Abstract
Background
The impact of physiotherapy on insulin sensitivity and peripheral glucose metabolism in critically ill patients is not well understood.
Methods
This pooled analysis investigates the impact of different physiotherapeutic strategies on insulin sensitivity in critically ill patients. We pooled data from two previous trials in adult patients with sequential organ failure assessment score (SOFA)≥ 9 within 72 h of intensive care unit (ICU) admission, who received hyperinsulinaemic euglycaemic (HE) clamps. Patients were divided into three groups: standard physiotherapy (sPT, n = 22), protocol‐based physiotherapy (pPT, n = 8), and pPT with added muscle activating measures (pPT+, n = 20). Insulin sensitivity index (ISI) was determined by HE clamp. Muscle metabolites lactate, pyruvate, and glycerol were measured in the M. vastus lateralis via microdialysis during the HE clamp. Histochemical visualization of glucose transporter‐4 (GLUT4) translocation was performed in surgically extracted muscle biopsies. All data are reported as median (25th/75th percentile) (trial registry: ISRCTN77569430 and ISRCTN19392591/ethics approval: Charité‐EA2/061/06 and Charité‐EA2/041/10).
Results
Fifty critically ill patients (admission SOFA 13) showed markedly decreased ISIs on Day 17 (interquartile range) 0.029 (0.022/0.048) (mg/min/kg)/(mU/L) compared with healthy controls 0.103 (0.087/0.111), P < 0.001. ISI correlated with muscle strength measured by medical research council (MRC) score at first awakening (r = 0.383, P = 0.026) and at ICU discharge (r = 0.503, P = 0.002). Different physiotherapeutic strategies showed no effect on the ISI [sPT 0.029 (0.019/0.053) (mg/min/kg)/(mU/L) vs. pPT 0.026 (0.023/0.041) (mg/min/kg)/(mU/L) vs. pPT+ 0.029 (0.023/0.042) (mg/min/kg)/(mU/L); P = 0.919]. Regardless of the physiotherapeutic strategy metabolic flexibility was reduced. Relative change of lactate/pyruvate ratio during HE clamp is as follows: sPT 0.09 (−0.13/0.27) vs. pPT 0.07 (−0.16/0.31) vs. pPT+ −0.06 (−0.19/0.16), P = 0.729, and relative change of glycerol concentration: sPT −0.39 (−0.8/−0.12) vs. pPT −0.21 (−0.33/0.07) vs. pPT+ −0.21 (−0.44/−0.03), P = 0.257. The majority of ICU patients showed abnormal localization of GLUT4 with membranous GLUT4 distribution in 37.5% (3 of 8) of ICU patients receiving sPT, in 42.9% (3 of 7) of ICU patients receiving pPT, and in 53.8% (7 of 13) of ICU patients receiving pPT+ (no statistical testing possible).
Conclusions
Our data suggest that a higher duration of muscle activating measures had no impact on insulin sensitivity or metabolic flexibility in critically ill patients with sepsis‐related multiple organ failure.
Keywords: Critical illness, Multiple organ failure, Glucose clamp technique, Microdialysis, Stress hyperglycaemia, Protocol‐based physiotherapy
Background
Resistance to anabolic signals, such as insulin, result in protein breakdown and mortality‐relevant hyperglycaemia in critically ill patients. 1 , 2 While it is well established that exercise improves insulin sensitivity and metabolic flexibility in healthy individuals and patients with type I and II diabetes, 3 , 4 , 5 this finding has yet to be investigated in critically ill patients suffering from stress hyperglycaemia. In a post hoc analysis of an interventional trial, Patel et al. showed that patients with mechanical ventilation receiving early mobilization required a lower daily insulin dose to reach similar blood glucose targets than patients receiving standard care. The authors draw the conclusion that their findings indicate an improvement in insulin sensitivity. 6
However, there is yet no prospective study investigating the impact of physiotherapy and muscle activity on insulin sensitivity in critically ill patients. Our study is the first to address this issue by using hyperinsulinaemic euglycaemic (HE) clamp studies as gold standard for measuring peripheral insulin sensitivity. We also conducted microdialysis of the vastus lateralis muscle during HE clamp to measure the metabolic flexibility. Metabolic flexibility refers to the ability of the muscle cell to switch between fatty acids and glucose. 7 In healthy individuals, the response to the insulin signal is a considerable increase in pyruvate concentration and mild increase of lactate concentration resulting in a distinct decrease of lactate/pyruvate ratio. This indicates the switch to aerobic glycolysis. A sharp decline in glycerol concentration indicates a suppression of free fatty acid metabolism.
In this study, we investigated the impact of physiotherapy on insulin sensitivity and peripheral glucose metabolism in critically ill patients with sepsis‐related multiple organ failure. We hypothesized that an increase in the level of physiotherapy results in an improvement of insulin sensitivity in critically ill patients.
Methods
Inclusion criteria and setting
We analysed data from critically ill patients ≥18 years with sepsis‐related multiple organ failure in whom we conducted HE clamps in the third week of intensive care unit (ICU) stay. Sepsis‐related multiple organ failure was defined as SOFA ≥ 9 within the first 72 h of ICU admission. The data were pooled from two previously published monocentric trials after ensuring there were no differences in baseline patient characteristics by principal component analysis (see statistics). Enrolment for both included studies is described in the original reports; inclusion criteria did not differ in between these two studies. 8 , 9 , 10
All patients received usual care according to locally established clinical standard operating procedures. To provide a reference and further context for the results, HE clamp and tissue metabolism data were compared with measurements performed on four healthy subjects. 9
Intervention
We compared the impact of three different types of physiotherapeutic strategies on insulin sensitivity. (A) The first group of patients received standard physiotherapy (sPT) as per standard of care. (B) The second group received protocol‐based physiotherapy (pPT), and (C) the third group received pPT with additional early muscle activating measures (pPT+), such as whole body vibration and/or neuromuscular electrostimulation (see also flow chart, Supporting Information, Figure S1).
Measurements
Hyperinsulinaemic euglycaemic clamp
During HE clamp, supraphysiological insulin level and external glucose supply result in suppression of endogenous glucose production. The glucose infusion rate needed to keep a stable blood glucose level is equivalent to the tissue uptake of glucose. The calculation of the insulin sensitivity index (ISI) is recognized as the gold standard to determine peripheral insulin sensitivity (for details, see Supporting Information). ISI, as primary outcome, is calculated as the ratio of the steady state glucose infusion rate per body weight and the steady state plasma insulin concentration. 11 , 12
Tissue metabolite measurements by microdialysis
We performed microdialysis of the vastus lateralis muscle as previously described. 13 Concentrations of glucose, pyruvate, lactate, and glycerol, reflecting regulation of metabolism in skeletal muscle, were measured from the microdialysate at baseline and steady state of the HE clamp 8 , 13 and averaged over three measurements.
Muscle biopsy and glucose transporter‐4 analysis
Open surgical muscle biopsies were obtained on Median Day 15 of ICU stay, as described previously. 10 Glucose transporter‐4 (GLUT4) was stained immunohistochemically and its distribution within the muscle cell was classified by an expert as either normal (membranous) or abnormal (partly membranous or perinuclear/diffuse). 8
Mobility level
According to study protocol, the mobility level was measured once daily by the treating physiotherapist on a six‐step scale (Level 0: no mobilization, Level 1: passive mobilization in bed, Level 2: assistive mobilization to the edge of the bed, Level 3: assistive mobilization to a chair, Level 4: stepping next the bed, Level 5: walking >3 m). It is reported as a mean before HE clamp.
Statistical analysis
The data from two trials were pooled. 8 , 9 , 10 To detect differences in baseline characteristics, we performed a univariate analysis and then a principal component analysis. The following baseline characteristics were included: age, sex, height, weight, severity of illness (SOFA, SAPS II, and Acute Physiology And Chronic Health Evaluation [APACHE II]), dosage of received medication (insulin, norepinephrine, and nutrition), blood glucose level, sedation level (Richmond Agitation‐Sedation scale RASS), fraction of days in septic shock, and ICU day of the HE clamp (Table S1). Data were pooled for analysis after we showed no difference in baseline characteristics between the three interventional groups and a good overlap and lack of clustering of all three patient groups in the principal component analysis.
All metric and ordinal data are reported using median (25th/75th percentile); all categorical data are reported using count (percentage). Relative changes are calculated as (steady state − baseline)/baseline. Due to small sample size, non‐parametric tests were used. Differences between two groups are tested using the Mann–Whitney test, and between more than two groups the Kruskal–Wallis test. For related samples, we used the Wilcoxon test. Univariate analyses were performed using the Spearman test. For multivariate analysis, a linear regression was performed. Significance level is set at P < 0.05, due to the exploratory nature significance is reported unadjusted for multiple testing. All analyses were performed in IBM SPSS Statistics Version 25. Figures are generated using Sigma Plot Version 12.0.
Results
Patient population
We included n = 50 ICU patients suffering from sepsis‐related multiple organ failure with median SOFA score of 13 at admission and median Simplified Acute Physiology Score (SAPS II) score of 50.5. This was not quickly reversible as shown by a high median SOFA score before HE clamp and a high share of days in septic shock (Table 1). There was no difference in baseline characteristics between the three interventional groups (shown in Table S1). A detailed flow chart is presented in Figure S1.
Table 1.
Baseline characteristics
| Pooled data | |
|---|---|
| n = 50 | |
| Event leading to ICU admission | |
| ARDS/sepsis | 30 (60%) |
| Polytrauma | 13 (26%) |
| Neurological/others | 7 (14%) |
| Sex | |
| Male | 36 (72%) |
| Female | 14 (28%) |
| Age (years) | 58.5 (42/68) |
| Weight (kg) | 81.5 (75/95) |
| Height (m) | 1.75 (1.7/1.8) |
| Illness severity scoring at ICU admission | |
| SOFA | 13 (10/14) |
| SAPS2 | 50.5 (39/63) |
| APACHE II | 23 (18/28) |
| ICU admission to HE clamp | |
| ICU stay (days) | 17 (15/21) |
| Fraction of these days with septic shock (%) | 20 (10/50) |
| Norepinephrine a (μg/kg/min) | 0.01 (0.001/0.067) |
| SOFA a | 9.9 (8.4/12.1) |
| Fraction of these days receiving insulin (%) | 92 (63/100) |
| Insulin a (IU/days) | 43.1 (26.4/64.2) |
| Blood glucose level (mg/dL) | 132.1 (124.9/138.9) |
| Caloric intake a (kcal/kg PBW/day) | 19.1 (15.2/22) |
| RASS score | −2.5 (−3.4/−1.8) |
ARDS, acute respiratory distress syndrome; HE, hyperinsulinaemic euglycaemic; ICU, intensive care unit.
Table showing baseline characteristics of pooled data; categorical variables are presented as count (percentage); metric variables are presented as median (25th/75th percentile); shown baseline characteristics show no significant differences between observational and interventional trials (Table S1).
Mean before HE clamp.
We performed HE clamp on Median Day 17 (15/21) after ICU admission. Patients showed group differences in the amount of physiotherapeutic intervention before HE clamp, as shown in Table 2.
Table 2.
Dose of physiotherapy prior to hyperinsulinaemic euglycaemic (HE) clamp
| Daily physiotherapy before HE clamp | sPT | pPT | pPT+ | P |
|---|---|---|---|---|
| n = 22 | n = 8 | n = 20 | ||
| Mean duration of daily physiotherapy (min) | 12.1 (6.5/13.8) | 20.4 (17.3/23.8) | 21.7 (17.6/24.8) | 0.110 |
| Days receiving protocol‐based physiotherapy within interventional trial before HE clamp (days) | 0 | 13.5 (11.5/19.5) | 15 (12.5/19.5) | 0.823 a |
| Mean duration of added physiotherapeutic measures (whole body vibration and neuromuscular electrical stimulation) | 0 | 0 | 16.1 (11.2/19.0) | — |
| Total daily time of muscle activating measures (min) | 12.1 (6.5/13.8) | 20.4 (17.3/23.8) | 37.9 (29.6/46.0) | <0.001* |
Patients in the protocol‐based groups (pPT and pPT+) received a significantly higher dose of physiotherapy than patients in the standard physiotherapy group (sPT). When adding the time of whole body vibration and neuromuscular electrical stimulation, patients in the pPT+ group had significantly higher total time of muscle activating measures. All variables are presented as median (25th/75th percentile); P‐value determined by Kruskal–Wallis.
Significance calculated between protocol‐based physiotherapy (pPT) and protocol‐based physiotherapy combined with added measures group (pPT+) by Mann–Whitney U.
Significant differences.
Effect of physiotherapy on peripheral insulin sensitivity—hyperinsulinaemic euglycaemic clamp
We measured a markedly decreased ISI in all our patients compared with healthy subjects [all ICU patients 0.029 (0.022/0.048) (mg/min/kg)/(mU/L); healthy 0.103 (0.087/0.111) (mg/min/kg)/(mU/L); P < 0.001]. However, there was no effect of the different physiotherapeutic strategies (sPT, pPT, pPT+) on the ISI [sPT 0.029 (0.019/0.053) vs. pPT 0.026 (0.023/0.041) vs. pPT+ 0.029 (0.023/0.042); P = 0.919]. The duration of daily physiotherapeutic intervention did not correlate with the ISI (r = 0.134, P = 0.354), as seen in Figure 1.
Figure 1.
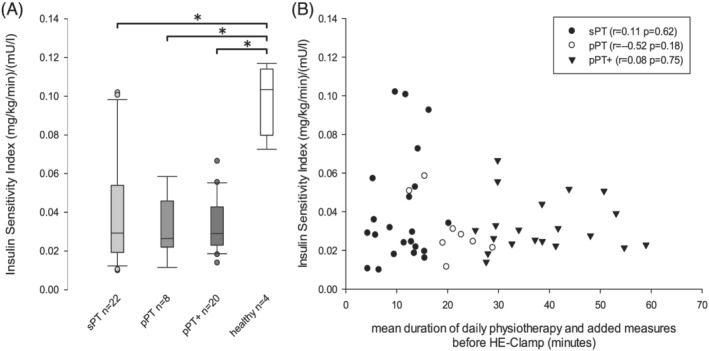
Scatterplot insulin sensitivity and time of daily physiotherapy. (A) (left) Boxplot depicting insulin sensitivity index measured by hyperinsulinaemic euglycaemic (HE) clamp in physiotherapeutic groups and healthy control. (B) (right) Scatterplot showing insulin sensitivity index against dose of daily physiotherapy and added muscle activating measures (electrical muscle stimulation, vibration therapy) respectively in critically ill sepsis patients. ‘pPT’, protocol‐based physiotherapy; ‘pPT+’, protocol‐based physiotherapy with additional muscle activating measures; ‘sPT’, standard physiotherapy. Significant group differences are indicated by asterisk.
In accordance with these results, we could not detect an influence of different levels of mobilization or of the time of muscle activating measures on required insulin dosage, mean blood glucose level, or achievement of glycaemic target as previously described by Patel and colleagues. 6 All three therapeutic groups showed similar dose of enteral and parenteral nutrition (Table S1).
Effect of physiotherapy on metabolic flexibility—measured by microdialysis of the vastus lateralis muscle during hyperinsulinaemic euglycaemic clamp
All baseline metabolite concentrations in our patients are comparable with healthy individuals.
During HE clamp, the healthy individuals showed a considerable increase of pyruvate concentration [relative change 1.84 (1.05/2.15)] and mild increase of lactate concentration [relative change 0.26 (0.14/0.4)] and a distinct decrease of lactate pyruvate ratio [relative change −0.52 (−0.56/−0.42)] and a decline of glycerol concentration of almost 75% [relative change −0.74 (−0.87/−0.58)] indicating an intact metabolic flexibility (Figure 2 and Table S3).
Figure 2.
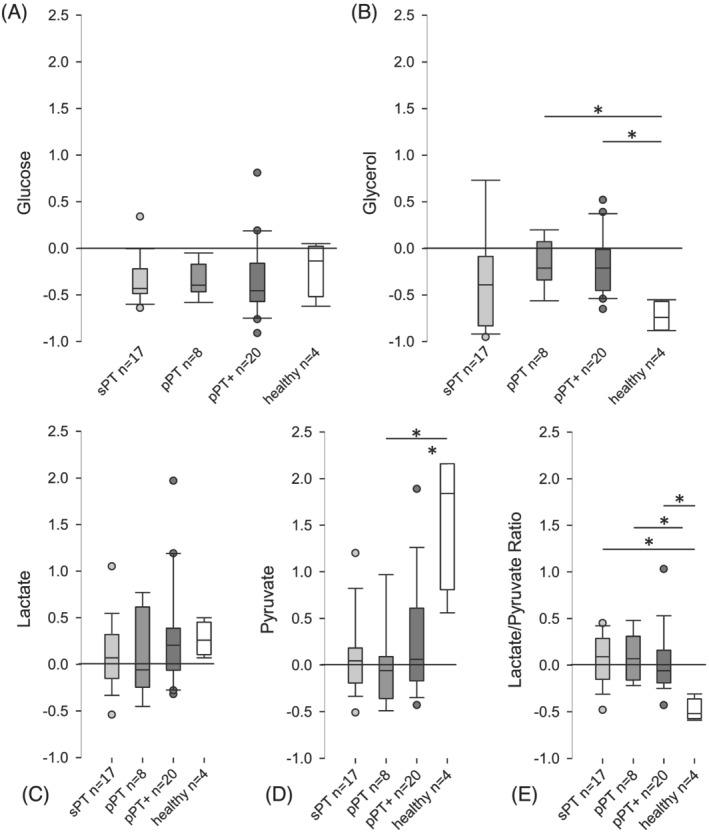
Relative changes of muscle metabolite concentrations during hyperinsulinaemic euglycaemic clamp. Boxplots showing relative changes of dialysate concentrations [(A) glucose, (B) glycerol, (C) lactate, (D) pyruvate, (E) lactate/pyruvate ratio] obtained by microdialysis of the vastus lateralis muscle during hyperinsulinaemic euglycaemic clamp. Relative changes are defined as the difference of steady state and baseline concentration, in relation to baseline concentration. Reference line shown at relative change of 0, indicating neither increase nor decrease of concentration (e.g. relative change of −0.50 is equal to a decrease by 50%). Grouped by physiotherapeutic intervention. ‘pPT’, protocol‐based physiotherapy; ‘pPT+’, protocol‐based physiotherapy with additional muscle activating measures; ‘sPT’, standard physiotherapy. Significant differences are indicated with an asterisk; significance is unadjusted for multiple testing due to exploratory nature of the analysis.
These changes in metabolite concentrations were almost absent in our patients, regardless of the therapeutic strategy they received (Figure 2). Relative changes in lactate concentration were small and showed no group difference [sPT 0.07 (−0.13/0.31) vs. pPT −0.06 (−0.22/0.59) vs. pPT+ 0.21 (−0.05/0.39); P = 0.669] as were changes in pyruvate concentration [sPT 0.05 (−0.18/0.17) vs. pPT −0.06 (−0.36/0.09) vs. pPT+ 0.06 (−0.17/0.61); P = 0.371]. This resulted in almost no changes of lactate/pyruvate ratio [sPT 0.09 (−0.13/0.27) vs. pPT 0.07 (−0.16/0.31) vs. pPT+ −0.06 (−0.19/0.16); P = 0.729].
The relative changes in glycerol concentration were also small and showed no group difference [sPT −0.39 (−0.8/−0.12) vs. pPT −0.21 (−0.33/0.07) vs. pPT+ −0.21 (−0.44/−0.03); P = 0.257]. No correlation of daily dose of physiotherapy and metabolic flexibility could be observed (Figure 2 and Table S3).
Association of dose of physiotherapy and level of mobility
We found a positive correlation between mean duration of physiotherapy including muscle activating measures and the mean level of mobilization prior HE clamp (r = 0.607, P < 0.001; Figure 3). The more time was put into physiotherapy and muscle activating measures, the higher the mean level of mobility before HE clamp was achieved. However, this failed to translate into higher muscle strength as the investigated population showed no association of physiotherapy dose and MRC score at discharge [MRC score: sPT 3.75 (3.44/4.38) vs. pPT 3.78 (2.88/4.00) vs. pPT+ 3.53 (2.06/4.00); P = 0.395, Kruskal–Wallis].
Figure 3.
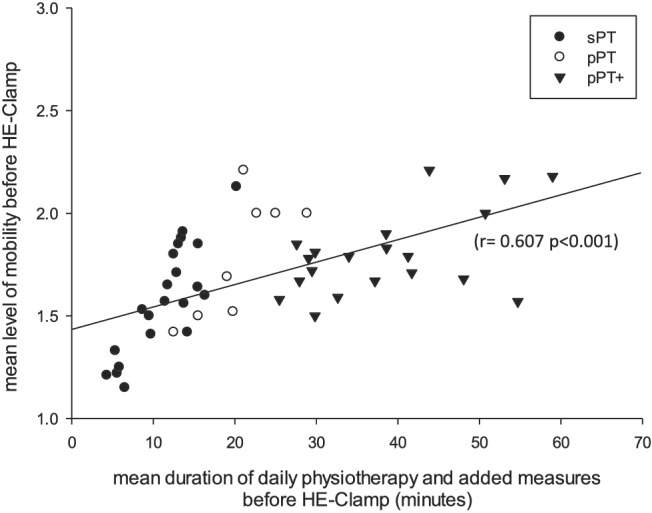
Mean duration of daily muscle activating measures and achieved mean level of physiotherapy divided by interventional groups. Scatter plot depicting mean level of physiotherapy measured by five‐step activity scale against duration of daily physiotherapy and added muscle activating measures such as electrical muscle stimulation or vibration therapy. Split up into the three therapeutic groups: ‘pPT’, protocol‐based physiotherapy; ‘pPT+’, protocol‐based physiotherapy with additional muscle activating measures; ‘sPT’, standard physiotherapy.
Association of insulin sensitivity and muscle strength
Insulin sensitivity index correlated with muscle strength measured by MRC score at first awakening (r = 0.383, P = 0.026) and at ICU discharge (r = 0.503, P = 0.002) (Figure 4). In the critically ill sepsis patients, ISI correlated inversely with age (Spearman: r = −0.444, P = 0.001) and body mass index (BMI) (Spearman: r = −0.285, P = 0.045). In addition, sex‐specific differences were observed. Female patients had a lower ISI than male patients [all female ICU patient: 0.024 (0.020/0.030) vs. male 0.032 (0.023/0.052); P = 0.026].
Figure 4.
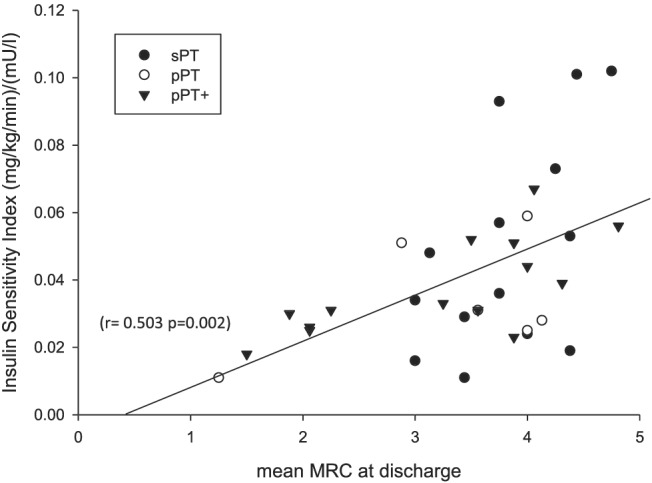
Association of mean MRC at discharge and insulin sensitivity index in critically ill sepsis patients with multiple organ failure. Scatterplot depicting insulin sensitivity index against mean MRC at discharge from ICU. ‘pPT’, protocol‐based physiotherapy; ‘pPT+’, protocol‐based physiotherapy with additional muscle activating measures; ‘sPT’, standard physiotherapy. Data are only available for those 34 patients, who regained adequate consciousness for muscle strength assessment and survived until discharge.
The multivariate analysis revealed ISI as an independent risk factor for muscle weakness (std. coefficient β = 0.484, P = 0.034). This correlation was independent of age, BMI, sex, SOFA score, insulin dosage, enteral nutrition, and time of physiotherapy in a multivariate regression (Table S4).
Immunohistochemical analysis
The immunohistochemical analysis of the muscle biopsies showed abnormally localized GLUT4 in almost all critically ill patients. There was no relevant impact of the amount of early muscle activating measures on GLUT4 distribution as shown in Table 3.
Table 3.
Glucose transporter‐4 (GLUT4) location in histochemical analysis
| GLUT4 location in histochemical analysis |
sPT n = 8 |
pPT n = 7 |
pPT+ n = 13 |
|---|---|---|---|
| Abnormal, nuclear or diffuse | 5 (62.5%) | 4 (57.1%) | 6 (46.2%) |
| Abnormal, partly membranous | 3 (37.5%) | 2 (28.6%) | 3 (23.1%) |
| Normal | 0 | 1 (14.2%) | 4 (30.7%) |
Distribution of GLUT4 location within the cell after immunohistochemical staining. Semiquantitative assessment of the histochemical staining. This finding is mainly observational, as no statistical significance could possibly be reached because of low sample numbers. Variables are presented as count (percentage). No significant group difference due to small sample number possible.
Discussion
This paper is the first to investigate the impact of physiotherapy dosage on insulin sensitivity measured by the gold standard HE clamp in patients with sepsis‐related multiple organ failure. We used the time‐consuming and invasive measurement of the HE clamp because surrogate indices such as HOMA or QUICKI do not sufficiently correlate with ISI and thus cannot be adequately used in critically ill patients. 12
As expected from our previous work, 8 , 9 we observed a highly reduced insulin sensitivity during HE clamp in all of our patients. In line with our previous work, a reduced ISI was associated with the occurrence of intensive care unit‐acquired weakness (ICUAW). Somewhat unexpected and in conflict with our previous work, we could not observe an improvement of insulin sensitivity and metabolic flexibility as a result of intensified physiotherapy and early mobilization in this prospectively investigated patient cohort.
For muscular glucose metabolization, a complex chain of mechanisms is necessary. It is best sorted into the three steps of supply, transmembraneous transport, and metabolism. 14 Results from microdialysis in our patients implicate a sufficient muscle perfusion and supply of glucose and oxygen to the muscle, as baseline interstitial glucose, lactate and pyruvate concentration, as well as lactate/pyruvate ratio were comparable with healthy controls. Our data are limited to the resting muscle, as we did not measure metabolite levels during exercise, when negative effects of sepsis‐related microcirculatory disturbance may become more severe due to higher metabolic activity.
Transmembraneous glucose uptake occurs through facilitated diffusion via GLUT4 located at the sarcolemmal membrane. There are two distinct mechanisms regulating GLUT4 translocation from intracellular vesicles to the cell membrane: one insulin‐dependent mechanism by activation of the insulin signalling cascade 15 and one insulin‐independent mechanism induced by muscle activation and mediated by AMPK and AS160 or calmodulin. However, the exact mechanism and the proportion of impact of key proteins remain uncertain. 15 , 16 , 17 , 18 It is established that this is one rate limiting step of glucose uptake. 19
In our previous work in critically ill patients, we showed a failure of the insulin‐dependent translocation of GLUT4 to the sarcolemmal membrane with GLUT4 remaining in the perinuclear region. In five patients within a pilot trial of severe H1N1 with multiple organ failure and a less prolonged course of sepsis activation of the muscle by electrical muscle stimulation led to a relocation of GLUT4 to the sarcolemmal membrane indicating the insulin‐independent regulation could be restored. 8 In this prospective randomized design in critically ill patients with sepsis‐related multiple organ failure, we could not reproduce this finding. However, a normal distribution of GLUT4 could only be observed in pPT and pPT+ groups indicating that intensifying muscle activation in critically ill patients may have an effect on the capacity of transmembraneous glucose uptake in the skeletal muscle. We hypothesize that this patient cohort with prolonged severe sepsis and a more intense and longer lasting cytokine effect on the muscle cell showed a more severe form of GLUT4 translocation failure than the patients with single organ failure we previously described.
The last step is the intracellular metabolism: the physiological reaction of the skeletal muscle to the high insulin and glucose concentration under HE clamp is a significant rise in lactate and pyruvate level with a concomitant decrease of glycerol concentration in the microdialysate of skeletal muscle as shown in our healthy controls. In our patient cohort with sepsis‐related multiple organ failure, this pattern of metabolic reactions was absent. This absence was independent from the dose of physiotherapy and muscle activating measures the patients received. This metabolic inflexibility is understood to be one of the most important mechanisms underlying obesity and type 2 diabetes. 20 A central hypothesis for the development of ICUAW is that decreased insulin sensitivity leads to a bioenergetics failure within the muscle cell. 21 It stands to reason that the metabolic inflexibility and restriction in glucose uptake due to limits of intracellular metabolism are part of this bioenergetic failure in severe sepsis.
It has to be pointed out that neither the glucose uptake nor the intracellular metabolic flexibility could be identified as the main defect of glucose metabolism in our patients. Both are shown to be impaired and neither could be mitigated by muscle activation, even when additional muscle activating measures were added to the therapeutic regimen.
Our findings need to be discussed in consideration of other literature investigating the impact of early mobilization in critically ill patients. Most of these trials do not report percentage of patients receiving insulin or daily insulin dose at all. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Interestingly, two influential trials on early mobilization, which did not primarily focus on glycaemic control, showed no impact of mobilization on insulin therapy within their interventional groups. 35 , 36
One post hoc analysis by Patel et al., 6 however, postulated that early mobilization may have led to an improvement of insulin sensitivity in critically ill patients. This was however a post hoc analysis of clinical routine data. The authors neither measured insulin sensitivity, for example, by using the gold standard of HE clamp, or surrogates nor measured metabolic flexibility on tissue level. Besides these methodological differences, the severity of illness or prolonged sepsis of the patients reported here may also explain the divergence of the results. In comparison, our patients had a higher incidence of sepsis with higher insulin dosages and a more prolonged course of critical illness, with more than twice the days on a ventilator.
Hence, it may be hypothesized that the effectiveness of mobilization to mitigate insulin resistance may be dependent on the severity of sepsis or the duration of the phase with high severity of sepsis and resulting cytokine storm.
Limitations
Most importantly, our patient collective is limited to severe sepsis with all patients suffering from multiple organ failure; critically ill patients with only single organ failure were not included. A significant share of ICU patients is not represented in this investigation. This may explain the incongruence with results published previously.
Several other limitations have to be addressed to put the results of this investigation into perspective; some of these are inherent to the field of ICU research. The number of patients included is small and these are split up into three groups only allowing an exploratory view on the results. The HE clamp and concomitant microdialysis cannot indicate the mechanisms behind insulin resistance in skeletal muscle tissue. No measurement of muscle perfusion or microcirculation was applicable at the time of the study, with the disruption of sepsis on microcirculation and tissue perfusion; a measure to quantify differences in perfusion and resulting differences in glucose supply would be highly beneficial. Furthermore, the high insulin levels during HE clamp may lead to a temporary increase in tissue perfusion, due to insulin‐mediated capillary recruitment. This may lead to a distortion of results. All of these aspects will need to be addressed in future trials.
Conclusions
In our study population of critically ill patients in sepsis‐related multiple organ failure, the severity of insulin resistance was associated with reduced muscle strength at first awakening and ICU discharge. A physiotherapeutic regimen including pPT and early muscle activating measures lead to a higher dose of physiotherapy delivered to these critically ill patients and a higher mean level of mobilization. However, the higher level of physiotherapy had no impact on peripheral insulin sensitivity or metabolic flexibility on muscle tissue level in these patients. The mechanism behind this finding seems to be a combination of transmembraneous glucose uptake and intracellular metabolism. Which of these two aspects precedes the other or has a stronger clinical implication cannot be determined by our work and needs further detailed investigation.
As we can only report on sepsis patients with multiple organ failure, authors of future studies investigating the effect of early mobilization in other critically ill patients should feel encouraged either to report on insulin dosage in their patients or to measure insulin blood levels and report QUICKI or HOMA, to gain further insight into the effect of early mobilization on insulin sensitivity.
These findings suggest that during severe sepsis, physiotherapy has a low impact in the prevention of insulin resistance and metabolic inflexibility. Clinicians should start these preventive measures as early as possible, before multiple organ failure can develop to achieve the highest effect possible.
Conflict of interest
The authors Niklas M. Carbon, Lilian J. Engelhardt, Tobias Wollersheim, Julius J. Grunow, Claudia D. Spies, Sven Märdian, Knut Mai, Joachim Spranger, and Steffen Weber‐Carstens declare that they have no conflict of interest.
Funding
The study personnel received funding from Deutsche Forschungsgemeinschaft within the Project ‘Critical illness myopathy and timely electrical muscle stimulation’, project number 34181657.
Niklas M. Carbon and Steffen Weber‐Carstens are participants in the Activity Project—Avoiding long term ventilation and immobility, funded by Dräger (Drägerwerk AG & Co. KGaA, Moislinger Allee 53–55, Lübeck 23558).
Tobias Wollersheim is participant in the BIH‐Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Julius J. Grunow is participant in the BIH‐Charité Junior Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Supporting information
Figure S1: Study enrollment scheme for observational and interventional trials.
Figure S2: Principal Component Analysis.
Table S1: Baseline characteristics.
Table S2: Hyperinsulinemic Euglycemic Clamp Setup.
Table S3: Microdialysis of the m. vastus lateralis during HE‐Clamp.
Table S4: Impact of predictors on Strength measured by MRC Score at discharge ‐ Results of the linear Regression Analysis.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 37
Carbon N. M., Engelhardt L. J., Wollersheim T., Grunow J. J., Spies C. D., Märdian S., Mai K., Spranger J., and Weber‐Carstens S. (2022) Impact of protocol‐based physiotherapy on insulin sensitivity and peripheral glucose metabolism in critically ill patients, Journal of Cachexia, Sarcopenia and Muscle, 13, 1045–1053, 10.1002/jcsm.12920
References
- 1. Preiser J‐C, Ichai C, Orban J‐C, Groeneveld ABJ. Metabolic response to the stress of critical illness. BJA Br J Anaesth 2014;113:945–954. [DOI] [PubMed] [Google Scholar]
- 2. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003;78:1471–1478. [DOI] [PubMed] [Google Scholar]
- 3. Landt KW, Campaigne BN, James FW, Sperling MA. Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care 1985;8:461–465. [DOI] [PubMed] [Google Scholar]
- 4. Castaneda C, Layne JE, Munoz‐Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002;25:2335–2341. [DOI] [PubMed] [Google Scholar]
- 5. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003;26:2977–2982. [DOI] [PubMed] [Google Scholar]
- 6. Patel BK, Pohlman AS, Hall JB, Kress JP. Impact of early mobilization on glycemic control and ICU‐acquired weakness in critically ill patients who are mechanically ventilated. Chest 2014;146:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdul‐Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010;2010:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber‐Carstens S, Schneider J, Wollersheim T, Assmann A, Bierbrauer J, Marg A, et al. Critical illness myopathy and GLUT4: significance of insulin and muscle contraction. Am J Respir Crit Care Med 2013;187:387–396. [DOI] [PubMed] [Google Scholar]
- 9. Wollersheim T, Woehlecke J, Krebs M, Hamati J, Lodka D, Luther‐Schroeder A, et al. Dynamics of myosin degradation in intensive care unit‐acquired weakness during severe critical illness. Intensive Care Med 2014;40:528–538. [DOI] [PubMed] [Google Scholar]
- 10. Wollersheim T, Grunow JJ, Carbon NM, Haas K, Malleike J, Ramme SF, et al. Muscle wasting and function after muscle activation and early protocol‐based physiotherapy: an explorative trial. J Cachexia Sarcopenia Muscle 2019;10:734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol‐Endocrinol Metab 1979;237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 12. Holzinger U, Kitzberger R, Fuhrmann V, Funk G‐C, Madl C, Ratheiser K. Correlation of calculated indices of insulin resistance (QUICKI and HOMA) with the euglycaemic hyperinsulinaemic clamp technique for evaluating insulin resistance in critically ill patients. Eur J Anaesthesiol 2007;24:966–970. [DOI] [PubMed] [Google Scholar]
- 13. Boschmann M, Engeli S, Moro C, Luedtke A, Adams F, Gorzelniak K, et al. LMNA mutations, skeletal muscle lipid metabolism, and insulin resistance. J Clin Endocrinol Metab 2010;95:1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013;93:993–1017. [DOI] [PubMed] [Google Scholar]
- 15. Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 2007;5:237–252. [DOI] [PubMed] [Google Scholar]
- 16. Garetto LP, Richter EA, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise in the rat: the two phases. Am J Physiol‐Endocrinol Metab 1984;246:E471–E475. [DOI] [PubMed] [Google Scholar]
- 17. Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci 1995;92:5817–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holloszy JO. Exercise‐induced increase in muscle insulin sensitivity. J Appl Physiol 2005;99:338–343. [DOI] [PubMed] [Google Scholar]
- 19. Kubo K, Foley JE. Rate‐limiting steps for insulin‐mediated glucose uptake into perfused rat hindlimb. Am J Physiol‐Endocrinol Metab 1986;250:E100–E102. [DOI] [PubMed] [Google Scholar]
- 20. Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed‐rest studies. J Appl Physiol 2011;111:1201–1210. [DOI] [PubMed] [Google Scholar]
- 21. Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf‐Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. The Lancet 2005;365:53–59. [DOI] [PubMed] [Google Scholar]
- 22. TEAM Study Investigators , Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, et al. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi‐national, multi‐centre, prospective cohort study. Crit Care 2015;19:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med 2005;33:2259–2265. [DOI] [PubMed] [Google Scholar]
- 24. Cheung AM, Tansey CM, Tomlinson G, Diaz‐Granados N, Matté A, Barr A, et al. Two‐year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:538–544. [DOI] [PubMed] [Google Scholar]
- 25. Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 2010;91:536–542. [DOI] [PubMed] [Google Scholar]
- 26. Pohlman MC, Schweickert WD, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med 2010;38:2089–2094. [DOI] [PubMed] [Google Scholar]
- 27. Winkelman C, Johnson KD, Hejal R, Gordon NH, Rowbottom J, Daly J, et al. Examining the positive effects of exercise in intubated adults in ICU: a prospective repeated measures clinical study. Intensive Crit Care Nurs Off J Br Assoc Crit Care Nurses 2012;28:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dinglas VD, Colantuoni E, Ciesla N, Mendez‐Tellez PA, Shanholtz C, Needham DM. Occupational therapy for patients with acute lung injury: factors associated with time to first intervention in the intensive care unit. Am J Occup Ther Off Publ Am Occup Ther Assoc 2013;67:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinglas VD, Parker AM, Reddy DRS, Colantuoni E, Zanni JM, Turnbull AE, et al. A quality improvement project sustainably decreased time to onset of active physical therapy intervention in patients with acute lung injury. Ann Am Thorac Soc 2014;11:1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrold ME, Salisbury LG, Webb SA, Allison GT, Australia and Scotland ICU Physiotherapy Collaboration . Early mobilisation in intensive care units in Australia and Scotland: a prospective, observational cohort study examining mobilisation practises and barriers. Crit Care 2015;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hickmann CE, Castanares‐Zapatero D, Bialais E, Dugernier J, Tordeur A, Colmant L, et al. Teamwork enables high level of early mobilization in critically ill patients. Ann Intensive Care 2016;6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaller SJ, Anstey M, Blobner M, Edrich T, Grabitz SD, Gradwohl‐Matis I, et al. Early, goal‐directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet Lond Engl 2016;388:1377–1388. [DOI] [PubMed] [Google Scholar]
- 33. Weeks A, Campbell C, Rajendram P, Shi W, Voigt L. A descriptive report of early mobilization for critically ill ventilated patients with cancer. Rehabil Oncol Am Phys Ther Assoc Oncol Sect 2017;35:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McWilliams D, Jones C, Atkins G, Hodson J, Whitehouse T, Veenith T, et al. Earlier and enhanced rehabilitation of mechanically ventilated patients in critical care: a feasibility randomised controlled trial. J Crit Care 2018;44:407–412. [DOI] [PubMed] [Google Scholar]
- 35. Morris P, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure*. Crit Care Med 2008;36:2238–2243. [DOI] [PubMed] [Google Scholar]
- 36. Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Study enrollment scheme for observational and interventional trials.
Figure S2: Principal Component Analysis.
Table S1: Baseline characteristics.
Table S2: Hyperinsulinemic Euglycemic Clamp Setup.
Table S3: Microdialysis of the m. vastus lateralis during HE‐Clamp.
Table S4: Impact of predictors on Strength measured by MRC Score at discharge ‐ Results of the linear Regression Analysis.


