Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with predominant antibody deficiency (PAD) is associated with high morbidity, yet data regarding the response to SARS-CoV-2 immunization in PAD patients, including additional dose vaccine, are limited.
Objective
To characterize antibody response to SARS-CoV-2 vaccine in PAD patients and define correlates of vaccine response.
Methods
We assessed the levels and function of anti-SARS-CoV-2 antibodies in 62 PAD patients compared with matched healthy controls at baseline, at 4 to 6 weeks after the initial series of immunization (a single dose of Ad26.COV2.S [Janssen] or two doses of BNT162b2 [Pfizer-BioNTech] or mRNA-1273 [Moderna]), and at 4 to 6 weeks after an additional dose immunization, if received.
Results
After the initial series of SARS-CoV-2 vaccination, PAD patients had lower mean anti-spike antibody levels compared with matched healthy controls (140.1 vs 547.3 U/mL; P = .02). Patients with secondary PAD (eg, B-cell depletion therapy was used) and those with severe primary PAD (eg, common variable immunodeficiency with autoinflammatory complications) had the lowest mean anti-spike antibody levels. Immune correlates of a low anti-spike antibody response included low CD4+ T helper cells, low CD19+ total B cells, and low class-switched memory (CD27+IgD/M–) B cells. In addition, a low (<100 U/mL) anti-spike antibody response was associated with prior exposure to B-cell depletion therapy, both at any time in the past (odds ratio = 5.5; confidence interval, 1.5-20.4; P = .01) and proximal to vaccination (odds ratio = 36.4; confidence interval, 1.7-791.9; P = .02). Additional dose immunization with an mRNA vaccine in a subset of 31 PAD patients increased mean anti-spike antibody levels (76.3 U/mL before to 1065 U/mL after the additional dose; P < .0001).
Conclusions
Patients with secondary and severe primary PAD, characterized by low T helper cells, low B cells, and/or low class-switched memory B cells, were at risk for low antibody response to SARS-CoV-2 immunization, which improved after an additional dose vaccination in most patients.
Key words: SARS-CoV-2, COVID-19, Vaccine response, Humoral immunodeficiency, Predominant antibody deficiency, Common variable immunodeficiency, CVID, Hypogammaglobulinemia, Specific antibody deficiency, IgG subclass deficiency, Anti-spike antibody, Anti-nucleocapsid antibody, Neutralization assay, Additional dose
What is already known about this topic? Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with predominant antibody deficiency is associated with high morbidity; however, understanding of the response to SARS-CoV-2 immunization in these patients is limited.
What does this article add to our knowledge? Patients with secondary and severe primary predominant antibody deficiency, characterized by low B cells, low T helper cells, and/or low class-switched memory B cells, had low antibody response to SARS-CoV-2 immunization, which improved after additional dose vaccination.
How does this study impact current management guidelines? These data identify patient factors associated with low response to SARS-CoV-2 vaccination and support recommendations regarding additional doses of COVID-19 vaccines in patients with moderate or severe forms of immune deficiency.
Introduction
With the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, there has been an unparalleled rapid development of vaccines. This includes the use of mRNA vaccines, including BNT162b2 (Pfizer-BioNTech, New York, NY and Cambridge, MA) and mRNA-1273 (Moderna, Cambridge, MA), and adenoviral vector vaccines, including Ad26.COV2.S (Janssen, Beerse, Belgium). However, patients with underlying immune deficiencies including predominant antibody deficiency (PAD) were excluded from clinical trials assessing SARS-CoV-2 vaccine efficacy,1, 2, 3 and data regarding the response to vaccination in patients across the clinical spectrum of PAD are limited to case series.4, 5, 6, 7
Studies to date on coronavirus disease 2019 (COVID-19) among patients with immunodeficiency demonstrated high morbidity and mortality relative to the general population.8 In one study, 63% of immunodeficient patients with COVID-19 required hospitalization, with a case-fatality rate of approximately 10%.9 In addition, severe COVID-19 disease has been associated with specific primary and/or secondarily acquired defects in underlying immune signaling pathways that are critical in the defense against viral pathogens.10 , 11 Among patients with primary antibody deficiencies, there are limited data to suggest worse outcomes among patients with common variable immunodeficiency (CVID) compared with agammaglobulinemia.12 However, data regarding the severity of COVID-19 infection in patients with immunodeficiency vary widely by patient demographics.9 , 13, 14, 15 Together, these data suggest underlying immunophenotypic correlates of both risk for and protection against naturally acquired SARS-CoV-2. Whether underlying immunophenotypic factors also determine response to the novel SARS-CoV-2 vaccines is largely unknown.
Patients with PAD demonstrate increased susceptibility to infections and impaired vaccine responses. The response to vaccination can be used as a correlate of the immune system’s ability to fight natural infections and is a component of the diagnosis for several types of PAD disorders.16 Although the application of vaccines and interpretation of antibody responses can be complex, there are guidelines regarding the interpretation of vaccine responses in patients with immunodeficiency such as for the pneumococcal polysaccharide and tetanus toxoid vaccines.17 However, given the recent development of SARS-CoV-2 vaccines, the response of patients with PAD has not been fully elucidated.
To understand the immunogenicity of the SARS-CoV-2 vaccines better in patients across the clinical spectrum of PAD, we evaluated anti-SARS-CoV-2 antibody levels and neutralization capacity in patients who had an initial course of vaccination as well as in those who received an additional dose vaccination.
Methods
This study was performed at Mass General Brigham under an institutional review board–approved protocol (No. 2021P002414). Antibody response to the SARS-CoV-2 vaccine in patients with known PAD was evaluated. Inclusion criteria were adult PAD patients longitudinally observed at Mass General Brigham who underwent initial series SARS-CoV-2 vaccination between December 16, 2020 and June 9, 2021 as well as PAD patients who had clinically obtained testing during this same period. Patients who received additional SARS-CoV-2 vaccines after the primary series were assessed longitudinally. Exclusion criteria were PAD patients with prior positive polymerase chain reaction testing for SARS-CoV-2. The PAD diagnoses were confirmed by manual chart review by a clinical immunologist and met consensus definitions.16 , 18 , 19 Patients with confounding variables at the time of immunodeficiency diagnosis (eg, clonal lymphocyte population or ongoing immunosuppression without the potential for discontinuation) were considered to be secondary PAD. Patients with primary PAD were further subclassified as mild (IgG subclass deficiency, specific antibody deficiency, and primary hypogammaglobulinemia), moderate (uncomplicated CVID, defined as an absence of co-occurring autoinflammatory clinical features20), and severe (complicated PAD that encompassed the diagnoses of activated PI3K-δ syndrome, TACI deficiency, nuclear factor-κB1 deficiency, and complicated CVID/specific antibody deficiency (SAD), defined as the presence of co-occurring autoinflammatory clinical features20 but without a known genetic etiology). We evaluated demographic information and clinical characteristics including the type of PAD; the vaccine type received; previous genetic testing if performed or available; and previous immune testing performed, including native antibody levels, native antibody responses to vaccines (eg, pneumovax23; Hemophilus influenza B [HIB]; and tetanus, diphtheria, and pertussis), peripheral blood lymphocyte counts, and T-cell functional studies, when available. We evaluated previous and current treatment regimens with a focus on immunoglobulin replacement type, if received, and other immunosuppressants or biologics received in the past or in close proximity to vaccination (defined as 6 months before to 1 month after immunization).
Serologic assays were performed through Massachusetts General Pathology Laboratory using the Roche (Basel, Switzerland) Elecsys Anti-SARS-CoV-2 S-antibody test (evaluating antibodies to the SARS-CoV-2 spike (S) protein receptor binding domain; anti-spike antibody) and the Roche Elecsys Anti-SARS-CoV-2 N-antibody test (evaluating antibodies to the SARS-CoV-2 nucleocapsid domain; anti-nucleocapsid antibody). These tests are semiquantitative and have been correlated with neutralizing immunity.21 , 22 The Roche S-antibody assay reports in absorbance units per milliliter with values of 0.8 U/mL or greater considered reactive.23 We further delineated a minimum threshold protective anti-spike antibody response as 100 U/mL or greater, which has been correlated with a detectable level of pseudovirus neutralization in healthy control subjects.24 The Roche N-antibody assay reports a cutoff index (COI), with values of 1.00 or greater COI considered reactive.
Neutralization was measured using a SARS-CoV-2 pseudovirus neutralization assay that was previously described.25 Briefly, lentiviral particles encoding both luciferase and ZsGreen reporter genes were pseudotyped with SARS-CoV-2 spike protein and produced in 293T cells, titered using ZsGreen expression by flow cytometry and used in an automated neutralization assay with 50 to 250 infectious units of pseudovirus coincubated with threefold serial dilutions of serum for 1 hour. Neutralization was determined on 293T-ACE2 cells. The percent neutralization was determined by subtracting background luminescence measured in cell control wells (cells only) from sample wells and dividing by the virus control wells (virus and cells only). We calculated pseudovirus neutralization function (pNT50) values by taking the inverse of the 50% inhibitory concentration.
Quantitative detection of total (IgA, IgM, and IgG) and individual isotype (IgG, IgA, or IgM) antibodies to the SARS-CoV-2 receptor binding domain (RBD) was performed by enzyme linked immunosorbent assay, as previously described.24 , 26
The PAD participants were matched according to age (±10 years) and the time from the most recent vaccination (±14 days) at a ratio of 1:1 with healthy controls. The control population was healthy ambulatory adults sampled in August 2020 or early 2021, who provided consent under institutional review board protocols (Nos. 2020P001081 and 2020P002274), as described previously.24 The comparator cohort of 62 healthy control volunteers had anti-spike and anti-nucleocapsid antibodies to SARS-CoV-2 evaluated on identical Roche Elecsys platforms through the Massachusetts General Pathology Laboratory.
We used repeated-measures ANOVA for continuous variables and conditional logistic regression for categorical variables for matched participants and those who received additional vaccination after the initial series. One-way ANOVA with Tukey’s post hoc correction and simple logistic regression were used to compare SARS-CoV-2 antibody levels among subgroups of patients with different PAD types. To account for extreme heteroscedasticity, all antibody responses to SARS-CoV-2 vaccine were reported as geometric means (95% confidence interval [CI]), and log transformations were used to transform all antibody measures before statistical analyses to estimate P values. Statistical analyses were completed with SAS software (version 9.4, SAS Institute, Cary, NC) and Prism software (version 7.01, Reston, Va); two-tailed P less than .05 was considered significant.
Results
Antibody response to SARS-CoV-2 vaccine is lower among patients with PAD compared with healthy controls
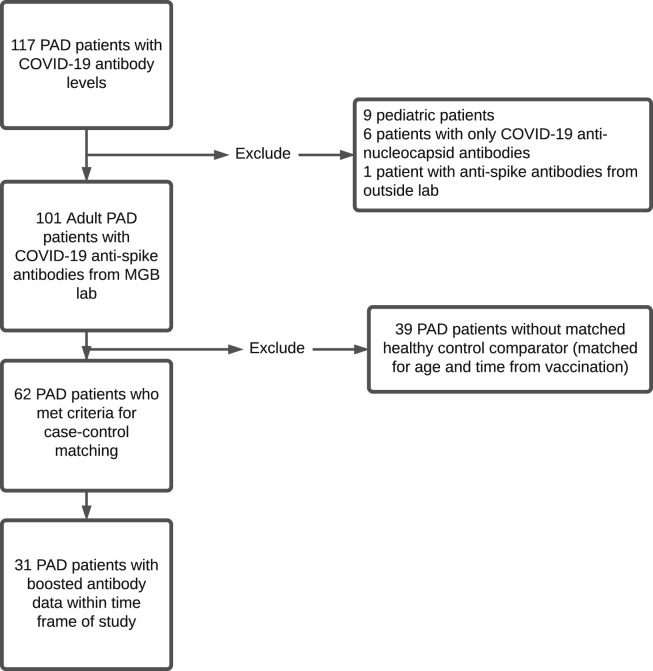
A total of 101 individuals with PAD met criteria for this study (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org). Of the 101, 62 met criteria for case-control matching based on age (±10 years) and time from the most recent vaccination (±14 days) at a ratio of 1:1 case to control; these 62 patients were used for all subsequent analyses (Table I ). Mean age of the 62-patient PAD cohort was 52.5 years. There were 43 women (69.4%) and 19 men (30.6%). There were no statistically significant differences between PAD and healthy control groups in terms of age, sex, and time between blood draw and the most recent vaccination. The PAD group consisted of more non-Hispanic White patients (95.2%) than did the control population (P < .01). Among both groups, most participants had received the mRNA-1273 (Moderna) vaccine (PAD: 53.2% vs healthy controls: 54.8%), followed by the BNT162b2 (Pfizer-BioNTech) vaccine (PAD: 40.3% vs healthy controls: 24.9%), followed by the Ad26.COV2.S (Janssen) vaccine (PAD: 6.5% vs. healthy controls: 20.9%) (P = .05).
Figure E1.
Flow diagram illustrating cohort inclusion criteria. COVID-19, Coronavirus disease 2019; MGB, Mass General Brigham; PAD, predominant antibody deficiency.
Table I.
Demographic characteristics of cases and controls
| Characteristic | Predominant antibody deficiency (n = 62) | Healthy controls (n = 62) | P |
|---|---|---|---|
| Age, y (mean) | 52.5 | 52.6 | .93 |
| Sex (% female) | 69.4 | 56.5 | .14 |
| Non-Hispanic White (%) | 95.2 | 61.1 | <.01 |
| Missing | — | 8 | |
| Vaccine (% [n]) | |||
| mRNA-1273 (Moderna) | 53.2 (33) | 54.8 (34) | |
| BNT162b2 (Pfizer) | 40.3 (25) | 24.9 (15) | |
| Ad26.COV2.S (Janssen) | 6.5 (4) | 20.9 (13) | .05 |
| Time from most recent vaccination to blood draw, d | 36.6 | 35.1 | .16 |
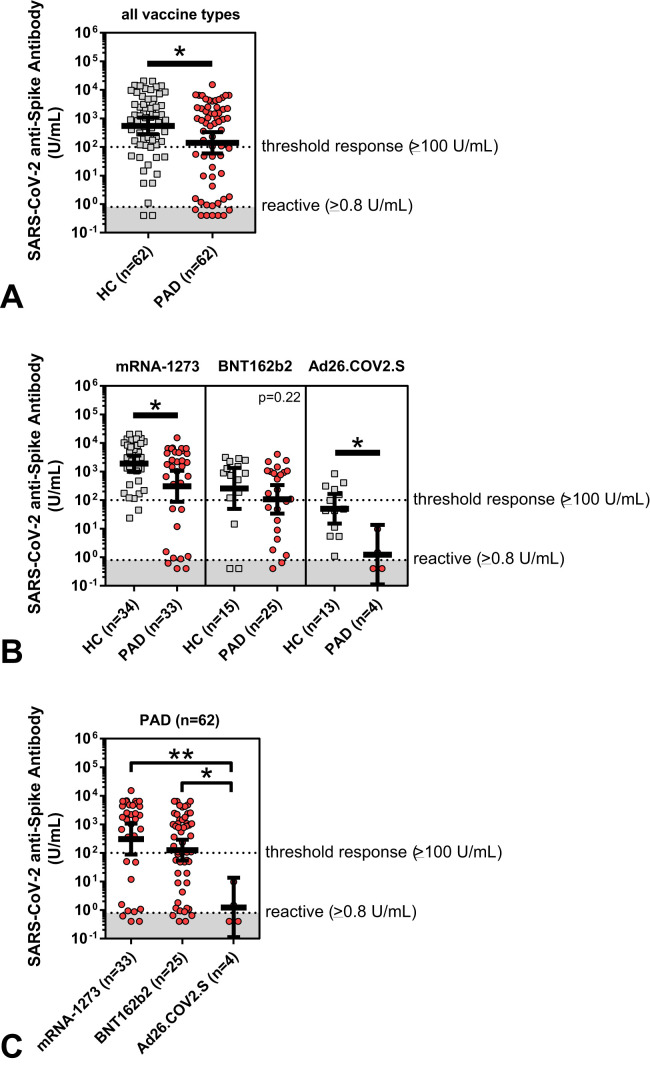
We observed significantly lower mean anti-spike antibody levels in PAD patients compared with matched healthy controls after the initial series SARS-CoV-2 vaccination (anti-spike antibody level for all vaccine types: 140.1 vs 547.3 U/mL; P = .02) (Table II and Figure 1 , A). The odds of mounting a protective anti-spike antibody response of 100 U/mL or greater were 2.5 times higher in healthy controls than in those with a diagnosis of PAD. Regarding the response to the specific SARS-CoV-2 vaccine received, PAD patients had significantly lower antibody responses compared with matched healthy controls after immunization with mRNA-1273 (Moderna) and Ad26.COV2.S (Janssen) ( Table II and Figure 1, B). Overall, SARS-CoV-2 anti-spike antibody titers were significantly higher in PAD patients who had received either mRNA vaccine platform compared with the Ad26.COV2.S (Janssen) vaccine (Figure 1, C).
Table II.
Anti-spike antibody levels in predominant antibody deficiency patients compared with matched healthy controls
| Variable | Predominant antibody deficiency (n = 62) | Healthy controls (n = 62) | P |
|---|---|---|---|
| Anti-spike antibody, U/mL (geometric mean [95% CI]) | 140.1 (59.2-331.5) | 547.3 (280.2-1,069.0) | .02 |
| Anti-spike antibody (%) | |||
| ≥100 | 59.7 | 79.0 | |
| OR (95% CI) | Reference | 2.5 (1.1-5.7) | .03 |
| Anti-spike antibody (%) | |||
| <100 | 40.3 | 21.0 | |
| 100-1,000 | 21.0 | 29.0 | |
| ≥1,000 | 38.7 | 50.0 | |
| OR 100-1,000 vs <100 (95% CI) | Reference | 2.5 (0.95-6.8) | .06 |
| OR ≥1,000 vs <100 (95% CI) | Reference | 2.5 (1.02-6) | .046 |
| Anti-spike antibody, U/mL (geometric mean [95% CI]) | |||
| mRNA-1273 (Moderna) | 305.2 (87.4-1,065) | 1,905 (988.9-3,669) | .03 |
| BNT162b2 (Pfizer) | 106.9 (33.9-337.3) | 258 (49.3-1,349) | .22 |
| Ad26.COV2.S (Janssen) | 1.2 (0.1-13.6) | 50.0 (15.2-164.7) | .03 |
CI, confidence interval; OR, odds ratio.
Figure 1.
SARS-CoV-2 anti-spike antibody levels (U/mL), shown in log scale and compared between matched healthy controls (HC) (gray squares; n = 62) and patients with predominant antibody deficiency (PAD) (red circles; n = 62). Shown by all vaccine types (A) and by specific initial series SARS-CoV-2 vaccine type received (B, C). Symbols represent unique individuals, bars represent geometric means (±95% confidence intervals) of total indicated patients (n), and shading represents the assay lower limit of reactivity. ∗P < .05; ∗∗P < .01.
Antibody response to SARS-CoV-2 vaccine is lower among PAD patients with secondary and severe primary immunodeficiency
To determine whether anti-spike antibody responses correlated with the clinical diagnosis, we subcategorized the PAD cohort (Table III ). Ten patients met criteria for secondary PAD owing to the presence of a potentially confounding immunosuppressive variable at the time of immune deficiency diagnosis. Fifty-two patients had no confounding variables at the time of diagnosis and met criteria for primary PAD. We further subcategorized primary PAD patients by the underlying degree of humoral immune dysfunction. Immunologic testing including native immunoglobulin levels (before immunoglobulin replacement therapy), antibody titers to T cell–dependent and T cell–independent immunizations, peripheral lymphocyte flow cytometry (including analysis of B-cell and T-cell maturation), and T-cell functional testing of T-cell receptor, mitogen, and antigen stimuli for this cohort are detailed in Table E1 (in this article’s Online Repository at www.jaci-inpractice.org). Primary PAD participants were classified as mild (IgG subclass deficiency, SAD, and primary hypogammaglobulinemia; n = 12), moderate (CVID without autoinflammatory clinical features [CVID]; n = 21), or severe (complicated PAD encompassing the diagnoses of activated PI3K-δ syndrome, TACI deficiency, nuclear factor-κB1 deficiency, and complicated CVID/SAD with autoinflammatory clinical features but without a known genetic etiology [complicated CVID/SAD]; n = 19).
Table III.
Subcategorization of PAD cases
| Primary (n = 52) |
Secondary (n = 10) | P | ||||
|---|---|---|---|---|---|---|
| Subtype | Mild (n = 12) | Moderate (n = 21) | Severe (n = 19) | P | ||
| PAD diagnosis | ||||||
| Clinical entities | Immunoglobulin subclass deficiency (3) Specific antibody deficiency (5) PHG (4) |
Common variable immunodeficiency (21) | Complicated PAD (19): Activated PI3K-δ syndrome (4) TACI deficiency (3) Nuclear factor-κB1 deficiency (1) Complicated common variable immunodeficiency/specific antibody deficiency (gene not known) (11) |
Diagnosis confounded by: Clonal suppression (3) Immunosuppression (7) |
||
| PID genetic testing | ||||||
| Yes (% [n]) | 33.3 (4) | 42.8 (9) | 84.2 (16) | .005 | 10.0 (1) | .0074 |
| Pathogenic variant (% [n]) | 0 | 0 | 42.1 (8): PIK3CD (3) TNFRSF13B (3) PIK3AP1 (1) NFκB1 (1) |
<.0001 | 0 | .19 |
| IgR | ||||||
| Yes (% [n]) | 33.3 (4) | 85.7 (18) | 84.2 (16) | .0012 | 40.0 (4) | .041 |
| Immunosuppression (ever) | ||||||
| Yes (% [n]) | 75.0 (9) | 90.5 (19) | 78.9 (15) | .47 | 90.0 (9) | .57 |
| Intermittent prednisone or hydroxychloroquine only (% [n]) | 66.7 (8) | 52.4 (11) | 15.8 (3) | .0082 | 0 | .01 |
| B-cell depletion before diagnosis (% [n]) | 8.7 (1, >5 y) | 0 | 5.3 (1, >5 y) | .47 | 50.0 (5, ongoing) | <.0001 |
| Receiving therapy at diagnosis (% [n]) | 0 | 0 | 0 | — | 70.0 (7) | <.0001 |
| Immunosuppression (around SARS-CoV-2 vaccine) | ||||||
| Yes: any, ≤1 mo before (% [n]) | 25.0 (3) | 23.8 (5) | 36.8 (7) | .64 | 40.0 (4) | .49 |
| Yes: any, ≤1 mo after (% [n]) | 16.7 (2) | 23.8 (5) | 31.6 (6) | .65 | 20.0 (2) | .74 |
| Yes: B-cell depletion, ≤6 mo before to ≤1 mo after (% [n]) | 0 | 0 | 15.8 (3) | .0003 | 50.0 (5) | <.0001 |
IgR, Immunoglobulin replacement; PAD, predominant antibody deficiency; PID, primary immunodeficiency.
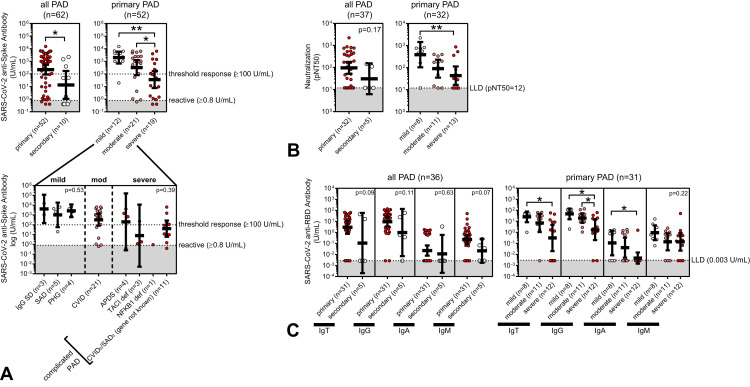
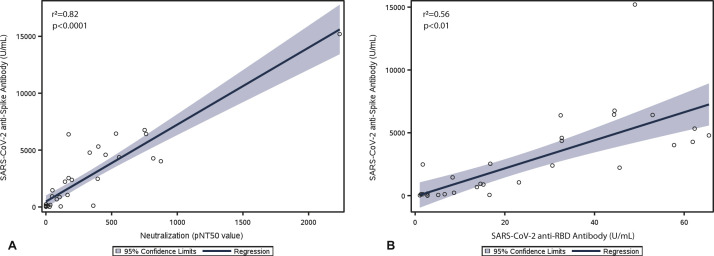
Patients with secondary PAD had significantly lower mean anti-spike antibody levels compared with patients with primary PAD (13.4 vs 219.8 U/mL; P = .02) (Table IV and Figure 2 , A). We also analyzed pseudovirus neutralization in postimmunization serum available in 37 PAD patients and SARS-CoV-2 anti-RBD-specific antibody levels, including IgG, IgA, and IgM, in postimmunization serum available in 36 PAD patients. Overall, we observed linear correlations between anti-spike antibody levels and pseudovirus neutralization function and anti-RBD antibody levels, respectively, among PAD patients (Figure 3 ). Similar to the observed difference in anti-spike antibody levels, patients with secondary PAD trended toward lower SARS-CoV-2 vaccine response by pseudovirus neutralization and total anti-RBD antibody levels compared with patients with primary PAD (Table IV and Figure 2, B, C).
Table IV.
Antibody response to SARS-CoV-2 vaccine in PAD patients by clinical subtype
| Variable | Primary PAD (n = 52) |
All primary PAD (n = 52) (total) | Secondary PAD (n = 10) (total) | P | |||
|---|---|---|---|---|---|---|---|
| Mild (n = 12) | Moderate (n = 21) | Severe (n = 19) | P | ||||
| Anti-spike antibody, U/mL (geometric mean [95% CI], n) | 2,003 (677.1-5,922) n = 12 | 321.8 (81-1,279) n = 21 | 35.7 (7.7-166.2) n = 19 | .001 | 219.8 (90.1-536.2) n = 52 | 13.4 (1.1-170.8) n = 10 | .02 |
| Neutralization (pNT50) (geometric mean [95% CI], n) | 389 (103.8-1,458) n = 8 | 90.2 (35.3-230.1) n = 11 | 43.4 (16.6-113.7) n = 13 | .01 | 96.6 (52.0-179.3) n = 32 | 30.9 (6.2-154.4) n = 5 | .17 |
| Anti-receptor binding domain antibody (IgT), U/mL (geometric mean [95% CI], n) | 25.6 (8.4-78.2) n = 8 | 7.1 (1.1-47.5) n = 11 | 0.3 (0.02-4.9) n = 12 | .01 | 3.0 (0.8-11.3) n = 31 | 0.1 (0.0-58.5) n = 5 | .09 |
CI, confidence interval; PAD, predominant antibody deficiency.
Figure 2.
SARS-CoV-2 anti-spike antibody levels (U/mL) (A), SARS-CoV-2 pseudovirus neutralization values (pNT50) (B), and SARS-CoV-2 anti-RBD antibody titers (U/mL) (C), shown in log scale and compared between predominant antibody deficiency (PAD) diagnoses as indicated. Symbols represent unique individuals, bars represent geometric means (±95% confidence intervals) of total indicated patients (n), and shading represents the assay lower limit of detection (LLD) or reactivity, respectively. ∗P < .05; ∗∗P < .01. APDS, Activated PI3K Delta Syndrome; CVID, common variable immunodeficiency; def, deficiency; PHG, primary hypogammaglobulinemia; SAD, specific antibody deficiency.
Figure 3.
SARS-CoV-2 anti-spike antibody level (U/mL) correlates linearly with pseudovirus neutralization function (pNT50) (A) and total anti-receptor binding domain (RBD) antibody level (U/mL) (B) in patients with predominant antibody deficiency (PAD). Linear regression analysis from 37 (A) and 36 (B) PAD patients with correlation coefficients (r2) and significance (P) is shown. Shaded area represents 95% confidence limits.
Among primary PAD patients, those classified as having severe disease had significantly lower mean anti-spike antibody levels compared with those classified as having moderate disease and those classified as having mild disease (severe: 35.7 U/mL; moderate: 321.8 U/mL; mild: 2003 U/mL; P = .001) (Table IV and Figure 2, A). There was no statistically significant difference in anti-spike antibody responses further delineated by subcategorized clinical entity within the mild and severe disease subtypes (Figure 2, A). Analysis of pseudovirus neutralization showed a similar trend, with significantly lower mean neutralization function in severe compared with mild PAD patients (severe: 43.4 pNT50 vs mild: 389 pNT50; P = .01) (Table IV and Figure 2, B). Finally, analysis of anti-RBD–specific antibody responses showed a similar trend, with significantly lower mean anti-RBD antibodies observed in severe compared with mild PAD patients (severe: 0.3 vs mild: 25.6 U/mL IgT; P = .01) (Table IV and Figure 2, B, C). An exception was the IgM-specific anti-RBD response, which was not statistically different among mild, moderate, and severe primary PAD groups.
Immunophenotypic risk factors for low anti-spike antibody response among patients with PAD
Within the PAD cohort, we analyzed underlying immunophenotypic correlates of a severely low antibody response to SARS-CoV-2 immunization, defined as an anti-spike antibody level less than 100 U/mL. The PAD patients with anti-spike antibody levels less than 100 U/mL had lower native antibody levels, including IgG, IgA, and IgM, and lower native HIB vaccine levels (Table V ). In addition, PAD patients with anti-spike antibody levels less than 100 U/mL had lower absolute circulating counts of total CD3+ T cells, CD4+ T helper cells, and total CD19+ B cells. Finally, PAD patients with anti-spike antibody levels less than 100 U/mL demonstrated impaired B-cell maturation. Specifically, patients with less than 5% memory (CD27+ as a percentage of CD19+) B cells in circulation and less than 2% class-switched memory (CD27+IgM/D– as a percentage of CD19+) B cells in circulation were at increased risk for having an anti-spike antibody level less than 100 U/mL (odds ratio [OR] = 9.7; 95% CI, 1.9-49.9; P < .01, and OR = 2.3; 95% CI, 0.6-9; P < .01, respectively).
Table V.
Antibody response to SARS-CoV-2 vaccine in predominant antibody deficiency patients by underlying immunophenotype
| Variable | Anti-spike antibody <100 U/mL (n = 25) |
Anti-spike antibody ≥100 U/mL (n = 37) |
P |
|---|---|---|---|
| Native immunoglobulin levels, mg/dL (mean) | |||
| IgG | 443 | 678 | <.01 |
| IgA | 45 | 171 | <.01 |
| IgM | 60 | 103 | .03 |
| IgG1 | 311 | 392 | .07 |
| IgG2 | 146 | 182 | .36 |
| IgG3 | 32 | 34 | .93 |
| IgG4 | 13 | 16 | .19 |
| Missing, n | (3-16) | (7-12) | |
| IgG antibody levels (mean) | |||
| Streptococcus pneumoniae (% >1.3 μg/mL) | 47 | 56 | .29 |
| Haemophilus influenzae, mg/L | 0.3 | 1.6 | <.01 |
| Tetanus, IU/mL | 0.98 | 1.4 | .42 |
| Diphtheria, IU/mL | 0.28 | 0.25 | .85 |
| Missing, n | (10-13) | (7-16) | |
| Flow cytometry (mean absolute count of cells/μL) | |||
| CD3+ | 986 | 1,287 | .04 |
| CD4+ | 559 | 810 | .02 |
| CD8+ | 356 | 416 | .3 |
| CD3–CD16+56+ | 190 | 200 | .71 |
| CD4+CD45RA+ | 234 | 348 | .05 |
| CD4+CD45RO+ | 309 | 393 | .37 |
| CD8+CD45RA+ | 179 | 240 | .41 |
| CD8+CD45RO+ | 145 | 134 | .71 |
| CD19+ | 129 | 256 | <.01 |
| CD19+CD27+ | 32 | 46 | .09 |
| CD19+CD27+IgM/IgD– | 4 | 12 | <.01 |
| CD19+CD27+IgM/IgD+ | 26 | 33 | .26 |
| Missing, n | (5-9) | (7-11) | |
| Severity markers | |||
| <20% CD4+CD45RA+ (% CD4+) | |||
| Odds ratio | Reference | 1 (0.16-6.1) | .64 |
| Missing (n = 14) | |||
| <5% CD19+CD27+ (% CD19+) | |||
| Odds ratio | Reference | 9.7 (1.9-49.9) | <.01 |
| Missing (n = 18) | |||
| <10% CD19+CD27+ (% CD19+) | |||
| Odds ratio | Reference | 2.3 (0.6-9) | .22 |
| Missing (n = 18) | |||
| <2% CD19+CD27+IgM/IgD– (% CD19+) | |||
| Odds ratio | Reference | 11 (2-60) | <.01 |
| Missing (n = 18) |
Passive transfer of anti-spike antibodies in patients receiving immunoglobulin replacement therapy occurred at a low level
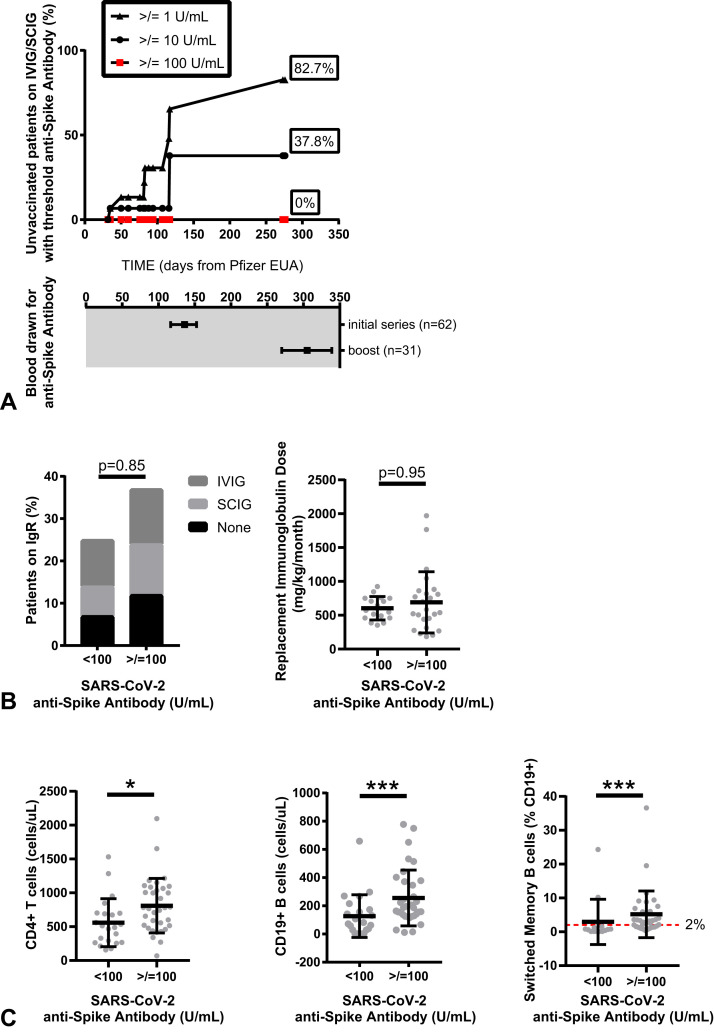
Despite exclusion in this study of PAD patients with prior positive polymerase chain reaction testing for SARS-CoV-2, mean anti-nucleocapsid antibody levels were higher in PAD patients who actively received intravenous immunoglobulin therapy (1.15 COI) compared with subcutaneous immunoglobulin therapy (0.19 COI) and compared with no replacement immunoglobulin therapy (0.09 COI) (P = .047) (see Table E2 in this article’s Online Repository at www.jaci-inpractice.org). These data were consistent with low levels of passively transferred anti-SARS-CoV-2 antibodies in immunoglobulin replacement products, as previously described.27
To determine whether passive antibody transfer could be confounding the anti-spike antibody analysis, we analyzed anti-spike antibodies at prevaccination time points in PAD patients who were receiving replacement immunoglobulin therapy, as available (n = 16) (Figure 4 , A). At the median time of final blood draw for this study, the detection of passively transferred anti-spike antibodies was extremely low (anti-spike antibody levels greater than 1 U/mL, greater than 10 U/mL, and greater than 100 U/mL were seen in 82.7%, 37.8%, and 0.0% of unvaccinated PAD subjects receiving immunoglobulin replacement, respectively). These data suggest limited confounding of passively received anti-spike antibodies, particularly using a threshold response anti-spike antibody level of 100 U/mL or greater. Moreover, PAD patients who mounted an anti-spike level of 100 U/mL or greater did not differ by immunoglobulin treatment status or dose per body weight (milligrams per kilogram) per month of immunoglobin therapy, if received (Figure 4, B). Instead, as noted earlier, differences in response to vaccine correlated with underlying immunophenotypes including lower native immunoglobulin levels, lower native HIB vaccine titer, lower absolute CD3+ T cells, CD4+ T cells, and CD19+ B cells, as well as lower absolute and percent class-switched memory B cells in circulation (Figure 4, C).
Figure 4.
Evaluation of timing of SARS-CoV-2 anti-spike antibody testing in relation to potential for passive antibody transfer (A). Detection of threshold anti-spike antibodies (greater than 1 U/mL, greater than 10 U/mL, or greater than 100 U/mL) in vaccine-naive predominant antibody deficiency (PAD) patients receiving intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG) therapy. Data are shown as percent positive by Kaplan-Meier curve; symbols indicate events over 276 days after emergency use authorization (EUA) of the Pfizer vaccine (top) with corresponding dates of blood draw for all PAD patients included in this study shown as median (± interquartile range) days (bottom). Threshold vaccine response, defined in the study as an anti-spike antibody level of 100 U/mL or greater, is shown in relation to immunoglobulin replacement therapy (B) and underlying immunophenotype (C). Symbols represent unique individuals; bars represent means (±SD) of total indicated patients (n). ∗P < .05; ∗∗∗P < .001. IgR, Immunoglobulin replacement
Immunosuppression associated with low anti-spike antibody response among patients with PAD
The PAD patients who received any immunosuppression in the 1 month before SARS-CoV-2 immunization had a lower mean anti-spike antibody response (30.1 vs 276.5 U/mL; P = .02) (Table VI ). Receiving any immunosuppression in the 1 month after SARS-CoV-2 immunization also trended toward a lower mean anti-spike antibody level among PAD patients (39.1 vs 258.4 U/mL; P = .07). Finally, PAD patients who had any previous use of a B-cell depletion agent (eg, rituximab) had a lower mean anti-spike antibody response (11.6 vs 289.8 U/mL; P < .01) and increased odds of mounting an anti-spike antibody response of less than 100 U/mL (OR = 5.5; 95% CI, 1.5-20.4; P = .01). This association became more pronounced when we accounted for patients who received a B-cell depletion agent proximal to the time of immunization, which we defined as 6 months before to 1 month after the initial immunization. Specifically, PAD patients who had proximal use of a B-cell depletion agent had a lower mean anti-spike antibody response (0.67 vs 308.8 U/mL; P < .01) and increased odds of mounting an anti-spike antibody response of less than 100 U/mL (OR = 36.4; 95% CI, 1.7-791.9; P = .02).
Table VI.
Antibody response to SARS-CoV-2 vaccine in predominant antibody deficiency patients by secondary immunosuppression
| Variable | Anti-spike antibody, U/mL (geometric mean [95% confidence interval]) | P | Anti-spike antibody, <100 U/mL (odds ratio [95% confidence interval]) | P |
|---|---|---|---|---|
| Immune suppression (ever) | ||||
| Yes | 142 (55.3-367.8) | |||
| No | 123.9 (9.9-1,543) | .91 | 0.35 (0.08-1.6) | .18 |
| Immune suppression (≤1 mo before) | ||||
| Yes | 30.1 (5.6-162.4) | |||
| No | 276.5 (104.9-728.9) | .02 | 1.5 (0.5-4.5) | .45 |
| Immune suppression (≤1 mo after) | ||||
| Yes | 39.1 (6-253.1) | |||
| No | 258.4 (87.1-766.3) | .07 | 1.4 (0.4-4.6) | .61 |
| B cell depletion therapy | ||||
| Ever | ||||
| Yes | 11.6 (1.3-94.7) | |||
| No | 289.8 (121.9-688.7) | <.01 | 5.5 (1.5-20.4) | .01 |
| Recent (≤6 mo before to ≤1 mo after) | ||||
| Yes | 0.67 (0.39-1.1) | |||
| No | 308.8 (140.9-676.7) | <.01 | 36.4 (1.7-791.9) | .02 |
Response to additional dose of SARS-CoV-2 vaccine among PAD patients
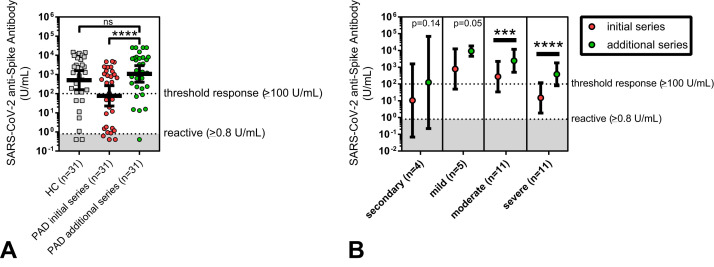
Of the initial 62 PAD patients, 31 received an additional dose of SARS-CoV-2 vaccine beyond the initial series, with follow-up anti-SARS-CoV-2 antibody testing performed. Most (90.3%) received one additional mRNA vaccine dose after the initial series mRNA immunization. In contrast, three patients (9.7%) received additional mRNA vaccine doses after the initial series Ad26.COV2.S (Janssen) immunization. The additional dose SARS-CoV-2 vaccine in the 31-patient PAD cohort had significantly increased mean anti-spike antibody levels (76.3 U/mL before to 1,065 U/mL after the additional dose; P < .0001) (Table VII and Figure 5 , A). Overall, the additional dose SARS-CoV-2 vaccine in PAD subjects improved anti-spike antibodies to the level of the matched healthy controls after the primary series immunization. The fold increase in anti-spike antibodies after the additional dose SARS-CoV-2 vaccine was similar across risk factors including the clinical diagnosis (eg, secondary PAD and severe primary PAD), initial receipt of the Ad26.COV2.S (Janssen) vaccine, severe immunophenotype (eg, less than 2% class-switched memory B cells), and secondary immunosuppression (eg, use of a B-cell depletion agent). The observed increase in anti-spike antibodies after the additional series immunization was statistically significant for patients with moderate and severe primary PAD, specifically (Figure 5, B). Six patients (19.4%) had persistently low (less than 100 U/mL) anti-spike antibodies after the additional dose SARS-CoV-2 vaccine. Analysis of the variables associated with low anti-spike antibodies after the initial series immunization (Tables V and VI) identified that the only persistent correlation was the recent use of a B cell–depleting therapy from the 6 months before to the 1 month after the additional dose vaccine (OR = 23; 95% CI, 2.5-213.7; P = .006).
Table VII.
Response to additional dose of SARS-CoV-2 vaccine in predominant antibody deficiency patients
| Variable | Initial series vaccine anti-spike antibody, U/mL |
Additional dose vaccine anti-spike antibody, U/mL |
Fold change | P | ||
|---|---|---|---|---|---|---|
| Geometric mean | (95% confidence interval) | Geometric mean | (95% confidence interval) | |||
| Clinical subtype | ||||||
| All predominant antibody deficiency (n = 31) | 76.3 | (22.5-259) | 1,065 | (395-2,871) | 14-fold | <.0001 |
| Secondary (n = 4) | 10.5 | (0.07-1,603) | 124.1 | (0.2-69,396) | 12-fold | |
| Primary (n = 27) | 102.3 | (27.6-379.2) | 1,464 | (565-3,795) | 14-fold | .36 |
| Mild (n = 5) | 786.9 | (49.9-12,414) | 9,188 | (4,558-18,522) | 12-fold | |
| Moderate (n = 11) | 277.5 | (34.0-2,251) | 2,441 | (507.6-11,742) | 9-fold | |
| Severe (n = 11) | 14.9 | (1.9-119.3) | 381.1 | (78.8-1,844) | 25-fold | .59 |
| Type of vaccination series | ||||||
| Janssen plus mRNA (n = 3) | 0.6 | (0.1-4.0) | 15.0 | (0.002-143,901) | 25-fold | |
| mRNA plus mRNA (n = 28) | 127.8 | (38.8-420.7) | 1,682 | (714.6-3,959) | 13-fold | .34 |
| Immunophenotype | ||||||
| <20% CD45RA+ (%CD4+) | 38.6 | (0.06-25,476) | 2,140 | (72.6-63,064) | 55-fold | |
| ≥20% CD45RA+ (%CD4+) | 136.6 | (32.9-565.6) | 1,554 | (549.1-4,399) | 11-fold | .12 |
| <2% CD27+IgM/IgD– (%CD19+) | 66.6 | (14.0-316.9) | 1,337 | (464.8-3,848) | 20-fold | |
| ≥2% CD27+IgM/IgD– (%CD19+) | 1,289 | (428.7-3,877) | 6,759 | (2,282-20,019) | 5-fold | .81 |
| IgG <500 mg/dL | 16.5 | (2.0-135.2) | 286.6 | (40.6-2,025) | 17-fold | |
| IgG ≥500 mg/dL | 167.7 | (27.1-1,040) | 2,694 | (950.6-7,637) | 16-fold | .17 |
| B cell depletion therapy | ||||||
| Ever | ||||||
| Yes | 12.7 | (0.86-187.4) | 248.2 | (19.7-3,127) | 20-fold | |
| No | 159.2 | (41.4-612.7) | 1,932 | (715.4-5,220) | 12-fold | .33 |
| Recent (≤6 mo before to ≤1 mo after) | ||||||
| Yes | 0.89 | (0.34-2.0) | 26.6 | (2.1-335.1) | 30-fold | |
| No | 222.5 | (71.1-696.2) | 2,583 | (1,168-5,712) | 12-fold | .21 |
Figure 5.
SARS-CoV-2 anti-spike antibody titers (U/mL), shown in log scale and compared between initial series SARS-CoV-2 vaccination (red circles) and additional dose SARS-CoV-2 vaccination (green circles). Data are shown for the total boosted predominant antibody deficiency (PAD) cohort (n = 31) and compared with the matched healthy controls (n = 31) (A) and by PAD diagnosis (B). Symbols (A) represent unique individuals and bars represent geometric means (±95% confidence intervals) of total indicated patients (n). Symbols (B) represent geometric means (±95% confidence intervals) of total indicated patients (n). Shading represents the assay lower limit of reactivity. ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Discussion
To our knowledge, this is the largest case–control matched immunodeficiency patient cohort evaluating the response to SARS-CoV-2 vaccination to date and the first study evaluating additional vaccine doses in patients with PAD. Because SARS-CoV-2 infection in patients with PAD is associated with high morbidity, an improved understanding of the effectiveness of SARS-CoV-2 immunization in immunodeficient patients is critical.
In this study, we found that approximately 60% of PAD patients were able to develop anti-spike antibody responses of 100 U/mL or greater after the initial series SARS-CoV-2 vaccination. However, compared with healthy controls matched for age and time from immunization, PAD patients had significantly lower mean anti-spike antibody levels. The underlying PAD diagnosis and immunophenotypic markers of disease severity correlated with the response to vaccination. Specifically, anti-spike antibody levels were lowest in patients with secondary and severe primary PAD (such as complicated CVID) compared with those with mild PAD (such as IgG subclass deficiency, SAD, and primary hypogammaglobulinemia). Certain immunophenotypic markers correlated with a lower anti-spike antibody response, including low native antibody levels (IgG, IgA, and IgM), low native IgG antibodies for HIB, low CD4+ T helper cells, low CD19+ total B cells, and low class-switched memory (CD27+IgD/M–) B cells. These clinical diagnostic and immunophenotypic risk factors may help clinicians to stratify patients with PAD better in terms of identifying patients who are at highest risk for a low antibody response after SARS-CoV-2 immunization.
Many patients with PAD will require secondary immunosuppression to manage autoimmune and/or autoinflammatory disease comorbidity.28 Here, secondary immunosuppression, in particular the use of a B cell–depleting agent, most frequently rituximab, was associated with a decreased humoral immune response to SARS-CoV-2 vaccination. Prior B cell–depleting agent use correlated with severely low (less than 100 U/mL) mean anti-spike antibody levels in this study. These data are consistent with prior reports of lower SARS-CoV-2 vaccine antibody responses after B cell–depleting therapy in other immunodeficient patient demographics4 , 29 and suggest that this patient population should maintain increased precautions and vigilance regarding potential COVID-19 exposure. Overall, these data highlight the unique risk for patients with primary immunodeficiency related to SARS-CoV-2 vaccination: the potential for diminished immune response owing to both a congenital immunodeficiency and the use of secondary immunosuppression.
In PAD patients who received an additional SARS-CoV-2 vaccine, specifically one or more additional mRNA vaccine doses, anti-spike antibody levels increased significantly. These data support recommendations from the Centers for Disease Control and Prevention30 regarding additional doses of COVID-19 vaccine as a part of the primary series and then as booster doses in patients with moderate or severe forms of immune deficiency. Increased anti-spike antibody levels after additional dose immunization were observed even in PAD patients with an at-risk immunophenotype for poor response to initial series immunization (eg, low class-switched memory [CD27+IgM/D–] B cells). These data suggest a significant benefit to additional dose vaccination in patients with moderate to severe immune deficiency phenotypes. This additional dose vaccine increased anti-spike antibodies to the level of matched controls after the initial series immunization. However, the optimal timing and number of vaccine doses needed to prevent or mitigate disease in patients with PAD require future study. In addition, there are not yet data addressing the SARS-CoV-2 vaccine memory response in the PAD patient demographic. Trends toward different isotype anti-RBD antibody responses between PAD diagnoses suggest that specific PAD patients are more predisposed to short-lived antibody responses after SARS-CoV-2 vaccine. Data from patients with CVID suggested an extrafollicular or incomplete germinal center response to SARS-CoV-2 vaccination, yielding a marked reduction in RBD-specific B cells.31 However, a dedicated follow-up study of the SARS-CoV-2 humoral immune response over time in PAD patients is needed to address the question of vaccine durability in this patient demographic. Finally, secondary immunosuppression, specifically recent B-cell depletion therapy, was the only persistent risk factor for a low (less than 100 U/mL) anti-spike antibody response after additional dose immunization. These data suggest that additional doses of immunization may not be an adequate strategy in this particular patient demographic, and consideration of alternate options such as tixagevimab/cilgavimab (Evusheld, AstraZeneca, Cambridge, UK) for prophylaxis may be warranted.
A limitation to this study includes potential confounding from immunoglobulin replacement that may contain antibodies to SARS-CoV-2.27 Our analysis demonstrated that no patients who received immunoglobulin replacement had prevaccine anti-spike antibodies that met criteria for a minimal threshold response to vaccination, which was defined in our study as 100 U/mL or greater. These data suggest that passive antibody transfer from immunoglobulin replacement therapy occurred, but at very low levels, at the time of this analysis. These data also highlight the importance of effective vaccine counseling in this patient demographic, because at the time of this study, immunoglobulin replacement alone did not confer large amounts of antibodies against SARS-CoV-2. It is expected that confounding from passive transfer of SARS-CoV-2 antibodies in immunoglobulin replacement will increase over time, making future studies more challenging. Other potential limitations were that this analysis was performed using data from a large but single health care system, so these findings may not be generalizable to other settings. In addition, there may be sampling bias in that differences may have existed between patients who consented to be a part of this study and those who did not. We found that SARS-CoV-2 anti-spike antibody levels trended toward higher in PAD patients who had received the mRNA-1273 (Moderna) vaccine compared with the other vaccine platforms. However, this was a retrospective analysis and patients were not assigned to vaccination platforms; therefore, there may have been selection bias in patients who chose specific vaccines.
Additional studies are needed to characterize the immune response of PAD patients after vaccination. In addition to the antibody responses analyzed here, T-cell response to vaccination may provide important cellular immune protection against severe infection, which we are unable to assess with these serologic data. T cells may confer long-lasting immune memory against coronavirus, which was reported in SARS-CoV-1 survivors.32 Moreover, even in the absence of neutralizing antibodies, there were reports of cellular immune response without seroconversion.33 Longitudinal studies are needed to evaluate the duration of response to vaccine to determine the optimal vaccination strategy, because antibody responses can wane over time.17
Our data provide new insights into the immune response to SARS-CoV-2 vaccination in patients with PAD. Patients with secondary and severe primary PAD developed lower antibody responses to SARS-CoV-2 vaccination, which improved after additional dose immunization for SARS-CoV-2. Certain immunophenotypic risk factors were associated with low response to vaccine (including low native antibody levels [IgG, IgA, and IgM], low native IgG antibodies for HIB, low CD4+ T helper cells, low CD19+ total B cells, and low class-switched memory [CD27+IgD/M–] B cells); however, after an additional dose vaccination, these patients reached anti-spike antibody levels comparable to those of the matched healthy control population after the initial series vaccination. This highlights the importance of careful monitoring in this particular subset of patients with a moderate to severe immune deficiency phenotype. It also underscores the importance of additional and booster dose vaccination in this patient population. Given the high morbidity from COVID-19 infection in this population, strategies to improve host immunity using booster vaccination should be considered in addition to maintaining precautions regarding COVID-19 infection.
Footnotes
S. Barmettler is supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award no. K23AI163350. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: J.R. Farmer holds investigator-initiated grants from Bristol Myers Squibb and Pfizer with no direct relation to the work presented. The rest of the authors declare that they have no relevant conflicts of interest.
Online Repository
.
Table E1.
Immunophenotype of predominant antibody deficiency patients
| Variable | IgG subclass deficiency (n = 3) | Specific antibody deficiency (n = 5) | Primary hypogammaglobulinemia (n = 4) | Common variable immunodeficiency (n = 21) | Complicated predominant antibody deficiency (n = 19) | Secondary hypogammaglobulinemia (n = 10) | P |
|---|---|---|---|---|---|---|---|
| Immunoglobulins, mg/dL (mean) | |||||||
| IgG | 662 | 795 | 659 | 465 | 570 | 648 | .38 |
| IgA | 155 | 144 | 158 | 68 | 33 | 320 | <.01 |
| IgM | 74 | 78 | 64 | 26 | 187 | 37 | .08 |
| IgG1 | 334 | 493 | 365 | 281 | 514 | 342 | .27 |
| IgG2 | 259 | 323 | 179 | 118 | 148 | 191 | .21 |
| IgG3 | 37 | 52 | 30 | 30 | 37 | 28 | .48 |
| IgG4 | 24 | 37 | 17 | 5 | 17 | 20 | .20 |
| Missing, n | (0) | (1-2) | (1-2) | (4-9) | (4-12) | (1-4) | |
| Antibody titers (mean) | |||||||
| Streptococcus pneumoniae (% >1.3 g/mL) | 81 | 62 | 58 | 43 | 40 | 73 | .054 |
| Haemophilus influenzae, mg/L | 0.6 | 0.9 | 1.1 | 0.7 | 2.3 | 0.6 | .59 |
| Tetanus, IU/mL | 1.5 | 2.2 | 1.4 | 1.2 | 0.8 | 1.2 | .18 |
| Diphtheria, IU/mL | 0.3 | 0.5 | 0.5 | 0.3 | 0.1 | 0.3 | .49 |
| Missing, n | (0) | (0-2) | (1-2) | (5-10) | (7-10) | (2-4) | |
| Flow cytometry (count of cells/μL, %) | |||||||
| CD3+ (% CD45+) | 1,569, 71 | 1,670, 71 | 1,271, 72 | 1,181, 71 | 983, 73 | 1,118, 71 | .31 |
| CD4+ (% CD45+) | 1,183, 54 | 928, 44 | 915, 52 | 819, 48 | 475, 37 | 612, 39 | <.01 |
| CD8+ (% CD45+) | 333, 15 | 599, 22 | 310, 29 | 317, 20 | 439, 31 | 431, 27 | .85 |
| CD3–CD16+56+ (%CD45+) | 188, 8 | 158, 10 | 269, 15 | 223, 12 | 143, 11 | 252, 22 | .33 |
| CD4+CD45RA+ (%CD4+) | 507, 43 | 473, 50 | 343, 40 | 385, 43 | 139, 27 | 326, 47 | <.01 |
| CD4+CD45RO+ (%CD4+) | 599, 51 | 360, 39 | 416, 49 | 413, 49 | 306, 67 | 265, 48 | .19 |
| CD8+CD45RA+ (%CD8+) | 202, 63 | 416, 64 | 133, 44 | 137, 50 | 246, 52 | 262, 59 | .53 |
| CD8+CD45RO+ (%CD8+) | 111, 30 | 130, 26 | 122, 40 | 109, 42 | 172, 39 | 147, 35 | .8 |
| CD19+ (% CD45+) | 439, 19 | 378, 15 | 230, 12 | 223, 14 | 170, 12 | 57, 4 | <.01 |
| CD19+CD27+ (%CD19+) | 25, 6 | 88, 17 | 36, 16 | 40, 18 | 42, 29 | 21, 14 | .69 |
| CD19+CD27+IgM/IgD– (%CD19+) | 9, 2 | 29, 5 | 13, 6 | 10, 5 | 4,4 | 9, 6 | .13 |
| CD19+CD27+IgM/IgD+ (%CD19+) | 16 | 60 | 6 | 32 | 33 | 15 | .65 |
| Missing, n | 0 | (1) | (1-2) | (0-7) | (0-6) | (2-5) | |
| Severity markers (n, % severe) | |||||||
| <20% CD45RA+ (%CD4+) | 1, 33 | 0 | 0 | 1, 6 | 5, 31 | 0 | .19 |
| Missing (n = 12) | |||||||
| <5% CD27+ (%CD19+) | 1, 33 | 1, 25 | 0 | 3, 18 | 2, 13 | 2, 50 | .45 |
| Missing (n = 16) | |||||||
| <10% CD27+ (%CD19+) | 3, 100 | 1, 25 | 2, 67 | 6, 35 | 7, 44 | 2, 50 | .39 |
| Missing (n = 16) | |||||||
| <2% CD27+IgM/IgD– (%CD19+) | 1, 33 | 2, 50 | 0, 0 | 7, 41 | 10, 63 | 2, 50 | .51 |
| Missing (n = 16) | |||||||
| <70% S pneumoniae (% >1.3 μg/mL) | 1, 33 | 2, 50 | 2, 67 | 14, 88 | 8, 73 | 2, 29 | .049 |
| Missing (n = 18) | |||||||
| <500 IgG (mg/dL) | 0 | 1, 25 | 1, 25 | 9, 53 | 6, 40 | 4, 44 | .67 |
| Missing (n = 10) | |||||||
| T cell function (n, % abnormal) | |||||||
| Anti-CD3 | 0 | 0 | 0 | 0 | 2, 20 | — | 0.67 |
| Missing (n = 41) | |||||||
| PHA | 0 | 0 | 0 | 0 | 2, 17 | — | 0.65 |
| Missing (n = 38) | |||||||
| PWM | 0 | 0 | 0 | 0 | 0 | — | — |
| Missing (n = 39) | |||||||
| Candida | 0 | 0 | 0 | 1, 13 | 1, 10 | — | 1 |
| Missing (n = 40) | |||||||
| Tetanus | 0 | 0 | 0 | 3, 38 | 6, 60 | — | 0.46 |
| Missing (n = 40) |
PHA, Phytohemagglutinin; PWM, Pokeweed mitogen.
Table E2.
Nucleocapsid antibody testing in predominant antibody deficiency patients (n = 24)
| Variable | Mean nucleocapsid antibody (cutoff index) | P |
|---|---|---|
| Intravenous immunoglobulin (n = 11) | 1.15 | |
| (Q1-Q3) | (0.09-2.2) | |
| Subcutaneous immunoglobulin (n = 14) | 0.19 | .047 |
| (Q1-Q3) | (0.09-0.27) | |
| No replacement immunoglobulin (n = 7) | 0.09 | |
| (Q1-Q3) | (0.086-0.095) |
Q1-Q3, Quartile 1 to Quartile 3.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delmonte O.M., Bergerson J.R.E., Burbelo P.D., Durkee-Shock J.R., Dobbs K., Bosticardo M., et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148:1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abo-Helo N., Muhammad E., Ghaben-Amara S., Panasoff J., Cohen S. Specific antibody response of patients with common variable immunodeficiency to BNT162b2 coronavirus disease 2019 vaccination. Ann Allergy Asthma Immunol. 2021;127:501–503. doi: 10.1016/j.anai.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squire J., Joshi A. Seroconversion after coronavirus disease 2019 vaccination in patients with immune deficiency. Ann Allergy Asthma Immunol. 2021;127:383–384. doi: 10.1016/j.anai.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delavari S., Abolhassani H., Abolnezhadian F., Babaha F., Iranparast S., Ahanchian H., et al. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J Clin Immunol. 2021;41:345–355. doi: 10.1007/s10875-020-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W., Wang W. Auto-antibodies against type I IFNs are associated with severe COVID-19 pneumonia. Signal Transduct Target Ther. 2021;6:96. doi: 10.1038/s41392-021-00514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I., et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.04.013. 211-3.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucciol G., Tangye S.G., Meyts I. Coronavirus disease 2019 in patients with inborn errors of immunity: lessons learned. Curr Opin Pediatr. 2021;33:648–656. doi: 10.1097/MOP.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus N., Frizinsky S., Hagin D., Ovadia A., Hanna S., Farkash M., et al. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol. 2020;11:614086. doi: 10.3389/fimmu.2020.614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudouris E.S., Pinto-Mariz F., Mendonca L.O., Aranda C.S., Guimaraes R.R., Kokron C., et al. Outcome of SARS-CoV-2 infection in 121 patients with inborn errors of immunity: a cross-sectional study. J Clin Immunol. 2021;41:1479–1489. doi: 10.1007/s10875-021-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonilla F.A., Barlan I., Chapel H., Costa-Carvalho B.T., Cunningham-Rundles C., de la Morena M.T., et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4:38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orange J.S., Ballow M., Stiehm E.R., Ballas Z.K., Chinen J., De La Morena M., et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 suppl):1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Bonilla F.A., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Hsu J.T., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186–1205. doi: 10.1016/j.jaci.2015.04.049. e1-78. [DOI] [PubMed] [Google Scholar]
- 19.Bousfiha A., Jeddane L., Picard C., Al-Herz W., Ailal F., Chatila T., et al. Human inborn errors of immunity: 2019 update of the IUIS Phenotypical Classification. J Clin Immunol. 2020;40:66–81. doi: 10.1007/s10875-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee T.K., Gereige J.D., Maglione P.J. State-of-the-art diagnostic evaluation of common variable immunodeficiency. Ann Allergy Asthma Immunol. 2021;127:19–27. doi: 10.1016/j.anai.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel E.U., Bloch E.M., Clarke W., Hsieh Y.H., Boon D., Eby Y., et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02257-20. e02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Acero R., Castelletti N., Fingerle V., Olbrich L., Bakuli A., Wolfel R., et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021;10:1505–1518. doi: 10.1007/s40121-021-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suhandynata R.T., Bevins N.J., Tran J.T., Huang D., Hoffman M.A., Lund K., et al. SARS-CoV-2 serology status detected by commercialized platforms distinguishes previous infection and vaccination adaptive immune responses. J Appl Lab Med. 2021;6:1109–1122. doi: 10.1093/jalm/jfab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naranbhai V., Garcia-Beltran W.F., Chang C.C., Mairena C.B., Thierauf J.C., Kirkpatrick G., et al. Comparative Immunogenicity and Effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 Vaccines. J Infect Dis. 2022;225:1141–1150. doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2523. doi: 10.1016/j.cell.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephenson K.E., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Truyers C., et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero C., Diez J.M., Gajardo R. Anti-SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products. Lancet Infect Dis. 2021;21:765–766. doi: 10.1016/S1473-3099(21)00059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer J.R., Ong M.S., Barmettler S., Yonker L.M., Fuleihan R., Sullivan K.E., et al. Common variable immunodeficiency non-infectious disease endotypes redefined using unbiased network clustering in large electronic datasets. Front Immunol. 2017;8:1740. doi: 10.3389/fimmu.2017.01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Moller B., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases COVID-19 vaccines for moderately to severely immunocompromised people. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html Accessed February 9, 2022.
- 31.Salinas A.F., Mortari E.P., Terreri S., Quintarelli C., Pulvirenti F., Di Cecca S., et al. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J Clin Immunol. 2021;41:1709–1722. doi: 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng O.W., Chia A., Tan A.T., Jadi R.S., Leong H.N., Bertoletti A., et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34:2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallais F., Velay A., Nazon C., Wendling M.J., Partisani M., Sibilia J., et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27:113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]