Abstract
Objectives
There are positive correlations between subjective health reports and episodic memory performance in older adults. However, previous studies have not evaluated the scope of such complex relationships, nor the potentially nonlinear magnitude of these correlations across age and time. We employed multiple subjective heath indices to evaluate the scope and nonlinearity of such relationships with memory performance.
Methods
We utilized a cross-sectional (N = 2,783 at baseline) and longitudinal sample (N = 311) of healthy older adults aged 65 and older from the Advanced Cognitive Training for Independent and Vital Elderly study. We used time-varying effects modeling (TVEM) to assess potential differences in relationship magnitudes between memory and 3 subjective health subscales (general health, role physical function, and physical function, from the Short Form Health Survey) across 5 years.
Results
Episodic memory positively predicted all subjective health measures cross-sectionally and longitudinally in our sample. TVEM revealed the relationships between all subjective health measures and episodic memory were stable across age. While role physical function and physical function maintained stable relationships with episodic memory across time, general health became increasingly coupled with memory 5 years following baseline.
Discussion
Together, our findings highlight stable and varying relationships between episodic memory and multiple subjective health indicators across metrics of time in older adults.
Clinical Trials Registration Number: NCT00298558
Keywords: Episodic memory, Subjective health, Subjective physical function, TVEM
Subjective health reports are frequently used in gerontology and in clinical settings given their ease of administration, face validity, and relationship to objective health outcomes (Hunt & McEwen, 1980). Declining health is a leading concern for older adults, and addressing older adults’ assessments of their health results in improving their daily functioning and experiences (Coll-Planas et al., 2017; McNeil, 1995). Such subjective health reports correlate moderately with objective physical health in older adults (Jylhä, 2009; Pinquart, 2001). For example, poorer subjective health predicts increased occurrence of diseases and bodily declines such as back problems, rheumatoid arthritis, and heart conditions (Kempen et al., 1997). Relatedly, poorer subjective health predicts more physician visits (Miilunpalo et al., 1997), increased levels of detrimental biomarkers (Jylhä et al., 2006), and increased risk of mortality (Singh-Manoux et al., 2007). Subjective health is therefore posited to have a biological basis and is useful in evaluating older adults’ health status.
Interestingly, subjective health evaluations moderately and positively correlate with cognition in older adults, particularly episodic memory (Small et al., 2011). While this relationship is present in both cross-sectional and longitudinal analyses (Bendayan et al., 2017; Farias et al., 2009; Luszcz et al., 2015; Small et al., 2011; Wahlin et al., 2003), the temporal dynamics and strength of such relationships vary. For example, mild cognitive impairment (MCI) is a period during which deficits in episodic memory become more pronounced and is a meaningful predictor of future cognitive impairment. In one study while subjective health predicted conversion to MCI, subjective memory evaluations were not significantly associated with future MCI conversion within the same participants, highlighting the sensitivity of subjective health to future MCI (Sargent-Cox et al., 2011). Relatedly, other work demonstrated that older adults with poorly rated subjective health depicted steeper declines in episodic memory compared to those with excellent self-rated health (Bendayan et al., 2017). Additionally, Infurna and colleagues (2011) found that declines in episodic memory predicted subsequent declines in subjective health limitations among older adults while declines in health limitations failed to predict episodic memory performance. Together, there is clear evidence that subjective health and cognition, or episodic memory in particular, are related and demonstrate complex temporal dynamics. However, prior work has not fully disentangled how the strength of such relationships varies across time metrics.
While prior work has provided useful information about these complex relationships, several gaps need addressing. First, prior work generally only included a single measure to assess subjective health, asking participants to rate their general health ordinally (Lee et al., 2012; Liang et al., 2010). Subjective health is posited to be a complex construct tapping into multiple metacognitive domains (French, Browning, et al., 2012; Sargent-Cox et al., 2010). Although single measures of subjective health are informative and useful, older adults’ beliefs of their health vary depending on the question asked (Sargent-Cox et al., 2008). Second, prior work assumed the relationship between subjective health and episodic memory differed linearly across time. That is, a one-unit decrease in subjective health measures results in a one-unit decrease in episodic memory measures, on average. However, evidence suggests that the relationship between subjective health and episodic memory may be variable across time metrics. Specifically, older-old adults often display worse episodic memory performance than younger-old adults, and the rate of episodic memory decline increases as older adults approach death or the onset of dementia (Laukka et al., 2004; Wilson et al., 2007, 2012). Older adults also exhibit increased variability in episodic memory performance with increasing time (Nyberg et al., 2012). Additionally, longitudinal declines in subjective health are greater in older-old adults than in younger-old adults (Pinquart, 2001). The relationship between subjective health and cognitive status is present in younger-old adults but is reduced in older-old adults (French, Sargent-Cox, et al., 2012). Together, this highlights a need to examine these relationships with multiple time metrics while also addressing the possibility that the relationship between the two constructs may vary nonlinearly.
The current study addresses both limitations. First, we utilized multiple subjective health indicators including general health, role physical functioning (the role physical health affects daily life), and physical functioning (or the amount one is limited in completing daily activities). Examining relationships across several domains of subjective health will better capture the breadth of possible relationships with episodic memory. Next, we used time-varying effects modeling (TVEM) both cross-sectionally and longitudinally to address the second limitation. TVEM relaxes the assumption of linearity across various time metrics (e.g., age and time). TVEM is an extension of regression which examines how the association between two variables differ (when cross-sectional) or change (when longitudinal) with a time-varying metric. TVEM offers advantages over linear regression models utilizing age or time as a moderator as interaction terms assume linearity. Even when the age or time interaction term is quadratic, the effect is assumed to be constant. Such moderator terms in linear models may fail to capture subtle differences in the magnitude of associations between variables across age or time in later adulthood. TVEM is traditionally used in intensive longitudinal data; however, it can be used cross-sectionally as well when there is variability in the time-varying variable of interest (e.g., adequate coverage of different ages) and a dense collection of data points across the time metric (i.e., adequate power). While TVEM has largely been used in longitudinal addiction, epidemiological, and developmental research (Lanza et al., 2017; Linden-Carmichael et al., 2017), TVEM has recently been utilized to answer novel gerontological questions (Sprague et al., 2019).
To examine the possibility that episodic memory and subjective health vary across time metrics, we explored the differences in relationship magnitude between episodic memory and subjective health in both a cross-sectional and longitudinal sample of healthy older adults. Given the increased variability of episodic memory performance among older adults, and greater declines in memory and subjective health in the oldest-old compared to younger-old adults, we first hypothesized the relationship magnitude between subjective health metrics and episodic memory would be strongest for the oldest-old (those 85+) at baseline. We further hypothesized that the relationship magnitude would be greater 5 years following baseline than at the start of the study. As general health has been implicated in having the most variable relationship with memory, we hypothesized that general health in particular would exhibit different relationship magnitudes with episodic memory cross-sectionally and longitudinally.
Method
Participants
Older adults who participated in the baseline assessment of the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE; https://doi.org/10.3886/ICPSR36036.v1) trial were analyzed in the cross-sectional analysis (analytic N = 2,783; Table 1 for participant characteristics). The ACTIVE study was a large randomized-control trial cognitive training study designed to examine the impact of cognitive training on future cognition, health, and everyday function. Participants were randomized to a no-contact control group or one of three possible cognitive interventions (memory, processing speed, or reasoning training). Participants in the no-contact control group did not receive any intervention (or placebo control) and completed the same follow-up assessments as the intervention group. Longitudinal changes in construct relationship magnitudes (N = 698; Table 1 for participant characteristics) were examined using data from the follow-up assessments at Years 1, 2, and 3, and 5-year follow-up for participants in the no-contact control group. Participants were excluded from ACTIVE if they: were less than 65 years old at the time of screening, received a score of 23 or less on the Mini-Mental State Examination, had medical conditions resulting in rapid health decline or mortality within an estimated 2 years, had severe sensory losses, recently underwent cognitive training, were unavailable for experiment participation during testing and training portions of the study, had severe sensory loss, or required significant assistance with self-care or communication. See Jobe and colleagues (2001) for more details regarding the ACTIVE study design. Participants from the cross-sectional analyses were removed if they were missing the memory composite score (n = 16). Participants assigned to the control group were used in the longitudinal analysis. Those missing any data at any time point between baseline and 5 years post-test were removed, leaving a longitudinal analytic sample of 311 participants.
Table 1.
Participant Demographic Information
| Variable | Full control group (n = 2,783) | Analytic sample (n = 311) | Longitudinal drop out vs remained | |
|---|---|---|---|---|
| M (SD) or n (%) | Range | M (SD) or n (%) | p Value | |
| Male, n (%) | 673 (24.16%) | — | 80 (25.7%) | .102 |
| White race, n (%) | 2,029 (72.83%) | — | 241 (77.5%) | .012 |
| Age, M (SD) | 73.63 (5.90) | 65–93 | 72.90 (5.44) | <.001 |
| Education, n (SD) | 13.53 (2.70) | 6–20 | 13.71 (2.59) | <.001 |
| SF-36 General Health Subscale, M (SD) | 68.23 (19.56) | 0–100 | 67.94 (19.85) | <.001 |
| SF-36 Role Physical Function Subscale, M (SD) | 57.69 (40.69) | 0–100 | 53.68 (41.55) | <.001 |
| SF-36 Physical Function Subscale, M (SD) | 66.21 (26.55) | 0–100 | 63.23 (28.02) | <.001 |
| Memory Composite, M (SD) | −0.07 (0.94) | −4.12–2.06 | −0.01 (0.93) | <.001 |
Notes: SD = standard deviation; SF-36 = 36-item Short Form.
Measures
Covariates
Gender (women = 0, men = 1), race (non-White = 0, White = 1), and years of education (0–20, indicating no formal education to a doctoral degree) served as time-invarying covariates. The sample was predominantly women (n = 2,113; 75.84%), White (n = 2,029; 72.83%), and had at least 12 years of education (M = 13.53, SD = 2.70) at baseline.
Verbal episodic memory (predictor)
The Hopkins Verbal Learning Test (HVLT) and Rey Auditory Verbal Learning Test (AVLT) assessed episodic memory (Brandt, 1991; Rey, 1941). During the HVLT, participants were asked to recall lists of 12 words within 2 min over three trials. For the AVLT, participants were asked to recall a list of 15 words within 2 min across seven trials. A memory composite score was created using each task’s average z-scores following procedures validated by Jobe et al. (2001) and Rebok et al. (2014); the baseline Cronbach’s α = 0.80. Higher scores indicated better memory performance.
Subjective health (outcome)
The general health, role physical function, and physical function subscales of the Medical Outcome Survey 36-item Short Form (SF-36) were used to assess subjective health (Ware & Sherbourne, 1992). The general health subscale consists of five items measuring health, frequency of illness in relation to others, and prospective health. Baseline scores ranged from 0 to 100. The role physical function subscale consists of four items measuring the degree to which participants have problems with daily life because of physical health. Baseline scores ranged from 0 to 100. The physical function subscale consists of 10 items measuring the degree to which participants are limited in completing physical activities. Baseline scores ranged from 0 to 100. All scores were recoded according to the SF-36 scoring manual using the general equation: ((Raw Score − Lowest Possible Raw Score)/Possible Raw Score Range) * 100. Higher scores on all subscales indicated better health (McHorney et al., 1993). Correlations between subjective indices and memory task scores are reported in Table 2.
Table 2.
Correlations Between Variables of Interest
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. HVLT | — | ||||
| 2. AVLT | 0.67* | — | |||
| 3. SF-36 Role Physical Function | 0.18* | 0.16* | — | ||
| 4. SF-36 Physical Function | 0.18* | 0.16* | 0.59* | — | |
| 5. SF-36 General Health | 0.17* | 0.15* | 0.48* | 0.53* | — |
Notes: AVLT = Auditory Verbal Learning Test; HVLT = Hopkins Verbal Learning Test; SF-36 = 36-item Short Form.
*p < .001.
Analytic Strategy
Data were analyzed using SAS 9.4 and the TVEM macro version 3.1.1 (available from https://methodology.psu.edu/downloads/tvem). TVEM is an extension of regression modeling, specifically in which regression coefficients can be estimated as a function of a time-varying metric (e.g., age, time). These regression coefficient functions are allowed to vary smoothly across the time-varying metric. Specifically, we utilized the p-spline method to estimate our parameter functions, which allows the coefficient to vary nonparametrically across the time-varying metric. P-spline estimation smooths these coefficient functions by penalizing exaggerations of minor fluctuations due to randomness within a given age or time range. Regression coefficients are presented in figures representing the coefficient function, as opposed to tables, as the time-varying metrics are treated as continuous. Previous studies have found that older adults who are women, are White, and with greater education tend to exhibit greater subjective health (Spuling et al., 2015; Yao & Robert, 2008). For both the cross-sectional and longitudinal analyses, models were therefore analyzed adjusted for gender, race, and education. Covariates were treated as static vectors across the time-varying metrics, and the effects of non-time-varying covariates are reported numerically as opposed to a figure of a time-varying coefficient estimate. We also assessed the impact of participant attrition in the longitudinal sample. We compared demographics of those who dropped out of the study compared to those who remained, and adjusted for significant factors in the longitudinal TVEM analysis.
Power
Current statistical packages cannot estimate power for TVEM. The number of participants per age group (e.g., 66, 67, etc.) in the cross-sectional analyses ranged from 45 to 192. The power analysis for a multiple linear regression with five parameters (covariates, memory, and age) revealed that with 90% power and an effect size of 0.285 (adjusted R2 obtained from regression), a minimum sample size of 64 was necessary. Because TVEM relies on nearby ages to obtain its coefficient curve, there is sufficient power for this analysis. Participants aged 85 years and older were collapsed for analyses due to insufficient coverage (i.e., power) and to aid with interpretation.
Interpretation
The output of this relationship is represented graphically such that the estimate of the relationship between the two variables is depicted as a solid line across the time-varying metric (age for cross-sectional and time since baseline for longitudinal) and the gray areas represent the 95% confidence bands. P-values are not reported in TVEM, but regions where the confidence band intersects the x-axis denotes regions where p > .05. Importantly, 95% confidence intervals are not constrained to be constant across the time-varying metric using the TVEM method. This differs from traditional regression in which 95% confidence intervals are assumed to be constant across all ages. Instances of “fanning” for the 95% confidence interval are likely due to fewer participants for a given age range (e.g., >90 years old, n = 16), resulting in a wider confidence interval. For additional interpretation, please see (Shiyko et al., 2012; Sprague et al., 2019; Tan et al., 2012).
Results
Cross-Sectional
General health
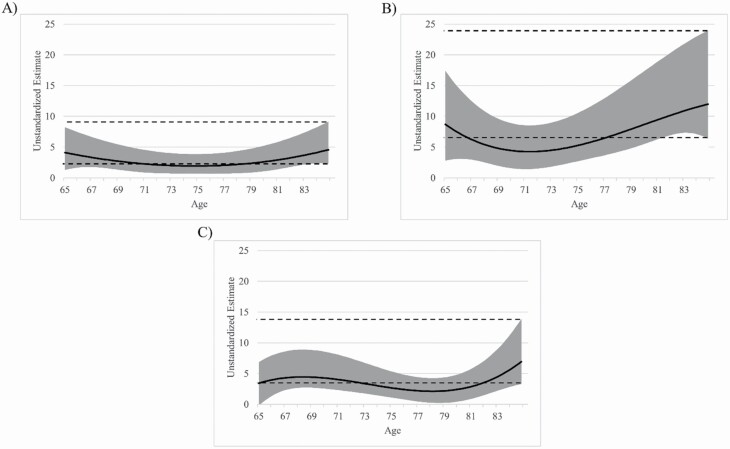
TVEM results indicated that the relationship between episodic memory and general health was significant (p < .05) across older adulthood (Figure 1A) such that higher memory function was associated with higher general health. TVEM also indicated that the relationship magnitude was stable across all ages in our sample (p > .05). Higher educational attainment was associated with better subjective health (b = 0.837, p < .001) while gender and race were not (ps > .05).
Figure 1.
Coefficient curve of episodic memory composite on (A) general health, (B) role physical function, and (C) physical function across age. Models are adjusted for race, sex, and education. The solid line represents the coefficient estimate and the light gray band represents the 95% confidence interval. Dashed lines represent the 95% confidence interval for older adults aged 85+ years old. Areas where the dashed lines intersect the gray band indicate the magnitude for that age group is not significantly different from the 85+-year-olds (p > .05). Areas where the coefficient band intersects x = 0 indicate a nonsignificant relationship (p > .05). Larger estimate scores indicate greater coefficient magnitude.
Role physical function
The relationship between memory and role physical function was significant (p < .05) across our sample as higher memory performance predicted greater role physical function (Figure 1B). The relationship magnitude did not significantly vary as a function of age (p > .05). Higher educational attainment (b = 0.817, p < .01) and gender (men > women; b = 6.04, p < .001) were associated with better subjective health while race was not (p > .05).
Physical function
TVEM revealed that the relationship between episodic memory and physical function was significant (p < .05) across older adulthood such that increased memory predicted better physical function (Figure 1C). TVEM revealed that the relationship magnitude was stable across all ages (p > .05). Higher educational attainment (b = 0.802, p < .01) and gender (men > women; b = 6.106, p < .001) were associated with better subjective health while race was not (p > .05).
Longitudinal
General health
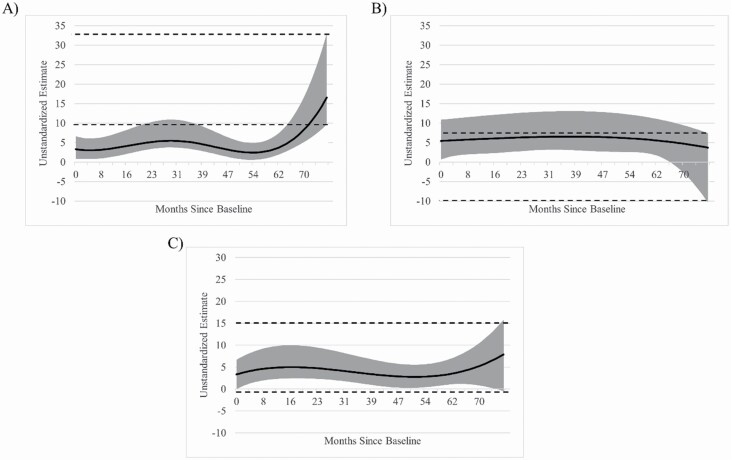
TVEM indicated that the relationship between memory and general health was significant (p < .05) across all months following baseline assessment in our sample such that higher memory function was associated with greater general health across time (Figure 2A). TVEM revealed a significant curvilinear relationship such that the relationship magnitude between memory and general health was greater at 77 months follow-up compared to earlier time points (e.g., 77-month estimate = 16.55, baseline estimate = 3.32). Higher educational attainment (b = 0.870, p < .001) was associated with better subjective health while race and gender were not (ps > .05).
Figure 2.
Coefficient curve of episodic memory composite on (A) general health, (B) role physical function, and (C) physical function across time following baseline assessment. Models are adjusted for race, sex, and education. The solid line represents the coefficient estimate and the light gray band represents the 95% confidence interval. Dashed lines represent the 95% confidence interval for 6 years following baseline. Areas where the dashed lines intersect the gray band indicate the magnitude for that time is not significantly different from 6 years following baseline (p > .05). Areas where the coefficient band intersects x = 0 indicate a nonsignificant relationship (p > .05). Larger estimate scores indicate greater coefficient magnitude.
Role physical function
The relationship between memory and role physical function was significant (p < .05) across the majority of months following baseline as greater memory performance predicted greater role physical function. We observed the relationship magnitude between role physical function and episodic memory was largely stable across time, but weakened such that the relationship became nonsignificant (p > .05) at 72 months (6 years) following baseline, although this is likely due to “fanning” or increased variability in the coefficient estimate due to fewer data points (Figure 2B). Higher educational attainment (b = 1.807, p < .05) was associated with subjective health while race and gender were not (ps > .05).
Physical function
TVEM results indicated that the relationship between physical function and episodic memory was significant (p < .05) across all months following baseline such that greater memory was associated with increased physical function (Figure 2C). The relationship magnitude was stable across time (e.g., 77-month estimate = 7.90, baseline estimate = 3.33). Higher educational attainment (b = 1.968, p < .01), race (White > Black; b = 7.804, p < .001), and gender (men > women; b = 4.967, p < .05) were all associated with better subjective health.
Discussion
The current study investigated potential age- and time-varying relationships between three subjective health subscales and episodic memory. Memory performance significantly predicted all three of our subscales across age, and across time, in our sample of older adults. Contrary to our predictions, we observed the relationships between episodic memory and subjective health scales of general health, role physical function, and physical function remained stable across the age range of our sample. While the relationship magnitude between episodic memory and role physical function and physical function remained largely stable across time, the magnitude between memory and general health increased significantly over time, suggesting the two constructs became more tightly coupled in the years following baseline assessment. Together, these results provide important information regarding the scope of relationships between episodic memory and various subjective health metrics across both age and time.
Episodic memory significantly predicted all three subjective health subscales both across age and across time in our sample. While previous reports have often included a single general measure of subjective health (Liang et al., 2010), here we found that episodic memory was related to multiple subjective health measures both cross-sectionally and longitudinally. Although subjective health measures assess different personal health assessments (i.e., physical health, limitations due to health, and general health), our findings suggest they may maintain a generalized relationship with episodic memory performance among older adults.
Our cross-sectional results demonstrate that the relationship magnitude between episodic memory and our subjective health indices did not differ as a function of age. This suggests that the ability of episodic memory performance to predict subjective health is equivalent for younger-old adults and older-old adults, despite episodic memory differences. This information may be useful to clinicians or researchers who wish to investigate age-invariant coupling between memory and subjective health throughout later adulthood as the relationship magnitude between the two constructs is constant. Additionally, our cross-sectional results provide important information that subjective health indices covering multiple distinct domains all maintain age-invariant relationships with episodic memory, even after controlling for factors such as education (Spuling et al., 2017).
Our longitudinal results suggest that our health measures may tap into related, yet slightly different, mechanisms as time progresses. Although episodic memory predicted all three subscales across time, the relationship magnitude became more tightly coupled with general health while remaining stable with role physical function and physical function. As the general health construct includes questions relating health to other older adults that the participants know, observed relationship changes with this construct may reflect changes in health perceptions relative to older adults’ peers as they age. Older adults tend to rate their health as better than their peers as they become older (Sargent-Cox et al., 2008), and this positive health bias may become more tightly coupled with episodic memory performance with time.
In addition to age and physical function, higher education was consistently associated with better subjective health across all subscales both cross-sectionally and longitudinally. This finding corroborates previous findings that higher education relates to better subjective health in later adulthood (Spuling et al., 2017). Education is a proxy of “cognitive reserve” (Stern, 2002), and it is possible that other behavioral contributors related to education and enhanced cognitive reserve (e.g., physical activity engagement, baseline engagement in cognitively stimulating activities) were responsible for the consistent association. These other indicators, however, were not assessed in the ACTIVE trial. Furthermore, we found race and gender were inconsistently associated with subjective health. This highlights the potentially complex relationships among such factors and different domains of subjective health assessments in older adults. These results indicate the need for future studies to disentangle how race and gender contribute not just to subjective health generally, but also to more subtle differences among subjective health domains.
It should be noted that subjective health and verbal episodic memory are likely related bidirectionally, and it is probable that not one factor has a solely causal influence on the other in aging. On one hand, the ability to subjectively evaluate one’s own health has been posited to utilize metacognitive processes, and metamemory specifically (Wahlin et al., 2003). Previous reports have found that objective memory performance (verbal recall) correlates with subjective memory evaluations longitudinally in older adults (Johansson et al., 1997; Jones et al., 2019), suggesting that the ability to evaluate aspects of one’s memory declines alongside objective memory performance in later adulthood. It is therefore likely that the ability to recall one’s health status also declines. On the other hand, older adults who engage in healthier lifestyle activities, such as physical exercise, exhibit greater verbal episodic memory performance (Frith & Loprinzi, 2019). And conversely, negative health status, such as obesity, has been related with worse verbal episodic memory performance (Loprinzi & Frith, 2018). As previous research has observed a positive correspondence between objective health measures and subjective health measures in aging (Wu et al., 2013), older adults who are objectively healthier also tend to provide more positive subjective health reports, and likely maintain greater verbal episodic memory performance.
Differences in brain structure likely underly the relationship between subjective health measures and episodic memory. Neural contributors could arise from reduced cortical thickness in frontal, temporal, and occipital brain regions, which has been shown to alter the rate of episodic memory decline (Verfaillie et al., 2018). Such cortical thinning in regions responsible for processing episodic memory correlates with general, physical, and prospective subjective health evaluations in older adults, making this possibility promising (Düzel et al., 2018; Hahm et al., 2019). Such structural differences may reflect differences in processes necessary for recalling verbal information as well as evaluating one’s health history. While we were unable to account for such factors in the current study, further research should investigate the central nervous system contributors (e.g., differences in brain structure) to the relationship between subjective physical health and episodic memory in older adults.
A possible limitation is the generalizability of our study as our sample was primarily White and healthy. Additionally, we were limited in sample size in the oldest-old range (85+ years, cross-sectional n = 136). This likely contributed to “fanning” observed in the longitudinal relationship between physical function and memory as fewer participants had complete data for all subsequent follow-up assessments. Because of this limitation we cannot make strong claims regarding the far end of the age spectrum, a period during which differences in relationship magnitude are likely to occur. Additionally, the current study evaluated only one aspect of episodic memory, specifically verbal memory across a brief time interval. It is possible that other aspects of memory, such as aspects of encoding, recollection or familiarity, retrieval at longer time delays, or memory in other modalities may maintain differential relationship magnitudes with subjective health. This should be examined in future studies.
Although not a limitation per se as we contend that subjective health ratings are used widely in research and medical care, future work should also consider relationships between objective health indicators and memory (Zajacova & Woo, 2016). Subjective health ratings are posited to capture systematically different information regarding older adults’ self-evaluated health with increasing age as indicated by a reduced ability to predict mortality in older-old adults. However, subjective health measures have been found to correspond with objective measures in old age (Liang et al., 2010; Pinquart, 2001; Wu et al., 2013), converging evidence suggests subjective health correlates with episodic memory in older adults (Small et al., 2011), and subjective health metrics are becoming more valid as time progresses (Schnittker & Bacak, 2014), so this concern is attenuated.
This study had several important strengths. To our knowledge, it is the first to examine the coupling of memory function and multiple subjective health indicators across age and time in a regionally diverse sample of older adults. Additionally, our investigation is among the first to utilize TVEM in gerontological research and demonstrates both stable and varying relationships between episodic memory and subjective health in older adults when linearity is not assumed. Furthermore, by utilizing longitudinal data we were able to examine age-related changes rather than solely age-related differences in relationship magnitude. Future work should utilize more racially and age-diverse samples to better generalize these results. Additional research should also examine the potential coupling of subjective health with other cognitive domains, such as processing speed or executive function which have been shown to positively correlate with subjective health indices in later adulthood (McHugh & Lawlor, 2016; van Boxtel et al., 1996).
In conclusion, using TVEM we observed invariant relationships between episodic memory and subjective health indicators across age, and both varying (general health) and invariant relationships (role physical function and physical function) across time in our study of older adults depending on the outcome. Results strengthen the predictive validity of episodic memory on subjective health indicators, and inform our understanding of the stability of such relationships across multiple subjective health domains in aging. Future investigations incorporating neuroimaging approaches, in tandem with environmental factors, will assist in further elucidating the processes by which these health–cognition relationships may remain stable or become more tightly coupled in later adulthood.
Funding
This work was supported by grants from the National Institute of Aging (U01AG14260; U01AG14263; U01AG14276; U01AG14282; U01AG14289) and the National Institute of Nursing Research (U01NR04507; U01NR04508) of the National Institutes of Health. B. N. S. was supported by a National Institute on Aging Kirschstein Institutional National Research Service Award (T32-AG055381) awarded to the University of Pittsburgh (PIs: Drs. Mary Ganguli and Caterina Rosano).
Conflict of Interest
None declared.
Acknowledgments
Special thanks to Dr. Stephanie Lanza for her feedback. All data and study materials are available upon request. The current study was not preregistered.
References
- Bendayan, R., Piccinin, A. M., Hofer, S. M., & Muniz, G. (2017). Are changes in self-rated health associated with memory decline in older adults? Journal of Aging and Health, 29(8), 1410–1423. doi: 10.1177/0898264316661830 [DOI] [PubMed] [Google Scholar]
- Brandt, J. (1991). The Hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clinical Neuropsychologist, 5(2), 125–142. doi: 10.1080/13854049108403297 [DOI] [Google Scholar]
- Coll-Planas, L., Nyqvist, F., Puig, T., Urrútia, G., Solà, I., & Monteserín, R. (2017). Social capital interventions targeting older people and their impact on health: A systematic review. Journal of Epidemiology and Community Health, 71(7), 663–672. doi: 10.1136/jech-2016-208131 [DOI] [PubMed] [Google Scholar]
- Düzel, S., Drewelies, J., Gerstorf, D., Demuth, I., Kühn, S., & Lindenberger, U. (2018). Facets of subjective health horizons are differentially linked to brain volume. GeroPsych, 31(3), 127–136. doi: 10.1024/1662-9647/a000191 [DOI] [Google Scholar]
- Farias, S. T., Cahn-Weiner, D. A., Harvey, D. J., Reed, B. R., Mungas, D., Kramer, J. H., & Chui, H. (2009). Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. The Clinical Neuropsychologist, 23(3), 446–461. doi: 10.1080/13854040802360558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, D. J., Browning, C., Kendig, H., Luszcz, M. A., Saito, Y., Sargent-Cox, K., & Anstey, K. J. (2012). A simple measure with complex determinants: Investigation of the correlates of self-rated health in older men and women from three continents. BMC Public Health, 12, 649. doi: 10.1186/1471-2458-12-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, D. J., Sargent-Cox, K., & Luszcz, M. A. (2012). Correlates of subjective health across the aging lifespan: Understanding self-rated health in the oldest old. Journal of Aging and Health, 24(8), 1449–1469. doi: 10.1177/0898264312461151 [DOI] [PubMed] [Google Scholar]
- Frith, E., & Loprinzi, P. D. (2019). Association of physical activity on memory and executive function: Population-based national sample of older adults. Journal of Cognitive Enhancement, 3(4), 425–435. doi: 10.1007/s41465-019-00127-6 [DOI] [Google Scholar]
- Hahm, S., Lotze, M., Domin, M., & Schmidt, S. (2019). The association of health-related quality of life and cerebral gray matter volume in the context of aging: A voxel-based morphometry study with a general population sample. NeuroImage, 191, 470–480. doi: 10.1016/j.neuroimage.2019.02.035 [DOI] [PubMed] [Google Scholar]
- Hunt, S. M., & McEwen, J. (1980). The development of a subjective health indicator. Sociology of Health & Illness, 2(3), 231–246. doi: 10.1111/j.1467-9566.1980.tb00213.x [DOI] [PubMed] [Google Scholar]
- Infurna, F. J., Gerstorf, D., Ryan, L. H., & Smith, J. (2011). Dynamic links between memory and functional limitations in old age: Longitudinal evidence for age-based structural dynamics from the AHEAD study. Psychology and Aging, 26(3), 546–558. doi: 10.1037/a0023023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, B., Allen-Burge, R., & Zarit, S. H. (1997). Self-reports on memory functioning in a longitudinal study of the oldest old: Relation to current, prospective, and retrospective performance. The Journals of Gerontology: Series B, 52B(3), P139–P146. doi: 10.1093/geronb/52B.3.P139 [DOI] [PubMed] [Google Scholar]
- Jobe, J. B., Smith, D. M., Ball, K., Tennstedt, S. L., Marsiske, M., Willis, S. L., Rebok, G. W., Morris, J. N., Helmers, K. F., Leveck, M. D., & Kleinman, K. (2001). ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials, 22(4), 453–479. doi: 10.1016/s0197-2456(01)00139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. W., Fauth, E. B., Ernsth Bravell, M., Johansson, B., & Ledermann, T. (2019). Longitudinal correspondence between subjective and objective memory in the oldest old: A parallel process model by gender. European Journal of Ageing, 16(3), 317–326. doi: 10.1007/s10433-019-00500-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhä, M. (2009). What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science & Medicine (1982), 69(3), 307–316. doi: 10.1016/j.socscimed.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Jylhä, M., Volpato, S., & Guralnik, J. M. (2006). Self-rated health showed a graded association with frequently used biomarkers in a large population sample. Journal of Clinical Epidemiology, 59(5), 465–471. doi: 10.1016/j.jclinepi.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Kempen, G. I., Ormel, J., Brilman, E. I., & Relyveld, J. (1997). Adaptive responses among Dutch elderly: The impact of eight chronic medical conditions on health-related quality of life. American Journal of Public Health, 87(1), 38–44. doi: 10.2105/ajph.87.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, S. T., Russell, M. A., & Braymiller, J. L. (2017). Emergence of electronic cigarette use in US adolescents and the link to traditional cigarette use. Addictive Behaviors, 67, 38–43. doi: 10.1016/j.addbeh.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukka, E. J., Jones, S., Small, B. J., Fratiglioni, L., & Bäckman, L. (2004). Similar patterns of cognitive deficits in the preclinical phases of vascular dementia and Alzheimer’s disease. Journal of the International Neuropsychological Society, 10(3), 382–391. doi: 10.1017/S1355617704103068 [DOI] [PubMed] [Google Scholar]
- Lee, H. L., Huang, H. C., Lee, M. D., Chen, J. H., & Lin, K. C. (2012). Factors affecting trajectory patterns of self-rated health (SRH) in an older population—A community-based longitudinal study. Archives of Gerontology and Geriatrics, 54(3), e334–e341. doi: 10.1016/j.archger.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Liang, J., Quiñones, A. R., Bennett, J. M., Ye, W., Xu, X., Shaw, B. A., & Ofstedal, M. B. (2010). Evolving self-rated health in middle and old age: How does it differ across Black, Hispanic, and White Americans? Journal of Aging and Health, 22(1), 3–26. doi: 10.1177/0898264309348877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden-Carmichael, A. N., Vasilenko, S. A., Lanza, S. T., & Maggs, J. L. (2017). High-intensity drinking versus heavy episodic drinking: Prevalence rates and relative odds of alcohol use disorder across adulthood. Alcoholism, Clinical and Experimental Research, 41(10), 1754–1759. doi: 10.1111/acer.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi, P. D., & Frith, E. (2018). Obesity and episodic memory function. The Journal of Physiological Sciences, 68(4), 321–331. doi: 10.1007/s12576-018-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszcz, M. A., Anstey, K. J., & Ghisletta, P. (2015). Subjective beliefs, memory and functional health: Change and associations over 12 years in the Australian Longitudinal Study of Ageing. Gerontology, 61(3), 241–250. doi: 10.1159/000369800 [DOI] [PubMed] [Google Scholar]
- McHorney, C. A., Ware, J. E., Jr., & Raczek, A. E. (1993). The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31(3), 247–263. doi: 10.1097/00005650-199303000-00006 [DOI] [PubMed] [Google Scholar]
- McHugh, J. E., & Lawlor, B. A. (2016). Executive functioning independently predicts self-rated health and improvement in self-rated health over time among community-dwelling older adults. Aging & Mental Health, 20(4), 415–422. doi: 10.1080/13607863.2015.1018866 [DOI] [PubMed] [Google Scholar]
- McNeil, J. K. (1995). Effects of nonprofessional home visit programs for subclinically unhappy and unhealthy older adults. Journal of Applied Gerontology, 14(3), 333–342. doi: 10.1177/073346489501400307 [DOI] [Google Scholar]
- Miilunpalo, S., Vuori, I., Oja, P., Pasanen, M., & Urponen, H. (1997). Self-rated health status as a health measure: The predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. Journal of Clinical Epidemiology, 50(5), 517–528. doi: 10.1016/s0895-4356(97)00045-0 [DOI] [PubMed] [Google Scholar]
- Nyberg, L., Lövdén, M., Riklund, K., Lindenberger, U., & Bäckman, L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Pinquart, M. (2001). Correlates of subjective health in older adults: A meta-analysis. Psychology and Aging, 16(3), 414–426. doi: 10.1037//0882-7974.16.3.414 [DOI] [PubMed] [Google Scholar]
- Rey, A. (1941). L’examen psychologique dans les cas d’encéphalopathie traumatique. (Les problems.) [The psychological examination in cases of traumatic encepholopathy. Problems.] Archives de Psychologie, 28, 215–285. [Google Scholar]
- Rebok, G. W., Ball, K., Guey, L. T., Jones, R. N., Kim, H.-Y., King, J. W., Marsiske, M., Morris, J. N., Tennstedt, S. L., Unverzagt, F. W., & Willis, S. L. (2014). Ten-year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62(1), 16–24. doi: 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox, K. A., Anstey, K. J., & Luszcz, M. A. (2008). Determinants of self-rated health items with different points of reference: Implications for health measurement of older adults. Journal of Aging and Health, 20(6), 739–761. doi: 10.1177/0898264308321035 [DOI] [PubMed] [Google Scholar]
- Sargent-Cox, K. A., Anstey, K. J., & Luszcz, M. A. (2010). The choice of self-rated health measures matter when predicting mortality: Evidence from 10 years follow-up of the Australian Longitudinal Study of Ageing. BMC Geriatrics, 10(1), 18. doi: 10.1186/1471-2318-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox, K., Cherbuin, N., Sachdev, P., & Anstey, K. J. (2011). Subjective health and memory predictors of mild cognitive disorders and cognitive decline in ageing: The Personality and Total Health (PATH) through Life Study. Dementia and Geriatric Cognitive Disorders; Basel, 31(1), 45–52. doi: 10.1159/000322373 [DOI] [PubMed] [Google Scholar]
- Schnittker, J., & Bacak, V. (2014). The increasing predictive validity of self-rated health. PLoS One, 9( 1) :e84933. doi: 10.1371/journal.pone.008493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyko, M. P., Lanza, S. T., Tan, X., Li, R., & Shiffman, S. (2012). Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: Differences between successful quitters and relapsers. Prevention Science, 13(3), 288–299. doi: 10.1007/s11121-011-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux, A., Guéguen, A., Martikainen, P., Ferrie, J., Marmot, M., & Shipley, M. (2007). Self-rated health and mortality: Short- and long-term associations in the Whitehall II study. Psychosomatic Medicine, 69(2), 138–143. doi: 10.1097/PSY.0b013e318030483a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, B. J., Dixon, R. A., & McArdle, J. J. (2011). Tracking cognition–health changes from 55 to 95 years of age. The Journals of Gerontology, Series, B: Psychological Sciences and Social Sciences, 66B(Suppl. 1), i153–i161. doi: 10.1093/geronb/gbq093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague, B. N., Phillips, C. B., & Ross, L. A. (2019). Age-varying relationships between physical function and cognition in older adulthood. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74(5), 772–784. doi: 10.1093/geronb/gbx126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuling, S. M., Huxhold, O., & Wurm, S. (2017). Predictors of self-rated health: Does education play a role above and beyond age? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 72(3), 415–424. doi: 10.1093/geronb/gbv057 [DOI] [PubMed] [Google Scholar]
- Spuling, S. M., Wurm, S. C. G., Tesch-Roemer, C., & Huxhold, O. (2015). Changing predictors of self-rated health: Disentangling age and cohort effects. Psychology and Aging, 30(2), 462–474. doi: 10.1037/a0039111 [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. doi: 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- Tan, X., Shiyko, M. P., Li, R., Li, Y., & Dierker, L. (2012). A time-varying effect model for intensive longitudinal data. Psychological Methods, 17(1), 61–77. doi: 10.1037/a0025814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel, M. P., Langerak, K., Houx, P. J., & Jolles, J. (1996). Self-reported physical activity, subjective health, and cognitive performance in older adults. Experimental Aging Research, 22(4), 363–379. doi: 10.1080/03610739608254017 [DOI] [PubMed] [Google Scholar]
- Verfaillie, S. C. J., Slot, R. E., Tijms, B. M., Bouwman, F., Benedictus, M. R., Overbeek, J. M., Koene, T., Vrenken, H., Scheltens, P., Barkhof, F., & van der Flier, W. M. (2018). Thinner cortex in patients with subjective cognitive decline is associated with steeper decline of memory. Neurobiology of Aging, 61, 238–244. doi: 10.1016/j.neurobiolaging.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Wahlin, A., Maitland, S. B., Bäckman, L., & Dixon, R. A. (2003). Interrelations between subjective health and episodic memory change in Swedish and Canadian samples of older adults. The International Journal of Aging and Human Development, 57(1), 21–35. doi: 10.2190/9VAA-KMYV-U2HU-PVAW [DOI] [PubMed] [Google Scholar]
- Ware, J. E., Jr., & Sherbourne, C. D. (1992). The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30(6), 473–483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- Wilson, B. T. L., Bienias, J. L., & Bennett, D. A. (2007). Terminal cognitive decline: Accelerated loss of cognition in the last years of life. Psychosomatic Medicine, 69(2), 131–137. doi: 10.1097/PSY.0b013e31803130ae [DOI] [PubMed] [Google Scholar]
- Wilson, S. E., Hizel, L. P., Boyle, P. A., & Bennett, D. A. (2012). Terminal dedifferentiation of cognitive abilities. Neurology, 78(15), 1116–1122. doi: 10.1212/WNL.0b013e31824f7ff2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S., Wang, R., Zhao, Y., Ma, X., Wu, M., Yan, X., & He, J. (2013). The relationship between self-rated health and objective health status: A population-based study. BMC Public Health, 13(1), 320. doi: 10.1186/1471-2458-13-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, L., & Robert, S. A. (2008). The contributions of race, individual socioeconomic status, and neighborhood socioeconomic context on the self-rated health trajectories and mortality of older adults. Research on Aging, 30(2), 251–273. doi: 10.1177/0164027507311155 [DOI] [Google Scholar]
- Zajacova, A., & Woo, H. (2016). Examination of age variations in the predictive validity of self-rated health. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71(3), 551–557. doi: 10.1093/geronb/gbv050 [DOI] [PMC free article] [PubMed] [Google Scholar]