Summary
Background
Severe COVID-19 T-cell lymphopenia is more common among older adults and entails poor prognosis. Offsetting the decline in T-cell count during COVID-19 demands fast and massive T-cell clonal expansion, which is telomere length (TL)-dependent.
Methods
We developed a model of TL-dependent T-cell clonal expansion capacity with age and virtually examined the relation of T-cell clonal expansion with COVID-19 mortality in the general population.
Findings
The model shows that an individual with average hematopoietic cell TL (HCTL) at age twenty years maintains maximal T-cell clonal expansion capacity until the 6th decade of life when this capacity rapidly declines by more than 90% over the next ten years. The collapse in the T-cell clonal expansion capacity coincides with the steep increase in COVID-19 mortality with age.
Interpretation
Short HCTL might increase vulnerability of many older adults, and some younger individuals with inherently short HCTL, to COVID-19 T-cell lymphopenia and severe disease.
Funding
A full list of funding bodies that contributed to this study can be found in the Acknowledgements section.
Keywords: Telomeres, T-cells, COVID-19, SARS-CoV-2, Ageing, Vaccines
Research in context.
Evidence before this study
We used the terms “telomere”, “COVID-19”, “SARS-CoV-2” for a search in PubMed and retrieved 37 papers. None of the papers examined nor considered the role of hematopoietic cell telomere length (HCTL) dynamics and T-cell clonal expansion in the pathogenesis of COVID-19.
Added value of this study
We integrate age-dependent HCTL shortening with T-cell clonal expansion into a model that links HCTL with T-cell lymphopenia and severe COVID-19 in older adults and individuals with inherently short telomeres.
Implications of all the available evidence
Our model suggests that short HCTL contributes to the development of COVID-19 T-cell lymphopenia— a potential mechanism linking telomere biology with COVID-19.
Alt-text: Unlabelled box
Introduction
COVID-19 is confronting health-care workers with mortality from ageing-related diseases.1,2 Short telomere length (TL) in hematopoietic cells contributes to mortality from these diseases3 and it might play a role in severe COVID-19.4, 5, 6, 7 A study of adults whose hematopoietic cell TL (HCTL) had been measured several years before the COVID-19 pandemic observed that among individuals who went on to contract COVID-19, those with short HCTL were more likely to have severe disease.7 This finding is consistent with a causal role of HCTL in some ageing-related diseases.8,9 Although the mechanisms that link short HCTL with severe COVID-19 are partially understood, COVID-19 lymphopenia is a potential explanation.10, 11, 12
Transient lymphopenia is a common feature of acute viral respiratory infections.13 The drastic and prolonged lymphopenia of COVID-19, however, is distinctive and largely stems from falling counts of T cells.14, 15, 16 Regardless of the still poorly understood primary causes of this T-cell lymphopenia, the decline in T-cell count in COVID-19 demands fast and massive T-cell clonal expansion, which is TL-dependent.17,18 As HCTL shortens with age,19 T-cells from many older adults might lack the TL-dependent clonal expansion capacity required to offset the development of COVID-19 lymphopenia. These individuals, and some younger adults with inherently short HCTL, might be at a higher risk of developing COVID-19 T-cell lymphopenia and severe disease.
In absence of an acute infection, TL might exert a minimal effect on the slow T- cell turnover because the biological half-lives of circulating naïve T cells and recently formed memory T cells are ∼ 5 years and ∼ 5 months, respectively.20 In the face of SARS-CoV-2 infection, however, diminished TL-dependent T-cell proliferative capacity in older adults could result in a T cell shortfall.10, 11, 12 Moreover, the clearance of SARS-CoV-2 requires clonal expansion and differentiation of naïve T cells into SARS-CoV-2 antigen-specific effector/memory (henceforth memory) T cells.15,16 Short naïve T-cell telomeres might thus limit adaptive immunity against the virus even without infection-mediated T-cell lymphopenia. We therefore modelled TL-dependent T-cell clonal expansion capacity with age and virtually examined its relation to COVID-19 mortality in the general population.
Methods
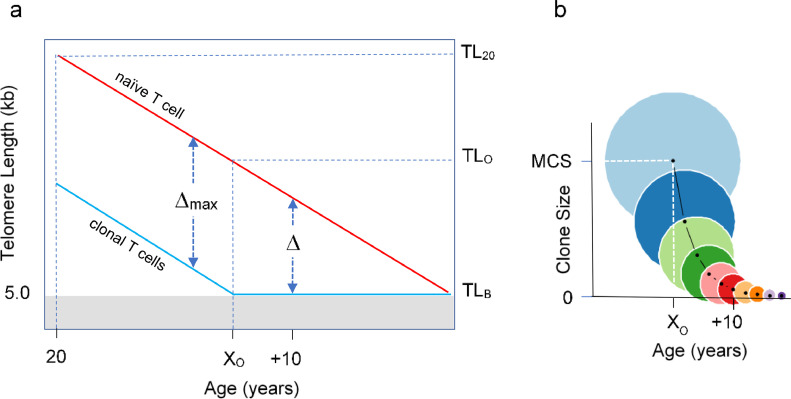
The following assumptions on T-cell replication, TL, and T-cell clone size (CS) drive our model (Figure 1): (i) T-cell TL-dependent cessation of replication is defined by a “telomeric brink” (TLB) that stops replication at 5 kb.19 (ii) TL of a naïve T cell at age 20 years (TL20) progressively shortens at a rate of 0.03 kb/year21,22 until it reaches the TLB. (iii) A single naïve T cell can clonally expand and through 20 successive replications it generates a maximal CS (MCS) of 220 (∼ one million) memory T cells; this value was estimated based on the ∼ 1.4 kb TL difference between circulating naïve and memory T cells and the ∼ 0.07 kb telomere shortening per replication of T cells.23 (iv) Most memory T cells are formed in response to new antigens during childhood and early adulthood,24 when HCTL is comparatively long,25, 26, 27 enabling the achievement of MCS. (v) Due to age-dependent TL shortening, a naïve T-cell TL reaches the “telomeric onset” (TLO = 6.4 kb) at “age of onset” (XO). Until XO, a naïve T cell can generate MCS. After XO, a naïve T cell can generate only a limited clonal size (LCS < MCS), as the TL of the clonal cells converges to the TLB.
Figure 1.
Age-dependent T-cell telomere length (TL) and its relation to T-cell clonal expansion. (a) displays age-dependent TL before ( ) and after (
) and after ( ) clonal expansion. Naïve T-cell clonal expansion shortens telomeres by Δ, where Δmax is T-cell telomere shortening resulting from expansion to form the maximal clonal size (MCS). The telomeric brink (TLB) of 5 kb is TL that increases the risk of cessation of replication. TL20 is TL at 20 years, TLO is telomeric onset, which indicates the shortest T-cell TL that enables attaining MCS. XO is age of onset of clonal expansion limitation. (b) displays T-cell clonal expansion size vs age from XO. Circle areas depict relative clonal size at and after XO. Light blue circle is MCS.
) clonal expansion. Naïve T-cell clonal expansion shortens telomeres by Δ, where Δmax is T-cell telomere shortening resulting from expansion to form the maximal clonal size (MCS). The telomeric brink (TLB) of 5 kb is TL that increases the risk of cessation of replication. TL20 is TL at 20 years, TLO is telomeric onset, which indicates the shortest T-cell TL that enables attaining MCS. XO is age of onset of clonal expansion limitation. (b) displays T-cell clonal expansion size vs age from XO. Circle areas depict relative clonal size at and after XO. Light blue circle is MCS.
Table 1 displays the parameters used to construct the model and examine its ramifications. We denote the maximal TL shortening due to clonal expansion as Δmax and the TL shortening per replication as r. We define CS by the number (N) of T-cell replications producing . As a clone expands, telomeres of its T cells progressively shorten, i.e., , where r is the telomere shortening due to T-cell replication. Prior to XO, the maximum number of T-cell replications in clonal expansion is . After XO, the number of T-cell replications in clonal expansion is , where X designates age and the corresponding age-dependent TL of a naïve T cell is , where g is the TL shortening in the naïve T cell each year. The resulting XO is the number of years it takes for a naïve T cell to reach TLO. These onset measures are defined:
| (1) |
Table 1.
Abbreviations, parameters, units, and values.
| TL20 | TL at age 20 years (kb) |
| TLX | TL at x years older than 20 years (kb) |
| TLB | Telomeric brink, stopping cell replication (5 kb) |
| TLO | TL at onset of clone size limitation (6.4 kb) |
| XO | Age in years when TL0 is reached |
| CS | Clone Size |
| MCS | Maximal Clone Size (∼ 106 T cells) |
| LCS | Limited Clone Size (< MCS) |
| Δmax | TL shortening required for achieving MCS (∼ 1.4 kb) |
| g | TL shortening rate with age (∼ 0.03 kb/year) |
| r | TL shortening rate per T-cell replication (∼ 0.07 kb) |
| N | Number of T-cell replications in LCS (after TLO, XO) |
| Nmax | Number of T-cell replications in MCS (before TLO, XO) |
The clone size depends on age relative to XO as follows:
| (2) |
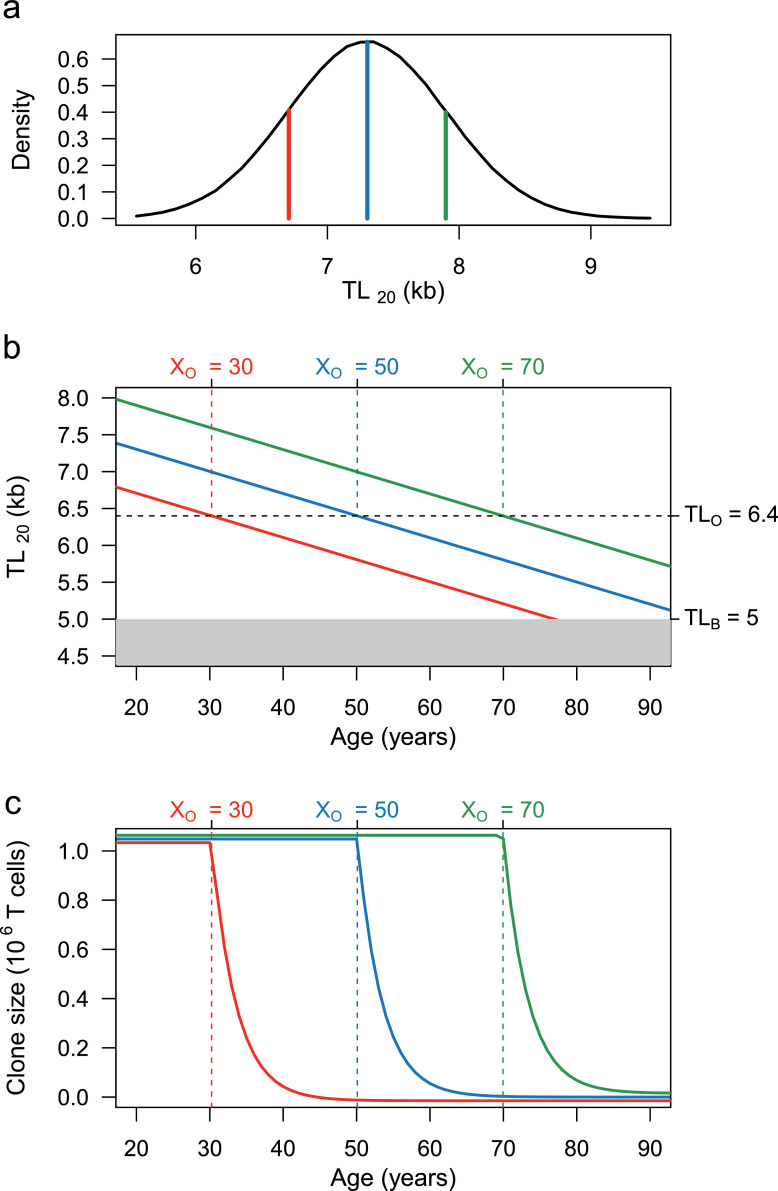
Age-specific density plots of HCTL18 were used to extrapolate the age-dependent T-cell TL and CS with age (Figure 2), which was then used to describe the relative proportion of CS in a population (Figure 3). In Figure 3a, the T-cell TL distribution for age 20 was derived from 1,000,000 random generations from a normal distribution with a mean = 7.3 kb and SD = 0.6 kb.
Figure 2.
Population distribution of T-cell TL at age 20 (TL20), T-cell TL shortening with age, and age-dependent change in T-cell clone size (CS). (a) displays the TL20 distribution, showing mean TL = 7.3 kb ( ), long TL (mean + SD) = 7.9 kb (
), long TL (mean + SD) = 7.9 kb ( ), and short TL (mean – SD) = 6.7 kb (
), and short TL (mean – SD) = 6.7 kb ( ). (b) displays age-dependent change in T-cell for mean, long and short TL20. Past the telomeric onset (TLO = 6.4 kb), TL is insufficient to produce MCS because a full clonal expansion drops TL below the telomeric brink (TLB = 5 kb). The TLO is reached at different ages of onset (XO), i.e., an older age for T-cells with long T-cell telomeres and younger with T-cells with short telomeres. The age-dependent T-cell TL shortening (0.03 kb/year) for T cells with mean, long, and short telomeres at TL20 is shown by the lines. (c) shows that the T-cell CS is partitioned by the XO into plateau and slope regions. T cells with mean, long, or short TL20 achieve MCS on the CS plateau, but their CS exponentially collapses (slope) once their TLs shorten below TLO and exceed XO (at different ages).
). (b) displays age-dependent change in T-cell for mean, long and short TL20. Past the telomeric onset (TLO = 6.4 kb), TL is insufficient to produce MCS because a full clonal expansion drops TL below the telomeric brink (TLB = 5 kb). The TLO is reached at different ages of onset (XO), i.e., an older age for T-cells with long T-cell telomeres and younger with T-cells with short telomeres. The age-dependent T-cell TL shortening (0.03 kb/year) for T cells with mean, long, and short telomeres at TL20 is shown by the lines. (c) shows that the T-cell CS is partitioned by the XO into plateau and slope regions. T cells with mean, long, or short TL20 achieve MCS on the CS plateau, but their CS exponentially collapses (slope) once their TLs shorten below TLO and exceed XO (at different ages).
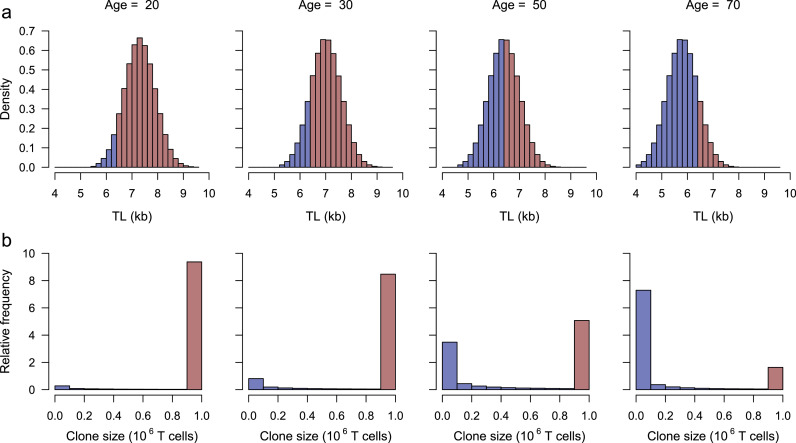
Figure 3.
Shifts by age in naïve T-cell TL distribution and relative frequency (0 to 10) of T-cell clone size (CS) in the population. (a) displays the shift in TL20 distribution (Figure 2a) resulting from age-dependent shortening of 0.03 kb/year. It depicts TL < TLO (6.4 kb) by blue bars ( ) and TL > TLO by red bars (
) and TL > TLO by red bars ( ). (b) displays relative frequency of CS generated by naïve T-cell clonal expansion corresponding to the categories of TL below or above TLO. It shows that maximal CS (MCS) of ∼ 106 cells occurs in individuals with naïve T-cell TL > TLO, while limited CS (LCS) occurs in those with naïve T-cell TL ≤ TLO. At age 20, naïve T cells of nine out of ten individuals can generate MCS. At age 70, naïve T cells of less than two out of ten individuals can generate MCS, and seven out of ten generate clone sizes that are less than 0.1 MCS. At age 50 the population is approximately equally divided between the MCS and LCS groups.
). (b) displays relative frequency of CS generated by naïve T-cell clonal expansion corresponding to the categories of TL below or above TLO. It shows that maximal CS (MCS) of ∼ 106 cells occurs in individuals with naïve T-cell TL > TLO, while limited CS (LCS) occurs in those with naïve T-cell TL ≤ TLO. At age 20, naïve T cells of nine out of ten individuals can generate MCS. At age 70, naïve T cells of less than two out of ten individuals can generate MCS, and seven out of ten generate clone sizes that are less than 0.1 MCS. At age 50 the population is approximately equally divided between the MCS and LCS groups.
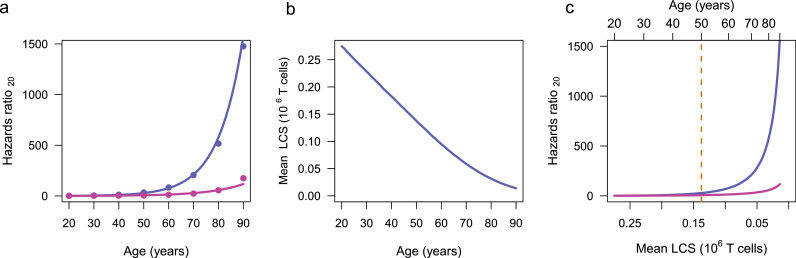
The link of the mean LCS to COVID-19 and general mortality in the population (Figure 4) was developed by calculating age-specific COVID-19-linked mortalities and total non-COVID-19 linked mortalities normalized by the age-specific US population. The hazards ratio, defined hazards ratio20 = (mortalityage/populationage) / (mortality20/population20), is based on the CDC records of 494,234 provisional COVID-19 deaths and 3,845,819 total deaths between January 1, 2020, through March 8, 202128 and the 2019 US Census.29 Non-COVID-19 mortalities were estimated by subtracting the COVID-19 mortalities from the total mortalities for each age group. The comparison of the COVID-19 and non-COVID-19 hazards ratios20 (Figure 4a) assumes that TL-dependent COVID-19 mortality only occurred for CS < MCS. Effect of other LCS cut-off levels, e.g., < 0.5 MCS or < 0.15 MCS on the relationship of LCS, hazards ratio20 and age is explored in Supplementary Information A1 and the uncertainty in the onset of clonal expansion limitation is explored in A2.
Figure 4.
Steps linking mean limited clone size (LCS) to COVID-19 mortality and general mortality hazards ratios20 in the population. (a) displays data based on COVID-19 mortality ( ) and non-COVID-19 mortality (
) and non-COVID-19 mortality ( ), and corresponding exponential fitted relationships for hazards ratios20 (
), and corresponding exponential fitted relationships for hazards ratios20 ( and
and  ). (b) displays the relationship of mean LCS in units of 106 cells with age, generated with Eq. 2, using the TL20 distribution of Figure 2a. (c) displays the relationships of hazards ratios20 generated from COVID-19 mortality and non-COVID-19 mortality plotted against mean LCS obtained from Figure 4b. The top of the panel also displays age. The divergence between the COVID-19 and non-COVID-19 mortalities occurs at mean LCS of ∼ 0.13×106 T cells. At the corresponding age, 50 years, the population is about evenly divided into the LCS and MCS groups (Figure 3b). After this age, increased proportion of the population is in the LCS group, which is susceptible to COVID-19 mortality, whereas the MCS group is not.
). (b) displays the relationship of mean LCS in units of 106 cells with age, generated with Eq. 2, using the TL20 distribution of Figure 2a. (c) displays the relationships of hazards ratios20 generated from COVID-19 mortality and non-COVID-19 mortality plotted against mean LCS obtained from Figure 4b. The top of the panel also displays age. The divergence between the COVID-19 and non-COVID-19 mortalities occurs at mean LCS of ∼ 0.13×106 T cells. At the corresponding age, 50 years, the population is about evenly divided into the LCS and MCS groups (Figure 3b). After this age, increased proportion of the population is in the LCS group, which is susceptible to COVID-19 mortality, whereas the MCS group is not.
The following considerations are relevant for appraising the model's parameters and assumptions: First, while the MCS is based on the in vivo TL difference between naïve and memory T cells, the data on telomere shortening per T-cell replication are from cultured cells.23 A similar approach (based on data from circulating hematopoietic cells and telomere shortening in cultured cells) was previously used to generate consistent information on hematopoietic cell replicative kinetics.30,31 Second, the model's TL population distribution is derived from a large population-based study19 that measured HCTL by Southern blotting.32 Its telomere data are consistent with another large-scale study that used Flow-FISH to measure HCTL.25 Third, the model is based on age-dependent shortening of HCTL and not T-cell TL. As TL differences among leukocyte lineages within the individual are far smaller than the inter-individual HCTL variation,33 T-cell TL largely mirrors HCTL. Fourth, the TL signal for cessation of cell replication originates from the shortest telomeres in the nucleus and not their mean TL.34,35 Using the Telomeres Shortest Length Assay (TeSLA), a method that tallies and measures the shortest telomeres,36 a recent study showed that in patients with COVID-19 the shortest telomeres in peripheral blood mononuclear cells were associated with low lymphocyte counts.12 The principles that drive our model thus likely apply to the T cell's shortest telomeres.
Ethics statement
The paper used data from publicly available databases and involved no animal or human experiments or studies.
Statistics statement
Analysis of model sensitivity and parameter uncertainty is provided in Supplementary Information A1.
Role of the funding source
The study sponsors had no role in study design, collection, analysis, and interpretation of the data. Funding sources had no contribution to writing the report or decisions on publication.
Results
The core conclusion of the model is as follows: Until reaching the age of onset, XO, age-dependent shortening of T-cell telomeres exert little influence on the ability of naïve T cells to generate the MCS of 220 memory T cells in response to antigen stimulation (Figure 1a). However, once XO is reached, the ability of T cells to expand clonally declines in an exponential manner, i.e., 220 → 219→ 218 → 217, etc. Since T-cell TL shortens at a pace of 0.03 kb/year and by 0.07 kb/replication, the T-cell clonal expansion capacity drops by half every 2.3 years past XO. Thus, in one decade after XO, the clonal expansion capacity of naive T cells is about 5% of the MCS (Figure 1b).
HCTL tracks with age, meaning that as they get older, individuals maintain their TL ranking at any given age.37 To examine the effect of inter-individual TL variation and age on T-cell clonal expansion, consider three individuals with average, long (one SD above the mean) and short (one SD below the mean) naïve T-cell TL20 (Figure 2a): The individual with average TL20 can attain the MCS of ∼106 cells up to age 50 years, i.e., the individual's XO (Figure 2b). Thereafter, while the individual's naïve T cells continue their slow age-related telomere shortening (Figure 2b), their clonal expansion capacity declines exponentially from the MCS (Figure 2c). Next consider the individual with long T-cell TL20 (Figure 2a). The ability of naive T-cells of this individual to achieve MCS extends XO to 70 years (Figure 2c). In contrast, naïve T cells of the individual with short T-cell TL20 are only able to achieve MCS to the XO of 30 years (Figure 2c). Our model thus showcases the high XO variability across the population.
The proportion of the population with T-cell TL > TLO slowly and continuously declines from 90% at age 20 to 10% at age 70 (Figure 3a). In contrast, after reaching XO, the T-cell clonal expansion capacity rapidly declines with age, and therefore the general population divides into two groups when the mean population XO is 50 years: a MCS group that can generate a full clonal expansion of naïve T cells and a LCS group that can generate only a fraction of the MCS (Figure 3b).
What then might be the minimal TL-dependent T-cell CS that enables survival of an individual contracting COVID-19? The definitive answer awaits telomere and T-cell data in populations of COVID-19 patients. That said, we infer this CS from a comparison of age-dependent hazards ratio of mortality relative to age 20 years (hazards ratio20) from COVID-19 and from general causes other than COVID-19. The hazards ratio20 increased exponentially with age for both COVID-19 mortality and non-COVID-19 mortality, but mortality from COVID-19 increased much faster than non-COVID-19 mortality (Figure 4a).
We assumed that, as a group, individuals who generate MCS experience no T-cell TL-related COVID-19 mortality and accordingly focused on the mean CS for the LCS group, i.e., individuals older than XO (Figure 3a). The TL-limited clonal expansion of these individuals, we assumed, might contribute to their propensity to die from COVID-19, given the association between T-cell lymphopenia and COVID-19 mortality. Figure 4b shows that the mean LCS in individuals older than XO decreases in a near linear manner with age. Plots of the mean LCS vs. hazards ratio20 of COVID-19 mortality and non-COVID-19 mortality suggest a divergence between the two trajectories during the 6th decade (Figure 4c). The figure also displays the mean LCS at the age of 50 years, when the size of LCS group is equivalent to that of the MCS group (Figure 3b). Mean LCS at this age amounts to ∼ 0.13 × 10−6 T cells.
Discussion
Apart from heritability,38 no other single factor so profoundly affects HCTL as does ageing, explaining the key conclusion of our model: Age-dependent telomere shortening might drain T cells of much needed clonal expansion capacity in the face of SARS-CoV-2 infection. As SARS-CoV-2 memory T cells play a greater role than neutralizing antibodies in recovering from the infection,15 the ageing effect on HCTL could impede adaptive immunity and heighten the risk for severe COVID-19. Moreover, we assume that the MCS applies not only for naïve T cells that clonally expand to produce memory T cells but also formation of new naïve T cells. This means that regardless of the primary cause of COVID-19 T-cell lymphopenia, the T cell response after XO will be compromised on two levels, i.e., formation of SARS-CoV-2-specific memory T-cells and replenishing the loss of naïve T-cells.
Mortality (yes/no) is a clear outcome of COVID-19. In contrast, ‘severe’ COVID-19 is an amorphous outcome that is categorized differently in various studies. Moreover, data of severity of COVID-19 are not uniformly accessible from public records as are mortality data. Therefore, we have elected to use mortality as an ‘endpoint’ in our model. The divergence of COVID-19 mortality from non-COVID-19 mortality when the mean LCS is about one tenth of MCS suggests the following: In the absence of COVID-19, ∼ 10% MCS is generally sufficient to accommodate the low turnover of T-cells.20 This LCS, however, might contribute to mortality in the face of SARS-CoV-2, because the infection demands massive T-cell clonal expansion to offset the primary cause of the dropping naïve T-cell count, and to generate memory T-cells that clear the virus.
As illustrated in Figs. 2 and 3, our model might also apply to naïve T cells of a subset of younger adults, whose HCTL is ranked at the lower range of the HCTL distribution in the general population.19,25 Comparatively short HCTL might also diminish naïve T-cell clonal expansion in response to SARS-CoV-2 infection in males, whose HCTL is shorter than in females from birth onwards,19,25,27,38,39 persons with atherosclerotic cardiovascular disease,39, 40, 41 obese persons42,43 and smokers43, 44, 45 whose HCTL is respectively shorter than that in healthy, lean and non-smoking individuals. These groups of individuals are at a higher risk of severe COVID-19 and death from the disease.46, 47, 48, 49 There are, of course, factors other than HCTL that contribute to the propensity of these groups to severe COVID-19. The XO/TLO concept provides, however, the framework for testing the role of HCTL in the pathogenesis of COVID-19 regardless of age and comorbidities.
Humans have comparatively short telomeres relative to their long lifespan,50,51 and therefore our model may not apply to most terrestrial mammals, including laboratory animal models that are used for viral research. For instance, TL-mediated replicative ageing is probably inconsequential during the 2, 3-year lifespan of mice, considering their long telomeres (mean TL > 30 kb) and robust activity in their somatic cells of telomerase, the reverse transcriptase that elongates telomeres. In contrast, the average human TL at birth is only ∼ 9.5 kb.27 As telomerase activity is repressed in replicating human somatic cells, their short telomeres experience further age-dependent shortening after birth. Although naïve T cells have some telomerase activity, it is insufficient to prevent their age-dependent telomere shortening, and ageing may thus undermine the T-cell clonal expansion in many older humans.
Relatedly, the model shows that individuals with naïve T-cells whose TL20 is one SD below the mean might be unable to achieve MCS as early as the third decade of life. This unexpected finding suggests that these individuals might (a) develop severe COVID-19 T-cell lymphopenia despite their young age, or (b) tap more naïve T cells for clonal expansion in response to exposure to pathogens.52 Older adults may not have sufficient naïve T cells, particularly, naïve CD8 T cells, for this purpose.53 Empirical data based on HCTL measurements in otherwise healthy adults who developed COVID-19 will help testing these alternatives.
We acknowledge limitations: The model draws on HCTL data from populations comprising principally whites of European ancestry in high-income countries. It should also be tested in populations of different ancestries and in low- and middle-income countries. That said, we anticipate that the principles of the model will hold, although the XO and TLO might shift up or down depending on specific populations and geographical regions. The TL difference between naïve T cells and memory T cells likely reflects the clonal expansion in response to not only a single encounter but multiple encounters with a given antigen and its cross-reactive antigens. Thus, the MCS and LCS definitions in absolute T-cell numbers might be off the mark. Of note, however, the MCS and LCS can be expressed in the model in relative units of MCS (i.e., 0.5 MCS, 0.25 MCS, etc.) rather than absolute units (numbers of T cells), yielding identical results. Therefore, the principles of our model are likely to hold notwithstanding the above limitations. Finally, our model focuses on telomere length dynamics in limiting T-cell clonal expansion through mechanisms that trigger replicative senescence. However, independent mechanisms might limit T-cell clonal expansion through pathways that lead to T-cell exhaustion. Typically, this exhaustion is associated with the upregulation of the programmed cell death (PD-1) pathway,54 but de novo DNA-methylation might also promote T cell exhaustion.55
In conclusion, while our model is clearly an oversimplification, it highlights an overlooked effect of ageing within the very complex system of T-cell regulation.53 The insight generated by our model might set the stage for measurement of TL parameters not only in older adults but also the general population, helping to identify, regardless of age, individuals vulnerable to severe COVID-19 because of short T-cell telomeres. These individuals might show, in addition, an early waning immunity after SARS-CoV-2 vaccination, facilitating the evolution of novel variants of the virus.56 Finally, the ramifications of these conclusions go beyond the influence of TL on T-cell response to SARS Cov-2 infection and vaccination against the virus. They suggest that TL might be a limiting factor in immunotherapies whose efficacies depend on clonally expanding (in vivo and in vitro) transplanted hematopoietic cells, chimeric antigen receptor T cells, and tumour-infiltrating lymphocytes.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
Contributors
Conceptualization: JJA, AA; Model development: JJA; Visualization: JJA, KGA, AIY; Supervision: AA; Writing original draft: JJA, AA; Writing review and editing: JJA, AA, ES, KGB, AIY, DL, SV. All had full access to all the data in the study and accept responsibility to submit for publication.
Acknowledgments
Funding provided by the following grants: NIH RF1AG046860 (AIY, KGA); NIH 5U24AG066528 (SV); 1R56AG073226-01, U01AG066529 Norwegian Research Council (NFR) ES562296 (AA).
Data sharing
All data are available in the main text or the supplementary materials.
Footnotes
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103978.
Appendix. Supplementary materials
Supplementary Information: Supplementary Information A contains Text, figures, and table. Supplementary Information B contain R programming language scripts for generating the model and Figs. Figure 2, Figure 3, Figure 4, S1, and S2.
References
- 1.Promislow D.E.L. A geroscience perspective on COVID-19 Mortality. J Gerontol A Biol Sci Med Sci. 2020;75(9):e30. doi: 10.1093/gerona/glaa094. -e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegelhalter D. Use of "normal" risk to improve understanding of dangers of covid-19. BMJ Clin Res Ed. 2020;370:m3259. doi: 10.1136/bmj.m3259. [DOI] [PubMed] [Google Scholar]
- 3.Arbeev K.G., Verhulst S., Steenstrup T., et al. Association of leukocyte telomere length with mortality among adult participants in 3 longitudinal studies. JAMA Netw Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2020.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froidure A., Mahieu M., Hoton D., et al. Short telomeres increase the risk of severe COVID-19. Aging. 2020;12(20):19911–19922. doi: 10.18632/aging.104097. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGroder C.F., Zhang D., Choudhury M.A., et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021 doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez-Vazquez R., Guío-Carrión A., Zapatero-Gaviria A., Martínez P., Blasco M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging. 2021;13(1):1–15. doi: 10.18632/aging.202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q., Codd V., Raisi-Estabragh Z., et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: a cohort study in UK Biobank. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aviv A., Shay J.W. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1741) doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codd V., Wang Q., Allara E., et al. Polygenic basis and biomedical consequences of telomere length variation. Nat Genet. 2021;53(10):1425–1433. doi: 10.1038/s41588-021-00944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aviv A. Telomeres and COVID-19. FASEB J. 2020;34(6):7247–7252. doi: 10.1096/fj.202001025. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aviv A. Short telomeres and severe COVID-19: the connection conundrum. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benetos A., Lai T.P., Toupance S., et al. The nexus between telomere length and lymphocyte count in seniors hospitalized With COVID-19. J Gerontol Ser A Biol Sci Med Sci. 2021;76(8):e97–e101. doi: 10.1093/gerona/glab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClain M.T., Park L.P., Nicholson B., et al. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J Clin Virol. 2013;58(4):689–695. doi: 10.1016/j.jcv.2013.09.015. the official publication of the Pan American Society for Clinical Virology. [DOI] [PubMed] [Google Scholar]
- 14.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodes R.J., Hathcock K.S., Weng N.P. Telomeres in T and B cells. Nat Rev Immunol. 2002;2(9):699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 18.Patrick M., Weng N.P. Expression and regulation of telomerase in human T cell differentiation, activation, aging and diseases. Cell Immunol. 2019;345 doi: 10.1016/j.cellimm.2019.103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steenstrup T., Kark J.D., Verhulst S., et al. Telomeres and the natural lifespan limit in humans. Aging. 2017;9(4):1130–1142. doi: 10.18632/aging.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrisekoop N., den Braber I., de Boer A.B., et al. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc Natl Acad Sci USA. 2008;105(16):6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nettle D., Gadalla S.M., Lai T.P., Susser E., Bateson M., Aviv A. Measurement of telomere length for longitudinal analysis: implications of assay precision. Am J Epidemiol. 2021;190(7):1406–1413. doi: 10.1093/aje/kwab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steenstrup T., Hjelmborg J.V., Kark J.D., Christensen K., Aviv A. The telomere lengthening conundrum–artifact or biology? Nucleic Acids Res. 2013;41(13):e131. doi: 10.1093/nar/gkt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng N.P., Hathcock K.S., Hodes R.J. Regulation of telomere length and telomerase in T and B cells: a mechanism for maintaining replicative potential. Immunity. 1998;9(2):151–157. doi: 10.1016/s1074-7613(00)80597-x. [DOI] [PubMed] [Google Scholar]
- 24.Farber D.L., Yudanin N.A., Restifo N.P. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14(1):24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aubert G., Baerlocher G.M., Vulto I., Poon S.S., Lansdorp P.M. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLos Genet. 2012;8(5) doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benetos A., Verhulst S., Labat C., et al. Telomere length tracking in children and their parents: implications for adult onset diseases. FASEB J: official publication of the Federation of American Societies for Experimental Biology. 2019;33(12):14248–14253. doi: 10.1096/fj.201901275R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Factor-Litvak P., Susser E., Kezios K., et al. Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics. 2016;137(4) doi: 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deaths involving coronavirus disease 2019 (COVID-19) reported to NCHS by sex and age group and week ending date [Internet]. 2021 [cited March 3, 2021]. Available from: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/.
- 29.2019: ACS 1-year estimates subject tables [Internet]. Survey/Program: American Community Survey TableID: S0101. 2019. Available from: https://www.census.gov/programs-surveys/acs/.
- 30.Shepherd B.E., Guttorp P., Lansdorp P.M., Abkowitz J.L. Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Exp Hematol. 2004;32(11):1040–1050. doi: 10.1016/j.exphem.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 31.Sidorov I., Kimura M., Yashin A., Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37(4):514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Kimura M., Stone R.C., Hunt S.C., et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5(9):1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 33.Kimura M., Gazitt Y., Cao X., Zhao X., Lansdorp P.M., Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38(10):854–859. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemann M.T., Strong M.A., Hao L.Y., Greider C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 35.Zou Y., Sfeir A., Gryaznov S.M., Shay J.W., Wright W.E. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15(8):3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai T.P., Zhang N., Noh J., et al. A method for measuring the distribution of the shortest telomeres in cells and tissues. Nat Commun. 2017;8(1):1356. doi: 10.1038/s41467-017-01291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benetos A., Kark J.D., Susser E., et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12(4):615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hjelmborg J.B., Dalgård C., Möller S., et al. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52(5):297–302. doi: 10.1136/jmedgenet-2014-102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benetos A., Toupance S., Gautier S., et al. Short leukocyte telomere length precedes clinical expression of atherosclerosis: the blood-and-muscle model. Circ Res. 2018;122(4):616–623. doi: 10.1161/CIRCRESAHA.117.311751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Mello M.J., Ross S.A., Briel M., Anand S.S., Gerstein H., Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8(1):82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 41.Haycock P.C., Heydon E.E., Kaptoge S., Butterworth A.S., Thompson A., Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ Clin Res Ed. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gielen M., Hageman G.J., Antoniou E.E., et al. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr. 2018;108(3):453–475. doi: 10.1093/ajcn/nqy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdes A.M., Andrew T., Gardner J.P., et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. (London, England) [DOI] [PubMed] [Google Scholar]
- 44.Bateson M., Aviv A., Bendix L., et al. Smoking does not accelerate leucocyte telomere attrition: a meta-analysis of 18 longitudinal cohorts. R Soc Open Sci. 2019;6(6) doi: 10.1098/rsos.190420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nawrot T.S., Staessen J.A., Gardner J.P., Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363(9408):507–510. doi: 10.1016/S0140-6736(04)15535-9. (London, England) [DOI] [PubMed] [Google Scholar]
- 46.Bae S., Kim S.R., Kim M.N., Shim W.J., Park S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107(5):373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 47.Nimgaonkar I., Valeri L., Susser E., Hussain S., Sunderram J., Aviv A. The age pattern of the male-to-female ratio in mortality from COVID-19 mirrors that of cardiovascular disease in the general population. Aging. 2021;13(3):3190–3201. doi: 10.18632/aging.202639. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popkin B.M., Du S., Green W.D., et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calado R.T., Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol. 2013;50(2):165–174. doi: 10.1053/j.seminhematol.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomes N.M., Ryder O.A., Houck M.L., et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10(5):761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer A., Zhang Y., Perelson A.S., Wingreen N.S. Regulation of T cell expansion by antigen presentation dynamics. Proc Natl Acad Sci USA. 2019;116(13):5914–5919. doi: 10.1073/pnas.1812800116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goronzy J.J., Weyand C.M. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573–583. doi: 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghoneim H.E., Fan Y., Moustaki A., et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell. 2017;170(1):142–157. doi: 10.1016/j.cell.2017.06.007. .e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saad-Roy C.M., Morris S.E., Metcalf C.J.E., et al. Epidemiological and evolutionary considerations of SARS-CoV-2 vaccine dosing regimes. Science. 2021;372(6540):363–370. doi: 10.1126/science.abg8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information: Supplementary Information A contains Text, figures, and table. Supplementary Information B contain R programming language scripts for generating the model and Figs. Figure 2, Figure 3, Figure 4, S1, and S2.