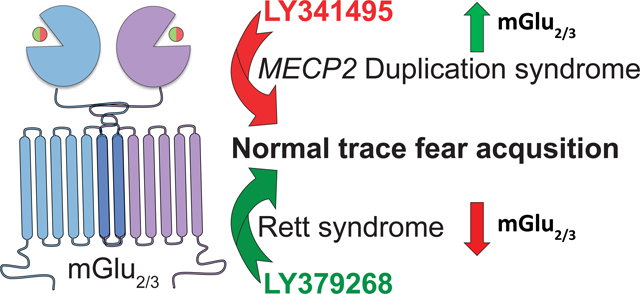
Abstract
Rett syndrome (RTT) and MECP2 Duplication syndrome (MDS) have opposing molecular origins in relation to expression and function of the transcriptional regulator Methyl-CpG-binding protein 2 (MeCP2). Several clinical and preclinical phenotypes, however, are shared between these disorders. Modulation of MeCP2 levels has recently emerged as a potential treatment option for both of these diseases. However, toxicity concerns remain with these approaches. Here, we focus on pharmacologically modulating the group II metabotropic glutamate receptors (mGlu), mGlu2 and mGlu3, which are two downstream targets of MeCP2 that are bidirectionally affected in expression in RTT patients and mice (Mecp2Null/+) versus an MDS mouse model (MECP2Tg1/o). Mecp2Null/+ and MECP2Tg1/o animals also exhibit contrasting phenotypes in trace fear acquisition, a form of temporal associative learning and memory, with trace fear deficiency observed in Mecp2Null/+ mice and abnormally enhanced trace fear acquisition in MECP2Tg1/o animals. In Mecp2Null/+ mice, treatment with the mGlu2/3 agonist LY379268 reverses the deficit in trace fear acquisition, and mGlu2/3 antagonism with LY341495 normalizes the abnormal trace fear learning and memory phenotype in MECP2Tg1/o mice. Altogether, these data highlight the role of group II mGlu receptors in RTT and MDS and demonstrate that both mGlu2 and mGlu3 may be potential therapeutic targets for these disorders.
Keywords: mGlu2, mGlu3, Rett syndrome, MECP2 Duplication syndrome, MECP2, trace fear
Graphical Abstract
1. Introduction
MECP2-associated disorders are X-linked monogenic neurodevelopmental diseases that are caused by abnormal expression and/or function of the protein Methyl-CpG-binding protein 2 (MeCP2), which is encoded on the X chromosome. Rett syndrome (RTT) is caused by loss-of-function (LOF) mutations in MECP2, and is observed most often in females (Amir et al., 1999; Lombardi et al., 2015). The multi-domain clinical symptoms of RTT, including motor dysfunction, impaired social skills, cognitive decline, and breathing abnormalities, overlap with another MECP2-associated disorder, MECP2 Duplication syndrome (MDS), which is caused by multiple copies of the MECP2 gene. Due to random X chromosome inactivation in females, patients diagnosed with MDS are predominantly male (Ramocki et al., 2009; Van Esch et al., 2005). The symptoms observed in RTT patients are recapitulated in mice that ubiquitously or cell type-specifically lack Mecp2 or express functionally mutated Mecp2 (Adachi et al., 2009; Ballinger et al., 2019; Brown et al., 2016; Chen et al., 2001; Collins et al., 2021; Fyffe et al., 2008; Gemelli et al., 2006; Goffin et al., 2011; Guy et al., 2001; Heckman et al., 2014; Jentarra et al., 2010; Kerr et al., 2008; Lamonica et al., 2017; Lawson-Yuen et al., 2007; Luikenhuis et al., 2004; Moretti et al., 2006, 2005; Pelka et al., 2006; Pitcher et al., 2015; Samaco et al., 2013, 2009, 2008; Schaevitz et al., 2013; Shahbazian et al., 2002; Vermudez et al., 2021). Similarly, clinical characteristics of MDS are observed in mouse models that either have global or neuronal-specific MECP2 overexpression (Collins et al., 2004; Na et al., 2014). Interestingly, many phenotypes in RTT mice are antiparallel to those observed in MDS animal models, including anxiety, motor abnormalities, and cognitive function. For example, RTT mice display deficits in associative learning and memory in a fear conditioning task; in contrast, MDS mice exhibit abnormally enhanced learning in this task in addition to impairments in extinction of fear-learned behavior (Collins et al., 2004; Moretti et al., 2006; Na et al., 2012; Stansley et al., 2018; Stearns et al., 2007).
Previous studies in RTT models have shown that genetic normalization of MECP2, even after disease onset, can rescue many phenotypes in Mecp2 mutant mice, supporting the feasibility of treating the disorder (Gadalla et al., 2017, 2013; Garg et al., 2013; Guy et al., 2007; Luoni et al., 2020; Matagne et al., 2021, 2017; Powers et al., 2019; Sinnett et al., 2021, 2017; Tillotson et al., 2017). Promisingly, recent studies have also demonstrated that genetic manipulations after symptom onset, including normalization of MeCP2 dosage with antisense oligonucleotides, can improve abnormal phenotypes in MDS mice (Koerner et al., 2018; Sztainberg et al., 2015). Pharmacological approaches targeting genes/pathways downstream of MeCP2 could also have potential in treating symptom domains of RTT and MDS. For example, in mice with MeCP2 over-expression in neurons (Tau-Mecp2), pharmacological antagonism of GABAA receptors can alleviate symptoms (Na et al., 2014). Comparably, studies have determined that modulation of receptors involved in neurotransmission and that are sensitive to MeCP2 dosage can improve phenotypes in RTT mice (Bittolo et al., 2016; Degano et al., 2014; Gogliotti et al., 2018, 2017, 2016; Li et al., 2017; Ogier et al., 2007; Roux et al., 2007; Scaramuzza et al., 2021; Zanella et al., 2008). These include the Trkb, glutamatergic AMPA, metabotropic glutamate (mGlu), and muscarinic acetylcholine receptors, which were initially identified in expression studies in RTT patient and mouse samples (Bedogni et al., 2016; Ben-Shachar et al., 2009; Chahrour et al., 2008; Gogliotti et al., 2018, 2017, 2016; Lin et al., 2016; Pacheco et al., 2017). The association of these receptors with neurological and neurodevelopmental disorders, as well as the advent of selective modulators, make these receptors potential therapeutic targets for new drug candidates.
The expression of the group II mGlu receptors, mGlu2 and mGlu3, has been consistently demonstrated to be affected by MeCP2 dosage in patient and preclinical samples (Bedogni et al., 2016; Ben-Shachar et al., 2009; Chahrour et al., 2008; Gogliotti et al., 2018; Lin et al., 2016; Pacheco et al., 2017). Additionally, these dimeric receptors have been implicated in neuropsychiatric disorders such as schizophrenia and depression (Chaki, 2017; Egan et al., 2004; Harrison et al., 2008; Maksymetz et al., 2017; Saini et al., 2017). Studies using subtype-selective modulators, preclinical knockout mouse models, and clinical genetic associations have shown that mGlu3 has a vital role in cognition, specifically in hippocampal and prefrontal cortical function (Dogra et al., 2021; Egan et al., 2004; Fujioka et al., 2014; Joffe et al., 2020, 2019; Lainiola et al., 2014; Pöschel et al., 2005; Rosenberg et al., 2016; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; Walker et al., 2017, 2015). mGlu2 has also been shown to be involved in cognition, as mGlu2-selective positive allosteric modulators (PAMs) improve learning and memory in rodent models of schizophrenia (Griebel et al., 2016; Nikiforuk et al., 2010). Given the molecular studies demonstrating the relationship of MeCP2 and the group II mGlu receptors, as well as the association of these receptors with cognition, we posited that mGlu2 and mGlu3 may play critical roles in the etiology or treatment of the cognitive phenotypes observed in RTT and MDS.
In this study, we show that mGlu2 and mGlu3 receptor levels are decreased in temporal cortex samples from RTT patient autopsies. Expression of these receptors is also reciprocally decreased and increased in the hippocampus of Mecp2Null/+ and MECP2Tg1/o mice, respectively. Based on these findings, we tested the hypothesis that an mGlu2/3 agonist or antagonist would positively affect behavior in Mecp2Null/+ and MECP2Tg1/o mice in a hippocampal-dependent behavioral cognitive assay. We show here that activation of mGlu2/3 receptors reverses deficient trace fear acquisition in Mecp2Null/+ animals, whereas mGlu2/3 antagonism normalizes the abnormal enhanced trace fear acquisition phenotype in MECP2Tg1/o mice. Collectively, these data demonstrate that both mGlu2 and mGlu3 receptors are implicated in the cognitive function of RTT and MDS model mice, and that modulation of mGlu2/3 activity may be beneficial in alleviating cognitive symptoms of these two disorders.
2. Materials and Methods
2.1. Animals
All animals used in the present study were group housed with food and water given ad libitum and maintained on a 12hr light/dark cycle. Animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All studies were approved by the Vanderbilt Institutional Animal Care and Use Committee and took place during the light phase. MECP2Tg1/o mice (FVB-Tg(MECP2)1Hzo/J, stock no. 008679) were cryorecovered and Mecp2Null/+ (B6.129P2(C)-Mecp2tm1.1Bird/J, stock no. 003890) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Given that MECP2Tg1/o mice on the FVB/N background are prone to retinal degeneration, we utilized the F1 generation or hybrid mice (FVB/N x C57BL/6) for our studies as described previously (Samaco et al., 2012; Zhang et al., 2017). These F1 hybrid mice were generated by crossing MECP2Tg1/o mice (FVB/N background) with wild-type (WT) C57BL/6J mice (The Jackson Laboratory, stock no. 000664). To reflect the predominantly male clinical population in MDS (Ramocki et al., 2009), male MECP2Tg1/o mice and WT littermates were used for all experiments, and at 8–12 weeks old, an age range at which MECP2Tg1/o mice were previously observed to exhibit abnormal phenotypes (Samaco et al., 2012; Zhang et al., 2017). Similarly, as a reflection of the predominantly female RTT patient population, female Mecp2Null/+ mice and WT littermates (Mecp2+/+) were utilized and aged to at least 20 weeks of age prior to experiments to reflect the symptomatic age of female Mecp2Null/+ animals (Guy et al., 2007, 2001). Mecp2Null/+ animals were maintained on a C57BL/6J background by breeding Mecp2Null/+ with WT C57BL/6J mice (The Jackson Laboratory, stock no. 000664).
2.2. Total Protein Preparation
The cortex and hippocampus were microdissected from 8–9-week-old male WT littermates and MECP2Tg1/o mice, and 20–25-week-old female littermates, Mecp2+/+ and Mecp2Null/+ animals. Total protein was prepared as previously described in (Fisher et al., 2018). Briefly, tissue samples were homogenized using a hand-held motorized mortar and pestle in radioimmunoprecipitation assay buffer (RIPA) containing 10mM Tris-HCl, 150mM NaCl, 1mM ethylenediaminetetraacetic acid (EDTA), 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, and 1% deoxycholate. After homogenization, samples were centrifuged and the supernatant was collected. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay (Pierce).
2.3. SDS-Page and Western Blotting
As previously described in (Fisher et al., 2018), 50μg of total protein was electrophoretically separated using a 4–20% SDS polyacrylamide gel and transferred onto a nitrocellulose membrane (iBlot2, ThermoFisher (for mGlu2 and mGlu3); Criterion™ Blotter, Bio-Rad (for MeCP2)). Membranes were blocked in TBS Odyssey blocking buffer (LI-COR) for 1hr at room temperature. Membranes were probed with primary antibodies overnight at 4°C: mouse anti-mGlu2 (1:1000 Abcam, cat. no. ab15672), rabbit anti-mGlu3 (1:1000, Alomone, cat. no. AGC_012), rabbit anti-MeCP2 (1:1000, Millipore, cat. no. 07–013), rabbit anti-vGlut2 (1:1000, Cell Signaling Technology, cat. no. 71555) and mouse anti-Gapdh (1:1000, ThermoFisher, cat. no. MA5–15738), followed by the fluorescent secondary antibodies: goat anti-rabbit (800nm, 1:5000, LI-COR, cat. no. 926–32211) and goat anti-mouse (680nm, 1:10,000, LI-COR, cat. no. 926–68020). Fluorescence was detected using the Odyssey (LI-COR) imaging system at the Vanderbilt University Medical Center Molecular Cell Biology Resource (MCBR) Core and then quantified using the Image Studio Lite software (LI-COR). Values were normalized to Gapdh and compared relative to littermate controls (WT littermates or Mecp2+/+).
2.4. Total RNA Extraction and cDNA Synthesis
For total RNA extraction of human temporal cortex samples, frozen samples were obtained from the University of Maryland Brain and Tissue Bank and the Harvard Brain Tissue Resource Center, which are Brain and Tissue Repositories of the National Institutes of Health NeuroBioBank (neurobiobank.nih.gov). For mouse total RNA extraction, the cortex and hippocampus were microdissected from 8–9-week-old male WT littermates and MECP2Tg1/o mice, and 20–25-week-old female littermates, Mecp2+/+ and Mecp2Null/+ animals. Total RNA was prepared from tissue samples using TRIzol Reagent (ThermoFisher) and isolated using a RNeasy Mini Kit (Qiagen) in accordance with manufacturer’s instructions. Total RNA was DNase-treated with RNase-Free DNase Set (Qiagen), and cDNA from 2μg of total RNA was synthesized using a SuperScript™ VILO™ cDNA Synthesis Kit (ThermoFisher, cat. no. 11754050).
2.5. Quantitative Real-Time PCR (qRT-PCR)
qRT-PCR (CFX96, Bio-Rad, Vanderbilt University Medical Center MCBR Core) on 50ng/9μL cDNA was run in duplicate using TaqMan™ Fast Universal PCR Master Mix (2X), no AmpErase™ UNG (Life Technologies, cat. no. 4352042) and Life Technologies gene expression assays for human GRM2 (Hs00968358_m1), GRM3 (Hs00932301_m1) and G6PD (Hs00166169_m1), and mouse Grm2 (Mm01235831_m1), Grm3 (Mm00725298_m1) and Gapdh (Mm99999915_g1). Similar qRT-PCR was performed using PowerUp™ SYBR™ Green Master Mix (ThermoFisher, cat. no. A25742) with the following primers (5’ to 3’): Mecp2 (exon 4, forward: ATGAGACTGTGCTCCCCATC, reverse: TTTTCTCACCAAGGGTGGAC) and Gapdh (exon 6, forward: CGACTTCAACAGCAACTCCC, reverse: GCCGTATTCATTGTCATACCAGG). All primers used for SYBR qRT-PCR were designed using Primer3 and constructed by Sigma through the Vanderbilt University Medical Center MCBR Core. Ct values for each sample were normalized to G6PD/Gapdh expression and analyzed using the delta–delta Ct method as described in (Gogliotti et al., 2017). Values exceeding two times the standard deviation were classified as outliers. Each value was compared to the average delta-Ct value acquired for control human samples or wild-type littermate control mice (WT littermate or Mecp2+/+) and calculated as percent-relative to the average control delta-Ct.
2.6. Drugs
LY379268 (mGlu2/3 agonist) and LY341495 (mGlu2/3 antagonist) were purchased from Tocris (Minneapolis, MN). All drugs used for behavioral experiments were diluted in 10% Tween-80.
2.7. Behavioral Assays
All behavioral experiments were conducted at predicted symptomatic ages (8–12-week-old MECP2Tg1/o and 20–25-week-old Mecp2Null/+ mice) and using sex- and age-matched littermate controls (WT littermates or Mecp2+/+ mice) at the Vanderbilt Mouse Neurobehavioral Lab (MNL) Core. All experiments were preceded by intraperitoneal (i.p.) injections of the following drugs (Tmax in parentheses): vehicle (10% Tween-80), LY379268 (1mg/kg, 30 min) or LY341495 (3mg/kg, 20 min pre-LY379268 administration or 30 min). Quantification was performed either by a researcher blinded to the genotype and treatment and/or by automated software.
2.7.1. Open Field
Mice were placed in the activity chamber for 30 min and locomotor activity was quantified as beam breaks in the X, Y and Z axis using Activity Monitor software (Med Associates Inc).
2.7.2. Trace Fear Conditioning
Mice were habituated to the room for 1 hour before all tests. On acquisition or conditioning day, mice were treated with compounds or vehicle prior to being placed into an operant chamber with a shock grid (Med Associates Inc.) in the presence of a 10% vanilla odor cue. Modified from previous studies (Dogra et al., 2021; Xu et al., 2014), mice were acclimated for 1 min and exposed to a mild 1 sec foot shock (0.5 mA for WT littermate and MECP2Tg1/o mice, and 0.7mA for Mecp2+/+ and Mecp2Null/+ animals) that was preceded by a 15 sec tone. A precise 30 sec interval or “trace” separated the tone and shock. Three or four tone-trace-shock pairings were applied, 240 sec apart, for the MECP2Tg1/o or Mecp2Null/+ mouse lines, respectively. Percentage of time spent freezing during each trace was measured by Video Freeze software (Med Associates Inc.).
2.8. Statistical Analyses
Statistics were carried out using Prism 9 (GraphPad) and Excel (Microsoft). All data shown represent mean ± SEM. Statistical significance between genotypes was determined using Student’s t-test, or 2-way ANOVA with Sidak’s or Tukey’s post-hoc. Sample size (denoted as “n”), statistical test and results of statistical analyses are specified in each figure legend.
3. Results
3.1. Group II mGlu receptor expression is decreased in clinical and preclinical RTT samples
We first investigated the expression of mGlu2 and mGlu3 in post-mortem brain tissue from female patients clinically diagnosed with RTT. Complementing our previous studies in the motor cortex and cerebellum (Gogliotti et al., 2018, 2017, 2016), we obtained fourteen temporal cortex samples (Brodmann area 20 or 38, BA 20 or BA 38) from female RTT patients characterized as having truncation mutations in MECP2 (R168X, R255X, and R270X) and fourteen age-, sex- and post-mortem interval (PMI)-matched controls (Supplementary Table 1). qRT-PCR analyses revealed decreased levels of GRM2 and GRM3 mRNA in the temporal cortex of RTT patients (Figure 1A), which is in agreement with previous transcriptomic analyses of other brain regions, including the cerebellum and frontal, motor and temporal cortices (Ben-Shachar et al., 2009; Gogliotti et al., 2018; Lin et al., 2016).
Figure 1: mGlu2 and mGlu3 expression is decreased in RTT patients and mice.
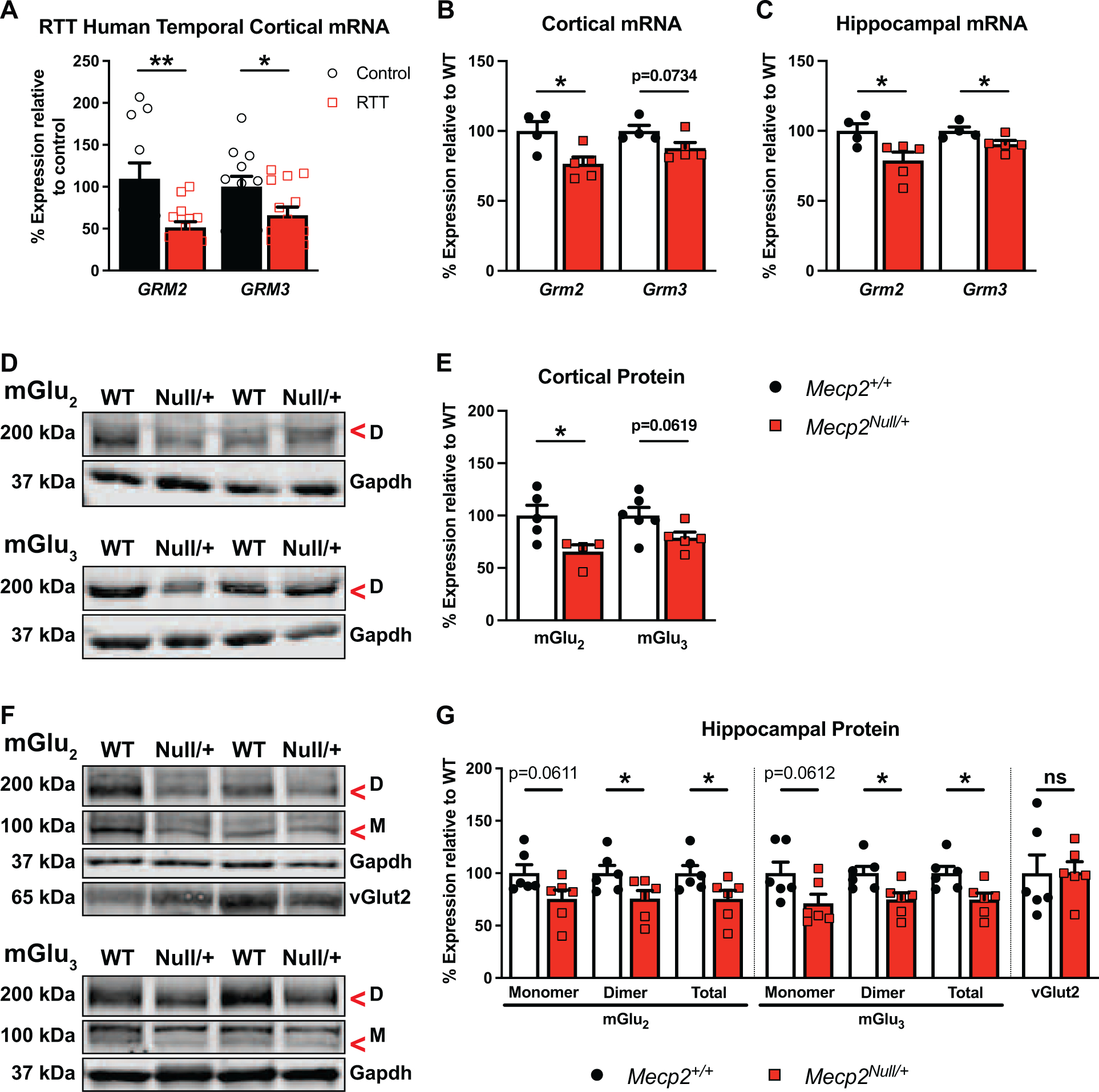
(A) Compared to controls (black bars / white circles, n=11–12), GRM2 and GRM3 mRNA are both decreased in RTT patients in temporal cortex autopsy samples (red bars / white squares, n=14) (GRM2: t(23)=3.144, p=0.0045; GRM3: t(24)=2.196, p=0.0380). RTT patient samples used in this study have truncated MeCP2 mutations, specifically R168X, R255X and R270X (see Supplementary Table 1). (B-C) Compared to littermate controls, Mecp2+/+ (white bars / black circles, n=4–5), Grm2 and/or Grm3 mRNA levels are decreased in 20–25-week-old Mecp2Null/+ mice (red bars / squares, n=4–5) (cortex: Grm2: t(7)=2.897, p=0.0231; Grm3: t(7)=2.104, p=0.0734; hippocampus: Grm2: t(7)=2.599, p=0.0355; Grm3: t(7)=2.460, p=0.0435). (D) Representative immunoblots illustrating cortical dimeric (“D”, 200 kDa) form of mGlu2 or mGlu3 and Gapdh (37 kDa) loading control in Mecp2+/+ (“WT”) and Mecp2Null/+ (“Null/+”) animals. (E) Cortical mGlu2 protein expression is significantly decreased in Mecp2Null/+ mice (n=4–5) relative to Mecp2+/+ animals (n=5–6) (mGlu2: t(7)=2.715, p=0.0300; mGlu3: t(9)=2.131, p=0.0619). (F) Representative immunoblots for hippocampal proteins as in (D) with the addition of the mGlu2 or mGlu3 monomeric protein (“M”, 100 kDa) and vGlut2 (65 kDa). (G) Compared to Mecp2+/+ animals (n=5–6), mGlu2 and mGlu3 proteins (total = monomer + dimer) are reduced in the hippocampus of Mecp2Null/+ mice (n=5–6) (mGlu2: Monomer: t(10)=2.109, p=0.0611; Dimer: t(10)=2.285, p=0.0454; Total: t(10)=2.250, p=0.0482; mGlu3: Monomer: t(10)=2.108, p=0.0612; Dimer: t(10)=2.798, p=0.0188; Total: t(10)=2.811, p=0.0185). vGlut2 is unchanged between genotypes (t(10)=0.04986, p=0.9612). Student’s t-test. ns (not significant), *p<0.05, **p<0.01.
Decreased expression of mGlu2 and mGlu3 mRNA has also been observed in preclinical RTT mouse models, particularly in the cortex of male Mecp2 null mice (Bedogni et al., 2016; Chahrour et al., 2008; Pacheco et al., 2017). Consistent with these transcriptomic studies, we found significantly reduced Grm2 mRNA expression in the cortex of naïve 20–25-week-old female Mecp2Null/+ animals compared to the wild-type (WT) littermate controls, Mecp2+/+ (Figure 1B). Interestingly, cortical Grm3 mRNA was not statistically different between the mutant and control mice. Given that mGlu2 and mGlu3 have both been linked to cognitive function, specifically hippocampal-dependent cognition (De Filippis et al., 2015; Lyon et al., 2011), we assessed Grm2 and Grm3 transcript levels in the hippocampus, and found that hippocampal Grm2 and Grm3 mRNA levels were also decreased in Mecp2Null/+ animals (Figure 1C).
Next, we determined whether altered expression of mGlu2 and mGlu3 in Mecp2Null/+ mice, which express ~50% less MeCP2 than littermate controls Mecp2+/+ (Supplementary Figure 1), was maintained at the protein level. As illustrated in the representative immunoblots, only mGlu2 was significantly decreased in the cortex of Mecp2Null/+ animals compared to their WT counterparts, which is consistent with the transcript expression (Figure 1D-E, antibody validation in Supplementary Figure 2; mGlu3 global knockout animals have been previously characterized in (Dogra et al., 2021); monomer level in the cortex of both mGlu2 and mGlu3 was too low to be accurately quantified). Also in agreement with the transcript expression data was the observed decreased expression of mGlu2 and mGlu3 protein in the hippocampus of Mecp2Null/+ mice (Figure 1F-G). Notably, for both receptors, the dimer and total (sum of monomer and dimer) protein levels were significantly reduced in Mecp2Null/+ animals without differences in the control synaptic protein vGlut2 (Figure 1G). These clinical and preclinical data provided rationale for investigating the role and therapeutic potential of group II mGlu receptors in RTT and related disorders.
3.2. Group II mGlu receptor expression is increased in MECP2Tg1/o mice
We further explored the molecular relationship of mGlu2 and mGlu3 with MeCP2 using a rodent MDS model, MECP2Tg1/o, and tested the hypothesis that increased MeCP2 expression in the MECP2Tg1/o context also enhances mGlu2 and mGlu3 expression. Similar to the use of symptomatic female Mecp2Null/+ animals to reflect the clinical population, we utilized 8–9-week-old male MECP2Tg1/o mice and WT littermates to assess protein and mRNA expression in the hippocampus. In agreement with previous reports (Collins et al., 2004; Fisher et al., 2018; Na et al., 2012; Sztainberg et al., 2015), MeCP2 mRNA and protein expression were increased in MECP2Tg1/o mice compared to WT littermates (Figure 2A-C). Comparably, Grm2 and Grm3 mRNA levels were also significantly increased in MECP2Tg1/o animals (Figure 2A). To determine if this effect was also observed at the protein level, we evaluated mGlu2 and mGlu3 protein expression. As illustrated in the representative immunoblots, expression of both receptors was increased in MECP2Tg1/o animals (Figure 2B). In Figure 2C, quantification of expression showed that statistical significance was observed for the expression of the monomer, dimer and total (sum of monomer and dimer) protein for mGlu2, and the level of the dimer and total mGlu3 protein was significantly different between MECP2Tg1/o mice and WT littermates. These overexpression data of group II mGlu receptors suggested that these proteins could potentially contribute to the abnormal phenotypes in MDS mice, which could be amenable to receptor modulation.
Figure 2: Hippocampal mGlu2 and mGlu3 expression is increased in MDS mice.
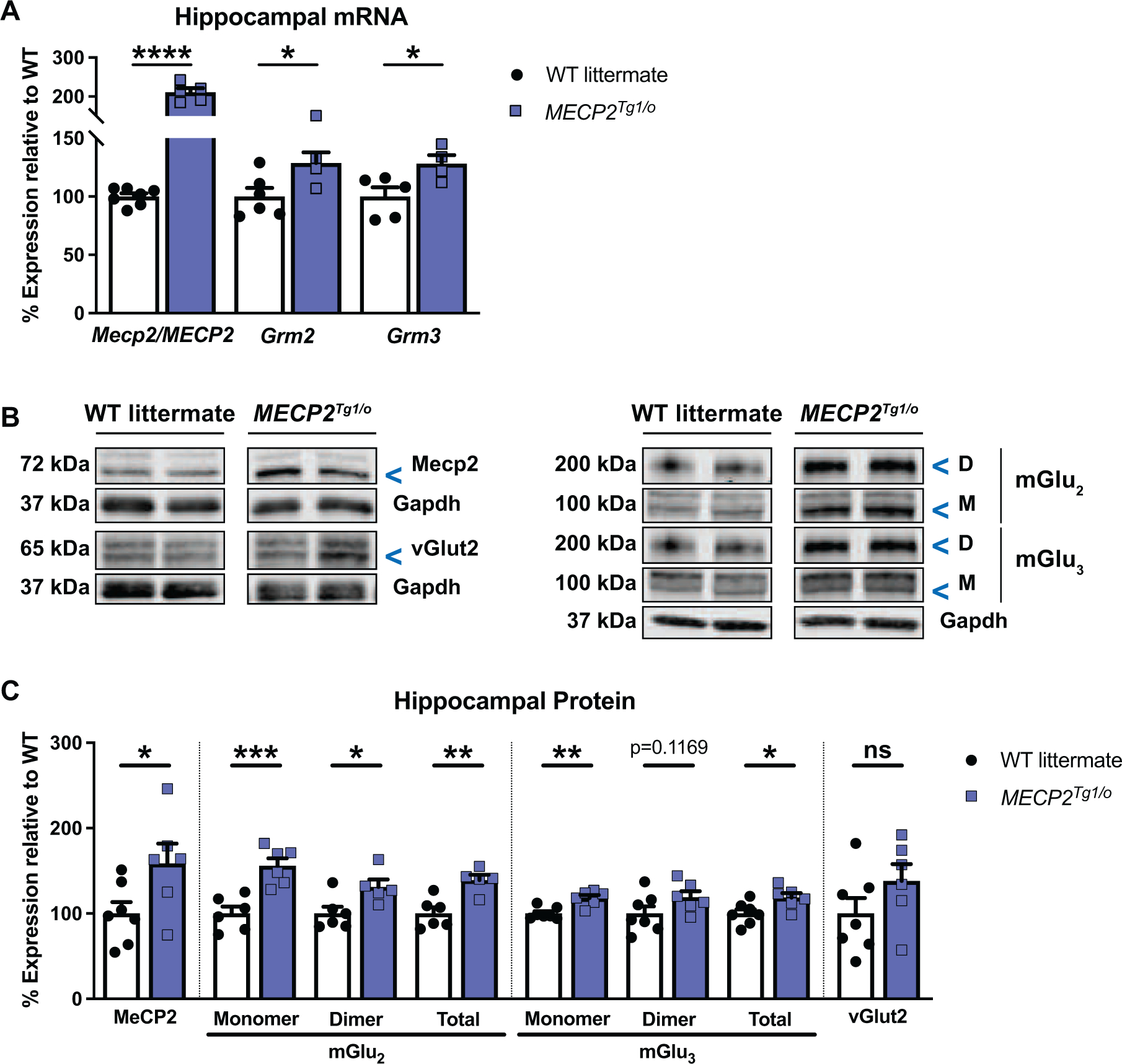
(A) Compared to WT littermates (white bars / black circles, n=5–7), Mecp2/MECP2, Grm2 and Grm3 transcripts are increased in the hippocampus of 8–9-week-old MECP2Tg1/o mice (blue bars / squares, n=4–5) (Mecp2/MECP2: t(10)=11.89, p<0.0001; Grm2: t(8)=2.476, p=0.0384; Grm3: t(7)=2.586, p=0.0362). (B) Representative immunoblots illustrating MeCP2 (72 kDa), mGlu2 or mGlu3 dimer (“D”, 200 kDa) and monomer (“M”, 100 kDa), vGlut2 (65 kDa), and Gapdh (37 kDa) loading control in hippocampal samples of WT littermates and MECP2Tg1/o mice. (C) Protein expression of MeCP2, mGlu2, and mGlu3 (total = monomer + dimer) is increased in MECP2Tg1/o mice (n=5–6) relative to WT littermates (n=6–7) (MeCP2: t(11)=2.269, p=0.0444; mGlu2: Monomer: t(10)=4.669, p=0.0009; Dimer: t(9)=2.642, p=0.0268; Total: t(9)=4.028, p=0.0030; mGlu3: Monomer: t(11)=4.140, p=0.0016; Dimer: t(11)=1.701, p=0.1169; Total: t(11)=2.597, p=0.0248). vGlut2 is unchanged between genotypes (t(11)=1.433, p=0.1797). Student’s t-test. ns (not significant), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.3. RTT and MDS mice exhibit abnormal and bidirectional phenotypes in trace fear acquisition
Numerous studies have characterized RTT model mice as exhibiting abnormal phenotypes in cognition, specifically in associative learning and memory, which is often assessed using a cued or contextual fear conditioning assay (Collins et al., 2021; Gogliotti et al., 2017, 2016; Lamonica et al., 2017; Merritt et al., 2020; Moretti et al., 2006; Pelka et al., 2006; Pitcher et al., 2015; Samaco et al., 2013; Schaevitz et al., 2013; Stearns et al., 2007; Vermudez et al., 2021). Interestingly, a recent study highlighted the role of mGlu3 in mediating a temporal- and hippocampal-dependent associative learning and memory phenotype known as trace fear acquisition or conditioning (Dogra et al., 2021). Prior to investigating the relationship of mGlu2/3 receptors and trace fear conditioning in a RTT mouse model, we first characterized the trace fear behavior of Mecp2Null/+ animals. In this task, the mice were trained to associate their environment and presented tone with an aversive stimulus in the form of a mild foot shock (0.7 mA); the “trace” period that separates the tone and shock presentations is thought to enhance learning (Figure 3A). As shown in Figure 3B, increases in percent freezing in Mecp2+/+ animals paralleled the increase in tone-trace-shock pairings (black symbols), reflecting learning behavior. However, we demonstrate here that Mecp2Null/+ mice exhibit abnormal trace fear acquisition, as illustrated by the attenuated percent freezing compared to their Mecp2+/+ counterparts. In particular, freezing behavior was significantly different between Mecp2+/+ and Mecp2Null/+ animals at the last trace period (T4), suggesting a deficit in trace fear acquisition.
Figure 3: Contrasting phenotypes in trace fear conditioning between Mecp2Null/+ and MECP2Tg1/o animals.
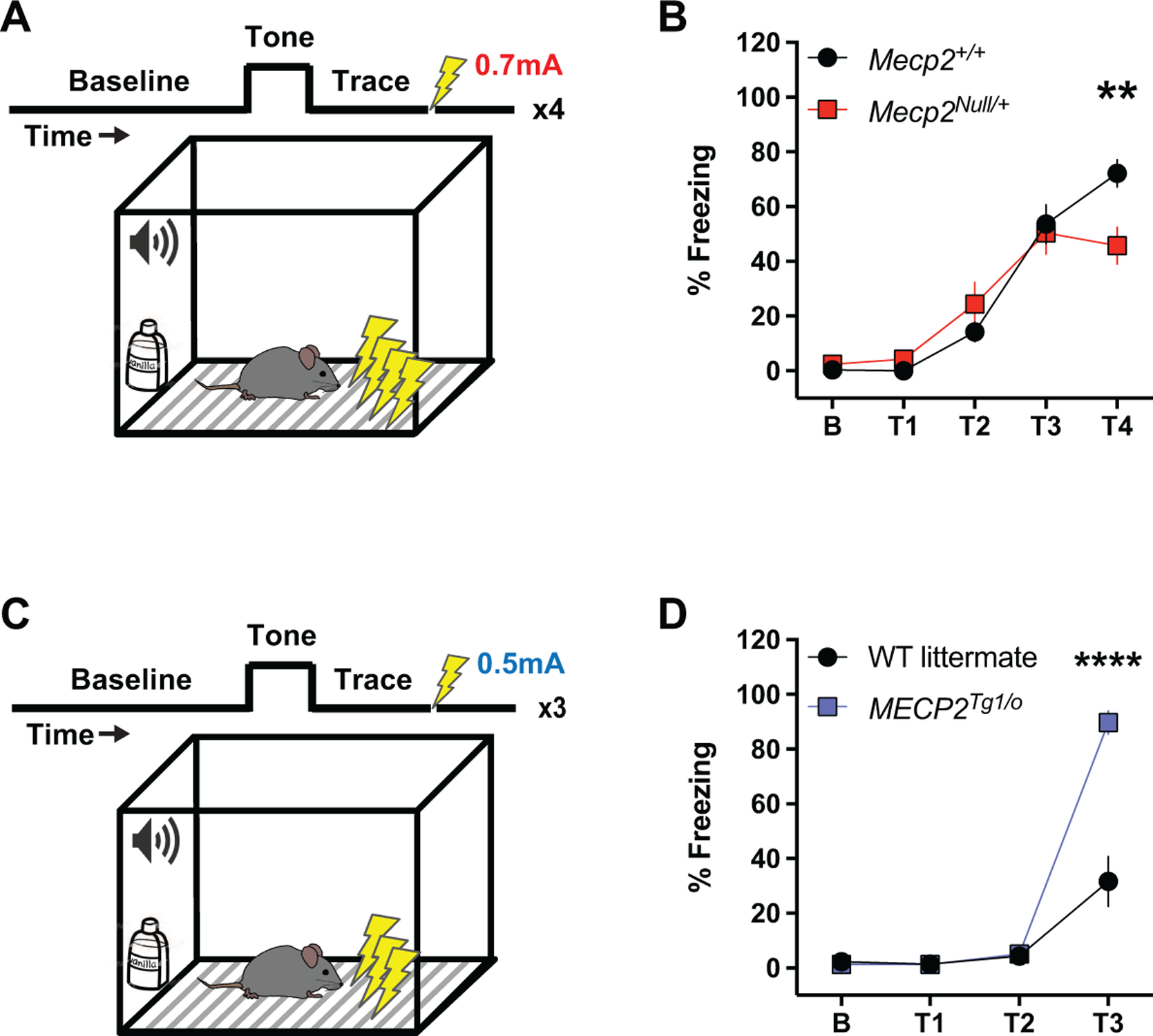
(A) Diagram illustrating the trace fear conditioning paradigm. Mice are trained to associate their environment (context and tone) with an aversive stimulus in the form of a mild foot shock (0.7 mA), with a temporal component (trace) separating the tone and shock. Four tone-trace-shock pairings are presented and percent freezing during the trace period is measured as a proxy for fear learning behavior. (B) Compared to littermate controls, Mecp2+/+ (black circles, n=17), 20–25-week-old Mecp2Null/+ animals (red squares, n=12) display attenuated percent freezing during the fourth trace period (T4) (F(4,108)=4.276; p=0.0016). (C) Diagram illustrating the trace fear conditioning paradigm in WT littermates and MECP2Tg1/o animals. The protocol mentioned above in RTT mice is followed with notable differences in the foot shock intensity (0.5 mA) and the number of tone-trace-shock pairings (three). (D) Compared to WT littermates (black circles, n=11), 8–12-week-old MECP2Tg1/o animals (blue squares, n=10) exhibit increased percent freezing during the third trace period (T3) (F(3,57)=35.14; p<0.0001). 2-way ANOVA with Sidak’s post-hoc test. **p<0.01, ****p<0.0001.
Similar to RTT model mice, MDS mice have been shown to exhibit abnormal phenotypes in cued or contextual fear associative learning and memory (Collins et al., 2004; Fisher et al., 2018; Na et al., 2014, 2012; Stansley et al., 2018). However, as in RTT model animals, phenotypes in trace fear conditioning have not been assessed in mice modeling MDS. WT littermate and MECP2Tg1/o animals were subjected to a similar trace fear acquisition paradigm as RTT mice, with the notable differences of a milder foot shock intensity (0.5 mA) and reduced number of tone-trace-shock pairings (Figure 3C). These changes were performed to account for the high percent freezing phenotype that MDS mice exhibit in fear conditioning assays (Collins et al., 2004; Fisher et al., 2018; Na et al., 2014, 2012; Stansley et al., 2018). Compared to WT littermates, MECP2Tg1/o mice displayed enhanced trace fear acquisition during the last trace period measured (T3, Figure 3D). This abnormal enhancement in trace fear acquisition was in contrast to the deficient phenotype in Mecp2Null/+ animals; coupled with the increases in expression of mGlu2 and mGlu3 in the hippocampus of MECP2Tg1/o mice, we hypothesized that these changes in trace fear behavior might be sensitive to mGlu2/3 receptor modulation.
3.4. mGlu2/3 activation reverses deficits in trace fear acquisition in Mecp2Null/+ mice
Detection and quantitation of a trace fear acquisition deficit in RTT mice allowed us to then test the hypothesis that administration of a nonselective mGlu2/3 agonist, LY379268, to increase receptor activity would improve this abnormal cognitive phenotype in Mecp2Null/+ animals. Animals were intraperitoneally administered with either vehicle (10% Tween-80) or 1mg/kg LY379268 30 minutes prior to trace fear conditioning (Figure 4A). LY379268 treatment reversed the trace fear acquisition deficit in vehicle-treated Mecp2Null/+ mice with statistical significance observed between vehicle- and LY379268-treated Mecp2Null/+ mice at trace periods 2 (T2) and 4 (T4) (Figure 4B-D). The increased freezing behavior at period T2 was only observed in Mecp2Null/+ animals (Figure 4C), suggesting an increased sensitivity to LY379268 in the mutant mice compared to their WT counterparts. In contrast, Mecp2+/+ animals did not exhibit a significant change in response to LY379268 at any of the trace periods measured. To eliminate the possibility that the increased percent freezing in Mecp2Null/+ animals was due to hypolocomotive effects of LY379268, as has previously been shown in WT animals (Imre, 2007; Imre et al., 2006; Woolley et al., 2008), we placed the mice in an open field chamber and monitored spontaneous locomotor activity. As illustrated in Supplementary Figure 2, 1mg/kg LY379268 did not change the distance traveled of Mecp2+/+ or Mecp2Null/+ animals, which already have reduced locomotion at baseline.
Figure 4: Attenuated trace fear acquisition in Mecp2Null/+ mice is improved with group II mGlu receptor activation.
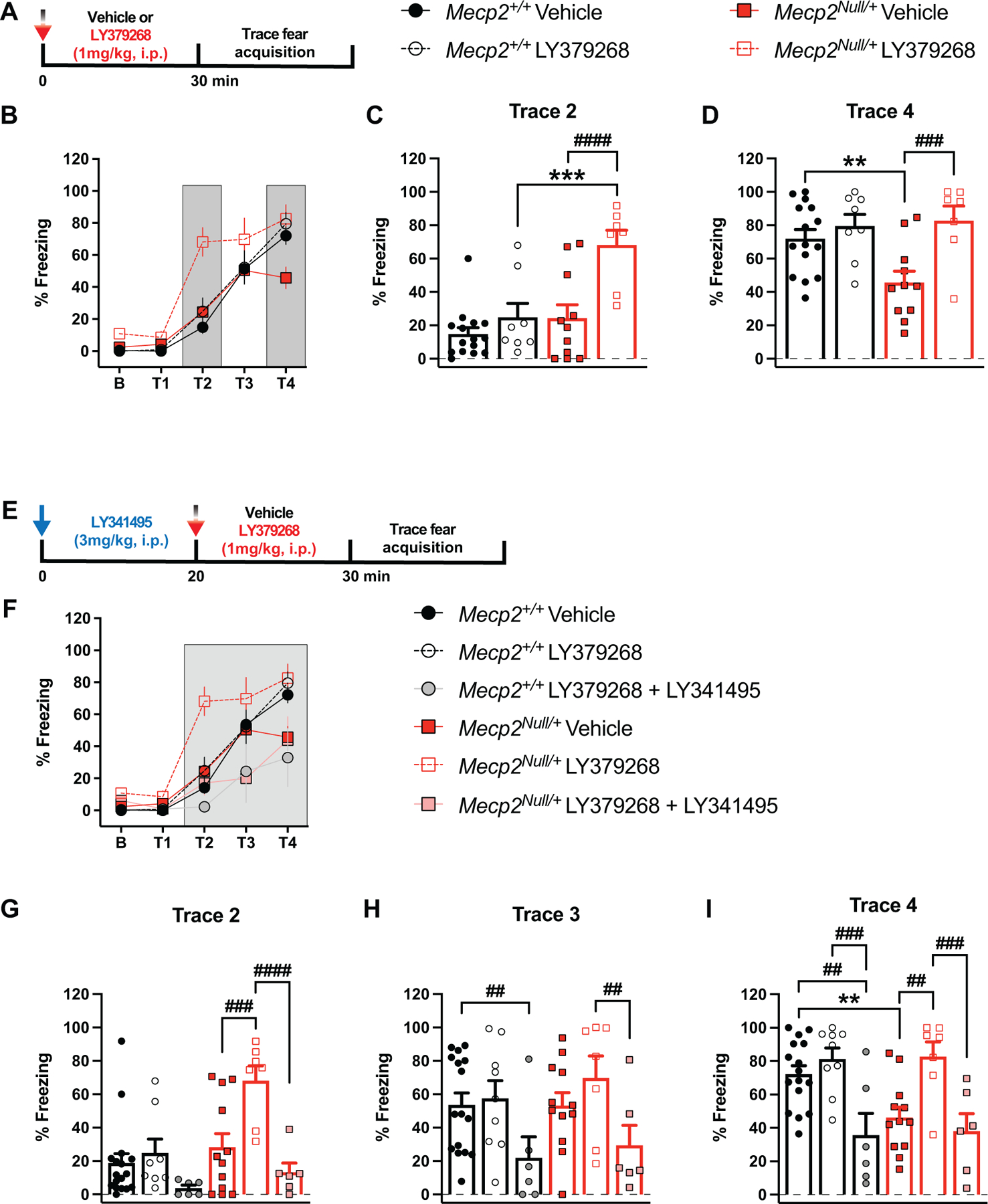
(A) Diagram illustrating timeline of drug administration prior to trace fear conditioning. Mice are treated intraperitoneally (i.p.) with vehicle (10% Tween-80) or 1mg/kg LY379268 (mGlu2/3 agonist) 30 minutes prior to placement in the fear conditioning box. (B-D) Treatment with LY379268 increases trace fear acquisition in Mecp2Null/+ mice (red bars, vehicle: closed red squares, n=15; LY379268: open red squares, n=7). Freezing behavior in littermate controls, Mecp2+/+(black bars) is not different between the treatment groups, vehicle (closed black circles, n=15) and LY379268 (open black circles, n=8). Analysis of trace periods T2 and T4 (dark grey bars in B) are shown in (C) and (D), respectively. (F(12,148)=3.314; ***p=0.0001 (T2, LY379268: Mecp2+/+ vs Mecp2Null/+), ####p<0.0001 (T2, Mecp2Null/+: Vehicle vs LY379268), **p=0.0034 (T4, Vehicle: Mecp2+/+ vs Mecp2Null/+), ###p=0.0005 (T4, Mecp2Null/+: Vehicle vs LY379268)). (E) Diagram illustrating timeline of administration of LY341495 (mGlu2/3 antagonist) and LY379268 prior to trace fear conditioning. LY341495 (3mg/kg, i.p.) is administered 20 minutes prior to LY379268 (1mg/kg, i.p.). (F-I) Co-administration of LY341495 and LY379268 blocked the reversal effect of LY379268 in Mecp2Null/+ animals (red bars, vehicle: closed red squares, n=12; LY341495 + LY379268: pink squares, n=6) at trace periods T2-T4. Percent freezing in Mecp2+/+ mice (black bars) is decreased with co-administration of LY341495 and LY379268, particularly at trace periods T3-T4 (vehicle: closed black circles, n=17; LY341495 + LY379268: grey circles, n=6). Analysis of trace periods T2-T4 (dark grey) is shown in (G-I). (F(20,200)=3.423; ###p=0.0005 (T2, Mecp2Null/+: Vehicle vs LY379268), ####p<0.0001 (T2, Mecp2Null/+: LY37968 vs LY379268 + LY341495), ##p=0.0044 (T3, Mecp2+/+: Vehicle vs LY379268 + LY341495), ##p=0.0043 (T3, Mecp2Null/+: LY379268 vs LY379268 + LY341495), **p=0.0042 (T4, Vehicle: Mecp2+/+ vs Mecp2Null/+), ##p=0.0011 (T4, Mecp2+/+: Vehicle vs LY379268 + LY341495), ###p=0.0008 (T4, Mecp2+/+: LY379268 vs LY379268 + LY341495), ##p=0.0020 (T4, Mecp2Null/+: Vehicle vs LY379268), ###p=0.0010 (T4, Mecp2Null/+: LY37968 vs LY379268 + LY341495)). 20–25-week-old mice. 2-way ANOVA with Tukey’s post-hoc test. *between-genotypes. #within-genotypes. **p<0.01, ***p<0.001, ##p<0.01, ###p<0.001, ####p<0.0001.
To further validate that the reversal effects in trace fear conditioning were due to mGlu2/3 modulation by LY379268 and not an off-target result, we administered the mGlu2/3 orthosteric antagonist LY341495 (3mg/kg) prior to LY379268 treatment (Figure 4E). LY341495 blocked the effect of LY379268 in Mecp2Null/+ mice, specifically at trace periods T2-T4 (Figure 4F-I). In these trace periods, the freezing behavior of vehicle-treated Mecp2Null/+ animals was indistinguishable from Mecp2Null/+ mice that received both LY341495 and LY379268. Importantly, the ability of LY341495 to block LY379268’s increased freezing effect at period T2 in Mecp2Null/+ animals suggests that the left-shifted response of the mutant animals to LY379268 is a consequence of mGlu2/3 modulation (Figure 4G). Interestingly, co-administration of LY379268 and LY341495 to Mecp2+/+ animals decreased percent freezing at trace periods T3-T4 (Figure 4H-I). Again, this could potentially be due to alterations in locomotor activity; however, assessment of distance traveled in an open field assay revealed no locomotor effects of LY341495 and LY379268 co-administration in either the Mecp2+/+ and Mecp2+/− mice (Supplementary Figure 3). Overall, these data support the ability of mGlu2/3 activation to enhance the trace fear acquisition response in Mecp2Null/+ animals.
3.5. mGlu2/3 antagonism normalizes the abnormal trace fear acquisition phenotype in MECP2Tg1/o mice
Given the beneficial effects of activating mGlu2/3 in RTT mice, which display opposite trace fear conditioning phenotypes from MDS mice, we then posited the converse hypothesis: that inhibiting mGlu2/3 could alleviate the abnormal increase in trace fear behavior in MECP2Tg/o animals. Prior to trace fear conditioning, animals were intraperitoneally administered either vehicle (10% Tween-80) or the nonselective orthosteric mGlu2/3 antagonist LY341495 (3mg/kg) (Figure 5A). LY341495 treatment did not affect trace fear behavior in WT littermates (Figure 5B-C). However, MECP2Tg1/o animals administered LY341495 exhibited significantly reduced percent freezing at the last tone-trace-shock pairing (T3) compared to vehicle-treated MECP2Tg1/o mice (Figure 5C). Notably, LY341495-treated MECP2Tg1/o mice displayed comparable freezing behavior to vehicle-treated WT counterparts. To rule out potential locomotor effects of mGlu2/3 inhibition with the nonselective antagonist, we placed vehicle- and LY341495-treated animals in an open field chamber. At baseline, MECP2Tg1/o mice presented with hypolocomotor activity compared to WT littermates (Supplementary Figure 4). Importantly, regardless of genotype, LY341495 administration did not affect distance traveled in the open field task, which is in agreement with previous studies using systemic administration of LY341495 (Fukumoto et al., 2016; Gleason et al., 2013). Overall, these data suggest that mGlu2/3 antagonism has positive effects in normalizing enhanced trace fear behavior in MECP2Tg1/o animals.
Figure 5: Group II mGlu receptor antagonism normalizes enhanced trace fear acquisition in MECP2Tg1/o mice.
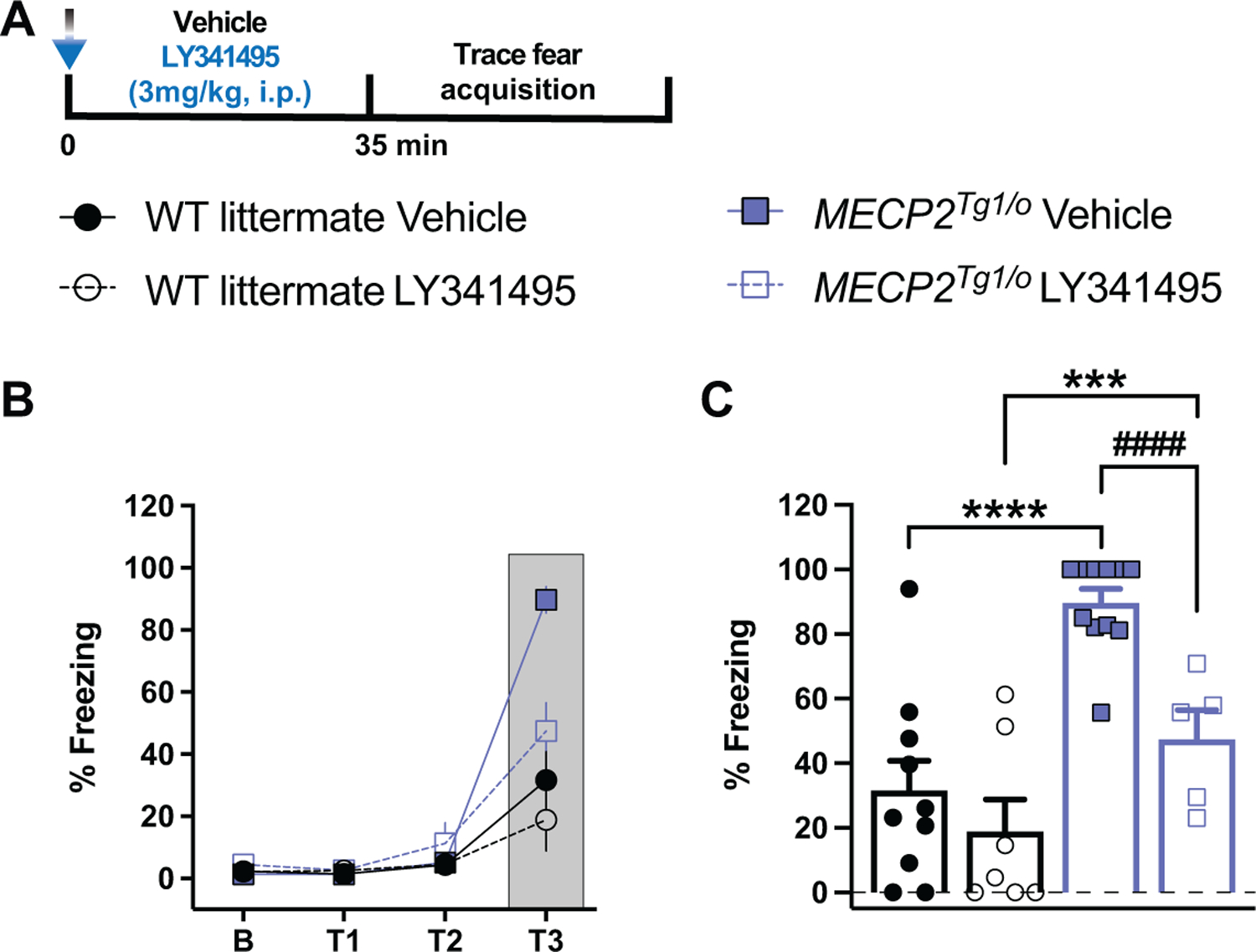
(A) Diagram illustrating the timeline of LY341495 administration prior to trace fear conditioning. Mice are treated intraperitoneally (i.p.) with vehicle (10% Tween-80) or 3mg/kg LY341495 (mGlu2/3 antagonist) 35 minutes prior to placement in the fear conditioning box. (B-C) LY341495 treatment significantly decreases the enhanced trace fear acquisition phenotype in MECP2Tg1/o mice (blue bars, vehicle: closed blue squares, n=11; LY341495: open blue squares, n=5) but not in WT littermates (white bars, vehicle: closed black circles, n=10; LY341495: open black circles, n=7). Analysis of trace period T3 (dark grey) is shown in (C). (F(9,87)=16.87; ****p<0.0001 (Vehicle: WT vs MECP2Tg1/o), ***p=0.0008 (LY341495: WT vs MECP2Tg1/o), ####p<0.0001 (MECP2Tg1/o: Vehicle vs LY341495)). 8–12-week-old mice. 2-way ANOVA with Tukey’s post-hoc test. *between-genotypes. #within-genotypes. ***p<0.001, ****p<0.0001, ####p<0.0001.
4. Discussion
Our understanding of MECP2-associated disorders has developed tremendously in the past 20 years. However, despite the monogenicity of these disorders and the link of MeCP2 protein expression and/or function to each disease, no effective treatment is currently available for RTT or MDS. Promisingly, recent studies have demonstrated that genetic manipulations of MECP2 and pharmacological modulations of downstream targets of the protein improve phenotypes in preclinical rodent models of RTT (Bittolo et al., 2016; Degano et al., 2014; Gadalla et al., 2017, 2013; Garg et al., 2013; Gogliotti et al., 2018, 2017, 2016; Li et al., 2017; Luoni et al., 2020; Matagne et al., 2021, 2017; Ogier et al., 2007; Powers et al., 2019; Roux et al., 2007; Scaramuzza et al., 2021; Sinnett et al., 2017; Tillotson et al., 2017; Zanella et al., 2008) and MDS (Koerner et al., 2018; Na et al., 2014; Sztainberg et al., 2015). In this study, we aimed to capitalize on previous reports by investigating two potential therapeutic targets, mGlu2 and mGlu3, and employing pharmacological modulators of these receptors to evaluate effects in RTT (Mecp2Null/+) and MDS (MECP2Tg1/o) mouse models.
mGlu2 and mGlu3 are group II mGlu receptors that have been robustly implicated in neuropsychiatric disorders (Chaki, 2017; Joffe and Conn, 2019; Maksymetz et al., 2017; Swanson et al., 2005; Walker and Conn, 2015). Their role in neurodevelopmental disorders is limited; however, mGlu3 has been shown to be involved in the cognitive phenotypes of Fragile X syndrome (FXS) (Choi et al. 2011) and mGlu2/3 receptors have been implicated in autism-like phenotypes in a rat model of autism (Chen et al. 2014). Moreover, expression studies demonstrate that both mGlu2 and mGlu3 are decreased in male Mecp2Null/y mice and patients diagnosed with RTT (Bedogni et al. 2016; Chahrour et al. 2008; Pacheco et al. 2017; Ben-Shachar et al. 2009; Gogliotti et al. 2018; Lin et al. 2016). Here, we further support these data by showing attenuated mGlu2 and mGlu3 expression in the temporal cortex of RTT autopsy samples from patients with truncating mutations in MeCP2. Altered levels of these receptors are conserved in a female RTT mouse model, Mecp2Null/+, specifically in the cortex and hippocampus. Since RTT and MDS are due to loss-of-function mutations and multiple copies of MECP2, respectively, and given the role of MeCP2 as a transcriptional activator (Chahrour et al. 2008), we posited that mGlu2 and mGlu3 expression would be increased in MDS. In the hippocampus of MECP2Tg1/o mice, we confirmed previous studies showing increased MeCP2 expression (Collins et al. 2004; Fisher et al. 2018; Na et al. 2012) and also observed increased mGlu2 and mGlu3 mRNA and protein expression. These findings suggest that group II mGlu receptors may be targets of MeCP2 transcriptional regulation, whether through direct or indirect mechanisms.
These molecular data provided our rationale to further explore mGlu2 and mGlu3 in the behavioral phenotypes of RTT and MDS, focusing on the learning and memory phenotypes given the role of group II mGlu receptors in cognition. Numerous studies have established that RTT and MDS mice have contrasting characteristics in contextual fear conditioning, a hippocampal-dependent learning and memory paradigm (Na et al. 2012; Na et al. 2014; Collins et al. 2004; Fisher et al. 2018; Gogliotti et al. 2017; Stansley et al. 2018; Moretti et al. 2006; Samaco et al. 2012; Stearns et al. 2007). In particular, RTT mice exhibit deficits in contextual fear conditioning, whereas MDS mice display an abnormal enhancement of this behavior. Several studies have described a similar hippocampal-dependent cognitive task that relies on both the spatial and temporal factors of learning and memory called trace fear conditioning (Huerta et al. 2000; Xu et al. 2014; Zhao et al. 2005; McEchron et al. 1998). Excitingly, our group has demonstrated a specific role for mGlu3 in trace fear acquisition (Dogra et al. 2021). Before exploring the relationship of mGlu2/3 receptors and trace fear conditioning in mouse models of MECP2-associated disorders, we first characterized the behavior of both Mecp2Null/+ and MECP2Tg1/o animals in this cognitive task, which has not yet been investigated. Here, we extend previous studies in cognitive domains to show that Mecp2Null/+ mice exhibit a deficit in trace fear acquisition. Conversely, MECP2Tg1/o animals have an abnormal enhanced trace fear acquisition phenotype, which supports the bidirectional behavioral phenotypes of cognition in mouse models of these two disorders.
These data prompted us to test our hypothesis that mGlu2/3 modulation could improve abnormal cognitive phenotypes in Mecp2Null/+ and MECP2Tg1/o mice using the trace fear paradigm. Previous preclinical studies have shown that mGlu3 activation and mGlu2 positive allosteric modulators improve deficits in cognitive tasks (Griebel et al. 2016; Nikiforuk et al. 2010; Walker et al. 2017). Given that both receptors are decreased in Mecp2Null/+ mice, it is likely that the function of both mGlu2 and mGlu3 is also disrupted. Therefore, we activated both receptors using a nonselective mGlu2/3 agonist, LY379268. Excitingly, in Mecp2Null/+ animals, LY379268 rescued deficiencies in trace fear acquisition. Interestingly, we observed a left-shifted response to LY379268 that was specific to Mecp2Null/+ mice. This could be attributed to a ceiling effect of the trace fear paradigm, for example the strong shock intensity, on the baseline trace fear behavior of Mecp2+/+ animals, which has also been previously observed in WT animals (Dogra et al. 2021), or to a potential sensitivity of the receptors to agonist in the Mecp2Null/+ context. Importantly, LY379268’s effect in augmenting freezing behavior in Mecp2Null/+ was completely blocked by the mGlu2/3 antagonist, LY341495, suggesting that LY379268 mediates its effects via mGlu2 and mGlu3 in the trace fear acquisition paradigm in RTT mice.
In contrast to the effects seen in Mecp2Null/+ animals, inhibition of mGlu2/3 receptors with LY341495 reversed the abnormally enhanced trace fear acquisition of MECP2Tg1/o mice. The lack of LY341495’s effect in WT animals suggests that the heightened trace fear phenotype in MECP2Tg1/o mice is driven, in part, by the increased expression of the mGlu2 and/or mGlu3 receptors. The bidirectionality in mGlu2/3 modulation to alleviate the abnormal behavioral cognition in Mecp2Null/+ and MECP2Tg1/o animals parallels the contrasting trace fear phenotypes. These together may indicate that, in both the RTT and MDS contexts where both receptors are affected, one or both of the group II mGlu receptors are needed to mediate this form of associative learning and memory.
In MECP2Tg1/o and Mecp2Null/+ mice, the functional consequences of altered mGlu2 and mGlu3 receptor expression, such as in impacting long-term synaptic plasticity in the hippocampal Schaffer collateral-CA1 (SC-CA1) synapse, remains to be elucidated. Previous reports have demonstrated that SC-CA1 long-term potentiation (LTP) is bidirectionally altered in Mecp2 mutant and MECP2Tg1/o animals (Asaka et al. 2006; Collins et al. 2004; Guy et al. 2007; Moretti et al. 2006; Sztainberg et al. 2015). Additionally, in Mecp2 mutant mice, NMDA antagonism has been shown to reverse LTP deficits (Weng et al. 2011). Although no studies have yet shown whether NMDA function is implicated in MDS, NMDA dysregulation has been associated with the enhanced LTP phenotype of mice modeling a related disorder, Pitt-Hopkins syndrome (Kennedy et al. 2016; Thaxton et al. 2018). Coupling these data with previous evidence illustrating that mGlu2/3 receptors play a role in NMDA-mediated LTP at the SC-CA1 synapses (Rosenberg et al. 2016), it is therefore possible that mGlu2/3 modulation may correct abnormal SC-CA1 LTP in RTT and MDS animals. However, empirical studies are needed to test this theory.
In conclusion, we have demonstrated that the expression of mGlu2 and mGlu3 is altered in RTT clinical samples and bidirectionally affected in Mecp2Null/+ and MECP2Tg1/o animals, indicating a potential role of group II mGlu receptors in RTT and MDS phenotypes. Correspondingly, we establish that Mecp2Null/+ and MECP2Tg1/o mice present abnormal and contrasting phenotypes in trace fear conditioning, a temporal-dependent form of associative learning and memory. Pharmacological mGlu2/3 activation or antagonism exerted efficacy in modulating trace fear behavior of Mecp2Null/+ and MECP2Tg1/o mice, respectively. Altogether, our data provide novel evidence that mGlu2 and mGlu3 are implicated in neurodevelopmental disorders, particularly in RTT and MDS.
Supplementary Material
Highlights.
mGlu2/mGlu3 receptors are decreased in Rett syndrome patients and mice, Mecp2Null/+
mGlu2/mGlu3 receptors are increased in MECP2 Duplication syndrome mice, MECP2Tg1/o
mGlu2/3 activation rescues trace fear acquisition deficits in Mecp2Null/+ mice
mGlu2/3 inhibition reverses heightened trace fear acquisition in MECP2Tg1/o mice
Acknowledgments
We acknowledge the donation of samples from RTT patient families, and we thank the University of Maryland Brain and Tissue Bank, the Harvard Brain Tissue Resource Center, the National Institutes of Health NeuroBioBank and Rettsyndrome.org, for their assistance in obtaining the valuable resource of autopsy samples. We also thank Dr. John Allison at the Vanderbilt Mouse Neurobehavioral Lab (MNL) Core for his assistance in the execution of the behavioral assays, and the Vanderbilt Medical Center Molecular Cell Biology Resource (MCBR) Core for providing the immunoblot imaging and qRT-PCR detection systems.
Funding
This work was supported by grant number 3503 from Rettsyndrome.org and R01MH062646 from the National Institutes of Mental Health (NIMH). S.A.D.V. was supported by a National Institutes of Health (NIH) training grant T32 GM007628 and a pre-doctoral fellowship, F31MH119699, from NIMH. R.G.G. was supported by NIMH K01MH112983 and National Institute of Neurological Disorders and Stroke (NINDS) R01NS112171.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Colleen Niswender reports financial support was provided by Rettsyndrome.org. Colleen Niswender reports financial support was provided by National Institute of Mental Health. Sheryl Vermudez reports financial support was provided by National Institute of Mental Health. Rocco Gogliotti reports financial support was provided by National Institute of Mental Health. Colleen Niswender reports financial support was provided by National Institute of Neurological Disorders and Stroke. Colleen Niswender reports a relationship with Boehringer Ingelheim Pharmaceuticals Inc that includes: funding grants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Autry AE, Covington HE, Monteggia LM, 2009. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J. Neurosci. 29, 4218–4227. doi: 10.1523/JNEUROSCI.4225-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY, 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188. doi: 10.1038/13810 [DOI] [PubMed] [Google Scholar]
- Asaka Y, Jugloff DGM, Zhang L, Eubanks JH, Fitzsimonds RM, 2006. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 21, 217–227. doi: 10.1016/j.nbd.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Ballinger EC, Schaaf CP, Patel AJ, de Maio A, Tao H, Talmage DA, Zoghbi HY, Role LW, 2019. Mecp2 Deletion from Cholinergic Neurons Selectively Impairs Recognition Memory and Disrupts Cholinergic Modulation of the Perirhinal Cortex. eNeuro 6. doi: 10.1523/ENEURO.0134-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni F, Cobolli Gigli C, Pozzi D, Rossi RL, Scaramuzza L, Rossetti G, Pagani M, Kilstrup-Nielsen C, Matteoli M, Landsberger N, 2016. Defects during mecp2 null embryonic cortex development precede the onset of overt neurological symptoms. Cereb. Cortex 26, 2517–2529. doi: 10.1093/cercor/bhv078 [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY, 2009. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet. 18, 2431–2442. doi: 10.1093/hmg/ddp181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittolo T, Raminelli CA, Deiana C, Baj G, Vaghi V, Ferrazzo S, Bernareggi A, Tongiorgi E, 2016. Pharmacological treatment with mirtazapine rescues cortical atrophy and respiratory deficits in MeCP2 null mice. Sci. Rep. 6, 19796. doi: 10.1038/srep19796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Selfridge J, Lagger S, Connelly J, De Sousa D, Kerr A, Webb S, Guy J, Merusi C, Koerner MV, Bird A, 2016. The molecular basis of variable phenotypic severity among common missense mutations causing Rett syndrome. Hum. Mol. Genet. 25, 558–570. doi: 10.1093/hmg/ddv496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY, 2008. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320, 1224–1229. doi: 10.1126/science.1153252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, 2017. mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends Pharmacol. Sci. 38, 569–580. doi: 10.1016/j.tips.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R, 2001. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 27, 327–331. doi: 10.1038/85906 [DOI] [PubMed] [Google Scholar]
- Chen Y-W, Lin H-C, Ng M-C, Hsiao Y-H, Wang C-C, Gean P-W, Chen PS, 2014. Activation of mGluR2/3 underlies the effects of N-acetylcystein on amygdala-associated autism-like phenotypes in a valproate-induced rat model of autism. Front. Behav. Neurosci. 8, 219. doi: 10.3389/fnbeh.2014.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, Woo NH, Tranfaglia MR, Bear MF, Zukin RS, McDonald TV, Jongens TA, McBride SMJ, 2011. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 1380, 106–119. doi: 10.1016/j.brainres.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY, 2004. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 13, 2679–2689. doi: 10.1093/hmg/ddh282 [DOI] [PubMed] [Google Scholar]
- Collins BE, Merritt JK, Erickson KR, Neul JL, 2021. Safety and efficacy of genetic MECP2 supplementation in the R294X mouse model of Rett syndrome. Genes Brain Behav. e12739. doi: 10.1111/gbb.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degano AL, Park MJ, Penati J, Li Q, Ronnett GV, 2014. MeCP2 is required for activity-dependent refinement of olfactory circuits. Mol. Cell. Neurosci. 59, 63–75. doi: 10.1016/j.mcn.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Valenti D, Chiodi V, Ferrante A, de Bari L, Fiorentini C, Domenici MR, Ricceri L, Vacca RA, Fabbri A, Laviola G, 2015. Modulation of Rho GTPases rescues brain mitochondrial dysfunction, cognitive deficits and aberrant synaptic plasticity in female mice modeling Rett syndrome. Eur. Neuropsychopharmacol. 25, 889–901. doi: 10.1016/j.euroneuro.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Dogra S, Stansley BJ, Xiang Z, Qian W, Gogliotti RG, Nicoletti F, Lindsley CW, Niswender CM, Joffe ME, Conn PJ, 2021. Activating mGlu3 Metabotropic Glutamate Receptors Rescues Schizophrenia-like Cognitive Deficits Through Metaplastic Adaptations Within the Hippocampus. Biol. Psychiatry 90, 385–398. doi: 10.1016/j.biopsych.2021.02.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR, 2004. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci USA 101, 12604–12609. doi: 10.1073/pnas.0405077101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher NM, Gogliotti RG, Vermudez SAD, Stansley BJ, Conn PJ, Niswender CM, 2018. Genetic Reduction or Negative Modulation of mGlu7 Does Not Impact Anxiety and Fear Learning Phenotypes in a Mouse Model of MECP2 Duplication Syndrome. ACS Chem. Neurosci. 9, 2210–2217. doi: 10.1021/acschemneuro.7b00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R, Nii T, Iwaki A, Shibata A, Ito I, Kitaichi K, Nomura M, Hattori S, Takao K, Miyakawa T, Fukumaki Y, 2014. Comprehensive behavioral study of mGluR3 knockout mice: implication in schizophrenia related endophenotypes. Mol. Brain 7, 31. doi: 10.1186/1756-6606-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S, 2016. The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology 41, 1046–1056. doi: 10.1038/npp.2015.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe SL, Neul JL, Samaco RC, Chao H-T, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, Zoghbi HY, 2008. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron 59, 947–958. doi: 10.1016/j.neuron.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla KKE, Bailey MES, Spike RC, Ross PD, Woodard KT, Kalburgi SN, Bachaboina L, Deng JV, West AE, Samulski RJ, Gray SJ, Cobb SR, 2013. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. Mol. Ther. 21, 18–30. doi: 10.1038/mt.2012.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla KKE, Vudhironarit T, Hector RD, Sinnett S, Bahey NG, Bailey MES, Gray SJ, Cobb SR, 2017. Development of a Novel AAV Gene Therapy Cassette with Improved Safety Features and Efficacy in a Mouse Model of Rett Syndrome. Mol. Ther. Methods Clin. Dev. 5, 180–190. doi: 10.1016/j.omtm.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, Foust KD, Kaspar BK, Bird A, Mandel G, 2013. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J. Neurosci. 33, 13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM, 2006. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol. Psychiatry 59, 468–476. doi: 10.1016/j.biopsych.2005.07.025 [DOI] [PubMed] [Google Scholar]
- Gleason SD, Li X, Smith IA, Ephlin JD, Wang XS, Heinz BA, Carter JH, Baez M, Yu J, Bender DM, Witkin JM, 2013. mGlu2/3 agonist-induced hyperthermia: an in vivo assay for detection of mGlu2/3 receptor antagonism and its relation to antidepressant-like efficacy in mice. CNS Neurol. Disord. Drug Targets 12, 554–566. doi: 10.2174/18715273113129990079 [DOI] [PubMed] [Google Scholar]
- Goffin D, Allen M, Zhang L, Amorim M, Wang I-TJ, Reyes A-RS, Mercado-Berton A, Ong C, Cohen S, Hu L, Blendy JA, Carlson GC, Siegel SJ, Greenberg ME, Zhou Z, 2011. Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci. 15, 274–283. doi: 10.1038/nn.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Fisher NM, Stansley BJ, Jones CK, Lindsley CW, Conn PJ, Niswender CM, 2018. Total RNA sequencing of rett syndrome autopsy samples identifies the M4 muscarinic receptor as a novel therapeutic target. J. Pharmacol. Exp. Ther. 365, 291–300. doi: 10.1124/jpet.117.246991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Fisher NM, Adams J, Zamorano R, Walker AG, Blobaum AL, Engers DW, Hopkins CR, Daniels JS, Jones CK, Lindsley CW, Xiang Z, Conn PJ, Niswender CM, 2017. mGlu7 potentiation rescues cognitive, social, and respiratory phenotypes in a mouse model of Rett syndrome. Sci. Transl. Med. 9. doi: 10.1126/scitranslmed.aai7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, Stauffer SR, Bridges TM, Bartolome JM, Daniels JS, Jones CK, Lindsley CW, Conn PJ, Niswender CM, 2016. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum. Mol. Genet. 25, 1990–2004. doi: 10.1093/hmg/ddw074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Pichat P, Boulay D, Naimoli V, Potestio L, Featherstone R, Sahni S, Defex H, Desvignes C, Slowinski F, Vigé X, Bergis OE, Sher R, Kosley R, Kongsamut S, Black MD, Varty GB, 2016. The mGluR2 positive allosteric modulator, SAR218645, improves memory and attention deficits in translational models of cognitive symptoms associated with schizophrenia. Sci. Rep. 6, 35320. doi: 10.1038/srep35320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A, 2007. Reversal of neurological defects in a mouse model of Rett syndrome. Science 315, 1143–1147. doi: 10.1126/science.1138389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A, 2001. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322–326. doi: 10.1038/85899 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PWJ, Lane TA, 2008. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol (Oxford) 22, 308–322. doi: 10.1177/0269881108089818 [DOI] [PubMed] [Google Scholar]
- Heckman LD, Chahrour MH, Zoghbi HY, 2014. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. eLife 3. doi: 10.7554/eLife.02676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S, 2000. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 25, 473–480. doi: 10.1016/s0896-6273(00)80909-5 [DOI] [PubMed] [Google Scholar]
- Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ, 2006. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol. Biochem. Behav. 84, 392–399. doi: 10.1016/j.pbb.2006.05.021 [DOI] [PubMed] [Google Scholar]
- Imre G, 2007. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 13, 444–464. doi: 10.1111/j.1527-3458.2007.00024.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentarra GM, Olfers SL, Rice SG, Srivastava N, Homanics GE, Blue M, Naidu S, Narayanan V, 2010. Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation. BMC Neurosci. 11, 19. doi: 10.1186/1471-2202-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Conn PJ, 2019. Antidepressant potential of metabotropic glutamate receptor mGlu2 and mGlu3 negative allosteric modulators. Neuropsychopharmacology 44, 214–236. doi: 10.1038/s41386-018-0192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Santiago CI, Engers JL, Lindsley CW, Conn PJ, 2019. Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol. Psychiatry 24, 916–927. doi: 10.1038/s41380-017-0015-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, Lindsley CW, Winder DG, Conn PJ, 2020. mGlu2 and mGlu3 Negative Allosteric Modulators Divergently Enhance Thalamocortical Transmission and Exert Rapid Antidepressant-like Effects. Neuron 105, 46–59.e3. doi: 10.1016/j.neuron.2019.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AJ, Rahn EJ, Paulukaitis BS, Savell KE, Kordasiewicz HB, Wang J, Lewis JW, Posey J, Strange SK, Guzman-Karlsson MC, Phillips SE, Decker K, Motley ST, Swayze EE, Ecker DJ, Michael TP, Day JJ, Sweatt JD, 2016. Tcf4 regulates synaptic plasticity, DNA methylation, and memory function. Cell Rep. 16, 2666–2685. doi: 10.1016/j.celrep.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Alvarez-Saavedra M, Sáez MA, Saona A, Young JI, 2008. Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Hum. Mol. Genet. 17, 1707–1717. doi: 10.1093/hmg/ddn061 [DOI] [PubMed] [Google Scholar]
- Koerner MV, FitzPatrick L, Selfridge J, Guy J, De Sousa D, Tillotson R, Kerr A, Sun Z, Lazar MA, Lyst MJ, Bird A, 2018. Toxicity of overexpressed MeCP2 is independent of HDAC3 activity. Genes Dev. 32, 1514–1524. doi: 10.1101/gad.320325.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainiola M, Procaccini C, Linden A-M, 2014. mGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behav. Brain Res. 266, 94–103. doi: 10.1016/j.bbr.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Lamonica JM, Kwon DY, Goffin D, Fenik P, Johnson BS, Cui Y, Guo H, Veasey S, Zhou Z, 2017. Elevating expression of MeCP2 T158M rescues DNA binding and Rett syndrome-like phenotypes. J. Clin. Invest. 127, 1889–1904. doi: 10.1172/JCI90967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A, Liu D, Han L, Jiang ZI, Tsai GE, Basu AC, Picker J, Feng J, Coyle JT, 2007. Ube3a mRNA and protein expression are not decreased in Mecp2R168X mutant mice. Brain Res. 1180, 1–6. doi: 10.1016/j.brainres.2007.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Nicholls L, Assareh H, Fang Z, Amos TG, Edwards RJ, Assareh AA, Voineagu I, 2016. Transcriptome analysis of human brain tissue identifies reduced expression of complement complex C1Q Genes in Rett syndrome. BMC Genomics 17, 427. doi: 10.1186/s12864-016-2746-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Bellot-Saez A, Phillips ML, Yang T, Longo FM, Pozzo-Miller L, 2017. A small-molecule TrkB ligand restores hippocampal synaptic plasticity and object location memory in Rett syndrome mice. Dis. Model. Mech. 10, 837–845. doi: 10.1242/dmm.029959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi LM, Baker SA, Zoghbi HY, 2015. MECP2 disorders: from the clinic to mice and back. J. Clin. Invest. 125, 2914–2923. doi: 10.1172/JCI78167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikenhuis S, Giacometti E, Beard CF, Jaenisch R, 2004. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci USA 101, 6033–6038. doi: 10.1073/pnas.0401626101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoni M, Giannelli S, Indrigo MT, Niro A, Massimino L, Iannielli A, Passeri L, Russo F, Morabito G, Calamita P, Gregori S, Deverman B, Broccoli V, 2020. Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome. eLife 9. doi: 10.7554/eLife.52629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon L, Burnet PWJ, Kew JNC, Corti C, Rawlins JNP, Lane T, De Filippis B, Harrison PJ, Bannerman DM, 2011. Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and cognition. Neuropsychopharmacology 36, 2616–2628. doi: 10.1038/npp.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymetz J, Moran SP, Conn PJ, 2017. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol. Brain 10, 15. doi: 10.1186/s13041-017-0293-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne V, Borloz E, Ehinger Y, Saidi L, Villard L, Roux J-C, 2021. Severe offtarget effects following intravenous delivery of AAV9-MECP2 in a female mouse model of Rett syndrome. Neurobiol. Dis. 149, 105235. doi: 10.1016/j.nbd.2020.105235 [DOI] [PubMed] [Google Scholar]
- Matagne V, Ehinger Y, Saidi L, Borges-Correia A, Barkats M, Bartoli M, Villard L, Roux J-C, 2017. A codon-optimized Mecp2 transgene corrects breathing deficits and improves survival in a mouse model of Rett syndrome. Neurobiol. Dis. 99, 1–11. doi: 10.1016/j.nbd.2016.12.009 [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF, 1998. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 8, 638–646. doi: [DOI] [PubMed] [Google Scholar]
- Merritt JK, Collins BE, Erickson KR, Dong H, Neul JL, 2020. Pharmacological readthrough of R294X Mecp2 in a novel mouse model of Rett Syndrome. Hum. Mol. Genet. doi: 10.1093/hmg/ddaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY, 2005. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 14, 205–220. doi: 10.1093/hmg/ddi016 [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY, 2006. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 26, 319–327. doi: 10.1523/JNEUROSCI.2623-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Morris MJ, Nelson ED, Monteggia LM, 2014. GABAA receptor antagonism ameliorates behavioral and synaptic impairments associated with MeCP2 overexpression. Neuropsychopharmacology 39, 1946–1954. doi: 10.1038/npp.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, Monteggia LM, 2012. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J. Neurosci. 32, 3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P, Drescher KU, van Gaalen M, Relo A-L, Mezler M, Marek G, Schoemaker H, Gross G, Bespalov A, 2010. Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J. Pharmacol. Exp. Ther. 335, 665–673. doi: 10.1124/jpet.110.170506 [DOI] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM, 2007. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. 27, 10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco NL, Heaven MR, Holt LM, Crossman DK, Boggio KJ, Shaffer SA, Flint DL, Olsen ML, 2017. RNA sequencing and proteomics approaches reveal novel deficits in the cortex of Mecp2-deficient mice, a model for Rett syndrome. Mol. Autism 8, 56. doi: 10.1186/s13229-017-0174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PPL, 2006. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain 129, 887–898. doi: 10.1093/brain/awl022 [DOI] [PubMed] [Google Scholar]
- Pitcher MR, Herrera JA, Buffington SA, Kochukov MY, Merritt JK, Fisher AR, Schanen NC, Costa-Mattioli M, Neul JL, 2015. Rett syndrome like phenotypes in the R255X Mecp2 mutant mouse are rescued by MECP2 transgene. Hum. Mol. Genet. 24, 2662–2672. doi: 10.1093/hmg/ddv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöschel B, Wroblewska B, Heinemann U, Manahan-Vaughan D, 2005. The metabotropic glutamate receptor mGluR3 is critically required for hippocampal long-term depression and modulates long-term potentiation in the dentate gyrus of freely moving rats. Cereb. Cortex 15, 1414–1423. doi: 10.1093/cercor/bhi022 [DOI] [PubMed] [Google Scholar]
- Powers S, Miranda C, Dennys-Rivers C, Huffenberger A, Braun L, Rinaldi F, Wein N, Meyer KC, Solano S, Nguyen K, Lang E, Kaspar AA, Foust KD, Thomsen G, Fugere M, Kaspar BK, 2019. Rett syndrome gene therapy improves survival and ameliorates behavioral phenotypes in MeCP2 null (S51.002). Neurology. [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CMB, Schaaf CP, Richman R, Fang P, Glaze DG, Lupski JR, Zoghbi HY, 2009. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 66, 771–782. doi: 10.1002/ana.21715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N, Gerber U, Ster J, 2016. Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. J. Neurosci. 36, 11521–11531. doi: 10.1523/JNEUROSCI.1519-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux J-C, Dura E, Moncla A, Mancini J, Villard L, 2007. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur. J. Neurosci. 25, 1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x [DOI] [PubMed] [Google Scholar]
- Saini SM, Mancuso SG, Mostaid MS, Liu C, Pantelis C, Everall IP, Bousman CA, 2017. Meta-analysis supports GWAS-implicated link between GRM3 and schizophrenia risk. Transl. Psychiatry 7, e1196. doi: 10.1038/tp.2017.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao H-T, Sun Y, Greer JJ, Zoghbi HY, Neul JL, 2008. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 17, 1718–1727. doi: 10.1093/hmg/ddn062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao H-T, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, Greer JJ, Percy A, Glaze DG, Zoghbi HY, Neul JL, 2009. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci USA 106, 21966–21971. doi: 10.1073/pnas.0912257106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY, 2012. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat. Genet. 44, 206–211. doi: 10.1038/ng.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, McGraw CM, Ward CS, Sun Y, Neul JL, Zoghbi HY, 2013. Female Mecp2(+/−) mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet. 22, 96–109. doi: 10.1093/hmg/dds406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramuzza L, De Rocco G, Desiato G, Cobolli Gigli C, Chiacchiaretta M, Mirabella F, Pozzi D, De Simone M, Conforti P, Pagani M, Benfenati F, Cesca F, Bedogni F, Landsberger N, 2021. The enhancement of activity rescues the establishment of Mecp2 null neuronal phenotypes. EMBO Mol. Med. 13, e12433. doi: 10.15252/emmm.202012433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz LR, Gómez NB, Zhen DP, Berger-Sweeney JE, 2013. MeCP2 R168X male and female mutant mice exhibit Rett-like behavioral deficits. Genes Brain Behav. 12, 732–740. doi: 10.1111/gbb.12070 [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H, 2002. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35, 243–254. doi: 10.1016/s0896-6273(02)00768-7 [DOI] [PubMed] [Google Scholar]
- Sinnett SE, Boyle E, Lyons C, Gray SJ, 2021. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain 144, 3005–3019. doi: 10.1093/brain/awab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett SE, Hector RD, Gadalla KKE, Heindel C, Chen D, Zaric V, Bailey MES, Cobb SR, Gray SJ, 2017. Improved MECP2 Gene Therapy Extends the Survival of MeCP2-Null Mice without Apparent Toxicity after Intracisternal Delivery. Mol. Ther. Methods Clin. Dev. 5, 106–115. doi: 10.1016/j.omtm.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley BJ, Fisher NM, Gogliotti RG, Lindsley CW, Conn PJ, Niswender CM, 2018. Contextual fear extinction induces hippocampal metaplasticity mediated by metabotropic glutamate receptor 5. Cereb. Cortex 28, 4291–4304. doi: 10.1093/cercor/bhx282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns NA, Schaevitz LR, Bowling H, Nag N, Berger UV, Berger-Sweeney J, 2007. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience 146, 907–921. doi: 10.1016/j.neuroscience.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden A-M, Monn JA, Schoepp DD, 2005. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 4, 131–144. doi: 10.1038/nrd1630 [DOI] [PubMed] [Google Scholar]
- Sztainberg Y, Chen H, Swann JW, Hao S, Tang B, Wu Z, Tang J, Wan Y-W, Liu Z, Rigo F, Zoghbi HY, 2015. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature 528, 123–126. doi: 10.1038/nature16159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Kloth AD, Clark EP, Moy SS, Chitwood RA, Philpot BD, 2018. Common Pathophysiology in Multiple Mouse Models of Pitt-Hopkins Syndrome. J. Neurosci. 38, 918–936. doi: 10.1523/JNEUROSCI.1305-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson R, Selfridge J, Koerner MV, Gadalla KKE, Guy J, De Sousa D, Hector RD, Cobb SR, Bird A, 2017. Radically truncated MeCP2 rescues Rett syndrome-like neurological defects. Nature 550, 398–401. doi: 10.1038/nature24058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, Lugtenberg D, Bienvenu T, Jensen LR, Gecz J, Moraine C, Marynen P, Fryns J-P, Froyen G, 2005. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 77, 442–453. doi: 10.1086/444549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermudez SAD, Gogliotti RG, Arthur B, Buch A, Morales C, Moxley Y, Rajpal H, Conn PJ, Niswender CM, 2021. Profiling beneficial and potential adverse effects of MeCP2 overexpression in a hypomorphic Rett syndrome mouse model. Genes Brain Behav. e12752. doi: 10.1111/gbb.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AG, Conn PJ, 2015. Group I and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics. Curr. Opin. Pharmacol. 20, 40–45. doi: 10.1016/j.coph.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AG, Sheffler DJ, Lewis AS, Dickerson JW, Foster DJ, Senter RK, Moehle MS, Lv X, Stansley BJ, Xiang Z, Rook JM, Emmitte KA, Lindsley CW, Conn PJ, 2017. Co-Activation of Metabotropic Glutamate Receptor 3 and Beta-Adrenergic Receptors Modulates Cyclic-AMP and Long-Term Potentiation, and Disrupts Memory Reconsolidation. Neuropsychopharmacology 42, 2553–2566. doi: 10.1038/npp.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, Lindsley CW, Conn PJ, 2015. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci USA 112, 1196–1201. doi: 10.1073/pnas.1416196112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SM, McLeod F, Bailey MES, Cobb SR, 2011. Synaptic plasticity deficits in an experimental model of rett syndrome: long-term potentiation saturation and its pharmacological reversal. Neuroscience 180, 314–321. doi: 10.1016/j.neuroscience.2011.01.061 [DOI] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DNC, 2008. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 196, 431–440. doi: 10.1007/s00213-007-0974-x [DOI] [PubMed] [Google Scholar]
- Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A, 2014. Hippocampal metaplasticity is required for the formation of temporal associative memories. J. Neurosci. 34, 16762–16773. doi: 10.1523/JNEUROSCI.2869-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Mebarek S, Lajard A-M, Picard N, Dutschmann M, Hilaire G, 2008. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir. Physiol. Neurobiol. 160, 116–121. doi: 10.1016/j.resp.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Zhang D, Yu B, Liu J, Jiang W, Xie T, Zhang R, Tong D, Qiu Z, Yao H, 2017. Altered visual cortical processing in a mouse model of MECP2 duplication syndrome. Sci. Rep. 7, 6468. doi: 10.1038/s41598-017-06916-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M-G, Toyoda H, Ko SW, Ding H-K, Wu L-J, Zhuo M, 2005. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J. Neurosci. 25, 7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


