Abstract
Background/objectives:
Pregnant women are exposed to multiple phthalates and their replacements, which are endocrine disrupting chemicals associated with adverse maternal and child health outcomes. Identifying maternal characteristics associated with phthalate/replacement exposure during pregnancy is important.
Methods:
We evaluated 13 maternal sociodemographic and lifestyle factors, enrollment year, and conception season as determinants of exposure biomarkers of phthalates and their replacements in 482 pregnant women from the Illinois Kids Development Study (I-KIDS, enrolled 2013–2018). We quantified 19 phthalate/replacement metabolites in pools of five first-morning urines collected across pregnancy. K-means clustering identified women with distinct patterns of biomarker concentrations and principal component analysis (PCA) identified principal component (PC) profiles of biomarkers that exist together. We used multivariable regression models to evaluate associations of predictors with identified k-means clusters and PCs.
Results:
K-means clustering identified two clusters of women: 1) low phthalate/di(2-ethylhexyl) terephthalate (∑DEHTP) and 2) high phthalate/∑DEHTP biomarker concentrations. PCA identified four PCs with loadings heaviest for biomarkers of plasticizer phthalates [di-isononyl, di-isodecyl, di-n-octyl phthalates] (PC1), of other phthalates [dibenzyl, di-n-butyl, di-iso-butyl phthalates] (PC2), of phthalate replacements [∑DEHTP, di(isononyl) cyclohexane-1,2-dicarboxylate (∑DiNCH)] (PC3), and of monoethyl phthalate (PC4). Overall, age, marital status, income, parity, pre-pregnancy BMI, caffeine intake, enrollment year, and conception season were independently associated with k-means cluster membership and at least one PC. Additionally, race/ethnicity, education, employment, pregnancy intention, smoking status, alcohol intake, and diet were associated with at least one PC. For instance, women who conceived in the spring, summer, and/or fall months had lower odds of high phthalate/∑DEHTP cluster membership and had lower plasticizer phthalate, phthalate replacement, and MEP PC scores.
Conclusions:
Conception season, enrollment year, and several sociodemographic/lifestyle factors were predictive of phthalate/replacement biomarker profiles. Future studies should corroborate these findings, with a special focus on replacements to which pregnant women are becoming increasingly exposed.
Keywords: Pregnancy, determinants, endocrine disruptors, phthalates, DiNCH, DEHTP
Graphical Abstract
1. INTRODUCTION
Ortho-phthalate diesters, or phthalates, are a class of chemicals widely used in the production of plastics for food contact materials and in some personal care products to which humans are exposed through ingestion, dermal absorption, and inhalation (National Research Council, 2008; Woodruff et al., 2011). An increasing body of evidence points to phthalates as endocrine disrupting chemicals that interact with multiple hormones and hormone-regulated processes (Diamanti-Kandarakis et al., 2009; Engel et al., 2017; Gore et al., 2015; Johns et al., 2016; Johns et al., 2015b), which is concerning given that pregnancy is a hormonally sensitive window and pregnant women are ubiquitously exposed to these chemicals (Woodruff et al., 2011). Studies have shown that higher maternal urinary phthalate metabolite concentrations are associated with adverse pregnancy outcomes, including preeclampsia and pregnancy hypertensive disorders (Cantonwine et al., 2016; Ferguson et al., 2015), glucose intolerance and gestational diabetes (James-Todd et al., 2018; James-Todd et al., 2016; Shaffer et al., 2019), and preterm birth (Ferguson et al., 2014). In response to the growing concerns over the endocrine disrupting properties and subsequent regulation of ortho-phthalate diesters (Kamrin, 2009), purportedly safe replacements were developed and introduced into the U.S. market before or in the early 2000s, including di(isononyl) cyclohexane-1,2-dicarboxylate (DiNCH) (Wadey, 2003) and a terephthalate diester, di(2-ethylhexyl) terephthalate (DEHTP) (Abe et al., 2012; McCombie et al., 2017). Recent observational evidence indicates that DiNCH and DEHTP exposure may be associated with adverse health outcomes, including increased risk of uterine fibroids and pre-term birth (Lee et al., 2020; Yland et al., 2022), as well as altered sex steroid hormone and oxidative stress levels (Derakhshan et al., 2021; Long et al., 2021; Pacyga et al., 2021; van et al., 2019). Therefore, additional studies are needed to identify maternal characteristics associated with phthalate and replacement exposures, which can be used as covariates in studies evaluating the health implications of increasing and decreasing exposure to these chemicals during pregnancy.
To identify sub-populations of pregnant women with higher phthalate/replacement exposures, numerous prior studies evaluated important seasonal, sociodemographic, and lifestyle predictors of phthalate metabolite (and to a lesser extent phthalate replacement) concentrations. In general, these studies evaluated bivariable and/or multivariable associations between predictors and concentrations of individual phthalate biomarkers (as individual biomarkers or molar sums of biomarkers from common parents) and found that the following characteristics most often remained as important determinants of phthalate biomarker concentrations: age, race/ethnicity, education, income, marital status, social class, parity, pre-pregnancy body mass index (BMI), smoking status, diet, and study year (Cantonwine et al., 2014; Casas et al., 2011; He et al., 2019; James-Todd et al., 2017; Philips et al., 2018; Shu et al., 2018; Valvi et al., 2015; Wenzel et al., 2018). However, a major limitation of these studies is that they only assessed determinants of individual biomarkers (or molar sums representing single parent compounds) in single-pollutant models. Given that pregnant women are exposed to numerous phthalates and their replacements, some studies also assessed predictors of maternal exposure to numerous chemicals (including phthalates) using unsupervised learning methods such as k-means clustering and principal component analysis (PCA) (Chen et al., 2021; Kalloo et al., 2018; Lee et al., 2017; Montazeri et al., 2019). One study using k-means paired with logistic regression analyses found that race/ethnicity and diet were associated with clusters of women who had higher biomarker concentrations of personal care product and plasticizer phthalates, respectively (Kalloo et al., 2018). Another study observed associations of parity, pre-pregnancy BMI, and job type with high phthalate cluster membership (Chen et al., 2021). The few studies using PCA paired with linear regression analyses reported that age, birthplace, race/ethnicity, income, job type, parity, pre-pregnancy BMI, smoking status, and diet were associated with principal components (PCs) heavily loaded for phthalate metabolites, including mono(3-carboxypropyl) phthalate (MCPP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-iso-butyl phthalate (MiBP), and monoethyl phthalate (MEP), and metabolites of di(2-ethylhexyl) phthalate (DEHP) (Chen et al., 2021; Kalloo et al., 2018; Lee et al., 2017). Such approaches that identify determinants of biomarker concentration patterns/profiles may better identify unique characteristics of women who may benefit most from interventions targeted at decreasing phthalate/replacement exposure.
Given that phthalates and their replacements are a diverse class of chemicals with multiple exposure sources, our study focused on identifying whether maternal sociodemographic characteristics, lifestyle factors, enrollment year, and conception season are predictors of phthalate/replacement biomarker concentrations. Our first objective was to identify patterns of phthalate/replacement biomarker concentrations using both k-means clustering and PCA, which can identify groups of pregnant women with similar phthalate/replacement biomarker concentration profiles (k-means) and groups of phthalates/replacements that likely exist together (PCA). Our second objective was to assess associations of maternal sociodemographic characteristics, early gestation lifestyle factors, conception season, and study enrollment year with the patterns of phthalate/replacement biomarker concentrations identified in k-means and PCA.
2. MATERIALS AND METHODS
2.1. Illinois Kids Development Study (I-KIDS) recruitment and enrollment
The current study includes pregnant women from I-KIDS, an ongoing prospective pregnancy cohort designed to evaluate the impacts of prenatal environmental chemical exposures on infant neurodevelopment. Pregnant women were recruited at their first prenatal care appointment from two local obstetric clinics in Champaign-Urbana, IL. Women who expressed interest in the study were eligible to participate if they were ≥ 10 but < 15 weeks pregnant, 18–40 years old, fluent in English, in a low-risk singleton pregnancy, living within a 30-minute drive of the University of Illinois campus, and not planning to move out of the area before their child’s first birthday. The current study includes the first 482 women who enrolled in I-KIDS between December 2013 and August 2018, and remained in the study through the birth of their infant. These women provided written informed consent and the study was approved by the Institutional Review Board at the University of Illinois. The analysis of de-identified specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
2.2. Collection of maternal sociodemographic, lifestyle, and conception season information
Immediately after enrollment, an I-KIDS staff member visited each participant’s home to obtain information about sociodemographic and lifestyle characteristics. We collected information about the following sociodemographic characteristics using an interviewer-administered questionnaire: age, race/ethnicity, education level, marital status, employment status, and household annual income. Women additionally reported whether they planned their current pregnancy. To determine conception season, we used the estimated due date based on the first day of the last menstrual period collected at baseline and confirmed after the first trimester ultrasound. Each woman reported the following information since conception: smoking status, the number of eight-ounce cups of caffeinated beverages consumed on a typical day, and the number of servings of alcoholic beverages consumed per week. Self-reported pre-pregnancy weight and height were used to calculate pre-pregnancy BMI (in kg/m2). Self-reported pre-pregnancy BMI is highly correlated with first trimester measured BMI in other pregnant populations (Bannon et al., 2017; Holland et al., 2013; Natamba et al., 2016), as well as ours (r = 0.99, data not shown). Participants completed a semi-quantitative food frequency questionnaire (FFQ) at enrollment that was adapted for pregnant women from the full-length Block-98 FFQ (NutritionQuest, Berkeley, CA) and asked about maternal diet during the previous three months (Boucher et al., 2006). Reported dietary intakes were used to calculate first trimester Alternative Healthy Eating Index 2010 (AHEI-2010) – an 11-component diet quality measure (scored out of 110) based on food/nutrients predictive of chronic disease risk and mortality; higher scores reflect better diet quality (Chiuve et al., 2012; McCullough et al., 2002).
2.3. Assessment of urinary phthalate/replacement biomarker concentrations
I-KIDS participants provided up to five first-morning urine samples at the following gestational timepoints: 8–15, 13–22, 19–28, 25–33, 32–40 weeks gestation (median 13, 17, 23, 28, and 34 weeks gestation, respectively) as described previously (Pacyga et al., 2021), which corresponded with study home visits (at median 13, 17, and 34 weeks gestation) or routine prenatal care visits (median 23 and 28 weeks gestation). Most women contributed all five urine samples (94.4%), whereas 5.2% and 0.4% contributed four and three urine samples, respectively. Urine samples were collected in polypropylene urine cups and refrigerated immediately. Within 24 hours of collection, urine samples were aliquoted for long-term storage or pooled from each timepoint. Beginning with the first visit’s sample, we added 900 μL of urine to a 5 mL cryovial tube. Each time a women provided a sample, we layered fresh urine onto frozen urine from prior gestational timepoints before immediately freezing it at −80° C. At the end of pregnancy, we thawed and vortex all pooled samples to measure specific gravity. For quality assurance and control, we also collected duplicates and purified water blanks every 10 samples to be analyzed at the CDC. We stored all aliquoted urine at −80° C and sent pooled samples on dry ice to the CDC laboratory in three batches in chronological order of enrollment (batch one enrolled December 2013 - February 2015, batch two enrolled February 2015 - July 2016, and batch three enrolled July 2016 - August 2018). The following phthalate/replacement metabolites were quantified in all batches using previously published methods (Silva et al., 2013; Silva et al., 2007): mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), monoisononyl phthalate (MiNP), monocarboxyoctyl phthalate (MCOP), monocarboxynonyl phthalate (MCNP), MCPP, MBzP, MEP, MBP, mono-hydroxybutyl phthalate (MHBP), MiBP, mono-hydroxy-isobutyl phthalate (MHiBP), cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester (MCOCH), and cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester (MHiNCH). Three additional metabolites were added to the CDC analytical panel for women in batches two and three (monooxononyl phthalate (MONP), mono(2-ethyl-5-hydroxyhexyl) terephthalate (MEHHTP), and mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP)) (Silva et al., 2007; Silva et al., 2019). The CDC laboratory has rigorous quality control/quality assurance protocols with excellent long-term reproducibility of most phthalate metabolite biomarkers over 3 and 8 month periods and intra- and inter-day coefficients of variation < 14% for most biomarkers (Silva et al., 2013; Silva et al., 2007; Silva et al., 2019).
2.4. Statistical Analysis
For phthalate/replacement metabolite concentrations below the limit of detention (LOD), we used instrumental-reading values to avoid bias associated with imputing values below the LOD (Succop et al., 2004). Across the individual and molar sum biomarkers described below, only one woman had a zero concentration for the sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites (∑DiNCH) (meaning that her urinary concentrations of both MCOCH and MHiNCH were zero). In final statistical models we added a constant (1.0) to ∑DiNCH before ln-transformation to avoid undefined estimates (Weiss et al., 2015). To account for urine dilution, we adjusted all urinary phthalate/replacement metabolite concentrations using the following formula: Pc = P[(1.016 − 1)/(SG − 1)], where Pc is the specific gravity-adjusted metabolite concentration, P is the measured metabolite concentration (ng/mL), 1.016 is the population median specific gravity, and SG is the specific gravity of each individual urine sample (Meeker et al., 2009). We summed the molar concentrations (in nmol/mL) of the following metabolites to create biomarkers of exposure to phthalate/replacement parent compounds that are metabolized and excreted as multiple urinary metabolites (Pacyga et al., 2021): MEHP, MEHHP, MEHOP, and MECPP for the sum of di(2-ethylhexyl) phthalate metabolites (∑DEHP); MiNP and MCOP for the sum of di-isononyl phthalate metabolites (∑DiNP); MBP and MHBP for the sum of di-n-butyl phthalate metabolites (∑DBP); MiBP and MHiBP for the sum of di-iso-butyl phthalate metabolites (∑DiBP); MHiNCH and MCOCH for ∑DiNCH; and MEHHTP and MECPTP for the sum of di(2-ethylhexyl) terephthalate metabolites (∑DEHTP). We also created another ∑DiNP (∑DiNP2) limited to women enrolled between February 2015 and August 2018 to include MONP. These molar concentrations were converted to ng/mL by multiplying ∑DEHP, ∑DiNP (both versions), ∑DBP, ∑DiBP, ∑DiNCH, and ∑DEHTP by the molecular weights of MECPP, MCOP, MBP, MiBP, MHiNCH, and MECPTP, respectively. We estimated exposure to di-isodecyl phthalate, di-n-octyl phthalate, benzylbutyl phthalate, and diethyl phthalate using ng/mL concentrations of their corresponding urinary metabolites MCNP, MCPP, MBzP, and MEP, respectively.
To understand how phthalate/replacement metabolite concentrations in I-KIDS compare to those in the general U.S. population, we used data from the National Health and Nutrition Examination Survey (NHANES) survey cycles 2013–14, 2015–16, and 2017–18 (NHANES, 2013–2014; NHANES, 2015–2016; NHANES, 2017–2018). These NHANES survey cycles correspond with urine collection years in I-KIDS. Though most women in NHANES were not pregnant, we subset the NHANES sample to only include 18 – 40 year-old females with data on urinary phthalate/replacement metabolite concentrations. Finally, because NHANES does not provide specific gravity information, we reported median (25th, 75th percentiles) unadjusted phthalate/replacement metabolite concentrations for both samples (Table 2).
Table 2.
Unadjusted phthalate/replacement metabolite concentrations (ng/mL).
| I-KIDS (n=482) | NHANES (n=1076) | |||
|---|---|---|---|---|
| Parent Compound | Metabolite(s) | % ≥ LOD | Median (25th, 75th pctl) 2013–2018 | Median (25th, 75th pctl) 2013–2018 |
| DEHP | MEHP | 74.3 | 1.3 (0.8, 2.2) | 1.1 (0.6, 2.3) |
| MEHHP | 100.0 | 6.0 (3.8, 9.2) | 5.3 (2.5, 10.8) | |
| MEOHP | 100.0 | 4.6 (3, 6.9) | 3.7 (1.7, 7.3) | |
| MECPP | 100.0 | 9.2 (6.1, 14.8) | 8.6 (4.1, 16.8) | |
| DiNP | MCOP | 100.0 | 11.0 (5.4, 25.7) | 8.0 (3.5, 23.6) |
| MiNP | 41.7 | 0.7 (0.4, 1.5) | 0.6 (0.6, 1.1) | |
| MONP | 100.0 | 2.7 (1.7, 4.7)1 | 1.6 (0.7, 3.2)3 | |
| DiDP | MCNP | 100.0 | 2.1 (1.4, 3.3) | 1.6 (0.8, 3.4) |
| DOP | MCPP | 97.1 | 1.5 (0.9, 2.6) | 1.1 (0.5, 2.6) |
| BBzP | MBzP | 99.6 | 5.3 (2.8, 12) | 4.6 (1.6, 12.0) |
| DEP | MEP | 100.0 | 25.0 (12.6, 46.5) | 34.4 (14.6, 85.8) |
| DBP | MBP | 100.0 | 12.6 (8.1, 19.5) | 11.2 (5.1, 20.2) |
| MHBP | 90.0 | 1.2 (0.7, 2) | 0.9 (0.3, 1.7)2 | |
| DiBP | MiBP | 99.8 | 9.1 (5.5, 14.1) | 8.7 (4.0, 17.9) |
| MHiBP | 99.8 | 3.3 (2, 5.1) | 2.9 (1.4, 6.0)2 | |
| DiNCH | MHiNCH | 77.4 | 0.8 (0.4, 1.6) | 0.4 (0.3, 1.1) |
| MCOCH | 50.4 | 0.5 (0.3, 1) | 0.4 (0.4, 0.8)3 | |
| DEHTP | MEHHTP | 100.0 | 8.7 (3.7, 19.7)1 | 6.0 (2.2, 17.1)3 |
| MECPTP | 100.0 | 60.5 (24.3, 140)1 | 20.7 (8.5, 67.6)3 | |
Urinary phthalate/replacement metabolite concentrations were obtained for 18–40 year-old pregnant and non-pregnant females from NHANES survey years 2013–14, 2015–16, and 2017–18 I-KIDS reports numeric values for all concentrations below the LOD, while NHANES replaces all values below the LOD with the LOD/√2 for that metabolite. Concentrations do not account for urine dilution.
n=309,
n=1074,
n=682.
I-KIDS, Illinois Kids Development Study; NHANES, National Health and Nutrition Examination Survey.
Our analyses included 15 maternal characteristics that have been previously shown to predict phthalate/replacement biomarker concentrations or were hypothesized to be critical determinants of phthalate/replacement exposure in our population (Gao et al., 2017; He et al., 2019; James-Todd et al., 2017; Lyden et al., 2020; Rodriguez-Carmona et al., 2020; Shu et al., 2018). These included age, race/ethnicity, education, marital status, employment status, household annual income, parity, conception season, enrollment year, smoking in the first trimester, consumption of alcohol and caffeine in the first trimester, pregnancy intention, pre-pregnancy BMI, and diet quality. Almost all predictors were assessed as categorical variables, with the exception of enrollment year, which we evaluated as a continuous variable that can be interpreted for every 1 year increase. Details about variable categorization are provided in Table 1. Of note, an additional category for smoking in the first trimester (“unknown”) was created to account for missingness due to an ambiguous skip pattern in the first iteration of the survey. Pre-pregnancy BMI was categorized based on standard U.S. clinical cut-offs (Weir and Jan, 2021).
Table 1.
Characteristics of I-KIDS women in the full and sub-samples.
| Full sample enrolled 12/2013 – 8/2018 (n=482) | Sub-sample enrolled 2/2015 – 8/2018 (n=309) | |
|---|---|---|
| n (%) | n (%) | |
| Maternal age | ||
| < 30 years | 197 (41.9) | 115 (37.2) |
| ≥ 30 years | 285 (59.1) | 194 (62.8) |
| Race/ethnicity | 1 missing | |
| Non-Hispanic white | 385 (80.0) | 251 (81.2) |
| Non-Hispanic black | 26 (5.4) | 16 (5.2) |
| Asian | 26 (5.4) | 20 (6.5) |
| Other1 | 44 (9.2) | 22 (7.1) |
| Education | ||
| Some college or less | 90 (18.7) | 52 (16.8) |
| College grad or higher | 392 (81.3) | 257 (83.2) |
| Marital status | ||
| Married | 426 (88.4) | 273 (88.4) |
| Unmarried | 56 (11.6) | 36 (11.7) |
| Employment status | ||
| Unemployed | 67 (13.9) | 39 (12.6) |
| Employed | 415 (86.1) | 270 (87.4) |
| Household income | 4 missing | |
| <$60,000 | 138 (28.6) | 84 (27.2) |
| $60,000-$99,999 | 182 (37.8) | 114 (36.9) |
| ≥$100,000 | 158 (32.8) | 109 (35.3) |
| Enrollment year | ||
| 12/2013 – 02/2015 | 173 (35.9) | -- |
| 02/2015 – 07/2016 | 174 (36.1) | 174 (56.3) |
| 07/2016 – 08/2018 | 135 (28.0) | 135 (43.7) |
| Conception season | ||
| Winter | 122 (25.3) | 80 (25.9) |
| Spring | 134 (27.8) | 101 (32.7) |
| Summer | 107 (22.2) | 78 (25.2) |
| Fall | 119 (24.7) | 50 (16.2) |
| Parity | ||
| 0 children | 246 (51.0) | 164 (53.1) |
| ≥ 1 child | 236 (49.0) | 145 (46.9) |
| Active smoker | ||
| No | 423 (87.8) | 294 (95.2) |
| Yes | 24 (5.0) | 15 (4.9) |
| Missing | 35 (7.3) | -- |
| Alcohol intake | 1 missing | |
| No serving/week | 281 (58.3) | 180 (58.3) |
| ≥ 1 servings/week | 200 (41.5) | 129 (41.8) |
| Pregnancy intention | ||
| Planned | 321 (66.6) | 207 (67.0) |
| Unplanned | 161 (33.4) | 102 (33.0) |
| Pre-pregnancy BMI | ||
| < 25 kg/m2 | 258 (53.5) | 162 (52.4) |
| ≥ 25 kg/m2 | 224 (46.5) | 147 (47.6) |
| Caffeine intake | ||
| None | 196 (40.7) | 124 (40.1) |
| < 1 cups/week | 145 (30.1) | 93 (30.1) |
| ≥ 1 cups/week | 141 (29.3) | 92 (29.7) |
| Overall diet quality | 3 missing | 2 missing |
| AHEI < 55.1 | 239 (49.6) | 153 (49.5) |
| AHEI ≥ 55.1 | 240 (49.8) | 154 (49.8) |
Hispanic white, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiracial, and other.
I-KIDS, Illinois Kids Development Study
We selected methods to evaluate chemical mixtures appropriate for the specific research question (Braun et al., 2016). We used the following two unsupervised methods (objective 1): k-means clustering and PCA (Hotelling, 1933; MacQueen, 1967). K-means clustering identifies subgroups of participants with distinct biomarker concentration profiles, which is useful for identifying pregnant women who may experience relatively high or low chemical exposures. We used k-means clustering to group pregnant women into k number of distinct, non-overlapping clusters (identified using Euclidean geometry) based on their similarities across all individual phthalate/replacement biomarker concentrations. To identify the optimal number of clusters, we compared 1, 2, 3, and 4 cluster solutions using the pseudo f-statistic index (the ratio of between-cluster variance to within cluster variance) and confirmed the ideal number of clusters using elbow plots of R2 values. PCA identifies linear combinations of biomarker concentration patterns among highly correlated biomarker that explain most of the variance in biomarker concentrations in a population. These resulting patterns can be related to common exposure sources or behaviors in the study population. We used PCA with a Varimax rotation to identify biomarkers of highly correlated phthalate/replacements to which pregnant women are likely exposed and created distinct, uncorrelated PC scores that explain most of the variance in phthalate/replacement biomarker concentrations in our participants. To determine the ideal number of PCs, we assessed elbow plots of eigenvalues (total variance explained by each component) and used the total variance explained to confirm the optimal number of components that best represents the data. We considered biomarkers with loadings ≥ 0.3 to be notable. For both k-means and PCA, we included specific gravity-adjusted phthalate/replacement biomarker concentrations in ng/mL that were ln-transformed and z-transformed.
We used logistic and linear regression models to evaluate associations of 15 maternal characteristics with the identified clusters and PCs, respectively (objective 2). Evaluating associations of characteristics with identified k-means clusters using logistic regression models provides information about characteristics of pregnant women with specific phthalate/replacement biomarker concentration profiles. Assessing associations of maternal characteristics with identified PCs using linear regression models provides information about characteristics that likely result in exposure to certain phthalates/replacements from common exposure sources or behaviors. We evaluated both unadjusted models (bivariable analyses) and models simultaneously adjusted for all 15 predictors (multivariable analyses). A total of 9 women had missing data on at least one predictor. Therefore, 473 women who enrolled between December 2013 and August 2018 (referred to as the full sample) were included in final multivariable analyses. To assess MONP and both DEHTP metabolites, we conducted additional analyses limited to women enrolled between February 2015 and August 2018 (referred to as the sub-sample). A total of 305 women were included in these multivariable analyses. There was high agreement between the full and sub-samples with regards to k-means cluster membership (Kappa statistic = 0.82) and PC scores (r > 0.8). However, we reported results from both samples to provide information about phthalate/∑DiNCH biomarker concentrations across the whole study period and to report results related to phthalate replacement DEHTP.
We used SAS 9.4 (version 15.1, SAS Institute) for all statistical analyses. We used PROC FASTCLUS and PROC LOGISTIC to assign k-means clusters and for bivariable and multivariable logistic regression models, respectively. We used PROC FACTOR for the PCA and PROC GENMOD for bivariable and multivariable linear regression models. Based on recommendations from the American Statistical Association and others (Amrhein et al., 2019; Wasserstein and Lazar, 2016), rather than using P-values, we used the magnitude of associations and 95% confidence intervals (CIs) to identify potentially meaningful results. We used RStudio Version 1.3.1093 (RStudio, Boston, MA) to generate figures.
3. RESULTS
3.1. I-KIDS population characteristics and urinary phthalate/replacement biomarker concentrations
Sociodemographic and lifestyle characteristics of I-KIDS women have been previously described (Pacyga et al., 2021) and are outlined in Table 1. Briefly, most women were non-Hispanic white, of high socioeconomic status, and engaged in healthy lifestyle behaviors. Greater than 97% of I-KIDS women had detectable urinary concentrations of at least one metabolite per phthalate parent compounds (including DEHTP), while only 77% had detectable urinary concentrations of at least one DiNCH metabolite (Table 2). Most phthalate/replacement biomarkers were weakly-to-moderately correlated (r < 0.4), although strong correlations were observed between MCPP, ∑DiNP, and ∑DiNP2 (r > 0.8; Supplemental Figure 1). I-KIDS pregnant women had similar median urinary phthalate and DiNCH metabolite concentrations as those from a nationally representative sample of 18 – 40 year-old pregnant or non-pregnant U.S. women from the 2013 – 2018 National Health and Nutrition Examination Survey (NHANES) cycles (Table 2). However, I-KIDS women had higher median concentrations of DEHTP metabolites, but lower median concentrations of MEP (with overlapping 25th and 75th percentiles) compared to NHANES women.
3.2. K-means clusters of women with distinct phthalate/replacement biomarker concentration profiles
Our goal with using k-means clustering was to identify groups of women with distinct profiles of urinary phthalate/replacement biomarker concentrations. In the full sample (excluded MONP and the DEHTP metabolites), we identified the following two clusters: cluster 1 included women with concentrations of all phthalate biomarkers below the sample median, while cluster 2 included women with concentrations of all phthalate biomarkers above the sample median (Figure 1 and Supplemental Table 1). ∑DiNCH concentrations were similar between the two clusters, and therefore did not drive cluster membership. In the sub-sample (includes MONP and DEHTP metabolites), the k-means procedure identified similar clusters as those identified in the full sample. Therefore, the two clusters of women in this sub-sample included women with all phthalate biomarker concentrations (including ∑DEHTP) below the sample median (cluster 1) and those with all phthalate biomarker concentrations (including ∑DEHTP) above the sample median (cluster 2) (Figure 1 and Supplemental Table 1).
Figure 1. Multivariable associations of sociodemographic, lifestyle, enrollment year, and conception season predictors with k-means clusters.
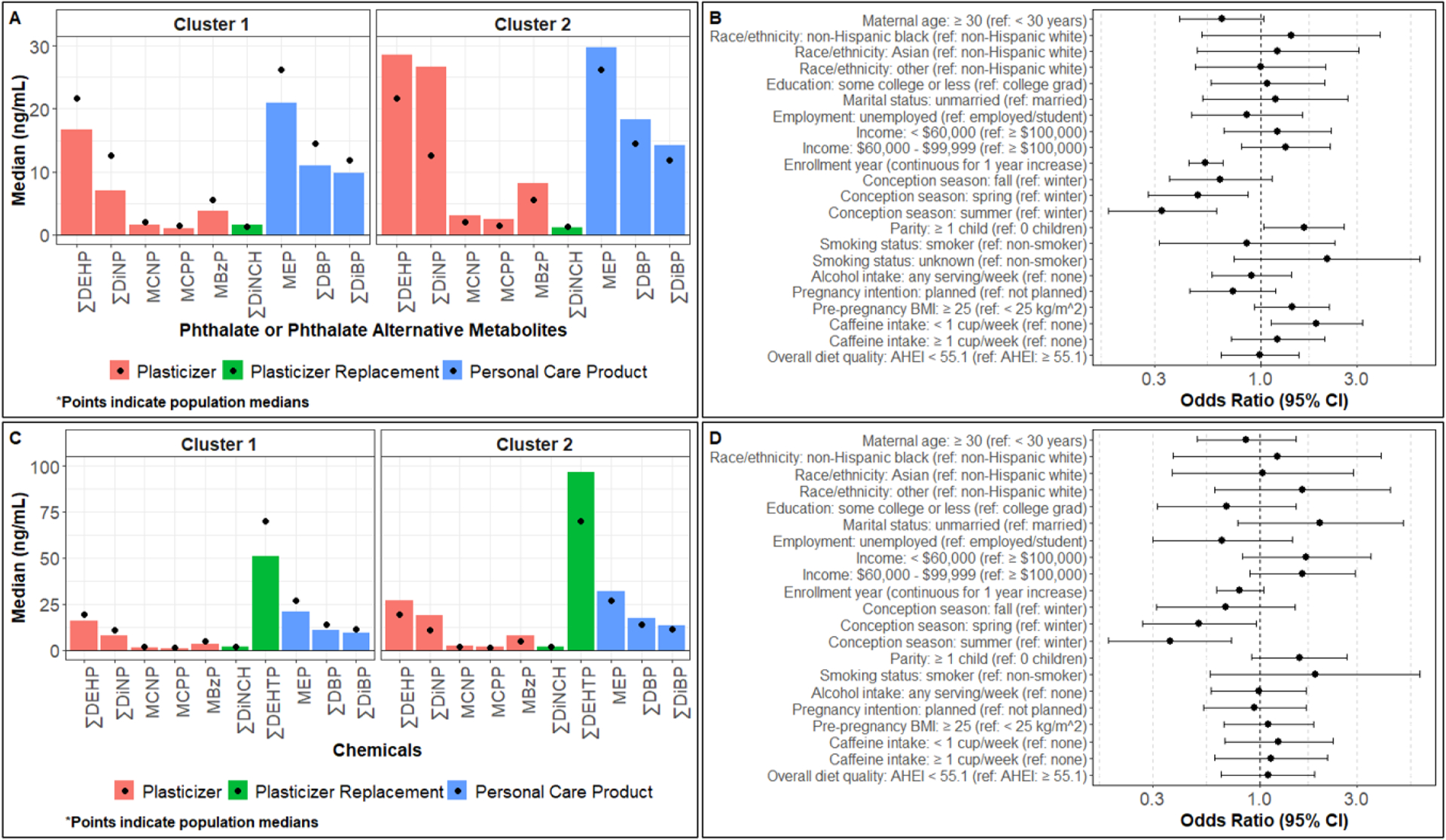
Median urinary specific gravity-adjusted phthalate/replacement biomarker concentrations by k-means cluster in the A) full sample (enrolled between 12/2013 and 8/2018) or C) sub-sample (enrolled between 2/2015 and 8/2018). Logistic regression models simultaneously adjusted for all listed predictors evaluated associations of 15 predictors with the odds of having B) high phthalate (cluster 2, n=230) than low phthalate (cluster 1, n=243) in the full sample or D) high phthalate including ∑DEHTP (cluster 2, n=131) than low phthalate including ∑DEHTP (cluster 1, n=174) biomarker concentrations in the sub-sample. BMI, body mass index; CI, confidence interval.
3.3. Associations of maternal characteristics with identified k-means clusters
Bivariable analyses evaluating associations of characteristics with k-means clusters for the full and sub-samples are presented in Supplemental Table 2. In multivariable logistic regression models simultaneously adjusted for all characteristics, women had higher odds of high phthalate cluster membership if they had ≥ 1 child prior to the I-KIDS pregnancy (ref = no children; OR: 1.6; 95% CI: 1.0, 2.6), had overweight or obesity before pregnancy (ref = under-/normal weight; OR: 1.4; 95% CI: 0.9, 2.2), and consumed < 1 cup of caffeine per week (ref = no caffeine consumption; OR: 1.9; 95% CI: 1.1, 3.2) (Figure 1 and Supplemental Table 2). Conversely, women had lower odds of high phthalate cluster membership if they were ≥ 30 years old (ref = < 30 years; OR: 0.6; 95% CI: 0.4, 1.0), enrolled earlier in the study (for every 1 year increase in study year; OR: 0.5; 95% CI: 0.4, 0.7), and conceived in the spring (OR: 0.5; 95% CI: 0.3, 0.9), summer (OR: 0.3; 95% CI: 0.2, 0.6), or fall (OR: 0.6; 95% CI: 0.4, 1.1) compared to winter. In multivariable logistic regression models in the sub-sample, associations of enrollment year, conception season, and parity with cluster membership were similar to those observed in the full sample (Figure 1 and Supplemental Table 2). However, additional associations of marital status and annual household income with cluster membership emerged in the multivariable logistic regression models in the sub-sample (Figure 1 and Supplemental Table 2). Specifically, women had a higher odds of high phthalate (including ∑DEHTP) cluster membership if they were unmarried (ref = married, OR: 2.0; 95% CI: 0.8, 5.1) and had annual household incomes < $100,000 (ref = ≥ $100,000; < $60,000 OR: 1.7; 95% CI: 0.8, 3.5; $60,000 - $99,999 OR: 1.6; 95% CI: 0.9, 2.9).
3.4. PCs of phthalate/replacement biomarker concentrations
Our goal with PCA was to identify phthalate/replacement biomarker concentrations that exist together due to common exposure sources or lifestyle factors. In the full sample, four PCA components accounted for 71.2% of the total variance (32.2%, 17.2%, 11.2%, and 10.6% of the total variance explained by components 1–4, respectively). The heaviest loadings for each PC are as follows: ∑DiNP, MCNP, and MCPP with component 1 (referred to as phthalate plasticizer component); MBzP, ∑DBP, and ∑DiBP with component 2 (referred to as other phthalate component); ∑DEHP and ∑DiNCH with component 3 (referred to as ∑DEHP/∑DiNCH component); and MEP with component 4 (referred to as MEP component) (Supplement Table 3). In the sub-sample, four PCA components accounted for 66.9% of the total variance (27.6%, 16.3%, 13.1%, and 9.9% of the total variance explained by components 1–4, respectively). Components 2 (other phthalate component) and 4 (MEP component) were similar to those discussed above, whereas component 1 was heavily loaded by ∑DiNP2, MCNP, and MCPP (referred to as plasticizer phthalate component) and component 3 was heavily loaded by ∑DiNCH and ∑DEHTP (referred to as phthalate replacement component) (Supplemental Table 3). In the full sample and the sub-sample, the biomarkers that loaded most heavily were positively correlated with the four component scores indicating that as urinary concentrations of those biomarkers increase, component scores increase (Supplemental Table 3).
3.5. Associations of maternal characteristics with identified PCs
Results of bivariable analyses evaluating the relationships between characteristics and PC scores for the full and sub-samples are presented in Supplemental Tables 4 and 5, respectively. In multivariable linear regression models in the full sample (Figure 2 and Supplemental Table 4), phthalate plasticizer component scores were lower in Asian women (ref = non-Hispanic white), those with annual household incomes < $60,000 (ref = ≥ $100,000), those who conceived in the spring, summer, or fall (ref = winter), and those who planned their pregnancy (ref = unplanned pregnancy). Conversely, plasticizer component scores were higher in women who had lower educational attainment (ref = college graduates), enrolled earlier in the study, those who had overweight or obesity before pregnancy (ref = under-/normal weight), and who consumed < 1 cups of caffeine/week (ref = no caffeine consumption). Other phthalate scores were lower in women ≥ 30 years old (ref = < 30 years old) and those with lower educational attainment (ref = college graduates), but higher in Asian women or women of other race/ethnicity (ref = non-Hispanic white), those with annual household incomes < $100,000 (ref = ≥ $100,000), and those who had ≥ 1 child prior to the I-KIDS pregnancy (ref = no children). ∑DEHP/∑DiNCH component scores were lower in women who conceived in the spring or summer months (ref = winter), in those with annual household incomes < $60,000 (ref = ≥ $100,000), and in women with unknown smoking status (ref = non-smokers). However, ∑DEHP/∑DiNCH component scores were higher in Asian women or those of other race/ethnicity (ref = non-Hispanic white), in women who enrolled later in the study, in those who smoked in the first trimester (ref = non-smokers), and in those that had overweight or obesity before pregnancy (ref = under-/normal weight). Lastly, MEP scores were higher in black women or those of other race/ethnicity (ref = non-Hispanic white), in unmarried women (ref = married), and among those who consumed some amount of caffeine/week (ref = no caffeine consumption), while MEP scores were lower in women who conceived in the spring or fall months (ref = winter), who consumed ≥ 1 servings/week of alcohol in the first trimester (ref = no alcohol consumption), who had overweight or obesity before pregnancy (ref = under, and had poor first trimester diet quality (ref = better diet quality).
Figure 2. Multivariable associations of sociodemographic, lifestyle, enrollment year, and conception season predictors with principal component scores.
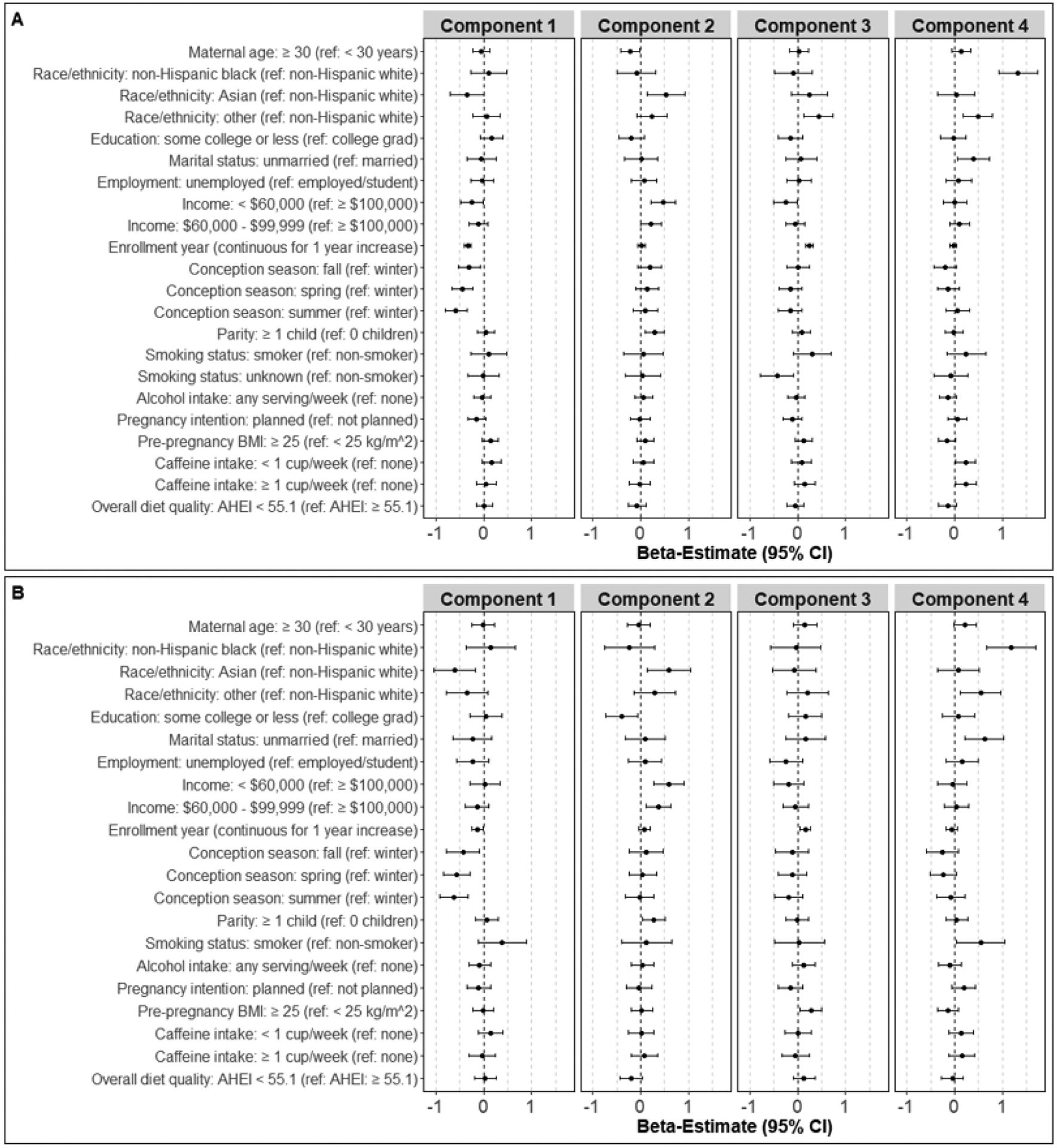
Linear regression models simultaneously adjusted for all listed predictors evaluated associations of 15 maternal predictors with change in component scores in the A) full sample (enrolled between 12/2013 and 8/2018, n=473) and B) sub-sample (enrolled between 2/2015 and 8/2018, n=305). Component 1 = phthalate plasticizer component, component 2 = other phthalate component, component 3 = ∑DEHP/∑DEHTP (full sample) or phthalate replacement (sub-sample) component, and component 4 = MEP component. BMI, body mass index; CI, confidence interval.
In multivariable analyses in the sub-sample (Figure 2 and Supplemental Table 5), associations of race/ethnicity, enrollment year, and conception season with the phthalate plasticizer component, of race/ethnicity, annual household income, and parity with the other phthalate component, of annual household income, enrollment year, conception season, and pre-pregnancy BMI with the phthalate replacement component, and of race/ethnicity, marital status, and conception season with the MEP component were similar to those reported above in the full sample. However, unique associations of maternal characteristics with each component also emerged in this sub-sample. Phthalate plasticizer scores were higher among women who were unemployed (ref = employed) and those who smoked in the first trimester (ref = non-smoker). Other phthalate scores were higher among women who enrolled later in the study, but were lower in those who had lower educational attainment (ref = college graduates) and had poor first trimester diet quality (ref = better diet quality). Phthalate replacement scores were lower among women who were unemployed (ref = employed), but higher among women ≥ 30 years old (ref = < 30 years old). Lastly, MEP scores were higher among women ≥ 30 years old (ref = < 30 years old), those who enrolled earlier in the study, those who smoked in the first trimester (ref = non-smoker), and those who planned their current pregnancy (ref = unplanned pregnancy).
4. DISCUSSION
In the current study, we identified two distinct clusters of women: those with low phthalate (including ∑DEHTP) and those with high phthalate (including ∑DEHTP) biomarker concentrations. We also identified four components representative of phthalate/replacement biomarker concentrations from common exposure sources or those that track with certain behaviors. We identified age, marital status, annual household income, parity, pre-pregnancy BMI, caffeine intake, conception season, and enrollment year as important predictors of k-means clusters and at least one PC. Additionally, race/ethnicity, education, employment, pregnancy intention, smoking and consuming alcohol in the first trimester, and first trimester diet quality were identified as important determinants of at least one PC. Overall, our findings contribute information about predictors of phthalate/replacement mixtures that may be important confounding factors in studies evaluating associations of chemical mixtures with pregnancy-related health outcomes. Furthermore, our results may inform future perinatal health recommendations by providing insights into characteristics of pregnant women who are most likely to be exposed to phthalates and their replacements.
4.1. K-means clustering identified two groups of women with distinct phthalate/replacement biomarker profiles
K-means clustering is useful for identifying subgroups of pregnant women with distinct patterns of biomarker concentrations. A major strength of this approach is that the identified clusters of women can be used in future studies to evaluate the relationships between population exposure patterns and adverse health outcomes. In our population, we identified two groups of women: those who had low phthalate (including ∑DEHTP) and those who had high phthalate (including ∑DEHTP) biomarker concentrations. A study of pregnant women from Ohio used k-means clustering to characterize patterns of exposure to numerous chemical classes, including phthalates, and identified three clusters of women that had different phthalate biomarker concentration patterns than those identified in our population (Kalloo et al., 2018). However, somewhat consistent with our results, another study of pregnant women from Wuhan, China evaluated trimester-specific population profiles of multiple chemical classes and observed that in any one trimester, groups of pregnant women had either high or low phthalate biomarker concentrations (Chen et al., 2021). This highlights a limitation of k-means because identified biomarker concentration patterns are population-specific and may not be generalizable to other cohorts, and models that account for multiple classes of chemicals may yield different conclusions than models that focus on one chemical class. Therefore, additional studies using these approaches are needed to determine whether these patterns persist in other populations. Nevertheless, in our relatively homogenous population, k-means identified two relatively even clusters of women with concentrations of all phthalate biomarkers (including DEHTP) that were consistently higher or lower than the sample median. Interestingly, our results also suggests that the plasticizer replacement biomarker ∑DiNCH was not related to high vs. low chemical cluster membership. In other words, DiNCH was uniformly distributed in our population. Because DiNCH was developed specifically for use in so-called sensitive applications, including medical tubing and children’s toys, it is possible that sources of DiNCH are less avoidable in our population than other phthalates/replacements.
4.2. Several maternal characteristics were important predictors of k-means clusters
Although previous studies evaluated predictors of phthalate/replacement biomarker concentrations, most studies did not account for exposures to chemical mixtures. Therefore, we paired k-means clustering with logistic regression to identify characteristics associated with the two identified clusters of women. Overall, we observed that age, marital status, annual household income, parity, pre-pregnancy BMI, caffeine intake, enrollment year, and conception season remained the strongest important independent predictors of phthalate/replacement biomarker concentrations. Our findings that pregnant women who are younger, unmarried, have lower incomes, and had pre-pregnancy overweight/obesity have higher phthalate biomarker concentrations are in line with findings from several previous studies of pregnant women from the contiguous U.S. and Puerto Rico, Canada, Mexico, Europe, and China (Cantonwine et al., 2014; Lewin et al., 2017; Li et al., 2019; Philips et al., 2018; Polinski et al., 2018; Valvi et al., 2015; Wenzel et al., 2018; Wu et al., 2020). To our knowledge, this is the first study to identify caffeine consumption as a predictor of phthalate biomarker concentrations in pregnant women, although recent coffee consumption was associated with higher MCPP biomarker concentrations in an adolescent population (Smith et al., 2021) and higher MEHHP-to-MECPP and MEOHP-to-MECPP ratios in U.S adults from NHANES 2001 – 2012 survey cycles (Yaghjyan et al., 2016). Whether this relationship is confounded by lifestyle factors that track with caffeine consumption in pregnancy, to the diuretic nature of caffeine, or possible contamination by phthalates in food packaging used in the preparation or consumption of caffeinated beverages will need to be further investigated. Our findings that women with at least one child have higher phthalate concentrations are consistent with some prior studies, but the literature related to phthalates and parity is generally mixed (Arbuckle et al., 2014; Cantonwine et al., 2014; Philips et al., 2018; Zhu et al., 2016). We also observed that women who conceived in the spring/summer/fall had lower odds of high phthalate/∑DEHTP. Given that women who conceived in the spring/summer provided most of their urine samples in fall/winter, our findings are somewhat consistent with studies of Swedish and Chinese pregnant women reporting higher phthalate biomarker concentrations in urine samples collected during spring/summer than fall/winter (Gao et al., 2017; Shu et al., 2018). These studies suggest that these trends are likely due to seasonal differences in diet or personal care product use. Our findings related to enrollment period are supported by those from NHANES and other U.S. biomonitoring studies showing that phthalate biomarker concentrations are decreasing, especially phthalate plasticizer biomarker concentrations, while DiNCH and DEHTP metabolite concentrations are increasing over time (CDC, 2021; Lessmann et al., 2016). These trends have also been confirmed in studies that include more recent sampling periods (Qu et al., 2022; Runkel et al., 2022) and suggest that women may be choosing to change their product use over time or that the use of phthalates/replacements in consumer products may be changing.
4.3. PCA identified four components of phthalate/replacement biomarker concentrations
PCA is a dimension reduction method useful for identifying distinct, uncorrelated patterns of biomarker concentrations among highly correlated chemicals. Consistent with previous human biomonitoring studies (Koch et al., 2013), we observed that phthalate/replacement biomarkers were correlated along exposure sources, as the four identified components were heavily loaded by phthalates from plastics (∑DiNP or ∑DiNP2, MCNP, and MCPP), other phthalates (MBzP, ∑DBP, and ∑DiBP), major plasticizer phthalate and its replacements (∑DEHP, ∑DiNCH, and ∑DEHTP), and a major personal care product-related phthalate metabolite, MEP. While we only assessed phthalates, previous studies included additional chemical classes to identify broader exposure patterns during pregnancy (Chen et al., 2021; Kalloo et al., 2018; Lee et al., 2017; Montazeri et al., 2019; Rosofsky et al., 2017). In PCA analyses, a few studies in pregnant populations from the USA (Ohio), Canada, and Europe reported that MEP always represented a unique component (Kalloo et al., 2018; Lee et al., 2017; Rosofsky et al., 2017), which is consistent with our findings. In most populations, including ours, pregnant women have highest MEP concentrations relative to other phthalate metabolites likely due to their use of fragranced/perfumed products or cosmetics that contain diethyl phthalate, MEP’s parent compound (Hsieh et al., 2019; Parlett et al., 2013). Though a major limitation of PCA is that the identified components are unique to each population (similar to k-means), these consistent MEP findings in predominately white Western populations suggest that there may be certain exposure patterns that are consistent in pregnant women, although this needs to be further corroborated in non-Western populations. Most importantly, PCA in our population also identified a separate component heavily weighted for biomarkers of replacement compounds, DiNCH and DEHTP. This suggests that the replacements either have shared exposure sources or exist together through behaviors aimed at reducing exposure to phthalates. These results are concerning since phthalate replacement concentrations are increasing over time (CDC, 2021), and their health impacts during pregnancy are poorly understood (Campioli et al., 2017; Woodward et al., 2020). Additional studies in more diverse populations are needed to confirm these exposure biomarker patterns.
4.4. Several maternal characteristics were important predictors of PCs
Pairing PCA results with linear regression helped us identify maternal characteristics that best tracked with the four identified exposure biomarker profiles/clusters. Similar to our results from k-means clustering, this approach also identified age, marital status, income, parity, pre-pregnancy BMI, caffeine intake, enrollment year, and conception season as important independent predictors of phthalate/replacement biomarker concentrations. However, these analyses additionally identified race/ethnicity, education, employment status, pregnancy intention, smoking status, alcohol intake, and diet quality as determinants of at least one PC. Our findings pertaining to sociodemographic characteristics are consistent with previous studies from the contiguous U.S. and Puerto Rico showing that women who are younger, have lower income, and are unmarried have higher other phthalate metabolite concentrations, and that women who are older, have higher incomes, and are employed have higher phthalate replacement biomarker concentrations (James-Todd et al., 2017; Polinski et al., 2018; Rodríguez-Carmona et al., 2020; Wenzel et al., 2018). Our findings also confirmed that black women, those who were older, and those who smoked during pregnancy have higher MEP concentrations, those with lower educational attainment have lower other phthalate (MBzP, ∑DBP and ∑DiBP) biomarker concentration, and that women who had overweight or obesity before pregnancy had higher phthalate plasticizer biomarker concentrations (Arbuckle et al., 2014; He et al., 2019; Polinski et al., 2018; Rodríguez-Carmona et al., 2020; Valvi et al., 2015; Wenzel et al., 2018; Wu et al., 2020). However, inconsistent with results the Puerto Rican study (Rodriguez-Carmona et al., 2020), we observed that women with pre-pregnancy overweight or obesity had higher phthalate replacement biomarker concentrations. Urinary DEHTP metabolite concentrations in our population are at least two times higher than those in the Puerto Rico cohort, which may explain discrepancies in findings. Additionally, our findings regarding pregnancy intention are somewhat in line with those from a multi-center cohort of U.S. pregnant women finding that women with unplanned pregnancies generally had higher MCPP and lower MEP first trimester biomarker concentrations (Lyden et al., 2020). We also observed that women with lower diet quality had lower other phthalate PC scores (strongest correlations with MBzP, ∑DBP and ∑DiBP). One possible explanation is that women with lower diet quality may be less likely to take supplements (Dickinson and MacKay, 2014), which are a source of DBP (Kelley et al., 2012). Alternatively, it is possible that our diet quality measure does not account for the use of plastic food storage containers or the use of plastic water bottles – dietary habits that have been associated with urinary BBzP and DBP metabolite biomarker concentrations (Yan et al., 2009).
With regard to other studies using PCA, our findings are somewhat in line with those in pregnant women from Ohio, Canada, and China (discussed above). Race/ethnicity, household income, educational attainment, parity, pre-pregnancy BMI and diet were important predictors of phthalate biomarker PCs in the Ohio cohort (Kalloo et al., 2018), parity and smoking status were predictors of phthalate biomarker PCs in the Canadian cohort (Lee et al., 2017), while income, pre-pregnancy BMI, and employment status were important predictors of phthalate biomarkers PCs in the China cohort (Chen et al., 2021). However, two of these studies also reported that birthplace was associated with phthalate biomarker PCs (Kalloo et al., 2018; Lee et al., 2017), which was not evaluated in our study. Conversely, the multi-cohort European study found no associations of education or employment status with phthalate biomarker PCs (Montazeri et al., 2019). Given that only a few studies paired PCA with linear regression models, additional studies are needed to corroborate our findings. Nevertheless, our results show that phthalate/replacement exposure biomarker patterns in pregnant women are associated with sociodemographic-related lifestyle characteristics that may predict consumption of products containing phthalates/replacements.
4.5. Strengths and limitations
Our study has several strengths. First, we systematically assessed a large number of a priori hypothesized maternal characteristics, some of which are not extensively studied in the literature (e.g. caffeine consumption, pregnancy intention, overall diet quality), providing novel information about predictors of phthalate/replacement biomarker concentrations in pregnancy. Second, we focused only on phthalates and their replacements to better understand biomarker profiles and predictors of this large class of endocrine disrupting chemicals. Additionally, we assessed associations of predictors with phthalate/replacement metabolites using mixture methods. It has been demonstrated that the use of mixture methods to evaluate association of phthalate biomarkers with preterm birth identified preterm birth risk beyond what was expected based on single-pollutant models. Our approach may help us better identify potential confounding factors of associations between cumulative phthalate/replacement exposure and pregnancy-related health outcomes (Boss et al., 2018). Third, phthalate/replacement biomarker concentrations were quantified in pooled samples of up five first-morning urines, which is considered best practice for the assessment of non-persistent chemicals (Fromme et al., 2007; Johns et al., 2015a; Preau et al., 2010). Additionally, most women (94%) contributed all five urine samples, indicating that chemical biomarkers measured in pooled samples for our population likely represents exposure across gestation. Lastly, our study is one of few to evaluate maternal predictors of DiNCH and DEHTP metabolite concentrations – plasticizer replacements to which pregnant women are becoming increasingly exposed. Interestingly, women in our study had higher DEHTP concentrations than women from NHANES, but also higher concentration than those reported by other pregnancy cohorts (Rodríguez-Carmona et al., 2020; Wu et al., 2020). We are either capturing more recent trends or I-KIDS women (who represent women with higher socioeconomic status than the representative sample in NHANES) may be choosing products labeled as “phthalate free” that may contain replacements. This will need to be investigated in future studies.
However, our study also has limitations. First, data on three urinary metabolites (DiNP metabolite MONP and DEHTP metabolites MEHHTP and MECPTP) were not available from our earliest participants, which limits our available sample size for assessing predictors of ∑DiNP (with the additional MONP metabolite) and ∑DEHTP, as well as ∑DiNCH that had lower detection frequencies compared to the other phthalate/replacement metabolites evaluated in our study. The loss of power and introduction of additional metabolites in multivariable analyses may account for differences in associations in the full versus sub-samples. Second, the timing for assessing early pregnancy lifestyle factors, which may change across a pregnancy, relative to phthalate/replacement biomarker concentrations analyzed from a pooled sample to represent total gestational concentrations, may not be appropriate given the non-persistent nature of these chemicals. Third, the I-KIDS population is primarily comprised of non-Hispanic white women of high socioeconomic status, which may limit the generalizability of our findings to more diverse populations and our capacity to use refined categories for our sociodemographic variables. For example, our Asian category falsely homogenizes a diverse set of ethnicities, so additional studies may be needed to expand on this category. Lastly, although k-means clustering and PCA can be informative, the number and types of identified k-means clusters or PCs varies by population, making it challenging to use these data to generate universal recommendations for reducing prenatal phthalate exposure.
5. CONCLUSIONS
In this midwestern U.S. population, we identified two distinct groups of pregnant women with specific phthalate/replacement biomarker concentration profiles, and four uncorrelated profiles of phthalate/replacement biomarkers that likely track together due to shared exposure sources. We also observed that several sociodemographic characteristics, early pregnancy lifestyle characteristics, enrollment year, and seasonality were associated with biomarker concentration profiles identified in k-means clustering and PCA. These findings contribute to the growing body of literature reporting confounding factors that should be considered in statistical models evaluating associations of biomarkers of phthalate/replacement mixtures with pregnancy-related health outcomes. Additionally, pairing unsupervised pattern identification methods like k-means clustering and PCA with analyses evaluating predictors of phthalate/replacement biomarker concentration patterns are valuable for making recommendations targeted at limiting women’s consumption of phthalate-containing products during pregnancy. Future studies in more diverse populations are needed to confirm our findings (especially the magnitude of associations for each predictor) and to continue evaluating replacements such as DEHTP and DiNCH to fill in knowledge gaps about the determinants of these supposedly safer alternatives during and beyond pregnancy.
Supplementary Material
Funding sources:
This publication was made possible by the National Institute for Environmental Health Sciences (NIH/NIEHS) grants ES024795, ES032227, ES022848, the U.S. Environmental Protection Agency grant RD83543401, and National Institute of Health Office of the Director grant OD023272. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or NIH. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. This project was also supported by the USDA National Institute of Food and Agriculture and Michigan AgBioResearch.
Statement of ethics:
Dr. Braun served as an expert witness in litigation related to perfluorooctanonic acid contamination in drinking water in New Hampshire. Any funds he received from this arrangement were/are paid to Brown University and cannot be used for his direct benefit (e.g., salary/fringe, travel, etc.). The other authors have no conflicts of interest to disclose.
Abbreviations:
- BMI
body mass index
- DEHTP
di(2-ethylhexyl) terephthalate
- DiNCH
di(isononyl) cyclohexane-1,2-dicarboxylate
- I-KIDS
Illinois Kids Development Study
- MBP
mono-n-butyl phthalate
- MBzP
monobenzyl phthalate
- MCNP
monocarboxynonyl phthalate
- MCOCH
cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester
- MCOP
monocarboxyoctyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MECPTP
mono(2-ethyl-5-carboxypentyl) terephthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEHHTP
mono(2-ethyl-5-hydroxyhexyl) terephthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MEP
monoethyl phthalate
- MHBP
mono-hydroxybutyl phthalate
- MHiBP
mono-hydroxy-isobutyl phthalate
- MHiNCH
cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester
- MiBP
mono-isobutyl phthalate
- MiNP
mono-isononyl phthalate
- MONP
monooxononyl phthalate
- ∑DBP
sum of di-n-butyl phthalate metabolites
- ∑DEHP
sum of di(2-ethylhexyl) phthalate metabolites
- ∑DEHTP
sum of di(2-ethylhexyl) terephthalate metabolites
- ∑DiBP
sum of di-iso-butyl phthalate metabolites
- ∑DiNCH
sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites
- ∑DiNP
sum of di-isononyl phthalate metabolites
- NHANES
National Health and Nutrition Examination Survey
- PC
principal component
- PCA
principal component analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
CRediT AUTHOR STATEMENT
Diana C. Pacyga: Validation, Formal Analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization; Diana K. Haggerty: Validation, Formal Analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization; Megan Nicol: Writing – Original Draft, Writing - Review & Editing; Melissa Henning: Writing – Original Draft, Writing - Review & Editing; Antonia M. Calafat: Methodology, Validation, Writing - Review & Editing; Susan L. Schantz: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition; Rita S. Strakovsky: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Funding Acquisition.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abe Y, et al. , 2012. Survey of Plasticizers in Polyvinyl Chloride Toys. Food Hygiene and Safety Science. 53, 19–27. [DOI] [PubMed] [Google Scholar]
- Amrhein V, et al. , 2019. Scientists rise up against statistical significance. Nature. 567, 305–307. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, et al. , 2014. Phthalate and bisphenol A exposure among pregnant women in Canada--results from the MIREC study. Environ Int. 68, 55–65. [DOI] [PubMed] [Google Scholar]
- Bannon AL, et al. , 2017. Comparison of Self-reported and Measured Pre-pregnancy Weight: Implications for Gestational Weight Gain Counseling. Matern Child Health J. 21, 1469–1478. [DOI] [PubMed] [Google Scholar]
- Boss J, et al. , 2018. Associations between mixtures of urinary phthalate metabolites with gestational age at delivery: a time to event analysis using summative phthalate risk scores. Environ Health. 17, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher B, et al. , 2006. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 9, 84–93. [DOI] [PubMed] [Google Scholar]
- Braun JM, et al. , 2016. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect. 124, A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioli E, et al. , 2017. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Scientific Reports. 7, 11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, et al. , 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int. 62, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, et al. , 2016. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environmental health perspectives. 124, 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L, et al. , 2011. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 37, 858–66. [DOI] [PubMed] [Google Scholar]
- CDC, Fourth National Report on Human Exposure to Environmental Chemicals (Updated Tables, March 2021). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Georgia, 2021. [Google Scholar]
- Chen H, et al. , 2021. Characteristics of exposure to multiple environmental chemicals among pregnant women in Wuhan, China. Sci Total Environ. 754, 142167. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, et al. , 2012. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 142, 1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council, N. R., National Research Council (US) Committee on the Health Risks of Phthalates. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Phthalate Exposure Assessment in Humans. National Academies Press, Washington, DC, 2008. [PubMed] [Google Scholar]
- Derakhshan A, et al. , 2021. Association of phthalate exposure with thyroid function during pregnancy. Environ Int. 157, 106795. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, et al. , 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, MacKay D, 2014. Health habits and other characteristics of dietary supplement users: a review. Nutr J. 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, et al. , 2017. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicology Letters. 277, 54–63. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2015. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 36, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2014. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 70, 118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, et al. , 2007. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. International Journal of Hygiene and Environmental Health. 210, 21–33. [DOI] [PubMed] [Google Scholar]
- Gao H, et al. , 2017. Season-dependent concentrations of urinary phthalate metabolites among Chinese pregnant women: Repeated measures analysis. Environ Int. 104, 110–117. [DOI] [PubMed] [Google Scholar]
- Gore AC, et al. , 2015. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, et al. , 2019. Distribution and Dietary Predictors of Urinary Phthalate Metabolites among Pregnant Women in Shanghai, China. Int J Environ Res Public Health. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E, et al. , 2013. Self-reported pre-pregnancy weight versus weight measured at first prenatal visit: effects on categorization of pre-pregnancy body mass index. Matern Child Health J. 17, 1872–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotelling H, 1933. Analysis of a complex of statistical variables into principal components. Journal of Educational Psychology. 24, 417–441. [Google Scholar]
- Hsieh CJ, et al. , 2019. Personal care products use and phthalate exposure levels among pregnant women. Sci Total Environ. 648, 135–143. [DOI] [PubMed] [Google Scholar]
- James-Todd TM, et al. , 2018. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health. 17, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, et al. , 2016. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment International. 96, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, et al. , 2017. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J Expo Sci Environ Epidemiol. 27, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, et al. , 2015a. Exposure assessment issues in epidemiology studies of phthalates. Environment international. 85, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, et al. , 2016. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environ Health Perspect. 124, 1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, et al. , 2015b. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloo G, et al. , 2018. Profiles and Predictors of Environmental Chemical Mixture Exposure among Pregnant Women: The Health Outcomes and Measures of the Environment Study. Environmental Science & Technology. 52, 10104–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrin MA, 2009. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 12, 157–74. [DOI] [PubMed] [Google Scholar]
- Kelley KE, et al. , 2012. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ Health Perspect. 120, 379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, et al. , 2013. Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health. 216, 672–81. [DOI] [PubMed] [Google Scholar]
- Lee G, et al. , 2020. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ Res. 189, 109874. [DOI] [PubMed] [Google Scholar]
- Lee W-C, et al. , 2017. Identification of chemical mixtures to which Canadian pregnant women are exposed: The MIREC Study. Environment International. 99, 321–330. [DOI] [PubMed] [Google Scholar]
- Lessmann F, et al. , 2016. Determination of metabolites of di(2-ethylhexyl) terephthalate (DEHTP) in human urine by HPLC-MS/MS with on-line clean-up. J Chromatogr B Analyt Technol Biomed Life Sci. 1011, 196–203. [DOI] [PubMed] [Google Scholar]
- Lewin A, et al. , 2017. Univariate predictors of maternal concentrations of environmental chemicals: The MIREC study. Int J Hyg Environ Health. 220, 77–85. [DOI] [PubMed] [Google Scholar]
- Li J, et al. , 2019. Determinants of exposure levels, metabolism, and health risks of phthalates among pregnant women in Wuhan, China. Ecotoxicol Environ Saf. 184, 109657. [DOI] [PubMed] [Google Scholar]
- Long SE, et al. , 2021. Urinary phthalate metabolites and alternatives and serum sex steroid hormones among pre- and postmenopausal women from NHANES, 2013–16. Sci Total Environ. 769, 144560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden GR, et al. , 2020. Pregnancy intention and phthalate metabolites among pregnant women in The Infant Development and Environment Study cohort. Paediatr Perinat Epidemiol. 34, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen J, Some methods for classification and analysis of multivariate observations. Proceedings of 5th Berkeley Symposium on Mathematical Statistics and Probability, 1967, pp. 281–297. [Google Scholar]
- McCombie G, et al. , 2017. Survey on plasticizers currently found in PVC toys on the Swiss market: Banned phthalates are only a minor concern. J Environ Sci Health A Tox Hazard Subst Environ Eng. 52, 491–496. [DOI] [PubMed] [Google Scholar]
- McCullough ML, et al. , 2002. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 76, 1261–71. [DOI] [PubMed] [Google Scholar]
- Meeker JD, et al. , 2009. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 117, 1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri P, et al. , 2019. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int J Hyg Environ Health. 222, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natamba BK, et al. , 2016. Concordance between self-reported pre-pregnancy body mass index (BMI) and BMI measured at the first prenatal study contact. BMC Pregnancy Childbirth. 16, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHANES, National Health and Nutrition Examination Survey Data. In: N. C. f. H. S. (NCHS), (Ed.). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, Maryland, 2013–2014. [Google Scholar]
- NHANES, National Health and Nutrition Examination Survey Data. In: N. C. f. H. S. (NCHS), (Ed.). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, Maryland, 2015–2016. [Google Scholar]
- NHANES, National Health and Nutrition Examination Survey Data. In: N. C. f. H. S. (NCHS), (Ed.). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, Maryland, 2017–2018. [Google Scholar]
- Pacyga DC, et al. , 2021. Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ Int. 155, 106676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlett LE, et al. , 2013. Women's exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 23, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, et al. , 2018. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ Res. 161, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polinski KJ, et al. , 2018. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res. 162, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau JL Jr., et al. , 2010. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect. 118, 1748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, et al. , 2022. Geographic distribution and time trend of human exposure of Di(2-ethylhexyl) phthalate among different age groups based on global biomonitoring data. Chemosphere. 287, 132115. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Carmona Y, et al. , 2020. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J Expo Sci Environ Epidemiol. 30, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, et al. , 2020. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. Journal of Exposure Science & Environmental Epidemiology. 30, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosofsky A, et al. , 2017. Exposure to multiple chemicals in a cohort of reproductive-aged Danish women. Environ Res. 154, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkel AA, et al. , 2022. Exposure of men and lactating women to environmental phenols, phthalates, and DINCH. Chemosphere. 286, 131858. [DOI] [PubMed] [Google Scholar]
- Shaffer RM, et al. , 2019. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int. 123, 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, et al. , 2018. Temporal trends of phthalate exposures during 2007–2010 in Swedish pregnant women. J Expo Sci Environ Epidemiol. 28, 437–447. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2013. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000–2012). Environ Res. 126, 159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 860, 106–12. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2019. Exposure to di-2-ethylhexyl terephthalate in the U.S. general population from the 2015–2016 National Health and Nutrition Examination Survey. Environ Int. 123, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, et al. , 2021. Dietary intake and household exposures as predictors of urinary concentrations of high molecular weight phthalates and bisphenol A in a cohort of adolescents. J Expo Sci Environ Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succop PA, et al. , 2004. Imputation of data values that are less than a detection limit. J Occup Environ Hyg. 1, 436–41. [DOI] [PubMed] [Google Scholar]
- Valvi D, et al. , 2015. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health. 218, 220–31. [DOI] [PubMed] [Google Scholar]
- van TETJ, et al. , 2019. Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women. Environ Sci Technol. 53, 3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadey BL, 2003. An innovative plasticizer for sensitive applications. Journal of Vinyl & Additive Technology. 9, 172–176. [Google Scholar]
- Wasserstein RL, Lazar NA, 2016. The ASA's Statement on p-Values: Context, Process, and Purpose. American Statistician. 70, 129–131. [Google Scholar]
- Weir CB, Jan A, BMI Classification Percentile And Cut Off Points. StatPearls, Treasure Island (FL), 2021. [PubMed] [Google Scholar]
- Weiss L, et al. , 2015. Temporal variability and sources of triclosan exposure in pregnancy. Int J Hyg Environ Health. 218, 507–13. [DOI] [PubMed] [Google Scholar]
- Wenzel AG, et al. , 2018. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 193, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, et al. , 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 119, 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward MJ, et al. , 2020. Phthalates and Sex Steroid Hormones Among Men From NHANES, 2013–2016. J Clin Endocrinol Metab. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, et al. , 2020. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ Sci Technol. 54, 1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghjyan L, et al. , 2016. Associations of individual characteristics and lifestyle factors with metabolism of di-2-ethylhexyl phthalate in NHANES 2001–2012. Environ Res. 149, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, et al. , 2009. Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Hum Ecol Risk Assess. 15, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yland JJ, et al. , 2022. Phthalate and DINCH urinary concentrations across pregnancy and risk of preterm birth. Environ Pollut. 292, 118476. [DOI] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2016. Free and total urinary phthalate metabolite concentrations among pregnant women from the Healthy Baby Cohort (HBC), China. Environ Int 88, 67–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


