Abstract
We previously showed that the mouse inorganic phosphate transporter Npt1 operates in the hepatic sinusoidal membrane transport of anionic drugs such as benzylpenicillin and mevalonic acid. In the present study, the mechanism of renal secretion of penem antibiotics was examined by using a Xenopus oocyte expression system. Faropenem (an oral penem antibiotic) was transported via Npt1 with a Michaelis-Menten constant of 0.77 ± 0.34 mM in a sodium-independent but chloride ion-sensitive manner. When the concentration of chloride ions was increased, the transport activity of faropenem by Npt1 was decreased. Since the concentration gradient of chloride ions is in the lumen-to-intracellular direction, faropenem is expected to be transported from inside proximal tubular cells to the lumen. So, we tested the release of faropenem from Xenopus oocytes. The rate of efflux of faropenem from Npt1-expressing oocytes was about 9.5 times faster than that from control water-injected Xenopus oocytes. Faropenem transport by Npt1 was significantly inhibited by β-lactam antibiotics such as benzylpenicillin, ampicillin, cephalexin, and cefazolin to 24.9, 40.5, 54.4, and 26.2% of that for the control, respectively. Zwitterionic β-lactam antibiotics showed lesser inhibitory effects on faropenem uptake than anionic derivatives, indicating that Npt1 preferentially transports anionic compounds. Other anionic compounds, such as indomethacin and furosemide, and the anion transport inhibitor 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid significantly inhibited faropenem uptake mediated by Npt1. In conclusion, our results suggest that Npt1 participates in the renal secretion of penem antibiotics.
From the pharmacokinetic point of view, β-lactam antibiotics are classified into renal and biliary excretion types in terms of elimination pathway (1, 17), presumably due to the differences in their affinities to membrane transporters responsible for the renal and hepatic cell membrane transport processes among derivatives (21–23, 26–29). In renal tubular secretion there are two membrane transport processes, i.e., extraction of the antibiotics from blood across the basolateral membrane and release from the epithelial cells to the tubular lumen across the luminal brush-border membrane. Accordingly, it is essential to identify the transporters at both the basolateral and luminal membranes to understand the renal secretion mechanism of the antibiotics.
Recent molecular biological studies identified an organic anion-dicarboxylic acid exchange transporter, OAT1 (ROAT1), as the renal basolateral membrane transporter (19, 20). It exhibits a broad substrate specificity for organic anions, including benzylpenicillin, cephaloridine, p-aminohippuric acid, and others (10). Although at present an organic anion transporting polypeptide (oatp1) that transports bile salts and sulfobromophthalein (2, 9) and a kidney-specific anion transporter (OAT-K1) which specifically mediates transport of methotrexate (14, 18) are known as the luminal anion transporters, little is known about transporters for β-lactam antibiotics that exist at the renal luminal membrane.
We have reported (31) that mouse Npt1, which was originally cloned as the renal sodium-dependent phosphate transporter and which functions in the reabsorption of phosphate (6), is present at the hepatic sinusoidal membrane and accepts a variety of β-lactam antibiotics. Furthermore, rabbit NaPi-1 was shown to transport organic anions such as benzylpenicillin and probenecid (4). Accordingly, Npt1, which is localized at the luminal membrane in the kidney, may be a pharmacologically important transporter in the renal excretion of β-lactam antibiotics.
Faropenem, which is a penem antibiotic with a previous code of SUN5555, is eliminated exclusively through the kidney and is expected to be secreted actively into urine (11, 30). In the present study, to clarify the renal secretion mechanism of faropenem, we examined the relevance of the renal luminal anion transporter Npt1 on the renal secretion of faropenem using an Npt1 gene expression system.
MATERIALS AND METHODS
Materials.
[14C]faropenem (specific activity, 52 mCi/mmol) and unlabeled faropenem were gifts from Suntory Co. Ltd. (Tokyo, Japan). T7 RNA polymerase was purchased from Gibco BRL Life Technologies (Gaithersburg, Md.). All other chemicals were commercial products of reagent grade.
Expression of mouse Npt1 in Xenopus laevis oocytes.
Mouse Npt1 was cloned from a mouse kidney cDNA library by using the human NPT1 cDNA fragment (5, 16) as the probes as described elsewhere (31). Capped cRNA for mouse Npt1 was synthesized in vitro by using T7 RNA polymerase. Oocytes from X. laevis were defolliculated and injected with Npt1 cRNA (15 ng) or with water as the control. After injection, the oocytes were incubated for 4 days in modified Barth's solution (88 mmol of NaCl, 1 mmol of KCl, 0.33 mmol of Ca(NO3)2, 0.41 mmol of CaCl2, 0.82 mmol of MgSO4, 2.4 mmol of NaHCO3, and 10 mmol of HEPES-NaOH [pH 7.4] per liter) containing gentamicin at 18°C and were used for the transport study.
Transport assay.
Four days after cRNA injection, the oocytes were transferred to the Cl−-free uptake solution (100 mmol of sodium gluconate, 2 mmol of potassium gluconate, 1 mmol of calcium gluconate, 1 mmol of magnesium gluconate, and 10 mmol of HEPES-NaOH [pH 7.4] per liter) containing 1 μCi of radiolabeled faropenem per ml and were incubated for 60 min at 25°C unless otherwise noted. For the inhibition study, oocytes expressing Npt1 were incubated in the uptake solution described above with or without 5 mM test compound. For the efflux study, oocytes expressing Npt1 were loaded by microinjection of 50 nl of [14C]faropenem (1 μCi/μl) and were allowed to recover for 30 min in modified Barth's solution (13). Then, the oocytes were washed twice with Cl−-free uptake solution, and efflux was initiated by resuspending the oocytes in 150 μl of uptake solution in the presence or absence of 1 mM test compound. After 30 min of incubation, 125 μl of incubation medium was removed from each well and was mixed with the same volume of 20% sodium dodecyl sulfate (SDS) to estimate the drug concentration in the medium. The oocytes were transferred to scintillation vials and were solubilized with 10% SDS. The radioactivity in each incubation medium and the corresponding oocyte was quantified with a liquid scintillation counter (Aloka, Tokyo, Japan). Efflux of [14C]faropenem was estimated from the radioactivity in the medium as a percentage of the total injected radioactivity, i.e., radioactivity in medium/sum of radioactivities in medium and oocyte.
Statistical methods.
Results are given as the mean ± standard deviation (SD). Statistical analysis was performed by the Mann-Whitney U test. The criterion of statistical significance was deemed to be a P value of less than 0.05.
RESULTS
Time course and monovalent ion dependence of faropenem transport.
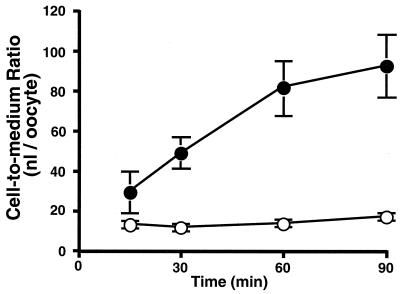
The uptake of [14C]faropenem by oocytes expressing mouse Npt1 was significantly higher than that by water-injected oocytes and increased linearly up to 60 min (Fig. 1). The uptake rate of [14C]faropenem was five times higher than that of water-injected control oocytes at 60 min.
FIG. 1.
Time course of [14C]faropenem uptake by Xenopus oocytes expressing mouse Npt1. [14C]faropenem (20 μM) uptake was measured in chloride ion-free medium. Closed and open symbols represent the results obtained with Xenopus oocytes injected with mouse Npt1 and water, respectively. Data are expressed as the means ± SDs for six to eight uptake measurements.
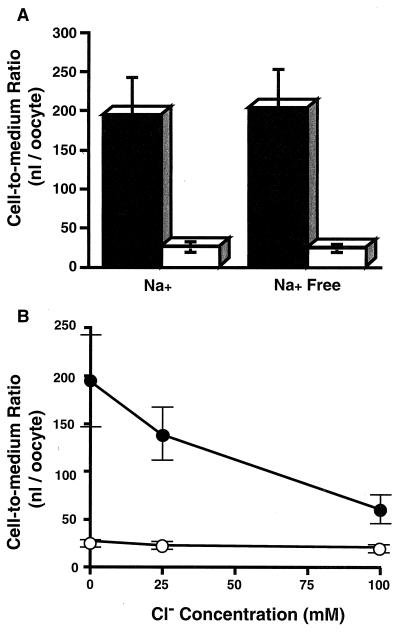
The replacement of sodium ions in the medium by potassium ions had no effect on [14C]faropenem uptake (Fig. 2A). Chloride ions in the medium, however, had a significant effect on the uptake rate of [14C]faropenem (Fig. 2B). When the concentration of chloride ions was increased, the rate of faropenem transport was decreased. So, faropenem transport by mouse Npt1 was suggested to be sodium independent and chloride sensitive.
FIG. 2.
Sodium and chloride ion dependences of [14C]faropenem uptake by Xenopus oocytes expressing mouse Npt1. (A) Uptake of [14C]faropenem was measured in the presence or absence of sodium ions. In the sodium ion-free experiment, sodium ions were replaced with potassium ions. (B) Uptake of [14C]faropenem was measured in medium containing various concentrations of chloride ions. The chloride ion concentration was adjusted by replacing sodium chloride with sodium gluconate to give a constant sodium ion concentration. Closed and open symbols represent the results obtained with Xenopus oocytes injected with mouse Npt1 cRNA and water, respectively. Data are expressed as the means ± SDs for six to eight uptake measurements.
Concentration dependence of faropenem transport.
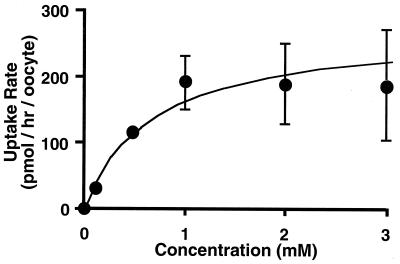
Kinetic parameters of faropenem transport via mouse Npt1 were evaluated from the concentration dependence of faropenem uptake. The faropenem uptake by mouse Npt1 was estimated after subtracting the uptake by water-injected oocytes from that by Npt1 cRNA-injected oocytes (Fig. 3). The faropenem uptake was saturable with a Michaelis-Menten constant (Km) of 0.77 ± 0.34 mM and a maximum uptake rate (Vmax) of 271 ± 45.6 pmol/h/oocyte (mean ± standard error).
FIG. 3.
Concentration dependence of [14C]faropenem uptake by Xenopus oocytes expressing mouse Npt1. [14C]faropenem uptake was measured in chloride ion-free medium. Uptake of faropenem via Npt1 was estimated by subtraction of the uptake by water-injected oocytes from that of mouse Npt1 cRNA-injected oocytes. Data are expressed as the means ± SDs for six to eight uptake measurements.
Faropenem efflux from the cell via Npt1.
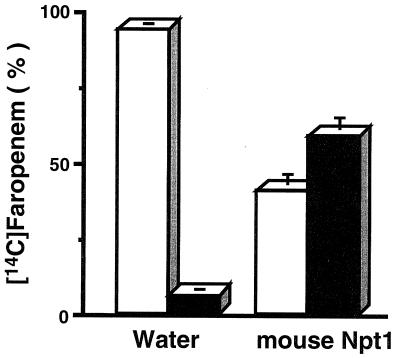
To confirm the movement of [14C]faropenem in the cell-to-extracellular medium direction via mouse Npt1, an efflux study was performed. Efflux of preloaded [14C]faropenem from oocytes expressing mouse Npt1 was significantly greater than that of water-injected control oocytes. As shown in Fig. 4, in the case of oocytes expressing mouse Npt1, 58.9% ± 4.6% (mean ± SD; n = 4) of preloaded [14C]faropenem was released into the medium within 30 min, whereas only 6.2% ± 0.6% (n = 4) was released from control oocytes.
FIG. 4.
Efflux of [14C]faropenem from Xenopus oocytes via mouse Npt1. Efflux of preloaded [14C]faropenem was measured in water- or mouse Npt1 cRNA-injected oocytes in chloride ion-free medium. Open and closed bars represent the fractional amounts in oocytes and extracellular medium after 30 min of efflux, respectively.
This result indicates that mouse Npt1 mediates the transport of faropenem bidirectionally, from cell to medium as well as from medium to cell.
Substrate specificity of mouse Npt1.
An inhibition study was performed to characterize further the Npt1-mediated [14C]faropenem uptake (Table 1). The addition of 5 mM unlabeled β-lactam antibiotics, including faropenem, benzylpenicillin, ampicillin, cefazolin, and cephalexin, significantly inhibited the uptake of [14C]faropenem (P < 0.05). Among them, zwitterionic ampicillin and cephalexin were relatively weak inhibitors compared with the anionic derivatives. Anionic compounds such as indomethacin, furosemide, benzoic acid, lactic acid, and probenecid significantly reduced the uptake of faropenem. On the other hand, 2-ketoglutaric acid was not inhibitory.
TABLE 1.
Inhibitory effect on [14C]faropenem uptake by mouse Npt1-expressing oocytesa
| Inhibitor | Concn (mM) | Relative uptake (% of that for control) |
|---|---|---|
| Control | 100.0 | |
| Faropenem | 5 | 18.7 ± 4.8b |
| Benzylpenicillin | 5 | 24.9 ± 5.6b |
| Ampicillin | 5 | 40.5 ± 29.2b |
| Cephalexin | 5 | 54.4 ± 30.2b |
| Cefazolin | 5 | 26.2 ± 10.3b |
| Indomethacin | 1 | 8.3 ± 0.4b |
| Furosemide | 1 | 15.6 ± 4.3b |
| Benzoic acid | 5 | 32.1 ± 22.2b |
| Lactic acid | 5 | 51.1 ± 22.2b |
| Probenecid | 5 | 6.4 ± 0.5b |
| DIDS | 0.01 | 39.9 ± 14.2b |
| DIDS | 0.1 | 19.9 ± 2.3b |
| DIDS | 1 | 6.0 ± 0.8b |
| 2-Ketoglutaric acid | 5 | 83.9 ± 6.2 |
The uptake was measured in chloride ion-free medium in the absence or presence of each inhibitor. Each value represents the mean ± SD for six to eight oocytes. The data were obtained by subtraction of the uptake by water-injected oocytes from that by Npt1 cRNA-injected oocytes.
Significantly different from the control uptake by Mann-Whiney U test (P < 0.05).
Considering the effect of chloride ions, Npt1-mediated transport of faropenem was thought to occur via an anion-exchange mechanism. Hence, the effect of 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), a classical anion-exchange inhibitor, on faropenem transport was examined. The uptake of [14C]faropenem was strongly inhibited by a low concentration of DIDS (0.01 mM), with comparable levels of inhibition occurring at higher concentrations (0.1 and 1 mM). Thus, DIDS is a potent inhibitor of Npt1-mediated transport of faropenem, therefore implying that transport of faropenem via mouse Npt1 proceeds through an anion-exchange mechanism.
DISCUSSION
The mechanism of elimination of β-lactam antibiotics from the body are of pharmacological and therapeutic interest. Although some β-lactam antibiotics are excreted in part via the liver (12, 15), most of them are excreted via the kidney (1, 23, 29). However, the secretion pathway of β-lactam antibiotics at the apical membrane of renal proximal tubule cells has not been clarified at the molecular level, although basolateral transporter OAT1 was shown to participate (19, 20). Organic anion transporters such as oatp1 and OAT-K1 have been identified as renal luminal membrane transporters (2, 14), but they are unlikely to accept β-lactam antibiotics as substrates.
Recently, it was revealed that the mouse and rabbit sodium-dependent inorganic phosphate transporter Npt1 exhibits transport activities for organic anions such as benzylpenicillin and phenol red (4, 31). To investigate the relevance of this phosphate transporter to β-lactam antibiotic transport, we cloned the mouse sodium-dependent phosphate transporter (Npt1) and used faropenem as a test substrate. Faropenem, a novel penem antibiotic, is eliminated mainly through the renal tubules rather than through the glomeruli in animals (10, 30), and the process seems to involve an active secretion mechanism sensitive to probenecid (11).
As has been demonstrated in the present study, mouse Npt1 transported faropenem with an apparent Km value of 0.77 mM (Fig. 3). Interestingly, faropenem transport via mouse Npt1 was chloride ion sensitive (Fig. 2B). Since the concentrations of chloride ions in proximal tubule cells and outside the proximal tubule cells are about 7.9 and 110 mM, respectively (8), faropenem is expected to be transported from proximal tubular cells into urine via mouse Npt1. To confirm this, we examined the direction of the Npt1-mediated transport of faropenem. We observed accelerated release of faropenem from oocytes expressing mouse Npt1 (Fig. 4). Therefore, we conclude that faropenem transport by Npt1 is bidirectional and the drug is expected to be transported across the renal epithelial luminal membrane depending on the chloride ion concentration and/or other factors.
The molecular recognition characteristics of membrane transporters form one of our fields of interest. A peptide transporter (PepT1) recognizes several β-lactam antibiotics such as cefadroxil and cefixime (23, 24) but does not recognize faropenem as a substrate (unpublished data). As regards the substrate selectivity for mouse Npt1, we showed that anionic β-lactam antibiotics are more potent inhibitors than zwitterionic derivatives. These results suggest that the molecular recognition of β-lactam antibiotics is different between Npt1 and PepT1. PepT1 has a crucial role in the intestinal absorption of β-lactam antibiotics (24, 25), while Npt1 could have an important role in their secretion in the kidney. Thus, our results should be helpful in understanding the pharmacological role of physiological transporters in the absorption and secretion processes of β-lactam antibiotics at the molecular level.
In addition to β-lactam antibiotics, several organic anions such as probenecid, indomethacin (a nonsteroidal anti-inflammatory drug), and furosemide (a diuretic) reduced the level of uptake of faropenem by Npt1. We have already reported that benzylpenicillin and foscarnet (an antibiotic that contains a phosphate moiety) are transported via mouse Npt1 (31). These observations suggest that mouse Npt1 has a broad substrate specificity and could make a significant contribution to the renal excretion of antibiotics.
Localization of mouse Npt1 in the kidney is predominantly at the luminal membrane in proximal tubules (3, 7). Mouse Npt1 transports inorganic phosphate in a sodium ion-dependent manner (6). In this study, however, we found that Npt1 transports faropenem in a sodium ion-independent manner. In the basolateral membrane, β-lactam antibiotics are expected to be commonly transported via the organic anion transporter OAT1 (19, 20). An outwardly directed dicarboxylate gradient is essential to energize the transport activity. Accumulation of β-lactam antibiotics from blood into proximal tubule cells via OAT1 and the elimination of these anions into urine via Npt1 across the luminal membrane may cooperate to achieve efficient renal secretion. In the present study, 2-ketoglutarate had no effect on faropenem transport, so Npt1 seems to transport faropenem in a manner different from that in which OAT1 transports faropenem.
In conclusion, mouse Npt1, initially cloned as a phosphate transporter, was identified as the transporter responsible for faropenem secretion in renal proximal tubules. The physiological direction of faropenem transport is considered to be from proximal tubular cells to urine across the brush-border membrane because of the gradient of chloride ion and the accumulation of anions from the basolateral side. These results should be helpful in understanding the secretion of antibiotics at the molecular level.
REFERENCES
- 1.Barza M, Brusch J, Bergeron M G, Kemmotsu O, Weinstein L. Extraction of antibiotics from the circulation by liver and kidney: effect of probenecid. J Infect Dis. 1975;131:S86–S97. doi: 10.1093/infdis/131.supplement.s86. [DOI] [PubMed] [Google Scholar]
- 2.Bergwerk A J, Shi X, Ford A C, Kanai N, Jacquemin E, Burk R D, Bai S, Novikoff P M, Stieger B, Meier P J, Schuster V L, Wolkoff A W. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am J Physiol. 1996;271:G231–G238. doi: 10.1152/ajpgi.1996.271.2.G231. [DOI] [PubMed] [Google Scholar]
- 3.Biber J, Custer M, Werner A, Kaissling B, Murer H. Localization of NaPi-1, a Na/Pi cotransporter, in rabbit kidney proximal tubules. II. Localization by immunohistochemistry. Pflugers Arch. 1993;424:210–215. doi: 10.1007/BF00384344. [DOI] [PubMed] [Google Scholar]
- 4.Busch A E, Schuster A, Waldegger S, Wagner C A, Zempel G, Broer S, Biber J, Murer H, Lang F. Expression of a renal type I sodium/phosphate transporter (NaPi-1) induces a conductance in Xenopus oocytes permeable for organic and inorganic anions. Proc Natl Acad Sci USA. 1996;93:5347–5351. doi: 10.1073/pnas.93.11.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong S S, Kristjansson K, Zoghbi H, Hughes M. Molecular cloning of the cDNA encoding a human renal sodium phosphate transport protein and its assignment to chromosome 6p21.3-p23. Genomics. 1993;18:355–359. doi: 10.1006/geno.1993.1476. [DOI] [PubMed] [Google Scholar]
- 6.Chong S S, Kozak C A, Liu L, Kristjansson K, Dunn S T, Bourdeau J E, Hughes M R. Cloning, genetic mapping, and expression analysis of a mouse renal sodium-dependent phosphate cotransporter. Am J Physiol. 1995;268:F1038–F1045. doi: 10.1152/ajprenal.1995.268.6.F1038. [DOI] [PubMed] [Google Scholar]
- 7.Custer M, Meier F, Schlatter E, Greger R, Garcia-Perez A, Biber J, Murer H. Localization of NaPi-1, a Na-Pi cotransporter, in rabbit kidney proximal tubules. I. mRNA localization by reverse transcription/polymerase chain reaction. Pflugers Arch. 1993;424:203–209. doi: 10.1007/BF00384343. [DOI] [PubMed] [Google Scholar]
- 8.Fromter E. Viewing the kidney through microelectrodes. Am J Physiol. 1984;247:F695–F705. doi: 10.1152/ajprenal.1984.247.5.F695. [DOI] [PubMed] [Google Scholar]
- 9.Jacquemin E, Hagenbuch B, Stieger B, Wolkoff A W, Meier P J. Expression cloning of a rat liver Na+-independent organic anion transporter. Proc Natl Acad Sci USA. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jariyawat S, Sekine T, Takeda M, Apiwattanakul N, Kanai Y, Sophsan S, Endou H. The interaction and transport of β-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther. 1999;290:672–677. [PubMed] [Google Scholar]
- 11.Kanai Y, Morozumi N, Yonemoto Y, Sugita O, Ohmura N, Kikuchi Y. Pharmacokinetics of SY5555, a novel oral penem antibiotic, in animals. Chemotherapy (Tokyo) 1994;42:243–253. [Google Scholar]
- 12.Kind A C, Tupasi T E, Standiford H C, Kirby W M. Mechanisms responsible for plasma levels of nafcillin lower than those of oxacillin. Arch Intern Med. 1970;125:685–690. [PubMed] [Google Scholar]
- 13.Li L, Lee T K, Meier P J, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem. 1998;273:16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- 14.Masuda S, Saito H, Nonoguchi H, Tomita K, Inui K. mRNA distribution and membrane localization of the OAT-K1 organic anion transporter in rat renal tubules. FEBS Lett. 1997;407:127–131. doi: 10.1016/s0014-5793(97)00314-1. [DOI] [PubMed] [Google Scholar]
- 15.Matsui H, Yano K, Okuda T. Pharmacokinetics of the cephalosporin SM-1652 in mice, rats, rabbits, dogs, and rhesus monkeys. Antimicrob Agents Chemother. 1982;22:213–217. doi: 10.1128/aac.22.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto K, Tatsumi S, Sonoda T, Yamamoto H, Minami H, Taketani Y, Takeda E. Cloning and functional expression of a Na+-dependent phosphate co-transporter from human kidney: cDNA cloning and functional expression. Biochem J. 1995;305:81–85. doi: 10.1042/bj3050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nightingale C H, Greene D S, Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975;64:1899–1926. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- 18.Saito H, Masuda S, Inui K. Cloning and functional characterization of a novel rat organic anion transporter mediating basolateral uptake of methotrexate in the kidney. J Biol Chem. 1996;271:20719–20725. doi: 10.1074/jbc.271.34.20719. [DOI] [PubMed] [Google Scholar]
- 19.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- 20.Sweet D H, Wolff N A, Pritchard J B. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- 21.Tamai I, Terasaki T, Tsuji A. Evidence for the existence of a common transport system of beta-lactam antibiotics in isolated rat hepatocytes. J Antibiot (Tokyo) 1985;38:1774–1780. doi: 10.7164/antibiotics.38.1774. [DOI] [PubMed] [Google Scholar]
- 22.Tamai I, Tsuji A. Transport mechanism of cephalexin in isolated hepatocytes. J Pharmacobiodyn. 1987;10:632–638. doi: 10.1248/bpb1978.10.632. [DOI] [PubMed] [Google Scholar]
- 23.Tamai I, Tsuji A, Kin Y. Carrier-mediated transport of cefixime, a new cephalosporin antibiotic, via an organic anion transport system in the rat renal brush-border membrane. J Pharmacol Exp Ther. 1988;246:338–344. [PubMed] [Google Scholar]
- 24.Tamai I, Tomizawa N, Takeuchi T, Nakayama K, Higashida H, Tsuji A. Functional expression of transporter for beta-lactam antibiotics and dipeptides in Xenopus laevis oocytes injected with messenger RNA from human, rat and rabbit small intestines. J Pharmacol Exp Ther. 1995;273:26–31. [PubMed] [Google Scholar]
- 25.Tamai I, Nakanishi T, Hayashi K, Terao T, Sai Y, Shiraga T, Miyamoto K, Takeda E, Higashida H, Tsuji A. The predominant contribution of oligopeptide transporter PepT1 to intestinal absorption of beta-lactam antibiotics in the rat small intestine. J Pharm Pharmacol. 1997;49:796–801. doi: 10.1111/j.2042-7158.1997.tb06115.x. [DOI] [PubMed] [Google Scholar]
- 26.Terasaki T, Tamai I, Takanosu K, Nakashima E, Tsuji A. Kinetic evidence for a common transport route of benzylpenicillin and probenecid by freshly prepared hepatocytes in rats. Influence of sodium ion, organic anions, amino acids and peptides on benzylpenicillin uptake. J Pharmacobiodyn. 1986;9:18–28. doi: 10.1248/bpb1978.9.18. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji A, Terasaki T, Tamai I, Nakashima E, Takanosu K. A carrier-mediated transport system for benzylpenicillin in isolated hepatocytes. J Pharm Pharmacol. 1985;37:55–57. doi: 10.1111/j.2042-7158.1985.tb04931.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji A, Terasaki T, Takanosu K, Tamai I, Nakashima E. Uptake of benzylpenicillin, cefpiramide and cefazolin by freshly prepared rat hepatocytes. Evidence for a carrier-mediated transport system. Biochem Pharmacol. 1986;35:151–158. doi: 10.1016/0006-2952(86)90508-3. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji A, Terasaki T, Tamai I, Takeda K. In vivo evidence for carrier-mediated uptake of beta-lactam antibiotics through organic anion transport systems in rat kidney and liver. J Pharmacol Exp Ther. 1990;253:315–320. [PubMed] [Google Scholar]
- 30.Tsuji A, Sato H, Tamai I, Adachi H, Nishihara T, Ishiguro M, Ohnuma N, Noguchi T. Physiologically based pharmacokinetics of a new penem, SUN5555, for evaluation of in vivo efficacy. Drug Metab Dispos. 1990;18:245–252. [PubMed] [Google Scholar]
- 31.Yabuuchi H, Tamai I, Morita K, Kouda T, Miyamoto K, Takeda E, Tsuji A. Hepatic sinusoidal membrane transport of anionic drugs mediated by anion transporter Npt1. J Pharmacol Exp Ther. 1998;286:1391–1396. [PubMed] [Google Scholar]