine protein‐to‐creatinine ratio, and species‐specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc. 2010;236:874‐879. [DOI] [PubMed] [Google Scholar]
- 111. Hanzlicek AS, Roof CJ, Sanderson MW, Grauer GF. Comparison of urine dipstick, sulfosalicylic acid, urine protein‐to‐creatinine ratio and a feline‐specific immunoassay for detection of albuminuria in cats with chronic kidney disease. J Feline Med Surg. 2012;14:882‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lee SY, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol. 2015;30:1063‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mackinnon B, Shakerdi L, Deighan CJ, Fox JG, O'Reilly DSJ, Boulton‐Jones M. Urinary transferrin, high molecular weight proteinuria and the progression of renal disease. Clin Nephrol. 2003;59:252‐258. [DOI] [PubMed] [Google Scholar]
- 114. Kjeldsen L, Johnsen AH, Sengelov H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Bio Chem. 1993;268:10425‐10432. [PubMed] [Google Scholar]
- 115. Schmidt‐Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase‐associated lipocalin. J Am Soc Nephrol. 2007;18:407‐413. [DOI] [PubMed] [Google Scholar]
- 116. Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase‐associated lipocalin in normal and neoplastic human tissues. Cell type‐specific pattern of expression. Histochem J. 1999;31:433‐441. [DOI] [PubMed] [Google Scholar]
- 118. Christensen EI, Verroust PJ, Nielsen R. Receptor‐mediated endocytosis in renal proximal tubule. Pflug Arch Eur J Phy. 2009;458:1039‐1048. [DOI] [PubMed] [Google Scholar]
- 119. Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase‐associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307‐315. [DOI] [PubMed] [Google Scholar]
- 120. Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase‐associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase‐associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285‐294. [DOI] [PubMed] [Google Scholar]
- 122. Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in children with chronic kidney disease. Ped Nephrol. 2007;22:101‐108. [DOI] [PubMed] [Google Scholar]
- 123. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase‐Fielitz A, NGAL Meta‐analysis Investigator Group . Accuracy of neutrophil gelatinase‐associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta‐analysis. Am J Kidney Dis. 2009;54:1012‐1024. [DOI] [PubMed] [Google Scholar]
- 124. De Silva PM, Mohammed Abdul KS, Eakanayake EM, et al. Urinary biomarkers KIM‐1 and NGAL for detection of chronic kidney disease of uncertain etiology (CKDu) among agricultural communities in Sri Lanka. PLoS Negl Trop Dis. 2016;10:e0004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nabity MB, Lees GE, Cianciolo R, Boggess MM, Steiner JM, Suchodolski JS. Urinary biomarkers of renal disease in dogs with X‐linked hereditary nephropathy. J Vet Intern Med. 2012;26:282‐293. [DOI] [PubMed] [Google Scholar]
- 126. Kim YM, Polzin DJ, Rendahl A, Granick JL. Urinary neutrophil gelatinase‐associated lipocalin in dogs with stable or progressive kidney disease. J Vet Intern Med. 2019;33:654‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Scheemaeker S, Meyer E, Schoeman JP, Defauw P, Duchateau L, Daminet S. Urinary neutrophil gelatinase‐associated lipocalin as an early biomarker for acute kidney injury in dogs. Vet J. 2020;255:105423. [DOI] [PubMed] [Google Scholar]
- 128. Hsu WL, Lin YS, Hu YY, Wong ML, Lin FY, Lee YJ. Neutrophil gelatinase‐associated lipocalin in dogs with naturally occurring renal diseases. J Vet Intern Med. 2014;28:437‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. van de Vrie M, Deegens JK, van der Vlag J, Hilbrands LB. Effect of long‐term storage of urine samples on measurement of kidney injury molecule 1 (KIM‐1) and neutrophil gelatinase‐associated lipocalin (NGAL). Am J Kidney Dis. 2014;63:573‐576. [DOI] [PubMed] [Google Scholar]
- 130. Schuh MP, Nehus E, Ma Q, et al. Long‐term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis. 2016;67:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Daure E, Belanger MC, Beauchamp G, Lapointe C. Elevation of neutrophil gelatinase‐associated lipocalin (NGAL) in non‐azotemic dogs with urinary tract infection. Res Vet Sci. 2013;95:1181‐1185. [DOI] [PubMed] [Google Scholar]
- 132. Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5:2229‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Langelueddecke C, Roussa E, Fenton RA, Wolff NA, Lee WK, Thévenod F. Lipocalin‐2 (24p3/neutrophil gelatinase‐associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J Biol Chem. 2012;287:159‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hsu WL, Chiou HC, Tung KC, et al. The different molecular forms of urine neutrophil gelatinase‐associated lipocalin present in dogs with urinary diseases. BMC Vet Res. 2014;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yamamoto T, Noiri E, Ono Y, et al. Renal L‐type fatty acid‐binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894‐2902. [DOI] [PubMed] [Google Scholar]
- 136. Xu Y, Xie Y, Shao X, Ni Z, Mou S. L‐FABP: a novel biomarker of kidney disease. Clin Chim Acta. 2015;445:85‐90. [DOI] [PubMed] [Google Scholar]
- 137. Smathers RL, Petersen DR. The human fatty acid‐binding protein family: evolutionary divergences and functions. Hum Genomics. 2011;5:170‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sweetser DA, Heuckeroth RO, Gordon JI. The metabolic significance of mammalian fatty‐acid‐binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337‐359. [DOI] [PubMed] [Google Scholar]
- 139. Ek‐Von Mentzer BA, Zhang F, Hamilton JA. Binding of 13‐HODE and 15‐HETE to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. Implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J Biol Chem. 2001;276:15575‐15580. [DOI] [PubMed] [Google Scholar]
- 140. Kamijo A, Sugaya T, Hikawa A, et al. Urinary excretion of fatty acid‐binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kamijo A, Sugaya T, Hikawa A, et al. Clinical evaluation of urinary excretion of liver‐type fatty acid‐binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med. 2005;145:125‐133. [DOI] [PubMed] [Google Scholar]
- 142. Sasaki H, Kamijo‐Ikemori A, Sugaya T, et al. Urinary fatty acids and liver‐type fatty acid binding protein in diabetic nephropathy. Nephron Clin Pract. 2009;112:c148‐c156. [DOI] [PubMed] [Google Scholar]
- 143. Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P. Urinary liver‐type fatty acid‐binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care. 2010;33:1320‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Susantitaphong P, Siribamrungwong M, Doi K, Noiri E, Terrin N, Jaber BL. Performance of urinary liver‐type fatty acid‐binding protein in acute kidney injury: a meta‐analysis. Am J Kidney Dis. 2013;61:430‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Matsui K, Kamijo‐Ikemori A, Imai N, et al. Clinical significance of urinary liver‐type fatty acid‐binding protein as a predictor of ESRD and CVD in patients with CKD. Clin Exp Nephrol. 2016;20:195‐203. [DOI] [PubMed] [Google Scholar]
- 146. Hikasa S, Shimabukuro S, Hideta K, et al. Urinary liver‐type fatty acid‐binding protein levels as a potential risk factor for renal dysfunction in male HIV‐infected Japanese patients receiving antiretroviral therapy: a pilot study. Int J STD AIDS. 2018;29:1424‐1431. [DOI] [PubMed] [Google Scholar]
- 147. Plesinski K, Adamczyk P, Swietochowska E, et al. Evaluation of liver‐type fatty acid binding protein (L‐FABP) and interleukin 6 in children with renal cysts. Adv Clin Exp Med. 2019;28:1675‐1682. [DOI] [PubMed] [Google Scholar]
- 148. Kamijo‐Ikemori A, Sugaya T, Sekizuka A, Hirata K, Kimura K. Amelioration of diabetic tubulointerstitial damage in liver‐type fatty acid‐binding protein transgenic mice. Nephrol Dial Transplant. 2009;24:788‐800. [DOI] [PubMed] [Google Scholar]
- 149. Ichikawa D, Kamijo‐Ikemori A, Sugaya T, et al. Renoprotective effect of renal liver‐type fatty acid binding protein and angiotensin II type 1a receptor loss in renal injury caused by RAS activation. Am J Physiol Renal Physiol. 2014;306:F655‐F663. [DOI] [PubMed] [Google Scholar]
- 150. Parikh CR, Butrymowicz I, Yu A, et al. Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis. 2014;63:567‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu KD, Siew ED, Reeves WB, et al. Storage time and urine biomarker levels in the ASSESS‐AKI study. PloS One. 2016;11:e0164832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kamijo A, Sugaya T, Hikawa A, et al. Urinary liver‐type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem. 2006;284:175‐182. [DOI] [PubMed] [Google Scholar]
- 153. Holzscheiter L, Beck C, Rutz S, et al. NGAL, L‐FABP, and KIM‐1 in comparison to established markers of renal dysfunction. Clin Chem Lab Med. 2014;52:537‐546. [DOI] [PubMed] [Google Scholar]
- 154. Imai N, Yasuda T, Kamijo‐Ikemori A, Shibagaki Y, Kimura K. Distinct roles of urinary liver‐type fatty acid‐binding protein in non‐diabetic patients with anemia. PloS One. 2015;10:e0126990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Asada T, Isshiki R, Hayase N, et al. Impact of clinical context on acute kidney injury biomarker performances: differences between neutrophil gelatinase‐associated lipocalin and L‐type fatty acid‐binding protein. Sci Rep. 2016;6:33077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nishida M, Kawakatsu H, Hamaoka K. Urinary liver‐type fatty acid‐binding protein in pediatric nephrotic syndrome and tubular dysfunction. Pediatr Int. 2018;60:442‐445. [DOI] [PubMed] [Google Scholar]
- 157. Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule‐1 (KIM‐1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up‐regulated in renal cells after injury. J Biol Chem. 1998;273:4135‐4142. [DOI] [PubMed] [Google Scholar]
- 158. Castillo‐Rodriguez E, Fernandez‐Prado R, Martin‐Cleary C, et al. Kidney injury marker 1 and neutrophil gelatinase‐associated lipocalin in chronic kidney disease. Nephron. 2017;136:263‐267. [DOI] [PubMed] [Google Scholar]
- 159. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule‐1 (KIM‐1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237‐244. [DOI] [PubMed] [Google Scholar]
- 160. Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule‐1: a tissue and urinary biomarker for nephrotoxicant‐induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552‐F563. [DOI] [PubMed] [Google Scholar]
- 161. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule‐1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517‐F529. [DOI] [PubMed] [Google Scholar]
- 162. Satirapoj B, Aramsaowapak K, Tangwonglert T, Supasyndh O. Novel tubular biomarkers predict renal progression in type 2 diabetes mellitus: a prospective cohort study. J Diabetes Res. 2016;2016:3102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hsu CY, Xie D, Waikar SS, et al. Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int. 2017;91:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Seibert FS, Sitz M, Passfall J, et al. Prognostic value of urinary calprotectin, NGAL and KIM‐1 in chronic kidney disease. Kidney Blood Press Res. 2018;43:1255‐1262. [DOI] [PubMed] [Google Scholar]
- 165. Wagoner MP, Yang Y, McDuffie JE, et al. Evaluation of temporal changes in urine‐based metabolomic and kidney injury markers to detect compound induced acute kidney tubular toxicity in beagle dogs. Curr Top Med Chem. 2017;17:2767‐2780. [DOI] [PubMed] [Google Scholar]
- 166. Lippi I, Perondi F, Meucci V, Bruno B, Gazzano V, Guidi G. Clinical utility of urine kidney injury molecule‐1 (KIM‐1) and gamma‐glutamyl transferase (GGT) in the diagnosis of canine acute kidney injury. Vet Res Commun. 2018;42:95‐100. [DOI] [PubMed] [Google Scholar]
- 167. Bland SK, Cote O, Clark ME, et al. Characterization of kidney injury molecule‐1 in cats. J Vet Intern Med. 2014;28:1454‐1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sabbisetti VS, Ito K, Wang C, Yang L, Mefferd SC, Bonventre JV. Novel assays for detection of urinary KIM‐1 in mouse models of kidney injury. Toxicol Sci. 2013;131:13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pennemans V, Rigo JM, Faes C, Reynders C, Penders J, Swennen Q. Establishment of reference values for novel urinary biomarkers for renal damage in the healthy population: are age and gender an issue? Clin Chem Lab Med. 2013;51:1795‐1802. [DOI] [PubMed] [Google Scholar]
- 170. Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol. 2015;30:677‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. McWilliam SJ, Antoine DJ, Sabbisetti V, et al. Reference intervals for urinary renal injury biomarkers KIM‐1 and NGAL in healthy children. Biomark Med. 2014;8:1189‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Huang Y, Tian Y, Likhodii S, Randell E. Baseline urinary KIM‐1 concentration in detecting acute kidney injury should be interpreted with patient pre‐existing nephropathy. Pract Lab Med. 2019;15:e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bland SK, Schmiedt CW, Clark ME, DeLay J, Bienzle D. Expression of kidney injury molecule‐1 in healthy and diseased feline kidney tissue. Vet Pathol. 2017;54:490‐510. [DOI] [PubMed] [Google Scholar]
- 174. Kang DH, Hughes J, Mazzali M, et al. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448‐1457. [DOI] [PubMed] [Google Scholar]
- 175. El Awad B, Kreft B, Wolber EM, et al. Hypoxia and interleukin‐1beta stimulate vascular endothelial growth factor production in human proximal tubular cells. Kidney Int. 2000;58:43‐50. [DOI] [PubMed] [Google Scholar]
- 176. Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol J Am Soc Nephrol. 2011;26:1132‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Shulman K, Rosen S, Tognazzi K, Manseau EJ, Brown LF. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996;7:661‐666. [DOI] [PubMed] [Google Scholar]
- 178. Bobkova IN, Kozlovskaia LV, Rameeva AS, Varshavskiĭ VA, Golitsyna EP. Clinical implication of urine test for markers of endothelial dysfunction and angiogenesis factors in assessment of tubulointerstitial fibrosis in chronic glomerulonephritis. Ter Arkh. 2007;79:10‐15. [PubMed] [Google Scholar]
- 179. Rudnicki M, Perco P, Enrich J, et al. Hypoxia response and VEGF‐A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest. 2009;89:337‐346. [DOI] [PubMed] [Google Scholar]
- 180. Chakrabarti S, Syme HM, Elliott J. Urinary vascular endothelial growth factor as a prognostic marker in feline chronic kidney disease [abstract]. J Vet Intern Med. 2012;26:1524. [Google Scholar]
- 181. Uchida K, Gotoh A. Measurement of cystatin‐C and creatinine in urine. Clin Chim Acta. 2002;323:121‐128. [DOI] [PubMed] [Google Scholar]
- 182. Monti P, Benchekroun G, Berlato D, Archer J. Initial evaluation of canine urinary cystatin C as a marker of renal tubular function. J Small Anim Pract. 2012;53:254‐259. [DOI] [PubMed] [Google Scholar]
- 183. Herget‐Rosenthal S, Feldkamp T, Volbracht L, Kribben A. Measurement of urinary cystatin C by particle‐enhanced nephelometric immunoassay: precision, interferences, stability and reference range. Ann Clin Biochem. 2004;41:111‐118. [DOI] [PubMed] [Google Scholar]
- 184. Thielemans N, Lauwerys R, Bernard A. Competition between albumin and low‐molecular‐weight proteins for renal tubular uptake in experimental nephropathies. Nephron. 1994;66:453‐458. [DOI] [PubMed] [Google Scholar]
- 185. Paepe D, Ghys LF, Smets P, et al. Routine kidney variables, glomerular filtration rate and urinary cystatin C in cats with diabetes mellitus, cats with chronic kidney disease and healthy cats. J Feline Med Surg. 2015;17:880‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Henderson B. Integrating the cell stress response: a new view of molecular chaperones as immunological and physiological homeostatic regulators. Cell Biochem Funct. 2010;28:1‐14. [DOI] [PubMed] [Google Scholar]
- 187. Musial K, Zwolinska D. Heat shock proteins in chronic kidney disease. Pediatr Nephrol. 2011;26:1031‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Goering PL, Fisher BR, Noren BT, Papaconstantinou A, Rojko JL, Marler RJ. Mercury induces regional and cell‐specific stress protein expression in rat kidney. Toxicol Sci. 2000;53:447‐457. [DOI] [PubMed] [Google Scholar]
- 189. Mueller T, Bidmon B, Pichler P, et al. Urinary heat shock protein‐72 excretion in clinical and experimental renal ischemia. Pediatr Nephrol. 2003;18:97‐99. [DOI] [PubMed] [Google Scholar]
- 190. Bruchim Y, Avital Y, Horowitz M, Mazaki‐Tovi M, Aroch I, Segev G. Urinary heat shock protein 72 as a biomarker of acute kidney injury in dogs. Vet J. 2017;225:32‐34. [DOI] [PubMed] [Google Scholar]
- 191. Montero A, Munger KA, Khan RZ, et al. F(2)‐isoprostanes mediate high glucose‐induced TGF‐beta synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int. 2000;58:1963‐1972. [DOI] [PubMed] [Google Scholar]
- 192. Morrow JD. The isoprostanes ‐ unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr Pharm Des. 2006;12:895‐902. [DOI] [PubMed] [Google Scholar]
- 193. Montuschi P, Barnes PJ, Roberts LJ 2nd. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791‐1800. [DOI] [PubMed] [Google Scholar]
- 194. Badr KF, Abi‐Antoun TE. Isoprostanes and the kidney. Antioxid Redox Signal. 2005;7:236‐243. [DOI] [PubMed] [Google Scholar]
- 195. Lim PS, Chang YM, Thien LM, et al. 8‐iso‐prostaglandin F2alpha as a useful clinical biomarker of oxidative stress in ESRD patients. Blood Purif. 2002;20:537‐542. [DOI] [PubMed] [Google Scholar]
- 196. Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009‐1016. [DOI] [PubMed] [Google Scholar]
- 197. Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42‐50. [DOI] [PubMed] [Google Scholar]
- 198. Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752‐760. [DOI] [PubMed] [Google Scholar]
- 199. Upadhyay A, Larson MG, Guo CY, et al. Inflammation, kidney function and albuminuria in the Framingham offspring cohort. Nephrol Dial Transplant. 2011;26:920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Nerpin E, Helmersson‐Karlqvist J, Riserus U, et al. Inflammation, oxidative stress, glomerular filtration rate, and albuminuria in elderly men: a cross‐sectional study. BMC Res Notes. 2012;5:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Soffler C, Campbell VL, Hassel DM. Measurement of urinary F2‐isoprostanes as markers of in vivo lipid peroxidation: a comparison of enzyme immunoassays with gas chromatography‐mass spectrometry in domestic animal species. J Vet Diag Invest. 2010;22:200‐209. [DOI] [PubMed] [Google Scholar]
- 202. Choi‐Miura NH, Oda T. Relationship between multifunctional protein "clusterin" and Alzheimer disease. Neurobiol Aging. 1996;17:717‐722. [PubMed] [Google Scholar]
- 203. Rodriguez‐Pineiro AM, de la Cadena MP, Lopez‐Saco A, et al. Differential expression of serum clusterin isoforms in colorectal cancer. Mol Cell Proteomics. 2006;5:1647‐1657. [DOI] [PubMed] [Google Scholar]
- 204. Garcia‐Martinez JD, Martinez‐Subiela S, Tvarijonaviciute A, et al. Urinary ferritin and cystatin C concentrations at different stages of kidney disease in leishmaniotic dogs. Res Vet Sci. 2015;99:204‐207. [DOI] [PubMed] [Google Scholar]
- 205. Zhou X, Ma B, Lin Z, et al. Evaluation of the usefulness of novel biomarkers for drug‐induced acute kidney injury in beagle dogs. Toxicol Appl Pharmacol. 2014;280:30‐35. [DOI] [PubMed] [Google Scholar]
- 206. Gordin E, Gordin D, Viitanen S, et al. Urinary clusterin and cystatin B as biomarkers of tubular injury in dogs following envenomation by the European adder. Res Vet Sci. 2021;134:12‐18. [DOI] [PubMed] [Google Scholar]
- 207. Chen H, Avital Y, Peterson S, et al. Evaluation of cystatin B as a marker of acute kidney injury in dogs and cats [Research Communications of the 30th ECVIM‐CA Online Congress. 2‐5 September 2020, abstract ESVNU‐O‐4]. J Vet Intern Med. 2020;34:3104. [Google Scholar]
- 208. Garcia‐Martinez JD, Tvarijonaviciute A, Ceron JJ, et al. Urinary clusterin as a renal marker in dogs. J Vet Diag Invest. 2012;24:301‐306. [DOI] [PubMed] [Google Scholar]
- 209. KuKanich K, George C, Roush JK, et al. Effects of low‐dose meloxicam in cats with chronic kidney disease. J Feline Med Sur. 2020;23:138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973‐1984). J Am Vet Med Assoc. 1987;190:1196‐1202. [PubMed] [Google Scholar]
- 211. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213‐217. [DOI] [PubMed] [Google Scholar]
- 212. Gressner AM, Weiskirchen R, Breitkopf K, et al. Roles of TGF‐beta in hepatic fibrosis. Front Biosci. 2002;7:d793‐d807. [DOI] [PubMed] [Google Scholar]
- 213. Bottinger EP, Bitzer M. TGF‐beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600‐2610. [DOI] [PubMed] [Google Scholar]
- 214. Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754‐765. [DOI] [PubMed] [Google Scholar]
- 215. Euler‐Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15‐25. [DOI] [PubMed] [Google Scholar]
- 216. Verrecchia F, Mauviel A. Transforming growth factor‐beta and fibrosis. World J Gastroenterol. 2007;13:3056‐3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495‐2508. [DOI] [PubMed] [Google Scholar]
- 218. Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a TGF‐β1‐independent mechanism. Kidney Int. 1997;52:637‐647. [DOI] [PubMed] [Google Scholar]
- 219. Wolf G. Renal injury due to renin‐angiotensin‐aldosterone system activation of the transforming growth factor‐beta pathway. Kidney Int. 2006;70:1914‐1919. [DOI] [PubMed] [Google Scholar]
- 220. Shin DM, Jeon JH, Kim CW, et al. TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008;22:2498‐2507. [DOI] [PubMed] [Google Scholar]
- 221. Branton MH, Kopp JB. TGF‐beta and fibrosis. Microb Infect. 1999;1:1349‐1365. [DOI] [PubMed] [Google Scholar]
- 222. Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217‐224. [DOI] [PubMed] [Google Scholar]
- 223. Edwards DR, Murphy G, Reynolds JJ, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6:1899‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Border WA, Noble NA. Cytokines in kidney disease: the role of transforming growth factor‐beta. Am J Kidney Dis. 1993;22:105‐113. [DOI] [PubMed] [Google Scholar]
- 225. Serini G, Bochaton‐Piallat ML, Ropraz P, et al. The fibronectin domain ED‐A is crucial for myofibroblastic phenotype induction by transforming growth factor‐beta1. J Cell Biol. 1998;142:873‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Gentle ME, Shi S, Daehn I, et al. Epithelial cell TGFbeta signaling induces acute tubular injury and interstitial inflammation. J Am Soc Nephrol. 2013;24:787‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Xu X, Zheng L, Yuan Q, et al. Transforming growth factor‐β in stem cells and tissue homeostasis. Bone Res. 2018;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Murakami K, Takemura T, Hino S, Yoshioka K. Urinary transforming growth factor‐beta in patients with glomerular diseases. Pediatr Nephrol. 1997;11:334‐336. [DOI] [PubMed] [Google Scholar]
- 229. Đukanović L, Ležaić V, Miljković Đ, et al. Transforming growth factor‐beta 1 in Balkan endemic nephropathy. Nephron Clin Pract. 2009;111:c127‐c132. [DOI] [PubMed] [Google Scholar]
- 230. De Muro P, Faedda R, Fresu P, et al. Urinary transforming growth factor‐β1 in various types of nephropathy. Pharmacol Res. 2004;49:293‐298. [DOI] [PubMed] [Google Scholar]
- 231. Ruiz‐Torres MP, Bosch RJ, O'Valle F, et al. Age‐related increase in expression of TGF‐beta1 in the rat kidney: relationship to morphologic changes. J Am Soc Nephrol. 1998;9:782‐791. [DOI] [PubMed] [Google Scholar]
- 232. Lawson JS, Liu HH, Syme HM, Purcell R, Wheeler‐Jones CPD, Elliott J. The cat as a naturally occurring model of renal interstitial fibrosis: characterisation of primary feline proximal tubular epithelial cells and comparative pro‐fibrotic effects of TGF‐beta1. PLoS One. 2018;13:e0202577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Wootton CI, Murphy R. Type III procollagen amino terminal propeptide: what does it measure in children? Br J Dermatol. 2012;167:1391‐1392. [DOI] [PubMed] [Google Scholar]
- 234. Soylemezoglu O, Wild G, Dalley AJ, et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant. 1997;12:1883‐1889. [DOI] [PubMed] [Google Scholar]
- 235. Hezzell MJ, Boswood A, Chang YM, Moonarmart W, Elliott J. Associations among serum N‐terminal procollagen type III concentration, urinary aldosterone‐to‐creatinine ratio, and ventricular remodeling in dogs with myxomatous mitral valve disease. Am J Vet Res. 2012;73:1765‐1774. [DOI] [PubMed] [Google Scholar]
- 236. Glinska‐Suchocka K, Orlowska A, Jankowski M, et al. Serum concentrations of PIIINP aminopeptide in dogs with liver fibrosis. Pol J Vet Sci. 2016;19:365‐369. [DOI] [PubMed] [Google Scholar]
- 237. Mosca A, Comparcola D, Romito I, et al. Plasma N‐terminal propeptide of type III procollagen accurately predicts liver fibrosis severity in children with non‐alcoholic fatty liver disease. Liver Int. 2019;39:2317‐2329. [DOI] [PubMed] [Google Scholar]
- 238. Duprez DA, Gross MD, Ix JH, et al. Collagen biomarkers are associated with decline in renal function independently of blood pressure and other cardiovascular risk factors: the Multi‐Ethnic Study of Atherosclerosis Study. J Hypertens. 2019;37:2398‐2403. [DOI] [PubMed] [Google Scholar]
- 239. Rasmussen DGK, Boesby L, Nielsen SH, et al. Collagen turnover profiles in chronic kidney disease. Sci Rep. 2019;9:16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Teppo AM, Tornroth T, Honkanen E, et al. Urinary amino‐terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003;75:2113‐2119. [DOI] [PubMed] [Google Scholar]
- 241. Ghoul BE, Squalli T, Servais A, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010;5:205‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Ix JH, Biggs ML, Mukamal K, et al. Urine collagen fragments and CKD progression‐the cardiovascular health study. J Am Soc Nephrol. 2015;26:2494‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Yilmaz R, Yildirim T, Baydar DE, et al. Urinary type III procollagen is associated with chronic allograft dysfunction and predicts graft survival. Transplant Proc. 2017;49:281‐287. [DOI] [PubMed] [Google Scholar]


