Supplemental Digital Content is available in the text.
Keywords: rotavirus, genotype, vaccine, effectiveness, strain
Background:
Rotavirus causes 215,000 deaths from severe childhood diarrhea annually. Concerns exist that a monovalent vaccine (RV1) and a pentavalent vaccine (RV5) may be less effective against rotavirus strains not contained in the vaccines. We estimated the vaccine effectiveness (VE) of RV1 and RV5 against severe rotavirus gastroenteritis caused by vaccine (homotypic) and nonvaccine (partially and fully heterotypic) strains.
Methods:
After conducting a systematic review, we meta-analyzed 31 case-control studies (N = 27,293) conducted between 2006 and 2020 using a random-effects regression model.
Results:
In high-income countries, RV1 VE was 10% lower against partially heterotypic (P = 0.04) and fully heterotypic (P = 0.10) compared with homotypic strains (homotypic VE: 90% [95% confidence intervals (CI): 82–94]; partially heterotypic VE: 79% [95% CI: 71–85]; fully heterotypic VE: 80% [95% CI: 65–88]). In middle-income countries, RV1 VE was 14–16% lower against partially heterotypic (P = 0.06) and fully heterotypic (P = 0.04) compared with homotypic strains (homotypic VE: 81% [95% CI: 69–88]; partially heterotypic VE: 67% [95% CI: 54–76]; fully heterotypic VE: 65% [95% CI: 51–75]). Strain-specific RV5 VE differences were less pronounced, and primarily derived from high-income countries. Limited data were available from low-income countries.
Conclusions:
Vaccine effectiveness of RV1 and RV5 was somewhat lower against nonvaccine than vaccine strains. Ongoing surveillance is important to continue long-term monitoring for strain replacement, particularly in low-income settings where data are limited.
Rotavirus is the leading cause of severe childhood diarrhea globally, causing 215,000 deaths annually.1 In 2009, the World Health Organization recommended rotavirus vaccine introduction for all national childhood immunization programs.2 Since then, 2 live-attenuated rotavirus vaccines, RV1 and RV5, have been introduced in over 100 countries.3 The global impact of rotavirus vaccination has been profound, with a 59% decrease in the proportion of hospital admissions and a 36% decrease in diarrhea deaths due to rotavirus among children younger than 5 years old in countries that have introduced rotavirus vaccine.4 The vaccine effectiveness of RV1 and RV5 varies by setting, with diminished vaccine effectiveness in low-and-middle-income countries compared with high-income countries.5,6
Six rotavirus strains predominantly cause disease in humans, G1P[8], G2P[4], G3P[8], G4P[8], G9P[8], and G12P[8].7 The nomenclature for these strains is based on the antigenic and genetic differences of 2 outer-capsid proteins: VP7, a glycoprotein (G), and VP4, a protease-cleaved protein (P). These proteins are responsible for initiating neutralization activity and are primary targets for vaccine development. Differences between strains may influence naturally acquired immunity, as suggested by birth cohort studies indicating that the risk of reinfection may depend on the strains of prior infections.8,9
RV1 is a monovalent vaccine composed of a single human strain, G1P[8], and RV5 is a pentavalent vaccine composed of reassortment of G1, G2, G3, G4, and P[8] strains. Given the strain composition of these vaccines, especially RV1, relative to the full genetic diversity of rotaviruses, concerns exist that these vaccines may be less effective against severe rotavirus disease caused by strains not contained in the vaccines (heterotypic strains) compared with vaccine-type strains (homotypic). Even a small difference in the strain-specific vaccine effectiveness (VE) could cause selective pressure over time toward strains that are able to evade vaccine-induced immunity and hinder the benefits of rotavirus vaccination, although natural fluctuations in circulating strains are expected to continue postvaccine introduction.10 While clinical trials of RV1 and RV5 showed evidence of good homotypic and heterotypic protection, a lower VE of RV1 was seen in a large Latin American trial.11 Additionally, initial postintroduction data from Latin American countries and from Australia showed a dominance of heterotypic strains after implementation of RV1, although this may have been a result of natural fluctuations in strain distribution rather than selective pressure.12
A 2014 meta-analysis of postlicensure studies reassuringly supported evidence from clinical trials suggesting that RV1 and RV5 were highly effective against both homotypic and heterotypic strains. However, that prior meta-analysis was limited to early adopter countries and lacked data from low- and middle-income countries. Thus, an updated evaluation of strain-specific VE is warranted. Since the previous meta-analysis in 2014, more than 40 countries have introduced a rotavirus vaccine, including many low- and middle-income countries. Given the high strain diversity and lower VE of rotavirus vaccines in low- and middle-income countries, concerns about broad protection against heterotypic strains are even greater in these settings.
Here, we updated the literature search and meta-analysis conducted in the 2014 with data through October 2020. Our primary objective was to assess RV1 and RV5 VE against homotypic and heterotypic strains by examining data from published postlicensure case-control studies among children under 5 years of age conducted in high-, middle-, and low-income countries globally.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guided the design of this systematic review and meta-analysis.13 To supplement articles included in the prior 2014 literature review,6 reports published between January 24, 2014, and October 16, 2020, were identified from MEDLINE, EMBASE, CAB Abstracts, and Global Health databases. Eligibility criteria differed slightly for this updated review, so studies included in the 2014 systematic review were rereviewed for eligibility.6 The exact search strategy used in the 2014 literature review was implemented, using search terms including “rotavirus,” “surveillance,” “genotype,” “strain,” “case control studies,” and “effectiveness studies” (see Text, Supplemental Digital Content 1, http://links.lww.com/INF/E493). No language restrictions were imposed on the search.
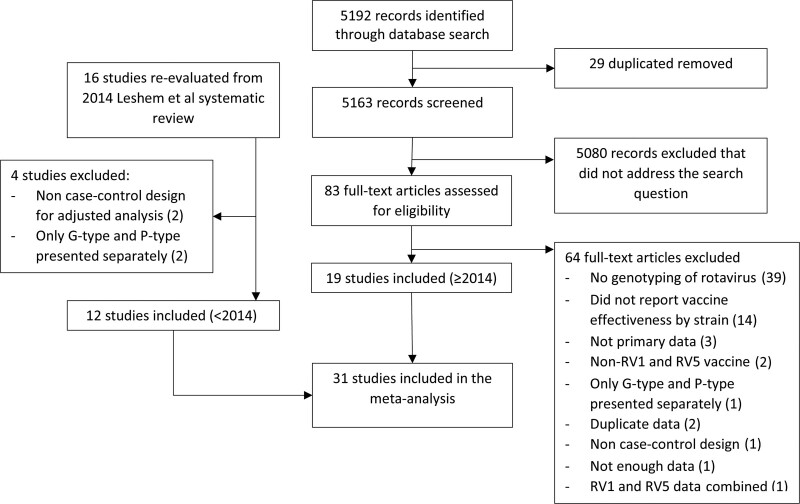
Studies were eligible if they were observational case-control studies that reported VE of RV1 or RV5 against acute gastroenteritis caused by specific rotavirus strains (strain-specific VE). Studies were also eligible if they reported overall VE but 1 rotavirus strain was detected in over 80% of rotavirus cases. For these studies, the overall VE was used as a proxy for the strain-specific VE for the predominant strain; studies eligible per these criteria were excluded in a sensitivity analysis (SA1). Studies were also eligible if they reported VE for RV1 and RV5 combined, but 1 vaccine type accounted for >80% of vaccinations. Studies eligible per this 80% threshold were categorized based on the predominant vaccine type but were excluded in a sensitivity analysis (SA2). Studies were excluded if they were clinical trials, duplicate data, calculated VE using study designs other than a case-control methodology, did not provide enough data, or did not report VE by strain (Fig. 1). Non-case-control studies, such as cohort studies or studies using a screening method, were excluded in the primary analysis to reduce methodologic heterogeneity but included in a sensitivity analysis (SA3). The prior literature review included studies with VE by G-type or P-type only, while this review only included studies with full G-P typing.
FIGURE 1.
Study selection.
Data Extraction and Variable Definition
Covidence software (covidence.org) was used to manage article screening, full-text review, and data extraction by 2 of 5 independent reviewers (JC, AA, JP, AR, JW). Disagreements between reviewers were resolved by consensus or consultation with an additional reviewer (JT, UP, or BL). General study characteristics were extracted, including first author, publication date, year(s) of study, type of rotavirus vaccine (RV1 or RV5) and country. The primary outcome of interest was the adjusted odds ratio or VE (1-odds ratio) and corresponding confidence intervals (CI) for the odds of rotavirus vaccination (RV1 or RV5) comparing cases of acute gastroenteritis caused by specific rotavirus strains to controls. If there was more than 1 type of control, estimates were abstracted based on the following a priori hierarchy to minimize heterogeneity: test-negative controls, community-based controls, then hospital-based controls. Test-negative controls are patients with acute gastroenteritis who test-negative for rotavirus and are used extensively to measure rotavirus VE.14 VE of a full course of rotavirus vaccination was prioritized for extraction, and if not available, data on at least 1 dose of rotavirus vaccine compared with no vaccination was extracted. Rotavirus strains were categorized based on their alignment with the vaccine strains; specifically, strains were categorized as fully homotypic if both G-type and P-type antigens were in the vaccine, fully heterotypic if neither the G-type nor the P-type antigens were in the vaccine, or partially heterotypic if either the G-type or P-type antigen was in the vaccine. Methodologic details of each study were extracted to assess heterogeneity between studies. Data were extracted on the age criteria, laboratory processes, location where acute gastroenteritis cases were identified (in-patient ward or emergency department), and the method of vaccine confirmation. Consistent with the prior 2014 meta-analysis, countries were categorized as high-, middle-, and low-income based on level of economic development (gross national income per person) at the time of study implementation, using the World Bank’s classification.15 Also consistent with the prior 2014 meta-analysis, 2 studies done in Australia and 1 study done in Israel were classified as a middle-income setting because most cases belonged to subpopulations of lower socioeconomic, with a sensitivity analysis (SA4) conducted classifying them per their national income level.16–18
Statistical Analysis
Study-specific effect estimates (log of the odds ratio) were pooled using a single DerSimonian and Laird restricted maximum likelihood method random-effects model. We a priori decided to estimate strain-specific VE by vaccine type and the country’s income level because of the substantial heterogeneity of overall VE of rotavirus vaccines by income level5 and plausible variability in strain-specific VE by vaccine type due to vaccine strain composition. We conducted a meta-regression analysis using a mixed-effects model including strain type (homotypic, partially, or fully heterotypic), vaccine type (RV1 or RV5), and income level (high, middle, or low) and interactions between these modifiers to compare partially and fully heterotypic VE against homotypic VE. Confidence intervals for the absolute difference in the VE were obtained by bootstrap using 1000 resamples. Statistical heterogeneity was evaluated with the I2 statistic. Potential publication bias was assessed by evaluating asymmetry of “funnel plots,” plots of the effect estimates by the inverse of the standard error, using the Eggers test for funnel plot asymmetry.
RESULTS
We identified 5192 studies and after abstract screening, 29 duplicate records and 5080 irrelevant publications were excluded. Of the remaining 83 studies assessed by full-text review, 64 studies were excluded for the following reasons: no rotavirus genotyping data, no VE by strain, not primary data, non-RV1 or RV5 vaccine, only presented G-type and P-type results separately, duplicate data, not a case-control study, not enough data; 1 study reported combined VE for RV1 and RV5 and did not meet the threshold for >80% of 1 vaccine type (Fig. 1). Sixteen publications included in the 2014 systematic review were re-evaluated for eligibility; 2 studies were deemed ineligible for inclusion because they were not case-control studies19,20 and 2 studies were excluded that only presented G-type and P-type results separately.21,22 Therefore, a total of 31 case-control studies were included in this meta-analysis (12 publications included in Leshem et al and 19 new publications).16–18,21,23–49 There were 12 studies in high-income countries, 17 studies classified as middle-income, and 2 studies from low-income countries, for a total of 27,293 participants (5219 cases and 22,207 controls) (Table 1).
TABLE 1.
Characteristics of 31 Studies Included in the Meta-analysis of Strain-specific Vaccine Effectiveness of RV1 and RV5
| Country | Country Income Level | Vaccine Type | Study Period | Strains | Sample Size Included in Strain-specific Analyses | Author, Publication Year |
|---|---|---|---|---|---|---|
| Belgium | High | RV1 | 2008–2010 | G1P[8], G2P[4] | 121 cases, 156 controls | Braeckman, 2012 |
| Belgium | High | RV1 | 2008–2010 | G3P[8], G4P[8] | 28 cases, 30 controls | Matthijnssens, 2014 |
| Japan | High | RV1, RV5 | 2014–2015 | G1P[8], G9P[8], G2P[4] | 440 cases, 903 controls | Araki, 2018 |
| Japan | High | RV1 and RV5 combined* | 2017 | G8P[8] | 41 cases, 24 controls | Hoque, 2018 |
| Taiwan | High | RV1, RV5 | 2009–2011 | G1P[8] | 127 cases, 893 controls | Chang, 2014 |
| Taiwan | High | RV1, RV5 | 2014–2017 | G1P[8], G2P[4], G3P[8], G9P[8] | 312 cases, 1212 controls | Huang, 2020 |
| United States | High | RV1, RV5 | 2010–2011 | G1P[8], G2P[4] | 145 cases, 353 controls | Cortese, 2013 |
| United States | High | RV1, RV5 | 2009–2011 | G3P[8], G12P[8], G1P[8], G2P[4] | 373 cases, 1966 controls | Payne, 2013 |
| United States | High | RV1, RV5 | 2012–2013 | G1P[8], G2P[4], G3P[8], G12P[8] | 433 cases, 2852 controls | Payne, 2015 |
| United States | High | RV1, RV5 | 2013 | G12P[8]† | 73 cases, 103 controls | Immergluck, 2016 |
| United States | High | RV5 | 2008 | G3P[8] | 31 cases, 79 controls | Boom, 2010 |
| Israel | High | RV5 | 2011–2015 | G1P[8] | 20 cases, 628 controls | Muhsen, 2018 |
| Australia | Middle | RV1 | 2007 | G9P[8]† | 21 cases, 83 controls | Snelling, 2009 |
| Australia | Middle | RV1 | 2008–2009 | G2P[4]† | 36 cases, 94 controls | Snelling, 2011 |
| Bolivia | Middle | RV1 | 2010–2011 | G2P[4], G3P[8], G9P[6], G9P[8] | 189 cases, 586 controls | Patel, 2013 |
| Bolivia | Middle | RV1 | 2013–2014 | G3P[8], G9P[8] | 109 cases, 419 controls | Pringle, 2016 |
| Botswana | Middle | RV1 | 2013–2015 | G2P[4] | 45 cases, 317 controls | Gastanaduy, 2016 |
| Brazil | Middle | RV1 | 2008–2011 | G1P[8], G2P[4] | 82 cases, 1682 controls | Ichihara, 2014 |
| Brazil | Middle | RV1 | 2006–2008 | G2P[4] | 22 cases, 183 controls | Corriera, 2010 |
| Brazil | Middle | RV1 | 2008–2009 | G2P[4] | Matched pairs: 222 neighborhood controls, 286 hospital controls | Justino, 2011 |
| El Salvador | Middle | RV1 | 2007–2009 | G1P[8]† | 251 cases, 770 controls | De Palma, 2010 |
| Guatemala | Middle | RV1, RV5 | 2012–2013 | G12P[8]† | 228 cases, 263 controls | Gastanaduy, 2016 |
| Kenya | Middle | RV1 | 2014–2017 | G1P[8], G2P[4] | 50 cases, 365 controls | Khagayi, 2019 |
| Lebanon | Middle | RV1 and RV5 combined* | 2011–2013 | G1P[8], G9P[8], G2P[4], G4P[8] | 409 cases, 930 controls | Ali, 2016 |
| Mexico | Middle | RV1 | 2010 | G9P[4] | 9 cases, 17 controls | Yen, 2011 |
| South Africa | Middle | RV1 | 2010–2012 | G12P[8] | 230 cases, 1100 controls | Groome, 2014 |
| Israel | Middle | RV5 | 2011–2013 | G1P[8] | 63 cases, 228 controls | Leshem, 2016 |
| Nicaragua | Middle | RV5 | 2007–2008 | G2P[4]† | 231 cases, 1044 controls | Patel, 2009 |
| Nicaragua | Middle | RV5 | 2008–2010 | G2P[4], G1P[8], G3P[8] | 733 cases, 3749 controls | Patel, 2016 |
| Malawi | Low | RV1 | 2012–2015 | G1P[8], G2P[4] | 79 cases, 692 controls | Bar-Zeev, 2016 |
| Tanzania | Low | RV1 | 2014–2015 | G1P[8]† | 66 cases, 131 controls | Platts-Mills, 2017 |
*Both RV1 and RV5 combined for VE effectiveness by strain, but >80% received RV1 so classified as RV1 in meta-analysis. Excluded in sensitivity analysis (Table, Supplemental Digital Content 3, http://links.lww.com/INF/E495).
†Overall VE used, but 1 strain was >80% dominant so VE classified as that strain. Excluded in sensitivity analysis (Table, Supplemental Digital Content 3, http://links.lww.com/INF/E495).
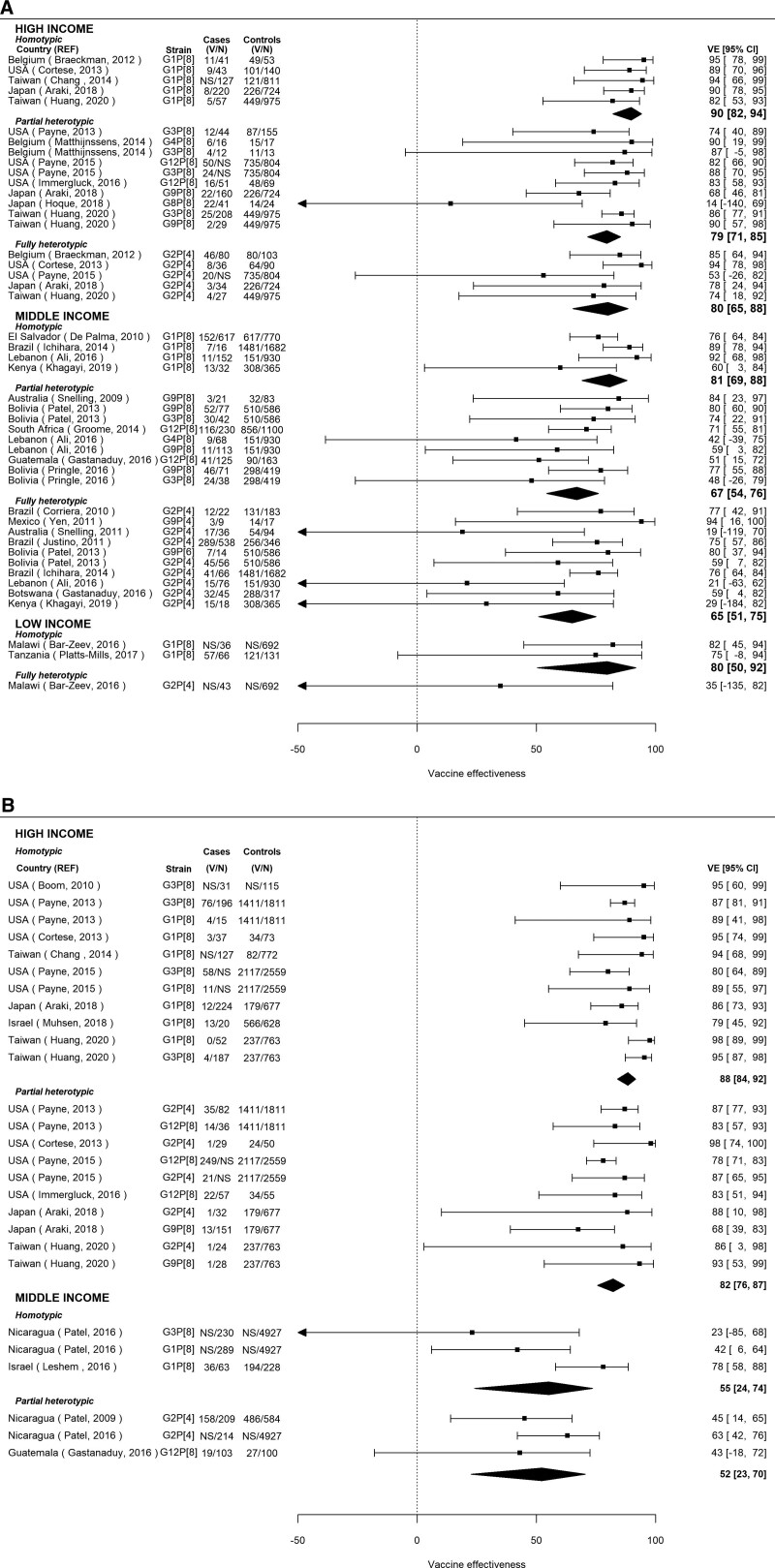
For RV1, VE was highest in high-income countries, and there was a consistently reduced VE (increased odds ratio) for partially or fully heterotypic compared with homotypic strains (Fig. 2A). In high-income countries, VE calculated from the pooled OR was 90% (95% CI: 82–94; Number of studies [N] = 5) against homotypic strains, 79% (95% CI: 71–85; N = 10) against partially heterotypic strains, and 80% (95% CI: 65–88; N = 5) against fully heterotypic strains, translating into an approximately 10% lower VE against partially and fully heterotypic compared with homotypic strains (VE difference = –10.2 [95% CI: –20.8 to –2.6] and –9.6 [95% CI: –27.0 to 1.4]). When combining partially and fully heterotypic strains, the VE was 80% (95% CI: 73–85; N = 15), for a VE difference of –10.0 (95% CI: –17.7 to –2.0) compared with homotypic strains (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/E494). In middle-income countries, RV1 VE was 81% (95% CI: 69–88; N = 4), 67% (95% CI: 54–76; N = 9), and 65% (95% CI: 51–75; N = 10) against homotypic, partially heterotypic, and fully heterotypic strains, translating into an approximately 14–16% lower VE against partially and fully heterotypic compared with homotypic strains (VE difference = –13.9 [95% CI: –29.8 to 5.2] and –15.8 [95% CI: –34.6 to 0.6]). When combining partially and fully heterotypic strains, the VE was 66% (95% CI: 58–73; N = 19), for a VE difference of –14.6 (95% CI: –29.4 to –0.1) compared with homotypic strains (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/E494). In 2 low-income countries, RV1 VE was 80% (95% CI: 50–92) against homotypic strains, and 1 low-income study estimated an RV1 VE of 35% (95% CI: –135 to 82) against heterotypic strains.
FIGURE 2.
A: Vaccine effectiveness by country income level and strain type for RV1. The 95% confidence intervals may differ slightly from original publications due to small differences in back-calculation of odds ratios from abstracted vaccine effectiveness estimates or truncation of lower limits at 0 (REF Ali 2016). B: Vaccine effectiveness by country income level and strain type for RV5. NS indicates not specified, i.e., raw numbers not specified in the paper.
For RV5, VE was also highest in high-income countries, but the difference between VE against partially heterotypic and homotypic strains was minimal (Fig. 2B; Table 2). In high-income countries, VE was 88% (95% CI: 84–92; N = 11) and 82% (95% CI: 76–87; N = 10) against fully homotypic and partially heterotypic. In middle-income countries, VE was 55% (95% CI: 24–74; N = 3) and 52% (95% CI: 23–70; N = 3) against fully homotypic and partially heterotypic strains. In high- and middle-income countries respectively, the VE was approximately 6% and 3% lower against partially heterotypic compared with homotypic strains (VE difference = –6.2 [95% CI: –13.0 to 0.01] and –3.0 [95% CI: –28.1 to 32.1]). No studies on RV5 VE in low-income countries met inclusion criteria. No fully heterotypic VE estimates for RV5 were identified.
TABLE 2.
Meta-regression model results estimating the effect of strain type, comparing partially heterotypic and fully heterotypic to homotypic.
| RV1/ RV5 | Income level | Strain category | Specific strains (No. of studies) | Odds ratio (95% CI) | Vaccine effectiveness (95% CI) | Absolute difference in the VE (95%*) | Ratio of the pooled ORs (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| RV1 | High | Homotypic | G1P[8] (N = 5) | 0.10 | 90 | REF | REF | |
| (0.06, 0.18) | (82, 94) | |||||||
| Partially heterotypic | G3P[8] (N = 4), G4P[8] (N = 1), G8P[8] (N = 1), G9P[8] (N = 2), G12P[8] (N = 2) | 0.20 | 79 | –10.2% | 1.98 | 0.04 | ||
| (0.14, 0.29) | (71, 85) | (–20.8, –2.6) | (1.04, 3.77) | |||||
| Fully heterotypic | G2P[4] (N = 5) | 0.20 | 80 | –9.6% | 1.93 | 0.10 | ||
| (0.11, 0.35) | (65, 88) | (–27.0, 1.4) | (0.88, 4.22) | |||||
| Middle | Homotypic | G1P[8] (N = 4) | 0.19 | 81 | REF | REF | ||
| (0.12, 0.31) | (69, 88) | |||||||
| Partially heterotypic | G3P[8] (N = 2), G4P[8] (N = 1), G9P[8] (N = 4), G12P[8] (N = 2) | 0.33 | 67 | –13.9% | 1.71 | 0.06 | ||
| (0.24, 0.46) | (54, 76) | (–29.8, 5.2) | (0.97, 3.01) | |||||
| Fully heterotypic | G2P[4] (N = 10) | 0.35 | 65 | –15.8% | 1.81 | 0.04 | ||
| (0.25, 0.49) | (51, 75) | (–34.6, 0.6) | (1.02, 3.21) | |||||
| Low | Homotypic | G1P[8] (N = 2) | 0.20 | 80 | REF | REF | ||
| (0.08, 0.50) | (50, 92) | |||||||
| Fully heterotypic | G2P[4] (N = 1) | 0.65 | 35 | –44.7% (NC†) | 3.18 | 0.19 | ||
| (0.18, 2.35) | (–135, 82) | (0.57, 17.80) | ||||||
| RV5 | High | Homotypic | G1P[8] (N = 7), G3P[8] (N = 4) | 0.12 | 88 | REF | REF | |
| (0.08, 0.16) | (84, 92) | |||||||
| Partially heterotypic | G2P[4] (N = 5), G9P[8] (N = 2), G12P[8] (N = 3) | 0.18 | 82 | –6.2% | 1.54 | 0.08 | ||
| (0.13, 0.24) | (76, 87) | (–13.0, 0.01) | (0.94, 2.52) | |||||
| Middle | Homotypic | G1P[8] (N = 2), G3P[8] (N = 1) | 0.45 | 55 | REF | REF | ||
| (0.26, 0.76) | (24, 74) | |||||||
| Partially heterotypic | G2P[4] (N = 2), G12P[8] (N = 1) | 0.48 | 52 | –3.0% | 1.06 | 0.86 | ||
| (0.30, 0.77) | (23, 70) | (–28.1, 32.1) | (0.54, 2.10) |
*Confidence intervals for the absolute difference in the VE calculated using bootstrapping estimation with 1000 resamples.
†NC = not calculable due to limited number of studies
There was some evidence of methodologic and statistical heterogeneity identified between the pooled studies. Cases were primarily identified in hospital settings or the emergency department, resulting in VE estimates against severe acute gastroenteritis for most studies. We extracted VE estimates calculated from test-negative controls in 23 studies (74%), community-based controls in 5 studies (16%), and hospital-based controls in 3 studies (10%). Thirteen studies restricted to <5 years, 14 had a lower age cutoff, and 2 had higher cutoffs. Additionally, the overall VE was abstracted from 7 studies without strain-specific estimates but with a prevalence of greater than 80% for 1 strain.16,17,31,32,37,43,47 Two studies reported combined VE for RV1 and RV5,23,35 but greater than 80% of vaccinated patients were documented to have RV1 and estimates were classified as such. However, the sensitivity analyses found similar results to the primary meta-regression model (see Table, Supplemental Digital Content 3, http://links.lww.com/INF/E495). The estimate of statistical residual heterogeneity, I2, in the mixed effect model was 35%. A qualitative assessment of methodologic heterogeneity is presented in Table, Supplemental Digital Content 4, http://links.lww.com/INF/E496. The funnel plot assessment (see Figure, Supplemental Digital Content 5, http://links.lww.com/INF/E497) and Eggers test (P = 0.01) suggested asymmetry, with an absence of estimates with larger variances and smaller VE, even after accounting for heterogeneity by strain type, country income level, and vaccine type.
DISCUSSION
In this updated systematic review and meta-analysis of 31 studies examining strain-specific rotavirus VE across a range of country income-levels, we found that RV1 and RV5 VE varied expectedly by country income level, and VE was somewhat lower against nonvaccine-type than vaccine-type genotypes. At all country-level income strata, the effectiveness was higher against homotypic strains than partially or fully heterotypic strains, although data from low-income countries was limited. These differences in VE by strain were more pronounced for RV1 than RV5. Among high- and middle-income countries, which comprised the majority of data, RV1 VE was approximately 10–16% lower against heterotypic compared with homotypic strains, and RV5 VE was approximately 3–6% lower against heterotypic compared with homotypic strains. The differences in RV1 VE were statistically significant for 2 comparisons, partially heterotypic compared with homotypic strains in high-income countries and fully heterotypic compared with homotypic strains in middle-income countries.
The results of this meta-analysis differ from those of the prior meta-analysis conducted in 2014.6 The 2014 meta-analysis reported a lower RV1 VE for partially and fully heterotypic strains compared with fully homotypic strains in high-income countries, but these differences were imprecise given the prior limited number of studies, and this trend was not observed in middle-income countries, leading the authors to conclude that RV1 and RV5 exerted similar effectiveness against homotypic and heterotypic rotavirus strains. Here, the inclusion of twice as many studies increased the power for detecting small differences in effectiveness. This change in overall conclusion regarding the strain-specific VE of RV1 highlights the importance of updating meta-analyses as more studies are published. Furthermore, it emphasizes the need for individual studies to publish genotype-specific findings even if the individual study is underpowered for genotype-specific assessment, as these data can be combined in future meta-analyses.
While the updated findings from this meta-analysis indicate slight variation in RV1 VE by strain, the concern that mass vaccination with rotavirus vaccines, particularly RV1, leads to selective pressure and strain replacement has not been supported by years of surveillance data.12 Early surveillance data from some countries which introduced RV1, including Australia, Brazil, and some countries in Europe, indicated a predominance of G2P[4] (heterotypic) strains, but this strain evolution was transient and continuous surveillance from multiple countries have not been seen a clear pattern of sustained predominant strains postintroduction.12 Temporal and regional fluctuations in strain distribution appear to occur independently of vaccination implementation.12 Additionally, there has been no conclusive evidence of rotavirus vaccine escape or newly emerging strains since worldwide vaccine introduction.12 Our findings point toward the need for continued strain surveillance to ensure that these slight strain-specific variations in VE do not slowly lead to long-term changes in strain prevalence. Indeed, it could take many years before small strain-specific differences in VE could result in accumulation of strains and drive changes in the genotype distribution.10
Two additional rotavirus vaccines, Rotavac and ROTASIIL, were recently approved and recommended for global use.50 As countries begin to introduce these vaccines and evaluate vaccine effectiveness, a continued effort to present VE estimates by genotype should be a priority for these newer vaccines. Rotavac is also a monovalent vaccine based on G9P[11], and ROTASIIL is a pentavalent vaccine based on G-type gene substitutions, G1-4 and G9. These vaccines have the potential for dramatic impact on the burden of rotavirus disease, particularly in low-income countries, given their lower cost and a greater heat-stability for ROTASIIL.50
Several limitations of this analysis must be considered when evaluating these findings. First, while many studies were designed using a generic protocol for postlicensure evaluation of rotavirus vaccines,50 there remained methodologic heterogeneity between studies. There was slight discrepancy in the eligibility criteria for cases and controls, although most studies enrolled hospitalized patients or patients seen within the emergency department for acute gastroenteritis, reported test-negative controls, and restricted enrollment to children under 5 years of age. While all studies reported VE estimates adjusted for potential confounders, the analysis methods and choice of confounders varied by study (see Table, Supplemental Digital Content 4, http://links.lww.com/INF/E496). The asymmetry in the funnel plot could be caused by heterogeneity in effect size according to study size due to reporting bias, methodologic differences between studies, true heterogeneity of effect according to study size or chance. Second, 7 studies did not report strain-specific VE, but >80% of rotavirus cases were of the same strain; a sensitivity analysis excluding these 7 studies found consistent results. Third, there may be selection bias if there are differences between the studies included and excluded from the literature review because they did not report VE by strain. A recent meta-analysis of overall RV1 and RV5 VE by country child mortality level and age at vaccination included 60 studies,5 while we identified only 31 eligible studies over a similar time period with VE by strain. Fourth, while we almost doubled the number of studies included in the 2014 meta-analysis, we were still limited in the number of studies from low-income countries, with only 2 low-income studies reporting strain-specific VE. Even after more than 10 years of vaccine introduction, data on strain-specific VE from low-income countries remain scarce, particularly for RV5, highlighting the need to continue evaluating strain-specific VE in these settings. Fifth, while G and P proteins are used to distinguish genotypes of rotavirus, there could be other gene segments not routinely characterized that impact vaccine effectiveness. Additionally, VE estimates were pooled based on categorization of G and P proteins into homotypic and heterotypic, so there may be variability in the protection of rotavirus vaccines against specific strains that this categorization does not capture, particularly for RV5, which is composed of multiple G-types. Despite these limitations, this review was comprehensive of the literature spanning 14 years and including 31 studies, used robust approaches for primary and secondary evaluation of study eligibility, pooled adjusted VE estimates to account for confounding in the observational studies and implemented statistical evaluation of strain-specific VE using a mixed-effects meta-regression model.
In summary, this comprehensive systematic review and meta-analysis of 31 case-control studies from a variety of countries between 2006 and 2020 found an overall high effectiveness of RV1 and RV5 vaccines against rotavirus hospitalization with an expected gradient in effectiveness by country income level and provided new evidence of lower VE against nonvaccine-type than vaccine-type strains, particularly for RV1. Ongoing surveillance and vaccine-effectiveness studies with genotype-specific VE results presented are crucial to continue long-term monitoring for strain replacement due to vaccine pressure, particularly in low-income settings where data are limited and the potential impact of rotavirus vaccines is high.
ACKNOWLEDGMENTS
We acknowledge Junaid Panjwani (JP), Alexia Rodriguez (AR), and Joanne Wu (JW) for their support with article screening, full-text review, and data extraction.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
B.A.L. acknowledges funding support from NIH/NIGMS R01GM124280 NIH/NIAID R01AI112970 and the Vaccine Impact Modeling Consortium.
B.A.L. reports grants and personal fees from Takeda Pharmaceuticals, personal fees from World Health Organization outside the submitted work. The remaining authors have no conflicts of interest to disclose.
All authors contributed to study design, interpretation of data, and writing of the report. J.C. conducted all analysis of the data. All authors had access to data and commented on and approved the final version. J.C. and A.B.A., as well acknowledged collaborators J.P., A.R., and J.W., reviewed potentially eligible studies.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com)
REFERENCES
- 1.Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(suppl 2):S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotavirus Vaccines. WHO position paper—January 2013. Releve Epidemiologique Hebdomadaire. 2013;88:49–64. [PubMed] [Google Scholar]
- 3.International Vaccine Access Center (IVAC) JHBSoPH. VIEW-hub Report: Global Vaccine Introduction and Implementation. 2020. Available at: https://view-hub.org/sites/default/files/2020-05/VIEW-hub_Report_Mar2020.pdf. Accessed December 28, 2020.
- 4.Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006-2019. J Infect Dis. 2020;222:1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett E, Parashar UD, Tate JE. Real-world effectiveness of rotavirus vaccines, 2006-19: a literature review and meta-analysis. Lancet Glob Health. 2020;8:e1195–e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:847–856. [DOI] [PubMed] [Google Scholar]
- 7.Bányai K, László B, Duque J, et al. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012;30(suppl 1):A122–A130. [DOI] [PubMed] [Google Scholar]
- 8.Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. [DOI] [PubMed] [Google Scholar]
- 9.Fischer TK, Valentiner-Branth P, Steinsland H, et al. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, West Africa. J Infect Dis. 2002;186:593–597. [DOI] [PubMed] [Google Scholar]
- 10.Pitzer VE, Patel MM, Lopman BA, et al. Modeling rotavirus strain dynamics in developed countries to understand the potential impact of vaccination on genotype distributions. Proc Natl Acad Sci U S A. 2011;108:19353–19358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vesikari T. Rotavirus vaccination: a concise review. Clin Microbiol Infect. 2012;18(suppl 5):57–63. [DOI] [PubMed] [Google Scholar]
- 12.Bibera GL, Chen J, Pereira P, et al. Dynamics of G2P[4] strain evolution and rotavirus vaccination: a review of evidence for Rotarix. Vaccine. 2020;38:5591–5600. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua H, Feng S, Lewnard JA, et al. The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology. 2020;31:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The World Bank Group. World Bank Country and Lending Groups. 2020. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed December 2, 2019.
- 16.Snelling TL, Andrews RM, Kirkwood CD, et al. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in central Australia. Clin Infect Dis. 2011;52:191–199. [DOI] [PubMed] [Google Scholar]
- 17.Snelling TL, Schultz R, Graham J, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49:428–431. [DOI] [PubMed] [Google Scholar]
- 18.Leshem E, Givon-Lavi N, Tate JE, et al. Real-world effectiveness of pentavalent rotavirus vaccine among Bedouin and Jewish children in Southern Israel. Clin Infect Dis. 2016;62(suppl 2):S155–S160. [DOI] [PubMed] [Google Scholar]
- 19.Gurgel RQ, Cuevas LE, Vieira SC, et al. Predominance of rotavirus P[4]G2 in a vaccinated population, Brazil. Emerg Infect Dis. 2007;13:1571–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurgel RG, Bohland AK, Vieira SC, et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology. 2009;137:1970–1975. [DOI] [PubMed] [Google Scholar]
- 21.Staat MA, Payne DC, Donauer S, et al. ; New Vaccine Surveillance Network (NVSN). Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128:e267–e275. [DOI] [PubMed] [Google Scholar]
- 22.Mast TC, Khawaja S, Espinoza F, et al. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J. 2011;30:e209–e215. [DOI] [PubMed] [Google Scholar]
- 23.Ali Z, Harastani H, Hammadi M, et al. Rotavirus genotypes and vaccine effectiveness from a Sentinel, Hospital-Based, Surveillance Study for Three Consecutive Rotavirus Seasons in Lebanon. PLoS One. 2016;11:e0161345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki K, Hara M, Tsugawa T, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Japanese children. Vaccine. 2018;36:5187–5193. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Zeev N, Jere KC, Bennett A, et al. ; Vaccine Effectiveness and Disease Surveillance Programme, Malawi (VACSURV) Consortium. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis. 2016;62(suppl 2):S213–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199–e207. [DOI] [PubMed] [Google Scholar]
- 27.Braeckman T, Van Herck K, Meyer N, et al. ; RotaBel Study Group. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ. 2012;345:e4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang WC, Yen C, Wu FT, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J. 2014;33:e81–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363–369. [DOI] [PubMed] [Google Scholar]
- 30.Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013;132:e25–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhea in El Salvador: case-control study. BMJ. 2010;340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gastañaduy PA, Contreras-Roldán I, Bernart C, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Guatemala. Clin Infect Dis. 2016;62(suppl 2):S121– S126. [DOI] [PubMed] [Google Scholar]
- 33.Gastañaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis. 2016;62(suppl 2):S161–S167. [DOI] [PubMed] [Google Scholar]
- 34.Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14:1096–1104. [DOI] [PubMed] [Google Scholar]
- 35.Hoque SA, Kobayashi M, Takanashi S, et al. Role of rotavirus vaccination on an emerging G8P[8] rotavirus strain causing an outbreak in central Japan. Vaccine. 2018;36:43–49. [DOI] [PubMed] [Google Scholar]
- 36.Ichihara MY, Rodrigues LC, Teles Santos CA, et al. Effectiveness of rotavirus vaccine against hospitalized rotavirus diarrhea: a case-control study. Vaccine. 2014;32:2740–2747. [DOI] [PubMed] [Google Scholar]
- 37.Immergluck LC, Parker TC, Jain S, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr. 2016;172:116–120.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Justino MC, Linhares AC, Lanzieri TM, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J. 2011;30:396–401. [DOI] [PubMed] [Google Scholar]
- 39.Khagayi S, Omore R, Otieno GP, et al. Effectiveness of monovalent rotavirus vaccine against hospitalization with acute rotavirus gastroenteritis in Kenyan children. Clin Infect Dis. 2020;70:2298–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthijnssens J, Zeller M, Heylen E, et al. ; RotaBel Study Group. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin Microbiol Infect. 2014;20:O702–O710. [DOI] [PubMed] [Google Scholar]
- 41.Muhsen K, Anis E, Rubinstein U, et al. Effectiveness of rotavirus pentavalent vaccine under a universal immunization programme in Israel, 2011-2015: a case-control study. Clin Microbiol Infect. 2018;24:53–59. [DOI] [PubMed] [Google Scholar]
- 42.Patel M, Pedreira C, De Oliveira LH, et al. Effectiveness of pentavalent rotavirus vaccine against a diverse range of circulating strains in Nicaragua. Clin Infect Dis. 2016;62(suppl 2):S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. [DOI] [PubMed] [Google Scholar]
- 44.Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ. 2013;346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009-2011. Clin Infect Dis. 2013;57:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012-2013. Clin Infect Dis. 2015;61:1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platts-Mills JA, Amour C, Gratz J, et al. Impact of rotavirus vaccine introduction and postintroduction etiology of diarrhea requiring hospital admission in Haydom, Tanzania, a rural African setting. Clin Infect Dis. 2017;65:1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013-2014. Clin Infect Dis. 2016;62(suppl 2):S115–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yen C, Figueroa JR, Uribe ES, et al. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis. 2011;204:783–786. [DOI] [PubMed] [Google Scholar]
- 50.Generic Protocol for Monitoring Impact of Rotavirus Vaccination on Gastroenteritis Disease Burden and Viral Strains. World Health Organization; 2008. Available at: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.16_eng.pdf. Accessed December 28, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.