Abstract
In recent years, the clinical impact of intestinal microbiota–kidney interaction has been emerging. Experimental evidence highlighted a bidirectional evolutionary correlation between intestinal microbiota and kidney diseases. Nonetheless, acute kidney injury (AKI) is still a global public health concern associated with high morbidity, mortality, healthcare costs, and limited efficient therapy. Several studies on the intestinal microbiome have improved the knowledge and treatment of AKI. Therefore, the present review outlines the concept of the gut–kidney axis and data about intestinal microbiota dysbiosis in AKI to improve the understanding of the mechanisms of the intestinal microbiome on the modification of kidney function and response to kidney injury. We also introduced the future directions and research areas, emphasizing the intervention approaches and recent research advances of intestinal microbiota dysbiosis during AKI, thereby providing a new perspective for future clinical trials.
Keywords: Intestinal microbiota, kidney diseases, AKI, gut–kidney axis, intestinal microbiome dysbiosis, therapies
Introduction
Acute kidney injury (AKI) is a major and life-threatening kidney disease resulting from pathogenic conditions, such as hemodynamic instability, sepsis, and drug toxicity [1,2]. The current understanding of AKI involves alteration of the microcirculation, renal tubular epithelial cell (RTEC) injury, and intrarenal inflammation. AKI causes severe complications with uremic toxins building up in the body, resulting in a decline of renal function [3]. Furthermore, AKI has been recognized as a non-self-limited process strongly linked to an increased risk of chronic kidney disease (CKD) [4]. However, no therapeutic strategies reliably improve survival except renal replacement therapies (long-term dialysis and kidney transplantation). However, renal replacement presents inherent disadvantages such as side effects, high cost, and increased risk of infections [5].
In the past few years, intestinal microbiome has gained increasing attention with respect to human health and diseases. Intestinal microbiota composition is in a certain proportion after birth, with beneficial bacteria as predominant species, thus forming a symbiosis of quality and quantity. Reportedly, intestinal microbiota dysbiosis is associated with colorectal cancer, liver diseases, obesity, and renal diseases [6–9]. A recent study showed changes in intestinal microbiota composition in animal models of ischemic AKI [10]. Increasing evidence suggested that intestinal microbiota and its metabolites contribute substantially to the pathogenesis of AKI [11]. A permanent alteration in the microbiota composition or function alters intestinal motility, and permeability initiates the immune response, thus improving visceral adaptivity [12,13]. In this review, we focused on the potential mechanisms of intestinal microbiota dysbiosis in AKI and summarized the recent research progress and promising therapeutic strategies.
Role of the microbiota in gut–kidney axis
The human intestinal tract, especially the large intestine, harbors an extremely diverse population of microorganisms, termed intestinal microbiota. The human endogenous intestinal microbiota is recognized as an essential ‘organ’ in healthy individuals with a dynamic and beneficial association with the human host [14]. The most dominant bacterial phyla of the intestinal microbiota are Firmicutes and Bacteroidetes, while Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia phyla are in the minority [15]. The intestinal microbiota composition of humans changes continuously over the life of the host, based on lifestyle, including eating habits, age, administration of antibiotics, and presence or absence of diseases [16]. The balance of the intestinal microbiota is essential for the integrity of the intestinal epithelial barrier [17], executing energy metabolism [18,19], and regulating immune health [20]. It also promotes the maturation of the intricate enteric nervous system (ENS) [21]. Colonization of germ-free (GF) mice with microbiota from conventional specific pathogen-free (SPF) mice releases 5-HT and activates 5-HT4 receptors, which in turn alter the neuroanatomy of the ENS and improve the rate of intestinal transit [22].
Recently, the gut–kidney axis has gained increasing attention, but the studies have mainly focused on CKD [23]. The basic principle of the gut–kidney axis is the crosstalk between the imbalance of intestinal microbiota and the decreased renal function [24]. In CKD, renal injury causes the accumulation of metabolic waste in the body, which could infiltrate into the intestinal cavity, causing intestinal microbiota dysbiosis. In addition, the intestinal epithelial barrier is impaired, facilitating the passage of opportunistic pathogens and enterogenic urinary toxins into the blood circulation, which triggers a systemic inflammatory response and deteriorates renal disease [25,26]. Furthermore, intestinal dysbiosis and its metabolites play a critical role in blood pressure control. For example, Nα-acetyl-L-arginine, stearic acid, phosphatidic acid, and glucoside are abundant in hypertension samples [27]. Similarly, metabolomics illustrated that gut microbial metabolites are involved in the pathogenesis and progression of diabetic kidney disease (DKD), including short-chain fatty acids (SCFAs), trimethylamine-N-oxide (TMAO), bile acids (BAs), polyphenols, tryptophan-derived metabolites, branched-chain amino acids (BCAAs), and other metabolites [28]. However, intestinal microbiome dysbiosis is also crucial in AKI-to-CKD transition. Changes in the intestinal microbiota associated with these metabolites are also detected in early kidney disease [29]. Such as, SCFAs were shown to decrease the inflammatory response, reduce the infiltration of damaged tissue by leukocytes and affect chemotaxis and cytokine production in a murine IRI model of AKI.
Interestingly, renal tubular damage is also frequently observed in patients with inflammatory bowel disease (IBD), and the morbidity of IBD-related renal manifestations is up to 20% [30]. In addition, intestinal immune tolerance deficiencies induce the absorption of antigens and activate the mucosal lymphoid tissue (MALT), causing excessive deposition of abnormal IgA1 in the glomerular region, eventually developing IgA nephropathy (IgAN) [31]. Recent advancements in basic and clinical research have demonstrated that dysfunction of other organs underlies the poor outcomes of AKI [32]. Significant progress has been made in renal therapy based on the gut–kidney axis, including modulation of the gut environment, improvement of the systemic inflammatory response, and decline in toxin accumulation; together, these factors reverse the renal injury [33,34].
Intestinal microbiota and AKI
Recent evidence from studies on AKI-to-CKD transition and end-stage renal disease (ESRD) indicated that changes in intestinal microbiota occur in kidney diseases [35]. A previous study on AKI has focused on the analysis of intestinal microbiota composition by sequencing the V4 region of the 16S rRNA gene for the fecal samples collected from mouse induction of kidney ischemia–reperfusion (I/R) [19]. Results showed that the bacterial community was dominated by increasing abundance of Clostridium and Ruminococcus and decreasing abundance of Bifidobacterium and TM7 after I/R injury [36]. Another study demonstrated the hallmarks of I/R injury-induced dysbiosis mainly were increase of Enterobacteriacea, decrease of Lactobacilli and Ruminococacceae. Colonizing GF mice with post-AKI microbiota aggravated I/R injury severity with worsened inflammation in recipient mice compared to colonizing with microbiota from sham-operated mice [37]. Intestinal microbial dysbiosis is known as the consequence of AKI since several metabolites, including endotoxin and uremia toxins, are absorbed into the blood without clearing by the kidney [38].
Reportedly, serum lipopolysaccharide (LPS) level is a major indicator for verifying the occurrence of intestinal dysbiosis. Serum LPS circulates in the kidney, thus triggering inflammation and the oxidative stress pathway to promote kidney injury [39]. Low levels of endotoxemia and intestinal bacterial translocation were observed in mice with renal I/R injury [40]. Gut-derived uremic toxins (GDUT) consist of protein-bound toxins, p-cresyl sulfate (PCS), indoxyl sulfate (IS), and TMAO, with a detrimental effect on kidney function through the gut–kidney axis [41,42]. Furthermore, the serum level of GDUT and related metabolites affect kidney functions and are associated with an increased risk of kidney diseases [43]. With an increase in the abundance of bacteria that produce GDUT, a concurrent decrease was detected in the abundance of beneficial bacteria that produce SCFAs [44], which regulate the inflammation and energy metabolism in AKI. Moreover, many D-amino acids have been tested in the excrement of renal I/R mice, and only D-serine can be detected in the kidney [36]. These results indicated that the intestinal microbiota produces D-serine in response to AKI injury of renal I/R mice.
Previous studies have shown that intestinal dysfunction during kidney injury is due to the disruption of the intestinal barrier, which is partially mediated by the permeation of noxious molecules [40,45]. The key components that determine intestinal barrier function are the intestinal epithelial cells with tight intercellular junctions (TJs) [46]. Restoring epithelial TJ proteins in vivo reverses microbiota dysbiosis preventing toxin and pathogen translocation through intestinal barrier transported to the systemic circulation, which contributes to inflammation and progression of AKI [47,48]. In severe acute pancreatitis (SAP), tumor necrosis factor-alpha (TNF-α) elevates the intestinal permeability and promotes bacterial translocation from the epithelium, which further stimulates the excessive release of inflammatory cytokines and aggravates AKI [49]. During AKI sepsis, the disruption of the mucus membrane barrier may worsen systemic inflammation and potentiation of AKI [50]. Moreover, increased inflammatory cytokines disrupt the gut barrier function and increase the permeability by acting on the junctional complexes, such as apical TJs and junctional adhesion molecules (JAMs) [51] or via activation of myosin light chain kinase (MLCK) [52]. On the other hand, impaired clearance of water and metabolic products causes gut barrier injury and hyperpermeability [53].
Microbial stimuli influence the phenotype of renal lymphocytes and the expression of cytokines in kidneys and also modulate the outcome of AKI [20]. The equilibrium between Th1 and Th2 responses disrupts renal I/R injury, and GF mice exhibit abundant NKT cells and fewer Th2 cells with lower interleukin-4 (IL-4) levels than SPF mice [54]. Intestinal Th17 cells may migrate to the kidney through the S1P receptor pathway, thus promoting the development of autoimmune nephritis [55]. Also, Th17 cells play a major role in the chronic process of AKI induced by high salinity [56]. In renal I/R injury, AKI was associated with elevated Th17 and Th1 responses [57]. The depletion of microbiota significantly attenuated renal damage, maintained tubular integrity by reducing the maturation status of F4/80+ renal resident macrophages and bone marrow monocytes, as well as decreased the migratory capacity toward CX3CL1 and CCL2 ligands compared to the controls [58]. In addition, in kidney tissue after I/R, the activation of the costimulatory molecules CD80 and CD40 of bone marrow dendritic cells can be inhibited via products generated by the intestinal microbiota [59]. Previous studies demonstrated that GF mice are protected from intestinal IRI due to increased production of IL-10 [60]. The lack of intestinal microbiota is accompanied by IL-10-mediated inflammation, indicating the essential role of intestinal microbiota in facilitating acute renal inflammatory responses [61].
Therapies targeting the intestinal microbiota in AKI
The composition, dynamics, and stability of gut microbiota are critical for diagnosing, treating, and preventing specific diseases. The gut microbiome transitioned from being a ‘missing’ organ to a potential target for therapeutic applications. Thus, targeting the intestinal microbiota might provide a novel therapeutic strategy in AKI.
Probiotics and prebiotics
Probiotics are living microorganisms beneficial to the host health by improving the intestinal microbial balance, and the most studied probiotics are Saccharomyces cerevisiae, Streptococci, Lactobacillus, and Bifidobacterium species [62,63]. Prebiotics are usually defined as non-digestible carbohydrates, such as lactulose and fructooligosaccharides, that selectively stimulate the growth and activity of beneficial intestinal bacteria [64]. Hitherto, various probiotics and prebiotics have been widely popularized among the general public because of their robust therapeutic effects and fewer side effects [65]. These conventional treatments may be a valid alternative to drug-based therapy in the prevention or amelioration of intestinal microbiome-associated kidney diseases [66–68].
The present study suggested that preventive Lactobacillus casei treatment affects the inflammatory state by decreasing the expression of proinflammatory cytokines, TNF-α and IL-6, in an LPS-induced endotoxic AKI mouse model [69]. The administration of Lactobacillus paracasei significantly reduced the serum LPS levels in obese-insulin resistance-induced kidney injury model [70]. Typically, Lactobacillus salivarius BP121 suppresses the generation of uremic toxins and regulates AMPK- and TLR4-dependent TJ assembly, thus protecting against cisplatin-induced AKI by downregulating renal inflammatory mediators and decreasing the oxidative stress [71]. Lactobacillus mix addition also decreases the production of proinflammatory cytokines (TNF-α and IL-6) and prevents renal proximal tubular cell apoptosis in a cisplatin-induced renal injury in pig model [72]. Moreover, Bifidobacteria is another widely used probiotic along with Lactobacilli in kidney diseases [73,74]. Bifidobacteria pretreatment is a valid strategy to prevent intestinal barrier dysfunction and reduce bacterial translocation in mice following liver or intestinal I/R injury [75,76]. However, whether Bifidobacteria is beneficial to kidney I/R injury by improving microbiota dysbiosis is yet uncertain. A randomized controlled trial indicated that prebiotics is valuable in the modification of the stool microbiome with improved inflammatory indices during CKD.
Recently, the probiotic L. casei Zhang has been confirmed to slow the progression of acute and CKD through animal experiments and clinical trials [77]. The study showed that L. casei Zhang improved intestinal dysbacteriosis and elevated the useful metabolites (such as SCFAs and niacinamide) in mice AKI and CKD models. Also, oral L. casei Zhang postponed the decline in kidney function in patients with stage 3–5 CKD. Hitherto, only a few studies have discussed whether prebiotic supplementation is effective in AKI. Taken together, oral probiotics might alter the intestinal microbiome dysbiosis in AKI.
SCFAs supplementation
SCFAs belong to fermentation metabolic end-products from complex polysaccharides produced by the intestinal microbiota and are comprised of butyrate, propionate, and acetate [78]. Moreover, the metabolic end-products are key candidate mediators for gut–kidney crosstalk. These metabolites also bind the G-protein membrane receptors (GPR) on intestinal epithelium or epigenetically modify histone deacetylase (HDAC) [79] and directly penetrate the epithelial membrane through transporter channels to affect kidney function [80]. The importance of SCFAs with respect to anti-inflammatory properties to protect against AKI has been utilized [81,82]. Treatment with SCFAs, especially acetate, inhibits inflammation and apoptosis processes and reduces NF-κB activation after kidney I/R injury [83]. The supplementation of dietary SCFAs in I/R AKI mouse model improves kidney dysfunction and protects from other complications [59]. Furthermore, SCFAs exert a strong influence and role via the immunomodulatory effects on the polarization of T-cell subsets through GPR expression [84]. In sepsis-induced AKI, SCFAs ameliorate immune function by inhibiting NADPH oxidase signaling in T cells [85]. SCFAs also upregulate serotonin (5-HT) [86], which modulates the immune homeostasis by either enhancing dendritic cell-mediated T-cell activation or affecting macrophage polarization and phagocytosis [87]. In addition, SCFAs are known as HDAC inhibitors [88] and can suppress high glucose-induced apoptosis of renal tubular epithelial cells by inhibiting HDAC2 due to its anti-oxidative property [89].
A recent study suggested that a high-fiber (HF) diet or SCFA supplementation prevents the development of AKI and subsequent CKD [90]. In experimental murine folic-acid nephropathy (FAN) mice models, HF improves the kidney function, such as less tubular injury on day 2 and less interstitial fibrosis and chronic inflammation on day 28 compared to normal chow-fed mice. Moreover, the study also found that the HF diet reduces AKI-induced dysbiosis, fosters the expansion of SCFAs-producing bacteria, and increases SCFA concentrations in fecal and serum. The main mechanisms are associated with HDAC inhibition and activation of GPR by SCFAs. However, studies have failed to report the application of SCFAs in clinical AKI patients. Overall, in vitro and in vivo, exogenous SCFA supplements may be a new therapeutic tool for preventing the progression of renal inflammation and the potential direction for translational clinical studies.
Immunomodulator
With respect to the immune dysfunction related to the gut–kidney axis, dampening the immune response by targeting microbiota-derived mediators is an accepted strategy for limiting kidney damage. The animal models of kidney I/R injury showed that depletion of intestinal microbiota protected against AKI via reduced Th17, Th1 response, and expansion of Tregs and M2-polarized macrophages [57]. Th17 cell expansion and maintenance depend on the proinflammatory cytokine IL-23, which can be blocked with monoclonal antibodies against IL-23 [91] and IL-23/IL-12 [92]. In addition, IL-17A, secreted by Th17 cells, might be the most promising target and can be disrupted with monoclonal anti-IL-17A antibodies, such as secukinumab and ixekizumab [93]. Alternatively, monoclonal antibodies, such as brodalumab, can also inhibit IL-17 receptor signaling [94].
Selective decontamination of the digestive tract (SDD)
SDD is an infection intervention strategy by topical administration of antibiotics to the oropharynx and the gastrointestinal tract, which was proposed about 30 years ago [95]. A recent study demonstrated that oral vancomycin reduces the levels of uremic toxins produced in the intestinal microbiota of CKD patients [96]. After hemodialysis, each participant took a single 250 mg capsule of vancomycin for 4 weeks. These results showed that the pre-dialysis mean plasma concentrations of both IS and PCS were elevated. However, following the administration of vancomycin, the IS and PCS concentrations decreased on day 2 or 5 and returned to baseline by day 28. Moreover, the primary change in the gut microbiome was the persistent decrease in diversity, which needs to be elucidated further.
Oral vancomycin not only disrupts the translocation of uremic toxins to the kidney by interfering with intestinal mucosal permeability but also decreases the amount of intestinal Th17 cells, which in turn alleviates renal inflammatory damage [55,97]. Several studies have shown that the addition of SDD reduces the infection rate and mortality in the intensive care unit (ICU), wherein AKI was a serious complication [98]. Thus, this is a promising method to break down the intestinal microbiota dysbiosis, followed by inhibition of inflammation and rebuilding the intestinal barrier [59,99]. However, recent studies have revealed that antibiotic-induced microbiome depletion (AIMD) disrupts host glucose homeostasis with reduced renal glucose and pyruvate levels, causing severe tubular injury after I/R [100]. Therefore, the rational application of antibiotics for the maintenance of intestinal flora homeostasis is challenging for AKI treatment.
Other therapeutic approaches
The oral administration of spherical carbon adsorbent reduces the absorption of harmful metabolites through gastrointestinal sequestration. The oral administration of activated charcoal AST-120 upregulates the expression of the colonic epithelial TJ proteins, such as ZO-1, occludin, and claudin-1, which was associated with the intestinal epithelial barrier [101]. AST-120 reduces the level of IS-derived from tryptophan metabolism in plasma and uric levels and ameliorates CKD-induced endotoxemia and systemic inflammation [42,102]. Furthermore, CharXgen is a new activated and safe charcoal that lowers the level of protein-bound uremic toxins [103]. Thus, the application of carbon adsorbent might slow the chronic process of AKI.
Another study demonstrated the renoprotective effects of gut-derived D-serine in mediating AKI [36]. In a mouse kidney I/R model, AKI-induced gut dysbiosis contributed to the altered metabolism of D-amino acids, which decreased the activity of D-amino acid oxidase and increased the activity of serine racemase. In this study, oral D-serine administration repaired the kidney injury in this AKI mouse model. It also suppressed hypoxia-induced tubular damage during the early phase of injury and promoted post-hypoxic tubular cell proliferation, thereby deeming it as a potential novel therapeutic target for AKI.
In addition to the above drug treatment, traditional Chinese medicine (TCM) is gaining increasing attention to maintain intestinal microbiota balance [104]. Oral administration of high-dose of emodin (TCM extract) in gentamicin sulfate-induced AKI rats restores the disrupted microbiome compared to the control group [105]. Similarly, oral emodin protects the intestinal epithelial barrier and reduces uremic toxin accumulation in CKD patients [106]. Based on these results, TCM application in AKI might be one of the key therapeutic methods in the future.
Future directions and perspectives
Fecal microbiota transplantation (FMT) has been explored as an effective and safe intervention for intestinal microbiota reprogramming [107]. It has also been shown to be a promising treatment in other diseases, including recurrent Clostridium difficile infection [108], neurological disorders [109], ulcerative colitis [110], and metabolic syndrome [111]. Interestingly, a recent study demonstrated that FMT alleviates tubulointerstitial inflammation in diabetic nephropathy rats by decreasing serum IL-6 levels. Furthermore, the desquamation and necrosis of tubular epithelial cells in the FMT group were significantly attenuated compared to the diabetic rats [112]. In addition, FMT has been confirmed to modulate renal phenotype and alleviate inflammation, including reduction of albuminuria immediately and a decreased expression of KC chemokine in the humanized mouse model of IgA nephropathy [113]. Another study indicated that FMT was efficacious in treating IgAN in two patients with refractory IgAN [114]. Strikingly, FMT treatment improved the gut microbiota disturbance in the adenine-induced CKD mice model, further limiting the accumulation of uremic toxins issued from the intestinal cresol pathway [115].
Intriguingly, the main pathological feature of AKI is characterized by acute tubular epithelial cells injury. Maladaptive or incomplete repair of renal tubules increases the profibrotic signaling leading to the transition of AKI to subsequent CKD [116]. Thus, the early use of FMT prevents renal function deterioration. However, the benefits and risks of FMT need to be assessed in clinical practice. A study reported that a patient died from infection because of drug-resistant Escherichia coli bacteria in donor stool samples [117]. The published literature on FMT in kidney diseases is limited; thus, future studies should explore this possibility in AKI [34]. Table 1 summarizes the recent advances in novel methods on intestinal microbiota for kidney diseases.
Table 1.
Therapies targeting the intestinal microbiota in kidney diseases.
| Methods | Detailed strain | Effects | Applications |
|---|---|---|---|
| Probiotics | Lactobacillus, Bifidobacteria | Anti-inflammatory, decrease oxidative stress and apoptosis, and limit absorption across intestine | LPS-AKI, CIS-AKI, CKD |
| Prebiotics | Lactulose | Stimulate the growth and activity of beneficial intestinal bacteria | CKD |
| SCFAs | Butyrate, propionate, and acetate | Modulate the immune homeostasis | IRI-AKI, Sepsis-AKI |
| Immune modulator | Monoclonal antibodies | Dampen the immune response | IRI-AKI |
| SDD | Vancomycin | Reduce uremic toxins, rebuild the intestinal barrier | AKI |
| Others | Charcoal adsorbent, D-serine, TCM | Protect the intestinal barrier, reduce uremic toxins | AKI, CKD |
| FMT | Intestinal microbiota reprogramming | Anti-inflammatory, repair tubular epithelial cells injury | CKD |
Conclusion
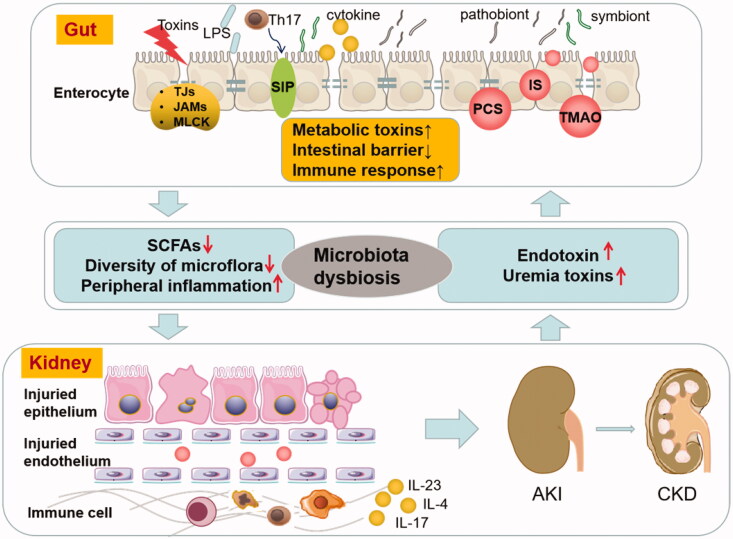
The crosstalk of gut and kidney is a hot topic in human multiorgan diseases. A significant effort has been taken to understand the complex and abnormal correlation between AKI and intestinal microbiota dysbiosis to create a ‘vicious circle’ (Figure 1). AKI development alters enteric microbial compositional disruption, while the kidney can also be the direct target of intestinal microbiota dysbiosis by intestinal barrier disruption and excessive secretion of uremic toxins, which might trigger immune dysfunction that damages the renal tubular cells. Due to intestine microbiota dysbiosis’s critical role in AKI, novel preventive or therapeutic targets are under investigation. The present review summarized the treatment options on the regulation of intestinal microbiota in AKI, including the intake of probiotics and prebiotics, administration of beneficial intestinal metabolic products, such as SCFAs, the addition of the necessary modulator for microbiota immune, and selective decontamination of the digestive tract as oral antibiotics. In addition, activated charcoal, D-serine, and effective TCM are promising strategies to modulate intestinal microbiota in AKI. Despite the limited data available on FMT in kidney diseases, FMT might provide a reference for further research. To date, there is still a lack of clinical intervention trials investigating these novel AKI therapies. Therefore, further studies are urgently required to elucidate the underlying mechanisms and the clinical application of these treatments on intestine microbiota that would ameliorate AKI. Also, additional knowledge of intestinal microbiota-AKI is necessary as it may open new avenues for AKI therapy.
Figure 1.
Vicious circle of AKI and intestinal microbiota dysbiosis. AKI profoundly alters enteric microbial compositional disruption, and the kidney can also be the direct target of intestinal microbiota dysbiosis by intestinal barrier disruption and excessive secretion of uremic toxins and triggers immune response. AKI profoundly alters enteric microbial compositional disruption, and the kidney is also the direct target of intestinal microbiota dysbiosis by intestinal barrier disruption and excessive secretion of uremic toxins and triggers immune response. (LPS: lipopolysaccharide; TJs: tight intercellular junctions; JAMs: junctional adhesion molecules; MLCK: myosin light chain kinase; SCFAs: short-chain fatty acids; PCS: p-cresyl sulfate; IS: indoxyl sulfate; TMAO: trimethylamine-N-oxide; AKI: acute kidney injury; CKD: chronic kidney disease).
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant no. 82100719), the Natural Science Foundation of Jiangsu Province (grant no. BK20210982), and the Science and Technology Development Foundation of Nanjing Medical University (grant no. NMUB2019040).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Agarwal A, Dong Z, Harris R, et al. ;Acute Dialysis Quality Initiative XIII Working Group . Cellular and molecular mechanisms of AKI. J Am Soc Nephrol. 2016;27(5):1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Cai J, Tang C, et al. Mitophagy in acute kidney injury and kidney repair. Cells. 2020;9(2):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He L, Wei Q, Liu J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92(5):1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson FP, Yang W, Machado CA, et al. Dialysis versus nondialysis in patients with AKI: a propensity-matched cohort study. Clin J Am Soc Nephrol. 2014;9(4):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilg H, Adolph TE, Gerner RR, et al. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33(6):954–964. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H, Cani PD, Mayer EA.. Gut microbiome and liver diseases. Gut. 2016;65(12):2035–2044. [DOI] [PubMed] [Google Scholar]
- 8.Torres-Fuentes C, Schellekens H, Dinan TG, et al. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747–756. [DOI] [PubMed] [Google Scholar]
- 9.Bao N, Chen F, Dai D.. The regulation of host intestinal microbiota by polyphenols in the development and prevention of chronic kidney disease. Front Immunol. 2019;10:2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Ma L, Fu P.. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des Dev Ther. 2017;11:3531–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Ankawi G, Sun J, et al. Gut–kidney crosstalk in septic acute kidney injury. Crit Care. 2018;22(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrieta M-C, Stiemsma LT, Amenyogbe N, et al. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng HJ, Guo J, Jia Q, et al. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;142:303–313. [DOI] [PubMed] [Google Scholar]
- 14.Consortium THMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meddings J. The significance of the gut barrier in disease. Gut. 2008;57(4):438–440. [DOI] [PubMed] [Google Scholar]
- 17.Paone P, Cani PD.. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishima E, Ichijo M, Kawabe T, et al. Germ-free conditions modulate host purine metabolism, exacerbating adenine-induced kidney damage. Toxins (Basel). 2020;12(9):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrianova NV, Popkov VA, Klimenko NS, et al. Microbiome-metabolome signature of acute kidney injury. Metabolites. 2020;10(4):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knauf F, Brewer JR, Flavell RA.. Immunity, microbiota and kidney disease. Nat Rev Nephrol. 2019;15(5):263–274. [DOI] [PubMed] [Google Scholar]
- 21.Yarandi SS, Kulkarni S, Saha M, et al. Intestinal bacteria maintain adult enteric nervous system and nitrergic neurons via toll-like receptor 2-induced neurogenesis in mice. Gastroenterology. 2020;159(1):200–213.e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vadder F, Grasset E, Mannerås Holm L, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115(25):6458–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahl MV, Vaziri ND.. The chronic kidney disease – colonic axis. Semin Dial. 2015;28(5):459–463. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y-Y, Chen D-Q, Chen L, et al. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med. 2019;17(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung S, Barnes JL, Astroth KS.. Gastrointestinal microbiota in patients with chronic kidney disease: a systematic review. Adv Nutr. (Bethesda, MD), 2019;10(5):888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, et al. The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother. 2017;93:412–419. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Yang X, Zhou X, et al. The role and mechanism of intestinal flora in blood pressure regulation and hypertension development. Antioxid Redox Signal. 2021;34(10):811–830. [DOI] [PubMed] [Google Scholar]
- 28.Fang Q, Liu N, Zheng B, et al. Roles of gut microbial metabolites in diabetic kidney disease. Front Endocrinol. 2021;12:636175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrios C, Beaumont M, Pallister T, et al. Gut-microbiota-metabolite axis in early renal function decline. PloS One. 2015;10(8):e0134311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambruzs JM, Larsen CP.. Renal manifestations of inflammatory bowel disease. Rheum Dis Clin North Am. 2018;44(4):699–714. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy DD, Kujawa J, Wilson C, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121(10):3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SA, Cozzi M, Bush EL, et al. Distant organ dysfunction in acute kidney injury: a review. Am J Kidney Dis. 2018;72(6):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramezani A, Raj DS.. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong J, Noel S, Pluznick JL, et al. Gut microbiota-kidney cross-talk in acute kidney injury. Semin Nephrol. 2019;39(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noel S, Martina-Lingua MN, Bandapalle S, et al. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract. 2014;127(1–4):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakade Y, Iwata Y, Furuichi K, et al. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight. 2018;3(20):e97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Kim CJ, Go YS, et al. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020;98(4):932–946. [DOI] [PubMed] [Google Scholar]
- 38.Rabb H, Pluznick J, Noel S.. The microbiome and acute kidney injury. Nephron. 2018;140(2):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassenius MI, Pietiläinen KH, Kaartinen K, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34(8):1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SW, Chen SWC, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91(1):63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoh M, Hayashi H, Watanabe M, et al. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp Nephrol. 2003;95(3):e111–e118. [DOI] [PubMed] [Google Scholar]
- 42.Lano G, Burtey S, Sallée M.. Indoxyl sulfate, a uremic endotheliotoxin. Toxins. 2020;12(4):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. JASN. 2016;27(1):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meijers BKI, Evenepoel P.. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. 2011;26(3):759–761. [DOI] [PubMed] [Google Scholar]
- 45.Pluznick JL. Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int. 2016;90(6):1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karczewski J, Troost FJ, Konings I, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G851–G859. [DOI] [PubMed] [Google Scholar]
- 48.Lau WL, Savoj J, Nakata MB, et al. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci. 2018;132: 509–522. [DOI] [PubMed] [Google Scholar]
- 49.Ruan Q, Lu H, Zhu H, et al. A network-regulative pattern in the pathogenesis of kidney injury following severe acute pancreatitis. Biomed Pharmacother. 2020;125:109978. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Ankawi G, Sun J, et al. Gut-kidney crosstalk in septic acute kidney injury. Crit Care. 2018;22(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. [DOI] [PubMed] [Google Scholar]
- 52.Zahs A, Bird MD, Ramirez L, et al. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G705–G712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaziri ND, Yuan J, Norris K.. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang HR, Gandolfo MT, Ko GJ, et al. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297(5):F1457–F1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krebs CF, Paust H-J, Krohn S, et al. Autoimmune renal disease is exacerbated by S1P-Receptor-1-dependent intestinal Th17 cell migration to the kidney. Immunity. 2016;45(5):1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehrotra P, Patel JB, Ivancic CM, et al. Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by at-1R antagonism. Kidney Int. 2015;88(4):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Kim CJ, Go YS, et al. Intestinal microbiota controls acute kidney injury severity by immune modulation. Kidney Int. 2020;98(4):932–946. [DOI] [PubMed] [Google Scholar]
- 58.Emal D, Rampanelli E, Stroo I, et al. Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2017;28(5):1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrade-Oliveira V, Amano MT, Correa-Costa M, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26(8):1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souza DG, Vieira AT, Soares AC, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173(6):4137–4146. [DOI] [PubMed] [Google Scholar]
- 61.Andrade-Oliveira V, Foresto-Neto O, Watanabe IKM, et al. Inflammation in renal diseases: new and old players. Front Pharmacol. 2019;10:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senok AC, Ismaeel AY, Botta GA.. Probiotics: facts and myths. Clin Microbiol Infect. 2005;11(12):958–966. [DOI] [PubMed] [Google Scholar]
- 63.Di Cerbo A, Palmieri B, Aponte M, et al. Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol. 2016;69(3):187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 65.Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30(1):17–25. [DOI] [PubMed] [Google Scholar]
- 66.Lee E-S, Song E-J, Nam Y-D, et al. Probiotics in human health and disease: from nutribiotics to pharmabiotics. J Microbiol (Seoul, Korea). 2018;56(11):773–782. [DOI] [PubMed] [Google Scholar]
- 67.Tsai Y-L, Lin T-L, Chang C-J, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.García-Arroyo FE, Gonzaga G, Muñoz-Jiménez I, et al. Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS ONE. 2018;13(8):e0202901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haro C, Mónaco ME, Medina M.. Lactobacillus casei beneficially modulates immuno-coagulative response in an endotoxemia model. Blood Coagul Fibrinolysis. 2018;29(1):104–110. [DOI] [PubMed] [Google Scholar]
- 70.Wanchai K, Yasom S, Tunapong W, et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin Sci (Lond). 2018;132(14):1545–1563. [DOI] [PubMed] [Google Scholar]
- 71.Lee T-H, Park D, Kim YJ, et al. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int J Mol Med. 2020;45(4):1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y-J, Li K-Y, Wang P-J, et al. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced Lanyu pig model. J Food Drug Anal. 2020;28(1):103–114. [DOI] [PubMed] [Google Scholar]
- 73.Vitetta L. Probiotics can break the toxic relationship between the intestinal microbiome and the kidney. Dig Dis Sci. 2019;64(2):297–299. [DOI] [PubMed] [Google Scholar]
- 74.Simeoni M, Citraro ML, Cerantonio A, et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur J Nutr. 2019;58(5):2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Zhang W, Zuo L, et al. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr. 2013;109(11):1990–1998. [DOI] [PubMed] [Google Scholar]
- 76.Xing H-C, Li L-J, Xu K-J, et al. Protective role of supplement with foreign bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J Gastroenterol Hepatol. 2006;21(4):647–656. [DOI] [PubMed] [Google Scholar]
- 77.Zhu H, Cao C, Wu Z, et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021;33(10):2091–2093. [DOI] [PubMed] [Google Scholar]
- 78.Morrison DJ, Preston T.. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 80.Rossi M, Johnson DW, Xu H, et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovas Dis. 2015;25(9):860–865. [DOI] [PubMed] [Google Scholar]
- 81.Carney EF. Acute kidney injury. Protective role of gut microbial SCFAs. Nat Rev Nephrol. 2015;11(3):127. [DOI] [PubMed] [Google Scholar]
- 82.Felizardo RJF, Watanabe IKM, Dardi P, et al. The interplay among gut microbiota, hypertension and kidney diseases: the role of short-chain fatty acids. Pharmacol Res. 2019;141:366–377. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi M, Mikami D, Kimura H, et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. 2017;486(2):499–505. [DOI] [PubMed] [Google Scholar]
- 84.Huang W, Zhou L, Guo H, et al. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism. 2017;68:20–30. [DOI] [PubMed] [Google Scholar]
- 85.Al-Harbi NO, Nadeem A, Ahmad SF, et al. Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int Immunopharmacol. 2018;58:24–31. [DOI] [PubMed] [Google Scholar]
- 86.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. JI. 2013;190(5):2301–2310. [DOI] [PubMed] [Google Scholar]
- 88.Fellows R, Varga-Weisz P.. Chromatin dynamics and histone modifications in intestinal microbiota-host crosstalk. Mol Metab. 2020;38:100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du Y, Tang G, Yuan W.. Suppression of HDAC2 by sodium butyrate alleviates apoptosis of kidney cells in db/db mice and HG-induced NRK52E cells. Int J Mol Med. 2020;45(1):210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Li YJ, Loh YW, et al. Fiber derived microbial metabolites prevent acute kidney injury through G-Protein coupled receptors and HDAC inhibition. Front Cell Dev Biol. 2021;9:648639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–1040. [DOI] [PubMed] [Google Scholar]
- 92.Jauregui-Amezaga A, Somers M, De Schepper H, et al. Next generation of biologics for the treatment of Crohn’s disease: an evidence-based review on ustekinumab. Clin Exp Gastroenterol. 2017;10:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nirula A, Nilsen J, Klekotka P, et al. Effect of IL-17 receptor a blockade with brodalumab in inflammatory diseases. Rheumatology (Oxford). 2016;55(suppl 2):ii43–ii55. [DOI] [PubMed] [Google Scholar]
- 94.Baker KF, Isaacs JD.. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77(2):175–187. [DOI] [PubMed] [Google Scholar]
- 95.de Smet AMGA, Kluytmans JAJW, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. [DOI] [PubMed] [Google Scholar]
- 96.Nazzal L, Roberts J, Singh P, et al. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol Dial Transplant. 2017;32(11):1809–1817. [DOI] [PubMed] [Google Scholar]
- 97.Mu Q, Tavella VJ, Kirby JL, et al. Antibiotics ameliorate lupus-like symptoms in mice. Sci Rep. 2017;7(1):13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sánchez-Ramírez C, Hípola-Escalada S, Cabrera-Santana M, et al. Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit Care. 2018;22(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hiippala K, Jouhten H, Ronkainen A, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(8):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Osada Y, Nakagawa S, Ishibe K, et al. Antibiotic-induced microbiome depletion alters renal glucose metabolism and exacerbates renal injury after ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2021;321(4):F455–F465. [DOI] [PubMed] [Google Scholar]
- 101.Vaziri ND, Yuan J, Khazaeli M, et al. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am J Nephrol. 2013;37(6):518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Evenepoel P, Meijers BKI, Bammens BRM, et al. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009;76(114):S12–S19. [DOI] [PubMed] [Google Scholar]
- 103.Lin C-J, Sun C-Y, Wu C-J, et al. CharXgen-activated bamboo charcoal encapsulated in sodium alginate microsphere as the absorbent of uremic toxins to retard kidney function deterioration. IJMS. 2020;21(4):1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng W, Ao H, Peng C, et al. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. [DOI] [PubMed] [Google Scholar]
- 105.Sun J, Luo J-W, Yao W-J, et al. Effect of emodin on gut microbiota of rats with acute kidney failure. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2019;44(4):758–764. [DOI] [PubMed] [Google Scholar]
- 106.Han W-B, Liu Y-L, Wan Y-G, et al. Pathomechanism and treatment of gut microbiota dysbiosis in chronic kidney disease and interventional effects of chinese herbal medicine. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2017;42(13):2425–2432. [DOI] [PubMed] [Google Scholar]
- 107.Gupta A, Khanna S.. Fecal microbiota transplantation. JAMA. 2017;318(1):102. [DOI] [PubMed] [Google Scholar]
- 108.Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kelly CP. Fecal microbiota transplantation-an old therapy comes of age. N Engl J Med. 2013;368(5):474–475. [DOI] [PubMed] [Google Scholar]
- 110.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149(1):110–118.e114. [DOI] [PubMed] [Google Scholar]
- 111.Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611–619.e616. [DOI] [PubMed] [Google Scholar]
- 112.Hu ZB, Lu J, Chen PP, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10(6):2803–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lauriero G, Abbad L, Vacca M, et al. Fecal microbiota transplantation modulates renal phenotype in the humanized mouse model of IgA nephropathy. Front Immunol. 2021;12:694787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao J, Bai M, Yang X, et al. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: the first case reports. Ren Fail. 2021;43(1):928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barba C, Soulage CO, Caggiano G, et al. Effects of fecal microbiota transplantation on composition in mice with CKD. Toxins (Basel). 2020;12(12):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Venkatachalam MA, Weinberg JM, Kriz W, et al. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. [DOI] [PubMed] [Google Scholar]