Abstract
The clinical and fundamental exploration of patients suffering from disorders of consciousness (DoC) is commonly used by researchers both to test some of their key theoretical predictions and to serve as a unique source of empirical knowledge about possible dissociations between consciousness and cognitive and/or neural processes. For instance, the existence of states of vigilance free of any self-reportable subjective experience [e.g. “vegetative state (VS)” and “complex partial epileptic seizure”] originated from DoC and acted as a cornerstone for all theories by dissociating two concepts that were commonly equated and confused: vigilance and conscious state. In the present article, we first expose briefly the major achievements in the exploration and understanding of DoC. We then propose a synthetic taxonomy of DoC, and we finally highlight some current limits, caveats and questions that have to be addressed when using DoC to theorize consciousness. In particular, we show (i) that a purely behavioral approach of DoC is insufficient to characterize the conscious state of patients; (ii) that the comparison between patients in a minimally conscious state (MCS) and patients in a VS [also coined as unresponsive wakefulness syndrome (UWS)] does not correspond to a pure and minimal contrast between unconscious and conscious states and (iii) we emphasize, in the light of original resting-state positron emission tomography data, that behavioral MCS captures an important but misnamed clinical condition that rather corresponds to a cortically mediated state and that MCS does not necessarily imply the preservation of a conscious state.
Keywords: disorders of consciousness, minimally conscious state, vegetative state, electroencephalography, positron emission tomography
Introduction
Sustained impairments of consciousness obviously constitute a devastating condition that requires a better understanding of the corresponding physiopathology in order to cure patients and to take care of them optimally. This clinical goal converges with the scientific goal aiming at elaborating a solid biological theory of consciousness. It is of special interest that the exploration of consciousness in patients affected with disorders of consciousness (DoC) has been extremely fruitful during the last decades. This clinical source of knowledge was and is still valuable for at least two main reasons. First, it enabled one to make use of the neuropsychological dissociation approach, which through the fractionation of a complex mental phenomenon into distinct cognitive processes proved its value in all domains of cognition (i.e. episodic memory, language, decision-making,perception,… and now consciousness). Second, it stimulated theorization of consciousness by revealing the existence of very challenging extreme situations such as comatose, “vegetative,” minimally conscious and related pathological states. Converging evidence suggests that this avenue of research still holds rich perspectives to inspire and to constrain all theoretical biological models of consciousness. For all these reasons, the science of DoC is not reserved to experts of DoC but should rather be familiar to most neuroscientists tackling the questions related to consciousness. However, getting acquainted with this knowledge is not obvious for many distinct reasons. In this paper, we aimed at providing such a synthetic introductory overview of neuroscience of DoC. We will first present the major achievements of the field and clarify the blooming and complex terminology that emerged to describe most of the unusual and complex cognitive states. Then, we will explain the major limits and caveats of this branch as well as the recent important revisions that modified the overall theoretical and clinical landscape of DoC. Then, we will explain why a recent consensual view emerged to call for the urgent need to build a new classification of DoC combining behavioral with structural and functional brain data. We will finally close this overview by listing some future goals that appear to us as key problems to solve. We also make explicit that in this paper we will not address at length the debated question of the definition of consciousness, which we have addressed elsewhere (e.g. Dehaene and Naccache 2001; Naccache 2018a, b). More specifically, we will use here the “self-reportability” criterion of the conscious state defined in previous studies and adopted by various theories such as the Global Neuronal Workspace theory (GNWT) of consciousness or Higher-order Thought (HOT) theories (Rosenthal 1986; Dehaene and Naccache 2001). While there is a large theoretical consensus that self-reportability is specific to conscious contents and to conscious states, the possibility of conscious states in the absence of self-reportability and of non self-reported conscious contents remains debated (Block 1995; Lamme 2006; Bayne 2018). In this article, we will mention, whenever needed, the impact of this definition choice on the topics that will be discussed here.
Major behavioral achievements in the DoC literature
Comatose state
Long before consciousness became the subject of theoretical considerations, in ancient Greece, altered states of consciousness were described empirically with the term coma, from the greek word “koma,” which means “deep sleep” (Koehler and Wijdicks 2008). In this condition, patients seem asleep and have their eyes closed but are unresponsive and unarousable, even with strong stimulation. This state of the apparent complete loss of both vigilance and consciousness, along with milder forms of vigilance impairments such as stupor or lethargy, long resumed the nosography of consciousness alterations (Plum and Posner 1972). Recent studies questioned the systematic value of eyes opening as evidence of the preserved activity of arousal structures including the ascending reticular activating system (ARAS). In particular, a case report of unilateral partial eye opening in a patient with confirmed brain death suggested the possibility to observe eye opening through the residual activity of sympathetic circuit within the low cervical/upper thoracic cord (Santamaria et al. 1999; Kondziella and Frontera 2021) located below the brain stem ARAS. Except these very rare situations, other case reports and anatomical considerations suggested that pathways controlling the main palpebrae elevator muscle (Levator Palpebrae Superioris) run in close association with the ARAS through the paramedian tegmentum of the upper brain stem. Therefore, one could predict the possibility of dissociations between these closely related pathways with an impaired ARAS activity and a preserved eye opening. This would correspond to very rare description of eyes-open comatose (Kondziella and Frontera 2021).
Vegetative state
However, in the middle of the 20th century, the development of intensive care units with mechanical ventilation along with the progress of other resuscitation techniques led to the survival of severely brain-injured patients beyond the acute stage. Consequently, physicians discovered new post-comatose states characterized by the recovery of some arousal, i.e. spontaneous eyes opening behavior, and some reflexive motor behaviors. In some of these patients, however, the recovery seemed to be limited to automatic and reflexive processes. The accumulation of such cases prompted Jennett and Plum to name this condition the “persistent vegetative state” (VS), in their famous Lancet paper in 1972: “Persistent Vegetative State after Brain Injury. A syndrome in search for a name” (Jennett and Plum 1972). The syndrome was initially described as follows: “[..] the absence of any adaptive response to the external environment, the absence of any evidence of a functioning mind which is either receiving or projecting information, in a patient who has long periods of wakefulness.” Patients in this state thus have preserved autonomic regulation and vegetative functions (originating mainly in the brainstem) and exhibit spontaneous or induced arousal, as evidenced by eyes opening and sleep–wake cycles (Bekinschtein et al. 2009a; Landsness et al. 2011; Rossi Sebastiano et al. 2018). Regarding the latter (i.e. sleep–wake cycles preservation in the VS), one should first be aware that alternating periods of eyes-open/eyes-closed behaviors do not necessarily correspond to genuine sleep–wake cycles (Bekinschtein et al. 2009a; Cologan et al. 2013). Nevertheless, several studies reported true sleep–wake cycles in both MCS and VS (Landsness et al. 2011; Cologan et al. 2013; de Biase et al. 2014; Forgacs et al. 2014; Aricò et al. 2016; Arnaldi et al. 2016; Rossi Sebastiano et al. 2018; Gibson et al. 2020). As noted by Saper and Fuller (2017), the preservation of organized sleep cycles including sleep electroencephalogram (EEG) patterns such as sleep spindles and slow-wave sleep would indicate the functionality of thalamo-cortical loops, beyond brain stem structures, which do not necessarily imply conscious processing. Rapid eye movement (REM) sleep, however, corresponds to a cortical wakefulness stage associated with the more complex, narrative and sustained conscious dreaming activities. The exploration of its preservation in DoC patients, and in particular in the VS, is thus of prime interest. Interestingly, while a majority of MCS and VS patients seem to show at least partial preservation of sleep–wake cycles, it seems that REM sleep is much more frequent in the MCS than in the VS (see Table 1 of Pan et al. (2021).
Table 1.
Sketch of a new classification and taxonomy of DoC [adapted from Naccache (2018a)]
| State # | State name | Source of evidence |
|---|---|---|
| Evidence of unconsciousness | ||
| 1a | Comatose state | Behavior and functional brain imaging |
| 1b | Comatose state | Behavior |
| 2a | VS/UWS | Behavior and functional brain imaging |
| 2b | VS/UWS | Behavior |
| Evidence of consciousness | ||
| 3a | Cortically mediated state | Functional brain imaging |
| 3b | Cortically mediated state | Behavior |
| 4a | Conscious state | Functional brain imaging |
| 4b | Conscious state | Behavior |
However, they completely lack the behavioral evidence of self or environmental awareness and, apparently, their behavior can be entirely explained by reflexes stemming from subcortical structures, notably the brainstem and medulla, as reflected by another term used at that time to label this condition, the “apallic syndrome,” which means the “absence of cortex.”
Interestingly, since Jennett and Plum described this syndrome, its definition did not substantially change, except for the abandon of the term “permanent” to the profit of “chronic” for states lasting more than 12 months in traumatic cases and 3 months in nontraumatic ones (Giacino et al. 2018a). Also, a new denomination has been proposed, the unresponsive wakefulness syndrome (UWS), mainly because of the perceived pejorative connotation associated with the word vegetative in the public opinion (Laureys et al. 2010). Regarding this matter, the term vegetative refers both to the preservation of the autonomic and vegetative functions and to “an organic body capable of growth and development but devoid of sensation and thought” as stated in the original Jennett paper. In this view, the new proposed term is more descriptive and neutral as it relates to the behavioral description of an unresponsive patient who shows signs of wakefulness. While interesting, this new name still suffers from a lack of recognition and from a common confusion between wakefulness and awareness even among the medical community, as evidenced by the different perception of patient’s prognosis when the same reality is described by either of the two labels (Kondziella et al. 2019). As such, the term VS is still recommended by some scientific societies (Turner-Stokes 2014). While agreeing on the inadequacy of VS terminology, Naccache also raised additional concerns related to UWS phrasing (Naccache 2018a): (i) first, the adjective “unresponsive” is not univocal because many unconscious behavioral responses can be observed and (ii) by focusing on the absence of overt voluntary and conscious responses, one may incorrectly bias perception of UWS toward the one associated to the locked-in syndrome (LIS), leading to the false belief according to which the problem of a patient in a UWS would mostly be due to impairments in overt responses (unresponsive), whereas consciousness is probably preserved. However, this is of course not the case in the majority of patients. In the subsequent work, we will refer to this state using both names, with the following abbreviation: VS/UWS.
Minimally conscious state
In 2002, Joseph Giacino and his colleagues proposed to delineate a new syndrome, the minimally conscious state (MCS), in order to explicitly describe the case of patients who, despite not being fully conscious, do not meet the VS/UWS criteria. These patients, previously labeled as minimally responsive, present “a condition of severely altered consciousness in which minimal but definite behavioral evidence of self or environmental awareness is demonstrated” (Giacino et al. 2002). Evidence of such awareness lies in the demonstration of cognitively mediated behaviors, which, although inconsistent, are reproducible and sustained long enough to be differentiated from reflexive behaviors. A list of proposed behaviors fulfilling these criteria was provided in the original publication:
following simple verbal motor commands;
gestural or verbal yes/no responses (regardless of accuracy);
intelligible verbalization;
purposeful behavior, including movements or affective behaviors that occur in contingent relation to relevant environmental stimuli and are not due to reflexive activity.
Among the qualifying purposeful behaviors, one may mention:
appropriate smiling or crying response to the auditory or visual content of emotional but not to neutral topics or stimuli;
vocalizations or gestures that occur in direct response to the verbal questions or instructions;
reaching for objects that demonstrates a clear relationship between object location and direction of reach;
touching or holding objects in a manner that accommodates the size and shape of the object;
pursuit eye movement or sustained fixation that occurs in direct response to moving or salient stimuli.
This important effort stemmed from the design of a new behavioral scale: the JFK Coma-Recovery-Scale [CRS (Giacino et al. 1991)], revised in 2005 [CRS-r (Giacino et al. 2004; Kalmar and Giacino 2005)]. The CRS-r circumscribed the exact characterization of each and every behavior qualifying for an MCS diagnosis, together with precise instructions on how to look for them. This hierarchical and rigorous scale designed to probe MCS in various cognitive and sensory-motor domains rapidly became the gold standard to differentiate MCS from VS/UWS states. The CRS-r constitutes a very good compromise between examination time (usually from 30 to 45 min) and sensitivity and presents a satisfactory inter-rater stability. Several studies demonstrated the utility of identifying MCS from VS/UWS: all other things being equal, being in an MCS is associated with a better prognosis of overt consciousness recovery and with a better overall clinical evolution (Luauté et al. 2010; Faugeras et al. 2018; Perez et al. 2020a).
It should be noted, however, that from the beginning MCS was quite a heterogeneous syndrome, regrouping patients exhibiting behaviors encompassing various cognitive processes. To address this issue, some authors proposed a further distinction of MCS– and MCS+ patients, on the basis of the absence/presence of signs of language function, respectively (Bruno et al. 2011, 2012). In that frame, MCS+ patients are patients able to exhibit command following, intelligible verbalization and intentional although non-functional communication, while MCS– patients only show contextualized motor and emotional behaviors such as visual pursuit or fixation, orientation to noxious stimuli and object reaching or manipulation (Thibaut et al. 2020).
At the same time, the upper limits of the MCS condition that distinguish it from the conscious state have been defined as (i) the existence of a functional communication (the ability for a subject to reliably answer simple questions, through verbal or nonverbal output) and/or as (ii) the ability to use objects functionally with an intentional behavior. It should be noted that this emergence from the MCS (EMCS, with E for emergence or exit) can still be (and usually is) accompanied by various disabling cognitive deficits that have been described under different labels, notably in the traumatic brain injury literature. Initial descriptions focused on memory and orientation disturbances grouped under the acronym post-traumatic amnesia (Symonds and Ritchie Russell 1943). More recently, the term post-traumatic confusional state (Stuss et al. 1999; Sherer et al. 2020) was proposed to underline the wide range of potential neurobehavioral deficits observed after a traumatic injury, including attention, memory and orientation, along with emotional, behavioral, perceptual or sleep–wake disturbances (Sherer et al. 2020). These neurocognitive disorders are not only reminiscent of the global cognitive disturbances described in delirium (Oldham and Holloway 2020) but can also encompass some specific cognitive domain deficits secondary to focal lesions since traumatic brain injuries are highly heterogeneous.
Neurological pitfalls when probing consciousness in patient behavior
Moreover, states of altered consciousness are to be differentiated from unresponsiveness due to sensory deficits (blindness and deafness), sensorimotor impairments and/or other primary deficits such as aphasia, agnosia or apraxia (Smart et al. 2008; Majerus et al. 2009; Bruno et al. 2012; Rohaut et al. 2017; Pincherle et al. 2020). One of the most compelling illustrations of this fact is to be found in the LIS (Plum and Posner 1966; Bauer et al. 1979), a condition in which patients are fully conscious but lack the ability to communicate due to impaired motor function. Other differential diagnoses of DoC are conditions characterized by a disruption of intentional behavior, such as akinetic mutism, loss of psychic self-activation syndrome or catatonia (Young and Rund 2010; Riveros et al. 2018; Walther et al. 2019). As a consequence, clinicians are trained to be aware of such difficulties and caveats when examining noncommunicating patients (Rohaut et al. 2013).
From an elusive MCS to a reliable CMS
Of special interest to theories of consciousness, one may note that if each of the MCS behaviors do require some degree of cortical network engagement (e.g. visual pursuit demonstrates the functionality of an occipital-parieto-frontal network, involving notably the frontal eye field), they do not necessarily correspond to conscious behaviors [(Naccache 2018a) and see below]. This issue is particularly important considering that the presence of a single MCS item of the CRS-r is sufficient to label the patient as being in an MCS. For instance, a patient presenting only visual fixation is currently labeled as MCS even if all other behavioral data point to reflexive functioning. Naccache framed an anagram of the acronym of MCS, in order to keep the very useful relevant behavioral criteria used in the CRS-r, while proposing a completely different interpretation of its meaning. Patients in MCS are not “minimally conscious” given that none of the MCS items does translate in univocal evidence for the conscious state, but they are beyond any doubt in a cortically mediated state (CMS). The exclusive observation of their behavior enables the unmistakable following conclusion: their behavior necessarily implies an overt contribution of cortical networks. In contrast, patients in VS/UWS do not show any obvious contribution of their cortical networks to behavior.
In order to illustrate and strengthen the relevance of this CMS interpretation of MCS, we present here an original analysis of 18F-fluoro-deoxyglucose positron emission tomography (FDG-PET) resting-state imaging data of patients in a behavioral VS/UWS or MCS state. PET studies revealed that unconsciousness across various conditions (anesthesia, sleep and DoC) was associated with a global reduction of brain metabolism to approximately 50% of normal (Stender et al. 2014a, 2015; Hermann et al. 2021). PET was then successfully used to diagnose MCS from VS/UWS patients, with optimal performances obtained with a clever normalization procedure by extracerebral tissue resulting in a single measure of cerebral metabolic activity, the metabolic index of the best preserved hemisphere [MIBH (Stender et al. 2015)]. We recently validated MIBH as an accurate and robust procedure to diagnose MCS in a cohort of 52 patients (21 VS/UWS and 31 MCS) (Hermann et al. 2021) and here present regional metabolism and voxel-wise analyses of this dataset supporting the CMS hypothesis (see Hermann et al. 2021 and Supplementary Material for details regarding methods).
We first investigated whether a classification based on regional metabolism would outperform the MIBH (which measures the hemispheric cortical average) by extracting the average metabolic index values for each patient within 41 cortical regions (Tzourio-Mazoyer et al. 2002) and contrasting the VS/UWS from MCS. We found that all cortical regions significantly discriminated VS/UWS from MCS patients (all false-discovery rate corrected P-values <0.05) and that several regions had similar (or even slightly better) performances than the MIBH (Fig. 1 and Supplementary Table and Fig. S1). Importantly, the latter included primary or secondary specialized cortical areas, not specifically associated with consciousness: the left paracentral lobule [AUC 0.835 (0.730–0.919)], the left lingual and calcarine regions from the occipital cortex [AUCs 0.834 (0.731–0.918) and 0.832 (0.728–0.922) respectively] as well as the left and right supplementary motor areas (SMAs) [AUCs 0.817 (0.699–0.911) and 0.816 (0.701–0.909) respectively]. Actually, among the regions traditionally associated with consciousness, only the left precuneus (Boly et al. 2008; Vanhaudenhuyse et al. 2010; Crone et al. 2015) ranked in the top 10 discriminative regions [AUC 0.821 (0.705–0.912)]. These results are in accordance with a previous FDG-PET report of the VS/UWS vs MCS contrast (Stender et al. 2014a) and with a previous demonstration that several functional magnetic resonance imaging (fMRI) resting-state networks, including auditory, sensorimotor and visual networks were also accurate in discriminating MCS from VS/UWS (Demertzi et al. 2015; Wu et al. 2015). Noteworthy, an overall left/right asymmetry was observed with a higher AUC for right hemisphere AAL regions (paired t-test P value = 0.017). This asymmetry may be explained by the following hypothesis that takes into account a classical caveat of clinical examination of DoC patients due to unilateral neglect syndrome following right-hemispheric lesions (Rohaut et al. 2013): the reference criterion for the MCS/VS-UWS distinction relies on behavioral examination (i.e. CRS-r score). Therefore, some patients with right-hemispheric lesions inducing unilateral neglect impairments may be classified as behavioral VS/UWS whereas their brain metabolism and activity correspond to MCS. When computing the AUC of left hemispheric AAL regions in such patients, such a mismatch between behavioral categorization and PET would be maximal (i.e. MCS metabolism for VS behavior), whereas using right-hemispheric AAL regions would minimize such a mismatch due to lower metabolism in the right hemisphere. Accordingly, such an effect could explain the observed better ranking of right AAL regions than left ones. Note, however, that in any case, we deal here with a subtle effect given that AAL regions did show significant AUC values.
Figure 1.
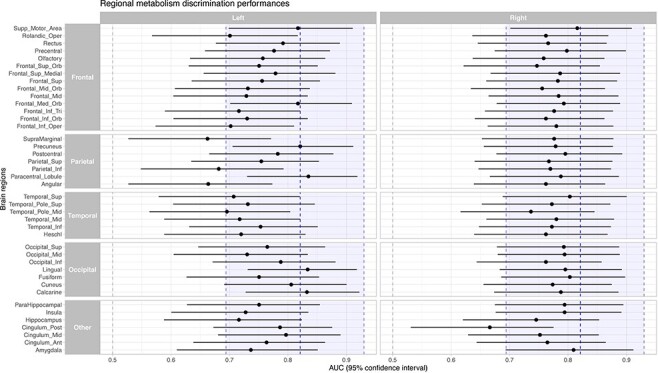
FDG-PET regional discrimination performance
Legend: Respective AUCs and 10 000 bootstrapped 95% confidence intervals for the VS/UWS vs MCS discrimination of the 41 cortical regions of the AAL atlas in both hemispheres. 10 000 permutation testing against 0.5, all false-discovery rate corrected P-values < 0.05. Blue dashed line and shaded region represent the AUC and 95% confidence interval of the metabolic index of the best preserved hemisphere.
We then investigated the voxel-wise metabolic correlates of the CRS-r, by dichotomizing each CRS-r subscale according to the presence or absence of an MCS item in each individual and using parametric statistical mapping to investigate the specific metabolic pattern associated with each subscale. This analysis showed that the presence of either a visual MCS item or a motor MCS’ item, which are the most prevalent MCS items (Wannez et al. 2018), was significantly associated with metabolism restricted within specialized first-order cortical areas, occipital cortex and motor and premotor cortices, respectively, without activation in associative prefrontal or parietal networks typically observed during conscious states (Maquet et al. 1997; Nofzinger et al. 2002; Laureys et al. 2004a; Boveroux et al. 2010; Laureys and Schiff 2012). On the contrary, the presence of response to command MCS item, i.e. closer to the reportability criteria definition of consciousness, was significantly associated with a higher metabolism in widespread cortical areas. Notably this network was not restricted to language-related regions (left-lateralized inferior frontal and temporo-parietal junction) but also included the default-mode network (dorsolateral prefrontal cortex and posterior cingulate/precuneus), which is closely related to conscious processing (Fig. 2 and Supplementary Fig. S2).
Figure 2.
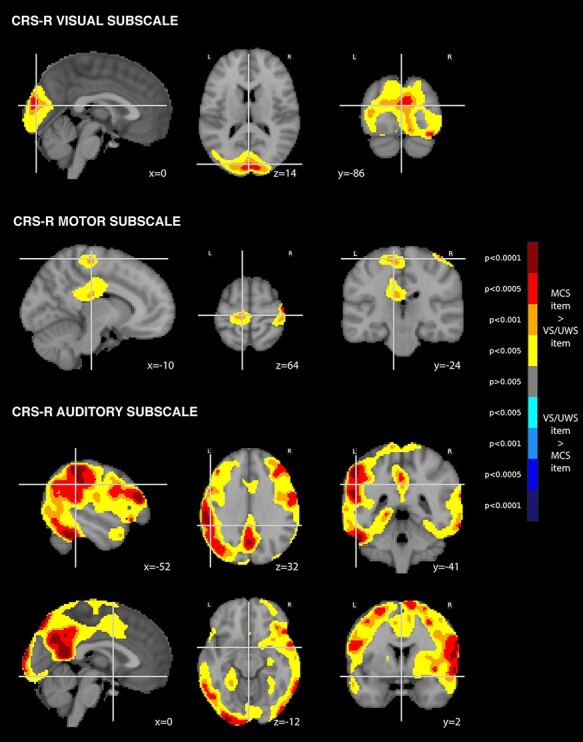
Metabolic correlates of CRS-R MCS items
Legend: Independent FDG-PET metabolic correlates of the CRS-R MCS items in the visual subscale, the auditory subscale and the motor subscale [P < 0.005 uncorrected, cluster extent 100 voxels, superimposed on sagittal, axial and coronal slices (from left to right) of the MNI 152 T1 brain template with related y, x and z MNI coordinates]. L = left; R = right.
These findings suggest that the VS/UWS vs MCS contrast reflect a mosaic of cortical network activity, across a multitude of brain functions, rather than a pure minimal contrast between a conscious and unconscious state. By better interpreting the meaning of MCS as CMS, it becomes also clear that CMS is a very heterogeneous category including patients more or less close to the conscious state. Note also that in addition to this reinterpretation of MCS as CMS, this new formulation is not incompatible with the fact that most behaviorally VS/UWS patients do show cortical activity and cortical responses to external stimuli, although predominantly in primary cortices (somatosensory or auditory for instance; Laureys et al. 2002a, b). Indeed, in these cases, the cortical activations do not translate into overt behavior that are used to compute a CRS-r behavioral score. Therefore, in addition to interpreting MCS as CMS, this new taxonomy also further emphasizes the importance of combining functional brain-imaging data to behavioral observation when assessing residual cognitive and conscious abilities. Indeed, as we will show it below, some patients in a behavioral VS even show much richer cognitive processing revealed by functional brain imaging. Altogether this highlights the notion that current behavioral measures are insufficient to answer the crucial question: is the patient still holding a conscious self-reportable subjective experience?
The break-in and rise of functional brain imaging to explore DoC patients
A new source of information to define cognitive and conscious status
In 2006, Owen and colleagues published in Science a breakthrough case report: a young noncommunicating patient in a behavioral VS/UWS after severe TBI showed task-related fMRI activation similar to those of conscious volunteers following verbal instructions. More precisely, when asked to imagine playing tennis or moving around her home, the patient activated the predicted cortical areas (i.e. SMA vs parahippocampal place area, posterior parietal cortex and premotor cortex respectively) in a manner indistinguishable from that of healthy volunteers engaged in the same task (Owen et al. 2006). The most impressive aspect of this case report relied on the time-sustained attribute of the observed modulations of brain activity: after each single verbal instruction, specific brain patterns were activated and maintained for 30 s. This active maintenance of task-related patterns supported the idea of intentional responses under executive control rather than transient automatic activations elicited automatically by unconscious semantic processing of verbal instructions. In other terms, one could at least infer intentional response to verbal command in this patient using fMRI, whereas no such ability translated into overt behavioral responses. While the genuine interpretation of these results raised many questions (i.e. was the patient conscious?), this paper turned to be a landmark in the field: the use of functional brain-imaging data can add new information to better describe the current cognitive and conscious status of DoC patients (Naccache 2006). Since then, a multitude of studies reported new results supporting this general principle, using various functional brain-imaging techniques (i.e. PET, fMRI and EEG) (Comanducci et al. 2020; Kondziella et al. 2020) or other physiological measures (i.e. pupillometry, heart rate, …) (Raimondo et al. 2017; Arzi et al. 2020; Perez et al. 2020b). Some authors even used this brain-activity response to command to open a bilateral communication channel with DoC patients using a binary code (e.g. imagine playing tennis to answer “YES” vs imagine moving in your home to answer “NO”) with fMRI or EEG (Monti et al. 2010; Cruse et al. 2011; Goldfine et al. 2011).
Cognitive motor dissociation (CMD) and related variations
Once this new era of the DoC literature was launched, more and more reports of dissociations between fine behavioral examination and brain-activity findings led to the formulation of the following new principle: some patients may actually be in a richer cognitive and conscious state than the one inferred from a strict behavioral point of view. This concept of dissociation between motor behavior and cognitive abilities was framed by Schiff et al. under the name cognitive motor dissociation (CMD) (Schiff 2015): some patients show univocal response to verbal command in their brain activity, in spite of the absence of any reliable corresponding overt behavior and in the absence of trivial peripheral or central motor pathway impairments. In other terms, the CMD points to a more conceptually challenging state than severe syndromes affecting the motor pathways such as amyotrophic lateral sclerosis, Guillain-Barré or LIS for instance. A recent EEG paradigm enabled the detection of CMD in 15% of ICU patients (Claassen et al. 2019).
Of special interest, various independent studies using resting-state or task-related recordings with different techniques (e.g. PET, fMRI, EEG, Transcranial magnetic stimulation coupled with EEG (TMS-EEG)) converged on the average proportion of ∼15% of patients who are behaviorally in a VS/UWS but who would be at least in an MCS once taking into account brain-activity findings (Monti et al. 2010; Sarasso et al. 2014; Kondziella et al. 2016; Claassen et al. 2019; Gui et al. 2020; Edlow and Naccache 2021; Hermann et al. 2021; Sokoliuk et al. 2021).
Once CMD dissociation was framed, several other acronyms and dissociations appeared in the literature, such as higher-order cortex motor dissociation (HMD) (Edlow et al. 2017) or MCS-star (MCS*) (Thibaut et al. 2021) that aimed at defining less demanding dissociations than CMD, that is higher-order brain activation in response to passive stimuli or in resting-state paradigm, higher than those expected from VS/UWS patients, but without univocal response to verbal command.
The schematic evolution of DoC achievements is represented as a historical timeline in Fig. 3.
Figure 3.
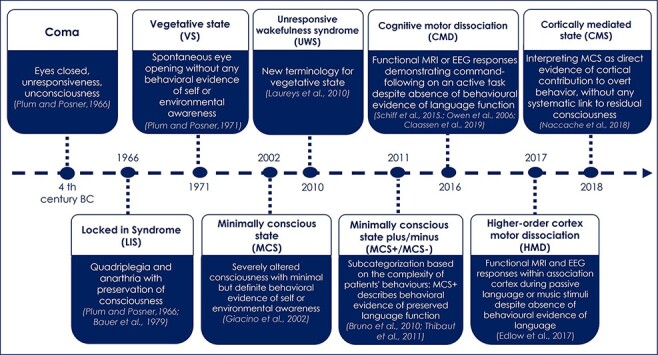
Schematic timeline of the syndromic taxonomy of disorders of consciousness
Legend: This timeline illustrates the progressive enrichment of this taxonomy as well as the reinterpretation of previously described entities (e.g. VS reframed as a UWS and MCS interpreted as a CMS). Note that the first four key categories of this taxonomy (coma, VS, LIS and MCS) set up the general landscape of DoC, whereas the more recent ones aim at reinterpreting them and at enriching them with data originating from functional brain imaging including electrophysiology.
Toward a new classification of DoC patients combining behavior and functional brain-imaging data
Converging toward the need for such a new classification
In addition to the evidence listed in the previous section that demonstrated the added value of taking into account brain activity to improve the diagnostic stage, several studies revealed a prognostic value of these findings. For instance, Sitt and colleagues showed that patients in a behavioral VS/UWS labeled as MCS by a multivariate EEG-based classifier had a significant better prognosis of consciousness improvement (improving to behavioral MCS or better within the next 6 weeks) as compared to those confirmed by the classifier as being in a VS/UWS (Sitt et al. 2014) A similar finding was reported in the EEG-based CMD study mentioned above (Claassen et al. 2019). In the same line, (Perez et al. 2020b) showed that patients with a “global effect” (that corresponds to a late, sustained and brain-scale neural response indexing conscious detection of violations of auditory regularities; Bekinschtein et al. 2009b) have a better prognosis of behaviorally overt consciousness recovery. Given that previous studies established that being in a behavioral MCS is associated with a better prognosis of consciousness recovery (Luauté et al. 2010; Faugeras et al. 2018), the break-in of functional brain imaging and these first reports led to the following conclusion: it is highly probable that patients diagnosed in a richer cognitive state by functional brain imaging than by behavioral observation will also have a better prognosis of overt consciousness recovery. This mode of reasoning as well as the cumulative evidence confirming the added value of functional brain imaging converged on explicit calls for a new classification (Laureys et al. 2004b; Bayne et al. 2018; Naccache 2018a; Giacino et al. 2018b; Comanducci et al. 2020; Kondziella et al. 2020; Provencio et al. 2020), while recognizing the lack of consensus about a specific protocol that could enrich the current behavioral gold-standard method.
Looking for names beyond a “Byzantine” taxonomy: VS/UWS, MCS/CMS, MCS+/MCS–, CMD, HMD, MCS*, …
The stimulating profusion of new studies led to the formulation of many acronyms and names discussed above, which need to be compared to each other in order to introduce more homogeneity and to extract the key factors that should be used to build this new classification. We propose here such a comparison of the polymorph terminology (see Fig. 4 and Table 1 for an explanation of the number/letter code used) and a corresponding list of key factors at work. In particular, we propose to distinguish the two possible sources of information: behavior versus brain activity as well as four levels that can be observed either in behavior or in brain-activity: (i) VS/UWS; (ii) CMS; (iii) response to command; and (iv) functional communication. Such a 4 × 4 matrix enables the delineation of three zones: a univocal zone of the conscious state that includes all eight cases with a functional communication that demonstrates the existence of self-reported subjective states. In contrast, the single case corresponding to VS/UWS confirmed both in the behavior and in brain activity would label a unmistakable nonconscious state. The remaining eight cases would define a gray zone in which it is not possible to define the conscious status with certainty. As explained above and elsewhere in greater details (Naccache 2018a), we prefer the use of CMS than MCS to remove the ambiguity of associating MCS with consciousness: if all MCS patients are in CMS, it is not true that all MCS patients are conscious. In this gray zone, however, CMS states would be associated with higher probabilities of consciousness and most importantly with larger chance of recovering a behaviorally overt conscious state.
Figure 4.
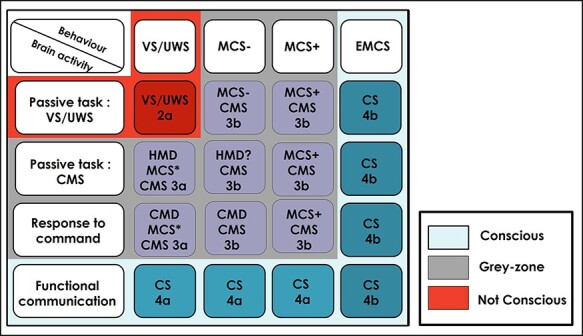
Comparison of the polymorph terminology of disorder of consciousness
Legend:; 2a-4b:classification adapted from Naccache (2018a). See paragraph Looking for names beyond a “Byzantine” taxonomy: VS/UWS, MCS/CMS, MCS+/MCS–, CMD, HMD, MCS*, … for a full description of this 4 × 4 matrix.
Proposal of a sketch of a new classification of DoC
In light of the ideas discussed above, we close this first part by presenting a minimal sketch of such a new classification capitalizing on most ideas developed here could be. This sketch was introduced in a previous paper (see Table 1).
Some current limits, pitfalls and caveats of the DoC literature for science of consciousness
Finally, we list a nonexhaustive series of current issues and problems related to the use of this rich neurological literature to enlighten neuroscientific theories of consciousness.
MCS versus VS/UWS contrast is not a minimal contrast of consciousness but rather a blurry contrast
In the search of the neural bases, correlates (NCC) or signatures of conscious states, it is obvious that contrasting extreme clinical conditions such as comatose states versus behaviorally overt conscious states, defined for instance by the EMCS criteria of the CRS-r, will not be specific enough to capture specific neural properties of being in a conscious state. Indeed, comatose states are conceived not only as an alteration of consciousness but also of vigilance or arousal. Therefore, such an extreme contrast comparing brain activity recorded during conscious wakefulness versus comatose states will not specifically isolate the neural signatures of the conscious state. With this logic in mind, several groups explicitly or implicitly chose the VS/UWS versus MCS contrast as a supposedly much better comparison. By equating vigilance between the two populations of patients and by defining MCS as a minimal but conscious state, this choice seemed indeed to enable such a minimal and pure contrast to isolate specific neural correlates of conscious states. Crucially, the interpretation of all fMRI, quantitative EEG, PET imaging, TMS-EEG and other brain activity or behavioral measures used to contrast VS/UWS with MCS relies on this premise. However, several problems weaken this assumption.
Once it is made clear that MCS does not necessarily correspond to a conscious state (even “minimal,” whatever that may mean), but rather to a CMS, it is clear that contrasting VS/UWS with MCS/CMS will not be the ideal contrast to isolate ultimate neural signatures of consciousness.
Second, once one is aware of the ∼15% proportion of patients in a behavioral VS/UWS who are actually in a much richer state (MCS/CMS or even EMCS and conscious), the behavioral VS/UWS versus MCS/CMS contrast loses in purity. The temptation of “cleaning” the behavioral VS/UWS database from the patients in a richer state would obviously lead to a circularity bias, because brain-activity cutoff was first obtained through the lens of the behavioral gold standard.
For these two reasons, although this contrast is clinically very relevant, it remains very blurry to isolate genuine neural signatures of the conscious state and conscious processing. Still, it conveys precious information but should be completed by other efforts to confirm the relevance of the proposed neural signatures of conscious state and conscious processing. One of the possible tracks to follow would be to confront the potential signatures of consciousness identified in Doc patients, to several other unconscious conditions such as epileptic absence seizures, anesthesia and sleep.
Circularity of gold-standard behavioral criteria of consciousness
Back to the arguments mentioned above, one should also be aware of the circularity of the behavioral gold-standard criterion of consciousness. We defined (and still define) consciousness according to behavioral criterion, then we explored neural correlates of this state and discovered substantial dissociations at the single-patient level between these brain-activity measurements and their behavioral counterparts (e.g. the case of ∼15% CMD among behaviorally VS/UWS patients). This dialectic evolution of knowledge necessarily means that behavioral observation that we chose as the gold standard is obviously not the best definition we should end up with: the ideal and perfect criterion of consciousness should get rid of the limits of behavioral observation and will therefore not reach the highest levels of performance as long as the reference measure is the behavior. Given that we do not yet converge on a new gold standard, we have to be aware of this key limit.
One of the best ways to escape, at least partially, from this circularity consists in moving from instantaneous consciousness diagnosis to study the neural dynamics of overt consciousness recovery (Edlow et al. 2021). This change of focus from diagnosis to prognosis offers a solid basis that could lead to important theoretical and pragmatic discoveries. Note, however, that this prognosis-based approach will probably enable the identification of factors related to the recovery of conscious processing rather than the direct identification of neural signatures of conscious processing (see Perez et al. (2020b)).
A last word concerning this issue. There are nevertheless two solid reasons not to drop behavioral definition of consciousness too fast. First, behavioral observations and interactions are the major mode of communication humans use together through social cognition. In other terms, behavior is also the gold standard because it is the way we live and interact together. This parameter may evolve with the digital revolution and the probable development of efficient brain–computer interfaces, but so far behavior still has the lead (Luauté et al. 2010; Chatelle et al. 2012; Eliseyev et al. 2021). Not to mention the importance of brain–body interactions and social cognition in the individual development of many cognitive functions. Second, a pragmatic reason to adopt or drop a gold standard relies on its availability and cost: behavioral interaction with DoC patients in everyday life is obviously much more available and inexpensive than using functional brain-imaging tools in a permanent way.
Lack of a consensual definition of consciousness at bedside
Readers familiar with current cognitive neuroscience of consciousness perfectly know that distinct, and sometimes mutually exclusive, theories coexist. Actually, these divergences originate in part from the lack of a common definition of the word: consciousness. Ranging from phenomenal consciousness to self-reflexive subjective reports, these divergences are out of the scope of the present paper. However, there is no reason to expect that such massive conceptual and theoretical divergences would not affect the world of neurology and DoC patients. As a direct consequence, the clinical definition of consciousness is also open to these differences. While this discussion does not impact most of neurological conditions, they clearly impact the field of DoC patients. The seminal clinical definition used by neurologists has been stated by Plum and Posner in 1971: “Consciousness means awareness of self and environment” (Plum and Posner 1972). Being “aware of something” means being able to self-report it as a conscious content and therefore coincides with the reportability definition used, for instance, by GNWT and by HOT theories. However this definition departs from other theories such as phenomenal consciousness theory (Block 1995), microconsciousness theory (Zeki and Bartels 1999) or local-recurrent consciousness theory (Lamme 2006). In other terms and obviously, the lack of consensual definition of consciousness does not spare the field of DoC patients.
Individual, technical, methodological and statistical caveats of brain-activity signatures of consciousness
A weakness of the DoC literature relies on a series of intrinsic and methodological difficulties. First, many of these patients show substantial fluctuations, whose time constant remains difficult to characterize (from a few minutes to several days). Observable clinical signs of consciousness fluctuate across the same day (Bekinschtein et al. 2009a; Cortese et al. 2015) and a single clinical evaluation can result in up to 30% of misdiagnoses as compared with multiple assessments (Wannez et al. 2017; Wang et al. 2020). The frequency of misdiagnoses of DOC patients by clinical consensus compared to behavior-scale assessments is up to 40% in VW/UWS (Childs et al. 1993; Schnakers et al. 2009; Stender et al. 2014b; Wang et al. 2020). Even when standardized scoring systems are used, the lack of training and experience may influence the reliability of evaluations (Løvstad et al. 2010). Clinical assessments are highly dependent on the personal relevance (personal history and preferences) and complexity of the stimuli used to elicit behaviors (Stefano et al. 2012; Perrin et al. 2015; Magliacano et al. 2019). For instance, a higher percentage of patients demonstrate the ability of locating sounds when probed with their own names as compared to neutral sounds (Cheng et al. 2013). Family caregivers of DoC patients often report higher interactions with the environment in their relatives than care professionals (Formisano et al. 2011; Moretta et al. 2017). This may not only be linked to family optimistic biases and prolonged time of observation but also to the use of emotionally competent stimuli (Damasio 2001).
In addition to these intrinsic fluctuations related to patients, there are also technical, methodological and statistical sources of variability that complicate the interpretation of collected data (Bardin et al. 2011; Boly 2011; Cruse et al. 2011, 2014; King et al. 2011; Goldfine et al. 2013; Tzovara et al. 2015; Gabriel et al. 2016). The diversity of brain-imaging techniques, paradigms (resting-state, passive, active) and analyses pipelines across teams and studies also limits the generalizability and interpretability of the findings. Moreover, several results were discussed according to the reliability of their statistical methodology. Finally, it is noteworthy that it can be challenging to obtain sensitive brain measures of cognitive processing at the single-subject level even in healthy conscious individuals (e.g. Cruse et al. 2014; Rohaut et al. 2015; Kallionpää et al. 2019).
Conclusion
We conclude by an obvious remark: the evolution of DoC concepts is far from being achieved. While the development of functional brain-imaging data is only beginning and should dramatically impact our knowledge about patients’ consciousness and cognition as we largely discussed above, it is not less true and important that behavioral observation is also open to revolutionary developments that should equally impact our understanding. We can illustrate this last comment by citing “en vrac” a few recent and promising examples of such a “Behavioral 2.0” period of the exploration of DoC patients: (i) behavioral EMG recordings can be used to detect infra-threshold responses to command (Bekinschtein et al. 2008); (ii) trace conditioning effects, that require working memory resources postulated to be a specific property of conscious processing, can be probed at the bedside (Bekinschtein et al. 2009b); (iii) the habituation of the auditory startle response that relies on the preservation of the frontal cortex inhibition linked to conscious processing was explored at the bedside and demonstrated a powerful diagnostic value to identify MCS or CS patients as well as a prognostic value to predict consciousness recovery (Hermann et al. 2020); (iv) olfactory sniffing response that requires cognitive resources was tested at the bedside and demonstrated both a diagnostic and a prognostic value (Arzi et al. 2020), etc. For all these reasons, the medical and scientific field of DoC patients, although complex, should continue to be a unique source of knowledge and intuitions to test, correct and improve neuroscientific models and theories of consciousnes.
Supplementary Material
Contributor Information
Aude Sangaré, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France; Department of Neurophysiology, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France.
Esteban Munoz-Musat, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France.
Amina Ben Salah, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France.
Pauline Perez, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France.
Mélanie Valente, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France; Department of Neurophysiology, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France.
Frédéric Faugeras, Department of Neurology, AP-HP, Hôpital Henri-Mondor-Albert Chenevier, Université Paris Est Creteil, Créteil 94 000, France; Département d’Etudes Cognitives, École normale supérieure, PSL University, Paris 75005, France; Inserm U955, Institut Mondor de Recherche Biomédicale, Equipe E01 NeuroPsychologie Interventionnelle, Créteil 94000, France.
Vadim Axelrod, Gonda Multidisciplinary Brain Research Center, Bar-Ilan University, Ramat Gan 5290002, Israel.
Sophie Demeret, Department of Neurology, Neuro-ICU, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France.
Clémence Marois, Department of Neurology, Neuro-ICU, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France.
Nadya Pyatigorskaya, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France; Department of Neuroradiology, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France.
Marie-Odile Habert, Department of Nuclear Medicine, AP-HP, Pitié-Salpêtrière Hospital, Paris, France; Laboratoire d’Imagerie Biomédicale, LIB, INSERM, CNRS, Sorbonne Université, Paris, France.
Aurélie Kas, Department of Nuclear Medicine, AP-HP, Pitié-Salpêtrière Hospital, Paris, France; Laboratoire d’Imagerie Biomédicale, LIB, INSERM, CNRS, Sorbonne Université, Paris, France.
Jacobo D Sitt, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France.
Benjamin Rohaut, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France; Department of Neurology, Neuro-ICU, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France.
Lionel Naccache, Brain institute-ICM, Inserm U1127, CNRS UMR 7225, Sorbonne Université, Paris 75013, France; Department of Neurophysiology, AP-HP, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris 75006, France; Medical Intensive Care Unit, AP-HP, Hôpital Européen Georges Pompidou, Paris 75015, France.
Supplementary data
Supplementary data is available at NCONSC online.
Data availability
Ethic committee approval does not allow the open sharing of raw human patients data (notably of brain-imaging data). However, post-processed anonymized data are available upon reasonable request.
Funding
A.S. and B.H. were funded by the “Bourse poste d’Accueil” grant from “Inserm” and L.N. was funded by an “FRM Equipe 2015 and by UNIM.”
Conflict of interest statement
None declared.
References
- Aricò I, Naro A, Pisani LR et al. Could combined sleep and pain evaluation be useful in the diagnosis of disorders of consciousness (DOC)? Preliminary findings. Brain Inj 2016;30:159–63. [DOI] [PubMed] [Google Scholar]
- Arnaldi D, Terzaghi M, Cremascoli R et al. The prognostic value of sleep patterns in disorders of consciousness in the sub-acute phase. Clin Neurophysiol 2016;127:1445–51. [DOI] [PubMed] [Google Scholar]
- Arzi A, Rozenkrantz L, Gorodisky L et al. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. Nature 2020;581:428–33. [DOI] [PubMed] [Google Scholar]
- Bardin JC, Fins JJ, Katz DI et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain 2011;134:769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G, Gerstenbrand F, Rumpl E Varieties of the locked-in syndrome. J Neurol 1979;221:77–91. [DOI] [PubMed] [Google Scholar]
- Bayne T On the axiomatic foundations of the integrated information theory of consciousness. Neurosci Conscious 2018;2018:niy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne T, Hohwy J, Owen AM Response to “Minimally conscious state or cortically mediated state?” Brain 2018;141:e26. [DOI] [PubMed] [Google Scholar]
- Bekinschtein TA, Coleman MR, Niklison J et al. Can electromyography objectively detect voluntary movement in disorders of consciousness? J Neurol Neurosurg Psychiatry 2008;79:826–8. [DOI] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B et al. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci U S A 2009b;106:1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein TA, Golombek DA, Simonetta SH et al. Circadian rhythms in the vegetative state. Brain Inj 2009a;23:915–9. [DOI] [PubMed] [Google Scholar]
- Block N On a confusion about a function of consciousness. Behav Brain Sci 1995;18:227–47. [Google Scholar]
- Boly M Measuring the fading consciousness in the human brain. Curr Opin Neurol 2011;24:394–400. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Balteau E et al. Consciousness and cerebral baseline activity fluctuations. Hum Brain Mapp 2008;29:868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, Bruno M-A et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010;113:1038–53. [DOI] [PubMed] [Google Scholar]
- Bruno M-A, Majerus S, Boly M et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol 2012;259:1087–98. [DOI] [PubMed] [Google Scholar]
- Bruno M-A, Vanhaudenhuyse A, Thibaut A et al. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011;258:1373–84. [DOI] [PubMed] [Google Scholar]
- Chatelle C, Chennu S, Noirhomme Q et al. Brain-computer interfacing in disorders of consciousness. Brain Inj 2012;26:1510–22. [DOI] [PubMed] [Google Scholar]
- Cheng L, Gosseries O, Ying L et al. Assessment of localisation to auditory stimulation in post-comatose states: use the patient’s own name. BMC Neurol 2013;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs NL, Mercer WN, Childs HW Accuracy of diagnosis of persistent vegetative state. Neurology 1993;43:1465–7. [DOI] [PubMed] [Google Scholar]
- Claassen J, Doyle K, Matory A et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019;380:2497–505. [DOI] [PubMed] [Google Scholar]
- Cologan V, Drouot X, Parapatics S et al. Sleep in the unresponsive wakefulness syndrome and minimally conscious state. J Neurotrauma 2013;30:339–46. [DOI] [PubMed] [Google Scholar]
- Comanducci A, Boly M, Claassen J et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: review of an IFCN-endorsed expert group. Clin Neurophysiol 2020;131:2736–65. [DOI] [PubMed] [Google Scholar]
- Cortese MD, Riganello F, Arcuri F et al. Coma recovery scale-r: variability in the disorder of consciousness. BMC Neurol 2015;15:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone JS, Schurz M, Höller Y et al. Impaired consciousness is linked to changes in effective connectivity of the posterior cingulate cortex within the default mode network. Neuroimage 2015;110:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse D, Beukema S, Chennu S et al. The reliability of the N400 in single subjects: implications for patients with disorders of consciousness. Neuroimage Clin 2014;4:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2011;378:2088–94. [DOI] [PubMed] [Google Scholar]
- Damasio A Fundamental feelings. Nature 2001;413:781–781. [DOI] [PubMed] [Google Scholar]
- de Biase S, Gigli GL, Lorenzut S et al. The importance of polysomnography in the evaluation of prolonged disorders of consciousness: sleep recordings more adequately correlate than stimulus-related evoked potentials with patients’ clinical status. Sleep Med 2014;15:393–400. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001;79:1–37. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Antonopoulos G, Heine L et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 2015;138:2619–31. [DOI] [PubMed] [Google Scholar]
- Edlow BL, Chatelle C, Spencer CA et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140:2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Claassen J, Schiff ND et al. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol 2021;17:135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Naccache L. Unmasking covert language processing in the intensive care unit with electroencephalography. Ann Neurol 2021;89:643–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseyev A, Gonzales IJ, Le A et al. Development of a brain-computer interface for patients in the critical care setting. PLoS One 2021;16:e0245540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Valente M et al. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Inj 2018;32:72–7. [DOI] [PubMed] [Google Scholar]
- Forgacs PB, Conte MM, Fridman EA et al. Preservation of EEG organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann Neurol 2014;76:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano R, D’Ippolito M, Risetti M et al. Vegetative state, minimally conscious state, akinetic mutism and Parkinsonism as a continuum of recovery from disorders of consciousness: an exploratory and preliminary study. Funct Neurol 2011;26:15–24. [PMC free article] [PubMed] [Google Scholar]
- Gabriel D, Muzard E, Henriques J et al. Replicability and impact of statistics in the detection of neural responses of consciousness. Brain 2016;139:e30–e30. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58:349–53. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated. Arch Phys Med Rehabil 2004;85:2020–9. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Katz DI, Schiff ND et al. Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018a;91:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Katz DI, Schiff ND et al. Comprehensive systematic review update summary: disorders of consciousness. Neurology 2018b;91:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Kezmarsky MA, DeLuca J et al. Monitoring rate of recovery to predict outcome in minimally responsive patients. Arch Phys Med Rehabil 1991;72:897–901. [DOI] [PubMed] [Google Scholar]
- Gibson RM, Ray LB, Laforge G et al. 24-h polysomnographic recordings and electrophysiological spectral analyses from a cohort of patients with chronic disorders of consciousness. J Neurol 2020;267:3650–63. [DOI] [PubMed] [Google Scholar]
- Goldfine AM, Bardin JC, Noirhomme Q et al. Reanalysis of “Bedside detection of awareness in the vegetative state: a cohort study”. Lancet 2013;381:289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AM, Victor JD, Conte MM et al. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophysiol 2011;122:2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui P, Jiang Y, Zang D et al. Assessing the depth of language processing in patients with disorders of consciousness. Nat Neurosci 2020;23:761–70. [DOI] [PubMed] [Google Scholar]
- Hermann B, Salah AB, Perlbarg V et al. Habituation of auditory startle reflex is a new sign of minimally conscious state. Brain 2020;143:2154–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Stender J, Habert M-O et al. Multimodal FDG-PET and EEG assessment improves diagnosis and prognostication of disorders of consciousness. Neuroimage Clin 2021;30:102601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennett B, Plum F Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972;1:734–7. [DOI] [PubMed] [Google Scholar]
- Kallionpää RE, Pesonen H, Scheinin A et al. Single-subject analysis of N400 event-related potential component with five different methods. Int J Psychophysiol 2019;144:14–24. [DOI] [PubMed] [Google Scholar]
- Kalmar K, Giacino JT. The JFK coma recovery scale—revised. Neuropsychol Rehabil 2005;15:454–60. [DOI] [PubMed] [Google Scholar]
- King J-R, Bekinschtein T, Dehaene S. Comment on “Preserved feedforward but impaired top-down processes in the vegetative state”. Science 2011;334:1203; author reply 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler PJ, Wijdicks EFM. Historical study of coma: looking back through medical and neurological texts. Brain 2008;131:877–89. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Bender A, Diserens K et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol 2020;27:741–56. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Cheung MC, Dutta A. Public perception of the vegetative state/unresponsive wakefulness syndrome: a crowdsourced study. PeerJ 2019;7:e6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella D, Friberg CK, Frokjaer VG et al. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016;87:485–92. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Frontera JA. Pearls & oy-sters: eyes-open coma. Neurology 2021;96:864–7. [DOI] [PubMed] [Google Scholar]
- Lamme VAF. Towards a true neural stance on consciousness. Trends Cogn Sci 2006;10:494–501. [DOI] [PubMed] [Google Scholar]
- Landsness E, Bruno M-A, Noirhomme Q et al. Electrophysiological correlates of behavioural changes in vigilance in vegetative state and minimally conscious state. Brain 2011;134:2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Antoine S, Boly M et al. Brain function in the vegetative state. Acta Neurol Belg 2002a;102:177–85. [PubMed] [Google Scholar]
- Laureys S, Celesia GG, Cohadon F et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 2010;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Faymonville ME, Peigneux P et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage 2002b;17:732–41. [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004a;3:537–46. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004b;3:537–46. [DOI] [PubMed] [Google Scholar]
- Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 2012;61:478–91. [DOI] [PubMed] [Google Scholar]
- Løvstad M, Frøslie KF, Giacino JT et al. Reliability and diagnostic characteristics of the JFK coma recovery scale-revised: exploring the influence of rater’s level of experience. J Head Trauma Rehabil 2010;25:349–56. [DOI] [PubMed] [Google Scholar]
- Luauté J, Maucort-Boulch D, Tell L et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010;75:246–52. [DOI] [PubMed] [Google Scholar]
- Magliacano A, De Bellis F, Galvao-Carmona A et al. Can salient stimuli enhance responses in disorders of consciousness? A systematic review. Curr Neurol Neurosci Rep 2019;19:98. [DOI] [PubMed] [Google Scholar]
- Majerus S, Bruno M-A, Schnakers C et al. The problem of aphasia in the assessment of consciousness in brain-damaged patients. Prog Brain Res 2009;177:49–61. [DOI] [PubMed] [Google Scholar]
- Maquet P, Degueldre C, Delfiore G et al. Functional neuroanatomy of human slow wave sleep. J Neurosci 1997;17:2807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–89. [DOI] [PubMed] [Google Scholar]
- Moretta P, Trojano L, Masotta O et al. Family caregivers’ opinions about interaction with the environment in consciousness disorders. Rehabil Psychol 2017;62:208–13. [DOI] [PubMed] [Google Scholar]
- Naccache L Psychology. Is she conscious? Science 2006;313:1395–6. [DOI] [PubMed] [Google Scholar]
- Naccache L Minimally conscious state or cortically mediated state? Brain 2018a;141:949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L. Why and how access consciousness can account for phenomenal consciousness. Philos Trans R Soc Lond B Biol Sci 2018b;373:20170357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Miewald JM et al. Human regional cerebral glucose metabolism during non‐rapid eye movement sleep in relation to waking. Brain 2002;125:1105–15. [DOI] [PubMed] [Google Scholar]
- Oldham MA, Holloway RG. Delirium disorder: integrating delirium and acute encephalopathy. Neurology 2020;95:173–8. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M et al. Detecting awareness in the vegetative state. Science 2006;313:1402. [DOI] [PubMed] [Google Scholar]
- Pan J, Wu J, Liu J et al. A systematic review of sleep in patients with disorders of consciousness: from diagnosis to prognosis. Brain Sci 2021;11:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P, Madsen J, Banellis L et al. Conscious processing of narrative stimuli synchronizes heart rate between individuals. Cell Rep 2020a;36:109692. [DOI] [PubMed] [Google Scholar]
- Perez P, Valente M, Hermann B et al. Auditory event-related “global effect” predicts recovery of overt consciousness. Front Neurol 2020b;11:588233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Castro M, Tillmann B et al. Promoting the use of personally relevant stimuli for investigating patients with disorders of consciousness. Front Psychol 2015;6:1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincherle A, Rossi F, Jöhr J et al. Early discrimination of cognitive motor dissociation from disorders of consciousness: pitfalls and clues. J Neurol 2020;268:178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum F, Posner JB. The Diagnosis of Stupor and Coma. F. A. Davis Company, Philadelphia: F.A. Davis Co, 1966. [Google Scholar]
- Plum F, Posner JB. The diagnosis of stupor and coma. Contemp Neurol Ser 1972;10:1–286. [PubMed] [Google Scholar]
- Provencio JJ, Hemphill JC, Claassen J et al. The curing coma campaign: framing initial scientific challenges-proceedings of the first curing coma campaign scientific advisory council meeting. Neurocrit Care 2020;33:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo F, Rohaut B, Demertzi A et al. Brain-heart interactions reveal consciousness in noncommunicating patients. Ann Neurol 2017;82:578–91. [DOI] [PubMed] [Google Scholar]
- Riveros R, Bakchine S, Pillon B et al. Fronto-subcortical circuits for cognition and motivation: dissociated recovery in a case of loss of psychic self-activation. Front Psychol 2018;9:2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaut B, Faugeras F, Chausson N et al. Probing ERP correlates of verbal semantic processing in patients with impaired consciousness. Neuropsychologia 2015;66:279–92. [DOI] [PubMed] [Google Scholar]
- Rohaut B, Faugeras F, Naccache L. Neurology of consciousness impairments. Brain Disorders in Critical Illness Chapter 7 Neurology of consciousness impairments from Section 2 - Behavioral Neurology in the ICU. Cambridge University Press, 2013;59–67. [Google Scholar]
- Rohaut B, Raimondo F, Galanaud D et al. Probing consciousness in a sensory-disconnected paralyzed patient. Brain Inj 2017;31:1398–403. [DOI] [PubMed] [Google Scholar]
- Rosenthal DM Two concepts of consciousness. Philos Stud 1986;49:329–59. [Google Scholar]
- Rossi Sebastiano D, Visani E, Panzica F et al. Sleep patterns associated with the severity of impairment in a large cohort of patients with chronic disorders of consciousness. Clin Neurophysiol 2018;129:687–93. [DOI] [PubMed] [Google Scholar]
- Santamaria J, Orteu† N, Iranzo A et al. Eye opening in brain death. J Neurol 1999;246:720–2. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol 2017;44:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S, Rosanova M, Casali AG et al. Quantifying cortical EEG responses to TMS in (un)consciousness. Clin EEG Neurosci 2014;45:40–9. [DOI] [PubMed] [Google Scholar]
- Schiff ND Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015;72:1413–5. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer M, Katz DI, Bodien YG et al. Post-traumatic confusional state: a case definition and diagnostic criteria. Arch Phys Med Rehabil 2020;101:2041–50. [DOI] [PubMed] [Google Scholar]
- Sitt JD, King J-R, El Karoui I et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014;137:2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart CM, Giacino JT, Cullen T et al. A case of locked-in syndrome complicated by central deafness. Nat Clin Pract Neurol 2008;4:448–53. [DOI] [PubMed] [Google Scholar]
- Sokoliuk R, Degano G, Banellis L et al. Covert speech comprehension predicts recovery from acute unresponsive states. Ann Neurol 2021;89:646–56. [DOI] [PubMed] [Google Scholar]
- Stefano CD, Cortesi A, Masotti S et al. Increased behavioural responsiveness with complex stimulation in VS and MCS: preliminary results. Brain Inj 2012;26:1250–6. [DOI] [PubMed] [Google Scholar]
- Stender J, Gosseries O, Bruno M-A et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014a;384:514–22. [DOI] [PubMed] [Google Scholar]
- Stender J, Gosseries O, Bruno M-A et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014b;384:514–22. [DOI] [PubMed] [Google Scholar]
- Stender J, Kupers R, Rodell A et al. Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J Cereb Blood Flow Metab 2015;35:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Binns MA, Carruth FG et al. The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg 1999;90:635–43. [DOI] [PubMed] [Google Scholar]
- Symonds CP, Ritchie Russell W. Accidental head injuries: prognosis in service patients. Lancet 1943;241:7–10. [Google Scholar]
- Thibaut A, Bodien YG, Laureys S et al. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol 2020;267:1245–54. [DOI] [PubMed] [Google Scholar]
- Thibaut A, Panda R, Annen J et al. Preservation of brain activity in unresponsive patients identifies MCS star. Ann Neurol 2021;90:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Stokes L Prolonged disorders of consciousness: new national clinical guidelines from the Royal College of Physicians, London. Clin Med (Lond) 2014;14:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89. [DOI] [PubMed] [Google Scholar]
- Tzovara A, Simonin A, Oddo M et al. Neural detection of complex sound sequences in the absence of consciousness. Brain 2015;138:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ-F et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010;133:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Wilson JE et al. Structure and neural mechanisms of catatonia. Lancet Psychiatry 2019;6:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu X, Hu Z et al. The misdiagnosis of prolonged disorders of consciousness by a clinical consensus compared with repeated coma-recovery scale-revised assessment. BMC Neurol 2020;20:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannez S, Gosseries O, Azzolini D et al. Prevalence of coma-recovery scale-revised signs of consciousness in patients in minimally conscious state. Neuropsychol Rehabil 2018;28:1350–9. [DOI] [PubMed] [Google Scholar]
- Wannez S, Heine L, Thonnard M et al. Coma Science Group collaborators . The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol 2017;81:883–9. [DOI] [PubMed] [Google Scholar]
- Wu X, Zou Q, Hu J et al. Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci 2015;35:12932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Rund D Psychiatric considerations in patients with decreased levels of consciousness. Emerg Med Clin North Am 2010;28:595–609. [DOI] [PubMed] [Google Scholar]
- Zeki S, Bartels A Toward a theory of visual consciousness. Conscious Cogn 1999;8:225–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Ethic committee approval does not allow the open sharing of raw human patients data (notably of brain-imaging data). However, post-processed anonymized data are available upon reasonable request.


