Abstract
Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Over 220 countries and territories have been affected by this virus, and the infection rate has continued to rise. As patients recover from the virus, many are experiencing lingering symptoms. Understanding the impact of demographics and comorbidities on symptom prevalence, manifestations, and severity is not only relevant during acute infection, it is critical to the clinical management of patients with post-acute sequelae of COVID-19, also known as PASC. Herein, we provide a comprehensive review on the most recent research related to PASC. Specifically, we focus on the description of the disorder itself, compared to acute COVID-19, and which types of patients are most affected by long-term sequelae. Further, we share recommendations for management of the most common complications of PASC.
Keywords: Post-acute sequelae of COVID-19, Complications, Management
Introduction
Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also known as the novel coronavirus (2019-nCoV). It was first reported in December 2019 within the Hubei Province in Wuhan, China. By March 11, 2020, the World Health Organization (WHO) had declared a global pandemic [1, 2]. Over 220 countries and territories have been affected globally. As of March 1, 2022, there are 437.5 million confirmed cases of COVID-19, with a total of 5.9 million deaths worldwide [3]. The transmission is primarily through large respiratory droplets and aerosols from infected people coughing, sneezing, laughing, and singing, but the virus has also been found in stool and urine [1, 4, 5]. The virus can be spread through mucous membranes [6]. Countries have undertaken measures to control outbreaks and to support the increased demand for COVID-19 care [1, 2]. A collective global effort has been launched to advance potential therapeutics and preventative care for COVID-19. This includes novel vaccines, curative agents, and supportive measures with varying degrees of success. This demand for care also includes many patients who suffer from sequelae of COVID-19 beyond the acute phase of infection.
Post-acute sequelae of COVID-19 (PASC) is a developing complication of SARS-CoV-2 infection, causing ongoing symptoms in patients who are beyond the acute phase yet have not completely recovered. Understanding the clinical manifestations of PASC is important for early detection and mitigation efforts. This also supports clinicians, with the goal of returning patients to pre-disease function.
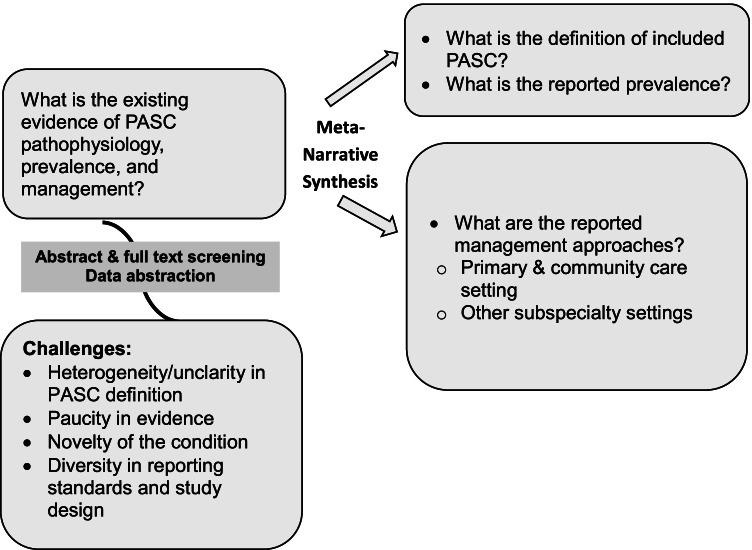
This review summarizes the best available evidence that explores, potential complications, and management plans of PASC following the Realist and MEta-Narrative Evidence Syntheses: Evolving Standards (RAMESES) [7]. In this meta-narrative systematic review, we present descriptive statistics and narratively without quantitative pooling. Figure 1 summarizes the framework used to explore and synthesize this evidence.
Fig. 1.
Mapping analysis
Background
Post-Acute and Long-Term Covid-19 Complications and Manifestations
The acute phase of COVID-19 infection is defined by the symptoms occurring the first 4 weeks after initial symptom onset [8, 9]. While evidence about stages beyond the acute phase is still evolving, some suggest the presence of symptoms beyond 12 weeks from onset of illness comprises post-acute COVID-19 syndrome as one group [10]; on the other hand, others have categorized post-acute COVID-19 patients with lingering symptoms into 4 groups [8]: 1) patients who have severe manifestations such as ARDS (acute respiratory distress syndrome), 2) those who require intensive care unit admission (ICU), 3) those not admitted who later present with signs of end-organ damage, such as cardiac or respiratory disease, and 4) those who do not require hospitalization but have prolonged symptoms without end-organ damage.
We propose the following simplified symptom course-based categorization:
Subacute or ongoing COVID-19 (PACS, post-acute COVID-19 syndrome) defined as symptoms continuing beyond the 4 weeks of acute infection, up to 12 weeks.
Post-COVID-19 syndrome / conditions (PCS, PCC, long-COVID, long-haulers’ syndrome) chronic ongoing COVID-19 symptoms beyond 12 weeks from acute infection.
Post-acute sequelae of COVID-19 (PASC) symptoms persisting beyond the 4-week period of acute infection. Both PACS (i.e., 4–12 weeks) and PCS (i.e., > 12 weeks) are identified as two different chronological stages of Post-COVID Conditions.
Methods
Literature Search
We conducted a literature search that included Cochrane Central Register of Controlled Trials October 2021, EBM Reviews—Cochrane Database of Systematic Reviews 2020 to December 03, 2020, Embase from January 2020 to December 2021, Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily from January 2020 to January 2022. The search included any studies that have enrolled patients who were treated for post-acute COVID-19 care. Our search terms were developed to attain all relatable publications on the given topics knowing that terminology is evolving and depending on current state of knowledge; these included post-acute syndrome, long hauler, long COVID, persistent COVID-19 syndrome, post-acute sequelae of COVID-19 (PASC), and chronic COVID-19.
Data Extraction and Synthesis
Data were extracted by a pair of independent reviewers and synthesized using a metanarrative approach. We extracted data on patients’ demographics, baseline characteristics, sample size, study type (pathophysiology, prevalence, management) and findings when meta-narratively synthesized, when reported. After the initial search and prior knowledge of existing paucity in the literature, while thoroughly observing the methodological heterogeneity between studies, and the non-standardized use of the defining PASC across the literature, insufficient reporting and diversity in range of evidence quality and sources regarding PASC etiology and outcomes, we synthesized the evidence using a meta-narrative assessment approach according to the RAMESES guidelines [7] and did not pursue meta-analysis as summarized in Fig. 1.
This type of systematic reviews follows a framework that includes planning, search, mapping, appraisal, and narrative synthesis. The narrative synthesis phase consists of identifying the key dimensions of the question of interest; providing a narrative account of the contribution of each dimension; explaining concordant and discordant findings; and considering the higher order data through overview of bias risk. Data then are presented using descriptive statistics and narratively without quantitative pooling as used in systematic reviews with meta-analysis.
Results
The initial search included 513 articles of which 117 eligible references were used to synthesize and summarize this narrative evidence.
Prevalence
While data remain scarce due to the novelty of this disease, it is estimated that PASC affects at least 10–30% of people who test positive for COVID-19 [9, 10]. Some patients have had 20 or more different symptoms, indicating COVID-19 is a multisystem disease. Acute COVID-19 has a worse prognosis in older patients (> 65), those with lower socioeconomic status, and those of certain ethnic groups including American Indian, Alaska Native, Hispanic/Latino, South Asian, and African American populations [11, 12]; there is not enough data to determine if this pattern extends to PASC, although Halpin et al. noted 42.1% of Black Asian Minority Ethnic (BAME) participants reported moderate to severe breathlessness vs. 25% of white patients [13]. Compared to South Asian women, fewer white women and men died than would be expected: (95% CI): white women 0.9 (0.8 to 1.0) and white men 0.9 (0.8 to 1.0) For those of black ethnicity, death rates were not different from the expected rates in the standard population [12].
Sex
Both men and women may present with any PASC symptom(s). Men are at greater risk for increased symptom severity and death [1, 14]; however, PASC is more common in women [15], and women are more likely to have persistent fatigue, anxiety, and depression at 6-month follow-up [15]. A study of 128 participants demonstrated 54% of women had fatigue at median of 10 weeks after initial COVID-19 symptoms [16].
Pathophysiological Mechanisms
The main mechanisms of acute COVID-19 include direct viral toxicity, endothelial damage and microvascular injury, hypercoagulability resulting in thrombosis and micro-thrombosis, immune system dysregulation and stimulation of a hyperinflammatory state, and maladaption of the angiotensin-converting enzyme 2 (ACE-2) pathway [9]. ACE-2 (angiotensin-converting enzyme 2) is an important regulator of the renin-angiotensin system (RAS), and SARS-CoV-2, which has a high affinity for ACE-2, enters human cells by binding to host ACE-2 receptors and entering through endocytosis or membrane fusion [17]. This direct tropism onto ACE-2 receptors of various tissues (endothelium, lung parenchyma, heart, kidneys, and intestines) is thought to cause multi-organ injury [17, 18].
There is little known about the pathophysiology of post-acute COVID-19, but it is thought to be multifactorial as multiple organs are involved [19]. Mechanisms which potentially contribute to the pathophysiology of post-acute COVID-19 include viral-specific pathophysiologic changes, immunologic aberrations, and inflammation in response to the acute infection, and sequelae of post-intensive care syndrome [9]. SARS-CoV-2 infection in patients with comorbidities may result in excessive cytokine release, cytokine storm, resulting in a destructive immune response, leading to acute respiratory distress syndrome (ARDS), multi-organ dysfunction syndrome (MODS), activation of coagulation pathways causing an imbalance of procoagulant and anticoagulant factors resulting in micro-thrombosis, disseminated intravascular coagulation (DIC), septic shock, multiorgan failure, and death [19]. Immune destruction can also result in autoimmune phenomena and molecular mimicry which lead to autoimmunity [20–24].
Homeostasis between the systemic inflammatory response syndrome (SIRS), and the compensatory anti-inflammatory response syndrome (CARS) determines clinical recovery or viral reactivation, secondary infections (bacterial, pulmonary aspergillosis), and death [9, 18].
Smoking Status
There is limited data on the contribution of tobacco use to poor outcomes in COVID-19; however, a few studies have found smoking was most likely associated with progression and negative outcomes of COVID-19 [25, 26]. This can be understood to be closely linked to the many known adverse effects of smoking.
Laboratory Findings
Blood tests should be ordered selectively, based on concern. Research is still needed to refine indications and interpretation of laboratory testing in post-acute COVID-19. Routine laboratory with a complete blood count (CBC) and comprehensive metabolic panel (CMP) should be considered in all patients with PASC. Significantly elevated white blood cell counts, marked leukopenia, high neutrophil counts, thrombocytopenia, decreased CD3, CD4, CD 8 T-lymphocyte counts, and elevations in certain inflammatory biomarkers (C-reactive protein, procalcitonin, cytokine IL-6, ferritin, and serum amyloid A) have been noted in the literature to be associated with severe disease [27]. It is unclear if laboratory findings with acute COVID-19 infection, may be prognosticators for PASC risk.
Comorbidities
Patients with underlying pulmonary conditions, obesity, and older age are at increased risk for developing PASC [28].
PASC and Management
Persistent viremia is thought to occur in patients who have a weak or absent antibody response, inflammatory or immune disorders, relapse, reinfection, deconditioning, or mental health disorders, such as post-traumatic stress disorder [29, 30]. General principles for the management of PASC include controlling comorbid conditions, such as diabetes, hypertension, kidney disease, respiratory diseases, and ischemic heart disease, and treating specific complications as necessary. Vital signs including temperature, heart rate, blood pressure, respiratory rate, and pulse oximetry should be measured routinely. Comprehensive physical, cognitive, and psychological assessments should be completed. Symptom-guided testing should be utilized for disease-specific evaluation of persistent symptoms. Antibiotics for secondary infections should be considered. Self-monitoring with FDA (Federal Drug Administration)-approved devices including pulse oximetry, blood glucose and blood pressure monitoring should be encouraged. Patients should also be encouraged to limit alcohol use, discontinue tobacco use, practice good sleep hygiene, and eat a well-balanced diet [10]. Mental health concerns should be addressed by connecting patients to support groups, mental health services, and community services, as job loss, financial stress, and food insecurity may negatively impact recovery [10]. Pediatric studies have been similar to adults in this regard, multiple and varied symptoms show that a multicomponent intervention will be required, and that mental and physical health symptoms occur concurrently [11, 31].
A summary of PASC management recommendations is noted in Table 1 [10, 15, 32–49].
Table 1.
Symptomatic management of PASC
| PASC symptoms that may be managed by primary care providers and generally do not require referral | |
| Constitutional |
• 80–90% of hospitalized with COVID-19 are successfully discharged • Management of patients with mild PASC should focus on functional status • There can be light aerobic exercise such as walking or Pilates, with a gradually increased intensity over a 4-to-6 week period, and with a phased return to work [10] • Fever can be treated with antipyretics such as acetaminophen or NSAIDs • Patients should be encouraged to monitor their general health and nutrition, get adequate sleep, and limit substances such as tobacco, alcohol, and caffeine • Patients should check daily pulse oximetry if indicated • Set SMART goals to manage activities of daily living -Specific, Measurable, Attainable, Realistic and Timely goals [32] |
| Gastrointestinal | • Symptomatic management includes that for nausea, vomiting, anorexia, diarrhea, GERD, and loss of appetite |
| Olfactory |
• For patients with persistent anosmia, olfactory training has been proposed as a self-management strategy by using essential oils or other strong odors on a regular basis for several weeks • Patients should minimize external distractions and focus at least 20 s on a single scent, and then move on to another to amplify the body’s natural mechanism of recovery by altering neural pathways [33] • Intranasal steroids (Mometasone furoate as example) have also been used in patients with loss of smell for more than 2 weeks associated with nasal symptoms [34] • Omega 3 fatty acids (no specific dose recommendation) shown to be beneficial in patients with isolated anosmia > 2 weeks, with resolution of other symptoms [34] |
| Ocular | • Symptomatic management of conjunctivitis, keratoconjunctivitis, or ocular irritation |
| Skin | • Non-specific erythematous rash, maculopapular rash, urticaria, vesicles, and chilblain-like lesions on extremities, sometimes called “COVID toe” are all managed per standard of care |
| Ability to exercise |
• The Stanford Hall consensus for returning to exercise recommends [35]: 1. Mild disease-One week of low grade stretching and strengthening before cardiovascular sessions 2. Mild disease-Limited activity such as slow walking, increased rest periods if symptoms worsen 3. If persistence of fatigue, breathlessness, cough, fever-limited activity to 60% maximum heart rate, until 2–3 weeks after symptoms resolve 4. If lymphopenia or oxygen requirement-requires respiratory assessment before resuming exercise 5. If cardiac involvement-requires cardiac assessment before resuming exercise |
| PASC symptoms that may require referral to subspecialties | |
| Chronic fatigue | • There is a potential role for Cognitive Behavioral Therapy (CBT) and Health & Wellness Coaching (HWC) programs focused nutritional status, graded exercise, mindfulness, and sleep hygiene, with the goal of improved quality of life |
| Respiratory complications |
• Chest imaging is necessary in patients with current respiratory symptoms and previous abnormal imaging • For most patients, a chest radiography is sufficient • Chest computed tomography (CT), if needed, should be based upon concern for underlying pathology, such as contrast-enhanced CT if malignancy is suspected, non-contrast high resolution CT for concern of interstitial lung disease, especially in patients who had ARDS • A chest radiograph is recommended at 12 weeks in patients who have had COVID pneumonia, as patients may have persistence of signs of lung damage, including ground-glass opacities, consolidation, and interlobular septal thickening • If the chest radiograph is abnormal, then a CT chest and consultation with pulmonologist is recommended • Lung abnormalities have been noted to persist on chest CT for > 6 months in 50% of previously hospitalized patients, even those with non-severe respiratory disease [36] • For patients with a normal chest radiograph, unexplained respiratory symptoms, and hypoxia, there should be a high index of suspicion for venous thromboembolism. These patients should be evaluated with CT-chest angiogram • Rarely, chest or neck discomfort related to venous stenosis from previous central venous catheterization is present and an ultrasound or phlebography may be needed [37] • Patients with persistent, progressive, new respiratory symptoms, or those who were hospitalized for respiratory symptoms, including COVID-19-related ARDS, may benefit from treatment for restrictive or obstructive disorders if uncovered after pulmonary function testing is completed. Pulmonary fibrosis noted on CT chest of patients after hospitalization, may be treated with oral steroid therapy and antifibrotic drugs (pirfenidone and nintedanib are currently being evaluated for use) [38] • This testing may include spirometry, lung volumes, and diffusion capacity • Patients recovering from severe lung infection, such as those requiring oxygen by high flow nasal canula or mechanical ventilation have been found to have impaired diffusing capacity for carbon monoxide in up to 56% of cases, and impaired exercise capacity during the first 6 months after discharge [15] • Referral to pulmonology for persistent abnormal chest x-ray, abnormal oximetry, or unexplained dyspnea is appropriate, as complete cardiopulmonary exercise testing may identify those who would benefit from pulmonary or physical rehabilitation; however, more data about appropriate timing of pulmonary function tests (PFTs) and follow-up PFTs is needed • For patients with chronic cough, if all other causes of cough have been ruled out, the treatment is supportive. Patients may also experience breathlessness after infection. In the absence of other underlying complications such as pleuritis or infection, patients should be instructed on breathing techniques to control or manage their symptoms • Breathing techniques include diaphragmatic breathing, slow deep breathing, yoga breathing, pursed lip breathing, or breathing through the nose and out through the mouth, slowly, aiming for a ratio of 1:2, in 5–10-min bursts throughout the day [10] |
| Hypoxia |
• Hypoxia associated with decreased diffusion capacity may occur as a sequela of COVID-19. It may be silent, which is to say asymptomatic, or be associated with increased work of breathing • Patients with hypoxia during the subacute phase of COVID-19 do not necessarily require pulmonary rehabilitation but should be monitored closely and supported with supplemental oxygen if necessary [39] • Monitoring oxygen saturation in post COVID-19 patients without “red flags” can be reassuring in those experiencing persistent breathlessness • Oxygen saturations of 96% or above in patients without chronic lung disease are expected. For those with chronic lung disease, saturations in the 88 to 92% range may be acceptable |
| Neurological |
• Patients should be evaluated for focal symptoms and sensory deficits • Unexplained muscle weakness may indicate the need for neurologic consult with electromyography and nerve conduction studies. Neurologic imaging is necessary only in the face of a neurologic deficit • Headache management includes amitriptyline, venlafaxine, and mirtazapine for tension-type headaches [40] • Migraine-like headaches may benefit from beta-blockers, neuromodulators, antidepressants, calcium channel blockers, or ACE inhibitors/angiotensin II receptor blockers [40] • Address mood, sleep, and stress disorders [40] |
| Dysautonomia and orthostatic intolerance |
Patient education regarding physiologic changes associated with orthostatic intolerance syndrome, and management may provide reassurance [41–43]. This includes: • Structured non-upright exercise such as swimming, or recumbent exercise bicycle should be encouraged • Fluid and salt repletion with 2–3 L of water daily, one to two teaspoons of salt daily, and limiting alcohol and caffeine are recommended • Avoiding exacerbating factors such as prolonged standing, warm environments, dehydration, and sudden changes in position. Consume small and frequent meals to avoid splanchnic vasodilatation • Compression garments extending to waist, or abdominal binders • Isometric exercises with sustained tensing of muscles to improve venous return to the heart and raise blood pressure may be beneficial. Counter-pressure maneuvers include tensing thigh, buttock muscles, crossing arms and legs, folding arms and leaning forward, squatting, or raising a leg and putting foot on a stool • Patients with POTS symptomology may benefit from pharmacological therapy such as Fludrocortisone, a fluid expander; Midodrine, a sympathomimetic alpha-1-agonist that increases vasoconstriction and venous return to heart; and Clonidine and Methyldopa, which improve hyperadrenergic symptoms caused by catecholamine surge on standing |
| Cardiovascular complications |
• Patients should be questioned about ongoing or intermittent dyspnea occurring with both rest and exertion; fatigue, cough, chest pain or discomfort experienced at rest, with exertion, or positionally; orthopnea, peripheral edema, palpitations, dizziness, orthostasis, syncope or presyncope; as well as any supplemental oxygen needs • It is important to determine if the symptoms are worsening, persistent, or new as late complications such as secondary bacterial pneumonia, empyema, pulmonary embolism, myocardial inflammation, and injury may occur • A 12-lead ECG is recommended to evaluate patients with cardiac symptoms, and if unremarkable, in the setting of persistent palpitations or dysautonomia symptoms, Holter monitoring is recommended • Patients with orthostatic symptoms may benefit from tilt table testing • An echocardiogram, chest CT, or CMR is recommended in patients with history of myocardial injury, myocarditis, dyspnea, or cardiac symptoms and signs such as chest pain not typical for pleuritic or musculoskeletal conditions, edema, and new murmurs • Left ventricular systolic function and heart failure should be managed according to standards of care • If echocardiography is normal, patients may need cardiopulmonary exercise testing • Patients should avoid intense cardiovascular exercise for three months after myocarditis or pericarditis; athletes should rest from cardiovascular training and competitive sports for 3–6 months until resolution of myocardial inflammation by CMR or troponin normalization and be followed by a cardiologist to guide them in their return to exercise [44] • Patients with significant cardiac injury and functional limitations would benefit from cardiac rehabilitation • Training and high-level sport may resume following myocarditis if left ventricular systolic function is normal, serum biomarkers of myocardial injury are normal, and if relevant arrhythmias are ruled out on 24-h ECG monitoring and exercise testing [42] • If returning to high–level sport or physically demanding occupation following myocarditis, patients are required to undergo periodic reassessment, in particular during the first 2 years [44] • There is insufficient evidence of long-term cardiac function after patients seems to have recovered, or for how long patients remain in a hypercoagulable state after acute infection [45] |
| Thromboembolic complications |
• Patients hospitalized typically receive prophylactic anticoagulation • Higher-risk patients may be discharged with 10 additional days of thromboprophylaxis [46], but there is no consensus on the benefit, or duration of prolonged prophylaxis with low molecular weight heparin after hospital discharge • If a patient has a thrombotic event, standard guidelines for anticoagulation should be followed • No data are available on the duration of hypercoagulability post-acute COVID-19 |
| Multisystem Inflammatory Syndrome (MIS) | • Intravenous immunoglobulins, adjunctive glucocorticoids, and low dose aspirin until coronary arteries are confirmed normal at least 4 weeks after diagnosis [47] |
| Post-Intensive Care Syndrome |
• Patients with protracted mental health disorders, should be referred for psychiatric care if experiencing moderate to severe symptoms • Therapy for PTSD may focus on trauma-based CBT, cognitive processing therapy, and/or eye movement desensitization and reprocessing [48] |
| Additional considerations |
• There are currently no studies showing any definite benefit of vitamin and mineral supplementation in the management of acute, subacute, or chronic COVID-19 symptoms • A healthy diet including vegetables, fruits, whole grains, legumes, nuts, and moderate amounts of fish, dairy, and poultry is recommended to aid in recovery • Patients should limit red and processed meats, as well as refined carbohydrates and sugar • Many supplements have been recommended for managing acute symptoms of COVID-19, such as vitamins A, C, D, and E, along with zinc, omega-3 fatty acids, and probiotics. Modest results have been noted with supplements, except vitamin C and zinc which showed no benefit [49]; these may be especially beneficial in older patients who often do not ingest enough of these nutrients due to changes in appetite, limited access to healthy foods, cost of groceries, and chronic health conditions • Bone density studies to evaluate for osteopenia and osteoporosis may be indicated in patients with prolonged illness and immobilization |
PASC Symptoms that may be Managed by Primary Care
The authors propose the following PASC symptoms may be managed by primary care providers if there is access, and generally do not require subspecialty referral.
-
A.
Constitutional
One-third or more of patients with PASC experience more than one symptom [48] and the most common of these are waxing and waning fatigue (15–87%), dyspnea (10–71%), chest pain or tightness (12–44%) cough (17–34%), and sleep disturbance (24%-26%) [15, 38, 50–52]. Patients may also report low-grade fever (< 101F), myalgias and weakness, arthralgias, sicca symptoms, rhinitis, headaches, loss of appetite, dysgeusia, brain fogginess/poor concentration, anosmia/hyposmia, alopecia, diarrhea, anxiety, depression, and posttraumatic stress disorder [15, 19, 50, 53]. Autonomic symptoms of night sweats, temperature dysregulation, dizziness, palpitations at rest or with mild exertion, and postural tachycardia may occur [38, 54]. A significant number of patients with mild disease experience symptoms months after acute infection [55], and symptoms may be cyclical [54].
Approximately 10 to 20% of patients require rehospitalization within 30 to 60 days, but most patients hospitalized with COVID-19 are successfully discharged [56, 57]. Patients with moderate to severe COVID-19 admitted to the hospital may experience persistence of symptoms for months post hospitalization (52–87%) [19, 28, 50].
-
B.
Gastrointestinal
The most common gastrointestinal symptoms include nausea, vomiting, diarrhea, anorexia, and loss of appetite [58–60]. Less frequent complaints include abdominal pain, distension, or gastrointestinal bleeding [58, 61, 62]. Gastroesophageal reflux symptoms also occur and may be treated with anti-reflux medications, such as proton-pump inhibitors or H2 blockers. Patients may develop gustatory dysfunction or dysgeusia, absent other symptoms, that persist beyond the acute phase of COVID-19 infection [58, 63]. Dysregulation of gut motility increased visceral hypersensitivity, dysbiosis, and alterations of gut autocrine axis are proposed mechanisms [64]. Symptomatic management and supportive care are recommended.
-
C.
Anosmia
Anosmia has been reported in 79.7% of patients during acute COVID-19, particularly in patients who also experienced fever, may occur in the absence of other symptoms, and may persist after recovery from acute infection in up to 56% of patients [58, 65, 66]. One systematic review and metanalysis of 24 studies (N 8438) showed persistence of anosmia in 41% of patients and ageusia in 38.2% [67]. Whether these symptoms are permanent, or transient in all patients is unknown.
-
D.
Ocular
Ocular manifestations are not well described. One meta-analysis and systematic review involving 1533 patients, revealed 11.2% had ocular symptoms, primarily conjunctivitis (86.4%), ocular pain, dry eye, and floaters [68]. SARS-CoV-2 is rarely associated with inflammatory responses of the ocular surface and the natural history is rapid self-limited conjunctivitis, without complications, or effect on visual acuity. However, case reports of episcleritis, and keratoconjunctivitis from herpes-like pseudodendritic infiltration on the cornea, resulting in a decline in vision have been reported [69], and may require the care of an eye specialist.
-
E.
Skin
Skin manifestations are noted in up to 20% of patients, and include exanthematous, vesicular, and papulosquamous eruptions, urticarial and erythema multiforme-like reactions, confluent erythematous maculopapular-morbilliform rashes, thrombotic purpura, livedo reticularis, and acral purpuric nodules similar to idiopathic perniosis (chilblains), sometimes called “COVID toe” [10, 58, 70].
-
F.
Endocrine
Cases of diabetic ketoacidosis, subacute thyroiditis, Hashimoto’s thyroiditis, bone demineralization from steroid use, vitamin D deficiency, and immobilization have been noted in patients with PASC [19].
PASC Symptoms that may Require Referral to Subspecialties
It is the authors’ opinion that patients with persistent multisystem symptoms, especially if lasting > 12 weeks, may benefit from being evaluated in a COVID-19 recovery clinic or subspecialty clinic for specific symptoms. We summarize the most observed presentations.
-
A.
Chronic Fatigue
Some patients experience persistent fatigue, similar to chronic fatigue syndrome or central sensitization syndrome with chronic fatigue [71]. Other possible causes including nutritional deficiencies should be investigated and corrected if possible. There is a paucity of information on the management of patients with fatigue, myalgias, and exercise intolerance. Some multidisciplinary centers, such as Mayo Clinic, have implemented cognitive behavioral treatment programs to assist patients in recovering from these symptoms [72, 73].
-
B.
Respiratory Complications
Patients may have persistent, progressive, or new respiratory symptoms. Those hospitalized for respiratory symptoms, may have other complications, such as COVID-19 related acute respiratory distress syndrome (ARDS) [72]. Studies have shown patients with severe acute COVID-19 (requiring high-flow nasal cannula, non-invasive, or invasive mechanical ventilation) are at greatest risk for long-term pulmonary complications including persistent diffusion impairment and pulmonary fibrosis [9, 15].
Dyspnea with or without oxygen dependence is a common persistent symptom beyond acute COVID-19, occurring in 42–66% of patients 60–100 days after acute infection [9, 13, 50, 74, 75]. In addition to respiratory causes, patients should also be evaluated for anemia, evidence of pulmonary embolism, and cardiac causes, such as heart failure [38]. Self-monitoring over three to five days with normal baseline saturations may provide reassurance to patients with dyspnea for which no other cause has been found.
Dyspnea after COVID-19 pneumonia is multifactorial-associated with resolving pneumonia (including scarring from inflammation), deconditioning, neuromuscular weakness, heart failure, post-intubation tracheal stenosis, and exacerbation of underlying lung disease (chronic obstructive lung disease, asthma, interstitial lung disease). Chronic cough persisting > 8 weeks is not uncommon.
Persistent need for supplemental oxygen for hypoxemia or need for continuous positive airway pressure (CPAP) or other breathing support while sleeping has been reported in 6.6% and 6.9% of patients, respectively, at 60 days post-acute COVID-19, and patients’ average six-minute walking distance has been noted to be lower because of dyspnea [9, 38].
Diffusion capacity reduction has been noted in post-acute COVID-19 and the more severe the acute illness, the more significant the reduction [9, 39]. Hospitalized patients have been noted to have restrictive lung disease at 3 and 6 months post-acute COVID-19 [9, 39, 76]. Studies have demonstrated ground-glass opacities by computed tomography at 6 months after acute infection [9, 15]. Fibrotic changes, including reticulations and traction bronchiectasis have also been noted 3 months after hospitalization in 25–65% of patients [9, 76, 77].
-
C.
Monitoring of Post-acute COVID-19 Hypoxia
Pulse oximetry is beneficial to the assessment and management of acute COVID-19 resulting in pneumonia, exacerbations of underlying lung disease, and thromboembolic disease. Prolonged hypoxia prior to and during hospitalization for COVID-19 is a risk factor for venous thromboembolism [78].
The British Thoracic Society defines the target range of oxygen saturation as 94–98%, and a level less than or equal to 92% as requiring oxygen in the setting of non-chronic respiratory failure [10]. Medicare guidelines use 88% as the level below which patients qualify for home oxygen.
An exertional desaturation test in patients with normal oxygen saturations greater than or equal to 96%, may be beneficial in patients with lightheadedness or severe breathlessness with exercise [10, 79]. A decline in saturation by 3% after walking 40 steps on a flat surface, and after one minute of sit-to-stand as fast as possible, is abnormal and requires further evaluation [10, 39].
Monitoring oxygen saturation in post COVID-19 patients without “red flags” can be reassuring in those experiencing persistent breathlessness. Oxygen saturations of 96% or above in patients without chronic lung disease are expected. For those with chronic lung disease, saturations in the 88 to 92% range may be acceptable. Patients with chest pain and significant breathlessness should be evaluated for pulmonary embolism [38].
Interstitial lung disease is unlikely in patients without hypoxia; however, long-term outcomes are not available, and patients who survive ARDS need to be monitored closely for persistent impairment of lung function.
Patients with hypoxia during the subacute phase of COVID-19 do not necessarily require pulmonary rehabilitation but should be monitored closely and supported with supplemental oxygen if necessary [39].
-
D.
Cardiovascular Complications
Underlying cardiovascular conditions may result in both severe and persistent disease, and atrial fibrillation triggered by hypoxia. The most prevalent cardiovascular complication of COVID-19 (8–12% of patients) is acute myocardial injury [58, 80, 81].
Cardiovascular PASC disorders that persist include myocarditis, fibrosis, scarring, and pericarditis [58, 82–87]. A study of 26 competitive college athletes with mild to asymptomatic disease who had cardiac magnetic resonance imaging (MRI) showed myocarditis in 15% of these patients. Interestingly, there was also evidence of previous myocardial injury noted in 30.8% [82–87].
Myocardial infarction, congestive heart failure, cardiomyopathy, and arrhythmias occur in approximately 20% of patients with underlying heart disease [15, 50, 88]. Evidence from cardiac MRI suggests myocardial inflammation may be as high as 60%, greater than 60 days after diagnosis of infection [88]. Chest pain has been noted in 20% of patients 60 days post-infection [50].
-
E.
Hematologic and Thromboembolic Complications
Elevated D-dimer levels have been associated with hypercoagulability, increased risk for pulmonary vasculature micro-thrombosis in patients admitted to the intensive care unit, and poorer prognosis in patients with COVID-19 [89, 90]. There is evidence for empiric anticoagulation among patients with COVID-19 with elevated D-dimer levels [91].
Many patients are at increased risk of arterial and venous thromboembolic events, because of the hyper-inflammatory and hypercoagulable nature of COVID-19 [46, 58, 90, 92–94]. These complications may present several weeks after acute COVID-19.
-
F.
Neurologic Complications
Many studies reported the presence of SARS-CoV-2 in cerebrospinal fluid and brain tissue of infected patients on autopsy [95]. Over 80% of hospitalized patients will have neurologic symptoms during their illness [96]. Neurological manifestations involve the central nervous system, peripheral nervous system, or skeletal muscles [21, 97].
Neurologic symptoms may persist after acute illness has resolved; patients have been found to experience meningitis, hypoxic encephalopathy (from decreased cardiac/pulmonary function), ischemic or hemorrhagic stroke (from coagulation disorders or depressed cardiac function), seizures, or neuromuscular weakness [58, 98, 99]. Autoimmune/para-infectious processes can result in Guillain–Barre, transverse myelitis, acute inflammatory demyelinating polyneuropathy, and autoimmune encephalitis [97, 99–101]. There may be movement disorders, such as Parkinsonism, myoclonus, and ataxia [101]. The most common symptoms are headache, and dizziness [99].
Encephalopathy and delirium have been described in almost 70% of ICU patients and can result in long-term cognitive impairment or brain fog [96, 99]. Post-COVID-19 brain fog can also occur because of dysautonomia, deconditioning, and posttraumatic stress disorder [19]. Cognitive dysfunction is noted to occur in approximately 88% of patients with PASC [101]. Psychiatric disorders, such as psychosis, obsessive–compulsive disorder (OCD), anxiety, depression, post-traumatic stress disorder (PTSD) and sleep disturbances, have been reported in 30–40% of COVID-19 survivors [102].
The true prevalence of post-COVID headache is unknown but is estimated to be 14–43% [103]. Medication overuse headaches, exacerbation of preexisting migraine headaches, and daily persistent headaches without prior headache history are potential etiologies [40, 103, 104]). COVID-associated headaches have been described as bifrontal, holocranial, frequently last for > 24 h, worse in the evening, exacerbated by exercise and coughing, associated with photophobia and phonophobia) [40, 103, 104]. One percent of patients will present with trigeminal symptoms [103].
-
G.
Dysautonomia
Cytokine storm, direct viral mediation, autoantibodies to B-adrenoceptors, and muscarinic receptors have all been proposed for the relationship between COVID-19 and its effect on the autonomic nervous system. Patients presenting with persistent palpitations, chest pain, fatigue, breathlessness, presyncope, or syncope > 4 weeks after acute infection should have blood pressure and heart rate monitoring after five minutes lying down and 3 min standing to evaluate for orthostatic hypotension, defined as a fall of > 20 mmHg systolic and > 10 mmHg diastolic pressure after standing for 3 min [105]. Patients who have an increase in heart rate of ≥ 30 beats/minute when standing for > 30 s, or ≥ 40 beats/minute in the age group of 12–19 years, in the absence of orthostatic hypotension, may have postural orthostatic tachycardia syndrome (POTS) [106]. Deconditioning, hypovolemia, and prolonged bedrest may exacerbate orthostatic intolerance and POTS. The release of epinephrine and norepinephrine causes tachycardia in orthostatic intolerance, resulting in palpitations, breathlessness and chest pain sometimes seen in PASC. High catecholamines can cause paradoxical vasodilatation, sympathetic activity withdrawal, activation of the vagus nerve, and result in dizziness, hypotension, and possibly syncope [41–43].
-
H.
Multisystem Inflammatory Syndrome (MIS)
Although seen more commonly in pediatric population, MIS can also occur in adults (MIS-A) [21].
Diagnostic criteria include age > = 21 years, with subjective or documented fever (> = 38.0 C) for > = 24 h prior to or within 3 days of hospitalization and at least 3 clinical criteria occurring prior to or within the first 3 days of hospitalization. (At least one primary criterion), current or recent SARS-CoV-2 infection, elevated levels of at least two laboratories (CRP, ferritin, IL-6, ESR, procalcitonin), and the exclusion of other diagnoses [107]. The diagnosis is confirmed with (At least one primary criterion), current or recent SARS-CoV-2 infection, elevated levels of at least two laboratory tests (CRP, ferritin, IL-6, ESR, procalcitonin), and the exclusion of other diagnoses [107].
Primary clinical criteria are rash, non-purulent conjunctivitis, and severe cardiac illness (myocarditis, pericarditis, coronary artery dilatation/aneurysm, new-onset right or left ventricular dysfunction (LVEF < 50%), 2nd/3rd degree AV block, or ventricular tachycardia [107].
Secondary criteria include new-onset neurologic signs and symptoms (encephalopathy without prior cognitive impairment, seizures, meningeal signs, or peripheral neuropathy including Guillain–Barre syndrome), abdominal pain, vomiting or diarrhea, thrombocytopenia (platelet count < 150,000/microliters), shock, or hypotension unrelated to medical therapy [107]. Recent reports on 6-month follow up showed few organ-specific sequelae in pediatric patients with MIS, but still ongoing concern for poor exercise tolerance and mental health support [107, 108].
-
I.
Post-Intensive Care Syndrome (PICS)
Some PASC patients may experience neurocognitive dysfunction (poor memory and concentration), and mental health disorders such as anxiety, depression, or post-traumatic stress disorder (PTSD) [109] that are similar to the syndrome experienced in patients who have been admitted to intensive care environments. In a study of 100 patients discharged from the hospital, 24% reported PTSD, 18% had new or worsened memory problems, and 16% had new or worsened concentration problems [56]. Almost 50% of COVID-19 survivors reported a worsened quality of life [110], 22% had anxiety and/or depression [110], and 30–34% had persistent symptoms of PTSD at three months post infection [111].
After the first 6 months, patients suffer considerably from depression (21%) and anxiety (28%) [111]. These mental health conditions are thought to occur due to loneliness, social isolation, and changes in routine. It is important to note that the impact on physical health can affect responses to assessment tools such as the PHQ-9, designed to measure anxiety and depression in physically healthy populations.
-
J.
Nutrition
Patients with diabetes, hypertension, older age, and cancer tend to have more severe COVID-19 infections and are more likely to have long-term complications from COVID-19 and therefore may benefit from a nutritional evaluation. Observational studies have shown deficiencies in micronutrients, particularly Vitamin D [112]. However, there is no data showing that reversing such deficits improves COVID-19 outcomes. Also, there is no clear evidence about the benefits of vitamin and mineral supplementation in the management of acute, subacute, or chronic COVID-19 symptoms.
Discussion
There is ambiguity in defining differences between post-COVID syndrome (PCS), PACS, and PASC in the published literature [113–115]. We have given our own refinement for this categorization. PASC is becoming a recognizable and crucial clinical entity as data continue to inform of its pathogenesis, presentation, and clinical course. Anecdotal reporting has also raised questions about the implications of receiving a COVID-19 vaccine and the improvement in PASC. One out of five patients report improvement, mainly brain fog and anosmia, after SARS-CoV-2 vaccination [116]. Two-thirds of patients with PASC saw no worsening of symptoms after vaccination; fever, chills, and gastro-intestinal symptoms were frequently reported, similar to the general population [116]. This will require further research.
The current understanding suggests PASC is caused by ACE2 receptor-binding, immune-dysregulation, and stimulation of hyper-inflammatory and hyper-coagulation pathways [9]. Preexisting conditions and the relationship to PASC remain largely unclear. Demographic data show higher prevalence and severity in females, with significant impact on under-resourced and disparate communities.
The long-term management of PASC remains largely unknown. The most reported follow-up assessments include quality of life, functional neurocognitive and psychological evaluations, pulmonary function screening, and occupational rehabilitation. The multiorgan and heterogenous manifestation of PASC requires the need for multidisciplinary, coordinated care management that includes peer and community support for patients presenting with PACS.
The strength of this review study is the succinct, up-to-date information it provides, including a table summarizing current management recommendations from literature, focused to general physicians, and primary care providers who are most likely to see PASC patients front-line. The meta-narrative review approach is deemed feasible, robust, and appropriate at this stage to outline existing evidence and gaps in knowledge for future direction. Limitations of this review study include the paucity of information regarding the underlying etiology of PASC, limited data on patient symptoms and duration, limited information in the literature on the management of PASC, and little data on the prevalence of PASC in unvaccinated vs. vaccinated individuals. By structuring our review on specialty referrals, generalizability is limited to higher income countries where this is the norm for health care systems. Further data are still yet to be seen on the prevalence of PASC in lower income regions.
Ongoing studies to improve our understanding and ability to treat PASC are underway and seek to elucidate the clinical spectrum and biology of those that have recovered from COVID-19 infection, as well as understand the phenotype of those patients who do not fully recover. Further studies are also needed to understand the long-term implications of COVID-19 and PASC as it is currently uncertain if the SARS-CoV-2 infection initiates or promotes the pathogenesis of conditions or findings previously discussed or increases the risk of development of other disorders. There are many ongoing efforts to further understand PASC, including the National Institute of Health (NIH) initiative launch [117]. Open and collaborative efforts may significantly provide pathways to better report, understand, and explore best practices for managing PASC.
Conclusion
The lasting effect of COVID-19 involves multiple organ systems requiring ongoing medical attention well after acute illness has passed. Adequate treatment in most cases requires a multi-disciplinary approach. Much research has been gained on PASC, but more is still needed to examine the self-reported phenomena of PASC, and to reduce healthcare treatment and disease burden on these patients. This will move clinicians forward to better deliver evidence-based and patient-centered care.
Abbreviations
- ICU
(Intensive care unit)
- ARDS
(Acute respiratory distress syndrome)
- COVID-19
(Coronavirus Disease 2019)
- SARS-CoV-2
(Severe acute respiratory syndrome coronavirus 2)
- PASC
(Post-acute sequelae of COVID-19)
- 2019-nCoV
(Novel coronavirus)
- WHO
(World Health Organization)
- PACS
(post-acute COVID-19 syndrome)
- PCS
(post-COVID syndrome)
- PCC
(post-COVID conditions)
- MIS
(Multisystem Inflammatory Syndrome)
- PICS
(Post-intensive care syndrome)
- PTSD
(Post-traumatic stress disorder)
- PHQ-9
(Patient Health Questionnaire-9)
- RAMESES
(Realist and MEta-Narrative Evidence Syntheses: Evolving Standards)
- BAME
(Black Asian Minority Ethnic)
- ACE-2
(angiotensin-converting enzyme 2)
- RAS
(renin-angiotensin system)
- ARDS
(acute respiratory distress syndrome)
- MODS
(multi-organ dysfunction syndrome)
- FDA
(Federal Drug Administration)
- DIC
(disseminated intravascular coagulation)
- SIRS
(systemic inflammatory response syndrome)
- CARS
(compensatory anti-inflammatory response syndrome)
- CBC
(complete blood count)
- CMP
(comprehensive metabolic panel)
- MRI
(magnetic resonance imaging)
- POTS
(postural orthostatic tachycardia syndrome)
- CRP
(C-reactive protein)
- ESR
(erythrocyte sedimentation rate)
- LVEF
(left ventricular ejection fraction)
- AV
(atrioventricular)
- NIH
(National Institute of Health)
- CPAP
(continuous positive airway pressure)
- OCD
(obsessive–compulsive disorder)
Authors' Contributions
BM and AMAD contributed to the hypothesis and generation. All co-authors contributed to study protocol and input about the literature search. BM, LS, and AMAD contributed to data abstraction and synthesis. BM, LS, and AMAD contributed to the main draft. BM, AMAD, DK, ND reviewed the second draft. All co-authors contributed to finalizing the manuscript.
Availability of Data and Material (Data Transparency)
This is a meta-narrative review and availability of data and material is not applicable.
Code Availability (Software Application or Custom Code)
Not applicable.
Declarations
Ethics Approval (Include Appropriate Approvals or Waivers)
This is a meta-narrative review and ethical approval is not applicable.
Consent to Participate (Include Appropriate Statements)
This is a meta-narrative review and consent to participate is not applicable.
Consent for Publication (Include Appropriate Statements)
This is a meta-narrative review. All co-authors consent to being part of this work and no other consent for publication is required or applicable.
Conflicts of Interest/Competing Interests (Include Appropriate Disclosures)
The authors report no conflict of interest.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barek A, Aziz A, Safiquel Islam M. Impact of age, sex, comorbidities, and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6(12):e05684. doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2020. WHO Director-General’s Opening Remarks at the media Briefing on COVID-19. March 11, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar] .
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar M, Al Khodor S. Pathophysiology and treatment strategies for COVID-19. J Transl Med. 2020;18:353. doi: 10.1186/s12967-020-02520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: meta-narrative reviews. BMC Med. 2013;11:20. doi: 10.1186/1741-7015-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long COVID” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144. doi: 10.1186/212913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 11.Wilder JM. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapey E, Gallier S, Mainey C, et al. Ethnicity and risk of death in patients hospitalized for COVID-19 infection in the UK: an observational cohort study in an urban catchment area. BMJ Open Respir Res. 2020;7(1):e000644. doi: 10.1136/bmjresp-2020-000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman, NAP, Peddu, V, Xie, H. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLos Biol. 2020; 18(9). [DOI] [PMC free article] [PubMed]
- 15.Huang C, Huang L, Wang Y, et al. 6 month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathangey G, Fadudu PP, Hospodr AR, Abbas AE. Angiotensin-converting enzymes 2 and COVID-19: patients, comorbidities, and therapies. Am J Physiol Lung Cell Mol Physiol. 2021;320(3):L301–L330. doi: 10.1152/ajplung.00259.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chippa, V, Aleem, A, Anjum, F. Post Acute Coronavirus (COVID-19) Syndrome. StatPearls.2021 Oct.PMID:34033370. [PubMed]
- 20.Gasparotto M, Framba V, Piovella C, et al. Post-COVID-19 arthritis: a case reports and literature review. Clin Rheumatol. 2021;40:3357–3362. doi: 10.1007/s10067-020-05550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson LA, Canna SW, Griedman KG, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020;72(11):1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharias H, Dubey S, Koduri G, D’Cruz D. Rheumatological complications of Covid 19. Autoimmun Rev. 2021;20(9):102883. doi: 10.1016/j.autrev.2021.102883.Epub2021Jul5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roongta R, Chattopadhyay A, Ghosh A. Correspondence on ‘Onset of rheumatoid arthritis after COVID-19: coincidence or connected?’. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220479. [DOI] [PubMed] [Google Scholar]
- 24.Derksen VFAM, Kissel T, Lamers-Karnebeek FBG, et al. Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-219859. [DOI] [PubMed] [Google Scholar]
- 25.Smoking and COVID-19. World Health Organization. 2020. https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19. Accessed 19 Mar 2022.
- 26.Alqahtani, JS, Oyelade, T, Aldhahir, AM. Prevalence, severity, and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020; 15(5). [DOI] [PMC free article] [PubMed]
- 27.Tjendra Y, Al Mana AF, Espejo AP, et al. Predicting disease severity and outcome in COVID-19 patients: a review of multiple biomarkers. Arch Pathol Lab Med. 2020;144(2):1465–1474. doi: 10.5858/arpa.2020-0471-SA. [DOI] [PubMed] [Google Scholar]
- 28.Halpin S, O’Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242–1243. doi: 10.1002/jmv.26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desimmie BA, Raru YY, Awadh HM, et al. Insights into SARS-CoV-2 persistence and its relevance. Viruses. 2021;13(6):1025. doi: 10.3390/v13061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukaetova-Ladinska EB, Kronenberg G, Raha-Chowdhury R. COVID-19 and neurocognitive disorders. Curr Opin Psychiatry. 2021;34(2):149–156. doi: 10.1097/YCO.0000000000000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson T, Shafran R, De Stavola B, et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open. 2021;11(8): e052838. Published 2021 Aug 26. 10.1136/bmjopen-2021-052838. [DOI] [PMC free article] [PubMed]
- 32.Rubin R. Will the Real SMART Goals Please Stand Up? 2002; http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.523.6999&rep=rep1&type=pdf, 2020. Accessed 19 Mar 2022.
- 33.Whitcroft KL, Hummel T. Olfactory Dysfunction in COVID-19 Diagnosis and Management. JAMA. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 34.Neta FI, Fernandes ACL, Vale AJM, et al. Pathophysiology and possible treatments for olfactory-gustatory disorders in patients affected by COVID-19. Curr Res Pharmacol Drug Discov. 2021;2:100035. doi: 10.1016/j.crphar.2021.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker-Davies, RM, O’Sullivan, O, Senaratne, KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020. 0:1–11. (https://bjsm.bmj.com/content/54/16/949). [DOI] [PMC free article] [PubMed]
- 36.Chen, Y, Ding, C, Yu, L, et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Medicine. 2021; 191. [DOI] [PMC free article] [PubMed]
- 37.Mickely V. Central vein obstruction in vascular access. Eur J Vasc Endovasc Surg. 2006;32(4):439–444. doi: 10.1016/j.ejvs.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Al-Jahdhami I, Al-Naamani K, Al-Mawali A. The Post-acute COVID-19 Syndrome (Long COVID) Oman Med J. 2021;36(1):e220. doi: 10.5001/omj.2021.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenhalgh, T, Javid, B, Knight, M, et al. What is the efficacy and safety of rapid exercise tests for exertional desaturation in covid-19? Oxford COVID-19 Evidence Service 2020. [DOI] [PMC free article] [PubMed]
- 40.Membrilla JA, Caronnac J, Trigo-Lopez A, et al. Persistent headache after COVID-19: Pathophysiology, clinical, and treatment. Neurol Perspect. 2021;1(1):S31–S36. doi: 10.1016/j.neurop.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman R, Abuzinadah AR, Gibbons C, et al. Orthostatic hypotension: JACC State-of-the-Art review. J Am Coll Cardiol. 2018;72:1294–1309. doi: 10.1016/j.jacc.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 42.Jardine DL, Wieling W, Brignole M, et al. The pathophysiology of the vasovagal response. Heart Rhythm. 2018;15:9221–9929. doi: 10.1016/j.hrthm.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology, and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 44.Pelliccia A, Solberg EE, Papadakis M, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology. Eur Heart J. 2019;40:19–33. doi: 10.1093/eurheartj/ehy730. [DOI] [PubMed] [Google Scholar]
- 45.Mitrani RD, Dabas N, et al. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17(11):1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.British Thoracic Society. BTS guidance on venous thromboembolic disease in patients with COVID-19, 2020.
- 47.Henderson, LA, Canna, SW, Friedman, KG, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19. Version 2.2021; 73 (4): e13-e29. [DOI] [PMC free article] [PubMed]
- 48.Watkins LE, Sprang KR, Rothbaum BO. Treating PTSD: a review of evidence-based psychotherapy interventions. Front Behav Neurosci. 2018;12:258. doi: 10.3389/fnbeh.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Louca, P, Murray, B, Klaser, K, et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445, 850 users of the COVID-19 Symptom study app. BMJ Nutr Prev Health. 2021; 4(1). 10.1136/bmjnph-2021-000250 [DOI] [PMC free article] [PubMed]
- 50.Carfi A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold DT, Hamilton FW, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knight D, Munipalli B, Logvinov II, Halkar MG, Mitri G, Dabrh A and Hines SL. Perception, Prevalence, and Prediction of Severe Infection and Post-acute Sequelae of COVID-19. The American journal of the medical sciences. 2022; S0002–9629(22)00002–7. Advance online publication. [DOI] [PMC free article] [PubMed]
- 53.COVID Symptom Study. How long does COVID-19 last? Kings College London, 2020. https://covid19.joinzoe.com/post/covid-long-term?fbclid=lwAR1RxlcmmdL-EFjh_al.
- 54.Nath A. Long-Haul COVID. Neurology. 2020;95:559560. doi: 10.1212/WNL.0000000000010640. [DOI] [PubMed] [Google Scholar]
- 55.Tenforde MW, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, March-June 2020. Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowles KH, McDonald M, Barron Y, et al. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174:316. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325:304. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baj J, Karakula-Juchnowicz H, Teresinski G, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6):1753–1784. doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, Z, Zhao, N, Shu, Y, et al. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology 2020. [DOI] [PMC free article] [PubMed]
- 62.Guotao L, Xingpeng Z, Zhihui D, et al. SARS-CoV-2 infection presenting with hematochezia. Med Mal Infect. 2020;50:293–296. doi: 10.1016/j.medmal.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aroniadis, OC, DiMaio, CJ, Dixon, RE, et al. Current knowledge and research priorities in the digestive manifestations of COVID-19. Clin Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed]
- 64.Schmulson M, Ghoshal UC, Barbara G. Managing the inevitable surge of post-COVID-19 functional gastrointestinal disorders. Am J Gastroenterol. 2021;116(1):4–7. doi: 10.14309/ajg.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 65.Luers JC, Rokohl AC, Loreck N, et al. Olfactory and gustatory dysfunction in coronavirus disease 19 (COVID-19) Clin Infect Dis. 2020;10:1093. doi: 10.1093/cid/ciaa525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lechien, JR, Chiesa-Estombia, CM, De Siati, DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Oto-Rhino-Layngol. 2020:1–11. [DOI] [PMC free article] [PubMed]
- 67.Agyeman AA, Chin KL. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inomata T, Kitazawa K, Kuno T, et al. Clinical and prodromal ocular symptoms in coronavirus disease: a systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(10):29. doi: 10.1167/iovs.61.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitazawa K, Deinhardt-Emmer S, Inomata T, et al. The Transmission of SARS-CoV-2 infection on the ocular surface and prevention strategies. Cells. 2021;10(4):796. doi: 10.3390/cells10040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magro C, Nuovo G, Mulvey J, et al. The Skin as a critical window in unveiling the pathophysiologic principles of COVID-19. Clin Dermatol. 2021 doi: 10.1016/j.clindermatol.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bierle DM, Aakre CA, Grach SL, et al. Central sensitization phenotypes in post acute sequelae of SARS-CoV-2 infection (PASC): defining the Post COVID syndrome. J Prim Care Community Health. 2021;12:21501327211030826. doi: 10.1177/21501327211030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraser, E. Long term respiratory complications of covid-19. BMJ. 2020; 370:m3001. [DOI] [PubMed]
- 73.Post-COVID Recovery. Mayo Clinic. https://connect.mayoclinic.org/blog/post-covid-recovery/tab/next-steps. Accessed 19 Mar 2022.
- 74.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chopra V, Flanders SA, O’Malley M. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalizations. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 77.Zhao, YM, Shang, YM, Song, WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine.2020: 100463.10.1016/j.eclinm.2020. 100463.Epub2020 Jul 15. [DOI] [PMC free article] [PubMed]
- 78.Borvik T, Evensen LH, Morelli V, et al. Impact of respiratory symptoms and oxygen saturation on the risk of incident venous thromboembolism-the Tromso study. Res Pract Thromb Haemost. 2020;4(2):255–262. doi: 10.1002/rth2.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tobin, MJ, Laghi, F, Jubran, A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 2020. [DOI] [PMC free article] [PubMed]
- 80.Lippi, G, Lavie, CJ, Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed]
- 81.Kang, Y, Chen, T, Mui, D, et al. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020. [DOI] [PMC free article] [PubMed]
- 82.Rajpal S, Tong M, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from covid-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aghagoli G, Marin BG, Soliman LB, et al. Cardiac involvement in COVID-19 patients: risk factors, predictors, and complications: a review. J Card Surg. 2020;35(6):1302–1305. doi: 10.1111/jocs.14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 86.Atri D, Siddiqi HK, Lang JP, et al. COVID-19 for the Cardiologist. Basic Virology, Epidemiology, Cardiac Manifestations, and Potential Therapeutic Strategies. JACC Basic Transl Sci. 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res. 2020;126(10):1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puntmann VO, Carerj ML, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang, N, Li, D, Wang, X, Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020; 18(4):844–847. [PMC free article] [PubMed] [Google Scholar]. [DOI] [PMC free article] [PubMed]
- 90.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poggiali, E, Bastoni, D, Ioannilli, E, et al. Deep Vein Thrombosis and Pulmonary Embolism: Two Complications of COVID-19 Pneumonia? Eur J Case Rep Intern. Med. 2020; 7. 94. [DOI] [PMC free article] [PubMed]
- 94.Wichmann, D, Sperhake, JP, Lutgehetmann, M, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020. [DOI] [PubMed]
- 95.Li Y-C, Zhang Y, Bai-Hong T. What can cerebrospinal fluid testing and brain autopsies tell us about viral neuroinvasion of SARS-CoV-2. J Med Virol. 2021;93(7):4247–4257. doi: 10.1002/jmv.26943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020 doi: 10.1002/mv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. E Clin Med. 2021;15:101019. doi: 10.1016/j.eclinm2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Padroni M, Mastrangelo V, Asioli GM, et al. Guillain- Barre syndrome following COVID-19: new infection, old complication? J Neurol. 2020;1:1–3. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martelletti P, Bentivegna E, Luciani M, Spuntarelli V. Headache as a prognostic factor for COVID-19. Time to re-evaluate. SN Compr Clin Med. 2020;26:1–2. doi: 10.1007/s42399-020-00657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martelletti P, Bentivegna E, Spuntarelli V, Luciani M. Long-COVID Headache. SN Compr Clin Med. 2021;3(8):1704–1706. doi: 10.1007/s42399-021-00964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930–936. doi: 10.1111/j.1468-1331.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 106.Sheldon RS, Grubb BP, 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.https://www.cdc.gov/mis/mis-a/hcp.html
- 108.Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health 2021. [DOI] [PubMed]
- 109.Xiong J, Lipsitz O, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong AW, Shah AS, et al. Patient-reported outcome measures after COVID-19: a prospective cohort study. Er Respir J. 2020;56(5):2003276. doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamamoto V, Bolanos JF, Fiallos J, et al. COVID-19: review of a 21st century pandemic from Etiology to neuro-psychiatric implications. J Alzheimers Dis. 2020;77(2):459–504. doi: 10.3233/JAD-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Munshi R, Hussein MH, Toraih EA, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93:733. doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 113.Sivan M, Taylor S. NICE guidelines on long covid. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 114.Rando, HM, Bennett, TD, Byrd, JB, et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv. Version 1. Preprint.2021 Mar. 10.1101/2021.03.20.21253896. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8010765.
- 115.Peluso, MJ, Kelly, JD, Lu, S, et al. Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19.medRxiv. 2021 Mar 12; 2021.03.11.212522311.10.1101/2021.03.11.21252311.
- 116.Scherlinger, M, Pijnenburg, L, Chatelus, E, et al. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines (Basel). 2022 Jan; 10(1): 46. Published online 2021 Dec 30. 10.3390/vaccines10010046. [DOI] [PMC free article] [PubMed]
- 117.https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a meta-narrative review and availability of data and material is not applicable.
Not applicable.