Sarcopenia, the age‐related loss of skeletal muscle mass and function, is associated with increasing burden of frailty, disability, and mortality for our aging society. Nevertheless, the underlying cellular and molecular mechanisms and the role of life‐style factors are insufficiently understood. Exercise is one such factor and recent evidence supports the potential of strength training alone or combined with aerobic exercise to mitigate sarcopenia. 1 Gut microbiota are implicated in the development of muscle loss during aging as well, as recently systematically reviewed in this journal. 2 Consequently, the combination of appropriate exercise programmes and dietary interventions aimed at modifying gut microbiota hold great promise to counteract sarcopenia. However, the evaluation of such combined approaches led to ambiguous results, with one recent meta‐analysis supporting favourable effects on aging‐related sarcopenia 3 and another not. 4 Herein, we discuss potential reasons for those discrepancies, elucidate the complex interactions between exercise, gut microbiota and skeletal muscle health, and suggest appropriate intervention strategies to prevent aging‐related sarcopenia.
Type and dose of exercise in the prevention of aging‐related sarcopenia
Various exercise programmes, including single or combined exercise types, for example, aerobic and/or resistance exercise, have been demonstrated to reduce aging‐related sarcopenia in healthy and diseased people. 5 , 6 In obese individuals aged >64 years old, weight loss combined with both aerobic and resistance training (RT) most efficiently preserved lean mass, physical function, and reduced frailty. 7 This combination is likely the most promising strategy to maintain and/or improve muscle mass and strength. 1
Evidence‐based guidelines recommend the adoption of either a combination of RT and power training or high‐intensity interval training (HIIT) against age‐related sarcopenia. 8 , 9 , 10 Moderate to high (60% to 80% of 1‐RM) intensities are optimal for RT, while low to moderate (e.g. lighter loading and high movement velocity) intensities are preferable for power training to stimulate the speed component (≤60% of 1‐RM) for adults that are already physically strong as a prerequisite for power performance. 11 The volume of RT should be adapted to preexisting muscular fitness: from 3 to 6 sets per muscle group per week (for beginners) to a maximum of 10 sets per muscle group per week (for advanced) of 10–15 repetitions per set. Conversely, 85% to 95% of the maximum heart rate should be the target for HIIT, which should be performed for 4 × 4′ intervals or at intensities greater than peak aerobic capacity for 5 × 1′ intervals to improve muscle mass and physical function in a short time frame. 10 Exercise schedules need to be adapted to the individual performance status. Risks for power training include exaggerated exhausting, especially for sedentary older people, increased blood pressure, and during the first weeks of training, joint limitations, and pain. Regarding HIIT, untrained older people may be unable to adhere to the target heart rate. Despite the potential of exercise to counteract sarcopenia, poor adherence and lack of sufficient physical fitness may indeed represent the main barriers for the implementation of exercise programmes in older people. 2
Effects of exercise on gut microbiota
Growing evidence suggests that physical activity (including exercise) can trigger favourable changes in the qualitative and quantitative gut microbial composition and metabolic function, resulting in health benefits for the host. 12 These changes are independent of diet and may depend on type and intensity of exercise. 13
Athletes generally exhibit higher biodiversity and representation of bacterial taxa with anti‐inflammatory properties and capacity to synthetize short‐chain fatty acids (SCFAs) in their faecal microbiota than sedentary controls. 14 , 15 Compared with community‐dwelling older adults, master athletes display a more homogeneous composition of gut microbiota, which was associated with positive health benefits, such as psychological well‐being, most likely due to changes in the gut–brain axis. 16
Both aerobic training and RT also showed significant modifications of faecal microbiota composition after the implementation of an exercise programme in both younger and older individuals. 17 , 18 These modifications included an increased representation of Bifidobacteria and Faecalibacterium prausnitzii and were associated with higher stool levels of butyrate. 17 , 18 Bifidobacteria can positively modulate the host immunity through the up‐regulation of anti‐inflammatory cytokines, and T cell regulation, while SCFAs, and particularly butyrate, a microbial metabolite synthetized by F. prausnitzii among others, is a well‐known regulator of the host metabolic balance. 19 , 20 Interestingly, such changes were influenced by the pre‐existing obesity status, but were independent of diet, and rapidly disappeared after the exercise intervention. 17
The effects of exercise programmes on the aging gut microbiota are less clear, because in older individuals overweight, chronic inflammatory states, multimorbidity, and polypharmacy progressively can promote gut microbiota dysbiosis with increased representation of opportunistic pathogens. 21 , 22 In addition, exercise‐induced microbiota alterations seem to be more substantial in earlier life compared with later life. 23 However, recent findings from the American Gut Project revealed that chronic exercise benefits especially overweight elderly individuals by maintaining gut microbiota stability (composition and function). 24 Importantly, excessive exercise, for example, disproportionate to training levels, or exercise in hot environments, can induce unfavourable changes in gut microbiota composition and disrupt the gut mucosal barrier, resulting in a paradox pro‐inflammatory effect for the host. 25 , 26
Effects of gut microbiota on skeletal muscle and aging‐related sarcopenia
Several in vitro experiments and preclinical and clinical studies provide direct and indirect evidence for the interplay between gut microbiota and muscle mass. 27 , 28 Age‐related decline in muscle mass and function was suggested to be associated with a distinct gut microbiota composition towards dysbiosis. 29 , 30 The composition of gut microbiota has further been linked to obesity and various metabolic diseases, including type 2 diabetes. 31 , 32
The emerging concept of the gut‐muscle axis assumes a reciprocal effect between these organs. While the mechanistic underpinnings of gut and muscles interactions are still poorly understood, the influence of gut microbiota on the general regulation of the host metabolism is well established and a promising research field. The synthesis of SCFAs by gut microbiota, resulting from microbial metabolism of ingested plant fibres, is thought to be favourable for the host metabolism, including via increased insulin sensitivity, muscle anabolism and modulation of age‐related chronic inflammation. 19 These effects are particularly pronounced for butyrate and have a relevant influence on fuel availability and exercise capacity. 33 On the other hand, gut microbiota dysbiosis resulting from sedentary lifestyle and unhealthy dietary patterns can be associated with increased intestinal mucosa permeability and absorption of bacterial metabolites and endotoxins that promote low‐grade systemic inflammation and insulin resistance. 15 , 31 , 32
Animal experimentations indicate a bidirectional communication between gut and skeletal muscle and point out that gut microbiota is critical for optimal muscle function. 34 , 35 In fact, the presence of an intact gut microbiome is necessary for normal muscle adaptations to exercise 36 and to promote adequate dietary protein digestion and amino acid absorption, a critical processes to counteract sarcopenia‐associated muscle protein wasting. 37 , 38
A putative role of mitochondria in exercise–microbiota–muscle interactions
Mitochondrial dysfunction has emerged as a central factor in the pathogenesis of age‐related sarcopenia. 39 While the integral role of mitochondrial deficits in muscle degeneration 39 as well as the benefits of various types of exercise on skeletal muscle mitochondria 40 are widely accepted, it is still poorly understood whether exercise‐induced benefits on the muscle‐gut axis are also partially mediated via mitochondria.
Among the numerous direct effects of regular exercise on muscle mitochondria are improved energy metabolism, mitochondrial biogenesis, as well as antioxidative and immune capacities. 40 However, exercise also affects mitochondria in tissues remote from skeletal muscle, such as the brain 41 and possibly the gut. 42 How exercise‐induced muscle mitochondria benefits are communicated to (mitochondria in) other tissues is a topic of intense investigation and involves signalling via myo/mitokines, micro‐RNAs, and metabolites. 41
On the other hand, gut microbiota are increasingly recognized to also exert direct and indirect effects on mitochondria, 43 in particular during exercise. Mediators of these interactions may be SCFAs and secondary bile acids, but also gut hormones and redox or inflammatory signalling. A recent study demonstrated that germ‐free mice had atrophic skeletal muscles with impaired mitochondrial functions. 44 Transplantation of gut microbiota in these mice increased both skeletal muscle mass and mitochondrial function, supporting an important role of gut microbiota on skeletal muscle mitochondria. In horses, it was shown that specifically butyrate‐producing bacteria of the gut microbiome were involved in modulating mitochondria‐related gene expression, possibly impacting energy metabolism, oxidative stress, and inflammation. 45 Conversely, mitochondria may also modulate gut microbiota, via mechanisms including redox signalling, immune system activation and intestinal barrier function modulation. 42
In summary, although it is likely that mitochondria are involved in the interplay of exercise, skeletal muscle, and gut microbiota (Figure 1), more research is required to elucidate the multidirectional signalling between different tissues, mitochondrial populations (i.e. in the skeletal muscle and in gut tissues), and microbiota. Furthermore, growing evidence supports the notion that different exercise modalities (such as type, duration, frequency, and intensity) elicit differential benefits for muscle mitochondria. 40 It remains to be investigated, whether specific exercise recommendations to prevent age‐related sarcopenia differ in their effects on muscle (and potentially gut) mitochondria. In one recent study, HIIT increased markers of mitochondrial biogenesis, mitochondrial fusion, and mitophagy in obese older adults (Gouspillou et al. JCSM, in press) and acted synergistically with protein ingestion and L‐citrulline supplementation for increasing myocellular protein synthesis, muscle hypertrophy and strength. 46 Based on these promising results more comparative studies on the efficiency, mode of action and mediating role of mitochondria of different exercise regimes in combination with relevant dietary strategies are required.
Figure 1.
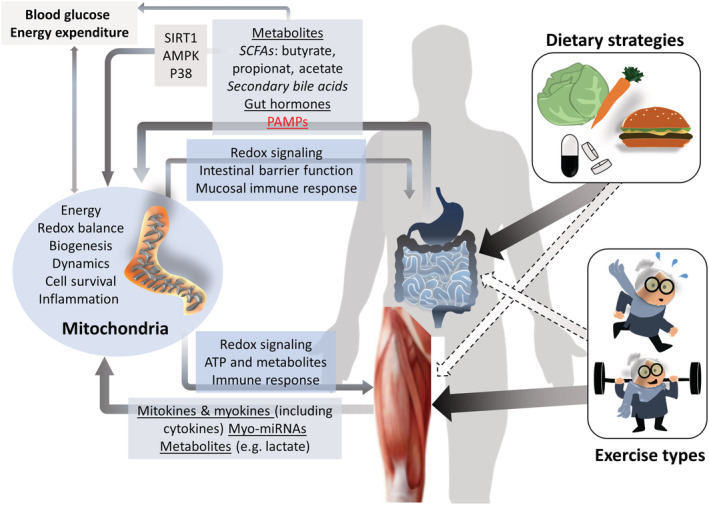
Potential mediation of the gut‐muscle axis by mitochondria. Metabolites and pathogen associated molecular patterns (PAMPs) are released by gut microbiota in response to dietary input and impact on host metabolism and mitochondria. Binding of short chain fatty acids (SCFAs) to G‐protein‐receptor coupled receptors on enteroendocrine L‐cells results in the secretion of metabolism‐modulating gut hormones. SCFAs also control mitochondrial biogenesis and ATP‐production and fatty acid oxidation via sirtuin 1 (SIRT1), AMP‐activated protein kinase (AMPK) and p38. Exercise induces the release of a variety of signalling molecules, including myo‐/mitokines, myo‐micro RNAs (myo‐miRNAs), many of which modulate mitochondrial functions. Mitochondria in turn regulate skeletal muscle and gut functions.
Dietary measures to support resistance training and gut microbiota
Although a systematic review does not support important benefits of dietary supplementation combined with exercise training in the prevention and treatment of sarcopenia in subjects aged 65 or older, 47 sophisticated nutritional strategies are expected to promote protein biosynthesis, growth, and/or maintenance of skeletal muscle 48 as well as favourable effects on gut microbiota. 21 While benefits of various dietary strategies—including protein, essential amino acid, polyunsaturated fatty acids, and antioxidant supplementation—on muscle mass and function in healthy elderly are widely accepted, the effects for frail populations are less clear. 2
An increase in protein intake is generally considered the cornerstone dietary measure for preventing and treating age‐related sarcopenia, in association with exercise. 49 However, a shift towards high‐protein diets can be associated with alterations of gut microbiota composition, and reduction of synthesis of important mediators of the gut‐muscle axis, such as SCFAs. 50 , 51 Such changes are exacerbated by a specifically increased ingestion of proteins of animal, and not vegetal, origin. 51 Notably, in contrast to moderate protein supplementation, a very high protein intake is not advantageous for muscle strength enhancement during RT and this is likely due to its effects on gut microbiome function. 52
Thus, microbiome‐centred dietary strategies to counteract age‐related sarcopenia should include not only moderate amino acid and protein supplementation but also balanced levels of polyunsaturated fatty acids, fibres, and antioxidants. 20 Recent data from the US National Health and Nutrition Examination Survey suggest that increasing dietary fibre intake towards recommended levels (∼28–34 g/day) is associated with improvements in muscle mass and strength in adults aged 40 years and older. 53 Plant and fibre‐rich dietary choices are in fact associated with a more diverse and compositionally distinct microbiota and with a greater potential to produce SCFAs. 54 The Mediterranean‐style diet fulfils these criteria and can indeed induce positive changes in gut microbiota composition and function that are associated with reduced frailty and improved physical performance. 55
Conclusions and future perspectives
The gut microbiota has emerged as a powerful modulator of musculoskeletal health and disease and exercise likely is an important mediator. Exercise is associated with increased microbiota biodiversity and favours, for example, butyrate‐producing taxa with beneficial metabolic functions, which may contribute to the benefits of regular physical activity on human health. Effective strategies aimed at counteracting age‐related sarcopenia should consider the effects of exercise and nutrition on the gut microbiota. RT and balanced dietary intake of proteins and fibres are the interventions with the highest potential of inducing favourable changes in the gut microbiota, through mediation of microbial metabolites including SCFAs that have a known modulatory effect on muscle anabolism and chronic inflammation.
Exercise training also improves muscle mitochondria functions, which in turn regulate skeletal muscle and possibly gut functions. Mechanistically, mitochondria likely are key players in exercise–microbiota–muscle interactions. They are essential in skeletal muscle function during and following exercise and both regulate and are regulated by the gut microbiome. How mitochondrial populations (e.g. in the gut and the muscle) communicate and which effects physical activity and exercise exert on the reciprocal interactions of gut mitochondria and microbiota requires more research.
In conclusion, dietary strategies have the potential to support exercise‐induced adaptations and prevent age‐related microbiota dysbiosis and thus may be effective against age‐related sarcopenia. However, the—potentially synergistic—interaction between dietary interventions and exercise programmes against sarcopenia is insufficiently understood, especially from a clinical point of view. 56 Traditionally, the investigation of dietary and exercise strategies on aging‐related factors in humans is complex and outcomes are determined by individual predispositions (genetic make‐up, general health status, dietary and physical activity habits, etc.) and variations to exercise adaptations and dietary interventions. Differences in microbiota likely are among these determining factors. Thus, future trials investigating the combination of nutritional and exercise interventions against age‐related sarcopenia should consider also gut microbiota composition and function among their endpoints, to disentangle the complex mechanisms of the gut‐muscle axis.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was financially supported by grants from the Austrian Federal Ministry of Education, Science and Research represented by the Austrian Research Promotion Agency (FFG) as part of the ERA‐Net Cofund HDHL‐INTIMIC (grant number BW000017276).
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for editorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 57
Burtscher J., Ticinesi A., Millet G. P., Burtscher M., and Strasser B. (2022) Exercise–microbiota interactions in aging‐related sarcopenia, Journal of Cachexia, Sarcopenia and Muscle, 13, 775–780, 10.1002/jcsm.12942
References
- 1. Barajas‐Galindo DE, González Arnáiz E, Ferrero Vicente P, Ballesteros‐Pomar MD. Effects of physical exercise in sarcopenia. A systematic review. Endocrinol Diabetes Nutr (Engl Ed). 2021;68:159–169. [DOI] [PubMed] [Google Scholar]
- 2. Liu C, Cheung WH, Li J, Chow SK, Yu J, Wong SH, et al. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle 2021. 10.1002/jcsm.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liao CD, Wu YT, Tsauo JY, Chen PR, Tu YK, Chen HC, et al. Effects of protein supplementation combined with exercise training on muscle mass and function in older adults with lower‐extremity osteoarthritis: a systematic review and meta‐analysis of randomized trials. Nutrients 2020;12. 10.3390/nu12082422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi M, Kim H, Bae J. Does the combination of resistance training and a nutritional intervention have a synergic effect on muscle mass, strength, and physical function in older adults? A systematic review and meta‐analysis. BMC Geriatr 2021;21:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen HT, Chung YC, Chen YJ, Ho SY, Wu HJ. Effects of different types of exercise on body composition, muscle strength, and IGF‐1 in the elderly with sarcopenic obesity. J Am Geriatr Soc 2017;65:827–832. [DOI] [PubMed] [Google Scholar]
- 6. Geirsdottir OG, Arnarson A, Ramel A, Briem K, Jonsson PV, Thorsdottir I. Muscular strength and physical function in elderly adults 6–18 months after a 12‐week resistance exercise program. Scand J Public Health 2015;43:76–82. [DOI] [PubMed] [Google Scholar]
- 7. Villareal DT, Waters DL, Qualls C. Exercise type in dieting obese older adults. N Engl J Med 2017;377:599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coelho‐Júnior HJ, Uchida MC, Picca A, Bernabei R, Landi F, Calvani R, et al. Evidence‐based recommendations for resistance and power training to prevent frailty in community‐dwellers. Aging Clin Exp Res 2021;33:2069–2086. [DOI] [PubMed] [Google Scholar]
- 9. Strasser B. Importance of assessing muscular fitness in secondary care. Front Genet 2020;11:583810. 10.3389/fgene.2020.583810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blackwell JEM, Gharahdaghi N, Brook MS, Watanabe S, Boereboom CL, Doleman B, et al. The physiological impact of high‐intensity interval training in octogenarians with comorbidities. J Cachexia Sarcopenia Muscle 2021;12:866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Askow AT, McKenna CF, Box AG, Khan NA, Petruzzello SJ, De Lisio M, et al. Of sound mind and body: exploring the diet‐strength interaction in healthy aging. Front Nutr 2020;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017;2017;3831972. 10.1155/2017/3831972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev 2019;47:75–85. [DOI] [PubMed] [Google Scholar]
- 14. Barton W, Penney NC, Cronin O, Garcia‐Perez I, Molloy MG, Holmes E, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018;67:625–633. [DOI] [PubMed] [Google Scholar]
- 15. Ticinesi A, Lauretani F, Tana C, Nouvenne A, Ridolo E, Meschi T. Exercise and immune system as modulators of intestinal microbiome: implications for the gut‐muscle axis hypothesis. Exerc Immunol Rev 2019;25:84–95. [PubMed] [Google Scholar]
- 16. Fart F, Rajan SK, Wall R, Rangel I, Ganda‐Mall JP, Tingö L, et al. Differences in gut microbiome composition between senior orienteering athletes and community‐dwelling older adults. Nutrients 2020;12. 10.3390/nu12092610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018;50:747–757. [DOI] [PubMed] [Google Scholar]
- 18. Erlandson KM, Liu J, Johnson R, Dillon S, Jankowski CM, Kroehl M, et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis 2021;8:20499361211027067. 10.1177/20499361211027067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes 2020;11:411–455. [DOI] [PubMed] [Google Scholar]
- 20. Prokopidis K, Witard OC. Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr Res Rev 2021;1–10, 10.1017/s0954422421000135 [DOI] [PubMed] [Google Scholar]
- 21. Strasser B, Wolters M, Weyh C, Krüger K, Ticinesi A. The effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrients 2021;13. 10.3390/nu13062045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep 2017;7:11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mika A, Van Treuren W, González A, Herrera JJ, Knight R, Fleshner M. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS One 2015;10:e0125889, 10.1371/journal.pone.0125889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu Q, Jiang S, Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiology 2020;9:e1053. 10.1002/mbo3.1053 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, et al. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol (1985) 2015;118:1059–1066. [DOI] [PubMed] [Google Scholar]
- 26. Chantler S, Griffiths A, Matu J, Davison G, Jones B, Deighton K. The effects of exercise on indirect markers of gut damage and permeability: a systematic review and meta‐analysis. Sports Med 2021;51:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaźmierczak‐Siedlecka K, Folwarski M, Skonieczna‐Żydecka K, Ruszkowski J, Makarewicz W. The use of Lactobacillus plantarum 299v (DSM 9843) in cancer patients receiving home enteral nutrition—study protocol for a randomized, double‐blind, and placebo‐controlled trial. Nutr J 2020;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen YM, Wei L, Chiu YS, Hsu YJ, Tsai TY, Wang MF, et al. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016;8:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ticinesi A, Mancabelli L, Tagliaferri S, Nouvenne A, Milani C, Del Rio D, et al. The gut‐muscle axis in older subjects with low muscle mass and performance: a proof of concept study exploring fecal microbiota composition and function with shotgun metagenomics sequencing. Int J Mol Sci 2020;21. 10.3390/ijms21238946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho‐Junior HJ, et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients 2019;12. 10.3390/nu12010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 32. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019;129:4050–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hawley JA. Microbiota and muscle highway—two way traffic. Nat Rev Endocrinol 2020;16:71–72. [DOI] [PubMed] [Google Scholar]
- 34. Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, et al. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab 2019;316:E956–e66. [DOI] [PubMed] [Google Scholar]
- 35. Nay K, Jollet M, Goustard B, Baati N, Vernus B, Pontones M, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab 2019;317:E158–e71. [DOI] [PubMed] [Google Scholar]
- 36. Valentino TR, Vechetti IJ, Jr. , Mobley CB, Dungan CM, Golden L, Goh J, et al. Dysbiosis of the gut microbiome impairs mouse skeletal muscle adaptation to exercise. J Physiol 2021;599:4845–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Marco Castro E, Murphy CH, Roche HM. Targeting the gut microbiota to improve dietary protein efficacy to mitigate sarcopenia. Front Nutr 2021;8:656730. 10.3389/fnut.2021.656730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ticinesi A, Tana C, Nouvenne A. The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care 2019;22:4–12. [DOI] [PubMed] [Google Scholar]
- 39. Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B. Role of age‐related mitochondrial dysfunction in sarcopenia. Int J Mol Sci 2020;21:5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burtscher J, Burtscher M, Millet GP. The central role of mitochondrial fitness on antiviral defenses: an advocacy for physical activity during the COVID‐19 pandemic. Redox Biol 2021;43:101976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burtscher J, Millet GP, Place N, Kayser B, Zanou N. The muscle‐brain axis and neurodegenerative diseases: the key role of mitochondria in exercise‐induced neuroprotection. Int J Mol Sci 2021;22. 10.3390/ijms22126479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol 2017;8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yardeni T, Tanes CE, Bittinger K, Mattei LM, Schaefer PM, Singh LN, et al. Host mitochondria influence gut microbiome diversity: a role for ROS. Sci Signal 2019;12. 10.1126/scisignal.aaw3159 [DOI] [PubMed] [Google Scholar]
- 44. Lahiri S, Kim H, Garcia‐Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 2019;11. 10.1126/scitranslmed.aan5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mach N, Moroldo M, Rau A, Lecardonnel J, Le Moyec L, Robert C, et al. Understanding the Holobiont: crosstalk between gut microbiota and mitochondria during long exercise in horse. Front Mol Biosci 2021;8:656204. 10.3389/fmolb.2021.656204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Callahan MJ, Parr EB, Hawley JA, Camera DM. Can high‐intensity interval training promote skeletal muscle anabolism? Sports Med. 2021;51:405–421. [DOI] [PubMed] [Google Scholar]
- 47. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McKendry J, Currier BS, Lim C, Mcleod JC, Thomas ACQ, Phillips SM. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients 2020;12. 10.3390/nu12072057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bauer J, Morley JE, Schols A, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. an SCWD position paper. J Cachexia Sarcopenia Muscle 2019;10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blachier F, Beaumont M, Portune KJ, Steuer N, Lan A, Audebert M, et al. High‐protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clin Nutr 2019;38:1012–1022. [DOI] [PubMed] [Google Scholar]
- 52. McKenna CF, Salvador AF, Hughes RL, Scaroni SE, Alamilla RA, Askow AT, et al. Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle‐aged adults: a randomized control trial. Am J Physiol Endocrinol Metab 2021;320:E900–e13. [DOI] [PubMed] [Google Scholar]
- 53. Frampton J, Murphy KG, Frost G, Chambers ES. Higher dietary fibre intake is associated with increased skeletal muscle mass and strength in adults aged 40 years and older. J Cachexia Sarcopenia Muscle 2021;2134–2144. 10.1002/jcsm.12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koponen KK, Salosensaari A, Ruuskanen MO, Havulinna AS, Männistö S, Jousilahti P, et al. Associations of healthy food choices with gut microbiota profiles. Am J Clin Nutr 2021;114:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU‐AGE 1‐year dietary intervention across five European countries. Gut 2020;69:1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silva R, Pizato N, da Mata F, Figueiredo A, Ito M, Pereira MG. Mediterranean diet and musculoskeletal‐functional outcomes in community‐dwelling older people: a systematic review and meta‐analysis. J Nutr Health Aging 2018;22:655–663. [DOI] [PubMed] [Google Scholar]
- 57. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019 . J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


