Abstract
Background:
U.S. women who have been incarcerated report high rates of sexual risk behavior and sexually transmitted infections (STIs).
Materials and Methods:
We estimated the effect of incarceration on the time to first incident STI in a multicenter cohort of U.S. women with or at risk for HIV. We used marginal structural models to compare time to first self-reported gonorrhea, chlamydia, or trichomonas infection for nonincarcerated women and incarcerated women. Covariates included demographic factors, HIV status, sex exchange, drug/alcohol use, and prior incarceration.
Results:
Three thousand hundred twenty-four women contributed a median of 4 at-risk years and experienced 213 first incident STI events. The crude incidence of STIs was 3.7 per 100 person-years for incarcerated women and 1.9 per 100 person-years for nonincarcerated women. The weighted hazard ratio for incident STIs was 4.05 (95% confidence interval: 1.61–10.19).
Conclusion:
Women with or at risk for HIV in the United States who have recently experienced incarceration may be at increased STI risk.
Keywords: incarceration, women, gonorrhea, chlamydia, trichomonas, STI
Introduction
Women in the United States who have experienced incarceration bear a disproportionate burden of sexually transmitted infections (STIs). The rates of gonorrheal, chlamydial, and trichomonal infections reported among incarcerated women (5.7%–9.2%, 11.3%–21.5%, and 14%, respectively) are approximately double the rates among women in the general U.S. population.1–7 Gonorrhea and chlamydia are most common among young women; however, women ages 35–55 who experience incarceration are diagnosed with chlamydia and gonorrhea at higher rates than their never-incarcerated counterparts.1,2,4
Incarceration is a significant risk factor for trichomonas across age groups, and studies of trichomonas among incarcerated women demonstrate clinically significant rates of infection in women older than the age of 35 years.7,8 For women in this age group, which is generally considered to be at lower risk of STIs, incarceration may be an important risk factor.
The high prevalence of STIs among incarcerated women is due, in part, to their preincarceration behaviors, including high rates of multiple partnerships, sexual partners who are themselves at high risk of STIs, condomless sex, and sex exchange.9–14 The majority of women in prisons and jails used drugs before incarceration, and drug use is associated with both sex exchange and sex with partners who inject drugs.15,16 Preincarceration STI risk may be heightened further because many women who experience incarceration have sexual networks with high rates of STIs and have overlapping sexual and drug-use networks.13,16,17
The experience of incarceration may act as a structural force across the life course that increases future risk for STIs, as it disrupts relationships and causes economic hardship that may be associated with a subsequent increase in STI risk.13 The disruptive effects of incarceration persist among women who are older than the age of 35 years; we have previously demonstrated that incarceration is associated with subsequent increases in the numbers of total and new sexual partners among a longitudinal cohort of women with median age of 44.18 Evidence of a significant association between incarceration and subsequent STIs from cross-sectional studies has been mixed, not only due to the difficulty of assessing temporal relationships in cross-sectional data, but also due to the variable omission of drug use and sex exchange in analyses.9–11
The objective of this study was to estimate the effect of incarceration on the combined incidence of self-reported gonorrhea, chlamydia, and trichomonas among a longitudinal cohort of women with HIV or at risk for HIV in the United States, accounting for confounding using marginal structural models (MSM). We hypothesized that if an episode of incarceration increased risk for STIs, the time to STI diagnosis following incarceration would be shorter compared with women who did not experience incarceration.
Materials and Methods
Women's Interagency HIV Study
The Women's Interagency HIV Study (WIHS) is a geographically diverse multicenter cohort study of women with or at risk for HIV; recruitment, retention, and participant characteristics are described elsewhere.19 Since the initiation of the cohort in 1993, women aged 25 to 60 years have been recruited in four waves and then participated in biannual study visits. Eligibility criteria have been similar across waves. Women were considered at risk for HIV and eligible if they had at least one high-risk exposure in the preceding 5 years.
High-risk exposures were defined as an STI diagnosis, sex without a condom with three or more men, sex with a condom with six or more men, trading sex, sex with a man with HIV, injection drug use, use of crack cocaine, cocaine, heroin, or methamphetamine, or having a partner who had any of these high-risk exposures. Questions regarding incarceration were added to the WIHS in October 2007. Women consented to the use of their data as part of their overall WIHS participation, and the Institutional Review Board at our institution approved this secondary data analysis. The STROBE checklist for this cohort analysis is included as Supplementary Data.
Eligibility
To be considered at-risk for incident STIs in this analysis, WIHS participants were required to contribute two STI-free study visits (with no more than one missed visit between), starting from the first visit, in which they were asked the incarceration questions. We allowed one missed visit because we assumed women were not incarcerated at missed visits, unless study staff noted specifically that the visit was missed due to incarceration. These two “run-in” visits meant that women were free of self-reported STIs at the start of follow-up and provided historical values for incarceration measures and the covariates.
Our analytic timescale was visits in calendar time. The study period included October 2007 through September 2017. Women were followed until one of the following events: (1) report of an STI, (2) death, (3) loss to follow-up (defined as missing two consecutive visits), or (4) administrative censoring after 10 visits. Women who died, were lost to follow-up, or were administratively censored were censored at their last attended visit; see Supplementary Appendix SA for additional details.
Measures
Time-varying incarceration exposures were based on reporting “yes” or “no” to being incarcerated in either a prison or a jail in the past 6 months. Study staff also indicated visits missed due to incarceration based on information obtained from the participant. The STI outcome was based on participant's first report of a diagnosis by a health care professional of gonorrhea, chlamydia, or trichomonas in the prior 6 months during the study period. No STI testing was performed for confirmation. We included these three infections together since they often have similar clinical presentations (e.g., vaginal discharge) and community practitioners frequently test for all three in symptomatic women.
To maintain accurate temporal relationships between the outcome reported at a given visit and previous exposures, we considered the exposure at the visit before outcome ascertainment (with confounders reported at the visit before the exposure).
We considered both baseline and time-varying confounders based on the criminal legal and STI literature. We accounted for historical incarceration at baseline (participants were asked about incarceration before the start of the study period).10,20,21 Baseline sociodemographic data included age at first visit during the study period, race, which was coded as “Black,” “White,” or “other,” and Hispanic ethnicity.21,22
We classified women at their first visit during the study period as enrolled in Bronx, NY, Brooklyn, NY, Washington, DC, San Francisco, CA, Los Angeles, CA, Chicago, IL, Chapel Hill, NC, Atlanta, GA, Miami, FL, Birmingham, AL, or Jackson, MS.23,24 The two NY sites were grouped together as were the Southern sites (NC, GA, FL, AL, and MS), due to small sample sizes. We dichotomized level of education into completion of less than or at least high school.21,25 Baseline HIV status was used; the four women who seroconverted during the study period were considered HIV-seronegative.26,27
Our time-varying confounders were sex exchange and substance use.10 Women were asked at each visit whether they had sex for drugs, money, or shelter in the past 6 months. We included three variables describing substance use in the 6 months before each visit: (1) any illicit drug use (crack cocaine, cocaine, heroin, methamphetamines, other opioids, or any injection drug use); (2) alcohol use (none, 1–7, or >7 drinks/week); and (3) any marijuana use.16,28
Missing data
We encountered missing data in our exposure, outcome, and covariates. We handled covariate missing data other than the baseline incarceration history covariate by carrying forward the last nonmissing value or, if there were no previous values, by carrying backwards from the first future, nonmissing value. Multiple imputation with 50 multiply-imputed data sets was used to handle missingness in the incarceration exposure and history, and the STI outcome; details can be found in Supplementary Appendix SA.
Statistical approach
Traditionally, the effect of a time-varying exposure on a time-to-event outcome is estimated using a time-dependent Cox proportional hazards model. This approach may be biased if there are time-dependent confounders that are affected by past exposure but may also affect future exposure.29 For our analysis, the literature suggests that substance use and sex exchange meet these criteria. Both are risk factors for STIs and are affected by prior incarceration due to the collateral consequences of incarceration on housing and employment; substance use and sex exchange also affect future incarceration as they are some of the most common reasons that women are arrested.14,30,31
Under certain assumptions (e.g., conditional exchangeability, positivity, and counterfactual consistency), inverse-probability (IP)-weighted MSM allow for consistent estimation of the causal effect of a time-varying exposure on an outcome, controlling for time-varying confounders.32 With a correctly specified weight model, the IP weights create a pseudo-population where time-varying confounding is no longer present.
To estimate the effect of the exposure on the outcome, the final weighted outcome model need only include the exposure and time. Once the weights are applied in this way, the interpretation of the final model is similar to a Cox proportional hazards model. Supplementary Appendix SA provides details of the modeling approach, and Supplementary Figure S1 shows a conceptual diagram of the relationships between exposure, outcome, and time-varying and static confounders.
We used two methods to define the incarceration exposure. First, we specified that a woman stayed in the incarcerated status once she became incarcerated for the first time; exposure E1 captures the effects of a single incarceration episode on all visits afterward. Second, we specified that a woman could become incarcerated at one visit but could switch back to the “nonincarcerated” status at her next study visit. Exposure (2) captures the effect of being newly incarcerated at every visit. This exposure was used to demonstrate the effects of each new episode of incarceration on risk of STI in the following 6 months.
To control for confounding, we created IP of treatment weights for both exposures, and we controlled for potential nonignorable loss to follow-up using IP of censoring weights. Detailed descriptions of the creation of the weights are contained in Supplementary Appendix SA.
We calculated the crude incidence of any self-reported STI using a data set without multiple imputation. We estimated weighted survival curves for first report of one or more STIs by incarceration exposure using the nonparametric Nelson-Aalen survival estimator.33 At each time point, the average probability from the 50 multiple imputation data sets was plotted on the survival curves. We estimated weighted hazard ratios comparing the hazard of first report of one or more STIs using pooled logistic regression. Estimated hazard ratios based on the 50 imputation data sets were combined using Rubin's method,34 and corresponding 95% confidence intervals (CIs) were estimated using robust variances. Statistical analyses were conducted in SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
There were 3,124 women who contributed a median of 4 at-risk years (interquartile range [IQR] 2–5) during the study period. The median age at the start of the study period was 44 years (IQR 37–50). The majority of participants identified as black (n = 2,166; 67.7%) and had completed high school or more (n = 2,036; 65.2%). There were 635 (20.3%) participants of Hispanic ethnicity. Of all participants, 71.5% (n = 2,234) were women with HIV.
There were 1,186 women (39.1%) who reported incarceration before the study period. Four hundred thirty-six women (14.0%) reported drinking >7 drinks per week, 603 (19.3%) reported marijuana use, and 373 (11.9%) reported illicit drug use at the first visit during the study period. Only 3.1% of women (n = 98) reported engaging in sex exchange at the first visit during the study period. Participant characteristics are shown in Table 1.
Table 1.
Baseline Characteristics of Participants by Incarceration Status, 2007–2017, Women's Interagency HIV Study
| Overall (N = 3,124) | Incarcerated during study period (N = 212) | Not incarcerated during study period (N = 2,912) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Category | n | n | n | |||
| Median age, years (IQR) | 3,124 | 44 (37–50) | 212 | 42 (35–48) | 2,912 | 44 (37–50) | |
| High school education or more (%) | 2,036 | 65.2 | 119 | 56.1 | 1,917 | 65.8 | |
| Race (%) | White | 582 | 18.6 | 23 | 10.9 | 559 | 19.2 |
| Black | 2,116 | 67.7 | 163 | 76.9 | 1,953 | 67.1 | |
| Other | 426 | 13.6 | 26 | 12.3 | 400 | 13.7 | |
| Hispanic ethnicity (%) | 635 | 20.3 | 33 | 15.6 | 602 | 20.7 | |
| Positive HIV status (%) | 2,234 | 71.5 | 128 | 60.4 | 2,106 | 72.3 | |
| Site (%) | Bronx, NY | 422 | 13.5 | 16 | 7.6 | 406 | 13.9 |
| Brooklyn, NY | 449 | 14.4 | 22 | 10.4 | 427 | 14.7 | |
| Washington, DC | 346 | 11.1 | 20 | 9.4 | 326 | 11.2 | |
| Los Angeles, CA | 406 | 13.0 | 22 | 10.4 | 384 | 13.2 | |
| San Francisco, CA | 373 | 11.9 | 40 | 18.9 | 333 | 11.4 | |
| Chicago, IL | 353 | 11.3 | 37 | 17.5 | 316 | 10.9 | |
| Chapel Hill, NC | 186 | 6.0 | 19 | 9.0 | 167 | 5.7 | |
| Atlanta, GA | 247 | 7.9 | 19 | 9.0 | 228 | 7.8 | |
| Miami, FL | 130 | 4.2 | 8 | 3.8 | 122 | 4.2 | |
| Birmingham, AL | 104 | 3.3 | 5 | 2.4 | 99 | 3.4 | |
| Jackson, MS | 108 | 3.5 | 4 | 1.9 | 104 | 3.6 | |
| Incarceration before study period (%) | 1,186 | 39.1 | 159 | 77.9 | 1,027 | 36.3 | |
| Exchanged sexa in past 6 months (%) | 98 | 3.1 | 23 | 10.9 | 75 | 2.6 | |
| Alcohol use in past 6 months (%) | None | 1,701 | 54.5 | 87 | 41.0 | 1,614 | 55.4 |
| >0–7 drinks/week | 987 | 31.6 | 66 | 31.1 | 921 | 31.6 | |
| >7 drinks/week | 436 | 14.0 | 59 | 27.8 | 377 | 13.0 | |
| Marijuana use in past 6 months (%) | 603 | 19.3 | 74 | 34.9 | 529 | 18.2 | |
| Illicit drug useb in past 6 months (%) | 373 | 11.9 | 80 | 37.7 | 293 | 10.1 | |
Missing values for these variables are excluded.
Exchanged sex for drugs, money, or shelter.
Illicit drug use includes any crack cocaine, cocaine, heroin, methamphetamine, injection drugs, or nonprescription narcotics.
IQR, interquartile range.
There were 1,179 visits at which the incarceration exposure was missing (5.2%) and 1,342 at which the STI outcome was missing (5.9%). There were 1,163 visits missing either the sex exchange or the substance use variable at the visit before measurement of the exposure (5.1%), and 1,177 visits that were missing one of these covariates two visits before measurement of the exposure (5.2%). There was minimal missing data for the other covariates (0%–5.9%).
Before imputing missing data, the study period included 10,661 at-risk years. There were 298 total incarceration events and 212 first incarceration events during the study period. There were 213 first incident STI events with one or more self-reported STIs during the study period. Women reported only one STI at 96.7% of visits with self-reported STIs. This equated to a crude incidence rate of 3.7 first STIs per 100 person-years for women who were incarcerated at any time during the study period and 1.9 first STIs per 100 person-years for women who were not incarcerated during the study period.
The prevalence of gonorrhea, chlamydia, and trichomonas during the study period were 0.6%, 1.2%, and 5.3%, respectively. The prevalence of each infection among women who were incarcerated was higher than among women who were not incarcerated, as shown in Table 2. There were 645 women lost to follow-up during the study period.
Table 2.
Exposure and Outcome Frequencies and Prevalence, and Incidence of First STI by Incarceration During the Study Period, 2007–2017, Women's Interagency HIV Study
| Overall (N = 3,124 women) | Incarcerated (N = 212 women) | Not incarcerated (N = 2,912 women) | |
|---|---|---|---|
| Total self-reports of at least one STIa | 213 | 27 | 186 |
| Self-reported gonorrhea | 19 | 3 | 16 |
| Self-reported chlamydia | 37 | 6 | 31 |
| Self-reported trichomonas | 164 | 18 | 146 |
| At-risk years | 10,661 | 727 | 9,935 |
| First self-reported STI prevalenceb | 6.9 | 12.7 | 6.4 |
| Self-reported gonorrhea prevalence | 0.6 | 1.4 | 0.6 |
| Self-reported chlamydia prevalence | 1.2 | 2.8 | 1.1 |
| Self-reported trichomonas prevalence | 5.3 | 8.5 | 5.1 |
| First STI incidence rateb | 2.0 | 3.7 | 1.9 |
| First incarceration eventsc | 212 | 212 | 0 |
| Censoredd | 654 | 45 | 609 |
STIs include the first self-report during the study period of one or more of gonorrhea, chlamydia, and trichomonas.
Period prevalence per 100 participants who were active at any point during the study period and crude incidence per 100 person-years was calculated using data from the 3,096 women with nonmissing self-reported STI data who reported one or more STIs during the study period.
There were a total of 298 incarceration events among the 212 women who experienced incarceration during the study period.
Women who missed at least two consecutive visits during the study period.
STIs, sexually transmitted infections.
Table 3.
Crude and Inverse-Probability-Weighted Results Comparing Hazard of Incident STIs Among Incarcerated Participants Compared to Those Not Incarcerated, for Each Incarceration Exposure, 2007–2017, Women's Interagency HIV Study
| Exposure definition | Crude |
Weighted |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| First incarceration (E1) | 3.71 | 2.45–5.60 | 1.44 | 0.69–3.03 |
| Incarceration at each visit (E2) | 4.41 | 2.47–7.87 | 4.08 | 1.61–10.30 |
CI, confidence interval; HR, hazard ratio.
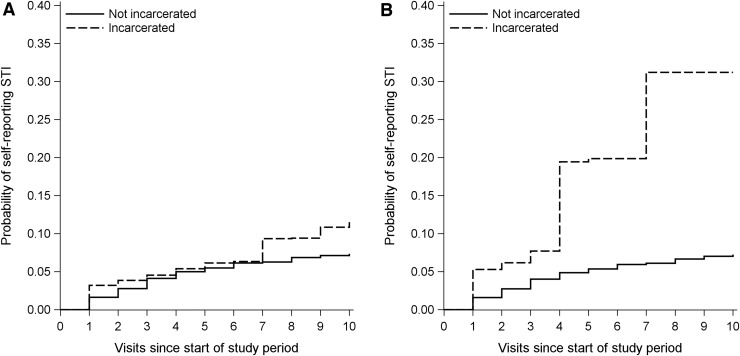
The IP-weighted survival curves for each exposure definition are shown in Figure 1. They are not statistically significantly different. The IP-weighted hazard ratio for incident STIs for exposure E1was 1.44 (95% CI: 0.69–3.03) and for exposure E2 was 4.08 (95% CI: 1.61–10.30). The crude and IP-weighted hazard ratios for report of one or more STIs among incarcerated participants compared to those not incarcerated using both exposure definitions are shown in Table. The distribution of weights is shown in Supplementary Appendix SA.
FIG. 1.
Inverse-probability-weighted cumulative incidence of first sexually transmitted infection for two incarceration exposures, 2007–2017, Women's Interagency HIV Study. (A) Displays results for the exposure defined by first incarceration (E1). (B) Displays results for the exposure defined by incarceration at each visit (E2). The curves are not statistically significantly different; 95% CIs (not pictured) were wide enough that the never-incarcerated curves and their 95% CIs were almost entirely contained within the CIs for the incarcerated curves. CIs, confidence intervals.
Discussion
Accounting for measured confounders and loss to follow-up, we demonstrate that an episode of incarceration may increase women's risk of gonorrhea, chlamydia, or trichomonas during the subsequent 6 months. There may be a smaller increase in risk at time points further from the episode of incarceration compared to immediately following incarceration. Our results extend and strengthen the findings of previous cross-sectional studies suggesting that women who experienced incarceration were 1.3–2 times as likely to experience an STI.9–11 Despite wide CIs around our estimates, they are consistent with the magnitude of previously reported effects of incarceration on STIs.
Results of earlier studies suggested that sex exchange and drug use may mediate some or all of the effects of incarceration on STIs. The findings from our complex, time-varying approach allowed for these variables to act as mediators while accounting for any confounding these variables might be introducing.
Prior work with this cohort shows that those women may have new sexual partners following an episode of incarceration.18 The current findings demonstrate that this postincarceration change in behavior can result in increased risk for STIs. Through various mechanisms, gonorrhea, chlamydia, and trichomonas can all facilitate acquisition of HIV.35 While increases in new sexual partnerships postincarceration reflect a potential increase in exposure to HIV, an increase in STIs postincarceration may result in increased susceptibility to HIV. These results provide additional evidence for incarceration as a structural force in women's lives, potentially driving STI acquisition, with important subsequent implications for HIV acquisition.
As such, approaches to decreasing the burden of STIs and reducing HIV acquisition among women who have experienced incarceration will require tailoring of integrated multilevel interventions that not only address specific risk behaviors, but also aim to decrease exposure to the criminal legal system.
Programs that provide behavioral risk reduction education and skills training use trauma-informed approaches to service provision, facilitate access to safe housing and employment, connect participants to medications for opioid use disorder or other treatments for substance use disorders, and increase awareness of and access to preexposure prophylaxis for HIV will have the most impact.36–39 There is increasing evidence of the value of interventions that specifically address the social and economic collateral consequences of incarceration for women and are developed in collaboration with women who have lived experiences of incarceration.40,41
Although the WIHS is a multicenter, geographically diverse cohort study, our findings may not be broadly generalizable. The potential accrual of benefits from behavioral or other changes related to duration of WIHS participation may attenuate our estimates by buffering the effects of incarceration.42 In addition, women with HIV may have decreased their risk behaviors after diagnosis, whereas women at risk for HIV were recruited on the basis of their risk behaviors. The pooling of data from these groups was a limitation of our study.
The WIHS cohort also represents an older group of women compared with those with the highest rates of incarceration or STIs. Questions about incarceration were not introduced into the WIHS cohort until 2007, when the median age of participants was 44 years. Of women imprisoned nationally in 2016, nearly half (46.3%) were younger than the age of 35.43,44 Among incarcerated women, the highest rates of gonorrhea and chlamydia are in women aged younger than 35 years, although older age is a risk factor for trichomonas.
Our estimates of rates of STIs are not likely to be generalizable to all women with or at risk for HIV in the United States or to all women who experience incarceration in the United States. However, the consistent association between incarceration and STIs across age groups and settings suggests that the underlying causal relationship between experiencing incarceration and increased rates of STIs may also be consistent across age groups. Our findings highlight incarceration as an important risk factor for STIs among women in older age groups.
Another limitation to this study is the use of self-reported STI outcomes. This potentially introduces bias due to STI testing for women who were symptomatic versus not, and it is likely that there were asymptomatic STI outcomes that were not captured in our data. Women who have been incarcerated may also have been tested during an episode of incarceration, with longer incarcerations and prison incarcerations more likely to include STI testing.45,46 We addressed this to the extent possible by temporally separating the incarceration exposures and the reports of STI outcomes. This approach also would have caused us to miss STI events that occurred after an episode of incarceration, but before the subsequent 6-month period of data ascertainment.
Also, reassuring is the evidence from biologic testing for STIs that was performed at each participant's baseline visit. Although some of these tests were performed many years in the past (e.g., the first wave of the WIHS in 1994–1995) and is not an ideal comparison, the laboratory data place the self-reported data in context. The prevalences of gonorrhea, chlamydia, and trichomonas at baseline were 0.4%, 1.5%, and 8.5%, respectively. These are similar to the prevalences of each of the self-reported STIs during our study period (0.6%, 1.2%, and 5.3%).47
This study does have important strengths. The longitudinal structure of the WIHS allowed us to temporally separate measurement of the incarceration exposure and measurement of the STI outcome by 6 months, decreasing the chances that the observed effects were due solely to testing during an episode of incarceration. Our approach using MSM with IP weights produced estimates of the effect of incarceration while accounting for sex exchange, drug use, other confounders, and loss to follow-up.
The relatively few missing values in the dataset allowed for use of the selected multiple imputation approach. The final analysis featured a large sample size and long duration of follow-up where rare incarceration and STI events could be observed. In addition, the WIHS intentionally recruited women at risk for HIV who were socioeconomically and racially similar to its participants with HIV and the general U.S. population of women with HIV.19
Conclusion
Our findings have important implications for STI and HIV prevention among women who have experienced incarceration. Women are more likely to have new sexual partners after incarceration, and we now demonstrate that this behavior change may translate to increased risk of STIs, which in turn may result in increased HIV acquisition.18 We note that experiences of incarceration are not evenly distributed throughout the population, and our study sample reflects the disproportionate burden of incarceration among black women and women who had not competed high school.21 Mass decarceration and decriminalization of women of color, poor women, and women who use drugs have the potential to reduce disparities in STIs and HIV.
In addition to secondary prevention of STIs and HIV through multilevel interventions to reduce risk for women returning to the community after incarceration, there is a critical need for primary prevention of criminal legal involvement. Decreasing women's exposure to the criminal legal system is a critical public health intervention for the prevention of STIs and HIV.
Availability of Data and Material
Access to individual-level data from the MACS/WIHS Combined Cohort Study Data (MWCCS) may be obtained upon review and approval of a MWCCS concept sheet. Links and instructions for online concept sheet submission are on the study website (https://mwccs.org/).
Supplementary Material
Acknowledgments
The authors thank Professor Stephen Cole for his methodological expertise.
Authors' Contributions
A.K.K. conceived and supervised the study and wrote the article. J.E.R. and B.E.S.-S. completed the analyses. A.E. and C.R. assisted with the study design and analyses. M.C., A.A., T.T., K.G.M., J.M., J.C., J.D.D., A.F., M.A.F., and D.M.L. participated in the primary data collection and assisted with the study. A.A.A. participated in the primary data collection and assisted with conceiving and supervising the study. All the authors critically reviewed the article.
Author Disclosure Statement
A.A.A. has received consulting fees from Merck, Viiv, and Gilead, and UNC has received funds from Gilead for her research. The other authors declare no conflicts of interest.
Funding Information
This work was supported by funding from the University of North Carolina at Chapel Hill Center for AIDS Research (A.K.K., BESS—P30 AI50410), the National Institute of Environmental Health Sciences (NIEHS) (J.E.R.—T32 ES007018), and the Gillings School of Public Health (J.E.R.—Innovation Laboratory Award).
Data in this article were collected by the Women's Interagency HIV Study, now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; and Data Analysis and Coordination Center (Gypsyamber D'Souza, Stephen Gange and Elizabeth Golub), U01-HL146193.
Also, Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Connie Wofsy Women's HIV Study, Northern California CRS (Bradley Aouizerat, Phyllis Tien, and Jennifer Price), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (A.A.A.), U01-HL146194.
The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR).
MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Supplementary Material
References
- 1. Mertz KJ, Schwebke JR, Gaydos CA, Beidinger HA, Tulloch S, Levine WC. Screening women in jails for chlamydial and gonococcal infection using urine tests: Feasibility, acceptability, prevalence, and treatment rates. Sex Transm Dis 2002;29:271–276. [DOI] [PubMed] [Google Scholar]
- 2. Kouyoumdjian FG, Leto D, John S, Henein H, Bondy S. A systematic review and meta-analysis of the prevalence of chlamydia, gonorrhoea and syphilis in incarcerated persons. Int J STD AIDS 2012;23:248–254. [DOI] [PubMed] [Google Scholar]
- 3. Joesoef MR, Weinstock HS, Kent CK, et al. Sex and age correlates of chlamydia prevalence in adolescents and adults entering correctional facilities, 2005: Implications for screening policy. Sex Transm Dis 2009;36(Suppl 2):S67–S71. [DOI] [PubMed] [Google Scholar]
- 4. Hardick J, Hsieh Y-H, Tulloch S, Kus J, Tawes J, Gaydos CA. Surveillance of Chlamydia trachomatis and Neisseria gonorrhoeae infections in women in detention in Baltimore, Maryland. Sex Transm Dis 2003;30:64–70. [DOI] [PubMed] [Google Scholar]
- 5. Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex Transm Dis 2012;39:92–96. [DOI] [PubMed] [Google Scholar]
- 6. Fox KK, Whittington WL, Levine WC, Moran JS, Zaidi AA, Nakashima AK. Gonorrhea in the United States, 1981–1996: Demographic and geographic trends. Sex Transm Dis 1996;25:386–393. [DOI] [PubMed] [Google Scholar]
- 7. Nijhawan AE, Chapin KC, Salloway R, et al. Prevalence and predictors of trichomonas infection in newly incarcerated women. Sex Transm Dis 2012;39:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nijhawan AE, DeLong AK, Celentano DD, et al. The association between Trichomonas infection and incarceration in HIV-seropositive and at-risk HIV-seronegative women. Sex Transm Dis 2011;38:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers SM, Khan MR, Tan S, Turner CF, Miller WC, Erbelding E. Incarceration, high-risk sexual partnerships and sexually transmitted infections in an urban population. Sex Transm Infect 2012;88:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wise A, Finlayson T, Nerlander L, Sionean C, Paz-Bailey G, for the NHBS Study Group. Incarceration, sexual risk-related behaviors, and HIV infection among women at increased risk of HIV infection, 20 United States cities. J Acquir Immune Defic Syndr 2017;75(Suppl 3):S261–S267. [DOI] [PubMed] [Google Scholar]
- 11. Khan MR, Epperson MW, Mateu-Gelabert P, Bolyard M, Sandoval M, Friedman SR. Incarceration, sex with an STI- or HIV-infected partner, and infection with an STI or HIV in Bushwick, Brooklyn, NY: A social network perspective. Am J Public Health 2011;101:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan MR, Wohl DA, Weir SS, et al. Incarceration and risky sexual partnerships in a southern US city. J Urban Health 2008;85:100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fogel CI, Gelaude DJ, Carry M, et al. Context of risk for HIV and sexually transmitted infections among incarcerated women in the south: Individual, interpersonal, and societal factors. Women Health 2014;54:694–711. [DOI] [PubMed] [Google Scholar]
- 14. Hankel J, Heil M, Dewey S, Martinez N. Characteristics of women seeking services at a transitional housing facility for women leaving street-based sex work: Implications for social service providers. J Soc Serv Res 2015;42:41–56. [Google Scholar]
- 15. James DJ, Glaze LE. Mental health problems of prison and jail inmates. BJS Bull 2006; (NCJ 213600). US Department of Justice, Office of Justice Programs, September, 2006. pp. 1–11. [Google Scholar]
- 16. Cotten-Oldenburg NU, Jordan BK, Martin SL, Kupper L. Women inmates' risky sex and drug behaviors: Are they related? Am J Drug Alcohol Abuse 1999;25:129–149. [DOI] [PubMed] [Google Scholar]
- 17. Walters SM, Rivera AV, Reilly KH, et al. Exchange sex among persons who inject drugs in the New York metropolitan area: The importance of local context, gender and sexual identity. AIDS Behav 2018;22:2773–2787. [DOI] [PubMed] [Google Scholar]
- 18. Knittel AK, Shook-Sa BE, Rudolph J, et al. Incarceration and number of sexual partners after incarceration among vulnerable US women, 2007–2017. Am J Public Health 2020;110(Suppl 1):S100–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women's Interagency HIV Study (WIHS). Int J Epidemiol 2018;47:393i–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Twenge JM, Sherman RA, Wells BE. Changes in American adults' sexual behavior and attitudes, 1972–2012. Arch Sex Behav 2015;44:2273–2285. [DOI] [PubMed] [Google Scholar]
- 21. Brown MM, Chesney-Lind M. Women's incarceration in the United States. In: Griffin OH, III, Woodward VH, eds., Routledge, NY: Routledge handbook of corrections in the United States. 2017. [Google Scholar]
- 22. Aholou TM, McCree DH, Oraka E, et al. Sexual risk and protective behaviors among reproductive-aged women in the United States. J Womens Health (Larchmt) 2017;26:1150–1160. [DOI] [PubMed] [Google Scholar]
- 23. Kajstura A. Women's Mass Incarceration: The Whole Pie 2019. Prison Policy Initiative. 2019. Available at: https://www.prisonpolicy.org/reports/pie2019women.html. Published 2019. Updated October 29. Accessed December, 2020.
- 24. Koblin BA, Grant S, Frye V, et al. HIV sexual risk and syndemics among women in three urban areas in the United States: Analysis from HVTN 906. J Urban Health 2015;92:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adimora AA, Schoenbach VJ, Bonas DM, Martinson FE, Donaldson KH, Stancil TR. Concurrent sexual partnerships among women in the United States. Epidemiology 2002;13:321–372. [DOI] [PubMed] [Google Scholar]
- 26. Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilitities, 2006: Declining share of epidemic but persistent public health opportunity. PLoS One 2009;4:e7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005;39:446–453. [DOI] [PubMed] [Google Scholar]
- 28. Lambdin BH, Comfort M, Kral AH, Lorvick J. Accumulation of jail incarceration and hardship, health status, and unmet health care need among women who use drugs. Womens Health Issues 2018;28:470–475. [DOI] [PubMed] [Google Scholar]
- 29. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 30. Lorvick J, Comfort M, Kral AH, Lambdin BH. Exploring lifetime accumulation of criminal justice involvement and associated health and social outcomes in a community-based sample of women who use drugs. J Urban Health 2017;95:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams J, Moody J, Morris M. Sex, drugs, and race: How behaviors differentially contribute to the sexually transmitted infection risk network structure. Am J Public Health 2013;103:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernán MÁ, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 33. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 34. Rubin DB. Multiple imputation for nonresponse in surveys. Vol 81. New York, New York: John Wiley & Sons, 2004. [Google Scholar]
- 35. Mwatelah R, McKinnon LR, Baxter C, Abdool Karim Q, Abdool Karim SS. Mechanisms of sexually transmitted infection-induced inflammation in women: Implications for HIV risk. J Int AIDS Soc 2019;22(Suppl 6):e25346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutledge R, Madden L, Ogbuagu O, Meyer JP. HIV risk perception and eligibility for pre-exposure prophylaxis in women involved in the criminal justice system. AIDS Care 2018;30:1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilbert L, Raj A, Hien D, Stockman J, Terlikbayeva A, Wyatt G. Targeting the SAVA (substance abuse, violence, and AIDS) syndemic among women and girls: A global review of epidemiology and integrated interventions. J Acquir Immune Defic Syndr 2015;69:S118–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Springer SA, Larney S, Alam-Mehrjerdi Z, Altice FL, Metzger D, Shoptaw S. Drug treatment as HIV prevention among women and girls who inject drugs from a global perspective: Progress, gaps, and future directions. J Acquir Immune Defic Syndr 2015;69(Suppl 2):S155–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller NA, Najavits LM. Creating trauma-informed correctional care: A balance of goals and environment. Eur J Psychotraumatol 2012;3:17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilbert L, Goddard-Eckrich D, Chang M, et al. Effectiveness of a culturally tailored HIV and sexually transmitted infection prevention intervention for Black women in community supervision programs: A randomized clinical trial. JAMA Netw Open 2021;4:e215226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramsey SE, Ames EG, Uber J, et al. Linking women experiencing incarceration to community-based HIV pre-exposure prophylaxis care: A qualitative study. AIDS Educ Prev 2021;33:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tross S, Pinho V, Lima JE, et al. Participation in HIV behavioral research: Unanticipated benefits and burdens. AIDS Behav 2018;22:2258–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pettit B, Western B. Mass imprisonment and the life course: Race and class inequality in U.S. incarceration. Am Sociol Rev 2004;69:151–169. [Google Scholar]
- 44. Carson EA. Prisoners in 2016. BJS Bull 2018; (NCJ 251149). US Department of Justice, Office of Justice Programs, January 2018. pp. 1–35. [Google Scholar]
- 45. Nijhawan AE, Salloway R, Nunn AS, Poshkus M, Clarke JG. Preventive healthcare for underserved women: Results of a Prison Survey. J Womens Health (Larchmt) 2010;19:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flanigan TP, Zaller N, Beckwith CG, et al. Testing for HIV, sexually transmitted infections, and viral hepatitis in jails: Still a missed opportunity for public health and HIV prevention. J Acquir Immune Defic Syndr 2010;55(Suppl 2):S78–S83. [DOI] [PubMed] [Google Scholar]
- 47. Greenblatt RM, Bacchetti P, Barkan S, et al. Lower genital tract infections among HIV-infected and high-risk uninfected women: Findings of the Women's Interagency HIV Study (WIHS). Sex Transm Dis 1999;26:143–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to individual-level data from the MACS/WIHS Combined Cohort Study Data (MWCCS) may be obtained upon review and approval of a MWCCS concept sheet. Links and instructions for online concept sheet submission are on the study website (https://mwccs.org/).