Abstract
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder in which recurrent abdominal pain is associated with defecation or a change in bowel habits (constipation, diarrhea, or both), and it is often accompanied by symptoms of abdominal bloating and distension. IBS is an important health care issue because it negatively affects the quality of life of patients and places a considerable financial burden on health care systems. Despite extensive research, the etiology and underlying pathophysiology of IBS remain incompletely understood. Proposed mechanisms involved in its pathogenesis include increased intestinal permeability, changes in the immune system, visceral hypersensitivity, impaired gut motility, and emotional disorders. Recently, accumulating evidence has highlighted the important role of the gut microbiota in the development of IBS. Microbial dysbiosis within the gut is thought to contribute to all aspects of its multifactorial pathogenesis. The last few decades have also seen an increasing interest in the impact of antibiotics on the gut microbiota. Moreover, antibiotics have been suggested to play a role in the development of IBS. Extensive research has established that antibacterial therapy induces remarkable shifts in the bacterial community composition that are quite similar to those observed in IBS. This suggestion is further supported by data from cohort and case-control studies, indicating that antibiotic treatment is associated with an increased risk of IBS. This paper summarizes the main findings on this issue and contributes to a deeper understanding of the link between antibiotic use and the development of IBS.
Keywords: Gut microbiota, Irritable bowel syndrome, Antibiotics, Intestinal barrier, Gut motility, Gut sensitivity
Core Tip: Irritable bowel syndrome (IBS) is among the most common gastrointestinal disorders; however, its etiology and underlying pathophysiology have yet to be fully elucidated. The present review focuses on the existing evidence on the pathogenic role of the gut microbiota in the development of IBS. Moreover, it provides a comprehensive review on the magnitude of changes in the gut microbiota in response to antibiotics. The paper contributes to a deeper understanding of the link between antibiotic use and the development of IBS.
INTRODUCTION
Recent advances in culture-independent techniques have greatly expanded our understanding of the human gut microbiota and its functionalities. It is becoming increasingly recognized that gut bacteria play a pivotal role in host homeostasis and are involved in the progression and development of numerous human diseases.
The gut microbiota is established early in life, remains relatively stable thereafter, and is subject to shaping by environmental and host factors (e.g., age, diet, lifestyle, and medications)[1,2]. With regard to the environment, antibiotics have been reported to play a particularly important role in the modulation of the gut microbial community. However, most studies in this area were undertaken 30 to 40 years ago and relied on culture-based techniques. Global antibiotic use has grown 66% since 2000 and continues to grow at a high rate[3,4]. This fact, along with rapid technological advancements for culture-independent analysis, has reinforced the need to take a fresh look at antibiotic-induced changes in the human gut microbiota and clinical consequences of antibiotic intervention. Several studies have reported that antibiotic treatment is associated with an increased risk of irritable bowel syndrome (IBS)[5-8].
IBS is a common gastrointestinal disorder affecting 10%–15% of the population in Europe and North America[9]. This condition negatively affects the quality of life of patients and imposes a significant socioeconomic burden[10]. Over the past few decades, the gut microbiota has emerged as a potential factor that contributes to the pathophysiology of IBS[11,12]. Microbial dysbiosis within the gut has been implicated in intestinal barrier dysfunction, visceral hypersensitivity, impaired gastrointestinal motility, and altered immune response[13-17]. Moreover, various studies have consistently shown the efficacy of microbiota-directed therapies, including prebiotics, probiotics, nonabsorbable antibiotics, dietary changes, and fecal microbial transplantation, in alleviating IBS symptoms[18].
In this paper, we provide a brief overview of the human gut microbiota and its impact on host homeostasis. We highlight what is currently known regarding the role of gut bacteria in the pathophysiology of IBS. Furthermore, we provide an overview of the most up-to-date literature about the impact of antibiotics on gut microbiota composition and discuss a possible link between antibiotic use and the development of IBS. Finally, we identify knowledge gaps and uncertainties that must be filled to orient future research in this area.
GUT MICROBIOTA AND ITS ROLE IN HOST HOMEOSTASIS
The human gut microbiota is a community of microorganisms that inhabit the gastrointestinal tract and is composed of approximately 1014 bacterial cells[19,20]. In healthy adults, more than 90% of gut bacteria belong to four dominant phyla, namely, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, whereas other phyla are far less abundant[21,22].
Currently, the gut microbiota is considered an indispensable “organ” within the body with distinct metabolic and immune functions (Table 1). Most of its effects are mediated through metabolites.
Table 1.
Gut microbiota functions
|
Bacterial phylum
|
Key representatives
|
Functions
|
| Firmicutes | Members of the genera Enterococcus, Ruminococcus, Clostridium, Lactobacillus, Faecalibacterium, Roseburia, and Eubacterium | Metabolism of amino acids[23,24], carbohydrates[25], bile acids, and their salts[22]. Lipid metabolism and cholesterol synthesis[25]. Synthesis of vitamins К2, B1, B2, B6, B7, B9, and B12[26]. Maintenance of a proper immune response[28,29] and intestinal epithelial barrier integrity[31,32]. Protection against enteric pathogens[33] |
| Bacteroidetes | Members of the genera Bacteroides and Prevotella | Metabolism of amino acids[24], carbohydrates[25,141], bile acids, and their salts[22,142]. Synthesis of vitamin К2[27]. Regulation of appetite[143]. Maintenance of a proper immune response[28-29] and intestinal epithelial barrier integrity[31]. Protection against enteric pathogens[33] |
| Actinobacteria | Members of the genera Bifidobacterium and Corynebacterium | Metabolism of bile acids and their salts[22]. Synthesis of vitamins К2, B1, B2, B6, B7, B9, and B12[26]. Protection against enteric pathogens[33] |
| Proteobacteria | Members of the genera Desulfovibrio, Escherichia, and Shigella | Metabolism of amino acids[144] |
Thus, some of the most important roles of the gut microbiota include metabolism of dietary compounds[23-25], synthesis of vitamins[26,27], regulation of the immune response[28-30], maintenance of intestinal epithelial barrier integrity[25,31,32], and protection against enteric pathogens[33].
MODERN CONCEPT OF IBS: EVOLVING ROLE OF GUT MICROBIOME
Despite extensive research, the etiology and underlying pathophysiology of IBS remain incompletely understood. Proposed mechanisms involved in its pathogenesis include visceral hypersensitivity, impaired gut motility[13,34], increased intestinal permeability[34-36], emotional disorders[11,37], and changes in the immune system[34,37,38].
Over the past decade, there has been an increasing amount of literature on the role of the gut microbiota in the pathogenesis of IBS. The concept of the “microbiota-gut-brain” axis has been proposed[14-17], supporting the crucial role of microbial dysbiosis in the development of IBS symptoms. It is thought that, in genetically predisposed individuals, environmental factors alter the composition of the gut microbiota, leading to disruption of intestinal epithelial barrier integrity[13]. Once the intestinal barrier is breached, bacteria interact with the immune system of the host, provoke a series of immune reactions, and lead to low-grade mucosal inflammation in the gut wall. Collectively, these changes result in sensitivity and motility abnormalities, emotional disorders, and the development of IBS symptoms (abdominal pain, bloating, and alterations in bowel habits)[35]. Interestingly, the gut microbiota not only initiates such a pathological cascade in IBS but also contributes to all aspects of its multifactorial pathogenesis through the release of metabolites[11,12]. These provisions will be discussed below.
Microbiota and motility/sensitivity abnormalities
The enteroendocrine system modulates gut motor and sensory functions through the secretion of neuropeptides and neurotransmitters[39].
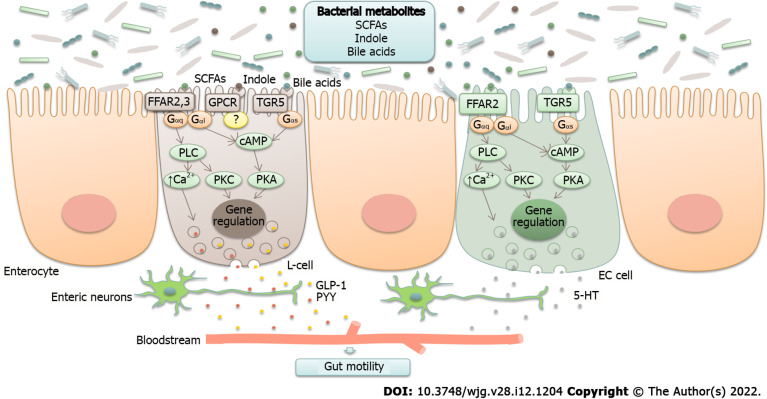
Bacterial metabolites are able to stimulate the production of several neuropeptides, including neuropeptide Y, peptide YY, glucagon-like peptide-1 (GLP-1)[40], cholecystokinin, and substance P (Figure 1)[15,41].
Figure 1.
Neurotransmitter modulation by gut microbiota (schematic illustration). Bacterial metabolites, such as short-chain fatty acids (SCFAs), secondary bile acids, and indole, are able to stimulate the production of neurotransmitters, including glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and serotonin (5-HT). They act through G-protein coupled receptors (GPCRs) FFAR2, FFAR3, and TGR5 that are coupled to different types of G proteins (Gαs, Gαq, and Gαi) and activate different pathways known to regulate gene expression and promote exocytosis by raising intracellular Ca2+ levels. SCFAs are recognized by FFAR2 and FFAR3. Enteroendocrine L-cells express both of these proteins, whereas enterochromaffin (EC) cells have been reported to express FFAR2. Bile acids are recognized by TGR5 receptors expressed in L-cells and EC cells. The sensing of indole remains elusive, although it is thought to act through GPCR. Gαs stimulates adenylate cyclase and elevates cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA). Gαi inhibits the cAMP pathway. Gαq stimulates phospholipase C (PLC), resulting in the generation of diacylglycerol (DAG) and inositol triphosphate (IP3), which activate protein kinase C (PKC) and induce intracellular Ca2+ release[23,138-140]. SCFA: Short-chain fatty acids; GLP-1: Glucagon-like peptide-1; PYY: Peptide YY.
For instance, short-chain fatty acids (SCFAs), secondary bile acids, and indole, which are produced by members of the genera Clostridium, Bacteroides, and Ruminococcus[23,25], stimulate intestinal L-cells to secrete GLP-1[42]. GLP-1 reduces postprandial motility in the upper gastrointestinal tract (antrum, duodenum, and jejunum) and increases colonic transit[43,44]. A study conducted by Li et al[45] reported decreased serum GLP-1 levels and reduced mucosal expression of GLP-1 receptors in patients with constipation-predominant IBS (IBS-C). The authors suggested that lower GLP-1 levels lead to the loss of its prokinetic effects in the colon, resulting in constipation and abdominal pain. In a rat model of bowel dysfunction, administration of the GLP-1 receptor agonist exendin-4 alleviated stress-induced defecation and visceral pain sensitivity[46,47]. Clinical interventions in patients with IBS demonstrated that the synthetic GLP-1 analog ROSE-010 reduced abdominal pain and increased colonic transit[45,48]. The underlying molecular mechanisms responsible for the amelioration of symptoms remain unknown. The authors suggest that modulation of enteric neuronal function and tight junction expression, as well as the activation of serotonergic pathways in the colon, may play a role.
Secondary bile acids and SCFAs, which are mainly produced by Eubacterium, Bacteroides, and Clostridium (clusters IV, XI, XIII, and XIVa)[22], promote serotonin synthesis from colonic enterochromaffin cells[49]. Serotonin is an important neurotransmitter that, among its other functions, regulates gastrointestinal motility[50]. Serum serotonin levels were found to be increased in those with diarrhea-predominant IBS (IBS-D) and reduced in those with IBS-C[34].
The serotonin system represents a potential therapeutic target in IBS. The effects of serotonin are mediated through 5-HT receptors located on the surface of distinct cell types. Fourteen different serotonin receptor subtypes have been identified and classified into seven groups (5-HT1–7), with 5-HT3 and 5-HT4 being the most investigated receptors in the intestine. Both receptor subtypes are expressed on neurons within the myenteric and submucosal plexuses of the enteric nervous system, intrinsic and extrinsic sensory neurons, interstitial cells of Cajal, enterocytes, and enterochromaffin cells[51]. 5-HT3 receptors are involved in the contraction of intestinal smooth muscle and in gut-brain communication through vagal afferent fibers[52]. Activation of 5-HT4 receptors induces neuronal release of acetylcholine and accelerates the peristaltic reflex[53]. 5-HT3 receptor antagonists have been shown to improve abdominal pain and global IBS symptoms in patients with nonconstipated and IBS-D[54,55]. 5-HT4 agonists have been shown to relieve overall and individual symptoms (abdominal pain/discomfort, stool frequency, stool consistency, and straining during defecation) in patients with IBS-C[56-58]. However, cardiovascular side effects were seen with these drugs, and they were either withdrawn from the market (cisapride) or approved for a limited population (tegaserod). Therefore, new safe and well-tolerated 5-HT4 agonists are under development[59,60].
A number of animal studies have shown the prominent role of the gut microbiota in visceral hypersensitivity[41]. For example, colonization of germ-free rats with the gut microbiota from patients with IBS reduced the pain threshold to colorectal distension[42]. Furthermore, the beneficial effects of probiotic strains (e.g., Lactobacillus reuteri, Lactobacillus plantarum, Lactobacillus helveticus, and Bifidobacterium longum) in alleviating visceral sensitivity have been documented[61-63].
Thus, the microbiota influences the main pathogenetic factors of IBS (i.e., motility and sensitivity) both directly and through microbial metabolites.
Microbiota as a regulator of stress and emotional responses
The physiological response to stress is mediated through the hypothalamic-pituitary-adrenal (HPA) axis[64]. Activation of this axis results in the release of corticotropin releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus. CRH acts on the anterior pituitary and induces the production of adrenocorticotropic hormone (ACTH), which in turn stimulates the adrenal cortex to secrete cortisol.
Different types of stressors are known to contribute to the development, maintenance, and exacerbation of IBS symptoms[11]. The results of multiple studies suggest that there is HPA axis dysregulation in IBS. For instance, patients with IBS were found to have excess levels of ACTH in the plasma and cortisol in the serum in response to CRH infusion[65].
Growing evidence indicates that the gut microbiota is involved in the regulation of HPA axis activity. It has been shown that colonization with beneficial microorganisms in early life is of great importance for the normal development of this axis[66]. Moreover, alterations in the gut microbiota may influence the release of ghrelin and galanin, which are endocrine peptides contributing to the stress response through modulation of CRH, ACTH, and glucocorticoid secretion[40,67].
Dysfunction of the HPA axis, along with alterations in neurotransmitter metabolism, appear to be crucial factors in the development of psychiatric disorders, such as anxiety and depression[68,69]. A recent meta-analysis of 27 studies have reported elevated levels of anxiety and depression in patients with IBS as compared to those in healthy controls[70]. Comorbid emotional disorders lead to persistence of symptoms, drive patients to seek medical care, and contribute to poor outcomes[11].
A growing body of literature supports the association between microbial dysbiosis and the development of anxiety and depression. For instance, certain species within the Lactobacillaceae and Bifidobacteriaceae families are known to produce gamma-aminobutyric acid (GABA). GABA is the main inhibitory neurotransmitter of the central nervous system, playing an important role in the pathogenesis of mood disorders[49,71]. Interestingly, a specific type of GABA receptor (GABA-b) is localized on submucosal and myenteric neurons of the enteric nervous system[72] and is thought to be involved in the modulation of gut motility and sensitivity[37]. Furthermore, members of the genera Bacillus and Escherichia have been found to produce other neurotransmitters affecting mood and behavior, such as dopamine, serotonin, and norepinephrine[15,73]. In recent studies, germ-free mice have been widely used as a tool for assessing the role of intestinal microbes in brain function and behavior. Studies on germ-free and specific pathogen-free mice indicate that intestinal microbes can cause imbalances of the HPA axis, resulting in an anxiety-like behavioral phenotype[74]. Fecal microbiota transplantation studies have indicated the rodent-to-rodent and human-to-rodent transfer of anxiety-like behaviors[75,76]. Moreover, animal studies have shown that transplantation of the microbiota from depressed patients to rodents is able to induce depression-like behavior. The authors linked microbiota-induced depression in mice to alterations in the cAMP-response element binding protein (CREB) signaling pathway in the olfactory bulb[77] and alterations in carbohydrate and amino acid metabolism[78].
However, despite the data obtained, further research is needed to investigate the difference in emotional disorder levels in patients with postinfectious and other forms of IBS.
Microbiota and host immunity
Recently, considerable literature has grown around the theme of immune system activation in IBS. For instance, an increased number of mast cells located in close proximity to enteric nerve fibers have been found in colonic biopsies from patients with IBS and have been associated with the severity of symptoms[11,38,79]. Mast cells are thought to be key players in intestinal mucosal inflammation[79]. Their degranulation causes the release of inflammatory mediators (histamine, serotonin, and proteases), resulting in lymphocyte activation and cytokine imbalance[80]. Patients with IBS were found to have higher levels of proinflammatory interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor-α (TNF-α) and lower levels of anti-inflammatory IL-10 in both serum and the intestinal mucosa[81,82]. These changes result in altered pain thresholds and visceral hypersensitivity[38,83]. In addition, mast cell degranulation has been shown to reduce the expression of tight junction proteins, probably through tryptase release[13]. Apart from mast cells, increased numbers of eosinophils and intraepithelial lymphocytes have been observed in colonic biopsies from patients with IBS[11,79].
Gut bacteria play an important role in the modulation of the immune response. For example, butyrate produced by members of the phylum Firmicutes[25] induces the differentiation of regulatory T cells[29,84], thereby preventing an excessive immune response and autoimmunity[22,85]. Furthermore, Lactobacilli spp. metabolize dietary tryptophan into indole-3-aldehyde, which acts as an aryl hydrocarbon receptor (AHR) ligand[85]. AHR is a ligand-activated transcription factor that is expressed by immune cells and regulates the number of intraepithelial lymphocytes and IL-22 production[86]. Probiotic strains, such as Lactobacillus rhamnosus, Lactobacillus casei, and Bifidobacterium breve, were shown to induce IL-4 and IL-10 production, whereas L. reuteri and L. plantarum were found to downregulate the expression of TNF-α[87,88].
The importance of the interaction between the gut microbiota and host immune system in IBS is highlighted by a number of studies in patients with postinfectious IBS, indicating activation of the gastrointestinal immune system after acute gastroenteritis[89,90]. Moreover, animal studies have shown that stress-induced changes in the gut microbiota are associated with altered immune response and increased susceptibility to enteric pathogens[91,92].
Microbiota and intestinal barrier integrity
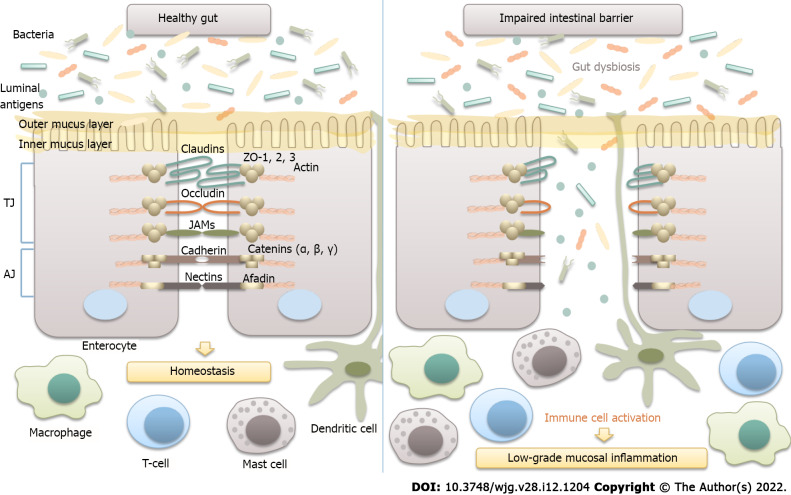
Intestinal epithelial barrier integrity is of great importance for gut homeostasis, as it prevents the translocation of luminal antigens to the mucosa, thus averting the development of low-grade mucosal inflammation in the gut wall (Figure 2).
Figure 2.
Microbiota and intestinal barrier integrity. The intestinal barrier plays an essential role in maintaining host homeostasis. It is mainly composed of the mucus layer, the epithelial layer, and the underlying lamina propria. Intestinal epithelial cells are tightly attached to each other by junctional complexes. Tight junctions (TJs) are composed of several proteins, including occludin, claudins, zonula occludens (ZOs), and junctional adhesion molecules (JAMs), which interact with each other, as well as with the cytoskeleton. The adherence junction is composed of the nectin-afadin system and the E-cadherin-catenin system. Intestinal epithelial barrier integrity prevents the translocation of bacteria and luminal antigens to the mucosa, thus averting their interaction with the host immune system and the development of low-grade mucosal inflammation in the gut wall. TJ: Tight junctions; AJ: Adherence junction; JAM: Junctional adhesion molecules.
An increased density of epithelial gaps has been shown by electron microscopy in gut biopsies of patients with IBS[93]. Furthermore, histological examination of colonic biopsies revealed decreased expression of tight junction proteins, such as occludin; claudins 1, 3, and 5; and zonula occludens-1[13,36,82,93]. Increased serum levels of anti-flagellin antibodies in patients with IBS further support the substantial role of intestinal permeability in the pathogenesis of IBS[94].
The gut microbiota is an important determinant of intestinal epithelial barrier integrity. In particular, certain gut bacteria, such as Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii, and Ruminococcus spp., were shown to affect the mucus layer thickness and composition[1,22,31]. Moreover, SCFAs, which are produced predominantly by members of the genera Eubacterium, Clostridium, Ruminococcus, and Faecalibacterium, have been demonstrated to augment the expression of claudins 3 and 4 and occludin. Polyamines (putrescine, spermidine, and spermine), which are produced by certain species within the Clostridium, Enterococcus, Streptococcus, and Lactobacillus genera, have been shown to stimulate the production of E-cadherin and zonula occludens-1[95]. There is also evidence that probiotic strains of Bifidobacterium and Lactobacillus promote intestinal barrier function and prevent bacterial translocation[32,96].
Most likely, the preservation of the optimal composition of the microbiota (e.g., a sufficient number of SCFA producers) may serve as a factor preventing the development of IBS.
GUT MICROBIAL COMPOSITION IN PATIENTS WITH IBS
A considerable amount of literature has been published on the compositional changes of the gut microbiota in patients with IBS. Although data from these studies are inconsistent and even conflicting, some common features can be found (Table 2). The discrepancy in findings is possibly due to differences in the population studied (e.g., age, lifestyle, initial microbiota composition, prior antibiotic and/or probiotic use, and diagnostic criteria for IBS) and methodological issues, such as study design and methods for microbiota assessment and data analysis.
Table 2.
Compositional changes in gut microbiota in patients with irritable bowel syndrome (common threads)
|
Ref.
|
Subjects
|
Method
|
Specimen
|
Diversity
|
Faecalibacterium
|
Enterobacteriaceae
|
Bifidobacterium
|
Lactobacillus
|
| Dior et al[145], 2016 | IBS-D (n = 16), IBS-C (n =15), Controls (n = 15) | Real-time PCR | Stool | No data | − | ↑ in IBS-D (Escherichia) | ↑ in IBS-C | − |
| Ringel-Kulka et al[108], 2016 | IBS (n = 56), Controls (n = 20) | 16S rRNA | Stool | No data | − | − | − | ↑ |
| Maharshak et al[102], 2018 | IBS-D (n = 23), Controls (n = 24) | 16S rRNA | Stool | ↓1 | ↓ | ↑ (unclassified genus) | − | − |
| Colonic biopsy | −1 | − | − | − | ↑ | |||
| Gobert et al[146], 2016 | IBS-C (n = 33), Controls (n = 58) | 16S rRNA | Stool | No data | − | ↑ | ↓ | − |
| Shukla et al[105], 2015 | IBS (n = 47), Controls (n = 30) | 16S rRNA; real-time PCR | Stool | No data | − | − | ↓ | − |
| Su et al[107], 2018 | IBS-D (n = 40), Controls (n = 20) | 16S rRNA; real-time PCR | Stool | No data | − | − | ↓ | ↓ |
| Zhuang et al[109], 2018 | IBS-D (n = 30), Controls (n = 13) | 16S rRNA | Stool | −2 | − | − | − | ↓ |
| Zhong et al[147], 2019 | IBS-D (n = 20), Controls (n = 16) | FISH | Colonic biopsy | No data | − | ↑ (E. coli) | ↓ | − |
| Jeffery et al[100], 2020 | IBS (n = 80), Controls (n = 65) | 16S rRNA, shotgun sequencing | Stool | ↓2 | − | − | − | − |
| Rangel et al[148], 2015 | IBS (n = 33), Controls (n = 16) | Microarray analysis | Stool | ↓2 | ↓ (F. prausnitzii) | − | − | − |
| Colonic biopsy | −2 | − | − | − | − |
Rarefaction analysis.
Shannon diversity index.
↓: Decreased abundance; ↑: Increased abundance; –: No significant differences found; IBS: Irritable bowel syndrome; IBS-D: Diarrhea-predominant irritable bowel syndrome; IBS-C: Constipation-predominant irritable bowel syndrome; FISH: Fluorescence in situ hybridization; E. coli: Escherichia coli; F. prausnitzii: Faecalibacterium prausnitzii.
The majority of authors report decreased microbial diversity in patients with IBS[97-101]. Furthermore, a substantial number of studies have shown a lower abundance of butyrate-producing bacteria from the genus Faecalibacterium, mainly F. prausnitzii,[97,98,102,103] as well as an increase in the abundance of the Enterobacteriaceae family, including pathogens such as Escherichia coli and Enterobacter spp.[98,104-106]. Moreover, patients with IBS were found to have a reduced prevalence of Bifidobacterium, providing a range of beneficial properties to the host[98,103,104,106,107]. Significant differences in Lactobacillus numbers were also observed between patients with IBS and healthy controls, but the findings of different studies were not consistent. Some authors reported an increased amount of Lactobacillus[98,99,102,108], while others documented a decrease in the abundance of this commensal[103,104,106,107,109].
Overall, there seems to be some evidence to indicate that patients with IBS have decreased numbers of bacteria contributing to the maintenance of host homeostasis and proper immune response, as well as increased numbers of microbes with proinflammatory properties.
ANTIBIOTICS, GUT MICROBIOTA, AND IBS
Effects of antibiotics on gut microbiota composition
The discovery of antibiotics in the early 20th century was a great milestone in the history of medicine, as it changed the natural course of most infectious diseases and saved countless lives[110,111]. However, a growing number of studies have shown that inappropriate use of antibiotics promotes the development of antibiotic resistance[112,113]. Furthermore, accumulating evidence indicates that antibiotic exposure in early life increases the risk of obesity and autoimmune and allergic diseases[114-117].
During the past four decades, there has been an increasing interest in the impact of antibiotics on the composition of the gut microbiota. A substantial number of studies in this area were conducted in the 1980s and 1990s and relied on culture-based techniques. However, researchers indicate that up to 80% of gut bacteria are nonculturable[118]. Therefore, the focus has shifted to culture-independent approaches mainly based on 16S rRNA gene sequence analysis.
Extensive research has established that antibiotic treatment induces a dramatic loss of diversity and remarkable shifts in community composition (Table 3), with the time of recovery varying substantially[119-123].
Table 3.
Effects of antibiotics on gut microbiota composition (based on culture-independent approaches)
|
Ref.
|
Method
|
Antibiotic
|
Dosing regimen
|
Diversity
|
Compositional changes
|
| Pallav et al[136], 2014 | Pyrosequencing | Amoxicillin | 250 mg 3 times daily for 7 d | −1,2 | ↑ Escherichia, Shigella |
| Kabbani et al[137], 2017 | 16S rRNA | Amoxicillin-Clavulanate | 875/125 mg twice daily for 7 d | ↓1,3 | ↑ Escherichia, Parabacteroides, Enterobacter ↓ Roseburia |
| Burdet et al[120], 2019 | 16S rRNA | Ceftriaxone | 1 g once daily for 3 d | ↓1,4 | ↓ Firmicutes, Actinobacteria, Bacteroidetes |
| Raymond et al[135], 2016 | Shotgun sequencing | Cefprozil | 500 mg twice daily for 7 d | ↓5 | ↑Flavonifractor, Lachnoclostridium, Parabacteroides, ↓Bifidobacteriaceae, Coriobacteriaceae, Eubacteriaceae, Oxalobacteraceae, Pasteurellaceae, Veillonellaceae |
| Rashid et al[121], 2015 | Pyrosequencing | Ciprofloxacin | 500 mg twice daily for 10 d | ↓1 | ↑ Bacteroides↓ Faecalibacterium, Alistipes, unculturable Ruminococcaceae |
| Clindamycin | 150 mg 4 times daily for 10 d | ↓1 | ↓ Roseburia, Lachospira, Coprococcus, Dorea, Ruminococcus | ||
| Isaac et al[122], 2017 | 16S rRNA | Vancomycin | 250 mg per os 4 times daily for 2 wk | ↓1,4 | ↑ Escherichia, Shigella, Klebsiella, ↓ Bacteroidetes, Faecalibacterium, Ruminococcus |
OTU analysis.
Rarefaction analysis.
Chao1 index.
Shannon index.
Simpson index.
The inconsistency in the results of various studies can be attributed to substantial heterogeneity in sample characteristics (age, ethnicity, diet, etc.) and study methodology. Furthermore, antibiotic characteristics, such as their class, pharmacokinetics (absorption and excretion), range of action, and dosing regimen, have been shown to shape the response of the gut microbiota to antibiotic perturbation[124]. For instance, vancomycin is poorly absorbed when administered orally, resulting in high fecal concentrations. Therefore, it significantly alters the composition of the gut microbiota by increasing pathogenic Proteobacteria, such as Klebsiella, Escherichia, and Shigella, and decreasing members of the Bacteroidetes phylum[122]. Lipophilic antibiotics (e.g., lincosamides and macrolides) are eliminated mainly by biliary excretion and therefore cause profound changes in the intestinal microbiota[125]. For example, treatment with clindamycin resulted in a reduction in microbial diversity and a decrease in Roseburia, Lachospira, Coprococcus, Dorea, and Ruminococcus. Changes in microbial composition were observed throughout 12 mo after clindamycin exposure[121]. In a recent study conducted by Haak et al[123], it was shown that treatment with broad-spectrum antibiotics (ciprofloxacin, vancomycin, and metronidazole) promotes the growth of Streptococcus and Lactobacillus. Furthermore, the authors found reduced numbers of anaerobes producing SCFAs, such as Bacteroides, Subdoligranulum, and Faecalibacterium. Interestingly, a return toward baseline was observed between 8 and 31 mo, but the composition of the microbiota often remained changed from its initial state.
There is some evidence that antibiotics can indirectly affect the composition of the gut microbiota. This is due to interdependence among different microbial taxa, as they have a variety of shared metabolic pathways[124,126]. Thus, the loss or reduction of certain taxa affects the growth of other members of the community. As an example, vancomycin treatment reduces the number of Gram-negative commensals, although this drug selectively targets Gram-positive bacteria[127].
In a recent systematic review, Zimmerman et al[128] summarized data from 129 studies on the effect of antibiotics on the composition of the gut microbiota. The authors concluded that the majority of antibiotics (amoxicillin, amoxicillin/clavulanate, cephalosporins, lipopolyglycopeptides, macrolides, ketolides, clindamycin, tigecycline, quinolones, and fosfomycin) increase the abundance of Enterobacteriaceae, mainly Citrobacter spp., Enterobacter spp., and Klebsiella spp. These bacteria contain molecules that directly enhance the inflammatory response of the host and may play a significant role in the alteration of bile acid metabolism[129]. Moreover, expansion of bacteria belonging to the Enterobacteriaceae family was associated with inflammatory bowel diseases, both in animal models and in humans[130,131]. Zimmerman et al[128] reported that amoxicillin, piperacillin, ticarcillin, cephalosporins (except fifth generation cephalosporins), carbapenems, and lipoglycopeptides facilitate the overgrowth of Enterococcus, while treatment with macrolides and doxycycline results in decreased numbers of these bacteria. It has conclusively been shown that piperacillin, ticarcillin, carbapenems, macrolides, clindamycin, and quinolones markedly reduce the abundance of anaerobic bacteria. Finally, the authors documented that the most long-lasting changes in the community structure are caused by ciprofloxacin (1 year), clindamycin (2 years), and clarithromycin plus metronidazole (4 years).
Another negative effect of antibiotic treatment is the loss of colonization resistance. Depletion of beneficial gut commensals, such as Lachnospiraceae, Ruminococcaceae, and Clostridium scindens, as well as changes in their metabolic activity promote overgrowth of Clostridium difficile, Enterococcus, and other pathogens[33,124].
Antibiotics as a risk factor for IBS
Data from large cohort and case-control studies indicate that antibiotics are a risk factor for functional gastrointestinal disorders and IBS in particular. A retrospective study on more than 26000 patients showed that exposure to macrolides and tetracyclines may be associated with the development of IBS[5]. Similarly, a prospective case-control study found that antibiotic treatment of nongastrointestinal infections was associated with the development of IBS [odds ratio (OR) = 2.30; 95% confidence interval (CI): 1.22-4.33; P = 0.01] and other functional gastrointestinal disorders (OR = 1.90; 95%CI: 1.21-2.98; P = 0.005)[6]. A longitudinal study by Krogsgaard et al[7] also identified that the use of antibiotics was a predictor for IBS (OR = 1.8; 95%CI: 1.0-3.2). Additionally, a recent meta-analysis showed that the use of antibiotics for infectious enteritis was associated with an increased risk of IBS (OR = 1.69; 95%CI: 1.20-2.37)[8].
However, nonabsorbable antibiotics can be used to treat IBS. In a double-blind, randomized, placebo-controlled study, treatment with neomycin resulted in a 35% improvement in composite scores of IBS symptoms, compared with only 11% for placebo (P < 0.05)[132]. Nonetheless, the use of this antibiotic is limited by the risk for C.difficile infection and systemic adverse events. A recent meta-analysis of four studies and 1803 patients showed that rifaximin was more effective than placebo in the overall improvement of IBS symptoms (OR = 1.19; 95%CI: 1.08-1.32 and OR = 1.36; 95%CI: 1.18–1.58, respectively, P < 0.05 for both). There was no difference in adverse events between rifaximin and placebo[133]. Due to its safety, rifaximin was approved by the Food and Drug Administration for the treatment of IBS-D.
Similarities in gut microbiota between patients with IBS and those after antibiotic exposure
Analysis of data on changes in the gut microbiota in patients with IBS and those after antibiotic exposure uncovers some common features and trends. For instance, decreased microbial diversity[97-99,121,128] and a reduction in the abundance of Faecalibacterium, particularly F. prausnitzii[97,98,102,121,122], have been observed in both cases. F. prausnitzii is one of the most abundant bacterial species in the gut, exhibiting anti-inflammatory effects through inhibition of IL-8 production, promotion of IL-10 secretion, and upregulation of regulatory T cells[134]. Moreover, patients with IBS were shown to have reduced numbers of Bifidobacterium[98,103,104,106,107]. Likewise, several studies have reported a decreased abundance of these commensals after antibiotic exposure[121,128,135]. Most members of the genus Bifidobacterium are known to exert beneficial effects on host health, including competitive exclusion of enteric pathogens, metabolism of dietary compounds, and regulation of the immune response[22,26,33]. Furthermore, both IBS and antibiotic exposure are characterized by overgrowth of Enterobacteriaceae[98,104,106,136,137]. The Enterobacteriaceae family includes pathogenic bacteria (e.g., Escherichia, Shigella, Klebsiella, and Enterobacter) with proinflammatory properties that may contribute to low-grade inflammation in the gut wall[98].
CONCLUSION
There is clear and consistent evidence from a variety of studies that patients with IBS have altered composition of the gut microbiota and that these alterations are related to the generation of gastrointestinal symptoms. However, studies comparing fecal microbiota in patients with IBS and healthy controls produced variable findings. To date, there is still no consensus on distinct microbiome signatures in IBS. Although some common threads reviewed here were found, prospective large-scale studies need to be carried out to shed light on this issue. Independent analysis of the gut microbiota and its metabolites will help to develop novel microbiota-based treatment strategies that target the underlying pathophysiology of IBS rather than focusing on symptom alleviation.
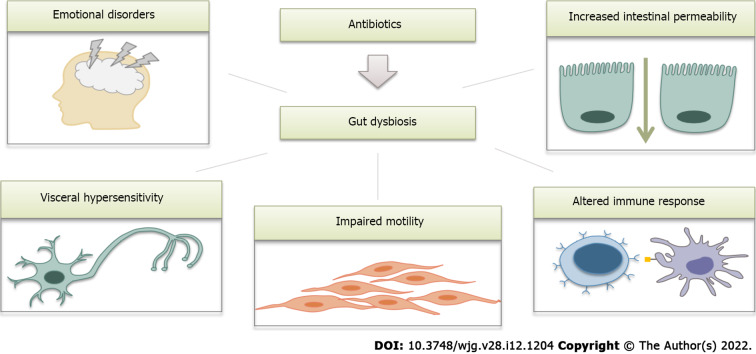
A number of recent studies have addressed the effects of antibiotics on gut microbiota composition, and these effects were found to be quite similar to those observed in IBS. We suggest that the Rome V criteria could provide a new definition of postantibiotic IBS. As major disruptors of the gut microbiota, antibiotics seem to contribute to all aspects of IBS pathogenesis (Figure 3). However, further research in this area is definitely warranted.
Figure 3.
Possible link between antibiotic use and the development of irritable bowel syndrome (schematic illustration). Antibiotics cause profound changes in the gut microbiota and therefore contribute to all mechanisms involved in the pathogenesis of irritable bowel syndrome.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 24, 2021
First decision: November 7, 2021
Article in press: February 22, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chen F, Mi Y, Ren JY, Wang JH S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
Contributor Information
Zarina Mamieva, Department of Internal Disease Propaedeutics, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia. mamievazarina@mail.ru.
Elena Poluektova, Department of Internal Disease Propaedeutics, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia.
Valery Svistushkin, Department of Ear, Throat and Nose Diseases, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia.
Vasily Sobolev, Department of Ear, Throat and Nose Diseases, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia.
Oleg Shifrin, Department of Internal Disease Propaedeutics, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia.
Francisco Guarner, Digestive System Research Unit, Vall d’Hebron Research Institute, Barcelona 08035, Spain.
Vladimir Ivashkin, Department of Internal Disease Propaedeutics, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia.
References
- 1.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miranda C, Silva V, Capita R, Alonso-Calleja C, Igrejas G, Poeta P. Implications of antibiotics use during the COVID-19 pandemic: present and future. J Antimicrob Chemother. 2020;75:3413–3416. doi: 10.1093/jac/dkaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villarreal AA, Aberger FJ, Benrud R, Gundrum JD. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17–20. [PubMed] [Google Scholar]
- 6.Paula H, Grover M, Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Non-enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neurogastroenterol Motil. 2015;27:1580–1586. doi: 10.1111/nmo.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krogsgaard LR, Engsbro AL, Bytzer P. Antibiotics: a risk factor for irritable bowel syndrome in a population-based cohort. Scand J Gastroenterol. 2018;53:1027–1030. doi: 10.1080/00365521.2018.1500638. [DOI] [PubMed] [Google Scholar]
- 8.Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, Singh S, Grover M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;152:1042–1054.e1. doi: 10.1053/j.gastro.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quigley EM, Fried M, Gwee KA, Khalif I, Hungin AP, Lindberg G, Abbas Z, Fernandez LB, Bhatia SJ, Schmulson M, Olano C, LeMair A Review Team: World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A Global Perspective Update September 2015. J Clin Gastroenterol. 2016;50:704–713. doi: 10.1097/MCG.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 10.Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 11.Raskov H, Burcharth J, Pommergaard HC, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365–383. doi: 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mari A, Abu Baker F, Mahamid M, Sbeit W, Khoury T. The Evolving Role of Gut Microbiota in the Management of Irritable Bowel Syndrome: An Overview of the Current Knowledge. J Clin Med. 2020;9 doi: 10.3390/jcm9030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133–146. doi: 10.1016/S2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 14.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 17.Quigley EMM. The Gut-Brain Axis and the Microbiome: Clues to Pathophysiology and Opportunities for Novel Management Strategies in Irritable Bowel Syndrome (IBS) J Clin Med. 2018;7 doi: 10.3390/jcm7010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon CC, Wang YP, Lu CL. Targeting the gut microbiota for the treatment of irritable bowel syndrome. Kaohsiung J Med Sci. 2020;36:160–170. doi: 10.1002/kjm2.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 20.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411–455.. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 26.Linares DM, Gómez C, Renes E, Fresno JM, Tornadijo ME, Ross RP, Stanton C. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front Microbiol. 2017;8:846. doi: 10.3389/fmicb.2017.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 2016;1372:53–64. doi: 10.1111/nyas.13145. [DOI] [PubMed] [Google Scholar]
- 28.Grigg JB, Sonnenberg GF. Host-Microbiota Interactions Shape Local and Systemic Inflammatory Diseases. J Immunol. 2017;198:564–571. doi: 10.4049/jimmunol.1601621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol (Oxf) 2016;217:300–310. doi: 10.1111/apha.12695. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Covington A, Pamer EG. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279:90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camilleri M, Ford AC. Irritable Bowel Syndrome: Pathophysiology and Current Therapeutic Approaches. Handb Exp Pharmacol. 2017;239:75–113. doi: 10.1007/164_2016_102. [DOI] [PubMed] [Google Scholar]
- 35.Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376:2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 36.D'Antongiovanni V, Pellegrini C, Fornai M, Colucci R, Blandizzi C, Antonioli L, Bernardini N. Intestinal epithelial barrier and neuromuscular compartment in health and disease. World J Gastroenterol. 2020;26:1564–1579. doi: 10.3748/wjg.v26.i14.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadjivasilis A, Tsioutis C, Michalinos A, Ntourakis D, Christodoulou DK, Agouridis AP. New insights into irritable bowel syndrome: from pathophysiology to treatment. Ann Gastroenterol. 2019;32:554–564. doi: 10.20524/aog.2019.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casado-Bedmar M, Keita ÅV. Potential neuro-immune therapeutic targets in irritable bowel syndrome. Therap Adv Gastroenterol. 2020;13:1756284820910630. doi: 10.1177/1756284820910630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018;11:3–20. doi: 10.1038/mi.2017.73. [DOI] [PubMed] [Google Scholar]
- 40.Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics. 2018;15:36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cani PD, Knauf C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol Metab. 2016;5:743–752. doi: 10.1016/j.molmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18:2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Malley D. Endocrine regulation of gut function - a role for glucagon-like peptide-1 in the pathophysiology of irritable bowel syndrome. Exp Physiol. 2019;104:3–10. doi: 10.1113/EP087443. [DOI] [PubMed] [Google Scholar]
- 44.Halim MA, Degerblad M, Sundbom M, Karlbom U, Holst JJ, Webb DL, Hellström PM. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018;103:575–585. doi: 10.1210/jc.2017-02006. [DOI] [PubMed] [Google Scholar]
- 45.Li ZY, Zhang N, Wen S, Zhang J, Sun XL, Fan XM, Sun YH. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin Res Hepatol Gastroenterol. 2017;41:459–465. doi: 10.1016/j.clinre.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Cui X, Chen Y, Wang Y, Li X, Lin L, Zhang H. Exendin-4, an analogue of glucagon-like peptide-1, attenuates hyperalgesia through serotonergic pathways in rats with neonatal colonic sensitivity. J Physiol Pharmacol. 2014;65:349–357. [PubMed] [Google Scholar]
- 47.O'Brien R, O'Malley D. The Glucagon-like peptide-1 receptor agonist, exendin-4, ameliorated gastrointestinal dysfunction in the Wistar Kyoto rat model of Irritable Bowel Syndrome. Neurogastroenterol Motil. 2020;32:e13738. doi: 10.1111/nmo.13738. [DOI] [PubMed] [Google Scholar]
- 48.Camilleri M, Vazquez-Roque M, Iturrino J, Boldingh A, Burton D, McKinzie S, Wong BS, Rao AS, Kenny E, Månsson M, Zinsmeister AR. Effect of a glucagon-like peptide 1 analog, ROSE-010, on GI motor functions in female patients with constipation-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G120–G128. doi: 10.1152/ajpgi.00076.2012. [DOI] [PubMed] [Google Scholar]
- 49.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge X, Pan J, Liu Y, Wang H, Zhou W, Wang X. Intestinal Crosstalk between Microbiota and Serotonin and its Impact on Gut Motility. Curr Pharm Biotechnol. 2018;19:190–195. doi: 10.2174/1389201019666180528094202. [DOI] [PubMed] [Google Scholar]
- 51.Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, Claeysen S, Cunningham KA, Fone KC, Gershon M, Di Giovanni G, Goodfellow NM, Halberstadt AL, Hartley RM, Hassaine G, Herrick-Davis K, Hovius R, Lacivita E, Lambe EK, Leopoldo M, Levy FO, Lummis SCR, Marin P, Maroteaux L, McCreary AC, Nelson DL, Neumaier JF, Newman-Tancredi A, Nury H, Roberts A, Roth BL, Roumier A, Sanger GJ, Teitler M, Sharp T, Villalón CM, Vogel H, Watts SW, Hoyer D. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol Rev. 2021;73:310–520. doi: 10.1124/pr.118.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gros M, Gros B, Mesonero JE, Latorre E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J Clin Med. 2021;10 doi: 10.3390/jcm10153429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y, Yu T, Tang Y, Xiong W, Shen X, Jiang L, Lin L. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0172846. doi: 10.1371/journal.pone.0172846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Black CJ, Burr NE, Ford AC. Relative Efficacy of Tegaserod in a Systematic Review and Network Meta-analysis of Licensed Therapies for Irritable Bowel Syndrome With Constipation. Clin Gastroenterol Hepatol. 2020;18:1238–1239.e1. doi: 10.1016/j.cgh.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev. 2007:CD003960. doi: 10.1002/14651858.CD003960.pub3. [DOI] [PubMed] [Google Scholar]
- 58.Shah ED, Lacy BE, Chey WD, Chang L, Brenner DM. Tegaserod for Irritable Bowel Syndrome With Constipation in Women Younger Than 65 Years Without Cardiovascular Disease: Pooled Analyses of 4 Controlled Trials. Am J Gastroenterol. 2021;116:1601–1611. doi: 10.14309/ajg.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukudo S, Nakamura M, Hamatani T, Kazumori K, Miwa H. Efficacy and Safety of 5-HT4 Receptor Agonist Minesapride for Irritable Bowel Syndrome with Constipation in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2021;19:538–546.e8. doi: 10.1016/j.cgh.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 60.Hamatani T, Noda N, Takagaki T, Yodo Y, Kawai H, Kakuyama H, Kaji Y, Fujio Y. Thorough QT/QTc Study Shows That a Novel 5-HT4 Receptor Partial Agonist Minesapride Has No Effect on QT Prolongation. Clin Pharmacol Drug Dev. 2020;9:938–951. doi: 10.1002/cpdd.778. [DOI] [PubMed] [Google Scholar]
- 61.Pusceddu MM, Gareau MG. Visceral pain: gut microbiota, a new hope? J Biomed Sci. 2018;25:73. doi: 10.1186/s12929-018-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ait-Belgnaoui A, Payard I, Rolland C, Harkat C, Braniste V, Théodorou V, Tompkins TA. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J Neurogastroenterol Motil. 2018;24:138–146. doi: 10.5056/jnm16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Song L, Wang Y, Liu C, Zhang L, Zhu S, Liu S, Duan L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34:1368–1376. doi: 10.1111/jgh.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei P, Keller C, Li L. Neuropeptides in gut-brain axis and their influence on host immunity and stress. Comput Struct Biotechnol J. 2020;18:843–851. doi: 10.1016/j.csbj.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kano M, Muratsubaki T, Van Oudenhove L, Morishita J, Yoshizawa M, Kohno K, Yagihashi M, Tanaka Y, Mugikura S, Dupont P, Ly HG, Takase K, Kanazawa M, Fukudo S. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7:12425. doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275.. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faravelli C, Lo Sauro C, Godini L, Lelli L, Benni L, Pietrini F, Lazzeretti L, Talamba GA, Fioravanti G, Ricca V. Childhood stressful events, HPA axis and anxiety disorders. World J Psychiatry. 2012;2:13–25. doi: 10.5498/wjp.v2.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee C, Doo E, Choi JM, Jang SH, Ryu HS, Lee JY, Oh JH, Park JH, Kim YS Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility. The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-analysis. J Neurogastroenterol Motil. 2017;23:349–362. doi: 10.5056/jnm16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Auteri M, Zizzo MG, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res. 2015;93:11–21. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 73.Kawase T, Nagasawa M, Ikeda H, Yasuo S, Koga Y, Furuse M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br J Nutr. 2017;117:775–783. doi: 10.1017/S0007114517000678. [DOI] [PubMed] [Google Scholar]
- 74.Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, Wang H, Zhou C, Fang L, Li W, Niu R, Wei H, Xie P. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, Surette MG, Verdu EF, Collins SM, Bercik P. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 76.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pigrau Pastor M, Sidani S, Pinto-Sanchez MI, Philip V, McLean PG, Hagelsieb MG, Surette MG, Bergonzelli GE, Verdu EF, Britz-McKibbin P, Neufeld JD, Collins SM, Bercik P. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 77.Huang C, Yang X, Zeng B, Zeng L, Gong X, Zhou C, Xia J, Lian B, Qin Y, Yang L, Liu L, Xie P. Proteomic analysis of olfactory bulb suggests CACNA1E as a promoter of CREB signaling in microbiota-induced depression. J Proteomics. 2019;194:132–147. doi: 10.1016/j.jprot.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 78.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 79.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 81.Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine. 2017;93:34–43. doi: 10.1016/j.cyto.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Barbalho SM, Goulart RA, Araújo AC, Guiguer ÉL, Bechara MD. Irritable bowel syndrome: a review of the general aspects and the potential role of vitamin D. Expert Rev Gastroenterol Hepatol. 2019;13:345–359. doi: 10.1080/17474124.2019.1570137. [DOI] [PubMed] [Google Scholar]
- 83.Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J Neurogastroenterol Motil. 2016;22:558–574. doi: 10.5056/jnm16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 85.Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol. 2017;198:572–580. doi: 10.4049/jimmunol.1601247. [DOI] [PubMed] [Google Scholar]
- 86.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azad MAK, Sarker M, Wan D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res Int. 2018;2018:8063647. doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yousefi B, Eslami M, Ghasemian A, Kokhaei P, Salek Farrokhi A, Darabi N. Probiotics importance and their immunomodulatory properties. J Cell Physiol. 2019;234:8008–8018. doi: 10.1002/jcp.27559. [DOI] [PubMed] [Google Scholar]
- 89.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swan C, Duroudier NP, Campbell E, Zaitoun A, Hastings M, Dukes GE, Cox J, Kelly FM, Wilde J, Lennon MG, Neal KR, Whorwell PJ, Hall IP, Spiller RC. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut. 2013;62:985–994. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 91.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 93.Cheng P, Yao J, Wang C, Zhang L, Kong W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol Med Rep. 2015;12:3257–3264. doi: 10.3892/mmr.2015.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Thiel IAM, de Jonge WJ, Chiu IM, van den Wijngaard RM. Microbiota-neuroimmune cross talk in stress-induced visceral hypersensitivity of the bowel. Am J Physiol Gastrointest Liver Physiol. 2020;318:G1034–G1041. doi: 10.1152/ajpgi.00196.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 97.Vich Vila A, Imhann F, Collij V, Jankipersadsing SA, Gurry T, Mujagic Z, Kurilshikov A, Bonder MJ, Jiang X, Tigchelaar EF, Dekens J, Peters V, Voskuil MD, Visschedijk MC, van Dullemen HM, Keszthelyi D, Swertz MA, Franke L, Alberts R, Festen EAM, Dijkstra G, Masclee AAM, Hofker MH, Xavier RJ, Alm EJ, Fu J, Wijmenga C, Jonkers DMAE, Zhernakova A, Weersma RK. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aap8914. [DOI] [PubMed] [Google Scholar]
- 98.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, Moayyedi P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology. 2019;157:97–108. doi: 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 99.Duan R, Zhu S, Wang B, Duan L. Alterations of Gut Microbiota in Patients With Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin Transl Gastroenterol. 2019;10:e00012. doi: 10.14309/ctg.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeffery IB, Das A, O'Herlihy E, Coughlan S, Cisek K, Moore M, Bradley F, Carty T, Pradhan M, Dwibedi C, Shanahan F, O'Toole PW. Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology. 2020;158:1016–1028.e8. doi: 10.1053/j.gastro.2019.11.301. [DOI] [PubMed] [Google Scholar]
- 101.Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, Guarner F, Azpiroz F, Manichanh C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maharshak N, Ringel Y, Katibian D, Lundqvist A, Sartor RB, Carroll IM, Ringel-Kulka T. Fecal and Mucosa-Associated Intestinal Microbiota in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Dig Dis Sci. 2018;63:1890–1899. doi: 10.1007/s10620-018-5086-4. [DOI] [PubMed] [Google Scholar]
- 103.Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49:331–337. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- 104.Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38. doi: 10.1111/jgh.13471. [DOI] [PubMed] [Google Scholar]
- 105.Shukla R, Ghoshal U, Dhole TN, Ghoshal UC. Fecal Microbiota in Patients with Irritable Bowel Syndrome Compared with Healthy Controls Using Real-Time Polymerase Chain Reaction: An Evidence of Dysbiosis. Dig Dis Sci. 2015;60:2953–2962. doi: 10.1007/s10620-015-3607-y. [DOI] [PubMed] [Google Scholar]
- 106.Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, Mullin GE. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J Acad Nutr Diet. 2020;120:565–586. doi: 10.1016/j.jand.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 107.Su T, Liu R, Lee A, Long Y, Du L, Lai S, Chen X, Wang L, Si J, Owyang C, Chen S. Altered Intestinal Microbiota with Increased Abundance of Prevotella Is Associated with High Risk of Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterol Res Pract. 2018;2018:6961783. doi: 10.1155/2018/6961783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ringel-Kulka T, Benson AK, Carroll IM, Kim J, Legge RM, Ringel Y. Molecular characterization of the intestinal microbiota in patients with and without abdominal bloating. Am J Physiol Gastrointest Liver Physiol. 2016;310:G417–G426. doi: 10.1152/ajpgi.00044.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhuang X, Tian Z, Li L, Zeng Z, Chen M, Xiong L. Fecal Microbiota Alterations Associated With Diarrhea-Predominant Irritable Bowel Syndrome. Front Microbiol. 2018;9:1600. doi: 10.3389/fmicb.2018.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Durand GA, Raoult D, Dubourg G. Antibiotic discovery: history, methods and perspectives. Int J Antimicrob Agents. 2019;53:371–382. doi: 10.1016/j.ijantimicag.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Aminov R. History of antimicrobial drug discovery: Major classes and health impact. Biochem Pharmacol. 2017;133:4–19. doi: 10.1016/j.bcp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 112.Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus. 2017;9:e1403. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sultan I, Rahman S, Jan AT, Siddiqui MT, Mondal AH, Haq QMR. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front Microbiol. 2018;9:2066. doi: 10.3389/fmicb.2018.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turta O, Rautava S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC Med. 2016;14:57. doi: 10.1186/s12916-016-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scheer S, Medina TS, Murison A, Taves MD, Antignano F, Chenery A, Soma KK, Perona-Wright G, Lupien M, Arrowsmith CH, De Carvalho DD, Zaph C. Early-life antibiotic treatment enhances the pathogenicity of CD4+ T cells during intestinal inflammation. J Leukoc Biol. 2017;101:893–900. doi: 10.1189/jlb.3MA0716-334RR. [DOI] [PubMed] [Google Scholar]
- 116.Gustafsson J, McDonald KG, Newberry R. Disruption of the gut microbiota by antibiotics exposure during early life promotes spontaneous Th2 responses and loss of tolerance to dietary antigens. e-pub ahead of print 2016. [Google Scholar]
- 117.Rasmussen SH, Shrestha S, Bjerregaard LG, Ängquist LH, Baker JL, Jess T, Allin KH. Antibiotic exposure in early life and childhood overweight and obesity: A systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1508–1514. doi: 10.1111/dom.13230. [DOI] [PubMed] [Google Scholar]
- 118.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Panda S, El khader I, Casellas F, López Vivancos J, García Cors M, Santiago A, Cuenca S, Guarner F, Manichanh C. Short-term effect of antibiotics on human gut microbiota. PLoS One. 2014;9:e95476. doi: 10.1371/journal.pone.0095476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burdet C, Grall N, Linard M, Bridier-Nahmias A, Benhayoun M, Bourabha K, Magnan M, Clermont O, d'Humières C, Tenaillon O, Denamur E, Massias L, Tubiana S, Alavoine L, Andremont A, Mentré F, Duval X CEREMI Group. Ceftriaxone and Cefotaxime Have Similar Effects on the Intestinal Microbiota in Human Volunteers Treated by Standard-Dose Regimens. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.02244-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rashid MU, Zaura E, Buijs MJ, Keijser BJ, Crielaard W, Nord CE, Weintraub A. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin Infect Dis. 2015;60 Suppl 2:S77–S84. doi: 10.1093/cid/civ137. [DOI] [PubMed] [Google Scholar]
- 122.Isaac S, Scher JU, Djukovic A, Jiménez N, Littman DR, Abramson SB, Pamer EG, Ubeda C. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72:128–136. doi: 10.1093/jac/dkw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haak BW, Lankelma JM, Hugenholtz F, Belzer C, de Vos WM, Wiersinga WJ. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J Antimicrob Chemother. 2019;74:782–786. doi: 10.1093/jac/dky471. [DOI] [PubMed] [Google Scholar]
- 124.Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906–1915. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 125.Baietto L, Corcione S, Pacini G, Perri GD, D'Avolio A, De Rosa FG. A 30-years review on pharmacokinetics of antibiotics: is the right time for pharmacogenetics? Curr Drug Metab. 2014;15:581–598. doi: 10.2174/1389200215666140605130935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Becattini S, Taur Y, Pamer EG. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471–489. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 129.Baldelli V, Scaldaferri F, Putignani L, Del Chierico F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms. 2021;9 doi: 10.3390/microorganisms9040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 133.Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 134.Lopez-Siles M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J. 2017;11:841–852. doi: 10.1038/ismej.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Raymond F, Ouameur AA, Déraspe M, Iqbal N, Gingras H, Dridi B, Leprohon P, Plante PL, Giroux R, Bérubé È, Frenette J, Boudreau DK, Simard JL, Chabot I, Domingo MC, Trottier S, Boissinot M, Huletsky A, Roy PH, Ouellette M, Bergeron MG, Corbeil J. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016;10:707–720. doi: 10.1038/ismej.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pallav K, Dowd SE, Villafuerte J, Yang X, Kabbani T, Hansen J, Dennis M, Leffler DA, Newburg DS, Kelly CP. Effects of polysaccharopeptide from Trametes versicolor and amoxicillin on the gut microbiome of healthy volunteers: a randomized clinical trial. Gut Microbes. 2014;5:458–467. doi: 10.4161/gmic.29558. [DOI] [PubMed] [Google Scholar]
- 137.Kabbani TA, Pallav K, Dowd SE, Villafuerte-Galvez J, Vanga RR, Castillo NE, Hansen J, Dennis M, Leffler DA, Kelly CP. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8:17–32. doi: 10.1080/19490976.2016.1267890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Covasa M, Stephens RW, Toderean R, Cobuz C. Intestinal Sensing by Gut Microbiota: Targeting Gut Peptides. Front Endocrinol (Lausanne) 2019;10:82. doi: 10.3389/fendo.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 140.Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70–83. doi: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cantarel BL, Lombard V, Henrissat B. Complex carbohydrate utilization by the healthy human microbiome. PLoS One. 2012;7:e28742. doi: 10.1371/journal.pone.0028742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fukiya S, Arata M, Kawashima H, Yoshida D, Kaneko M, Minamida K, Watanabe J, Ogura Y, Uchida K, Itoh K, Wada M, Ito S, Yokota A. Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. FEMS Microbiol Lett. 2009;293:263–270. doi: 10.1111/j.1574-6968.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- 143.Kimura I, Inoue D, Hirano K, Tsujimoto G. The SCFA Receptor GPR43 and Energy Metabolism. Front Endocrinol (Lausanne) 2014;5:85. doi: 10.3389/fendo.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Portune K, Beaumont M, Davila A, Tomé D, Blachier F, Sanz Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci Technol . 2016 [Google Scholar]
- 145.Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, Humbert L, Trugnan G, Seksik P, Sokol H, Rainteau D, Sabate JM. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28:1330–1340. doi: 10.1111/nmo.12829. [DOI] [PubMed] [Google Scholar]
- 146.Gobert AP, Sagrestani G, Delmas E, Wilson KT, Verriere TG, Dapoigny M, Del'homme C, Bernalier-Donadille A. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci Rep. 2016;6:39399. doi: 10.1038/srep39399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhong W, Lu X, Shi H, Zhao G, Song Y, Wang Y, Zhang J, Jin Y, Wang S. Distinct Microbial Populations Exist in the Mucosa-associated Microbiota of Diarrhea Predominant Irritable Bowel Syndrome and Ulcerative Colitis. J Clin Gastroenterol. 2019;53:660–672. doi: 10.1097/MCG.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 148.Rangel I, Sundin J, Fuentes S, Repsilber D, de Vos WM, Brummer RJ. The relationship between faecal-associated and mucosal-associated microbiota in irritable bowel syndrome patients and healthy subjects. Aliment Pharmacol Ther. 2015;42:1211–1221. doi: 10.1111/apt.13399. [DOI] [PubMed] [Google Scholar]