Abstract
Platelet-leukocyte crosstalk is commonly manifested by reciprocal links between thrombosis and inflammation. Platelet thrombus acts as a reactive matrix that recruits leukocytes to the injury site where their massive accumulation, activation and migration promote thrombotic events while triggering inflammatory responses. As a life-threatening condition with the associations between inflammation and thrombosis, COVID-19 presents diffuse alveolar damage due to exaggerated macrophage activity and cytokine storms. These events, together with direct intracellular virus invasion lead to pulmonary vascular endothelialitis, cell membranes disruption, severe endothelial injury, and thrombosis. The developing pre-alveolar thrombus provides a hyper-reactive milieu that recruits circulating leukocytes to the injury site where their activation contributes to thrombus stabilization and thrombosis propagation, primarily through the formation of Neutrophil extracellular trap (NET). NET fragments can also circulate and deposit in further distance where they may disseminate intravascular thrombosis in severe cases of disease. Thrombi may also facilitate leukocytes migration into alveoli where their accumulation and activation exacerbate cytokine storms and tissue damage, further complicating the disease. Based on these mechanisms, whether an effective anti-inflammatory protocol can prevent thrombotic events, or on the other hand; efficient antiplatelet or anticoagulant regimens may be associated with reduced cytokine storms and tissue damage, is now of interests for several ongoing researches. Thus shedding more light on platelet-leukocyte crosstalk, the review presented here discusses the detailed mechanisms by which platelets may contribute to the pathogenesis of COVID-19, especially in severe cases where their interaction with leukocytes can intensify both inflammatory state and thrombosis in a reciprocal manner.
Keywords: Antiplatelet drugs, Anti-inflammatory agents, COVID-19, Cytokine storms, Damage-associated molecular pattern molecules, Leukocyte Migration, NETs, Platelets, Thrombosis
1. Introduction
As a serious challenge for health providers around the globe, rapidly spreading coronavirus disease 2019 (COVID-19) has been considered the most devastating pandemic in current decades, with no definitive medication or therapeutic protocol. The causative agent of the disease, called SARS-CoV-2, is a highly contagious respiratory virus that can disrupt the immune system in various ways. In patients, depending on the adequacy and ability of the immune response, different levels of the disease are observed, ranging from a mild cold-like illness to severe cases of acute respiratory distress syndrome (ARDS) with multi-organ failure (MOF) and even death [1]. Accordingly, patients are classified into four groups based on their clinical symptoms and disease severity, including mild, moderate, severe, and critical status [2], [3]. Most patients present with mild to moderate symptoms (81%), whereas the severe and critical cases, which often require hospitalization, account for only 14% and 5% of all symptomatic patients, respectively [4]. About 20% of hospitalized patients may also need to be admitted to the intensive care unit (ICU) [5], which unfortunately has a mortality rate of more than 50% [6]. According to studies, one of the most critical factors associated with disease severity and worsening the patient's clinical condition that leads to hospitalization in the ICU is an intense immune response known as Cytokine Storm Syndrome (CSS) [1], [7], [8], [9]. CSS is a systemic inflammatory response characterized by the over-activation of alveolar macrophages leading to the overproduction of inflammatory cytokines and chemical mediators by immune cells [10], a condition that can lead to multi-organ failure (MOF) if left untreated [11], [12], [13], [14], [15].
Upon exposure, the SARS-CoV-2 interacts with the respiratory target cells via Angiotensin-converting enzyme 2 (ACE2). Mechanistically, here type II alveolar pneumocytes and endothelial cells lining blood vessels that express high levels of ACE2 are primarily involved with SARS-CoV-2, triggering the first rapid pro-inflammatory responses in the lower respiratory tract. In fact, type II pneumocytes are epithelial immune cells that also express high levels of Toll like receptor-7 (TLR7) and TLR8, which turn them into specialized virus responders. When these receptors interact with viruses or viral RNA, pneumocytes become activated and produce large amounts of pro-inflammatory cytokines and chemokines, including interferon-β (IFN-β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β, monocyte chemo-attractant protein-1 (MCP-1), IL-8, granulocyte-monocyte colony-stimulating factor (GM-CSF), and IL-12 [16], [17]. On the other hand, when the virus enters endothelial cells, the ACE2 receptor is down-regulated, and thus imbalancing ACE/ACE2 ratio leads to the amplification of TNF-α and soluble IL-6 receptors [18]. These events associated with pneumocytes' cytokines release eventually activate other hyper-inflammatory signals, which cause neutrophils, monocytes, and T cells infiltration into lung tissue [19], [20], [21], [22]. Most importantly, the chemokine slope induced by MCP-1 and IL-8 forces monocytes/macrophages and pro-inflammatory neutrophils to chemotaxis in the lungs. In their path, they accumulate near the capillary bed, from where they migrate to the alveolar space and play a role in cytokine production, microthrombi formation, and cell damage [1], [23], [24], [25]. In such a milieu, T-helper type 1 (Th1) lymphocytes promote the proliferation of inflammatory monocytes and macrophages by secreting large amounts of IFN-γ, and GM-CSF while on the other side, considerable production of IL-6, TNF-α, and other cytokines by activated CD14 + CD16+ inflammatory macrophages contribute to cytokine storms (Fig. 1 ) [26]. In addition, the accumulation of these hyper-activated macrophages in close proximity of capillary bed with the massive release of cytotoxic materials, including reactive oxygen species (ROS), can aggravate pulmonary vascular endothelialitis, which is already triggered by SARS-CoV-2-induced direct damages on endothelial cells [27], [28], [29]. Once an injury occurs, subsequent exposure of the sub-endothelial matrix to circulation establishes platelet aggregation and thrombus formation linking inflammatory and thrombotic events. On the other hand, cytokine storms per se may enhance platelet activation state, further potentiating thrombotic events. Platelets activated on thrombi can also recruit leukocytes to the injury site, a cytokine milieu in which neutrophils are highly stimulated by reacting with platelets and eventually releasing their chromatin as neutrophil extracellular traps (NETs) [1], [30], [31], [32]. These highly pro-coagulant materials propagate thrombosis, while the release of circulating NETs and their deposition in distant inflamed vessels may cause disseminated intravascular thrombosis (DIC), as already seen in severe cases of COVID-19 patients with evident CSS [33] (Fig. 1). These are the mechanisms that can associate COVID-19 with thrombosis and thromboembolism during a cytokine storm, a serious complication that is considered an important risk factor for predicting a poor prognosis.
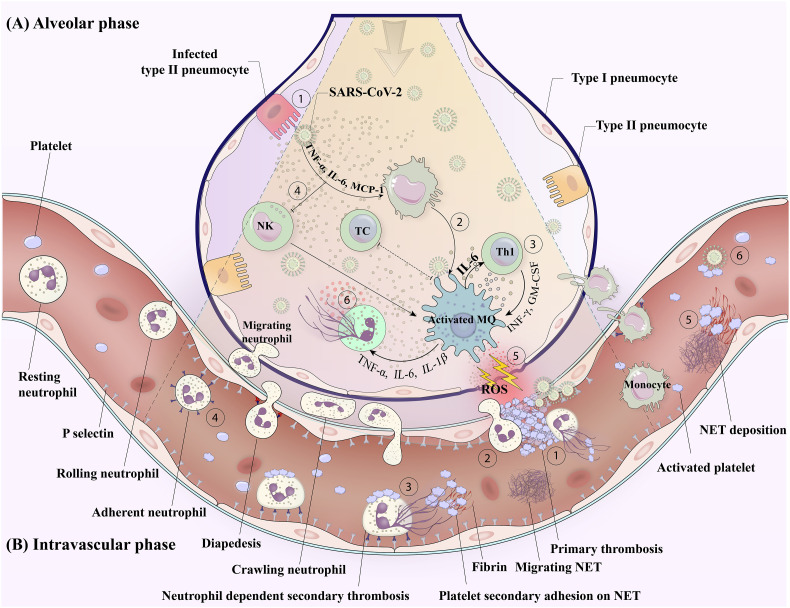
Fig. 1.
Platelet leukocyte crosstalk in COVID-19 a mutual link between thrombosis and inflammation.
A) Alveolar phase: SARS-CoV-2 invasion to type II peneumocyte induces cytokine release (1). These cytokines and the direct interaction of the virus with tissue-resident monocyte/macrophage turn them into a hyper-inflammatory phenotype with exaggerated activity that contributes to cytokine storms and tissue damage (2). The crosstalk between activated macrophage and other immune cells supports a robust “cytokine storm syndrome” in severe case of disease (3). On the one hand, under the influence of such an inflammatory milieu, exhausted NK cells (4) fail to eradicate infected macrophage, while on the other hand, the infected cells that express lower MCH class I, also evade from recognition by cytotoxic T cells. Under such a condition, hyper-activated macrophages freely contribute to tissue damage and vascular injury either by releasing ROS (5) and other toxic agents or by inducing NETs formation (6). These are events that link COVID-19-induced inflammatory responses to vascular thrombotic events.
B) Intravascular phase: Endothelitis due to direct invasion of SARS-CoV-2 or indirect damage by macrophages and hyperinflammatory condition may cause injury in adjacent alveolar vessels where the platelets recruitment creates a reactive thrombus attracting leukocytes (1). The recruited leukocytes may migrate to the alveolar tissue through the thrombus (2), or release the extracellular traps in a low shear pocket that propagate thrombosis (3). Cytokine milieu orchestrated by hyper-inflamed or damaged alveolar tissue, may also urge adjacent endothelium to express inflammatory molecules and receptors, which recruit circulating leukocytes (4). Produced NETs on endothelium or thrombi may be released from their original site and disseminated while their deposition at greater distances can create secondary foci of thrombosis (5). In severe condition of disease, blood-borne viruses may also directly affect circular platelets and leucocytes while causing their activation (6). Abbreviations: Th1: T-helper cell type 1; NK: natural killer cell; TC: cytotoxic T cell; MQ: macrophage; NET: neutrophil extracellular trap; ROS: reactive oxygen species.
Notwithstanding the foregoing, whether platelet activation state and thrombotic events can also feedback on cytokine storms, which exacerbate the disease severity and complexity, is another important topic to be discussed here. This hypothesis is based on the fact that as an important source, activated platelets release vast arrays of cytokines, chemokine, and chemo-attractants that can not only quantitatively raise cytokine levels but can also develop the condition of CSS by the increasing effects on other immune cells involved in cytokine production. On the other hand, as depicted in Fig. 1, the fact that alveolar thrombosis can serve as a gateway for a wide range of leukocytes to migrate to the lung tissue and thus actively accumulate in the center of the infection is also important, as this increased load of inflammatory cells may also play a significant role in worsening the prognosis of the disease.
Therefore, due to the importance of thrombotic events in developing poor clinical outcomes that can lead to increased mortality in complicated cases of COVID-19, the review presented here focuses on different mechanisms by which mutual interaction of platelet and leukocyte involves exacerbating cytokine storms, worsening thrombotic and inflammatory condition.
2. Mechanisms by which SARS-CoV-2 can cause endothelial injury leading to thrombo-inflammation
Histological analysis has demonstrated endothelial apoptosis in SARS-CoV-2 infection. This is also confirmed by autopsy case studies that showed the association of pulmonary endothelial injuries with intracellular virus penetration leading to endothelium disruption in COVID patients [34], [35]. These evidences support that the direct invasion of SARS-CoV-2 can cause endothelial injury in patients leading to diffuse pre-alveolar thrombosis as shown by several groups [36]. However, in severe cases of disease the widespread endothelial dysfunction and endotheliitis may also cause leukocyte recruitment which triggers inflammatory responses that can be associated with more generalized micro-thrombotic complications including deep vein thrombosis (DVT), pulmonary embolism (PE) and stroke [37]. In addition, the co-localization SARS-CoV-2-specific antigens with different components of complements (including C4d and C5b-9) within the thrombosed arteries also highlights the key role of virus-dependent activation of this cascade in the induction of endothelial injury and its subsequent thrombosis [38]. These are in addition to the indirect involvement of SARS-CoV-2 infection in thrombosis which is mediated by cytokine milieu in infected alveoli that attracts leukocytes to infiltrate in close proximity of surrounding vascular beds where the leukocytes, especially agitated macrophages [39] and neutrophils [40] damage blood lining integrity through their proteolytic released materials, ROS generation and complement activation while progressing the endothelial injury and thrombosis. More specifically, in an experimental model, the infection of human umbilical vein endothelial cells (HUVECs) with SARS-CoV-2 promoted mitochondrial dysfunction which manifested by increased levels of superoxide anion, mitochondrial membrane potential, and mitochondrial DNA (mtDNA) release as the major signals that trigger activation of TLR9 and NF-κB leading to endothelial cell dysfunction, inflammatory responses and cytokines releases. Notably, this study also showed the extracellular release of mtDNA by infected endothelial cells, an important DAMP that plays a key role in disseminating inflammatory immune response and cytokine release by TLR9-dependent mechanisms. On the other hand, in this model, SARS-CoV-2 infection was also associated with decreased levels of nitric oxide synthase (eNOS) expression [41] as key enzyme that generates NO to maintain the quiescent state of endothelium with anti-inflammatory, antithrombotic and antioxidant function [42]. Therefore, the diminished level of NO induced by SARS-CoV-2 invasion can aggravate endothelial lesion. Most recently, Robles et al. has shown that the interaction of spike protein through its RGD motif with integrin ⍺5β1 expressed on endothelial cells can also activate its downstream NF-κB target gene expression programs responsible for vascular leakage and leukocyte adhesion [43]. This is another way by which SARS-CoV-2 directly induces endothelial cell dysfunction leading to vascular inflammation and thrombotic events in COVID-19.
3. Mechanisms by which SARS-CoV-2 propagates thrombosis through the induction of platelets activation
3.1. Direct mechanism
3.1.1. Virus interaction with platelets and its possible effect on modulating of thrombotic events
It has been recently discovered that during infections, platelets play an active role in immune responses against various pathogens. It is believed that when a virus enters the bloodstream, abundant platelets can be at the forefront of neutralizing viral particles and initiating primary immune responses [44]. So far, several lines of evidence have shown that some surface molecules, including integrin αIIb/β3, collagen receptor glycoprotein (GP) VI, complement receptor 2 (CR2), TLRs, lectins such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) or C-type lectin-domain family-2 (CLEC-2), as well as several chemokine receptors can mediate direct binding of platelets to viruses including adenovirus, influenza, HIV and dengue virus [45], [46]. The interaction of SARS-CoV-2 with platelets is also thought to control platelet-mediated immunity while increasing the risk of thrombotic events and coagulation dysfunction in COVID-19 [47]. It is noteworthy that since platelets are not naturally leave bloodstream, this direct interaction can occur only in severe cases of disease associated with the viremia, a condition that directly exposes SARS-CoV-2 to circulating platelets [31], [48]. As depicted in Fig. 2 , the following are the main molecules that have been proposed as SARS-CoV-2 platelet receptors.
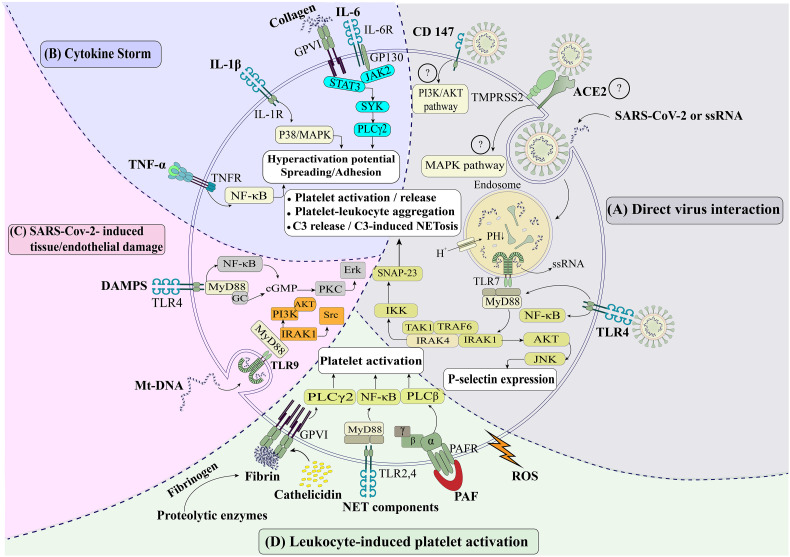
Fig. 2.
Different Pathways of platelet activation in COVID-19.
A) Direct interaction of virus with platelets: The SARS-CoV-2 - may engage with platelet receptors such as ACE2 and CD147 and activate various intracellular signaling pathways. Platelets also endocytose SARS-CoV-2 or its RNA, creating an inflammasome in which the AKT and P38-MAPK pathways are activated when SARS-CoV-2 RNA interacts with TLR7. Followed by TLR7 engagement with virus RNA, an adaptor protein, MYD88, activates other adaptor proteins such as IRAK1, IRAK4, TRAF6 (that activate AKT and P38-MAPK pathways), and TAK1, IKK, SNAP-23 (that lead to C3 release and C3-induced NETosis). Generally, these activating pathways lead to enhanced expression of P-selectin and CD40L, increased platelet-leukocyte interactions and PLAs formation. B). The effect of cytokine storms on platelets: Overexpression of inflammatory cytokines such as IL-6, IL-1, and TNF-α may activate jak2/STAT3 and its cross-talk with GPVI/collagen signaling, P38/MAPK, and NF-κB, respectively, leading to platelet hyperactivation. C) SARS-CoV-2-induced tissue/endothelial damage: DAMPs and mt-DNA released by damaged tissues and endothelial cells are sensed with TLR4 and TLR9 and activate NF-κB/PKC-ERK and IRAK1/PI3K-AKT respectively, leading to platelet activation D). Leukocyte-induced platelet activation: Leukocytes may activate platelets through their released mediators. Here, in a conserved milieu, leucocyte derived NET components and cathelicidin (interacting with TLR2, 4, and GPVI, respectively), chemokines/chemoattractants (particularly PAF interacting with GPCRs) as well as ROS and proteolytic enzymes (that induce the generation of fibrin interacting with GPVI), promote platelet activation and pro-coagulant function.
Abbreviations: ACE2: Angiotensin-converting enzyme-2; AKT: protein kinase B; GPCRs: G protein–coupled receptors; IKK: IκB kinase; IRAK: Interleukin-1 receptor-associated kinase; JAK: Janus kinase; JNK: c-Jun N-terminal kinase (a major signaling cassettes of MAPK signaling pathway); MAPK: mitogen-activated protein kinase (MAPK) MYD88: Myeloid differentiation primary response 88; NET: neutrophil extracellular trap; NF-κB: nuclear factor-kB; PAF: platelet activating factor; PLA: platelet-leukocyte aggregations; PLC: phospholipase C; PKC: Protein kinase C; ROS: reactive oxygen species; SNAP-23: Synaptosomal-associated protein 23; Src: sarcoma virus tyrosine kinase; SYK: spleen tyrosine kinase; TAK: transforming growth factor-β-activated kinase; TLR: toll like receptor; TNF: tumor necrosis factor; TRAF: Tumor necrosis factor receptor–associated factor.
3.1.1.1. Angiotensin-converting enzyme 2 (ACE2)
So far, ACE2 has been identified as a potential receptor for SARS-CoV-2 binding and entry into cells. In addition to ACE2, transmembrane serine protease-2 (TMPRSS2) is also required to facilitate virus entry into the cells (Fig. 2, section A). In fact, this enzyme cleaves the spike protein (S) on SARS-CoV-2 to allow viral and cell membranes to be fused well [49]. However, the expression of ACE2 and TMPRSS2 in platelets and megakaryocytes has not yet been fully elucidated. Since earlier studies found no evidence of ACE2 or TMPRSS2 expression in human platelets [47], [50], [51], [52], it has been suggested that SARS-CoV-2 may react with platelets through ACE2-independent mechanisms to possibly enter platelets. Nevertheless, a recent challenging discovery by Zhang et al. that indicates the expression of ACE2 and TMPRSS2 on platelets may change this theory [31].
3.1.1.2. CD147
In another scenario, CD147 was also introduced as an alternative receptor for SARS-CoV [53], SARS-CoV-2 [54], [55], HIV-1 and measles viruses to enter the cell [56], [57]. This is interesting because in people more vulnerable to COVID-19, including men (compared to women) or patients with asthma, chronic obstructive pulmonary disease and obesity, the expression of CD147 is higher, which may indicate a specific role for this molecule in virus infectivity [58]. CD147 or extracellular matrix metalloproteinase inducer (EMMPRIN) is a glycoprotein from the immunoglobulin superfamily which is widely expressed on blood cells while also existing as soluble biologically active molecule [58], [59]. CD147 homotypic interactions play different roles in normal development and pathological conditions including viral infections, Alzheimer's disease, cancer, and ischemia [60]. RNA-seq and proteomic analysis revealed that CD147 is highly expressed on platelets (approximately 2000 copies per cell) [61], [62] and can potentially interacts with GPVI, the central collagen receptor [63]. CD147 is generally recognized as a pro-inflammatory and pre-thrombotic molecule in the context of thrombo-inflammation, which enhances platelet activation and adhesion, leukocyte chemotaxis, and subsequent thrombus formation through the binding of multiple molecules [64]. Activated platelets express CD147 while its homotypic interactions induce platelet degranulation and release. On the other hand, platelet-expressed CD147 can activate nuclear factor κB (NF-κB) pathway in monocytes, resulting in the generation of metallo-matrix protease (MMP), specially MMP-9, and cytokines release (including IL-6, TNF-α, and IL-10), which can mutually enhance atherosclerosis and inflammation following monocyte–platelet interaction [59]. In vivo, CD147 improves platelet–monocyte interaction and promotes monocyte recruitment to the vascular wall [65]. Thus, taking into account the role of this molecule in SARS-CoV-2-induced platelet activation, Zhang et al. have suggested that this virus can increase P-selectin expression, CD40L release, and platelet aggregation through direct interaction between its spike protein and CD147 [55], [59], [63]. This research is in line with other studies suggesting that the PI3K/AKT signaling downstream of CD147 could be considered as a possible pathway of platelet activation by SARS-CoV-2 [66], [67].
3.1.1.3. Glucose-regulated protein 78
Glucose-regulated protein 78 (GRP78) is another platelet surface molecule that, due to its ability to bind to spike protein [47], may be involved in platelet contamination by SARS-CoV-2 [68], [69], [70], [71], [72]. This molecule contributes to critical protein folding in the endoplasmic reticulum (ER), from where it is translocated to the cell surface under pathophysiological conditions. Surface GRP78 functions as a co-receptor for viral entry while also interacting with numerous signaling molecules [73], [74], [75]. Previous research has shown that when the ER is stressed by viral infections, GRP78 overexpression and its subsequent internalization may be involved in the entry of viruses (such as Bat Coronavirus, MERS-CoV, Ebola Virus, Dengue Virus, Japanese Encephalitis Virus, Influenza Virus, and Zika Virus) into the host cell. Notably, both GRP78 expression and its serum levels have been shown to increase significantly during SARS-CoV infection [76], [77], [78], [79].
3.1.1.4. Toll-like receptors (TLRs)
TLRs are other important receptors that have been shown to interact with pathogen-associated molecular patterns (PAMPs) including viral antigens and their genomic materials. So far, different types of TLRs including TLR1, 2, 3, 4, 6, 7, and 9 have been reported to be functionally active in platelets, of which TLR4 is the best characterized surface PRRs on platelets and mainly involved in the interaction with gram-negative bacteria and their released components as well as in sepsis-dependent thrombosis [80]. In this regards, activation of TLR4 on platelets by PAMPs (sepsis, viraemia and LPS) induces a prothrombotic and proinflammatory state [81], which provides a potential explanation for the thrombotic events observed in COVID-19 patients. Especially, since computationally the spike protein binds more strongly to TLR4 than ACE2 [82], and also if COVID-19 has a secondary bacterial infection (which accounted for 10% of ICU patients in one study [1], TLR4 may react strongly with substances released from gram-negative bacteria, especially LPS, which may exacerbate thrombosis in these patients [83].
In addition to platelet-virus interaction, which occurs through surface receptors binding to antigens, platelets also express endosomal TLRs which can react with viral genomic materials including free RNA or DNA [84]. In platelets, endosomal TLR7 and 9 can sense ssRNA (single-stranded RNA) viruses and unmethylated CpG DNA, respectively [85]. In human platelets, the primary response to viral single-stranded RNA (ssRNA) is often mediated by the interaction of TLRs with endocytosed virions or their genomic materials. Although platelets can directly endocytose free ssRNA, internalization of virus-carrying endolysosomes and acidic pH also degrade endocytosed virions so that their ssRNA can be delivered to TLR7 [44], [84], [86]. Followed by ligand interaction, TLR7 signaling activates the protein kinase B/AKT via a MyD88-, Akt-, IRAK4-, IKK-involved pathway [84] as well as P38-mitogen-activated protein kinase (P38-MAPK) pathways, which results in the release of platelet α-granules, increased expression of P-selectin and CD40L, complement C3 release, platelets-mediated neutrophil DNA release, and platelet-leukocyte aggregations (PLAs) formation in patients [44], [86]. The limited presence of SARS-CoV-2 RNA in the blood circulation has been reported in some patients with COVID-19 [87]. Like other viruses, several lines of evidence have shown that the TLR3, TLR7, and TLR8 receptors can also sense the SARS-CoV-2 viral ss-RNA [88]. This is important evidence that suggests similar pathways for platelet activation induced by the interaction of SARS-CoV-2 genomic material and TLRs. Fig. 2, section A has depicted how TLR4 and TLR7 sense intact SARS-CoV-2 and its ssRNA respectively, while activating platelets through down-stream signaling pathways.
In this regard, the MAPK cascade is an important platelet signaling pathway which is significantly affected by COVID-19 [50]. The role of MAPK in platelet activation and thrombosis is well established. Manne et al. showed upregulated phosphorylation of ERK1/2, P38, eIF4E, and JNK of MAPK pathway in platelets obtained from patients with severe COVID-19 hospitalized in ICU, indicating extreme activation of the this signaling pathways in COVID-19 [31], [50]. Furthermore, it has been suggested that MAPK signaling induces thromboxane production in COVID-19 patients by phosphorylation and activation of cytoplasmic phospholipase A2 as a key enzyme which regulates thromboxane production [89]. In addition, it seems that increased MAPK activation in COVID-19 can also be due to elevated the Janus kinase-3 (JAK3) activity, which is known to act upstream of the MAPK pathway. Increased phosphorylation of other enzymes in platelets such as protein kinase C-δ (PKC-δ) has also been reported in COVID-19, which may highlight the important role of other signaling pathways in the induction of platelet activity [52].
3.2. Indirect mechanisms
3.2.1. The effects of CSS syndrome on platelet activation state and thrombotic events
In severe cases of infectious disease including COVID-19, an over-activation of inflammatory immune response, mainly described as “macrophage activation syndrome”, induces unleashed cytokine storms which result in subsequent immune exhaustion leading to severe clinical manifestations of diseases [90]. In COVID-19, CSS is an acute systemic inflammatory condition characterized by fever and multi-organ dysfunction [15]. Over-expression of MCP- 1, IFN- γ, IL-6, TNF-α, GM-CSF, and IL-1β have been identified in patients with a severe COVID-19 (ICU patients) [1], [31], [90], [91], [92], [93], [94]. This exaggerated inflammatory response also dramatically activates Th1 cells which are involved in subsequent specific immune reaction [1], [95].
In such a condition, inflammatory cytokines produced in large quantities by pneumocytes, endothelial cells, and leukocytes may act as potent triggers for platelet activation [31]. This is somewhat supported by the simultaneous observation of activated platelets (manifested with increased P-selectin and CD63 expression) and cytokine storms in patients with severe COVID-19 (ICU patients), especially since even in in vitro, the plasma of these patients can activate the platelets of healthy individuals [30]. In this regard, from mechanistic point of view, IL-6 appears to be a key cytokine driving platelet hyper-reactivity in COVID-19 [19]. Two molecules carry out IL-6 biological activities include the IL-6 receptor (IL-6R) and the membrane-bound β-receptor glycoprotein 130 (gp130) [96], [97], [98]. Platelets express gp130 on their membranes at rest condition and the platelet-derived IL-6 trans-signaling has suggested to be involved in both thrombogenicity [99] and inflammatory responses within a damaged vessel [100]. It has been also shown that IL-6 can potentiate GPVI-dependent platelet activation where IL-6 treated platelets demonstrated higher levels of convulxin-induced P–selectin expression and αIIbβ3 activation than those of vehicle controls. Fig. 2, section B depicted the cross-activations between IL-6, GP130, Janus tyrosine kinase 2 (JAK2), signal transducer and activator of transcription 3 (STAT3) and collagen receptor GPVI, that potentiate platelet activation through the Syk/phospholipaseCγ2 (PLCγ2) pathways [101], [102].
IL1-β is another key cytokine produced during COVID-19, and has been shown to activate platelets. IL-1β increases platelet activation and stimulates inflammatory platelet function. On IL1-β stimulation, the induction of p38/MAPK signaling pathway induces platelet activation and its pro-inflammatory function that contributes to the development of thrombosis. IL1-β also stimulates and increases platelet adhesion to different matrices [103].
Elevated TNF-α level may also regulate thrombotic events via megakaryocyte reprogramming and platelet activation. TNF-α causes molecular and metabolic changes in megakaryocyte and platelets respectively. Exposure to TNF-α promotes platelet hyperreactivity and increased mitochondrial mass. Thereby, affecting resident megakaryocytes in the bone marrow, the increased TNF-α level may significantly induce megakaryopoiesis and thrombopoiesis, leading to the production of hyper-reactive platelets [104]. This is evidence that can partly attribute the thrombogenic capacity of COVID-19 to the observed increase in TNF-α production during SARS-CoV-2 infection. Besides, a cohort study by Jorge et al. has also showed a direct correlation between elevated levels of IL-8 and increased severity of COVID-19 [105]. This is in line with some previous evidence that IL-8 induces platelet activity, which can lead to pro-coagulant function [106]. However, a direct association between elevated levels of this cytokine and the risk of thrombotic complications in COVID-19 patients has not yet been reported. Fig. 2, section B summarized different signaling pathways by which IL1-β and TNF-α can modulate platelet functional activity including adhesive capabilities [107], [108].
3.2.2. The effects of damage-associated molecular patterns (DAMPs) on platelet activation state and thrombotic events
During infections, platelets in addition to recognizing pathogen-associated molecular patterns (PAMPs), also sense various components derived from infected tissue, known as damage-associated molecular patterns (DAMPs), by pattern recognition receptors (PRRs) [85]. DAMPs include a range of different molecules with various properties, from proteins, enzymes and genomic materials, etc., the most studied of which are high mobility group box 1 (HMGB1), heat shock proteins (HSPs) and mitochondrial DNA (mt-DNA) released from dying or lytic cells during host tissue injury or viral infection [83], [109]. Platelets express different families of PRRs including TLRs (Toll-like receptors), CLRs (C-type lectin receptors), and NLRs (NOD [nucleotide-binding oligomerization domain]-like receptors) of which C-type lectin receptors DC-SIGN (dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin) and CLEC-2 (C-type lectin-like receptor 2) are mostly involved in the recognition of DAMPs [85]. However, in COVID-19, among all PRRs, TLR4 interaction with DAMPs and its down-stream activating signals into platelets (possibly through NF-κB or PKC/ERK activation [80], is suggested to be the most prominent route for induction of thrombotic complications manifested as myocardial infarction and embolism [83]. On the other hand, from endosomal platelet TLRs, TLR9 which typically senses dsDNA from bacteria or viruses can also recognize and interact with mt-DNA as an impotent DAMP molecule while activating platelets mainly through NF-κB [110] or signaling via a MyD88-, Akt-, IRAK4-, IKK-involved pathway [84]. Therefore, since SARS-CoV-2 invasion, especially to endothelial cells, causes cell damage and mt-DNA release [41], in COVID-19 patients these DAMP molecules may also play an important role in platelet activation and thrombosis. Fig. 2, section C summarized different pathways by which DAMPs associated with SARS-CoV-2 tissue damage may activate platelets and play a role in thrombotic events.
4. Leukocyte-dependent mechanisms of platelet activation in COVID-19
4.1. Neutrophil activation by SARS-CoV-2 and its direct effect on platelet function
4.1.1. Neutrophil activation in COVID-19
As key players in innate immunity, neutrophils are the first cells to be recruited in the infected area. The pathophysiology of severe forms of COVID-19 is associated with changes in the abundance, phenotype, and function of neutrophils. Following SARS-CoV-2 infection, induction of high levels of granulocyte-colony stimulating factor (G-CSF) by stimulation of emergency granulopoiesis [111] increases neutrophils in the bloodstream and nasopharyngeal area [112] and eventually spreads these cells through the lungs [113]. An increase in blood neutrophil count is also considered as a clinical symptom of COVID-19 [114], [115], [116]. In COVID-19 patients, neutrophilia predicts poor outcomes, and therefore an increased neutrophil to lymphocyte ratio (NLR) can be an independent risk factor for severe disease [31], [92]. In response to cytokine storms following SARS-CoV-2 infection, circulating neutrophils are activated, resulting in a complex set of receptors and adhesion molecules expressed on their surface [117]. Chemotactic gradient induced by the chemokine (C-X-C motif) ligand 8 (CXCL8 [IL-8]), CXCL1 (Gro-α), CXCL2 (Gro-β), chemokine (C—C motif) ligand 3 (CCL3 [MIP-1-α]), and CCL2 (MCP1) drive circulating CXCR1 + CXCR2+ neutrophils migration into the lungs and perhaps other tissues [118], [119], [120]. Binding of these chemokines not only directs the distribution of neutrophils but also activates them [121]. In response to gradient, rolling neutrophils may leave the vessels and migrate into the infected tissue through interaction with inflamed endothelium expressing P-selectin and other inflammatory molecules [105], the endothelium itself being affected either by direct infection or cytokines storm (Fig. 1) [19]. Although these neutrophils mainly contribute to cellular crosstalk and immunity by their released pro-inflammatory chemokines/cytokines, these tissue resident fully activated cells also release ROS [122], different range of proteolytic enzymes and other proteins with antimicrobial and inflammatory functions that, in addition to their antiviral effects, can cause significant damage to the lungs, arteries and other organs [111]. Viruses and pro-inflammatory cytokines such as TNF-α and IL-8 can also drive neutrophils to release NETs [123], [124], [125]. NETs are composed of extracellular DNA fibers that contain histones and granule-derived enzymes such as MPO (myeloperoxidase) and elastase [126]. Once neutrophils enter lung tissue, SARS-CoV-2 can bind to them directly via the ACE2 receptor. The ACE2/TMPRSS2 pathway is required for SARS-CoV-2 entry and neutrophil NET release [33]. TLR-7 may also be involved in a molecular mechanism by which this intracellular sensor helps detect single-stranded RNA generated by intracellular replication of SARS-CoV-2 [127]. Although neutrophil NET formation is originally considered as an innate defense mechanism against pathogenic invaders, its exaggerated formation in the lungs by overactive leukocytes may cause cellular apoptosis, tissue damage and fibrosis due to destructive effects of released DNA, histones and granular proteins (such as MPO) [128], [129]. In this regard, it is thought that higher levels of NETs released by circulating and lung-infiltrating neutrophils in patients are directly related to the severity of COVID-19. This is also supported by the observation of higher levels of NET biomarkers (MPO-DNA and citrullinated histone H3 complexes) in patients with severe disease (admitted to the ICU) than in those with mild disease [130], [131]. In addition, under circulatory conditions, due to pro-coagulate and pro-inflammatory nature of released NETs, as wells as its toxicity that causes endothelial activation/damage, a strong link between COVID-19-associated NETs formation and immune-thrombosis has also been discussed, which may increase the complexity of the disease [130]. Consequently, NETs and its components are likely to be considered as indicators of severity and the progression of disease in COVID-19 patients.
4.1.2. Neutrophil-induced platelet activation
As shown in Fig. 2, section D, neutrophil contributes to platelet activation by releasing a wide array of substances, including ROS, chemokines, and chemo-attractants especially platelet activating factor (PAF), as well as proteolytic enzymes such as elastase and cathepsin G [132], [133], [134], [135], which induce fibrin generation on the thrombi, linking pro-inflammatory responses to pro-coagulant state [136], [137]. In addition, NETs formation by activated leukocyte acts as a critical link between inflammation and thrombosis. NETs can be involved, directly or through platelet activation, in initiating and increasing thrombogenic events in various diseases [32]. As shown in Fig. 1, it has been suggested that, NETs act as a scaffold for platelet adhesion, propagating thrombus formation and pro-coagulant function [138], [139], [140]. Histones in NETs activate platelets by binding to TLRs [141], [142], [143]. Antimicrobial compounds in the NETs, such as cathelicidin (LL-37), can also activate platelets via GPVI, resulting in neutrophil-platelet aggregates (Fig. 2, section D) [144], [145]. In the vasculature, deposited NETs can disrupt endothelial integrity, causing inflammation and injury on endothelium, where in turn, platelets recruitment and activation, provoke aggregation [139] and coagulation via the activation of factor XII [146], [147]. As shown in Fig. 1, circulating NET components (citrullinated histone H3, cell-free DNA and neutrophil elastase) may also deposit in distant arteries and vessels, leading to thrombosis through endothelial cell destruction and platelet aggregation [130].
Recent research has found an association between circulating NETs and COVID-19 and their role as a prognostic indicator of disease [130], [148]. Studies have shown increased levels of NET-forming markers such as circulating DNA and citrullinated histone H3 in patients with COVID-19 [148]. In these patients, high levels of NET markers are associated with widespread inflammation, cytokine release, and thrombosis [149]. The evidence of NETs co-localization with platelet (staining with platelet factor 4 [PF4]) in addition to increased levels of soluble platelet-derived factors (including PF4 and regulated upon activation, normal T-cell expressed and secreted [RANTES]) that elicit NETosis, as well as elevated platelet-neutrophil aggregates in COVID-19 patients, support an important role for this platelet/NET positive-feedback loop in the pathogenesis of thrombosis in these patients [150], [151].
On the other hand, in severe cases of disease, excessive NETs generation may lead to the vascular deposition of their aggregates that damage the tissues by the occlusion of vessels and ducts. This is important evidence to suggest that NET formation and aggregation may be one of the causes of multi-organ damage in COVID-19 [148]. This is in line with previous researches that indicate circulating NETs and their toxic degradation compounds are elevated in patients suffering from transfusion-associated acute lung injury [152].
4.2. Monocyte activation by SARS-CoV-2 and its direct effect on platelet activation
The pathogenesis of SARS-CoV-2 is mainly related to the phenotypes and functions of macrophages including those which derived from migrating monocytes or tissue resident macrophages in lung and other organs. Upon infection, tissue resident alveolar macrophages bind to SARS-CoV-2 via the interaction between their lectin-like receptors, such as CD169 [153], or ACE2 receptor and the S protein [154]. Inflammatory gradient induced by the invasion of SARS-CoV-2 strongly attracts blood monocytes (as precursor of recruited tissue macrophages) to the site of infection in lung, where they differentiate into pro-inflammatory alveolar macrophages (AMs) under inflammatory conditions [155], [156]. AMs expand to alveolar cavities in severe COVID-19, forming a mixed infiltrate with neutrophils and lymphocytes [154]. Monocyte-derived and resident AMs are highly prone to polarize to pro-inflammatory M1 phenotype during the early stages of ARDS [157], [158]. ROS and several pro-inflammatory cytokines, including IL-1β, IL-6, IL-18, MCP-1, MIP-2, and TNFα are released by M1-polarized AMs [159], [160]. This systemic inflammatory reaction, which results from massive macrophage activation, and is called “macrophage activation syndrome” (MAS), plays a key role in SARS-CoV-2-associated complications including ARDS [161], [162], [163]. In general, the levels of peripheral monocytes (including CD14 + CD16++ and intermediate pro-inflammatory CD14++CD16+ subpopulations with increased production of IL-6) were also significantly higher in patients with COVID-19, particularly in those who developed ARDS. Notably, relative counts of pro-inflammatory monocytes in patients with severe COVID-19 increases to 45% of total blood monocytes. (Compared with 1–5% in healthy individuals). This finding emphasizes the critical role of monocytes in the generalization of the inflammatory responses caused by SARS-CoV-2 [164].
In addition to chemokine gradient induced by the invasion of SARS-CoV-2, two antimicrobial compounds in NET known as cathepsin G and cathelicidin cause monocytes recruitments into the infection site [165]. Cathelicidin-related antimicrobial peptides (CRAMP) are widely distributed in NETs. CRAMP is an antimicrobial polypeptide found in neutrophil secondary granules that increases monocyte uptake by creating an anchor on endothelial cells [166].
On the other hand, NETs also induce macrophage hyper-activation and their over-production of pro-inflammatory cytokines that facilitate Th1 cell differentiation under severe inflammatory condition [167], [168]. Actually, NETs internalization and degradation by macrophages significantly promote the release of pro-inflammatory cytokines [167], [169], [170]. For example in Behçet's disease, NETs stimulate macrophages to produce IL-8 and TNF-α, two important pro-inflammatory cytokines in BD [171].
Active macrophages are a major source of ROS which cause significant platelet activation at the inflammatory site [172], [173], [174]. ROS induce P-selectin [175], [176] [177] and CD40L [178] expression in platelets. ROS may also indirectly increase platelet reactivity by interfering with endogenous platelet inhibition mechanisms, such as scavenging of nitric oxide (NO) [179]. On the other hand, NO is also synthesized by endothelial cells to maintain endothelium integrity. Therefore, the imbalance between ROS production and NO availability disrupts intracellular antioxidant defense and cause endothelial damage [36]. This has been clearly shown in a model of ischemia/reperfusion-induced liver injury in which activated Kupffer cells (resident liver macrophages) release ROS, TNF-α, IL-1β, and other chemokines, that contribute to hepatocyte and endothelial damage as well as recruitment and activation of further leukocytes involved in a vicious circle of injury [180]. Given this mechanisms, in COVID-19, the presence of hyper-activated alveolar macrophages in close proximity of blood vessels seems to also cause endothelial damage leading to a progressive thrombosis (Fig. 1, Fig. 3B). Furthermore, similar to neutrophils, hyper activated macrophage itself may extrude their web-like chromatin DNA fibers to extracellular space and produce macrophage extracellular traps (METs) which not only cause direct endothelial activation and damage but also act as an important mediator of inflammation and likely thrombosis with the same mechanism that already described for NETs [181], [182], [183].
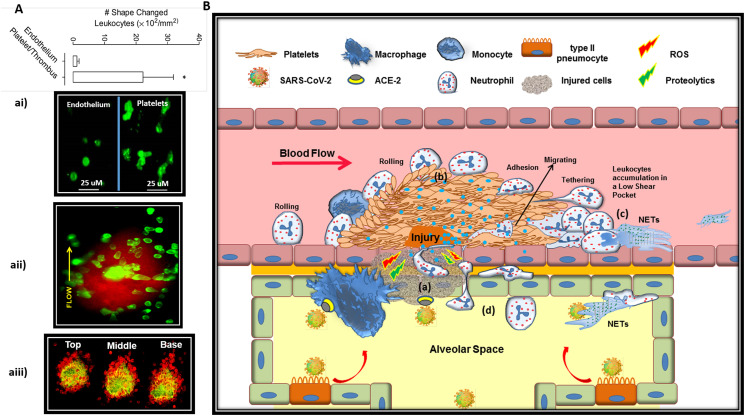
Fig. 3.
Thrombus effectively gates inflammatory leukocytes into the alveolar space.
A) Evidence for efficient leukocyte migration through thrombi. ai) Representative images depicting polarized and spread leukocytes (Gr-1 Ab, green) on C57Bl/6 mouse mesenteric veins after IR injury in mesenteric vasculature. As depicted, area with aggregated platelets were at least 20-fold more effective in inducing leukocyte shape change and migration compared to where leukocytes directly interacting with the endothelium free of platelets (Ghasemzadeh et al. Blood 2013). aii) Representative image at 10 min post injury (needle injury on mesenteric veins) depicting migrating leukocytes (Gr-1 Ab, green) at different positions between the margin and the center of a thrombus (platelet stained with DiOC6 in red). aiii) GFP-NOD mice were injected systemically with an anti Gr-1 Ab (for leukocyte staining in red), prior to induction of vascular injury via needle puncture. Thrombus formation (in yellow greenish) was monitored by confocal microscopy where the confocal sections through the “Top”, “Middle” and “Base” confirmed directed intravascular leukocyte migration into thrombi (Ghasemzadeh et al. Thrombosis & Hemostasis 2015).
B) Thrombi facilitate directed migration of circulating leucocytes from blood to infected alveolar tissue dictated by the cytokine gradient. (a) Alveolar vessel injured by direct invasion of SARS-CoV-2 or fully agitated macrophage under hyper-inflammatory state, recruits platelets that forms a developing thrombus at site of injury. (b) P-selectin expressing platelets on thrombus recruit leukocytes while their activation in pro-inflammatory milieu leads to cytokine-induced arrest and their subsequent adhesion and activation. Fully activated neutrophils may release NET that propagates thrombosis (c) or migrate to the source of inflammation through the thrombi (d), in a direction that is dictated by continuous release of cytokines/chemo-attractants (showing by blue dots) from the site of infection and alveolar damage.
Abbreviations: Ab: antibody; IR: ischemia-reperfusion; NET: neutrophil extracellular traps. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. How activated platelets feedback on COVID-19
5.1. Direct interaction of activated platelets with leukocytes
5.1.1. Activated platelets in thrombi recruit leukocytes while transferring them to the source of inflammation
There is mounting evidence that platelets play a key role in leukocyte recruitment to site of injury and inflammation. Platelet activation leads to P-selectin expression and the release of several pro-inflammatory molecules (e.g., neutrophil-activating peptide 2 [NAP-2], PF4, PAF, …) which can attract leucocytes to the site of injury and thrombus formation where they seriously get activated through the further engagement of their integrins with several adhesion molecules expressed on reactive thrombi. The initial interaction between platelet P-selectin and neutrophil P-selectin glycoprotein ligand 1 (PSGL-1) mediates their tethering and rolling on developing thrombi. PSGL-1 ligation here primes leukocyte β2 integrins (macrophage-1 antigen [Mac-1]), which their binding to platelet ligands such as GPIbα, ICAM-2 and fibrinogen (on thrombi) results in full activation of integrin. Once integrin gets activated, its ligation with fibrinogen induces potent outside-in signals that lead to the leukocyte degranulation and superoxide production as well as their shape change, crawling, migration and extravasation, all of which play critical roles in inflammatory responses. Neutrophil post-activation at the site of injury may also induce NET formation especially on thrombi low shear pocket area (Fig. 3B) [133]. It has been already showed that platelets thrombi can attract leukocytes while also gating them to the lower tissues via a directed migration mechanisms (Fig. 3 aii & aiii) [135]. More interestingly an observation indicated that compare to inflamed endothelium free of platelets, platelet aggregates can recruit 20 folds more leukocytes in the same area from the vascular surface (Fig. 3 ai). This finding indicates that platelet thrombi are highly reactive surfaces for leukocyte attraction and trans-migration through the vessels. Therefore, since the lung infection with SARS CoV-2 also involves the pre-alveolar vessels by either direct or indirect damage to endothelium [19], thrombotic events in this area may anchor masses of circulating leukocytes to penetrate tissues and exacerbate inflammatory state (Fig. 3B). Same scenario may also occur in other damaged organs affected by the virus invasion.
5.1.2. Platelet-induced neutrophil activation
Platelet activation increases mortality in patients suffering from a severe form of the COVID-19. Activated platelets reprogram cellular functions via specific cellular interactions [184], [185], [186]. In a positive feedback loop, activated platelets mediate significant leukocyte responses, including secretion [187], [188], adhesion and migration [189], [190], as well as NET formation [138], as a considerable initiator of coagulation and thrombosis. SARS-CoV-2 binding to TLR7 may induces P-selectin and CD40L expression on platelets that causes their increased interaction with neutrophils [86]. On the other hand, platelet-released chemokines, such as NAP-2, IL-8, growth-regulated oncogene-α (Gro-α) and PF4 attract neutrophils to the site of inflammation. All of these mediators enhance the activation state of integrins on neutrophils [151], [191]. P-selectin and GPIbα are key adhesion receptors on platelets that mediate a direct crosstalk between these cells and neutrophils via the interaction with PSGL-1 and MAC-1, respectively [133], [151], [192]. Platelet-induced neutrophil activation stimulates neutrophils to release their proteolytic enzymes including elastase and cathepsin-G in an inflammatory milieu where platelets present large quantities of connective tissue–activating peptide III (CTAP-III), an inactive precursor of NAP-2. Here cathepsin G, converts the abundance of these inactive CTAP-III on platelets to active NAP-2, a potent chemokine, and neutrophil activator that attracts and stimulates neutrophil through the engagement of CXCR1 and CXCR2. NAP-2 -induced Mac-1 activation on neutrophils, promotes leukocyte adhesion and migration into thrombi. Given the abundance of NAP-2 in platelets and its predominant role in inducing neutrophil shape change and motility, this chemokine is considered to be the major mediator of platelets that can promote directed intravascular leukocyte migration through a developing thrombi [134], [135].
On the other hand, activated platelet and its releasate showed to contribute to neutrophil NETs formation in various ways. In this regard, Middleton et al. found significantly elevated levels of platelet-derived soluble factors (including PF4 and CCL5 [RANTES]) in COVID-19 patients, which can cause NETosis. Platelet-derived PF4 has previously been shown to bind to NETs, compressing and making them resistant to DNase activity [150]. The presence of this PF4/NET positive feedback loop that maintains NET-induced coagulation cascade associated with immune thrombosis, may explain the perplexing observations of overwhelming inflammation with cytokine storm and widespread micro-/macrovascular thrombosis in COVID-19 patients [193], [194]. In addition, platelet binding to neutrophils can increase the expression of the receptor-interacting protein kinase-3 (RIPK3) and pseudo-kinase, mixed lineage kinase domain-like (MLKL) involved in the NETosis process in neutrophils where in a positive feedback, RIPK3 may activate platelets as well [195]. Platelets can also stimulate neutrophil NET production by secreting high mobility group box 1 (HMGB1), a nuclear nonhistone DNA-binding protein, which is over-expressed in COVID-19 and plays a key role in the cross-communication between thrombotic events, cellular stress and immune responses [196], [197], [198]. In this regard, while platelet-derived HMGB1 is considered as an important mediator of thrombosis [197], as a DAMP, it also exerts its potent inflammatory effects through TLR2 and TLR4 on neutrophils [199]. In some viral infections, such as influenza, virus interaction with platelet TLR7 can also induce the release of complement component C3 protein from platelet α-granules, which forces neutrophils to release their DNA content during the NETosis process. Intriguingly, C3-induced NETosis does not require neutrophils attachment to the vascular bed. Thus, these circulating NETed neutrophils can move through the bloodstream [44] while capturing pathogens [200], and wherever deposited, they may cause intravascular thrombosis due to thrombogenic properties of NETs [130]. The deposition of this circulating NETs or NETed neutrophils especially on inflamed endothelium of cytokine-affected vessels in areas farther from the main site of infection damage (Fig. 1), may act as a scaffold for initiation of a disseminated thrombosis and DIC in severe cases of COVID-19. In addition to C3, virus-exposed platelets secrete GM-CSF in neutrophil-depended manner which in turn, controls the levels of DNA released by activated neutrophils [44]. High levels of C3 and GM-CSF have been reported in patients with COVID-19 and as the disease progresses, the C3-NET-GM-CSF response may become out of control due to high levels of inflammatory cytokines and tissue damage [1], [38].
Circulating platelets with higher levels of P-selectin expression also promote the formation of platelet-leukocyte aggregates (PLAs) [50], [201]. Pulmonary damage and coagulopathy in COVID-19 could be relevant to platelet-neutrophil aggregate PNA- [202] and potentially the formation of platelet-induced NETs in these aggregations [149] (Fig. 1). Alexander et al. discovered that COVID-19 patients have a higher proportion of PNA than healthy donors. They showed that the levels of PNA in severe patients were substantially more significant than in moderate individuals. They discovered platelets are pre-activated and potentially contribute to micro-thrombotic complications in severe cases of COVID-19 [203]. Therefore, given the elevated levels PLAs and their links with inflammation and severity of disease in these patients, it can be suggested that PLAs may be involved in the etiology of COVID-19.
5.1.3. Platelet-induced monocyte activation
In patients with severe forms of the COVID-19, activated platelets forming platelet-monocyte aggregates (PMAs) can induce monocyte dependent tissue factor (TF) expression that causes hypercoagulability [31], [203]. P-selectin and integrin αIIb/β3 play important roles in platelet-monocyte interaction and the induction of TF expression by monocyte [204], [205]. Eugenio et al. showed that TF expression increases only in monocytes but not in platelets in critically ill COVID-19 patients. They also indicated that monocytes tethered with platelets can express significantly higher TF compared to monocytes alone, as TF expression was significantly higher in platelet-monocyte complexes (CD14 + CD41+) than in monocytes without platelet adhesion (CD14 + CD41-). On the other hand, given no increase in TF expressing platelets in COVID-19 patients, they suggested that this association could not be driven by platelets [30]. However, it is not clear that whether TF on PMAs is solely expressed by monocytes as platelets may also generate TF within their direct cross-talk with these cells. However, the lack of significant TF expression in PNAs compared to its high levels in PMAs may also highlight a significant role of monocytes as the main source of TF or its inducer [206], [207], [208].
5.1.4. PMPs activate neutrophils and monocytes
Platelet microparticles (PMPs) are extracellular vesicles released by platelets during activation [209]. Recent studies have found that PMP count is higher in hospitalized patients with COVID-19 than healthy controls [86], [210]. PMPs may enter other tissues, such as lymph nodes, affecting immune responses while these particles can transport diverse molecules, including miRNAs, mRNA, non-coding RNA, cytokines, and surface proteins to other cells, modulating their functional activities [211]. It has been shown that in patients with dengue virus, PMPs released by activated platelets can stimulate neutrophils and macrophages via the C-type lectin domain-family 5A (CLEC5A) receptor and TLR2 on neutrophils, resulting in the formation of NET and the release of pro-inflammatory cytokines [212]. It has been shown that in some pathologic condition, DAMPs released by inflammatory milieu can lead to platelet activation and its subsequent EVs release while IL-1β and caspase-1–carrying microparticles can bind to neutrophils and promote the formation of platelet–neutrophil aggregates which result in lung vasoocclusion and injury [213]. PMPs can also bind to monocyte subsets and activate these cells, resulting in the release of inflammatory mediators such as IL-1, IL-6 and TNF-α, as well as their adhesion to endothelial cells [214].
5.2. Activated platelets feedback on cytokine storms
Previous studies have shown that activated platelets secrete pro-thrombotic agents (e.g., TXA2 and ADP), and pro-inflammatory factors (e.g., IL-1β, RANTES, PAF and soluble P-selectin and CD40L) which can enhance both platelet activation and inflammation together [186], [215], [216]. While molecules like CD40L, PF4, and P-selectin are abundant in platelet granules and are released upon degranulation, cytokines like IL-1 may also be produced de novo after platelets activation. Although platelets are anucleate, they contain rich and diverse transcriptome that could explain their ability to produce and release inflammatory cytokines upon activation, independent of storage pool [217], [218]. In addition platelets have the potential to directly stimulate a wide range of cell types to release various cytokines [52], while their own released pro-inflammatory mediators can also promote leukocyte activation and NET formation which contribute to thrombotic events and other pathological condition.
Platelets per se are an important source of inflammatory mediators that significantly contribute to the cytokine load in severe COVID-19 [52]. In this regard, patients with severe and critical forms of COVID-19 have the highest plasma levels of PF4, serotonin and CD40L released from platelets while platelets from these patients are also more sensitive to releasing cytokines such as TGF-β, IL-1β, and IFN- γ [31]. Zaid et al. found increased levels of Groα, IL-7, macrophage-derived chemokine (MDC), platelet-derived growth factor-AA/BB (PDGF-AB/BB), and RANTES in patients with COVID-19 whereas on the other hand, they showed reduced levels of some of these specific cytokines or growth factors (e.g., eotaxin, IFN- γ, IL-1β, TGF-β) in platelets of these patients. Therefore, a significant increase of these mediators in the blood of COVID-19 patients may be attributed to the platelet involvement in their release [52]. According to previous in vitro and in vivo studies, platelets may also release microparticles containing IL-1β in response to some viral infection, which leads to an increase in endothelial permeability [219]. Serotonin release from platelets can also directly affect blood vessel integrity leading to leukocytes uptake, activation and their cytokine release [220], [221]. These responses associated with increased levels of IL-1β and other cytokines, could also play a role in COVID-19-related ARDS.
On the other hand, studies showed that activated platelets can directly regulate leukocyte function and their cytokine release during infections [222], where the direct interaction between platelet P-selectin and CD40L with neutrophil causes the release of their granules contents, complement C3, a variety of cytokines such as CCL2, CCL3, CCL7, IL-1, IL-7, IL-8, and hepatocyte growth factor [223], [224]. These cytokines are substantially higher in COVID-19 patients than in healthy individuals [225]. More specifically, the soluble CD40L released from platelets in COVID-19 may be involved in activating CD40-coated cells including different type of lymphocytes while regulating their function and affecting cytokine release [226].
6. Evidence for reciprocal treatment of thrombosis and inflammation in COVID-19
6.1. Antiplatelet therapies
So far, several studies have been performed or are still ongoing on the effect of antiplatelet therapies on the treatment of COVID-19. In this regard, some researchers believe that, given the specific role of platelet activity and thrombotic events in propagation of inflammatory conditions [227], it is very likely that antiplatelet therapies may control or help treat COVID-19, especially in hospitalized patients [201]. In a most recent publication resulted from a multicentre international registry (Health Outcome Predictive Evaluation Registry (HOPE-COVID-19), Santoro et al. concluded that without an increased risk of bleeding, antiplatelet therapies were associated with reduced mortality risk of COVID-19 while shortening duration of mechanical ventilation in hospitalized patients. This study included 7824 consecutive patients with COVID-19, of which 730 (9%) received antiplatelet regimen during hospitalization; including 680 patients (93%) received a single antiplatelet drug (645 patients with aspirin, 33 patients with clopidogrel, and 1 with ticlopidine and ticagrelor, respectively) and 50 patients (7%) received dual antiplatelet therapy (35 patients with aspirin and clopidogrel, 10 patients with aspirin and ticagrelor, and 5 patients with aspirin and prasugrel) [228]. With the advantage of evaluating different antiplatelet regimens, this study was consistent with an earlier research by Chow et al., who previously reported in a multicenter observational cohort study of 412 patients with COVID-19 that, the use of aspirin in hospital significantly decreases the risk of hospitalization in intensive care units, the need for mechanical ventilation and death [229]. Similarly, in a retrospective study of 2785 hospitalized patients with COVID-19, Meizlish et al. have also shown that aspirin therapy (n = 638) was associated with lower incidence of in-hospital death [230]. In addition to these observations, there were few studies that did not show a significant effect of aspirin therapy on the status or outcome of patients, however, these studies, while pointing to the uncertainty of their findings, also recommend continuing researches with larger cohorts and evaluating more antiplatelet drugs [231]. Among these studies, there was a Randomised Evaluation of COVID-19 Therapy (RECOVERY) performed on 14,892 patients, almost half of whom randomly received aspirin while the rest were under usual care alone. Although this trial indicated that aspirin was not associated with a reduction in 28-day mortality or the risk of progression to invasive mechanical ventilation or death, its administration was shown to be associated with a slight increase in the rate of being discharged alive within 28 days [232]. In this regard, in addition to the aforementioned study by Santoro et al. the results of two ongoing RECOVERY (NCT04381936) and ACTIV-4 (NCT04505774) clinical trials which are evaluating the benefit of P2Y12 treatment in COVID-19 are of particular interest. Especially that as a member of this group of antiplatelet drugs, ticagrelor prescription in pneumonia patients has shown a significant reduction of NET release, platelet–leukocyte interactions, and IL-6 levels [233], the inflammatory evidence, all of which are also prominent in the pathogenesis of COVID-19. Overall, most studies to date have pointed to the beneficiary roles of antiplatelet therapies in relieving the complications of COVID-19 in hospitalized patients, some of which, such as reducing the need for mechanical ventilation, indicate their significant importance in controlling of severe lung inflammation and ARDS [234]. Although these observations are indirect evidence that thrombosis can exacerbate inflammatory responses, especially cytokine storm in COVID-19 patients, more data are needed on the direct effects of antiplatelet therapies on the reduction of major inflammatory mediators in COVID-19.
6.2. Anti-inflammatory therapies
Despite the controversial use of steroids in COVID-19 [235], to date, antagonizing or inhibiting of major cytokines and other inflammatory compounds or their various signaling pathways have also been considered as important supportive therapies for controlling severe inflammatory conditions and cytokine storms, especially in critically ill patients. Steroids are commonly used anti-inflammatory drugs that quench inflammatory state interfering with NF-κB, the key inflammatory transcriptional regulator, while also suppressing immune responses [236]. However, due to the risk of thromboembolisms [237] and generalized immune suppression, their high dose and long prescription especially in critically ill patients are almost challenging therapeutic approaches [238]. Some earlier retrospective cohort studies have suggested tocilizumab (a monoclonal anti-soluble IL-6 receptor antibody) as an efficient treatment for COVID-19 [239]. Given these preliminary findings, in a larger randomised controlled trials (REMAP/CAP-RECOVERY trial platform) adding tocilizumab to standard dexamethasone treatment showed superior effect in patients treatment compared to dexamethasone alone [240] with increasing organ support-free days as well as a 1.6-fold increment in chance of survival compared to control. In this regard, some researchers believe that one of the reasons for the better performance of this anti-inflammatory combination is the possible role of tocilizumab in improving coagulation function and thrombotic conditions in patients, especially since recent studies have also showed a lower incidence of thromboembolic events in COVID-19 patients receiving tocilizumab [241] as well as reduced D-dimer levels during infection [242]. In addition to IL-6 inhibition, in some smaller cohorts, researchers also showed that both the blockade of IL-1 receptor with anakinra [243] and IL-1β blocking monoclonal antibodies (canakinumab) can attenuate some inflammatory signals leading to an improved oxygenation associated with survival [244]. Another ongoing clinical trial has also focused on whether neutralizing interleukin-8 (IL-8) with BMS-986253 can help improve the health condition of COVID-19 hospitalized patients (ClinicalTrials.gov Identifier: NCT04347226). However, to date, there is no reliable information that directly addresses the effect of anti-interleukin therapy on the modulation of platelet function and the improvement of thrombotic indices in COVID-19 patients, therefore, conducting such studies in the next steps will be considered and encouraged. In addition to anti-cytokine therapies in nonrandomized proof-of-concept study, Annane et al. showed that the administration of complement C5 inhibitor eculizumab, in patients with severe COVID-19 were associated with increased survival and accelerated improvement of biomarkers of tissue hypoxia and inflammation. More interestingly, these patients also showed an improvement in platelet count [245] which could be due to the inhibition of complement-mediated thrombotic microangiopathy as a known effect of eculizumab already shown in acute hemolytic-uremic syndrome [246]. Given these preliminary results, larger clinical studies are planned or ongoing now to investigate the efficacy and safety of C5 blockade (either with eculizumab or ravulizumab) in the treatment of severe COVID-19 patients (ClinicalTrials.gov Identifiers: NCT04288713, NCT04355494, NCT04346797 with eculizumab and NCT04369469, NCT04390464 with ravulizumab). It is also interesting to follow the effects of these anti-inflammatory drugs on the thrombotic event in COVID-19, which may shed more light on the importance of platelet-leukocyte cross-talk in the pathogenesis of the disease.
7. Conclusion
Exhausted immune responses in COVID-19 fail to control viral invasion and subsequent macrophage hyper-activation which is involved in both alveolar tissue damage and cytokine storms. Under this condition, pre-alveolar vascular damage caused by direct viral invasion, macrophage toxic activity or cytokine storms, recruits platelets that create developing thrombi at the site of injury. However, regardless of whether the thrombosis is due to infection or not, the developing thrombi in such a hyper-inflammatory milieu not only efficiently recruit the massive accumulation of circulatory leukocytes that exacerbate thrombotic events in the prealveolar area, but may also serve as potential gateway for the transfer of these active leukocytes to infected alveoli. Obviously, this resultant congestion of leukocytes in alveoli aggravates tissue damage while further enhancing cytokine storms, vascular damage and thrombotic events in a vicious circle. Therefore, review presented here hypothesizes that to control the severe cases of COVID-19, as much as anti-inflammatory therapies can be effective in relieving the cytokine storm, antithrombotic therapies can also be effective and vice versa.
To address this hypothesis, the review has presented all proven mechanisms by which the mutual interaction between platelets and leukocytes can intensify both inflammatory state and thrombotic events in a reciprocal manner, in particular, how cytokine storms can act as a prelude to thrombosis and why it is quite possible to expect thrombotic disorders following such inflammatory conditions. Given this, it may be advisable to start prophylactic antiplatelet therapy during cytokine storm. On the other hand, it has been noted that since the onset of thrombotic conditions can exacerbate the inflammatory state, therefore, if thrombotic events occur prior to this exacerbation, prompt and appropriate antiplatelet therapy may be helpful in controlling the inflammatory status and preventing the cytokine storm.
In conclusion, to better control the severe cases of COVID-19, this review suggests that as much as anti-inflammatory therapies can be effective in relieving the cytokine storm, antithrombotic therapies may also be effective and vice versa. The important suggestion that may help improve current therapeutic approaches to focus more on the prevention of thrombotic events, as it may be able to effectively control the inflammatory status as the Achilles heel of the disease.
Abbreviations
- ACE2
Angiotensin-converting enzyme-2
- AKT
protein kinase B
- AM
alveolar macrophages
- ARDS
acute respiratory distress syndrome
- BD
Behçet's disease
- CLEC5A
C-type lectin domain-family 5A
- CLEC-2
C-type lectin-domain family 2
- CCL
chemokine (C-C motif) ligand
- CR
complement receptor
- CSS
cytokine storm syndrome
- CRAMP
Cathelicidin-related antimicrobial peptides
- CTAP-III
connective tissue–activating peptide III
- CXCL
chemokine (C-X-C motif) ligand
- DIC
disseminated intravascular thrombosis
- DVT
deep vein thrombosis
- DC-SIGN
dendritic cell-Specific intercellular adhesion molecule-3-grabbing non-integrin
- DAMP
damage-associated molecular pattern molecule
- EMMPRIN
extracellular matrix metalloproteinase inducer
- ER
endoplasmic reticulum
- GM-CSF
granulocyte-monocyte colony-stimulating factor
- GP
glycoprotein
- GRP-78
Glucose-regulated protein 78
- GP 130
glycoprotein 130
- G-CSF
granulocyte-colony stimulating factor
- GRO
growth-regulated oncogene
- HMGB1
high mobility group box 1
- ICU
intensive care unit
- IFN
interferon
- IL
interleukin
- JAK3
Janus kinase-3
- MCP
monocyte chemo-attractant protein
- MMP
metallo-matrix protease
- MPO
myeloperoxidase
- MAC1
macrophage-1 antigen
- MLKL
mixed lineage kinase domain-like
- MDC
macrophage-derived chemokine
- MOF
multi-organ failure
- NET
neutrophil extracellular traps
- NF-κB
nuclear factor -κB
- NLR
neutrophil to lymphocyte ratio
- NAP-2
neutrophil-activating peptide 2
- PE
pulmonary embolism
- PLA
platelet-leukocyte aggregations
- PKC
protein kinase C
- PAF
platelet activating factor
- PF4
platelet factor 4
- PSGL
P-selectin glycoprotein ligand 1
- PNA
platelet-neutrophil aggregate
- PMAs
platelet-monocyte aggregates
- PMPs
Platelet Microparticles
- PDGF
platelet-derived growth factor
- P38-MAPK
P38-mitogen-activated protein kinase
- ROS
reactive oxygen species
- RANTES
regulated upon activation, normal T-cell expressed and secreted
- RIPK3
receptor-interacting protein kinase-3
- TLR
toll like receptor
- TNF
tumor necrosis factor
- Th1
T-helper type 1
- TMPRSS2
transmembrane Serine Protease-2
- TF
tissue factor
CRediT authorship contribution statement
Mehran Ghasemzadeh provided intellectual input, designed and wrote the paper as main author, depicted the figures.
Ehteramolsadat Hosseini provided intellectual input and helped with the writing of paper and depicted the figures.
Javad Ahmadi provided intellectual input and helped with the writing of paper and depicted the figures.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He F., Deng Y., Li W. Coronavirus disease 2019: what we know? J. Med. Virol. 2020;92(7):719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J. Med. Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y., Shen C., Li J., Yuan J., Wei J., Huang F., et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teijaro J.R., Walsh K.B., Rice S., Rosen H., Oldstone M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. 2014;111(10):3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens E.M., Koretzky G.A. Cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69(6):1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Wang F., Zhang P., Zhang Y., Chen Y., Fan X., et al. Management of cytokine release syndrome related to CAR-T cell therapy. Front. Med. 2019;13(5):610–617. doi: 10.1007/s11684-019-0714-8. [DOI] [PubMed] [Google Scholar]
- 13.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. Springer. [DOI] [PubMed] [Google Scholar]
- 14.Murthy H., Iqbal M., Chavez J.C., Kharfan-Dabaja M.A. Cytokine release syndrome: current perspectives. ImmunoTargets Ther. 2019;8:43. doi: 10.2147/ITT.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., et al. Cytokine release syndrome. J. Immunother. Cancer. 2018;6(1):1–14. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J., et al. Innate immune response of human alveolar type ii cells infected with severe acute respiratory syndrome–coronavirus. Am. J. Respir. Cell Mol. Biol. 2013;48(6):742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong M.H., Johnson M.D. Differential response of primary alveolar type I and type II cells to LPS stimulation. PLOS One. 2013;8(1) doi: 10.1371/journal.pone.0055545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. Basic Transl. Sci. 2021;6(3):202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiko G.E., Horvat J.C., Beagley K.W., Hansbro P.M. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami M., Kamimura D., Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50(4):812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Sokol C.L., Luster A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015;7(5) doi: 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S., Jiang L., Li X., Lin F., Wang Y., Li B., et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5(12) doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Liao W., Wan L., Xiang T., Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2021;34(5):330–335. doi: 10.1089/vim.2020.0062. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890. doi: 10.1016/j.chom.2020.04.017. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Sun R. BioRxiv; 2020. Aberrant Pathogenic GM-CSF+ T Cells and Inflammatory CD14+ CD16+ Monocytes in Severe Pulmonary Syndrome Patients of a New Coronavirus. [Google Scholar]
- 27.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;118102 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park M.D. Macrophages: a Trojan horse in COVID-19? Nat. Rev. Immunol. 2020;20(6):351. doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirai T., Hilhorst M., Harrison D.G., Goronzy J.J., Weyand C.M. Macrophages in vascular inflammation–from atherosclerosis to vasculitis. Autoimmunity. 2015;48(3):139–151. doi: 10.3109/08916934.2015.1027815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13(1):1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perdomo J., Leung H.H., Ahmadi Z., Yan F., Chong J.J., Passam F.H., et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019;10(1):1–14. doi: 10.1038/s41467-019-09160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217(12) doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beigee F.S., Toutkaboni M.P., Khalili N., Nadji S.A., Dorudinia A., Rezaei M., et al. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol. Res. Pract. 2020;216(10) doi: 10.1016/j.prp.2020.153228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giordo R., Paliogiannis P., Mangoni A.A., Pintus G. SARS-CoV-2 and endothelial cell interaction in COVID-19: molecular perspectives. Vasc. Biol. 2021;3(1):R15–R23. doi: 10.1530/VB-20-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFadyen J.D., Stevens H., Peter K. The emerging threat of (micro) thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127(4):571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aid M., Busman-Sahay K., Vidal S.J., Maliga Z., Bondoc S., Starke C. Vascular disease and thrombosis in SARS-CoV-2-infected rhesus macaques. Cell. 2020;183(5):1354–1366. doi: 10.1016/j.cell.2020.10.005. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iba T., Levy J. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018;16(2):231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 41.Costa T.J., Potje S.R., Fraga-Silva T.F., da Silva-Neto J.A., Barros P.R., Rodrigues D., et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vasc. Pharmacol. 2022;142 doi: 10.1016/j.vph.2021.106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 43.Robles J.P., Zamora M., Adan-Castro E., Siqueiros-Marquez L., de la Escalera G.M., Clapp C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J. Biol. Chem. 2022;101695 doi: 10.1016/j.jbc.2022.101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koupenova M., Corkrey H.A., Vitseva O., Manni G., Pang C.J., Clancy L., et al. The role of platelets in mediating a response to human influenza infection. Nat. Commun. 2019;10(1):1–18. doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pryzdial E.L., Lin B.H., Sutherland M.R. Platelets in Thrombotic and Non-thrombotic Disorders. Springer; 2017. Virus–platelet associations; pp. 1085–1102. [Google Scholar]
- 46.Bote J., Corkrey H.A., Koupenova M. Human platelets and influenza virus: internalization and platelet activation. Platelets. 2021:1–8. doi: 10.1080/09537104.2021.1961710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen S., Zhang J., Fang Y., Lu S., Wu J., Zheng X., et al. SARS-CoV-2 interacts with platelets and megakaryocytes via ACE2-independent mechanism. J. Hematol. Oncol. 2021;14(1):1–5. doi: 10.1186/s13045-021-01082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X., et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middleton E.A., Rowley J.W., Campbell R.A., Grissom C.K., Brown S.M., Beesley S.J., et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood. 2019;134(12):911–923. doi: 10.1182/blood.2019000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., et al. Platelets can associate with SARS-cov-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J., et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191(5):755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K., Chen W., Zhang Z., Deng Y., Lian J.-Q., Du P., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5(1):1–10. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P. BioRxiv; 2020. SARS-CoV-2 Invades Host Cells Via a Novel Route: CD147-spike Protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pushkarsky T., Zybarth G., Dubrovsky L., Yurchenko V., Tang H., Guo H., et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin a. Proc. Natl. Acad. Sci. 2001;98(11):6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe A., Yoneda M., Ikeda F., Terao-Muto Y., Sato H., Kai C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol. 2010;84(9):4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., Wawrzyniak P., et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75(11):2829–2845. doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt R., Bültmann A., Fischel S., Gillitzer A., Cullen P., Walch A., et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 2008;102(3):302–309. doi: 10.1161/CIRCRESAHA.107.157990. Epub 2007/12/01. [DOI] [PubMed] [Google Scholar]
- 60.Iacono K.T., Brown A.L., Greene M.I., Saouaf S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 2007;83(3):283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burkhart J.M., Vaudel M., Gambaryan S., Radau S., Walter U., Martens L., et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–e82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 62.Pennings G., Yong A., Kritharides L. Expression of EMMPRIN (CD147) on circulating platelets in vivo. J. Thromb. Haemost. 2010;8(3):472–481. doi: 10.1111/j.1538-7836.2009.03716.x. [DOI] [PubMed] [Google Scholar]
- 63.Seizer P., Borst O., Langer H.F., Bültmann A., Münch G., Herouy Y., et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI-EMMPRIN interaction. Thromb. Haemost. 2009;101(04):682–686. doi: 10.1160/th08-06-0368. [DOI] [PubMed] [Google Scholar]
- 64.Heinzmann D., Noethel M., von Ungern-Sternberg S., Mitroulis I., Gawaz M., Chavakis T., et al. CD147 is a novel interaction partner of integrin αMβ2 mediating leukocyte and platelet adhesion. Biomolecules. 2020;10(4):541. doi: 10.3390/biom10040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulz C., Von Brühl M.L., Barocke V., Cullen P., Mayer K., Okrojek R., et al. EMMPRIN (CD147/basigin) mediates platelet–monocyte interactions in vivo and augments monocyte recruitment to the vascular wall. J. Thromb. Haemost. 2011;9(5):1007–1019. doi: 10.1111/j.1538-7836.2011.04235.x. [DOI] [PubMed] [Google Scholar]
- 66.Maugeri N., De Lorenzo R., Clementi N., Antonia Diotti R., Criscuolo E., Godino C., et al. Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 2022;20(2):434–448. doi: 10.1111/jth.15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khezri M.R., Varzandeh R., Ghasemnejad-Berenji M. The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell. Mol. Biol. Lett. 2022;27(1):1–10. doi: 10.1186/s11658-022-00308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlos A.J., Ha D.P., Yeh D.-W., Van Krieken R., Gill P., Machida K. BioRxiv; 2021. GRP78 Binds SARS-CoV-2 Spike Protein and ACE2 and GRP78 Depleting Antibody Blocks Viral Entry and Infection in Vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elfiky A.A., Baghdady A.M., Ali S.A., Ahmed M.I. GRP78 targeting: hitting two birds with a stone. Life Sci. 2020;118317 doi: 10.1016/j.lfs.2020.118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elfiky A.A., Ibrahim I.M., Ismail A.M., Elshemey W.M. A possible role for GRP78 in cross vaccination against COVID-19. J. Infect. 2021;82(2):282–327. doi: 10.1016/j.jinf.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020;80(5):554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmeira A., Sousa E., Köseler A., Sabirli R., Gören T., Türkçüer İ., et al. Preliminary virtual screening studies to identify GRP78 inhibitors which may interfere with SARS-CoV-2 infection. Pharmaceuticals. 2020;13(6):132. doi: 10.3390/ph13060132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ha D.P., Van Krieken R., Carlos A.J., Lee A.S. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J. Infect. 2020;81(3):452. doi: 10.1016/j.jinf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nain M., Mukherjee S., Karmakar S.P., Paton A.W., Paton J.C., Abdin M., et al. GRP78 is an important host factor for japanese encephalitis virus entry and replication in mammalian cells. J. Virol. 2017;91(6):e02274-16. doi: 10.1128/JVI.02274-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsai Y.-L., Zhang Y., Tseng C.-C., Stanciauskas R., Pinaud F., Lee A.S. Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface. J. Biol. Chem. 2015;290(13):8049–8064. doi: 10.1074/jbc.M114.618736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan C.-P., Siu K.-L., Chin K.-T., Yuen K.-Y., Zheng B., Jin D.-Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80(18):9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chu H., Chan C.-M., Zhang X., Wang Y., Yuan S., Zhou J., et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J. Biol. Chem. 2018;293(30):11709–11726. doi: 10.1074/jbc.RA118.001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Köseler A., Sabirli R., Gören T., Türkçüer I., Kurt Ö. Endoplasmic reticulum stress markers in SARS-COV-2 infection and pneumonia: case-control study. In Vivo. 2020;34(3 suppl):1645–1650. doi: 10.21873/invivo.11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sabirli R., Koseler A., Goren T., Turkcuer I., Kurt O. High GRP78 levels in Covid-19 infection: a case-control study. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Da L.P., Schattner M. Platelet toll-like receptors in thromboinflammation. Front. Biosci. 2017;22(11):1867–1883. doi: 10.2741/4576. Epub 2017/04/15. [DOI] [PubMed] [Google Scholar]
- 81.Schattner M. Platelet TLR4 at the crossroads of thrombosis and the innate immune response. J. Leukoc. Biol. 2019;105(5):873–880. doi: 10.1002/JLB.MR0618-213R. [DOI] [PubMed] [Google Scholar]
- 82.Choudhury A., Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020;92(10):2105–2113. doi: 10.1002/jmv.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aboudounya M.M., Heads R.J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banerjee M., Huang Y., Joshi S., Popa G.J., Mendenhall M.D., Wang Q.J., et al. Platelets endocytose viral particles and are activated via TLR (toll-like receptor) signaling. Arterioscler. Thromb. Vasc. Biol. 2020;40(7):1635–1650. doi: 10.1161/ATVBAHA.120.314180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Portier I., Campbell R.A. Role of platelets in detection and regulation of infection. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):70–78. doi: 10.1161/ATVBAHA.120.314645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koupenova M., Vitseva O., MacKay C.R., Beaulieu L.M., Benjamin E.J., Mick E., et al. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124(5):791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manne B.K., Münzer P., Badolia R., Walker-Allgaier B., Campbell R.A., Middleton E., et al. PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf1 in the MAPK pathway. J. Thromb. Haemost. 2018;16(6):1211–1225. doi: 10.1111/jth.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petrey A.C., Qeadan F., Middleton E.A., Pinchuk I.V., Campbell R.A., Beswick E.J. Cytokine release syndrome in COVID-19: innate immune, vascular, and platelet pathogenic factors differ in severity of disease and sex. J. Leukoc. Biol. 2021;109(1):55–66. doi: 10.1002/JLB.3COVA0820-410RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bektas A., Schurman S.H., Franceschi C., Ferrucci L. A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short-and long-term inflammaging? Immun. Ageing. 2020;17(1):1–10. doi: 10.1186/s12979-020-00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao Y.M., Xu G., Wang B., Liu B.C. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J. Intern. Med. 2021;289(2):147–161. doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchingo J.M., Sinclair L.V., Howden A.J., Cantrell D.A. Quantitative analysis of how myc controls T cell proteomes and metabolic pathways during T cell activation. elife. 2020;9 doi: 10.7554/eLife.53725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 97.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012;8(9):1237. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 99.Tedgui A., Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 2006;86(2):515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 100.Marino M., Scuderi F., Ponte E., Maiuri M., De Cristofaro R., Provenzano C., et al. Novel path to IL-6 trans-signaling through thrombin-induced soluble IL-6 receptor release by platelets. J. Biol. Regul. Homeost. Agents. 2013;27(3):841–852. [PubMed] [Google Scholar]
- 101.Houck K.L., Yuan H., Tian Y., Solomon M., Cramer D., Liu K., et al. Physical proximity and functional cooperation of glycoprotein 130 and glycoprotein VI in platelet membrane lipid rafts. J. Thromb. Haemost. 2019;17(9):1500–1510. doi: 10.1111/jth.14525. [DOI] [PubMed] [Google Scholar]
- 102.Senchenkova E.Y., Russell J., Yildirim A., Granger D.N., Gavins F.N. Novel role of T cells and IL-6 (Interleukin-6) in angiotensin II–induced microvascular dysfunction. Hypertension. 2019;73(4):829–838. doi: 10.1161/HYPERTENSIONAHA.118.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pum A., Ennemoser M., Adage T., Kungl A.J. Cytokines and chemokines in SARS-CoV-2 infections—therapeutic strategies targeting cytokine storm. Biomolecules. 2021;11(1):91. doi: 10.3390/biom11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davizon-Castillo P., McMahon B., Aguila S., Bark D., Ashworth K., Allawzi A., et al. TNF-α–driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134(9):727–740. doi: 10.1182/blood.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Masso-Silva J.A., Moshensky A., Lam M.T., Odish M., Patel A., Xu L., et al. Increased peripheral blood neutrophil activation phenotypes and NETosis in critically ill COVID-19 patients: a case series and review of the literature. Clin. Infect. Dis. 2022;74(3):479–489. doi: 10.1093/cid/ciab437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Regnault V., de Maistre E., Carteaux J.-P., Gruel Y., Nguyen P., Tardy B., et al. Platelet activation induced by human antibodies to interleukin-8. Blood. 2003;101(4):1419–1421. doi: 10.1182/blood-2002-02-0620. [DOI] [PubMed] [Google Scholar]
- 107.Li J., Kim K., Barazia A., Tseng A., Cho J. Platelet–neutrophil interactions under thromboinflammatory conditions. Cell. Mol. Life Sci. 2015;72(14):2627–2643. doi: 10.1007/s00018-015-1845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beaulieu L.M., Lin E., Mick E., Koupenova M., Weinberg E.O., Kramer C.D., et al. Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler. Thromb. Vasc. Biol. 2014;34(3):552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panigrahi S., Ma Y., Hong L., Gao D., West X.Z., Salomon R.G., et al. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ. Res. 2013;112(1):103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meizlish M.L., Pine A.B., Bishai J.D., Goshua G., Nadelmann E.R., Simonov M., et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5(5):1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38(8):970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 113.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 114.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russell C.D., Unger S.A., Walton M., Schwarze J. The human immune response to respiratory syncytial virus infection. Clin. Microbiol. Rev. 2017;30(2):481–502. doi: 10.1128/CMR.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Capucetti A., Albano F., Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front. Immunol. 2020;11:1259. doi: 10.3389/fimmu.2020.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 120.Lacy P. Mechanisms of degranulation in neutrophils. Allergy, Asthma Clin. Immunol. 2006;2(3):1–11. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Domínguez-Luis M.J., Armas-González E., Herrera-García A., Arce-Franco M., Feria M., Vicente-Manzanares M., et al. L-selectin expression is regulated by CXCL8-induced reactive oxygen species produced during human neutrophil rolling. Eur. J. Immunol. 2019;49(3):386–397. doi: 10.1002/eji.201847710. [DOI] [PubMed] [Google Scholar]
- 122.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng O.Z., Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Keshari R.S., Jyoti A., Dubey M., Kothari N., Kohli M., Bogra J., et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLOS One. 2012;7(10) doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12(1):109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 126.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 127.Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., et al. Recognition of single-stranded RNA viruses by toll-like receptor 7. Proc. Natl. Acad. Sci. 2004;101(15):5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thammavongsa V., Missiakas D.M., Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342(6160):863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yadav R., Yoo D.-g., Kahlenberg J.M., Bridges S.L., Jr., Oni O., Huang H., et al. Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis. J. Cyst. Fibros. 2019;18(5):636–645. doi: 10.1016/j.jcf.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ng H., Havervall S., Rosell A., Aguilera K., Parv K., Von Meijenfeldt F.A., et al. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021;41(2):988–994. doi: 10.1161/ATVBAHA.120.315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Petito E., Falcinelli E., Paliani U., Cesari E., Vaudo G., Sebastiano M., et al. Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J. Infect. Dis. 2021;223(6):933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bazzoni G., Dejana E., Del Maschio A. Platelet-neutrophil interactions. Possible relevance in the pathogenesis of thrombosis and inflammation. Haematologica. 1991;76(6):491–499. [PubMed] [Google Scholar]
- 133.Ghasemzadeh M., Hosseini E. Platelet-leukocyte crosstalk: linking proinflammatory responses to procoagulant state. Thromb. Res. 2013;131(3):191–197. doi: 10.1016/j.thromres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 134.Ghasemzadeh M., Hosseini E. Intravascular leukocyte migration through platelet thrombi: directing leukocytes to sites of vascular injury. Thromb. Haemost. 2015;113(06):1224–1235. doi: 10.1160/TH14-08-0662. [DOI] [PubMed] [Google Scholar]
- 135.Ghasemzadeh M., Kaplan Z.S., Alwis I., Schoenwaelder S.M., Ashworth K.J., Westein E., et al. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121(22):4555–4566. doi: 10.1182/blood-2012-09-459636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goel M.S., Diamond S.L. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and-independent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2001;21(12):2093–2098. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 137.Goel M.S., Diamond S.L. Neutrophil cathepsin G promotes prothrombinase and fibrin formation under flow conditions by activating fibrinogen-adherent platelets. J. Biol. Chem. 2003;278(11):9458–9463. doi: 10.1074/jbc.M211956200. [DOI] [PubMed] [Google Scholar]
- 138.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 139.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., et al. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thålin C., Hisada Y., Lundström S., Mackman N., Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019;39(9):1724–1738. doi: 10.1161/ATVBAHA.119.312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014;123(18):2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Semeraro F., Ammollo C.T., Morrissey J.H., Dale G.L., Friese P., Esmon N.L., et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zucoloto A.Z., Jenne C.N. Platelet-neutrophil interplay: insights into neutrophil extracellular trap (NET)-driven coagulation in infection. Front. Cardiovasc. Med. 2019;6:85. doi: 10.3389/fcvm.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinod K., Deppermann C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets. 2021;32(3):314–324. doi: 10.1080/09537104.2020.1817360. [DOI] [PubMed] [Google Scholar]
- 145.Pircher J., Czermak T., Ehrlich A., Eberle C., Gaitzsch E., Margraf A., et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018;9(1):1–15. doi: 10.1038/s41467-018-03925-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Massberg S., Grahl L., von Bruehl M.-L., Manukyan D., Pfeiler S., Goosmann C., et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16(8):887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 147.von Brühl M.-L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209(4):819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rossaint J., Herter J.M., Van Aken H., Napirei M., Döring Y., Weber C., et al. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap–mediated sterile inflammation. Blood. 2014;123(16):2573–2584. doi: 10.1182/blood-2013-07-516484. [DOI] [PubMed] [Google Scholar]
- 152.Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bedin A.-S., Makinson A., Picot M.-C., Mennechet F., Malergue F., Pisoni A., et al. Monocyte CD169 expression as a biomarker in the early diagnosis of coronavirus disease 2019. J. Infect. Dis. 2021;223(4):562–567. doi: 10.1093/infdis/jiaa724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yona S., Kim K.-W., Wolf Y., Mildner A., Varol D., Breker M., et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Higgins D.M., Sanchez-Campillo J., Rosas-Taraco A.G., Higgins J.R., Lee E.J., Orme I.M., et al. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with mycobacterium tuberculosis. J. Immunol. 2008;180(7):4892–4900. doi: 10.4049/jimmunol.180.7.4892. [DOI] [PubMed] [Google Scholar]
- 158.Swiecki M., Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr. Opin. Virol. 2011;1(6):463–475. doi: 10.1016/j.coviro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bhatia M., Zemans R.L., Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012;46(5):566–572. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Laskin D.L., Malaviya R., Laskin J.D. Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol. Sci. 2019;168(2):287–301. doi: 10.1093/toxsci/kfy309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.İnandıklıoğlu N., Akkoc T. Immune responses to SARS-CoV, MERS-CoV and SARS-CoV-2. Cell Biol. Transl. Med. 2020;9:5–12. doi: 10.1007/5584_2020_549. [DOI] [PubMed] [Google Scholar]
- 162.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tufan A., Güler A.A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., et al. COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2020:1–10. doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J., Sjöberg S., Tang T.-T., Öörni K., Wu W., Liu C., et al. Cathepsin G activity lowers plasma LDL and reduces atherosclerosis. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2014;1842(11):2174–2183. doi: 10.1016/j.bbadis.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wantha S., Alard J.-E., Megens R.T., Van Der Does A.M., Döring Y., Drechsler M., et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ. Res. 2013;112(5):792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barrera-Vargas A., Gómez-Martín D., Carmona-Rivera C., Merayo-Chalico J., Torres-Ruiz J., Manna Z., et al. Differential ubiquitination in NETs regulates macrophage responses in systemic lupus erythematosus. Ann. Rheum. Dis. 2018;77(6):944–950. doi: 10.1136/annrheumdis-2017-212617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu Q., Shi H., Zeng T., Liu H., Su Y., Cheng X., et al. Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still’s disease. Arthritis Res. Ther. 2019;21(1):1–11. doi: 10.1186/s13075-018-1800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Farrera C., Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013;191(5):2647–2656. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 170.Mistry P., Carmona-Rivera C., Ombrello A.K., Hoffmann P., Seto N.L., Jones A., et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann. Rheum. Dis. 2018;77(12):1825–1833. doi: 10.1136/annrheumdis-2018-213746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li L., Yu X., Liu J., Wang Z., Li C., Shi J., et al. Neutrophil extracellular traps promote aberrant macrophages activation in Behçet’s disease. Front. Immunol. 2021;11:3838. doi: 10.3389/fimmu.2020.590622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krötz F., Sohn H.-Y., Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004;24(11):1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 173.Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirology. 2009;14(1):27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 174.Rahman I., Adcock I. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 175.Ay C., Dunkler D., Marosi C., Chiriac A.-L., Vormittag R., Simanek R., et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 176.Ay C., Jungbauer L.V., Sailer T., Tengler T., Koder S., Kaider A., et al. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin. Chem. 2007;53(7):1235–1243. doi: 10.1373/clinchem.2006.085068. [DOI] [PubMed] [Google Scholar]
- 177.Kim K., Li J., Tseng A., Andrews R.K., Cho J. NOX2 is critical for heterotypic neutrophil-platelet interactions during vascular inflammation. Blood. 2015;126(16):1952–1964. doi: 10.1182/blood-2014-10-605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pignatelli P., Sanguigni V., Lenti L., Ferro D., Finocchi A., Rossi P., et al. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110(10):1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 179.Tajima M., Sakagami H. Tetrahydrobiopterin impairs the action of endothelial nitric oxide via superoxide derived from platelets. Br. J. Pharmacol. 2000;131(5):958. doi: 10.1038/sj.bjp.0703648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ju C., Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 2016;13(3):316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goldmann O., Medina E. The expanding world of extracellular traps: not only neutrophils but much more. Front. Immunol. 2013;3:420. doi: 10.3389/fimmu.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pertiwi K.R. 7th International Conference on Research, Implementation, and Education of Mathematics and Sciences (ICRIEMS 2020) Atlantis Press; 2021. Potential roles of extracellular traps in the progression of Coronavirus Disease (Covid)-19. [Google Scholar]
- 183.Pertiwi K.R., de Boer O.J., Mackaaij C., Pabittei D.R., de Winter R.J., Li X., et al. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 2019;247(4):505–512. doi: 10.1002/path.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kapur R., Zufferey A., Boilard E., Semple J.W. Nouvelle cuisine: platelets served with inflammation. J. Immunol. 2015;194(12):5579–5587. doi: 10.4049/jimmunol.1500259. [DOI] [PubMed] [Google Scholar]
- 185.Popa M., Tahir S., Elrod J., Kim S.H., Leuschner F., Kessler T., et al. Role of CD40 and ADAMTS13 in von willebrand factor-mediated endothelial cell–platelet–monocyte interaction. Proc. Natl. Acad. Sci. 2018;115(24):E5556–E5565. doi: 10.1073/pnas.1801366115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Semple J.W., Italiano J.E., Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 187.Hottz E.D., Medeiros-de-Moraes I.M., Vieira-de-Abreu A., de Assis E.F., Vals-de-Souza R., Castro-Faria-Neto H.C., et al. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J. Immunol. 2014;193(4):1864–1872. doi: 10.4049/jimmunol.1400091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Weyrich A.S., Elstad M.R., McEver R.P., McIntyre T.M., Moore K.L., Morrissey J.H., et al. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Invest. 1996;97(6):1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Middleton E.A., Rondina M.T., Schwertz H., Zimmerman G.A. Amicus or adversary revisited: platelets in acute lung injury and acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2018;59(1):18–35. doi: 10.1165/rcmb.2017-0420TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pan D., Amison R.T., Riffo-Vasquez Y., Spina D., Cleary S.J., Wakelam M.J., et al. P-rex and vav rac-GEFs in platelets control leukocyte recruitment to sites of inflammation. Blood. 2015;125(7):1146–1158. doi: 10.1182/blood-2014-07-591040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kasper B., Brandt E., Bulfone-Paus S., Petersen F. Platelet factor 4 (PF-4)–induced neutrophil adhesion is controlled by src-kinases, whereas PF-4–mediated exocytosis requires the additional activation of p38 MAP kinase and phosphatidylinositol 3-kinase. Blood. 2004;103(5):1602–1610. doi: 10.1182/blood-2003-08-2802. [DOI] [PubMed] [Google Scholar]
- 192.McDonald B., Urrutia R., Yipp B.G., Jenne C.N., Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 193.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nakazawa D., Desai J., Steiger S., Müller S., Devarapu S.K., Mulay S.R., et al. Activated platelets induce MLKL-driven neutrophil necroptosis and release of neutrophil extracellular traps in venous thrombosis. Cell Death Dis. 2018;4(1):1–11. doi: 10.1038/s41420-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Maugeri N., Campana L., Gavina M., Covino C., De Metrio M., Panciroli C., et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014;12(12):2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 197.Vogel S., Bodenstein R., Chen Q., Feil S., Feil R., Rheinlaender J., et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 2015;125(12):4638–4654. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vogel S., Rath D., Borst O., Mack A., Loughran P., Lotze M.T., et al. Platelet-derived high-mobility group box 1 promotes recruitment and suppresses apoptosis of monocytes. Biochem. Biophys. Res. Commun. 2016;478(1):143–148. doi: 10.1016/j.bbrc.2016.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park J.S., Svetkauskaite D., He Q., Kim J.-Y., Strassheim D., Ishizaka A., et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 200.Jenne C.N., Wong C.H., Zemp F.J., McDonald B., Rahman M.M., Forsyth P.A., et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13(2):169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 201.Taus F., Salvagno G., Canè S., Fava C., Mazzaferri F., Carrara E., et al. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40(12):2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Le Joncour A., Biard L., Vautier M., Bugaut H., Mekinian A., Maalouf G., et al. Neutrophil-platelet and monocyte-platelet aggregates in COVID-19 patients. Thromb. Haemost. 2020;120(12):1733–1735. doi: 10.1055/s-0040-1718732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guo L., Rondina M.T. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front. Immunol. 2019;10:2204. doi: 10.3389/fimmu.2019.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ivanov I.I., Apta B.H., Bonna A.M., Harper M.T. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-49635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lösche W., Scholz T., Temmler U., Oberle V., Claus R.A. Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils. Platelets. 2004;15(2):109–115. doi: 10.1080/09537100310001649885. [DOI] [PubMed] [Google Scholar]
- 207.Scholz T., Zhao L., Temmler U., Bath P., Heptinstall S., Lösche W. The GPIIb/IIIa antagonist eptifibatide markedly potentiates platelet-leukocyte interaction and tissue factor expression following platelet activation in whole blood in vitro. Platelets. 2002;13(7):401–406. doi: 10.1080/0953710021000024367. [DOI] [PubMed] [Google Scholar]
- 208.Zhao L., Bath P.M., May J., Lösche W., Heptinstall S. P-selectin, tissue factor and CD40 ligand expression on platelet-leucocyte conjugates in the presence of a GPIIb/IIIa antagonist. Platelets. 2003;14(7–8):473–480. doi: 10.1080/09537100310001638562. [DOI] [PubMed] [Google Scholar]
- 209.Heijnen H.F., Schiel A.E., Fijnheer R., Geuze H.J., Sixma J.J. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and -granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 210.Cappellano G., Raineri D., Rolla R., Giordano M., Puricelli C., Vilardo B., et al. Circulating platelet-derived extracellular vesicles are a hallmark of sars-cov-2 infection. Cells. 2021;10(1):85. doi: 10.3390/cells10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Puhm F., Boilard E., Machlus K.R. Platelet extracellular vesicles: beyond the blood. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):87–96. doi: 10.1161/ATVBAHA.120.314644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sung P.-S., Huang T.-F., Hsieh S.-L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 2019;10(1):1–13. doi: 10.1038/s41467-019-10360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vats R., Brzoska T., Bennewitz M.F., Jimenez M.A., Pradhan-Sundd T., Tutuncuoglu E., et al. Platelet extracellular vesicles drive inflammasome–IL-1β–dependent lung injury in sickle cell disease. Am. J. Respir. Crit. Care Med. 2020;201(1):33–46. doi: 10.1164/rccm.201807-1370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Weiss R., Gröger M., Rauscher S., Fendl B., Eichhorn T., Fischer M.B., et al. Differential interaction of platelet-derived extracellular vesicles with leukocyte subsets in human whole blood. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-25047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bozza F.A., Shah A.M., Weyrich A.S., Zimmerman G.A. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am. J. Respir. Cell Mol. Biol. 2009;40(2):123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yun S.-H., Sim E.-H., Goh R.-Y., Park J.-I., Han J.-Y. Platelet activation: the mechanisms and potential biomarkers. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dittrich M., Birschmann I., Pfrang J., Herterich S., Smolenski A., Walter U., et al. Analysis of SAGE data in human platelets: features of the transcriptome in an anucleate cell. Thromb. Haemost. 2006;95(04):643–651. [PubMed] [Google Scholar]
- 218.Brown G.T., Narayanan P., Li W., Silverstein R.L., McIntyre T.M. Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop. J. Immunol. 2013;191(10):5196–5203. doi: 10.4049/jimmunol.1300354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hottz E.D., Lopes J.F., Freitas C., Valls-de-Souza R., Oliveira M.F., Bozza M.T. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122(20):3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cloutier N., Allaeys I., Marcoux G., Machlus K.R., Mailhot B., Zufferey A., et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl. Acad. Sci. 2018;115(7):E1550–E1559. doi: 10.1073/pnas.1720553115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Duerschmied D., Suidan G.L., Demers M., Herr N., Carbo C., Brill A., et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121(6):1008–1015. doi: 10.1182/blood-2012-06-437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chae W.-J., Ehrlich A.K., Chan P.Y., Teixeira A.M., Henegariu O., Hao L., et al. The wnt antagonist Dickkopf-1 promotes pathological type 2 cell-mediated inflammation. Immunity. 2016;44(2):246–258. doi: 10.1016/j.immuni.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fitch-Tewfik J., Flaumenhaft R. Platelet granule exocytosis: a comparison with chromaffin cells. Front. Endocrinol. 2013;4:77. doi: 10.3389/fendo.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sut C., Tariket S., Aubron C., Aloui C., Hamzeh-Cognasse H., Berthelot P., et al. The non-hemostatic aspects of transfused platelets. Front. Med. 2018;5:42. doi: 10.3389/fmed.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Andre P., Prasad K.S., Denis C.V., He M., Papalia J.M., Hynes R.O., et al. CD40L stabilizes arterial thrombi by a β 3 integrin–dependent mechanism. Nat. Med. 2002;8(3):247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 227.Müller K.A., Chatterjee M., Rath D., Geisler T. Platelets, inflammation and anti-inflammatory effects of antiplatelet drugs in ACS and CAD. Thromb. Haemost. 2015;114(09):498–518. doi: 10.1160/TH14-11-0947. [DOI] [PubMed] [Google Scholar]
- 228.Santoro F., Nuñez-Gil I.J., Vitale E., Viana-Llamas M.C., Reche-Martinez B., Romero-Pareja R., et al. Antiplatelet therapy and outcome in COVID-19: the health outcome predictive evaluation registry. Heart. 2022;108(2):130–136. doi: 10.1136/heartjnl-2021-319552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chow J.H., Khanna A.K., Kethireddy S., Yamane D., Levine A., Jackson A.M., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth. Analg. 2021;132(4):930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 230.Meizlish M.L., Goshua G., Liu Y., Fine R., Amin K., Chang E., et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am. J. Hematol. 2021;96(4):471–479. doi: 10.1002/ajh.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Spaetgens B., Nagy M., Ten Cate H. Antiplatelet therapy in patients with COVID-19—more is less? JAMA. 2022;327(3):223–224. doi: 10.1001/jama.2021.23866. [DOI] [PubMed] [Google Scholar]
- 232.Group RC Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (London, England) 2022;399(10320):143–151. doi: 10.1016/S0140-6736(21)01825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sexton T.R., Zhang G., Macaulay T.E., Callahan L.A., Charnigo R., Vsevolozhskaya O.A., et al. Ticagrelor reduces thromboinflammatory markers in patients with pneumonia. JACC. 2018;3(4):435–449. doi: 10.1016/j.jacbts.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Maes M., Higginson E., Pereira-Dias J., Curran M.D., Parmar S., Khokhar F., et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care. 2021;25(1):1–11. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chaharom F.E., Pourafkari L., Chaharom A.A.E., Nader N.D. Effects of corticosteroids on Covid-19 patients: a systematic review and meta-analysis on clinical outcomes. Pulm. Pharmacol. Ther. 2021;102107 doi: 10.1016/j.pupt.2021.102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. Epub 2005/10/21. [DOI] [PubMed] [Google Scholar]
- 237.Johannesdottir S.A., Horváth-Puhó E., Dekkers O.M., Cannegieter S.C., Jørgensen J.O.L., Ehrenstein V., et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern. Med. 2013;173(9):743–752. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- 238.Martínez-Taboada V.M., López-Hoyos M., Crespo J., Hernández J.L. COVID-19 and immune-mediated inflammatory diseases: why don't our patients get worse? Autoimmun. Rev. 2020;19(12) doi: 10.1016/j.autrev.2020.102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Levi M. Tocilizumab in severe COVID-19: a promise fulfilled. Eur. J. Intern. Med. 2022;95:38–39. doi: 10.1016/j.ejim.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M. 2021. Interleukin-6 Receptor Antagonists in Critically Ill Patients With Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chan K.H., Patel B., Podel B., Szablea M.E., Shaaban H.S., Guron G., et al. Tocilizumab and thromboembolism in COVID-19: a retrospective hospital-based cohort analysis. Cureus. 2021;13(5) doi: 10.7759/cureus.15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stallmach A., Kortgen A., Gonnert F., Coldewey S.M., Reuken P., Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure—a cautionary case series. Crit. Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020;2(5):276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ucciferri C., Auricchio A., Di Nicola M., Potere N., Abbate A., Cipollone F. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020;2(8) doi: 10.1016/S2665-9913(20)30167-3. e457-ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Annane D., Heming N., Grimaldi-Bensouda L., Frémeaux-Bacchi V., Vigan M., Roux A.-L., et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Legendre C., Licht C., Muus P., Greenbaum L., Babu S., Bedrosian C., et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N. Engl. J. Med. 2013;368(23):2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]