Abstract
Adult neurogenesis consists in the generation of newborn neurons from neural stem cells taking place in the adult brain. In mammals, this process is limited to very few areas of the brain, and one of these neurogenic niches is the subgranular layer of the dentate gyrus (DG) of the hippocampus. Adult newborn neurons are generated from quiescent neural progenitors (QNPs), which differentiate through different steps into mature granule cells (GCs), to be finally integrated into the existing hippocampal circuitry.
In animal models, adult hippocampal neurogenesis (AHN) is relevant for pattern discrimination, cognitive flexibility, emotional processing and resilience to stressful situations. Imaging techniques allow to visualize newborn neurons within the hippocampus through all their stages of development and differentiation. In humans, the evidence of AHN is more challenging, and, based on recent findings, it persists through adulthood, even if it declines with age. Whether this process has an important role in human brain function and how it integrates into the existing hippocampal circuitry is still a matter of exciting debate. Importantly, AHN deficiency has been proposed to be relevant in many psychiatric disorders, including mood disorders, anxiety, post-traumatic stress disorder and schizophrenia.
This review aims to investigate how AHN is altered in different psychiatric conditions and how pharmacological treatments can rescue this process. In fact, many psychoactive drugs, such as antidepressants, mood stabilizers and atypical antipsychotics (AAPs), can boost AHN with different results. In addition, some non-pharmacological approaches are discussed, as well.
Keywords: Adult hippocampal neurogenesis, antidepressants, mood stabilizers, atypical antipsychotics, receptor biased agonism, physical activity, restorative sleep
1. INTRODUCTION
Neurogenesis is a biological process of formation of new neurons from neural precursors, and it was traditionally considered a phenomenon circumscribed to neurodevelopmental stages [1]. In fact, as suggested originally by the neuroscientist Pasko Rakic, neurogenesis in adult humans would not be feasible because the higher functions of the brain require stability over plasticity. Conversely, in the last twenty years, many neuroscientists have been convinced otherwise, i.e., it is exactly the brain plasticity mediated by neurogenesis during the whole lifetime that may contribute to human’s flexibility and high functioning in many cognitive processes. In adult humans, this process seems mostly restricted in the hippocampus region, while in mammals, it also takes place in the subventricular zone (SVZ) of the lateral ventricles. In rodents, the newborn neurons migrate from the SVZ to the olfactory bulb (OB) through the rostral migratory stream (RMS). Surprisingly, a similar pathway has also been found in humans along with a putative lateral ventricular extension, though neural migration was not clearly demonstrated [2]. Intriguingly in humans, neurogenesis seems to occur in other areas, such as the striatum [3].
Altman's pioneering studies gave the first evidence on the presence of undifferentiated cells in the granular cell layer of the dentate gyrus (DG) in the hippocampus of adult rats. The 3H-thymidine tagged cells were numerous not only in neonates and infant rats DG, but in adult rats as well [4, 5]. This knowledge on rats was later extended to several species of mammals, where neurogenesis was detected in the postnatal period. Later on, Eriksson [6], using immunohistochemical techniques postmortem, was the first to demonstrate the generation of new neurons from dividing progenitor cells in the DG of the hippocampus in human brains.
Recently, two important studies have confirmed the existence of adult hippocampal neurogenesis (AHN) in humans at different ages. Boldrini et al. [7], by analyzing the whole autopsy hippocampi, found that hippocampal neurogenesis still persists throughout aging, which was confirmed by similar evidence under strictly controlled conditions by comparing healthy individuals with patients with Alzheimer’s disease (AD) [8].
Conversely, two other groups, namely Sorrells et al. [9] and Cipriani et al. [10], challenged AHN. Sorrells et al. describe a sharp decline of proliferating progenitor cells and young neurons in the DG of humans during early post-natal life, where neural progenitors could no longer be identified in subjects after the age of thirteen. Such a discrepancy is mostly due to prolonged fixation conditions in the analysis carried out in the Sorrells’ work, which causes a reduction in the antigen detection, as it was clearly demonstrated by the recent paper of Moreno-Jimenez et al. [8]. This and other issues have been nicely discussed in a review by Kempermann et al. [11]. This is a methodological issue, which needs to be taken into account when detecting the presence of AHN. This inherent limit may be circumvented in the future by using reliable markers to identify AHN in vivo, especially in humans. This is expected to solve a number of issues and to build established evidence in the field.
At present, despite controversial findings and various methodological approaches, the issue concerning the presence of neural progenitor cells in the human hippocampus remains an exciting and challenging topic. This is further emphasized when considering the potential physiological role of these cells in the complex DG network and its connection to other brain structures.
Intriguingly, a reduction of AHN has been considered across many psychiatric and neurological disorders, including mood disorders, post-traumatic stress disorder, schizophrenia, and AD [12].
This review aims to investigate the functional role of AHN in animal models and humans, and how it is altered in different psychiatric conditions. In addition, pharmacological treatments that can increase the process of neurogenesis will be taken into consideration, based on the evidence that many psychoactive drugs such as antidepressants, mood stabilizers and atypical antipsychotics (AAPs), can boost the proliferation of immature precursors, induce their maturation or prevent their cell death. This effect is expected to produce a gross increase in the number of mature neurons integrated within the hippocampus and to potentially improve the synaptic connections within the limbic system. Non-pharmacological approaches that may increase AHN, such as physical exercise and restorative sleep, are discussed as well.
2. AHN IN ANIMAL MODELS AND HUMANS
In murine species, AHN is spatially restricted to two specific neurogenic brain regions. The subgranular zone (SGZ) in the DG where newborn adult neurons migrate to a short distance radially into the granular cell layer and differentiate into glutamatergic granule cells (GCs), and the SVZ of the lateral ventricles where new neurons migrate through the rostral migratory stream to the olfactory bulb to become mostly GABAergic (GABA, γ-aminobutyric acid) interneurons [1]. In both niches, pluripotent neural stem cells persist and are able to give rise to the main lineages of the central nervous system, e.g., neurons, oligodendrocytes and astrocytes [13]. However, these reservoirs of stem cells decrease with aging [14].
In the DG of mice, the generation of new neurons starts from quiescent neural progenitors (QNPs), which are type I radial-glial-like cells (or type I intermediate neural progenitors (INPs)) and self-renewing multipotent neural stem cells, characterized by markers such as GFAP, nestin and Sox2. Once activated, type I INPs undergo asymmetric divisions and generate amplifying type II INPs, expressing proliferation markers, such as Ki-67 and nestin, which subsequently become type III INPs or neuroblasts characterized by the expression of DCX and polysialylated neural cell adhesion molecule (PSA-NCAM) [15].
Besides proliferation, adult neurogenesis is a multistep process that includes differentiation, migration, axonal elongation, axon branching, enlargement of synaptic zones, circuit integration and survival, where some of the new cells can be eliminated at any stage in their maturation progress [15, 16].
In the past, the only tools available for studying the process of adult neurogenesis were bromodeoxyuridine (BrdU), a thymidine analog that is incorporated in the nucleus in the S phase of cell cycle, and 14C labeling, which integrates into genomic DNA in a stable manner and allows dating the moment of cell birth [17]. At present, the development of novel imaging techniques in living animals, such as two-photon microscopy, advanced our knowledge and paved the way to understand how newborn cells integrate into the hippocampal circuitry. In 2016, Danielson et al. managed to monitor in vivo the activity of adult-born GCs using two-photon calcium imaging [18, 19].
The integration of new neurons in the DG can take several weeks in animals, where they may die, particularly during the first four weeks, depending on the environmental stimuli. Initially, adult-born neurons show increased excitability and are mostly influenced by local GABAergic interneurons, such as parvalbumin-positive basket cells and chandelier cells [15], responding to unusual GABA activation due to high intracellular chloride concentration that leads to membrane depolarization once GABA is released. Then, the chloride gradient switches because the NKCC1 transporter substitutes with KCC2. The first excitatory contacts come from local glutamatergic mossy fibers, which develop during the second week, whereby the new maturating neurons start to express specific N-methyl-D-aspartate (NMDA) receptors containing NR2B subunit that lowers the threshold for induction of long-term potentiation and enhanced plasticity [20].
Despite having enhanced excitability, immature cells are gifted with a modest excitatory innervation compared with their mature counterparts, which explains the different electrophysiological activity going on within these two neuronal populations occurring in the complex DG circuitry [21, 22].
Dendritic spines formation takes place during the first 2-3 weeks or even longer after neuronal birth [15,23], and it is controlled by local astrocytes. The block of vesicular release from astrocytes results in an impaired dendritic maturation, thus showing a fundamental modulatory role for surrounding astrocytes [24].
The synaptic output from adult-born GC is generally formed within the first three weeks. These neurons are excitatory cells reaching with their axons inhibitory interneurons of the DG, which in turn control GC activity. In fact, overall, the immature newborn neurons inhibit the activity of the DG. In addition, they also synapse mostly in the CA3 region and in part in the CA2 region with both inhibitory interneurons and pyramidal cells [20].
Finally, they become functionally mature GCs within eight weeks, despite strongly influencing the hippocampal circuitry during different phases of their maturation process [25]. In a twelve weeks old mouse, the amount of immature neurons is around 10% of the total GCs [26].
Long-distance monoaminergic and cholinergic projections coming from different nuclei influence adult-born GCs activity with relevant consequences for the use of compounds with therapeutic activity [27].
Though adult neurogenesis in rodents and mice is clearly demonstrated, this topic is more controversial in primates. In fact, AHN has been confirmed in several primate species, including marmosets [28], lemurs [29], macaques and baboons [30]. Gould et al. [31] were the first to perform a BrdU treatment in adult marmoset monkeys and found labeled cells in the DG; most of them showed characteristics of GC precursors. The adult generation of neurons in the DG was also observed in macaques, although the rate of neurogenesis was found to be lower than in rats [32]. Thus, despite the fact that differences exist among various species, the process of AHN has been found in many mammalian species [11].
In humans, the first proof of adult neurogenesis dates back to late 90’s when Eriksson et al. [6] using BrdU detected dividing neuronal cells in postmortem samples from five patients in the GC layer of the DG, owing to a morphology similar to that reported in other species [6].
Regarding neurogenesis, specific markers target neuronal precursors in different phases of the maturation process, and they are used to characterize the hippocampal neuronal population. Nevertheless, the relationship between animal and human studies remains a complex topic, particularly if we consider lifespan differences and the decline of neurogenesis with aging between these two species.
At present, it has been proposed that adult neurogenesis in the human hippocampus generates about 700 newly born neurons daily in the DG at a rate of 1.75%/year. Thus, most neurons can be replaced within the human lifespan, forming a self-renewing population in the hippocampus [33-35]. However, whether this process is sufficient to prevent hippocampal volume reduction with aging is still a matter of extensive debate, where several studies have reached opposite conclusions, thus underlining the complexity and variability of such phenomenon. In rodents, the hippocampal volume remains stable with age, or it might even increase as reported by some [36, 37].
Recently, Boldrini et al. [7] confirmed the presence of AHN in postmortem humans that did not decrease with age, despite a reduction of QNPs, especially in the anterior DG [7]. The use of stereology has strengthened these findings as this technique quantifies cells in a specific volume of tissue. Conversely, Sorrells et al. [9] could not find any neural precursor in the adult hippocampus by using markers for cell proliferation such as DCX, Ki-67 and PSA-NCAM.
The Sorrells’ study [9] attracted some methodological criticism. For instance, sample preparation with uncontrolled fixation conditions may alter the immune staining of protein markers. In fact, as it was clearly shown by Moreno-Jimenez et al. [8], the detection of AHN markers in humans critically depends on fixing procedure and further steps in sample processing. Persistent storage in a fixing solution condition may reduce antigen integrity, thereby weakening the power of detection. Furthermore, the fact that the study was carried in brain tissue from epileptic patients could have been confounding, since the hippocampal neurogenic niche is altered by epilepsy [11, 38].
On this topic, by using a strict methodological procedure, Moreno-Jimenez et al. identified thousands of DCX-positive neuronal precursors in the DG of several adults [8]. These data further confirm the persistence of neurogenesis in older healthy people, a phenomenon that is found decreased in patients with AD. In addition, this study confirmed that the detection of AHN markers in humans is critically dependent on fixation and histological preparations, where prolonged fixation conditions can drastically reduce the detection of neuronal precursors.
In addition, Boldrini et al. [7] found a reduction in angiogenesis and neural plasticity in older people, even though neurogenesis still persists.
3. NEUROPHYSIOLOGY OF AHN IN ANIMAL MODELS AND HUMANS
In order to establish a role of adult neurogenesis, a variety of experimental approaches in mice can be performed, such as low dose brain irradiation, treatment with anti-mitotic agents, or aging models. Lately, the development of transgenic mice has offered the possibility to induce a neurogenesis deficit in a temporally regulated manner [25, 39, 40]. It is now clear that newborn immature neurons regulate the excitability of DG and thus influence the information flow coming from the entorhinal cortex to DG and then to CA3, the so-called trisynaptic model. The number of principal cells in the DG of rats is around ten times higher than in the entorhinal cortex and CA3, which emphasizes the role of neurogenesis in the DG compared with other limbic cortex structures [25].
The alteration of AHN selectively affects hippocampus-dependent tasks such as spatial and contextual memory, and pattern discrimination [41].
One possible explanation is that the inhibitory control of immature neurons on the activity of DG cells determines selection and sparse activation of non-overlapping cellular specific circuits, also called engrams, which allow distinguishing similar experiences spatially and temporally by producing different outputs for similar inputs. In fact, the ablation of adult neurogenesis increases the activity of DG mature neurons, while the increase of neurogenesis reduces DG excitability. In the latter, the number of excitatory synaptic contacts of newborn neurons onto GABAergic hilar interneurons is increased [42].
On the one hand, young neurons are broadly tuned to novelty and time-related information due to their hyper-excitability, and on the other hand, mature GCs are supposed to be more critical to encode space-related novelties due to a higher excitation threshold. In fact, highly excitable young neurons may be ineffective in separating similar events, while mature GCs are proposed to be more efficient in the sparse encoding of contextual information due to their higher excitation level. In this scheme, young neurons act as pattern integrators to encode temporal associations and as indirect pattern separators for inhibiting mature GCs to facilitate sparse coding [15].
Adult neurogenesis is required for changing a previous memory (e.g., safety location) but not for learning the initial rule. In fact, in the Morris water maze test, intact neurogenesis is necessary for mice to learn the new position of the safety platform when it is changed (reverse learning), while it does not seem relevant to find the platform for the first time. In addition, neurogenesis is also implicated in avoiding merging between the novel and previously formed memories and/or in the process needed to clear unnecessary memories [26].
Based on this evidence, we might infer how adult neurogenesis seems important for cognitive flexibility to adopt successful strategies, especially in new situations. Although much of this function is attributable to the prefrontal cortex (PFC), hippocampus could also have a role [26]. Importantly, while the dorsal hippocampus may contribute to cognition, orientation and spatial learning, the ventral hippocampus seems relevant for emotional behavior, social interaction, mood and regulation of the neuroendocrine system [26].
Besides neutral situations, AHN also seems relevant in stressful and emotionally charged circumstances where it influences emotional engrams generated in the DG. Such function contributes to reduce anxiety and depression in stressful conditions, as demonstrated in animal models [43]. In fact, neurogenesis within the ventral hippocampus may exert inhibitory control over the hypothalamus and HPA axis, thus regulating the stress-related neuroendocrine response, which in turn has a negative feedback activity on the hippocampus itself [44].
As demonstrated for spatial and contextual memory, the inhibitory activity of newborn neurons over mature GCs may be important to modulate responses to fear experiences, e.g., to erase fear memories when necessary [45]. When mice learn to associate an experience with an aversive event, such as a foot-shock, this memory trace is encoded by a cellular representation (engram) in the hippocampus. Once the shock is removed in a fear extinction animal protocol, cognitive flexibility is subsequently required to learn that the same context has now become unharmful, and this process may need adult neurogenesis. In fact, in stressful conditions, where neurogenesis is inhibited, the discriminatory capacity can be reduced, and new safe conditions can still be perceived as dangerous [46]. Although the ablation of new neurons can impair the capability of reducing anxiety in stressful environments, it has a low impact on basal levels of anxiety in animal models [47].
The relevance of AHN in humans in vivo is more difficult to establish with respect to animal models because there are no straightforward tools to neither identify newborn neurons nor inhibit neurogenesis [48].
Neural precursors were identified in the human hippocampus using magnetic resonance spectroscopy markers. However, these findings need to be confirmed [49]. Currently, many efforts are being made to find new in vivo markers using magnetic resonance imaging (MRI) and positron emission tomography (PET) techniques, which could pave the way to finally confirm adult neurogenesis in humans [50]. Indirect evidence of adult neurogenesis in humans comes from hippocampal volume reports where it has been shown how physical exercise, enriched environment and antidepressant medications can either increase or prevent a decrease of hippocampal volume in healthy and pathological conditions. These activities seem to increase cerebral blood flow in the hippocampus, which is unfortunately, not a specific marker of neurogenesis [51]. To corroborate the potential relevance of AHN in humans, aging individuals show a reduced pattern separation capability that is associated with a weaker DG functional MRI signal [26], and patients treated with chemotherapy often report a decline in memory as a sign of hippocampal alteration [52].
4. PHARMACOLOGICAL TREATMENTS AND AHN IN ANIMAL MODELS AND HUMANS
4.1. Antidepressants
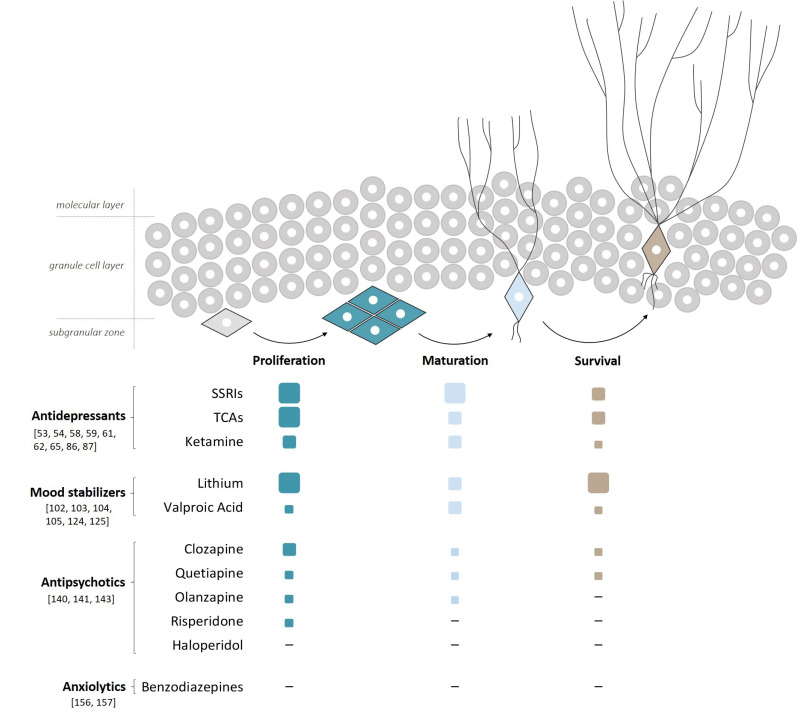
The strongest evidence of a correlation between pharmacological treatment and the occurrence of neurogenesis applies to antidepressants (Fig. 1).
Fig. (1).
Effects of pharmacological treatments on the different phases of AHN A schematic representation showing the stimulatory effects of the most widely used psychoactive drugs on the different phases of AHN. Based on the number of publications showing drug efficacy, values are reported as high ( ), medium (
), medium ( ), low (
), low ( ) and not effective (-). High (
) and not effective (-). High ( ) refers to many papers, medium (
) refers to many papers, medium ( ) to several papers, low (
) to several papers, low ( ) to few papers and (-) refers to no papers. Citations supporting our criteria are also included. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
) to few papers and (-) refers to no papers. Citations supporting our criteria are also included. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A large number of studies have pointed out how antidepressants increase AHN in different species, from rodents up to primates and humans [53]. Among these, mouse models are the most studied, and a recent meta-analysis has confirmed that chronic treatments involving most commonly used antidepressants significantly increase the hippocampal neurogenesis [54]. A pioneer study showed that when hippocampal neurogenesis was abolished by X-irradiation, the effects of the serotonin selective reuptake inhibitor (SSRI) antidepressant fluoxetine on depression-anxiety paradigms are abolished [55]. Following these early results, other studies reproduced these findings, showing that the occurrence of this effect is dependent on the specific mouse strain and the type of antidepressant being administered [56-58].
The sequential process of AHN includes division from neural precursors (e.g. QNPs or INPs) to generate newborn neurons (proliferation), development of neuronal morphology, axonal elongation and dendritic arborization (maturation), and finally prevention of cell death that normally could erase a fraction of adult newborn neurons (survival).
Antidepressants and lithium are the strongest pharmacological stimulants to increase AHN, where the former seem more powerful in the proliferative and maturation steps, while the latter is more potent in the survival phase. Valproic acid has less impact on AHN compared to lithium. AAPs, particularly clozapine, have a certain degree of increasing AHN, especially in the proliferative phase, whereas haloperidol has no effect. BDZs seem not to have any effect on AHN.
Different dosing of SSRIs, such as fluoxetine, appears to affect differently the various steps of neurogenesis, including an increase in cell proliferation and the rate of symmetric divisions of neural progenitors. In particular, fluoxetine administration in rats determines an increase in proliferation of type II and type III INPs, with no effect on type I cells [59,60]. In addition, chronic, but not sub-chronic, administration of fluoxetine accelerates the maturation of newly born neurons by enhanced dendritic arborization, thus increasing the numbers of mature neurons [58, 61].
Conversely, the ability of antidepressants to boost the survival of newly born neurons is less evident. One initial study showed that fluoxetine treatment was unable to alter cell survival [62], but later on, it was demonstrated that a four-week treatment was effective in prolonging cell survival [61, 63]. Fluoxetine also seems endowed with a specific anti-apoptotic effect, which appears to be associated with an increase of anti-apoptotic gene Bcl-2 in the rat hippocampus, frontal cortex and striatum [64].
Tricyclic antidepressants, such as imipramine, although less studied, have been shown to positively affect proliferation, maturation and survival [56, 65] (Fig. 1).
In postmortem human studies, in first work, Boldrini et al. [66] found that patients treated with SSRI and tricyclics antidepressants presented an increased number of neural precursor cells (NPCs) and dividing cells. Then, in a subsequent study, they discovered that treated patients have higher numbers of mature GCs and increased granular layer, but no expansion of neural progenitors. Therefore, they proposed that this enhanced neurogenesis could be related to the maturation and survival phases [67].
Besides the different phases of neurogenesis involved in the activity of antidepressants, the increase of brain-derived neurotrophic factor (BDNF) in the hippocampus is considered as one of the key elements in the mechanism of antidepressants action [68]. For example, the deletion of the BDNF receptor TrkB abolished the effect of antidepressants, decreased AHN and increased anxiety-like behavior in mice [47, 69].
Considering that antidepressants increase the serotonin (5-HT) levels, the serotoninergic terminals in the hippocampus should be determinant for this neurotrophic potentiation by acting through various 5-HT receptors. Among these receptors, 5-HT1A and 5-HT4 have received particular attention, as they increase BDNF expression through different mechanisms, e.g., the activation of the cAMP response element-binding protein (CREB) [70]. Blocking of 5-HT1A or 5-HT4 receptors strongly reduces the neurogenic effect of fluoxetine [71]. Similarly, the norepinephrine reuptake inhibitor reboxetine also increases the levels of BDNF in a CREB-dependent fashion [72].
DG neurons express different 5-HT receptors other than 5-HT1A and 5-HT4, such as 5-HT2A, 5-HT2C and 5-HT7. It has been proposed that if 5-HT1A receptors are involved in the acute stimulation of proliferation, then the 5-HT2C receptors may antagonize this effect and cause a delay in fluoxetine effect [73]. For this reason, the antagonism at 5-HT2C receptor shared by atypical antidepressants, such as mirtazapine, trazodone and agomelatine, is considered relevant for their efficacy.
Among the different 5-HT receptors involved in the mechanism of antidepressants, the 5-HT1A receptors surely have a special role that goes beyond the process of neurogenesis. In fact, 5-HT increase induced by SSRI activates 5-HT1A receptors on mature GCs, which are Gi protein-coupled receptors that silence neuronal activity by opening the G protein-coupled inwardly-rectifying potassium channels (GIRKs) and inhibit the DG function similar to newborn hippocampal neurons. This dual-action provided by 5-HT1A receptors may be relevant in the antidepressant and anxiolytic effects of SSRI. In fact, fluoxetine loses its efficacy in 5-HT1A knockout mice, while imipramine is still effective due to its influence on the noradrenergic system [55]. In addition, the 5-HT1A partial agonist buspirone is commonly used for treating generalized anxiety and depression, and its neurotrophic effect has already been demonstrated in animal models [74].
Regarding downstream effectors targeted by SSRIs in relation to neurogenesis, the p21 kinase and the bone morphogenetic protein 4 (BMP4) growth factor have been studied. Reduction of p21 levels in the SGZ of the DG was observed after treatment with fluoxetine, imipramine and desipramine, which was responsible for hippocampal neuronal proliferation [75]. Brooker et al. [76] demonstrated that fluoxetine suppresses BMP signaling that normally inhibits cell proliferation in the DG, leading to enhanced neurogenesis.
If we consider AHN across age, it is well-known that at an older age, neurogenesis seems to decrease, in terms of both the number of progenitor cells and maturation [8, 35, 77], as evidenced mostly in animal models. Still, in elderly people, a reduction of QNPs has been found in the anterior DG. In fact, fluoxetine was able to increase neurogenesis and survival in three-month-old mice, but not in older mice [78, 79]. This evidence might imply that in the elderly population, the antidepressant effect of SSRI, which is still present, might involve other mechanisms besides neurogenesis [80]. Conversely, if we consider the large variability of the number of neural precursors in the general population, especially in the elderly, one can speculate that this can be a relevant factor influencing the response to antidepressants [7]. Nonetheless, the hippocampus of aged mice is still sensitive to synaptic remodeling and an increase of dendritic spines induced by fluoxetine and other SSRIs. Synaptogenesis is active not only in the hippocampus, but also in other areas of the brain, such as the pre-frontal cortex (PFC) and amygdala, and it is relevant in the general mechanism of action of the antidepressants [79, 81].
In summary, AHN is relevant for the mechanism of action of antidepressants, at least in terms of improvement of certain cognitive domains and resilience to stress. These are some but not all the aspects of depression, a complex and heterogeneous disorder characterized by different phenotypes and etiologies, which at the moment are still not well characterized.
4.2. New Antidepressants: Ketamine
The 2-4 weeks of the lag period in the antidepressant response matches the time needed for the whole process of neurogenesis to become effective [82]. This characterizes both first- and second-generation antidepressants and represents a time limitation in getting a therapeutic response when it is needed.
With these premises, the discovery of ketamine, which at low dose is able to obtain an antidepressant response in 24h in humans lasting for several days, has been an extraordinary breakthrough in neuropharmacology.
In animal models, ketamine rapidly accelerates BDNF expression and release through different signaling shortcuts that promote rapid morphological changes. The mechanism of action involves NMDA receptor antagonism, especially on the GABAergic interneurons that control the glutamatergic activity. Subsequently, the ketamine-induced glutamate release stimulates postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which causes depolarization and activation of L-type voltage-dependent Ca2+ channels leading to a rapid BDNF release [83]. In addition, the ketamine NMDA receptor antagonism increases BDNF translation through the deactivation of eukaryotic elongation factor 2 (EEF2) signaling [84], and it reduces the production of reactive oxygen species (ROS) due to increase of calcium.
In the very short term, in mice, a single dose of ketamine rapidly increases, especially in the medial prefrontal cortex (mPFC) pyramidal neurons, the number of dendritic spines, the level of synaptic proteins such as postsynaptic density protein 95 (PSD95) and synapsin-1, and is able to reverse chronic stress induced by synaptic deficits [85]. At the same time, the rapid increase of BDNF in the hippocampus stimulates neurogenesis in the DG, which is important for the sustained antidepressant actions of ketamine [86].
Ketamine seems to accelerate the maturation phase of hippocampal immature neurons, but it has less effect on the numbers of neural progenitors [86, 87] (Fig. 1). However, a recent study found an increase in the densities of neuronal progenitors and newborn GCs in the hippocampal ventral region, together with an increase of expression of specific NMDA and AMPA subunits and mammalian target of rapamycin (mTOR) phosphorylation [87]. In addition, Grossert et al. [88], through an in vitro model based on human induced pluripotent stem cells (iPSCs), determined an increase of proliferation of NPCs induced by ketamine, where transcriptome analysis revealed significant upregulation of insulin-like growth factor 2 (IGF2) and p11, a member of the S100 EF-hand protein family, which are both implicated in the pathophysiology of depression.
Importantly, the antidepressant effects of ketamine are blocked in conditional BDNF knockout mice and are strongly reduced in BDNF Val66Met mice [84]. In addition, infusion of an anti-BDNF neutralizing antibody blocked the antidepressant effects of ketamine [89], while systemic administration of a TrkB inhibitor antagonized the antidepressant effects of both the (R)- and (S)-ketamine enantiomers [90], confirming the relevance of BDNF pathway in the mechanism of ketamine. In humans, the antidepressant actions of ketamine seem attenuated in major depressive disorder (MDD) patients with BDNF Val66Met allele [91], although this effect appears to be race-specific [92].
The rapid activation of the BDNF signaling pathway compared to the slower increase of expression induced by monoaminergic antidepressants might produce different kinetics on TrkB receptor activation and its downstream effectors, thereby generating diverse neural responses [93]. Among these effectors, mTOR surely has a relevant role. In fact, the administration of the mTOR inhibitor rapamycin into the mPFC seems to strongly reduce the antidepressant behavioral actions of ketamine [85]. Moreover, the blood-brain barrier-permeable molecule NV-5138, which activates mTORC1, produces ketamine-like changes and antidepressant behavioral responses [94]. These findings demonstrate the relevance of the BDNF-TrkB-mTORC1 signaling pathway in the mechanism of ketamine.
Interestingly, Ly et al. [95] demonstrated how psychedelic compounds such as lysergic acid diethylamide (LSD), N,N-dimethyltryptamine (DMT), and 2,5-dimethoxy-4-iodoamphetamine (DOI) may increase dendritic arbor complexity, promote dendritic spine growth and stimulate synapse formation, thereby highlighting the potential of psychedelics for treating depression and related disorders.
Together, all these studies indicate that neural hippocampal proliferation and maturation could be involved mostly in the sustained antidepressant action of ketamine, while synaptogenesis and neural plasticity, particularly in the PFC, seem to have a prominent role in the very early phases of ketamine actions. However, a recent study in mice found that some behavior changes induced by ketamine, like sucrose preference, were independent of synaptogenesis in the mPFC, implying that other mechanisms can be involved in the very short term [96].
4.3. Mood Stabilizers and AHN in Animal Models and Humans: Lithium and Valproic Acid
Mood stabilizers are well-known medications mostly used to treat bipolar disorder (BD). Postmortem studies on BD patients have found a significant decrease of volume in areas of the brain directly involved in emotions and cognitions. In addition, impaired neuroplasticity and neurodegeneration have been considered relevant in the etiopathogenesis and prognosis of this disease [97].
Despite many decades, lithium is still considered the first-line treatment for BD. In the last thirty years, numerous studies have examined the neurotrophic and neuroprotective effects of lithium. One of the most replicated findings is the increased grey matter volume in BD patients following lithium therapy in specific regions of the brain, such as PFC, anterior cingulate cortex (ACC), amygdala and the hippocampus [98]. However, not all human studies have consistently reported these findings. In fact, few studies did not find any changes in BD patients treated with lithium [99].
Interestingly, a human brain imaging longitudinal study found that lithium increases gray matter volume which was associated with positive clinical outcomes, while the anti-epileptic valproic acid (VPA) does not induce the same changes [90]. In addition, according to another study, decreased hippocampus and ACC volumes may be markers of non-response for BD patients under lithium treatment [91].
In animal models, Rajkowska et al. [102] demonstrated that the total number of both neurons and glial cells, especially astrocytes, was significantly increased in the DG of lithium-treated mice, but they remained unchanged in the mPFC. Since the volume of the hippocampus and its subfields remained unchanged in lithium-treated animals, this study demonstrated a change of density of both neurons and glia. Zanni et al. [103] showed that lithium accumulates in the hippocampus of young and adult mice and increases the Ki-67 staining of cells, but it has no effect on neural differentiation observed by a total number of DCX positive neural cells. Dong et al. [104] found that lithium enhanced both cell proliferation and differentiation of mesenchymal stem cells to neural cells in the rat spinal cord. In mice, lithium also increased the expression of anti-apoptotic gene Bcl-2 [105] and suppressed p53 and the pro-apoptotic gene Bax [106] (Fig. 1).
Regarding the role of BDNF, similar to antidepressants, chronic administration of lithium increased BDNF expression in the rodent brain [107], particularly in the hippocampus and frontal cortex.
The molecular mechanisms behind the neurogenic effects of lithium are complex and involve multiple targets. Among the many targets of lithium, the inhibition of glycogen synthase kinase 3 (GSK-3) and inositol monophosphatase (IMP) represent the most relevant mechanisms responsible of lithium neurotrophic and neuroprotective effects [108].
The inhibition of GSK-3 isoforms modulates downstream effectors such as β-catenin, CREB, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and other elements downstream the PI3k/Akt signaling pathway [98]. The transcription factor β-catenin is activated in the Wnt pathway that is important for cell proliferation and differentiation. After being phosphorylated by GSK-3, β-catenin undergoes proteasomal degradation [109]. GSK-3 has a multifunctional role relevant not only for cell proliferation but also for maturation and survival. GSK-3 activation has been linked to apoptotic cell death induced by a variety of neural insults, including glutamate excitotoxicity [110], and its inhibition contributes to the antiapoptotic effect of lithium.
These effects go beyond the actions of lithium on triggering neuronal progenitors and provide the basis to comprehend the neuroprotection exerted by lithium following a variety of insults [111-113].
The two isoforms GSK-3α and GSK-3β have distinct roles in transcriptional regulation and cell survival [114, 115], where GSK-3β is the main target of lithium responsible for its neurotrophic effects, although GSK-3α inhibition can be involved on these aspects.
Interestingly, modulation of the expression of histone deacetylase (HDAC) in specific areas of the brain, such as the hippocampus, has implications for neurogenesis, and this mechanism is shared by VPA as well.
In relation to novel mechanisms, it has been shown how miRNAs play an important role in neurogenesis and synaptogenesis [116, 117], and that the expression of several miRNAs are changed in humans and rats by common mood stabilizers, including lithium [118, 119]. For example, Chen et al. [119] showed that lithium increased the expression of four miRNAs (miR-34a, miR-152, miR-155 and miR-221) in lymphoblastoid cell lines derived from BD patients, while another study found that mood stabilizers tend to increase the expression of miR-128 and miR-378, which are involved in neuron maturation and synaptogenesis [120].
Besides neurogenesis and synaptogenesis, lithium has been proven to have additional properties, such as being neuroprotective, anti-inflammatory, anti-oxidative and autophagy inducer, encouraging its potential use beyond BD, especially for neurodegenerative diseases [121, 122].
After lithium, VPA is the most widely used drug to treat BD, and its mechanism involves blocking sodium and calcium voltage-dependent channels and increasing GABA-mediated inhibitory neurotransmission [123]. Besides these actions, VPA has been found to have other targets that contribute to its complex and diverse effects on neural proliferation and maturation.
VPA promotes the neuronal differentiation of neural stem cells in several in vitro culture systems and in vivo, and it drives NPCs into the neuronal lineage over the glial lineage by a process involving HDAC inhibition that modifies chromatin structure and gene expression [124, 125]. In addition, VPA has been shown to increase neuronal survival by inhibiting pro-apoptotic factors through the activation of extracellular signal-regulated kinase (ERK) and protein kinase C (PKC), an action similar to neurotrophic factors [126, 127] (Fig. 1). Moreover, VPA promotes the Wnt/β-catenin signaling by directly or indirectly inhibiting the activity of GSK3β [128]. The β-catenin transcription factor is involved in promoting neurogenesis and neuroprotection in mammalian neural stem cells [129].
In one mice study, VPA was able to increase BDNF expression in the PFC that was accompanied by the extinction of conditioned fear. Recently, VPA has also been demonstrated to promote cell proliferation, generation of newborn mature GCs and an increase in density of immature neurons in a mouse model of AD [130]. However, few studies have found opposite evidence where VPA behaves as an inhibitor of cell proliferation. In one mice study, VPA treatment decreased hippocampal neurogenesis and produced cognitive decline and impairment in spatial memory test [131]. On this matter, Welbalt et al. [132] demonstrated that fluoxetine prevents memory deficits and reduction in hippocampal cell proliferation caused by VPA. In addition, it was found that if prenatal exposure to VPA enhances embryonic neurogenesis, later on, it causes a depletion of NPCs and a decreased level of AHN with cognitive functional impairment [133].
For all these reasons, it is not easy to unequivocally determine the effects of VPA, probably due to its very complex and diverse mechanism of action.
In relation to the other mood stabilizers, we can mention that lamotrigine increases the number of BrdU-labeled cells in the rat hippocampus [134]. Boku et al. [135] showed that lithium and valproate, but not carbamazepine and lamotrigine, recovered adult rat DG NPC proliferation that was decreased by dexamethasone, an agonist of the glucocorticoid receptor. Nevertheless, all four compounds decreased NPC apoptosis. In addition, when compared to retinoic acid effects on differentiation of NPC, lithium and carbamazepine increased the ratio of neurons compared to astrocytes, while lamotrigine had an opposite effect.
4.4. Antipsychotics and AHN in Animal Models and Humans
Antipsychotics (APs) are medications used in mental disorders that are characterized by psychotic features such as schizophrenia and BD. APs are generally divided into typical antipsychotics (TAPs) and atypical antipsychotics (AAPs), based on the idea that AAPs have reduced motor side effects and better efficacy on the cognitive functions [136]. However, this classification is somehow controversial underling the fact that each AP has unique characteristics. TAPs are mainly antagonists at D2 and D3 receptors, while AAPs act beyond dopamine receptors by involving several targets [137-139].
As postulated for BD, schizophrenia also shows brain deficits in different areas, such as PFC and hippocampus, including alterations in hippocampal neurogenesis.
In several studies of rodent models, AAPs such as clozapine and olanzapine increased the number of proliferating cells in the SGZ, while TAP haloperidol did not (Fig. 1). Besides, the effect on the survival of newly generated neurons was less evident [140]. Similarly, Chikama et al. [141] also demonstrated that olanzapine, quetiapine, clozapine, risperidone, aripiprazole increased BrdU-positive cells, but unfortunately, the survival of newly generated cells was not analyzed. Another study found that while clozapine was able to rescue adult neurogenesis, neuronal survival and dendritic branching in the hippocampus and PFC in a stress-induced animal model, and most importantly, improved the depressive symptoms such as behavior despair and anhedonia, haloperidol aggravated them [142]. This and other studies confirmed the superiority of clozapine over the other AAPs. In addition, clozapine and quetiapine have been approved for the treatment of the resistant forms of major depression.
In a phencyclidine (PCP)-induced animal model of schizophrenia, the decrease in the number of BrdU-labeled cells in the DG was prevented by co-administration of clozapine, but again not with haloperidol [143]. However, few studies have questioned the neurogenic effects of AAPs, where the dose (low vs. high) and the duration of the treatment may be determinant for the final response [144].
In relation to neurotrophic factors, postmortem studies of schizophrenic patients have shown that BDNF mRNA is reduced in the hippocampus and PFC areas, where AAPs seem capable to significantly reverse this reduction. Several experiments in animal models of schizophrenia or depression have shown that clozapine and quetiapine, but not haloperidol, were able to reverse the reduction of BDNF expression in both the hippocampal and cortical regions [145, 146]. In mice, while chronic administration of haloperidol and high-dose of risperidone significantly downregulated hippocampal BDNF mRNA, lower doses of risperidone and clozapine did not obtain the same reduction [147]. In humans, following an eight months treatment of drug-naïve first-episode schizophrenic patients, olanzapine, quetiapine, risperidone, aripiprazole and amisulpride significantly increased serum BDNF levels that were associated to the volume of the left hippocampus [148].
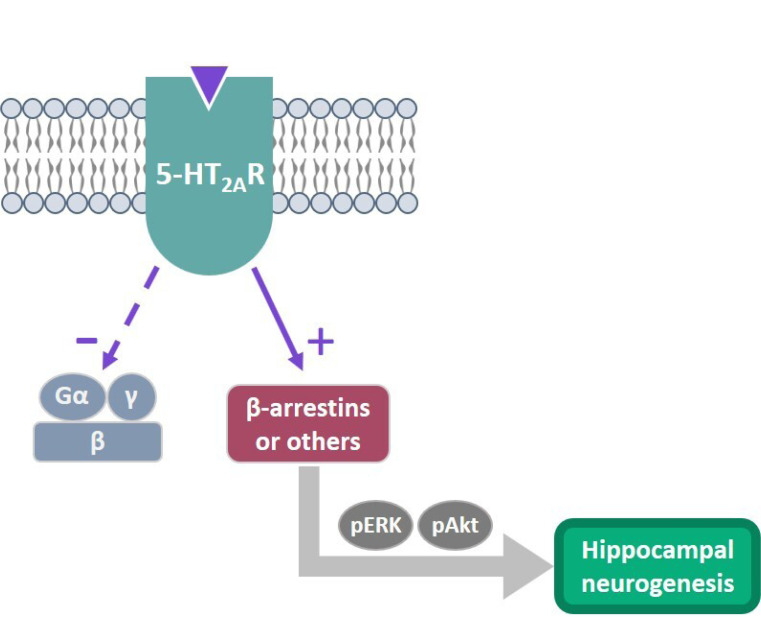
In relation to the mechanism of action of AAPs, the kinases ERK and protein kinase B (Akt) have received particular attention, considering their relevance in brain development, neurogenesis, neuronal differentiation, synaptogenesis, memory and cognition [149]. Clozapine, olanzapine and quetiapine are capable of activating these kinases, where clozapine results superior in comparison with the others both in vitro [150] and in vivo [151]. Intriguingly, ERK and Akt activation seem in part mediated by 5-HT2A receptor through a novel mechanism called ‘biased agonism’, where a ligand is able to activate specific receptor-mediated intracellular pathways dependent or independent from the G proteins that might involve other effectors, such as β-arrestin (Fig. 2). In different cell lines, clozapine behaves as a 5-HT2A receptor biased agonist via a G protein-independent pathway [150]. Similarly, it was found that clozapine-mediated suppression of MK-801 and PCP-induced hyperlocomotion in mice was dependent on 5-HT2A-induced Akt activation, thereby confirming the relevance of 5-HT2A biased agonism in the pharmacological activity of clozapine [151].
Fig. (2).
Biased agonism at the 5-HT2A receptor as a new mechanism to stimulate AHN The ability of a ligand to activate either G protein-dependent or G protein-independent signaling is called “biased agonism” or “functional selectivity”. AAPs, especially clozapine, act as biased agonists via G protein-independent signaling at the 5-HT2A receptor, activating ERK1/2 and Akt kinases through β-arrestin 2 or other effectors. This mechanism could be relevant to explain how AAPs stimulate AHN, with clozapine being superior among AAPs. This concept applies to other receptors, like µ-opioid receptors [210] and β-adrenergic receptors [211].
4.5. Benzodiazepines and AHN in Animal Models and Humans
A deficit of hippocampal neurogenesis leads to an increase in anxiety- and depressive-like behaviors in animal models [47]. Anxiety and depression are often clinically related, where the use of the anxiolytic drugs benzodiazepines (BDZs) and antidepressants, individually or in combination, is frequent. BDZs have the advantage of their immediate efficacy on anxiety symptoms compared to antidepressants. However, when chronically used, they may induce addiction and cognitive issues [152].
BDZs potentiate GABA activity by modulating as positive allosteric compounds receptor-channel GABAA. GABA neurotransmitter is fundamental in the neurodevelopment and in the adult brain, and it is also crucial for adult-born GCs maturation and synaptic integration. GABA exerts its activities through the pentameric receptor complex GABAA, formed by different subunits that can determine different and sometimes opposite biological responses [153].
Importantly, in the critical period of the first weeks of the newborn neurons in the DG, due to the peculiar chloride gradient, GABA induces depolarization and Ca2+ influx leading to CREB-induced gene expression and BDNF increase [154].
In relation to the electrophysiological properties of the newborn neurons, they do not seem to be influenced by zolpidem, while mature GCs were sensitive to this drug, most likely due to a different expression in the α1-subunit component of the GABAA [155].
In a previous study, the treatment of wild-type mice with diazepam or GABAA agonist did not change either the survival of young DG GCs or the number of fourteen-day-old BrdU-labeled cells [156]. Similar evidence was confirmed where diazepam did not have any effect on AHN [157]. Besides, diazepam has been shown to reduce the survival of young olfactory bulb GCs in the adult mouse [158].
Another study showed that mice treated with the BDZ midazolam in early postnatal life exhibited deficits in cognitive performances along with decreased AHN and synaptogenesis [159]. Similarly, diazepam inhibited the accelerated proliferation of hippocampal neural stem cells after focal cerebral ischemia in mice [160]. In addition, concomitant administration of diazepam and fluoxetine inhibited the increased rate of neurogenesis induced by fluoxetine in healthy mice and in animal models of depression [157,161]. This evidence was confirmed in postmortem human brains, where patients taking both BDZs and antidepressants had less GCs in their DG compared to patients taking only antidepressants [67].
Interestingly, one latest study shows that the use of 2,3-benzodiazepine derivatives instead of the classic 1,4-benzodiazepine results in improved cognitive performance, increased proliferation in SGZs and synaptic transmission in the hippocampus [162].
All these data indicate that BDZs do not seem to influence AHN activity, though they may have an inhibitory effect when AHN is increased in specific conditions (Fig. 1). However, considering the complex and diverse actions of GABA on AHN through different GABAA complexes, further studies are needed to reach a definitive conclusion.
4.6. Other Drugs and AHN in Animal Models and Humans
Interestingly, a number of other molecules have also shown some potential for targeting AHN, especially those with a psychotropic effect, which seems to play a role in neurogenesis. For example, the anti-epileptics and nerve pain treating pregabalin and gabapentin promote hippocampal neurogenesis in vivo through the interaction with α2δ subunit and the activation of the NF-κB pathway [163]. Another study demonstrated the effect of the anti-epileptic ethosuximide in inducing the proliferation and differentiation of NPCs in vitro. In a rat model of AD, ethosuximide was shown to activate PI3K/Akt and Wnt/β-catenin pathways in the hippocampus and counteract the inhibition of neurogenesis [164]. Cannabinoids can also promote AHN, according to Jiang et al. [165], where they show that HU210, a potent synthetic cannabinoid agonist, promotes proliferation but not differentiation of cultured embryonic hippocampal NPCs by CB1 receptors mediated activation of Gi proteins and subsequently of ERK signaling. Moreover, chronic administration of HU210 promoted neurogenesis in the hippocampal DG of adult rats [165].
Like ketamine, low doses of the psychedelic drug psilocybin also tend to increase hippocampal neurogenesis [166].
Similarly, some other chemicals with pro-neurogenic activity have been studied:
P7C3, an aminopropyl carbazole which is potentially useful for the treatment of AD and similar neurodegenerative disorders, was found to be the best performer among 1000 molecules screened in vivo [167].
BCI-838 and its metabolite BCI-632, mGluR2 and mGluR3 antagonists with potential AD therapeutic or prophylactic properties stimulated the proliferation of hippocampal progenitors and increased the number of newborn neurons in a mouse model of AD [168].
The anti-diabetic metformin, by activating atypical PKC-CBP pathway, promoted neurogenesis in rodents and humans in vitro and in adult mice in vivo [169].
The neurogenic hormone melatonin and its analog IQM316 induced hippocampal neurogenesis in mice [170].
Curcumin [171].
12-deoxyphorbols [172].
5. NON-PHARMACOLOGICAL TREATMENTS AND AHN IN ANIMAL MODELS AND HUMANS
Besides pharmacological treatments to increase neurogenesis, alternative approaches such as physical activity, diet, environmental enrichment, cognitive stimulation, and restorative sleep have been proven to be neurogenic enhancers [173, 174].
Among these, we decided to focus our attention on physical activity and restorative sleep. Physical activity has very extensive scientific evidence demonstrating its efficacy and it is nowadays part of several multidisciplinary therapeutic programs [175]. Restorative sleep not only is relevant for AHN but is clearly related to neuropsychiatric disorders [176]. In fact, sleep disturbances are often the core symptoms of mood disorders such as depression, and mood and sleep disorders are connected to impaired neurogenesis [177]. The presence of insomnia in depressed patients can be an aggravating factor for the prognosis, where restorative sleep can be determinant for their recovery [178].
5.1. Physical Exercise
Daily alternation of restorative sleep and physical activity is important for general wellness, and both activities have been reported to modulate hippocampal neurogenesis [179].
Scientific evidence, especially in animal models, has validated how physical exercise decreases depressive behaviors, enhances hippocampal neurogenesis and hippocampal-dependent learning, and increases dendritic plasticity [180]. MRI studies, in mice and humans, have also confirmed these effects induced by physical exercise together with an increase of cerebral blood flux, angiogenesis and improved cognitive functions [181]. Recently, Song et al. [182] found how physical exercise, in particular aerobic exercise, benefits global cognition in mild cognitive impairment (MCI) patients.
However, the understanding of the mechanisms involved in the functional and structural benefits of physical exercise has proven to be challenging. Interestingly, It has been hypothesized that exercise is able to restore normal brain neurotransmitter levels of animal models of depression by increasing the de novo synthesis of amino acid neurotransmitters, such as glutamate and GABA, especially in the anterior cingulate cortex (ACC), an observation consistent with the therapeutic role of physical activity in neuropsychiatric disorders [183]. In addition, while physical exercise increases plasma levels of the 5-HT precursor tryptophan in humans, in mice, it increases 5-HT synthesis and tryptophan transporter expression of the blood-brain barrier [184].
The increase of AHN induced by exercise was abolished in Tph2 knockout mice and in 5-HT3 receptor knockout mice, confirming the need for the 5-HT system to be functional [184]. In this context, So et al. [185] confirmed that while chronic moderate running in mice augments cell proliferation, survival, neuronal differentiation and migration, intense running promoted only neuronal differentiation and migration, which was accompanied with lower expressions of vascular endothelial growth factor (VEGF), BDNF, insulin-like growth factor 1 (IGF-1), and erythropoietin (EPO). Furthermore, they also observed that hippocampal neurogenesis induced by moderate, but not intense, exercise improves the animal's ability in spatial pattern separation.
Similarly, Nokia et al. [186] found a positive correlation between mild aerobic exercise and health improvement, including hippocampal neurogenesis, whereas high-intensity training did not show the same improvement. This difference has been confirmed in other studies, as well [187].
Yau et al. [188] proposed that differences between the two forms of exercise in activating certain genes related to lipid metabolism (e.g., adiponectin), protein synthesis and inflammation in the hippocampus, including a higher level of stress induced by intense exercise compared to mild exercise, may explain these results. Notably, the same group had previously observed an increase of hippocampal BDNF transcription [189] and hippocampal neurogenesis [187] in response only to mild exercise. On this topic, Suwabe et al. reported that acute moderate exercise improves DG-mediated pattern separation in humans, which enhances episodic memory [190]. The same group found that leptin acting in the hippocampus mediates this enhancement [173]. Interestingly, blocking mineralocorticoid and glucocorticoid receptors seems to attenuate the increase in hippocampal neurogenesis induced by mild exercise, suggesting that some level of corticosteroids is required to augment hippocampal neurogenesis [191]. In addition, the changes in neurogenesis and synaptogenesis after aerobic exercise are associated with an increase in blood flow in certain areas of the brain, such as the DG [192].
In conclusion, these studies have emphasized the relevance of physical activity for modulating AHN, which seems to correlate with improvements in hippocampus-related specific activities and certain cognitive functions. For these reasons, physical exercise has emerged as a potential therapeutic strategy for treating neuropsychiatric disorders, such as depression.
5.2. Restorative Sleep
Sleep is a biological and homeostatic process essential for individual’s health. Its role is crucial for brain functioning, body metabolism, and for immune, endocrine and cardiovascular systems. Sleep is characterized by the alternation of different stages of rapid eye movement (REM) and non-REM cycles, each one distinguished by specific electroencephalography (EEG) patterns [193]. In particular, some of these rhythms are observed in the hippocampus, like theta rhythm during the REM sleep [194] and sharp-wave ripples during the non-REM phase [195]. These oscillations, in which the hippocampus is involved, seem to promote consolidation of memories acquired during wakefulness [196].
Circadian rhythms are supervised by several clock genes. A master clock situated in the suprachiasmatic nucleus in the hypothalamus is the principal circadian pacemaker that affects NPCs homeostasis and adult neurogenesis by regulating the genes through epigenetic mechanisms [197].
Experimental paradigms of sleep deprivation have been performed in murine models to study the association between sleep and neurogenesis, and many studies have observed the repercussion on hippocampal cell proliferation [198]. Chronic sleep deprivation, sleep restriction, sleep fragmentation or selective REM deprivation studies have been shown to reduce AHN, synaptic plasticity and memory consolidation in the hippocampus [177, 199]. Whereas acute sleep deprivation studies did not report any significant differences in AHN between experimental and control groups [200], though further studies are required to make conclusive remarks.
Hippocampal neurogenesis was found to be significantly reduced when sleep deprivation was protracted for several days [201]. One study reported a reduction of neurogenesis only in the DG but not in the SVZ [200]. Studies that applied sleep fragmentation, which results in shorter REM stages and less consolidated non-REM stages, also show decrements in cell proliferation [202]. A selective REM sleep deprivation study found a reduction in cell proliferation similar to the total sleep deprivation paradigm, suggesting that REM sleep assumes a key role in hippocampal neurogenesis [203]; however, we cannot rule out that non-REM phase may also have a role.
Sleep deprivation alters not just adult hippocampal proliferation, but it also has detrimental effects on maturation and survival of newborn neurons [203]. Most studies have primarily examined the effects of sleep deprivation in the DG, while few studies have also considered SVZ, where they did not report any significant decrease in neurogenesis in this region [200]. Intriguingly, the ventral/posterior part of the DG, which is mostly related to emotional regulation and anxiety, is more affected compared to the dorsal/anterior portion [204].
Stress is another relevant factor known to reduce AHN. Stress and sleep deprivation are strongly associated. Prolonged sleep deprivation can lead to an increased release of stress hormones that reduces AHN [200]. In an interesting study, adrenal glands were surgically removed in mice to evaluate whether sleep effect on neurogenesis is actually a consequence of an increased level of glucocorticoids. After sleep deprivation, mice with and without adrenal glands, both displayed a reduction of neurogenesis. This confirms that the sleep effect on hippocampal neurogenesis is not merely dependent on glucocorticoids [198].
Regarding the mechanism of action, Akers et al. [193] proposed that AHN is influenced by sleep via epigenetic modifications. Acute and chronic deprivation both lead to changes in epigenetic markers in rodents and in humans [205]. In humans, sleep deprivation can lead to modifications in the methylation of genes implicated in Notch and Wnt signaling, both of which play a role in the regulation of neurogenesis [205]. Therefore, these epigenetic markers may represent a target for neurogenesis re-establishment in subjects with insufficient sleep [193].
MRI studies found hippocampal volume reduction in patients with primary insomnia [206], while others did not report the same [207, 208]. Hence, further studies are needed to establish an exact relationship between insomnia and loss of hippocampal volume in humans.
CONCLUSION
In the last decades, there has been a great interest in the field of psychiatric disorders to new concepts related to functional neuroanatomical deficits, such as reduced hippocampal neurogenesis.
From considerable scientific evidence, it has emerged that AHN is relevant in animal models for cognitive flexibility, emotional processing and pharmacological treatments. In humans, the evidence of AHN is based on postmortem studies, and its physiological role is still under investigation. On this topic, the discovery of in vivo markers for neurogenesis will be determinant to confirm the relevance of human AHN in health and disease. In addition, the interplay between AHN, hippocampus and PFC functions requires further investigation in animals and humans. Besides, though AHN is relevant across many different animal species, it does not seem to follow any evolutionistic path or hierarchical scale [209].
Antidepressants and lithium are the strongest pharmacological boosters to increase AHN, where the former seems more powerful in the proliferative and maturation steps, while the latter is more potent in the survival phase. Importantly, VPA has less impact on AHN compared to lithium, being most active in the neural differentiation mechanism. AAPs, mostly clozapine, have a certain degree of increasing AHN, especially in the proliferative phase, whereas TAPs (e.g., haloperidol) have no effect. However, these effects must be considered together with the multiple actions induced by AAPs. BDZs seem not to have any effect, but they may inhibit AHN when it is stimulated pharmacologically or in other particular conditions. From a clinical point of view, future research should try to understand the consequences of these underlying differences among the AHN boosters in terms of the number of new GCs and hippocampal-dependent cognitive and emotional functions.
Non-pharmacological approaches, such as physical activity and restorative sleep, have proved to improve general wellness and AHN, and therefore they should be considered as an augmentation therapy to pharmacological treatments.
In summary, AHN is relevant for the mechanism of action of several drugs in terms of the improvement of certain cognitive domains and resilience to stress. However, these are only some aspects of complex disorders, such as depression and schizophrenia, which are clearly characterized by different etiologies and phenotypes. Moreover, in order to obtain a whole picture of these complex mental disorders, one must include other fundamental neuroplastic processes, such as synaptogenesis and synaptic plasticity, in different areas of the brain, which may or may not be associated to AHN.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 5-HT
Serotonin
- AAP
Atypical Antipsychotic
- ACC
Anterior Cingulate Cortex
- AD
Alzheimer's Disease
- AHN
Adult Hippocampal Neurogenesis
- Akt
Protein kinase B
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
- AP
Antipsychotic
- BCAA
Branched Chain Amino Acid
- BD
Bipolar Disorder
- BDNF
Brain-derived Neurotrophic Factor
- BDZ
Benzodiazepine
- BMP4
Bone Morphogenetic Protein 4
- BrdU
Bromodeoxyuridine
- CREB
cAMP Response Element-binding Protein
- DG
Dentate Gyrus
- DISC-1
Disrupted in schizophrenia-1
- DMT N
N-Dimethyltryptamine
- DOI 2
5-Dimethoxy-4-iodoamphetamine
- EEF2
Eukaryotic Elongation Factor 2
- EEG
Electroencephalography
- EPO
Erythropoietin
- ERK
Extracellular Signal-regulated Kinase
- Fmr1
Fragile X Mental Retardation 1
- GABA
γ-aminobutyric Acid
- GC
Granule Cell
- GIRK
G Protein-coupled Inwardly-rectifying Potassium Channel
- GSK3
Glycogen Synthase Kinase 3
- HDAC
Histone Deacetylase
- HIT
High Intensive Interval Training
- HPA
Hypothalamic-pituitary-adrenal
- IGF-1
Insulin-like Growth Factor 1
- IGF-2
Insulin-like Growth Factor 2
- IMP
Inositol Monophosphatase
- INP
Intermediate Neural Progenitors
- iPSC
Induced Pluripotent Stem Cell
- LSD
Lysergic Acid Diethylamide
- MCI
Mild Cognitive Impairment
- MDD
Major Depressive Disorder
- mPFC
Medial Prefrontal Cortex
- MRI
Magnetic Resonance Imaging
- mTOR
Mammalian Target of Rapamycin
- NF-κB
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
- NMDA
N-Methyl-D-Aspartate
- NPC
Neural Precursor Cell
- PCP
Phencyclidine
- PET
Positron Emission Tomography
- PFC
Prefrontal Cortex
- PKC
Protein Kinase C
- PSA-NCAM
Polysialylated Neural Cell Adhesion Molecule
- PSD95
Postsynaptic Density Protein 95
- QNP
Quiescent Neural Progenitor
- REM
Rapid Eye Movement
- ROS
Reactive Oxygen Species
- SGZ
Subgranular Zone
- SSRI
Selective Serotonin Reuptake Inhibitor
- SVZ
Subventricular Zone
- TAP
Typical Antipsychotic
- VEGF
Vascular Endothelial Growth Factor
- VPA
Valproic Acid
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was funded by Fondazione ARPA, a non-profit organization founded in 1992 (http://www.fondazi- onea rpa.it). The resources of Fondazione ARPA are aimed towards basic and scientific research, mainly for oncology, transplants and new medical and surgical techniques.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
References
- 1.Ming G.L., Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis M.A., Kam M., Nannmark U., Anderson M.F., Axell M.Z., Wikkelso C., Holtås S., van Roon-Mom W.M.C., Björk-Eriksson T., Nordborg C., Frisén J., Dragunow M., Faull R.L.M., Eriksson P.S. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science (80-) 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 3.Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H., Frisén J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 5.Altman J., Das G.D. Post-natal origin of microneurones in the rat brain. Nature. 1965;207(5000):953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 7.Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J., Hen R., Mann J.J. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno-Jiménez E.P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N., Ávila J., Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 9.Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I., Chang E.F., Gutierrez A.J., Kriegstein A.R., Mathern G.W., Oldham M.C., Huang E.J., Garcia-Verdugo J.M., Yang Z., Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipriani S., Ferrer I., Aronica E., Kovacs G.G., Verney C., Nardelli J., Khung S., Delezoide A.L., Milenkovic I., Rasika S., Manivet P., Benifla J.L., Deriot N., Gressens P., Adle-Biassette H. Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer’s disease adults. Cereb. Cortex. 2018;28(7):2458–2478. doi: 10.1093/cercor/bhy096. [DOI] [PubMed] [Google Scholar]
- 11.Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A., Gould E., Hen R., Abrous D.N., Toni N., Schinder A.F., Zhao X., Lucassen P.J., Frisén J. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempermann G., Krebs J., Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry. 2008;21(3):290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann O., Liebl J., Bernard S., Alkass K., Yeung M.S.Y., Steier P., Kutschera W., Johnson L., Landén M., Druid H., Spalding K.L., Frisén J. The age of olfactory bulb neurons in humans. Neuron. 2012;74(4):634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Capilla-Gonzalez V., Herranz-Pérez V., García-Verdugo J.M. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front. Cell. Neurosci. 2015;9:365. doi: 10.3389/fncel.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonçalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167(4):897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Hochgerner H., Zeisel A., Lönnerberg P., Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 2018;21(2):290–299. doi: 10.1038/s41593-017-0056-2. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn H.G., Eisch A.J., Spalding K., Peterson D.A. Detection and phenotypic characterization of adult neurogenesis. Cold Spring Harb. Perspect. Biol. 2016;8(3):a025981. doi: 10.1101/cshperspect.a025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielson N.B.B., Kaifosh P., Zaremba J.D.D., Lovett-Barron M., Tsai J., Denny C.A.A., Balough E.M.M., Goldberg A.R.R., Drew L.J.J., Hen R., Losonczy A., Kheirbek M.A.A. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90(1):101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biane J.S., Kheirbek M.A. Imaging adult hippocampal neurogenesis In Vivo. Neuropsychopharmacology. 2017;42(1):373–374. doi: 10.1038/npp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepousez G., Nissant A., Lledo P.M. Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron. 2015;86(2):387–401. doi: 10.1016/j.neuron.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Mongiat L.A., Espósito M.S., Lombardi G., Schinder A.F. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4(4):e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieni C.V., Nietz A.K., Panichi R., Wadiche J.I., Overstreet-Wadiche L. Distinct determinants of sparse activation during granule cell maturation. J. Neurosci. 2013;33(49):19131–19142. doi: 10.1523/JNEUROSCI.2289-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C., Teng E.M., Summers R.G., Jr, Ming G.L., Gage F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultan S., Li L., Moss J., Petrelli F., Cassé F., Gebara E., Lopatar J., Pfrieger F.W., Bezzi P., Bischofberger J., Toni N. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron. 2015;88(5):957–972. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat. Rev. Neurosci. 2017;18(6):335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guthrie K.M., Tran A., Baratta J., Yu J., Robertson R.T. Patterns of afferent projections to the dentate gyrus studied in organotypic co-cultures. Brain Res. Dev. Brain Res. 2005;157(2):162–171. doi: 10.1016/j.devbrainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Bunk E.C., Stelzer S., Hermann S., Schäfers M., Schlatt S., Schwamborn J.C. Cellular organization of adult neurogenesis in the common marmoset. Aging Cell. 2011;10(1):28–38. doi: 10.1111/j.1474-9726.2010.00639.x. [DOI] [PubMed] [Google Scholar]
- 29.Fasemore T.M., Patzke N., Kaswera-Kyamakya C., Gilissen E., Manger P.R., Ihunwo A.O. The Distribution of Ki-67 and Doublecortin-immunopositive cells in the brains of three strepsirrhine primates: Galago demidoff, Perodicticus potto, and Lemur catta. Neuroscience. 2018;372:46–57. doi: 10.1016/j.neuroscience.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Jabès A., Lavenex P.B., Amaral D.G., Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur. J. Neurosci. 2010;31(2):273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould E., Tanapat P., McEwen B.S., Flügge G., Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornack D.R., Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laplagne D.A., Espósito M.S., Piatti V.C., Morgenstern N.A., Zhao C., van Praag H., Gage F.H., Schinder A.F. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4(12):e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knoth R., Singec I., Ditter M., Pantazis G., Capetian P., Meyer R.P., Horvat V., Volk B., Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A., Possnert G., Mash D.C., Druid H., Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ninkovic J., Mori T., Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J. Neurosci. 2007;27(40):10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., Mori K., Ikeda T., Itohara S., Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11(10):1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 38.Jessberger S., Parent J.M. Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 2015;7(12):1–10. doi: 10.1101/cshperspect.a020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaiss C.A., Yu T.S., Zhang G., Chen J., Dimchev G., Parada L.F., Powell C.M., Kernie S.G. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 2011;31(13):4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai C.Y., Tsai C.Y., Arnold S.J., Huang G.J. Ablation of hippocampal neurogenesis in mice impairs the response to stress during the dark cycle. Nat. Commun. 2015;6:8373. doi: 10.1038/ncomms9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandola A., Hendrikse J., Lucassen P.J., Yücel M. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: practical implications for mental health treatment. Front. Hum. Neurosci. 2016;10:373. doi: 10.3389/fnhum.2016.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikrar T., Guo N., He K., Besnard A., Levinson S., Hill A., Lee H.K., Hen R., Xu X., Sahay A. Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circuits. 2013;7:204. doi: 10.3389/fncir.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kheirbek M.A., Drew L.J., Burghardt N.S., Costantini D.O., Tannenholz L., Ahmari S.E., Zeng H., Fenton A.A., Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anacker C., Zunszain P.A., Cattaneo A., Carvalho L.A., Garabedian M.J., Thuret S., Price J., Pariante C.M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry. 2011;16(7):738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng W., Saxe M.D., Gallina I.S., Gage F.H. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009;29(43):13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahay A., Scobie K.N., Hill A.S., O’Carroll C.M., Kheirbek M.A., Burghardt N.S., Fenton A.A., Dranovsky A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Revest J.M., Dupret D., Koehl M., Funk-Reiter C., Grosjean N., Piazza P.V., Abrous D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry. 2009;14(10):959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 48.Aimone J.B., Li Y., Lee S.W., Clemenson G.D., Deng W., Gage F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scharfman H. E., Hen R. Is more neurogenesis always better? Science(80-) 2007;315(5810):336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sierra A., Encinas J.M., Maletic-Savatic M. Adult human neurogenesis: From microscopy to magnetic resonance imaging. Front. Neurosci. 2011;5:47. doi: 10.3389/fnins.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Consortium B. Train the brain consortium. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The train the brain study. Sci. Rep. 2017;7:39471. doi: 10.1038/srep39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietrich J., Prust M., Kaiser J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience. 2015;309:224–232. doi: 10.1016/j.neuroscience.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Boldrini M., Underwood M.D., Hen R., Rosoklija G.B., Dwork A.J., John Mann J., Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lino de Oliveira C., Bolzan J.A., Surget A., Belzung C. Do antidepressants promote neurogenesis in adult hippocampus? A systematic review and meta-analysis on naive rodents. Pharmacol. Ther. 2020;210:107515. doi: 10.1016/j.pharmthera.2020.107515. [DOI] [PubMed] [Google Scholar]
- 55.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (80-) 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 56.Surget A., Saxe M., Leman S., Ibarguen-Vargas Y., Chalon S., Griebel G., Hen R., Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry. 2008;64(4):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 57.David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I., Drew M., Craig D.A., Guiard B.P., Guilloux J.P., Artymyshyn R.P., Gardier A.M., Gerald C., Antonijevic I.A., Leonardo E.D., Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.David D.J., Wang J., Samuels B.A., Rainer Q., David I., Gardier A.M., Hen R. Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist. 2010;16(5):578–591. doi: 10.1177/1073858409360281. [DOI] [PubMed] [Google Scholar]
- 59.Encinas J.M., Vaahtokari A., Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA. 2006;103(21):8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Micheli L., Ceccarelli M., Gioia R., D’Andrea G., Farioli-Vecchioli S., Costanzi M., Saraulli D., Cestari V., Tirone F. Terminal differentiation of adult hippocampal progenitor cells is a step functionally dissociable from proliferation and is controlled by Tis21, Id3 and NeuroD2. Front. Cell. Neurosci. 2017;11:186. doi: 10.3389/fncel.2017.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J.W., David D.J., Monckton J.E., Battaglia F., Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 2008;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malberg J.E., Eisch A.J., Nestler E.J., Duman R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakagawa S., Kim J., Lee R., Malberg J.E., Chen J., Steffen C., Zhang Y., Nestler E.J., Duman R.S. Regulation of neurogenesis in adult mouse hippocampus by CAMP. J. Neurosci. 2002;22(9):1–10. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Réus G.Z., Abelaira H.M., Agostinho F.R., Ribeiro K.F., Vitto M.F., Luciano T.F., Souza C.T., Quevedo J. The administration of olanzapine and fluoxetine has synergistic effects on intracellular survival pathways in the rat brain. J. Psychiatr. Res. 2012;46(8):1029–1035. doi: 10.1016/j.jpsychires.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Keilhoff G., Becker A., Grecksch G., Bernstein H.G., Wolf G. Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology. 2006;31(6):1165–1176. doi: 10.1038/sj.npp.1300924. [DOI] [PubMed] [Google Scholar]
- 66.Boldrini M., Santiago A.N., Hen R., Dwork A.J., Rosoklija G.B., Tamir H., Arango V., John M.J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boldrini M., Butt T.H., Santiago A.N., Tamir H., Dwork A.J., Rosoklija G.B., Arango V., Hen R., Mann J.J. Benzodiazepines and the potential trophic effect of antidepressants on dentate gyrus cells in mood disorders. Int. J. Neuropsychopharmacol. 2014;17(12):1923–1933. doi: 10.1017/S1461145714000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Björkholm C., Monteggia L.M. BDNF -a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergami M., Berninger B., Canossa M. Conditional deletion of TrkB alters adult hippocampal neurogenesis and anxiety-related behavior. Commun. Integr. Biol. 2009;2(1):14–16. doi: 10.4161/cib.2.1.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pascual-Brazo J., Castro E., Díaz A., Valdizán E.M., Pilar-Cuéllar F., Vidal R., Treceño B., Pazos A. Modulation of neuroplasticity pathways and antidepressant-like behavioural responses following the short-term (3 and 7 days) administration of the 5-HT₄ receptor agonist RS67333. Int. J. Neuropsychopharmacol. 2012;15(5):631–643. doi: 10.1017/S1461145711000782. [DOI] [PubMed] [Google Scholar]
- 71.Mendez-David I., David D.J., Darcet F., Wu M.V., Kerdine-Römer S., Gardier A.M., Hen R. Rapid anxiolytic effects of a 5-HT₄ receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology. 2014;39(6):1366–1378. doi: 10.1038/npp.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen M.J., Russo-Neustadt A.A. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19(10):962–972. doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klempin F., Babu H., De Pietri Tonelli D., Alarcon E., Fabel K., Kempermann G. Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front. Mol. Neurosci. 2010;3:1–11. doi: 10.3389/fnmol.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grabiec M., Turlejski K., Djavadian R.L. The partial 5-HT1A receptor agonist buspirone enhances neurogenesis in the opossum (Monodelphis domestica). Eur. Neuropsychopharmacol. 2009;19(6):431–439. doi: 10.1016/j.euroneuro.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Pechnick R.N., Zonis S., Wawrowsky K., Cosgayon R., Farrokhi C., Lacayo L., Chesnokova V. Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS One. 2011;6(11):e27290. doi: 10.1371/journal.pone.0027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brooker S.M., Gobeske K.T., Chen J., Peng C.Y., Kessler J.A. Hippocampal bone morphogenetic protein signaling mediates behavioral effects of antidepressant treatment. Mol. Psychiatry. 2017;22(6):910–919. doi: 10.1038/mp.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao M.S., Hattiangady B., Shetty A.K. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 78.Couillard-Despres S., Wuertinger C., Kandasamy M., Caioni M., Stadler K., Aigner R., Bogdahn U., Aigner L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol. Psychiatry. 2009;14(9):856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- 79.Mcavoy K., Russo C., Kim S., Rankin G., Sahay A. Fluoxetine Induces input-specific hippocampal dendritic spine remodeling along the septo-temporal axis in adulthood and middle age. Hippocampus. 2015;25(11):1429–1446. doi: 10.1002/hipo.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zoric B., Jankovic L., Kuzmanovic P.J., Zidverc-Trajkovic J., Mijajlovic M., Stanimirovic D. The efficacy of fluoxetine in BMS-A cross-over study. Gerodontology. 2018;35(2):123–128. doi: 10.1111/ger.12332. [DOI] [PubMed] [Google Scholar]
- 81.Micheli L., Ceccarelli M., D’Andrea G., Tirone F. Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise. Brain Res. Bull. 2018;143:181–193. doi: 10.1016/j.brainresbull.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Schoenfeld T.J., Cameron H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40(1):113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duman R.S., Aghajanian G.K., Sanacora G., Krystal J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22(3):238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Autry A.E., Adachi M., Nosyreva E., Na E.S., Los M.F., Cheng P.F., Kavalali E.T., Monteggia L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li N., Liu R.J., Dwyer J.M., Banasr M., Lee B., Son H., Li X.Y., Aghajanian G., Duman R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma Z., Zang T., Birnbaum S.G., Wang Z., Johnson J.E., Zhang C.L., Parada L.F., Trk B. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat. Commun. 2017;8(1):1668. doi: 10.1038/s41467-017-01709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada J., Jinno S. Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology. 2019;158:107710. doi: 10.1016/j.neuropharm.2019.107710. [DOI] [PubMed] [Google Scholar]
- 88.Grossert A., Mehrjardi N.Z., Bailey S.J., Lindsay M.A., Hescheler J., Šarić T., Teusch N. Ketamine increases proliferation of human ipsc-derived neuronal progenitor cells via insulin-like growth factor 2 and independent of the NMDA receptor. Cells. 2019;8(10):E1139. doi: 10.3390/cells8101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lepack A.E., Fuchikami M., Dwyer J.M., Banasr M., Duman R.S. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 2014;18(1):1–6. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang J.J., Wang N., Yang C., Shi J.Y., Yu H.Y., Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol. Psychiatry. 2015;77(3):e19–e20. doi: 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 91.Laje G., Lally N., Mathews D., Brutsche N., Chemerinski A., Akula N., Kelmendi B., Simen A., McMahon F.J., Sanacora G., Zarate C., Jr Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry. 2012;72(11):e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su T.P., Chen M.H., Li C.T., Lin W.C., Hong C.J., Gueorguieva R., Tu P.C., Bai Y.M., Cheng C.M., Krystal J.H. Dose-related effects of adjunctive ketamine in taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482–2492. doi: 10.1038/npp.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lepack A.E., Bang E., Lee B., Dwyer J.M., Duman R.S. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kato T., Hahm S., Duman R. S., Kato T., Pothula S., Liu R., Duman C. H., Terwilliger R., Vlasuk G. P., Saiah E., Hahm S., Duman R. S. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mtorc1 activation graphical abstract find the latest version: sestrin modulator nv-5138 produces rapid antidepressant effects via direct mtorc1 activation. J. Clin. Invest. 2019;129(6):2542–2554. doi: 10.1172/JCI126859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ly C., Greb A.C., Cameron L.P., Wong J.M., Barragan E.V., Wilson P.C., Burbach K.F., Soltanzadeh Zarandi S., Sood A., Paddy M.R., Duim W.C., Dennis M.Y., McAllister A.K., Ori-McKenney K.M., Gray J.A., Olson D.E. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23(11):3170–3182. doi: 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hare B.D., Shinohara R., Liu R.J., Pothula S., DiLeone R.J., Duman R.S. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat. Commun. 2019;10(1):223. doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dell’Osso L., Del Grande C., Gesi C., Carmassi C., Musetti L. A new look at an old drug: neuroprotective effects and therapeutic potentials of lithium salts. Neuropsychiatr. Dis. Treat. 2016;12:1687–1703. doi: 10.2147/NDT.S106479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lyoo I.K., Dager S.R., Kim J.E., Yoon S.J., Friedman S.D., Dunner D.L., Renshaw P.F. Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacology. 2010;35(8):1743–1750. doi: 10.1038/npp.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McDonald C. Brain structural effects of psychopharmacological treatment in bipolar disorder. Curr. Neuropharmacol. 2015;13(4):445–457. doi: 10.2174/1570159X13666150403231654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun Y.R., Herrmann N., Scott C.J.M., Black S.E., Khan M.M., Lanctôt K.L. Global grey matter volume in adult bipolar patients with and without lithium treatment: A meta-analysis. J. Affect. Disord. 2018;225(225):599–606. doi: 10.1016/j.jad.2017.08.078. [DOI] [PubMed] [Google Scholar]
- 101.Selek S., Nicoletti M., Zunta-Soares G.B., Hatch J.P., Nery F.G., Matsuo K., Sanches M., Soares J.C. A longitudinal study of fronto-limbic brain structures in patients with bipolar I disorder during lithium treatment. J. Affect. Disord. 2013;150(2):629–633. doi: 10.1016/j.jad.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 102.Rajkowska G., Clarke G., Mahajan G., Licht C.M.M., van de Werd H.J.J.M., Yuan P., Stockmeier C.A., Manji H.K., Uylings H.B.M. Differential effect of lithium on cell number in the hippocampus and prefrontal cortex in adult mice: a stereological study. Bipolar Disord. 2016;18(1):41–51. doi: 10.1111/bdi.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zanni G., Michno W., Di Martino E., Tjärnlund-Wolf A., Pettersson J., Mason C.E., Hellspong G., Blomgren K., Hanrieder J. Lithium accumulates in neurogenic brain regions as revealed by high resolution ion imaging. Sci. Rep. 2016;2017(7):1–12. doi: 10.1038/srep40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong B.T., Tu G.J., Han Y.X., Chen Y. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int. J. Clin. Exp. Pathol. 2015;8(3):2473–2483. [PMC free article] [PubMed] [Google Scholar]
- 105.Chen G., Rajkowska G., Du F., Seraji-Bozorgzad N., Manji H.K. Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 2000;75(4):1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 106.Chen R.W., Chuang D.M. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 1999;274(10):6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 107.Machado-Vieira R., Manji H.K., Zarate C.A.Jr. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl. 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berridge M.J., Downes C.P., Hanley M.R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 109.Jope R. S., Johnson G. V. W. The glamour and gloom of glycogen synthase Kinase-3. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Grimes C. A., Jope R. S. The multifaceted roles of glycogen synthase kinase 3 b in cellular signaling. Prog. Neurobiol. 2001;65:391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 111.Ciftci E., Karacay R., Caglayan A., Altunay S., Ates N., Altintas M.O., Doeppner T.R., Yulug B., Kilic E. Neuroprotective effect of lithium in cold- induced traumatic brain injury in mice. Behav. Brain Res. 2020;392:112719. doi: 10.1016/j.bbr.2020.112719. [DOI] [PubMed] [Google Scholar]
- 112.Fabrizi C., Pompili E., Somma F., Vito S., De, Ciraci V., Artico M., Lenzi P., Fornai F., Fumagalli L. Lithium limits trimethyltin-induced cytotoxicity and proinflammatory response in microglia without affecting the concurrent autophagy impairment. J. Appl. Toxicol. 2017;37(2):207–213. doi: 10.1002/jat.3344. [DOI] [PubMed] [Google Scholar]
- 113.Busceti C. L., Biagioni F., Aronica E., Riozzi B., Storto M., Battaglia G., Giorgi F. S., Gradini R., Fornai F., Caricasole A., Nicoletti F., Bruno V. Induction of the Wnt Inhibitor , Dickkopf-1 , is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48(4):694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 114.Liang M., Chuang D. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation. J. Biol. Chem. 2006;281(41):30479–30484. doi: 10.1074/jbc.M607468200. [DOI] [PubMed] [Google Scholar]
- 115.Liang M., Chuang D. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J. Biol.Chem. 2007;282(6):3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- 116.Kim A.H., Reimers M., Maher B., Williamson V., McMichael O., McClay J.L., van den Oord E.J.C.G., Riley B.P., Kendler K.S., Vladimirov V.I. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010;124(1-3):183–191. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maffioletti E., Tardito D., Gennarelli M., Bocchio-Chiavetto L. Micro spies from the brain to the periphery: New clues from studies on microRNAs in neuropsychiatric disorders. Front. Cell. Neurosci. 2014;8(MAR):75. doi: 10.3389/fncel.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou R., Yuan P., Wang Y., Hunsberger J.G., Elkahloun A., Wei Y., Damschroder-Williams P., Du J., Chen G., Manji H.K. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34(6):1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen H., Wang N., Burmeister M., McInnis M.G. MicroRNA expression changes in lymphoblastoid cell lines in response to lithium treatment. Int. J. Neuropsychopharmacol. 2009;12(7):975–981. doi: 10.1017/S1461145709000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kidnapillai S., Wade B., Bortolasci C.C., Panizzutti B., Spolding B., Connor T., Crowley T., Jamain S., Gray L., Leboyer M., Berk M., Walder K. Drugs used to treat bipolar disorder act via microRNAs to regulate expression of genes involved in neurite outgrowth. J. Psychopharmacol. (Oxford) 2020;34(3):370–379. doi: 10.1177/0269881119895534. [DOI] [PubMed] [Google Scholar]
- 121.Forlenza O.V., De-Paula V.J.R., Diniz B.S.O. Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem. Neurosci. 2014;5(6):443–450. doi: 10.1021/cn5000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pasquali L., Busceti C.L., Fulceri F., Paparelli A., Fornai F. Intracellular pathways underlying the effects of lithium. Behav. Pharmacol. 2010;21(5-6):473–492. doi: 10.1097/FBP.0b013e32833da5da. [DOI] [PubMed] [Google Scholar]
- 123.Kwan P., Sills G.J., Brodie M.J. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol. Ther. 2001;90(1):21–34. doi: 10.1016/S0163-7258(01)00122-X. [DOI] [PubMed] [Google Scholar]
- 124.Hsieh J., Nakashima K., Kuwabara T., Mejia E., Gage F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Juliandi B., Abematsu M., Sanosaka T., Tsujimura K., Smith A., Nakashima K. Induction of superficial cortical layer neurons from mouse embryonic stem cells by valproic acid. Neurosci. Res. 2012;72(1):23–31. doi: 10.1016/j.neures.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 126.Hao Y., Creson T., Zhang L., Li P., Du F., Yuan P., Gould T.D., Manji H.K., Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 2004;24(29):6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell. Mol. Life Sci. 2007;64(16):2090–2103. doi: 10.1007/s00018-007-7079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gould T.D., Chen G., Manji H.K. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29(1):32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- 129.Beckervordersandforth R., Zhang C.L., Lie D.C. Transcription-factor-dependent control of adult hippocampal neurogenesis. Cold Spring Harb. Perspect. Biol. 2015;7(10):a018879. doi: 10.1101/cshperspect.a018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng Q., Long Z., Feng M., Zhao Y., Luo S., Wang K., Wang Y., Yang G., He G. Valproic acid stimulates hippocampal neurogenesis via Activating the Wnt/β-catenin signaling pathway in the APP/PS1/Nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2019;11:62. doi: 10.3389/fnagi.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Orczyk J.J., Banks M.K., Garraghty P.E. The effects of valproic acid on appetitive and aversive instrumental learning in adult rats. Front. Behav. Neurosci. 2014;8(APR):113. doi: 10.3389/fnbeh.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Welbat J.U., Sangrich P., Sirichoat A., Chaisawang P., Chaijaroonkhanarak W., Prachaney P., Pannangrong W., Wigmore P. Fluoxetine prevents the memory deficits and reduction in hippocampal cell proliferation caused by valproic acid. J. Chem. Neuroanat. 2016;78:112–118. doi: 10.1016/j.jchemneu.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 133.Juliandi B., Tanemura K., Igarashi K., Tominaga T., Furukawa Y., Otsuka M., Moriyama N., Ikegami D., Abematsu M., Sanosaka T., Tsujimura K., Narita M., Kanno J., Nakashima K. Reduced adult hippocampal neurogenesis and cognitive impairments following prenatal treatment of the antiepileptic drug valproic acid. Stem Cell Reports. 2015;5(6):996–1009. doi: 10.1016/j.stemcr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kondziella D., Strandberg J., Lindquist C., Asztely F. Lamotrigine increases the number of BrdU-labeled cells in the rat hippocampus. Neuroreport. 2011;22(2):97–100. doi: 10.1097/WNR.0b013e328342d2fa. [DOI] [PubMed] [Google Scholar]
- 135.Boku S., Nakagawa S., Masuda T., Nishikawa H., Kato A., Toda H., Song N., Kitaichi Y., Inoue T., Koyama T. Effects of mood stabilizers on adult dentate gyrus-derived neural precursor cells. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(1):111–117. doi: 10.1016/j.pnpbp.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 136.Aringhieri S., Carli M., Kolachalam S., Verdesca V., Cini E., Rossi M., McCormick P.J., Corsini G.U., Maggio R., Scarselli M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018;192:20–41. doi: 10.1016/j.pharmthera.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 137.Maggio R., Scarselli M., Capannolo M., Millan M.J. Novel dimensions of D3 receptor function: Focus on heterodimerisation, transactivation and allosteric modulation. Eur. Neuropsychopharmacol. 2015;25(9):1470–1479. doi: 10.1016/j.euroneuro.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 138.Rossi M., Fasciani I., Marampon F., Maggio R., Scarselli M. The first negative allosteric modulator for dopamine D2 and D3 receptors, SB269652 may lead to a new generation of antipsychotic drugs. Mol. Pharmacol. 2017;91(6):586–594. doi: 10.1124/mol.116.107607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scarselli M., Armogida M., Chiacchio S., DeMontis M.G., Colzi A., Corsini G.U., Maggio R. Reconstitution of functional dopamine D(2s) receptor by co-expression of amino- and carboxyl-terminal receptor fragments. Eur. J. Pharmacol. 2000;397(2-3):291–296. doi: 10.1016/S0014-2999(00)00272-7. [DOI] [PubMed] [Google Scholar]
- 140.Wang H.D., Dunnavant F.D., Jarman T., Deutch A.Y. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29(7):1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- 141.Chikama K., Yamada H., Tsukamoto T., Kajitani K., Nakabeppu Y., Uchimura N. Chronic atypical antipsychotics, but not haloperidol, increase neurogenesis in the hippocampus of adult mouse. Brain Res. 2017;1676:77–82. doi: 10.1016/j.brainres.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 142.Morais M., Patrício P., Mateus-Pinheiro A., Alves N.D., Machado-Santos A.R., Correia J.S., Pereira J., Pinto L., Sousa N., Bessa J.M. The modulation of adult neuroplasticity is involved in the mood-improving actions of atypical antipsychotics in an animal model of depression. Transl. Psychiatry. 2017;7(6):e1146. doi: 10.1038/tp.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maeda K., Sugino H., Hirose T., Kitagawa H., Nagai T., Mizoguchi H., Takuma K., Yamada K. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J. Pharmacol. Sci. 2007;103(3):299–308. doi: 10.1254/jphs.FP0061424. [DOI] [PubMed] [Google Scholar]
- 144.Meyer U., Knuesel I., Nyffeler M., Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology (Berl.) 2010;208(4):531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- 145.Park S.W., Lee S.K., Kim J.M., Yoon J.S., Kim Y.H. Effects of quetiapine on the brain-derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neurosci. Lett. 2006;402(1-2):25–29. doi: 10.1016/j.neulet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 146.Fumagalli F., Molteni R., Bedogni F., Gennarelli M., Perez J., Racagni G., Riva M.A. Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK-801. Neuroreport. 2004;15(13):2109–2112. doi: 10.1097/00001756-200409150-00022. [DOI] [PubMed] [Google Scholar]
- 147.Chlan-Fourney J., Ashe P., Nylen K., Juorio A.V., Li X.M. Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 2002;954(1):11–20. doi: 10.1016/S0006-8993(02)03215-8. [DOI] [PubMed] [Google Scholar]
- 148.Rizos E., Papathanasiou M.A., Michalopoulou P.G., Laskos E., Mazioti A., Kastania A., Vasilopoulou K., Nikolaidou P., Margaritis D., Papageorgiou C., Liappas I. A longitudinal study of alterations of hippocampal volumes and serum BDNF levels in association to atypical antipsychotics in a sample of first-episode patients with schizophrenia. PLoS One. 2014;9(2):e87997. doi: 10.1371/journal.pone.0087997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Samuels I.S., Saitta S.C., Landreth G.E. Maping CNS development and cognition: an ERKsome process. Neuron. 2009;61(2):160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aringhieri S., Kolachalam S., Gerace C., Carli M., Verdesca V., Brunacci M.G., Rossi C., Ippolito C., Solini A., Corsini G.U., Scarselli M. Clozapine as the most efficacious antipsychotic for activating ERK 1/2 kinases: Role of 5-HT2A receptor agonism. Eur. Neuropsychopharmacol. 2017;27(4):383–398. doi: 10.1016/j.euroneuro.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 151.Schmid C.L., Streicher J.M., Meltzer H.Y., Bohn L.M. Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801-induced behaviors through a βarrestin2-independent activation of Akt. Neuropsychopharmacology. 2014;39(8):1902–1913. doi: 10.1038/npp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schmitz A. Benzodiazepine use, misuse, and abuse: A review. Ment Health Clin. 2016;6(3):120–126. doi: 10.9740/mhc.2016.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Duveau V., Laustela S., Barth L., Gianolini F., Vogt K.E., Keist R., Chandra D., Homanics G.E., Rudolph U., Fritschy J.M. Spatiotemporal specificity of GABAA receptor-mediated regulation of adult hippocampal neurogenesis. Eur. J. Neurosci. 2011;34(3):362–373. doi: 10.1111/j.1460-9568.2011.07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005;25(5):1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Overstreet Wadiche L., Bromberg D.A., Bensen A.L., Westbrook G.L. GABAergic signaling to newborn neurons in dentate gyrus. J. Neurophysiol. 2005;94(6):4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 156.Karten Y.J.G., Jones M.A., Jeurling S.I., Cameron H.A. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16(3):312–320. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- 157.Sun Y., Evans J., Russell B., Kydd R., Connor B. A benzodiazepine impairs the neurogenic and behavioural effects of fluoxetine in a rodent model of chronic stress. Neuropharmacology. 2013;72:20–28. doi: 10.1016/j.neuropharm.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 158.Yamaguchi M., Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. USA. 2005;102(27):9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu J., Mathena R.P., Singh S., Kim J., Long J.J., Li Q., Junn S., Blaize E., Mintz C.D. Early developmental exposure to repetitive long duration of midazolam sedation causes behavioral and synaptic alterations in a rodent model of neurodevelopment. J. Neurosurg. Anesthesiol. 2019;31(1):151–162. doi: 10.1097/ANA.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nochi R., Kaneko J., Okada N., Terazono Y., Matani A., Hisatsune T. Diazepam treatment blocks the elevation of hippocampal activity and the accelerated proliferation of hippocampal neural stem cells after focal cerebral ischemia in mice. J. Neurosci. Res. 2013;91(11):1429–1439. doi: 10.1002/jnr.23264. [DOI] [PubMed] [Google Scholar]
- 161.Wu X., Castrén E. Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol. Psychiatry. 2009;66(1):5–8. doi: 10.1016/j.biopsych.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 162.Üçel U.İ., Can Ö.D., Demir Ö.Ü., Ulupinar E. Antiamnesic effects of tofisopam against scopolamine-induced cognitive impairments in rats. Pharmacol. Biochem. Behav. 2020;190(January):172858. doi: 10.1016/j.pbb.2020.172858. [DOI] [PubMed] [Google Scholar]
- 163.Valente M.M., Bortolotto V., Cuccurazzu B., Ubezio F., Meneghini V., Francese M.T., Canonico P.L., Grilli M. α2δ ligands act as positive modulators of adult hippocampal neurogenesis and prevent depression-like behavior induced by chronic restraint stress. Mol. Pharmacol. 2012;82(2):271–280. doi: 10.1124/mol.112.077636. [DOI] [PubMed] [Google Scholar]
- 164.Tiwari S.K., Seth B., Agarwal S., Yadav A., Karmakar M., Gupta S.K., Choubey V., Sharma A., Chaturvedi R.K. Ethosuximide induces hippocampal neurogenesis and reverses cognitive deficits in an amyloid-β Toxin-induced Alzheimer Rat model via the phosphatidylinositol 3-Kinase (PI3K)/Akt/Wnt/β-catenin pathway. J. Biol. Chem. 2015;290(47):28540–28558. doi: 10.1074/jbc.M115.652586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang W., Zhang Y., Xiao L., Cleemput J.Van., Shao-Ping J., Guang B, Xia Z. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 2005;115(11):3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Catlow B.J., Song S., Paredes D.A., Kirstein C.L., Sanchez-Ramos J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 2013;228(4):481–491. doi: 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- 167.Pieper A.A., Xie S., Capota E., Estill S.J., Zhong J., Long J.M., Becker G.L., Huntington P., Goldman S.E., Shen C.H., Capota M., Britt J.K., Kotti T., Ure K., Brat D.J., Williams N.S., MacMillan K.S., Naidoo J., Melito L., Hsieh J., De Brabander J., Ready J.M., McKnight S.L. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142(1):39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim S.H., Steele J.W., Lee S.W., Clemenson G.D., Carter T.A., Treuner K., Gadient R., Wedel P., Glabe C., Barlow C., Ehrlich M.E., Gage F.H., Gandy S. Proneurogenic Group II mGluR antagonist improves learning and reduces anxiety in Alzheimer Aβ oligomer mouse. Mol. Psychiatry. 2014;19(11):1235–1242. doi: 10.1038/mp.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang J., Gallagher D., DeVito L.M., Cancino G.I., Tsui D., He L., Keller G.M., Frankland P.W., Kaplan D.R., Miller F.D. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 170.Figueiro-Silva J., Antequera D., Pascual C., de la Fuente Revenga M., Volt H., Acuña-Castroviejo D., Rodríguez-Franco M.I., Carro E. The Melatonin analog IQM316 may induce adult hippocampal neurogenesis and preserve recognition memories in mice. Cell Transplant. 2018;27(3):423–437. doi: 10.1177/0963689717721217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tiwari S.K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L.K.S., Patel D.K., Srivastava V., Singh D., Gupta S.K., Tripathi A., Chaturvedi R.K., Gupta K.C. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 172.Geribaldi-Doldán N., Flores-Giubi E., Murillo-Carretero M., García-Bernal F., Carrasco M., Macías-Sánchez A.J., Domínguez-Riscart J., Verástegui C., Hernández-Galán R., Castro C. 12-Deoxyphorbols promote adult neurogenesis by inducing neural progenitor cell proliferation via PKC activation. Int. J. Neuropsychopharmacol. 2015;19(1):1–14. doi: 10.1093/ijnp/pyv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Soo J., Rakwal R., Shibato J., Takahashi K., Koizumi H., Shima T. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA. 2019;116(22):1–6. doi: 10.1073/pnas.1815197116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kirk-Sanchez N.J., McGough E.L. Physical exercise and cognitive performance in the elderly: current perspectives. Clin. Interv. Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cordes T., Bischoff L.L., Schoene D., Schott N., Voelcker-rehage C., Meixner C., Appelles L., Bebenek M., Berwinkel A., Hildebrand C., Jöllenbeck T., Johnen B., Kemmler W., Klotzbier T., Korbus H., Rudisch J., Vogt L., Weigelt M., Wittelsberger R., Zwingmann K., Wollesen B. A multicomponent exercise intervention to improve physical functioning, cognition and psychosocial well-being in elderly nursing home residents : A study protocol of a randomized controlled trial in the PROCARE; long-term care) project. BMC Geriatr. 2019;19(1):369. doi: 10.1186/s12877-019-1386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Walker M. P., Ph D. The role of slow wave sleep in memory processing. J. Clin. Sleep Med. 2009;5(2 Suppl):S20-6. doi: 10.5664/jcsm.5.2S.S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Navarro-Sanchis C., Brock O., Winsky-Sommerer R., Thuret S. Modulation of adult hippocampal neurogenesis by sleep: Impact on mental health. Front. Neural Circuits. 2017;11(October):74. doi: 10.3389/fncir.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Perlis G, D.E., Mendelson W.B., Bootzin R.R., Wyatt J. Psychophysiological Insomnia: The behavioural model and a neurocognitive perspective. J. Sleep. Res. 1997:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 179.Alkadhi K., Zagaar M., Alhaider I., Salim S., Aleisa A. Neurobiological consequences of sleep deprivation. Curr. Neuropharmacol. 2013;11(3):231–249. doi: 10.2174/1570159X11311030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Déry N., Pilgrim M., Gibala M., Gillen J., Martin W.J., MacQueen G., Becker S. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front. Neurosci. 2013;7(7):1–15. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pereira A.C., Huddleston D.E., Brickman A.M., Sosunov A.A., Hen R., McKhann G.M., Sloan R., Gage F.H., Brown T.R., Small S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song D., Yu D.S.F., Li P.W.C., Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018;79(79):155–164. doi: 10.1016/j.ijnurstu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 183.Maddock R.J., Casazza G.A., Fernandez D.H., Maddock M.I. Acute modulation of cortical glutamate and GABA content by physical activity. J. Neurosci. 2016;36(8):2449–2457. doi: 10.1523/JNEUROSCI.3455-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Klempin F., Beis D., Mosienko V., Kempermann G., Bader M., Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J. Neurosci. 2013;33(19):8270–8275. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.So J.H., Huang C., Ge M., Cai G., Zhang L., Lu Y., Mu Y. Intense exercise promotes adult hippocampal neurogenesis but not spatial discrimination. Front. Cell. Neurosci. 2017;11:13. doi: 10.3389/fncel.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nokia M.S., Lensu S., Ahtiainen J.P., Johansson P.P., Koch L.G., Britton S.L., Kainulainen H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016;594(7):1855–1873. doi: 10.1113/JP271552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Inoue K., Hanaoka Y., Nishijima T., Okamoto M., Chang H., Saito T., Soya H. Long-term mild exercise training enhances hippocampus-dependent memory in rats. Int. J. Sports Med. 2015;36(4):280–285. doi: 10.1055/s-0034-1390465. [DOI] [PubMed] [Google Scholar]
- 188.Yau S.Y., Li A., Hoo R.L., Ching Y.P., Christie B.R., Lee T.M., Xu A., So K.F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA. 2014;111(44):15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Soya H., Nakamura T., Deocaris C.C., Kimpara A., Iimura M., Fujikawa T., Chang H., McEwen B.S., Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem. Biophys. Res. Commun. 2007;358(4):961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 190.Suwabe K., Hyodo K., Byun K., Ochi G., Fukuie T. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: evidence for memory flexibility in young adults. Sci. Rep. 2017:7–5140. doi: 10.1038/s41598-017-04850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chang Y.T., Chen Y.C., Wu C.W., Yu L., Chen H.I., Jen C.J., Kuo Y.M. Glucocorticoid signaling and exercise-induced downregulation of the mineralocorticoid receptor in the induction of adult mouse dentate neurogenesis by treadmill running. Psychoneuroendocrinology. 2008;33(9):1173–1182. doi: 10.1016/j.psyneuen.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 192.Okamoto M., Yamamura Y., Liu Y-F., Min-Chul L., Matsui T., Shima T., Soya M., Takahashi K., Soya S., McEwen B.S., Soya H. Hormetic effects by exercise on hippocampal neurogenesis with glucocorticoid signaling. Brain Plast. 2015;1(1):149–158. doi: 10.3233/BPL-150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Akers K.G., Chérasse Y., Fujita Y., Srinivasan S., Sakurai T., Sakaguchi M. Concise review: regulatory influence of sleep and epigenetics on adult hippocampal neurogenesis and cognitive and emotional function. Stem Cells. 2018;36(7):969–976. doi: 10.1002/stem.2815. [DOI] [PubMed] [Google Scholar]
- 194.Buzsáki G., Csicsvari J., Dragoi G., Harris K., Henze D., Hirase H. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cereb. Cortex. 2002;12(9):893–899. doi: 10.1093/cercor/12.9.893. [DOI] [PubMed] [Google Scholar]
- 195.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 197.Papazyan R., Zhang Y., Lazar M.A. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat. Struct. Mol. Biol. 2016;23(12):1045–1052. doi: 10.1038/nsmb.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mueller A.D., Pollock M.S., Lieblich S.E., Epp J.R., Galea L.A.M., Mistlberger R.E. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(5):R1693–R1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- 199.Prince T.M., Abel T. The impact of sleep loss on hippocampal function. Learn. Mem. 2013;20(10):558–569. doi: 10.1101/lm.031674.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mirescu C., Peters J.D., Noiman L., Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc. Natl. Acad. Sci. USA. 2006;103(50):19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lucassen P.J., Meerlo P., Naylor A.S., van Dam A.M., Dayer A.G., Fuchs E., Oomen C.A., Czéh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur. Neuropsychopharmacol. 2010;20(1):1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 202.Guzman-Marin R., Suntsova N., Methippara M., Greiffenstein R., Szymusiak R., McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur. J. Neurosci. 2005;22(8):2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 203.Guzman-Marin R., Suntsova N., Bashir T., Nienhuis R., Szymusiak R., McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31(2):167–175. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tung A., Takase L., Fornal C., Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience. 2005;134(3):721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 205.Nilsson E.K., Boström A.E., Mwinyi J., Schiöth H.B. Epigenomics of total acute sleep deprivation in relation to genome-wide DNA methylation profiles and RNA expression. OMICS. 2016;20(6):334–342. doi: 10.1089/omi.2016.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Joo E.Y., Kim H., Suh S., Hong S.B. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep (Basel) 2014;37(7):1189–1198. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Winkelman J.W., Benson K.L., Buxton O.M., Lyoo I.K., Yoon S., O’Connor S., Renshaw P.F. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11(6):576–582. doi: 10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 208.Spiegelhalder K., Regen W., Baglioni C., Klöppel S., Abdulkadir A., Hennig J., Nissen C., Riemann D., Feige B. Insomnia does not appear to be associated with substantial structural brain changes. Sleep (Basel) 2013;36(5):731–737. doi: 10.5665/sleep.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kempermann G. Adult neurogenesis: an evolutionary perspective. Cold Spring Harb. Perspect. Biol. 2015;8(2):a018986. doi: 10.1101/cshperspect.a018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schmid C.L., Kennedy N.M., Ross N.C., Lovell K.M., Yue Z., Morgenweck J., Cameron M.D., Bannister T.D., Bohn L.M. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171(5):1165–1175.e13. doi: 10.1016/j.cell.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wisler J.W., DeWire S.M., Whalen E.J., Violin J.D., Drake M.T., Ahn S., Shenoy S.K., Lefkowitz R.J. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. USA. 2007;104(42):16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]