ABSTRACT
The association between dairy product consumption and cardiovascular health remains highly debated. We quantitatively synthesized prospective cohort evidence on the associations between dairy consumption and risk of hypertension (HTN), coronary heart disease (CHD), and stroke.
We systematically searched PubMed, Embase, and Web of Science through August 1, 2020, to retrieve prospective cohort studies that reported on dairy consumption and risk of HTN, CHD, or stroke. We used random-effects models to calculate the pooled RR and 95% CI for the highest compared with the lowest category of intake and for a 1-serving/d increase in consumption. We rated the quality of evidence using NutriGrade.
Fifty-five studies were included. Total dairy consumption was associated with a lower risk of HTN (RR for highest compared with lowest level of intake: 0.91, 95% CI: 0.86, 0.95, I2 = 73.5%; RR for 1-serving/d increase: 0.96, 95% CI: 0.94, 0.97, I2 = 66.5%), CHD (highest compared with lowest level of intake: 0.96, 95% CI: 0.92, 1.00, I2 = 46.6%; 1-serving/d increase: 0.98, 95% CI: 0.95, 1.00, I2 = 56.7%), and stroke (highest compared with lowest level of intake: 0.90, 95% CI: 0.85, 0.96, I2 = 60.8%; 1-serving/d increase: 0.96, 95% CI: 0.93, 0.99, I2 = 74.7%). Despite moderate to considerable heterogeneity, these associations remained consistent across multiple subgroups. Evidence on the relation between total dairy and risk of HTN and CHD was of moderate quality and of low quality for stroke. Low-fat dairy consumption was associated with lower risk of HTN and stroke and high-fat dairy with a lower risk of stroke. Milk, cheese, or yogurt consumption showed inconsistent associations with the cardiovascular outcomes in high compared with low intake and dose–response meta-analyses.
Total dairy consumption was associated with a modestly lower risk of hypertension, CHD, and stroke. Moderate to considerable heterogeneity was observed in the estimates, and the overall quality of the evidence was low to moderate.
Keywords: dairy, milk, cheese, yogurt, hypertension, coronary heart disease, stroke
Statement of significance: Total dairy consumption was associated with a modestly lower risk of hypertension, CHD, and stroke. The overall body of evidence on total dairy consumption and risk of cardiovascular outcomes was of low to moderate quality.
Introduction
Dietary intervention is one of the most important strategies for the prevention of cardiovascular diseases (CVDs), a significant cause of morbidity and mortality globally (1, 2). Although there is a strong consensus on the fact that dietary patterns emphasizing low intakes of red and processed meats, as well as high intakes of minimally processed fruits, vegetables, and whole grains, are protective against CVD (3), the place of dairy products as part of a heart-healthy diet remains debated (4–6). On the one hand, dairy products contain several bioactive compounds that may be protective against CVDs. Some are thought to reduce blood pressure (e.g., peptides, calcium, magnesium, potassium) (7), attenuate the cholesterol-raising effects of saturated fat (e.g., calcium, magnesium, potassium) (8), enhance insulin sensitivity (e.g., pentadecanoic acid, vitamin D, menaquinone) (9, 10), and promote the equilibrium of gut microbiota (e.g., lactic acid bacteria) (11). On the other hand, dairy products also contain a myriad of fatty acids, mostly saturated, with distinct effects on cardiometabolic and cardiovascular health (12). Long, even-chain saturated fatty acids are abundant in dairy fat and have been associated with insulin resistance (13). In contrast, medium- and odd-chain saturated fatty acids and ruminant trans fatty acids are less abundant in dairy fat, but they may improve insulin sensitivity (14, 15). Emerging evidence also suggests that milk-fat globule membranes have beneficial impacts on lipid cardiovascular risk factors (16). Besides, specific dairy foods, such as cheese, are rich in sodium, which increases blood pressure when consumed in excessive amounts (17). Finally, accumulating evidence suggests that the dairy matrix per se may also influence the impact of dairy consumption on cardiometabolic health parameters (18). The heterogeneity in the nutritional composition and matrices of dairy products highlights the need to evaluate how the consumption of these foods, both collectively and individually, is associated with cardiovascular outcomes.
The literature on dairy consumption and cardiovascular health is vast and has grown rapidly (19). To date, there is a substantial body of evidence reporting that, independent of dairy fat or sodium content, total dairy product consumption is not associated with a higher risk of developing hypertension (HTN), coronary heart disease (CHD), or stroke (20). With regard to specific dairy products, meta-evidence suggested that milk consumption is inversely associated with HTN risk (21), but mixed findings were reported on the potential association between milk intake and clinical outcomes such as CHD or stroke (22–24). For cheese, no association with HTN risk was reported from previous meta-analyses (21, 25). However, similar to milk, mixed findings were reported with regard to the risk of CHD or stroke, with some meta-analyses reporting inverse associations (26) and other null associations with higher cheese intakes (27). Finally, only a limited number of studies have reported on the relation between yogurt and HTN risk (28–32), and to our knowledge, no meta-analysis has been conducted on this specific relation. With regard to the relation between yogurt intake and risk of CHD or stroke, even though meta-evidence is available, these were also based on a limited number of studies (27). These issues highlight the importance of periodic review of meta-evidence on the associations between dairy product consumption and cardiovascular health outcomes.
Furthermore, to inform dietary guidelines and constructively contribute to the debate on the place of dairy products within a healthy diet, the quality of meta-evidence supporting the relation between dairy consumption and cardiovascular health outcomes needs to be assessed using validated grading systems that adequately take into consideration the inherent nature of nutrition science (e.g., reliance on observational data, dietary assessment methods and their validity, etc.) (33–36). In that regard, the validated NutriGrade approach to assess the quality of evidence in nutrition has been demonstrated to be more reliable than other tools such as the Grading of Recommendations Assessment, Development, and Evaluation approach (34, 36–38).
To have a comprehensive and up-to-date overview of the association between dairy consumption and cardiovascular health, we conducted a systematic review and meta-analysis of prospective cohort studies. We aimed to assess the associations between total and specific dairy consumption and the risk of HTN, CHD, and stroke, as well as to evaluate the quality of the evidence supporting the investigated relations using NutriGrade.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (39). The protocol was registered on the international prospective register of systematic reviews (PROSPERO; #CRD42019144618).
Data sources and search strategy
We searched PubMed, Web of Science, and Embase through August 1, 2020, using a combination of terms with relevance to dairy products and the cardiovascular outcomes of interest (i.e., HTN, CHD, stroke, CVD). The specific search terms and search strategy used for each database, along with the number of records retrieved, are presented in Supplemental Table 1. The search was restricted to studies published in English. Screening of titles and abstracts and subsequent full-text reviews of potentially eligible studies were conducted by 2 independent reviewers. The reference lists of included studies were also screened to identify additional relevant studies. Disagreement and discordance between the 2 reviewers were resolved by discussion.
Study eligibility criteria
Studies were included if they 1) were of prospective cohort design [i.e., the most appropriate study design for assessing the long-term relation between dairy consumption and cardiovascular health-related outcomes (33)], 2) assessed the association between dairy consumption (total dairy intake and/or intake of low-fat dairy and/or high-fat dairy and/or milk and/or cheese and/or yogurt) and incidence of HTN and/or CHD (total, fatal or nonfatal) and/or stroke (total, ischemic, hemorrhagic, fatal or nonfatal) and/or CVD (defined as a combination of stroke and CHD), 3) provided risk estimates for at least 2 levels of exposure, and 4) were free of the outcome of interest at baseline.
Data extraction
Data extraction was performed in duplicate so that at least 2 reviewers achieved consensus on which data to extract from included studies. For each included study, extracted data included first author's name, publication year, source(s) of funding, cohort name, the country where the study was conducted, number of participants, sex of participants, age range of participants at baseline, follow-up duration, method used to assess diet, endpoints, method used to ascertain cases, number of cases, statistical model used, types and categories of dairy product used as the exposure, risk estimates from the maximally adjusted multivariable model for each level of intake, and covariates included in the maximally adjusted multivariable model.
Risk-of-bias assessment
We used the Newcastle–Ottawa Scale (NOS) to assess the risk of bias (ROB) of included studies (40). The NOS contains 9 items. Each item is scored (0–1 point) according to specific criteria. The maximum possible score is 9. Scores from 7 to 9 reflect low ROB, scores from 4 to 6 indicate moderate ROB, and scores 0 to 3 indicate high ROB. Primary confounders included risk factors included in the Framingham risk score algorithm for the calculation of the 10-y risk of CVD [age, sex, weight, and/or BMI (in kg/m2), baseline blood lipids, baseline blood pressure, baseline diabetes status, smoking status] (41). Total energy intake was also considered a primary confounder because self-reported dairy intake is confounded by total energy intake (42). Secondary confounders included physical activity level, diet quality, and/or red meat intake (used as a proxy to diet quality) given the reported clustering of dairy consumption and healthy lifestyle habits (43).
Data synthesis and analysis
RRs were used as the common measure of association across studies. HRs and ORs were considered equivalent to RRs (44, 45). To harmonize exposures between studies, we used 200 g as the standard quantity for 1 serving of total, high-fat, and low-fat dairy intake, 242 g for milk, 184 g for yogurt, and 28 g for cheese (46). To determine the intake levels, we used the median of each dairy intake category if available or the midpoint between the lower and upper bound of each category of intake. When the highest category was open, we multiplied the lower bound of the highest category by 1.75 (47).
If a study reported only risk estimates for subtypes of a specific dairy food but not for total consumption of the specific dairy food (e.g., estimates available for reduced-fat milk and whole milk but not for total milk), the risk estimates of each subtype were pooled using fixed-effects meta-analysis (i.e., reduced-fat milk + whole milk = total milk) (36). The pooled RR was subsequently used in the meta-analysis pertaining to the specific dairy food. This specific process was used for the study by Duffey et al. (48), in which we pooled risk estimates for HTN risk associated with low-fat milk intake and whole-milk intake, as reported in the article, and used the pooled RR in the meta-analysis on milk consumption and HTN risk. Likewise, in studies in which only risk estimates for individual dairy foods or subtypes were available but no estimates for total dairy intake were included, we pooled risk estimates from all available individual dairy foods or subtypes using fixed-effects meta-analysis to obtain the risk estimate for total dairy intake. In the study by Beydoun et al. (31) in the Healthy Aging in Neighborhoods of Diversity across the Life Span Cohort (United States), estimates were available for the risk of HTN associated with intakes of cheese, milk, and yogurt individually. Importantly, no other dairy foods were assessed in this study (31). Therefore, the risk estimates of cheese, milk, and yogurt were pooled using fixed-effects meta-analysis to obtain the RR for total dairy consumption. Notably, milk, cheese, and yogurt are the most consumed dairy foods and account for most of total dairy intake in the United States (49). The same approach was used in the studies by Bernstein et al. (50) (low-fat dairy + high-fat dairy), Goldbohm et al. (51) (milk products + cheese), Johansson et al. (52) (nonfermented milk + fermented milk + cheese), and Key et al. (53) (cheese + yogurt + milk). Finally, we also harmonized stroke outcomes, when needed. Kinjo et al. (54) and Larsson et al. (55) both reported risk estimates for stroke subtypes but not total stroke. We thus pooled risk estimates for stroke subtypes and used the pooled RR in the meta-analysis on stroke (54, 55). Again, fixed-effects meta-analyses were used. For the harmonization process of both dairy exposures and stroke outcomes, we used fixed-effects meta-analyses because pooled data were obtained from the same population and under the same study settings (36). Last, we preferentially used sex-specific risk estimates, when available (36).
For each outcome of interest, we first conducted meta-analyses of high compared with low dairy intake by pooling the risk estimates from the highest category of intake compared with the lowest category of intake reported in each study using random-effects models. For 1 study that used the highest category of dairy intake as the reference category (56), we back-calculated risk estimates and CIs to set the lowest category of intake as the reference group (57). In studies with only estimates for continuous dairy intake, we used the provided estimate in the meta-analysis. We subsequently performed dose–response meta-analyses for a 1-serving/d increase in consumption in the subset of studies that provided sufficient data (i.e., at least 3 levels of intake and corresponding number of cases, person years, and risk estimates). We imputed person years based on available data when not provided in the original article. The dose–response relation was estimated by using generalized least squares trend estimation, according to the methods developed by Greenland and Longnecker (58). We used the 2-stage generalized least squares trend estimation method, which first estimated study-specific slope lines and then combined with studies in which the slopes were directly reported to obtain an overall average slope. We also used random-effects models for dose–response analyses.
Heterogeneity in the summary estimate was assessed using the I2 statistic and interpreted according to the Cochrane Handbook thresholds (0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75–100%: considerable heterogeneity) (59). To identify potential sources of heterogeneity, we first conducted influence analyses by systematically removing each study from the meta-analysis and recalculating the RR to evaluate if any single study contributed to the observed heterogeneity. We also conducted univariate meta-regressions by using study-level data to explore potential sources of heterogeneity. Predefined subgroup analyses were conducted by sex, country/region where the studies were conducted (e.g., America compared with Europe compared with Asia), adjustment for total energy intake, length of follow-up, and number of cases. We tested for the presence of publication bias using the Begg and Egger tests and visual inspection of a funnel plot. Furthermore, we conducted sensitivity analyses. We a priori determined to assess the association between dairy consumption and risk of CVD, defined as a combined outcome of CHD and stroke. This analysis included all studies that reported risk estimates on CHD, stroke, or CHD and stroke. If a study reported on CHD and stroke separately but provided no estimate for overall CVD, we pooled the risk estimates for CHD and stroke using fixed meta-analysis and used the pooled estimate in the overall meta-analysis. We treated this meta-analysis as a separate sensitivity analysis because substantial evidence suggests that the relations between some dairy subtypes and risk of CHD or stroke may differ (e.g., cheese) (20).
Post hoc sensitivity analyses (i.e., not included in the prespecified and registered analysis protocol) included the assessment of the relation between total dairy and risks of HTN and CHD while excluding the studies for which we calculated the risk estimates for total dairy using the procedure described above (i.e., by pooling estimates for dairy foods or subtypes using fixed-effects meta-analysis). We did not conduct such analysis for stroke as this process was not applied in any studies for stroke. We also repeated the main analysis on total dairy and risk of HTN, CHD, and stroke by excluding studies that did not control for any of the secondary confounders. Finally, we applied the trim-and-fill method when needed to assess and adjust for potential publication bias, when publication bias was suspected based on Begg and Egger tests and/or visual inspection of the funnel plot.
Statistical analyses for the meta-analysis were performed using Stata v.15.1 (StataCorp). Statistical significance was considered at P < 0.05.
Assessment of the quality of evidence
We used the NutriGrade scoring system for cohort studies to assess the quality of evidence supporting the observed relation between each dairy category and outcome we evaluated (34). The NutriGrade scoring system for meta-analyses of cohort studies includes 8 items as follows: 1) ROB, study quality, and study limitations (0–2 points); 2) precision (0–1 point); 3) heterogeneity (0–1 point); 4) directness (0–1 point); 5) publication bias (0–1 point); 6) funding bias (0–1 point); 7) effect size (0–2 points); and 8) dose–response (0–1 point) (34). NutriGrade scores ≥8/10 points indicate “high-quality evidence,” meaning that there is high confidence in the effect estimate, and further research probably will not change the confidence in the effect estimate (34). Other thresholds are 6–7.99 points for “moderate-quality evidence,” 4–5.99 points for “low-quality evidence,” and 0–3.99 for “very low-quality evidence.” Very low-quality evidence refers to very low confidence in the effect estimate (34). The assessment was conducted independently by 2 reviewers. Disagreement and discordance in the scoring were resolved by discussion.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Results
We screened a total of 1524 studies, and 55 articles met the inclusion criteria (Supplemental Figure 1). The list of studies that were excluded following full-text review and the reason for exclusion are presented in Supplemental Table 2. A total of 18 studies (including 24 risk estimates) reported data on dairy consumption and HTN risk (28–32, 48, 60–71), 24 studies (including 30 risk estimates) reported on CHD risk (51–53, 56, 72–91), and 20 studies (including 25 risk estimates) reported on stroke risk (50–52, 54–56, 72, 73, 75, 76, 78, 81–84, 90–94). A total of 16 additional studies (21 risk estimates) were included in further sensitivity analyses on total CVD risk as they reported data on the relation between dairy intake and risk of CVD, defined as CHD and stroke (56, 72, 73, 76, 78, 81, 82, 90, 91, 95–101). Included cohorts comprised between 337 and 409,885 adult participants followed from 5 to 32 y.
Hypertension risk
Characteristics of the 18 studies that assessed the relation between dairy consumption and risk of HTN are presented in Supplemental Table 3. These studies were conducted in the United States (n = 6), Europe (n = 6), and Asia (n = 5), and 1 was multicontinental. Inclusion of primary and secondary confounders in fully adjusted models from those studies is presented in Supplemental Table 4. No study controlled for all primary and/or secondary confounders. The assessment of ROB using the NOS is presented in Supplemental Table 5. All studies were considered to have a moderate ROB with NOS scores ranging between 4 and 6 points.
The meta-analysis on the association between total dairy product consumption and risk of incident HTN (highest compared with lowest level of intake) included 23 risk estimates. High total dairy consumption was associated with a 9% lower risk of HTN compared with low dairy consumption (pooled RR for highest compared with lowest level of intake: 0.91; 95% CI: 0.86, 0.95; I2 = 73.5%; Table 1 and Figure 1). In the dose–response meta-analysis, each additional daily serving of dairy products was associated with a 4% lower risk of HTN (pooled RR for a 1-serving/d increase: 0.96; 95% CI: 0.94, 0.97; I2 = 66.5%; Table 1). Among dairy subtypes, high compared with low intakes of low-fat dairy, milk, and yogurt were associated with a 5–12% lower risk of HTN (Table 1). No difference in HTN risk was found when high compared with low intakes of high-fat dairy or cheese were compared. In the dose–response meta-analysis, each additional serving of low-fat dairy, but not milk or yogurt, was associated with a 5% lower risk HTN (pooled RR: 0.95; 95% CI: 0.92, 0.98) (Table 1).
TABLE 1.
Meta-analysis of multivariable relative risks of the association between dairy product consumption and hypertension using random effects1
| High vs. low intake | Dose–response (1-serving/d increase) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy product | Risk estimates (n) | Participants (n) | Cases (n)2 | Pooled RR (95% CI) | I 2 (%) | Risk estimates (n) | Participants (n) | Cases (n)2 | Pooled RR (95% CI) | I 2 (%) | Quality of evidence3 |
| Total dairy | 23 | 414,148 | >136,025 | 0.91 (0.86, 0.95) | 73.5 | 19 | 314,954 | >120,254 | 0.96 (0.94, 0.97) | 66.5 | Moderate |
| High-fat dairy | 11 | 103,307 | >25,232 | 0.99 (0.94, 1.05) | 84.2 | 7 | 35,767 | >10,091 | 0.98 (0.95, 1.00) | 11.8 | Low |
| Low-fat dairy | 11 | 103,307 | >25,232 | 0.88 (0.80, 0.96) | 58.9 | 7 | 38,012 | >11,075 | 0.95 (0.92, 0.98) | 57.6 | Low |
| Milk | 13 | 340,403 | >118,481 | 0.94 (0.90, 0.97) | 77.6 | 7 | 121,507 | 34,524 | 0.96 (0.90, 1.02) | 78.0 | Low |
| Cheese | 9 | 264,514 | >95,066 | 0.97 (0.94, 1.01) | 41.8 | 6 | 50,502 | 12,702 | 1.00 (0.96, 1.04) | 54.0 | Very low |
| Yogurt | 10 | 263,473 | >102,792 | 0.95 (0.90, 1.00) | 65.6 | 8 | 232,435 | 94,100 | 0.95 (0.89, 1.02) | 84.7 | Low |
Pooled relative risks are from random-effects meta-analyses. I2 refers to the proportion of heterogeneity between studies.
Number of cases not available in Beydoun et al. (31), Engberink et al. (67), and Johansson et al. (70).
The quality of evidence was graded according to the NutriGrade scoring system.
FIGURE 1.
Association of total dairy consumption with hypertension risk, for high compared with low intake, using random-effects meta-analysis. Weights of each estimate are represented by the size of the square. Black diamonds represent the individual estimate effects, and black lines represent the 95% CI. The x-axis is the relative risk. The overall effect estimates and 95% CIs are represented by the diamond and dotted line. I2 refers to the proportion of heterogeneity between studies. M, risk estimate among men; W, risk estimate among women.
There was evidence of substantial heterogeneity in the pooled estimates for the high compared with low and dose–response analysis for total dairy and HTN risk (I2 > 65.0% for both). No single study appeared to cause heterogeneity according to the influence analysis (Supplemental Figure 2). In the stratified analyses (Supplemental Table 6), the relation between total dairy intake and HTN risk significantly differed between studies among which the fully adjusted model included total energy intake as a covariate and those without adjustment for total energy intake (P-interaction = 0.04). There was an inverse association between total dairy and HTN risk among studies for which the final multivariable model included total energy intake (n = 21; RR: 0.90; 95% CI: 0.86, 0.94; I2 = 70.0%) but not among those that did not control for total energy intake (n = 2; RR: 1.17; 95% CI: 0.79, 1.73; I2 = 73.5%). Still, heterogeneity was substantial within the 2 strata. No significant interactions were observed in the association between total dairy intake and HTN risk in other stratified analyses (i.e., by region, sex, duration of follow-up, and number of cases; P-interaction > 0.05 for all). However, heterogeneity was minimal (I2 = 0%) when we restricted the analysis among studies conducted in Europe (n = 7; RR: 0.96; 95% CI: 0.91, 1.02) and among studies that included both females and males (n = 9; RR: 0.91; 95% CI: 0.87, 0.94). Furthermore, excluding studies for which we calculated the risk estimates for total dairy had virtually no impact on the results (pooled RR for high compared with low total dairy intake: 0.90; 95% CI: 0.85, 0.96). Similarly, excluding the 1 study that did not adjust for any of secondary confounders had also little impact on the results (pooled RR for high compared with low total dairy intake: 0.90; 95% CI: 0.86, 0.94). Examination of the funnel plot (Supplemental Figure 3) and Egger (P = 0.85) and Begg (P = 0.83) tests suggest no publication bias with regard to the relation between total dairy intake and HTN risk.
According to the NutriGrade scoring system, the quality of evidence supporting the inverse association between total dairy consumption and HTN risk was moderate, whereas for other dairy subtypes, the quality was rated as low, with the exception of cheese, for which the quality of evidence was rated as very low (Table 1 and Supplemental Table 7). The quality of evidence for the relation between cheese consumption and HTN risk was lower than for other dairy subtypes because of the lack of association in the high compared with low meta-analysis (precision item) and the lack of dose–response relation (dose–response item).
CHD risk
Characteristics of the 24 studies that assessed the relation between dairy consumption and risk of CHD are presented in Supplemental Table 8. The cohorts on which these studies were based were from the United States (n = 4), Europe (n = 13), and Asia (n = 6), and 1 was multicontinental. Inclusion of primary and secondary confounders in adjusted models of studies that reported on CHD is presented in Supplemental Table 9. Only 1 study controlled for all primary confounders, and no study controlled for all primary and secondary confounders. The assessment of ROB using the NOS is presented in Supplemental Table 10. Of the 24 studies, 1 was at high ROB (NOS score = 2), 20 at moderate ROB (NOS score = 4–6), and 3 at low ROB (NOS score = 7).
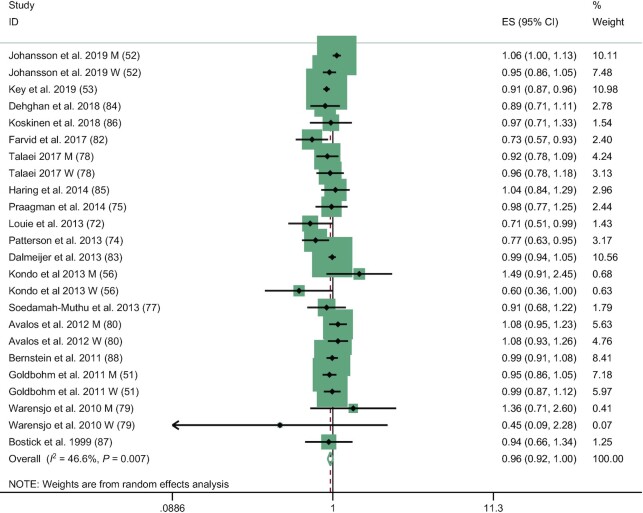
The meta-analysis on the association between total dairy product consumption and risk of incident CHD (high compared with low intake) included 24 risk estimates obtained from 18 publications. The pooled RR for highest compared with lowest category of intake was 0.96 (95% CI: 0.92, 1.00; I2 = 46.6%) (Table 2 and Figure 2). In the dose–response meta-analysis, the pooled RR for a 1-serving/d increase was 0.98 (95% CI: 0.95, 1.00; I2 = 56.7%; Table 2). Among dairy subtypes, high cheese consumption was associated with a lower risk of CHD compared with low intakes (pooled RR for highest compared with lowest category of intake: 0.90; 95% CI: 0.84, 0.97; I2 = 47.1%; Table 2). This association was, however, not supported by a dose–response relation. Finally, there were no differences in CHD risk associated with high compared with low intakes or dose–response relation with CHD risk for high-fat dairy, low-fat dairy, milk, or yogurt.
TABLE 2.
Meta-analysis of multivariable relative risks of the association between dairy product consumption and CHD using random effects1
| High vs. low intake | Dose–response (1-serving/d increase) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy product | Risk estimates (n) | Participants (n) | Cases (n) | Pooled RR (95% CI) | I 2 (%) | Risk estimates (n) | Participants (n) | Cases (n) | Pooled RR (95% CI) | I 2 (%) | Quality of evidence2 |
| Total dairy | 24 | 868,821 | 34,248 | 0.96 (0.92, 1.00) | 46.6 | 13 | 291,132 | 15,831 | 0.98 (0.95, 1.00) | 56.7 | Moderate |
| High-fat dairy | 8 | 218,139 | 6546 | 1.02 (0.95, 1.08) | 16.8 | 6 | 171,587 | 5325 | 1.00 (0.96, 1.03) | 34.9 | Low |
| Low-fat dairy | 9 | 204,435 | 9646 | 0.95 (0.87, 1.05) | 58.3 | 8 | 192,365 | 8599 | 0.99 (0.95, 1.04) | 54.9 | Low |
| Milk | 17 | 823,742 | 22,614 | 1.03 (0.97, 1.08) | 37.0 | 16 | 313,526 | 10,670 | 1.00 (1.00, 1.00) | 0.0 | Low |
| Cheese | 15 | 684,832 | 19,940 | 0.90 (0.84, 0.97) | 47.1 | 7 | 139,207 | 7299 | 0.97 (0.94, 1.01) | 62.2 | Low |
| Yogurt | 7 | 497,217 | 8938 | 1.02 (0.88, 1.18) | 64.7 | 5 | 119,209 | 34,925 | 1.00 (1.00, 1.00) | 0.0 | Low |
Pooled relative risks are from random-effects meta-analyses. I2 refers to the proportion of heterogeneity between studies.
The quality of evidence was graded according to the NutriGrade scoring system.-
FIGURE 2.
Association of total dairy consumption with coronary heart disease risk, for high compared with low intake, using random-effects meta-analysis. Weights of each estimate are represented by the size of the square. Black diamonds represent the individual estimate effects, and black lines represent the 95% CI. The x-axis is the relative risk. The overall effect estimates and 95% CIs are represented by the diamond and dotted line. I2 refers to the proportion of heterogeneity between studies. M, risk estimate among men; W, risk estimate among women.
There was evidence of moderate heterogeneity in the pooled estimate for total dairy intake and CHD risk (I2 for high compared with low meta-analysis: 46.6%; I2 for dose–response meta-analysis: 56.7%). No individual study had a major influence on the pooled estimate when removed (Supplemental Figure 4). In analyses stratified by region, sex, follow-up duration, number of cases, and adjustment for total energy intake (Supplemental Table 11), no significant interactions were detected. However, a statistical trend (P = 0.09) suggested potential differences according to whether the model was adjusted for total energy or not. Studies for which the fully adjusted model controlled for energy intake suggested a lower risk of CHD associated with high compared with low total dairy intakes (n = 18; RR: 0.95; 95% CI: 0.91, 0.99; I2 = 43.7%). However, the inverse association between total dairy intake and CHD risk was not observed when studies among which fully adjusted model did not include total energy intake were pooled (n = 6, RR: 1.06; 95% CI: 0.90, 1.25; I2 = 38.9%). The pooled RR of CHD for high compared with low total dairy intake following the exclusion of studies for which we calculated the risk estimates was 0.90 (95% CI: 0.85, 0.95). The pooled RR of CHD for high compared with low total dairy intake following the exclusion of the 7 studies that did not adjust for any of secondary confounders was 0.96 (95% CI: 0.92, 1.01). There was no evidence of publication bias from the funnel plot (Supplemental Figure 5) and Egger (P = 0.41) and Begg (P = 0.36) tests.
Based on the NutriGrade scoring system for cohort studies, the quality of the evidence on the relation between the consumption of total dairy and CHD risk was rated as moderate, whereas it was rated as low for dairy subtypes (Table 2 and Supplemental Table 12).
Stroke risk
Characteristics of the 20 studies that assessed the relation between dairy consumption and risk of stroke are presented in Supplemental Table 13. These studies were conducted in the United States (n = 1), Europe (n = 9), and Asia (n = 9), and 1 was multicontinental. Inclusion of primary and secondary confounders in adjusted models of studies that reported on stroke is presented in Supplemental Table 14. Only 1 study controlled for all primary confounders, and no study controlled for all primary and secondary confounders. The assessment of ROB using the NOS is presented in Supplemental Table 15. Of the 20 studies, 1 was at high ROB (NOS score = 3), 18 at moderate risk (NOS score = 5–6), and 1 at low ROB (NOS score = 7).
The meta-analysis on the association between total dairy product consumption and risk of stroke (high compared with low intake) included 19 risk estimates extracted from 13 publications. We found that high total dairy consumption was associated with a 10% lower risk of stroke compared with low dairy consumption (pooled RR for highest compared with lowest category of intake: 0.90; 95% CI: 0.85, 0.96; I2 = 60.8% Table 3 and Figure 3). In the dose–response meta-analysis, each additional daily serving of dairy products was associated with a 4% lower risk of stroke (pooled RR for a 1-serving/d increase: 0.96; 95% CI: 0.93, 0.99; I2 = 74.7%; Table 3). Among individual dairy products, high compared with low intakes of high-fat dairy, low-fat dairy, and cheese were associated with a 6–9% lower risk of stroke. Inverse relations were also observed in the dose–response meta-analyses for high-fat and low-fat dairy but not for cheese. High compared with low intakes of yogurt and milk were not associated with risk of stroke, but an inverse dose–response relation was found between milk consumption and stroke risk (pooled RR for a 1-serving/d increment in milk consumption: 0.94; 95% CI: 0.89, 0.99; I2 = 76.4%).
TABLE 3.
Meta-analysis of multivariable relative risks of the association between dairy product consumption and stroke using random effects1
| High vs. low intake | Dose–response (1-serving/d increase) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy product | Risk estimates (n) | Participants (n) | Cases (n) | Pooled RR (95% CI) | I 2 (%) | Risk estimates (n) | Participants (n) | Cases (n) | Pooled RR (95% CI) | I 2 (%) | Quality of evidence2 |
| Total dairy | 19 | 552,573 | 27,775 | 0.90 (0.85, 0.96) | 60.8 | 16 | 431,799 | 22,677 | 0.96 (0.93, 0.99) | 74.7 | Low |
| High-fat dairy | 9 | 412,206 | 14,869 | 0.93 (0.89, 0.98) | 0.0 | 7 | 285,046 | 10,839 | 0.99 (0.97, 1.00) | 0.0 | Low |
| Low-fat dairy | 11 | 432,988 | 15,923 | 0.91 (0.87, 0.96) | 0.0 | 9 | 305,828 | 11,893 | 0.97 (0.95, 1.00) | 14.0 | Low |
| Milk | 15 | 665,044 | 27,223 | 0.96 (0.88, 1.04) | 72.1 | 12 | 343,302 | 14,713 | 0.94 (0.89, 0.99) | 76.4 | Low |
| Cheese | 10 | 335,543 | 14,799 | 0.94 (0.88, 1.00) | 23.8 | 7 | 202,562 | 10,986 | 0.96 (0.91, 1.01) | 52.1 | Low |
| Yogurt | 4 | 107,603 | 5471 | 1.07 (0.97, 1.18) | 0.0 | 3 | 73,194 | 5312 | 1.08 (0.85, 1.39) | 79.8 | Very low |
Pooled relative risks are from random-effects meta-analyses. I2 refers to the proportion of heterogeneity between studies.
The quality of evidence was graded according to the NutriGrade scoring system.
FIGURE 3.
Association of total dairy consumption with stroke risk, for high compared with low intake, using random-effects meta-analysis. Weights of each estimate are represented by the size of the square. Black diamonds represent the individual estimate effects, and black lines represent the 95% CI. The x-axis is the relative risk. The overall effect estimates and 95% CIs are represented by the diamond and dotted line. I2 refers to the proportion of heterogeneity between studies. M, risk estimate among men; W, risk estimate among women.
There was evidence of substantial heterogeneity in the pooled estimate for the association between total dairy intake and stroke risk (I2 for high compared with low intake: 60.8%; I2 for a 1-serving/d increment: 74.7%). There was no evidence of undue influence from a single study (Supplemental Figure 6). In the stratified analyses (Supplemental Table 16), no significant interactions were found when stratified by region, sex, follow-up duration, number of cases, and adjustment for total energy intake. However, a significant interaction (P = 0.04) suggesting a differential relation between total dairy intake and stroke risk according to follow-up duration (±10 y) was detected. The inverse relation appeared, in fact, stronger among studies with shorter follow-up duration (RR for n = 1 study with <10 y of follow-up: 0.66; 95% CI: 0.53, 0.82), even though the inverse association was also observed among studies with longer follow-up (pooled RR n = 18 studies with >10 y of follow-up: 0.92; 95% CI: 0.87, 0.97). Besides, when we restricted the analyses for studies conducted in Asia (risk estimates, n = 8; pooled RR: 0.78; 95% CI: 0.70, 0.86) or the United States (n = 2; pooled RR: 0.90; 95% CI: 0.84, 0.97), among females only (n = 5; pooled RR: 0.91; 95% CI: 0.86, 0.97), or with <1000 cases (n = 9; pooled RR: 0.91; 95% CI: 0.85, 0.97), heterogeneity was minimal (I2 = 0%, for all). The pooled RR of stroke for high compared with low total dairy intake following the exclusion of the 6 studies that did not adjust for any of secondary confounders was 0.91 (95% CI: 0.85, 0.97). Examination of the funnel plot (Supplemental Figure 7) and Egger (P = 0.01) and Begg (P = 0.07) tests suggested potential publication bias favoring a lower risk of stroke associated with total dairy intake. We investigated this further using the trim-and-fill method. The RR of stroke increased from 0.89 (95% CI: 0.84, 0.96; main analysis based on 19 risk estimates) to 0.95 (95% CI: 0.88, 1.03; trim-and-fill analysis based on 25 risk estimates, of which 6 were imputed) (Supplemental Figure 8).
The quality of the evidence supporting the inverse association between total dairy consumption and stroke risk was of low quality according to the NutriGrade scoring system. The same level of evidence quality was attributed to the relations between stroke risk and high-fat dairy, low-fat dairy, milk, and cheese consumption. Data pertaining to the association between yogurt intake and stroke risk were of very low quality, mostly due to the lack of association in the high compared with low meta-analysis (precision item) and the lack of dose–response relation (dose–response item) (Table 3 and Supplemental Table 17).
Sensitivity analysis on overall CVD risk (i.e., coronary heart disease and/or stroke)
We conducted an additional sensitivity analysis on the association between dairy consumption and overall risk of CVD, defined as CHD and/or stroke. This analysis included all individual studies that reported on dairy and risk of CHD, stroke, or the combination of both. Characteristics of the 16 studies that we additionally included in this meta-analysis are presented in Supplemental Table 18.
The meta-analysis on the association between total dairy product consumption and risk of incident CVD (high compared with low intake) included 33 risk estimates extracted from 25 publications (Supplemental Table 19). We found that higher total dairy consumption was associated with a significant 5% lower risk of CVD compared with lower dairy consumption (pooled RR for highest compared with lowest category of intake: 0.95; 95% CI: 0.91, 0.98; I2 = 60.7%; Supplemental Figure 9). CVD risk was 3% lower for each dairy serving/d increment in consumption (pooled RR for a 1-serving/d increase: 0.97; 95% CI: 0.95, 0.99; I2 = 72.6%; Supplemental Table 19). Among individual dairy products, only cheese consumption was inversely associated with CVD risk, and this relation was significant in the high compared with low intake and the dose–response meta-analyses (Supplemental Table 19).
Discussion
In this meta-analysis of prospective cohort studies on dairy product consumption and cardiovascular outcomes, we found modest inverse associations between total dairy intake and risk of HTN, CHD, and stroke. Results were consistent in high compared with low intakes and dose–response meta-analyses as well as across multiple participant and study characteristics. However, these associations were marked with moderate to considerable heterogeneity. In that regard, we could not identify a clear source of between-study heterogeneity, with the exception of plausible effect modification due to adjusting for total energy intake in the fully adjusted models from the original studies. With regard to dairy fat content, we observed that total low-fat dairy consumption was associated with a slightly lower risk of HTN and stroke but not CHD. However, total high-fat dairy consumption was not associated with the risk of HTN and CHD but showed a modest inverse association with stroke risk. On the other hand, the consumption of milk or yogurt—2 dairy foods with predominately low-fat content—showed no consistent inverse associations with the risk of HTN or stroke, and consumption of cheese—a high-fat dairy food—showed no consistent inverse associations with the risk of stroke. Notably, no positive association between total dairy, high-fat dairy, or dairy subtypes and any cardiovascular outcome was observed. This systematic review and meta-analysis provides a comprehensive overview of the potential benefits and the lack of data supporting potential harms associated with dairy intake in the context of CVD prevention. Still, results of this meta-analysis on dairy and cardiovascular outcomes (HTN, CHD, stroke) should be interpreted in the context that they rely on observational evidence of very low to moderate quality according to the NutriGrade scoring system.
With regard to HTN risk, the first 2 meta-analyses on this topic were published in the early 2010s and reported inverse dose–response associations between total and low-fat dairy consumption and HTN risk (21, 25). Two additional meta-analyses, published in 2017 and 2018, obtained similar conclusions (102, 103). Although the current work includes more than double the number of studies and nearly 10 times the number of participants and cases, the pooled RRs for a 1-serving/d increase in consumption we obtained for the relations between HTN risk and total (RR: 0.96; 95% CI: 0.94, 0.97) and low-fat (RR: 0.95; 95% CI: 0.92, 0.98) dairy were nearly identical to those reported nearly 10 y ago (21, 25). This highlights that evidence generated over the past decade has been consistent with early observational data on dairy consumption and HTN risk but also demonstrates the robustness of the evidence body on dairy and HTN risk. The lack of a dose–response association between milk consumption and HTN risk we found is the only finding that differs from previous meta-analyses, as both previous meta-analyses reported significant inverse associations (21, 25). This suggests that most recent prospective cohort studies on milk consumption and HTN risk tended to report mostly neutral associations. Finally, even though previous meta-analyses on cheese and yogurt consumption and risk of HTN were based on limited data (21, 25), our updated work provides consistent conclusions, suggesting no differential risk of HTN associated with cheese or yogurt intake.
Previous meta-analyses on total dairy consumption and CHD risk have been less consistent than those on HTN risk (20, 26, 104, 105). Some meta-analyses reported no association (104–106), and others reported a modest inverse association (20, 26). Interestingly, meta-analyses that reported a modest inverse association between total dairy intake and CHD risk are the most recent ones (26). Conversely, meta-analyses that reported no significant association are among the first published on this topic (104, 105). The borderline inverse association we found between total dairy intake and CHD risk corroborates this trend, suggesting that early meta-analyses may have pooled studies that were biased toward neutral associations. Our results on stroke risk are also consistent with previous meta-analyses (107). Indeed, we found an inverse association between total dairy intake and stroke risk by pooling data from half a million individuals and >25,000 cases. Again, data on individual dairy foods were less consistent and suggested no association with stroke risk, which is consistent with previous meta-analyses (105, 108, 109).
Overall, even though findings from our meta-analyses are concordant with previous studies despite including up to 10 times the number of participants and cases, evidence generated from our analyses are, for the most, of low quality according to the standardized and validated NutriGrade scoring system (34). In that regard, it is important to stress that nearly all of the inverse relations between dairy intake, either total intakes or intakes of dairy subtypes, and risk of HTN, CHD, or stroke were of modest effect size (i.e., <10% difference in risk). In the NutriGrade scoring system, the effect size criteria account for 20% of the quality of evidence assessment (2/10 points), and NutriGrade considers any difference in risk estimates <20% between highest and lowest category of intake (RR: 0.80–1.20) as nonmeaningful. Therefore, it is unlikely that the body of evidence on the relation between dairy consumption and cardiovascular health will ever be considered of high quality despite the publication of additional studies in the future. Indeed, modest effect sizes as the ones we repeatedly observed could be attributable to residual confounding as substantial evidence shows that dairy consumption clusters with healthier lifestyle habits (43, 110). Also, a number of Mendelian randomization study showed no causal associations between dairy intake and cardiometabolic (111) and cardiovascular outcomes (112). For instance, a Mendelian randomization study based on 22 cohorts found no causal association between milk and blood pressure or HTN risk, using a genetic polymorphism located upstream of the lactase gene (LCT-13910 C/T, rs4988235) as an instrumental variable (112). Even though Mendelian randomization studies on dairy intake have to be interpreted with caution because dairy products such as yogurt and cheese contain little to no lactose and are well tolerated among lactose-intolerant individuals, such studies raise uncertainties with regard to potential causal relations between dairy intake and lower risk of cardiovascular outcomes. Similarly, high-quality fully controlled trials that investigated the impact of the consumption of dairy products on surrogate risk factors of cardiometabolic and CVDs reported, for the most, null results (113–116). Some trials also reported deleterious impacts of dairy intake on cardiometabolic health (117).
Still, despite the limitations related to the modest quality of evidence, our work has several important implications. First, with regard to the debate on the place of dairy products within a dietary pattern emphasizing low intakes of red and processed meats and high intakes of minimally processed plant foods (4), it is important to underscore that we found no evidence of a higher risk of cardiovascular outcomes associated with the consumption of total and subtypes of dairy products. These findings are of particular relevance with regard to dairy products with higher fat content (i.e., total high-fat dairy or cheese), given the predominant content of saturated fatty acids in dairy fat (118). One plausible explanation of the lack of a deleterious association between high-fat dairy product consumption and risk of CVD-related outcomes is related to the effect of the dairy matrix. Indeed, it has been previously demonstrated that the dairy matrix attenuates the cholesterol-raising effects of saturated fat from dairy: consuming dairy fat from hard cheese leads to smaller increases in plasma cholesterol compared with consuming the same amount of dairy fat but from butter (8, 114, 119). Besides, not only did we find no evidence of potential cardiovascular harm associated with high-fat dairy consumption, but we also observed that total high-fat dairy consumption was associated with a lower risk of stroke. Potential mechanisms that could explain inverse associations between total high-fat dairy consumption and cardiovascular outcome risk are related to the bioactive properties of dairy fat that have been previously described (120). For instance, milk-fat globule membrane polar lipids have been shown to reduce circulating lipid risk factors through potential reduced intestinal cholesterol absorption (16, 121). Such mechanisms could explain why the consumption of both high-fat and low-fat dairy products showed modest inverse associations with stroke risk. Still, no study has investigated the potential cardiovascular benefits associated with the substitution of low-fat for high-fat dairy products. In contrast, replacing high-fat with low-fat dairy products was associated with a slightly lower risk of type 2 diabetes in a pooled analysis of 3 large US cohorts (49). Similar analyses should be conducted on cardiovascular outcomes (HTN, CHD, stroke) to better delineate the place of low- and high-fat dairy in healthy dietary patterns and unravel the complex relation between dairy fat intake and cardiovascular outcomes. Beyond these aspects, the importance attributed to dairy products in healthy dietary patterns should also be delineated in full consideration of sustainability and planetary health considerations (122).
Our study has several strengths, including the comprehensive synthesis of all available prospective studies with large numbers of participants and cases of HTN, CHD, and stroke; broadly consistent findings using several analytic methods and across different subgroups; and assessment of dose–response associations. However, several limitations should be noted. First, the fact that this work is based on cohorts with diverse ethnic profiles, socioeconomic status, and dietary habits limited our ability to obtain homogeneous results. As is always the case in meta-analyses of nutritional epidemiology evidence, the quality of individual studies remains the cornerstone of meta-analyses (123). There are no randomized controlled trials with hard endpoints available on this topic, and such a trial is highly unlikely due to feasibility and cost constraints. Thus, the assessment of the association between dairy consumption and clinical outcomes needs to rely primarily on data from observational studies and will remain susceptible to residual or unmeasured confounding. In that regard, the known clustering of dairy intake with healthy lifestyles and diets may explain some of the discrepancies we observed between analyses on milk, yogurt, or cheese, as well as low-fat and high-fat dairy products (43). Moreover, most included studies were at moderate to high ROB, which suggests that the overall body of evidence on dairy consumption and cardiovascular health is susceptible to potential biases. The fact that the evidence was judged to vary between very low and moderate quality reflects the latter. More specific to the relation between dairy intake and stoke risk, the limitations associated with the fact that most studies reported on total stroke, rather than providing estimates for stroke subtypes, may also have caused heterogeneity in our analyses because the factors underpinning different stroke types may be differentially affected by nutritional components.
In summary, this systematic review and meta-analysis suggests that total dairy consumption is associated with a modestly lower risk of HTN, CHD, or stroke. Although results for total dairy consumption were consistent in high compared with low intakes and dose–response meta-analyses as well as across multiple participant and study characteristics, this was not the case for individual dairy foods. Therefore, results of the current study should be interpreted in the context that they rely on evidence of very low to moderate quality.
Supplementary Material
Acknowledgments
We thank Angela Eady and Rebecca Sedore for their contribution to the work.
The authors’ responsibilities were as follows—VH, VM, PABR, PZ, and J-PD-C: designed the research; ZC, MA, VH, KJ, VM, PABR, PZ, and JPDC: conducted the systemic review and data extraction; ZC and J-PD-C: performed the statistical analyses and drafted the manuscript; J-PD-C: had primary responsibility for final content; and all authors: made critical revisions to the manuscript for key intellectual content and read and approved the final version.
Notes
The authors reported no funding received for this study. Article processing charges were supported by Hypertension Canada. Hypertension Canada had no role in the study design, interpretation of the results, or manuscript writing.
Author disclosures: J-PD-C received speaker and consulting honoraria from the Dairy Farmers of Canada in 2016 and 2018 outside the submitted work. All other authors report no conflicts of interest.
Supplemental Figures 1–9 and Supplemental Tables 1–19 and are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CVD, cardiovascular disease; CHD, coronary heart disease; HTN, hypertension; NOS, Newcastle–Ottawa Scale; ROB, risk of bias.
Contributor Information
Zhangling Chen, Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Mavra Ahmed, Department of Nutritional Sciences, University of Toronto, Toronto, ON, Canada; Joannah and Brian Lawson Centre for Child Nutrition, University of Toronto, Toronto, ON, Canada.
Vanessa Ha, School of Medicine, Faculty of Health Sciences, Queen's University, Kingston, ON, Canada.
Katherine Jefferson, Faculty of Health Sciences, Ontario Tech University, Oshawa, ON, Canada.
Vasanti Malik, Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Department of Nutritional Sciences, University of Toronto, Toronto, ON, Canada.
Paula A B Ribeiro, Montreal Behavioural Medicine Centre, CIUSSS du Nord-de-l’Île-de-Montréal, Montréal, QC, Canada; Centre de recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM), Montréal, QC, Canada.
Priccila Zuchinali, Centre de recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM), Montréal, QC, Canada.
Jean-Philippe Drouin-Chartier, Centre Nutrition, santé et société (NUTRISS), Institut sur la Nutrition et les Aliments Fonctionnels (INAF), Université Laval, Quebec city, QC, Canada; Faculté de pharmacie, Université Laval, Quebec city, QC, Canada.
Data Availability
Data described in the manuscript, code book, and analytic code will not be made publicly available.
References
- 1. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JWet al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet North Am Ed. 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2020;120(12):1998–2031.e15. [DOI] [PubMed] [Google Scholar]
- 4. Willett WC, Ludwig DS. Milk and health. N Engl J Med. 2020;382(7):644–54. [DOI] [PubMed] [Google Scholar]
- 5. Astrup A. Milk and health. N Engl J Med. 2020;382(23):e86. [DOI] [PubMed] [Google Scholar]
- 6. Givens DI. Milk and health. N Engl J Med. 2020;382(23):e86. [DOI] [PubMed] [Google Scholar]
- 7. Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30(1):365–401. [DOI] [PubMed] [Google Scholar]
- 8. de Goede J, Geleijnse JM, Ding EL, Soedamah-Muthu SS. Effect of cheese consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73(5):259–75. [DOI] [PubMed] [Google Scholar]
- 9. Santaren ID, Watkins SM, Liese AD, Wagenknecht LE, Rewers MJ, Haffner SM, Lorenzo C, Hanley AJ. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr. 2014;100(6):1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Grantham N, Ebeling PR, Daly RM. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care. 2011;34(5):1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez MA, Panahi S, Daniel N, Tremblay A, Marette A. Yogurt and cardiometabolic diseases: a critical review of potential mechanisms. Adv Nutr. 2017;8(6):812–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr. 2015;35:517–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JWet al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tremblay BL, Rudkowska I. Nutrigenomic point of view on effects and mechanisms of action of ruminant trans fatty acids on insulin resistance and type 2 diabetes. Nutr Rev. 2017;75(3):214–23. [DOI] [PubMed] [Google Scholar]
- 15. Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Wong Ket al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15(10):e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vors C, Joumard-Cubizolles L, Lecomte M, Combe E, Ouchchane L, Drai J, Raynal K, Joffre F, Meiller L, Le Barz Met al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Gut. 2020;69(3):487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DGet al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 18. Drouin-Chartier JP, Tremblay AJ, Maltais-Giguere J, Charest A, Guinot L, Rioux LE, Labrie S, Britten M, Lamarche B, Turgeon SLet al. Differential impact of the cheese matrix on the postprandial lipid response: a randomized, crossover, controlled trial. Am J Clin Nutr. 2017;106(6):1358–65. [DOI] [PubMed] [Google Scholar]
- 19. Schmid D, Song M, Zhang X, Willett WC, Vaidya R, Giovannucci EL, Michels KB. Yogurt consumption in relation to mortality from cardiovascular disease, cancer, and all causes: a prospective investigation in 2 cohorts of US women and men. Am J Clin Nutr. 2020;111(3):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté M-È, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–7. [DOI] [PubMed] [Google Scholar]
- 22. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017.32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45(10):925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soedamah-Muthu SS, de Goede J. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analyses of prospective cohort studies. Curr Nutr Rep. 2018;7(4):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens. 2012;26(1):3–13. [DOI] [PubMed] [Google Scholar]
- 26. Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, Irvin SR, Miller PE, Watson H, Fryzek JP. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr. 2016;115(4):737–50. [DOI] [PubMed] [Google Scholar]
- 27. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buendia JR, Li Y, Hu FB, Cabral HJ, Bradlee ML, Quatromoni PA, Singer MR, Curhan GC, Moore LL. Long-term yogurt consumption and risk of incident hypertension in adults. J Hypertens. 2018;36(8):1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51(4):1073–9. [DOI] [PubMed] [Google Scholar]
- 30. Babio N, Becerra-Tomas N, Martinez-Gonzalez MA, Corella D, Estruch R, Ros E, Sayon-Orea C, Fito M, Serra-Majem L, Aros Fet al. Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J Nutr. 2015;145(10):2308–16. [DOI] [PubMed] [Google Scholar]
- 31. Beydoun MA, Fanelli-Kuczmarski MT, Beydoun HA, Dore GA, Canas JA, Evans MK, Zonderman AB. Dairy product consumption and its association with metabolic disturbance in a prospective study of urban adults. Br J Nutr. 2018;119(6):706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Villaverde P, Lajous M, MacDonald CJ, Fagherazzi G, Boutron-Ruault MC, Bonnet F. Dairy product consumption and hypertension risk in a prospective French cohort of women. Nutr J. 2020;19(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Satija A, Stampfer MJ, Rimm EB, Willett W, Hu FB. Perspective: are large, simple trials the solution for nutrition research?. Adv Nutr. 2018;9(4):378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwingshackl L, Knuppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal Ket al. Perspective: Nutrigrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tobias DK, Wittenbecher C, Hu FB. Grading nutrition evidence: where to go from here?. Am J Clin Nutr. 2021;113(6):1385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeraatkar D, Bhasin A, Morassut RE, Churchill I, Gupta A, Lawson DO, Miroshnychenko A, Sirotich E, Aryal K, Mikhail Det al. Characteristics and quality of systematic reviews and meta-analyses of observational nutritional epidemiology: a cross-sectional study. Am J Clin Nutr. 2021;113(6):1578–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qian F, Riddle MC, Wylie-Rosett J, Hu FB. Red and processed meats and health risks: how strong is the evidence?. Diabetes Care. 2020;43(2):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwingshackl L, Schlesinger S, Devleesschauwer B, Hoffmann G, Bechthold A, Schwedhelm C, Iqbal K, Knüppel S, Boeing H. Generating the evidence for risk reduction: a contribution to the future of food-based dietary guidelines. Proc Nutr Soc. 2018;77:432–44. [DOI] [PubMed] [Google Scholar]
- 39. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. [Internet]. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/PROGRAMS/CLINICAL_EPIDEMIOLOGY/OXFORD.ASP [Accessed May 1, 2021]
- 41. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 42. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S.; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 43. Panahi S, Fernandez MA, Marette A, Tremblay A. Yogurt, diet quality and lifestyle factors. Eur J Clin Nutr. 2017;71(5):573–9. [DOI] [PubMed] [Google Scholar]
- 44. Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead?. BMJ. 1998;316(7136):989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stare J, Maucort-Boulch D. Odds ratio, hazard ratio and relative risk. Metodoloski zvezki. 2016;13:59–67. [Google Scholar]
- 46. Schwingshackl L, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drouin-Chartier JP, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks F, Rosner B, Willett WC, Hu FB, Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ. 2020;368:m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR Jr, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr. 2010;92(4):954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drouin-Chartier JP, Li Y, Ardisson Korat AV, Ding M, Lamarche B, Manson JE, Rimm EB, Willett WC, Hu FB. Changes in dairy product consumption and risk of type 2 diabetes: results from 3 large prospective cohorts of US men and women. Am J Clin Nutr. 2019;110(5):1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bernstein AM, Pan A, Rexrode KM, Stampfer M, Hu FB, Mozaffarian D, Willett WC. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;43(3):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldbohm RA, Chorus AM, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. 2011;93(3):615–27. [DOI] [PubMed] [Google Scholar]
- 52. Johansson I, Esberg A, Nilsson LM, Jansson JH, Wennberg P, Winkvist A. Dairy product intake and cardiometabolic diseases in northern Sweden: a 33-year prospective cohort study. Nutrients. 2019;11(2):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Key TJ, Appleby PN, Bradbury KE, Sweeting M, Wood A, Johansson I, Kuhn T, Steur M, Weiderpass E, Wennberg Met al. Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease a prospective study of 7198 incident cases among 409 885 participants in the pan-European EPIC cohort. Circulation. 2019;139(25):2835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kinjo Y, Beral V, Akiba S, Key T, Mizuno S, Appleby P, Yamaguchi N, Watanabe S, Doll R. Possible protective effect of milk, meat and fish for cerebrovascular disease mortality in Japan. J Epidemiol. 1999;9(4):268–74. [DOI] [PubMed] [Google Scholar]
- 55. Larsson SC, Virtamo J, Wolk A. Dairy consumption and risk of stroke in Swedish women and men. Stroke. 2012;43(7):1775–80. [DOI] [PubMed] [Google Scholar]
- 56. Kondo I, Ojima T, Nakamura M, Hayasaka S, Hozawa A, Saitoh S, Ohnishi H, Akasaka H, Hayakawa T, Murakami Yet al. Consumption of dairy products and death from cardiovascular disease in the Japanese general population: the NIPPON DATA80. J Epidemiol. 2013;23(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Orsini N. From floated to conventional confidence intervals for the relative risks based on published dose-response data. Comput Methods Programs Biomed. 2010;98(1):90–3. [DOI] [PubMed] [Google Scholar]
- 58. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 59. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons; 2011. [Google Scholar]
- 60. Umesawa M, Kitamura A, Kiyama M, Okada T, Shimizu Y, Imano H, Ohira T, Nakamura M, Maruyama K, Iso H. Association between dietary behavior and risk of hypertension among Japanese male workers. Hypertens Res. 2013;36(4):374–80. [DOI] [PubMed] [Google Scholar]
- 61. Mirmiran P, Golzarand M, Bahadoran Z, Ataee M, Azizi F.. Paradoxical association of dairy intake between men and women with the incidence of hypertension: a three-year follow up in Tehran Lipid and Glucose Study. Nutr Diet. 2016;73(2):153–61. [Google Scholar]
- 62. Talaei M, Pan A, Yuan JM, Koh WP. Dairy food intake is inversely associated with risk of hypertension: the Singapore Chinese Health Study. J Nutr. 2017;147(2):235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR Jr. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr. 2005;82(6):1169–77.; quiz 363–4. [DOI] [PubMed] [Google Scholar]
- 64. Wang H, Fox CS, Troy LM, McKeown NM, Jacques PF. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: the Framingham Heart Study. Br J Nutr. 2015;114(11):1887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alonso A, Beunza JJ, Delgado-Rodriguez M, Martinez JA, Martinez-Gonzalez MA. Low-fat dairy consumption and reduced risk of hypertension: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2005;82(5):972–9. [DOI] [PubMed] [Google Scholar]
- 66. Engberink MF, Geleijnse JM, de Jong N, Smit HA, Kok FJ, Verschuren WM. Dairy intake, blood pressure, and incident hypertension in a general Dutch population. J Nutr. 2009;139(3):582–7. [DOI] [PubMed] [Google Scholar]
- 67. Engberink MF, Hendriksen MA, Schouten EG, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Inverse association between dairy intake and hypertension: the Rotterdam Study. Am J Clin Nutr. 2009;89(6):1877–83. [DOI] [PubMed] [Google Scholar]
- 68. Shin H, Yoon YS, Lee Y, Kim CI, Oh SW. Dairy product intake is inversely associated with metabolic syndrome in Korean adults: Anseong and Ansan cohort of the Korean Genome and Epidemiology Study. J Korean Med Sci. 2013;28(10):1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johansson I, Nilsson LM, Esberg A, Jansson JH, Winkvist A. Dairy intake revisited—associations between dairy intake and lifestyle related cardio-metabolic risk factors in a high milk consuming population. Nutr J. 2018;17(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim D, Kim J. Dairy consumption is associated with a lower incidence of the metabolic syndrome in middle-aged and older Korean adults: the Korean Genome and Epidemiology Study (KoGES). Br J Nutr. 2017;117(1):148–60. [DOI] [PubMed] [Google Scholar]
- 71. Bhavadharini B, Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, Gupta R, Lear S, Wentzel-Viljoen Eet al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Res Care. 2020;8(1):e000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Louie JC, Flood VM, Burlutsky G, Rangan AM, Gill TP, Mitchell P. Dairy consumption and the risk of 15-year cardiovascular disease mortality in a cohort of older Australians. Nutrients. 2013;5(2):441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ness AR, Smith GD, Hart C. Milk, coronary heart disease and mortality. J Epidemiol Community Health. 2001;55(6):379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Patterson E, Larsson SC, Wolk A, Akesson A. Association between dairy food consumption and risk of myocardial infarction in women differs by type of dairy food. J Nutr. 2013;143(1):74–9. [DOI] [PubMed] [Google Scholar]
- 75. Praagman J, Franco OH, Ikram MA, Soedamah-Muthu SS, Engberink MF, van Rooij FJ, Hofman A, Geleijnse JM. Dairy products and the risk of stroke and coronary heart disease: the Rotterdam Study. Eur J Nutr. 2015;54(6):981–90. [DOI] [PubMed] [Google Scholar]
- 76. Praagman J, Dalmeijer GW, van der Schouw YT, Soedamah-Muthu SS, Monique Verschuren WM, Bas Bueno-de-Mesquita H, Geleijnse JM, Beulens JW. The relationship between fermented food intake and mortality risk in the European Prospective Investigation into Cancer and Nutrition–Netherlands cohort. Br J Nutr. 2015;113(3):498–506. [DOI] [PubMed] [Google Scholar]
- 77. Soedamah-Muthu SS, Masset G, Verberne L, Geleijnse JM, Brunner EJ. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br J Nutr. 2013;109(4):718–26. [DOI] [PubMed] [Google Scholar]
- 78. Talaei M, Koh WP, Yuan JM, Pan A. The association between dairy product intake and cardiovascular disease mortality in Chinese adults. Eur J Nutr. 2017;56(7):2343–52. [DOI] [PubMed] [Google Scholar]
- 79. Warensjo E, Nolan D, Tapsell L. Dairy food consumption and obesity-related chronic disease. Adv Food Nutr Res. 2010;59:1–41. [DOI] [PubMed] [Google Scholar]
- 80. Avalos EE, Barrett-Connor E, Kritz-Silverstein D, Wingard DL, Bergstrom JN, Al-Delaimy WK. Is dairy product consumption associated with the incidence of CHD?. Public Health Nutr. 2013;16(11):2055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Elwood PC, Strain JJ, Robson PJ, Fehily AM, Hughes J, Pickering J, Ness A. Milk consumption, stroke, and heart attack risk: evidence from the Caerphilly cohort of older men. J Epidemiol Community Health. 2005;59(6):502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Farvid MS, Malekshah AF, Pourshams A, Poustchi H, Sepanlou SG, Sharafkhah M, Khoshnia M, Farvid M, Abnet CC, Kamangar Fet al. Dietary protein sources and all-cause and cause-specific mortality: the Golestan Cohort Study in Iran. Am J Prev Med. 2017;52(2):237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dalmeijer GW, Struijk EA, van der Schouw YT, Soedamah-Muthu SS, Verschuren WM, Boer JM, Geleijnse JM, Beulens JW. Dairy intake and coronary heart disease or stroke—a population-based cohort study. Int J Cardiol. 2013;167(3):925–9. [DOI] [PubMed] [Google Scholar]
- 84. Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, Gupta R, Lear S, Wentzel-Viljoen E, Avezum Aet al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet North Am Ed. 2018;392(10161):2288–97. [DOI] [PubMed] [Google Scholar]
- 85. Haring B, Gronroos N, Nettleton JA, von Ballmoos MC, Selvin E, Alonso A. Dietary protein intake and coronary heart disease in a large community based cohort: results from the Atherosclerosis Risk in Communities (ARIC) study [corrected]. PLoS One. 2014;9(10):e109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Koskinen TT, Virtanen HEK, Voutilainen S, Tuomainen TP, Mursu J, Virtanen JK. Intake of fermented and non-fermented dairy products and risk of incident CHD: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2018;120(11):1288–97. [DOI] [PubMed] [Google Scholar]
- 87. Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149(2):151–61. [DOI] [PubMed] [Google Scholar]
- 88. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bergholdt HK, Nordestgaard BG, Varbo A, Ellervik C. Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98 529 Danish adults. Int J Epidemiol. 2015;44(2):587–603. [DOI] [PubMed] [Google Scholar]
- 90. Talaei M, Hosseini N, van Dam RM, Sadeghi M, Oveisgharan S, Dianatkhah M, Sarrafzadegan N. Whole milk consumption and risk of cardiovascular disease and mortality: Isfahan Cohort Study. Eur J Nutr. 2019;58(1):163–71. [DOI] [PubMed] [Google Scholar]
- 91. Wang XY, Liu FC, Yang XL, Li JX, Cao J, Lu XF, Huang JF, Li Y, Chen JC, Zhao LCet al. Association of cardiovascular diseases with milk intake among general Chinese adults. Chin Med J. 2020;133(10):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sauvaget C, Nagano J, Allen N, Grant EJ, Beral V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol. 2003;32(4):536–43. [DOI] [PubMed] [Google Scholar]
- 93. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Dairy foods and risk of stroke. Epidemiology. 2009;20(3):355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lin PH, Yeh WT, Svetkey LP, Chuang SY, Chang YC, Wang C, Pan WH. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac J Clin Nutr. 2013;22(2):482–91. [PubMed] [Google Scholar]
- 95. Sonestedt E, Wirfalt E, Wallstrom P, Gullberg B, Orho-Melander M, Hedblad B. Dairy products and its association with incidence of cardiovascular disease: the Malmo Diet and Cancer Cohort. Eur J Epidemiol. 2011;26(8):609–18. [DOI] [PubMed] [Google Scholar]
- 96. von Ruesten A, Feller S, Bergmann MM, Boeing H.. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67(4):412–9. [DOI] [PubMed] [Google Scholar]
- 97. Bonthuis M, Hughes MC, Ibiebele TI, Green AC, van der Pols JC. Dairy consumption and patterns of mortality of Australian adults. Eur J Clin Nutr. 2010;64(6):569–77. [DOI] [PubMed] [Google Scholar]
- 98. Buziau AM, Soedamah-Muthu SS, Geleijnse JM, Mishra GD. Total fermented dairy food intake is inversely associated with cardiovascular disease risk in women. J Nutr. 2019;149(10):1797–804. [DOI] [PubMed] [Google Scholar]
- 99. Wang XJ, Jiang CQ, Zhang WS, Zhu F, Jin YL, Woo J, Cheng KK, Lam TH, Xu L. Milk consumption and risk of mortality from all-cause, cardiovascular disease and cancer in older people. Clin Nutr. 2020;39(11):3442–51. [DOI] [PubMed] [Google Scholar]
- 100. Ding M, Li J, Qi L, Ellervik C, Zhang XH, Manson JE, Stampfer M, Chavarro JE, Rexrode KM, Kraft Pet al. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ. 2019;367:l6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schmid D, Song MY, Zhang XH, Willett WC, Vaidya R, Giovannucci EL, Michels KB. Yogurt consumption in relation to mortality from cardiovascular disease, cancer, and all causes: a prospective investigation in 2 cohorts of US women and men. Am J Clin Nutr. 2020;111(3):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee M, Lee H, Kim J. Dairy food consumption is associated with a lower risk of the metabolic syndrome and its components: a systematic review and meta-analysis. Br J Nutr. 2018;120(4):373–84. [DOI] [PubMed] [Google Scholar]
- 104. Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93(1):158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qin LQ, Xu JY, Han SF, Zhang ZL, Zhao YY, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr. 2015;24(1):90–100. [DOI] [PubMed] [Google Scholar]
- 106. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fontecha J, Calvo MV, Juarez M, Gil A, Martinez-Vizcaino V. Milk and dairy product consumption and cardiovascular diseases: an overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(Suppl 2):S164–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hu D, Huang J, Wang Y, Zhang D, Qu Y. Dairy foods and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24(5):460–9. [DOI] [PubMed] [Google Scholar]
- 109. Alexander DD, Miller PE, Vargas AJ, Weed DL, Cohen SS. Meta-analysis of egg consumption and risk of coronary heart disease and stroke. J Am Coll Nutr. 2016;35(8):704–16. [DOI] [PubMed] [Google Scholar]
- 110. Fernandez MA, Marette A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr Rev. 2018;76(Suppl 1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vissers LET, Sluijs I, van der Schouw YT, Forouhi NG, Imamura F, Burgess S, Barricarte A, Boeing H, Bonet C, Chirlaque MDet al. Dairy product intake and risk of type 2 diabetes in EPIC-InterAct: a Mendelian randomization study. Diabetes Care. 2019;42(4):568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ. 2017;356:j1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rancourt-Bouchard M, Gigleux I, Guay V, Charest A, Saint-Gelais D, Vuillemard JC, Lamarche B, Couture P. Effects of regular-fat and low-fat dairy consumption on daytime ambulatory blood pressure and other cardiometabolic risk factors: a randomized controlled feeding trial. Am J Clin Nutr. 2020;111(1):42–51. [DOI] [PubMed] [Google Scholar]
- 114. Brassard D, Tessier-Grenier M, Allaire J, Rajendiran E, She Y, Ramprasath V, Gigleux I, Talbot D, Levy E, Tremblay Aet al. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: a randomized controlled trial. Am J Clin Nutr. 2017;105(4):800–9. [DOI] [PubMed] [Google Scholar]
- 115. Drouin-Chartier JP, Gagnon J, Labonte ME, Desroches S, Charest A, Grenier G, Dodin S, Lemieux S, Couture P, Lamarche B. Impact of milk consumption on cardiometabolic risk in postmenopausal women with abdominal obesity. Nutr J. 2015;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schmidt KA, Cromer G, Burhans MS, Kuzma JN, Hagman DK, Fernando I, Murray M, Utzschneider KM, Holte S, Kraft Jet al. Impact of low-fat and full-fat dairy foods on fasting lipid profile and blood pressure: exploratory endpoints of a randomized controlled trial. Am J Clin Nutr. 2021;114(3):882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schmidt KA, Cromer G, Burhans MS, Kuzma JN, Hagman DK, Fernando I, Murray M, Utzschneider KM, Holte S, Kraft Jet al. The impact of diets rich in low-fat or full-fat dairy on glucose tolerance and its determinants: a randomized controlled trial. Am J Clin Nutr. 2021;113(3):534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yu E, Hu FB. Dairy products, dairy fatty acids, and the prevention of cardiometabolic disease: a review of recent evidence. Curr Atheroscler Rep. 2018;20(5):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Drouin-Chartier JP, Tremblay A, Guinot L, Maltais-Giguère J, Rioux LE, Charest A, Labrie S, Britten M, Lamarche B, Turgeon SLet al. Differential impact of the cheese matrix on the postprandial lipid response: a randomized, crossover, controlled trial. Am J Clin Nutr. 2017;106(6):1358–65. [DOI] [PubMed] [Google Scholar]
- 120. Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122(2):369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Le Barz M, Vors C, Combe E, Joumard-Cubizolles L, Lecomte M, Joffre F, Trauchessec M, Pesenti S, Loizon E, Breyton AEet al. Milk polar lipids favorably alter circulating and intestinal ceramide and sphingomyelin species in postmenopausal women. JCI Insight. 2021;6(10):e145161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Willett WC, Hu FB, Rimm EB, Stampfer MJ. Building better guidelines for healthy and sustainable diets. Am J Clin Nutr. 2021;114(2):401–4. [DOI] [PubMed] [Google Scholar]
- 123. Barnard ND, Willett WC, Ding EL. The misuse of meta-analysis in nutrition research. JAMA. 2017;318(15):1435–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made publicly available.