Abstract
Cerebral palsy is the most prevalent physical disability in children; however, its inherent molecular mechanisms remain unclear. In the present study, we performed in-depth clinical and molecular analysis on 120 idiopathic cerebral palsy families, and identified underlying detrimental genetic variants in 45% of these patients. In addition to germline variants, we found disease-related postzygotic mutations in ∼6.7% of cerebral palsy patients. We found that patients with more severe motor impairments or a comorbidity of intellectual disability had a significantly higher chance of harbouring disease-related variants. By a compilation of 114 known cerebral-palsy-related genes, we identified characteristic features in terms of inheritance and function, from which we proposed a dichotomous classification system according to the expression patterns of these genes and associated cognitive impairments. In two patients with both cerebral palsy and intellectual disability, we revealed that the defective TYW1, a tRNA hypermodification enzyme, caused primary microcephaly and problems in motion and cognition by hindering neuronal proliferation and migration. Furthermore, we developed an algorithm and demonstrated in mouse brains that this malfunctioning hypermodification specifically perturbed the translation of a subset of proteins involved in cell cycling. This finding provided a novel and interesting mechanism for congenital microcephaly. In another cerebral palsy patient with normal intelligence, we identified a mitochondrial enzyme GPAM, the hypomorphic form of which led to hypomyelination of the corticospinal tract in both human and mouse models. In addition, we confirmed that the aberrant Gpam in mice perturbed the lipid metabolism in astrocytes, resulting in suppressed astrocytic proliferation and a shortage of lipid contents supplied for oligodendrocytic myelination. Taken together, our findings elucidate novel aspects of the aetiology of cerebral palsy and provide insights for future therapeutic strategies.
Keywords: cerebral palsy, TYW1, GPAM
Li et al. present an in-depth genetic and clinical analysis of 120 families with cerebral palsy. They identify detrimental genetic variants in 54 families, including in two genes not previously associated with cerebral palsy: TYW1 and GPAM. Functional studies reveal roles for these genes in neurogenesis and myelination, respectively.
Introduction
Cerebral palsy (CP) is an umbrella term spanning a group of disorders with compromised ambulant performances, and is attributed to non-progressive disturbances in developing foetal and infant brains.1 CP can be categorized according to motor signs (spasticity, dyskinesia, ataxia and hypotonia, among which spasticity manifests in ∼80% of CP cases), involvement of extremities (hemiplegia, monoplegia, diplegia, triplegia and quadriplegia), and anatomical sites of brain lesions (cerebral cortex, pyramidal tract, extrapyramidal system and cerebellum).2,3 In CP children who are 2 years or older, severity is reliably classified via the five-level Gross Motor Function Classification System (GMFCS).4 The common comorbidities of CP include intellectual disability (ID), epilepsy, vision and hearing problems, language deficits, autism spectrum disorders (ASDs), sleep disorders and secondary musculoskeletal defects such as scoliosis and hip dislocation.2,3 CP is the most prevalent physical disability of childhood, with a prevalence of 2.0–3.5 cases per 1000 live births in countries with varying degrees of socioeconomic development (>20 million patients around the world), and the incidence of CP at term has remained consistent for over the past 50 years.1-3,5–7 CP poses a critical problem for rehabilitation and welfare systems, and the lifetime costs for a CP child in the USA are estimated to be $1 million.5 The conventional risk factors of CP consist of gestational and perinatal insults, including birth asphyxia, neonatal infections and teratogens.1,5 However, two thirds of CP patients are born at term, and birth asphyxia happens in <10% of CP cases.2,6,7 Because the estimated upper limit of unconfirmed causality of CP comprises 80% of cases, it is reasonable that unknown mechanisms exist accounting for most CP events.3
A growing body of evidence has indicated prevailing genetic contributors to the aetiology of CP, including the reports of familial cases and the high concordance of monozygotic twins; furthermore, the positive correlation between CP incidence and parental ages suggests a genetic component, as does the prevalence of congenital malformation observed in CP patients.8–12 Large cohorts of case-control studies assessing polymorphisms in a variety of candidate genes did not identify any significantly associated variants.2,13,14 Therefore, the genetic underpinnings of CP are probably similar to those of other neurodevelopmental disorders (NDDs) such as ID and ASD, where causative variants are rarely detected in association studies based on common variants but with large effect sizes.15–17 In line with this hypothesis, clinically relevant copy number variation (CNV), both de novo and inherited, were recently identified in four collections of CP patients, resolving 4–31% of the cryptogenic cases.18–21 Meanwhile, gene-panel sequencing and whole-exome sequencing revealed pathogenic variants, mostly de novo, in an aggregate of six CP studies, yielding molecular diagnoses for ∼15% of cases.22–27
So far, the known genetic and molecular information on CP aetiology has been insufficient, which impedes improvements in prophylaxis, prognosis and treatment of CP patients in the era of precision medicine. Large cohorts need to be recruited to fully decipher the corresponding gene spectrum due to the high genetic heterogeneity of CP, and geographic and ethnic biases should be addressed. Whole-genome sequencing (WGS), in comparison with gene-panel sequencing or whole-exome sequencing, can render better coverage of predisposing alleles, and somatic mosaicism or postzygotic mutations (PZM) in CP patients must be highlighted as in other NDDs.28,29 Hence, targeted investigation into the common characteristics of CP-related genes, in addition to detailed mechanistic studies, is warranted before the introduction of a gene-based classification system that may have a profound impact on clinical practice when treating CP patients.
Materials and methods
Cohort recruitment
The CP families were recruited by the Department of Rehabilitation of GWCMC from August 2014 to June 2017 in a cohort study. This study was approved by the ethic committee of GWCMC (approval no. 2016061601), and the written informed consents were signed by all families involved in this study. The family history and clinical characteristics of CP individuals were documented regarding the CP phenotypic profiles. The motor signs (spastic, ataxic, dyskinetic etc.) and anatomical distribution (monoplegia, diplegia, triplegia, hemiplegia and quadriplegia) of movement disorders were evaluated and categorized. Motor severity was measured by the GMFCS on five levels.30 The common comorbidities, i.e. ID, vision/hearing impairments, epilepsy, dyslexia etc., were recorded for each patient. The fine motor skills were evaluated by the Manual Ability Classification System (MACS).31 Rehabilitation training outcome was determined by the Activity of Daily Living (ADL),32 and the Gross Motor Function Measure (GMFM),33 both of which were measured twice before and after treatments.
Magnetic Resonance Imaging (MRI) and diffusion tensor imaging (DTI)
All MRIs were performed on a 3.0 T Siemens scanner (Magnetom Systems, Skyra, Munich, Germany). For DTI, an echoplanar sequence with diffusion gradients (b = 1000 s/mm2) applied in 64 non-collinear directions was used. Basic parameters included: TR (10 000 ms)/TE (92 ms), voxel (2.0 × 2.0 × 2.0 mm), field of view (256 × 256) and layer thickness (2.0 mm). DTI measurement was three-dimensional reconstruction of colour-coded FA graphics through DTI Studio (DWI standard deviation).
Analysis pipeline of deep sequencing data
The variant prioritization and evaluation of etiologic involvement were performed by two pipelines, namely MERAP and ANNOVAR. In brief, MERAP pipeline first filters all identified variants through comparison with the disease-associated variants in the Human Gene Mutation Database (HGMD, 2020.2) and the Online Mendelian Inheritance in Man (OMIM) to collect those known disease-causing variants. To filter out neutral variants, MERAP uses entries from the dbSNP143 (http://www.ncbi.nlm.nih.gov/projects/SNP/), 1000 Genome (http://www.1000genomes.org/), NHLBI Exome Sequencing Project (ESP, http://evs.gs.washington.edu/EVS/), ExAc (http://exac.broadinstitute.org/) and the Chinese Millionome Database (CMDB) (https://db.cngb.org/cmdb/) as screening databases. In principle, candidate variants causing recessive traits should not occur in healthy controls as homozygotes, and the frequency of respective heterozygotes should not exceed 0.1%. MERAP uses the RefSeq genes (http://www.ncbi.nlm.nih.gov/refseq/) as a reference, and nonsynonymous changes are described in terms of gene ID, base change, protein change, genomic coordinate, transcript coordinate, protein coordinate, protein length, affiliated with gene description from the Human Gene Nomenclature Committee (http://www.genenames.org/). Changes destroying conventional splice sites or introducing novel splice sites are identified by MERAP’s module called SSFinder. To assess the pathogenicity of missense mutations, MERAP generates a single score integrating the results of seven different algorithms, including the Grantham score, PhyloP, GERP, SIFT, PolyPhen2, Mutation-Taster and the Conserved Domains Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). With empirical false discovery rate cut-offs, this score serves as dichotomized pathogenicity predictions even if any two of the seven algorithms might not coincide, as is often the case. MERAP rules out candidate genes reported to harbour homozygous loss-of-function (LOF) variants in healthy individuals, which applies to >1% of the human genes. Typically, if >3 independent truncating variants are observed in >10 of the exomes listed in the 1000 Genome, Exome Variant Server (ESP6500), NHLBI GO ESP (http://evs.gs.washington.edu/EVS/) and ExAC (http://exac.broadinstitute.org/) databases, the relevant gene is flagged as LOF tolerant. To facilitate the choice between few remaining candidate genes, MERAP also provides a list of ∼4500 known disease genes extracted from OMIM (http://www.ncbi.nlm.nih.gov/omim), ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/) and HGMD (http://www.hgmd.org/), as well as >8000 associated disorders and their symptoms. For novel candidate genes without a previous link to disease, MERAP offers information on their interaction with known disease genes, based on data from Biogrid (http://thebiogrid.org/) and IntAct (http://www.ebi.ac.uk/intact/), assuming that genes implicated in clinically similar disorders tend to cluster in gene or protein interaction networks. Variants were considered de novo if neither parent had the variant, and candidate variants were selected by segregation analysis. The pathogenic and probably pathogenic genes/variants were defined according to the standards and guidelines of ACMG.34
The ANNOVAR pipeline was also used, with additional considerations from the Residual Variation Intolerance Score (RVIS) and the Combined Annotation-Dependent Depletion (CADD) score.35 The former ranks genes in terms of intolerance to functional genetic variation and the latter integrates several well-known tools. We empirically set a cut-off RVIS score of 50th percentile for known and novel genes and a cut-off CADD score of 20 for novel candidate genes. When defining likely causal variants/genes, we followed the guidelines designated by ACMG.
All variants of putative clinical relevance were confirmed by the conventional PCR and Sanger sequencing. Parent-child relationships were confirmed by using the PLINK with SNPs drawn from WGS that matched the CytoScan SNP repertoire.36 The false positive rate of WGS was generated by the comparison between Sanger sequencing and WGS, and we found no false positive variant called by our pipeline. The false negative rate of WGS was evaluated by the SNP genotyping comparison between WGS and CytoScan high-density microarray, which resulted in an average false negative rate of <0.1%.
Zebrafish modelling
All zebrafish experimentation was carried out in accordance with the NIH Guidelines for the care and use of laboratory animals (http://oacu.od.nih.gov/regs/index.htm) and ethically approved by the Administration Committee of Experimental Animals, Jiangsu Province, China [Approval ID: SYXK (SU) 2007–0021].
Zebrafish lines and whole-mount in situ hybridization
The zebrafish embryos and adults were maintained in the Zebrafish Center of Nantong University under conditions in accordance with our previous protocols.37,38 The fish lines Tg(mnx1: GFP)ml,2Tg(huC: egfp), Tg(huC: mcherry) and Tg(gfap: egfp) have been described in the previous work.37,38 The whole-mount in situ hybridization (WISH) was performed as previously described.39 The templates for generating antisense RNA probes were amplified from the cDNA library, using specific primers targeting tyw1 and gpam listed as here: tyw1-forward: 5ʹ AAG GCC AGC TGC AAG AAT AA; tyw1-reverse: 5ʹ GTG TGG TGT CTC CAG CAG AA; gpam-forward: 5ʹ TCC TGT TCA CAA GTC CCA CA; gpam-reverse: 5ʹ AGT TCT TGC GGA GCA TCC TA.
Morpholino and mRNAs injections
tyw1 splice-blocking morpholino (MO; Gene Tools, OR, USA) sequence was 5′ AAC CTT ATT CCC ACT TAA TGT TAC C. gpam splice-blocking morpholino sequence was 5′ GGT GCT ACT TTT CTC CAA GCT TAC C. The sequence of a standard control MO oligo was 5′ CCT CTT ACC TCA GTT ACA ATT TAT A. tyw1 translation-blocking morpholino sequence was 5′ CAG CAT CTC ATG TAC TCT CTC CAT C. gpam translation-blocking morpholino sequence was 5′ ACG TCC ATC CCC TCT CTT CAA ACC A. The MOs were diluted to 0.3 mM with RNase-free water and injected into the yolk of one to two-cell stage embryos and then raised in E3 medium at 28.5°C. The wild-type and mutated cDNAs [TYW1 (NM_018264) mut1: p. R389Q; TYW1 mut2: p. R206C; GPAM (NM_001244949) mut1: p. G499R; GPAM mut2: p. P669S] containing the open reading frame of the zebrafish and human tyw1 or gpam genes were cloned into pCS2+ vector, respectively, and then were transcribed in vitro by using the mMESSAGE mMACHIN Kit (Thermo Fisher Scientific, MA, USA) after the recombinant plasmids linearized with NotI Restriction Enzyme (NEB, MA, USA), and then the capped mRNAs were purified by RNeasy Mini Kit (Qiagen, Frankfurt, Germany). Around 2 nl of mRNA were injected at 50 ng/µl into half-cell stage embryos.
RNA isolation, reverse transcription and PCR
Total RNA was extracted from zebrafish embryos by TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Thermo Fisher Scientific, MA, USA). The reverse transcription was carried out according to the standard protocols from manufacturer (Fermentas, Thermo Fisher Scientific, MA, USA). The PCR was carried out as previously described to validate the efficiency of splicing-blocking effect of specific morpholino 37 by using the primer sequencing listed as follows: tyw1-forward: 5ʹ TTA TCG GTG TTG TCG GGT TT; tyw1-reverse: 5ʹ CCT CTG CCA ATC TGT CTT CC; gpam-forward:5ʹ ACA GTG CGC AAA AAG AGG TC; gpam-reverse: 5ʹ TAA GCA CGT TCT CCA CCA CA.
Locomotion analysis in zebrafish larvae, microscopy and statistical analysis
The locomotion analysis of 5 dpf zebrafish larvae was carried out by using the DanioVision system (Noldus Information Technology, Wageningen, Netherlands). The confocal imaging was performed by using a TCS-SP8 LSM confocal imaging system (Leica, Wetzlar, Germany). The zebrafish embryos were embedded as we previously did.37 Photographs of in situ hybridization results were taken by using a DP70 camera on an Olympus stereomicroscope MVX10. Statistical comparisons of the data were carried out by Student’s t-test, and P < 0.05 was considered statistically significant.
Mice modelling
All mice experiments in this study were approved by the institutional animal care and use committee in the Guangzhou Medical University (registration no. 2019–436, 2019–694). In this study, all mice, either wild-type or mutant, were generated from the C57BL/6J strain and provided by the Cyagen Biosciences.
Generation of knockout mice by CRISPR–Cas9 system
Knockout mouse lines were created by CRISPR–Cas9-mediated genome engineering. To create a Tyw1 knockout mouse model, exon 2–3 was selected as the target site. gRNA was designed as the following sequences: gRNA1 (matching reverse strand of gene): GCCTAAGGACCACGTTTCGATGG. gRNA2 (matching reverse strand of gene): CATGTAAGACCTCACGATCAAGG.
To create a Gpam knockout mouse model, exon 2–4 was selected as the target site. gRNA was designed as the following sequences: gRNA1 (matching reverse strand of gene): CTCCCACGGGAAAACCACGCAGG. gRNA2 (matching reverse strand of gene): CCCAAGGGGTCACGCACCACAGG.
Cas9 mRNA and gRNA were co-injected into the cytoplasm of fertilized eggs. The microinjected zygotes were cultured in medium until the two-cell stage, and then were transferred into the oviducts of pseudopregnant ICR females at 0.5 d after mating with vasectomized males. The mutant F0 mice were identified by genomic PCR and the DNA sequence was confirmed by Sanger sequencing. Subsequent breeding of the mutant F0 mice generated offspring with desired genotypes for experiments. Gene knockout efficiency was identified using quantitative PCR (qPCR) (Supplementary Fig. 10).
Behavioural tests
All behavioural tests were carried out on both male and female mice at 8 weeks of age, and the balanced gender was kept for both wild-type and Tyw1−/− or Gpam−/− mice. Each test was conducted at fixed day time (between 8:30 and 18:30) on each training day. Mice were moved to the testing room 1 h before behavioural testing for acclimation, and those participating in multiple tests were allowed to rest for at least 3 days between two tests. All experimental areas were cleaned with 70% ethanol before the tests and between participants. All behavioural tests were carried out with the presence of two researchers blinded to the genotype.
Morris water maze test
The Morris water maze test was performed to investigate the learning and memory ability of mice. Briefly, a platform was placed at the central zone of one quadrant of the pool below the surface of water. The mice were trained to learn the position of the hidden platform for 4 days. At day 5, the platform was removed. The mice were released into water and allowed to swim for 60 s to search a virtual quadrant centred on the location of the platform. Duration in zone of platform refers to amount of time mice remained in this virtual zone. Frequency of crossing platform refers to number of times mice crossed the virtual zone, while latency to first arrival refers to the time needed for mice to reach the virtual zone at first time, while distance travelled in target quadrant refers to the distance mice swam in the virtual quadrant. Videos were recorded and analysed by using the SuperMaze V2.0 software (XinRuan, Shanghai, China).
Rotarod test
Rotarod test was performed to indicate motor coordination of mice in each group. First, mice were trained three times at the rate of 30 rpm before test, and each training maintained for 5 min. In the test, the rotation rate increased gradually to reach 40 rpm within 300 s. The test was over when a mouse fell off. Each mouse was detected three times and the mean latency time at rotarod was recorded.
Grip strength test
Grip strength of mice forelimbs was measured with a grip strength test meter (BIOSEB, EB Instruments). During the test, the mice were placed over the grid allowing only forepaws to attach to the grid. After the mice paws grasped the grid, their tails were pulled horizontally until they completely released hold. Each mouse was tested for three times. The readings of grip strength and duration of grasping the grid were recorded and analysed by using the SuperGSM software (XinRuan, Shanghai, China).
Tissue preparation
Mice were anaesthetized with 50 mg/kg sodium pentobarbital. After being perfused with 0.9% NaCl and 4% paraformaldehyde, brains were extracted and postfixed for 24 h, followed with dehydration by using sucrose. Brains were cut into two hemispheres, and then serial sagittal sections (30 μm thick) were sliced with freezing microtome (CM3050S, Leica, Germany).
Nissl staining
Sections were immersed into 75% ethanol (30 s), dH2O (30 s) and cresyl violet (2 min). Then, sections were dehydrated with gradient ethanol (75%, 95%, 100%) for 30 s, followed by incubation with xylene and mounted with neutral resins. The images were taken under an inverted microscope.
5-Ethynyl-2ʹ-deoxyuridine labelling
For proliferation assays, pregnant dams on E13.5 were intraperitoneally injected with 5-ethynyl-2ʹ-deoxyuridine (EdU, 20 mg/kg body weight) 2 h before euthanasia. Embryonic brains were harvested, fixed in paraformaldehyde and immersed in 30% sucrose for dehydration. Brain slices were then stained with Click-iT EdU Imaging Kits (C10339, Invitrogen, Thermo Fisher Scientific, USA). Cells incorporated with EdU were determined under a fluorescence microscope. Percentage of EdU+ fluorescence area (indicating the cell number of EdU+) of E13.5 mouse brain cortex was calculated in three random fields per coverslip. For migration assays, pregnant dams on E15.5 were intraperitoneally injected with EdU. Brains were harvested after 72 h and stained as before.
Immunostaining
To detect expression of TYW1 and GPAM in human brain tissue, the normal brain tissue microarrays (BNC17011, Biomax, MD, USA) were prepared. According to the instruction from Biomax official website, BNC17011 were derived from normal brain tissue of male or female at the age of 2 to 50, including brain regions such as frontal lobe, apical lobe, occipital lobe, temporal lobe, midbrain, pons, medulla oblongata, thalamus opticus, cerebellum, hippocampus, callositas, optic nerve and spinal cord. The slides were immersed in xylene and graded ethanol to deparaffinize and rehydrate. After antigen retrieval using microwave with sodium citrate buffer, 0.3% Triton X-100 was added to permeabilize the tissue. The slides were blocked with goat serum and incubated with primary antibodies at 4°C overnight. After washing with PBS, the sections were incubated with secondary antibodies.
For mouse brain tissues, the perfused brain samples were fixed within 4% paraformaldehyde (PFA) in 0.01 M PBS at 4°C for 24 h, then washed by PBS twice, cryoprotected with sucrose gradients, snap frozen and sectioned with a cryostat (Leica, Wetzlar, Germany) to thickness of 30 µm. Then the sections were immuno-stained by using aforementioned methods, except deparaffinization and rehydration.
Number of Cux1+ or Foxp2+ cells in frontal cortex and motor cortex were counted on three sagittal cerebrum sections, respectively. Gfap+ and NeuN+ cells in different brain regions were counted in three random fields per section. Data were obtained from at least three independent experiments and analysed with ImageJ software.
To visualize and measure the cholesterol level, Filipin staining was performed in tissue sections (10-µm thick) by using a cell-based Cholesterol Assay Kit (ab133116, Abcam, UK). Briefly, the tissue sections were washed (3 × 5 min) with Cholesterol Detection wash buffer, and then the Filipin III was added to each section and incubated in the dark for 60 min at room temperature. After washing, the fluorescence images were obtained by a Leica DMi8 (Leica Microsystems, Wetzlar, Germany) fluorescence microscope by using ×20 HC PL FLUOTAR objective.
Western blot analysis
Total protein was extracted from peripheral blood using cell lysate containing RIPA and protease inhibitor cocktail. And the brain tissues were lysed with 2% SDS in PBS with PMSF and proteinase inhibitor cocktail. The BCA assay was used to determine protein concentration. Proteins were resolved on 7.5, 10 or 15% tris-glycine gels based on different molecular weight and transferred to PVDF membranes. After blocking, the membranes were incubated with primary antibodies, followed by horseradish peroxidase (HRP)-labelled secondary antibodies. Then, blots were visualized by West Pico Plus Chemiluminescent Substrate (Thermo Fisher Scientific, MA, USA) and scanned using ChemiDocTM MP system (Bio-Rad, CA, USA). Densitometries of individual blot signals were quantified using ImageJ software.
qPCR with reverse transcription analysis
Total RNA was extracted from the mice brain tissues by using TRIZOL reagent (Invitrogen, Thermo Fisher Scientific, MA, USA), and cDNA was synthesized from 1 µg total RNA by using PrimeScript™ RT Master Mix (RR036Q, Takara, Shiga, Japan). qPCR was carried out by using PowerUp™ SYBR™ Green Master Mix (A25778, Applied Biosystems, Thermo Fisher Scientific, MA, USA) on Biosystems QuantStudio 6 Flex Real-time PCR system (Applied Biosystems, Thermo Fisher Scientific, MA, USA). β-Actin was used as a reference gene. For comparison, mRNA expression levels of knockout mice were normalized to those of wild-type mice for each participant. The primers were: Tyw1-forward: 5ʹ GTG GGA CTT GTC GCC TTT G; Tyw1-reverse: 5ʹ GGG AAC GAG TGA CCT GCT T; Stil-forward: 5ʹ GAC ACA ATT CAG GAC TGG TAG AC; Stil-reverse: 5ʹ GGC ATG ATC CAC TTT CTG TTC A; Sass6-forward: 5ʹ ATT CCT TTA CGC GGA CTT AGC; Sass6-reverse: 5ʹ AAG TAG GCT GAA GAC GAG GAG; Ncapd2-forward: 5ʹ AGC CAG ACA AGC CTC ATT GAC; Ncapd2-reverse: 5ʹ TCC ATA GGT GAC GGA TGT CCA; Cenpe-forward: 5ʹ CTT CAG TGG CTG TCT GTG TTC; Cenpe-reverse: 5ʹ CCA TCG CTC TGA TAA ATA GCG TT; β-Actin-forward: 5ʹ GGC TGT ATT CCC CTC CAT CG; β-Actin-reverse: 5ʹ CCA GTT GGT AAC AAT GCC ATG T.
Culture of primary neurons
E13.5 mouse embryo brains were taken out from a Tyw1+/− pregnant dam. Cortex in each brain was dissected separately and collected in Hibernate-E supplemented with 2% B27 on ice. Single cells were obtained by using 0.05% trypsin (containing 0.2 mM EDTA) digestion for 10 min at 37°C. After filtration with 70-μm strainer and centrifugation, cells resuspended in Neurobasal medium with 2% B27 Plus Supplement, 0.25% Glutamax and 25 μM glutamate were placed in poly-D-lysine coated plates. Adherent neurons were prepared for EdU and TUNEL staining. To prepare migration assay, isolated cells were initially cultured in ultra-low attachment plates to form neurospheres, which could be digested by using 0.05% trypsin without damage of neurites.
Proliferation and apoptosis assays of primary neurons
At two DIV, cells were labelled with 10 μM EdU solution and incubated for 4 h. Cells were treated by using 4% PFA, followed by 0.5% TritonX-100 permeabilization. After being washed twice with 3% BSA, cells were incubated with Click-iT reaction cocktail according to protocol of Click-iT EdU Imaging Kits (C10339, Invitrogen, Thermo Fisher Scientific, MA, USA) and stained with DAPI. Dead cells were labelled using In Situ Cell Death Detection Kit, POD (11684817910, Roche, Switzerland).
Migration test of primary neurons
Neurospheres were digested with 0.05% trypsin to obtain single neurons and resuspended in Neurobasal medium with 1% B27 Plus Supplement. Cells were seeded on the upper layer of a cell culture insert with PET track-etched membrane (8 μm pore size, 353097, Corning, NY, USA) at density of 1 × 105 cells per well. Neurobasal medium with 2% B27 Plus Supplement was added into the bottom of the lower chamber. After 16 h, the culture insert was taken out and the medium was removed carefully. Cells on the upper surface were wiped, while migrating cells in pore and on the lower surface of membrane were fixed, stained with 0.1% crystal violet and observed.
Flow cytometry
Whole-brain tissues from Gpam−/− mice on P1 (postnatal day 1) were collected, including brain tissues of their wild-type littermate. After digestion with 4 mg/ml papain and 0.1 mg/ml DNase in 37°C shaker for 30 min, suspension was diluted using DMEM/F12 medium containing 5% FBS and filtered with 70-μm strainers. Then, suspension was centrifuged and the cells were fixed in 4% PFA. 0.3% Triton-X100 was used for permeabilization. After being blocked with 5% goat serum, cells were incubated with mouse anti-Gfap antibody and rabbit anti-Ki67 antibody or rabbit anti-Caspase-3 antibody at 4°C for 1 h. Cells were washed with DPBS and incubated with Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 647 goat anti-rabbit IgG at 4°C for 30 min. After being washed twice, cells were suspended by using DPBS and analysed on the flow cytometer (BD FACSCanto, BD Biosciences, CA, USA).
Measurement of phosphatidic acid (PA)
Phosphatidic acid was measured by using the PicoProbeTM Phosphatidic Acid Assay Kit (BioVision, CA, USA). In brief, medulla of wild-type and Gpam−/− mice was homogenized in PA assay buffer. Lipid extraction was obtained according to the protocol and solubilized in 5% Triton X-100 solution. ‘Sample background control’ and ‘Sample’ were prepared in parallel. Standard curve was generated by using PA standard solution. Converter mix was only added in sample and standard wells. All wells were incubated at 45°C for 1 h. Reaction mix was added in each well and incubate at 37°C for 30 min. Fluorescence was recorded at excitation/emission = 535/587 nm and PA concentration was expressed as nmol PA per mg tissue weight. Value of PA concentration was normalized.
Measurement of phosphatidylcholine (PC)
Phosphatidylcholine was measured by using the phosphatidylcholine assay kit (Abcam, UK). For each individual, about 10 mg brain tissues were washed with cold PBS, resuspended in the assay buffer and homogenized on ice. After 10 min incubation, samples were centrifuged for 5 min at 4°C at 16 000g. The supernatant was incubated with the reaction mix including OxiRed Probe supplemented with hydrolysis enzyme for 30 min. The colorimetric reading was measured at an optical density of 570 nm (OD570 nm) on a microplate reader.
Measurement of Phosphatidylethanolamine (PE)
Phosphatidylethanolamine was measured by using the phosphatidylethanolamine assay kit (Abcam, UK) according to the manufacturer’s instructions. Briefly, solubilized lipids were extracted from brain tissues with 5% Triton X-100, incubated with converter mix at 45°C for 1 hour, and then with reaction mix at 40°C for 3 hours. Fluorescence was recorded at excitation/emission 535/587 nm.
Modelling the effect of TYW1 knockout on ribosomal frameshift
The modelling of TYW1-knockout effect on ribosomal frameshift was based on the fact that TYW1 is a critical enzyme involved in the synthesis of wybutosine, so the hypomorphic and null alleles of TYW1 could reduce and even remove the production of wybutosine accordingly.40,41 It is known that wybutosine at the 37 position of tRNAPhe near the 3ʹ base of anticodon can stabilize the codon–anticodon interaction thus drastically reduce the ribosomal frameshift on a specific UUU codon.42 We, therefore, hypothesized that the density and distribution of UUU codons along a specific mRNA should determine the extent of influence exerted by wybutosine, and in parallel, the relative abundance of wybutosine was another major effector during this process. Our modelling procedure went as follows:
Download the UCSC RefSeq (refGene) gene models of human, mouse and zebrafish from https://genome.ucsc.edu/cgi-bin/hgTables. Extract the coding domain sequences (CDS) of each protein-coding gene. If multiple isoforms exist, choose the longest ones.
Label the location of UUU codons, normalize the location values by the length of CDS. By doing this, we harvested a series of normalized location values, i.e. L(1), L(2), …, for each gene, where 0 < L(n) < 1, n = 1, 2, ….
- We made the following two assumptions: (a) for a UUU codon at any location, there is a fixed chance of ribosomal frameshift designated by Pf, given a fixed level of wybutosine; (2b) for a given UUU codon located at L(n), after frameshifting, the remaining activity of a protein is proportionate to L(n). Thus, the consequence of translating a mRNA holding multiple UUU codons, i.e. the remaining activity of a protein, designated by R, can be estimated as:
For the wild-type (WT) and TYW1-knockout mice (KO), we defined the R values for WT and KO to be R(WT) and R(KO), respectively. The R value is determined by the density and distribution of codon UUU in a given mRNA, also by Pf (the chance of ribosomal frameshift at a specific location of mRNA, given a fixed level of wybutosine). We set the values of Pf for R(WT) and R(KO) to be 0.12 and 0.35, respectively.42 Subsequently, we defined the attenuation coefficient to be R(KO)/R(WT). An attenuation coefficient reflected the extent of protein activity reduction with Tyw1-knockout, and the expected values of attenuation coefficient ranged from 0 to 1. The lower the value of attenuation coefficient, the more severe the ribosomal frameshift and protein degradation when TYW1 is knocked out.
After generating a list of attenuation coefficients for each human/mouse/zebrafish protein, we focused on proteins meeting with these criteria: (1) a protein with ortholog in all three species; (2) a protein with attenuation coefficient <0.34 (i.e. 0.12/0.35); (3) a protein with its corresponding gene matching TYW1 in terms of expression profiles in normal brains and (4) a protein known to be disease-related, with records in HGMD or OMIM.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. This study’s samples and data are available to the scientific community on request.
Results
CP cohort recruitment, medical evaluation and genetic screening
A total of 120 CP families, including 118 trios and two quartets, were recruited by the Department of Rehabilitation of the Guangzhou Women and Children’s Medical Center (GWCMC) from August 2014 to June 2017 in a cohort study. This study was approved by the ethics committee of GWCMC, and written informed consents were signed by the participating families. The following inclusion criteria were adopted: (i) a diagnosis of CP made by a board of paediatric neurologists, based on early-onset non-progressive motor disability; (ii) aged 2 years and older at the time of symptom ascertainment and (iii) uneventful pregnancy and delivery (109 CP patients were born at term, six at 36 gestational weeks, two at 35 weeks, three at 34 weeks, one at 33 weeks and one at 30 weeks). The following exclusion criteria were implemented: (i) perinatal insults including potential asphyxia with an Apgar score <7, traumatic brain injury (TBI),43 postencephalitis brain lesions44 and brain tumours; (ii) late-onset hereditary spastic paraplegia (HSP) or DOPA-responsive dystonia (DRD), due to the distinct progressive trajectory and featured fluctuation of manifestation; (iii) maternal infections with a history of fever during pregnancy or (iv) hyperbilirubinemia of the newborn with high bilirubin levels as a risk for kernicterus.45,46 The classification and summary of the CP cohort are shown in Fig. 1A and B. As expected, the majority of cohort patients belonged to spastic CP, followed by dyskinetic and ataxic CP. There were less patients with more severe motor impairments (GMFCS levels IV and V) than those who were mildly disabled in terms of motion (GMFCS levels I–III). There was a significant bias for males in this CP cohort, as observed previously in ID and ASD cohorts.47 The prevalent comorbidities included ID and microcephaly. More details of the CP patients can be found in Supplementary Table 1 and Supplementary Fig. 1.
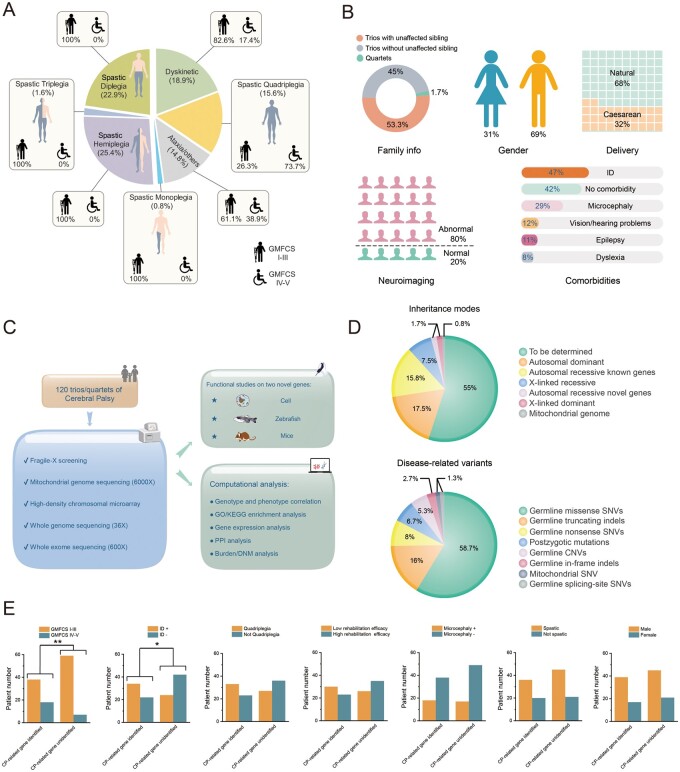
Figure 1.
CP cohort study information. (A) Classification of the cohort patients based on motor signs, anatomical distributions and motor severity. Spastic quadriplegia (19 patients, GMFCS levels I–III: 5 patients, GMFCS levels IV–V: 14 patients), dyskinetic (23 patients, GMFCS levels I–III: 19 patients, GMFCS levels IV–V: 4 patients), spastic diplegia (28 patients, GMFCS levels I–III: 28 patients, GMFCS levels IV–V: 0 patient), spastic triplegia (two patients, GMFCS levels I–III: 2 patients, GMFCS levels IV–V: 0 patient), spastic hemiplegia (31 patients, GMFCS levels I–III: 31 patients, GMFCS levels IV–V: 0 patient), spastic monoplegia (one patient, GMFCS levels I–III: 1 patient, GMFCS levels IV–V: 0 patient), ataxia and others (18 patients, GMFCS levels I–III: 11 patients, GMFCS levels IV–V: 7 patients). (B) Summary of the cohort patients in terms of family information (trios with unaffected sibling: 54 patients, trios without unaffected sibling: 64 patients, quartets: two patients), gender (male: 84 patients, female: 38 patients), delivery (natural: 81 patients, caesarean: 39 patients), neuroimaging (abnormal: 98 patients, normal: 24 patients), and comorbidities (no cormorbidity: 51 patients, ID: 57 patients, microcephaly: 35 patients, vison/hearing problems: 15 patients, epilepsy: 13 patients, dyslexia: 10 patients). (C) Project schematics, including cohort recruitment, genetic assessment, gene-oriented bioinformatics and functional analysis. (D) Inheritance modes and disease-related variants identified in this study. To be determined: 66, autosomal dominant genes: 21, autosomal recessive known genes: 19, autosomal recessive novel genes: 2, X-linked recessive: 9, X-linked dominant: 2, mitochondrial genome: 1. Germline missense SNVs: 44, germline truncating indels: 12, germline nonsense SNVs: 6, postzygotic mutations: 5, germline CNVs: 4, germline in-frame indels: 2, germline splicing-site SNV: 1, mitochondrial SNV: 1. (E) Correlations between diagnostic rates and clinical parameters. ID+: with intellectual disability. ID−: without intellectual disability. Microcephaly+: with microcephaly. Microcephaly−: without microcephaly.
As shown in Fig. 1C and Supplementary Table 2, we initiated a comprehensive genetic assessment via multiple platforms, including targeted PCR for fragile-X, mitochondrial genome sequencing (6000×), a high-density cytogenetic microarray, whole-exome sequencing (600×) and WGS (36×). In principle, we defined the disease-related variants by adopting the ACMG-recommended standards, reinforced by an in-house variation database of 2247 ethnic-matched unaffected individuals, and another database (https://db.cngb.org/cmdb/) of >50 000 individuals of East Asian descent.34,48
In sum, we identified CP-related candidate genes and variants in 54 families (∼45% of the 120 CP families), a comparable rate with that of other NDDs.16,49–52 Interestingly, the unexpected abundance of recessive variants (∼1/4) in the candidate genes, was notably higher than that of previous NDD investigations in outbred populations.50,53,54 Whether this was attributed to the specific genetic structure of the ethnic group in the present study or was due to the characteristic aetiology of CP per se, remains to be solved in future studies.55
Of the 75 CP-related variants identified in this cohort, germline point substitutions accounted for ∼68% of instances, followed by germline indels (∼18.7%) and CNVs (∼5.3%) (Fig. 1D). PZMs were, for the first time, described in a CP cohort, in which ∼6.7% of the CP-related variants were classified as PZMs. This finding met with the expectation derived from other NDD studies, and fills a gap in our understanding of CP aetiology.28
A closer look at the phenotype-genotype correlation in the patients revealed the following. Patients with more severe physical impairments (GMFCS level IV or V) had a higher chance of harbouring CP-related variants (P = 0.00613; Fisher exact test) than that of patients less disabled in movement (GMFCS levels I–III); patients with comorbidity of ID were more likely to have deleterious variants (P = 0.01065; Fisher exact test) compared with those without ID (Fig. 1E).
In this cohort, we found a total of 129 protein-changing de novo mutations (DNMs) in the CP patients, the rate of which was significantly higher than that of the in-house 2247 ethnic-matched unaffected individuals (P = 0.0083; bootstrap 1000 times). When counting private mutations of the CP families as the data input of a burden analysis, we concluded that the 146 private mutations found in the CP patients were significantly more than what we expected by counting the rate of private mutations in the in-house 2247 ethnic-matched controls (P = 0.0142; bootstrap 1000 times). Paternal ages were revealed to correlate positively with DNM counts of the patients (r = 0.63, P = 0.025; Pearson correlation test). We also detected 27 PZMs in 66 CP patients, but no significant correlation with paternal or maternal ages was established. The aforementioned statistical analyses were based on the protein-coding exons and conventional splice sites with >10× non-redundant coverage (31 856 576 bp) in all the CP families and the in-house controls.
The details of disease-related variants identified in this study are shown in Supplementary Tables 3 and 4.
Assessment of the role of de novo mutations
On aggregate, we characterized 26 CP-related de novo events in ∼1/5 of the cohort individuals, which included 18 autosomal dominant mutations (two postzygotic), two X-linked dominant mutations (one postzygotic), three de novo CNVs spanning multiple disease-related genes and one de novo mutation in the mitochondrial genome (Supplementary Figs 2 and 3).
It was noted that there was a PZM observed on a male patient’s (CP_102_1) X chromosome while the affected gene, TAF1 (OMIM: 313650), is known to be related to X-linked recessive ID. Also, interestingly, a patient (CP_098_1) had compound heterozygous variants in ATP8A2 (OMIM: 605870), albeit with one allele of postzygotic origin.
We found in two spastic diplegia patients (CP_050_1 and CP_095_1) DNMs of SPAST (OMIM: 604277), a gene frequently identified in CP patients that is related to early-onset autosomal dominant spastic paraplegia. The HSP comprises a large group of inherited neurological disorders, of which there have been multiple related genes or loci reported.56,57 When symptoms begin before the age of 2 years, the non-progressive spastic gait (toe walking) of early-onset HSP, such as SPG4 caused by defective SPAST, may closely resemble that of spastic diplegic CP, thus included in this cohort.
We identified in three CP patients (CP_039_1, CP_067_1, CP_094_1) DNMs of KIF1A (OMIM: 601255), which is known to cause autosomal dominant mental retardation and motor delay. These patients showed variable phenotypes (spastic diplegia and quadriplegia, GMFCS levels I–V). Nevertheless, all of the three patients manifested ID and severe microcephaly. Another recurrent gene in this cohort was COL4A1 (OMIM: 120130), in which we identified DNMs in two CP patients (CP_008_1 and CP_033_1). Both patients presented spastic CP, epilepsy and severe ID. In addition, one patient (CP_008_1) showed severe microcephaly and congenital blindness.
Some of the CP patients harboured disease-related variants in genes that are functionally relevant. For example, the patient CP_107_1 (dyskinetic CP with ID) had a postzygotic de novo mutation of BCL11A (OMIM: 606557), while the patient CP_096_1 [mixed-type CP (spasticity and dyskinesia) with ID] had a de novo truncating mutation of BCL11B (OMIM: 606558). BCL11A and BCL11B encode zinc-finger proteins that regulate transcription, highly expressed in brain and bone marrow, and were reported to be related to autosomal dominant developmental disorders. A similar scenario occurred in two CP patients (CP_055_1 and CP_061_1), where the former (spastic hemiplegia with severe ID and microcephaly) had a de novo missense mutation of TUBA1A (OMIM: 602529), and the latter (spastic hemiplegia with mild motor impairment [GMFCS level I]) had a postzygotic de novo mutation of TUBB2B (OMIM: 612850). TUBA1A and TUBB2B code for brain-enriched tubulin components as subunits of microtubules, and are known to be associated with autosomal dominant brain malformations.
Identification of variants involved in recessive disease
Altogether, we confirmed recessive-disease-related variants in 30 CP families (1/4 of the 120 cohort families), including 21 families with autosomal recessive disease, eight families with X-linked recessive disease, and one CNV located at Xq28 of a male patient (CP_119_1) inherited from the unaffected mother, where a variety of CNVs have been reported previously in patients with neuropsychiatric disorders and, in some cases, with gender bias.58,59
We noticed that, among the 21 CP families with autosomal recessive disease, the zygotic ratio of compound heterozygosity versus homozygosity was 3:1, which was acceptable in a largely outbred country. Interestingly, among the six homozygous variants, only one was found to be autozygous via CMA-based genotyping and family history consulting (CP_036_1, second-cousin consanguinity). This observation may indicate the existence of CP-related hotspot variants in a specific population, although other explanations exist and this hypothesis requires further evidence.
Defects of RARS2 (OMIM: 611524), a gene coding for mitochondrial arginyl-tRNA synthetase, led to manifestation in two patients (CP_007_1 and CP_110_1). The former patient had severe spastic quadriplegia with ID, epilepsy, strabismus and amblyopia, probably caused by the highly pathogenic compound heterozygous variants of RARS2; whereas the latter patient, with mild dyskinetic CP, moderate ID and hearing problem, was probably associated with a homozygous missense variant that may be less damaging.
The patient CP_105_1 presented ataxic CP with mild ID and microcephaly. A homozygous truncating variant was found in the gene of SQSTM1 (OMIM: 601530), encoding a ubiquitin-binding protein. According to a recent report, four families with autosomal recessive childhood-onset neurodegenerative disorders were found to have detrimental variants in SQSTM1.60 However, the onset age in our case was much younger (in the first year since birth) compared with that of the previous report (onset between 7–15 years of age); therefore, this finding expands the phenotype spectrum related to the gene of SQSTM1.
TARS (OMIM: 187790) codes for a threonyl-tRNA synthetase and plays dual roles both as an assembly scaffold of translation initiation components and as a target mRNA selector.61 A very recent report introduced two individuals carrying TARS variants with trichothiodystrophy,62 while we found a spastic hemiplagia patient (CP_014_1) with biallelic TARS variants, who presented an overlapping phenotype including developmental delay, but without a manifestation in the hair or skin (Supplementary Fig. 4).
PTK7 (OMIM: 601890) encodes a tyrosine kinase of the Wnt-signalling pathway, and altered PTK7 activity in mouse models induces perturbation of neural tube development.63 Recent studies have found an association of PTK7 variants with neural tube defects and foetal anomaly in humans.64,65 We found that the female patient CP_052_1 had compound heterozygous deleterious variants of this gene, and the patient showed spastic diplegia with mild motion impairment (GMFCS level I) without other comorbidities reported, although a possibility of spina bifida still remained due to the absence of spinal cord MRI (Supplementary Fig. 5).
Another two novel candidate genes, namely, TYW1 (OMIM: 611243) and GPAM (OMIM: 602395), were identified in two CP families with distinct phenotypes (CP_012 and CP_063, respectively). The former family had multiple patients, manifesting both CP and ID, while the latter family had a patient with CP but without ID. Because this dichotomous manifestation epitomized the two major subgroups of CP (with or without ID), we carried out detailed functional studies and present the results in the subsequent parts of this paper.
Common features of CP-related genes
Combining CP-related genes from our present cohort and from previous studies, a total of 114 CP-related genes have now been identified up till now (Supplementary Table 5). This number is far less than the 700+ genes involved in ID and related NDDs.47 About half of these CP-related genes are defined to be ID-related genes, and this is not unexpected since CP is on a diagnostic continuum with ID, although CP and ID are regarded as distinct disorders (Fig. 2A). The inheritance modes of the 114 CP-related genes included autosomal dominant (43.9%), autosomal recessive (41.2%), X-linked recessive (12.3%) and X-linked dominant (2.6%). Compared with those of ID-related genes, CP-related genes significantly lack autosomal recessive genes but have more autosomal dominant genes (P = 0.0006; Fisher exact test) (Fig. 2A). We thus speculate that there is a great chance for novel autosomal recessive CP-related genes to be identified in future studies.
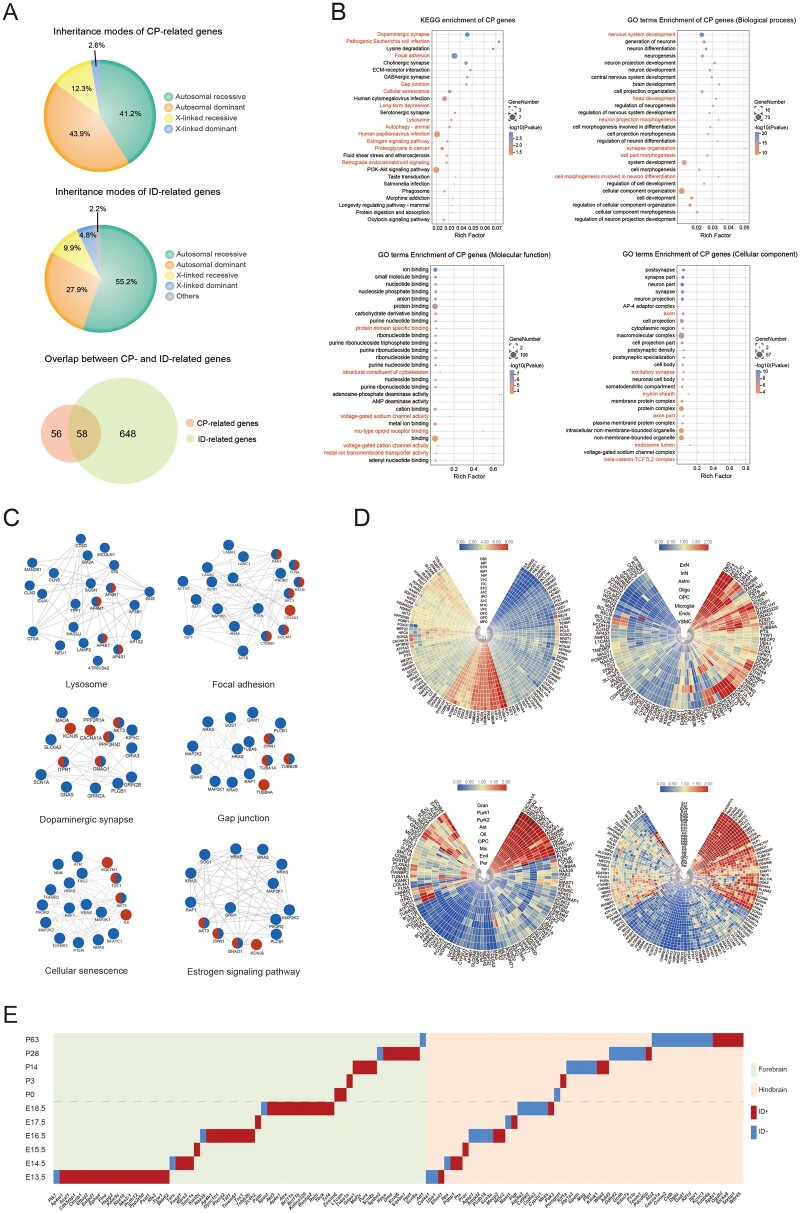
Figure 2.
CP-related gene summary. (A) Inheritance-mode comparisons between CP-related genes and ID-related genes. The data of CP-related genes were from this study and a collection of studies from the literature. The data of ID-related genes were retrieved from the literature.47 Inheritance modes of 114 CP-related genes included 47 autosomal recessive, 50 autosomal dominant, 14 X-linked recessive and three X-linked dominant. Inheritance modes of 706 ID-related genes included 390 autosomal recessive, 197 autosomal dominant, 70 X-linked recessive, 33 X-linked dominant and 16 others (AD/AR, XLD/XLR). (B) Enriched KEGG pathways and GO terms of the CP-related genes. Upper left: enriched KEGG pathways. Upper right: enriched GO terms of Biological Process. Bottom left: enriched GO terms of molecular function. Bottom right: enriched GO terms of Cellular Component. The red colour indicates pathways and GO terms that are also enriched in the ID-related genes. (C) Protein–protein interaction network modules linking CP-related genes (red), ID-related genes (blue) and shared genes (half red, half blue). (D) Expression of CP-related genes in different human brain regions and cell types. Upper left: expression heatmap in 16 human brain regions.127 MFC: medial prefrontal cortex. OFC: orbital prefrontal cortex. DFC: dorsolateral prefrontal cortex. VFC: ventrolateral prefrontal cortex. M1C: primary motor cortex. S1C: somatosensory cortex. IPC: posterior inferior parietal cortex. A1C: primary auditory cortex. STC: superior temporal cortex. ITC: inferior temporal cortex. V1C: primary visual cortex. HIP: hippocampus. AMY: amygdala. STR: striatum. MD: mediodorsal nucleus of thalamus. CBC: cerebellar cortex. Upper right: expression heatmap in eight cell types of human dorsolateral prefrontal cortex.127 ExN: excitatory neuron. InN: interneuron. Astro: astrocyte. Oligo: oligodendrocyte. OPC: oligodendrocyte progenitor cell. Endo: endothelial cell. VSMC: vascular smooth muscle cell. Bottom left: expression heatmap in nine cell types of human cerebellum.128 Gran: cerebellar granule cell. Purk1: Purkinje neuron, subtype 1. Purk2: Purkinje neuron, subtype 2. Ast: astrocyte. Oli: oligodendrocyte. OPC: oligodendrocyte precursor cell. Mic: microglia. End: endothelial cell. Per: pericyte. Bottom right: expression heatmap in 24 cell types of human frontal cortex.128 Ex1: excitatory neuron, subtype 1. Ex2: excitatory neuron, subtype 2. Ex3e: excitatory neuron, subtype 3e. Ex4: excitatory neuron, subtype 4. Ex5b: excitatory neuron, subtype 5 b. Ex6a: excitatory neuron, subtype 6a. Ex6b: excitatory neuron, subtype 6 b. Ex8: excitatory neuron, subtype 8. In1a: inhibitory neuron, subtype 1a. In1b: inhibitory neuron, subtype 1 b. In1c: inhibitory neuron, subtype 1c. In3: inhibitory neuron, subtype 3. In4a: inhibitory neuron, subtype 4a. In4b: inhibitory neuron, subtype 4 b. In6a: inhibitory neuron, subtype 6a. In6b: inhibitory neuron, subtype 6 b. In7: inhibitory neuron, subtype 7. In8: inhibitory neuron, subtype 8. Ast: astrocyte. Oli: oligodendrocyte. OPC: oligodendrocyte precursor cell. Mic: microglia. End: endothelial cell. Per: pericyte. (E) Expression climax of CP-related genes in mice, with regard to brain regions (forebrain and hindbrain), developmental stages (11 time points before and after birth), and comorbidity (with or without ID). x-coordinate: CP-related genes; y-coordinate: embryonic and postnatal days. Gene expression data were retrieved from https://www.ebi.ac.uk/gxa/home.
We found that the 114 CP-related genes were enriched in a variety of pathways and protein–protein interaction modules, involving multiple aspects of brain development and functioning (Fig. 2B). We noticed that there were at least six functional modules that reflected the essential and coordinated roles of CP-related genes in living organisms (i.e. lysosomes, gap junctions, dopaminergic synapses, focal adhesions, cellular senescence and oestrogen signalling modules) (Fig. 2C). Oestrogen signalling pathways regulate a plethora of physiological processes in mammals, including cellular homeostasis and behaviour. Given the significant gender bias in populations of CP, ID and ASD, it may be worthwhile to explore the involvement of oestrogen signalling pathways in the aetiology of NDDs. Furthermore, we identified the pathogen-related modules (pathogenic Escherichiacoli infection, human cytomegalovirus infection, and human papillomavirus infection) enriched in CP/ID/NDD genes, comprising ∼11% of the 114 CP-related genes, which occurred in 4% of CP cohort patients, such as TUBA1A, TUBB2B, CTNNB1 and COL4A1. Although it is well known that CP and ID can be sequelae of intracranial pathogenic infections, whether the variants of these genes increase relevant disease risk remains unclear.
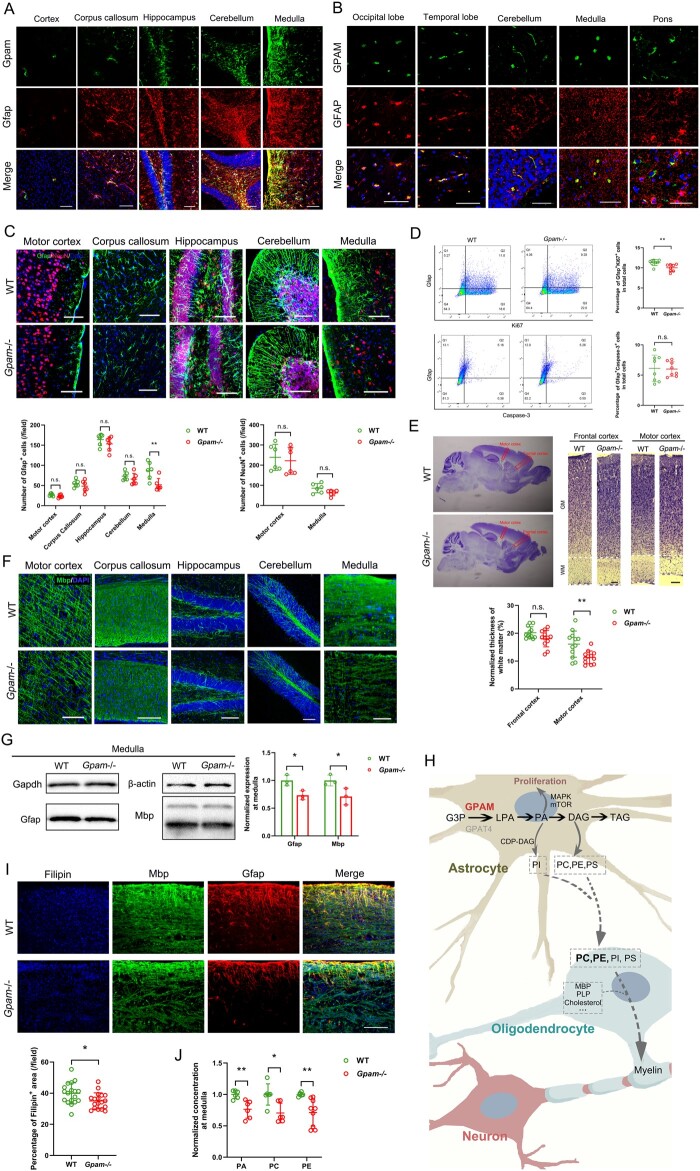
When data-mining in the public databases and literature of gene expression profiles in the brain, we were aware that CP-related genes are expressed in a variety of anatomic structures, including the prefrontal cortex, motor cortex, cerebellar cortex, hippocampus, striatum and somatosensory cortex; in addition, these CP-related genes are expressed in a variety of brain cells, including excitatory neurons, inhibitory neurons, interneurons, astrocytes, oligodendrocytes, cerebellar granule cells, Purkinje neurons and microglia (Fig. 2D). The top three brain regions for CP-related genes were cerebellum, primary motor cortex and striatum; the top three cell types were Purkinje cells, astrocytes and oligodendrocytes. For example, TYW1 showed enriched expression in neurons within each brain region, but not in pericytes or microglia. GPAM showed exclusively high expression in astrocytes, whereas it was untraceable in other brain cells. PTK7 showed higher expression in pericytes compared with that of other cell types, indicating a role in capillary formation. These specific expression patterns may serve as useful guidelines for further mechanistic studies on these genes.
CP-related genes reached their highest expression levels in different brain regions (forebrain, 57%; hindbrain, 43%) and at sequential developmental stages (embryonic, 58%; postnatal 42%) (Fig. 2E). Interestingly, we noticed that CP-related genes could be roughly divided into two subgroups with distinctive features, namely, forebrain-expressed genes and hindbrain-expressed genes. The former genes, such as TYW1, were highly expressed in the forebrain, including the motor cortex and striatum; furthermore, they had a significantly higher chance to have comorbidity of ID than that of the latter ones, such as GPAM, which were specifically expressed in the hindbrain, including the brainstem and cerebellum (P = 0.002; Fisher exact test). Another observation was that forebrain-expressed CP-related genes preferentially had their highest expression levels during embryonic periods, while those of hindbrain-expressed CP-related genes occurred throughout embryonic and postnatal periods, but with an inclination for postnatal days (P = 0.05; Fisher exact test). Hence, these results suggest that forebrain-expressed genes may participate in neurogenesis that is generally completed before birth, while hindbrain-expressed genes may take part in myelination that is not complete until 2 years after birth in humans.66,67 In the next subsection, we illustrate this dichotomous classification system by delving into two typical CP-related genes newly identified in this study, namely TYW1 and GPAM.
Null and hypomorphic alleles of TYW1 cause primary microcephaly and impairment in motion and cognition
The male proband CP_012_1, an ataxic CP patient, was the second of two children of a non-consanguineous, healthy Chinese couple with an unremarkable family history. He was born by normal delivery at 40 weeks of gestational age after an uneventful pregnancy with a birth weight of 3.8 kg (+1.17 SD). At conception, the mother was 31 years old and the father was 34 years old. By clinical examination at an age of 6 years, the patient’s height, body weight and head circumference were 103 cm (−3.20 SD), 16 kg (−2.06 SD) and 47.5 cm (−3.26 SD), respectively. At an age of 8 years and 7 months, the cranial MRI of the patient showed a slightly widened cerebral subarachnoid space, enlarged ventricle and prominence of cerebellar sulci. Cognitive evaluation (Wechsler Preschool and Primary Scale of Intelligence) was performed at an age of 7 years and 6 months, with a full-scale IQ of 40, indicating a moderate ID. The level of GMFCS was III, indicating that the patient could walk using a hand-held mobility device. His fine motor skills were poor; the MACS was evaluated at the level of IV (i.e. handles a limited selection of easily managed objects in adapted situations). Another patient CP_012_2, a 3-year-older sister of the proband, was diagnosed with mixed-type CP (spasticity and dyskinesia). The mother had a normal delivery after 40 weeks of uneventful gestation, and the birth weight of the patient was 3.2 kg (−0.02 SD). The parameters of clinical examination at an age of 14 years of the patient were as follows: height of 150 cm (−1.51 SD), weight of 41 kg (−1.03 SD) and head circumference of 49 cm (−3.64 SD). Cognitive evaluation (Estimated Cognitive Level of Children with CP) was performed at an age of 13 years and 1 month with an IQ of <50, indicating a moderate ID. The level of the GMFCS was IV, meaning self-mobility with limitations and needing powered mobility. Her fine motor skills were poor; the MACS was at level III (i.e. handles objects with difficulty, needs help to prepare and/or modify activities). The medical details of the two patients are presented in Fig. 3A and Supplementary Tables 1 and 6.
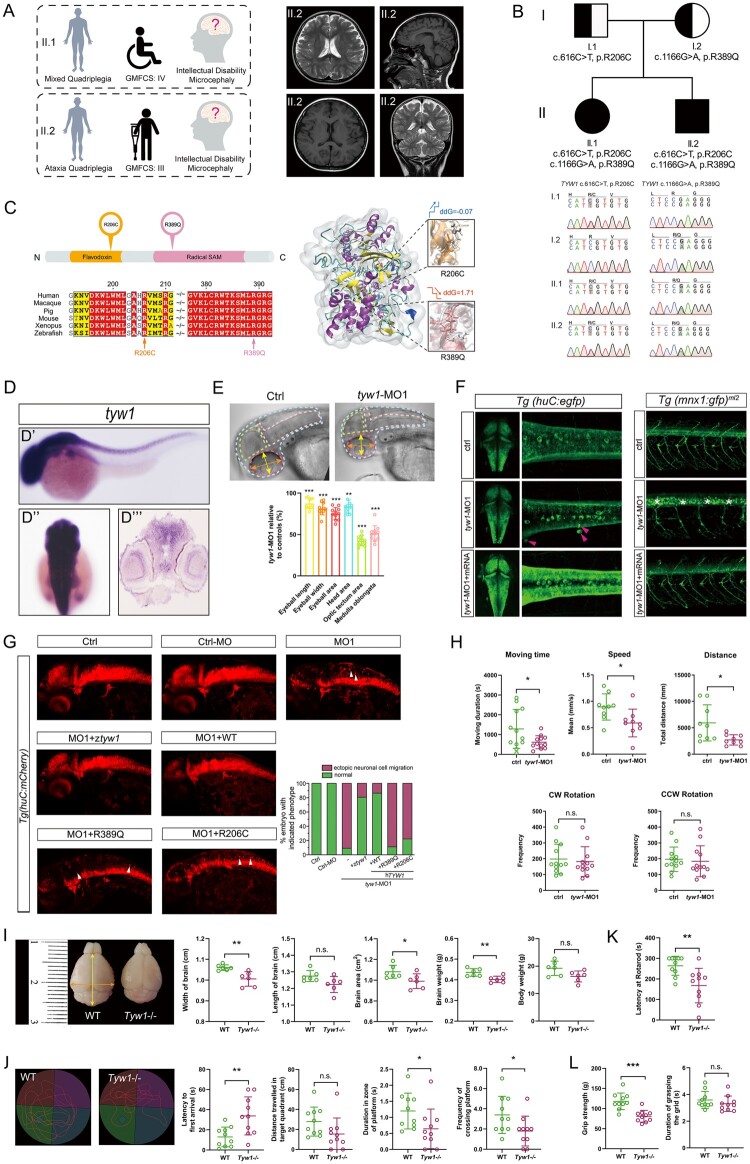
Figure 3.
Correlation between the phenotype and genotype of TYW1. (A) Clinical summary of the two index patients harbouring TYW1 variants, and the cranial MRI of the patient II.2 (CP_012_1). (B) Compound heterozygous variants of TYW1 were identified in the two index patients, which cosegregated with the phenotype in the family members. (C) The two identified amino-acid changes (i.e. R206C and R389Q) were located inside the protein domains of flavodoxin and radical SAM, respectively. The two changes took over the highly conserved amnio-acid positions throughout a series of species. A three-dimensional structural model of TYW1 protein displayed the stability alterations (ddG, Gibbs free energy) and local structural changes, before and after the introduction of two amino-acid changes. In the two zoomed-in views, the white bars denote wild-type amino acids and the colour bars denote mutated amino acids. (D–H) Morphological and behavioural assays in zebrafish models. (D) WISH of zebrafish embryos at 36 hours postfertilization (hpf) using a zebrafish antisense probe of tyw1 showing specific expression of tyw1 in the central nervous system, including the brain and spinal cord. D’: lateral view. D’’: dorsal view. D’’’: cross section. hpf: hours postfertilization. (E) Head area, optic tectum area, medulla oblongata area, eyeball area, eyeball length and eyeball width of the tyw1 knockdown (tyw1-MO1, a splice-blocking morpholino) zebrafish larvae at 5 dpf were significantly decreased compared with those of the controls. n = 10 for each test group. ** P < 0.01, *** P < 0.001, unpaired t-test. dpf: days postfertilization. (F) Confocal imaging of egfp positive cells in transgenic zebrafish Tg(huC: egfp) embryos at 48 hpf showing ectopic neuronal cells Figure 3: Continued (indicated by red arrowheads) in the tyw1-MO1 zebrafish group, which could be rescued by the wild-type tyw1 mRNA. Confocal imaging of gfp positive cells in the transgenic zebrafish Tg(mnx1: gfp)ml2 embryos at 72 hpf showed undifferentiated motor neuronal cells (indicated by white stars) in the tyw1-MO1 zebrafish group, which could be rescued by wild-type tyw1 mRNA. (G) The tyw1 knockdown (tyw1-MO1) brought about ectopic neuronal cells in the zebrafish Tg(huC: mCherry) embryos at 48 hpf, and there were only 9% of participants that remained normal. This abnormal phenotype could be rescued by zebrafish tyw1 mRNA (ztyw1), human TYW1 mRNA (WT), mutated human TYW1 leading to R389Q or mutated human TYW1 leading to R206C, to variable extents (81, 86, 11 and 22%, respectively). Ectopic neuronal cells are indicated by white arrowheads. The number of fish used ranged from 63 to 88 in the different test groups. (H) Swimming behaviour tests of tyw1-MO1 zebrafish larvae at 5 dpf compared with the controls, in terms of moving time, speed, distance, clockwise (CW) rotation and counterclockwise (CCW) rotation. The former three parameters revealed significant reductions in the tyw1-MO1 group compared with those in the control group, while the latter two parameters showed no significant change. n = 9 to 12 for each test group. * P < 0.05, n.s. = no significant difference, unpaired t-test. (I–L) Morphological and behavioural assays on Tyw1 knockout (Tyw1−/−) mouse models. (I) The Tyw1−/− mice at postnatal 8 weeks showed significantly reduced brain size and weight compared with those of wild-type (WT) mice. The orange arrow, yellow arrow and red-dashed lines indicate the width, length and area of brain, respectively. In parallel, measurements of body weight revealed no significant difference between the Tyw1−/− mice and the WT mice. N = 6 per genotype with equal numbers of male and female mice. * P < 0.05, ** P < 0.01, n.s.: no significant difference, unpaired t-test. (J) Track plots depicting the traces of mice during the probe test in the Morris water maze. The Tyw1−/− mice showed worse performance compared with that of WT mice in the test. n = 10 per genotype with equal numbers of male and female mice. * P < 0.05, ** P < 0.01, n.s.: no significant difference, unpaired t-test. (K) Rotarod tests showed significantly reduced motor coordination and balance of the Tyw1−/− mice compared with those of WT mice. n = 10 per genotype with equal numbers of male and female mice. ** P < 0.01, unpaired t-test. (L) Grip strength tests revealed significantly weaker grip strength of the Tyw1−/− mice compared with that of the WT mice. n = 10 per genotype with equal numbers of male and female mice. *** P < 0.001, n.s.: no significant difference, unpaired t-test.
In each of the two patients, we identified compound heterozygous variants in TYW1 (OMIM: 611243), which were cosegregating with the phenotype in the family and were confirmed by targeted PCR and Sanger sequencing (Fig. 3B). The pRecessive value of TYW1 (http://exac.broadinstitute.org/) was 0.9945, which was a strong indicator of a recessive-disease-related gene. The two variants (NM_018264: c.616C>T, p. R206C; c.1166G>A, p. R389Q) were absent from an in-house database with 2247 healthy controls, and were very rare in the public databases (http://exac.broadinstitute.org/; https://db.cngb.org/cmdb/) with allele frequencies below 1 × 10−5. Pathogenicity prediction classified the two variants as damaging or disease-causing (Supplementary Table 4). Multiple protein-sequence alignments among different species showed high conservation of both positions, i.e. R206 and R389, which were located in the functional domains (Fig. 3C). Protein structural modelling revealed the functional locations of both amino acids, and showed reduced protein stability (R389Q) and disturbed substrate binding (R206C) (Fig. 3C). Compatible with the prediction, we found a dramatically lower protein level of TYW1 in the patient’s peripheral blood sample, as compared with those of the parents and healthy children of the same age and gender (Supplementary Fig. 6).
Using WISH in zebrafish, we found that tyw1 expression was highly enriched in the developing central nervous system, including the brain and spinal cord (Fig. 3D). Morpholino-mediated tyw1 knockdown (tyw1-MO) zebrafish models were established by blocking mRNA splicing (MO1) and protein translation (MO2) of tyw1 (Supplementary Figs 7–9). We observed significant head size reduction in the tyw1-MO zebrafish compared with that of WT zebrafish (Fig. 3E). It was revealed that tyw1 deficiency in zebrafish resulted in ectopic neuronal cell migration in the brain and undifferentiated motor neuronal cells in the spinal cord (Fig. 3F and Supplementary Fig. 9). This phenotypic anomaly was effectively rescued by zebrafish tyw1 mRNA or human TYW1 mRNA, but not by TYW1 mRNAs with variants identified in the CP patients (Fig. 3G). This result substantiated the pathogenicity of the two variants identified in these patients. By performing swimming behaviour tests, we noticed that the swimming capacity of tyw1-MO zebrafish larvae was compromised significantly, specifically in terms of swimming speed, time and distance, but not in rotation (Fig. 3H).
By using CRISPR–Cas9 genome editing technology, we constructed a Tyw1-knockout mouse model (Supplementary Fig. 10). In the Tyw1−/− mice, the brain size and weight were significantly below the level of those of WT littermates, with abnormal intracranial morphology (Fig. 3I and Supplementary Fig. 11). All behavioural tests (i.e. the Morris water maze, rotarod test, and grip strength test) revealed significantly reduced performances of the Tyw1−/− group compared with those of the WT controls (Fig. 3J–L). The deterioration of both motion and cognition was compatible with the manifestation of the CP patients with hypomorphic TYW1 alleles, and reflected the underlying cerebral regions responsible for both functions.
Defective Tyw1 hinders neuronal proliferation and migration due to an increased ribosomal frameshift in a subset of cell-cycling-related proteins
The gene of TYW1 (OMIM: 611243) encodes a tRNA-wybutosine (tRNA-yW) synthesizing protein, localized on the cytosolic surface of the endoplasmic reticulum and which is responsible for producing a hypermodified guanosine (i.e. wybutosine) at position 37 of phenylalanine tRNA (tRNAPhe) adjacent to the 3ʹ of the anticodon in archaea and eukaryotes.40,41 This hypermodification at position 37 of tRNAPhe is known to play a critical role in stabilizing the appropriate interactions between codons and anticodons during protein translation, an absence of which promotes a ribosomal frameshift, thus reducing translational accuracy.68–71 Interestingly, although two codons exist for phenylalanine, i.e. UUU and UUC, it appears that wybutosine exerts an influence only on the UUU codon.42
The expression of TYW1 is enriched in neurons, especially during the embryonic development (Fig. 2D and E; Fig. 4A and B). We observed significantly decreased neurogenesis in the E13.5 brains of Tyw1−/− mice compared with those of WTs (Fig. 4C). In Tyw1−/− mouse brains, the migration of cortical neuronal precursors between E15.5 and E18.5 was hindered, resulting in an obvious reduction of superficial-layer neurons and a noticeable accumulation of deep-layer neurons (Fig. 4D). Consequently, in both the frontal cortex and motor cortex of adult mouse brains, the thickness of superficial layers (layers II–III) was significantly less in the Tyw1−/− group compared with that of WTs, while there was a non-significant reverse trend in the deep layer (layer VI) (Fig. 4E). The simultaneous abnormalities in both frontal and motor cortices in this mouse model provide mechanistic insights into the CP-plus-ID phenotype of patients with hypomorphic TYW1 alleles in the present study.
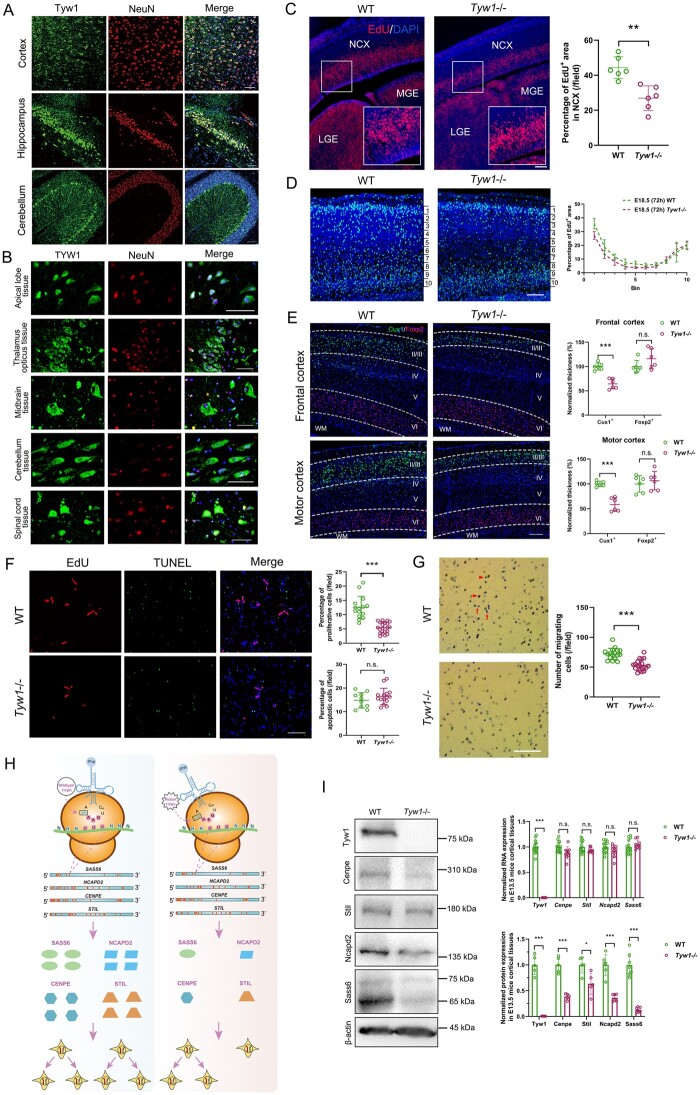
Figure 4.
Functional mechanism of defective TYW1. (A) Brain-section immunostaining of wild-type mice at postnatal 8 weeks, where Tyw1 (green) and NeuN (red) are colabelled, showing Tyw1 expression in neurons within the cortex, hippocampus and cerebellum. Blue: DAPI. Scale bar = 75 μm. (B) Immunostaining of normal human brain tissue microarrays, where TYW1 (green) and NeuN (red) are colabelled, revealing TYW1 expression in neurons within a variety of brain regions. Blue: DAPI. Scale bar = 50 μm. (C) EdU was intraperitoneally injected at 2 h into pregnant mice before euthanizing them to harvest embryos at E13.5. Cells incorporated with EdU were determined in the embryonic brain sections under fluorescent microscopy and were analysed with ImageJ software. Compared with those of WT littermates, significantly reduced percentages of EdU+ areas were observed in the neocortex (NCX) of Tyw1−/− embryonic brains, as well as in the medial ganglionic eminence (MGE) and lateral ganglionic eminence (LGE). Red: EdU. Blue: DAPI. n = 3 per genotype. For a specific sample, each measurement was performed twice in separate fields. ** P < 0.01, unpaired t-test. Scale bar = 100 μm. (D) EdU was injected into the pregnant mice carrying embryos at E15.5, which were observed after 72 h. The embryonic cortex was divided into 10 equally spaced bins along the vertical axis. The percentage of EdU+ area in each bin was measured. In Tyw1−/− mouse cortices, there were less EdU+ cells in superficial layers (layers II–III) than those in WT mice, while the number of EdU+ cells in the deep layer (layer VI) was greater than that in WT mice. Light blue: EdU. Blue: DAPI. n = 3 per genotype. Scale bar = 100 μm. (E) Brain-section immunostaining of mice at postnatal 8 weeks, where Cux1+ and Foxp2+ cells were labelled for neurons in superficial layers (layers II–III) and the deep layer (layer VI), respectively. Compared with those of WT mice, Tyw1−/− mouse brains showed a significantly reduced thickness of superficial layers at both frontal and motor cortices, while in the deep layers, the reverse phenomenon occurred, albeit without statistical significance. WM: white matter. Green: Cux1. Red: Foxp2. Blue: DAPI. n = 3 per genotype. For a specific sample, each measurement was performed twice in separate fields. *** P < 0.001, n.s.= no Figure 4: Continued significant difference, unpaired t-test. Scale bar = 200 μm. (F–G) Proliferation, apoptosis and migration analysis of primary neurons from the cortices of E13.5 mice. (F) Quantification of the proliferative and apoptotic primary neurons from E13.5 mouse brain cortices. The primary neurons from the Tyw1−/− mice showed significantly decreased proliferation (as indicated by EdU staining), while the intensity of apoptosis did not change significantly (as determined by TUNEL staining). *** P < 0.001, n.s.: no significant difference, unpaired t-test. Scale bar = 100 μm. (G) Quantification of migrating primary neurons from the E13.5 mouse brain cortex. In the transwell assay, the primary neurons from the Tyw1−/− mice showed significantly decreased migration. Red arrows indicate cells that already migrated through pores, while arrowheads indicate cells that were migrating in pores. *** P < 0.001, n.s. = no significant difference, unpaired t-test. Scale bar = 250 μm. (H) Schematics of the mechanism of ribosomal frameshift under the conditions of wild-type and mutant alleles of TYW1. GAA is the anticodon of tRNAPhe for phenylalanine, and UUU is the codon of mRNA for phenylalanine. In the four mRNAs, with normalized lengths, of SASS6, NCAPD2, CENPE and STIL, the positions of UUU codons are indicated by red bars. The introduction of mutated TYW1 led to promotion of a ribosomal frameshift at the interaction between tRNAPhe and mRNAs, resulting in the reduced production of a subset of proteins involved in cell cycling. (I) Measurements of mRNA and protein levels in the E13.5 mouse brain cortex. In the Tyw1−/− group, the mRNA levels of Cenpe, Stil, Ncapd2 and Sass6 did not change significantly compared with those in the WT group. However, the protein levels of Cenpe, Stil, Ncapd2 and Sass6 showed significant reductions compared with those in the WT group, with remaining levels of 0.38, 0.63, 0.36 and 0.14, respectively. β-actin was used as the internal control for both RNA and protein measurements. Neither RNA nor protein of Tyw1 could be detected in the Tyw1−/− group. MW (Tyw1) = 84 kDa. MW (Cenpe) =312 kDa. MW (Stil) =143 kDa. MW (Ncapd2) =157 kDa. MW (Sass6) =74 kDa and 65 kDa. MW (β-actin) = 43 kDa. Quantitative PCR with reverse transcription and western blotting data were from three independent experiments. * P < 0.05, *** P < 0.001, unpaired t-test.
Primary neurons extracted from E13.5 mouse brain cortices showed significantly decreased proliferation in the Tyw1−/− group compared with that in the WT group, while the apoptotic intensity showed no obvious increase in the Tyw1−/− group (Fig. 4F). Migration tests of primary neurons revealed a reduced migratory capacity of the Tyw1−/− group compared with that of the WT group (Fig. 4G). In parallel, we evaluated the performance of SH-SY5Y cells after introducing a TYW1 knockout (Supplementary Fig. 12). Interestingly, as observed in the primary cortical neurons from mice, TYW1-knockout SH-SY5Y cells presented significantly reduced abilities of proliferation, adhesion and migration, compared with those of WT cells. However, there was no observable change in neuron differentiation (Supplementary Fig. 13).
To explore the mechanism of epigenetic regulation by TYW1, we constructed a model to simulate how the levels of wybutosine affected the ribosomal frameshift (see the Materials and methods). We defined a parameter, namely, the attenuation coefficient, for each protein to depict the vulnerability of a specific protein-coding gene to the reduced level of wybutosine. Typically, if the mRNA sequence of a specific gene does not contain a UUU codon, this gene will not be affected by the ribosomal frameshift related to the wybutosine level. On the contrary, if the mRNA sequence of a gene holds many UUU codons, its protein translation will be sensitive to the wybutosine level, with the extent determined by the density and distribution of UUU codons. This algorithm thus generated a protein list with attenuation coefficients lower than expectation, due to a malfunctioning TYW1. Subsequently, we filtered the list by two criteria (Supplementary Table 7). First, since we observed microcephaly and neurological manifestation in humans, mice and zebrafish, we chose proteins with low attenuation coefficients in all three species. Second, we focused on proteins that are known to be disease-related and that bear resemblance to TYW1 in terms of gene expression patterns, especially in developing brains (Supplementary Fig. 14). In this way, four candidate proteins emerged, namely CENPE (OMIM: 117143), STIL (OMIM: 181590), NCAPD2 (OMIM: 615638) and SASS6 (OMIM: 609321) (Fig. 4H). Measurements in E13.5 mouse brain samples showed significantly reduced levels of these four proteins in the Tyw1−/− group compared with those in the WT group, while the corresponding RNA levels did not change significantly (Fig. 4I). Therefore, it is reasonable that the defective Tyw1 hindered the production of these four proteins in the brain, with an ensuing reduction of neuronal proliferation and migration. Interestingly, these four proteins are all involved in cell cycling and mitosis, the defects of which have been reported to be related to primary microcephaly. STIL and SASS6 are necessary for centriole duplication during the cell cycle.72–77 CENPE is required for spindle microtubule capture and attachment at the kinetochore during cell division.78–80 NCAPD2 is a component of the condensin multiprotein complex that participates in mitotic chromosomal condensation.81,82
Null and hypomorphic alleles of GPAM induce drastically reduced motor ability with unaffected cognition
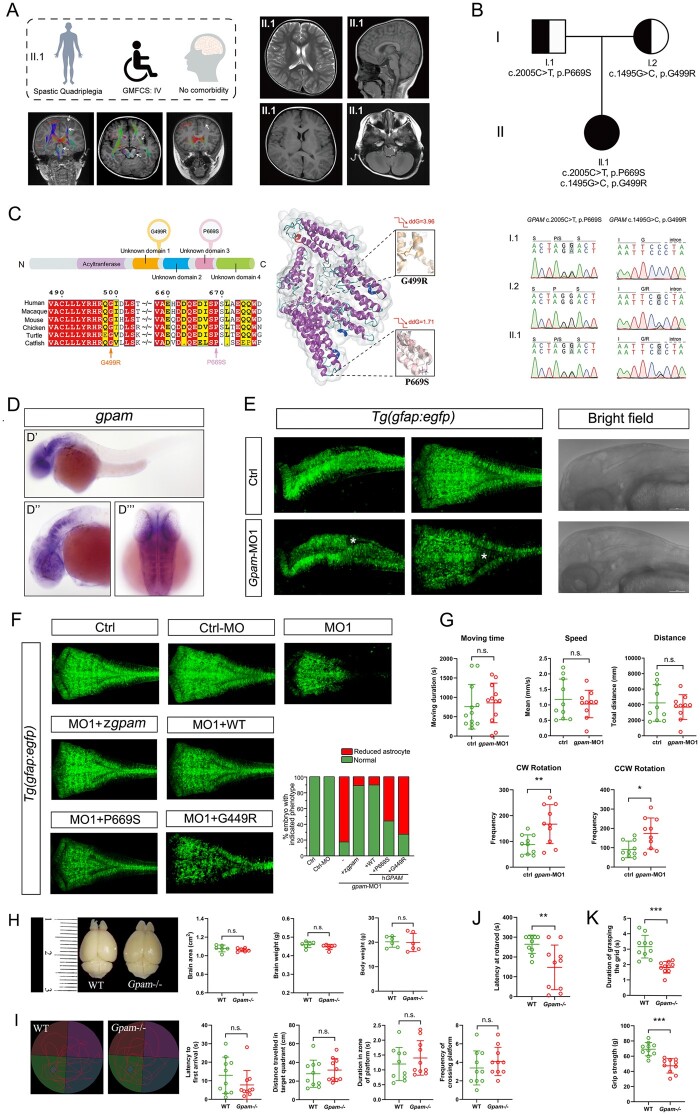
The female proband CP_063_1, a spastic quadriplegic CP patient, was the only child of unrelated healthy parents without a relevant family history. She was born by caesarean section at 39 gestational weeks when her mother was 30 years old and father was 32 years old. Her birth weight was 3.05 kg (−0.44 SD). The clinical examination at an age of 2 years and 4 months showed a body height of 83 cm (−2.45 SD), weight of 10 kg (−1.7 SD) and head circumference of 47 cm (−1.31 SD). The cranial neuroimaging taken at 4 years old revealed no apparent anatomical abnormality except for plagiocephaly, which was due to the elongated period of remaining in a supine position after birth. However, there was a reduction of white-matter fibre tracts, especially in the corticospinal tract. The level of GMFCS was IV, meaning ‘self-mobility with limitations and may use powered mobility’. Her fine motor skills were poor, as the MACS was evaluated at a level of III (i.e. handles objects with difficulty, need help to prepare and/or modify activities). The cognitive evaluation (Wechsler Preschool and Primary Scale of Intelligence) was performed at an age of three years and seven months, revealing a full-scale IQ of 116. This patient epitomized a major subgroup, comprising half of the CP population, who were impaired in motion albeit normal in intelligence. The medical information of this patient is presented in Fig. 5A and Supplementary Tables 1 and 6.
Figure 5.
Correlation between the phenotype and genotype of GPAM. (A) Brief medical description of the index patient II.1 (CP_063_1) harbouring GPAM variants, and the results of MRI and diffusion tensor imaging (DTI). No gross anatomical abnormality was observed except for plagiocephaly. In comparison with features of the DTI scan of a normal brain, there were relatively fewer white-matter fibre bundles observed in the patient brain, especially the left hemisphere. The most obvious change was at the left corticospinal tract, left front limb of the internal capsule and left white-matter junction bundle. Meanwhile, the FA values (including forward/backward, left/right and up/down directions) were lower on the left side than on the right side. These DTI findings were consistent with the clinical manifestations of the patient, such as high muscle tone of the limbs, hyperextension of the knee, scissoring gait and mirror movement of the hands. Furthermore, the right limb function of the patient was worse than that of the left. Red represents fibres from left to right; green represents fibres from front to back; blue represents fibres from top to bottom. The three images represent the distributions of fibres in different layers of the child with CP. Deterioration of the corticospinal tract is indicated by white arrows. (B) Compound heterozygous variants of GPAM were identified in the index patient, which cosegregated with the phenotype in the family members. (C) The two identified amino-acid changes (i.e. G499R and P669S) were both located inside the conserved protein domains. The two changes took over the amino-acid positions that were highly conserved among different species. A three-dimensional structural model of the GPAM protein displayed the reduced protein stability (ddG, Gibbs free energy), as well local structural alterations before and after the introduction of two amino-acid changes. In the two zoomed-in views, white bars denote wild-type amino acids and coloured bars denote mutated amino acids. (D–G) Morphological and behavioural assays in zebrafish models. (D) WISH of zebrafish embryos at 36 hpf by using a zebrafish antisense gpam probe showing specific expression Figure 5: Continued of gpam in the brain, especially in the hindbrain. D’: lateral view. D’’: lateral view with magnification of head region. D’’’: dorsal view. hpf: hours postfertilization. (E) Confocal imaging of transgenic zebrafish Tg(gfap: egfp) embryos at 48 hpf showing a reduced number of gfap+ cells in the gpam knockdown group (gpam-MO1, a splicing-blocking morpholino), particularly in the hindbrain, labelled by white stars. Brightfield lateral imaging showing no apparent general change in the brain. Left: lateral view. Middle: dorsal view. Right: lateral view. (F) The gpam knockdown (gpam-MO1) caused a reduction of astrocytes in Tg(gfap: egfp) embryos at 48 hpf, and there were only 17% of participants that remained normal. This abnormal phenotype could be rescued by zebrafish gpam mRNA (zgpam), human GPAM mRNA (WT), mutated human GPAM leading to P669S, or mutated human GPAM leading to G449R, to variable extents (86, 87, 44 and 28%, respectively). The numbers of fish that were used ranged from 36 to 72 in the different test groups. (G) Swimming behaviour tests of the gpam-MO1 zebrafish larvae at 5 dpf compared with the controls, in terms of moving time, speed, distance, clockwise (CW) rotation and counterclockwise (CCW) rotation. The former three parameters showed no significant changes, while the latter two parameters revealed significant changes between the two groups. n = 10 to 12 for each test group. * P < 0.05, ** P < 0.01, n.s.: no significant difference, unpaired t-test. dpf: days postfertilization. (H–K) Morphological and behavioural assays of Gpam knockout (Gpam−/−) mouse models. (H) The Gpam−/− mice at postnatal 8 weeks showed no significant alterations of brain size, brain weight or body weight compared with those of WT mice. The red-dashed line indicates the area of brain. n = 6 per genotype with equal number of males and females. n.s.: no significant difference, unpaired t-test. (I) Track plots depicting the traces of mice during the probe test in the Morris water maze. The Gpam−/− mice showed no difference in performance compared with that of WT mice. n = 10 per genotype with equal numbers of male and female mice. n.s.: no significant difference, unpaired t-test. (J) Rotarod tests showed significantly reduced motor coordination and balance of the Gpam−/− mice compared with those of WT mice. n = 10 per genotype with equal numbers of male and female mice. ** P < 0.01, unpaired t-test. (K) Grip strength tests revealed significantly shorter grip duration and weaker grip strength of the Gpam−/− mice compared with those of WT mice. n = 10 per genotype with equal numbers of male and female mice. *** P < 0.001, unpaired t-test.
Compound heterozygous variants of GPAM (OMIM: 602395) were identified in this patient, the association between which and the CP phenotype in this family was confirmed by using targeted PCR and Sanger sequencing (Supplementary Table 3 and Fig. 5B). The pRecessive value 0.9998 of GPAM (http://exac.broadinstitute.org/) rendered a very strong indicator for a recessive-disease-related gene. Allele frequencies of the two variants (NM_001244949: c.1495G>C, p. G499R; c.2005C>T, p. P669S) were both below 1 × 10−5, in the large-scale databases (https://db.cngb.org/cmdb/ and http://exac.broadinstitute.org/), and with no match in the in-house database of >2247 healthy individuals. Pathogenicity prediction labelled the two variants damaging or disease-causing (Supplementary Table 4). Multiple protein-sequence alignments in a variety of species showed high conservation of both positions (i.e. G499 and P669), which were located inside the important protein domains. Three-dimensional structural models revealed that G499 and P669 were positioned at the pivotal regions, and both alterations of amino acids led to a dramatic reduction of protein structural stability (Fig. 5C).
Using WISH analysis in zebrafish, we found that gpam was specifically expressed in the developing central nervous system, mainly in the hindbrain (Fig. 5D). Morpholino-mediated gpam knockdown (gpam-MO) zebrafish models were established by blocking mRNA splicing (MO1) and protein translation (MO2) of gpam (Supplementary Figs 7 and 15). There was no apparent head size difference between gpam-MO zebrafish and WTs, but we observed an obvious reduction of astrocytes in gpam-MO zebrafish, especially in the hindbrain (Fig. 5E and Supplementary Fig. 16). This reduction could be effectively rescued by the WT human GPAM or zebrafish gpam mRNA, but not by the GPAM mRNAs with variants causing G499R and P669S (Fig. 5F). This result corroborated the pathogenicity of the two variants identified in the patient. Furthermore, swimming behaviour tests showed that the swimming capacity of gpam-MO zebrafish was compromised significantly (Fig. 5G), mimicking the motor impairment of the respective CP patient. Interestingly, we noticed that the swimming capacity patterns of tyw1-MO and gpam-MO zebrafish were complementary to each other, such that the swimming distance, speed and moving time were abnormal in tyw1-MO zebrafish but normal in gpam-MO zebrafish; in contrast, the rotational movement was normal in tyw1-MO zebrafish but abnormal in gpam-MO zebrafish. This finding may reflect differential underlying mechanisms of these two genes, although more experiments are needed to confirm or refute this hypothesis.
Meanwhile, we constructed Gpam knockout mice models via CRISPR–Cas9 genome editing technology (Supplementary Fig. 10). In Gpam knockout (Gpam−/−) mice, there was no significant alteration of brain size or weight compared with that of wild-type littermates (Fig. 5H), compatible with our observations in humans and zebrafish. Similarly, results from the Morris water maze showed no significant differences between Gpam−/− mice and wild-type mice (Fig. 5I). However, the rotarod test and the grip strength test revealed significant reductions in motor capacities of Gpam−/− mice compared with those of wild-type mice (Fig. 5J and K).
Defective Gpam interferes with astrocytic proliferation and oligodendrocytic myelination by perturbing lipid metabolism
The gene of GPAM encodes a mitochondrial enzyme that catalyses the production of lysophosphatidic acid (LPA) by using glycerol-3-phosphate (G3P) as a substrate, which is the rate-limiting step in the synthesis of glycerolphospholipids and triacylglycerol (TAG).83 There are four isoenzymes in this step, among which GPAM occupies most of the activity in the liver, and hepatic knockdown of Gpam in mice reduces levels of TAG, diacylglycerol, fatty acids and cholesterol.83–85 In the central nervous system, GPAM was specifically expressed in astrocytes, and reached its highest expression level on P14 in mouse hindbrain (Fig. 2D and E; Fig. 6A and B). Enzymatic activity in astrocytes is maintained mainly by GPAM, supplemented by GPAT4, an isoenzyme ubiquitously expressed in a variety of brain cells including neurons and oligodendrocytes.86,87
Figure 6.
Functional mechanism of defective GPAM. (A) Brain-section immunostaining of wild-type mice at postnatal 8 weeks, where Gpam (green) and Gfap (red) are colabelled, showing Gpam expression in astrocytes within many brain regions. Blue: DAPI. Scale bar = 100 μm. (B) Immunostaining of the normal human brain tissue microarrays, where GPAM (green) and GFAP (red) are colabelled, revealing GPAM expression in astrocytes within a variety of brain regions. Blue: DAPI. Scale bar = 50 μm. (C) Brain-section immunostaining of the Gpam−/− mice at postnatal 8 weeks revealing a significantly reduced number of Gfap+ cells in the medulla, as compared with that in WT mice. The same tendency was observed in other brain regions albeit without statistical significance. Green: Gfap. Red: NeuN. Blue: DAPI. Scale bar = 100 μm. n = 3 per genotype. For a specific sample, each measurement was performed twice in separate fields. ** P < 0.01, n.s.: no significant difference, unpaired t-test. (D) Flow cytometry of whole-brain cell suspension from P1 mice stained with Gfap, Ki67 and Caspase-3. Percentages of the Gfap+Ki67+ cells were significantly lower in the Gpam−/− mice brain compared with those in WT mice, indicating inhibited proliferation of astrocytes. Percentages of Gfap+Caspase-3+ cells showed no difference between the two groups, indicating no activated apoptosis. n = 8 per genotype with equal numbers of male and female mice. ** P < 0.01, n.s.: no significant difference, unpaired t-test. (E) Nissl staining of sagittal brain sections of mice at postnatal 8 weeks showing a significantly thinner white matter in the motor cortex of the Gpam−/− group, as compared with that of the WT group. The same tendency was observed in the frontal cortex, Figure 6: Continued albeit without statistical significance. Scale bar = 200 μm. n = 3 per genotype. For a specific sample, each measurement was performed four times in separate fields. ** P < 0.01, n.s.: no significant difference, unpaired t-test. (F) Brain-section immunostaining of mice at postnatal 8 weeks showing a lower density of myelin binding protein (Mbp) in the medulla of the Gpam−/− mice, compared with that of WT mice. Green: Mbp. Blue: DAPI. Scale bar = 100 μm. (G) Western blot of the mouse brain medulla showing significant reductions of Gfap and Mbp in the Gpam−/− group compared with those in the WT group. Gapdh and β-actin were used as controls. MW (Gfap) = 50 kDa; MW (Mbp) = 19 and 26 kDa, respectively. For a specific sample, each measurement was performed three times independently. * P < 0.05, unpaired t-test. (H) Schematics of the mechanism of GPAM involvement in lipid metabolism and its influence on astrocytic proliferation and oligodendrocytic myelination, by regulating the production of PA, PI, PC, PE and PS. GPAT4: glycerol-3-phosphate acyltransferase 4. G3P: glycerol-3-phosphate. LPA: lysophosphatidic acid. PA: phosphatidic acid. DAG: diacylglycerol. TAG: triacylglycerol. CDP-DAG: cytidine diphosphate diacylglycerol. PI: phosphatidylinositol. PC: phosphatidylcholine. PE: phosphatidylethanolamine. PS: phosphatidylserine. MBP: myelin binding protein. PLP: proteolipid protein. (I) Brain-medulla-section immunostaining showing significant reductions of Filipin, as well as Mbp and Gfap, in Gpam−/− mice compared with those in WT mice. The percentage of the Filipin+ area per imaging field was proportional to the cholesterol abundance. Scale bar = 100 μm. n = 3 per genotype. For a specific sample, each measurement was performed four times in separate fields. * P < 0.05, unpaired t-test. (J) Quantification of PA, PC and PE levels in the brain medullas of mice at postnatal 8 weeks revealing a significant reduction in the Gpam−/− group compared with those in the WT group. n = 3 per genotype. For a specific sample, each measurement was performed twice or thrice. * P < 0.05, ** P < 0.01, unpaired t-test.
In Gpam−/− mice, we observed a reduced number of astrocytes, especially in the medulla regions (Fig. 6C). Whole-brain single-cell suspension of P1 mice showed significantly reduced proliferation of astrocytes in Gpam−/− mice, but no apparent increase in the intensity of apoptosis was observed (Fig. 6D). Interestingly, brain-section staining revealed an obviously decreased white-matter thickness of the motor cortex in Gpam−/− mice (Fig. 6E). In parallel with the decrease of astrocytic density, a myelin marker, namely myelin binding protein (Mbp), was revealed to be significantly reduced in the medulla regions of Gpam−/− mouse brains (Fig. 6F and G). Given the phenotypic similarities between the respective CP patient and the Gpam−/− mice, we hypothesize that the defective Gpam may lead to hypomyelination of the corticospinal tract that originates from the primary motor cortex, passes through and modulates in the medulla and terminates in the spinal cord.88 However, this interpretation requires further testing in future studies.
Myelin is a specialized form of oligodendrocytic membrane that tightly wraps and insulates neuronal axons to accelerate conduction of action potentials.66,89 Compared with the properties of other membranes, myelin has a much higher lipid content that consists of up to 80% of the total dry weight, and the most abundant lipid groups in myelin are cholesterol, phospholipids and glycolipids.89–91 It has been reported that in mouse models, lipid synthesis blockage in oligodendrocyte causes instant onset of severe demyelination and neurological symptoms; however, this damage can be spontaneously remedied to a great extent after a period of months.92 Because the blood–brain barrier shields the central nervous system from circulating lipids, an exogenous lipid supply in the parenchyma of brain may account for this phenomenon; fortuitously, recent studies have discovered that the major suppliers of lipids in the brain are astrocytes.93–95 Actually, it has been revealed that horizontal lipid flux from astrocytes to oligodendrocytes is a major feature of myelination, and that disruption of lipid synthesis in astrocytes leads to lasting and more severe hypomyelination than inactivation of that in oligodendrocytes.96–98 In astrocytes, GPAM is committed to a rate-limiting step from G3P to LPA, the subsequent lipid products of which include PA, diacylglycerol (DAG), TAG, phosphatidylinositol (PI), phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylserine (PS). PI, PE, PC and PS are the major components of myelin99,100 (Fig. 6H). Noticeably, a large body of evidence has implicated an association between GPAM polymorphisms and plasma levels of cholesterol.101–106 In our present study, experiments in brain sections and extracts confirmed this hypothesis, with significantly reduced contents of lipids detected in medullary tissues of Gpam−/− mice (Fig. 6I and J). Interestingly, we also observed a significant reduction of 1,2-diacyl-G3P (PA), which is a downstream product of GPAM-involved reactions (Fig. 6H and J). PA is not only a precursor of PI, a component of myelin, but also promotes cell proliferation in astrocytes by activating the MAPK and mTOR signalling pathways (Fig. 6H).107–109 In addition, inhibition of PA is known to reduce the number of astrocytes and to cause neurodevelopmental disorders.110–113
Discussion
This work is a large-scale, multi-dimensional, clinical and genetic investigation on the aetiology of CP. We identified, in 45% of 120 CP families, detrimental variants of established and novel genes related to CP and other NDDs. Importantly, future studies should address whether our adjustment of clinical exclusions in the selection of this cohort may have affected the diagnostic yield. The meta-analysis on the 114 CP-related genes found in both the present study and previous studies created an updated compendium spanning a variety of attributes of CP-related genes, which may be a useful reference for subsequent research. Intriguingly, we proposed a novel classification system for CP-related genes according to their dichotomous spatiotemporal expression patterns. This may serve as a useful guideline for exploring the underlying mechanisms of CP-related genes and for designing targeted treatments. In future studies, identification of more CP-related genes from larger cohorts is needed to substantiate this classification system.
TYW1 is a novel CP-related gene, defects of which were confirmed in the present study to result in primary microcephaly and deterioration of both motion and cognition by disturbing proliferation and migration of neurons in the developing brain, including the motor cortex and frontal cortex. Blockage of neuronal proliferation and/or migration during brain development is a common theme in the aetiology of primary microcephaly.114,115 Interestingly, most of the known MCPH (i.e. microcephaly primary hereditary) genes encode components of machineries of cell division and cell cycling, while our present results of the mechanisms by which TYW1 influences a subset of MCPH genes is in line with previous findings. TYW1 plays a critical role in generating wybutosine, a hypermodification at position 37 of tRNAPhe responsible for stabilizing codon–anticodon binding, and this epigenetic procedure can be grouped into one of the three aspects of tRNA activation, namely, biogenesis, charging and modification of tRNAs.70,71 We noticed that a large proportion of genes involved in tRNA charging have been found to be associated with disease, especially NDDs; however, merely ∼10% of genes participating in tRNA modifications are related to human illness.68 Because of the enduring existence of most tRNA modification throughout species evolution, we posit that tRNA modifications may be underestimated in their roles in disease onset and progression. Future studies in this field can be modelled after this work on TYW1, and since most tRNA modifications were identified at position 34 and 37 in the anticodon stem loop, the algorithm that we developed for wybutosin at position 37 may be useful for application in a multitude of scenarios.
GPAM encodes a mitochondrial enzyme specifically expressed in astrocytes and plays a crucial role in lipid metabolism in the brain. Aberrant GPAM found in the present study reduced a variety of lipid contents in astrocytes and oligodendrocytes, causing hypomyelination in the corticospinal tract and suppression of proliferation of astrocytes, the latter of which is known to deteriorate the interplay between oligodendrocytes and astrocytes thus aggravating the hypomyelination.98,116 Dysfunction of lipid metabolism is present in a variety of neurodevelopmental disorders, including ID, ASD, attention deficit hyperactivity disorder and schizophrenia.117–121 Additionally, genetic defects in astrocytes, such as GFAP and NPC1, are known to be related to lesions in brain white matter.122–125 Our present work helped to elucidate the complex relationship between astrocytes and myelination, which may guide the development of novel therapeutic strategies. Intriguingly, there have been reports in mouse models that a high-fat diet promotes circulating lipid contents and ameliorates hypomyelination in the brain if lipid synthesis in astrocytes is blocked; therefore, it is tantalizing to apply this high-fat diet intervention to Gpam−/− mice and human patients with similar lipid metabolic problems.97,126
Funding
We are very grateful to the families participating in this study. This work was supported by the Major Medical Collaboration and Innovation Program of Guangzhou Science Technology and Innovation Commission (201604020020), the National Natural Science Foundation of China (81671067, 81974163, 81672253, 31871063, 81701451, 51971236 and 32070977), the China Postdoctoral Science Foundation (2019M662852), the Natural Science Foundation of Guangdong Province of China (2019A1515010420, 2018A030313538 and 2021A1515012543) and the Key-Area Research and Development Program of Guangdong Province (2019B020227001).
Competing interests
The authors report no competing interests.
Supplementary Material
Abbreviations
- ADL
Activity of Daily Living
- ASDs
autism spectrum disorders
- CNV
copy number variation
- CP
cerebral palsy
- DAG
diacylglycerol
- DNMs
de novo mutations
- DRD
DOPA-responsive dystonia
- DTI
diffusion tensor imaging
- EdU
5-ethynyl-2’-deoxyuridine
- GMFCS
Gross Motor Function Classification System
- GWCMC
Guangzhou Women and Children’s Medical Center
- G3P
glycerol-3-phosphate
- HSP
hereditary spastic paraplegia
- ID
intellectual disability
- LPA
lysophosphatidic acid
- MACS
Manual Ability Classification System
- Mbp
myelin binding protein
- MCPH
microcephaly primary hereditary
- NDD
neurodevelopmental disorders
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- PZM
postzygotic mutations
- TAG
triacylglycerol
- TBI
traumatic brain injury
- tRNA-yW
tRNA-wybutosine
- tRNAPhe
phenylalanine tRNA
- WGS
whole-genome sequencing
- WISH
whole-mount in situ hybridization
References
- 1. Colver A, Fairhurst C, Pharoah PO.. Cerebral palsy. Lancet. 2014;383(9924):1240–1249. [DOI] [PubMed] [Google Scholar]
- 2. Moreno-De-Luca A, Ledbetter DH, Martin CL.. Genetic [corrected] insights into the causes and classification of [corrected] cerebral palsies. Lancet Neurol. 2012;11(3):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Novak I, Morgan C, Adde L, et al. . Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, Russell DJ.. Development of the gross motor function classification system for cerebral palsy. Dev Med Child Neurol. 2008;50(4):249–253. [DOI] [PubMed] [Google Scholar]
- 5. Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N.. The complex aetiology of cerebral palsy. Nat Rev Neurol. 2018;14(9):528–543. [DOI] [PubMed] [Google Scholar]
- 6. Graham HK, Rosenbaum P, Paneth N, et al. . Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacLennan AH, Thompson SC, Gecz J.. Cerebral palsy: Causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015;213(6):779–788. [DOI] [PubMed] [Google Scholar]
- 8. Petterson B, Stanley F, Henderson D.. Cerebral palsy in multiple births in Western Australia: Genetic aspects. Am J Med Genet. 1990;37(3):346–351. [DOI] [PubMed] [Google Scholar]
- 9. Fletcher NA, Foley J.. Parental age, genetic mutation, and cerebral palsy. J Med Genet. 1993;30(1):44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costeff H. Estimated frequency of genetic and nongenetic causes of congenital idiopathic cerebral palsy in west Sweden. Ann Hum Genet. 2004;68(Pt 5):515–520. [DOI] [PubMed] [Google Scholar]
- 11. Hemminki K, Li X, Sundquist K, Sundquist J.. High familial risks for cerebral palsy implicate partial heritable aetiology. Paediatr Perinat Epidemiol. 2007;21(3):235–241. [DOI] [PubMed] [Google Scholar]
- 12. Garne E, Dolk H, Krageloh-Mann I, Holst Ravn S, Cans C, Group SC.. Cerebral palsy and congenital malformations. Eur J Paediatr Neurol. 2008;12(2):82–88. [DOI] [PubMed] [Google Scholar]
- 13. O'Callaghan ME, MacLennan AH, Haan EA, Dekker G.. South Australian Cerebral Palsy Research G. The genomic basis of cerebral palsy: A HuGE systematic literature review. Hum Genet. 2009;126(1):149–172. [DOI] [PubMed] [Google Scholar]
- 14. Fahey MC, Maclennan AH, Kretzschmar D, Gecz J, Kruer MC.. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59(5):462–469. [DOI] [PubMed] [Google Scholar]
- 15. Turner TN, Coe BP, Dickel DE, et al. . Genomic patterns of de novo mutation in simplex autism. Cell. 2017;171(3):710–722.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu H, Kahrizi K, Musante L, et al. . Genetics of intellectual disability in consanguineous families. Mol Psychiatry. 2018;24(7):1027–1039. [DOI] [PubMed] [Google Scholar]
- 17. Satterstrom FK, Kosmicki JA, Wang J, et al. . Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(3):568–584.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Segel R, Ben-Pazi H, Zeligson S, et al. . Copy number variations in cryptogenic cerebral palsy. Neurology. 2015;84(16):1660–1668. [DOI] [PubMed] [Google Scholar]
- 19. Oskoui M, Gazzellone MJ, Thiruvahindrapuram B, et al. . Clinically relevant copy number variations detected in cerebral palsy. Nat Commun. 2015;6:7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zarrei M, Fehlings DL, Mawjee K, et al. . De novo and rare inherited copy-number variations in the hemiplegic form of cerebral palsy. Genet Med. 2018;20(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corbett MA, van Eyk CL, Webber DL, et al. . Pathogenic copy number variants that affect gene expression contribute to genomic burden in cerebral palsy. NPJ Genom Med. 2018;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMichael G, Bainbridge MN, Haan E, et al. . Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20(2):176–182. [DOI] [PubMed] [Google Scholar]
- 23. Parolin Schnekenberg R, Perkins EM, Miller JW, et al. . De novo point mutations in patients diagnosed with ataxic cerebral palsy. Brain. 2015;138(Pt 7):1817–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthews AM, Blydt-Hansen I, Al-Jabri B, et al. ; On behalf of TIDE BC, United for Metabolic Diseases and the CAUSES Study. Atypical cerebral palsy: Genomics analysis enables precision medicine. Genet Med. 2018;21(7):1621–1628. doi:10.1038/s41436-018-0376-y [DOI] [PubMed] [Google Scholar]
- 25. Takezawa Y, Kikuchi A, Haginoya K, et al. . Genomic analysis identifies masqueraders of full-term cerebral palsy. Ann Clin Transl Neurol. 2018;5(5):538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Eyk CL, Corbett MA, Frank MSB, et al. . Targeted resequencing identifies genes with recurrent variation in cerebral palsy. NPJ Genom Med. 2019;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin SC, Lewis SA, Bakhtiari S, et al. . Mutations disrupting neuritogenesis genes confer risk for cerebral palsy. Nat Genet. 2020;52(10):1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D'Gama AM, Walsh CA.. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci. 2018;21(11):1504–1514. [DOI] [PubMed] [Google Scholar]
- 29. Campbell IM, Shaw CA, Stankiewicz P, Lupski JR.. Somatic mosaicism: Implications for disease and transmission genetics. Trends Genet. 2015;31(7):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B.. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. [DOI] [PubMed] [Google Scholar]
- 31. Eliasson AC, Krumlinde-Sundholm L, Rosblad B, et al. . The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–554. [DOI] [PubMed] [Google Scholar]
- 32. Xu K, Wang L, Mai J, He L.. Efficacy of constraint-induced movement therapy and electrical stimulation on hand function of children with hemiplegic cerebral palsy: A controlled clinical trial. Disabil Rehabil. 2012;34(4):337–346. [DOI] [PubMed] [Google Scholar]
- 33. Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S.. The gross motor function measure: A means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31(3):341–352. [DOI] [PubMed] [Google Scholar]
- 34. Richards S, Aziz N, Bale S, et al. . Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang K, Li M, Hakonarson H.. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Purcell S, Neale B, Todd-Brown K, et al. . PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu M, Liu D, Dong Z, et al. . Kinesin-12 influences axonal growth during zebrafish neural development. Cytoskeleton (Hoboken). 2014;71(10):555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gong J, Wang X, Zhu C, et al. . Insm1a regulates motor neuron development in Zebrafish. Front Mol Neurosci. 2017;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong J, Chai L, Xu G, Ni Y, Liu D.. The expression of natriuretic peptide receptors in developing zebrafish embryos. Gene Expr Patterns. 2018;29:65–71. [DOI] [PubMed] [Google Scholar]
- 40. Young AP, Bandarian V.. TYW1: A radical SAM enzyme involved in the biosynthesis of wybutosine bases. Methods Enzymol. 2018;606:119–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noma A, Kirino Y, Ikeuchi Y, Suzuki T.. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006;25(10):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlson BA, Kwon SY, Chamorro M, Oroszlan S, Hatfield DL, Lee BJ.. Transfer RNA modification status influences retroviral ribosomal frameshifting. Virology. 1999;255(1):2–8. [DOI] [PubMed] [Google Scholar]
- 43. Gelineau-Morel RN, Zinkus TP, Le Pichon JB.. Pediatric head trauma: A review and update. Pediatr Rev. 2019;40(9):468–481. [DOI] [PubMed] [Google Scholar]
- 44. Fraley CE, Pettersson DR, Nolt D.. Encephalitis in previously healthy children. Pediatr Rev. 2021;42(2):68–77. [DOI] [PubMed] [Google Scholar]
- 45. Lauer BJ, Spector ND.. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32(8):341–349. [DOI] [PubMed] [Google Scholar]
- 46. Wu YW, Kuzniewicz MW, Wickremasinghe AC, et al. . Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: A population-based study. JAMA Pediatr. 2015;169(3):239–246. [DOI] [PubMed] [Google Scholar]
- 47. Vissers LE, Gilissen C, Veltman JA.. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17(1):9–18. [DOI] [PubMed] [Google Scholar]
- 48. South ST, Lee C, Lamb AN, et al. . ACMG Standards and Guidelines for constitutional cytogenomic microarray analysis, including postnatal and prenatal applications: Revision 2013. Genet Med. 2013;15(11):901–909. [DOI] [PubMed] [Google Scholar]
- 49. Najmabadi H, Hu H, Garshasbi M, et al. . Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478(7367):57–63. [DOI] [PubMed] [Google Scholar]
- 50. Doan RN, Lim ET, De Rubeis S, et al. . Recessive gene disruptions in autism spectrum disorder. Nat Genet. 2019;51(7):1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ruzzo EK, Perez-Cano L, Jung JY, et al. . Inherited and de novo genetic risk for autism impacts shared networks. Cell. 2019;178(4):850–866.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gilissen C, Hehir-Kwa JY, Thung DT, et al. . Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511(7509):344–347. [DOI] [PubMed] [Google Scholar]
- 53. Vissers LE, de Ligt J, Gilissen C, et al. . A de novo paradigm for mental retardation. Nat Genet. 2010;42(12):1109–1112. [DOI] [PubMed] [Google Scholar]
- 54. Martin HC, Jones WD, McIntyre R, et al. . Quantifying the contribution of recessive coding variation to developmental disorders. Science. 2018;362(6419):1161–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kahrizi K, Hu H, Hosseini M, et al. . Effect of inbreeding on intellectual disability revisited by trio sequencing. Clin Genet. 2019;95(1):151–159. [DOI] [PubMed] [Google Scholar]
- 56. Kara E, Tucci A, Manzoni C, et al. . Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain. 2016;139(Pt 7):1904–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pearson TS, Pons R, Ghaoui R, Sue CM.. Genetic mimics of cerebral palsy. Mov Disord. 2019;34(5):625–636. [DOI] [PubMed] [Google Scholar]
- 58. El-Hattab AW, Schaaf CP, Fang P, et al. . Clinical characterization of int22h1/int22h2-mediated Xq28 duplication/deletion: New cases and literature review. BMC Med Genet. 2015;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yon DK, Park JE, Kim SJ, Shim SH, Chae KY.. A sibship with duplication of Xq28 inherited from the mother; genomic characterization and clinical outcomes. BMC Med Genet. 2017;18(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haack TB, Ignatius E, Calvo-Garrido J, et al. . Absence of the autophagy adaptor SQSTM1/p62 causes childhood-onset neurodegeneration with ataxia, dystonia, and gaze palsy. Am J Hum Genet. 2016;99(3):735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jeong SJ, Park S, Nguyen LT, et al. . A threonyl-tRNA synthetase-mediated translation initiation machinery. Nat Commun. 2019;10(1):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Theil AF, Botta E, Raams A, et al. . Bi-allelic TARS mutations are associated with brittle hair phenotype. Am J Hum Genet. Aug 1 2019;105(2):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Williams M, Yen W, Lu X, Sutherland A.. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev Cell. 2014;29(1):34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lei Y, Kim SE, Chen Z, et al. . Variants identified in PTK7 associated with neural tube defects. Mol Genet Genomic Med. 2019;7(4):e00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meier N, Bruder E, Lapaire O, et al. . Exome sequencing of fetal anomaly syndromes: Novel phenotype-genotype discoveries. Eur J Hum Genet. 2019;27(5):730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nave KA, Werner HB.. Myelination of the nervous system: Mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. [DOI] [PubMed] [Google Scholar]
- 67. Bercury KK, Macklin WB.. Dynamics and mechanisms of CNS myelination. Dev Cell. Feb 23 2015;32(4):447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. El Yacoubi B, Bailly M, de Crecy-Lagard V.. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet. 2012;46:69–95. [DOI] [PubMed] [Google Scholar]
- 69. Landgraf BJ, McCarthy EL, Booker SJ.. Radical S-adenosylmethionine enzymes in human health and disease. Annu Rev Biochem. 2016;85:485–514. [DOI] [PubMed] [Google Scholar]
- 70. Rak R, Dahan O, Pilpel Y.. Repertoires of tRNAs: The couplers of genomics and proteomics. Annu Rev Cell Dev Biol. 2018;34:239–264. [DOI] [PubMed] [Google Scholar]
- 71. Schaffer AE, Pinkard O, Coller JM.. tRNA metabolism and neurodevelopmental disorders. Annu Rev Genomics Hum Genet. 2019;20:359–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Izraeli S, Lowe LA, Bertness VL, et al. . The SIL gene is required for mouse embryonic axial development and left-right specification. Nature. 1999;399(6737):691–694. [DOI] [PubMed] [Google Scholar]
- 73. Kumar A, Girimaji SC, Duvvari MR, Blanton SH.. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet. 2009;84(2):286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vulprecht J, David A, Tibelius A, et al. . STIL is required for centriole duplication in human cells. J Cell Sci. 2012;125(Pt 5):1353–1362. [DOI] [PubMed] [Google Scholar]
- 75. Leidel S, Delattre M, Cerutti L, Baumer K, Gonczy P.. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat Cell Biol. 2005;7(2):115–125. [DOI] [PubMed] [Google Scholar]
- 76. Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P.. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell. 2007;13(2):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van Breugel M, Hirono M, Andreeva A, et al. . Structures of SAS-6 suggest its organization in centrioles. Science. 2011;331(6021):1196–1199. [DOI] [PubMed] [Google Scholar]
- 78. Putkey FR, Cramer T, Morphew MK, et al. . Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev Cell. 2002;3(3):351–365. [DOI] [PubMed] [Google Scholar]
- 79. Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW.. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol. 2000;2(8):484–491. [DOI] [PubMed] [Google Scholar]
- 80. Yen TJ, Li G, Schaar BT, Szilak I, Cleveland DW.. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992;359(6395):536–539. [DOI] [PubMed] [Google Scholar]
- 81. Schmiesing JA, Gregson HC, Zhou S, Yokomori K.. A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol Cell Biol. 2000;20(18):6996–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martin CA, Murray JE, Carroll P, et al. . Mutations in genes encoding condensin complex proteins cause microcephaly through decatenation failure at mitosis. Genes Dev. 2016;30(19):2158–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Karasawa K, Tanigawa K, Harada A, Yamashita A.. Transcriptional regulation of acyl-CoA:glycerol-sn-3-phosphate acyltransferases. Int J Mol Sci. 2019;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xu H, Wilcox D, Nguyen P, et al. . Hepatic knockdown of mitochondrial GPAT1 in ob/ob mice improves metabolic profile. Biochem Biophys Res Commun. 2006;349(1):439–448. [DOI] [PubMed] [Google Scholar]
- 85. Hammond LE, Gallagher PA, Wang S, et al. . Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol. 2002;22(23):8204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Papatheodorou I, Moreno P, Manning J, et al. . Expression Atlas update: From tissues to single cells. Nucleic Acids Res. 2020;48(D1):D77–D83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tabula Muris C, Overall C, Logistical C, et al. . Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562(7727):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Iwasaki Y, Saito Y, Mori K, et al. . An autopsied case of adult-onset bulbospinalform Alexander disease with a novel S393R mutation in the GFAP gene. Clin Neuropathol. 2015;34(4):207–214. [DOI] [PubMed] [Google Scholar]
- 89. Aggarwal S, Yurlova L, Simons M.. Central nervous system myelin: Structure, synthesis and assembly. Trends Cell Biol. 2011;21(10):585–593. [DOI] [PubMed] [Google Scholar]
- 90. O'Brien JS. Stability of the myelin membrane. Science. 1965;147(3662):1099–1107. [DOI] [PubMed] [Google Scholar]
- 91. Ozgen H, Baron W, Hoekstra D, Kahya N.. Oligodendroglial membrane dynamics in relation to myelin biogenesis. Cell Mol Life Sci. 2016;73(17):3291–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Saher G, Brugger B, Lappe-Siefke C, et al. . High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8(4):468–475. [DOI] [PubMed] [Google Scholar]
- 93. Saher G, Stumpf SK.. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta. 2015;1851(8):1083–1094. [DOI] [PubMed] [Google Scholar]
- 94. Pfrieger FW, Ungerer N.. Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res. 2011;50(4):357–371. [DOI] [PubMed] [Google Scholar]
- 95. Wang H, Eckel RH.. What are lipoproteins doing in the brain? Trends Endocrinol Metab. 2014;25(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Molofsky AV, Deneen B.. Astrocyte development: A guide for the perplexed. Glia. 2015;63(8):1320–1329. [DOI] [PubMed] [Google Scholar]
- 97. Camargo N, Goudriaan A, van Deijk AF, et al. . Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017;15(5):e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Molina-Gonzalez I, Miron VE.. Astrocytes in myelination and remyelination. Neurosci Lett. 2019;713:134532. [DOI] [PubMed] [Google Scholar]
- 99. Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST.. Neuronal lipid metabolism: Multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci. 2018;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chrast R, Saher G, Nave KA, Verheijen MH.. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52(3):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Teslovich TM, Musunuru K, Smith AV, et al. . Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Johansen CT, Wang J, Lanktree MB, et al. . An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2011;31(8):1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tikkanen E, Tuovinen T, Widen E, et al. . Association of known loci with lipid levels among children and prediction of dyslipidemia in adults. Circ Cardiovasc Genet. 2011;4(6):673–680. [DOI] [PubMed] [Google Scholar]
- 104. Qi Q, Liang L, Doria A, Hu FB, Qi L.. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes. 2012;61(3):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Surakka I, Horikoshi M, Magi R, et al. . The impact of low-frequency and rare variants on lipid levels. Nat Genet. 2015;47(6):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang S, Yin RX, Miao L, Zhang QH, Zhou YG, Wu J.. Association between the GPAM rs1129555 SNP and serum lipid profiles in the Maonan and Han populations. Int J Clin Exp Pathol. 2018;11(3):1484–1498. [PMC free article] [PubMed] [Google Scholar]
- 107. Brandenburg LO, Pufe T, Koch T.. Role of phospholipase d in g-protein coupled receptor function. Membranes (Basel). 2014;4(3):302–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Foster DA, Salloum D, Menon D, Frias MA.. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J Biol Chem. 2014;289(33):22583–22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Guizzetti M, Costa LG.. Effect of ethanol on protein kinase Czeta and p70S6 kinase activation by carbachol: A possible mechanism for ethanol-induced inhibition of glial cell proliferation. J Neurochem. 2002;82(1):38–46. [DOI] [PubMed] [Google Scholar]
- 110. Samson HH. Microcephaly and fetal alcohol syndrome: Human and animal studies. Alcohol Brain Dev. 1986;167–183. [Google Scholar]
- 111. Perez-Torrero E, Duran P, Granados L, Gutierez-Ospina G, Cintra L, Diaz-Cintra S.. Effects of acute prenatal ethanol exposure on Bergmann glia cells early postnatal development. Brain Res. 1997;746(1-2):305–308. [DOI] [PubMed] [Google Scholar]
- 112. Guizzetti M, Zhang X, Goeke C, Gavin DP.. Glia and neurodevelopment: Focus on fetal alcohol spectrum disorders. Front Pediatr. 2014;2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Burkhardt U, Klein J.. Chapter 26 - ethanol impairs phospholipase D signaling in astrocytes. In: Patel VB, ed. Molecular aspects of alcohol and nutrition. Academic Press; 2016:325–335. [Google Scholar]
- 114. Hu WF, Chahrour MH, Walsh CA.. The diverse genetic landscape of neurodevelopmental disorders. Annu Rev Genomics Hum Genet. 2014;15:195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jayaraman D, Bae BI, Walsh CA.. The genetics of primary microcephaly. Annu Rev Genomics Hum Genet. 2018;19:177–200. [DOI] [PubMed] [Google Scholar]
- 116. Kiray H, Lindsay SL, Hosseinzadeh S, Barnett SC.. The multifaceted role of astrocytes in regulating myelination. Exp Neurol. 2016;283(Pt B):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Irons M, Elias ER, Salen G, Tint GS, Batta AK.. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341(8857):1414. [DOI] [PubMed] [Google Scholar]
- 118. Brookes KJ, Chen W, Xu X, Taylor E, Asherson P.. Association of fatty acid desaturase genes with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60(10):1053–1061. [DOI] [PubMed] [Google Scholar]
- 119. Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC.. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;71(4):201–204. [DOI] [PubMed] [Google Scholar]
- 120. Pinto D, Delaby E, Merico D, et al. . Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94(5):677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jungerius BJ, Hoogendoorn ML, Bakker SC, et al. . An association screen of myelin-related genes implicates the chromosome 22q11 PIK4CA gene in schizophrenia. Mol Psychiatry. 2008;13(11):1060–1068. [DOI] [PubMed] [Google Scholar]
- 122. Namekawa M, Takiyama Y, Aoki Y, et al. . Identification of GFAP gene mutation in hereditary adult-onset Alexander's disease. Ann Neurol. 2002;52(6):779–785. [DOI] [PubMed] [Google Scholar]
- 123. Patel SC, Suresh S, Kumar U, et al. . Localization of Niemann-Pick C1 protein in astrocytes: Implications for neuronal degeneration in Niemann- Pick type C disease. Proc Natl Acad Sci USA. 1999;96(4):1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schmitt A, Gofferje V, Weber M, Meyer J, Mossner R, Lesch KP.. The brain-specific protein MLC1 implicated in megalencephalic leukoencephalopathy with subcortical cysts is expressed in glial cells in the murine brain. Glia. 2003;44(3):283–295. [DOI] [PubMed] [Google Scholar]
- 125. Dietrich J, Lacagnina M, Gass D, et al. . EIF2B5 mutations compromise GFAP+ astrocyte generation in vanishing white matter leukodystrophy. Nat Med. 2005;11(3):277–283. [DOI] [PubMed] [Google Scholar]
- 126. Yazdi M, Ahnmark A, William-Olsson L, et al. . The role of mitochondrial glycerol-3-phosphate acyltransferase-1 in regulating lipid and glucose homeostasis in high-fat diet fed mice. Biochem Biophys Res Commun. 2008;369(4):1065–1070. [DOI] [PubMed] [Google Scholar]
- 127. Li M, Santpere G, Imamura Kawasawa Y, et al. BrainSpan Consortium† . Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science. 2018;362(6420):eaat7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lake BB, Chen S, Sos BC, et al. . Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat Biotechnol. 2018;36(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. This study’s samples and data are available to the scientific community on request.