ABSTRACT
Background
The relationship between intake of sugar-sweetened beverages (SSBs) and the risk of death in patients with chronic kidney disease (CKD) is unclear. We evaluated the association between SSB intake and subsequent overall mortality in CKD patients.
Methods
We included data from 3996 CKD patients who participated in the 1999–2014 National Health and Nutrition Examination Survey (NHANES). SSB intake was assessed by a 24-h dietary recall, grouped as none, >0 to <1 serving/day, 1 to <2 servings/day and ≥2 servings/day. After adjusting for demographic variables, lifestyle, diet and comorbidities, Cox proportional risk regressions were applied to analyze the associations between the daily intake of SSBs as well as added sugar from beverages and all-cause mortality.
Results
In the whole research population, the median age at baseline was 67 years, 22% were Black and 54% were female. A total of 42% had stage 3 CKD. During an average follow-up period of 8.3 years, a sum of 1137 (28%) deaths from all causes was recorded. The confounder-adjusted risk of mortality was associated with an increase of 1 serving/day of SSBs, with all-cause mortality of 1.18 [95% confidence interval (95% CI)1.08–1.28], and intakes of increased 20-g added sugar/1000 kcal of total energy per day were associated with all-cause mortality of 1.14 (1.05–1.24). Equivalently substituting 1 serving/day of SSBs with unsweetened coffee [HR (95% CI) 0.82 (0.74–0.91)], unsweetened tea [HR (95% CI) 0.86 (0.76–0.98)], plain water [HR (95% CI) 0.79 (0.71–0.88)], or non- or low-fat milk [HR (95% CI) 0.75 (0.60–0.93)] were related to a 14–25% reduced risk of all-cause mortality.
Conclusion
Findings suggest that in the CKD population, increased SSB intake was associated with a higher risk of mortality and indicated a stratified association with dose. Plain water and unsweetened coffee/tea might be possible alternatives for SSBs to avert untimely deaths.
Keywords: CKD, mortality, National Health and Nutrition Examination Survey, SSBs
INTRODUCTION
In recent years, beverages have become a notable contributor to the sugar intake in the modern diet [1]. The largest single source of added sugar in the American diet are sugar-sweetened beverages (SSBs) [2], which comprise sodas, soft drinks, sugary coffees/teas, fruit-flavored drinks, vitamin-water drinks and energy drinks with added caloric sweeteners such as fruit juice concentrates, sucrose and high fructose corn syrup [3]. A characteristic 12-oz serving of soda contains 140–150 calories and 35–38 g of sugar. In 2001, approximately 21% of the total energy in the American diet was consumed as beverages, the majority of which are sugary sodas and soft drinks [4]. Although consumption of SSBs in America has declined over the past decade [5], national survey data revealed that in these years the consumption of SSBs has rebounded slightly among adults of most age groups [6]. In the rest of the world, especially in developing countries, the intake of SSBs shows an escalating trend due to extensive urbanization and beverage marketing [7].
So far, much scientific attention has been aroused to the positive correlation between SSB intake and weight gain [3], diabetes [8] and coronary heart disease [9, 10]. Previous longitudinal studies have focused on the female population [11], elderly population [12], the population with gastrointestinal tumors [13] or populations in certain occupations [14], and several studies have been conducted in the last century [15, 16]. Two previous studies from the National Health and Nutrition Examination Survey (NHANES) declared that higher SSB intake was associated with higher all-cause mortality and mortality of cardiovascular disease (CVD) [15, 17]. However, two Asian cohorts reported the opposite conclusion, i.e. SSBs showed no significant association with all-cause mortality [18, 19]. Diabetes, hypertension and CVD are associated with a higher prevalence of chronic kidney disease (CKD) [20, 21]. Besides, in addition to these well-known adverse consequences of SSB intake, recent clinical studies [22–25] showed that sugary soda intake was associated with kidney damage. The underlying renal lesions in CKD patients directly affect glycometabolism in vivo, which might cause further metabolic disorders and uncontrollable chronic inflammation and eventually lead to death from hypertonic non-ketoacidosis or ketoacidosis in CKD patients. However, there is limited research on the effect of SSB intake on the prognosis of the CKD population. In the current analysis, we consulted the 1999–2014 NHANES database to analyze the dose–response relationship between SSB consumption and all-cause mortality in the CKD population, and to assess whether this relationship is independent of other dietary factors related to SSBs and acknowledged CKD risk factors.
MATERIALS AND METHODS
Study design and population
NHANES is a periodic survey of a sample of the non-institutionalized civilian population of the USA conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC). It is essentially a multi-stage probability sampling design that has released data in 2-year cycles since 1999 [26]. After providing informed consent, randomly selected participants undergo a personal questionnaire at home, followed by a physical and laboratory examination and a 24-h dietary recall at a mobile screening center. More details about the survey are available on the NHANES website [27].
This research used NHANES data from 1999 to 2014 (82 091 people). Non-pregnant individuals aged 20 years or older (42 377 subjects) were selected and included only CKD participants (4696 subjects). CKD was defined as estimated glomerular filtration rate (eGFR) <60 and >15 mL/min/1.73 m2 (applying the CKD Epidemiology Collaboration equation) and/or urinary albumin/creatinine ratio (ACR) >30 mg/g [28]. To ensure the completeness and analyzability of the dietary recall questionnaire, we only included those survey participants for whom the variable “dietary recall status” was classified as “reliable and conforming to minimum standards”. We also excluded individuals who lacked covariate information (demographic, lifestyle or comorbidities) or information on mortality status and follow-up time. Finally, the remaining 3996 participants with complete data were enrolled in the analysis (Figure 1).
FIGURE 1:
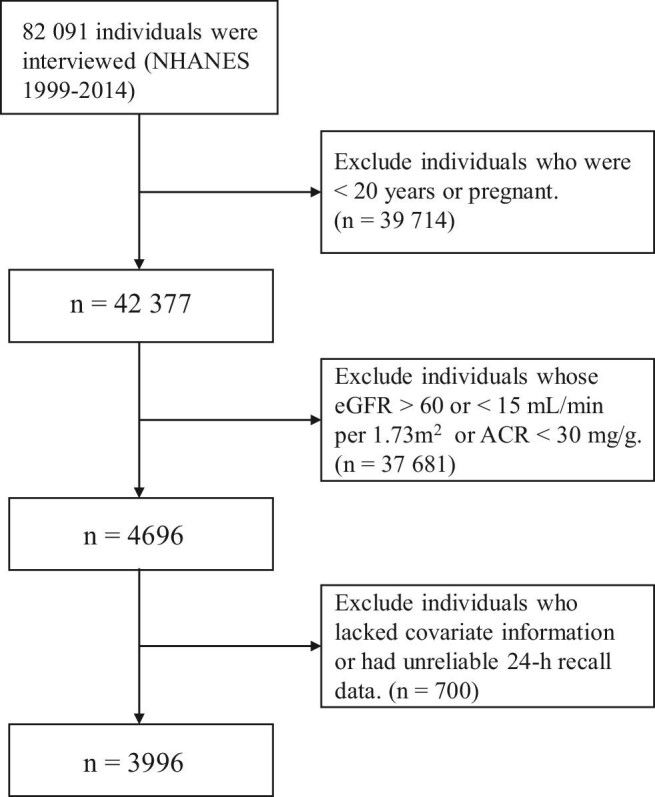
Flow diagram of the selection of eligible participants, National Health and Nutrition Examination Survey, 1999–2014.
Assessments of beverage intakes and covariates
In all NHANES cycles, a 24-h dietary recall questionnaire was used to assess individual dietary intakes. In the first two cycles, 1999–2002, only one in-person 24-h dietary recall was conducted. Starting from 2003, the cycles included two dietary recalls, the primary interviewed by trained investigators at a mobile examination center based on the USDA's Automated Multiple-Pass Method of 24-h recalls and the other followed up by telephone after 3–10 days [29]. Based on the previous analysis [30], the beverages, nutrients and energy intakes of participants were calculated using the results of a 24-h dietary recall from the 1999–2002 cycles and the average of nutritional information from both recalls in the 2003–2014 cycles.
Similar to previous NHANES reports [31], SSBs were defined as sodas, sugary fruit juices, fruit-flavored drinks, sport and energy drinks, sweetened coffee and tea, and other sugary drinks. Daily consumption of artificially sweetened beverages, pure fruit juices, unsweetened tea and coffee, plain water, non- or low-fat milk, and sweetened milk were also computed for substitution analysis. One serving of beverage was defined as 12 oz standard [11, 14, 32, 33]. Moreover, the US Department of Agriculture (USDA) Dietary Research Nutrition Database and Food Patterns Equivalence Database were used to determine added sugars from SSBs, total daily energy intake, vegetables, fruits, grains, red meat and processed meat [14, 17]. The dietary intake of protein, sodium, magnesium, potassium, calcium and phosphorus was calculated using the food information from the 24-h dietary recall, which was used to calculate the dietary acid load, according to the Remer and Manz equations of the potential renal acid load [34].
According to the current articles, the following variables were selected as confounders and measured at baseline. The demographic characteristics included age, sex, race/ethnicity, education level and marital status. Leisure-time physical activity, smoking status and alcohol consumption status were categorized as behaviors related to lifestyle. Details of data collection and definition have been stated elsewhere [26].
The participants had their height, weight, blood pressure, blood and urine specimen taken at the mobile examination center (MEC). Glycohemoglobin was measured by the A1c 2.2 Plus Glycohemoglobin Analyzer (Tosoh Medics, Inc.), the Primus CLC330 and Primus CLC385 (Primus Corporation), and the A1c G7 HPLC Glycohemoglobin Analyzer (Tosoh Medics, Inc.). Fasting plasma glucose was measured by the Roche/Hitachi 911, Roche Cobas Mira and Roche Modular P Chemistry Analyzers (Roche Diagnostics). Blood total cholesterol and high-density lipoprotein (HDL) levels were measured by the Roche Modular P and Roche Cobas 6000 Chemistry Analyzers (Roche Diagnostics) [26]. Serum creatinine was measured using a Jaffé rate method. The CKD Epidemiology Collaboration equation was used to calculate serum creatinine-based eGFR [35]. Urine albumin was measured by a solid-phase fluorescence immunoassay, and urine creatinine was measured by the modified Jaffé kinetic method.
Diabetes was defined as self-reported diabetes (or reported use of anti-hyperglycemic medications), or glycol hemoglobin ≥6.5% or measuring fasting glucose ≥126 mg/dL [36]. Hypertension was defined as self-reported hypertension (or reported use of anti-hypertensive medications) or measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg [36]. High cholesterol was defined as the ratio of total cholesterol to HDL of >5.9 [37]. Participants were considered to have a history of CVD if they reported having been diagnosed by a doctor as having had a heart attack, congestive heart failure, angina, coronary heart disease or stroke [37].
Since the current study was a secondary analysis of NHANES data, which are publicly available, no institutional review board approval was necessary or obtained.
Outcome ascertainment
All-cause mortality during follow-up time was the study outcome. Prior to December 31, 2015, NHANES records were linked to National Death Index records using a probability matching algorithm based on name, social security number, birth date, ethnicity, etc. [38]. The cause of death was determined according to the 10th edition of the International Statistical Classification of Diseases.
Statistical analysis
In consideration of the complex sampling design of the NHANES data, all analyses followed the CDC analytical reporting guidelines to calculate the combined weight for the 1999–2014 period using ‘2 days dietary weight’ as the sample weight. According to SSB consumption, participants were divided into four groups: none, >0 to <1 serving/day, 1 to <2 servings/day and ≥2 servings/day. Furthermore, we divided them into five groups based on the intake of added sugars in SSBs, taking into account that added sugars from SSBs may be a crucial link in the adverse health effects of SSBs. The differences between groups were examined by analysis of variance and Rau–Scott χ2 test with adjusted sample weights for baseline continuous variables and categorical variables, respectively.
Adjusting for potential confounders, we conducted a multivariate Cox regression model to estimate the relationship between SSB intake and all-cause mortality in CKD patients. Schoenfeld residuals were used to test the proportional risk hypothesis, and no violations were found. Model 1 was adjusted for age, gender, race, family poverty-to-income ratio (PIR), education, marital status, vigorous/moderate recreational activities for at least 10 min continuously per week, alcohol consumption status and cigarette smoking status. Model 2 additionally adjusted a range of dietary factors, including total energy intake, intake of whole grains, fruit, vegetables, red and processed meat, artificially sweetened beverages, dietary acid load, dietary sodium and total fat. Model 3 further incorporated baseline eGFR, body mass index (BMI), and history of chronic diseases, including high cholesterol levels, hypertension, diabetes, CVD and cancer. We repeated analyses for all models by substituting SSB intake with added sugar from SSBs. We adapted restricted cubic splines (RCSs) to determine the potential non-linear association between SSB intake (as a continuous variable) and mortality.
Subgroup analyses were conducted using a Wald test based on age (<60 versus ≥60 years), gender, race (non-Hispanic White versus others), BMI (<25 versus ≥25 kg/m2), history of hypertension, diabetes, CVD or cancer, and CKD stage to evaluate whether there was a statistical difference of the correlation of SSB intake and mortality because of demographic factors, behavioral habits and health conditions of CKD patients. The cross-product terms of SSB intake and stratified variable were added into the model accordingly, and the likelihood ratio test was applied to assess whether the interaction was statistically significant.
We conducted the substitution analysis by decreasing SSB consumption and increasing other beverage consumption instantaneously by one daily serving. Hazard ratio (HR) was calculated based on the difference in beta coefficient of changes in beverage intake, and 95% confidence interval (CI) was calculated based on the corresponding variance and covariance [39]. All analyses were performed in R version 3.5.2, and a bilateral P-value below 0.05 was regarded as statistically significant.
RESULTS
In the whole research population, the median age at baseline was 67 (52–78) years, 22% were Black and 54% were female. A total of 42% had stage 3 CKD. Participants with higher SSB intake were more possible to be younger, male, non-Hispanic White, unmarried, less physically active with a lower household PIR and lower education level. Meanwhile, SSB consumption was involved with a higher intake of total energy, dietary acid load, red and processed meat, and lower whole-grain consumption. The prevalence of diabetes, hypertension, CVD and cancer was lower as SSB intake increased (Table 1). Supplementary Data, Table S1 displays the baseline characteristic distribution of added sugars in SSBs, consistent with the distribution of SSB intake for each group.
Table 1.
Baseline characteristics of CKD participants according to daily SSB intake
| Characteristics | 0 serving | >0 to <1 serving | 1 to <2 servings | ≥2 servings |
|---|---|---|---|---|
| Participants, n | 1320 (33.0) | 1285 (32.2) | 777 (19.4) | 614 (15.4) |
| Age, years | 69.0 (57.0, 78.0) | 72.0 (60.0, 80.0) | 64.0 (49.0, 76.0) | 49.0 (35.0, 64.0) |
| Male (%) | 598 (45.3) | 537 (41.8) | 375 (48.3) | 326 (53.1) |
| Self-reported race/ethnicity (%) | ||||
| Mexican American | 180 (13.6) | 151 (11.8) | 133 (17.1) | 106 (17.3) |
| Others | 148 (11.2) | 142 (11.1) | 85 (10.9) | 66 (10.7) |
| Non-Hispanic White | 797 (60.4) | 736 (57.3) | 331 (42.6) | 239 (28.9) |
| Non-Hispanic Black | 195 (14.8) | 256 (19.9) | 228 (29.3) | 203 (33.1) |
| Married (%) | 702 (53.2) | 662 (51.5) | 390 (50.2) | 294 (47.9) |
| Education (%) | ||||
| Less than high school | 410 (31.1) | 397 (30.9) | 257 (33.1) | 211 (34.4) |
| High school graduates or equivalent | 303 (23.0) | 342 (26.6) | 176 (22.7) | 169 (27.5) |
| Some college or above | 607 (46.0) | 546 (42.5) | 344 (44.3) | 234 (38.1) |
| Family PIR level (%) | ||||
| ≥4 | 346 (26.2) | 295 (23.0) | 156 (20.1) | 93 (15.1) |
| >1 to <4 | 735 (55.7) | 772 (60.1) | 463 (59.6) | 366 (59.6) |
| ≤1 | 239 (18.1) | 218 (17.0) | 158 (20.3) | 155 (25.2) |
| Alcohol drinking (%) | ||||
| Non-drinkers | 485 (36.7) | 484 (37.7) | 269 (34.6) | 183 (29.8) |
| Moderate drinkers | 507 (38.4) | 542 (42.2) | 281 (36.2) | 201 (32.7) |
| Binge drinkers | 224 (17.0) | 196 (15.3) | 138 (17.8) | 115 (18.7) |
| Heavy drinkers | 104 (7.9) | 63 (4.9) | 89 (11.5) | 115 (18.7) |
| Cigarette smoking (%) | ||||
| Never smoking | 659 (49.9) | 714 (55.6) | 423 (54.4) | 288 (46.9) |
| Former smoking | 458 (34.7) | 420 (32.7) | 204 (26.3) | 133 (21.7) |
| Current smoking | 203 (15.4) | 151 (11.8) | 150 (19.3) | 193 (31.4) |
| >10 min of vigorous/moderate recreational activity per week (%) | 110 (8.3) | 92 (7.2) | 83 (10.7) | 78 (12.7) |
| BMI, kg/m2 | 28.4 (24.8, 33.5) | 28.3 (24.8, 32.6) | 28.5 (25.1, 32.6) | 28.8 (24.7, 34.7) |
| Total-to-HDL cholesterol ratio ≥5.9 (%) | 115 (8.7) | 118 (9.2) | 88 (11.3) | 95 (15.5) |
| Prevalent hypertension (%) | 982 (74.4) | 941 (73.2) | 534 (68.7) | 353 (57.5) |
| Prevalent diabetes (%) | 463 (35.1) | 350 (27.2) | 152 (19.6) | 104 (16.9) |
| History of CVD (%) | 354 (26.8) | 369 (28.7) | 180 (23.2) | 95 (15.5) |
| History of cancer (%) | 224 (17.0) | 261 (20.3) | 109 (14.0) | 58 (9.4) |
| eGFR, mL/min/1.73 m2 | 60.3 (49.9, 90.0) | 57.8 (47.9, 85.7) | 67.1 (50.3, 98.5) | 88.8 (57.4, 112.5) |
| ACR, mg/g | 42.6 (14.7, 100.1) | 39.6 (11.8, 89.8) | 43.9 (18.2, 94.9) | 53.6 (33.3, 124.6) |
| CKD stage (%) | ||||
| 1 | 316 (23.9) | 276 (21.5) | 258 (33.2) | 303 (49.3) |
| 2 | 349 (26.4) | 306 (23.8) | 162 (20.8) | 126 (20.5) |
| 3 | 605 (45.8) | 635 (49.4) | 310 (39.9) | 161 (26.2) |
| 4 | 50 (3.8) | 68 (5.3) | 47 (6.0) | 24 (3.9) |
| Dietary acid load, mEq/day | 7.3 (−3.8, 19.1) | 5.0 (−5.2, 16.1) | 7.9 (−3.6, 20.3) | 15.9 (0.5, 30.4) |
| Daily intake | ||||
| Energy intake, kcal/day | 1549.5 (1155.3, 2007.4) | 1609.0 (1259.3, 2058.5) | 1747.0 (1361.0, 22 213.5) | 2283.3 (1820.0, 2930.3) |
| Dietary sodium intake, mg/day | 2570.0 (1841.0, 3676.5) | 2645.0 (1825.5, 3533.5) | 2666.0 (1904.0, 3655.5) | 3284.7 (2389.8, 4565.75) |
| Total fat intake, g/day | 57.0 (38.3, 84.4) | 58.4 (39.3, 82.8) | 63.0 (42.5, 87.7) | 78.5 (54.7, 110.1) |
| Fresh fruit, servings/day | 0.5 (0.0, 1.4) | 0.9 (0.1, 1.7) | 0.8 (0.0, 1.6) | 0.4 (0.0, 1.6) |
| Vegetables, servings/day | 1.2 (0.5, 2.1) | 1.2 (0.6, 2.0) | 1.1 (0.5, 1.9) | 0.4 (0.0, 1.6) |
| Red and processed meat, g/day | 97.4 (47.6, 159.7) | 91.0 (50.2, 149.7) | 97.2 (51.6, 165.5) | 128.9 (70.7, 212.4) |
| Whole grains, g/day | 11.8 (0.0, 36.9) | 11.3 (0.0, 36.3) | 2.8 (0.0, 27.8) | 0.0 (0.0, 14.9) |
All analyses involved complex sampling designs. Categorical variables were given as number (percentage), and continuous variables as median with interquartile range due to their skewed distributions.
During an average period of 8.3 years' follow-up, 1137 (28%) deaths of all causes were recorded. Table 2 shows the relationship of daily SSB intake with mortality. After adjusting for demographic and lifestyle-related factors and dietary factors, for all-cause mortality, those who consumed ≥2 servings/day of SSBs had an HR (95% CI) of 1.80 (1.27–2.55) compared with those who did not consume SSBs. Additional adjustment for baseline eGFR, BMI, high total to HDL cholesterol level, hypertension, diabetes, CVD and cancer further strengthened the association [HR (95% CI) was 1.90 (1.36–2.66), P-value for trend <0.001]. For each additional serving of SSB intake per day, there was an 18% higher risk of all-cause mortality [Model 3, HR (95% CI) was 1.18 (1.08−1.28)] in the continuous analysis. RCSs indicated a linear relationship between SSB intake and all-cause mortality (Supplementary Data, Figure S1).
Table 2.
The associations of daily intakes of SSBs with mortality
| Sugar-sweetened beverages | 0 serving/day | >0 to <1 serving/day | 1 to <2 servings/day | ≥2 servings/day | Each serving/day |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Deaths, n | 398 | 430 | 198 | 111 | 1137 |
| Model 1 | 1 | 1.05 (0.87–1.27) | 0.94 (0.75–1.17) | 1.15 (0.83–1.59) | 1.02 (0.94–1.10) |
| Model 2 | 1 | 1.20 (1.00–1.45) | 1.12 (0.90–1.40) | 1.80 (1.27–2.55) | 1.16 (1.06–1.26) |
| Model 3 | 1 | 1.24 (1.02–1.49) | 1.19 (0.95–1.49) | 1.90 (1.36–2.66) | 1.18 (1.08–1.28) |
All analyses involved complex sampling designs. Model 1 was adjusted for age, gender, family PIR, self-reported race, education, marital status, alcohol consumption, smoking status and vigorous/moderate recreational activity. Model 2 was additionally adjusted for total energy intake, intake of whole grains, fruit, vegetables, red and processed meat, artificially sweetened beverages, dietary acid load, dietary sodium and total fat. Model 3 was additionally adjusted for baseline eGFR, BMI, total-to-HDL cholesterol ratio, hypertension, diabetes, CVD and cancer.
Table 3 shows the relationship between added sugar in SSBs and mortality. Compared with the quintile with the lowest added sugar intake from SSBs, the highest quintile was associated with higher all-cause mortality [HR (95% CI) was 1.69 (1.2–2.36)]. The RCSs showed that added sugar in SSBs was linearly associated with all-cause mortality (Supplementary Data, Figure S1), with an HR (95% CI) of 1.14 (1.05–1.24) for each increased 20-g added sugar/1000 kcal of total energy intake.
Table 3.
The associations of daily added sugar intakes from SSBs with mortality
| Add sugar | Q1 | Q2 | Q3 | Q4 | Q5 | Each 20 g added sugar/1000 kcal of total energy intake |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Deaths, n | 430 | 159 | 241 | 180 | 127 | 1137 |
| Model 1 | 1 | 0.83 (0.63–1.10) | 0.99 (0.80–1.22) | 1.01 (0.80–1.28) | 1.11 (0.81–1.53) | 1.01 (0.92–1.09) |
| Model 2 | 1 | 0.96 (0.73–1.25) | 1.15 (0.93–1.43) | 1.27 (1.01–1.60) | 1.60 (1.13–2.25) | 1.12 (1.03–1.22) |
| Model 3 | 1 | 0.98 (0.75–1.27) | 1.20 (0.97–1.47) | 1.30 (1.02–1.66) | 1.69 (1.20–2.36) | 1.14 (1.05–1.24) |
Data are presented as HR (95% CI). All analyses involved complex sampling designs. Model 1 was adjusted for age, gender, family PIR, self-reported race, education, marital status, alcohol consumption, smoking status and vigorous/moderate recreational activity. Model 2 was additionally adjusted for total energy intake, intake of whole grains, fruit, vegetables, red and processed meat, artificially sweetened beverages, dietary acid load, dietary sodium and total fat. Model 3 was additionally adjusted for baseline eGFR, body mass index, total-to-HDL cholesterol ratio, hypertension, diabetes, CVD and cancer.
Q: quintile.
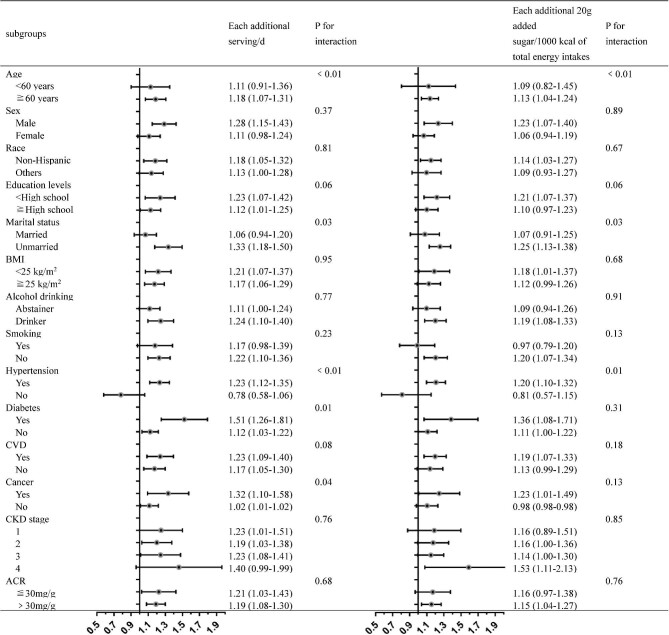
Figure 2 displays the subgroup analysis results according to different demographics and life-related characteristics as well as health conditions. Individuals who were male and >60 years old at baseline were associated with a higher risk of mortality with SSB intake. Moreover, deleterious associations were discovered among some subgroups (unmarried, non-Hispanic White, lower education levels, nonsmoker and drinker) but not in others (married, other races, higher education levels, smoker and abstainer) in the analysis. When stratified by comorbidities, associations were marginally stronger among those with diabetes, hypertension, CVD and cancer, and not significant among those without diseases, and the interaction for hypertension, diabetes and cancer was statistically significant (P-value for interaction <0.05 for all). The associations between added sugar intake in SSBs and all-cause mortality were chiefly paralleled with SSBs in subgroup analyses.
FIGURE 2:
Subgroup analyses of the associations between SSB intake and mortality. Adjusted covariates: age, gender, family PIR, self-reported race, education, marital status, alcohol consumption, smoking status, vigorous/moderate recreational activity, total energy intake, intake of whole grains, fruit, vegetables, red and processed meat, artificially sweetened beverages, dietary acid load, dietary sodium, total fat, baseline eGFR, BMI, total-to-HDL cholesterol ratio, hypertension, diabetes, CVD and cancer.
Table 4 demonstrates HRs from substitution models by decreasing SSB intake and simultaneously increasing intake of another beverage by 1 serving/day. There was no crucial association with mortality when replacing 1 serving of SSBs with an equivalent amount of artificially sweetened beverages or pure juice or sweetened milk. However, we estimated that substituting SSBs with an equivalent amount of unsweetened coffees [HR (95% CI) 0.82 (0.74–0.91)], unsweetened teas [HR (95% CI) 0.86 (0.76–0.98)], plain water [HR (95% CI) 0.79 (0.71–0.88)] or non- or low-fat milk [HR (95% CI) 0.75 (0.60–0.93)] was related to a 14–25% reduced risk of all-cause mortality.
Table 4.
HR (95% CI) of all-cause mortality in substitution analysis
| Substituted by | All-cause mortality |
|---|---|
| Artificially sweetened beverage | 0.92 (0.81–1.03) |
| Pure juice | 0.79 (0.59–1.07) |
| Unsweetened coffee | 0.82 (0.74–0.91) |
| Unsweetened tea | 0.86 (0.76–0.98) |
| Plain water | 0.79 (0.71–0.88) |
| Non- or low-fat milk | 0.75 (0.60–0.93) |
| Sweetened milk | 0.90 (0.40–2.05) |
Adjusted covariates: age, gender, family PIR, self-reported race, education, marital status, alcohol consumption, smoking status, vigorous/moderate recreational activity, total energy intake, intake of whole grains, fruit, vegetables, red and processed meat, artificially sweetened beverages, dietary acid load, dietary sodium, total fat, baseline eGFR, BMI, total-to-HDL cholesterol ratio, hypertension, diabetes, CVD and cancer.
DISCUSSION
Our study showed a positive stratified association with dose between SSB intake and all-cause mortality in the CKD population after adjusting for confounding factors. Each serving increase in daily SSB consumption was associated with an 8% higher risk of all-cause mortality, which is a stronger association observed among men compared with women, although no significant interaction with sex was observed. Compared with adults in the lowest quintile of added sugar in SSBs, those in the top quintile had a 69% higher risk of all-cause mortality. Additionally, we estimated that substituting 1 serving of SSBs with alternative plain water and unsweetened coffee/tea was associated with a 14–25% lower risk of all-cause mortality. On the whole, our study demonstrated that increased consumption of SSBs was associated with higher mortality among CKD patients.
It was worth noting that there was a lack of research on the effect of SSB intake on mortality in people with CKD. The effects of SSBs on mortality have been contradictory in studies of the general population and different racial groups. Several studies [12–15, 40, 41], including data from 10 European countries [41] and two US large cohorts [14], have illustrated that higher SSB intake was related to higher all-cause mortality. However, other studies have found an invalid association between consumption of sugary beverages and all-cause mortality [16, 18, 19, 42, 43], which might be explained by the relatively average low intake level of SSBs in some populations [16, 18, 19]. Besides, higher SSB intake may represent higher socioeconomic status in two Asian cohort studies [18, 19], and it was still likely that there existed residual confounding, even though education attainment or income was adjusted. In the present analysis, our study found that higher SSB consumption is positively associated with increased all-cause mortality for CKD patients, with each serving increase in daily SSB consumption associated with an 8% higher risk of mortality, and the HR of those who consumed ≥2 servings/day of SSBs was statistically significant compared with those who did not consume SSBs for all-cause mortality. Compared with the general population, CKD patients had abnormal metabolic status, including imbalance of glucose metabolism and acid–base metabolism, and more severe inflammatory status [44], making it difficult to clear the effect of SSBs on physiological and pathological activities in vivo, which led to a vicious cycle and greatly increased the risk of death due to unbalanced metabolism. Meanwhile, in combination with the results of RCS, it is reasonable to speculate that the association between higher SSB intake and higher mortality might be primarily driven by CKD patients who consume >2 servings of SSBs per day. Therefore, we suggest that future intervention studies of higher SSB intake should be conducted in the CKD population. By quantifying SSB intake in a hierarchical manner and assessing the association between SSBs and risk of death in the CKD population, our results complemented and extended previous studies and are easier to translate into clinical recommendations. In addition, our study showed that the effect of added sugar from SSBs on mortality in the CKD population approximately corresponded to that of SSBs, suggesting that it might be more sensible to focus on the added sugar intake from SSBs rather than SSB intake.
The following mechanisms could explain our finding that higher intake of added sugars from SSBs is associated with a higher risk of mortality. It was reported that fructose, as the main component of sweeteners of SSBs, was associated with increased serum uric acid concentrations and excessive secretion of renin, which could cause vascular disease and renal interstitial fibrosis [45] as well as kidney stones, directly or indirectly [46]. In animal models, fructose-related hyperuricemia resulted in metabolic syndrome, which was associated with renal hypertrophy, glomerular hypertension, cortical vasoconstriction and renal vascular arterioles [47]. Furthermore, the phosphate additives from SSBs increase dietary acid load [34, 48] and possibly affect the serum fibroblast growth factor-23 level [49], thus further reducing the kidney function. In CKD patients, these mechanisms could explain the situation that increased SSB intake worsened renal function and made it more difficult to control. Overall, the relation to survival shown in the study further emphasizes the significance of reducing SSB intake, especially added sugar intake from SSBs, in secondary prevention for CKD.
In subgroup analysis, we found that an additional daily serving of SSBs was associated with higher all-cause mortality in CKD patients >60 years old than that of those <60 years old, which might be attributed to slower physiological activities and metabolic rates, as well as poorer kidney function of older patients, making it more difficult to clear the damage caused by SSBs.
The results of the relationship between SSBs and all-cause mortality in CKD patients with other comorbidities are worth discussing. We observed a higher risk of mortality in CKD subgroups with a history of chronic diseases, especially in the hypertension subgroup, diabetes subgroup and cancer subgroup. In CKD patients with underlying diabetes or hypertension, high consumption of SSBs potentially contributed to metabolic disorders and chronic inflammation [50], as well as making it difficult to control blood glucose or blood pressure, which might result in increased adiposity [51, 52] and further deterioration of kidney function [53], ultimately leading to hypertonic non-ketoacidosis or ketoacidosis and cardiovascular death. Likewise, high intake of SSBs in CKD patients with a history of cancer may lead to poor tumor treatment and increased mortality. The comparison might be diluted when considering that in the subgroup analysis the situation of patients with CKD was more complex and that high SSB intake at baseline did not represent high SSB intake in the past or future. Therefore, we recommended further interventional studies or clinical trials in CKD patients with diversiform chronic diseases. In summary, clinicians and health care professionals should encourage dietary interventions among CKD patients with multiple chronic diseases to reduce SSB intake, which slows down the progression of established CKD and improves quality of life.
In substitution analysis, we estimated that substituting 1 serving of SSBs per day with alternative plain water and unsweetened coffee/tea, as well as non- or low-fat milk, was associated with a lower risk of all-cause mortality. Our findings are consistent with the previous studies of mortality of the general population [17] and risk of type 2 diabetes mellitus [54] that have found that replacing SSBs with water, unsweetened coffee/tea or low-fat milk was associated with attenuated risk of death or diabetes. In agreement with these studies, our observations emphasized the significance of limiting SSB consumption and substituting SSBs with other beneficial alternatives.
The present study has some limitations. First, the 24-h dietary recall information collected at baseline was not updated during the follow-up period, and we could only infer that participants' SSB consumption habits were maintained to be stable over time. In addition, other variables that could influence renal function, including blood pressure, blood glucose status, salt intake and other dietary information, might also change during follow-up. Since dietary information and some comorbidities were based on self-report, measurement errors in SSB intake and other dietary items are inevitable, or there may exist reporting bias. Second, the presence of a dose–response relationship did not inevitably connote causation, as was the case with other observational studies. Residual confounders might also be present, even after adjustment for sociodemographic, lifestyle-related and diet-related factors, and comorbidities.
In conclusion, we found that in the CKD population, increased SSB intake was associated with a higher risk of mortality and indicated a stratified association with dose. Plain water and unsweetened coffee/tea might be possible alternatives for SSBs to avert untimely deaths, which could be a simple, economical, clinically safe and effective choice for CKD patients. In future studies, researchers should take advantage of the latest dietary information to further investigate the life-course relationship between SSB consumption and mortality, as well as to discover the mechanism of SSB damage to the kidney.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge and thank members of the National Center for Health Statistics of the Centers for Disease Control and Prevention and the participants who contributed time and data to the National Health and Nutrition Examination Survey.
Contributor Information
Xiao-Yu Cai, Division of Internal Medicine, Department of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Nan-Hui Zhang, Department of Nephrology, Xiangyang No.1 People's Hospital, Hubei University of Medicine, Xiangyang, Hubei Province, China.
Yi-Chun Cheng, Division of Internal Medicine, Department of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Shu-Wang Ge, Division of Internal Medicine, Department of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Gang Xu, Division of Internal Medicine, Department of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
FUNDING
This work was financially supported by the International (Regional) Cooperation and Exchange Projects (NSFC-DFG Grant No. 81761138041); National Natural Science Foundation of China (Grant No. 81570667); Major Research Plan of the National Natural Science Foundation of China (Grant No. 91742204); and the National Key R&D Program of China (Grant No. 2018YFC1314003-1).
AUTHORS’ CONTRIBUTIONS
X.-Y.C. designed the study, conducted the statistical analyses and wrote the paper; N.-H.Z. conducted the statistical analyses and contributed to the critical review of the manuscript; Y.-C.C. contributed to data interpretation and the critical review of the manuscript; S.-W.G. and G.X. designed the study, supervised the study process, and contributed to data interpretation and the critical review of the manuscript; and all authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
REFERENCES
- 1. Popkin BM. Patterns of beverage use across the lifecycle. Physiol Behav 2010; 100: 4–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slining MM, Popkin BM. Trends in intakes and sources of solid fats and added sugars among U.S. children and adolescents: 1994–2010. Pediatr Obes 2013; 8: 307–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 2013; 14: 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med 2004; 27: 205–210 [DOI] [PubMed] [Google Scholar]
- 5. Welsh JA, Sharma AJ, Grellinger L et al. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011; 94: 726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosinger A, Herrick K, Gahche J et al. Sugar-sweetened beverage consumption among U.S. adults, 2011–2014. NCHS Data Brief 2017; 270: 1–8 [PubMed] [Google Scholar]
- 7. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013; 9: 13–27 [DOI] [PubMed] [Google Scholar]
- 8. Malik VS, Popkin BM, Bray GA et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010; 33: 2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fung TT, Malik V, Rexrode KM et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009; 89: 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Koning L, Malik VS, Kellogg MD et al. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012; 125: 1735–1741, S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vyas A, Rubenstein L, Robinson J et al. Diet drink consumption and the risk of cardiovascular events: a report from the Women's Health Initiative. J Gen Intern Med 2015; 30: 462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrington WE, White E. Mortality outcomes associated with intake of fast-food items and sugar-sweetened drinks among older adults in the Vitamins and Lifestyle (VITAL) study. Public Health Nutr 2016; 19: 3319–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miles FL, Chang SC, Morgenstern H et al. Association of sugary beverages with survival among patients with cancers of the upper aerodigestive tract. Cancer Causes Control 2016; 27: 1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik VS, Li Y, Pan A et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019; 139: 2113–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Q, Zhang Z, Gregg EW et al. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014; 174: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med 2007; 44: 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang YB, Chen JX, Jiang YW et al. Association of sugar-sweetened beverage and artificially sweetened beverage intakes with mortality: an analysis of US National Health and Nutrition Examination Survey. Eur J Nutr 2021; 60: 1945–1955 [DOI] [PubMed] [Google Scholar]
- 18. Odegaard AO, Koh WP, Yuan JM et al. Beverage habits and mortality in Chinese adults. J Nutr 2015; 145: 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu ZM, Tse LA, Chan D et al. Dietary sugar intake was associated with increased body fatness but decreased cardiovascular mortality in Chinese elderly: an 11-year prospective study of Mr and Ms OS of Hong Kong. Int J Obes 2018; 42: 808–816 [DOI] [PubMed] [Google Scholar]
- 20. Haroun MK, Jaar BG, Hoffman SC et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 2003; 14: 2934–2941 [DOI] [PubMed] [Google Scholar]
- 21. Perneger TV, Brancati FL, Whelton PK et al. End-stage renal disease attributable to diabetes mellitus. Ann Intern Med 1994; 121: 912–918 [DOI] [PubMed] [Google Scholar]
- 22. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol 2011; 6: 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saldana TM, Basso O, Darden R et al. Carbonated beverages and chronic kidney disease. Epidemiology 2007; 18: 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shoham DA, Durazo-Arvizu R, Kramer H et al. Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999–2004. PLoS One 2008; 3: e3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuzbashian E, Asghari G, Mirmiran P et al. Sugar-sweetened beverage consumption and risk of incident chronic kidney disease: Tehran lipid and glucose study. Nephrology 2016; 21: 608–616 [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention/National Center for Health Statistics (2017) About the National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm#data (27 April 2021, date last accessed) [Google Scholar]
- 27. Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm (27 April 2021, date last accessed) [Google Scholar]
- 28. Chen J, Muntner P, Hamm LL et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004; 140: 167–174 [DOI] [PubMed] [Google Scholar]
- 29. Moshfegh AJ, Rhodes DG, Baer DJ et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008; 88: 324–332 [DOI] [PubMed] [Google Scholar]
- 30. Sun Y, Wang D, Zhou Q. Caffeine intake and the risk of recurrent kidney stones in adults, an analysis of 2007–2014 National Health and Nutrition Examination Surveys. Eur J Nutr 2020; 59: 2683–2692 [DOI] [PubMed] [Google Scholar]
- 31. Kit BK, Fakhouri TH, Park S et al. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr 2013; 98: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guercio BJ, Zhang S, Niedzwiecki D et al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: results from CALGB 89803 (Alliance). PLoS One 2018; 13: e0199244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mossavar-Rahmani Y, Kamensky V, Manson JE et al. Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women's Health Initiative. Stroke 2019; 50: 555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banerjee T, Crews DC, Wesson DE et al. High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol 2015; 26: 1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He S, Ryan KA, Streeten EA et al. Prevalence, control, and treatment of diabetes, hypertension, and high cholesterol in the Amish. BMJ Open Diabetes Res Care 2020; 8: e000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saydah SH, Siegel KR, Imperatore G et al. The cardiometabolic risk profile of young adults with diabetes in the U.S. Diabetes Care 2019; 42: 1895–1902 [DOI] [PubMed] [Google Scholar]
- 38. National Center for Health Statistics . 2019. 2015 Public-Use Linked Mortality Files. https://www.cdc.gov/nchs/data-linka ge/morta lity-public.htm (27 April 2021, date last accessed) [Google Scholar]
- 39. Chiuve SE, Fung TT, Rimm EB et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012; 142: 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anderson JJ, Gray SR, Welsh P et al. The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: a prospective cohort study. BMC Med 2020; 18: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mullee A, Romaguera D, Pearson-Stuttard J et al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern Med 2019; 179: 1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fuchs MA, Sato K, Niedzwiecki D et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS One 2014; 9: e99816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keller A, O'Reilly EJ, Malik V et al. Substitution of sugar-sweetened beverages for other beverages and the risk of developing coronary heart disease: results from the Harvard Pooling Project of Diet and Coronary Disease. Prev Med 2020; 131: 105970. [DOI] [PubMed] [Google Scholar]
- 44. Tyson CC, Luciano A, Modliszewski JL et al. Effect of bicarbonate on net acid excretion, blood pressure, and metabolism in patients with and without CKD: the acid base compensation in CKD study. Am J Kidney Dis 2021; 78: 38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang DH, Nakagawa T, Feng L et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 2002; 13: 2888–2897 [DOI] [PubMed] [Google Scholar]
- 46. Asselman M, Verkoelen CF. Fructose intake as a risk factor for kidney stone disease. Kidney Int 2008; 73: 139–140 [DOI] [PubMed] [Google Scholar]
- 47. Nakagawa T, Hu H, Zharikov S et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006; 290: F625–F631 [DOI] [PubMed] [Google Scholar]
- 48. Rebholz CM, Coresh J, Grams ME et al. Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am J Nephrol 2015; 42: 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rebholz CM, Grams ME, Coresh J et al. Serum fibroblast growth factor-23 is associated with incident kidney disease. J Am Soc Nephrol 2015; 26: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DiNicolantonio JJ, O'Keefe JH, Lucan SC. Added fructose: a principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin Proc 2015; 90: 372–381 [DOI] [PubMed] [Google Scholar]
- 51. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013; 98: 1084–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonzalez-Palacios S, Navarrete-Munoz EM, Garcia-de-la-Hera M et al. Sugar-containing beverages consumption and obesity in children aged 4–5 years in Spain: the INMA study. Nutrients 2019; 11: 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin J, Fung TT, Hu FB et al. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses' Health Study. Am J Kidney Dis 2011; 57: 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drouin-Chartier JP, Zheng Y, Li Y et al. Changes in consumption of sugary beverages and artificially sweetened beverages and subsequent risk of type 2 diabetes: results from three large prospective U.S. cohorts of women and men. Diabetes Care 2019; 42: 2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.