Abstract
Excited-state palladium catalysis has emerged as a promising strategy for developing novel and valuable reactions. Herein, we report the first excited-state Pd-catalyzed radical migratory Mizoroki-Heck reaction that enables C2-alkenylation of carbohydrates using readily available 1-bromosugars and alkenes. The reaction tolerates a wide variety of functional groups and complex molecular architectures, including derivatives of natural products and marketed drugs. Preliminary mechanistic studies and DFT calculations suggest the involvement of visible-light-induced photoexcitation of Pd species, 1,2-spin-centered shift (SCS) process, and Heck-type cross-coupling reaction. The reaction expands the reactivity profile of excited-state Pd catalysis and provides a streamlined protocol for the preparation of a wide variety of C2-alkenylated carbohydrate mimetics to aid the discovery and development of new therapeutics, agrochemicals, and materials.
Graphical Abstract
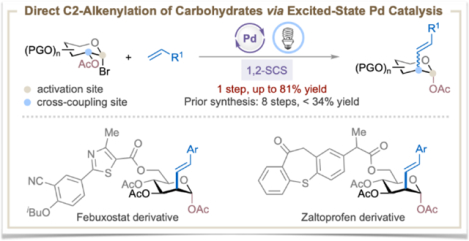
Carbohydrates are involved in a wide range of biological processes, including their role in glycolipids and glycoproteins, where they serve as ligands for cell-cell interactions or as receptors for toxins, antibodies, viruses, and bacteria.1 A growing body of literature links the sugar composition of various glycoconjugates to numerous diseases such as cancer, viral infections, diabetes, and neurological disorders.2 The selective modification of carbohydrate scaffolds to enhance or alter the biochemical properties of the parent glycoconjugates is, therefore, an appealing strategy with which to develop novel therapeutics.3 Indeed, many pharmaceuticals, vaccines, cell surface engineering agents, and imaging probes contain carbohydrate moieties and mimetics, such as C2-functionalized 2-deoxy sugars.3a–c, 4 However, synthesis of these glycomimetics is often labor-intensive and time-consuming. For example, the current state-of-the-art synthesis of C2-alkenylated carbohydrates starting from 1-bromosugars requires 8 steps with less than 34% overall yield (Figure 1A).5 Consequently, a strategy that enables one-step access to C2-substituted carbohydrates from readily available starting materials would be of considerable value to medicinal and process chemists.
Figure 1.
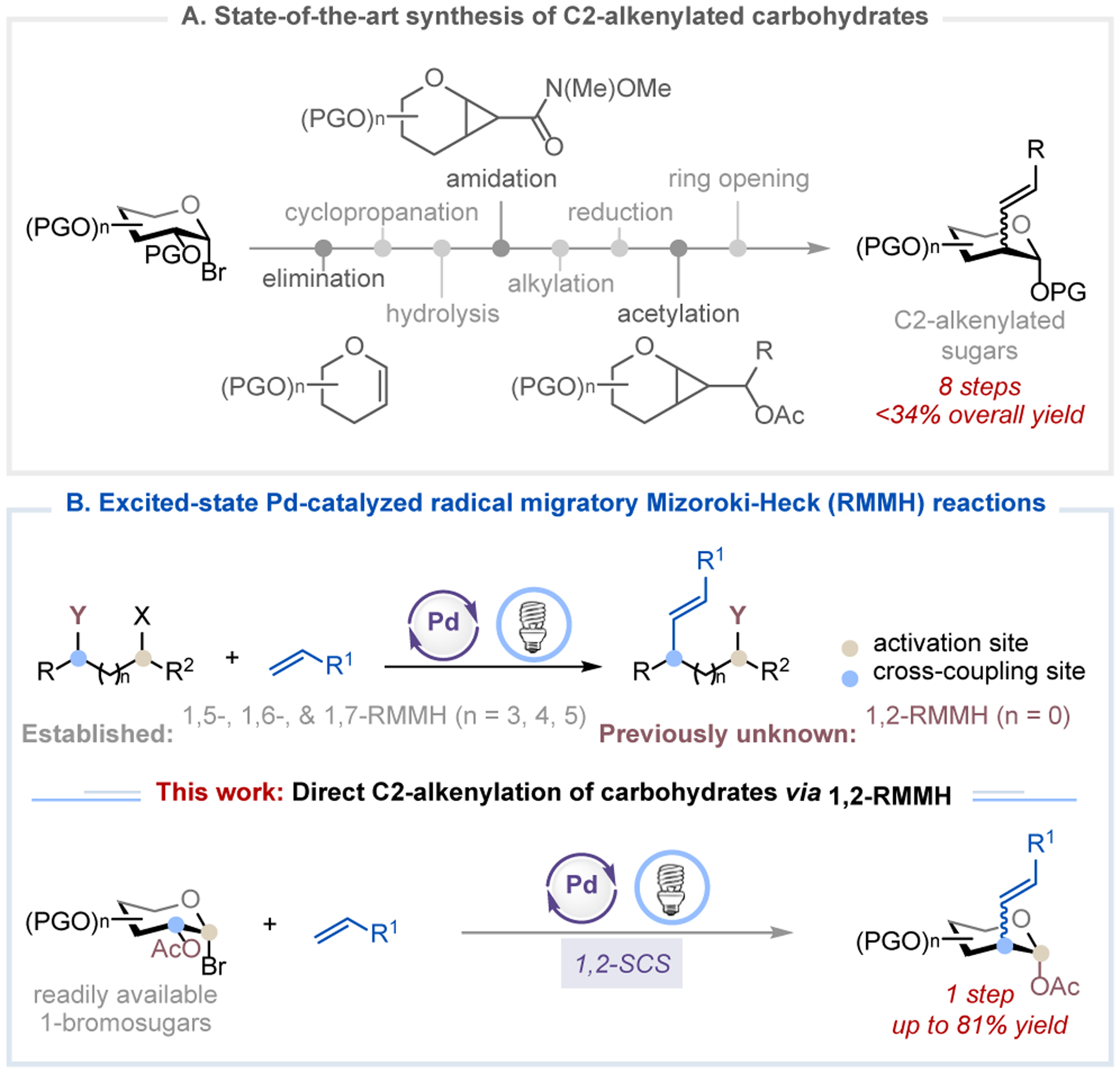
Excited-state Pd-catalyzed radical migratory Mizoroki-Heck reaction via 1,2-SCS pathway enabling C2-alkenylation of carbohydrates. PG = protecting group.
Excited-state palladium catalysis has emerged as a powerful tool in organic synthesis because of its ability to access both open-shell (one-electron) and closed-shell (two-electron) reactivities under irradiation of visible light.6 Exploitation of this type of hybrid reactivity has led to a wide range of carbon-carbon and carbon-heteroatom bond forming reactions, including radical Mizoroki-Heck reactions.7 Although elegant excited-state Pd-catalyzed 1,5-, 1,6-, and 1,7-radical migratory Mizoroki-Heck (RMMH) reactions have been developed to remotely install an alkenyl group,7n, 7p the corresponding 1,2-RMMH reaction remains elusive (Figure 1B). Inspired by the seminal work of Giese, who showed that 1-glycosyl radical could undergo a 1,2-spin-center shift (SCS) with concomitant acyloxy migration to form the deoxypyranosan-2-yl radical,8,9 we questioned whether we could merge the excited-state Pd-catalyzed radical Mizoroki-Heck reaction with the 1,2-SCS process to achieve a direct, catalytic C2-alkenylation of carbohydrates from readily accessible 1-bromosugars (Figure 1B). Accomplishment of such a reaction would be significant and novel because it (i) represents the first example of excited-state Pd-catalyzed RMMH reaction that proceeds through a 1,2-SCS mechanism, (ii) significantly streamlines the synthesis of C2-alkenylated carbohydrates from an 8-step procedure to a single step protocol, (iii) expands the reactivity profile of excited-state Pd catalysis, and (iv) provides a new approach to the synthesis of unnatural carbohydrates and late-stage functionalization of glycoconjugates, which will be useful for modern glycomimetic synthesis and drug discovery.
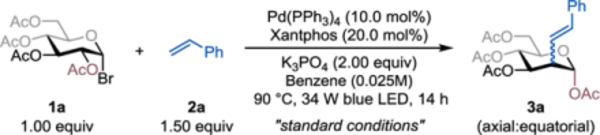
Achieving the proposed reaction is, however, challenging because it requires precise control of the kinetics of the Mizoroki-Heck coupling reaction and the 1,2-SCS process to minimize premature C1-alkenylation.7q Indeed, initial attempts using 1-glucosyl bromide (1a) and styrene (2a) as model substrates and our reported reaction conditions7y gave only the C1-alkenylated products. Nevertheless, after further optimization of the reaction, we found that exposing 1a (1.00 equiv) and 2a (2.00 equiv) to visible light (34 W blue LEDs) in the presence of Pd(PPh3)4 (10.0 mol%), xantphos (20.0 mol%) and K3PO4 (2.00 equiv) in benzene at 0.025 M at 90 °C for 14 h afforded the desired C2-alkenylated 2-deoxyglucoside (3a) in 83% yield with an axial/equatorial (ax:eq) selectivity of 4.2:1 (Table 1, entry 1). The Pd(PPh3)4 catalyst is essential for the desired reactivity: when it was replaced with Pd(OAc)2, only 19% of the desired product was formed, and in its absence the reaction failed completely (entries 2 & 3). Conventional Ru- and Ir-based photocatalysts also failed to give the desired products (entries 4 & 5). The xantphos (9,9-dimethyl-9H-xanthene-4,5-diyl)bis(di-phenylphosphane) ligand also plays an important role in promoting the reaction and its removal significantly decreased the reaction yield (entry 6), suggesting that xantphos-ligated Pd complexes may possibly be active catalysts. The identity of the base was critical because the absence of K3PO4 or its replacement with DIPEA or Cs2CO3 dramatically diminished the reaction efficiency (entries 7–9). Conducting the reaction at room temperature (rt) gave the undesired C1-alkenylated product, indicating that elevated reaction temperatures promote the 1,2-SCS process (entry 10). Control experiments showed that both light and an oxygen-free environment were crucial for the success of the reaction (entries 11–12).
Table 1.
Selected Optimization Experiments.a
| |||
|---|---|---|---|
| Entry | Deviation from the standard conditions | Yield (%) | ax:eq |
| 1 | none | 83 | 4.2:1 |
| 2 | Pd(OAc)2 instead of Pd(PPh3)4 | 19 | 4.8:1 |
| 3 | no Pd(PPh3)4 | <2 | - |
| 4 | Ru(bpy)3(PF6)2 | <2 | - |
| 5 | Ir(PPy)2(dtbbpy)PF6 | <2 | - |
| 6 | no Xantphos | 30 | 4.2:1 |
| 7 | DIPEA instead of K3PO4 | 52 | 4.2:1 |
| 8 | Cs2CO3 instead of K3PO4 | 67 | 4.2:1 |
| 9 | no Base | 8 | 4.2:1 |
| 10 | room temperature | <2 | - |
| 11 | with air | <2 | - |
| 12 | no light | <2 | - |
See the Supporting Information (SI) for experimental details. Yields of 3a and axial:equatorial (ax:eq) ratios were determined by 1H NMR analysis using dibromomethane as the internal standard.
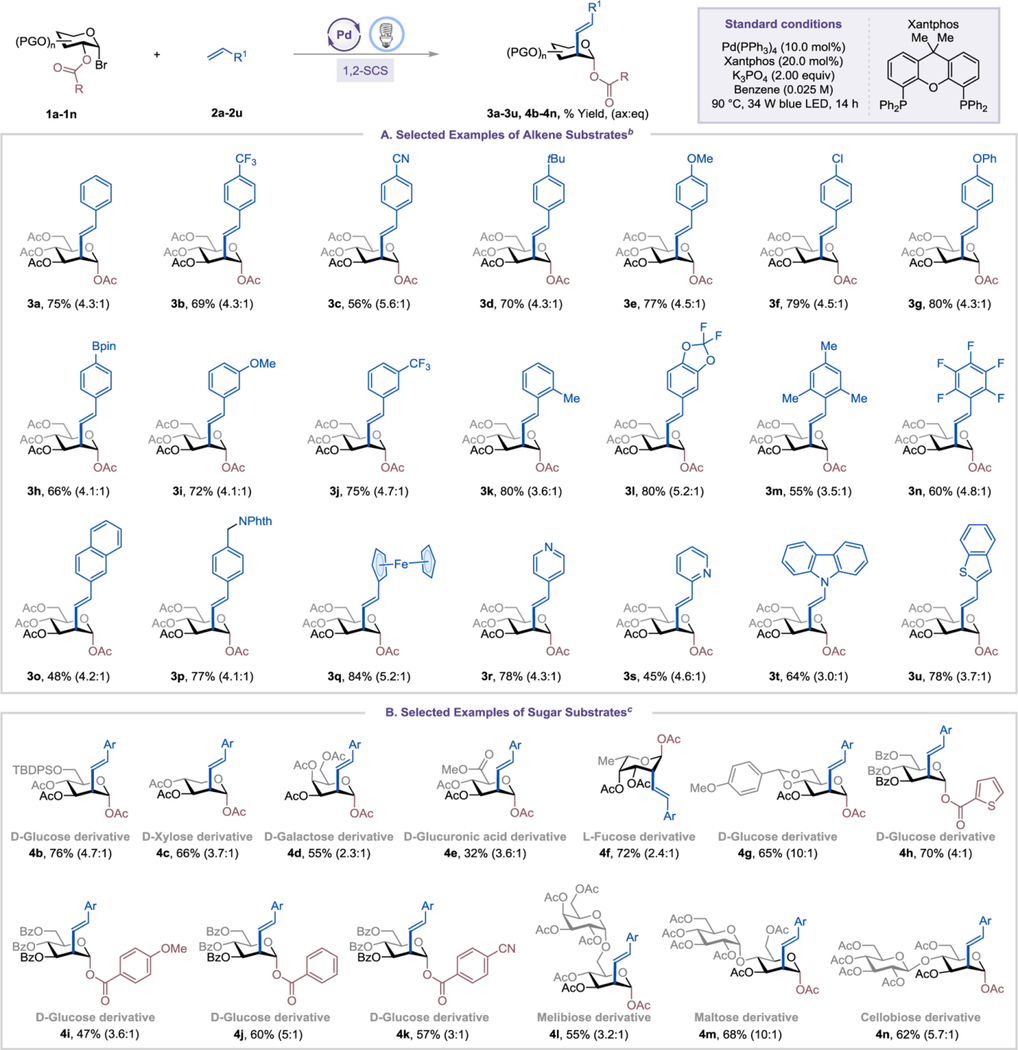
With the optimized conditions in hand, we first investigated the substrate scope of alkene derivatives (Table 2A). Styrenes with both electron-withdrawing and electron-donating substituents, including trifluoromethyl, cyano, tert-butyl, methoxy, and methyl groups on different positions of the phenyl ring were well tolerated under the standard conditions, affording the corresponding products 3a-3e and 3i-3k in 56–80% yield with moderate ax:eq selectivity. Reactions of styrenes with other substituents such as chloro (2f), phenoxy (2g), boronic ester (2h), and N-methylphthalimide (2p) also gave good yields. Multi-substituted substrates showed good compatibility under the reaction system and gave the desired products 3l-3n in yields of 55–80%. An extended aromatic ring, such as 2-vinylnaphthalene (2o) was a viable substrate and furnished the desired product 3o in a moderate yield. Alkenes bearing ferrocene and heterocyclic moieties, including pyridine, carbazole, and benzothiophene, gave the corresponding products 3q-3u in 45–84% yield.
Table 2.
Substrate Scope of Alkene and Sugar Derivativesa
|
See SI for experimental details. The isolated yield and axial:equatorial (ax:eq) ratio are indicated below each entry.
Use 2a as bromosugar substrate.
Use 1-methyl-2-vinylbenzene (2k) as coupling partner. Ar = 2-MePh.
We next examined the scope of 1-bromosugars using 2-methylstyrene (2k) as a coupling partner (Table 2B). D-Glucoside derivatives protected with acetyl or tert-butyldimethylsilyl groups were well tolerated and formed the desired products 3k and 4b in 76–80% yield. D-Xylose, D-galactoside, D-glucuronic acid, and L-fucoside derivatives reacted under the standard conditions, affording the corresponding products 4c-4f in moderate to good yields. A substrate with a fused ring structure at C4 and C6 positions was also compatible, forming product 4g in 65% yield. The ester migrating group could be extended beyond an acetoxy group to (hetero)aryl ester groups with different electronic properties, delivering the desired products 4h-4k in 47–70% yield. Disaccharides, such as D-melibiose, D-maltose, and D-cellobiose derivatives, could also be used (4l-4n), further establishing the utility of the transformation.
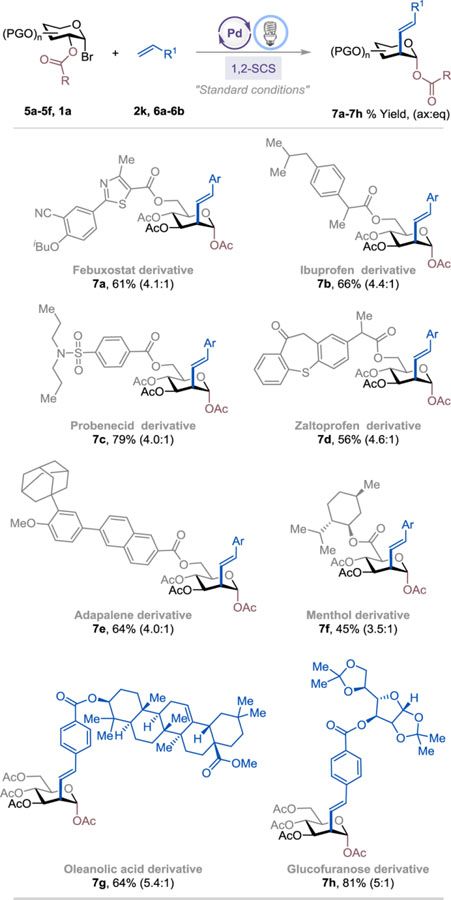
Late-stage modifications of biologically active molecules are often a key to identifying medicinal agents.10 To demonstrate the use of the excited Pd-catalyzed C2-alkenylation of carbohydrates in late-stage synthetic applications, a range of natural product- and drug-glycoconjugates were subjected to the standard conditions (Table 3). 1-Bromoglucosyl-conjugated drug molecules such as Febuxostat (an anti-hyperuricemic drug), Ibuprofen (non-steroidal anti-inflammatory drug, NSAID), Probenecid (anti-gout), Zaltoprofen (NSAID), Adapalene (antiacne agent), and L-Menthol (decongestant and analgesic) were all successfully alkenylated at the C2 position, affording the corresponding products 7a-7f in 45–79% yield with moderate stereoselectivity. Alkenes bearing natural products such as oleanolic acid and glucofuranose were also viable substrates, furnishing the desired products 7g and 7h in 64% and 81% yield, respectively.
Table 3.
Selected Examples of Late-Stage Functionalization of Natural Product/Drug Glycoconjugates.a
|
See SI for experimental details. The isolated yield and axial:equatorial (ax:eq) ratio are indicated below each entry.
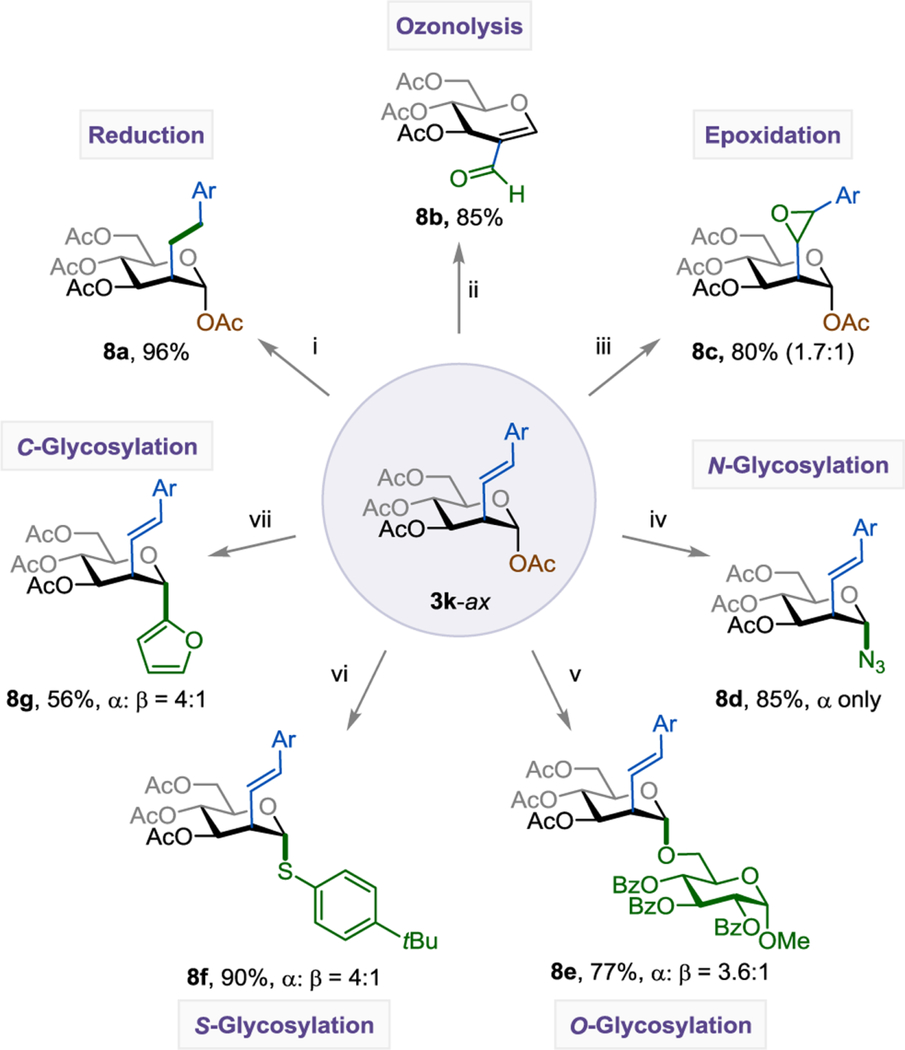
The C2-alkenylated carbohydrate products are synthetic intermediates useful for the preparation of a wide array of glycomimetics (Table 4). For example, alkene in product 3k could be fully reduced to the saturated alkane derivative 8a in a 96% yield. Under ozonolysis conditions, C2-enal 8b was formed. Treatment of 3k with 3-chloroperoxybenzoic (m-CPBA) in DCM at rt gave the desired epoxide 8c in 80% yield. C2-alkenylated carbohydrate products are also good glycosyl donors for N-, O-, S-, and C-glycosylation, forming the corresponding products 8d-8g in 56%−90% yield with up to >99% α-selectivity.
Table 4.
Post-Functionalization of C2-Alkenyl Sugar.a
|
See SI for experimental details. The isolated yield and axial:equatorial (ax:eq) ratio are indicated below each entry. Reaction conditions: (i) Pd/C (10 mol%), EtOAc (0.100 M), H2 (1 atm), rt, 3h; (ii) 1. O3 (1 atm), DCM (0.100 M), –78 °C, 45 min; 2. PPh3 (2.00 equiv); (iii) m-CPBA (2.00 equiv), DCM (0.100 M), rt. 24h; (iv) BF3•Et2O (1.20 equiv), TMSN3 (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h; (v) BF3•Et2O (1.20 equiv), (2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triyl tribenzoate (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h; (vi) BF3•Et2O (1.20 equiv), 4-tert-butylbenzenethiol (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h; (vii) BF3•Et2O (1.20 equiv), furan (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h.
To gain a better understanding of the reaction mechanism, we performed a series of mechanistic studies. Stern-Volmer luminescence quenching experiments revealed that a 1-bromosugar quenches the excited Pd catalyst more efficiently than styrene (Figure S2). The addition of a radical scavenger such as 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) or butylated hydroxytoluene (BHT) inhibited the reaction (Figure 2A). These results and the isolated 1-TEMPO-glucosyl adduct 3a’ indicate the formation of a 1-glycosyl radical intermediate during the reaction. Subjecting substrates 1,2-cis- and 1,2-trans-2-iodosugars (9a-eq and 9a-ax, respectively), to the standard reaction conditions gave the desired product 3a in similar yield and stereoselectivity (Figure 2B) as the parent reaction (Table 1, entry 1), suggesting that a C2-radical species is the common intermediate. Kinetic isotope effect (KIE) studies using either a mixture of 2e and 2e-d2 or 2e-d1 afforded a value of 1.84 and 2.14, respectively, showing a primary kinetic isotope effect (Figure 2C).11 Light ON/OFF experiments and the measured quantum yield (ϕ = 0.15) (Figure 2D) suggested that an extended radical chain mechanism is unlikely. Alkene formation could proceed through three different reaction mechanisms: (i) Pd-catalyzed β-hydride elimination, (ii) palladoradical hydrogen atom abstraction, or (iii) bromine atom transfer followed by HBr elimination. DFT calculations showed that the recombination of benzylic radical IV with [PdI]Br followed by the β-hydride elimination is the most favorable reaction pathway for this transformation (Figure 2E and Figures S9–S13 in the SI), which is consistent with our experimental data.
Figure 2.
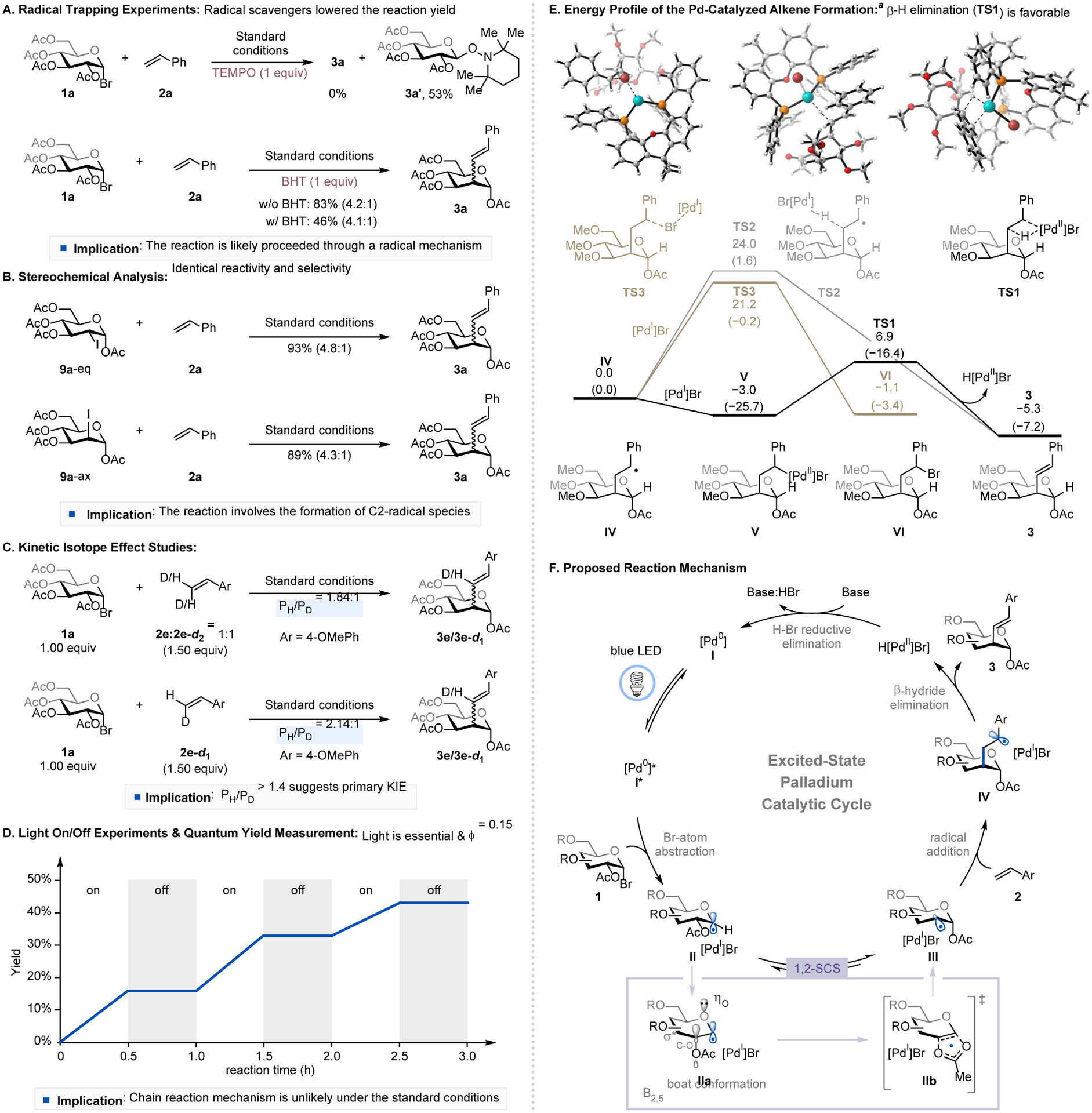
Mechanistic studies and a proposed reaction mechanism. aDFT calculations were performed at the M06/SDD-6-311+G(d,p)/SMD//B3LYP-D3/SDD-6-31G(d) level of theory using a simplified model of the glucosyl radical(1), where the OMe groups were used in place of the OAc groups at the C3, C4, and C6 of the pyranose ring.
Based on these results, a plausible catalytic cycle is proposed in Figure 2F. Photoexcitation of the [Pd0] catalyst furnishes excited *[Pd0] that abstracts a bromine atom from 1-glycosyl bromide 1, forming alkyl radical/Pd(I) species II. This radical species II undergoes 1,2-spin-center shift (SCS) via a conformational change (IIa) and a concerted [2,3]-acyloxy rearrangement (IIb),7y affording C2-radical/Pd(I) intermediate III. Subsequent radical addition of III to alkene substrate 2 produces radical intermediate IV, which recombines with Pd(I) followed by β-hydride elimination, furnishing the desired product 3 and H[PdII]Br complex. Base-assisted HBr reductive elimination of H[PdII]Br regenerates the active [Pd0] catalyst, closing the catalytic cycle.
In summary, we report the first excited-state Pd-catalyzed radical migratory Mizoroki-Heck reaction proceeding through the 1,2-SCS pathway, enabling the direct C2-alkenylation of carbohydrates from readily available 1-bromosugars and alkene derivatives. The reaction (i) significantly streamlines the synthesis of C2-alkenylated glycomimetics, (ii) has high functional group tolerance and broad substrate scope, and (iii) is amenable to late-stage functionalization of complex molecules such as natural product- and drug-glycoconjugates. The resulting C2-alkenylated carbohydrates can serve as versatile synthetic intermediates and glycosyl donors. Preliminary mechanistic studies and DFT calculations suggest a mechanism involving photoexcited Pd species, 1,2-SCS process, and Mizoroki-Heck cross-coupling reaction. We anticipate this excited Pd-catalyzed radical migratory cross-coupling strategy can be extended to other related C2-functionalization of carbohydrates and reactions beyond carbohydrate chemistry.
Supplementary Material
ACKNOWLEDGMENT
The research reported in this publication was supported by the National Institutes of Health (R35-GM119652 to M.-Y.N and R35-GM128779 to P.L.). DFT calculations were performed at the Center for Research Computing at the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation grant number ACI1548562. The Shimadzu UPLC/MS used for portions of this work was purchased with funds from NIGMS equipment administrative supplement (R35-GM119652-04S1), Shimadzu Scientific Instruments grant, and Office of the Vice President for Research at Stony Brook University.
Footnotes
Supporting Information
Experimental details and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCE
- (1).Varki A Essentials of Glycobiology; Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, Eds.; Cold Spring Harbor Press: 2017. [PubMed] [Google Scholar]
- (2).(a) Werz DB; Seeberger PH, Carbohydrates as the Next Frontier in Pharmaceutical Research. Chem. - Eur. J 2005, 11, 3194–3206; [DOI] [PubMed] [Google Scholar]; (b) Seeberger PH; Werz DB, Synthesis and Medical Applications of Oligosaccharides. Nature 2007, 446, 1046–1051. [DOI] [PubMed] [Google Scholar]
- (3).(a) Jackman JE; Fierke CA; Tumey LN; Pirrung M; Uchiyama T; Tahir SH; Hindsgaul O; Raetz CR, Antibacterial Agents That Target Lipid a Biosynthesis in Gram-Negative Bacteria Inhibition of Diverse Udp-3-O-(R-3-Hydroxymyristoyl)-N-Acetylglucosamine Deacetylases by Substrate Analogs Containing Zinc Binding Motifs. J. Biol. Chem 2000, 275, 11002–11009; [DOI] [PubMed] [Google Scholar]; (b) Hang HC; Bertozzi CR, Ketone Isosteres of 2-N-Acetamidosugars as Substrates for Metabolic Cell Surface Engineering. J. Am. Chem. Soc 2001, 123, 1242–1243; [DOI] [PubMed] [Google Scholar]; (c) Li X; Uchiyama T; Raetz CR; Hindsgaul O, Synthesis of a Carbohydrate-Derived Hydroxamic Acid Inhibitor of the Bacterial Enzyme (Lpxc) Involved in Lipid a Biosynthesis. Org. Lett 2003, 5, 539–541; [DOI] [PubMed] [Google Scholar]; (d) Bernardi A; Cheshev P, Interfering with the Sugar Code: Design and Synthesis of Oligosaccharide Mimics. Chem. - Eur. J 2008, 14, 7434–7441; [DOI] [PubMed] [Google Scholar]; (e) Koester DC; Holkenbrink A; Werz DB, Recent Advances in the Synthesis of Carbohydrate Mimetics. Synthesis 2010, 2010, 3217–3242; [Google Scholar]; (f) Lepenies B; Yin J; Seeberger PH, Applications of Synthetic Carbohydrates to Chemical Biology. Curr. Opin. Chem. Biol 2010, 14, 404–411. [DOI] [PubMed] [Google Scholar]
- (4).(a) Flasch H, Bioavailability of Beta-Acetyldigoxin and Digoxin. Wien. Klin. Wochenschr 1975, 53, 873–877; [DOI] [PubMed] [Google Scholar]; (b) Rossini A; Like A; Dulin W; Cahill G, Pancreatic Beta Cell Toxicity by Streptozotocin Anomers. Diabetes 1977, 26, 1120–1124; [DOI] [PubMed] [Google Scholar]; (c) Som P; Atkins H; Bandoypadhyay D; Fowler J; MacGregor R; Matsui K; Oster Z; Sacker D; Shiue C; Turner H, A Fluorinated Glucose Analog, 2-Fluoro-2-Deoxy-D-Glucose (F-18): Nontoxic Tracer for Rapid Tumor Detection. J. Nucl. Med 1980, 21, 670–675; [PubMed] [Google Scholar]; (d) Lu S; Li X; Wang A, A New Chiral Diphosphine Ligand and Its Asymmetric Induction in Catalytic Hydroformylation of Olefins. Catal. Today 2000, 63, 531–536. [Google Scholar]
- (5).(a) Timmers CM; Leeuwenburgh MA; Verheijen JC; van der Marel GA; van Boom JH, Rhodium (II) Catalyzed Asymmetric Cyclopropanation of Glycals with Ethyl Diazoacetate. Tetrahedron: Asymmetry 1996, 7, 49–52; [Google Scholar]; (b) Munyololo M; Gammon DW; Mohrholz I, Extending the Scope of the Ferrier Reaction: Fragmentation-Rearrangement Reactions of Selectively Substituted 1, 2-Cyclopropanated Glucose Derivatives. Carbohydr. Res 2012, 351, 49–55. [DOI] [PubMed] [Google Scholar]
- (6).For selected reviews, see:; (a) Chuentragool P; Kurandina D; Gevorgyan V, Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem. Int. Ed 2019, 58, 11586–11598; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Chuentragool P; Gevorgyan V, Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis 2019, 51, 985; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kancherla R; Muralirajan K; Arunachalam S; Rueping M, Visible Light-Induced Excited-State Transition-Metal Catalysis. Trends Chem. 2019, 1, 510–523; [Google Scholar]; (d) Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G, Visible Light-Induced Palladium-Catalysis in Organic Synthesis. Chem. Lett 2019, 48, 181–191; [Google Scholar]; (e) Cheng W-M; Shang R, Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS Catal. 2020, 10, 9170–9196; [Google Scholar]; (f) Shing Cheung KP; Sarkar S; Gevorgyan V, Visible Light-Induced Transition Metal Catalysis. Chem. Rev 2022, 122, 1543–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).For selected excited-state Pd-catalyzed reactions, see:; (a) Parasram M; Chuentragool P; Sarkar D; Gevorgyan V, Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-Hat: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers. J. Am. Chem. Soc 2016, 138, 6340–6343; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Parasram M; Gevorgyan V, Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem. Int. Ed 2017, 56, 14212–14216; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V, General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhou WJ; Cao GM; Shen G; Zhu XY; Gui YY; Ye JH; Sun L; Liao LL; Li J; Yu DG, Visible-Light-Driven Palladium-Catalyzed Radical Alkylation of C–H Bonds with Unactivated Alkyl Bromides. Angew. Chem. Int. Ed 2017, 56, 15683–15687; [DOI] [PubMed] [Google Scholar]; (e) Wang G-Z; Shang R; Cheng W-M; Fu Y, Irradiation-Induced Heck Reaction of Unactivated Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2017, 139, 18307–18312; [DOI] [PubMed] [Google Scholar]; (f) Cheng WM; Shang R; Fu Y, Irradiation-Induced Palladium-Catalyzed Decarboxylative Desaturation Enabled by a Dual Ligand System. Nat. Commun 2018, 9, 5215; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chuentragool P; Parasram M; Shi Y; Gevorgyan V, General, Mild, and Selective Method for Desaturation of Aliphatic Amines. J. Am. Chem. Soc 2018, 140, 2465–2468; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Sun L; Ye J-H; Zhou W-J; Zeng X; Yu D-G, Oxy-Alkylation of Allylamines with Unactivated Alkyl Bromides and Co2 Via Visible-Light-Driven Palladium Catalysis. Org. Lett 2018, 20, 3049–3052; [DOI] [PubMed] [Google Scholar]; (i) Jiao Z; Lim LH; Hirao H; Zhou JS, Palladium-Catalyzed Para-Selective Alkylation of Electron-Deficient Arenes. Angew. Chem. Int. Ed 2018, 57, 6294–6298; [DOI] [PubMed] [Google Scholar]; (j) Wang G-Z; Shang R; Fu Y, Irradiation-Induced Palladium-Catalyzed Direct C–H Alkylation of Heteroarenes with Tertiary and Secondary Alkyl Bromides. Synthesis 2018, 50, 2908–2914; [Google Scholar]; (k) Wang G-Z; Shang R; Fu Y, Irradiation-Induced Palladium-Catalyzed Decarboxylative Heck Reaction of Aliphatic N-(Acyloxy) Phthalimides at Room Temperature. Org. Lett 2018, 20, 888–891; [DOI] [PubMed] [Google Scholar]; (l) Kurandina D; Rivas M; Radzhabov M; Gevorgyan V, Heck Reaction of Electronically Diverse Tertiary Alkyl Halides. Org. Lett 2018, 20, 357–360; [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Koy M; Sandfort F; Tlahuext-Aca A; Quach L; Daniliuc CG; Glorius F, Palladium-Catalyzed Decarboxylative Heck-Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light. Chem. - Eur. J 2018, 24, 4552–4555; [DOI] [PubMed] [Google Scholar]; (n) Chuentragool P; Yadagiri D; Morita T; Sarkar S; Parasram M; Wang Y; Gevorgyan V, Aliphatic Radical Relay Heck Reaction at Unactivated C (Sp3)–H Sites of Alcohols. Angew. Chem. Int. Ed 2019, 58, 1794–1798; [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) He L; Jia C; Zhang Y; He J, Visible Light Catalyzed Step-Growth Polymerization through Mizoroki–Heck Coupling Reaction. Macromol. Rapid Commun 2020, 41, 1900640; [DOI] [PubMed] [Google Scholar]; (p) Feng L; Guo L; Yang C; Zhou J; Xia W, Visible-Light-Induced Palladium-Catalyzed Intermolecular Narasaka–Heck Reaction at Room Temperature. Org. Lett 2020, 22, 3964–3968; [DOI] [PubMed] [Google Scholar]; (q) Li M; Qiu Y-F; Wang C-T; Li X-S; Wei W-X; Wang Y-Z; Bao Q-F; Ding Y-N; Shi W-Y; Liang Y-M, Visible-Light-Induced Pd-Catalyzed Radical Strategy for Constructing C-Vinyl Glycosides. Org. Lett 2020, 22, 6288–6293; [DOI] [PubMed] [Google Scholar]; (r) Adamik R; Földesi T; Novák Z, Photocatalytic Palladium-Catalyzed Fluoroalkylation of Styrene Derivatives. Org. Lett 2020, 22, 8091–8095; [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Huang H-M; Bellotti P; Pflueger PM; Schwarz JL; Heidrich B; Glorius F, A Three-Component, Interrupted Radical Heck/Allylic Substitution Cascade Involving Unactivated Alkyl Bromides. J. Am. Chem. Soc 2020, 142, 10173–10183; [DOI] [PubMed] [Google Scholar]; (t) Huang H-M; Koy M; Serrano E; Pflüger PM; Schwarz JL; Glorius F, Catalytic Radical Generation of Π-Allylpalladium Complexes. Nat. Catal 2020, 3, 393–400; [Google Scholar]; (u) Shing Cheung KP; Kurandina D; Yata T; Gevorgyan V, Photoinduced Palladium-Catalyzed Carbofunctionalization of Conjugated Dienes Proceeding Via Radical-Polar Crossover Scenario: 1,2-Aminoalkylation and Beyond. J. Am. Chem. Soc 2020, 142, 9932–9937; [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Torres GM; Liu Y; Arndtsen BA, A Dual Light-Driven Palladium Catalyst: Breaking the Barriers in Carbonylation Reactions. Science 2020, 368, 318–323; [DOI] [PubMed] [Google Scholar]; (w) Kvasovs N; Iziumchenko V; Palchykov V; Gevorgyan V, Visible Light-Induced Pd-Catalyzed Alkyl-Heck Reaction of Oximes. ACS Catal. 2021, 11, 3749–3754; [DOI] [PMC free article] [PubMed] [Google Scholar]; (x) Lee GS; Kim D; Hong SH, Pd-Catalyzed Formal Mizoroki–Heck Coupling of Unactivated Alkyl Chlorides. Nat. Commun 2021, 12, 991; [DOI] [PMC free article] [PubMed] [Google Scholar]; (y) Zhao G; Yao W; Mauro JN; Ngai MY, Excited-State Palladium-Catalyzed 1,2-Spin-Center Shift Enables Selective C-2 Reduction, Deuteration, and Iodination of Carbohydrates. J. Am. Chem. Soc 2021, 143, 1728–1734; [DOI] [PMC free article] [PubMed] [Google Scholar]; (z) Meyer T; Rabeah J; Brückner A; Wu X-F, Visible-Light-Induced Palladium-Catalyzed Dehydrogenative Carbonylation of Amines to Oxalamides. Chem. - Eur. J 2021, 27, 5642–5647; [DOI] [PubMed] [Google Scholar]; (aa) Yang Z; Koenigs RM, Photoinduced Palladium-Catalyzed Dicarbofunctionalization of Terminal Alkynes. Chem. - Eur. J 2021, 27, 3694–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Giese B; Gröninger KS; Witzel T; Korth HG; Sustmann R, Synthesis of 2-Deoxy Sugars. Angew. Chem. Int. Ed 1987, 26, 233–234; [Google Scholar]; (b) Korth HG; Sustmann R; Groeninger KS; Leisung M; Giese B, Electron Spin Resonance Spectroscopic Investigation of Carbohydrate Radicals. 4. 1, 2-Acyloxyl Migration in Pyranosyl Radicals. J. Org. Chem 1988, 53, 4364–4369; [Google Scholar]; (c) Beckwith ALJ; Crich D; Duggan PJ; Yao Q, Chemistry of Β-(Acyloxy)Alkyl and Β-(Phosphatoxy)Alkyl Radicals and Related Species: Radical and Radical Ionic Migrations and Fragmentations of Carbon−Oxygen Bonds. Chem. Rev 1997, 97, 3273–3312. [DOI] [PubMed] [Google Scholar]
- (9).For reviews, see:; (a) Wessig P; Muehling O, Spin-Center Shift (Scs)–a Versatile Concept in Biological and Synthetic Chemistry. Eur. J. Org. Chem 2007, 2007, 2219–2232; [Google Scholar]; (b) Marino C; Bordoni AVV, Deoxy Sugars. General Methods for Carbohydrate Deoxygenation and Glycosidation. Org. Biomol. Chem 2022; 20, 934–962; [DOI] [PubMed] [Google Scholar]; (c) Suh CE; Carder HM; Wendlandt AE, Selective Transformations of Carbohydrates Inspired by Radical-Based Enzymatic Mechanisms. ACS Chem. Biol 2021, 16, 1814–1828; [DOI] [PubMed] [Google Scholar]; for selected examples of applications of 1,2-SCS in organic synthesis, see:; (d) Wessig P; Mühling O, A New Photochemical Route to Cyclopropanes. Angew. Chem. Int. Ed 2001, 40, 1064–1065; [DOI] [PubMed] [Google Scholar]; (e) Jin J; MacMillan DW, Alcohols as Alkylating Agents in Heteroarene C–H Functionalization. Nature 2015, 525, 87–90; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nacsa ED; MacMillan DWC, Spin-Center Shift-Enabled Direct Enantioselective Alpha-Benzylation of Aldehydes with Alcohols. J. Am. Chem. Soc 2018, 140, 3322–3330; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Dimakos V; Gorelik D; Su HY; Garrett GE; Hughes G; Shibayama H; Taylor MS, Site-Selective Redox Isomerizations of Furanosides Using a Combined Arylboronic Acid/Photoredox Catalyst System. Chem. Sci 2020, 11, 1531–1537; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Masuda Y; Tsuda H; Murakami M, C1 Oxidation/C2 Reduction Isomerization of Unprotected Aldoses Induced by Light/Ketone. Angew. Chem. Int. Ed 2020, 59, 2755–2759; [DOI] [PubMed] [Google Scholar]; (i) Carder HM; Suh CE; Wendlandt AE, A Unified Strategy to Access 2-and 4-Deoxygenated Sugars Enabled by Manganese-Promoted 1, 2-Radical Migration. J. Am. Chem. Soc 2021, 143, 13798–13805; [DOI] [PubMed] [Google Scholar]; (j) Turner JA; Rosano N; Gorelik DJ; Taylor MS, Synthesis of Ketodeoxysugars from Acylated Pyranosides Using Photoredox Catalysis and Hydrogen Atom Transfer. ACS Catal. 2021, 11, 11171–11179; [Google Scholar]; (k) Zhao G; Yao W; Kevlishvili I; Mauro JN; Liu P; Ngai M-Y, Nickel-Catalyzed Radical Migratory Coupling Enables C-2 Arylation of Carbohydrates. J. Am. Chem. Soc 2021, 143, 8590–8596; [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Zhu Q; Nocera DG, Catalytic C (Β)–O Bond Cleavage of Lignin in a One-Step Reaction Enabled by a Spin-Center Shift. ACS Catal. 2021, 11, 14181–14187. [Google Scholar]
- (10).Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW, The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-Like Molecules. Chem. Soc. Rev 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- (11).Shmidt A; Smirnov V, Kinetic Study of the Heck Reaction by the Method of Competing Reactions. Kinetics and catalysis 2001, 42, 800–804. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


