Abstract
An increasing number of physicians realize that chronic graft-versus-host disease (cGVHD) is not just dominated by T cells and that B cells also play a vital role in cGVHD development. It has been reported that altered B cell subsets, aberrant B cell signaling pathways, antibody deposition, and abnormal T-B interactions can be observed in many cGVHD patients. Studies of B cells in cGVHD development are now mainly focused on B cell subsets and GC destruction. These two aspects describe the process of B cell evolution in cGVHD patients and are associated with some original treatments. In this review, we summarize recent literature and discuss mechanisms and novel ideas of therapeutic strategies regarding the two aspects mentioned above.
Keywords: B cell subset, Breg, cGVHD, GC, pathogenesis
1. INTRODUCTION
Chronic graft versus host disease (cGVHD) is a major complication affecting the long-term survival of patients after allogeneic haematopoietic stem cell transplantation (allo-HSCT). This condition occurs in 30% to 70% of patients who suffer from HSCT.1 Traditional cGVHD therapies of corticosteroids and calcineurin inhibitors cannot help even 50% of patients,2 and prophylaxis is much more important than treatment.
Unlike T cells, which have been deeply studied in both aGVHD and cGVHD, our understanding of B cells in cGVHD pathogenesis is still at an early stage, which means there remains much to be discovered. Many studies have demonstrated that B cells are involved in the generation of cGVHD. Immunoglobulin (Ig) deposition was observed in liver and lung tissue from human biopsies and in cGVHD mouse models,3 and the use of the CD20 monoclonal antibody (mAb) rituximab for some steroid-refractory cGVHD patients also showed good efficacy.4 Our previous review summarized four obvious changes in B cells in cGVHD patients: altered B cell subsets, aberrant B cell pathways, autoantibodies, and T-B interaction.5 Recent studies of B cells in cGVHD development have mainly focused on altered B cell subsets and GC destruction. The most observed presentation of cGVHD is aberrant B cell subsets, which are the cause and consequence of cGVHD. It is generally believed that cGVHD is often driven by the germinal center (GC) reaction, in which T follicular helper (Tfh) cells interact with GC B cells that produce antibodies that bind to and destroy related target organs. GC formation is disrupted in cGVHD patients and results in a substantially abnormal B cell reconstitution.6 In this review, we summarized recent research and presented our assessment of cGVHD treatment strategies from these two aspects.
2. B CELL SUBSETS
Reconstitution of B cells is slow in patients after allo-HSCT. It takes nearly 1 year for B cells to accomplish reconstitution and maintain stability.7 For normal patients undergoing allo-HSCT, sufficient numbers of naive B cells and circulating transitional B cells are needed to neutralize B cell activating factor (BAFF) and promote the deletion of alloreactive and autoreactive B cells. BAFF levels gradually decrease following B cell recovery.8 Excessive BAFF levels can be regarded as a marker of B cell autoreactivity. In cGVHD patients, relatively decreased naive B cells and persistently increased BAFF concentrations were observed, which led to the generation of autoantibodies.9 Sarantopoulos reported that in cGVHD patients, circulating pre-GC B cells (IgD+CD38+CD27+) and post-GC “plasmablast-like” cells (IgDloCD38hiCD27+) were significantly increased in a BAFF-dependent manner.10 The pre-GC B cells and post-GC “plasmablast-like” cells isolated from cGVHD patients are able to secrete antibodies in the absence of BCR signal stimulation.9 We speculated that the two cell types might mediate the anti-host response without antigen stimulation.
Except for naive B cells, increased transitional B cells (CD21−) and decreased memory B cells (CD27+) are widely observed in cGVHD patients.10 Wang et al showed that in effective extracorporeal photopheresis (ECP) treatment, CD19+CD21lo B cells are decreased.11 Some laboratories even demonstrated that CD19+CD21lo transitional B cells can be used as a biomarker for cGVHD diagnosis and as an evaluation of efficacy after treatment.12 Gao et al reported that prophylaxis of cGVHD with mesenchymal stem cell (MSC) infusion is linked with an increased number of CD27+ memory B cells,13 but these CD27+ memory B cells have not been used in clinic. Effectively monitoring B cell subset variations may be a good way to predict and intervene early in this disease.
Regulatory B cells (Bregs) are a series of negative-immunoregulatory B cells with the ability to secrete the anti-inflammatory cytokine interleukin 10 (IL-10).14 The phenotypic definition of Bregs has not been confirmed. Several types of Bregs have been identified in humans with negative-immunoregulatory functions. Blair et al described CD19+CD24+CD38hi Bregs enriched within CD19+IgM+CD27+ memory and CD19+CD24hiCD38hi transitional B cells.15 van de Veen et al reported that CD73−CD25+CD71+ human B regulatory cells could produce IL-10.16 Iwata identified another type of Breg enriched in CD24hiCD27+ and CD27hiCD38hi plasmablast B cells.17 Sarvaria et al described a kind of Bregs within cord blood that protects against cGVHD by suppressing CD4+ T cell activities, while these Bregs were deficient in cGVHD patients.18 As regulatory T cells (Tregs) infusion has been taken on in many mouse and even preclinical experiments, Breg infusion may be a potential strategy for cGVHD prophylaxis and treatment in the future, but the definition and validation of Bregs still remains an obstacle.
3. GC DESTRUCTION
Traditional opinions suggest that aberrant GC formation, follicular T-helper cells, and GC B cells are involved in cGVHD. GCs are located in the secondary lymphoid organs, and GC formation is usually associated with Ig somatic hypermutation.6 Tfh cells promote proliferation and differentiation of B cells through their surface molecules, such as inducible T-cell co-stimulator (ICOS) and CD40 ligand (CD40L).19,20 In addition, the secretion of IL-21 by Tfh cells also helps B cells undergo class switching and enhances their Ig affinity.21 In a mouse model, enlarged GCs and expanding Tfh cells were observed in the spleen. The increased B cells in GCs were shown to be necessary to induce a bronchial obliterans (BO) cGVHD model.3 Little about GCs has been reported in human patients because of difficulty of biopsy.22 GCs are the key link between immature B cells and specific mature B cells. Destruction of GC formation results in the development of cGVHD. In recent years, studies of B cells in cGVHD have mainly focused on targeting GC reactions, Tfh cells and related signaling pathways.
A checkpoint regulator named signaling lymphocyte activation molecule family 3 (SLAMF3) was found to negatively regulate B cell homeostasis and modulate the activation thresholds of B cell subsets. In the cGVHD mouse model, administering SLAMF3 impaired antibody responses and GC B cell numbers but not CXCR5+PD-1+ICOS+ Tfh cells. By contrast, anti-SLAMF3 accelerated the differentiation of both GC B cells and donor-derived Tfh cells initiated by cGVHD and decreased the numbers of donor-derived Tregs and T follicular regulatory (Tfr) cells. Decreasing anti-SLAMF3-related signaling is effective in preventing autoimmune responses during cGVHD.23 Paz et al demonstrated that lipid kinase phosphoinositide-3-kinase-δ (PI3Kδ) is required in donor T cells for their normal function and survival it supports donor T cells to differentiate into pathogenic effector T cells and Tfh cells. Targeting PI3Kδ with its specific inhibitor compound GS-649443 reduces pathogenic Tfh/GC B cells and effector T cells. Additionally, inhibiting PI3Kδ decreases antibody deposition in the lungs and GC reaction in secondary lymphoid organs and ultimately ameliorates cGVHD in mouse model. Clinical experiment is to be expected for better treatment of cGVHD. B cell lymphoma 6 (BCL6), expressed in donor T and B cells, is essential for the development and function of Tfh, Tfr, and GC B cells. BCL6 is a master regulator of GC reactions.24 Mice receiving BCL6-knockout T or B cells failed to develop BO cGVHD.25 Paz et al demonstrated that a small-molecule BCL6 inhibitor (79-6) is effective for BO cGVHD mice with efficacy of better pulmonary function and less Ig deposition in lung biopsy. Small molecular inhibitors are promising cGVHD therapies for future development.
Interestingly, Deng et al found a GC-independent method for the development of cGVHD. They showed that extrafollicular CD44hiCD62loPSGL-1loCD4+ T cell (PSGL-1loCD4+ T cells) and B cell interactions are sufficient to induce this disease. PSGL-1loCD4+ T cells can be blocked through ICOS/ICOSL interactions and BCL6 and signal transducer and activator of transcription 3 (stat3) deficiency in donor CD4+ T cells, resulting in cGVHD amelioration.26 This point is in contrast with our previous recognition of supra-activated GC reactions in cGVHD, which are alternative pathways of the classical theory of GC destruction.
4. DISCUSSION
Due to the early prevention and timely control of aGVHD, the incidence of aGVHD gradually decreased, while the proportion of cGVHD has gradually increased as a major complication after transplantation. Unlike aGVHD, cGVHD is more likely to start slowly, involve a wide range of organs and tissues and seriously affect the quality of life of allo-HSCT patients. The pathogenesis of cGVHD is unclear, and a majority of studies have mainly focused on the role of T cells. In recent years, an increasing number of studies have found that B cells also play an important role in the pathogenesis of cGVHD and affect the course of the disease. Our previous paper summarized four types of B cell changes in cGVHD patients: altered B cell subsets, aberrant B cell signaling pathways, autoantibodies, and T–B interactions. In this review, we concentrated on the research progress in recent years on changes in B cell subsets, the induction of cGVHD by GC destruction and related treatment strategies. The variation of the B cell subsets is the change that is most seen in cGVHD patients. We elucidated several B cell subset changes in detail, and long-term monitoring of B cell subsets may be beneficial for controlling cGVHD, but it is limited by difficult follow-up and vague recognition of the B cell subsets. With the development of single-cell sequencing technology, more sophisticated B cell subsets will be distinguished and identified in the future. By understanding more detailed subsets, we can specifically target one of the subsets and achieve precise treatment.
GC destruction is a common occurrence in cGVHD pathogenesis. It has been found in many animal studies that GC B cell and Tfh cell numbers are increased. Targeting their related pathways is a novel method to treat cGVHD, but these methods are still in the laboratory or preclinical stage. Except for the small molecule inhibitors mentioned above, blocking surface markers on Tfh cells such as IL21/IL21R, ICOS, and CD40L with mAbs may provide new approaches for cGVHD therapy.27,28 Deng et al presented a supplementary pathway of extrafollicular CD4+ T and B cell interactions in inducing cGVHD.26 Jin et al measured extrafollicular CD4+ T cells in the peripheral blood of cGVHD patients and found that they were significantly expanded compared to patients who did not develop cGVHD.29 Monitoring or targeting these circulating extrafollicular Th-like cells may help us prevent or attenuate cGVHD (Fig. 1).
Figure 1.
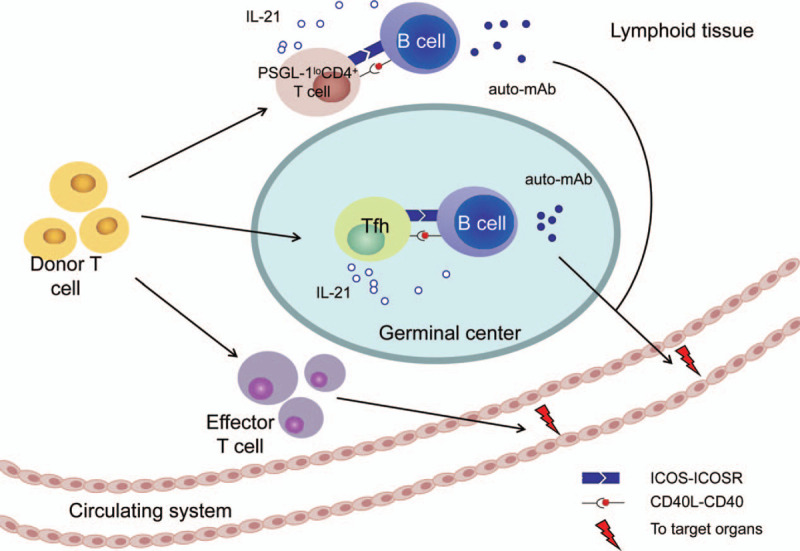
Hypothesis of Tfh-B cell interaction in GC and the extrafollicular T-B pathway. auto-mAb = autoreactive monoclonal antibodies; CD40L = CD40 ligand; ICOS = inducible T-cell co-stimulator; IL-21 = interleukin-21; PSGL-1 = P-selectin glycoprotein ligand 1.
ACKNOWLEDGMENTS
This paper is supported by Army Key Foundation(No. AWS14C014), National Key research and development plan(No. 2017YFA0105502), Chinese National Natural Science Foundation(No. 81570097), National Natural Youth Science Foundation of China (No. 81400081), Foundation of Xinqiao Hospital(2016D413) and Clinical Foundation of Xinqiao Hospital(2015LYC02).
REFERENCES
- [1].Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 2015;21(2):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martin PJ, Lee SJ, Przepiorka D, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol Blood Marrow Transplant 2015;21(8):1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood 2012;119(6):1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006;108(2):756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li X, Gao Q, Feng Y, Zhang X. Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Brit J Haematol 2019;184(3):323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood 2014;123(25):3988–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol 2003;24(6):343–349. [DOI] [PubMed] [Google Scholar]
- [8].Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood 2009;113(16):3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood 2015;125(11):1703–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Greinix HT, Pohlreich D, Kouba M, et al. Elevated numbers of immature/transitional CD21-B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant 2008;14(2):208–219. [DOI] [PubMed] [Google Scholar]
- [11].Wang L, Ni M, Huckelhoven-Krauss A, et al. Modulation of B cells and homing marker on NK cells through extracorporeal photopheresis in patients with steroid-refractory/resistant graft-vs.-host disease without hampering anti-viral/anti-leukemic effects. Front Immunol 2018;9:2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Greinix HT, Kuzmina Z, Weigl R, et al. CD19+CD21low B cells and CD4+CD45RA+CD31+ T cells correlate with first diagnosis of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2015;21(2):250–258. [DOI] [PubMed] [Google Scholar]
- [13].Gao L, Zhang Y, Hu B, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol 2016;34(24):2843–2850. [DOI] [PubMed] [Google Scholar]
- [14].Young JS, Wu T, Chen Y, et al. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol 2012;189(1):222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010;32(1):129–140. [DOI] [PubMed] [Google Scholar]
- [16].van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M. Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol 2016;138(3):654–665. [DOI] [PubMed] [Google Scholar]
- [17].Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117(2):530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sarvaria A, Basar R, Mehta RS, et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood 2016;128(10):1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 2011;34(6):932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr Opin Immunol 2013;25(3):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zotos D, Coquet JM, Zhang Y, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med 2010;207(2):365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Forcade E, Kim HT, Cutler C, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood 2016;127(20):2489–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang N, Yigit B, van der Poel CE, et al. The checkpoint regulator SLAMF3 preferentially prevents expansion of auto-reactive B cells generated by graft-vs.-host disease. Front Immunol 2019;10:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Paz K, Flynn R, Du J, et al. Targeting PI3Kdelta function for amelioration of murine chronic graft-versus-host disease. Am J Transplant 2019;19(6):1820–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paz K, Flynn R, Du J, et al. Small-molecule BCL6 inhibitor effectively treats mice with nonsclerodermatous chronic graft-versus-host disease. Blood 2019;133(1):94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Deng R, Hurtz C, Song Q, et al. Extrafollicular CD4(+) T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease. Nat Commun 2017;8(1):978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang M, Wu Y, Bastian D, et al. Inducible T-cell co-stimulator impacts chronic graft-versus-host disease by regulating both pathogenic and regulatory T cells. Front Immunol 2018;9:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weiss JM, Chen W, Nyuydzefe MS, et al. ROCK2 signaling is required to induce a subset of T follicular helper cells through opposing effects on STATs in autoimmune settings. Sci Signal 2016;9(437):ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jin H, Yang K, Zhang H, et al. Expansion of circulating extrafollicular helper T-like cells in patients with chronic graft-versus-host disease. J Autoimmun 2019;100:95–104. [DOI] [PubMed] [Google Scholar]


