Abstract
Study Objectives:
To examine the association between obstructive sleep apnea (OSA) risk and cognitive disorders among US adults.
Methods:
Data from the 2016 wave of the Health and Retirement Study (HRS) were utilized. Probable OSA cases were identified with survey items that resembled critical elements of a clinically validated OSA screen (STOP-Bang questionnaire). Weighted prevalences of cognitive impairment not dementia (CIND) and dementia among individuals with and without probable OSA were assessed. Cross-sectional analyses of associations between OSA risk and cognitive outcomes, along with effect modification by race and ethnicity, were estimated using imputed data.
Results:
Of the 20,910 HRS participants, 60% had probable OSA. CIND and dementia were more common among adults with probable OSA as compared with those without (12.7% vs 8.0% for CIND; 3.2% vs 2.0% for dementia). Probable OSA was associated with CIND (odds ratio [OR] = 1.22, 95% confidence interval [CI] = 1.08–1.37) and dementia (OR = 1.27, 95% CI = 1.04–1.54). Race/ethnicity significantly modified the association between probable OSA and CIND, with a higher risk for CIND in Whites (OR = 1.35, 95% CI = 1.17–1.57) as compared with non-Whites (OR = 0.98, 95% CI = 0.81–1.19).
Conclusions:
CIND and dementia are more common among older adults who are at high risk for OSA, as compared with low-risk individuals. These data highlight the importance of consideration of OSA risk in large-scale studies of OSA and cognitive disorders.
Citation:
Shieu MM, Dunietz GL, Paulson HL, Chervin RD, Braley TJ. The association between obstructive sleep apnea risk and cognitive disorders: a population-based study. J Clin Sleep Med. 2022;18(4):1177–1185.
Keywords: obstructive sleep apnea, cognitive disorders, dementia, effect modification
BRIEF SUMMARY
Current Knowledge/Study Rationale: To date, evidence regarding the association between OSA and cognitive disorders has relied on diagnosed cases. Given the under-diagnosis of OSA, the magnitude of these associations could be significantly different than current estimates. This study utilized a nationally representative cohort with a diverse OSA risk profile to address this gap.
Study Impact: These data highlight the potential impact of undiagnosed OSA in cognitive disorders in middle-aged and older adults. The observed relationships between OSA risk factors and cognitive disorders may have a dose-response pattern.
INTRODUCTION
Approximately 5.7 million Americans are living with dementia.1 The most common types of dementia, Alzheimer disease (AD) and vascular dementia, account for 70% of cases.2 In addition, 15% of older Americans are estimated to experience mild cognitive impairment (MCI; or “cognitive impairment, not dementia” [CIND]), a condition that progresses to AD in approximately 80% of cases.3 The lack of effective treatments to prevent, cure, or slow dementia progression has galvanized the need to identify modifiable risk factors for these conditions.
Sleep disturbances, and obstructive sleep apnea (OSA) in particular, have gained substantial attention recently as potentially modifiable risk factors for AD and MCI/CIND.4,5 Despite these intriguing findings, prior research on OSA and cognitive outcomes has been restricted to examination of OSA cases diagnosed by polysomnography, or self-reported OSA cases.5–7 Given that up to 80% of those with OSA remain undiagnosed,8,9 the more comprehensive potential impact of missed OSA cases on neurodegeneration is unknown, highlighting a critical gap in sleep and dementia research. This gap may be particularly germane for older adults. Although older work estimates OSA to affect approximately 20% of adults aged 65 years and older,7 more recent data from our group suggest that a substantially higher proportion (56%) of older adults may have undiagnosed OSA.10 These data illuminate the value of research methods that more inclusively evaluate associations between OSA risk (including probable cases) and cognitive decline. Specifically, population-based research that utilizes nationally representative samples, measures that capture undiagnosed OSA cases, and algorithms that reliably identify cognitive impairment and dementia are necessary to fully understand the scope and magnitude of the relationship between OSA and cognitive disorders. In addition, a better understanding of effect modifications of demographic variables on OSA and cognitive disorders on a population level is sorely needed.
The objective of this study, therefore, was to examine relationships between probable OSA and cognitive disorders among midlife and older adults, by leveraging objective cognitive and health data collected from the Health and Retirement Study (HRS). The HRS is an ideal dataset to study this relationship, given its large and diverse sample that is representative of US adults aged over 50. The 2016 HRS wave included detailed data regarding features of OSA risk and objective cognitive testing.11 An evaluation of key health and demographic effect modifiers on this relationship was also conducted.
METHODS
The HRS
The HRS is a large, nationally representative, racially and ethnically diverse, prospective cohort study of US adults aged over 50, designed to investigate the health, social, and economic implications of the aging of the American population. The present study utilized health data from the core dataset of the 2016 wave of the HRS when detailed sleep- and OSA-related questions first became available. This biennial survey, which began in 1992, collects a wide-range of data on health, cognition, family, employment, and wealth, with oversampling of Black, Hispanic, and minority-headed households. The HRS follows respondents longitudinally until death. If a respondent is unable or unwilling to participate in the survey, the HRS attempts to identify a proxy respondent (usually a spouse or adult child) to complete the survey for them.11
This study was deemed “nonregulated” by the Medical School Institutional Review Board at the University of Michigan, as only publicly available, de-identified data were used.
Study variables
Identification of high-risk (probable) OSA cases
In the 2016 wave, the HRS included items that allowed for the assessment of OSA risk; specifically, HRS items that closely resembled 7 out of 8 items from the STOP-Bang questionnaire were adapted for use in this study. This approach has proven useful previously in a large-scale epidemiological study10 and effective as the original version when used as a patient-administered tool only (no health care provider administration).12 The STOP-Bang questionnaire is a validated, 8-item screening instrument that assesses characteristics known to confer OSA risk that form the acronym “STOP-Bang” (Snoring, Tiredness, Observed apneas, high blood Pressure, BMI, Age, Neck circumference, Gender).13 Item scores (1/0) are based on yes/no answers. In general, a total score of 0 to 2 is considered low risk, 3 to 4 moderate risk, and 5 or greater high risk. Furthermore, a modified STOP-Bang score that includes 2 positive “STOP” items plus confirmation of male sex and/or body mass index (BMI) >35 kg/m2 has been reported to reflect higher OSA risk, and to be more sensitive than total score alone.14 The utility of the STOP-Bang has been widely demonstrated in a variety of large samples, many of which included high proportions of older adults.10,15
In this study, we defined the exposure (probable OSA) based on these adapted STOP-Bang items derived from the HRS (see Table 1 for details and cutoffs). For primary analyses, to enhance specificity, high-risk (probable OSA) cases were defined by the presence of either (1) a STOP-Bang score of ≥ 3, in which at least 2 of the positive items came from the “STOP” portion of the questionnaire, plus a positive score for male sex or BMI > 35,14 or (2) a total STOP-Bang score of ≥ 4, in which case any combination of at least 4 positive items was permissible. Participants who were not probable OSA were considered as low OSA risk.
Table 1.
Probable OSA (high OSA risk) corresponding to STOP-Bang questionnaire items.
| Questions from the HRS Physical Health File | Cutoffs of the HRS Questions Considered as a Positive Result | Corresponding STOP-Bang Item |
|---|---|---|
| In the past 12 months, how often did you snore while you were sleeping? | ≥ 3 | Do you snore loudly? |
| Have you had any of the following persistent or troublesome problems? Severe fatigue or exhaustion. | Yes | Do you often feel tired, fatigued, or sleepy during the daytime? |
| In the past 12 months, how often did you snort, gasp, or stop breathing while you were sleeping? | Rarely, occasionally, and frequently | Has anyone observed you stop breathing during sleep? |
| Has a doctor ever told you that you have high blood pressure or hypertension? In order to lower your blood pressure, are you now taking any medication? | Yes | Do you have (or are you being treated for) high blood pressure? |
| About how much do you weigh (lbs)? How tall are you (feet, inches)? | > 35 kg/m2 | BMI |
| Year born | > 50 years | Age |
| N/A | N/A | Neck circumference |
| Sex | Men | Sex |
BMI = body mass index, HRS = Health and Retirement Study, N/A = Not available, OSA = obstructive sleep apnea.
Identification of CIND and dementia cases
CIND and dementia cases were identified through the application of a validated, HRS-based algorithm applied to objective cognitive test scores from the HRS core dataset. The HRS “core” interview objectively assesses cognitive function in self-respondents with a range of tests. Cognitive testing includes assessment of immediate and delayed 10-noun recall, serial 7 subtraction test, backward number counting, vocabulary (crystallized knowledge), and mental status (see Table 2 for details). Using an algorithm described in Iwashyna et al16 and developed by Crimmins et al,17 scores from the immediate and delayed 10-noun recall test, serial 7 subtraction test, and a backward count from 20 test were converted to a 27-point cognitive scale, which allowed for the classification of respondents into 3 categories: dementia (score 0 to 6), CIND (score 7 to 11), and normal cognition (score 12 to 27). These assessments have been validated to reliably identify cognitively normal individuals in 87% of cases, and conversely, > 80% of adults with CIND and/or dementia, when compared with more formal neuropsychological testing applied to a specialized HRS cohort.17 Individuals with proxy scores who scored from 6 to 11 were classified as having dementia, 3–5 as having CIND, and 0–2 as having normal cognition. To account for missing data, cognitive measurements were imputed as described in Langa et al.18
Table 2.
Cognitive tests included in the study.
| Name | Description | Score Range |
|---|---|---|
| Immediate word recall | The interviewer read 1 of 4 possible lists of 10 nouns to the respondent. The lists do not overlap in word content and the initial list was randomly assigned to the respondent, in a longitudinal manner such that each respondent was assigned a different set of words in each of four successive waves of data collection. | 0–10 |
| Delayed word recall | After approximately 5 minutes of asking other survey questions (eg, depression, and cognition items including backward count, and serial 7’s) the respondent was asked to recall the nouns previously presented as part of the immediate recall task. | 0–10 |
| Serial 7’s task | The interviewer asked the respondent to subtract 7 from 100, and continue subtracting 7 from each subsequent number for a total of 5 trials. | 0–5 |
| Backward count | Respondents were asked to count backward for 10 continuous numbers beginning with the number 20. | 0–2 |
Covariates
Demographic information (age, sex, and race/ethnicity) was collected during the 2016 interview. Race/ethnicity was categorized into 4 groups: Hispanic, non-Hispanic White, non-Hispanic Black/African American, and non-Hispanic other race. Hypertension, diabetes, and depression were defined by either of 2 criteria: self-report of each condition as being told of this condition by a doctor and self-reported use of condition-specific medications. Marital status was classified for married or cohabiting respondents. Smoking status was categorized as current smoker, ex-smoker, and nonsmoker. Similarly, alcohol consumption was divided into 3 categories: current, past, and none.
We created a metabolic equivalent (MET) score, accounting for the relative energy expended across levels of intensity and frequency reported for 3 physical activity questions (frequency of mild activity, moderate activity, and vigorous activity).19,20 This approach approximates MET by combining the 3 questions and weighting the responses to reflect the relative energy expended. The final MET score ranged from 0 to 16.33.
Statistical analysis
Descriptive statistics were calculated as proportions for categorical variables and as means and standard errors for continuous variables. The weighted prevalence, accounting for HRS weights, of CIND and dementia and mean score of each objective cognitive test were calculated for the entire sample and by OSA risk.
Cross-sectional associations between high OSA risk and cognitive outcomes were examined. Weighted logistic regression was used to estimate the crude and adjusted prevalence odds ratios (ORs) and 95% confidence intervals (CIs). In this analysis, we implemented multinomial logistic regression to estimate the effects of probable OSA on both CIND and dementia, defined as categorical outcomes, vs normal cognition as the reference. Models were adjusted for age, race/ethnicity, education, marital status, smoking, alcohol consumption, and physical activity. Sex and hypertension were not included in the model since they were binary items that are included in the STOP-Bang questionnaire (exposure). Finally, we examined whether there is a dose–response association between the number of OSA risk factors (as an ordinal variable) and CIND or dementia.
To examine potential mediation of diabetes and depression, we further adjusted for diabetes and depression in the models. BMI was taken into account to investigate its effect on the relationship between OSA risk and cognitive disorders. To examine race/ethnicity as a possible modifier of the associations between OSA risk and cognitive function, an interaction term for White/non-White and OSA risk was added to the model. Prevalence ORs were reported for Whites and non-Whites.
Identification of CIND by algorithms is more challenging in comparison to dementia or normal cognition.17 We conducted sensitivity analyses to adjust for possible misclassification of CIND by restricting the sample to HRS participants who obtained concordant classifications of CIND during the survey waves of 2012, 2014, and 2016. That is, we classified participants with CIND if their cognitive function scores corresponded to CIND in at least 2 HRS waves.
Common approaches to addressing the presence of missing data include multiple imputation, which may help reduce bias or increase precision. Multiple imputation methods impute or fill in multiple plausible values of a given variable for each study participant with missing data. Implementation of multiple imputation creates several completed datasets that are utilized in the statistical analysis. We conducted multiple imputation to account for missing values of the following items: snoring (4.8% missing), fatigue (0.4% missing), snort (42.3% missing), BMI (3.4% missing), smoking (46.0% missing), and alcohol consumption (0.6% missing), assuming data were missing at random.21 First, we imputed values for the missing data 25 times by sampling from the chained equations using PROC MI procedures within SAS software (SAS Institute, Cary, NC). We included auxiliary variables that may contain information about the missing data, variables and outcomes involved in the planned analysis, and variables accounting for the clusters and strata in the process.22 From the complete set of variables and the imputed set, we created 25 complete datasets. Second, we analyzed the 25 complete datasets using SURVEY procedures within SAS. Finally, we combined the 25 parameter estimates and standard errors to calculate pooled estimates and standard errors using PROC MIANALYZE procedures, which reflect the variability of the imputed data along with the HRS survey design.23
Population attributable risk percent (PARP) for CIND and dementia was calculated using a model-based method developed by Greenland and Drescher24 and SAS macros provided by Rückinger et al.25 The method was shown to generate the most plausible results comparing with some other methods of calculating PARP when confounders appear in a dataset.25 We estimated PARP from adjusted logistic models for age, race/ethnicity, marital status, education, smoking categories, drinking categories, and physical activity. All analyses were performed in SAS version 9.4 and accounted for the HRS survey weights.
RESULTS
Data from 20,910 respondents were utilized. The weighted prevalence of probable OSA was 59.7%. Weighted summary statistics of demographic and characteristics are presented in Table 3. The mean age of HRS participants was 65.2 years. Nearly 47% were men and 80% were non-Hispanic Whites. In comparison to low OSA risk participants, those with high OSA risk were more likely to be older, men, and have high BMI, diabetes, hypertension, depression, and lower physical activity. Those with high OSA risk were also less likely to be married or to have obtained at least a college education.
Table 3.
Summary statistics of demographic and characteristics.
| Total (n = 20,910) | Percentage of Missing | High OSA Risk (n = 6,612) | Low OSA Risk (n = 4,361) | |
|---|---|---|---|---|
| Men (%) | 46.5 | 0.0 | 65.3 | 26.7 |
| Age (years) | 65.2 (0.09) | 0.0 | 63.6 (0.1) | 63.2 (0.2) |
| Race/ethnicity (%) | 0.0 | |||
| White | 79.5 | 77.6 | 83.0 | |
| Black | 10.7 | 12.2 | 8.3 | |
| Hispanic | 4.2 | 4.4 | 3.2 | |
| Othera | 5.6 | 5.8 | 5.5 | |
| Education (%) | 5.2 | |||
| Below college | 60.8 | 62.7 | 55.2 | |
| College | 26.1 | 26.0 | 29.0 | |
| Above college | 13.1 | 11.3 | 15.8 | |
| Married (%) | 65.2 | 0.04 | 71.7 | 74.3 |
| BMI (kg/m2) | 29.0 (0.06) | 3.4 | 31.2 (0.1) | 27.3 (0.09) |
| Diabetes (%) | 22.3 | 0.1 | 29.3 | 13.5 |
| Hypertension (%) | 54.8 | 0.2 | 76.0 | 25.0 |
| Depression (%) | 25.1 | 0.2 | 29.3 | 22.0 |
| Smoking (%) | 46.0 | |||
| Nonsmoker | 64.2 | 61.7 | 60.4 | |
| Ex-smoker | 10.1 | 12.0 | 12.1 | |
| Current smoker | 25.7 | 26.3 | 27.5 | |
| Alcohol (%) | 0.6 | |||
| Nondrinker | 36.6 | 35.0 | 31.0 | |
| Past drinker | 17.9 | 17.6 | 18.0 | |
| Current drinker | 45.5 | 47.4 | 51.0 | |
| MET scoreb | 4.5 (0.04) | 1.0 | 4.1 (0.06) | 5.2 (0.08) |
| STOP-Bang score | 3.8 (0.02) | 47.5 | 4.6 (0.01) | 2.7 (0.01) |
HRS survey weighted proportion (%) or HRS survey weighted mean (and standard error) of selected variables, by OSA risk; total study population. aIncludes American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, and other race/ethnicity. bMetabolic equivalent of task accounting for mild, moderate, and vigorous activities. Ranged from 0 to 16.33. BMI = body mass index, MET = metabolic equivalent of task, OSA = obstructive sleep apnea.
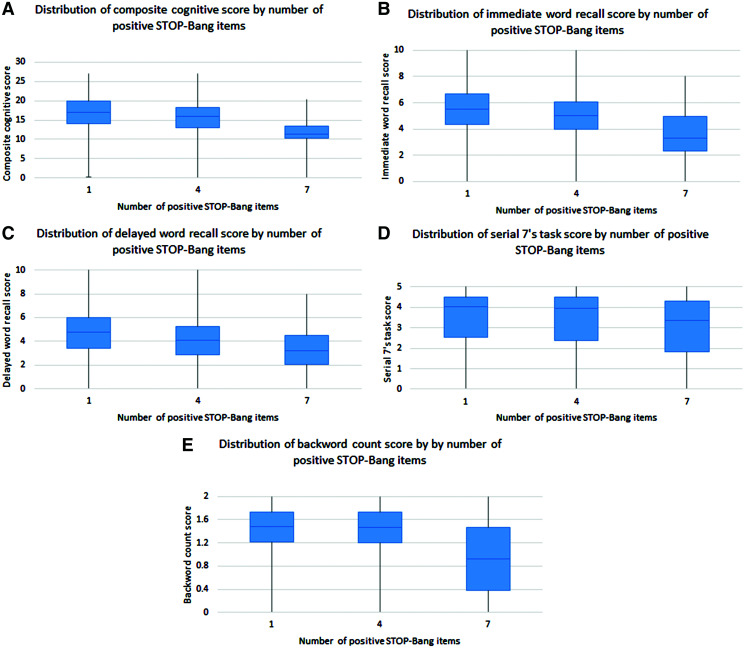
The weighted prevalence, mean score, and distribution for each cognitive test are shown in Table 4 and Figure 1. Overall, the prevalences of CIND and dementia were 13.0% and 3.7%, respectively, and higher for those at high risk for OSA (12.7% for CIND and 3.2% for dementia) compared with those at low risk for OSA (8.0% for CIND and 2.0% for dementia). The prevalences of CIND and dementia for those with missing exposure data are not shown. Participants at high risk for OSA, in comparison to those at low risk, had lower scores on all cognitive tests except for the backward count. The inverse association between positive STOP-Bang items and cognitive test score was seen in the composite cognitive score, immediate word recall, and delayed word recall (Figure 1).
Table 4.
Prevalence of cognitive disorders and mean of cognitive test score.
| Total (n = 20,910) | High OSA Risk (n = 6,612) | Low OSA Risk (n = 4,361) | |
|---|---|---|---|
| Normal cognition (n, weighted %) | 15,110 (83.3) | 4,932 (84.1) | 3,362 (90.0) |
| CIND (n, weighted %) | 3,446 (13.0) | 1,089 (12.7) | 474 (8.0) |
| Dementia (n, weighted %) | 1,065 (3.7) | 292 (3.2) | 134 (2.0) |
| Immediate word recall (weighted mean score, SE) | 5.6 (0.02) | 5.5 (0.03) | 6.0 (0.03) |
| Delayed word recall (weighted mean score, SE) | 4.6 (0.02) | 4.5 (0.03) | 5.0 (0.04) |
| Serial 7’s task (weighted mean score, SE) | 3.8 (0.01) | 3.8 (0.02) | 3.9 (0.03) |
| Backward count (weighted mean score, SE) | 1.9 (0.004) | 1.9 (0.07) | 1.9 (0.008) |
HRS survey weighted prevalence (%) of each cognitive disorder and HRS survey weighted mean (and SE) of each objective cognitive test score, by OSA risk. CIND = cognitive impairment not dementia, OSA = obstructive sleep apnea, SE = standard error.
Figure 1. Distribution of composite cognitive scores and each individual test score by number of positive STOP-Bang items.
Table 5 displays the cross-sectional associations between high OSA risk and cognitive disorders among all respondents. This analysis utilized imputed data generated to account for the 47.5% of values that were missing in 1 question of the adapted STOP-Bang score. Overall, high OSA risk was significantly associated with CIND (OR = 1.22, 95% CI = 1.08–1.37) and dementia (OR = 1.27, 95% CI = 1.04-1.54). After adding diabetes and depression (potential mediators) to the models, the associations for CIND (OR = 1.18, 95% CI = 1.05–1.33) and dementia (OR = 1.21, 95% CI = 0.99–1.48) remained significant. When we further adjusted for BMI in the models, associations increased in magnitude for dementia (OR = 1.46, 95% CI = 1.18–1.81) (data not shown in Table 5). Increased age; Black, Hispanic, and “other” racial categories; and current/prior tobacco use were also significantly and positively associated with CIND and dementia. Conversely, having a college or above college degree, married/cohabitating status, and current/prior alcohol use were associated with lower prevalence odds of having CIND and dementia. When the STOP-Bang score was analyzed as an ordinal variable, for every additional STOP-Bang item that was positive, the odds for CIND (adjusted OR = 1.10, 95% CI = 1.05–1.15) and dementia (adjusted OR = 1.11, 95% CI = 1.02–1.21) increased by 10%, on average, respectively (data not shown).
Table 5.
Association between OSA and cognitive disorders.
| CIND (n = 3,446) | Dementia (n = 1,065) | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Adjustedb | Unadjusted | Adjusteda | Adjustedb | |
| High vs low OSA risk | 1.21 (1.09, 1.34) | 1.22 (1.08, 1.37) | 1.18 (1.05, 1.33) | 1.12 (0.94, 1.34) | 1.27 (1.04, 1.54) | 1.21 (0.99, 1.48) |
| Age | 1.05 (1.05, 1.06) | 1.06 (1.06, 1.07) | 1.07 (1.06, 1.07) | 1.11 (1.10, 1.12) | 1.11 (1.10, 1.12) | 1.11 (1.10, 1.13) |
| Race/ethnicityc | ||||||
| Black | 2.90 (2.59, 3.27) | 2.99 (2.62, 3.41) | 3.14 (2.75, 3.59) | 3.29 (2.75, 3.92) | 3.97 (3.19, 4.93) | 4.20 (3.37, 5.24) |
| Hispanic | 2.65 (2.19, 3.21) | 3.19 (2.57, 3.95) | 3.27 (2.63, 4.06) | 2.89 (2.16, 3.88) | 5.75 (4.10, 8.06) | 5.86 (4.23, 8.18) |
| Otherd | 1.28 (1.02, 1.61) | 2.07 (1.62, 2.65) | 2.13 (1.66, 2.73) | 0.84 (0.54, 1.31) | 2.06 (1.28, 3.32) | 2.15 (1.33, 3.47) |
| Education | ||||||
| College | 0.27 (0.23, 0.31) | 0.37 (0.32, 0.44) | 0.37 (0.32, 0.44) | 0.16 (0.12, 0.22) | 0.29 (0.21, 0.40) | 0.30 (0.22, 0.41) |
| Above college | 0.16 (0.12, 0.21) | 0.22 (0.17, 0.28) | 0.22 (0.17, 0.28) | 0.20 (0.13, 0.32) | 0.37 (0.23, 0.60) | 0.38 (0.23, 0.61) |
| Married | 0.54 (0.49, 0.60) | 0.85 (0.76, 0.95) | 0.89 (0.80, 0.99) | 0.31 (0.26, 0.36) | 0.66 (0.55, 0.80) | 0.70 (0.58, 0.85) |
| Diabetes | 1.54 (1.38, 1.71) | 1.10 (0.88, 1.12) | 1.80 (1.52, 2.12) | 1.00 (0.84, 1.20) | ||
| Depression | 1.49 (1.33, 1.67) | 1.54 (1.36, 1.74) | 1.53 (1.29, 1.81) | 1.67 (1.38, 2.01) | ||
| Smoking | 0.99 (0.92, 1.07) | 1.28 (1.17, 1.40) | 1.27 (1.17, 1.39) | 0.77 (0.68, 0.86) | 1.31 (1.14, 1.51) | 1.30 (1.13, 1.50) |
| Alcohol | 0.62 (0.59, 0.66) | 0.81 (0.76, 086) | 0.81 (0.76, 0.86) | 0.41 (0.37, 0.46) | 0.63 (0.56, 0.71) | 0.64 (0.57, 0.72) |
| Physical activitye | 0.88 (0.87, 0.90) | 0.95 (0.94, 0.97) | 0.96 (0.94, 0.97) | 0.72 (0.69, 0.76) | 0.84 (0.80, 0.87) | 0.85 (0.81, 0.88) |
Cross-sectional association (adjusted prevalence odds ratio and 95% confidence interval) between OSA risk (exposure) and CIND or dementia (outcome) (imputed dataset). aAdjusted for age, race/ethnicity, education, marital status, smoking, alcohol, and physical activity. bAdjusted for age, race/ethnicity, education, marital status, smoking, alcohol, physical activity, diabetes, and depression. cWhite is the reference. dIncludes American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, and other race/ethnicity. eAccounted for mild, moderate, and vigorous activities. Ranged from 0 to 16.33. CIND = cognitive impairment not dementia, OSA = obstructive sleep apnea.
Sensitivity analyses restricted HRS participants to those who obtained at least 2 concordant classifications of CIND during the survey waves of 2012, 2014, and 2016 (15,721 study participants with normal cognition, 2,585 participants with CIND, and 1,065 participants with dementia). These adjusted analyses showed a very similar magnitude of association between high OSA risk and cognitive disorders (CIND: OR = 1.23, 95% CI = 1.07–1.40; dementia: OR = 1.26, 95% CI = 1.03–1.53).
We also conducted formal interaction analysis to examine whether race/ethnicity modified the association between high OSA risk and CIND or dementia. As the study population was predominantly White, we reported the imputed effect estimates of OSA risk on cognitive disorders stratified by White and non-White participants (Table 6). The weighted prevalence of probable OSA was 58% for Whites and 66% for non-Whites. Adjusted analyses demonstrated increased prevalence ORs of CIND in Whites with OSA (OR = 1.35, 95% CI = 1.17–1.57), but not in non-Whites with OSA (OR = 0.98, 95% CI = 0.81–1.19). This reflects a positive interaction between race and OSA risk (P for interaction < .001). In contrast, probable OSA was associated with an increased prevalence OR of dementia in both Whites (OR = 1.24, 95% CI = 0.96–1.60) and non-Whites (OR = 1.23, 95% CI =0.91–1.66), with no significant interaction between race and OSA risk (P for interaction = 0.14).
Table 6.
Association between OSA and cognitive disorders by race.
| Prevalence of probable OSA (%) | CIND | Dementia | |||
|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted ORa (95% CI) | Crude OR (95% CI) | Adjusted ORa (95% CI) | ||
| Total sample | 59.7 | ||||
| High vs low OSA risk | 1.21 (1.09, 1.34) | 1.22 (1.08, 1.37) | 1.12 (0.94, 1.34) | 1.27 (1.04, 1.54) | |
| Whites | 58.1 | ||||
| High vs low OSA risk | 1.25 (1.10, 1.42) | 1.35 (1.17, 1.57) | 1.04 (0.83, 1.32) | 1.24 (0.96, 1.60) | |
| Non-whites | 66.3 | ||||
| High vs low OSA risk | 1.01 (0.84, 1.21) | 0.98 (0.81, 1.19) | 1.13 (0.86, 1.48) | 1.23 (0.91, 1.66) | |
Association (adjusted OR and 95% CI) between OSA risk (exposure) and CIND or dementia (outcome), by race (Whites and non-Whites) (imputed dataset). aAdjusted for age, education, marital status, smoking, alcohol, and physical activity. The models for the total population were further adjusted for race/ethnicity. CI = confidence interval, CIND = cognitive impairment not dementia, OR = odds ratio, OSA = obstructive sleep apnea.
Finally, given the apparent increased prevalence odds for CIND and dementia when probable OSA is present, we estimated the PARP for each cognitive disorder using a model-based method. The assumption, although not yet proven, is that probable OSA may contribute to CIND and dementia onset. The PARP from adjusted models for OSA and CIND is 12% and 16% for OSA and dementia. Under the above assumption, these results suggest that successful identification and treatment of OSA could eliminate up to 12% and 16% of CIND and dementia cases, respectively.
DISCUSSION
Among a nationally representative cohort of 20,901 middle-aged and older Americans, more than half (60%) had probable OSA and the prevalence of cognitive impairment was > 1.5-fold higher among US adults at high risk for OSA, in comparison to those at low risk. In addition, probable OSA was associated with 22% increased odds of CIND and 27% increased odds of dementia. These findings suggest an increased risk of cognitive disorders among adults with probable OSA. Whether OSA contributes in a causal manner to the risk of CIND and dementia cannot be proven in a cross-sectional study. If so, however, the magnitude of contribution suggested by the current findings would represent a very substantial public health burden, and potential opportunity for intervention.
Our findings build upon existing data that suggest a positive association between claims-based or diagnostically confirmed cases of OSA and poor cognitive outcomes. A prior case-control study that utilized national claims data reported a 1.7 times higher risk of developing dementia over the 5-year study period among adults over age 40 with an OSA diagnosis, in comparison to controls.26 Similarly, increased AD and dementia risk were observed in veteran men aged 55 and over with an OSA claims diagnosis relative to veterans without an OSA diagnosis.6 Studies of objectively confirmed OSA have yielded similar results. For example, a cohort study that used polysomnography found that severe OSA (defined as an apnea-hypopnea index ≥ 30 events/h) was associated with a 1.7 times higher risk of AD and a 2.4 times higher risk of dementia among individuals with OSA, compared with those without OSA.27 In a prior study that assessed OSA through self-report, a significant association between sleep-disordered breathing and earlier age of AD onset was observed.28 If confirmed, and again if an underlying causal relationship exists, this association could have important treatment implications. In a recent Medicare claims analysis of beneficiaries with OSA, positive airway pressure treatment was associated with lower odds of incident diagnoses of Alzheimer and nonspecified forms of dementia (OR = 0.78, 95% CI = 0.69–0.89; OR = 0.69, 95% CI = 0.55–0.85). Lower odds of MCI, approaching statistical significance, were also observed among positive airway pressure users.29
While the aforementioned studies have offered important insight into associations between OSA and dementia risk, the national scope and public health impact of OSA—including effects on cognitive outcomes—must be informed by large, population-based studies that minimize referral bias. Furthermore, current studies do not account for undiagnosed OSA cases, as the majority of those with OSA, up to 80% of cases, remain undiagnosed.9 Symptoms of OSA may not be mentioned or recognized during routine clinical assessments. In this regard, nationally representative datasets that assess clinical factors associated with OSA risk, such as survey items that closely resembled STOP-Bang questionnaires, offer a useful means to minimize bias and misclassification issues that arise from claims data and self-report.
Interestingly, an increased OR of CIND was seen in Whites with probable OSA, relative to non-Whites with OSA (P for interaction < .001). In contrast, there was little difference in the prevalence OR of dementia between Whites and non-Whites (P for interaction = .14). This could result from the fact that algorithms to identify CIND have lower sensitivity and specificity in comparison to those for normal cognition or dementia.30 We performed a sensitivity analysis by restricting the HRS sample to participants who had concordant classification of cognitive function across assessment time points and showed a slightly stronger association between OSA risk and cognitive disorders, further suggesting the role of OSA risk in CIND.
Our study also highlights race/ethnicity as a potential moderator, between OSA and cognitive disorders, which warrants further study. Blacks, Hispanics, and Asians are particularly more likely to have OSA.31 Moreover, the prevalence of AD is also higher among non-Whites as compared with Whites.32 While further studies are necessary to understand this relationship, biological and also cultural risk factors can impact diagnosis and treatment.33 Yet, despite the higher prevalence of both conditions among non-Whites, moderation by age, sex, and race/ethnicity has been only rarely been examined for associations between OSA and cognitive outcomes. One study examining moderation found that women with sleep apnea, but not men, were more likely to develop dementia (hazard ratio = 2.4).26 These data suggest that age and sex are moderators on the association of sleep apnea and dementia. We did not examine age and sex as moderators of OSA and cognitive disorders in our study, as both variables are items in the STOP-Bang score utilized to define the exposure (probable OSA). Future studies focusing on interaction of demographic characteristics, such as sex, age, and race/ethnicity, with OSA could help uncover differential impact of OSA on cognitive outcomes in a specific subgroup.
Our study has several strengths. First, this study consisted of a large, racially and ethnically diverse, and nationally representative sample. Furthermore, the inclusion of study participants aged over 50 allow us to examine the association between OSA and cognitive outcomes among not only the older population but also among middle-aged individuals. Second, we utilized survey items that closely resembled those of the validated and commonly used clinical screening tool to define probable OSA (exposure). In addition, cognitive outcomes were defined by objective cognitive testing. Finally, rich information on covariates including comorbidities and socioeconomic status was available in the study. Hence, we were able to adjust for covariates in the models and stratify the dataset to examine effect modifications of certain variables.
Some limitations of the study should be noted. Missing values in OSA and cognitive outcomes were observed. However, we used the imputed cognitive outcome data described in Langa et al18 and imputed the survey items for probable OSA. In fact, missing data issues often arise in studies that contain cognitive measurement in older populations. This could result in decreased power and biased results. Second, probable OSA was based, in part, on self-reported questions. Nevertheless, similar survey items in the validated questionnaire (STOP-Bang questionnaire) have been shown to reliably identify probable OSA. Further, when subjecting another nationally representative sample of Medicare beneficiaries to a similar analysis, 94% of participants with high surrogate STOP-Bang scores and concomitant polysomnography claims had a corresponding OSA diagnosis.10 Our data did not include information about daytime sleepiness, patient’s chorotype, and time of the day when cognitive tests were administered that could influence the association between OSA and cognitive disorders. Finally, the study reported cross-sectional associations of probable OSA and cognitive outcomes that could lead to temporal ambiguity. Despite the cross-sectional design, epidemiological evidence of OSA as a determinant of dementia appears more biologically plausible than that of dementia as a cause of OSA.34
In short, the high frequencies of cognitive impairment, OSA, and cognitive impairment among persons with OSA raise several important implications. Most OSA remains undiagnosed and probable OSA ascertained in a manner similar to our approach may be an essential complement to more objective data, especially in large-scale studies where definitive diagnosis is not feasible. Future research must further address the likelihood that OSA causes or contributes to neurodegeneration, especially among minority groups, and study the effects of treatment for OSA on cognitive decline. Our estimates of the PARP for OSA and CIND (12%) and OSA and dementia (16%) suggest that if cause-and-effect associations can be proven, and middle-aged or older individuals with OSA can be treated effectively for OSA, then this strategy may be the single most effective thus far identified to alter trajectories, toward CIND and dementia, that so far remain largely unalterable.
ABBREVIATIONS
- AD
Alzheimer disease
- CI
confidence interval
- CIND
cognitive impairment not dementia
- HRS
Health and Retirement Study
- MET
metabolic equivalent of task
- OR
odds ratio
- OSA
obstructive sleep apnea
- PARP
population attributable risk percent
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the University of Michigan. Dr. Shieu is supported by a T32 grant from National Heart, Lung, and Blood Institute (T32 HL110952). Drs. Braley and Dunietz report funding from the National Institute on Aging, Award Number R01AG074342. Dr. Braley also receives funding from the National Center for Complementary and Integrative Health (Award Number R01AT011341) and the Patient-Centered Outcomes Research Institute (Award Number MS-1610-36980). She is named in a patent, held by the University of Michigan, concerning a treatment for sleep apnea. She completed a sleep apnea clinical trial that received material support, but no financial support, from Biogen-Idec. She is also site principal investigator for several industry-funded studies of MS immunotherapeutics at the University of Michigan (Genentech-Roche). She has done consulting work for Greenwich Biosciences. Dr. Chervin has received research funding from the National Institutes of Health (HL105999 and NS099043). He is named in or has developed materials, patented and copyrighted by the University of Michigan, and designed to assist with assessment or treatment of sleep disorders. He has served on the boards of the American Academy of Sleep Medicine, Associated Professional Sleep Societies, American Board of Sleep Medicine, American Academy of Sleep Medicine Foundation (which funded the current research), International Pediatric Sleep Society, and the nonprofit Sweet Dreamzzz. He is an author and editor for UpToDate, has edited a book for Cambridge University Press, and has consulted for Zansors. Dr. Paulson reports no conflicts of interest.
REFERENCES
- 1. Plassman BL , Langa KM , Fisher GG , et al . Prevalence of dementia in the United States: the aging, demographics, and memory study . Neuroepidemiology. 2007. ; 29 ( 1–2 ): 125 – 132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzheimer Association . 2020 Alzheimer’s disease facts and figures . Alzheimers Dement. 2020. ; 16 ( 3 ): 391 – 460 . [Google Scholar]
- 3. Albert MS , DeKosky ST , Dickson D , et al . The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease . Alzheimers Dement. 2011. ; 7 ( 3 ): 270 – 279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mubashir T , Abrahamyan L , Niazi A , et al . The prevalence of obstructive sleep apnea in mild cognitive impairment: a systematic review . BMC Neurol. 2019. ; 19 ( 1 ): 195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JE , Yang SW , Ju YJ , Ki SK , Chun KH . Sleep-disordered breathing and Alzheimer’s disease: a nationwide cohort study . Psychiatry Res. 2019. ; 273 : 624 – 630 . [DOI] [PubMed] [Google Scholar]
- 6. Yaffe K , Nettiksimmons J , Yesavage J , Byers A . Sleep quality and risk of dementia among older male veterans . Am J Geriatr Psychiatry. 2015. ; 23 ( 6 ): 651 – 654 . [DOI] [PubMed] [Google Scholar]
- 7. Ancoli-Israel S , Kripke DF , Klauber MR , Mason WJ , Fell R , Kaplan O . Sleep-disordered breathing in community-dwelling elderly . Sleep. 1991. ; 14 ( 6 ): 486 – 495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kapur V , Blough DK , Sandblom RE , et al . The medical cost of undiagnosed sleep apnea . Sleep. 1999. ; 22 ( 6 ): 749 – 755 . [DOI] [PubMed] [Google Scholar]
- 9. Silverberg DS , Oksenberg A , Iaina A . Sleep related breathing disorders are common contributing factors to the production of essential hypertension but are neglected, underdiagnosed, and undertreated . Am J Hypertens. 1997. ; 10 ( 12 Pt 1 ): 1319 – 1325 . [DOI] [PubMed] [Google Scholar]
- 10. Braley TJ , Dunietz GL , Chervin RD , Lisabeth LD , Skolarus LE , Burke JF . Recognition and diagnosis of obstructive sleep apnea in older Americans . J Am Geriatr Soc. 2018. ; 66 ( 7 ): 1296 – 1302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sonnega A , Faul JD , Ofstedal MB , Langa KM , Phillips JWR , Weir DR . Cohort profile: the Health and Retirement Study (HRS) . Int J Epidemiol. 2014. ; 43 ( 2 ): 576 – 585 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boynton G , Vahabzadeh A , Hammoud S , Ruzicka DL , Chervin RD . Validation of the STOP-Bang questionnaire among patients referred for suspected obstructive sleep apnea . J Sleep Disord Treat Care. 2013. ; 2 ( 4 ):10.4172/2325-9639.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung F , Yegneswaran B , Liao P , et al . STOP questionnaire: a tool to screen patients for obstructive sleep apnea . Anesthesiology. 2008. ; 108 ( 5 ): 812 – 821 . [DOI] [PubMed] [Google Scholar]
- 14. Chung F , Yang Y , Brown R , Liao P . Alternative scoring models of STOP-bang questionnaire improve specificity to detect undiagnosed obstructive sleep apnea . J Clin Sleep Med. 2014. ; 10 ( 9 ): 951 – 958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagappa M , Patra J , Wong J , et al . Association of STOP-Bang questionnaire as a screening tool for sleep apnea and postoperative complications: a systematic review and Bayesian meta-analysis of prospective and retrospective cohort studies . Anesth Analg. 2017. ; 125 ( 4 ): 1301 – 1308 . [DOI] [PubMed] [Google Scholar]
- 16. Iwashyna TJ , Ely EW , Smith DM , Langa KM . Long-term cognitive impairment and functional disability among survivors of severe sepsis . JAMA. 2010. ; 304 ( 16 ): 1787 – 1794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crimmins EM , Kim JK , Langa KM , Weir DR . Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study . J Gerontol B Psychol Sci Soc Sci. 2011. ; 66 ( Suppl 1 ): i162 – i171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langa-Weir Classification of Cognitive Function (1995 Onward) https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/Data_Description_Langa_Weir_Classifications2016.pdf .
- 19. He XZ , Baker DW . Differences in leisure-time, household, and work-related physical activity by race, ethnicity, and education . J Gen Intern Med. 2005. ; 20 ( 3 ): 259 – 266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen M , Li L , Su D . Physical activity and mortality among middle-aged and older adults in the United States . J Phys Act Health. 2014. ; 11 ( 2 ): 303 – 312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin DB . Multiple Imputation for Nonresponse in Surveys. New York, NY: Jon Wiley & Sons; 1987. https://www.wiley.com/en-us/Multiple+Imputation+for+Nonresponse+in+Surveys-p-9780471655749 . Accessed May 1, 2021.
- 22. Azur MJ , Stuart EA , Frangakis C , Leaf PJ . Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011. ; 20 ( 1 ): 40 – 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berglund PA . Multiple imputation using the fully conditional specification method: a comparison of SAS, Stata, IVEware, and R. SAS Glob Forum Proceedings, Pap 2081-2015. Published online 2015. :1–17. https://support.sas.com/resources/papers/proceedings15/2081-2015.pdf . Accessed April 20, 2021.
- 24. Greenland S , Drescher K . Maximum likelihood estimation of the attributable fraction from logistic models . Biometrics. 1993. ; 49 ( 3 ): 865 – 872 . [PubMed] [Google Scholar]
- 25. Rückinger S , von Kries R , Toschke AM . An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors . BMC Med Res Methodol. 2009. ; 9 ( 1 ): 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang WP , Liu ME , Chang WC , et al . Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan . PLoS One. 2013. ; 8 ( 10 ): e78655 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lutsey PL , Misialek JR , Mosley TH , et al . Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study . Alzheimers Dement. 2018. ; 14 ( 2 ): 157 – 166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osorio RS , Gumb T , Pirraglia E , et al. ; Alzheimer’s Disease Neuroimaging Initiative . Sleep-disordered breathing advances cognitive decline in the elderly . Neurology. 2015. ; 84 ( 19 ): 1964 – 1971 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dunietz GL , Chervin RD , Burke JF , Conceicao AS , Braley TJ . Obstructive sleep apnea treatment and dementia risk in older adults . Sleep. 2021. ; 44 ( 9 ): zsab076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plassman BL , Langa KM , Fisher GG , et al . Prevalence of cognitive impairment without dementia in the United States . Ann Intern Med. 2008. ; 148 ( 6 ): 427 – 434 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jackson CL , Powell-Wiley TM , Gaston SA , Andrews MR , Tamura K , Ramos A . Racial/ethnic disparities in sleep health and potential interventions among women in the United States . J Womens Health (Larchmt). 2020. ; 29 ( 3 ): 435 – 442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim ASP , Fleischman DA , Dawe RJ , et al . Regional neocortical gray matter structure and sleep fragmentation in older adults . Sleep. 2016. ; 39 ( 1 ): 227 – 235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chin AL , Negash S , Hamilton R . Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease . Alzheimer Dis Assoc Disord. 2011. ; 25 ( 3 ): 187 – 195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liguori C , Maestri M , Spanetta M , et al . Sleep-disordered breathing and the risk of Alzheimer’s disease . Sleep Med Rev. 2021. ; 55 : 101375 . [DOI] [PubMed] [Google Scholar]