Highlights
-
•
Extensive studies confirmed the role of adenosine in pain.
-
•
All four subtypes of adenosine receptors contribute to antinociception, especially in neuropathic pain.
-
•
Positive allosteric modulator of A1R and high selective A3R, which are developments in recent years, present potent antinociceptive effects.
Keywords: Adenosine, Adenosine receptor, Positive allosteric adenosine modulator, Pain, Antinociception, Opioid
Abstract
Physical and emotional pain deteriorates the quality of well-being. Also, numerous non-invasive and invasive treatments for diagnosed diseases such as cancer medications and surgical procedures cause various types of pain. Despite the multidisciplinary approaches available to manage pain, the unmet needs for medication with minimal side effects are substantial. Especially with the surge of opioid crisis during the last decades, non-opioid analgesics may reduce life-threatening overdosing and addictive liability. Although many clinical trials supported the potential potency of cannabis and cannabidiol (CBD) in pain management or treatment, the long-term benefits of cannabis or CBD are still not evident. At the same time, growing evidence shows the risk of overusing cannabis and CBD. Therefore, it is urgent to develop novel analgesic medications that minimize side effects. All four well-identified adenosine receptors, A1, A2A, A2B, and A3, are implicated in pain. Recently, a report demonstrated that an adenosine A1R-specific positive allosteric modulator (PAM) is a potent analgesic without noticeable side effects. Also, several A3R agonists are being considered as promising analgesic agent. However, the importance of adenosine in pain is relatively underestimated. To help readers understand, first, we will summarize the historical perspective of the adenosine system in preclinical and clinical studies. Then, we will discuss possible interactions of adenosine and opioids or the cannabis system focusing on pain. Overall, this review will provide the potential role of adenosine and adenosine receptors in pain treatment.
1. Introduction
Adenosine (ADO) is a purine nucleoside and a ubiquitous endogenous neurotransmitter (Dunwiddie and Masino, 2001). ADO exerts a function through four G protein-coupled adenosine receptors (ARs), A1R, A2AR, A2BR, and A3 R, which had been cloned since the early 1990s and characterized pharmacologically with completed nomenclature in 2001 (Fredholm et al., 2001, Klinger et al., 2002, Mahan et al., 1991, Meng et al., 1994, Sajjadi and Firestein, 1993, Stehle et al., 1992). These receptors are widely distributed in the human body. Therefore, ADO also becomes a ubiquitous extracellular signaling molecule, and thus serves as a diverse pharmacological target, paradoxically, which is why it is difficult to achieve the pharmacological target (Borea et al., 2016). So, there are still a few ADO-related drugs used in clinical practice: ADO, theophylline, and istradefylline. Of note, ADO as A1R and A2AR agonist is well-known medicine in clinical practice as a treatment for paroxysmal supraventricular tachycardia, theophylline is an A1R antagonist and is usually prescribed for asthma patients, and recently approved istradefylline is an A2AR antagonist which compensates the dopamine-off symptoms in Parkinson’s disease (Borea et al., 2018, Shang et al., 2021). ADO is mainly metabolized from ATP intracellularly and extracellularly. Also, ADO can be a precursor for ATP synthesis. Thus, ADO levels and function are closely related to energy homeostasis (Lindberg et al., 2015).
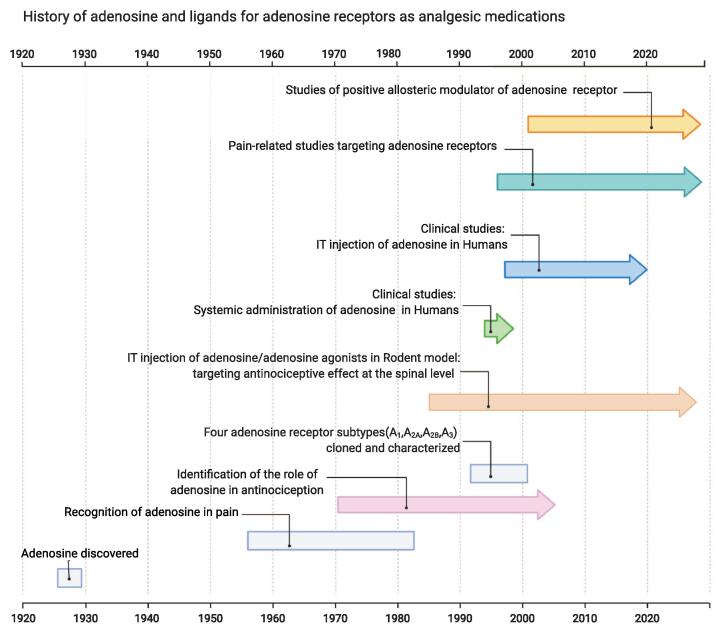
In 1927, Drury and Szent-Gyorgyi noticed that the heart rate slowed by intravenous (IV) administration of a substance extracted from cardiac tissue (Drury and Szent-Gyorgyi, 1929). After then it took nearly 50 years until ADO was chosen for arrhythmias in clinical practice. Although the exact time when ADO was recognized as being related to pain is not known, many studies have been conducted to confirm the role of ADO in antinociception in the 1980 s. With the advent of many pharmacological ligands targeting ARs, ADO and agonists of ARs emerged as novel analgesics through substantial preclinical studies until the end of the 1990 s. However, despite the potential utility of ADO and ARs agonists in pain treatment, no subsequent clinical studies were available. Since the 2000 s, pain-related ADO studies moved to target every four types of ARs, but studies centered only on ADO itself as analgesia until recently. For example, in 2020, a pilot study of intrathecal (IT) ADO injection to patients with neuropathic pain demonstrated that the effect of acupuncture was due to the elevation of ADO levels (Aghamohammadi et al., 2020, Takano et al., 2012). Recently, amid legalizing the medical and recreational use of cannabis and CBD, several studies showed a pharmacological interaction between ADO with opioids and cannabis in pain. We summarized the milestone of pain-related ADO studies over time in Fig. 1. A1R is recognized as the most promising pharmacological target as a non-opioid analgesic among many candidates. Especially, a very recent study discovered that a novel positive allosteric modulator (PAM) of A1R exhibits analgesic effects without notable side effects in rats (Draper-Joyce et al., 2021). This review will update the development of pain-related ADO studies and discuss the possibility of developing ARs targeting agents as new analgesics in the future.
Fig. 1.
The history of pain-related ADO studies and developing as analgesia over time. Developing PAM and highly selective AR agonists in various animal pain models has been active in recent years.
2. Recognizing that adenosine is involved in pain mechanisms
ADO has been implicated in inflammatory events and is thought to play a role in stressful events in the periphery and central nervous system (Hasko and Cronstein, 2004). The pain usually accompanies inflammation which raises the question of how ADO can be involved in pain management. Despite the low extracellular concentration of ADO, it can be dramatically increased in response to stressful situations and cellular damage events (Cronstein et al., 1994, Hasko and Cronstein, 2013). In basal conditions, it has been shown that the extracellular concentration of ADO can be as low as 1 µM because of rapid metabolism and uptake. However, in stressful and inflammatory conditions like sepsis and rheumatoid arthritis, ADO levels can reach up to 100 µM (Martin et al., 2000, Sottofattori et al., 2001). In other conditions of stress like epilepsy, pain, and inflammation, ADO levels are also found to be altered (Borea et al., 2016). A litany of literature reported that administration of ADO analogs either spinally or systemically has been associated with decreased pain perception in response to aversive and painful stimuli. For example, Sumida et al have demonstrated that administration of ADO analog R(-)N6-(2-phenylisopropyl) adenosine (R-PIA) on spinal dorsal horn neurons in the context of noxious heating on the hind paw was associated with decreased pain perception (Sumida et al., 1998).
Similarly, another experiment that utilized a chronic central pain rat model induced spinal cord injury photochemically via laser irradiation. The rat model exhibited chronic allodynia like-behavior. In the Von Frey test, they then administered ADO analog intrathecally, which decreased mechanical and cold allodynia-like symptoms for up to 5 h post-injection. To further confirm the effect of ADO, they administered ADO antagonist theophylline which reversed the pain-alleviating effect of ADO (Sjolund et al., 1998). With these findings, ADO plays an essential role in pain and nociception. However, it is critical to understand that ADO conducts its actions via four ARs (Klotz, 2000). Therefore, it is imperative to understand the unique effect of ADO on each of these receptors.
3. Identification of the role of adenosine in nociception
3.1. A1R
Around the 1970s, that agonism of the A1R receptor premiered as a pain-alleviating target mainly in preclinical models of neuropathic pain and inflammation exhibiting allodynia or hyperalgesia (Sawynok, 2016). Compared to other AR subtypes, A1R possesses a relatively high affinity for ADO (EC50, 0.31 μM), with numerous studies suggesting that large portions of the function of ADO within the brain are mediated through the A1R (Fredholm et al., 2001). A1R is signaling through Giα/o and is expressed in many tissues, including the brain (Dunwiddie and Masino, 2001), with its stimulation leading to the inhibition of adenylyl cyclase (AC) and thereby reducing cyclic adenosine monophosphate (cAMP) production (Burnstock et al., 2008, Fredholm et al., 2005), an important secondary messenger involved in numerous biological functions. Reduced cAMP produced from A1R stimulation inhibits cAMP-dependent protein kinase A (PKA) in neurons thereby modulating neuropeptide and neurotransmitter release (Yabuuchi et al., 2006). In addition, the dissociation of the Gα-subunit from heterotrimeric G-protein in response to the stimulation of the A1R also engages the phospholipase C (PLC) pathway through the release of Gβγ dimers in specific cell types (Biber et al., 1997). Simply, activation of the A1R decreases the AC-cAMP-PKA signaling cascade while synergistically potentiating the PLC signaling cascade (Biber et al., 1997). Within the periphery, A1R are localized on sensory nerve endings within the dorsal horn of the spinal cord and specific supraspinal locales within the pain signaling neuraxis (Fredholm et al., 2001, Katz et al., 2015). More specifically, A1R in the spinal cord is distributed along with postsynaptic neuronal cell bodies and processes of the dorsal superficial layers (lamina II), an area important for ADO signaling and an area highly involved in ADO production (Yamaoka et al., 2013).
Interestingly, Goldman et al. highlighted that the antinociceptive effects of acupuncture are mediated through the A1R (Goldman et al., 2010, Zylka et al., 2010). When the investigators stimulated the Zusanli point (located superficial to the peroneal nerve innervating the hind paw) with acupuncture needles, a large and localized release of adenine nucleotides and ADO occurred (Goldman et al., 2010, Zylka et al., 2010, Zylka et al., 2011). To further determine whether activation of the A1R induces pain relief in acupuncture, Goldman et al. induced chronic pain through sensitization of the hind paw and then injected the A1R agonist 2-chloro-N6-cyclopentyladenosine (CCPA) ipsilaterally to the Zusanli point, a pressure point on a lower leg, one of the most frequently used points in acupressure and acupuncture, which resulted in brief antinociceptive effects. In contrast, contralateral acupuncture to the Zusanli point or acupuncture within A1R knockout mice prevented pain relief (Goldman et al., 2010).
3.2. A2aR
A2AR is widely distributed throughout different body organs, both centrally and peripherally (Lee et al., 2003). Therefore, it is vital to understand the underlying mechanism of how A2AR work. A2AR is mainly coupled to the Gs family (Wang and Zhou, 2019). Thus, the primary signaling pathway for this receptor is through activation of AC, generation cAMP, and later downstream activation of PKA (Nemeth et al., 2003, Peters and Scott, 2009). Interestingly, A2AR is expressed on neurons and immune cells in the peripheral and central nervous systems (Dare et al., 2007). More importantly, it is expressed on both neurons and glial cells in the central and peripheral nervous systems (Gomes et al., 2013). Moreover, A2AR and A2BR are significantly co-localized in the same brain areas (Shaw et al., 2020). Several investigators reported the anti-inflammatory effect of A2AR activation in both the peripheral and central nervous systems. However, in regard to A2AR implication in pain, several studies show contradicting results (Sawynok, 2016). For example, mice lacking A2AR exhibited decreased sensitivity to nociceptive stimuli. Consistently, administrating A2AR selective antagonist SCH58261 had antinociceptive effects (Hussey et al., 2007, Hussey et al., 2010). Also, a different A2AR antagonist ZM241385 showed a reduction in nociceptive behavior (Li et al., 2010). Varano et al recently conducted a study testing an emerging inverse agonist for A2AR where they reported equal effect and, in some cases, more significant effect in antinociception than morphine (Varano et al., 2016). A finding further confirms the possible role A2AR can play in pain mechanisms. In further studies, activation of A2AR has pro-algogenic effects (Bura et al., 2008). On the other hand, a recent study has reported that IT injection of A2AR agonist 2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamido adenosine HCl (CGS21680) alleviated neuropathic pain in spinal cord injury rats (Kwilasz et al., 2018). Consequently, IT injection of A2AR agonist has antinociceptive effect on neuropathic pain (Loram et al., 2009). The discrepancies described between the effects of activation and deactivation of A2AR may be due to different actions of A2AR regarding pain, centrally, in contrast, to peripherally (Sawynok, 2016).
3.3. A2bR
The expression of A2BR is similar to A2AR. It is widely expressed in body organs and immune cells and is thought to play a role in inflammatory conditions (Aherne et al., 2011). Another supporting fact for the role of A2BR in mediating inflammatory reactions is the low affinity to ADO compared to AR subtypes. Consequently, A2BR is activated only when ADO levels are high in inflammatory and stressful events (Borea et al., 2017, Feoktistov and Biaggioni, 2011). There is limited literature on A2BR in pain, mainly due to a lack of selective agonists and antagonists for this receptor. In a study, they used a non-selective A2BR antagonist DMPX which prefers A2BR to A2AR. They used the hot plate test to produce an acute pain model of mice, and they reported the antinociceptive effect of the compound at a 30 mg/kg dose. To further confirm this, they tested other selective A2BR antagonist compounds (PSB-50, PSB-53, PSB-1115, PSB-55, and enprofylline) and reported dose-dependent analgesic effects in a hot plate test (Abo-Salem et al., 2004). Since it has been implicated in inflammatory responses, Hu et al studied the effect of A2BR on chronic pain. They reported that A2BR could be a connecting link between neurons and microglia in inflammatory conditions. Moreover, when activated by high ADO levels, it induces nociceptive hyperexcitability and promotes chronic pain (Hu et al., 2016). All these findings seem to confirm that A2BR is involved in promoting nociception. With different studies reporting the antinociceptive effect of A2BR blockade. With a definite need for more studies to confirm the role, A2BR plays in pain management and nociception as a possible therapeutic target for pain.
3.4. A3R
Like other receptor subtypes of its class, the A3R is generally coupled to Gi/o, and activation of A3R and Gi/o coupling leads to inhibition of AC and decreased cAMP concentrations with occasional extracellular signal-regulated kinase 1/2 (ERK1/2) activation being documented (Barkan et al., 2020, Schulte and Fredholm, 2002). For a large portion of time, the enigmatic nature of the A3R led to its omission as a potential antinociceptive target reportedly due to low expression profiles in the CNS as well as non-specific ligands for the receptor. However, it has become apparent that the A3R is highly expressed on various immune cells, the lumbar spinal cord, and within supraspinal areas such as the rostral ventral medial medulla (RVM) (Durante et al., 2021, Jacobson et al., 2019, Janes et al., 2016, Little et al., 2014). Without question, peripheral A3R afferent projections (ie: spinal cord and RVM) are functionally important in pain, with many studies indicating selective A3R agonists dose-dependently modulate neuropathic pain. Some of the first evidence of the antinociceptive actions of the A3R arose from an investigation by Chen et al. in which, using the well-characterized model of mechano-allodynia, dose-dependent agonists of the A3R receptor with N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA) rapidly reversed neuropathic pain (Chen et al., 2012). Most importantly, the effects of IB-MECA were insensitive to naloxone, suggesting the activity of this compound was not opioid-mediated (Chen et al., 2012). Interestingly, in addition to its standalone ability to modulate pain, the effects of IB-MECA were equal to already established antinociceptives gabapentin or amitriptyline but respectively > 350- and > 75-fold more potent (Chen et al., 2012).
In a more recent study, the expression of the A3R on immune cells and their role in neuropathic pain was deduced (Durante et al., 2021). Due to the high protein expression of the A3R on T cells (especially CD4+ and CD8+) (Borea et al., 2016), Durante et. al questioned whether these receptors play a role in the beneficial agonist effects in mechano-allodynia modeled neuropathic pain. Using mice deficient for both T and B cells (Rag-KO mice) compared to WT-counterparts, investigators discovered Rag-KO mice were insensitive to the anti-allodynic effects of A3R agonism (Durante et al., 2021). Significantly, transplantation of CD4+ cells from WT mice restored A3R agonist-mediated anti-allodynia within Rag-KO mice. In contrast, transplantation of CD4+ T cells from Adora3-KO or interleukin-10 (IL-10) receptor knockout mice into Rag-KO had no effects, highlighting the role of A3R expression on CD4+ T cells and its role in neuropathic pain (Durante et al., 2021).
4. Interaction of adenosine receptors with opioid or cannabis receptors
4.1. Interaction between adenosine receptors and opioid receptors
In the late 1980s, several studies confirmed that morphine act on opioid receptors to release ADO within primary afferent nerve terminals in the dorsal horn of the spinal cord, and the ADO subsequent activation of A1R and A2AR to produce spinal analgesia (Sawynok et al., 1989, Sweeney et al., 1987, Sweeney et al., 1989). Furthermore, the additive interaction between ARs agonists and opioid mu receptors and the synergistic interaction with delta and kappa was noticed (De Lander and Keil, 1994). In the 2000s, since the synergistic effect and relationship of ADO and opioids were confirmed in the laboratory, animal experiments were conducted considering its clinical application. Hence, more detailed studies focusing on each ARs agonist and antagonists in pain were conducted together. In the mid-2000s, studies confirmed the anti-allodynic effect of ARs agonist with morphine in various nerve injury animal models that include spinal cord injury and peripheral nerve injury. In addition, AR agonist has an additive effect with morphine in reducing mechanical allodynia-like behavior and tactile allodynia, suggesting a synergistic interaction between A1R and mu-opioid receptor (Hwang et al., 2005, von Heijne et al., 2000, Zhang et al., 2005).
Eisenach et al. studied IT injection of ADO in humans performed studies to determine whether opioid administration also induces ADO release in humans. A total of 32 patients and volunteers were enrolled for this study, and their clinical data suggest that local opioid receptor stimulation in the spinal cord releases ADO (Eisenach et al., 2004). However, until recently, no notable clinical studies validate the potential interaction between A1R and mu-opioid receptors. Recently, several studies focused on opioid-induced hyperalgesia (OIH; increased pain sensitivity), tolerance, and withdrawal, which can contribute to dependence and abuse. Interestingly, in rats, morphine-induced OIH and antinociceptive tolerance is associated with spinal A3R signaling, and A3R activation potentiates morphine antinociception in the opioid-tolerant state (Doyle et al., 2020, Leduc-Pessah et al., 2021). Collectively, the interaction of opioids and ADO/ARs have a synergistic or additive effect in antinociceptive mechanism, and A3R agonist may have a protective or reliving role against opioid side effects, including tolerance, OIH, and withdrawal.
4.2. Interaction between adenosine receptors and cannabis receptors
Cannabinoids produce more than 100 naturally occurring chemicals, the most abundant of which are Δ-9-tetrahydrocannabinol (THC), cannabidiol (CBD). THC and CBD bind with cannabinoid receptors (CB1R and CB2R) of endocannabinoids in the body. THC is a psychotropic chemical that makes people feel “high,” whereas CBD is a non-psychotropic chemical. CBD was recently shown to have therapeutic potential in various medical disorders such as epilepsy. The endocannabinoid system is present throughout the pain pathways as cannabinoid receptor agonists have antinociceptive effects in animal models of acute, inflammatory, and neuropathic pain (Chayasirisobhon et al., 2020, Starowicz and Finn, 2017). According to studies about the relationship between ADO and cannabis, colocalization of A1R and CB1R and their coupling suggest that these receptors may interact each other.
Furthermore, a variety of heteromers of A2AR, CB1R, and D2R in different elements of striatal spine modules raised, and interaction of A2AR and CB1R have been consistently reported to occur in the brain striatum (Ferre et al., 2010, Franco et al., 2019, Hoffman et al., 2010, Martire et al., 2011, Moreno et al., 2018, Tebano et al., 2012). Furthermore, a result revealed that A2AR and CB1R heteromers function at the hippocampus's presynaptic level, suggesting that CBD may interact with A2AR and CB1R in the brain (Aso et al., 2019). Nevertheless, only a few studies revealed pain-related interactions of ADO and cannabis, while most studies centered on a neuropsychiatric perspective. However, A2AR is known to participate in some anti-inflammatory effects of CBD, suggesting a possible interaction between A2AR and CB1R in pain regulation (Mecha et al., 2013).
5. Developing adenosine and adenosine analogs as analgesic agents
5.1. Intrathecal injection of adenosine and adenosine receptor agonists in rodent models
Previous studies have confirmed that ADO has a role in antinociception at the spinal cord level (Doi et al., 1987, Sweeney et al., 1987, Sweeney et al., 1989). Hence, during the 1980s, research centered on developing ADO analogs and ARs agonists. In particular, IT injection of ADO and ARs agonists in rodent models revealed the antinociceptive properties targeting ARs, as summarized in Table 1 (Jacobson et al., 2019). In 1984, a study with IT injection of 5′-N-Ethylcarboxamido adenosine (NECA), an AR agonist, revealed a dose-dependent antinociception effect (Post, 1984). In addition, in 1987, Doi et al. injected ATP, ADP, AMP, ADO, and adenine in mice IT space, also they noticed these compounds except adenine have a dose-dependent antinociceptive response and suggest the existence of ARs which modulate spinal nociceptive sensory processing (Doi et al., 1987). From the 1980s to the 1990s, several studies focused on the efficacy and safety of ADO and ARs agonists in rodent models with the IT approach. Despite some dose-dependent motor impairment in rats, the safety of IT administration is warranted at antinociceptive doses of ADO and ARs agonists (Karlsten et al., 1993, Sosnowski et al., 1989). These preclinical results prompted the clinical use of IT-injected ADO in humans. Prior to the clinical tests, at the end of the 1990s, preclinical toxicity screening of IT ADO in rats and dogs reported no neurotoxicity from eighteen rats with 4 days of administration. Additionally, a study with nine beagle dogs with 26 days of IT administration of ADO enabled the phase I safety trials of IT ADO administration in humans (Chiari et al., 1999).
Table 1.
The list of ARs agonists and antagonists in pain research.
| Agonists | Pain model | Administration | References | |
|---|---|---|---|---|
| A1R | NECA | Colonic inflammation | IT/SC | Sohn et al., 2008 |
| CPA | Diabetic neuropathy | IT/SC | Katz et al., 2015 | |
| R-PIA | SCI | IT | Heijne et al., 2000 | |
| PAM: | ||||
| T62 | Acutepain/carrageenin inflammation | IT | Li et al., 2003 | |
| SNL | PO | Li et al., 2004 | ||
| MIPS521 | PNL /SNI | IT | Joyce et al., 2021 | |
| VCP171 | PNL /SNI | IT | Joyce et al., 2021 | |
| Antagonists | ||||
| DPCPX | PNL /SNI | IT | Joyce et al., 2021 | |
| CPT | SC formalin injection | IT | Yoon et al., 2006 | |
| A2AR | Agonists | |||
| CGS21680 | Plantar incision | IT | Zahn et al., 2007 | |
| ATL313 | CCI/PNL | IT | Loram et al., 2009 | |
| Antagonists | ||||
| ZM241385 | CCI/PNL | IT | Loram et al., 2009 | |
| CSC | SC formalin injection | IT | Yoon et al., 2006 | |
| Agonists | ||||
| BAY606583 | CCI | IT | Loram et al., 2013 | |
| Antagonist | ||||
| A2BR | ATL801 | IBS | PO | Kolachala et al., 2008 |
| MRS 1754 | IBS | IP | Aano et al., 2017 | |
| PSB1115 | IBS | IP | Aano et al, 2017 | |
| alloxazine | SC formalin injection | IT | Yoon et al., 2006 | |
| Agonists | ||||
| MRS5698 | Opioid analgesic tolerance | IT | Pessah et al., 2021 | |
| IB-MECA | CIPN | IP | Janes et al., 2014 | |
| A3R | CCI | IP | Ford et al., 2015 | |
| CI-IB-MECA | Diabetic neuropathy | IP | Yan et al., 2016 | |
| CCI | IP | Chen et al., 2012 | ||
| MRS5980 | Collitis | IP | Lucarini et al., 2020 | |
| Antagonist | ||||
| MRS1220 | SC formalin injection | IT | Yoon et al., 2006 | |
| MRS1523 | CINP | IP | Janes et al., 2014 |
Abbreviation: PNL: partial nerve ligation; SNI: sciatic nerve injury; SCI: spinal cord injury; CCI: chronic constriction injury; IBS: irritable bowel syndrome; CINP: Chemotherapy induced neuropathy; IT: intrathecal; SC: subcutaneous; PO: per oral; IP: intraperitoneal.
5.2. Clinical trials of systemic adenosine as antinociceptive agent
In the 1990s, a series of clinical trials proved the efficacy of systemic ADO as an analgesic agent in humans (Belfrage et al., 1995, Segerdahl et al., 1995a, Segerdahl et al., 1995b, Segerdahl et al., 1994, Segerdahl et al., 1996, Sollevi et al., 1995). In 1994, the first clinical trial of IV constant infusion of 70 µg/kg/min dose of ADO in nine healthy volunteers with a single-blind, randomized, placebo-controlled study, showed an analgesic effect of ADO measure by visual analog scale (VAS, 0–100 mm). Furthermore, a co-administration of ADO with morphine or ketamine exhibited an additive effect. An additional study on six healthy volunteers attenuated tactile allodynia. Subsequently, a preliminary report for ADO treatment in two patients with peripheral neuropathic pain and the following seven neuropathic patients raised the need for a controlled study verifiable whether ADO is potentially relieving effect in neuropathic pain. Unfortunately, these studies had limitations due to a small number of subjects. However, soon after, perioperative ADO infusion in 43 patients who underwent abdominal hysterectomy showed that ADO infusion reduced the requirements of volatile anesthetic and postoperative opioid analgesic (Segerdahl et al., 1997).
Nevertheless, there were no clinically significant results directly applied to clinical practice. Furthermore, a relatively large study showed the no analgesic effect of the IV ADO phase 2 trial on 166 gynecologic postoperative patients (Habib et al., 2008). In addition, a recent meta-analysis that included 757 patients from 9 studies for the analgesic effect of perioperative ADO infusion concluded that systemic ADO has no pain-reducing effect or prophylactic effect against postoperative nausea and vomiting (Jin and Mi, 2017). Therefore, based on the recent negative outcome of using systemic ADO administration in humans, ADO is no longer a clinical option for analgesics. Moreover, it seems reasonable to view it as currently having no benefits due to other effects such as cardiovascular side effects.
5.3. Clinical trials of intrathecal adenosine as antinociceptive agent
The studies for systemic administration of ADO in humans failed to show a satisfactory clinical outcome for reducing acute pain. On the other hand, a study showed an interesting finding that patients with neuropathic pain had reduced blood and CSF ADO levels, demonstrating that ADO deficiency is associated with neuropathic pain (Guieu et al., 1996).
In the late-1990 s, IT injection of ADO with clinical application considering in humans performed actively, following previous rodents’ successful results. The phase 1 clinical safety study of 500 ∼ 2000 µg dose of IT ADO on 12 healthy volunteers evaluated the side and analgesic effects. The results showed the safety of IT ADO and statistically significant reduction of pain in sensory skin test and tourniquet ischemic test (Rane et al., 1998). Furthermore, an open-label, dose-escalating trial and a double-blind, placebo-controlled trial on 65 volunteers showed that IT ADO does not produce a high incidence of severe side effects and advocated further investigation of IT ADO for analgesia in humans. Additional study with 0.5 mg, 2.0 mg dose of IT ADO injection on 30 volunteers, using topical capsaicin for causing pain, showed ADO had no dose-dependent effect for reducing pain thus, recommended 0.5 mg or fewer doses of IT ADO should be considered for the treatment of pain (Eisenach et al., 2002a, Eisenach et al., 2002b). A similar study with IT ADO, whether perioperative IT ADO injection reduced aesthetic requirements and postoperative opioid requirements in 40 female patients who underwent a hysterectomy. Despite positive results of the previous phase 1 study, replicated experiments showed no anesthetic drugs or postoperative analgesic effects of ADO after hysterectomy (Rane et al., 2000). According to this comparative study, the additional negative results on 90 patients who underwent an abdominal hysterectomy were divided into early, late, control groups, IT ADO 1,000 µg was not effective as an analgesic for postoperative pain relief without pre-emptive effect (Sharma et al., 2006). In addition, Eisenach et al. conducted a further study following their previous study in seven patients with chronic neuropathic pain, which showed that IT ADO reduces allodynia. However, they concluded that IT ADO as a sole agent for treating neuropathic pain might be limited due to modest and common side effects (Eisenach et al., 2003). Then recently, research on 40 patients who had radicular limb pain since underwent lumbar discectomy suggested that 1000 µg of IT ADO is a safe and effective method for postoperative neuropathic pain management after uni-level disk surgeries (Aghamohammadi et al., 2020). Taken together, IT ADO in humans appears to have the possibility of future application in neuropathic related pain rather than acute or postoperative pain.
6. Targeting adenosine receptors for future analgesia
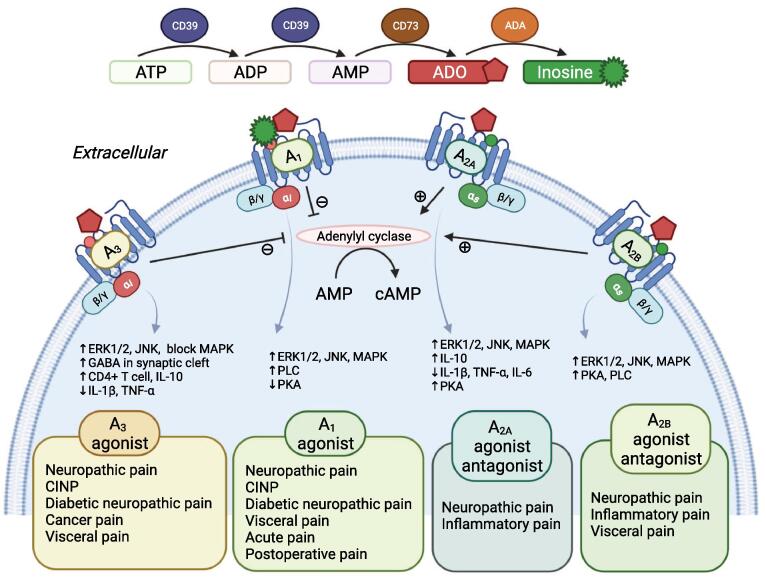
According to the research, all four ARs are involved in the process of antinociception in the spinal cord (Yoon et al., 2006). The schematic mechanism of each receptor is shown in Fig. 2.
Fig. 2.
The schematic mechanism of each receptor. Gi subunit coupled A1R and A3R are activated, cAMP is reduced, which induces several inhibitory responses in the body. And all four subtypes of AR are thought to be involved in pain regulation by inducing activation through phosphorylation of p38 MAPK (mitogen-activated protein kinase), ERK1/2 (extracellular signal-regulated), and JNK (Jun-N-terminal kinase) which are involved in inflammation pathways and apoptosis.
6.1. Targeting A1R and positive allosteric modulator in pain
Early on, many studies have shown that A1Rs play a significant role in the antinociception mechanisms thus selective A1R agonists were considered as the most promising new analgesic candidates (Sawynok et al., 2016, Wu et al., 2005, Zahn et al., 2007). In addition, researchers conducted studies on various types of pain targeting A1R, including neuropathic pain, visceral pain, and postoperative pain (Horiuchi et al., 2010, Sohn et al., 2008). In particular, activation of A1Rs contributes to reducing neuropathic pain significantly. Recently, A1R has also been known to contribute to regulation pain by TRPV1, transient receptor potential cation channel subfamily V member 1, also known as capsaicin and vanilloid receptors (Kan et al., 2018, Katz et al., 2015, Yamaoka et al., 2013).
To date, although the precise mechanisms are not well studied how A1R involves in pain, when Gi subunit coupled A1R is activated, dampening the cAMP levels may induce several inhibitory responses in the body, which may result in pain reduction through the phosphorylation of p38 MAPK (mitogen-activated protein kinase), ERK1/2 (extracellular signal-regulated) and JNK (Jun-N-terminal kinase) (Shaw et al., 2020). Furthermore, the role of A1R, which is more prominent than other AR in pain, is due to their location on peripheral sensory nerve endings in the spinal cord dorsal horn and at supraspinal pain-processing structures (Sawynok et al., 2016, Schulte et al., 2003, Vincenzi et al., 2020). Another advantage of A1R is that inosine, a metabolite of ADO, binds to A1R with a similar affinity to ADO and induces antinociceptive properties, anti-allodynia, and anti-hyperalgesia. However, despite the advantages of targeting A1R, its development as a new analgesic was limited due to the possibility of inducing serious adverse effects on the cardiovascular system and motor function. Therefore, the development of A1R-positive allosteric modulators (PAM) which improve efficacy and limit side effects, enhancing the effect of endogenous ADO has emerged (Vincenzi et al., 2020).
In the early 2000s, researchers performed experiments validating T62 as the first PAM of A1AR in animal models.These studies confirmed that T62 produces antinociception and also suggested it lacks efficacy against acute nociceptive stimuli under normal conditions, but reduces hypersensitivity during inflammation through a central mechanism. In addition, these authors observed that chronically administered T62 lose efficacy over time, partly due to receptor down-regulation (Li et al., 2004, Li et al., 2003, Pan et al., 2001). Despite the early identification of 2A3BTs (T62, PD81,723, LUF5484) as PAM of A1R, their mechanism of action appears complex and is not fully understood. Furthermore, 2A3BTs recognize an allosteric site at low concentrations while binding to the orthosteric site at high concentrations; thereby, these agents interact with both an allosteric and the orthosteric site of A1R. Therefore, removing the competitive properties of 2A3BTs while retaining allosteric properties is vital for developing the PAM of A1R (Valant et al., 2010). Hence, VCP 171, next-generation PAM of the A1R is expected that could be an effective treatment for neuropathic pain. However, VCP 171 has a weak in vivo effect and did not contribute to PAM activity under physiological conditions (Draper-Joyce et al., 2021, Imlach et al., 2015). In 2021, Joyce et al hypothesized that cooperation between the PAM and ADO is more critical than the A1R affinity of PAM in antinociception. Finally, these researchers successfully developed the new PAM of the A1R, MIPS521, which exhibits analgesic efficacy in rats in vivo through modulation of the increased levels of endogenous ADO. In addition, these authors reported the structure of the A1R co-bound to ADO, MIPS521, and a Gi2 heterotrimer (Draper-Joyce et al., 2021). These results provide a step toward a non-opioid analgesic development and support additional validating studies following preclinical and clinical studies.
6.2. Targeting A2AR, or A2BR in pain
The role of A2AR and A2BR in pain is controversial since both pronociceptive and antinociceptive effects have been reported, A2AR agonists show some peripheral pronociceptive effects. However also exhibit antinociceptive effect through acting on immune cells to suppress inflammation and on spinal glia to suppress pain signaling and A2BR agonists contribute to the peripheral proinflammatory process of immune cells, but also exhibit spinal antinociceptive effects (Gomes et al., 2013, Sawynok et al., 2016, Vincenzi et al., 2020). For this reason, these A2AR and A2BR are considered to have a role in inflammatory and neuropathic pain. Furthermore, a study suggested that peripheral A2AR mediated anti-inflammatory actions can relieve perioperative pain (Hayashida et al., 2005). In the inflammation process, activation of A2AR decreases proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 and increases the release of the IL-10, which is a potent anti-inflammatory cytokine. This process contributes to reducing neuropathic pain. Indeed, elevated IL-10 mRNA in the CSF was observed with a single IT injection of the A2AR agonists, and it produced a long-duration reversal of mechanical allodynia and thermal hyperalgesia for at least 4 weeks (Loram et al., 2009). In addition, several studies described the relationship between A2AR and Derived Neurotropic Factor (BDNF), which regulate microglial activity in neuropathic pain. Based on these results, studies convinced IT A2AR agonists probably be a novel target for treating neuropathic pain (Kwilasz et al., 2018, Loram et al., 2009, Loram et al., 2013). Furthermore, a recent study with the monoarthritis mice model suggested A2AR agonists have an analgesic effect in inflammatory pain; thus they can be a potential therapeutic agent of inflammatory pain (Eisenach et al., 2002b; Ravani et al., 2017). Interestingly, several papers showed that systemic and IT injection of selective A2R antagonists has antinociceptive effects and sex differences in pain response. A2AR antagonist reduced the nociceptive behavior in female wild-type mice, but not in males (Luongo and Salvemini, 2018, Varano et al., 2016).
On the other hand, in comparison to other AR subtypes, ADO exhibit a lower affinity for A2BR, and lack of its selective agonist makes it difficult in conducting related research; therefore, limited studies were conducted based on this receptor and their involvement in the pain are poorly understood (Borea et al., 2017, Vincenzi et al., 2020). Nonetheless, a study reported that A2BR antagonists reduce pain in the visceral hypersensitivity rat model (Asano and Takenaga, 2017, Asano et al., 2017, Kolachala et al., 2008). Furthermore, another study noticed that A2BR selective agonist also has an analgesic role in the neuropathic pain model (Loram et al., 2013). Bottom line, both A2AR and A2BR lead to increased cAMP accumulation, and this cAMP activates PKA of downstream pathways. Of note, all ARs are coupled to MAPK pathways, ERK1/2, p38 MAPK, and JNK (Borea et al., 2016, Coppi et al., 2021, Shaw et al., 2020).
6.3. Targeting A3R in pain
To date, studies about A3R have shown promise for the treatment of various diseases such as cancer and autoimmune disease. In addition, the activation of the A3R in humans does not cause cardiac or hemodynamic side effects and even A3R agonists are potent, selective, and orally bioavailable. Therefore, clinical research for developing A3R agonists is intensively underway(Silverman et al., 2008). Like A1R, Gi-coupled A3R activation inhibits AC, thereby reducing intracellular cAMP levels, which is considered a first step in the antinociception. Of course, the pronociceptive role of peripheral A3R was reported, but recent studies showed results on the antinociceptive role of A3R in neuropathic pain with mechanistic actions on glial cells (Sawynok et al., 2016, Vincenzi et al., 2020). Notably, A3R on immune cells modulates cytokine release such as CD4+ T cells and IL-10, thereby reducing DRG neuron excitability (Durante et al., 2021). In addition, enhancing ERK1/2 and p38 by A3R activation results in a reduced level of proinflammatory cytokines TNF-α and IL-1β. Furthermore, A3R agonists counteracting extracellular GABA decrease through prevented GAT-1 and GAD65 inactivation, as the result, preventing mechanical allodynia. Variously developed agonists of A3R revealed the role of A3R in various preclinical pain models and suggested antinociception effects in neuropathic pains, including chronic constriction injury-induced, chemotherapy-induced, and diabetic neuropathy. In chemotherapy-induced pain, A3R agonists prevent paclitaxel-induced neuropathic pain via modulating spinal redox-dependent signaling pathways (Janes et al., 2014). And a study shows that IB-MECA, one of A3R alleviated mechanical hyperalgesia and thermal hypoalgesia in diabetic neuropathy mice (Yan et al., 2016). In addition, some studies suggested the association of A3R with colitis-induced visceral pain control (Lucarini et al., 2020). Interestingly, all the effects of A3R agonists were prevented by the spinal administration of the selective A3R antagonist MRS1523(Chen et al., 2012, Coppi et al., 2021, Ford et al., 2015). A3R agonists also promise novel non-opioid analgesia for pain control.
7. Conclusions
Extensive studies confirmed the role of ADO in pain. All four subtypes of ARs contribute to antinociception, especially in neuropathic pain. Following the preclinical studies, AR agonist emerged as one of the most promising non-opioid analgesics, but cardiovascular side effects have yet to be overcome. However, PAM of A1R and high selective A3R, which are developments in recent years, present potent antinociceptive effects with fewer side effects in animal models. Consequently, additional well-designed clinical trials should validate the potential use of AR agonists alone or a combination of other existing analgesic medications depending on the sub-category of pain.
Declaration of Competing Interest
D-S Choi is a scientific advisory board member to Peptron Inc. Peptron had no role in preparing, reviewing, or approving the manuscript. All the other authors declare no biomedical financial interests or potential conflicts of interest.
Acknowledgments
We thank all the laboratory members for their helpful discussion and comments. This work was supported by the Samuel C. Johnson Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, and the National Institute on Alcohol Abuse and Alcoholism (AA028968, AA029258) and Aging (AG072898). National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03032817). Figures 1 and 2 were created with BioRender.com.
References
- Abo-Salem O.M., et al. Antinociceptive effects of novel A2B adenosine receptor antagonists. J. Pharmacol. Exp. Ther. 2004;308:358–366. doi: 10.1124/jpet.103.056036. [DOI] [PubMed] [Google Scholar]
- Aghamohammadi D., et al. Pilot prospective open-label one-arm trial investigating intrathecal Adenosine in neuropathic pain after lumbar discectomy. BMC Res. Notes. 2020;13:284. doi: 10.1186/s13104-020-05133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne C.M., et al. The resurgence of A2B adenosine receptor signaling. Biochim. Biophys. Acta (BBA)-Biomembranes. 2011;1808:1329–1339. doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Takenaga M. Adenosine A2B receptors: an optional target for the management of irritable bowel syndrome with diarrhea? J. Clin. Med. 2017;6 doi: 10.3390/jcm6110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., et al. Aminophylline suppresses stress-induced visceral hypersensitivity and defecation in irritable bowel syndrome. Sci. Rep. 2017;7:40214. doi: 10.1038/srep40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E., et al. Adenosine A2A-cannabinoid CB1 receptor heteromers in the hippocampus: cannabidiol blunts delta(9)-tetrahydrocannabinol-induced cognitive impairment. Mol. Neurobiol. 2019;56:5382–5391. doi: 10.1007/s12035-018-1456-3. [DOI] [PubMed] [Google Scholar]
- Barkan K., et al. Pharmacological characterisation of novel adenosine A3 receptor antagonists. Sci. Rep. 2020;10:20781. doi: 10.1038/s41598-020-74521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfrage M., et al. Systemic adenosine infusion alleviates spontaneous and stimulus evoked pain in patients with peripheral neuropathic pain. Anesth. Analg. 1995;81:713–717. doi: 10.1097/00000539-199510000-00010. [DOI] [PubMed] [Google Scholar]
- Biber K., et al. Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J. Neurosci. 1997;17:4956–4964. doi: 10.1523/JNEUROSCI.17-13-04956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea P.A., et al. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016;37:419–434. doi: 10.1016/j.tips.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Borea P.A., et al. Pathological overproduction: the bad side of adenosine. Br. J. Pharmacol. 2017;174:1945–1960. doi: 10.1111/bph.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea P.A., et al. Pharmacology of adenosine receptors: the state of the art. Physiol. Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- Bura A.S., et al. A 2A adenosine receptor regulates glia proliferation and pain after peripheral nerve injury. Pain. 2008;140:95–103. doi: 10.1016/j.pain.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Chayasirisobhon S. Mechanisms of action and pharmacokinetics of cannabis. Perm J. 2020;25:1–3. doi: 10.7812/TPP/19.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., et al. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012;26:1855–1865. doi: 10.1096/fj.11-201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiari A., et al. Preclinical toxicity screening of intrathecal adenosine in rats and dogs. Anesthesiology. 1999;91:824–832. doi: 10.1097/00000542-199909000-00035. [DOI] [PubMed] [Google Scholar]
- Coppi E., et al. Uncovering the mechanisms of adenosine receptor-mediated pain control: focus on the A3 receptor subtype. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22157952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein, B. N., 1994. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. (1985). 76, 5-13. [DOI] [PubMed]
- Dare E., et al. Modulation of glial cell functions by adenosine receptors. Physiol. Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- De Lander G.E., Keil G.J., 2nd Antinociception induced by intrathecal coadministration of selective adenosine receptor and selective opioid receptor agonists in mice. J. Pharmacol. Exp. Ther. 1994;268:943–951. [PubMed] [Google Scholar]
- Doi T., et al. Spinal antinociceptive effects of adenosine compounds in mice. Eur. J. Pharmacol. 1987;137:227–231. doi: 10.1016/0014-2999(87)90226-3. [DOI] [PubMed] [Google Scholar]
- Doyle T.M., et al. Chronic morphine-induced changes in signaling at the A3 adenosine receptor contribute to morphine-induced hyperalgesia, tolerance, and withdrawal. J. Pharmacol. Exp. Ther. 2020;374:331–341. doi: 10.1124/jpet.120.000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper-Joyce C.J., et al. Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature. 2021;597:571–576. doi: 10.1038/s41586-021-03897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury A.N., Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T.V., Masino S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Durante M., et al. Adenosine A3 agonists reverse neuropathic pain via T cell-mediated production of IL-10. J Clin Invest. 2021;131 doi: 10.1172/JCI139299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach J.C., et al. Dose response of intrathecal adenosine in experimental pain and allodynia. Anesthesiology. 2002;97:938–942. doi: 10.1097/00000542-200210000-00028. [DOI] [PubMed] [Google Scholar]
- Eisenach J.C., et al. Phase I safety assessment of intrathecal injection of an American formulation of adenosine in humans. Anesthesiology. 2002;96:24–28. doi: 10.1097/00000542-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Eisenach J.C., et al. Intrathecal but not intravenous opioids release adenosine from the spinal cord. J. Pain. 2004;5:64–68. doi: 10.1016/j.jpain.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Eisenach J.C., et al. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- Feoktistov I., Biaggioni I. Role of adenosine A2B receptors in inflammation. Adv. Pharmacol. 2011;61:115–144. doi: 10.1016/B978-0-12-385526-8.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S., et al. Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br. J. Pharmacol. 2010;160:443–453. doi: 10.1111/j.1476-5381.2010.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A., et al. Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J. Neurosci. 2015;35:6057–6067. doi: 10.1523/JNEUROSCI.4495-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R., et al. Potentiation of cannabinoid signaling in microglia by adenosine A2A receptor antagonists. Glia. 2019;67:2410–2423. doi: 10.1002/glia.23694. [DOI] [PubMed] [Google Scholar]
- Fredholm B.B., et al. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm B.B., et al. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Goldman N., et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes C., et al. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guieu R., et al. Adenosine and neuropathic pain. Pain. 1996;68:271–274. doi: 10.1016/s0304-3959(96)03214-9. [DOI] [PubMed] [Google Scholar]
- Habib A.S., et al. Phase 2, double-blind, placebo-controlled, dose-response trial of intravenous adenosine for perioperative analgesia. Anesthesiology. 2008;109:1085–1091. doi: 10.1097/ALN.0b013e31818db88c. [DOI] [PubMed] [Google Scholar]
- Hasko G., Cronstein B. Regulation of inflammation by adenosine. Front. Immunol. 2013;4:85. doi: 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Cronstein B.N. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hayashida M., et al. Clinical application of adenosine and ATP for pain control. J Anesth. 2005;19:225–235. doi: 10.1007/s00540-005-0310-8. [DOI] [PubMed] [Google Scholar]
- Hoffman A.F., et al. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J. Neurosci. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., et al. Adenosine A1 receptor agonists reduce hyperalgesia after spinal cord injury in rats. Spinal Cord. 2010;48:685–690. doi: 10.1038/sc.2009.194. [DOI] [PubMed] [Google Scholar]
- Hu X., et al. Sustained elevated adenosine via ADORA2B promotes chronic pain through neuro-immune interaction. Cell Reports. 2016;16:106–119. doi: 10.1016/j.celrep.2016.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey M.J., et al. Reduced response to the formalin test and lowered spinal NMDA glutamate receptor binding in adenosine A2A receptor knockout mice. Pain. 2007;129:287–294. doi: 10.1016/j.pain.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Hussey M.J., et al. Genetic deletion of the adenosine A(2A) receptor in mice reduces the changes in spinal cord NMDA receptor binding and glucose uptake caused by a nociceptive stimulus. Neurosci. Lett. 2010;479:297–301. doi: 10.1016/j.neulet.2010.05.084. [DOI] [PubMed] [Google Scholar]
- Hwang J.H., et al. Morphine can enhance the antiallodynic effect of intrathecal R-PIA in rats with nerve ligation injury. Anesth. Analg. 2005;100:461–468. doi: 10.1213/01.ANE.0000143561.68417.70. [DOI] [PubMed] [Google Scholar]
- Imlach W.L., et al. A positive allosteric modulator of the adenosine A1 receptor selectively inhibits primary afferent synaptic transmission in a neuropathic pain model. Mol. Pharmacol. 2015;88:460–468. doi: 10.1124/mol.115.099499. [DOI] [PubMed] [Google Scholar]
- Jacobson K.A., et al. Historical and current adenosine receptor agonists in preclinical and clinical development. Front. Cell. Neurosci. 2019;13:124. doi: 10.3389/fncel.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes K., et al. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155:2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes K., et al. Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics. Br. J. Pharmacol. 2016;173:1253–1267. doi: 10.1111/bph.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Mi W. Adenosine for postoperative analgesia: a systematic review and meta-analysis. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0173518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H.W., et al. Downregulation of adenosine and adenosine A1 receptor contributes to neuropathic pain in resiniferatoxin neuropathy. Pain. 2018;159:1580–1591. doi: 10.1097/j.pain.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsten R., et al. A neurotoxicologic evaluation of the spinal cord after chronic intrathecal injection of R-phenylisopropyl adenosine (R-PIA) in the rat. Anesth. Analg. 1993;77:731–736. doi: 10.1213/00000539-199310000-00013. [DOI] [PubMed] [Google Scholar]
- Katz N.K., et al. Central or peripheral delivery of an adenosine A1 receptor agonist improves mechanical allodynia in a mouse model of painful diabetic neuropathy. Neuroscience. 2015;285:312–323. doi: 10.1016/j.neuroscience.2014.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger M., et al. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell. Signal. 2002;14:99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- Klotz K.-N. Adenosine receptors and their ligands. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- Kolachala V., et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br. J. Pharmacol. 2008;155:127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz A.J., et al. Sustained reversal of central neuropathic pain induced by a single intrathecal injection of adenosine A2A receptor agonists. Brain Behav. Immun. 2018;69:470–479. doi: 10.1016/j.bbi.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Leduc-Pessah H., et al. Spinal A3 adenosine receptor activation acutely restores morphine antinociception in opioid tolerant male rats. J. Neurosci. Res. 2021 doi: 10.1002/jnr.24869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.C., et al. Characterization of the rat A2A adenosine receptor gene: a 4.8-kb promoter-proximal DNA fragment confers selective expression in the central nervous system. Eur. J. Neurosci. 2003;18:1786–1796. doi: 10.1046/j.1460-9568.2003.02907.x. [DOI] [PubMed] [Google Scholar]
- Li L., et al. Peripheral adenosine A2A receptors are involved in carrageenan-induced mechanical hyperalgesia in mice. Neuroscience. 2010;170:923–928. doi: 10.1016/j.neuroscience.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Li X., et al. Repeated dosing with oral allosteric modulator of adenosine A1 receptor produces tolerance in rats with neuropathic pain. Anesthesiology. 2004;100:956–961. doi: 10.1097/00000542-200404000-00028. [DOI] [PubMed] [Google Scholar]
- Li X., et al. Allosteric adenosine receptor modulation reduces hypersensitivity following peripheral inflammation by a central mechanism. J. Pharmacol. Exp. Ther. 2003;305:950–955. doi: 10.1124/jpet.102.047951. [DOI] [PubMed] [Google Scholar]
- Lindberg D., et al. Purinergic signaling and energy homeostasis in psychiatric disorders. Curr. Mol. Med. 2015;15:275–295. doi: 10.2174/1566524015666150330163724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J.W., et al. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain. 2014;138:28–35. doi: 10.1093/brain/awu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram L.C., et al. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J. Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram L.C., et al. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav. Immun. 2013;33:112–122. doi: 10.1016/j.bbi.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarini E., et al. Acute visceral pain relief mediated by A3AR agonists in rats: involvement of N-type voltage-gated calcium channels. Pain. 2020;161:2179–2190. doi: 10.1097/j.pain.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo L., Salvemini D. Targeting metabotropic adenosine receptors for neuropathic pain: Focus on A2A. Brain Behav. Immun. 2018;69:60–61. doi: 10.1016/j.bbi.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Mahan L.C., et al. Cloning and expression of an A1 adenosine receptor from rat brain. Mol. Pharmacol. 1991;40:1–7. [PubMed] [Google Scholar]
- Martin C., et al. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit. Care Med. 2000;28:3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- Martire A., et al. Pre-synaptic adenosine A2A receptors control cannabinoid CB1 receptor-mediated inhibition of striatal glutamatergic neurotransmission. J. Neurochem. 2011;116:273–280. doi: 10.1111/j.1471-4159.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Mecha M., et al. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol. Dis. 2013;59:141–150. doi: 10.1016/j.nbd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Meng F., et al. Cloning and expression of the A2a adenosine receptor from guinea pig brain. Neurochem. Res. 1994;19:613–621. doi: 10.1007/BF00971338. [DOI] [PubMed] [Google Scholar]
- Moreno E., et al. Singular Location and Signaling Profile of Adenosine A2A-Cannabinoid CB1 Receptor Heteromers in the Dorsal Striatum. Neuropsychopharmacology. 2018;43:964–977. doi: 10.1038/npp.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth Z.H., et al. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochem. Biophys. Res. Commun. 2003;312:883–888. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Pan H.L., et al. Allosteric adenosine modulation to reduce allodynia. Anesthesiology. 2001;95:416–420. doi: 10.1097/00000542-200108000-00025. [DOI] [PubMed] [Google Scholar]
- Peters M.F., Scott C.W. Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J. Biomol. Screen. 2009;14:246–255. doi: 10.1177/1087057108330115. [DOI] [PubMed] [Google Scholar]
- Post C. Antinociceptive effects in mice after intrathecal injection of 5'-N-ethylcarboxamide adenosine. Neurosci. Lett. 1984;51:325–330. doi: 10.1016/0304-3940(84)90397-5. [DOI] [PubMed] [Google Scholar]
- Rane K., et al. Intrathecal adenosine administration: a phase 1 clinical safety study in healthy volunteers, with additional evaluation of its influence on sensory thresholds and experimental pain. Anesthesiology. 1998;89:1108–1115. doi: 10.1097/00000542-199811000-00010. discussion 9A. [DOI] [PubMed] [Google Scholar]
- Rane K., et al. Intrathecal adenosine administration in abdominal hysterectomy lacks analgesic effect. Acta Anaesthesiol. Scand. 2000;44:868–872. doi: 10.1034/j.1399-6576.2000.440714.x. [DOI] [PubMed] [Google Scholar]
- Ravani A., et al. Role and function of A2A and A(3) adenosine receptors in patients with ankylosing spondylitis, psoriatic arthritis and rheumatoid arthritis. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi F.G., Firestein G.S. cDNA cloning and sequence analysis of the human A3 adenosine receptor. Biochim. Biophys. Acta. 1993;1179:105–107. doi: 10.1016/0167-4889(93)90077-3. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338:1–18. doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Sawynok J., et al. Adenosine release may mediate spinal analgesia by morphine. Trends Pharmacol. Sci. 1989;10:186–189. doi: 10.1016/0165-6147(89)90235-6. [DOI] [PubMed] [Google Scholar]
- Schulte G., Fredholm B.B. Signaling pathway from the human adenosine A<sub>3</sub>receptor expressed in chinese hamster ovary cells to the extracellular signal-regulated kinase 1/2. Mol. Pharmacol. 2002;62:1137. doi: 10.1124/mol.62.5.1137. [DOI] [PubMed] [Google Scholar]
- Schulte G., et al. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience. 2003;121:907–916. doi: 10.1016/s0306-4522(03)00480-9. [DOI] [PubMed] [Google Scholar]
- Segerdahl M., et al. Peroperative adenosine infusion reduces the requirements for isoflurane and postoperative analgesics. Anesth. Analg. 1995;80:1145–1149. doi: 10.1097/00000539-199506000-00013. [DOI] [PubMed] [Google Scholar]
- Segerdahl M., et al. Systemic adenosine attenuates touch evoked allodynia induced by mustard oil in humans. NeuroReport. 1995;6:753–756. doi: 10.1097/00001756-199503270-00012. [DOI] [PubMed] [Google Scholar]
- Segerdahl M., et al. The influence of adenosine, ketamine, and morphine on experimentally induced ischemic pain in healthy volunteers. Anesth. Analg. 1994;79:787–791. doi: 10.1213/00000539-199410000-00029. [DOI] [PubMed] [Google Scholar]
- Segerdahl M., et al. Antinociceptive effect of perioperative adenosine infusion in abdominal hysterectomy. Acta Anaesthesiol. Scand. 1997;41:473–479. doi: 10.1111/j.1399-6576.1997.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Segerdahl M., et al. Peroperative adenosine infusion reduces isoflurane concentrations during general anesthesia for shoulder surgery. Acta Anaesthesiol. Scand. 1996;40:792–797. doi: 10.1111/j.1399-6576.1996.tb04534.x. [DOI] [PubMed] [Google Scholar]
- Shang P., et al. Emerging nondopaminergic medications for parkinson's disease: focusing on A2A receptor antagonists and GLP1 receptor agonists. J Mov Disord. 2021;14:193–203. doi: 10.14802/jmd.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., et al. Efficacy of intrathecal adenosine for postoperative pain relief. Eur. J. Anaesthesiol. 2006;23:449–453. doi: 10.1017/S0265021506000342. [DOI] [PubMed] [Google Scholar]
- Shaw S., et al. Adenosine receptor signalling: Probing the potential pathways for the ministration of neuropathic pain. Eur. J. Pharmacol. 2020;889 doi: 10.1016/j.ejphar.2020.173619. [DOI] [PubMed] [Google Scholar]
- Silverman M.H., et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J. Rheumatol. 2008;35:41–48. [PubMed] [Google Scholar]
- Sjolund K.F., et al. Intrathecal administration of the adenosine A1 receptor agonist R-phenylisopropyl adenosine reduces presumed pain behaviour in a rat model of central pain. Neurosci. Lett. 1998;243:89–92. doi: 10.1016/s0304-3940(98)00092-5. [DOI] [PubMed] [Google Scholar]
- Sohn C.I., et al. Adenosine receptor agonists modulate visceral hyperalgesia in the rat. Gut Liver. 2008;2:39–46. doi: 10.5009/gnl.2008.2.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollevi A., et al. Systemic adenosine infusion: a new treatment modality to alleviate neuropathic pain. Pain. 1995;61:155–158. doi: 10.1016/0304-3959(94)00187-J. [DOI] [PubMed] [Google Scholar]
- Sosnowski M., et al. Assessment of the role of A1/A2 adenosine receptors mediating the purine antinociception, motor and autonomic function in the rat spinal cord. J. Pharmacol. Exp. Ther. 1989;250:915–922. [PubMed] [Google Scholar]
- Sottofattori E., et al. HPLC determination of adenosine in human synovial fluid. J. Pharm. Biomed. Anal. 2001;24:1143–1146. doi: 10.1016/s0731-7085(00)00574-4. [DOI] [PubMed] [Google Scholar]
- Starowicz K., Finn D.P. Cannabinoids and pain: sites and mechanisms of action. Adv. Pharmacol. 2017;80:437–475. doi: 10.1016/bs.apha.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Stehle J.H., et al. Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol. Endocrinol. 1992;6:384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- Sumida T., et al. Spinal R-phenyl-isopropyl adenosine inhibits spinal dorsal horn neurons responding to noxious heat stimulation in the absence and presence of sensitization. Pain. 1998;74:307–313. doi: 10.1016/s0304-3959(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Sweeney M.I., et al. Involvement of adenosine in the spinal antinociceptive effects of morphine and noradrenaline. J. Pharmacol. Exp. Ther. 1987;243:657–665. [PubMed] [Google Scholar]
- Sweeney M.I., et al. Morphine, capsaicin and K+ release purines from capsaicin-sensitive primary afferent nerve terminals in the spinal cord. J. Pharmacol. Exp. Ther. 1989;248:447–454. [PubMed] [Google Scholar]
- Takano T., et al. Traditional acupuncture triggers a local increase in adenosine in human subjects. J. Pain. 2012;13:1215–1223. doi: 10.1016/j.jpain.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano M.T., et al. Adenosine A(2A)-cannabinoid CB(1) receptor interaction: an integrative mechanism in striatal glutamatergic neurotransmission. Brain Res. 2012;1476:108–118. doi: 10.1016/j.brainres.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Valant C., et al. Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol. Pharmacol. 2010;78:444–455. doi: 10.1124/mol.110.064568. [DOI] [PubMed] [Google Scholar]
- Varano F., et al. Design, synthesis, and pharmacological characterization of 2-(2-furanyl)thiazolo[5,4-d]pyrimidine-5,7-diamine derivatives: new highly potent A2A adenosine receptor inverse agonists with antinociceptive activity. J. Med. Chem. 2016;59:10564–10576. doi: 10.1021/acs.jmedchem.6b01068. [DOI] [PubMed] [Google Scholar]
- Vincenzi F., et al. Targeting adenosine receptors: a potential pharmacological avenue for acute and chronic pain. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21228710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne M., et al. Marked enhancement of anti-allodynic effect by combined intrathecal administration of the adenosine A1-receptor agonist R-phenylisopropyladenosine and morphine in a rat model of central pain. Acta Anaesthesiol. Scand. 2000;44:665–671. doi: 10.1034/j.1399-6576.2000.440606.x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhou F.M. cAMP-producing chemogenetic and adenosine A2a receptor activation inhibits the inwardly rectifying potassium current in striatal projection neurons. Neuropharmacology. 2019;148:229–243. doi: 10.1016/j.neuropharm.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Wu W.P., et al. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Yabuuchi K., et al. Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2a receptor signaling in the neostriatum. Neuroscience. 2006;141:19–25. doi: 10.1016/j.neuroscience.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Yamaoka G., et al. Different analgesic effects of adenosine between postoperative and neuropathic pain. J. Orthop. Sci. 2013;18:130–136. doi: 10.1007/s00776-012-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., et al. Role of A3 adenosine receptor in diabetic neuropathy. J. Neurosci. Res. 2016;94:936–946. doi: 10.1002/jnr.23774. [DOI] [PubMed] [Google Scholar]
- Yoon M.H., et al. Roles of adenosine receptor subtypes in the antinociceptive effect of intrathecal adenosine in a rat formalin test. Pharmacology. 2006;78:21–26. doi: 10.1159/000094762. [DOI] [PubMed] [Google Scholar]
- Zahn P.K., et al. Adenosine A1 but not A2a receptor agonist reduces hyperalgesia caused by a surgical incision in rats: a pertussis toxin-sensitive G protein-dependent process. Anesthesiology. 2007;107:797–806. doi: 10.1097/01.anes.0000286982.36342.3f. [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. Intrathecal morphine reduces allodynia after peripheral nerve injury in rats via activation of a spinal A1 adenosine receptor. Anesthesiology. 2005;102:416–420. doi: 10.1097/00000542-200502000-00027. [DOI] [PubMed] [Google Scholar]
- Zylka M.J. Needling adenosine receptors for pain relief. Nat. Neurosci. 2010;13:783–784. doi: 10.1038/nn0710-783. [DOI] [PubMed] [Google Scholar]
- Zylka M.J. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 2011;17:188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]