Key Points
Question
What are the effects of estradiol or vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12-week treatment in postmenopausal women?
Findings
In this secondary analysis of 144 participants in a randomized clinical trial of postmenopausal women with moderate to severe vulvovaginal symptoms, women using vaginal 10 μg estradiol tablet demonstrated larger changes in the vaginal microbiota and metabolome compared with women using vaginal moisturizer or placebo, despite a decrease in pH within each intervention group.
Meaning
These findings suggest that treatment of genitourinary symptoms of menopause with topical estradiol changes the vaginal microenvironment in ways that may promote genitourinary health.
This secondary analysis of a random clinical trial compares changes in the vaginal microbiota, metabolome, and pH among women using a vaginal estradiol tablet, moisturizer, or placebo.
Abstract
Importance
Postmenopausal women with genitourinary symptoms of menopause are often prescribed vaginal estradiol or moisturizer for symptom improvement, but the impact of these treatments on the local microenvironment is poorly understood.
Objective
To compare changes in the vaginal microbiota, metabolome, and pH among women using low-dose vaginal estradiol tablet or low pH moisturizer gel for 12-weeks vs low pH placebo.
Design, Setting, and Participants
This is a post hoc prespecified secondary analysis of a 12-week multicenter randomized clinical trial among postmenopausal women with moderate to severe genitourinary symptoms. Women were enrolled between April 2016 and February 2017; final follow-up visits occurred in April 2017. Data were analyzed from November 2018 to July 2021.
Interventions
Ten-μg vaginal estradiol plus placebo gel vs placebo tablet plus vaginal moisturizer vs dual placebo.
Main Outcomes and Measures
The main outcome measures were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH.
Results
Of 302 postmenopausal women from the parent trial, 144 women (mean [SD] age, 61 [4] years) were included in this analysis. After 12 weeks, the microbiota was dominated with Lactobacillus and Bifidobacterium communities among 36 women (80%) in the estradiol group, compared with 16 women (36%) using moisturizer and 13 women (26%) using placebo (P < .001). The composition of vaginal fluid metabolites also varied after 12-weeks among women in the estradiol group with significant changes in 90 of 171 metabolites measured (53%) (P < .001), including an increase in lactate. The 12-week pH among women in the estradiol group was lower vs placebo (median [IQR] pH, 5 [4.5-6.0] vs 6 [5.5-7.0]; P = .005) but not the moisturizer group vs placebo (median [IQR] pH, 6 [5.5-6.5]; P = .28). There was a decrease in pH from baseline to 12-weeks within the moisturizer (median [IQR] pH, 7 [6.0-7.5] vs 6 [5.5-6.5]; P < .001) and placebo (median [IQR] pH, 7 [7.0-7.5] vs 6 [5.5-7.0]; P < .001) groups. Women with high-diversity bacterial communities at baseline exhibited greater median change in pH compared with women with low-diversity communities (median [IQR] change, −1 [−2 to −0.5] vs −0.3 [−1.1 to 0], P = .007).
Conclusions and Relevance
This secondary analysis of a randomized clinical trial found that use of vaginal estradiol tablets resulted in substantial changes in the vaginal microbiota and metabolome with a lowering in pH, particularly in women with high-diversity bacterial communities at baseline. Low pH moisturizer or placebo did not significantly impact the vaginal microbiota or metabolome despite lowering the vaginal pH. Estradiol use may offer additional genitourinary health benefits to postmenopausal women.
Trial Registration
ClinicalTrials.gov Identifier: NCT02516202
Introduction
In the Menopause Strategies–Finding Lasting Answers for Symptoms and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, no significant difference in symptom reduction was seen between estradiol vaginal tablet or vaginal moisturizer and placebo: all 3 groups had similar improvement.1 While both hormonal and nonhormonal treatments have been shown to improve postmenopausal vaginal symptoms,1,2,3,4,5,6 the lack of difference between estradiol and placebo was surprising. The placebo gel used in the trial had high lubricity and low pH, while the moisturizer also had low pH; both properties may contribute to decreased symptoms via biological effects that differ from estradiol. Some interpreted these findings as a statement that estrogen treatment was ineffective, rather than a recognition that different types of interventions may reduce postmenopausal vaginal discomfort.
The Food and Drug Administration (FDA) guidance for outcome measures in clinical trials of treatment for moderate to severe genitourinary syndrome of menopause (GSM) recommends 3 coprimary end points: change in most bothersome symptom (MBS) severity, pH, and vaginal maturation index (VMI).7 In our primary analysis, more participants in the estrogen group had pH less than 5 and more than 5% superficial cells on VMI than placebo at 12 weeks, although the placebo gel was acidic.1 The regulatory guidance weights these changes in the vaginal mucosal environment equally with improvement in MBS, but it is unclear whether these mucosal changes reflect any additional benefits to genitourinary health beyond symptom improvement.
We conducted a subset post hoc analysis of participants from the MsFLASH Vaginal Health Trial to evaluate the effect of topical interventions for GSM on the microbiota and metabolome, and to assess whether larger changes in pH and VMI in the estradiol group reflect other changes in the vaginal microbiota and metabolome that might impact genitourinary health.
Methods
This secondary analysis of a randomized clinical trial was approved by the institutional review board at Fred Hutchinson Cancer Research Center. All participants provided written informed consent. The primary trial and this prespecified post hoc secondary analysis followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The primary trial protocol and statistical analysis plan are provided in Supplement 1.
Study Design and Population
The MsFLASH Vaginal Health Trial was a multicenter, double-blind randomized clinical trial of vaginal estradiol tablet 0.01 mg (plus placebo gel) or vaginal moisturizing gel (plus placebo tablet) vs dual placebo in 302 postmenopausal women with moderate to severe vulvovaginal discomfort.1 Women were enrolled between April 2016 and February 2017; final follow-up visits occurred in April 2017. The primary outcome was change over 12-weeks in severity of MBS selected by participants at enrollment, including pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, or pain. Symptom severity was rated from 0 to 3, signifying none, mild, moderate, or severe.
A subset of 144 women eligible for this post hoc analysis used at least 80% of study product doses and were not diagnosed with yeast, bacterial vaginosis (BV), or trichomoniasis by culture or wet mount. We randomly selected 15 eligible participants from each group to evaluate changes in the vaginal microbiota and metabolome in response to treatment. We also selected 20 eligible participants from each treatment group who experienced a decrease in MBS of at least 2 points from enrollment to 12 weeks and matched them with 20 eligible participants with a decrease in MBS of 1 point or less by treatment group, race (dichotomized as White or other race, including Black, American Indian, Asian, or Pacific Islander), and MBS selection (pain with vaginal penetration or other), and were frequency-matched by study site, age, and baseline MBS severity. The 2 selection processes were not mutually exclusive. Race was self-identified by the trial participants. Race was included as a factor in matching participants in higher and lower response categories to reduce heterogeneity across the response groups.
At enrollment, 4 weeks, and 12 weeks, vaginal swabs were collected for pH, VMI, microbiota, and metabolomic analyses. Primary outcome measures included changes in diversity and composition of the vaginal microbiota, composition of small molecule metabolites, and pH within the estradiol and moisturizer groups vs placebo across 12-weeks. Microbiota and metabolome characterization details are provided in the eMethods in Supplement 2. Vaginal pH was measured using pH paper. We evaluated change in VMI and symptom improvement when we stratified women based on the diversity of their bacterial communities at baseline. Smears stained with the standard Papanicolau stain were used to evaluate VMI and defined as less than 5% or 5% or greater superficial cells. Symptom improvement was measured as change in the symptom severity scale, and clinically relevant improvement was defined as a decrease of at least 2 points in MBS severity.
Molecular Methods
The vaginal microbiota was characterized by 16S rRNA gene sequencing; taxonomy was assigned to sequence variants by placing on a custom phylogenetic tree.8,9,10 Sequences have been deposited in the National Center for Biotechnology Information Sequence Read Archive (PRJNA788936). Further details are provided in the eMethods in Supplement 2.
Metabolomic Profiling
Broad-based metabolomic profiling was performed on a liquid-chromatography mass spectrometry (LC-MS/MS) platform at Northwest Metabolomics Research Center.11 Further details are provided in the eMethods in Supplement 2.
Estradiol Concentrations in Serum Samples
Serum estradiol levels were measured at the Brigham Research Assay Core Laboratory using LC-MS/MS on the Triple Quad 5500+ system (SCIEX).12,13 Further details are provided in the eMethods in Supplement 2.
Statistical Analysis
Sequence data were normalized using centered-log-ratio transformation.14 We used the Shannon Diversity Index (SDI) to calculate α diversity, and Aitchison distances were used to estimate β diversity. The metabolome data were quantile normalized,15 with missing values imputed to the minimum detected value per metabolite divided by √2 and log2-transformed. Changes in the microbiota and metabolome were evaluated across 12 weeks using linear mixed models with the placebo group as the referent and incorporating visit- and participant-specific random effects. Linear mixed models were adjusted for age and time since menopause. False discovery rate correction was conducted using the Benjamini-Hochberg method. Two-sided P = .05 and q < .20 were considered nominally statistically significant. Correlations among and between metabolite and microbiota abundances were assessed using Spearman rank correlation. Euclidean distances were used for unsupervised hierarchical clustering of microbiota and metabolite data. All statistical analyses were conducted using R version 3.6.0 (R Project for Statistical Computing). Extension packages used included gplots, vegan, MiRKAT, lme4 and ade4. Data were analyzed from November 2018 to July 2021.
Results
Study Population
Of 302 postmenopausal women from the parent trial, 144 women (mean [SD] age, 61 [4] years) were included in this analysis. Demographic characteristics of participants in this post hoc secondary analysis were similar to those of the broader original trial population. Most participants were White (129 participants [90%]), 6 participants (4%) were Black, and 9 participants (6%) identified as another race (including American Indian, Asian or Pacific Islander, or unknown) (Table). By design, distribution of women by study group was roughly equivalent: 45 women (31%) were in the estradiol with placebo gel group, 48 women (33%) were in the moisturizer and placebo tablet group, and 51 women (35%) were in the dual placebo group.
Table. Baseline Demographic Characteristics of Participants Included in Subset Analysis.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| Estradiol + placebo gel (n = 45) | Moisturizer + placebo tablet (n = 48) | Dual placebo (n = 51) | |
| Age at screening, mean (SD), y | 61 (4) | 60 (4) | 61 (5) |
| Race | |||
| Black | 5 (11) | 1 (2) | 0 (0) |
| White | 37 (82) | 43 (90) | 49 (96) |
| Othera | 3 (7) | 4 (8) | 2 (4) |
| MBS | |||
| Pain with sex | 19 (42) | 32 (67) | 28 (55) |
| Dryness | 11 (24) | 8 (17) | 14 (27) |
| Itch, burn, or irritation | 15 (33) | 8 (17) | 9 (18) |
| Vaginal maturation index, <5% superficial cells | 37 (82) | 37 (77) | 40 (78) |
| pH, mean (SD) | 6.8 (1.0) | 6.6 (1.1) | 6.9 (0.9) |
| Subsequent MBS symptom improvement ≥2 points | 24 (53) | 21 (44) | 25 (49) |
Abbreviation: MBS, most bothersome symptom.
Other race includes 2 American Indian individuals, 6 Asian or Pacific Islander individuals, and 1 unknown individual with unknown race.
Impact of Moisturizer and Estradiol Treatment on the Vaginal Microbiota
Median (IQR) SDI at 12 weeks showed decreases in both estradiol (baseline: 1.7 [0.8-2.7]; 12 weeks: 0.5 [0.2-1.0]; P < .001) and moisturizer (baseline: 1.3 [0.4-2.6]; 12 weeks: 0.8 [0.2-1.3]; P = .03) groups compared with the placebo group (baseline: 1.2 [0.5-2.5]; 12 weeks: 1.5 [0.7-2.5]) (Figure 1A). At 12 weeks, 36 women (80%) in the estradiol group had bacterial communities dominated by Lactobacillus and Bifidobacterium, compared with 13 women (26%) in the placebo group (P < .001) (Figure 1B; eFigure 1 in Supplement 2). Analyses of β diversity showed significant shifts in composition of bacterial communities by 12 weeks among women in the estradiol group compared with the placebo group (P < .001) but not moisturizer vs placebo groups (P = .08) (Figure 2A). Relative abundances of 31 individual bacterial taxa changed after 12 weeks of estradiol treatment (eTable 1 in Supplement 2). Among the top 10 significant taxa, abundances of lactobacilli and bifidobacteria were higher, while abundances of Streptococcus mitis, Peptoniphilus spp, Anaerococcus vaginalis, and Finegoldia magna decreased from baseline with estradiol use (eTable 1 in Supplement 2). In contrast, abundances of 11 bacterial taxa were significantly changed at 12 weeks among women in the moisturizer group, including increase in Lactobacillus acidophilus, or Lactobacillus kitasatonis and decreases in S mitis, Prevotella timonensis, Peptoniphilus coxii, and A vaginalis (eTable 2 in Supplement 2). We did not note increases in abundance of other lactobacilli or bifidobacteria in the moisturizer group (eTable 2 in Supplement 2). Instead, there was an increase in Enterococcus faecalis (eTable 2 in Supplement 2), which was not observed with estradiol use (eTable 1 in Supplement 2).
Figure 1. Changes in the Vaginal Microbiota With Estradiol, Moisturizer, or Placebo Use.
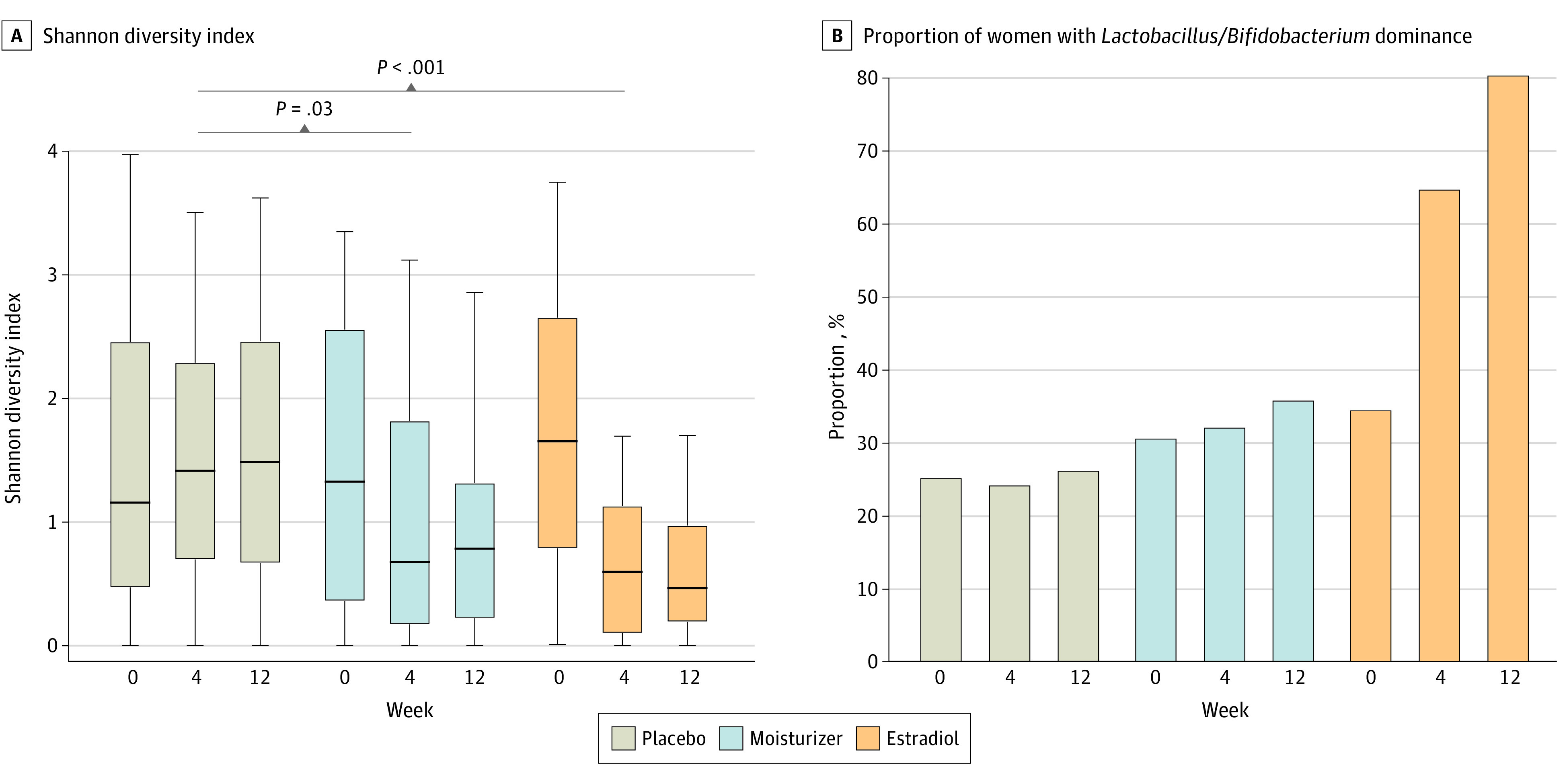
Figure 2. β Diversity Plots Depicting Shifts in the Vaginal Microbiota and Metabolome With Estradiol, Moisturizer, or Placebo Use.
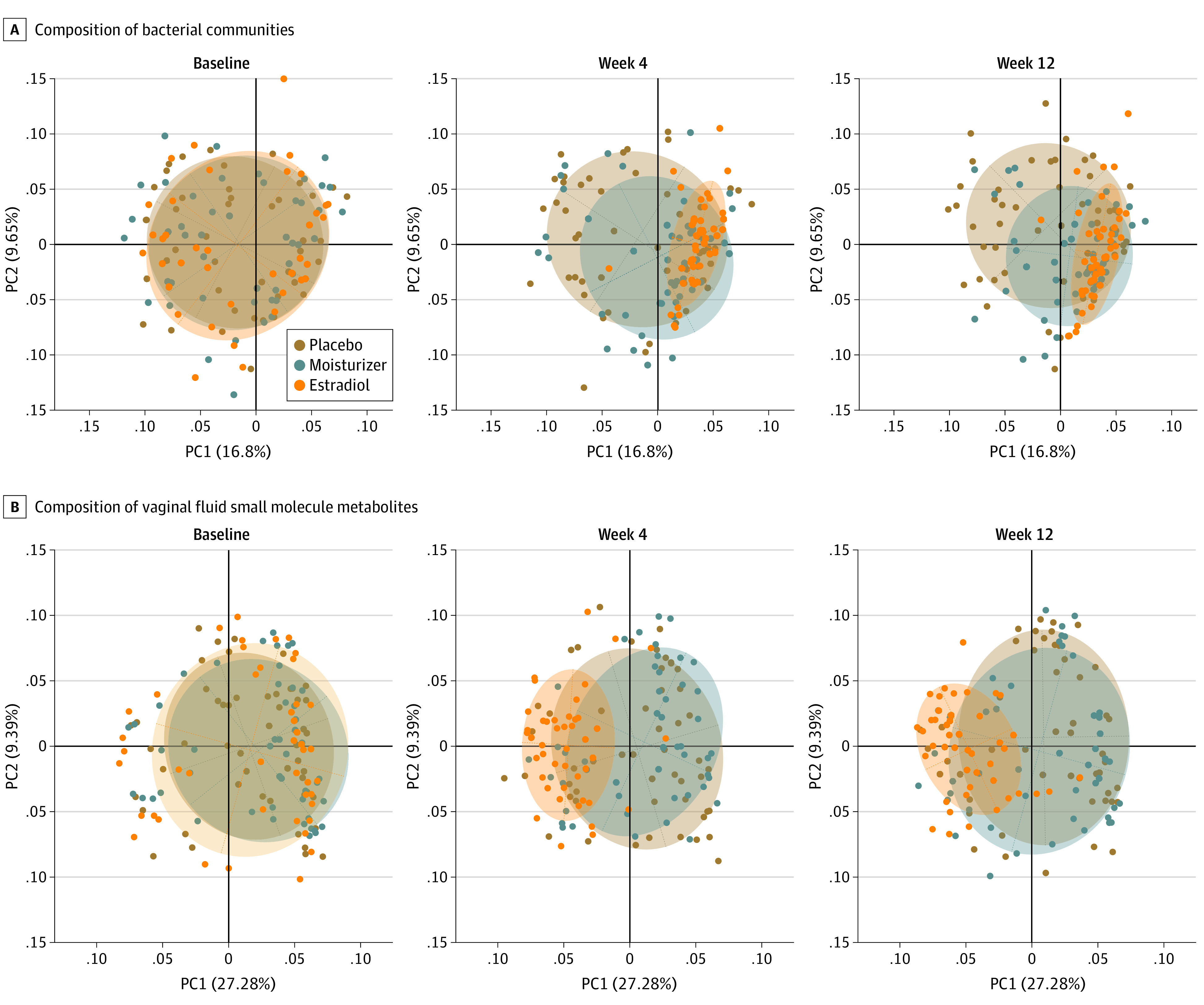
Each dot represents the vaginal bacterial community or metabolic profile in a single participant. Overlapping circles indicate no differences between the groups. By week 4, women in the estradiol group had different vaginal bacterial communities (P < .001) and metabolic profiles (P < .001) compared with women in the placebo group. These changes were maintained at week 12. Such significant shifts in the microbiota (P = .08) or metabolic profiles was not observed among women in the moisturizer group (P = .72). PC indicates principal coordinates.
Impact of Moisturizer and Estradiol Treatment on the Vaginal Metabolome and pH
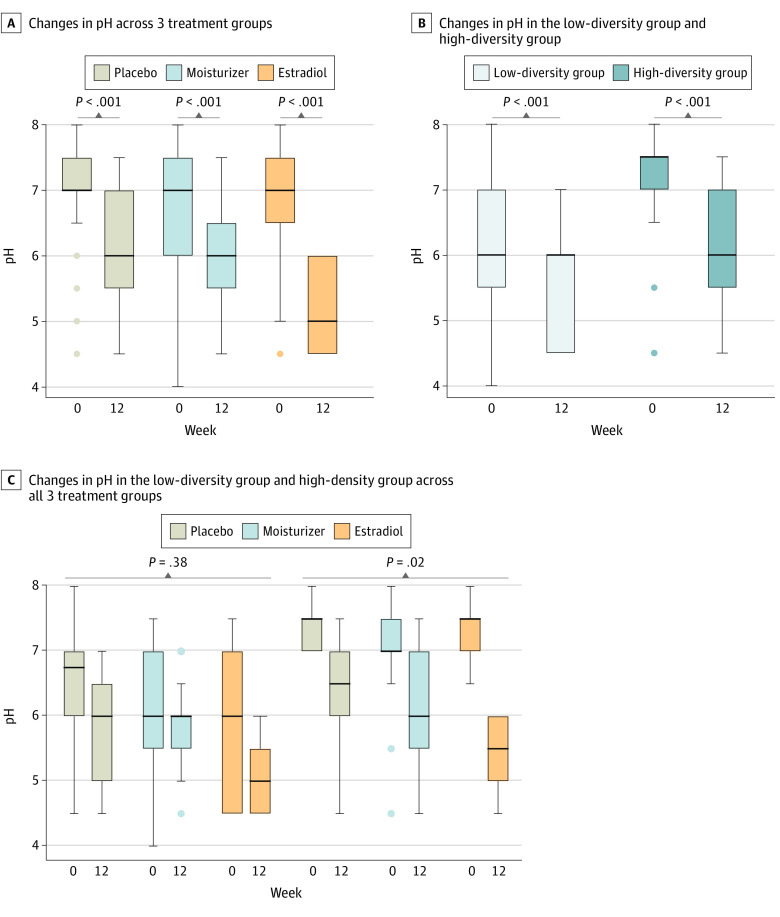
The composition of vaginal fluid small molecule metabolites significantly changed from baseline to 12 weeks with estradiol treatment (P < .001) but not with moisturizer use (P = .72) (Figure 2B). Of 171 metabolites measured, 90 (53%), from multiple metabolic pathways, were significantly changed in women using estradiol vs placebo: 41 metabolites were higher and 49 metabolites were lower (eTable 3 in Supplement 2). We noted significant increases in abundances of lactate, indole-3-lactate, and phenyl-lactate metabolites in the estradiol group vs placebo group, which can contribute to low pH (eTable 3 in Supplement 2), but which were not observed in the moisturizer vs placebo groups (eTable 4 in Supplement 2). Consistent with shifts in metabolites, vaginal pH at 12 weeks among women using estradiol was significantly lower vs the placebo group (median [IQR] pH, 5 [4.5-6.0] vs 6 [5.5-7.0]; P = .005), but there was no statistically significant difference for women in the moisturizer group (median [IQR] pH, 6 [5.5-6.5]; P = .28). However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low pH formulations of both the moisturizer and the placebo (Figure 3A).
Figure 3. Vaginal pH Among Women Using Estradiol, Moisturizer, or Placebo.
Lines indicate medians; boxes, IQRs; error bars, ranges.
Association Between Changes in the Vaginal Microbiota and Metabolites
Given the consistent shifts across 12 weeks with estradiol use, we examined the association between changes in the microbiota and small molecule metabolites. Hierarchical clustering of the vaginal microbiota at baseline identified 2 subgroups of study participants: 1 subgroup of women had vaginal microbiotas with lower SDI compared with women in the second subgroup (median [IQR] SDI, 0.6 [0.1-1.1] vs 2.5 [1.7-3.0]; P < .001) (eFigure 2 in Supplement 2). Similarly, the metabolites at baseline largely separated according to the microbiota groups noted previously (eFigure 3 in Supplement 2). The median pH at baseline of the low-diversity subgroup was lower than the pH of the high-diversity subgroup, and those participants were more likely to have more than 5% superficial cells on VMI (eTable 5 in Supplement 2). Among 6 Black women included in our analysis, all were in the low-diversity subgroup. Overall, the microbiota and metabolites were highly correlated at baseline (eFigure 4 in Supplement 2). Furthermore, α diversity and pH were highly correlated: samples with low SDI also had lower pH (eFigure 4 in Supplement 2). Median change in pH was significantly greater over 12 weeks for women in the high-diversity subgroup compared with the low-diversity subgroup (median [IQR] pH, −1.0 [−2.0 to −0.5] vs −0.3 [−1.1 to 0]; P = .007); however, the reduction in pH over 12 weeks was significant within both diversity subgroups (Figure 3B). Decreases in pH were observed in women in the high-diversity subgroup across all 3 intervention groups (Figure 3C). Moreover, similar proportions of women in the high- vs low-diversity subgroups had a shift to more than 5% superficial cells on VMI (15 of 49 women [31%] vs 12 of 45 women [27%]).
We noted strong positive correlations between changes in the microbiota and changes in metabolites across 12 weeks among women using estradiol; maximal changes occurred with 4 weeks of estradiol use (eFigure 4 in Supplement 2). Importantly, there was an orchestrated shift in the microbiota (Figure 4A) and metabolites (Figure 4B) with estradiol use from the high-diversity subgroup toward the low-diversity subgroup, while such concerted shifts were not noted with moisturizer or placebo use. Women in the low-diversity subgroup maintained these communities and metabolites across all 3 intervention groups over 12 weeks (Figure 4). Moreover, there was no difference in the proportion of women with a decrease of 2 points or more in symptom severity between high- vs low-diversity baseline subgroups (eTable 5 in Supplement 2).
Figure 4. Metabolite and Microbiota Shifts Among Women in the High- and Low-Diversity Subgroups.
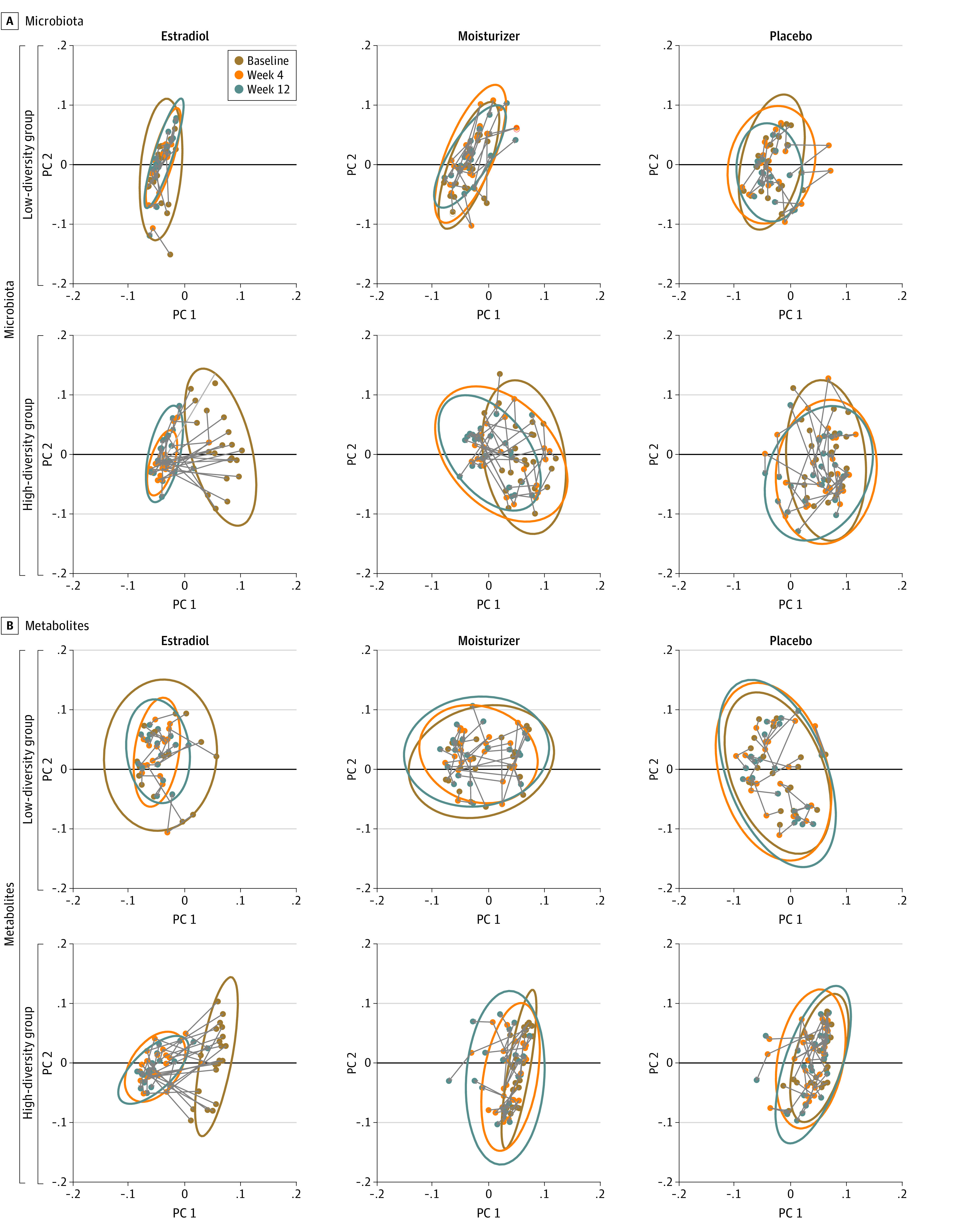
Each dot represents the vaginal bacterial community (A) or metabolic profile (B) in a single study participant and by time in study. PC indicates principal coordinates.
Impact of Serum Estradiol Levels on the Vaginal Microbiota and pH
We measured serum estradiol concentrations in the estradiol and placebo groups to determine whether overall differences or changes noted in the vaginal microenvironment were a response to circulating estradiol. We noted a significant increase in serum estradiol concentrations with estradiol treatment (median [IQR] estradiol, 4.0 [3.1-5.4] pg/mL at 12 weeks vs 3.6 [2.6-4.5] pg/mL at baseline; P = .02) but not placebo (median [IQR] estradiol, 3.1 [2.2-4.4] pg/mL at 12 weeks vs 3.1 [2.1-4.7] pg/mL at baseline; P = .48) (eFigure 5 in Supplement 2). No changes were noted across 12 weeks in estradiol concentrations in the low- or high-diversity subgroups (eFigure 5 in Supplement 2). Estradiol levels at baseline also did not vary significantly between estradiol and placebo groups (median [IQR] 3.6 [3.1-4.7] pg/mL vs 3.1 [2.1-4.7] pg/mL; P = .14), nor did estradiol levels vary between high- vs low-diversity subgroups at baseline (median [IQR], 3.6 [2.6-4.7] pg/mL vs 3.1 [2.3-4.2] pg/mL; P = .23) or 12 weeks (median [IQR], 3.4 [2.7-4.8] pg/mL vs 3.4 [2.6-5.0] pg/mL, P = .73). Interestingly, increase in serum estrogen concentration between week 12 compared with baseline (median [IQR] increase, 0.4 [−0.3 to 1.2] pg/mL) was not associated with change in SDI (median [IQR] change, –1.31 [–1.96 to –0.06]; P = .71) or pH (median [IQR] change, –1.5 [–2.0 to –1.0]; P = .27) among women using estradiol, despite significant decreases in SDI and pH in this group, suggesting that local concentrations of estradiol may play a greater role than systemic concentrations in mediating changes in the vagina.
Discussion
In this post hoc secondary analysis of participants in a randomized clinical trial of topical treatment for moderate to severe postmenopausal vaginal symptoms, we demonstrated a significantly greater impact of vaginal estradiol on the vaginal microbiota, metabolome, and pH than low-pH vaginal moisturizer or low-pH placebo gel. Although participants in all treatment groups had significant decreases in vaginal pH over 12 weeks, participants in the estradiol group had the largest decrease, associated with changes in the composition of the vaginal microbiota and metabolites. The effect of estradiol was greatest in women with a high-diversity vaginal microbial community, high pH, and low VMI at study entry and was not associated with changes in serum levels of estrogen.
Many treatment trials of postmenopausal vaginal discomfort measure clinician-driven metrics, such as visual appearance on examination, pH, or vaginal epithelial cell maturation.16,17 In a meta-analysis of randomized trials, vaginal estradiol was associated with significant improvement in these findings to a greater extent than placebo, but not necessarily women’s report of symptoms.3 Our in-depth analysis demonstrates the same disconnect: estradiol was associated with significant, profound changes in microbiota, small molecule metabolites, and pH of the vagina but did not confer a significantly greater symptom benefit than placebo.1 The placebo gel had high lubricity, which likely conferred the symptom benefit without microbiota and metabolite changes in the vaginal microenvironment.
Change in pH is 1 of 3 FDA-recommended primary outcomes for treatment trials in GSM. A significant decrease in vaginal pH has been seen in trials of vaginal estrogen,3,18 vaginal DHEA,19 vaginal laser,20 hyaluronic acid, and vaginal gels containing lactic acid.2,21,22 Our findings demonstrate that a significant drop in pH with treatment does not necessarily reflect the same underlying biological effect in all cases. In premenopausal women, pH is often linked to levels of vaginal lactic acid. The estradiol group alone had significant changes in metabolic features that contribute to low pH compared with placebo. In the popular press, advertising language touts “pH-balanced” products for women, and there have been many attempts to promote vaginal health by decreasing pH alone.23,24,25,26 However, none of the low-pH products studied in premenopausal women have decreased incidence of BV.27,28 In fact, a 2019 study by van der Veer et al26 found that use of 1 lactic acid–based douche in premenopausal women increased risk for having diverse communities of vaginal anaerobes. Our analysis shows that a decrease only in vaginal pH is insufficient to change the microbiota in postmenopausal women. Additionally, the metabolites in vaginal fluid did not shift significantly with low-pH interventions, even among women with high-diversity microbial communities, suggesting that lowering pH alone may not alter underlying microbial or host metabolic processes.
It is not surprising that participants in our trial with a high-diversity microbiota at enrollment had a greater change in diversity of the microbial community with vaginal estrogen. What is of interest is that vaginal estrogen contributed to an additional reduction in pH even among women with a low-diversity community, suggesting that estradiol facilitates an increase in metabolic activity of lactic acid–producers, such as lactobacilli and bifidobacteria. Moreover, even among women who started with high-diversity communities, there was no difference in the proportion with a large reduction in symptom severity (ie, ≥2 points) among treatment groups, confirming a lack of causal association between microbiota and postmenopausal vaginal symptom severity. In our original analysis, participants in the estradiol group were more likely to report meaningful benefit from the estradiol intervention than the other 2 groups. It is possible that the microbial and metabolic changes with estradiol use may contribute to overall well-being in ways not measured by the symptom questionnaire.
In premenopausal women, higher relative abundance of lactobacilli in the vagina is associated with lower levels of proinflammatory cytokines and chemokines.29 The inflammation associated with diverse microbes (and higher pH) is proposed as 1 contributor to increased risks for cervical dysplasia and HIV acquisition. While a few studies have demonstrated differences in vaginal fluid immune markers between premenopausal and postmenopausal women, the contribution of the vaginal microbiota or metabolites to these differences has not been evaluated.30,31 A small randomized clinical trial of a vaginal probiotic in postmenopausal women found short-term decreases in proinflammatory gene pathways with increased vaginal lactobacilli.32 Together, these data suggest that increasing vaginal lactobacilli in postmenopausal women may improve vaginal health regardless of impact on symptoms and should be an area of future investigation.
Limitations
This study has some limitations. This is a subset analysis of a limited number of trial participants; hence, our findings may not be generalizable. However, we included participants who reported greatest adherence to study intervention and thus likely reflect biological effects of adherent use. Participants were selected to represent a range of response to treatment, providing additional breadth to our analysis. We only collected samples at 3 points during the trial, so a detailed time course of the kinetics of the noted changes is not possible. Additionally, we do not know if these changes are durable over longer periods of therapy, nor whether longer-term treatment might lead to additional or different shifts in microbes or metabolites. However, our data suggest that much of the change occurred within 4 weeks. The low-pH products used in our trial achieved low pH using sorbic acid and not lactic acid, and it is possible that the 2 acids might have different effects on microbial communities. As noted in our initial report,1 our trial participants mostly identified as White, limiting generalizability. The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities.9,33
Conclusions
The findings of this secondary analysis of a randomized clinical trial suggest that a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal. The metric of lowering of vaginal pH per FDA guidance for primary efficacy analyses in clinical trials in GSM is a blunt measure.7 In the era of precision medicine, trial outcomes should reflect patient-reported outcomes and specific biological effects. The MsFLASH Vaginal Health Trial demonstrated that although the biological effects of estrogen on the vaginal microenvironment may not be linked to symptom improvement, they may reflect an important change in vaginal mucosal characteristics not observed with products that simply lower pH.
Trial Protocol and Statistical Analysis Plan
eMethods.
eFigure 1. Relative Abundance and α Diversity in the Placebo, Moisturizer and Estradiol Groups at Baseline, Week 4 and Week 12
eFigure 2. Unsupervised Hierarchical Clustering of Bacterial Communities at Baseline
eFigure 3. Unsupervised Hierarchical Clustering of Metabolic Profiles at Baseline
eFigure 4. Vaginal Microbiota and Metabolites are Highly Correlated
eFigure 5. Small but Significant Increase in Serum Estradiol Concentrations of Women Using Vaginal Estradiol
eTable 1. Comparison of Change in Individual Bacterial Taxa in the Estradiol Group vs the Placebo Group
eTable 2. Comparison of Change in Individual Bacterial Taxa in the Moisturizer vs the Placebo Group
eTable 3. Comparison of Change in Individual Metabolites in the Estradiol Group vs the Placebo Group
eTable 4. Comparison of Change in Individual Metabolites in the Moisturizer Group vs the Placebo Group
eTable 5. Demographic and Clinical Characteristics of Participants by Microbial Diversity Grouping at Baseline
Data Sharing Statement
References
- 1.Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms: a randomized clinical trial. JAMA Intern Med. 2018;178(5):681-690. doi: 10.1001/jamainternmed.2018.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YH, Park S, Lee M, Hahn S, Jeon MJ. Effect of a pH-balanced vaginal gel on dyspareunia and sexual function in breast cancer survivors who were premenopausal at diagnosis: a randomized controlled Trial. Obstet Gynecol. 2017;129(5):870-876. doi: 10.1097/AOG.0000000000001988 [DOI] [PubMed] [Google Scholar]
- 3.Biehl C, Plotsker O, Mirkin S. A systematic review of the efficacy and safety of vaginal estrogen products for the treatment of genitourinary syndrome of menopause. Menopause. 2019;26(4):431-453. doi: 10.1097/GME.0000000000001221 [DOI] [PubMed] [Google Scholar]
- 4.Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23(3):259-263. doi: 10.1016/0378-5122(95)00955-8 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Geng L, Song X, Li H, Giordan N, Liao Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10(6):1575-1584. doi: 10.1111/jsm.12125 [DOI] [PubMed] [Google Scholar]
- 6.Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril. 1994;61(1):178-180. doi: 10.1016/S0015-0282(16)56474-7 [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration . Guidance for industry: estrogen and estrogen/progestin products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms—recommendation for clinical evaluation. Updated August 2018. Accessed February 17, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estrogen-and-estrogenprogestin-drug-products-treat-vasomotor-symptoms-and-vulvar-and-vaginal-atrophy
- 8.Golob JL, Pergam SA, Srinivasan S, et al. Stool microbiota at neutrophil recovery is predictive for severe acute graft vs host disease after hematopoietic cell transplantation. Clin Infect Dis. 2017;65(12):1984-1991. doi: 10.1093/cid/cix699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan S, Chambers LC, Tapia KA, et al. Urethral microbiota in men: association of Haemophilus influenzae and Mycoplasma penetrans with nongonococcal urethritis. Clin Infect Dis. 2021;73(7):e1684-e1693. doi: 10.1093/cid/ciaa1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan S, Morgan MT, Fiedler TL, et al. Metabolic signatures of bacterial vaginosis. mBio. 2015;6(2):e00204-15. doi: 10.1128/mBio.00204-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder PJ, Bhasin S, Cunningham GR, et al. ; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624. doi: 10.1056/NEJMoa1506119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasuja GK, Travison TG, Davda M, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 2013;68(6):733-740. doi: 10.1093/gerona/gls216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egozcue J, Pawlowsky-Gahn V, Mateu-Figueras G, Barcelo-Vidal C. Isometric logratio transformations for compositional data analysis. Math Geol. 2003;35(3):279-300. doi: 10.1023/A:1023818214614 [DOI] [Google Scholar]
- 15.Liu X, Ser Z, Locasale JW. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem. 2014;86(4):2175-2184. doi: 10.1021/ac403845u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christmas MM, Song B, Bell RJ, Iliodromiti S, Mitchell C, Hickey M; International COMMA (Core Outcomes in Menopause) Consortium . Variation in outcome reporting and measurement tools in clinical trials of treatments for genitourinary symptoms in peri- and postmenopausal women: a systematic review. Menopause. 2020;27(9):1070-1080. doi: 10.1097/GME.0000000000001570 [DOI] [PubMed] [Google Scholar]
- 17.Lima SMRR, Honorato JV. Critical analysis of methods for assessing genitourinary syndrome of menopause used in clinical trials. Menopause. 2019;26(12):1436-1442. doi: 10.1097/GME.0000000000001406 [DOI] [PubMed] [Google Scholar]
- 18.Galhardo CL, Soares JM Jr, Simões RS, Haidar MA, Rodrigues de Lima G, Baracat EC. Estrogen effects on the vaginal pH, flora and cytology in late postmenopause after a long period without hormone therapy. Clin Exp Obstet Gynecol. 2006;33(2):85-89. [PubMed] [Google Scholar]
- 19.Labrie F, Archer DF, Koltun W, et al. ; VVA Prasterone Research Group . Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243-256. doi: 10.1097/GME.0000000000000571 [DOI] [PubMed] [Google Scholar]
- 20.Politano CA, Costa-Paiva L, Aguiar LB, Machado HC, Baccaro LF. Fractional CO2 laser versus promestriene and lubricant in genitourinary syndrome of menopause: a randomized clinical trial. Menopause. 2019;26(8):833-840. doi: 10.1097/GME.0000000000001333 [DOI] [PubMed] [Google Scholar]
- 21.Buzzaccarini G, Marin L, Noventa M, et al. Hyaluronic acid in vulvar and vaginal administration: evidence from a literature systematic review. Climacteric. 2021;24(6):560-571. doi: 10.1080/13697137.2021.1898580 [DOI] [PubMed] [Google Scholar]
- 22.Nappi RE, Kotek M, Brešt’anský A, Giordan N, Beriotto I, Tramentozzi E. Treatment of vulvo-vaginal atrophy with hyaluronate-based gel: a randomized controlled study. Minerva Obstet Gynecol. 2021. doi: 10.23736/S2724-606X.21.04841-7 [DOI] [PubMed] [Google Scholar]
- 23.Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19(2):151-161. doi: 10.3109/13697137.2015.1124259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simoes JA, Bahamondes LG, Camargo RP, et al. A pilot clinical trial comparing an acid-buffering formulation (ACIDFORM gel) with metronidazole gel for the treatment of symptomatic bacterial vaginosis. Br J Clin Pharmacol. 2006;61(2):211-217. doi: 10.1111/j.1365-2125.2005.02550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasnopolsky VN, Prilepskaya VN, Polatti F, et al. Efficacy of vitamin C vaginal tablets as prophylaxis for recurrent bacterial vaginosis: a randomised, double-blind, placebo-controlled clinical trial. J Clin Med Res. 2013;5(4):309-315. doi: 10.4021/jocmr1489w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Veer C, Bruisten SM, van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19(1):168. doi: 10.1186/s12866-019-1545-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holley RL, Richter HE, Varner RE, Pair L, Schwebke JR. A randomized, double-blind clinical trial of vaginal acidification versus placebo for the treatment of symptomatic bacterial vaginosis. Sex Transm Dis. 2004;31(4):236-238. doi: 10.1097/01.OLQ.0000118423.20985.E7 [DOI] [PubMed] [Google Scholar]
- 28.Petersen EE, Magnani P. Efficacy and safety of vitamin C vaginal tablets in the treatment of non-specific vaginitis: a randomised, double blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2004;117(1):70-75. doi: 10.1016/j.ejogrb.2004.02.032 [DOI] [PubMed] [Google Scholar]
- 29.Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965-976. doi: 10.1016/j.immuni.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chappell CA, Isaacs CE, Xu W, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015;213(2):204.e1-204.e6. doi: 10.1016/j.ajog.2015.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy K, Keller MJ, Anastos K, et al. Impact of reproductive aging on the vaginal microbiome and soluble immune mediators in women living with and at-risk for HIV infection. PLoS One. 2019;14(4):e0216049. doi: 10.1371/journal.pone.0216049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisanz JE, Enos MK, Mwanga JR, et al. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio. 2014;5(5):e01580-e14. doi: 10.1128/mBio.01580-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(suppl 1):4680-4687. doi: 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods.
eFigure 1. Relative Abundance and α Diversity in the Placebo, Moisturizer and Estradiol Groups at Baseline, Week 4 and Week 12
eFigure 2. Unsupervised Hierarchical Clustering of Bacterial Communities at Baseline
eFigure 3. Unsupervised Hierarchical Clustering of Metabolic Profiles at Baseline
eFigure 4. Vaginal Microbiota and Metabolites are Highly Correlated
eFigure 5. Small but Significant Increase in Serum Estradiol Concentrations of Women Using Vaginal Estradiol
eTable 1. Comparison of Change in Individual Bacterial Taxa in the Estradiol Group vs the Placebo Group
eTable 2. Comparison of Change in Individual Bacterial Taxa in the Moisturizer vs the Placebo Group
eTable 3. Comparison of Change in Individual Metabolites in the Estradiol Group vs the Placebo Group
eTable 4. Comparison of Change in Individual Metabolites in the Moisturizer Group vs the Placebo Group
eTable 5. Demographic and Clinical Characteristics of Participants by Microbial Diversity Grouping at Baseline
Data Sharing Statement