Abstract
The skin forms a crucial, dynamic barrier between an animal and the external world. In mammals, three stem cell populations possess robust regenerative potential to maintain and repair the body's protective surface: epidermal stem cells, which maintain the stratified epidermis; hair follicle stem cells, which power the cyclic growth of the hair follicle; and melanocyte stem cells, which regenerate pigment-producing melanocytes to color the skin and hair. These stem cells reside in complex microenvironments (“niches”) comprising diverse cellular repertoires that enable stem cells to rejuvenate tissues during homeostasis and regenerate them upon injury. Beyond their niches, skin stem cells can also sense and respond to fluctuations in organismal health or changes outside the body. Here, we review these diverse cellular interactions and highlight how far-reaching signals can be transmitted at the local level to enable skin stem cells to tailor their actions to suit the particular occasion and optimize fitness.
The formation, maintenance, and regeneration of tissues and organs is a wonder of molecular and cellular choreography, and nowhere is this more intricate than in the mammalian skin. The skin serves as a physical barrier protecting organisms from insults, infection, and dehydration. It regulates body temperature and enables remarkably complex arrays of sensations. It contains multiple stem cell populations that maintain and repair tissues throughout life. And it does so in an ever-changing environment. These multifaceted functions are enabled by a rich array of signals and cell types that converge on stem cell niches (Fig. 1).
Figure 1.
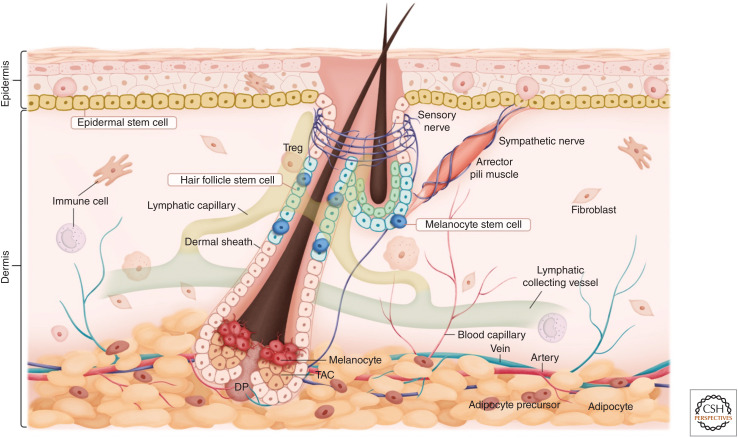
Skin stem cells and their niches. The skin is a complex organ composed of cell types from diverse lineages. With the exception of higher primates, whose eccrine sweat glands and associated stem cells are highly abundant, most mammals have three major skin stem cell populations—epidermal stem cells, hair follicle stem cells, and melanocyte stem cells. These stem cells regenerate in rich microenvironments (niches) replete with diverse cell types, including distinct fibroblast populations, sensory and sympathetic innervations, a vast array of immune cells, blood vessels, lymphatic capillaries, and subcutaneous adipocytes. In addition to their specialized functions, complex interactions among these cell types underlie tissue formation, homeostasis, and repair in the skin. (TAC) Transit-amplifying cell, (DP) dermal papilla.
The establishment of mammalian skin stem cells and their niches occurs over the course of embryonic development. Shortly after gastrulation, the epidermis begins as a single layer of multipotent epithelial progenitors. As mesenchymal cells begin to populate the skin, the epithelial progenitors produce and secrete extracellular matrix (ECM) proteins that assemble into a “basement membrane” demarcating epidermis from dermis. During this time, specialized epithelial–mesenchymal communications begin to diversify the epithelial progenitors, leading to the specification of hair follicles and stratification of the epidermis. Melanoblasts, the embryonic precursors of melanocytes, migrate from the neural crest and populate the epidermis and hair follicles. As development proceeds, additional cell types populate the dermis to form blood vessels, lymphatic vessels, muscles, and adipose tissue. Sensory and sympathetic neurons begin to innervate distinct regions of the skin, while a complex array of immune cells take up residence in the skin with some cells moving into the epidermis and others staying in the dermis. Altogether, the mature skin is composed of >50 distinct cell types (Joost et al. 2020).
BOX 1. GLOSSARY.
Extracellular matrix (ECM): Extracellular depositions produced and secreted by cells. It consists of collagen fibers, proteoglycans, fibronectin, and laminin, which together form a structurally stable network that protects and supports tissues and modulates signaling pathways by sequestering secreted proteins.
Basement membrane: A sheet-like structure composed of ECM that separates the epithelium from the underlying mesenchyme within an organ.
Lymphatic vessels: Vascular structures crucial for carrying lymphatic fluids (lymph) and immune cells. Lymphatic vessels connect with lymphatic capillaries in peripheral tissues and transport lymph to and from the lymph nodes.
Bulge: A protrusion of the hair follicle that hosts hair follicle stem cells and melanocyte stem cells.
Transit-amplifying cells (TACs): A population of highly proliferative stem cell progeny responsible for the majority of tissue production. Stem cells proliferate sparingly to produce TACs, which then undergo rapid proliferation to produce downstream differentiated progeny. In contrast to stem cells, which are long-lived, TACs are short-lived and can only undergo a finite number of divisions before differentiation or death.
Adrenal glands: Triangular-shaped endocrine glands located on top of each kidney. Adrenal glands produce several hormones crucial for body physiology and stress responses. The key hormones include cortisol (corticosterone in mice), epinephrine (also known as adrenaline), norepinephrine (also known as noradrenaline), and aldosterone. Cortisol, epinephrine, and norepinephrine all become elevated under stress. Cortisol suppresses immune responses and regulates blood sugar levels. Epinephrine and norepinephrine trigger the “adrenaline rush” that works together with the sympathetic nervous system (see “autonomic nervous system” below) to increase heart rate and redirect blood flow to the brain and muscle. Aldosterone regulates salt balance and blood pressure.
Autonomic nervous system: A component of the peripheral nervous system that regulates involuntary processes and unconscious responses. The autonomic nervous system consists of the sympathetic nervous system and the parasympathetic nervous system. The sympathetic nervous system innervates essentially all organs and is active constantly at a basal level to maintain body physiology. Under stress, the sympathetic nervous system becomes highly activated, triggering the so-called “fight-or-flight” response, which includes increased heart rate, pupal dilation, and increased blood pressure. These responses allow animals to respond quickly to threats. By contrast, the parasympathetic nervous system counteracts the effect from the sympathetic nervous system and is responsible for the “rest-and-digest” response, which brings the stress response back to a baseline.
Arrector pili muscle (APM): A bundle of smooth muscle cells in the skin responsible for the “goosebump” reaction.
Macrophages: Specialized immune cells involved in engulfing bacteria and clearing tissue debris.
T regulatory cells (Treg): A subset of T cells crucial for suppressing T-cell-mediated immune attack, thereby preventing autoimmunity and establishing self-tolerance.
Major histocompatibility complex (MHC class I) molecules: One class of cell surface recognition molecules that are expressed in all nucleated somatic cells. MHC class I molecules present peptides to cytotoxic T cells to trigger immune responses.
pKa: The negative base 10 logarithm of the acid dissociation constant (Ka) of a solution (pKa = −log Ka). pKa indicates how strong or weak an acid is. The lower the pKa, the stronger the strength of the acid.
With this dazzling array of cellular interactions emerging spatially and temporally, localized specialized microenvironments (“niches”) begin to form and specify the long-lived tissue resident stem cells of the adult epidermis, hair follicle, and melanocyte lineage, which as a cohort are among the most highly regenerative tissues in adult mammals. These stem cells reside along the basement membrane at the interface with the dermis. Epidermal stem cells (EpdSCs) reside in the innermost “basal” layer and are responsible for generating the terminally differentiating stratified layers that constitute the barrier that keeps microbes out and retains body fluids. Hair follicle stem cells (HFSCs) reside as a single layer within an anatomical niche referred to as the “bulge” that is responsible for fueling cyclic hair growth. Melanocyte stem cells (McSCs) intermingle with EpdSCs and HFSCs in their niches and are responsible for generating the pigment-producing melanocytes that color the skin and hair (Fig. 1).
Beyond their niches, skin stem cells are also sensitive to systemic changes in body physiology and dynamic changes in the external environment. Over the past decade, the field of skin stem cell biology has rapidly advanced, establishing the skin as a paradigm to understand fundamental principles in tissue regeneration and wound repair. These findings demonstrate that tissue regeneration is a highly collaborative process across lineages and cell types. Here, we discuss diverse mechanisms by which stem cells communicate with and respond to their niches, to physiological changes, and to the external environment. We also demonstrate how these interactions shape and modulate tissue turnover, regeneration, and injury repair.
HAIR FOLLICLE STEM CELLS
Hair—the key trait that characterizes mammals—grows out of the hair follicle. The hair follicle is an epithelial tissue that cycles through bouts of growth (anagen), regression (catagen), and rest (telogen) throughout an organism's life, a process known as the hair cycle (Fig. 2A). In humans, the anagen phase of the hair cycle can last for 3–7 years, while in mice it is typically 2 weeks. In mice, the first two hair cycles are synchronized, in which HFSCs display coordinated cycles of activation, destruction, and quiescence. In part, this synchrony is governed by an intricate array of lymphatic capillaries, which interconnect the bulge of each hair follicle and control their activity (Gur-Cohen et al. 2019; Peña-Jimenez et al. 2019).
Figure 2.
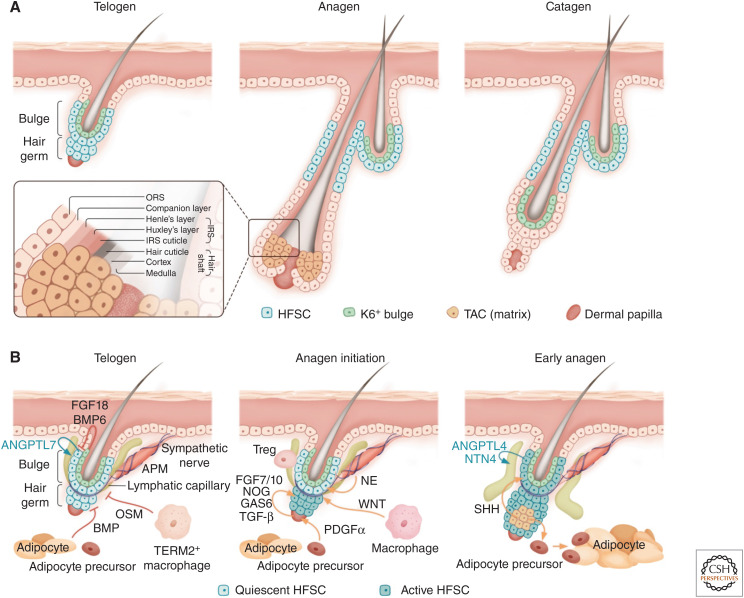
The hair follicle lineage and niche signals that regulate hair follicle stem cells (HFSCs). (A) Overview of the hair cycle. The telogen hair follicle contains HFSCs that are located at the outer bulge layer and the hair germ, as well as the K6 (keratin 6)+ inner bulge layer, which is made up of differentiated progeny of HFSCs. During anagen, the HFSCs proliferate to generate transit-amplifying cells (TACs), which undergo rapid proliferation and expansion to make all the differentiated layers of the anagen hair follicle (see insert)—the three layers of the hair shaft, the three layers of its channel (the inner root sheath [IRS]), and the companion layer sandwiched between ORS (outer root sheath) and IRS. In catagen, most of the hair follicle cells produced in anagen are destroyed or remolded, sparing some ORS cells to make a new bulge and hair germ that fuels the next hair cycle. (B) Niche signals regulate HFSC quiescence and activation. In telogen, signals from the K6+ bulge, mature adipocytes, and TERM2+ macrophages maintain HFSCs in a quiescent state. During the telogen to anagen transition, activating signals become highly up-regulated in the dermal papilla (DP). Signals from sympathetic neurons, Regulatory T cells (Tregs), adipocyte precursors, and macrophages have all been reported to contribute to HFSC activation in the hair germ. In early anagen, Sonic Hedgehog (SHH) secreted from the TACs further activates HFSCs located in the bulge. SHH also modulates activation signals expressed in the DP and promotes adipocyte precursors to make mature adipocytes. HFSCs are normally wrapped around tightly by lymphatic capillaries, a process mediated by HFSC-derived Angiopoietin-like 7 (ANGPTL7). During early anagen, HFSCs transiently switch to expressing Angiopoietin-like 4 (ANGPTL4) and Netrin-4 (NTN4), which promotes a transient dissociation between HFSCs and lymphatic capillaries to promote hair growth. (APM) Arrector pili muscle, (OSM) Oncostatin M, (NE) norepinephrine, (BMP) bone morphogenetic protein.
The hair follicle operates in a classical hierarchical model of regeneration, in which stem cells do not produce differentiated cells directly, but instead generate transit-amplifying cells (TACs) that proliferate rapidly to generate downstream differentiated progeny. Similar hierarchical models are commonly seen in regenerative tissues such as the hematopoietic system and the intestinal epithelium. At anagen onset, HFSCs in the hair germ proliferate transiently to produce short-lived TACs, which then undergo rapid proliferation and differentiation to produce seven morphologically and molecularly distinct differentiated cell types downstream—the three layers of the hair shaft, the three layers of the inner root sheath (IRS), and the companion layer sandwiched between the outer root sheath (ORS) and IRS (Fig. 2A; Greco et al. 2009; Rompolas et al. 2013). A specialized cluster of mesenchymal cells—the dermal papilla (DP)—regulates TAC fate choices, which become more restricted as anagen proceeds (Yang et al. 2017). This hierarchy enables HFSCs to be used sparingly, leaving the bulk of hair follicle regeneration and hair growth to the TACs. The design also means that stem cells, TACs, and differentiated progeny all reside in spatially defined compartments within a hair follicle—analogous to the entire hematopoietic lineage (stem cells—progenitors—differentiated cells) and yet packed in defined space within one tiny hair follicle. These features, in conjunction with the visible and trackable nature of hair growth, have established the hair follicle as a powerful model system that roots much of our fundamental understanding of current mammalian stem cell biology.
HFSCs stay in quiescence for most of the hair cycle but become activated to proliferate transiently during early anagen to regenerate new hair follicles (Fig. 2B). The stereotypic and highly predictable activation timing allows the identification of diverse niche cell types that can influence quiescence and activation decisions of stem cells—one of the most critical decisions for all somatic stem cells.
The Stem Cells in Control: Establishing and Shaping Their Own Niche
HFSCs in the bulge of the hair follicle are flanked internally by a layer of inner bulge cells that are differentiated progeny of HFSCs and marked by an intermediate filament keratin 6 (K6) expression (Hsu et al. 2011). K6+ bulge expresses high levels of inhibitory signals including fibroblast growth factor 18 (FGF18) and bone morphogenetic protein 6 (BMP6) to keep HFSCs in quiescence (Fig. 2B). Removal of the K6 bulge through plucking or genetic ablation leads to HFSC activation (Hsu et al. 2011). Besides sending inhibitory signals, the intercellular, cadherin-mediated adherens junctions between K6+ bulge and bulge HFSCs also forms a tightly sealed barrier to prevent bacteria from entering the skin through the hair orifice. Removal of E-cadherin in HFSCs compromises the tight seal between K6+ bulge and HFSCs, leading to a breach in this barrier and triggering HFSCs to release C-C motif chemokine ligand 1 (CCL1) and C-C motif chemokine ligand 2 (CCL2) to recruit dendritic cells and activate regulatory T cells, which protect against infection (Lay et al. 2018). In turn, the stimulated regulatory T cells feed back on the HFSCs to promote their proliferation and bandage the damaged niche. Increasingly, the field is appreciating that immune stem cell cross talk is a two-way street of checks and balances.
Similar to what is seen in the hematopoietic stem cells and in the intestine, HFSCs can also be subdivided into two anatomically distinct groups—a more quiescent population (located in the bulge), and a small “primed” cluster that is more readily activated (located at the bulge base or “hair germ”) (Cotsarelis et al. 1990; Blanpain et al. 2004; Morris et al. 2004; Tumbar et al. 2004; Greco et al. 2009; Yang et al. 2017). At anagen onset, HFSCs in the hair germ always become activated before HFSCs located at the bulge become activated (Fig. 2B).
While multiple niche cell types participate in activating HFSCs within the hair germ (see below for discussions on dermal cell types), HFSCs within the bulge rely on early progeny—the TACs—for their activation. As the hair germ grows to produce an early pool of TACs, the TACs produce Sonic Hedgehog (SHH), a secreted protein that can act long range to activate bulge HFSCs, prompting them to transiently self-renew before returning to quiescence again as the hair follicle grows downward (Hsu et al. 2014).
In another interesting twist of shaping their niches, telogen bulge HFSCs produce a secreted factor Angiopoietin-like 7 (ANGPTL7), which maintains a tight connection between lymphatic capillaries and HFSCs (Gur-Cohen et al. 2019). Just after anagen begins, bulge HFSCs transiently reduce ANGPTL7 expression and switch to briefly producing Angiopoietin-like 4 (ANGPTL4) and Netrin-4 (NTN4), which cause lymphatics to transiently disconnect from the bulge. This lymphangiogenic switch, governed by the HFSCs themselves, is in turn essential for the transient bulge proliferation during early anagen that restocks the HFSC pool.
The extent to which lymphatics and TACs regulate the balance between quiescence and tissue regeneration is still unfolding. However, it is intriguing that immediately after the bulge proliferation ceases in mid-anagen, ANGPTL7 levels rise again and lymphatics reconnect to the bulge, while the SHH-expressing TACs and their associated DP are progressively distanced from the bulge as the hair follicle grows downward.
Finally, during catagen, while most of the hair follicle cells are destroyed or differentiated, some cells in the lower ORS survive, returning back to the bulge area but become the differentiated inner K6+ bulge that inhibits HFSC activation (Hsu et al. 2011). Stem cells in the upper ORS, which reentered quiescence early in the hair cycle, also survive catagen and become the HFSCs for the next hair cycle. In this sense, HFSCs are essentially architects of their own niche—HFSCs produce and regulate progeny that impact their own behavior in return—stimulating their proliferation early in the hair cycle and promoting a prolonged quiescence phase following a regenerative bout. Feedback regulations from progeny to stem cells is now an established theme across vertebrate and invertebrate regenerative systems (for review, see Hsu and Fuchs 2012).
Epithelial–Mesenchymal Interactions
Although the hair follicle is separated from the dermal compartment by a basement membrane, it grows deep into the dermis as it regenerates. In so doing, it encounters multiple mesenchymal populations (such as adipocytes and diverse fibroblasts), providing an opportunity to study reciprocal interactions between epithelium and mesenchyme.
Dermal Papilla
During telogen, the hair germ is adjacent to the DP. Upon laser-mediated DP ablation in mice, hair follicles arrest at telogen, underscoring the importance of DP for anagen entry (Sennett and Rendl 2012; Rompolas et al. 2013). While HFSCs remain phenotypically quiescent throughout telogen, molecular cross talk between the hair germ and the DP undergoes dynamic changes transitioning from early (refractory) to late (competent) telogen (Greco et al. 2009). Secreted factors that activate HFSCs become up-regulated in DP during late telogen compared to early telogen, contributing to the timing at which quiescent HFSCs become reactivated to initiate the next hair cycle (Greco et al. 2009; Oshimori and Fuchs 2012; Rezza et al. 2016; Yang et al. 2017).
Reciprocal interactions between the hair follicle and the DP also control how long anagen lasts, offering a model to delineate how interactions between epithelium and mesenchyme regulate the length of the regeneration phase. Fgf5 becomes up-regulated in the lower ORS and TACs in late anagen hair follicles (Hébert et al. 1994). Moreover, Fgf5 mutant mice have significantly extended anagen and significantly longer hair, a phenotype that is recapitulated by knocking out receptors for FGF (both Fgfr1 and Fgfr2) in the DP (Hébert et al. 1994; Harshuk-Shabso et al. 2020). Collectively, these data are consistent with a model, whereby hair follicle–derived FGF5 acts on DP to initiate catagen.
In addition to FGF signaling, WNT and transforming growth factor β (TGF-β) signaling are also implicated in catagen control. During late anagen, secreted WNT agonists R-spondins gradually decrease in DP, while WNT antagonists Dickkopf2 (DKK2) and Notum increase in DP, which together lead to a decrease in WNT signaling in the hair follicle (Harshuk-Shabso et al. 2020). Thus, conditionally ablating β-catenin (Ctnnb1, a downstream component of WNT signaling) in HFSCs during telogen results in a perpetual state of HFSC quiescence and failure to reenter anagen (Lien et al. 2014), while ablation after anagen entry leads to a shortened anagen and accelerated catagen entry (Choi et al. 2013). These findings underscore the importance of WNT signaling for cell proliferation and fate determination in both HFSCs and TACs. In the hair follicle, the process is finely tuned through a WNT-regulated switch in β-catenin's DNA-binding partners (Yang et al. 2017; Adam et al. 2018). For TGF-β signaling, both lower ORS and DP have been suggested to be the source for TGF-β1 (Foitzik et al. 2000). Tgfb1-null mice enter catagen early (Foitzik et al. 2000), a phenotype that can be recapitulated when the TGF-β receptor is knocked out in the hair follicle (Mesa et al. 2015).
Dermal Adipocytes and Dermal Sheath
In addition to DP, additional mesenchymal cell types also participate in HFSC regulation. Dermal adipocyte precursors, a specialized fibroblast population responsible for dermal adipogenesis, secrete platelet-derived growth factor α (PDGFα), which likely acts on DP to modulate anagen entry (Festa et al. 2011). Adipocyte precursor–derived PDGFα also regulates the self-renewal of adipocyte precursors themselves (Rivera-Gonzalez et al. 2016). Mature dermal adipocytes, located at the lower dermis, have been suggested to be a source of BMPs that inhibit HFSC activation (Plikus et al. 2008; Keyes et al. 2013).
Signals from the hair follicle also orchestrate dermal changes, showing an example of how the growth of epithelium and mesenchyme can be coupled. Anagen and telogen hair follicles differ greatly in length. The anagen hair follicle is one of the most highly proliferative tissues in mammals—within 7 days, the hair follicle grows from a small cluster that contains just the HFSCs in telogen to a multilineage structure that is >60 times its original length in anagen. Such growth compels the surrounding dermis to undergo concurrent and rapid adipogenesis, which greatly expands the dermal space to accommodate rapidly down-growing hair follicles in anagen (Donati et al. 2014; Rivera-Gonzalez et al. 2016; Zhang et al. 2016). SHH, secreted from the TACs of the hair follicle, not only act on the bulge HFSCs in early anagen, but also on the shorter-lived progeny and DP to regulate hair follicle downgrowth (Woo et al. 2012; Hsu et al. 2014). Additionally, SHH acts on adipocyte precursors to trigger dermal adipogenesis (Fig. 2B). In catagen, TACs are destroyed, and the growth of the hair follicle and dermal adipogenesis both ceases. In this sense, the growth of both the epithelium and mesenchyme can be coupled with just one signal (Zhang et al. 2016).
Dermal sheath—a smooth muscle cloak that encases the hair follicle—also undergoes remodeling at different hair cycle stages. Anagen hair follicles are enclosed by dermal sheath throughout the entire hair follicle. During catagen, contraction of dermal sheath is activated by the calcium–calmodulin–myosin light chain kinase pathway and functions to draw the DP upward to meet the HFSCs again. In telogen, this dermal sheath is no longer visible, but its progenitors remain; in the next round of anagen, the progenitors regenerate a new dermal sheath and also may contribute to some of the DP cells (Rahmani et al. 2014; Heitman et al. 2020; Shin et al. 2020).
Reaching Outside the Skin: Connectivity with Organismal Physiology
Besides local changes within the skin, the hair cycle is also modulated by systemic changes of the body. For example, estrogens such as 17β-estradiol inhibit anagen initiation and promote premature catagen entry (Fraser et al. 1953; Movérare et al. 2002; Ohnemus et al. 2005). In addition, both pregnancy and lactation can change the hair cycle profoundly, and these changes are in part mediated by the hormone prolactin, which becomes up-regulated during both pregnancy and lactation (Craven et al. 2006). Direct administration of prolactin prolongs HFSC quiescence via Janus kinase (JAK)-signal transducer and activator of transcription 5 (STAT5) signaling (Goldstein et al. 2014).
Recently, the adrenal gland–derived stress hormone corticosterone (the cortisol equivalent in rodents) was found to be a key regulator of telogen length. Corticosterone inhibits the expression of growth arrest–specific 6 (Gas6, a secreted factor expressed in DP), which normally activates HFSCs. Under chronic stress, corticosterone levels rise, suppressing Gas6 expression and inhibiting hair follicle regeneration. By contrast, when corticosterone is removed, Gas6 becomes up-regulated, allowing the hair follicle to enter anagen constantly throughout life. These discoveries not only uncover how chronic, long-lasting stress inhibits tissue production, but also demonstrate an interesting example of how local tissue changes are controlled by systemic bodily changes via a circulating hormone (Choi et al. 2021). It is tempting to speculate that under duress, elicited in the above examples by pregnancy and emotional trauma, respectively, organisms exploit hormonal circulation as a means of instructing stem cell populations in the body to halt nonessential regenerative processes and conserve body energy until the stress has passed.
Sensing and Responding to the External Environment
The skin lies at the interface between the organism and its external environment. The sensory nervous system (the afferent arm) receives diverse stimuli from the outside world and endows the organisms with remarkable complexity in sensations (Zimmerman et al. 2014). In addition to their neurological functions, emerging evidence suggests that the sensory nerves play a key role in modulating the immune cell landscape in the skin (for review, see Chu et al. 2020). By contrast, the sympathetic nervous system transmits signals to the periphery (the efferent arm). Sympathetic neurons are a part of our autonomic nervous system, which innervates all organs and regulates our unconscious actions in responding to both physiological changes and external stimuli. As such, sympathetic nervous system represents a particularly attractive system connecting the skin, the body, and the external world (Botchkarev et al. 1999; Fan et al. 2018).
In the skin, piloerection, or goosebumps, relies on the action of three interconnected cell types—the hair follicle, the arrector pili muscle (APM), and the sympathetic innervation. HFSCs express Nephronectin, an ECM protein that attracts APMs, which express the receptor for Nephronectin (α8β1 integrin) (Fujiwara et al. 2011). APMs and sympathetic innervations act as a dual component niche to regulate HFSCs. APMs serve as an anchor to attract and maintain sympathetic innervations to HFSCs, while sympathetic nerves are the signaling component. With the help of APMs, sympathetic nerves innervate HFSCs directly and regulate HFSCs via the neurotransmitter norepinephrine (Shwartz et al. 2020).
Under basal conditions, nerve-derived norepinephrine primes HFSCs for activation by keeping inhibitory signals such as FGF18 low in the HFSCs. When the external temperature drops, sympathetic neuronal activity becomes elevated, triggering the contraction of APMs and leading to goosebumps formation. When cold stimuli last for a while, elevated sympathetic nerve activity triggers HFSCs to become activated precociously to regenerate a new hair coat. In this sense, sympathetic neurons and APMs allow organisms to respond to cold temperature in two ways: a first rapid goosebump response that traps a layer of warm air close to the skin surface, and a second response that initiates new hair growth via activating HFSCs (Shwartz et al. 2020).
Connecting with the Vasculature—Interactions with Lymphatic Vessels and Blood Vessels
The lymphatic capillaries that wrap around the bulge niche also connect to collecting lymphatic vessels, which function dually in transporting immune cells to the lymph nodes and regulating tissue fluid homeostasis. Whether either of these two established functions account for why transient lymphatic detachment from the niche promotes hair follicle regeneration remains unknown. It is also possible that the lymphatic capillaries participate in draining certain metabolites and/or growth factors essential for regeneration.
In addition to the lymphatic vessels, blood vessels in the dermis also undergo substantial remodeling during hair cycle stages (Xiao et al. 2013; Li et al. 2019). In contrast to the lymphatic vasculature, blood vessels are important in bringing nutrients, hormones, and oxygen to the skin. That said, blood vessel endothelial cells are a rich source of BMP4, an inhibitory factor for HFSC activation, and during telogen, when the dermis is at its thinnest, increased BMP4 levels could contribute to the extended quiescence (Li et al. 2019).
Influence from Immune Cells
Traditionally studied for their roles in host defense and inflammation, immune cells have emerged as critical regulators in development, tissue regeneration, and repair. While inflammation following microbial infection and/or wounding entails a surge of infiltrating immune cells from the circulation, the homeostatic skin also displays an array of resident immune cell populations (for review, see Niec et al. 2021). Resident macrophages play diverse roles in HFSC regulation. They are a source of WNTs (Castellana et al. 2014)—key signals required to stabilize β-catenin and activate HFSCs at the start of a new hair cycle. Interestingly, a subset of resident macrophages, marked by TREM2 (Triggering Receptor Expressed on Myeloid Cells 2), has a different function—during telogen, they participate in maintaining quiescence by secreting Oncostatin M, which acts on JAK-STAT signaling in the stem cells (Wang et al. 2019). The K6+ inner bulge is also a potent source of HFSC inhibitory factors, including BMP6 and FGF18 (Hsu et al. 2011). Hair plucking removes this layer of cells and potently triggers anagen. Plucking also induces local tissue damage, and it is interesting to speculate that the macrophage milieu is altered as a consequence. Indeed, tumor necrosis factor α (TNF-α), secreted mainly by activated macrophages following injury was shown to stimulate new hair growth even beyond the plucked area (Chen et al. 2015).
Besides myeloid lineages, T regulatory cells (Tregs) also influence HFSCs. Tregs have long been known for their suppressive action on pathogen-induced inflammatory immune cells (e.g., cytotoxic T lymphocytes) to enable tissue repair once infections subside. However, emerging evidence also suggests interesting communication circuits between Treg and stem or progenitor cells in diverse tissues including the skeletal muscle and visceral fat (for review, see Munoz-Rojas and Mathis 2021). In response to damage within the telogen bulge, but independent of pathogen infection, HFSCs transmit signals that culminate in increased local density of activated Tregs, which in turn stimulate HFSCs to locally proliferate and reinforce the barrier (Lay et al. 2018). In response to hair plucking, activated Tregs can also stimulate HFSCs to proliferate by a mechanism involving Notch signaling (Ali et al. 2017). In this case, anagen is triggered and the proliferative effects are funneled into hair cycling rather than patching the niche. Based upon the ability of Tregs to communicate with both tissue stem cells and the immune system, and to change their properties in response to tissue damage and pathogens, their functions in homeostasis and wound repair will continue to be a focus for future research.
There is a long-standing interest in understanding the immunogenicity of stem cells due to their critical importance in renewing tissues long term and their translational potential in tissue replacement. Agudo et al. address this problem by engineering a mouse model in which cytotoxic T cells are engineered to kill GFP-positive (GFP+) cells. Whereas many GFP+ cells throughout the body are sensitive to cytotoxic T-cell immune attack in this model, HFSCs survive. Interestingly, rather than involving Tregs to dampen immune responses, homeostatic HFSCs express low levels of major histocompatibility complex (MHC) class I molecules to avoid cytotoxic T cells. In addition, HFSCs also evade natural killer cells that often attack cells with low MHC class I levels (Agudo et al. 2018). Many stem cells also keep protein synthesis rates low (Signer et al. 2014; Blanco et al. 2016; Sendoel et al. 2017), which further imparts a survival advantage under nutrient-limiting or other stressful conditions that may otherwise make them vulnerable. Getting to the heart of how stem cells communicate beneficially with immune cells and resist immune attack will be an exciting area to watch in the future.
MELANOCYTE STEM CELLS
McSCs are neural crest–derived cells that reside within the lower bulge and hair germ region of the hair follicle (Nishimura et al. 2002; Infarinato et al. 2020). Like HFSCs, McSCs stay in a strict quiescent phase during telogen and become activated transiently in anagen to produce differentiated melanocytes that migrate downward to color the newly regenerated hair follicles from the root. During catagen, the differentiated melanocytes are destroyed, leaving only McSCs when the hair follicle remodels back to telogen (Fig. 3A).
Figure 3.
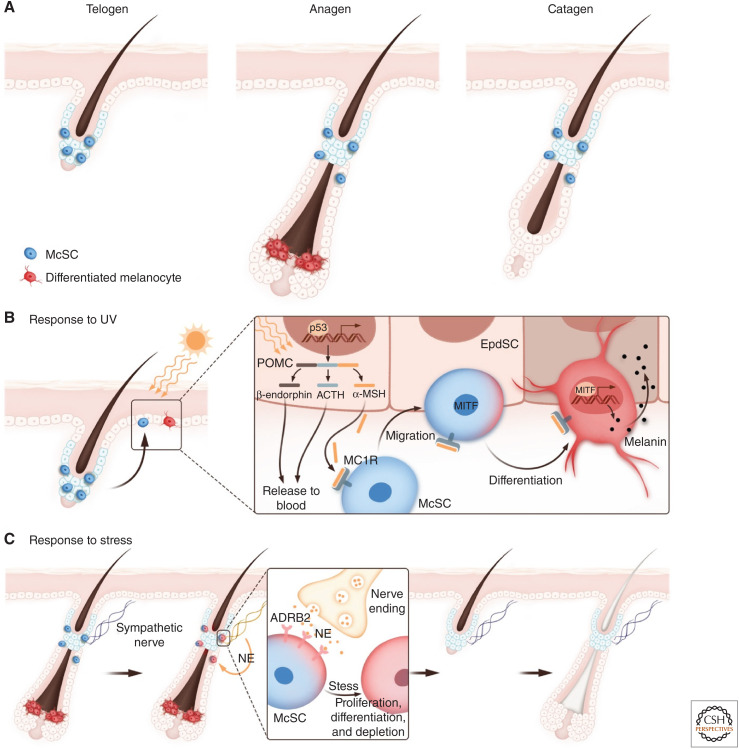
Melanocyte stem cells (McSCs) and their responses to ultraviolet (UV) and stress. (A) Schematic of the melanocyte lineage during the hair cycle. Differentiated melanocytes are made during anagen to color the newly regenerated hair shaft. (B) UV irradiation leads to DNA damage in the epidermis and up-regulation of p53 and POMC (proopiomelanocortin), a precursor peptide that is processed to form β-endorphin, Adrenocorticotropic hormone (ACTH), and α-MSH (α-melanocyte-stimulating hormone). α-MSH binds to MC1R (melanocortin 1 receptor) to promote McSC migration toward the epidermis. MC1R signaling further up-regulates a transcription factor, MITF, which promotes melanocyte differentiation and melanin production. (EpdSC) Epidermal stem cells. (C) Under stress, sympathetic neurons become highly activated, releasing large amounts of norepinephrine (NE), which binds to the β2 adrenergic receptor (ADRB2) on McSCs to drive their hyperproliferation, differentiation, and permanent depletion.
Coordination between Two Different Types of Stem Cells within a Shared Niche
Concurrent activation of both HFSCs and McSCs is crucial for producing a pigmented hair, and it provides a model to study how two distinct populations of stem cells located in the same niche coordinate their behaviors. At the onset of anagen, WNT signaling is important for the activation of both HFSCs and McSCs. This occurs predominantly within the hair germ, which is a particularly rich source of WNT-activating factors that build up toward the end of telogen (Greco et al. 2009; Rabbani et al. 2011; Harshuk-Shabso et al. 2020). Other factors appear to be tailored for each stem cell population. For example, the endothelin pathway regulates the activation and differentiation of McSCs (Takeo et al. 2016). Interestingly, however, mutations in an HFSC-specific transcription factor, Nfib (nuclear factor I B), lead to up-regulation of EDN2 (endothelin 2), which triggers ectopic McSC differentiation in the bulge (Rabbani et al. 2011; Chang et al. 2013; Takeo et al. 2016). KIT ligand (KITL, also known as stem cell factor [SCF]) is another factor important for McSC activation (Botchkareva et al. 2001) and that is expressed by TACs and DP (Chang et al. 2013; Liao et al. 2017).
Once the hair cycle has been activated, both McSCs and HFSCs must differentiate synchronously to allow a smooth transfer of melanin pigment to the differentiating hair follicle cells. Recent studies suggest that in part, this is mediated by BMP signaling. Once the hair cycle has been launched by WNT signaling, BMP signaling in activated McSCs promotes their differentiation to fully mature melanocytes (Infarinato et al. 2020). On the hair follicle side, BMP signaling in the TACs preferentially function on promoting their differentiation into the medulla (Fig. 2A), the lineage whose cells accept the melanin to form pigmented hair (Genander et al. 2014).
Responding to Ultraviolet Radiation
A fascinating example of how melanocyte and epithelial stem cells can rapidly adjust and coordinate their behavior is unveiled with ultraviolet (UV) radiation. Exposure to UV leads to DNA damage in the epidermis and induction of P53, which up-regulates proopiomelanocortin (POMC) expression in epidermal cells. POMC is cleaved into smaller peptides including α-melanocyte-stimulating hormone (α-MSH), which then binds to and directly stimulates melanocortin receptor (MC1R) to trigger McSC migration into the epidermis (Cui et al. 2007; Chou et al. 2013). MC1R stimulation also functions in melanogenesis, as exemplified by the polymorphisms in Mc1r that are found in red-headed and fair-skinned individuals, who have reduced melanogenesis and are more susceptible to UV-induced damage (Valverde et al. 1995). UV-induced P53 expression in the epidermis also up-regulates two additional secreted factors known to regulate melanogenesis—KITL and EDN1 (Hyter et al. 2013; Moore et al. 2013; Chang et al. 2017). Together, these pathways lead to hyperpigmentation of the epidermis, which in turn protects the EpdSCs from further UV radiation (Fig. 3B).
Interestingly, another cleavage biproduct of POMC is β-endorphin, which is an endogenous opioid with affinity for μ-opioid receptors (Fig. 3B). Elevation of β-endorphin drives sun-seeking behavior and tanning addiction, ironically linking a love of sunshine to harmful UV rays (Fell et al. 2014).
Responding to Stress
Loss of McSCs leads to production of unpigmented hair or hair graying—a phenotype associated with aging (Nishimura et al. 2005)—and has been anecdotally linked to physiological or psychological stress. Under acute stress in mice, hyperactivation of the sympathetic nervous system releases a large amount of norepinephrine, which drives McSCs into a rapid proliferation state, followed by migration, differentiation, and permanent depletion from the bulge (Fig. 3C; Zhang et al. 2020b). By contrast, circulating stress hormones from the hypothalamic–pituitary–adrenal (HPA) axis do not play a major role in stress-induced hair graying (Zhang et al. 2020a,b). Norepinephrine triggers analogous changes in McSCs located within the human hair follicle, suggesting that a similar mechanism might occur in humans as well (Rachmin et al. 2021). Moreover, patients undergoing partial sympathectomy (ablation of sympathetic neurons) often develop fewer gray hairs on the sympathectomized side with time, raising the intriguing possibility that norepinephrine signaling might also in part be related to McSC loss seen during aging (Lerner 1966; Ortonne et al. 1982).
These findings suggest that while both HFSCs and McSCs are sensitive to stress, the mechanisms leading to their changes under stress are distinct (Sendoel et al. 2017; Zhang et al. 2020b; Choi et al. 2021). Overall, by diversifying both the stem cells and their niche ecosystems, the skin has evolved to receive a myriad of different cues and delegate responsibilities with remarkable precision. In this regard, it is intriguing that despite the symbiotic relationship of McSCs and HFSCs, each stem cell population still retains at least some control of its own destiny and appears to invoke distinct ways of responding to stresses.
EPIDERMAL STEM CELLS
EpdSCs regenerate the stratified epidermis, which forms a physical barrier that protects organisms from insults and dehydration. The skin surface is also colonized by a vast number of microorganisms that exert diverse functions on health and disease. Moreover, a repertoire of immune cell populations—including Langerhans cells, CD8+ resident memory T cells, innate lymphoid cells (ILCs), and dendritic epidermal γδ T cells (DETCs)—further form an immunological barrier to protect organisms from infection (Pasparakis et al. 2014; Kobayashi et al. 2019b). We refer the readers to several other excellent reviews on the topics of skin microbiota (Grice and Segre 2011; Belkaid and Tamoutounour 2016; Chen et al. 2018), skin immunity (Kobayashi et al. 2019a), and stem cell–immune cell interactions (Naik et al. 2018). Here, we limit our focus to signals and cell–cell interactions crucial for EpdSCs to maintain the constantly renewing epidermis.
Balancing Growth and Differentiation
EpdSCs are located at the basal layer of the epidermis. They secrete their own basement membrane, which can sequester growth factors coming from diverse cell types within the skin (Fig. 4). Dermal fibroblasts produce FGFs, IGFs (insulin growth factors), and ligands for EGFRs (epidermal growth factor receptors), which are all important signals for epidermal proliferation (Rheinwald and Green 1975; Sadagurski et al. 2006). EpdSCs are also responsive to WNTs, which can come from EpdSCs and other nearby niche cells (Choi et al. 2013; Lim et al. 2013). Which WNTs are key and who transmits them remains unclear.
Figure 4.
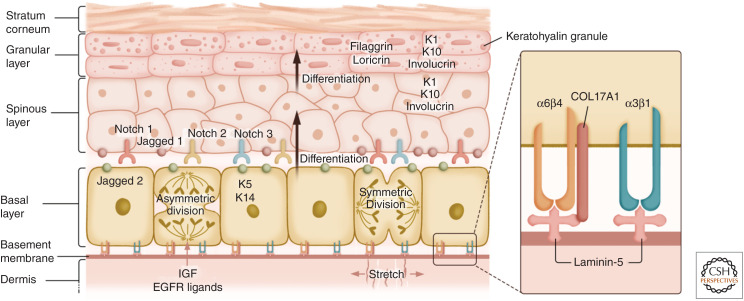
The stratified skin epidermis. Epidermal stem cells (EpdSCs) are located at the basal layer of the epidermis. EpdSCs adhere to the extracellular matrix rich basement membrane through interactions between α3β1 and α6β4 integrins with the laminin-5. EpdSCs can both self-renew and generate progeny that are committed to terminal differentiate. In some cases, differentiation is triggered when a stem cell divides parallel to the plane of the basal layer, but then one of its daughters detaches and moves into the suprabasal layer. In other cases, perpendicular divisions can result in one basal cell remaining basally as a stem cell, while another committed daughter cell is displaced into the suprabasal layer. Irrespective, cells committed to terminal differentiation transition through the spinous layer, the granular layer, and eventually into the dead stratum corneum that sheds from the skin surface. Until the granular:stratum corneum transition, each layer expresses different sets of proteins, including different keratin proteins. Secreted proteins such as insulin-like growth factors (IGFs) and ligands for epidermal growth factor receptors (EGFRs) promote EpdSC proliferation, while Notch signaling regulates the differentiation process.
Through ECM-integrin and growth factor receptor engagements, EpdSCs proliferate to renew themselves. This process is sensitive to neighbor cell density, which governs cell shape and, hence, the EpdSC surface ratio of proliferation-promoting basement membrane contacts versus proliferation-dampening intercellular adhesion contacts (Box et al. 2019; Dekoninck et al. 2020). EpdSCs undergo two types of division—symmetric division, which produces two EpdSCs that stay at the basal layer, and asymmetric division, which produces one basal cell and one differentiated suprabasal cell. Differentiating daughter cells move upward in a constant flux out of the basal layer to produce the spinous layer, the granular layer, and finally the stratum corneum suprabasal layers, whose dead cells are eventually shed from the skin surface, replaced by inner cells moving outward (Fig. 4).
During embryonic development when the skin first begins to stratify from the single-layered epidermis, more than half of the EpdSCs orient their mitotic spindle perpendicularly to the basement membrane, positioning one daughter cell away from the basement membrane to form the suprabasal layer (Lechler and Fuchs 2005; Williams et al. 2011). Notch signaling becomes elevated in the suprabasal daughters and drives their differentiation; gain and loss of function studies underscore the importance of the Notch pathway in epidermal differentiation (Lowell et al. 2000; Rangarajan et al. 2001; Blanpain et al. 2006; Williams et al. 2011).
Protection for the EpdSCs
The cells of the epidermis are subjected to a barrage of stresses from the external environment including mechanical forces. In part, they protect themselves by producing an elaborate mechanical infrastructure composed of keratins, proteins that assemble into 10 nm intermediate filaments. EpdSCs are marked by filaments composed of keratins K5 and K14, creating a cytoskeleton compatible with cell division. Upon withdrawal from the cell cycle and commitment to terminally differentiate, cells switch to a more robust mechanical framework composed of K1 and K10. In the later stages of terminal differentiation, even transcription halts, and all organelles including the nucleus are lost. This creates layers of flattened dead cells in the stratum corneum—cellular skeletons that are packed with keratin fibrils and sealed to each other by lipid bilayers—a veritable fortress to the external world.
Exploiting the Extremes at the Body Surface
The sudden destructive phase at the end of terminal differentiation has long remained a puzzle. Recent clues came from studying the protein deposits (“keratohyalin granules”) in the last transcriptionally active cells near the skin surface in the granular layer. During terminal differentiation, filaggrin and its paralogs, which are large proteins composed of histidine-rich protein repeats, form these granules when they reach a critical concentration and undergo dynamic conformational changes (liquid–liquid phase transitions) (Quiroz et al. 2020). These oil-like granules continue to grow and increase in viscosity as their concentration increases. The long head and tail domains of K1 and K10 that protrude along the surface of the terminally differentiating filaments also undergo these conformational transitions. With their ability to bind to filaggrin, this results in a stiff cytoplasmic web that cages the granules.
The skin surface is very acidic in contrast to the basal layer. As cells transition through this pH gradient, they reach the pH of filaggrin's pKa, causing the proteins to revert their conformation and disperse the granules. It remains to be addressed whether organelle disappearance results from mechanical distortion due to the viscous granule–keratin fibril infrastructure of the granular layer cytoplasm, or from release of potentially sequestered destructive enzymes during granule dispersion. However, it is noteworthy that the filaggrin mutations that are associated with the human inflammatory disorder, atopic dermatitis, are compromised in their ability to undergo liquid–liquid phase transitions, leaving a defective, vulnerable skin barrier whose outermost cells retain their organelles (Quiroz et al. 2020). Proteins optimal at undergoing liquid–liquid phase transitions are typically sensitive to concentration, pH, and temperature (Quiroz and Chilkoti 2015), and they abound in the differentiating layers of the epidermis, primed at taking advantage of this harsh environment.
Growth by Stretching—How Mechanical Force Regulates Stem Cell Behavior
As a whole, the epidermis is considerably stiffer and more elastic than the dermis, with the basal layer being ∼5× stiffer than papillary dermis (3 vs. 0.6 kPa) but 3–4× less than the underlying basement membrane (>10 kPa) (Fiore et al. 2020). Moreover, the terminally differentiating spinous and granular layers combined are 4× stiffer than the basal layer. Harboring flattened layers of dead, enucleated squames, the outermost stratum corneum displays the greatest stiffness (>20 kPa). Thus, in addition to providing protection from environmental extremes, these barrier cells can provide feedback regulation to the basal EpdSCs by exerting overlying mechanical forces or changes in contractility that can provide resistance to and slow the outward flux of terminally differentiating cells (Fiore et al. 2020; Ning et al. 2021).
Another tantalizing facet of the skin is its remarkable capacity to adapt and grow when stretched. Mechanical stretching of the skin is a common procedure to grow extra skin for reconstructive surgeries. Stretch is sensed by a subpopulation of EpdSCs, triggering a transient shift of these EpdSCs toward self-renewal but maintaining their ability to differentiate. Regulators of the actomyosin cytoskeleton, such as formin-like proteins and nonmuscle myosin, are crucial for EpdSCs to respond to stretching by promoting the translocation of canonical mechanotransducers YAP1 (Yes-associated protein 1), TAZ (transcriptional coactivator with PDZ-binding motif), and MKL1 (megakaryoblastic leukemia 1) into the nucleus to coordinate downstream transcriptional changes (Aragona et al. 2020).
The skin also undergoes significant expansion during pregnancy, and increased angiogenesis is thought to drive this expansion in part by regulating a population of Tbx3+ (T-box transcription factor 3) EpdSCs (Ichijo et al. 2021). In cultured cells, stretch triggers Piezo 1 (Piezo-type mechanosensitive ion channel component 1)-mediated calcium signaling and a genome-wide decrease in H3K9me3-marked heterochromatin regions to “soften” the nuclei and buffer against potential DNA damage due to mechanical changes. Similar changes in chromatin occur when embryonic skin is stretched (Nava et al. 2020).
Survival of the Fittest—How Cell Competition Shapes Epidermal Differentiation and Turnover
Integrins α3β1 coordinate the EpdSC migration and basement membrane assembly (Raghavan et al. 2000), while integrins α6β4 form the core of specialized integrin–ECM complexes, called hemidesmosomes, which link to the basal keratin cytoskeleton inside the cells and to the transmembrane collagen XVII (COL17A1) on the outside, enabling EpdSCs to adhere tightly to the basement membrane (Wang et al. 2020). Mutations in the genes encoding α6β4, COL17A1, or laminin-5 all lead to epidermolysis bullosa—blistering of the skin due to compromised adhesion of the basal progenitors to the basement membrane. Using the pioneering methods of the late Howard Green to culture and engraft human EpdSCs (Rheinwald and Green 1975; Green et al. 1979), such devastating disorders can now be corrected through gene-editing and engraftment approaches (Hirsch et al. 2017). A tiny skin biopsy is sufficient to produce the amount of epidermis necessary to cover the whole body, underscoring the remarkable regenerative capacity harbored within EpdSCs.
In cultured human keratinocytes, higher expression of β1 integrin tracks with a subpopulation of epidermal cells, EpdSCs, that have greater stem cell potential (Jones and Watt 1993; Jones et al. 1995). In mice, ablation of β1 integrin impairs EpdSC proliferation (Raghavan et al. 2000). Recently, COL17A1 levels were shown to correlate with whether an epidermal progenitor preferentially chooses to divide symmetrically or asymmetrically. When COL17A1 levels were high, EpdSCs underwent symmetric divisions preferentially to generate two basal EpdSCs. By contrast, when Col17a1 was depleted in a subset of EpdSCs, the Col17a1-depleted EpdSCs appeared to divide asymmetrically and over time were outcompeted by their wild-type counterparts (Liu et al. 2019).
Studies in Drosophila have established that the growth and survival of cells within tissues are shaped by competitions between neighboring cells (for review, see Baker 2020). Interestingly, this type of cell competition also plays a crucial role in shaping embryonic epidermal development in mammals. When epidermal progenitors heterozygous for the proto-oncogene Mycn mutation were placed in an otherwise wild-type epidermis, they acted as less fit “loser” cells but when placed in an epidermis of homozygous Mycn mutant cells, they acted as more fit “winner” cells. An important facet of classical cell competition is that more fit cells rid the tissue of neighbors that are less fit. As shown by Ellis et al. (2019), at early embryonic stages when the epidermis is only a single-layered epithelium, this occurs by actively promoting the death of unhealthy neighbors and engulfing them. Once the epidermis begins to stratify, the more fit progenitors shift to expelling their less fit basal layer neighbors by promoting their delamination and differentiation.
WOUND HEALING
After injury, the integrity of the skin must be repaired and restored quickly. Wound healing is a highly collaborative process involving interactions and communication among diverse cell types in the skin. The repair process is separated into three stages—inflammation, tissue formation, and tissue remodeling. During inflammation, diverse immune cells (both skin-resident and circulating immune cells) are attracted to the wound site to clear cellular debris and fight microbial infections. During tissue formation, granulation tissues (a mixture of immune cells, fibroblasts, and blood vessels) are formed in the dermis, and some fibroblasts differentiate to form highly contractile myofibroblasts. In addition, both EpdSCs and HFSCs participate in re-epithelialization to make a new epidermis to cover the wound and tentatively restore the barrier. During tissue remodeling, granulation tissues resolve, and the ECM undergoes reorganization and reformation. Additional collagen fibers are produced, which add tensile strength to strengthen the wound site and form scar tissues. A myriad of different cell–cell interactions across epithelium, fibroblasts, blood vessels, and immune cells are essential for different phases of wound healing and have been nicely reviewed (Eming et al. 2014). Here, we focus on the wound healing process from the lens of stem cells.
Stem Cell Behaviors during Wound Repair
EpdSCs and their differentiated suprabasal daughters both participate in re-epithelialization and are major contributors to the processes (Aragona et al. 2017; Park et al. 2017). Cells closest to the wound form a migratory front, followed by a proliferation ring that is further away from the wound. Although both proliferation and migration of EpdSCs occur during wound healing, inhibition of migration leads to a more significant delay in re-epithelization than inhibition of proliferation, suggesting a particularly crucial role of migration during re-epithelization (Heller et al. 2014; Aragona et al. 2017; Park et al. 2017).
Cells in the hair follicles also participate in re-epithelialization. Lineage-tracing experiments with full-thickness (punch biopsy) wounds have suggested that bulge HFSCs contribute to re-epithelization but do not stay in the epidermis for the long term (Ito et al. 2005), while cells above the bulge, located at the junctional zone and isthmus regions, although mostly contributing to the renewal of the isthmus and sebaceous glands under steady state, can give rise to long-lasting basal epidermal cells upon wounding (Brownell et al. 2011; Page et al. 2013). By contrast, lineage tracings on partial-thickness wounds suggest that bulge HFSCs contribute long term when the epidermis and junctional zone/isthmus are missing (Ge et al. 2017). These findings collectively point to the view that it is the closest stem cell population to the wound site that performs the lion's share of wound repair.
In yet another twist on the plasticity of the skin epithelium, it was shown that a group of sebaceous duct GATA6+ cells normally only contribute to cells at the junctional zone and sebaceous ducts, but can migrate to the epidermis upon wounding. Remarkably, the progeny of these GATA6+ cells first appeared to contribute to the differentiated suprabasal layer of the epidermis, but then dedifferentiated to form EpdSCs within the basal layer of epidermis (Donati et al. 2017). The circumstances, frequencies, and mechanisms by which stem cell progeny can revert to a stem cell fate are still unfolding. It seems likely that the ability is unleashed only under dire conditions when tissue fitness is threatened. Similar observations have been made by studies on the epithelia of the lung and intestine (Tata et al. 2013; Murata et al. 2020), and in all these cases, tissue injury and/or stem cell death appear to be a prerequisite to triggering this behavior.
Epithelium-Immune Cell Interactions during Wounding
Most of the T cells residing in mouse epidermis are DETCs. Upon wounding, DETCs produce and secrete factors such as IGF1, FGF7, and FGF10, to which EpdSCs respond to promote their proliferation and/or migration (Havran 2000; Sharp et al. 2005). Interestingly, the number and behavior of DETCs are also influenced by EpdSCs. EpdSCs near the wound edge up-regulate SKINTs (selection and upkeep of intraepithelial T-cell proteins), members of the PD1 family of immune interacting proteins (Keyes et al. 2016). Tregs also play a role in wound healing in part through limiting the accumulation of interferon γ (IFN-γ)-producing T cells and proinflammatory macrophages. Depletion of Tregs using a Treg-specific diphtheria toxin receptor mouse model during the inflammation phase leads to a delay in re-epithelization and increased inflammation (Nosbaum et al. 2016). Interestingly, long after the wound has healed, EpdSCs appear to bear a “memory” of their interactions with inflammatory immune cells, as evidenced by their ability to retain certain chromatin changes that occurred at the time of inflammation months before (Naik et al. 2017). Although mechanisms are still unfolding, epigenetic memory of inflammatory experiences sensitizes the cells to react more rapidly when challenged the second time (Niec et al. 2021).
De Novo Formation of Cell Types after Large Wounds
Surgeons have long known that epidermis removed from a healthy body site and engrafted into a third-degree burn site can sustain epidermis long term, but cannot regenerate hair follicles or sweat glands. The burn-repaired skin's ability to sense and stretch is also compromised. After decades of research, it has become increasingly clear that there are different stem cells for hair follicles and sweat glands, and each has their own niches. The ability of stem cells to regenerate these different appendages depends upon an array of complex, tissue-tailored niche interactions with other skin cell types, including melanocytes, innervations, lymphatics, dermal fibroblasts, adipocytes, and immune cells.
Studies on mice have shown that a large wound can sometimes lead to de novo formation of the hair follicle in the center of the wound (Ito et al. 2007). This wound-induced hair follicle formation recapitulates some features similar to embryonic hair follicle development and can undergo hair cycling, but it lacks McSCs or melanocytes. Similar to the hair follicle development process, regeneration of hair follicles in these large wounds requires WNT signaling and SHH signaling (Ito et al. 2007; Lim et al. 2018; Phan et al. 2020), and involves dermal γδ T cells and skin microbiota (Gay et al. 2013; Wang et al. 2021).
An additional hurdle in wound repair is scarring. Interestingly, dermal myofibroblasts can arise from multiple cell populations within injured skin, some of which produce more fibrotic proteins than others (Plikus et al. 2017; Shook et al. 2018). Moreover, inhibiting dermal YAP activity in myofibroblasts reduces scarring, providing further insights that in the future might be useful for treating wounds (Mascharak et al. 2021).
CONCLUDING REMARKS
In recent decades, we have witnessed an exciting blossoming in the field of skin stem cells. Advances in lineage tracing, imaging, mouse genetics, and high throughput sequencing have delineated many interesting examples of diverse interactions between stem cells and their local and systemic environments. These discoveries have also opened new lines of inquiry at the crossroads of cell biology, genomics, epigenetic regulation, neurobiology, immunology, and physiology. Moving forward, we expect new discoveries will begin to connect these different levels of understanding, leading to a comprehensive view of dynamic stem cell behaviors from physiology, niche, down to molecular and metabolic changes within stem cells. We also expect new medical breakthroughs based on this rich body of discoveries. In the 1980s, Howard Green's pioneering approach to culture and expand keratinocytes began to revolutionize stem cell biology and regenerative medicine. Four decades later, we are now nearing the goal of being able to regenerate a fully functional skin with rich arrays of cell types after severe injury.
ACKNOWLEDGMENTS
We thank Yi Zheng for illustrations and Shuri Gur-Cohen and Yulia Shwartz for comments. We are grateful for our many colleagues in the community whose work has established a vibrant research field and inspired us to write this review. Due to space constraints, we regret that we were not able to discuss all the relevant and exciting articles in the field. Y.-C.H. is a Pew Scholar and a New York Stem Cell Foundation – Robertson Investigator. E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the NIH (R01-AR070825 to Y.-C.H; R01-AR050452 and R01-AR27883 to E.F.), Starr Foundation-Tri-SCI (to E.F.), Harvard Stem Cell Institute (to Y.-C.H.), the New York Stem Cell Foundation (to Y.-C.H.), and American Cancer Society (to Y.-C.H.).
Footnotes
Editors: Kenneth D. Poss and Donald T. Fox
Additional Perspectives on Regeneration available at www.cshperspectives.org
REFERENCES
- Adam RC, Yang H, Ge Y, Lien WH, Wang P, Zhao Y, Polak L, Levorse J, Baksh SC, Zheng D, et al. 2018. Temporal layering of signaling effectors drives chromatin remodeling during hair follicle stem cell lineage progression. Cell Stem Cell 22: 398–413.e7. 10.1016/j.stem.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, et al. 2018. Quiescent tissue stem cells evade immune surveillance. Immunity 48: 271–285.e5. 10.1016/j.immuni.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. 2017. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169: 1119–1129.e11. 10.1016/j.cell.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascré G, Simons BD, Blanpain C. 2017. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun 8: 14684. 10.1038/ncomms14684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M, Sifrim A, Malfait M, Song Y, Van Herck J, Dekoninck S, Gargouri S, Lapouge G, Swedlund B, Dubois C, et al. 2020. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584: 268–273. 10.1038/s41586-020-2555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. 2020. Emerging mechanisms of cell competition. Nat Rev Genet 21: 683–697. 10.1038/s41576-020-0262-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Tamoutounour S. 2016. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol 16: 353–366. 10.1038/nri.2016.48 [DOI] [PubMed] [Google Scholar]
- Blanco S, Bandiera R, Popis M, Hussain S, Lombard P, Aleksic J, Sajini A, Tanna H, Cortés-Garrido R, Gkatza N, et al. 2016. Stem cell function and stress response are controlled by protein synthesis. Nature 534: 335–340. 10.1038/nature18282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648. 10.1016/j.cell.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. 2006. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev 20: 3022–3035. 10.1101/gad.1477606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Peters EM, Botchkareva NV, Maurer M, Paus R. 1999. Hair cycle-dependent changes in adrenergic skin innervation, and hair growth modulation by adrenergic drugs. J Invest Dermatol 113: 878–887. 10.1046/j.1523-1747.1999.00791.x [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Khlgatian M, Longley BJ, Botchkarev VA, Gilchrest BA. 2001. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J 15: 645–658. 10.1096/fj.00-0368com [DOI] [PubMed] [Google Scholar]
- Box K, Joyce BW, Devenport D. 2019. Epithelial geometry regulates spindle orientation and progenitor fate during formation of the mammalian epidermis. eLife 8: e47102. 10.7554/eLife.47102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. 2011. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8: 552–565. 10.1016/j.stem.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana D, Paus R, Perez-Moreno M. 2014. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol 12: e1002002. 10.1371/journal.pbio.1002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. 2013. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature 495: 98–102. 10.1038/nature11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Kuo CJ, Ito T, Su YY, Jiang ST, Chiu MH, Lin YH, Nist A, Mernberger M, Stiewe T, et al. 2017. CK1α ablation in keratinocytes induces p53-dependent, sunburn-protective skin hyperpigmentation. Proc Natl Acad Sci 114: E8035–E8044. 10.1073/pnas.1702763114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. 2015. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161: 277–290. 10.1016/j.cell.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YE, Fischbach MA, Belkaid Y. 2018. Skin microbiota–host interactions. Nature 553: 427–436. 10.1038/nature25177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, et al. 2013. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 13: 720–733. 10.1016/j.stem.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Zhang B, Ma S, Gonzalez-Celeiro M, Stein D, Jin X, Kim S, Kang Y, Besnard A, Rezza A, et al. 2021. Corticosterone inhibits Gas6 to govern hair follicle stem-cell quiescence. Nature 592: 428–432. 10.1038/s41586-021-03417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. 2013. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med 19: 924–929. 10.1038/nm.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Artis D, Chiu IM. 2020. Neuro-immune interactions in the tissues. Immunity 52: 464–474. 10.1016/j.immuni.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. 1990. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329–1337. 10.1016/0092-8674(90)90696-C [DOI] [PubMed] [Google Scholar]
- Craven AJ, Nixon AJ, Ashby MG, Ormandy CJ, Blazek K, Wilkins RJ, Pearson AJ. 2006. Prolactin delays hair regrowth in mice. J Endocrinol 191: 415–425. 10.1677/joe.1.06685 [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, et al. 2007. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 128: 853–864. 10.1016/j.cell.2006.12.045 [DOI] [PubMed] [Google Scholar]
- Dekoninck S, Hannezo E, Sifrim A, Miroshnikova YA, Aragona M, Malfait M, Gargouri S, de Neunheuser C, Dubois C, Voet T, et al. 2020. Defining the design principles of skin epidermis postnatal growth. Cell 181: 604–620.e22. 10.1016/j.cell.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Proserpio V, Lichtenberger BM, Natsuga K, Sinclair R, Fujiwara H, Watt FM. 2014. Epidermal Wnt/β-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proc Natl Acad Sci 111: E1501–E1509. 10.1073/pnas.1312880111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E, Kar G, Kayikci M, Russell R, Kretzschmar K, Mulder KW, et al. 2017. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat Cell Biol 19: 603–613. 10.1038/ncb3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SJ, Gomez NC, Levorse J, Mertz AF, Ge Y, Fuchs E. 2019. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature 569: 497–502. 10.1038/s41586-019-1199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6: 265sr6. 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SM, Chang YT, Chen CL, Wang WH, Pan MK, Chen WP, Huang WY, Xu Z, Huang HE, Chen T, et al. 2018. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci 115: E6880–E6889. 10.1073/pnas.1719548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. 2014. Skin β-endorphin mediates addiction to UV light. Cell 157: 1527–1534. 10.1016/j.cell.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. 2011. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761–771. 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore VF, Krajnc M, Quiroz FG, Levorse J, Pasolli HA, Shvartsman SY, Fuchs E. 2020. Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature 585: 433–439. 10.1038/s41586-020-2695-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, et al. 2000. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J 14: 752–760. 10.1096/fasebj.14.5.752 [DOI] [PubMed] [Google Scholar]
- Fraser AS, Nay T, Turner HN. 1953. Growth of the mouse coat. II: Effect of sex and pregnancy. Aust J Biol Sci 6: 645–656. 10.1071/BI9530645 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. 2011. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144: 577–589. 10.1016/j.cell.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, et al. 2013. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med 19: 916–923. 10.1038/nm.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, Lu CP, Polak L, Yuan S, Elemento O, et al. 2017. Stem cell lineage infidelity drives wound repair and cancer. Cell 169: 636–650.e14. 10.1016/j.cell.2017.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Cook PJ, Ramsköld D, Keyes BE, Mertz AF, Sandberg R, Fuchs E. 2014. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell 15: 619–633. 10.1016/j.stem.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Fletcher S, Roth E, Wu C, Chun A, Horsley V. 2014. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev 28: 983–994. 10.1101/gad.236554.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. 2009. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169. 10.1016/j.stem.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H, Kehinde O, Thomas J. 1979. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci 76: 5665–5668. 10.1073/pnas.76.11.5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. 2011. The skin microbiome. Nat Rev Microbiol 9: 244–253. 10.1038/nrmicro2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, Fuchs E. 2019. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366: 1218–1225. 10.1126/science.aay4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshuk-Shabso S, Dressler H, Niehrs C, Aamar E, Enshell-Seijffers D. 2020. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat Commun 11: 5114. 10.1038/s41467-020-18643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran WL. 2000. A role for epithelial γδ T cells in tissue repair. Immunol Res 21: 63–70. 10.1385/IR:21:2-3:63 [DOI] [PubMed] [Google Scholar]
- Hébert JM, Rosenquist T, Gotz J, Martin GR. 1994. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell 78: 1017–1025. 10.1016/0092-8674(94)90276-3 [DOI] [PubMed] [Google Scholar]
- Heitman N, Sennett R, Mok KW, Saxena N, Srivastava D, Martino P, Grisanti L, Wang Z, Ma'ayan A, Rompolas P, et al. 2020. Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science 367: 161–166. 10.1126/science.aax9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller E, Kumar KV, Grill SW, Fuchs E. 2014. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev Cell 28: 617–632. 10.1016/j.devcel.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, et al. 2017. Regeneration of the entire human epidermis using transgenic stem cells. Nature 551: 327–332. 10.1038/nature24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E. 2012. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol 13: 103–114. 10.1038/nrm3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. 2011. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105. 10.1016/j.cell.2010.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. 2014. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157: 935–949. 10.1016/j.cell.2014.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyter S, Coleman DJ, Ganguli-Indra G, Merrill GF, Ma S, Yanagisawa M, Indra AK. 2013. Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell Melanoma Res 26: 247–258. 10.1111/pcmr.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo R, Kabata M, Kidoya H, Muramatsu F, Ishibashi R, Abe K, Tsutsui K, Kubo H, Iizuka Y, Kitano S, et al. 2021. Vasculature-driven stem cell population coordinates tissue scaling in dynamic organs. Sci Adv 7: eabd2575. 10.1126/sciadv.abd2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infarinato NR, Stewart KS, Yang Y, Gomez NC, Pasolli HA, Hidalgo L, Polak L, Carroll TS, Fuchs E. 2020. BMP signaling: at the gate between activated melanocyte stem cells and differentiation. Genes Dev 34: 1713–1734. 10.1101/gad.340281.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354. 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- Ito M, Yang ZX, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. 2007. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320. 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM. 1993. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73: 713–724. 10.1016/0092-8674(93)90251-K [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. 1995. Stem cell patterning and fate in human epidermis. Cell 80: 83–93. 10.1016/0092-8674(95)90453-0 [DOI] [PubMed] [Google Scholar]
- Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, Sequeira I, Sandberg R, Kasper M. 2020. The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 26: 441–457.e7. 10.1016/j.stem.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Keyes BE, Segal JP, Heller E, Lien WH, Chang CY, Guo X, Oristian DS, Zheng D, Fuchs E. 2013. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci 110: E4950–E4959. 10.1073/pnas.1320301110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, Fuchs E. 2016. Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell 167: 1323–1338.e14. 10.1016/j.cell.2016.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Naik S, Nagao K. 2019a. Choreographing immunity in the skin epithelial barrier. Immunity 50: 552–565. 10.1016/j.immuni.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo JH, Shih HY, Truong A, Doebel T, Sakamoto K, Cui CY, et al. 2019b. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 176: 982–997.e16. 10.1016/j.cell.2018.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay K, Yuan S, Gur-Cohen S, Miao Y, Han T, Naik S, Pasolli HA, Larsen SB, Fuchs E. 2018. Stem cells repurpose proliferation to contain a breach in their niche barrier. eLife 7: e41661. 10.7554/eLife.41661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280. 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AB. 1966. Gray hair and sympathectomy. Report of a case. Arch Dermatol 93: 235–236. 10.1001/archderm.1966.01600200091018 [DOI] [PubMed] [Google Scholar]
- Li KN, Jain P, He CH, Eun FC, Kang S, Tumbar T. 2019. Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. eLife 8: e45977. 10.7554/eLife.45977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CP, Booker RC, Morrison SJ, Le LQ. 2017. Identification of hair shaft progenitors that create a niche for hair pigmentation. Genes Dev 31: 744–756. 10.1101/gad.298703.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. 2014. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol 16: 179–190. 10.1038/ncb2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. 2013. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 342: 1226–1230. 10.1126/science.1239730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CH, Sun Q, Ratti K, Lee SH, Zheng Y, Takeo M, Lee W, Rabbani P, Plikus MV, Cain JE, et al. 2018. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat Commun 9: 4903. 10.1038/s41467-018-07142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Matsumura H, Kato T, Ichinose S, Takada A, Namiki T, Asakawa K, Morinaga H, Mohri Y, De Arcangelis A, et al. 2019. Stem cell competition orchestrates skin homeostasis and ageing. Nature 568: 344–350. 10.1038/s41586-019-1085-7 [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. 2000. Stimulation of human epidermal differentiation by δ-notch signalling at the boundaries of stem-cell clusters. Curr Biol 10: 491–500. 10.1016/S0960-9822(00)00451-6 [DOI] [PubMed] [Google Scholar]
- Mascharak S, des Jardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, et al. 2021. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372: eaba2374. 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa KR, Rompolas P, Zito G, Myung P, Sun TY, Brown S, Gonzalez DG, Blagoev KB, Haberman AM, Greco V. 2015. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature 522: 94–97. 10.1038/nature14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, Parekh P, Lee SH, Kontchou NA, Yeh I, et al. 2013. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci 110: E3225–E3234. 10.1073/pnas.1312933110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. 2004. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417. 10.1038/nbt950 [DOI] [PubMed] [Google Scholar]
- Movérare S, Lindberg MK, Faergemann J, Gustafsson JA, Ohlsson C. 2002. Estrogen receptor α, but not estrogen receptor β, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J Invest Dermatol 119: 1053–1058. 10.1046/j.1523-1747.2002.00637.x [DOI] [PubMed] [Google Scholar]
- Munoz-Rojas AR, Mathis D. 2021. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol 10.1038/s41577-021-00519-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Jadhav U, Madha S, van Es J, Dean J, Cavazza A, Wucherpfennig K, Michor F, Clevers H, Shivdasani RA. 2020. Ascl2-dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell Stem Cell 26: 377–390.e6. 10.1016/j.stem.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, Fuchs E. 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550: 475–480. 10.1038/nature24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Cowley CJ, Fuchs E. 2018. Two to tango: dialog between immunity and stem cells in health and disease. Cell 175: 908–920. 10.1016/j.cell.2018.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava MM, Miroshnikova YA, Biggs LC, Whitefield DB, Metge F, Boucas J, Vihinen H, Jokitalo E, Li X, Garcia Arcos JM, et al. 2020. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181: 800–817.e22. 10.1016/j.cell.2020.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niec R, Rudensky AY, Fuchs E. 2021. Inflammatory adaptation in barrier tissues. Cell 184: 3361–3375. 10.1016/j.cell.2021.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning W, Muroyama A, Li H, Lechler T. 2021. Differentiated daughter cells regulate stem cell proliferation and fate through intra-tissue tension. Cell Stem Cell 28: 436–452.e5. 10.1016/j.stem.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. 2002. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416: 854–860. 10.1038/416854a [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. 2005. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307: 720–724. 10.1126/science.1099593 [DOI] [PubMed] [Google Scholar]
- Nosbaum A, Prevel N, Truong HA, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, Rosenblum MD. 2016. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol 196: 2010–2014. 10.4049/jimmunol.1502139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnemus U, Uenalan M, Conrad F, Handjiski B, Mecklenburg L, Nakamura M, Inzunza J, Gustafsson JA, Paus R. 2005. Hair cycle control by estrogens: catagen induction via estrogen receptor (ER)-α is checked by ER-β signaling. Endocrinology 146: 1214–1225. 10.1210/en.2004-1219 [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Thivolet J, Guillet R. 1982. Graying of hair with age and sympathectomy. Arch Dermatol 118: 876–877. 10.1001/archderm.118.11.876a [DOI] [PubMed] [Google Scholar]
- Oshimori N, Fuchs E. 2012. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10: 63–75. 10.1016/j.stem.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Göttgens B, Jensen KB. 2013. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13: 471–482. 10.1016/j.stem.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Gonzalez DG, Guirao B, Boucher JD, Cockburn K, Marsh ED, Mesa KR, Brown S, Rompolas P, Haberman AM, et al. 2017. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat Cell Biol 19: 155–163. 10.1038/ncb3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Haase I, Nestle FO. 2014. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 14: 289–301. 10.1038/nri3646 [DOI] [PubMed] [Google Scholar]
- Peña-Jimenez D, Fontenete S, Megias D, Fustero-Torre C, Graña-Castro O, Castellana D, Loewe R, Perez-Moreno M. 2019. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J 38: e101688. 10.15252/embj.2019101688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QM, Fine GM, Salz L, Herrera GG, Wildman B, Driskell IM, Driskell RR. 2020. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. eLife 9: e60066. 10.7554/eLife.60066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. 2008. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451: 340–344. 10.1038/nature06457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, et al. 2017. Regeneration of fat cells from myofibroblasts during wound healing. Science 355: 748–752. 10.1126/science.aai8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz FG, Chilkoti A. 2015. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat Mater 14: 1164–1171. 10.1038/nmat4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz FG, Fiore VF, Levorse J, Polak L, Wong E, Pasolli HA, Fuchs E. 2020. Liquid–liquid phase separation drives skin barrier formation. Science 367: eaax9554. 10.1126/science.aax9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou WC, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. 2011. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145: 941–955. 10.1016/j.cell.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmin I, Lee JH, Zhang B, Sefton J, Jung I, Lee YI, Hsu YC, Fisher DE. 2021. Stress-associated ectopic differentiation of melanocyte stem cells and ORS amelanotic melanocytes in an ex vivo human hair follicle model. Exp Dermatol 30: 578–587. 10.1111/exd.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. 2000. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150: 1149–1160. 10.1083/jcb.150.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani W, Abbasi S, Hagner A, Raharjo E, Kumar R, Hotta A, Magness S, Metzger D, Biernaskie J. 2014. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev Cell 31: 543–558. 10.1016/j.devcel.2014.10.022 [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. 2001. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20: 3427–3436. 10.1093/emboj/20.13.3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza A, Wang Z, Sennett R, Qiao W, Wang D, Heitman N, Mok KW, Clavel C, Yi R, Zandstra P, et al. 2016. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep 14: 3001–3018. 10.1016/j.celrep.2016.02.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. 1975. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6: 331–343. 10.1016/S0092-8674(75)80001-8 [DOI] [PubMed] [Google Scholar]
- Rivera-Gonzalez GC, Shook BA, Andrae J, Holtrup B, Bollag K, Betsholtz C, Rodeheffer MS, Horsley V. 2016. Skin adipocyte stem cell self-renewal is regulated by a PDGFA/AKT-signaling axis. Cell Stem Cell 19: 738–751. 10.1016/j.stem.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. 2013. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502: 513–518. 10.1038/nature12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Yakar S, Weingarten G, Holzenberger M, Rhodes CJ, Breitkreutz D, Leroith D, Wertheimer E. 2006. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol Cell Biol 26: 2675–2687. 10.1128/MCB.26.7.2675-2687.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendoel A, Dunn JG, Rodriguez EH, Naik S, Gomez NC, Hurwitz B, Levorse J, Dill BD, Schramek D, Molina H, et al. 2017. Translation from unconventional 5′ start sites drives tumour initiation. Nature 541: 494–499. 10.1038/nature21036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett R, Rendl M. 2012. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol 23: 917–927. 10.1016/j.semcdb.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LL, Jameson JM, Cauvi G, Havran WL. 2005. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol 6: 73–79. 10.1038/ni1152 [DOI] [PubMed] [Google Scholar]
- Shin W, Rosin NL, Sparks H, Sinha S, Rahmani W, Sharma N, Workentine M, Abbasi S, Labit E, Stratton JA, et al. 2020. Dysfunction of hair follicle mesenchymal progenitors contributes to age-associated hair loss. Dev Cell 53: 185–198.e7. 10.1016/j.devcel.2020.03.019 [DOI] [PubMed] [Google Scholar]
- Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, López-Giráldez F, Dash BC, Muñoz-Rojas AR, Aultman KD, Zwick RK, Lei V, et al. 2018. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362: eaar2971. 10.1126/science.aar2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwartz Y, Gonzalez-Celeiro M, Chen CL, Pasolli HA, Sheu SH, Fan SM, Shamsi F, Assaad S, Lin ET, Zhang B, et al. 2020. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182: 578–593.e19. 10.1016/j.cell.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer RA, Magee JA, Salic A, Morrison SJ. 2014. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509: 49–54. 10.1038/nature13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo M, Lee W, Rabbani P, Sun Q, Hu H, Lim CH, Manga P, Ito M. 2016. EdnrB governs regenerative response of melanocyte stem cells by crosstalk with Wnt signaling. Cell Rep 15: 1291–1302. 10.1016/j.celrep.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. 2013. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503: 218–223. 10.1038/nature12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. 2004. Defining the epithelial stem cell niche in skin. Science 303: 359–363. 10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. 1995. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet 11: 328–330. 10.1038/ng1195-328 [DOI] [PubMed] [Google Scholar]
- Wang ECE, Dai Z, Ferrante AW, Drake CG, Christiano AM. 2019. A subset of TREM2+ dermal macrophages secretes Oncostatin M to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell 24: 654–669.e6. 10.1016/j.stem.2019.01.011 [DOI] [PubMed] [Google Scholar]
- Wang W, Zuidema A, Te Molder L, Nahidiazar L, Hoekman L, Schmidt T, Coppola S, Sonnenberg A. 2020. Hemidesmosomes modulate force generation via focal adhesions. J Cell Biol 219: e201904137. 10.1083/jcb.201904137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Sweren E, Liu H, Wier E, Alphonse MP, Chen R, Islam N, Li A, Xue Y, Chen J, et al. 2021. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe 29: 777–791.e6. 10.1016/j.chom.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E. 2011. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470: 353–358. 10.1038/nature09793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. 2012. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev 26: 1235–1246. 10.1101/gad.187401.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Woo WM, Nagao K, Li W, Terunuma A, Mukouyama YS, Oro AE, Vogel JC, Brownell I. 2013. Perivascular hair follicle stem cells associate with a venule annulus. J Invest Dermatol 133: 2324–2331. 10.1038/jid.2013.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Adam RC, Ge Y, Hua ZL, Fuchs E. 2017. Epithelial–mesenchymal micro-niches govern stem cell lineage choices. Cell 169: 483–496.e13. 10.1016/j.cell.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tsai PC, Gonzalez-Celeiro M, Chung O, Boumard B, Perdigoto CN, Ezhkova E, Hsu YC. 2016. Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes Dev 30: 2325–2338. 10.1101/gad.285429.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, He M, Rachmin I, Yu X, Kim S, Fisher DE, Hsu YC. 2020a. Melanocortin 1 receptor is dispensable for acute stress induced hair graying in mice. Exp Dermatol 30: 572–577. 10.1111/exd.14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S, Goncalves WA, Shwartz Y, Fast EM, Su Y, et al. 2020b. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577: 676–681. 10.1038/s41586-020-1935-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD. 2014. The gentle touch receptors of mammalian skin. Science 346: 950–954. 10.1126/science.1254229 [DOI] [PMC free article] [PubMed] [Google Scholar]