Abstract
Volume overload, and its attendant increase in acute care utilization and cardiovascular morbidity and mortality, represents a critical challenge for the practicing nephrologist. This is particularly true among patients with ESKD on HD, where predialysis volume overload and intradialytic and postdialytic hypovolemia account for almost a third of all cost for the Medicare dialysis benefit. Quantitative lung ultrasound is a tool for assessing the extent of extravascular lung water that outperforms physical exam and plain chest radiography. B-lines are vertical hyperechoic artifacts present in patients with increased extravascular lung water. B-lines have been shown to decrease dynamically during the hemodialysis treatment in proportion to ultrafiltration volume. Among patients with chronic heart failure, titration of diuretics on the basis of the extent of pulmonary congestion noted on lung ultrasonography has been shown to decrease recurrent acute care utilization. Early data from randomized controlled trials of lung ultrasound–guided ultrafiltration therapy among patients with ESKD on HD have shown promise for potential reduction in recurrent episodes of decompensated heart failure and cardiovascular events. Ultimately, lung ultrasound may predict those who are ultrafiltration tolerant and could be used to decrease acute care utilization and, thus, cost in this population.
Keywords: chronic kidney disease, cardiovascular outcomes, ESKD, heart failure, hemodialysis, intradialytic hypotension, POCUS, point-of-care ultrasound, pulmonary edema, quantitative lung ultrasonography
Introduction
Volume overload is one of the most vexing problems in nephrology and its assessment is the most difficult. Nephrologists and patients with ESKD are locked in a constant, cataclysmic struggle to control volume overload and mitigate complications of relative hypovolemia complicating judicious ultrafiltration.
Volume overload is a mediator of adverse cardiovascular outcomes in patients with ESKD on hemodialysis (HD) and is attended by increased and recurrent acute care utilization (1,2). In the United States, ESKD care is covered under Medicare and, although patients with ESKD constitute only 1% of Medicare beneficiaries, they constitute 7% of all costs. At $36 billion per year, this is close to the total annual budget of the National Institutes of Health and nearly 1% of the annual budget of the US Federal Government (3). Acute care utilization constitutes about 30% of the cost of ESKD care, representing the largest modifiable cost for patients who will remain on in-center HD. Cardiovascular events, especially heart failure admissions, are responsible for an outsized proportion of these events (4). Cost is especially dear for local institutions as the Centers for Medicare and Medicaid Services reins in spending for repeat heart failure admissions.
It stands to reason that intimate knowledge of the presence of pulmonary congestion could be used to presage acute care utilization and mitigate its risk with extra ultrafiltration, additional ultrafiltration sessions, or titration of antihypertensives (5). However, physical examination signs of volume overload, such as rales and edema, are insensitive for the prediction of the presence of pulmonary congestion (6). Indeed, the presence of pedal edema correlates better with cardiac risk factors, particularly body mass index, rather than being indicative of volume expansion (7). Even chest x-ray is insensitive for the detection of pulmonary congestion (8). Dry weight probing is limited by other factors influencing weight.
Technical Aspects
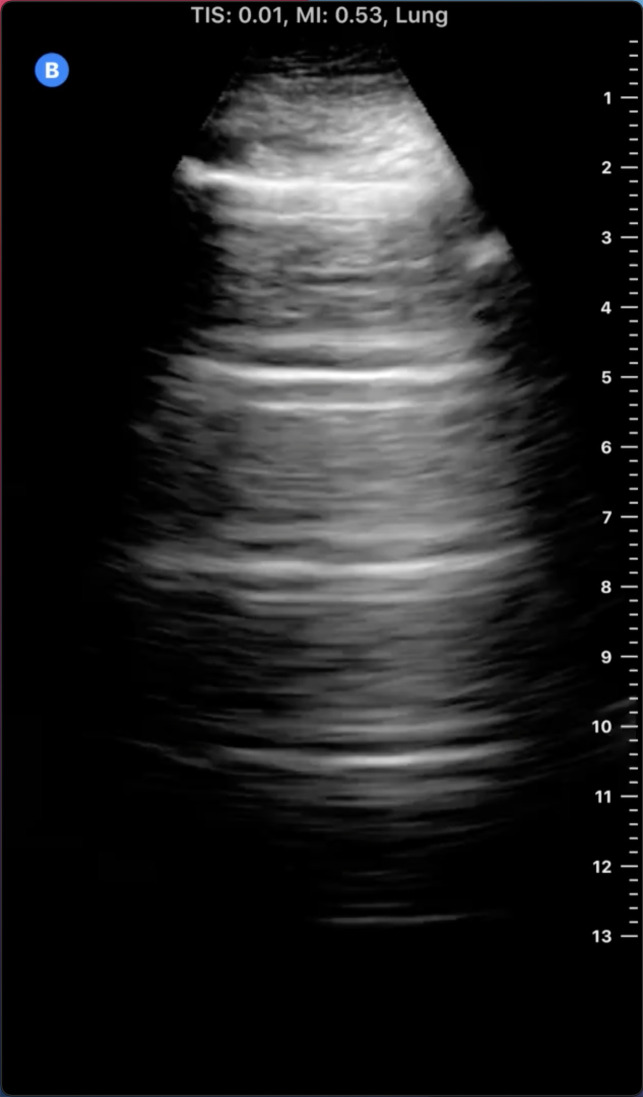
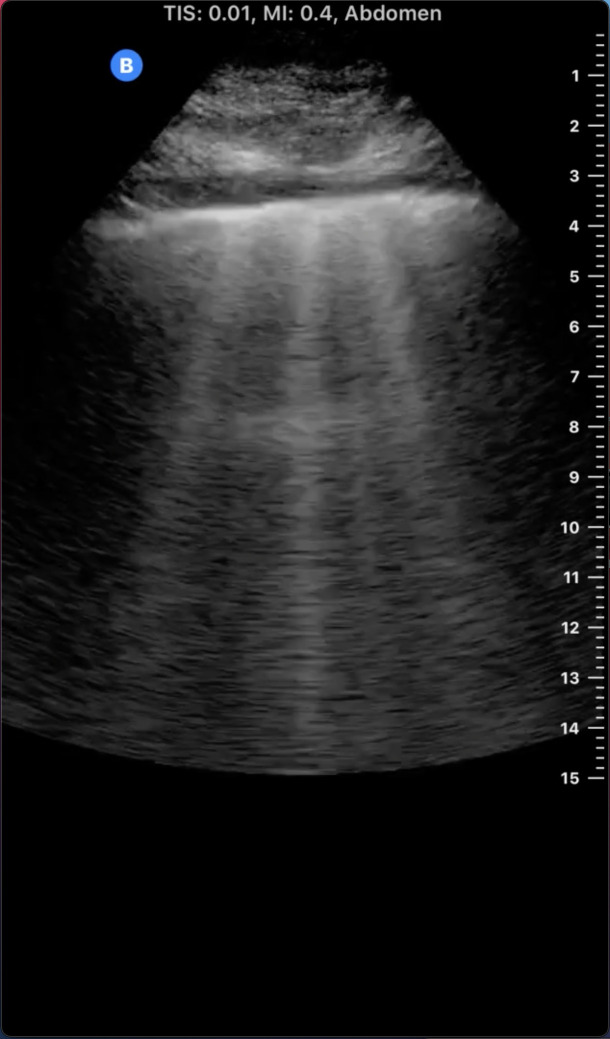
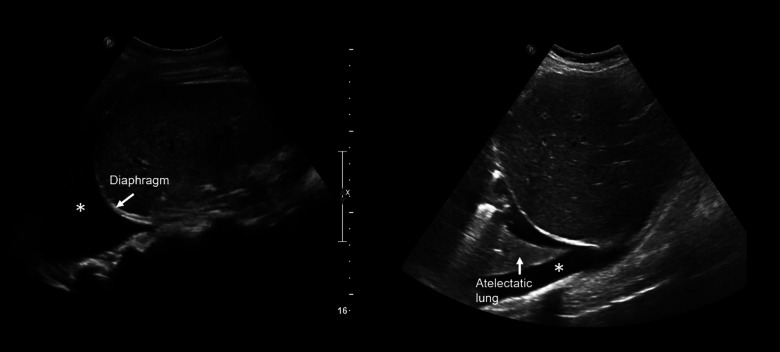
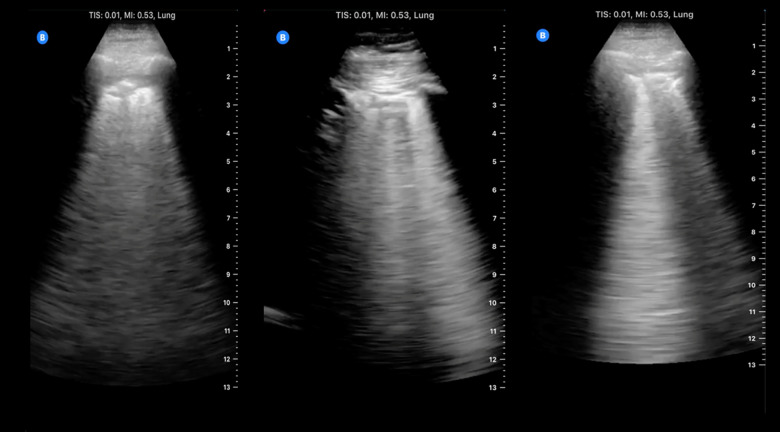
The appearance of normal lung parenchymal architecture is obscured by the presence of reverberation artifacts arising from high acoustic impedance mismatch at the pleural-alveolar interface as sound waves pass from water density (skin and soft tissue) to air density (in the alveolar sac) (9). In patients without pathology, the usual appearance of the lung is termed the A-line pattern, which consists of horizontally oriented reflections of the hyperechoic pleural line at integer multiples of the pleural depth (Figure 1). With increasing pulmonary congestion, there is a shift from a normal horizontally oriented A-line pattern to a vertically oriented B-line pattern. B-lines are discrete, laser-like hyperechoic lines that radiate to the edge of the ultrasound field and move with respiration (Figure 2) (10). B-lines can be tallied serially across multiple intercostal spaces across the chest, allowing quantification of pulmonary congestion, with the 28-zone anterior lung ultrasound the most validated for research purposes (11). The more B-lines counted across the chest, the more the pulmonary congestion correlating with extravascular lung water, as measured by thermodilution (12–14). Quantitative lung ultrasound has been reliably shown to have good inter-rater reliability and concordance using different ultrasound transducers (15). Any transducer can be used, but the most optimal images are captured when the focal depth is set at the pleural line with increasing gain in the far field and harmonics turned off (16). Quantitative lung ultrasound is predictive of acute cardiogenic pulmonary edema in the emergency department, and it outperforms the physical exam and even the chest x-ray (8,17). Lung ultrasound is also sensitive for the presence of pleural effusions, which appear as anechoic structures that allow passage of the ultrasound beam and interdigitate between lung and diaphragm (Figure 3) (18). Lung ultrasound can be taught easily using a remote, web-based application and can be incorporated in the outpatient setting with an abbreviated eight-zone protocol that can be obtained in <2 minutes (19,20).
Figure 1.
Lung ultrasound A-line pattern. Note the bright horizontal lines at integer multiples of the pleural depth which diminish in intensity with increasing depth.
Figure 2.
Lung ultrasound B-lines. Note the hyperechoic vertical lines extending from the pleural line radiating to the edge of the ultrasound field.
Figure 3.
Pleural effusion by ultrasound. Note the anechoic/space (*) separating lung at left from the diaphragm at right.
Lung ultrasound measures pulmonary congestion, volume overload in the organ of interest, and is posited to identify those tolerant of ultrafiltration (21). This stands in contrast to other putative markers of volume overload, such as bioimpedance or blood volume monitoring devices, which can only estimate pulmonary congestion on the basis of total extracellular and intracellular volumes or intravascular volume, respectively (21).
Comparison with Physical Examination
In a study performed by Torino and colleagues (6) writing in CJASN in 2016, 79 patients were enrolled and serial examinations with 28-zone lung ultrasound and physical examinations with assessment for lung crackles and pedal edema were undertaken, representing 1106 paired examinations. Overall, detection of pulmonary congestion by assessment for lung crackles or pedal edema was very poor with low sensitivity and specificity: 65% of lung ultrasound examinations demonstrated significant pulmonary congestion, whereas crackles were present in only 21% and pedal edema was present in only 10% of patients. Crackles were absent in fully half of those with severe pulmonary congestion and were present in 5% of those with no pulmonary congestion. Edema was absent in 80% of evaluations with severe pulmonary congestion. Even a composite score from the combination of crackles and edema only improved the area under the receiver operating characteristic curve for prediction of mild, moderate, and severe pulmonary congestion to 0.60, 0.65, and 0.68, respectively. The sensitivity of lung crackles and pedal edema to detect severe pulmonary congestion was 9% and 3%, respectively. This dramatic demonstration of the poor sensitivity of traditional physical exam markers of volume overload in patients on HD would seem to indicate the need for a paradigm shift in volume status assessment in the dialysis unit. However, as Sherman (22) points out in an accompanying editorial, pulmonary congestion may occur in patients on HD irrespective of volume status.
Abbreviated Lung Ultrasound
Despite providing excellent diagnostic and prognostic data, the reference standard 28-zone lung ultrasound has been criticized by Koratala and Ross (23) as cumbersome and inconvenient. Efforts to simplify the 28-zone study have been undertaken by Torino and colleagues (20). They reanalyzed data from 303 of the original 392 patients on HD studied by Zoccali et al. (24) and found that a simplified eight-zone lung ultrasound had good agreement with the 28-zone lung ultrasound and remained predictive of mortality (P<0.01) and cardiovascular events (P≤0.05) (20). The overall time needed to perform the study was cut from 3.05 (interquartile range [IQR], 2.22–5.00) minutes for the 28-zone lung ultrasound to 1.35 (IQR, 1.16–2.00) minutes for the eight-zone lung ultrasound (20). Buessler and colleagues (25) demonstrated that, among patients with suspected acute decompensated heart failure, simplified six-zone or eight-zone lung ultrasound studies improve diagnosis accuracy over a prediction score on the basis of clinical parameters. Finally, in a study of 98 patients on HD presenting to acute care, Reisinger and coauthors (26) showed good agreement of 12 different simplified scanning patterns, including four four-zone, four six-zone, and four eight-zone lung ultrasound studies with the 28-zone lung ultrasound study. They posit that a simplified heuristic of a B-line every other lung zone is suggestive of at least moderate pulmonary congestion and they advocate the use of the four-zone lung ultrasound, which has an area under the receiver operating characteristic curve of up to 0.91 for prediction of the total B-line score (26).
Limitations
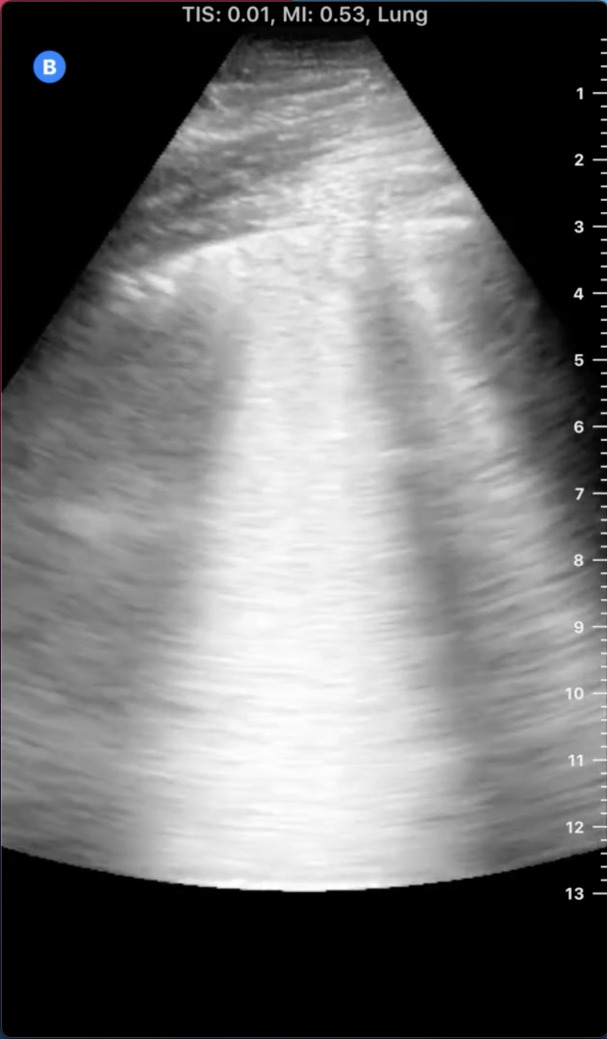
Quantitative lung ultrasonography has limitations in assessing extent of pulmonary congestion. Patients with diffuse parenchymal lung diseases, such as idiopathic pulmonary fibrosis, typically have findings of increased B-lines thought to originate from fibrosis at the level of the pulmonary alveolar interstitium (10). Other alveolar filling processes apart from pulmonary congestion can present with increased B-lines, such as the acute respiratory distress syndrome (ARDS). Although B-lines are common in both ARDS and pulmonary congestion, ARDS can often be distinguished from pulmonary congestion on the basis of the presence of pleural line abnormalities, reduction in lung sliding, spared areas, and consolidations (27). Viral pneumonias, notably including coronavirus disease 2019, present with similar lung ultrasound findings with thickened or irregular pleural lines, subpleural consolidations, and patches of confluent B-lines with spared areas limiting interpretations of quantitative lung ultrasound (Figures 4 and 5) (28,29). Patients with severe lung disease preventing interpretation of lung ultrasound have been excluded from large clinical trials, limiting generalizability to these patients (30,31). Finally, as with any physical exam or clinical ultrasound finding, lung ultrasound should never be interpreted in isolation and the addition of focused cardiac assessment and abdominal venous Doppler studies may add significantly to knowledge of the patient’s hemodynamic status (32).
Figure 4.
Confluent B-lines on ultrasound. Note the confluence of the hyperechoic lines radiating from the pleural line and extending to the edge of the ultrasound field.
Figure 5.
Acute respiratory distress syndrome and viral pneumonia by ultrasound. Note the thickening, irregularity, and interruptions in the pleural line with hyperechoic artifacts radiating posteriorly, giving the appearance of B-lines.
Observational Studies
The first documented use of lung ultrasound among patients on dialysis was by Noble and colleagues (33) writing in Chest in 2009. They presented an observational cohort of 40 patients with ESKD chronically on HD admitted at a tertiary care center and performed 28-zone lung ultrasound before, during, and after HD. They found that asymptomatic pulmonary congestion is highly prevalent (34 of 40 patients) despite low prevalence of dyspnea (three of 34 patients with significant pulmonary congestion). Further, they demonstrated that the B-line score decreases quantitatively and dynamically in real time during HD in proportion to ultrafiltration volume. All patients with an initial B-line score of greater than one (34 of 40) had a statistically significant reduction in B-line score during a single HD treatment (P<0.001) (33).
Since the original demonstration, multiple studies have confirmed the relationship of pulmonary congestion, as measured by lung ultrasound among patients with ESKD on chronic dialysis (34). Further, pulmonary congestion, as measured by lung ultrasound in patients with ESKD on HD, was found to be an excellent prognostic marker correlating with poor physical functioning and adverse cardiovascular outcomes in observational studies (34–36). Siriopol and colleagues (37) were the first to demonstrate an association with increasing pulmonary congestion, as measured by quantitative lung ultrasound among patients with ESKD on HD, outperforming bioimpedance-derived parameters. To date, Zoccali and coauthors (24) reported the largest observational cohort, with 392 patients on HD, demonstrating that patients with very severe pulmonary congestion have a hazard ratio (HR) of 4.2 (95% confidence interval [95% CI], 2.45 to 7.23) for death and an HR of 3.2 (95% CI, 1.75 to 5.88) for cardiovascular events compared with patients with mild to no pulmonary congestion. They further demonstrated dose dependence, with patients with the most severe congestion having the highest risk of adverse cardiovascular outcomes or death, and those with moderate to severe pulmonary congestion having intermediate elevations in risk (HR, 1.70; 95% CI, 1.00 to 2.80) compared with those with mild to no pulmonary congestion (P<0.001). They demonstrated improved risk reclassification when adding extent of pulmonary congestion to traditional risk factors for cardiac events for patients with CKD (10%; P=0.02), but not for predicting all-cause mortality.
Interventional Studies
In the first interventional study of its kind, Siriopol and colleagues (38) evaluated the effect of a lung ultrasound–guided dry weight probing strategy on a low-cardiovascular-risk subset of patients with ESKD on HD. This study enrolled 250 patients, excluding those with New York Heart Association (NYHA) class 3–4 heart failure and coronary disease, and randomized them 1:1 to usual care versus a dry weight probing strategy using lung ultrasound. At an average follow-up of 21.3±5.6 months, there was no difference in the primary composite end point (all-cause death and cardiovascular events) or secondary end points, including intradialytic hypotension (IDH) and hospitalizations. Patients in the active arm did have lower rates of predialytic dyspnea (HR, 0.81; 95% CI, 0.68 to 0.96), however, they had an increased rate of intradialytic cramping (HR, 1.26; 95% CI, 1.16 to 1.37). Notably, the median (IQR) B-line score in the active group was seven (three to 12), and a B-line score ≥15 occurred in only 15% of patients. Moreover, for every 1 dry weight decrease there were 3 dry weight increases in response to evidence of hypovolemia detected by bioimpedance. In other words, patients were three times more likely to be assessed as hypovolemic on the basis of bioimpedance data than they were to be assessed as hypervolemic on the basis of lung ultrasound data. Most patients were volume depleted rather than volume overloaded. This limits the interpretability of this negative result because the intervention of interest only occurred in only 15% of patients, and most patients had minimal pulmonary congestion to start with on the basis of lung ultrasound.
More recently, Loutradis and coauthors (39) described a single-blind, randomized study in which 71 patients were randomized to usual care versus lung ultrasound–guided dry weight reduction. Ambulatory BP monitoring (48 hour) was undertaken before the intervention and 8 weeks after. In the primary outcome, the authors demonstrated statistically significant reductions in 48-hour systolic BP in the interventional group versus in the control group (−6.61±9.57 versus −0.67±13.07 mm Hg, respectively; P=0.03) and diastolic BP in the interventional group versus in the control group (−3.85±6.34 versus −0.55±8.28 mm Hg, respectively; P=0.03) (39). Exploratory outcomes of changes in echocardiographic parameters also showed improvement, including reductions in inferior vena cava (IVC) diameter (−0.43±4.00 versus 0.71±4.82; P=0.03), left atrial surface area (−1.09±4.61 versus 0.93±3.06 cm2; P=0.03), and left ventricular filling pressures early transmitral diastolic velocities ratio (E/e′) (−0.38±3.14 versus 1.36±3.54; P=0.03) in the control group compared with standard of care (40). As expected, more patients in the interventional group achieved greater dry weight reduction (54% versus 14%; P<0.001), and over the course of the study the total B-line score fell (−5.31±12.53 versus 2.17±7.62; P<0.001). The study was not powered to explore outcomes such as hospital admission or mortality; however, in the follow-up period, one patient from the intervention group and one from the control group died of central nervous system infection and lung infection, respectively. There were five hospitalizations: two in the intervention group (for hematuria and mechanical central venous catheter failure), and three in the control group (for hematuria, gastrointestinal bleeding, and lung infection) (39). None of these are expected complications of hypovolemia and no access thromboses were observed, which was increased in the Crit-Line Intradialytic Monitoring Benefit Study evaluating dry weight probing using a blood volume monitoring device (41).
Agarwal and colleagues (42) have criticized this study because the control group failed to conform to the standard of care of initial dry weight probing for reduction in BP. Despite achieving less net ultrafiltration, more patients in the control arm developed IDH than the intervention group, although this was not statistically significant (56% versus 34%; P=0.07). This trend reversal may lend credence to the idea that lung ultrasound detection of pulmonary congestion predicts ultrafiltration tolerance. Although this study was not powered to predict adverse cardiovascular outcomes or mortality, the Frequent Hemodialysis Network study demonstrated an HR of 0.61 (95% CI, 0.53 to 0.92) in the primary composite outcome of all-cause mortality and left ventricular hypertrophy, a finding that is presaged by echocardiographic indicators of filling pressure (43).
After a delay due to enrollment kinetics, the results of the Lung Water by Ultrasound Guided Treatment in Hemodialysis Patients (LUST) study were recently reported by Zoccali and colleagues (44) writing in Kidney International. The LUST study was a single-blind, randomized controlled trial of a lung ultrasound–guided ultrafiltration strategy versus usual care in patients with ESKD on HD with comorbid cardiovascular disease, including prior myocardial infarction and cardiomyopathy with NYHA class 3–4 heart failure. Patients in the interventional group underwent an initial and monthly 28-zone lung ultrasound. Those identified to have moderate to severe pulmonary congestion (B-line score of ≥15) were targeted for ultrafiltration intensification. Lung ultrasound was repeated weekly until the goal B-line score <15 (representing mild to no pulmonary congestion) was achieved. Patients not achieving the target B-line score within 3–4 weeks had intensification of antihypertensive therapy according to a prespecified formulary. The study was powered to detect a 33% risk reduction in its primary composite outcome, including all-cause death, nonfatal myocardial infarction, or decompensated heart failure.
The study was concluded and published having enrolled 363 patients out of a targeted enrollment of 500. A total of 307 patients completed the study, including 152 in the intervention group and 155 in the control group. At a mean follow-up of 1.5 years, there was no significant difference in the primary composite end point (HR, 0.88; 95% CI, 0.63 to 1.24). A total of 51 (28%) patients in the interventional arm died versus 59 (33%) in the usual care group (HR, 0.99; 95% CI, 0.61 to 1.29). Despite not meeting the target enrollment, the study demonstrated reduction in B-line score in the intervention arm from a baseline of 15 (95% CI, 12 to 19) B-lines on enrollment to nine (95% CI, give to 12) at study end, compared with those in the control arm who had worsening pulmonary congestion with a baseline B-line score of 16 (95% CI, 13 to 20) rising to 30 (95% CI, 20 to 39) at study end. Although there were no differences in pre- or postdialysis BPs or left ventricular mass index, ambulatory BP monitoring was not undertaken as in the study by Loutradis et al. (39). The intervention was demonstrated to be safe with less IDH in the interventional group versus the usual care group (320 [95% CI, 300 to 342] events per 100 patient-years versus 473 [95% CI, 448 to 500] events per 100 patient-years, respectively). There were no differences in rates of HD vascular access dysfunction or arrhythmias. Indeed, the key finding of the study is that a lung ultrasound–guided ultrafiltration strategy safely resolved lung congestion when compared with standard of care among patients with ESKD on HD.
In a reflection on completion of the study, given the incidence rate of the primary outcomes, the authors realized the power calculation was overly optimistic. A post hoc analysis of the study was undertaken that demonstrated reductions in recurrent episodes of decompensated heart failure (HR, 0.37; 95% CI, 0.15 to 0.93) and cardiovascular events (HR, 0.63; 95% CI, 0.41 to 0.97) among patients in the interventional group (44). This nominally represents a 12% decrease in the incidence rate of the composite outcome. If realized, these reductions in recurrent episodes of decompensated heart failure and cardiovascular events would dramatically decrease acute care utilization among patients with ESKD on HD, proving ultrafiltration guided by lung ultrasound a useful intervention. Studies with higher enrollment and with extended length are needed to ascertain whether resolution of lung congestion improves cardiovascular outcomes (30,45).
Prediction of IDH
The LUST study demonstrated less IDH among outpatients with ESKD on HD. Among critically ill patients with AKI requiring intermittent HD, da Hora Passos and coauthors (46) conducted a single-center, prospective, observational study of obtaining 28-zone lung ultrasound and IVC diameters on 248 patients before each dialysis session. Patients with pulmonary congestion on lung ultrasound and hypervolemia on the basis of IVC distention were less likely to have IDH (odds ratio, 0.08; 95% CI, 0.04 to 0.18; P=0.001) and were less likely to have dialysis discontinued (odds ratio, 0.07; 95% CI, 0.01 to 0.57; P=0.01). In contrast, patients without pulmonary congestion and IVC distention had the highest incidence of IDH, although, in general, patients with IVC distention had less IDH, irrespective of the presence or absence of pulmonary congestion. Following up on this result, Khanin and colleagues (47) conducted a retrospective analysis in an intensive care setting of 113 patients undergoing HD with lung ultrasound pattern documented by the treating team on the same day. They found that patients with documentation of an A-line pattern (indicating a dry lung) had a higher incidence of IDH than those with an overriding B-line pattern (indicating a wet lung), with an odds ratio of 3.63 (95% CI, 1.40 to 9.40) and odds ratio of 3.01 (95% CI, 1.10 to 8.22) after adjustment for the Acute Physiology and Chronic Health Evaluation (APACHE II) score (47). These studies suggest that clinical ultrasound may be helpful in identifying patients tolerant of volume removal.
Quantitative Lung Ultrasound in Heart Failure
Similar to what is seen among patients on HD, pulmonary congestion is the number one driver of acute care utilization among patients with heart failure (48). Pulmonary congestion, as quantified by an eight-zone lung ultrasound among 185 ambulatory patients with chronic heart failure, predicted a worse outcome for those in the highest tertile of B-lines, with a four-fold increase in a composite outcome, including hospitalization for acute decompensated heart failure and all-cause mortality (adjusted HR, 4.08; 95% CI, 1.95 to 8.54; P<0.001) (49). In a study of 349 patients hospitalized with acute decompensated heart failure, pulmonary congestion, as quantified using a further-simplified four-zone lung ultrasound, decreased with diuretic therapy and predicted risk of recurrent heart failure hospitalization and all-cause death as far out as 180 days (HR, 2.01; 95% CI, 1.11 to 3.64) (50).
Interventional studies of lung ultrasound–guided diuretic therapy have demonstrated reductions in composite outcomes consisting of recurrent acute care utilization, rehospitalization, and mortality are mainly driven by reduced urgent visits for heart failure. In a single-blind trial of 123 patients admitted with acute decompensated heart failure who were randomized to usual care versus diuretic therapy guided by an eight-zone lung ultrasound study, patients in the study group had an HR of 0.52 (95% CI, 0.27 to 1.00; P=0.05) for a composite outcome of mortality, time to an urgent visit, and hospitalization for acute decompensated heart failure (30). This was primarily driven by reductions in urgent visits for worsening heart failure, because in individual analysis there were no differences in mortality or heart failure hospitalization. A larger, unblinded study of 244 patients with chronic heart failure on optimal medical regimens comparing physical examination–guided therapy to physical examination–guided therapy augmented with lung ultrasound demonstrated reduction in hospitalization for acute decompensated heart failure among the lung ultrasound–enhanced group, with a relative risk of 0.44 (95% CI, 0.23 to 0.84; P=0.01). Again, there was no difference in mortality (45). Most recently, a single-blinded, randomized controlled trial of 126 patients comparing usual care to usual care with an eight-zone lung ultrasound showed reduction in the composite outcome of urgent heart failure visits, rehospitalization for acute decompensated heart failure, and all-cause death (HR, 0.55; 95% CI, 0.31 to 0.98; P=0.04). This was primarily driven by a reduction of urgent heart failure visits, and there was no difference in rehospitalization rates or all-cause death (31). These studies primarily enrolled patients with heart failure with reduced ejection fraction, and the largest effect was seen in the study by Marini et al. (45), which had the most patients with NYHA class 3–4 heart failure. These results are of critical importance to practicing nephrologists who comanage patients with heart failure and the cardiorenal syndrome and are increasingly assessed on metrics of acute care utilization in the context of value-based care models.
Conclusion
Among patients with ESKD chronically on dialysis, pulmonary congestion is accurately measured by quantitative lung ultrasound and has been repeatedly demonstrated to predict adverse cardiovascular events. Quantification of pulmonary congestion may identify those patients on dialysis who will respond well to ultrafiltration intensification. Post hoc analysis of the largest interventional study to date revealed decreases in recurrent acute decompensated heart failure and cardiovascular events, but whether a lung ultrasound–guided ultrafiltration strategy reduces acute care utilization in this population is an active area of study. Among patients with chronic heart failure, adding lung ultrasound to usual care has been demonstrated in randomized controlled trials to reduce recurrent acute care utilization. If benefits of lung ultrasound–guided ultrafiltration therapies can be proven among patients with ESKD on chronic dialysis, it represents significant potential for improvements in patient care and cost savings.
Disclosures
The authors have nothing to disclose.
Funding
None.
Author Contributions
A. Koratala and N. Reisinger reviewed and edited the manuscript; and N. Reisinger wrote the original draft.
References
- 1.Mathew AT, Rosen L, Pekmezaris R, Kozikowski A, Ross DW, McGinn T, Kalantar-Zadeh K, Fishbane S: Potentially avoidable readmissions in United States hemodialysis patients. Kidney Int Rep 3: 343–355, 2017. 10.1016/j.ekir.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S: Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 28: 2491–2497, 2017. 10.1681/ASN.2016121341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V: US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 75: A6–A7, 2020. 10.1053/j.ajkd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Arneson TJ, Liu J, Qiu Y, Gilbertson DT, Foley RN, Collins AJ: Hospital treatment for fluid overload in the Medicare hemodialysis population. Clin J Am Soc Nephrol 5: 1054–1063, 2010. 10.2215/CJN.00340110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoccali C: Lung ultrasound in the management of fluid volume in dialysis patients: Potential usefulness. Semin Dial 30: 6–9, 2017. 10.1111/sdi.12559 [DOI] [PubMed] [Google Scholar]
- 6.Torino C, Gargani L, Sicari R, Letachowicz K, Ekart R, Fliser D, Covic A, Siamopoulos K, Stavroulopoulos A, Massy ZA, Fiaccadori E, Caiazza A, Bachelet T, Slotki I, Martinez-Castelao A, Coudert-Krier MJ, Rossignol P, Gueler F, Hannedouche T, Panichi V, Wiecek A, Pontoriero G, Sarafidis P, Klinger M, Hojs R, Seiler-Mussler S, Lizzi F, Siriopol D, Balafa O, Shavit L, Tripepi R, Mallamaci F, Tripepi G, Picano E, London GM, Zoccali C: The agreement between auscultation and lung ultrasound in hemodialysis patients: The LUST study. Clin J Am Soc Nephrol 11: 2005–2011, 2016. 10.2215/CJN.03890416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal R, Andersen MJ, Pratt JH: On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3: 153–158, 2008. 10.2215/CJN.03650807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maw AM, Hassanin A, Ho PM, McInnes MDF, Moss A, Juarez-Colunga E, Soni NJ, Miglioranza MH, Platz E, DeSanto K, Sertich AP, Salame G, Daugherty SL: Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: A systematic review and meta-analysis. JAMA Netw Open 2: e190703, 2019. 10.1001/jamanetworkopen.2019.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O: The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 156: 1640–1646, 1997. 10.1164/ajrccm.156.5.96-07096 [DOI] [PubMed] [Google Scholar]
- 10.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) : International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38: 577–591, 2012. 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 11.Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E: Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 93: 1265–1270, 2004. 10.1016/j.amjcard.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 12.Picano E, Scali MC, Ciampi Q, Lichtenstein D: Lung ultrasound for the cardiologist. JACC Cardiovasc Imaging 11: 1692–1705, 2018. 10.1016/j.jcmg.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 13.Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, Kruse JM: Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care 19: 36, 2015. 10.1186/s13054-015-0756-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E: Ultrasound comet-tail images: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest 127: 1690–1695, 2005. 10.1378/chest.127.5.1690 [DOI] [PubMed] [Google Scholar]
- 15.Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, Picano E, Zoccali C: Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 3: 586–594, 2010. 10.1016/j.jcmg.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Matthias I, Panebianco NL, Maltenfort MG, Dean AJ, Baston C: Effect of machine settings on ultrasound assessment of B-lines. J Ultrasound Med 40: 2039–2046, 2020. 10.1002/jum.15581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D: Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: A systematic review and meta-analysis. Acad Emerg Med 21: 843–852, 2014. 10.1111/acem.12435 [DOI] [PubMed] [Google Scholar]
- 18.Brogi E, Gargani L, Bignami E, Barbariol F, Marra A, Forfori F, Vetrugno L: Thoracic ultrasound for pleural effusion in the intensive care unit: A narrative review from diagnosis to treatment. Crit Care 21: 325, 2017. 10.1186/s13054-017-1897-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gargani L, Sicari R, Raciti M, Serasini L, Passera M, Torino C, Letachowicz K, Ekart R, Fliser D, Covic A, Balafa O, Stavroulopoulos A, Massy ZA, Fiaccadori E, Caiazza A, Bachelet T, Slotki I, Shavit L, Martinez-Castelao A, Coudert-Krier MJ, Rossignol P, Kraemer TD, Hannedouche T, Panichi V, Wiecek A, Pontoriero G, Sarafidis P, Klinger M, Hojs R, Seiler-Mußler S, Lizzi F, Onofriescu M, Zarzoulas F, Tripepi R, Mallamaci F, Tripepi G, Picano E, London GM, Zoccali C: Efficacy of a remote web-based lung ultrasound training for nephrologists and cardiologists: A LUST trial sub-project. Nephrol Dial Transplant 31: 1982–1988, 2016. 10.1093/ndt/gfw329 [DOI] [PubMed] [Google Scholar]
- 20.Torino C, Tripepi R, Loutradis C, Sarafidis P, Tripepi G, Mallamaci F, Zoccali C: Can the assessment of ultrasound lung water in haemodialysis patients be simplified? [published online ahead of print December 29, 2020] Nephrol Dial Transplant 10.1093/ndt/gfaa285 [DOI] [PubMed] [Google Scholar]
- 21.Zoccali C, Mallamaci F: Mapping progress in reducing cardiovascular risk with kidney disease: Managing volume overload. Clin J Am Soc Nephrol 13: 1432–1434, 2018. 10.2215/CJN.01360118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman RA: Crackles and comets: Lung ultrasound to detect pulmonary congestion in patients on dialysis is coming of age. Clin J Am Soc Nephrol 11: 1924–1926, 2016. 10.2215/CJN.09140816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koratala A, Ross DW: Lung ultrasound in hemodialysis patients: Is it practical to scan 28 zones? Am J Kidney Dis 75: 815, 2020. 10.1053/j.ajkd.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 24.Zoccali C, Torino C, Tripepi R, Tripepi G, D’Arrigo G, Postorino M, Gargani L, Sicari R, Picano E, Mallamaci F; Lung US in CKD Working Group : Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 24: 639–646, 2013. 10.1681/ASN.2012100990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buessler A, Chouihed T, Duarte K, Bassand A, Huot-Marchand M, Gottwalles Y, Pénine A, André E, Nace L, Jaeger D, Kobayashi M, Coiro S, Rossignol P, Girerd N: Accuracy of several lung ultrasound methods for the diagnosis of acute heart failure in the ED: A multicenter prospective study. Chest 157: 99–110, 2020. 10.1016/j.chest.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 26.Reisinger N, Lohani S, Hagemeier J, Panebianco N, Baston C: Lung ultrasound to diagnose pulmonary congestion among patients on hemodialysis: Comparison of full versus abbreviated scanning protocols [published online ahead of print June 3, 2021]. Am J Kidney Dis 10.1053/j.ajkd.2021.04.007 [DOI] [PubMed] [Google Scholar]
- 27.Copetti R, Soldati G, Copetti P: Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 6: 16, 2008. 10.1186/1476-7120-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group (CCUSG) : Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med 46: 849–850, 2020. 10.1007/s00134-020-05996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisinger N, Koratala A: Lung ultrasound: A valuable tool for the assessment of dialysis patients with COVID-19. Clin Exp Nephrol 24: 850–852, 2020. 10.1007/s10157-020-01903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J: Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail 21: 1605–1613, 2019. 10.1002/ejhf.1604 [DOI] [PubMed] [Google Scholar]
- 31.Araiza-Garaygordobil D, Gopar-Nieto R, Martinez-Amezcua P, Cabello-López A, Alanis-Estrada G, Luna-Herbert A, González-Pacheco H, Paredes-Paucar CP, Sierra-Lara MD, Briseño-De la Cruz JL, Rodriguez-Zanella H, Martinez-Rios MA, Arias-Mendoza A: A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study). Am Heart J 227: 31–39, 2020. 10.1016/j.ahj.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 32.Argaiz ER, Koratala A, Reisinger N: Comprehensive assessment of fluid status by point-of-care ultrasonography. Kidney360 2: 1326–1338, 2021. 10.34067/KID.0006482020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJR, Liteplo A: Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 135: 1433–1439, 2009. 10.1378/chest.08-1811 [DOI] [PubMed] [Google Scholar]
- 34.Covic A, Siriopol D, Voroneanu L: Use of lung ultrasound for the assessment of volume status in CKD. Am J Kidney Dis 71: 412–422, 2018. 10.1053/j.ajkd.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 35.Enia G, Tripepi R, Panuccio V, Torino C, Garozzo M, Battaglia GG, Zoccali C: Pulmonary congestion and physical functioning in peritoneal dialysis patients. Perit Dial Int 32: 531–536, 2012. 10.3747/pdi.2010.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enia G, Torino C, Panuccio V, Tripepi R, Postorino M, Aliotta R, Bellantoni M, Tripepi G, Mallamaci F, Zoccali C; Lung Comets Cohort Working Group : Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol 8: 1343–1348, 2013. 10.2215/CJN.11111012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siriopol D, Hogas S, Voroneanu L, Onofriescu M, Apetrii M, Oleniuc M, Moscalu M, Sascau R, Covic A: Predicting mortality in haemodialysis patients: A comparison between lung ultrasonography, bioimpedance data and echocardiography parameters. Nephrol Dial Transplant 28: 2851–2859, 2013. 10.1093/ndt/gft260 [DOI] [PubMed] [Google Scholar]
- 38.Siriopol D, Onofriescu M, Voroneanu L, Apetrii M, Nistor I, Hogas S, Kanbay M, Sascau R, Scripcariu D, Covic A: Dry weight assessment by combined ultrasound and bioimpedance monitoring in low cardiovascular risk hemodialysis patients: A randomized controlled trial. Int Urol Nephrol 49: 143–153, 2017. 10.1007/s11255-016-1471-0 [DOI] [PubMed] [Google Scholar]
- 39.Loutradis C, Sarafidis PA, Ekart R, Papadopoulos C, Sachpekidis V, Alexandrou ME, Papadopoulou D, Efstratiadis G, Papagianni A, London G, Zoccali C: The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: A randomized controlled trial. Kidney Int 95: 1505–1513, 2019. 10.1016/j.kint.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 40.Loutradis C, Papadopoulos CE, Sachpekidis V, Ekart R, Krunic B, Karpetas A, Bikos A, Tsouchnikas I, Mitsopoulos E, Papagianni A, Zoccali C, Sarafidis P: Lung ultrasound-guided dry weight assessment and echocardiographic measures in hypertensive hemodialysis patients: A randomized controlled study. Am J Kidney Dis 75: 11–20, 2020. 10.1053/j.ajkd.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 41.Reddan DN, Szczech LA, Hasselblad V, Lowrie EG, Lindsay RM, Himmelfarb J, Toto RD, Stivelman J, Winchester JF, Zillman LA, Califf RM, Owen WF Jr: Intradialytic blood volume monitoring in ambulatory hemodialysis patients: A randomized trial. J Am Soc Nephrol 16: 2162–2169, 2005. 10.1681/ASN.2004121053 [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Toto RD, Weir MR: Extravascular lung water assessment by ultrasound to guide dry weight changes: Ready for prime time? Am J Kidney Dis 75: 1–3, 2020. 10.1053/j.ajkd.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010. 10.1056/NEJMoa1001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zoccali C, Torino C, Mallamaci F, Sarafidis P, Papagianni A, Ekart R, Hojs R, Klinger M, Letachowicz K, Fliser D, Seiler-Mußler S, Lizzi F, Wiecek A, Miskiewicz A, Siamopoulos K, Balafa O, Slotki I, Shavit L, Stavroulopoulos A, Covic A, Siriopol D, Massy ZA, Seidowsky A, Battaglia Y, Martinez-Castelao A, Polo-Torcal C, Coudert-Krier MJ, Rossignol P, Fiaccadori E, Regolisti G, Hannedouche T, Bachelet T, Jager KJ, Dekker FW, Tripepi R, Tripepi G, Gargani L, Sicari R, Picano E, London GM: A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int 100: 1325–1333, 2021. 10.1016/j.kint.2021.07.024 [DOI] [PubMed] [Google Scholar]
- 45.Marini C, Fragasso G, Italia L, Sisakian H, Tufaro V, Ingallina G, Stella S, Ancona F, Loiacono F, Innelli P, Costantino MF, Sahakyan L, Gabrielyan S, Avetisyan M, Margonato A, Agricola E: Lung ultrasound-guided therapy reduces acute decompensation events in chronic heart failure. Heart 106: 1934–1939, 2020. 10.1136/heartjnl-2019-316429 [DOI] [PubMed] [Google Scholar]
- 46.da Hora Passos R, Caldas J, Ramos JGR, Dos Santos Galvão de Melo EB, Ribeiro MPD, Alves MFC, Batista PBP, Messeder OHC, de Carvalho de Farias AM, Macedo E, Rouby JJ: Ultrasound-based clinical profiles for predicting the risk of intradialytic hypotension in critically ill patients on intermittent dialysis: a prospective observational study. Crit Care 23: 389, 2019. 10.1186/s13054-019-2668-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khanin Y, Hirsch JS, Stalbow D, Zhang M, Hasan Z, Ross DW: Intradialytic hypotension in critically ill patients on hemodialysis with A-line versus B-line pattern on lung ultrasonography. Kidney Int Rep 6: 1969–1972, 2021. 10.1016/j.ekir.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L; EuroHeart Survey InvestigatorsHeart Failure Association, European Society of Cardiology : EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur Heart J 27: 2725–2736, 2006. 10.1093/eurheartj/ehl193 [DOI] [PubMed] [Google Scholar]
- 49.Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD: Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 37: 1244–1251, 2016. 10.1093/eurheartj/ehv745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray JJV: Lung ultrasound in acute heart failure. JACC Heart Fail 7: 849–858, 2019. 10.1016/j.jchf.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]