1 |. INTRODUCTION
It is widely accepted that organisms, including microbes, are not distributed in a spatially independent manner within ecosystems and that this nonrandom distribution is observable only at scales relevant to the organisms in question.1–4 In this review, we discuss three levels of spatial scale at which basic biochemical and biophysical principles determine the function of dental plaque communities: the macro, meso, and microscale (Figure 1).
FIGURE 1.

The significance of scale in dental plaque biology. The structure of dental plaque can be studied at three scales: the micro, meso, and macroscales. Different fundamental forces contribute to the structure of dental plaque at each scale. Micro and macroscale processes that structure dental plaque contribute to the unique emergent properties of dental plaque at the mesoscale. These properties include the creation of ecologic niches, metabolic cooperation, and persistence in its environment
2 |. SIGNIFICANCE OF SCALE IN ORAL MICROBIAL ECOLOGY
Ecologists have long acknowledged the importance of scale for understanding community structure-function relationships.5 In ecology, two fundamental determinants of spatial scales are grain and extent.6 The grain of an ecologic system is defined as the breadth of the individual units of observation.6 For example, in the oral microbiome, if the teeth of some number of human volunteers are scraped over a 10 mm2 area to collect dental plaque and the microbial deoxyribonucleic acid (DNA) from each scraping is pooled and sequenced, the grain of the measurement would be 10 mm2 in area. In contrast to grain, the extent is defined as the breadth of the entire study.6 For example, if the different oral surfaces (eg, the buccal mucosa, gingiva, tongue dorsum, and teeth) from one human volunteer are each swabbed and the DNA collected from each site is sequenced, the extent of the measurements would be roughly equivalent to the surface area of the human oral cavity, or approximately 214 cm2.7 Unique mechanisms dominate community assembly at different scales.6 In the oral microbiome, intercellular interactions are hypothesized to dominate at small spatial scales, whereas environmental filtering (eg, the nature of the surface available for attachment or the oxygen availability at different sites in the oral cavity) is hypothesized to dominate community structuring at larger spatial scales.8,9 Here, we identify three levels of spatial scale in the human dental plaque microbial community using biological and ecologic principles to justify studying these communities at three ordinal levels.
We define the macroscale level of dental plaque structure to be on the order of 1 mm to 1 cm or more. This is the scale of the relevant microbially colonized human structures in the mouth; for example, the tooth, tongue, gingiva, and cheek.9 This is, in fact, the scale at which the first direct observation of bacteria was made by van Leeuwenhoek, when he scraped his teeth and used a microscope of his own fashioning to produce remarkably realistic drawings of bacterial cells.10,11 In true Linnaean fashion, van Leeuwenhoek took great pains to dissect his dental plaque to classify and catalog the different organisms that he saw using phenotypic characteristics, resulting in the first description of dental plaque community structure.10 Although the sampling procedure has remained largely unchanged (ie, tooth scraping), modern studies of the macroscale structure of oral microbial communities are achieved through molecular sequencing or other omics approaches.12,13 The power of sequencing approaches is that they enable phylogenomic comparisons between the different surfaces or environments in the mouth; for example, differences in taxonomic composition and abundances in supra and subgingival dental plaque communities have been robustly detected.14,15 However, the spatial resolution afforded by sequencing approaches is limited by the sampling technology. Scraping or swabbing oral surfaces to collect microbial populations and homogenizing the sample to extract DNA unavoidably limits spatial resolution to the centimeter scale.
At the other end of the spatial scale relevant to dental plaque is that from 1 to 10 μm, which we call the microscale. This is the scale of the individual cells themselves that comprise dental plaque.16 Relevant processes at this scale involve intercellular interactions, both physical and chemical, among the microbes in the dental plaque community and between microbial cells and host.17 These interactions are best studied with biochemical and genetic approaches and by microscopy, including electron microscopy. What remains is the spatial scale between the two extremes of macro and microscale, from 10 to 1000 μm, which we call the mesoscale. This is the scale of the dental plaque biofilm itself, which, in one dimension, may extend tens to 100 or more micrometers outward from the saliva-coated tooth surface.18 Electron and light microscopy imaging of intact dental plaque has revealed that oral microbes assemble into highly nonrandom structures that qualitatively resemble the spatial patterning governed by the phenotypically different cells in metazoan tissues and organs.19 In this review, we focus our analysis on the mesoscale structure of dental plaque, and we discuss how the micro and macroscale features of the microbial community contribute to the emergent properties of the dental plaque biofilm at this scale, which include metabolic heterogeneity, persistence, and community function in health and disease.
3 |. MACROSCALE STRUCTURE OF DENTAL PLAQUE
The human oral cavity includes a number of different habitats for microbes. These habitats include the epithelial mucosa of the cheek and gingiva, the papillary surface of the tongue dorsum, and the nonshedding, hard surfaces of teeth, which themselves consist of two distinct compartments: the supragingival surface (above the gum line) and the subgingival surface (that below the gum line).20 Site-specific sequencing studies of these different habitats have revealed that they support different microbial communities mediated by the characteristics of the surfaces available for attachment, oxygen availability, and exposure to host products delivered by saliva and gingival crevicular fluid.12 Numerous studies have described the structure of oral microbial communities in terms of the taxonomic composition and abundances within those communities.12,21,22 The limitations of sampling at the macroscale necessitate a compositional definition for community structure, in contrast to the spatial definition of structure we define for the micro and mesoscales. On the millimeter to centimeter scale, differences in community composition are readily observed through mechanical sampling, pooling of extracted DNA, and sequencing of supragingival and subgingival plaque communities.
Numerous molecular-based sequencing studies have resulted in a consensus among researchers that approximately 700 species or phylotypes comprise the bacterial component of the oral microbiome.20,21 Approximately one half to two thirds of these 700 species have been cultivated in the laboratory; the remaining organisms are known only from molecular sequencing surveys, primarily of 16S ribosomal ribonucleic acid (RNA) sequence, but increasingly through metagenomic analysis.20,23 Like the human microbiome in general, the dental plaque microbiome of healthy subjects is dominated by members of the phyla Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes, as well as the additional orally abundant Fusobacteria and Spirochaetes.24 Meta-analysis of supra and subgingival dental plaque 16S sequence data from the Human Microbiome Project revealed that 13 genera are both highly abundant and have high prevalence within the sampled population; these genera include Corynebacterium, Streptococcus, Fusobacterium, Prevotella, Leptotrichia, Haemophilus, and Aggregatibacter.25 At the genus level, the taxonomic composition of both the supra and subgingival communities in states of health are similar, with marked differences in relative abundance; for example, the genus Prevotella is more abundant in subgingival communities, a reflection of the different environmental conditions (eg, oxygen tension experienced by the supra and subgingival microbial communities).24,25
The oral microbiome is known to mediate diseases in the mouth, including dental caries and periodontal disease, and a tremendous effort has been made to characterize the macroscale structure of health and disease-associated dental plaque communities.17,26 Periodontitis is a chronic, progressive disease, characterized by expansion of the microbial biofilm at the gingival margin with the formation of an inflammatory infiltrate that contributes to destruction of connective tissue attachment to the tooth, alveolar bone resorption and may result in eventual tooth loss.14,27 In addition, periodontal disease status is correlated with certain comorbid systemic diseases, including cardiovascular disease, rheumatoid arthritis, adverse pregnancy outcome, and cancer, through cellular and molecular mechanisms that are not well understood.27–33 No single organism is implicated in the transition from health to periodontal disease; rather, the subgingival microbial community present in states of periodontal health undergoes a dysbiosis, or transition, in which the community structure (ie, species composition and abundance) shifts toward a pathogenic state.34 DNA-DNA checkerboard hybridization first identified a three-member consortium of gram-negative organisms (Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia), called the "red complex," enriched in the subgingival microbiomes of patients with periodontal disease.35 These organisms have been termed pathobionts rather than pathogens because, although implicated in disease, they are normally present at lower abundances in the microbiota of subjects with no clinical markers of periodontitis.36 The list of pathobionts has recently been expanded through modern culture-independent molecular surveys to include the gram-positive Filifacter alocis and many other species.14,24,37
The gingival pocket increases in volume during the progression of periodontal disease, which may drive higher bacterial load within this niche.17,38 Rather than replacing earlier colonizers, the total bacterial load is increased in patients with periodontal disease as newly dominant members of the community emerge and accumulate. This suggests a dynamic physical interplay between biofilms and host tissue to create this new space.14 Even a cursory visualization of supra and subgingival dental plaque (eg, with light microscopy of gram-stained tooth specimens) reveals the microbial cells within these communities to be highly ordered and nonrandomly distributed.39 This mesoscale structure is the subject of an exhaustive body of literature dating to the mid-20th century, and both qualitative and quantitative analyses of dental plaque at varying levels of specificity have been achieved.
4 |. MESOSCALE STRUCTURE OF DENTAL PLAQUE
4.1 |. Early morphologic analyses of plaque development and community structure with electron and light microscopy
The microscope is a tool uniquely well suited for the study of dental plaque mesoscale structure. The development of imaging and labeling technologies has enabled the observation of dental plaque mesoscale structure and the formation and testing of hypotheses regarding the development of these communities and their function in health and disease.
The acquired salivary pellicle is a layer of salivary proteins and glycoproteins that permanently coats the surface of oral tissues, including teeth, and which is acquired by exogenously introduced oral surfaces within hours.40 This glycoprotein-rich pellicle was observed in early transmission electron microscopy and light microscopy imaging of natural teeth, dental crowns, and substrates including hydroxyapatite chips.41 Early transmission electron microscopy and histological imaging of resin crowns worn by human volunteers and replaced at various intervals allowed visualization and qualitative analysis of dental plaque development over time (Figure 2).42 Toluidine blue-methylene blue staining of 1-week-old supragingival dental plaque revealed that the initial community was composed almost entirely of coccoid cells arranged in morphologically distinct, palisading columns with respect to the saliva-coated substrate. This observation led to the conclusion that early plaque structure is formed through the expansion and division of early coccoid colonizers.18 In 2-month-old supragingival plaque, the biofilm structure was dominated by gram-negative filamentous cells, with many "corncob" structures present at the apical surface of the biofilms in which central filamentous cells are surrounded by coccoid cells. This observation supported the hypothesis that dental plaque communities undergo a process of ecologic succession over time, in which early colonizing gram-positive facultative aerobic cocci and rods initially establish and expand and are then replaced to some degree by filamentous and fusiform gram-negative anaerobes, especially below the apical, oxygen-bathed surface.43
FIGURE 2.

Early histochemical light micrograph of dental plaque on an epon strip and worn for 2 days by a healthy volunteer. Visible are palisading columns of phenotypically similar organisms, an abundance of cocci with some rods, and the salivary pellicle. Reprinted with permission from Listgarten et al42
The removable resin crowns used in the aforementioned study also allowed the microscopic observation of subgingival plaque in patients with periodontal disease. Histologic imaging revealed these subgingival communities to be less organized than supragingival ones, with an abundance of gram-negative filaments.42 A striking arrangement of cells that the authors called test-tube brush formations were frequently observed, consisting of one or more central filamentous cells surrounded by numerous thin filamentous cells. Transmission electron microscopy revealed the presence of numbers of spirochetes, sometimes arranged around a thick central filament.42
Transmission electron microscopy of extracted incisors, canines, premolars, and molars from patients with severe chronic periodontal disease revealed complex microbial communities. This allowed the formulation of multiple hypotheses regarding the development of dental plaque biofilm structure and the role of this structure in periodontal disease.38 The observation of numerous spirochetes of different morphologies at the apical surface of subgingival biofilms implicated their role in promoting periodontal disease. Furthermore, the lack of an extensive carbohydrate-rich extracellular matrix in subgingival plaque led the authors to conclude that intercellular interactions were a major driver of biofilm structure. Although conspicuous in their morphology, the corncob structures at the apical surface were observed infrequently. This led to the hypothesis that these structures were not responsible for disease progression.38
In fact, the lack of any single morphologically identified organism that was overrepresented in periodontal disease–associated biofilms led to the formulation of the nonspecific plaque hypothesis. According to this hypothesis, periodontal disease is mediated by expansion of the entire subgingival community, not any single pathogen. The nonspecific plaque hypothesis has been refined in the light of new knowledge indicating that not all microbes in the community are equivalent in terms of their impact on health. This refinement, called the ecologic plaque hypothesis, states that periodontal disease results from an imbalance in the microbial community mediated by ecologic stress that results in enrichment of disease-associated organisms.44,45 Recently, the ecologic plaque hypothesis has been further refined to become the polymicrobial synergy hypothesis, according to which synergistic physical and metabolic interactions among plaque microbes shape the community toward a pathogenic state and disrupt host homeostasis.26 According to this hypothesis, periodontal disease results from reciprocally reinforced interactions between plaque microbes and a dysregulated host immune system.17
4.2 |. Biofilm matrix interactions and mesoscale structure
Conventional electron microscopy necessitates dehydration of the sample to be imaged under vacuum, which degrades the extracellular matrix material of microbial biofilms. Recently, field emission scanning electron microscopy has been applied to image dental plaque biofilms on extracted teeth from periodontal disease patients.46 In the images acquired, fine strands of extracellular polymeric material can be seen intercalated among bacterial cells (Figure 3).
FIGURE 3.

Field emission-scanning electron microscopy image of subgingival dental plaque. Mesoscale structure of the subgingival biofilm consists of loosely arranged cells with multiple different morphologies, including rods, filaments, and spirochetes. Also present are extrapolymeric substances and putative outer membrane vesicles arranged in chains, both of which contribute to the spatial structure of plaque biofilms. Image credit: Richard Holliday. Reproduced from Holliday et al46
The extracellular matrix of dental plaque biofilms provides a structural and biochemical scaffold that functions over mesoscale lengths. The oral biofilm matrix is composed of polymeric substances, including exopolysaccharides and extracellular DNA. The function of extracellular DNA in the biofilm matrix is yet to be determined, but treatment of nascent in vitro oral biofilms with the DNA-degrading enzyme NucB inhibited biofilm formation and reduced species diversity, suggesting a role for this extracellular DNA in biofilm structural development.47 Exopolysaccharides are synthesized by the glucosyltransferases, exoenzymes that are ubiquitous among streptococci. The glucosyltransferases of Streptococcus mutans have been studied extensively for their role in dental caries pathogenesis. The glucans synthesized by S. mutans afford adherence mechanisms for S. mutans through membrane-associated glucan-binding proteins and interactions with surface glucans. A feedforward loop takes place in which repeated dietary intake of carbohydrate provides substrates for glucan synthesis, which increases the bulk and integrity of the exopolysaccharides matrix and provides a diffusion barrier to the pH-buffering saliva. A critical consequence is a localized drop in pH and changes in community composition to favor acidogenic and aciduric organisms that may ultimately lead to the formation of dental caries.48
Early childhood caries is an aggressive, rapidly progressing form of dental caries that affects toddlers and is associated with carriage of Candida albicans. Though S. mutans does not coaggregate with C. albicans directly, S. mutans glucosyltransferase B binds C. albicans and nucleates glucan synthesis on the surface of C. albicans cells, thereby recruiting S. mutans attachment.49 In the presence of repeated dietary sugar intake, C. albicans and S. mutans thus function synergistically to create a dense biofilm matrix, prone to acidification. Importantly, interaction between S. mutans and other streptococci stimulates physical changes in C. albicans from yeast to hyphae morphology, providing a long-range scaffold for glucan nucleation and physical interaction with streptococci.
In addition to exopolysaccharides matrix, putative outer membrane vesicles are also visualized in field emission scanning electron microscopy images of subgingival dental plaque (Figure 3). Outer membrane vesicles are produced by a wide variety of gram-negative bacteria and are implicated in intercellular communication.50 The outer membrane vesicles of P. gingivalis mediate horizontal gene transfer and contain adhesive surface molecules, including fimbrial proteins and gingipains.51,52 P. gingivalis outer membrane vesicles have been observed to mediate aggregation of Fusobacterium, Streptococcus, and Actinomyces and coaggregation of Treponema denticola with Lachnoanaerobaculum saburreum in vitro.53,54 In summary, modern advances in electron microscopy have allowed visualization of the mesoscale structure of dental plaque biofilms in a manner that preserves extracellular matrix materials.46 Modern field emission scanning electron microscopy images reveal highly complex subgingival biofilm communities; however, the extent of the taxonomic complexity in these communities is implicit only from the observation of morphologically distinct microbial cells, without specific information for molecular or taxonomic identity.
4.3 |. Fluorescence imaging of natural plaque communities with taxonomic resolution
Fluorescence in situ hybridization with probes directed against the 16S ribosomal RNA allows the culture-independent visualization of bacterial cells with taxonomic resolution.55,56 Fluorescence in-situ hybridization is therefore an extraordinary tool that links the molecular information provided by 16S sequencing with the spatial resolution of light microscopy. The further use of spectrally variant reporters allows multiple different taxa to be visualized simultaneously.57
Fluorescence in-situ hybridization labeling has been performed on a variety of artificial substrates worn in the mouths of human volunteers. These qualitative studies have been used in a number of ways: to localize early colonizers, including Actinomyces spp and Streptococcus spp, at the basal surface of supragingival biofilms grown on glass substrates58; to determine the distribution of Actinomyces, Fusobacterium nucleatum, Streptococcus, and Veillonella on enamel chips59; and to determine the distribution of Streptococcus sp, Rothia sp, Pasteurellaceae sp, and Veillonella sp in 8-hour plaque biofilms on enamel chips.60 Together, these studies have confirmed the localization of early colonizers within supragingival plaque and the localization of Actinomyces and Streptococcus at the salivary pellicle.61 Fluorescence in-situ hybridization imaging has not been performed extensively on subgingival biofilms in states of health; however, artificial substrates inserted in the periodontal pocket of human volunteers allowed observation and localization of Treponema sp cells within subgingival biofilms.62
Extensive fluorescence in-situ hybridization labeling with simultaneous use of two or three probes of subgingival biofilms on teeth extracted from patients with periodontal disease has provided a qualitative visual atlas of the spatial distributions of some dominant organisms, including putative periodontal pathobionts (Figure 4).63 Importantly, the multiplex labeling of dental plaque with taxon-specific probes in combination with domain-level probes for Bacteria and Eukarya (Fungi) allowed the localization of specific microbial taxa within the greater context of the intact biofilm; that is, observation of the mesoscale structure of dental plaque with limited taxonomic resolution.
FIGURE 4.
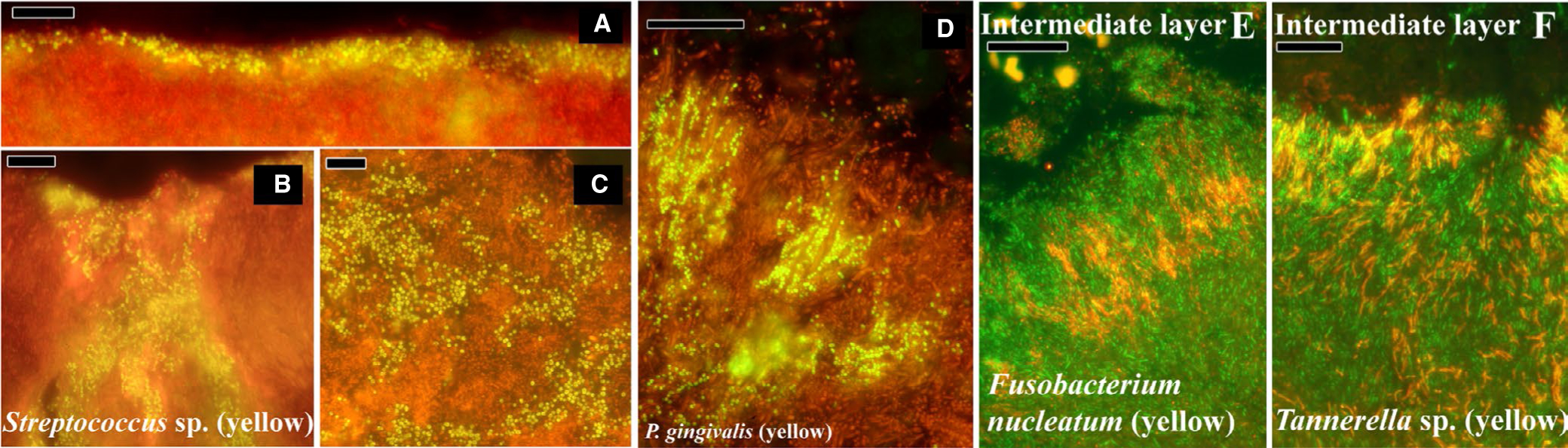
Fluorescence in-situ hybridization–labeled supra and subgingival dental plaque on teeth from donors with periodontal disease. A-C, Supragingival plaque labeled with a probe for all bacteria; EUB-338 in red, and streptococci in yellow. D, Subgingival plaque labeled with EUB red and a probe for Porphyromonas gingivalis in yellow. E, Subgingival plaque intermediate layer labeled with EUB in green and a probe for Fusobacterium nucleatum in red. F, Intermediate layer of subgingival plaque labeled with EUB in green and a genus-level probe for Tannerella in red. Reprinted from Zijnge et al63
Systematic analysis of hundreds of thin sections from seven teeth carefully extracted from four different donors so as to preserve associated biofilms allowed Zijnge et al to identify some common features of supra and subgingival plaque.63 Differential fluorescence intensity of the bacterial domain–level probe EUB combined with morphologic analysis revealed four distinct layers in subgingival plaque, which the authors called basal, intermediate, top, and outside. The basal layer contained Actinomyces and other cells that displayed weak EUB fluorescence, which the authors interpreted as representative of low metabolic activity. The intermediate layer contained Fusobacterium and Tannerella species. The top layer of the biofilm consisted of many cells of the Cytophaga-Flavobacterium-Bacteroides cluster, including Tannerella sp and Prevotella sp; and Porphyromonas and Prevotella were distributed in the intermediate and top layers, sometimes in clusters, suggesting a role for cell growth and division in biofilm development. The top layer also contained large cells of the Synergistetes group A, and these cells were frequently observed in close association with host polymorphonuclear lymphocytes. Spirochaetes were observed outside the densely packed biofilm structure, as well as examples of the previously described aggregate termed "test-tube brushes" comprised of Tannerella sp, Fusobacterium sp, Synergistetes group A species, and filamentous cells from the Cytophaga-Flavobacterium-Bacteroides cluster.
Zijnge et al further described supragingival plaque, which, in contrast to subgingival plaque samples, displayed a high amount of heterogeneity in its mesoscale structure. The authors identified two layers in supragingival biofilm structures. The basal layer was composed of taxonomically and morphologically diverse consortia in different biofilm samples, including Actinomyces, Streptococcus, Lactobacillus, and fungi, while the apical layer frequently consisted of Streptococcus sp, sometimes aligned as a thin coat and sometimes as constituents of "corncob" structures with filamentous fungi.
In summary, fluorescence in-situ hybridization labeling of carefully preserved dental plaque with taxon-specific and general bacterial and fungal probes allowed the first qualitative description of the mesoscale spatial structure of dental plaque with taxonomic specificity. The utility of fluorescence in-situ hybridization with its exquisite molecular specificity is offset by the spectral cross talk of available fluorescence reporters,64 which, until recently, has restricted the multiplexing capability of fluorescence in-situ hybridization to the use of only a handful of different probes in any single sample.57,65
4.4 |. Systems-level fluorescence in-situ hybridization imaging of dental plaque biofilms
The application of combinatorial labeling and spectral imaging to microbial fluorescence in-situ hybridization combines the major strength of DNA sequencing (namely its extraordinary breadth) with that of imaging (ie, its ability to provide spatial information on the microscale). Combinatorial labeling and spectral imaging–fluorescence in-situ hybridization allowed the first simultaneous visualization of 15 different genera of oral microbes in dental plaque that had been scraped from healthy volunteers.66 Although self-removal of plaque biofilms with dental floss followed by vortex mixing of the sample resulted in loss of mesoscale structure, quantitative analysis of intergenus pairings in the observed plaque aggregates allowed the identification of putative biofilm associations. These statistically significant pairings were then used to inform a network analysis of the plaque biofilm informed by direct spatial contacts and led to the surprising observation that Fusobacterium did not make intertaxon associations with a large number of other genera, as previously predicted from in-vitro coaggregation assays.67
Recently, multispectral fluorescence in-situ hybridization was applied to supragingival plaque extracted from healthy volunteers in a manner that preserved the three-dimensional structure of large regions of the biofilm.25 Within these undisturbed regions, large, annular, multitaxon structures, termed hedgehogs, were repeatedly observed. These structures consisted of a mass of radially oriented Corynebacterium filaments with Streptococcus at the periphery. Seven other taxa were consistently observed within hedgehog structures: Porphyromonas, Haemophilus/Aggregatibacter, Neisseriaceae, Fusobacterium, Leptotrichia, Capnocytophaga, and Actinomyces (Figure 5). Although Fusobacterium cells were present within the hedgehog structures, a filamentous Corynebacterium species dominated the community in volume, with cellular filaments anchored at the presumed tooth-associated base and extending through the structure to its apex, where Streptococcus decorated the Corynebacterium filament tips. Also present in this apical layer were Haemophilus/Aggregatibacter and Porphyromonas. Capnocytophaga cells occupied a wide band just inside the periphery along with Fusobacterium and Leptotrichia, which together comprised a dense, filament-rich annulus. Neisseriaceae formed clusters just inside the periphery, and Actinomyces could be found at the base of the hedgehog structure. The apical portions of the Corynebacterium filaments were frequently involved in corncob structures with cells of the genera Streptococcus and Porphyromonas. Corncob structures had been described in early electron microscopy images of supragingival dental plaque, and in early fluorescence in-situ hybridization–labeled plaque with limited taxonomic identification19,63 (Figure 6). Sometimes, a second layer of kernels was present on Streptococcus-involved corncobs which included Haemophilus/Aggregatibacter. At their basal anchor, the Corynebacterium filaments were sometimes embedded within cells of the genera Actinomyces, consistent with these organisms acting as salivary-pellicle binding, early colonizers of the developing biofilm.
FIGURE 5.
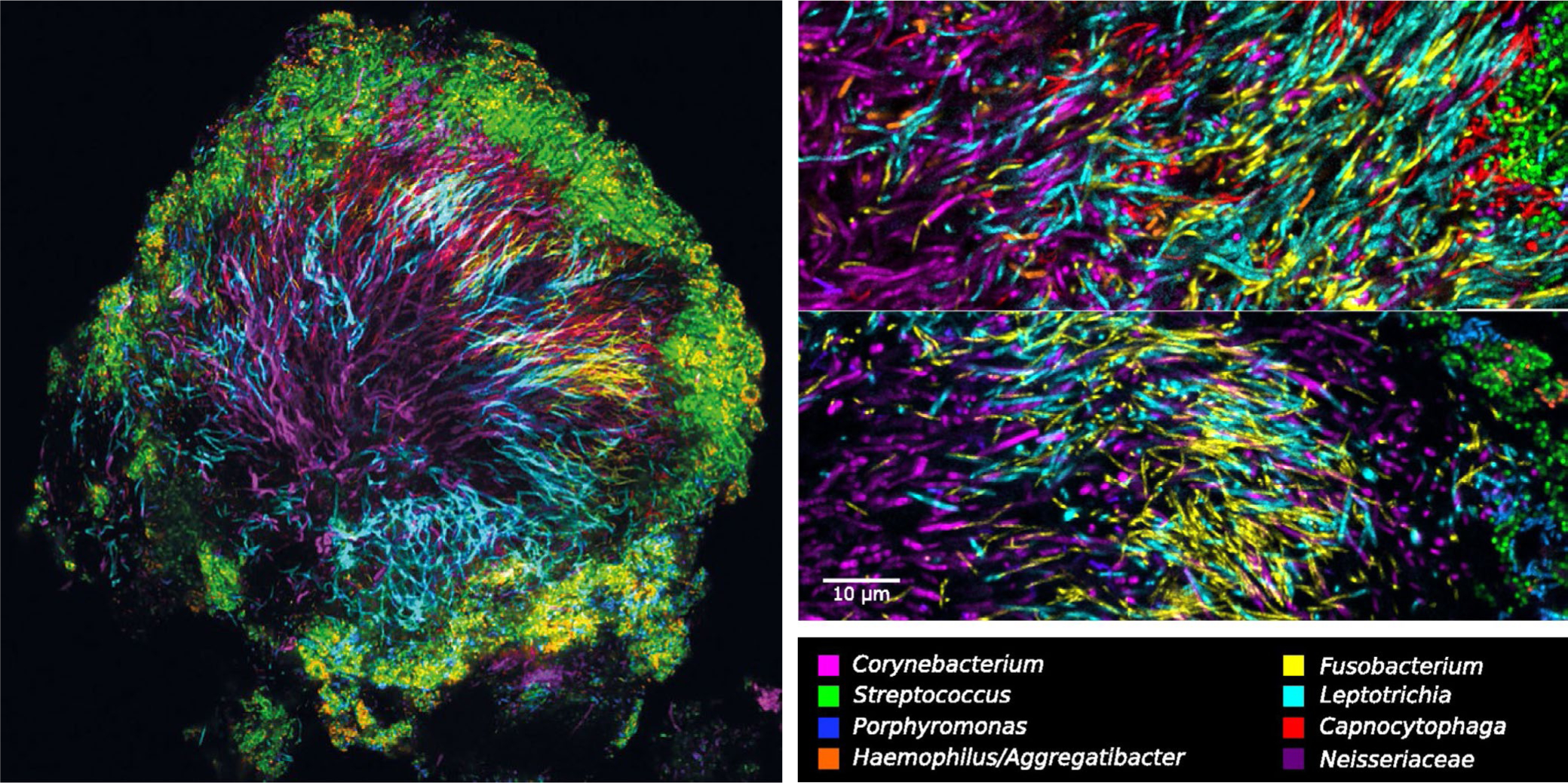
Multispectral fluorescence in-situ hybridization images of a hedgehog structure in supragingival plaque carefully scraped from the tooth of a healthy volunteer. Visible are filamentous organisms in the center of the structure and streptococci and other facultative anaerobes at the saliva-exposed surface of the structure. Zoom images show the abundance of filamentous organisms in the center of the hedgehog structure, which include Corynebacterium, Leptotrichia, and Fusobacterium. Reprinted from Mark Welch et al25
FIGURE 6.

Corncob structures in supragingival plaque. A, B, Scanning electron microscope images of corncob structures showing a central filament encased at the tip with cocci. C, Multispectral fluorescence in-situ hybridization images of corncob structures observed at the periphery of hedgehog structures. Fluorescence in-situ hybridization allows taxonomic identification of the component cells and reveals the central filament to be either unlabeled or labeled with a probe for Corynebacterium. The cocci that decorate the filament show some diversity and include cells of the genera Streptococcus, Haemophilus or Aggregatibacter, and Porphyromonas. A, B, Previously unpublished images courtesy of Professor Charles M. Cobb, see Cobb and Killoy.19 C, Reprinted from Mark Welch et al25
Taken together, these observations led Mark Welch et al to propose a model in which environmental pressures and synergistic intercellular interactions–including oxygen tension, the production and local accumulation of lactic acid and hydrogen peroxide by streptococci, and the host-supplied nonshedding tooth surface and saliva–contribute to the ecologic succession and remodeling of the hedgehog community to its climax state.25 This model further assigned a central role to filamentous Corynebacterium spp as the central organizer of the hedgehog structure.
5 |. MICROSCALE STRUCTURE OF DENTAL PLAQUE
Small-scale processes in microbial communities underlie large-scale functions. Taken together, the integrated action of microscale processes contributes to the mesoscale biofilm structure and the emergent function of the community.4 In dental plaque, these processes include synergistic interactions, both physical and biochemical, between microorganisms, micro-environmental inputs, and host contributions (ie, diet and immune system interactions).17 Importantly, these microscale processes do not act independently, but rather function together as a complex, interactive system. For example, the chemical interactions between organisms are mediated by physical proximity, including those mediated by direct physical contacts, whereas both chemical and physical interactions underlie the pattern of ecologic succession observed in dental plaque development. Therefore, although the discussion of microscale interactions in dental plaque could begin with any type of interaction, the consideration of dental plaque formation as a developmental process provides a logical framework for structuring this discussion. Lastly, it should be noted that the microscale forces that structure dental plaque biofilms have been studied mostly in the context of oral diseases; that is, dental caries and periodontal disease. A need for future research is observation of the changes in environment and microbial communities that cooccur during the transition from health to disease, as these will provide an opportunity for a mechanistic understanding of oral biofilm development.
5.1 |. Physical interactions between microbes and host
Early, culture-based studies of bacteria isolated at different time points from artificial substrates worn by human volunteers led to the hypothesis that a process of ecologic succession occurs in the oral microbial community.68 These initial observations have been confirmed with modern molecular approaches.69 Additionally, early electron microscopic and nonspecific histologic imaging of substrates worn for different times42 corroborated this hypothesis and have been further confirmed with taxon-specific fluorescence imaging of worn substrates and in vitro systems.60 Early imaging studies also revealed that the initial colonizers do not bind directly to tooth enamel or artificial substrates, but rather to a salivary pellicle.70
The salivary pellicle is a thin (~100–200 nm thick) layer of salivary proteins and glycoproteins that permanently coats the teeth and quickly absorbs to artificial tooth substrates within minutes, depending upon the substrate material.71 Of the 700 bacterial species that comprise the oral microbiome, a very small number are able to bind directly to the salivary pellicle and mostly belong to the genus Streptococcus.67 In fact, within the first 4 hours of colonization, streptococci comprise upward of 80% of the pellicle-bound community.60,72 Members of the salivarius and mitis groups have demonstrated the ability to act as founder organisms. Species including S. mutans, Streptococcus sobrinus, Streptococcus gordonii, and Streptococcus oralis express the antigen I/II proteins that recognize human gp 340, other salivary glycoproteins, and proline-rich proteins.73 Streptococcal pili and fimbriae of Streptococcus parasanguinis, Streptococcus salivarius, and S. gordonii, as well as many as yet uncharacterized surface proteins, recognize other salivary components, including alpha amylase.74 Other potential founder organisms that have been isolated from or observed in early colonizing aggregates include members of the genera Veillonella, Actinomyces, and Haemophilus.60 Recently, cells of the genus Rothia were observed to nucleate multispecies microcolonies on fluorescence in-situ hybridization–labeled enamel chips worn by healthy volunteers.60
Other early colonizing organisms include species of the genera Veillonella, Gemella, and Haemophilus. Early colonization and development of plaque biofilms is driven by growth of the early colonizing organisms that initially adhere to the salivary pellicle. Evidence for the importance of growth in plaque development comes from early imaging studies in which columnar palisades of morphologically and phenotypically similar cocci were observed, suggesting a vertical growth pattern in 2-day-old supragingival dental plaque.42 Subsequent light-imaging studies revealed further growth patterns, including pyramidal expansion of antibody-labeled Streptococcus sanguinis and closely apposed restriction of morphologically similar organisms.75 This pattern of growth is strongly indicative of competition and ecologic advantage in biofilms.76 After the initial growth of early colonizing organisms, new species take up residence in the biofilm, including strict anaerobes, such as F. nucleatum and others, as succession proceeds toward the climax community.
5.2 |. Physical interactions among dental plaque microbes
Coaggregation is the specific, molecularly mediated adhesion between cells of two different species. The specificity of physical interactions between oral microbes was first described by Gibbons and Nygaard and was greatly expanded by Kolenbrander and coworkers with the systematic testing of over 1000 binary coaggregation interactions among available cultivable oral isolates.67,77 In fact, many of these coaggregation interactions have been confirmed by fluorescence microscopy and in vitro microbial coculture in flow systems, including that between S. gordonii and Veillonella atypica, between Aggregatibacter actinomycetemcomitans and F. nucleatum, and between F. nucleatum, Actinomyces naeslundii, and S. oralis, among many others.78–80 The molecular cell surface mediators of coaggregation generally consist of a protein adhesin, which recognizes a specific carbohydrate-motif receptor. Coaggregation functions to physically connect different species in oral biofilms and is necessary in the oral environment to counteract the effect of salivary flow, which could dislodge cells that are not tightly adhered to their neighbors. In fact, attachment of S. gordonii to a saliva-coated substrate was observed to increase in response to increasing shear forces in an in vitro flow system.81 More recently, the three streptococcal adhesins GspB, Hsa of S. gordonii, and SrpA of S. sanguinis were all found to mediate shear-enhanced adhesion of streptococci to immobilized platelet receptors.82
In addition to binding of salivary components of the acquired pellicle, the antigen I/II homologue SspB of S. gordonii mediates coaggregation with A. naeslundii83 and P. gingivalis via its minor fimbrial protein, Mfa1,84 while the long fimbriae protein FimA and other P. gingivalis surface proteins have been shown to interact with S. gordonii glyceraldehyde 3-phosphate dehydrogenase.85,86 CshA is a structural component of mitis-group streptococci fibrils and has been demonstrated to mediate streptoccoal coaggregation with Actinomyces and Candida.73
Among the oral isolates tested, F. nucleatum demonstrated a unique ability to coaggregate with multiple different species, including both early and late colonizers. This led to the hypothesis that F. nucleatum functions as a physical bridge in dental plaque biofilms to unite early and late colonizers and drive the community to its climax state. Several cell-surface adhesins have been identified in F. nucleatum, including an arginine-inhibitable adhesin, RadD,87 implicated in coaggregation with gram-positive organisms, including S. gordonii and A. naeslundii, as well as S. mutans, Staphylococcus aureus,88 and C. albicans.89 Adherence-inducing determinant 1 was subsequently identified as a putative accessory protein that contributes to RadD-mediated coaggregation with streptococci.90 The F. nucleatum surface protein FadA and the outer membrane autotransporter protein Fap2 have been demonstrated to mediate binding to host cells,91,92 and Fap2 further mediates coaggregation with P. gingivalis.93 The F. nucleatum porin protein FomA has demonstrated binding to the salivary protein statherin, as well as coaggregation with P. gingivalis.94,95 Recently, another F. nucleatum 23726 outer membrane protein, coaggregation-mediating protein A, was identified as a mediator of F. nucleatum coaggregation with S. gordonii.96 Although these culture and molecular studies identified multiple Fusobacterium proteins as candidates for mediating interspecies interactions, culture-independent imaging studies led to somewhat different conclusions. In our initial combinatorial labeling and spectral imaging–fluorescence in-situ hybridization study of 15 genera of oral microbes in semidisrupted dental plaque from healthy donors, Fusobacterium was observed to physically associate with only two other genera, Prevotella and Actinomyces, at frequencies that were higher than would be expected from random associations.66 Thus, the role that Fusobacterium plays in structuring the biofilm community in vivo remains a problem warranting further study.
5.3 |. Biochemical interactions among dental plaque microbes
Chemical interaction among oral biofilm members may be either synergistic or antagonistic.97 Although secreted molecules have the potential to exert their effects over long distances due to the dispersive effect of diffusion, we include these interactions in our discussion of microscale inputs because, in practice, the close apposition of microbial cells98 and the dense extrapolymeric matrix greatly restrict the diffusion of molecules within dental plaque biofilms.99 In contrast to human gut microbial communities, in which inferred co-occurrence patterns and known metabolic capacities suggest high levels of competition for overlapping resources that result in niche partitioning, there is strong evidence for metabolic cooperation in dental plaque communities.100 The strongest line of evidence for metabolic cooperation within oral biofilms comes from the observation that in vitro consortia of oral microbes grow as luxurious biofilms in stark contrast to mono-species cultures, which grow sparingly, if at all, when given dilute saliva as the sole nutritional source.101–103 Metabolic cooperation is exemplified by the interaction of V. atypica and S. gordonii. Lactic acid produced by S. gordonii as a waste product of glucose fermentation is used by V. atypica as a carbon source. In vitro coculture of these two organisms resulted in increased transcription of amyB, a gene for alpha amylase in S. gordonii.78 Thus, V. atypica is hypothesized to induce S. gordonii to hydrolyze glycogen and subsequently release lactic acid.104
In addition to syntrophic metabolism, other forms of metabolic cooperativity have been observed in oral communities. For example, increased biofilm growth of P. gingivalis in an in vitro coculture model with S. gordonii was recently shown to be mediated in part by 4-aminobenzoate/para-amino benzoic acid, produced by S. gordonii.105 Evidence suggests the metabolic cross talk mediated by para-amino benzoic acid's role in folate biosynthesis results in increased expression of P. gingivalis fimbriae and increased adhesion.106
Metabolic cooperation has also been observed in the form of oxidative protection. F. nucleatum has been shown to support the growth of P. gingivalis in oxygenated and carbon dioxide–depleted environments,107 and metabolic cross talk in the form of increased electron acceptor bioavailability (oxygen) has been observed between S. gordonii and A. actinomycetemcomitans in a coinfection model.108 Thus, metabolic cooperativity has been observed in many forms in oral microbe coculture. Since many oral microbes are auxotrophic for one or more amino acids, this suggests that many more metabolic interactions remain to be identified.102
In addition to synergistic interactions, biochemical interactions among oral microbes may also be antagonistic.109,110 Bacteriocins are ribosomally synthesized short antimicrobial peptides secreted by many oral bacteria and include the lantibiotics.111 The mutacins produced by S. mutans have antimicrobial activity against many other streptococci, including closely related strains.112 S. gordonii and S. sanguinis of the mitis group streptococci produce hydrogen peroxide as a by-product of carbohydrate metabolism.113 Though some oral microbes possess catalase genes, and therefore resistance to oxidative stress (eg, A. naeslundii, A actinomycetemcomitans, Corynebacterium spp, and others), streptococci do not, and strains of S. mutans display a range of susceptibility to hydrogen peroxide.114 Thus, microscale gradients of hydrogen peroxide concentration within plaque biofilms may regulate the mesoscale biogeography of susceptible strains. Many commensal oral microbes possess an arginine deaminase system and produce ammonia through arginine metabolism, which raises the pH of the biofilm environment and inhibits growth of aciduric organisms, including S. mutans.115 Taken together, the microscale, synergistic and antagonistic interactions among oral microbes contribute dynamic biochemical scaffolds for shaping the mesoscale architecture of the plaque biofilm.
6 |. MICROSCALE ENVIRONMENTAL PROCESSES
The physicochemical contributions of the oral environment are hypothesized to have a major influence on dental plaque macroscale structure.6 In contrast to the epithelial tissues that comprise the gingiva, hard and soft palate, and cheeks, the hard, nonshedding surface of the tooth is a unique platform for oral biofilms to develop upon.116 Epithelial cells are periodically sloughed off, along with their attached microbial aggregates or biofilms, which then leave the mouth via swallowing.117 The complexity and spatial structure of supra and subgingival oral biofilms may be a product of extended time for development. The nonshedding teeth provide a permanent surface for bacterial colonization and biofilm development in the absence of host behavioral interventions (ie, oral hygiene and tooth brushing).118 In contrast to teeth, host epithelial tissues are characterized by constant shedding and sloughing off of dead cells that is hypothesized to inhibit permanent bacterial colonization.119 In an in vitro monoculture system seeded with S mutans, stochastic processes, including random initial attachment of cells and early microcolony development among monogenic populations, were observed, suggesting a role for early stochastic and self-reinforcing dynamics in the assembly of multispecies plaque communities.120 In a different microbiome system, a 15-member model gut community in mice, community organization was characterized as having a highly mixed, almost random distribution of cells, with no strong taxonomic spatial pattern. This less ordered community was hypothesized to result from the peristaltic flow and mixing forces operating in the gut.121
The pH and oxygen tension are two chemical characteristics of the oral environment that each have a strong influence on dental plaque structure. This is because oral microbes have different and highly specific tolerances for oxygen concentration and pH.17 Whereas the oxygen concentration and pH of saliva and gingival crevicular fluid are relatively stable, dental plaque microbes interact with one another to create microscale gradients in pH and oxygen tension.48 For example, the early colonizers are invariably facultative anaerobes (eg, Streptococcus sp).122 During ecologic succession, anaerobes establish in the biofilm after a sufficient period of facultative anaerobe growth, which presumably creates an anaerobic niche within the biofilm structure as facultative anaerobes at the apical surface sequester oxygen. Indeed, in hedgehog structures, anaerobes such as Fusobacterium, Leptotrichia, and Prevotella are localized to the interior portions of the biofilm, whereas Streptococcus and Pasteurellaceae decorate the apical, saliva, and air-exposed surfaces.25 In addition, the apical surfaces of hedgehog structures are regularly decorated with corncob structures. These well-described structures consist of a central filamentous cell decorated with numerous cocci. With taxonomic resolution provided by fluorescence in-situ hybridization labeling, the central filament is frequently identified as a species of the anaerobic Corynebacterium genus, and the cocci are either Streptococcus, Pasteurellaceae or Porphyromonas. The co-occurence of all three cocci taxa in a single corncob structure suggests a complex interaction between the four taxa, whereas the existence of Streptoccocus or Porphyromonas-only corncobs suggests functional equivalence between these two genera in terms of their interaction with Corynebacterium. Importantly, P. gingivalis is a strict anaerobe; therefore, the Porphyromonas species that periodically localizes to the saliva-exposed apical layer of hedgehogs including comprising corncobs is most likely a less well characterized oral species (eg, Porphyromonas catoniae or Porphyromonas pasteri) that is presumed to have some degree of aerotolerance.25
7 |. HOST IMMUNE SYSTEM INTERACTIONS WITH SUBGINGIVAL ORAL MICROBES
The synergistic interactions between host and oral microbes are a final factor that is hypothesized to influence the mesoscale structure of dental plaque.123 These interactions are most frequently studied in the context of the oral diseases, especially periodontal disease. The spatial scales at which dental plaque microbes interact with the host immune system are varied: Inflammation of the host gingiva and its associated tissue destruction occur throughout the space of the periodontal pocket and beyond, the scale at which we call macro for the purpose of this discussion, but the cellular and molecular interactions involved occur at microscales. For convenience, we discuss immune system interactions here.
That subgingival dental plaque communities require inflammation for their nutritional support seems paradoxical because a localized inflammatory response by the host normally functions to inhibit microbial growth. This paradox is resolved by the demonstrated ability of P. gingivalis to manipulate the host immune response in such a fashion as to uncouple tissue destruction from microbicide during inflammation, through co-activation and manipulation of the complement and toll-like receptor pathways.17 P. gingivalis surface molecules activate toll-like receptors, and the excreted gingipains HgpA and RgpB induce complement C5 catalysis to generate C5a, the ligand for the C5a receptor of host immune cells, leading to a proinflammatory signal.124 At the same time, P. gingivalis inactivates bactericidal activity through downregulation of immune cell MyD88 signaling to achieve a proinflammatory, anti-phagocytic response in neutrophils and macrophages.125 P gingivalis also has been demonstrated to down-regulate expression in epithelial cells of interleukin-8, a chemokine that recruits phagocytic neutrophils and Th1 cells to the gingiva. Additionally, P. gingivalis regulates T-cell production of interferon gamma to achieve localized and intermittent chemokine paralysis.124,126–128 Thus, P. gingivalis acts as a keystone species in the developing plaque community to create an environment that favors the growth and expansion of anaerobic organisms that utilize peptides, amino acids, and iron liberated into the gingival crevice through the action of tissue hydrolases.129 In this way, the synergistic interactions between oral microbes and the host immune system contribute to the physical structure of the subgingival biofilm through expansion of the anaerobic subgingival space and growth of the biofilm community.
8 |. ASSIGNING THE CENTRAL ORGANIZER ROLE IN PLAQUE MESOSCALE STRUCTURE
The studies of Kolenbrander and coworkers identified F. nucleatum as a coaggregating partner of multiple different species. Because of this property, they proposed that Fusobacterium bridged early and late colonizers and served the role of central organizer in dental plaque structure.67,130 One may ask why Corynebacterium was missing from this picture and how our understanding of dental plaque structure has evolved in the light of new observations. hypothesis-testing approaches with holistic discovery-based methods for understanding the structure and function of any biological system, including dental plaque.
Recognition of Corynebacterium in dental plaque dates back to the pioneering studies of Gibbons et al.131 Using culture-based assays and careful quantitation, they identified the predominant cultivable microbiota of dental plaque. Among these were gram-positive rods, referred to at that time as “diphtheroids,” but which Gibbons and Nygaard suggested were probably members of the Corynebacterium genus.77 Significantly, one isolate displayed strong coaggregation with two strains of Streptococcus sanguis (now S. sanguinis). Curiously, after these initial observations, investigations of the presence and function of Corynebacterium species in supragingival plaque are surprisingly absent from the literature until after ca 2008. Corynebacterium species probes were not included in the seminal DNA checkerboard hybridization assays of healthy and disease-associated dental plaque communities of Socransky and coworkers.132,133 Although Kolenbrander et al134 systematically tested the coaggregation profiles of dozens of dental plaque isolates, Corynebacterium spp were not systematically tested in this initial work. Thus, their observation of promiscuous coaggregation between F. nucleatum and other species led to their model in which F. nucleatum functions as a central bridge linking early and late colonizers in dental plaque biofilms.130 At about the same time, Khemaleelakul et al135 tested Corynebacterium species in in vitro coaggregation assays with oral and skin taxa and identified positive associations with multiple strains of Actinomyces, Fusobacterium, Prevotella, and Streptococcus, albeit not Veillonella, and Rickard et al136 reported in vitro corncob-like structures between Streptococcus cristatus and Corynebacterium matruchotii in phase-contrast images. However, neither of these results were incorporated into the colonization model of Kolenbrander.
Regarding "corncobs," early in vitro coculture experiments with F nucleatum and S. anguis (now sanguinis) showed striking corncob-like associations that may have contributed to consensus in the field that the corncob structures previously observed in scanning electron micrographs of intact supragingival dental plaque involved F. nucleatum as the nucleating filament.137 Probes for C. matruchotii were included among the 272 species that comprised the Human Oral Microbiome Identification Microarray phylochip, and this innovation led to the recognition of Corynebacterium as a member of dental plaque.138 However, it was not until the revolution afforded by culture-independent approaches to study plaque composition and structure–namely, next-generation sequencing and combinatorial labeling and spectral imaging–fluorescence in-situ hybridization imaging and the systematic application of these methods to the study of dental plaque structure in states of health—that the newly appreciated role of Corynebacterium was realized, both in terms of its abundance and prevalence in supragingival plaque and its structural role as an organizing scaffold.25 The primary lesson learned here may be the importance of complementing reductionist
The large size and filamentous morphology of Corynebacterium cells immediately suggested its role in mediating the long-range spatial structure of supragingival plaque biofilms. The striking morphology of Corynebacterium filaments in hedgehog structures and their involvement in "corncob" arrangements are particularly conspicuous. However, Corynebacterium was not the sole filamentous organism that was abundantly observed in hedgehogs. Filaments of Fusobacterium, and Capnocytophaga and Leptotrichia as well, were also highly prevalent and abundant. The roles of these other filamentous organisms remain to be established. A possible central organizing role for Corynebacterium in supragingival biofilm structure and assembly does not exclude a key role for Fusobacterium or the other filamentous organisms. It could be that Fusobacterium is important in the development of supragingival plaque before assembly of the climax community, perhaps through initial establishment of an anaerobic niche. Especially important is that, although Corynebacterium species are abundant and highly prevalent in both supragingival and subgingival plaque,25 Fusobacterium dominates in abundance in subgingival plaque,14 and its demonstrated coaggregation potential with periodontal disease–associated anaerobic late colonizers may belie its particular significance in structuring the subgingival community.
9 |. CONCLUSIONS AND FUTURE DIRECTIONS
The structure of dental plaque microbial communities may be studied at multiple spatial scales, and unique processes contribute to the structure and function of these biological systems at different scales. The processes that contribute to the macroscale structure of dental plaque are mostly environmental characteristics of the community habitat. On the other extreme, the microscale intercellular interactions, both physical and chemical, dominate the assembly of dental plaque communities. At the mesoscale, both environmental processes and intercellular interactions combine to shape the dental plaque biofilm with its emergent properties. Environmental factors–such as the permanent, nonshedding nature of the tooth surface upon which early colonizers adhere–shape the stability and longevity of dental plaque, whereas the concentration of oxygen gives rise to highly structured anaerobic niches in supragingival plaque (formed by the encapsulation of biofilms by fastidious aerobes) or less restricted distributions of anaerobes in subgingival plaque. In addition, the metabolic cooperation of organisms over microscales sums across the dental plaque biofilm and gives rise to community metabolic resiliency. Our understanding of dental plaque structure and function at different scales is informed by the use of different types of tools: DNA sequencing or other omics approaches for macroscale, genetic manipulation and biochemical readouts for microscale, and light and electron microscope imaging for microscale and especially mesoscale.
Our understanding of dental plaque structure at all scales has been enabled by the development of new technologies. The first direct observation of bacteria was preceded and made possible by the development of the technology used to make that observation, namely van Leeuwenhoek's remarkable single-lens microscope.10 Other technological advances that allowed new observations and facilitated the asking of new kinds of questions included improved DNA sequencing tools, improved tools for genetic manipulation of microbes, and especially new imaging tools.139–143 The development of new technologies and the new kinds of observations they afford are not independent. Improved DNA sequencing technologies allowed the creation of near-complete databases of 16S ribosomal RNA sequences, which then allowed the development of systems-level fluorescence in-situ hybridization–labeling technologies.144,145
A recent study not only illustrates the importance of technology development in the scientific process but also provides a clear example of how microscale cell-cell interactions might contribute to mesoscale structure. Many of the organisms that comprise dental plaque and which are localized to specific niches within the biofilms are not motile.18,146 How do these organisms take up residence in their niche? Recently, a mechanism for cellular transport within plaque biofilms was proposed in which cells, including Prevotella oris, Parvimonas micra, F. nucleatum, S. sanguinis, Veillonella parvula, and others, are transported as cargo along the length of the bacterium Capnocytophaga gingivalis mediated by the ability of capsulate-producing C. gingivalis to undergo gliding motility.143 The authors observed long-scale transport of organisms within an in vitro consortium of oral microbes and proposed this as a possible mechanism for long-range facilitated organismal transport within dental plaque.143 This study is not only a clear example of how microscale cell-cell interactions might contribute to mesoscale structure, but also serves to highlight the importance of technology development in the scientific process. The further development of live-cell imaging tools such as these will allow a detailed understanding of the dynamic, ecological process that occurs during plaque development.
The correlation of structure with function is a basic tenet of biology. Just as the development of multispectral fluorescence in-situ hybridization has expanded the utility of 16S sequencing to allow systems-level mapping of dental plaque biofilms for taxonomic identification, the development of systems-level imaging tools that harness the utility and power of meta-transcriptomic and metabolomic studies will further our understanding of oral community structure and function. A major challenge for future research is to extend the power of multispectral imaging to living communities. Imaging fixed biofilm samples via fluorescence microscopy limits the interpretation of community structure to static snapshots in time. To begin to probe the dynamics of community assembly, live-cell imaging of model biofilms (in vitro or animal) comprised of organisms with genetically encoded fluorescence reporters for taxonomic identity and metabolic activity will greatly improve our understanding of community structure, assembly, and function. Meeting this challenge will require the multidisciplinary efforts of scientists with different specialized expertise. Achieving such synthesis will lead to greater understanding of the fundamental biological processes that underlie dental plaque community structure and function.
ACKNOWLEDGMENTS
The work of the authors is supported by National Institutes of Health grant DE022586 to GGB and DE028042 to AMV We thank Dr Jessica Mark Welch for a critical reading of the manuscript.
REFERENCES
- 1.Earle KA, Billings G, Sigal M, et al. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe. 2015;18(4):478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards J, Johnson C, Santos-Medellín C, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci USA. 2015;112(8):E911–E920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flintrop CM, Rogge A, Miksch S, Thiele S, Waite AM, Iversen MH. Embedding and slicing of intact in situ collected marine snow. Limnol Oceanogr Methods. 2018;16(6):339–355. [Google Scholar]
- 4.Nunan N, Wu K, Young IM, Crawford JW, Ritz K. Spatial distribution of bacterial communities and their relationships with the micro-architecture of soil. FEMS Microbiol Ecol. 2003;44(2):203–215. [DOI] [PubMed] [Google Scholar]
- 5.Wiens JA. Spatial scaling in ecology. Funct Ecol. 1989;3(4):385–397. [Google Scholar]
- 6.Ladau J, Eloe-Fadrosh EA. Spatial, temporal, and phylogenetic scales of microbial ecology. Trends Microbiol. 2019;27(8):662–669. [DOI] [PubMed] [Google Scholar]
- 7.Collins LM, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 1987;66(8):1300–1302. [DOI] [PubMed] [Google Scholar]
- 8.Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8(7):471–480. [DOI] [PubMed] [Google Scholar]
- 9.Proctor DM, Relman DA. The landscape ecology and microbiota of the human nose, mouth, and throat. Cell Host Microbe. 2017;21(4):421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobell C Antony van Leeuwenhoek and His ‘Little Animals’. New York, NY: Harcourt, Brace and Co.; 1932. [Google Scholar]
- 11.Listgarten MA, Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978;5(2):115–132. [DOI] [PubMed] [Google Scholar]
- 12.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milani C, Duranti S, Bottacini F, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):e00036–17. 10.1128/MMBR.00036-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7(5):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinoza JL, Harkins DM, Torralba M, et al. Supragingival plaque microbiome ecology and functional potential in the context of health and disease. MBio. 2018;9(6):e01631–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Li Y. Atlas of Oral Microbiology: From Healthy Microflora to Disease. London: Academic Press; 2015. [Google Scholar]
- 17.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Listgarten MA. The structure of dental plaque. Periodontol 2000. 1994;5:52–65. [DOI] [PubMed] [Google Scholar]
- 19.Cobb CM, Killoy WJ. Microbial colonization in human periodontal disease: an illustrated tutorial on selected ultrastructural and ecologic considerations. Scanning Microsc. 1990;4(3):675–690; discussion 690–691. [PubMed] [Google Scholar]
- 20.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaura E, Keijser BJF, Huse SM, Crielaard W. Defining the healthy ‘core microbiome’ of oral microbial communities. BMC Microbiol. 2009;9(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vartoukian SR. Cultivation strategies for growth of uncultivated bacteria. J Oral Biosci. 2016;58(4):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6(6):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci USA. 2016;113(6):E791–E800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajishengallis G Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Börnigen D, Ren B, Pickard R, et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci Rep. 2017;7(1):17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92(6):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26(6):315–321. [DOI] [PubMed] [Google Scholar]
- 31.Kriebel K, Hieke C, Müller-Hilke B, Nakata M, Kreikemeyer B. Oral biofilms from symbiotic to pathogenic interactions and associated disease–connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front Microbiol. 2018;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee A, Jantsch V, Khan R, et al. Rheumatoid arthritis-associated autoimmunity due to Aggregatibacter actinomycetemcomitans and its resolution with antibiotic therapy. Front Immunol. 2018;9:2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(10):606–620. [DOI] [PubMed] [Google Scholar]
- 34.Hajishengallis G Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. [DOI] [PubMed] [Google Scholar]
- 36.Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23(4):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dabdoub SM, Ganesan SM, Kumar PS. Comparative metagenomics reveals taxonomically idiosyncratic yet functionally congruent communities in periodontitis. Sci Rep. 2016;6(1):38993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman HN. The apical border of plaque in chronic inflammatory periodontal disease. Br Dent J. 1976;141(4):105–113. [DOI] [PubMed] [Google Scholar]
- 39.Moutsopoulos NM, Chalmers NI, Barb JJ, et al. Subgingival microbial communities in leukocyte adhesion deficiency and their relationship with local immunopathology. PLoS Pathog. 2015;11(3):e1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakubovics NS. Intermicrobial interactions as a driver for community composition and stratification of oral biofilms. J Mol Biol. 2015;427(23):3662–3675. [DOI] [PubMed] [Google Scholar]
- 41.Carrassi A, Santarelli G, Abati S. Early plaque colonization on human cementum. J Clin Periodontol. 1989;16(4):265–267. [DOI] [PubMed] [Google Scholar]
- 42.Listgarten MA, Mayo HE, Tremblay R. Development of dental plaque on epoxy resin crowns in man. A light and electron microscopic study. J Periodontol. 1975;46(1):10–26. [DOI] [PubMed] [Google Scholar]
- 43.Adriaens PA, Edwards CA, De Boever JA, Loesche WJ. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1988;59(8):493–503. [DOI] [PubMed] [Google Scholar]
- 44.Marsh PD. Dental diseases—are these examples of ecological catastrophes? Int J Dent Hyg. 2006;4(Suppl 1):3–10; discussion 50–52. [DOI] [PubMed] [Google Scholar]
- 45.Rosier BT, De Jager M, Zaura E, Krom BP. Historical and contemporary hypotheses on the development of oral diseases: are we there yet? Front Cell Infect Microbiol. 2014;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holliday R, Preshaw PM, Bowen L, Jakubovics NS. The ultrastructure of subgingival dental plaque, revealed by high-resolution field emission scanning electron microscopy. BDJ Open. 2015;1(1):15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rostami N, Shields RC, Yassin SA, et al. A critical role for extracellular DNA in dental plaque formation. J Dent Res. 2017;96(2):208–216. [DOI] [PubMed] [Google Scholar]
- 48.Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol. 2019;431(16):2957–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D, Sengupta A, Niepa THR, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7(1):41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schertzer JW, Whiteley M. Bacterial outer membrane vesicles in trafficking, communication and the host-pathogen interaction. J Mol Microbiol Biotechnol. 2013;23(1–2):118–130. [DOI] [PubMed] [Google Scholar]
- 51.Gui MJ, Dashper SG, Slakeski N, Chen Y-Y, Reynolds EC. Spheres of influence: Porphyromonas gingivalis outer membrane vesicles. Mol Oral Microbiol. 2016;31(5):365–378. [DOI] [PubMed] [Google Scholar]
- 52.Ho M-H, Chen C-H, Goodwin JS, Wang B-Y, Xie H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS One. 2015;10(4):e0123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grenier D Porphyromonas gingivalis outer membrane vesicles mediate coaggregation and piggybacking of Treponema denticola and Lachnoanaerobaculum saburreum. Int J Dent. 2013;2013(6):305476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamaguchi A, Nakayama K, Ichiyama S, et al. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. 2003;47(6):485–491. [DOI] [PubMed] [Google Scholar]
- 55.Amann R, Fuchs BM. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat Rev Microbiol. 2008;6(5):339–348. [DOI] [PubMed] [Google Scholar]
- 56.DeLong EF, Wickham GS, Pace NR. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243(4896):1360–1363. [DOI] [PubMed] [Google Scholar]
- 57.Karygianni L, Hellwig E, Al-Ahmad A. Multiplex fluorescence in situ hybridization (M-FISH) and confocal laser scanning microscopy (CLSM) to analyze multispecies oral biofilms. Microbial Biofilms. Methods in Molecular Biology. New York: Humana Press; 2014;1147:65–72. [DOI] [PubMed] [Google Scholar]
- 58.Dige I, Raarup MK, Nyengaard JR, Kilian M, Nyvad B. Actinomyces naeslundii in initial dental biofilm formation. Microbiology (Reading, Engl). 2009;155(7):2116–2126. [DOI] [PubMed] [Google Scholar]
- 59.Al-Ahmad A, Wunder A, Auschill TM, et al. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J Med Microbiol. 2007;56(5):681–687. [DOI] [PubMed] [Google Scholar]
- 60.Palmer RJ, Shah N, Valm A, et al. Interbacterial adhesion networks within early oral biofilms of single human hosts. Appl Environ Microbiol. 2017;83(11):e00407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zijnge V, Ammann T, Thurnheer T, Gmür R. Subgingival biofilm structure. Front Oral Biol. 2012;15:1–16. [DOI] [PubMed] [Google Scholar]
- 62.Wood SR, Kirkham J, Marsh PD, Shore RC, Nattress B, Robinson C. Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. J Dent Res. 2000;79(1):21–27. [DOI] [PubMed] [Google Scholar]
- 63.Zijnge V, van Leeuwen MBM, Degener JE, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5(2):e9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters JC, Wittmann T. Concepts in quantitative fluorescence microscopy. Quantitative Imaging in Cell Biology. Methods in Cell Biology. San Diego: Academic Press; 2014;123:1–18. [DOI] [PubMed] [Google Scholar]
- 65.Thurnheer T, Gmür R, Guggenheim B. Multiplex FISH analysis of a six-species bacterial biofilm. J Microbiol Methods. 2004;56(1):37–47. [DOI] [PubMed] [Google Scholar]
- 66.Valm AM, Mark Welch JL, Rieken CW, et al. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci USA. 2011;108(10):4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolenbrander PE, Palmer RJ, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42(1):47–79. [DOI] [PubMed] [Google Scholar]
- 68.Sterberg SK, Sudo SZ, Folke LE. Microbial succession in subgingival plaque of man. J Periodont Res. 1976;11(5):243–255. [DOI] [PubMed] [Google Scholar]
- 69.Uzel NG, Teles FR, Teles RP, et al. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol. 2011;38(7):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedman MT, Barber PM, Mordan NJ, Newman HN. The ‘plaque-free zone’ in health and disease: a scanning electron microscope study. J Periodontol. 1992;63(11):890–896. [DOI] [PubMed] [Google Scholar]
- 71.Friedman MT, Barber P, Newman HN. Ultrastructure and histochemistry of the dental cuticle in adult periodontitis. J Periodontol. 1993;64(6):520–528. [DOI] [PubMed] [Google Scholar]
- 72.Dige I, Nyengaard JR, Kilian M, Nyvad B. Application of stereological principles for quantification of bacteria in intact dental biofilms. Oral Microbiol Immunol. 2009;24(1):69–75. [DOI] [PubMed] [Google Scholar]
- 73.Nobbs AH, Jenkinson HF, Everett DB. Generic determinants of Streptococcus colonization and infection. Infect Genet Evol. 2015;33:361–370. [DOI] [PubMed] [Google Scholar]
- 74.Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol Rev. 2009;73(3):407–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2(13):1599–1607. [DOI] [PubMed] [Google Scholar]
- 76.Mitri S, Clarke E, Foster KR. Resource limitation drives spatial organization in microbial groups. ISME J. 2016;10(6):1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibbons RJ, Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970;15(12):1397–1400. [DOI] [PubMed] [Google Scholar]
- 78.Egland PG, Palmer RJ, Kolenbrander PE. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA. 2004;101(48):16917–16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Periasamy S, Chalmers NI, Du-Thumm L, Kolenbrander PE. Fusobacterium nucleatum ATCC 10953 requires Actinomyces naeslundii ATCC 43146 for growth on saliva in a three-species community that includes Streptococcus oralis 34. Appl Environ Microbiol. 2009;75(10):3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Periasamy S, Kolenbrander PE. Aggregatibacter actinomycetemcomitans builds mutualistic biofilm communities with Fusobacterium nucleatum and Veillonella species in saliva. Infect Immun. 2009;77(9):3542–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding AM, Palmer RJ, Cisar JO, Kolenbrander PE. Shear-enhanced oral microbial adhesion. Appl Environ Microbiol. 2010;76(4):1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yakovenko O, Nunez J, Bensing B, et al. Serine-rich repeat adhesins mediate shear-enhanced streptococcal binding to platelets. Infect Immun. 2018;86(6):e00160–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jakubovics NS, Strömberg N, van Dolleweerd CJ, Kelly CG, Jenkinson HF. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol Microbiol. 2005;55(5):1591–1605. [DOI] [PubMed] [Google Scholar]
- 84.Park Y, Simionato MR, Sekiya K, et al. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2005;73(7):3983–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maeda K, Nagata H, Kuboniwa M, et al. Identification and characterization of Porphyromonas gingivalis client proteins that bind to Streptococcus oralis glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. 2013;81(3):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maeda K, Nagata H, Yamamoto Y, et al. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect Immun. 2004;72(9):1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edwards AM, Grossman TJ, Rudney JD. Association of a high-molecular weight arginine-binding protein of Fusobacterium nucleatum ATCC 10953 with adhesion to secretory immunoglobulin A and coaggregation with Streptococcus cristatus. Oral Microbiol Immunol. 2007;22(4):217–224. [DOI] [PubMed] [Google Scholar]
- 88.Lima BP, Hu LI, Vreeman GW, Weibel DB, Lux R. The oral bacterium Fusobacterium nucleatum binds Staphylococcus aureus and alters expression of the staphylococcal accessory regulator sarA. Microb Ecol. 2018;339(8):520–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu T, Cen L, Kaplan C, et al. Cellular components mediating coadherence of Candida albicans and Fusobacterium nucleatum. J Dent Res. 2015;94(10):1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaplan A, Kaplan CW, He X, McHardy I, Shi W, Lux R. Characterization of aid1, a novel gene involved in Fusobacterium nucleatum interspecies interactions. Microb Ecol. 2014;68(2):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fardini Y, Wang X, Témoin S, et al. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol Microbiol. 2011;82(6):1468–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem. 2007;282(34):25000–25009. [DOI] [PubMed] [Google Scholar]
- 93.Coppenhagen-Glazer S, Sol A, Abed J, et al. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect Immun. 2015;83(3):1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kinder SA, Holt SC. Localization of the Fusobacterium nucleatum T18 adhesin activity mediating coaggregation with Porphyromonas gingivalis T22. J Bacteriol. 1993;175(3):840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu W, Røder HL, Madsen JS, Bjarnsholt T, Sørensen SJ, Burmølle M. Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization. Front Microbiol. 2016;7(258):1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lima BP, Shi W, Lux R. Identification and characterization of a novel Fusobacterium nucleatum adhesin involved in physical interaction and biofilm formation with Streptococcus gordonii. Microbiologyopen. 2017;6(3):e00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLean JS. Advancements toward a systems level understanding of the human oral microbiome. Front Cell Infect Microbiol. 2014;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mutha NVR, Mohammed WK, Krasnogor N, et al. Transcriptional profiling of coaggregation interactions between Streptococcus gordonii and Veillonella parvula by dual RNA-seq. Sci Rep. 2019;9(1):7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92(12):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levy R, Borenstein E. Metabolic modeling of species interaction in the human microbiome elucidates community-level assembly rules. Proc Natl Acad Sci USA. 2013;110(31):12804–12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diaz PI. Microbial diversity and interactions in subgingival biofilm communities. Front Oral Biol. 2012;15:17–40. [DOI] [PubMed] [Google Scholar]
- 102.Jakubovics NS. Saliva as the sole nutritional source in the development of multispecies communities in dental plaque. In: Conway T, Cohen PS eds. Metabolism and Bacterial Pathogenesis. Washington, DC: Microbiol Spectrum, ASM Press; 2015:263–278. 10.1128/microbiolspec.MBP-0013-2014 [DOI] [PubMed] [Google Scholar]
- 103.Kolenbrander PE. Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source. Int J Oral Sci. 2011;3(2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson BP, Jensen BJ, Ransom EM, et al. Interspecies signaling between Veillonella atypica and Streptococcus gordonii requires the transcription factor CcpA. J Bacteriol. 2009;191(17):5563–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuboniwa M, Lamont RJ. Subgingival biofilm formation. Periodontol 2000. 2010;52(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuboniwa M, Houser JR, Hendrickson EL, et al. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat Microbiol. 2017;2(11):1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diaz PI, Zilm PS, Rogers AH. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology (Reading, Engl). 2002;148(2):467–472. [DOI] [PubMed] [Google Scholar]
- 108.Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. A Commensal bacterium promotes virulence of an opportunistic pathogen via cross-respiration. MBio. 2016;7(3):e00782–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang X, Browngardt CM, Jiang M, Ahn S-J, Burne RA, Nascimento MM. Diversity in antagonistic interactions between commensal oral streptococci and Streptococcus mutans. Caries Res. 2018;52(1–2):88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qi F, Kreth J. Methods to study antagonistic activities among oral bacteria. Oral Biology. Methods in Molecular Biology. New York: Humana Press; 2016;1537:203–218. [DOI] [PubMed] [Google Scholar]
- 111.Mathur H, Field D, Rea MC, Cotter PD, Hill C, Ross RP. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes. 2018;4(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merritt J, Qi F. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol. 2012;27(2):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamon CB, Klebanoff SJ. A peroxidase-mediated, Streptococcus mitis-dependent antimicrobial system in saliva. J Exp Med. 1973;137(2):438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Redanz S, Cheng X, Giacaman RA, Pfeifer CS, Merritt J, Kreth J. Live and let die: hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol Oral Microbiol. 2018;33(5):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193(1):1–6. [DOI] [PubMed] [Google Scholar]
- 116.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. [DOI] [PubMed] [Google Scholar]
- 117.Nobbs AH, Jenkinson HF, Jakubovics NS. Stick to your gums: mechanisms of oral microbial adherence. J Dent Res. 2011;90(11):1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jakubovics NS, Kolenbrander PE. The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 2010;16(8):729–739. [DOI] [PubMed] [Google Scholar]
- 119.Dongari-Bagtzoglou A Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev Anti Infect Ther. 2008;6(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paula AJ, Hwang G, Koo H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat Commun. 2020;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mark Welch JL, Hasegawa Y, McNulty NP, Gordon JI, Borisy GG. Spatial organization of a model 15-member human gut microbiota established in gnotobiotic mice. Proc Natl Acad Sci USA. 2017;114(43):E9105–E9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95(5):369–380. [DOI] [PubMed] [Google Scholar]
- 123.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maekawa T, Krauss JL, Abe T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15(6):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Makkawi H, Hoch S, Burns E, et al. Porphyromonas gingivalis stimulates TLR2-PI3K signaling to escape immune clearance and induce bone resorption independently of MyD88. Front Cell Infect Microbiol. 2017;7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dutzan N, Kajikawa T, Abusleme L, et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10(463):eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moutsopoulos NM, Moutsopoulos HM. The oral mucosa: a barrier site participating in tissue-specific and systemic immunity. Oral Dis. 2018;24(1–2):22–25. [DOI] [PubMed] [Google Scholar]
- 128.Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-κB RelA/p65. PLoS Pathog. 2013;9(4):e1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66(3):486–505; tableofcontents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gibbons RJ, Socransky SS, de Araujo WC, van Houte J. Studies of the predominant cultivable microbiota of dental plaque. Arch Oral Biol 1964;9(3):365–370. [DOI] [PubMed] [Google Scholar]
- 132.Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30(7):644–654. [DOI] [PubMed] [Google Scholar]
- 133.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. ‘Checkerboard’ DNA-DNA hybridization. Biotechniques. 1994;17(4):788–792. [PubMed] [Google Scholar]
- 134.Kolenbrander PE, Andersen RN, Moore LV. Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect Immun. 1989;57(10):3194–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khemaleelakul S, Baumgartner JC, Pruksakom S. Autoaggregation and coaggregation of bacteria associated with acute endodontic infections. J Endod. 2006;32(4):312–318. [DOI] [PubMed] [Google Scholar]
- 136.Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11(2):94–100. [DOI] [PubMed] [Google Scholar]
- 137.Lancy P, Dirienzo JM, Appelbaum B, Rosan B, Holt SC. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect Immun. 1983;40(1):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Colombo APV, Boches SK, Cotton SL, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80(9):1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Inoue S Collected Works of Shinya Inoue. Singapore: World Scientific; 2008. [Google Scholar]
- 140.Luo ML, Mullis AS, Leenay RT, Beisel CL. Repurposing endogenous type I CRISPR-Cas systems for programmable gene repression. Nucleic Acids Res. 2015;43(1):674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mardis ER. DNA sequencing technologies: 2006–2016. Nat Protoc. 2017;12(2):213–218. [DOI] [PubMed] [Google Scholar]
- 142.Mark Welch JL, Utter DR, Rossetti BJ, Mark Welch DB, Eren AM, Borisy GG. Dynamics of tongue microbial communities with single-nucleotide resolution using oligotyping. Front Microbiol. 2014;5:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shrivastava A, Patel VK, Tang Y, Yost SC, Dewhirst FE, Berg HC. Cargo transport shapes the spatial organization of a microbial community. Proc Natl Acad Sci USA. 2018;115(34):8633–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford). 2010;2010:baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Valm AM, Mark Welch JL, Borisy GG. CLASI-FISH: principles of combinatorial labeling and spectral imaging. Syst Appl Microbiol. 2012;35(8):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smeda-Pienaar K, Kaambo E, Africa CWJ. Bacterial morphotype grading for periodontal disease assessment. BDJ Open. 2017;3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]


