Abstract
Every year, 2 million women reach menopause in the United States, and they may spend 40% or more of their life in a postmenopausal state. In the years immediately preceding menopause—known as the menopause transition (or perimenopause)—changes in hormones and body composition increase a woman’s overall cardiometabolic risk. In this narrative review, we summarize the changes in weight, body composition, and body fat distribution, as well as the changes in energy intake, energy expenditure, and other cardiometabolic risk factors (lipid profile, glucose metabolism, sleep health, and vascular function), that occur during the menopause transition. We also discuss the benefits of lifestyle interventions in women in the earlier stages of menopause before these detrimental changes occur. Finally, we discuss how to include perimenopausal women in research studies so that women across the life-span are adequately represented.
INTRODUCTION
Obesity is a major public health concern that affects women disproportionately more than men (1). In the United States, the prevalence of obesity in women was 39.7% among those aged 20 to 39 years, 43.3% among those aged 40 to 59 years, and 43.3% among those aged 60 years and over (2). During the menopause transition, women experience dramatic decreases in circulating estrogens—particularly estradiol (E2)—and increases in the gonadotropin follicle-stimulating hormone (FSH) (3). These hormonal changes are associated with changes in energy expenditure and energy intake that promote a positive energy balance, leading to weight gain (3–5). This weight gain is due to increased fat mass and, in particular, increased abdominal fat deposition (3–5), which contribute to increased cardiometabolic risk. Despite these known detrimental consequences of the menopause transition, many researchers avoid combining premenopausal, perimenopausal, and postmenopausal women in their studies or avoid enrolling perimenopause-aged women. Yet the menopause transition itself has much to teach us about how these adverse cardiometabolic profiles arise. Because all women will experience menopause and have the potential of spending much of their lives in the postmenopausal phase, understanding how the menopause transition affects body composition and cardiometabolic health is critically important to understanding the evolution of women’s health across the life-span.
This narrative review details how the menopause transition imposes a significant health burden on women and affects total body weight, body composition, and body fat distribution, as well as how these changes impact cardiometabolic health. We also briefly review lifestyle interventions to improve cardiometabolic health in women. Finally, we call for further research during the menopause transition to identify the inflection points of risk accrual, as they are likely to be the time points at which interventions have the best chance of being effective.
DEFINING THE MENOPAUSE TRANSITION: STAGING AND STUDY DESIGN CONSIDERATIONS
The menopause transition—a brief overview
The menopause transition (i.e., perimenopause) is characterized by an increased rate of attrition of ovarian follicles. The initial result of this decrease in follicle number is reduced inhibin-B release from the ovaries. This reduction in inhibin-B signals the anterior pituitary to upregulate secretion of FSH which, in turn, stimulates ovarian E2 production, with FSH continuing to increase as ovarian function declines. As women transition through perimenopause, the ovarian follicle supply becomes critically low, and the ovary can no longer respond to the increased FSH signaling with consistent output of E2. Eventually, all functioning follicles are lost, and E2 production by the ovary ceases, accompanied by a steady state of high FSH output. Natural menopause is typically defined after amenorrhea of 12 months in a woman aged 45 or older (6). Median age of natural menopause is 51.4 years (7), with an approximate 95% CI of 45 to 55 years, and it has been reported to be slightly higher at 52.5 years among longitudinally studied women (8). Although the length of the menopause transition typically encompasses 4 to 5 years, the length of the transition is highly variable and it can last from <1 to 10 years or longer (9). While 12% of women report sudden amenorrhea, the remainder of women experience changes in cycle length and variability in cycles during the transition (9). Approximately 5% of women experience early menopause, defined as having a final menstrual cycle between ages 40 and 45 years. A minority of women (~1%) experience primary ovarian insufficiency (POI), defined as hypergonadotropic amenorrhea before 40 years of age. A woman can also undergo iatrogenic premature menopause after having bilateral oophorectomy surgery or iatrogenic ablation of ovarian function (e.g., chemotherapy, pelvic radiation) prior to natural menopause (10).
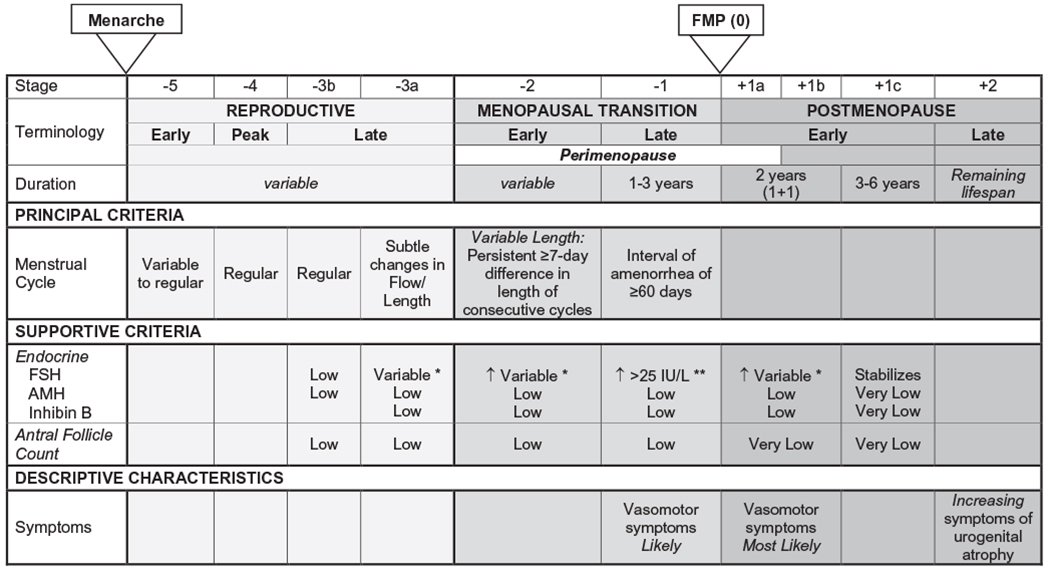
When classifying menopause stages, it is important to know that the menopause transition can be divided into early or late perimenopause stages (11). Early perimenopause is marked by increases in menstrual cycle length of 7 days or more, and some women report small but noticeable increases in common menopausal symptoms, such as vasomotor symptoms (e.g., hot flashes, night sweats). Late perimenopause is characterized by intervals of amenorrhea for at least 60 days and an elevated FSH >25 mIU/mL with a more sharply increased prevalence of menopausal symptoms. The Stages of Reproductive Aging Workshop (STRAW)+10 guidelines provide a comprehensive and easy to understand staging system for determining where a woman is in her menopause transition (Table 1) (11).
TABLE 1.
The Stages of Reproductive Aging Workshop +10 staging system for reproductive aging in women
|
This table was adapted with permission from the previously published STRAW+10 staging guidelines (11).
Abbreviations: AMH, anti-Müllerian hormone; FMP, final menstrual period; FSH, follicle-stimulating hormone.
↑ = elevated.
Blood draws during early follicular phase (cycle days 2-5).
Approximate expected concentration based on assays using current international pituitary standard.
Considerations when studying the stages of the menopause transition
The menopause transition is difficult to study because of the inherent variability in the process of ovarian failure (initiation and duration), the confounding effects of chronological aging, and the variability of hormones (ovarian and gonadotropins) both between cycles and between women. These highly variable characteristics might explain why many studies exclude women in the perimenopausal age range. However, classification systems such as STRAW+10 criteria assist in identifying stages of the transition without relying on spot-check levels of hormones, which can lead to misclassification. It is helpful, in longitudinal studies, to organize changes around the final menstrual period (FMP) to better elucidate how and when changes in the parameters of interest relate to menopause. This approach does not, however, adequately account for variability in transition duration, which can be meaningful.
Even though the STRAW+10 criteria has simplified classification of women as they progress through the menopause transition, early perimenopause remains more difficult to identify than late perimenopause. Entry into the menopause transition is defined by the STRAW+10 criteria as increased variability in cycle length (i.e., a difference of ±7 days within 10 cycles). This change can be challenging to accurately measure as it requires a well-documented cycle history. Most women notice more obvious changes, such as a missed menstrual cycle. With the evolution of new menstrual period tracking apps, it may become easier for women to observe and report changes in menstrual cycle length and regularity (e.g., tracking of deviation in more than 7 days from “usual” menstrual cycle length) (12). Researchers often rely on self-reported cycle lengths, elevated early follicular phase FSH (cycle days 1 to 5), or serum E2 to help define early perimenopause (11). Because of cycle-to-cycle variability in FSH and E2, single measurements of these hormones are of limited value for classifying women in the early stage of the menopause transition. Anti-Müllerian hormone (AMH), which is secreted by small antral follicles (granulosa cells), is also a reliable marker of ovarian follicle reserve, with lower AMH indicative of being closer to the FMP. Although AMH can be predictive of age at FMP, there are several caveats. First, AMH is most predictive in women in their late 40s or early 50s. It is possible for women aged 35 to 39 years to have an undetectable concentration of AMH but still have regular menstrual cycles (13). Additionally, the lack of an international standard for AMH means that measurement techniques can vary (13). Nonetheless, AMH is one of the best predictors of FMP (13–15). With greater population data, AMH could be a useful qualitative measure of the early menopause transition.
Late perimenopause is easier to characterize than early perimenopause, given that it is defined by the presence of at least 60 days of amenorrhea (11). An FSH concentration greater than 25 IU/L is now also considered to be characteristic of the late perimenopausal period (11). Although listed in the STRAW+10 criteria, using the presence of vasomotor symptoms to define the late perimenopausal period is not recommended because their appearance does not strictly follow changes in the menstrual cycle or hormones across the menopause transition (16). Additionally, not all women will experience vasomotor symptoms (16).
CHANGES IN WEIGHT AND BODY COMPOSITION
On average, women gain approximately 5 to 7 pounds (or 2 to 3 kg) over the course of the menopause transition (17,18), yet there is substantial interindividual variability. Increased weight and particularly abdominal fat are associated with more severe vasomotor symptoms (i.e., hot flashes and night sweats) and insomnia (19,20), as well as increased fatigue and decreased quality of life (21,22). Some women may not experience weight gain even though changes in body composition occur (i.e., increased fat mass, decreased fat-free mass, and decreased bone mineral density). The following section details the changes in body composition and fat distribution that have been reported in both animal and human (observational and clinical) studies focused on the loss of ovarian function. A summary of the known changes in body composition and cardiometabolic risk that occur during the menopause transition and into the postmenopausal years is included (Figure 1).
Figure 1.
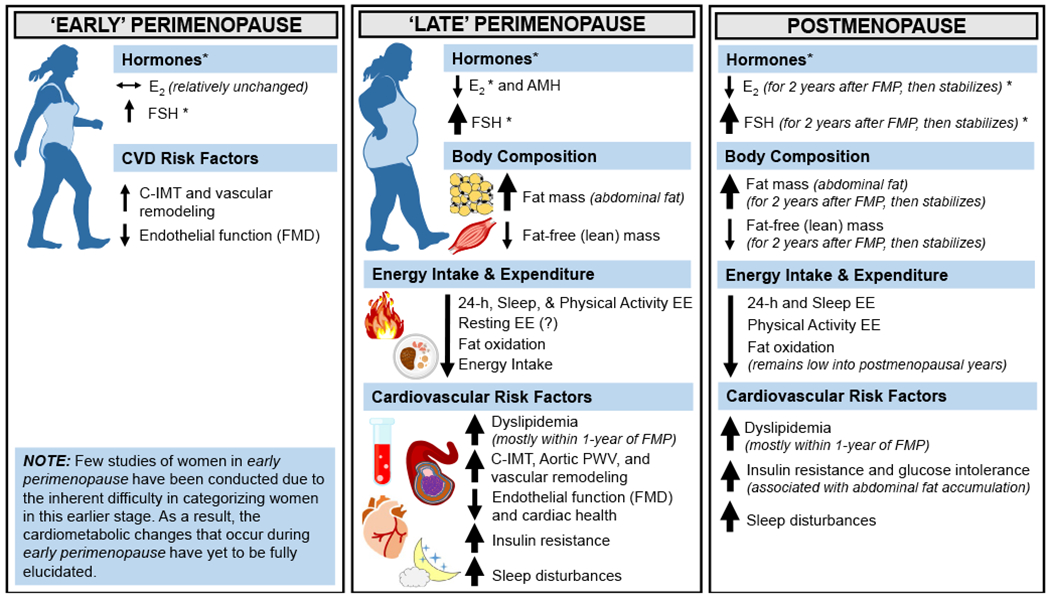
Associated changes in cardiometabolic risk during the menopause transition. Changes in cardiometabolic risk factors occur during the menopause transition, which is separated into two subcategories (early perimenopause and late perimenopause) and into the postmenopausal years. Horizontal arrows (↔) indicate stability, and smaller or larger/thicker directional arrows (↑ or ↓) indicate smaller or larger changes that occur. *Although E2 concentrations are lower at menopause onset compared with premenopausal concentrations, the patterns of E2 decline and FSH rise during perimenopause are heterogenous across women. AMH, anti-Müllerian hormone; C-IMT, carotid intima-media thickness; E2, estradiol; EE, energy expenditure; FSH, follicle-stimulating hormone; FMP, final menstrual period; PWV, pulse wave velocity
Findings from animal studies
Ovariectomy (OVX) is used as a model of menopause in laboratory animals to study how the loss of ovarian function causes fat mass accumulation. OVX eliminates ovarian estrogens and raises FSH due to the loss of negative feedback from E2. OVX is associated with increased body weight and abdominal adiposity when compared with animals that undergo sham surgery (23–25). Although increased body weight and abdominal adiposity is caused by a combination of increased energy intake and/or decreased energy expenditure, the regulation of energy intake by E2 appears to differ in mice and rats, such that OVX increased energy intake in rats but not mice. The increase in energy intake (+20%) in rats can last for several weeks (26–29), but in one study, returned to the level of energy intake of that in sham-operated controls (29). OVX also caused marked decreases in spontaneous physical activity (locomotor activity), metabolic rate, and energy expenditure in both mice (26–28,30) and rats (26,29,31). Another study reported that energy expenditure is lower in OVX mice than OVX+E2 mice when activity is similar (28). Evidence also supports a strong, protective effect of E2 against weight gain in OVX rodents, as weight gain was prevented in OVX rats treated with E2 add-back or estrogen receptor (ER)-α agonist (25,32).
The metabolic actions of estrogens that cause alterations in body weight and adiposity are mediated through the ER. As a member of the nuclear receptor superfamily, ER regulates gene expression by binding to the estrogen response element (ERE) located on the promotor sequence of target genes. ER has two main subtypes, ERα and ERβ, which are widely expressed through the body (e.g., brain, blood vessels, and adipose tissue). Much of our understanding on the molecular pathways that ER action regulates cardiovascular health and metabolism has been derived exclusively from rodent models (33). Evidence suggests that ERα is the primary mediator of the estrogen suppression of adiposity, as ERβ knockout (ERβKO) mice have stable body weight and percent fat (34). In contrast, ERα knockout (ERαKO) mice have increased fat mass, as well as adipocyte number and size (35). Brain-specific ERαKO mice have abdominal obesity mediated through hyperphagia and hypometabolism, produced by decreased heat production and decreased physical activity (36). There is a third known estrogen receptor that binds E2 at the cell surface. This G-protein coupled estrogen receptor (GPER, formerly GPCR30) induces rapid, nongenomic signaling and, like the classical ERs, is expressed widely through the body (37). Mice lacking GPER are reported to have increased body weight and visceral adiposity (38), although this is not a consistent observation in female mice (39,40).
Although most differences in body composition between males and females have been attributed to E2 and testosterone, more recently, FSH has been implicated as a mediator of abdominal adiposity. Liu and colleagues demonstrated that animals treated with an FSH beta-subunit-blocking antibody had a marked reduction in adiposity (41). The anti-obesity response to blocking FSH activity occurred in both sham and OVX animals, suggesting an effect of FSH even when FSH is in the normal range. Indeed, the anti-obesity effect of the FSH antibody was most pronounced in the abdominal visceral region (~70% difference in visceral fat volume vs. the comparator group), but differences were also apparent in abdominal subcutaneous fat volume (~30%) and total fat mass (~30%) (41).
Findings from human studies
Observational studies
Observational studies have shown that body weight and adiposity increase across the menopause transition. The Study of Women’s Health Across the Nation (SWAN) provided very compelling evidence that an accelerated gain in fat mass and loss of fat-free (lean) mass were related to the menopause transition (42) rather than aging (43,44). Although an increase in fat mass and a decline in fat-free mass were observed during pre-menopause (prior to the menopause transition), these changes accelerated during perimenopause before stabilizing in the postmenopausal years (42). Other observational studies confirmed the increase in fat mass, particularly attributed to increased abdominal fat (subcutaneous and visceral), during the menopause transition (3–5,18). Changes in body composition may also vary depending on race or initial level of adiposity on entering perimenopause (18). These observed changes in body composition are partially the result of reductions in energy expenditure and fat oxidation (3), with the reduction in energy expenditure possibly explained by a reduction in regular physical activity during the perimenopausal years (45). The Healthy Transitions longitudinal study comprehensively phenotyped women across menopause and was the first study to demonstrate that women who completed the menopause transition had a greater reduction in 24-hour energy expenditure and sleep energy expenditure compared with women who did not complete the transition (3). Although resting energy expenditure (REE) was not measured in Healthy Transitions, average REE remained stable through menopause in the Montreal-Ottawa New Emerging Team (MONET) study (46). Data from SWAN also indicated that bone mineral density declines at the greatest rate beginning 1 year before the FMP and decelerates (but does not cease) 2 years after the FMP at both the lumbar spine (7.38% loss) and femoral neck (5.8% loss) sites (47). Another study demonstrated that bone loss begins 2 to 3 years before the FMP and ends 3 to 4 years after the FMP, with spine, total body bone mineral, and femoral neck declining by 10.5%, 7.7%, and 5.3%, respectively (48). Supportive evidence suggests that the loss of estrogens is associated with or triggers the decreases in energy expenditure and physical activity (49,50), as well as bone mineral density (51). Because low rates of energy expenditure and fat oxidation at rest predict future fat gain (52–54), understanding the impact of menopause on human bioenergetics is critical. Although increased energy intake can also contribute to weight gain, longitudinal studies that assessed changes in energy intake across the menopause transition are limited (both by the lack of research examining energy intake across menopause, as well as the pitfalls surrounding self-reported energy intake (55)), but suggested energy intake decreased over the 3 to 4 years leading up to menopause onset (3).
An increase in fat mass (predominantly in the abdominal region) and decrease in fat-free mass during the menopause transition may result in little or no change in body weight yet will subsequently lead to increased cardiometabolic risk. The Women’s Health Initiative demonstrated that central obesity (defined by a waist circumference >88 cm (56)) was detrimental regardless of weight status, as normal-weight central obesity (defined by BMI 18.5 to 24.9 kg/m2 and waist circumference >88 cm) was associated with excess risk of mortality that was similar to women with BMI-defined obesity and central obesity (57). Thus, body fat distribution, rather than weight status alone, is a good determinant of cardiometabolic risk during the menopause transition.
Intervention studies
Several interventional studies indicated that hormone therapy initiated in the perimenopausal or early postmenopausal years attenuated weight gain (adiposity). In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, a randomized, placebo-controlled trial, women who received oral conjugated equine estrogens (CEE) gained less body weight and had smaller increases in waist girth than women randomized to placebo (58). The Danish Osteoporosis Prevention Study was a partially randomized study in which women who received sequential oral estrogen and progestogen gained less body weight, fat mass, and trunk fat than untreated women (59). In the Kronos Early Estrogen Prevention Study (KEEPS), increases in weight and waist circumference were attenuated in women randomized to either transdermal E2 or CEE for 4 years (60).
The administration of gonadotropin-releasing hormone agonist (GnRHAG) or GnRH antagonist (GnRHANT) in combination with E2 versus placebo therapy is useful for isolating specific effects of the loss of ovarian E2 in premenopausal women. Both GnRHAG and GnRHANT reversibly suppresses ovarian function via the suppression of the hypothalamic-pituitary-ovarian axis, essentially creating a “medical menopause.” Studies have shown that both acute and chronic ovarian hormone suppression with GnRHAG and GnRHANT alters REE and total energy expenditure. In a small group of premenopausal women, REE was measured during the mid-luteal phase of the menstrual cycle (when E2 was elevated), during the early follicular phase (when E2 was low), and after 6 days of GnRHANT treatment (when E2 was even lower) (49). REE across these 3 conditions paralleled the changes in E2, with higher REE when E2 was higher. In another study, REE decreased in premenopausal women randomized to 5 months of GnRHAG therapy, but this decrease in REE was attenuated in women who received transdermal E2 add-back (50). Conversely, total energy expenditure measured by whole-room indirect calorimetry decreased in response to GnRHAG (−128 kcal/d) but was not prevented by E2 add-back (50).
GnRHAG and GnRHANT therapy also alters body composition. In brief, 4 months of GnRHAG therapy in premenopausal women resulted in no change in total body weight, but increased total body fat and trunk fat mass (61–63). Administering GnRHAG for shorter durations also resulted in no change in total body weight; however, visceral fat mass was increased in as little as 4 weeks, with estrogen add-back attenuating this effect (64). Althought body weight, fat mass, and trunk mass were all increased after 6 months of GnRHAG suppression in one study (65), some studies do not observe changes in overall fat mass after 3 to 5 months of suppression (51,66). Despite no change in total fat mass, one of these studies found that 5 months of GnRHAG resulted in an increase in visceral fat area measured by computerized tomography (CT) (51). Collectively, these studies suggest that the loss of ovarian E2 promotes abdominal fat accumulation and a decline in energy expenditure.
CHANGES IN CARDIOMETABOLIC HEALTH
Changes in reproductive hormones and body composition across the menopause transition are associated with increased overall metabolic and cardiovascular disease (CVD) risk. These changes in cardiometabolic risk have led some researchers to suggest that obesity in women may be more appropriately diagnosed by a BMI ≥25 kg/m2 (rather than 30 kg/m2) later in life (67,68). Furthermore, the presence of central obesity is likely more detrimental to overall health and cardiometabolic risk than BMI-defined weight status alone (57). The following section details the changes in metabolic and cardiovascular health observed during the menopause transition and into the postmenopausal years and highlights how menopausal hormone therapy modifies cardiometabolic risk.
Metabolic health
Insulin sensitivity and glucose tolerance
Although the physiological effects of ovarian hormones on insulin metabolism are not clear, a role for estrogens (particularly E2) in maintaining glucose homeostasis through effects on insulin secretion and clearance has been suggested (69). It is well-established that E2 promotes peripheral (vs. central) fat distribution and improves insulin sensitivity in women (70).
In cross-sectional studies, evidence linking menopausal status and abdominal adiposity with insulin resistance and prevalent diabetes is mixed. Several of these studies found no associations between menopausal status and metabolic factors, including fasting glucose and insulin, insulin secretion rate, insulin sensitivity, and diabetes risk (71–74). Longitudinal studies, including the 6-year Pizarra Study (75), the 8-year Australian Longitudinal Study on Women’s Health (76), and the 3-year Diabetes Prevention Program (77), also found no association between natural postmenopausal status and diabetes risk. Conversely, data from SWAN indicated a progressive increase in the risk of metabolic syndrome development with menopause; this increased risk was primarily driven by worsening lipid profile rather than deleterious changes in glucose (78). In postmenopausal women without obesity, increase in abdominal fat during menopause was associated with decreased tissue insulin sensitivity and glucose tolerance (79). In a longitudinal study across the menopause transition, no changes in fasting glucose, fasting insulin, lipids, or insulin sensitivity were observed (3). However, a secondary analysis found that insulin sensitivity was reduced in women who gained the most abdominal adiposity (18). Although it was hypothesized that menopause status is associated with reduced insulin secretion and clearance (80), these associations are complicated by numerous factors that may be different between premenopausal and postmenopausal women. Increased BMI and abdominal adiposity, however, has always been strongly linked to insulin resistance and consequently, is associated with an increased risk for type 2 diabetes and CVD (81). Therefore, the increase in abdominal adiposity observed during the menopause transition is likely to be associated with insulin resistance and reduced glucose homeostasis.
Clinical studies involving exogenous E2 administration provide insight into the mechanistic effects of menopause on insulin sensitivity and glucose tolerance. E2 administration to postmenopausal women suggests a role for estrogens in maintaining glucose homeostasis through effects on insulin secretion and clearance (82). The risk of developing type 2 diabetes was significantly reduced in postmenopausal women who used hormone therapy in several randomized clinical trials, including the Women’s Health Initiative and Heart and Estrogen/Progestin Replacement Study (HERS) (83–85). Compared with transdermal estrogens, the effects of oral estrogens are difficult to interpret because of the influences of first-pass metabolism in the liver (discussed in greater detail in this publication (83)). One recent study found that a short, 8-week treatment of orally administered CEE and the selective estrogen receptor modulator (SERM), bazedoxifene, did not alter insulin sensitivity or ectopic fat in postmenopausal women with obesity (86). Still, the effects of transdermal E2 on glucose metabolism in postmenopausal women are conflicting. Although some studies of daily transdermal E2 reported reduction in insulin concentrations during oral glucose tolerance testing (OGTT) (87,88), others did not (89–91). C-peptide concentrations during an OGTT have also been shown to be increased (89,91) or decreased (90) in response to transdermal E2 therapy. One study found that acute administration (2.5-mg intravenous bolus) of conjugated estrogens increased insulin clearance and action in post-menopausal women during a constant rate of insulin infusion (92). However, a follow-up study revealed that 24-hour transdermal E2 administration did not alter insulin secretion or clearance in postmenopausal women, yet longer time since menopause did reveal a reduced effect of transdermal E2 to increase glucose uptake (93). It is likely that estrogen therapy has a beneficial effect on β-cell function and insulin secretion in postmenopausal women (83). Specifically, increased glucose-stimulated insulin secretion, as assessed by secretion of C-peptide and HOMA-β, and enhanced hepatic insulin clearance have been reported (94–96).
Although study findings do not currently support that menopausal hormone therapy consistently improves insulin sensitivity or glucose tolerance, the full extent of its potential risks and benefits needs further investigation. Importantly, the variability in treatments (estrogen only, estrogen plus progestins or SERM, etc.), treatment duration (weeks, months, or years), method of administration (oral vs. transdermal), and differences in metabolic status on administration (normal vs. obesity weight status, insulin resistant vs. type 2 diabetic, etc.) is important to consider when evaluating the effect of such therapies on metabolic health.
Dyslipidemia
Postmenopausal abdominal fat gain is associated with an adverse lipid profile (97). Dyslipidemia develops 10 to 15 years later in women than in men (98), presumably due to the protective effect of ovarian hormones (particularly E2). In SWAN, women had sharp increases in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein (Apo)B concentrations within a 1-year interval surrounding the FMP (99). Furthermore, the menopause-related increase in LDL-C was associated with greater risk of carotid plaque later in life in follow-up analysis (99). Postmenopausal women also experience elevated concentrations of LDL-C and triglycerides, which contribute to the atherosclerotic process (99). In contrast to epidemiological studies supporting that high-density lipoprotein cholesterol (HDL-C) is cardioprotective, a number of studies have reported higher HDL-C as a CVD risk factor after menopause (100). Future longitudinal studies are needed to further evaluate the effect of HDL-C on CVD across menopause. Studies have shown that oral estrogen therapy resulted in decreased LDL-C and increased HDL-C (101–103); however, this was at the expense of an increase in triglycerides (102).
Sleep disruption
Disruptions in sleep health also become more prevalent in women as they age and particularly around the menopause transition with the decline of E2 and progesterone. Sleep health is characterized by a number of sleep parameters that are affected by menopause, including changes in sleep duration, sleep times, awakenings, sleepiness, and sleep disorders (104). In SWAN, the prevalence of sleep disturbances ranged from 16% to 42% in premenopausal women and increased to 39% to 47% and 35% to 60% in perimenopausal and postmenopausal women, respectively (104). Vasomotor symptoms are associated with reduced sleep quality and increased waking, yet late perimenopausal and postmenopausal women still report more sleep difficulties compared with premenopausal women regardless of vasomotor symptom presence (99). Importantly, sleep disruption promotes increased energy intake (105) likely due to a combination of altered appetite hormones (i.e., decreased leptin and increased ghrelin, hunger, and appetite) (106), as well as altered sensitivity to food reward and disinhibited eating (105). Although sleep disruption is a well-recognized risk factor for metabolic abnormalities, including insulin resistance and glucose tolerance (107,108), the regulatory mechanisms linking sleep disruption and the changes in metabolic homeostasis that coincide with menopause have yet to be fully elucidated. Given the current breadth of literature, it is likely that women who experience sleep disruption during menopause may experience even greater weight and abdominal fat gain compared with women who do not.
Cardiovascular health
Postmenopausal women experience an increase in CVD risk that is primarily thought to be due to ovarian failure and the loss of E2 that occurs across the menopause transition. CVD is the leading cause of mortality in postmenopausal women. The CVD risk factors that become more prevalent after menopause include vascular dysfunction, hypertension, and cardiac dysfunction.
Vascular dysfunction and hypertension
Aging is associated with vascular dysfunction, featuring endothelial dysfunction and large elastic arterial stiffening. The vascular endothelium is a single-cell layer that lines arterial walls and becomes dysfunctional during the aging process due, in part, to reduced nitric oxide (NO) availability, secondary to increased oxidative stress and inflammation. Oxidative stress is characterized by an excessive production of reactive oxygen species that scavenge NO and subsequently impairs endothelial function and increases large elastic arterial stiffness (109). Vascular inflammation and increased reactive oxygen species can also impair endothelial nitric oxide synthase (eNOS), further reducing NO bioavailability and worsening endothelial function and arterial stiffening.
In women, vascular function appears to be preserved up until the menopause transition, after which vascular function progressively deteriorates, possibly due to a shift in redox balance and inflammatory status. In postmenopausal women, the loss of the antioxidant and anti-inflammatory effects of E2 are linked with a pro-oxidant and low-grade pro-inflammatory condition (110,111). Early observations noted that endothelial function, measured via brachial artery flow-mediated dilation (FMD), was preserved in women ≤40 years of age, decreased by 0.21% per year after age 40 years, and lowest in women ages 50+ years (by 0.49% per year) (112). A subsequent study found that the magnitude of difference in FMD between premenopausal women and late perimenopausal women was twice as great as the difference between premenopausal and early perimenopausal women (113); the greater difference was attributed to increased oxidative stress due to declines in E2 (114). In postmenopausal women, FMD was improved with E2 treatment, whereas FMD in placebo-treated women remained unchanged (115). Women with premature ovarian failure also had lower FMD compared with controls, which was rescued with oral CEE plus progestogen cyclic therapy (116). Oral CEE plus progestogen therapy did not improve FMD in postmenopausal women who already had coronary artery disease (117), in agreement with speculation that women who already have established coronary artery disease may not benefit from hormone therapy (118). Perimenopausal and postmenopausal women also exhibited greater arterial stiffness as indicated by an increase in pulse wave velocity (PWV) and reduced carotid artery compliance (inverse of stiffness) (119,120). However, adjusting for age eliminated the effect of menopausal status on arterial stiffness, suggesting that chronological aging may be more of a contributing factor to arterial stiffening than ovarian aging (121). This observation highlights the difficulty in separating the effects of chronological aging from the effects of ovarian aging and demonstrates the need for controlled interventions that can isolate the effects of age from female sex hormones.
Epidemiological data suggest that estrogen-based hormone therapy mitigates many risk factors for CVD (122), although the relative benefit of hormone therapy remains unclear and is the subject of ongoing debate. A current prevailing hypothesis is that postmenopausal women more than 10 years past menopause do not derive cardiovascular benefit from estrogen (122). The “timing hypothesis” posits that women who start menopausal hormone therapy within 10 years of their FMP derive protection from CVD, presumably because the intervention occurs before aging and lack of estrogen has resulted in too much vascular dysfunction to preclude the beneficial effects of estrogen (122). The “timing hypothesis” for menopausal hormone therapy may also apply to effects of estrogens on body composition and cardiometabolic risk factors. Menopausal hormone therapy has been shown to increase NO production, decrease inflammation, and increase vascular smooth muscle cell growth and proliferation (118). The Early versus Late Estrogen Trial (ELITE) demonstrated that, compared with placebo, women randomized to E2 who were less than 6 years past menopause had less progression of subclinical atherosclerosis as measured by carotid intimal-medial thickening (C-IMT), whereas there was no difference in atherosclerosis progression between the conditions in women who were 10 years past menopause (103). In contrast to ELITE, there were no changes in C-IMT or coronary artery calcium progression between early postmenopausal women (i.e., <6 years since menopause onset) randomized to estrogen or placebo in KEEPS. The reasons for the differential responses of C-IMT to estrogen may be related to the dose and type of estrogen used (i.e., 0.45 mg of conjugated equine estrogen and 0.05 ug/d of transdermal E2 in KEEPS vs. 1.0 mg/d of oral E2 in ELITE) and/or study population (103,123).
Endothelial dysfunction and arterial stiffness is also prognostic of hypertension in postmenopausal women (124). Age is a risk factor for hypertension as women experience increases in blood pressure as they get older. However, like vascular dysfunction, the age-related increases in blood pressure are more rapid in women, coinciding with the menopause transition (125). Pinpointing the mechanism behind the increase in blood pressure during this time is difficult because it is likely multifactorial; women gain weight and have increased incidence of metabolic syndrome, both of which increase the likelihood of hypertension and influence the response to traditional hypertensive treatments (124). Examining each of these facets is outside the scope of this review (more in depth review in (125)); however, estrogens are theorized to play a role.
Cardiac dysfunction
Concurrent with the shift in body composition, vascular dysfunction can contribute to pathophysiological changes to the heart that increase CVD risk. For example, arterial stiffening of the large elastic arteries can lead to increases in systolic and pulse pressures and aortic impedance (i.e., the resistance imposed on left ventricular [LV] ejection by the vasculature), consequently increasing the afterload and the amount of work performed by the LV to eject blood. These changes can contribute to LV hypertrophy, altered diastolic filling, and decrease LV systolic reserve (126). Evidence supporting a role for estrogens on LV hypertrophy induced by pressure overload come from studies of OVX mice that developed LV hypertrophy, which was rescued with E2 replacement (127,128). Furthermore, women in heart failure are more likely to have heart failure with preserved ejection fraction (HFpEF) than men, which often has better outcomes than heart failure with reduced ejection fraction (HFrEF) (129). Compared with men, women also have increased LV mass as they age and thus, have increased diastolic dysfunction (130,131). Nonetheless, the extent to which menopause per se affects heart failure prevalence is still unclear.
Although the role of exogenous estrogens or other hormones in maintaining cardiovascular health remains to be elucidated, what is not controversial is that women experience an increase in CVD risk factors around the time of menopause and should be monitored and counseled accordingly. It has been suggested that menopause represents an ideal time point to assess cardiovascular health and risk (132) and a perfect time to consider initiation of lifestyle interventions and careful monitoring of conventional risk factors for CVD including but not limited to cholesterol, diabetes, and hypertension.
LIFESTYLE INTERVENTIONS TO IMPROVE OVERALL HEALTH DURING MENOPAUSE
Current clinical guidelines do not recommend the use of menopausal hormone therapy for preventive indications; it is indicated only for the treatment of menopausal symptoms (133–135). Although menopausal hormone therapy has benefits—many of which (body composition, cardiovascular and metabolic health, and bioenergetics) have been reviewed here—not all women can or wish to take hormone therapy at the time of menopause transition. Even those who can take hormone therapy may experience a variety of side effects from different hormone treatment regimens. Introducing behavioral modifications that include diet (calorie restriction) and exercise (aerobic and/or resistance training) can help women limit weight gain and reduce cardiometabolic risk that arises during the menopause transition. Indeed, clinical trials provide ample evidence that diet and exercise—resistance or aerobic—can reduce body weight and notably, visceral fat mass accumulation, in many different populations. Even 5% weight loss in individuals with obesity has been shown to improve adipose tissue, liver and muscle insulin sensitivity, and β-cell function (136).
Despite the well-established evidence base that diet and exercise help limit weight gain, the vast majority of diet and exercise interventions have been conducted in postmenopausal women, and perimenopausal women have scarcely been studied (137). Longitudinal data from the SWAN study in women 47 to 57 years of age revealed an inverse association between percent body fat increased moderate or vigorous physical activity, particularly among White women (138). Similarly among postmenopausal women, there is a significant dose response for greater total body weight, fat, and intra-abdominal fat loss with increased exercise duration (139,140). Several studies addressed whether exercise or caloric restriction is more effective in reducing weight and targeting abdominal fat in postmenopausal women. The Sex Hormones and Physical Exercise (SHAPE)-2 study randomized postmenopausal women with overweight to either calorie restriction alone, calorie restriction plus exercise, or control (141). In brief, the SHAPE-2 exercise program combined both moderate-to-vigorous aerobic and resistance training 4 h/week (including two 1-hour fitness sessions plus 2 additional hours of walking), resulting in ~350 kcal/d exercise energy expenditure. Both intervention groups targeted 5 to 6 kg of body weight loss over a 10-to-14-week period and observed that subcutaneous fat loss was larger in the calorie restriction plus exercise group with no differences in intra-abdominal fat loss (141). In the Diet, Exercise, and Metabolism for Older Women Study, postmenopausal women with overweight were randomized to either a reduced calorie diet or a reduced calorie diet with aerobic exercise (3 d/week for 45-minutes at >85% heart rate reserve) for 6 months. Both groups lost similar amounts of weight, including similar subcutaneous abdominal and gluteal fat loss (142). Compared with premenopausal women, early postmenopausal women were equally capable of losing weight and reducing android fat when following a 3-month high-intensity exercise training program (3 d/week for 1 hour of instructor-led spinning/cycling intervals) (143). Another important study from the Women’s Healthy Lifestyle Project, a 4.5-year randomized clinical trial using long-term, dietary restriction (1,300 kcal/d) and increased physical activity (1,000 to 1,500 kcal/week of programmed moderate-intensity aerobic activity and additional activities of daily living) during peri- and post-menopause, demonstrated that waist circumference and fat mass were reduced, and fat-free mass was maintained (144). Subclinical markers of atherosclerosis, particularly C-IMT, were slowed with diet and exercise (145). Importantly, this long-term study demonstrated that continued behavioral modification among populations most susceptible to weight gain, such as perimenopausal women, is possible to implement.
A recent systematic review compared weight loss success across different lifestyle interventions and reported that postmenopausal women with obesity who were randomized to either diet or exercise modification alone had greater weight and fat mass loss compared with controls, whereas combining both diet and exercise resulted in the greatest weight and fat mass loss (137). Although only three of the aggregated studies had an exercise-only group as part of the lifestyle intervention, weight and fat mass loss appeared to be the greatest among women performing both aerobic and resistance training (146) versus aerobic or resistance training alone (147,148). Indeed, resistance training may help attenuate the loss in bone density that occurs with menopause, or the potential weight loss-associated bone loss with lifestyle interventions (149). In summary, initiating lifestyle modification programs that incorporate diet and exercise (aerobic and resistance training) for women during perimenopause may be timelier and have a higher yield in terms of reducing future risk of cardiometabolic than waiting until the postmenopausal years, after substantial weight gain and fat mass accrual have already occurred.
FUTURE DIRECTIONS AND CONSIDERATIONS
The menopause transition is accompanied by distinct changes in reproductive hormones, body composition, energy intake and expenditure, and other CVD risk factors. Intervention strategies that mitigate the negative effects that arise during the menopause transition are important to ensure that women maintain overall health as they age. First, behavioral (lifestyle) interventions targeting the perimenopausal period—possibly before detrimental changes in body composition and cardiometabolic health occur—are warranted. Few perimenopausal interventions exist that target weight loss (144,145), with most interventions focusing on the postmenopausal period (137). Second, given sleep disruption is one of the primary reasons why women seek medical care during menopause, future studies need to disentangle the degree to which sleep disruption (e.g., shortened sleep, interrupted sleep) alters individual behavior (i.e., energy intake and physical activity) and metabolism (i.e., whole-body and tissue-specific) across menopause and possibly exacerbates metabolic dysfunction and weight gain (107). Third, investigating racial and ethnic disparities in women across the menopause transition is also important and is lacking in the current literature. Black women have less visceral fat and intrahepatic lipid yet are paradoxically more insulin resistant compared with White women (150–152), suggesting that Black or non-White women are at greater risk of cardiometabolic dysfunction with menopause. Investigating racial and ethnic disparities in menopause-related health outcomes is particularly important in the quest to maximize the “health span” and not just the life-span of women. Finally, although research is currently limited, studies of how the female gut microbiome modifies energy balance, calorie absorption, and fat storage during menopause should also be undertaken (153,154).
The North American Menopause Society (NAMS) is a resource to assist medical professionals in the management of menopause and it provides a body of evidence-based recommendations for the clinical care of women across the menopause transition (17). These recommendations range from managing common body changes (particularly weight gain) and disease risk to complementary and alternative medicine and prescription-based treatment approaches. If weight loss is indicated (i.e., BMI ≥ 30kg/m2 or BMI ≥27 kg/m2 with a comorbidity), NAMS recommends combining caloric restriction alongside an intensive behavioral lifestyle intervention (≥14 in-person counseling sessions in 6 months) (17,155). Of course, the prescribed caloric deficit is not a one-size-fits-all dictum and is particularly difficult to prescribe because of changes in body composition and a declining metabolic rate. Instead, caloric deficit targets (via diet, exercise, or both) need to be individualized to weight loss goals, current body composition, and health status. Furthermore, because women experience many changes in cardiometabolic health during the menopause transition, caring for women on an individual level is increasingly important—lifestyle changes may be best for some, whereas pharmacotherapy or pharmacogenomic analysis might be better for others. Readiness for initiating changes in lifestyle or treatment strategies should be considered.
Researchers looking to incorporate perimenopausal women into their investigations should consider:
Characterizing women by menopause stage (11), including menstrual cycle tracking and measurement of sex steroids, FSH concentrations, and AMH;
Documenting FMP to help guide menopause staging;
Conducting a thorough review of medical history, including past surgeries (e.g., cesarean delivery, bariatric, hysterectomy, oophorectomy);
Documenting sleep health, including relevant sleep parameters such as sleep duration, sleep times, awakenings, sleepiness, and specific sleep disorder symptoms;
Documenting all medications, including current, recent, or past use of menopausal hormone therapy (or determining if these medications are deemed exclusion criteria) and medications used to treat menopause symptoms (e.g., depression, vasomotor symptoms, sleep disturbances);
How the research findings will delineate the effect of aging from any changes in sex steroids or gonadotropins associated with menopause.O
Study Importance.
Study Importance What is already known?
The menopause transition (i.e., perimenopause) is characterized by changes in hormones and body composition that increase overall cardiometabolic risk.
What does this review add?
This review summarizes the changes in cardiometabolic health that occur during the menopause transition because of changes in hormones and body composition.
This review provides considerations when incorporating perimenopausal women into clinical research studies.
How might these results change the direction of research?
Researchers should consider including perimenopausal women in their future investigations to ensure adequate representation of women across the life-span.
Research in perimenopausal women must properly characterize women by menopause stage, document current sleep health, and document current or past surgeries or medications used to treat menopausal symptoms.
Funding information
KL Marlatt is supported by U54 GM104940. DRPM is supported by T32 AG000279. KMG is supported by K01 DK109053 and the Boettcher Foundation. KL Moreau is supported by AG049762, HL146558,and HL136601. ELM is supported by R01 DK112260, U54 AG062319, P30 DK048520,and UL1 TR002535. NS is supported by R01 HD100343, R01 HD087314, R25 HD075737, and R13 AG069384. WMK is supported R01 DK112260, U54 AG062319, U01 AR071124, P30 DK048520, and the VA Eastern Colorado Geriatric Research, Education, and Clinical Center.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 3.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Load). 2008;32(6):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric. 2004;7(4):375–389. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring). 2010;18(3):604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace RB, Sherman BM, Bean JA, Treloar AE, Schlabaugh L. Probability of menopause with increasing duration of amenorrhea in middle-aged women. Am J Obstet Gynecol. 1979;135(8):1021–1024. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. [DOI] [PubMed] [Google Scholar]
- 8.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harlow SD. Menstrual cycle changes as women approach the final menses: what matters? Obstet Gynecol Clin North Am. 2018;45(4):599–611. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N Research on the mechanisms of premature ovarian failure. J Soc Gynecol Investig. 2001;8(suppl 1):S10–S12. [DOI] [PubMed] [Google Scholar]
- 11.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15(4):603–612. [DOI] [PubMed] [Google Scholar]
- 13.Aydogan B, Mirkin S. The utility of measuring anti-Mullerian hormone in predicting menopause. Climacteric. 2015;18(6):777–789. [DOI] [PubMed] [Google Scholar]
- 14.Sowers MFR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertone-Johnson ER, Manson JE, Purdue-Smithe AC, et al. Anti-Müllerian hormone levels and incidence of early natural menopause in a prospective study. Hum Reprod. 2018;33(6):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001;4(4):267–272. [PubMed] [Google Scholar]
- 17.Shifren JL, Gass MLS, Kagan R, et al. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21(10):1038–1062. [DOI] [PubMed] [Google Scholar]
- 18.Marlatt KL, Redman LM, Beyl RA et al. Racial differences in body composition and cardiometabolic risk during the menopause transition: a prospective, observational cohort study. Am J Obstet Gynecol. 2020;222(4):365.e1–365.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurston RC, Sowers MR, Sutton-Tyrrell K, et al. Abdominal adiposity and hot flashes among midlife women. Menopause. 2008;15(3):429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women’s health across the nation. Am J Epidemiol. 2009;170(6):766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones GL, Sutton A. Quality of life in obese postmenopausal women. Menopause Int. 2008;14(1):26–32. [DOI] [PubMed] [Google Scholar]
- 22.Wadden T, Butryn M, Sarwer D, et al. Comparison of psychosocial status in treatment-seeking women with class III vs. class I-II obesity. Surg Obes Relat Dis. 2006;2(2):138–145. [DOI] [PubMed] [Google Scholar]
- 23.Wohlers LM, Spangenburg EE. 17β-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Biochem. 2010;110(2):420–427. [DOI] [PubMed] [Google Scholar]
- 24.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229(11):1127–1135. [DOI] [PubMed] [Google Scholar]
- 25.Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51(7):861–870. [DOI] [PubMed] [Google Scholar]
- 26.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166(3):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camporez JPG, Jornayvaz FR, Lee H-Y, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154(3):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers NH, Li JWP, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira JA, Foley AM, Brown M. Sex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male rats. Eur J Appl Physiol. 2012;112(8):3007–3018. [DOI] [PubMed] [Google Scholar]
- 30.Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39(2):248–256. [DOI] [PubMed] [Google Scholar]
- 31.Hertrampf T, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. Analysis of the effects of oestrogen receptor a (ERα)- and ERβ-selective ligands given in combination to ovariectomized rats. Br J Pharmacol. 2008;153(7):1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigt C, Hertrampf T, Zoth N, Fritzemeier KH, Diel P. Impact of estradiol, ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity. Mol Cell Endocrinol. 2012;351(2):227–238. [DOI] [PubMed] [Google Scholar]
- 33.Frank A, Brown LM, Clegg DJ. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol. 2014;35(4):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640–645. [DOI] [PubMed] [Google Scholar]
- 35.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Nedungadi T, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma G, Prossnitz ER. G-protein-coupled estrogen receptor (GPER) and sex-specific metabolic homeostasis. Adv Exp Med Biol. 2017;1043:427–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas E, Bhattacharya I, Brailoiu E, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104(3):288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mårtensson UEA, Salehi SA, Windahl S, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. [DOI] [PubMed] [Google Scholar]
- 40.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154(11):4136–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greendale GA, Sternfeld B, Huang M et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4(5):e124865. doi: 10.1172/jci.insight.124865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquali R, Casimirri LF, Labate AMM, et al. Body weight, fat distribution and the menopausal status in women. The VMH Collaborative Group. Int J Obes Relat Metab Disord. 1994;18(9):614–621. [PubMed] [Google Scholar]
- 44.Matthews KA, Abrams B, Crawford S, et al. Body mass index in mid-life women: Relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord. 2001;25(6):863–873. [DOI] [PubMed] [Google Scholar]
- 45.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med. 2010;28(5):426–434. [DOI] [PubMed] [Google Scholar]
- 46.Duval K, Prud’homme D, Rabasa-Lhoret R, et al. Effects of the menopausal transition on energy expenditure: a MONET Group Study. Eur J Clin Nutr. 2013;67(4):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greendale GA, Sowers MF, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Recker R, Lappe J, Davies K, Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000;15(10):1965–1973. [DOI] [PubMed] [Google Scholar]
- 49.Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and β-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90(6):3312–3317. [DOI] [PubMed] [Google Scholar]
- 50.Melanson EL, Gavin KM, Shea KL, et al. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol. 2015;119(9):975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shea KL, Gavin KM, Melanson EL, et al. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015;22(10):1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Froidevaux F, Schutz Y, Christin L, Jéquier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57(1):35–42. [DOI] [PubMed] [Google Scholar]
- 53.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–472. [DOI] [PubMed] [Google Scholar]
- 54.Zurlo F, Lillioja S, Puente AED, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol Endocrinol Metab. 1990;259(5):E650–E657. [DOI] [PubMed] [Google Scholar]
- 55.Hill RJ, Davies PSW. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85(4):415–430. [DOI] [PubMed] [Google Scholar]
- 56.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
- 57.Sun Y, Liu B, Snetselaar LG, et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open. 2019;2:e197337. doi: 10.1001/jamanetworkopen.2019.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Espeland MA, Stefanick ML, Kritz-Silverstein D, et al. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. J Clin Endocrinol Metab. 1997;82(5):1549–1556. [DOI] [PubMed] [Google Scholar]
- 59.Jensen LB, Vestergaard P, Hermann AP, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish osteoporosis prevention study. J Bone Miner Res. 2003;18(2):333–342. [DOI] [PubMed] [Google Scholar]
- 60.Cintron D, Beckman JP, Bailey KR, Lahr BD, Jayachandran M, Miller VM. Plasma orexin A levels in recently menopausal women during and 3 years following use of hormone therapy. Maturitas. 2017;99:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Douchi T, Kuwahata R, Yamasaki H, et al. Inverse relationship between the changes in trunk lean and fat mass during gonadotropin-releasing hormone agonist therapy. Maturitas. 2002;42(1):31–35. [DOI] [PubMed] [Google Scholar]
- 62.Douchi T, Kuwahata T, Yoshimitsu N, Iwamoto I, Yamasaki H, Nagata Y. Changes in serum leptin levels during GnRH agonist therapy. Endocr J. 2003;50(3):355–359. [DOI] [PubMed] [Google Scholar]
- 63.Yamasaki H, Douchi T, Yamamoto S, Toshimichi O, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet Gynecol. 2001;97(3):338–342. [DOI] [PubMed] [Google Scholar]
- 64.Santosa S, Bonnes SL, Jensen MD. Acute female hypogonadism alters adipose tissue fatty acid storage factors and chylomicronemia. J Clin Endocrinol Metab. 2016;101(5):2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Revilla R, Revilla M, Villa LF, Cortés J, Arribas I, Rico H. Changes in body composition in women treated with gonadotropin-releasing hormone agonists. Maturitas. 1998;31(1):63–68. [DOI] [PubMed] [Google Scholar]
- 66.Nowicki M, Adamkiewicz G, Bryc W, Kokot F. The influence of luteinizing hormone-releasing hormone analog on serum leptin and body composition in women with solitary uterine myoma. Am J Obstet Gynecol. 2002;186(3):340–344. [DOI] [PubMed] [Google Scholar]
- 67.Banack HR, Wactawski-Wende J, Hovey KM, Stokes A. Is BMI a valid measure of obesity in postmenopausal women? Menopause. 2018;25(3):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blew RM, Sardinha LB, Milliken LA, et al. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res. 2002;10(8):799–808. [DOI] [PubMed] [Google Scholar]
- 69.Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48(11):2213–2220. [DOI] [PubMed] [Google Scholar]
- 70.Bruns CM, Kemnitz JW. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J. 2004;45(2):160–169. [DOI] [PubMed] [Google Scholar]
- 71.Muscelli E, Kozakova M, Flyvbjerg A, et al. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. Am J Hypertens. 2009;22(4): 364–370. [DOI] [PubMed] [Google Scholar]
- 72.Toth MJ, Sites CK, Eltabbakh GH, Poehlman ET. Effect of menopausal status on insulin-stimulated glucose disposal: comparison of middle-aged premenopausal and early postmenopausal women. Diabetes Care. 2000;23(6):801–806. [DOI] [PubMed] [Google Scholar]
- 73.Sites CK, Toth MJ, Cushman M, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002;77(1):128–135. [DOI] [PubMed] [Google Scholar]
- 74.Monterrosa-Castro A, Blümel JE, Portela-Buelvas K, et al. Type II diabetes mellitus and menopause: a multinational study. Climacteric. 2013;16(6):663–672. [DOI] [PubMed] [Google Scholar]
- 75.Soriguer F, Morcillo S, Hernando V, et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause. 2009;16(4):817–821. [DOI] [PubMed] [Google Scholar]
- 76.Mishra GD, Carrigan G, Brown WJ, Barnett AG, Dobson AJ. Short-term weight change and the incidence of diabetes in midlife: results from the Australian longitudinal study on women’s health. Diabetes Care. 2007;30(6):1418–1424. [DOI] [PubMed] [Google Scholar]
- 77.Kim C, Edelstein SL, Crandall JP, et al. Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause. 2011;18(8):857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: The Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168(14): 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sites CK, Calles-Escandon J, Brochu M, Butterfield M, Ashikaga T, Poehlman ET. Relation of regional fat distribution to insulin sensitivity in postmenopausal women. Fertil Steril. 2000;73(l):61–65. [DOI] [PubMed] [Google Scholar]
- 80.Walton C, Godsland IF, Proudler AJ, Wynn V, Stevenson JC. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur J Clin Invest. 1993;23(8):466–473. [DOI] [PubMed] [Google Scholar]
- 81.Westphal SA. Obesity, abdominal obesity, and insulin resistance. Clin Cornerstone. 2008;9(1):23–31. [DOI] [PubMed] [Google Scholar]
- 82.Manassiev NA, Godsland IF, Crook D, Proudler AJ, Whitehead Ml, Stevenson JC. Effect of postmenopausal oestradiol and dydrogesterone therapy on lipoproteins and insulin sensitivity, secretion and elimination in hysterectomised women. Maturitas. 2002;42(3):233–242. [DOI] [PubMed] [Google Scholar]
- 83.Mauvais-Jarvis F, Manson JAE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the heart and estrogen/progestin replacement study: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. [DOI] [PubMed] [Google Scholar]
- 85.Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. [DOI] [PubMed] [Google Scholar]
- 86.Marlatt KL, Lovre D, Beyl RA, et al. Effect of conjugated estrogens and bazedoxifene on glucose, energy and lipid metabolism in obese postmenopausal women. Eur J Endocrinol. 2020;183(4):439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB. Effects of low doses of transdermal 17 β-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab. 1992;74(6):1396–1400. [DOI] [PubMed] [Google Scholar]
- 88.Cagnacci A, Tuveri F, Cirillo R, Setteneri AM, Melis GB, Volpe A. The effect of transdermal 17-β-estradiol on glucose metabolism of postmenopausal women is evident during the oral but not the intravenous glucose administration. Maturitas. 1997;28(2):163–167. [DOI] [PubMed] [Google Scholar]
- 89.Cucinelli F, Paparella P, Soranna L, et al. Differential effect of transdermal estrogen plus progestagen replacement therapy on insulin metabolism in postmenopausal women: Relation to their insulinemic secretion. Eur J Endocrinol. 1999;140(3):215–223. [DOI] [PubMed] [Google Scholar]
- 90.Karjalainen A, Paassilta M, Heikkinen J, Bäckström AC, Savolainen M, Kesäniemi YA. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrinol (Oxf). 2001;54(2):165–173. [DOI] [PubMed] [Google Scholar]
- 91.Paoletti AM, Pilloni M, Orrù M, et al. Efficacy and safety of oral and transdermal hormonal replacement treatment containing levonorgestrel. Maturitas. 2002;42(2):137–147. [DOI] [PubMed] [Google Scholar]
- 92.Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285(2):E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Pelt RE, Schwartz RS, Kohrt WM. Insulin secretion and clearance after subacute estradiol administration in postmenopausal women. J Clin Endocrinol Metab. 2008;93:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Godsland IF, Gangar K, Walton C, et al. Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy. Metabolism. 1993;42:846–853. [DOI] [PubMed] [Google Scholar]
- 95.Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead MI, Stevenson JC. Effects of oral and transdermal 17β-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion, and elimination in postmenopausal women. Metabolism. 2000;49:742–747. [DOI] [PubMed] [Google Scholar]
- 96.Lovre D, Peacock E, Katalenich B, et al. Conjugated estrogens and bazedoxifene improve β cell function in obese menopausal women. J Endocr Soc. 2019;3:1583–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franklin RM, Ploutz-Snyder L, Kanaley JA. Longitudinal changes in abdominal fat distribution with menopause. Metabolism. 2009;58(3):311–315. [DOI] [PubMed] [Google Scholar]
- 98.Sutton-Tyrrell K, Lassila HC, Meilahn E, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29(6):1116–1121. [DOI] [PubMed] [Google Scholar]
- 99.El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health across the Nation (SWAN). Menopause. 2019;26(10):1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El Khoudary SR. HDL and the menopause. Curr Opin Lipidol. 2017;28(4):328–336. [DOI] [PubMed] [Google Scholar]
- 101.Maclaran K, Stevenson JC. Primary prevention of cardiovascular disease with HRT. Womens Health (Lond). 2012;8(1):63–74. [DOI] [PubMed] [Google Scholar]
- 102.Mebane Sims IL. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273(13):199–208. [PubMed] [Google Scholar]
- 103.Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kravitz HM, Joffe H. Sleep during the perimenopause: A SWAN Story. Obstet Gynecol Clin North Am. 2011;38(3):567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014;134:86–91. [DOI] [PubMed] [Google Scholar]
- 106.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. [DOI] [PubMed] [Google Scholar]
- 107.Arble DM, Bass J, Behn CD, et al. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep. 2015;38(12):1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Cauter E Sleep disturbances and insulin resistance. Diabet Med. 2011;28(12):1455–1462. [DOI] [PubMed] [Google Scholar]
- 109.Moreau KL. Intersection between gonadal function and vascular aging in women. J Appl Physiol. 2018;125(6):1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91(11):5212–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abu-Taha M, Rius C, Hermenegildo C, et al. Menopause and ovariectomy cause a low grade of systemic onflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol. 2009;183(2):1393–1402. [DOI] [PubMed] [Google Scholar]
- 112.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. [DOI] [PubMed] [Google Scholar]
- 113.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45(6):1107–1112. [DOI] [PubMed] [Google Scholar]
- 114.Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. GeroScience. 2020;42(6):1699–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98(11):4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kalantaridou SN, Naka KK, Papanikolaou E, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89(8):3907–3913. [DOI] [PubMed] [Google Scholar]
- 117.Kelemen M, Vaidya D, Waters DD, et al. Hormone therapy and antioxidant vitamins do not improve endothelial vasodilator function in postmenopausal women with established coronary artery disease: a substudy of the Women’s Angiographic Vitamin and Estrogen (WAVE) trial. Atherosclerosis. 2005;179(1):193–200. [DOI] [PubMed] [Google Scholar]
- 118.Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Rev Recent Clin Trials. 2012;7(1):47–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19(12):2205–2212. [DOI] [PubMed] [Google Scholar]
- 120.Staessen JA, van der Heijden-Spek JJ, Safar ME, et al. Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens. 2001;15(8):511–518. [DOI] [PubMed] [Google Scholar]
- 121.O’Neill SM, Liu J, O’Rourke MF, Khoo SK. The menopausal transition does not appear to accelerate age-related increases in arterial stiffness. Climacteric. 2013;16(1):62–69. [DOI] [PubMed] [Google Scholar]
- 122.Lobo RA, Pickar JH, Stevenson JC, Mack WJ, Hodis HN. Back to the future: hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis. 2016;254:282–290. [DOI] [PubMed] [Google Scholar]
- 123.Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161(4):249–260. [DOI] [PubMed] [Google Scholar]
- 124.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51(10):997–1002. [DOI] [PubMed] [Google Scholar]
- 125.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24(7):740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–354. [DOI] [PubMed] [Google Scholar]
- 127.Van Eickels M, Grohé C, Cleutjens JPM, Janssen BJ, Wellens HJJ, Doevendans PA. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104(12):1419–1423. [DOI] [PubMed] [Google Scholar]
- 128.Patten RD, Pourati I, Aronovitz MJ, et al. 17 beta-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail. 2008;14(3):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lam CSP, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Regitz-Zagrosek V Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5(5):425–439. [DOI] [PubMed] [Google Scholar]
- 131.Lieb W, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119(24):3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Collins P, Webb CM, de Villiers TJ, Stevenson JC, Panay N, Baber RJ. Cardiovascular risk assessment in women – an update. Climacteric. 2016;19(4):329–336. [DOI] [PubMed] [Google Scholar]
- 133.Pinkerton JAV, Aguirre FS, Blake J, et al. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24(7):728–753. [DOI] [PubMed] [Google Scholar]
- 134.ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216. [DOI] [PubMed] [Google Scholar]
- 135.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. [DOI] [PubMed] [Google Scholar]
- 136.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng C-C, Hsu C-Y, Liu J-F. Effects of dietary and exercise intervention on weight loss and body composition in obese postmenopausal women. Menopause. 2018;25:772–782. [DOI] [PubMed] [Google Scholar]
- 138.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc. 2005;37(7):1195–1202. [DOI] [PubMed] [Google Scholar]
- 139.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–330. [DOI] [PubMed] [Google Scholar]
- 140.Dieli-Conwright CM, Parmentier J-H, Sami N, et al. Adipose tissue inflammation in breast cancer survivors: effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res Treat. 2018;168(1):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Gemert WA, Peeters PH, May AM, et al. Effect of diet with or without exercise on abdominal fat in postmenopausal women - a randomised trial. BMC Public Health. 2019;19:174. doi: 10.1186/S12889-019-6510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serra MC, Blumenthal JB, Addison OR, Miller AJ, Goldberg AP, Ryan AS. Effects of weight loss with and without exercise on regional body fat distribution in postmenopausal women. Ann Nutr Metab. 2017;70(4):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mandrup CM, Egelund J, Nyberg M, et al. Effects of high-intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am J Obstet Gynecol. 2017;216(4):384.e1–384.e11. doi: 10.1016/j.ajog.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 144.Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26(3):212–220. [DOI] [PubMed] [Google Scholar]
- 145.Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44(3):579–585. [DOI] [PubMed] [Google Scholar]
- 146.van Gemert WAM, van der Palen J, Monninkhof EM, et al. Quality of life after diet or exercise-induced weight loss in overweight to obese postmenopausal women: the SHAPE-2 randomised controlled trial. PLoS One. 2015;10(6):e0127520. doi: 10.1371/journal.pone.0127520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012;20(8):1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16(1):66–72. [DOI] [PubMed] [Google Scholar]
- 149.Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): a meta-analysis. Bone. 2021;143:115697. doi: 10.1016/j.bone.2020.115697 [DOI] [PubMed] [Google Scholar]
- 150.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11(8):755–762. [DOI] [PubMed] [Google Scholar]
- 152.Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in β-cell function. Diabetes Care. 2000;23(9):1353–1358. [DOI] [PubMed] [Google Scholar]
- 153.Schreurs MPH, Van SPJDV, Romano A, Dieleman S, Werner HMJ. How the gut microbiome links to menopause and obesity, with possible implications for endometrial cancer development. J Clin Med. 2021;10(13):2916. doi: 10.3390/jcm10132916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions—but do we have the will? Fertil Steril. 2017;107(4):833–839. [DOI] [PubMed] [Google Scholar]
- 155.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]


