Abstract
Introduction
The benefits of physical activity for glycaemic control in type 2 diabetes (T2D) are well-known. However, whether established glycaemic and cardiovascular benefits can be maximised by exercising at a certain time of day is unknown. Given postprandial glucose peaks contribute to worsening glycated haemoglobin (HbA1c) and cardiovascular risk factors, and that exercise immediately lowers blood glucose, prescribing exercise at a specific time of day to attenuate peak hyperglycaemia may improve glycaemic control and reduce the burden of cardiovascular disease in people with T2D.
Methods and analysis
A single-centre randomised controlled trial will be conducted by the University of Wollongong, Australia. Individuals with T2D (n=70, aged 40–75 years, body mass index (BMI): 27–40 kg/m2) will be recruited and randomly allocated (1:1), stratified for sex and insulin, to one of three groups: (1) exercise at time of peak hyperglycaemia (ExPeak, personalised), (2) exercise not at time of peak hyperglycaemia (NonPeak) or (3) waitlist control (WLC, standard care). The trial will be 5 months, comprising an 8-week intervention and 3-month follow-up. Primary outcome is the change in HbA1c preintervention to postintervention. Secondary outcomes include vascular function (endothelial function and arterial stiffness), metabolic control (blood lipids and inflammation) and body composition (anthropometrics and dual-energy X-ray absorptiometry (DEXA)). Tertiary outcomes will examine adherence.
Ethics and dissemination
The joint UOW and ISLHD Ethics Committee approved protocol (2019/ETH09856) prospectively registered at the Australian New Zealand Clinical Trials Registry. Written informed consent will be obtained from all eligible individuals prior to commencement of the trial. Study results will be published as peer-reviewed articles, presented at national/international conferences and media reports.
Trial registration number
ACTRN12619001049167.
Keywords: diabetic nephropathy & vascular disease, hypertension, clinical physiology, complementary medicine, physiology, sports medicine
Strengths and limitations of this study.
A strength of this randomised controlled trial is the use of continuous glucose monitoring for personalising exercise timing to attenuate peak hyperglycaemia, as well as the inclusion of an active placebo control condition.
This study will employ a variety of data collection methods (in-lab and free-living) to measure changes in cardiovascular and metabolic health, physical activity and behaviour change.
Recruitment of participants across Australia (urban and rural) with remote delivery is both a strength in diversity and inclusion and a limitation given the reliance on dried blood spot home collection, and vascular/body composition measures will not be available for those unable to attend the university visits.
This study is robust in its adaptation to the COVID-19 pandemic, while retaining high-quality study design and data collection with strong external validity.
Introduction
Approximately 463 million adults are living with type 2 diabetes (T2D) and this number is expected to increase to 700 million by 2045.1 Individuals with T2D have a twofold greater risk of developing atherosclerotic cardiovascular disease (CVD; eg, myocardial infarction, stroke, etc) and CVD accounts for ~70% of deaths in patients with T2D.2 T2D is characterised by elevated fasting and postprandial blood glucose levels.3 Large excursions in blood glucose, especially during the postprandial period (ie, postprandial hyperglycaemia) cause oxidative stress, inflammation and endothelial dysfunction, which mechanistically links impaired glucose regulation with the development of CVD in people with T2D.4 5 Acute and chronic exercise training improve blood glucose regulation and reduce cardiovascular risk factors. The benefits of exercise training on glycaemic control are largely attributed to the accumulated effects of individual exercise sessions6 7 increasing contraction-mediated and insulin-mediated glucose uptake7 8 consistently and overtime. The current guidelines for physical activity recommend adults to accumulate ~150–300 min of moderate intensity aerobic activity throughout the week to improve or maintain health,9 including glycaemic control (ie, glycated haemoglobin (HbA1c)) in people with T2D.10 However, mounting evidence11–15 indicates that exercise timing (eg, premeal vs postmeal or morning vs afternoon) influences glycaemic responses, yet there are no consistent guidelines on exercise timing in any current physical activity recommendations globally.
Multiple systematic reviews have recently examined the effects of exercise timing on measures of glycaemic control in people with T2D and suggest the best time to exercise is within the first few hours after a meal.11–13 However, performing exercise at different times of the day (ie, morning vs afternoon) has also shown to influence glycaemic responses.14 15 For example, Savikj et al (2019) recently demonstrated that 2 weeks of high-intensity interval training (HIIT; 3 days/week) performed in the afternoon improved 24 hours glucose concentration by −0.6 mmol/L more than HIIT in the morning,14 whereas a separate study by Teo et al (2019) found no significant differences in any glycaemic outcomes (HbA1c, fasting or postprandial glucose) after 12 weeks of exercise (3 days/week) performed in the morning versus afternoon.15 Given the inconsistent findings and broad recommendations in the current literature (ie, exercise timing relative to time of day or meal consumption), a more personalised approach may be needed to target CVD and for practitioners to prescribe exercise timing for people with T2D. Postprandial hyperglycaemia is linked to CVD and timing exercise to specifically target the largest postprandial excursion (ie, peak hyperglycaemia) of the day may lead to greater glycaemic benefits and reduced cardiovascular risk.
It is unknown if prescribing daily exercise at a specific time of day, to attenuate peak hyperglycaemia, will lead to greater improvements in HbA1c compared with the current physical activity guidelines of accumulating ~150–300 min/week at any time. Further, the vascular effects of exercising specifically to attenuate peak hyperglycaemia are unknown. The endothelium is a key regulator of vascular homeostasis and endothelial function is an early risk factor for CVD.16 17 Hyperglycaemia increases production of reactive oxygen species18 and the resulting oxidative stress reduces vascular homeostasis (ie, by increasing vasoconstriction and decreasing vasodilation) which can lead to endothelial dysfunction and CVD over time. A longer-term intervention of daily exercise is now warranted to garner a better understanding of exercise timing on glycaemic control and to examine whether exercising at the time of peak hyperglycaemia improves HbA1c and reduces cardiovascular risk factors.
The aim of this trial is to determine whether exercising to attenuate peak hyperglycaemia (exercise beginning ~30 min before peak hyperglycaemia) improves glycaemic control (HbA1c and 24 hours mean, fasting and postprandial glucose) and reduces cardiovascular risk factors (including lipids, C reactive protein (CRP), vascular function), more than exercising not at time of peak hyperglycaemia (exercise ~90 min after peak hyperglycaemia) or at any time of the day (no prescribed exercise time that is, physical activity guidelines) in people with T2D. The efficacy, feasibility and adherence to prescribing an exercise time will also be explored during a 3-month follow-up. Given that postprandial hyperglycaemia is associated with worsening HbA1c19 and endothelial dysfunction20 in T2D, we hypothesise that exercising to attenuate peak hyperglycaemia will lead to the greatest improvements in glycaemic control, which in turn will improve vascular function and reduce cardiovascular risk.
Methods and analysis
A single-centre randomised controlled trial will be conducted at the University of Wollongong, Australia from July 2019 to December 2022 (figure 1). Participants will be recruited through online advertising using a clinical trials recruitment company (Trial Facts). A medical screening questionnaire and informed consent (online supplemental material) will be obtained from all eligible individuals prior to participation. Study data will be collected and managed using the secure online REDCap (Research Electronic Data Capture) tools hosted at the University of Wollongong, Australia.21 22
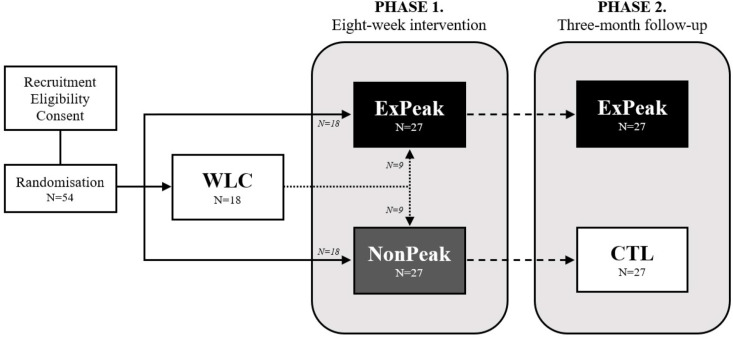
Figure 1.
Study design and flow chart. Eligible participants will be randomised (n=54) to one of three groups: (1) exercise at peak hyperglycaemia (ExPeak; n=18), (2) exercise after peak hyperglycaemia (NonPeak; n=18) or (3) waitlist control (WLC; n=18). Participants randomised to WLC will be rerandomised to ExPeak or NonPeak after the waitlist period. Following the 8-week intervention (phase 1), the ExPeak (n=27) group will continue to exercise at peak hyperglycaemia, whereas the non-peak (n=27) group will become the control (CTL; n=27) group for the 3-month follow-up (phase 2). Participants in the WLC and CTL groups will receive standard care advice to exercise in accordance with the WHO physical activity guidelines.
bmjopen-2021-057183supp001.pdf (940.8KB, pdf)
Participants
Inclusion criteria:
Physician diagnosed T2D (registration with the National Diabetes Services Scheme).
HbA1c between 6.5% and 9.0%.
Aged between 40 and 75 years.
BMI between 27 and 40 kg/m2.
Diabetes treated with lifestyle, oral medications and/or intermediate/long-acting insulin
Stable weight for previous 3 months (±4 kg)
Stable medications for previous 3 months
Able to speak and understand English
Exclusion criteria:
Any absolute contraindications to exercise (ie, musculoskeletal/joint injury, etc)
Presence or history of CVD, kidney or liver disease
Diagnosed diabetes complications that is, neuropathy, retinopathy and so on.
Diabetes treated with short-acting insulin
Uncontrolled hypertension (>160/90 mm Hg)
>150 min of moderate to vigorous intensity exercise/week (per Godin leisure time physical activity questionnaire)
Study design
Seventy males and females (aged 40–75 years, BMI: 27–40 kg/m2) will be recruited and randomised to one of three groups for 8 weeks: (1) ExPeak, (2) NonPeak or (3) WLC. Participants allocated to the WLC group will be rerandomised to the ExPeak or NonPeak intervention group following the waitlist period. During the 8-week intervention (Phase 1), all groups will be prescribed ~150 min/week of physical activity as per the current guidelines. The intervention groups will be prescribed daily exercise at a specific time. During the exercise intervention, participants will have five telehealth consults with an accredited exercise physiologist, in line with Australia’s Medicare health plan for people with diabetes. An automatic computer-generated random number table will be used to perform random allocation of participants (1:1 ratio), stratified for sex and exogenous insulin usage. A sealed envelope system will be used to blind researchers from group allocations. Allocations will be sealed in an opaque envelope (by a person independent to the clinical trial) until a participant is enrolled and needing to commence the intervention.
Participants will undergo a 3-month follow-up (Phase 2), where adherence to exercising at a prescribed time (with minimal contact from the research team) will be assessed. During Phase 2, participants in the ExPeak group will be advised to continue exercising daily at their time of peak hyperglycaemia and participants in the NonPeak group will be advised to exercise in accordance with the World Health Organization 2020 guidelines for physical activity, that is, accumulate ~150–300 min of physical activity per week at any time of day,9 thus becoming the control group.
Interventions
All exercise sessions will be performed in a free-living setting (home-based) for the duration of this trial. Participants in the ExPeak and NonPeak groups will be prescribed ~22 min of daily moderate-intensity physical activity (aerobic exercise, eg, walking, cycling, swimming, etc) for 8 weeks, to align with the physical activity guidelines of accumulating at least 150 min of aerobic activity per week. The preintervention Continuous Glucose Monitoring (CGM) data (outlined below) will be used to determine time of peak hyperglycaemia. The ExPeak group will begin exercising ~30 min before their peak hyperglycaemia typically occurs and the NonPeak group will begin exercising ~90 min after their peak hyperglycaemia typically occurs. Participants in the control groups will exercise in accordance with the physical activity guidelines.9 Exercise intensity will be determined using the Borg Scale to indicate Rate of Perceived Exertion, which uses numbered categories from 6 to 20 (ie, no exertion at all to maximal exertion) to gauge how hard a person ‘feels’ they are working.23 Daily exercise should be completed as one continuous bout but may be accumulated over a 30-min period depending on individual needs (ideally accumulated in bouts of >10 min, interspersed with short periods of rest). Participants will have two phone consults and five telehealth video consults with an accredited exercise physiologist on alternate weeks throughout the 8-week exercise intervention, in addition to maintaining standard care treatment with healthcare professionals and habitual medication and diet.
Experimental protocol
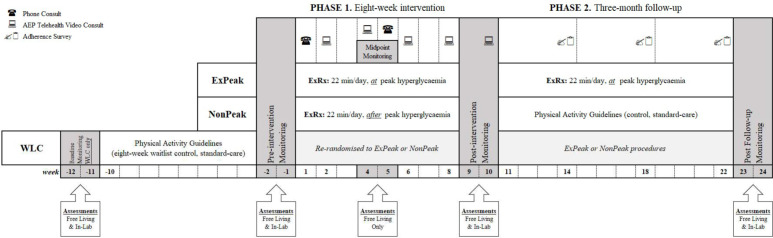
The intervention period will be 5 months in total, with the 8-week intervention (Phase 1) commencing after 2 weeks of preintervention monitoring, and the 3-month follow-up (Phase 2) commencing after 2 weeks of postintervention monitoring. Preassessments and postassessments will be conducted at the University of Wollongong to evaluate glycaemic and metabolic control, vascular function and body composition (figure 2). Participants will be instructed to abstain from physical activity for >24 hours and to fast for ~10 hours before each in-lab assessment.
Figure 2.
Timeline of study protocol. Participants randomised to the waitlist control (WLC) group will undergo measures before and after an 8-week waitlist control period. Then are randomised to one of two intervention groups for 8 weeks: (1) exercise at peak hyperglycaemia ((ExPeak) ExRx: begin exercise ~30 min before peak hyperglycaemia) or (2) exercise after peak hyperglycaemia ((NonPeak) ExRx: begin exercise ~90 min after peak hyperglycaemia). All groups undergo preintervention CGM to measure time of peak hyperglycaemic prior to interventions. Phase 1—8-week intervention: both intervention groups will perform ~22 min of daily exercise at their prescribed time. Participants will receive two phone consults and five telehealth video consults (via zoom or skype) with an accredited exercise physiologist. Phase 2—3-month follow-up: the ExPeak group will continue to exercise for ~22 min/day at peak hyperglycaemia and the NonPeak group will exercise according to the physical activity guidelines. Three adherence surveys will be conducted (at the end of each month), but no formal contact. Free Living Assessments: 14-day CGM, 2-hour MMTT, 7-day ActiGraph activity monitoring, 7-day HR monitoring (midpoint only; polar Bluetooth HR monitor worn on same days as ActiGraph, only during prescribed exercise), 7-day diet record, quality of life survey and self-regulatory efficacy and physical activity questionnaire. In-lab assessments: (1) blood sample HbA1c, CRP and blood lipids (TG, Tc, HDL and LDL); (2) vascular measures FMD and arterial stiffness via PWV/PWA; and (3) anthropometrics (height and weight) and body composition DEXA. AEP, accredited exercise physiologist; CGM, continuous glucose monitoring; CRP, C reactive protein; DEXA, dual X-ray absorptiometry; ExPeak, exercise at peak hyperglycaemia (intervention group); ExRx, exercise prescription; FMD, flow-mediated dilation; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; MMTT, mixed meal tolerance test; NonPeak, exercise after peak (intervention group); PWA, pulse wave analysis; PWV, pulse wave velocity; Tc, total cholesterol; TG, triglyceride; WLC, waitlist control.
A 2-week monitoring period will be conducted preintervention, midway through, postintervention and after the 3-month follow-up. Participants in the WLC group will have two additional weeks of baseline monitoring before the waitlist period commences. Participants will maintain normal daily activity and dietary patterns during each monitoring period, except for the midpoint assessment where they will continue to follow intervention protocol. During the 3-month follow-up, participants will complete three short surveys (one at the end of each month, seven questions each) to assess adherence to the exercise prescription but will otherwise have no formal contact with the research team (figure 2). Other than the prescribed exercise, participants will be asked to maintain normal dietary habits and medication usage throughout the study period.
Determination of peak hyperglycaemia
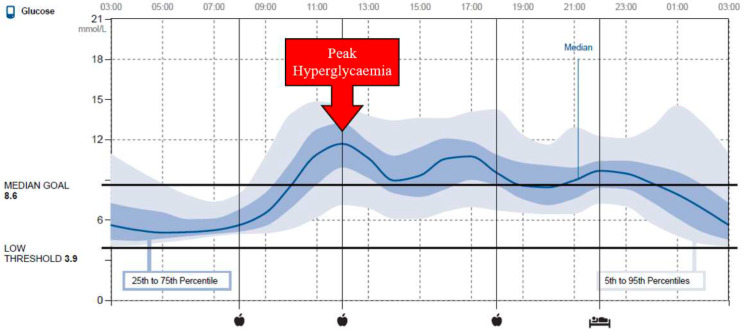
The ‘Glucose Pattern Insights’ report (automatically generated via LibreView software), for the 2-week preintervention CGM (Freestyle Libre, Abbott), will be used to determine the average time that peak hyperglycaemia occurs for each participant (figure 3). Trained researchers will verify time of peak hyperglycaemia by analysing the raw CGM data using the following methods. After the CGM data is cleaned and separated into full days (ie, >24 hours of uninterrupted data), maximum glucose and the time it occurs will be calculated for each day of the 2-week monitoring period. The average time of day that peak hyperglycaemia occurs will be then determined for each participant—if peak hyperglycaemia occurs at the same time of day (or within ~30 min) on five or more occasions over the 14 days CGM period, that time of day will be identified as the time of peak hyperglycaemia. Alternatively, time of peak hyperglycaemia will be calculated as an average from 14 days of continuous glucose measurements. Exercise for the ExPeak group will be prescribed in relation to the highest peak (ie, greatest glucose excursion); if there are multiple glucose excursions throughout the day with the same peak level, participants will be given an option of the times to exercise, but must stick with one time for the duration of the intervention. Time of peak hyperglycaemia will be reassessed following the waitlist period for participants initially randomised to the WLC group and again in the ExPeak group for the 3-month follow-up.
Figure 3.
Example ‘Glucose Pattern Insights’ report. Via LibreView, of a 24 hours blood glucose curve averaged from 14 days of continuous glucose measurements.
Outcome measures
The primary outcome is the change in HbA1c following the 8-week intervention. Secondary outcome measures will examine additional indices of glycaemic control (via CGM-derived variables (including 24 hours mean, area under the curve (AUC), glycaemic variability, time in range, etc) and a mixed meal tolerance test (MMTT)), vascular function (endothelial function and arterial stiffness), metabolic control (blood lipids and inflammation) and body composition (BMI, total and regional fat and fat-free mass). Tertiary outcome measures will focus on the efficacy, feasibility and adherence to exercise prescription (accelerometer and surveys). Apart from the midintervention assessment, participants will resume normal daily living (not exercise at their prescribed time) to assess training effects.
Glycaemic control
The primary outcome of glycaemic control will be assessed by measuring HbA1c. A finger-prick blood sample will be collected using a HbA1c (~2 µL) specific test disc and immediately analysed with the Cobas b 101 System (Roche Diagnostics).
Secondary glycaemic outcomes will also be assessed with CGM and a MMTT (low glycaemic index, Glucerna). From each 2-week CGM, we will calculate mean 24 hours glucose, 24 hours and 3 hours postprandial AUC and incremental area under the curve (iAUC) calculated using the trapezoid method,24 hyperglycaemia (time spent ≥10 mmol/L), glycaemic variability (mean amplitude of glycaemic variability) and nocturnal glucose profiles. We will also calculate mean glucose, total AUC and iAUC for 2 hours following the MMTT. The MMTT will begin after an overnight fast (>10 hours), and blood glucose will be measured with the CGM and finger pricks (0, 15, 30, 60, 90 and 120 min) following drink consumption.
Metabolic control
Metabolic control will be assessed by measuring blood lipids (triglyceride, total cholesterol, high-density lipoprotein and low-density lipoprotein) and inflammation (CRP). Finger-prick blood samples will be collected via lipid (~19 µL) or inflammation (~12 µL) specific test discs and immediately analysed with the Cobas b 101 System.
Body composition
Waist to hip ratio, height and weight will be measured to the nearest 0.1 cm and 0.1 kg, respectively, using standard scales, a stadiometer and measuring tape. Total and regional fat and fat-free mass will be measured by DEXA (MedixDR Whole Body DEXA, Sydney, Australia).
Vascular function
Endothelial function will be assessed by measuring endothelium-dependent flow-mediated dilation (FMD). This technique uses ultrasound imaging (Terason uSmart 3300) of the brachial artery. Following 10–15 min of laying supine (at rest), a longitudinal section of the brachial artery, 2–3 cm above the antecubital fossa, will be imaged using B-mode ultrasound imaging (insonation angle of 60°). A blood pressure cuff placed around the forearm, 1–2 cm below the olecranon process, will then be rapidly inflated to ~60 mm Hg above resting systolic blood pressure for 5 min. Brachial artery diameter and blood flow velocity will be recorded for 1 min before cuff inflation (baseline), ~30 s prior to cuff release (ischaemic stimulus), and 3 min following cuff release (recovery).25 26 The ~5 min recording will then be analysed with custom-designed edge-detection and wall-tracking software (Cardiovascular Suite, Quipu, Italy) which reduces user bias and increases accuracy. FMD will be reported as an absolute change in artery diameter (absolute FMD = postocclusionmean diameter − preocclusionmean diameter), and a relative change in artery diameter from baseline (%FMD=100 × (absolute FMD/preocclusionmean diameter)). Allometric scaling will be used to account for potential confounders from baseline diameter.26 27
Blood flow (mL/min) will be measured using non-invasive Doppler from the cross-sectional area and blood velocity (velocity × π × (diameter2/4)×60). Shear rate (s-1) will then be determined from the diameter and velocity measures (four times velocity/diameter).28 Shear rate area under the curve (SRAUC) will automatically be calculated from the diameter and velocity measures from the time of cuff release to peak dilation of the artery. Antegrade and retrograde mean blood velocities will be used to calculate baseline antegrade and retrograde shear rates (four times mean baseline antegrade or retrograde velocity ÷ mean baseline diameter), and the mean blood flow to mean arterial pressure ratio will be used to measure vascular conductance (mL/min/mmHg).25 26
Central arterial stiffness will be assessed via pulse wave analysis (PWA) and pulse wave velocity (PWV) measurements (SphygmoCor XCEL System, AtCor Medical). PWA will be used to measure central blood pressure. A brachial blood pressure cuff will be inflated and the central aortic pressure waveform, derived from pulsations at the brachial artery, will be recorded for 5 s and then automatically analysed through the SphygmoCor software. Key parameters of central blood pressure and arterial stiffness will be determined from the aortic waveform including systolic pressure, diastolic pressure, pulse pressure, aortic pressure, augmentation index and mean arterial pressure. PWV will be measured by holding a tonometer on the carotid artery for 10–15 s, while a femoral blood pressure cuff is automatically inflated. Once fully inflated, the femoral cuff and carotid tonometer will simultaneously record a 10 s capture of the carotid and femoral pressure waveforms. PWV will then be calculated by dividing the carotid–femoral distance by the pulse transit time; the carotid–femoral distance will be calculated by subtracting the proximal distance (distance between the carotid artery and sternal notch) from the distal distance (distance between the sternal notch and proximal edge of the femoral cuff) (PWV (m/s) = (distal – proximaldistance)/transit time).29 Measurements will be performed in duplicate. A third measurement will be taken if the difference between the two PWV values is >0.5 m/s and the average of the three values will be used.
An automatic blood pressure monitor (Oscar2 Ambulatory Blood Pressure Monitor with SphygmoCor interfacing, SunTech Medical) will also be used to continuously assess blood pressure and pulse wave analyses every hour for 24 hours. We will report 24 hours blood pressure as an average of 24 measurements.
Diet and physical activity monitoring
Participants will complete a 7-day diet record during each 2-week monitoring period. Food diaries will be analysed (using FoodWorks10 Nutrition Software) to confirm macronutrient composition and total energy intake are consistent throughout the study period. Physical activity will be monitored during the same 7-day period using an accelerometer (ActiGraph Bluetooth Smart wGT3X-BT), worn around the waist during wake hours. Physical activity and sedentary time will be compared between groups at each timepoint during wake hours. The accelerometers will also be used to confirm exercise intensity and compliance to the exercise prescription. A heart rate monitor (Polar H7 Bluetooth Heart Rate Monitor) will be worn during the midpoint monitoring period on the same days as the accelerometer, only during the exercise sessions, to assess exercise intensity.
Adherence and lifestyle questionnaires
Participants will complete a quality of life (SF-36) survey and a self-regulatory efficacy and physical activity questionnaire during each 2-week monitoring period. Participants will also complete three surveys during the follow-up period, one at the end of each month that is specific to their exercise group, to assess adherence to exercise prescription between the ExPeak and control groups. Surveys will include questions on known perceived facilitators and barriers to filling the exercise prescription and, in turn, support or aggravation of intervention efficacy. Such factors will include the availability of nearby green and open spaces (eg, beaches, parks)30 and levels of felt safety to exercise outdoors during the day and evening hours.31
Remote participants
Participants who cannot attend the university (eg, due to COVID-19 restrictions) for in-lab assessments will receive a home-based testing kit (via mail) which includes a dried blood spot test kit (ZRT Laboratory kit for measurement of HbA1c, lipids, CRP and insulin), CGM, accelerometer and Glucerna MMTT drink. Instructions will be provided and followed-up via a phone or video call. All other study protocols will be the same, however data for the DEXA and vascular assessments will not be available.
Statistical analysis
Sample size
Sample size was calculated based on a previous study investigating the effect of exercise timing in people with T2D, where they reported a difference of −0.6 mmol/L in 24 hours blood glucose between exercise performed in the morning versus afternoon.14 To detect a clinically meaningful change in HbA1c between groups, with a moderate effect size of 0.2, statistical power of 80%, and an alpha level of 0.05 (two-sided), a total of ~54 participants (27 per intervention group) is required for this trial. The power calculation is based on the change in HbA1c from a previous trial in our lab in people with T2D.32 To account for an expected 30% drop-out rate, 70 participants will be recruited.
Statistics
This study will be reported according to the CONSORT 2010 Statement and the CONSERVE 2021 Statement for randomised controlled trials. Descriptive statistics will be assessed (means, SD and frequencies), and histograms, Q–Q plots and the Shapiro-Wilk test will be used to identify outliers and test for normality. Linear mixed models (with time × intervention, and main effect of time) will be used to assess differences between groups, for primary (HbA1c) and secondary (CGM, MMTT, vascular function, metabolic control and body composition) outcomes. Tertiary outcomes (eg, adherence to the exercise prescription) will be analysed from the accelerometer and follow-up surveys (QualtricsXM). Attention to treat analyses will be performed for primary analyses (Phase 1) and per protocol analyses will be undertaken for secondary and tertiary outcomes (Phase 2). Data with skewed distribution will be log-transformed or square-rooted prior to the statistical analysis. For the 3-month follow-up, intention to treat analysis will be used and missing data will not be imputed.
Patient and public involvement
No patient involved.
Ethics and dissemination
This research has been reviewed and approved by the University of Wollongong Human Research Ethics Committee (2019/ETH09856). This trial was prospectively registered at the Australian New Zealand Clinical Trials Registry (ACTRN12619001049167). Written informed consent will be obtained from all eligible individuals prior to commencement of the trial. Participants will remain anonymous, and all collected data will be deidentified and coded. An alpha-numerical code (stored on a password-protected central spreadsheet) will be allocated to each participant and used for identification on all subsequent paperwork. All results from the study will be published as peer-reviewed articles in international journals, presented at international conferences and promoted through social media. Changes to the protocol due to COVID-19 will be reported according to the CONSERVE 2021 Statement.33
Discussion
The primary objective of this trial is to determine if strategically timing exercise, to reduce daily peak hyperglycaemia, will improve glycaemic control and lower cardiovascular risk factors in people with T2D. This is the first study to investigate whether prescribing exercise that is personalised to target daily peak hyperglycaemia, using CGM, can improve cardiovascular risk factors in T2D. Based on evidence from prior research,11 34–36 it is hypothesised that strategically timing daily exercise to attenuate peak hyperglycaemia will improve glycaemic control (HbA1c), and the reduction in peak glycaemia will improve vascular function (endothelial function and arterial stiffness), blood lipids and CRP, more than exercising not at peak hyperglycaemia or control standard care (ie, physical activity guidelines).
Recent evidence suggests exercise timing may be important to offset circadian rhythms14 and to target postprandial hyperglycaemia11 in T2D. However, there are no recommendations for exercise timing in the current physical activity guidelines (ie, physical activity can be accumulated at any time throughout the week). Further, adherence to the current recommendations is notoriously poor. Regardless of the effectiveness for an intervention to improve diabetes management, findings will only be translatable if patients comply with and adopt to the treatment over the long-term. Therefore, adherence to prescribed daily exercise time (ie, creating more of a habit) will be assessed for 3 months following the 8-week intervention. Exercising at the time of peak hyperglycaemia may improve self-efficacy to the exercise prescription, as results from the CGM data (pre/mid/post 8-week intervention) will allow participants to see the direct impact of exercise on blood glucose levels. Use of CGM in this trial not only offers the distinct advantage of determining time of peak hyperglycaemia, but will also allow us to examine any changes in daily glycaemic patterns, such as glycaemic variability, which are more closely related to cardiovascular risk than HbA1c.37 If strategically timing exercise to attenuate peak hyperglycaemia improves long-term glycaemic control (HbA1c), reduces cardiovascular risk (endothelial dysfunction and arterial stiffness) and improves exercise adherence then this may be an alternative recommendation for physical activity prescription in people with T2D.
Strengths and limitations
This is the first randomised controlled trial to examine the effects of personalising exercise timing to attenuate peak hyperglycaemia (determined via CGM technology) on cardiometabolic and vascular health outcomes in individuals with T2D. This study will be conducted in free-living conditions, with exercise performed at home and contact/delivery of Phase 1 (8-week exercise intervention) mirroring standard care (five telehealth calls with an exercise physiologist), while Phase 2 (3-month follow-up) will assess adherence to the exercise prescription (with minimal contact from the research team); thus informing us of the real-world applicability of the proposed exercise prescription. In addition, this study will use a variety of data collection methods (in-lab and free-living) to objectively measure cardiometabolic health, vascular function, physical activity and behaviour change across the trial. Due to the COVID-19 pandemic, remote participants from across rural and urban Australia will be included, allowing for a wider range of individuals to be recruited while adhering to the COVID-19 restrictions. However, this is also a limitation as the vascular and body composition measures will be excluded for those who cannot attend university assessments, and dried blood spot testing kits will be used rather than the gold-standard plasma measurement of HbA1c. Finally, a limitation of the waitlist control group is the potential overestimation of intervention effects and bias in favour of the treatment group. Nevertheless, inclusion of the waitlist control group will provide insight on the cause–effect relationship between the intervention and subsequent health outcomes/behaviour changes, as these participants will follow a delayed-start design (ie, will receive treatment following the waitlist period), thus allowing for direct comparisons to be made under various conditions with reduced error variance and not withholding treatment to individuals.
Supplementary Material
Footnotes
Contributors: CRC drafted the manuscript. MEF, CRC, BMR and TA-B conceived and contributed to the design of the study and plan for analysis. MEF and CRC will conduct the study, collect data and analyse data. MEF, CRC and TA-B will analyse and interpret the data. All authors reviewed and approved the final manuscript.
Funding: This trial was funded by a University of Wollongong Small Grant and Revitialise Research Grant. MEF’s time was supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (APP1177234). TA-B’s time was supported by a NHMRC Boosting Dementia Research Leader Fellowship (GNT1140317).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani M, DeFronzo RA, Del Prato S, et al. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? Diabetes Care 2017;40:813–20. 10.2337/dc16-2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heden TD, Kanaley JA. Syncing exercise with meals and circadian clocks. Exerc Sport Sci Rev 2019;47:22–8. 10.1249/JES.0000000000000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Defronzo RA. Banting lecture. from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95. 10.2337/db09-9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A. The glucose triad and its role in comprehensive glycaemic control: current status, future management. Int J Clin Pract 2010;64:1705–11. 10.1111/j.1742-1241.2010.02517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson PD, Crouse SF, Goodpaster B, et al. The acute versus the chronic response to exercise. Med Sci Sports Exerc 2001;33:S438–45. 10.1097/00005768-200106001-00012 [DOI] [PubMed] [Google Scholar]
- 7.Horowitz JF. Exercise-Induced alterations in muscle lipid metabolism improve insulin sensitivity. Exerc Sport Sci Rev 2007;35:192–6. 10.1097/jes.0b013e318156e084 [DOI] [PubMed] [Google Scholar]
- 8.Richter EA, Derave W, Wojtaszewski JF. Glucose, exercise and insulin: emerging concepts. J Physiol 2001;535:313–22. 10.1111/j.1469-7793.2001.t01-2-00313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull FC, Al-Ansari SS, Biddle S, et al. World Health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–9. 10.1001/jama.2011.576 [DOI] [PubMed] [Google Scholar]
- 11.Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med 2018;48:1479–91. 10.1007/s40279-018-0864-x [DOI] [PubMed] [Google Scholar]
- 12.Teo SYM, Kanaley JA, Guelfi KJ, et al. Exercise timing in type 2 diabetes mellitus: a systematic review. Med Sci Sports Exerc 2018;50:2387–97. 10.1249/MSS.0000000000001732 [DOI] [PubMed] [Google Scholar]
- 13.Aqeel M, Forster A, Richards E, et al. The effect of timing of exercise and eating on postprandial response in adults: a systematic review. Nutrients 2020;12:221. 10.3390/nu12010221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 2019;62:233–7. 10.1007/s00125-018-4767-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teo SYM, Kanaley JA, Guelfi KJ, et al. The effect of exercise timing on glycemic control: a randomized clinical trial. Med Sci Sports Exerc 2020;52:323–34. 10.1249/MSS.0000000000002139 [DOI] [PubMed] [Google Scholar]
- 16.Kapuku GK, Harshfield GA, Davis HC, et al. Early markers of cardiovascular disease. Vascul Pharmacol 2006;45:277–80. 10.1016/j.vph.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Jones H, Thijssen D, et al. Flow-Mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 2011;57:363–9. 10.1161/HYPERTENSIONAHA.110.167015 [DOI] [PubMed] [Google Scholar]
- 18.Colette C, Monnier L. Acute glucose fluctuations and chronic sustained hyperglycemia as risk factors for cardiovascular diseases in patients with type 2 diabetes. Horm Metab Res 2007;39:683–6. 10.1055/s-2007-985157 [DOI] [PubMed] [Google Scholar]
- 19.Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health 2015;73:1–9. 10.1186/s13690-015-0088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceriello A. Point: postprandial glucose levels are a clinically important treatment target. Diabetes Care 2010;33:1905–7. 10.2337/dc10-0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer KA, Pivarnik JM, Womack CJ, et al. Reliability and validity of the Borg and Omni rating of perceived exertion scales in adolescent girls. Med Sci Sports Exerc 2002;34:2057–61. 10.1097/00005768-200212000-00029 [DOI] [PubMed] [Google Scholar]
- 24.Le Floch JP, Escuyer P, Baudin E, et al. Blood glucose area under the curve. methodological aspects. Diabetes Care 1990;13:172–5. 10.2337/diacare.13.2.172 [DOI] [PubMed] [Google Scholar]
- 25.Francois ME, Durrer C, Pistawka KJ, et al. Resistance-based interval exercise acutely improves endothelial function in type 2 diabetes. Am J Physiol Heart Circ Physiol 2016;311:H1258–67. 10.1152/ajpheart.00398.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019;40:2534–47. 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- 27.Atkinson G, Batterham AM. The percentage flow-mediated dilation index: a large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med 2013;18:354–65. 10.1177/1358863X13508446 [DOI] [PubMed] [Google Scholar]
- 28.Green D, Cheetham C, Henderson C, et al. Effect of cardiac pacing on forearm vascular responses and nitric oxide function. Am J Physiol Heart Circ Physiol 2002;283:H1354–60. 10.1152/ajpheart.00050.2002 [DOI] [PubMed] [Google Scholar]
- 29.Butlin M, Qasem A. Large artery stiffness assessment using SphygmoCor technology. Pulse 2017;4:180–92. 10.1159/000452448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astell-Burt T, Feng X, Kolt GS. Green space is associated with walking and moderate-to-vigorous physical activity (mvpA) in middle-to-older-aged adults: findings from 203 883 Australians in the 45 and up study. Br J Sports Med 2014;48:404–6. 10.1136/bjsports-2012-092006 [DOI] [PubMed] [Google Scholar]
- 31.Dendup T, Feng X, O'Shaughnessy P, et al. Perceived built environment and type 2 diabetes incidence: exploring potential mediating pathways through physical and mental health, and behavioural factors in a longitudinal study. Diabetes Res Clin Pract 2021;176:108841. 10.1016/j.diabres.2021.108841 [DOI] [PubMed] [Google Scholar]
- 32.Francois ME, Durrer C, Pistawka KJ, et al. Combined interval training and post-exercise nutrition in type 2 diabetes: a randomized control trial. Front Physiol 2017;8:528. 10.3389/fphys.2017.00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other Extenuating circumstances. JAMA 2021;326:257. 10.1001/jama.2021.9941 [DOI] [PubMed] [Google Scholar]
- 34.Ceriello A, Esposito K, Piconi L, et al. Glucose "peak" and glucose "spike": Impact on endothelial function and oxidative stress. Diabetes Res Clin Pract 2008;82:262–7. 10.1016/j.diabres.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 35.Ceriello A, Taboga C, Tonutti L, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation 2002;106:1211–8. 10.1161/01.CIR.0000027569.76671.A8 [DOI] [PubMed] [Google Scholar]
- 36.Lunder M, Janić M, Japelj M, et al. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol 2018;17:1–8. 10.1186/s12933-018-0797-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005;54:1–7. 10.2337/diabetes.54.1.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057183supp001.pdf (940.8KB, pdf)