Summary
Background
Older adults with type 1 diabetes have distinct characteristics that can make optimising glycaemic control challenging. We sought to test our hypothesis that hybrid closed-loop glucose control is safe and more effective than sensor-augmented pump (SAP) therapy in older adults with type 1 diabetes.
Methods
In an open-label, multicentre, multinational (UK and Austria), randomised, crossover study, adults aged 60 years and older with type 1 diabetes using insulin pump therapy underwent two 16-week periods comparing hybrid closed-loop (CamAPS FX, CamDiab, Cambridge, UK) and SAP therapy in random order. Block randomisation by means of central randomisation software to one of two treatment sequences was stratified by centre. The primary endpoint was the proportion of time sensor glucose was in target range between 3·9 and 10·0 mmol/L. Analysis for the primary endpoint and adverse events was by intention-to-treat. The study has completed and is registered at ClinicalTrials.gov NCT04025762.
Findings
38 participants were enrolled. One participant withdrew during run-in because of difficulties with the study pump infusion sets. 37 participants (median [IQR] age 68 [63–70] years, mean [SD] baseline glycated haemoglobin [HbA1c]; 7·4% [0·9%]; 57 [10] mmol/mol) were randomly assigned between Sept 4, 2019, and Oct 2, 2020. The proportion of time with glucose between 3·9 and 10·0 mmol/L was significantly higher in the closed-loop group compared to the SAP group (79·9% [SD 7·9] vs 71·4% [13·2], difference 8·6 percentage points [95% CI 6·3 to 11·0]; p<0·0001). Two severe hypoglycaemia events occurred during the SAP period. There were two non-treatment related serious adverse events: cardiac arrest from pulmonary embolism associated with COVID-19 during the SAP period resulting in death, and a hospital presentation for parenteral hydrocortisone because of COVID-19 in a participant with adrenal insufficiency during the run-in period.
Interpretation
Hybrid closed-loop insulin delivery is safe and achieves superior glycaemic control to SAP therapy in older adults with long duration of type 1 diabetes. Importantly this was achieved without increasing the risk of hypoglycaemia in this population with risk factors for severe hypoglycaemia. This suggests that hybrid closed-loop therapy is a clinically important treatment option for older adults with type 1 diabetes.
Introduction
The population of older adults with type 1 diabetes is growing because of rising incidence and advancements in diabetes care leading to increased longevity.1 Older adults present distinct challenges for management of type 1 diabetes. Impaired awareness of hypoglycaemia is more common with increasing age and diabetes duration, with as many as 28% of adults with diabetes duration of 30 years or more having impaired awareness of hypoglycaemia as determined by Gold score.2 The WISDM study showed a high burden of undetected hypoglycaemia, particularly overnight, when adults aged over 60 years used a masked glucose sensor.3 Time spent with glucose concentrations of less than 3·9 mmol/L was 4·9% (70 min/day) when participants used standard blood glucose monitoring.
The prevalence of diabetes related complications and other significant comorbidities also rises with increasing age and duration of diabetes, with older adults having the highest rates of major lower-extremity amputation, cardiovascular events, visual impairment, and end-stage renal disease in addition to potential problems with hearing, dexterity, mobility, and cognition.4–7
Although recommended glycaemic targets are different for older adults with type 1 diabetes, with emphasis on minimising hypoglycaemia,8 older adults remain at risk of developing the vascular complications associated with suboptimal glycaemic control. Many older adults wish to pursue the standard recommended targets to avoid complications associated with persistent hyperglycaemia. Severe hypoglycaemia remains a common occurrence in this population.9,10
Hybrid closed-loop systems are transforming the management of type 1 diabetes for children and adults, and there is good evidence to support the safety and efficacy of this approach.11 The ability to manage technology and devices in older adults might not be as widespread as in younger people and high-quality evidence for the safety and efficacy of closed-loop technology in older adults is scarce. In the present study, we sought to test our hypothesis that use of the Cambridge closed-loop algorithm in older adults with type 1 diabetes is safe and improves glucose control compared with sensor augmented pump (SAP) therapy.
Methods
Study design and participants
The study adopted an open-label, multicentre, multinational, two-period, randomised, crossover design contrasting 16 weeks of hybrid closed-loop insulin delivery with 16 weeks of sensor augmented pump therapy. Participants were recruited from diabetes outpatient clinics at three centres in the UK (Cambridge, Manchester, and Birmingham) and one centre in Austria (Graz).
Key inclusion criteria were age 60 years and above, type 1 diabetes for 12 months or more, insulin pump therapy for 3 months or more, and screening glycated haemoglobin (HbA1c) 86 mmol/mol or less (≤10·0%). Key exclusion criteria included current use of a closed-loop system, use of any glucose-lowering agent commenced in the 3 months before enrolment, and more than one episode of severe hypoglycaemia during the preceding 6 months. Complete inclusion and exclusion criteria are in the appendix (p 2). There was no screening for dexterity, vision, or hearing impairment. There was no screening for cognitive impairment but given the inclusion criteria of insulin pump use with good knowledge of insulin self-adjustment, individuals with substantial cognitive impairment would probably not have been included in the study cohort. Participant withdrawal criteria are in the appendix (p 9). Eligible participants were identified by clinical teams at each centre.
Approval was received from an independent research ethics committee in the UK (17/NW/0394) and Austria (31–446 ex 18/19), and regulatory authorities in the UK (Medicines and Healthcare products Regulatory Agency), and in Austria (Austrian Agency for Health and Food Safety). Safety aspects were overseen by an independent data safety monitoring board which met every 6 months. Participants gave written informed consent before any study procedures. The study protocol and statistical analysis plan are available in the appendix.
Randomisation and masking
Participants were assigned following the run-in period by means of block randomisation and central randomisation software (Randomizer version 2.1.0, Medical University Graz, Graz, Austria) to one of two treatment sequences; 16 weeks of closed-loop insulin delivery followed by 16 weeks of sensor augmented pump therapy or vice versa. There was a 4 week washout period between treatment periods. Randomisation was stratified by centre and done by the site study team.
Procedures
Participants were screened for eligibility including blood tests. Following enrolment, participants had bodyweight and height measured, a 12-lead electrocardiogram (ECG) done, baseline questionnaires administered, and were trained on the study devices which were used as sensor augmented pump therapy (auto mode disabled) for a 3–4 week run-in period at home following baseline screening. A minimum of 10 days of sensor data during the run-in period, and demonstration of safe use of the insulin pump was required for randomisation.
Study visit schedules are in the appendix (pp 4–7). Following random assignment to the closed-loop insulin delivery system group, participants were trained on the closed-loop system and then used this at home over 16 weeks with no study restrictions to activities or travel. For the sensor augmented pump (SAP) therapy period participants used the same study devices as during the closed loop period but with the auto mode function disabled. Participants used the devices for 16 weeks with no study restrictions to activities or travel. There was no low glucose suspend or predictive low glucose suspend functionality during the SAP period. For those randomly assigned to SAP therapy first, this sequence was reversed.
For both periods, after the three telephone or email contacts in the first 2 weeks after treatment period initiation, all participants were contacted by the study team monthly to record adverse events, device deficiencies, and other relevant information. Throughout the study, participants or their clinical team were free to adjust diabetes therapy, but no active treatment optimisation was done by the research team outside of planned study contacts. Study contacts were scheduled more frequently than would be provided in standard clinical care to apply study devices (Actiwatch [Philips Respironics, Murrysville, PA, USA] and Lifecard Holter monitor [Spacelabs Healthcare, Snoqualmie, WA, USA]). All participants were able to contact a 24-h telephone helpline to the local research team throughout the study, but there was no remote monitoring by the study team. During the washout period, participants could continue to use the study devices with auto mode disabled or they could continue to use their prestudy devices. There were no study procedures during the washout period.
HbA1c was measured locally at enrolment, at treatment initiation and at the end of each study group by means of an International Federation of Clinical Chemistry reference HbA1c method.
The CamAPS FX hybrid closed-loop system comprises an unlocked android smartphone (Samsung Galaxy S8, South Korea) hosting the CamAPS FX app (CamDiab, Cambridge, UK) running the Cambridge adaptive model predictive control algorithm (version 0.3.71), which receives sensor data from the Dexcom G6 continuous glucose monitor (Dexcom, San Diego, CA, USA) and directs insulin delivery on a Dana Diabecare RS insulin pump (Sooil, Seoul, South Korea). Both pump and sensor communicate via Bluetooth with the CamAPS FX app hosted on the smartphone. Participants could use the CamAPS FX app on their own smartphone provided this was compatible.
The control algorithm was initialised by use of total daily insulin dose and bodyweight. Algorithm directed insulin delivery is automatically adjusted every 8–12 minutes, with the app-based control algorithm communicating the current insulin infusion rate to the insulin pump wirelessly. Insulin sensitivity and active insulin time are automatically calculated and adjusted over time by the adaptive algorithm. When auto mode is not operational, the insulin pump reverts to preprogrammed basal rates. The treat-to-target control algorithm had a nominal glucose target concentration of 5·8 mmol/L, which was adjustable between 4·4 and 11·0 mmol/L across different times of day and night. Participants were free to adjust their targets during different times of the day throughout the study period. The closed-loop system includes an optional exercise mode (Ease-off function), which temporarily raises the glucose target and suspends algorithm-directed insulin delivery if sensor glucose is less than 7·0 mmol/L and a so-called Boost function to intensify algorithm-driven insulin delivery. No information on the use of Ease-off and Boost was collected.
Outcomes
The primary endpoint was the between-group difference in time in target glucose range from 3·9 to 10·0 mmol/L over the 16-week intervention periods. Key secondary endpoints were time in hyperglycaemia (>10·0 mmol/L), mean sensor glucose, HbA1c, and time in hypoglycaemia (<3·9 mmol/L) over the 16 week treatment periods and at the 16-week treatment period. Additional secondary efficacy endpoints were SD and coefficient of variation of glucose, time with glucose less than 3·5 mmol/L, less than 3·0 mmol/L, greater than 16·7 mmol/L, and insulin metrics (appendix). Sensor-use and closed-loop use were assessed over the 16-week treatment periods. A subset of secondary endpoints (time in the target range, time <3·9 mmol/L, time >10·0 mmol/L, mean sensor glucose, SD of glucose, and total insulin dose) were also tabulated separately for daytime (0600 h–2359 h) and night-time (0000 h–0559 h) over the 16-week period.
Safety evaluation comprised the frequency of severe hypoglycaemia and diabetic ketoacidosis, and other adverse events or serious adverse events.
Statistical analysis
The power calculation is based on improvements in time in target glucose range (3·9–10·0 mmol/L). A sample size of 31 participants was established to have 80% power to detect a between-group difference in time in target, assuming a population difference of 10 percentage points,12 an SD of 18 percentage points at baseline, and a two-sided type 1 error rate of 0·05. Up to 40 participants were to be recruited aiming for 36 completed participants to allow for drop-outs.
Analyses were done on an intention-to-treat basis. All randomly assigned participants were included in the primary analysis. The primary endpoint (time in target glucose range) and secondary endpoints were compared between treatment groups by means of a linear mixed model. Analysis included the fixed, categorical effects of treatment period and the random, categorical effect of site. For highly skewed data a rank-based transformation was used. Missing data were not imputed.
Significance tests were based on a two-sided α=0·05 (two-sided 95% CIs). For the primary endpoint and other key endpoints, the familywise type I error rate was controlled at two-sided α=0·05 by means of a gatekeeping strategy. The primary endpoint (ie, time spent with sensor glucose concentrations between 3·9 and 10·0 mmol/L, was tested first, and if it passed significance testing, other key endpoints were tested in the order listed below by means of the fixed-sequence method at α=0·05 in the following order: time spent above target glucose (10·0 mmol/L), HbA1c, average of glucose concentrations, and time spent below target glucose (3·9 mmol/L).
Secondary endpoints were adjusted for multiple comparisons to control the false discovery rate by means of the Benjamini-Hochberg method.13 Outcomes were calculated by means of GStat software, version 2.3 (University of Cambridge, Cambridge, UK), and statistical analyses were done with SPSS version 27.
The study is registered with clinicaltrials.gov (NCT04025762).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Sept 4, 2019 and Oct 2, 2020, 38 participants were enrolled. One participant withdrew during run-in because of difficulties with the study pump infusion sets. Overall, 37 participants were randomly assigned (median [IQR] age 68 [63–70] years, 57% [n=21] male, mean [SD] baseline HbA1c 7·4% [0·9%]; 57 [10] mmol/mol]; table 1). One participant died during the first study period (sensor-augmented pump therapy) following cardiac arrest caused by a pulmonary embolism associated with COVID-19 infection. Two participants from Graz had reduced duration of the second study period (90 days and 92 days) owing to Brexit-related sponsorship issues. The flow of participants through the study is shown in the appendix (p 14).
Table 1:
Baseline characteristics
| Overall (n=37) | Closed-loop first group (n=20) | Sensor-augmented pump therapy first group (n=17) | |
|---|---|---|---|
|
| |||
| Age, years | 68 (63–70) | 68(63–70) | 67 (62–70) |
| Sex | |||
| Female | 16 (43%) | 8 (40%) | 8 (47%) |
| Male | 21 (57%) | 12 (60%) | 9 (53%) |
| Ethnic | |||
| White | 36 (97%) | 20 (100%) | 16 (94%) |
| Black African, Caribbean | 1 (3%) | 0 | 1 (6%) |
| Body-mass index, kg/m2 | 27·4 (25·2–30·0) | 28·2 (25·4–31·7) | 27·4 (24·9–38·5) |
| Duration of diabetes, years | 38 (32–47) | 38 (32–48) | 38 (32–48) |
| Duration of pump therapy, years | 10 (7–15) | 11 (8–16) | 9 (5–14) |
| Presence of diabetes related complications | |||
| Macrovascular disease | 4 (11%) | 2 (10%) | 2 (12%) |
| Nephropathy | 4 (11%) | 2 (10%) | 2 (12%) |
| Retinopathy | 9 (24%) | 5 (25%) | 4 (24%) |
| Neuropathy | 10 (27%) | 5 (25%) | 5 (29%) |
| Foot disease | 4 (11%) | 3 (15%) | 1 (6%) |
| Charlson comorbidity index | 4 (1) | 4 (2) | 4 (1) |
| Continuous glucose monitor user | 25 (68%) | 13 (65%) | 12 (71%) |
| HbA1c, mmol/mol | 57 (10) | 58 (10) | 57 (9) |
| HbA1c, % | 7·4% (0·9%) | 7·5% (1·0%) | 7·4% (0·9%) |
| Percentage of time with glucose | |||
| 3·9–10·0 mmol/L | 70·0 (13·8) | 69·6 (14·1) | 70·3 (13·7) |
| >10·0 mmol/L | 25·5 (15·2–41·0) | 25·5 (15·1–41·9) | 25·5 (15·9–39·8) |
| >16·7 mmol/L | 0·6 (0·0–1·8) | 0·7 (0·2–1·9) | 0·6 (0·0–2·0) |
| <3·9 mmol/L | 1·8 (0·8–3·2) | 1·6 (0·4–2·7) | 1·8 (1·1–4·2) |
| <3·0 mmol/L | 0·1 (0·0–0·4) | 0·1 (0·0–0·4) | 0·1 (0·0–0·4) |
| Mean glucose, mmol/L | 8·5(1·2) | 8·6 (1·3) | 8·5 (1·2 |
| Glucose, mmol/L | 2·8 (0·5) | 2·8 (0·6) | 2·8 (0·5) |
| Total daily insulin, units per day | 45·1 (36·8–57·2) | 45·8 (38·3–51·1) | 40·0 (35·4–62·4) |
| Total daily basal insulin, units per day | 21·8 (16·0–27·4) | 22·0 (17·1–27·0) | 21·8 (14·2–28·2) |
| Total daily bolus insulin, units per day) | 23·2 (18·7–32·3) | 24·0 (19·3–32·3) | 23·2 (16·5–32·0) |
| TDD (units per kg/day) | 0·5 (0·5–0·6) | 0·5 (0·4–0·6) | 0·6 (0·5–0·7) |
Data are n (%), mean (SD), or median (IQR) unless otherwise indicated. Glucose data are based on sensor glucose measurements, HbA1c=glycated haemoglobin.
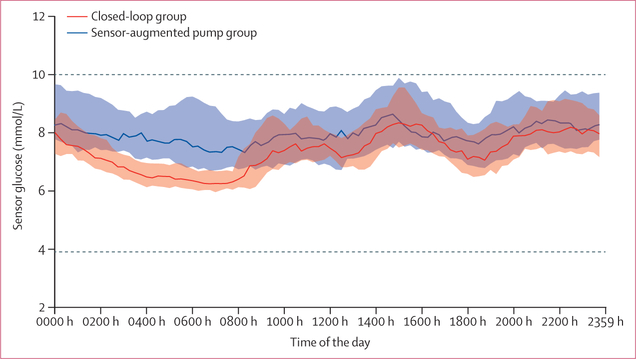
Primary, key, and secondary endpoints for all randomly assigned participants are shown in table 2. The time in target glucose range 3·9 to 10·0 mmol/L was 8·6 percentage points (95% CI 6·3–11·0; p<0·0001) higher in 16-week closed-loop period compared with the 16-week sensor-augmented pump (SAP) period. The time spent in hyperglycaemia (>10·0 mmol/L), HbA1c, and mean sensor glucose were all significantly lower during closed-loop compared with during the SAP period (table 2). The figure shows the 24-h glucose profiles. There was no difference in time spent in hypoglycaemia <3·9 mmol/L, between interventions (table 2). Type 1 error has not been controlled outside the hierarchical analysis given the final p value of 0·54.
Table 2:
Glucose control, insulin delivery, and usage endpoints in the intention-to-treat analysis population
| Closed-loop group (n=36) | Sensor-augmented pump therapy group (n=37) | Treatment difference (95% CI) | p value* | |
|---|---|---|---|---|
|
| ||||
| Primary endpoint † | ||||
| Time with glucose 3·9 to 10·0 mmol/L, % | 79·9% (7·9) | 71·4% (13·2) | 8·6 (6·3 to 11·0) | <0·0001 |
| Key secondary endpoints† | ||||
| Time with glucose >10·0 mmol/L, % | 16·7% (11·4 to 23·9) | 21·4% (16·9 to 36·5) | −8·5% (−10·9 to −6·1) | <0·0001 |
| Mean glucose, mmol/L | 7·8 (0·7) | 8·5 (1·1) | −0·7 (−0·9 to −0·5) | <0·0001 |
| HbA1c, mmol/mol | 49·3 (7·9) | 52·1 (9·2) | −2·7 (−4·2 to −1·2) | 0·0008 |
| HbA1c, % | 6·7% (0·7%) | 6·9% (0·9%) | −0·2% (−0·4 to −0·1) | 0·0008 |
| Time with glucose <3·9 mmol/L, % | 1·7 (1·3 to 2·4) | 1·7 (0·9 to 2·7) | −0·1 (−0·3 to 0·2) | 0·54 |
| Other secondary endpoints ‡ | ||||
| Time with glucose | ||||
| <3·5 mmol/L, % | 0·7% (0·5 to 1·1) | 0·7% (0·4 to 1·2) | 0·0% (−0·2 to 0·1) | 0·69 |
| <3·0 mmol/L, % | 0·2% (0·1 to 0·3) | 0·2% (0·1 to 0·3) | 0·0% (−0·1 to 0·1) | 0·69 |
| >l6·7 mmol/L, % | 0·5% (0·2 to 0·8) | 0·8% (0·2 to 2·8) | −0·7% (−1·0 to −0·3) | <0·0001 |
| Glucose, mmol/L | 2·6 (0·5) | 2·8 (0·6) | −0·2 (−0·3 to −0·1) | <0·0001 |
| Glucose coefficient of variation, % | 32·5 (4·2) | 32·7 (4·5) | −0·3 (−1·2 to 0·6) | 0·49 |
| Total daily insulin, units per day | 46·3 (36·9 to 53·5) | 42·9 (36·6 to 53·0) | 1·2 (−0·6 to 3·0) | 0·20 |
| Total daily basal insulin, units per day | 27·7 (18·9 to 32·0) | 21·5 (15·9 to 27·0) | 4·7 (3·2 to 6·1) | <0·0001 |
| Total daily bolus insulin, units per day | 20·2 (13·5 to 26·1) | 23·4 (17·0 to 29·6) | −3·5 (−4·9 to −2·0) | <0·0001 |
| Total daily dose, units per kg/day | 0·5 (0·5 to 0·6) | 0·5 (0·4 to 0·6) | 0·0 (0·0 to 0·0) | 0·35 |
| Time using continuous glucose monitoring, % | 99·7 (99·3–99·9) | 99·4 (98·8–99·9) | 0·45 (0·06–0·85) | 0·026 |
| Time using closed-loop, % | 96·7% (95·1–98·0) | .. | .. | .. |
Data are mean (SD) or median (IQR). Endpoints calculated from all randomised subjects with at least 168 h of CGM data in at least one period. Glucose data are based on sensor glucose measurements. Treatment difference is calculated as closed loop minus sensor augmented pump therapy. One participant randomised to initial use of sensor-augmented pump therapy did not cross over to closed-loop insulin delivery.
Based on a linear mixed model adjusting for period as a fixed effect and site as a random effect.
Tested in hierarchy as listed to control the type 1 error using the fixed-sequence method.
Adjusted for multiple comparisons using Benjamini-Hochberg procedure to control false discovery rate. HbA1c=glycated haemoglobin
Figure: Sensor glucose concentrations.
Median sensor glucose concentrations and IQRs during closed-loop insulin delivery (solid red line and red shaded area, n=36) and sensor-augmented pump therapy (dashed dark blue line and blue shaded area, n=37). Dashed horizontal lines indicate the target glucose range between 3·9 and 10 mmol/L.
The percentage times spent with glucose less than 3·5 mmol/L and less than 3·0 mmol/L were not significantly different between interventions (table 2). Time spent with glucose concentrations in significant hyperglycaemia (>16·7 mmol/L) was significantly lower in the closed-loop compared with the SAP period. Glucose variability as measured by SD of glucose was significantly lower in the closed-loop period compared to SAP, but there was no difference in the coefficient of variation of glucose between the two periods (table 2). Although total daily insulin dose was similar between treatment periods, basal insulin dose was significantly higher during the closed-loop compared to the SAP period, with a significant reduction in bolus insulin dose (table 2).
A per-protocol analysis of the primary outcome is shown in the appendix (p 11) confirming findings observed applying the intention-to-treat analysis. Efficacy and safety analysis by treatment sequence is shown in the appendix (p 12). There was no carryover effect between interventions (p=0·99).
Closed-loop glucose control overnight was superior to the daytime with time spent in target glucose range overnight 86·8% (8·7%) compared with 77·6% (8·0%) during the daytime period and time spent in hypoglycaemia (<3·9 mmol/L 2·0% [1·5 to 2·7]) during the day and 0·9% (0·5 to 1·6) during the night with closed-loop (appendix p 13). There was an appreciable difference in treatment effect between daytime and night-time across all key glycaemic metrics.
Glucose sensor use was high during both treatment periods at 98% (7%) in the closed-loop and 99% (7%) in the SAP period. In the closed-loop period, the closed loop was operational for 96·7% of the time (table 2).
Safety-related events are summarised in table 3. Two severe hypoglycaemia events occurred during the sensor augmented pump period. There were no diabetic ketoacidosis events. There were two non-treatment related serious adverse events: cardiac arrest from pulmonary embolism associated with COVID-19 during the SAP period resulting in death, and a hospital presentation for parenteral hydrocortisone because of COVID-19 in a participant with adrenal insufficiency during the run-in period. A total of 18 other adverse events (six closed-loop, nine SAP, two run-in, one washout) of which four were study procedure related (skin reactions to Holter electrodes or sensors) and eight device deficiencies (five closed-loop, one SAP, two run-in) were reported. No device deficiency led to an adverse event.
Table 3:
Adverse events and safety analyses in the intention-to-treat analysis population
| Prerandomisation (n=38) | Closed-loop group (n=36) | Sensor-augmented pump therapy group (n=37) | Washout (n=36) | |
|---|---|---|---|---|
|
| ||||
| Severe hypoglycaemia* events | 0 | 0 | 2 | 0 |
| Participants with severe hypoglycaemia | 0 | 0 | 2 (5%) | 0 |
| Incidence rate of severe hypoglycaemia/100 person years | .. | 0 | 17·6 | .. |
| Serious adverse events (not study related) | 1 | 0 | 1 | 0 |
| Participants with serious adverse events | 1 (3%) | 0 | 1 (3%) | 0 |
| Adverse events | 2 | 6 | 9 | 1 |
| Study related | 0 | 3 | 1 | 0 |
| Other | 2 | 3 | 8 | 1 |
| Participants with adverse events | 2 (5%) | 4 (11%) | 7 (19%) | 1 (3%) |
| Number of device deficiencies | ||||
| Pump related | 2 | 3 | 0 | 0 |
| Sensor related | 0 | 1 | 0 | 0 |
| App related | 0 | 1 | 0 | 0 |
| Phone related | 0 | 0 | 1 | 0 |
| Participants with device deficiencies | 2 (5%) | 5 (14%) | 1 (3%) | 0 |
Defined as requiring assistance of another person.
Discussion
This study shows that the Cambridge hybrid closed-loop algorithm is safe, and significantly improves glycaemic control compared with sensor-augmented pump therapy, without increasing hypoglycaemia in older adults with type 1 diabetes.
The time spent in target glucose range (3·9–10·0 mmol/L) with closed-loop in this study population was high at 80%, and the 8·6 percentage point additional time in range compared to SAP therapy equates to an additional 2 h each day in target glucose range. Importantly this improvement in glycaemic control with closed-loop was achieved without any increase in hypoglycaemia and in the context of a population with tight glycaemic control at baseline (baseline HbA1c 7·4%; 57 mmol/mol).
The guidelines suggest relaxation of glycaemic targets in older adults owing to increased risk of hypoglycaemia in older adults with long duration of diabetes.8 However many older adults with type 1 diabetes wish to pursue the standard recommended glycaemic targets to avoid complications associated with persistent hyperglycaemia, but might increase the risk of severe hypoglycaemia. The present study shows that it is possible to achieve excellent glycaemic control by means of a hybrid closed-loop system without increasing the risk of hypoglycaemia above that of sensor augmented pump therapy in older adults. The adjustable target glucose in the Cambridge closed-loop algorithm allows the user to customise the algorithm to suit their personal needs and risks, which is particularly useful in the older adult population. There were no episodes of severe hypoglycaemia during closed-loop period compared with two episodes during sensor augmented pump period.
Hybrid closed-loop use was associated with a significant reduction in time spent in hyperglycaemia greater than 16·7 mmol/L, which is a risk factor for development of acute hyperglycaemic emergencies. The glycaemic outcomes shown with hybrid closed-loop in the present study compare favourably with those of studies of younger adults and children with type 1 diabetes,14–16 probably reflecting the high degree of motivation and engagement with diabetes management in this population as can be observed by the glycaemic control attained during the sensor-augmented pump period.
Although total daily insulin requirements between treatment periods was similar, there was a significant increase in basal insulin delivery during the closed-loop period with a corresponding reduction in bolus insulin delivery. Other commercially available closed-loop systems use auto-corrections to manage hyperglycaemia as their ability to step up basal insulin delivery is more constrained. The CamAPS FX closed-loop system does not have such limitations on basal insulin delivery. The automated basal adjusts for insulin under-delivery and reflects the totality of basal and auto-corrections applied by Medtronic 780G [Northridge, CA, USA] and Tandem Control-IQ systems [San Diego, CA, USA].
Glucose sensor and closed-loop usage were high suggesting trust in the devices and automation of insulin delivery in this population, and the longer-term usability of the hybrid closed-loop system. Retention of participants in the present study was also good, supporting acceptability of this technology. Psychosocial, cognitive, and sleep outcomes will be reported separately.
There is little data regarding the use of closed-loop systems by older adults with which to compare our findings. A small non-randomised pilot study of 15 older adults (aged ≥65 years) compared 4 weeks of SAP therapy to 4 weeks of closed-loop therapy with Tandem Control IQ and showed improved glucose control with 79·6% time in range and 0·8% time in hypoglycaemia (<3·9 mmol/L) during closed-loop use.17 Use of the closed-loop system was associated with high scores in ease of use, trust, and usability. In a real-world analysis of 33 older adults (aged ≥50 years) using the Medtronic 670G closed-loop system, time in target glucose range was between 73% and 75% during 12 months of follow-up,18 whereas in a retrospective analysis of 48 older adults (aged ≥65 years) using Control IQ, time in range was 76% over 3 months of follow-up.19 Assessment of the efficacy and safety of hybrid closed-loop system from these studies is limited by their design.
The strengths of our study include the multicentre, multinational, randomised, crossover design. The control sensor-augmented pump therapy period included the same devices with the closed-loop functionality disabled allowing any differences in glycaemic control to be attributed to algorithm-directed insulin delivery alone. There was very high treatment adherence in both study periods. Despite the study taking place during the COVID-19 pandemic, there were few protocol deviations which could have affected glycaemic outcomes. The study limitations include enrolling participants that might not be fully representative of the general population of older adults with type 1 diabetes owing to the requirement for insulin pump therapy and the low baseline HbA1c, although this is in keeping with reported epidemiological data in this age-group.10 There was little ethnic diversity in the study population which reflects study site demographics and registry demographics in this age group.10 Although there were no issues with training or management of the technology by participants during the study, the study participants had a relatively high level of educational attainment (appendix p 10) and might have had a higher level of technological proficiency than an age-matched population which might limit generalisability of the results to the wider population of older adults with type 1 diabetes. However, our study cohort has a similar level of educational attainment to that reported of older adults in the type 1 diabetes exchange.10
The type 1 diabetes exchange data shows that older adults have lower HbA1c than younger adults, but greater burden of hypoglycaemia.10,20 Real-world data from the US reports a similarly low prevalence of microvascular complications in older adults with similar HbA1c to that of our study cohort (neuropathy 12–16%, nephropathy 11–14%).20 It is possible that those reaching older adulthood with a long duration of type 1 diabetes are characterised by a history of optimal glycaemic control and low burden of complications. These older adults have not succumbed to diabetes-related complications and are therefore over-represented in this age-group.
In conclusion, hybrid closed-loop insulin delivery by means of the CamAPS FX app applying the Cambridge algorithm was safe and led to clinically significant improvements in glycaemic control compared with sensor-augmented pump therapy in older adults with type 1 diabetes over 16 weeks. Closed-loop usage was high, suggesting high acceptability of this therapy in this age group.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles published from database inception up to Sep 30, 2021, with no language restrictions, using the terms (“artificial pancreas” OR “closed-loop”) AND (“type 1 diabetes” OR “diabetes”) AND (“older adults”) AND (“randomised” OR “randomised controlled trial”), for reports of randomised controlled trials. We identified no randomised trials in this population.
A small non-randomised pilot study of 15 older adults (age ≥65 years) compared 4 weeks of closed-loop therapy with Control IQ to 4 weeks of sensor augmented pump therapy and reported 79·6% time in target glucose range and 0·8% of time spent in hypoglycaemia (<3·9 mmol/L) during closed-loop use. In a retrospective real-world analysis of 48 older adults (aged ≥65 years), which used the Control IQ closed-loop system, time in range was 76% during 3 months of follow-up. In a real-world analysis of 33 adults aged 50 years or older, which used the Medtronic 670G closed-loop system, time in target glucose range was between 73% and 75% during 12 months of follow-up. Assessment of the efficacy of the hybrid closed-loop system in this age-group from these non-randomised studies is limited by their design.
Added value of this study
To our knowledge, our study is the only multinational randomised study of hybrid closed-loop use specifically in older adults. Baseline HbA1c in our cohort was low at 7·4% (>57 mmol/mol), reflecting the high degree of motivation and engagement with diabetes management in this population. We showed that compared with sensor augmented pump therapy, hybrid closed-loop insulin delivery led to a clinically significant increase in time in range of 8·6 percentage points and a reduction in HbA1c of 2·7 mmol/mol. Importantly, this was achieved without increasing the time in hypoglycaemia. Closed-loop usage in the present study was high, suggesting high acceptability of this therapy in this age group.
Implications of all the available evidence
The use of the Cambridge hybrid closed-loop algorithm is safe and leads to clinically significant improvements in glycaemic control in older adults with type 1 diabetes compared with sensor augmented pump therapy. Our study shows that it is possible to achieve excellent glycaemic control by means of a hybrid closed-loop system without increasing the risk of hypoglycaemia above that of sensor augmented pump therapy in older adults. Results from this study support the adoption of closed-loop therapy in older adults with type 1 diabetes in clinical practice.
Acknowledgments
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases under the grant number 1DP3DK112176-01. Additiona lsuppor tfo rth eartificia lpancrea swork from National Institute for Health Research Cambridge Biomedical Research Centre, and JDRF. Dexcom supplied discounted continuous glucose monitoring devices. The views expressed are those of the author(s) and not necessarily those of the funders. We are grateful to study volunteers for their participation. Josephine Hayes (University of Cambridge) provided administrative support. We acknowledge support by the staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility. We are grateful for the expertise of the data safety and monitoring board.
Funding National Institute of Diabetes and Digestive and Kidney Diseases.
Declaration of interests
CKB has received consulting fees from CamDiab. SH serves as a member of Medtronic advisory board, is a director of Ask Diabetes providing training and research support in health-care settings, and reports having received training honoraria from Medtronic, Dexcom, and Sanofi. HT receives consulting fees and speaker honoraria from Eli Lilly, and reports having received research support from Dexcom. MEW reports patents related to closed-loop and being a consultant at CamDiab. KKH has received consulting fees from Cecelia Health, Insulet, and Havas Health. JKM is a member on the advisory board of Boehringer Ingelheim, Becton-Dickinson, Eli Lilly, Medtronic, Prediktor A/S, Roche Diabetes Care, and Sanofi-Aventis and received speaker honoraria from Abbott Diabetes Care, AstraZeneca, Becton-Dickinson, Dexcom, Eli Lilly, Mercke Sharp & Dohme, NovoNordisk, Roche Diabetes Care, Sanofi, and Servier. LL has received personal fees from Abbott Diabetes Care, Dexcom, Insulet, Medtronic, Novo Nordisk, and Sanofi. MLE is a clinical triallist with or has served on advisory boards or received speakers or writers fees from Medtronic, Dexcom, Abbott Diabetes Care, Roche, AstraZeneca, Novo Nordisk, Eli Lilly, Zucara, Pila Pharma, and Imcyse Pharma. RH reports receiving speaker honoraria from Eli Lilly, Dexcom, and Novo Nordisk; receiving license or consultancy fees from B Braun and Abbott Diabetes Care; patents related to closed-loop; and being director at CamDiab. WMM, KD, TP, and PN declare no competing financial interests exist.
Footnotes
Data sharing
The study protocol and statistical analysis plan are available in the appendix. A de-identified data set of the outcomes presented in this manuscript will be made available 6 months after publication on a case-by-case basis on reasonable request by email to the corresponding author for research purposes.
Contributor Information
Charlotte K Boughton, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK; Cambridge University Hospitals NHS Foundation Trust, Wolfson Diabetes and Endocrine Clinic, Cambridge, UK.
Sara Hartnell, Cambridge University Hospitals NHS Foundation Trust, Wolfson Diabetes and Endocrine Clinic, Cambridge, UK.
Hood Thabit, Diabetes, Endocrinology and Metabolism Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK; Division of Diabetes, Endocrinology and Gastroenterology, Faculty of Biology, Medicine and Health, University of Manchester, UK.
Womba M Mubita, Diabetes, Endocrinology and Metabolism Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK.
Katharine Draxlbauer, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Tina Poettler, Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Austria.
Malgorzata E Wilinska, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK.
Korey K Hood, Division of Pediatric Endocrinology, Stanford University, Stanford Diabetes Research Center, CA, USA.
Julia K Mader, Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Austria.
Parth Narendran, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK; Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
Lalantha Leelarathna, Diabetes, Endocrinology and Metabolism Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK; Division of Diabetes, Endocrinology and Gastroenterology, Faculty of Biology, Medicine and Health, University of Manchester, UK.
Mark L Evans, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK; Cambridge University Hospitals NHS Foundation Trust, Wolfson Diabetes and Endocrine Clinic, Cambridge, UK.
Roman Hovorka, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK.
References
- 1.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 2012; 61: 2987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen SE, Åsvold BO, Frier BM, Aune SE, Hansen LI, Bjørgaas MR. Hypoglycaemia symptoms and impaired awareness of hypoglycaemia in adults with Type 1 diabetes: the association with diabetes duration. Diabet Med 2014; 31: 1210–17. [DOI] [PubMed] [Google Scholar]
- 3.Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020; 323: 2397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ólafsdóttir AF, Svensson A-M, Pivodic A, et al. Excess risk of lower extremity amputations in people with type 1 diabetes compared with the general population: amputations and type 1 diabetes. BMJ Open Diabetes Res Care 2019; 7: e000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingstone SJ, Looker HC, Hothersal l EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012; 9: e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK Renal Registry. UK Renal registry 23rd annual report—data to Dec 31, 2019. Bristol, UK: UK Renal Registry, 2021. [Google Scholar]
- 7.Weinstock RS, Schütz-Fuhrmann I, Connor CG, et al. Type 1 diabetes in older adults: comparing treatments and chronic complications in the United States T1D Exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract 2016; 122: 28–37. [DOI] [PubMed] [Google Scholar]
- 8.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013; 98: 3411–19. [DOI] [PubMed] [Google Scholar]
- 10.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019; 21: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and metaanalysis. BMJ 2018; 361: k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015; 373: 2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300. [Google Scholar]
- 14.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019; 381: 1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tauschmann M, Allen JM, Wilinska ME, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016; 39: 1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021; 397: 208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisio A, Gonder-Frederick L, McFadden R, et al. The impact of a recently approved automated insulin delivery system on glycemic, sleep, and psychosocial outcomes in older adults with type 1 diabetes: a pilot study. J Diabetes Sci Technol 2021; published online Jan 15, 2021. 10.1177/1932296820986879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berget C, Akturk HK, Messer LH, et al. Real-world performance of hybrid closed loop in youth, young adults, adults and older adults with type 1 diabetes: identifying a clinical target for hybrid closed-loop use. Diabetes Obes Metab 2021; 23: 2048–57. [DOI] [PubMed] [Google Scholar]
- 19.Toschi E, Atakov-Castillo A, Slyne C, Munshi M. Closed-loop insulin therapy in older adults with type 1 diabetes: real-world data. Diabetes Technol Ther 2021; 24: 140–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019; 42: 2220–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.