Abstract
We developed a single tube multiplex real-time PCR assay that allows for the rapid detection and typing of 9 nonpneumophila Legionella spp. isolates that are clinically relevant. The multiplex assay is capable of simultaneously detecting and discriminating L. micdadei, L. bozemanii, L. dumoffii, L. longbeachae, L. feeleii, L. anisa, L. parisiensis, L. tucsonensis serogroup (sg) 1 and 3, and L. sainthelensis sg 1 and 2 isolates. Evaluation of the assay with nucleic acid from each of these species derived from both clinical and environmental isolates and typing strains demonstrated 100% sensitivity and 100% specificity when tested against 43 other Legionella spp. Typing of L. anisa, L. parisiensis, and L. tucsonensis sg 1 and 3 isolates was accomplished by developing a real-time PCR assay followed by high-resolution melt (HRM) analysis targeting the ssrA gene. Further typing of L. bozemanii, L. longbeachae, and L. feeleii isolates to the serogroup level was accomplished by developing a real-time PCR assay followed by HRM analysis targeting the mip gene. When used in conjunction with other currently available diagnostic tests, these assays may aid in rapidly identifying specific etiologies associated with Legionella outbreaks, clusters, sporadic cases, and potential environmental sources.
Keywords: Real-time PCR, Legionella spp., Detection, Typing, HRM Analysis
1. Introduction
Legionellae are facultative intracellular gram-negative bacteria found ubiquitously in water and soil environments (Fields et al., 2002; Mercante and Winchell, 2015). Being an intracellular organism, Legionellae are capable of replicating in environmental protozoa as well as within alveolar macrophages and epithelial cells (Cianciotto et al., 1990; Fields, 1996; Newton et al., 2010). Legionellae are common contaminates of natural and man-made water systems, including cooling towers, air conditioning systems, fountains, and whirlpools, where conditions may be ideal for growth and propagation (Kozak et al., 2013; Steinert et al., 2002). Once aerosolized, the bacteria can enter the human respiratory tract and cause disease manifesting as Legionnaires' disease, a severe form of pneumonia, or Pontiac fever, a self-limiting flu-like illness (Fields et al., 2002).
Over 60 Legionella spp. comprising 70 distinct serogroups have been identified to date (http://www.bacterio.cict.fr/l/legionella.html). Although the majority of cases of Legionnaires' disease are caused by Legionella pneumophila, nearly one-half of all Legionella spp. have been associated with human disease. It is possible that under the appropriate conditions, immunocompromised individuals can be infected with any Legionella spp. (Fields et al., 2002). Infections caused by Legionella spp. other than L. pneumophila are probably not diagnosed regularly due to limitations of current diagnostic methods which are biased toward the detection of L. pneumophila. L. pneumophila is the species most frequently isolated from water distribution systems, but Legionella micdadei, Legionella bozemanii, Legionella dumoffii, Legionella anisa, and Legionella feeleii are also isolated relatively frequently (Best et al., 1983; Bornstein et al., 1985; Joly et al., 1986; Lowry et al., 1991; Palutke et al., 1986; Parry et al., 1985). Worldwide, of the reported nonpneumophila infections, L. micdadei accounts for 60% of the cases; L. bozemanii, for 15%; L. dumoffii, for 10%; Legionella longbeachae, for 5%; and other species, for 10% (Amodeo et al., 2010; Benson et al., 1990; Fang et al., 1989; Herwaldt et al., 1984; Lee et al., 2009; Reingold et al., 1984; Yu et al., 2002). However, global incidence of Legionella infection surveillance data should be interpreted carefully as it is underrecognized in many countries because of the lack of diagnostics and surveillance systems.
The current gold standard for typing of isolated Legionella spp. is based on PCR amplification followed by sequencing of the macrophage infectivity potentiator gene (mip) (Ratcliff et al., 1998). Although reliable, this method is time consuming and requires specialized training. In this study, we developed a rapid, single-tube real-time multiplex PCR assay capable of identifying 9 nonpneumophila Legionella spp.: L. micdadei, L. bozemanii, L. dumoffii, L. longbeachae, L. feeleii, L. anisa, Legionella parisiensis, Legionella tucsonensis sg 1 and 3, and Legionella sainthelensis sg 1 and 2 isolates. The assay was designed to identify 5 of the most prevalent nonpneumophila species involved in human disease (L. micdadei, L. bozemanii, L. dumoffii, L. longbeachae, and L. feeleii). However, we were able to exploit primer/probe cross-reactivity to identify an additional 4 nonpneumophila species (L. anisa, L. parisiensis, L. tucsonensis sg 1 and 3, and L. sainthelensis sg 1 and 2) that, although rare, have also been identified as cause of human disease. Additional follow-up high-resolution melt (HRM) assays were developed to discriminate nucleotide polymorphisms within specific targets to differentiate L. anisa, L. parisiensis, and L tucsonensis sg 1 and sg 3 isolates and serogroups for L. bozemanii, L. longbeachae, and L. feeleii isolates. Identification of specific serogroups could be valuable when trying to pinpoint the source of infection during outbreak investigations.
2. Materials and methods
2.1. Primer and probe design
PrimerQuest® Software (Integrated DNA Technologies, Coralville, IA, USA) was used to design multiple TaqMan® primer-probe sets targeting gyrB (L. bozemanii), legS2 (L. dumoffii), figA (L. feeleii), ligB (L. longbeachae), and the migB genes (L. micdadei). Primer sets for real-time PCR-HRM typing assays were designed to target the mip gene (L. bozemanii, L. feeleii, and L. longbeachae) and the ssrA gene (L. anisa, L. parisiensis, and L. tucsonensis sg 1 and 3). Primer and probe sequences, GenBank accession number, final concentrations, and the distinct fluorophores for each probe used in the multiplex and HRM assays are listed in Table 1. Primers and probe sets were initially tested and optimized in singleplex format (data not shown).
Table 1.
Primers and probes for multiplex real-time and HRM detection/typing of nonpneumophila Legionella spp.
| Primer/probe | Sequence (5′→3′) | Species target | Gene target | GenBank accession no. |
Primer/probe final concentration |
|---|---|---|---|---|---|
| bozemanii-F bozemanii-R bozemanii-P3 |
TCCGCTGCTGAAGTGATTATG CATGCAAACCACCCGATACT Q705-AAATTTACCACCGGCGTGAAGCAC-BHQ3 |
L. bozemanii, L. anisa, L. parisiensis, L. tucsonensis sg 1 and 2 |
gyrB | HQ717438 | 125 nmol/L 125 nmol/L 25 nmol/L |
| dumoffii-F dumoffii-R dumoffii-P |
CAGGAAAGCGCGACATCTAT ATCCAGCTCGTTCGCAATAA HEX-TGGAAACCCTCAATGGTCCGTTCT-BHQ1 |
L. dumoffii | legS2 | EU107519 | 250 nmol/L 250 nmol/L 50 nmol/L |
| feeleii-F feeleii-R feeleii-P2 |
AACCGGTTTATCGGTCTTT ATCAACCAGCTTGTCTCG TEX615-AGCTTGAGAATTTGATGGATTATCACTCGC-BHQ2 |
L. feeleii | figA | AY753535 | 125 nmol/L 125 nmol/L 25 nmol/L |
| LLB-F1 LLB-R1 LLB-P1 |
CTGCAGAAGTTGCTGATTGTG GACGTGGCGAATGACTTATCT Q670-TGTCGCCAAGAAGTTGTATCTCATGCT-BHQ3 |
L. longbeachae, L. sainthelensis sg 1 and 2 |
ligB | AY512558 | 125 nmol/L 125 nmol/L 25 nmol/L |
| micdadei-F micdadei-R micdadei-P |
TGACAAGTGAGAGCAAGAGTT GTATCTATTCCGACAGCGATAGG FAM-ACAGAAGGAGAACCTTCCGGTGTG-BHQ1 |
L. micdadei | migB | AY512559 | 125 nmol/L 125 nmol/L 25 nmol/L |
| LLB-HRM-F LLB-HRM-R |
TGGCTAAGCGTAGTGCTG CAACAGTTACAGTATCTGATTTAC |
L. longbeachae | mip | X83036 | 250 nmol/L 250 nmol/L |
| bozemanii-HRM-F1 bozemanii-HRM-R1 |
CCAAGCGGCTTGCAATATAA CAAATACAGTACCATCAATTAAAGTACC |
L. bozemanii | mip | U91609 | 250 nmol/L 250 nmol/L |
| feeleii-HRM-F1 feeleii-HRM-R |
GACCTAATGGCAAAACGAAATG CGCCTTCTTTGGCCTTATTC |
L. feeleii | mip | U92205 | 250 nmol/L 250 nmol/L |
| tmRNA-HRM-F tmRNA-HRM-R |
GGCGACCTGGCTTC GGTCATCGTTTGCATTTATATTTA |
L. anisa, L. parisiensis, L. tucsonensis sg 1 and 2 |
ssrA | HG525464 | 250 nmol/L 250 nmol/L |
2.2. Multiplex real-time PCR assay
The multiplex reaction contained 12.5 μL of Quanta PerfeCTa™ Multiplex qPCR SuperMix (Quanta Biosciences, Gaithersburg, MD, USA), 0.5 μL of each primer and probe (Table 1), 5 ng of template, and nuclease-free water to a final volume of 25 μL. The assay was performed on the Rotor-Gene Q (Rotor-Gene 6000) (Qiagen, Valencia, CA, USA) instrument with the following thermocycling conditions: 95 °C for 5 minutes followed by 45 cycles of 95 °C for 15 seconds and 60 °C for 1 minute (data acquisition in all 5 channels). Channel gain settings were set at green (Fields, 1996), yellow (Cianciotto et al., 1990), orange (Newton et al., 2010), red (Mercante and Winchell, 2015), and crimson (Cianciotto et al., 1990). All samples were tested in duplicate. Primers and probe sets were initially tested and optimized in singleplex format (data not shown).
2.3. Real-time PCR-HRM assay
The ssrA and mip real-time PCR-HRM assays were prepared using the Universal SYBR GreenER qPCR kit (Life Technologies, Grand Island, NY, USA), containing the following components per reaction: 12.5 μL of 2× master mix, 0.5 μL of each primer (Table 1), 1 μL 10 mmol/L dNTP mix, 0.25 μL of Platimun Taq polymerase, 5 ng of template, and nuclease-free water to a total final volume of 25 μL. All HRM assays were performed on the Rotor-Gene Q instrument with the following thermocycling conditions: 95 °C for 5 minutes followed by 35 cycles of 95 °C for 15 seconds and 60 °C for 1 minute (data acquisition in green channel). Following amplification, HRM was performed between 74 °C and 84 °C (ssrA) and 68 °C and 82 °C (mip) at a rate of 0.03 °C per step. HRM normalizing regions for each assay were as follows: ssrA 77 °C–78 °C/81 °C–82 °C, L. bozemanii (mip) 70.5 °C–71.5 °C/79 °C–80 °C, L. feeleii (mip) 71.5 °C–72.5 °C/78.5 °C–79.5 °C., and L. longbeachae (mip) 73.5 °C–74.5 °C/78.5 °C–79.5 °C. All samples were tested in duplicate. Direct fluorescent antibody (DFA) and mip sequencing procedures to confirm HRM results were performed as previously described (Katz, 1985; Ratcliff et al., 1998).
2.4. Analytical specificity, sensitivity, isolates, and nucleic acid extraction
Analytical specificity was verified using a comprehensive panel of 52 Legionella spp. type strains representing most species and serogroups along with 21 L. micdadei, 28 L. bozemanii, 21 L. dumoffii, 80 L. longbeachae, 11 L. feeleii, 43 L. anisa, 2 L. parisiensis, 2 L. tucsonensis sg 1 and sg 3, and 20 L. sainthelensis sg 1 and sg 2 clinical and environmental isolates (Table 2) previously identified by serological methods and/or mip sequencing following previously described methods (Katz, 1985; Ratcliff et al., 1998). Analytical sensitivity was established by testing 10-fold DNA dilutions from 100 pg/μL down to 1 fg/μL from each of the 9 Legionella spp. detected by the assay. Each dilution was tested in 10 replicates, and limits of detection (LODs) were established for each assay, defined as the lowest dilution in which ≥50% of replicates had positive crossing threshold (Ct) values. The Ct values were plotted against nucleic acid concentration to determine slope and assay efficiencies (%). This was calculated by determining the percentage of difference either above or below the perfect slope of −3.3 and efficiency of 100%. Isolates were selected from the CDC reference diagnostics library and grown on buffered charcoal yeast extract agar plates at 35 °C with 2.5% CO2 for 48–72 h. Total nucleic acid was extracted using the Roche MagNA Pure Compact and/or the MagNA Pure LC instruments (Roche Applied Science, Indianapolis, IN, USA) and normalized to 1 ng/μL using the NanoDrop® ND-1000 V3.5.2 Spectrophotometer (NanoDrop products, Wilmington, DE, USA).
Table 2.
Strains used for evaluating specificity and sensitivity of the real-time PCR multiplex assay.
| Strains | No. of isolates tested |
|---|---|
| L. pneumophila sg. 1–17 | 22 |
| L. adelaidensis | 1 |
| L. anisa | 43 |
| L. beliardensis | 1 |
| L. birmighamensis | 2 |
| L. bozemanii | 28 |
| L. brunensis | 1 |
| L. busanensis | 1 |
| L. cherrii | 1 |
| L. cinannatiensis | 1 |
| L. drozanskii | 1 |
| L. dumoffii | 21 |
| L. erythra | 1 |
| L. fairfieldensis | 1 |
| L. fallonii | 1 |
| L. feeleii | 11 |
| L. geestiana | 1 |
| L. genomo species | 1 |
| L. gormanii | 1 |
| L. gratiana | 1 |
| L. gresilensis | 1 |
| L. hackleliae | 2 |
| L. impletisoli | 1 |
| L. israelensis | 1 |
| L. jamestowniensis | 1 |
| L. jordanis | 1 |
| L. lansingensis | 1 |
| L. longbeachae | 80 |
| L. londiniensis | 1 |
| L. lytica | 1 |
| L. maceachernii | 1 |
| L. micdadei | 21 |
| L. moravica | 1 |
| L. nagasakiensis | 2 |
| L. nautarum | 1 |
| L. oakridgensis | 2 |
| L. parisiensis | 2 |
| L. quateirensis | 1 |
| L. quinlavanii | 2 |
| L. rowbowthamii | 1 |
| L. rubriluscens | 1 |
| L. sainthelensis | 20 |
| L. santicrucis | 2 |
| L. shakespearei | 1 |
| L. spiritensis | 2 |
| L. steigerwaltii | 1 |
| L. taurinensis | 1 |
| L. tucsonensis | 3 |
| L. wadsworthii | 1 |
| L. waltersii | 1 |
| L. worsleiensis | 1 |
| L. yabuchiae | 1 |
| Total | 300 |
3. Results
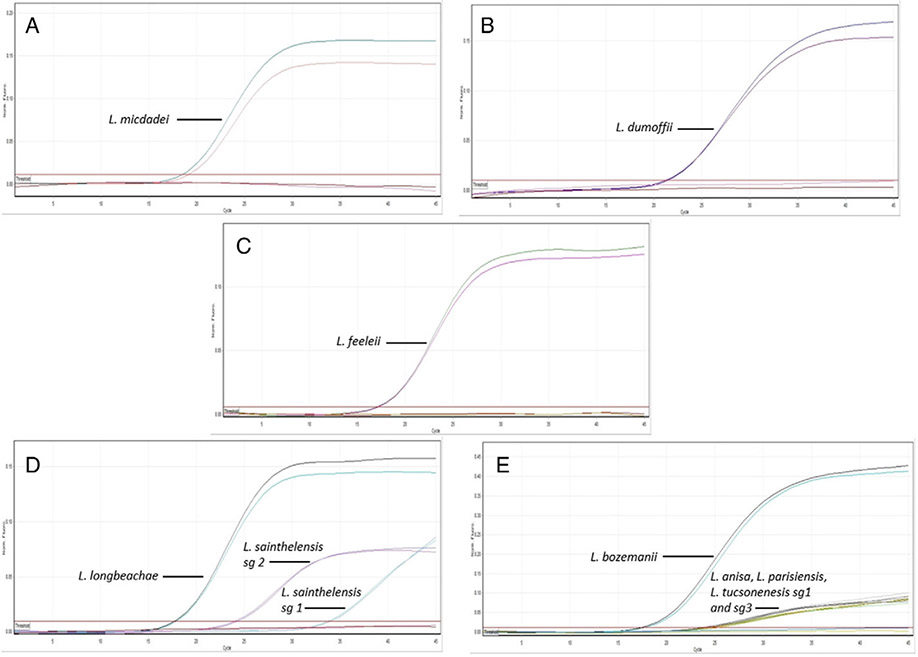
3.1. Multiplex real-time PCR analysis
Real-time PCR amplification curves for detection of 9 pathogenic nonpneumophila Legionella spp. typing strain isolates are shown in Fig. 1. Specific amplification of L. micdadei (Fig. 1A), L. dumoffii (Fig. 1B), and L. feeleii (Fig. 1C) typing strains is displayed in the green (FAM), yellow (HEX), and orange (TX-RED 615) channels, respectively. Fig. 1D displays amplification curves for L. longbeachae and L. sainthelensis sg 1 and sg 2 type strains in the red (Quasar670) channel. Ct values of 17–20 are observed for L. longbeachae isolates, whereas L. sainthelensis sg 1 and L. sainthelensis sg 2 isolates display Ct values of 32–35 and 24–27, respectively. Fig. 1E displays amplification curves for L. bozemanii (Ct 17–20), L. anisa, L. pansiensis, and L. tucsonensis sg 1 and sg 3 (Ct 24–27) in the crimson (Quasar705) channel. The multiplex assay achieved 100% analytical specificity when tested against a panel composed of 300 Legionella spp. type strains and clinical/environmental isolates (data not shown) (Table 2). An LOD of 50 fg per reaction was established for the L. micdadei, L. dumoffii, L. feeleii, L. longbeachae, and L. bozemanii targets. An LOD of 500 fg/reaction was established for the L. anisa, L. pansiensis, and L. tucsonensis sg 1 and sg 3 targets and 500 pg and 5 pg for L. sainthelensis sg 1 and L. sainthelensis sg 2 targets, respectively. The LODs were equivalent for the singleplex and multiplex Legionella spp.–specific assays (data not shown). Assay efficiencies ranged from 80% to 110% depending on the target (data not shown).
Fig. 1.
Multiplex assay for detection and identification of 9 clinically relevant nonpneumophila Legionella isolates. (A) L. micdadei, (B) L. dumoffii, (C) L. feeleii, (D) L. longbeachae, L. sainthelensis sg 1 and sg 2, and (E) L. bozemanii, L. anisa, L. parisiensis, and L. tucsonensis sg 1 and sg 3. All samples were run in duplicate.
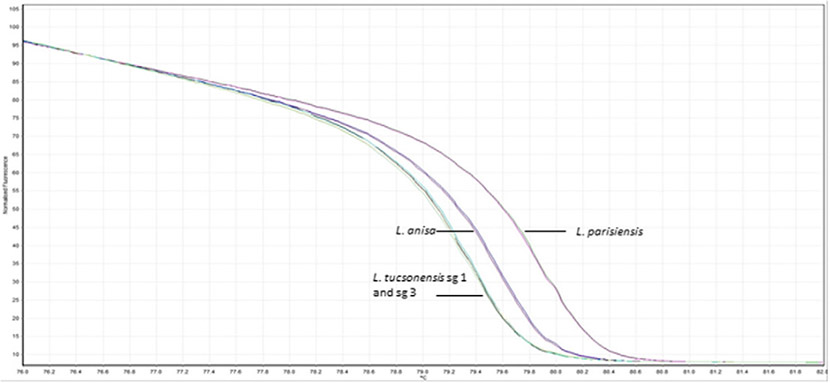
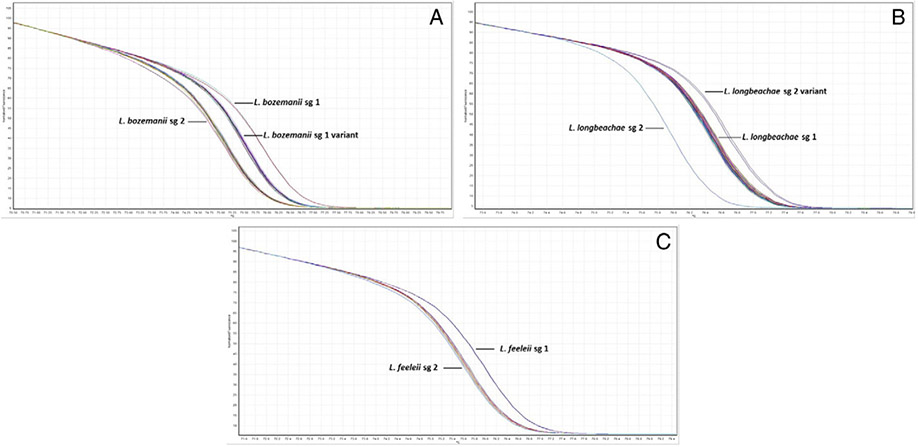
3.2. Real-time PCR and HRM analysis
Speciation of L. anisa, L. parisiensis, and L. tucsonensis sg 1 and sg 3 isolates is shown in Fig. 2. HRM analysis allows for detection of each species based on the melting pattern of the 101-bp amplicon targeting the ssrA gene. Specific nucleotide differences at 4 different positions within the ssrA amplicon dictate the specific melting pattern of each species. L. anisa isolates displayed nucleotide bases T, T, C, and A; L. tucsonensis sg 1 and 3 displayed A, A, A, and A; and L. parisiensis displayed A, T, and C and a base pair deletion at positions 50, 67, 68, and 77 within the amplicon, respectively. A total of 43 L. anisa, 2 L. parisiensis, 1 L. tucsonensis sg 1, and 1 L. tucsonensis sg 3 isolates were tested with 100% specificity. Fig. 3A displays HRM analysis of the 112-bp amplicon targeting the mip gene of 26 L. bozemanii isolates previously serotyped. The curve melting pattern shows clear discrimination between 1 sg 1 and 13 sg 2 isolates which targets a 2-bp difference at positions 22 and 81 within the amplicon, G and C (sg1) and A and T (sg2), respectively. An additional distinct melting pattern was observed in 12/26 isolates (sg 1 variant) previously typed by DFA as sg 1, and nucleotides A and C were identified after mip sequencing analysis at positions 22 and 81 within the amplicon (data not shown). Typing of L. longbeachae serogroups was accomplished by targeting a 2-bp difference within a 180-bp region in the mip gene. L. longbeachae sg 1 displayed nucleotide bases C and C at positions 139 and 145 within the amplicon, whereas sg 2 isolates displayed nucleotide bases A and T, respectively. Fig. 3B displays HRM analysis of 77 L. longbeachae isolates [sg 1 (73), sg 2 (Cianciotto et al., 1990), and sg 2 variant (Fields et al., 2002)]. Results were confirmed by DFA and mip sequencing analysis (data not shown). HRM analysis of a 100-bp amplicon within the mip gene region of 11 L. feeleii isolates is displayed in Fig. 3C. A single base pair nucleotide difference was targeted, and clear discrimination between 1 L. feeleii sg 1 isolate with nucleotide G at position 24 and 10 sg 2 isolates with nucleotide T at position 24 within the amplicon was observed. Results were confirmed by mip sequencing analysis (data not shown).
Fig. 2.
Real-time PCR-HRM assay for discrimination of L. anisa, L. parisiensis, and L. tucsonensis sg 1 and sg 3 isolates targeting the ssrA gene.
Fig. 3.
HRM profiles for subtyping of Legionella spp., targeting nucleotide differences in the mip gene. (A) Subtyping of 26 L. bozemanii isolates, (B) subtyping of 77 L. longbeachae isolates, and (C) subtyping of 11 L. feeleii isolates.
4. Discussion
Although L. pneumophila causes the majority of cases of Legionnaires' disease, the number of cases caused by other Legionella spp. is not well established, as the infections are difficult to diagnose due to limitations of the current diagnostic methods, which are biased toward the detection of L. pneumophila (Svarrer and Uldum, 2012). A study of community-acquired Legionnaires' disease identified culture-confirmed cases where L. pneumophila was responsible for the greatest percentage of cases (91.5%), followed by L. longbeachae (3.9%) and L. bozemanii (2.4%). The remainder of cases were due to L. micdadei, L. feeleii, L. dumoffii, Legionella wadsworthii, and L. anisa (Yu et al., 2002). Due to the lack of molecular detection diagnostics for nonpneumophila Legionella, we developed a multiplex real-time PCR assay for detection of 9 clinically relevant nonpneumophila Legionella spp. isolates, as well as real-time HRM assays to rapidly subtype L. longbeachae, L bozemanii, and L. feeleii isolates to the serogroup level. Detection of L. longbeachae is of particular importance outside of the United States. In Australia and New Zealand, L. longbeachae has been reported to be more prevalent in clinical cases than in other parts of the world (Murdoch, 2003; Whiley and Bentham, 2011). In addition to its detection capabilities, the assay allows for a time and cost-efficient alternative to mip sequencing, which can often take 8+ hours to complete and requires specialized equipment and training.
The current multiplex assay uses hydrolysis probe-based chemistry and targets distinct regions within the L. dumoffii, L. feeleii, and L. micdadei genomes, allowing for specific identification of these species. Primers and probes were designed to be specific for both L. longbeachae and L. bozemanii targets. Mismatches of primers and/or probes to target sequences of other Legionella spp. were exploited to enable additional identification. The lower assay efficiency due to primer and/or probe mismatches, along with the use of normalized nucleic acid, allows for successful discrimination of the cross-reactive species within each assay based on a differential Ct value and fluorescence levels of the amplification curve. Lower efficiency in oligo binding in the L. longbeachae assay allows for detection of L. sainthelensis sg 1 and sg 2 isolates, while the L. bozemanii assay can also detect L. anisa, L. parisiensis, and L. tucsonensis sg 1 and sg 3 isolates. Although rarely isolated from clinical cases, L. anisa, L. parisiensis, and L. tucsonensis have been identified in the past as direct causes of human disease, including pneumonia and Pontiac fever (Fallon and Stack, 1990; Fenstersheib et al., 1990; Jones et al., 2003; Lo Presti et al., 1997; Thacker et al., 1989). In addition, L. anisa is the most frequently isolated species from hospital water systems, in conjunction with L. pneumophila (van der Mee-Marquet et al., 2006).
Although widely used for subtyping of Legionella spp., DFA assay analysis is limited by cross-reactions among Legionella spp. and serogroups (Mercante and Winchell, 2015; Thacker et al., 1985). Since L. anisa, L. parisiensis, and L. tucsonensis sg 1 and sg 3 amplification curves fall within the same Ct value, a follow-up real-time PCR-HRM assay was developed in order to discriminate these species based on nucleotide differences within a 101-bp amplicon in the ssrA gene, as seen in Fig. 2. We were able to rapidly distinguish L. longbeachae–, L. bozemanii–, and L. feeleii–specific serogroups by designing an additional real-time PCR assay followed by HRM analysis targeting a specific region of the mip gene (Fig. 3). This increased resolution methodology has also been used to detect single-nucleotide polymorphisms in human genes (Gundry et al., 2003; Herrmann et al., 2006) and to subtype and identify antibiotic resistance in other bacteria (Bidet et al., 2012; Mitchell et al., 2009; Wolff et al., 2008).
Although limited to detection of isolates, this assay complements existing methods for detection and could serve as a tool for identification of clinically relevant nonpneumophila Legionella spp. in a clinical microbiology lab. The assay allows for rapid identification of 9 Legionella spp., along with subtyping of L. longbeachae, L. bozemanii, and L. feeleii isolates using HRM. The assay builds upon our recently developed multiplex assay (Benitez and Winchell, 2013), thus expanding the field of molecular diagnostics for Legionella detection. These assays can be used to complement bacteriological culture and antigen detection, allowing rapid and specific diagnosis, especially during outbreak investigations.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Amodeo MR, Murdoch DR, Pithie AD. Legionnaires' disease caused by Legionella longbeachae and Legionella pneumophila: comparison of clinical features, host-related risk factors, and outcomes. Clin Microbiol Infect 2010;16:1405–7. [DOI] [PubMed] [Google Scholar]
- Benitez AJ, Winchell JM. Clinical application of a multiplex real-time PCR assay for simultaneous detection of Legionella species, Legionella pneumophila, and Legionella pneumophila serogroup 1. J Clin Microbiol 2013;51:348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson RF, Thacker WL, Fang FC, Kanter B, Mayberry WR, Brenner DJ. Legionella sainthelensi serogroup 2 isolated from patients with pneumonia. Res Microbiol 1990;141:453–63. [DOI] [PubMed] [Google Scholar]
- Best M, Yu VL, Stout J, Goetz A, Muder RR, Taylor F. Legionellaceae in the hospital water-supply. Epidemiological link with disease and evaluation of a method for control of nosocomial Legionnaires' disease and Pittsburgh pneumonia. Lancet 1983;2:307–10. [DOI] [PubMed] [Google Scholar]
- Bidet P, Liguori S, Plainvert C, Bonacorsi S, Courroux C, d'Humieres C, et al. Identification of group A streptococcal emm types commonly associated with invasive infections and antimicrobial resistance by the use of multiplex PCR and high-resolution melting analysis. Eur J Clin Microbiol Infect Dis 2012;31:2817–26. [DOI] [PubMed] [Google Scholar]
- Bornstein N, Vieilly C, Marmet D, Surgot M, Fleurette J. Isolation of Legionella anisa from a hospital hot water system. Eur J Clin Microbiol 1985;4:327–30. [DOI] [PubMed] [Google Scholar]
- Cianciotto NP, Bangsborg JM, Eisenstein BI, Engleberg NC. Identification of mip-like genes in the genus Legionella. Infect Immun 1990;58:2912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon RJ, Stack BH. Legionnaires' disease due to Legionella anisa. J Infect 1990;20:227–9. [DOI] [PubMed] [Google Scholar]
- Fang GD, Yu VL, Vickers RM. Disease due to the Legionellaceae (other than Legionella pneumophila). Historical, microbiological, clinical, and epidemiological review. Medicine 1989;68:116–32. [DOI] [PubMed] [Google Scholar]
- Fenstersheib MD, Miller M, Diggins C, Liska S, Detwiler L, Werner SB, et al. Outbreak of Pontiac fever due to Legionella anisa. Lancet 1990;336:35–7. [DOI] [PubMed] [Google Scholar]
- Fields BS. The molecular ecology of legionellae. Trends Microbiol 1996;4:286–90. [DOI] [PubMed] [Google Scholar]
- Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 2002;15:506–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundry CN, G.V. J, H.R. G, J.P. R, Chen J, Wittwer CT. Amplicon melting analysis with labeled primers: a closed-tube method for differentiating homozygotes and heterozygotes. Clin Chem 2003;49:396. [DOI] [PubMed] [Google Scholar]
- Herrmann MG, Durtschi JD, Bromley LK, Wittwer CT, Voelkerding KV. Amplicon DNA melting analysis for mutation scanning and genotyping: cross-platform comparison of instruments and dyes. Clin Chem 2006;52:494–503. [DOI] [PubMed] [Google Scholar]
- Herwaldt LA, Gorman GW, McGrath T, Toma S, Brake B, Hightower AW, et al. A new Legionella species, Legionella feeleii species nova, causes Pontiac fever in an automobile plant. Ann Intern Med 1984;100:333–8. [DOI] [PubMed] [Google Scholar]
- Joly JR, Dery P, Gauvreau L, Cote L, Trepanier C. Legionnaires' disease caused by Legionella dumoffii in distilled water. CMAJ 1986;135:1274–7. [PMC free article] [PubMed] [Google Scholar]
- Jones TF, Benson RF, Brown EW, Rowland JR, Crosier SC, Schaffner W. Epidemiologic investigation of a restaurant-associated outbreak of Pontiac fever. Clin Infect Dis 2003;37:1292–7. [DOI] [PubMed] [Google Scholar]
- Katz SM. Legionellosis. Boca Raton, Fla: CRC Press; 1985. [Google Scholar]
- Kozak NA, Lucas CE, Winchell JM. Identification of Legionella in the environment. Methods Mol Biol 2013;954:3–25. [DOI] [PubMed] [Google Scholar]
- Lee J, Caplivski D, Wu M, Huprikar S. Pneumonia due to Legionella feeleii: case report and review of the literature. Transpl Infect Dis 2009;11:337–40. [DOI] [PubMed] [Google Scholar]
- Lo Presti F, Riffard S, Vandenesch F, Reyrolle M, Ronco E, Ichai P, et al. The first clinical isolate of Legionella parisiensis, from a liver transplant patient with pneumonia. J Clin Microbiol 1997;35:1706–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry PW, Blankenship RJ, Gridley W, Troup NJ, Tompkins LS. A cluster of Legionella sternal-wound infections due to postoperative topical exposure to contaminated tap water. N Engl J Med 1991;324:109–13. [DOI] [PubMed] [Google Scholar]
- Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev 2015;28:95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SL, Wolff BJ, Thacker WL, Ciembor PG, Gregory CR, Everett KD, et al. Genotyping of Chlamydophila psittaci by real-time PCR and high-resolution melt analysis. J Clin Microbiol 2009;47:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch DR. Diagnosis of Legionella infection. Clin Infect Dis 2003;36:64–9. [DOI] [PubMed] [Google Scholar]
- Newton HJ, Ang DK, van Driel IR, Hartland EL. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 2010;23:274–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palutke WA, Crane LR, Wentworth BB, Geiger JG, Cardozo L, Singhakowinta A, et al. Legionella feeleii-associated pneumonia in humans. Am J Clin Pathol 1986;86:348–51. [DOI] [PubMed] [Google Scholar]
- Parry MF, Stampleman L, Hutchinson JH, Folta D, Steinberg MG, Krasnogor LJ. Waterborne Legionella bozemanii and nosocomial pneumonia in immunosuppressed patients. Ann Intern Med 1985;103:205–10. [DOI] [PubMed] [Google Scholar]
- Ratcliff RM, Lanser JA, Manning PA, Heuzenroeder MW. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J Clin Microbiol 1998;36:1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold AL, Thomason BM, Brake BJ, Thacker L, Wilkinson HW, Kuritsky JN. Legionella pneumonia in the United States: the distribution of serogroups and species causing human illness. J Infect Dis 1984:149:819. [DOI] [PubMed] [Google Scholar]
- Steinert M, Hentschel U, Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol Rev 2002;26:149–62. [DOI] [PubMed] [Google Scholar]
- Svarrer CW, Uldum SA. The occurrence of Legionella species other than Legionella pneumophila in clinical and environmental samples in Denmark identified by mip gene sequencing and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect 2012;18:1004–9. [DOI] [PubMed] [Google Scholar]
- Thacker WL, Plikaytis BB, Wilkinson HW. Identification of 22 Legionella species and 33 serogroups with the slide agglutination test. J Clin Microbiol 1985;21:779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker WL, Benson RF, Schifman RB, Pugh E, Steigerwalt AG, Mayberry WR, et al. Legionella tucsonensis sp. nov. isolated from a renal transplant recipient. J Clin Microbiol 1989;27:1831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mee-Marquet N, Domelier AS, Arnault L Bloc D, Laudat P, Hartemann P, et al. Legionella anisa, a possible indicator of water contamination by Legionella pneumophila. J Clin Microbiol 2006;44:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley H, Bentham R. Legionella longbeachae and legionellosis. Emerg Infect Dis 2011;17:579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff BJ, Thacker WL Schwartz SB, Winchell JM. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high resolution melt analysis. Antimicrob Agents Chemother 2008;52:3542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis 2002;186:127–8. [DOI] [PubMed] [Google Scholar]