Abstract
Background
A European League Against Rheumatism (EULAR) taskforce was convened to develop recommendations for lifestyle behaviours in rheumatic and musculoskeletal diseases (RMDs). This paper reviews the literature on the effects of physical exercise and body weight on disease-specific outcomes of people with RMDs.
Methods
Three systematic reviews were conducted to summarise evidence related to exercise and weight in seven RMDs: osteoarthritis, rheumatoid arthritis, systemic lupus erythematosus, axial spondyloarthritis (axSpA), psoriatic arthritis, systemic sclerosis and gout. Systematic reviews and original studies were included if they assessed exercise or weight in one of the above RMDs, and reported results regarding disease-specific outcomes (eg, pain, function, joint damage). Systematic reviews were only included if published between 2013–2018. Search strategies were implemented in the Medline, Embase, Cochrane Library of systematic reviews and CENTRAL databases.
Results
236 articles on exercise and 181 articles on weight were included. Exercise interventions resulted in improvements in outcomes such as pain and function across all the RMDs, although the size of the effect varied by RMD and intervention. Disease activity was not influenced by exercise, other than in axSpA. Increased body weight was associated with worse outcomes for the majority of RMDs and outcomes assessed. In general, study quality was moderate for the literature on exercise and body weight in RMDs, although there was large heterogeneity between studies.
Conclusion
The current literature supports recommending exercise and the maintenance of a healthy body weight for people with RMDs.
Keywords: epidemiology, arthritis, patient reported outcome measures, physical therapy modalities
Key messages.
What is already known about this subject?
Body weight and physical exercise are important for health.
It is unclear whether body weight or change in body weight and exercise influence outcomes in rheumatic and musculoskeletal diseases (RMDs).
What does this study add?
This study summarises the literature on the association between exercise and weight with disease outcomes of seven RMDs, concluding that performing exercise and a healthy weight are associated with better outcomes in people with RMDs.
How might this impact on clinical practice or further developments?
People with RMDs should be encouraged to perform exercise if they do not currently perform exercise, or maintain exercise if performing sufficient quantities.
People with RMDs should be encouraged and supported to attain and maintain a healthy body weight.
Introduction
Rheumatic and musculoskeletal diseases (RMDs) comprise a wide range of conditions characterised by pain, disability and poorer quality of life (QoL).1–3 Globally, these conditions comprise a significant burden which is continuing to increase. For instance, the Global Burden of Disease study reported that the percentage increase of disability adjusted life years driven by RMDs (other than lower back pain) was 128.9% (95% CI 122.0% to 136.3%) between 1990 and 2019 across all age groups.4 While some RMDs have many effective pharmacological treatments (eg, rheumatoid arthritis (RA)5), for some the treatment options are limited (eg, systemic lupus erythematosus (SLE)6) and for others there are no effective disease modifying treatments (eg, osteoarthritis (OA)7). However, there is room for additional improvements in outcomes in all RMDs, potentially through modification of lifestyle behaviour.
Physical activity (including exercise)8 is clearly beneficial for health, regardless of the presence of chronic diseases such as RMDs. The WHO and American institutions recommend that all adults aged 18–65 years should participate in at least 150 min per week of moderate-intensity aerobic physical activity, or do at least 75 min of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity activity (moderate intensity=3.0 to <6.0 METs (metabolic equivalent of task), vigorous intensity =≥6 METs, where 1 MET is the rate of energy expenditure at rest). Additionally, all adults aged 18–65 years should perform muscle-strengthening activities (eg, resistance training and weight lifting) involving major muscle groups on two or more days a week.9 10 In 2018, the European League Against Rheumatism (EULAR) recommended physical activity for people with inflammatory arthritis and OA,11 after a systematic review illustrating the benefits of exercise on strength, flexibility and cardiovascular fitness.12 Furthermore, exercise is closely linked to body weight. The prevalence of global obesity is increasing and obesity, as well as physical inactivity, is associated with poor health, comorbidity (eg, cardiovascular disease,13 diabetes,14) and increased risk of mortality.13 Therefore, there is an urgent need for strategies to ameliorate the obesity epidemic for the benefit of global health.
However, it is unclear whether exercise is effective at improving RMD-specific outcomes (eg, pain, disability), or whether excess weight is associated with worse RMD outcomes. Therefore, a EULAR taskforce was convened in 2018 to develop recommendations for lifestyle improvements in RMDs, with the focus on lifestyles that affect disease progression. The taskforce decided to focus on six lifestyle factors: diet, exercise, body weight, alcohol, smoking and (paid) work-participation, in seven diseases: RA, OA, SLE, axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), systemic sclerosis (SSc) and gout (henceforth referred to collectively as RMDs). For each of these lifestyle factors, systematic reviews were performed, aiming to collate all relevant literature on each factor in order to formulate evidence-based recommendations. This article presents the results of the systematic reviews assessing the impact of exercise and body weight on the disease-specific outcomes of people with RMDs.
Methods
Design
These reviews were conducted following EULAR’s standard operating procedure for EULAR endorsed recommendations15 and are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.16
Search strategy
A two-step process was used to identify studies to include in each review. Initially, a review of systematic reviews was conducted using the MEDLINE, EMBASE and Cochrane Library databases, aiming to identify existing systematic reviews on the included exposures and RMD progression (online supplemental tables 1 and 2, defined a priori by the study team) that were published from 1 January 2013 to 18 September 2018. Two reviewers screened the titles and abstracts (JMG and MW) and then a team of four reviewers screened the eligible full texts (JMG, MW, JR-C and GC; each full text screened by two reviewers). Only existing systematic reviews relating to exercise and weight are presented here.
rmdopen-2021-002168supp001.pdf (7.5MB, pdf)
Following this, separate systematic reviews of original studies of exercise and weight in RMDs were conducted (from inception to search date). It was decided that there were sufficient numbers of published systematic reviews regarding exercise and OA and therefore OA was not included in the systematic review of original studies of exercise. Search strategies for each review were developed based on a predefined PICO strategy (participants, intervention/exposure, comparison, outcome) (online supplemental tables 3 and 4 for search strategies) and implemented in the MEDLINE, EMBASE and CENTRAL databases (dates when strategies were implemented: exercise: 18 March 2019; weight: 14 March 2019). Titles and abstracts, followed by full texts, were screened by two reviewers (exercise: JMG and GC; weight: JMG and SMMV).
Inclusion and exclusion criteria
Systematic reviews were included if they:
Included adults with an RMD (OA, RA, SLE, axSpA, PsA, SSc, gout).
Studied the relationship between exercise or weight and disease-specific outcomes (online supplemental table 5 for list of included outcomes).
Published in English, French, Spanish or Italian.
No restrictions were implemented regarding the included study designs of studies in systematic reviews.
Original studies were included if they:
Used a longitudinal study design (randomised controlled trials (RCTs), non-randomised trials, single-arm intervention studies, longitudinal observational studies)
Included adults with an RMD (RA, SLE, axSpA, PsA, SSc, gout (and OA for the weight review)).
Studied the relationship between exercise or weight and outcomes (online supplemental table 5 for list of included outcomes).
Published in English, French, Spanish or Italian.
Conference abstracts were excluded.
Risk of bias assessment
To assess the risk of bias of included systematic reviews and meta-analyses, the AMSTAR-2 tool was used.17 Each included review or meta-analysis was rated as critically low, low, moderate or high quality. For included RCTs, an abbreviated version of the Cochrane Risk of Bias tool was used,18 assessing four criteria: randomisation procedure, allocation concealment procedure, blinding of participants and blinding of assessors. Each aspect was rated as either low or high/unclear risk of bias. A machine learning algorithm was used to assist the process,19 which identifies passages of text from included manuscripts and assigns grades for each criteria. A reviewer (JMG) checked each of the algorithm’s grades and if there was disagreement, changed the grade. The QUIPS tool was used to assess the quality of observational studies across six domains: study population, attrition, exposure measurement, outcome measurement, confounding and statistical analysis.20
Synthesis of data
Data from articles were extracted into prespecified tables, including study design, demographics, and results of outcomes and follow-up. The data from the individual articles are presented in the form of a narrative summary. Where possible, the mean and SD were extracted. SDs were estimated from 95% CIs or SEs when not reported. Means and SDs were estimated from medians and range/IQR when only these summary statistics were presented using published formulas.21 Furthermore, standardised mean differences (SMD) were calculated for individual studies as this allows results measured on different instruments to be compared and combined (SMDs provided in online supplemental materials). The SMD was estimated as the difference between the scores of the intervention and control group at follow-up divided by the pooled SD. Meta-analysis was used to combine the results of RCTs where possible. An SMD ≥0.2 was considered a small effect, ≥0.5 as a medium-sized effect and ≥0.8 as a large effect.22 Heterogeneity was quantified using the I2 statistic. All statistical analyses were performed using Stata V.14 (StataCorp).
Grades of Recommendations, Assessment, Development and Evaluation (GRADE) defines high-quality evidence as evidence where further research is very unlikely to change our confidence in the estimate of effect.23 Therefore, evidence was rated as high quality if supported by meta-analyses of ≥5 RCTs at low-moderate risk of bias reporting consistent results without important limitations.24 GRADE defines moderate quality evidence as evidence where further research is likely to have an important impact on the confidence of the estimate of effect, or may change the estimate.23 Evidence was rated as moderate if supported by meta-analyses of ≥3 RCTs or supported by a single RCT with a sample size ≥100 and at low-moderate risk of bias or multiple large observational studies. GRADE defines low quality evidence as evidence where further research is very likely to have an important influence on our confidence in the estimates, or likely to change the estimate.23 Evidence was rated as low if supported by multiple RCTs of small sample size or high risk of bias or by single observational studies only. GRADE defines very low quality of evidence as evidence where the estimate of the effect is very uncertain.23 Evidence was rated as very low if supported by single small RCTs, or non-randomised trials or single arm intervention studies. Evidence could be downgraded in the event of other potential biases (such as study limitations, inconsistency of results, imprecision, publication bias24 or conflicts of interest).
Results
Search strategy and study characteristics
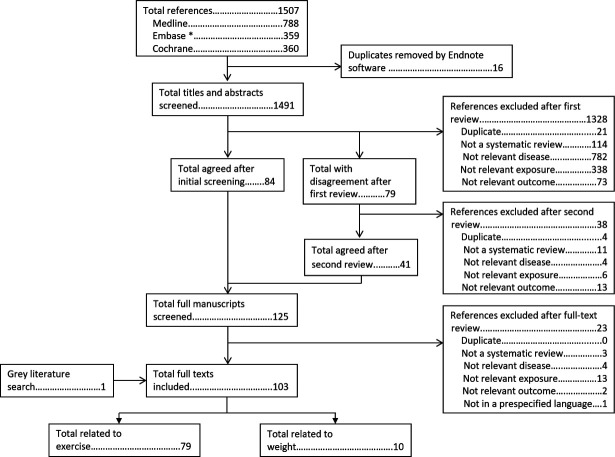
The search strategy to identify published systematic reviews identified 1507 abstracts, of which 16 were duplicates and were removed by reference managing software. After title and abstract screening, 1366 abstracts were excluded and the full manuscripts of the remaining 125 were screened. Seventy-nine of these assessed exercise and 10 assessed weight (figure 1).
Figure 1.
Flow chart of search strategy to identify published systematic reviews and meta-analyses. *EMBASE search excluded journals included in MEDLINE.
The search strategy to identify original articles assessing exercise in RMDs identified 4031 abstracts. After removal of 597 duplicates, 3434 titles and abstracts were screened. Of these 223 full manuscripts were screened, of which 157 are included in this review (figure 2). The search strategy to identify original articles assessing weight identified 3973 abstracts. Once duplicates were removed, 3625 abstracts were screened, followed by screening of 304 full manuscripts. In total, 171 studies on weight were included (figure 3). Results are summarised in tables 1 and 2, with additional information on demographics, specific interventions and control groups (typically usual care or wait-list control), and results of meta-analyses provided in online supplemental material.
Figure 2.
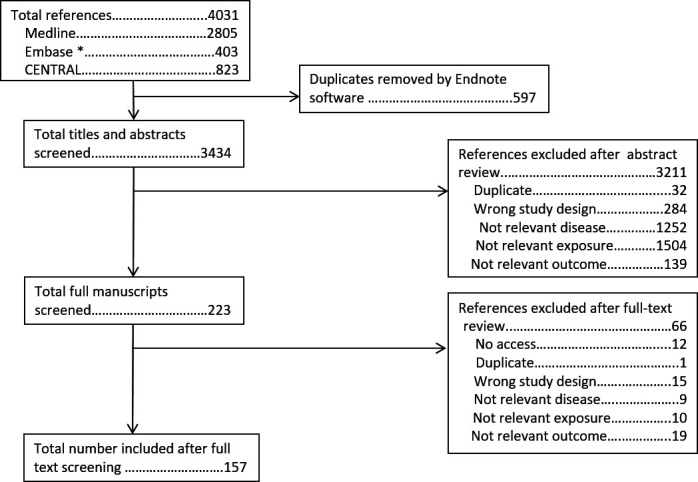
PRISMA flow chart for review of individual studies of exercise. *EMBASE search excluded journals included in MEDLINE. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Figure 3.
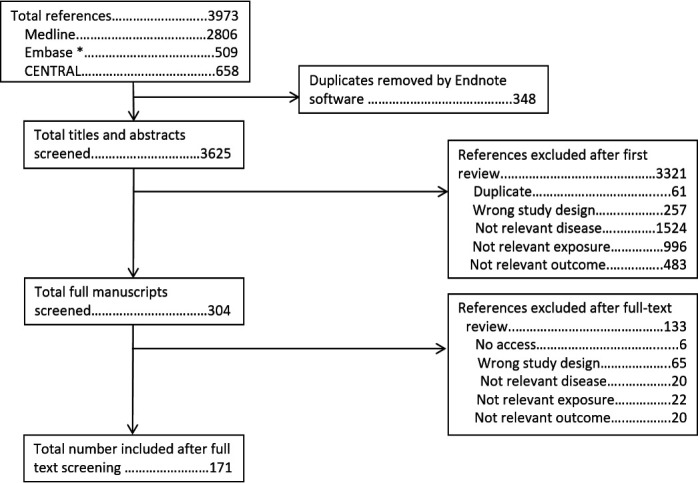
PRISMA flow chart for review of individual studies of weight. *EMBASE search excluded journals included in MEDLINE. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Summary table of results regarding exercise
| Level of evidence | Disease | Effect size | |||
| None | Small | Medium | Large | ||
| Very low |
OA | ||||
| RA | |||||
| axSpA | |||||
| Other |
SLE Muscle strengthening (disease activity) |
PsA Muscle strengthening (function, disease activity) SSc Muscle strengthening (function) |
|||
| Low |
OA | Yoga (function) | |||
| RA | Tai chi (pain, function, disease activity); Yoga (disease activity) |
Yoga (function) | |||
| axSpA | Aquatic (pain, function, disease activity) | Aerobic (pain, function) | |||
| Other |
SLE Aerobic (disease activity) SSc Aerobic + muscle strengthening (pain) |
SSc Aerobic + muscle strengthening (pain) |
|||
| Moderate |
OA | ||||
| RA | Aerobic (disease activity); Aerobic + muscle strengthening (disease activity) Muscle strengthening (disease activity) |
Aerobic + muscle strengthening (pain) Aquatic (pain, function) Muscle strengthening (pain, function) |
|||
| axSpA | Muscle strengthening (pain, function, disease activity) | ||||
| Other |
SLE Aerobic (fatigue) |
||||
| High |
OA | Aquatic (pain, function, HR-QoL); Exercise therapy (pain, function); Muscle strengthening (pain, function) Tai chi (stiffness, HR-QoL) |
Aerobic (pain, function); Land-based (pain, function) Tai chi (pain, function) |
||
| RA | Aerobic (pain, function); Aerobic + muscle strengthening (function) |
||||
| axSpA | Aerobic + muscle strengthening (pain, function, disease activity) | ||||
| Other | |||||
axSpA, axial spondyloarthritis; HR-QoL, health-related quality of life; OA, osteoarthritis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Table 2.
Summary table of results from observational studies within the weight systematic review
| Outcome | RMD | ||||||
| OA | RA | SLE | axSpA | PsA | SSc | Gout | |
| Pain | ✔✔✔ | ✔✔ | – | ✔ | ✔ | – | – |
| Function | ❌ | ✔✔✔ | ✔ | – | ✔ | – | ❌ |
| Radiographic progression | ❌ | ✔✔† | – | ✔ | – | – | – |
| Disease activity | – | ✔✔✔ | ❌ | ✔✔ | ✔✔ | – | ✔ (serum uric acid, gout attacks) |
| Fatigue | – | ✔ | ❌ | ✔ | – | – | – |
| Comorbidities | – | ✔✔ | ✔✔ | ✔ | – | – | – |
| Other | – | – | – | – | ✔(Enthesitis) ✔(Psoriasis score) |
– | – |
| Mortality | – | ✔✔✔* | – | – | – | ✔* | – |
Level of evidence of an association between weight and outcome: ✔=very low quality, ✔✔=low quality, ✔✔✔=moderate quality, ✔✔✔✔=high quality, ❌=No evidence of association between weight and outcome from included observational studies, –=No information from included observational studies
This table refers to results where increasing weight is associated with worse scores on outcome measures or higher risk of poor outcomes, other than where noted as follows
*Higher weight associated with lower risk of mortality.
†Higher weight associated with lower radiographic progression in RA.
axSpA, axial spondyloarthritis; OA, osteoarthritis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RMD, rheumatic and musculoskeletal diseases; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Exercise
Osteoarthritis
Aerobic exercise
Four meta-analyses of RCTs,25–28 four systematic reviews of RCTs29–32 and three systematic reviews of observational studies33–35 assessed aerobic exercise for OA. Aerobic exercise improved pain25–27 30–33 and function25 29–32 34 with small-medium effect sizes (pain SMD ranges −0.24 (95% CI −0.50 to 0.02)30 to -0.61 (95% CI −0.75 to –0.48)25; function −0.30 (95% CI −1.53 to 0.92)28 to −0.58 (95% CI −0.75 to –0.40)25) as well as one study reporting an association with health-related QoL (HR-QoL).31 Another review reported no association with radiographic damage35 (online supplemental tables 6 and 7).
Aquatic exercise
Five meta-analyses of RCTs28 36–39 and three systematic reviews of RCTs7 32 40 studied aquatic exercise for OA. Small effects on pain7 28 32 36 37 39 (SMDs ranging −0.26 (95% CI −0.41 to –0.11)39 to −1.16 (95% CI -3.03 to 0.71)38) function7 32 37–39 (SMDs ranging −0.22 (95% CI −0.38 to –0.07)39 to −0.55 (95% CI −0.94 to –0.16)38) and HR-QoL37–39 (SMDs ranging −0.21 (95% CI −0.59 to 0.18)38 to −0.25 (95% CI −0.49 to –0.01)37)were reported (online supplemental tables 8 and 9).
Guidelines / recommendations
Two systematic reviews41 42 summarised recent guidelines and evidence for OA and exercise, both concluding that exercise is strongly recommended for OA. A third paper43 contained a literature review supporting EULAR recommendations for the non-pharmacological management of OA, also recommending that patients with OA have regular, individualised exercise regimens (online supplemental tables 10 and 11).
High vs low intensity exercise
One Cochrane review44 reported that high intensity exercise was more beneficial than low intensity exercise for pain (meta-MD in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scale −0.84 (95% CI −1.63 to –0.04)) and function (meta-MD WOMAC function −2.65 (95% CI −5.29 to –0.01)) in OA. A systematic review45 identified one prospective study46 that reported no exercise or low intensity exercise was associated with deterioration in physical function (online supplemental tables 12 and 13).
Home exercise
One meta-analysis47 reported that home exercise interventions were superior to no exercise in terms of pain (SMD −0.46 (95% CI −0.68 to –0.24)) and function (SMD −0.35 (95% CI −0.56 to –0.15)) in OA, but home exercise programmes were inferior to supervised exercise (online supplemental tables 14 and 15).
Land-based exercise
Several articles assessed the efficacy of any type of land-based exercise (as opposed to aquatic) for OA: five meta-analyses of RCTs,36 48–51 one systematic review of meta-analyses, reviews and RCTs7 and one systematic review of RCTs.40 The meta-analyses all reported small effects in favour of land based exercise in terms of pain (SMDs ranging from −0.24 (95% CI −0.42 to –0.06)48 to −0.49 (95% CI −0.59 to –0.39)51) and function (SMDs ranging from −0.34 (95% CI −0.50 to –0.18)48 to -0.52 (95% CI −0.64 to –0.39)51), with the systematic reviews in agreement. One meta-analysis also reported an improvement in HR-QoL following exercise (SMD 0.28 (95% CI 0.15 to 0.40))51 (online supplemental tables 16 and 17).
Multidisciplinary interventions
One meta-analysis52 and one systematic review53 reported multimodal interventions were superior to exercise only for pain, function and HR-QoL (online supplemental tables 18 and 19).
Muscle strengthening exercise
Nine meta-analyses of RCTs,25–28 54–58 four systematic reviews of RCTs,29 59 60 and one review of meta-analyses, reviews and RCTs7 assessed muscle strengthening exercise for OA. Small26 55 56 58 to medium25 27 28 sized effects on pain7 59 60 (SMDs ranging from −0.23 (95% CI −0.42 to –0.04)55 to −1.19 (95% CI −1.67 to –0.70)57), as well as small28 56 to medium25 57 58 effects on function were reported7 59 60 (SMDs ranging from −0.10 (95% CI −0.33 to 0.13)55 to −0.60 (95% CI −0.83 to –0.37)25). One meta-analysis reported no effect of muscle strengthening exercise on HR-QoL for hand OA,56 whereas a systematic review reported improvements in HR-QoL in knee OA59 (online supplemental tables 20 and 21).
Physical exercise therapy
In total, five meta-analyses of RCTs,61–65 two systematic reviews of RCTs,60 66 and one systematic review of reviews and RCTs67 assessed exercise therapy for OA. Small effects for pain60 62–64 66 (SMDs ranging from −0.20 (95% CI −0.28 to –0.11)62 to -0.71 (95% CI −1.60 to 0.19)64) and function were reported60 62 63 65 66 (SMDs ranging from −0.07 (95% CI −0.28, 0.15)64 to −0.33 (95% CI −0.50 to –0.15)63), and no effect on anxiety (SMD −0.11 (95% CI −0.26 to 0.05)) and depression (SMD −0.16 (95% CI −0.29 to –0.02)).62 One meta-analysis reported a medium-sized effect on HR-QoL in knee OA (SMD 0.70 (95% CI 0.20 to 1.20)),61 but another reported no effect in hip OA (SMD −0.06 (95% CI −0.27 to 0.16))63 (online supplemental tables 22 and 23).
Tai chi
Four meta-analyses of RCTs26 68–70 and one systematic review of RCTs71 assessed tai chi for OA. Medium-sized effects were reported in terms of pain26 68–70 (SMDs ranged from −0.45 (95% CI −0.70 to –0.20)70 to −0.77 (95% CI −1.13 to –0.41)68) and function68–70 (SMDs ranged from −0.61 (95% CI −0.85 to –0.37)70 to −0.75 (95% CI −0.98 to –0.52)68), and small effects on stiffness68 70 and HR-QoL68 69 (online supplemental tables 24 and 25).
Yoga
One meta-analysis of RCTs,72 two systematic reviews of RCTs71 73 and one systematic review of RCTs and single arm interventions,74 assessed yoga in OA. Three systematic reviews reported benefits of yoga on pain and function.71 73 74 The meta-analysis reported a large effect of yoga on function (SMD −1.83 (95% CI −2.09 to –1.57)).72 One systematic review reported inconsistent findings in terms of the effect of yoga on HR-QoL74 (online supplemental tables 26 and 27).
OA summary
The majority of systematic reviews and meta-analyses reported medium-sized effects of physical activity on pain and function in OA, including both aerobic and muscle strengthening physical activity. The quality of the evidence was generally moderate to high (table 1). While the majority of studies on OA included people with knee or hip OA, several studies included people with hand OA.42 52 55 56 64 73
Rheumatoid arthritis
Aerobic exercise
One meta-analysis,75 three systematic reviews76–78 (which highlighted a 2010 meta-analysis,79) eight RCTs,80–87 four non-randomised studies,88–91 one case–control study92 and two prospective cohort studies93 94 assessing aerobic exercise in RA were included. Aerobic exercise improved pain76 77 79 80 82 84 87 (meta-analysis79: SMD 0.31 (95% CI 0.06 to 0.55)); and function76 79 80 84 (meta-analysis79: SMD 0.24 (95% CI 0.10 to 0.38)) in RA with small effect sizes, although some studies reported statistically non-significant results. One meta-analysis and four included RCTs reported no effect of aerobic exercise on disease activity79 80 82 83 85 (meta-analysis79: SMD 0.08 (95% CI −0.08 to 0.25)), while another reported a weak correlation.78 Furthermore, studies reported a small effect on HR-QoL.79 81 82 84 Small effects were also reported on fatigue (meta-analysis75: SMD −0.31 (95% CI –0.55 to –0.06)), anxiety, depression and self-efficacy75 81 82 85 (online supplemental tables 28–31).
Aerobic + muscle strengthening exercise
Twenty-one reports of RCTs,95–115 one non-randomised trial,116 and seven single arm studies117–123 reported on interventions containing both aerobic and muscle strengthening elements in RA. Small effects were reported for pain96 99 101 106 110–112 and function,95–100 102 106 108–111 115 with wide heterogeneity between studies potentially due to differences in the interventions and follow-up lengths. No effect was observed for disease activity102 106 108 110 and depression.97 101 Two RCTs reported a small effect on HR-QoL, which was not statistically significant.98 102 One recent RCT reported a medium-sized effect on fatigue,96 with two older RCTs reporting no effect101 115 (online supplemental tables 32 and 33).
Aquatic exercise
Two systematic reviews,77 124 four RCTs87 125–127 and one non-randomised trial89 studied aquatic exercises for RA. Small-medium effects on pain87 124 126 and function77 124–127 were reported (online supplemental tables 34–36).
High vs low intensity
Three RCTs128–130 and one long-term extension131 were included, with none reporting significant differences between high and low intensity exercise on pain or function (online supplemental tables 37 and 38).
Home exercise
Two systematic reviews,77 132 and five RCTs101 133–136 studied home exercise programmes. The reviews concluded that home exercise was beneficial for reducing pain and function.77 132 In studies comparing home-based unsupervised physical activity to supervised physical activity, inconsistent results were reported in terms of pain101 134 135 and function134–136 potentially due to differences in the interventions between studies (online supplemental tables 39–41).
Muscle strengthening exercise
One meta-analysis of hand muscle strengthening exercises,137 three systematic reviews (one including a 2012 meta-analysis138)77 139 140 17 reports of RCTs and long-term extensions,128 131 141–155 1 non-randomised trial156 and 1 single-arm study157 assessed muscle strengthening exercise in RA. Small effects from muscle strengthening exercise were reported on pain77 137 138 140 141 143 144 153 (meta-analysis138: MD pain Visual Analogue Scale −4.13 (95% CI −10.97 to 2.71)). Inconsistent results were reported in terms of function, with several studies reporting improvements in function77 137 138 143 148 149 151 (meta-analysis138: MD Health Assessment Questionnaire −0.22 (95% CI −0.35 to –0.10)), whereas other RCTs reported no benefit,128 144 147 152 153 potentially due to differences in the comparison group where some were given low-intensity exercise or exercise advice. No effect was reported on disease activity128 140 143 145 147 and HR-QoL142 144 145 147 (online supplemental tables 42–44).
Tai chi
One systematic review (which identified a 2004 review158)77 one RCT,159 one non-randomised trial160 and two single-arm studies161 162 assessed tai chi for RA. The reviews and RCT reported no benefit in terms of pain,159 function,77 158 159 disease activity77 158 or depression159 (online supplemental tables 45–47).
Yoga
One meta-analysis,72 one systematic review,73 three RCTs163–165 and two non-randomised trials166 167 studied yoga for RA. The meta-analysis reported a large effect of yoga on pain (but included some patients with OA)72 as did one RCT,165 whereas two other RCTs reported no effect, and the systematic review graded the evidence as very low.73 163 164 The meta-analysis (only including studies of RA)72 and one RCT reported medium-sized effects on function,163 whereas another RCT reported no effect.164 No effect on disease activity was reported by two RCTs.163 164 Studies were generally small therefore potentially some findings are due to chance (online supplemental tables 48–50).
RA summary
There was moderate-high quality evidence of a small effect of physical activity on pain and function in RA. Physical activity did not affect disease activity (table 1).
Systemic lupus erythematosus
Aerobic exercise
Two meta-analyses,168 169 three systematic reviews,170–172 four RCTs173–176 and three non-randomised trials177–179 assessed aerobic exercise in SLE. One RCT reported lower pain,173 whereas another did not174 although both studies had low sample sizes. No effect of disease activity was reported.168 173 174 A medium-sized effect on fatigue was also reported (MD −0.61 [95% CI −1.19 to –0.02]168; MD −0.52 [95% CI −0.92 to –0.13]169)168 169 171 172 175 176 (online supplemental tables 51–53).
Aerobic & muscle strengthening exercise
One meta-analysis,168 one systematic review171 and three RCTs173 180 181 compared aerobic and muscle strengthening exercise, whereas one RCT182 and one non-randomised trial183 assessed interventions combining both aerobic and muscle strengthening exercises. Aerobic exercise was reported to lead to less fatigue compared with muscle strengthening in two studies,168 171 but two other RCTs reported no difference,180 181 although again sample sizes were small. No difference was reported in terms of disease activity168 173 181 or depression168 173 180 (online supplemental tables 54–56).
Muscle strengthening exercise
One RCT compared muscle strengthening exercise to control in SLE,173 reporting no effect on disease activity, pain and fatigue, but a medium-sized effect on depression in favour of muscle strengthening exercise (online supplemental tables 57 and 58).
SLE summary
There is moderate quality evidence of a medium-sized effect of aerobic exercise on fatigue in SLE. There is low-quality evidence that physical activity does not affect disease activity in SLE (table 1).
Axial spondyloarthritis
Aerobic exercise
Two systematic reviews,184 185 four RCTs,186–189 one single arm study190 and three prospective cohort studies191–193 assessed aerobic exercise in axSpA. One RCT investigating exercising with the aid of a video game console reported a medium-sized effect on pain, function and disease activity,186 whereas other studies of traditional exercise interventions reported no effect184 187–189 (online supplemental tables 59–62).
Aerobic + muscle strengthening exercise
Six meta-analyses,194–199 3 systematic reviews,184 185 200 10 RCTs,201–210 3 non-randomised trials,211–213 5 single arm studies214–218 and 1 prospective cohort study219 assessed aerobic + muscle strengthening exercise in axSpA. Small effects were reported for pain,185 196 197 204 209 210 but with some inconsistency200 207 208 (SMDs ranging from −0.22 (95% CI −0.49 to 0.06)197 to −0.42 (95% CI −0.74 to –0.09),196 whereas consistent medium-sized improvements in function184 194–199 201 203 205 207 210 (SMDs ranging from −0.44 (95% CI −0.79 to –0.09)199 to −0.72 (−1.03 to –0.40),194 and disease activity184 194–199 201 205 206 (SMDs ranging from −0.47 (95% CI −0.84 to –0.09)196 to -0.90 (−1.52 to –0.27)194 were reported. Medium-sized effects on fatigue201 202 and spinal mobility195 198 199 205 were also reported (online supplemental tables 63–66).
Aquatic exercise
Three systematic reviews184 200 220 and two RCTs221 222 assessing aquatic exercise for axSpA were included. Small, inconsistent effects were reported in terms of pain,184 220–222 function184 200 220–222 and disease activity184 220–222 and no effect on spinal mobility184 221 222 (online supplemental tables 67–69).
Home-based exercise
One meta-analysis,197 one systematic review,185 eight RCTs,186 189 203 207 210 223–225 five non-randomised trials212 226–229 and one single arm study217 assessed home-based exercise. Compared with control, home exercise had a medium-sized effect on pain,186 197 210 224 function,186 189 197 210 223–225 disease activity186 197 223 224 and HR-QoL.186 224 However, several studies found that group-based exercise was more effective than home-based exercise in terms of function203 207 and disease activity203 (online supplemental tables 70–72).
Muscle strengthening exercise
Eight RCTs (and two long-term follow-ups),223 225 230–237 three non-randomised trials226–228 and three single arm studies238–240 assessed muscle strengthening exercise. Medium-sized effects of muscle strengthening exercise were reported in terms of pain,225 231–233 235 function223 225 230 232–235 and disease activity223 230 232–235 (online supplemental tables 73 and 74).
AxSpA summary
There is high-quality evidence of a medium-sized effect of aerobic + muscle strengthening exercise in axSpA. Muscle strengthening exercise results in larger and more consistent effects on pain, function and disease activity compared with aerobic exercise (table 1).
Psoriatic arthritis
Muscle strengthening exercise
One RCT241 and one single arm study242 assessed muscle strengthening exercise in PsA. The RCT reported better function and disease activity at 12 weeks compared with the control arm241 (online supplemental tables 75 and 76).
PsA Summary
There is little evidence of the effect of physical activity in PsA.
Systemic sclerosis
Aerobic exercise
One single arm study243 reported improvements in function between baseline and 4 months after the intervention (online supplemental tables 77 and 78).
Aerobic + muscle strengthening exercise
One systematic review,244 two RCTs245 246 and one single arm study247 assessed aerobic plus muscle strengthening interventions for SSc, reporting a small effect on function245 246 but no effect on pain246 (online supplemental tables 79–81).
Aquatic exercise
One RCT,248 which only reported data on the intervention group, showed improvements in function from baseline to 18 weeks follow-up (online supplemental tables 82 and 83).
Muscle strengthening exercise
One RCT,249 one non-randomised trial250 and three single arm studies251–253 assessed muscle strengthening exercise in SSc. The RCT reported a medium-sized effect on function249 and the non-randomised study reported better outcomes in terms of pain and function in the exercise arm250 (online supplemental tables 84 and 85).
SSc summary
There is very-low-quality evidence of a medium effect of physical activity on function in SSc (table 1).
Gout
Aerobic exercise
One case–control study254 reported that performing regular exercise ≥150 min per week was associated with reduced odds of tophus in gout (online supplemental tables 86 and 87).
Yoga
One RCT compared yoga to blood-letting in gout,255 reporting benefits of yoga in terms of pain and serum uric acid (online supplemental tables 88 and 89).
Gout summary
There is little research on the effect of physical activity in gout.
Body weight and weight reduction
Osteoarthritis
Two meta-analyses,26 33 5 systematic reviews,32 34 35 43 45 21 reports describing RCTs and long-term follow-up studies,256–276 2 non-randomised trials277 278 and 6 single-arm intervention studies assessing weight-loss interventions,279–284 as well as 44 observational studies285–328 assessing the association between body weight and outcomes in OA were identified. From studies assessing weight-loss interventions, small effects on pain,26 32 43 265–267 270 275 function,259 265–268 270 272 273 stiffness256 266 273 and walking tests,260 265 272 276 but no effect on HR-QoL260 269 or patient global assessment260 269 were reported. In observational studies, higher body weight was associated with higher pain33 291 292 299 300 302 307 but not with worse function45 287 297 303 319 or radiographic progression35 294 301 306 (online supplemental tables 90–115).
Rheumatoid arthritis
Three meta-analyses329–331 and 61 observational studies92 332–391 assessed the relationship between body weight and outcomes in RA. Higher weight was associated with worse pain,329 354 361 362 380 function,329 336 344 362 disease activity,329 330 336 338 339 342 344 347 357 361–363 366 384 389 and fatigue,351 as well as more comorbidities.331 355 360 365 374 390 Higher baseline weight was associated with lower risk of death.335 367 378 379 However, high rates of weight loss were associated with increased mortality risk.335 340 356 381 Higher weight was associated with lower radiographic progression337 341 346 347 368 371 375 376 (online supplemental tables 116–132).
Systemic lupus erythematosus
Eight prospective studies were identified assessing the relationship between body weight and outcomes in SLE.392–399 Higher weight was associated with worse function,394 comorbidity,396 398 399 employment status394 and mental health,392 but no associations between weight and disease activity395 or fatigue395 were reported (online supplemental tables 133–139).
Axial spondyloarthritis
Thirteen observational studies were identified assessing the relationship between body weight and outcomes in axSpA.192 359 400–410 Higher weight was associated with worse pain,410 disease activity,405 410 fatigue,405 radiographic progression,401 403 comorbidity359 404 and lower odds of meeting response criteria.405 409 One unadjusted analysis reported people with higher weight were more likely to discontinue anti-tumour necrosis factor treatment,406 but another study adjusting for confounders reported no association405 (online supplemental tables 140–149).
Psoriatic arthritis
One meta-analysis,330 one RCT,411 one single-arm study412 and seven observational studies413–419 were identified assessing the relationship between weight and outcomes in PsA. Higher weight was associated with worse pain,415 function,415 disease activity,330 414–417 joint counts,415 enthesitis occurrence413 and psoriasis score.415 Interventional studies reported improvements in pain,411 412 function,411 412 patient global assessment,411 412 and reductions in disease activity411 412 and C-reactive protein411 412 following weight loss (online supplemental tables 150–170).
Systemic sclerosis
Two observational studies reported that higher weight was associated with a lower risk of mortality in SSc420 421 (online supplemental tables 171 and 172).
Gout
One systematic review,422 one single-arm study423 and five observational studies424–428 were identified assessing the relationship between weight and outcomes in gout. One observational study reported no association between weight and function.428 Weight loss was associated with improvements in serum uric acid level,422 423 425 and less frequent gout attacks.422–425 Higher weight was associated with renal failure in one study426 but not another428 (online supplemental tables 173–180).
Discussion
These three systematic reviews summarise the current evidence regarding the effects of physical activity and body weight on disease outcomes of people with seven RMDs. The data from over 400 published reviews and original articles suggests there are benefits of exercising for a range of outcomes important to people with RMDs, and also indicate an association between heavier body weight and worse outcomes, although differences were noted across RMDs. There was moderate-to-high-quality evidence that exercise interventions resulted in less pain and better function in RMDs, and exercise was also associated with reductions in disease activity in axSpA. The size of the effect of exercise varied between RMDs. For instance, there were medium-sized effects demonstrated in people with axSpA and only small effects in those with RA. There was moderate-quality evidence that heavier body weight was associated with poorer outcomes, including pain, function and disease activity, with relatively consistent results across the seven RMDs. However, the amount of available evidence varied considerably between RMDs, with the majority of studies focusing on OA, RA, axSpA and to a lesser extent SLE, with only a few studies available focusing on PsA, SSc and gout.
The majority of studies of exercise across the RMDs focused on aerobic and muscle strengthening exercise. Both appeared to improve outcomes in the seven RMDs included in this review, and when combined they seemed to produce stronger effects, although relatively few studies compared aerobic plus muscle strengthening exercise to either form of exercise individually.173 A few studies indicated that supervised exercise was marginally more effective than unsupervised home-based exercise,47 134 203 although this should not deter individuals who do not wish to attend exercise classes from performing home-based exercise. Overall, the current evidence base indicates that exercising results in better outcomes in people with RMDs, when compared with groups assigned to control groups with no exercise intervention. People with RMDs should be encouraged by their health professionals to start exercising if they currently perform no exercise, or maintain their exercise if they already perform a sufficient amount of exercise. Health professionals should also recognise that there are potentially large barriers to initiating and continuing to exercise for people with RMDs, including time, resources and lack of motivation, as well as disease specific barriers.429–431
Heavier body weight was consistently associated with poorer outcomes, including pain, function, disease activity, fatigue and comorbidities. The included studies evaluating weight-loss interventions typically reported improved outcomes in the weight-loss groups compared with controls.260 265 411 Additionally, multiple observational studies have reported an association between heavier baseline weight and worse disease outcomes. While these observational studies typically have longer follow-up compared with RCTs, causality between heavier body weight and worse outcomes is difficult to establish, given the complex interrelated associations between heavy weight and other potential sociodemographic and biological factors that could be influencing outcomes.432 433 Furthermore, the majority of research focused on OA and RA, with few research studies published on the other RMDs. Despite this, given the deleterious effect of heavier weight on other outcomes (eg, cardiovascular disease13 and type 2 diabetes14) and the association between weight and poor outcomes in RMDs, people with RMDs should be encouraged to attain and maintain a healthy weight (what consists a healthy weight will be person-specific and should be decided in consultation between the person with an RMD and their health professional).
This review has several limitations. While the large scope and high number of studies included means that a large proportion of the literature has been surveyed, due to the breadth of the research questions, some studies may have been missed. However, the extensive search strategies which were implemented in several databases should mean that these missing studies comprise a low proportion of the total evidence base. Due to the large number of studies, data extraction and risk of bias assessment were not performed in duplicate. Furthermore, due to the nature of the method of identifying studies, some selection bias could be influencing the results, particularly in the body weight systematic review. Many studies will assess multiple factors influencing progression of outcomes, and will often only report the significant associations in their abstracts. As our search strategy identified studies reporting that they studied the association between weight and outcomes in their abstracts, this potentially means we missed studies that assessed the association, found no relationship, and therefore did not report they had assessed weight in their abstract. The majority of studies on exercise were RCTs, with strict inclusion criteria, meaning the samples in these studies were often highly selected, thus reducing generalisability. The majority of studies on weight were longitudinal observational studies. While these studies typically had longer follow-up periods than the few interventional studies included, there is the possibility of differential attrition leading to biased results. For instance, those with higher weight may be more likely to have worse outcomes, which could lead them to leave the study before these outcomes are assessed. Lastly, as the evidence within this review was used to formulate the taskforce’s recommendations, an update was not deemed appropriate. However, this means that some relevant papers have been published in the interim between implementing the search strategy and publishing this paper which are not included.434 435
In conclusion, these reviews inform the 2021 EULAR recommendations which advise people with RMDs to perform sufficient amounts of exercise, given the beneficial effects of exercise on numerous outcomes. Furthermore, the 2021 EULAR recommendations also advise people with RMDs to maintain a healthy weight, given the association between heavier weight and poor outcomes, as detailed in this review.
Footnotes
Twitter: @james_gwinnutt
Contributors: Study concept and design: JMG, MW, JR-C, GC, FG and SMMV; Acquisition of data: JMG, MW, JR-C, GC and SMMV; Analysis and interpretation of data: JMG, MW, JR-C, ABa, HB-F, ABo, GC, SdS, AdT, TED, RHM, PP, LS-F, TAS, KW-B, JW, MZ-S, FG and SMMV; Review of manuscript: JMG, MW, JR-C, ABa, HB-F, ABo, GC, SdS, AdT, TED, RHM, PP, LS-F, TAS, KW-B, JW, MZ-S, FG and SMMV; Guarantor: SMMV.
Funding: This work was funded by the European League Against Rheumatism. SMMV and JMG are supported by Versus Arthritis (grant number 21755) and the NIHR Manchester Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. 10.1038/nrdp.2018.1 [DOI] [PubMed] [Google Scholar]
- 2.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers 2016;2:16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 3.Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. 10.1038/nrdp.2016.39 [DOI] [PubMed] [Google Scholar]
- 4.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JA, Christensen R, Wells GA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev 2009;4:CD007848. 10.1002/14651858.CD007848.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 7.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 8.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services . Physical activity guidelines for Americans. 2nd edn. Washington, DC, 2018. [Google Scholar]
- 10.World Health Organisation . Global recommendations on physical activity for health, 2010. [PubMed] [Google Scholar]
- 11.Rausch Osthoff A-K, Niedermann K, Braun J, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018;77:1251–60. 10.1136/annrheumdis-2018-213585 [DOI] [PubMed] [Google Scholar]
- 12.Rausch Osthoff A-K, Juhl CB, Knittle K, et al. Effects of exercise and physical activity promotion: meta-analysis Informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis. RMD Open 2018;4:e000713. 10.1136/rmdopen-2018-000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13., Afshin A, Forouzanfar MH, GBD 2015 Obesity Collaborators, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdullah A, Peeters A, de Courten M, et al. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 2010;89:309–19. 10.1016/j.diabres.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D, Aletaha D, Carmona L, et al. 2014 update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis 2015;74:8-13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soboczenski F, Trikalinos TA, Kuiper J, et al. Machine learning to help researchers evaluate biases in clinical trials: a prospective, randomized user study. BMC Med Inform Decis Mak 2019;19:96. 10.1186/s12911-019-0814-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280-6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Kunz R, et al. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336:995–8. 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol 2014;66:622–36. 10.1002/art.38290 [DOI] [PubMed] [Google Scholar]
- 26.Corbett MS, Rice SJC, Madurasinghe V, et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthritis Cartilage 2013;21:1290–8. 10.1016/j.joca.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2013;27:1059–71. 10.1177/0269215513488898 [DOI] [PubMed] [Google Scholar]
- 28.Uthman OA, van der Windt DA, Jordan JL, et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ 2013;347:f5555. 10.1136/bmj.f5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wijnen A, Bouma SE, Seeber GH, et al. The therapeutic validity and effectiveness of physiotherapeutic exercise following total hip arthroplasty for osteoarthritis: a systematic review. PLoS One 2018;13:e0194517. 10.1371/journal.pone.0194517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alrushud AS, Rushton AB, Kanavaki AM, et al. Effect of physical activity and dietary restriction interventions on weight loss and the musculoskeletal function of overweight and obese older adults with knee osteoarthritis: a systematic review and mixed method data synthesis. BMJ Open 2017;7:e014537. 10.1136/bmjopen-2016-014537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clin Rehabil 2017;31:612–24. 10.1177/0269215517691085 [DOI] [PubMed] [Google Scholar]
- 32.Quintrec J-LL, Verlhac B, Cadet C, et al. Physical exercise and weight loss for hip and knee osteoarthritis in very old patients: a systematic review of the literature. Open Rheumatol J 2014;8:89–95. 10.2174/1874312901408010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzobon D, Ferreira PH, Blyth FM, et al. Can obesity and physical activity predict outcomes of elective knee or hip surgery due to osteoarthritis? A meta-analysis of cohort studies. BMJ Open 2018;8:e017689. 10.1136/bmjopen-2017-017689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Rooij M, van der Leeden M, Heymans MW, et al. Prognosis of pain and physical functioning in patients with knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res 2016;68:481–92. 10.1002/acr.22693 [DOI] [PubMed] [Google Scholar]
- 35.Bastick AN, Belo JN, Runhaar J, et al. What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin Orthop Relat Res 2015;473:2969–89. 10.1007/s11999-015-4349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beumer L, Wong J, Warden SJ, et al. Effects of exercise and manual therapy on pain associated with hip osteoarthritis: a systematic review and meta-analysis. Br J Sports Med 2016;50:458–63. 10.1136/bjsports-2015-095255 [DOI] [PubMed] [Google Scholar]
- 37.Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev 2016;3:CD005523. 10.1002/14651858.CD005523.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu M, Su Y, Zhang Y, et al. Effectiveness of aquatic exercise for treatment of knee osteoarthritis: systematic review and meta-analysis. Z Rheumatol 2015;74:543–52. 10.1007/s00393-014-1559-9 [DOI] [PubMed] [Google Scholar]
- 39.Waller B, Ogonowska-Slodownik A, Vitor M, et al. Effect of therapeutic aquatic exercise on symptoms and function associated with lower limb osteoarthritis: systematic review with meta-analysis. Phys Ther 2014;94:1383–95. 10.2522/ptj.20130417 [DOI] [PubMed] [Google Scholar]
- 40.Romeo A, Parazza S, Boschi M, et al. Manual therapy and therapeutic exercise in the treatment of osteoarthritis of the hip: a systematic review. Reumatismo 2013;65:63–74. 10.4081/reumatismo.2013.63 [DOI] [PubMed] [Google Scholar]
- 41.Gay C, Chabaud A, Guilley E, et al. Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Ann Phys Rehabil Med 2016;59:174–83. 10.1016/j.rehab.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 42.Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum 2014;43:701–12. 10.1016/j.semarthrit.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 43.Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 44.Regnaux J-P, Lefevre-Colau M-M, Trinquart L, et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev 2015:CD010203. 10.1002/14651858.CD010203.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Rooij M, van der Leeden M, Heymans MW, et al. Course and predictors of pain and physical functioning in patients with hip osteoarthritis: systematic review and meta-analysis. J Rehabil Med 2016;48:245–52. 10.2340/16501977-2057 [DOI] [PubMed] [Google Scholar]
- 46.Juhakoski R, Malmivaara A, Lakka TA, et al. Determinants of pain and functioning in hip osteoarthritis - a two-year prospective study. Clin Rehabil 2013;27:281–7. 10.1177/0269215512453060 [DOI] [PubMed] [Google Scholar]
- 47.Anwer S, Alghadir A, Brismée J-M. Effect of home exercise program in patients with knee osteoarthritis: a systematic review and meta-analysis. J Geriatr Phys Ther 2016;39:38–48. 10.1519/JPT.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 48.Moseng T, Dagfinrud H, Smedslund G, et al. The importance of dose in land-based supervised exercise for people with hip osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 2017;25:1563–76. 10.1016/j.joca.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 49.Fernandopulle S, Perry M, Manlapaz D, et al. Effect of Land-Based generic physical activity interventions on pain, physical function, and physical performance in hip and knee osteoarthritis: a systematic review and meta-analysis. Am J Phys Med Rehabil 2017;96:773–92. 10.1097/PHM.0000000000000736 [DOI] [PubMed] [Google Scholar]
- 50.Henriksen M, Hansen JB, Klokker L, et al. Comparable effects of exercise and analgesics for pain secondary to knee osteoarthritis: a meta-analysis of trials included in Cochrane systematic reviews. J Comp Eff Res 2016;5:417–31. 10.2217/cer-2016-0007 [DOI] [PubMed] [Google Scholar]
- 51.Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2015;1:CD004376. 10.1002/14651858.CD004376.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aebischer B, Elsig S, Taeymans J. Effectiveness of physical and occupational therapy on pain, function and quality of life in patients with trapeziometacarpal osteoarthritis - A systematic review and meta-analysis. Hand Ther 2016;21:5–15. 10.1177/1758998315614037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finney A, Healey E, Jordan JL, et al. Multidisciplinary approaches to managing osteoarthritis in multiple joint sites: a systematic review. BMC Musculoskelet Disord 2016;17:266. 10.1186/s12891-016-1125-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartholdy C, Juhl C, Christensen R, et al. The role of muscle strengthening in exercise therapy for knee osteoarthritis: a systematic review and meta-regression analysis of randomized trials. Semin Arthritis Rheum 2017;47:9–21. 10.1016/j.semarthrit.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 55.Magni NE, McNair PJ, Rice DA. The effects of resistance training on muscle strength, joint pain, and hand function in individuals with hand osteoarthritis: a systematic review and meta-analysis. Arthritis Res Ther 2017;19:131. 10.1186/s13075-017-1348-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Østerås N, Kjeken I, Smedslund G, et al. Exercise for hand osteoarthritis. Cochrane Database Syst Rev 2017;1:CD010388. 10.1002/14651858.CD010388.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coudeyre E, Jegu AG, Giustanini M, et al. Isokinetic muscle strengthening for knee osteoarthritis: a systematic review of randomized controlled trials with meta-analysis. Ann Phys Rehabil Med 2016;59:207–15. 10.1016/j.rehab.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Su Y, Chen S, et al. The effects of resistance exercise in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 2016;30:947–59. 10.1177/0269215515610039 [DOI] [PubMed] [Google Scholar]
- 59.Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil 2017;31:596–611. 10.1177/0269215517691084 [DOI] [PubMed] [Google Scholar]
- 60.Brosseau L, Wells GA, Pugh AG, et al. Ottawa panel evidence-based clinical practice guidelines for therapeutic exercise in the management of hip osteoarthritis. Clin Rehabil 2016;30:935–46. 10.1177/0269215515606198 [DOI] [PubMed] [Google Scholar]
- 61.Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med 2018;52:1031–8. 10.1136/bjsports-2017-098099 [DOI] [PubMed] [Google Scholar]
- 62.Hurley M, Dickson K, Hallett R, et al. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev 2018;4:CD010842. 10.1002/14651858.CD010842.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sampath KK, Mani R, Miyamori T, et al. The effects of manual therapy or exercise therapy or both in people with hip osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 2016;30:1141–55. 10.1177/0269215515622670 [DOI] [PubMed] [Google Scholar]
- 64.Bertozzi L, Valdes K, Vanti C, et al. Investigation of the effect of conservative interventions in thumb carpometacarpal osteoarthritis: systematic review and meta-analysis. Disabil Rehabil 2015;37:2025–43. 10.3109/09638288.2014.996299 [DOI] [PubMed] [Google Scholar]
- 65.Desveaux L, Beauchamp M, Goldstein R, et al. Community-based exercise programs as a strategy to optimize function in chronic disease: a systematic review. Med Care 2014;52:216–26. 10.1097/MLR.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 66.Ferreira GE, Robinson CC, Wiebusch M, et al. The effect of exercise therapy on knee adduction moment in individuals with knee osteoarthritis: a systematic review. Clin Biomech 2015;30:521–7. 10.1016/j.clinbiomech.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 67.Fehring TK, Fehring K, Odum SM, et al. Physical therapy mandates by Medicare administrative contractors: effective or wasteful? J Arthroplasty 2013;28:1459–62. 10.1016/j.arth.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Huang L, Su Y, et al. The effects of traditional Chinese exercise in treating knee osteoarthritis: a systematic review and meta-analysis. PLoS One 2017;12:e0170237. 10.1371/journal.pone.0170237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y-W, Hunt MA, Campbell KL, et al. The effect of Tai Chi on four chronic conditions-cancer, osteoarthritis, heart failure and chronic obstructive pulmonary disease: a systematic review and meta-analyses. Br J Sports Med 2016;50:397–407. 10.1136/bjsports-2014-094388 [DOI] [PubMed] [Google Scholar]
- 70.Yan J-H, Gu W-J, Sun J, et al. Efficacy of Tai Chi on pain, stiffness and function in patients with osteoarthritis: a meta-analysis. PLoS One 2013;8:e61672. 10.1371/journal.pone.0061672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part one: introduction, and mind-body exercise programs. Clin Rehabil 2017;31:582–95. 10.1177/0269215517691083 [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Lu S, Wang R, et al. Integrative effect of yoga practice in patients with knee arthritis: a PRISMA-compliant meta-analysis. Medicine 2018;97:e11742. 10.1097/MD.0000000000011742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cramer H, Lauche R, Langhorst J, et al. Yoga for rheumatic diseases: a systematic review. Rheumatology 2013;52:2025–30. 10.1093/rheumatology/ket264 [DOI] [PubMed] [Google Scholar]
- 74.Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med 2016;2016:6016532. 10.1155/2016/6016532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rongen-van Dartel SAA, Repping-Wuts H, Flendrie M, et al. Effect of aerobic exercise training on fatigue in rheumatoid arthritis: a meta-analysis. Arthritis Care Res 2015;67:1054–62. 10.1002/acr.22561 [DOI] [PubMed] [Google Scholar]
- 76.Hernández-Hernández MV, Díaz-González F. Role of physical activity in the management and assessment of rheumatoid arthritis patients. Reumatol Clin 2017;13:214–20. 10.1016/j.reuma.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 77.Siegel P, Tencza M, Apodaca B, et al. Effectiveness of occupational therapy interventions for adults with rheumatoid arthritis: a systematic review. Am J Occup Ther 2017;71:7101180050. 10.5014/ajot.2017.023176 [DOI] [PubMed] [Google Scholar]
- 78.Larkin L, Kennedy N. Correlates of physical activity in adults with rheumatoid arthritis: a systematic review. J Phys Act Health 2014;11:1248–61. 10.1123/jpah.2012-0194 [DOI] [PubMed] [Google Scholar]
- 79.Baillet A, Zeboulon N, Gossec L, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care Res 2010;62:984–92. 10.1002/acr.20146 [DOI] [PubMed] [Google Scholar]
- 80.Katz P, Margaretten M, Gregorich S, et al. Physical activity to reduce fatigue in rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res 2018;70:1–10. 10.1002/acr.23230 [DOI] [PubMed] [Google Scholar]
- 81.Baxter SV, Hale LA, Stebbings S, et al. Walking is a feasible physical activity for people with rheumatoid arthritis: a feasibility randomized controlled trial. Musculoskeletal Care 2016;14:47–56. 10.1002/msc.1112 [DOI] [PubMed] [Google Scholar]
- 82.Feldthusen C, Dean E, Forsblad-d'Elia H, et al. Effects of Person-Centered physical therapy on Fatigue-Related variables in persons with rheumatoid arthritis: a randomized controlled trial. Arch Phys Med Rehabil 2016;97:26–36. 10.1016/j.apmr.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 83.Sjöquist ES, Brodin N, Lampa J, et al. Physical activity coaching of patients with rheumatoid arthritis in everyday practice: a long-term follow-up. Musculoskeletal Care 2011;9:75–85. 10.1002/msc.199 [DOI] [PubMed] [Google Scholar]
- 84.Brodin N, Eurenius E, Jensen I, et al. Coaching patients with early rheumatoid arthritis to healthy physical activity: a multicenter, randomized, controlled study. Arthritis Rheum 2008;59:325–31. 10.1002/art.23327 [DOI] [PubMed] [Google Scholar]
- 85.Li LC, Davis AM, Lineker SC, et al. Effectiveness of the primary therapist model for rheumatoid arthritis rehabilitation: a randomized controlled trial. Arthritis Rheum 2006;55:42–52. 10.1002/art.21692 [DOI] [PubMed] [Google Scholar]
- 86.Melikoglu MA, Karatay S, Senel K, et al. Association between dynamic exercise therapy and IGF-1 and IGFBP-3 concentrations in the patients with rheumatoid arthritis. Rheumatol Int 2006;26:309–13. 10.1007/s00296-005-0605-y [DOI] [PubMed] [Google Scholar]
- 87.Hansen TM, Hansen G, Langgaard AM, et al. Longterm physical training in rheumatoid arthritis. A randomized trial with different training programs and blinded observers. Scand J Rheumatol 1993;22:107–12. 10.3109/03009749309099253 [DOI] [PubMed] [Google Scholar]
- 88.Nordström DC, Konttinen YT, Solovieva S, et al. In- and out-patient rehabilitation in rheumatoid arthritis. A controlled, open, longitudinal, cost-effectiveness study. Scand J Rheumatol 1996;25:200–6. 10.3109/03009749609069988 [DOI] [PubMed] [Google Scholar]
- 89.Minor MA, Hewett JE. Physical fitness and work capacity in women with rheumatoid arthritis. Arthritis Care Res 1995;8:146–54. 10.1002/art.1790080306 [DOI] [PubMed] [Google Scholar]
- 90.Noreau L, Martineau H, Roy L, et al. Effects of a modified dance-based exercise on cardiorespiratory fitness, psychological state and health status of persons with rheumatoid arthritis. Am J Phys Med Rehabil 1995;74:19–27. 10.1097/00002060-199501000-00004 [DOI] [PubMed] [Google Scholar]
- 91.Ekblom B, Lövgren O, Alderin M, et al. Effect of short-term physical training on patients with rheumatoid arthritis. a six-month follow-up study. Scand J Rheumatol 1975;4:87–91. 10.3109/03009747509095620 [DOI] [PubMed] [Google Scholar]
- 92.Nadareishvili Z, Michaud K, Hallenbeck JM, et al. Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: a nested, case-control study. Arthritis Rheum 2008;59:1090–6. 10.1002/art.23935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum 2008;58:2612–21. 10.1002/art.23811 [DOI] [PubMed] [Google Scholar]
- 94.Stenström CH. Radiologically observed progression of joint destruction and its relationship with demographic factors, disease severity, and exercise frequency in patients with rheumatoid arthritis. Phys Ther 1994;74:32–9. 10.1093/ptj/74.1.32 [DOI] [PubMed] [Google Scholar]
- 95.Lange E, Kucharski D, Svedlund S, et al. Effects of aerobic and resistance exercise in older adults with rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res 2019;71:61–70. 10.1002/acr.23589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Durcan L, Wilson F, Cunnane G. The effect of exercise on sleep and fatigue in rheumatoid arthritis: a randomized controlled study. J Rheumatol 2014;41:1966–73. 10.3899/jrheum.131282 [DOI] [PubMed] [Google Scholar]
- 97.Breedland I, van Scheppingen C, Leijsma M, et al. Effects of a group-based exercise and educational program on physical performance and disease self-management in rheumatoid arthritis: a randomized controlled study. Phys Ther 2011;91:879–93. 10.2522/ptj.20090010 [DOI] [PubMed] [Google Scholar]
- 98.Hurkmans EJ, van den Berg MH, Ronday KH, et al. Maintenance of physical activity after Internet-based physical activity interventions in patients with rheumatoid arthritis. Rheumatology 2010;49:167–72. 10.1093/rheumatology/kep285 [DOI] [PubMed] [Google Scholar]
- 99.Flint-Wagner HG, Lisse J, Lohman TG, et al. Assessment of a sixteen-week training program on strength, pain, and function in rheumatoid arthritis patients. J Clin Rheumatol 2009;15:165–71. 10.1097/RHU.0b013e318190f95f [DOI] [PubMed] [Google Scholar]
- 100.Bulthuis Y, Drossaers-Bakker KW, Taal E, et al. Arthritis patients show long-term benefits from 3 weeks intensive exercise training directly following hospital discharge. Rheumatology 2007;46:1712–7. 10.1093/rheumatology/kem236 [DOI] [PubMed] [Google Scholar]
- 101.Neuberger GB, Aaronson LS, Gajewski B, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum 2007;57:943–52. 10.1002/art.22903 [DOI] [PubMed] [Google Scholar]
- 102.van den Berg MH, Ronday HK, Peeters AJ, et al. Using internet technology to deliver a home-based physical activity intervention for patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2006;55:935–45. 10.1002/art.22339 [DOI] [PubMed] [Google Scholar]
- 103.Munneke M, de Jong Z, Zwinderman AH, et al. Effect of a high-intensity weight-bearing exercise program on radiologic damage progression of the large joints in subgroups of patients with rheumatoid arthritis. Arthritis Rheum 2005;53:410–7. 10.1002/art.21165 [DOI] [PubMed] [Google Scholar]
- 104.de Jong Z, Munneke M, Lems WF, et al. Slowing of bone loss in patients with rheumatoid arthritis by long-term high-intensity exercise: results of a randomized, controlled trial. Arthritis Rheum 2004;50:1066–76. 10.1002/art.20117 [DOI] [PubMed] [Google Scholar]
- 105.de Jong Z, Munneke M, Zwinderman AH, et al. Long term high intensity exercise and damage of small joints in rheumatoid arthritis. Ann Rheum Dis 2004;63:1399–405. 10.1136/ard.2003.015826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Häkkinen A, Sokka T, Hannonen P. A home-based two-year strength training period in early rheumatoid arthritis led to good long-term compliance: a five-year followup. Arthritis Rheum 2004;51:56–62. 10.1002/art.20088 [DOI] [PubMed] [Google Scholar]
- 107.Häkkinen A, Sokka T, Kautiainen H, et al. Sustained maintenance of exercise induced muscle strength gains and normal bone mineral density in patients with early rheumatoid arthritis: a 5 year follow up. Ann Rheum Dis 2004;63:910–6. 10.1136/ard.2003.013003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum 2003;48:2415–24. 10.1002/art.11216 [DOI] [PubMed] [Google Scholar]
- 109.Westby MD, Wade JP, Rangno KK, et al. A randomized controlled trial to evaluate the effectiveness of an exercise program in women with rheumatoid arthritis taking low dose prednisone. J Rheumatol 2000;27:1674–80. [PubMed] [Google Scholar]
- 110.Häkkinen A, Sokka T, Kotaniemi A, et al. Dynamic strength training in patients with early rheumatoid arthritis increases muscle strength but not bone mineral density. J Rheumatol 1999;26:1257–63. [PubMed] [Google Scholar]
- 111.Lyngberg KK, Harreby M, Bentzen H, et al. Elderly rheumatoid arthritis patients on steroid treatment tolerate physical training without an increase in disease activity. Arch Phys Med Rehabil 1994;75:1189–95. 10.1016/0003-9993(94)90003-5 [DOI] [PubMed] [Google Scholar]
- 112.Ekdahl C, Andersson SI, Moritz U, et al. Dynamic versus static training in patients with rheumatoid arthritis. Scand J Rheumatol 1990;19:17–26. 10.3109/03009749009092618 [DOI] [PubMed] [Google Scholar]
- 113.Lyngberg K, Danneskiold-Samsøe B, Halskov O. The effect of physical training on patients with rheumatoid arthritis: changes in disease activity, muscle strength and aerobic capacity. A clinically controlled minimized cross-over study. Clin Exp Rheumatol 1988;6:253–60. [PubMed] [Google Scholar]
- 114.Nordemar R, Ekblom B, Zachrisson L, et al. Physical training in rheumatoid arthritis: a controlled long-term study. I. Scand J Rheumatol 1981;10:17–23. [PubMed] [Google Scholar]
- 115.Nordemar R. Physical training in rheumatoid arthritis: a controlled long-term study. II. Functional capacity and general attitudes. Scand J Rheumatol 1981;10:25–30. 10.1080/03009748109095266 [DOI] [PubMed] [Google Scholar]
- 116.Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJJCS, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819–25. 10.1136/annrheumdis-2012-202075 [DOI] [PubMed] [Google Scholar]
- 117.Löfgren M, Opava CH, Demmelmaier I, et al. Long-term, health-enhancing physical activity is associated with reduction of pain but not pain sensitivity or improved exercise-induced hypoalgesia in persons with rheumatoid arthritis. Arthritis Res Ther 2018;20:262. 10.1186/s13075-018-1758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nordgren B, Fridén C, Demmelmaier I, et al. An outsourced health-enhancing physical activity programme for people with rheumatoid arthritis: exploration of adherence and response. Rheumatology 2015;54:1065–73. 10.1093/rheumatology/keu444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Gioia L, Zincarelli C, Di Minno MND, et al. Effectiveness of a rehabilitative programme in improving fatigue and function in rheumatoid arthritis patients treated with biologics: a pilot study. Clin Exp Rheumatol 2013;31:285–8. [PubMed] [Google Scholar]
- 120.Strasser B, Leeb G, Strehblow C, et al. The effects of strength and endurance training in patients with rheumatoid arthritis. Clin Rheumatol 2011;30:623–32. 10.1007/s10067-010-1584-2 [DOI] [PubMed] [Google Scholar]
- 121.van der Giesen FJ, van Lankveld W, Hopman-Rock M, et al. Exploring the public health impact of an intensive exercise program for patients with rheumatoid arthritis: a dissemination and implementation study. Arthritis Care Res 2010;62:865–72. 10.1002/acr.20138 [DOI] [PubMed] [Google Scholar]
- 122.de Jong Z, Munneke M, Kroon HM, et al. Long-term follow-up of a high-intensity exercise program in patients with rheumatoid arthritis. Clin Rheumatol 2009;28:663–71. 10.1007/s10067-009-1125-z [DOI] [PubMed] [Google Scholar]
- 123.Neuberger GB, Press AN, Lindsley HB, et al. Effects of exercise on fatigue, aerobic fitness, and disease activity measures in persons with rheumatoid arthritis. Res Nurs Health 1997;20:195–204. [DOI] [PubMed] [Google Scholar]
- 124.Al-Qubaeissy KY, Fatoye FA, Goodwin PC, et al. The effectiveness of hydrotherapy in the management of rheumatoid arthritis: a systematic review. Musculoskeletal Care 2013;11:3–18. 10.1002/msc.1028 [DOI] [PubMed] [Google Scholar]
- 125.Siqueira US, Orsini Valente LG, de Mello MT, et al. Effectiveness of aquatic exercises in women with rheumatoid arthritis: a randomized, controlled, 16-Week Intervention-The HydRA trial. Am J Phys Med Rehabil 2017;96:167–75. 10.1097/PHM.0000000000000564 [DOI] [PubMed] [Google Scholar]
- 126.Eversden L, Maggs F, Nightingale P, et al. A pragmatic randomised controlled trial of hydrotherapy and land exercises on overall well being and quality of life in rheumatoid arthritis. BMC Musculoskelet Disord 2007;8:23. 10.1186/1471-2474-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bilberg A, Ahlmén M, Mannerkorpi K. Moderately intensive exercise in a temperate pool for patients with rheumatoid arthritis: a randomized controlled study. Rheumatology 2005;44:502–8. 10.1093/rheumatology/keh528 [DOI] [PubMed] [Google Scholar]
- 128.Lemmey AB, Marcora SM, Chester K, et al. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1726–34. 10.1002/art.24891 [DOI] [PubMed] [Google Scholar]
- 129.van den Ende CH, Breedveld FC, le Cessie S, et al. Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2000;59:615–21. 10.1136/ard.59.8.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van den Ende CH, Hazes JM, le Cessie S, et al. Comparison of high and low intensity training in well controlled rheumatoid arthritis. Results of a randomised clinical trial. Ann Rheum Dis 1996;55:798–805. 10.1136/ard.55.11.798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lemmey AB, Williams SL, Marcora SM, et al. Are the benefits of a high-intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3-year followup study. Arthritis Care Res 2012;64:71–5. 10.1002/acr.20523 [DOI] [PubMed] [Google Scholar]
- 132.Hammond A, Prior Y. The effectiveness of home hand exercise programmes in rheumatoid arthritis: a systematic review. Br Med Bull 2016;119:49–62. 10.1093/bmb/ldw024 [DOI] [PubMed] [Google Scholar]
- 133.Zernicke J, Kedor C, Müller A, et al. A prospective pilot study to evaluate an animated home-based physical exercise program as a treatment option for patients with rheumatoid arthritis. BMC Musculoskelet Disord 2016;17:351. 10.1186/s12891-016-1208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seneca T, Hauge EM, Maribo T. Comparable effect of partly supervised and self-administered exercise programme in early rheumatoid arthritis--a randomised, controlled trial. Dan Med J 2015;62:A5127. [PubMed] [Google Scholar]
- 135.Hsieh L-F, Chen S-C, Chuang C-C, et al. Supervised aerobic exercise is more effective than home aerobic exercise in female Chinese patients with rheumatoid arthritis. J Rehabil Med 2009;41:332–7. 10.2340/16501977-0330 [DOI] [PubMed] [Google Scholar]
- 136.Stenström CH. Home exercise in rheumatoid arthritis functional class II: goal setting versus pain attention. J Rheumatol 1994;21:627–34. [PubMed] [Google Scholar]
- 137.Williams MA, Srikesavan C, Heine PJ, et al. Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev 2018;7:CD003832. 10.1002/14651858.CD003832.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baillet A, Vaillant M, Guinot M, et al. Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials. Rheumatology 2012;51:519–27. 10.1093/rheumatology/ker330 [DOI] [PubMed] [Google Scholar]
- 139.Daien CI, Hua C, Combe B, et al. Non-pharmacological and pharmacological interventions in patients with early arthritis: a systematic literature review informing the 2016 update of EULAR recommendations for the management of early arthritis. RMD Open 2017;3:e000404. 10.1136/rmdopen-2016-000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bergstra SA, Murgia A, Te Velde AF, et al. A systematic review into the effectiveness of hand exercise therapy in the treatment of rheumatoid arthritis. Clin Rheumatol 2014;33:1539–48. 10.1007/s10067-014-2691-2 [DOI] [PubMed] [Google Scholar]
- 141.Lo C-N, Xia G, Leung BP. The effect of nerve mobilization exercise in patients with rheumatoid arthritis: a pilot study. Reumatismo 2017;69:111–8. 10.4081/reumatismo.2017.918 [DOI] [PubMed] [Google Scholar]
- 142.Williamson E, McConkey C, Heine P, et al. Hand exercises for patients with rheumatoid arthritis: an extended follow-up of the SARAH randomised controlled trial. BMJ Open 2017;7:e013121. 10.1136/bmjopen-2016-013121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lourenzi FM, Jones A, Pereira DF, et al. Effectiveness of an overall progressive resistance strength program for improving the functional capacity of patients with rheumatoid arthritis: a randomized controlled trial. Clin Rehabil 2017;31:1482–91. 10.1177/0269215517698732 [DOI] [PubMed] [Google Scholar]
- 144.Lamb SE, Williamson EM, Heine PJ, et al. Exercises to improve function of the rheumatoid hand (SARAH): a randomised controlled trial. Lancet 2015;385:421–9. 10.1016/S0140-6736(14)60998-3 [DOI] [PubMed] [Google Scholar]
- 145.Manning VL, Hurley MV, Scott DL, et al. Education, self-management, and upper extremity exercise training in people with rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res 2014;66:217–27. 10.1002/acr.22102 [DOI] [PubMed] [Google Scholar]
- 146.Dogu B, Sirzai H, Yilmaz F, et al. Effects of isotonic and isometric hand exercises on pain, hand functions, dexterity and quality of life in women with rheumatoid arthritis. Rheumatol Int 2013;33:2625–30. 10.1007/s00296-013-2787-z [DOI] [PubMed] [Google Scholar]
- 147.Baillet A, Payraud E, Niderprim V-A, et al. A dynamic exercise programme to improve patients' disability in rheumatoid arthritis: a prospective randomized controlled trial. Rheumatology 2009;48:410–5. 10.1093/rheumatology/ken511 [DOI] [PubMed] [Google Scholar]
- 148.Masiero S, Boniolo A, Wassermann L, et al. Effects of an educational-behavioral joint protection program on people with moderate to severe rheumatoid arthritis: a randomized controlled trial. Clin Rheumatol 2007;26:2043–50. 10.1007/s10067-007-0615-0 [DOI] [PubMed] [Google Scholar]
- 149.O'Brien AV, Jones P, Mullis R, et al. Conservative hand therapy treatments in rheumatoid arthritis--a randomized controlled trial. Rheumatology 2006;45:577–83. 10.1093/rheumatology/kei215 [DOI] [PubMed] [Google Scholar]
- 150.Buljina AI, Taljanovic MS, Avdic DM, et al. Physical and exercise therapy for treatment of the rheumatoid hand. Arthritis Rheum 2001;45:392–7. [DOI] [PubMed] [Google Scholar]
- 151.Scholten C, Brodowicz T, Graninger W, et al. Persistent functional and social benefit 5 years after a multidisciplinary arthritis training program. Arch Phys Med Rehabil 1999;80:1282–7. 10.1016/S0003-9993(99)90030-8 [DOI] [PubMed] [Google Scholar]
- 152.Boström C, Harms-Ringdahl K, Karreskog H, et al. Effects of static and dynamic shoulder rotator exercises in women with rheumatoid arthritis: a randomised comparison of impairment, disability, handicap, and health. Scand J Rheumatol 1998;27:281–90. 10.1080/030097498442398 [DOI] [PubMed] [Google Scholar]
- 153.Komatireddy GR, Leitch RW, Cella K, et al. Efficacy of low load resistive muscle training in patients with rheumatoid arthritis functional class II and III. J Rheumatol 1997;24:1531–9. [PubMed] [Google Scholar]
- 154.Hoenig H, Groff G, Pratt K, et al. A randomized controlled trial of home exercise on the rheumatoid hand. J Rheumatol 1993;20:785–9. [PubMed] [Google Scholar]
- 155.Dellhag B, Wollersjö I, Bjelle A. Effect of active hand exercise and wax bath treatment in rheumatoid arthritis patients. Arthritis Care Res 1992;5:87–92. 10.1002/art.1790050207 [DOI] [PubMed] [Google Scholar]
- 156.Marcora SM, Lemmey AB, Maddison PJ. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. J Rheumatol 2005;32:1031–9. [PubMed] [Google Scholar]
- 157.Göksel Karatepe A, Günaydin R, Türkmen G, et al. Effects of home-based exercise program on the functional status and the quality of life in patients with rheumatoid arthritis: 1-year follow-up study. Rheumatol Int 2011;31:171–6. 10.1007/s00296-009-1242-7 [DOI] [PubMed] [Google Scholar]
- 158.Han A, Robinson V, Judd M, et al. Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev 2004;3:CD004849. 10.1002/14651858.CD004849 [DOI] [PubMed] [Google Scholar]
- 159.Wang C. Tai Chi improves pain and functional status in adults with rheumatoid arthritis: results of a pilot single-blinded randomized controlled trial. Med Sport Sci 2008;52:218–29. 10.1159/000134302 [DOI] [PubMed] [Google Scholar]
- 160.Shin J-H, Lee Y, Kim SG, et al. The beneficial effects of Tai Chi exercise on endothelial function and arterial stiffness in elderly women with rheumatoid arthritis. Arthritis Res Ther 2015;17:380. 10.1186/s13075-015-0893-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee H-Y, Hale CA, Hemingway B, et al. Tai Chi exercise and auricular acupressure for people with rheumatoid arthritis: an evaluation study. J Clin Nurs 2012;21:2812–22. 10.1111/j.1365-2702.2011.04058.x [DOI] [PubMed] [Google Scholar]
- 162.Uhlig T, Fongen C, Steen E, et al. Exploring Tai Chi in rheumatoid arthritis: a quantitative and qualitative study. BMC Musculoskelet Disord 2010;11:43. 10.1186/1471-2474-11-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ward L, Stebbings S, Athens J, et al. Yoga for the management of pain and sleep in rheumatoid arthritis: a pilot randomized controlled trial. Musculoskeletal Care 2018;16:39–47. 10.1002/msc.1201 [DOI] [PubMed] [Google Scholar]
- 164.Evans S, Moieni M, Lung K, et al. Impact of iyengar yoga on quality of life in young women with rheumatoid arthritis. Clin J Pain 2013;29:988–97. 10.1097/AJP.0b013e31827da381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh VK, Bhandari RB, Rana BB. Effect of yogic package on rheumatoid arthritis. Indian J Physiol Pharmacol 2011;55:329–35. [PubMed] [Google Scholar]
- 166.Badsha H, Chhabra V, Leibman C, et al. The benefits of yoga for rheumatoid arthritis: results of a preliminary, structured 8-week program. Rheumatol Int 2009;29:1417–21. 10.1007/s00296-009-0871-1 [DOI] [PubMed] [Google Scholar]
- 167.Bosch PR, Traustadóttir T, Howard P, et al. Functional and physiological effects of yoga in women with rheumatoid arthritis: a pilot study. Altern Ther Health Med 2009;15:24–31. [PubMed] [Google Scholar]
- 168.O'Dwyer T, Durcan L, Wilson F. Exercise and physical activity in systemic lupus erythematosus: a systematic review with meta-analyses. Semin Arthritis Rheum 2017;47:204–15. 10.1016/j.semarthrit.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 169.Wu M-L, Yu K-H, Tsai J-C. The effectiveness of exercise in adults with systemic lupus erythematosus: a systematic review and meta-analysis to guide evidence-based practice. Worldviews Evid Based Nurs 2017;14:306–15. 10.1111/wvn.12221 [DOI] [PubMed] [Google Scholar]
- 170.Andrades C, Fuego C, Manrique-Arija S, et al. Management of cardiovascular risk in systemic lupus erythematosus: a systematic review. Lupus 2017;26:1407–19. 10.1177/0961203317704710 [DOI] [PubMed] [Google Scholar]
- 171.del Pino-Sedeño T, Trujillo-Martín MM, Ruiz-Irastorza G, et al. Effectiveness of nonpharmacologic interventions for decreasing fatigue in adults with systemic lupus erythematosus: a systematic review. Arthritis Care Res 2016;68:141–8. 10.1002/acr.22675 [DOI] [PubMed] [Google Scholar]
- 172.Yuen HK, Cunningham MA. Optimal management of fatigue in patients with systemic lupus erythematosus: a systematic review. Ther Clin Risk Manag 2014;10:775–86. 10.2147/TCRM.S56063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Abrahão MI, Gomiero AB, Peccin MS, et al. Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: a randomized controlled trial. Scand J Rheumatol 2016;45:197–201. 10.3109/03009742.2015.1094126 [DOI] [PubMed] [Google Scholar]
- 174.Boström C, Elfving B, Dupré B, et al. Effects of a one-year physical activity programme for women with systemic lupus erythematosus - a randomized controlled study. Lupus 2016;25:602–16. 10.1177/0961203315622817 [DOI] [PubMed] [Google Scholar]
- 175.Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology 2003;42:1050–4. 10.1093/rheumatology/keg289 [DOI] [PubMed] [Google Scholar]
- 176.Robb-Nicholson LC, Daltroy L, Eaton H, et al. Effects of aerobic conditioning in lupus fatigue: a pilot study. Br J Rheumatol 1989;28:500–5. 10.1093/rheumatology/28.6.500 [DOI] [PubMed] [Google Scholar]
- 177.Soriano-Maldonado A, Morillas-de-Laguno P, Sabio JM, et al. Effects of 12-week aerobic exercise on arterial stiffness, inflammation, and cardiorespiratory fitness in women with systemic lupus erythematosus: Non-Randomized controlled trial. J Clin Med 2018;7. 10.3390/jcm7120477. [Epub ahead of print: 24 11 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.dos Reis-Neto ET, da Silva AE, Monteiro CMdeC, et al. Supervised physical exercise improves endothelial function in patients with systemic lupus erythematosus. Rheumatology 2013;52:2187–95. 10.1093/rheumatology/ket283 [DOI] [PubMed] [Google Scholar]
- 179.Carvalho MRPde, Sato EI, Tebexreni AS, et al. Effects of supervised cardiovascular training program on exercise tolerance, aerobic capacity, and quality of life in patients with systemic lupus erythematosus. Arthritis Rheum 2005;53:838–44. 10.1002/art.21605 [DOI] [PubMed] [Google Scholar]
- 180.Bogdanovic G, Stojanovich L, Djokovic A, et al. Physical activity program is helpful for improving quality of life in patients with systemic lupus erythematosus. Tohoku J Exp Med 2015;237:193–9. 10.1620/tjem.237.193 [DOI] [PubMed] [Google Scholar]
- 181.Ramsey-Goldman R, Schilling EM, Dunlop D, et al. A pilot study on the effects of exercise in patients with systemic lupus erythematosus. Arthritis Care Res 2000;13:262–9. [DOI] [PubMed] [Google Scholar]
- 182.Miossi R, Benatti FB, Lúciade de Sá Pinto A, et al. Using exercise training to counterbalance chronotropic incompetence and delayed heart rate recovery in systemic lupus erythematosus: a randomized trial. Arthritis Care Res 2012;64:1159–66. 10.1002/acr.21678 [DOI] [PubMed] [Google Scholar]
- 183.Timóteo RP, Silva AF, Micheli DC, et al. Increased flexibility, pain reduction and unaltered levels of IL-10 and CD11b + lymphocytes in patients with systemic lupus erythematosus were associated with kinesiotherapy. Lupus 2018;27:1159–68. 10.1177/0961203318768880 [DOI] [PubMed] [Google Scholar]
- 184.Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological and non-biological pharmacological treatment: a systematic literature review Informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 2017;3:e000397. 10.1136/rmdopen-2016-000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O'Dwyer T, O'Shea F, Wilson F. Exercise therapy for spondyloarthritis: a systematic review. Rheumatol Int 2014;34:887–902. 10.1007/s00296-014-2965-7 [DOI] [PubMed] [Google Scholar]
- 186.Karahan AY, Tok F, Yildirim P, et al. The effectiveness of Exergames in patients with ankylosing spondylitis: a randomized controlled trial. Adv Clin Exp Med 2016;25:931–6. 10.17219/acem/32590 [DOI] [PubMed] [Google Scholar]
- 187.Jennings F, Oliveira HA, de Souza MC, et al. Effects of aerobic training in patients with ankylosing spondylitis. J Rheumatol 2015;42:2347–53. 10.3899/jrheum.150518 [DOI] [PubMed] [Google Scholar]
- 188.Niedermann K, Sidelnikov E, Muggli C, et al. Effect of cardiovascular training on fitness and perceived disease activity in people with ankylosing spondylitis. Arthritis Care Res 2013;65:1844–52. 10.1002/acr.22062 [DOI] [PubMed] [Google Scholar]
- 189.Sweeney S, Taylor G, Calin A. The effect of a home based exercise intervention package on outcome in ankylosing spondylitis: a randomized controlled trial. J Rheumatol 2002;29:763–6. [PubMed] [Google Scholar]
- 190.Ajeganova S, Wörnert M, Hafström I. A four-week team-rehabilitation programme in a warm climate decreases disability and improves health and body function for up to one year: a prospective study in Swedish patients with inflammatory joint diseases. J Rehabil Med 2016;48:711–8. 10.2340/16501977-2122 [DOI] [PubMed] [Google Scholar]
- 191.Brophy S, Cooksey R, Davies H, et al. The effect of physical activity and motivation on function in ankylosing spondylitis: a cohort study. Semin Arthritis Rheum 2013;42:619–26. 10.1016/j.semarthrit.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ward MM. Predictors of the progression of functional disability in patients with ankylosing spondylitis. J Rheumatol 2002;29:1420–5. [PubMed] [Google Scholar]
- 193.Uhrin Z, Kuzis S, Ward MM. Exercise and changes in health status in patients with ankylosing spondylitis. Arch Intern Med 2000;160:2969–75. 10.1001/archinte.160.19.2969 [DOI] [PubMed] [Google Scholar]
- 194.Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2018;99:383–9. 10.1016/j.apmr.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 195.Chang W, Tsou Y, Lee C. Comparison between specific exercises and physical therapy for managing patients with ankylosing spondylitis: a meta-analysis of randomized controlled trials. Int J Clin Experiment Med 2016;9:17028–39. [Google Scholar]
- 196.Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum 2016;45:411–27. 10.1016/j.semarthrit.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 197.Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol 2015;34:1737–44. 10.1007/s10067-015-2913-2 [DOI] [PubMed] [Google Scholar]
- 198.Liang H, Li W-R, Zhang H, et al. Concurrent intervention with exercises and stabilized tumor necrosis factor inhibitor therapy reduced the disease activity in patients with ankylosing spondylitis: a meta-analysis. Medicine 2015;94:e2254. 10.1097/MD.0000000000002254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martins NA, Furtado GE, Campos MJ, et al. Exercise and ankylosing spondylitis with New York modified criteria: a systematic review of controlled trials with meta-analysis. Acta Reumatol Port 2014;39:298–308. [PubMed] [Google Scholar]
- 200.Sharan D, Rajkumar JS. Physiotherapy for ankylosing spondylitis: systematic review and a proposed rehabilitation protocol. Curr Rheumatol Rev 2017;13:121–5. 10.2174/1573397112666161025112750 [DOI] [PubMed] [Google Scholar]
- 201.Sveaas SH, Bilberg A, Berg IJ, et al. High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): a multicentre randomised trial of 100 patients. Br J Sports Med 2020;54:292–7. 10.1136/bjsports-2018-099943 [DOI] [PubMed] [Google Scholar]
- 202.Sveaas SH, Berg IJ, Fongen C, et al. High-intensity cardiorespiratory and strength exercises reduced emotional distress and fatigue in patients with axial spondyloarthritis: a randomized controlled pilot study. Scand J Rheumatol 2018;47:117–21. 10.1080/03009742.2017.1347276 [DOI] [PubMed] [Google Scholar]
- 203.Aydın T, Taşpınar Özgür, Sarıyıldız MA, et al. Evaluation of the effectiveness of home based or hospital based calisthenic exercises in patients with ankylosing spondylitis. J Back Musculoskelet Rehabil 2016;29:723–30. 10.3233/BMR-160677 [DOI] [PubMed] [Google Scholar]
- 204.Roşu MO, Ţopa I, Chirieac R, et al. Effects of Pilates, McKenzie and Heckscher training on disease activity, spinal motility and pulmonary function in patients with ankylosing spondylitis: a randomized controlled trial. Rheumatol Int 2014;34:367–72. 10.1007/s00296-013-2869-y [DOI] [PubMed] [Google Scholar]
- 205.Sveaas SH, Berg IJ, Provan SA, et al. Efficacy of high intensity exercise on disease activity and cardiovascular risk in active axial spondyloarthritis: a randomized controlled pilot study. PLoS One 2014;9:e108688. 10.1371/journal.pone.0108688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kjeken I, Bo I, Rønningen A, et al. A three-week multidisciplinary in-patient rehabilitation programme had positive long-term effects in patients with ankylosing spondylitis: randomized controlled trial. J Rehabil Med 2013;45:260–7. 10.2340/16501977-1078 [DOI] [PubMed] [Google Scholar]
- 207.Analay Y, Ozcan E, Karan A, et al. The effectiveness of intensive group exercise on patients with ankylosing spondylitis. Clin Rehabil 2003;17:631–6. 10.1191/0269215503cr658oa [DOI] [PubMed] [Google Scholar]
- 208.Hidding A, van der Linden S, Gielen X, et al. Continuation of group physical therapy is necessary in ankylosing spondylitis: results of a randomized controlled trial. Arthritis Care Res 1994;7:90–6. 10.1002/art.1790070208 [DOI] [PubMed] [Google Scholar]
- 209.Hidding A, van der Linden S, Boers M, et al. Is group physical therapy superior to individualized therapy in ankylosing spondylitis? A randomized controlled trial. Arthritis Care Res 1993;6:117–25. 10.1002/art.1790060303 [DOI] [PubMed] [Google Scholar]
- 210.Kraag G, Stokes B, Groh J, et al. The effects of comprehensive home physiotherapy and supervision on patients with ankylosing spondylitis--a randomized controlled trial. J Rheumatol 1990;17:228–33. [PubMed] [Google Scholar]
- 211.Levitova A, Hulejova H, Spiritovic M, et al. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res Ther 2016;18:275. 10.1186/s13075-016-1180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aytekin E, Caglar NS, Ozgonenel L, et al. Home-based exercise therapy in patients with ankylosing spondylitis: effects on pain, mobility, disease activity, quality of life, and respiratory functions. Clin Rheumatol 2012;31:91–7. 10.1007/s10067-011-1791-5 [DOI] [PubMed] [Google Scholar]
- 213.Viitanen JV, Heikkilä S. Functional changes in patients with spondylarthropathy. A controlled trial of the effects of short-term rehabilitation and 3-year follow-up. Rheumatol Int 2001;20:211–4. 10.1007/s002960100101 [DOI] [PubMed] [Google Scholar]
- 214.Lubrano E, D'Angelo S, Parsons WJ, et al. Effectiveness of rehabilitation in active ankylosing spondylitis assessed by the ASAS response criteria. Rheumatology 2007;46:1672–5. 10.1093/rheumatology/kem247 [DOI] [PubMed] [Google Scholar]
- 215.Band DA, Jones SD, Kennedy LG, et al. Which patients with ankylosing spondylitis derive most benefit from an inpatient management program? J Rheumatol 1997;24:2381–4. [PubMed] [Google Scholar]
- 216.Viitanen JV, Lehtinen K, Suni J, et al. Fifteen months' follow-up of intensive inpatient physiotherapy and exercise in ankylosing spondylitis. Clin Rheumatol 1995;14:413–9. 10.1007/BF02207674 [DOI] [PubMed] [Google Scholar]
- 217.Kraag G, Stokes B, Groh J, et al. The effects of comprehensive home physiotherapy and supervision on patients with ankylosing spondylitis--an 8-month followup. J Rheumatol 1994;21:261–3. [PubMed] [Google Scholar]
- 218.Viitanen JV, Suni J, Kautiainen H, et al. Effect of physiotherapy on spinal mobility in ankylosing spondylitis. Scand J Rheumatol 1992;21:38–41. 10.3109/03009749209095061 [DOI] [PubMed] [Google Scholar]
- 219.Escalas C, Dalichampt M, Dougados M, et al. Evaluation of physiotherapy in a prospective cohort of early axial spondyloarthritis. Data from the DESIR cohort. Joint Bone Spine 2016;83:185–90. 10.1016/j.jbspin.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 220.Zão A, Cantista P. The role of land and aquatic exercise in ankylosing spondylitis: a systematic review. Rheumatol Int 2017;37:1979–90. 10.1007/s00296-017-3829-8 [DOI] [PubMed] [Google Scholar]
- 221.Dundar U, Solak O, Toktas H, et al. Effect of aquatic exercise on ankylosing spondylitis: a randomized controlled trial. Rheumatol Int 2014;34:1505–11. 10.1007/s00296-014-2980-8 [DOI] [PubMed] [Google Scholar]
- 222.Karapolat H, Eyigor S, Zoghi M, et al. Are swimming or aerobic exercise better than conventional exercise in ankylosing spondylitis patients? A randomized controlled study. Eur J Phys Rehabil Med 2009;45:449–57. [PubMed] [Google Scholar]
- 223.Hsieh L-F, Chuang C-C, Tseng C-S, et al. Combined home exercise is more effective than range-of-motion home exercise in patients with ankylosing spondylitis: a randomized controlled trial. Biomed Res Int 2014;2014:398190. 10.1155/2014/398190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rodríguez-Lozano C, Juanola X, Cruz-Martínez J, et al. Outcome of an education and home-based exercise programme for patients with ankylosing spondylitis: a nationwide randomized study. Clin Exp Rheumatol 2013;31:739–48. [PubMed] [Google Scholar]
- 225.Lim H-J, Moon Y-I, Lee MS. Effects of home-based daily exercise therapy on joint mobility, daily activity, pain, and depression in patients with ankylosing spondylitis. Rheumatol Int 2005;25:225–9. 10.1007/s00296-004-0536-z [DOI] [PubMed] [Google Scholar]
- 226.Yigit S, Sahin Z, Demir SE, et al. Home-based exercise therapy in ankylosing spondylitis: short-term prospective study in patients receiving tumor necrosis factor alpha inhibitors. Rheumatol Int 2013;33:71–7. 10.1007/s00296-011-2344-6 [DOI] [PubMed] [Google Scholar]
- 227.Durmus D, Alayli G, Cil E, et al. Effects of a home-based exercise program on quality of life, fatigue, and depression in patients with ankylosing spondylitis. Rheumatol Int 2009;29:673–7. 10.1007/s00296-008-0756-8 [DOI] [PubMed] [Google Scholar]
- 228.Durmuş D, Alaylı G, Uzun O, et al. Effects of two exercise interventions on pulmonary functions in the patients with ankylosing spondylitis. Joint Bone Spine 2009;76:150–5. 10.1016/j.jbspin.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 229.Karapolat H, Akkoc Y, Sari I, et al. Comparison of group-based exercise versus home-based exercise in patients with ankylosing spondylitis: effects on Bath ankylosing spondylitis indices, quality of life and depression. Clin Rheumatol 2008;27:695–700. 10.1007/s10067-007-0765-0 [DOI] [PubMed] [Google Scholar]
- 230.Souza MCde, Jennings F, Morimoto H, et al. Swiss ball exercises improve muscle strength and walking performance in ankylosing spondylitis: a randomized controlled trial. Rev Bras Reumatol Engl Ed 2017;57:45–55. 10.1016/j.rbre.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 231.Kasapoglu Aksoy M, Birtane M, Taştekin N, et al. The effectiveness of structured group education on ankylosing spondylitis patients. J Clin Rheumatol 2017;23:138–43. 10.1097/RHU.0000000000000499 [DOI] [PubMed] [Google Scholar]
- 232.Rosu OM, Ancuta C. McKenzie training in patients with early stages of ankylosing spondylitis: results of a 24-week controlled study. Eur J Phys Rehabil Med 2015;51:261–8. [PubMed] [Google Scholar]
- 233.Masiero S, Poli P, Bonaldo L, et al. Supervised training and home-based rehabilitation in patients with stabilized ankylosing spondylitis on TNF inhibitor treatment: a controlled clinical trial with a 12-month follow-up. Clin Rehabil 2014;28:562–72. 10.1177/0269215513512214 [DOI] [PubMed] [Google Scholar]
- 234.Altan L, Korkmaz N, Dizdar M, et al. Effect of Pilates training on people with ankylosing spondylitis. Rheumatol Int 2012;32:2093–9. 10.1007/s00296-011-1932-9 [DOI] [PubMed] [Google Scholar]
- 235.Masiero S, Bonaldo L, Pigatto M, et al. Rehabilitation treatment in patients with ankylosing spondylitis stabilized with tumor necrosis factor inhibitor therapy: a randomized controlled trial. J Rheumatol 2011;38:1335–42. 10.3899/jrheum.100987 [DOI] [PubMed] [Google Scholar]
- 236.Fernández-de-Las-Peñas C, Alonso-Blanco C, Alguacil-Diego IM, et al. One-year follow-up of two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2006;85:559–67. 10.1097/01.phm.0000223358.25983.df [DOI] [PubMed] [Google Scholar]
- 237.Fernández-de-Las-Peñas C, Alonso-Blanco C, Morales-Cabezas M, et al. Two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2005;84:407–19. 10.1097/01.phm.0000163862.89217.fe [DOI] [PubMed] [Google Scholar]
- 238.Gyurcsik ZN, András A, Bodnár N, et al. Improvement in pain intensity, spine stiffness, and mobility during a controlled individualized physiotherapy program in ankylosing spondylitis. Rheumatol Int 2012;32:3931–6. 10.1007/s00296-011-2325-9 [DOI] [PubMed] [Google Scholar]
- 239.Hulejová H, Levitová A, Kuklová M, et al. No effect of physiotherapy on the serum levels of adipocytokines in patients with ankylosing spondylitis. Clin Rheumatol 2012;31:67–71. 10.1007/s10067-011-1773-7 [DOI] [PubMed] [Google Scholar]
- 240.Ortancil O, Sarikaya S, Sapmaz P, et al. The effect(s) of a six-week home-based exercise program on the respiratory muscle and functional status in ankylosing spondylitis. J Clin Rheumatol 2009;15:68–70. 10.1097/RHU.0b013e31819b5ed0 [DOI] [PubMed] [Google Scholar]
- 241.Roger-Silva D, Natour J, Moreira E, et al. A resistance exercise program improves functional capacity of patients with psoriatic arthritis: a randomized controlled trial. Clin Rheumatol 2018;37:389–95. 10.1007/s10067-017-3917-x [DOI] [PubMed] [Google Scholar]
- 242.Chimenti MS, Triggianese P, Conigliaro P, et al. Self-reported adherence to a home-based exercise program among patients affected by psoriatic arthritis with minimal disease activity. Drug Dev Res 2014;75(Suppl 1):S57–9. 10.1002/ddr.21197 [DOI] [PubMed] [Google Scholar]
- 243.Antonioli CM, Bua G, Frigè A, et al. An individualized rehabilitation program in patients with systemic sclerosis may improve quality of life and hand mobility. Clin Rheumatol 2009;28:159–65. 10.1007/s10067-008-1006-x [DOI] [PubMed] [Google Scholar]
- 244.Moran ME. Scleroderma and evidence based non-pharmaceutical treatment modalities for digital ulcers: a systematic review. J Wound Care 2014;23:510–6. 10.12968/jowc.2014.23.10.510 [DOI] [PubMed] [Google Scholar]
- 245.Rannou F, Boutron I, Mouthon L, et al. Personalized physical therapy versus usual care for patients with systemic sclerosis: a randomized controlled trial. Arthritis Care Res 2017;69:1050–9. 10.1002/acr.23098 [DOI] [PubMed] [Google Scholar]
- 246.Schouffoer AA, Ninaber MK, Beaart-van de Voorde LJJ, et al. Randomized comparison of a multidisciplinary team care program with usual care in patients with systemic sclerosis. Arthritis Care Res 2011;63:909–17. 10.1002/acr.20448 [DOI] [PubMed] [Google Scholar]
- 247.Pinto ALS, Oliveira NC, Gualano B, et al. Efficacy and safety of concurrent training in systemic sclerosis. J Strength Cond Res 2011;25:1423–8. 10.1519/JSC.0b013e3181d6858b [DOI] [PubMed] [Google Scholar]
- 248.Maddali Bongi S, Del Rosso A, Galluccio F, et al. Efficacy of a tailored rehabilitation program for systemic sclerosis. Clin Exp Rheumatol 2009;27:44–50. [PubMed] [Google Scholar]
- 249.Stefanantoni K, Sciarra I, Iannace N, et al. Occupational therapy integrated with a self-administered stretching program on systemic sclerosis patients with hand involvement. Clin Exp Rheumatol 2016;34(Suppl 100):157–61. [PubMed] [Google Scholar]
- 250.Horváth J, Bálint Z, Szép E, et al. Efficacy of intensive hand physical therapy in patients with systemic sclerosis. Clin Exp Rheumatol 2017;35(Suppl 106):159–66. [PubMed] [Google Scholar]
- 251.Mugii N, Matsushita T, Oohata S, et al. Long-term follow-up of finger passive range of motion in Japanese systemic sclerosis patients treated with self-administered stretching. Mod Rheumatol 2019;29:484–90. 10.1080/14397595.2018.1466635 [DOI] [PubMed] [Google Scholar]
- 252.Landim SF, Bertolo MB, Marcatto de Abreu MF, et al. The evaluation of a home-based program for hands in patients with systemic sclerosis. J Hand Ther 2019;32:313–21. 10.1016/j.jht.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 253.Mugii N, Hasegawa M, Matsushita T, et al. The efficacy of self-administered stretching for finger joint motion in Japanese patients with systemic sclerosis. J Rheumatol 2006;33:1586–92. [PubMed] [Google Scholar]
- 254.Ma L, Sun R, Jia Z, et al. Clinical characteristics associated with subcutaneous tophi formation in Chinese gout patients: a retrospective study. Clin Rheumatol 2018;37:1359–65. 10.1007/s10067-017-3969-y [DOI] [PubMed] [Google Scholar]
- 255.Adithya Acharya K, Sharma A. Evaluation of the efficacy of siravyadha and guduchi siddha yoga basti in the management of vatarakta with special reference to gout. Int J Res Ayuveda Pharm 2019;4:402–9. [Google Scholar]
- 256.Sadeghi A, Rad ZA, Sajedi B, et al. Effect of weight losing on the clinical status improvement of patients with knee osteoarthritis. Reumatol Clin 2019;15:73–6. 10.1016/j.reuma.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 257.O'Brien KM, Wiggers J, Williams A, et al. Telephone-based weight loss support for patients with knee osteoarthritis: a pragmatic randomised controlled trial. Osteoarthritis Cartilage 2018;26:485–94. 10.1016/j.joca.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 258.Allen KD, Oddone EZ, Coffman CJ, et al. Patient, provider, and combined interventions for managing osteoarthritis in primary care: a cluster randomized trial. Ann Intern Med 2017;166:401–11. 10.7326/M16-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Allen KD, Yancy WS, Bosworth HB, et al. A combined patient and provider intervention for management of osteoarthritis in veterans. Ann Intern Med 2016;164:73–83. 10.7326/M15-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Christensen R, Henriksen M, Leeds AR, et al. Effect of weight maintenance on symptoms of knee osteoarthritis in obese patients: a twelve-month randomized controlled trial. Arthritis Care Res 2015;67:640–50. 10.1002/acr.22504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hunter DJ, Beavers DP, Eckstein F, et al. The intensive diet and exercise for arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage 2015;23:1090–8. 10.1016/j.joca.2015.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Saraboon Y, Aree-Ue S, Maruo SJ. The effect of multifactorial intervention programs on health behavior and symptom control among community-dwelling overweight older adults with knee osteoarthritis. Orthop Nurs 2015;34:296–308. 10.1097/NOR.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 263.Beavers DP, Beavers KM, Loeser RF, et al. The independent and combined effects of intensive weight loss and exercise training on bone mineral density in overweight and obese older adults with osteoarthritis. Osteoarthritis Cartilage 2014;22:726–33. 10.1016/j.joca.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Henriksen M, Christensen R, Hunter DJ, et al. Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage 2014;22:639–46. 10.1016/j.joca.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 265.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263–73. 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Somers TJ, Blumenthal JA, Guilak F, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain 2012;153:1199–209. 10.1016/j.pain.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bliddal H, Leeds AR, Stigsgaard L, et al. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis 2011;70:1798–803. 10.1136/ard.2010.142018 [DOI] [PubMed] [Google Scholar]
- 268.Gudbergsen H, Boesen M, Christensen R, et al. Radiographs and low field MRI (0.2T) as predictors of efficacy in a weight loss trial in obese women with knee osteoarthritis. BMC Musculoskelet Disord 2011;12. 10.1186/1471-2474-12-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Riecke BF, Christensen R, Christensen P, et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage 2010;18:746–54. 10.1016/j.joca.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 270.Ravaud P, Flipo RM, Boutron I. ARTIST (osteoarthritis intervention standardized) study of standardised consultation versus usual care for patients with osteoarthritis of the knee in primary care in France: pragmatic randomised controlled trial. BMJ 2009;338:b421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Miller GD, Nicklas BJ, Loeser RF. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J Am Geriatr Soc 2008;56:644–51. 10.1111/j.1532-5415.2007.01636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Miller GD, Nicklas BJ, Davis C, et al. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity 2006;14:1219–30. 10.1038/oby.2006.139 [DOI] [PubMed] [Google Scholar]
- 273.Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage 2005;13:20–7. 10.1016/j.joca.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 274.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum 2004;50:1501–10. 10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- 275.Rejeski WJ, Focht BC, Messier SP, et al. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol 2002;21:419–26. 10.1037/0278-6133.21.5.419 [DOI] [PubMed] [Google Scholar]
- 276.Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc 2000;48:1062–72. 10.1111/j.1532-5415.2000.tb04781.x [DOI] [PubMed] [Google Scholar]
- 277.Toda Y. The effect of energy restriction, walking, and exercise on lower extremity lean body mass in obese women with osteoarthritis of the knee. J Orthop Sci 2001;6:148–54. 10.1007/s007760100063 [DOI] [PubMed] [Google Scholar]
- 278.Huang MH, Chen CH, Chen TW, et al. The effects of weight reduction on the rehabilitation of patients with knee osteoarthritis and obesity. Arthritis Care Res 2000;13:398–405. [DOI] [PubMed] [Google Scholar]
- 279.Bartholdy C, Christensen R, Kristensen LE, et al. Association between weight loss and spontaneous changes in physical inactivity in overweight/obese individuals with knee osteoarthritis: an 8-week prospective cohort study. Arthritis Care Res 2020;72:397–404. 10.1002/acr.23868 [DOI] [PubMed] [Google Scholar]
- 280.Aree-Ue S, Saraboon Y, Belza B. Long-Term adherence and effectiveness of a multicomponent intervention for community-dwelling overweight Thai older adults with knee osteoarthritis: 1-year follow up. J Gerontol Nurs 2017;43:40–8. 10.3928/00989134-20170111-09 [DOI] [PubMed] [Google Scholar]
- 281.Atukorala I, Makovey J, Lawler L, et al. Is there a dose-response relationship between weight loss and symptom improvement in persons with knee osteoarthritis? Arthritis Care Res 2016;68:1106–14. 10.1002/acr.22805 [DOI] [PubMed] [Google Scholar]
- 282.Bartels EM, Christensen R, Christensen P, et al. Effect of a 16 weeks weight loss program on osteoarthritis biomarkers in obese patients with knee osteoarthritis: a prospective cohort study. Osteoarthritis Cartilage 2014;22:1817-25. 10.1016/j.joca.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 283.Paans N, van den Akker-Scheek I, Dilling RG, et al. Effect of exercise and weight loss in people who have hip osteoarthritis and are overweight or obese: a prospective cohort study. Phys Ther 2013;93:137–46. 10.2522/ptj.20110418 [DOI] [PubMed] [Google Scholar]
- 284.Gudbergsen H, Boesen M, Lohmander LS, et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage 2012;20:495–502. 10.1016/j.joca.2012.02.639 [DOI] [PubMed] [Google Scholar]
- 285.Bihlet AR, Byrjalsen I, Bay-Jensen A-C, et al. Identification of pain categories associated with change in pain in patients receiving placebo: data from two phase 3 randomized clinical trials in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 2018;19:17. 10.1186/s12891-018-1938-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Han A, Gellhorn AC. Trajectories of quality of life and associated risk factors in patients with knee osteoarthritis: findings from the osteoarthritis initiative. Am J Phys Med Rehabil 2018;97:620–7. 10.1097/PHM.0000000000000926 [DOI] [PubMed] [Google Scholar]
- 287.Jacobs CA, Vranceanu A-M, Thompson KL, et al. Rapid progression of knee pain and osteoarthritis biomarkers greatest for patients with combined obesity and depression: data from the osteoarthritis initiative. Cartilage 2020;11:38–46. 10.1177/1947603518777577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pelletier J-P, Raynauld J-P, Abram F, et al. Exploring determinants predicting response to intra-articular hyaluronic acid treatment in symptomatic knee osteoarthritis: 9-year follow-up data from the osteoarthritis initiative. Arthritis Res Ther 2018;20:40. 10.1186/s13075-018-1538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Eymard F, Chevalier X, Conrozier T. Obesity and radiological severity are associated with viscosupplementation failure in patients with knee osteoarthritis. J Orthop Res 2017;35:2269–74. 10.1002/jor.23529 [DOI] [PubMed] [Google Scholar]
- 290.Moyer R, Wirth W, Eckstein F. Longitudinal changes in magnetic resonance imaging-based measures of femorotibial cartilage thickness as a function of alignment and obesity: data from the osteoarthritis initiative. Arthritis Care Res 2017;69:959–65. 10.1002/acr.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bastick AN, Verkleij SPJ, Damen J, et al. Defining hip pain trajectories in early symptomatic hip osteoarthritis--5 year results from a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage 2016;24:768-75. 10.1016/j.joca.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 292.de Rezende MU, Hissadomi MI, de Campos GC, et al. One-Year results of an educational program on osteoarthritis: a prospective randomized controlled trial in Brazil. Geriatr Orthop Surg Rehabil 2016;7:86–94. 10.1177/2151458516645634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Beavers KM, Beavers DP, Newman JJ, et al. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis Cartilage 2015;23:249–56. 10.1016/j.joca.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chatterjee D, McGee A, Strauss E, et al. Subchondral calcium phosphate is ineffective for bone marrow edema lesions in adults with advanced osteoarthritis. Clin Orthop Relat Res 2015;473:2334–42. 10.1007/s11999-015-4311-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Karsdal MA, Bihlet A, Byrjalsen I, et al. OA phenotypes, rather than disease stage, drive structural progression--identification of structural progressors from 2 phase III randomized clinical studies with symptomatic knee OA. Osteoarthritis Cartilage 2015;23:550–8. 10.1016/j.joca.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 296.Kobayashi N, Inaba Y, Yukizawa Y, et al. Use of 18F-fluoride positron emission tomography as a predictor of the hip osteoarthritis progression. Mod Rheumatol 2015;25:925–30. 10.3109/14397595.2015.1045257 [DOI] [PubMed] [Google Scholar]
- 297.Magnusson K, Slatkowsky-Christensen B, van der Heijde D, et al. Body mass index and progressive hand osteoarthritis: data from the Oslo hand osteoarthritis cohort. Scand J Rheumatol 2015;44:331–6. 10.3109/03009742.2014.994560 [DOI] [PubMed] [Google Scholar]
- 298.Gudbergsen H, Boesen M, Christensen R, et al. Changes in bone marrow lesions in response to weight-loss in obese knee osteoarthritis patients: a prospective cohort study. BMC Musculoskelet Disord 2013;14:106. 10.1186/1471-2474-14-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Perrot S, Bertin P. "Feeling better" or "feeling well" in usual care of hip and knee osteoarthritis pain: determination of cutoff points for patient acceptable symptom state (PASS) and minimal clinically important improvement (MCII) at rest and on movement in a national multicenter cohort study of 2414 patients with painful osteoarthritis. Pain 2013;154:248–56. 10.1016/j.pain.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 300.Coffman CJ, Allen KD, Woolson RF. Mixed-effects regression modeling of real-time momentary pain assessments in osteoarthritis (OA) patients. Health Serv Outcomes Res Method 2012;12:200–18. 10.1007/s10742-012-0085-y [DOI] [Google Scholar]
- 301.Miyazaki T, Uchida K, Sato M, et al. Knee laxity after staircase exercise predicts radiographic disease progression in medial compartment knee osteoarthritis. Arthritis Rheum 2012;64:3908–16. 10.1002/art.34662 [DOI] [PubMed] [Google Scholar]
- 302.Rabago D, Zgierska A, Fortney L, et al. Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: results of a single-arm uncontrolled study with 1-year follow-up. J Altern Complement Med 2012;18:408–14. 10.1089/acm.2011.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sands GH, Brown PB, Essex MN. The Efficacy of Continuous Versus Intermittent Celecoxib Treatment in Osteoarthritis Patients with Body Mass Index ≥30 and <30 kg/m(2.). Open Rheumatol J 2013;7:32–7. 10.2174/1874312901307010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bartlett SJ, Ling SM, Mayo NE, et al. Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthritis Care Res 2011;63:1722–8. 10.1002/acr.20614 [DOI] [PubMed] [Google Scholar]
- 305.Bingham CO, Smugar SS, Wang H, et al. Predictors of response to cyclo-oxygenase-2 inhibitors in osteoarthritis: pooled results from two identical trials comparing etoricoxib, celecoxib, and placebo. Pain Med 2011;12:352–61. 10.1111/j.1526-4637.2011.01060.x [DOI] [PubMed] [Google Scholar]
- 306.Nishimura A, Hasegawa M, Kato K, et al. Risk factors for the incidence and progression of radiographic osteoarthritis of the knee among Japanese. Int Orthop 2011;35:839–43. 10.1007/s00264-010-1073-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Richette P, Poitou C, Garnero P, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis 2011;70:139–44. 10.1136/ard.2010.134015 [DOI] [PubMed] [Google Scholar]
- 308.Woollard JD, Gil AB, Sparto P, et al. Change in knee cartilage volume in individuals completing a therapeutic exercise program for knee osteoarthritis. J Orthop Sports Phys Ther 2011;41:708–22. 10.2519/jospt.2011.3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage 2011;19:1117–22. 10.1016/j.joca.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 310.Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT study. J Gerontol A Biol Sci Med Sci 2010;65:519–25. 10.1093/gerona/glp217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Eckstein F, Maschek S, Wirth W, et al. One year change of knee cartilage morphology in the first release of participants from the osteoarthritis initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis 2009;68:674–9. 10.1136/ard.2008.089904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Le Graverand M-PH, Brandt K, Mazzuca SA, et al. Progressive increase in body mass index is not associated with a progressive increase in joint space narrowing in obese women with osteoarthritis of the knee. Ann Rheum Dis 2009;68:1734–8. 10.1136/ard.2007.085530 [DOI] [PubMed] [Google Scholar]
- 313.Botha-Scheepers S, Dougados M, Ravaud P, et al. Effect of medial tibial plateau alignment on serial radiographs on the capacity to predict progression of knee osteoarthritis. Osteoarthritis Cartilage 2008;16:272–6. 10.1016/j.joca.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 314.Davies-Tuck ML, Wluka AE, Wang Y, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage 2008;16:337–42. 10.1016/j.joca.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 315.Pelletier J-P, Raynauld J-P, Berthiaume M-J, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther 2007;9:R74. 10.1186/ar2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Reijman M, Pols HAP, Bergink AP, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam study. Ann Rheum Dis 2007;66:158–62. 10.1136/ard.2006.053538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Raynauld J-P, Martel-Pelletier J, Berthiaume M-J, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther 2006;8:R21. 10.1186/ar1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wluka AE, Forbes A, Wang Y, et al. Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res Ther 2006;8:R90. 10.1186/ar1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum 2003;48:3359–70. 10.1002/art.11420 [DOI] [PubMed] [Google Scholar]
- 320.Cicuttini F, Wluka A, Wang Y, et al. The determinants of change in patella cartilage volume in osteoarthritic knees. J Rheumatol 2002;29:2615–9. [PubMed] [Google Scholar]
- 321.Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol 2002;29:139–46. [PubMed] [Google Scholar]
- 322.Detora LM, Krupa D, Bolognese J, et al. Rofecoxib shows consistent efficacy in osteoarthritis clinical trials, regardless of specific patient demographic and disease factors. J Rheumatol 2001;28:2494–503. [PubMed] [Google Scholar]
- 323.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 2000;43:995. [DOI] [PubMed] [Google Scholar]
- 324.Harris PA, Hart DJ, Dacre JE, et al. The progression of radiological hand osteoarthritis over ten years: a clinical follow-up study. Osteoarthritis Cartilage 1994;2:247–52. 10.1016/S1063-4584(05)80076-7 [DOI] [PubMed] [Google Scholar]
- 325.Ledingham J, Dawson S, Preston B, et al. Radiographic progression of hospital referred osteoarthritis of the hip. Ann Rheum Dis 1993;52:263–7. 10.1136/ard.52.4.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Schouten JS, van den Ouweland FA, Valkenburg HA. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis 1992;51:932–7. 10.1136/ard.51.8.932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Berkhout B, Macfarlane JD, Cats A. Symptomatic osteoarthrosis of the knee: a follow-up study. Br J Rheumatol 1985;24:40–5. 10.1093/rheumatology/24.1.40 [DOI] [PubMed] [Google Scholar]
- 328.Ahn JH, Kang HW, Yang TY, et al. Risk factors for radiographic progression of osteoarthritis after meniscus allograft transplantation. Arthroscopy 2016;32:2539–46. 10.1016/j.arthro.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 329.Liu Y, Hazlewood GS, Kaplan GG, et al. Impact of obesity on remission and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2017;69:157–65. 10.1002/acr.22932 [DOI] [PubMed] [Google Scholar]
- 330.Lupoli R, Pizzicato P, Scalera A, et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther 2016;18:297. 10.1186/s13075-016-1194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Baghdadi LR, Woodman RJ, Shanahan EM, et al. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 2015;10:e0117952. 10.1371/journal.pone.0117952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Baker JF, Stokes A, Mikuls TR, et al. Current and early life weight and associations with mortality in rheumatoid arthritis. Clin Exp Rheumatol 2019;37:768–73. [PubMed] [Google Scholar]
- 333.Hirose W, Harigai M, Uchiyama T, et al. Low body mass index and lymphocytopenia associate with Mycobacterium avium complex pulmonary disease in patients with rheumatoid arthritis. Mod Rheumatol 2019;29:105–12. 10.1080/14397595.2018.1452334 [DOI] [PubMed] [Google Scholar]
- 334.Lechtenboehmer CA, Jaeger VK, Kyburz D, et al. Brief report: influence of disease activity in rheumatoid arthritis on radiographic progression of concomitant interphalangeal joint osteoarthritis. Arthritis Rheumatol 2019;71:43–9. 10.1002/art.40684 [DOI] [PubMed] [Google Scholar]
- 335.England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res 2018;70:11–18. 10.1002/acr.23258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Nikiphorou E, Norton S, Young A, et al. The association of obesity with disease activity, functional ability and quality of life in early rheumatoid arthritis: data from the early rheumatoid arthritis Study/Early rheumatoid arthritis network UK prospective cohorts. Rheumatology 2018;57:1194–202. 10.1093/rheumatology/key066 [DOI] [PubMed] [Google Scholar]
- 337.Rydell E, Forslind K, Nilsson Jan-Åke, et al. Smoking, body mass index, disease activity, and the risk of rapid radiographic progression in patients with early rheumatoid arthritis. Arthritis Res Ther 2018;20:82. 10.1186/s13075-018-1575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Schulman E, Bartlett SJ, Schieir O, et al. Overweight, obesity, and the likelihood of achieving sustained remission in early rheumatoid arthritis: results from a multicenter prospective cohort study. Arthritis Care Res 2018;70:1185–91. 10.1002/acr.23457 [DOI] [PubMed] [Google Scholar]
- 339.Smolen JS, Szumski A, Koenig AS, et al. Predictors of remission with etanercept-methotrexate induction therapy and loss of remission with etanercept maintenance, reduction, or withdrawal in moderately active rheumatoid arthritis: results of the PRESERVE trial. Arthritis Res Ther 2018;20:8. 10.1186/s13075-017-1484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sparks JA, Chang S-C, Nguyen U-S, et al. Weight change during the early rheumatoid arthritis period and risk of subsequent mortality in women with rheumatoid arthritis and matched comparators. Arthritis Rheumatol 2018;70:18–29. 10.1002/art.40346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.van der Heijde D, Durez P, Schett G, et al. Structural damage progression in patients with early rheumatoid arthritis treated with methotrexate, baricitinib, or baricitinib plus methotrexate based on clinical response in the phase 3 RA-BEGIN study. Clin Rheumatol 2018;37:2381–90. 10.1007/s10067-018-4221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Bird P, Nicholls D, Barrett R, et al. Longitudinal study of clinical prognostic factors in patients with early rheumatoid arthritis: the PREDICT study. Int J Rheum Dis 2017;20:460–8. 10.1111/1756-185X.13036 [DOI] [PubMed] [Google Scholar]
- 343.D'Agostino M-A, Alten R, Mysler E, et al. Body mass index and clinical response to intravenous or subcutaneous abatacept in patients with rheumatoid arthritis. Clin Rheumatol 2017;36:2655–65. 10.1007/s10067-017-3788-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.George MD, Østergaard M, Conaghan PG, et al. Obesity and rates of clinical remission and low MRI inflammation in rheumatoid arthritis. Ann Rheum Dis 2017;76:1743–6. 10.1136/annrheumdis-2017-211569 [DOI] [PubMed] [Google Scholar]
- 345.Iannone F, Courvoisier DS, Gottenberg JE, et al. Body mass does not impact the clinical response to intravenous abatacept in patients with rheumatoid arthritis. Analysis from the "pan-European registry collaboration for abatacept (PANABA). Clin Rheumatol 2017;36:773–9. 10.1007/s10067-016-3505-5 [DOI] [PubMed] [Google Scholar]
- 346.Joo YB, Bang S-Y, Ryu JA, et al. Predictors of severe radiographic progression in patients with early rheumatoid arthritis: a prospective observational cohort study. Int J Rheum Dis 2017;20:1437–46. 10.1111/1756-185X.13054 [DOI] [PubMed] [Google Scholar]
- 347.Levitsky A, Brismar K, Hafström I, et al. Obesity is a strong predictor of worse clinical outcomes and treatment responses in early rheumatoid arthritis: results from the SWEFOT trial. RMD Open 2017;3:e000458. 10.1136/rmdopen-2017-000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mariette X, Alten R, Nüßlein HG, et al. The effect of body mass index on clinical response to abatacept as a first-line biologic for rheumatoid arthritis: 6-month results from the 2-year, observational, prospective ACTION study. Joint Bone Spine 2017;84:571–6. 10.1016/j.jbspin.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 349.Miwa Y, Saito M, Furuya H, et al. Clinical characteristics of rheumatoid arthritis patients achieving functional remission after six months of non-tumor necrosis factor biological disease-modifying antirheumatic drugs (DMARDs) treatment. Intern Med 2017;56:2271–5. 10.2169/internalmedicine.8723-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ramírez J, Narváez JA, Ruiz-Esquide V, et al. Clinical and sonographic biomarkers of structural damage progression in RA patients in clinical remission: a prospective study with 12 months follow-up. Semin Arthritis Rheum 2017;47:303–9. 10.1016/j.semarthrit.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 351.Feldthusen C, Grimby-Ekman A, Forsblad-d'Elia H, et al. Explanatory factors and predictors of fatigue in persons with rheumatoid arthritis: a longitudinal study. J Rehabil Med 2016;48:469–76. 10.2340/16501977-2090 [DOI] [PubMed] [Google Scholar]
- 352.Gardette A, Ottaviani S, Sellam J, et al. Body mass index and response to abatacept in rheumatoid arthritis. Eur J Clin Invest 2016;46:1048–52. 10.1111/eci.12691 [DOI] [PubMed] [Google Scholar]
- 353.Gardette A, Ottaviani S, Sellam J, et al. Body mass index and response to tocilizumab in rheumatoid arthritis: a real life study. Clin Rheumatol 2016;35:857–61. 10.1007/s10067-016-3183-3 [DOI] [PubMed] [Google Scholar]
- 354.McWilliams DF, Walsh DA. Factors predicting pain and early discontinuation of tumour necrosis factor-α-inhibitors in people with rheumatoid arthritis: results from the British Society for rheumatology biologics register. BMC Musculoskelet Disord 2016;17:337. 10.1186/s12891-016-1192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Tantayakom P, Koolvisoot A, Arromdee E, et al. Metabolic syndrome is associated with disease activity in patients with rheumatoid arthritis. Joint Bone Spine 2016;83:563–7. 10.1016/j.jbspin.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 356.Baker JF, Billig E, Michaud K, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol 2015;67:1711–7. 10.1002/art.39136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Iannone F, Fanizzi R, Notarnicola A, et al. Obesity reduces the drug survival of second line biological drugs following a first TNF-α inhibitor in rheumatoid arthritis patients. Joint Bone Spine 2015;82:187–91. 10.1016/j.jbspin.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 358.Pers Y-M, Godfrin-Valnet M, Lambert J, et al. Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J Rheumatol 2015;42:580–4. 10.3899/jrheum.140673 [DOI] [PubMed] [Google Scholar]
- 359.Kim HW, Park JK, Yang J-A, et al. Comparison of tuberculosis incidence in ankylosing spondylitis and rheumatoid arthritis during tumor necrosis factor inhibitor treatment in an intermediate burden area. Clin Rheumatol 2014;33:1307–12. 10.1007/s10067-013-2387-z [DOI] [PubMed] [Google Scholar]
- 360.Ochi K, Go Y, Furuya T, et al. Risk factors associated with the occurrence of distal radius fractures in Japanese patients with rheumatoid arthritis: a prospective observational cohort study. Clin Rheumatol 2014;33:477–83. 10.1007/s10067-013-2415-z [DOI] [PubMed] [Google Scholar]
- 361.Sandberg MEC, Bengtsson C, Källberg H, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis 2014;73:2029–33. 10.1136/annrheumdis-2013-205094 [DOI] [PubMed] [Google Scholar]
- 362.Ajeganova S, Andersson ML, Hafström I, et al. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term followup from disease onset. Arthritis Care Res 2013;65:78–87. 10.1002/acr.21710 [DOI] [PubMed] [Google Scholar]
- 363.Gremese E, Carletto A, Padovan M, et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res 2013;65:94–100. 10.1002/acr.21768 [DOI] [PubMed] [Google Scholar]
- 364.Kanecki K, Tyszko P, Wisłowska M, et al. Preliminary report on a study of health-related quality of life in patients with rheumatoid arthritis. Rheumatol Int 2013;33:429–34. 10.1007/s00296-012-2421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Dirven L, Huizinga TWJ, Allaart CF. Risk factors for reported influenza and influenza-like symptoms in patients with rheumatoid arthritis. Scand J Rheumatol 2012;41:359–65. 10.3109/03009742.2012.670729 [DOI] [PubMed] [Google Scholar]
- 366.Wevers-de Boer K, Visser K, Heimans L, et al. Remission induction therapy with methotrexate and prednisone in patients with early rheumatoid and undifferentiated arthritis (the IMPROVED study). Ann Rheum Dis 2012;71:1472–7. 10.1136/annrheumdis-2011-200736 [DOI] [PubMed] [Google Scholar]
- 367.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res 2012;64:1471–9. 10.1002/acr.21627 [DOI] [PubMed] [Google Scholar]
- 368.de Rooy DPC, van der Linden MPM, Knevel R, et al. Predicting arthritis outcomes--what can be learned from the Leiden Early Arthritis Clinic? Rheumatology 2011;50:93–100. 10.1093/rheumatology/keq230 [DOI] [PubMed] [Google Scholar]
- 369.Klaasen R, Wijbrandts CA, Gerlag DM, et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum 2011;63:359–64. 10.1002/art.30136 [DOI] [PubMed] [Google Scholar]
- 370.Liao KP, Weinblatt ME, Cui J, et al. Clinical predictors of erosion-free status in rheumatoid arthritis: a prospective cohort study. Rheumatology 2011;50:1473–9. 10.1093/rheumatology/ker129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tekaya R, Sahli H, Zribi S. [Obesity has a protective effect on radiographic joint damage in rheumatoid arthritis]. Tunis Med 2011;89. [PubMed] [Google Scholar]
- 372.Pye SR, Marshall T, Gaffney K, et al. Influence of arthritis and non-arthritis related factors on areal bone mineral density (BMDa) in women with longstanding inflammatory polyarthritis: a primary care based inception cohort. BMC Musculoskelet Disord 2010;11:106. 10.1186/1471-2474-11-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Verstappen SMM, Bakker MF, Heurkens AHM, et al. Adverse events and factors associated with toxicity in patients with early rheumatoid arthritis treated with methotrexate tight control therapy: the CAMERA study. Ann Rheum Dis 2010;69:1044–8. 10.1136/ard.2008.106617 [DOI] [PubMed] [Google Scholar]
- 374.Furuya T, Yamagiwa K, Ikai T, et al. Associated factors for falls and fear of falling in Japanese patients with rheumatoid arthritis. Clin Rheumatol 2009;28:1325–30. 10.1007/s10067-009-1229-5 [DOI] [PubMed] [Google Scholar]
- 375.Hashimoto J, Garnero P, van der Heijde D, et al. A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod Rheumatol 2009;19:273–82. 10.1007/s10165-009-0170-4 [DOI] [PubMed] [Google Scholar]
- 376.van der Helm-van Mil AHM, van der Kooij SM, Allaart CF, et al. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis 2008;67:769–74. 10.1136/ard.2007.078832 [DOI] [PubMed] [Google Scholar]
- 377.Cohen J-D, Dougados M, Goupille P, et al. Health assessment questionnaire score is the best predictor of 5-year quality of life in early rheumatoid arthritis. J Rheumatol 2006;33:1936–41. [PubMed] [Google Scholar]
- 378.Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 2005;165:1624–9. 10.1001/archinte.165.14.1624 [DOI] [PubMed] [Google Scholar]
- 379.Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 2005;52:722–32. 10.1002/art.20878 [DOI] [PubMed] [Google Scholar]
- 380.Sköldstam L, Brudin L, Hagfors L, et al. Weight reduction is not a major reason for improvement in rheumatoid arthritis from lacto-vegetarian, vegan or Mediterranean diets. Nutr J 2005;4:15. 10.1186/1475-2891-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum 2004;50:3450–7. 10.1002/art.20612 [DOI] [PubMed] [Google Scholar]
- 382.Hoekstra M, van Ede AE, Haagsma CJ, et al. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann Rheum Dis 2003;62:423–6. 10.1136/ard.62.5.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Krishnan E, Lingala B, Bruce B, et al. Disability in rheumatoid arthritis in the era of biological treatments. Ann Rheum Dis 2012;71:213–8. 10.1136/annrheumdis-2011-200354 [DOI] [PubMed] [Google Scholar]
- 384.Kreps DJ, Halperin F, Desai SP, et al. Association of weight loss with improved disease activity in patients with rheumatoid arthritis: a retrospective analysis using electronic medical record data. Int J Clin Rheumtol 2018;13:1–10. 10.4172/1758-4272.1000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Mori S, Yoshitama T, Hidaka T, et al. Comparative risk of hospitalized infection between biological agents in rheumatoid arthritis patients: a multicenter retrospective cohort study in Japan. PLoS One 2017;12:e0179179. 10.1371/journal.pone.0179179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Rashid N, Lin AT, Aranda G, et al. Rates, factors, reasons, and economic impact associated with switching in rheumatoid arthritis patients newly initiated on biologic disease modifying anti-rheumatic drugs in an integrated healthcare system. J Med Econ 2016;19:568–75. 10.3111/13696998.2016.1142448 [DOI] [PubMed] [Google Scholar]
- 387.Ottaviani S, Gardette A, Roy C, et al. Body mass index and response to rituximab in rheumatoid arthritis. Joint Bone Spine 2015;82:432–6. 10.1016/j.jbspin.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 388.Ottaviani S, Gardette A, Tubach F, et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol 2015;33:478–83. [PubMed] [Google Scholar]
- 389.Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res 2015;67:1619–26. 10.1002/acr.22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 2008;67:64–9. 10.1136/ard.2006.059980 [DOI] [PubMed] [Google Scholar]
- 391.Kent PD, Luthra HS, Michet C. Risk factors for methotrexate-induced abnormal laboratory monitoring results in patients with rheumatoid arthritis. J Rheumatol 2004;31:1727–31. [PubMed] [Google Scholar]
- 392.Figueiredo-Braga M, Cornaby C, Bernardes M, et al. Correlation between physical markers and psychiatric health in a Portuguese systemic lupus erythematosus cohort: the role of suffering in chronic autoimmune disease. PLoS One 2018;13:e0195579. 10.1371/journal.pone.0195579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Jacobs J, Korswagen L-A, Schilder AM, et al. Six-year follow-up study of bone mineral density in patients with systemic lupus erythematosus. Osteoporos Int 2013;24:1827–33. 10.1007/s00198-012-2157-9 [DOI] [PubMed] [Google Scholar]
- 394.Katz P, Yazdany J, Julian L, et al. Impact of obesity on functioning among women with systemic lupus erythematosus. Arthritis Care Res 2011;63:1357–64. 10.1002/acr.20526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chaiamnuay S, Bertoli AM, Fernández M, et al. The impact of increased body mass index on systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA XLVI) [corrected]. J Clin Rheumatol 2007;13:128–33. 10.1097/RHU.0b013e3180645865 [DOI] [PubMed] [Google Scholar]
- 396.Chaiamnuay S, Bertoli AM, Roseman JM, et al. African-American and Hispanic ethnicities, renal involvement and obesity predispose to hypertension in systemic lupus erythematosus: results from LUMINA, a multiethnic cohort (LUMINAXLV). Ann Rheum Dis 2007;66:618–22. 10.1136/ard.2006.059311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Uaratanawong S, Deesomchok U, Hiransuttikul N, et al. Four years follow-up of bone mineral density change in premenopausal women with systemic lupus erythematosus. J Med Assoc Thai 2004;87:1374–9. [PubMed] [Google Scholar]
- 398.Bruce IN, Gladman DD, Urowitz MB. Detection and modification of risk factors for coronary artery disease in patients with systemic lupus erythematosus: a quality improvement study. Clin Exp Rheumatol 1998;16:435–40. [PubMed] [Google Scholar]
- 399.Petri M, Perez-Gutthann S, Spence D, et al. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 1992;93:513–9. 10.1016/0002-9343(92)90578-Y [DOI] [PubMed] [Google Scholar]
- 400.Hernández-Breijo B, Plasencia-Rodríguez C, Navarro-Compán V, et al. Association between concomitant csDMARDs and clinical response to TNF inhibitors in overweight patients with axial spondyloarthritis. Arthritis Res Ther 2019;21:66. 10.1186/s13075-019-1849-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Jeong H, Eun YH, Kim IY, et al. Effect of tumor necrosis factor α inhibitors on spinal radiographic progression in patients with ankylosing spondylitis. Int J Rheum Dis 2018;21:1098–105. 10.1111/1756-185X.13270 [DOI] [PubMed] [Google Scholar]
- 402.Pedersen SJ, Weber U, Said-Nahal R. Structural progression rate decreases over time on serial radiography and magnetic resonance imaging of sacroiliac joints and spine in a five-year follow-up study of patients with ankylosing spondylitis treated with tumour necrosis factor inhibitor. Scand J Rheumatol 2018:1–13. [DOI] [PubMed] [Google Scholar]
- 403.Maas F, Arends S, Wink FR, et al. Ankylosing spondylitis patients at risk of poor radiographic outcome show diminishing spinal radiographic progression during long-term treatment with TNF-α inhibitors. PLoS One 2017;12:e0177231. 10.1371/journal.pone.0177231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Maas F, Spoorenberg A, van der Slik BPG, et al. Clinical risk factors for the presence and development of vertebral fractures in patients with ankylosing spondylitis. Arthritis Care Res 2017;69:694–702. 10.1002/acr.22980 [DOI] [PubMed] [Google Scholar]
- 405.Micheroli R, Hebeisen M, Wildi LM, et al. Impact of obesity on the response to tumor necrosis factor inhibitors in axial spondyloarthritis. Arthritis Res Ther 2017;19:164. 10.1186/s13075-017-1372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Hwang J, Kim H-M, Jeong H, et al. Higher body mass index and anti-drug antibodies predict the discontinuation of anti-TNF agents in Korean patients with axial spondyloarthritis. Rev Bras Reumatol Engl Ed 2017;57:311–9. 10.1016/j.rbre.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 407.van Weely SFE, Kneepkens EL, Nurmohamed MT, et al. Continuous improvement of physical functioning in ankylosing spondylitis patients by tumor necrosis factor inhibitors: three-year followup and predictors. Arthritis Care Res 2016;68:1522–9. 10.1002/acr.22869 [DOI] [PubMed] [Google Scholar]
- 408.Maas F, Spoorenberg A, Brouwer E, et al. Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-α blocking therapy: a prospective longitudinal observational cohort study. PLoS One 2015;10:e0122693. 10.1371/journal.pone.0122693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Gremese E, Bernardi S, Bonazza S, et al. Body weight, gender and response to TNF-α blockers in axial spondyloarthritis. Rheumatology 2014;53:875–81. 10.1093/rheumatology/ket433 [DOI] [PubMed] [Google Scholar]
- 410.Ottaviani S, Allanore Y, Tubach F, et al. Body mass index influences the response to infliximab in ankylosing spondylitis. Arthritis Res Ther 2012;14:R115. 10.1186/ar3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Di Minno MND, Peluso R, Iervolino S, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis 2014;73:1157–62. 10.1136/annrheumdis-2012-202812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther 2019;21. 10.1186/s13075-019-1810-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Polachek A, Li S, Chandran V, et al. Clinical Enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res 2017;69:1685–91. 10.1002/acr.23174 [DOI] [PubMed] [Google Scholar]
- 414.Højgaard P, Glintborg B, Kristensen LE, et al. The influence of obesity on response to tumour necrosis factor-α inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology 2016;55:2191–9. 10.1093/rheumatology/kew326 [DOI] [PubMed] [Google Scholar]
- 415.Eder L, Thavaneswaran A, Chandran V, et al. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis 2015;74:813–7. 10.1136/annrheumdis-2013-204448 [DOI] [PubMed] [Google Scholar]
- 416.Mease PJ, Collier DH, Saunders KC, et al. Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open 2015;1:e000181. 10.1136/rmdopen-2015-000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.di Minno MND, Peluso R, Iervolino S, et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res 2013;65:141–7. 10.1002/acr.21711 [DOI] [PubMed] [Google Scholar]
- 418.Iannone F, Fanizzi R, Scioscia C, et al. Body mass does not affect the remission of psoriatic arthritis patients on anti-TNF-α therapy. Scand J Rheumatol 2013;42:41–4. 10.3109/03009742.2012.715186 [DOI] [PubMed] [Google Scholar]
- 419.Haddad A, Thavaneswaran A, Toloza S, et al. Diffuse idiopathic skeletal hyperostosis in psoriatic arthritis. J Rheumatol 2013;40:1367–73. 10.3899/jrheum.121433 [DOI] [PubMed] [Google Scholar]
- 420.Marini C, Formichi B, Bauleo C, et al. Survival protection by bodyweight in isolated scleroderma-related pulmonary artery hypertension. Intern Emerg Med 2016;11:941–52. 10.1007/s11739-016-1446-2 [DOI] [PubMed] [Google Scholar]
- 421.Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009;61:1403–11. 10.1002/art.24734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Nielsen SM, Bartels EM, Henriksen M, et al. Weight loss for overweight and obese individuals with gout: a systematic review of longitudinal studies. Ann Rheum Dis 2017;76:1870–82. 10.1136/annrheumdis-2017-211472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Dessein PH, Shipton EA, Stanwix AE, et al. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539–43. 10.1136/ard.59.7.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Nguyen U-SDT, Zhang Y, Louie-Gao Q, et al. Obesity paradox in recurrent attacks of gout in observational studies: clarification and remedy. Arthritis Care Res 2017;69:561–6. 10.1002/acr.22954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Romero-Talamás H, Daigle CR, Aminian A, et al. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis 2014;10:1161–5. 10.1016/j.soard.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 426.Su BY-J, Lai H-M, Chen C-J, et al. Ischemia heart disease and greater waist circumference are risk factors of renal function deterioration in male gout patients. Clin Rheumatol 2008;27:581–6. 10.1007/s10067-007-0750-7 [DOI] [PubMed] [Google Scholar]
- 427.Abhishek A, Valdes AM, Zhang W, et al. Association of serum uric acid and disease duration with frequent gout attacks: a case-control study. Arthritis Care Res 2016;68:1573–7. 10.1002/acr.22855 [DOI] [PubMed] [Google Scholar]
- 428.Alvarez-Nemegyei J, Cen-Pisté JC, Medina-Escobedo M, et al. Factors associated with musculoskeletal disability and chronic renal failure in clinically diagnosed primary gout. J Rheumatol 2005;32:1923–7. [PubMed] [Google Scholar]
- 429.Gwinnutt JM, Verstappen SM, Humphreys JH. The impact of lifestyle behaviours, physical activity and smoking on morbidity and mortality in patients with rheumatoid arthritis. Best Pract Res Clin Rheumatol 2020;34:101562. 10.1016/j.berh.2020.101562 [DOI] [PubMed] [Google Scholar]
- 430.Veldhuijzen van Zanten JJCS, Rouse PC, Hale ED, et al. Perceived barriers, facilitators and benefits for regular physical activity and exercise in patients with rheumatoid arthritis: a review of the literature. Sports Med 2015;45:1401–12. 10.1007/s40279-015-0363-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Gwinnutt JM, Alsafar H, Hyrich KL, et al. Do people with rheumatoid arthritis maintain their physical activity level at treatment onset over the first year of methotrexate therapy? Rheumatology 2021;60:4633–42. 10.1093/rheumatology/keab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Klein-Wieringa IR, van der Linden MPM, Knevel R, et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum 2011;63:2567–74. 10.1002/art.30449 [DOI] [PubMed] [Google Scholar]
- 433.Shin A, Shin S, Kim JH, et al. Association between socioeconomic status and comorbidities among patients with rheumatoid arthritis: results of a nationwide cross-sectional survey. Rheumatology 2019;58:1617–22. 10.1093/rheumatology/kez081 [DOI] [PubMed] [Google Scholar]
- 434.Björk M, Dragioti E, Alexandersson H, et al. Inflammatory arthritis and the effect of physical activity on quality of life and self-reported function: a systematic review and meta-analysis. Arthritis Care Res 2022;74:31–43. 10.1002/acr.24805 [DOI] [PubMed] [Google Scholar]
- 435.Ortolan A, Lorenzin M, Felicetti M, et al. Do obesity and overweight influence disease activity measures in axial spondyloarthritis? A systematic review and meta-analysis. Arthritis Care Res 2021;73:1815–25. 10.1002/acr.24416 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002168supp001.pdf (7.5MB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study.