Highlights
-
•
Breast tissue composition differences were observed between benign and malignant disease states.
-
•
Immune cell infiltrates were more marked in the setting of breast cancer.
-
•
Microbial communities, by deep sequencing of breast tissue, varied with tissue composition.
-
•
Alpha and beta diversity of the tissue microbiome varied with percentage fat and fibrosis in tissue.
-
•
Observed effects were independent of patient BMI.
Keywords: Breast neoplasms, Microbiome, Tissue microenvironment, Fibrosis, Adipocytes, Bacteria
Abstract
Background
Stromal and immune cell composition alterations in benign breast tissue associate with future cancer risk. Pilot data suggest the innate microbiome of normal breast tissue differs between women with and without breast cancer. Microbiome alterations might explain tissue microenvironment variations associated with disease status.
Methods
Prospectively-collected sterile normal breast tissues from women with benign (n=16) or malignant (n=17) disease underwent 16SrRNA sequencing with Illumina MiSeq and Hybrid-denovo pipeline processing. Breast tissue was scored for fibrosis and fat percentages and immune cell infiltrates (lobulitis) classified as absent/mild/moderate/severe. Alpha and beta diversity were calculated on rarefied OTU data and associations analyzed with multiple linear regression and PERMANOVA.
Results
Breast tissue stromal fat% was lower and fibrosis% higher in benign disease versus cancer (median 30% versus 60%, p=0.01, 70% versus 30%, p=0.002, respectively). The microbiome varied with stromal composition. Alpha diversity (Chao1) correlated with fat% (r=0.38, p=0.02) and fibrosis% (r=-0.32, p=0.05) and associated with different microbial populations as indicated by beta diversity metrics (weighted UniFrac, p=0.08, fat%, p=0.07, fibrosis%). Permutation testing with FDR control revealed taxa differences for fat% in Firmicutes, Bacilli, Bacillales, Staphylococcaceae and genus Staphylococcus, and fibrosis% in Firmicutes, Spirochaetes, Bacilli, Bacillales, Spirochaetales, Proteobacteria RF32, Sphingomonadales, Staphylococcaceae, and genera Clostridium, Staphylococcus, Spirochaetes, Actinobacteria Adlercreutzia. Moderate/severe lobulitis was more common in cancer (73%) than benign disease (13%), p=0.003, but no significant microbial associations were seen.
Conclusion
These data suggest a link between breast tissue stromal alterations and its microbiome, further supporting a connection between the breast tissue microenvironment and breast cancer.
Introduction
Worldwide, breast cancer is both the most common cancer and leading cause of cancer death in women [1]. Most cases occur in the absence of a clear strong predisposing risk factor such as a deleterious germline mutation, prior radiation or atypical hyperplasia [2]. Thus identification of modifiable factors that might prevent breast cancer in women thought to be of average risk would have great societal value. The importance of the stool microbiome, its relationship to immunity and cancer, and its role as a therapeutic target to overcome resistance to immunotherapies to treat cancers, has been established and is an area of active investigation [3]. Much less is known about both the role of the microbiome in cancer prevention and the microbiome of other tissue niches. Prior studies have established the existence of a distinct breast tissue microbiome in samples collected under surgically aseptic conditions [4], [5], [6], [7]. However, a reproducible characterization of the breast tissue microbiome in health and disease remains to be established, as do the mechanistic links between this microbial niche and breast carcinogenesis.
The microenvironment of breast epithelial cells, or the tumor microenvironment when breast cancer is present, is composed of stroma (including matrix, adipocytes, fibroblasts and blood vessels) and immune cells. Stromal changes, as well as alterations in the immune cell composition of benign breast lobules, encompassing both innate and adaptive immune effectors, have been described in association with future cancer risk [8], [9], [10]. These changes may be manifest by changes in the radiographic and histologic appearance of breast tissue [11,12]. Mammographic breast density is a well-described risk factor for breast cancer. This risk extends beyond the masking effect hindering detection of cancers, supporting an underlying biologic mechanism inherent to the tissue composition [13,14].
Obesity is a similar well-described risk factor for post-menopausal breast cancer, yet somewhat paradoxically, is inversely associated with mammographic breast density. This suggests there may be differences in local versus systemic effects attributable to adipocytes in relation to breast carcinogenesis [15,16]. Dysregulated adipocyte production of adipokines such as adiponectin and leptin are observed in obese women and these biologically active compounds influence cell metabolism and proliferation. The secretion of adiponectin, which has anti-inflammatory, anti-proliferative and pro-apoptotic effects, generally is inversely correlated with BMI, whereas leptin, which has been shown to activate cell signaling pathways that promote cell migration, invasion and survival, correlates positively with BMI, yet epidemiologic studies cannot uncouple the effect of diet and other factors from adipocyte presence or function within specific tissues [17,18].
Perturbation of the microbiome influences cancer risk both locally and at distant sites; proposed mechanistic links include induction of inflammation, alteration of the tissue microenvironment, metabolic effects, and direct toxic effects, but much remains to be explored, especially beyond the gut microbiome [19], [20], [21], [22]. In tandem, pilot data suggests that the composition of the innate microbiome of sterilely obtained histologically normal breast tissues is different between women with and without breast cancer [4], [5], [6]. However, there is as yet no data linking breast tissue microbial communities to specific histologic features of breast tissue, particularly those associated with breast cancer risk. Thus we undertook this study to explore whether microbiome alterations might explain variation in the tissue microenvironment in women with and without breast cancer and provide clues to mechanistic hypotheses linking stromal and immune cell characteristics and breast cancer development. Our aim was to evaluate characteristics of the stroma and immune cell tissue microenvironment in association with the microbiome profile of sterilely obtained normal breast tissue adjacent to either a benign or malignant breast lesion.
Materials and Methods
With IRB approval, we prospectively collected surgically sterile adjacent normal breast tissue samples from women undergoing lumpectomy for benign or malignant breast disease. We studied 33 women, median age 60 years (range 33 to 84 years), 16 with benign disease and 17 with breast cancer, as previously characterized [4]. After intraoperative pathology confirmation of complete removal of the targeted lesion, we collected adjacent normal breast tissue under surgically aseptic conditions which was snap-frozen in liquid nitrogen in the operating room. From this group of patients, a subset of 27 women in whom diagnostic histopathology slides could be reviewed for estimated percentages of fibrosis and fat and for extent of immune cell infiltrates (absent/mild versus moderate/severe) in the adjacent normal breast lobules form the basis of this report. The latter was defined based on numbers of mononuclear cells per lobule as follows: none: <10 mononuclear cells per lobule, mild: 10-50 cells per lobule; moderate: 50-100 cells per lobule; marked: >100 cells per lobule. The clinical characteristics of these women are summarized in Supplemental Table 1. 16S rRNA sequencing was done using the Illumina MiSeq platform and processed with the Hybrid-denovo pipeline [23]. Average coverage was more than 135,000 reads per sample. Alpha diversity (Observed OTU numbers, Chao1 estimate, Shannon index and inverse Simpson index) and beta diversity measures (unweighted, generalized, weighted UniFrac and Bray-Curtis distance) were calculated on the rarefied OTU data, and associated with histologic phenotypes using multiple linear regression and PERMANOVA, respectively. The arcsine square root transformation has been applied to percentage fat/fibrosis before association testing. Differentially abundant taxa were identified using a linear model-based permutation test, where the taxa relative abundance data after square-root transformation were treated as the outcomes, and false discovery rate (FDR) control was used to correct for multiple testing [24]. Taxa with an FDR less than 20% were reported. Analyses were adjusted for both final diagnosis (benign versus malignant) and sequencing batch. As the benign and malignant groups had distinct age distributions, adjusting for the final diagnosis also controlled for the effect of age, thus we did not additionally adjust for age in the analysis. Comparisons were performed with the Wilcoxon rank sum test, Fisher's exact test or Spearman rank correlation as appropriate.
Results
Clinical and Histologic Features
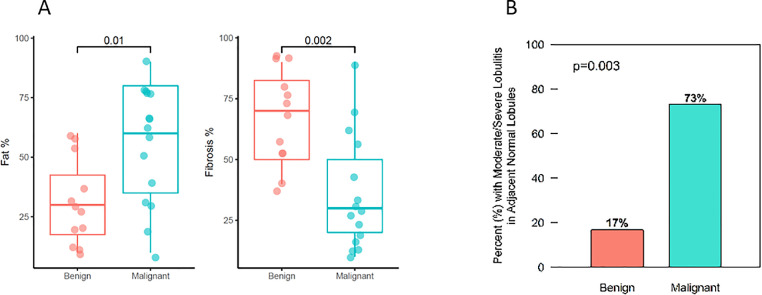
Median patient age was 60 years (range 33 to 84 years), and median BMI was 27.2 (range 20.7 to 48.1). Of the 27 patients included in the full analysis, 12 (44%) had benign breast disease and 15 (56%) had breast cancer. Those with benign breast disease had a diagnosis of atypical ductal hyperplasia [2], lobular carcinoma in situ [1], papilloma with atypia [1], and lower-risk benign lesions in the remaining 8. Among patients with breast cancer, 13 had invasive breast cancer and 2 had ductal carcinoma in situ; all 15 breast cancers were estrogen receptor-positive. Patients with malignant disease were significantly older (median 65 versus 50 years, p=0.006) and had lower mammographic breast density compared to patients with benign breast disease (73% versus 25% D1/D2, p=0.009), but BMI did not vary significantly by diagnosis (median 27.7 versus 25.9, p=0.27), Supplemental Table 1. Comparing patients with a benign diagnosis versus those with cancer with respect to the histologic characteristics of the lesion adjacent normal breast tissue, we found that the stromal fat percentage was lower (median 30% versus 60%, p=0.01) and the fibrosis percentage was higher (median 70% versus 30%, p=0.002) in samples from patients with benign disease versus cancer, Figs. 1A and 2. In terms of immune cell infiltrates, moderate/severe lobulitis was more frequent in patients with cancer (73%) than among those with benign disease (17%), p=0.003, Figs. 1B and 2. We found no statistically significant correlations between BMI and the degree of lobulitis (r = 0.09, p=0.65), while we saw a trend with moderate correlation between BMI and fat percentage (r = 0.37, p=0.06) and BMI and fibrosis percentage (r = -0.31, p = 0.12).
Fig. 1.
Stromal fat and fibrosis percentage and immune cell infiltrates in benign and malignant disease states. (A) Association of adjacent normal breast tissue fat and fibrosis percentage with disease state and (B) frequency of moderate/severe lobulitis in adjacent normal breast tissue with disease state.
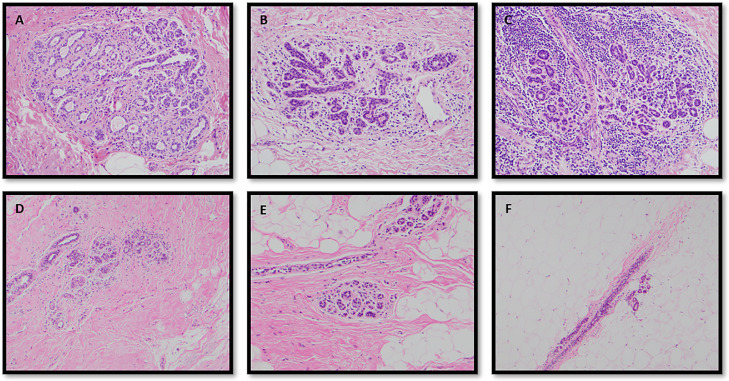
Fig. 2.
Histologic categorization of immune cell infiltrates, fat and fibrosis in breast tissue. Degrees of immune cell infiltrate (lobulitis) in adjacent normal breast tissues, panel A, mild, panel B, moderate, panel C, severe (20X magnification). Examples of histologic assessment of tissue composition, panel D, 10% fat and 90% fibrosis, panel E, 50% fat and 50% fibrosis, panel E, 90% fat and 10% fibrosis (10X magnification).
Microbiome Diversity Correlations with Histologic Tissue Composition
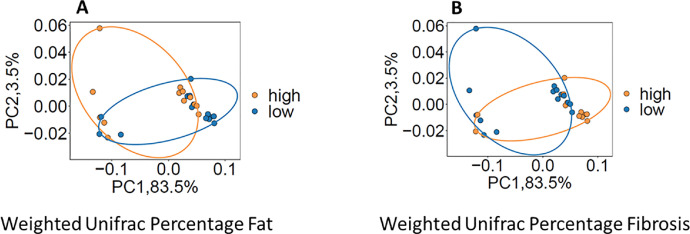
The breast tissue microbiome varied with stromal composition based on percentage fat and percentage fibrosis in the tissue. Among 27 patients in whom both the stromal composition and the breast tissue microbiome could be assessed, we found evidence of association with both alpha and beta diversity for stromal fat percentage and stromal fibrosis percentage. Specifically, alpha diversity metrics that measure species richness, such as observed OTU number or Chao1, correlated positively with stromal fat percentage (observed OTU number: r=0.32, p=0.06; Chao1 estimate: r = 0.38, p = 0.02) and correlated inversely with fibrosis percentage (observed OTU number: r=-0.24, p=0.09; Chao1 estimate: r = -0.32, p = 0.05)). No significant associations were found with alpha diversity measures that capture evenness such as Shannon (Fat %: p=0.95; Fibrosis %: p=0.77) and Inverse-Simpson indices (Fat %: p=0.77; Fibrosis %: p=0.97). In terms of beta diversity, only weighted UniFrac distance associated with fat percentage (p = 0.08) and fibrosis percentage (p = 0.07), Fig. 3. Other beta-diversity metrics, such as UniFrac, generalized UniFrac, and Bray-Curtis distance did not show evidence of association. When we explored these histologic impressions as a ratio, the fat/fibrosis ratio was also found to be associated with alpha diversity (Chao1: r = 0.34, p = 0.04; Observed OTU number: r= 0.3 p = 0.07) and beta diversity (Weighted UniFrac p = 0.07).
Fig. 3.
PCoA plots illustrating beta diversity in association with percentage fat and percentage fibrosis. The percentage fat (A) and percentage fibrosis (B) were dichotomized into high and low groups based on the median.
Microbiota Correlations with Histologic Tissue Composition
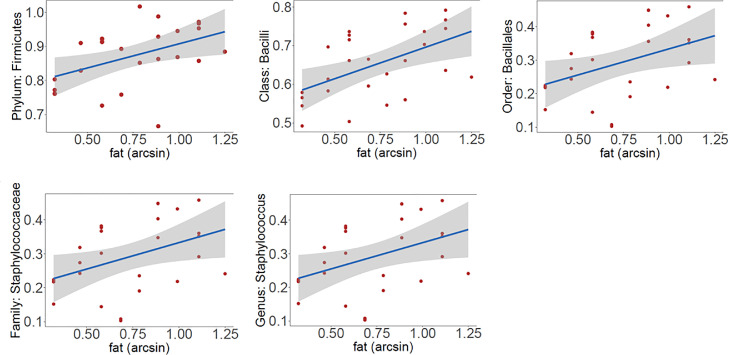
Permutation testing with multiple testing correction (FDR control) revealed breast tissue microbiome associations with stromal composition both for percentage fat, Table 1, and percentage fibrosis, Table 2, at 20% FDR.
Table 1.
Breast tissue microbiome associations with percentage fat estimates.
| Mean relative abundance | Spearman Correlation Coefficient | p- value | FDR-adjusted p-value | Direction | ||
|---|---|---|---|---|---|---|
| Phylum | ||||||
| Firmicutes | 0.357 | 0.545 | 0.003 | 0.027 | Positive | |
| Class | ||||||
| Firmicutes;Bacilli | 0.214 | 0.673 | 0.003 | 0.042 | Positive | |
| Order | ||||||
| Firmicutes;Bacillales | 0.109 | 0.471 | 0.003 | 0.072 | Positive | |
| Family | ||||||
| Firmicutes;Staphylococcaceae | 0.109 | 0.471 | 0.003 | 0.144 | Positive | |
| Genus | ||||||
| Firmicutes;Staphylococcus | 0.109 | 0.471 | 0.003 | 0.192 | Positive | |
Table 2.
Breast tissue microbiome associations with percentage fibrosis estimates.
| Mean relative abundance | Spearman Correlation Coefficient | p- value | FDR-adjusted p-value | Direction | ||
|---|---|---|---|---|---|---|
| Phyla | ||||||
| Firmicutes | 0.357 | -0.495 | 0.008 | 0.072 | Negative | |
| Spirochaetes | 1.32E-3 | 0.053 | 0.029 | 0.131 | Positive | |
| Class | Firmicutes;Bacilli | 0.214 | -0.640 | 0.003 | 0.042 | Negative |
| Order | Firmicutes;Bacillales | 0.109 | -0.479 | 0.004 | 0.096 | Negative |
| Spirochaetes;Spirochaetales | 1.33E-3 | 0.053 | 0.029 | 0.198 | Positive | |
| Proteobacteria;Sphingomonadales | 7.48E-4 | -0.365 | 0.029 | 0.198 | Negative | |
| Proteobacteria;RF32 | 9.11E-4 | 0.323 | 0.033 | 0.198 | Positive | |
| Family | Firmicutes;Staphylococcaceae | 0.109 | -0.479 | 0.003 | 0.144 | Negative |
| Genera | Firmicutes;Staphylococcus | 0.109 | -0.479 | 0.003 | 0.128 | Negative |
| Actinobacteria;Adlercreutzia | 4.33E-4 | 0.626 | 0.004 | 0.128 | Positive | |
| Firmicutes;Clostridium | 1.80E-3 | -0.131 | 0.006 | 0.128 | Negative | |
| Spirochaetes;Spirochaetes | 1.33E-3 | 0.053 | 0.029 | 0.203 | Positive | |
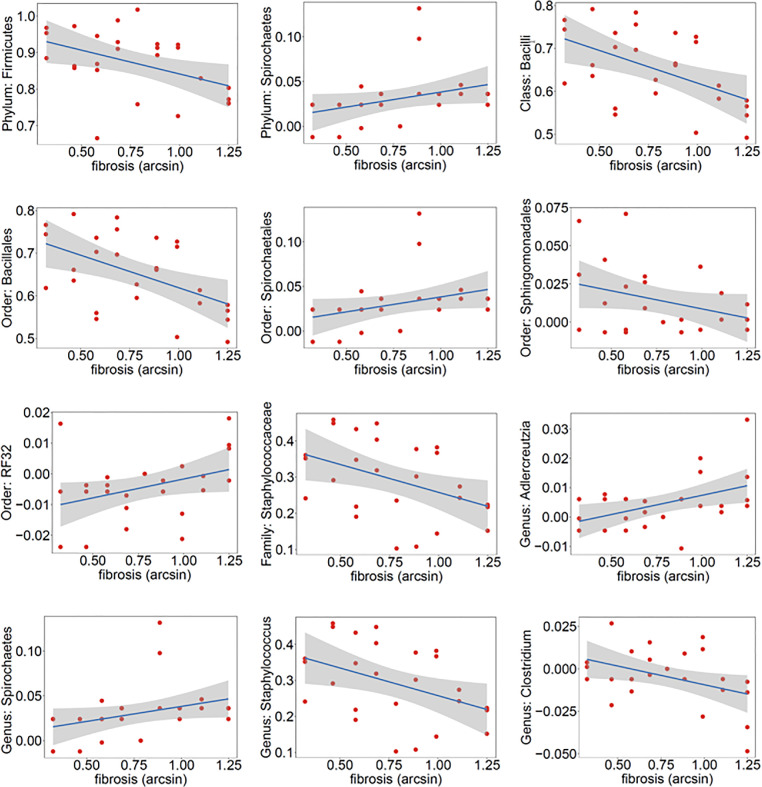
Taxa associations with percentage fat are illustrated in Fig. 4A. At the phyla level, Firmicutes was positively associated with percentage fat, as were the class Firmicutes Bacilli, order Firmicutes Bacillales, and family Firmicutes Staphylococcaceae. We identified one genus that positively correlated with percentage fat, Staphylococcus. Taxa associations with percentage fibrosis are illustrated in Fig. 4B. In terms of associations with percentage fibrosis, at the phyla level we observed a negative association with Firmicutes and positive association with Spirochaetes. Negative correlations with fibrosis percentage were seen at the class level with Firmicutes Bacilli, order level with Firmicutes Bacillales and Proteobacteria Sphingomonadales, the family level with Firmicutes Staphylococcaceae, and with the genera Clostridium and Staphylococcus. We saw positive associations with fibrosis percentage at the order level with Spirochaetes Spirochaetales and Proteobacteria RF32 and with the genera Spirochaeta and Actinobacteria Adlercreutzia.
Fig. 4A.
Differentially abundant taxa associated with stomal fat percentage. Y-axis shows the residuals of the taxa relative abundance data after removing the effect of final diagnosis and sequencing batch. X-axis shows the arcsine square root transformed percentage data.
Fig. 4B.
Differentially abundant taxa associated with stromal fibrosis percentage. Y-axis shows the residuals of the taxa relative abundance data after removing the effect of final diagnosis and sequencing batch. X-axis shows the arcsine square root transformed percentage data.
Despite the more frequent finding of moderate/severe lobulitis in adjacent normal tissue in patients with cancer than in patients with benign disease, we found no significant associations between microbial composition or diversity and lobulitis.
Discussion
In this study evaluating correlations between deep sequencing of the breast tissue microbiome and histopathology findings in a prospective cohort of patients undergoing breast surgery for benign disease or cancer we observed several key findings. First, we found significantly greater percentage fat and lower percentage fibrosis in the adjacent breast tissue of patients with breast cancer versus patients with a benign diagnosis. Secondly, the immune cell infiltrate in the adjacent breast tissue was significantly more marked in patients with cancer than in patients with a benign diagnosis. Third, regarding the microbiota, we observed significant associations of 1) alpha diversity 2) beta diversity and 3) distinct microbial communities in the lesion adjacent breast tissue in relation to fat percentage, fibrosis percentage and their ratio in terms of tissue composition assessed histologically. Interestingly, these effects were independent of patient BMI. We did not identify a clear relationship between the degree of immune cell infiltrate and the tissue microbiota. While prior studies have evaluated microbiome dysbiosis in the context of cancer and cancer risk, most have focused on the gut microbiome, and ours is the first to explore the relationship between the tissue microenvironment assessed histologically in terms of stromal and immune cell composition and the breast tissue microbiome.
Our findings of a greater percentage fat and lower percentage fibrosis in the adjacent breast tissue of patients with breast cancers is congruent with epidemiologic data reporting elevated risk of hormone receptor-positive breast cancer in post-menopausal women with obesity [15,16] although these studies do not provide information on the histologic composition of breast tissue nor the function of the adipocytes in breast tissue. While little data exists on adipocyte dysfunction within breast tissue, obesity, manifest frequently by increased number and altered functionality of adipocytes is linked to chronic inflammation, a marker of which might be the increased immune cell infiltrate we observed in the adjacent breast tissue of women with cancer in the present study [16]. Further, the relative composition of the adjacent normal breast might be different in our cancer patients than in age-matched controls without either cancer or benign breast disease and/or the spatial geographic distribution of adipocytes versus other matrix components may affect cancer development and progression. While there are beneficent adipokines such as adiponectin that can act by upregulation of their receptors and ERβ in the local tissue milieu to decrease proliferation and exert tumor suppressive effects, other adipokines, such as leptin, can exert local proliferative and pro-angiogenic effects [16,25].
Immune cells are another component of the tissue lesional or tumor microenvironment along with adipocytes, stroma, blood vessels and microbiota. Previous work has demonstrated the presence of a mucosal immune system in normal breast tissue with both T cells and dendritic cells intimately associated with the breast epithelial cells [8]. Additionally, subsequent work has shown that specific immune cell subsets in normal breast lobules varied across age-matched women with and without benign breast disease, with an overall increase in immune cell infiltrates compared to volunteer healthy donors [9]. In most studies, the predominant type of immune cells in benign breast tissues is CD8+ T cells and their presence and location along with dendritic cells suggest a role for antigen presentation and immune effector function to mitigate stressors and maintain lobular integrity [10]. These studies are aligned with our observation of a greater frequency of moderate to marked immune cell infiltrates in the lobules of patients with versus without cancer, although in the present study we evaluated immune cells in aggregate and not with markers to delineate immune cell subsets. As we have previously shown, data support the existence of an endogenous microbiome in normal breast tissue [4]. Crosstalk between immune cells and microbiota as well as other components of the tissue microenvironment provide the potential conditions for cancer suppression or development via suppression or promotion of anti-tumor immunity.
We observed increased microbial alpha diversity associated with higher fat percentage in breast tissue, despite a positive association with a current malignant diagnosis. When assessed by mammographic breast density, many epidemiologic and a few histologic studies have shown that a lower density, or higher proportion of fat in breast tissue confers a lower risk of future breast cancer in unaffected women [13,14,26]. While increased microbial alpha diversity is generally reported to be favorable with respect to cancer risk, as noted above, here we examined adjacent histologically normal tissue in women with cancer and benign disease. Thus we are unable to assess whether these observations may reflect a geographic phenomenon or might be different than in normal breast tissue from age-matched controls without breast disease. We also found differences in the breast tissue microbiome in terms of beta diversity by weighted unifrac which considers both branch distance on the phylogenetic tree and weighted relative abundance, with a positive correlation in terms of both absolute percentage fat percentage and the ratio of fat to fibrosis seen histologically in the breast tissue. Obesity is well-established as a risk factor for post-menopausal breast cancer, felt to be driven largely by increased aromatase activity of adipose tissue and hyperinsulinemic effects acting to increase unbound estrogen bioavailability [16]. Microbiota can act directly and indirectly via bacterial components, secreted bioactive products and derived metabolites and affect signaling pathways governing cell proliferation, differentiation, cytokines, growth factors and immunosurveillance [3,6,16,27]. Short-chain fatty acids (including acetate, propionate, and butyrate) are major metabolites of the gut microbiome which have cancer preventive effects via direct and immunologic effects [28]. Underlying mechanisms remain to be characterized and little is known about the role of microbiota in the tissue microenvironment in terms of cancer initiation and progression especially in niches outside the lung and gastrointestinal tract which are naturally colonized by commensals.
In the local breast tissue environment, perhaps influenced by commensal local microbiota, adipocytes may preferentially produce proliferation-inducing, pro-angiogenic adipokines such as leptin, or antiproliferative adipokines such as adiponectin. Adiponectin is an insulin-sensitizing hormone with anti-inflammatory and antiangiogenic as well as other antiproliferative properties [16]. Bacterial cell wall components affect the secretion of adipokines, depending on the presence of antigens from gram positive or gram negative bacteria. Lipopolysaccharide markedly inhibits adiponectin, leptin, and resistin secretion, whereas peptidoglycan (e.g. the antigens from Staphylococci) increase adiponectin secretion and decrease resistin secretion in vitro [29]. A recent study evaluated lipid signatures with chromatography and mass spectometry in association with the breast tissue microbiome in samples taken from clinically normal breast tissue from women with cancer or undergoing breast reduction [30]. The investigators found decreased ceramides, lipids with tumor suppressive antiproliferative effects, in tissues from women with breast cancer while in samples from the women without cancer there was increased relative abundance of bacteria genera with the capacity to synthesize these lipids including diacylglycerols, second messengers that drive activation, proliferation, migration, and effector function of both adaptive and innate immune cells. To the best of our knowledge no prior work has evaluated the histologic composition of breast tissues adjacent to benign or malignant lesions in relation to the breast tissue microbiome.
While we found increased relative abundance of Firmicutes at the phylum level and Staphylococci at the genus level with increasing fat percentage in breast tissue, this finding has not been reported previously regarding breast tissue composition. Pre-clinical and human studies evaluating the gut microbiome show associations between adipocyte-derived bacteria, including Firmicutes, and chronic inflammation and cancer risk as well as lower diversity and increased Firmicutes to Bacteroidetes ratio in obesity and with female sex [16,31]. One study using 16S sequencing demonstrated the presence of bacterial DNA in adipose tissue samples obtained under conditions of surgical sterility from omentum, mesentery and subcutaneous locations in obese subjects (median BMI 47.9) and found a predominance of the phyla Proteobacteria and Firmicutes as well as positive correlation between bacterial load and immune cell infiltration among omental samples [31]. Within the phyla Firmicutes, the genus Staphylococcus comprises at least 40 species, some of which can act directly on tissue or indirectly by toxin or enzyme production. A recent study showed an increased relative abundance of Staphylococcus in non-tumor breast tissues across adjacent normal tissue in patients with cancer, healthy controls without cancer and in high risk patients without prior or current cancer [7]. One species, Staphylococcus epidermidis was recently shown to have probiotic effects via fermentation of glycerol to butyrate via short chain fatty acid (SCFA) receptors to decrease pro-inflammatory cytokine production [32]. In turn, other preclinical investigation showed that microbiota are required for activated CD8+ T cells to transition into memory cells and that the microbiota-produced SCFA butyrate promoted memory potential of activated CD8+ T cells in vitro [33]. Thus, immune surveillance might be the mechanistic link connecting our finding of a potentially beneficent microbiome in the background breast tissue to both a current benign disease state and lower future risk of breast cancer.
We observed a negative correlation between percentage fibrosis in breast tissue and alpha diversity as well as differences in beta diversity assessed by weighted unifrac distance. Percentage fibrosis in breast tissue was associated with microbial community differences with a positive correlation with the phyla Spirochaetes and genera Spirochaeta and Actinobacteria Adlercreutzia and an inverse correlation with the phyla Firmicutes and with the genera Clostridium and Staphylococcus. While no data exists on histologic assessment of breast tissue fibrosis in association with the tissue microbiome, gut microbiome studies have demonstrated microbiome associations with tissue fibrosis in the liver, heart, kidney, lung and intestine with variations in bacterial effects by anatomic site [34]. For example, one study reported a decrease in the relative abundance of Actinobacteria in the gut microbiome of patients with pulmonary fibrosis and others showed tissue-specific associations with increased or decreased relative abundance of various members of the phyla Firmicutes in association with fibrosis of various organs [34,35]. Another study evaluated the gut microbiome with 16S sequencing of V1-V2 in healthy post-menopausal women undergoing negative screening mammography [36]. They reported increased alpha diversity and an elevated Firmicutes to Bacteroidetes ratio in the stool of women with higher mammographic breast density, which may be manifest as excess collagen with or without fibrosis in the breast tissue from the mammographically dense area [11].
Species of the Spirochaeta genus are common inhabitants of a variety of predominantly aquatic environments, exhibit mobility behaviors even through high viscosity environments, facilitated by their helical shape with axial filaments. Recent genomic and proteomics analysis suggests this genus clusters with the genera Treponema and Sphaerochaeta [37]. Associations with tissue fibrosis or collagen production are unknown. Adlercreutzia, an obligate anaerobe coccobacillus, has saccharolytic properties and when present in the gut appears to have a role in the breakdown of isoflavones, which serve as phytoestrogens, into daidzein and genistein, and subsequently to further metabolize daidzein to equol. Genistein has tumor inhibitory properties in vivo, although it's role in breast tissue is unstudied [38]. In animal studies, dietary modulation affects Adlercreutzia abundance in the stool where it may serve as a biomarker of the efficacy of anti-cancer dietary supplements [39]. Adlercreutzia abundance in the gut microbiome was positively correlated with BMI and inflammation as measured by serum leptin and adipsin concentrations in one study of obese patients [40]. The role of Adlercreutzia within breast tissue and any effect on collagen production or fibrosis remains to be explored. Within the current classification of the genus Clostridium, there are more than 200 species, including those that make collagenases such as Clostridium histolyticum which produces a mixture of collagenases and other proteinases that exhibits potent hydrolytic activity on connective tissue [41]. Although we did not find significant associations at the species level in the present study, collagenase-producing Clostridium may represent the functional link between breast tissue fibrosis and the presence of this taxa within breast tissue.
Limitations of this study include the relatively small sample size and those inherent to analysis of low microbial biomass tissues. However, this work was performed under stringent collection and assay conditions with appropriate negative controls as detailed previously. Strengths of our work include the comprehensive histologic evaluation of the tissues in concert with deep sequencing microbiome data from a prospective study in which intraoperative sterile fresh tissue collection was performed, uncontaminated by pathologic processing.
These data suggest there is a link between stromal alterations in breast tissue and its microbiome, further supporting the connection between the breast tissue microenvironment and breast cancer. Our observations suggest that variation in the breast tissue microbiome might provide a mechanistic link between stromal alterations in the microenvironment and risk factors for future breast cancer. A “healthy” core breast tissue microbiome remains to be established, and the exact nature and sequence of events underpinning the role of the microbiota in conjunction with other elements of the tissue microbiome in cancer prevention and development remain to be determined. Nonetheless, our data suggest possible hypotheses to test regarding the influence of the breast tissue microbiome and stromal composition on the development of breast cancer.
Disclosures
This work was supported by the Arnold and Kit Palmer Career Development Award in Cancer Research (TJH), the Mayo Clinic CTSA through grant number UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) (TJH), grants from the American Society of Breast Surgeons Foundation (TJH), Fraternal Order of Eagles Cancer Research Fund (TJH), and Mayo Center for Individualized Medicine (JC, NC, MW).
CRediT authorship contribution statement
Tina J Hieken: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. Jun Chen: Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Beiyun Chen: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – review & editing. Stephen Johnson: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Tanya L Hoskin: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Amy C Degnim: Data curation, Investigation, Resources, Writing – review & editing. Marina R Walther-Antonio: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Writing – review & editing. Nicholas Chia: Data curation, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing.
Footnotes
None of the authors have relevant financial disclosures related to this work.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lukasiewicz S., Czeczelewski M., Forma A., Baj J., Sitarz R., Stanislawek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021;13(17) doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch E.N., Wang J., Wargo JA. Gut microbiota and antitumor immunity: potential mechanisms for clinical effect. Cancer Immunol Res. 2021;9(4):365–370. doi: 10.1158/2326-6066.CIR-20-0877. [DOI] [PubMed] [Google Scholar]
- 4.Hieken T.J., Chen J., Hoskin T.L., Walther-Antonio M., Johnson S., Ramaker S., et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016;6:30751. doi: 10.1038/srep30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbaniak C., Gloor G.B., Brackstone M., Scott L., Tangney M., Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82(16):5039–5048. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslami S.Z., Majidzadeh A.K., Halvaei S., Babapirali F., Esmaeili R. Microbiome and breast cancer: new role for an ancient population. Front Oncol. 2020;10:120. doi: 10.3389/fonc.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzeng A., Sangwan N., Jia M., Liu C.C., Keslar K.S., Downs-Kelly E., et al. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021;13(1):60. doi: 10.1186/s13073-021-00874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degnim A.C., Brahmbhatt R.D., Radisky D.C., Hoskin T.L., Stallings-Mann M., Laudenschlager M., et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res Treat. 2014;144(3):539–549. doi: 10.1007/s10549-014-2896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degnim A.C., Hoskin T.L., Arshad M., Frost M.H., Winham S.J., Brahmbhatt R.A., et al. Alterations in the immune cell composition in premalignant breast tissue that precede breast cancer development. Clin Cancer Res. 2017;23(14):3945–3952. doi: 10.1158/1078-0432.CCR-16-2026. [DOI] [PubMed] [Google Scholar]
- 10.Goff S.L., Danforth D.N. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer. 2021;21(1):e63–e73. doi: 10.1016/j.clbc.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo C.W., Chew G., Hill P., Huang D., Ingman W., Hodson L., et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. doi: 10.1186/s13058-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T., Sun L., Miller N., Nicklee T., Woo J., Hulse-Smith L., et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 13.McCormack V.A., dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 14.Kleinstern G., Scott C.G., Tamimi R.M., Jensen M.R., Pankratz V.S., Bertrand K.A., et al. Association of mammographic density measures and breast cancer "intrinsic" molecular subtypes. Breast Cancer Res Treat. 2021;187(1):215–224. doi: 10.1007/s10549-020-06049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argolo D.F., Hudis C.A., Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep. 2018;20(6):47. doi: 10.1007/s11912-018-0688-8. [DOI] [PubMed] [Google Scholar]
- 16.Marzullo P., Bettini S., Menafra D., Aprano S., Muscogiuri G., Barrea L., et al. Spot-light on microbiota in obesity and cancer. Int J Obes. 2021;45(11):2291–2299. doi: 10.1038/s41366-021-00866-7. [DOI] [PubMed] [Google Scholar]
- 17.Macis D., Guerrieri-Gonzaga A., Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int J Epidemiol. 2014;43(4):1226–1236. doi: 10.1093/ije/dyu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19(8):1926–1932. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francescone R., Hou V., Grivennikov SI. Microbiome, inflammation, and cancer. Cancer J. 2014;20(3):181–189. doi: 10.1097/PPO.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xavier J.B., Young V.B., Skufca J., Ginty F., Testerman T., Pearson A.T., et al. The cancer microbiome: distinguishing direct and indirect effects requires a systemic view. Trends Cancer. 2020;6(3):192–204. doi: 10.1016/j.trecan.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepich-Poore G.D., Zitvogel L., Straussman R., Hasty J., Wargo J.A., Knight R. The microbiome and human cancer. Science. 2021;371(6536) doi: 10.1126/science.abc4552. eabc4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan A.A., Sirsat A.T., Singh H., Cash P. Microbiota and cancer: current understanding and mechanistic implications. Clin Transl Oncol. 2022;24(2):193–202. doi: 10.1007/s12094-021-02690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X., Johnson S., Jeraldo P., Wang J., Chia N., Kocher J.A., et al. Hybrid-denovo: a de novo OTU-picking pipeline integrating single-end and paired-end 16S sequence tags. Gigascience. 2018;7(3):1–7. doi: 10.1093/gigascience/gix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hale V.L., Chen J., Johnson S., Harrington S.C., Yab T.C., Smyrk T.C., et al. Shifts in the fecal microbiota associated with adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 2017;26(1):85–94. doi: 10.1158/1055-9965.EPI-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu D.T., Phuong T.N.T., Tien N.L.B., Tran D.K., Nguyen T.T., Thanh V.V., et al. The effects of adipocytes on the regulation of breast cancer in the tumor microenvironment: an update. Cells. 2019;8(8):857. doi: 10.3390/cells8080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh K., Brandt K.R., Reynolds C., Scott C.G., Pankratz V.S., Riehle D.L., et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res Treat. 2012;131(1):267–275. doi: 10.1007/s10549-011-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazar V., Ditu L.M., Pircalabioru G.G., Gheorghe I., Curutiu C., Holban A.M., et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., Liu B., Wei Y., Kuang DM. Influence of gut and intratumoral microbiota on the immune microenvironment and anti-cancer therapy. Pharmacol Res. 2021;174 doi: 10.1016/j.phrs.2021.105966. [DOI] [PubMed] [Google Scholar]
- 29.Taira R., Yamaguchi S., Shimizu K., Nakamura K., Ayabe T., Taira T. Bacterial cell wall components regulate adipokine secretion from visceral adipocytes. J Clin Biochem Nutr. 2015;56(2):149–154. doi: 10.3164/jcbn.14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giallourou N., Urbaniak C., Puebla-Barragan S., Vorkas P.A., Swann J.R., Reid G. Characterizing the breast cancer lipidome and its interaction with the tissue microbiota. Commun Biol. 2021;4(1):1229. doi: 10.1038/s42003-021-02710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massier L., Chakaroun R., Tabei S., Crane A., Didt K.D., Fallmann J., et al. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut. 2020;69(10):1796–1806. doi: 10.1136/gutjnl-2019-320118. [DOI] [PubMed] [Google Scholar]
- 32.Keshari S., Balasubramaniam A., Myagmardoloonjin B., Herr D.R., Negari I.P., Huang C.M. Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int J Mol Sci. 2019;20(18):4477. doi: 10.3390/ijms20184477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachem A., Makhlouf C., Binger K.J., de Souza D.P., Tull D., Hochheiser K., et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51(2):285–297. doi: 10.1016/j.immuni.2019.06.002. e5. [DOI] [PubMed] [Google Scholar]
- 34.Zhan S., Li N., Liu C., Mao R., Wu D., Li T., et al. Intestinal fibrosis and gut microbiota: clues from other organs. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.694967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z., Zhong W. Targeting the gut barrier for the treatment of alcoholic liver disease. Liver Res. 2017;1(4):197–207. doi: 10.1016/j.livres.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaghjyan L., Mai V., Wang X., Ukhanova M., Tagliamonte M., Martinez Y.C., et al. Gut microbiome, body weight, and mammographic breast density in healthy postmenopausal women. Cancer Causes Control. 2021;32(7):681–692. doi: 10.1007/s10552-021-01420-6. [DOI] [PubMed] [Google Scholar]
- 37.Gupta R.S., Mahmood S., Adeolu M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: proposal for a taxonomic revision of the phylum. Front Microbiol. 2013;4:217. doi: 10.3389/fmicb.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Constantinou A.I., Krygier A.E., Mehta R.R. Genistein induces maturation of cultured human breast cancer cells and prevents tumor growth in nude mice. Am J Clin Nutr. 1998;68(6 Suppl) doi: 10.1093/ajcn/68.6.1426S. 1426S-30S. [DOI] [PubMed] [Google Scholar]
- 39.Sharma M., Arora I., Stoll M.L., Li Y., Morrow C.D., Barnes S., et al. Nutritional combinatorial impact on the gut microbiota and plasma short-chain fatty acids levels in the prevention of mammary cancer in Her2/neu estrogen receptor-negative transgenic mice. PLoS ONE. 2020;15(12) doi: 10.1371/journal.pone.0234893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nitert M.D., Mousa A., Barrett H.L., Naderpoor N., de Courten B. Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front Endocrinol. 2020;11:605. doi: 10.3389/fendo.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jozwiak J., Komar A., Jankowska E., Martirosian G. Determination of the cytotoxic effect of Clostridium histolyticum culture supernatant on HeLa cells in the presence of protease inhibitors. Fems Immunol Med Mic. 2005;45(2):137–142. doi: 10.1016/j.femsim.2005.03.005. [DOI] [PubMed] [Google Scholar]