Abstract
Background and Objectives
Epidemiologic studies have suggested a link between rheumatoid arthritis and Parkinson disease (PD). Disease-modifying antirheumatic drugs (DMARDs) might explain this association. The aim of this work was to evaluate the association between DMARDs and risk of PD in persons with rheumatoid arthritis.
Methods
This nested nationwide case-control study was conducted within the Finnish Parkinson's Disease (FINPARK) cohort, which includes 22,189 Finnish persons with clinically verified PD diagnosed in 1996 to 2015. The cases had recorded diagnosis of PD in the Special Reimbursement Register and had no exclusion diagnoses with symptoms that may be confused with PD within 2 years of PD diagnosis. This study included cases with PD diagnosed during 1999 to 2015 and rheumatoid arthritis diagnosed >3 years before PD. Rheumatoid arthritis was identified from the Finnish Care Register for Health Care and Special Reimbursement Register. Cases were matched with up to 7 controls by age, sex, duration of rheumatoid arthritis, and region. DMARDs were categorized into 5 classes, and data on purchased prescriptions were identified from the Prescription Register since 1995. Associations were studied with conditional logistic regression adjusted for confounders.
Results
Altogether, 315 cases with PD and 1,571 matched controls were included. The majority (>60%) were women, and the median duration of rheumatoid arthritis on matching date was 11.6 years for controls and 12.6 years for cases. Use of DMARDs was not associated with risk of PD with a 3-year lag period applied between exposure and outcome except chloroquine/hydroxychloroquine, which associated with decreased risk (adjusted odds ratio [OR] 0.74, 95% confidence interval [CI] 0.56–0.97). Other DMARDs, including sulfasalazine, methotrexate, gold preparations, and immunosuppressants, were not associated with PD.
Discussion
Our results suggest that the lower risk of PD in people with rheumatoid arthritis is not explained by DMARD use because these drugs in general did not modify the risk of PD among persons with rheumatoid arthritis. Association between chloroquine/hydroxychloroquine and lower risk of PD and the possible underlying mechanisms should be further investigated.
Classification of Evidence
This study provides Class II evidence that in individuals with rheumatoid arthritis using DMARDs, only chloroquine/hydroxychloroquine was associated with a potentially decreased risk of developing PD (adjusted OR 0.74, 95% CI 0.56–0.97).
Rheumatoid arthritis has been linked to lower risk of Parkinson disease (PD),1-3 although some studies have also observed an increased risk of PD in people with rheumatoid arthritis4 or no association between rheumatoid arthritis and PD.5
One suggested explanation for the protective association is the medications used to treat rheumatoid arthritis.1 Disease-modifying antirheumatic drugs (DMARDs) such as methotrexate and sulfasalazine inhibit the rheumatic inflammation and progression of structural joint damage,6 and they could modify the risk of PD by interfering with immune system dysfunction, which has been suggested to be present in PD.7 However, there is very little research on the association of DMARDs with risk of PD. Although 1 study demonstrated lower risk of PD in users and nonusers of DMARDs with rheumatoid arthritis compared to people without rheumatoid arthritis,1 that study did not compare the risk between DMARD users and nonusers with rheumatoid arthritis. A case-control study that investigated several DMARDs reported that the use of azathioprine, leflunomide, or mycophenolate was associated with lower risk of PD.8
The primary research question of our study is whether the use of different DMARDs is associated with the risk of PD. We investigated this in a nested nationwide case-control study restricted to persons with rheumatoid arthritis diagnosed at least 3 years before clinically verified PD. This restriction of study population allowed us to minimize confounding by indication of DMARD treatment. To control for increased contact with health care professionals due to diagnostic process of PD, which could differentially affect the exposure in cases, we applied a 3-year lag period between DMARD use and PD diagnosis. In addition, due to the long onset period of PD, it is unlikely that DMARDs initiated within close proximity to PD diagnosis would affect the risk.
Methods
Finnish Parkinson's Disease Study Population
A nested case-control study was conducted within the Finnish Parkinson's Disease (FINPARK) cohort, which contains all community-dwelling Finnish persons who received special reimbursement for PD drugs in 1996 to 2015 (n = 22,189). These persons were identified with the Special Reimbursement Register, which includes information on entitlements to higher reimbursements for drugs because of chronic diseases. PD diagnosis was based on United Kingdom Parkinson's Disease Society Brain Bank criteria,9 and exclusion diagnoses for FINPARK cohort have been reported previously.10 For every person with PD, up to 7 comparison persons without PD were identified from the Social Insurance Institution database covering all residents, and they were matched for age, sex, and region of residence (n = 148,009). Each Finnish resident is given a unique personal identification number that enables data linkage across several registers. The FINPARK study has been described in detail previously.10
Identification of Cases and Controls for This Study
Formation of the study population is described in Figure 1. Persons diagnosed with PD in 1999 to 2015 (n = 19,568) were included in this study because drug exposure data were available since 1995 and we used a 3-year lag period in exposure assessment. To control for confounding by indication, we restricted the study to people who had been diagnosed with rheumatoid arthritis at least 3 years before PD diagnosis. Rheumatoid arthritis was defined from the Finnish Care Register for Health Care (1987–2012) and Special Reimbursement Register (1972–2012) with the use of ICD-9 and ICD-10 codes as described in eTable 1. In addition, ICD-8 codes (1972–1986) were used to get the earliest diagnosis date of rheumatoid arthritis for those who had rheumatoid arthritis based on ICD-9 or ICD-10 codes. Final diagnosis date for rheumatoid arthritis was defined either as the earliest date of the hospitalization or specialized healthcare outpatient visit or as the first date of the entitlement to reimbursement for drugs used to treat rheumatoid arthritis, whichever occurred first.
Figure 1. Flowchart of Formation of PD Cases and Controls.
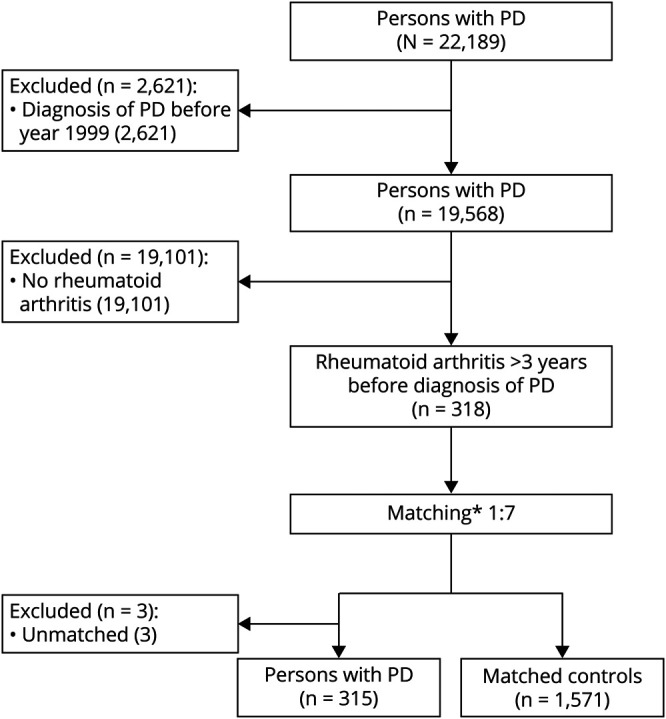
PD = Parkinson disease. *Age ± 2 years, sex, time since rheumatoid arthritis diagnosis ± 2 years, and university hospital district.
For each PD case with rheumatoid arthritis (n = 318), up to 7 controls without PD but with rheumatoid arthritis were matched from the controls of the FINPARK study. Date of the PD diagnosis was defined as the index date. Controls were matched according to sex, age ( ±2 years) on index date, time since rheumatoid arthritis diagnosis on index date ( ±2 years), and university hospital district. If no controls were identified from the same district, controls were allowed to come from a neighboring district. The same exclusion criteria were applied for cases and controls. In addition, controls were not allowed to have the diagnosis of dementia in PD (ICD-10 code F02.3). The final study population included 315 cases and 1,571 controls. Three cases without matched controls were excluded.
Drug Exposure
Data on DMARD and corticosteroid purchases were extracted from the Prescription Register from 1995 until the index date. The Prescription Register includes data on all reimbursed drug purchases; drug use during hospital stays or in public nursing homes is not recorded in this register. Drugs are categorized according to the Anatomical Therapeutic Chemical classification system. Drug use was defined with Anatomical Therapeutic Chemical codes (eTable 2, links.lww.com/WNL/B754), and DMARDs were categorized as follows: sulfasalazine (A07EC01), chloroquine (P01BA01) or hydroxychloroquine (P01BA02), gold preparations (M01CB), including auranofin and sodium aurothiomalate, and immunosuppressants (L04A), which consist of azathioprine, certolizumab pegol, ciclosporin, mycophenolic acid, and biological DMARDs (bDMARDs): abatacept, adalimumab, anakinra, etanercept, golimumab, and leflunomide. Methotrexate (L04AX03) was studied separately from immunosuppressants throughout the study due to its common use in the treatment of rheumatoid arthritis. Due to small number of bDMARD users during the study period, they were combined with users of immunosuppressants in the main analysis. In addition, we performed sensitivity analysis investigating bDMARDs as a separate category.
Corticosteroids (H02AB) covered prednisolone, prednisone, and methylprednisolone. Dexamethasone was excluded due to the low number of reimbursed purchases. All the above-mentioned drugs are available only as prescription drugs, and all reimbursed purchases can be identified reliably from the register. The first date of purchase was determined for each drug or drug group for each person, and person was defined as user if there was at least 1 purchase.
To control for biases caused by (1) prodromal symptoms or ongoing diagnosis process of PD affecting drug exposure or (2) newly diagnosed rheumatoid arthritis or changes in rheumatoid arthritis pharmacotherapy increasing the likelihood of being diagnosed with PD, we applied a 3-year lag period for assessing drug exposure. Three years was chosen on the basis of our previous study demonstrating that the incidence of use of muscle relaxants, an indicator of motor symptoms of PD, occurs within this 3-year period in FINPARK cohort.11 In the main analysis, drug use was determined before the 3-year lag period, indicating that exposure had occurred at least 3 years before the index date. Time before the lag period refers to the exposure assessment period. In addition, drug exposure was measured within the lag period only (within 3 years of index date) or ever before index date.
Furthermore, exposure histories based on the different types of DMARDs used during the exposure assessment period were derived.
Covariates
Comorbid conditions that were considered to be associated with exposure and outcome were used as covariates (eTable 3, links.lww.com/WNL/B754). History of asthma or chronic obstructive pulmonary disease, stroke, diabetes, cardiovascular diseases (including any of the following: hypertension, coronary artery disease, chronic heart failure, and chronic arrhythmias), substance abuse, and head injury were identified from the Special Reimbursement Register, Care Register for Health Care Register or Prescription Register. Cancer history was derived from the Cancer Register with the use of ICD-10 codes from International Agency for Research on Cancer Cancer Collaborative Research Group Tools. All covariates were defined until the start of the 3-year lag period and ever before the index date.
Statistical Analyses
Characteristics of cases and controls were compared with a χ2 test for categorical variables. For continuous variables, the t test was applied for normally distributed data and the Mann-Whitney U test for nonnormally distributed data. Conditional logistic regression was used to estimate the unadjusted odds ratios (ORs) and adjusted ORs (aORs) with 95% confidence intervals (CIs) for the association between exposures and PD. Analyses were conducted for different exposure periods, that is, without the lag period, with the 3-year lag, and use only during the 3-year lag period. We analyzed the association between individual DMARD categories (use vs no use) to assess the association of specific DMARDs. To account for changes in pharmacotherapy for rheumatoid arthritis (i.e., 1 person using more than just 1 type of DMARDs during the exposure assessment period), we grouped the persons according to the types of drugs they had purchased (sulfasalazine, methotrexate, chloroquine/hydroxychloroquine, gold preparations, and immunosuppressants). The association between different exposure histories for DMARDs was investigated compared to the most common exposure type category. Only categories with >5% frequency were reported.
The minimum detectable ORs for different exposure prevalence levels among controls are shown in eFigure 1, links.lww.com/WNL/B754. We had 80% power to detect ORs ≥1.41 (or ≤0.71) with an exposure prevalence of 50% and ORs ≥1.92 (or ≤0.52) with an exposure prevalence of 5% (α = 0.05).
Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). Power calculations were performed with Stata MP14.0 using power mcc-function (StataCorp, College Station, TX).
Standard Protocol Approvals, Registrations, and Patient Consents
According to Finnish legislation, ethics committee approval or informed consent is not needed because included persons cannot be identified due to pseudonymized register data, and the persons were not contacted.
Data Availability
The data used to conduct this research are not publicly available due to restrictions by the register maintainers and Finnish legislation. However, the data are available from the corresponding author, provided that appropriate permission of the register maintainers is sought and demonstrated.
Results
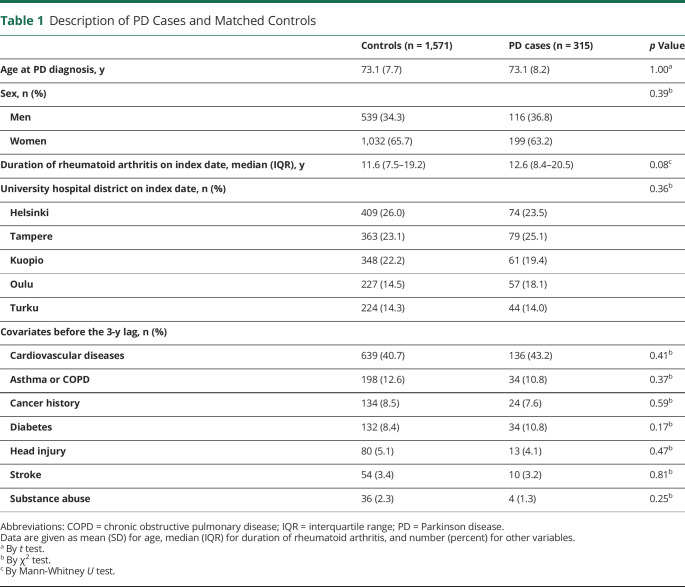
The characteristics of cases (n = 315) and matched controls (n = 1,571) are described in Table 1. The age ranged between 46 and 93 years (mean 73.1 years). Most of the study participants were women. Median duration of rheumatoid arthritis on the index date was 11.6 years for controls and 12.6 years for cases. The prevalence of different comorbid conditions was comparable between PD cases and controls, and cardiovascular diseases were the most common comorbid conditions.
Table 1.
Description of PD Cases and Matched Controls
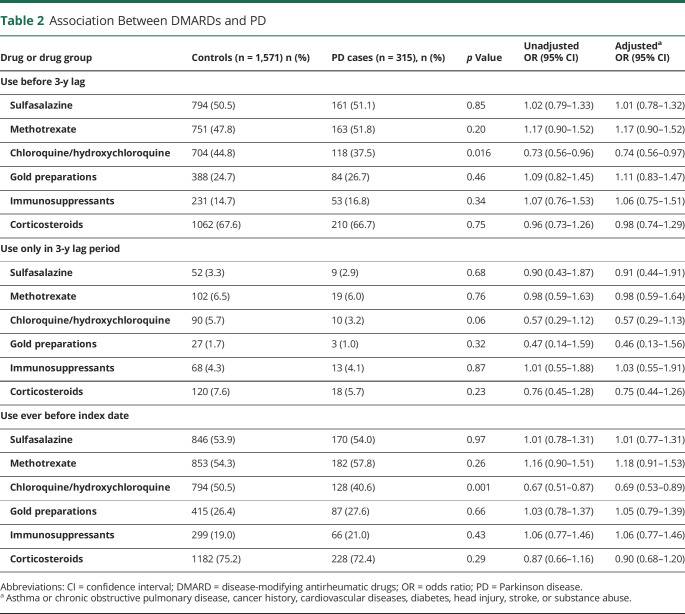
Use of DMARDs and corticosteroids in different time periods is summarized in Table 2. The 3 most commonly used DMARDs in both cases and controls were sulfasalazine, methotrexate, and chloroquine/hydroxychloroquine (Table 2). Gold preparations were used by approximately one-quarter of cases and controls during the exposure assessment period, and immunosuppressants were the least commonly used DMARD. Corticosteroids were used by nearly two-thirds of cases and controls during the exposure assessment period.
Table 2.
Association Between DMARDs and PD
Use of DMARDs or corticosteroids during the exposure assessment was not associated with risk of PD except for use of chloroquine/hydroxychloroquine, which was associated with decreased risk (aOR 0.74, 95% CI 0.56–0.97) (Table 2). The use of bDMARDs was infrequent in the study period, with <3% of cases and controls having used them before the 3-year lag time. They were not associated with risk of PD (aOR 0.98, 95% CI 0.46–2.09).
When any use before the index date was considered, regardless of whether it was initiated during the actual exposure assessment or during the lag time, the associations were similar. When initiations in the 3-year lag period were considered, no associations were observed.
The negative association of chloroquine/hydroxychloroquine was stronger when any use before the index date was considered (aOR 0.69, 95% CI 0.53–0.89) than in the main analysis with exposure that had occurred before the 3-year lag period (aOR 0.74, 95% CI 0.56–0.97).
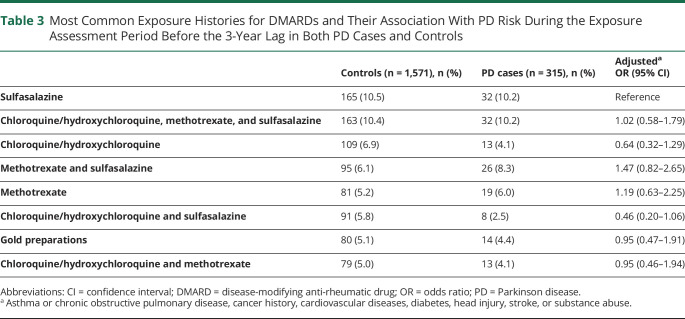
When changes in DMARDs during exposure assessment period (i.e., exposure histories) were considered, the most common exposure type was sulfasalazine, with 10% prevalence in both cases and controls (Table 3). Second most common was the combination of chloroquine/hydroxychloroquine, methotrexate, and sulfasalazine. The frequency of other types was <10% in cases and controls. No associations were observed between different exposure histories for DMARDs and PD risk when adjusted for different covariates (Table 3).
Table 3.
Most Common Exposure Histories for DMARDs and Their Association With PD Risk During the Exposure Assessment Period Before the 3-Year Lag in Both PD Cases and Controls
Classification of Evidence
This study provides Class II evidence that in individuals with rheumatoid arthritis using DMARDs, only chloroquine/hydroxychloroquine was associated with a potentially decreased risk of developing PD (adjusted OR 0.74, 95% CI 0.56–0.97).
Discussion
Studies on the association between DMARDs and risk of PD in a population restricted to rheumatoid arthritis are lacking, although they would aid in understanding whether the inverse association between rheumatoid arthritis and PD is explained by DMARD treatment of rheumatoid arthritis. Our nationwide nested case-control study of people with rheumatoid arthritis found no association between the use of DMARDs or corticosteroids and risk of PD on a general level. However, the use of chloroquine/hydroxychloroquine was associated with lower risk of PD, even when the analyses were restricted to exposure that had occurred at least 3 years before PD diagnosis.
These results extend the findings of earlier studies that have implied the role of immune system in PD pathogenesis.7 Genome-wide association studies have shown that autoimmune diseases, including rheumatoid arthritis, and PD share genetic pathways.12 Lower risk of PD has been observed in people with rheumatoid arthritis in some1-3 but not all studies,4,5 and similarly conflicting findings have been reported for systemic lupus erythematosus,4,13 another autoimmune disease. Given these inconsistent findings, it is difficult to conclude whether autoimmune diseases alter the PD pathophysiology and to what extent. However, the evidence of involvement of immune system dysfunction in PD pathogenesis7 supports the presumption that long-term use of DMARDs, which have anti-inflammatory properties, could explain the reduced risk of PD in individuals with rheumatoid arthritis. It is surprising that the number of pharmacoepidemiologic studies on DMARDs is still small.
Differences in our study design prevent direct comparison to earlier studies that have investigated mainly how rheumatoid arthritis as a disease is associated with risk of PD1-5 or how DMARDs are associated with risk of PD without restricting the study population to persons with rheumatoid arthritis.8 We wanted to focus on DMARDs and to avoid confounding by indication by restricting the study to people with rheumatoid arthritis. This allowed us to evaluate the association of DMARDs and PD instead of rheumatoid arthritis and PD. Second, we considered drug exposure that had occurred at least 3 years before PD diagnosis because PD has a long latency period before actual diagnosis and because potentially increased contact with health care, as evident from the initiation of muscle relaxants already 3 years before PD diagnosis,11 can affect drug exposure. In contrast, a previous case-control study8 applied only a 1-year lag between drug exposure and PD diagnosis. We also conducted sensitivity analyses considering exposure that had occurred until PD diagnosis and during the lag period. The results of these additional analyses were in line with the main analyses.
In Finland, we have a long-standing tradition to aim for remission in the treatment of rheumatoid arthritis. Thus, DMARDs have been used actively during the whole study period. Sulfasalazine was the most commonly used DMARD in our study, followed by methotrexate and chloroquine/hydroxychloroquine. These 3 DMARDs, so-called triple therapy, have been the recommended treatment according to Finnish guidelines for rheumatoid arthritis.14 In addition to individual drugs, we assessed whether exposure histories for different types of DMARDs at least 3 years before PD diagnosis would differ in the risk of PD but observed no differences compared to sulfasalazine.
Some of the DMARDs have previously been associated with reduced risk of PD. In an earlier case-control study,8 users of azathioprine, leflunomide, or mycophenolate had reduced risk of PD compared to nonusers with or without a 1-year lag between drug exposure and outcome. However, we did not observe a risk reduction for the immunosuppressant class in which these drugs, along with bDMARDs, were defined in our study. In a cohort study, people with rheumatoid arthritis had a lower risk of PD than those without rheumatoid arthritis regardless of DMARD use.1 The relative risk reduction was similar in DMARD users and nonusers with rheumatoid arthritis compared to persons without rheumatoid arthritis, meaning that use of DMARDs did not explain the protective association of rheumatoid arthritis. Despite differences in study setting, this reflects our results because in general we found no association between DMARDs use and risk of PD among people with rheumatoid arthritis.
In a previous case-control study, corticosteroids were associated with a lower risk of PD when the exposure had occurred at least 1 year before or up to PD diagnosis,8 while we did not observe an association between corticosteroids and PD. One explanation for our null result might be that despite their immunosuppressive effects, corticosteroids are aimed to be used at a low dose and for only a relatively short time in the treatment of rheumatoid arthritis due to the possible adverse effects in their long-term use such as osteoporosis.15 It is possible to reach remission with active use of DMARDs, even without long-term use of systemic corticosteroids.
Our finding of the association between chloroquine/hydroxychloroquine and lower risk of PD is, however, consistent with the previous case-control study8 that reported a protective association for hydroxychloroquine when any exposure before PD diagnosis was considered (relative risk 0.77, 95% CI 0.65–0.90) and a weaker association when exposure occurring at least 1 year before the outcome was investigated (relative risk 0.83, 95% CI 0.68–1.00). This attenuation may imply that the association in that study was due partially to increased health care contact in close proximity to PD diagnosis. Our findings provide additional support for this earlier observation because we were able to use a longer lag time in exposure assessment.
The neuroprotective potential of chloroquine and hydroxychloroquine has been speculated previously.16,17 Both chloroquine and hydroxychloroquine interfere with lysosomal activity and autophagy, can inhibit both innate and adaptive immune processes, and can reduce the production of inflammatory cytokines such as interleukin-6 and tumor necrosis factor.18 These immunomodulatory effects could have a role in modulating inflammatory processes in PD. However, methotrexate, the first-line DMARD for rheumatoid arthritis that is a more powerful immunosuppressant than hydroxychloroquine,19 was not associated with risk of PD in our study. Therefore, the protective association of chloroquine/hydroxychloroquine might be explained by reasons other than its immunosuppressive effects. Hydroxychloroquine has been implied to have pleiotropic effects; it was recently shown to improve lipid profiles and to reduce diabetes incidence in rheumatoid arthritis,20 a population at increased risk of cardiovascular diseases.21 It is possible that the effects on metabolic and cardiovascular risk factors22 might partly explain our findings. It should be noted that hydroxychloroquine is better tolerated than chloroquine; thus, it is currently used more commonly in the treatment of rheumatoid arthritis.23 Chloroquine was included in our study because we used drug exposure data since 1995.
The protective association of chloroquine/hydroxychloroquine is also supported by experimental models of PD; chloroquine protected dopaminergic neurons against 6-hydroxydopamine–induced neurotoxicity,17 and hydroxychloroquine ameliorated motor functions of rotenone-induced parkinsonian rats in behavioral tests.16 Neuroprotective effects of these drugs were suggested to be mediated through orphan nuclear receptor Nurr1, which is important in the development and maintenance of midbrain dopaminergic neurons24 and the expression of which was activated by both chloroquine17 and hydroxychloroquine.16 In terms of other neurodegenerative diseases, hydroxychloroquine did not, however, slow the progression of dementia in persons with Alzheimer disease compared to placebo in a double-blind clinical trial.25
Our study has several strengths. The definition of PD is based on clinically verified diagnosis. Using large nationwide registries, we could restrict the study population to persons who have the indication to use DMARDs, thereby controlling for confounding by indication. Due to long (up to 17 years) exposure assessment time, we were able to apply 3-year lag period. A short lag period between drug exposure and outcome of PD has been a key limitation in previous studies. If drug exposure is measured too close to the diagnosis of PD, it is more likely to reflect different contact density with prescribers than an actual risk factor. Furthermore, because PD progresses slowly over time, immediate exposure just before diagnosis is unlikely to have a significant effect on PD pathophysiology.
Our study was based on data on purchased drugs, in which case medication adherence is based on presumption. On the other hand, all the drugs included are available only as prescription drugs, which minimizes classification bias. DMARDs administered in hospitals, for example, infliximab, are not included in the Prescription Register. However, these drugs are never used as the first or only DMARD, and every patient receiving infliximab is also treated with other DMARD(s). Therefore, the drugs administered in hospital should not have a major impact on our results.
Although we had nationwide data, restriction of analyses to those with rheumatoid arthritis and at least 3 years of exposure assessment decreased the sample size. This means that we had limited power to detect weak associations. However, the power issue is not likely to explain the null findings because we were able to observe the association between chloroquine/hydroxychloroquine and PD risk and because the point estimates for other DMARDs in the main analyses were close to the null. Furthermore, on the basis of the exposure prevalence, we do not think that a clinically relevant signal was missed because of a lack of power, and restriction of study population helped us to avoid indication bias. We did not perform dose-response or duration of treatment analyses due to limitations posed by the sample size. Some of the newer immunosuppressants included in our study, mainly bDMARDs such as golimumab, entered the market at the end of the study period; therefore, they have been used to a lesser extent compared to older DMARDs such as sulfasalazine, methotrexate, and chloroquine/hydroxychloroquine. Findings of additional analysis regarding bDMARDs should be interpreted cautiously due to limited number of users in our study.
A possible limitation of our study is the lack of information on the severity of rheumatoid arthritis, which could affect chosen pharmacotherapy and have a differential impact on developing PD regardless of DMARD use. Our data included both seropositive and seronegative rheumatoid arthritis. This is a common approach in register-based studies on rheumatoid arthritis and unlikely to have a major impact on our results.
The sex distribution of our study population may appear surprising considering that PD is more common in men.26 However, rheumatoid arthritis is more common in women than in men,15 which explains the sex distribution in our study. Because the earlier studies on rheumatoid arthritis or other autoimmune rheumatic diseases and the risk of PD1,4 have reported sex distribution comparable to that in our study and the findings on the association between rheumatoid arthritis and PD have been inconsistent, it is difficult to speculate whether sex has implications for our results. We matched cases and controls by sex, and thus, our results are unlikely explained by sex.
Linkage of several registers enables us to account for multiple confounding factors, although adjustment with comorbid conditions did not change the results. We lacked data on smoking, which has been associated with an increased risk of rheumatoid arthritis15 and oppositely with a decreased risk of PD.27 However, we used smoking-associated comorbid conditions, including cancer from the Cancer registry, as proxies, but residual confounding is still possible. In addition, the association between chloroquine/hydroxychloroquine and lower risk of PD can be confounded by another variable that was not identified in our study. The association may also be explained by survival bias: both chloroquine and hydroxychloroquine are old drugs, and persons treated with them, especially in monotherapy, might have less severe rheumatoid arthritis and better overall health status than those treated with other DMARDs. However, hydroxychloroquine is also included in the drug combination with methotrexate and sulfasalazine, which is widely used also on moderate and severe rheumatoid arthritis.
The hypothesis that decreased risk of PD among patients with rheumatoid arthritis could be explained by use of DMARDs was not confirmed in our study. Further studies on newer DMARDs, especially on bDMARDs such as tumor necrosis factor-α inhibitors and target-specific DMARDs (JAK inhibitors), and assessment of dose-response relations between DMARDs and risk of PD are needed. The potential ability of chloroquine/hydroxychloroquine to modify the PD disease process should be studied further.
Glossary
- aOR
adjusted odds ratio
- bDMARD
biological DMARD
- CI
confidence interval
- DMARD
disease-modifying antirheumatic drug
- FINPARK
Finnish Parkinson's Disease
- ICD
International Classification of Diseases
- OR
odds ratio
- PD
Parkinson disease
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This project was funded by the Michael J. Fox Foundation for Parkinson's Research (grant MJFF-008834 to Anna-Maija Tolppanen, which paid salaries for Anne Paakinaho and Marjaana Koponen).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Sung YF, Liu FC, Lin CC, et al. Reduced risk of Parkinson disease in patients with rheumatoid arthritis: a nationwide population-based study. Mayo Clin Proc. 2016;91(10):1346-1353. [DOI] [PubMed] [Google Scholar]
- 2.Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH. Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology. 2009;73(18):1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacelis J, Compagno M, George S, et al. . Decreased risk of Parkinson's disease after rheumatoid arthritis diagnosis: a nested case-control study with matched cases and controls. J Parkinsons Dis 2021;11(2):821-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CC, Lin TM, Chang YS, et al. . Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Ann Med. 2018;50(1):83-90. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Sundquist J, Sundquist K. Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegener Dis. 2012;10(1-4):277-284. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023-2038. [DOI] [PubMed] [Google Scholar]
- 7.Tan E-, Chao Y-, West A, Chan L-, Poewe W, Jankovic J. Parkinson disease and the immune system: associations, mechanisms and therapeutics. Nat Rev Neurol. 2020;16(6):303-318. [DOI] [PubMed] [Google Scholar]
- 8.Racette BA, Gross A, Vouri SM, Camacho-Soto A, Willis AW, Searles Nielsen S. Immunosuppressants and risk of Parkinson disease. Ann Clin Transl Neurol. 2018;5(7):870-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentilä E, Tiihonen M, Taipale H, Hartikainen S, Tolppanen AM. Incidence of antidepressant use among community dwellers with and without Parkinson's disease: a nationwide cohort study. BMC Geriatr. 2021;21(1):202-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paakinaho A, Karttunen N, Koponen M, et al. . Incidence of muscle relaxant use in relation to diagnosis of Parkinson's disease. Int J Clin Pharm. 2020;42(2):336-340. [DOI] [PubMed] [Google Scholar]
- 12.Witoelar A, Jansen IE, Wang Y, et al. . Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurol. 2017;74(7):780-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu FC, Huang WY, Lin TY, et al. . Inverse association of Parkinson disease with systemic lupus erythematosus: a nationwide population-based study. Medicine. 2015;94(46):e2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möttönen T, Hannonen P, Leirisalo-Repo M, et al. . Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353(9164):1568-1573. [DOI] [PubMed] [Google Scholar]
- 15.Smolen JS, Aletaha D, Barton A, et al. . Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 16.Hedya SA, Safar MM, Bahgat AK. Hydroxychloroquine antiparkinsonian potential: Nurr1 modulation versus autophagy inhibition. Behav Brain Res. 2019;365:82-88. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Han BS, Moon J, et al. . Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson's disease. Proc Natl Acad Sci USA. 2015;112(28):8756-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155-166. [DOI] [PubMed] [Google Scholar]
- 19.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360-1372. [DOI] [PubMed] [Google Scholar]
- 20.Rempenault C, Combe B, Barnetche T, et al. . Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2018;77(1):98-103. [DOI] [PubMed] [Google Scholar]
- 21.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potashkin J, Huang X, Becker C, Chen H, Foltynie T, Marras C. Understanding the links between cardiovascular disease and Parkinson's disease. Mov Disord. 2020;35(1):55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shippey EA, Wagler VD, Collamer AN. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018;85(6):459-467. [DOI] [PubMed] [Google Scholar]
- 24.Jang Y, Kim W, Leblanc P, Kim CH, Kim KS. Potent synthetic and endogenous ligands for the adopted orphan nuclear receptor Nurr1. Exp Mol Med. 2021;53(1):19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Gool WA, Weinstein HC, Scheltens P, Walstra GJ. Effect of hydroxychloroquine on progression of dementia in early Alzheimer's disease: an 18-month randomised, double-blind, placebo-controlled study. Lancet. 2001;358(9280):455-460. [DOI] [PubMed] [Google Scholar]
- 26.Cerri S, Mus L, Blandini F. Parkinson's disease in women and men: what's the difference? J Parkinsons Dis. 2019;9(3):501-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo V, Vineis P, Cancellieri M, et al. . Exploring causality of the association between smoking and Parkinson's disease. Int J Epidemiol. 2019;48(3):912-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to conduct this research are not publicly available due to restrictions by the register maintainers and Finnish legislation. However, the data are available from the corresponding author, provided that appropriate permission of the register maintainers is sought and demonstrated.