Abstract
Aortic stiffness increases with advancing age, more than doubling during the human life span, and is a robust predictor of cardiovascular disease (CVD) clinical events independent of traditional risk factors. The aorta increases in diameter and length to accommodate growing body size and cardiac output in youth, but in middle and older age the aorta continues to remodel to a larger diameter, thinning the pool of permanent elastin fibers, increasing intramural wall stress and resulting in the transfer of load bearing onto stiffer collagen fibers. Whereas aortic stiffening in early middle age may be a compensatory mechanism to normalize intramural wall stress and therefore theoretically “good” early in the life span, the negative clinical consequences of accelerated aortic stiffening beyond middle age far outweigh any earlier physiological benefit. Indeed, aortic stiffness and the loss of the “windkessel effect” with advancing age result in elevated pulsatile pressure and flow in downstream microvasculature that is associated with subclinical damage to high-flow, low-resistance organs such as brain, kidney, retina, and heart. The mechanisms of aortic stiffness include alterations in extracellular matrix proteins (collagen deposition, elastin fragmentation), increased arterial tone (oxidative stress and inflammation-related reduced vasodilators and augmented vasoconstrictors; enhanced sympathetic activity), arterial calcification, vascular smooth muscle cell stiffness, and extracellular matrix glycosaminoglycans. Given the rapidly aging population of the United States, aortic stiffening will likely contribute to substantial CVD burden over the next 2–3 decades unless new therapeutic targets and interventions are identified to prevent the potential avalanche of clinical sequelae related to age-related aortic stiffness.
Keywords: aging, arterial stiffness, blood pressure, cardiovascular disease, hypertension, pulse wave velocity
Introduction
The human aorta is a large conduit artery that exerts a critical cushioning function during the cardiac cycle by converting highly pulsatile flow from left ventricular (LV) ejection into steady, nonpulsatile flow in downstream capillaries while also limiting large fluctuations in pulse pressure (PP). This well-known “windkessel effect” occurs because of the unique mechanical properties of the proximal ascending aortic wall, which is a highly organized structure with an intimal single layer of endothelial cells and a mixed, complex medial layer of smooth muscle and extracellular matrix proteins. The matrix proteins include elastic proteins organized into a concentric elastic lamellar (40–70 in the ascending aorta) mesh consisting of cross-linked elastin and an outer layer of fibrillin microfibrils that contribute to the dynamic cushioning properties (1) and collagen fibers that contribute to the load bearing functions of the arterial wall at high distending blood pressures (2). Thus, this elastic-rich phenotype allows the aorta to distend and store energy of the stroke volume during systole and recoil during diastole, using the stored energy to drive blood flow downstream in a continuous fashion (1, 3).
The highly elastic phenotype of the proximal aorta changes in the thoracic and abdominal aorta, with the number of elastic lamellae decreasing and vascular smooth muscle cells increasing in the medial wall. This pattern continues in the medium-sized conduit arteries, with the medial layer wall dominated by vascular smooth muscle and collagen and lower abundance of elastic lamellae until the level of smaller resistance arteries and arterioles consisting largely of vascular smooth muscle (1, 4). This is important because elastin protein laid down in the medial wall is highly regulated and occurs in utero during fetal growth and in the first postnatal year before the genetic program for elastin synthesis is silenced (1, 5). The result is a fixed amount of medial elastin fibers for the remainder of the human life span (1, 6). However, the chronic cyclic stress of the pulsatile load of the systolic stroke volume leads to gradual fragmentation and loss of elastin protein with advancing age (7–9), contributing in part to the increase in mechanical intrinsic stiffness of the aortic wall.
With somatic growth early in the life span (preadolescence and adolescence) the aorta increases in diameter and length to accommodate growing body size and cardiac output, but in middle and older age the aorta continues to remodel to larger diameter at the expense of thinning the pool of permanent elastin fibers. This dilation and thinning of the aorta with aging increases intramural wall stress, resulting in the transfer of load bearing from thinning elastin onto stiffer collagen fibers and thus leading to aortic stiffening. Thus, aortic stiffening in early middle age may be a compensatory mechanism to normalize intramural wall stress; therefore aortic stiffening theoretically may be “good” at this stage of the life span. Indeed, aortic characteristic impedance (Zc), which is 5 times more sensitive to diameter than stiffness, declines up until early middle age despite a monotonic increase in wall stiffness because aortic dilation/remodeling to a larger diameter offsets the effects of increasing stiffness on Zc (3, 10). However, in late middle and older age, accelerated aortic stiffening likely overwhelms aortic dilation, evidenced by the parallel rise in Zc and carotid-femoral pulse wave velocity (PWV) after the age of 60 yr (3, 10, 11). Therefore, the negative clinical consequences of aortic stiffening in older age (see aortic stiffness and target organ damage) far outweigh any earlier physiological benefit to normalizing wall stress.
The influence of traditional risk factors (lipids, smoking, glycemia, adiposity) on the age-related increase in carotid-femoral PWV is surprisingly modest, with age and systolic blood pressure as the strongest determinants of aortic stiffness in older adults (12), and chronic exposure to hypertension (13) can accelerate aortic stiffness, leading to an early vascular aging phenotype (14, 15). But does a stiffer aorta portend an independent risk of CVD events beyond age and blood pressure? What are the physiological and hemodynamic sequelae of higher aortic stiffness that are associated with and contribute to CVD events or target organ damage that are not attributable to these risk factors? Incidentally, the aorta itself is a target organ that is continually exposed to hemodynamic cyclic stress and blood pressure that lead to vascular damage with advancing age. This review covers basic principles of aortic stiffness and related central pulsatile pressure and flow hemodynamics, the physiological and clinical consequences of aortic stiffness, sex differences in aortic stiffness and pulsatile hemodynamics, and basic mechanisms and modulators of aortic stiffness that lead us to conclude that it is, in fact, “bad” to have a stiff aorta.
Basic Principles and Measurement of Aortic Stiffness and Pulsatile Hemodynamics
Arterial wall stiffness, expressed as incremental elastic modulus (Einc) (also known as Young’s modulus), is defined as the resistance of wall material (in this case mainly in the tunica media) to deformation and can be quantified by the slope of the stress/strain relation ex vivo in isolated vessel preparations (2, 3). Hence, a stiffer arterial wall will have a higher Einc. Although this can be performed experimentally in ex vivo rodent aortic segments, measuring wall stiffness directly in vivo in humans can be estimated with the use of high-resolution ultrasound to assess local distensibility and wall thickness (see below for calculation on Einc in humans) (16). Thus, indirect expressions of arterial wall stiffness can be approximated by the Moens–Korteweg equation for PWV as the square root of the ratio of the Einc and wall (e.g., intimal-medial-adventitial) thickness (h) to vessel diameter (D) and blood density (ρ) assuming a constant h, D, and ρ.
Whereas total arterial compliance involves the cushioning properties of the aorta and entire arterial tree and can be approximated by the ratio of stroke volume to PP or estimated from the diastolic pressure decay, the local area compliance (AC) of an artery can be quantified from the ratio of the absolute change in arterial volume (or diameter) with concomitant changes of local pressure (or local PP) for each cardiac cycle and expressed in square millimeters per millimeter of mercury. The AC is defined as the slope of the pressure-area curve and can be computed in vivo as
where Dmin is the diastolic lumen diameter, Dmax is the systolic lumen diameter, and ΔP is the PP. Because this relation is nonlinear, at low pressure the AC is high because the medial elastin bears the distending pressure load, whereas at high pressures the load on the medial vascular wall is absorbed by stiffer collagen fibers, resulting in lower compliance (i.e., higher stiffness). However, this pressure-area nonlinearity is often ignored in clinical assessments because the pressure change over one cardiac cycle occurs over a narrow range (i.e., local PP). Furthermore, AC adjusted for baseline area (or diameter), which provides more useful clinical information, is the distensibility coefficient (DC), defined as the fractional change in AC, and can be computed as
For a uniform tube (artery), the PWV of a propagating pressure wave can also be approximated by the link between the DC of the artery and speed of the traveling pressure wave along the artery via the Bramwell–Hill equation, expressed as
where the DC is calculated from the relative change in lumen cross-sectional area (diameter) per unit change in local PP or the AC normalized for arterial cross-sectional area (2). Furthermore, Einc can be computed from local arterial DC and wall intimal-medial thickness (IMT), the latter used as a surrogate for wall cross-sectional area (16):
In simple terms, PWV of the propagating pressure wave can be computed from speed (or transit time, τ) of the pressure pulse along a specific arterial segment (path length, L) via the equation
In general, a pressure wave recording with an invasive catheter or noninvasive applanation tonometer at two discrete points along an arterial segment can be used to calculate PWV. In rodents, pressure waveforms (via tonometry) (17) or flow waveforms (via ultrasound Doppler) (18–20) can be measured at the aortic arch and abdominal aorta to calculate aortic PWV and are well documented to increase with advancing age. In humans, because access to the aortic arch is difficult, the contemporary measurement of aortic stiffness is via carotid-femoral PWV, whereby pressure waveforms are recorded at the common carotid artery and the femoral artery. This is done by recoding the two pressure waveforms simultaneously and calculating the τ between the diastolic foot of the carotid and femoral waveforms or sequentially by R-wave gating the diastolic foot of each pressure waveform to the ECG, in which τ is computed as the time difference between each pressure waveform and the R wave as a fiducial point on the ECG. One major limitation in humans is that the carotid-femoral PWV does not take the highly elastic proximal ascending aorta into account. This occurs because the pressure wave generated by systolic ejection has already propagated past the proximal segment of the aorta when the common carotid pressure waveform is captured with the applanation tonometer. To account for this parallel transmission, the aortic L can be estimated by measuring the body surface distance between the suprasternal notch and the femoral sampling site and subtracting out the distance between the suprasternal notch and carotid sampling site. Alternatively, the direct carotid-to-femoral distance can be used for L and then multiplying (τ/L) × 0.8 (21).
In general, a stiffer aorta results in a faster forward-traveling pressure wave and subsequently higher carotid-femoral PWV. However, the PWV is highly influenced by the distending pressure and is the average of a wide range of regional PWVs from arterial segments differing in diameter and mechanical stiffness. For example, the ascending aorta, proximal descending aorta, and arch are more elastic segments containing high amounts of elastin protein, whereas more distal, muscular aortic segments and its branches (e.g., distal descending aorta, iliac and femoral conduit arteries) have less elastic wall properties. Of note, distending pressure historically has been expressed as the mean arterial pressure, and studies generally statistically adjust for differences in mean arterial pressure (22), but recently some have argued that diastolic blood pressure is more appropriate to approximate distending pressure (23). Indeed, diastolic blood pressure may better represent distending pressure because the pulse transit time for PWV calculation is estimated from the diastolic foot of the pressure wave, and the heart rate dependence of PWV is less pressure dependent and smaller than when corrected for mean arterial pressure (23).
High aortic stiffness is also associated with greater forward pressure amplitude (Pf), and subsequently a faster reflected or backward (Pb) pressure wave with greater amplitude returning from the periphery at the same velocity, thus adding to the second pressure peak in late systole at the ascending aorta. This late systolic boost, known as augmentation pressure, can be expressed as the augmentation index computed as the ratio of augmentation pressure to the pulse pressure and expressed as a percentage (augmentation pressure/PP × 100) (24). However, the aortic augmentation index has multiple limitations that make it a questionable index of aortic stiffness and/or wave reflections (25). Augmentation index is strongly inversely related to heart rate, height, and LV contractility and ejection duration (26). Indeed, heart failure patients who have stiff aortas generally have lower augmentation index than age-matched control subjects because of the short ejection period and reduced contractility (27, 28). Additionally, augmentation index increases with age up until middle age but then plateaus and even decreases in the 7th and 8th decades because the PP (denominator in the augmentation index equation) increases to a greater extent (as a result of greater Pf) than the augmentation pressure (11, 29, 30). It should be noted that the dogma that backward-traveling waves return to the ascending aorta during diastole in individuals with a very compliant aorta (e.g., young, healthy adults) and then shift the early arrival in diastole to later in systole as the aorta stiffens with aging and/or hypertension has been called into question (31). A meta-analysis of timing of reflected waves revealed that most reflected waves return in systole, even in younger persons, and that the reflected wave shifts only a small duration per decade during systole (31). However, the amplitude of Pb in systole likely does increase as the aorta stiffens (whether or not the timing of the return shifts), consistent with the augmentation of the second systolic peak of aortic systolic blood pressure waveform in late systole.
Alternatively, a more reliable way to characterize the amount of reflection in the aorta is to calculate the reflection coefficient (RC) as the ratio of Pb to Pf using wave separation analysis (32). By estimating aortic flow with standard echocardiographic methods using LV outflow tract area and pulse Doppler waveforms and obtaining central pressure waveforms in the common carotid artery (as a surrogate for aorta), the proximal aortic Zc, which describes the change in pressure generated for a given change in flow in the proximal ascending aorta in early systole before arrival of reflected pressure waves, can be computed in the time domain as the ratio of aortic Pf and flow (Qf), expressed as Zc = ΔPf/ΔQf. Aortic Zc is determined by the aortic diameter (inversely related) and mechanical wall stiffness (directly related) but, as mentioned above, is exquisitely more sensitive to lumen diameter than wall stiffness (33). The RC determines the amount of pressure wave reflection when the forward-traveling pressure wave encounters changes in Zc along the aorta, known as “impedance mismatch.” Higher local RCs result in more reflection at that particular site, but it is important to note that there are multiple sites of reflection (impedance mismatch) along the arterial tree and hence numerous reflected waves are generated along any arterial segment rather than at one discrete reflection site (34). It is hypothesized that although at each site of impedance mismatch a portion of the forward wave is reflected back toward the ascending aorta, when the backward-traveling wave reaches a site of impedance mismatch that wave is rereflected forward, resulting in dissipation of the reflected waves, especially those from the most distal reflecting sites (34). Nonetheless, the rise in PP that occurs with advancing age, in particular the exponential increase beyond middle age, is driven largely by augmented aortic Pf as a result of higher Zc rather than any effect from Pb (11, 30). Moreover, differences in aortic diameter and Zc may account in part for the higher PP in older women compared with men, thus explaining the well-described sex differences in PP with advancing age (see sex differences in aortic stiffness and pulsatile hemodynamics) (30, 33, 35).
Clinical and Physiological Consequences of Aortic Stiffness
Aortic stiffness, measured by carotid-femoral PWV, is a robust independent predictor of future clinical CVD events (e.g., nonfatal myocardial infarction, angina, stroke, mortality) in adults with hypertension (36, 37) and end-stage kidney disease (38, 39) and healthy middle-aged and older adults in the community (40–42). Consistent with these individual studies, meta-analyses of individual data from 17,635 participants from 16 studies demonstrate that aortic PWV was associated with 23%, 28%, and 30% higher risk of coronary heart disease, stroke, and nonfatal CVD events, respectively, after adjustment for all conventional CVD risk factors (43). Interestingly, the predictive value of carotid-femoral PWV was stronger in younger compared with older adults. This may be because of a “healthy survivor effect” (older adults with stiff aorta die younger) or because other CVD risk factors such as systolic hypertension become a stronger contributor to CVD risk that parallels aortic stiffness, whereas in younger adults with normal systolic blood pressure a stiffer aorta for age and sex may contribute to higher CVD risk. Moreover, carotid-femoral PWV improved reclassification of individuals at intermediate risk to either low or higher 10-yr risk, thus indicating that carotid-femoral PWV adds clinical value to CVD risk stratification (43). Furthermore, one recent study from the Framingham Heart Study not included in the meta-analysis demonstrated that aortic Pf was the best predictor of CVD events even after carotid-femoral PWV and systolic blood pressure were included in the model, whereas mean arterial pressure and wave reflection were not predictors (44). Thus, these intriguing data suggest that aortic Pf may be a novel hemodynamic marker of CVD risk, illustrating that pulsatile components of the hemodynamic pressure load (determined by aortic stiffness and function), rather than the steady component (determined by small artery resistance), may be the best determinants of CVD risk in middle-aged and older adults (44). Taken together, there is strong evidence that aortic stiffness and the resulting abnormal pulsatile hemodynamics from inadequate pressure buffering correlate with, and possibly contribute to, risk of developing CVD.
So how might higher aortic stiffness lead to a greater propensity to develop clinical CVD? The physiological consequences of aortic stiffening with aging can best be demonstrated by the changes in systolic, diastolic, and pulse pressure over the life span. Data from the Framingham Heart Study (11, 45) and others (29) clearly demonstrate a slow monotonic increase in systolic, diastolic, and mean blood pressure from adolescence through middle age, followed by an accelerated increase in systolic blood pressure after age 60 yr into the 7th and 8th decades and a parallel decrease in diastolic blood pressure, while mean arterial pressure plateaus. Subsequently, these patterns of systolic and diastolic blood pressure result in an exponential rise in PP beyond middle age and occur in parallel with increases in carotid-femoral PWV and aortic Zc (11). Therefore, loss of windkessel properties of the aorta, and possibly subtle changes in resistance, preload, and LV contractility (46), contribute cumulatively to the widening of PP observed with advancing age, likely explaining in part the high prevalence of the isolated systolic hypertension phenotype (45, 47) and the strong association of PP with CVD (48) among older adults (FIGURE 1).
FIGURE 1.
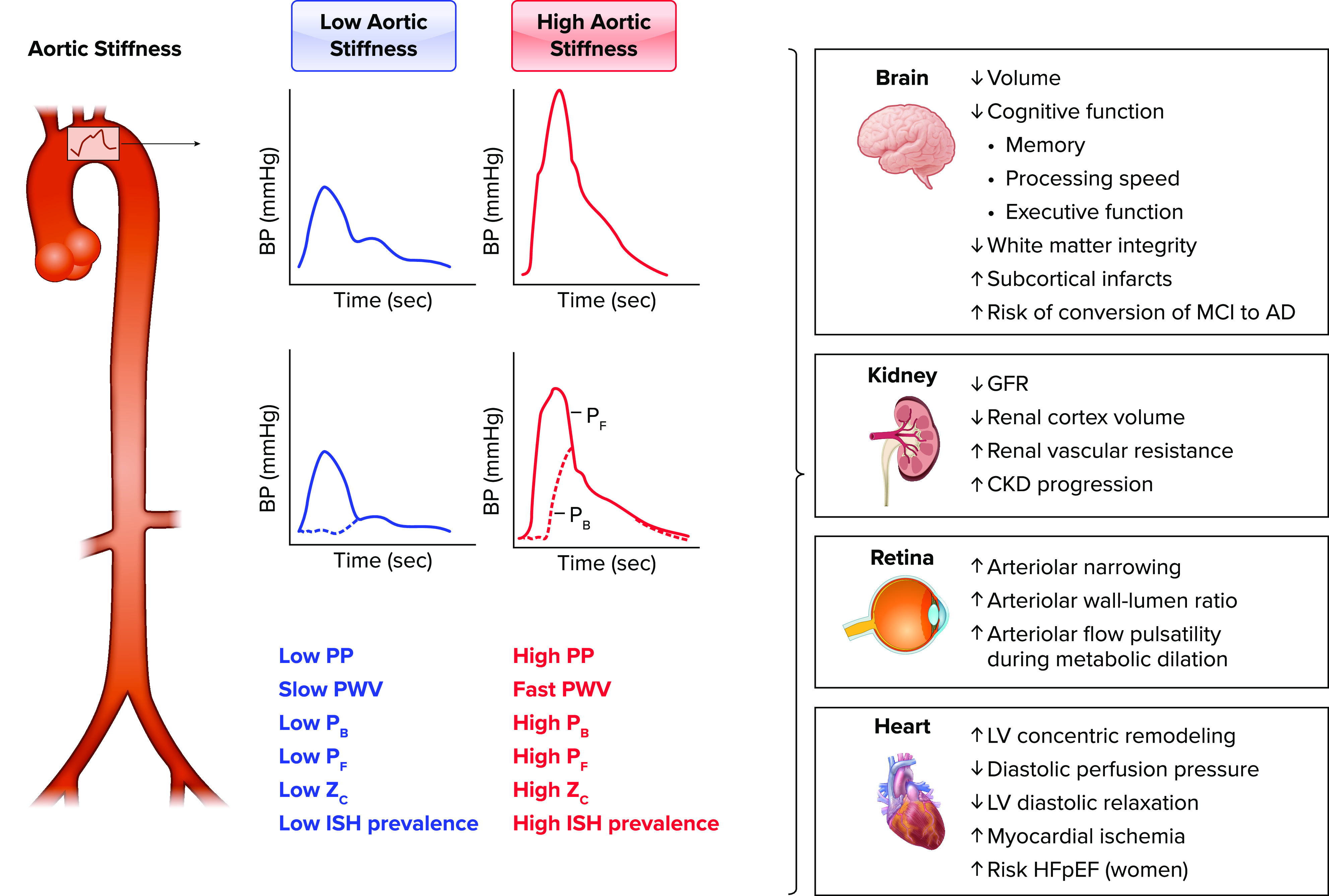
Elevated aortic stiffness is associated with target organ damage to brain, kidney, retina, and heart Low aortic stiffness is associated with low aortic pulse pressure (PP), slow carotid-femoral pulse wave velocity (PWV), low amplitude of reflected or backward reflected waves (Pb), low forward pressure wave amplitude (Pf), low aortic characteristic impedance (Zc), and low prevalence of isolated systolic hypertension (ISH), whereas high aortic stiffness is associated with high aortic PP, fast carotid-femoral PWV, high Pb, high Pf, high aortic Zc, and higher prevalence of ISH. Higher aortic stiffness is associated with decreases in brain volume, decline in cognitive functions including memory, processing speed, and executive function, reduced white matter integrity, increased subcortical infarcts, and higher risk of conversion of mild cognitive impairment (MCI) to Alzheimer’s disease (AD); kidney damage including lower glomerular filtration rate (GFR), decreased renal cortex volume, elevated renal vascular resistance, and progression of chronic kidney disease (CKD); retinal remodeling including increased arteriolar narrowing, increased arteriolar wall-lumen ratio, and increased flow pulsatility during local metabolic vasodilation; and adverse heart outcomes including increased left ventricular (LV) concentric remodeling, decreased diastolic perfusion pressure, increased myocardial ischemia, and higher risk of heart failure with preserved ejection fraction (HFpEF) among women. BP, blood pressure.
Aortic Stiffness and Target Organ Damage
Higher aortic stiffness is associated with subclinical target organ damage specifically of high-flow, low-resistance organs, such as the brain, kidney, retina, and heart (2) (FIGURE 1). The excessive pulsatile energy (pulsatile pressure × flow) resulting from a stiffer aorta with diminished pressure buffering capabilities leads to an augmented Pf that penetrates into the downstream microvasculature, resulting in both barotrauma (from high pressure pulsatility) and excessive blood flow shear forces. This excessive pulsatile energy is detrimental to the fragile microvasculature, and organs most susceptible to these deleterious effects are characterized by high-flow and low-impedance circulations (brain, kidney, retina, and heart, as above), allowing a wide-open path for the highly pulsatile energy to enter these end-organs. In addition, pulsatility in small arteries and arterioles may impact the ability of the myogenic response to properly regulate high and low pressure shifts putting capillaries and small arteries at risk of barotrauma. One popular hypothesis that has been advanced is that the relatively compliant aorta (and hence low Zc) results in substantial impedance mismatch with stiffer, narrower distal muscular arteries including first-order generation branches (for example, common carotid or renal artery) that prevents dangerous forward pulsatile power into the target organ microvasculature (49, 50). However, this theory has been called into question recently because local amplification of the PP into target organs is proportional to the local RC, which is low at these bifurcations and therefore should result in little wave reflection, not more (2, 51). Also, impedance matching between aorta and these first-order daughter vessels appears to be well optimized for forward wave transmission because of similar geometry (lumen diameter), and subsequently wave reflection at first-order branches appears to be minimal (2, 51, 52). Nonetheless, with aortic stiffening increased pulsatile power does occur and the excessive pressure and flow pulsatility results in microvascular remodeling and damage in these high-flow organs (2, 10). How higher aortic stiffness and pulsatile hemodynamics may result in adverse alterations to brain structure and cognitive function, kidney function, and retinal artery and LV structure and function is summarized below.
The Brain
The cerebral microvasculature is particularly sensitive to the transmission of excessive pulsatile power because it is characterized by low vascular resistance to maintain a constant high rate of perfusion (∼20% of cardiac output) to the highly metabolic brain. As described above, elevated aortic stiffness and increased pulsatile hemodynamics are associated with lower cognitive performance, including reductions in global cognition, processing speed, memory, and executive function (53–66) as well as greater longitudinal declines in cognition (62, 66–70) (FIGURE 1). Reductions in cognitive performance associated with large central artery (i.e., aorta and/or common carotid artery) stiffness are mediated, in part, through the development of cerebrovascular dysfunction and neuropathology. Elevated PP rises in parallel with age-related increases in carotid-femoral PWV and cerebral blood flow velocity pulsatility (71). Increased pulsatile pressure transmitted into the brain microvasculature is not fully buffered by cerebral autoregulation (72) and is the primary stimulus for cerebrovascular remodeling (increased wall thickness-to-lumen ratio) rather than mean pressure (73, 74). Indeed, in a reduced large artery elastin (haploinsufficient in Eln+/−) mouse model of large artery stiffening in the absence of comorbidities (aging, diabetes), large central artery stiffness selectively induces microvascular endothelial dysfunction and augmented vasoconstrictor responsiveness in cerebral arteries that could be responsible for diminished autoregulation in the brain (75, 76). As such, this remodeling likely occurs to increase cerebrovascular resistance (77) in an attempt to limit downstream capillary exposure to chronic pulsatile energy, at the expense of reductions in vascular endothelial function (78) and cerebral blood flow regulation that promotes cerebral hypoperfusion manifested as reduced basal cerebral blood flow and impaired cerebrovascular reserve in aging (79–81) (FIGURE 1).
Importantly, although the majority of research examining relations between large central artery stiffness and the brain have focused on aortic stiffness measured by carotid-femoral PWV or aortic Zc, evidence supports the importance of the evaluation of both aortic and common carotid artery stiffness as a “hemodynamic unit” to consider the relative stiffness or impedance gradient (or degree of mismatch) between the proximal aorta and common carotid arteries (57, 82). The stiffness or Zc of the common carotid arteries is higher compared with the proximal aorta when the aorta is relatively compliant (for example, in youth) because of differences in vessel wall properties (carotid has less elastin than proximal aorta), smaller arterial diameter, and differences in trajectories of stiffening with aging (83, 84), which produces a relative impedance mismatch at the proximal aortic-common carotid artery junction (82). This relative impedance gradient at the aorta-carotid interface, as well as rereflected waves returning from the distal carotid circulation (circle of Willis) that encounter strong negative rereflection RC at the proximal carotid, acts to reduce the transmission of pulsatile power into the first-generation branch artery (e.g., common carotid arteries) and into the downstream cerebrovasculature when the aorta is compliant, thus protecting the brain from harmful pulsatile pressure and flow (57, 82). With aortic stiffening and therefore increase in RC of the proximal aorta, the impedance gradient between the aorta-carotid interface is attenuated, allowing for more forward pulsatile power to penetrate the cerebral microvasculature. Collectively, these data underscore the importance of the relative contributions of both the aortic and common carotid arteries and related pulsatile hemodynamics necessary to comprehensively evaluate central artery stiffness contributions to brain aging.
Subcortical regions of the brain including the deep white matter and hippocampus, a region important for memory consolidation, are particularly sensitive to the effects of aortic stiffness because they are perfused by short penetrating vessels that branch directly off the circle of Willis. Barotrauma associated with greater transmission of pulsatile flow and pressure to the subcortical microvasculature increases the risk of microvascular ischemic injury (85) and increased white matter hyperintensity burden, e.g., subcortical infarcts (65, 77, 86), a hallmark pathology of cerebral small vessel disease (FIGURE 1). However, mounting evidence also supports a link between aortic stiffness and an increased risk for the development of Alzheimer’s disease and for the conversion from mild cognitive impairment to Alzheimer’s disease (87, 88). Interestingly, individuals with a carotid-femoral PWV > 11.4 m/s are at highest risk for converting from mild cognitive impairment to Alzheimer’s disease, consistent with the Framingham Heart Study data that demonstrate a markedly increase in overall CVD risk above this threshold of carotid-femoral PWV (41, 88) (FIGURE 1). Additionally, increased aortic stiffness is associated with increased odds for the development of cerebral small vessel disease and a positive β-amyloid scan (89). Although the presence of cerebral small vessel disease lowers the threshold of β-amyloid required to induce cognitive dysfunction (89, 90), in vitro work suggests that exposure of cerebral endothelial cells to increasing physiological levels of pulsatile stretch, such as occurs with increased aortic stiffness, upregulates the production of β-amyloid and its upstream regulators (91). This highlights a potentially independent role of aortic stiffness in Alzheimer’s disease neuropathology. Taken together, these studies demonstrate a clear link between elevated aortic stiffness, pulsatile hemodynamics and cognitive impairment, white matter subcortical disease, and potentially faster progression to Alzheimer’s disease. Further mechanistic work is needed not only to confirm these associations but also to identify potential targets for screening, early detection, and prevention of cognitive impairment and Alzheimer’s disease in older adults.
Kidney
The kidney has the highest flow rate and low resistance of any large organ, averaging ∼400 mL/min/100 g tissue or 25% of the cardiac output (2). The kidney allows for filtration of 180 L of blood per day through the glomerular capillaries to remove waste and produce urine in the renal tubules. Vascular resistance from the afferent to efferent arterioles increases minimally across the glomerulus to promote high glomerular filtration rate (GFR) but occurs at the risk of exposing the glomerulus to high mean and pulsatile pressures under healthy physiological conditions that are exaggerated in the presence of increased aortic stiffness (92). Accordingly, increased aortic stiffness is associated with lower estimated GFR and greater longitudinal declines in GFR (93), and this is related to reduced afferent arteriole autoregulation (94) and rarefaction of the microvasculature in the renal cortex (93, 95, 96) in patients with hypertension and chronic kidney disease (CKD) (FIGURE 1). Data from the AGES-Reykjavik cohort indicate that higher carotid-femoral PWV was associated with greater renal artery flow pulsatility measured by phase-contrast MR and lower microvascular volume in the renal cortex (96). Moreover, mediation analysis demonstrated that about one-third of the relation between carotid-femoral PWV and reduced GFR was mediated through higher renal artery flow pulsatility and another 20–30% by lower cortex volume and increased renal vascular resistance. Taken together, these data are consistent with the idea that aortic stiffness-associated flow pulsatility in the renal artery leads to downstream microvascular damage/remodeling in the renal cortex, such as rarefaction, vasoconstriction, and glomerulus damage, resulting in worsening of GFR (FIGURE 1).
Consistent with these physiological findings, epidemiology data show that higher aortic stiffness and PP are associated with a steeper decline in GFR over an 11-yr follow-up and 16% higher risk of incident CKD (adjusted for age, sex, mean arterial pressure, GFR) in middle-aged and older adults without CKD at baseline (97). Furthermore, among middle-aged and older patients with CKD at baseline, higher carotid-femoral PWV was an independent predictor of CKD progression and death after 5 yr of follow-up in the Chronic Renal Insufficiency Cohort study (98) (FIGURE 1). Furthermore, it is important to note that CKD can also augment aortic stiffness through dysregulation of bone metabolism and vascular calcification, impaired sodium regulation, and increased inflammation and sympathetic nerve activity, which likely further exacerbate kidney dysfunction in a feedforward vicious cycle (2, 99).
Retina
The retinal and optic nerve head circulation maintains constant blood flow over a wide range of perfusion pressures. They alter flow via metabolic autoregulation to meet the retinal and optic nerve head metabolic demands and in response to changes in arterial oxygen and carbon dioxide content (100). The retinal microcirculation is also highly sensitive to chronic elevations in blood pressure and aortic stiffness. Chronic hypertension can lead to hypertensive retinopathy characterized by general arteriolar narrowing in early stages on routine dilated fundoscopic exam, followed by more advanced pathophysiological alterations such as retinal hemorrhage, cotton wool spots, arterio-venous nicking, and retinal edema (101) (FIGURE 1). Ex vivo examination of small arteries and arterioles demonstrates two characteristic structural phenotypes in response to hypertension and reflex chronic vasoconstriction and vascular resistance, 1) “inward eutropic” remodeling, characterized by inward remodeling of vascular smooth muscle cells around a smaller lumen and external diameter (without hypertrophy), or 2) “outward hypertrophic” remodeling, where hyperplasia of vascular smooth muscle cells encroaches on lumen diameter, perhaps to reduce wall stress, where both mechanisms result in increased wall-to-lumen ratio (74, 101). Interestingly, the higher PP, not mean arterial pressure, is tightly correlated with cerebrovascular arteriole wall hypertrophy (e.g., cross-sectional area) in rodent hypertension models (74), suggesting that pressure pulsatility rather than mean pressure is the primary hemodynamic stimulus for microvascular remodeling in cerebral and, likely, retinal arterioles. In humans, more advanced retinal imaging via scanning laser Doppler flowmetry demonstrates that central PP, and not mean arterial pressure, is an independent determinant of retinal arteriolar remodeling (expressed as retinal media-to-lumen ratio) among middle-aged and older adults (102). In addition, both aortic stiffness (103–105) and carotid stiffness (106, 107) are associated with greater retinal arteriolar narrowing in healthy middle-aged and older adults with and without hypertension independent of blood pressure and other CVD risk factors. Finally, although penetration of pulsatile pressure and flow into retinal microcirculation is the purported hemodynamic link between aortic stiffness and retinal microvascular remodeling, no studies had directly tested whether pulsatile flow in retinal circulation is directly linked with aortic stiffness until recently. Using laser speckle flowgraphy, Holwerda et al. (108) found that although aortic stiffness was not associated with basal retinal arteriole flow pulsatility, aortic stiffness became associated with higher flow pulsatility following local metabolic vasodilation of the retinal circulation during a light flicker stimulus (FIGURE 1). Thus, these data suggest that adverse effects of aortic stiffness are unmasked in the retinal circulation only when local vascular resistance is acutely decreased via metabolic vasodilation. Taken together, aortic and carotid stiffness, perhaps through elevated PP, are associated with and possibly contribute to retinal arteriolar remodeling (narrowing) and flow pulsatility (only under local metabolic vasodilation) independent of mean arterial pressure and other CVD risk factors.
Heart
Stiffening of the proximal aorta, leading to higher aortic Zc, increases the pulsatile hemodynamic load on the heart by impairing optimal ventricular-arterial coupling through 1) augmenting the early systolic pressure rise in LV and aorta, which is a critical determinant of peak myocardial wall stress and subsequently myocardial oxygen consumption, and 2) shifting the timing of the arrival of reflected waves to the ascending aorta during mid-to-late systole instead of diastole, which raises mid-to-late systolic afterload and decreases diastolic perfusion pressure. It is important to recall that myocardial wall stress is a time-varying phenomenon during the cardiac cycle and peaks in early systole because wall stress determinants such as LV chamber size and wall thickness are maximal and minimal, respectively, so that an augmented early systolic LV pressure rise from an elevated proximal aortic Zc leads to greater myocardial oxygen consumption even before the arrival of reflected waves in mid-to-late systole. In this regard, the shift in arrival of reflected waves from diastole to mid-to-late systole (although this concept is debated in the field, as mentioned above) or greater amplitude of Pf, augments aortic systolic and PP and therefore increases late systolic pulsatile load. Indeed, both aortic stiffness and PP are predictive of LV mass independent of mean arterial pressure (109) and incident heart failure among middle-aged/older adults in most (110, 111), but not all (112), studies. Specifically, elevated late systolic pulsatile load is associated with LV “concentric” hypertrophy and fibrosis in preclinical models (113) and human studies (114, 115), with impaired LV diastolic relaxation and diastolic dysfunction (116–118) and increased risk of incident heart failure (119, 120), particularly heart failure with preserved ejection fraction (HFpEF) in women (FIGURE 1). Finally, the potential shift of reflected waves from diastole to earlier into systole in part from aortic stiffness, along with a steeper decline in diastolic pressure decay from lower total arterial compliance (121), contributes to a reduction in diastolic perfusion pressure and hence increasing risk of myocardial ischemia (122) (FIGURE 1). Taken together, augmentation of early systolic myocardial wall stress, elevated late systolic pulsatile load, and diminished diastolic perfusion pressure lead to a “hemodynamic milieu” primed for promoting LV hypertrophy/remodeling, myocardial ischemia, and heart failure with aging (123) (FIGURE 1).
Sex Differences in Aortic Stiffness and Pulsatile Hemodynamics
Current evidence suggests that significant sex differences exist in aortic stiffness and pulsatile hemodynamics, varying through the life span (35, 124, 125). This variation suggests some relation to endogenous female sex hormones, which are known to promote endothelial and vascular health, although the physiological basis for these sex differences is likely to be more complex than what can be attributed to sex hormones alone. Supporting the hormonal theory, previous studies report that prepubertal girls have stiffer aortas (e.g., higher carotid-femoral PWV) and higher PP than age-matched boys. However, once puberty occurred, carotid-femoral PWV of the girls decreased while it increased in the boys, abolishing any prepubertal differences. PP in the girls also became lower than that in the boys (126). Through the reproductive years (i.e., before age 50 yr, which is the average age at onset of menopause), adult women have similar carotid-femoral PWV and aortic Zc and lower brachial and central systolic and pulse pressures compared with men (11). Although one smaller study found lower Zc in young women compared with men, women showed an accelerated increase with aging (127); in general, women enjoy lower prevalence of hypertension up to the 6th decade of life (128). During these premenopausal years, use of oral contraceptives is associated with greater carotid-femoral PWV and PP in women compared with no contraceptive use (129), supporting the theory that suppression of endogenous female sex hormones might affect arterial function and hemodynamics.
With the onset of nonsurgical menopause, marked by natural withdrawal of female sex hormones, women start to experience hemodynamic consequences of arterial aging at a faster rate than age-matched men. Although carotid-femoral PWV increases similarly in aging men and women (11, 30), differences in aortic pulsatile arterial load and wave reflection become more prominent in women than men after the age of 50–60 yr (11, 35). Changes in the contour of the central arterial pulse between men and women can be observed from the 4th decade on, when women start to develop a more prominent second systolic peak and higher augmentation index compared with men (130). In addition, age-related increases in Zc and PP become exacerbated in women during this time (11, 35). Suggesting a relation with hormonal status, hormonal replacement therapy increases aortic compliance (131) and lowers blood pressure (132) in postmenopausal women, with a notable decrease in aortic compliance again after hormone replacement therapy is withdrawn for 4 weeks (133).
These more prominent age-related increases in aortic pulsatile load and PP in women have direct clinical implications that are intrinsically related to cardiovascular conditions that predominantly affect women. Given the exacerbated increases in systolic blood pressure and PP, middle-aged and older women have greater prevalence of hypertension, specifically isolated systolic hypertension (ISH), than men (134). Interestingly, recent data suggest that CVD event risk is increased in women at lower systolic blood pressure thresholds compared with men, as demonstrated by a similar hazard ratio in women with a systolic blood pressure of 100–109 mmHg compared with 130–139 mmHg in men (135). This has important clinical implications because ISH is present in 77% of individuals with refractory hypertension (136) and uncontrolled hypertension is nearly twice as common in women than men (30% vs. 17%, respectively) (137). In addition, in older women only, abnormalities in aortic stiffness and pulsatile arterial load such as lower arterial compliance and higher carotid-femoral PWV and aortic Zc are associated with several cardiovascular abnormalities of the HFpEF syndrome, such as alterations in diastolic function and ventricular-arterial coupling (124), increased concentric LV remodeling (138), and greater burden of epicardial (125, 139) and microvascular (125, 139) coronary artery disease. This is important because HFpEF is more than twice as common in women than men (140) (FIGURE 1). The sex differences in age-related pulsatile hemodynamics also play a role in paradoxical low-flow, low-gradient, normal-ejection fraction severe aortic stenosis. This is a condition that is most commonly found in older women with hypertension (141) and characterized by low aortic compliance and high PP (141, 142), as some of the factors limiting transaortic gradients in affected patients.
Several mechanisms for the sex differences in arterial aging and pulsatile hemodynamics have been postulated. Mitchell et al. (35) have proposed that women have greater mismatch between aortic flow and diameter than men, resulting in higher aortic Zc contributing to greater PP in older women. However, additional studies suggest that sex differences in pulsatile hemodynamics go beyond a simple comparison of aortic size between men and women. Although absolute aortic diameters are lower in women, when indexed to body surface area, women actually have proportionally greater increases in aortic size with aging than men (143). Additionally, sex differences in aortic Zc and PP in older individuals persist despite model adjustment for body surface area and ascending aortic diameter, confirming intrinsic differences in pulsatile hemodynamics that are independent from sex differences in aortic size. Alternatively, several other mechanisms for sex differences in aortic stiffness and pulsatile hemodynamics have been proposed, including a more rapid decrease in endothelium-dependent nitric oxide (NO) bioavailability in older women. Importantly, although the age-related decline in endothelial function is delayed by about a decade in women compared with men, once menopause ensues the deterioration in endothelial function is steeper in women than in men (144). Indeed, endothelial function progressively declines across the stages of menopause transition with greater reductions in the late perimenopausal and early postmenopausal phases, and is related to increased oxidative stress in part from the loss of estradiol (145). In addition, increased oxidative stress may also contribute to vascular aging in women; ascorbic acid, a potent free radical scavenger, can partially reverse cross-sectional differences in carotid compliance by menopause stage (146) but has no effect on carotid compliance or aortic stiffness (carotid-femoral PWV) in older men (147) (see more details on mechanisms below). Importantly, although it has been debated whether the postmenopausal ovary continues to secrete androgens, the decline in androgens appears to be associated with aging rather than menopause per se (148), and increased availability of unopposed androgens in the postmenopausal period may also contribute to accelerated arterial aging, as increased androgenicity promotes extracellular matrix remodeling with deterioration of the elastin-to-collagen ratio in the aortic wall (149). This concept has been confirmed by Georgiopoulos et al. (150), who studied 180 postmenopausal women without CVD or diabetes followed for 29 mo and demonstrated that increased free androgen index at baseline was associated with greater increases in carotid-femoral PWV and PP, decreases in endothelial function, and 2.7-fold increased odds for development of hypertension. However, separating the effects of menopause from aging is difficult because they coincide with each other and menopause and aging both produce changes in other CVD risk factors that affect arterial stiffness. Moreover, the menopause transition is characterized by changes in multiple sex hormones that may affect arterial function with aging in women.
In summary, sex differences in aortic stiffness and pulsatile hemodynamics are present throughout the life span, hemodynamically benefiting women during the postpubertal and premenopausal years and then adversely affecting women in the postmenopausal period. The accelerated deteriorations in aortic pulsatile load measures and PP in older women have direct relations to several cardiovascular abnormalities that predominantly affect women and can be partially explained by several factors including alterations in sex hormones on vascular function including the deterioration in endothelial function and mismatch between aortic flow (e.g., stroke volume) and aortic size. Improved understanding of the mechanisms contributing to the accelerated deterioration of aortic stiffness and pulsatile hemodynamics after menopause will have significant potential in identifying methods for screening, early detection, and prevention of CVDs in women.
Mechanisms and Modulators of Aortic Stiffness
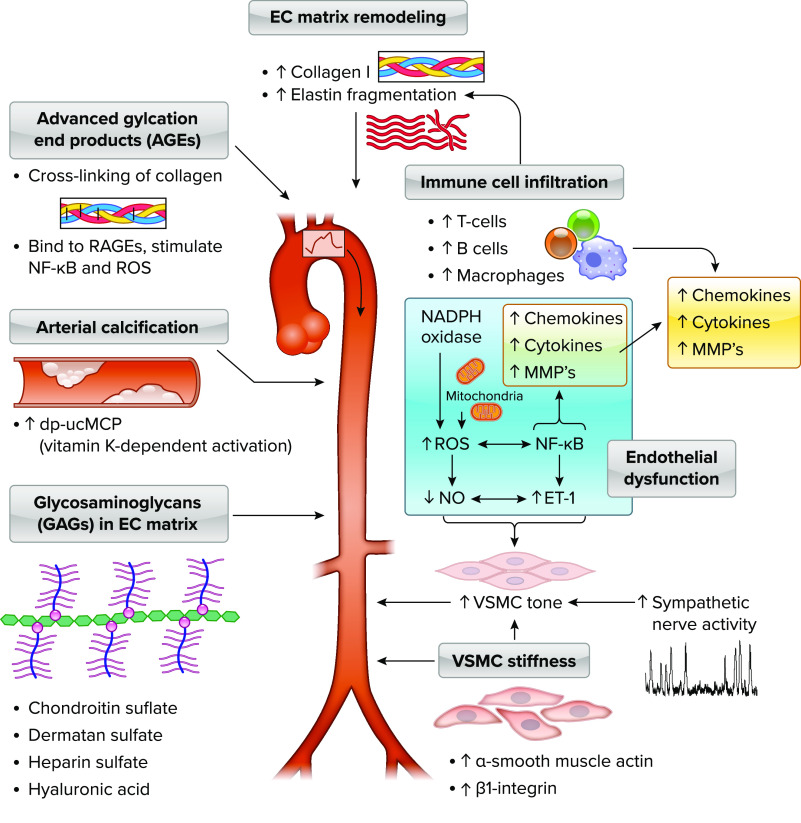
The cellular mechanisms and modulators that influence aortic stiffness can be classified into five general categories: 1) alterations in the extracellular matrix in the arterial wall, 2) modulators of arterial tone (i.e., vasodilators vs. vasoconstrictors), 3) intrinsic vascular smooth muscle cell stiffness, 4) arterial calcification, and 5) glycosaminoglycans (GAGs). Alterations in arterial smooth muscle tone can occur acutely or gradually, whereas the other four mechanisms are typically chronic changes that can take months or years to occur but may be influenced by a variety of lifestyle or physiological stressors or pathologies (151–155).
Alterations in the Extracellular Matrix in the Arterial Wall
Extracellular matrix factors that contribute to aortic stiffness have been reviewed in detail previously (156) but include accumulation and reorganization of fibrotic proteins such as collagen I and III and degradation and fragmentation of elastin. In addition, advanced glycation end products (AGEs), proteins or lipids that are formed from nonenzymatic glycation or oxidation after exposure to aldose sugars, accumulate in the vessel wall, leading to cross-links in extracellular matrix proteins with slow turnover rate (i.e., collagen I), and promote arterial stiffening (157). Furthermore, AGEs also bind to several different receptors for AGEs (RAGEs), which results in upregulation of the proinflammatory transcription factor nuclear factor-κB (NF-κB) and subsequently numerous proinflammatory and prooxidant genes that contribute to endothelial dysfunction (157).
Modulators of Arterial Tone
Alterations in vasodilators and constrictors such as NO and endothelin-1 (ET-1) likely contribute to age-related changes because NO is attenuated and circulating ET-1 is elevated with advancing age. Indeed, functional components of aortic stiffness are regulated in part by NO (158, 159) and ET-1 (160) in in vivo animal and human models, indicating that the endothelium-derived vasoactive molecules NO and ET-1 modulate arterial stiffness in part through altering vascular smooth muscle tone. However, vasodilators may play less of a role when hypertension is present. For example, intravenous nitroglycerin infused into adults with hypertension, leading to a large decrease in mean arterial pressure (–20 mmHg), resulted in no change in carotid-femoral PWV or carotid distensibility, whereas the same magnitude decrease in mean arterial pressure in normotensive adults resulted in a significant reduction in aortic and carotid stiffness (161). These data suggest that structural changes in arterial wall among adults with hypertension may render the arterial wall less sensitive to acute changes in mean arterial pressure; thus arterial stiffness becomes more blood pressure independent in chronic hypertension. Furthermore, recent evidence supports the idea that sympathetic nerve activity also modulates aortic stiffness likely from increased tone (162–164) and tonically elevated sympathetic activity contributes to arterial wall remodeling/thickening of common carotid arteries (165).
Cellular Mechanisms That Modulate Extracellular Matrix and Arterial Tone
Two of the major cellular/molecular mechanisms that contribute to age-related arterial stiffening that are involved with alterations in extracellular matrix and arterial tone are oxidative stress and inflammation. Elevations in plasma markers of oxidative stress in older adults (166–168) suggest that aging is a state of chronic, systemic oxidative stress (169), a condition that ensues when the bioavailability of reactive oxygen species (ROS) is increased relative to antioxidant defenses (170, 171). Oxidative stress in the vasculature, characterized by increases in arterial ROS, including superoxide anion (172) and hydrogen peroxide (173), is a primary mechanism underlying age-associated reductions in vascular NO bioavailability with aging (174, 175), thus acutely increasing vascular smooth muscle cell tone. Indeed, greater superoxide-associated oxidative stress contributes to in vivo measures aortic stiffening in rodent models of aging (176, 177) and common carotid stiffness in older postmenopausal women (146, 178). In contrast, oxidative stress does not appear to be a key mechanism contributing to aortic stiffness in older men (147).
The sources of oxidative stress with advanced age are multifocal, including increased production of superoxide associated with both elevated expression and activity of NADPH oxidase (179), increased endothelial nitric oxide (eNOS) uncoupling (180), and mitochondrial electron transport chain leakage (181, 182). NADPH oxidase-derived superoxide directly contributes to age-associated arterial dysfunction, as apocynin inhibition of NADPH leads to restoration of NO after in vitro inhibition of this enzyme by apocynin in arteries from aged mice (179). Supporting a role for eNOS uncoupling in age-related elevations in arterial superoxide, ex vivo administration of tetrahydrobiopterin reduces superoxide in arterioles from old rats (183). In contrast to NADPH oxidase, other oxidant enzyme systems such as xanthine oxidase or cytochrome P-450 appear to be less critical to age-related arterial oxidative stress, as no age-related increase in expression and/or activity in these oxidant enzymes have been found in arteries from aged mice or human endothelial cells of older adults (166, 167, 179, 184, 185). Emerging evidence points strongly to mitochondrial ROS as a major source of both superoxide and hydrogen peroxide in older rodent and human arteries (186–188). Interestingly, supplementation of the mitochondrion-specific antioxidant MitoQ ameliorates the age-related large artery stiffness in both rodents (186, 189) and humans (190), being the only ROS-specific antioxidant to have been shown to have a direct impact. This appears to be through improvements in endothelial function but also via normalization of the age-related changes in collagen and elastin (189).
To counter age-related changes in superoxide production and maintain appropriate redox balance, elevations in superoxide production with aging should signal an increase in antioxidant defenses. However, total antioxidant capacity is reduced (191) and the expression and/or activity of critical antioxidant enzymes do not increase in arteries of old rodents (179). Specifically, SODs are critical antioxidant enzymes that convert superoxide to H2O2 and thus play a primary role in reactive oxygen removal (192). In the face of elevated superoxide production with aging, neither copper zinc SOD, the intracellular isoform, nor manganese SOD, the mitochondrial isoform, increases in arteries of old mice (172, 179, 193). Although the expression of extracellular SOD appears to be either unchanged (193, 194) or increased (179), there is no increase in enzyme activity of any of these SOD isoforms in arteries of mice with advancing age (172, 179, 193).
Chronic, low-grade or “sterile” inflammation also has been implicated in age-associated stiffening of large elastic arteries. Age-associated inflammation is characterized by increases in circulating C-reactive protein, the acute-phase reactant linked to CVD risk, as well as proinflammatory cytokines such as interleukin (IL)-6 (166, 184). Importantly, both have been positively related to aortic stiffness (195, 196), and anti-tumor necrosis factor-α (TNF-α) therapy improved aortic stiffness in patients with rheumatoid arthritis (195, 197). As described above, activation of NF-κB is a central regulator of age-related vascular inflammation, with increased expression of NF-κB observed in endothelial cells from older human subjects (166, 184). In response to ROS (198) or inflammatory stimuli (199), NF-κB translocates to the nucleus and activates gene transcription of proinflammatory cytokines (199). Indeed, inhibition of NF-κB ameliorates aortic stiffness in older adults (200) and restores age-related impaired endothelium-dependent dilation of carotid arteries in mice (201), suggesting that NF-κB contributes at least in part to arterial aging.
Both vascular inflammation, via the cytokines IL-1β and TNF-α, and oxidative stress impact the primary mechanisms influencing extracellular matrix changes with aging (FIGURE 2), including matrix metalloproteinase (MMP)-mediated elastin breakdown and transforming growth factor-beta (TGF-β)-associated collagen accumulation (202, 203). Circulating plasma concentrations of MMP-9 are associated with increased large elastic artery stiffness in both healthy subjects and patients with CVD (204). MMP-9 can be directly transcribed via NF-κB (205) or indirectly via ROS-mediated activation of NF-κB (206), and there is a substantial increase in MMP-9 in mouse aortic tissue with aging (193). Also, overexpression of TGF-β is sufficient to induce collagen accumulation in many tissues (207, 208), and older mice express elevated arterial TGF-β in carotid tissue (18). Thus, TGF-β is associated with greater collagen I and III content in addition to augmented arterial superoxide in those older arteries (18). Furthermore, both MMPs and TGF-β also appear to be influenced by NO bioavailability, such that shear stress-induced NO downregulates MMP-2 expression in cultured endothelial cells and treatment of endothelial cells with a NO donor under static culture conditions will mimic this shear stress-induced reduction in MMP-2 (209). Likewise, inducible NOS-produced NO inhibits MMP-9, as activity of this MMP is induced after inhibition of NOS (210). Conversely, NO suppresses tissue inhibitor of matrix metalloproteinase (TIMP)-1, leading to increased MMP-9 (211). Thus, increases in oxidative stress and inflammation and decreases in NO bioavailability with aging may act in concert to alter both TGF-β and MMPs, important determinants of the extracellular matrix and fibrosis in the development of arterial stiffness.
FIGURE 2.
Mechanisms and modulators of aortic stiffness The cellular mechanisms and modulators that influence aortic stiffness can be classified into alterations in the extracellular (EC) matrix in the arterial wall, modulators of arterial tone, intrinsic vascular smooth muscle cell (VSMC) stiffness, arterial calcification, and glycosaminoglycans (GAGs). Alterations in arterial tone can occur acutely, whereas the other mechanisms are typically chronic changes that can take months or years to occur but may be influenced by a variety of lifestyle or physiological stressors or pathologies. Extracellular matrix remodeling includes accumulation and reorganization of fibrotic proteins such as collagen I and III and degradation and fragmentation of elastin. Advanced glycation end products (AGEs), proteins or lipids that are formed from nonenzymatic glycation or oxidation after exposure to aldose sugars, accumulate in the vessel wall, leading to cross-links in extracellular matrix proteins with slow turnover rate such as collagen I and promotion of arterial stiffening. AGEs also bind to several different receptors for AGEs (RAGEs), which results in upregulation of the proinflammatory transcription factor nuclear factor-κB (NF-κB) and prooxidant genes leading to reactive oxygen species (ROS) production. Calcification of the aortic wall occurs in both the media and intima, and activation of unphospho-decarboxylated matrix Gla-protein (dp-ucMGP), a protein secreted by VSMCs and chondrocytes, is a vitamin K-dependent process, so that elevated levels of circulating inactive dp-ucMGP are associated with higher aortic stiffness. Extracellular matrix of elastin sheets and collagen fiber proteins, the medial layer of the aorta also contains GAGs, including chondroitin sulfate, followed by dermatan sulfate, heparin sulfate, and hyaluronan, that play some role in modulating elastic properties of the arterial wall. α-Smooth muscle actin, the most abundant isoform of actin in VSMCs, and β1-integrin, a key adhesion molecule that couples vascular smooth muscle and extracellular matrix, mediate a significant component of age-related increase in VSMC stiffness. Endothelial dysfunction, through elevated ROS and NF-κB-mediated reductions in nitric oxide (NO) and increased endothelin 1 (ET-1) and increased sympathetic nerve activity, converges with vascular smooth muscle stiffness to raise vascular smooth muscle tone. ROS and cytokines/chemokines such as transforming growth factor-beta (TGF-β) and matrix metalloproteinases (MMPs) work synergistically to alter extracellular matrix and fibrosis in the development of arterial stiffness. Advancing age is associated with increases in T and B cells and macrophages in the perivascular adipose tissue of the aorta in mice, and T and B cell knockout mice (RAG−/−) have a lower rate of age-related aortic stiffening across the life span.
Although ROS will act on NF-κB to stimulate proinflammatory signaling, proinflammatory signaling itself can stimulate the local production of superoxide by inducing transcription of redox-sensitive genes, such as those encoding subunits of NADPH oxidase (212), and through the local recruitment of immune cells. Indeed, advancing age is associated with increases in T and B cells as well as macrophages in the perivascular adipose tissue of both the aorta and mesenteric vascular arcade. Interestingly, mice that lack T and B cells (RAG−/− knockout) have a lower rate of age-related aortic stiffening across the life span (213). Additionally, if immune-normal older mice have T cells depleted, thus reducing the amount of T cells in and around the arterial circulation, there is an amelioration of aortic PWV in these old mice (213). Therefore, it appears that resident T cells play a critical role in the inflammatory state of the aorta either directly or via cross talk with other resident immune cells and influence age-related aortic stiffness. Therefore, it appears that inflammatory and oxidative pathways interact with select immune cells advancing age to induce and perpetuate large central artery stiffness.
Vascular Smooth Muscle Stiffness
Intrinsic stiffness of vascular smooth muscle cells has been identified as a previously underappreciated contributor to aortic wall stiffness with aging (8, 214) and hypertension (9, 215). Indeed, about one-half of the stiffness in the aorta of aged mice can be attributed to active stiffness of the vascular smooth muscle cells (214). Primary vascular smooth muscle cells isolated from older nonhuman primates demonstrate higher in vivo elastic modulus (stiffness) with atomic force microscopy nanoindentation and a reconstituted in vitro tissue model compared with cells from young monkeys (8). However, stiffness of the older isolated vascular smooth muscle cells was abolished when they were treated with an actin cytoskeleton disrupter or myosin light chain kinase inhibitor. In addition, α-smooth muscle actin, the most abundant isoform of actin in smooth muscle cells, and β1-integrin, a key adhesion molecule that couples vascular smooth muscle and extracellular matrix, were both higher in older vascular smooth muscle cells. In summary, these data suggest that the actin component of cytoskeleton may be involved in a significant component of age-related increase in vascular smooth muscle cell stiffness.
Arterial Calcification
Calcification of the aortic wall occurs in both the media and intima, whereas the latter is associated with atherosclerosis mediated by chondrocyte-like cells of bone marrow origin and the former is relevant to arteriosclerosis and aging mediated by osteochondrocyte differentiation of vascular smooth muscle cells (2). Indeed, arterial calcification in humans has also been linked to higher aortic stiffness with aging and hypertension (216). McEniery et al. (216) found that total aortic calcium, quantified by high-resolution computed tomography in the ascending, descending, and abdominal aorta, was positively associated with carotid-femoral PWV in middle-aged and older healthy adults, and in multiple regression models only age, calcium phosphate, and carotid-femoral PWV were independently associated with aortic calcification. Moreover, older adults in the cohort with controlled isolated systolic hypertension had higher aortic stiffness and abdominal and descending aortic calcium than the similar-age healthy control subjects, with those with resistant systolic hypertension having the highest of both aortic stiffness and calcification. Finally, matrix Gla-protein (MGP) is a protein secreted by vascular smooth muscle cells and chondrocytes. In its inactivated form, unphospho-decarboxylated MGP (dp-ucMGP) is an inhibitor of vascular calcification by blocking osteogenic differentiation of vascular smooth muscle cells (217). However, the glutamate carboxylation and serine phosphorylation activation of dp-ucMGP is a vitamin K-dependent process, so that elevated levels of circulating inactive dp-ucMGP are associated with higher aortic stiffness independent of other risk factors in healthy adults (217) and adults with hypertension (218), diabetes (219), and kidney disease (220). Thus, additional data are needed to determine whether vitamin K supplementation is a novel preventive or treatment strategy for slowing aortic calcification-related stiffness.
Glycosaminoglycans
In addition to smooth muscle cells embedded in the extracellular matrix of elastin sheets and collagen fiber proteins, the medial layer of the aorta also contains GAGs and proteoglycans. In particular, GAGs are negatively charged polysaccharides made up of repeating disaccharides and bound to a protein core known as a proteoglycan (221). There are six types of GAG families including hyaluronic acid (i.e., hyaluronan), dermatan sulfate, keratan sulfate, chondroitin sulfate, heparin sulfate, and heparin. Hyaluronan is the only GAG not bound to a proteoglycan, and proteoglycans in the extracellular matrix usually include dermatan sulfate and chondroitin sulfate GAG chains. Furthermore, out of total GAGs, the aorta intima and media contain up to 50% chondroitin sulfate, followed by dermatan sulfate, heparin sulfate, and hyaluronan (222).
GAGs represent only 2–5% of thoracic aorta dry mass; this is likely why it receives less attention than extracellular matrix proteins elastin and collagen (223). Because GAGs are negatively charged molecules in the extracellular matrix, they attract cations such as sodium from interstitial fluid into the arterial wall. This creates an osmotic gradient promoting water influx into regions of the arterial wall containing GAGs, which can increase tissue volume (arterial wall swelling) in the vascular media (221). However, the exact nature of the distribution of the swelling in the arterial wall (e.g., uniform or more localized in the wall) is unclear, but more uniform swelling could possibly alter arterial extracellular matrix function (224) whereas more localized swelling might increase pressure at discrete locations, increasing risk of dissection (221).
To directly test the role of GAGs in aortic stiffness, ex vivo enzymatic digestion of 65% of chondroitin and dermatan sulfate resulted in a leftward shift in stress-strain curves or increased elastic modulus (stiffness) of rat mesenteric arteries (225) consistent with a significant functional role of GAGs in contribution to arterial stiffness. In the porcine aorta, ex vivo enzymatic removal of GAGs (via hyaluronidase, heparinase, chondroitinase) resulted in earlier transition points of the stress-strain curves, indicating earlier engagement of stiffer collagen fibers at lower levels of strain, but did not alter slopes of the stress-strain relation (226). Thus, taken together these findings suggest that GAGs in the extracellular matrix likely play some role in modulating elastic properties of the arterial wall, but the precise contribution to age- and hypertension-related aortic stiffness remains unclear.
Conclusions
Aortic stiffness is a robust independent risk factor for the future development of CVD and subclinical target organ damage to the brain, kidney, retina, and heart. The physiological consequences of aortic stiffness include increased early systolic load and myocardial wall stress on the heart through elevated Zc and late systolic load through premature wave reflections arriving during systole, augmenting PP and reducing diastolic coronary perfusion pressure. Importantly, there are sex differences in aortic pulsatile load that manifest in women during the postmenopausal years that may lead to differences in LV remodeling and higher risk for developing HFpEF. The mechanisms that contribute to aortic stiffness include cellular mechanisms that alter extracellular matrix proteins and arterial tone, the latter by mediators such as NO and ET-1, while intrinsic vascular smooth muscle stiffness, calcification, and GAGs likely play a complementary or synergistic role in the development and progression of aortic stiffness. There are numerous lifestyle interventions that have been studied to test efficacy on slowing or decreasing aortic stiffness in middle-aged and older adults with or without hypertension. Prospective interventions such as aerobic exercise (227–229), dietary-restricted weight loss (230, 231), dietary sodium restriction (232), and passive heat therapy (233) have generally reported beneficial effects on aortic stiffness with normotensive aging, but results are mixed when hypertension is present and are limited by small sample sizes, short treatment durations, and lack of long-term follow-up. The reader is referred to some excellent reviews on these topics (234–239). So, in summary, the subclinical and clinical consequences of premature aortic stiffening are not good, and given that the population of adults >65 yr of age in the United States will increase to ∼72 million by the year 2050, therapeutic targets and interventions are desperately needed to prevent the substantial future CVD burden related to aortic stiffness in the aging population.
Acknowledgments
The authors thank Teresa Ruggle for graphical assistance in developing the figures.
G.L.P. is supported by grants from the National Institutes of Health (AG063790) and the American Heart Association (19TPA34910016). A.J.D. is supported by grants from the National Institutes of Health (AG060395, AG050238). L.E.D. is supported by training grants from the National Institutes of Health (T32 AG000279, F32 AG071273-01A1). T.A.C. is supported by a Clinician Scientist Stage I Award from Heart and Stroke Foundation of Ontario and holds the Chair for Women’s Heart Health at the University of Ottawa Heart Institute.
No conflicts of interest, financial or otherwise, are declared by the authors.
G.L.P. conceived and designed research; G.L.P. performed experiments; G.L.P. and A.J.D. analyzed data; G.L.P., L.E.D., and A.J.D. interpreted results of experiments; G.L.P. prepared figures; T.A.C., L.E.D., and A.J.D. drafted manuscript; G.L.P., T.A.C., L.E.D., and A.J.D. edited and revised manuscript; G.L.P., T.A.C., L.E.D., and A.J.D. approved final version of manuscript.
References
- 1.Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr) 31: 305–325, 2009. doi: 10.1007/s11357-009-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 74: 1237–1263, 2019. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res 128: 864–886, 2021. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 4.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 39: 13–20, 1977. doi: 10.1016/S0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DJ, Robson P, Hew Y, Keeley FW. Decreased elastin synthesis in normal development and in long-term aortic organ and cell cultures is related to rapid and selective destabilization of mRNA for elastin. Circ Res 77: 1107–1113, 1995. doi: 10.1161/01.res.77.6.1107. [DOI] [PubMed] [Google Scholar]
- 6.Powell JT, Vine N, Crossman M. On the accumulation of D-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis 97: 201–208, 1992. doi: 10.1016/0021-9150(92)90132-z. [DOI] [PubMed] [Google Scholar]
- 7.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 97: 1555–1617, 2017. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 8.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol 305: H1281–H1287, 2013. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell GF. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J Appl Physiol (1985) 125: 1871–1880, 2018. doi: 10.1152/japplphysiol.00108.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation 122: 1379–1386, 2010. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEniery CM, Yasmin, Maki-Petaja KM, McDonnell BJ, Munnery M, Hickson SS, Franklin SS, Cockcroft JR, Wilkinson IB; Anglo-Cardiff Collaboration Trial Investigators. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo-Cardiff Collaborative Trial (ACCT III). Hypertension 56: 591–597, 2010. doi: 10.1161/HYPERTENSIONAHA.110.156950. [DOI] [PubMed] [Google Scholar]
- 13.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 62: 934–941, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag 4: 547–552, 2008. doi: 10.2147/vhrm.s1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens 26: 1049–1057, 2008. doi: 10.1097/HJH.0b013e3282f82c3e. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 17.Leloup AJ, Fransen P, Van Hove CE, Demolder M, De Keulenaer GW, Schrijvers DM. Applanation tonometry in mice: a novel noninvasive technique to assess pulse wave velocity and arterial stiffness. Hypertension 64: 195–200, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03312. [DOI] [PubMed] [Google Scholar]
- 18.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gioscia-Ryan RA, Clayton ZS, Fleenor BS, Eng JS, Johnson LC, Rossman MJ, Zigler MC, Evans TD, Seals DR. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. Geroscience 43: 423–432, 2021. doi: 10.1007/s11357-020-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartley CJ, Taffet GE, Michael LH, Pham TT, Entman ML. Noninvasive determination of pulse-wave velocity in mice. Am J Physiol Heart Circ Physiol 273: H494–H500, 1997. doi: 10.1152/ajpheart.1997.273.1.H494. [DOI] [PubMed] [Google Scholar]
- 21.Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 31: 2338–2350, 2010. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spronck B, Heusinkveld MH, Vanmolkot FH, Roodt JO, Hermeling E, Delhaas T, Kroon AA, Reesink KD. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens 33: 330–338, 2015. doi: 10.1097/HJH.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 23.Spronck B, Tan I, Reesink KD, Georgevsky D, Delhaas T, Avolio AP, Butlin M. Heart rate and blood pressure dependence of aortic distensibility in rats: comparison of measured and calculated pulse wave velocity. J Hypertens 39: 117–126, 2021. doi: 10.1097/HJH.0000000000002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 18: 3S–10S, 2005. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 525: 263–270, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O’Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension 38: 1433–1439, 2001. doi: 10.1161/hy1201.098298. [DOI] [PubMed] [Google Scholar]
- 28.Schofield RS, Pierce GL, Nichols WW, Klodell CT, Aranda JM Jr, Pauly DF, Hill JA, Braith RW. Arterial-wave reflections are increased in heart failure patients with a left-ventricular assist device. Am J Hypertens 20: 622–628, 2007. doi: 10.1016/j.amjhyper.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 29.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR; ACCT Investigators. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46: 1753–1760, 2005. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 31.Baksi AJ, Treibel TA, Davies JE, Hadjiloizou N, Foale RA, Parker KH, Francis DP, Mayet J, Hughes AD. A meta-analysis of the mechanism of blood pressure change with aging. J Am Coll Cardiol 54: 2087–2092, 2009. doi: 10.1016/j.jacc.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res 6: 648–656, 1972. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 108: 1592–1598, 2003. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 34.Davies JE, Alastruey J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, Mayet J. Attenuation of wave reflection by wave entrapment creates a “horizon effect” in the human aorta. Hypertension 60: 778–785, 2012. doi: 10.1161/HYPERTENSIONAHA.111.180604. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age Gene/Environment Susceptibility-Reykjavik Study. Hypertension 51: 1123–1128, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10–15, 2002. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 37.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 34: 1203–1206, 2003. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 38.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999. doi: 10.1161/01.CIR.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 39.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 40.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation 131: 354–361, 2015. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96: 308–315, 1997. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 46.Berger DS, Robinson KA, Shroff SG. Wave propagation in coupled left ventricle-arterial system. Implications for aortic pressure. Hypertension 27: 1079–1089, 1996. doi: 10.1161/01.HYP.27.5.1079. [DOI] [PubMed] [Google Scholar]
- 47.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 37: 869–874, 2001. doi: 10.1161/01.HYP.37.3.869. [DOI] [PubMed] [Google Scholar]
- 48.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 103: 1245–1249, 2001. doi: 10.1161/01.CIR.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res 3: 56–64, 2009. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li JK. Pulse wave reflections at the aorto-iliac junction. Angiology 36: 516–521, 1985. doi: 10.1177/000331978503600807. [DOI] [PubMed] [Google Scholar]
- 52.Li JK. Dominance of geometric over elastic factors in pulse transmission through arterial branching. Bull Math Biol 48: 97–103, 1986. doi: 10.1007/BF02460065. [DOI] [PubMed] [Google Scholar]
- 53.DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane-Cordova A, Sigurdsson G, Schmid P, Barlow PB, Pierce GL. Carotid beta-stiffness index is associated with slower processing speed but not working memory or white matter integrity in healthy middle-aged/older adults. J Appl Physiol (1985) 122: 868–876, 2017. doi: 10.1152/japplphysiol.00769.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension 53: 668–673, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geijselaers SL, Sep SJ, Schram MT, van Boxtel MP, van Sloten TT, Henry RM, Reesink KD, Kroon AA, Koster A, Schaper NC, Dagnelie PC, van der Kallen CJ, Biessels GJ, Stehouwer CD. Carotid stiffness is associated with impairment of cognitive performance in individuals with and without type 2 diabetes. The Maastricht Study. Atherosclerosis 253: 186–193, 2016. doi: 10.1016/j.atherosclerosis.2016.07.912. [DOI] [PubMed] [Google Scholar]
- 56.Menezes ST, Giatti L, Colosimo EA, Ribeiro AL, Brant LC, Viana MC, Cunha RS, Mill JG, Barreto SM. Aortic stiffness and age with cognitive performance decline in the ELSA-Brasil Cohort. J Am Heart Assoc 8: e013248, 2019. doi: 10.1161/JAHA.119.013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palta P, Sharrett AR, Wei J, Meyer ML, Kucharska-Newton A, Power MC, Deal JA, Jack CR, Knopman D, Wright J, Griswold M, Tanaka H, Mosley TH, Heiss G. Central arterial stiffness is associated with structural brain damage and poorer cognitive performance: the ARIC Study. J Am Heart Assoc 8: e011045, 2019. doi: 10.1161/JAHA.118.011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: the Framingham Third Generation Cohort Study. Hypertension 67: 513–519, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pase MP, Pipingas A, Kras M, Nolidin K, Gibbs AL, Wesnes KA, Scholey AB, Stough C. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens 28: 1724–1729, 2010. doi: 10.1097/HJH.0b013e32833b1ee7. [DOI] [PubMed] [Google Scholar]
- 62.Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke 38: 888–892, 2007. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- 63.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens 23: 1211–1216, 2005. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 64.Tarumi T, Gonzales MM, Fallow B, Nualnim N, Pyron M, Tanaka H, Haley AP. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens 31: 2400–2409, 2013. doi: 10.1097/HJH.0b013e328364decc. [DOI] [PubMed] [Google Scholar]
- 65.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81: 984–991, 2013. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson NL, Sutton-Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM, Najjar SS, Launer LJ, Yaffe K, Atkinson HH, Satterfield S, Newman AB. Arterial stiffness and cognitive decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci 66: 1336–1342, 2011. doi: 10.1093/gerona/glr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Araghi M, Shipley MJ, Wilkinson IB, McEniery CM, Valencia-Hernandez CA, Kivimaki M, Sabia S, Singh-Manoux A, Brunner EJ. Association of aortic stiffness with cognitive decline: Whitehall II longitudinal cohort study. Eur J Epidemiol 35: 861–869, 2020. doi: 10.1007/s10654-019-00586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, Manckoundia P, Agnoletti D, Labat C, Gautier S; PARTAGE Study Investigators. Pulse wave velocity is associated with 1-year cognitive decline in the elderly older than 80 years: the PARTAGE study. J Am Med Dir Assoc 13: 239–243, 2012. doi: 10.1016/j.jamda.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens 25: 1035–1040, 2007. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 70.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51: 99–104, 2008. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 71.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Tseng BM, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab 35: 527–530, 2015. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baumbach GL. Is pulse pressure a stimulus for altered vascular structure in chronic hypertension? Hypertension 18: 728–729, 1991. doi: 10.1161/01.hyp.18.6.728. [DOI] [PubMed] [Google Scholar]
- 74.Baumbach GL, Heistad DD. Adaptive changes in cerebral blood vessels during chronic hypertension. J Hypertens 9: 987–991, 1991. doi: 10.1097/00004872-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Walker AE, Henson GD, Reihl KD, Morgan RG, Dobson PS, Nielson EI, Ling J, Mecham RP, Li DY, Lesniewski LA, Donato AJ. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol 593: 1931–1943, 2015. doi: 10.1113/jphysiol.2014.285338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker AE, Kronquist EK, Chinen KT, Reihl KD, Li DY, Lesniewski LA, Donato AJ. Cerebral and skeletal muscle feed artery vasoconstrictor responses in a mouse model with greater large elastic artery stiffness. Exp Physiol 104: 434–442, 2019. doi: 10.1113/EP087453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension 67: 176–182, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu W, Chen Z, Ortega D, Liu X, Huang X, Wang L, Chen L, Sun J, Hatsukami TS, Yuan C, Li H, Yang J. Arterial elasticity, endothelial function and intracranial vascular health: a multimodal MRI study. J Cereb Blood Flow Metab 41: 1390–1397, 2021. doi: 10.1177/0271678X20956950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DuBose LE, Boles Ponto LL, Moser DJ, Harlynn E, Reierson L, Pierce GL. Higher aortic stiffness is associated with lower global cerebrovascular reserve among older humans. Hypertension 72: 476–482, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jefferson AL, Liu D, Gupta DK, Pechman KR, Watchmaker JM, Gordon EA, Rane S, Bell SP, Mendes LA, Davis LT, Gifford KA, Hohman TJ, Wang TJ, Donahue MJ. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology 89: 2327–2334, 2017. doi: 10.1212/WNL.0000000000004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 24: 1108–1113, 2011. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- 82.Haidar MA, van Buchem MA, Sigurdsson S, Gotal JD, Gudnason V, Launer LJ, Mitchell GF. Wave reflection at the origin of a first-generation branch artery and target organ protection: the AGES-Reykjavik Study. Hypertension 77: 1169–1177, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kröner ES, Lamb HJ, Siebelink HM, Cannegieter SC, van den Boogaard PJ, van der Wall EE, de Roos A, Westenberg JJ. Pulse wave velocity and flow in the carotid artery versus the aortic arch: effects of aging. J Magn Reson Imaging 40: 287–293, 2014. doi: 10.1002/jmri.24470. [DOI] [PubMed] [Google Scholar]
- 84.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens 26: 1411–1419, 2008. doi: 10.1097/HJH.0b013e3282ffac00. [DOI] [PubMed] [Google Scholar]
- 85.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Aortic stiffness, increased white matter free water, and altered microstructural integrity a continuum of injury. Stroke 48: 1567–1573, 2017. doi: 10.1161/STROKEAHA.116.016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, Venkatraman V, Harris TB, Barinas-Mitchell E, Sutton-Tyrrell K. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension 61: 160–165, 2013. doi: 10.1161/HYPERTENSIONAHA.112.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui C, Sekikawa A, Kuller LH, Lopez OL, Newman AB, Kuipers AL, Mackey RH. Aortic stiffness is associated with increased risk of incident dementia in older adults. J Alzheimers Dis 66: 297–306, 2018. doi: 10.3233/JAD-180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rouch L, Cestac P, Sallerin B, Andrieu S, Bailly H, Beunardeau M, Cohen A, Dubail D, Hernandorena I, Seux ML, Vidal JS, Hanon O. Pulse wave velocity is associated with greater risk of dementia in mild cognitive impairment patients. Hypertension 72: 1109–1116, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11443. [DOI] [PubMed] [Google Scholar]
- 89.Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, Wong D, Zhou Y, Knopman D, Mosley TH, Gottesman RF. Arterial stiffness and dementia pathology: atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology 90: e1248–e1256, 2018. doi: 10.1212/WNL.0000000000005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8: 448–460, 2002. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- 91.Gangoda SV, Avadhanam B, Jufri NF, Sohn EH, Butlin M, Gupta V, Chung R, Avolio AP. Pulsatile stretch as a novel modulator of amyloid precursor protein processing and associated inflammatory markers in human cerebral endothelial cells. Sci Rep 8: 1689, 2018. doi: 10.1038/s41598-018-20117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension 43: 163–168, 2004. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- 93.Huang N, Foster MC, Mitchell GF, Andresdottir MB, Eiriksdottir G, Gudmundsdottir H, Harris TB, Launer LJ, Palsson R, Gudnason V, Levey AS, Inker LA. Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol Dial Transplant 32: 677–684, 2017. doi: 10.1093/ndt/gfw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 95.Michener KH, Mitchell GF, Noubary F, Huang N, Harris T, Andresdottir MB, Palsson R, Gudnason V, Levey AS. Aortic stiffness and kidney disease in an elderly population. Am J Nephrol 41: 320–328, 2015. doi: 10.1159/000431332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 26: 1181–1187, 2015. doi: 10.1681/ASN.2014050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol 10: 2190–2197, 2015. doi: 10.2215/CJN.03000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, Roy J, Weir MR, Wright JT Jr, Bansal N, Hsu CY; CRIC Study Investigators. Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 71: 1101–1107, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chirinos JA, Townsend RR. Reducing arterial stiffness in CKD: revising the paradigms. Clin J Am Soc Nephrol 10: 547–550, 2015. doi: 10.2215/CJN.01900215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harris A, Ciulla TA, Chung HS, Martin B. Regulation of retinal and optic nerve blood flow. Arch Ophthalmol 116: 1491–1495, 1998. doi: 10.1001/archopht.116.11.1491. [DOI] [PubMed] [Google Scholar]
- 101.Lehmann MV, Schmieder RE. Remodeling of retinal small arteries in hypertension. Am J Hypertens 24: 1267–1273, 2011. doi: 10.1038/ajh.2011.166. [DOI] [PubMed] [Google Scholar]
- 102.Ott C, Raff U, Harazny JM, Michelson G, Schmieder RE. Central pulse pressure is an independent determinant of vascular remodeling in the retinal circulation. Hypertension 61: 1340–1345, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00617. [DOI] [PubMed] [Google Scholar]
- 103.Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, Cotch MF, Klein BE, Wong TY. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension 50: 617–622, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 104.Salvetti M, Agabiti Rosei C, Paini A, Aggiusti C, Cancarini A, Duse S, Semeraro F, Rizzoni D, Agabiti Rosei E, Muiesan ML. Relationship of wall-to-lumen ratio of retinal arterioles with clinic and 24-hour blood pressure. Hypertension 63: 1110–1115, 2014. doi: 10.1161/HYPERTENSIONAHA.113.03004. [DOI] [PubMed] [Google Scholar]
- 105.Triantafyllou A, Anyfanti P, Gavriilaki E, Zabulis X, Gkaliagkousi E, Petidis K, Triantafyllou G, Gkolias V, Pyrpasopoulou A, Douma S. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am J Hypertens 27: 1472–1478, 2014. doi: 10.1093/ajh/hpu074. [DOI] [PubMed] [Google Scholar]
- 106.Liao D, Wong TY, Klein R, Jones D, Hubbard L, Sharrett AR. Relationship between carotid artery stiffness and retinal arteriolar narrowing in healthy middle-aged persons. Stroke 35: 837–842, 2004. doi: 10.1161/01.STR.0000120310.43457.AD. [DOI] [PubMed] [Google Scholar]
- 107.Paini A, Muiesan ML, Agabiti-Rosei C, Aggiusti C, De Ciuceis C, Bertacchini F, Duse S, Semeraro F, Rizzoni D, Agabiti-Rosei E, Salvetti M. Carotid stiffness is significantly correlated with wall-to-lumen ratio of retinal arterioles. J Hypertens 36: 580–586, 2018. doi: 10.1097/HJH.0000000000001595. [DOI] [PubMed] [Google Scholar]
- 108.Holwerda SW, Kardon RH, Hashimoto R, Full JM, Nellis JK, DuBose LE, Fiedorowicz JG, Pierce GL. Aortic stiffness is associated with changes in retinal arteriole flow pulsatility mediated by local vasodilation in healthy young/middle-age adults. J Appl Physiol (1985) 129: 84–93, 2020. doi: 10.1152/japplphysiol.00252.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, Mitchell GF. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility–Reykjavik Study. Circ Cardiovasc Imaging 8: e003039, 2015. doi: 10.1161/CIRCIMAGING.114.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA 281: 634–639, 1999. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 111.Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc 4: e002189, 2015. doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandey A, Khan H, Newman AB, Lakatta EG, Forman DE, Butler J, Berry JD. Arterial stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the Health ABC Study (Health, Aging, and Body Composition). Hypertension 69: 267–274, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation 94: 3362–3368, 1996. doi: 10.1161/01.CIR.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 114.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens 26: 1017–1024, 2008. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 115.Zamani P, Bluemke DA, Jacobs DR Jr, Duprez DA, Kronmal R, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, Segers P, Hannan P, Chirinos JA. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: the multi-ethnic study of atherosclerosis. Hypertension 65: 85–92, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC; Asklepios Investigators. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension 61: 296–303, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 117.Fukuta H, Ohte N, Wakami K, Asada K, Goto T, Mukai S, Tani T, Kimura G. Impact of arterial load on left ventricular diastolic function in patients undergoing cardiac catheterization for coronary artery disease. Circ J 74: 1900–1905, 2010. doi: 10.1253/circj.CJ-10-0283. [DOI] [PubMed] [Google Scholar]
- 118.Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens 21: 1194–1202, 2008. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 119.Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol 60: 2170–2177, 2012. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chirinos JA, Segers P, Duprez DA, Brumback L, Bluemke DA, Zamani P, Kronmal R, Vaidya D, Ouyang P, Townsend RR, Jacobs DR Jr.. Late systolic central hypertension as a predictor of incident heart failure: the Multi-ethnic Study of Atherosclerosis. J Am Heart Assoc 4: e001335, 2015. doi: 10.1161/JAHA.114.001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoffman JI, Buckberg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc 3: e000285, 2014. doi: 10.1161/JAHA.113.000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ohtsuka S, Kakihana M, Watanabe H, Sugishita Y. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol 24: 1406–1414, 1994. doi: 10.1016/0735-1097(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 123.Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension 74: 218–228, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12655. [DOI] [PubMed] [Google Scholar]
- 124.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 61: 96–103, 2013. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coutinho T, Yam Y, Chow BJ, Dwivedi G, Inácio J. Sex differences in associations of aortic compliance with coronary artery plaque and calcification burden. J Am Heart Assoc 6: e006079, 2017. doi: 10.1161/JAHA.117.006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab 88: 5375–5380, 2003. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 127.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens 19: 2205–2212, 2001. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 128.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 31: 1247–1254, 2018. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hickson SS, Miles KL, McDonnell BJ, Yasmin, Cockcroft JR, Wilkinson IB, McEniery CM; ENIGMA Study Investigators. Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: the ENIGMA study. J Hypertens 29: 1155–1159, 2011. doi: 10.1097/HJH.0b013e328346a5af. [DOI] [PubMed] [Google Scholar]
- 130.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol 30: 1863–1871, 1997. doi: 10.1016/S0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 131.Rajkumar C, Kingwell BA, Cameron JD, Waddell T, Mehra R, Christophidis N, Komesaroff PA, McGrath B, Jennings GL, Sudhir K, Dart AM. Hormonal therapy increases arterial compliance in postmenopausal women. J Am Coll Cardiol 30: 350–356, 1997. doi: 10.1016/S0735-1097(97)00191-5. [DOI] [PubMed] [Google Scholar]
- 132.Kawecka-Jaszcz K, Czarnecka D, Olszanecka A, Rajzer M, Jankowski P. The effect of hormone replacement therapy on arterial blood pressure and vascular compliance in postmenopausal women with arterial hypertension. J Hum Hypertens 16: 509–516, 2002. doi: 10.1038/sj.jhh.1001431. [DOI] [PubMed] [Google Scholar]
- 133.Waddell TK, Rajkumar C, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Withdrawal of hormonal therapy for 4 weeks decreases arterial compliance in postmenopausal women. J Hypertens 17: 413–418, 1999. doi: 10.1097/00004872-199917030-00015. [DOI] [PubMed] [Google Scholar]
- 134.Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med 4: 10–13, 20, 2001. [PubMed] [Google Scholar]
- 135.Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, Claggett B, Merz CN, Cheng S. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation 143: 761–763, 2021. doi: 10.1161/CIRCULATIONAHA.120.049360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alam MG, Barri YM. Systolic blood pressure is the main etiology for poorly controlled hypertension. Am J Hypertens 16: 140–143, 2003. doi: 10.1016/s0895-7061(02)03198-9. [DOI] [PubMed] [Google Scholar]
- 137.Wilkins K, Gee M, Campbell N. The difference in hypertension control between older men and women. Health Rep 23: 33–40, 2012. [PubMed] [Google Scholar]
- 138.Coutinho T, Pellikka PA, Bailey KR, Turner ST, Kullo IJ. Sex differences in the associations of hemodynamic load with left ventricular hypertrophy and concentric remodeling. Am J Hypertens 29: 73–80, 2016. doi: 10.1093/ajh/hpv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coutinho T, Srivaratharajah K, Beanlands R, deKemp R, Mielniczuk L. Associations of hemodynamic load with impaired myocardial flow reserve: role of sex and hypertension (Abstract). Eur Heart J 36: 854, 2015. [Google Scholar]
- 140.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 123: 2006–2013, 2011. doi: 10.1161/CIRCULATIONAHA.110.954388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pibarot P, Dumesnil JG. Low-flow, low-gradient, normal ejection fraction aortic stenosis. Curr Cardiol Rep 12: 108–115, 2010. doi: 10.1007/s11886-010-0090-0. [DOI] [PubMed] [Google Scholar]
- 142.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 115: 2856–2864, 2007. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 143.Rylski B, Desjardins B, Moser W, Bavaria JE, Milewski RK. Gender-related changes in aortic geometry throughout life. Eur J Cardiothorac Surg 45: 805–811, 2014. doi: 10.1093/ejcts/ezt597. [DOI] [PubMed] [Google Scholar]
- 144.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 145.Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 42: 1699–1714, 2020. doi: 10.1007/s11357-020-00236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 21: 624–632, 2014. doi: 10.1097/GME.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 286: H1528–H1534, 2004. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- 148.Moreau KL, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ 11: 18, 2020. doi: 10.1186/s13293-020-00294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 46: 1129–1134, 2005. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 150.Georgiopoulos GA, Lambrinoudaki I, Athanasouli F, Armeni E, Rizos D, Kazani M, Karamanou M, Manios E, Augoulea A, Stellos K, Papamichael C, Stamatelopoulos K. Free androgen index as a predictor of blood pressure progression and accelerated vascular aging in menopause. Atherosclerosis 247: 177–183, 2016. doi: 10.1016/j.atherosclerosis.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 151.Barnes JN, Trombold JR, Dhindsa M, Lin HF, Tanaka H. Arterial stiffening following eccentric exercise-induced muscle damage. J Appl Physiol (1985) 109: 1102–1108, 2010. doi: 10.1152/japplphysiol.00548.2010. [DOI] [PubMed] [Google Scholar]
- 152.Lim LS, Ling LH, Cheung CM, Ong PG, Gong L, Tai ES, Mathur R, Wong D, Foulds W, Wong TY. Relationship of systemic endothelial function and peripheral arterial stiffness with diabetic retinopathy. Br J Ophthalmol 99: 837–841, 2015. doi: 10.1136/bjophthalmol-2014-306075. [DOI] [PubMed] [Google Scholar]
- 153.Machin DR, Park W, Alkatan M, Mouton M, Tanaka H. Effects of non-fat dairy products added to the routine diet on vascular function: a randomized controlled crossover trial. Nutr Metab Cardiovasc Dis 25: 364–369, 2015. doi: 10.1016/j.numecd.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 154.Vidal F, Cordovil I, Figueredo CM, Fischer RG. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol 40: 681–687, 2013. doi: 10.1111/jcpe.12110. [DOI] [PubMed] [Google Scholar]
- 155.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112: 2193–2200, 2005. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 156.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 157.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114: 597–605, 2006. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 158.Schmitt M, Avolio A, Qasem A, McEniery CM, Butlin M, Wilkinson IB, Cockcroft JR. Basal NO locally modulates human iliac artery function in vivo. Hypertension 46: 227–231, 2005. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- 159.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation 105: 213–217, 2002. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 160.McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol 42: 1975–1981, 2003. doi: 10.1016/j.jacc.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 161.Stewart AD, Jiang B, Millasseau SC, Ritter JM, Chowienczyk PJ. Acute reduction of blood pressure by nitroglycerin does not normalize large artery stiffness in essential hypertension. Hypertension 48: 404–410, 2006. doi: 10.1161/01.HYP.0000237669.64066.c5. [DOI] [PubMed] [Google Scholar]
- 162.Holwerda SW, Luehrs RE, DuBose L, Collins MT, Wooldridge NA, Stroud AK, Fadel PJ, Abboud FM, Pierce GL. Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle-age/older adults. Hypertension 73: 1025–1035, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nardone M, Incognito AV, Millar PJ. Evidence for pressure-independent sympathetic modulation of central pulse wave velocity. J Am Heart Assoc 7: e007971, 2018. doi: 10.1161/JAHA.117.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens 28: 979–984, 2010. doi: 10.1097/HJH.0b013e328336ed9a. [DOI] [PubMed] [Google Scholar]
- 165.Holwerda SW, Luehrs RE, DuBose LE, Majee R, Pierce GL. Sex and age differences in the association between sympathetic outflow and central elastic artery wall thickness in humans. Am J Physiol Heart Circ Physiol 317: H552–H560, 2019. doi: 10.1152/ajpheart.00275.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 167.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 169.Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann NY Acad Sci 854: 102–117, 1998. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- 170.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 171.Price DT, Vita JA, Keaney JF Jr.. Redox control of vascular nitric oxide bioavailability. Antioxid Redox Signal 2: 919–935, 2000. doi: 10.1089/ars.2000.2.4-919. [DOI] [PubMed] [Google Scholar]
- 172.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou X, Bohlen HG, Unthank JL, Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am J Physiol Heart Circ Physiol 297: H2227–H2233, 2009. doi: 10.1152/ajpheart.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. doi: 10.1161/01.HYP.38.2.274. [DOI] [PubMed] [Google Scholar]
- 176.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13: 576–578, 2014. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 179.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Santhanam AV, d’Uscio LV, Smith LA, Katusic ZS. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J Neurochem 122: 1211–1218, 2012. doi: 10.1111/j.1471-4159.2012.07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY) 8: 2897–2914, 2016. doi: 10.18632/aging.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 183.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol (1985) 105: 1359–1363, 2008. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park SY, Kwon OS, Andtbacka RH, Hyngstrom JR, Reese V, Murphy MP, Richardson RS. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf) 222: e12893, 2018. doi: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 188.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123: 2533–2542, 2010. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2018. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71: 1056–1063, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J Gerontol A Biol Sci Med Sci 61: 211–217, 2006. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- 192.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12: 772–783, 2013. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 16: 17–26, 2017. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mäki-Petäjä KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J, Wilkinson IB. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 114: 1185–1192, 2006. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 196.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, Levy D, Keaney JF Jr, Vasan RS, Mitchell GF, Benjamin EJ. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 51: 1651–1657, 2008. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mäki-Petäjä KM, Elkhawad M, Cheriyan J, Joshi FR, Östör AJ, Hall FC, Rudd JH, Wilkinson IB. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126: 2473–2480, 2012. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 198.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun 17: 221–237, 1992. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 199.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol 12: 85–98, 2000. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 200.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor kappa B signalling. J Hypertens 33: 2477–2482, 2015. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci 66: 409–418, 2011. 10.1093/gerona/glq233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10: 1077–1083, 1996. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 203.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda) 28: 391–403, 2013. doi: 10.1152/physiol.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yasmin, McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25: 372, 2005. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 205.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res 50: 556–565, 2001. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 206.Okamoto OK, Robertson DL, Fagan TF, Hastings JW, Colepicolo P. Different regulatory mechanisms modulate the expression of a dinoflagellate iron-superoxide dismutase. J Biol Chem 276: 19989–19993, 2001. doi: 10.1074/jbc.M101169200. [DOI] [PubMed] [Google Scholar]
- 207.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, Vanpelt CS, Geng YJ, Deng JM, Behringer RR, de Crombrugghe B. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum 56: 334–344, 2007. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- 209.Milkiewicz M, Kelland C, Colgan S, Haas TL. Nitric oxide and p38 MAP kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol 208: 229–237, 2006. doi: 10.1002/jcp.20658. [DOI] [PubMed] [Google Scholar]
- 210.Knipp BS, Ailawadi G, Ford JW, Peterson DA, Eagleton MJ, Roelofs KJ, Hannawa KK, Deogracias MP, Ji B, Logsdon C, Graziano KD, Simeone DM, Thompson RW, Henke PK, Stanley JC, Upchurch GR Jr.. Increased MMP-9 expression and activity by aortic smooth muscle cells after nitric oxide synthase inhibition is associated with increased nuclear factor-kappaB and activator protein-1 activity. J Surg Res 116: 70–80, 2004. doi: 10.1016/S0022-4804(03)00306-8. [DOI] [PubMed] [Google Scholar]
- 211.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci USA 104: 16898–16903, 2007. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem 113: 163–172, 2007. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 213.Trott DW, Machin DR, Phuong TT, Adeyemo AO, Bloom SI, Bramwell RC, Sorensen ES, Lesniewski LA, Donato AJ. T cells mediate cell non-autonomous arterial ageing in mice. J Physiol 599: 3973–3991, 2021. doi: 10.1113/JP281698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gao YZ, Saphirstein RJ, Yamin R, Suki B, Morgan KG. Aging impairs smooth muscle-mediated regulation of aortic stiffness: a defect in shock absorption function? Am J Physiol Heart Circ Physiol 307: H1252–H1261, 2014. doi: 10.1152/ajpheart.00392.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 65: 370–377, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki-Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB; Anglo-Cardiff Collaboration Trial Investigators. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension 53: 524–531, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126615. [DOI] [PubMed] [Google Scholar]
- 217.Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen JA, Vogt B, Martin PY, Burnier M, Bochud M. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension 66: 85–92, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05177. [DOI] [PubMed] [Google Scholar]
- 218.Chirinos JA, Sardana M, Syed AA, Koppula MR, Varakantam S, Vasim I, Oldland HG, Phan TS, Drummen NEA, Vermeer C, Townsend RR, Akers SR, Wei W, Lakatta EG, Fedorova OV. Aldosterone, inactive matrix gla-protein, and large artery stiffness in hypertension. J Am Soc Hypertens 12: 681–689, 2018. doi: 10.1016/j.jash.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sardana M, Vasim I, Varakantam S, Kewan U, Tariq A, Koppula MR, Syed AA, Beraun M, Drummen NE, Vermeer C, Akers SR, Chirinos JA. Inactive matrix Gla-protein and arterial stiffness in type 2 diabetes mellitus. Am J Hypertens 30: 196–201, 2017. doi: 10.1093/ajh/hpw146. [DOI] [PubMed] [Google Scholar]
- 220.Puzantian H, Akers SR, Oldland G, Javaid K, Miller R, Ge Y, Ansari B, Lee J, Suri A, Hasmath Z, Townsend R, Chirinos JA. Circulating dephospho-uncarboxylated matrix Gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens 31: 988–994, 2018. doi: 10.1093/ajh/hpy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ghadie N, St-Pierre JP, Labrosse MR. The contribution of glycosaminoglycans/proteoglycans to aortic mechanics in health and disease: a critical review. IEEE Trans Biomed Eng 68: 3491–3500, 2021. doi: 10.1109/TBME.2021.3074053. [DOI] [PubMed] [Google Scholar]
- 222.Aikawa J, Munakata H, Isemura M, Ototani N, Yosizawa Z. Comparison of glycosaminoglycans from thoracic aortas of several mammals. Tohoku J Exp Med 143: 107–112, 1984. doi: 10.1620/tjem.143.107. [DOI] [PubMed] [Google Scholar]
- 223.Wight TN. Vessel proteoglycans and thrombogenesis. Prog Hemost Thromb 5: 1–39, 1980. [PubMed] [Google Scholar]
- 224.Beenakker JW, Ashcroft BA, Lindeman JH, Oosterkamp TH. Mechanical properties of the extracellular matrix of the aorta studied by enzymatic treatments. Biophys J 102: 1731–1737, 2012. doi: 10.1016/j.bpj.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gandley RE, McLaughlin MK, Koob TJ, Little SA, McGuffee LJ. Contribution of chondroitin-dermatan sulfate-containing proteoglycans to the function of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 273: H952–H960, 1997. doi: 10.1152/ajpheart.1997.273.2.H952. [DOI] [PubMed] [Google Scholar]
- 226.Mattson JM, Turcotte R, Zhang Y. Glycosaminoglycans contribute to extracellular matrix fiber recruitment and arterial wall mechanics. Biomech Model Mechanobiol 16: 213–225, 2017. doi: 10.1007/s10237-016-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Collier SR, Kanaley JA, Carhart R Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens 22: 678–686, 2008. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- 228.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55: 235–239, 2005. doi: 10.2170/jjphysiol.S2116. [DOI] [PubMed] [Google Scholar]
- 229.Pierce GL, Harris SA, Seals DR, Casey DP, Barlow PB, Stauss HM. Estimated aortic stiffness is independently associated with cardiac baroreflex sensitivity in humans: role of ageing and habitual endurance exercise. J Hum Hypertens 30: 513–520, 2016. doi: 10.1038/jhh.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cooper JN, Buchanich JM, Youk A, Brooks MM, Barinas-Mitchell E, Conroy MB, Sutton-Tyrrell K. Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atherosclerosis 223: 485–490, 2012. doi: 10.1016/j.atherosclerosis.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 55: 855–861, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol 38: 506–513, 2001. doi: 10.1016/S0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 233.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brunt VE, Minson CT. Heat therapy: mechanistic underpinnings and applications to cardiovascular health. J Appl Physiol 130: 1684–1704, 2021. doi: 10.1152/japplphysiol.00141.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens 24: 8–13, 2015. doi: 10.1097/MNH.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol 65: 1042–1050, 2015. doi: 10.1016/j.jacc.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Petersen KS, Clifton PM, Lister N, Keogh JB. Effect of weight loss induced by energy restriction on measures of arterial compliance: a systematic review and meta-analysis. Atherosclerosis 247: 7–20, 2016. doi: 10.1016/j.atherosclerosis.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 238.Pierce GL. Aortic stiffness in aging and hypertension: prevention and treatment with habitual aerobic exercise. Curr Hypertens Rep 19: 90, 2017. doi: 10.1007/s11906-017-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol 597: 4901–4914, 2019. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]