Abstract
Non-invasive brain stimulation (NIBS), including transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS), is a potentially effective treatment strategy for a number of mental conditions. However, no quantitative evidence synthesis of randomized controlled trials (RCTs) of TMS or tDCS using the same criteria including several mental conditions is available. Based on 208 RCTs identified in a systematic review, we conducted a series of random effects meta-analyses to assess the efficacy of NIBS, compared to sham, for core symptoms and cognitive functioning within a broad range of mental conditions. Outcomes included changes in core symptom severity and cognitive functioning from pre- to post-treatment. We found significant positive effects for several outcomes without significant heterogeneity including TMS for symptoms of generalized anxiety disorder (SMD = −1.8 (95% CI: −2.6 to −1), and tDCS for symptoms of substance use disorder (−0.73, −1.00 to −0.46). There was also significant effects for TMS in obsessive-compulsive disorder (−0.66, −0.91 to −0.41) and unipolar depression symptoms (−0.60, −0.78 to −0.42) but with significant heterogeneity. However, subgroup analyses based on stimulation site and number of treatment sessions revealed evidence of positive effects, without significant heterogeneity, for specific TMS stimulation protocols. For neurocognitive outcomes, there was only significant evidence, without significant heterogeneity, for tDCS for improving attention (−0.3, −0.55 to −0.05) and working memory (−0.38, −0.74 to −0.03) in individuals with schizophrenia. We concluded that TMS and tDCS can benefit individuals with a variety of mental conditions, significantly improving clinical dimensions, including cognitive deficits in schizophrenia which are poorly responsive to pharmacotherapy.
Subject terms: Addiction, ADHD, Depression, Schizophrenia, Psychiatric disorders
Introduction
Mental ill-health affects more than 1 billion people globally and causes ~19% of years lived with disability [1], with numbers rising following the outbreak of Covid-19 [2–4]. Non-invasive brain stimulation (NIBS) has been proposed as an intervention strategy for mental disorders. NIBS has immediate effects on neural excitability but also after effects [5], which makes it a potentially suitable therapeutic tool for mental disorders. NIBS encompasses transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). During TMS, a brief electrical current flows through a wire coil, creating a magnet field that passes through the skull and induces a current on the surface of the cortex, depolarizing neurons or their axons [6]. This leads to alterations in the activation patterns of neural populations and can be most effectively achieved using repetitive TMS (rTMS) or theta-burst stimulation (TBS). tDCS is another non-invasive neurostimulation method that uses direct electrical currents to stimulate a targeted cortical area. The neurobiological basis for the longer lasting effects of tDCS is similar to the one found in TMS [7–9], and likely involves inducing long-term potentiation (LTP)-like plasticity [10, 11].
As many mental disorders are associated with imbalances in excitability [12, 13], NIBS is a potentially effective treatment strategy for a number of mental conditons. While meta-analyses of randomized controlled trials (RCTs) of TMS or tDCS for individual mental disorders are available, to date no meta-analytic synthesis using the same criteria across a large number of mental disorders has been published. Therefore, we conducted a series of meta-analyses of RCTs of TMS and/or tDCS using the same criteria across a broad range of mental conditions. Additionally, one key issue in assessing the effectiveness of NIBS is that their size and durability depends on the stimulation site, frequency, intensity, the number of stimulation sessions, and the shape of the magnetic pulse [14]. Therefore, we conducted additional analyses according to stimulation site, frequency, and number of stimulation sessions.
Methods
The study was registered with PROSPERO (number CRD42021250057), and followed PRISMA guidelines [15].
Search strategy and selection process
PubMed, OVID, and Web of Knowledge databases were systematically searched, from inception until April 26th 2021, with no language/document type restrictions. We used the Pubmed search syntax “(random*) AND (“TMS” OR rTMS OR tDCS OR TMS)” combined with a list of ICD-11 mental health conditions, adapted for each database (see Supplementary Materials for full list of search terms). References of each relevant retrieved meta-analysis were hand-searched for additional eligible studies. All reports were screened for eligibility by two independent screeners. Conflicts were resolved by discussion with a senior author.
Inclusion criteria
Studies were included if they met the following criteria: (1) randomized, sham-controlled trials using TMS and/or tDCS, (2) including children and/or adults with a primary diagnosis of a mental health condition using standardized diagnostic criteria (DSM-III/IV/5, ICD-9/10/11 or based on other standardized diagnostic tools), and (3) using standardized scales assessing core symptom severity and/or tasks measuring cognitive functioning (executive function, attention/vigilance, processing speed, and working memory).
Studies were excluded if: (1) data for core symptom severity or cognitive functioning were unavailable, (2) patients were in remission, (3) there was another concomitant intervention (e.g., pharmacotherapy/cognitive training), and (4) a crossover design was used and data for the first phase was not available.
Outcomes
Change in core symptom severity in each mental disorder was the primary outcome. Secondary outcomes were score changes in standard cognitive functioning tasks. Change was defined as the difference in scores between baseline and after the last treatment session. Four neurocognitive domains (attention/vigilance, executive functioning, processing speed, and working memory) were chosen, and associated tasks/constructs were defined based on guidelines from the MATRICS cognitive test battery for schizophrenia [16], as in previous analyses of neurostimulation for cognitive enhancement [17, 18]. Data for follow-up assessments were beyond the scope of the present paper.
Data extraction
Data were extracted by JH and independently checked by HC, RS, and VP Where outcome data were not available, corresponding authors were systematically contacted. Wherever available, data were extracted as baseline and endpoint means and standard deviations, or mean change scores and standard deviations. Where continuous outcome data were not available, response rates (≥50% score improvement) were extracted and pooled separately. Other extracted information included: participant demographics/baseline characteristics and intervention parameters, i.e., stimulation site, intensity/frequency, and number of sessions.
Risk of bias assessment
Risk of bias was assessed independently by two investigators with the Cochrane risk of bias for randomized trials version 2 (RoB2) assessment tool [19]. Items include whether the allocation sequence was random, whether participants or experimenters were aware of their assigned intervention, whether an appropriate analysis was planned and used, and whether the results may have been biased by missing data.
Statistical analysis
All analyses were conducted using Comprehensive Meta-Analysis software, version 3 [20], when two or more eligible TMS/tDCS RCTs on the same outcome were available. Data were grouped by disorder, stimulation technique (TMS/tDCS), and outcome (symptoms/cognitive domain); and pooled using random effects models based on standardized mean difference (SMD). SMD values of 0.2–0.5 were considered small, values of 0.5–0.8 medium, and values >0.8 were considered large, according to the commonly reported thresholds by Cohen (even though Cohen himself urged caution in this interpretation) [21]. Heterogeneity was assessed with Cochran’s Q test and the I2 statistic, which estimate the presence of significant heterogeneity, and the proportion of total variability due to between-study heterogeneity, respectively. Publication bias was assessed visually via funnel plots and quantitatively with the Egger’s test where at least ten studies were available. To examine sources of heterogeneity in core symptom severity outcomes among TMS trials, subgroup analyses were conducted based on: (a) stimulation technique, (b) stimulation site, (c) stimulation frequency, and (d) number of sessions. For subgroup analyses, rTMS at frequencies ≤1 Hz was defined as low frequency (LF), and rTMS at frequencies ≥5 Hz was defined as high frequency (HF). Where applicable, cTBS was grouped with LF rTMS and iTBS was grouped with HF rTMS due to the small number of available TBS trials. Sensitivity analyses were conducted excluding studies with a risk of bias assessment rated as “high”.
Post hoc analyses and changes to the pre-registred protocol are reported in the Supplementary Materials.
Results
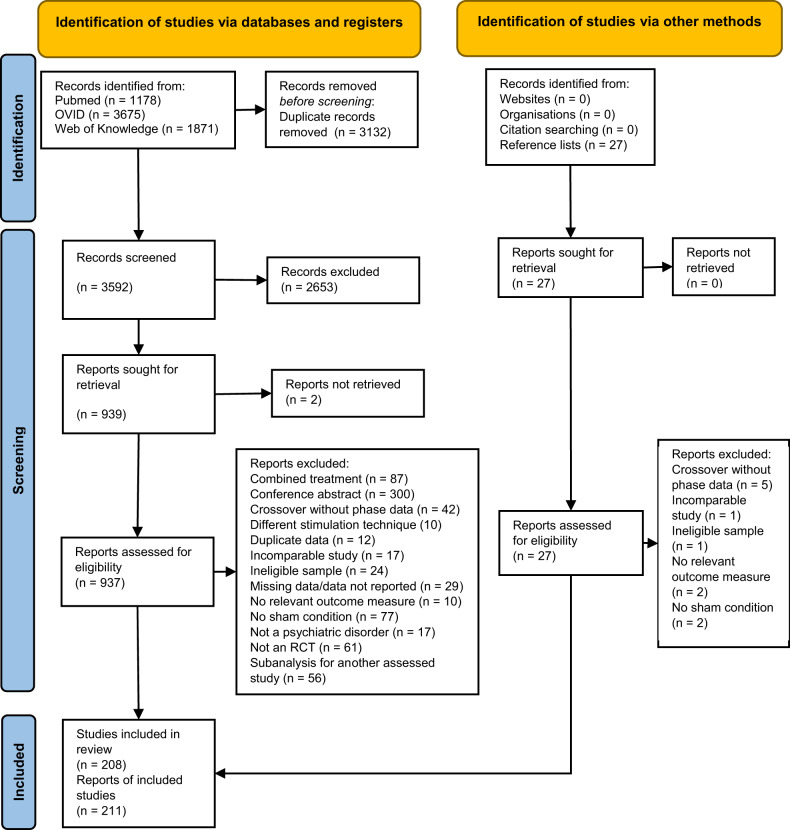
The systematic search yielded 3592 references from databases and 27 articles from bibliographies. After screening, 208 RCTs reported in 211 articles were included (Fig. 1; study characteristics in Supplementary Table 1). The majority investigated current depressive episodes in patients with major depressive disorder (MDD) or bipolar disorder (n = 99), and schizophrenia or schizoaffective disorder (n = 59), followed by obsessive-compulsive disorder (OCD, n = 27), substance use disorder (SUD, n = 10), posttraumatic stress disorder (PTSD, n = 8), generalized anxiety disorder (GAD, n = 5), attention-deficit/hyperactivity disorder (ADHD, n = 2), and tourettes/tic disorders (n = 2). For schizophrenia, data were available to assess the efficacy on positive, negative, and total core symptoms, as well as auditory hallucinations. The full list of included and excluded references (with reasons) are reported in the Supplementary Materials.
Fig. 1. PRISMA flow diagram.
The chart illustrates literature search process and how many studies were excluded at each stage.
Risk of bias
Around 23% RCTs were considered overall high risk of bias, most commonly due to inappropriate analysis (15%) and/or reporting of missing data (16%). Overall, the risk of bias was typically of some concerns (69%), or low (10%; Supplementary Table 2).
Meta-analyses results-efficacy on core symptoms-continuous outcomes
Active TMS was significantly superior to sham for the treatment of symptoms of depression, GAD, OCD, PTSD, total symptoms, negative symptoms and auditory hallucinations in schizophrenia but not for symptoms of ADHD, SUD, and overall positive symptoms in schizophrenia (Table 1; Supplementary Figs. 1–12, Funnel plots in Supplementary Figs. 13–20). Regarding tDCS, active stimulation was significantly bettter than sham for symptoms of depression, SUD, total, negative symptoms and auditory hallucinations in schizophrenia but not for symptoms of GAD, OCD, and overall positive symptoms in schizophrenia (Table 1; Supplementary Figs. 21–28).
Table 1.
Summary of the meta-analyses results: core symptoms severity—continuous outcomes.
| Heterogeneity | Egger’s test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | N | SMD (95% CI) | Z | p values | Q | p values | I2 | t | P values | |
| Core symptom severity | ||||||||||
| TMS | ||||||||||
| ADHD | 2 | 51 | −0.50 (−1.33 to 0.33) | 1.18 | 0.237 | 2.11 | 0.146 | 52 | ||
| Depression | 76 | 3366 | −0.45 (−0.57 to −0.33) | 7.16 | <0.001 | 197.91 | <0.001 | 62 | 1.95 | 0.055 |
| Unipolar | 42 | 2336 | −0.60 (−0.78 to −0.42) | 6.45 | <0.001 | 154.91 | <0.001 | 74 | 2.85 | 0.007 |
| Bipolar | 4 | 145 | −0.20 (−0.52 to 0.11) | 1.26 | 0.209 | 1.84 | 0.606 | 0 | ||
| GAD | 3 | 111 | −1.80 (−2.60 to −1.00) | 4.40 | <0.001 | 5.37 | 0.068 | 63 | ||
| OCD | 26 | 760 | −0.66 (−0.91 to −0.41) | 5.10 | <0.001 | 72.18 | <0.001 | 65 | 3.31 | 0.003 |
| PTSD | 10 | 255 | −1.09 (−1.61 to −0.57) | 4.10 | <0.001 | 42.44 | <0.001 | 79 | 0.59 | 0.572 |
| Schizophrenia | ||||||||||
| Positive symptoms | 33 | 1474 | −0.11 (−0.33 to 0.11) | 0.96 | 0.338 | 153.20 | <0.001 | 77 | 2.27 | 0.029 |
| Negative symptoms | 31 | 1266 | −0.49 (−0.73 to −0.26) | 4.07 | <0.001 | 133.98 | <0.001 | 78 | 2.45 | 0.020 |
| Total symptoms | 29 | 1334 | −0.50 (−0.66 to −0.33) | 5.81 | <0.001 | 58.67 | <0.001 | 52 | 2.42 | 0.022 |
| Auditory hallucinations | 16 | 545 | −0.19 (−0.36 to −0.02) | 2.19 | 0.029 | 12.62 | 0.632 | 0 | 2.64 | 0.020 |
| SUD | 4 | 100 | −1.46 (−3.35 to 0.42) | 1.52 | 0.128 | 49.44 | <0.001 | 92 | ||
| TDCS | ||||||||||
| Depression | 9 | 419 | −0.87 (−1.51 to −0.24) | 2.70 | 0.007 | 67.89 | <0.001 | 88 | ||
| Unipolar | 5 | 148 | −1.04 (−2.17 to 0.08) | 1.82 | 0.069 | 40.39 | <0.001 | 90 | ||
| GAD | 2 | 42 | −0.55 (−1.17 to 0.07) | 1.74 | 0.083 | 0.55 | 0.457 | 0 | ||
| OCD | 2 | 46 | −0.37 (−0.95 to 0.22) | 1.23 | 0.218 | 0.003 | 0.953 | 0 | ||
| Schizophrenia | ||||||||||
| Positive symptoms | 8 | 367 | −0.12 (−0.33 to 0.08) | 1.18 | 0.237 | 3.59 | 0.826 | 0 | ||
| Negative symptoms | 7 | 267 | −0.54 (−0.95 to −0.14) | 2.61 | 0.009 | 14.98 | 0.020 | 60 | ||
| Total symptoms | 9 | 386 | −0.63 (−1.03 to −0.23) | 3.10 | 0.002 | 26.14 | 0.001 | 69 | ||
| Auditory hallucinations | 7 | 312 | −0.42 (−0.81 to −0.02) | 2.06 | 0.040 | 16.50 | 0.011 | 64 | ||
| SUD | 7 | 224 | −0.73 (−1.00 to −0.46) | 5.29 | <0.001 | 2.95 | 0.815 | 0 | ||
| Cognitive functioning | ||||||||||
| TMS | ||||||||||
| Attention | ||||||||||
| Depression | 3 | 146 | −0.10 (−0.44 to 0.23) | 0.67 | 0.538 | 0.97 | 0.617 | 0 | ||
| Schizophrenia | 3 | 126 | −0.18 (−0.64 to 0.29) | 0.74 | 0.457 | 3.26 | 0.196 | 39 | ||
| Executive functioning | ||||||||||
| Depression | 8 | 292 | −0.41 (−0.39 to 0.08) | 1.35 | 0.176 | 7.46 | 0.383 | 6 | ||
| Schizophrenia | 5 | 142 | −0.28 (−0.74 to 0.18) | 1.19 | 0.233 | 6.82 | 0.146 | 41 | ||
| Processing speed | ||||||||||
| Depression | 7 | 276 | 0.07 (−0.17 to 0.31) | 0.59 | 0.553 | 4.71 | 0.582 | 0 | ||
| Schizophrenia | 5 | 168 | −0.26 (−0.57 to 0.04) | 1.70 | 0.090 | 1.84 | 0.765 | 0 | ||
| Working memory | ||||||||||
| Depression | 7 | 306 | 0.02 (−0.21 to 0.25) | 0.19 | 0.848 | 3.88 | 0.694 | 0 | ||
| Schizophrenia | 10 | 313 | −0.65 (−0.39 to 0.06) | 1.42 | 0.156 | 9.18 | 0.421 | 2 | 1.86 | |
| SUD | 2 | 69 | −0.66 (−1.87 to 0.55) | 1.07 | 0.285 | 5.95 | 0.015 | 83 | ||
| TDCS | ||||||||||
| Attention | ||||||||||
| Schizophrenia | 6 | 247 | −0.30 (−0.55 to −0.05) | −2.31 | 0.021 | 6.15 | 0.292 | 19 | ||
| Executive functioning | ||||||||||
| Depression | 3 | 154 | −0.19 (−0.51 to 0.12) | 1.20 | 0.231 | 0.12 | 0.942 | 0 | ||
| Schizophrenia | 7 | 261 | −0.13 (−0.37 to 0.12) | 1.00 | 0.317 | 3.76 | 0.710 | 0 | ||
| Processing speed | ||||||||||
| Depression | 3 | 123 | 0.05 (−0.31 to 0.41) | 0.29 | 0.771 | 1.34 | 0.512 | 0 | ||
| Schizophrenia | 7 | 261 | −0.38 (−0.78 to 0.18) | 1.87 | 0.061 | 14.23 | 0.027 | 58 | ||
| Working memory | ||||||||||
| Depression | 5 | 198 | −0.11 (−0.42 to 0.19) | 0.73 | 0.465 | 4.54 | 0.338 | 12 | ||
| Schizophrenia | 7 | 279 | −0.38 (−0.74 to −0.03) | 2.14 | 0.032 | 12.24 | 0.057 | 51 | ||
K number of studies included, N overall number of participants, SMD standardized mean difference, ADHD attention deficit hyperactivity disorder, GAD Generalized Anxiety Disorder, OCD obsessive-compulsive disorder, PTSD Posttraumatic stress disorder, SUD substance use disorders, TMS transcranial magnetic stimulation, tDCS transcranial direct current stimulation.
Meta-analyses results-efficacy on core symptoms-dicothomous outcomes
Pooled odds ratios for depression response rates showed that there were significantly more responders to active TMS than sham (Table 2).
Table 2.
Summary of dichotomous outcomes TMS depression meta-analyses results.
| Heterogeneity | Egger’s test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | N | OR (95% CI) | Z | p values | Q | P values | I2 | t | P values | |
| Overall | 25 | 1326 | 3.66 (2.38 to 5.63) | 5.90 | <0.001 | 42.94 | 0.010 | 44 | 2.66 | 0.014 |
| Stimulation type | ||||||||||
| BLDLPFC | 5 | 161 | 3.43 (1.20 to 9.80) | 2.30 | 0.022 | 11.47 | 0.022 | 65 | ||
| HF-LDLPFC | 6 | 101 | 6.03 (1.80 to 20.16) | 2.92 | 0.004 | 10.81 | 0.055 | 54 | ||
| HF-VC | 2 | 97 | 1.68 (0.52 to 5.42) | 0.87 | 0.386 | 2.02 | 0.156 | 50 | ||
| iTBS-LDLPFC | 4 | 151 | 3.84 (1.02 to 14.49) | 2.00 | 0.047 | 8.16 | 0.043 | 63 | ||
| TBS-BLDLPFC | 2 | 53 | 9.15 (3.29 to 25.41) | 4.25 | <0.001 | 0.20 | 0.651 | 0 | ||
| Treatment period | ||||||||||
| 10 | 15 | 754 | 5.08 (2.97 to 8.71) | 5.92 | <0.001 | 22.70 | 0.065 | 38 | 1.50 | 0.157 |
| 15 | 4 | 195 | 4.85 (1.90 to 12.35) | 3.31 | 0.001 | 1.10 | 0.777 | 0 | ||
| 20 | 3 | 190 | 1.29 (0.35 to 4.79) | 0.39 | 0.700 | 3.70 | 0.157 | 46 | ||
| 30 | 3 | 187 | 1.75 (0.75 to 4.10) | 1.30 | 0.194 | 2.62 | 0.270 | 24 | ||
K number of studies included, N overall number of participants, OR odds ratio, BL bilateral DLPFC stimulation, HF-L high frequency left DLPFC stimulation, HF-VC high frequency stimulation over the visual cortex, iTBS-L intermittent theta-burst stimulation over the left DLPFC, TBS-BLDLPFC bilateral DLPFC theta-burst stimulation.
Meta-analyses results-efficacy on cognitive functioning
We found that active TMS was not superior to sham for cognitive enhancement in any mental condition. tDCS significantly enhanced attention and working memory in patients with schizophrenia (Table 1; Supplementary Figs. 29–44).
A number of analyses were characterized by significant heterogeneity (significant Q test, Tables 1, 2).
Subgroup analyses for TMS trials of core symptoms
Stimulation site and frequency
Across disorders, the most common TMS stimulation site was the unilateral left (L) or right (R) dorsolateral prefrontal cortex (DLPFC) or bilateral DLPFC. For unipolar depression, BLDLPFC and HF-LDLPFC TMS were superior to sham. For OCD, BLDLPFC, LF-RDLPFC and LF supplementary motor area (LF-SMA) TMS were each superior to sham. For PTSD, HF-RDLPFC, and LF-RDLPFC TMS were superior to sham. For schizophrenia negative symptoms HF-LDLPFC was superior to sham (Table 3).
Table 3.
Summary subgroup meta-analyses: core symptoms, continuous outcome, TMS.
| Heterogeneity | Egger’s test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N Sess | K | N | SMD (95% CI) | Z | p values | Q | P values | I2 | t | P values | |
| Depression unipolar | |||||||||||
| Stimulation type | |||||||||||
| BLDLPFC | 15–20 | 4 | 206 | −0.27 (−0.55 to 0.01) | 1.92 | 0.055 | 0.63 | 0.889 | 0 | ||
| HF-LDLPFC | 5–30 | 26 | 1908 | −0.66 (−0.91 to −0.40) | 5.01 | <0.001 | 124.85 | <0.001 | 80 | 2.35 | 0.028 |
| 5 | 3 | 42 | −0.23 (−0.80 to 0.33) | 0.81 | 0.418 | 0.27 | 0.875 | 0 | |||
| 10 | 7 | 228 | −0.90 (−1.54 to −0.27) | 2.78 | 0.006 | 21.24 | 0.002 | 72 | |||
| 12–15 | 6 | 450 | −0.46 (−1.01 to 0.09) | 1.63 | 0.103 | 30.31 | <0.001 | 83 | |||
| 20 | 7 | 705 | −0.46 (−0.78 to −0.15) | 2.86 | 0.004 | 18.46 | 0.005 | 67 | |||
| 30 | 2 | 105 | −2.14 (−4.21 to −0.08) | 2.03 | 0.042 | 13.94 | <0.001 | 93 | |||
| LF-LDLPFC | 2 | 37 | −0.01 (−0.68 to 0.66) | 0.02 | 0.985 | 0.64 | 0.425 | 0 | |||
| LF-RDLPFC | 3 | 92 | −1.35 (−2.47 to −0.22) | 2.33 | 0.020 | 12.28 | 0.002 | 84 | |||
| sTMS | 2 | 165 | −0.55 (−1.13 to 0.02) | 1.89 | 0.059 | 3.41 | 0.061 | 71 | |||
| Bipolar | |||||||||||
| HF-LDLPFC | 3 | 99 | −0.28 (−0.67 to 0.11) | 1.43 | 0.154 | 1.37 | 0.504 | 0 | |||
| OCD | |||||||||||
| Stimulation type | |||||||||||
| BLDLPFC | 10–20 | 4 | 87 | −1.10 (−1.54 to −0.65) | 4.80 | <0.001 | 1.93 | 0.059 | 0 | ||
| HF-LDLPFC | 10–15 | 2 | 58 | −0.13 (−0.62 to 0.36) | 0.52 | 0.604 | 0.00 | 0.955 | 0 | ||
| HF-RDLPFC | 10–30 | 3 | 99 | −0.65 (−1.48 to 0.18) | 1.54 | 0.124 | 8.50 | 0.014 | 76 | ||
| LF-RDLPFC | 10–20 | 4 | 95 | −0.54 (−1.00 to −0.18) | 2.83 | 0.005 | 3.23 | 0.352 | 8 | ||
| LF-SMA | 10–25 | 6 | 183 | −1.24 (−2.23 to −0.25) | 2.46 | 0.014 | 43.92 | <0.001 | 89 | ||
| PTSD | |||||||||||
| Stimulation type | |||||||||||
| HF-RDLPFC | 10 | 3 | 73 | −1.32 (−2.50 to −0.14) | 2.20 | 0.028 | 10.04 | 0.007 | 80 | ||
| LF-RDLPFC | 10–15 | 4 | 70 | −0.83 (−1.43 to −0.23) | 2.72 | 0.006 | 4.90 | 0.179 | 38 | ||
| Schizophrenia negative symptoms | |||||||||||
| Stimulation type | |||||||||||
| BLDLPFC | 3 | 77 | 0.06 (−0.39 to 0.50) | 0.24 | 0.811 | 1.36 | 0.507 | 0 | |||
| HF-LDLPFC | 5–40 | 19 | 1029 | −0.61 (−0.92 to −0.30) | 3.86 | <0.001 | 96.74 | <0.001 | 81 | 3.06 | 0.007 |
| 5 | 3 | 107 | −0.23 (−0.61 to 0.16) | 1.16 | 0.244 | 1.00 | 0.605 | 0 | |||
| 10 | 2 | 139 | −0.11 (−0.46 to 0.24) | 0.62 | 0.537 | 1.00 | 0.318 | 0 | |||
| 15 | 5 | 284 | −0.83 (−1.42 to −0.25) | 2.79 | 0.005 | 17.07 | 0.002 | 77 | |||
| 20 | 6 | 319 | −1.12 (−1.99 to −0.25) | 2.52 | 0.012 | 60.44 | <0.001 | 92 | |||
| 40 | 3 | 185 | −0.10 (−0.39 to 0.19) | 0.68 | 0.495 | 0.23 | 0.892 | 0 | |||
| LF-LDLPFC | 10 | 2 | 43 | −0.32 (0.92 to 0.29) | 1.02 | 0.307 | 0.81 | 0.368 | 0 | ||
K number of studies included, N overall number of participants, SMD standardized mean difference, OCD obsessive-compulsive disorder, PTSD Posttraumatic stress disorder, TMS transcranial magnetic stimulation, sTMS synchronized TMS, BLDLPFC dorsolateral prefrontal cortex, LDLPFC left dorsolateral prefrontal cortex, RDLPFC right dorsolateral prefrontal cortex, SMA supplementary motor area, HF high-frequency stimulation (≥5 Hz), LF low-frequency stimulation (≤1 Hz).
Number of treatment sessions
For unipolar depression, HF-LDLPFC trials of 10, 20 and 30 sessions were superior to sham. For OCD, BLDLPFC, and LF-RDLPFC trials of 10–20 treatment sessions and LF-SMA trials of 10–25 sessions were superior to sham. For PTSD, 10–20 sessions of LF-RDLPFC were superior to sham. For schizophrenia, HF-LDLPFC trials of 10, 15, and 20 sessions were superior to sham (Table 3).
Sensitivity analyses of core symptoms
Sensitivity analyses excluding high risk of bias RCTs did not show substantial differences for the core symptom domains. The only change was the finding of TMS inducing significant improvements in executive functioning in depression (Table 4).
Table 4.
Sensitivity analyses of continuous outcome primary meta-analyses results.
| Heterogeneity | Egger’s test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | N | SMD (95% CI) | Z | p values | Q | p values | I2 | t | P values | |
| Core symptom severity | ||||||||||
| TMS | ||||||||||
| Depressiona | 56 | 1877 | −0.44 (−0.56 to −0.31) | 6.94 | <0.001 | 204.04 | <0.001 | 63 | 1.73 | 0.088 |
| GADa | 2 | 86 | −1.95 (−3.11 to −0.80) | 3.31 | 0.001 | 4.53 | 0.033 | 78 | ||
| OCDa | 18 | 536 | −0.65 (−0.95 to −0.36) | 4.28 | <0.001 | 48.18 | <0.001 | 65 | 1.89 | 0.078 |
| PTSDa | 8 | 225 | −1.03 (−1.61 to −0.45) | 3.47 | 0.001 | 40.00 | <0.001 | 82 | ||
| Schizophrenia | ||||||||||
| Positive symptomsa | 33 | 1242 | −0.24 (−0.51 to 0.03) | 1.75 | 0.080 | 147.45 | <0.001 | 81 | 2.49 | 0.019 |
| Negative symptomsa | 24 | 1034 | −0.54 (−0.84 to −0.24) | 3.48 | 0.001 | 125.85 | <0.001 | 82 | 2.24 | 0.036 |
| Total symptomsa | 29 | 1024 | −0.52 (−0.72 to −0.32) | 5.15 | <0.001 | 50.41 | 0.001 | 56 | 1.96 | 0.063 |
| Hallucinationsa | 13 | 459 | −0.21 (−0.39 to −0.03) | 2.32 | 0.021 | 10.75 | 0.550 | 0 | 3.71 | 0.003 |
| SUD | 4 | 100 | −1.46 (−3.35 to 0.42) | 1.52 | 0.128 | 49.44 | <0.001 | 92 | ||
| TDCS | ||||||||||
| Depressiona | 8 | 355 | −0.59 (−1.32 to 0.15) | 1.56 | 0.118 | 72.33 | <0.001 | 90 | ||
| GAD | 2 | 42 | −0.55 (−1.17 to 0.07) | 1.74 | 0.083 | 0.55 | 0.457 | 0 | ||
| OCD | 2 | 46 | −0.37 (−0.95 to 0.22) | 1.23 | 0.218 | 0.003 | 0.953 | 0 | ||
| Schizophrenia | ||||||||||
| Positive symptoms | 8 | 367 | −0.12 (−0.33 to 0.08) | 1.18 | 0.237 | 3.59 | 0.826 | 0 | ||
| Negative symptoms | 6 | 252 | −0.44 (−0.82 to −0.06) | 2.26 | 0.024 | 11.11 | 0.049 | 55 | ||
| Total symptomsa | 8 | 371 | −0.55 (−0.94 to −0.15) | 2.73 | 0.006 | 22.94 | 0.002 | 69 | ||
| Hallucinations | 7 | 312 | −0.42 (−0.81 to −0.02) | 2.06 | 0.040 | 16.50 | 0.011 | 64 | ||
| SUDa | 5 | 174 | −0.44 (−0.79 to −0.10) | 2.55 | 0.011 | 2.95 | 0.815 | 0 | ||
| Cognitive functioning | ||||||||||
| TMS | ||||||||||
| Attention | ||||||||||
| Depressiona | 2 | 62 | −0.05 (−0.45 to 0.56) | 0.21 | 0.837 | 0.37 | 0.545 | 0 | ||
| Schizophreniaa | 2 | 101 | 0.03 (−0.39 to 0.44) | 0.12 | 0.906 | 1.13 | 0.288 | 11 | ||
| Executive functioning | ||||||||||
| Depressionb | 7 | 229 | −0.27 (−0.53 to 0.01) | 2.05 | 0.040 | 4.18 | 0.652 | 0 | ||
| Schizophreniaa | 3 | 96 | 0.08 (−0.31 to 0.47) | 0.40 | 0.688 | 0.68 | 0.713 | 0 | ||
| Processing speed | ||||||||||
| Depressiona | 6 | 213 | 0.04 (−0.18 to 0.26) | 0.39 | 0.700 | 5.75 | 0.570 | 0 | ||
| Schizophreniaa | 4 | 143 | −0.25 (−0.59 to 0.08) | 1.51 | 0.132 | 1.96 | 0.580 | 0 | ||
| Working memory | ||||||||||
| Depressiona | 5 | 159 | 0.002 (−0.33 to 0.33) | 0.01 | 0.991 | 4.29 | 0.368 | 7 | ||
| Schizophreniaa | 8 | 267 | −0.12 (−0.38 to 0.15) | 0.88 | 0.381 | 8.07 | 0.326 | 13 | ||
| SUD | 2 | 69 | −0.66 (−1.87 to 0.55) | 1.07 | 0.285 | 5.95 | 0.015 | 83 | ||
| TDCS | ||||||||||
| Attention | ||||||||||
| Schizophrenia | 6 | 247 | −0.30 (−0.55 to −0.05) | −2.31 | 0.021 | 6.15 | 0.292 | 19 | ||
| Executive functioning | ||||||||||
| Depression | 3 | 154 | −0.19 (−0.51 to 0.12) | 1.20 | 0.231 | 0.12 | 0.942 | 0 | ||
| Schizophrenia | 7 | 261 | −0.13 (−0.37 to 0.12) | 1.00 | 0.317 | 3.76 | 0.710 | 0 | ||
| Processing speed | ||||||||||
| Depression | 3 | 123 | 0.05 (−0.31 to 0.41) | 0.29 | 0.771 | 1.34 | 0.512 | 0 | ||
| Schizophrenia | 7 | 261 | −0.38 (−0.78 to −0.18) | 1.87 | 0.061 | 14.23 | 0.027 | 58 | ||
| Working memory | ||||||||||
| Depression | 5 | 198 | −0.11 (−0.42 to 0.19) | 0.73 | 0.465 | 4.54 | 0.338 | 12 | ||
| Schizophrenia | 7 | 279 | −0.38 (−0.74 to −0.03) | 2.14 | 0.032 | 12.24 | 0.057 | 51 | ||
GAD generalized anxiety disorder, OCD obsessive-compulsive disorder, PTSD posttraumatic stress disorder, SUD substance use disorders, TMS transcranial magnetic stimulation, tDCS transcranial direct current stimulation.
aRe-calculated with papers including at least one “high risk” category removed.
bChange to significance.
Again a number of results were limited by significant heterogeneity (Tables 1, 2).
Discussion
We conducted the first series of meta-analyses of RCTs investigating the efficacy of NIBS for the treatment of core symptoms and improvement of cognitive functioning using the same criteria within a broad range of mental disorders. We found that TMS and tDCS had significant effects on the core symptom severity of several disorders, although significant heterogeneity limits the confidence of some results. We discuss here the effects of NIBS grouped by mental disorder.
In line with previous evidence synthesis [22], TMS significantly reduced GAD symptoms, with a large effect size and no significant heterogeneity. However, only three RCTs were available for analysis. Each study utilized a different stimulation protocol, with high-frequency right DLPFC (HF-R), low-frequency right DLPFC (LF-R), and low-frequency right parietal stimulation, all producing significant positive effects. Therefore, despite this positive finding, more studies are needed to better understand the therapeutic mechanisms and optimal treatment parameters of NIBS for GAD.
PTSD symptom severity significantly decreased following rTMS, yielding large effect sizes and significant improvements in the majority of RCTs, albeit with significant heterogeneity. As in previous reports [23], LF-RDLPFC rTMS yielded a significant effect without heterogeneity, indicating robust symptom improvement.
OCD symptoms were significantly reduced with TMS, with a medium effect size. Although heterogeneity for the overall findings was high, seven RCTs showed significant improvements of OCD symptoms. When taking into account different stimulation parameters, BLDLPFC rTMS produced the largest effect size without significant heterogeneity, indicating robust symptom improvement. Consistent with a previous analysis [24], LF-RDLPFC rTMS was also independently effective, whereas HF-RDLPFC stimulation was not. However, the low number of available RCTs suggests that these results should be considered cautiously. In line with previous literature, low-frequency SMA stimulation yielded large and significant effects but no robust conclusions can be drawn due to high heterogeneity [24].
Overall, TMS was effective in treating depressive episodes with a medium effect size, but high heterogeneity. Notably, when depressive episodes were split by polarity, TMS was effective in treating unipolar but not bipolar depression although for the latter there were only four available RCTs. Considering stimulation parameters as a possible source of variability across unipolar depression TMS studies, our subgroup analysis on BLDLPFC stimulation yielded a small but consistent effect, with no significant heterogeneity. A subgroup analysis on LF-RDLPFC stimulation also yielded a positive effect, although this was characterized by significant heterogeneity and comprised only three studies. In addition, we also found a positive effect of HF-LDLPFC stimulation, which was the most commonly implemented stimulation in RCTs for depression overall (n = 46). HF-LDLPFC rTMS has been shown to increase activity in the left PFC [25], an area that shows abnormal activity in patients with MDD [26]. Accordingly, large HF-LDLPFC TMS RCTs have found significant antidepressant effects [27, 28], and this stimulation type is currently recommended by treatment guidelines [29]. However, in our subgroup analysis on HF-LDLPFC stimulation for unipolar depression, heterogeneity remained significant, suggesting that the efficacy cannot be assumed to be robust across studies or participants, although heterogeneous findings should be expected with a large number of studies. Overall, the results of the current meta-analysis suggest that BLDLPFC rTMS might have a more consistent, small effect on symptoms of unipolar depression, while HF-LDLPFC stimulation could achieve larger effects but with more variability. Notably, effect sizes for tDCS overall depression symptom improvement were higher than those reported in previous studies [30–32], and are comparable to those recently reported for psycho- and pharmacotherapies [33, 34]. However, while the effect sizes are promising, the results should be interpreted with caution due to high heterogeneity.
In patients with schizophrenia, NIBS did not appear effective for reducing overall positive symptoms. Negative symptoms were significantly improved by both TMS and tDCS protocols with a medium effect size. However, these results were characterized by high heterogeneity and therefore some caution is warranted when interpreting the results of NIBS on negative symptoms in schizophrenia. For auditory hallucinations, tDCS yielded a small positive effect but with high heterogeneity and TMS yielded a negligible but homogenous positive effect. These findings were similar to a previous study found that TMS was effective for auditory hallucinations but not overall positive symptoms, which could even worsen [35]. The most common cortical targets for schizophrenia are the left DLPFC and the left temporoparietal junction (TPJ). Dysfunctional PFC activation has been associated with negative symptoms and cognitive deficits [36], and auditory hallucinations are thought to originate from spontaneous activity in hyperactive temporal regions, which is not adequately inhibited due to prefrontal hypoactivity [37]. Accordingly, our subgroup analyses showed that TMS protocols targeting hallucinations typically employed low-frequency stimulation over the left TPJ [38–45], while those targeting negative symptoms commonly used HF-LDLPFC stimulation [46–50]. Typically, tDCS protocols positioned the anode to excite neural activity in the left DLPFC and the cathode to inhibit neural activity in the left TPJ, which allowed both negative symptoms and hallucinations to be targeted [51–55]. In summary, it appears that neurostimulation could be used in patients with schizophrenia, especially for negative symptoms.
In the current analysis, TMS was not effective for the treatment of SUD symptoms but tDCS yielded a large effect size without significant heterogeneity. This is in contrast with a previous study that found both tDCS and TMS to be effective [56]. However, in the previous study, TMS and tDCS were grouped, and several TMS RCTs were excluded from the current analysis due to lacking a formal diagnosis [57], absent sham condition [58], non-standardized symptom measurement [59] and crossover designs without report of first phase data [60–62]. The reliable finding for tDCS as a treatment for SUD shows promise but was based on seven RCTs, so more trials are needed to draw robust conclusions.
We did not find TMS to be superior to sham for ADHD core symptoms. This is in line with a recent meta-analysis that found no effect of TMS or tDCS on ADHD clinical and cognitive symptoms [63]. The present analysis of ADHD was limited by RCT availability, and the exclusion of studies combining NIBS with cognitive training (CT). As per protocol, studies combining NIBS with CT were excluded from our analysis, as the focus was specifically on NIBS, and disentangling the specific contribution of NIBS when combined with CT would be challenging. However, while there is some evidence that the positive effects of tDCS can be enhanced when combined with CT by priming the brain regions that mediate the cognitive function being trained [64, 65], recent well-designed RCTs have not replicated these findings in ADHD [66]. Further RCTs combining tDCS and CT for ADHD should be conducted, to allow future meta-analyses assessing the potential of combining tDCS with CT for individuals with ADHD.
Finally, two RCTs investigating the efficacy of TMS for tic disorders were included in the systematic review but not the meta-analysis. One RCT was in adults [67], the other in children and young adults [68]. Both trials used inhibitory TMS over the supplementary motor area (SMA) but neither found positive effects of TMS on tic symptoms. Several small open label studies have found positive effects of low fequency TMS over the SMA for tic symptoms [69–72], but these findings have not yet been replicated in RCTs.
In terms of the impact of the duration of treatment, within disorder subgroup analyses of stimulation protocols with sufficient studies suggested that 10–20 sessions of TMS was typically most effective for reducing symptoms. Longer trial durations did not appear to increase effect sizes, although within most disorders the number of trials with more than ten sessions was limited.
Regarding improvement in cognitive functioning, small to moderate effect sizes without significant heterogeneity were found for tDCS on attention and working memory performance in patients with schizophrenia, and executive functioning performance in patients with depression after sensitivity analyses. RCTs for schizophrenia are of particular importance, as a previous evidence synthesis of pharmacological treatments for cognitive deficits in schizophrenia showed limited efficacy [73]. The effects for all other cognitive domains across disorders were either non-significant or could not be calculated due to lack of data in the studies. Overall, these results are consistent with a previous meta-analysis investigating the effects of NIBS on several cognitive functioning domains across several mental health disorders [18], with limited evidence for the overall effectiveness of NIBS on cognitive functioning but potential for positive effects on attention and working memory. A possible explanation for this finding may be provided by recent empirical evidence demonstrating that bifrontal tDCS can increase dopamine release in the ventral striatum [74]. It is hypothesized that dopamine activity in the striatum has associations with prefrontal functioning and more specifically with higher-order cognition including working memory updating and attention shifting [75]. Thus, increased dopamine activity in this area could be a potential mechanism behind these positive tDCS effects. However, we did not find any positive effect of tDCS for other cognitive domains across mental disorders, suggesting that tDCS could only be used to target specific cognitive functions in specific patient groups.
Strengths and limitations
Strengths of the current meta-analysis are the comprehensive search strategy, no limitations in language or type of document, the inclusion of unpublished infomation/data gathered by study authors, and inclusion of the most rigorous study design (RCT) only. The overall quality of included RCTs was also good. Although many RCTs were of “some concerns” according to the RoB2, this was due largely to a lack of a prespecified analysis plan rather than issues with RCT design. Another strength is the exploration of subgroups based on stimulation site and number of sessions, which allowed for identification of particularly strong treatment paradigms. Furthermore, only studies with formally diagnosed patients and those involving NIBS as a monotherapy or augmentation of stable treatment were retained, thus avoiding heterogeneity related to different diagnostic methods and confounding effects of additional therapies. However, although the effects of each treatment are difficult to disentangle in combined trials, previous studies have shown that NIBS can be more effective when co-initiated with other treatments such as pharmacotherapy [76], cognitive therapies [77, 78], exposure-based therapy [79] or cognitive training [64, 65]. Thus, our analysis was also limited by the exclusion of combined trials. We suggest that future RCTs should consider neurotherapies combined with other strategies, particularly in individuals with anxiety-related disorders, who can benefit from exposure-based techniques [80] and in those with ADHD, who can benefit from cognitive training in terms of improvement in some executive functions [81]. Furthermore, it should be noted that, given the nature of available data, we could not control for the effect of concomitant medication. Therefore, our results should be interpreted with caution, especially in relation to trials in individuals with schizophrenia, in which the majority of patients were medicated during the course of neurotherapy. Our meta-analysis was also limited by the small number of available RCTs for some disorders and stimulation types. For example, no subgroup analyses could be run within ADHD or GAD due to a lack of studies, and fewer than 10 tDCS RCTs were available for any mental disorder. Furthermore, as it is recommended to conduct meta-regression analyses with at least ten studies per regressor [82], and data on potential regressors were not consistently reported across studies, it was not possible to explore the planned regressors. We also planned to include RCTs in children/adolescents however, all the retained studies were in adults, which prevented us from assessing possible developmental differences in efficacy. We also could not examine the longer term effects of NIBS as data from follow-up periods were not analyzed. Additionally, funnel plots showed the possibility of some publication bias in the results regarding TMS in patients with OCD and schizophrenia. Finally, for the present report, our primary analysis was based on standardized mean difference as this was calculable from data provided by the majority of included studies. We did not plan to extract any data pertaining to NIBS safety, tolerability or individual patient responses as this was beyond the scope of the present study. Both TMS and tDCS have been widely reported as safe techniques with minimal adverse effects [83, 84], and as such the focus of this study was on efficacy. However, future studies should consider analysis of individual patient data as this would provide a reliable assessment of the percentage of responders and the acceptability of NIBS across mental disorders.
Conclusions
Overall, TMS was found to be superior to sham for GAD, and tDCS to be superior to sham for cravings in SUD. We also found significant medium to large effect sizes for TMS for reducing symptom severity of unipolar and overall depressive episodes, OCD, PTSD, and negative symptoms in patients with schizophrenia and TDCS for reducing symptom severity of overall depressive episodes, auditory hallucinations, and negative symptoms of schizophrenia. However, these results were characterized by significant heterogeneity, so must be interpreted with caution. In contrast to TMS, tDCS was effective for the enhancement of attention and working memory in patients with schizophrenia. In order to be most effective, TMS should entail 10–20 sessions. Further high quality NIBS trials are needed within understudied disorders and novel stimulation techniques. Additionally, further exploration of heterogeneity among trials within well-researched disorders is warranted to identify sources of variability in treatment effects.
Supplementary information
Author contributions
JH screened papers, extracted data, ran analyses, and wrote the first draft of the paper. HC screened papers, extracted data, did quality ratings, and critically revised the paper. NK extracted data, did quality ratings, and critically revised the paper. RS screened papers, extracted data, did quality ratings, and critically revised the paper. CR screened papers, did quality ratings, and critically revised the paper. VP extracted data, did quality ratings, and critically revised the paper. MG designed the study, provided supervision, and critically revised the paper. MS designed the study, and critically revised the paper. SR designed the study, and critically revised the paper. SC designed the study, provided supervision, and critically revised the paper. VB designed the study, provided supervision, ran analyses, and critically revised the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Samuele Cortese, Valerie Brandt.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01524-8.
References
- 1.Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21:10. doi: 10.1007/s11920-019-0997-0. [DOI] [PubMed] [Google Scholar]
- 2.Abbott A. COVID’s mental-health toll: how scientists are tracking a surge in depression. Nature. 2021;590:194–5. doi: 10.1038/d41586-021-00175-z. [DOI] [PubMed] [Google Scholar]
- 3.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–20. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–40. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann Phys Rehabil Med. 2015;58:208–13. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–50. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 7.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 8.Stafford J, Brownlow ML, Qualley A, Jankord R. AMPA receptor translocation and phosphorylation are induced by transcranial direct current stimulation in rats. Neurobiol Learn Mem. 2018;150:36–41. doi: 10.1016/j.nlm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Martins CW, de Melo Rodrigues LC, Nitsche MA, Nakamura-Palacios EM. AMPA receptors are involved in prefrontal direct current stimulation effects on long-term working memory and GAP-43 expression. Behav Brain Res. 2019;362:208–12. doi: 10.1016/j.bbr.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, et al. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107:1868–80. doi: 10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39:358–65. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, et al. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage. 2020;206:116287. doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10:1–18. doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583.. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Lage C, Wiles K, Shergill SS, Tracy DK. A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition. J Neural Transm. 2016;123:1479–90. doi: 10.1007/s00702-016-1592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begemann MJ, Brand BA, Curcic-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. 2020;50:2465–86. doi: 10.1017/S0033291720003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis. 3rd ed. Englewood, NJ: Biostat; 2013.
- 21.Correll J, Mellinger C, McClelland GH, Judd CM. Avoid Cohen’s ‘small’,‘medium’, and ‘large’for power analysis. Trends Cogn Sci. 2020;24:200–7. doi: 10.1016/j.tics.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Cirillo P, Gold AK, Nardi AE, Ornelas AC, Nierenberg AA, Camprodon J, et al. Transcranial magnetic stimulation in anxiety and trauma-related disorders: a systematic review and meta-analysis. Brain Behav. 2019;9:e01284. doi: 10.1002/brb3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kan RLD, Zhang BBB, Zhang JJQ, Kranz GS. Non-invasive brain stimulation for posttraumatic stress disorder: a systematic review and meta-analysis. Transl Psychiatry. 2020;10:168. doi: 10.1038/s41398-020-0851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perera MPN, Mallawaarachchi S, Miljevic A, Bailey NW, Herring SE, Fitzgerald PB. Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: a meta-analysis of randomized, sham-controlled trials. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:947–60. doi: 10.1016/j.bpsc.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Speer AM, Benson BE, Kimbrell TK, Wassermann EM, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. J Affect Disord. 2009;115:386–94. doi: 10.1016/j.jad.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George M, Nahas Z, Speer AM, Kimbrell T, Wassermann E, Teneback C, et al. Transcranial magnetic stimulation: a new method for investigating the neuroanatomy of depression. Adv Biol Psychiatry. 1998;19:94–122. doi: 10.1159/000058883. [DOI] [Google Scholar]
- 27.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 28.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Arns M, Bervoets C, van Eijndhoven P, Baeken C, van den Heuvel OA, Aleman A, et al. Consensus statement on the application of rTMS in depression in the Netherlands and Belgium. Tijdschr Psychiatr. 2019;61:411–20. [PubMed] [Google Scholar]
- 30.Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CHY, Young AH. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:l1079. doi: 10.1136/bmj.l1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Lam CL, Peng X, Zhang D, Zhang C, Huang R, et al. Efficacy and acceptability of transcranial direct current stimulation for treating depression: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021;126:481–90. doi: 10.1016/j.neubiorev.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24:256–313. doi: 10.1093/ijnp/pyaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuijpers P, Karyotaki E, Eckshtain D, Ng MY, Corteselli KA, Noma H, et al. Psychotherapy for depression across different age groups: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77:694–702. doi: 10.1001/jamapsychiatry.2020.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuijpers P, Noma H, Karyotaki E, Vinkers CH, Cipriani A, Furukawa TA. A network meta-analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry. 2020;19:92–107. doi: 10.1002/wps.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy NI, Lee WH, Frangou S. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: A meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69–77. doi: 10.1016/j.eurpsy.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Fan L, Qiu C, Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull. 2015;31:207–19. doi: 10.1007/s12264-014-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hugdahl K. Auditory hallucinations: a review of the ERC “VOICE” project. World J Psychiatry. 2015;5:193–209. doi: 10.5498/wjp.v5.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bais L, Vercammen A, Stewart R, van Es F, Visser B, Aleman A, et al. Short and long term effects of left and bilateral repetitive transcranial magnetic stimulation in schizophrenia patients with auditory verbal hallucinations: a randomized controlled trial. PLoS ONE. 2014;9:e108828. doi: 10.1371/journal.pone.0108828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blumberger DM, Christensen BK, Zipursky RB, Moller B, Chen R, Fitzgerald PB, et al. MRI-targeted repetitive transcranial magnetic stimulation of Heschl’s gyrus for refractory auditory hallucinations. Brain Stimul. 2012;5:577–85. doi: 10.1016/j.brs.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Brunelin J, Poulet E, Bediou B, Kallel L, Dalery J, D’Amato T, et al. Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res. 2006;81:41–5. doi: 10.1016/j.schres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 41.de Jesus DR, Gil A, Barbosa L, Lobato MI, Magalhães PV, Favalli GP, et al. A pilot double-blind sham-controlled trial of repetitive transcranial magnetic stimulation for patients with refractory schizophrenia treated with clozapine. Psychiatry Res. 2011;188:203–7. doi: 10.1016/j.psychres.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald PB, Benitez J, Daskalakis JZ, Brown TL, Marston NA, de Castella A, et al. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol. 2005;25:358–62. doi: 10.1097/01.jcp.0000168487.22140.7f. [DOI] [PubMed] [Google Scholar]
- 43.Lee SH, Kim W, Chung YC, Jung KH, Bahk WM, Jun TY, et al. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376:177–81. doi: 10.1016/j.neulet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 44.Saba G, Verdon CM, Kalalou K, Rocamora JF, Dumortier G, Benadhira R, et al. Transcranial magnetic stimulation in the treatment of schizophrenic symptoms: a double blind sham controlled study. J Psychiatr Res. 2006;40:147–52. doi: 10.1016/j.jpsychires.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Slotema CW, Blom JD, de Weijer AD, Diederen KM, Goekoop R, Looijestijn J, et al. Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry. 2011;69:450–6. doi: 10.1016/j.biopsych.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 46.Gan J, Duan H, Chen Z, Shi Z, Gao C, Zhu X, et al. Effectiveness and safety of high dose transcranial magnetic stimulation in schizophrenia with refractory negative symptoms: a randomized controlled study. Zhonghua Yi Xue Za Zhi. 2015;95:3808–12. [PubMed] [Google Scholar]
- 47.Kumar N, Vishnubhatla S, Wadhawan AN, Minhas S, Gupta P. A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2020;13:840–9. doi: 10.1016/j.brs.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007;95:151–7. doi: 10.1016/j.schres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Prikryl R, Ustohal L, Prikrylova Kucerova H, Kasparek T, Venclikova S, Vrzalova M, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophrenia Res. 2013;149:167–73. doi: 10.1016/j.schres.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Wobrock T, Guse B, Cordes J, Wölwer W, Winterer G, Gaebel W, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77:979–88. doi: 10.1016/j.biopsych.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Bose A, Shivakumar V, Agarwal SM, Kalmady SV, Shenoy S, Sreeraj VS, et al. Efficacy of fronto-temporal transcranial direct current stimulation for refractory auditory verbal hallucinations in schizophrenia: a randomized, double-blind, sham-controlled study. Schizophr Res. 2018;195:475–80. doi: 10.1016/j.schres.2017.08.047. [DOI] [PubMed] [Google Scholar]
- 52.Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–24. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- 53.Chang C-C, Tzeng N-S, Chao C-Y, Yeh C-B, Chang H-A. The effects of add-on fronto-temporal transcranial direct current stimulation (tDCS) on auditory verbal hallucinations, other psychopathological symptoms, and insight in schizophrenia: A randomized, double-blind, sham-controlled trial. Int J Neuropsychopharmacol. 2018;21:979–87. doi: 10.1093/ijnp/pyy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fröhlich F, Burrello TN, Mellin JM, Cordle AL, Lustenberger CM, Gilmore JH, et al. Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur Psychiatry. 2016;33:54–60. doi: 10.1016/j.eurpsy.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Valiengo LDCL, Goerigk S, Gordon PC, Padberg F, Serpa MH, Koebe S, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77:121–9. doi: 10.1001/jamapsychiatry.2019.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma T, Sun Y, Ku Y. Effects of non-invasive brain stimulation on stimulant craving in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. Front Neurosci. 2019;13:1095. doi: 10.3389/fnins.2019.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Q, Shen Y, Cao X, Li Y, Chen Y, Yang W, et al. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am J Addict. 2017;26:776–9. doi: 10.1111/ajad.12638. [DOI] [PubMed] [Google Scholar]
- 58.Liu T, Li Y, Shen Y, Liu X, Yuan T-F. Gender does not matter: add-on repetitive transcranial magnetic stimulation treatment for female methamphetamine dependents. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:70–5. doi: 10.1016/j.pnpbp.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 59.Bolloni C, Panella R, Pedetti M, Frascella AG, Gambelunghe C, Piccoli T, et al. Bilateral transcranial magnetic stimulation of the prefrontal cortex reduces cocaine intake: a pilot study. Front Psychiatry. 2016;7:133. doi: 10.3389/fpsyt.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shahbabaie A, Ebrahimpoor M, Hariri A, Nitsche MA, Hatami J, Fatemizadeh E, et al. Transcranial DC stimulation modifies functional connectivity of large‐scale brain networks in abstinent methamphetamine users. Brain Behav. 2018;8:e00922. doi: 10.1002/brb3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V, et al. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol. 2014;17:1591–8. doi: 10.1017/S1461145714000686. [DOI] [PubMed] [Google Scholar]
- 62.Hanlon CA, Dowdle LT, Correia B, Mithoefer O, Kearney-Ramos T, Lench D, et al. Left frontal pole theta burst stimulation decreases orbitofrontal and insula activity in cocaine users and alcohol users. Drug Alcohol Depend. 2017;178:310–7. doi: 10.1016/j.drugalcdep.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46:E14–33. doi: 10.1503/jpn.190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Au J, Katz B, Buschkuehl M, Bunarjo K, Senger T, Zabel C, et al. Enhancing working memory training with transcranial direct current stimulation. J Cogn Neurosci. 2016;28:1419–32. doi: 10.1162/jocn_a_00979. [DOI] [PubMed] [Google Scholar]
- 65.Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain stimulation. 2017;10:51–8. doi: 10.1016/j.brs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westwood SJ, Criaud M, Lam SL, Lukito S, Wallace-Hanlon S, Kowalczyk OS, et al. Transcranial direct current stimulation (tDCS) combined with cognitive training in adolescent boys with ADHD: a double-blind, randomised, sham-controlled trial. Psychol Med. 2021:1-16. [DOI] [PMC free article] [PubMed]
- 67.Landeros-Weisenberger A, Mantovani A, Motlagh MG, de Alvarenga PG, Katsovich L, Leckman JF, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe tourette syndrome. Brain Stimul. 2015;8:574–81. doi: 10.1016/j.brs.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu SW, Maloney T, Gilbert DL, Dixon SG, Horn PS, Huddleston DA, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7:212–8. doi: 10.1016/j.brs.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Mantovani A, Leckman JF, Grantz H, King RA, Sporn AL, Lisanb SH. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118:2314–5. doi: 10.1016/j.clinph.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS) Int J Neuropsychopharmacol. 2006;9:95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- 71.Kwon HJ, Lim WS, Lim MH, Lee SJ, Hyun JK, Chae JH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492:1–4. doi: 10.1016/j.neulet.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Le K, Liu L, Sun ML, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20:251–6.. doi: 10.1016/j.jocn.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 73.Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51. doi: 10.1016/S0140-6736(19)31135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fonteneau C, Redoute J, Haesebaert F, Le Bars D, Costes N, Suaud-Chagny M-F, et al. Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb Cortex. 2018;28:2636–46. doi: 10.1093/cercor/bhy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cools R. Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol. 2011;21:402–7. doi: 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 77.Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimul. 2014;7:325–31. doi: 10.1016/j.brs.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Kozel FA, Motes MA, Didehbani N, DeLaRosa B, Bass C, Schraufnagel CD, et al. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. J Affect Disord. 2018;229:506–14. doi: 10.1016/j.jad.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 79.Cobb AR, O’Connor P, Zaizar E, Caulfield K, Gonzalez-Lima F, Telch MJ. tDCS-augmented in vivo exposure therapy for specific fears: a randomized clinical trial. J Anxiety Disord. 2021;78:102344. [DOI] [PubMed]
- 80.Van Dis EA, Van Veen SC, Hagenaars MA, Batelaan NM, Bockting CL, Van Den Heuvel RM, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265–73. doi: 10.1001/jamapsychiatry.2019.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54:164–74. doi: 10.1016/j.jaac.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16:e1002742. doi: 10.1371/journal.pmed.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao H, Qiao L, Fan D, Zhang S, Turel O, Li Y, et al. Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): clinical applications and safety concerns. Front Psychol. 2017;8:685. doi: 10.3389/fpsyg.2017.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.