Abstract
Nutrition is one of the most important factors affecting pubertal development. Increasing demands for energy proteins and micronutrients are necessary to cope with the rapid linear pubertal growth and development, change in body composition, and increased physical activity. Adequate nutrition is a key permissive factor for the normal timing and tempo of pubertal development. Severe primary or secondary malnutrition also can adversely delay the onset and progression of puberty. The higher incidence of anorexia nervosa and bulimia in adolescents imposes a nutritional risk on pubertal development. Here we provide an overview of nutritional requirements (macronutrients and micronutrients) necessary to cope with these changes. In addition, we discuss possible nutritional interventions trials and their effects on several aspects of growth and development in undernourished and stunted adolescents, in low- and middle-income countries (LMIC), who require nutritional rehabilitation. This mini review sums up some important findings in this important complex that link between nutrition, nutritional interventions, and pubertal development. (www.actabiomedica.it)
Keywords: Nutrition, intervention, adolescents, growth, malnutrition, RUTF, supplements
Introduction
Puberty entails a progressive nonlinear process starting from prepubescent to full sexual maturity through the interaction and cooperation of biological, physical, and psychological changes. Nutrition is one of the most important factors affecting the onset and progression of pubertal development. Adequate nutrition is a key permissive factor for the normal timing and tempo of pubertal development. Puberty triggers a growth spurt, which increases nutritional needs including macro and micronutrients. Increased caloric, protein, iron, calcium, zinc, and folate needs must be provided during this critical period of rapid linear growth and bone accretion. Moderate and severe primary or secondary malnutrition can adversely modulate the onset and progression of puberty. The higher incidence of nutritional deficiencies and anorexia nervosa in adolescents imposes a nutritional risk on pubertal development and final adult stature.
Here we provide an overview of nutritional requirements (macronutrients and micronutrients) necessary to cope with these changes. In addition, we discuss possible nutritional interventions trials and their effects on several aspects of growth and development in undernourished and stunted adolescents, in low- and middle-income countries, who require nutritional rehabilitation (1).
Pubertal growth spurt and nutritional requirements
Nutrition during adolescence should meet the following objectives:
Provide the necessary nutrients to meet the demands of physical and cognitive growth and development.
Provide adequate stores for illness or pregnancy.
Prevent adult onset of diseases related to nutrition e.g., cardiovascular diseases, diabetes, osteoporosis, and cancer
Encourage healthy eating habits and lifestyle.
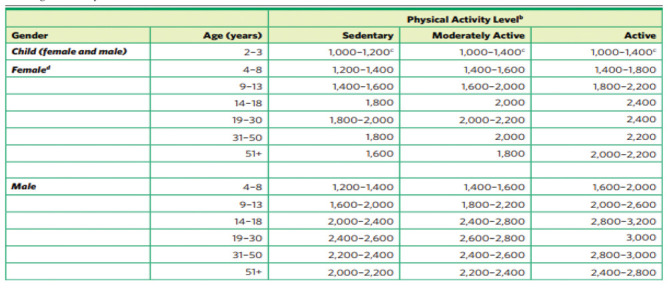
According to the Dietary Guidelines for Americans 2010, girls ages 9 to 13 generally require
1.400 to 2.200 calories and teen boys ages 14 to 18 require 2000 to 3200 calories per day to maintain healthy body weights. Girls ages 14 to 18 usually need 1.800 to 2.400 calories/daily during puberty. Active pubescent girls require more calories than those with low activity levels
(Table 1). Teenage athletes who regularly participate in vigorous sports training may require up to 5,000 calories per day (2).
Table 1.
Dietary Guidelines in adolescents (by the Advisory Committee. Dietary Guidelines for Americans 2010. (Source: https://health.gov/Dietary Guidelines 2010).
|
Tracking changes in dietary intake during puberty and their determinants in different populations
Understanding changes in dietary intake during puberty could aid the mapping of dietary interventions for primary prevention. Few studies available describing habitual dietary intake during puberty, differ in terms of study design, follow-up period, data collection methods, age of subjects, and study location. Dietary behaviors observed, range from specific food items or food groups to broader dietary patterns, including a range of foods (3-9).
An assessment of the current diet and nutritional status in many Asian and African countries proved deficient intake of macro and/or micronutrients in a large proportion of adolescents (Table 2) (10-13).
Table 2.
Summary of dietary intake of Adolescents in some LMIC Asian and African countries.
| Study and Ref. | Findings |
|---|---|
| National Institute of Nutrition, 2006. (10) | In India, 12,124 adolescents in villages of 10 states consumed grossly inadequate green leafy vegetables, fruits, pulses, and milk. The mean nutrient intakes were below the RDA in all adolescent age groups irrespective of sex, and 50% were not getting even 70% of their daily requirements of energy |
| Doku et al. 2013. (11) | In Ghana, 31% of those ages 12–18 years ate breakfast fewer than four days a week, 56 percent rarely ate fruits, 48 percent rarely ate vegetables, and boys were more physically active than girls. |
| Barugahara et al. 2013. (12) | In Uganda, girls ages 11–14 years achieved only 30% for folate, 36 % for energy, 54 %for iron and riboflavin, 59% for protein, 61% for vitamin A, 89% for vitamin C, and 92% for fiber of the WHO daily requirements. |
| Alam et al, 2010. (13) | In Bangladesh, 32% of adolescent girls were stunted. The overall dietary knowledge was low, and 36% were not aware of the importance of taking extra nutrients during adolescence for growth spurt. |
Review of optimal Mediterranean diet (MD) adherence in children and adolescents in southern European countries (Spain, Italy, Cyprus, Greece) showed that the major determinants of adherence to a traditional MD pattern in southern European countries rely on social and demographic factors. Children and adolescents may be the age-groups with the most deteriorated MD profile. In Spain, 49.4% of adolescents demonstrated intermediate adherence. In Greece, in the greater Athens area the overall adherence to the MD was poor. In Cyprus, more than one third of the children followed a poor-quality diet. The authors highlighted a need for nutrition education programs are not limited to children and adolescents but also include parents, teachers, and physicians (14,15).
Dietary patterns and timing of puberty: Effects of quantitatively high-calorie diet on puberty onset
Several lines of evidence support the interplay of nutritional status, energy balance, and hormones in the regulation of growth and pubertal development. Frequently, children with obesity are taller for their age, with accelerated linear growth and advanced skeletal maturation, and tend to mature earlier than lean children. Secular trends toward a declining age at puberty onset with correlated changes in body weight have been reported in economically advanced countries. This has been attributed to excess calorie intake along with reduced physical activity in children (16,17).
Feeding juvenile female rhesus monkeys born and raised with a high-calorie diet results in acceleration of body growth (weight, length, and BMI) and precocious menarche. These changes were associated with increased leptin and IGF-I levels throughout the experiment. These findings supported the importance of the quantity of caloric intake as an important factor in determining the onset and progression of puberty (18).
In a prospective study conducted in the United States, intake of sugar-sweetened beverages (SSBs) was associated with early sexual development in 5,583 girls. Premenarcheal girls who consumed >1.5 servings of SSBs per day at ages 9–14 years had a statistically significant 24% higher probability of menarche during follow-up than did girls who consumed ≤2 servings of SSBs weekly after adjusting for sociodemographic and maternal characteristics, physical activity, and total caloric intake. In another prospective study of girls from the United States, every standard deviation of caffeine every SD of aspartame intake at 10 years of age was related to a 22% and 20 % higher risk of early menarche. (<11 years of age) after adjusting for potential confounding variables. Neither fructose nor sucrose intake was related to the timing of puberty (19, 20).
In a Chinese study, three distinct dietary patterns, “traditional diet”, “unhealthy diet”, and “protein diet” were established. Neither the “traditional diet” pattern nor the “protein diet” pattern showed any association with precocious puberty. The “unhealthy diet” pattern was significantly positively associated with precocious puberty in both and girls. The relationship remained positive only for girls (OR = 1.25, 95% CI = 1.04–1.49) after adjustment for age and BMI but statistically non-significant after further adjustment for socioeconomic factors in both boys and girls (21).
In an Iranian study on 568 girls, three dietary patterns were defined, i.e., balanced pattern, high calorie and fat pattern and high protein diet pattern. Data from the multivariate regression analysis showed that both balanced dietary pattern and high protein diet pattern were protective factors for precocious puberty, while high calorie and fat pattern was risk factors (22)
Effects of different quantity and quality of protein intake on puberty
Some evidence indicates that animal protein intake during childhood may be related to the earlier onset of puberty. In a small study of girls from the United States, total animal protein intake at 3–8 years of age was related to earlier menarche and age at peak height velocity (APHV). Among German boys and girls, intakes of total and animal protein at 5–6 years of age were related to early APHV, menarche in girls, and voice break in boys (23,24).
Gunther et al. (24) reported that protein intake from cow milk and other dairy was related to earlier pubertal growth spurts and APHV among 112 German boys and girls. Similarly, in a prospective study of 134 Iranian girls, those with milk intake at ≥ 34 g per day, at 9 years of age, had higher odds of early menarche at or before age 12 years than did girls with milk intake at <34 g per day. Using data from the National Health and Nutrition Examination Survey, Wiley et al. (25) found that higher milk consumption at ages 5–12 years among 2,057 girls from the United States was associated with an earlier age at menarche. However, these findings were not supported in other studies (26,27).
Other studies suggested that animal protein increased the risk and plant protein decreased the risk of early menarche. Of protein-containing food groups, intakes of poultry and low-fat dairy were marginally associated with a higher risk of early menarche. The odds of early menarche were reduced with higher intake of plant protein. These findings suggested that partly substitution of animal protein with plant sources during childhood may postpone menarche’s timing. (28-30).
The potential effects of animal foods, especially dairy, on the timing of puberty have been attributed to a protein-mediated stimulation of IGF-1 secretion. Higher circulating levels of this hormone during childhood have been related in some studies to an earlier onset of puberty (31).
Micronutrients and a possible effect on pubertal development
Micronutrient requirements increase during puberty; thus, prepubertal micronutrient status may influence the timing of sexual maturation. Evidence on the effects of micronutrients on the timing of puberty is insufficient and inconsistent. In a randomized controlled trial among 160 Gambian boys, those supplemented with calcium for 12 months had an earlier APHV and shorter adult stature than did boys in the control group (32). In a randomized study among 144 Swiss girls, those supplemented with calcium from ages 7.9 to 8.9 years reached menarche earlier than did girls who received the placebo (33).
Kissinger and Sanchez reported a statistically significant, positive association between iron intake and age at menarche among 230 girls from the United States (34). In a small, randomized trial of 17 Iranian children, those supplemented with zinc had an earlier initiation of puberty compared with children assigned to the placebo group (35). In another randomized controlled trial of 102 boys ages 13.6 to 15.5 years with short stature and delayed puberty, supplementation with iron and vitamin A resulted in faster testicular growth (36). In the study by Maclure et al. (37), vitamin A intake was strongly associated with earlier menarche.
Dietary Fat and Polyunsaturated Fatty Acid Intakes during Childhood may affect Puberty Timing Independent of protein intake.
Among 3425 girls and 2495 boys, children with higher intakes of total fat and PUFA have been more likely to reach their puberty at an earlier age. Associations were not attenuated on additional adjustment for childhood dietary protein intake. (38)
Global prevalence of under-nutrition and nutritional stunting in adolescents
Under-nutrition is the most important cause of growth retardation worldwide. Poverty in the poor countries and self-induced food restriction in the rich countries or malabsorption and chronic systemic diseases are the main causes. There are an estimated 1.8 billion adolescents in the world, with 90% residing in low- and middle-income countries (LMIC). Nutritional deficiencies, suboptimal linear growth, and undernutrition are major public health problems (38,39).
The prevalence of moderate and severe underweight (undernutrition) is highest in South Asia; one in 5 girls aged 5–19 years and nearly one-third of their male peers are underweight. According to the Global School-Based Student Health Survey, more than 10% of surveyed girls were underweight in Mauritius, Sudan, Bangladesh, Maldives, Cambodia, and Vietnam.
In 2016, the mean BMI estimates for youths aged 10–19 in South Asia, Southeast Asia, East Africa, West Africa, and Central Africa were <20 for both male and female adolescents. The lowest BMI were seen in Ethiopia, Niger, Senegal, India, Bangladesh, Myanmar, and Cambodia (40,41).
Health surveys in 58 countries showed that the distribution of height-for-age z-scores (HAZ) for these adolescents a quarter of girls are 2 or more SDs below the mean height-for-age as compared to the WHO/CDC reference population (42). The limited published estimates of stunting in girls aged 15–19 ranged from 52% in Guatemala and 44% in Bangladesh to 8% in Kenya and 6% in Brazil (43).
The effect of chronic childhood malnutrition on pubertal growth and development
Chronic primary or secondary malnutrition leads to serious consequences including impaired growth, delayed pubertal development, osteopenia, osteomalacia, anemia, and different syndromes caused by deficiency of vitamins, minerals, essential fatty acids and amino acids, and trace elements (Table 3 and figure 1) (1,44).
Table 3.
Possible negative consequences of undernutrition during adolescence (From Ref. 50, modified).
Potentially may:
|
Figure 1.
Effect of dietary patterns on pubertal timing and growth
Pubertal growth and development were compared in 342 privileged, urban children and 347 impoverished rural adolescents from Kenya. Measurements of height, weight, upper arm circumference, and triceps skinfolds revealed marked differences between the two study groups just before the onset of sexual maturation. These differences were also found in the early stages of puberty but notable catch-up was evident throughout the later period of the maturational process. Early stages of sexual maturity were delayed by 3 yr. in malnourished boys with a 2.1 years lag in the age of onset of menarche in rural girls. Derived estimates of body fat as well as direct anthropometry revealed that the onset of puberty is not size related under the circumstances of chronic childhood malnutrition (45).
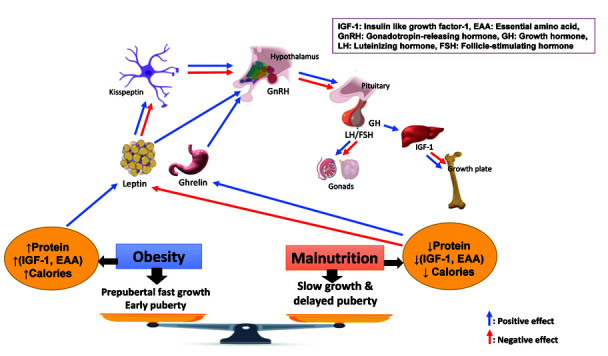
Hormonal mediation of delayed puberty during chronic malnutrition (Figure 1)
Hormonal adaptation mediates delayed puberty in situations with suboptimal nutrition to prolong growth of the individual and to delay reproductive ability until conditions to support reproduction exist (46). Current understanding of energy sufficiency or insufficiency signalling pathways considers the HPG-axis to re-activate. Research using animal models revealed new factors important in energy homeostasis. Of particular importance are leptin, ghrelin, kisspeptin, adenosine monophosphate-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) (47).
Abnormalities of the growth hormone (GH-IGF-1) axis and gonadotrophin secretion have been described in patients with primary and secondary malnutrition (chronic renal failure, cystic fibrosis and Crohn’s disease, AN). In addition, increased cytokines produced during chronic diseases such as juvenile idiopathic arthritis and CF may affect the GH-IGF-1 axis (48-52).
Leptin levels are directly related to adipose stores and act within the hypothalamus to adjust energy requirements. The gradual increases in GnRH are highly sensitive to body energy reserves that are influenced by leptin. In chronic undernutrition, leptin levels are low, and there is a loss of leptin permissive action (53). Ghrelin is secreted from the stomach because of energy insufficiency and acts as an inhibitory control on puberty onset in girls (54).
Kisspeptin also stimulates directly and indirectly the GnRH neurons that drive HPG axis maturation and puberty onset. Elucidation of the leptin/Kiss pathways require further study, but recent discoveries support a direct connection in undernutrition between leptin deficiency, reduced Kiss1 mRNA expression and reduced kisspeptin immunoreactivity (55-57).
Required interventions to manage nutritional problems in adolescents
Adolescent health and development require many interventions including health education; supportive parenting; nutrition; immunization; psychosocial support; prevention of injuries, violence, harmful practices and substance abuse; sexual and reproductive health information and services; management of communicable and non-communicable diseases.
To respond to the diverse needs of adolescents, different interventions are needed in different countries and communities. A review of the situation in selected South-East Asian countries showed acute scarcity of programs targeted at adolescents in the Region. The major underlying reason for the widespread lack of policies and programs for improving health and nutritional status of adolescents in the Region is a lack of age and sex-disaggregated data on health and nutritional status of adolescents at the national level. Scarcity of trained health providers and adolescent-friendly health centers to deal with the special needs of adolescents are important reasons for the neglect of adolescents in public health programs (58,59).
Strategies for managing adolescent undernutrition
The main strategies suggested for improving adolescent nutrition include food-based strategies like dietary modification and food fortification, for ensuring adequate nutrition at household level; addressing behaviour modification to bring about dietary change in adolescents. This can be achieved through school-based nutrition interventions, using a social marketing approach, behaviour change through communication and mobilizing families and communities; control of micronutrient deficiencies; regular nutrition assessment and counselling of adolescents; care of adolescents during pregnancy and postnatal period; intersectoral linkages at community level and building linkages with adolescent friendly health services (60).
Catch up growth in in low- and middle-income countries without intervention
Catch-up growth (CUG) from longitudinal cohorts LMIC without intervention showed inconsistent CUG and stunting recovery.
In a longitudinal study in Bangladesh, Svefors et al. (61) enrolled 1094 children at birth and followed their growth until age 10. The prevalence of stunting was highest at 2 years (50%) decreasing to 29% at 10 years.
In South Africa, Stein et al. (62) followed up 1918 subjects from birth to twenty years. They reported that progression through puberty modifies the relation between prepubertal and adult anthropometry.
Teivaanmaki et al. (63), in Malawi, enrolled 767 at birth and followed 522children up to the age of 15 years. Prevalence of stunting was highest at 2 years (80%) decreasing to 37% at 15 years. 84.7% of those stunted at 2 years recovered at least once by 15 years. By 15 years of age, only 9.0% of boys and 19.6% of girls reached advanced puberty.
Fink et al. (64) studied children in Vietnam, India, Ethiopia, and Peru (n = 976, 976, 974 and 678 respectively) from birth till 15 years. At 8 years of age, 31% of children were stunted. Of children classified as stunted at 8 years of age, 36% recovered from stunting by age of 15 years.
Lundeen et al. (65) studied a large cohort of children in Brazil, Guatemala, South Africa, Philippines and India from birth to adulthood. The prevalence of stunting was highest at 24 months. The prevalence of stunting decreased from 24 months to adulthood 3.2% (Brazil) to 41.1% (Philippines)]. By adulthood, a small proportion also became stunted (15% in the Philippines to 3% in Brazil). By adulthood, many recovered from stunting (82% in Brazil and 35% in the Philippines].
Bosch et al. (66), in Bangladesh, followed 707 children from birth to 12–16 years. They reported that adolescent stunting was associated with childhood stunting and childhood underweight.
Adair et al. (67), in Philippines enrolled 2011 children at birth; and followed them to age 12. Of the 1,252 children stunted at 2 years of age, 379 (30.3%) were not stunted at age 8.5, and 407 (32.5%) were not stunted at age 11–12. Only 1.3% of 11-year-old and 5.1% of 12-year-old girls achieve menarche. Among girls who achieved menarche, there was less stunting.
Effects of nutritional interventions in adolescents with undernutrition (Figure 2)
Figure 2.
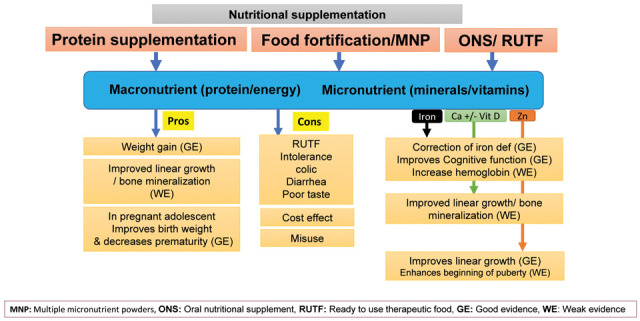
Pros and Cons of nutritional supplementation
The evidence for effective interventions to address nutritional problems in LMICs is mostly weak. Few studies have systematically examined nutritional interventions in settings where dietary inadequacy and micronutrient deficiencies exist. From high income countries (HICs), there is some evidence on interventions for eating disorders, although much further work is needed in these settings as well (68).
Protein based supplementation intervention in adolescents living in LMIC
A total of 7 protein-based intervention trials provided data to calculate an effect on linear growth were identified. Five studies contained multiple treatment groups, each providing either 2 or 3 data sets. Most of the studies were conducted in LMICs, including 1 in Africa, 2 in East Asia and the Pacific, and 3 in South Asia. The number of subjects in each study ranged from 35 to 190, with mean ages of subjects ranging from 33 to 161 mo. At baseline, 6 study populations had a mean HAZ <-2. Protein supplements were administered as either meat, cow milk (liquid or powder), amino acid–supplemented rice or wheat, casein, whey, or high-protein diets. The duration of supplementation ranged from 0.69 to 24 mo. Most datasets (85%) had a positive effect on size (69-78).
Micronutriments and protein supplementation (Figure 2)
The most studied micronutrient is calcium, as calcium absorption and bone mineralization have been shown to increase during early pubertal development among girls with consequences for long-term bone health. Supplementation with calcium, however, has been found to be of limited benefit even in settings where calcium intake was low. For example, in a Gambian cohort of children 10.3 years (Tanner stage 1) at enrolment, calcium supplementation (1,000 mg/day) increased bone mineral content of digital radius but had no impact on bone size or linear growth (79-81).
In a systematic review, several effective nutritional interventions were identified across a wide range of ages, duration, and baseline status; few studies were from LMICs. The overall findings were that interventions with zinc, vitamin A, multiple micronutrients, and protein had a significant impact on improving height; the effect size increases in linear growth ranged from 0.05 HAZ for vitamin A to 0.68 HAZ for protein. Interventions including iron, calcium, iodine, and food supplements showed no significant benefit, although sample sizes for the pooled studies for these were low (approximately 500–1,100). The sample size for the protein studies was low (n = 939), and yet the effect size was the highest. The multiple micronutrient intervention had modest significant benefits and may be a better way to combine provision of individual nutrients (82).
Randomized trials of iron supplementation have been shown to significantly improve hemoglobin concentrations among adolescents based on pooled analysis of 7 studies. However, there was a lack of significant impact on linear growth. This nutritional intervention may be worth considering, given the benefit of iron supplementation on cognition in school age children (83,84).
Home fortification of foods with multiple micronutrient powders (MNP) for health and nutrition (Figure 2)
Food fortification is defined as the practice of adding vitamins and minerals to commonly consumed foods during processing to increase their nutritional value. It is a proven, safe, and cost-effective strategy for improving diets and for the prevention and control of micronutrient deficiencies. In 2008 and 2012, the Copenhagen Consensus ranked food fortification as one of the most cost-effective development priorities. (85,86)
While mandatory food fortification has been used as a strategy to prevent micronutrient deficiencies in HIC, it is still less common in LMICs where food systems are not delivering nutritionally adequate diets due the production and consumption of just a few major starchy food crops (maize, rice, wheat) with low micronutrient content and/or bioavailability (phytate). In the past two decades, food fortification has become increasingly popular in LMICs for several reasons, including rapid urbanization and increasing household purchasing power, leading to a greater proportion of the population relying on processed foods (87,88).
Numerous country-level studies on the impact of food fortification on micronutrient status have shown positive results. A review in 2013 assessed the effects and safety of home (point-of-use) fortification of foods with multiple micronutrient powders on nutritional, health, and developmental outcomes in children. The intervention was consumption of food fortified at the point of use with multiple micronutrient powders formulated with at least iron, zinc and vitamin A compared with placebo, no intervention or the use of iron containing supplements, which is the standard practice. They included eight trials (3748 participants) conducted in LIC countries in Asia, Africa and the Caribbean, where anaemia is a public health problem. Home fortification with MNP reduced anemia by 31% and iron deficiency by 51% in infants and young children when compared with no intervention or placebo. However, authors did not find an effect on growth. In comparison with daily iron supplementation, the use of MNP produced similar results on anaemia and hemoglobin concentrations (89). In Costa Rica, an evaluation of the impact of iron fortification on anemia prevalence found a significant decrease at the national level in the prevalence of anemia among children and adolescents from 19 to 4% (90).
In Indonesia, a study conducted in two districts of West Java from 2011 to 2012 assessed the effects of large-scale fortification on the vitamin A status of women and children and found that fortified oil increased vitamin A intake close to the recommended nutrient intakes, contributing on average 38–40% among older children and adolescents (91).
A meta-analysis that included 43 studies reviewed the effects of food fortification with multiple micronutrients. Compared with placebo/no intervention, multiple micronutrients (MMN) fortification reduced anaemia by 32% (low-quality evidence (LQE)), iron deficiency anaemia by 72% (LQE), iron deficiency by 56% (LQE); vitamin A deficiency by 58% (low-quality evidence), vitamin B2 deficiency by 64% (LQE), vitamin B6 deficiency by 91% (LQE), vitamin B12 deficiency by 58% (LQE), weight-for-age z-scores (WAZ) (LQE) and weight-for-height/length Z-score (WHZ/WLZ) (LQE). The authors were uncertain about the effect of MMN fortification on zinc deficiency and height for-age Z-score (92).
A systemic review in 2014 suggested that micronutrient supplementation among adolescents (Predominantly females) can significantly decrease anemia prevalence. Interventions to improve nutritional status among “pregnant adolescents” showed statistically significant improved birth weight, decreased low birth weight, and preterm birth (93).
The WHO recommends iron-folic acid (IFA) supplementation for non-pregnant menstruating girls in contexts with high rates of anemia (94). On the other hand, another systematic review performed (2017) in both HICs and LIMCs did not find significant association between the effect of multiple micronutrient fortification on child growth outcomes such height/length-for-age z-score (HAZ/LAZ) in 8 studies (n =2889 participants; low-quality evidence) (95).
In addition, two recent reviews on fortified complementary foods (iron, folic acid, calcium, and vitamin D) in adolescents were uncertain of the effect of MMN fortification on hemoglobin concentrations, calcium supplementation on total body bone mineral content (BMC), calcium plus vitamin D supplementation on total body BMC, and zinc supplementation on zinc levels.
Fortified foods were associated with more diarrhea episodes (96,97).
Protein energy supplementation
Adolescent pregnancy is common in LMIC. In pregnant women in general, especially if they are undernourished, PES increased mean birth weight and decreased the risk of low birth weight, small-for-gestational-age births, and stillbirths. However, no studies have been conducted specifically on adolescent mothers (98).
Oral nutritional supplements (ONS) and ready to use therapeutic food (RUTF)
RUTF is an energy-dense, mineral- and vitamin-enriched food that requires no preparation and is specifically designed to treat severe acute malnutrition (SAM). RUTF has a similar nutrient composition to F-100 therapeutic milk, which is used to treat SAM in hospital settings. RUTF has significant advantages over liquid-based diets. The paste is oil-based with low water activity and, as such, can be stored at home with little risk of microbial contamination. It is easy to use, digestible and affordable at acceptable cost (99-101).
Ready-made oral nutritional supplements (ONS) are designed for children and available on prescription typically provide 2.4 kcal/ml or 1.5 kcal/ml in 125 ml and 200 ml bottles. One possible strategy to improve nutrient intake in children and adolescents with faltering growth is to reduce ONS volume by increasing the energy and nutrient density. Therefore, these formulas may be used in underweight children and adolescents with volume sensitivity or those with poor appetite (102).
ONS and RUTF proved to be effective in the management of underweight infants and young children with undernutrition. However, the major global focus of health has been on children under the age of 5 years, while older children (aged 6–9 years) and adolescents (aged 10–19 years) have not received the due attention until lately (103-105).
A controlled trial investigated the effects of energy dense pediatric ONS versus standard ONS in old children and adolescents with low BMI or low weight gain per day (WGD) (n= 34). ONS were randomized to high caloric ONS (cONS) (n =22) or standard ONS (sONS) (n = 12) for a year. Results showed that the WGD, height growth velocity (GV: cm/year), and Ht-SDS increased significantly, in both groups, during the year of ONS. The use of the cONS resulted in significantly greater mean total WGD and BMI-SDS after 6 months and 1 year, compared to the sONS group. The increase in insulin-like growth factor 1 (IGF1-SDS) was significantly higher in the cONS groups versus the sONS group. Moreover, the WGD was correlated significantly with the height GV during the year of ONS intake (106).
However, the cost of current standard RUTF and OSN is one of the major obstacles to their use in community-based management programs. In addition, the high prevalence of undesirable effects (nausea, vomiting and diarrhea) attributed to RUTF may hinder RUTF acceptability and adherence in some participants (107).
Supplement (mis)use in healthy adolescents
Although supplements may be necessary and safely consumed in certain specific situations, most healthy adolescents do not need them. Studies have indicated high prevalence of dietary supplement usage by adolescents that ranged from 10% to 74%. These supplements with no or little evidence of beneficial effects in healthy adolescents include proteins, amino acids, beta-hydroxy-beta-methyl butyrate, carnitine, creatine, vitamins, caffeine, and bicarbonate. The widespread use of these unnecessary dietary supplements among adolescents predisposes them to significant health risks (108-111).
Conclusion
Consuming an adequate and balanced healthy diet during the fast phase of pubertal growth spurt is necessary to assure proper growth and normal pubertal development. Excessive intake of some types of proteins, fat, minerals, and vitamins may lead to early puberty. Whereas, malnutrition of macro (proteins essential amino acids) , calories, essential fatty acids) and some micronutrients (iron, zinc, vitamin D ) can markedly delay the onset and/or delay the progress of growth and bone accretion during this critical period of growth (Figure 1). Although some studies suggested positive effect/s of food fortification with micronutrients and supplying more proteins and calories (RUTF and/or ONS) in malnourished children and adolescents (Figure 2), however long term-controlled studies are still highly required to support their positive effect on linear growth and pubertal development.
Acknowledgement:
We would like to present our appreciation and thanks to Prof. Vincenzo De Sanctis for suggesting the idea of this review, his kind guidance, expert editing, and continuous encouragement throughout the preparation of this manuscript.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Soliman A, De Sanctis V, Elalaily R. Nutrition and pubertal development. Indian J Endocrinol Metab. 2014;(18 (Suppl 1)):S39–S47. doi: 10.4103/2230-8210.145073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprio S, Cline G, Boulware S, et al. Effects of puberty and diabetes on metabolism of insulin-sensitive fuels. Am J Physiol. 1994;266:E885–91. doi: 10.1152/ajpendo.1994.266.6.E885. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bentley ME, Zhai F, Popkin BM. Tracking of dietary intake patterns of Chinese from childhood to adolescence over a six-year follow-up period. J Nutr. 2002;132:430–8. doi: 10.1093/jn/132.3.430. [DOI] [PubMed] [Google Scholar]
- Totland TH, Gebremariam MK, Lien N, et al. Does tracking of dietary behaviours differ by parental education in children during the transition into adolescence? Public Health Nutr. 2013;16:673–82. doi: 10.1017/S1368980012003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E, Warnberg J, Kearney J, Sjostrom M. The tracking of dietary intakes of children and adolescents in Sweden over six years: the European Youth Heart Study. Int J Behav Nutr Phys Act. 2009;6:91. doi: 10.1186/1479-5868-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PJ, Gallagher AM, Livingstone MB, et al. Tracking of nutrient intakes in adolescence: the experiences of the Young Hearts Project, Northern Ireland. Br J Nutr. 2000;84:541–8. [PubMed] [Google Scholar]
- Von Post-Skagegard M, Samuelson G, Karlstrom B, Mohsen R, Berglund L, Bratteby LE. Changes in food habits in healthy Swedish adolescents during the transition from adolescence to adulthood. Eur J Clin Nutr. 2002;56:532–8. doi: 10.1038/sj.ejcn.1601345. [DOI] [PubMed] [Google Scholar]
- Pearson N, Salmon J, Campbell K, Crawford D, Timperio A. Tracking of children’s body-mass index, television viewing and dietary intake over five-years. Prev Med. 2011;53:268–70. doi: 10.1016/j.ypmed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Keats EC, Rappaport A, Jain R, Oh C, Shah S, Bhutta ZA. Diet and Eating Practices among Adolescent Girls in Low-and Middle-Income Countries: A Systematic Review. Nutrients. 2018;10(12):1978. doi: 10.3390/nu10121978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Nutrition. Technical Report No.24. Indian Council of Medical Research, Hyderabad - 500 007, India. 2006 [Google Scholar]
- Doku D, Koivusilta L, Raisamo S, Rimpelä A. Socio-Economic Differences in Adolescents’ Breakfast Eating, Fruit and Vegetable Consumption and Physical Activity in Ghana. Public Health Nutr. 2013;16:864–72. doi: 10.1017/S136898001100276X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barugahara EI, Kikafunda J, Gakenia W M. Prevalence and Risk Factors of Nutritional Anaemia among Female School Children in Masindi District, Western Uganda. Afr J Food, Agric Nutr Dev. 2013;13:7679–92. [Google Scholar]
- Alam N, Roy S K, Ahmed T, Ahmed A M. Nutritional Status, Dietary Intake, and Relevant Knowledge of Adolescent Girls in Rural Bangladesh.”. J Health Popul Nutr. 2010;28:86–94. doi: 10.3329/jhpn.v28i1.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognon G, Moreno LA, Mouratidou T, et al. Adherence to a Mediterranean-like dietary pattern in children from eight European countries. The IDEFICS study. Int. J. Obes. 2014;38:108–14. doi: 10.1038/ijo.2014.141. [DOI] [PubMed] [Google Scholar]
- Novak D, Štefan L, Prosoli R, et al. Mediterranean Diet and Its Correlates among Adolescents in Non-Mediterranean European Countries: A Population-Based Study. Nutrients. 2017;9:177. doi: 10.3390/nu9020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14(10):1266. doi: 10.3390/ijerph14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath EW, Li J, Sun SS, et al. Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. Am J Clin Nutr. 2004;80:441–6. doi: 10.1093/ajcn/80.2.441. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Kurian JR, Keen KL, Shiel NA, Colman RJ, Capuano SV. Body weight impact on puberty: effects of high-calorie diet on puberty onset in female rhesus monkeys. Endocrinology. 2012;153:1696–1705. doi: 10.1210/en.2011-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Willett WC, Spiegelman D, et al. Sugar-sweetened beverage consumption and age at menarche in a prospective study of US girls. Hum. Reprod. 2015;30:675–83. doi: 10.1093/humrep/deu349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NT, Jacobs DR Jr, MacLehose RF, et al. Consumption of caffeinated and artificially sweetened soft drinks is associated with risk of early menarche. Am. J. Clin. Nutr. 2015;102:648–54. doi: 10.3945/ajcn.114.100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Y, Zhang Y, et al. Association between Dietary Patterns and Precocious Puberty in Children: A Population-Based Study. Int J Endocrinol. 2018;2018:4528704. doi: 10.1155/2018/4528704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani Tehrani F, Moslehi N, Asghari G, Gholami R, Mirmiran P, Azizi F. Intake of dairy products, calcium, magnesium, and phosphorus in childhood and age at menarche in the Tehran Lipid and Glucose Study. PLoS One. 2013;8(2):e57696. doi: 10.1371/journal.pone.0057696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diethelm K, Günther AL, Schulze MB, Standl M, Heinrich J, Buyken AE. Prospective relevance of dietary patterns at the beginning and during the course of primary school to the development of body composition. Br J Nutr. 2014 Apr 28;111(8):1488–98. doi: 10.1017/S0007114513004017. doi: 10.1017/S0007114513004017. Epub 2014 Jan. [DOI] [PubMed] [Google Scholar]
- Gunther AL, Karaolis-Danckert N, Kroke A, Remer T, Buyken AE. Dietary protein intake throughout childhood is associated with the timing of puberty. J Nutr. 2010;140:565–71. doi: 10.3945/jn.109.114934. [DOI] [PubMed] [Google Scholar]
- Wiley AS. Mil. k intake and total dairy consumption: associations with early menarche in NHANES 1999–2004. PLoS One. 2011;6(2):e14685. doi: 10.1371/journal.pone.0014685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Willett WC, Wang M, Rich-Edwards J, Frazier AL, Michels KB. Milk consumption after age 9 years does not predict age at menarche. J Nutr. 2015;145:1900–8. doi: 10.3945/jn.115.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C, Flexeder C, Thiering E, et al. Changes in dietary intake during puberty and their determinants: results from the GINIplus birth cohort study. BMC Public Health. 2015;15(1):841. doi: 10.1186/s12889-015-2189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslehi N, Asghari G, Mirmiran P, Azizi F. Longitudinal association of dietary sources of animal and plant protein throughout childhood with menarche. BMC Pediatr. 2021;21(1):206. doi: 10.1186/s12887-021-02670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers IS, Northstone K, Dunger DB, Cooper AR, Ness AR, Emmett PM. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 2010;13:2052–63. doi: 10.1017/S1368980010001461. [DOI] [PubMed] [Google Scholar]
- Kerver JM, Gardiner JC, Dorgan JF, Rosen CJ, Velie EM. Dietary predictors of the insulin-like growth factor system in adolescent females: results from the Dietary Intervention Study in Children (DISC) Am J Clin. Nutr. 2010;91:643–50. doi: 10.3945/ajcn.2009.28205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JM, Dunger DB. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J. Clin. Endocrinol. Metab. 2012;97:E786–90. doi: 10.1210/jc.2011-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Dibba B, Sawo Y, Cole TJ. The effect of prepubertal calcium carbonate supplementation on the age of peak height velocity in Gambian adolescents. Am J Clin Nutr. 2012;96:1042–50. doi: 10.3945/ajcn.112.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalley T, Rizzoli R, Hans D, Ferrari S, Bonjour JP. Interaction between calcium intake and menarcheal age on bone mass gain: an eight-year follow-up study from prepuberty to postmenarche. J Clin Endocrinol Metab. 2005;90:44–51. doi: 10.1210/jc.2004-1043. [DOI] [PubMed] [Google Scholar]
- Kissinger DG, Sanchez A. The association of dietary factors with age at menarche. Nutr Res. 1987;7:471–9. [Google Scholar]
- Halsted JA, Ronaghy HA, Abadi P, et al. Zinc deficiency in man. The Shiraz experiment. Am J Med. 1972;53:277–84. doi: 10.1016/0002-9343(72)90169-6. [DOI] [PubMed] [Google Scholar]
- Zadik Z, Sinai T, Zung A, Reifen R. Vitamin A and iron supplementation is as efficient as hormonal therapy in constitutionally delayed children. Clin. Endocrinol. 2004;60:682–87. doi: 10.1111/j.1365-2265.2004.02034.x. [DOI] [PubMed] [Google Scholar]
- Maclure M, Travis LB, Willett W, MacMahon B. A prospective cohort study of nutrient intake and age at menarche. Am J Clin Nutr. 1991;54:649–56. doi: 10.1093/ajcn/54.4.649. [DOI] [PubMed] [Google Scholar]
- Christian P, Smith ER. Adolescent Undernutrition: Global Burden, Physiology, and Nutritional Risks. Ann Nutr Metab. 2018;72:316–28. doi: 10.1159/000488865. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xiong J, Gao W, Wang X, et al. Dietary Fat and Polyunsaturated Fatty Acid Intakes during Childhood Are Prospectively Associated with Puberty Timing Independent of Dietary Protein. Nutrients. 2022;14:275. doi: 10.3390/nu14020275. https://doi.org/10.3390/nu14020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akseer N, Al-Gashm S, Mehta S, Mokdad A, Bhutta ZA. Global and regional trends in the nutritional status of young people: a critical and neglected age group. Ann N Y Acad Sci. 2017;1393:3–20. doi: 10.1111/nyas.13336. [DOI] [PubMed] [Google Scholar]
- Christian P, Smith ER. Adolescent Undernutrition: Global Burden, Physiology, and Nutritional Risks. Ann Nutr Metab. 2018;72:316–28. doi: 10.1159/000488865. [DOI] [PubMed] [Google Scholar]
- Kulin HE, Bwibo N, Mutie D, Santner SJ. The effect of chronic childhood malnutrition on pubertal growth and development. Am J Clin Nutr. 1982;36:527–36. doi: 10.1093/ajcn/36.3.527. [DOI] [PubMed] [Google Scholar]
- Black RE, Victora CG, Walker SP, et al. Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Prentice A, Schoenmakers I, Laskey MA, de Bono S, Ginty F, Goldberg GR. Nutrition and bone growth and development. Proc Nutr Soc. 2006;65(4):348–360. doi: 10.1017/s0029665106005192. doi: 10.1017/s0029665106005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi SC, Carducci B, Söder O, Bhutta ZA. The Intricate Relationship between Chronic Undernutrition, Impaired Linear Growth and Delayed Puberty: Is ‘catch-up’ growth possible during adolescence? UNICEF, Innocenti Working Paper. 2018-12:1–32. [Google Scholar]
- Campisi SC, Carducci B, Söder O, Bhutta ZA. The Intricate Relationship between Chronic Undernutrition, Impaired Linear Growth and Delayed Puberty: Is ‘catch-up’ growth possible during adolescence? Office of Research - Innocenti WP-sccessed July 2018. https://www.unicef-irc.org/publications. [Google Scholar]
- Roa J, Garcia-Galiano D, Varela L, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Neuroendocrinology. 2009;150:5016–26. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- Whiteford H A, Degenhardt L, Rehm J, et al. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolescent Health. 2002;31:192–200. doi: 10.1016/s1054-139x(02)00485-8. [DOI] [PubMed] [Google Scholar]
- Forbes GB. Influence of nutrition. In: Forbes GB, editor. Human body composition: Growth, aging, nutrition and activity. New York: Springer-Verlag; 1987. pp. 209–47. [Google Scholar]
- Kulin HE, Bwibo N, Mutie D, Santner S. The effect of chronic childhood malnutriton on pubertal growth and development. Am J Clin Nutr. 1982;36:527–36. doi: 10.1093/ajcn/36.3.527. [DOI] [PubMed] [Google Scholar]
- Adolescent Nutrition. A Review of the Situation in Selected South-East Asian Countries. WHO. 2006 https://apps.who.int/iris/bitstream/handle. Accessed October 18,2021. [Google Scholar]
- Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–93. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4:254–64. doi: 10.1016/S2213-8587(15)00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SH, Colledge WH. The Role of Kiss1 Neurons As Integrators of Endocrine, Metabolic, and Environmental Factors in the Hypothalamic-Pituitary-Gonadal Axis. Front Endocrinol (Lausanne) 2018;9:188. doi: 10.3389/fendo.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin S, Kiess W. Putative Effects of Obesity on Linear Growth and Puberty. Horm Res Paediatr. 2017;88:101–10. doi: 10.1159/000455968. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Tena-Sempere M. Metabolic control of female puberty: potential therapeutic targets. Expert Opin Ther Targets. 2016;20:1181–93. doi: 10.1080/14728222.2016.1212015. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Schwarz SW, Skinner C. The economic impact of adolescent health promotion policies and programs. Adolesc Med State of the Art Rev. 2011;22:367–86. [PubMed] [Google Scholar]
- Editorial. Putting adolescents at the centre of health and development. Lancet. 2012;379:1563. doi: 10.1016/S0140-6736(12)60536-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Regional Office for South-East Asia. Adolescent nutrition: a review of the situation in selected South-East Asian Countries. WHO Regional Office for South-East Asia. 2006 https://apps.who.int/iris/handle/10665/204764. [Google Scholar]
- Svefors P, Rahman A, Ekstrom EC, et al. Stunted at 10 Years. Linear Growth Trajectories and Stunting from Birth to Pre-Adolescence in a Rural Bangladeshi Cohort. PLoS One. 2016;11(3):e0149700. doi: 10.1371/journal.pone.0149700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AD, Lundeen EA, Martorell R, et al. Pubertal Development and Prepubertal Height and Weight Jointly Predict Young Adult Height and Body Mass Index in a Prospective Study in South Africa. J Nutr. 2016;146:1394–401. doi: 10.3945/jn.116.231076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teivaanmaki T, Cheung YB, Kortekangas E, Maleta K, Ashorn P. Transition between stunted and nonstunted status: both occur from birth to 15 years of age in Malawi children. Acta Paediatr. 2015;104:1278–85. doi: 10.1111/apa.13060. [DOI] [PubMed] [Google Scholar]
- Fink G, Rockers PC. Childhood growth, schooling, and cognitive development: further evidence from the Young Lives study. Am J Clin Nutr. 2014;100:182–8. doi: 10.3945/ajcn.113.080960. [DOI] [PubMed] [Google Scholar]
- Lundeen EA, Stein AD, Adair LS, et al. Height-for-age z scores increase despite increasing height deficits among children in 5 developing countries. Am J Clin Nutr. 2014;100:821–5. doi: 10.3945/ajcn.114.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch AM, Baqui AH, van Ginneken JK. Early-life determinants of stunted adolescent girls and boys in Matlab, Bangladesh. J Health Popul Nutr. 2008;26:189–99. [PMC free article] [PubMed] [Google Scholar]
- Adair LS. Filipino children exhibit catch-up growth from age 2 to 12 years. J Nutr. 1999;129:1140–8. doi: 10.1093/jn/129.6.1140. [DOI] [PubMed] [Google Scholar]
- Lassi Z, Moin A, Bhutta Z. Nutrition in Middle Childhood and Adolescence. In: Bundy DAP, Silva Nd, Horton S, et al., editors. Child and Adolescent Health and Development. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 20. Chapter 11. https://www.ncbi.nlm.nih.gov/ [PubMed] [Google Scholar]
- Grillenberger M, Neumann CG, Murphy SP, et al. Food supplements have a positive impact on weight gain and the addition of animal source foods increases lean body mass of Kenyan school children. J Nutr. 2003;(133 (Suppl 2)):3957S–64. doi: 10.1093/jn/133.11.3957S. [DOI] [PubMed] [Google Scholar]
- Kabir I, Rahman MM, Haider R, Mazumder RN, Khaled MA, Mahalanabis D. Increased height gain of children fed a high-protein diet during convalescence from shigellosis: a six-month follow-up study. J Nutr. 1998;128:1688–91. doi: 10.1093/jn/128.10.1688. [DOI] [PubMed] [Google Scholar]
- Lampl M, Johnston FE, Malcolm LA. The effects of protein supplementation on the growth and skeletal maturation of new Guinean school children. Ann Hum Biol. 1978;5:219–27. doi: 10.1080/03014467800002841. [DOI] [PubMed] [Google Scholar]
- Larnkjær A, Arnberg K, Michaelsen KF, Jensen SM, Mølgaard C. Effect of milk proteins on linear growth and IGF variables in overweight adolescents. Growth Horm IGF Res. 2014;24:54–9. doi: 10.1016/j.ghir.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Malcolm LA. Growth retardation in a new Guinea boarding school and its response to supplementary feeding. Br J Nutr. 1970;24:297–305. doi: 10.1079/bjn19700029. [DOI] [PubMed] [Google Scholar]
- Pereira SM, Begum A, Jesudian G, Sundararaj R. Lysine-supplemented wheat and growth of preschool children. Am J Clin Nutr. 1969;22:606–11. doi: 10.1093/ajcn/22.5.606. [DOI] [PubMed] [Google Scholar]
- Pereira SM, Jones S, Jesudian G, Begum A. Feeding trials with lysine and threonine-fortified rice. Br J Nutr. 1973;30:241–50. doi: 10.1079/bjn19730030. [DOI] [PubMed] [Google Scholar]
- Alarcon PA, Lin L-H, Noche M, Hernandez VC, Cimafranca L, Lam W, Comer GM. Effect of oral supplementation on catch-up growth in picky eaters. Clin Pediatr (Phila) 2003;42:209–17. doi: 10.1177/000992280304200304. [DOI] [PubMed] [Google Scholar]
- Maleta K, Kuittinen J, Duggan MB, Briend A, Manary M, Wales J, Kulmala T, Ashorn P. Supplementary feeding of underweight, stunted Malawian children with a ready-to-use food. J Pediatr Gastroenterol Nutr. 2004;38:152–8. doi: 10.1097/00005176-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Prasad Mp R, Benhur D, Kommi K, Madhari R, Rao MV, Patil JV. Impact of Sorghum supplementation on growth and micronutrient status of school going children in Southern India — a randomized trial. Indian J Pediatr. 2016;83:9–14. doi: 10.1007/s12098-015-1782-7. [DOI] [PubMed] [Google Scholar]
- Abrams SA, Copeland KC, Gunn SK, Gundberg CM, Klein KO, Ellis KJ. Calcium absorption, bone mass accumulation, and kinetics increase during early pubertal development in girls. J Clin Endocrinol Metab. 2000;85:1805–9. doi: 10.1210/jcem.85.5.6508. [DOI] [PubMed] [Google Scholar]
- Dibba B, Prentice A, Ceesay M, Mendy M, Darboe S, Stirling DM, Cole TJ, Poskitt EM. Bone mineral contents and plasma osteocalcin concentrations of Gambian children 12 and 24 mo after the withdrawal of a calcium supplement. Am J Clin Nutr. 2002;76:681–6. doi: 10.1093/ajcn/76.3.681. [DOI] [PubMed] [Google Scholar]
- Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EM. Effect of calcium supplementation on bone mineral accretion in gambian children accustomed to a low-calcium diet. Am J Clin Nutr. 2000;71:544–9. doi: 10.1093/ajcn/71.2.544. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Stein AD. The impact of nutritional interventions beyond the first 2 years of life on linear growth: a systematic review and meta-analysis. Adv Nutr. 2017;8:323–36. doi: 10.3945/an.116.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi ZS, Moin A, Das JK, Salam RA, Bhutta ZA. Systematic review on evidence-based adolescent nutrition interventions. Ann N Y Acad Sci. 2017;1393:34–50. doi: 10.1111/nyas.13335. [DOI] [PubMed] [Google Scholar]
- Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:501–8. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- Horton S, Mannar V, Wesley A. Copenhagen Consensus Center: Copenhagen, Denmark; 2008. Best Practice Paper Food Fortification with Iron and Iodine; Copenhagen Consensus Center Working Paper October; pp. 1–26. [Google Scholar]
- Hoddinott J, Rosegrant M, Torero M. Copenhagen Consensus Center: Copenhagen, Denmark; 2012. Investments to Reduce Hunger and Undernutrition; Copenhagen Consensus Center Working Paper March; pp. 1–69. [Google Scholar]
- Spohrer R, Larson M, Maurin C, Laillou A, Capanzana M, Garrett GS. The growing importance of staple foods and condiments used as ingredients in the food industry and implications for large-scale food fortification programs in Southeast Asia. Food Nutr Bull. 2013;34((2 Suppl)):50–61. doi: 10.1177/15648265130342S107. [DOI] [PubMed] [Google Scholar]
- Rowe LA, Dodson DM. Addressing Micronutrient Malnutrition in Urban Settings. In: Ahn R, Burke T, McGahan A, editors. Innovating for Healthy Urbanization. Boston, MA: Springer; 2015. [Google Scholar]
- De-Regil LM, Suchdev PS, Vist GE, Walleser S, Peña-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review) Evid Based Child Health. 2013;8:112–201. doi: 10.1002/ebch.1895. [DOI] [PubMed] [Google Scholar]
- Martorell R, Ascencio M, Tacsan L, et al. Effectiveness evaluation of the food fortification program of Costa Rica: impact on anemia prevalence and hemoglobin concentrations in women and children. Am J Clin Nutr. 2015;101:210–7. doi: 10.3945/ajcn.114.097709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandjaja , Jus’at I, Jahari AB, et al. Vitamin A-fortified cooking oil reduces vitamin A deficiency in infants, young children and women: results from a programme evaluation in Indonesia. Public Health Nutr. 2015;18:2511–22. doi: 10.1017/S136898001400322X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das JK, Salam RA, Mahmood SB, et al. Food fortification with multiple micronutrients: impact on health outcomes in general population. Cochrane Database Syst Rev. 2019;12(12):CD011400. doi: 10.1002/14651858.CD011400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam RA, Hooda M, Das JK, Arshad A, Lassi ZS, Middleton P, Bhutta ZA. Interventions to Improve Adolescent Nutrition: A Systematic Review and Meta-Analysis. J Adolesc Health. 2016;59:S29–S39. doi: 10.1016/j.jadohealth.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Guideline: implementing effective actions for improving adolescent nutrition. World Health Organization. 2018 https://apps.who.int/iris/handle/10665/260297. [Google Scholar]
- Osendarp SJM, Martinez H, Garrett GS, et al. Large-Scale Food Fortification and Biofortification in Low-and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps. Food Nutr Bull. 2018;39:315–31. doi: 10.1177/0379572118774229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidkamp RA, Piwoz E, Gillespie S, et al. Mobilising evidence, data, and resources to achieve global maternal and child undernutrition targets and the Sustainable Development Goals: an agenda for action. Lancet. 2021;397:1400–18. doi: 10.1016/S0140-6736(21)00568-7. [DOI] [PubMed] [Google Scholar]
- Salam RA, Das JK, Ahmed W, Irfan O, Sheikh SS, Bhutta ZA. Effects of preventive nutrition interventions among adolescents on health and nutritional status in low-and middle-income countries: a systematic review and meta-analysis. Nutrients. 2019;12(1):49. doi: 10.3390/nu12010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A, Bhutta ZA. Maternal nutrition and birth outcomes: effect of balanced protein-energy supplementation. Paediatr Perinat Epidemiol. 2012;(26 (Suppl 1)):178–90. doi: 10.1111/j.1365-3016.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- Schoonees A, Lombard M, Musekiwa A, Nel E, Volmink J. Ready-to-use therapeutic food for home-based treatment of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst Rev. 2013;2013(6):CD009000. doi: 10.1002/14651858.CD009000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh VD. Ready to Use Therapeutic Food [RUTF] formulation and packaging for malnutrition: an Overview. Nutri Food Sci Int J. 2018;4(5):555648. [Google Scholar]
- Fleet A, Kshirsagar R, Margit Bach M, Forteza ME. Expert Meeting on Ready-to-Use-Therapeutic Foods (RUTF) UNICEF. 2019; Report 1:1–20. [Google Scholar]
- Brown B, Roehl K, Betz M. Enteral nutrition formula selection: current evidence and implications for practice. Nutr Clin Pract. 2015;30:72–85. doi: 10.1177/0884533614561791. [DOI] [PubMed] [Google Scholar]
- Sato W, Furuta C, Matsunaga K, et al. Amino-acid-enriched cereals ready-to-use therapeutic foods (RUTF) are as effective as milk-based RUTF in recovering essential amino acid during the treatment of severe acute malnutrition in children: An individually randomized control trial in Malawi. PLoS One. 2018;13(8):e0201686. doi: 10.1371/journal.pone.0201686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahwere P, Balaluka B, Wells JC, et al. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk-and peanut paste-based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am J Clin Nutr. 2016;103:1145–61. doi: 10.3945/ajcn.115.119537. [DOI] [PubMed] [Google Scholar]
- Schoonees A, Lombard MJ, Musekiwa A, Nel E, Volmink J. Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst Rev. 2019;5(5):CD009000. doi: 10.1002/14651858.CD009000.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A, De Sanctis V, Elsiddig S, et al. Impact of oral nutritional supplements (ONS) on growth outcomes and IGF-1 level in underweight older children and young adolescents (5-14 years) with short stature and no systemic disease: High versus normal calories density formula. Acta Biomed. 2021;92(4):e2021320. doi: 10.23750/abm.v92i4.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahwere P, Balaluka B, Wells JC, et al. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk-and peanut paste-based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am J Clin Nutr. 2016;103:1145–61. doi: 10.3945/ajcn.115.119537. [DOI] [PubMed] [Google Scholar]
- Tiwari K. Supplement (mis)use in adolescents. Curr Opin Pediatr. 2020;32:471–5. doi: 10.1097/MOP.0000000000000912. [DOI] [PubMed] [Google Scholar]
- Stierman B, Mishra S, Gahche JJ, Potischman N, Hales CM. Dietary Supplement Use in Children and Adolescents Aged ≤19 Years — United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69:1557–62. doi: 10.15585/mmwr.mm6943a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch KD, Bell A. Dietary supplement use in adolescents. Curr Opin Pediatr. 2005;17:653–7. doi: 10.1097/01.mop.0000172819.72013.5d. [DOI] [PubMed] [Google Scholar]
- Alves C, Lima RV. Dietary supplement use by adolescents. J Pediatr (Rio J) 2009;85:287–94. doi: 10.2223/JPED.1907. [DOI] [PubMed] [Google Scholar]


