Abstract
Physical and mental health and hormonal imbalance are associated with the problems related to infertility and reproductive disorders. The rate of infertility has increased globally over the years, due to various reasons. Given the psychosocial implications of infertility and its effects on the life of the affected people, there has been an increased focus on its treatment over the last several years. Assisted reproductive technology can only solve about 50% of the cases. Moreover, it contains significant risks and does not solve the fundamental problem of infertility. As pluripotent stem cells have the potential to differentiate into almost any type of cell, they have been widely regarded as a promising option in the development of stem cell-based fertility treatments, which could even correct genetic diseases in offspring. These advancements in reproductive biotechnology present both challenges and possibilities for solving infertility problems caused by various unexplainable factors. This review briefly presents the different types of infertility disorders and the potential applications of stem cells in the treatment of these reproductive diseases.
Keywords: infertility, stem cells therapy, proliferation, differentiation, gametogenesis
Introduction
Infertility, which may be caused by genetic factors or an unhealthy lifestyle, has become a global concern. The main causes of male infertility include impaired spermatogenesis and abnormal sperm quality in males. In females, pelvic or inguinal surgery, impairment of follicle development, ovulation disorders, ovarian dysfunction, damaged fallopian tube or fallopian tube obstruction, and so on, could give rise to female infertility. Assisted reproductive technologies (ART) have shown promise in increasing the pregnancy rate; however, these methods have issues related to gametes regeneration and immune system intervention. 1 There are consistent reports that the application of ART for infertility treatment carries the risk of adverse perinatal outcomes and an increase in birth defects. After a cycle of intracytoplasmic sperm injection (ICSI), the risk of major birth defects associated with the cardiovascular, genitourinary, and musculoskeletal systems has been reported to have doubled. This is very concerning as ICSI accounts for 70% of all treatment cycles worldwide 2 . The stem cell technique alleviates the shortcomings of the above methods. Stem cells can proliferate extensively, produce similar copies of cells, and also differentiate into specific cells of a variety of tissues. They are commonly categorized as adult stem cells, early embryonic stem cells, and induced pluripotent stem cells (iPSCs) according to their derivations. Adult stem cells have been widely used in clinical treatment, but controversies are surrounding their ethical and safe use 3 . Umbilical cord stem cells (UCSTCs) have been successfully used in Phase I clinical trial to treat infertility due to intrauterine adhesions (IUA). No serious adverse events related to the treatment were found during the trial, and some subjects successfully gave birth to healthy offspring 4 . This article reviews the progress in the development and application of stem cell therapy as a new and effective method of treatment of infertility, in the clinical setting (Fig. 3 and Table 1).
Figure 3.
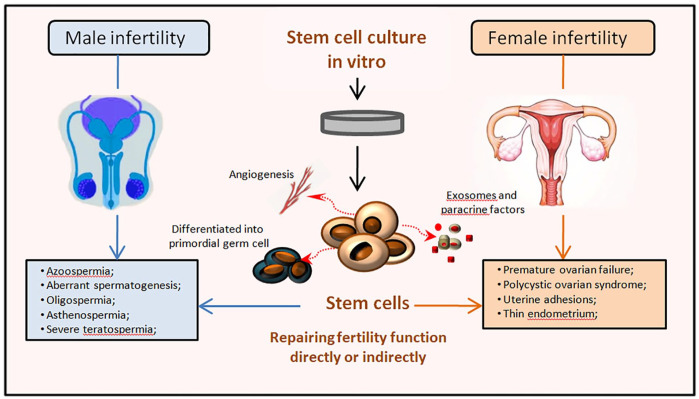
Potential applications of Stem cells on various reproductive diseases. Infertility globally affects approximately 10–15% of couples. Refractory infertility diseases, such as non-obstructive azoospermia (NOA), oligospermia, asthenospermia, severe teratozoospermia, and female’s premature ovarian failure (POF), polycystic ovary syndrome (PCOS), intrauterine adhesions (IUA), and thin endometrium, which bring great distress to both doctors and patients. The pluripotency, self-renewal and tissue repair characteristics of stem cells are regarded as great prospects for the treatment of male and female infertility and have been used in research and treatment of various infertility diseases. Various types of stem cells are isolated and cultured in vitro, which can regulate anti-oxidation, anti-apoptosis, angiogenesis and maintain immune balance by secreting cytokines and exosomes to improve microenvironment and repair the function of reproductive organs. In addition, these exogenous stem cells can differentiate into germline stem cells and participate in improving reproductive capacity directly.
Table 1.
A Variety of Stem Cells Technologies Provide the Promise for Curing Reproductive Diseases.
| Gender | Organs | Infertility diseases | Stem cells types | Applications | Effects and reference |
|---|---|---|---|---|---|
| Male | Testis | Azoospermia Apsermia Oligospermia Varicocele |
ADSCs | Rat | Spermatogenesis↑, Testis morphology↑, Birth↑ 5 |
| UCSCs | Mouse | Germ cells↑, Testicular tissue↑ 6 | |||
| iPSCs | Human | Spermatogenesis↑ 7 | |||
| SSCs | Macaque | Spermatogenesis↑ 8 | |||
| Asthenozoospermia | UCSCs | Hypothesis | Germ cells↑ 6 | ||
| Female | Endometrium | Asherman’s syndrome | MenSCs | Human | Endometrium↑ 9 |
| Intrauterine adhesions | UCSCs | Human | Endometrium↑, Birth↑ 4 | ||
| Thin endometrium and recurrent pregnancy losses | EPCs | Mouse | Endometrium↑, Birth↑ 10 | ||
| Ovary | Polycystic Ovarian Syndrome | ADSCs | Mouse, Rat | Follicles↑, Estradiol↑ 11 | |
| Premature Ovarian Failure | AFSCSs | Mouse | Follicles↑ 12 | ||
| Premature Ovarian Failure | BMSCS | Mouse | Follicles↑, Follicular stimulated hormone↓, Estradiol↑ 13 | ||
| Premature Ovarian Failure | OSCs | Mouse | Oocytes↑, Birth↑14,15 | ||
| Premature Ovarian Failure | ESCs | Mouse | Oocytes↑14,16 |
ADSCs: adipose tissue-derived MSCs; AFSCs: amniotic fluid stem cells; iPSCs: induced pluripotent stem cells; menSCs: menstrual blood-derived stromal cells; OSCs: oogonial stem cells; SSCs: spermatogonial stem cells; UCSCs: umbilical cord stem cells; BMSCs: bone marrow stem cells; EPCs: endometrial progenitor cells; ESCs: embryonic stem cells.
Human Infertility Defects
Male Infertility and Therapeutic Strategies
Spermatogenesis is the process by which mature sperm cells are produced through cellular differentiation within the testes. It is a continuous process that occurs throughout the entire reproductive life of a man, and every ejaculation produces millions of sperm. Various processes of reproductive function are regulated by gonadal hormones and subtle molecular circuits during development. Maintaining reproductive health is the key to successful fertility and a healthy relationship between partners. There are several factors, such as genetic mutations, infections, anatomical change, hormonal imbalances, and psychological stress, which directly or indirectly affect the normal development and quality of sperm and prevent men from being able to father offspring 1 . Routine semen evaluations provide information about pertaining to semen volume and concentration, as well as sperm motility and morphology. According to reports, up to 30% of men have fertility problems. The main causes of male infertility are the absence of sperm in the ejaculate (azoospermia), a decrease in sperm quantity and motility (oligoasthenospermia), and poor sperm quality (lack of active sperm with normal morphology), induced by various factors. These constitute up to 90% of the problems related to male infertility 17 (Fig. 1).
Figure 1.
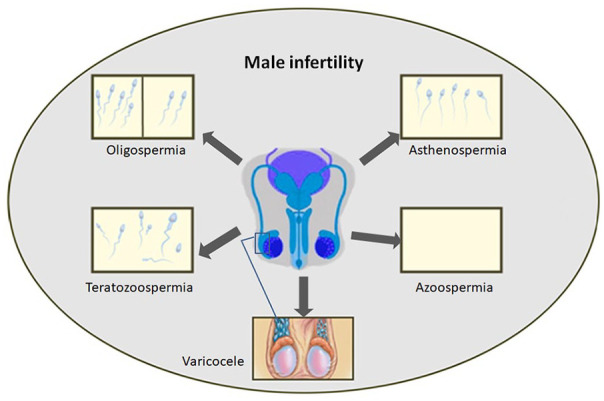
The common types of male infertility issues.
According to previous medical research, patients with low sperm counts should undergo ICSI, or orchiectomy, or non-operative therapy in accordance with established guidelines. For example, clinical trials have shown that microsurgical varicocelectomy can increase sperm concentration and motility. Therefore, improvement in the sperm quality through surgical treatment can increase the chances of infertile men to father offspring, and these men have the least risk of developing hydrocele or its recurrence 18 . Due to the latest developments in microsurgical methods and in vitro fertilization (IVF)/ICSI, an increasing number of patients with azoospermia, oligospermia (low sperm count), and poor sperm quality have obtained successful results 19 . In clinical practice, testicular sperm aspiration (TESA) and percutaneous epididymal sperm aspiration (PESA) combined with ICSI technology can solve most of the infertility problems, and the use of ART can result in successful conception. However, many studies have reported that although the possibility of residual confounding cannot be ruled out, the risk of ICSI-induced defects in the offspring increases even after multi-factor adjustment20,21. In previous studies, stem cells with self-renewal capacity were considered as a potential source for sperm generation through the formation of germ cells or the restoration of testicular tissue. However, further studies are required to understand the precise signal pathway, which will likely pave the way from theory to clinic trials and treatment 22 .
Azoospermia
Although testicular dysgenesis syndrome is usually caused by genetic defects and polymorphisms, there are reports of links to environmental and lifestyle factors resulting from rapid temporal changes which are capable of affecting the endocrine system and causing epigenetic modifications. Azoospermia is defined as the absence of sperm in the ejaculate after centrifugation during semen analysis. It is categorized into obstructive azoospermia (OA) and non-obstructive azoospermia (NOA). OA accounts for 40% of azoospermia cases and can occur in the seminal tubules, but it does not appear in post-ejaculated semen due to obstruction along the ejaculatory duct or the vas deferens duct. Patients with NOA frequently present with severe testicular failure which is diagnosed through testicular biopsy. Due to the failure of spermatogenesis, sperms are absent in the semen of men with NOA. It has been reported that there are at least 2000 genes related to spermatogenesis, and azoospermia accounts for 25% of the genetic causes of male infertility 23 . NOA is more frequently reported to be genetically abnormal than OA24,25. Some investigations have shown that chromosomal and genetic abnormalities can also cause different types of male infertility 25 . Klinefelter syndrome with a 47, XXY chromosomal complement is the most frequent chromosomal abnormality in patients with azoospermia. Recent studies have revealed that microdeletions on the long arm of the Y chromosome (Yq), especially at the azoospermic factor(AZF) region result in impaired spermatogenesis and are the genetic cause of the most common sperm failure resulting in male infertility. All microdeletions of AZFa, AZFb, and AZFc sub-regions on the Y chromosome can result in NOA although there is the possibility of the presence of sperms if only the AZFc sub-region is deleted 26 . Some cases of NOA are mainly diagnosed due to abnormalities in gonadotropin hormone release and function caused by hypothalamic or pituitary diseases, or intrinsic testicular abnormalities that may affect spermatogenesis. In primary testicular failure resulting from abnormalities in the functional elements of the testes, it is impossible to induce spermatogenesis through hormonal stimulation.
Low sperm quantity
Spermatogenesis is a complex network of physiological processes such as spermatogonial proliferation, spermatocyte meiosis, and spermatid morphogenesis occurring in the seminiferous tubules 27 and eventually forming mature male gametes. The differentiation of spermatogonia into spermatozoa requires the participation of multiple cell types, hormones, paracrine and autocrine factors, genes, and epigenetic regulators28,29. Some diseases and conditions, such as obesity, psychological factors, and environmental exposure, can affect spermatogenesis and affect the offspring28,30–32.
Oligospermia, asthenospermia, teratospermia, and high sperm DNA fragmentation rates are the most common phenotypes of poor sperm quality. Studies have confirmed that these low-quality sperms can cause infertility or poor pregnancy outcomes. Research on the genes and molecular mechanisms related to sperm abnormalities has been receiving increasing attention as genetic investigations could provide clues for treatment 33 . The various genes linked to the different types of sperm-related abnormalities are as follows: oligospermia:GSTM1, DNMT3L and CYP1A1; asthenospermia:CATSPER1, CRISP2, SEPT4, TCTE3, TEKT4, DNAH1, and so on; teratozoospermia:DPY19L2 and AURKC 34 . Metabolic and transcriptional abnormalities have been observed in the semen of men with as then ozoospermia. Studies have revealed that the expression levels of the genes encoding fructokinase citrate synthase, succinate dehydrogenase, and spermine synthase, which are associated with enzyme metabolism, were reduced in the semen of patients with asthenospermia 35 . Moreover, sperm motility and viability are affected by the exosomes in the male reproductive tract. Exosomes derived from individuals with normal sperms can increase sperm motility and trigger capacitation 36 . Varicocele with the etiology of intrinsic testicular impairment also induces testicular spermatogenesis by damaging sperm DNA, such that the reproductive function in patients gets severely affected 37 . Factors such as infection, oxidative stress, smoking, and an unhealthy lifestyle can also result in poor sperm quality.
Female Infertility and Therapeutic Strategies
Development of the ovarian follicles containing oocytes (mature ova or egg cells) is regulated by the endocrine and paracrine systems. Normal maturation of the oocytes is a prerequisite for a successful pregnancy. The hypothalamic-pituitary-gonadal axis (HPG) plays a critical role in the regulation of reproductive events 38 . Paracrine and autocrine signals between oocytes, cumulus granulosa, and mural granulosa are responsible for ovulation. Many of the signaling molecules involved in this process belong to the transforming growth factor beta (TGF-β) super family. Eppig et al. reported that, other than the bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9) belonging to TGF-β, fibroblast growth factors (FGFs) derived from oocytes are also involved in cumulus glycolysis for promoting ovarian development39,40. The causes of ovarian dysfunction remain complicated and controversial. Hormonal imbalances, such as primary or secondary hypergonadotropic amenorrhea, and also cholesterol concentration physiologically affect the oocyte survival 41 . Gene sequencing technology has identified chromosomal abnormalities and genetic mutations as the cause of infertility in females. Infertility may also be caused by immune system dysfunction, resulting from innate/adaptive immune response to viral infection42,43. Genetic and environmental factors can influence the rate of loss of primordial follicles, but exposure to harmful stimuli such as radiation, chemotherapy, cigarette smoking, and so on, can accelerate this process 44 . Furthermore, psychological impairments, predominately low self-confidence, have also been identified in cross-sectional research as one of the causes 45 . The most common causes of female infertility are premature ovarian failure, polycystic ovary syndrome (PCOS), and endometrial dysfunction including intrauterine adhesions and thin endometrium (Fig. 2). Females with abnormal ovarian function and endometrial dysfunction neither become pregnant during their child-bearing age nor successfully conceive through in vitro fertilization.
Figure 2.
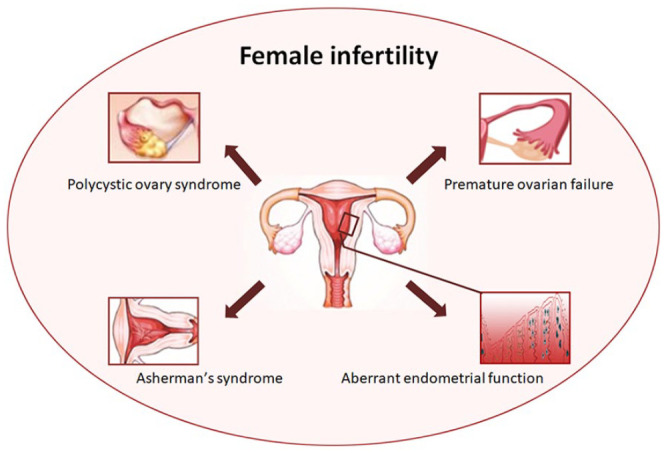
The common types of female infertility issues.
Potential therapeutic strategies might include targeting the mechanisms that control the HPO axis, improving the histological morphology of the uterus, and managing the expression and failure of genes related to the regulation of the nervous-endocrine-immune systems, and modulating the signal pathway associated with infertility. Previous therapeutic remedies, such as hormone replacement and psychological support, have been applied in the treatment of female infertility. Furthermore, in vivo fertilization treatment might prove beneficial severalinfertile couples 46 . Although IVF has a low risk of long-term adverse outcomes and involves limited usage of drugs, it requires mature oocytes of high quality 47 . The stem cell-based transplantation technique might effectively alleviate such problems and restore fertility.
Premature ovarian failure
Premature ovarian failure (POF) is one of the most common causes of female infertility. It is a heterogeneous disorder characterized by ovarian atrophy, diminished ovarian reserve, menstrual disorder, and ovarian dysfunction, along with elevated follicle stimulating hormone (FSH) and relatively low estradiol (E2) levels in women under 40 years of age 48 . Anti-Mullerian hormone (AMH) is exclusively produced by the ovarian follicular granulosa cells during the early stages of follicle development and is a reliable method for diagnosing ovarian follicular dysplasia. The genetic causes of follicular dysfunction include abnormal chromosomal structure and mutations in specific genes related to the ovarian function or metabolic regulation 48 . Chemotherapy and radiation therapy could also damage the DNA, leading to the loss of pre-antral follicles and eventually disrupting ovarian function1,44. Granulosa cells (GCs) dysfunction causes follicular atresia and has been implicated as the main cause of premature ovarian insufficiency (POI) 49 . Duan et al. found that lncRNA LINC02690 or GCAT1 (granulosa cell-associated transcript 1) is down regulated in the GCs of patients with biochemical POI (bPOI), which is demonstrated as a novel form of lncRNA-mediated epigenetic regulation of GC function that contributes to the pathogenesis of POI 50 . Studies have shown that stem cells from different sources play an important role in ovarian recovery through multiple mechanisms such as migration, anti-apoptosis, anti-fibrosis, angiogenesis, anti-inflammatory, immune regulation, and oxidative stress. Several articles have pointed out that MSCs can reduce cumulus cell apoptosis and restore sex hormone levels, whereas germline stem cells can produce new oocytes, showing female imprinting patterns14,51,52.
Polycystic ovarian syndrome
Polycystic ovary syndrome (PCOS) is one of the most common reproductive and metabolic dysfunctions in women of child-bearing age, with a prevalence rate of up to 10% 53 . Environmental and genetic factors play an important role in causing this disorder. Patients with PCOS are infertile due to the poor quality of the oocytes 54 . A review of publications available on this subject reveals that women with infertility are vulnerable to this condition, and women with PCOS are more likely to develop cardiovascular disease and metabolic disorders, such as diabetes, than the general population55,56. Insulin resistance and obesity associated with metabolic syndrome are prevalent in PCOS but are not common. Besides, clinical trials have shown that weight loss interventions before infertility treatment initiation are beneficial for reproduction and metabolism in obese women 57 . Immune disorders may play an important role in the pathogenesis of PCOS. Ingestion of glucose and saturated fat triggers mononuclear cells (MNCs) to secrete pro-inflammatory cytokines such as TNF-a, IL-6, and IL-1β, which subsequently mediate insulin resistance and hyperandrogenism in PCOS58,59. Based on the analysis of follicular fluid lymphocytes, the Th1 response of PCOS patients was significantly higher when compared with the control group 47 . Human MSCs can suppress human Th1 response, which could influence the internal inflammatory environment in PCOS 60 . The ovarian function may recover after the cessation of autoimmune reaction and the regression of concurrent endocrine disease in accordance with these alterations. Human mesenchymal stem cells preserve the capacity to suppress human Th1 response that may influence the internal inflammatory environment of PCOS 61 . It is interesting to note that stem-cell technology has the potential to generate functional oocytes for treating patients with PCOS.
Endometrial disorders
The endometrium is a dynamic and complex tissue composed of basal and functional layers, and is vital for the implantation of the embryo and menstruation 62 . A successful pregnancy involves a synchronized and coordinated cross-talk between a high-quality embryo with implantation capability and the endometrium. Repeated implantation failure (RIF), as the name suggests, refers to the process wherein good-quality embryos fail to undergo implantation even after repeated transfers. Its cause might lie in the embryo itself, the mother, or in some cases, both. However, in recent years, it has been reported to be closely related to abnormal endometrial function63–65. The temporal and spatial variation of the endometrial function is influenced by the circulation of the hormones produced by the various cellular components of the endometrium, including epithelial cells, stromal cells, local immune cells, and the vascular system. These endocrine hormones are regulated through the hypothalamic-pituitary-ovarian axis and paracrine morphological factors, cytokines, growth factors, etc66–68. Any paracrine- and autocrine-related changes can cause endometrial dysfunction. Adhesions, a thin endometrium, as well as insufficient endometrial growth are the most common uterine factors that affect the endometrial function and decrease the chances of pregnancy 69 . The Asherman’s syndrome(AS) is caused by intrauterine adhesions as a result of injury to the basal layer, as well as endometriosis, and occurs frequently during uterine cavity surgeries 70 . In patients with severe uterine adhesions, the viable endometrial surface may be reduced, leading to negative effects on fertility and recurrent miscarriage. Stem cells can directly or indirectly promote endometrial regeneration and have been proven to be an effective strategy for the treatment of intrauterine adhesions. Tan et al. cultured the menstrual blood-derived stromal cells (menSCs) of 7 patients with AS and performed autologous transplantation back into the uterus 9 . They observed that the morphology of the endometrium returned to normal, and its thickness increased significantly. Furthermore, three patients with refractory AS had successful pregnancies after being transplanted with the menSCs 9 .
Application of Stem Cell Therapies in Infertility
Stem cells have become a promising treatment option for infertility due to their numerous remarkable characteristics such as multi-directional differentiation, angiogenesis, immune regulation, and paracrine stimulation. The proposed mechanism of stem cells in the repair of reproductive dysfunction includes the following steps: stem cells migrate to the injured reproductive tissues caused by chemokines, and then differentiate and integrate somatic cells with non-tumorigenic properties. These cells, especially in the GCs, participate in follicle development and regulate ovarian physiology, including ovulation and luteal regression71,72. They may reside in the reproductive tissue and help ameliorate the damaged microenvironment by producing paracrine factors. Subsequent stem cell transplantations can restore the endocrine function by secreting anti-inflammatory factors or increasing the number of Treg cell population, leading to immune suppression 73 . Adult stem cells are isolated from a variety of tissues, including bone marrow, adipose tissue, Wharton’s jelly, umbilical cord blood, human amniotic fluid, and peripheral blood. Current reports have proposed that these stem cells could rescue unexplained infertility74–76. MSCs, the most common adult stem cells, have the highest multi-lineage differentiation potential among human stem cells to date 77 . Stem cells derived from the bone marrow, amniotic fluid, embryos, iPSCs, spermatogonia, and oocytes can all be reprogrammed to generate germ cells. Tissue-specific resident stem cells are sources of regenerative gametes, which could assist future research in reproductive medicine. In this section, we discuss the various features of stem cells, including migration, anti-apoptosis, anti-fibrosis, angiogenesis, anti-inflammation, immunoregulation, and oxidative stress, which provide the theoretical basis for further reproductive medicine research and clinical infertility treatment (Fig. 3).
Bone Marrow Stem Cells
Bone marrow stem cells are rich in hematopoietic cells (HSCs) and mesenchymal stem cells (BMMSCs). Both of them were extensively used for specific clinical applications in the fields of regenerative medicine and tissue engineering. This section further discusses the role of these two types of stem cells in the field of infertility. This will provide potential ideas for finding effective infertility treatments.
Haematopoietic stem cells
It is well know that hematopoietic stem cells (HSCs) transplantation can cure leukemia. HSCs have rarely been suggested, individually, to restore reproduction function by improving the local microenvironment. Many studies have found that ovarian failure is a serious late complication of allogeneic hematopoietic stem cell transplantation78,79. Sonoko Shimoji et al. have demonstrated donor T-cell-mediated graft-versus-host disease (GVHD) could target the ovary and impair ovarian function and fertility in mice after allogeneic hematopoietic stem cell transplantation 80 . However, the pre-transplant conditioning regimen has been appreciated as a cause of ovarian failure 81 . Mobilization of stem cells with G-CSF was found to decreased apoptosis, and increased proliferation of spermatogenic cells, so that to maintain testicular histology at the beginning of busulfan treatment 82 . Thus, the safety and effectiveness of HSCs for repairmen of reproductive organs function and infertility needs more research evidence.
Bone marrow mesenchymal stem cells (BMMSCs)
In recent decades, the biomedical applications of mesenchymal stem cells (MSCs) have attracted more and more attention. Since MSCs are easily extracted from bone marrow, fat and synovium, and differentiate into various cell lineages according to the requirements of specific biomedical applications, they are considered to have great potential in the treatment of various diseases. Results obtained from completed and on-going clinical studies indicate huge therapeutic potential of MSCs-based therapy in the treatment of degenerative, autoimmune, and genetic disorders. However, the ability to promote tumor growth and metastasis and overestimated therapeutic potential of MSCs still provide concerns for the field of regenerative medicine 83 . Thus, we should be more cautious in using the multi-differentiation potential of MSCs in the field of reproductive medicine.
Bone marrow-derived MSCs are adherent and heterogeneous assembled fibroblasts, which may be involved in angiogenesis, immunoregulation, and regeneration of gametes 84 . It has been reported in animal models that BMSCscan differentiate into hepatocytes, muscle cells, astrocytes, neurons, and endothelial cells, while human-derived BMSCs can differentiate into osteoblasts, adipocytes, chondrocytes, cardiomyocytes, muscle cells, neurons, and gastrointestinal cells83,85–88.
BMSC transplantation could improve folliculogenesis in PCOS mice through anti-inflammatory, anti-oxidative, and anti-apoptotic processes 89 . Past researchers have reported that miR-644-5p carried by BMSC-derived exosomes inhibited the apoptosis of ovarian granulosa cells by targeting p53 of cells to treat POF and restore ovarian function 90 . BMSCs exert functions mainly by inhibiting apoptosis of granulosa cells and promoting the activity of residual ovarian cells proliferation 91 . The Al-Hendy research group introduced the FSHR (-/-) FOR KO mouse POF model, proving that MSCs could restore follicular maturation and steroid hormone production, which could reveal the signal pathway for the treatment of female infertility 13 . A pilot study investigated the beneficial effects of the CD133+ BMSCs integrated with hormone replacement therapy in patients with endometrial atrophy (EA). Among the 5 patients with EA, 4 cases of endometrial thickness increased from 4.2 mm (2.7–5) to 5.7 mm (5–12), and 1 case successfully delivered a baby 92 . BMSCs demonstrated the potential to trans-differentiate into spermatogenic-like cells and enhance endogenous fertility recovery in busulfan-induced azoospermia model 93 . Thus, bone marrow-derived MSCs serve as a treatment option for both male and female infertility by improving the injured tissue or forming germ cells.
Adipose-Derived Stem Cells
Adipose tissue-derived MSCs (ADSCs) can be applied in future clinical therapy as are available in abundance following liposuction or lipectomy, with very little donor site discomfort 5 . ADSCs seem to improve vascular remodeling and tissue recovery indirectly through the secretion of paracrine cytokines or directly by differentiating into the injured cells 94 . Furthermore, autologous stem cells, the promising strategies for future tissue engineering, are more immunocompatible than allograft substitutions related to clinical use 95 . Autologous cells replacement of ADSCs was proven to elicit low alloimmune reaction when transplanted into patients without major histocompatibility complex-II expression.
ADSCs have been successfully used in the clinical treatment of traumatic calvarial defects, Buerger’s disease, and cartilage/bone regeneration96–98. They have been used to address reproductive problems in animal models of infertility. It has been revealed that ADSCs can differentiate into endometrial epithelial cells, thereby repairing the injured endometrium in vivo 99 . In addition, studies have also shown that autologous ADSC mitochondria can promote oocyte quality, embryonic development, and fertility in elderly mice, which could be a promising strategy for the treatment of reduced fertility or infertility in elderly women 100 . Yali Hu and colleagues injected green fluorescent protein (GFP)-ADSCs combined with collagen scaffolds, as degradable biomaterials, into ovaries. GFP signals were mainly observed in the ovarian interstitial cells and were retained longer with collagen than without it. In the study, the microenvironment constituting ADSCs and collagen aggregated in the ovary, which elevated the estradiol levels and increased the number and size of the antral follicles 11 . In addition, ADSCs can produce a mass of exosomes. Studies have shown that exosomal miR-323-3p was collected from modified ADSCs can promote cell proliferation and inhibit apoptosis in cumulus cells (CCs) in PCOS 101 . Moreover, ADSCs can be differentiated into primordial germ cell (PGC)-like cells through several treatment approaches 102 . The activation of Integrin-β3-TGF-β and MAPK pathways may be involved in the differentiation of MSCs into germ-like cells 103 . These recent studies could indicate the tendency of collagen/ADSCs to treat POF in the future. Erdal et al. reported that after injecting ADSCs into the seminiferous tubules in a rat model of azoospermia, spermatogenesis was completely restored, and the successive generations continued to have normal spermatogenesis. ADSCs initiate spermatogenesis in the testis and differentiate into spermatogonial stem cells (SSC) 5 . ADMSCs co-cultured with Sertoli cells (SCs) during retinoic acid and testosterone treatment stimulated the generation of male germ-like cells (MGLC) in vitro by activating theTGFβ-SMAD2/3, JAK2-STAT3, and AKT pathways 104 . ADSCs are considered to be effective and important candidate cells for the treatment of male infertility as they contain pluripotent stem cells that can differentiate into any cell of the three germ layers.
Umbilical Cord Stem Cells
The umbilical cord is a promising mesenchymal stem cell bank. UCSCs) are a sub-set of primitive stem cells with long-term self-renewal ability. They are involved in restoring injured tissues, and can be derived from the growing tissue fragments of Wharton’s jelly, amniotic membrane, cord lining, and perivascular region 105 . Human UCMSCs are recognized by monoclonal antibodies, and are mainly positive for CD29, CD44, CD90, CD105, and HLA-I surface markers, but negative for antigen expressions associated to CD106, CD133, hematopoietic stem cell CD34, leucocyte CD45, and HLA-DR87,106. A large number of studies have reported that human UCSC therapy can rescue the structure and function of injured tissues. One of the underlying mechanisms is proposed to be the activation of paracrine cytokines. Studies have confirmed that the ovarian function in aging mice was significantly improved after treatment with human UCSCs 107 . In female mice, superovulation and ozone inhalation were used to establish an accelerated model in which the number and quality of oocytes decreased. Another group of researchers observed that UCSCs survived in the testes for at least 120 days. They transplanted UCSCs into the tubules of germ cells-deficient mice and noted that they showed characteristics similar to sperms 6 . A phase I clinical trial enrolled 26 patients with IUA, who met the treatment criteria, and assessed the clinical characteristics following the transplantation of UCSC/collagen scaffold into their uterine cavity during a 30-month follow-up period. They reported that the uterine cavity of 20 patients improved with no surgical complications and 8 patients gave birth successfully 4 . These studies show that UCSCs have strong application prospects for the treatment of infertility.
Human Amniotic Fluid Stem Cells
The amniotic fluid is characterized by a light yellow transparent liquid, which protects and cushions the fetus in the amniotic sac throughout pregnancy 108 . Human pregnancy is generally divided into 3 phases, and the amniotic fluid contains different types of cells in each phase. As amniotic fluid stem cells (AFSCs) originated from the fetus of the same individual undergoing treatment, they may be used for regenerative therapy with low immunogenicity. It is recommended to obtain AFSCs from amniocentesis during full-term pregnancy or delivery. The stem cells of the amniotic fluid during mid-term and third term (full-term) pregnancy have a higher differentiation potential. Several studies have shown that AFSCs have surface markers (CD29, CD73, CD90, CD105, and CD117), pluripotent cell markers (Oct-4, C-myc, Ssea4+), and other characteristics after immune selection 12 .
In a study on the treatment of infertile mice with human AFSCs (hAFSCs), it was observed that the medium containing germ cell maturation factor cocktail could induce embryonic bodies from hAFSCs into germ-like cells in vitro, which showed elevated levels of meiotic germ cell markers Blimp1, Stella, Dazl, Vasa, Stra8, Scp3, and c-Mos but decreased levels of stem cell markers Oct4 and Nanog 109 . After transplanting hAFSCs into the ovaries of chemically damaged mice, immunohistochemistry was performed with human-specific nuclei antigen which was observed to bemainly co-localized with human follicle stimulating hormone receptor (FSHR) in the antral follicles around the oocytes. As a result, AMH was restored to normal levels 2 months after the hAFSCs treatment. Transplanting AFSCs into an ovarian model of POF caused by chemotherapy could save the reproductive ability by maintaining healthy follicles and preventing follicular atresia, but they could not differentiate into germ cells 12 . Gundacker et al. extensively discussed that AFSCs could be used to study the genetic regulation of spermatogenesis and screen male reproductive toxicity as they can be derived from amniocentesis with pathogenic mutations and effectively transfected 27 . These results suggest that hAFSCs can restore the morphology of chemically damaged follicles in mice. However, further testing of indicators is needed to determine its efficacy.
Embryonic Stem Cells
ESCs were first isolated and identified in mouse blastocysts by Kaufman in 1981 110 . In 1998, Thomson et al. studied the effect of human ESCs on severe combined immunodeficiency (SCID) mice and noted that both in vivo and in vitro, they could produce trophoblast cells and three embryonic germ layer derivatives 111 . ESCs have been developed as a new clinical strategy, as they can exert their differentiation potential to generate the required cells or reconstruct the transplanted tissue in vitro 112 . In vitro stem cell transplantation may provide effective treatment for severe diseases. However, the immune barriers, side effects, as well as ethical concerns usually limit the development and use of the ESCs transplantation technique.
The differentiation of human and mouse ESCs into putative primordial germ cells (PGCs) has been confirmed by multiple studies 113 . Mouse ESCs can form spermatozoa, and human ESCs have been successfully differentiated into SSCs114,115. Studies have shown that mouse ESCs can develop into oocyte-like cells when cultured. These oocytes undergo the first stage of meiosis and develop into a structure similar to embryonic cells 16 . Liu et al. reported in their study that small extracellular vesicles derived from ESCs were capable of improving the ovarian function in patients with POF by regulating the PI3K/AKT signaling pathway 116 . These applications of ESCs in the field of reproduction are beneficial for the study of fertility treatment and help to analyze the interaction and differentiation of germ cells and somatic cells.
Induced Pluripotent Stem Cells
Differentiated cells can be reprogrammed to an embryo-like state by transferring the nuclear contents into oocytes or fusing with ESCs. Induced pluripotent stem cells (iPSCs) were originally derived from mouse embryos or adult fibroblasts and cultured in a medium expressing the transcription factors Oct3/4, Klf4, Sox2, and c-Myc. The clones acquired ESC-like properties and possessed three germ layers 117 . Renee et al. indicated in their study that compared with hESCs, human adult and fetal somatic cell-derived iPSC lines have a significantly increased expression of germ cell-specific markers VASA in human spermatids 118 . In addition, the VASA and DAZL expression levels were increased in bone morphogenetic proteins(BMPs) induced cultures via initiation and progression to lengthen complex formations rather than contribute to the number of meiotic cells directly. Yang et al. reported that iPSC-derived male germ cells achieved integration into seminiferous tubules but failed to undergo meiosis as the germ cell marker SCP3 of EGFP-positive germ cells was negatively expressed 119 . Mouka et al. generated iPSCs by transducing erythroblasts, which were derived from peripheral blood mononuclear cells purified from patients with azoospermia, with Sendai viruses expressing the four mentioned factors 7 . It has also been reported that specific iPSCs of patients with POF successfully expressed female germ cell markers 120 . The patient-specific iPSCs achieved the ability to differentiate into three germ layers with pluripotency, which provides a potential method for repairing spermatogenesis and oogenesis in patients with complex chromosomal rearrangements.
Tissue-Specific Stem Cells (Resident Stem Cells)
A tissue has the natural ability to replace dead cells and heal wounds. Stem cells support tissue maintenance by balancing self-renewal and differentiation. This ability exists in resident stem cells, which renew themselves to preserve and repair damaged tissues to maintain homeostasis and tissue functions. In this section, we will explore the existence and application of tissue-specific stem cells in reproductive tissues.
Spermatogonial stem cells
SSCs, a type of pluripotent stem cells, maintain spermatogenesis in the seminiferous tubules over the entire reproductive life cycle of men. In the mouse testis, the most primitive sub-set of spermatogonia consists of A-single (Asingle), which may have the greatest potential with regard to function, A-paired (Apaired), and A-aligned (Aaligned). However, the fate of the single spermatogonia population changed significantly during the regeneration process after injury 121 . There is limited information about how it supports steady-state spermatogenesis within a sub-population of single spermatogonia. Similarly, spermatogenic lineage in humans contains A-dark(Adark) and A-pale 36 spermatogonia, which are considered to represent reserve and active stem cells, respectively 122 . Sertoli cells from the blood-testis barrier supply SSCs with hormones and growth factors. Further studies have demonstrated that transplantation of Sertoli cells with specific factors such as LIF, FGF, EGF, and GDNF can revive the propagation of SSCs in azoospermic patients123,124. SSCs from OA and NOA patients can proliferate in vitro and maintain their characteristics for more than 12 generations 124 . Hence, in immature testicular tissue, the interaction between transplanted SSCs and the original microenvironment is beneficial for long-term self-renewal and complete spermatogenesis 125 . Kyle et al. implanted autologous and allogeneic SSCs through ultrasound guidance into macaques that had undergone busulfan chemotherapy in 2012. The results revealed that the allogeneic recipient transplanted with SSCs from the donor successfully produced functional sperms and developed into an embryo, which was proved by IVF and ICSI 8 . Further clinical research aims to apply spermatogonial stem cell autotransplantation technology to humans, especially in pre-puberty males who suffer from infertility caused by radiotherapy or chemotherapy.
Ovarian stem cells
The previously entrenched dogma of reproductive biology that the generation of oocytes ceases from birth due to the fixed oocyte reserves, has been challenged by several recent studies. Joshua et al. used immunohistochemical analysis of the combination of VASA and Brdu to study the proliferation of germ cells and follicle regeneration in post-partum mammalian ovaries. Their results confirmed that after injecting GFP-positive ovarian fragments into wild-type female hosts, the infiltrated transgenic germ cells and scattered wild-type somatic cells supported follicle formation 126 . During further investigation, ovarian stem cells with the GFP virus were transplanted into the ovaries of infertile mice. The transplanted cells underwent oogenesis, and the mice produced normal GFP-labeled offspring 14 . Based on a previous study, Jonathan et al. stably isolated DEAD box polypeptide 4(DDX4)-positive cells from a donor ovary and introduced these mitotically active oogonial stem cells (OSCs) with a GFP expression vector into human ovarian tissue in vitro as well as NOD-SCID female mice in vivo, both of which demonstrated discernible follicles 15 . Although the results of using OSC xenotransplantation to obtain offspring theoretically imply potential and significant advancements in the treatment of age-related infertility, iatrogenic POF, and infertile female xenogeneic problems, their applicability is still controversial.
Endometrial progenitor cells
The human endometrium regenerates every month, which may be mediated by endometrial stem cells/progenitor cells. The hypothesis that stem cells/progenitor cells exist in the endometrium was proposed several years ago, but it was not until 2004 that the first functional evidence was published 127 . Several types of stem/progenitor cells have been identified: CD140b + CD146 + or SUSD2 + endometrial mesenchymal stem cells (eMSCs), N-cadherin + endometrial epithelial progenitor cells (eEPs) and lateral cells (SP). They are heterogeneous cells mainly composed of endothelial cells 128 . Park et al. observed that the Sonic hedgehog (SHH) pathway played a key role in stimulating endometrial stem cells via FAK/ERK1/2 and/or phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways to significantly enhance the in vivo differentiation and migration capabilities and subsequent therapeutic effects in an animal model of endometrial ablation 10 . Stimulating the local endometrial stem cells may play an important role in regulating the endometrial thickness. Endometrial stem cells are expected to become an important intervention strategy for promoting successful embryo implantation and improving pregnancy outcomes.
Conclusion and Future Perspectives
General causes of infertility include failure in germ cells production and transmission, psychological pressure and physical abnormalities, as well as environmental and inheritance factors. Although with the development of ART technology and innovations, over 8 million babies have been born following IVF; however, the impact of ART on the health of both patients and their offspring continues to cause concern. Here, we have described the pathological characteristics and treatment strategies of various refractory infertility diseases at present and focused on the application and prospects of stem cells in the treatment of infertility. Considering their advantages in pluripotent capacity, stem cells therapies are considered as an alternative approach to ART to improve infertility treatment outcomes in humans. In this review, we discussed clinical translational research studies on stem cells in the assistance of fertility, mainly indicating two mechanisms the direct differentiation and indirect secretion of cytokines to illustrate stem cell-based treatments in accordance with different conditions. For age-related or irreversible injured issues, researchers induced germ line stem cells to retrieve meiotic active gametes in culture or in post-natal mouse ovaries. Instead of GSCs, mesenchymal stem cells predominantly play an instrumental role in the immunoregulatory function and the recovery of failing reproductive organs. On the other hand, stemcells therapies remain largely in the preclinical investigational phase, and several ethical challenges created by new scientific developments in this field have aroused widespread conflicting opinions. The progress of clinical practice of stem cell therapy requires further long-term planning under strict evaluation and supervision to ensure accuracy, quality and safety. Since autologous stem cells are more ethical, safe and non-immune, the clinical application of autologous stem cells has more potential in the future.
Acknowledgments
This work is supported by the Second Affiliated Hospital of Fujian Medical University. We would like to thank Editage for English language editing. The manuscript has been reviewed and approved by all authors and is neither in submission nor under consideration for publication by any other journal. All authors have read the authorship agreement and policy on disclosure of potential conflicts of interest of Translational Research and have no disclosures to declare.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Science and Technology Bureau of Quanzhou (2020CT003 to S.L.) and National Natural Science Foundation of China (81901481 to J.W.).
ORCID iD: Jin-Xiang Wu  https://orcid.org/0000-0002-5231-7603
https://orcid.org/0000-0002-5231-7603
References
- 1. Sheikhansari G, Aghebati-Maleki L, Nouri M, Jadidi-Niaragh F, Yousefi M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed Pharmacother. 2018;102:254–62. [DOI] [PubMed] [Google Scholar]
- 2. Davies MJ, Rumbold AR, Moore VM. Assisted reproductive technologies: a hierarchy of risks for conception, pregnancy outcomes and treatment decisions. J Dev Orig Health Dis. 2017;8(4):443–47. [DOI] [PubMed] [Google Scholar]
- 3. Pourmoghadam Z, Aghebati-Maleki L, Motalebnezhad M, Yousefi B, Yousefi M. Current approaches for the treatment of male infertility with stem cell therapy. J Cell Physiol. 2018;233(10):6455–69. [DOI] [PubMed] [Google Scholar]
- 4. Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L, Zhou Q, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, Uludag O, Ustun H, Subası C, Karaoz E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: the sperm generation. Biomed Res Int. 2013;2013:529589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H, Tang QL, Wu XY, Xie LC, Lin LM, Ho GY, Ma L. Differentiation of human umbilical cord mesenchymal stem cells into germ-like cells in mouse seminiferous tubules. Mol Med Rep. 2015;12(1):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mouka A, Izard V, Tachdjian G, Brisset S, Yates F, Mayeur A, Drevillon L, Jarray R, Leboulch P, Maouche-Chretien L, Tosca L. Induced pluripotent stem cell generation from a man carrying a complex chromosomal rearrangement as a genetic model for infertility studies. Sci Rep. 2017;7:39760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hermann B, Sukhwani M, Winkler F, Pascarella J, Peters K, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–29. [DOI] [PubMed] [Google Scholar]
- 10. Park SR, Kim SR, Park CH, Lim S, Ha SY, Hong IS, Lee HY. Sonic hedgehog, a novel endogenous damage signal, activates multiple beneficial functions of human endometrial stem cells. Mol Ther. 2020;28(2):452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su J, Ding L, Cheng J, Yang J, Li XA, Yan G, Sun H, Dai J, Hu Y. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod. 2016;31(5):1075–86. [DOI] [PubMed] [Google Scholar]
- 12. Xiao G-Y, Liu IH, Cheng C-C, Chang C-C, Lee Y-H, Cheng WT-K, Wu S-C. Amniotic fluid stem cells prevent follicle atresia and rescue fertility of mice with premature ovarian failure induced by chemotherapy. PLoS One. 2014;9(9):e106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghadami M, El-Demerdash E, Zhang D, Salama SA, Binhazim AA, Archibong AE, Chen X, Ballard BR, Sairam MR, Al-Hendy A. Bone marrow transplantation restores follicular maturation and steroid hormones production in a mouse model for primary ovarian failure. PLoS One. 2012;7(3):e32462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–36. [DOI] [PubMed] [Google Scholar]
- 15. White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karin H, Guy F, Christenson LK, James K, Reinbold R, De La Fuente R, Wood J, Strauss JF, Boiani M, Schöler HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–56. [DOI] [PubMed] [Google Scholar]
- 17. Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs. 2016;25(18):S35–40. [DOI] [PubMed] [Google Scholar]
- 18. Lundy SD, Sabanegh ES., Jr. Varicocele management for infertility and pain: a systematic review. Arab J Urol. 2018;16(1):157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wosnitzer MS, Goldstein M. Obstructive azoospermia. Urol Clin North Am. 2014;41(1):83–95. [DOI] [PubMed] [Google Scholar]
- 20. Giorgione V, Parazzini F, Fesslova V, Cipriani S, Candiani M, Inversetti A, Sigismondi C, Tiberio F, Cavoretto P. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):33–42. [DOI] [PubMed] [Google Scholar]
- 21. Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–13. [DOI] [PubMed] [Google Scholar]
- 22. Spiller C, Koopman P, Bowles J. Sex determination in the mammalian germline. Annual Review of Genetics. 2017;51(1). [DOI] [PubMed] [Google Scholar]
- 23. Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15(6):369–84. [DOI] [PubMed] [Google Scholar]
- 24. Huyghe E, Izard V, Rigot JM, Pariente JL, Tostain J; les membres du Comité d’andrologie de l’association française d’urologie (CCAFU). [Optimal evaluation of the infertile male. 2007 French urological association guidelines]. Prog Urol. 2008;18(2):95–101. [DOI] [PubMed] [Google Scholar]
- 25. Peña VN, Kohn TP, Herati AS. Genetic mutations contributing to non-obstructive azoospermia. Best Pract Res Clin Endocrinol Metab. 2020;34(6):101479. [DOI] [PubMed] [Google Scholar]
- 26. Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660–65. [DOI] [PubMed] [Google Scholar]
- 27. Gundacker C, Dolznig H, Mikula M, Rosner M, Brandau O, Hengstschläger M. Amniotic fluid stem cell-based models to study the effects of gene mutations and toxicants on male germ cell formation. Asian J Androl. 2012;14(2):247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 2016;59:10–26. [DOI] [PubMed] [Google Scholar]
- 29. Yang T, Yang WX. The dynamics and regulation of microfilament during spermatogenesis. Gene. 2020;744:144635. [DOI] [PubMed] [Google Scholar]
- 30. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, Mortensen B, Appel EV, Jørgensen N, Kristiansen VB, Hansen T, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23(2):369–78. [DOI] [PubMed] [Google Scholar]
- 32. Stuppia L, Franzago M, Ballerini P, Gatta V, Antonucci I. Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin Epigenetics. 2015;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, Krausz C; European Association of Urology Working Group on Male Infertility. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62(2):324–32. [DOI] [PubMed] [Google Scholar]
- 34. Gao K, Wang ZQ, Liu XC, Dong ZL, Shan WS. [Genetic genes associated with oligospermia, asthenospermia and teratospermia: advances in studies]. Zhonghua Nan Ke Xue. 2017;23(4):367–71. [PubMed] [Google Scholar]
- 35. Chen L, Wen CW, Deng MJ, Ping L, Zhang ZD, Zhou ZH, Wang X. Metabolic and transcriptional changes in seminal plasma of asthenozoospermia patients. Biomed Chromatogr. 2020;34(3):e4769. [DOI] [PubMed] [Google Scholar]
- 36. Murdica V, Giacomini E, Alteri A, Bartolacci A, Cermisoni GC, Zarovni N, Papaleo E, Montorsi F, Salonia A, Viganò P, Vago R. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil Steril. 2019;111(5):897–908.e2. [DOI] [PubMed] [Google Scholar]
- 37. Santana VP, Miranda-Furtado CL, Pedroso DCC, Eiras MC, Vasconcelos MAC, Ramos ES, Calado RT, Ferriani RA, Esteves SC, Dos Reis RM. The relationship among sperm global DNA methylation, telomere length, and DNA fragmentation in varicocele: a cross-sectional study of 20 cases. Syst Biol Reprod Med. 2019;65(2):95–104. [DOI] [PubMed] [Google Scholar]
- 38. Matzuk M, Lamb D. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134(14):2593–603. [DOI] [PubMed] [Google Scholar]
- 40. Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23(6):787–823. [DOI] [PubMed] [Google Scholar]
- 41. Schisterman EF, Browne RW, Barr DB, Chen Z, Mumford SL, Louis GMB. Lipid levels and couple fecundity: the life study. J Clin Endocrinol Metab. 2014;99:2786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khan KN, Kitajima MK. Toll-like receptors in innate immunity: role of bacterial endotoxin and toll-like receptor 4 in endometrium and endometriosis. Gynecol Obstet Invest. 2009;68(1):40–52. [DOI] [PubMed] [Google Scholar]
- 43. Chen S. Expression of the T regulatory cell transcription factor FoxP3 in peri-implantation phase endometrium in infertile women with endometriosis. Reprod Biol Endocrinol. 2012;10(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mark-Kappeler CJ, Hoyer PB, Devine PJ. Xenobiotic effects on ovarian preantral follicles. Biol Reprod. 2011;85(5):871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singer D, Mann E, Hunter MS, Pitkin J, Panay N. The silent grief: psychosocial aspects of premature ovarian failure. Climacteric. 2011;14(4):428–37. [DOI] [PubMed] [Google Scholar]
- 46. Nargund G, Frydman R. Towards a more physiological approach to IVF. Reprod Biomed Online. 2007;14(5):550–52. [DOI] [PubMed] [Google Scholar]
- 47. Lim JH, Yang SH, Xu Y, Yoon SH, Chian RC. Selection of patients for natural cycle in vitro fertilization combined with in vitro maturation of immature oocytes. Fertil Steril. 2009;91(4):1050–55. [DOI] [PubMed] [Google Scholar]
- 48. Chapman C, Cree L, Shelling AN. The genetics of premature ovarian failure: current perspectives. Int J Womens Health. 2015;7:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103(2):303–16. [DOI] [PubMed] [Google Scholar]
- 50. Li D, Wang X, Dang Y, Zhang X, Zhao S, Lu G, Chan WY, Leung PCK, Qin Y. LncRNA GCAT1 is involved in premature ovarian insufficiency by regulating p27 translation in GCs via competitive binding to PTBP1. Mol Ther Nucleic Acids. 2021;23:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He Y, Chen D, Yang L, Hou Q, Ma H, Xu X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu R, Zhang X, Fan Z, Wang Y, Yao G, Wan X, Liu Z, Yang B, Yu L. Human amniotic mesenchymal stem cells improve the follicular microenvironment to recover ovarian function in premature ovarian failure mice. Stem Cell Res Ther. 2019;10(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31. [DOI] [PubMed] [Google Scholar]
- 54. Fournier A, Torre A, Delaroche L, Gala A, Mullet T, Ferrières A, Hamamah S. [Quality of oocytes and embryos from women with polycystic ovaries syndrome: state of the art]. Gynecol Obstet Fertil Senol. 2017;45(7-8):429–38. [DOI] [PubMed] [Google Scholar]
- 55. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas-Sun E, Ryckman KK, et al. Reproductive risk factors and coronary heart disease in the women’s health initiative observational study. Circulation. 2016;133(22):2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krentowska A, Łebkowska A, Jacewicz-Święcka M, Hryniewicka J, Leśniewska M, Adamska A, Kowalska I. Metabolic syndrome and the risk of cardiovascular complications in young patients with different phenotypes of polycystic ovary syndrome. Endocrine. 2021;72:400–10. [DOI] [PubMed] [Google Scholar]
- 57. Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, Gnatuk CL, Estes SJ, Fleming J, Allison KC, Sarwer DB, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(11):4048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gonzalez F, Sia CL, Shepard MK, Rote NS, Minium J. Hyperglycemia-induced oxidative stress is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. Hum Reprod. 2012;27(12):3560–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frank G, Ling SC, Shepard MK, Rote NS, Judi M. The altered mononuclear cell-derived cytokine response to glucose ingestion is not regulated by excess adiposity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(11):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qin L, Xu W, Li X, Meng W, Hu L, Luo Z, Wang Y, Luo S, Li S. Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome: analysis by flow cytometry. Eur J Obstet Gynecol Reprod Biol. 2016;197:136–41. [DOI] [PubMed] [Google Scholar]
- 61. Xie Q, Xiong X, Xiao N, He K, Chen M, Peng J, Su X, Mei H, Dai Y, Wei D, Lin G, et al. Mesenchymal stem cells alleviate DHEA-induced Polycystic Ovary Syndrome (PCOS) by inhibiting inflammation in mice. Stem Cells Int. 2019;2019:9782373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garry R, Hart R, Karthigasu KA, Burke C. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod. 2009;24(6):1393–1401. [DOI] [PubMed] [Google Scholar]
- 63. Kliman HJ, Frankfurter D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil Steril. 2019;111(4):618–28. [DOI] [PubMed] [Google Scholar]
- 64. Chen X, Man GCW, Liu Y, Wu F, Huang J, Li TC, Wang CC. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am J Reprod Immunol. 2017;78(2):e12693. [DOI] [PubMed] [Google Scholar]
- 65. Sheikhansari G, Pourmoghadam Z, Danaii S, Mehdizadeh A, Yousefi M. Etiology and management of recurrent implantation failure: a focus on intra-uterine PBMC-therapy for RIF. J Reprod Immunol. 2020;139:103121. [DOI] [PubMed] [Google Scholar]
- 66. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. [DOI] [PubMed] [Google Scholar]
- 67. Zhang S, Kong S, Lu J, Wang Q, Chen Y, Wang W, Wang B, Wang H. Deciphering the molecular basis of uterine receptivity. Mol Reprod Dev. 2013;80(1):8–21. [DOI] [PubMed] [Google Scholar]
- 68. Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med. 2013;34(5):939–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: stem cell- based therapy. Biomed Pharmacother. 2018;102:333–43. [DOI] [PubMed] [Google Scholar]
- 70. Salazar CA, Isaacson K, Morris S. A comprehensive review of Asherman’s syndrome: causes, symptoms and treatment options. Curr Opin Obstet Gynecol. 2017;29(4):249–56. [DOI] [PubMed] [Google Scholar]
- 71. Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4(5):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yin N, Zhao W, Luo Q, Yuan W, Luan X, Zhang H, Yin N, Zhao W, Luo Q, Yuan W. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by treg cells and associated cytokines. Reprod Sci. 2018;25:1073–1082. [DOI] [PubMed] [Google Scholar]
- 74. Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1307–14. [DOI] [PubMed] [Google Scholar]
- 75. Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, Yao Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yawno T, Schuilwerve J, Moss TJ, Vosdoganes P, Westover AJ, Afandi E, Jenkin G, Wallace EM, Miller SL. Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Dev Neurosci. 2013;35(2-3):272–82. [DOI] [PubMed] [Google Scholar]
- 77. Yagi H, Kitagawa Y. The role of mesenchymal stem cells in cancer development. Front Genet. 2013;4(4):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bhatia S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev Hematol. 2011;4(4):437–52; quiz 453–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guida M, Castaldi MA, Rosamilio R, Giudice V, Orio F, Selleri C. Reproductive issues in patients undergoing Hematopoietic Stem Cell Transplantation: an update. J Ovarian Res. 2016;9(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shimoji S, Hashimoto D, Tsujigiwa H, Miyawaki K, Kato K, Takahashi S, Ogasawara R, Jiromaru T, Iwasaki H, Miyamoto T, Akashi K, et al. Graft-versus-host disease targets ovary and causes female infertility in mice. Blood. 2017;129(9):1216–25. [DOI] [PubMed] [Google Scholar]
- 81. Schubert MA, Sullivan KM, Schubert MM, Nims J, Hansen M, Sanders JE, O’Quigley J, Witherspoon RP, Buckner CD, Storb R. Gynecological abnormalities following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1990;5(6):425–30. [PubMed] [Google Scholar]
- 82. Aboul Fotouh GI, Abdel-Dayem MM, Ismail DI, Mohamed HH. Histological study on the protective effect of endogenous stem cell mobilization in busulfan-induced testicular injury in albino rats. J Microsc Ultrastruct. 2018;6(4):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Im GI. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: their comparative efficacies and synergistic effects. J Biomed Mater Res A. 2017;105(9):2640–48. [DOI] [PubMed] [Google Scholar]
- 85. Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, Inoue M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201(6):608.e601–608.e608. [DOI] [PubMed] [Google Scholar]
- 86. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. [DOI] [PubMed] [Google Scholar]
- 87. Gao Q, Guo M, Jiang X, Hu X, Wang Y, Fan Y. A cocktail method for promoting cardiomyocyte differentiation from bone marrow-derived mesenchymal stem cells. Stem Cells Int. 2014;2014:162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ye Y, Zeng YM, Wan MR, Lu XF. Induction of human bone marrow mesenchymal stem cells differentiation into neural-like cells using cerebrospinal fluid. Cell Biochem Biophys. 2011;59(3):179–84. [DOI] [PubMed] [Google Scholar]
- 89. Kalhori Z, Azadbakht M, Soleimani Mehranjani M, Shariatzadeh MA. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy. 2018;20(12):1445–58. [DOI] [PubMed] [Google Scholar]
- 90. Sun B, Ma Y, Wang F, Hu L, Sun Y. MiR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther. 2019;10(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bao R, Xu P, Wang Y, Wang J, Xiao L, Li G, Zhang C. Bone marrow derived mesenchymal stem cells transplantation rescues premature ovarian insufficiency induced by chemotherapy. Gynecol Endocrinol. 2017;34(4):320–26. [DOI] [PubMed] [Google Scholar]
- 92. Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96. [DOI] [PubMed] [Google Scholar]
- 93. Zhang D, Liu X, Peng J, He D, Lin T, Zhu J, Li X, Zhang Y, Wei G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int J Mol Sci. 2014;15(8):13151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mirzaei H, Salehi H, Oskuee RK, Mohammadpour A, Mirzaei HR, Sharifi MR, Salarinia R, Darani HY, Mokhtari M, Masoudifar A. The therapeutic potential of human adipose-derived mesenchymal stem cells producing CXCL10 in a mouse melanoma lung metastasis model. Cancer Lett. 2018;419:30–39. [DOI] [PubMed] [Google Scholar]
- 95. Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, Benhaim P, Lorenz H, Hedrick M. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. [DOI] [PubMed] [Google Scholar]
- 96. Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32(6):370–73. [DOI] [PubMed] [Google Scholar]
- 97. Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ra JC, Jeong EC, Kang SK, Lee SJ, Choi KH. A prospective, nonrandomized, no placebo-controlled, phase I/II clinical trial assessing the safety and efficacy of intramuscular injection of autologous adipose tissue-derived mesenchymal stem cells in patients with severe Buerger’s disease. Cell Med. 2016;9(3):87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shao X, Ai G, Wang L, Qin J, Li Y, Jiang H, Zhang T, Zhou L, Gao Z, Cheng J, Cheng Z. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27(6):367–74. [DOI] [PubMed] [Google Scholar]
- 100. Wang ZB, Hao JX, Meng TG, Guo L, Dong MZ, Fan LH, Ouyang YC, Wang G, Sun QY, Ou XH, Yao Y-Q. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging. 2017;9(12):2480–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhao Y, Tao M, Wei M, Du S, Wang H, Wang X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif Cells Nanomed Biotechnol. 2019;47(1):3804–13. [DOI] [PubMed] [Google Scholar]
- 102. Wei Y, Fang J, Cai S, Lv C, Zhang S, Hua J. Primordial germ cell-like cells derived from canine adipose mesenchymal stem cells. Cell Prolif. 2016;49(4):503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fang J, Wei Y, Lv C, Peng S, Zhao S, Hua J. CD61 promotes the differentiation of canine ADMSCs into PGC-like cells through modulation of TGF-beta signaling. Sci Rep. 2017;7:43851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Luo Y, Xie L, Mohsin A, Ahmed W, Xu C, Peng Y, Hang H, Zhuang Y, Chu J, Guo M. Efficient generation of male germ-like cells derived during co-culturing of adipose-derived mesenchymal stem cells with Sertoli cells under retinoic acid and testosterone induction. Stem Cell Res Ther. 2019;10(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–47. [DOI] [PubMed] [Google Scholar]
- 106. Zhu SF, Hu HB, Xu HY, Fu XF, Peng DX, Su WY, He YL. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J Cell Mol Med. 2015;19(9):2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang J, Xiong J, Fang L, Lu Z, Wu M, Shi L, Qin X, Luo A, Wang S. The protective effects of human umbilical cord mesenchymal stem cells on damaged ovarian function: a comparative study. Biosci Trends. 2016;10(4):265–76. [DOI] [PubMed] [Google Scholar]
- 108. Hamid AA, Joharry MK, Mun-Fun H, Hamzah SN, Rejali Z, Yazid MN, Thilakavathy K, Nordin N. Highly potent stem cells from full-term amniotic fluid: a realistic perspective. Reprod Biol. 2017;17(1):9–18. [DOI] [PubMed] [Google Scholar]
- 109. Lai D, Wang F, Chen Y, Wang L, Wang Y, Cheng W. Human amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterility. BMC Dev Biol. 2013;13(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–56. [DOI] [PubMed] [Google Scholar]
- 111. Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones J. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–47. [DOI] [PubMed] [Google Scholar]
- 112. Yuval D, Juliana B, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. [DOI] [PubMed] [Google Scholar]
- 113. Tilgner K, Atkinson SP, Golebiewska A, Stojković M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2009;26(12):3075–85. [DOI] [PubMed] [Google Scholar]
- 114. Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Wang X, Wang L, Zeng F, Zhou Q. Viable fertile mice generated from fully pluripotent iPS cells derived from adult somatic cells. Stem Cell Rev Rep. 2010;6(3):390–97. [DOI] [PubMed] [Google Scholar]
- 115. Easley CA, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2(3):440–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X, Yuan J, Wang Y, Wu Q. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020;11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;51(15):2346–51. [DOI] [PubMed] [Google Scholar]
- 118. Sarita P, Medrano JV, Kehkooi K, Rosita BM, Ha Nam N, Blake B, Wilson KD, Wu JC, Carlos S, Outi H. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20(4):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yang S, Bo J, Hu H, Guo X, Tian R, Sun C, Zhu Y, Li P, Liu P, Zou S. Derivation of male germ cells from induced pluripotent stem cells in vitro and in reconstituted seminiferous tubules. Cell Prolif. 2012;45(2):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wen Y, He W, Jiang M, Zeng M, Cai L. Deriving cells expressing markers of female germ cells from premature ovarian failure patient-specific induced pluripotent stem cells. Regen Med. 2017;12(2):143–52. [DOI] [PubMed] [Google Scholar]
- 121. Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328(5974):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139(3):479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, Rafieian SH. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44(Suppl 1):41–55. [DOI] [PubMed] [Google Scholar]
- 124. Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, Kim JK, Kim KS, Lee DR. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43(4):405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, Ito J, Kashiwazaki N, Kikuchi K. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction. 2010;139(2):331–35. [DOI] [PubMed] [Google Scholar]
- 126. Joshua J, Jacqueline C, Tomoko K, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–50. [DOI] [PubMed] [Google Scholar]
- 127. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cousins FL, Dorien FO, Gargett CE. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:27–38. [DOI] [PubMed] [Google Scholar]