Abstract
Background
Electronic cigarette (e-cigarette) vaping, containing nicotine and/or Δ8, Δ9 or Δ10 or Δo tetrahydrocannabinol (Δn-THC), is associated with an outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI). Despite thousands being hospitalised with EVALI, much remains unknown about diagnosis, treatment and disease pathogenesis. Biomarkers of inflammation, oxidative stress and lipid mediators may help identify e-cigarette users with EVALI.
Methods
We collected plasma and urine along with demographic and vaping-related data of EVALI subjects (age 18–35 years) and non-users matched for sex and age in a pilot study. Biomarkers were assessed by ELISA/EIA and Luminex-based assays.
Results
Elevated levels of THC metabolite (11-nor-9-carboxy-Δ9-THC) were found in plasma from EVALI subjects compared to non-users. Levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), an oxidative DNA damage biomarker, and 8-isoprostane, an oxidative stress marker, were slightly increased in urine samples from EVALI subjects compared to non-users. Conversely, plasma levels of lipid mediators, including resolvin D1 (RvD1) and prostaglandin E2 (PGE2), were significantly lower in EVALI subjects compared to non-users. Both pro-inflammatory biomarkers, such as tumour necrosis factor-α, macrophage inflammatory protein-1β, RANTES (regulated on activation, normal T-cell expressed and secreted) and granulocyte–macrophage colony-stimulating factor, as well as anti-inflammatory biomarkers, such as interleukin-9 and CC10/16, were decreased in plasma from EVALI subjects compared to non-users, supportive of a possible dysregulated inflammatory response in EVALI subjects.
Conclusions
Significant elevations in urine and plasma biomarkers of oxidative stress, as well as reductions in lipid mediators, were shown in EVALI subjects. These noninvasive biomarkers (8-OHdG, 8-isoprostane, RvD1 and CC10/16), either individually or collectively, may serve as tools in diagnosing future EVALI subjects.
Short abstract
Biomarkers 8-OHdG, 8-isoprostane, RvD1 and CC10/16 are associated with electronic cigarettes and vaping https://bit.ly/3tJJV71
Introduction
Electronic cigarettes (e-cigarettes) or electronic nicotine delivery systems (ENDS), invented in 2003, were originally conceived as smoking cessation aids but are now a major avenue of nicotine consumption that is rapidly rising in popularity amongst young people [1, 2]. Cannabis e-cigarettes (CECs) are an adaptation of the same technology used to deliver Δ9-tetrahydrocannabinol (Δ9-THC) and have become popular among adolescents and young adults [3]. In 2019, the Centres for Disease Control and Prevention (CDC) described an outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI), which as of 18 February 2020, resulted in 2807 hospitalisations/cases and 68 deaths in the USA [4]. The disease presents with symptoms of cough, shortness of breath, chest pain, nausea, vomiting, diarrhoea, abdominal pain, fever and chills [4–7]. The majority of patients/subjects with EVALI have reported the use of THC-based, counterfeit e-cigarette products [8]. In spite of thousands hospitalised with EVALI, there remains a debate on the harmfulness of e-cigarettes as well as their potential long-term health effects [9, 10].
Commonly used as a cutting agent in vaping products, vitamin E acetate (VEA) has been implicated as a key agent in the occurrence of EVALI since its discovery in the bronchoalveolar lavage fluid (BALF) of 48 participants in a study of 51 known lung injury cases [11]. However, VEA was not found in the healthy participants, including 18 e-cigarette users [11]. Hence, there is some doubt over whether VEA is the sole causative agent. Chemical analyses of illicitly sourced CECs obtained from EVALI subjects demonstrated that these products can contain many different ingredients and adulterants, such as glycerol fatty acid esters, long-chain hydrocarbons, plasticisers, terpenes, metals and more [12, 13]. Aerosols generated from CECs contain concerning levels of irritants and carcinogens including benzene, isoprene, methyl vinyl ketone, butadiene, toluene, xylenes and more [3, 14]. Muthumalage et al. [13] showed the molecular mechanisms of injurious responses by inhaled illicit cartridges that are shown to cause EVALI. There is also debate on the protective effects of VEA against toxicity and vice versa [15–18]. Considering this, it is important to investigate further the mechanisms of EVALI disease development and progression among e-cigarette users.
Several potential mechanisms have been proposed for the pathogenesis of EVALI [19]. In vivo murine inhalation studies using EVALI subject-sourced CECs demonstrate that these products can cause cytotoxicity, epithelial barrier dysfunction and inflammation [8]. Mice exposed to EVALI subject-sourced CECs showed increased levels of eicosanoid inflammatory mediators and leukotrienes in BALF, as compared to the mice exposed to VEA or other cutting agents [8]. To date, no specific biomarkers for EVALI have been identified, and diagnoses are primarily achieved through a process of elimination with the appropriate history of recent e-cigarette use, new physical exam findings and clinical imaging [4–7].
Systemic biomarkers of oxidative stress, inflammation and lipid mediators have been studied in smokers [20–22], COPD patients [23–25] and ENDS users [8, 9, 26]. However, most studies at best can only imply and extrapolate to EVALI clinical cases [8, 26–29]. Little is known to assess potential biomarkers of EVALI. While attempting to bridge this gap in the research literature, the aim of this study is to identify potential biomarkers of oxidative stress, lipid mediators and inflammatory responses that may play a role in the pathogenesis of EVALI.
Methods
Participants
This study was conducted at the University of Rochester Medical Center with the help of the Clinical Research Center (Rochester, NY, USA). Participants were recruited through various local newspapers, magazine advertisements and flyers around Monroe County along with word of mouth (IRB approval #CR00003968), or at the time of admission at Strong Memorial Hospital during initial diagnosis for EVALI. Subjects in the EVALI group met clinical criteria with: 1) recent use (within 90 days of hospitalisation) of e-cigarette or vaping products; 2) bilateral ground-glass opacities on radiographic imaging; and 3) exclusion of other common causes for changes on chest imaging, including community-acquired pneumonia [30, 31]. The majority (5 out of 6) of samples from EVALI subjects were obtained prior to corticosteroid treatment. Participants were selected based on a self-reported questionnaire. To be eligible, all participants had to be between the ages of 18 and 35 years. Additionally, all participants were screened for a history of chronic illness such as heart and lung disease, diabetes, cancer and/or current viral flu/pneumonia infections. For non-users, participants also had to disclose if they were currently taking any anti-inflammatories or corticosteroid drugs and if so, were removed from the study. Participants were excluded if pregnant or breastfeeding. Participants in the non-user group were required to never have smoked any tobacco products. Participants in the EVALI group were screened for history of e-cigarette use/vaping in addition to their diagnosis. Written informed consent was obtained from all study participants.
Demography
During the questionnaire, participants provided their age, sex and ethnicity. E-cigarette use duration, e-cigarette duration per session, e-cigarette smoking frequency, flavour type and approximate amount of nicotine (high/low) in each flavour were also disclosed. The participants were categorised into two groups: non-user and EVALI subjects (table 1).
TABLE 1.
Demographic and vaping status characteristics of non-users and e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects
| Characteristics | Non-users | EVALI subjects |
| Subjects n | 6 | 6 |
| Age, years | ||
| Mean±sd | 22±2.44 | 22.3±6.4 |
| Range | 18–25 | 19–35 |
| Sex n (%) | ||
| Male | 3 (50) | 3 (50) |
| Female | 3 (50) | 3 (50) |
| Demography n | ||
| Caucasian | 5 | 3 |
| African American | 1 | 2 |
| Asian | 0 | 0 |
| Not Hispanic/Latino/Black (not White) | 0 | 1 |
| E-cigarette use n (%) | ||
| Vaping frequency, times per day | N/A | |
| ≥10 | 3 (50) | |
| 3–5 | 2 (33.3) | |
| <1 | 1 (16.6) | |
| Duration of vape per session, min | N/A | |
| ≥20 | 0 | |
| 10–14 | 2 (33.3) | |
| 5–9 | 0 | |
| <5 | 4 (66.6) | |
| Vaping years | N/A | |
| >5 | 0 | |
| 2–5 | 2 (33.3) | |
| 1–2 | 2 (33.3) | |
| <1 | 2 (33.3) | |
| Hospitalisations n (%) | 0 | 6 (100) |
N/A: not applicable.
Sample collection
Whole venous blood (approximately 20–25 mL) was collected from participants in vacutainer tubes containing EDTA, then spun at 1000 g for 10 min to obtain plasma and stored immediately at −80°C until further use. Participants provided urine, and samples were immediately stored at −80°C for further use.
Measurement of biomarkers by multiplex panel assay
Cytokine/mediator levels in plasma from non-users and EVALI subjects were quantified by Bio-Plex Pro Human 27-Plex assay (M500KCAF0Y; Bio-Rad, Hercules, CA, USA) with a 1:4 sample dilution per manufacturer's instructions on a FLEXMAP 3D instrument (Luminex, Austin, TX, USA).
Measurement of biomarkers by ELISA/EIA
Commercially available kits were used for quantifying resolvin D1 (RvD1; Cat #500380; Cayman Chemical, Ann Arbor, MI, USA), resolvin D2 (RvD2; Cat#501120; Cayman Chemical), resolvin E1 (RvE1; Cat#MBS286046; MyBioSource, San Diego, CA, USA), lipoxin B4 (LXB4; Cat#MBS9380211; MyBioSource), prostaglandin E2 (PGE2; Cat#514010; Cayman Chemical), cotinine (Cat#1-2002; Salimetrics, Carlsbad, CA, USA) and CC10/16 (Cat# DUGB00; R&D Systems, Minneapolis, MN, USA), in plasma. In urine samples, 8-isoprostane (Cat #516351; Cayman Chemical), 8-hydroxy-2′-deoxyguanosine (8-OHdG; Cat#4380-096K; R&D Systems) and THC metabolite, predominantly 11-nor-9-carboxy-Δ9-THC, i.e. Δ9-THC (Cat#701570; Cayman Chemical), were run according to the manufacturers’ directions, respectively.
Statistical analysis
Data from all assays were analysed and graphed using GraphPad Prism9. An unpaired t-test as well as an outlier's test was used to determine statistical significance. A p-value <0.05 was considered significant.
Results
Six age- and sex-matched subjects with confirmed EVALI (n=6) and non-users (n=6) were included for biomarker testing. Baseline demographics, and if applicable, vaping status and hospitalisations details were obtained and summarised in table 1. The average age of non-users and EVALI subjects was 22 years. The gender breakdown for both groups was 50% female to male. Non-users and EVALI subjects were mostly identified as Caucasian (table 1). The clinical presentation and median peak laboratory values for EVALI subjects were collected and summarised in tables 2 and 3, respectively. All EVALI participants required hospitalisation and met clinical criteria for “confirmed” EVALI [4, 6]. Primary chief complaints were gastrointestinal-related (3 out of 6), respiratory (2 out of 6), or combined (3 out of 6) for EVALI subjects. Maximal respiratory support included supplemental oxygen (5 out of 6) or invasive positive pressure (1 out of 6). One EVALI subject disclosed known drug abuse and reported hypertriglyceridaemia in the past medical history. All subjects received systemic steroids (median: 9 days). Based on the self-reported questionnaire, EVALI subjects had a preference for fruit-flavoured e-cigarettes, followed by mint and candy flavours. Beverage and no flavour were also reported (figure 1).
TABLE 2.
Clinical presentation of e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects
| Subjects n | 6 |
| Chief complaint n (%) | |
| Gastrointestinal | 3 (50) |
| Respiratory | 2 (33) |
| Combination | 3 (50) |
| Respiratory support n (%) | |
| Positive pressure ventilation | 1 (17) |
| Supplemental oxygen | 5 (83) |
| No respiratory support | 1 (17) |
| Antibiotics n (%) | 6 (100) |
| Duration of hospitalisation, days, median (interquartile range) | 5.5 (3.0–6.8) |
| Duration of systemic steroids, days, median (interquartile range) | 9.0 (6.5–15.7) |
TABLE 3.
Median peak laboratory values for e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects (n=6)
| Median (interquartile range) | Reference range | |
| C-reactive protein, mg·L−1 | 332.5 (210.0–390.3) | <5.0 |
| Erythrocyte sedimentation rate, mm·h−1 | 71.5 (39.0–95.3) | 0–20 |
| Total white blood cell count, ×103 per µL | 15.3 (12.4–18.5) | 3.8–10.5 |
| Neutrophils % | 91.5 (89.8–92.3) | 40–60 |
| Neutrophil number, ×103 per µL | 14.0 (11.1–17.1) | 2.5–7.0 |
FIGURE 1.
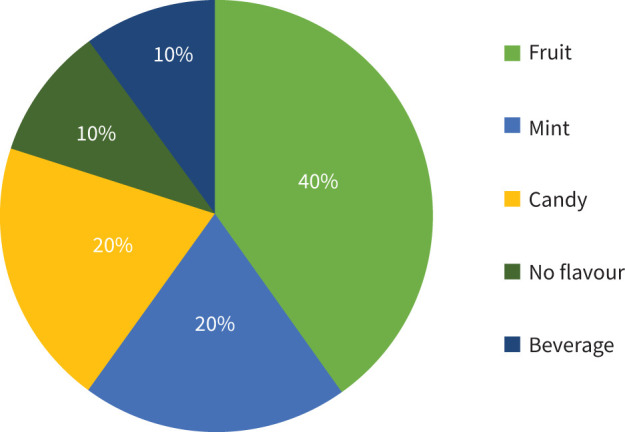
Flavour preferences of e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects. Self-reported survey data based on favourite flavour (n=6).
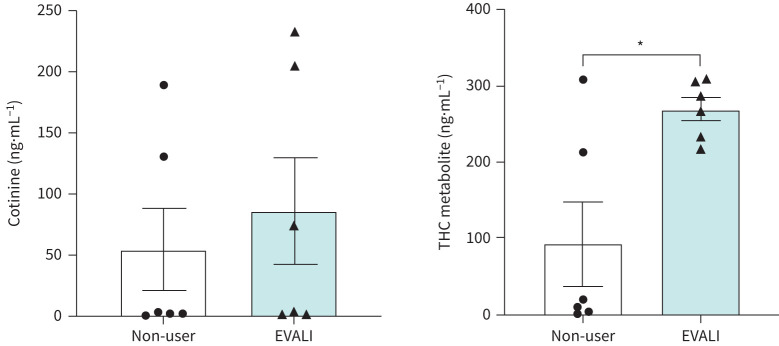
Plasma cotinine and THC metabolite levels in plasma and urine samples from EVALI subjects
Previous studies report high incidences of THC-based cartridges and associated e-cigarette products among those hospitalised with EVALI [7–9]. Considering this, all urine and plasma samples were evaluated for THC metabolite, i.e. 11-nor-9-carboxy-Δ9-THC, and the nicotine metabolite, i.e. cotinine, respectively. Consistent with these prior reports, urinary levels of the THC metabolite were significantly elevated in EVALI subjects compared to non-users (p=0.0112; figure 2). Plasma cotinine levels were also elevated in EVALI subjects, but not statistically different from non-users (p=0.5815), showing relatively low amount in EVALI users, but also suggesting that EVALI subjects are either dual users of e-cigarettes and tobacco cigarettes or vaped nicotine-containing e-cigarettes.
FIGURE 2.
Elevated plasma cotinine and tetrahydrocannabinol (THC) levels in e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects. The level of cotinine (in plasma) and THC metabolites (in urine) from non-users and EVALI subjects was quantified using ELISA. n=6 per group. Data are shown as mean±sem. *: p<0.05 as per unpaired t-test.
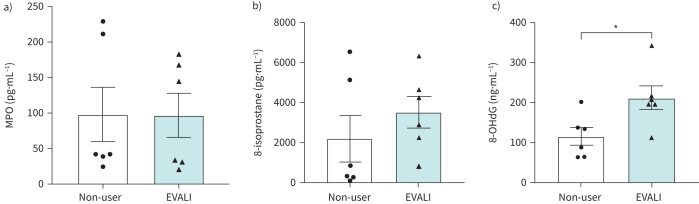
Oxidative stress markers in plasma and urine from EVALI subjects
To determine if elevated reactive oxygen species (ROS)/oxidative stress secondary to vaping contributed to the hospitalisation in EVALI subjects, we quantitated the levels of three oxidative stress markers (myeloperoxidase (MPO), 8-isoprostane and 8-OHdG) in the biological samples from all subjects. In plasma, MPO was not significantly different between groups (figure 3a). Likewise, 8-isoprostane was insignificantly elevated in the urine of EVALI subjects when compared with non-users (figure 3b). However, the concentration of 8-OHdG demonstrated a statistically significant increase in EVALI subjects as compared to non-users (p=0.0258; figure 3c).
FIGURE 3.
Oxidative stress markers in the plasma and urine from e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects. The levels of a) myeloperoxidase (MPO), b) 8-isoprostane and c) 8-hydroxy-2′-deoxyguanosine (8-OHdG) were measured in plasma (MPO) and urine (8-isoprostane and 8-OHdG) from non-user and EVALI subjects using ELISA-based assays. n=6 per group. Data are shown as mean±sem. *: p<0.05 as per unpaired t-test.
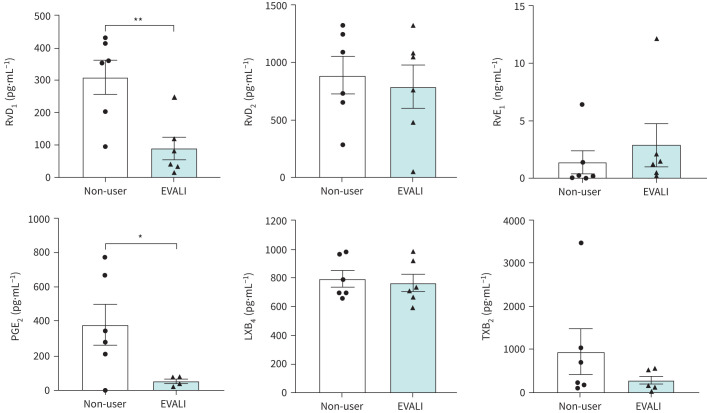
Pro-resolving lipid mediators in plasma of EVALI subjects
Since many mediators of inflammation are derived from phospholipids or polyunsaturated fatty acids, levels of lipid-derived mediators, including PGE2, RvD1, RvD2, LXB4, thromboxane B2 (TXB2) and RvE1, were measured in plasma from EVALI subjects and non-users. PGE2 levels were significantly decreased in plasma from EVALI subjects compared with non-users (p=0.0342; figure 4). Similarly, RvD1 demonstrated a significantly lower concentration in EVALI subjects compared with non-users (p=0.0062). However, other mediators, such as RvD2, RvE1, LXB4 and TXB2, exhibited non-significant changes between the groups.
FIGURE 4.
Changes in the levels of pro-resolving lipid mediators in plasma samples from e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects. Plasma levels of resolvins (RvD1, RvD2 and RvE1), prostaglandin E2 (PGE2), lipoxin B4 (LXB4) and thromboxane B2 (TXB2) in non-users and EVALI subjects were quantitated using ELISA-based assays. n=6 per group. Data are shown as mean±sem. *: p<0.05; **: p<0.01 as per unpaired t-test.
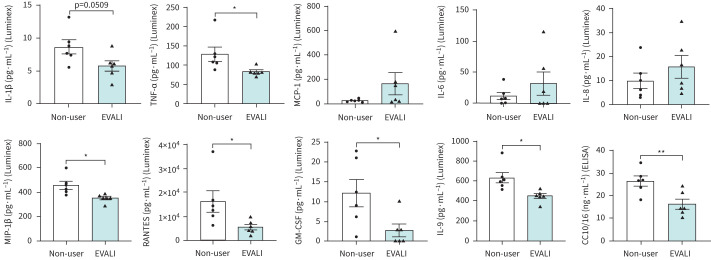
Inflammatory biomarkers in plasma
The levels of pro- and anti-inflammatory cytokines/chemokines were also measured in the plasma. Plasma levels of tumour necrosis factor-α (TNF-α), macrophage inflammatory protein-1β (MIP-1β), RANTES (regulated on activation, normal T-cell expressed and secreted), granulocyte–macrophage colony-stimulating factor, interleukin-9 (IL-9) and CC10/16 demonstrated significantly lower levels in EVALI subjects than the non-users (figure 5). IL-1β levels, although not statistically significant, were lower in EVALI subjects compared to non-users (p=0.0509). Contrarily, we found non-significant increases between groups for monocyte chemoattractant protein-1 (MCP-1) (p=0.1528), IL-8 (p=0.3296) and IL-6 (p=0.3329). Other targets,like IL-1rα and eotaxin saw insignificant alterations in levels between EVALI subjects and non-users as well (table 4).
FIGURE 5.
Dysregulated levels of inflammatory mediators in plasma from e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects. Plasma levels of inflammatory cytokines/chemokines/mediators in non-users and EVALI subjects were quantitated using Luminex (27-plex) and ELISA (CC10/16)-based assays. IL: interleukin; TNF-α: tumour necrosis factor-α; MCP-1: monocyte chemoattractant protein-1; MIP-1β: macrophage inflammatory protein-1β; GM-CSF: granulocyte–macrophage colony-stimulating factor. n=6 per group. Data are shown as mean±sem. *: p<0.05; **: p<0.01 as per unpaired t-test.
TABLE 4.
Mean values for various biomarkers quantitated in plasma or urine from e-cigarette, or vaping, product use-associated lung injury (EVALI) subjects and healthy subjects
| Parameters | Non-user pg·mL−1 | EVALI pg·mL−1 | p-value |
| THC metabolite (11-nor-9-carboxy-Δ9-THC) | (93.1±123.4)×103 | (271.2±35.5)×103 | 0.0112* |
| Cotinine | (54.6±76.6)×103 | (86.3±97.9)×103 | 0.5815 |
| Oxidative stress | |||
| MPO | 98.3±87.2 | 97.0±69.7 | 0.9786 |
| 8-OHdG | (115.4±49.0)×103 | (212.5±66.9)×103 | 0.0258* |
| 8-Isoprostane | (2.20±2.62)×103 | (3.52±1.77)×103 | 0.3720 |
| Lipid mediators | |||
| RvD1 | 310.3±120.1 | 88.8±78.5 | 0.0062** |
| RvD2 | 887.1±366.3 | 790.2±423.6 | 0.7071 |
| RvE1 | (1.36±2.32)×103 | (2.90±4.17)×103 | 0.4880 |
| LXB4 | 796.3±129.4 | 766.0±136.8 | 0.7264 |
| PGE2 | 379.3±265.7 | 52.4±24.2 | 0.0342* |
| TXB2 | 937.3±1179.6 | 266.8±204.9 | 0.2390 |
| Inflammatory mediators | |||
| TNF-α | 128.4±41.5 | 83.0±10.1 | 0.0389* |
| IL-1β | 8.66±2.35 | 5.75±1.76 | 0.0509 |
| MCP-1 | 26.7±9.1 | 167.0±202.6 | 0.1528 |
| IL-8 | 10.0±7.1 | 15.8±10.5 | 0.3296 |
| IL-6 | 11.9±13.1 | 32.0±42.1 | 0.3329 |
| CC10/16 | (26.6±4.9)×103 | (16.3±4.9)×103 | 0.0083#,* |
| MIP-1β | 455.4±71.5 | 352.3±31.3 | 0.0145* |
| RANTES | 16309.0±9912.3 | 5507.1±2573.93 | 0.0401* |
| GM-CSF | 12.2±7.7 | 2.8±3.5 | 0.0330* |
| Basic FGF | 124.0±14.3 | 99.2±25.5 | 0.0861 |
| VEGF | 182.9±356.6 | 2.1±0 | 0.2833 |
| PDGF-BB | 4726.4±3330.7 | 1419.5±1414.1 | 0.0682 |
| IL-1rα | 193.1±367.3 | 403.5±502.3 | 0.4671 |
| IL-2 | 5.39±4.98 | 3.76±2.35 | 0.5213 |
| IL-4 | 5.54±1.47 | 4.54±1.06 | 0.2473 |
| IL-5 | 24.6±26.4 | 5.6±6.5 | 0.1482 |
| IL-7 | 50.9±18.0 | 56.8±4.5 | 0.4961 |
| IL-9 | 635.2±118.0 | 451.4±53.8 | 0.0100* |
| IL-10 | 1.26±1.91 | 1.86±1.33 | 0.5731 |
| IL-13 | 3.58±0.59 | 4.25±0.93 | 0.2078 |
| IL-15 | 219.4±289.7 | 9.0±0 | 0.1354 |
| IL-17 | 22.2±4.2 | 19.5±5.1 | 0.3804 |
| Eotaxin | 71.3±27.7 | 43.6±23.0 | 0.1167 |
| IFN-γ | 29.4±11.3 | 24.2±6.5 | 0.3979 |
| G-CSF | 163.1±62.7 | 130.8±22.9 | 0.3051 |
| IL-12p70 | 8.21±4.61 | 6.32±4.09 | 0.5087 |
| MIP-1α | 3.47±1.43 | 3.97±1.79 | 0.6326 |
| IP10 | 756.0±267.9 | 2293.5±2177.0 | 0.1481 |
Data are presented as mean±sd unless otherwise indicated. THC metabolite, 8-OHdG and 8-isoprostane were measured in urine, whereas all other mediators were measured in plasma. THC: tetrahydrocannabinol; MPO: myeloperoxidase; 8-OHdG: 8-hydroxy-2′-deoxyguanosine; RvD1: resolvin D1; RvD2: resolvin D2; RvE1: resolvin E1; LXB4: lipoxin B4; PGE2: prostaglandin E2; TXB2: thromboxane B2; TNF-α: tumour necrosis factor-α; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MIP: macrophage inflammatory protein; GM-CSF: granulocyte–macrophage colony-stimulating factor; FGF: fibroblast growth factor; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor; IFN-γ: interferon-γ; G-CSF: granulocyte–colony-stimulating factor; IP10: interferon gamma-induced protein 10. #: p<0.00125 (FDR–p<0.05/40). *: p<0.05; **: p<0.01.
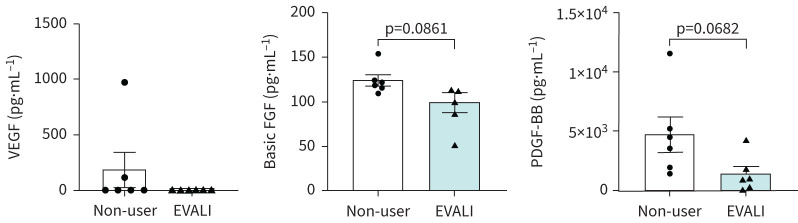
Plasma growth factor levels
Platelet-derived growth factor (PDGF)-BB and basic fibroblast growth factor (b-FGF) concentrations in plasma, while not statistically significant, were lower in EVALI subjects than their non-user counterparts (figure 6). In contrast, vascular endothelial growth factor (VEGF) levels were decreased, albeit insignificantly, for EVALI subjects when compared with non-users.
FIGURE 6.
Altered levels of growth factors in subjects with e-cigarette, or vaping, product use-associated lung injury (EVALI). Plasma levels of growth factors (vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF)-BB) in non-users and EVALI subjects were determined using Luminex. n=6 per group. Data are shown as mean±sem. p-values calculated per unpaired t-test.
Discussion
In this study, plasma and urine from EVALI subjects and non-users were used in an effort to identify potential biomarkers of disease. We identified multiple inflammatory cytokines, pro-resolving lipid mediators, oxidative stress and DNA damage markers to be significantly different between EVALI subjects and non-user controls.
Evidence from the literature suggest that THC-based cartridge and associated e-cigarette product users are more susceptible to hospitalisation due to EVALI [8]. In our self-reporting survey, only one of the subjects with EVALI admitted to using THC- or cannabidiol-based products for vaping. However, on urine analysis, multiple EVALI subjects, as well as non-user controls, demonstrated elevated levels of THC metabolite 11-nor-9-carboxy-Δ9-THC. These findings are in agreement with a previous self-reported public survey that shows that EVALI subjects typically use THC-containing products more than e-cigarette users who did not develop lung injury [31]. They also found nicotine use to be more prevalent in those who did not develop EVALI [31]. However, multiple EVALI subjects in our study had slightly higher cotinine levels as compared to non-users. In fact, previous reports have also found significantly higher levels of cotinine amongst e-cigarette users when compared to non-users, with levels comparable to traditional smokers [26, 32, 33]. Our results confirm that dual use of both THC- and nicotine-containing products may place oneself at greater risk for hospitalisation due to EVALI.
We found a significant increase in the levels of 8-OHdG in urine from EVALI subjects when compared to non-users. Similar findings were reported in e-cigarette users [26, 34] and cigarette smokers [35]. High levels of 8-OHdG indicate increased oxidative DNA damage and is a factor in the promotion of carcinogenesis [36]. Previous work by our group found an increase in the levels of 8-isoprostane in the urine from e-cigarette users as compared to non-users [26]. However, we did not see any significant change in the levels of MPO and 8-isoprostane (though there was a trend for increase) in the biological samples from EVALI subjects and non-users. The presence of increased levels of 8-OHdG supports that DNA damage plays a potentially important role in the pathogenesis of EVALI.
Resolvins, such as RvD1 and RvD2, play a major role in dampening inflammation. Singh et al. [26] previously demonstrated a significant decrease in the levels of RvD1 in the plasma from e-cigarette users. The levels of plasma RvD1 were significantly decreased in EVALI subjects compared to non-user controls. RvD1 is a lipid mediator that functions to dampen polymorphonuclear leukocytes infiltration and transmigration. Thus, lower levels of RvD1 are in line with prominent serum neutrophilia seen in the majority of EVALI subjects hospitalised with respiratory insufficiency and may potentially contribute to failed lung repair after e-cigarette exposure and/or vaping THC products [26]. In contrast, we also found a significant decrease in PGE2 levels in EVALI subjects compared to controls. PGE2 induces inflammation through mast cell activation. One potential explanation for these dichotomous findings is the concurrent use of non-steroid anti-inflammatory drugs (NSAIDs) in those hospitalised with EVALI, which would falsely lower the PGE2 levels seen in this group.
Cigarette smoke-induced oxidative stress is shown to activate the inflammatory response by upregulating cytokines, such as IL-6 and IL-8. IL-6 plays a key role in mediating acute phase response and is a prognostic biomarker in various acute organ injuries, including the lung. IL-8 functions in the chemotaxis and eventual phagocytosis of neutrophils and other granulocytes. Neutrophils are linked to inflammatory lung diseases, including COPD, asthma, bronchiolitis, respiratory distress syndrome and interstitial pneumonia [37]. With multiple assays, we found IL-6 and IL-8 levels to be comparable between EVALI and non-users. When BEAS2B cells were exposed to counterfeit cartridges, there were significantly higher levels of IL-6 and IL-8 compared to cells exposed to air, thus suggesting the eliciting of immune response of e-cigarette exposure [8]. Similarly, Singh et al. [26] also reported a significant increase in IL-6 and IL-8 production among e-cigarette users when comparing the plasma inflammatory profiles with non-users. Multiple explanations may account for these differences. One explanation is that immune suppression occurs through different cytokine pathways than that of e-cigarette exposure alone. A second possibility is that hospitalised EVALI subjects often presented later in the course of the disease and after their treatments, and peak IL-6 and IL-8 levels may have dissipated at the time of blood collection.
IL-9 is an activator of mast cells [38] and is significantly lowered in EVALI subjects compared to non-users. This interleukin has been linked to allergic asthma in mice [39], cancer [40], allergic lung inflammation, and contributes to autoimmune disease in humans [38]. IL-9 also has the ability to activate the mast cells that produce IL-13 and therefore affect epithelial cells of the lung and gut [38].
IL-1β, a pro-inflammatory cytokine, is linked to many acute and chronic inflammatory diseases, including acute lung injury. In this study, plasma IL-1β levels were lower, though not significant, for EVALI subjects when compared to the non-users. Our results are contrary to previous literature where IL-1β levels were significantly higher in BALF and saliva from e-cigarette users as compared to never-smokers [9, 26]. However, when analysed in plasma samples, there was a non-significant decrease in e-cigarette users [26]. This proves that the measurement of various pro-inflammatory mediators might vary based on the biological sample being tested as well as the time of collection.
MIP-1β, also known as CCL4, is a chemokine known to play a critical role in the chemotactic activity of monocytes through the CCR5 receptor, which has been connected to diverse immune responses. MIP-1β levels were found to be increased in natural killer cells, CD8+ T-cells and CD4+ T-cells in pregnant women exposed to the influenza A virus. Additionally, their levels correlate with the severity of influenza symptoms and viral replication along with a similar rise in kinetics after influenza infection like other chemokines. As a whole, members of the MIP family are implicated as important mediators of lung disease. RANTES is a chemokine involved in leukocyte influx and bronchial hyperresponsiveness [41]. It has been established to play an important role in allergic lung inflammation and leukocyte infiltration [41]. In our study, MIP-1β and RANTES were significantly lowered in EVALI than in non-users.
Club cell secreted protein (CC10/16) has several immune-regulatory activities, including inhibition of phospholipase A2 [42, 43]. In past studies of COPD, asthma, idiopathic pulmonary fibrosis, sarcoidosis and other pulmonary issues, CC16 has shown promise as a potential biomarker of lung epithelial injury [42, 44]. Kropski et al. [42] found lower levels of CC16 in the plasma of acute lung injury patients when compared to acute cardiogenic pulmonary oedema patients. Not unexpectedly, when previously analysed in e-cigarette users, there was only a minimal difference compared to normal subjects in both plasma and urine [26]. In our study, we found EVALI subjects to have significantly lower CC16 levels than their non-user counterparts. This is most likely due to the difference in severity at the time of presentation, with most EVALI subjects requiring hospitalisation. It is possible that the lower levels of CC16 are secondary to alterations in alveolar epithelial permeability, club cell death or changes in transcriptional activity within the remaining club cells [42]. However, CC16 remains a significant and biologically relevant biomarker for future investigation.
A potent inflammatory cytokine, TNF-α, has been implicated in various pulmonary diseases like asthma and COPD/emphysema. In a previous study, we found smaller concentrations of TNF-α in e-cigarette users compared to non-users, albeit non-significant [26]. This has concurred in the present study, where we found this to be true of EVALI subjects with the same level of non-significance.
MCP-1, which is also known as CCL2, is a chemokine that regulates the migration and infiltration of monocytes and macrophages in innate immunity [45]. This chemokine has been linked to atherosclerosis, inflammatory bowel disease, asthma and arthritis [45]. It was also connected to interstitial lung disease in paediatric patients with high levels, negatively correlating to restrictive lung function, forced vital capacity, total lung capacity and other lung disease severity scores [46]. In our previous study, the levels of MCP-1 in non-users and e-cigarette users were indistinguishable from each other [26]. We observed non-significant higher concentrations in EVALI subjects compared to non-users in Luminex-based assays.
PDGF mediates airway inflammation and remodelling in asthma, and plays a significant role in blood vessel formation [47]. Cucina et al. [48] established that nicotine enhanced the release of PDGF-BB in endothelial cells. Concurring with this, Singh et al. [26] found the AA isoform to be significantly elevated in e-cigarette users, which established high levels of cotinine as well. In contrast, our study found that the PDGF-BB isoform was insignificantly reduced in EVALI subjects compared to controls when we also showed an increase in nicotine amongst EVALI subjects.
To our knowledge, this is the first study to report changes in oxidative stress, lipid mediators and inflammatory markers from blood and urine of EVALI subjects, and these biomarkers are differentially regulated as compared to e-cigarette users as shown by us recently [26]. There are some limitations. The first is that EVALI samples were collected after the EVALI subjects were admitted to the hospital, and nearly all subjects received drugs, including NSAIDs and antibiotics, prior to blood and urine collection. Most of the subjects received blood draws prior to receiving treatment for EVALI with systemic steroids. The second limitation is the small sample size. Furthermore, comparing the systemic biomarkers of acute lung injury (i.e. adult respiratory distress syndrome) would have strengthened the study; however, such an investigation is beyond the scope of this pilot project. While thousands of young adults have been affected by EVALI, the incidence has remained low since 2019. Subjects were recruited from a single institution, limiting sample size; however each sample was matched for age and sex.
Overall, this pilot study identified multiple potential EVALI biomarkers, including markers of oxidative stress, such as 8-OHdG and 8-isoprostane as well as reduced levels of pro-resolving lipid mediator RvD1 and anti-inflammatory airway epithelial marker CC10/16. These findings provide a strong basis for the use of these potential biomarkers in the diagnosis of EVALI.
Acknowledgements
We thank our participants, research assistants and nurses. We thank Carl J. Johnston, University of Rochester Medical Center, for technical support in subject recruitment. We also thank Jiries Meehan-Atrash for his scientific input, and Shikha Sharma and Nashae Prout for technical assistance. We thank Daniel Croft and Nicholas Nacca for scientific input on EVALI subjects.
Provenance: Submitted article, peer reviewed.
Author contributions: S. Podguski, G. Kaur, T. Muthumalage, M.D. McGraw and I. Rahman conceived and designed the experiments, and wrote and edited the manuscript. I. Rahman obtained research funding, and designed the study and experimental plans/assays. I. Rahman and M.D. McGraw recruited the volunteers. S. Podguski, T. Muthumalage and G. Kaur performed the experiments, and S. Podguski and T. Muthumalage analysed data. All authors contributed to manuscript preparation and approved the final version before submission.
Conflict of interest: S. Podguski has nothing to disclose.
Conflict of interest: G. Kaur has nothing to disclose.
Conflict of interest: T. Muthumalage has nothing to disclose.
Conflict of interest: M.D. McGraw reports a grant from NIH/NHLBI outside the submitted work.
Conflict of interest: I. Rahman has nothing to disclose.
Support statement: This study was supported by National Institutes of Health (NIH) grants R01 HL135613 and HL135613-S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Kaur G, Gaurav A, Lamb T, et al. Current perspectives on characteristics, compositions, and toxicological effects of e-cigarettes containing tobacco and menthol/mint flavors. Front Physiol 2020; 11: 613948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omaiye EE, McWhirter KJ, Luo W, et al. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep 2019; 9: 2468. doi: 10.1038/s41598-019-39550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meehan-Atrash J, Luo W, McWhirter KJ, et al. The influence of terpenes on the release of volatile organic compounds and active ingredients to cannabis vaping aerosols. RSC Adv 2021; 11: 11714–11723. doi: 10.1039/D1RA00934F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html Date last updated: 25 February 2020. Date last accessed: 9 November 2021.
- 5.Chand HS, Muthumalage T, Maziak W, et al. Pulmonary toxicity and the pathophysiology of electronic cigarette, or vaping product, use associated lung injury. Front Pharmacol 2019; 10: 1619. doi: 10.3389/fphar.2019.01619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalininskiy A, Kittel J, Nacca NE, et al. E-cigarette exposures, respiratory tract infections, and impaired innate immunity: a narrative review. Pediatr Med 2021; 4: 5. doi: 10.21037/pm-20-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhikari R, Koritala T, Gotur R, et al. EVALI – e-cigarette or vaping product use-associated lung injury: a case report. Cureus 2021; 13: e13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthumalage T, Lucas JH, Wang Q, et al. Pulmonary toxicity and inflammatory response of e-cigarette vape cartridges containing medium-chain triglycerides oil and vitamin E acetate: implications in the pathogenesis of EVALI. Toxics 2020; 8: 46. doi: 10.3390/toxics8030046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song M-A, Freudenheim JL, Brasky TM, et al. Biomarkers of exposure and effect in the lungs of smokers, nonsmokers, and electronic cigarette users. Cancer Epidemiol Biomarkers Prev 2020; 29: 443–451. doi: 10.1158/1055-9965.EPI-19-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques P, Piqueras L, Sanz MJ. An updated overview of e-cigarette impact on human health. Respir Res 2021; 22: 151. doi: 10.1186/s12931-021-01737-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med 2020; 382: 697–705. doi: 10.1056/NEJMoa1916433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy B, Li L, Lu S, et al. Analysis of cannabinoid-containing fluids in illicit vaping cartridges recovered from pulmonary injury patients: identification of vitamin E acetate as a major diluent. Toxics 2020; 8: 8. doi: 10.3390/toxics8010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthumalage T, Friedman MR, McGraw MD, et al. Chemical constituents involved in e-cigarette, or vaping product use-associated lung injury (EVALI). Toxics 2020; 8: 25. doi: 10.3390/toxics8020025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meeh-Atrash J, Luo W, McWhirter KJ, et al. Aerosol gas-phase components from cannabis e-cigarettes and dabbing: mechanistic insight and quantitative risk analysis. ACS Omega 2019; 4: 16111–16120. doi: 10.1021/acsomega.9b02301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Sun NN, Zhang J, et al. Immunomodulatory effects of high-dose alpha-tocopherol acetate on mice subjected to sidestream cigarette smoke. Toxicology 2002; 175: 235–245. doi: 10.1016/S0300-483X(02)00064-1 [DOI] [PubMed] [Google Scholar]
- 16.Hybertson BM, Chung JH, Fini MA, et al. Aerosol-administered alpha-tocopherol attenuates lung inflammation in rats given lipopolysaccharide intratracheally. Exp Lung Res 2005; 31: 283–294. doi: 10.1080/01902140590918560 [DOI] [PubMed] [Google Scholar]
- 17.Meehan-Atrash J, Rahman I. Cannabis vaping: existing and emerging modalities, chemistry, and pulmonary toxicology. Chem Res Toxicol 2021; 34: 2169–2179. doi: 10.1021/acs.chemrestox.1c00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H. Vitamin E acetate as linactant in the pathophysiology of EVALI. Med Hypotheses 2020; 144: 110182. doi: 10.1016/j.mehy.2020.110182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman R, Stanton M, Suelzer EM. Compiling evidence for EVALI: a scoping review of in vivo pulmonary effects after inhaling vitamin E or vitamin E acetate. J Med Toxicol 2021; 17: 278–288. doi: 10.1007/s13181-021-00823-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Liang Q, Frost-Pineda K, et al. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol Biomarkers Prev 2011; 20: 1760–1769. doi: 10.1158/1055-9965.EPI-10-0987 [DOI] [PubMed] [Google Scholar]
- 21.Shiels MS, Katki HA, Freedman ND, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst 2014; 106: dju294. doi: 10.1093/jnci/dju294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman I, Morrison D, Donaldson K, et al. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996; 154: 1055–1060. doi: 10.1164/ajrccm.154.4.8887607 [DOI] [PubMed] [Google Scholar]
- 23.Stockley RA, Halpin DMG, Celli BR, et al. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med 2019; 199: 1195–1204. doi: 10.1164/rccm.201810-1860SO [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rio F, Miravitlles M, Soriano JB, et al. Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir Res 2010; 11: 63. doi: 10.1186/1465-9921-11-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvarajah S, Todd I, Tighe PJ, et al. Multiple circulating cytokines are coelevated in chronic obstructive pulmonary disease. Mediators Inflamm 2016; 2016: 3604842. doi: 10.1155/2016/3604842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh KP, Lawyer G, Muthumalage T, et al. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res 2019; 5: 00182-2019. doi: 10.1183/23120541.00182-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonough SR, Rahman I, Sundar IK. Recent updates on biomarkers of exposure and systemic toxicity in e-cigarette users and EVALI. Am J Physiol Lung Cell Mol Physiol 2021; 320: L661–L679. doi: 10.1152/ajplung.00520.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrini V, Panettieri RA, Gennaro ML. Lipid-laden macrophages as biomarkers of vaping-associated lung injury. Lancet Respir Med 2020; 8: e6. doi: 10.1016/S2213-2600(19)30476-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol 2015; 209: 52–58. doi: 10.1016/j.resp.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 30.Kalininskiy A, Bach C, Nacca N, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med 2019; 7: 1017–1026. doi: 10.1016/S2213-2600(19)30415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navon L, Jones CM, Ghinai I, et al. Risk factors for e-cigarette, or vaping, product use-associated lung injury (EVALI) among adults who use e-cigarette, or vaping, products – Illinois, July–October 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 1034–1039. doi: 10.15585/mmwr.mm6845e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas CE, Wang R, Adams-Haduch J, et al. Urinary cotinine is as good a biomarker as serum cotinine for cigarette smoking exposure and lung cancer risk prediction. Cancer Epidemiol Biomarkers Prev 2020; 29: 127–132. doi: 10.1158/1055-9965.EPI-19-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park MB, Choi JK. Differences between the effects of conventional cigarettes, e-cigarettes and dual product use on urine cotinine levels. Tob Induc Dis 2019; 17: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamaki-Ching S, Williams M, Hua M, et al. Correlation between biomarkers of exposure, effect and potential harm in the urine of electronic cigarette users. BMJ Open Respir Res 2020; 7: e000452. doi: 10.1136/bmjresp-2019-000452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Wang D, Liu X, et al. Oxidative DNA damage is involved in cigarette smoke-induced lung injury in rats. Environ Health Prev Med 2015; 20: 318–324. doi: 10.1007/s12199-015-0469-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qing X, Shi D, Lv X, et al. Prognostic significance of 8-hydroxy-2′-deoxyguanosine in solid tumors: a meta-analysis. BMC Cancer 2019; 19: 997. doi: 10.1186/s12885-019-6189-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimori Y, Kataoka M, Tada S, et al. The role of interleukin-8 in interstitial pneumonia. Respirology 2003; 8: 33–40. doi: 10.1046/j.1440-1843.2003.00420.x [DOI] [PubMed] [Google Scholar]
- 38.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol 2010; 10: 683–687. doi: 10.1038/nri2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temann U-A, Geba GP, Rankin JA, et al. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med 1998; 188: 1307–1320. doi: 10.1084/jem.188.7.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerlach K, Weigmann B. The dichotomous function of interleukin-9 in cancer diseases. J Mol Med 2019; 97: 1377–1383. doi: 10.1007/s00109-019-01826-5 [DOI] [PubMed] [Google Scholar]
- 41.Conti P, DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc 2001; 22: 133–137. doi: 10.2500/108854101778148737 [DOI] [PubMed] [Google Scholar]
- 42.Kropski JA, Fremont RD, Calfee CS, et al. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest 2009; 135: 1440–1447. doi: 10.1378/chest.08-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes PJ. Club cells, their secretory protein, and COPD. Chest 2015; 147: 1447–1448. doi: 10.1378/chest.14-3171 [DOI] [PubMed] [Google Scholar]
- 44.Almuntashiri S, Zhu Y, Han Y, et al. Club cell secreted protein CC16: potential applications in prognosis and therapy for pulmonary diseases. J Clin Med 2020; 9: 4039. doi: 10.3390/jcm9124039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–326. doi: 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartl D, Griese M, Nicolai T, et al. A role for MCP-1/CCR2 in interstitial lung disease in children. Respir Res 2005; 6: 93. doi: 10.1186/1465-9921-6-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kardas G, Daszynska-Kardas A, Marynowski M, et al. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front Pharmacol 2020; 11: 47. doi: 10.3389/fphar.2020.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cucina A, Sapienza P, Borrelli V, et al. Nicotine reorganizes cytoskeleton of vascular endothelial cell through platelet-derived growth factor BB. J Surg Res 2000; 92: 233–238. doi: 10.1006/jsre.2000.5894 [DOI] [PubMed] [Google Scholar]