Abstract
Purpose
To assess the effectiveness and safety of primary-needling in eyes who underwent a XEN45 implant.
Methods
Retrospective and single center study. Consecutive patients with early-to-moderate open-angle glaucoma (OAG) or ocular hypertension, who underwent XEN45 surgery, either alone or combined with phacoemulsification, and had at least a valid 12-month post-operative visit, were included in the study. Primary needling was performed by using a 30-gauge needle without viscoelastic. Subjects were divided in two groups: Eyes who underwent XEN+primary-needling (needling) and those who underwent XEN without primary-needling (no-needling). The primary end-point was the mean change in IOP from baseline to the last follow-up visit.
Results
Sixty-three eyes, 19 (30.2%) in the needling group and 44 (69.8%) in the no-needling one, were included in the study. There were not significant differences in mean IOP lowering between needling and no-needling groups at month-12 (mean difference −2.5±5.3 mm Hg, p=0.0926). No significant differences in mean reduction of ocular-hypotensive medications were observed between needling and no-needling groups, p=0.8690. At month-12, 50 (80.6%) had blebs considered as functioning, with no difference between groups, p = 0.5631. At month-12, 59 (93.7%) eyes were classified as success, with no significant differences between needling (17/19) and no-needling (42/44) groups, p=0.3754. Secondary needling was performed in 8 (12.7%) eyes, without differences between groups (p=0.6333).
Conclusion
Primary needling, at the time of surgery, was a safe procedure in OAG patients who underwent a XEN implant, although it was not associated with a lower postoperative IOP or less ocular hypotensive medications.
Keywords: open-angle glaucoma, MIGS, XEN, primary needling
Introduction
Micro-invasive glaucoma surgery (MIGS) has emerged as a safer and less traumatic approach for lowering intraocular pressure (IOP) in patients with glaucoma.1–3 Although MIGS procedures have been usually performed on mild-to-moderate glaucoma patients,1–4 due mainly to their IOP-lowering effect is slightly lower than that provided by traditional glaucoma surgery, there is evidence suggesting their effectiveness in advanced open-angle glaucoma (OAG) patients.5
Though MIGS mechanisms vary, some approaches include trabecular meshwork reshaping, trabecular meshwork stenting, and subconjunctival stenting. Among the different MIGS devices, XEN45 gel stent (Allergan, an AbbVie company, Irvine, CA, USA) allows a controlled aqueous humor flow from the anterior chamber to the subconjunctival space.1–3
XEN45 gel stent has usually been delivered using an ab-interno approach through a corneal incision.5–13 However, as surgeons have been gaining experience with the device, different changes, which aim to provide better clinical outcomes, have been introduced in the implantation technique.14–16
XEN45 has been shown to be effective for lowering IOP, although, achieving successful outcomes may require an intensive postoperative bleb management.5–13
Bleb fibrosis has been reported as a common complication after XEN implantation, with rates as high as 45%.17 Needling is a minimally invasive procedure that is commonly used for restoring the functionality of failed filtering blebs.18
Needling is a common procedure in eyes who have undergone XEN implant, with rates ranging between 10% and 60%.5–13 However, this needling, known as secondary needling, is considered as a rescue strategy for those cases in which a failure of the filtering bleb occurs.
Primary needling, at the time of ab-interno XEN implantation, has been proposed as a technique that may reduce the number of postoperative interventions. Kerr et al,19 in a retrospective study, observed that primary needling at the time of XEN insertion was associated with a significant reduction in the number of bleb interventions and, therefore, the subsequent postoperative visits. Additionally, as compared to preoperative values, this technique has provided a significant reduction in both IOP and ocular hypotensive medication.19
Although primary needling may be a promising strategy for eyes undergoing XEN implant, information about its effectiveness and safety is limited to only one study.19
The main purpose of our study is to assess the effectiveness, in terms of IOP lowering, and safety of primary needling in eyes who underwent a XEN45 gel stent implant. Additionally, the impact of primary needling on bleb morphology was also evaluated.
Methods
Design
Retrospective and single center study conducted on consecutive patients who underwent a XEN45 implant, either alone or in combination with phacoemulsification.
The study protocol was approved by the Ethic Committee of the University Hospital of Fuenlabrada (APR 20/05). Since, it was a retrospective revision of the medical charts, the Ethic Committee waived the need of written inform consent to participate in the study. In order to ensure patients confidentiality, any information that could lead to an individual being identified has been encrypted or removed, as appropriate. The study protocol adhered to the tenets of the Declaration of Helsinki and the Good Clinical Practice/International Council for Harmonization Guidelines.
Participants
Patients, aged ≥18 years, with insufficiently controlled early-to-moderate OAG or ocular hypertension; intolerance to topical hypotensive treatments; or poor treatment adherence were included in the study.
Patients with any form of glaucoma other than OAG; patients with any abnormality preventing reliable applanation tonometry in study eye(s); progressive retinal or optic nerve disease due to any cause, or patients with a conjunctival scarring, which, in the surgeon’s opinion could compromise the procedure outcomes, were excluded from the analysis.
Patients were instructed to withdraw topical and systemic ocular hypotensive medications on the day of surgery.
Surgical Technique
All procedures were performed under local anesthesia by a well-trained and experienced surgeon. XEN implant was placed, using an ab-interno approach, in the superior nasal quadrant using a standard technique (5–7).
Intraoperatively, 0.1 mL of MMC 0.01% or 0.02%, depending on the surgeon’s preference and patient characteristics, was injected subconjunctival.
Primary Needling Procedure
Initially, primary needling was performed when the XEN implant was twisted, trapped, or did not appear appeared free and mobile in the subconjunctival space for breaking any possible conjunctival adhesion. Therefore, the need for primary needling was determined by the surgeon at the time of surgery. Subsequently, primary needling became a common practice in our XEN45 device implant procedure and was performed routinely in all cases. Primary needling was performed by means of a 30-gauge needle, which was routinely slid above and below the implant by a sweeping motion for breaking any possible conjunctival adhesion, even where the stent appeared free and mobile. For primary needling, the 30-gauge needle was bended at a 90-degree angle and viscoelastic is not used during the procedure.
Anterior Segment Optical Coherence Tomography
A swept-source (SS) anterior segment optical coherence tomography (AS-OCT) Topcon DRI OCT Triton Swept source OCT, Topcon, Japan) was performed after surgery at six and twelve months for assessing bleb morphology.
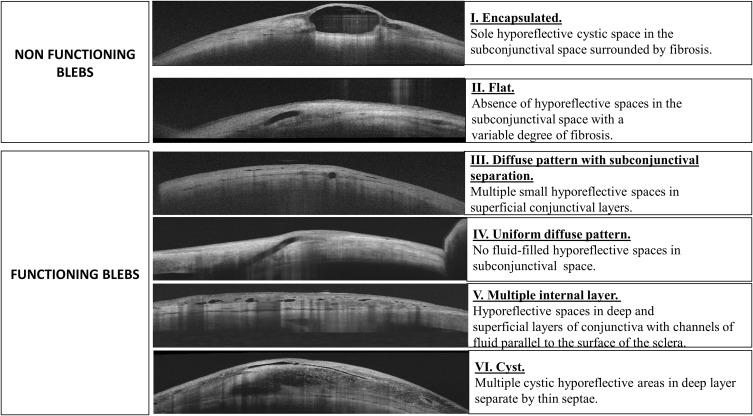
Blebs were classified, according to their functionality, in two main groups: Functioning and Non-functioning blebs (Figure 1). Functioning blebs were, in turn, sub-classified in five different patterns, namely Diffuse pattern; Subconjunctival separation (multiple small hyporeflective spaces in superficial conjunctival layers); Uniform (no fluid‐filled hyporeflective spaces in subconjunctival space); Multiple internal layer (hyporeflective spaces in deep and superficial layers of conjunctiva with channels of fluid parallel to the surface of the sclera); and Cyst (multiple cystic hyporeflective areas in deep layer separated by thin septae) (Figure 1). Non-functional blebs were classified in Encapsulated (Sole hyporeflective cystic space in the subconjunctival space surrounded by fibrosis) and Flat (absence of hyporeflective spaces in the subconjunctival space with a variable degree of fibrosis) (Figure 1).
Figure 1.
Overview of the bleb morphology classification system.
Regarding fibrosis, filtering blebs have been classified in blebs with subepithelial fibrosis and those without subepithelial fibrosis.
Photograph Bleb Assessment
Bleb photographs were reviewed and graded by two well-trained ophthalmologists (NMA and MBM) using the Indiana Bleb Appearance Grading Scale (IBAGS) (20). The IBAGS classifies blebs according to 4 features: Height (from H0 to H3), Bleb extend or bleb area (from E0 to E3), Bleb vascularity (from V0 to V3), and Seidel test (from S0 to S2).20
Study Groups
Eyes were divided in two different groups: Eyes who underwent a XEN45 device + primary needling (Needling group) and eyes who underwent XEN45 implantation without primary needling (No-Needling), regardless of whether the patient underwent the implant alone or in combination with cataract surgery (phacoemulsification).
Definitions
Success was defined as a month 12 IOP ≤ 18 mm Hg without (Complete success) or with hypotensive medication (Qualified success). Patients with an IOP < 6 mm Hg for more than 2 consecutive visits; those who experienced a severe loss of visual acuity (light perception or worse); or those who needed further glaucoma surgery were also considered as failure.
Glaucoma stage was defined according to the criteria described by Hodapp et al.21
Blebs were classified as functioning and non-functioning. Non-functioning bleb was defined as an IOP higher than 18 mm Hg without there being a justified cause for it.
Secondary needling or surgical bleb revision, as needed, was indicated in those cases of failure of the procedure due to fibrosis or encapsulation of the bleb that did not respond to massage in the slit lamp and topical hypotension medications.
Outcomes
The primary end-point was the mean change in IOP from baseline to the last follow-up visit.
Secondary end-points included mean IOP at the end of the follow-up period, the mean number of antiglaucoma medications and its changes from baseline; proportion of patients classified as success; proportion of secondary needling; bleb morphology; changes in bleb morphology after secondary needling; and incidence of adverse events.
Statistical analysis
The statistical software package (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021) was used for the analysis.
Descriptive statistics number (percentage), mean [standard deviation (SD)], mean [95% confidence interval (95% CI)], mean [standard error (SE)], median (interquartile range), or median (95% CI) were used, as appropriate.
Data were tested for normal distribution using a D’Agostino-Pearson test.
The changes in IOP and the number of ocular hypotensive medications over the course of the study were evaluated with the repeated measures ANOVA or a Friedman’s two-way analysis test, as appropriate.
The Mann–Whitney U-test was used to compare baseline and evolutive continuous variables, from baseline to month 12, between Needling and No-Needling groups.
Repeated analysis of covariance (ANCOVA) was used to assess the changes in IOP between eyes who underwent needling and those who did not. The model included Needling regime as factor and type of surgery (XEN alone or XEN+Phaco), age, preoperative IOP, number of ocular hypotensive medications, MMC dose, type of glaucoma, mean defect, and reason for performing surgery as covariates.
Categorical variables were compared using a Chi-square test and a Fisher’s exact test, as appropriate.
P value of less than 0.05 was considered significant.
Results
Fifty-five patients (63 eyes) were included in the analysis. Nineteen (30.2%) eyes underwent surgery with primary needling and 44 (69.8%) ones without primary needling. Eleven (17.5%) eyes underwent XEN45 alone and 52 (82.5%) ones underwent XEN45 + Phaco.
The main baseline demographic and clinical variables are shown in Table 1.
Table 1.
Overview of the Demographic and Clinical Characteristics of the Study Sample
| Overall (n=63) | No-Primary Needling (n=44) | Primary Needling (n=19) | p | |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 71.0 (7.2) | 71.1 (7.7) | 70.7 (6.3) | 0.9164a |
| 95% CI | 69.2 to 72.8 | 68.8 to 73.1 | 67.7 to 73.8 | |
| Sex, n (%) | ||||
| Men | 31 (49.2) | 21 (47.7) | 10 (52.6) | 0.7879b |
| Women | 32 (50.8) | 23 (52.3) | 9 (47.4) | |
| Eye, n (%) | ||||
| Right | 37 (58.7) | 26 (59.1) | 11 (57.9) | 1.000b |
| Left | 26 (41.3) | 18 (40.9) | 8 (42.1) | |
| Surgery | ||||
| XEN | 11 (17.5) | 9 (20.5) | 2 (10.5) | 0.4799b |
| XEN+PHACO | 52 (82.5) | 35 (79.5) | 17 (89.5) | |
| Type of glaucoma, n (%) | ||||
| NTG | 1 (1.6) | 0 (0.0) | 1 (5.3) | 0.4508c |
| POAG | 48 (76.2) | 33 (75.0) | 15 (78.9) | |
| PreP POAG | 2 (3.2) | 2 (4.5) | 0 (0.0) | |
| HTO | 11 (17.5) | 8 (18.2) | 3 (15.8) | |
| PXG | 1 (1.6) | 1 (2.3) | 0 (0.0) | |
| Main reason for surgery, n (%) | ||||
| Insufficient IOP control | 19 (30.2) | 11 (25.0) | 8 (42.1) | 0.1048c |
| VF progression | 21 (33.3) | 18 (40.9) | 3 (15.8) | |
| Intolerance to TOHM | 22 (34.9) | 15 (34.1) | 7 (36.8) | |
| Poor adherence | 1 (1.6) | 0 (0.0) | 1 (5.3) | |
| Secondary reason for surgery, n (%) | ||||
| Insufficient IOP control | 7 (36.8) | 6 (40.0) | 1 (25.0) | 0.1716c |
| VF progression | 1 (5.3) | 0 (0.0) | 1 (25.0) | |
| Intolerance to TOHM | 10 (52.6) | 9 (60.0) | 1 (25.0) | |
| Poor adherence | 1 (5.3) | 0 (0.0) | 1 (25.0) | |
| IOP, mm Hg | ||||
| Mean (SD) | 17.6 (5.3) | 17.3 (5.2) | 18.2 (5.5) | 0.4851a |
| 95% CI | 16.3 to 18.9 | 15.7 to 18.9 | 15.5 to 20.8 | |
| Number of OHM | ||||
| Mean (SD) | 2.1 (0.7) | 2.1 (0.6) | 2.0 (0.9) | 0.5854a |
| 95% CI | 1.9 to 2.3 | 1.9 to 2.3 | 1.6 to 2.5 | |
| Type of OHM, n (%)* | ||||
| PA | 50 (79.4) | 38 (86.4) | 12 (63.2) | 07289c |
| BB | 44 (69.8) | 31 (70.5) | 13 (68.4) | |
| CAI | 26 (41.3) | 17 (38.6) | 9 (47.4) | |
| AA | 11 (17.5) | 7 (15.9) | 4 (21.1) | |
| BCVA, logMAR test | ||||
| Mean (SD) | 0.54 (0.21) | 0.55 (0.23) | 0.54 (0.13) | 0.7847a |
| 95% CI | 0.49 to 0.60 | 0.48 to 0.62 | 0.48 to 0.60 | |
| RNFL thickness, µm** | ||||
| Mean (SD) | 77.4 (16.9) | 76.5 (18.3) | 79.7 (12.7) | 0.5029a |
| 95% CI | 73.0 to 81.9 | 70.8 to 82.2 | 76.2 to 86.3 | |
| VF, dB | ||||
| MD | ||||
| Mean (SD) | −5.6 (5.0) | −6.0 (4.7) | −4.7 (5.6) | 0.0790a |
| 95 CI | −6.9 to −4.3 | −7.5 to −4.5 | −7.5 to −2.0 | |
| PSD | ||||
| Mean (SD) | 4.9 (3.3) | 5.1 (3.4) | 45 (3.1) | 0.5236a |
| 95% CI | 4.1 to 5.8 | 4.0 to 6.2 | 3.0 to 6.0 | |
| VFI | ||||
| Mean (SD) | 87.3 (14.7) | 86.3 (13.8) | 89.3 (16.4) | 0.1166a |
| 95% CI | 83.4 to 91.1 | 81.8 to 90.7 | 81.4 to 97.2 |
Notes: aMann–Whitney U-test. bFisher exact test. cChi-squared test. *Percentage may be greater than 100%, because one eye may be taken more than one drug.
Abbreviations: OCT, **measure with optical coherence tomography; MMC, mitomycin-C; SD, standard deviation; CI, confidence interval; PHACO, phacoemulsification; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma; HTO, ocular hypertension; PXG, pseudoexfoliative glaucoma; IOP, intraocular pressure; VF, visual field; TOHM, topical ocular hypotensive medications; OHM, ocular hypotensive medications; PA, prostaglandin analogue; BB, betablocker; CAI, carbonic anhydrase inhibitor; AA, alfa agonist; BCVA, best corrected visual acuity; RNFL, retinal nerve fiber layer; MD, mean effect; PSD, pattern standard deviation; VFI, visual field index.
In the overall study sample, the mean (95% CI) IOP decreased significantly from 17.6 (16.3 to 18.9) mm Hg at baseline to 8.4 (7.2 to 9.7) mm Hg; 11.1 (9.7 to 12.5) mm Hg; 13.5 (12.1 to 15.0) mm Hg; 13.6 (12.6 to 14.5) mm Hg; 13.8 (12.8 to 14.8) mm Hg; and 12.6 (12.0 to 13.3) mm Hg at 1 day, 1 week, 1, 3, 6, and 12 months of follow-up, respectively (p<0.001, p<0.0001, p=0.0029, p<0.0001, p=0.0001, and p<0.0001, respectively).
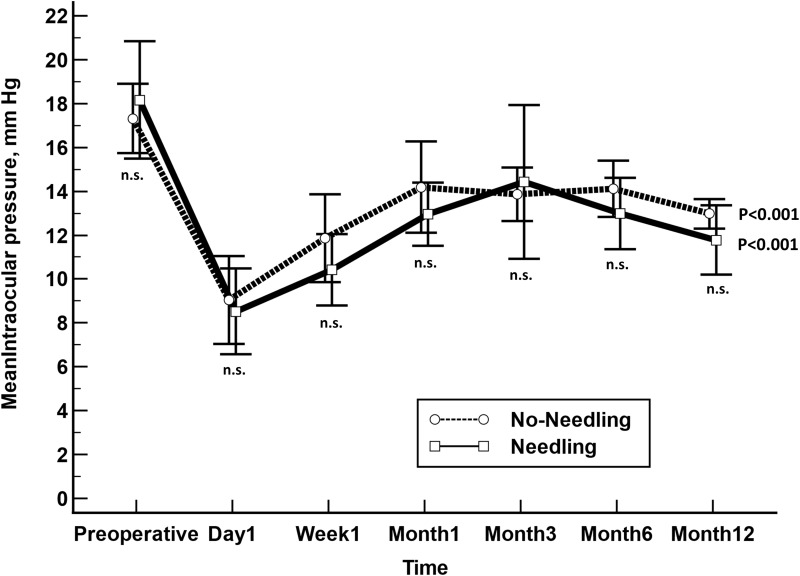
In the Needling group, the mean (SE) IOP was significantly lowered from 18.5 (1.3) at baseline mm Hg to 11.8 (0.8) mm Hg at month 12 (p=0.0002, Bonferroni corrected) and from 17.2 (0.8) mm Hg at baseline to 13.0 (0.3) mm Hg at month 12 in the No-Needling group (p<0.0001, Bonferroni corrected; without significant differences between groups at any of the time point measured (Figure 2).
Figure 2.
Unadjusted comparison of mean intraocular pressure (IOP) between XEN implant + primary needling (Needling) and XEN implant without primary needling (No-Needling). The vertical bars represent the 95% confidence interval. Statistical significance, at the different time point measurements, was determined using the one-way ANOVA test with the Scheffé’s method. As compared to baseline, the mean IOP was significantly reduced, at every time point measured, p<0.01 (repeated measures ANOVA and the Greenhouse–Geisser correction).
Abbreviation: n.s., not significant.
Mean IOP lowering was markedly greater in the Needling than in the No-needling group (mean difference: −2.5 mm Hg; 95% CI: −5.5 to 0.4 mm Hg), although this difference was not statistically significant (p=0.0926).
The adjusted model did not show significant differences in mean IOP lowering between Needling and No-Needling groups at any of the time point measured (Table 2).
Table 2.
Adjusted Mean Changes in Intraocular Pressure (IOP) in Eyes Who Underwent Xen Implant with Primary Needling (Needling) and Those Who Did It Without Primary Needling (No-Needling)
| Needling (n=19) | No-Needling (n=44) | Difference | Pa | |
|---|---|---|---|---|
| MCIOPD1, mm Hg | ||||
| Mean (SE) | 8.3 (1.6) | 8.3 (1.0) | 0.0 (2.0) | 0.9909 |
| 95% CI | 5.2 to 11.4 | 6.3 to 10.3 | −4.1 to 4.1 | |
| MCIOPW1, mm Hg | ||||
| Mean (SE) | 7.1 (1.6) | 5.4 (1.0) | 1.7 (2.0) | 0.4103 |
| 95% CI | 4.0 to 10.2 | 3.4 to 7.4 | −2.4 to 5.8 | |
| MCIOPM1, mm Hg | ||||
| Mean (SE) | 3.4 (1.8) | 3.8 (11) | −0.4 (2.3) | 0.8512 |
| 95% CI | −0.2 to 6.9 | 1.6 to 6.1 | −5.1 to 4.2 | |
| MCIOPM3, mm Hg | ||||
| Mean (SE) | 4.3 (1.1) | 3.7 (0.7) | 0.6 (1.5) | 0.7057 |
| 95% CI | 2.0 to 6.6 | 2.3 to 5.1 | −2.5 to 3.6 | |
| MCIOPM6, mm Hg | ||||
| Mean (SE) | 4.3 (1.2) | 3.7 (0.7) | 0.6 (1.5) | 0.6866 |
| 95% CI | 2.0 to 6.6 | 2.2 to 5.1 | −2.5 to 3.7 | |
| MCIOPM12, mm Hg | ||||
| Mean (SE) | 4.9 (0.7) | 4.9 (0.4) | 0.0 (0.9) | 0.9691 |
| 95% CI | 3.5 to 6.2 | 4.0 to 5.8 | −1.9 to 1.8 |
Notes: aAnalysis of covariance (ANCOVA). The model included “Primary needing” as a factor and age, preoperative IOP and number of ocular hypotensive medications, type of glaucoma, mitomycin-C dose, type of surgery (XEN alone or XEN + phacoemulsification), mean defect, and reason for performing surgery as covariates.
Abbreviations: MCIOP, mean change in intraocular pressure; SE, standard error; CI, confidence interval; D, day; W, week; M, month.
At the last follow-up visit, there were no significant differences in the percentage of IOP reduction between Needling (29.9%±29.4%) and No-Needling (18.5%±26.5%) groups, p=0.1463.
The mean number of antiglaucoma medications significantly decreased from 2.1 (1.9 to 2.3) at baseline to 0.2 (0.04 to 0.3) at month 12 (mean difference: −1.9 drugs, 95% CI: −2.1 to −1.7, p<0.0001, Bonferroni corrected). There was not significant difference in the mean reduction of ocular-hypotensive medications between Needling [1.9 (1.5 to 2.3), p<0.0001] and No-Needling [1.9 (1.7 to 2.2), p<0.0001], p=0.8690.
At the last follow-up visit 59 (93.7%) eyes were classified as success, 53 as complete success and 6 as qualified one, with no significant differences between Needling (17/19) and No-Needling (42/44) groups, p=0.3754.
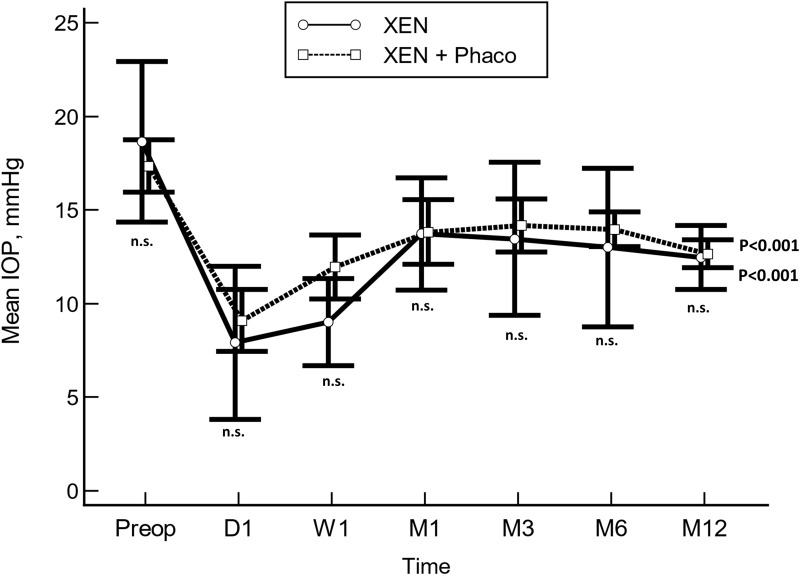
Regarding the type of surgery, IOP was significantly lowered in both XEN alone and XEN+Phacoemulsification, without significant differences between them at any of the different time-point measured (Figure 3).
Figure 3.
Unadjusted comparison of mean intraocular pressure (IOP) between XEN implant alone (XEN) and XEN implant in combination with phacoemulsification (XEN+Phaco). The vertical bars represent the 95% confidence interval. Statistical significance, at the different time point measurements, was determined using the one-way ANOVA test with the Scheffé’s method. As compared to baseline, the mean IOP was significantly reduced, at every time point measured, p<0.01 (repeated measures ANOVA and the Greenhouse–Geisser correction).
Abbreviation: n.s., not significant.
At month-12, 50 (80.6%) had blebs considered as functioning without any additional intervention. Four eyes, who were initially classified as “non-functioning” blebs, achieved a “functioning bleb” after undergoing a secondary needling procedure.
Table 3 shows bleb morphology according to AS-OCT and the IBAGS grade system in the overall study sample and in the two study groups.
Table 3.
A Comparison of the Bleb Morphology Assessed by Swept-Source (SS) Anterior Segment-Optical Coherence Tomography (as-OCT) and the Indiana Bleb Appearance Grading Scale (IBAGS) (18) Between Eyes Who Underwent Primary Needling (Needling) and Those Who Did Not (No-Needling)
| AS-OCT | Overall (n=62) | Needling (n=19) | No-Needling (n=43) | p |
|---|---|---|---|---|
| Bleb Morphology, n (%) | ||||
| Encapsulated | 2 (3.2) | 0 (0.0) | 2 (4.7) | 0.5665a |
| Flat | 9 (14.5) | 2 (10.5) | 7 (16.3) | |
| Subconjunctival separation | 8 (12.9) | 3 (15.8) | 5 (11.6) | |
| Uniform | 11 (17.7) | 3 (15.8) | 8 (18.6) | |
| Multiple internal layer | 19 (30.6) | 8 (42.1) | 11 (25.6) | |
| Cyst | 13 (21.0) | 3 (15.8) | 10 (23.3) | |
| Subepithelial fibrosis, n (%) | ||||
| Yes | 4 (6.5) | 0 (0.0) | 4 (9.3) | 0.3029b |
| No | 58 (93.5) | 19 (100.0) | 39 (90.7) | |
| Cyst, n (%) | ||||
| No | 31 (50.0) | 12 (63.2) | 19 (44.2) | 0.2993a |
| Subepithelial | 23 (37.1) | 6 (31.6) | 17 (39.5) | |
| Epithelial/subepithelial | 8 (12.9) | 1 (5.3) | 7 (16.3) | |
| IBAGS | ||||
| Heigh, n (%) | ||||
| H0 | 26 (41.9) | 8 (42.1) | 18 (41.9) | 0.7050a |
| H1 | 22 (35.5) | 8 (42.1) | 14 (32.6) | |
| H2 | 12 (19.4) | 3 (15.8) | 9 (20.9) | |
| H3 | 2 (3.2) | 0 (0.0) | 2 (4.7) | |
| Bleb extend, n (%) | ||||
| E0 | 16 (25.8) | 3 (15.8) | 13 (30.2) | 0.1325a |
| E1 | 21 (33.9) | 5 (26.3) | 16 (37.2) | |
| E2 | 23 (37.1) | 11 (57.9) | 12 (27.9) | |
| E3 | 2 (3.2) | 0 (0.0) | 2 (4.7) | |
| Bleb vascularity, n (%) | ||||
| V0 | 5 (8.1) | 0 (0.0) | 5 (11.6) | 0.3769a |
| V1 | 1 (1.6) | 0 (0.0) | 1 (2.3) | |
| V2 | 52 (83.9) | 18 (94.7) | 34 (79.1) | |
| V3 | 4 (6.5) | 1 (5.3) | 3 (7.0) | |
| Seidel test, n (%) | ||||
| S0 | 60 (96.8) | 17 (89.5) | 43 (100.0) | 0.0319a |
| S1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| S2 | 2 (3.2) | 2 (10.5) | 0 (0.0) |
Notes: aChi-squared test for trend. bFisher exact test.
According to AS-OCT examinations, the most prevalent pattern of functioning bleb was Multiple internal layer (19/62) and Cyst (13/62). There were no significant differences in bleb morphology between Needling and No-Needling groups.
Eight (12.7%) eyes underwent a secondary needling procedure, 3 (15.8%) eyes in the Needling group and 5 (11.4%) in the No-Needling one, p=0.6333. There were no significant changes in bleb morphology after secondary needling.
Eighteen (28.6%) eyes have experienced, at least, one adverse event. Inadequate IOP control (11/63), hyphema (3/63), and ocular hypertensive spike (3/63), with no significant differences between Needling and No-Needling groups (Table 4).
Table 4.
Overview of the Different Adverse Events in the Overall Study Sample and in Those Eyes Who Underwent Primary Needling (Needling) and Those Who Did Not (No-Needling)
| Complication*, n (%) | Overall | Needling | No-Needling | p |
|---|---|---|---|---|
| Inadequate IOP control | 11 (17.5) | 3 (15.8) | 8 (18.2) | 0.8194 |
| Hyphema | 3 (4.8) | 1 (5.3) | 2 (4.5) | 0.8918 |
| Ocular hypertensive spike† | 3 (4.8) | 0 (0.0) | 3 (6.8) | 0.2479 |
| Hypotonic maculopathy | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Iris touch | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Big bleb (360°) | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Prominent filtering bleb | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Hemovitreus | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Visual field progression | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Wipe-out | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
| Partial migration to AC | 1 (1.6) | 0 (0.0) | 1 (2.3) | 0.5085 |
Notes: *Percentages have been calculated according to the total sample. †Due to hyphema in two eyes.
Three (4.8%) eyes required surgical bleb revision (all of them in the No-Needling group), two (3.2%) eyes required a subsequent trabeculectomy (one eye in each group), and one (1.6%) eye (in the No-Needling group) required XEN extraction and reconversion to not-penetrating deep sclerectomy.
Discussion
The results of this study suggested that performing primary needling in eyes who underwent a XEN45 implant did not provided better clinical outcomes, although eyes who underwent primary needling were associated with fewer postoperative interventions.
Additionally, no significant differences in bleb morphology were observed between those eyes who underwent primary needling and those who did not.
Finally, as expected, XEN45 implant, either alone or in combination with phacoemulsification, was associated with a significantly IOP lowered and a reduction of the number of ocular hypotensive medications.
In eyes who underwent a XEN implant, bleb fibrosis associated with raised IOP is the most commonly reported bleb-related complication, which entails the need to carry out a greater number of postoperative needling, with rates that ranged between 33% and 45%.6–13,22,23 This relatively high rate of needling may be due to the fact that the implant becomes surrounded by Tenon’s capsule tissue.14
Role of bleb needling with anti-fibrotic agents like MMC and 5-fluorouracil (5-FU) has been well established in post-trabeculectomy eyes.24,25 Needling with antimetabolites for restoring function in failed or failing blebs is becoming the standard of care after XEN implantation.22,23
The ab interno approach may be associated with less tissue manipulation, since it does not need conjunctival dissection and suturing. However, determining whether the implant is free of Tenon’s adhesions is anything but easy. To this end, performing primary needling at the time of stent implantation may be a feasible strategy for achieving better clinical outcomes and reducing the need of subsequent bleb interventions.19
Although it may be rational to assume that removing, at the time of XEN implantation, possible conjunctival fibrotic tissue may allow a free and mobile implant and a way to improve filtration, which would provide lower postoperative IOP, the question of whether performing needling at the time of XEN implantation would provide better clinical outcomes has not been clarified.
Up to now, there is only one paper evaluating the impact of primary needling at the time of XEN implantation.19 Kerr et al19 in a retrospective, single-center, and cohort study evaluated the effect of primary needling on the rate of postoperative needling and surgical success. In agreement with our results, they did not find significant differences in both IOP lowering and reduction in number of ocular hypotensive medications between eyes who underwent XEN implant with primary needling and those who did without primary needling.19 Regarding success, as reported by Kerr et al,19 our study did not find significant differences between eyes who underwent primary needling and those who received the standard of care. However, unlike Kerr et al, who reported significantly lower rates of secondary needling (p=0.003), our study did not observe such a difference. Nevertheless, the median time to secondary needling was higher in the eyes who underwent primary needling (median: 8.0 months, 95% CI: 4.0 to 12.0 months) than in the eyes who received the standard of care (median: 2.0 months, 95% CI: 1.0 to 5.0 months), although this difference was not statistically significant (Hodges-Lehmann median difference: 6.0 months, p=0.0512), maybe because our study was underpowered for detecting it.
Bleb revisions rate was lower in the needling group (0/19) than in the No-needling one (3/44), but this difference was not statistically significant.
Because of primary needling aims to break potential Tenon’s tissue adhesions, it might be hypothesized that primary needling may improve bleb morphology, which therefore, would reduce the bleb encystment rates and bleb-related complications. The current study did not find significant differences in either bleb morphology assessed by AS-OCT or bleb photographs grading system between eyes who underwent primary needling and those who did not. These findings may speak in favor on primary needling performed in those cases where there are tissue adhesions and clinical outcomes may be compromised.
The IOP lowering effect and the reduction in the number of ocular hypotensive medications were statistically significant and in line with the current evidence.5–13,19
Regarding safety, reported adverse events were in line with the currently available scientific evidence5–13,19,26,27 and no unexpected adverse events were observed in the eyes who underwent primary needling.
Several limitations should be taken into consideration when interpreting the results of this study. The first one its retrospective design, which carry inherent biases. Nevertheless, in order to minimize these issues, inclusion and exclusion criteria have been carefully selected. Additionally, this was single-center study with a limited number of patients. We recognized that the study may be underpowered for detecting significant differences in some variables. The power for detecting the observed differences in IOP lowering and reduction of hypotensive medication, between Needling and No-Needling groups, was 42% and 4%, respectively. Furthermore, the difference in subjects between both populations could suppose an added bias.
In addition, a non-validated AS-OCT grading scales were used to evaluate bleb morphology and bleb fibrosis. These scales need to be validated in further studies.
Conclusions
The results of our study found that primary needling, at the time of surgery, was a safe procedure in OAG patients who underwent a XEN implant.
Although in our study primary needling was associated with a greater IOP lowering (mean difference: −2.5 mm Hg; 95% CI: −5.5 to 0.4 mm Hg), it was not statistically significant. Therefore, since we have not observed a higher success rate either, our recommendation, from a clinical point of view, would be to reserve the technique for those cases in which the XEN implant was twisted, trapped, or did not appear appeared free and mobile in the subconjunctival space. In addition, primary needling may be also recommended in those patients who have undergone a failed surgery due to fibrosis in the fellow eye or were more prone to fibrosis or excessive scarring.
Additionally, the larger time to needling observed in those eyes who underwent primary needling may entail fewer postoperative visits, and therefore lower burden for patients, ophthalmologists, and Health Systems.
Although in theory primary needling might have a positive impact on bleb morphology, our study did not confirm this hypothesis.
Acknowledgment
Editorial assistance in the preparation of this article was provided by Antonio Martinez (MD) of Ciencia y Deporte ltd.
Funding Statement
Support for editorial assistance was funded by Allergan, an AbbVie company. It should be noted that Allergan was not involved in the preparation of the manuscript nor did the company influence in any way the scientific conclusions reached.
Disclosure
Susana Perucho-Martínez reports grants from Allergan, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillunat LE, Erb C, Jünemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi: 10.2147/OPTH.S135316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah M. Micro-invasive glaucoma surgery - an interventional glaucoma evolution. Eye Vis. 2019;6:29. doi: 10.1186/s40662-019-0154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichani P, Popovic MM, Schlenker MB, Park J, Ahmed IIK. Microinvasive glaucoma surgery: a review of 3476 eyes. Surv Ophthalmol. 2021;66(5):714–742. doi: 10.1016/j.survophthal.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 5.Laborda-Guirao T, Cubero-Parra JM, Hidalgo-Torres A. Efficacy and safety of XEN 45 gel stent alone or in combination with phacoemulsification in advanced open angle glaucoma patients: 1-year retrospective study. Int J Ophthalmol. 2020;13(8):1250–1256. doi: 10.18240/ijo.2020.08.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. doi: 10.1016/j.ajo.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 7.Hengerer FH, Kohnen T, Mueller M, Conrad-Hengerer I. Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J Glaucoma. 2017;26(12):1130–1136. doi: 10.1097/IJG.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 8.Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579–1588. doi: 10.1016/j.ophtha.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Reitsamer H, Sng C, Vera V, Lenzhofer M, Barton K, Stalmans I; Apex Study Group. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):983–996. doi: 10.1007/s00417-019-04251-z [DOI] [PubMed] [Google Scholar]
- 10.Marcos Parra MT, Salinas López JA, López Grau NS, Ceausescu AM, Pérez Santonja JJ. XEN implant device versus trabeculectomy, either alone or in combination with phacoemulsification, in open-angle glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1741–1750. doi: 10.1007/s00417-019-04341-y [DOI] [PubMed] [Google Scholar]
- 11.Karimi A, Lindfield D, Turnbull A, et al. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye. 2019;33(3):469–477. doi: 10.1038/s41433-018-0243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenzhofer M, Kersten-Gomez I, Sheybani A, et al. Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol. 2019;47(5):581–587. doi: 10.1111/ceo.13463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibáñez-Muñoz A, Soto-Biforcos VS, Rodríguez-Vicente L, et al. XEN implant in primary and secondary open-angle glaucoma: A12-month retrospective study. Eur J Ophthalmol. 2020;30(5):1034–1041. doi: 10.1177/1120672119845226 [DOI] [PubMed] [Google Scholar]
- 14.Panarelli JF, Yan DB, Francis B, Craven ER. XEN gel stent open conjunctiva technique: a practical approach paper. Adv Ther. 2020;37(5):2538–2549. doi: 10.1007/s12325-020-01278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vera V, Gagne S, Myers JS, Ahmed IIK. Surgical approaches for implanting Xen gel stent without conjunctival dissection. Clin Ophthalmol. 2020;14:2361–2371. doi: 10.2147/OPTH.S265695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan NE, Tracer N, Terraciano A, Parikh HA, Panarelli JF, Radcliffe NM. Comparison of safety and efficacy between ab interno and ab externo approaches to XEN gel stent placement. Clin Ophthalmol. 2021;15:299–305. doi: 10.2147/OPTH.S292007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Midha N, Gillmann K, Chaudhary A, Mermoud A, Mansouri K. Efficacy of needling revision after XEN gel stent implantation: a prospective study. J Glaucoma. 2020;29(1):11–14. doi: 10.1097/IJG.0000000000001394 [DOI] [PubMed] [Google Scholar]
- 18.Feldman RM, Tabet RR. Needle revision of filtering blebs. J Glaucoma. 2008;17(7):594–600. doi: 10.1097/IJG.0b013e318181283e [DOI] [PubMed] [Google Scholar]
- 19.Kerr NM, Lim S, Simos M, Ward T. Primary needling of the ab interno gelatin microstent reduces postoperative needling and follow-up requirements. Ophthalmol Glaucoma. 2021;4(6):581–588. doi: 10.1016/j.ogla.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma. 2003;12(3):266–271. doi: 10.1097/00061198-200306000-00015 [DOI] [PubMed] [Google Scholar]
- 21.Hodapp E, Parrish R, Anderson D. Clinical Decisions in Glaucoma. St. Louis: Mosby-Year Book, Inc.; 1993. [Google Scholar]
- 22.Midha N, Rao HL, Mermoud A, Mansouri K. Identifying the predictors of needling after XEN gel implant. Eye. 2019;33(3):353–357. doi: 10.1038/s41433-018-0206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vera V, Sheybani A, Lindfield D, Stalmans I, Ahmed IIK. Recommendations for the management of elevated intraocular pressure due to bleb fibrosis after XEN gel stent implantation. Clin Ophthalmol. 2019;13:685–694. doi: 10.2147/OPTH.S195457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005;4:CD002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holló G. Wound healing and glaucoma surgery: modulating the scarring process with conventional antimetabolites and new molecules. Dev Ophthalmol. 2017;59:80–89. [DOI] [PubMed] [Google Scholar]
- 26.Lewczuk K, Konopińska J, Jabłońska J, et al. XEN Glaucoma Implant for the Management of Operated Uncontrolled Glaucoma: results and Complications during a Long-Term Follow-Up. J Ophthalmol. 2021;2021:2321922. doi: 10.1155/2021/2321922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewczuk K, Konopińska J, Jabłońska J, et al. XEN glaucoma implant for the management of glaucoma in NaïvePatients versus patients with previous glaucoma surgery. J Clin Med. 2021;10(19):4417. doi: 10.3390/jcm10194417 [DOI] [PMC free article] [PubMed] [Google Scholar]