Abstract
Planar cell polarity (PCP) refers to the coordinated orientation of cells in tissue plane. Originally discovered and studied in Drosophila melanogaster, PCP is now widely recognized in vertebrates, where it has been implicated in organogenesis. Specific sets of PCP genes have been identified. The proteins encoded by these genes are organized into a dedicated signaling pathway, become asymmetrically distributed to opposite sides of each cell within a tissue plane and guide many processes that include changes in cell shape and polarity, collective cell movements or uniform distribution of cell appendages. A unifying characteristic of these changes is that they often involve actomyosin rearrangement. Mutations in PCP genes cause congenital malformations of multiple organs in many animals and, importantly, in humans.
In the last decade, strong evidence has accumulated for a role of the PCP pathway in kidney development. It has been proposed that defective PCP signaling contributes to polycystic kidney disease and that specific PCP gene mutations lead to Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). In this review, we describe the origins, molecular constituents and cellular targets of PCP, with a special focus on the involvement of PCP molecules in normal kidney development and how their dysfunction leads to kidney disease.
Introduction
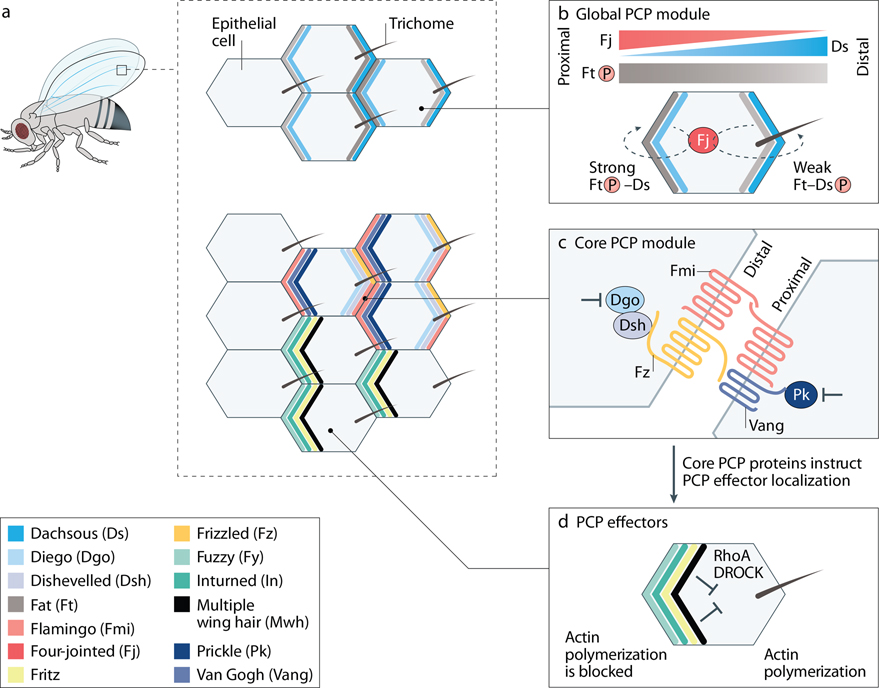
Unlike the easily appreciated apical-basal polarity of epithelial cells that refers to the asymmetry between top and bottom surfaces, planar cell polarity (PCP) reflects coordinated cell orientation in the plane of the tissue, i. e. in the direction perpendicular to the apical-basal axis. PCP has been recognized for decades by polarized arrays of bristles or hairs on the insect cuticle 1. Genetic screens in Drosophila uncovered mutants with disorganized wing hairs or eye photoreceptors 2, 3, leading to the identification of a set of conserved “core” PCP genes 4. These include Frizzled (fz/Fzd), Dishevelled (Dsh/Dvl), Flamingo or Starry night (fmi or stan/Celsr), Van Gogh or Strabismus (vang or stan/Vangl), Prickle (pk/Pk) and Diego (dgo/Diversin or Inversin) (fly name is followed by the vertebrate homologue name separated by “/”) 5, 6. The unifying characteristic of these genes is that encoded proteins are organized into complexes at opposing faces of epithelial cells 4. For example, in the Drosophila wing, the Vang/Pk complex is seen at the proximal side of the cell, whereas the Fz/Dsh/Diego complex is located distally 7–10 (Fig. 1). An exception is Fmi, which partners with both the proximal Vang/Pk complex and the distal Dsh/Fz complex 11, 12. Core PCP constituents are interdependent: mutations in any of them disrupt the localization of other core PCP proteins. The asymmetric localization of PCP protein complexes is crucial to the mechanisms by which they establish polarity along the epithelial plane and serves as a convincing marker of PCP signaling activity within the cell.
Figure 1: Planar cell polarity in Drosophila wing.
Left upper: Instructed by long-range cues, wing hexamer cells generate a single actin-based trichome (hair) at the most distal aspect of each cell. Right upper: Fat (Ft) mRNA is evenly expressed along the tissue, whereas Dachsous (Ds) transcript is expressed in a distal-proximal gradient and Four-jointed (Fj) kinase is expressed in a proximal-distal gradient. Fj phosphorylates both Ft (stronger phosphorylation at proximal side) and Ds (stronger phosphorylation at distal side). Strong Ft phosphorylation translates into a strong Ft-Ds protein-protein interactions at the proximal side, whereas Ds phosphorylation weakens Ft-Ds interactions at the distal part, thereby generating a shallow gradient of Ft-Ds activity across the tissue plane. Right middle: core PCP protein complexes are asymmetrically distributed: Vang-Pk to the proximal and Fz-Dsh-Dg to the distal sides. Cadherin Fmi is localized at both proximal and distal sides and forms homodimers between the extracellular domains of molecules expressed by adjacent cells. Interactions between Fmi and Vang or Fmi and Fz, as well as between extracellular domains of Vang and Fz expressed on surfaces of neighboring cells stabilize Vang-Pk and Fz-Dsh complexes on the opposite cell membranes. Inside the cell, mutual antagonism between Pk and Dsh creates “exclusion” zones where the proteins of the opposite core PCP complex cannot function. Right lower: Core PCP proteins control localization of PCP effectors via direct interactions (e.g. Vang interacts with In and Fy). In Drosophila wing cells, PCP effectors inhibit generation of trichome at the proximal side of the cell, whereas positive actin regulators, such as RhoA GTPase and Drosophila RhoA kinase (DROCK) accumulate at the distal side of the cell where they promote actin polymerization and hair formation.
PCP has been associated with a specific signaling pathway regulating cell shape and behavior. Whereas PCP as a phenomenon refers to tissue-wide subcellular asymmetry that can be conferred by diverse mechanisms, we will largely discuss the mechanism mediated by core PCP proteins. Besides core PCP proteins, this pathway includes a large number of other players. Mutations in Drosophila genes encoding the atypical cadherins Fat (ft/Fat) and Dachsous (ds/Dchs) and the Golgi kinase Four-jointed (ft/Fjt1) 13–16 produce long-range defects in tissue polarization. The initial analysis of ft and ds fly mutants suggested that the encoded proteins function as upstream regulators of core PCP module, however, existing genetic evidence is also consistent with their independent role in PCP, at least in some context 17. Overall, the upstream mechanisms that initiate asymmetric organization of core proteins may involve molecular gradients and mechanical forces, yet remain subject to debate.
Once the asymmetric distribution of core PCP proteins has been established, a set of downstream molecules encoded by PCP “effector” genes brings about changes in the cytoskeletal organization, cell adhesion and vesicular trafficking that drive directional movement and patterned tissue growth during organogenesis. Fly PCP effectors Fuzzy (fy/Fuz)18), Inturned (in/In) 19, Fritz (frt/Wdpcp) 20 and Multiple wing hairs (mwh; vertebrate homolog is unknown) 3, 21, 22 control planar polarity of actin-based hairs, specifying the position and orientation of a single hair on each wing cell (Fig 1). In addition, certain house-keeping molecules, such as myosins, protein kinases and small GTPases, act downstream of Fz/Dsh to control cell shape and behavior, however, they may also regulate morphogenesis independently of core PCP- or Ft/Ds modules 23–26. Recently, it has become clear that the initial classification of global, core and effector PCP genes does not fully reflect the complexity of the PCP pathway. For example, there is now evidence that Myosin II and other ‘downstream’ PCP effectors may act upstream of the core PCP proteins and influence their localization 16, 27, 22, 28, 29. This feedback regulation is an essential feature of PCP signaling.
Another layer of complexity derives from the evolution of PCP homologs. While there are only two frizzled and fat genes and single copies of other core PCP genes in Drosophila, vertebrate genomes contain at least 10 Frizzled 30, 4 Prickle, 2 Vang-like 31, 3 Fmi/Celsr and 3 Dsh/Dvl, as well as 4 Fat and 2 Dachsous genes. As a result, the analysis of PCP in vertebrates may be hampered by genetic redundancies and overlapping functional drift. In contrast, the mammalian PCP effectors Inturned, Fuzzy, and Fritz(WDPCP) are encoded by single-copy genes (reviewed in 6). For some vertebrate PCP players, such as Daam1, Shroom3 32–34 or noncanonical receptor tyrosine kinases Ror1/2 35–37, Ryk 38, 39 and Ptk7 40, the corresponding Drosophila mutants lack obvious PCP phenotypes. Other vertebrate PCP regulators have acquired novel functions that may relate to the PCP pathway indirectly; for example, regulation of ciliogenesis 41–45. Therefore, one goal of future studies is to understand whether the PCP molecules define a specific signaling pathway that orchestrates planar polarity or whether they also participate in a variety of events that are unrelated to PCP.
Importantly, loss of PCP signaling disturbs key morphogenetic processes such as cell migration, cell intercalation, constriction of apical surfaces, and oriented cell division (OCD) 46–51. Other vertebrate-specific processes that require PCP components and contribute to three-dimensional body architecture are generation and function of motile and primary cilia as well as left-right patterning 52–57. Thus, PCP signaling provides cells in a tissue with directional information that is needed for collective cell behaviors during organogenesis.
In the last 10–15 years, strong evidence has accumulated for a role of the PCP pathway in kidney development. Mutations of PCP genes have been associated with congenital malformations of the kidney and urinary tract in mice and humans 58 (Table). This review describes the origins of PCP signaling, its activity and cellular targets, with a particular emphasis on the function of the PCP pathway in normal kidney development and the consequences of PCP misregulation for kidney disease.
Origins of PCP
Phenotypic analysis of embryos carrying mutations in PCP genes has revealed the essential role of PCP components in many morphogenetic processes, such as neural tube closure or cardiac morphogenesis (reviewed in 6). However, despite intense research for almost three decades, how PCP is established remains uncertain. In principle, since planar polarity can be visualized at the subcellular level 6, orientation of individual cells in embryonic tissues could originate from the intrinsic polarity of the zygote. However, PCP is normally detected in different tissues rather late in embryonic development and spreads over large distances that are comparable to embryo size. This suggests that PCP forms in response to a long-range cue(s) that is sensed and interpreted within each cell of the tissue. The small differences between individual cells are then amplified to allow robust planar polarization.
Global PCP cues
Molecular gradients are traditionally invoked to explain long-range cues for PCP. These could be gradients of transcriptional or enzymatic activity, cell-cell adhesion, extracellular matrix, or diffusible growth factors. Secreted Wnt proteins have long been considered excellent candidates for this role, due to their apparent ability to trigger the enrichment of Frizzled receptors at the closest surface of each responding cell 30, 59 (Box 1). Supporting this notion, Wnt ligands were found to be essential for PCP, serving as directional guidance cues in both Drosophila and vertebrates 37, 60–64. Nevertheless, the involvement of Wnt ligands in PCP, especially in Drosophila, remains an ongoing debate 65–67. It has been challenging to formally prove the role of Wnt ligands in PCP. For example, morphogen gradients could influence PCP indirectly, by affecting growth and tissue shape, which, in turn, might modulate the PCP vector 68, 69. Alternatively, an initial positional cue in early embryogenesis might propagate through the developing tissue by interactions between neighboring cells. Further research is needed to distinguish these mechanisms.
BOX1: WNT SIGNALING AND PCP.
Wnt pathways promote cell proliferation, cell movements and cell fate specification during embryonic development 267. The canonical Wnt/β-catenin pathway results in the stabilization of β-catenin in the nucleus leading to target gene activation. Wnt proteins also activate noncanonical pathways that are independent of β-catenin but can modulate core PCP components (Fz, Dvl, Pk and Vang) to trigger changes in cell shape and behavior. Noncanonical Wnt signaling is often referred to as the “Wnt/PCP pathway”, based on several experimental observations. First, the core PCP component Frizzled is a Wnt ligand receptor 30. Second, Dvl functions as a core PCP protein and as an obligatory player in the Wnt/β-catenin pathway. Third, some Wnt proteins and core PCP pathway components regulate cilia functions and left-right patterning 45, 61. Finally, both Wnt and PCP signaling affect the localization or/and activity of apicobasal polarity components, such as apical Par proteins and the basolateral determinants Lgl/Scrib 92, 95.
Although these findings indicate that Wnt proteins can regulate PCP, the molecular mechanism is still lacking. Both canonical and noncanonical Wnt pathways, as well as core PCP protein stabilization, are accompanied by Dvl phosphorylation, but the modulation of cell shape and polarity involves RhoA GTPase, ROCK and Myosin activities, rather than β-catenin. Dvl phosphorylation leads to its association with the formin protein Daam1 and upregulation of RhoA and ROCK activity, followed by Myosin II phosphorylation 32, 114 268, 269. Alternatively, in mouse limb buds, Vangl2 phosphorylation by Casein kinase I is initiated in response to Wnt5a and Ror2, a receptor tyrosine kinase, may be a key step in the regulation of Vangl2 localization 37. The segregation of core protein complexes to opposite cell faces is achieved through positive and negative feedback loops to define cell and tissue polarity coordinates 6, 270.
Many Wnt proteins are expressed in the kidney in a precise temporal and spatial manner and are known to be the major regulators of kidney development. Thus, the crosstalk between Wnt and PCP pathways is one of the key mechanisms operating during different stages of kidney development.
Graded adhesiveness is an alternative to the diffusible factor hypothesis. This idea is consistent with the demonstration of opposing activity gradients for the atypical cadherins Fat and Ds (reviewed in 16, 70). The Golgi kinase Four-jointed phosphorylates the extracellular domains of these cadherins differently on each side of the cell, thereby increasing the affinity of Fat for Ds on one face and decreasing the affinity of Ds for Fat on the other. Thus, Four-jointed allows neighboring cells to polarize across the tissue (Fig 1). Gradients of Fat and Ds activity in the fly wing were originally thought to regulate fidelity of core PCP module-mediated signaling 13, but genetic evidence now argues that the pathway could also act independently of the core PCP module, in a context-restricted manner 71–73. Notably, Fat/Ds signaling not only regulates PCP but also affects cell and tissue growth by controlling the Hippo pathway through an independent mechanism 16, 70.
Mechanical strain across the tissue might also serve as a global PCP cue 69, 74. In the fly wing, planar polarized cells were proposed to realign in response to mechanical stress generated during the contraction of the hinge region 69. Similarly, the force generated during blastopore closure in gastrulating frog embryos was suggested to align microtubules in frog ectoderm 74. Consistent with this idea, artificially generated mechanical strain triggered microtubule polarization and altered PCP in frog ectoderm 74. Similarly, in mouse skin explants subjected to uniaxial stretch, Celsr1, a homolog of Fmi, polarized according to the direction of tissue deformation 75. Taken together, these studies suggest that PCP relies on mechanical forces. This hypothesis is further supported by the observations that the force generators, Myosin II and Rho-associated kinase (Rock), initially considered as PCP effectors 23, also function as key regulators of Vangl2 localization during neural tube closure in Xenopus 29 and the PCP during Drosophila germband extension; the latter involves a distinct molecular pathway 76, 77. Whether the mechanical forces regulating PCP originate within or outside of the polarizing cells is unknown and warrants further investigation.
Local PCP amplification
Whatever the global PCP cues are, PCP is amplified at the cellular level by feedback interactions among core PCP proteins in the cytoplasm and at the cell surface. Initially, core PCP complexes form clusters in the cells that lack visible tissue polarity. At the next stage, the cytoplasmic constituents of opposing PCP complexes antagonize each other within the same cell 78, whereas the transmembrane components positively reinforce each other’s localization across the junction between two neighboring cells 12, 79, 80. These interactions often rely on post-translational modifications of core PCP proteins, of which phosphorylation is the most common. Vangl or Dvl are phosphorylated in response to Wnt signals and these modifications appear essential for PCP in the mouse limb or frog neural plate 29, 37, 81, and are conserved in Drosophila 82, 83. Together, these mechanisms lead to the asymmetric distribution of core PCP proteins, a hallmark of active PCP signaling. Although the regulatory feedbacks are critical for initiation and maintenance of tissue polarity, they hinder our understanding of the sequence and causality of events which establish PCP.
In different epithelia, core PCP complexes initially form molecular clusters in a process reminiscent of membrane patch formation 84, 85. These clusters are stabilized by molecular interactions within each protein complex, so that Prickle associates with Vang 7, 85 and Dsh is recruited by Fz 86, 87. Recent data show that apicobasal polarity proteins enrich PCP complexes in the apical cell compartment and this may be essential for PCP signaling 29, 88, 89. The apical protein Par3 is planar polarized in the Xenopus neural plate and recruits Pk3 to the apical surface to promote anterior PCP complex formation 90. Interference with Par3 activity or with the binding between Par3 and Pk3 disturbs PCP in the neural plate. Notably, mutations of the basolateral determinant Scribble cause neural tube defects that are prototypical of core PCP mutants 91–93. Although many physical and functional interactions between apicobasal and PCP proteins have been reported 94–96, the extent of crosstalk between different polarity modules remains to be fully appreciated.
The Fz-Dvl and Vang-Pk complexes are not usually present in the same location in the cell, suggesting that they are mutually antagonistic 10, 78, 79, 97 In the fly wing, the Vang/Pk complex becomes proximal, whereas Fz/Dsh complex accumulates distally (Fig 1). This negative feedback regulation commonly involves proteasome-mediated degradation of one core PCP component triggered by its interaction with a protein from the opposing PCP complex 81, 98, 99. Specifically, Cullin1 and Smurf1 E3 ubiquitin ligases regulate Pk1 turnover in mice and flies in Fz- and Dvl-dependent manner, respectively 81, 98. Similarly, Drosophila Prickle and Fz have been reported to promote each other’s degradation 98, 100. Alternatively, intracellular partitioning of PCP clusters is achieved by vesicular trafficking 26, 101–104. In Drosophila, directional microtubule-dependent vesicular trafficking of Fz and Dsh to one side of the cell has been reported 105, 106. Fmi is internalized more rapidly in Fz or Vang mutant cells 99, indicating that Fz/Fmi and Vang/Fmi complexes are selectively stabilized at the relevant junctions and more resistant to endocytosis. The importance of vesicular trafficking is highlighted by the requirement of several basic components of the endocytic machinery for PCP 26, 101, 107.
Drosophila mutant PCP clones often affect the polarity of an adjacent wild-type tissue, a phenomenon called domineering nonautonomy 108, 109. This process is thought to reflect the formation of molecular bridges between the interacting cells due to a positive feedback. Extracellular domain of Fz on the distal side of one cell interacts with extracellular loops of Vang (likely in association with Fmi) on the proximal side of the neighboring cell, thereby coordinating polarity of individual cells in tissue plane 12, 79 (Fig 1). The asymmetric bridges may amplify PCP signaling by increasing the anisotropic adhesion of neighboring cells based on activities of Fmi/Celsr or Fat/Ds cadherins.
PCP signaling targets and mechanisms
Intracellular targets of PCP
Actomyosin complexes are crucial for cell contractility, directional membrane fusion and trafficking events underlying regulation of cell shape and motility; they are the key cellular targets of the PCP pathway. In Drosophila embryos, Myosin II activity is critical for the polarity of actin hairs in the wing as well as PCP during germband extension. In vertebrate embryos, actomyosin dynamics mediates most if not all PCP signaling effects on morphogenetic behaviors, such as mediolateral and radial cell intercalations, apical constriction and oriented cell divisions (OCD). Whereas core PCP proteins have been implicated in actin remodeling and Myosin II activation mostly indirectly, several PCP effectors are well known to influence actomyosin dynamics. Mwh is a formin-like protein that negatively controls actin polymerization during hair formation in fly wing and legs 110, 111. Inturned was proposed to function by binding to another formin protein, Daam1 112. Fritz/WDPCP is a WD40 domain protein 20 that affects F-actin by controlling septin localization 28, 113. Depletion of different PCP proteins in vertebrate tissues leads to a reduced F-actin and decreased phosphorylation of regulatory myosin light chain (MLC) staining 114–117. For example, reduction in phospho-MLC has been observed in Ptk7-mutant animals 117, 118. Similar analysis is warranted for other PCP proteins. Besides actomyosin, noncentrosomal microtubules frequently align in a polarized array in epithelial tissues, and can mediate directional trafficking of PCP components 105, 106. In frog ectoderm, this alignment has been associated with core PCP signaling 74. In the fly, the directionality of apical microtubule arrays is instructed by Prickle isoforms 119 120. Inactivation of ft and ds in Drosophila also leads to a loss of microtubule alignment and subsequent developmental phenotypes 14, 105.
Vesicular trafficking is another major intracellular target of PCP signaling 25, 26, 104, 107. Both core PCP proteins and their putative effectors have been reported to influence Rab-dependent trafficking processes 25, 26, 104, 121. Fuzzy contains a LONGIN domain, a common feature of SNARE proteins, that has been implicated in membrane fusion events 41, 42, 122. Consistent with the role of Fuzzy in PCP, mouse embryos lacking Fuzzy function developed neural tube defects 123. Cell junction remodeling coordinates PCP signaling-dependent cell movements within a tissue. Accordingly, PCP proteins have been physically linked to the apical and basolateral polarity regulators, including Par proteins, Lethal giant larvae and Scribble that regulate generation and maintenance of junctional complexes 90, 92, 94, 95. Rearranging cell junctions inevitably causes cells to undergo cell intercalations and shape changes. Furthermore, PCP signaling affects cell adhesion through Fmi/Celsr, an atypical cadherin that binds both Vangl2 and Fz. The binding of Vang/Vangl2 and Fz to classical cadherins has been also described 25, 124–126. The interaction of atypical cadherins Ft and Ds may also modulate adhesion strength between polarized cells in the plane of the tissue.
PCP regulation of coordinated cell behaviors
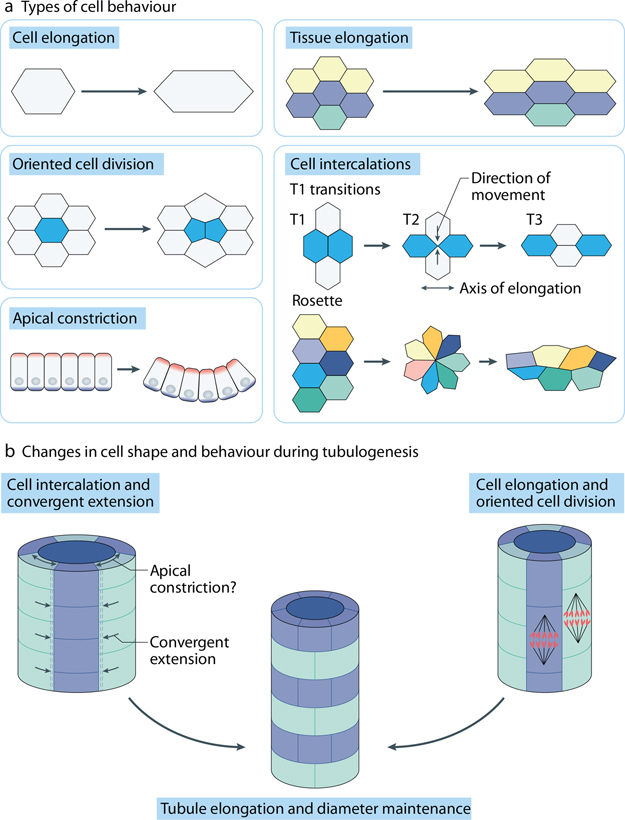
Whereas PCP proteins can control shape and motility of individual cells 36, 127–131, the primary function of PCP signaling is to regulate collective cell behaviors (Fig 2). Mediolateral cell intercalation, or convergent extension (CE), is a common mechanism of cell rearrangement 132–134. During CE, neighboring cells elongate in the direction perpendicular to the axis of extension, form mediolaterally directed actin-based protrusions and intercalate to produce a longer and narrower tissue array. These mediolateral intercalations are blocked in embryos deficient in PCP signaling 48, 130, 135. Both cell protrusive activity and junction remodeling are abnormal in the mutants, however, better understanding is hampered by the observations that overexpression and depletion of a PCP component often produce morphologically similar phenotypes (reviewed by 46). Moreover, since CE movements may be disrupted by defects in cell adhesion or growth factor signaling that is unrelated to PCP, the interpretation of these phenotypes can be ambiguous. Unlike CE, radial intercalations are oriented along the apicobasal axis. Radial intercalations are involved in lumen formation during tubulogenesis of multilayered epithelia (e. g. midgut or mammary gland)136, 137, early epiboly and epidermal differentiation in Xenopus embryos 138.
Figure 2: Cell behaviors during morphogenesis.
(A) Types of individual and collective cell behaviors that affect tissue architecture during morphogenesis. Cell elongation and oriented cell divisions act to promote tissue extension or orchestrate branching events. Cell intercalations are mediated by so called T1 transitions that involve four neighboring cells and by more complex intermediate ‘rosettes’ that include 5 or more cells. Apical constriction affects the curvature of the folding tissue and can promote neighbor cell exchanges. (B) Examples of cell behavior relevant to the formation of renal tubules. Both cell elongation and oriented cell divisions can stimulate tubule lengthening. Cell intercalations that accompany convergent extension rely on elongation and polarization of cells in the medio-lateral direction perpendicular to the tubular axis. Both cell intercalation and apical constriction reduce tubule diameter and lead to tubule elongation. Oriented cell divisions enable incorporation of a daughter cell along the tubular axis, facilitating tubule lengthening
Apical constriction, a process that involves shortening of apical cell junctions139, also appears to involve PCP signaling, at least in some cases 115. Apical constriction is a major cell behavior that is responsible for vertebrate neural fold formation 140, 141. Although actomyosin contractility plays key roles in both apical constriction and CE movements, it is currently unknown why Myosin II activation causes some cells to converge and extend, but leads to constriction in other cells. Differential phosphorylation of MLC has been argued to determine actomyosin activation at different locations, such as apical versus basolateral cell junctions in an ascidian embryo 142. Although PCP signaling takes place both apically and basolaterally, its spatially-restricted mechanisms remain to be further clarified 133, 142–144.
Oriented cell divisions (OCD) play a major role in axis elongation and organogenesis. OCD is an alignment of the mitotic spindles of dividing cells with the axis of tissue elongation. Since PCP dissolves during cell division, the dynamic regulation of protein localization is critical for PCP restoration at the end of each cell cycle. Indeed, Celsr1 phosphorylation in cells undergoing cell division was shown to be important for the dissolution and restoration of PCP 145, 146. OCD has been linked to PCP signaling in both Drosophila and vertebrates 147–150. In zebrafish embryos, PCP signaling-dependent OCD rather than convergent extension has been proposed to be a primary cause of body axis elongation 149, 150. Supporting this view, mutations in PCP components affect OCD in many models 6, 148, 151, 152. Although OCD phenotypes are frequently interpreted as a sign of deficient PCP signaling, dysfunction of apicobasal polarity components (reviewed by 136, 153) or IFT proteins 154 can also lead to randomized OCDs.
PCP components and cilia
Cilia are specialized organelles at the apical surface of most cells where they direct fluid movements (motile cilia) or detect environmental cues that coordinate cellular behaviors during tissue morphogenesis or homeostasis (primary cilia) 155. The PCP effectors Fuzzy, Inturned and Fritz/Wdpcp are critical for the formation of both motile cilia in Xenopus epidermis 41, 43 and primary cilia in frog and mouse tissues 28, 122, 156. Loss of PCP effectors results in shortened, sparse cilia and disturbance of Shh 41, 157 or Wnt signaling 158. PCP effectors were proposed to promote intraflagellar transport, possibly by regulating septin localization 28, 113 and the formation of the ciliary transition zone 159. A recent study discovered that Fuzzy and Inturned function as a heteromeric GTPase effector for Rab23, a Rab GTPase involved in ciliary traffic 160, directly linking these PCP effectors to membrane trafficking and ciliogenesis.
The involvement of core PCP proteins in cilia biology is more complex. Some PCP proteins, such as Vangl homologues, do not seem to contribute to the formation of primary cilia, but their loss affects function of motile cilia in the mouse node 52, gastrocoel roof plate in Xenopus 55 or Kupfer vesicle in zebrafish 54. In these tissues, the sole motile cilium on each cell is posteriorly tilted, enabling a leftward directional movement of extraembryonic fluid that serves to establish left-right asymmetry in embryos 53. Lack of Vangl function results in disorganized ciliary beating and randomized left-right axis 52, 54, 55. Mutations in Celsr1, Vangl2 or Fz3 disrupt both radial (location within the cell) and planar (uniform tilting) polarization of motile multicilia on mouse ependymal cells, associated with disorganization of actin and microtubule cytoskeleton 161. In some cases, the effects of PCP proteins on cilia have been linked to the centrosome/basal body that serves as a template for growing cilia 162. Interestingly, mutations in Celsr2 or Celsr3 affect assembly of ependymal cilia through loss of centrosomal/basal body structures, causing lethal hydrocephalus in mice 163. Inversin/Nephrocystin2 and Diversin (vertebrate homologues of Diego), and also Dvl2 and Prickle3 are all detected at the basal body in Xenopus or mammalian cells 164–167, where they may be required for normal centrosome/basal body structure and cilia formation 56, 57, 168–170. It is currently unclear to what extent these proteins are involved in core PCP signaling and whether their centrosomal and ciliary functions are relevant for PCP. As discussed below, some of these mutants exhibit kidney anomalies that, in some cases, might be attributed to ciliary dysfunction.
In summary, the PCP pathway controls various intracellular processes and collective cell behaviors that are necessary for normal morphogenesis and maintenance of various tissues. Below, we detail the role of PCP signaling in development of the kidney and how mutations in genes encoding various PCP constituents lead to diverse kidney defects in mice and humans.
The PCP pathway and kidney development
Overview of mammalian kidney development
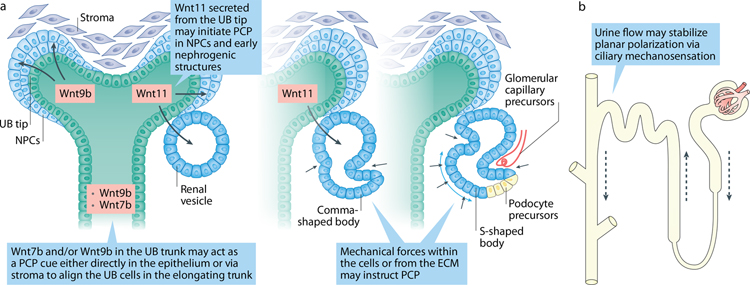
Development of the mammalian “metanephric” kidney starts at embryonic (E) day 9.5 in mice and at the 5th week of gestation in humans when a portion of the intermediate mesoderm on each side of the spinal cord undergoes mesenchymal-to-epithelial transition to form an epithelial tube, known as the nephric or Wolffian duct (ND), paralleled by a column of nephric cord 171. As development proceeds, the ND induces a specialized renal progenitor cell population within the nephric cord, to convert into tubules and glomerulus-like structures, sequentially forming the pronephros and then, more caudally, the mesonephros. Although both are transient structures in mammals, the genetic programs that govern their development are similar to the events underlying development of the metanephric kidney, the final functional kidney in higher vertebrates, including mammals. Because of its simplicity (a single nephron), the Xenopus pronephros has been an informative model of processes involved in formation of the metanephric kidney 172. The mammalian kidney is induced when caudal extension of the ND reaches the hindlimb level (Fig 3).There, signals from the nephric cord-derived metanephric mesenchyme (MM) stimulate lateral outgrowth of the ureteric bud (UB) from the ND 173, 174; the descending ND/Wolffian duct subsequently gives rise to the reproductive system in males or degenerates in females 175. As the UB contacts MM, it branches repeatedly to form a tree-like collecting system; a distal part of the collecting tree coalesces to form the renal pelvis and ureter. In a reciprocal fashion, Wnt molecules secreted from each UB tip cause nearby MM cells to condense into a cap mesenchyme (CM) 171, containing nephrogenic progenitor cells (NPCs). In response to inductive UB signals, the NPC cells undergo mesenchyme-to-epithelial transition (MET) and are transformed into a polarized epithelium lining an early renal vesicle with a central lumen. The renal vesicle twists and elongates to form a comma- and then an S-shaped body, as specialized segments of the nephron emerge. At its proximal end, interaction with blood vessels forms a glomerulus that filters fluid into the nephron; the tubular fluid is modified as it passes through successive nephron segments. At its distal end, the S-shaped body fuses with its parent UB tip to form a continuous lumen leading to a common collecting duct that carries tubular fluid out of the kidney towards the bladder. The glomerular filtrate begins to flow at about E15 in mice when the ureter connects with the bladder. Fluid flow may provide mechanical cues that give upstream/downstream information to further orient tubular epithelial cells 176.
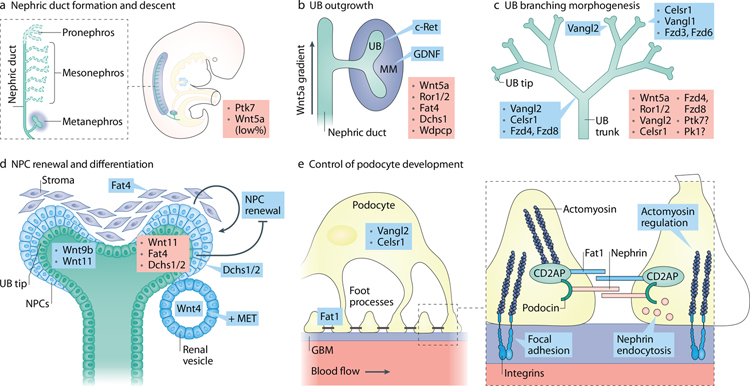
Figure 3: PCP signaling in kidney development.
(A). Nephric/Wolffian duct formation is affected by mutations in certain PCP genes; the known mutated PCP genes are shown in red on the right side of each panel. Expression of PCP genes participating in the discussed process is depicted when known. (B). Outgrowth of ureteric bud from nephric duct is largely controlled by c-Ret (expressed in the ND at the time of UB formation) and its ligand GDNF (expressed in the cells of metanephric mesenchyme. Loss or mutations in several PCP genes lead to abnormal UB outgrowth in both human and mice resulting in renal agenesis or kidney duplication. (C). Ureteric bud branching morphogenesis depends on timely and spatially coordinated changes in cell shape and movements. Mutations in PCP genes affect UB branching, branch shape and branching angles. (D). Nephrogenic progenitor cell renewal and differentiation depend on the crosstalk between stroma and UB tip. Loss of stroma-expressing Fat4 or of NPC-expressing Dchs1/2 leads to NPC expansion. The specific signaling events involving Fat4/Dchs1–2 are unclear. (E). Podocyte foot processes are interdigitated in a precise fashion along the glomerular capillary. Loss of core PCP protein Vangl2 and Celrs1 affects podocyte differentiation, nephrin internalization and glomerular maturation.
MM distant from the UB tip forms renal stroma that supports specification of the NPCs, development of nephrons and renal vasculature 177, 178. Cycles of UB branching, local nephrogenesis and vascular recruitment are reiterated until ~38 week of gestation in humans, generating ~ 1 million nephrons per kidney 179. In small rodents, nephrogenesis continues until postnatal day P7–10 generating ~ 10,000–40,000 nephrons per kidney 180. Although no new nephrons are formed thereafter, postnatal proliferation of tubular cells drives tubular elongation and final anatomy and function of the renal cortex and medulla.
Development of the mammalian kidney is controlled by a hierarchical gene regulatory network which governs precise spatial and timely changes in cell fate, shape, polarity, proliferation, adhesion, directional cell movements, and expression of specific genes 171, 181. Mutations in the key developmental genes that regulate these events lead to a wide spectrum of defects ranging from complete renal agenesis to hypoplastic/dysplastic kidneys to enlarged cystic kidneys (reviewed in 182, 183). These diverse defects are collectively referred to as “Congenital Anomalies of Kidneys and Urinary Tract (CAKUT) that occur in 1 in 500 children worldwide 183. The complexity of renal development makes it challenging to understand the molecular and cellular mechanisms that cause such diverse CAKUT phenotypes.
Expression of PCP constituents in the kidney
Many vertebrate homologs of the fly PCP genes are expressed in the developing mouse kidney. Core PCP proteins Vangl1, Vangl2, Pk1, Celsr1 as well as Ptk7 are all found in emerging nephrons and UB-derived structures 184, 185 and www.gudmap.org. Fat1 (a close homolog of Drosophila Fat2 gene that regulates PCP during oogenesis 186) is detected in UB187 and, together with Vangl2, is highly expressed in developing podocytes; Fat1 persists in differentiated podocytes 188, 189. Fat4 (the closest homolog of Drosophila gene ft) is found in the stroma surrounding CM while its interacting partner Dchs1 is expressed in progenitor cells at the outer edge of CM, where the interaction with Fat4 may take place 190, 191.
Several Wnt and Frizzled (Fzd) genes are expressed during kidney development, but it has been difficult to sort out the most important ligand/receptor pairs. In various cellular contexts, Wnt and Frizzled family members function in distinct canonical Wnt/β-catenin or PCP pathways, depending on the involvement of specific co-receptors 192, 193. The Wnt/β-catenin pathway plays critical roles in kidney development and disease (see Box 1, reviewed in 193, 194), but will not be detailed here. Among the Wnt ligands that may signal through the PCP pathway, Wnt5a acts via the co-receptor Ror2 195 and is expressed in a caudally-increasing gradient in intermediate mesenchyme 196, 197. Wnt11 is restricted exclusively to the UB tip 198. In contrast, Wnt9b is expressed in both the UB tip and the UB trunk, and Wnt7b is found only in the UB trunk 199, 200. Frizzled 3, 4, 6 and 8, are detected in the UB-derived collecting duct 201, 202. Thus, the distribution of specific Wnt and Frizzled molecules suggests that both short- and long-range signals may orchestrate PCP in the developing nephron.
Due to the existence of numerous PCP gene paralogs that are expressed in the kidney in complex patterns, it is difficult to infer their specific roles in nephrogenesis. However, the asymmetric distribution of PCP proteins in renal tubular cells indicates that the PCP pathway must be operative 201: Fzd3 and Fzd6 proteins are found at the distal side of the tubular cells whereas Vangl1 is sequestered at the proximal side of the cell in E18.5-P1 mouse renal tubules (Fig 4). This PCP hallmark suggests that PCP signaling is active during embryonic and early post-natal kidney development
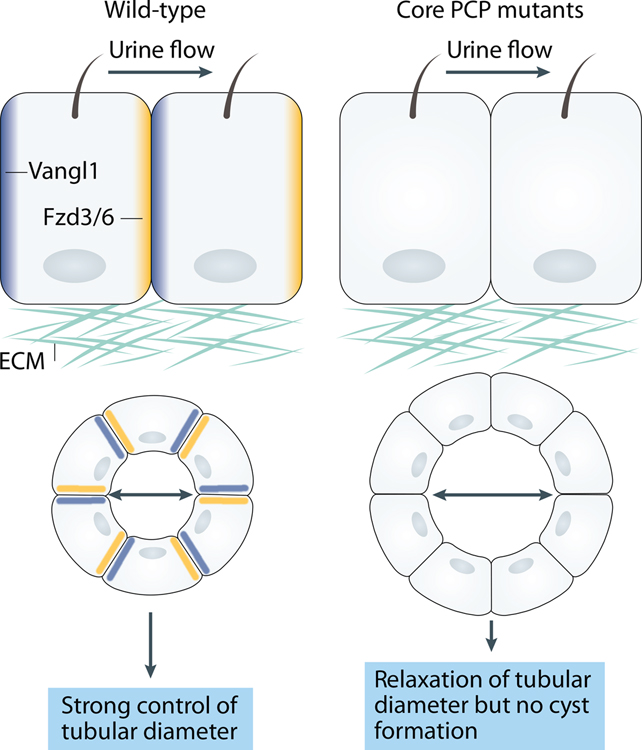
Figure 4. Relationship between PCP and cystogenesis.
Various mechanisms contribute to cyst formation in renal tubules including loss of cilia and/or ciliary function, increased cell proliferation or abnormal apical-basal polarity. It was also proposed that lack of PCP protein function might contribute to cystogenesis. Accumulated experimental data have shown that core PCP proteins are polarized along the tubular axis in the developing kidneys, and that PCP signaling tightly controls the tubular diameter via CE and OCD. However, loss of core PCP proteins does not lead to cyst formation.
PCP components and the initiation of kidney development
Careful analysis of the elongating nephric duct in amphibians, fish and mice revealed convergent extension (CE) cell behavior, implying planar polarization of the ND cells 203, 204. By injecting membrane-bound GFP, Lienkamp et al traced cell behavior in the proximal part of the pronephric duct in Xenopus tadpoles and showed that duct convergence and extension correlates with multicellular rosette formation 205. This process relies on coordinated constriction and stretching of apical cell surfaces. Four to eight cells form rosettes, in which the long apical cell surfaces form wider rosette axis is oriented perpendicularly to the tubular plane. This is followed by shrinking of the rosette’s wide axis. Subsequent stretching of apical surfaces of rosette cells occurs at the 90° angle, turning the rosette along tubular structure, thereby elongating the tubule longitudinally while narrowing its diameter (Fig 2). Importantly, expression of a mutant form of Disheveled (Xdd1) that can inhibit PCP signaling 48 disrupted directional rosette resolution resulting in wider and shorter proximal pronephros 205.
Xu et al implicated the mouse PCP gene Ptk7 in elongation of the ND/Wolffian duct after UB outgrowth, as the duct becomes a part of the male reproductive system 206. Loss of the Ptk7 gene affected CE in rapidly proliferating Wolffian duct cells, leading to a short, less coiled duct with reduced sperm motility. Of note, mice with targeted knockout of Ptk7 form kidneys, indicating that the ND has descended properly and UB outgrowth has occurred 40.
Wnt9b from the UB tip initiates metanephric mesenchymal cells to become nephrogenic progenitors 207. Wnt9b activates Wnt4 in the precursor-derived pretubular aggregates, where Wnt4 controls mesenchyme-to-epithelial transition (MET) crucial for subsequent tubule development 208, 209. Loss of Wnt4 blocks differentiation of renal epithelia precluding nephron formation 208. Interestingly, Wnt4 controls MET in a β-catenin-independent manner 210, yet its association with PCP and PCP signaling has not been directly investigated.
Mice with Wnt5a deletions exhibit a range of kidney phenotypes from duplex kidneys (common), to hydronephrosis, to unilateral or bilateral renal agenesis (rare) 196, 197, 211. Loss of the Wnt5a co-receptor Ror2 in collecting ducts causes duplex kidneys in ~ 50% of homozygous embryos, and over 85% of mice with Ror2+/−;Wnt5a+/− double heterozygous mutations exhibit duplicated ureter, unilateral or bilateral kidney agenesis. Ror1 also genetically interacts with Wnt5a, resulting in ureter duplication and ectopic kidney in double heterozygous mutants 212. The kidney phenotypes in Wnt5a/Ror1 (or Ror2) mice are consistent with defective UB outgrowth linked to aberrant c-ret/GDNF signaling 213, 214. Under guidance of GDNF from metanephric mesenchyme, ND expression of the GDNF receptor (c-Ret) is normally restricted to the site of UB outgrowth 174. In Wnt5a/Ror1–2 mice, ureter duplication is linked to elevated ectopic GDNF expression 197, 215. Similarly, duplex kidneys in up ~40% of Fat4−/− embryos and ~70% of double homozygous Fat4/Fjx1 embryos were reported 216. Detailed studies revealed that the duplex kidney defect in Fat4−/− kidneys was caused by excessive c-Ret/GDNF signaling and was rescued by genetically removing one GDNF allele 216. Unexpectedly, the researchers found that extracellular domains of Fat4 and c-Ret interact biochemically, providing an additional level of Fat4/c-Ret regulation 216. Mice with mutations in the PCP effector gene Wdpcp also have ectopic kidneys 28, however, the precise underlying mechanisms have not been elucidated. Taken together, these observations suggest that PCP molecules may localize or control activity of growth factors that drive early kidney development; loss of PCP may account for defects in ND patterning and misplaced UB outgrowth that lead to anomalies, ranging from duplicated ureters to renal agenesis. Whether these PCP components participate in PCP signaling as a single molecular pathway or act independently during kidney development remains an important question for future studies.
PCP molecules and UB branching morphogenesis
UB branching is central to kidney development and requires coordinated changes in cell behavior (reviewed in 174, 217, 218). Governed primarily by the GDNF/c-ret and FGF pathways, cells at the tip of each UB branch rapidly proliferate to form a UB ampoule 174, 219. Dichotomous branching of the ampoule requires remodeling of the cytoskeleton, cell-cell junctions and cell-ECM adhesion, culminating in profound cell shape changes. Several mouse PCP mutants exhibit defective UB branching morphogenesis, confirming that PCP signaling is required for cell behaviors implicated in UB bifurcation 185, 220. Hypo-dysplastic kidneys with fewer UB branches were found in homozygous E13.5 Vangl2Lp/Lp “Looptail” and Celsr1 “crash” mutants; this phenotype was more severe in double heterozygous Vangl2Lp/+;Celsr1Crsh/+ embryos 220, confirming that these two genes interact genetically during kidney development as they do in neural tube and inner ear development 92. Using optical projection tomography and 3D reconstruction, Brzoska et al showed that UB branching was particularly affected in the caudal aspects of E13.5 Vangl2Lp/Lp, Celsr1Crash/Crsh and Vangl2Lp/+;Celcr1Crsh/+ kidneys 220, although the mechanism(s) underlying this predilection is unclear and may reflect shortening of the embryonic rostral-caudal axis due to deficient CE in these mutants 91, 92. Vangl2Lp/Lp and Celsr1Crsh/Crsh animals also display reduced branching morphogenesis of the lung that is caused by defective cytoskeletal remodeling 51. Actin polymerization defects were reported in Vangl2Lp/Lp kidney cells as well 185, suggesting that actomyosin deregulation may contribute to reduced UB branching. Importantly, Vangl2 interacts with several PCP components, such as Dvl 221 and Ptk7, which have been linked to regulation of actomyosin contractility. Dvl activates the formin protein Daam1 that nucleates actin monomers and promotes actin polymerization 32, 222. Daam1 downregulation in Xenopus embryos disrupts pronephric tubulogenesis 223. Mice lacking the Ptk7 gene develop renal hypoplasia, and Ptk7 genetically interacts with Vangl2 40. Ptk7 was shown to activate Src-ROCK2 signaling and modulate Myosin II contractility at cell-cell junctions in the inner ear cells 117, 118. However, whether renal hypoplasia in Ptk7−/− mice is caused by actomyosin dysregulation in the UB cells is unclear.
PCP proteins remodel junctional complexes and ECM in flies 25, 224 and zebrafish 225, 226. Similar mechanisms may account for Wnt5a mouse mutants with renal hypoplasia and UB branching defects associated with disorganization of basement membrane and reduced expression of laminin and type IV collagen in collecting duct cells 211. Collectively, these studies indicate that PCP signaling is centrally involved in organizing cell shape changes and coordinated cell movements involved in UB branching morphogenesis.
PCP genes and nephrogenic progenitor cells (NPCs)
The NPC pool in condensing mesenchyme around each UB tip depends on cross-talk between the UB and its surrounding stroma 227, 228. Absence of Wnt11 in the UB tips results in decreased attachment of NPCs to the UB tip, loss of polarized distribution of several markers within NPCs and a disruption of polarized cell behavior 229. This causes a dispersion of NPCs from their niche and premature NPCs exhaustion leading to deficient UB branching and renal hypoplasia. Loss of stromal Fat4 gene results in a dramatic expansion of the NPC pool at E12.5-E13.5 228, 230, indicating that Fat4 non-autonomously inhibits NPC renewal (Fig 3). Similarly, loss of Fat4 ligands, Dchs1 or Dchs1/Dchs2, also expands the NPC pool 190, 191, 231. The abnormal CM compartment in Fat4−/− or Dchs1−/− mice might affect UB branching by disturbing positional cues. Indeed, these mutants exhibit reduced UB branch number and abnormal shape and orientation of branches 191. However, the signaling events contributing to NPC expansion are puzzling. Vangl2Lp/Lp mutant kidneys have normal NPC number; loss of one Vangl2 allele in Fat4−/− kidneys does not exacerbate Fat4−/− NPC phenotype 190. This argues against the involvement of the core PCP pathway in controlling NPC pool size. In various cellular contexts, Fat proteins activate both the PCP and Hippo pathways 16. However, genetic removal of a Yap allele did not rescue the excessive NPC phenotype in Fat4−/− mice 190, indicating that Hippo signaling was not involved. Canonical Wnt/b-catenin pathway activity drives cell proliferation of renal NPCs 232, but was unchanged in Fat4 and Dchs1/2 mutants 190. In conclusion, the core PCP pathway does not seem to control the NPC pool directly and the precise signaling events downstream of Fat4/Dchs in kidney progenitors require further investigation.
PCP molecules, tubulogenesis and pathogenesis of tubular cysts
In mice, renal tubule segments lengthen during embryogenesis, but there seems to be a secondary wave of tubular elongation in the early post-natal period. Tubular diameter of different segments is tightly controlled during this process. By carefully measuring tubular diameters in hundreds of circular transverse cross-sections of wildtype kidneys from embryonic day 13.5 to postnatal day P5, Thomas Carroll’s group has shown that the tubules become progressively narrower until late gestation; thereafter the tubular diameter is fixed 199. The phase of tubular narrowing (convergence) occurs through a reduction in the number of cells surrounding each tubular lumen; cells intercalate along the tubular axis to extend the tubule (Fig 2 and 4). In E13.5-E19.5 tubules, cell divisions are randomized, but become oriented only after tubular diameter has been established around the time of birth 199. Based on these results, the researchers proposed that tubular elongation is controlled by two temporally distinct, yet mechanistically connected, processes: CE during embryonic tubule narrowing and OCD after tubule diameter has been established postnatally. In Wnt9b−/− mice, tubules are dilated during the embryonic CE phase and become cystic postnatally. However, since Wnt9b acts in both β-catenin-dependent and β-catenin-independent pathways199, 232, the mechanisms underlying cystogenesis in Wnt9b−/− mouse are likely complex. Wnt7b−/− kidneys lack normal tubular elongation in the medullary tubules; this defect was attributed to randomized OCD 200. Notably, similar phenotype was found in the mice with deficiency of β-catenin in stroma 200, suggesting a paracrine action of Wnt7b through the Wnt/β-catenin pathway in the surrounding interstitium.
Mutations in several core PCP genes lead to tubular dilatation and occasional cysts. For example, embryonic kidneys of Pk1−/− mutant mice exhibit dilatation of renal cortical tubules while the medullary zone is hypoplastic. Marked cystic changes occur in ~ 5% of the mice 233. Similarly, tubular dilation and medullary zone hypoplasia were reported in the Ptk7−/− kidneys 234. E17.5–18.5 Vangl2Lp/Lp embryos display dilated proximal tubules and collecting ducts in the cortex, and significant loss of the medullary compartment 185, 220, 235. Jeff Axelrod’s group discovered that tubular diameters in E18.5 Vangl2Lp/Lp, double Fz3−/−;Fz6−/− or in double kidney-specific Vangl1−/−;Vangl2−/− (Vangl1,2DKO) mutants were not as tightly regulated as in control kidneys 201. Derish et al confirmed that tubular size was “relaxed” and the tubules were dilated due to defective CE in the E17.5 Vangl2Lp/Lp and Vangl2Δ/Δ (targeted knockout of Vangl2 exon4) kidneys 236. Whether cell rosette rearrangement controls tubular diameter and drives elongation of mammalian renal tubules was not studied, but Derish et al reported abnormal apical cell constriction and reduced phosphorylation of Myosin light chain in Vangl2 mutant tubular cells, consistent with such a possibility 236.
In 2006, Pontoglio’s group demonstrated that tubular elongation is associated with OCD along the tubular plane in wildtype postnatal mice 237. By measuring angles of mitotic spindles in neonatal Hnf1β mice (model of polycystic kidney disease (PKD) and Type I diabetes) and Pck rats (model of autosomal recessive PKD), the authors showed that OCD was randomized in pre-cystic dilated tubules of these animals and proposed that defective planar polarity underlies PKD 237 (Fig 4). This interesting idea was rapidly disseminated in several reviews 238, 239. In 2008, McNeil’s group showed that targeted deletion of Fat4 leads to tubule dilatation and some cysts in E16.5 mouse kidneys via randomized OCD 240. Removal of one Fat1 copy in Fat4−/− mice further enhanced cyst appearance 187, confirming a redundant Fat1/Fat4 function in this context. Importantly, loss of one Vangl2 allele on Fat4−/− background somewhat worsened the tubular phenotype, implying the potential involvement of PCP signaling However, since Fat proteins can also act via the Hippo pathway 16, 241, and there is now strong evidence that dysfunction of Hippo signaling causes severe cystogenesis in mice and humans 238, 242, 243, the cysts seen in Fat4−/− kidneys are likely due to complex mechanisms.
The relationship between core PCP genes and cystic kidney disease was recently addressed experimentally. Conditional Vangl1,2DKO mice generated by Axelrod’s group using Kif3a-Cre and Hoxb7-Cre deleters had a mild OCD defect at P1 201. Surprisingly, at 16 weeks of age, the mice displayed only minimally irregular tubules, with complete absence of cysts. Furthermore, cyst cells in conditional Kif3a−/− or ubiquitous Pkhd−/− mutants displayed asymmetric distribution of Vangl1 and Fzd6 proteins indicating that cystogenesis occurs despite normal core PCP module activity. Similarly, mutant mice with Hoxb7-Cre-driven excision of Vangl2 had tubular dilatation and cysts at E17.5 and some residual tubular dilatation at P1. However, tubular dilatation disappeared by P7 236 (Fig 4). Overall, these studies are consistent with a role for the core PCP pathway in establishing tubular diameter via CE, but do not provide a simple explanation for cystogenesis. The studies above suggest that additional molecular mechanisms operate around the time of birth to maintain renal tubule diameter.
What can drive the perinatal CE to OCD switch and what are the PCP pathway - independent mechanisms controlling tubular diameter? One possibility is postnatal ECM remodeling 244. This might lead to changes in the tension between ECM and tubular cells, modulating both cell shape and cell movements required for CE 245. With the onset of tubular flow at E15 in mice, mechanical forces on the primary cilium may also provide positional information that modulates ECM 246. Although PCP signaling appears to control OCD, loss of cilia (as in Ift88 zebrafish mutant 154) or ciliary function (as in Pck1 mutants 237) also leads to OCD randomization. However, loss of OCD does not seem by itself to be sufficient for cyst initiation: e.g. randomized OCD in the Pkhd1−/− mouse does not cause cystic transformation whereas OCD is normal in the dilated tubules prior to cyst formation in the Pkd1−/− or Pkd2−/− mice 247. Notably, the Pkd1-encoded protein, Polycystin1, binds to and stabilizes the junctional apicobasal polarity complex Par3/aPKC, regulating CE-like collective cell movements and cell polarity 248. Thus cilia-dependent mechanisms may instruct both CE and OCD within renal tubules, independent of the core PCP module, yet alternative factors and mechanisms are needed to maintain tubular diameter in the postnatal period 248.
PCP molecules and glomerular development
Tubular fluid, an ultrafiltrate of blood, is generated by intracapillary pressure which drives plasma through specialized sieve-like cell-cell junctions between podocytes. During nephrogenesis, podocyte precursor cells initially appear cuboidal but rapidly develop actin-based projections, foot processes (FPs), that envelop the underlying capillary. FPs from neighboring podocytes interdigitate along the capillary in the plane tangential to blood flow (reviewed in 249). Podocyte cell-cell junctions, initially found at the apical surface, descend toward the basal aspect of the cell to form highly-specialized slit diaphragms linked to the intracellular actin cytoskeleton. Each FP is anchored via focal adhesions to the underlying glomerular basement membrane. The highly polarized architecture of mature podocytes depends critically on actin cytoskeleton (Fig. 3).
Among the many fly PCP homologs expressed in podocytes 250, the giant cadherin Fat1 is detected at an early stage, as nephron progenitor cells differentiate into podocyte precursors 251. Fat1 was shown to participate in various cellular processes including PCP signaling together with Fat4 187. Fat1 depletion in cultured podocytes reduces cell adhesion and cell motility by decreasing activity of actin regulators Rac1/Cdc42, indicating its involvement in actin regulation 252. Fat1 is a part of the slit diaphragm complex. Consistent with this role, neonatal Fat1−/− mice die due to the complete lack of slit diaphragms and widespread loss of FPs 189. In rats recovering from puromycin aminonucleoside-induced nephrosis, elevated levels of Fat1 protein were found in the newly-formed contacts between podocytes 251, suggesting a role in recovery from injury by plausibly regulating actin cytoskeleton and FP assembly.
Vangl2 is highly expressed at the basolateral surface of podocyte precursors as they generate FPs 188. Its loss inappropriately traps nephrin (the major structural slit diaphragm protein) at the cell membrane due to a defect in nephrin internalization 235. Thus, Vangl2 is needed for the normal dynamics of cell junction remodeling and assembly of slit-diaphragms between newly formed FPs. As well, depletion of Vangl2 appears to change podocyte shape and reduces the number of filopodia and stress fibers, indicating that Vangl2 regulates podocyte actin cytoskeleton 235, 250. Embryonic Vangl2Lp/Lp glomeruli are immature, have reduced podocyte number and may show collapse of the glomerular tuft 185, 188. Adult mice with podocyte-specific excision of Vangl2 have fewer podocytes per glomerulus, yet they have no sign of glomerular dysfunction under basal conditions188. However, following experimental glomerular injury, the mutant mice develop severe, permanent damage compared to the normal recovery of glomeruli in wildtype animals 188. Thus, Vangl2 appears to contribute to podocyte survival and recovery, likely through its effects on actin cytoskeleton and slit-diaphragm remodeling.
PCP genes and human kidney disease
PCP gene mutations have been identified in a variety of human nephropathies (Table). Children born with neural tube defects (NTD) are at high risk for progressive renal dysfunction. For years, this was considered a secondary complication of the associated neurogenic bladder 253. However, NTD patients often have CAKUT phenotype such as renal hypoplasia, duplex or horseshoe kidneys 185; these congenital malformations arise in utero, well before bladder dysfunction could alter basic renal architecture. PCP gene mutations have been linked to human NTD 254. The mutant animal models of the same PCP genes also have NTDs (e. g., Vangl2−/− or Celsr1−/− mice 31, 255). Notably, these mutant animals display the spectrum of renal anomalies found in NTD patients. A plausible hypothesis is that the kidney abnormalities seen in NTD patients are attributable to abnormal PCP signaling.
Several PCP gene mutations cause Robinow syndrome that is characterized by pathognomonic facial dysmorphism and skeletal abnormalities 256–260; hydronephrosis or renal dysplasia have been reported in some patients 261. A WNT5a missense mutation was identified in a CAKUT patient with a unilateral duplex collecting system without skeletal defects 211, reflecting unilateral kidney duplication phenotype seen in Wnt5a mutant mice 197, 212. Mutations in both FAT4 or DCHS1 cause Van Maldergem syndrome featuring intellectual disability, craniofacial defects, hearing loss, skeletal malformations and, in some cases, renal hypoplasia 262; the latter phenotype is consistent with kidney anomalies seen in Fat4−/− 240 and Dchs1−/− mice191. Homozygous truncating FAT1 mutations were found in patients with a novel syndrome characterized by ocular abnormalities, nephropathy (glomerular- or glomerulotubular sclerosis), syndactyly and facial dysmorphism263, while milder recessive mutations were identified in patients with various isolated glomerulopathies. These patients showed podocyte foot process effacement 252 similar to the podocyte abnormalities and lack of slit diaphragm in Fat1−/− mice 189. Mutations in the PCP effectors INTU, FUZZY and WDPCP have been found in various ciliopathies, frequently with kidney involvement such as renal hypoplasia 43, 264, 265. Again, mouse PCP effector mutants faithfully recapitulate many phenotypic features found in the patients 28, 43, 122, 123, 266. Infantile Nephronopthisis type 2 (NPHP2, kidney microcysts and interstitial fibrosis) is due to mutations in INVERSIN 265; the disease is likely caused by complex mechanisms that involve abnormal ciliary functions as well as disrupted canonical and non-canonical Wnt signaling 165.
Conclusions and future directions
PCP signaling is centrally involved in kidney development -- from outgrowth and branching morphogenesis of the UB to the patterning of the glomerulus, to shaping proximal and distal nephron segments, to the postnatal changes that organize and maintain renal functions. PCP gene mutations cause human kidney malformations in the CAKUT spectrum; experimental excision of PCP genes in mice leads to similar malformations and implicates the pathway in recovery from acute kidney injury after birth. However, many of the fundamental properties of the PCP pathway in the kidney are still unclear. The global signals that engage this pathway during nephrogenesis are largely unknown and the mechanisms that coordinate PCP with the cytoskeleton and apicobasal polarity are unexplored (Fig 5). The relationship of planar cell organization to directional flow of tubular fluid and transduction of mechanical signals by primary cilia or extracellular matrix represent interesting areas for future study. Although the pathogenesis of PKD does not seem to involve loss of core PCP pathway function 201, it may be related to dysfunction of other PCP pathway components (e.g. a Fat or the PCP effector proteins) via links to the Hippo pathway or cilia signaling and actomyosin dynamics. Presence of multiple PCP gene homologs expressed in the developing kidney, their potential functional redundancies or involvement in the non-PCP-related processes accounts for the difficulties of studying PCP in this organ. Polycystin 1 appears to regulate renal tubular CE and OCD through mechanisms that are independent of PCP signaling 248. Thus, the PCP features may be disturbed indirectly in some nephropathies. To unravel these mechanisms, an approach that combines high-resolution live imaging in model organisms with ex vivo kidney explants or organoids carrying mutant PCP genes is needed. It is clear that more work is warranted to better understand the complexities of morphogenetic processes and PCP protein interactions involved in kidney development and the pathogenesis of the human disease. This review is just a starting point in that direction.
Figure 5. Potential instructive cues establishing PCP during kidney development.
The origin of PCP in early tubules is unknown, however, several molecules may potentially act as cues to initiate PCP in early nephrogenic structures. E.g. Wnt11 that expresses at the UB tip directs polarized NPC behaviors as they epithelize and transform into pre-tubular aggregates and renal vesicles. The link between apical-basal and PCP networks may coordinate establishment of planar polarization in the earliest structures. In the trunk, Wnt9b or Wnt7b may act locally in the UB trunk or non-autonomously on the cells adjacent to developing tubule to stabilize polarity of proliferating UB cells. Additionally, mechanical forces within the cells and/or from the ECM may provide polarity cues as comma- and S-shaped bodies undergo significant stretching and invagination. Onset of urine formation at E15.0 in the mouse nephron may provide further cues via ciliary mechanosensation to stabilize planar polarization along the growing tubule.
Supplementary Material
BOX2: PCP and the KIDNEY.
The human kidney has a highly complex architecture: it is made of ~ 1 mln nephrons populated by more than 20 cell types. Each nephron forms an ultrafiltrate of plasma that flows through a series of specialized tubular segments that absorb or secrete small molecules and electrolytes to regulate body homeostasis. The kidneys produce growth factors and hormones that adjust our physiology. Extensive changes in cell polarity, shape, adhesion and cell division during embryonic development enable collective cell behaviors that arrange and shape tubular segments (reviewed in 271). Apical-basal and planar cell polarity pathways in combination with mechanical forces set up the cell rearrangements and tissue coordinates that orchestrate morphogenesis.
Mutations in PCP genes have been associated with Congenital Anomalies of Kidney and Urinary Tract (CAKUT), including small, unilateral or horseshoe-shaped kidneys and polycystic kidney disease 185, 211, 261. Collectively, CAKUT represent a common group of human congenital defects, but only one third of the causative genes have been identified. Pathogenic mutations in several PCP genes have also been well-documented in human neural tube defects, NTDs (reviewed 254). Notably, NTDs are frequently associated with CAKUT, such as small or horseshoe kidneys 185. Consistent with the role of mutant PCP genes in causing human CAKUT and NTDs, mouse mutants of the PCP genes Vangl2, Ptk7, or Celsr1 exhibit both neural tube and kidney abnormalities 40, 185, 188, 220.
For many years, defective PCP signaling was thought to initiate cystogenesis in the developing kidney, a key feature of polycystic kidney disease (PKD) 237, 238. However, recent experimental data indicate that loss of core PCP genes leads to transient embryonic increase in nephric tubule diameter but does not result in postnatal kidney cysts201, 236. These observations largely refute the popular view that PCP defects are key to the pathogenesis of PKD in humans.
Bulleted points.
Planar cell polarity (PCP) refers to a coordinated cell organization across tissue plane and is appreciated in uniform patterns of scales on fish, trichomes (small hairs) on Drosophila wing or stereocilia in mammalian inner ear.
Evolutionarily conserved PCP proteins function in the specialized PCP signaling pathway that controls coordinated changes in cell shape and behavior and commonly involves actomyosin activation.
PCP proteins exhibit asymmetric subcellular localization along the tissue plane
Many vertebrate homologues of fly PCP components participate in PCP signaling, but the analysis is more complex due to functional redundancy and/or additional roles in non-PCP related processes.
Due to the essential roles of PCP proteins in morphogenetic processes, mutations in PCP genes cause congenital malformations of multiple organs and tissues.
Mutations in the core PCP genes cause various kidney abnormalities, including dilatation of renal tubules, but do not lead to cyst formation.
Acknowledgements
We would like to thank Luca Gusella for comments on the manuscript. We apologize to those investigators whose studies we could not cite due to the constraints for space. This work was supported by the grants from Kidney Foundation of Canada KFOC1719 and the Canadian Institute of Health Research MOP130315 and PJT-169082 to ET and the National Institutes of Health grants GM122492, HD092990, DE027665 and NS100759 to SYS.
References
- 1.Lawrence PA Gradients in the Insect Segment: The Orientation of Hairs in the Milkweed Bug Oncopeltus Fasciatus. Journal of Experimental Biology 44, 607–620 (1966). [DOI] [PubMed] [Google Scholar]
- 2.Wong LL & Adler PN Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol 123, 209–221 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubb D & Garcia-Bellido A A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol 68, 37–57 (1982). [PubMed] [Google Scholar]
- 4.Adler PN The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol 101, 1–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vladar EK, Antic D & Axelrod JD Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harb Perspect Biol 1, a002964 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodrich LV & Strutt D Principles of planar polarity in animal development. Development 138, 1877–1892 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastock R, Strutt H & Strutt D Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130, 3007–3014 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Strutt DI Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell 7, 367–375 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Axelrod JD Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev 15, 1182–1187 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenny A, Reynolds-Kenneally J, Das G, Burnett M & Mlodzik M Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol 7, 691–697 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Usui T et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98, 585–595 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Chen WS et al. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell 133, 1093–1105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma D, Yang CH, McNeill H, Simon MA & Axelrod JD Fidelity in planar cell polarity signalling. Nature 421, 543–547 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Harumoto T et al. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell 19, 389–401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matakatsu H & Blair SS Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development 131, 3785–3794 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Fulford AD & McNeill H Fat/Dachsous family cadherins in cell and tissue organisation. Curr Opin Cell Biol 62, 96–103 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Lawrence PA & Casal J Planar cell polarity: two genetic systems use one mechanism to read gradients. Development 145 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Collier S & Gubb D Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development 124, 4029–4037 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Adler PN, Zhu C & Stone D Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol 14, 2046–2051 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Collier S, Lee H, Burgess R & Adler P The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics 169, 2035–2045 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H & Adler PN The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics 160, 1535–1547 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Yan J, Lee H, Lu Q & Adler PN The proteins encoded by the drosophila planar polarity effector genes inturned, fuzzy and fritz interact physically and can re-pattern the accumulation of “upstream” planar cell polarity proteins. Dev Biol (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter CG et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Strutt DI, Weber U & Mlodzik M The role of RhoA in tissue polarity and Frizzled signalling. Nature 387, 292–295 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Classen AK, Anderson KI, Marois E & Eaton S Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 9, 805–817 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Ossipova O et al. Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nat Commun 5, 3734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zallen JA Planar polarity and tissue morphogenesis. Cell 129, 1051–1063 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Cui C et al. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol 11, e1001720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ossipova O, Kim K & Sokol SY Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biol Open 4, 722–730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhanot P et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Murdoch JN, Doudney K, Paternotte C, Copp AJ & Stanier P Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet 10, 2593–2601 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Habas R, Kato Y & He X Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854 (2001). [DOI] [PubMed] [Google Scholar]
- 33.McGreevy EM, Vijayraghavan D, Davidson LA & Hildebrand JD Shroom3 functions downstream of planar cell polarity to regulate myosin II distribution and cellular organization during neural tube closure. Biol Open 4, 186–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildebrand JD & Soriano P Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell 99, 485–497 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Hikasa H, Shibata M, Hiratani I & Taira M The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129, 5227–5239 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Nishita M et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 175, 555–562 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao B et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 20, 163–176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu W, Yamamoto V, Ortega B & Baltimore D Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119, 97–108 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Andre P et al. The Wnt coreceptor Ryk regulates Wnt/planar cell polarity by modulating the degradation of the core planar cell polarity component Vangl2. J Biol Chem 287, 44518–44525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430, 93–98 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Park TJ, Haigo SL & Wallingford JB Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 38, 303–311 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Zilber Y et al. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell 24, 555–565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toriyama M et al. The ciliopathy-associated CPLANE proteins direct basal body recruitment of intraflagellar transport machinery. Nat Genet 48, 648–656 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks ER & Wallingford JB Control of vertebrate intraflagellar transport by the planar cell polarity effector Fuz. J Cell Biol 198, 37–45 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adler PN & Wallingford JB From Planar Cell Polarity to Ciliogenesis and Back: The Curious Tale of the PPE and CPLANE proteins. Trends Cell Biol 27, 379–390 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray RS, Roszko I & Solnica-Krezel L Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell 21, 120–133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallingford JB, Niswander LA, Shaw GM & Finnell RH The continuing challenge of understanding, preventing, and treating neural tube defects. Science 339, 1222002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokol SY Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol 6, 1456–1467 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Ybot-Gonzalez P et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134, 789–799 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cirone P et al. A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11, 347–360 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yates LL et al. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet 19, 2251–2267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song H et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466, 378–382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashimoto M et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol 12, 170–176 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Borovina A, Superina S, Voskas D & Ciruna B Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol 12, 407–412 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Antic D et al. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One 5, e8999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mochizuki T et al. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 395, 177–181 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Yasunaga T, Itoh K & Sokol SY Regulation of basal body and ciliary functions by Diversin. Mech Dev 128, 376–386 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnell U & Carroll TJ Planar cell polarity of the kidney. Exp Cell Res 343, 258–266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adler PN, Krasnow RE & Liu J Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol 7, 940–949 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Qian D et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol 306, 121–133 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minegishi K et al. A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking. Dev Cell 40, 439–452 e434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gros J, Serralbo O & Marcelle C WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature 457, 589–593 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Chu CW & Sokol SY Wnt proteins can direct planar cell polarity in vertebrate ectoderm. Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu J, Roman AC, Carvajal-Gonzalez JM & Mlodzik M Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol 15, 1045–1055 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Navajas Acedo J et al. PCP and Wnt pathway components act in parallel during zebrafish mechanosensory hair cell orientation. Nat Commun 10, 3993 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu JJS et al. Frizzled-Dependent Planar Cell Polarity without Secreted Wnt Ligands. Dev Cell 54, 583–592 e585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ewen-Campen B, Comyn T, Vogt E & Perrimon N No Evidence that Wnt Ligands Are Required for Planar Cell Polarity in Drosophila. Cell Rep 32, 108121 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sagner A et al. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol 22, 1296–1301 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Aigouy B et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Matis M & Axelrod JD Regulation of PCP by the Fat signaling pathway. Genes Dev 27, 2207–2220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casal J, Lawrence PA & Struhl G Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133, 4561–4572 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casal J, Ibanez-Jimenez B & Lawrence PA Planar cell polarity: the prickle gene acts independently on both the Ds/Ft and the Stan/Fz systems. Development 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawrence PA, Struhl G & Casal J Planar cell polarity: one or two pathways? Nat Rev Genet 8, 555–563 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chien YH, Keller R, Kintner C & Shook DR Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr Biol 25, 2774–2784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aw WY, Heck BW, Joyce B & Devenport D Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr Biol 26, 2090–2100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bertet C, Sulak L & Lecuit T Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Simoes Sde M et al. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell 19, 377–388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tree DR et al. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109, 371–381 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Wu J & Mlodzik M The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell 15, 462–469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loza O et al. A synthetic planar cell polarity system reveals localized feedback on Fat4-Ds1 complexes. Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narimatsu M et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 137, 295–307 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Kelly LK, Wu J, Yanfeng WA & Mlodzik M Frizzled-Induced Van Gogh Phosphorylation by CK1epsilon Promotes Asymmetric Localization of Core PCP Factors in Drosophila. Cell Rep 16, 344–356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strutt H, Gamage J & Strutt D Reciprocal action of Casein Kinase Iepsilon on core planar polarity proteins regulates clustering and asymmetric localisation. Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strutt H, Gamage J & Strutt D Robust Asymmetric Localization of Planar Polarity Proteins Is Associated with Organization into Signalosome-like Domains of Variable Stoichiometry. Cell Rep 17, 2660–2671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jenny A, Darken RS, Wilson PA & Mlodzik M Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J 22, 4409–4420 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang-Snyder J, Miller JR, Brown JD, Lai CJ & Moon RT A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol 6, 1302–1306 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Strutt H, Warrington SJ & Strutt D Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell 20, 511–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahaffey JP, Grego-Bessa J, Liem KF Jr. & Anderson KV Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development 140, 1262–1271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu J, Klein TJ & Mlodzik M Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol 2, E158 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chuykin I, Ossipova O & Sokol SY Par3 interacts with Prickle3 to generate apical PCP complexes in the vertebrate neural plate. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kibar Z et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet 28, 251–255 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Montcouquiol M et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173–177 (2003). [DOI] [PubMed] [Google Scholar]
- 93.Murdoch JN et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet 12, 87–98 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Ossipova O, Dhawan S, Sokol S & Green JB Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell 8, 829–841 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Dollar GL, Weber U, Mlodzik M & Sokol SY Regulation of Lethal giant larvae by Dishevelled. Nature 437, 1376–1380 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Djiane A, Yogev S & Mlodzik M The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121, 621–631 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Park M & Moon RT The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol 4, 20–25 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Cho B, Pierre-Louis G, Sagner A, Eaton S & Axelrod JD Clustering and negative feedback by endocytosis in planar cell polarity signaling is modulated by ubiquitinylation of prickle. PLoS Genet 11, e1005259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strutt H & Strutt D Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol 18, 1555–1564 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warrington SJ, Strutt H, Fisher KH & Strutt D A Dual Function for Prickle in Regulating Frizzled Stability during Feedback-Dependent Amplification of Planar Polarity. Curr Biol 27, 2784–2797 e2783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wansleeben C et al. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development 137, 1067–1073 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Merte J et al. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol 12, 41–46; sup pp 41–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo Y, Zanetti G & Schekman R A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife 2, e00160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mottola G, Classen AK, Gonzalez-Gaitan M, Eaton S & Zerial M A novel function for the Rab5 effector Rabenosyn-5 in planar cell polarity. Development 137, 2353–2364 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Matis M, Russler-Germain DA, Hu Q, Tomlin CJ & Axelrod JD Microtubules provide directional information for core PCP function. Elife 3, e02893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimada Y, Yonemura S, Ohkura H, Strutt D & Uemura T Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell 10, 209–222 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Carvajal-Gonzalez JM et al. The clathrin adaptor AP-1 complex and Arf1 regulate planar cell polarity in vivo. Nat Commun 6, 6751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vinson CR & Adler PN Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329, 549–551 (1987). [DOI] [PubMed] [Google Scholar]
- 109.Adler PN, Taylor J & Charlton J The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev 96, 197–207 (2000). [DOI] [PubMed] [Google Scholar]
- 110.Lu Q, Schafer DA & Adler PN The Drosophila planar polarity gene multiple wing hairs directly regulates the actin cytoskeleton. Development 142, 2478–2486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan J et al. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 180, 219–228 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yasunaga T et al. The polarity protein Inturned links NPHP4 to Daam1 to control the subapical actin network in multiciliated cells. J Cell Biol 211, 963–973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim SK et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329, 1337–1340 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishimura T, Honda H & Takeichi M Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084–1097 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Ossipova O, Chuykin I, Chu CW & Sokol SY Vangl2 cooperates with Rab11 and Myosin V to regulate apical constriction during vertebrate gastrulation. Development 142, 99–107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shindo A & Wallingford JB PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 343, 649–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andreeva A et al. PTK7-Src signaling at epithelial cell contacts mediates spatial organization of actomyosin and planar cell polarity. Dev Cell 29, 20–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee J et al. PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium. Curr Biol 22, 956–966 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olofsson J, Sharp KA, Matis M, Cho B & Axelrod JD Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development 141, 2866–2874 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ayukawa T et al. Dachsous-dependent asymmetric localization of spiny-legs determines planar cell polarity orientation in Drosophila. Cell Rep 8, 610–621 (2014). [DOI] [PubMed] [Google Scholar]
- 121.Pataki C et al. Drosophila Rab23 is involved in the regulation of the number and planar polarization of the adult cuticular hairs. Genetics 184, 1051–1065 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gray RS et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol 11, 1225–1232 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seo JH et al. Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Hum Mol Genet 20, 4324–4333 (2011). [DOI] [PubMed] [Google Scholar]
- 124.Nagaoka T, Inutsuka A, Begum K, Bin hafiz K & Kishi M Vangl2 regulates E-cadherin in epithelial cells. Sci Rep 4, 6940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagaoka T, Inutsuka A, Begum K, Hafiz KM & Kishi M Vangl2 regulates e-cadherin in epithelial cells. Sci Rep 4, 6940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dos-Santos Carvalho S et al. Vangl2 acts at the interface between actin and N-cadherin to modulate mammalian neuronal outgrowth. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L et al. A lateral signalling pathway coordinates shape volatility during cell migration. Nat Commun 7, 11714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green JL, Inoue T & Sternberg PW Opposing Wnt pathways orient cell polarity during organogenesis. Cell 134, 646–656 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shafer B, Onishi K, Lo C, Colakoglu G & Zou Y Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell 20, 177–191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jessen JR et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol 4, 610–615 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davey CF, Mathewson AW & Moens CB PCP Signaling between Migrating Neurons and their Planar-Polarized Neuroepithelial Environment Controls Filopodial Dynamics and Directional Migration. PLoS Genet 12, e1005934 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wallingford JB, Fraser SE & Harland RM Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell 2, 695–706 (2002). [DOI] [PubMed] [Google Scholar]
- 133.Huebner RJ & Wallingford JB Coming to Consensus: A Unifying Model Emerges for Convergent Extension. Dev Cell 46, 389–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keller R Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950–1954 (2002). [DOI] [PubMed] [Google Scholar]
- 135.Wallingford JB et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405, 81–85 (2000). [DOI] [PubMed] [Google Scholar]
- 136.Walck-Shannon E & Hardin J Cell intercalation from top to bottom. Nat Rev Mol Cell Biol 15, 34–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith P et al. VANGL2 regulates luminal epithelial organization and cell turnover in the mammary gland. Sci Rep 9, 7079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ossipova O et al. The involvement of PCP proteins in radial cell intercalations during Xenopus embryonic development. Dev Biol (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sawyer JM et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol 341, 5–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nikolopoulou E, Galea GL, Rolo A, Greene ND & Copp AJ Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 144, 552–566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suzuki M, Morita H & Ueno N Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev Growth Differ 54, 266–276 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Sherrard K, Robin F, Lemaire P & Munro E Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr Biol 20, 1499–1510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williams M, Yen W, Lu X & Sutherland A Distinct Apical and Basolateral Mechanisms Drive Planar Cell Polarity-Dependent Convergent Extension of the Mouse Neural Plate. Dev Cell (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barlan K, Cetera M & Horne-Badovinac S Fat2 and Lar Define a Basally Localized Planar Signaling System Controlling Collective Cell Migration. Dev Cell 40, 467–477 e465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shrestha R et al. Mitotic Control of Planar Cell Polarity by Polo-like Kinase 1. Dev Cell 33, 522–534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Devenport D, Oristian D, Heller E & Fuchs E Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol 13, 893–902 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bellaiche Y, Beaudoin-Massiani O, Stuttem I & Schweisguth F The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development 131, 469–478 (2004). [DOI] [PubMed] [Google Scholar]
- 148.Baena-Lopez LA, Baonza A & Garcia-Bellido A The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15, 1640–1644 (2005). [DOI] [PubMed] [Google Scholar]
- 149.Gong Y, Mo C & Fraser SE Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689–693 (2004). [DOI] [PubMed] [Google Scholar]
- 150.Ciruna B, Jenny A, Lee D, Mlodzik M & Schier AF Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220–224 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Segalen M et al. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell 19, 740–752 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lake BB & Sokol SY Strabismus regulates asymmetric cell divisions and cell fate determination in the mouse brain. J Cell Biol 185, 59–66 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nance J & Zallen JA Elaborating polarity: PAR proteins and the cytoskeleton. Development 138, 799–809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Borovina A & Ciruna B IFT88 plays a cilia- and PCP-independent role in controlling oriented cell divisions during vertebrate embryonic development. Cell Rep 5, 37–43 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Gerdes JM, Davis EE & Katsanis N The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zeng H, Hoover AN & Liu A PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol 339, 418–428 (2010). [DOI] [PubMed] [Google Scholar]
- 157.Heydeck W, Zeng H & Liu A Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev Dyn 238, 3035–3042 (2009). [DOI] [PubMed] [Google Scholar]
- 158.Wallingford JB & Mitchell B Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 25, 201–213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu Q et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gerondopoulos A et al. Planar Cell Polarity Effector Proteins Inturned and Fuzzy Form a Rab23 GEF Complex. Curr Biol 29, 3323–3330 e3328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boutin C et al. A dual role for planar cell polarity genes in ciliated cells. Proc Natl Acad Sci U S A (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Conduit PT, Wainman A & Raff JW Centrosome function and assembly in animal cells. Nature Reviews Molecular Cell Biology 16, 611–624 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Tissir F et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci 13, 700–707 (2010). [DOI] [PubMed] [Google Scholar]
- 164.Watanabe D et al. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development 130, 1725–1734 (2003). [DOI] [PubMed] [Google Scholar]
- 165.Simons M et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37, 537–543 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schwarz-Romond T et al. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev 16, 2073–2084 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Itoh K, Jenny A, Mlodzik M & Sokol SY Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci 122, 3791–3798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Park TJ, Mitchell BJ, Abitua PB, Kintner C & Wallingford JB Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40, 871–879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cervenka I et al. Dishevelled is a NEK2 kinase substrate controlling dynamics of centrosomal linker proteins. Proc Natl Acad Sci U S A 113, 9304–9309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chu CW, Ossipova O, Ioannou A & Sokol SY Prickle3 synergizes with Wtip to regulate basal body organization and cilia growth. Sci Rep 6, 24104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dressler GR The cellular basis of kidney development. Annu Rev Cell Dev Biol 22, 509–529 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Krneta-Stankic V, DeLay BD & Miller RK Xenopus: leaping forward in kidney organogenesis. Pediatr Nephrol 32, 547–555 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Costantini F & Kopan R Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18, 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Costantini F Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol 1, 693–713 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shaw G & Renfree MB Wolffian duct development. Sex Dev 8, 273–280 (2014). [DOI] [PubMed] [Google Scholar]
- 176.Boletta A & Germino GG Role of polycystins in renal tubulogenesis. Trends Cell Biol 13, 484–492 (2003). [DOI] [PubMed] [Google Scholar]
- 177.Rowan CJ, Sheybani-Deloui S & Rosenblum ND Origin and Function of the Renal Stroma in Health and Disease. Results Probl Cell Differ 60, 205–229 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Sequeira-Lopez MLS & Torban E New insights into precursors of renal endothelium. Kidney Int 90, 244–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE & Bertram JF Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 63, 2113–2122 (2003). [DOI] [PubMed] [Google Scholar]
- 180.Baldelomar EJ, Charlton JR, Beeman SC & Bennett KM Measuring rat kidney glomerular number and size in vivo with MRI. Am J Physiol Renal Physiol 314, F399–F406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marcotte M, Sharma R & Bouchard M Gene regulatory network of renal primordium development. Pediatr Nephrol 29, 637–644 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Nicolaou N, Renkema KY, Bongers EM, Giles RH & Knoers NV Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11, 720–731 (2015). [DOI] [PubMed] [Google Scholar]
- 183.van der Ven AT, Vivante A & Hildebrandt F Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract. J Am Soc Nephrol 29, 36–50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Torban E et al. Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr Patterns 7, 346–354 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Yates LL et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet 19, 4663–4676 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Viktorinova I, Konig T, Schlichting K & Dahmann C The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development 136, 4123–4132 (2009). [DOI] [PubMed] [Google Scholar]
- 187.Saburi S, Hester I, Goodrich L & McNeill H Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development 139, 1806–1820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rocque BL et al. Deficiency of the planar cell polarity protein vangl2 in podocytes affects glomerular morphogenesis and increases susceptibility to injury. J Am Soc Nephrol 26, 576–586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ciani L, Patel A, Allen ND & ffrench-Constant C Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol 23, 3575–3582 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bagherie-Lachidan M et al. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development 142, 2564–2573 (2015). [DOI] [PubMed] [Google Scholar]
- 191.Mao Y, Francis-West P & Irvine KD Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development 142, 2574–2585 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y, Chang H, Rattner A & Nathans J Frizzled Receptors in Development and Disease. Curr Top Dev Biol 117, 113–139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang Y, Zhou CJ & Liu Y Wnt Signaling in Kidney Development and Disease. Prog Mol Biol Transl Sci 153, 181–207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Halt K & Vainio S Coordination of kidney organogenesis by Wnt signaling. Pediatr Nephrol 29, 737–744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nomachi A et al. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 283, 27973–27981 (2008). [DOI] [PubMed] [Google Scholar]
- 196.Huang L et al. Wnt5a is necessary for normal kidney development in zebrafish and mice. Nephron Exp Nephrol 128, 80–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yun K et al. Non-canonical Wnt5a/Ror2 signaling regulates kidney morphogenesis by controlling intermediate mesoderm extension. Hum Mol Genet (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Majumdar A, Vainio S, Kispert A, McMahon J & McMahon AP Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 (2003). [DOI] [PubMed] [Google Scholar]
- 199.Karner CM et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41, 793–799 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yu J et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136, 161–171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kunimoto K et al. Disruption of Core Planar Cell Polarity Signaling Regulates Renal Tubule Morphogenesis but Is Not Cystogenic. Curr Biol 27, 3120–3131 e3124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ye X, Wang Y, Rattner A & Nathans J Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development 138, 1161–1172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Drawbridge J, Meighan CM, Lumpkins R & Kite ME Pronephric duct extension in amphibian embryos: migration and other mechanisms. Dev Dyn 226, 1–11 (2003). [DOI] [PubMed] [Google Scholar]
- 204.Stewart K & Bouchard M Coordinated cell behaviours in early urogenital system morphogenesis. Semin Cell Dev Biol 36, 13–20 (2014). [DOI] [PubMed] [Google Scholar]
- 205.Lienkamp SS et al. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet 44, 1382–1387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu B et al. Protein tyrosine kinase 7 is essential for tubular morphogenesis of the Wolffian duct. Dev Biol 412, 219–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Carroll TJ, Park JS, Hayashi S, Majumdar A & McMahon AP Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9, 283–292 (2005). [DOI] [PubMed] [Google Scholar]
- 208.Kispert A, Vainio S & McMahon AP Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125, 4225–4234 (1998). [DOI] [PubMed] [Google Scholar]
- 209.Stark K, Vainio S, Vassileva G & McMahon AP Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994). [DOI] [PubMed] [Google Scholar]
- 210.Tanigawa S et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol 352, 58–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pietila I et al. Wnt5a Deficiency Leads to Anomalies in Ureteric Tree Development, Tubular Epithelial Cell Organization and Basement Membrane Integrity Pointing to a Role in Kidney Collecting Duct Patterning. PLoS One 11, e0147171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Qi X, Okinaka Y, Nishita M & Minami Y Essential role of Wnt5a-Ror1/Ror2 signaling in metanephric mesenchyme and ureteric bud formation. Genes Cells 21, 325–334 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F & Pachnis V Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994). [DOI] [PubMed] [Google Scholar]
- 214.Chi X et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell 17, 199–209 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nishita M et al. Role of Wnt5a-Ror2 signaling in morphogenesis of the metanephric mesenchyme during ureteric budding. Mol Cell Biol 34, 3096–3105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang H et al. FAT4 Fine-Tunes Kidney Development by Regulating RET Signaling. Dev Cell 48, 780–792 e784 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang S, Sekiguchi R, Daley WP & Yamada KM Patterned cell and matrix dynamics in branching morphogenesis. J Cell Biol 216, 559–570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Walker KA, Sims-Lucas S & Bates CM Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr Nephrol 31, 885–895 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhao H et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276, 403–415 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brzoska HL et al. Planar cell polarity genes Celsr1 and Vangl2 are necessary for kidney growth, differentiation, and rostrocaudal patterning. Kidney Int 90, 1274–1284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Torban E, Wang HJ, Groulx N & Gros P Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem 279, 52703–52713 (2004). [DOI] [PubMed] [Google Scholar]
- 222.Liu W et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A 105, 210–215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miller RK et al. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol 22, 1654–1664 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Warrington SJ, Strutt H & Strutt D The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development 140, 1045–1054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Williams BB et al. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J Cell Sci 125, 2141–2147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Coyle RC, Latimer A & Jessen JR Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp Cell Res 314, 2150–2162 (2008). [DOI] [PubMed] [Google Scholar]
- 227.Kopan R, Chen S & Little M Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr Top Dev Biol 107, 293–331 (2014). [DOI] [PubMed] [Google Scholar]
- 228.Das A et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15, 1035–1044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.O’Brien LL et al. Wnt11 directs nephron progenitor polarity and motile behavior ultimately determining nephron endowment. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ramalingam H et al. Disparate levels of beta-catenin activity determine nephron progenitor cell fate. Dev Biol 440, 13–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mao Y et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development 138, 947–957 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Karner CM et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu C et al. Null and hypomorph Prickle1 alleles in mice phenocopy human Robinow syndrome and disrupt signaling downstream of Wnt5a. Biol Open (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.San Agustin JT et al. Genetic link between renal birth defects and congenital heart disease. Nat Commun 7, 11103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Babayeva S et al. Planar cell polarity pathway regulates nephrin endocytosis in developing podocytes. J Biol Chem 288, 24035–24048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Derish I et al. Differential role of planar cell polarity gene Vangl2 in embryonic and adult mammalian kidneys. PLoS One 15, e0230586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fischer E et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet 38, 21–23 (2006). [DOI] [PubMed] [Google Scholar]
- 238.Happe H, de Heer E & Peters DJ Polycystic kidney disease: the complexity of planar cell polarity and signaling during tissue regeneration and cyst formation. Biochim Biophys Acta 1812, 1249–1255 (2011). [DOI] [PubMed] [Google Scholar]
- 239.Menezes LF & Germino GG Polycystic kidney disease, cilia, and planar polarity. Methods Cell Biol 94, 273–297 (2009). [DOI] [PubMed] [Google Scholar]
- 240.Saburi S et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40, 1010–1015 (2008). [DOI] [PubMed] [Google Scholar]
- 241.Matakatsu H & Blair SS Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development 139, 1498–1508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Happe H et al. Altered Hippo signalling in polycystic kidney disease. J Pathol 224, 133–142 (2011). [DOI] [PubMed] [Google Scholar]
- 243.Cai J et al. A RhoA-YAP-c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev 32, 781–793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sekiguchi R & Yamada KM Basement Membranes in Development and Disease. Curr Top Dev Biol 130, 143–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sutherland A, Keller R & Lesko A Convergent extension in mammalian morphogenesis. Semin Cell Dev Biol 100, 199–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Collins I & Wann AKT Regulation of the Extracellular Matrix by Ciliary Machinery. Cells 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nishio S et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21, 295–302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Castelli M et al. Polycystin-1 binds Par3/aPKC and controls convergent extension during renal tubular morphogenesis. Nat Commun 4, 2658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Quaggin SE & Kreidberg JA Development of the renal glomerulus: good neighbors and good fences. Development 135, 609–620 (2008). [DOI] [PubMed] [Google Scholar]
- 250.Babayeva S, Zilber Y & Torban E Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol 300, F549–560 (2011). [DOI] [PubMed] [Google Scholar]
- 251.Yaoita E et al. Role of Fat1 in cell-cell contact formation of podocytes in puromycin aminonucleoside nephrosis and neonatal kidney. Kidney Int 68, 542–551 (2005). [DOI] [PubMed] [Google Scholar]
- 252.Gee HY et al. FAT1 mutations cause a glomerulotubular nephropathy. Nat Commun 7, 10822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Montaldo P et al. Small renal size in newborns with spina bifida: possible causes. Clin Exp Nephrol 18, 120–123 (2014). [DOI] [PubMed] [Google Scholar]
- 254.Wang M, Marco P, Capra V & Kibar Z Update on the Role of the Non-Canonical Wnt/Planar Cell Polarity Pathway in Neural Tube Defects. Cells 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Murdoch JN et al. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics 78, 55–63 (2001). [DOI] [PubMed] [Google Scholar]
- 256.Person AD et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn 239, 327–337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bunn KJ et al. Mutations in DVL1 cause an osteosclerotic form of Robinow syndrome. Am J Hum Genet 96, 623–630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.White JJ et al. DVL3 Alleles Resulting in a −1 Frameshift of the Last Exon Mediate Autosomal-Dominant Robinow Syndrome. Am J Hum Genet 98, 553–561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Afzal AR et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 25, 419–422 (2000). [DOI] [PubMed] [Google Scholar]
- 260.van Bokhoven H et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet 25, 423–426 (2000). [DOI] [PubMed] [Google Scholar]
- 261.Brunetti-Pierri N et al. Robinow syndrome: phenotypic variability in a family with a novel intragenic ROR2 mutation. Am J Med Genet A 146A, 2804–2809 (2008). [DOI] [PubMed] [Google Scholar]
- 262.Mansour S et al. Van Maldergem syndrome: further characterisation and evidence for neuronal migration abnormalities and autosomal recessive inheritance. Eur J Hum Genet 20, 1024–1031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lahrouchi N et al. Homozygous frameshift mutations in FAT1 cause a syndrome characterized by colobomatous-microphthalmia, ptosis, nephropathy and syndactyly. Nat Commun 10, 1180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang W et al. Expanding the genetic architecture and phenotypic spectrum in the skeletal ciliopathies. Hum Mutat 39, 152–166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Otto EA et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34, 413–420 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Heydeck W & Liu A PCP effector proteins inturned and fuzzy play nonredundant roles in the patterning but not convergent extension of mammalian neural tube. Dev Dyn 240, 1938–1948 (2011). [DOI] [PubMed] [Google Scholar]
- 267.van Amerongen R & Nusse R Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 (2009). [DOI] [PubMed] [Google Scholar]
- 268.Marlow F, Topczewski J, Sepich D & Solnica-Krezel L Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol 12, 876–884 (2002). [DOI] [PubMed] [Google Scholar]
- 269.Weiser DC, Row RH & Kimelman D Rho-regulated myosin phosphatase establishes the level of protrusive activity required for cell movements during zebrafish gastrulation. Development 136, 2375–2384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Butler MT & Wallingford JB Planar cell polarity in development and disease. Nat Rev Mol Cell Biol 18, 375–388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Carroll TJ & Yu J The kidney and planar cell polarity. Curr Top Dev Biol 101, 185–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.