Abstract
Despite the impressive development of metal halide perovskites in diverse optoelectronics, progress on high-performance transistors employing state-of-the-art perovskite channels has been limited due to ion migration and large organic spacer isolation. Herein, we report high-performance hysteresis-free p-channel perovskite thin-film transistors (TFTs) based on methylammonium tin iodide (MASnI3) and rationalise the effects of halide (I/Br/Cl) anion engineering on film quality improvement and tin/iodine vacancy suppression, realising high hole mobilities of 20 cm2 V−1 s−1, current on/off ratios exceeding 107, and threshold voltages of 0 V along with high operational stabilities and reproducibilities. We reveal ion migration has a negligible contribution to the hysteresis of Sn-based perovskite TFTs; instead, minority carrier trapping is the primary cause. Finally, we integrate the perovskite TFTs with commercialised n-channel indium gallium zinc oxide TFTs on a single chip to construct high-gain complementary inverters, facilitating the development of halide perovskite semiconductors for printable electronics and circuits.
Subject terms: Electronic devices, Electrical and electronic engineering
Progress on high-performance transistor employing perovskite channels has been limited to date. Here, Zhu et al. report hysteresis-free tin-based perovskite thin-film transistors with high hole mobility of 20 cm2V–1S–1, which can be integrated with commercial metal oxide transistors on a single chip.
Introduction
As an emerging class of semiconductors with remarkable properties, metal halide perovskites have enabled unprecedented performance improvements in diverse optoelectronic devices, such as solar cells1–3, light-emitting diodes (LEDs)4–7, and photo/X-ray detectors8,9. In comparison, their prospects in high-performance transistors, which are fundamental building blocks for modern electronics, remain to be seen10,11. The dominant lead halide perovskites in state-of-the-art optoelectronic devices, e.g. methylammonium lead iodide (MAPbI3), exhibit small carrier effective masses comparable with silicon; however, their room-temperature charge-carrier mobilities are strongly limited by Fröhlich interactions due to the polar nature of Pb–halide bonds12,13. Furthermore, the severe ion migration induced by halide vacancies results in poor field-effect modulation and large current–voltage hysteresis14–18. After considerable efforts in ionic defect cleaning/healing, a MAPbI3 transistor with a room-temperature field-effect mobility (μFE) of ~4 cm2 V−1 s−1 was recently demonstrated19. In comparison, tin halide perovskites are predicated to possess higher room-temperature mobilities than their Pb-halide analogues owing to the reduced Fröhlich effect12. However, current Sn-perovskite transistors rely on two-dimensional (2D) layered perovskites or 2D/three-dimensional (3D) hybrids, in which carrier transport can be hindered by the bulky/insulating organic spacers, posing a critical barrier for further performance enhancement20–26.
Here, we demonstrate high-performance and hysteresis-free p-channel perovskite thin-film transistors (TFTs) with a 3D methylammonium tin iodide (MASnI3)-based channel layer via rational halide anion (I/Br/Cl) engineering. The co-substitution of small amounts of bromide and chloride for iodide enhances the film quality and vacancy passivation, enabling TFTs with excellent electrical characteristics, such as a high μFE of ~20 cm2 V−1 s−1, an on/off current ratio (Ion/Ioff) of over 107, and a threshold voltage (VTH) of 0 V. Unlike the dominant role of ion migration in causing the hysteresis of Pb-halide perovskite TFTs, we declare that the device hysteresis in Sn-based perovskite TFTs originates from minority carrier trapping at iodide vacancy sites. These deep traps are substantially reduced by proper Br and Cl co-substitution, which eliminates the hysteresis and provides high operational stability and reproducibility. By integrating the perovskite TFTs with n-channel indium gallium zinc oxide (IGZO) TFTs, we realised complementary inverters with high gain of 140 and noise margin of over 70%, suggesting great processability and compatibility for large-area electronic circuits.
Results
MASnX3 TFT performance
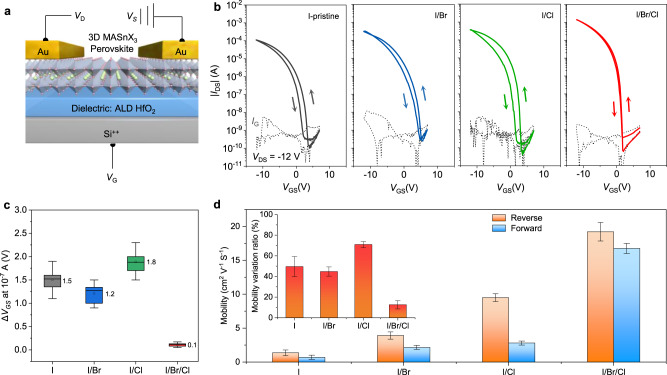
Perovskite films were spin-coated on hafnium (IV) oxide (HfO2) layers fabricated by atomic layer deposition (ALD) from precursors consisting of methylammonium iodide (MAI) and tin(II) halide (SnX2, X = Cl, Br, I), and then thermally annealed (see more details in Methods). Note that small amount of SnF2 addition in precursors is needed to get field-effect current modulation owing to the hole-suppression effect (Supplementary Fig. 1). Subsequently, gold source/drain electrodes were deposited, constructing the bottom-gate, top-contact TFTs (Fig. 1a). The TFTs obtained without halide engineering, denoted by ‘I-pristine’, exhibited typical p-channel transfer characteristics under continuous mode at room temperature. The representative I-pristine device exhibited a low gate leakage current of ~10−10 A, large Ion/Ioff ratio exceeding 106, and maximum μFE of 1.3 cm2 V−1 s−1 (Fig. 1b). This is the first demonstration of p-channel TFTs based on 3D MASnI3 perovskite films, demonstrating electrical parameters comparable to those of previously reported perovskite TFTs (Supplementary Table 1).
Fig. 1. Electrical characteristics of MASnX3 perovskite TFTs.
a TFT structure used in this work. b Transfer characteristics of the TFTs with different perovskite channel layers. IG: gate leakage current. c Hysteresis statistics of different TFTs. The error bars present standard errors calculated from ten devices per type, and the mean values are labelled. d Histogram of the extracted mobilities from the transfer characteristics under different scan directions. The error bars present standard errors calculated from ten devices per type. The inset shows the variation ratio of the mobility values extracted from reverse (μRev) and forward (μFor) scans, calculated by (μRev − μFor)/μRev × 100%.
We achieved TFTs with much improved performance by carefully engineering the halide compositions of the precursors for perovskite film deposition (Fig. 1b and Supplementary Fig. 2), inspired by the fact that recent breakthroughs in perovskite photovoltaics have been made mostly based on multiple compositions3,27–29. Specifically, partially substitution of the iodide source with bromide salt (2 mol%, the devices are denoted by ‘I/Br’) resulted in an over three-fold improvement in the μFE of the TFTs (4.3 cm2 V−1 s−1). With the channel films deposited from a precursor with 6 mol% chloride substitution, we obtained devices (denoted by ‘I/Cl’) exhibiting an even higher μFE (9.5 cm2 V−1 s−1). Surprisingly, a rational combination of the two halide engineering strategies, i.e. employing channels deposited from perovskite precursors with simultaneous Br and Cl substitution (2 mol% Br and 6 mol% Cl), led to greatly enhanced TFT (denoted by ‘I/Br/Cl’) performance. As shown in Fig. 1b, the optimised I/Br/Cl perovskite TFTs exhibited a μFE of 19.6 cm2 V−1 s−1 with an Ion/Ioff of 3 × 107, which is superior to reported Pb- and 2D Sn-based perovskite TFTs (Supplementary Table 1). Textbook-like output curves (IDS versus VDS) with clear linear and saturation currents were observed for all the devices (Supplementary Fig. 3), indicating an Ohmic contact between the channel films and electrodes and validating the reliability of mobility extraction30. Furthermore, the I/Br/Cl device operated in an ideal enhancement mode with a VTH of 0 V (Supplementary Fig. 4), suggesting that no applied bias voltage is needed to turn off the transistor, which is highly desirable for simplifying circuit design and minimising power consumption in practical applications31.
In addition to higher mobilities than those of I-pristine, I/Br, and I/Cl devices, the TFTs based on I/Br/Cl perovskite channels also exhibited significantly reduced, even negligible, current–voltage hysteresis. To quantitatively analyse the hysteresis for the I-pristine, I/Br, I/Cl, and I/Br/Cl TFTs, we calculated the difference in VGS (ΔVGS) at |IDS| = 10−7 A, halfway between the on and off states32, and presented the data in Fig. 1c. Notably, the I/Br/Cl devices exhibited an average ΔVGS of 0.1 V, which is less than 1/10 of that of the other three types of TFTs, in which ion migration and/or carrier trapping probably occurred (discussed later). The negligible hysteresis for the I/Br/Cl devices is comparable with commercialised amorphous metal oxide TFTs33. Similar to previous perovskite solar cells34, the hysteresis in the dual-sweep transfer curves of the TFTs also causes variations of the extracted performance parameters. We observed notable differences in the maximum mobility values extracted from the reverse (on-to-off, −12 to 7 V) and forward (off-to-on, 7 to −12 V) scans of the I-pristine, I/Br, and I/Cl TFTs (Supplementary Fig. 5), and presented the μFE statistics in Fig. 1d. The I/Cl TFTs demonstrated the largest mobility variations (>70%) because of their largest hysteresis, while the variations for the I-pristine and I/Br devices were slightly lower (50%). Notably, the I/Br/Cl devices exhibited the smallest mobility variations (12%) owing to their greatly reduced hysteresis. Because a strong mobility–hysteresis correlation exists but has been usually neglected in previous research on perovskite TFTs17, we recommend more information on the measurement methods, device hysteresis, and mobilities extracted from both the forward and reverse scans of devices be provided. The hysteresis-free character is desired for a wide range of electronic applications, such as logic circuits and backplanes in OLED displays35.
Channel film characterisations
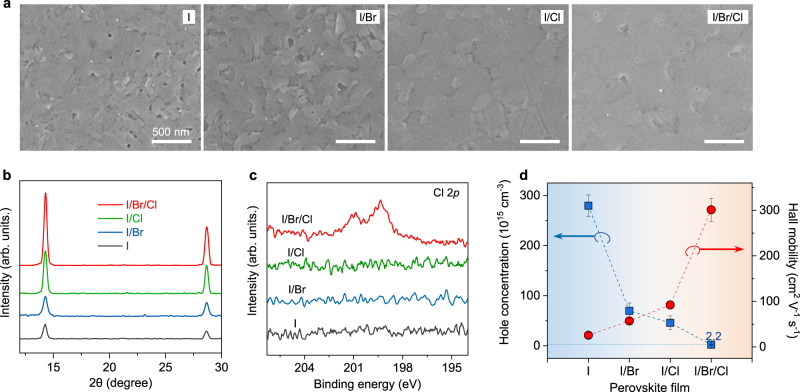
To comprehensively understand the benefits of halide engineering for perovskite TFTs, we performed a series of film characterisations. The scanning electron microscope (SEM) images in Fig. 2a reveal a few pinholes in the I-pristine perovskite film, and slight Br substitution supressed the pinholes. The incorporated Br anions could compete with I anions and coordinate more strongly with the metal ions (Sn2+), modulating the nucleation and crystallisation kinetics of the perovskite films36,37. In comparison, both the I/Cl and I/Br/Cl films exhibited a considerably smoother surface morphology, suggesting the incorporation of Cl in the precursor significantly promoted perovskite formation, similar to observations about Cl in lead halide perovskites for solar cells36,38,39. The results are consistent with X-ray diffraction (XRD) analyses, where the I/Cl and I/Br/Cl samples exhibited substantially increased intensities of the main diffraction peaks compared with those of the I-pristine and I/Br samples, suggesting improved crystallinity and/or improved grain orientation (Fig. 2b).
Fig. 2. Characterisation of MASnX3 films.
a SEM images, b XRD patterns, c Cl 2p core level XPS spectra, and d Hall mobilities and hole concentrations of the different perovskite films. The error bars present standard errors calculated from five films per type.
Interestingly, both the I/Br and I/Br/Cl films exhibited identifiable XRD peak shifts to higher angles compared with that of the I-pristine film. The peak shifts indicate a reduced d-spacing due to the incorporation of smaller Br and/or Cl ions into the I-based perovskite lattices. However, the I/Cl sample showed negligible peak shifts (Supplementary Fig. 6), which suggests that the Cl anions in the I/Cl perovskite film did not enter the perovskite lattice but only functioned to improve the film morphology and crystallinity40. The X-ray photoelectron spectroscopy (XPS) Cl 2p core level spectra further confirmed that Cl was undetectable in the I/Cl films but was successfully incorporated into the triple-halide I/Br/Cl films (Fig. 2c). These results are unsurprising considering the large discrepancy in ionic size between I and Cl anions and the potential volatilisation of Cl additives during film annealing41. However, in the I/Br/Cl perovskite film, the Br-substituted I-based lattice was capable of hosting Cl anions owing to the bridging effect of Br42,43, enabling the formation of the triple-halide MASn(I/Br/Cl)3 perovskite. We estimated the relative atomic concentration ratio of I:Br:Cl from the XPS data for the triple-halide sample. The relative ratio is 93.8%:2.4%:3.8% and the feeding ratio was 92%:2%:6%, consistent with above discussion.
We then conducted Hall-effect measurements to investigate the carrier concentrations and Hall mobilities of the perovskite films, which are important for understanding the performance of the resulting TFTs. As shown in Fig. 2d, the I-pristine films exhibited an average hole concentration of 2.8 × 1017 cm−3, which gradually decreased to 6.9 × 1016, 4.5 × 1016, and 2.2 × 1015 cm−3 for the I/Br, I/Cl, and I/Br/Cl films, respectively. This trend resulted from decreasing hole sources (tin vacancies). Generally, in 3D Sn-based perovskites, Sn2+ can easily oxidise into Sn4+ even under trace oxygen, and intrinsic tin vacancies (VSn) have a low formation energy, which both cause notoriously high hole concentrations. The Sn 3d5/2 XPS spectra (Supplementary Fig. 7) showed that the Sn4+ signal gradually decreased in the sequence of I > I/Br > I/Cl > I/Br/Cl, indicating suppressed Sn2+ oxidation. Furthermore, previous studies have suggested that the incorporation of anions with electronegativity stronger than that of I− raises the VSn formation energy during perovskite crystallisation, further reducing the hole concentration24,44. Additionally, for the I-pristine sample, a small shoulder peak appeared at a lower binding energy (~485.6 eV), which is ascribed to under-coordinated Sn with an oxidation state of δ < 2+ (Snδ< 2+)45. However, this shoulder peak was undetectable in other samples, indicating well-coordinated Sn sublattices and reduced structural imperfections with halide engineering.
The Hall mobilities (μHall) of the perovskite films showed a consistent trend with the μFE extracted from the corresponding TFTs, with the average μHall increasing from 25 cm2 V−1 s−1 (I-pristine) to 57 (I/Br) and 92 cm2 V−1 s−1 (I/Cl), respectively. The μHall for I/Br/Cl films was up to 301 cm2 V−1 s−1. According to the simple Drude model, the hole mobility of a p-type semiconductor is determined by the hole effective mass (mh*) and the average scattering time (τ):
| 1 |
where q is the elementary charge12. Considering the small halide substitution in the perovskite lattice, negligible changes to mh* were expected. Therefore, μHall should be mainly determined by the scattering time interval during carrier transport, which is dominated by scattering centres, e.g. ionised (negatively and positively charged) defects and crystal disorders, in the perovskite films. As revealed by the characterisations above, halide engineering effectively enhanced the film quality and reduced ionised defects, particularly in the I/Br/Cl film, significantly suppressing charge-carrier scattering and providing a rationale for the enhanced μHall of the halide-engineered perovskite films.
In addition to the improved perovskite film quality, the considerably improved performance of the I/Br/Cl devices is related to the properties of the dielectric–perovskite interfaces. Generally, the density of states (Ns) at the interface, which negatively affects the device performance of TFTs, can be estimated from the average subthreshold swing (SS, Supplementary Fig. 8):
| 2 |
where is the Boltzmann constant, e is the electron charge, and Ci is the areal capacitance of the dielectric layer46. Accordingly, of the I-pristine TFTs was calculated to be 1.5 × 1013 cm−2 eV−1, which reduced to ~3.3 × 1012 cm−2 eV−1 in the I/Br/Cl TFTs. This suggests that Br and Cl co-substitution enhanced not only the film quality but also the dielectric–perovskite interfaces of the TFTs.
Understanding of the hysteresis
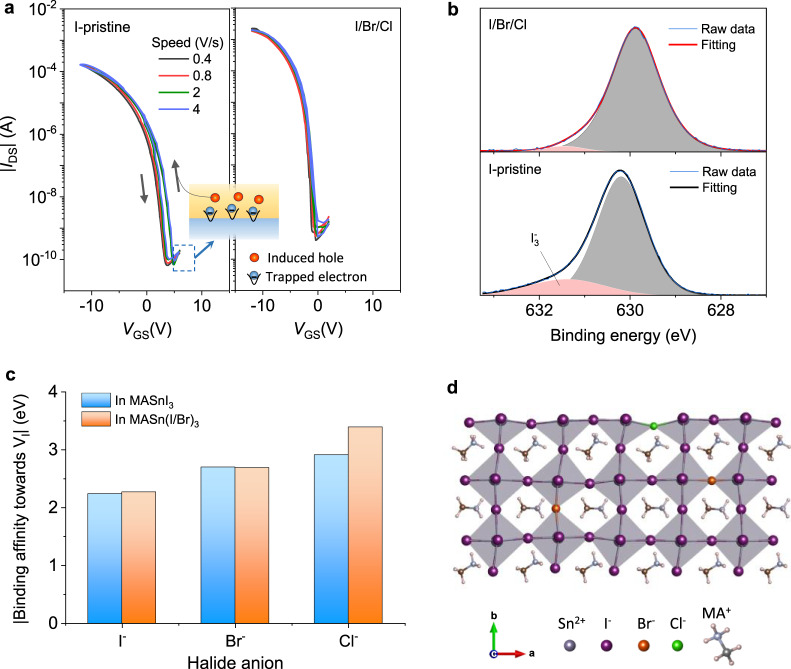
Having elucidated the benefits of rational halide engineering of MASnI3 precursors on the TFT performance, we attempted to gain an in-depth understanding of the hysteresis behaviour of the devices. The commonly observed hysteresis of transistors utilising 3D lead-halide perovskite films is typically attributed to ion migration in the perovskite channel47. Such ion-migration-induced hysteresis is strongly dependent on the sweep rate during device measurement. For example, transistors based on single crystalline MAPbX3 channels exhibited gradually expanded hysteresis when the sweep rate increased from 0.05 to 0.25 V s−1(ref. 48). However, we observed negligible changes to the hysteresis in the transfer curves of both the I-pristine and I/Br/Cl devices when the sweep rate increased from 0.4 to 4 V s−1 (Fig. 3a), suggesting that the ion migration in the MASnI3-based perovskite films did not contribute significantly to the TFT hysteresis. This can be partially explained by the different defect properties between Pb- and Sn-based perovskites28. In p-type MASnI3, tin vacancies (electron acceptors) are the dominant defects, whereas the iodine defects, e.g. iodine vacancies (VI) and interstitials (Ii), are much less (if even present)49. Consequently, the defect-associated migration of iodide ions, which have the lowest activation energy and move most easily, is less significant in MASnI3 than in Pb-based perovskites, greatly reducing the associated electric-field screening effects during TFT operation.
Fig. 3. Hysteresis and VI in the MASnX3 perovskite TFTs.
a Transfer characteristics of I-pristine and I/Br/Cl TFTs measured at different scan speeds. b I 3d3/2 core level spectra of the I-pristine and I/Br/Cl perovskites. c Calculated relative interaction strengths of halide anions with VI sites in MASnI3 and MASn(I/Br)3. d Illustration of the passivation effects of a VI defect by a Cl anion.
We then considered charge-carrier trapping as the primary reason for the hysteresis in our perovskite TFTs, which exhibited a higher current in the transfer curves during the off-to-on sweep than that during the on-to-off sweep. The established models indicate deep electron and hole traps in the semiconductor channels are possible causes for this type of hysteresis in TFTs50. Theoretical calculations have predicted that in MASnI3, hole traps induced by Ii and VSn defects are shallow, with thermodynamic ionisation levels close to or inside the valance band maximum49. Thus, they are expected to mainly affect the μFE and SS of the TFTs rather than induce hysteresis50. We postulated that VI defects, which possess the lowest formation energy among possible deep electron traps in MASnI3, were the root cause of the hysteresis in the p-channel TFTs. As shown in the inset of Fig. 3a, when VGS << VTH, negative charge accumulated in the channel, and VI-related long-lifetime electron traps were filled. The trapped electrons shifted the flat-band voltage; that is, the threshold voltage was reduced. When VGS swept towards negative potentials, more holes were induced, leading to a higher drain current. In comparison, the on-to-off sweep started directly from hole accumulation at VGS = −12 V without the influence of stored negative charge50, demonstrating a lower drain current. Therefore, the on-to-off measurement of the perovskite TFTs should more closely resemble the ideal field-effect transistor model, indicating the mobility extracted from the on-to-off transfer curve may be closer to the actual μFE.
With the hypothesis that deep electron traps dominate the hysteresis in our p-channel perovskite TFTs, we investigated the different VI properties of the I-pristine and I/Br/Cl perovskite films to unveil the mechanism that eliminated hysteresis in the optimised I/Br/Cl devices. In the I 3d3/2 core level XPS spectra (Fig. 3b), the peak for the I-pristine sample shifted by 0.4 eV towards higher binding energies compared with that of the I/Br/Cl perovskite. This peak shift was previously ascribed to iodine loss from the lattice51,52, indicating a higher probability of VI formation in the I-pristine perovskite film. In addition, a shoulder peak appeared at ~631.5 eV in the I 3d3/2 core level spectra of the I-pristine film, corresponding to the I3− species. These species were assigned to iodide interstitials/VI+ iodine Frenkel defects, which form preferentially under VSn-rich conditions53. The shoulder peak became negligible for the I/Br/Cl perovskite, which can be attributed to the reduced iodine loss from the perovskite lattice, along with the significantly reduced hole concentration (VSn defects) revealed by the Hall measurements. Density functional theory (DFT) calculations further confirmed the benefits of passivating VI sites in the I/Br/Cl perovskite film. As shown in Fig. 3c, Br or Cl anions, if successfully incorporated into the MASnI3 perovskite lattice, possess higher binding affinities towards VI sites than that of I anions (slab models in Supplementary Fig. 9), in agreement with recent Pb perovskites with double anions3. Based on the MASn(I/Br)3 perovskite, the calculated binding affinity of a third anion, Cl−, to VI was further enhanced (Figs. 3c, d), and hence the VI sites in the I/Br/Cl perovskite were expected to be greatly suppressed, rationalising the elimination of hysteresis in the resulting TFTs.
TFT stability and complementary inverter
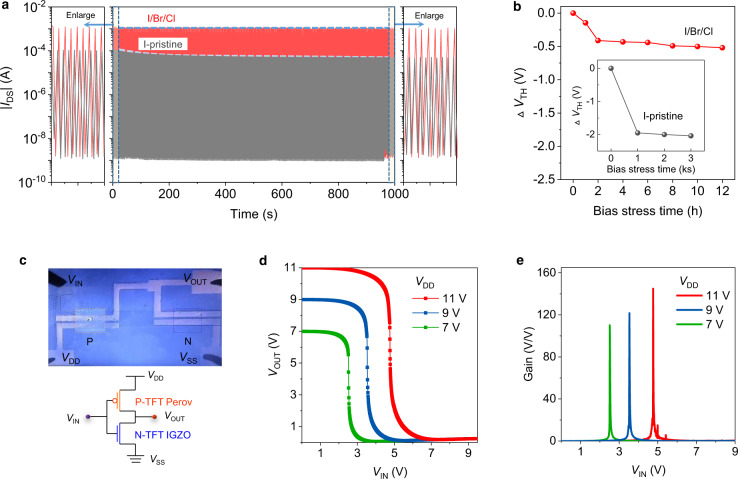
We then characterised the operational stability of our perovskite TFTs, which is another critical figure of merit for practical applications. We first monitored the on/off switching stability of the devices (Fig. 4a). The I-pristine device exhibited an obvious current decay during the consecutive on/off switching test, while the currents of both the on and off states of the I/Br/Cl device remained consistent. We also examined the device stability under dynamic VGS scans. The transfer characteristics of the I-pristine TFTs gradually shifted, while those of the I/Br/Cl TFTs overlapped completely over 100 cyclic sweeps (Supplementary Fig. 10), suggesting considerably enhanced reliability of the I/Br/Cl devices. To evaluate the stability of the TFTs more rigorously, we performed a bias stress test, during which −12 V was applied constantly, and the shift in VTH was monitored. As shown in Fig. 4b, the VTH of the I-pristine device shifted significantly by −2 V (~17% of the operating voltage) after only 1000 s during the bias test (Supplementary Fig. 11), reflecting the serious carrier trapping in the devices54. Encouragingly, the optimised I/Br/Cl TFT exhibited much improved stability with a small threshold voltage shift (ΔVTH) of 0.52 V even after biasing for 12 h, approaching the stability of previously demonstrated stable transistors based on organic and amorphous silicon channels47,55. In addition, we monitored the device air stability and found that the TFTs were unstable in air with fast device degradation due to the easy Sn2+ oxidisation. However, without air exposure (stored in vacuum, ~1 × 10−6 Torr), the device showed high stability with constant transfer characteristics after a test period for 60 days (Supplementary Fig. 12). We anticipate that effective encapsulations, such as those used in protecting organic electronics, and/or anti-oxidation additives for Sn-based perovskites would be helpful to improve the air stability in the near future56.
Fig. 4. Operational stability of perovskite TFTs and performance of the integrated inverters.
a On/off switching sweep of the I-pristine and I/Br/Cl TFTs. b VTH variation under bias (VGS = VDS = −12 V). c Optical image and diagram of an integrated perovskite/IGZO inverter. d Voltage transfer characteristics and e gain ((dVOUT)/(dVIN)).
With the reproducible and stable p-channel I/Br/Cl perovskite TFTs (Supplementary Fig. 13), we moved a step further to explore their compatibility with existing TFTs based on n-channel metal oxides for monolithic complementary circuit integration. We fabricated complementary inverters by integrating perovskite TFTs with IGZO TFTs on a single chip. Figure 4c shows an optical image and diagram of the complementary inverter. The standard rail-to-rail voltage transfer characteristics of the inverter at different VDD (Fig. 4d) show VOUT being either 0 V or the supplied VDD, suggesting an ideal logic ‘1’ to ‘0’ transfer. The inverter exhibited a high gain of over 100 at all measured VDD and a peak gain of 140 at VDD = 11 V (Fig. 4e), which is significantly higher than that of the wire-connected complementary inverter or CMOS-like inverters involving perovskite TFTs in previous studies24,57. To the best of our knowledge, this is also the first demonstration of the monolithic integration of a complementary circuit involving perovskite TFTs. Additionally, the noise margin of the inverter was 3.93 V, reaching 72% of the ideal value (VDD/2) (Supplementary Fig. 14), which is sufficient for most static logic applications58.
In conclusion, we have achieved high-performance and hysteresis-free MASnI3-based perovskite TFTs through rational halide engineering. We revealed the benefits of Br and Cl co-substitution in the precursor: enhanced film quality and reduced vacancy defects of the perovskite films, which enabled exceptional performance of the resulting TFTs. Moreover, we found ion migration had a negligible contribution to the hysteresis in our p-channel perovskite TFTs based on MASnI3 films. Alternatively, we correlated the hysteresis of our MASnI3 TFT with deep electron traps induced by VI defects, which are notably reduced by rational Br and Cl co-substitution in the precursor. By combining our operationally stable p-channel perovskite TFTs with n-channel IGZO TFTs, we demonstrated monolithically integrated high-gain complementary inverters, suggesting high compatibility and processability for electronic applications.
Methods
Precursor and device preparation
The precursor solutions were prepared by mixing MAX and SnX2 (X = I, Cl, Br) in DMF/DMSO binary solvents at a volume ratio of 4/1. The ratio of different composition is expressed as MASn(I1-yXy)3, X = Cl or Br, and y = 0~0.08. The precursor concentration was around 0.15 M. For example, to prepare the MASnI3 precursor, 102 mg of MAI, 216 mg of SnI2, 17 mg of PbI2, and 8.4 mg of SnF2 were dissolved in 4 mL of mixed solvent (3.2 mL of DMF and 0.8 mL of DMSO). The others were made similarly. Same amounts of SnF2 (8 mol% with respect to the Sn source) and lead substitution (6 mol%) were added in each precursor as anti-oxidation and stabiliser additives. Each precursor was stir-heated at 60 °C for 2 h before use. Dielectric layers were the reliable 40-nm HfO2 on Si substrates with capacitance of ~270 nF cm−2 grown by the mature atomic layer deposition (ALD) in National Institute for Nanomaterials Techanology, Pohang. For the perovskite film deposition, the HfO2/Si substrates (1.5 × 1.5 cm2) were first treated with UV ozone for 30 min, then the precursor solutions were dropped on the substrates and spin-coated at the speed of 4000 rpm for 25 s with 60 uL of chlorobenzene dripping after ~10 s from the beginning. Afterwards, the films were annealed at 70 °C for 10 min, constructing thin films with similar thickness of ~40 ± 5 nm. All precursor solutions and films were prepared in an N2-filled glove box (O2 and H2O levels: 1–2 ppm). Then thermal evaporation and a shadow mask were used for the Au source/drain electrode deposition. The channel width and length of the TFTs are 1000 and 150 um, respectively. For the complementary inverter integration, we used design-patterned indium tin oxide (ITO, 10 Ω/□) glass by photolithography as the bottom gate, ALD HfO2 as the dielectric layer, solution-processed self-patterned59 triple-halide perovskite as the p-channel and IGZO as the n-channel, and evaporated gold through a bespoke shadow mask as electrodes.
Film and device characterisations
The film XRD patterns were recorded using a Rigaku D/MAX 2600 V with Cu Kα (λ = 1.5406 Å) radiation. SEM images were obtained using a field-emission scanning electron microscope (Hitachi S4800). AFM height profile data were collected using an atomic force microscopy (Nanosurf Nanite AFM). XPS characterisations were conducted using a VersaProbe Scanning Microprobe under vacuum (10−8 Torr). The Hall measurements were performed using the van der Pauw method, using a 0.51T magnet and a bespoke sample holder in an N2-filled glove box at room temperature. The electrical signal during the Hall measurement was obtained using a Keithley 4200-SCS and probe station (MST-4000A, MS TECH, Korea). Transistor transfer characteristics were measured using a semiconductor parameter analyser (Keithley 4200-SCS) in an N2-filled glove box at room temperature in continuous mode. The saturation TFT mobility was calculated as60
| 3 |
where L, W, and Ci are the channel length and width and dielectric areal capacitance, respectively.
Supplementary information
Acknowledgements
This study was supported by the Ministry of Science and ICT through the National Research Foundation, funded by the Korean government (2021R1A2C3005401, and 2020R1A4A1019455). This research was also supported by the Samsung Display Corporation. H.H. Z. thanks Won-Tae Park, Ji-Young Go, and Gi-Seong Ryu for their help with the metal oxide TFTs.
Author contributions
H.H.Z. and Y.Y.N. conceived the study. H.H.Z. and A.L. performed the experiments. A.L., T.Z., Y.R., H.K., H.Y.C., J.H.L., H-J.K., and assisted with the characterisations and analyses. K.I.S. and J.W.H. performed the DFT calculations. H.J., T.Z., and A.L. assisted with the circuit mask design. H.H.Z., A.L., S.B., and Y.Y.N. wrote the manuscript. Y.C. assisted with manuscript revision. A.L. and S.B. contributed to the experimental design and data analysis. Y.Y.N. supervised the project. All the authors contributed to the discussion. All authors approved the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Funding
Open access funding provided by Linköping University.
Data availability
All data needed to evaluate the conclusion in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper can be available from the authors upon reasonable request.
Code availability
The custom software used for data acquisition and data analysis is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/15/2022
Missing Open Access funding information has been added in the Funding Note.
Contributor Information
Sai Bai, Email: sai.bai@liu.se.
Yong-Young Noh, Email: yynoh@postech.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29434-x.
References
- 1.Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 2.Bai S, et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature. 2019;571:245–250. doi: 10.1038/s41586-019-1357-2. [DOI] [PubMed] [Google Scholar]
- 3.Jeong J, et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature. 2021;592:381–385. doi: 10.1038/s41586-021-03406-5. [DOI] [PubMed] [Google Scholar]
- 4.Tan Z-K, et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014;9:687. doi: 10.1038/nnano.2014.149. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y-H, et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics. 2021;15:148–155. doi: 10.1038/s41566-020-00732-4. [DOI] [Google Scholar]
- 6.Karlsson M, et al. Mixed halide perovskites for spectrally stable and high-efficiency blue light-emitting diodes. Nat. Commun. 2021;12:361. doi: 10.1038/s41467-020-20582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin K, et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 percent. Nature. 2018;562:245–248. doi: 10.1038/s41586-018-0575-3. [DOI] [PubMed] [Google Scholar]
- 8.Dou L, et al. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014;5:5404. doi: 10.1038/ncomms6404. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, et al. All-inorganic perovskite nanocrystal scintillators. Nature. 2018;561:88–93. doi: 10.1038/s41586-018-0451-1. [DOI] [PubMed] [Google Scholar]
- 10.Shining a light on perovskite devices. Nat. Electron. 3, 657 https://www.nature.com/articles/s41928-020-00509-1 (2020).
- 11.Zhu H, Liu A, Noh Y-Y. Perovskite transistors clean up their act. Nat. Electron. 2020;3:662–663. doi: 10.1038/s41928-020-00470-z. [DOI] [Google Scholar]
- 12.Herz LM. Charge-carrier mobilities in metal halide perovskites: fundamental mechanisms and limits. ACS Energy Lett. 2017;2:1539–1548. doi: 10.1021/acsenergylett.7b00276. [DOI] [Google Scholar]
- 13.Wang K, Yang D, Wu CC, Sanghadasa M, Priya S. Recent progress in fundamental understanding of halide perovskite semiconductors. Prog. Mater. Sci. 2019;106:100580. doi: 10.1016/j.pmatsci.2019.100580. [DOI] [Google Scholar]
- 14.Labram JG, et al. Temperature-dependent polarization in field-effect transport and photovoltaic measurements of methylammonium lead iodide. J. Phys. Chem. Lett. 2015;6:3565–3571. doi: 10.1021/acs.jpclett.5b01669. [DOI] [PubMed] [Google Scholar]
- 15.Pininti AR, Ball JM, Albaqami MD, Petrozza A, Caironi M. Time-dependent field effect in three-dimensional lead-halide perovskite semiconductor thin films. ACS Appl. Energy Mater. 2021;4:10603–10609. doi: 10.1021/acsaem.1c01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y-H, Pattanasattayavong P, Anthopoulos TD. Metal-halide perovskite transistors for printed electronics: challenges and opportunities. Adv. Mater. 2017;29:1702838. doi: 10.1002/adma.201702838. [DOI] [PubMed] [Google Scholar]
- 17.Paulus F, Tyznik C, Jurchescu OD, Vaynzof Y. Switched-on: progress, challenges, and opportunities in metal halide perovskite transistors. Adv. Funct. Mater. 2021;31:2101029. doi: 10.1002/adfm.202101029. [DOI] [Google Scholar]
- 18.Zhu H, Liu A, Noh Y-Y. Recent progress on metal halide perovskite field-effect transistors. J. Inf. Disp. 2021;22:1–12. doi: 10.1080/15980316.2021.1957725. [DOI] [Google Scholar]
- 19.She XJ, et al. A solvent-based surface cleaning and passivation technique for suppressing ionic defects in high-mobility perovskite field-effect transistors. Nat. Electron. 2020;3:694–703. doi: 10.1038/s41928-020-00486-5. [DOI] [Google Scholar]
- 20.Kagan C, Mitzi D, Dimitrakopoulos C. Organic-inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors. Science. 1999;286:945–947. doi: 10.1126/science.286.5441.945. [DOI] [PubMed] [Google Scholar]
- 21.Matsushima T, et al. Solution-processed organic–inorganic perovskite field-effect transistors with high hole mobilities. Adv. Mater. 2016;28:10275–10281. doi: 10.1002/adma.201603126. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, et al. Highly stable lead-free perovskite field-effect transistors incorporating linear π-conjugated organic ligands. J. Am. Chem. Soc. 2019;141:15577–15585. doi: 10.1021/jacs.9b06276. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, et al. Perovskite and conjugated polymer wrapped semiconducting carbon nanotube hybrid films for high-performance transistors and phototransistors. ACS Nano. 2019;13:3971–3981. doi: 10.1021/acsnano.8b07567. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, et al. High-performance and reliable lead-free layered-perovskite transistors. Adv. Mater. 2020;32:2002717. doi: 10.1002/adma.202002717. [DOI] [PubMed] [Google Scholar]
- 25.Liang A, et al. Ligand-driven grain engineering of high mobility two-dimensional perovskite thin-film transistors. J. Am. Chem. Soc. 2021;143:15215–15223. doi: 10.1021/jacs.1c06337. [DOI] [PubMed] [Google Scholar]
- 26.Shao S, et al. Field-effect transistors based on formamidinium tin triiodide perovskite. Adv. Funct. Mater. 2021;31:2008478. doi: 10.1002/adfm.202008478. [DOI] [Google Scholar]
- 27.Xu J, et al. Triple-halide wide–band gap perovskites with suppressed phase segregation for efficient tandems. Science. 2020;367:1097. doi: 10.1126/science.aaz5074. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Poli I, Meggiolaro D, De Angelis F, Petrozza A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 2021;6:986–1002. doi: 10.1038/s41578-021-00331-x. [DOI] [Google Scholar]
- 29.Wang CB, et al. Illumination durability and high-efficiency Sn-based perovskite solar cell under coordinated control of phenylhydrazine and halogen ions. Matter. 2021;4:709–721. doi: 10.1016/j.matt.2020.11.012. [DOI] [Google Scholar]
- 30.Nikolka M, et al. High operational and environmental stability of high-mobility conjugated polymer field-effect transistors through the use of molecular additives. Nat. Mater. 2017;16:356–362. doi: 10.1038/nmat4785. [DOI] [PubMed] [Google Scholar]
- 31.Rim YS, et al. Interface engineering of metal oxide semiconductors for biosensing applications. Adv. Mater. Interfaces. 2017;4:1700020. doi: 10.1002/admi.201700020. [DOI] [Google Scholar]
- 32.Wan H, et al. Flexible carbon nanotube synaptic transistor for neurological electronic skin applications. ACS Nano. 2020;14:10402–10412. doi: 10.1021/acsnano.0c04259. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, et al. On the origin of improved charge transport in double-gate In–Ga–Zn–O thin-film transistors: a low-frequency noise perspective. IEEE Electron Device Lett. 2015;36:1040–1043. doi: 10.1109/LED.2015.2467164. [DOI] [Google Scholar]
- 34.Habisreutinger SN, Noel NK, Snaith HJ. Hysteresis index: a figure without merit for quantifying hysteresis in perovskite solar cells. ACS Energy Lett. 2018;3:2472–2476. doi: 10.1021/acsenergylett.8b01627. [DOI] [Google Scholar]
- 35.Zhu H, et al. Printable semiconductors for backplane TFTs of flexible OLED displays. Adv. Funct. Mater. 2020;30:1904588. doi: 10.1002/adfm.201904588. [DOI] [Google Scholar]
- 36.Li Z, et al. Scalable fabrication of perovskite solar cells. Nat. Rev. Mater. 2018;3:18017. doi: 10.1038/natrevmats.2018.17. [DOI] [Google Scholar]
- 37.Lee SJ, et al. Reducing carrier density in formamidinium tin perovskites and its beneficial effects on stability and efficiency of perovskite solar cells. ACS Energy Lett. 2018;3:46–53. doi: 10.1021/acsenergylett.7b00976. [DOI] [Google Scholar]
- 38.Yantara N, et al. Unravelling the effects of Cl addition in single step CH3NH3PbI3 perovskite solar cells. Chem. Mater. 2015;27:2309–2314. doi: 10.1021/cm502710r. [DOI] [Google Scholar]
- 39.Park B-W, et al. Stabilization of formamidinium lead triiodide α-phase with isopropylammonium chloride for perovskite solar cells. Nat. Energy. 2021;6:419–428. doi: 10.1038/s41560-021-00802-z. [DOI] [Google Scholar]
- 40.Qiao W-C, et al. Metastable alloying structures in MAPbI3−xClx crystals. NPG Asia Mater. 2020;12:68. doi: 10.1038/s41427-020-00249-w. [DOI] [Google Scholar]
- 41.Yu H, et al. The role of chlorine in the formation process of “CH3NH3PbI3-xClx” perovskite. Adv. Funct. Mater. 2014;24:7102–7108. [Google Scholar]
- 42.Protesescu L, et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015;15:3692–3696. doi: 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saidaminov MI, et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy. 2018;3:648–654. doi: 10.1038/s41560-018-0192-2. [DOI] [Google Scholar]
- 44.Lee SJ, et al. Reducing carrier density in formamidinium tin perovskites and its beneficial effects on stability and efficiency of perovskite solar cells. ACS Energy Lett. 2017;3:46–53. doi: 10.1021/acsenergylett.7b00976. [DOI] [Google Scholar]
- 45.Mundt LE, et al. Surface-activated corrosion in tin-lead halide perovskite solar cells. ACS Energy Lett. 2020;5:3344–3351. doi: 10.1021/acsenergylett.0c01445. [DOI] [Google Scholar]
- 46.Rolland A, Richard J, Kleider JP, Mencaraglia D. Electrical properties of amorphous silicon transistors and mis-devices: comparative study of top nitride and bottom nitride configurations. J. Electrochem. Soc. 1993;140:3679–3683. doi: 10.1149/1.2221149. [DOI] [Google Scholar]
- 47.Senanayak SP, et al. A general approach for hysteresis-free, operationally stable metal halide perovskite field-effect transistors. Sci. Adv. 2020;6:eaaz4948. doi: 10.1126/sciadv.aaz4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu W, et al. Single crystal hybrid perovskite field-effect transistors. Nat. Commun. 2018;9:5354. doi: 10.1038/s41467-018-07706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meggiolaro D, Ricciarelli D, Alasmari AA, Alasmary FAS, De Angelis F. Tin versus lead redox chemistry modulates charge trapping and self-doping in tin/lead iodide perovskites. J. Phys. Chem. Lett. 2020;11:3546–3556. doi: 10.1021/acs.jpclett.0c00725. [DOI] [PubMed] [Google Scholar]
- 50.Gu G, Kane MG, Doty JE, Firester AH. Electron traps and hysteresis in pentacene-based organic thin-film transistors. Appl. Phys. Lett. 2005;87:243512. doi: 10.1063/1.2146059. [DOI] [Google Scholar]
- 51.Zhao L, et al. Ultrasensitive heterojunctions of graphene and 2D perovskites reveal spontaneous iodide loss. Joule. 2018;2:2133–2144. doi: 10.1016/j.joule.2018.07.011. [DOI] [Google Scholar]
- 52.Liu A, et al. Room-temperature solution-synthesized p-type copper(I) iodide semiconductors for transparent thin-film transistors and complementary electronics. Adv. Mater. 2018;30:1802379. doi: 10.1002/adma.201802379. [DOI] [PubMed] [Google Scholar]
- 53.Ambrosio F, Meggiolaro D, Almutairi TM, De Angelis F. Composition-dependent struggle between iodine and tin chemistry at the surface of mixed tin/lead perovskites. ACS Energy Lett. 2021;6:969–976. doi: 10.1021/acsenergylett.1c00111. [DOI] [Google Scholar]
- 54.Liu A, et al. High-performance p-channel transistors with transparent Zn doped-CuI. Nat. Commun. 2020;11:4309. doi: 10.1038/s41467-020-18006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park S, Kim SH, Choi HH, Kang B, Cho K. Recent advances in the bias stress stability of organic transistors. Adv. Funct. Mater. 2020;30:1904590. doi: 10.1002/adfm.201904590. [DOI] [Google Scholar]
- 56.Ye T, et al. Ambient-air-stable lead-free CsSnI3 solar cells with greater than 7.5% efficiency. J. Am. Chem. Soc. 2021;143:4319–4328. doi: 10.1021/jacs.0c13069. [DOI] [PubMed] [Google Scholar]
- 57.Yusoff ARBM, et al. Ambipolar triple cation perovskite field effect transistors and inverters. Adv. Mater. 2017;29:1602940. doi: 10.1002/adma.201602940. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Kim H, Alshareef HNJAM. Oxide thin-film electronics using all-mxene electrical contacts. Adv. Mater. 2018;30:1706656. doi: 10.1002/adma.201706656. [DOI] [PubMed] [Google Scholar]
- 59.Park W-T, Noh Y-Y. A self-aligned high resolution patterning process for large area printed electronics. J. Mater. Chem. C. 2017;5:6467–6470. doi: 10.1039/C7TC01590A. [DOI] [Google Scholar]
- 60.Wang Z, Nayak PK, Caraveo-Frescas JA, Alshareef HN. Recent developments in p-type oxide semiconductor materials and devices. Adv. Mater. 2016;28:3831–3892. doi: 10.1002/adma.201503080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusion in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper can be available from the authors upon reasonable request.
The custom software used for data acquisition and data analysis is available from the corresponding author upon reasonable request.